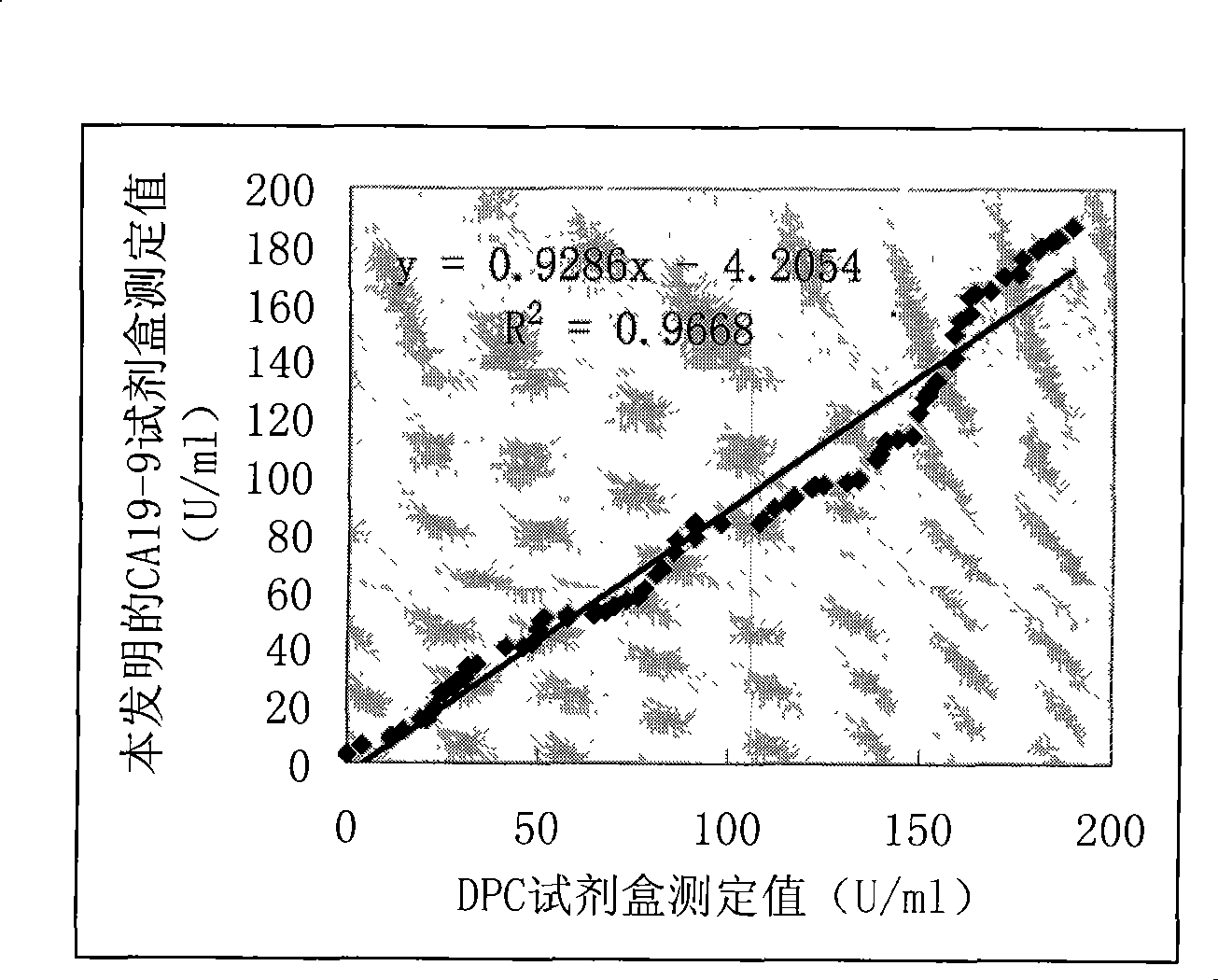
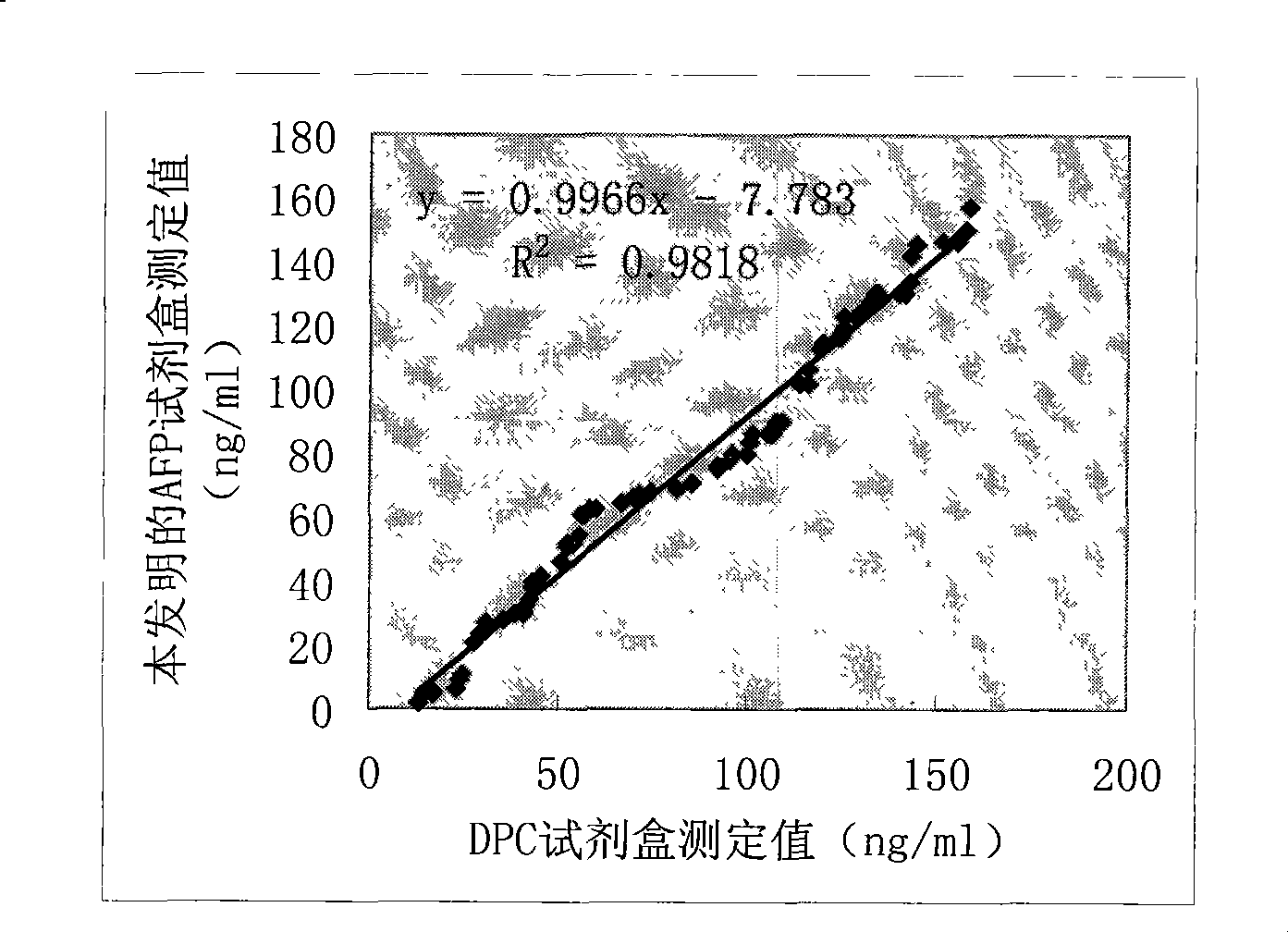
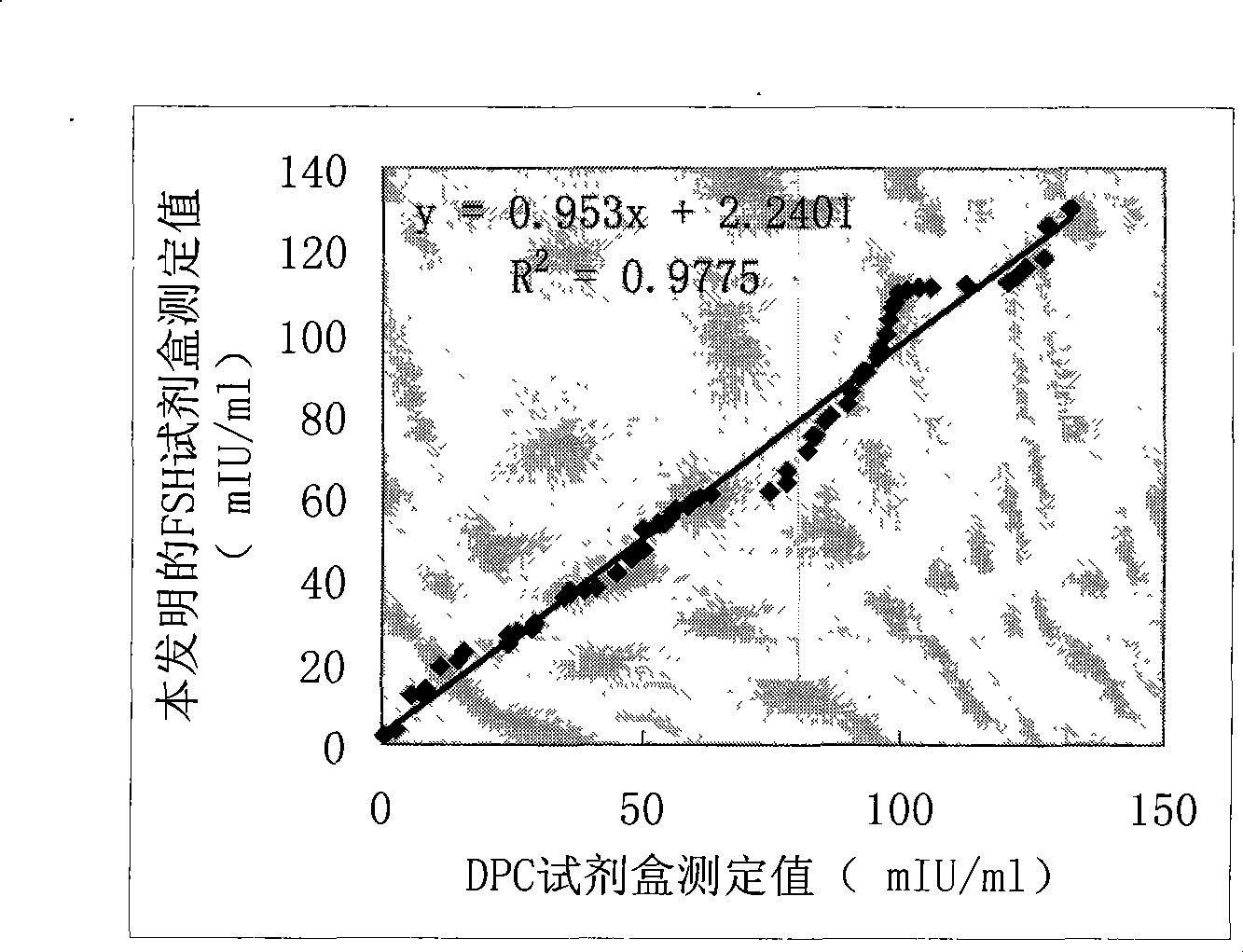
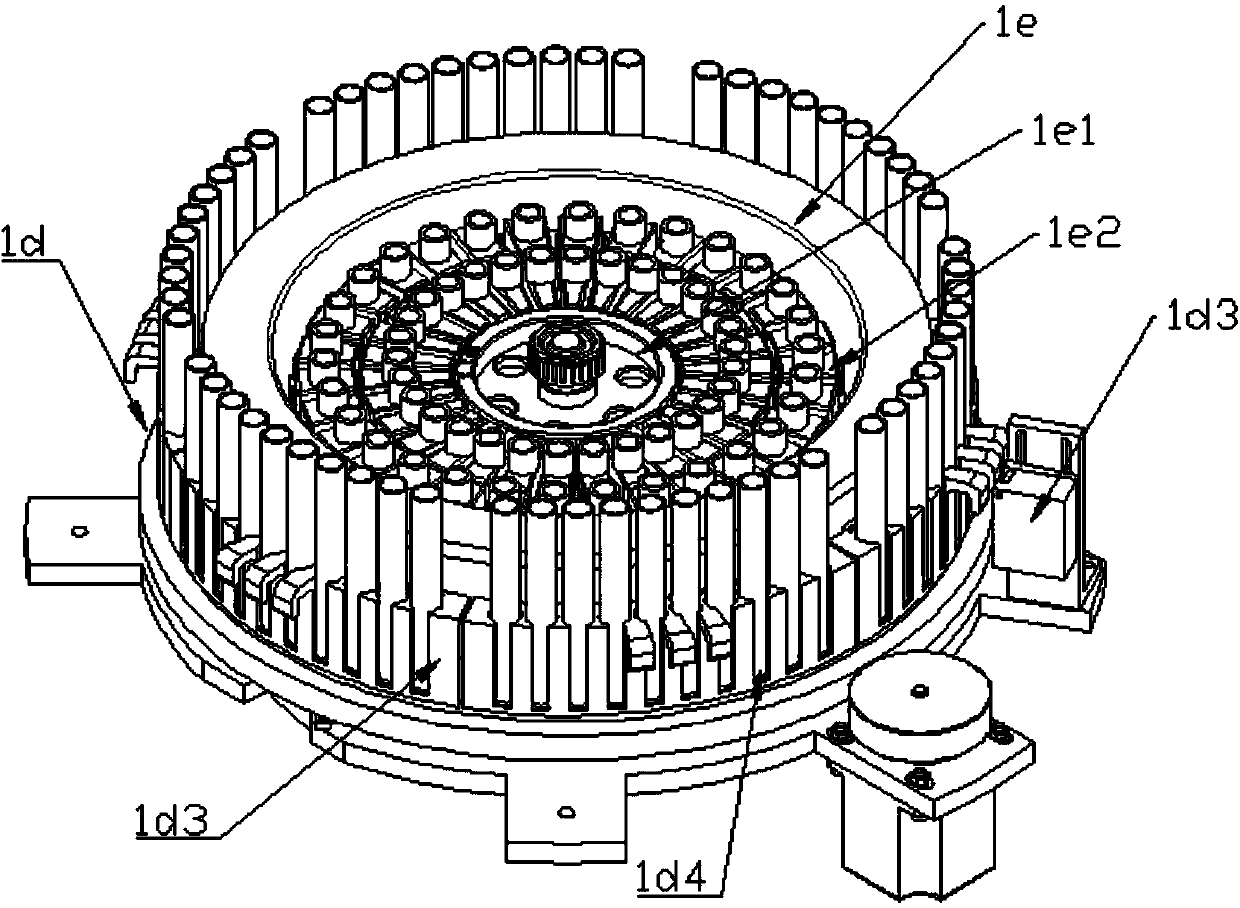
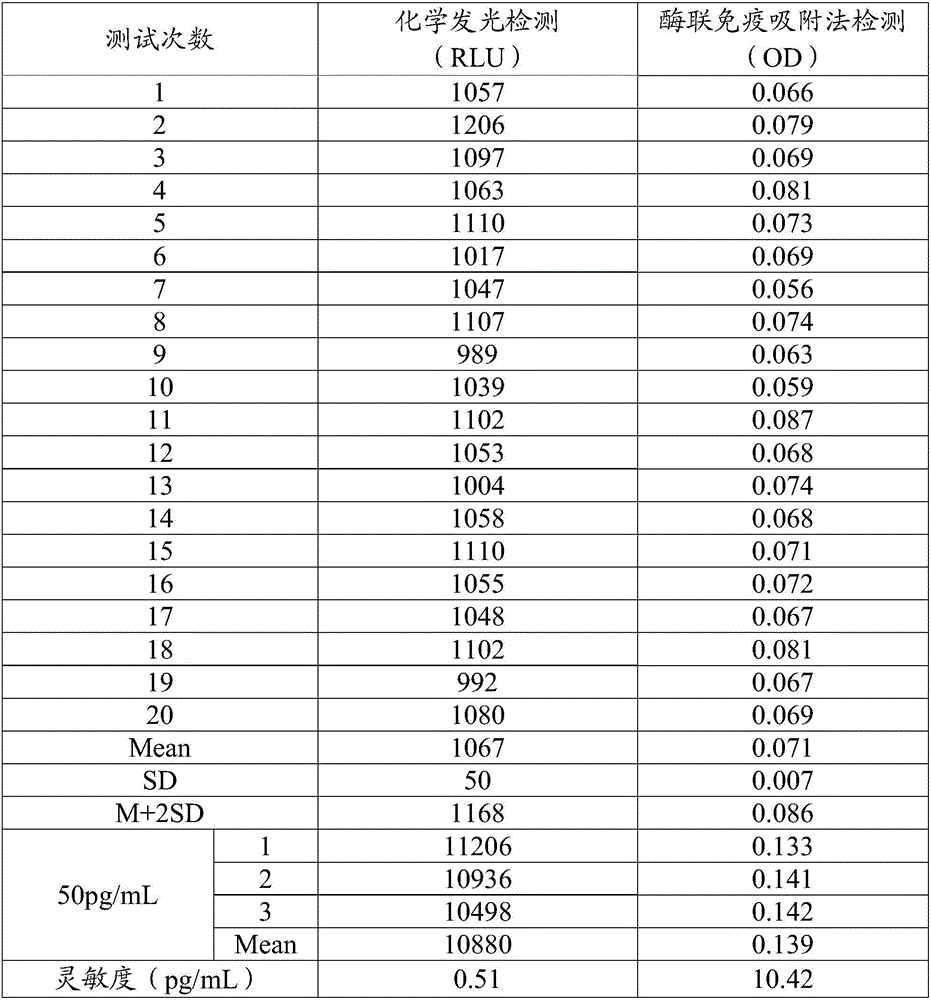
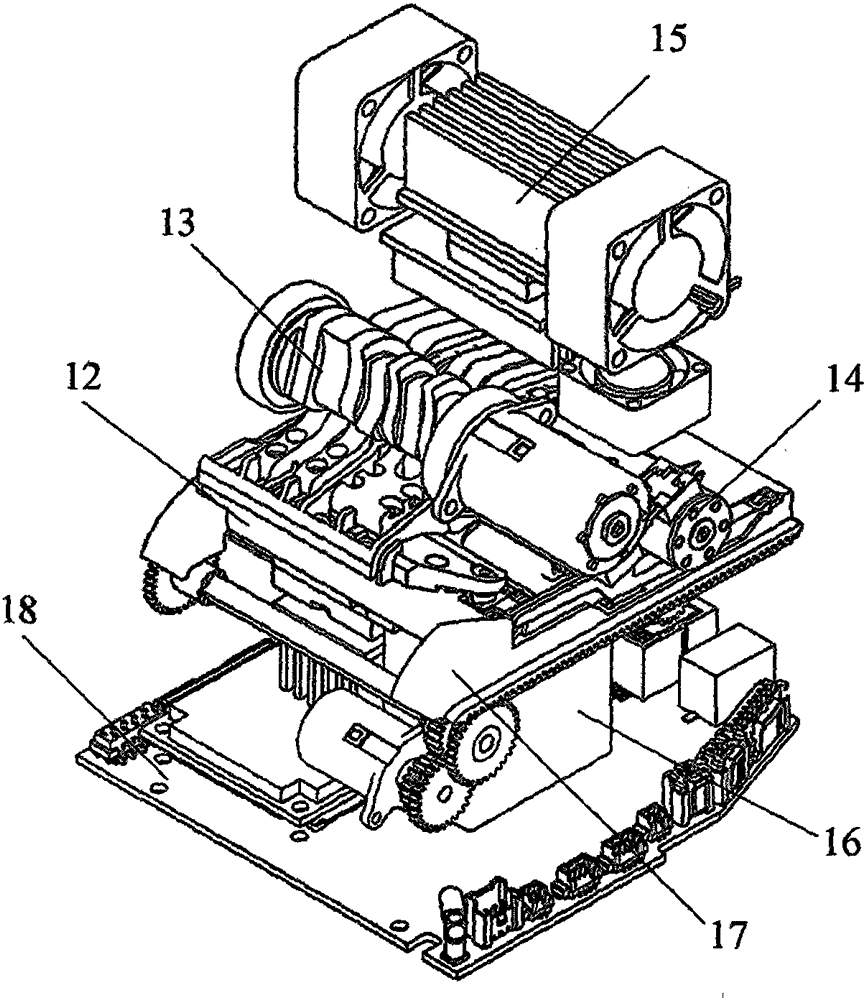
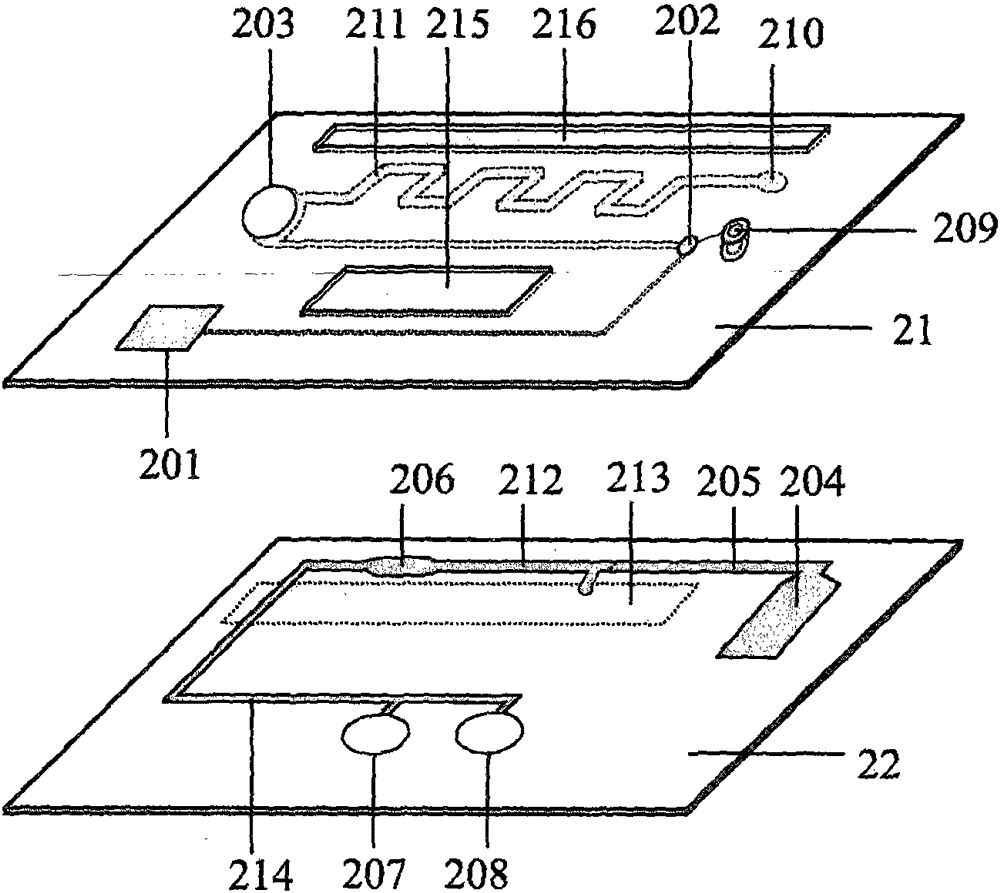
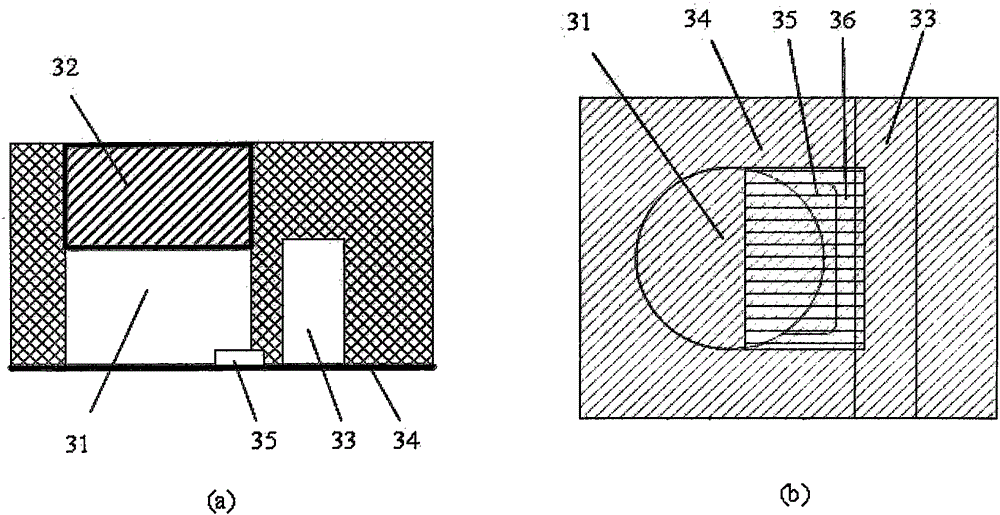
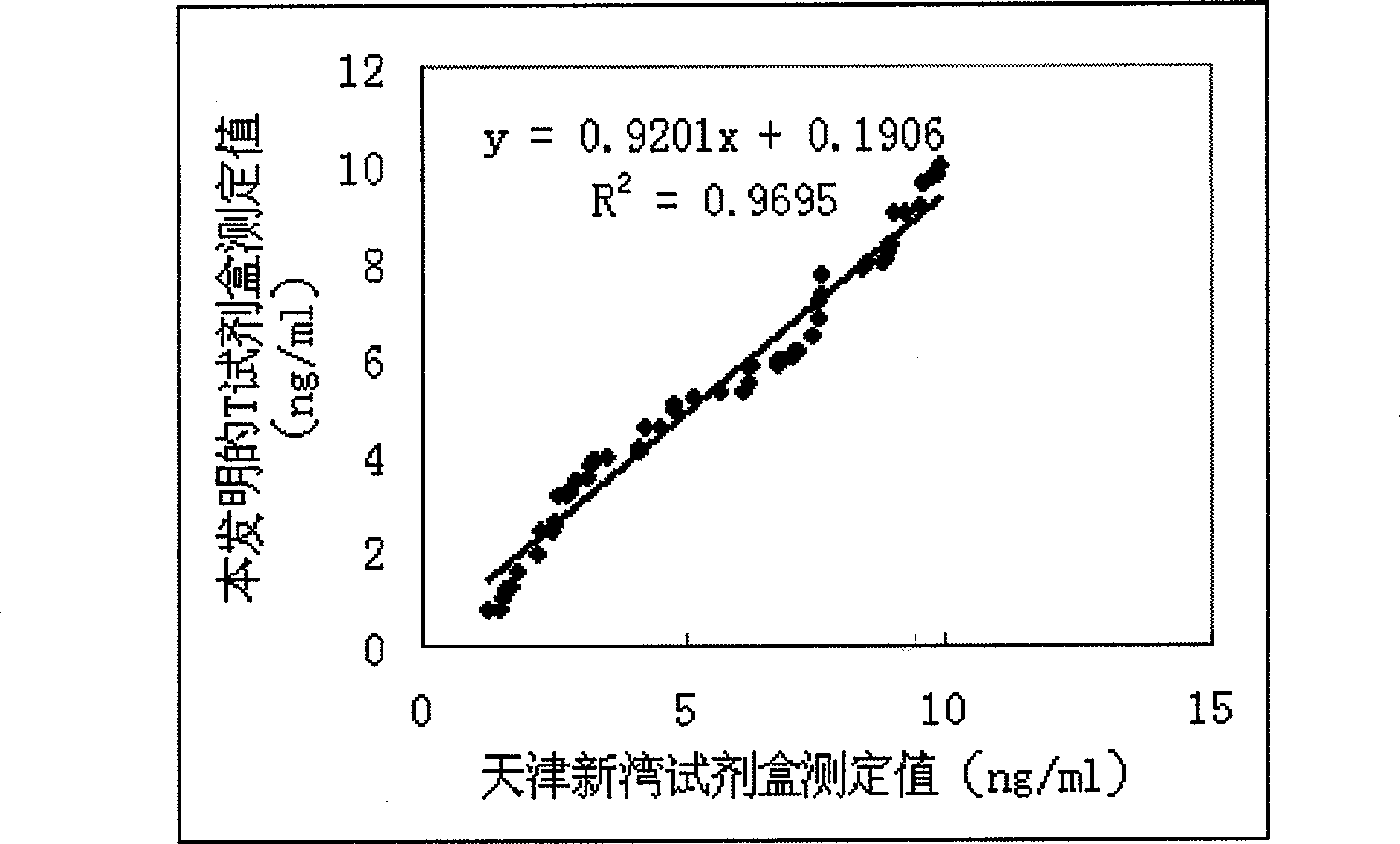
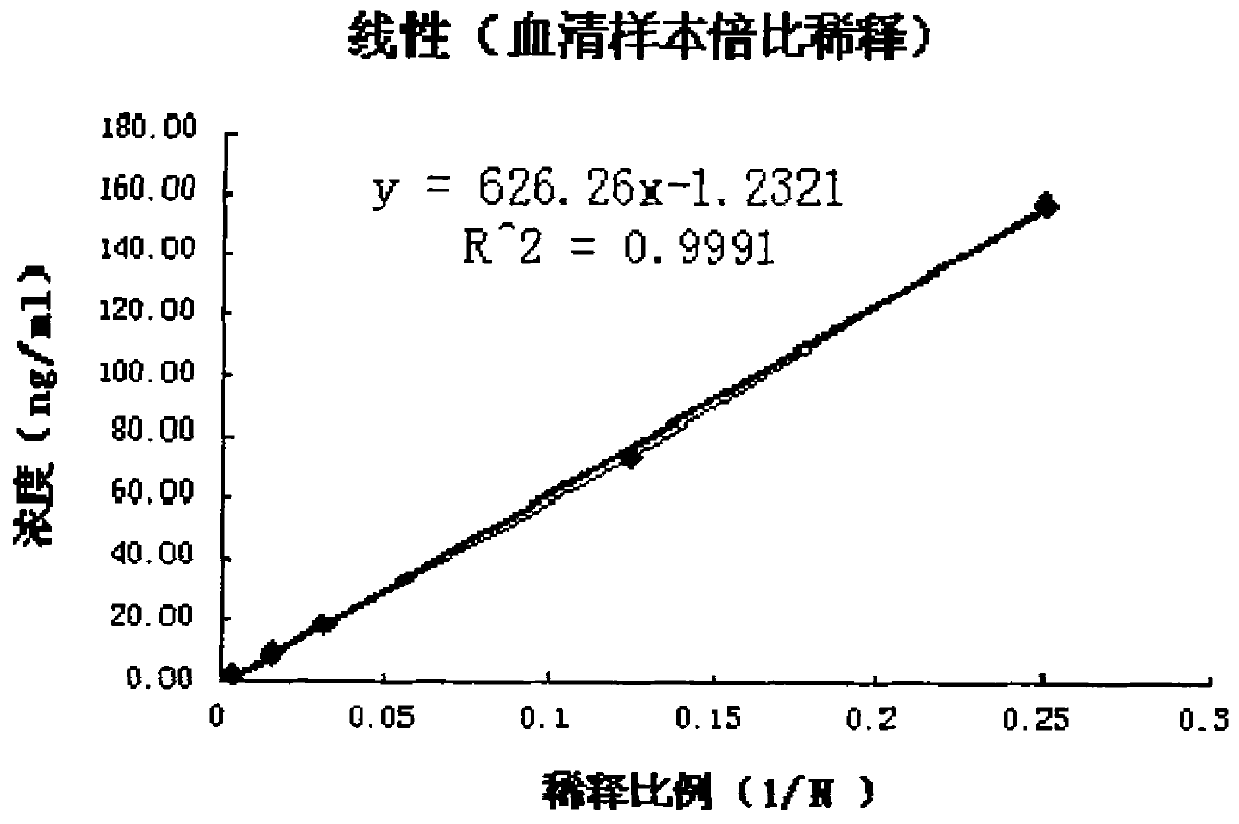
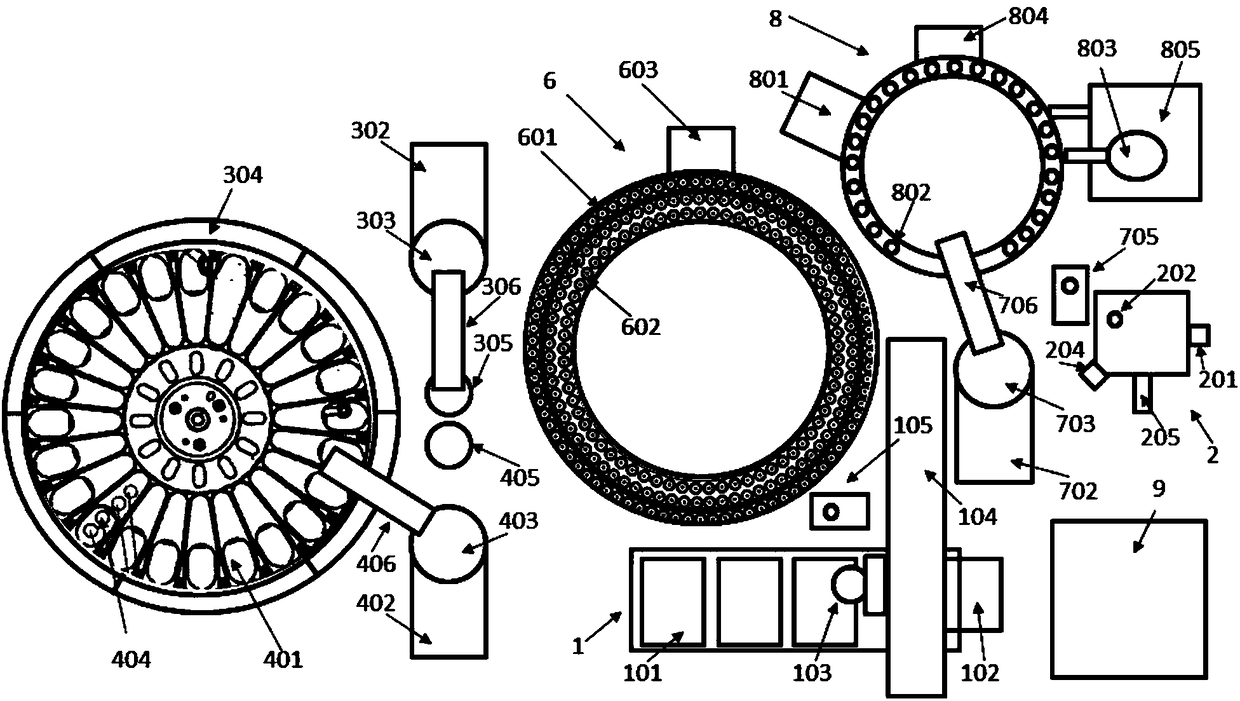
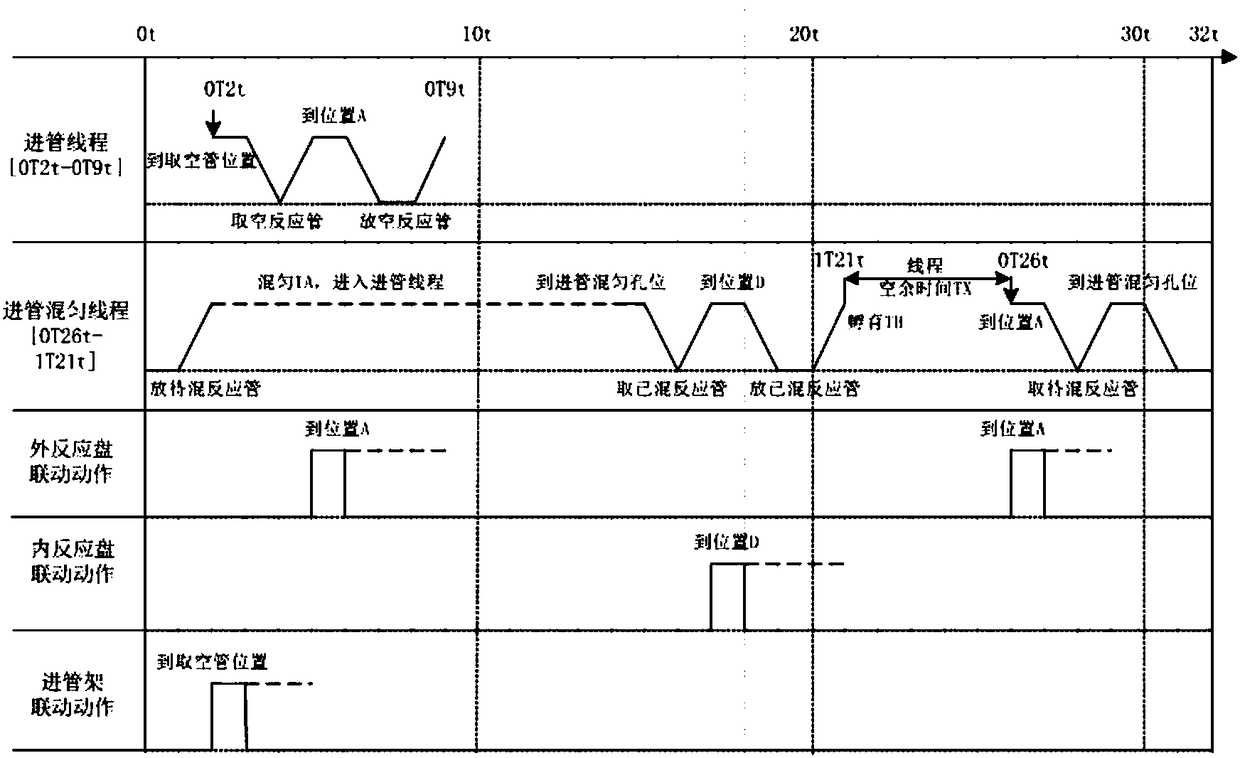
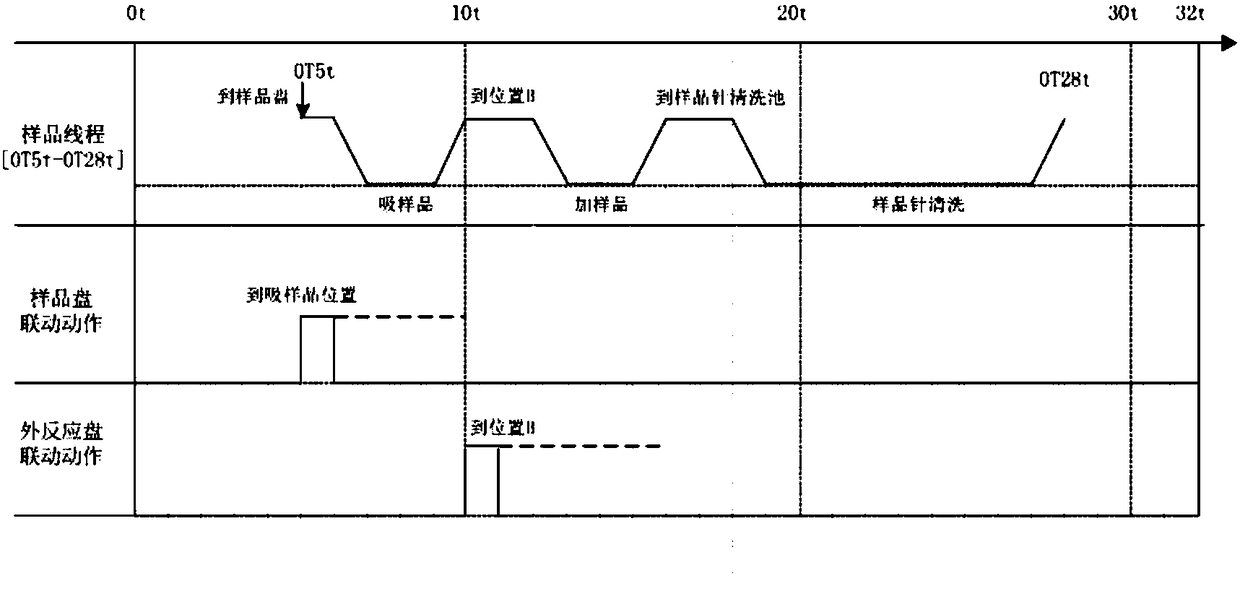
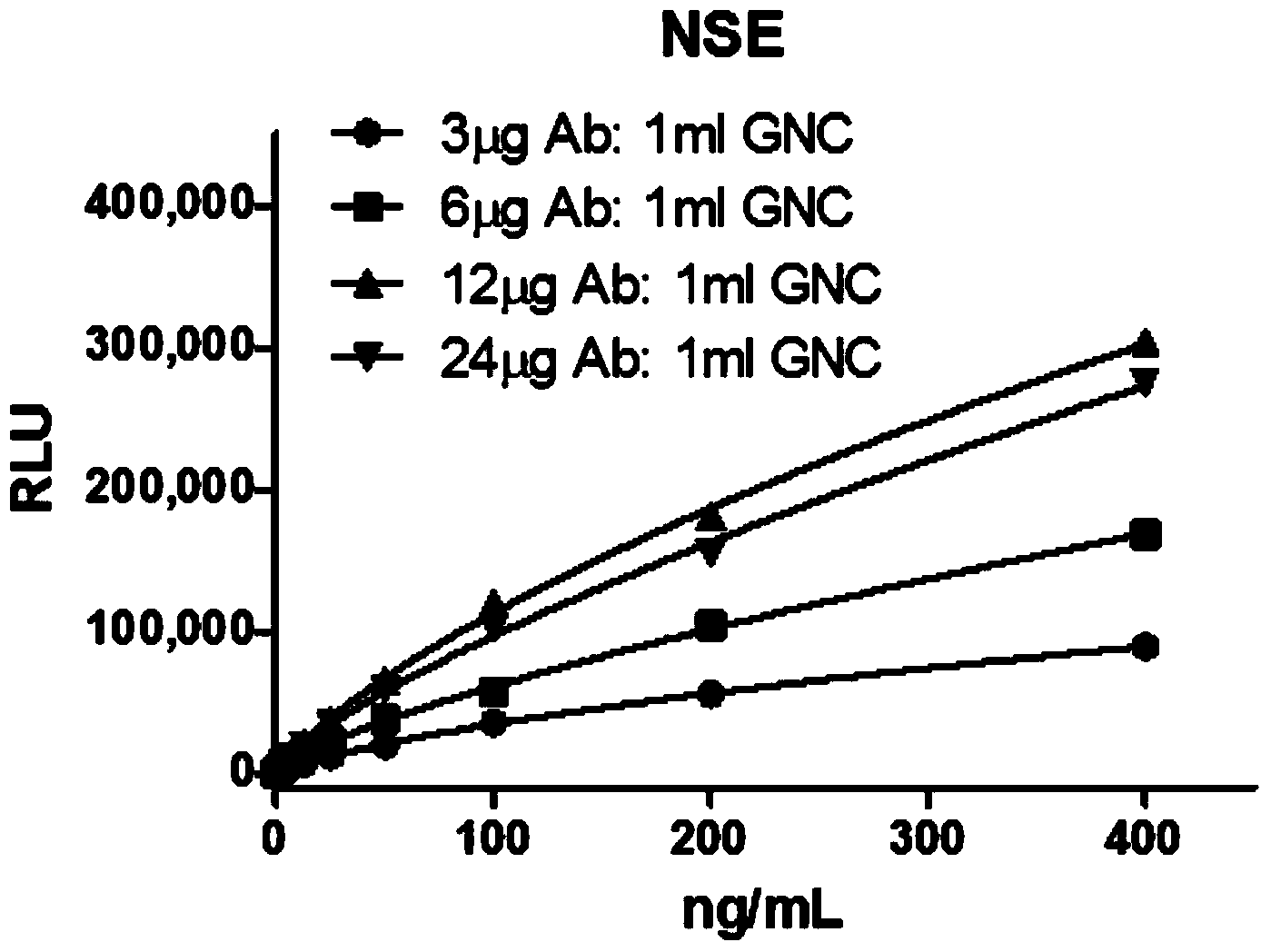
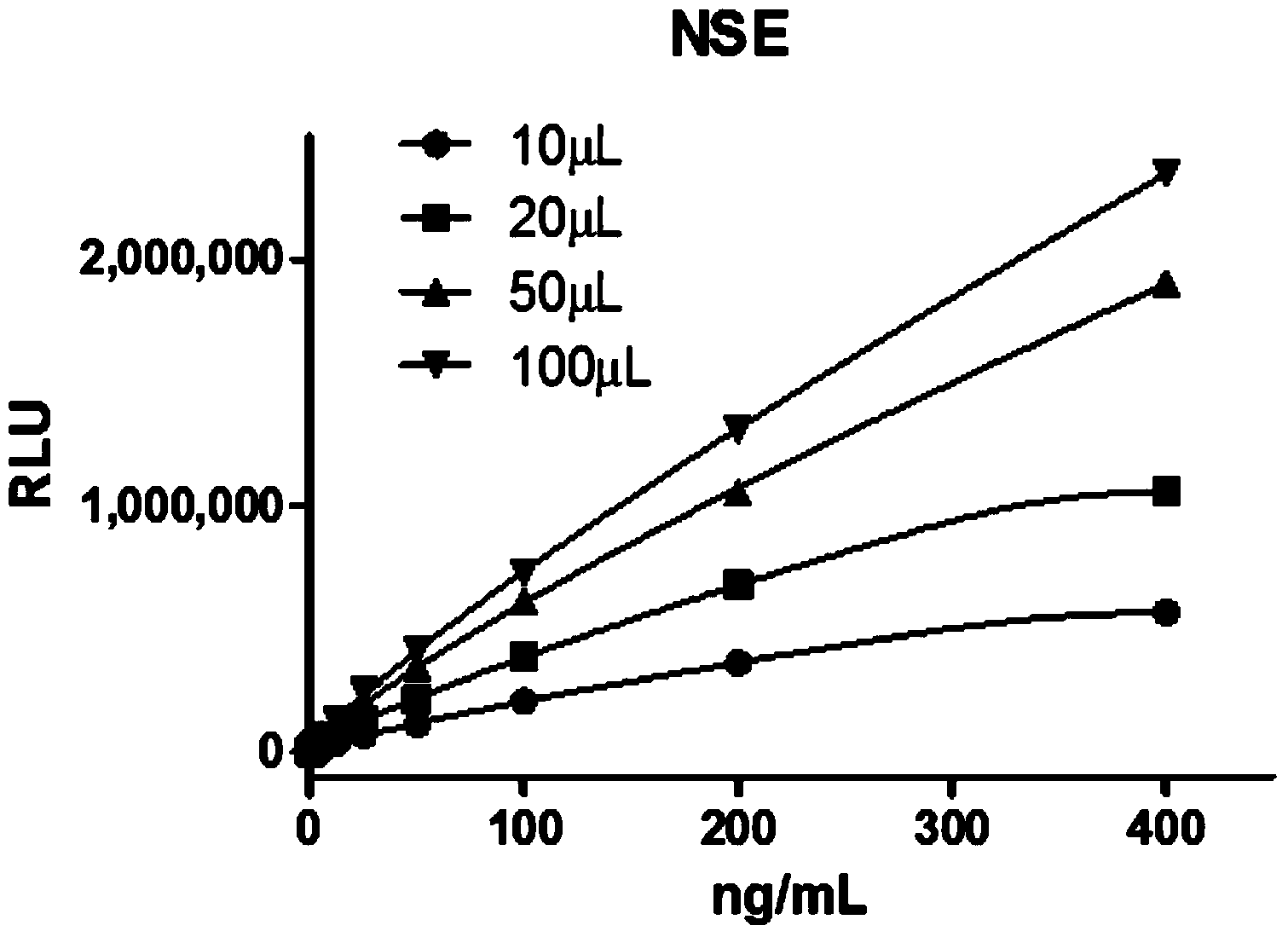
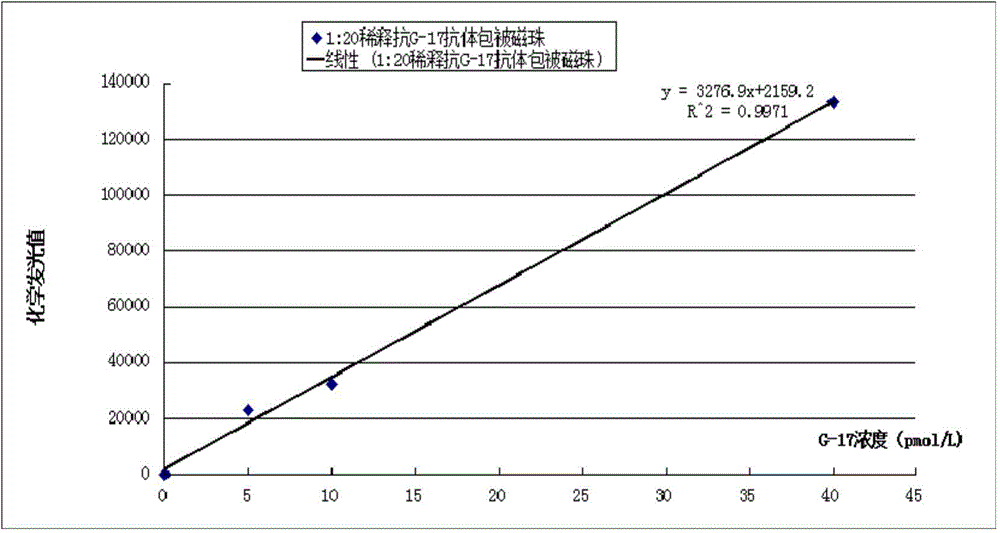
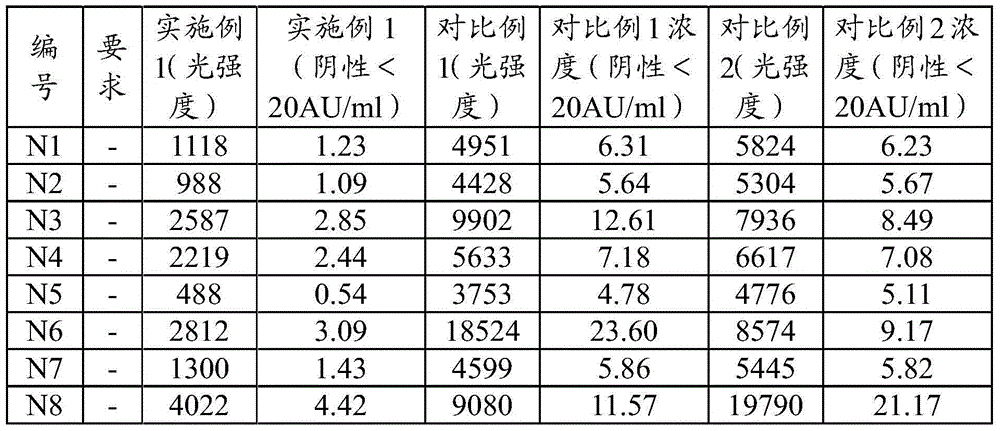
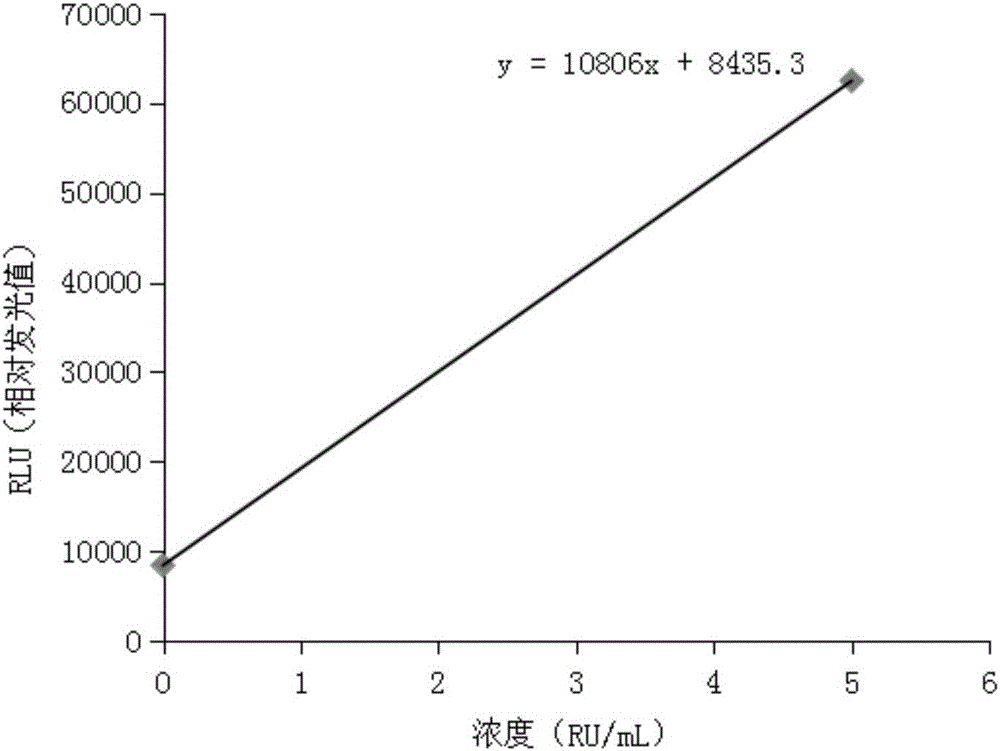
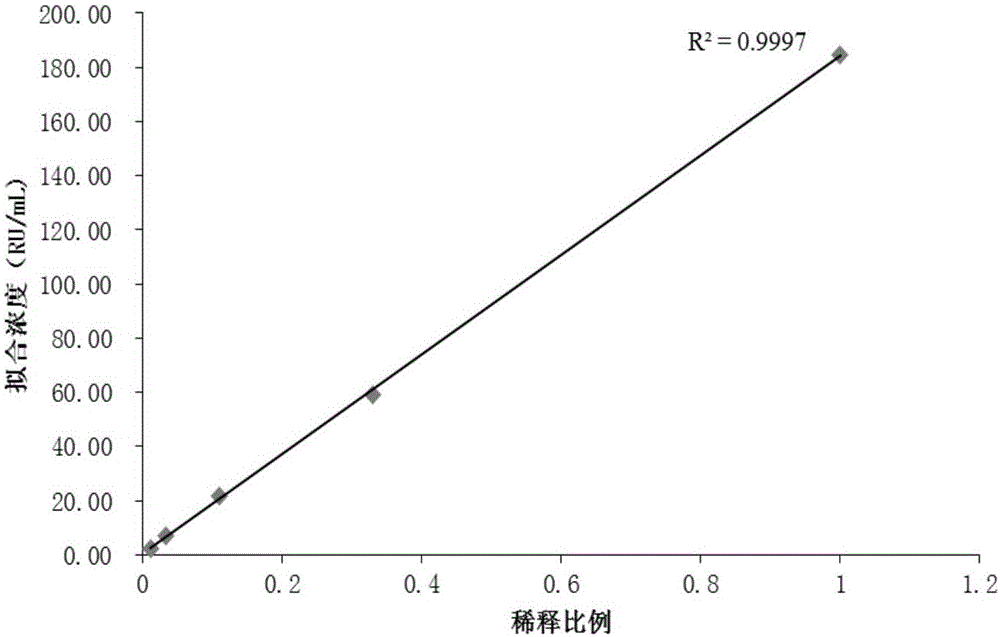
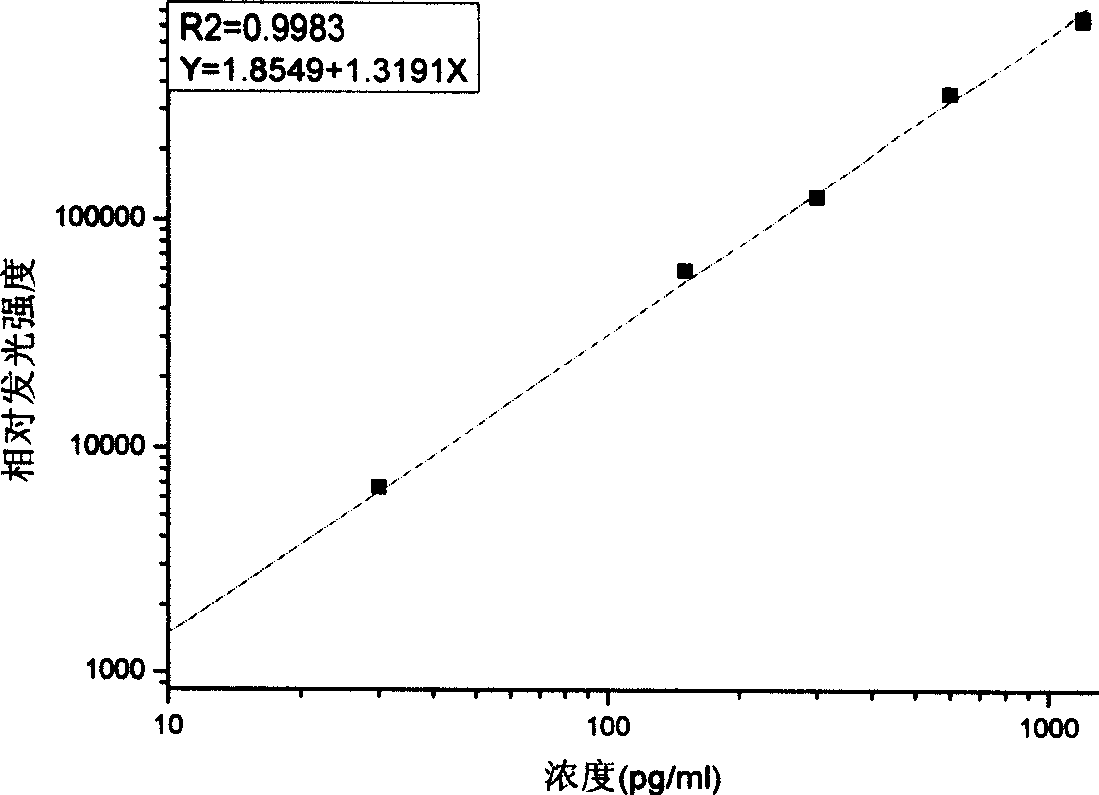
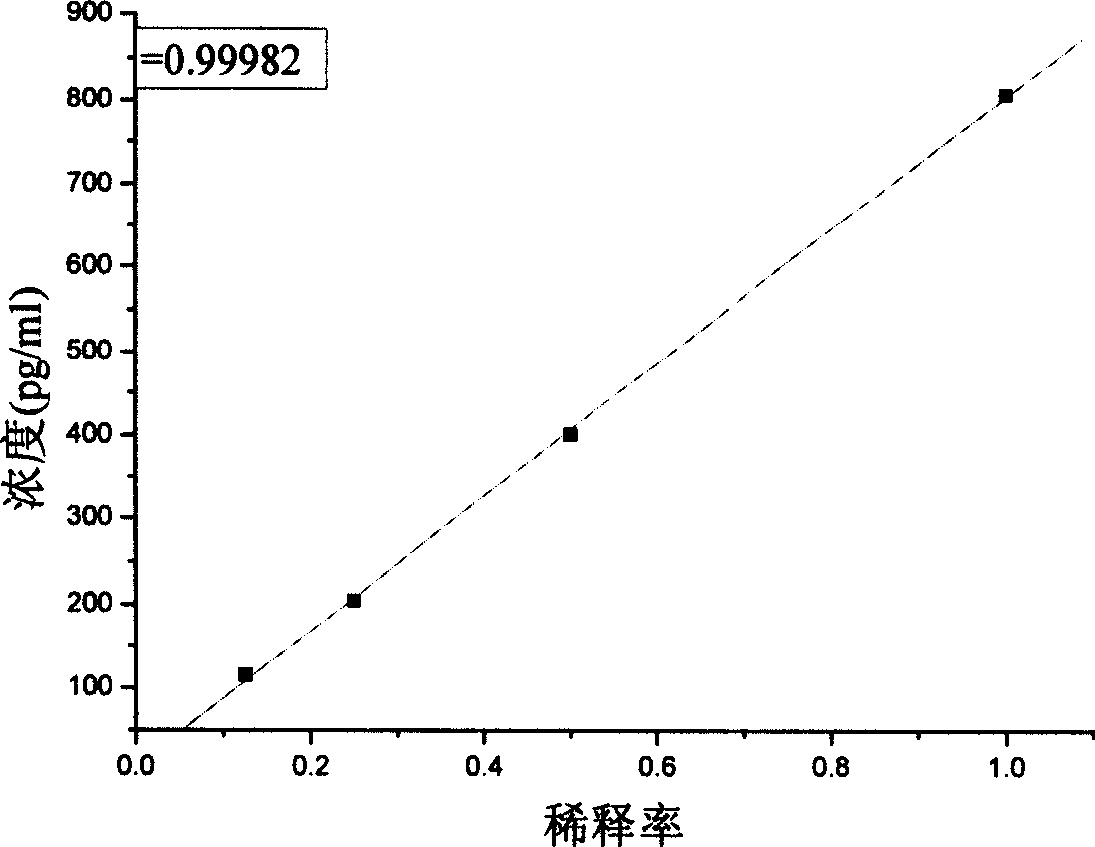
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
605 results about "Chemiluminescence immunoassay" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
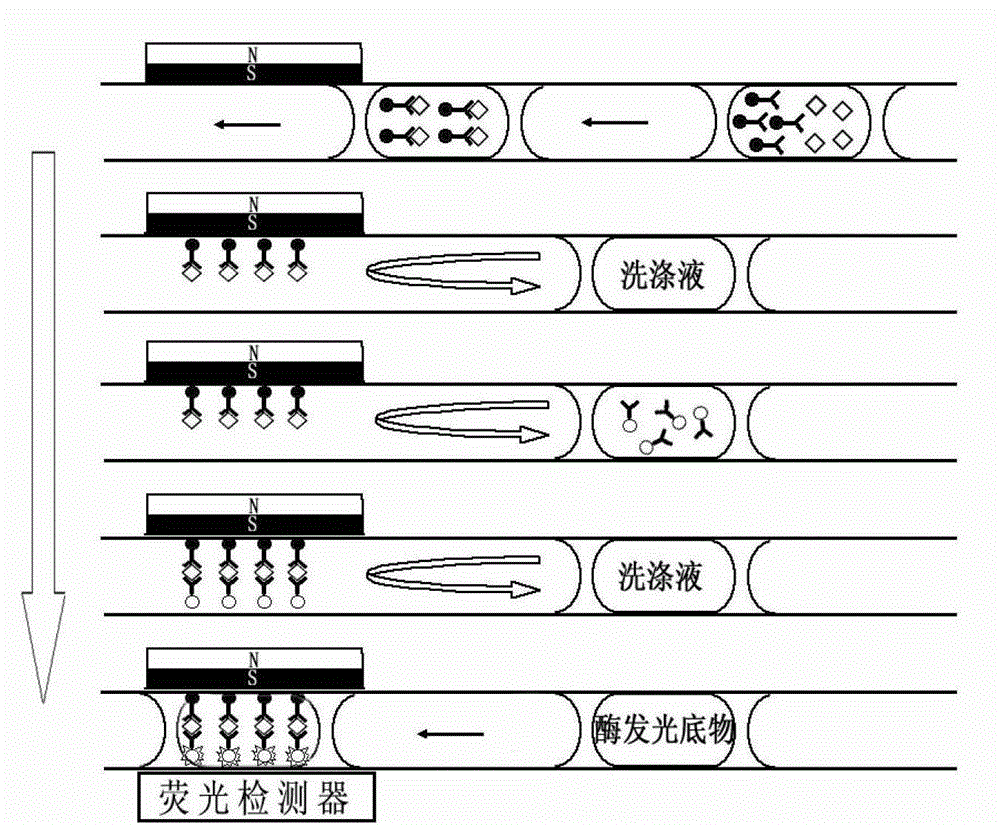
Magnetic microparticle separating chemiluminescence immune analysis determination reagent kit for detecting related sign object and preparing method thereof
ActiveCN101377490AEnsure sensitivityEnsure effectivenessChemiluminescene/bioluminescenceBiological testingDiseaseMicroparticle
The invention relates to the immunoassay medical field, particularly provides a magnetic particle separation chemiluminescence immunoassay assay kit and a preparation method thereof used for detecting disease related markers. The kit of the invention comprises: 1) a calibrator; 2) magnetic particles which are coated with streptavidin; 3) disease related marker antibodies of enzyme markers and biotin markers; and 4) a chemiluminescence substrate. Further, the method for preparing the kit according to the invention includes the following steps: 1) pure raw materials are used to prepare the calibrator; 2) the streptavidin is used to coat the magnetic particles; 3) the mixed liquid of the enzyme and the biotin markers are prepared; 4) the calibrator, the chemiluminescence substrate as well as the mixed liquid of the enzyme and the biotin markers are packaged in a separated way; and 5) a finished product is packaged. The kit has the advantages of convenience, rapidness, sensitivity, stability, and the like.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Full-automatic chemiluminescence immunoassay analyzer
ActiveCN103592450ARealize the refrigeration functionSimple structureMaterial analysisRefrigerationChemiluminescence immunoassay
The invention provides a full-automatic chemiluminescence immunoassay analyzer. The full-automatic chemiluminescence immunoassay analyzer comprises an operation control main body and a computer connected to the operation control main body. The operation control main body comprises a reagent sample module, an incubation module, a cleaning module and a detection module. The reagent sample module comprises a reagent sample tray and a sample arm between the reagent sample tray and the incubation module. The incubation module comprises a reaction disc and a reaction cup loading platform matching the reaction disc. The cleaning module comprises a cleaning disc and a cleaning device related to the cleaning disc. The detection module comprises a detection disc and a detection device related to the detection disc. The full-automatic chemiluminescence immunoassay analyzer realizes sample loading, incubation, cleaning and detection combination, and the reagent sample tray temperature is controlled so that reagent refrigeration is realized and work efficiency is improved; the detection disc temperature is constant by control so that a detection precision is improved; a structure design is reasonable so that operation is convenient and reagent, sample, substrate and waste replacement is convenient; and the full-automatic chemiluminescence immunoassay analyzer adopts a table-type design so that the structure is compact and an occupation space is reduced.
Owner:BIOSCIENCE (TIANJIN) DIAGNOSTIC TECH CO LTD
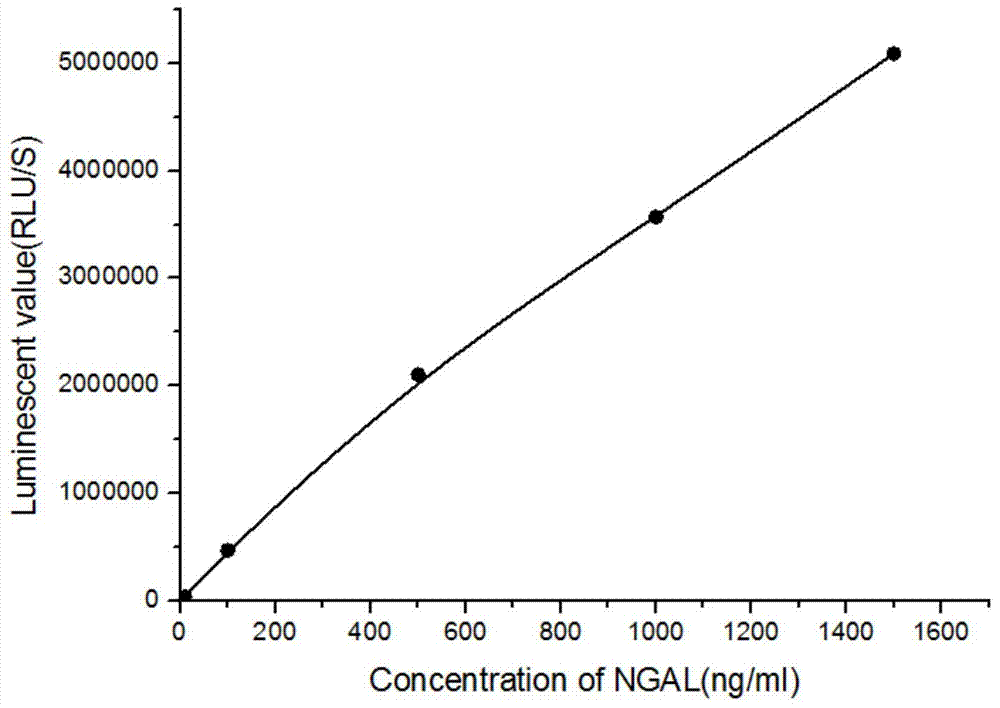
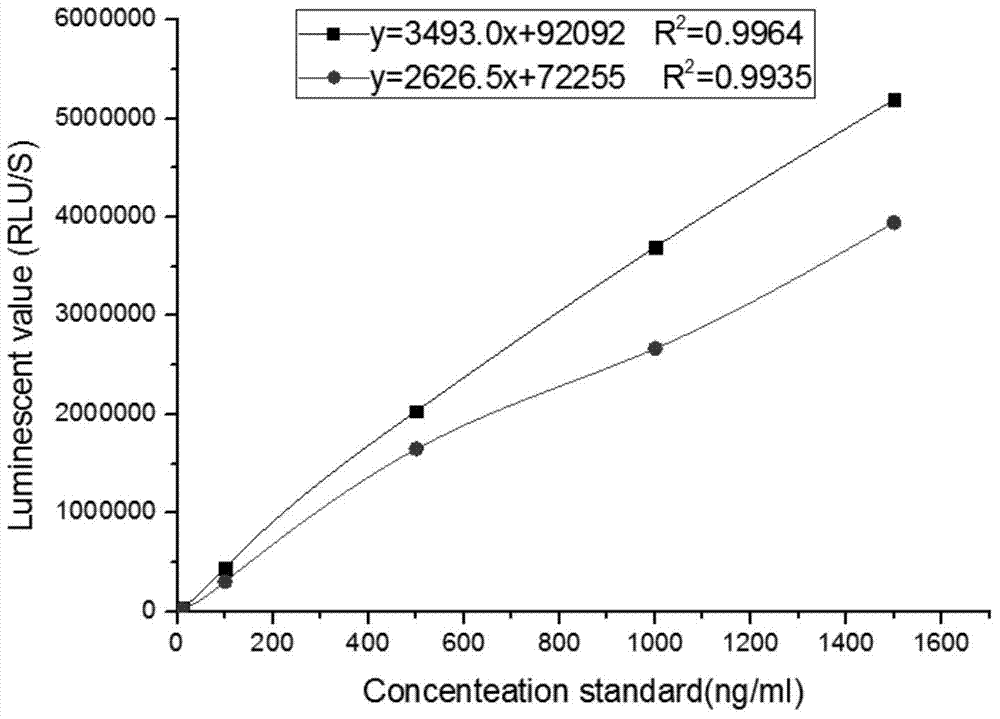
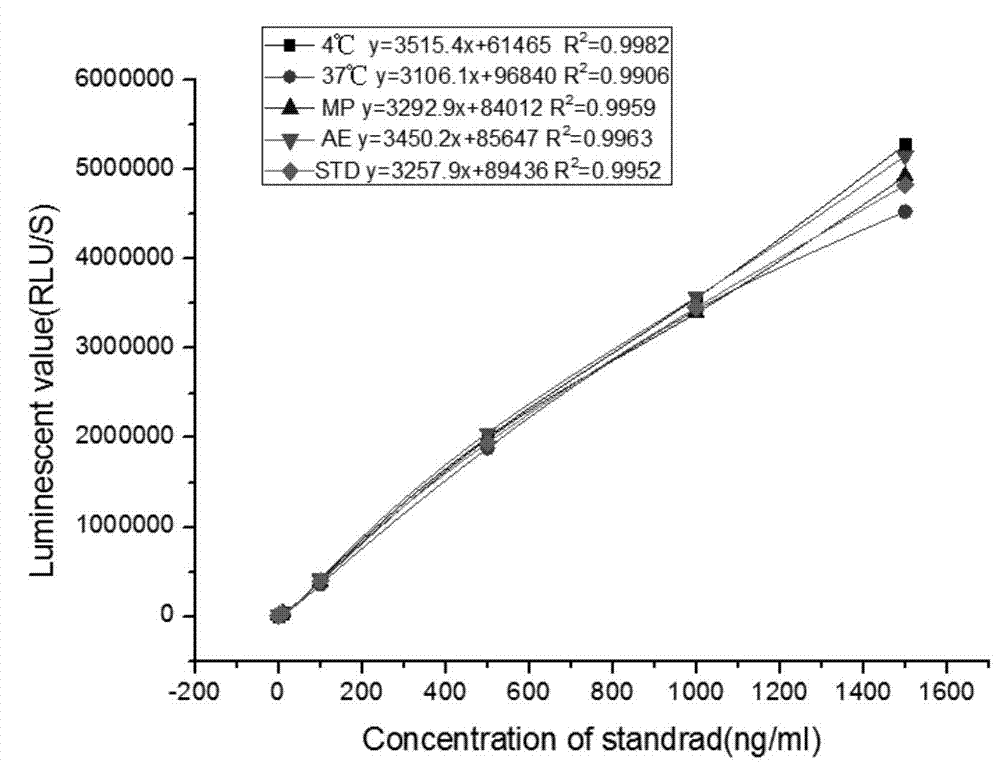
Kit for detecting NGAL content and preparation method thereof
The invention relates to a kit for detecting neutrophil gelatinase-associated lipocalin content based on chemiluminescence immunoassay. According to the invention, by employing a double-antibody sandwich immunization analysis method, a chemiluminescence magnetic microspheres immunization technology is used, anti-NGAL antibody-coated magnetic microspheres for specifically combining with NGAL antigen of a standard substance / sample in a reaction cup, then are reacted to another strain anti-NGAL antibody labelled with acridine salt to form an immunization compound, through an acid-base chemical reaction of a pre-Trigger and a Trigger, relative light unit (RLU / s) of the chemiluminescence reaction can be measured; the NGAL antigen content in the sample is in direct proportion to the relative light unit (RLU / s) measured by an optical system, determination of NGAL content in an urine specimen can be determined through standard curve fitting; and the method has the obvious advantages of high sensitivity, strong specificity, good stability, simple operation and low cost.
Owner:GUANGZHOU DARUI BIOTECH
Dry method photic stimulation chemiluminescence immunoassay reagent kit and preparation and application thereof
The invention relates to an immunoassay reagent kit, and discloses a dry method photic stimulation chemiluminescence immunoassay reagent kit and preparation and application thereof. The dry method photic stimulation chemiluminescence immunoassay reagent kit of the invention comprises a reaction vessel of an antigen or an antibody fixed with dry detection particles and dry biotin marks, wherein, the detection particles are luminous particles of the envelope antigen or the antibody. The kit of the invention can be used for quantitative or qualitative detection of the antigen or the antibody to be detected.
Owner:BEYOND DIAGNOSTICS (SHANGHAI) CO LTD
Anti-mullerian hormone chemiluminescence immunoassay kit and preparation method and application thereof
InactiveCN106053791AHigh detection sensitivityImprove detection accuracyMaterial analysisMonoclonal antibodyAssay sensitivity
The invention discloses an anti-mullerian hormone chemiluminescence immunoassay kit and a preparation method and application thereof. The anti-mullerian hormone chemiluminescence immunoassay kit comprises a solid-phase carrier enveloped by an anti-mullerian hormone monoclonal antibody, and an anti-mullerian hormone monoclonal antibody marked by a chemiluminescence marker. The anti-mullerian hormone chemiluminescence immunoassay kit can complete the assay of anti-mullerian hormone by taking a full-automatic chemiluminescence immunoassay analyzer as an assay tool. Through experiments, the assay sensitivity of the anti-mullerian hormone chemiluminescence immunoassay kit can reach 0.01ng / mL, and is improved by at last ten times compared with the traditional anti-mullerian hormone assay method, and the assay precision of the anti-mullerian hormone chemiluminescence immunoassay kit is higher.
Owner:SHENZHEN YHLO BIOTECH +1
POCT (Point of Care Testing) chemiluminescence immunoassay system and method
ActiveCN105203746ASimple structureReduce volumeBiological testingTemperature controlPoint-of-care testing
The invention relates to a POCT (Point of Care Testing) chemiluminescence immunoassay system and a POCT chemiluminescence immunoassay method. The POCT chemiluminescence immunoassay system comprises a testing card, an integrated fluid driving module, a magnet control module, a temperature control module, an optical signal detection module, an automatic testing card pushing device and a circuit analysis control module. Whole blood can be adopted as a detection sample of the system, and can be directly used without centrifugal treatment after being sampled, POCT is achieved beside a patient, and a diagnosis result is quickly obtained.
Owner:SHENZHEN HUAMAIXINGWEI MEDICAL TECH CO LTD
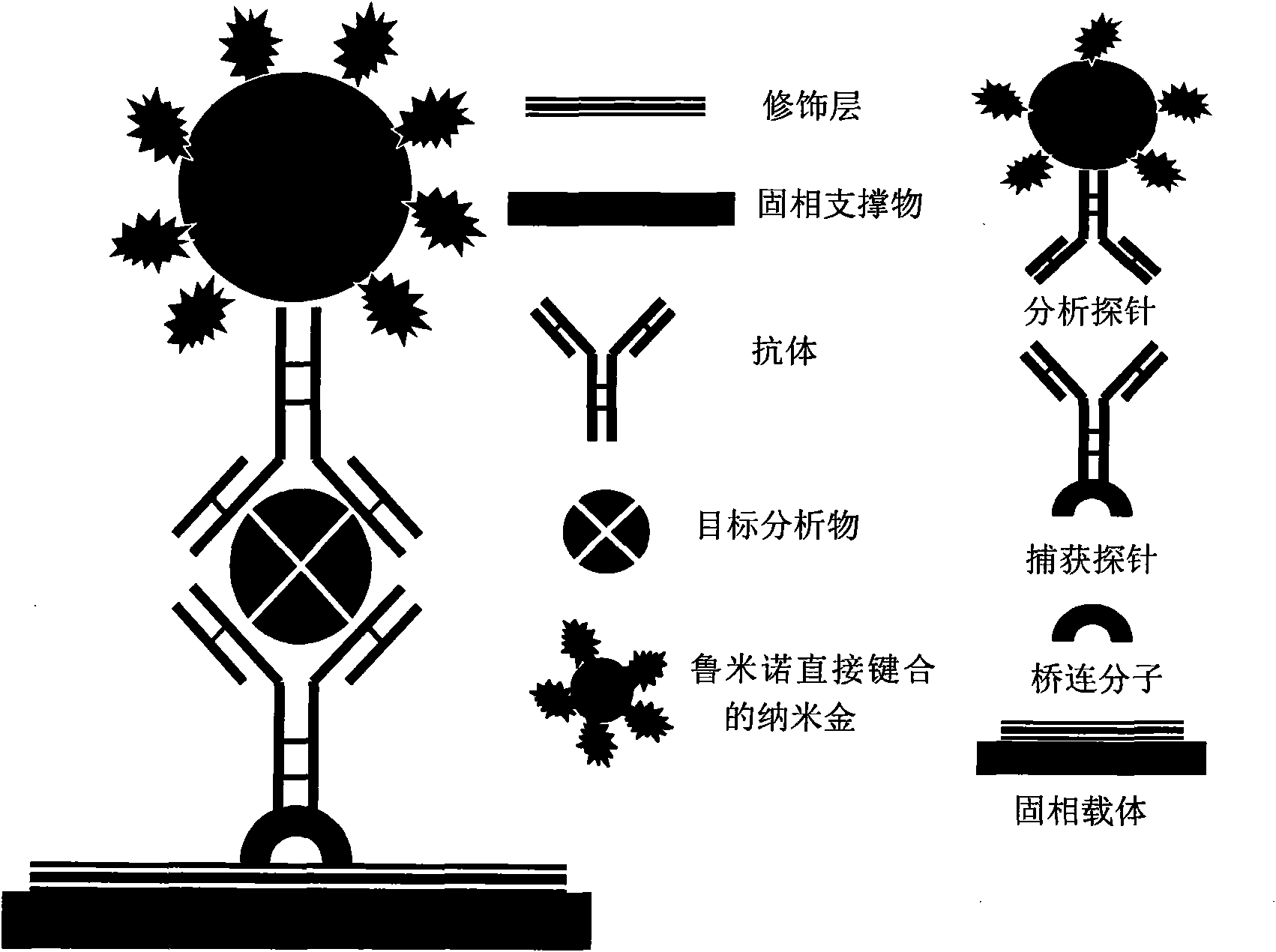
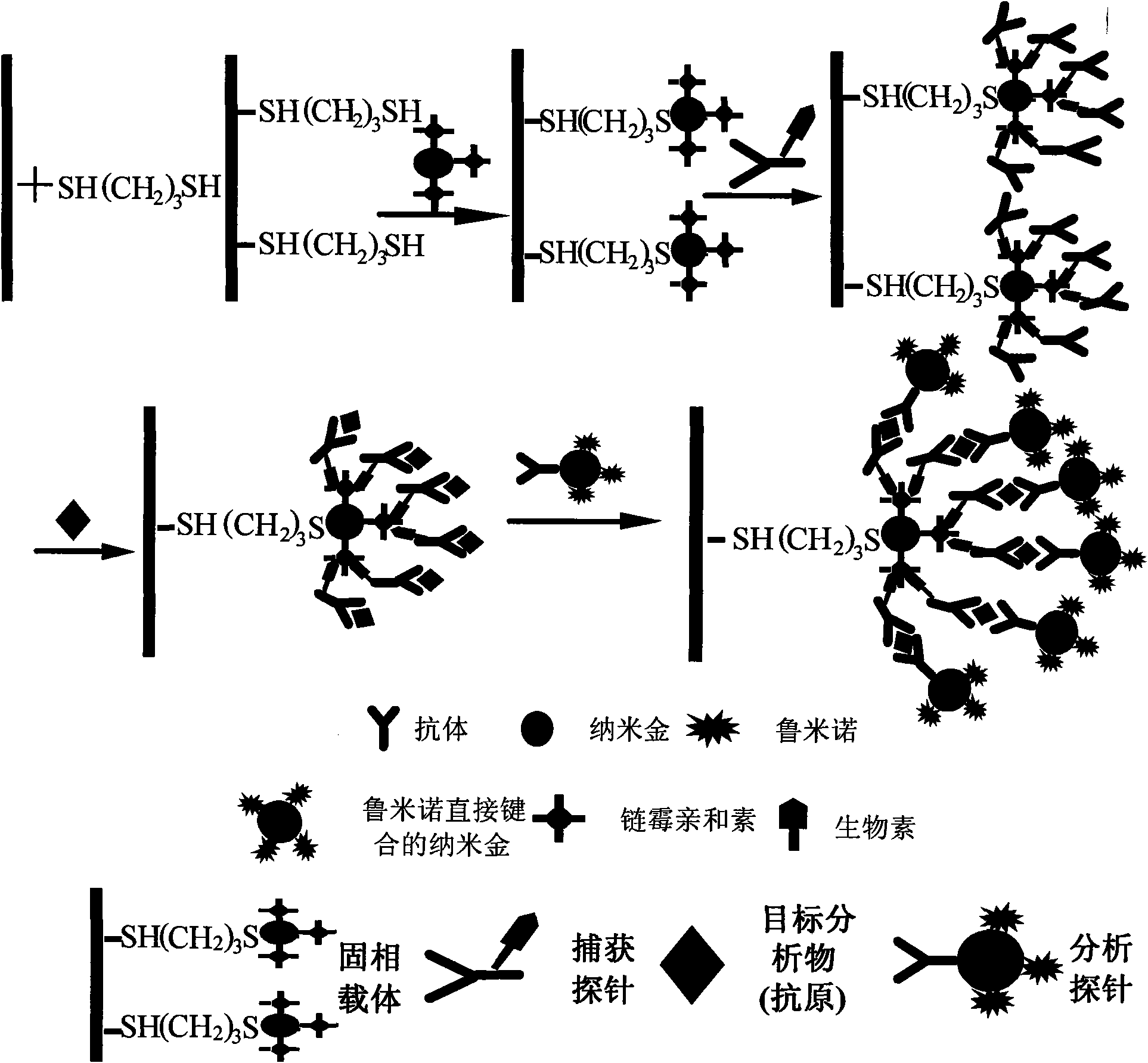
Application of nano-gold directly bonded with luminol in immunoassay
ActiveCN101900723ASimple methodFast wayAnalysis by electrical excitationBiological testingMulti analyteLinearity
The invention discloses an application of nano-gold directly bonded with luminol in an immunoassay. The invention is characterized in that the immunoassay probe of a nano-gold directly bonded with luminol comprises antibodies which are labeled by the nano-golds directly bonded with luminol, wherein the nano-gold directly bonded with luminol are prepared through reducing chloroauric acid by the luminol at a single step; a chemiluminescence immunoassay method is based on the immunoassay probe of the nano-gold directly bonded with luminol; and a kit is used for carrying out the immunoassay method. The chemiluminescence immunoassay method of the invention has the advantages of high sensitivity (for instance, the detection limit can reach 1.0pg / mL for detecting human IgG), wide range of linearity, good repeatability, simple operation, low cost and the like, can be applied to detect multi-analyte in various samples and has key application prospect in fields, such as clinical diagnosis and cure, drug analysis, food security detection, environmental monitoring and the like.
Owner:UNIV OF SCI & TECH OF CHINA
Magnetic granule competing method chemiluminescence immune analysis determination reagent kit for detecting hormone and preparing method thereof
InactiveCN101377491AGuaranteed SensitivityEasy to operateChemiluminescene/bioluminescenceBiological testingAntigenBiotin-streptavidin complex
The invention relates to the immunoassay medical field, particularly provides a magnetic particle competition method chemiluminescence immunoassay assay kit and a preparation method thereof used for detecting hormones. The kit according to the invention comprises: 1) a calibrator; 2) magnetic particles which are coated with streptavidin; 3) hormone antigens of enzyme markers; and 4) a chemiluminescence substrate. Further, the method for preparing the kit according to the invention has the following steps: 1) pure raw materials are used to prepare the calibrator; 2) the antigens are used to coat the magnetic particles; 3) the antigens of the enzyme markers are prepared; 4) the calibrator, the chemiluminescence substrate and the antigens of the enzyme markers are packaged in a separated way; and 5) a finished product is packaged. The kit has the advantages of convenience, rapidness, sensitivity, stability, and the like.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
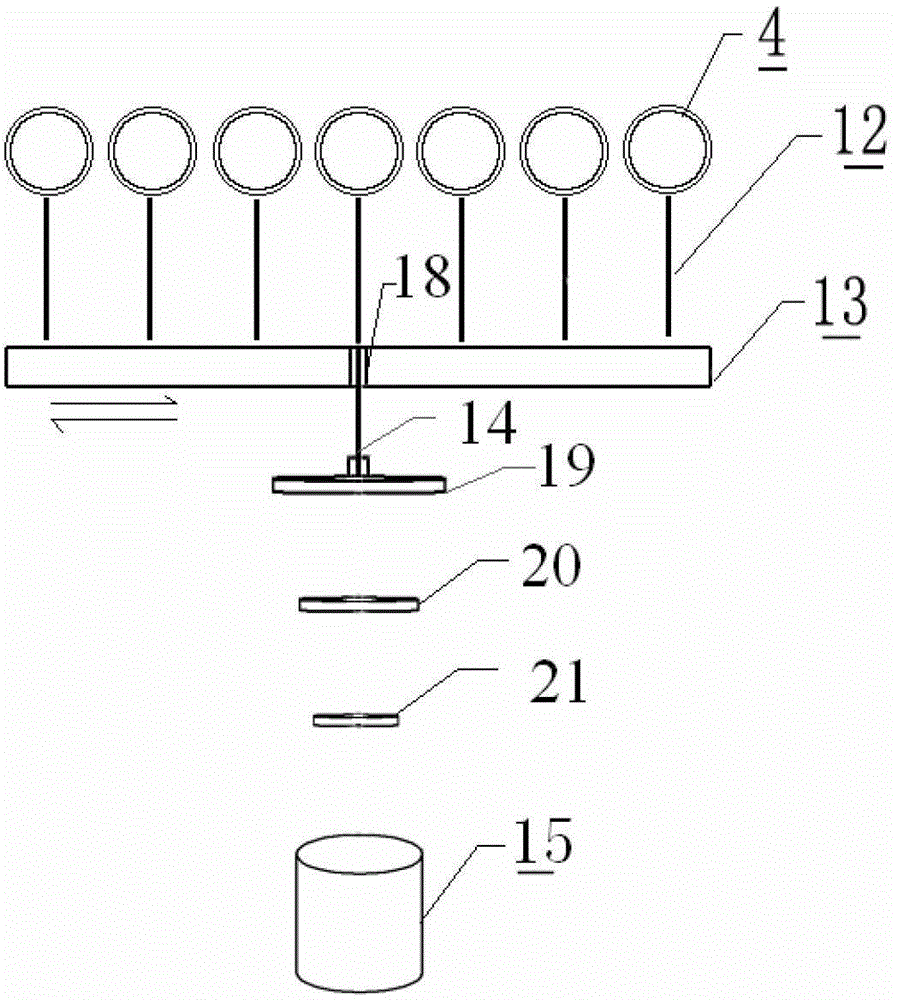
Reagent mixing and conveying device and reagent mixing method
ActiveUS20180252707A1Small sizeSmart structureRotating receptacle mixersTransportation and packagingMechanical engineeringChemiluminescence immunoassay
The present invention provides a reagent mixing device, which comprises a driving device, a transport device and a rotating part, wherein the transport device comprises a conveying mechanism for conveying a reagent kit and a mixing mechanism for mixing a reagent; the conveying mechanism is driven by the driving device to move relative to the mixing mechanism; the rotating part and mixing mechanism are in transmission matching; the conveying mechanism and the mixing mechanism are sleeved with each other to form a bearing structure. The present invention further provides a reagent mixing method. The reagent mixing device is small in size, smart in structure, easy to assemble and low in manufacturing cost. The reagent mixing method provided by the present invention is simple and reliable, high in overall operation reliability, and has very high application values in such analysis and test fields as full-automatic chemiluminescence immunoassay analyzers and biochemical analyzers.
Owner:LEADWAY HK
Testosterone detection reagent based on microparticle chemiluminescence immunoassay technology
ActiveCN103954779AFacilitate dissociationReduce the binding forceChemiluminescene/bioluminescenceBiological testingMicrosphereMicroparticle
The invention discloses a testosterone detection reagent based on a microparticle chemiluminescence immunoassay technology; the testosterone detection reagent comprises the following components: 0.002%-0.01% of paramagnetic microspheres, 0.4 mug / ml-1 mug / ml of acridinium ester labeled testosterone antibodies, a composite testosterone release agent, a luminescence liquid A, a luminescence liquid B, 0ng / dl-1500ng / dl of a testosterone calibrator and a cleaning fluid with a certain concentration; and the testosterone release agent is a mixture of dihydrotestosterone, danazol and heparin sodium. The testosterone detection reagent has the advantages of simple operation, high sensitivity, fast detection efficiency, low cost, easy automation and the like, and by combination of the selected composite release agent and different binding globulin, combined-state testosterone in serum can be fully released, so that the content detection of total testosterone is more accurate.
Owner:DIRUI MEDICAL TECH CO LTD
Kit for detecting estradiol by utilizing magnetic particle chemiluminescence immunoassay
InactiveCN101639478ASimple structureEasy to useChemiluminescene/bioluminescenceSerum igeHorse radish peroxidase
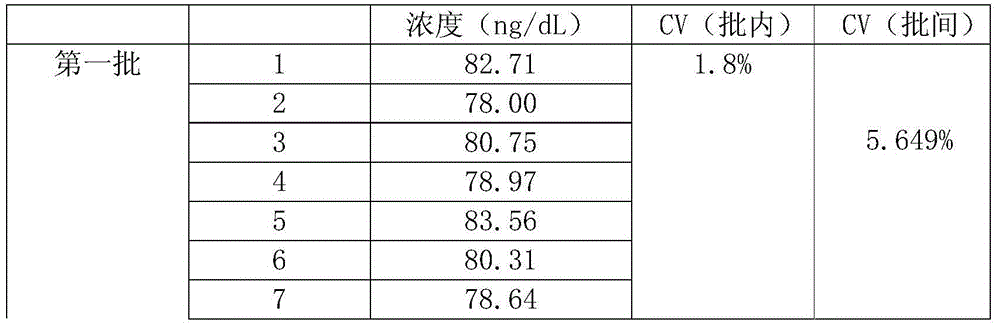
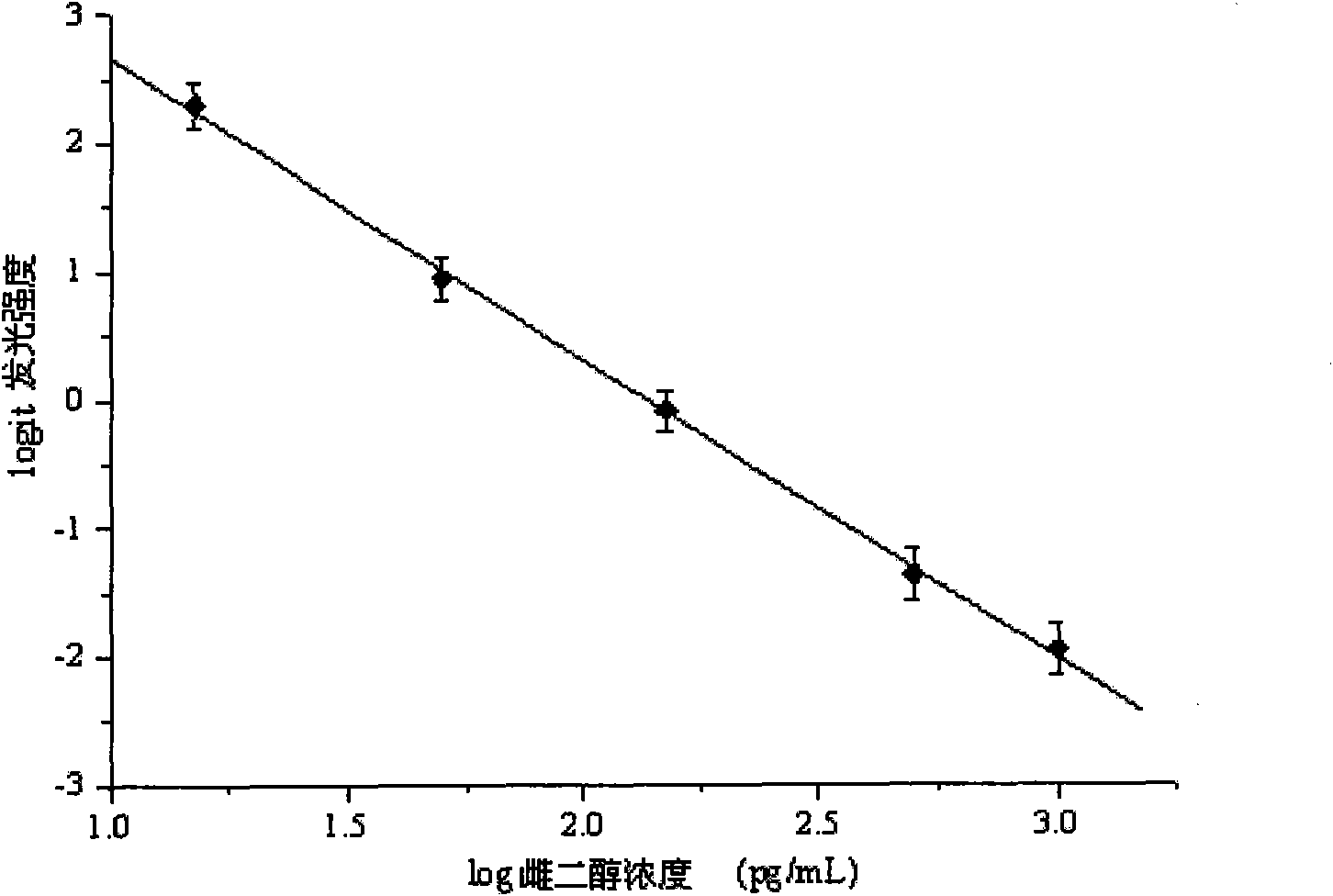
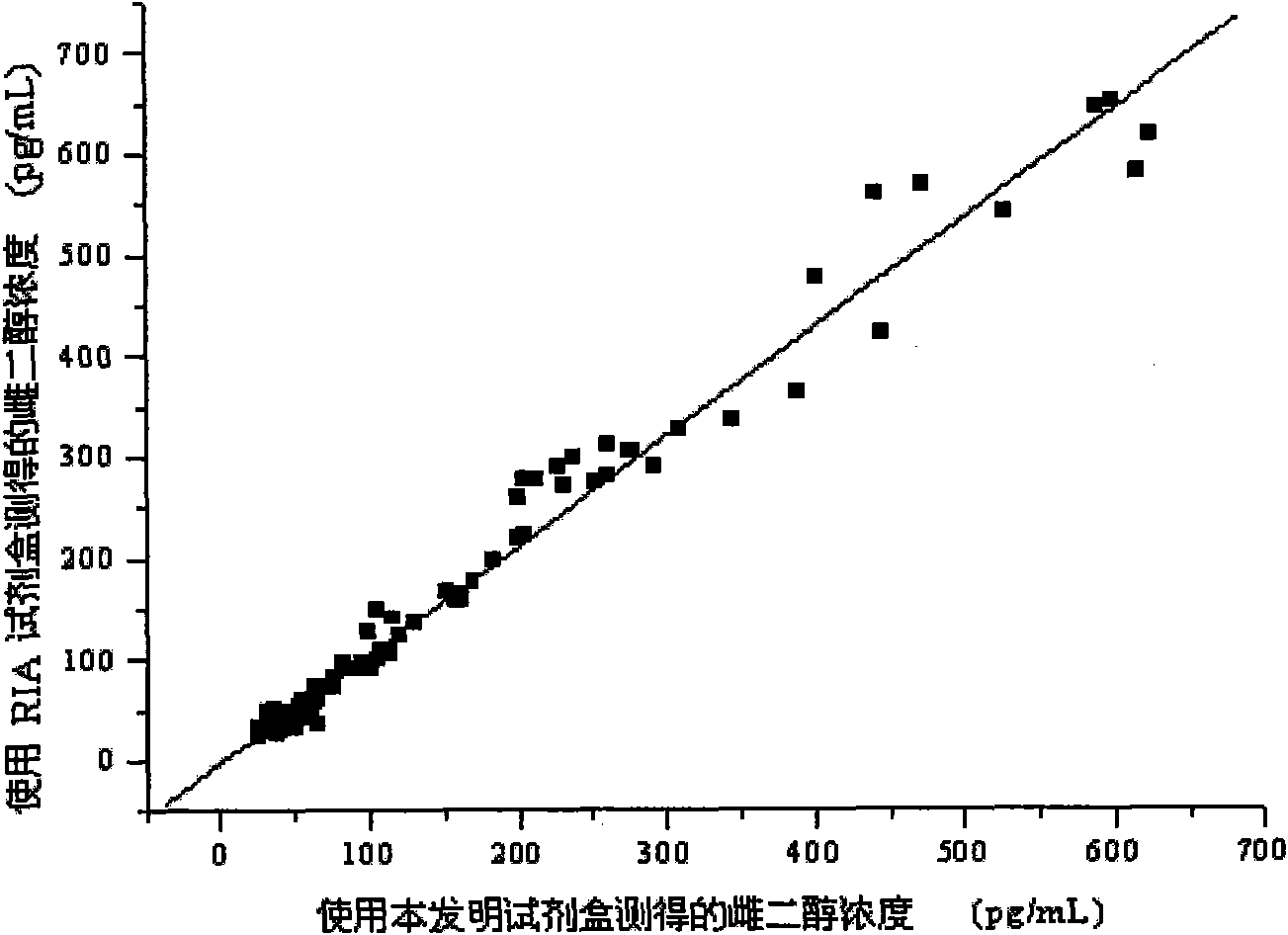
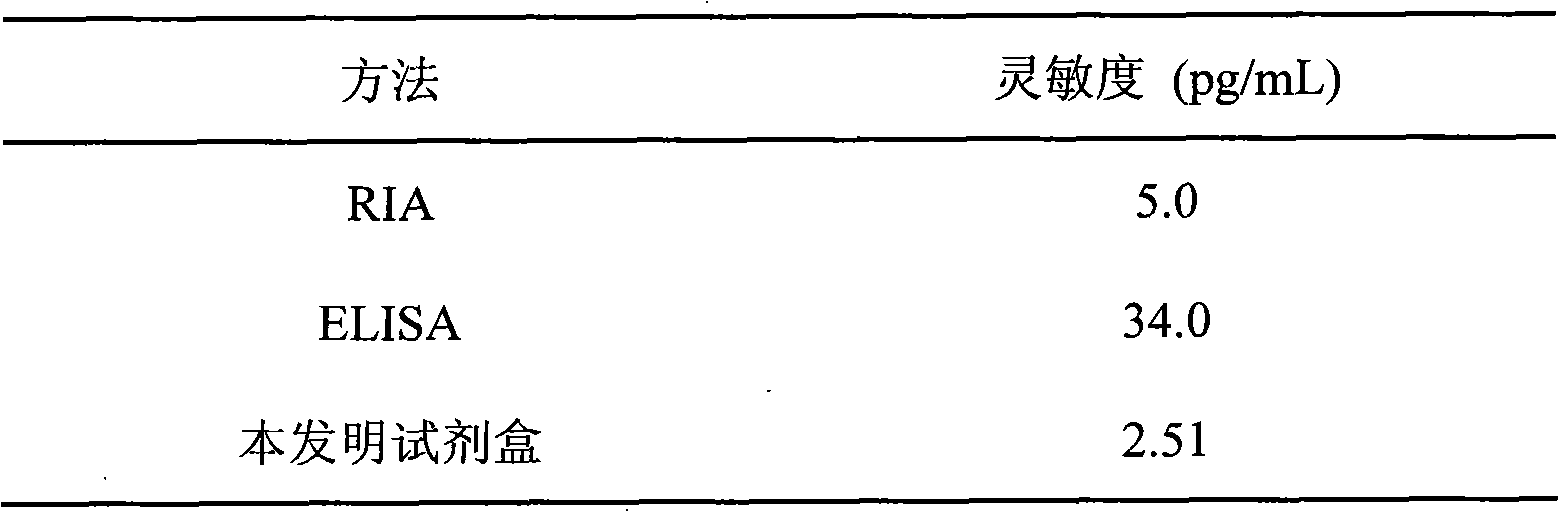
The invention discloses a kit for detecting the estradiol (E2) by utilizing magnetic particle chemiluminescence immunoassay. The kit comprises estradiol series calibrator, magnetic particle solution enveloped by the second antibody, estradiol marked by horse radish peroxidase, estradiol antibody, chemiluminescence substrate liquid and concentrated washing liquid. The invention further discloses apreparation method of the kit. The kit of the invention can quantitively detect the content of estradiol in the serum and blood plasma samples of human body, and can simultaneously detect a large number of samples, thus having advantages of simpleness, convenience, rapidness, stability, high sensitivity and the like, and providing a extremely valuable detection method for clinical diagnosis and scientific research.
Owner:TSINGHUA UNIV
Chemiluminescence immunity analysis detecting myocardium calcium protein T hypersensitization method for acridine ester and alkaline phosphatase
InactiveCN101226200AChemiluminescene/bioluminescenceBiological testingBiotin-streptavidin complexCalcium protein
The invention relates to a chemical illumination immunity analysis method for checking human cTnT, which uses acridiniumester and / or alkaline phosphatase as label. The immunity reaction uses two-site immunoassay and / or competition law. The immunity reaction can use streptavidin-biotin two-site immunoassay to improve sensitivity. The invention uses NaOH and H2O2 as acridiniumester illumination initiating agent, uses 1, 2-dioxo cyclohexane derivative (adamantine derivative) as the illumination substrate of alkaline phosphatase, and uses fluorescein derivative and surface activator as illumination renforcing agent. The solid carrier is orifice plate, macromolecule polymer tube (ball) and ferriferrous oxide magnetic particles or the like.
Owner:天津天美生物技术有限公司
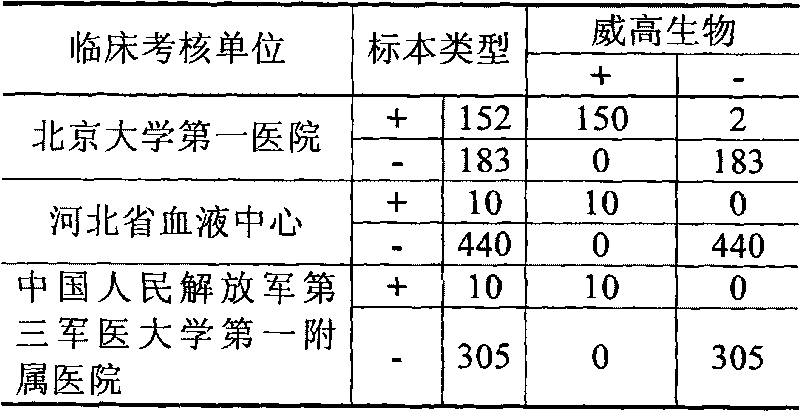
Hepatitis C virus antibody diagnostic kit and preparation method thereof
ActiveCN102072957AHigh sensitivityStrong specificityChemiluminescene/bioluminescenceSorbentQuality control
The invention belongs to the technical field of immunodiagnosis, in particular relates to a hepatitis C virus (HCV) antibody diagnostic kit adopting a micro particle chemiluminescence method, and a preparation method thereof. The kit consists of anti-HCV detecting magnetic micro particles, an anti-HCV detecting tracer conjugate, anti-HCV sample diluted solution and a quality control material. Theinvention also discloses the preparation method of the diagnostic kit, which adopts micro particle chemiluminescence immunoassay technology, has higher sensitivity and specificity than enzyme-linked immuno sorbent assay (ELISA), is suitable for clinical HCV auxiliary diagnosis and blood donor screening, and makes up the blank of the domestic production of the diagnostic kits for detecting the anti-HCV by the micro particle chemiluminescence method.
Owner:威海威高生物科技有限公司
Magnetic particle separation chemiluminescence immunoassay method of human cystatin C
InactiveCN101937000AHigh sensitivityStrong specificityChemiluminescene/bioluminescenceBiological testingFiltrationBlood plasma
The invention provides an in-vitro detection method of human cystatin C. The method adopts a magnetic particle separation chemiluminescence immunoassay technology which is a product integrating an enzyme labeling technology, a magnetic particle separation technology and a chemiluminescence detection technology and has the advantages of high sensitivity, good specificity, good repetitiveness, and the like. The method can be used for measuring the content of cystatin C in the serum, the plasma and the urine of a person, and the indexes are mainly used for clinically evaluating the renal function and mainly used for monitoring the filtration rate of glomerulus, the renal tubular dysfunction and various secondary nephropathies.
Owner:北京倍爱康生物技术有限公司
Time sequence control method and system for chemiluminescence immunoassay device
ActiveCN108226549AAvoid cycle conflictsAvoid time conflictsMaterial analysisControl systemEngineering
The invention discloses time sequence control method and system for a chemiluminescence immunoassay device. The system includes an input pipe module, a detection module, a sample module, a reagent module, a reaction plate module, a transfer pipe module, a cleaning disk module, and a liquid path control module. The control method includes flow line control to the units in the device according to time stamps and timing triggering, wherein a time sequence control part includes several key threads, which are completed in parallel in different zones simultaneously. In the method, actions of the device within 16 sec are defined as a time sequence period T, each 0.5 s defined as a timer t. The method, via reasonable hardware system arrangement in the CLIA device and systemic and complete time sequence control method design on the hardware layer, functional demands such as parallel control, seamless connection and abnormity treatment among different executing mechanisms in a bottom layer are achieved. The method achieves accurate control in a uniform period according to optimum time sequence flow, thus increasing test speed.
Owner:CHONGQING CAPITALBIO NEW VIEW DIAGNOSTIC TECH CO LTD
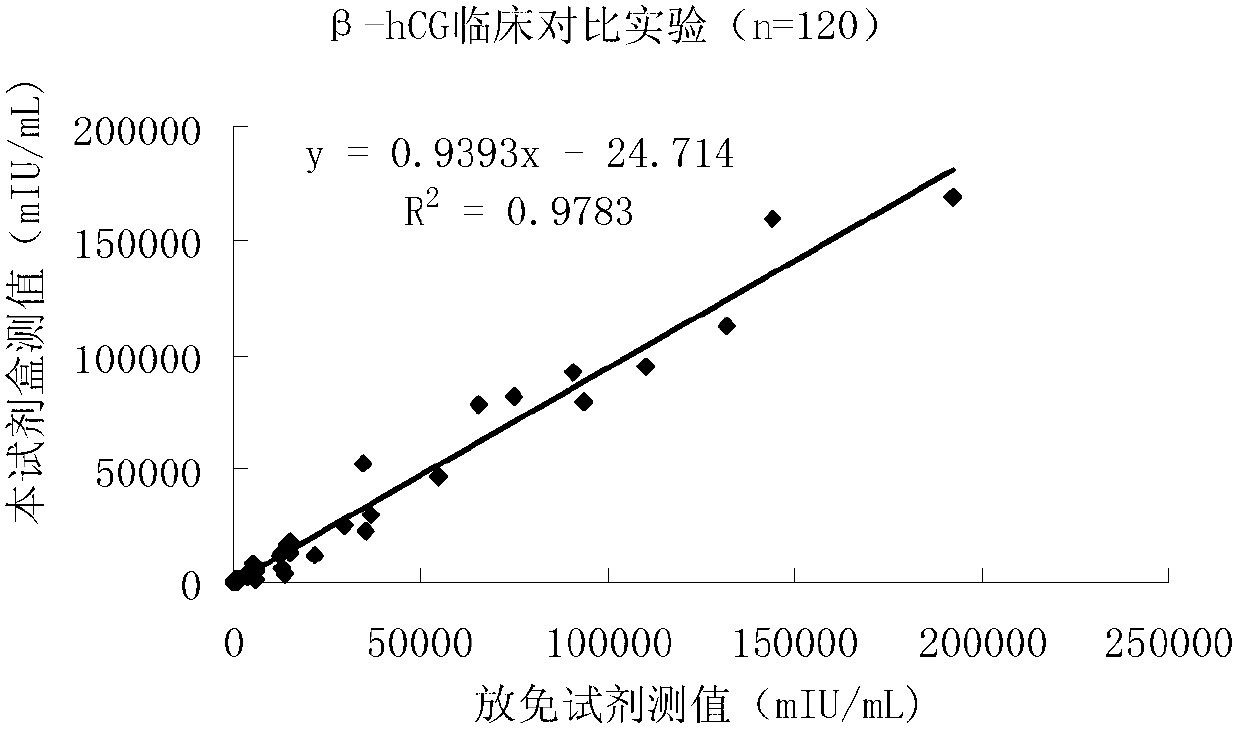
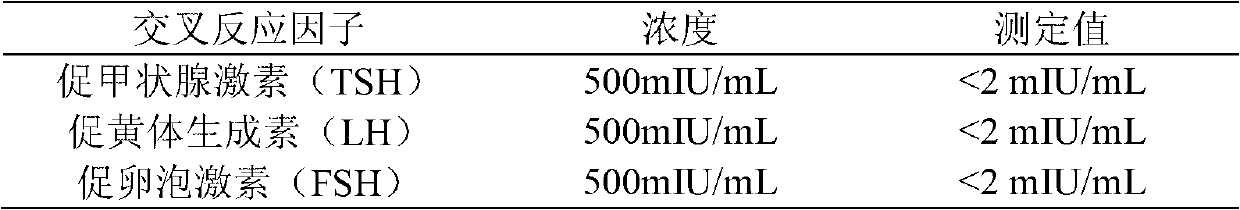
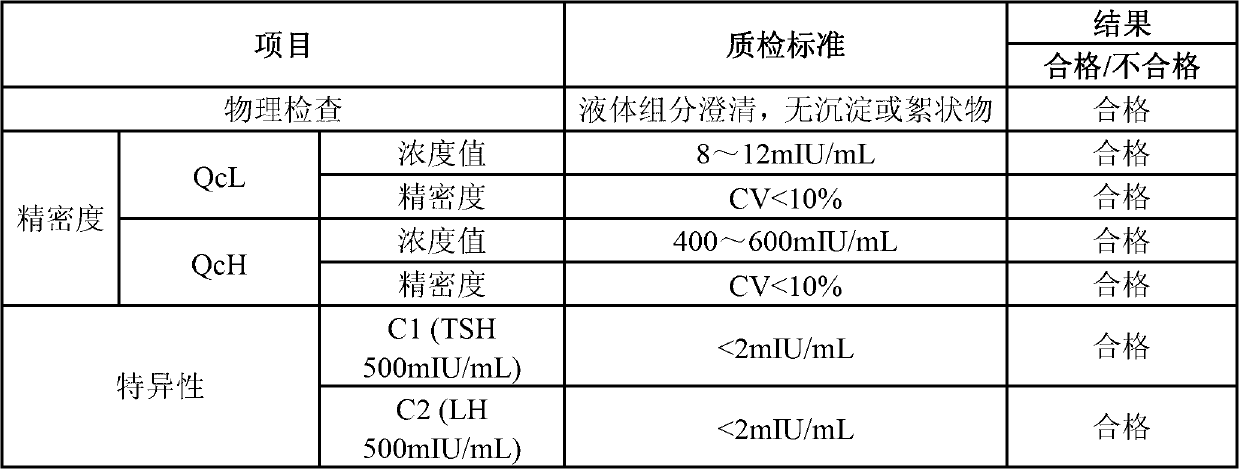
Quantitative detection kit combining magnetic particles with chemiluminescence immunoassay for beta human chorionic gonadotropin (beta-hCG), and preparation method of kit
ActiveCN102998467AImprove performanceLow cross-reaction coefficientBiological testingBiotin-streptavidin complexAbzyme
The invention discloses a quantitative detection kit combining magnetic particles with chemiluminescence immunoassay for beta human chorionic gonadotropin (beta-hCG). The kit comprises beta-hCG calibrators, magnetic particle suspension coupled with streptavidin, a beta-hCG antibody labeled with biotin, a beta-hCG abzyme combination, a beta-hCG quality controller, chemiluminescence liquor A, chemiluminescence liquor B, 20 times concentrated washing liquor, and a reaction tube, wherein enzyme adopted by the beta-hCG abzyme combination is horse radish peroxidase with the purity RZ being more than or equal to 3.0 and the activity being more than or equal to 250U / ml. The invention also discloses a preparation method of the kit. Compared with the conventional kit, the quantitative detection kit is simple and convenient to operate, is safe, does not cause environment pollution, and also has the advantages of wide concentration range, low cost, good stability and the like of detection samples.
Owner:BIOSCIENCE (TIANJIN) DIAGNOSTIC TECH CO LTD
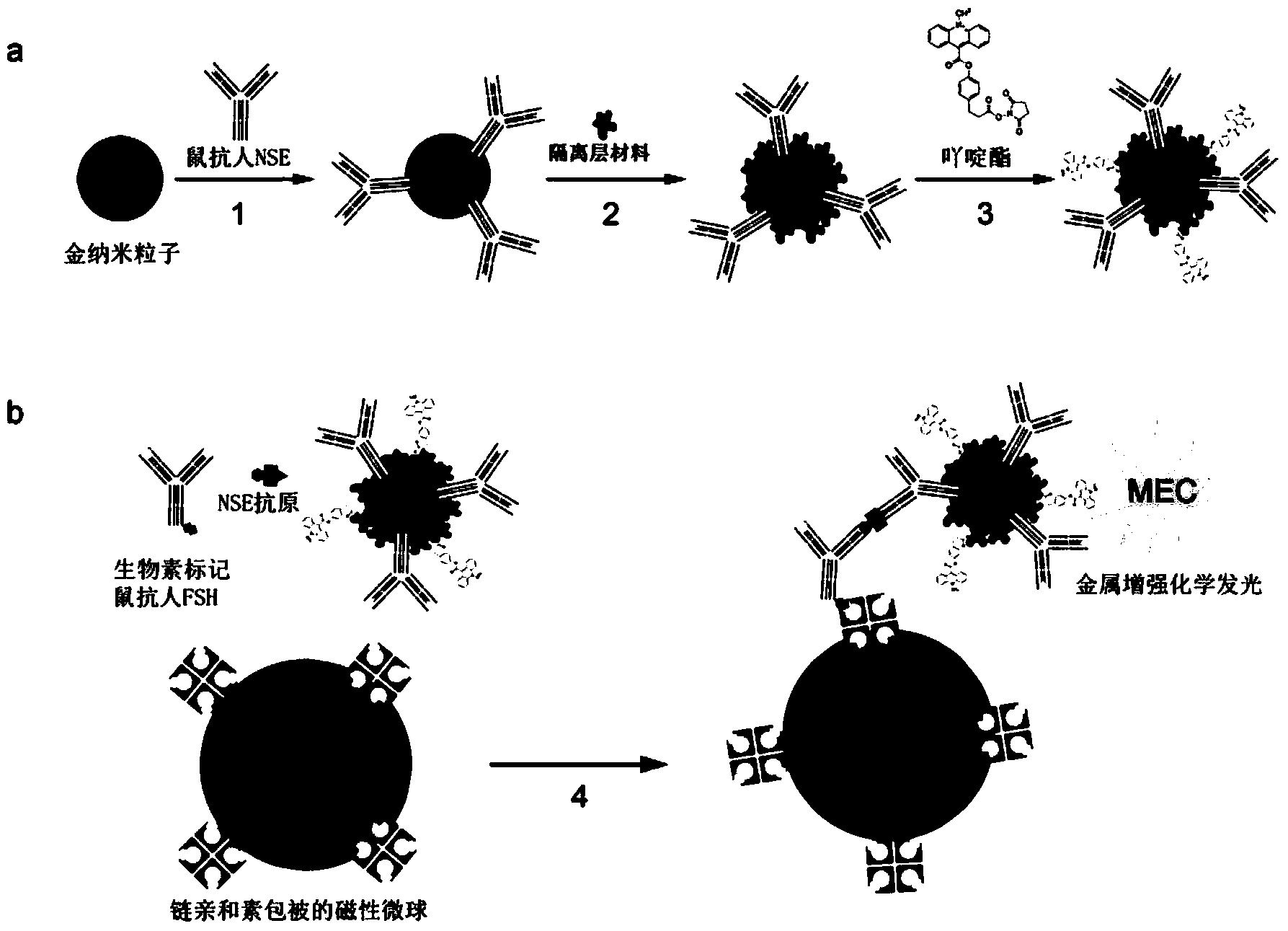
Dual-enhanced chemiluminescent immunoassay method based on metal enhanced luminescence and nano particle labelled amplification
ActiveCN104280542AHigh sensitivityThe detection process is fastChemiluminescene/bioluminescenceMicrosphereChemiluminescent immunoassay
The invention discloses a dual-enhanced chemiluminescent immunoassay method based on metal enhanced luminescence and nano particle labelled amplification. According to the dual-enhanced chemiluminescent immunoassay method based on metal enhanced luminescence and nano particle labelled amplification, metal nano particles replace conventional polystyrene nano microspheres, and luminescence substances are lebelled on the surface of an isolation layer spaced from the surfaces of the metal nano particles by 5-20nm; chemiluminescence of luminescence substances on sensitized metal nano particles is coupled to plasma waves on the surfaces of the metal nano particles; after resonance is generated, the chemiluminescence is emitted in the form of relatively high light intensity and relatively high attenuation speed. On the basis of a conventional chemiluminescence immunoassay technology, a metal enhanced luminescence technology and a nano particle labelled amplification technology are organically integrated; therefore, the dual-enhanced chemiluminescent immunoassay method has the advantages of high sensitivity, high detection speed and the like.
Owner:GETEIN BIOTECH
Gastrin-17 enzymatic chemiluminescence immunoassay kit
InactiveCN104914251ALittle variance between production batchesHigh affinityDisease diagnosisBiological testingMicrosphereImmune complex deposition
The invention discloses a gastrin-17 enzymatic chemiluminescence immunoassay kit and belongs to the technical field of chemiluminescence immunoassay analysis. The kit comprises an enzyme label liquid, a gastrin-17 standard, gastrin-17 monoclonal antibody-coated immunomagnetic beads, a sample diluent, a chemiluminescent substrate liquid and a washing liquid. The principle of the gastrin-17 enzymatic chemiluminescence immunoassay kit comprises that a gastrin-17 monoclonal antibody is connected to the surface of a magnetic bead so that a solid phase agent is obtained, and through capture of gastrin-17 in a sample and use of an enzyme-labeled anti-gastrin-17 monoclonal antibody, a solid phase-antibody-antigen-enzyme-labeled antibody sandwiched immune complex is formed. Through combination of a chemiluminescence technology and an immunomagnetic bead technology, the prepared kit has the advantages of high sensitivity, good specificity, wide linearity range and good stability and can satisfy clinical requirements on stomach function detection.
Owner:BIOHIT BIOTECH HEFEI +1
Kit for detecting hepatitis c virus antibody as well as detection method and application thereof
ActiveCN104697988AHigh sensitivityStrong specificityChemiluminescene/bioluminescenceAntigenMicrosphere
The invention discloses a kit for detecting a hepatitis c virus antibody as well as a detection method and an application thereof, and belongs to the technical field of in vitro diagnosis and detection. The kit consists of the following components: (1) a magnetic microsphere system: including a magnetic microsphere and an HCV antigen in indirect connection by virtue of a first bridged compound; and (2) a marker system: including an HCV fusion antigen and a marking tracer which are in indirect connection by virtue of a second bridged compound, wherein the HCV antigen and the HCV fusion antigen are combined with an HCV antibody on different sites. The kit and the detection method, by detecting the hepatitis c virus antibody through chemiluminescence immunoassay, have the advantages of high sensitivity, good specificity and broad detection scope.
Owner:SHENZHEN NEW INDS BIOMEDICAL ENG
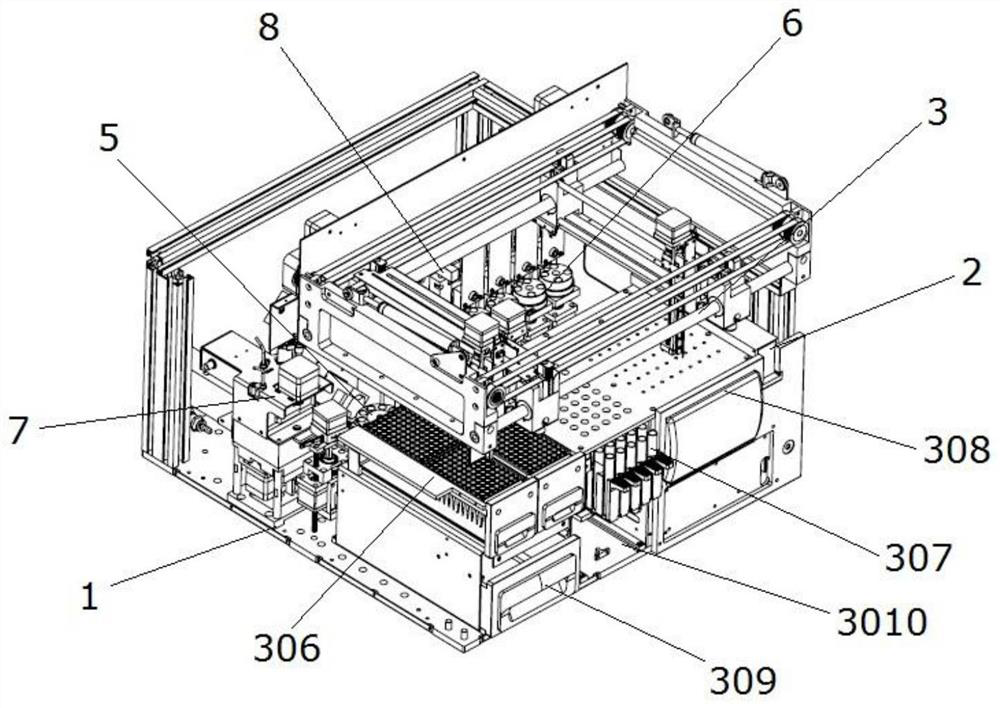
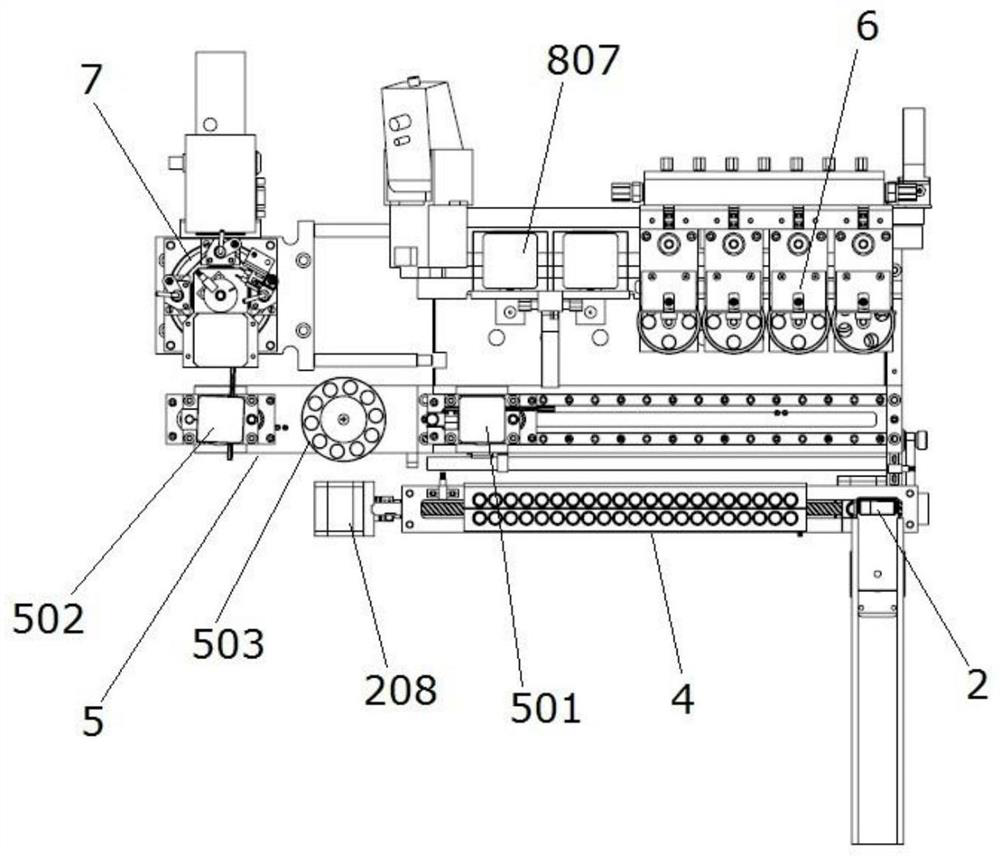
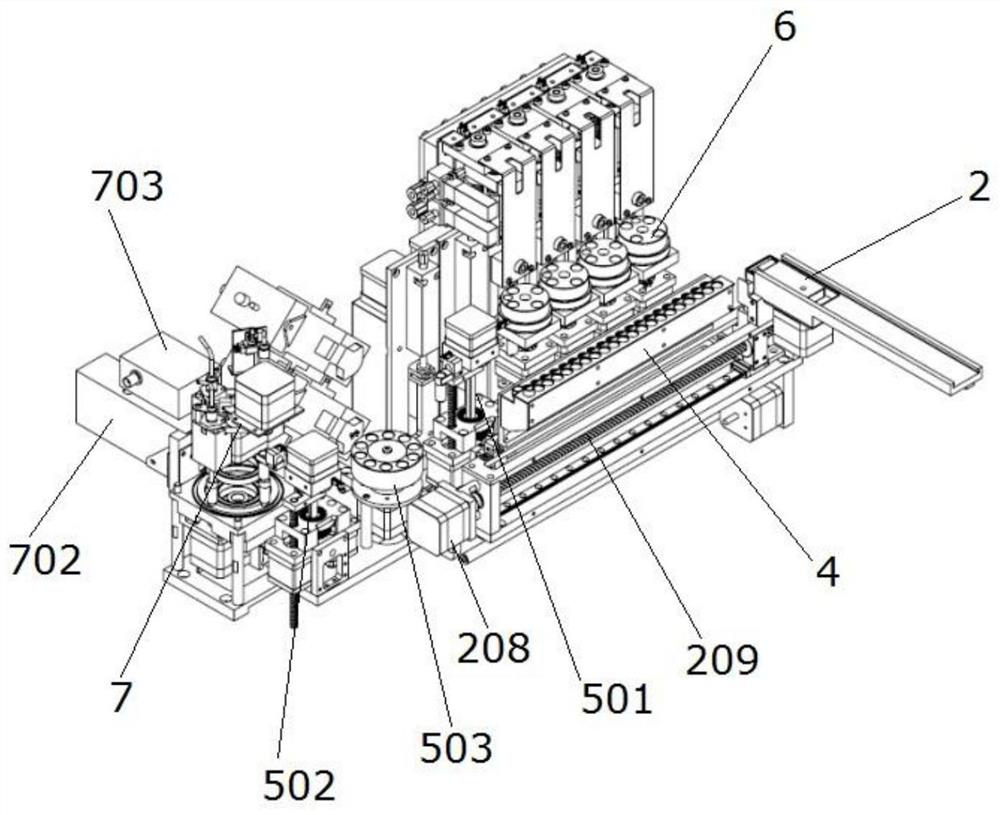
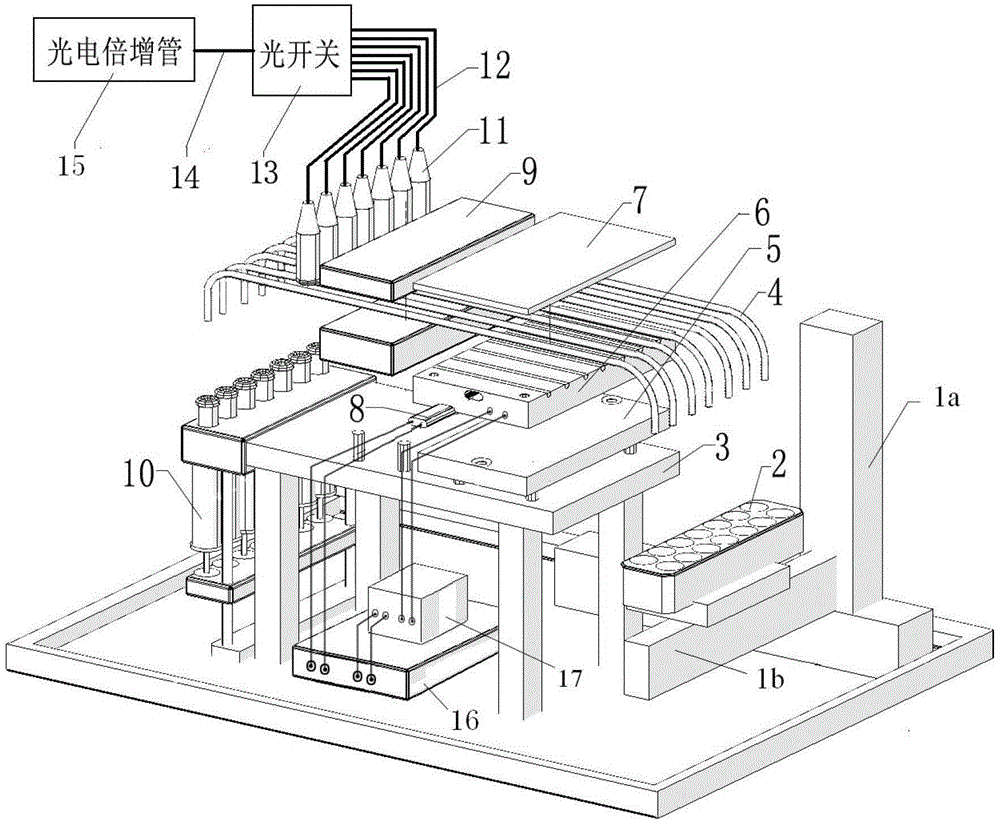
Full-automatic chemiluminescence immunoassay analyzer
PendingCN111735978AStructural integrationShort test timeChemiluminescene/bioluminescenceBiological testingRobot handEngineering
The invention discloses a full-automatic chemiluminescence immunoassay analyzer. The device comprises a base, and a reaction cup loading module, a mechanical arm sample injection module, an incubationmodule, a manipulator and transfer module, a magnetic separation cleaning module and a detection module which are independent of one another are integrally arranged on the base, wherein the reactioncup loading module is used for carrying reaction cups to preset positions; the manipulator and the transfer module are used for clamping the reaction cup into the incubation module, incubating the reaction cup and carrying out constant-temperature reaction; the reaction cup located in the incubation module is clamped to the magnetic separation cleaning module to be subjected to magnetic adsorptioncleaning, and the cleaned reaction cup is clamped to the detection module by the mechanical arm and the transfer module to be detected. A plurality of manipulators and a plurality of transfer devicescan be configured as required to connect all mutually independent module functional parts, and meanwhile, the instrument can also be configured with detection modules and process modules of various detection platforms to be matched with the manipulators and the transfer devices to realize desktop assembly line type diversified detection.
Owner:NANJING LANSION BIOTECH CO LTD
Treponema pallidum antibody diagnostic kit and preparation method thereof
The invention belongs to the technical field of immunologic diagnosis, in particular to a treponema pallidum antibody diagnostic kit by a chemiluminescence method and a preparation method thereof. The kit comprises an anti-TP test reaction plate, an anti-TP test enzyme complex, chemiluminescence substrate liquid, concentrated washing liquor, a negative contrast and a positive contrast. The invention also discloses a preparation method of the diagnostic kit, which adopts a chemiluminescence immunoassay technology; compared with ELISA (enzyme-linked immuno sorbent assay), the method has higher sensitivity and specificity, is suitable for the auxiliary diagnosis of clinical syphilis and screening of blood donors and fills a blank of the production of a treponema pallidum antibody diagnostic reagent detected by the domestic chemiluminescence method.
Owner:威海威高生物科技有限公司
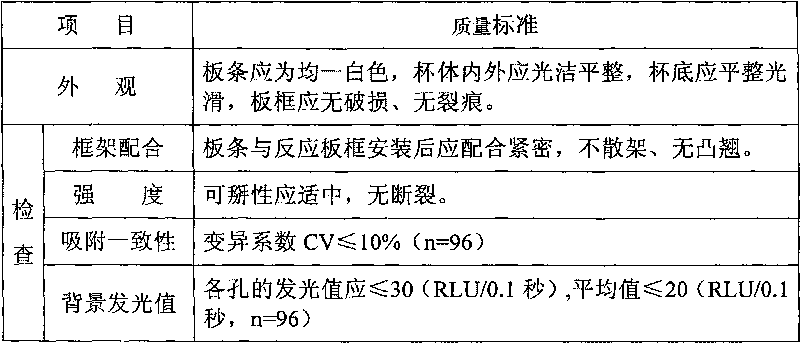
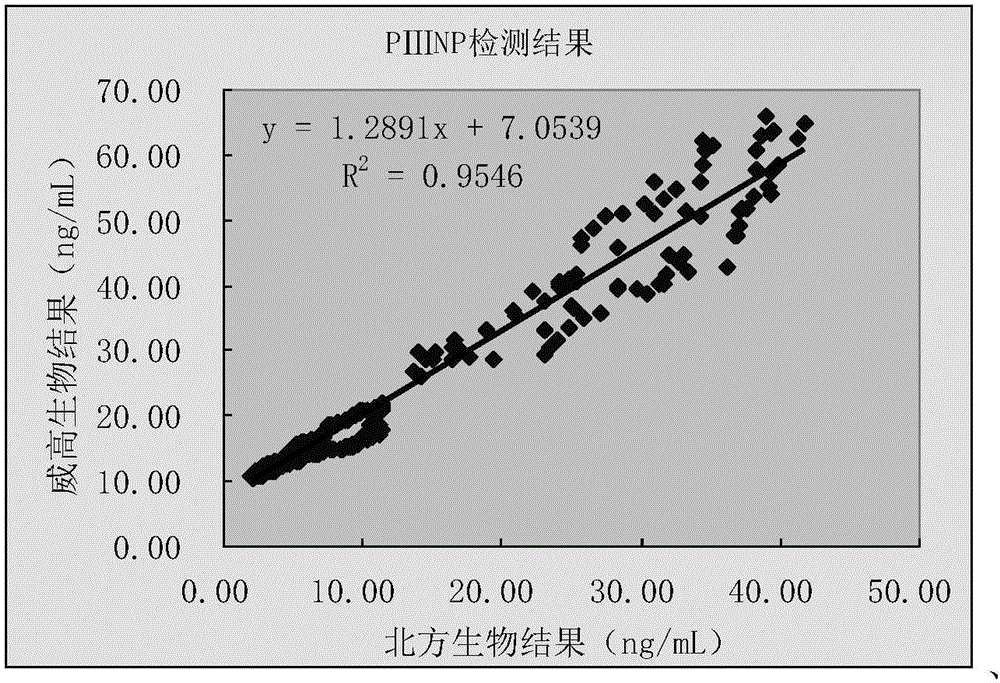
III type procollagen N-terminal peptide quantitative measurement kit and preparation method thereof
InactiveCN106053826AHigh precisionEasy to detectChemiluminescene/bioluminescenceBiological testingQuality controlHepatic fibrosis
The invention provides a magnetic particle chemiluminescence method III type procollagen N-terminal peptide quantitative measurement kit and a preparation method thereof. The kit is composed of PIIINP magnetic particles, tracing conjugate for measuring PIIINP, and a quality control substance. The invention also provides a preparation method of the quantitative measurement kit. The preparation method adopts a micro particle chemiluminescence immunoassay technology, an automatic chemiluminescence method is used to carry out detection, the operation time is reduced, the artificial operation error is reduced, the detection precision and accuracy are improved, and the kit is suitable for assisted diagnosis of early stage hepatic fibrosis in clinic.
Owner:威海威高生物科技有限公司
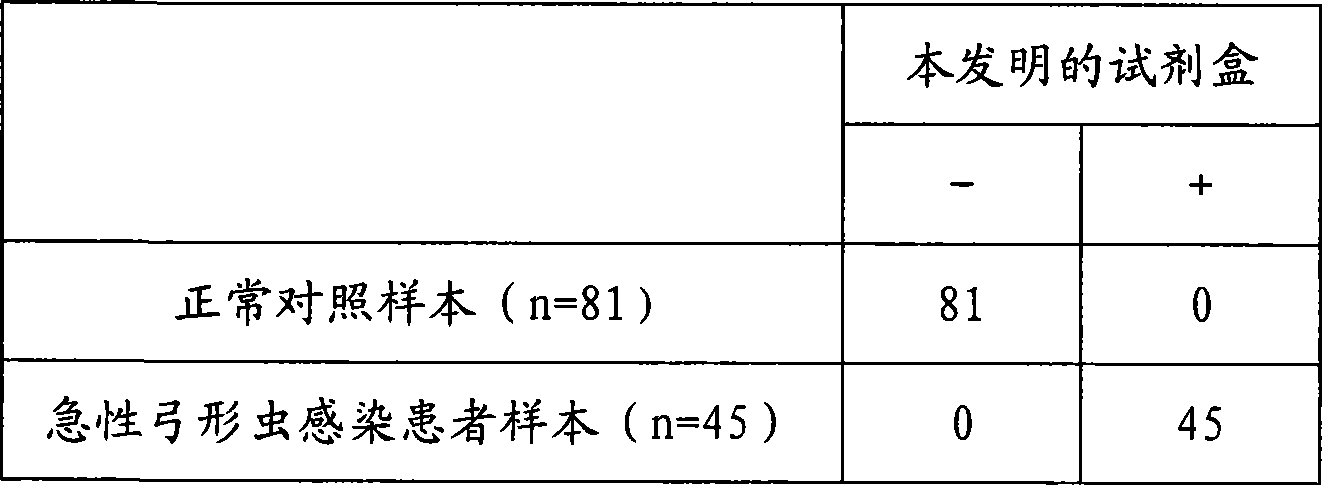
Chemiluminescence immune analysis determination reagent kit for detecting Toxoplasma Gondi IgM antibody
The invention discloses a toxoplasma gondii IgM antibody detection kit combined with the FITC-anti-FITC indirect coating technology and the chemiluminescent immunoassay technology, and a preparation method thereof. The kit of the invention is composed of a negative control, a positive control, solid-phase vectors for anti-FITC antibodies, anti-human Mu-chain monoclonal antibodies of FITC markers, toxoplasma gondii antigens which are marked by horse radish peroxidase, chemiluminescent substrates and concentrated washing solutions. The kit of the invention can be used as the aided detection index for prenatal prepotency diagnosis, and has vital significances for improving the birth population quality and doing the family planning and the prepotency well.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Chemiluminescence immunoassay system, as well as method and application thereof
ActiveCN102980996AReduce volumeFast analysisChemiluminescene/bioluminescenceFluid controlEngineering
The invention discloses a chemiluminescence immunoassay system, as well as a method and an application thereof. The chemiluminescence immunoassay system comprises a fluid control unit as well as a three-dimensional motion sample feeding platform, a temperature control unit, a magnetic field control module and an optical detecting module, wherein the three-dimensional motion sample feeding platform, the temperature control unit, the magnetic field control module and the optical detecting module are connected with each other in sequence; and the fluid control unit is connected with the temperature control unit. The chemiluminescence immunoassay system disclosed by the invention has the advantages as follows: with a capillary tube as a reactor, reagent transportation and reaction are both carried out in a water-in-oil type liquid drop by using a capillary tube liquid drop technology, so that a stable reaction condition is kept, and crossed pollution between samples and reaction suppression caused by surface adsorption are effectively avoided. The chemiluminescence immunoassay system disclosed by the invention has the advantages of small size, high assay speed, high testing flux, automatic operation and flexibility in application and is suitable for analyzing single sample, batch samples and field rapid assay; medical demands of the masses can be preferably satisfied; meanwhile, the purchasing and operation cost of equipment is obviously reduced, and excellent social and economic benefits are obtained.
Owner:GUANGDONG HEXIN BIOTECH
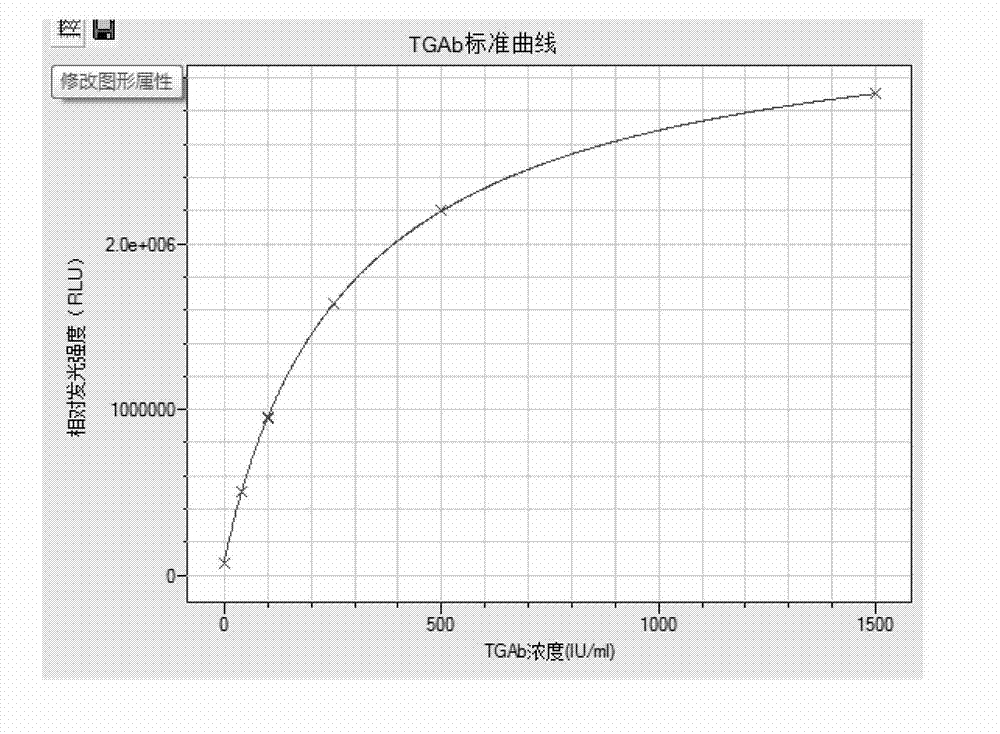
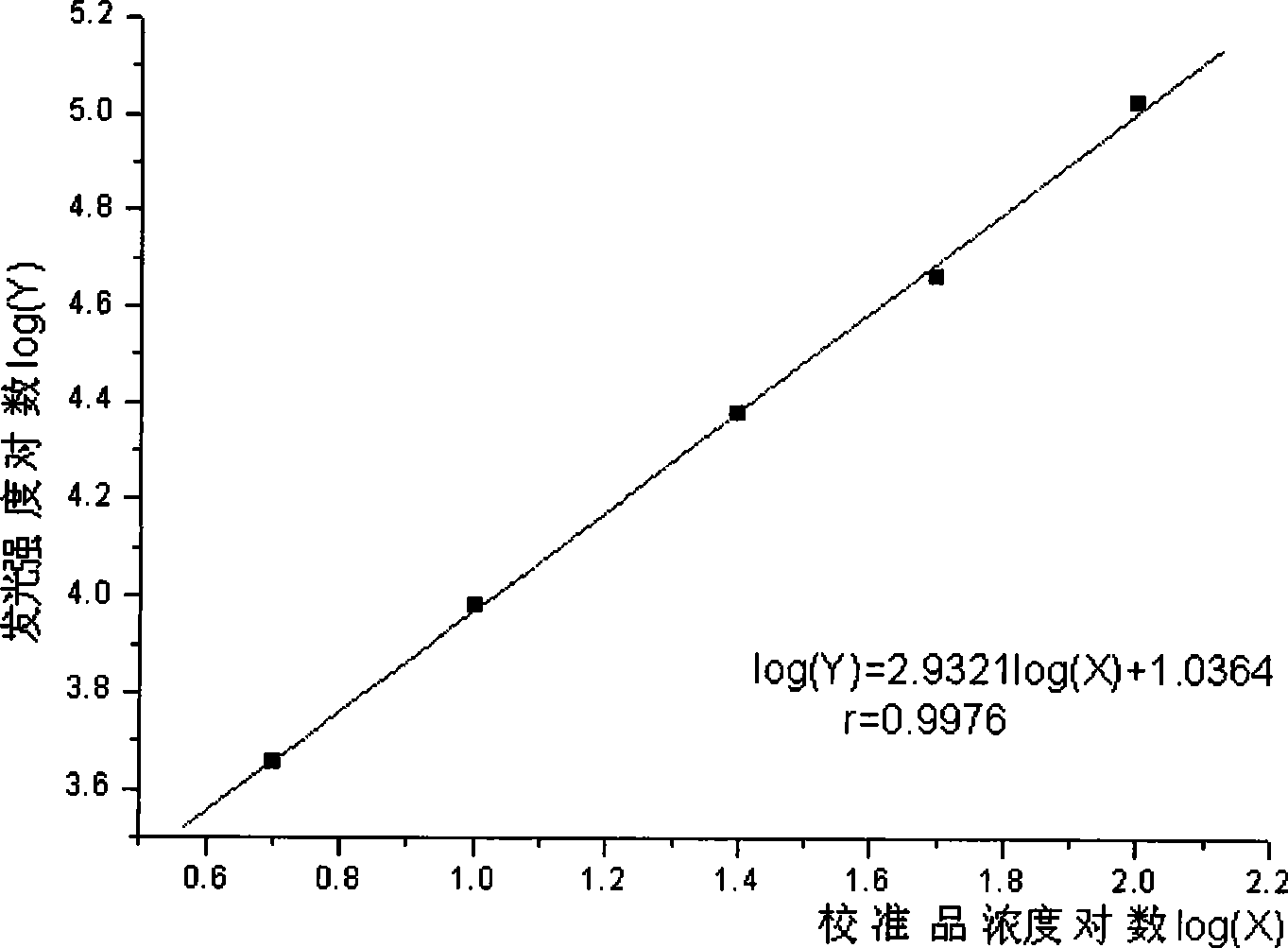
Magnetic particle chemiluminescence immunoassay kit and assay method for human thyroglobulin antibodies (TGAb)
InactiveCN102901812AEasy to separateAdequate and prompt responseChemiluminescene/bioluminescenceAbzymeImmune complex deposition
The invention relates to a magnetic particle chemiluminescence immunoassay method for human thyroglobulin antibodies (TGAb) and belongs to the technical field of immunoassay. TG antigens marked by fluorescein isothiocyanate (FITC) and human IgG antibodies marked by alkaline phosphatase are combined to form an antigen-antibody-second enzyme-labeled antibody sandwich immune complex with a sandwich-like structure. Then, magnetic particles connected with FITC antibodies are added, the antigen-antibody complex is connected to the magnetic particles through specific combination of the FITC antibodies and the FITC and directly deposited in an external magnetic field, and the complex formed by immune reaction can be separated from the other non-combined substances without centrifuging. A kit combines chemiluminescence with the magnetic particles to provide a reaction system close to a homogeneous phase. Compared with the prior art, the kit has the advantages of higher sensitivity, wide linear range, quickness and the like; the cost of a product is greatly reduced; and the kit has a broad application prospect on the aspects of clinical examination and the like.
Owner:JIANGSU ZECEN BIOTECH CO LTD
Chemical luminescence immune assay determination reagent kit for detecting human growth hormone
InactiveCN101368973AImprove performanceEasy to operateChemiluminescene/bioluminescenceBiological testingLuminescencePollution
The invention relates to the immunoassay field, in particular discloses a chemiluminescence immunoassay test kit and a preparing method thereof for detecting human growth hormone. The test kit uses principle of enzyme-catalyzed chemiluminescence, and adopts the micro hole plate as the solid-phase carrier so as to achieve batch detection. The monoclonal antibody can be labeled by alkaline phosphatase and horseradish peroxidase. Without radioactive pollution, the test kit has stable performance, simple operation, accurate and sensitive result and swift reaction speed.
Owner:北京科美东雅生物技术有限公司
Magnetic particle-based quantitative chemiluminescent assay kit for anti-ribosome P protein antibody IgG, and preparation and detection methods thereof
InactiveCN105954266AEasy to useGuaranteed detection effectChemiluminescene/bioluminescencePolyclonal antibodiesBovine serum albumin
The invention discloses a magnetic particle chemiluminescence quantitative assay kit for anti-ribosomal P protein antibody IgG. The kit includes: anti-ribosomal P protein antibody IgG calibrator; Ribosomal P protein antigen and bovine serum albumin-labeled Tris buffer; alkaline phosphatase-labeled goat anti-human polyclonal antibody and bovine serum albumin in Tris buffer; streptavidin-labeled magnetic particles and bovine serum albumin Serum albumin in Tris buffer; wash solution. Based on the traditional membrane strip immunoassay and enzyme-linked immunosorbent assay, the detection method of the kit increases the sensitivity and linear range by 3-5 orders of magnitude, and realizes quantitative detection in a real sense, with rapid response, reliable results, and It can be used in conjunction with a fully automatic chemiluminescence immunoassay analyzer to realize fully automatic use, and has irreplaceable important value for clinical diagnosis.
Owner:北京贝尔医疗设备有限公司
Chemical luminescence immune assay determination reagent kit for gastrin releasing peptide precursor
InactiveCN101368966AImprove linear rangeHas a set of practicabilityChemiluminescene/bioluminescenceGastrin-releasing peptideBiotin
The invention relates to the immunoassay medical field, in particularly provides a chemiluminescence immunoassay test kit and a preparing method thereof for pro gastrin releasing peptide (31-98). Through adopting Biotin-Streptoavidin system coated antibody, the invention can improve antibody coating efficiency, and can improve the sensitivity at the same time. The test kit provides diagnosis of small-cell lung cancer with accurate chemiluminescence test kit which has simple operation and sensitive result detecting, so as to meet the requirements in clinical diagnosis.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Magnetic particle chemiluminescence immunoassay kit of free thyroxine
InactiveCN101639481AGuaranteed SensitivitySuperparamagneticChemiluminescene/bioluminescenceMonoclonal antibodyHorseradish peroxidase
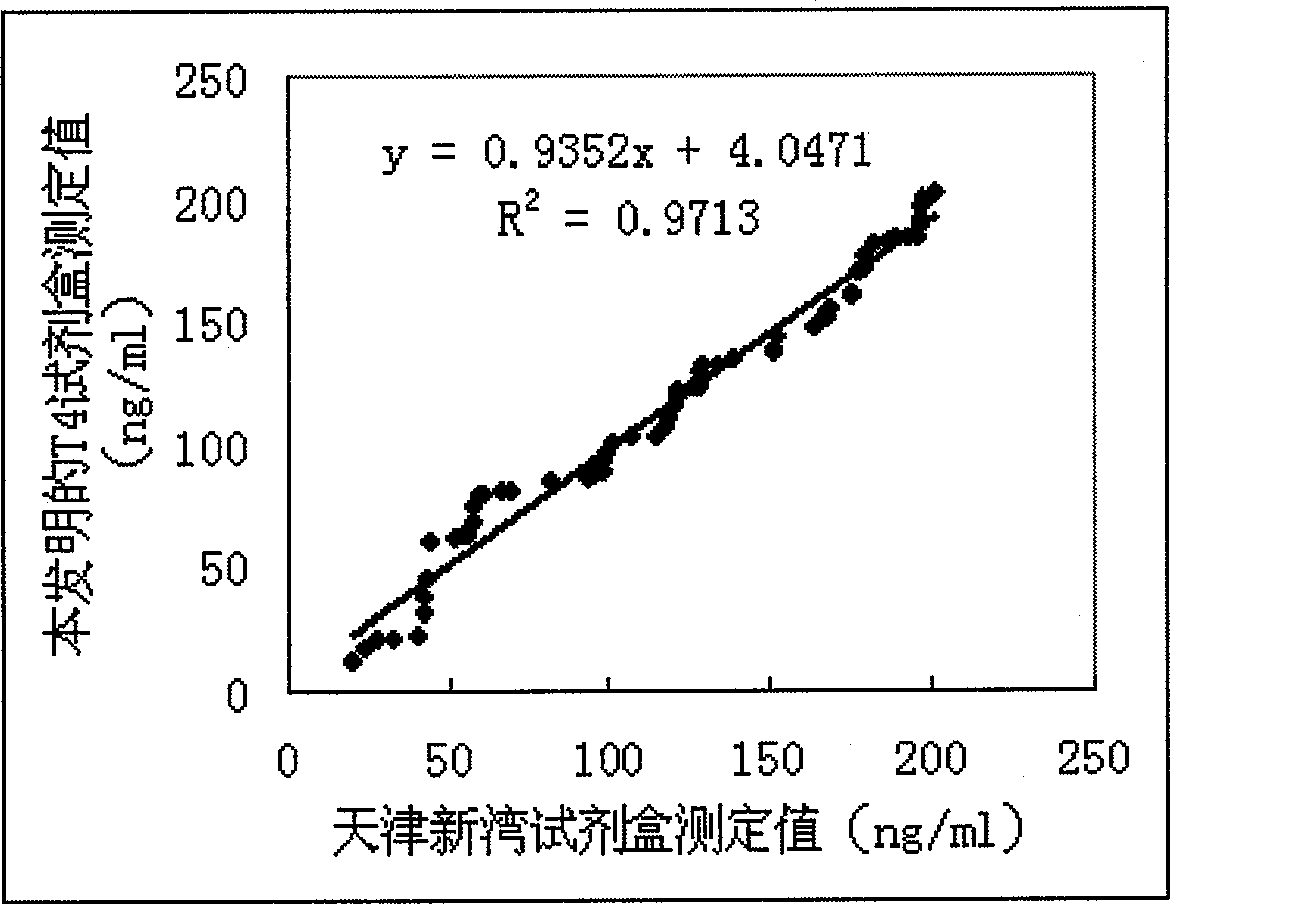
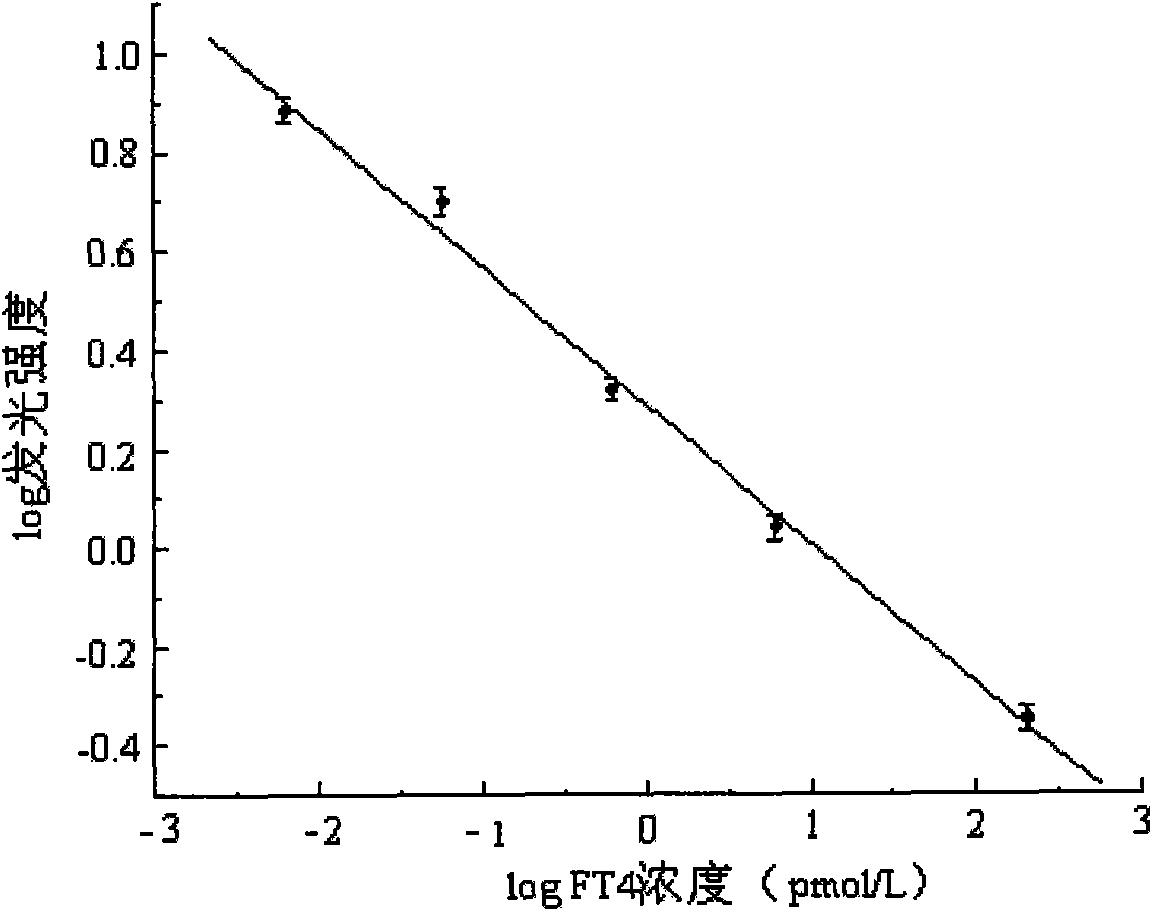
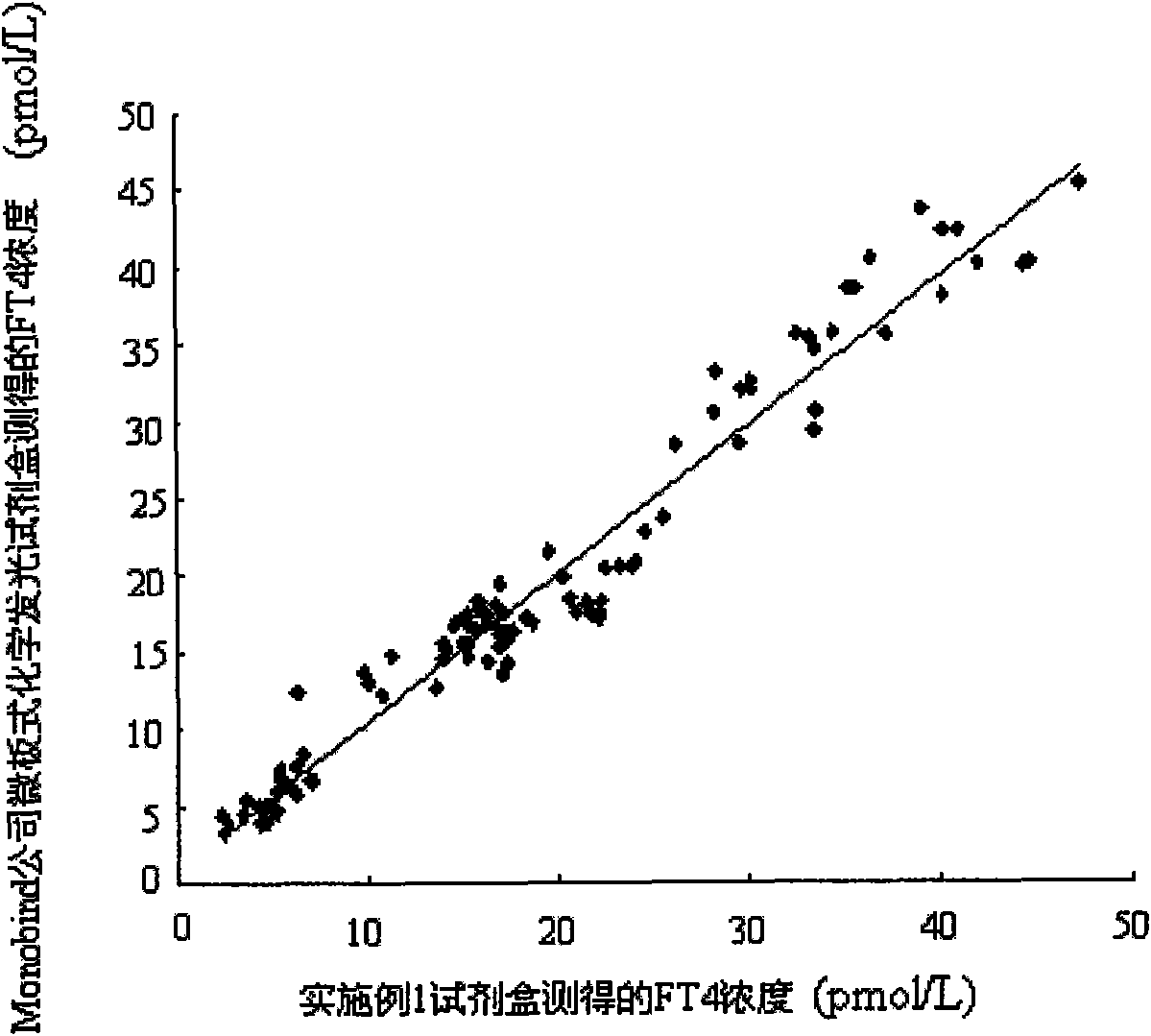
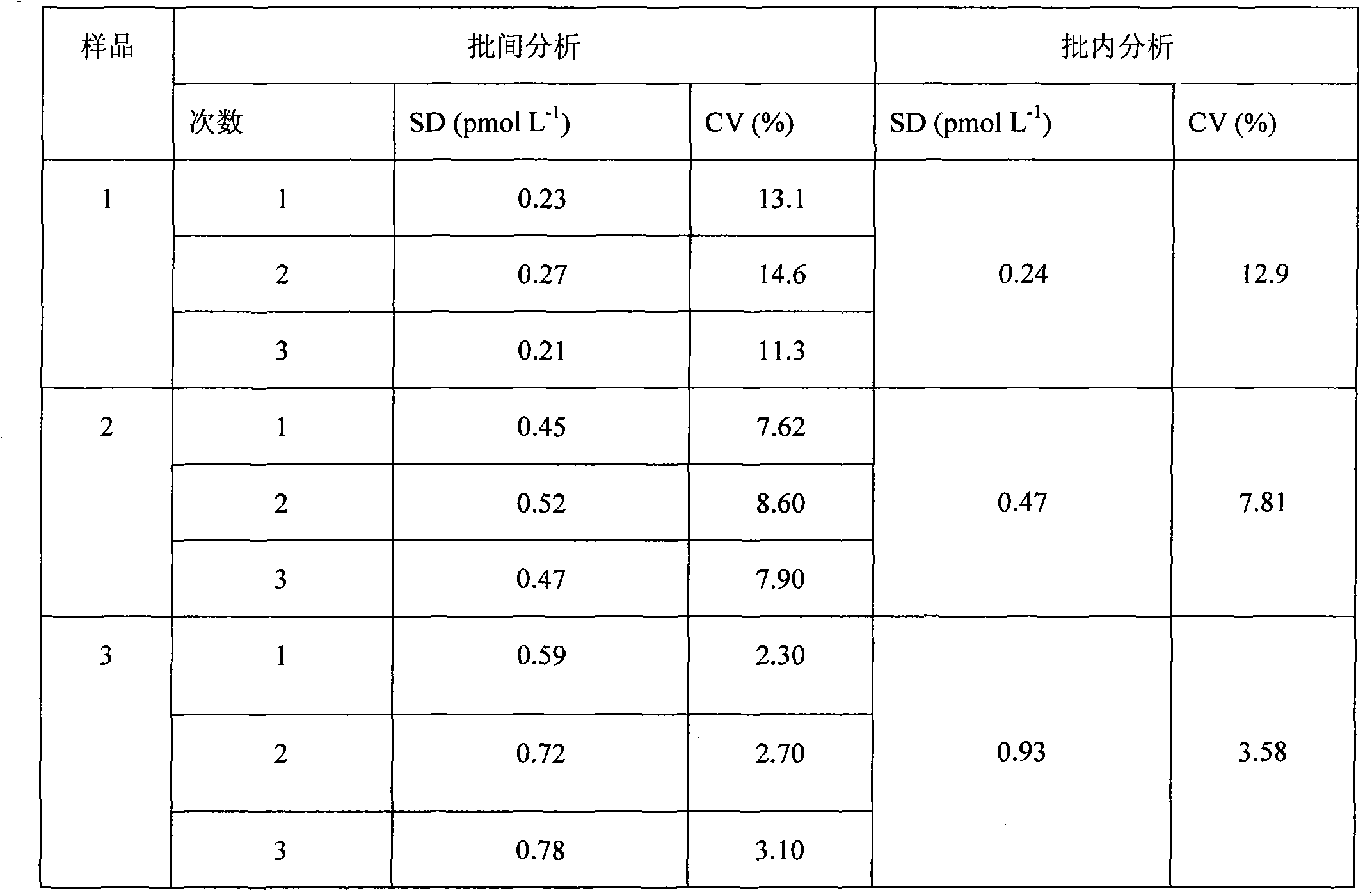
The invention discloses a magnetic particle chemiluminescence immunoassay kit of free thyroxine (FT4), belonging to the medical field of immunoassay. The kit comprises: 1) free thyroxine series calibrator; 2) magnetic particle solution enveloped by anti-fluorescein isothiocyanate monoclonal antibody; 3) free thyroxine antibody solution marked by fluorescein isothiocyanate; 4) free thyroxine markedby horse radish peroxidase; 5) chemiluminescence substrate liquid; and 6) concentrated washing liquid. The invention also discloses a preparation method of the kit. The kit in the invention has advantages of simpleness, convenience, rapidness, sensitivity, stability and the like.
Owner:TSINGHUA UNIV
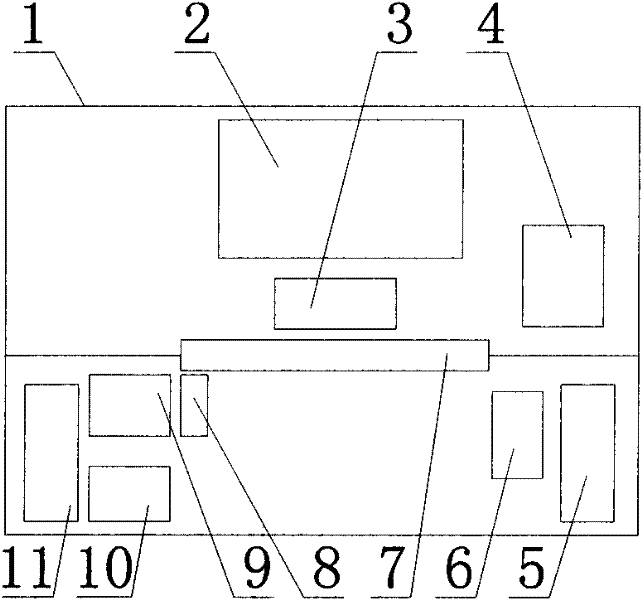
Automatic chemiluminescence immunoassay device
InactiveCN102253228ASimple structureSuitable for operationMaterial analysisPath switchingEngineering
An automatic chemiluminescence immunoassay device comprises a mechanical frame and a housing (1), a sampling work bench (11), a plate-washing work bench (5), a detection work bench (2), a light path switching device (3), a sampling pump (10), a plate-washing liquid pneumatic system (6), a reagent shelf (9), a needle-washing pool (8), a darkroom door (7), and a control part (4). The device of the invention can realize three-dimensional operation of sampling, plate washing, and detection, has a simple structure, is suitable for operation, has low cost, and facilitates industrial realization.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com