Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
86results about How to "Induce immune response" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for inducing mucosal humoral and cell-mediated immune responses by sublingual administration of antigens
InactiveUS20080112974A1Enhance immune responseInduce immune responseBacterial antigen ingredientsViral antigen ingredientsCell-mediated immune responsePathogen
Described are methods for inducing both a mucosal and a systemic immune response in the respiratory, digestive or urogenital tracts of a mammal to a microbial pathogen. The methods comprise topically administering onto the sublingual mucosa of the mammal an amount of an antigen effective to induce the mucosal and systemic immune responses and a pharmaceutically acceptable carrier or diluent. Pharmaceutical formulations and dosage forms for immunizing a mammal against a microbial pathogen to elicit a mucosal and systemic immune response in the respiratory, digestive or urogenital tracts are also described.
Owner:DUOTOL
Modified free-living microbes, vaccine compositions and methods of use thereof
InactiveUS20080248066A1Reduce microbesReduce spreadBacterial antigen ingredientsBacteriaHeterologous AntigensMicroorganism
Free-living microbes are provided in which the nucleic acid has been modified so that the microbe is attenuated for proliferation and / or which comprise genetic mutations that attenuate the ability of the microbe to repair its nucleic acid. Methods of using the modified microbes for the loading, activation, and / or maturation of antigen-presenting cells are also provided. Vaccine compositions comprising the modified microbes and / or the antigen-presenting cells and methods of using the vaccines are also provided. The microbes may be further modified to include heterologous antigens, such as tumor antigens or infectious disease antigens, for use as a vaccine against cancer or infectious diseases.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
H3 equine influenza A virus
ActiveUS20060153871A1Efficient yieldPrevent and inhibit infectionSsRNA viruses negative-senseMicroorganismsEquine influenza virusGene
The invention provides an isolated H3 equine influenza A virus, as well as methods of preparing and using the virus, and genes or proteins thereof.
Owner:WISCONSIN ALUMNI RES FOUND
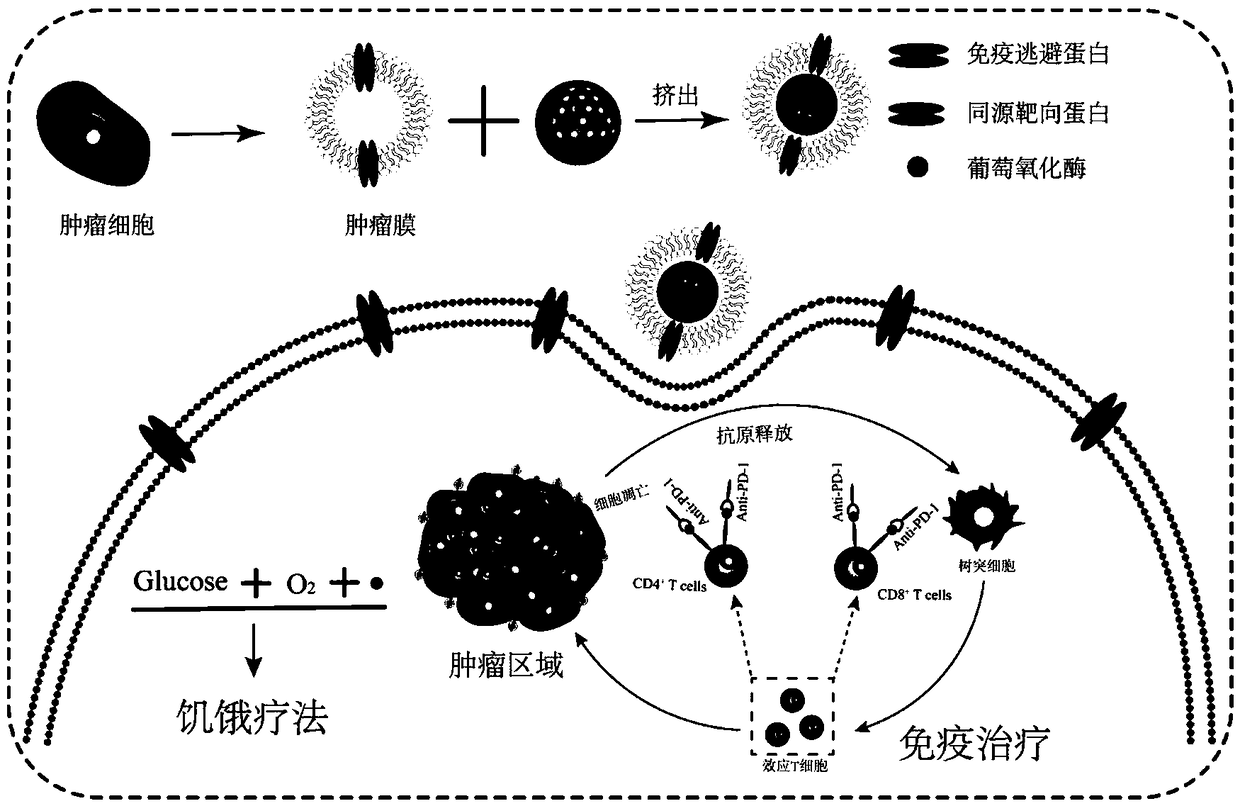
Tumor cell membrane coated nanometer material, method for preparing same and application of tumor cell membrane coated nanometer material
ActiveCN109078176AImprove enrichment capacityInhibit tumor growthPeptide/protein ingredientsInorganic non-active ingredientsBiocompatibility TestingBiomimetic materials
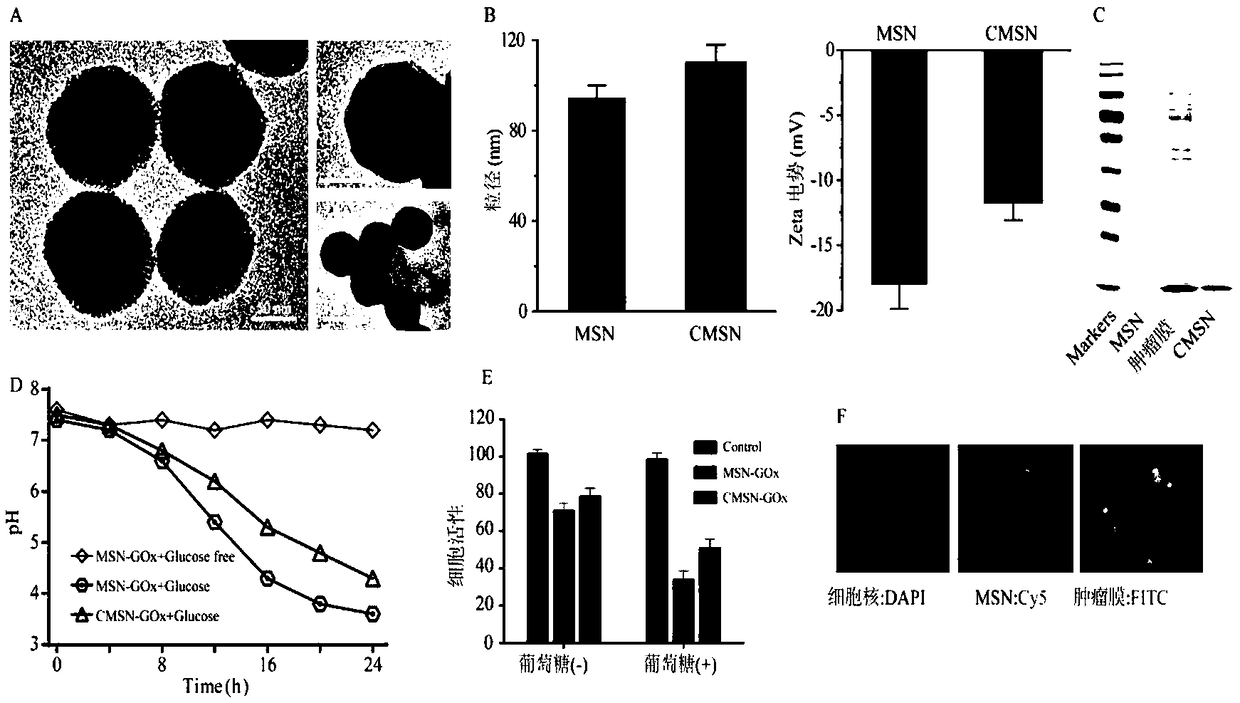
The invention discloses a tumor cell membrane coated nanometer material, a method for preparing the same and application of the tumor cell membrane coated nanometer material, and belongs to the fieldof nanometer materials. The tumor cell membrane coated nanometer material is particularly a tumor site targeting bionic nanometer material. Cancer cell membranes are coated on the surfaces of mesoporous silicon dioxide nanometer particles (MSN), and glucose oxidase (GOx) is loaded on the MSN, so that hunger treatment can be carried out. The tumor cell membrane coated nanometer material, the methodand the application have the advantages that the surfaces of bionic materials are functionalized, CMSN-GOx has immune escape and homologous targeting capability, and accordingly enrichment of nanometer particles on tumor sites can be obviously improved; tumor can be partially ablated by the prepared CMSN-GOx nanometer particles, and certain antitumor immune response can be generated by means of tumor membrane induction; the tumor can be effectively ablated by the aid of hunger therapy by the CMSN-GOx combined with PD-1 immunotherapy as compared with injection of only PD-1 immunosupressant orthe CMSN-GOx, and adaptive immune response can be induced; the tumor cell membrane coated nanometer material is excellent in biocompatibility and has great clinical application potential.
Owner:WUHAN UNIV
Modified free-living microbes, vaccine compositions and methods of use thereof
ActiveUS7833775B2Reduce microbesReduce proliferationBacterial antigen ingredientsNervous disorderTumour-associated antigenGene mutation
Free-living microbes are provided in which the nucleic acid has been modified so that the microbe is attenuated for proliferation and / or which comprise genetic mutations that attenuate the ability of the microbe to repair its nucleic acid. Methods of using the modified microbes for the loading, activation, and / or maturation of antigen-presenting cells are also provided. Vaccine compositions comprising the modified microbes and / or the antigen-presenting cells and methods of using the vaccines are also provided. The microbes may be further modified to include heterologous antigens, such as tumor antigens or infectious disease antigens, for use as a vaccine against cancer or infectious diseases.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
Novel vaccine for preventing COVID-19 and preparation method thereof
ActiveCN111939250AHighly conservativeAntibody induction ability is weakSsRNA viruses positive-senseAntibody mimetics/scaffoldsNucleotideReceptor
Provided is a novel vaccine for preventing COVID-19, the nucleotide sequence of an antigen of the novel vaccine is SEQ NO: 1, the amino acid sequence of the antigen of the novel vaccine is SEQ NO: 2,and the antigen of the vaccine comprises two functional parts: an S protein receptor binding structural domain capable of inducing a specific neutralizing antibody and a T cell related N protein truncated peptide fragment capable of inducing and activating effector T cells; The vaccine disclosed by the invention has the characteristics that the T cell related N protein truncated peptide fragment has weak capability of inducing the generation of the N protein antibody, so that a vaccine inoculator and a COVID-19 infected patient can be identified by using the N protein antibody, and the vaccineantigen does not induce the generation of the N protein antibody, so that lung injuries can be reduced, and the vaccine is safer. The cell vaccine disclosed by the invention is low in manufacturing cost, and can induce generation of virus-specific neutralizing antibodies and T cell immune response.
Owner:ZHENGZHOU UNIV
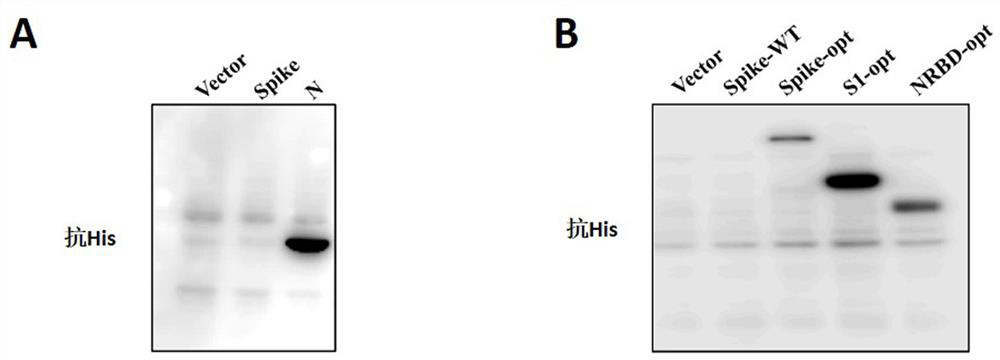
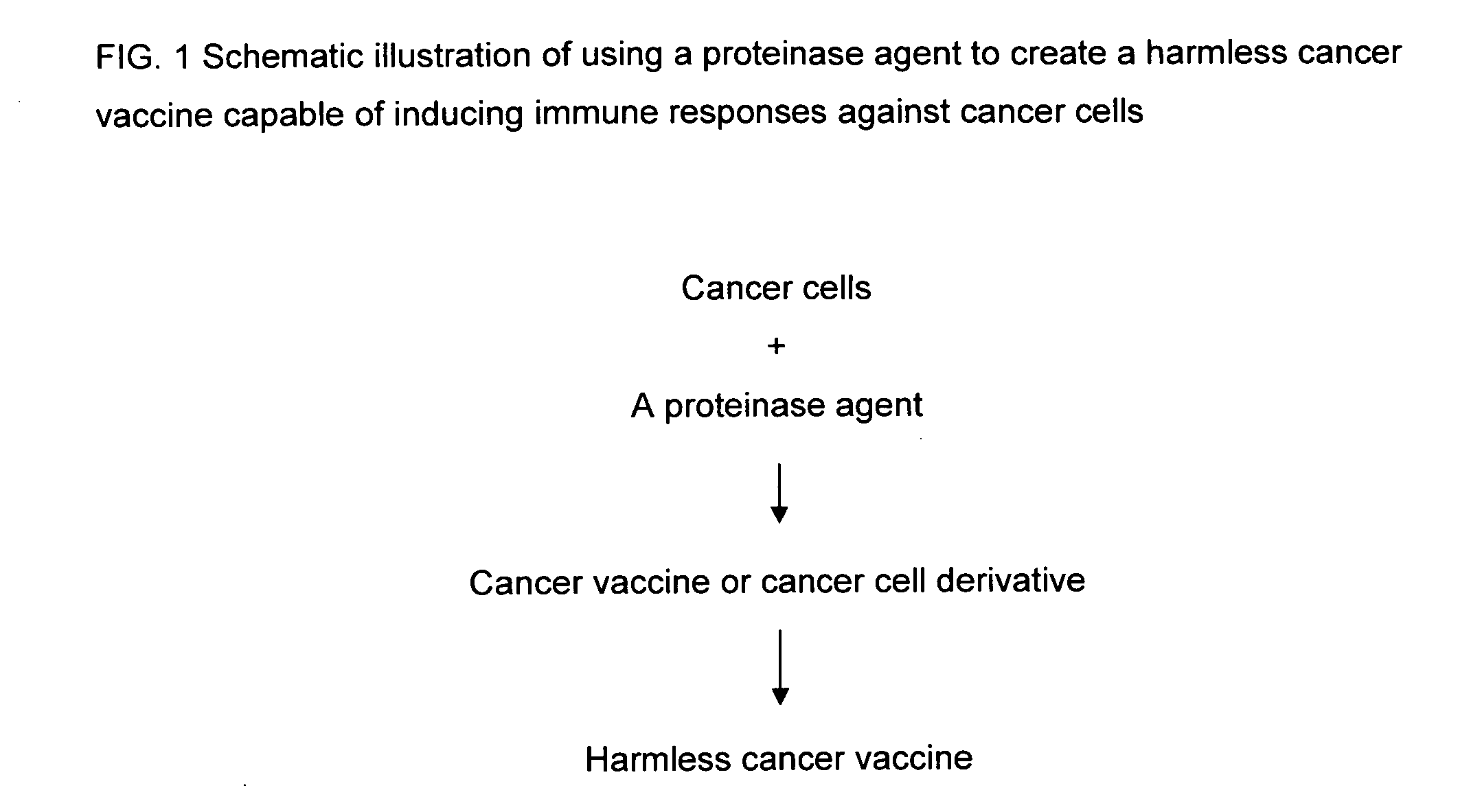
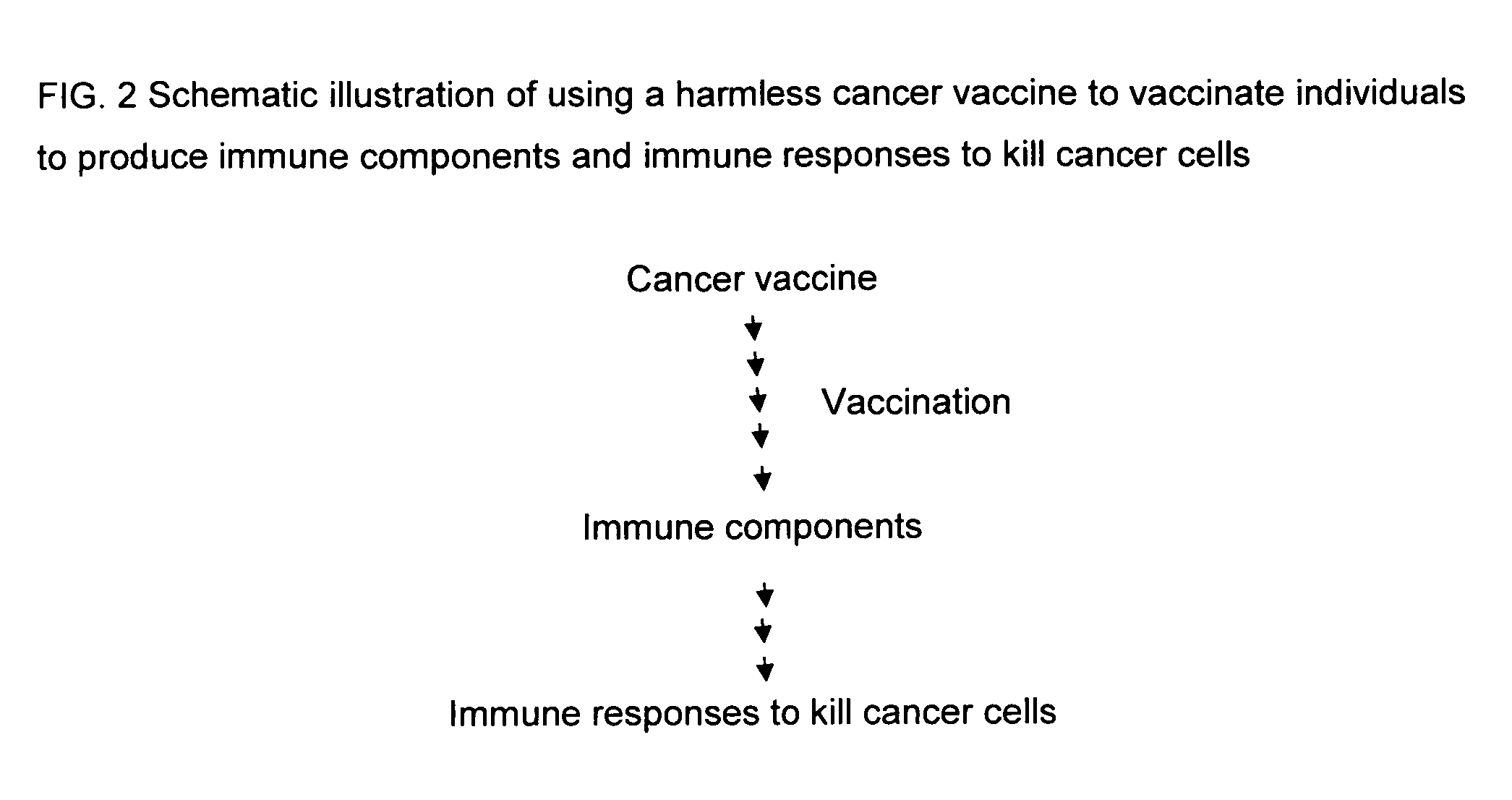
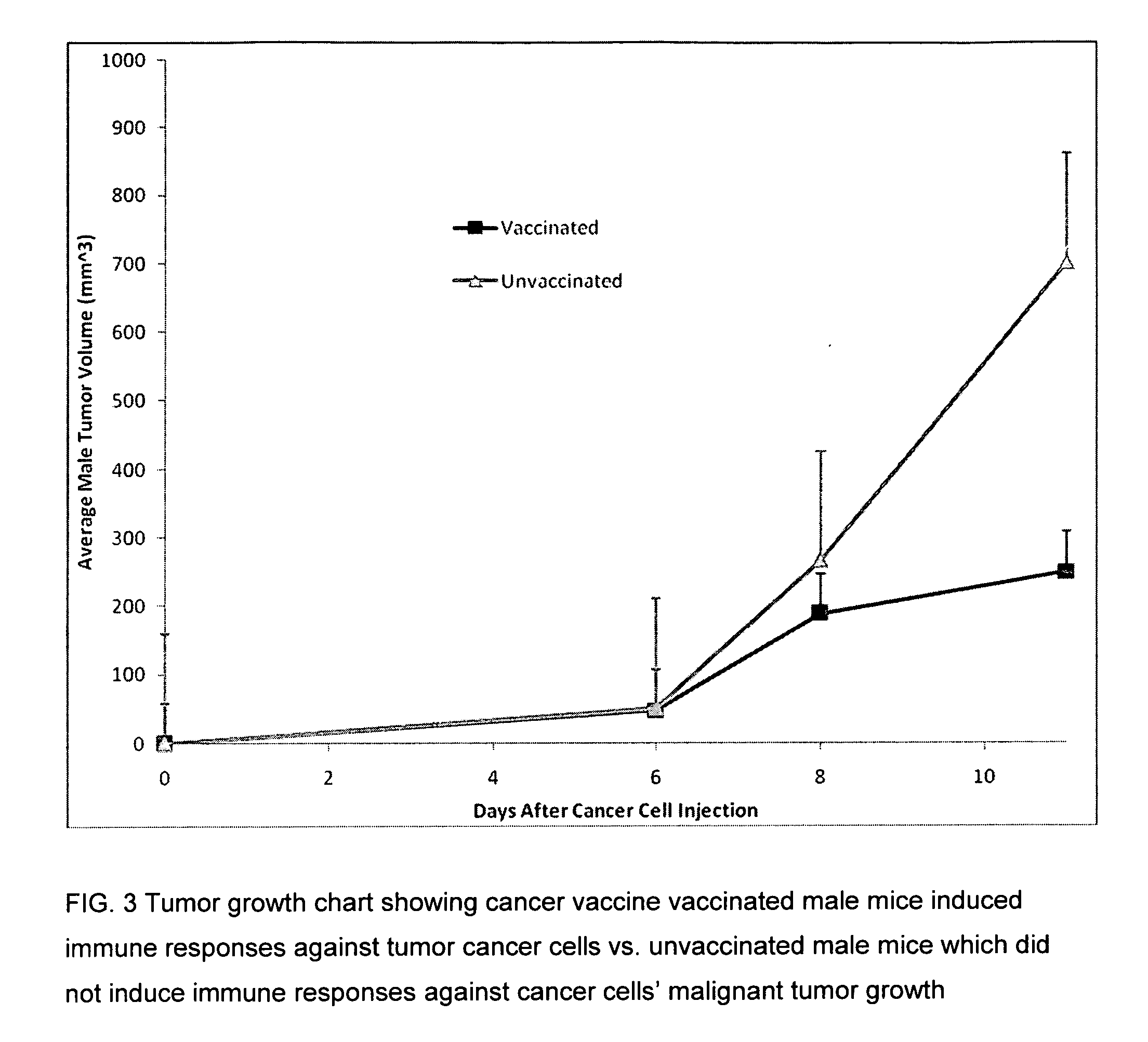
Proteinase-engineered cancer vaccine induces immune responses to prevent cancer and to systemically kill cancer cells
InactiveUS20090162405A1Induce immune responseVaccinesCancer antigen ingredientsCancer preventionCancer cell
A harmless cancer vaccine is made from cancer cells with extracellular proteins including self-recognition molecular patterns being digested by a proteinase. The cancer vaccine is used to vaccinate an individual to induce immune responses against cancer cells systemically. Cancer cells become harmless when they are digested by Tumorase™. Some proteinases including trypsin cannot kill cancer cells completely and treated cancer cells need to be further processed in order to be harmless and effective. Cancer cells may be from tissue-cultured human or animal cancer cell lines or cancer patients directly. Cancer vaccine vaccinated individuals produce cancer vaccine specific immune responses against cancer cells. Immune response components may be isolated and used to fight against cancer for a cancer patient with a suppressed immune system. Cancer vaccine specific immune components may include cancer vaccine specific polyclonal antibodies, B-cells, T-cells, natural killer cells, monocytes, macrophages and other lymphocytes.
Owner:QIAN YONG
Antigenic complex for the diagnosis and treatment of porphyromonas gingivalis infection
ActiveUS20110081358A1Induce immune responseAntibacterial agentsBacterial antigen ingredientsAntigenPorphyromonas gingivalis
Owner:UNIVERSITY OF MELBOURNE
Immunogenic compositions
InactiveUS7758869B2Induce immune responseEfficient responseAntibacterial agentsBacterial antigen ingredientsOral medicationImmunogenicity
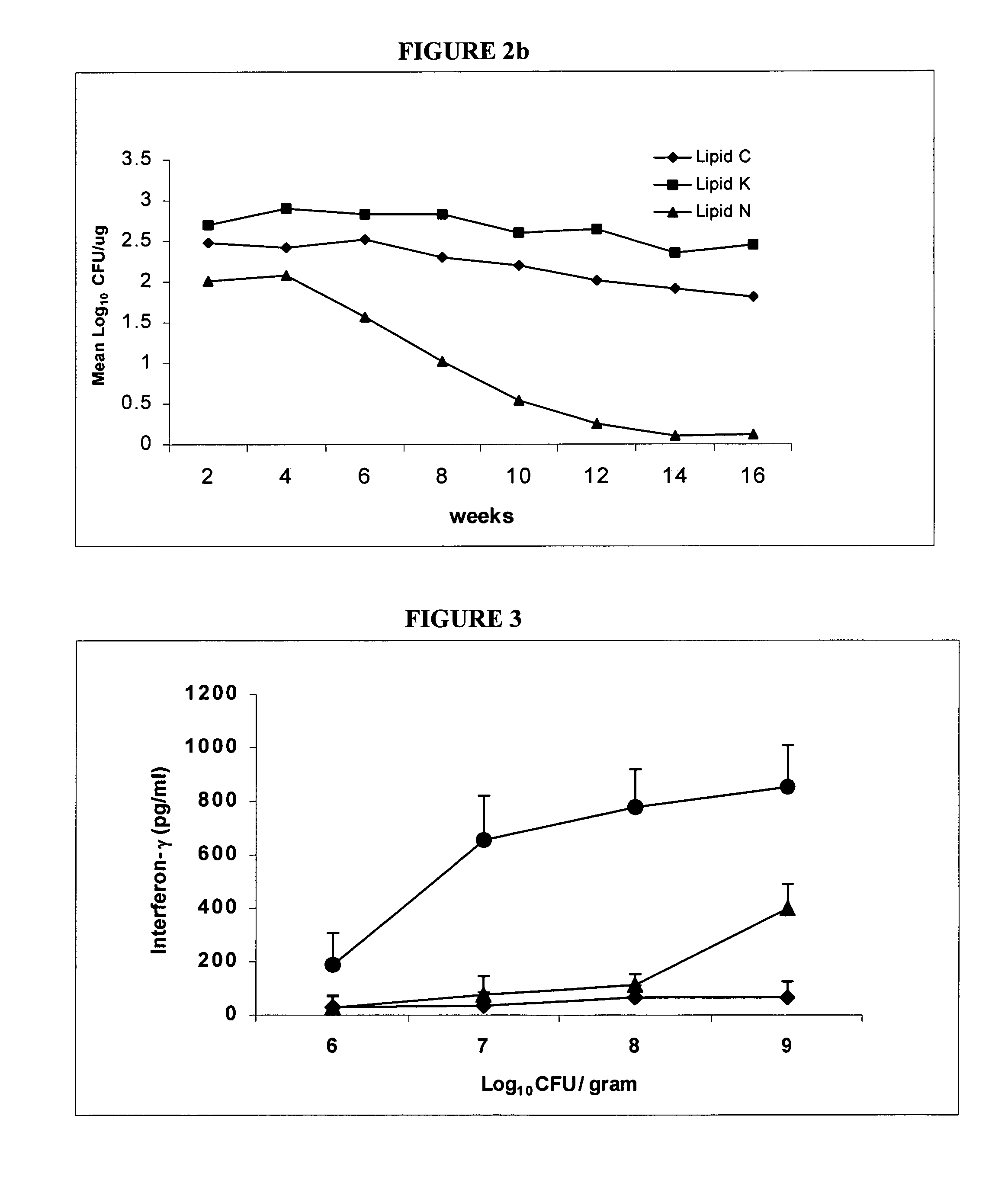
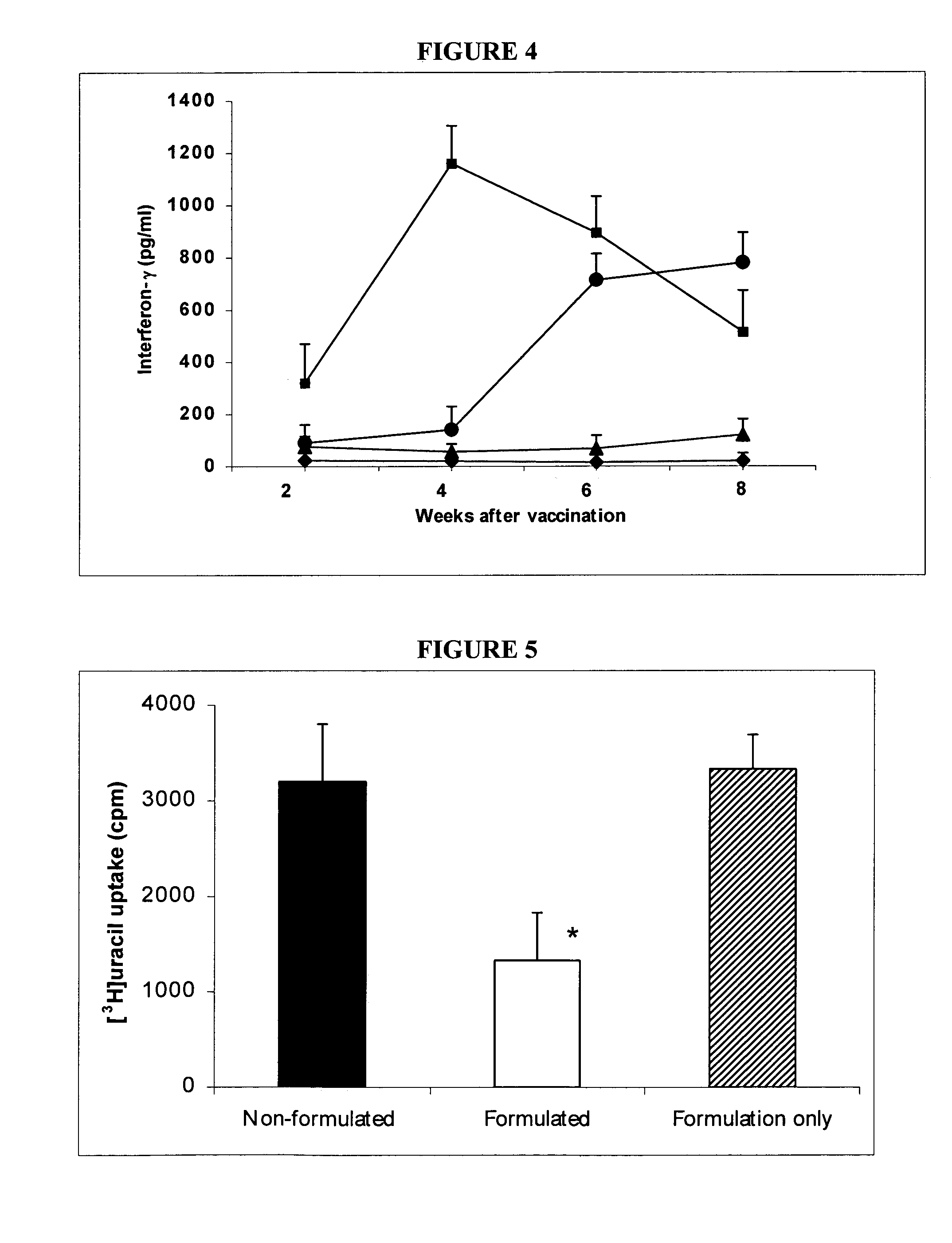
The invention relates to immunogenic compositions and to methods for immunizing animals using the same. The immunogenic composition comprises a lipid formulation most usually in solid form, and at least one immunogenic component. A preferred immunogenic component is a living organism. In a preferred embodiment the composition is formulated for oral administration.
Owner:OTAGO INNOVATION +2
Replication competent viruses capable of silencing virus inhibitory factor expression
ActiveUS20070122385A1Processing speedGood curative effectBiocideAntipyreticReplication competent virusGenome
Described is a replication-competent virus capable of replication and having lytic capacity in target cells. The virus comprises in the genome thereof, at least one DNA sequence coding for a silencing factor functional in reducing expression of a target gene in the target cells, operably linked to one or more expression control sequences, functional in the target cells. The use thereof in the preparation of a medicament and the use thereof in a method for lysing target cells expressing a virus inhibitory factor are also described.
Owner:STICHTING VUMC
Viruses with enhanced lytic potency
InactiveUS8052965B2Limited durationProcessing speedBiocidePeptide/protein ingredientsApoptosis pathwaysCell growth
Described is a replication competent recombinant virus, being capable to replicate and having lytic capacity in target cells, the said cell being hampered in the p53 dependent apoptosis pathway, the virus including in the genome thereof, the coding sequence of at least one restoring factor functional in restoring the p53 apoptosis pathway in the said target cells, operably linked to one or more expression control sequences, functional in the said target cells, as well as the use thereof in the preparation of a medicament, in particular for suppressing uncontrolled cell growth.
Owner:STICHTING VUMC
Methods of Using Phosphoantigen for the Treatment of Cancer
InactiveUS20100029674A1Effective treatmentGood effectUnknown materialsTissue cultureAbnormal tissue growthMammal
The present invention relates to compositions and methods useful for treating a cancer in mammals, including humans. The methods and compositions typically comprise use of a chemotherapeutic agent and a γδ T cell activator such that the composition is effective for treating a cancer. Preferably the composition enhances the effect of the γδ T cell activator and / or prevents or delays the escape of a tumor from control chemotherapy, particularly an anti-angiogenic chemotherapeutic agent.
Owner:INNATE PHARMA SA
Porcine pestvirus, vaccines, and assays
ActiveUS20180303926A1Induce immune responseSsRNA viruses positive-senseViral antigen ingredientsClinical researchPorcine pestivirus
Porcine pestivirus designated herein as atypical porcine pestivirus (“APPV”) (Genbank accession no. KR011347.1). Immunogenic compositions to induce an immune response against porcine pestivirus infection in a pig are described, which APPV antigenic agents (e.g., isolated whole virus, derivatives thereof, functional fragments thereof, and combinations of the foregoing). Methods of vaccinating against porcine pestivirus infection using the immunogenic compositions are also described. The methods can be also applied for clinical research and / or study, including diagnostic methods for detecting pestivirus infection using monoclonal antibodies specifically binding to APPV epitopes.
Owner:KANSAS STATE UNIV RES FOUND
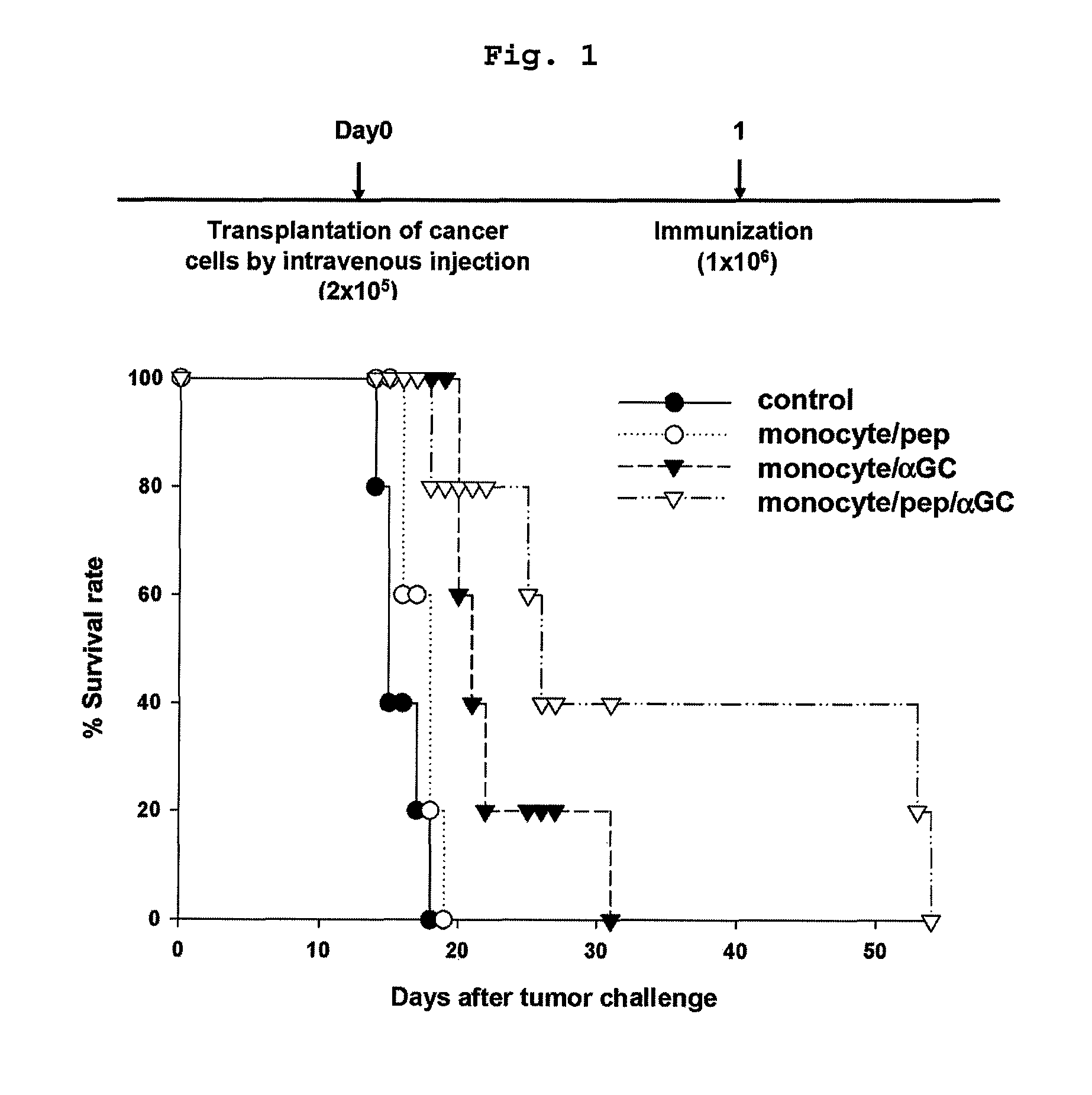
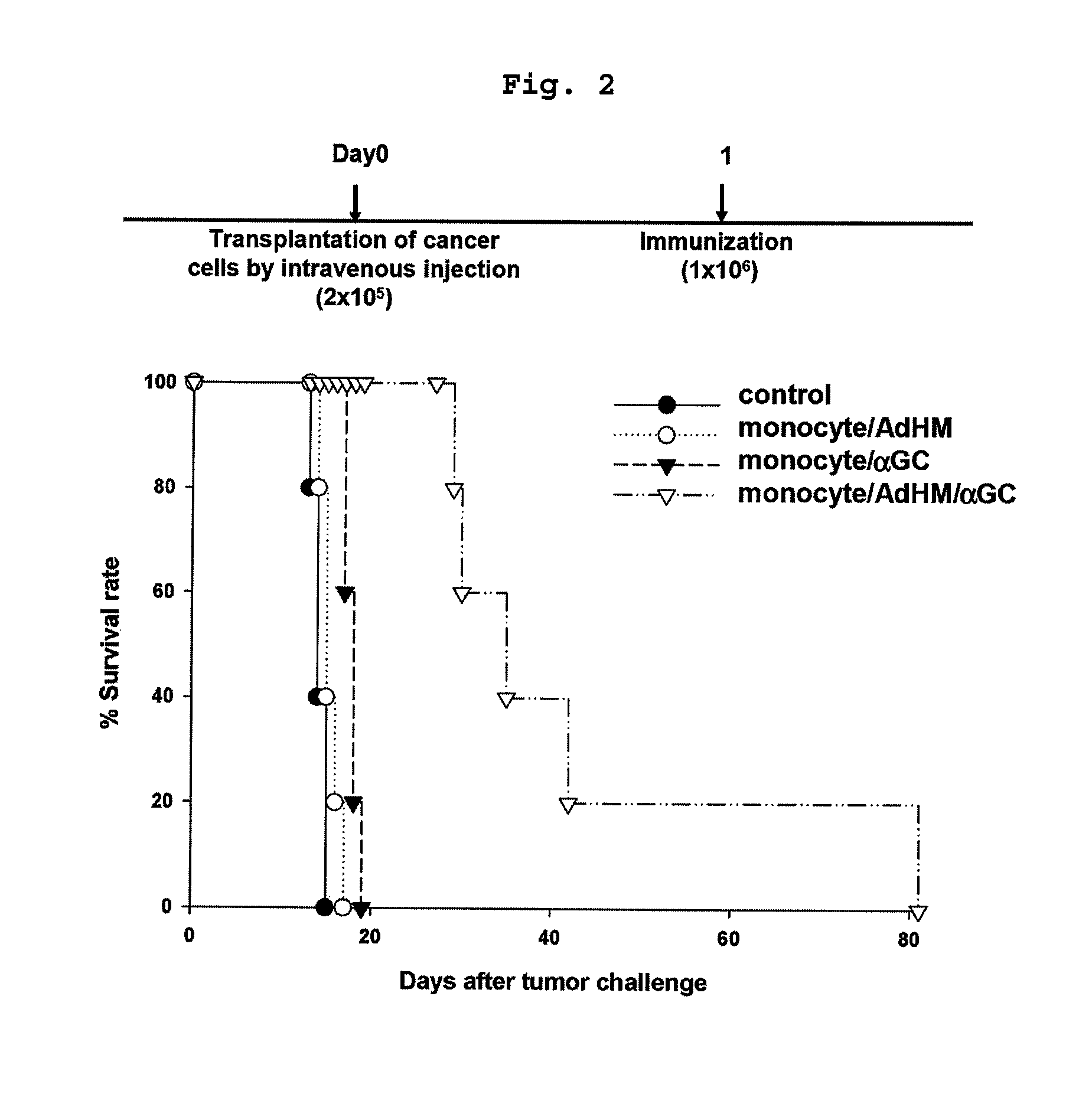
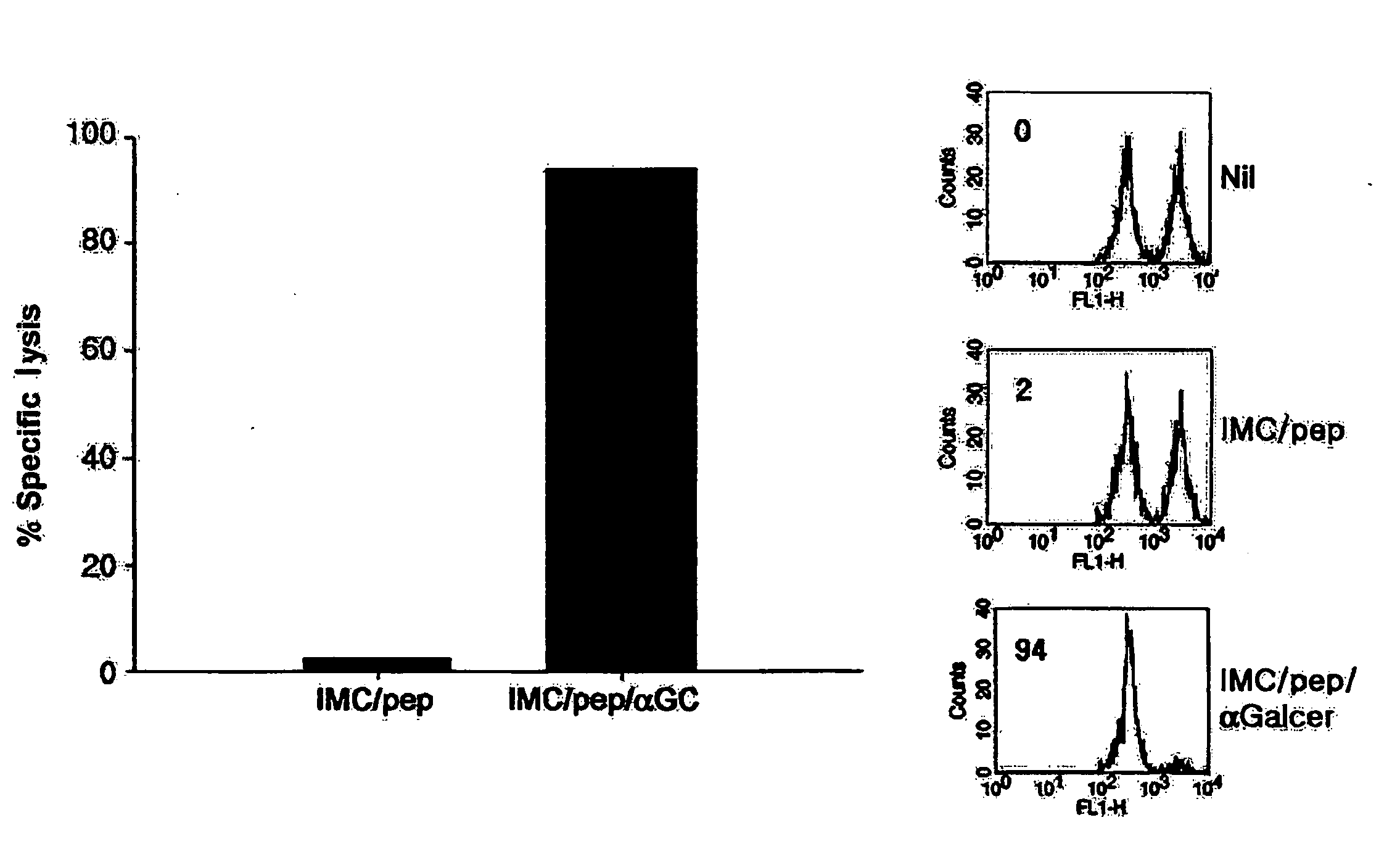
Vaccine comprising monocyte or immature myeloid cells (IMC) which were loaded with the ligand of natural killer T cell and antigen
ActiveUS9518126B2Improve isolationInduce immune responseAntibacterial agentsAntiviralsAbnormal tissue growthDendritic cell
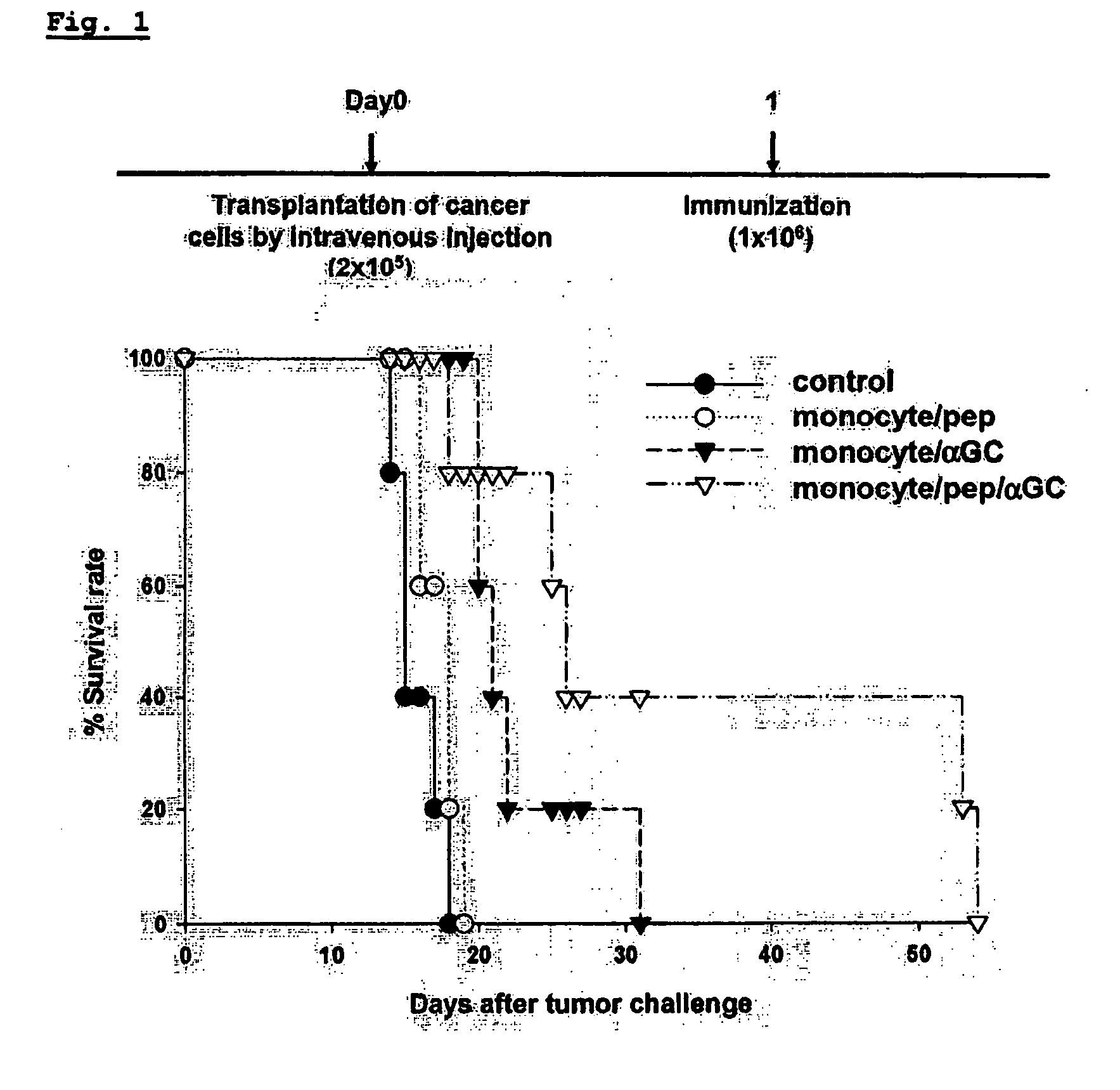
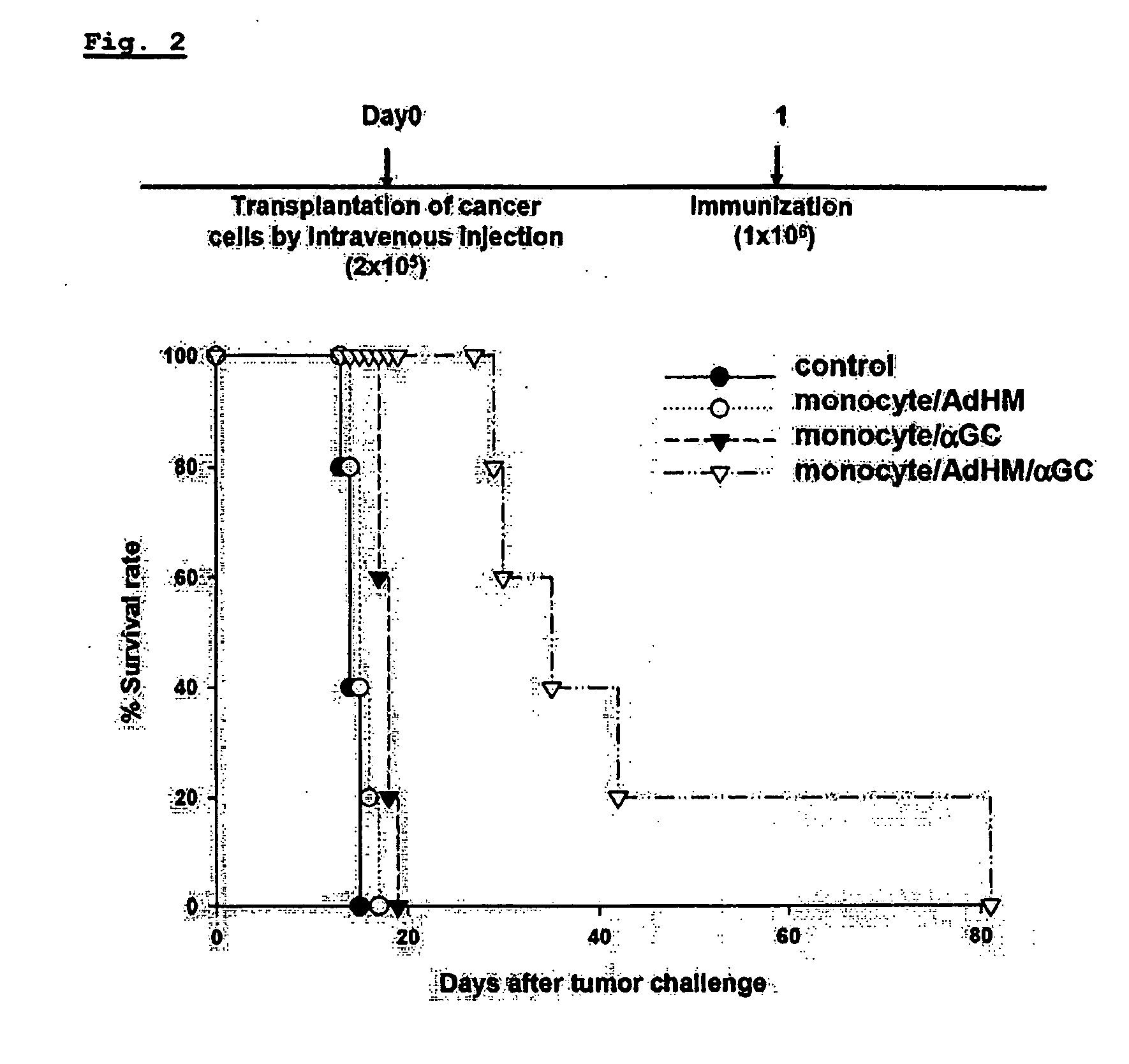
The present invention relates to an immuno-therapeutic and prophylactic vaccine comprising monocytes or immature myeloid cells (IMCs) loaded with the ligand of natural killer T cell and an antigen for the prevention and treatment of infectious disease or cancer, more precisely, an immuno-therapeutic and prophylactic vaccine comprising monocytes or IMCs loaded with α-galactosylceramide (αGalCer), a kind of glycolipid and a natural killer T cell ligand, and antigen. Monocytes or immature myeloid cells (IMCs) therein, which are easily obtainable, unlike dendritic cells, not only induce a significant level of cytotoxic T lymphocyte responses but also have a prophylactic and therapeutic effect on malignant tumor. Therefore, the immuno-therapeutic and prophylactic vaccine of the present invention can be effectively used as an immunotherapeutic agent.
Owner:CELLID
Antigenic Complex for the Diagnosis and Treatment of Porphyromonas Gingivalis Infection
InactiveUS20090169568A1Induce immune responseAntibacterial agentsBacterial antigen ingredientsAntigenPorphyromonas gingivalis
Owner:UNIVERSITY OF MELBOURNE
Application of streptococcus pneumoniae protein to resisting infection of S. pneumoniae
ActiveCN109456393AIncrease infectionReduced Colonization Protection ExperimentAntibacterial agentsBacterial antigen ingredientsPneumonia mrsaStreptococcus mitis
The invention provides application of S. pneumoniae protein to resisting infection of S. pneumoniae. The endopeptidase O (PepO) of S. pneumoniae is a subcutaneous immunologic adjuvant, and the prepared S. pneumoniae protein vaccines have the good protection effects on resisting infection of S. pneumoniae through mixing and fusing expression of the subcutaneous immunologic adjuvant and 673rd to 863rd amino acid peptide fragment of zinc metal protease B (ZmpB).
Owner:CHONGQING MEDICAL UNIVERSITY
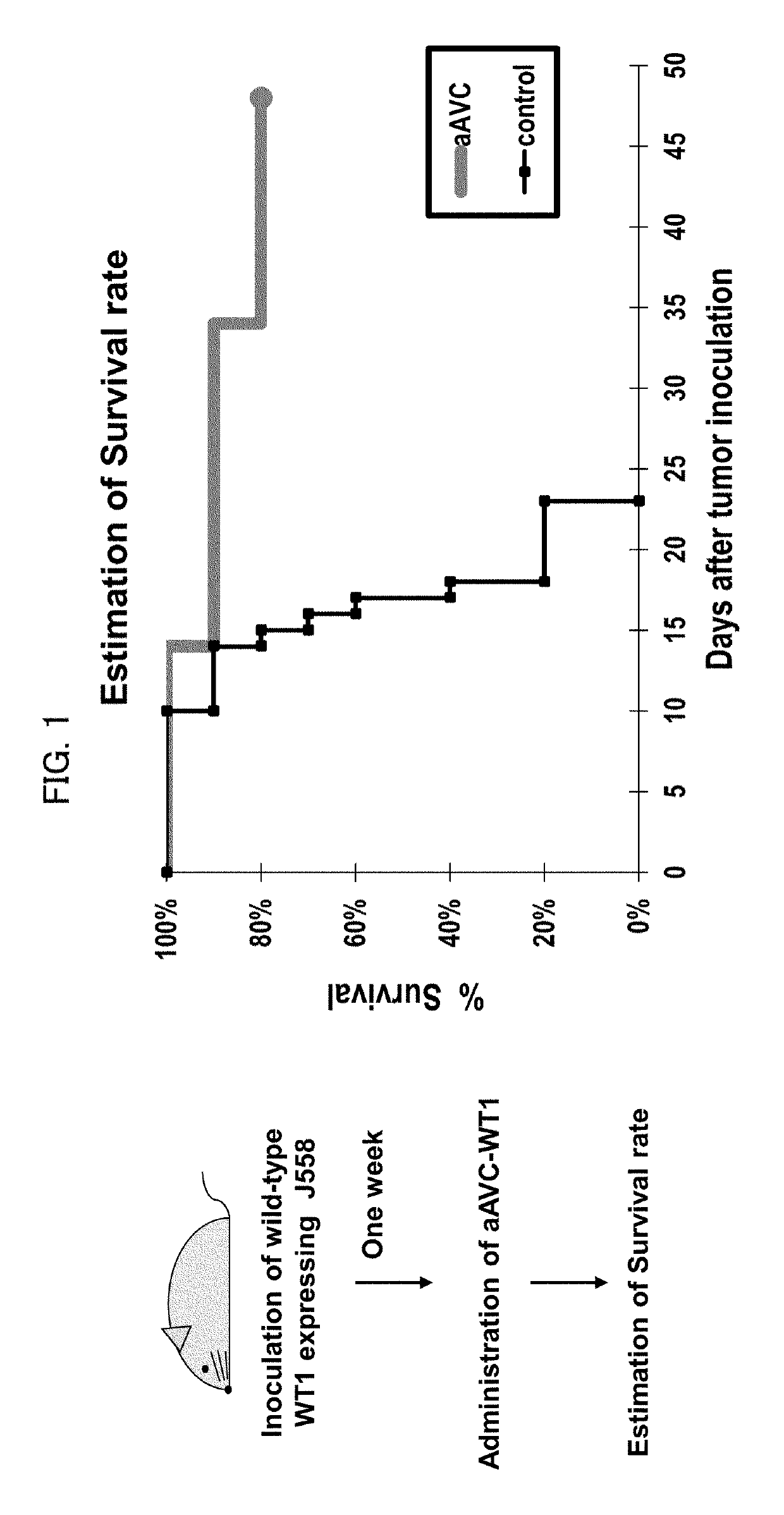
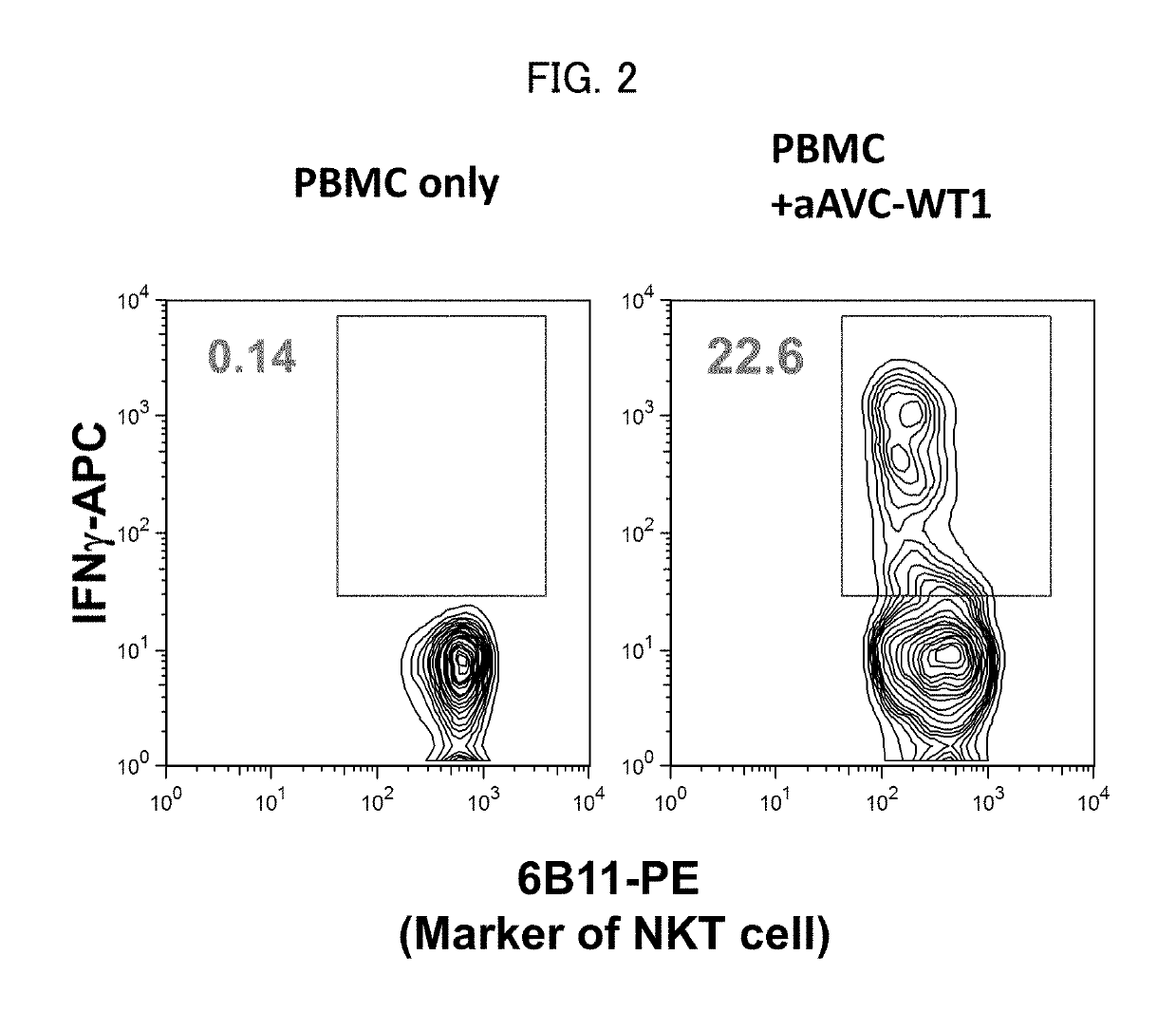
Cell for immunotherapy, including modified nucleic acid construct encoding Wilms tumor gene product
ActiveUS10316332B2Induce immune responseImprove expression levelTumor rejection antigen precursorsAntibody mimetics/scaffoldsAntigen receptorsWilms' tumor
A cell for immunotherapy of the present invention includes a nucleic acid encoding a Wilms tumor 1 gene product or a fragment of the Wilms tumor 1 gene product, wherein the nucleic acid including (i) a region encoding a fragment of the Wilms tumor 1 gene product, the fragment being indicated by positions 194 to 493 of amino acid sequence of SEQ ID NO:1 or by positions corresponding to the positions 194 to 493 of amino acid sequence corresponding to SEQ ID NO:1 and (ii) only one AUG as a functional start codon, connected to a 5′ terminal side of the region via 3m (m is 124-192) bases intervening between the AUG as the functional start codon and the 5′ terminal side of the region, and a nucleic acid encoding CD1d, wherein the cell has been loaded with a glycolipid recognized by antigen receptor of NKT cell.
Owner:RIKEN
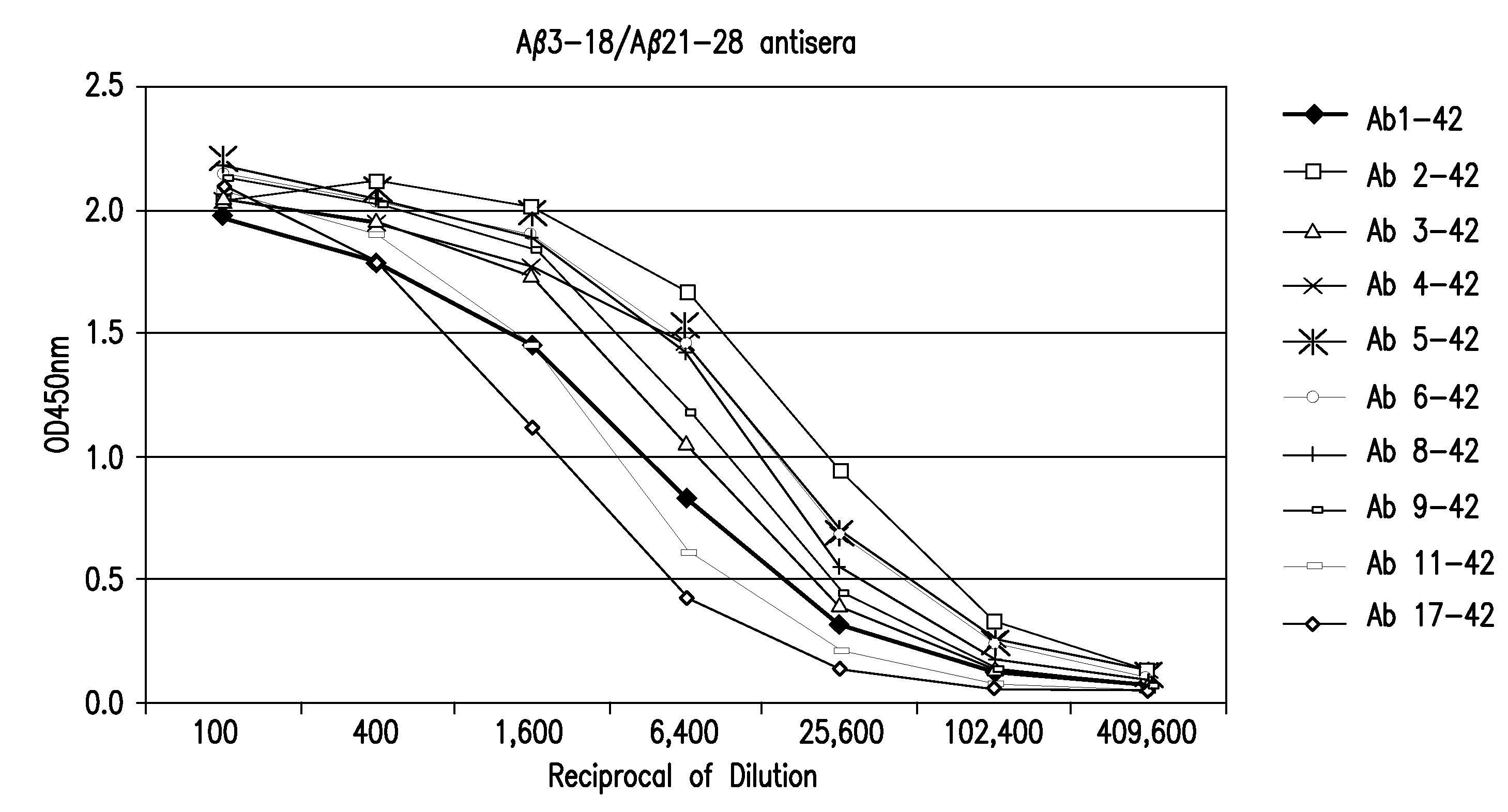
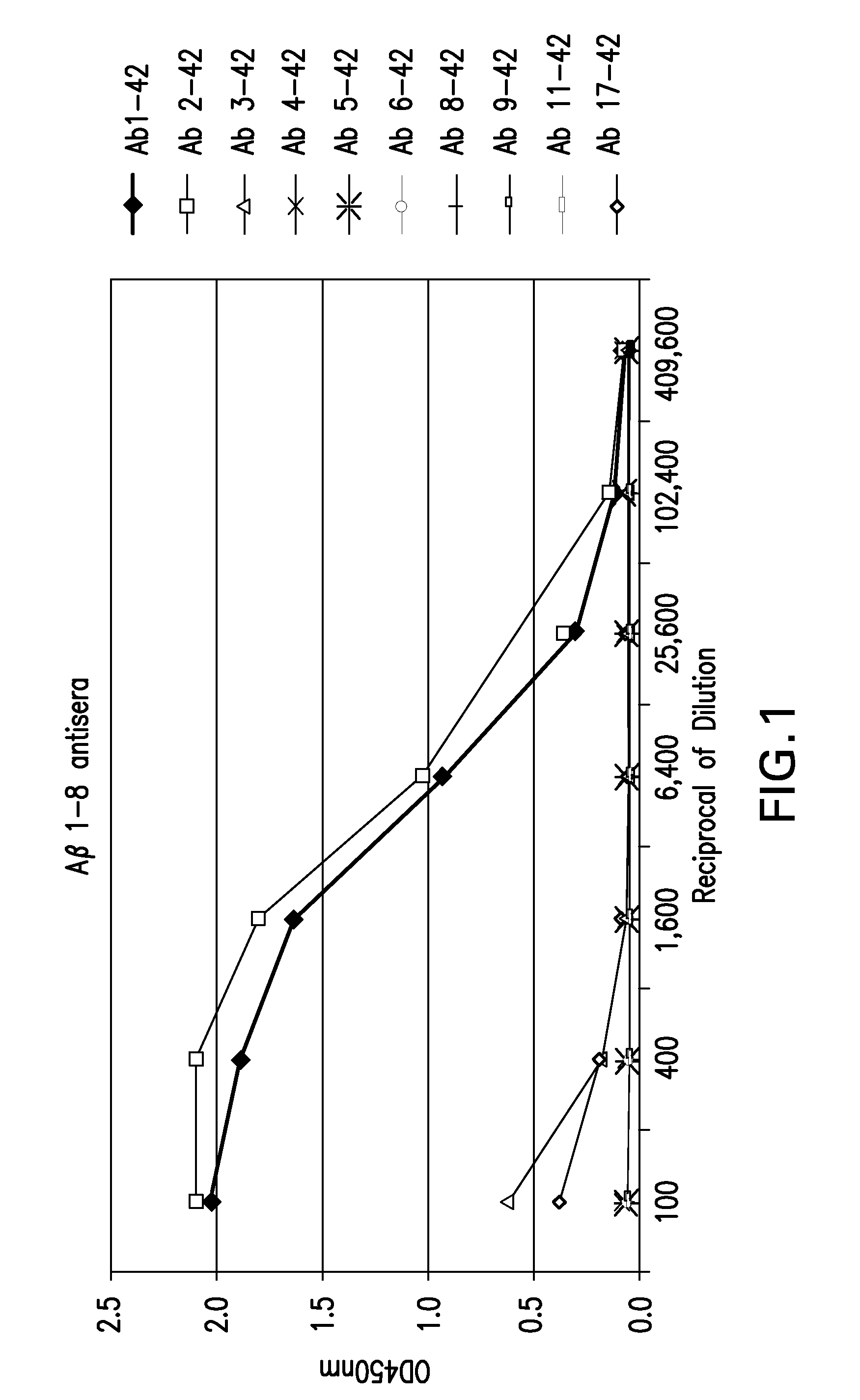
Vaccine for the treatment of alzheimer's disease
InactiveUS20110002949A1Induce immune responseEffective to induce immune responseNervous disorderSnake antigen ingredientsImmunogenicityAmyloid beta
The invention provides a method for the treatment of a patient having a more severe form of Alzheimer's disease (AD), where the severe form of AD is characterized by pathogenic deposits of amyloid beta peptide (Aβ), comprising the administration of an immunogenic fragment of Aβ capable of inducing an immune response in the form of antibodies to specific to the pathogenic deposits of Aβ and, in particular, to neurotoxic forms of Aβ including N-terminally truncated forms of Aβ. The invention further provides a method for selecting a suitable immunogenic fragment of Aβ for the treatment of a more severe form of AD.
Owner:MERCK SHARP & DOHME CORP
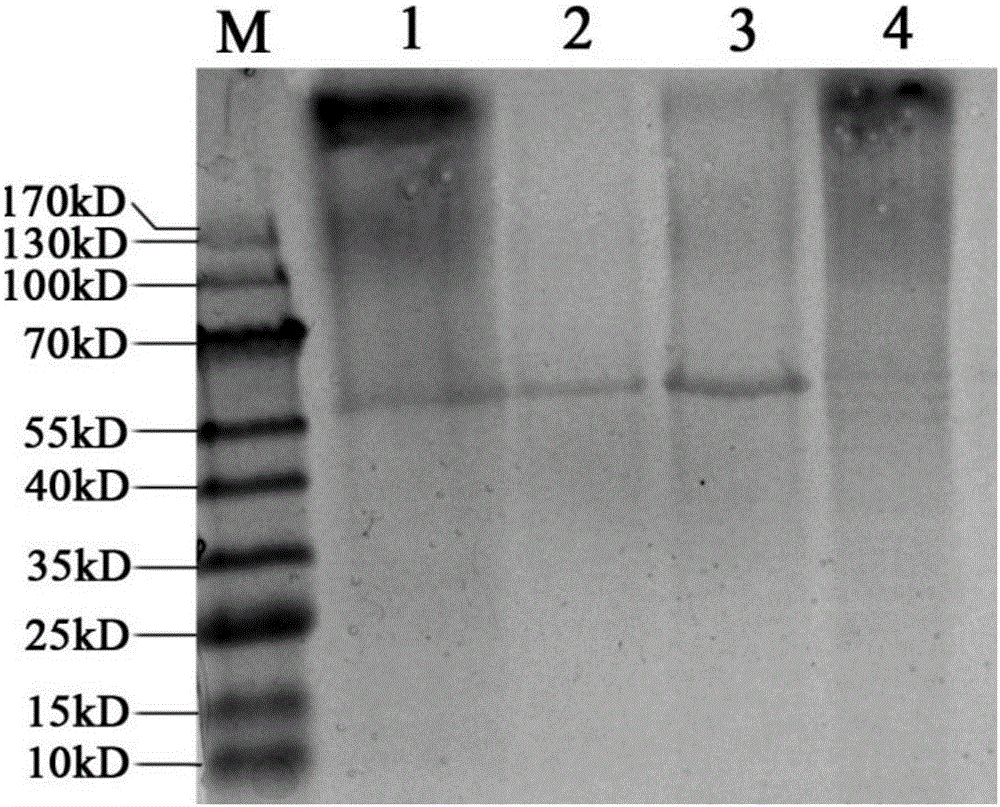
rBCG for expression of Br. Melitensis P39 and L7/L12 fusion gene and construction method thereof
ActiveCN106834331ASignificant immune adjuvant effectLow costAntibacterial agentsBacterial antigen ingredientsRibosomal proteinBCG vaccine
The invention provides rBCG for expression of Br. Melitensis P39 and L7 / L12 fusion gene. The rBCG is constructed by transferring an expression vector carrying codon-optimized Br. Melitensis P39 and L7 / L12 fusion gene into BCG. Brucellosis-generated cytoplasm binding protein PBP39 (coding gene is P39) and Brucellosis ribosomal protein L7 / L12 are both T-cell antigen. Bacillus Calmette-Guerin (BCG) vaccine is the only one commercial vaccine for preventing tuberculosis so far. The BCG vaccine has a remarkable immunologic adjuvant effect and is an exogenous gene expression host with good performance and high safety. By BCG expression of the codon-optimized Brucellosis P39 and L7 / L12 fusion gene, expression quantity of the target gene can be increased. The rBCG vaccine can simulate intracellur infection and parasitic characteristics of Brucellosis to more effectively induce body to generate immune response, can perform advantages of high safety, simple preparation, low cost, etc. of BCG as the expression host as well as the immunologic adjuvant effect of the BCG itself, and is expected to become a novel Brucellosis vaccine.
Owner:INNER MONGOLIA MEDICAL UNIV
Pneumococcal vaccine containing pneumococcal surface protein a
ActiveUS20150320851A1Induce immune responseInduce protective immunityAntibacterial agentsBacterial antigen ingredientsCoccidiaProtein s antigen
A pneumococcal vaccine comprising a fusion protein at least comprising a full-length family 1 pneumococcal surface protein A (PspA) or a fragment thereof, and a full-length family 2 PspA or a fragment thereof, in particular any one of the following fusion proteins (1) to (3):(1) a fusion protein at least comprising a family 1, clade 2 PspA and a family 2, clade 3 PspA,(2) a fusion protein at least comprising a family 1, clade 2 PspA and a family 2, clade 4 PspA, and(3) a fusion protein at least comprising a family 1, clade 2 PspA and a family 2, clade 5 PspA,is useful as a pneumococcal vaccine comprising a single protein antigen that has broadly cross-reactive immunogenicity and can induce immune response against a wide range of pneumococcal clinical isolates.
Owner:OSAKA UNIV
Adjuvant for Vaccines, Vaccine, and Immunity Induction Method
ActiveUS20180177867A1Induce immune responseOrganic active ingredientsPeptide/protein ingredientsTGE VACCINETlr agonists
Owner:THE UNIV OF TOKYO
Conditional replicating cytomegalovirus as a vaccine for CMV
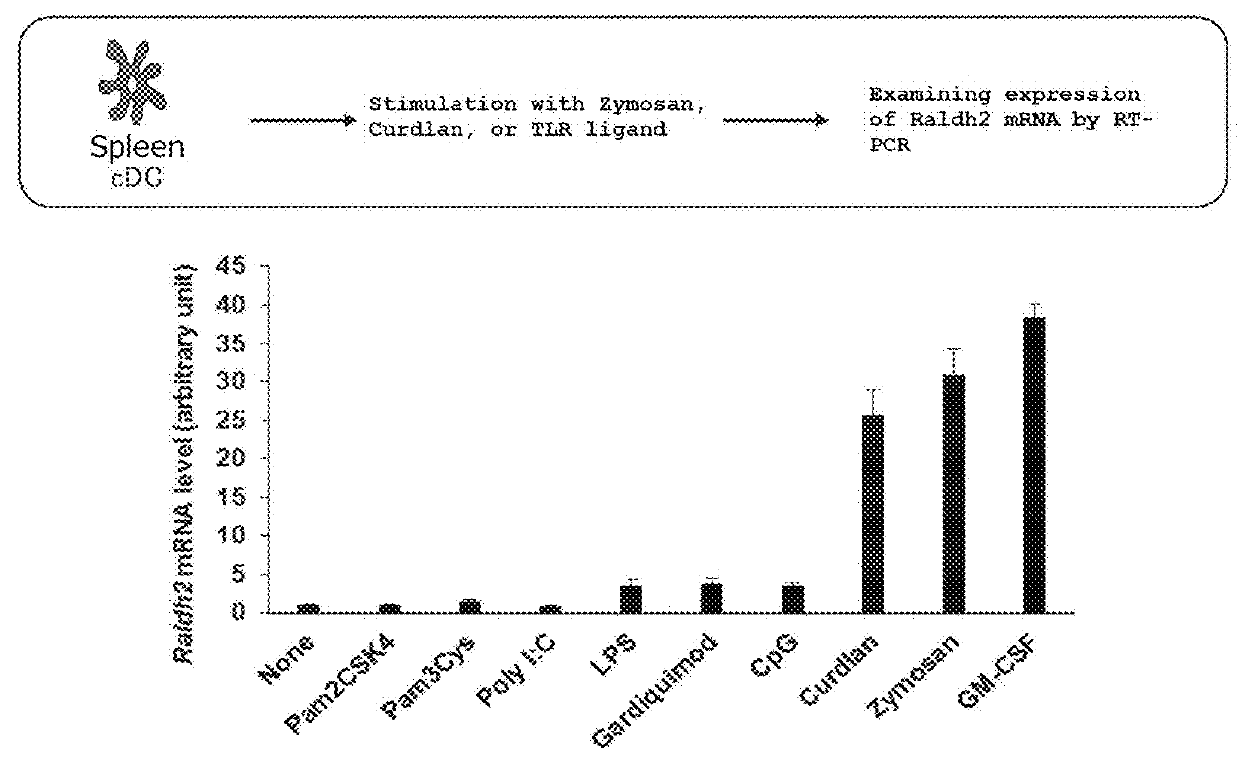
ActiveUS9546355B2Reduce the likelihood of infectionReduced likelihoodSugar derivativesViral antigen ingredientsCmv infectionsViral replication
The present invention relates to methods of inducing an immune response to cytomegalovirus (CMV) using a genetically modified CMV that is conditionally replication defective. The methods of the invention can be used to treat and / or prevent primary CMV infection, infection due to reactivation of a latent CMV and a super-infection of a different strain of CMV that had been previously encountered. The present invention also relates to a replication defective CMV which has been recombinantly altered to allow for external control of viral replication. Compositions comprising the replication defective CMV are also encompassed by the present invention.
Owner:MERCK SHARP & DOHME LLC
METHODS OF PREVENTING AND TREATING VIRAL INFECTIONS BY INHIBITING THE DelSGYLATION ACTIVITY OF OTU DOMAIN-CONTAINING VIRAL PROTEINS
InactiveUS20110033498A1Reduce capacityReduce and eliminate abilitySsRNA viruses negative-senseSsRNA viruses positive-senseISG15ADAMTS Proteins
Viruses having an impaired ability to deISGylate ISG15 conjugates, in particular, viral mutants comprising a mutation in the viral genome that reduces or eliminates the ability of the viral OTU domain-containing protein encoded by the viral genome to deISGylate ISG15 conjugates and / or deubiquitinate ubiquitinated proteins and / or deNeddylate Neddylated proteins are disclosed. Such viral mutants may be used in the formulation of immunogenic compositions for inducing an immune response and preventing, managing and / or treating a viral infection. Also disclosed are methods for identifying anti-viral compounds, in particular, methods of identifying compounds that reduce or inhibit the deISGylation activity and / or deubiquitination and / or deNeddylation activity of a viral OTU domain-containing protein. The compounds identified using such methods may be used as antiviral agents for the prevention, treatment and / or management of viral infections.
Owner:MT SINAI SCHOOL OF MEDICINE +1
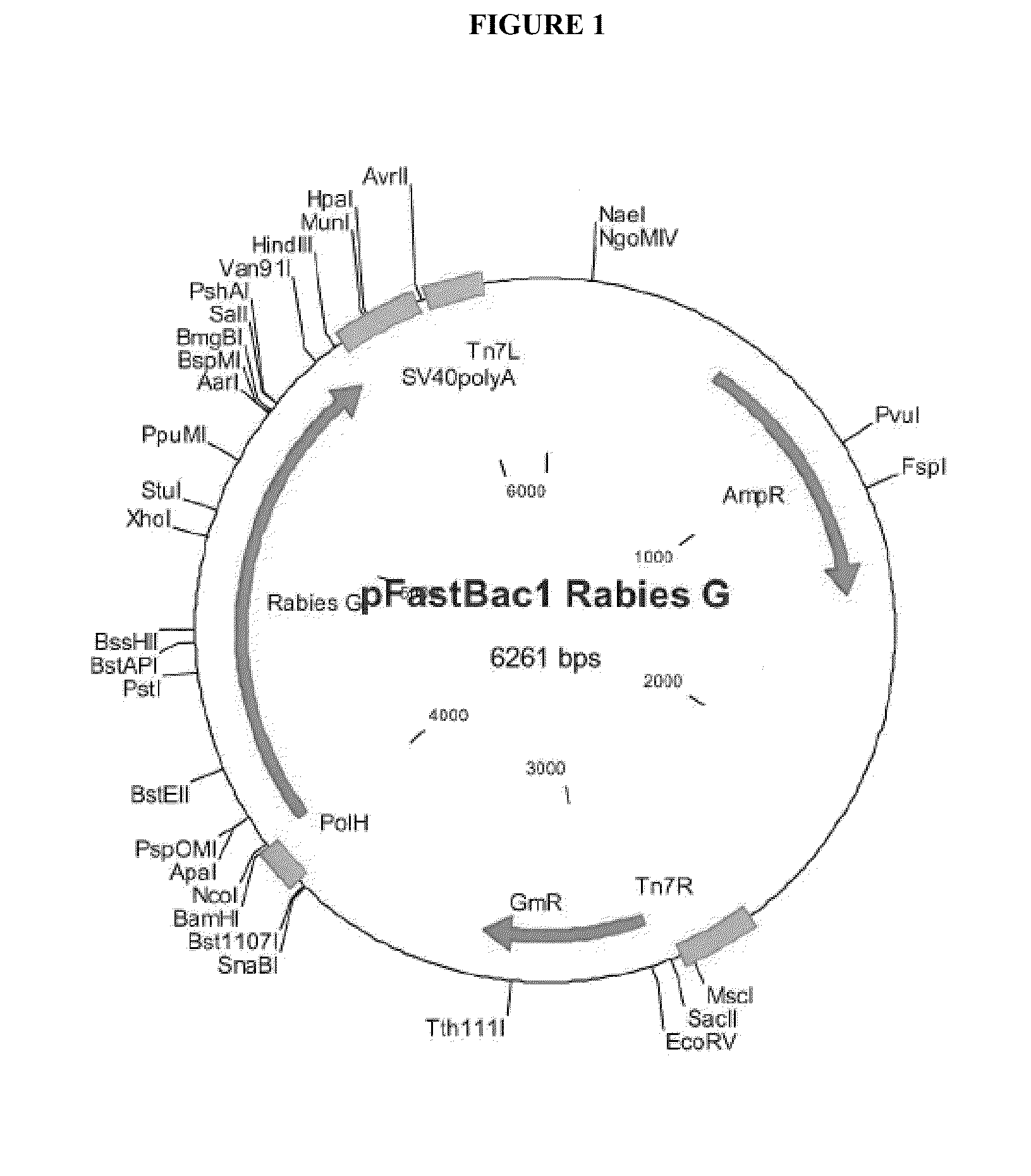
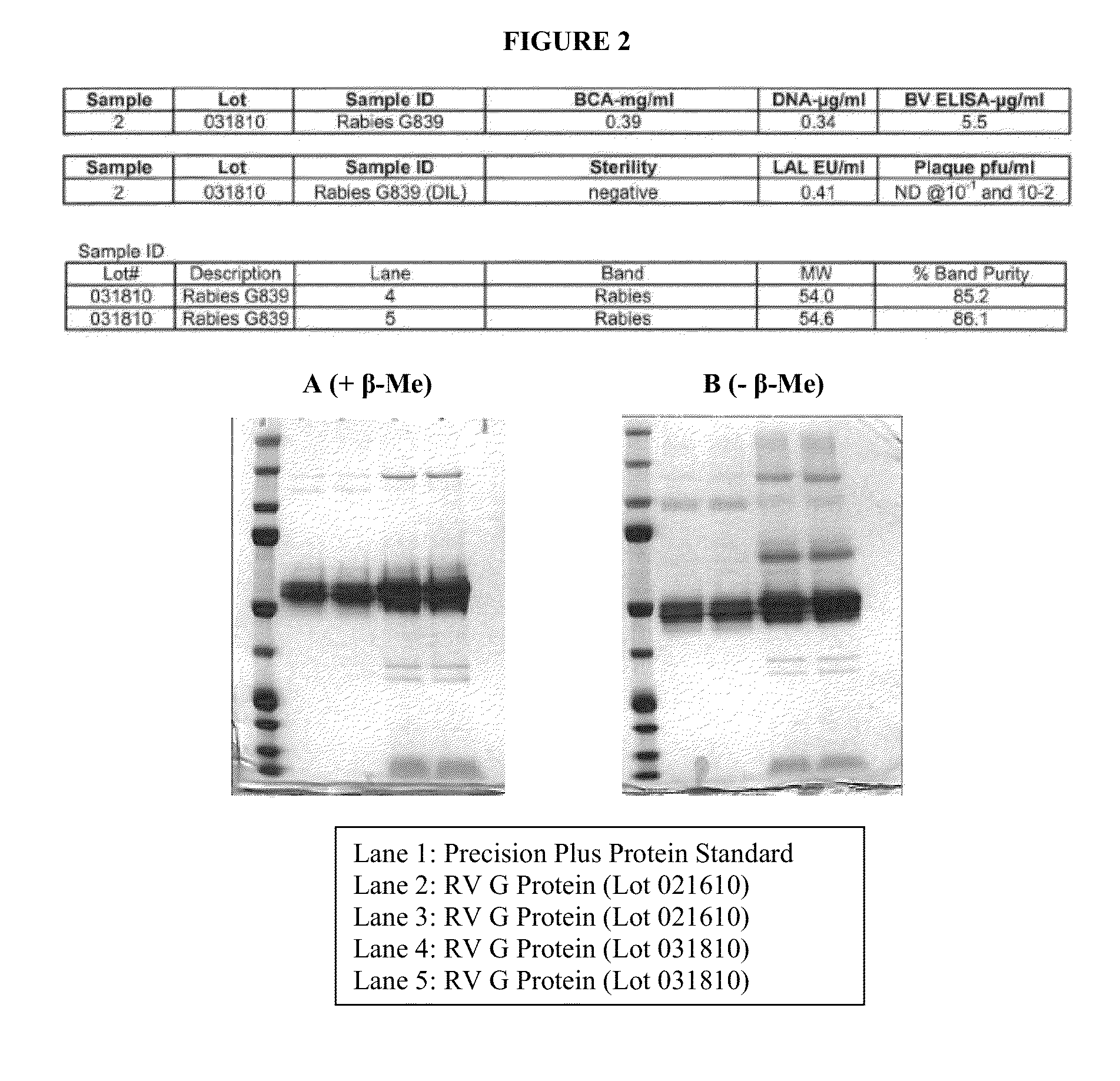
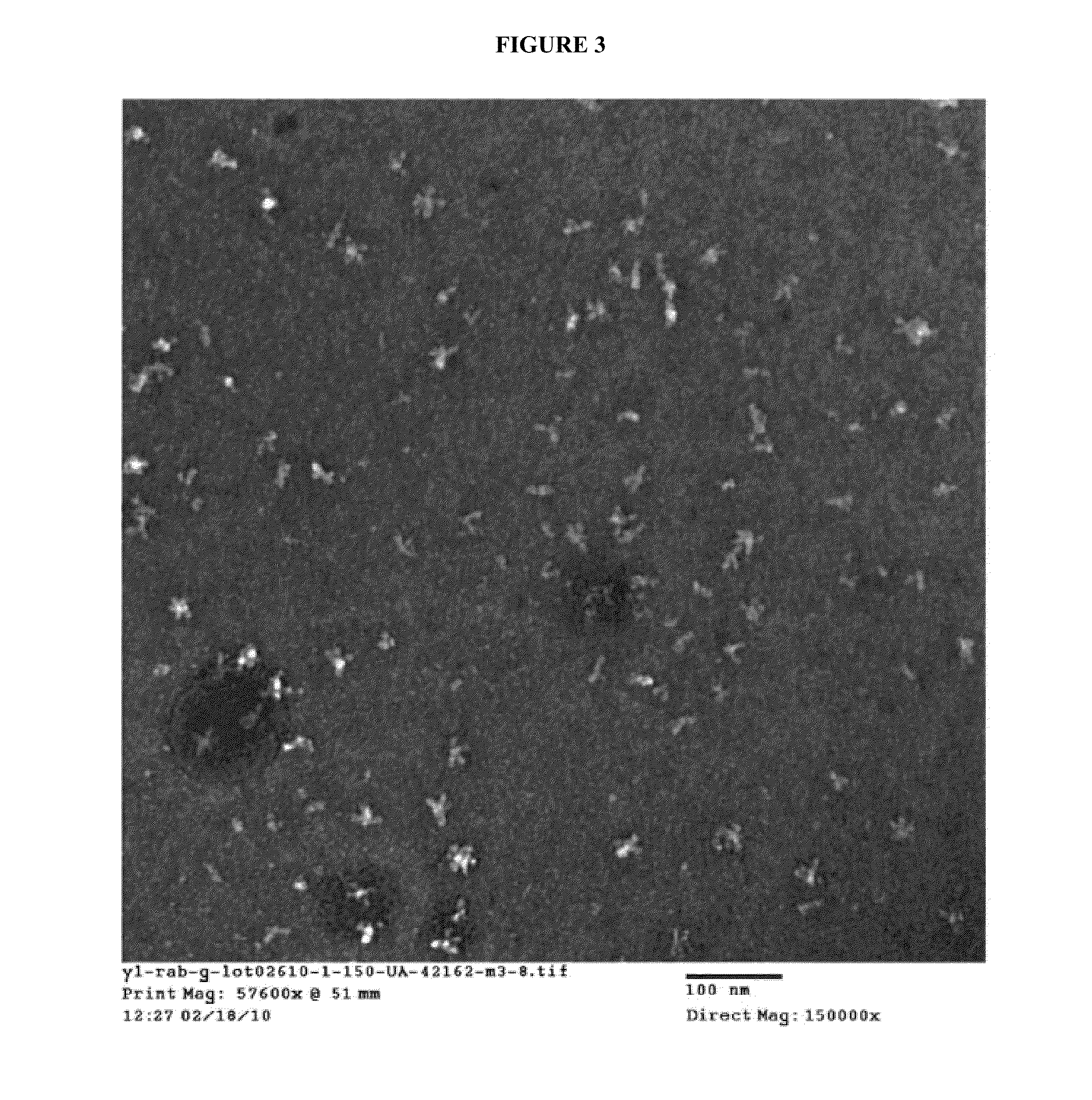
Rabies glycoprotein virus-like particles (VLPS)
ActiveUS20140178419A1Induce immune responsePotential immune responseSsRNA viruses negative-senseViral antigen ingredientsVirus-like particleViral infection
Owner:NOVAVAX
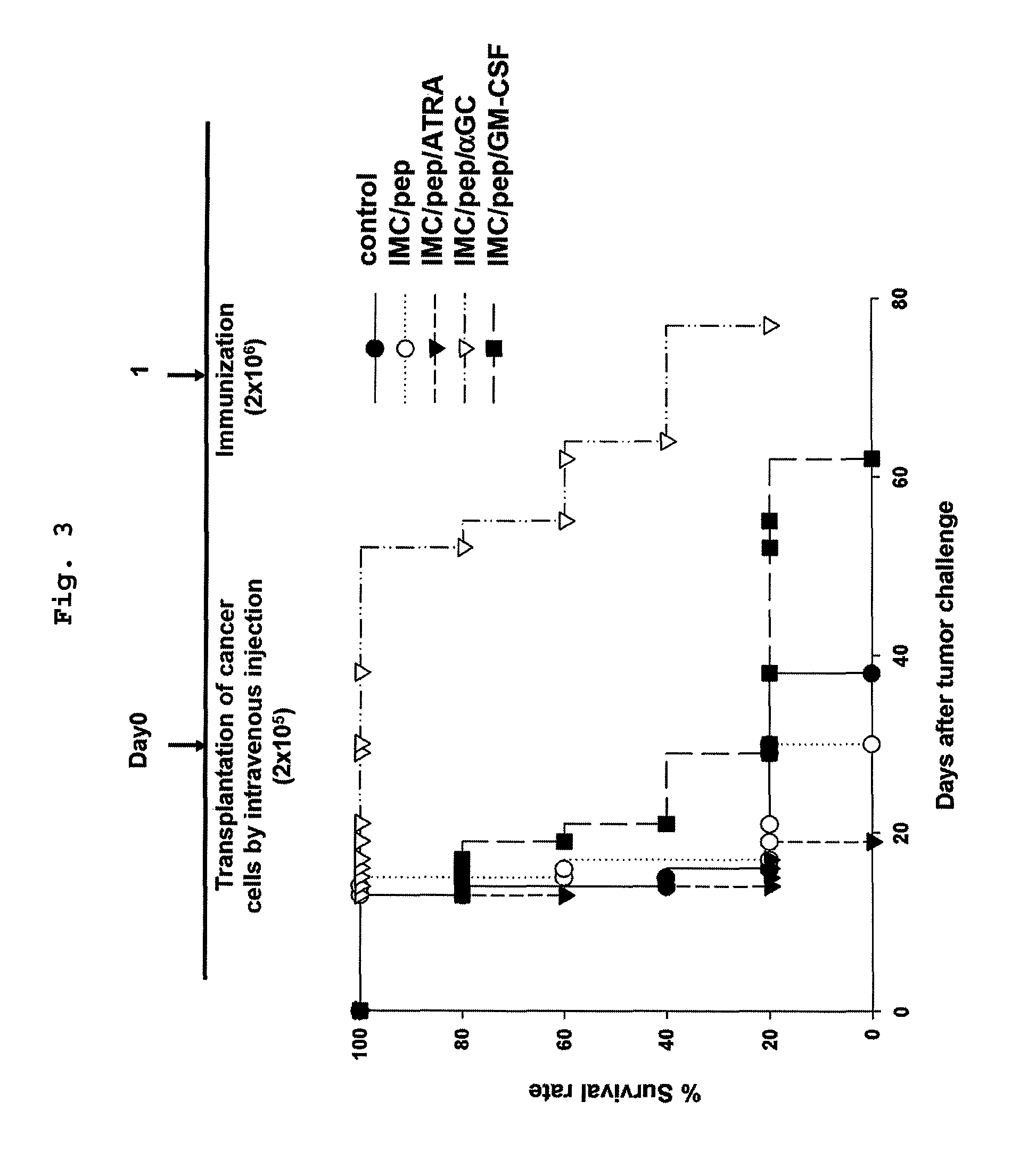
Vaccine comprising monocyte or immature myeloid cells (IMC) which were loaded with the ligand of natural killer t cell and antigen
ActiveUS20090285851A1Improve isolationInduce immune responseAntibacterial agentsOrganic active ingredientsDendritic cellImmunotherapeutic agent
The present invention relates to an immuno-therapeutic and prophylactic vaccine comprising monocytes or immature myeloid cells (IMCs) loaded with the ligand of natural killer T cell and an antigen for the prevention and treatment of infectious disease or cancer, more precisely, an immuno-therapeutic and prophylactic vaccine comprising monocytes or IMCs loaded with α-galactosylceramide (αGalCer), a kind of glycolipid and a natural killer T cell ligand, and antigen. Monocytes or immature myeloid cells (IMCs) therein, which are easily obtainable, unlike dendritic cells, not only induce a significant level of cytotoxic T lymphocyte responses but also have a prophylactic and therapeutic effect on malignant tumor. Therefore, the immuno-therapeutic and prophylactic vaccine of the present invention can be effectively used as an immunotherapeutic agent.
Owner:CELLID
Synthetic human immunodeficiency virus (HIV) envelope antigen, vectors, and compositions thereof
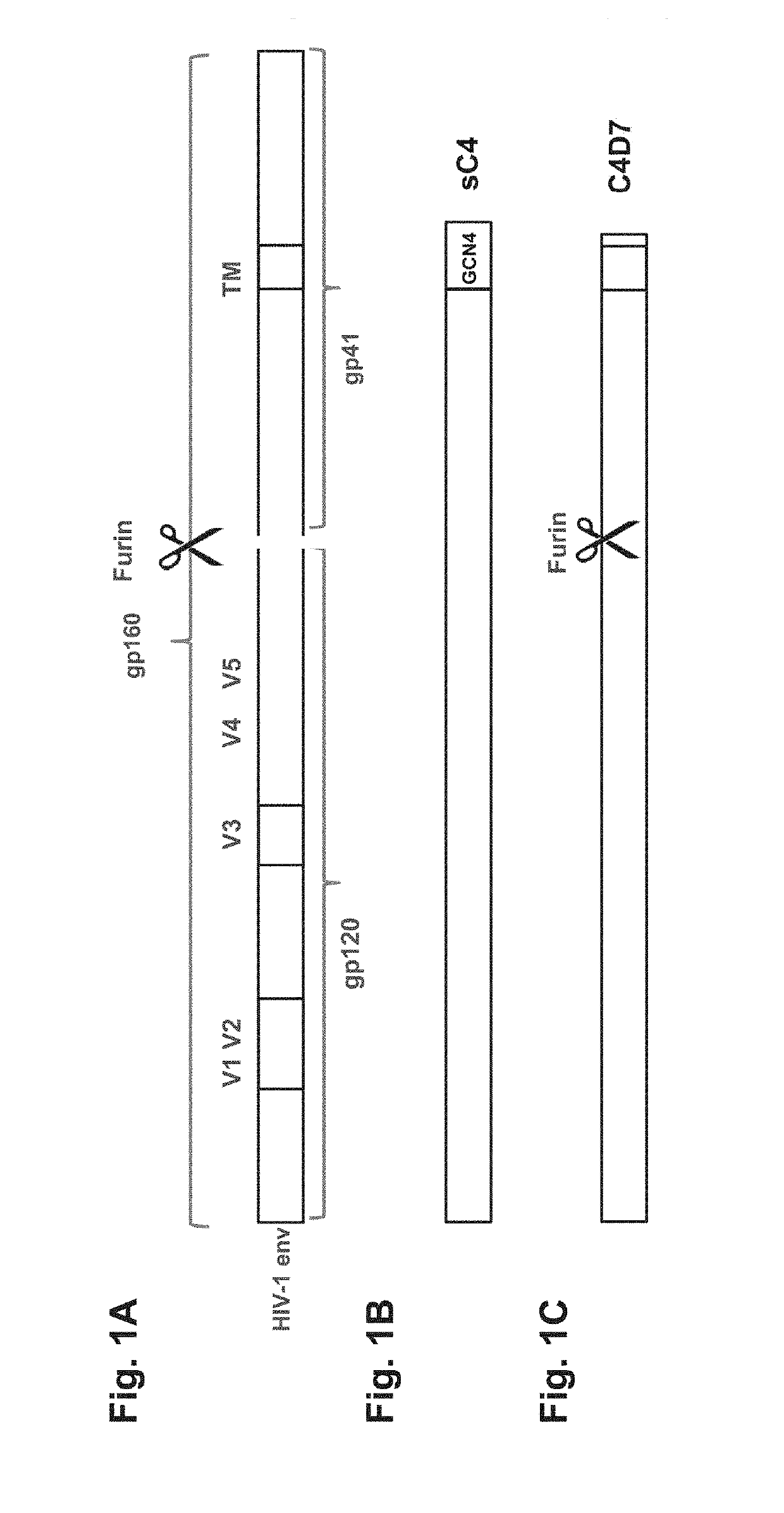
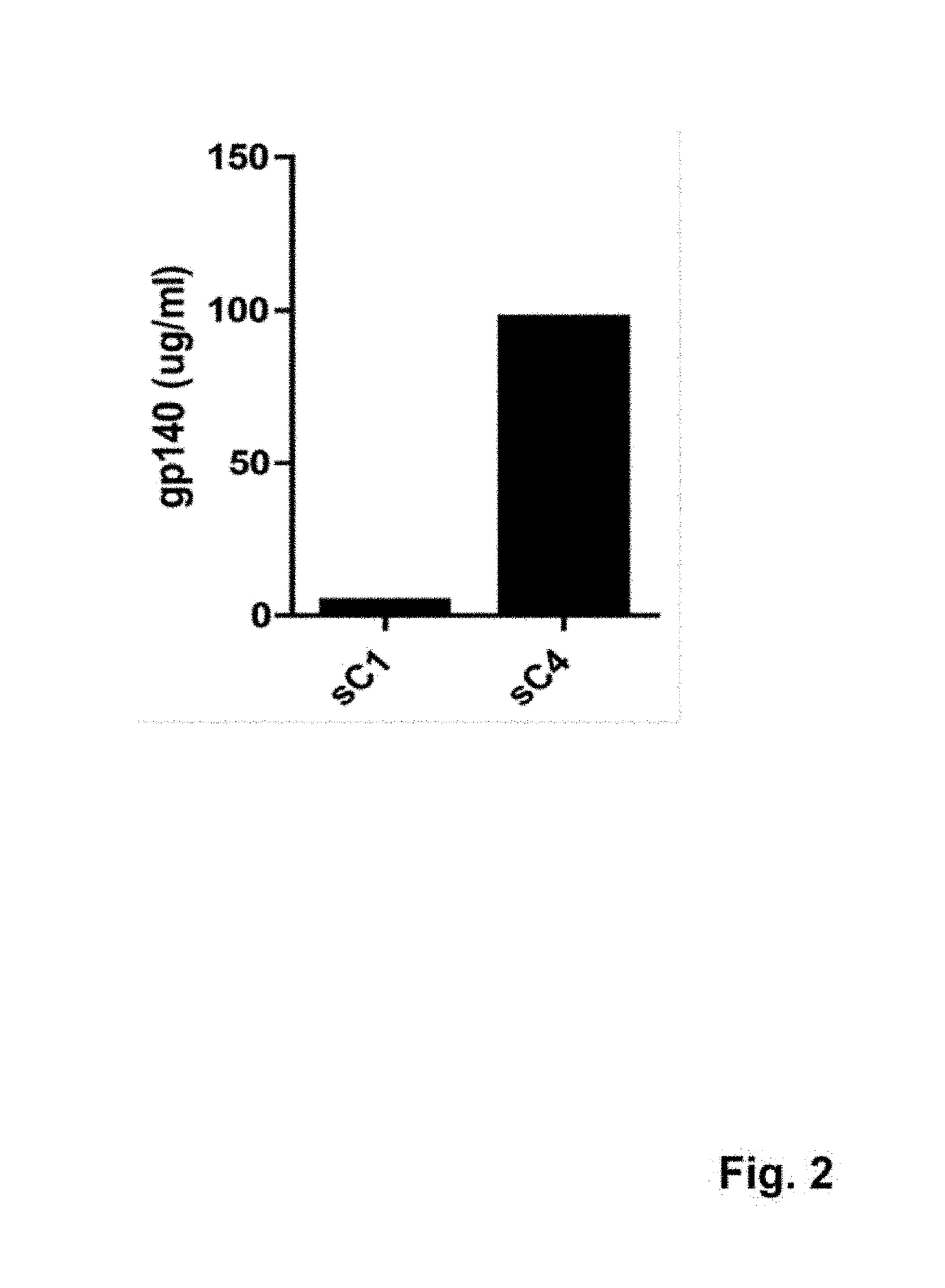
ActiveUS10369214B2High expressionImprove stabilityViral antigen ingredientsVirus peptidesAntigenHiv envelope
Synthetic HIV envelope proteins, vectors and compositions thereof, and methods for inducing protective immunity against human immunodeficiency virus (HIV) infection are described. Viral expression vectors encoding the synthetic HIV envelope proteins can be used in vaccines to provide improved protective immunity against HIV.
Owner:JANSSEN VACCINES & PREVENTION BV
Conditional replicating cytomegalovirus as a vaccine for cmv
ActiveUS20140220062A1Decrease likelihood of infectionReduce replicationSugar derivativesViral antigen ingredientsCytomegalovirus diseaseViral replication
The present invention relates to methods of inducing an immune response to cytomegalovirus (CMV) using a genetically modified CMV that is conditionally replication defective. The methods of the invention can be used to treat and / or prevent primary CMV infection, infection due to reactivation of a latent CMV and a super-infection of a different strain of CMV that had been previously encountered. The present invention also relates to a replication defective CMV which has been recombinantly altered to allow for external control of viral replication. Compositions comprising the replication defective CMV are also encompassed by the present invention.
Owner:MERCK SHARP & DOHME LLC
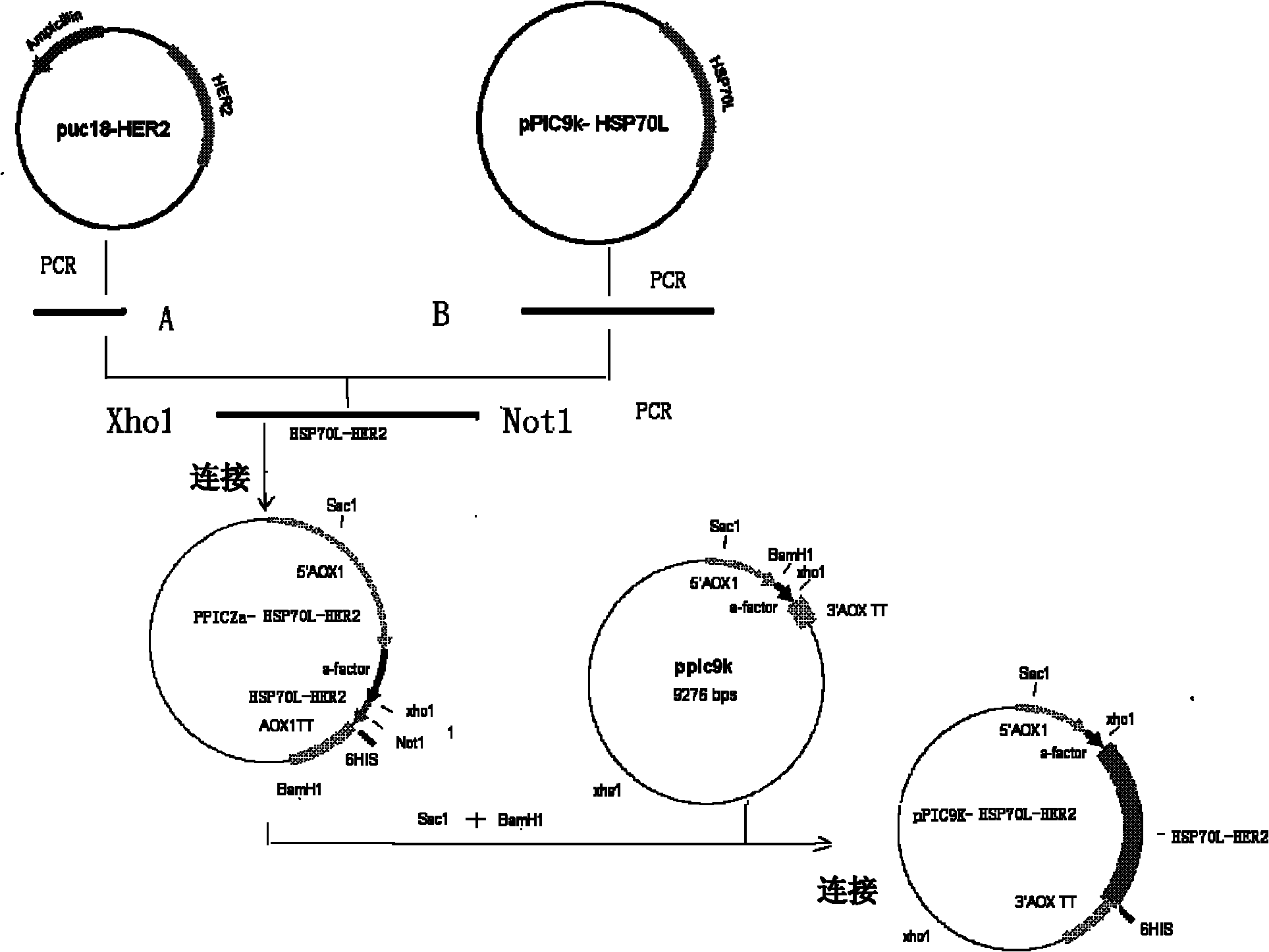
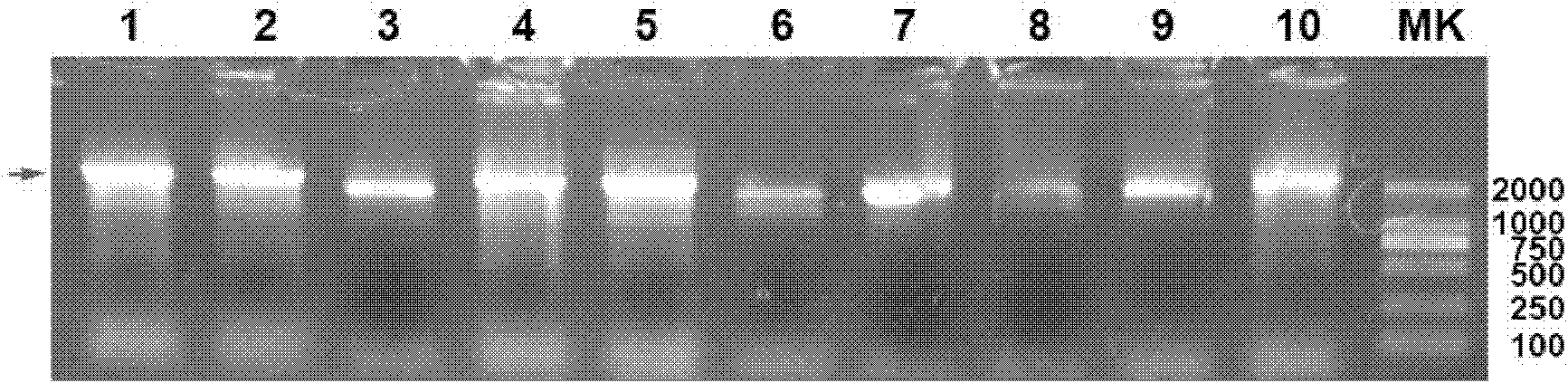
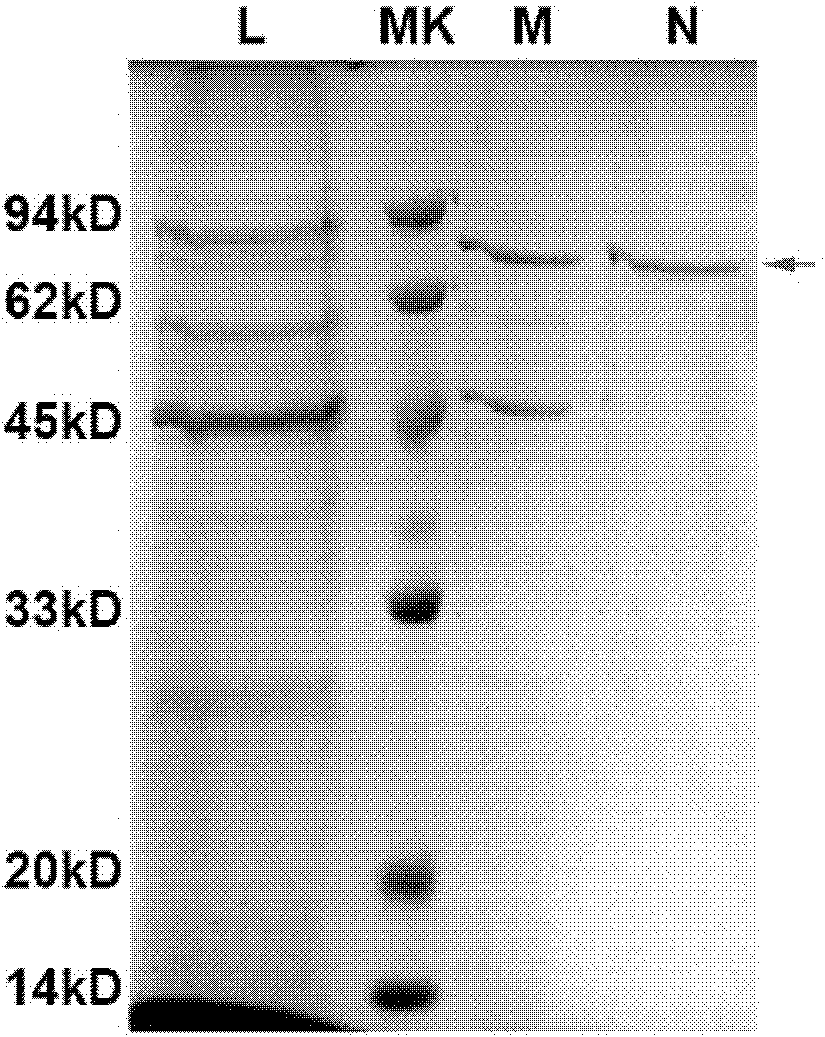
Preparation and application of Her2-neu antigen positive tumor therapeutic vaccine
The invention provides preparation and application of a Her2-neu antigen positive tumor therapeutic vaccine, and particularly provides a fusion protein. The fusion protein contains (a) a tumor antigen Her2-neu extracellular region element and (b) a heat shock protein element. The invention also provides a dendritic cell sensitized by fusion protein and a corresponding tumor therapeutic vaccine. Test proves Her2-neu antigen specific immunological response can be effectively activated by applying the vaccine in vivo, and the vaccine is effectively applied to Her2-neu positive tumor.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Nucleic Acid and Amino Acid Sequences, and Vaccine for the Control of Ectoparasite Infestations in Fish
ActiveUS20130280290A1Potential for inducing immune responseInduce immune responseFodderPeptide/protein ingredientsAntigenBiology
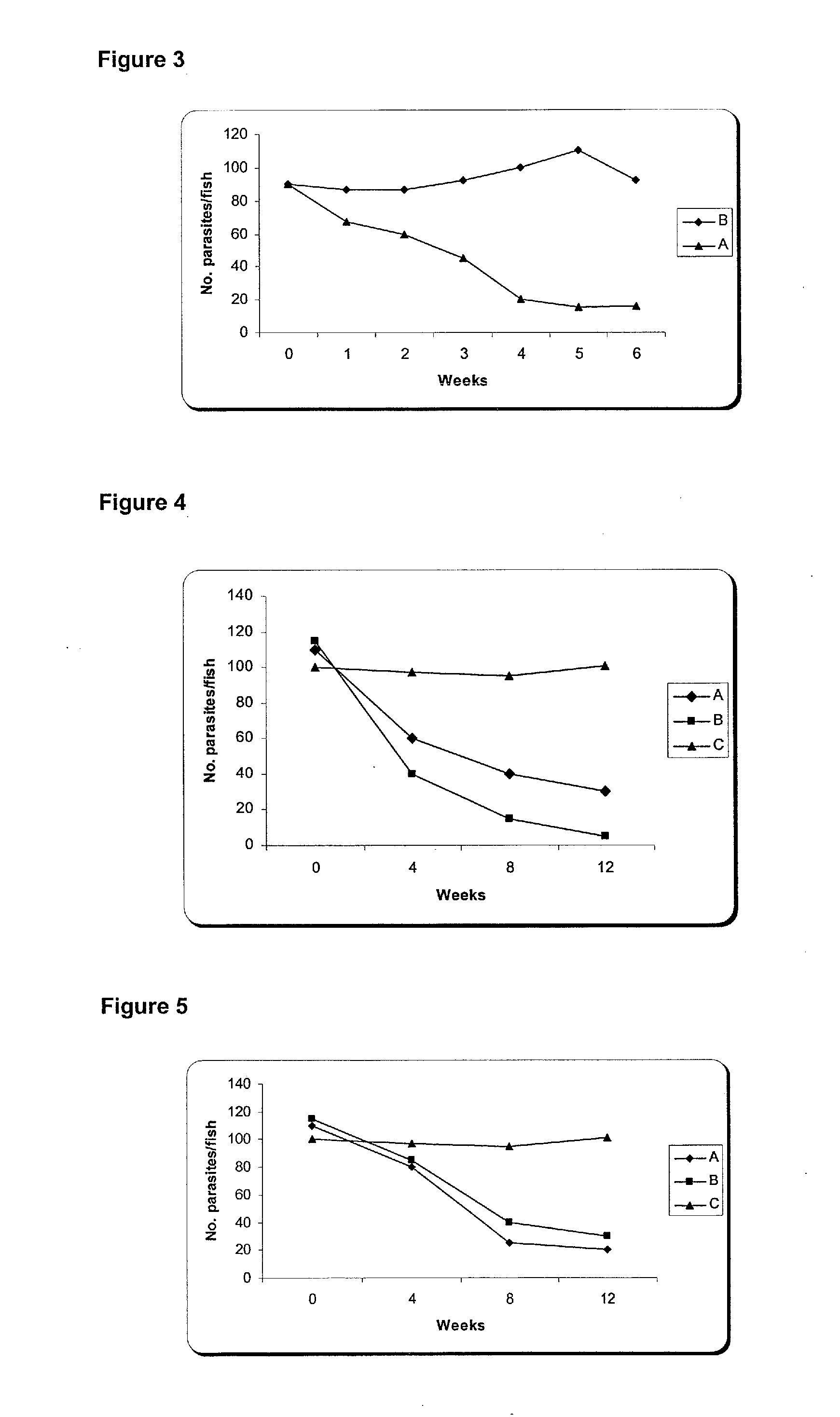
The present invention is related with the isolation and cloning of a new gene, the production of the protein encoded by this gene by using recombinant systems, and the use of this antigen in a vaccine formulation as a purified protein and / or naked DNA, to induce an immune response in aquatic organisms against different ectoparasite species, including the known as sea lice, and pathogens associated with these infestations. The vaccine preparations, administered by oral route, immersion bath or injection, demonstrated its efficacy by producing IgM humoral immune response and reducing the number of parasites per fish in the vaccinated fishes.
Owner:CENT DE ING GENETICA & BIOTECNOLOGIA
Messenger RNA nanoparticles and preparation method therefor
ActiveUS20170216457A1High expressionInduce immune responsePowder deliveryHydrolasesDiseaseGene delivery
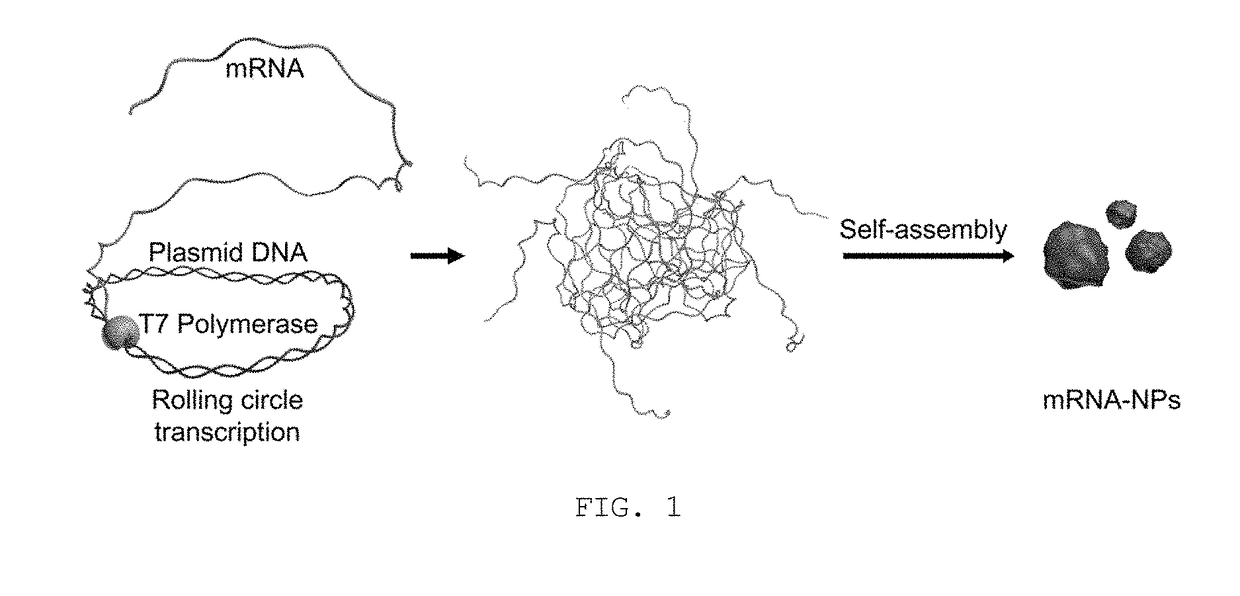
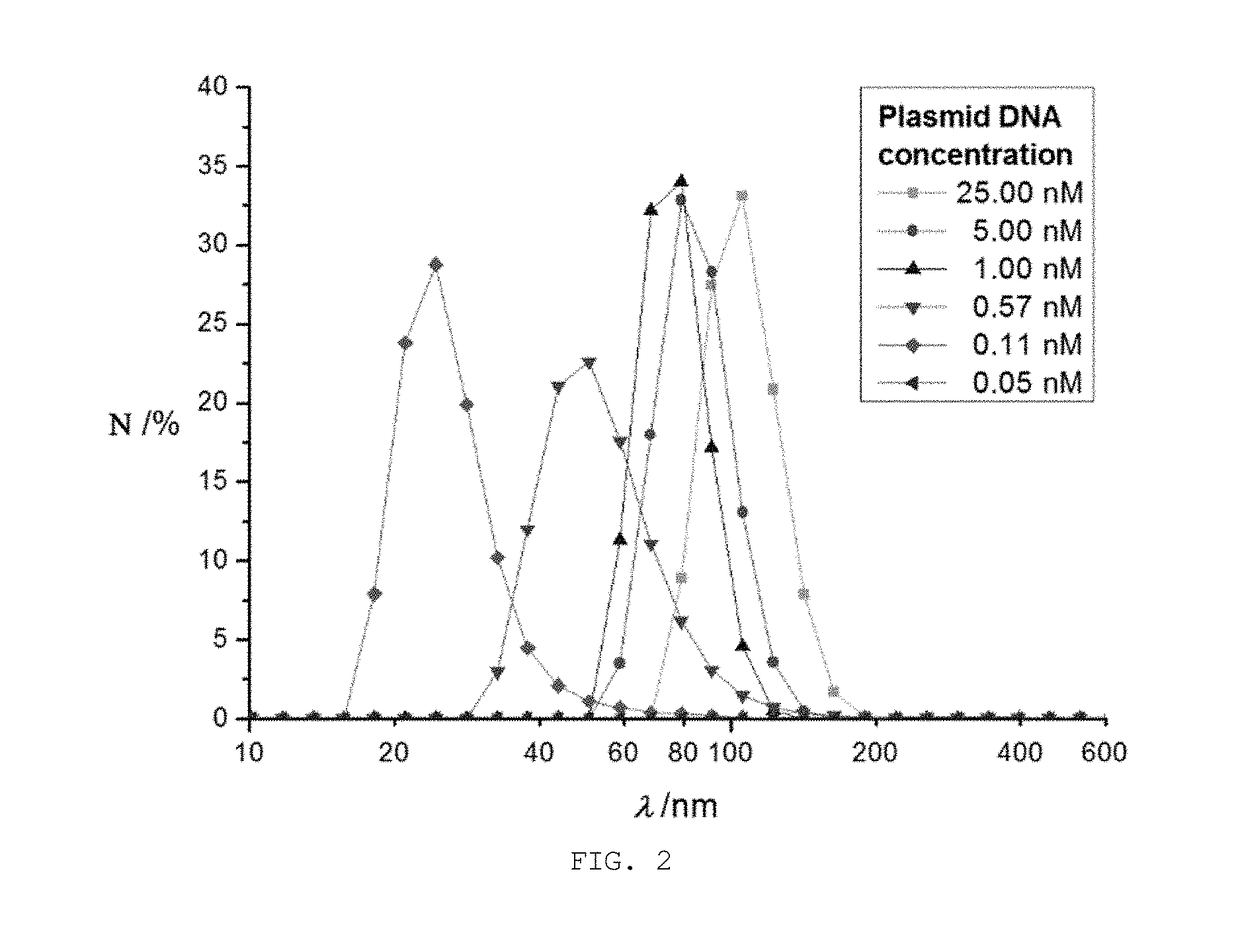
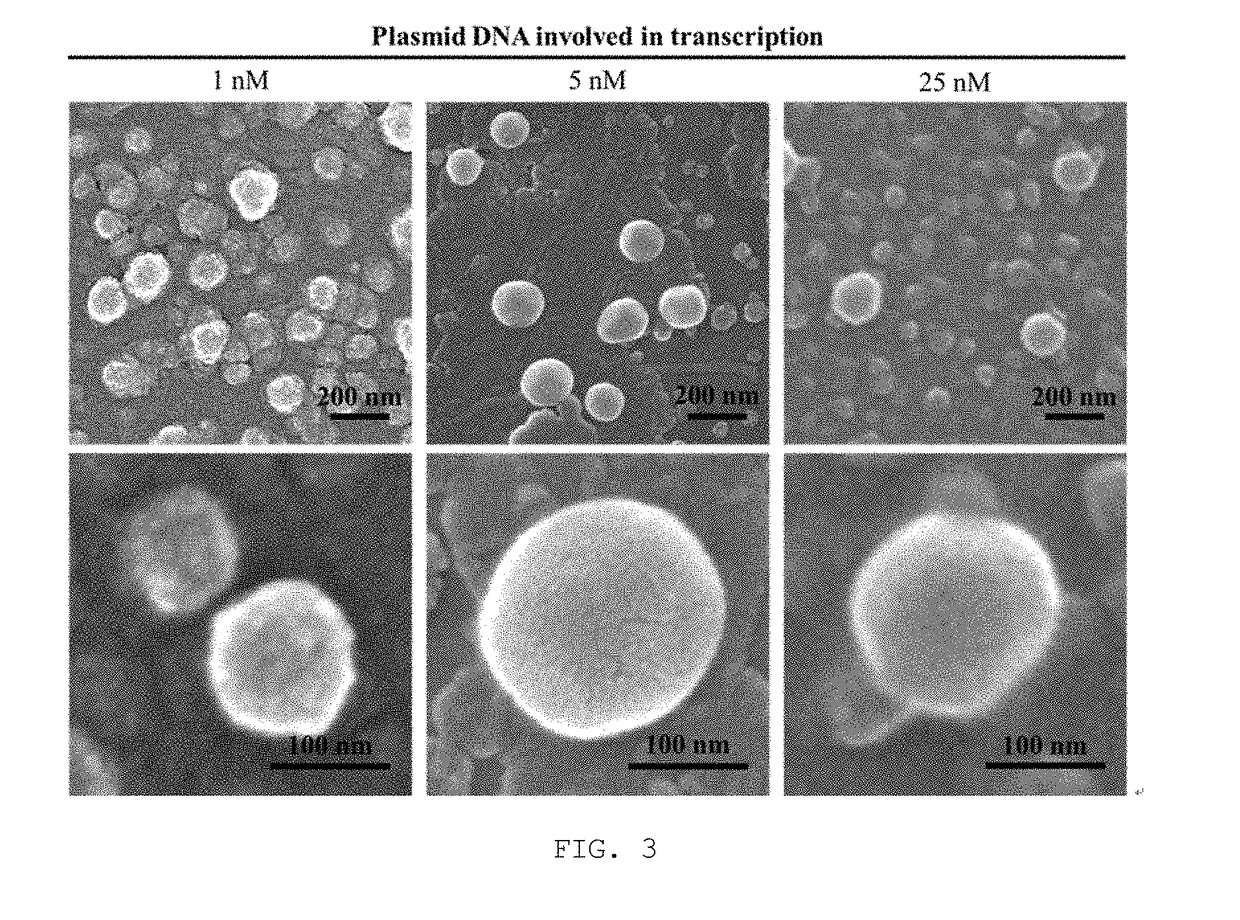
Disclosed are nanoparticles that are introduced into cells and express a specific protein and a manufacturing method thereof. More particularly, the present invention relates to mRNA nanoparticles, which increase the expression of a specific protein capable of stimulating the cellular immune system to induce cellular immune responses and are thus applicable to treat a variety of diseases, do not require passage across the nuclear envelope because a desired gene is delivered not as plasmid DNA itself but in the form of mRNA, thus improving the efficiency of protein expression, and the nanoparticles are generated through a one-step process with a relatively small amount of plasmid DNA via rolling circle transcription (RCT), thereby providing a simple and economical process for gene delivery. The present invention is also concerned with such mRNA nanoparticles.
Owner:UNIV OF SEOUL IND COOP FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com