Preparation method of HLA-G (Human Leukocyte Antigen G) antibody and application of HLA-G antibody in medicine
An HLA-G, 1. HLA-G technology, applied in the direction of antibody medical components, antibodies, anti-animal/human immunoglobulin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1: the result of clinical immunohistochemical test with the polyclonal antibody provided by the present invention
[0063] Table 1 uses the polyclonal antibody provided by the invention to do the result of immunohistochemical test
[0064] tumor type
[0065] Uterine cancer
Embodiment 2
[0066] Example 2: Establishment of a biotin-avidin double monoclonal antibody sandwich one-step method for detection of HLA-G chemiluminescence immunoassay:
[0067] A. Use the monoclonal antibody prepared by the present invention to prepare a complex of monoclonal antibody (1) with biotin, a complex of monoclonal antibody (2) with horseradish peroxidase, and bind streptavidin to a solid phase carrier superior.
[0068] B. Establish a CLIA method for detection of HLA-G by a one-step sandwich method of avidin-biotin double monoclonal antibody. Add 50 μl each of the serum to be tested (or HLA-G standard), the complex of monoclonal antibody (1) with biotin, and the complex of monoclonal antibody (2) with horseradish peroxidase Incubate the wells of the coated Nanc plate at 37°C for 60 minutes, wash with PBST three times, add the substrate luminol solution, detect the luminescence value at 425nm with a chemiluminescent immunoassay analyzer, and calculate the sample content by com...
Embodiment 3
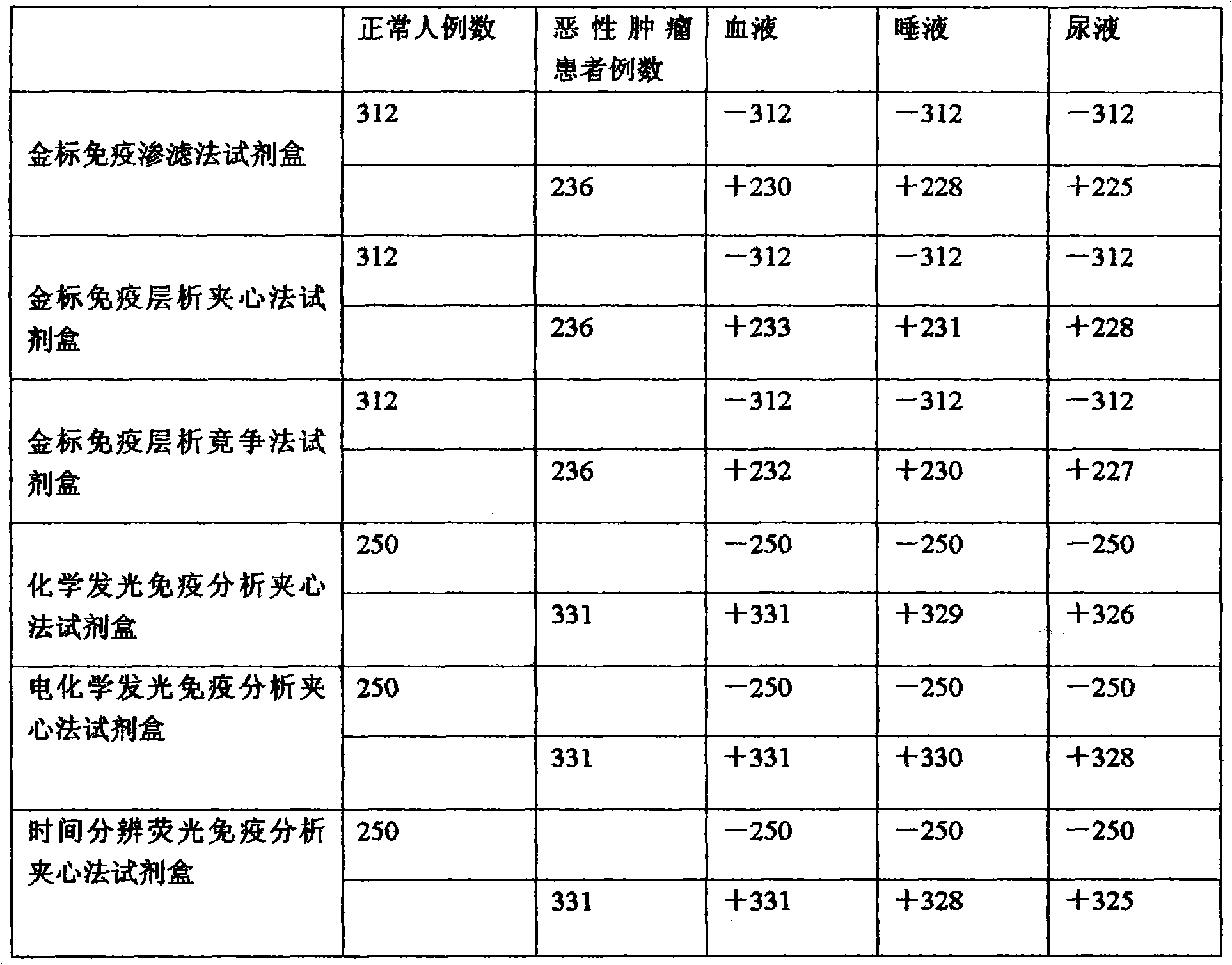
[0069] Example 3: The comparison of clinical detection HLA-G data of gold-labeled immunodiagnostic kit, chemiluminescence immunodiagnostic kit, electrochemiluminescence immunodiagnostic kit, and time-resolved immunoassay diagnostic kit is shown in the table below:
[0070] Table 2 The results of six kinds of kits detecting HLA-G in blood, saliva and urine of normal people and tumor patients
[0071]
[0072] Result analysis:
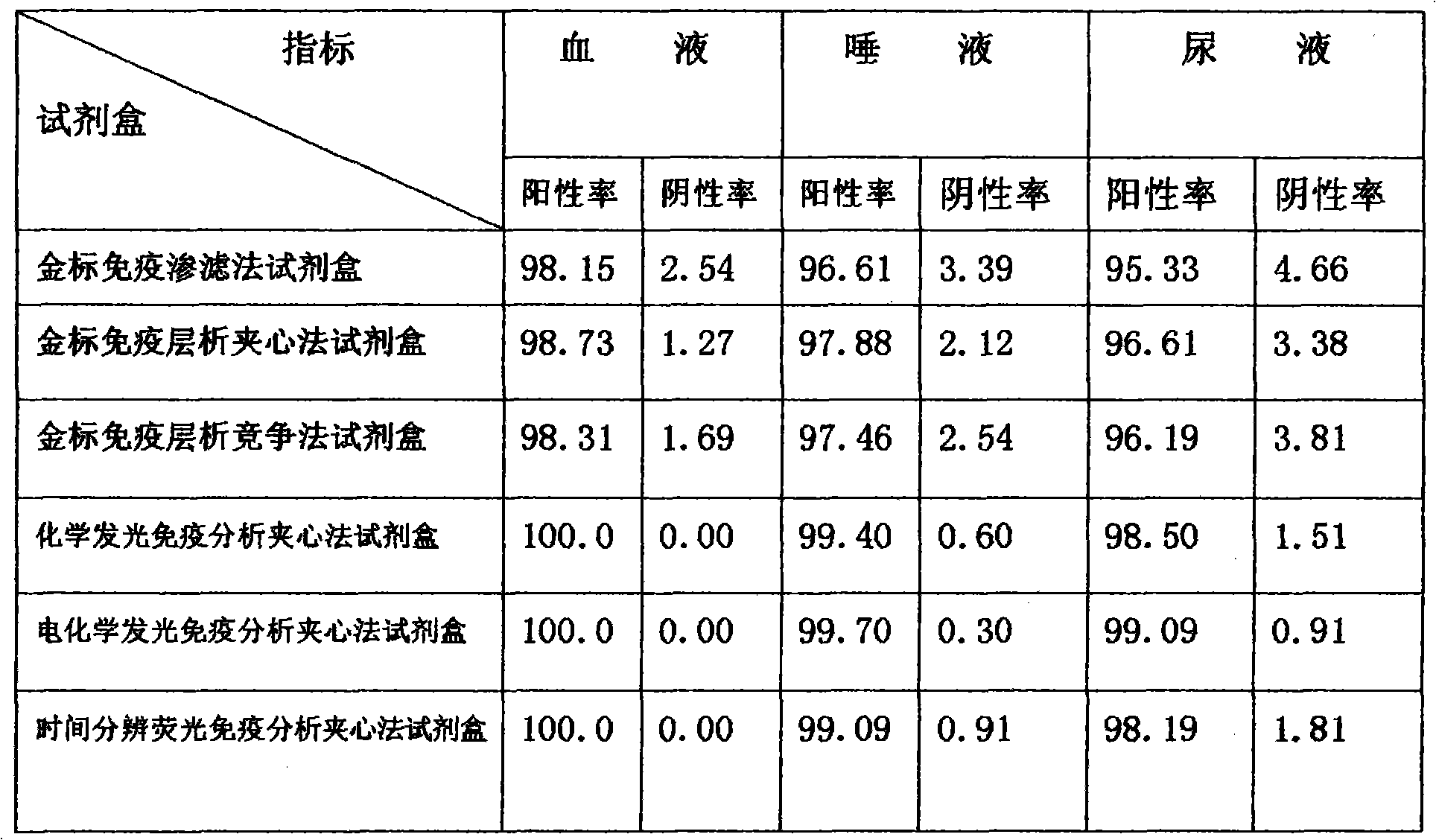
[0073] 1. The results of six kits for detecting HLA-G in blood, saliva and urine of tumor patients are shown in Table 3:
[0074] Table 3 Positive and negative rates of blood, saliva, and urine of tumor patients detected by six kits
[0075]
[0076] 2. The results of the six kits for detecting HLA-G in the blood, saliva and urine of normal people are all negative.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com