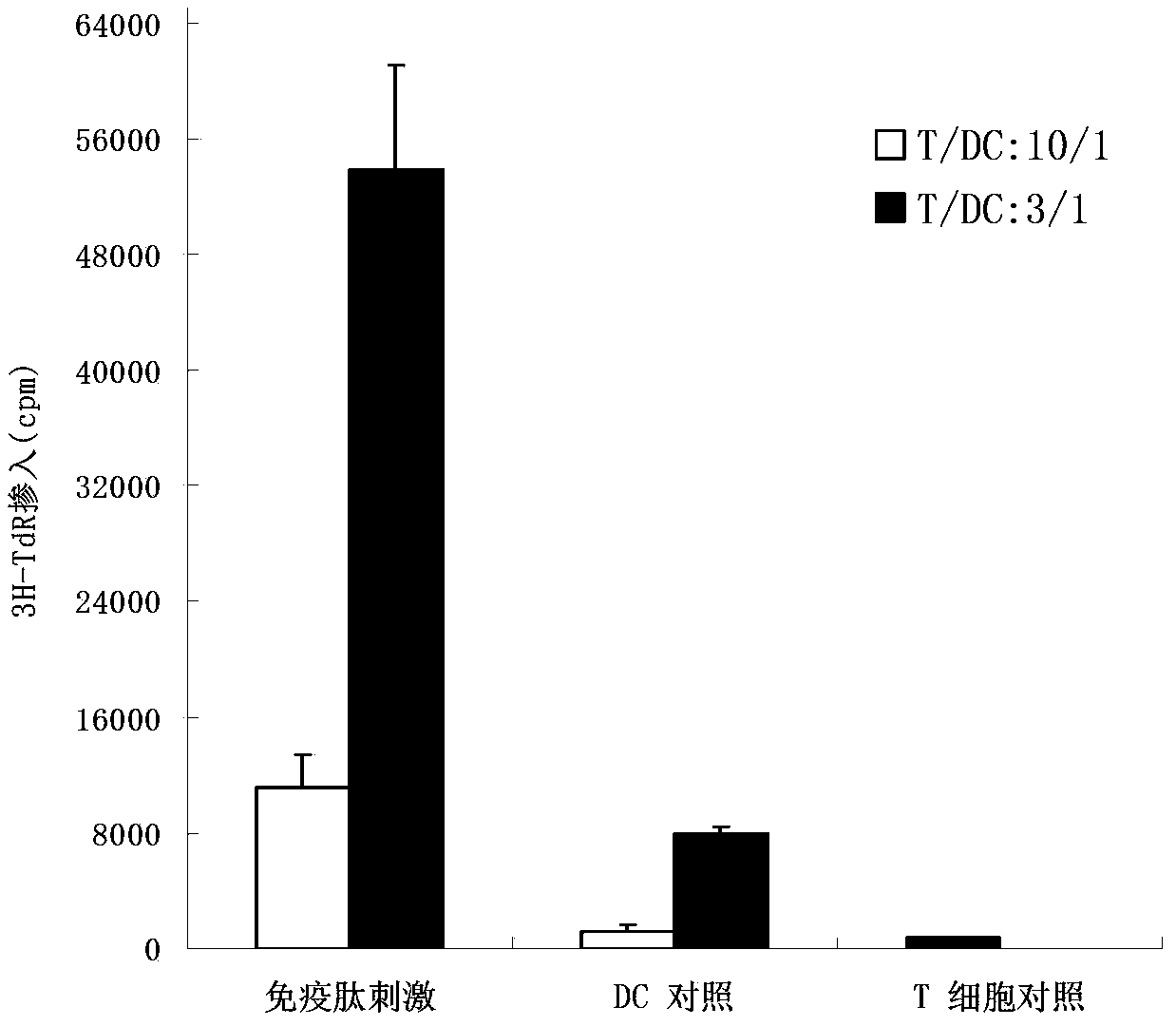
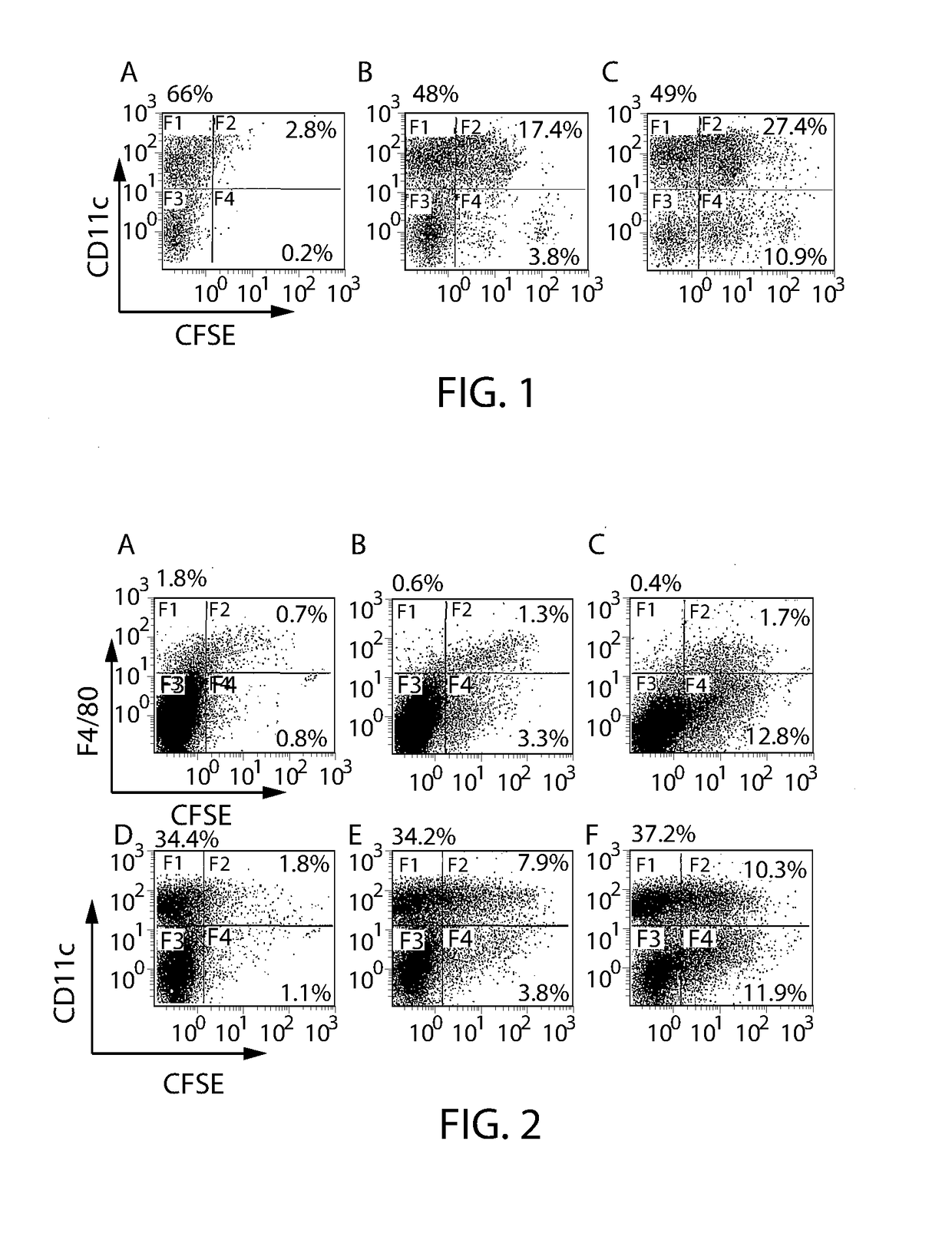
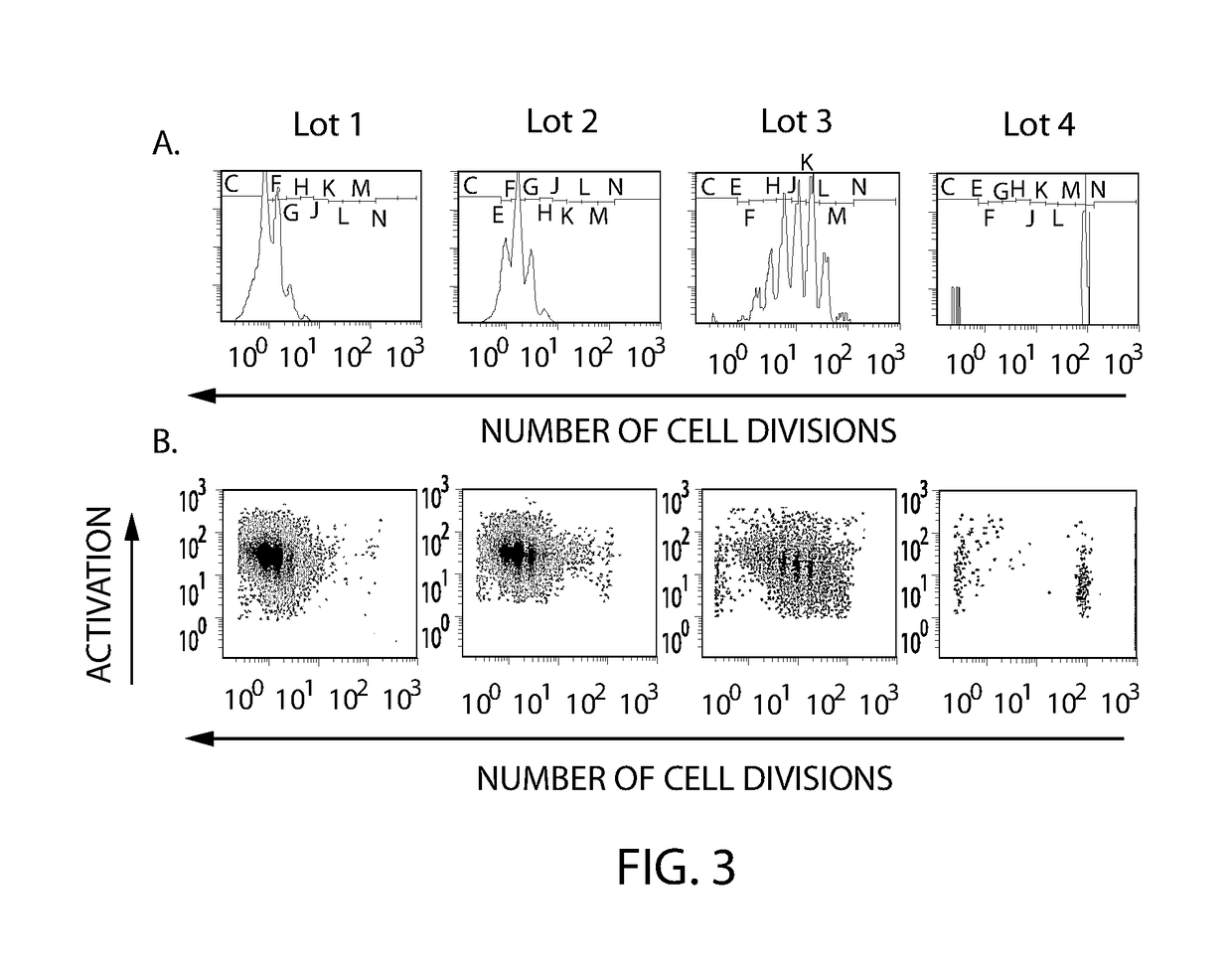
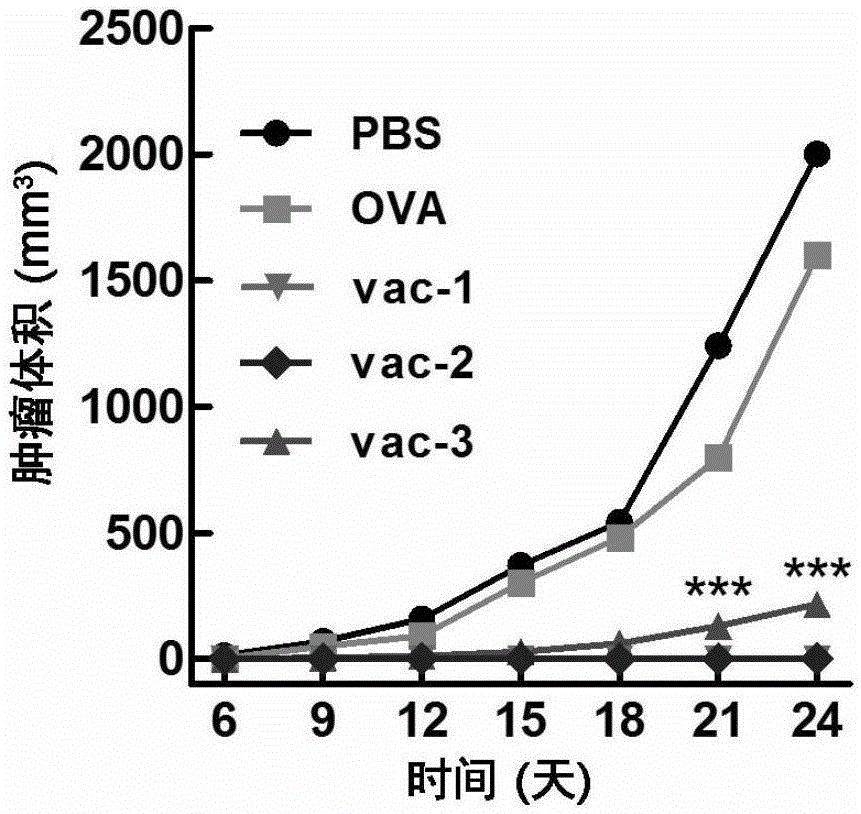
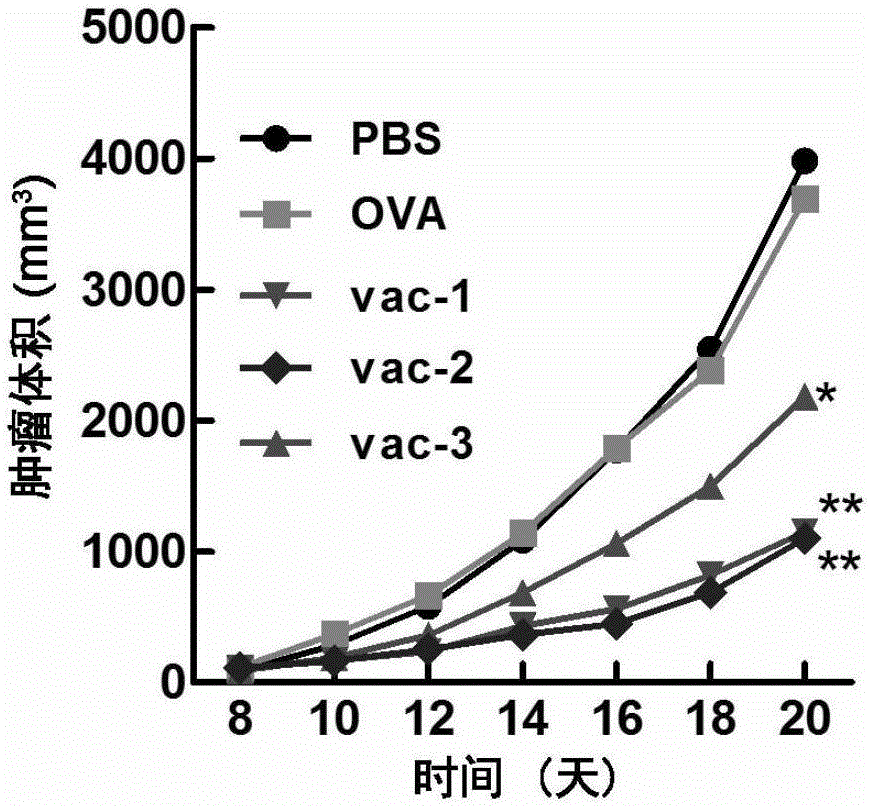
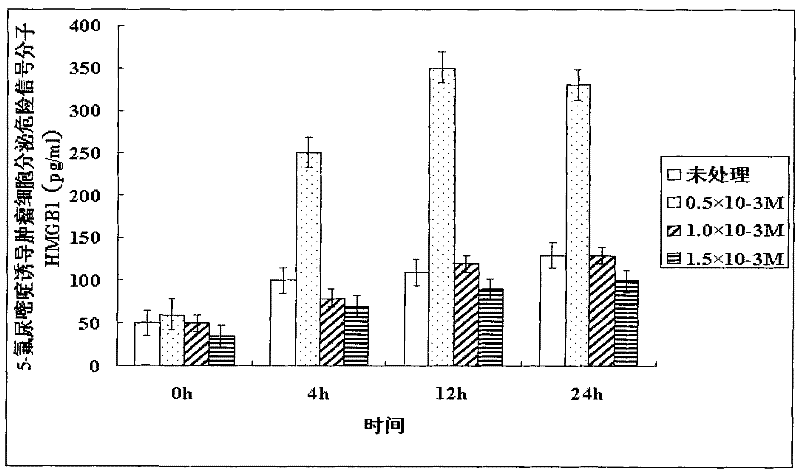
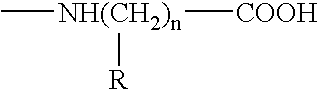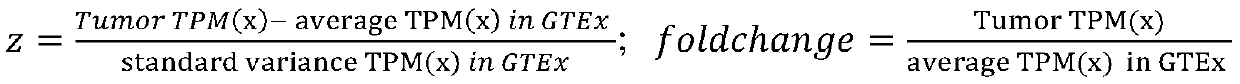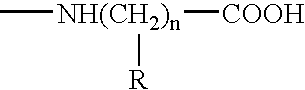Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
381 results about "Tumor vaccines" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Compositions and methods for treatment of tumors and metastatic diseases
InactiveUS6406689B1Stimulate immune responseSimple and reliable to useBiocideSnake antigen ingredientsDiseaseActive immunization
Owner:FALKENBERG FR W
Modulation of negative immune regulators and applications for immunotherapy
ActiveUS20060292119A1Easy to integrateBiocideSsRNA viruses positive-senseVaccinationImmunocompetence
The invention includes compositions and methods for enhancing immunopotency of an immune cell by way of inhibiting a negative immune regulator in the cell. The present invention provides vaccines and therapies in which antigen presentation is enhanced through inhibition of negative immune regulators. The present invention also provides a mechanism to break self tolerance in tumor vaccination methods that rely on presentation of self tumor antigens.
Owner:BAYLOR COLLEGE OF MEDICINE
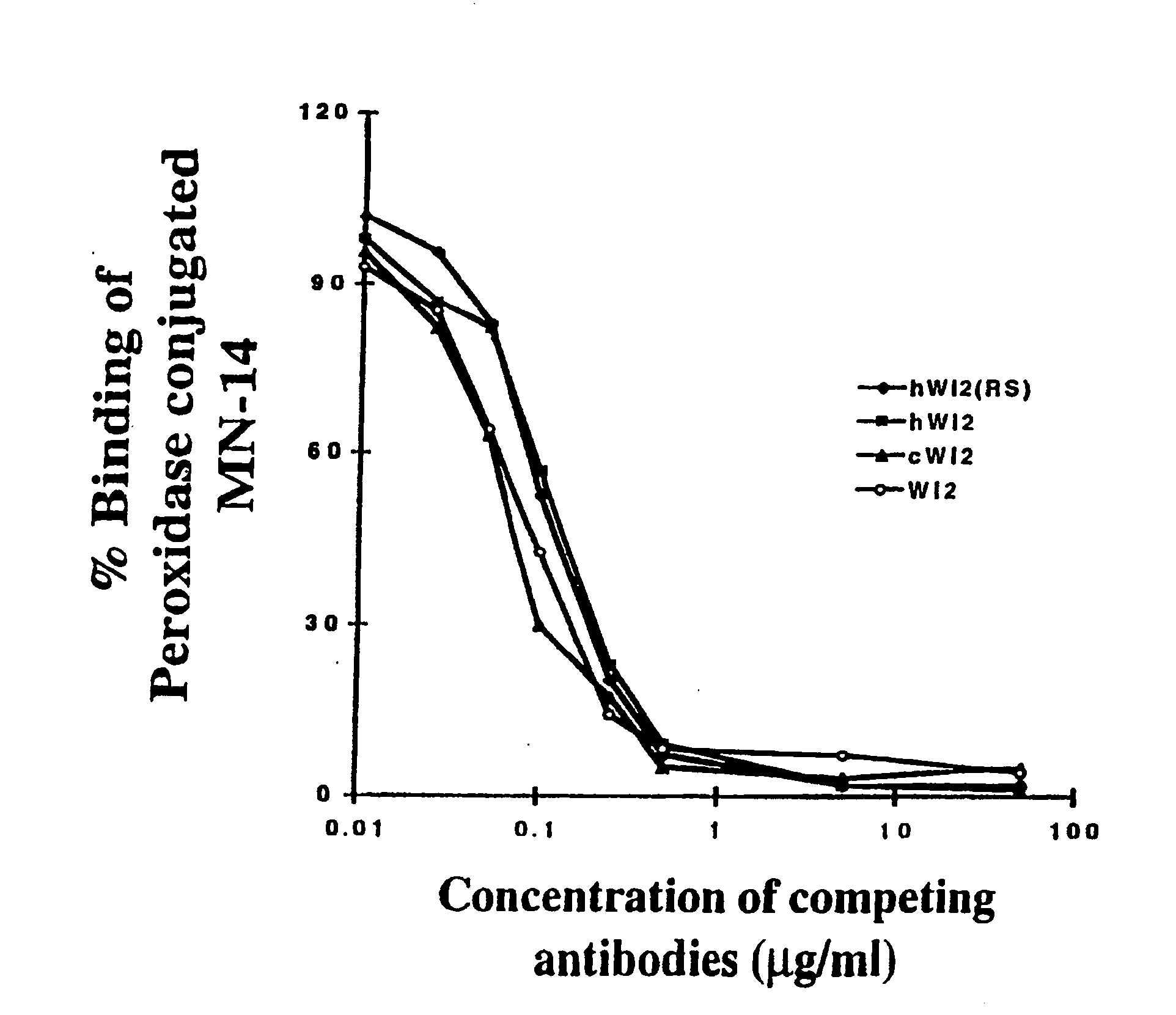
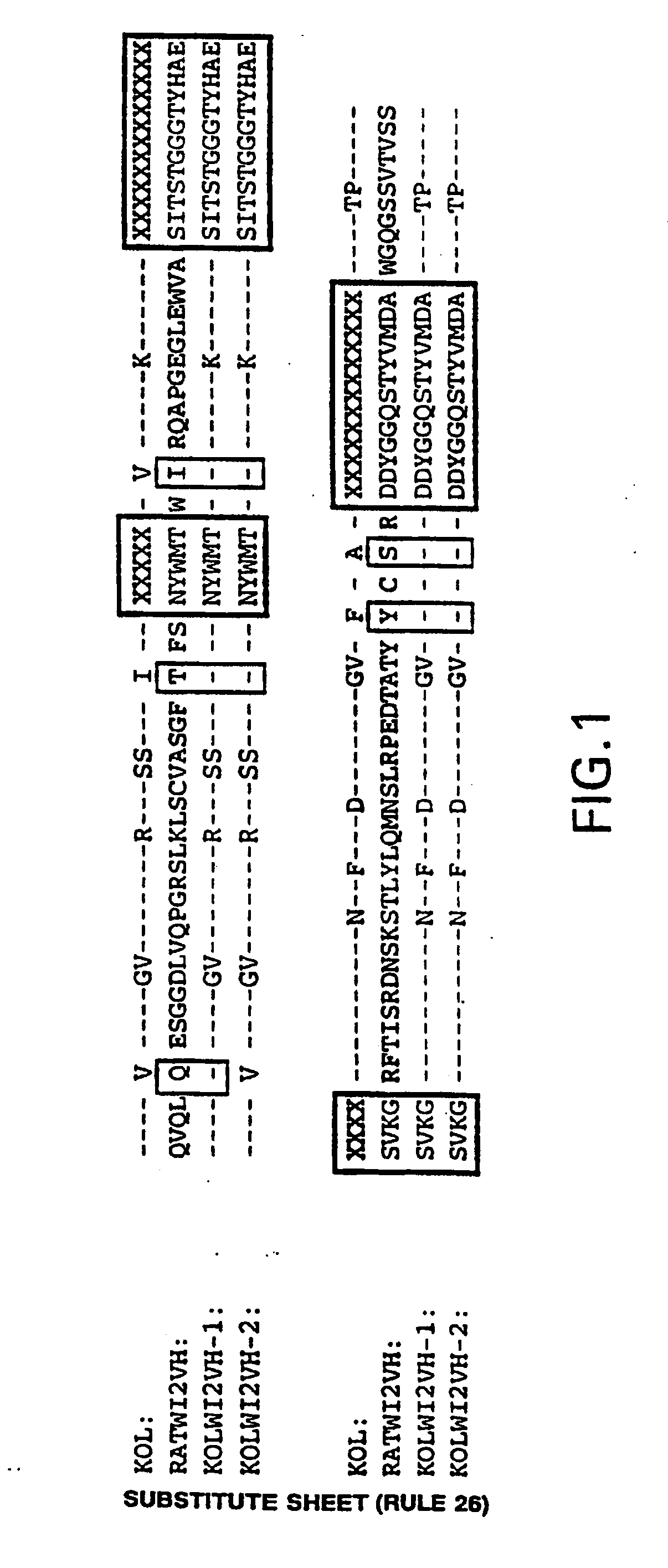
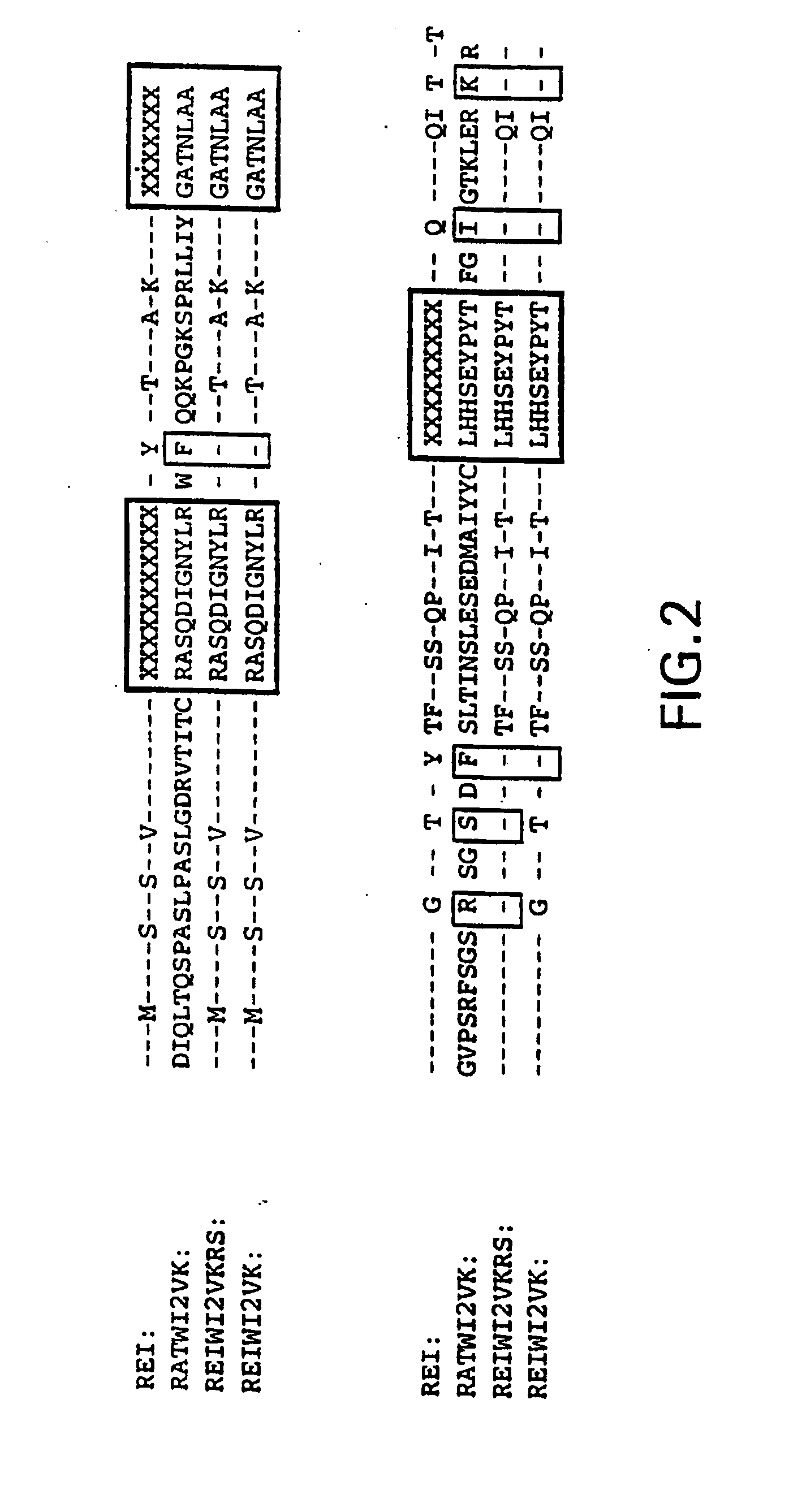
Humanization of an Anti-carcinoembryonic antigen Anti-idiotype antibody as a tumor vaccine and for targeting applications
InactiveUS20080069775A1Ultrasonic/sonic/infrasonic diagnosticsOrganic active ingredientsAntibody fragmentsAnti-CEA Antibody
A humanized form of an anti-idiotype antibody to CEA, e.g., hWI2, has conserved immunoreactivity. The clinical benefits of anti-CEA antibodies are maximized by using the humanized anti-idiotype as a clearing agent for anti-CEA antibodies or antibody fragments. The humanized anti-idiotype also can be used as an immunogenic vaccine.
Owner:IMMUNOMEDICS INC
Recombinant human Claudin18.2 tumor vaccine and preparation method thereof
InactiveCN101584860AImprove securitySimple manufacturing methodMicroorganism based processesAntibody medical ingredientsSide effectMouse Stomach
The invention belongs to the field of medicinal biotechnology, and in particular relates to a recombinant human Claudin18.2 tumor vaccine and a preparation method thereof. The invention aims to solve the problems of immunotherapy for stomach cancer, pancreatic cancer, esophagus cancer and metastatic and non-metastatic ovarian cancer, such as high after-excision recurrence rate, strong chemo-treatment and radiation treatment toxic side effect and high monoclonal antibody therapy cost. The invention adopts a technical proposal that the recombinant human Claudin18.2 tumor vaccine has a sequence of HMKSSQYIKANSKFIGEFDQWSTQDLYNNPVTAVFNYQGLWRSCVRESSGFTECRGYFTLLGLPAMLQAV. Animal experiments prove that rhClaudin18.2 fusion protein can induce high-titre neutralizing antibody in the bodies of tumor-bearing mice by over 1:10,000; the antibody can be combined with human KATOIII and PANC-1 tumor cells and mouse stomach cancer MFC and pancreatic cancer MPC-83 cells; and the protein serving as a tumor vaccine can suppress the growth of the stomach cancer MFC and pancreatic cancer MPC-83 cells in the bodies of the mice.
Owner:西安杰诺瓦生物科技有限公司
System and Method of Preparing and Storing Activated Mature Dendritic Cells
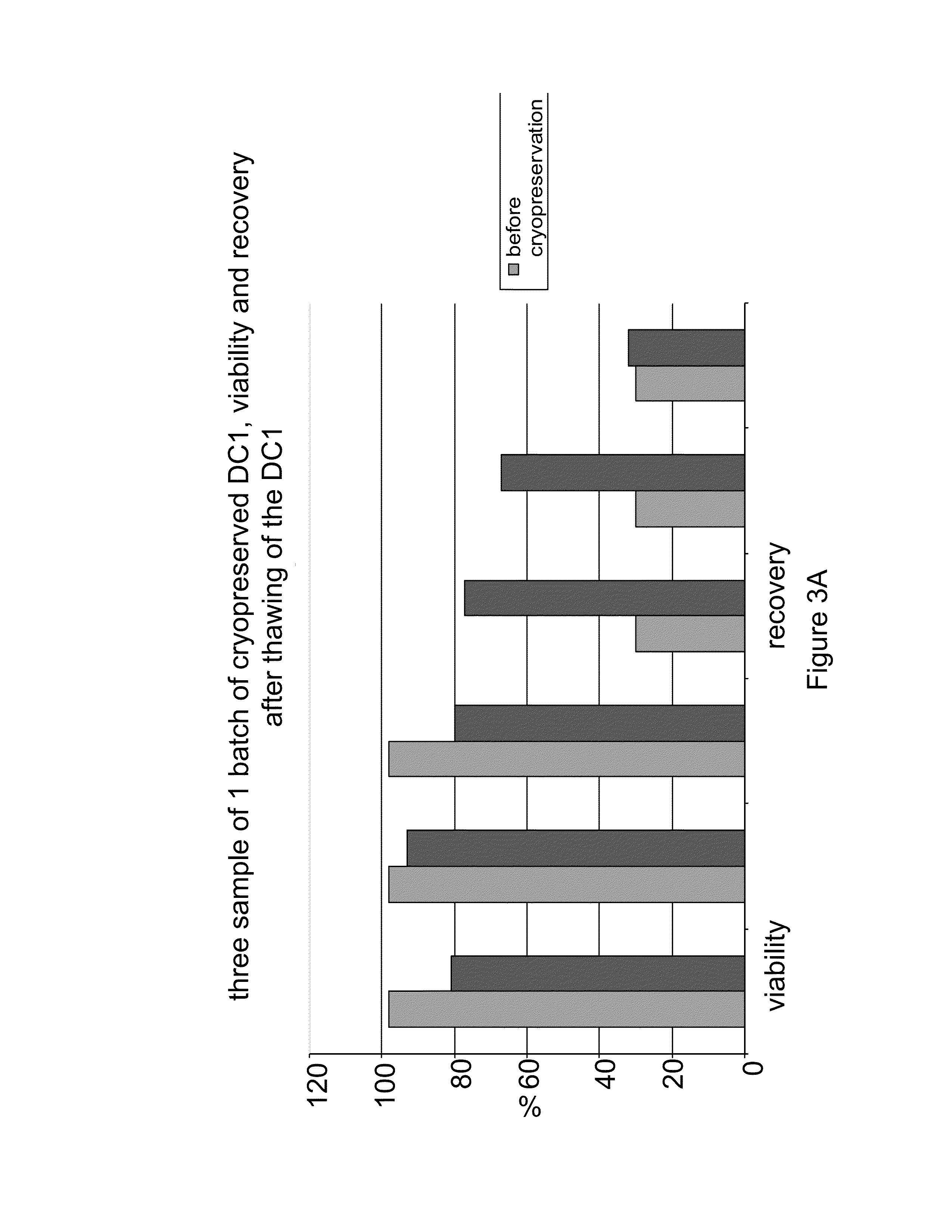
InactiveUS20130183343A1Promote recoveryImprove viabilityAntibacterial agentsBacterial antigen ingredientsDendritic cellVaccine Production
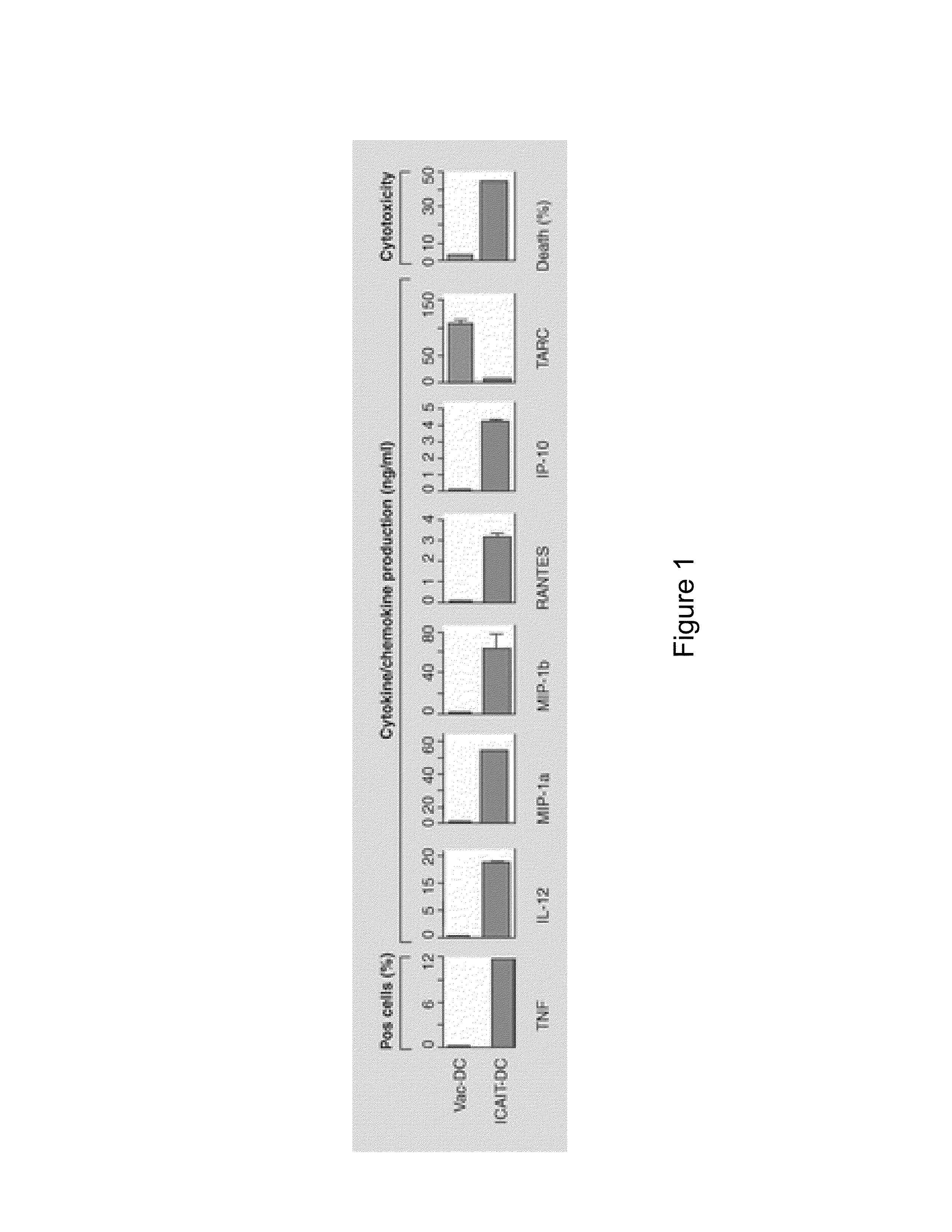
The present invention provides compositions and methods for generating and cryopreserving dendritic cells with superior functionality in producing stronger signals to T cells, resulting in a more potent DC-based anti-tumor vaccine. The present invention includes mature, antigen loaded DCs activated by Toll-like receptor agonists that induce clinically effective immune responses, preferably when used earlier in the disease process. The DCs of the present invention produce desirable levels of cytokines and chemokines, and further have the capacity to induce apoptosis of tumor cells. The cells can be cryopreserved and thawed for later use, thereby reducing the need for repeated pheresis and elutriation processes during vaccine production. These methods can also be utilized to directly target molecules involved in carcinogenetic signaling pathways and cancer stem cells.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Methods and compositions to enhance vaccine efficacy by reprogramming regulatory t cells
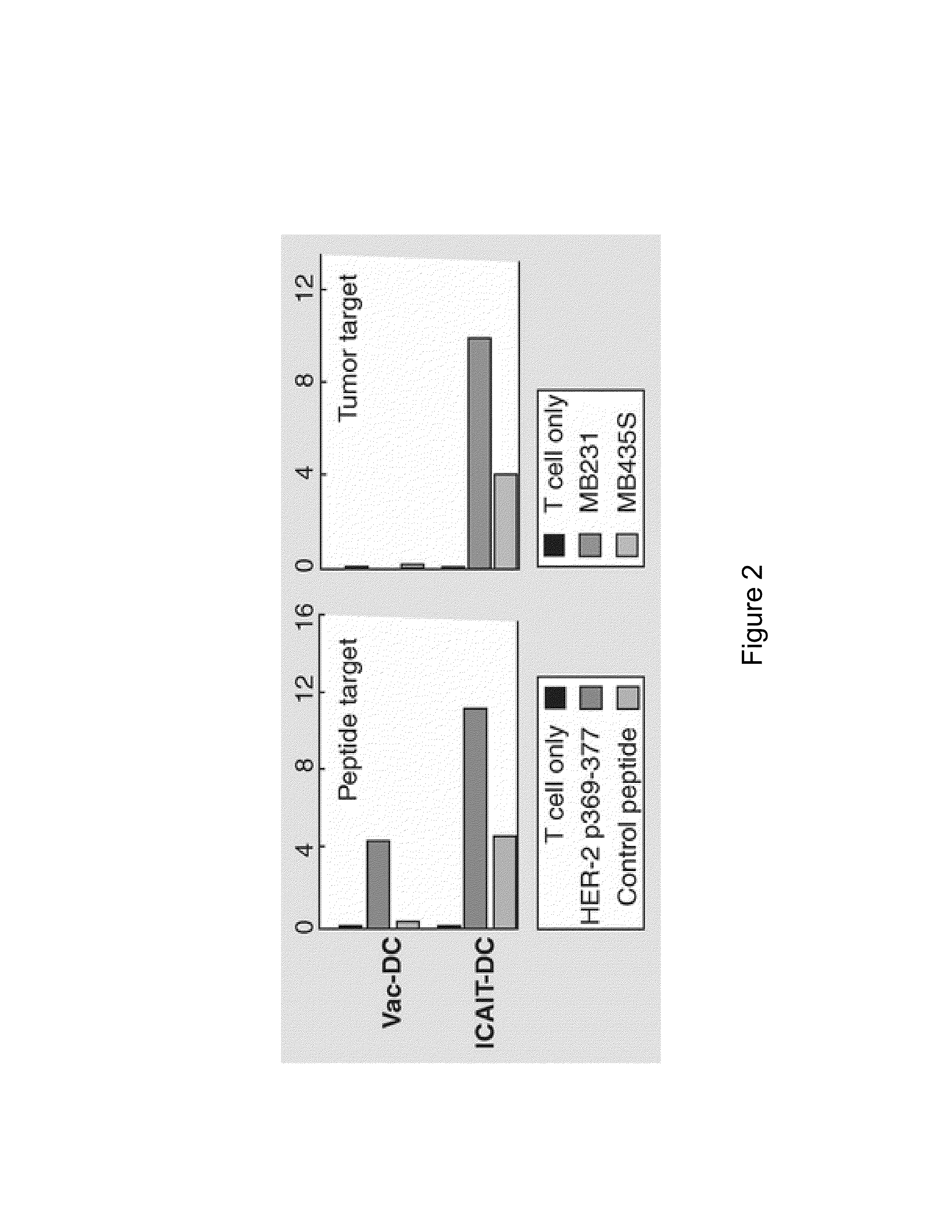
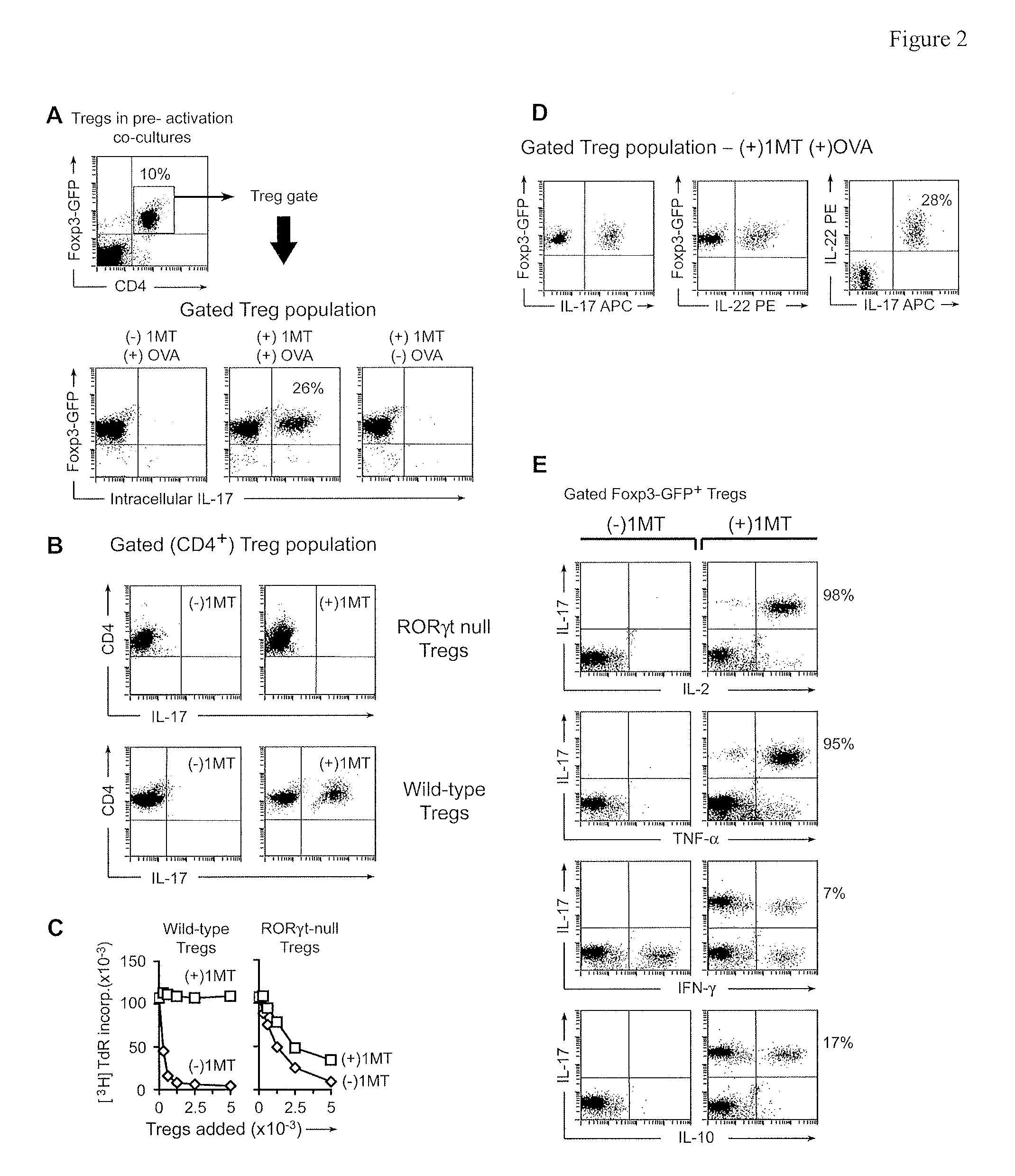
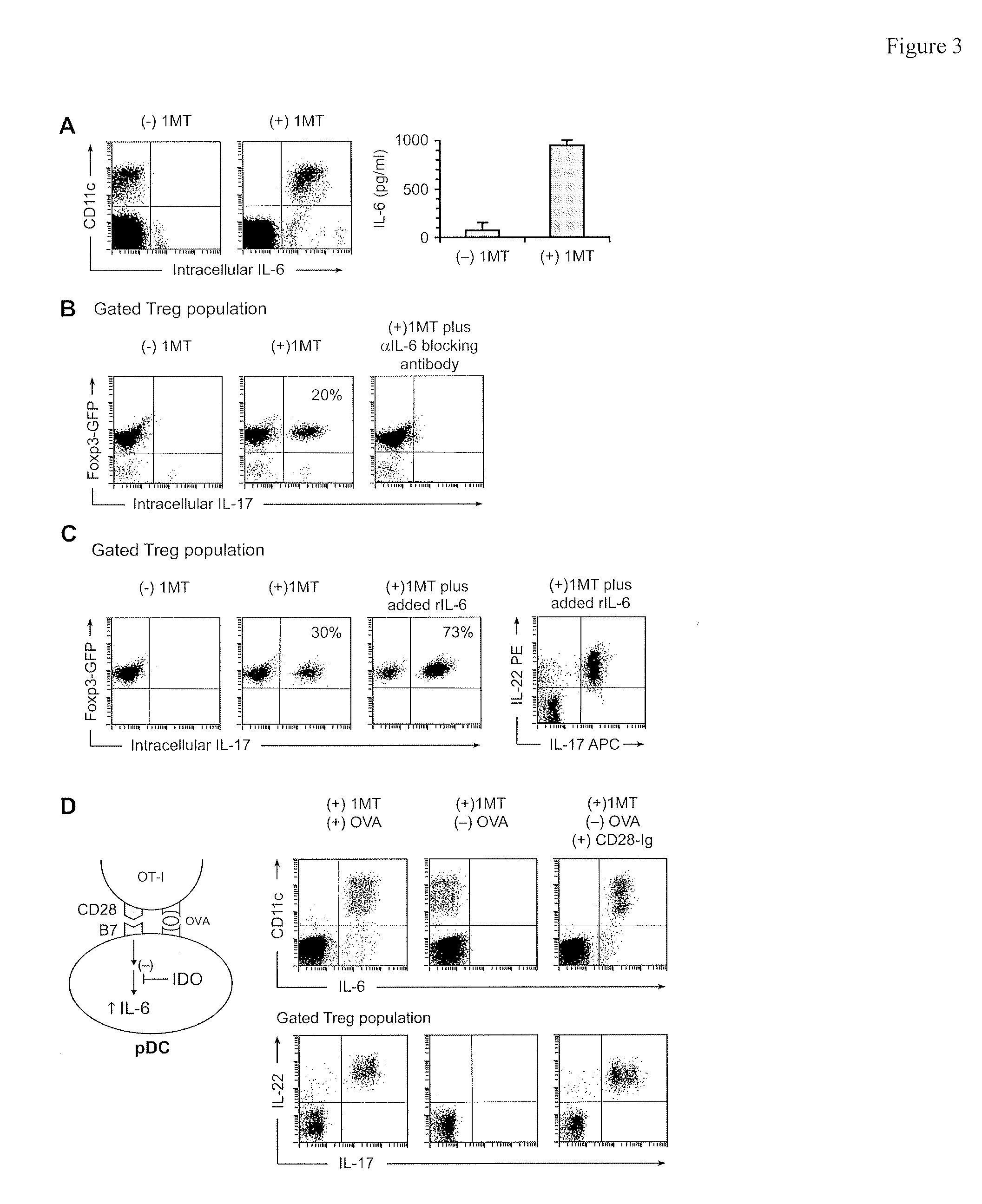
The immunoregulatory enzyme indoleamine 2,3-dioxygenase (IDO) is expressed by a subset of murine plasmacytoid DCs (pDCs) in tumor-draining LNs, where it can potently activate Foxp3 regulatory T cells (Tregs). We now show that IDO functions as a molecular switch in tumor-draining LNs, maintaining Tregs in their normal suppressive phenotype when IDO was active, but allowing inflammation-induced conversion of Tregs to a polyfunctional T-helper phenotype similar to proinflammatory TH17 cells when IDO was blocked. In vitro, conversion of Tregs to the TH17-like phenotype was driven by antigen-activated effector T cells, and required IL-6 produced by activated pDCs. IDO regulated this conversion by dominantly suppressing production of IL-6 in pDCs, in a GCN2-kinase dependent fashion. In vivo, using a model of established B16 melanoma, the combination of an IDO-inhibitor drug plus anti-tumor vaccine caused upregulation of IL-6 in pDCs and in situ conversion of a majority of Tregs to the TH17 phenotype, with marked enhancement of CD8+ T cell activation and anti-tumor efficacy. Thus, Tregs in tumor-draining LNs can be actively re-programmed in vitro and in vivo into T-helper cells, without the need for physical depletion, and IDO serves as a key regulator of this critical conversion.
Owner:GEORGIA HEALTH SCI UNIV RES INST
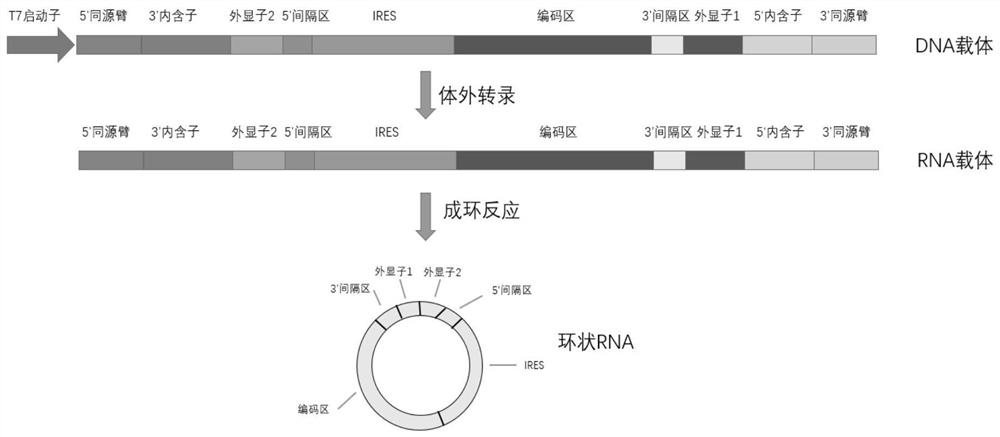
Recombinant nucleic acid molecule for transcribing circular RNA and application of recombinant nucleic acid molecule in protein expression
ActiveCN112481289AImprove expression levelImprove expression efficiencyTumor rejection antigen precursorsSsRNA viruses positive-senseDendritic cellTGE VACCINE
The invention relates to a recombinant nucleic acid molecule for transcribing circular RNA and application of the recombinant nucleic acid molecule in protein expression. Specifically, the invention relates to the recombinant nucleic acid molecule for transcribing circular RNA, a recombinant expression vector, linear RNA, circular RNA, a recombinant host cell, a pharmaceutical composition and a method for preparing protein. The recombinant nucleic acid molecule is transcribed to form circular RNA containing a specific IRES element, the IRES element can improve the protein expression level of the circular RNA in eukaryotic cells, efficient and durable expression of protein is achieved, and the recombinant nucleic acid molecule has important application values for preparing mRNA infectious disease vaccines, therapeutic mRNA tumor vaccines and mRNA-based dendritic cell tumor vaccines, and in the fields of gene therapy based on mRNA, chimeric antigen receptor T cell therapy based on mRNA,protein supplement therapy and the like.
Owner:SUZHOU CUREMED BIOMEDICAL TECH CO LTD
Recombination human Mucl-MBP fusion protein antitumour vaccine and production technology
InactiveCN1513556ATo achieve the purpose of anti-tumorLow costPeptide/protein ingredientsAntibody medical ingredientsEscherichia coliChemical synthesis
An anticancer vaccine of recombinant human MOC1-MBP fusion protein is disclosed, in which MBP is used as its adjuvant. The MBP gene and MUC1 gene are fused together. The MBP substituted for other fusion protein to induce CTL reaction. The pMAL-P2 is the carrier for effectively expressing maltose fusion protein. The serial repetitive sequence of MUC1 is inserted to downstream of malE gene.
Owner:台桂香
Methods and compositions for treating solid tumors and enhancing tumor vaccines
The present invention provides methods of treating and enhancing efficacy of immunotherapy for a solid tumor in a subject, comprising the step of contacting the subject with a compound or composition that modulates the expression or activity of ETRB, ET-1, ICAM-1, or another protein found herein to play a role in homing of T cells to a solid tumor. The present invention also provides methods of prognosticating a solid tumor in a subject, comprising the step of measuring an expression level of a protein found herein to play a role in homing of T cells to a solid tumor, or a nucleotide molecule encoding same.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Tumor vaccines
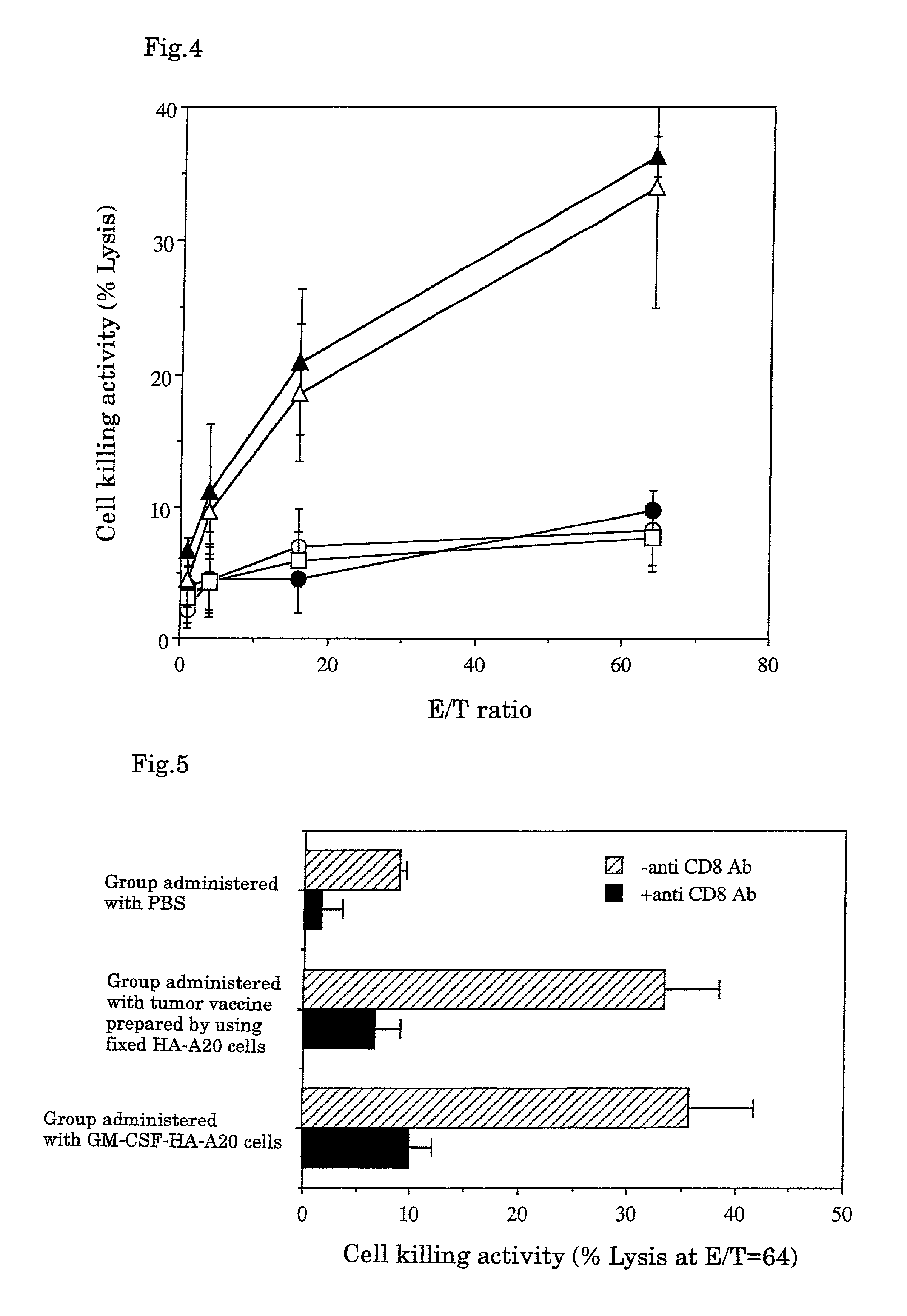
InactiveUS7247310B1Easy to handleImprove anti-tumor effectPeptide/protein ingredientsMammal material medical ingredientsAbnormal tissue growthGranulocyte colony-stimulating factor
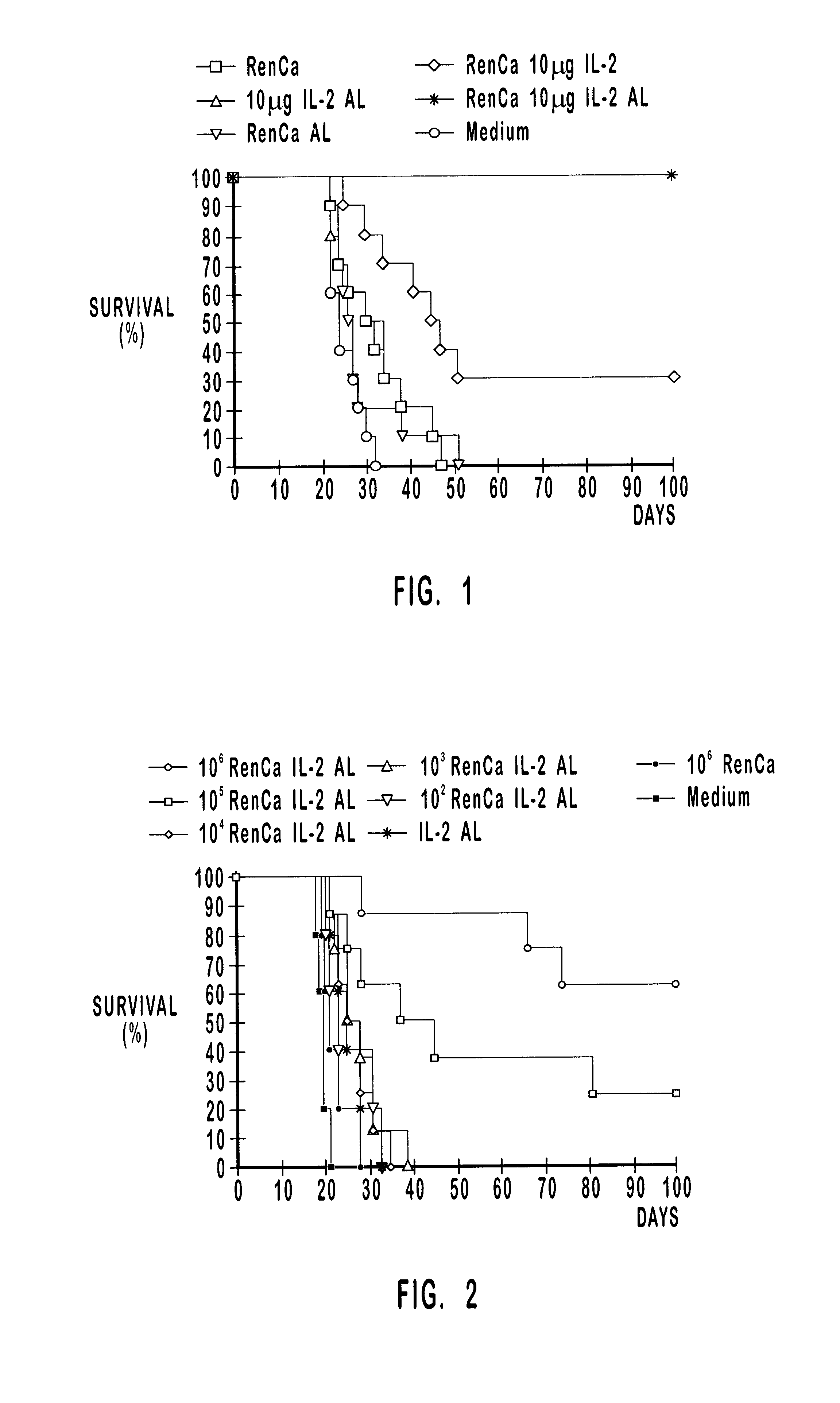
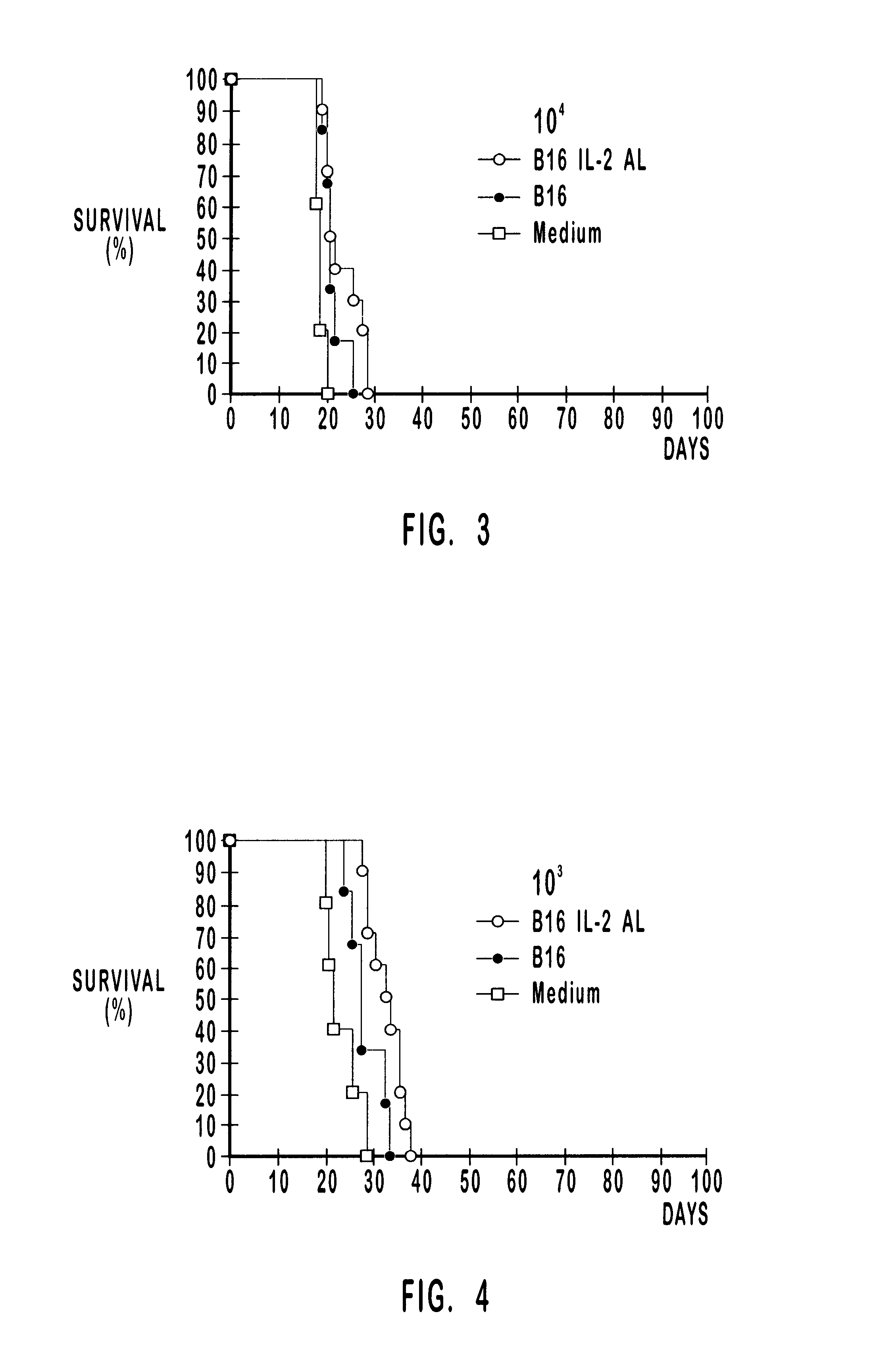
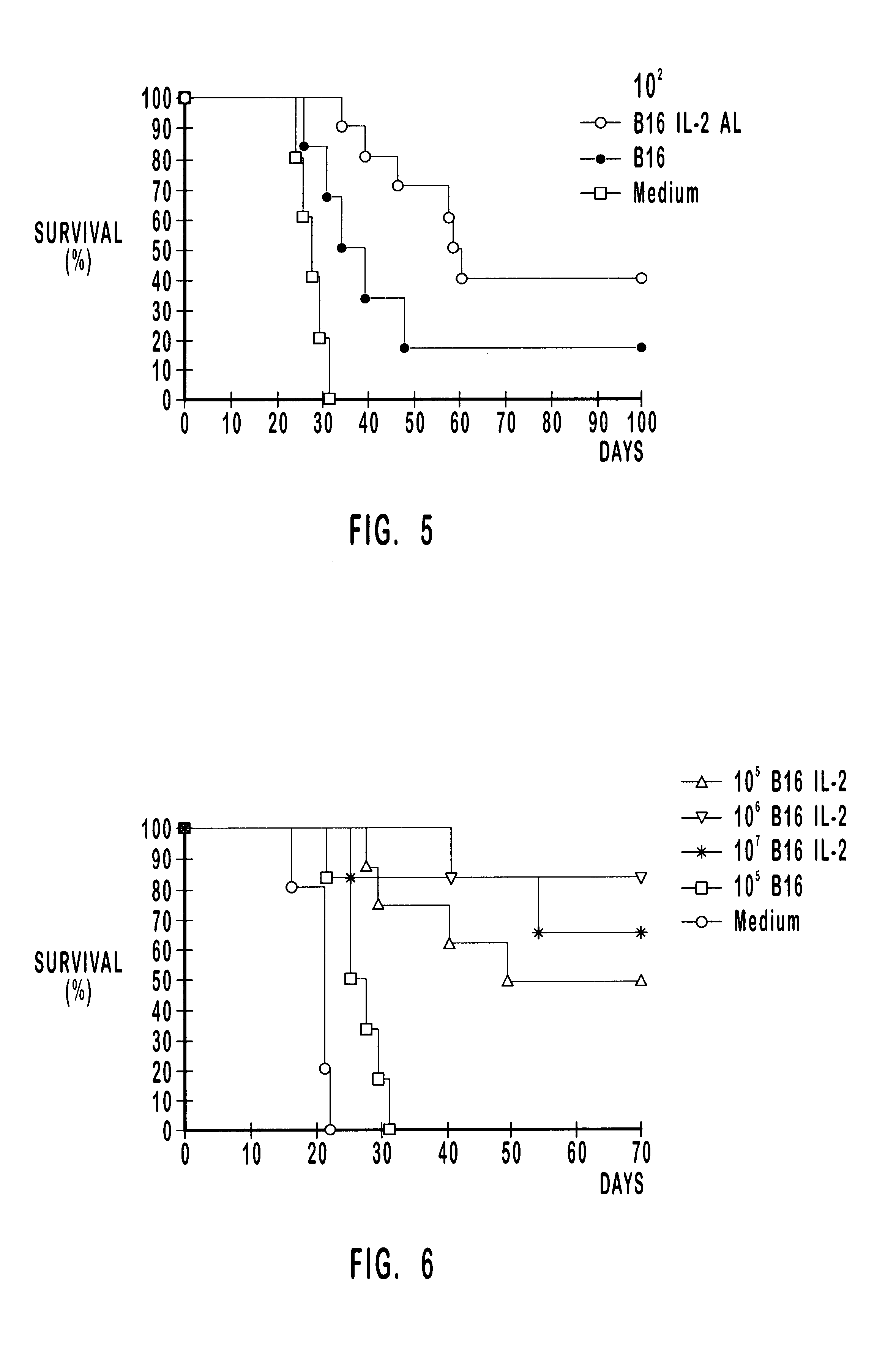
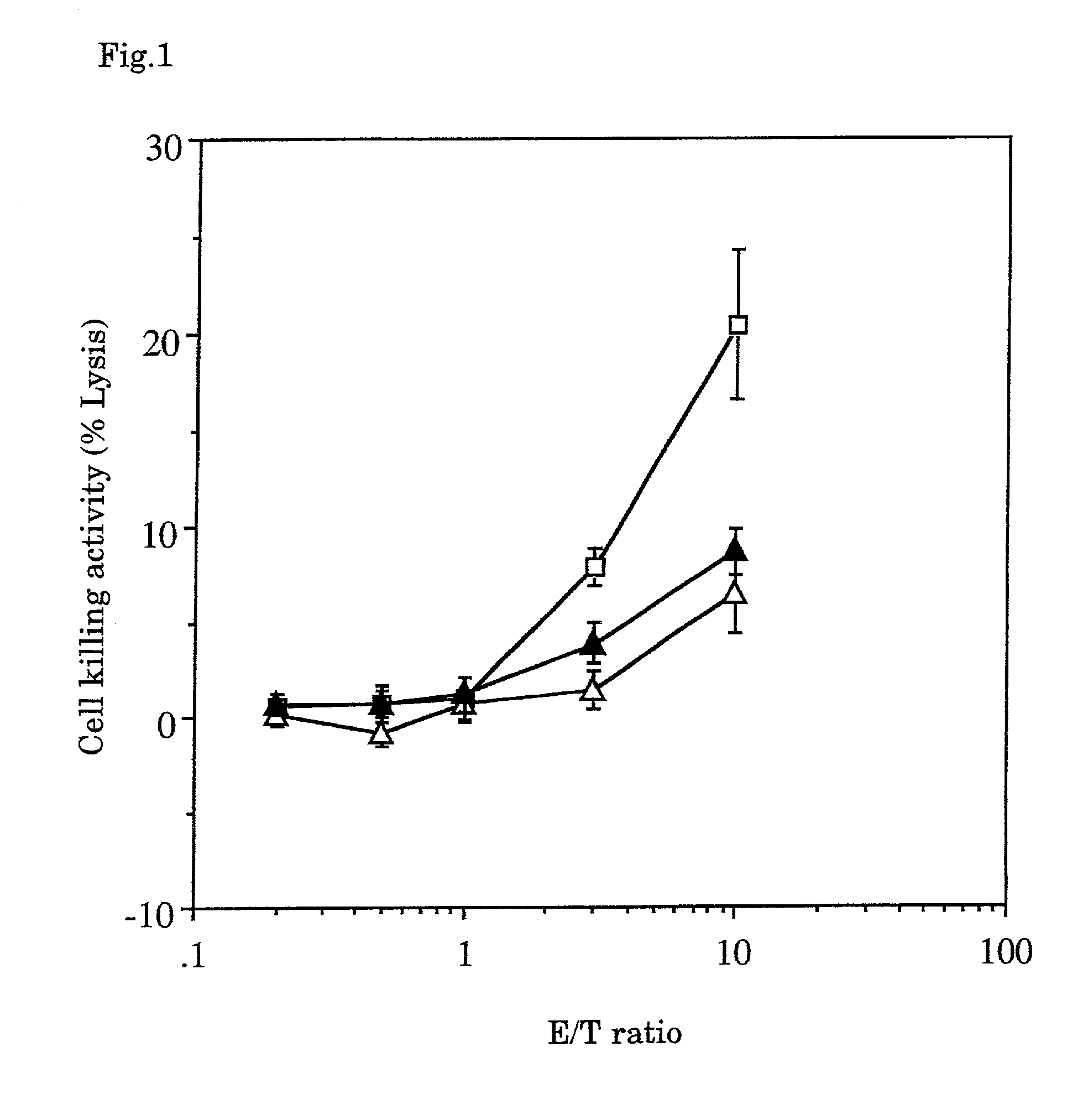
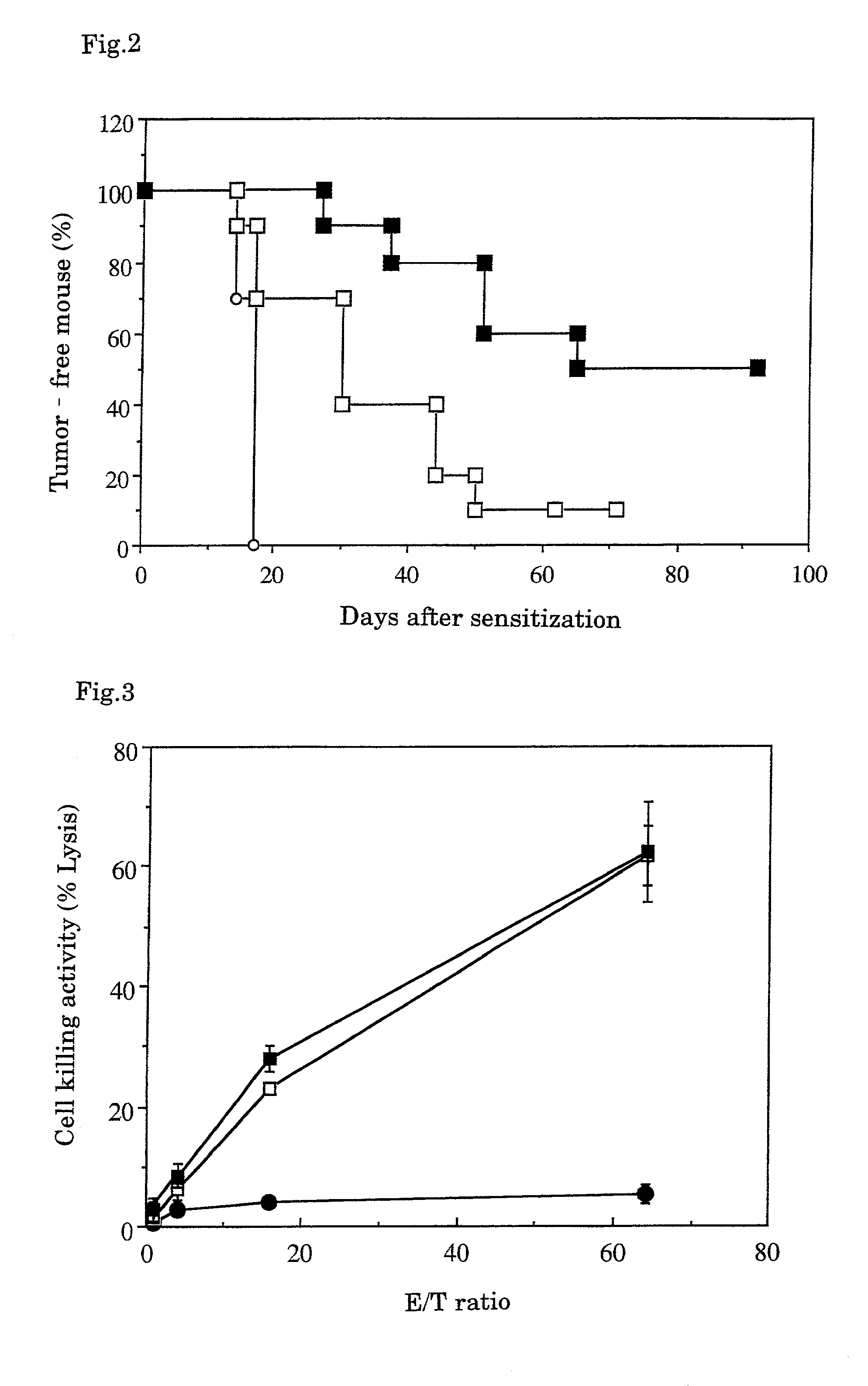
A tumor vaccine which comprises a microparticle or a lysate prepared from a solidified tumor material selected from the group consisting of a tumor tissue, a tumor cell, and a component thereof, and at least one cytokine and / or cytokine-inducing agent (e.g., a granulocyte-macrophage-colony stimulating factor and / or interleukin-2 and the like), and optionally an adjuvant. The vaccine can be easily prepared and widely applied for prevention of recurrence, inhibition of metastasis and therapeutic treatment regardless of a type of a tumor, and has excellent antitumor effect.
Owner:RIKEN +1
Tumor vaccine and preparation method thereof
ActiveCN103446580ASuitable for intakeImprove the efficiency of antigen presentationPowder deliveryVaccinesCell vesicleAdjuvant
The invention provides a tumor vaccine and a preparation method thereof. The tumor vaccine comprises cell vesicles from apoptosis tumor cells and an adjuvant. The invention also provides a preparation method of the tumor vaccine. The method comprises the steps of: irradiating tumor cells by ultraviolet ray to realize tumor cell apoptosis, collecting cell vesicles released by the tumor cells; and mixing the cell vesicles with the adjuvant to form the tumor vaccine. The tumor vaccine provided by the invention comprises wide and comprehensive tumor antigen spectrums, overcomes the defect of incapability to killing wide tumor cells in the prior art, and has good usage security and immunization targeting.
Owner:HUBEI SOUNDNY BIOLOGICAL TECH
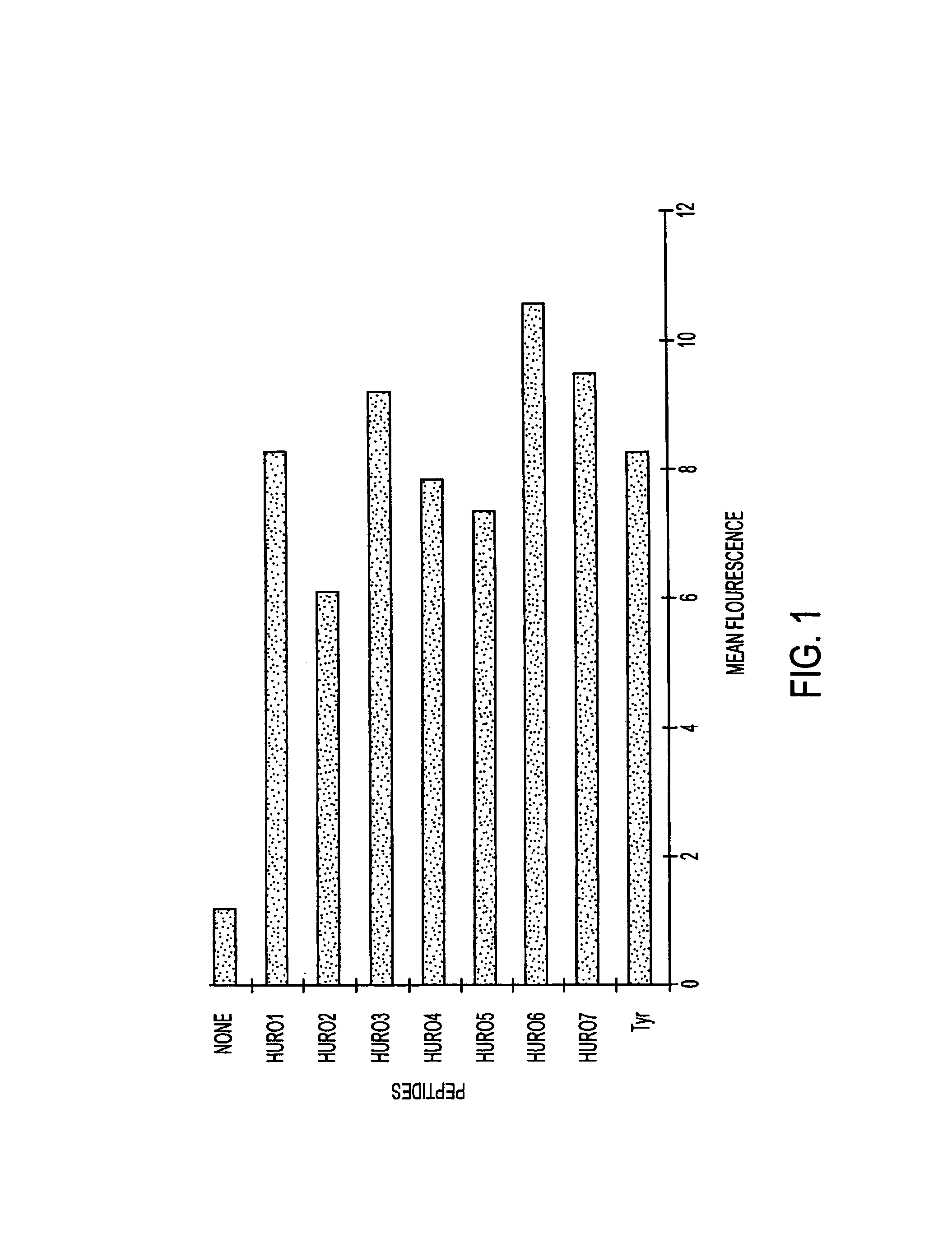
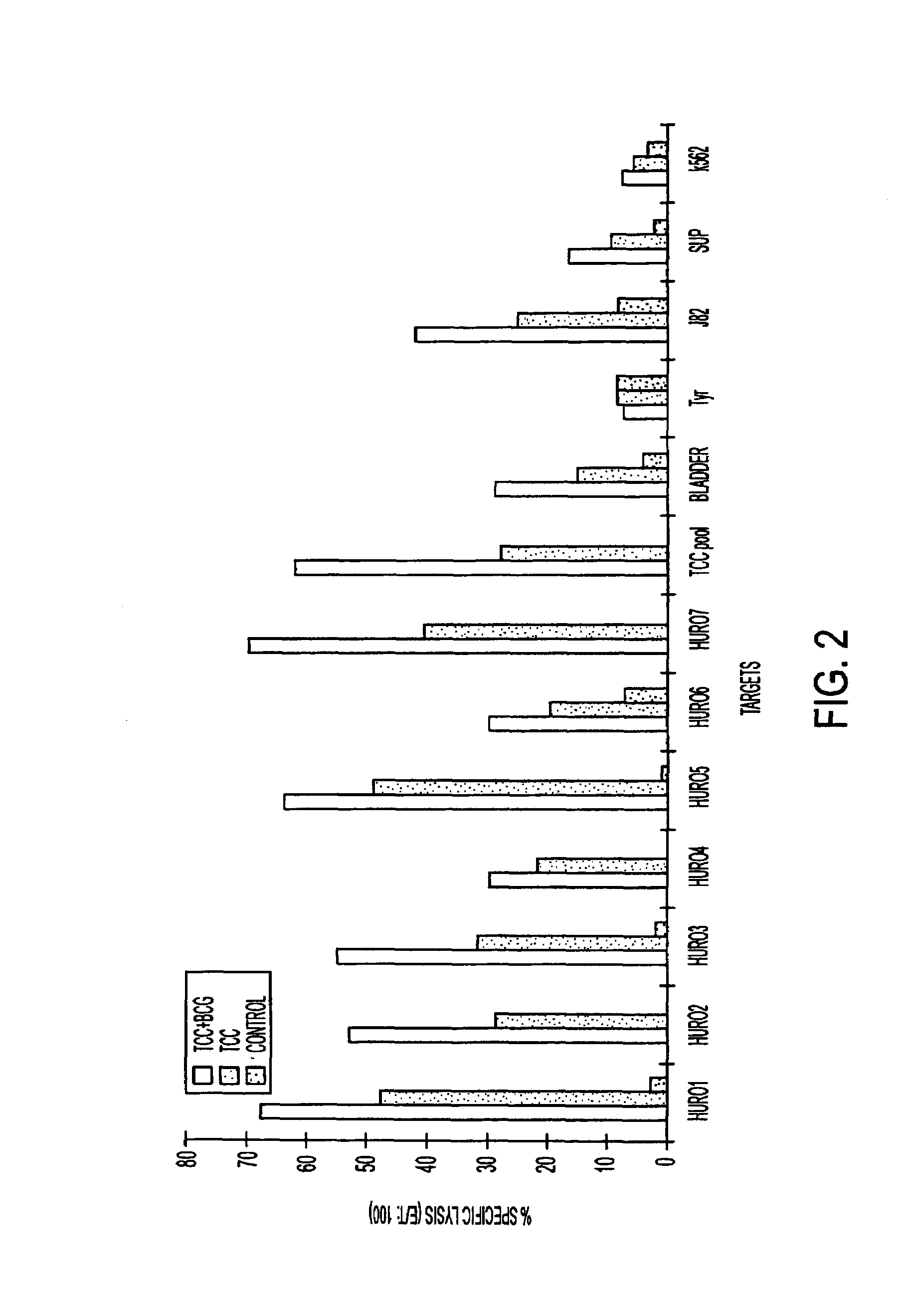
Tumor associated antigen peptides and use of same as anti-tumor vaccines
The present invention relates to tumor associated antigen (TAA) peptides and to use of same, of polynucleotides encoding same and of cells presenting same as anti-tumor vaccines. More particularly, the present invention relates to tumor associated antigen peptides derived from Uroplakin Ia, Ib, II and III, Prostate specific antigen (PSA), Prostate acid phosphatase (PAP) and Prostate specific membrane antigen (PSMA), BA-46 (Lactadherin), Mucin (MUC-1), and Teratocarcinoma-derived growth factor (CRIPTO-1) and the use of same as anti-tumor vaccines to prevent or cure bladder, prostate, breast or other cancers, carcinomas in particular. Most particularly, the present invention relates to tumor associated antigen peptides which are presentable to the immune system by HLA-A2 molecules.
Owner:YEDA RES & DEV CO LTD
Tumor antigenic polypeptide and application thereof as tumor vaccine
The invention provides a human mucin-1 tumor antigenic polypeptide with an amino acid sequence shown in SEQ ID NO: 1 or variants thereof, wherein the polypeptide can be combined with an HLA (Human Leukocyte Antigen) I and can be recognized by a cell CD8<+>T. The invention further provides nucleic acids coding the polypeptide. The invention further provides an antigen presenting cell capable of presenting the polypeptide on the surface of the cell and an immune effector cell capable of recognizing the polypeptide or antigen presenting cell. The invention further provides application of the polypeptide or variants thereof, the nucleic acids, the antigen presenting cell or the immune effector cell in the preparation of vaccines or pharmaceutical compositions for treating or preventing cancer. Tumor vaccines provided by the invention have a good treatment effect on relatively large crowds and are particularly applicable to the Asian, e.g. Chinese.
Owner:BEIJING ZHIFEI LVZHU BIOPHARM +1
Composition and therapeutic anti-tumour vaccine
ActiveUS9950049B2Peptide/protein ingredientsInorganic non-active ingredientsDendritic cellImmune complex deposition
The invention relates to a composition which induces, in a host, a cytotoxic cell response directed against cells expressing an antigen, in particular tumor cells, and which comprises red blood cells containing said antigen. These red blood cells may be in the form of an immune complex with an immunoglobulin, in particular IgG, which recognizes an epitope at the surface of the red blood cells, and / or be heat-treated or chemically treated so as to promote phagocytosis of said red blood cells by dendritic cells. As a variant, the red blood cells may be xenogenic red blood cells. The invention also relates to a therapeutic especially anti-tumor vaccine containing such a composition.
Owner:ERYTECH PHARMA
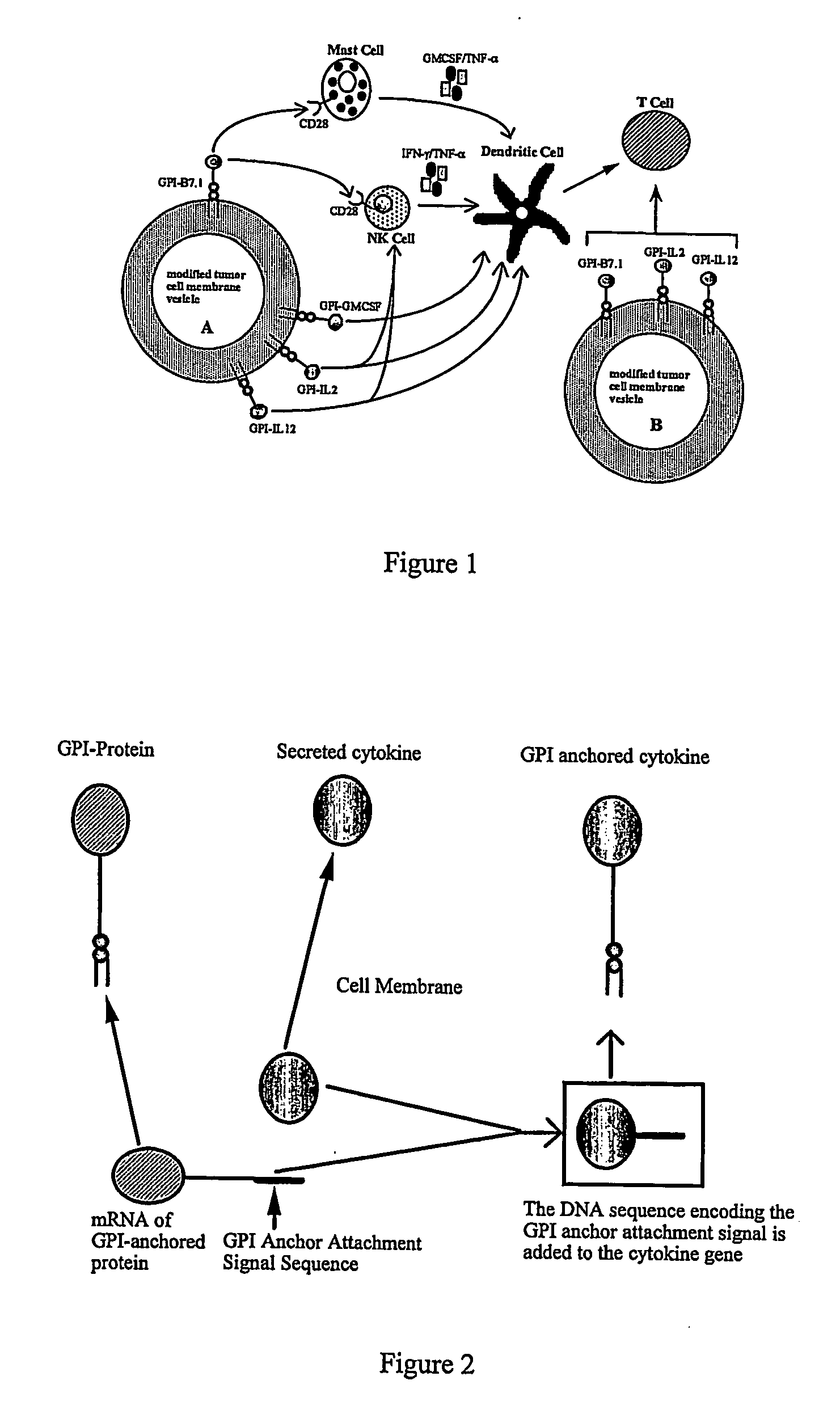
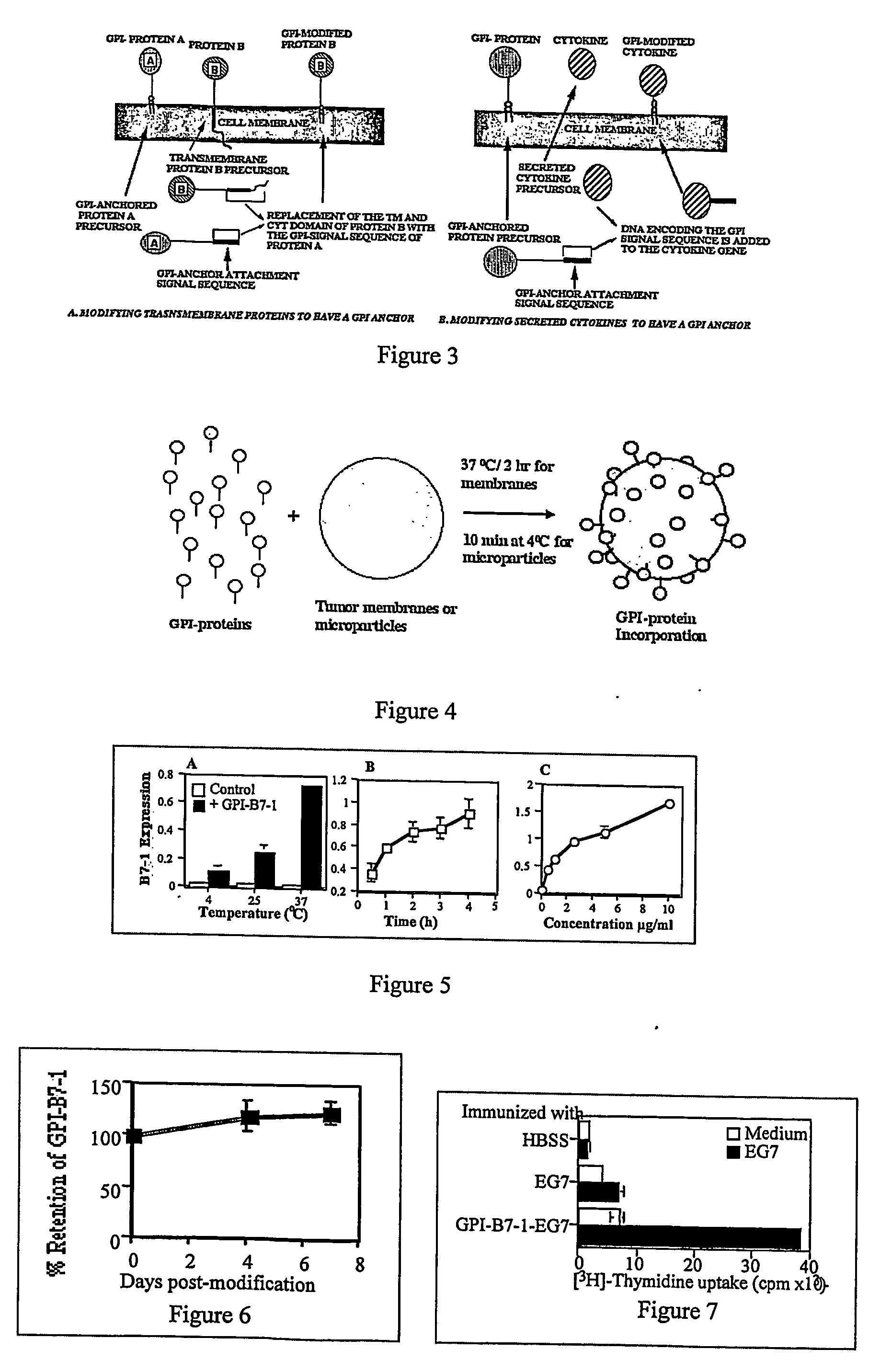
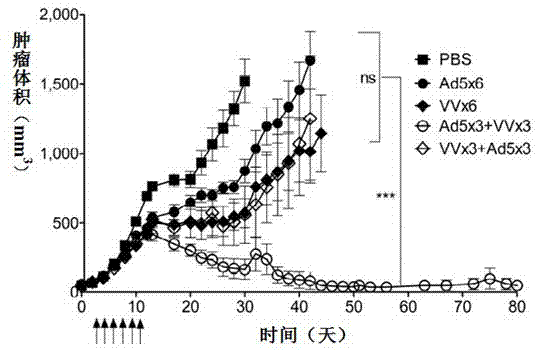
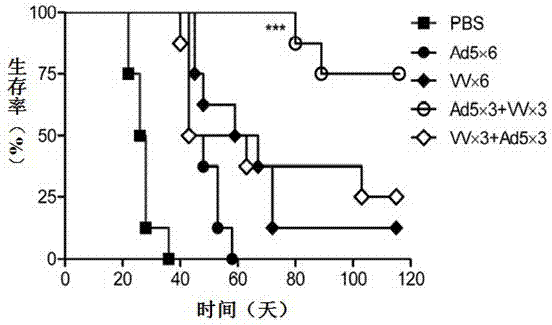
Therapeutic Compositions and Vaccines By Glycosyl-Phosphatidylinositol (Gpi)-Anchored Cytokines and Immunostimulatory Molecules
InactiveUS20070243159A1Induce toleranceSuppress immunityViral antigen ingredientsCancer antigen ingredientsAbnormal tissue growthGlycosyl-Phosphatidylinositol
A therapeutic composition or a vaccine comprising tumor membrane-anchored cytokines or other immunostimulatory or costimulatory molecules are provided. The therapeutic composition or a tumor vaccine can be used for treating a tumor or other disease such as autoimmune disorder, viral diseases, bacterial diseases, parasitic diseases, and transplant rejection.
Owner:IRM +1
Vaccine for inducing specific immunity of tumor and application thereof
InactiveCN103110939AEnhance tumor immunityStrong immune responseViral antigen ingredientsUnknown materialsOncolytic adenovirusSpecific immunity
The invention relates to a vaccine comprising an adenovirus vector and a vaccinia virus vector and an application of the vaccine in treating tumor. The vaccine comprises oncolytic adenovirus and vaccinia virus; the two viruses are used in sequence, wherein the oncolytic adenovirus is the reproductive human adenovirus type 5 and the vaccinia virus is the vaccinia virus for smallpox vaccine. According to the vaccine, the oncolytic adenovirus and the vaccinia virus are prepared into the vaccine by sequential combination, so the antineoplastic activity can be improved, the stronger cellular immune response can be generated compared with the effect of reversely associated virus or separate virus, the using effect is better and the long-term specific immunity of tumor is shown. The tumor vaccine can eradicate the tumor and generate the long-term specific immunity of tumor and can be used in the treatment of various tumors of the human or animals; and meanwhile, the vaccine can effectively prevent the tumor from relapsing and has a wide application prospect.
Owner:ZHENGZHOU UNIV +1
Compositions and methods for treatment of tumors and metastatic diseases
InactiveUS20020176845A1Effective treatmentStimulate immune responsePeptide/protein ingredientsCancer antigen ingredientsDiseaseActive immunization
Compositions and methods are provided which can be utilized in active immunization as a prophylactic treatment or a therapeutic treatment for tumors. The compositions are employed as injectable tumor vaccines or as preparations for intratumoral administration and are capable of stimulating immune responses to specific tumor antigens. The tumor vaccines are composed of an antigenic cellular material including a plurality of inactivated tumor cells or tumor cell portions, a depot material, and an immunostimulant adsorbed to the depot material. The depot material with absorbed immunostimulant is mixed with the tumor cells or tumor cell portions to form the vaccine compositions. The preparations for intratumoral administration include the depot material adsorbed immunostimulant without the antigenic cellular material. The immunostimulant adsorbed to the depot material permits release of biologically active quantities of the immunostimulant over a period of time rather than all at once.
Owner:FRANK W FALKENBERG
Immunotherapy against erbb-3 receptor
ActiveUS20140017259A1EfficaciousHigh affinityOrganic active ingredientsPeptide/protein ingredientsCancer cellMonoclonal antibody
The present invention describes methods and pharmaceutical compositions for the treatment of cancer in mammals, more particularly in human subjects. More specifically, the invention concerns anti-tumor vaccines based upon plasmid DNA and / or genetic vectors carrying a codon-usage optimized sequence and coding for a mutant form of the ErbB-3 receptor. Furthermore, the invention refers to monoclonal antibodies directed against the ErbB-3 receptor, obtained using these methods and capable to block its activity in cancer cells.
Owner:TAKIS
Preparation method of HLA-G (Human Leukocyte Antigen G) antibody and application of HLA-G antibody in medicine
InactiveCN101967191AWith sensitivityTo achieve the purpose of early detection and early treatmentImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigenUltra sensitive
The invention discloses an HLA-G (Human Leukocyte Antigen G) antigenic determinant and a sequence position thereof as well as a method for preparing an HLA-G antibody by utilizing the HLA-G antigenic determinant. An ultra-sensitive chemiluminescence immunoassay kit and the like are prepared by utilizing the HLA-G antibody and used for detecting an extremely low content of HLA-G in the body fluid of an early tumor patient so as to diagnose early tumors; a kit which has the advantages of easy and convenient operation without using an instrument and self-check and self-test function and detects the HLA-G through gold-marking immunoassay is prepared by utilizing the HLA-G antibody and used for basic screening and early tumor monitoring to eliminate the tumors in early stage so as to enhance the survival rate of tumor patients; and a broad-spectrum antitumor biological agent and a broad-spectrum tumor vaccine are prepared by utilizing the HLA-G antibody as an important raw material and used for treating and preventing malignant tumors.
Owner:GUANGZHOU TIANMEI BIOTECH
Cytokine gene modified antigen-presenting cell/tumor cell conjugate, its preparation and use
InactiveUS7067120B2Strong specificityLittle side effectsBiocideGenetic material ingredientsAntigenTumor vaccines
The present invention provides an antigen-presenting cell(APC) / tumor cell conjugate, wherein the antigen-presenting cell (APC) is modified by a cytokine gene selected from the group consisting of IL-2, IL-3, IL-4, IL-6, IL-12, IL-18, IFNα, IFNβ, IFNγ, TNF, TGF, GM-CSE, and the combination thereof. The conjugate is useful as a tumor vaccine to significantly induce an immunity specifically against the tumor cell The present invention also provides the method for preparing the conjugate and a pharmaceutical composition containing said conjugate.
Owner:SHANGHAI MEDIPHARM BIOTECH
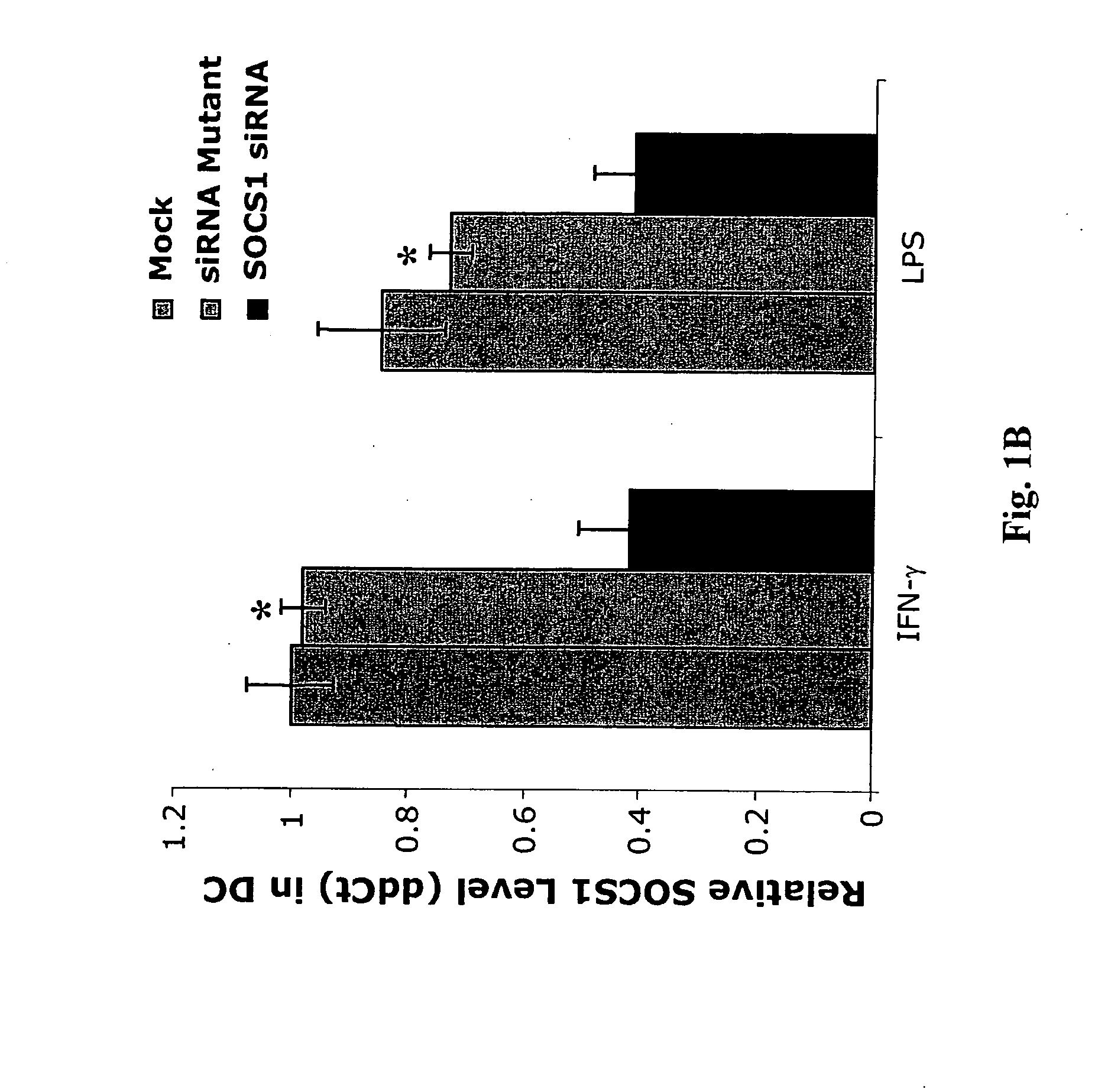
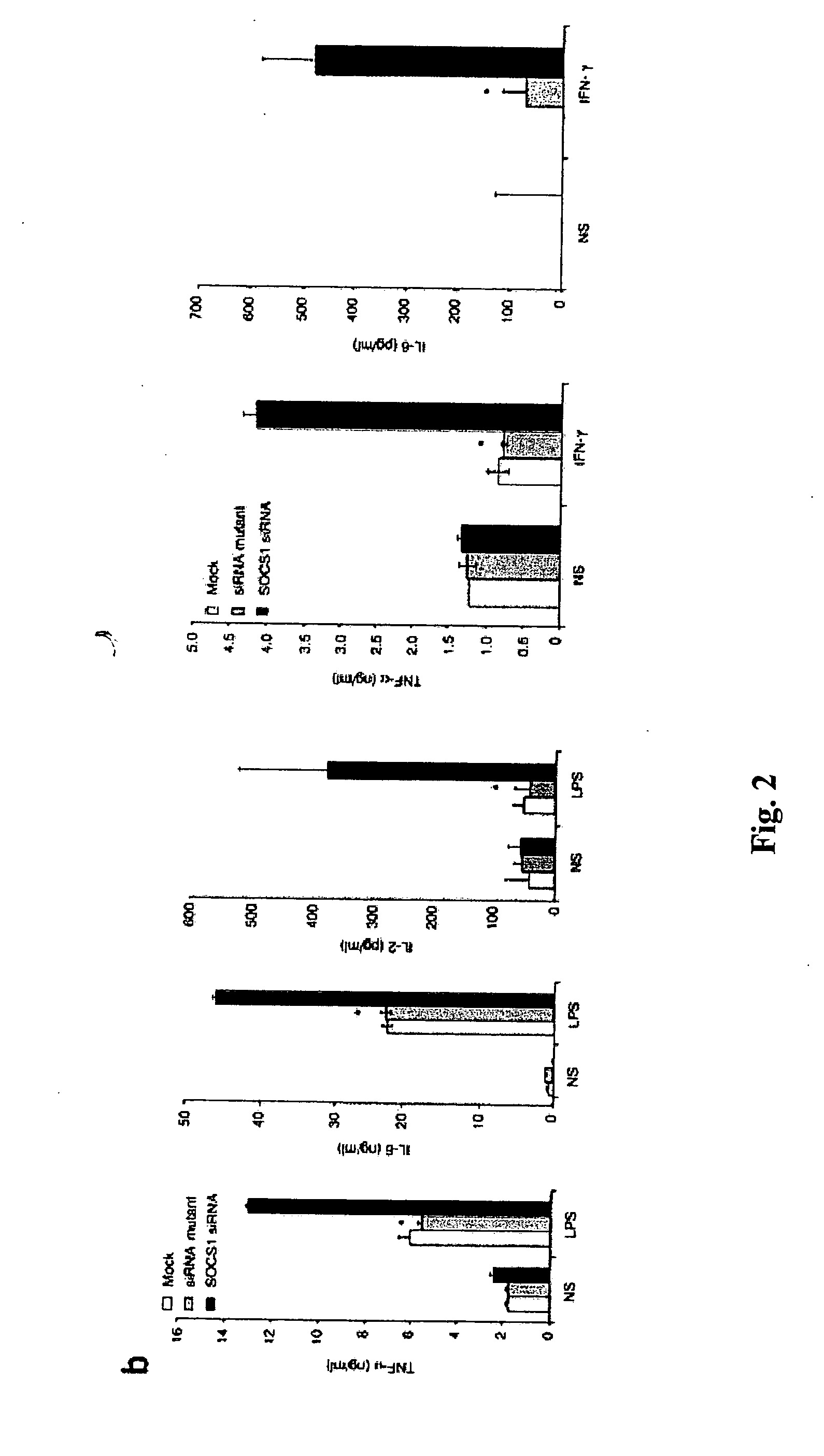
Modulation of cytokine signaling regulators and applications for immunotherapy
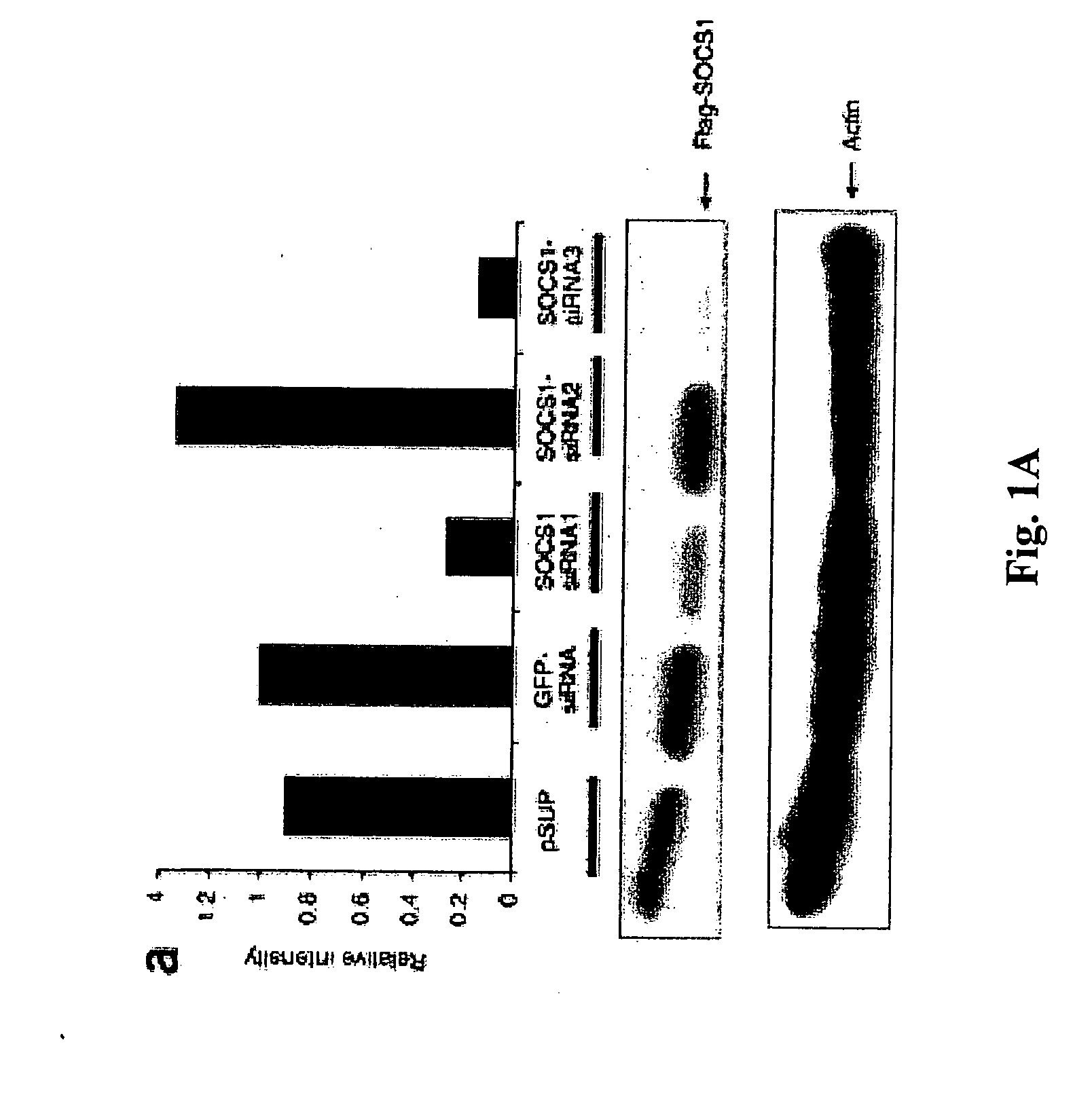
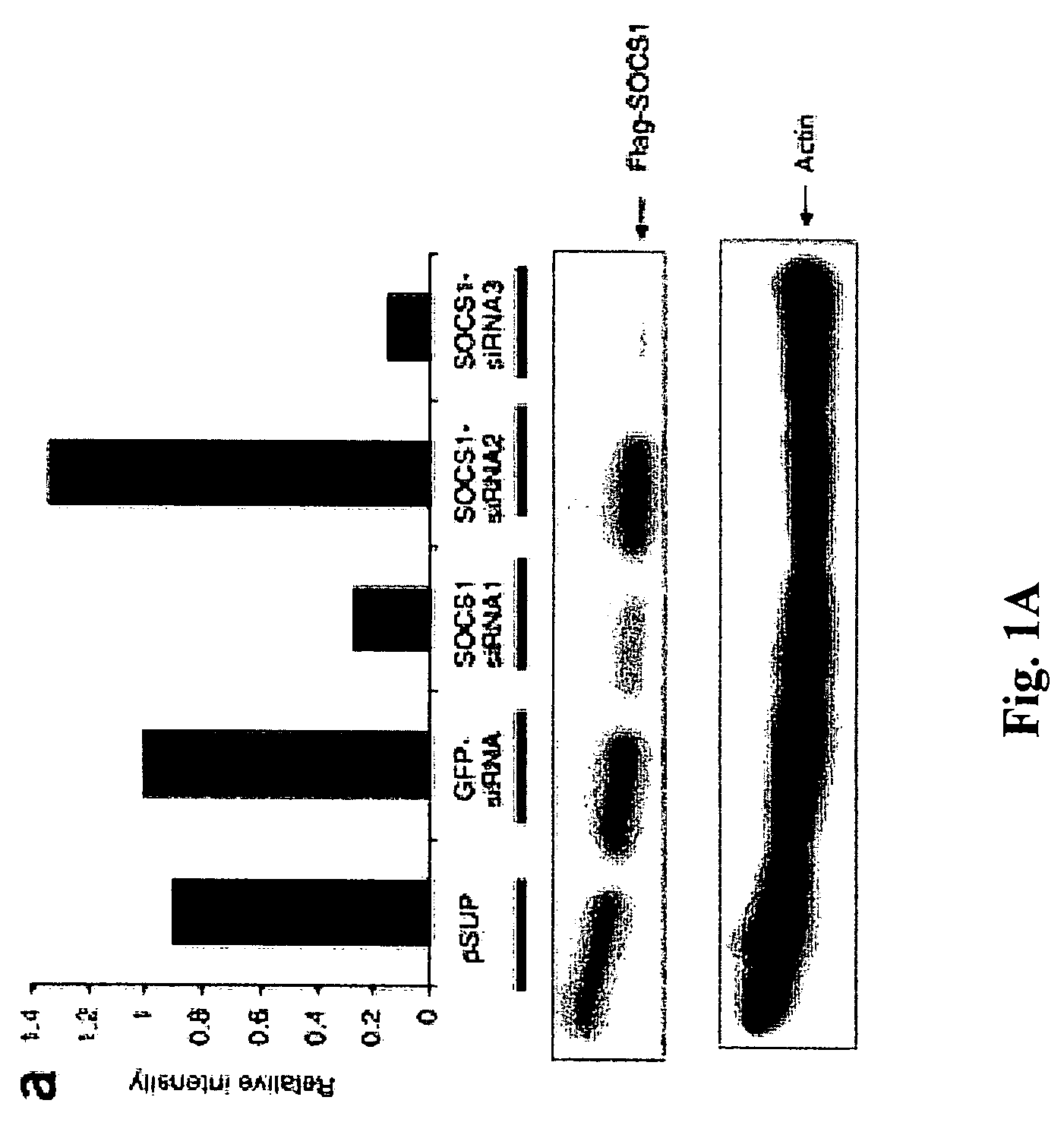
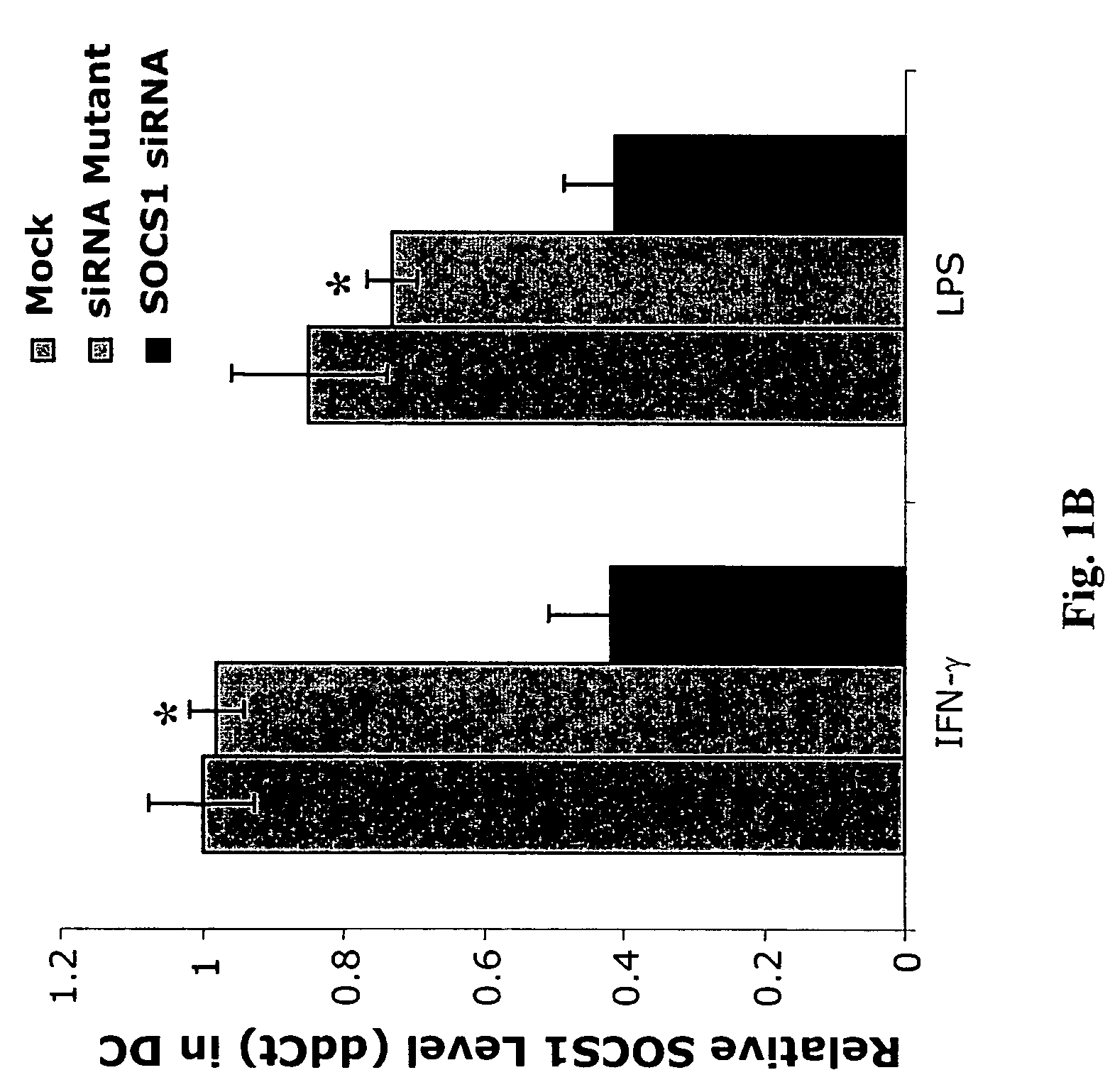
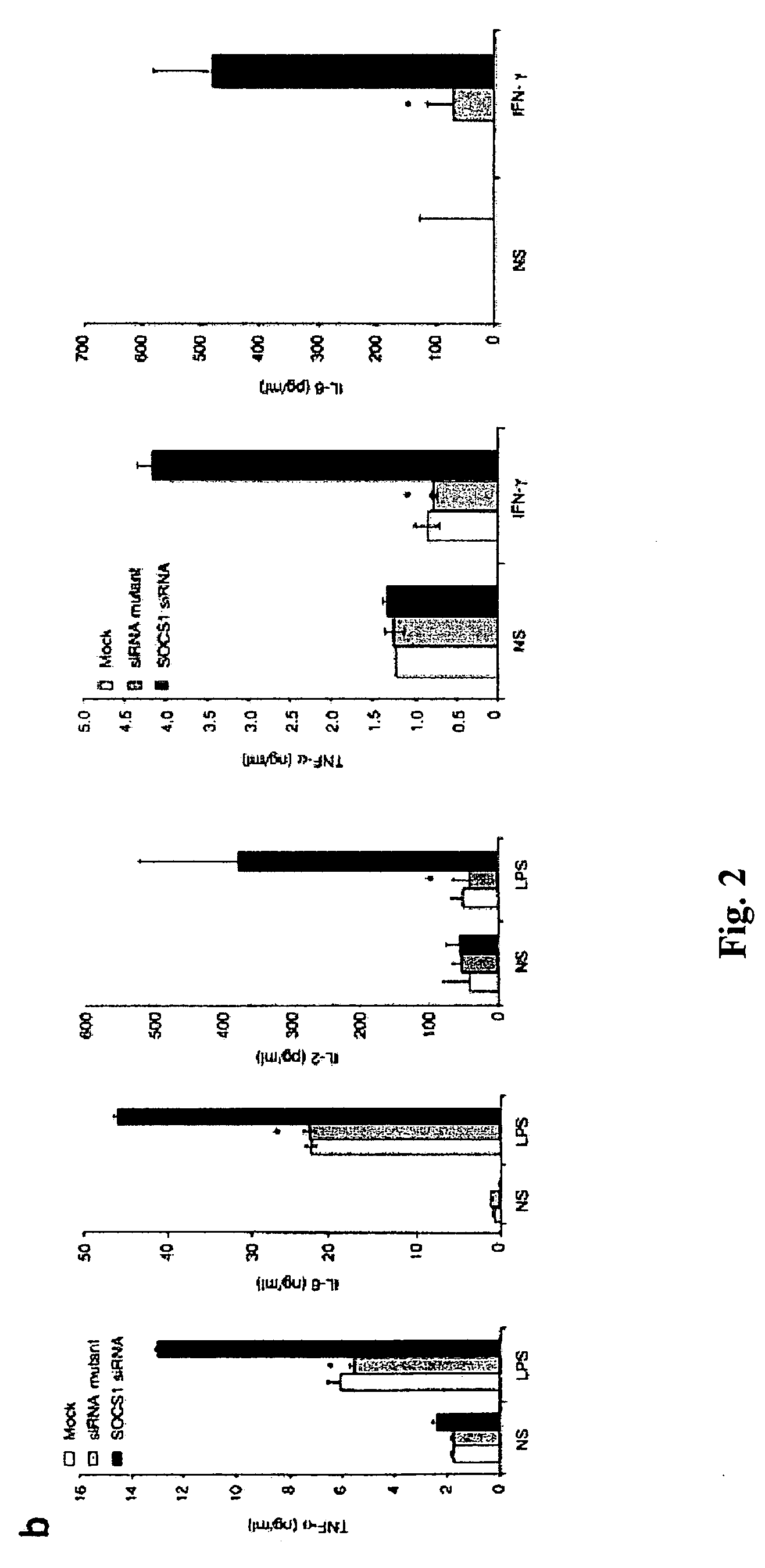
The present invention relates to regulation of antigen presentation by cytokine signaling regulators in antigen presenting cells, such as dendritic cells. The invention provides methods of modulating antigen presentation through modulation of cytokine signaling regulators, such as SOCS (SOCS1-7, CIS), SHP (SHP-1 and SHP-2) or PIAS (PIAS1, PIAS3, PIASx and PIASy). The present invention provides vaccines and therapies in which antigen presentation is enhanced through modulation of cytokine signaling regulators. The present invention also provides a mechanism to break self tolerance in tumor vaccination methods that rely on presentation of self tumor antigens.
Owner:BAYLOR COLLEGE OF MEDICINE
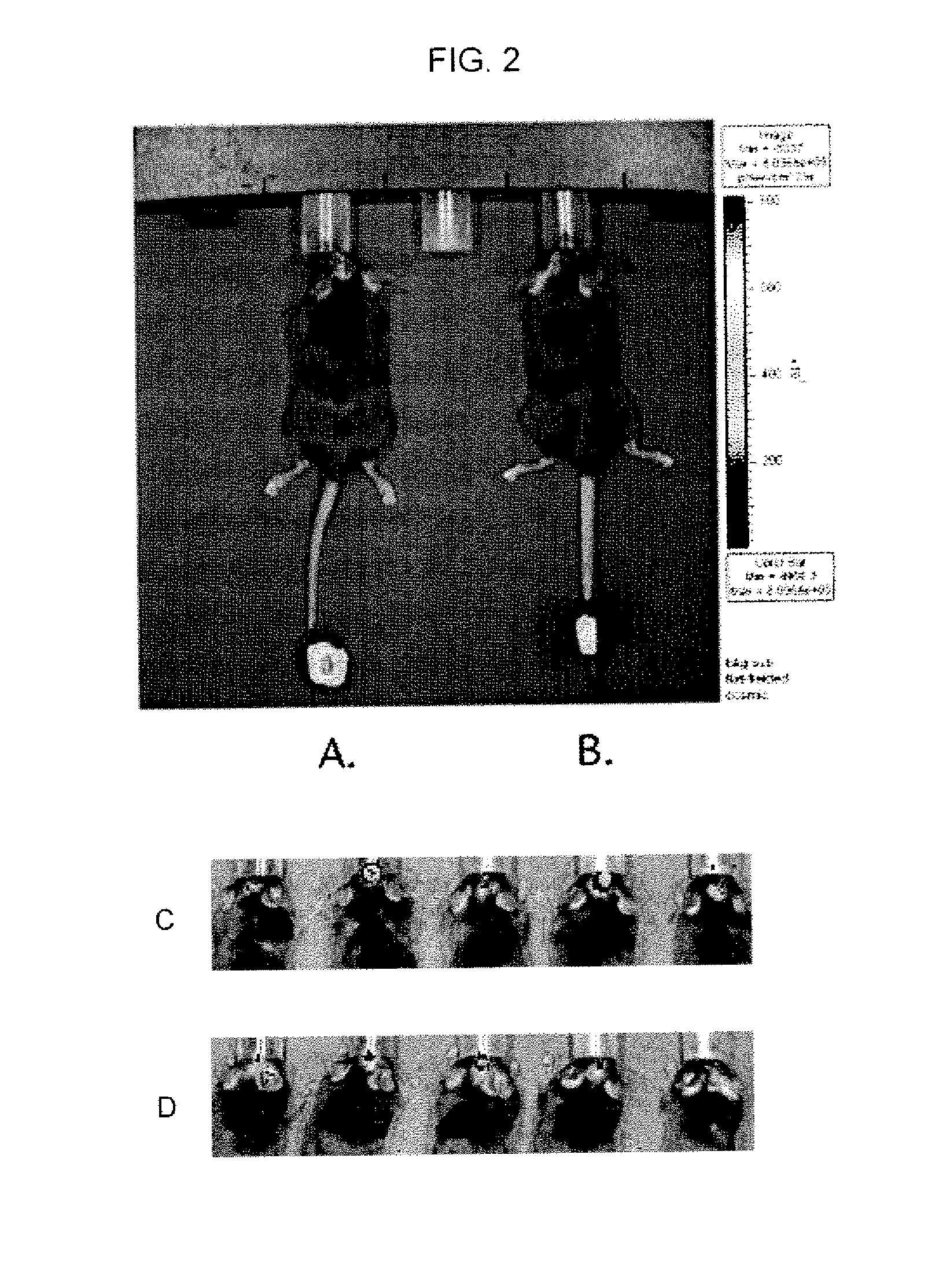
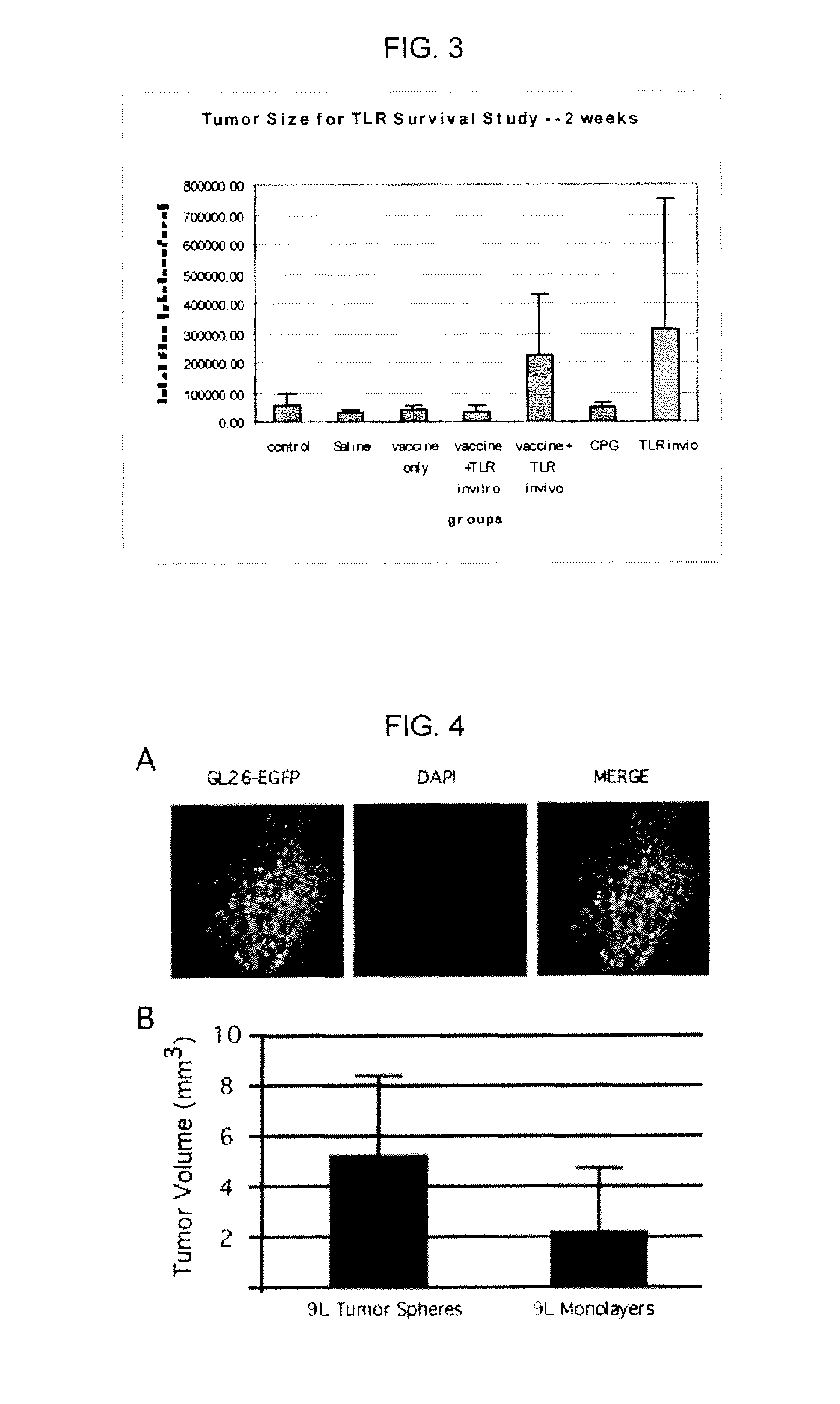
Use of toll-like receptor ligands as adjuvants to vaccination therapy for brain tumors
Compositions comprising dendritic cells pulsed with tumor lysate and at least one toll-like receptor (TLR) ligand which may be used for eliciting a specific immune response in a mammal in need thereof for treating diseases including a tumor are disclosed. Also disclose are methods of activating dendritic cells, comprising providing at least one toll-like receptor (TLR) ligand; and pulsing a dendentic cell with the at least one TLR ligand. A method further comprises pulsing the dendritic cell with a tumor lysate.
Owner:CEDARS SINAI MEDICAL CENT
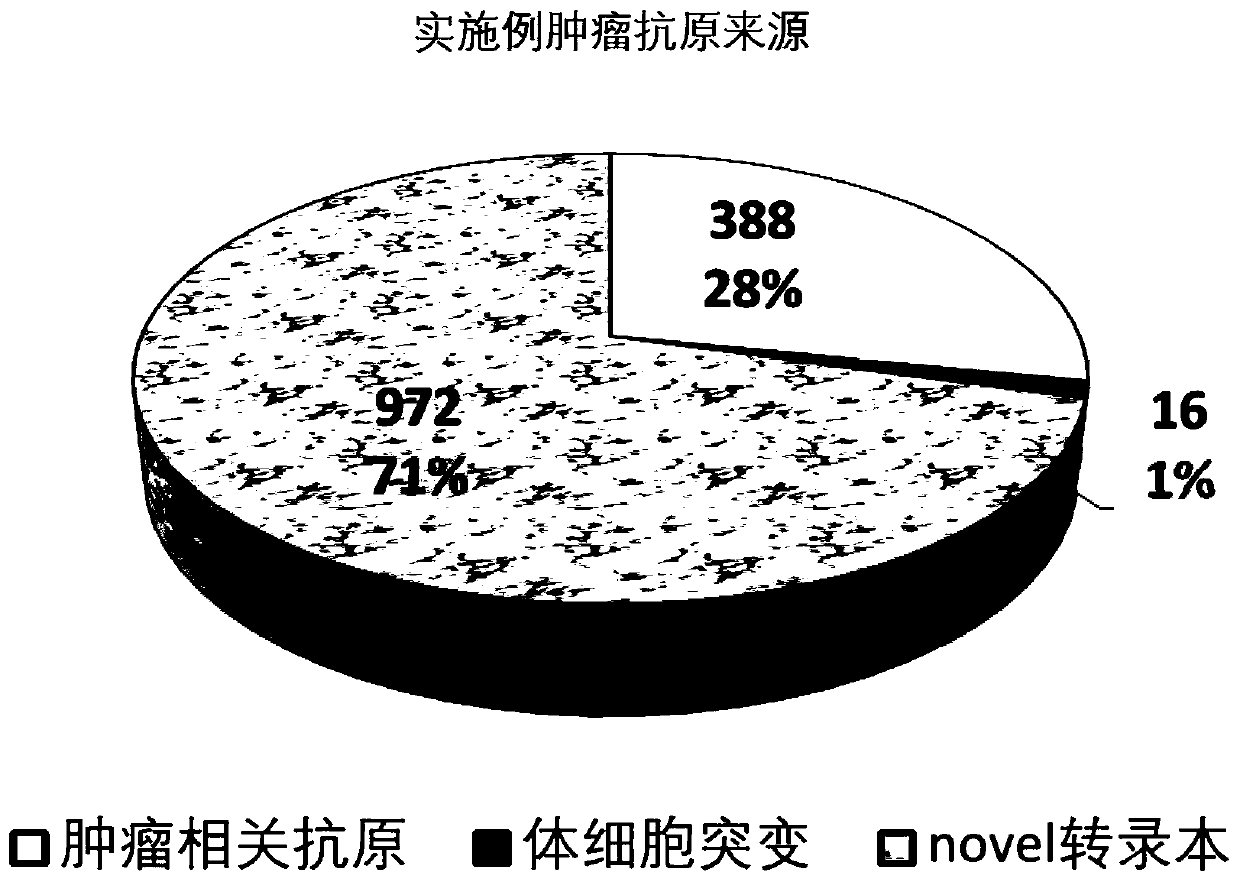
Tumor antigen prediction method based on whole transcriptome, and application of the same
The invention relates to a tumor antigen prediction method based on a whole transcriptome. The tumor antigen prediction method includes the steps: respectively performing protein generation and peptide fragment interception of tumor-associated antigen, detection of tumor somatic mutation and corresponding mutant peptide fragment interception, generation of tumor-specific novel transcript and peptide fragment interception, and gene fusion detection and fusion peptide fragment interception in tumor tissues, according to the whole transcriptome sequencing data of tumor tissues and corresponding adjacent para-carcinoma tissues, obtaining tumor-associated antigen, tumor somatic mutation, tumor novel transcript, tumor-specific peptide fragment of gene fusion, calculating affinity of the obtainedtumor-specific peptide fragment and the HLA molecule and the amount of expression in each transcript, and based on the affinity value of the tumor-specific peptide fragment and the amount of expression TPM (transcripts per million) value, evaluating the level of the candidate tumor antigen. The invention also provides an application of the same. The tumor antigen prediction method based on a whole transcriptome and the application of the same are conductive to accurate calculation of the tumor antigen load, evaluation of immunological therapy effect, and tumor vaccine design of the later stage of service.
Owner:上海鲸舟基因科技有限公司
Cell membrane tumor vaccine and preparation method and application thereof
InactiveCN110090298AImprove immunityGood biocompatibilityFused cellsCancer antigen ingredientsAbnormal tissue growthDendritic cell
The invention discloses a cell membrane tumor vaccine and a preparation method and an application thereof. For the first time, exogenous tumor antigens and adoptive cells are not used, a cell fusion technology is used to fuse dendritic cells and tumor cells, and after culture, the whole tumor antigen and costimulatory molecules are expressed on the cell membrane of the fused cells. A nano cell membrane vaccine prepared by coating the nanoparticle with the cell membrane subjected to peeling of the fused cell can directly activate the T cell like the antigen presenting cell, can also be recognized by the dendritic cell, processed and presented to the T cell, and indirectly activates the T cell. Two T cells are combined for activation of pathways, which can cause the strong anti-tumor immuneresponse. A cell membrane tumor vaccine strategy that mimics the tumor cells and the antigen presenting cells enables the development of the vaccines against a variety of tumor types, and has good biocompatibility and system safety. Therefore, the cell membrane vaccine is very suitable for the field of tumor vaccines.
Owner:WUHAN UNIV
Short chain polypeptide, application thereof as vaccine adjuvant and vaccine with short chain polypeptide serving as adjuvant
The invention provides short chain polypeptide, application thereof as a vaccine adjuvant and a vaccine with the short chain polypeptide serving as the adjuvant. A capping group having an anti-inflammatory effect is introduced into the short chain polypeptide, and can form hydrogel, and the hydrogel being simply and physically mixed with an antigen can effectively enhance the immune response ability of the antigen, and is used in a preventive tumor vaccine for completely inhibiting tumor growth. The sequence of the short chain polypeptide is X-GDFDFDY, wherein X is Fbp or Car.
Owner:NANKAI UNIV
Preparation method and application of a new tumor dendritic cell therapeutic vaccine
ActiveCN102258772APrevent relapseAvoid diversionBlood/immune system cellsAntibody medical ingredientsAbnormal tissue growthTumor therapy
The invention provides a preparation method and application of a new therapeutic vaccine for dendritic tumor cells. The therapeutic tumor vaccine is chemotherapeutic drug induced tumor antigen, wherein, the dendritic cells are loaded and activated. The chemotherapeutic medicament induced tumor antigen contains a plurality of immunostimulation molecules as well as tumor-associated and specific antigen, which can remarkably carry out chemotaxis and activation on immunocytes such as the dendritic cells, T-cells (thymus-dependent lymphocytes) and the like, stimulate the dendritic cells to be mature and express a plurality of cell factors and chemotactic factors, effectively induce immune response reaction with antigenic specificity and non-specificity and strengthen body immune function. The therapeutic vaccine provided by the invention can be used for preventing and treating tumors and has the characteristics of simple preparation process, low cost, strong specificity, obvious curative effect and the like.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
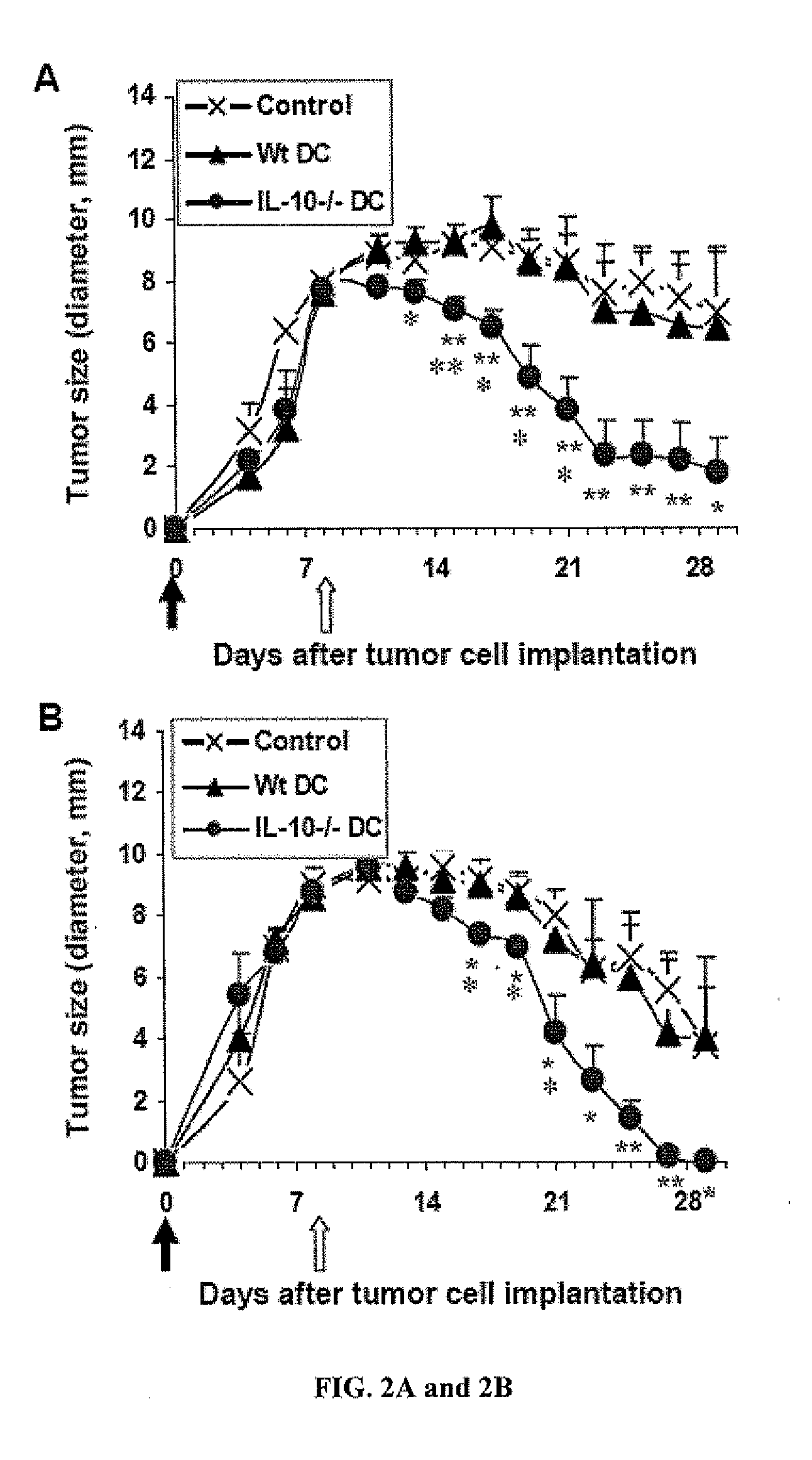
Anti-tumor vaccines delivered by dendritic cells devoid of interleukin-10
InactiveUS20090010948A1Highly effectiveBiocideGenetic material ingredientsDendritic cellInterleukin 10
It has been discovered that reducing, inhibiting or preventing the expression of immunosuppressive cytokines or tolergenic agents in antigen presenting cells improves the ability of the antigen presenting cell to promote an immune response. One embodiment provides a genetically engineered antigen presenting cell that has reduced or no expression of IL-10. Preferred antigen presenting cells are dendritic cells. Expression of IL-10 can be inhibited or blocked by genetically engineering the antigen presenting cell to express inhibitory nucleic acids that inhibit or prevent the expression mRNA encoding immunosuppressive cytokines. Inhibitory nucleic acids include siRNA, antisense RNA, antisense DNA, microRNA, and enzymatic nucleic acids that target mRNA encoding immunosuppressive cytokines. Immunosuppressive cytokines include, but are not limited to IL-10, TGF-β, IL-27, IL-35, or combinations thereof. Tolerogenic agents include but are not limited to indoleamine 2,3-dioxygenase.
Owner:THE UNIVERSITY OF HONG KONG
Tumor associated antigen, peptides thereof, and use of same as anti-tumor vaccines
InactiveUS20060263342A1Improve bindingBiocideTumor rejection antigen precursorsBULK ACTIVE INGREDIENTPolynucleotide
The invention relates to colon and prostate tumor associated antigen peptides obtainable from prostate specific G protein-coupled receptor (PSGR), six-transmembrane epithelial antigen of prostate (STEAP) and proteins encoded by genes found overexpressed in colon carcinoma cells, such as human 1-8D interferon induced transmembrane protein 2. The invention further relates to a polynucleotide encoding the tumor associated antigen peptides and to pharmaceutical compositions, which are preferably anti-tumor vaccine compositions, containing a tumor associated antigen, at least one tumor associated antigen peptide thereof, or encoding polynucleotide thereof as an active ingredient. The pharmaceutical compositions can be administered to a patient in need thereof to treat or inhibit the development of colon or prostate cancer.
Owner:YEDA RES & DEV CO LTD
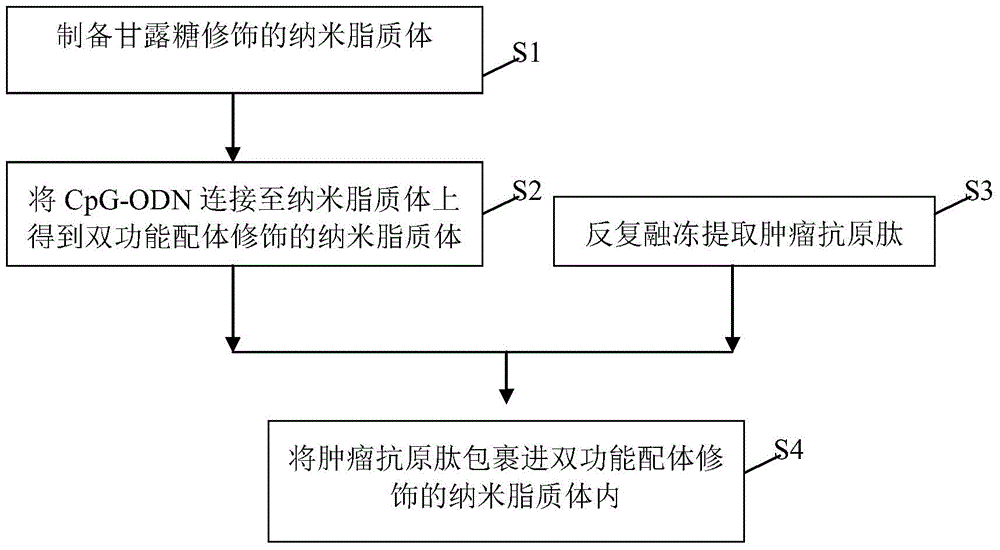
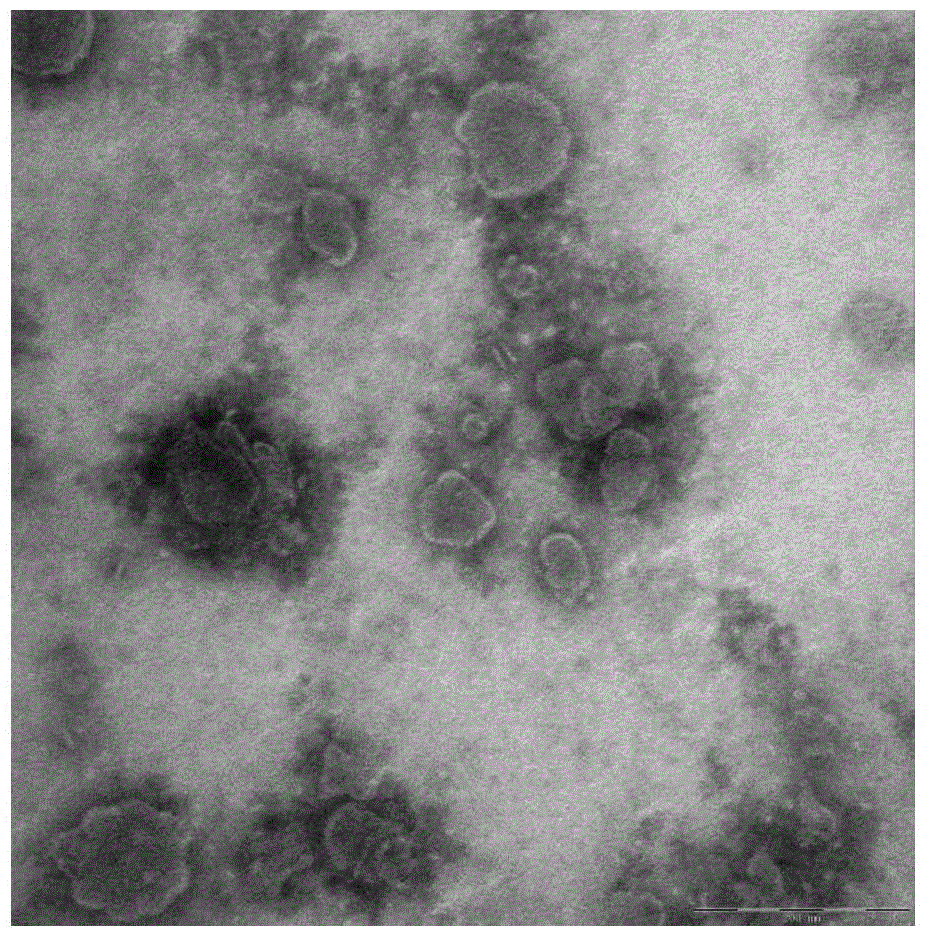
Bi-function ligand targeting dendritic cell tumor vaccine and preparation method thereof
InactiveCN104623646AReduced clearance rateExtended stayPharmaceutical non-active ingredientsAntibody medical ingredientsDendritic cellT lymphocyte
The invention relates to a bi-function ligand targeting dendritic cell tumor vaccine and a preparation method thereof. The method comprises the following steps: S1)preparing mannose modified nanoliposomes; S2)connecting CpG-ODN to the nanoliposomes to obtain the bi-function ligand modified nanoliposomes; S3)extracting tumour antigen peptide from tumor cells by a repeated thawing-freezing method; and S4) coating tumour antigen peptide by the ligand modified nanoliposomes by a freeze-drying-rehydration method. According to the invention, the bi-function ligand modified nanoliposomes can be taken as a carrier, mannose molecules on surface of liposome is specifically targeted to dendritic cells, CpG-ODN is combined to a Toll acceptor 9 in the dendritic cells for promoting the maturation of dendritic cells, matured dendritic cells enables high efficiency presentation of tumour antigen peptide released from inner part of nanoliposomes, T lymphocyte can be activated, and specific antineoplastic effect is generated.
Owner:GUANGXI MEDICAL UNIVERSITY
Dendritic cell tumor vaccine and its preparation and use
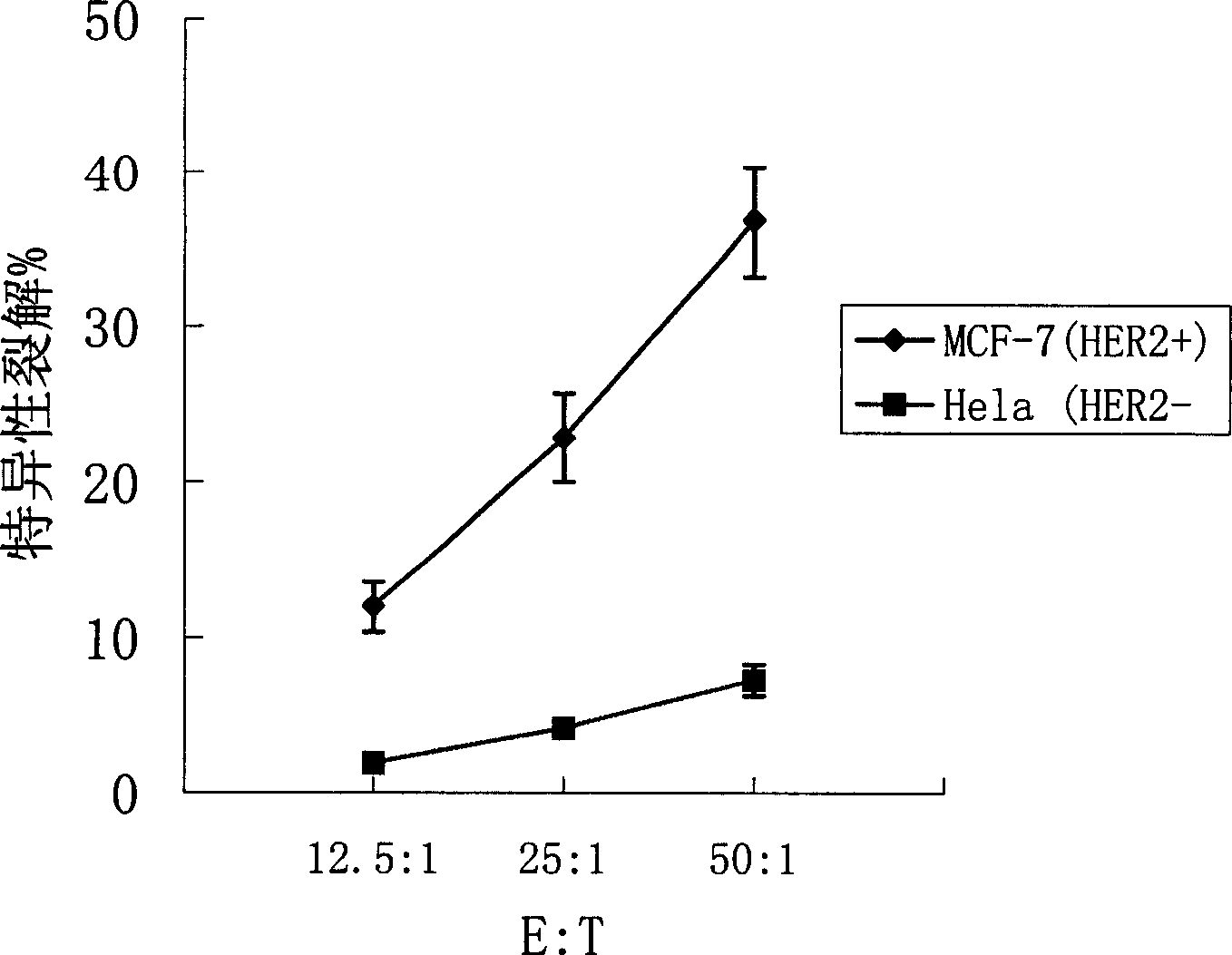
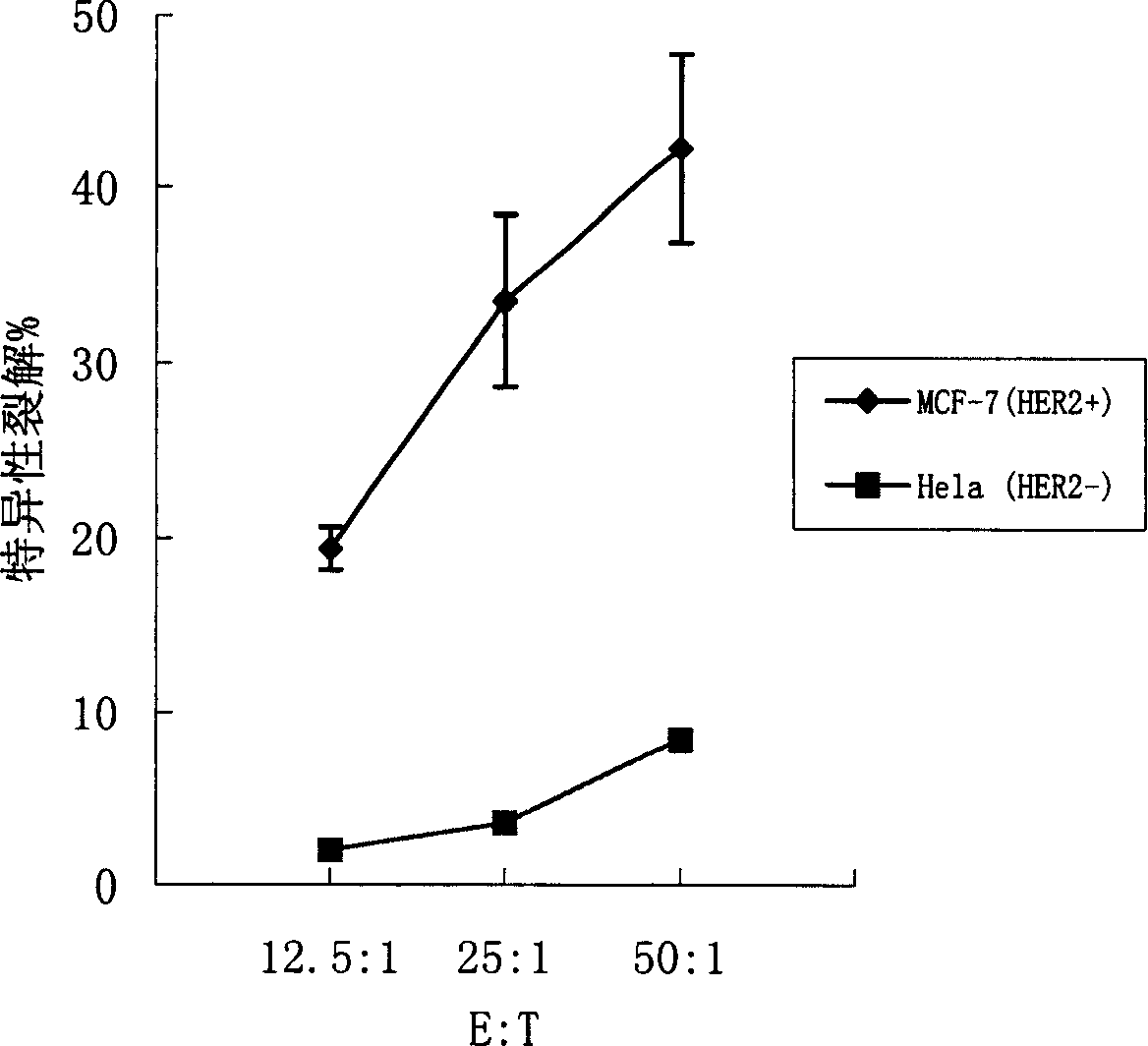
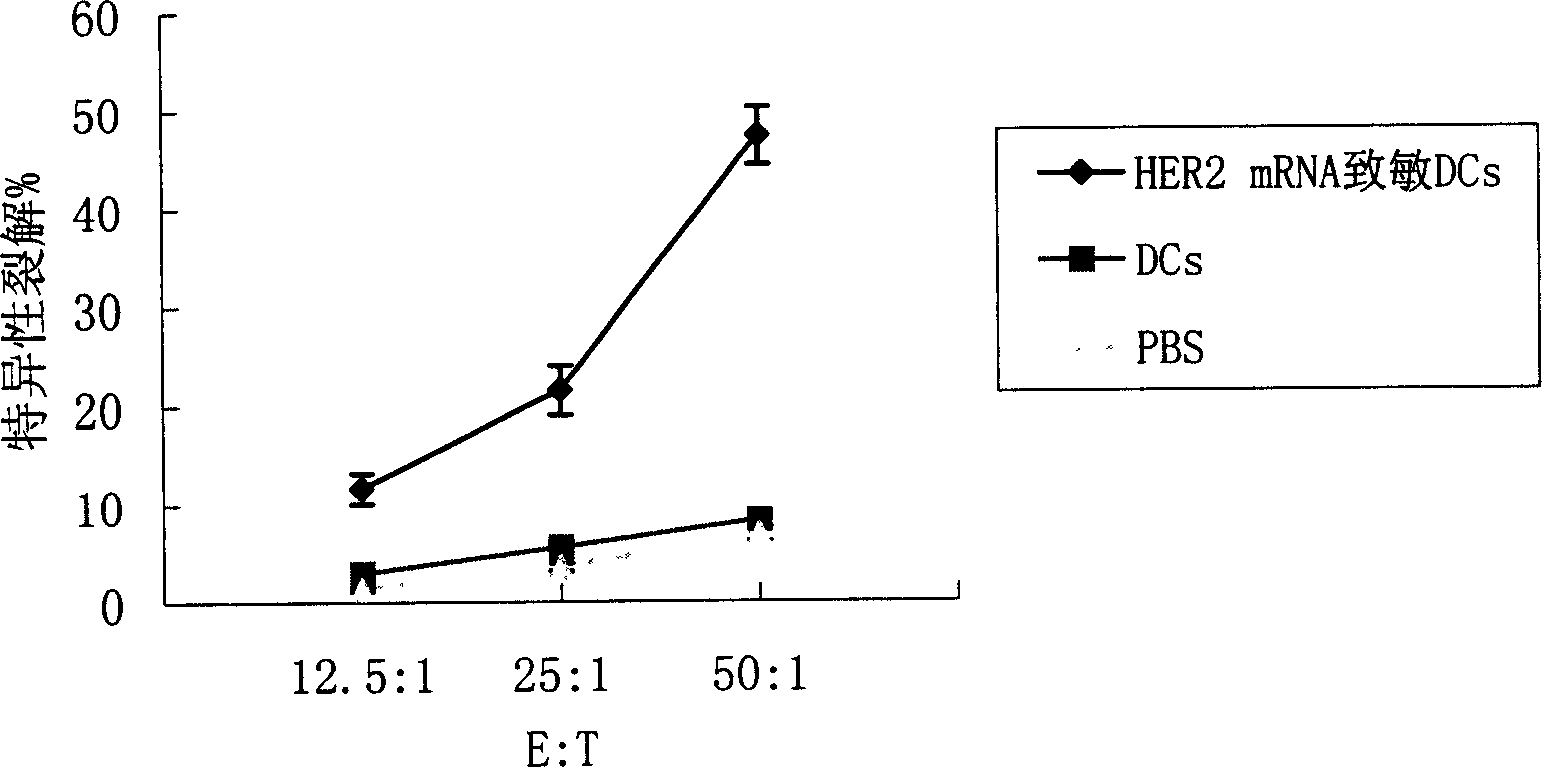
ActiveCN1607247AImprove efficiencyEasy to manufactureAntibody medical ingredientsAntineoplastic agentsSpecific immunityTumor antigen
Said invention provides tumor antigen mRNA sensitized dendritic cell tumor vaccine, preparation and use thereof, and medicinal composition of dendritic cell. Said tumor vaccine is specific mRNA modified dendritic cell vaccine which can stimulate body to produce specific immune response aiming at HER2 positive tumor, so it can be used in preventing and curing HER2 positive tumor such as breast cancer etc. said invention has high transfection efficiency, wide anti tumor gammarayspectrum and strong specificity.
Owner:上海海欣生物技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com