Preparation and application of Her2-neu antigen positive tumor therapeutic vaccine
A her2-neu and tumor antigen technology, which is applied in the field of preparation of Her2-neu antigen-positive tumor therapeutic vaccines, can solve the problems of not achieving satisfactory immune effects, inability to effectively induce immune responses, and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
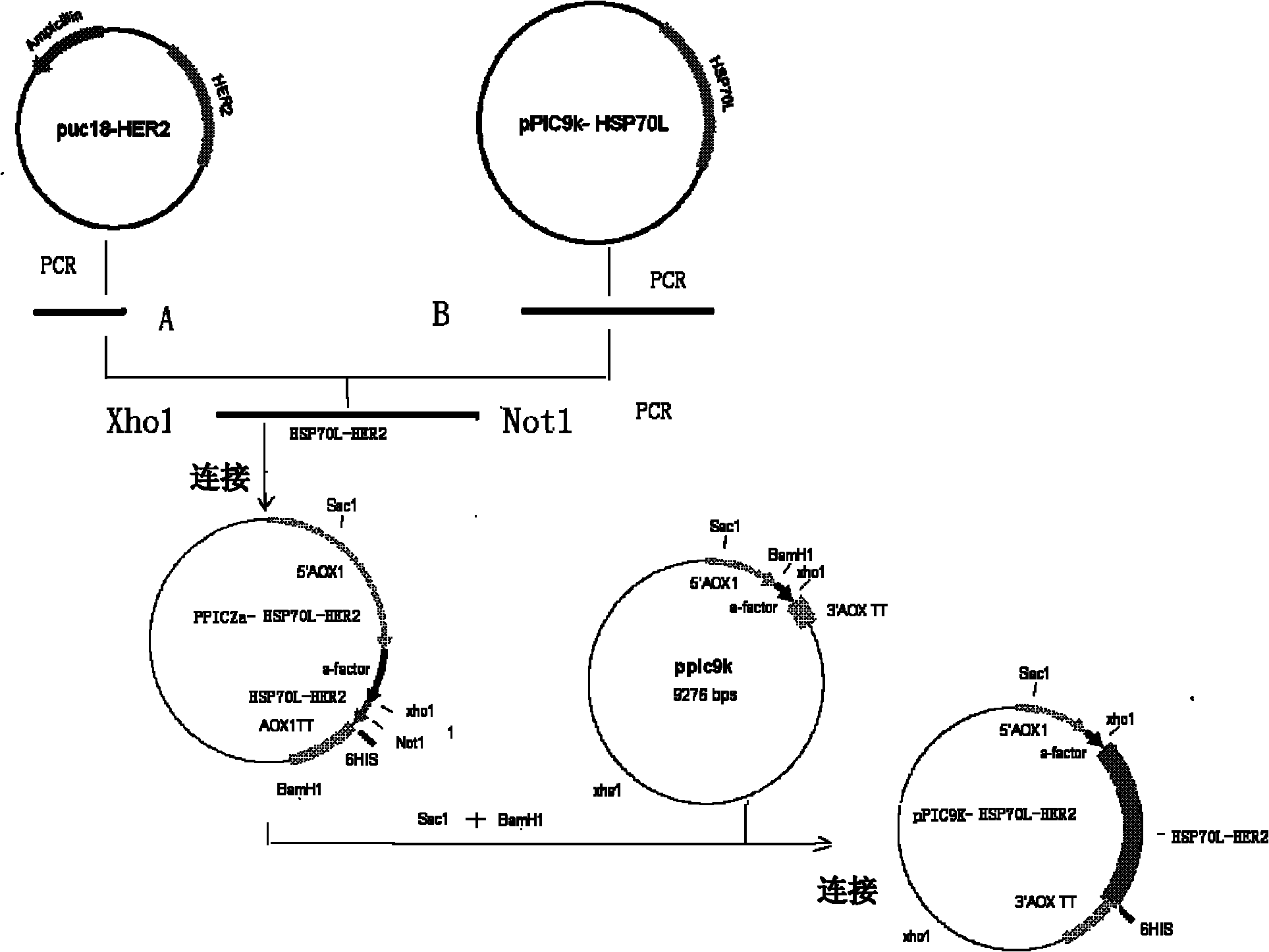
[0099] Her2 sequence design and optimization
[0100] According to the Her2 nucleic acid sequence design and synthesis of methanol yeast codon-optimized sequence SEQ ID NO: 1, and a Not I restriction site was added at the 3' end, the sequence was connected to the T vector pUC18 (purchased from Invitrogen), denoted as puc18-HER2 . The amino acid sequence of the Her2 / neu extracellular region element encoded by the optimized nucleotide sequence (SEQ ID NO: 1) is shown in SEQ ID NO: 2, with a length of 115, corresponding to positions 342-456.
Embodiment 2
[0102] Construction of recombinant expression plasmids
[0103] 1. Her2 342-456 and the acquisition of DNA fragments of Hsp70L1:
[0104]Using the plasmid puc18-HER2 as a template, and using M13R and HSP-HER2-F as primers, the encoding Her2 used to construct the fusion protein was obtained 342-456 DNA fragment (PCR product A, SEQ ID NO: 1).
[0105] The 5' end oligonucleotide primer M13R sequence used in the PCR reaction is:
[0106] 5'-CAGGAAACAGCTATGACC-3', (SEQ ID NO: 3).
[0107] The 3' end oligonucleotide primer HSP-HER2-F sequence is:
[0108] 5'-CTCTATTGAGATAGCATCTTGTTACGGTTTGGGTATG-3' (SEQ ID NO: 4).
[0109] At the same time, collect the HeLa cells (ATCC Number: CCL-2) that were heat-induced at 41°C for 1 h, use the total cellular RNA extraction reagent Trizol (Invitrogen Company) to prepare the total cellular RNA, and use AMV reverse transcriptase (Promega Company) to synthesize the first chain, using it as a template, amplified with the following α-factor and ...
Embodiment 3
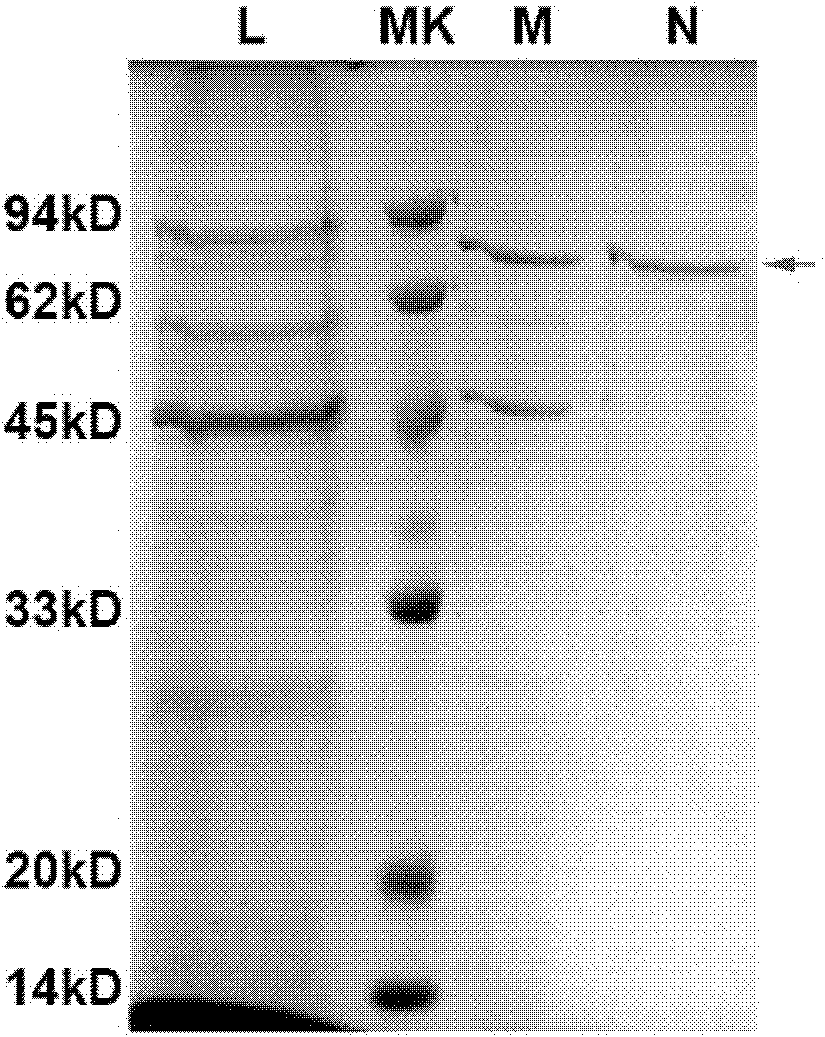
[0120] High expression of Hsp70L1-Her2 342-456 Construction of Engineering Bacteria and Identification of Positive Yeast Transformants
[0121] Kit (Qiagen) to extract a large amount of pPIC9k-Hsp70L1-Her2 342-456 The plasmid was linearized with SacI endonuclease, and electrotransformed into GS115 competent yeast cells under the transformation conditions of 2.0kV, 25μF, 200Ω to obtain yeast transformants.
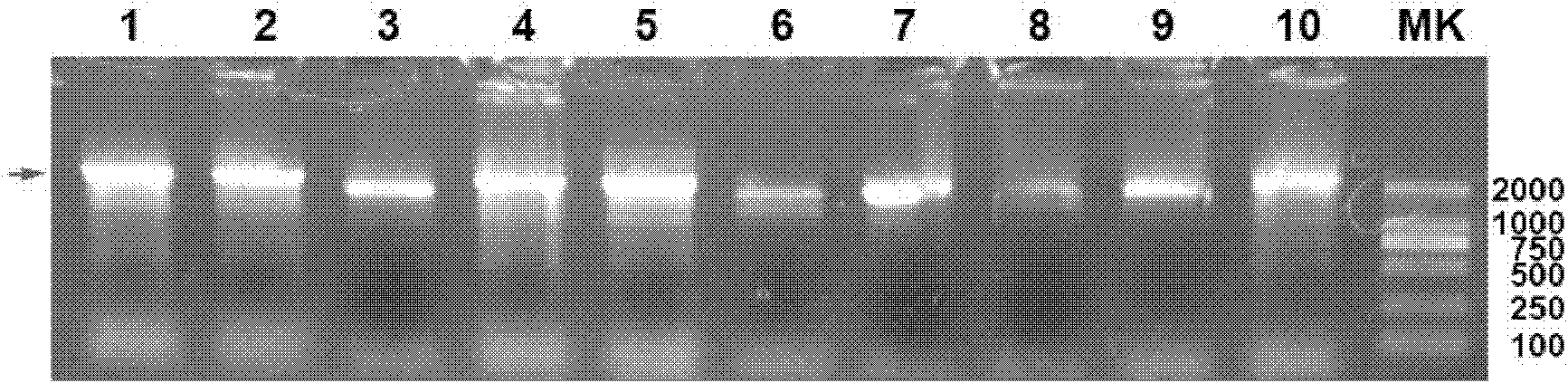
[0122] The single clones on the MD plate were inoculated into 3ml YPD medium respectively, and after culturing at 30°C for 24 hours, the yeast genomic DNA was extracted, and the α-factor primer (SEQ ID NO: 4) and 3'-aox primer (3'-AOX : 5'-GCAAATGGCATTCTGACATCC-3', (SEQ ID NO: 7)), yeast transformants were identified by PCR. Positive recombinants were identified by electrophoresis.
[0123] The results showed that for positive yeast transformants (referred to as pPIC9k-Hsp70L1-Her2 342-456 Yeast), a PCR band of about 2.1k was obtained ( figure 2 ), which is consistent...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com