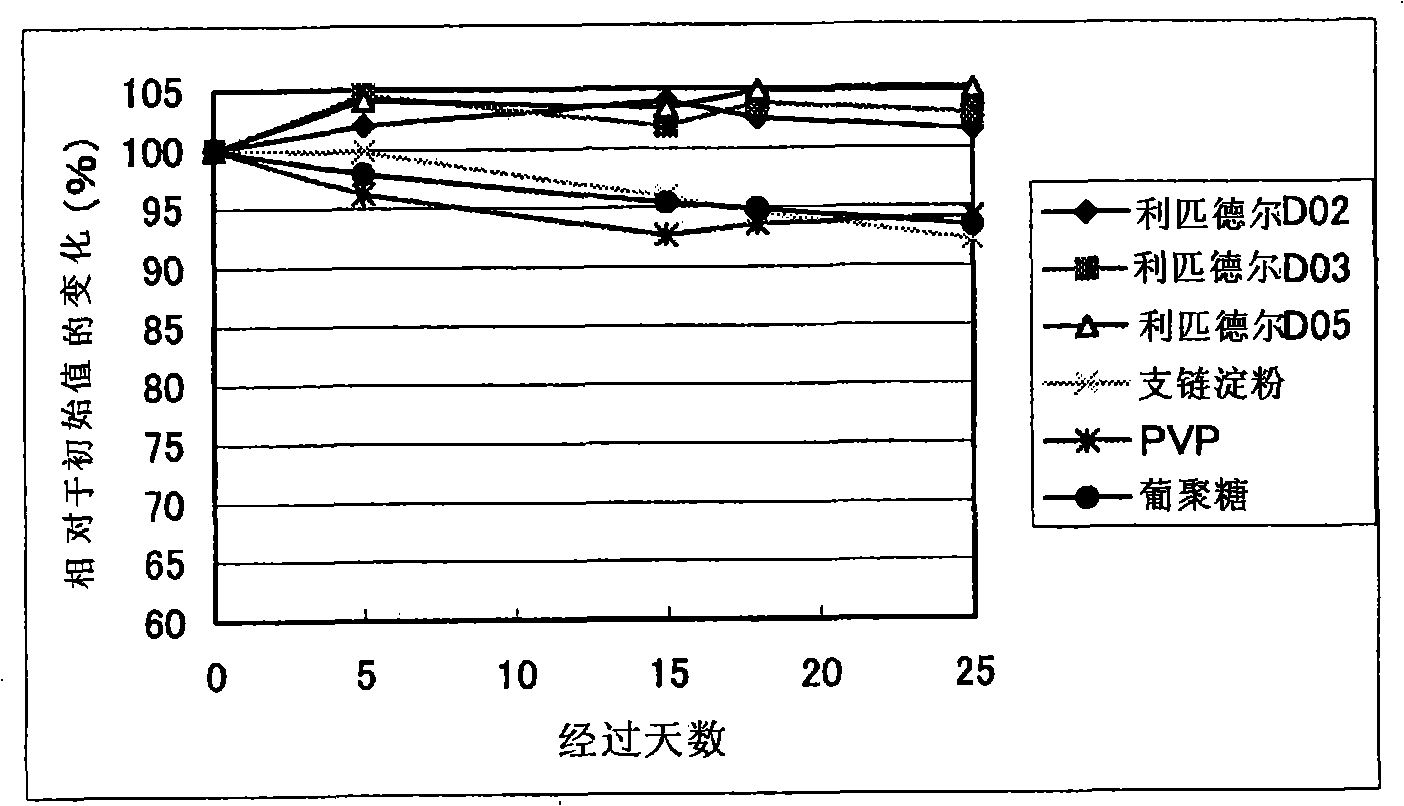
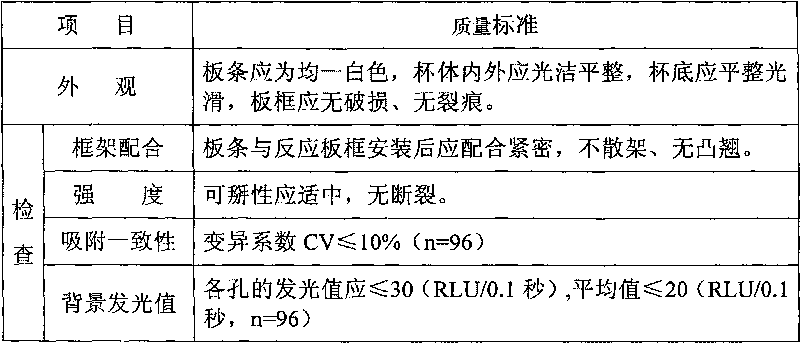
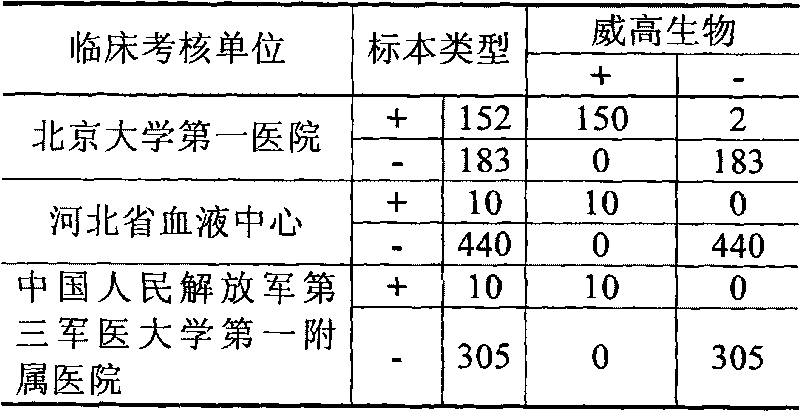
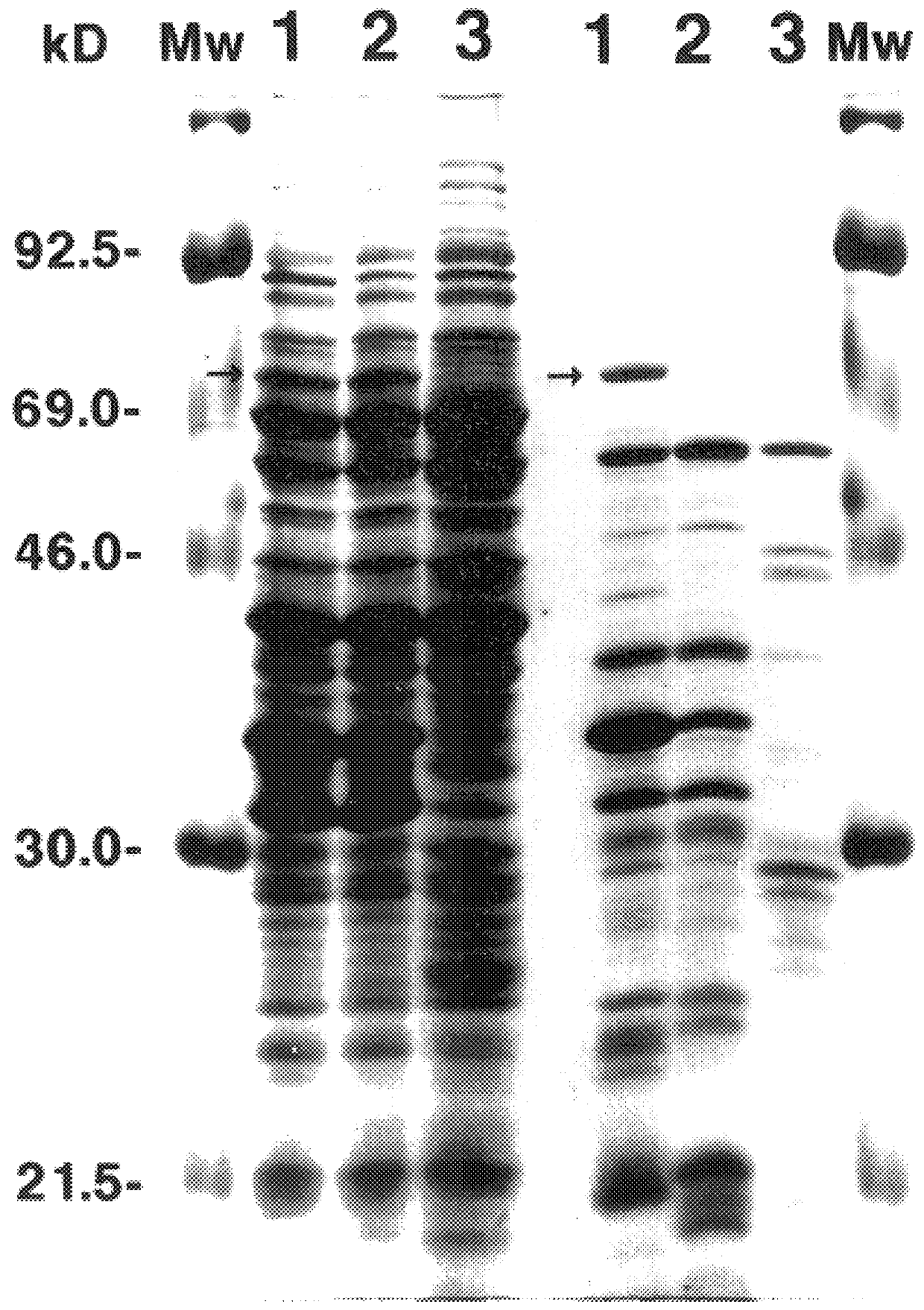
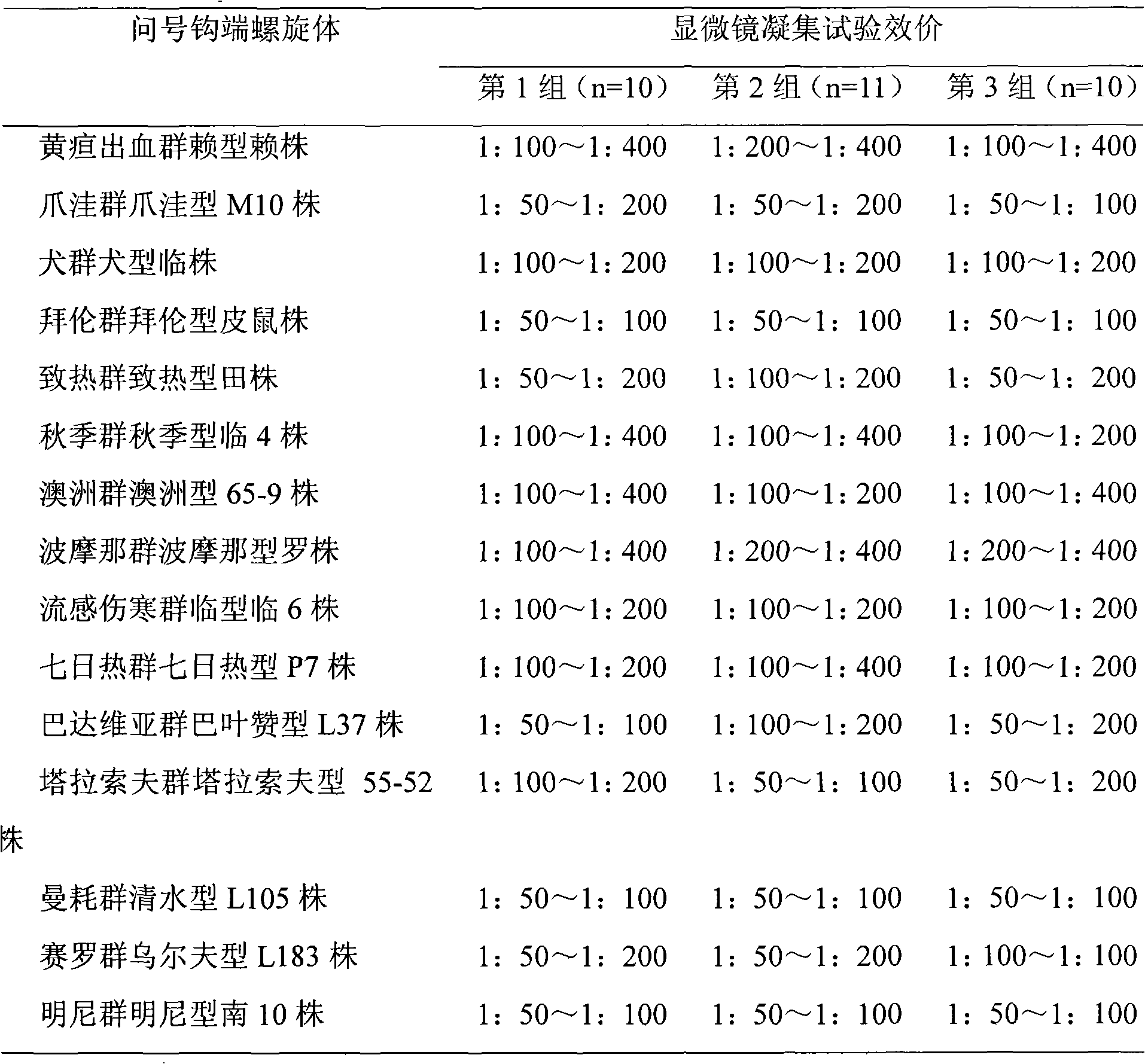
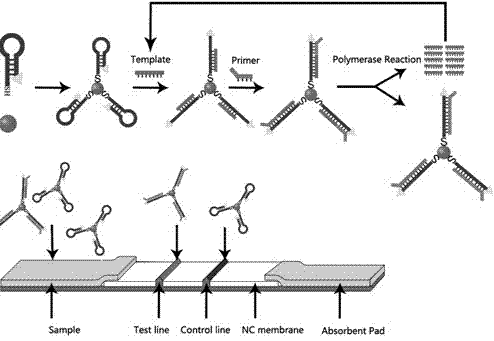
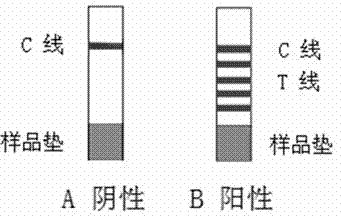
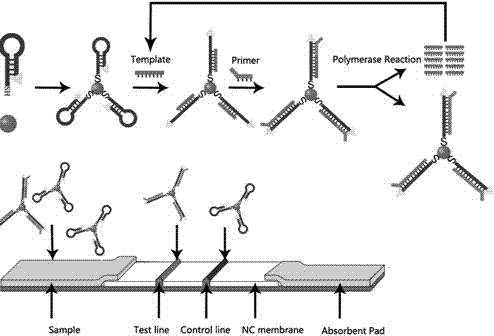
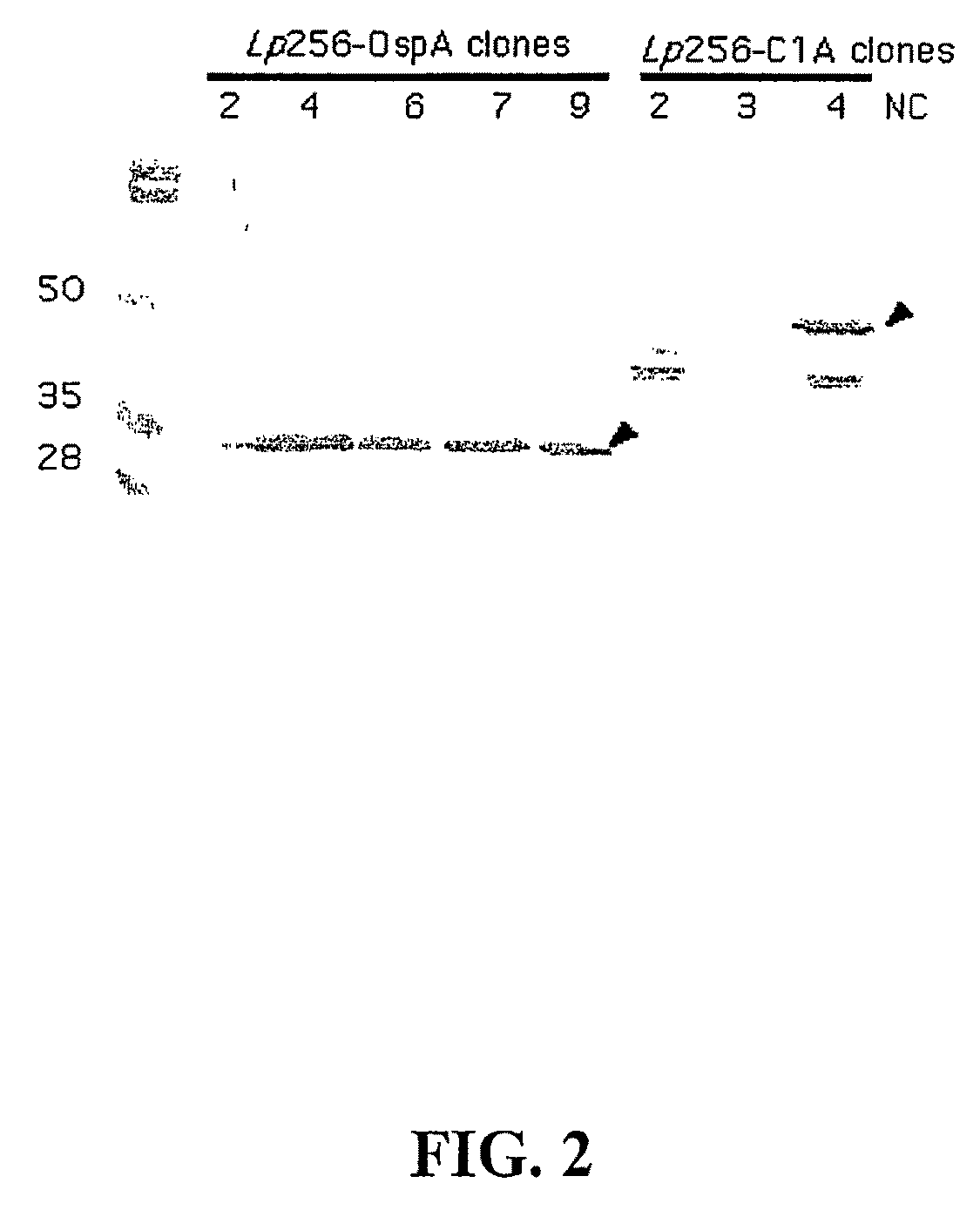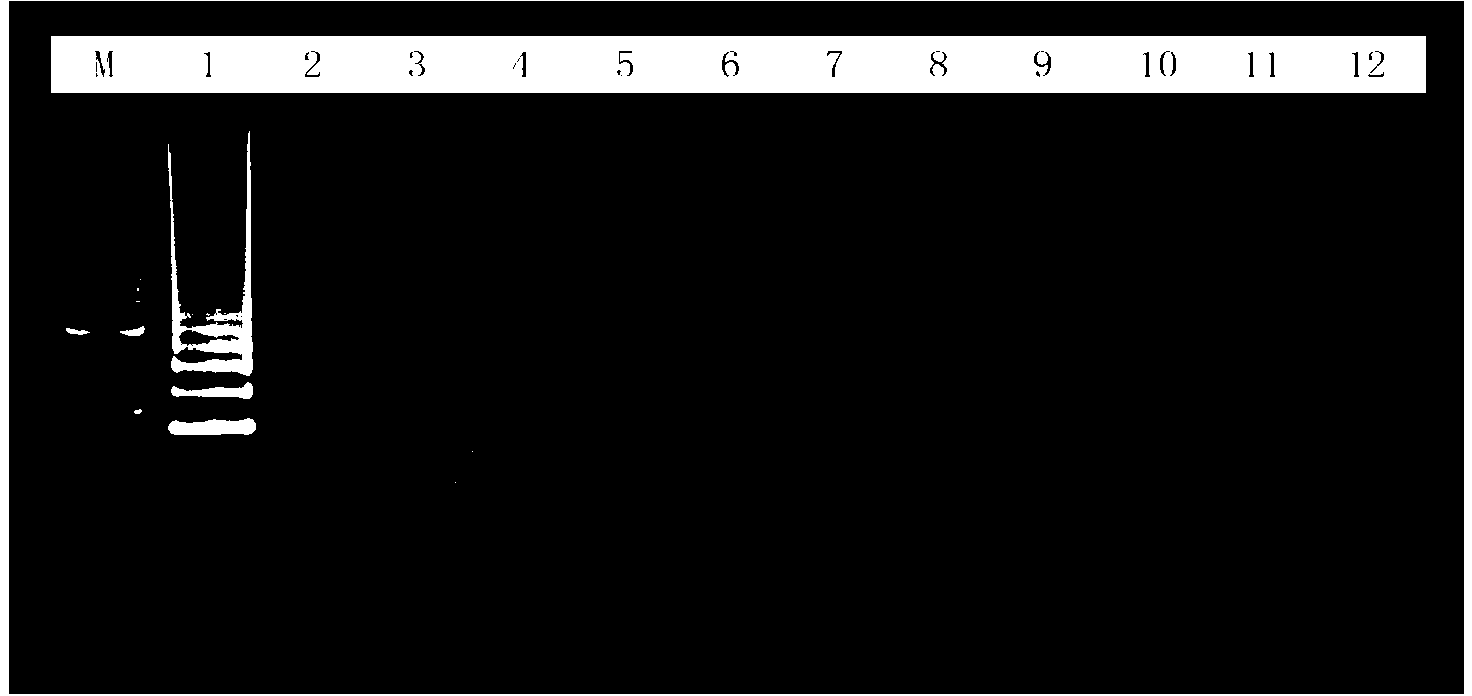Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
173 results about "Spiroplasma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Spiroplasma is a genus of Mollicutes, a group of small bacteria without cell walls. Spiroplasma shares the simple metabolism, parasitic lifestyle, fried-egg colony morphology and small genome of other Mollicutes, but has a distinctive helical morphology, unlike Mycoplasma. It has a spiral shape and moves in a corkscrew motion. Many Spiroplasma are found either in the gut or haemolymph of insects where they can act to manipulate host reproduction, or defend the host as endosymbionts. Spiroplasma are also disease-causing agents in the phloem of plants. Spiroplasmas are fastidious organisms, which require a rich culture medium. Typically they grow well at 30 °C, but not at 37 °C. A few species, notably Spiroplasma mirum, grow well at 37 °C (human body temperature), and cause cataracts and neurological damage in suckling mice. The best studied species of spiroplasmas are Spiroplasma poulsonii, a reproductive manipulator and defensive insect symbiont, Spiroplasma citri, the causative agent of citrus stubborn disease, and Spiroplasma kunkelii, the causative agent of corn stunt disease.
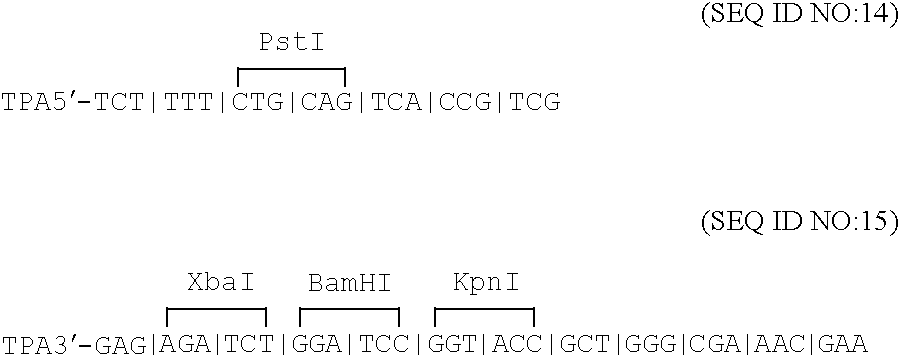
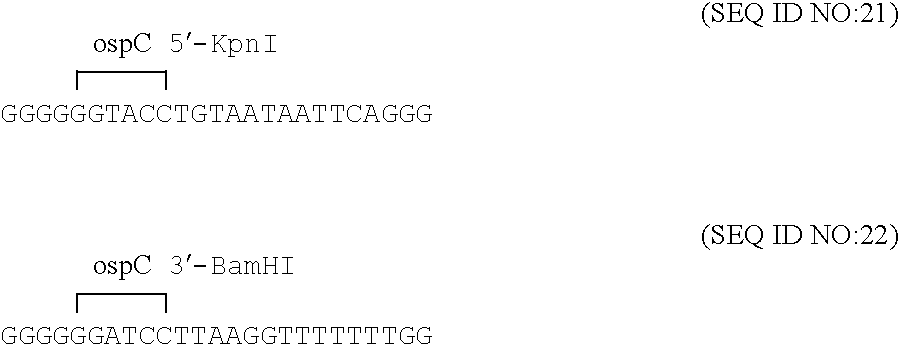
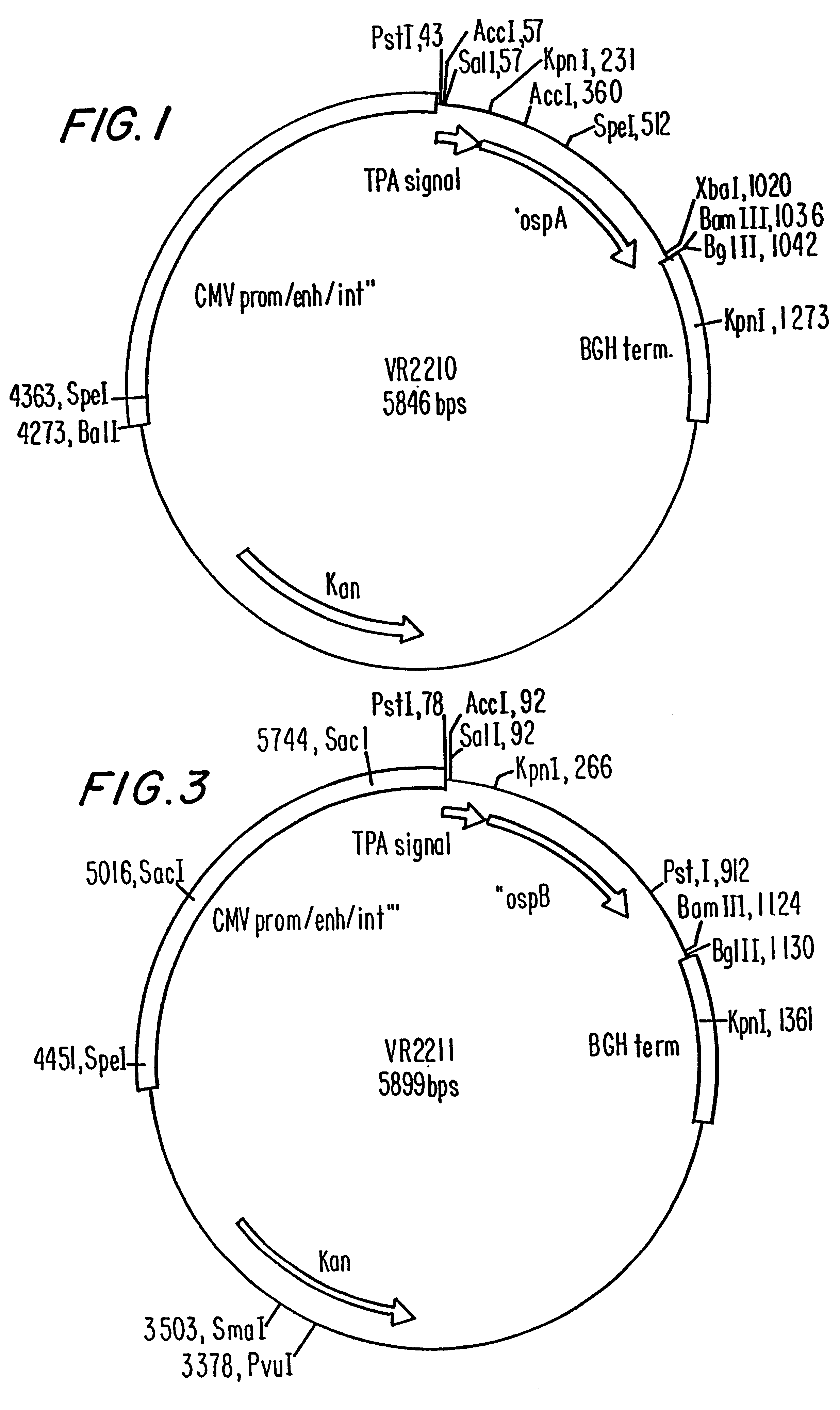
Compositions and methods for administering Borrelia DNA
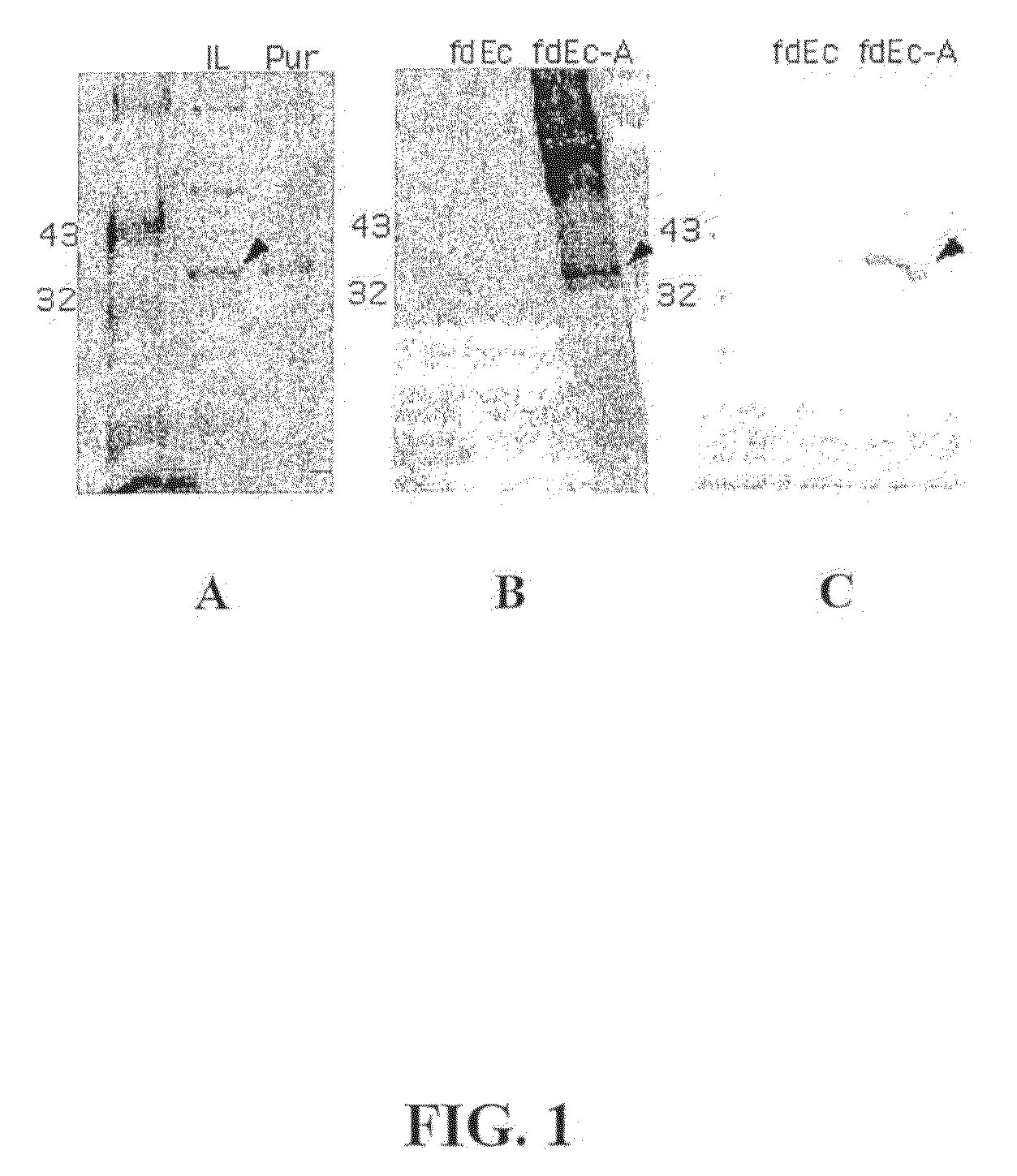
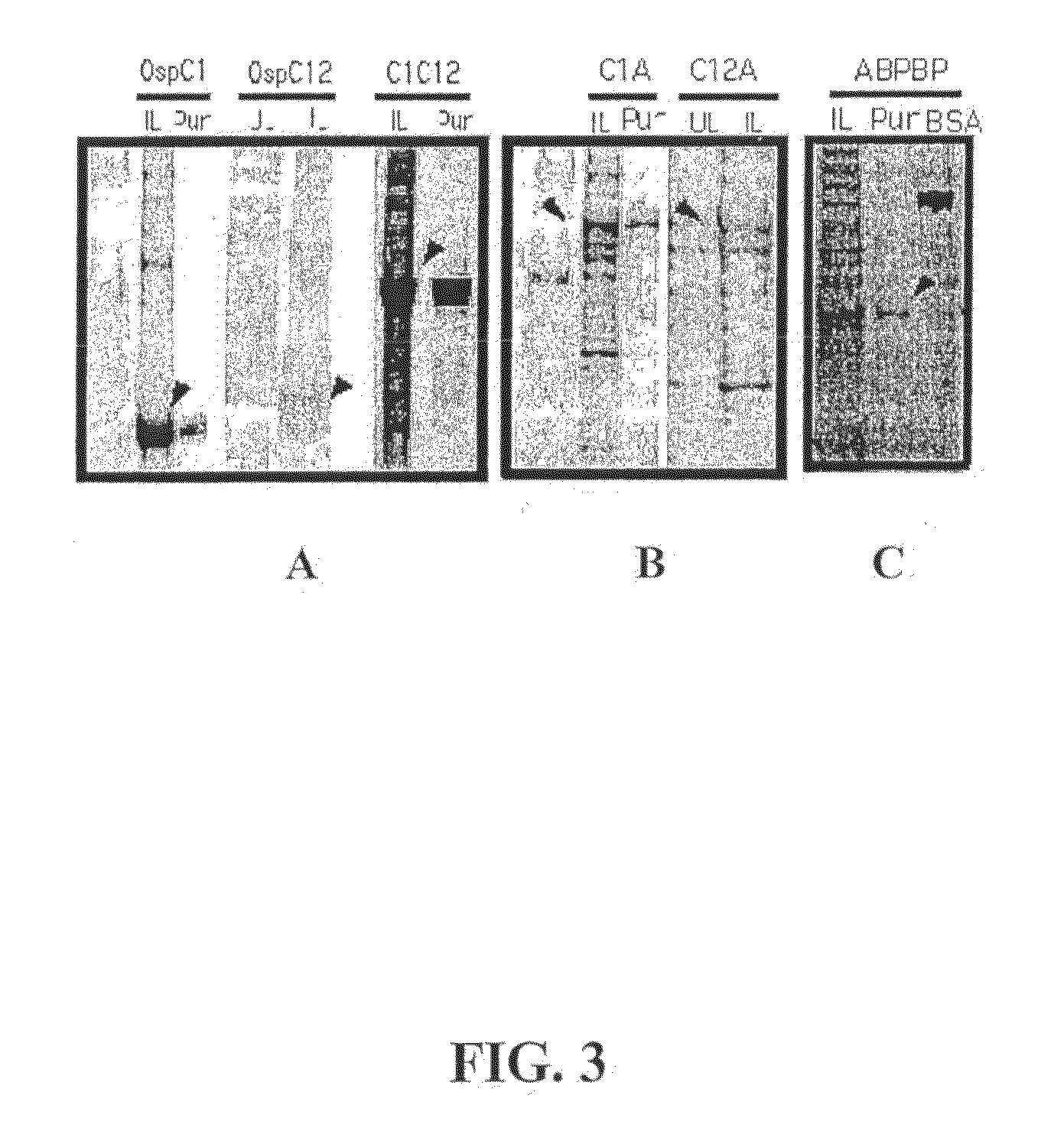
Disclosed is a vaccine against Lyme Disease or its causative agent Borrelia burgdorferi (sensu stricto or sensu lato) containing a plasmid a DNA encoding a promoter for driving expression in a mammalian cell, DNA encoding a leader peptide for facilitating secretion / release of a prokaryotic protein sequence from a mammalian cell, a DNA encoding Borrelia OspA or OspB, and a DNA encoding a terminator. Disclosed too is an immunogenic composition against Lyme Disease or its causative agent Borrelia burgdorferi (sensu stricto or sensu lato) containing a plasmid comprising a DNA encoding a promoter for driving expression in a mammalian cell, DNA encoding a leader peptide for facilitating secretion / release of a prokaryotic protein sequence from a mammalian cell, a DNA encoding a Borrelia OspC, and a DNA encoding a terminator. And, methods for making and using such vaccines and the immunogenic composition are also disclosed.
Owner:PASTEUR MERIEUX SERUMS & VACCINS SA
Lyme combination compositions and uses
InactiveUS6368603B1Safe and efficacious in dogNo exacerbation of diseaseAntibacterial agentsNanotechAntigenRabies
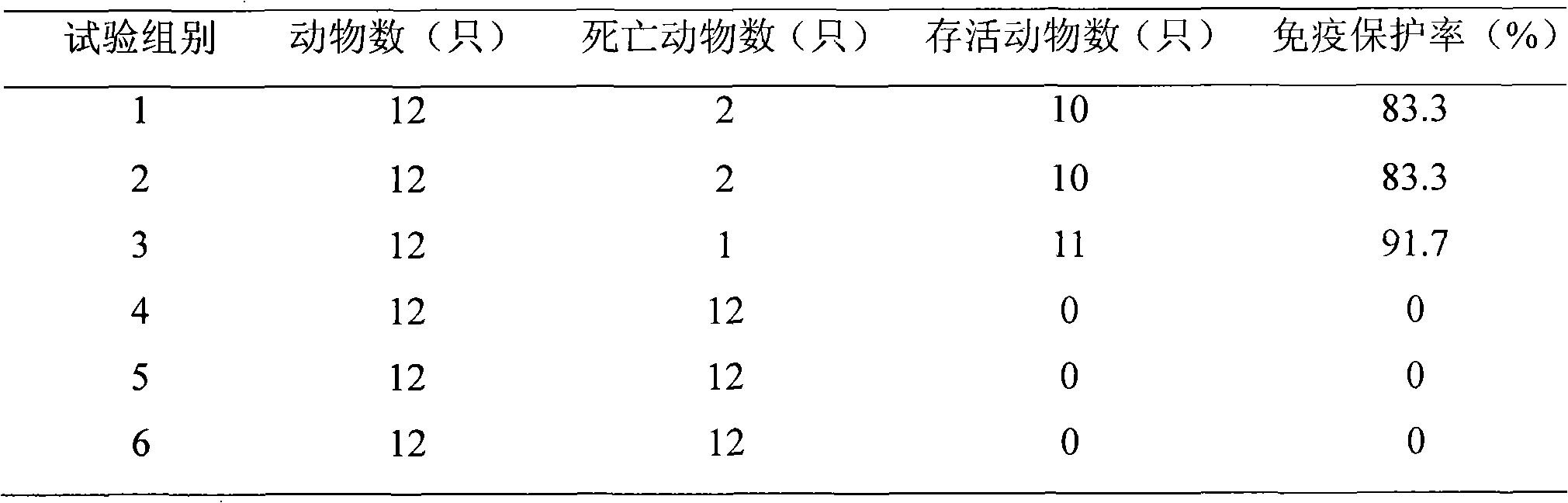
Disclosed and claimed are compositions containing a Borrelia burgdorferi antigen, and methods for making and using them. The antigen can be OspA. The compositions can contain at least one additional antigen from a pathogen other than Borrelia burgdorferi. The compositions are useful for eliciting an immunological response in a host mammal susceptible to Lyme Disease and to the mammalian pathogen other than Borrelia burgdorferi. Suitable host mammals include dogs, pups, horses, and, the additional antigen can be of a canine, equine or feline pathogen, such as rabies, canine distemper, adenovirus, coronavirus, parainfluenza and parvovirus. No significant efficacy interference is observed.
Owner:MERIAL LTD
Nucleic acid amplification oligonucleotides and probes to Lyme disease associated Borrelia
InactiveUS6074826AReduced thermal stabilityMaximize differenceSugar derivativesMicrobiological testing/measurementBorrelia gariniiHybridization probe
The present invention discloses hybridization assay probes, amplification primers, nucleic acid compositions and methods useful for detecting Borrelia nucleic acids. Hybridization assay probes and amplification primers that selectively detect Lyme disease-associated Borrelia and distinguish those Borrelia from Borrelia hermsii are disclosed. Other hybridization probes selectively detect Borrelia hermsii and not Lyme disease-associated Borrelia are also described.
Owner:GEN PROBE INC
Detection of transmissible spongiform encephalopathies
InactiveUS6033858ASugar derivativesMicrobiological testing/measurementSpiroplasmaTransmissible mink encephalopathy
Provided is a method of detecting transmissible spongiform encephalopathies. The method comprises: selecting a sample from a subject to determine whether the subject has a transmissible spongiform encephalopathy; and detecting spiroplasma-specific 16S rDNA indicative of transmissible spongiform encephalopathies in the sample. The spiroplasma-specific 16S rDNA is preferably detected by contacting the sample with a pair of oligonucleotide primers under polymerase chain reaction conditions and detecting the resulting polymerase chain reaction product, wherein each of the pair of the oligonucleotide primers is complementary to spiroplasma-specific 16S rDNA indicative of transmissible spongiform encephalopathies. Further provided is an oligonucleotide having a nucleotide sequence complementary to spiroplasma-specific 16S rDNA indicative of transmissible spongiform encephalopathies; as well as an oligonucleotide having a nucleotide sequence specific to spiroplasma-specific 16S rDNA indicative of transmissible spongiform encephalopathies.
Owner:BASTIAN FR O
Oral vaccine for Borrelia
The present invention relates to vaccines for control of Borrelia infections in animal and human populations. In particular, the present invention provides compositions and methods comprising recombinant bacteria engineered to express one or more Borrelia burgdorferi antigens for use as Lyme disease vaccines. In some embodiments, the recombinant bacteria are freeze-dried.
Owner:US BIOLOGIC INC
Canine combination vaccines
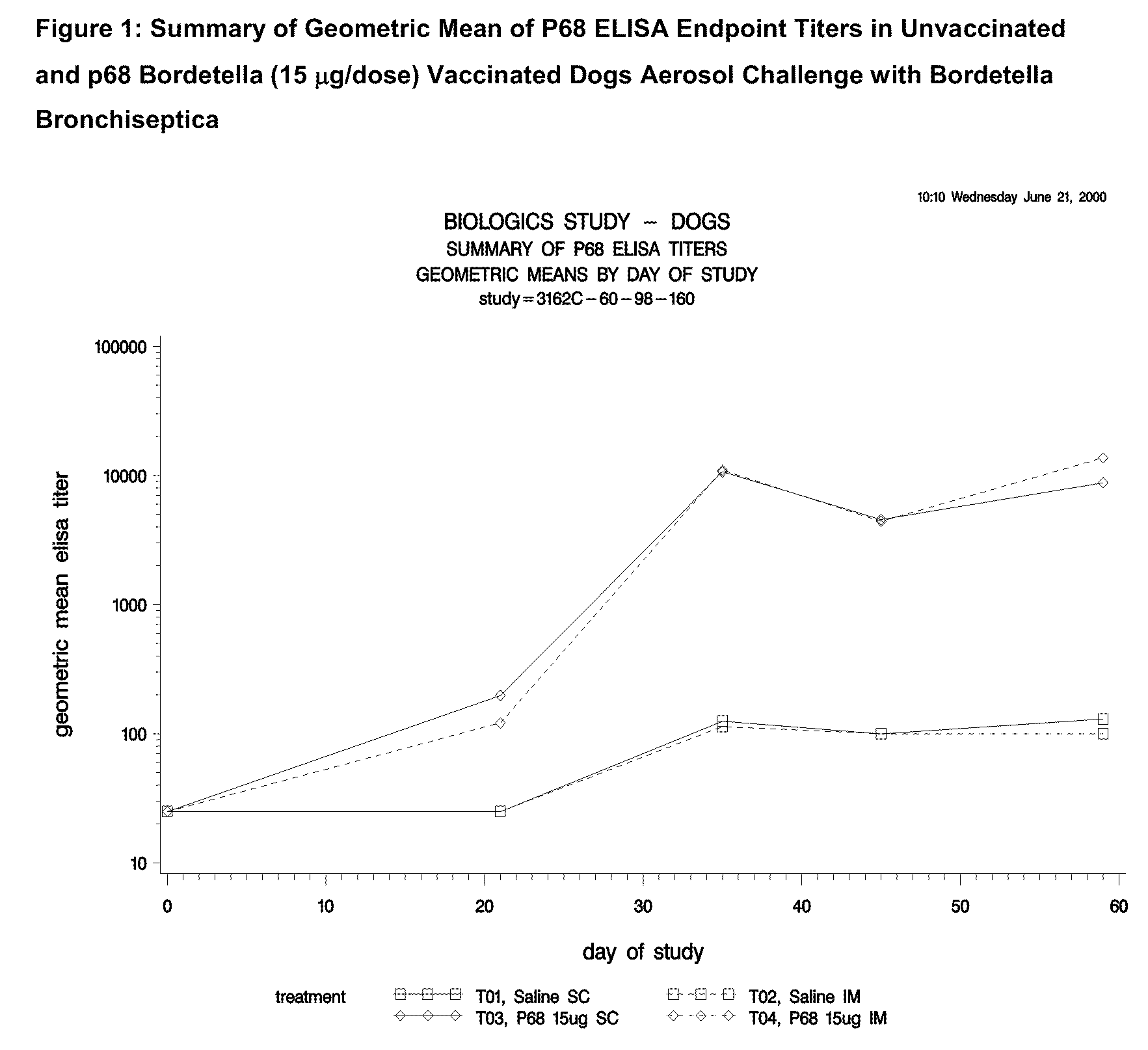
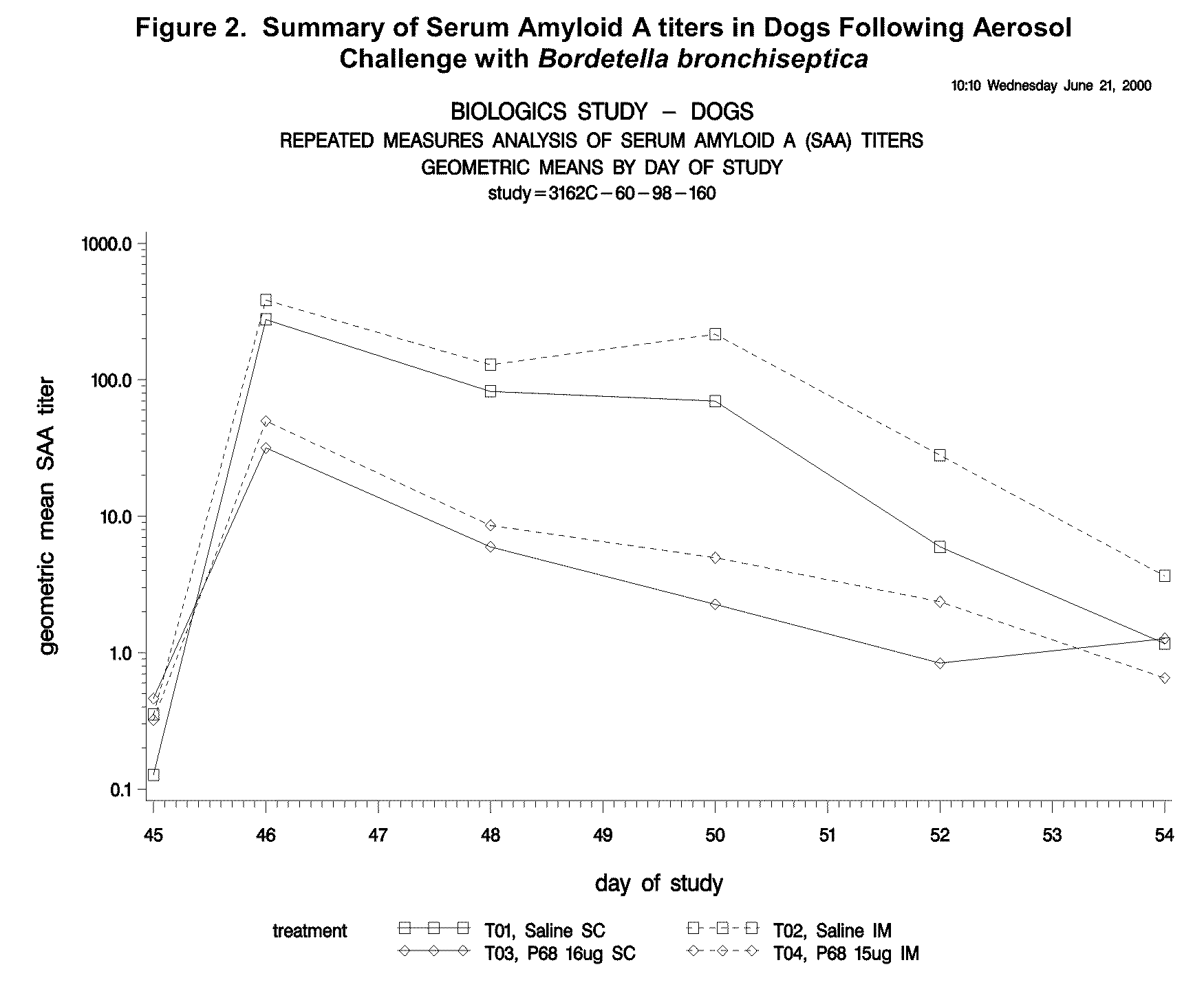
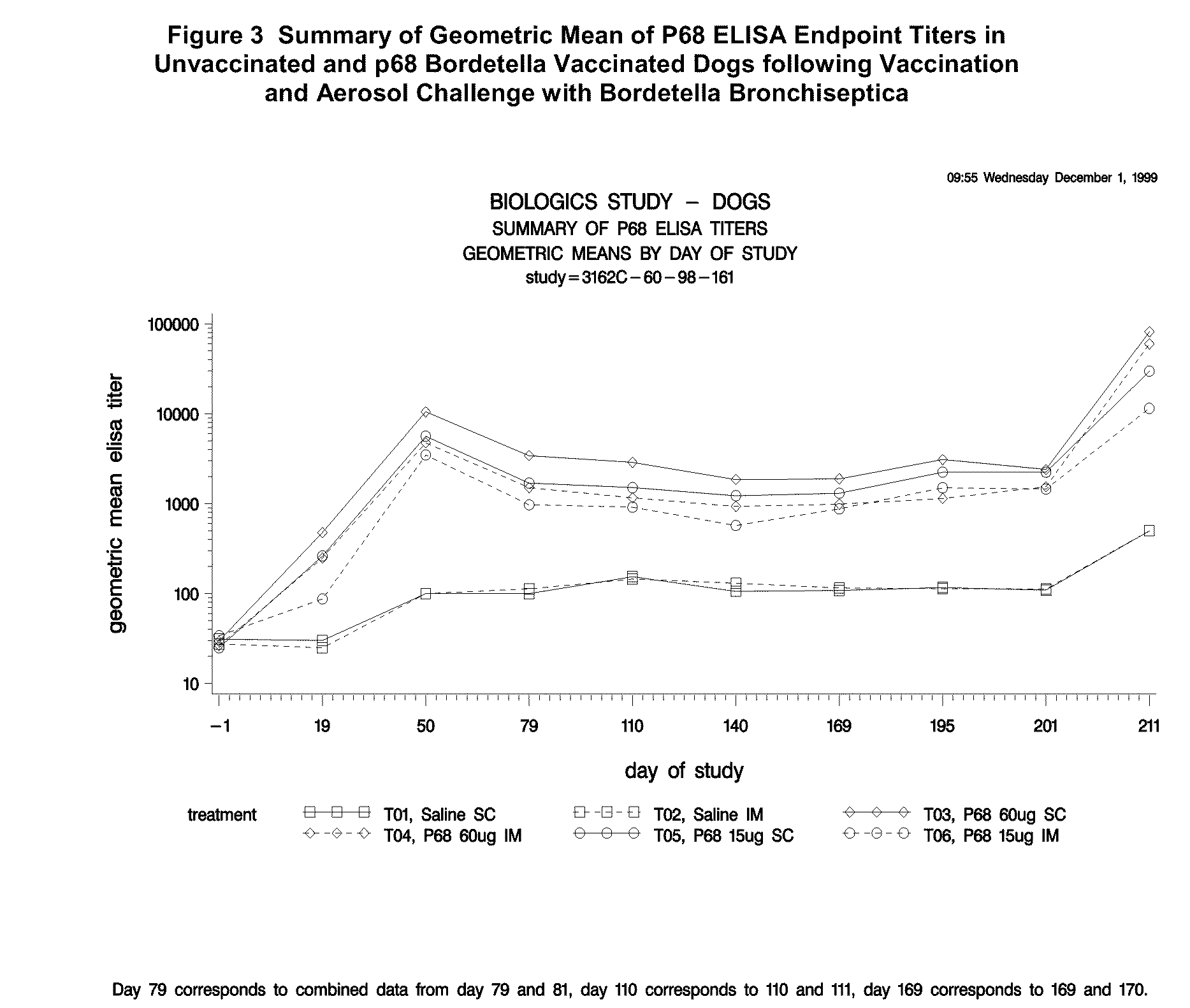
This invention relates to vaccines and methods for protecting dogs against disease caused by Bordetella bronchiseptica. This invention also relates to combination vaccines and methods for protecting dogs against disease or disorder caused by canine pathogens, for example, infectious tracheobronchitis caused by Bordetella bronchiseptica, canine distemper caused by canine distemper (CD) virus, infectious canine hepatitis (ICH) caused by canine adenovirus type 1 (CAV-1), respiratory disease caused by canine adenovirus type 2 (CAV-2), canine parainfluenza caused by canine parainfluenza (CPI) virus, enteritis caused by canine coronavirus (CCV) and canine parvovirus (CPV), and leptospirosis caused by Leptospira Bratislava, Leptospira canicola, Leptospira grippotyphosa, Leptospira icterohaemorrhagiae or Leptospira pomona. The vaccines of the present invention include a Bordetella bronchiseptica p68 antigen.
Owner:ZOETIS SERVICE LLC
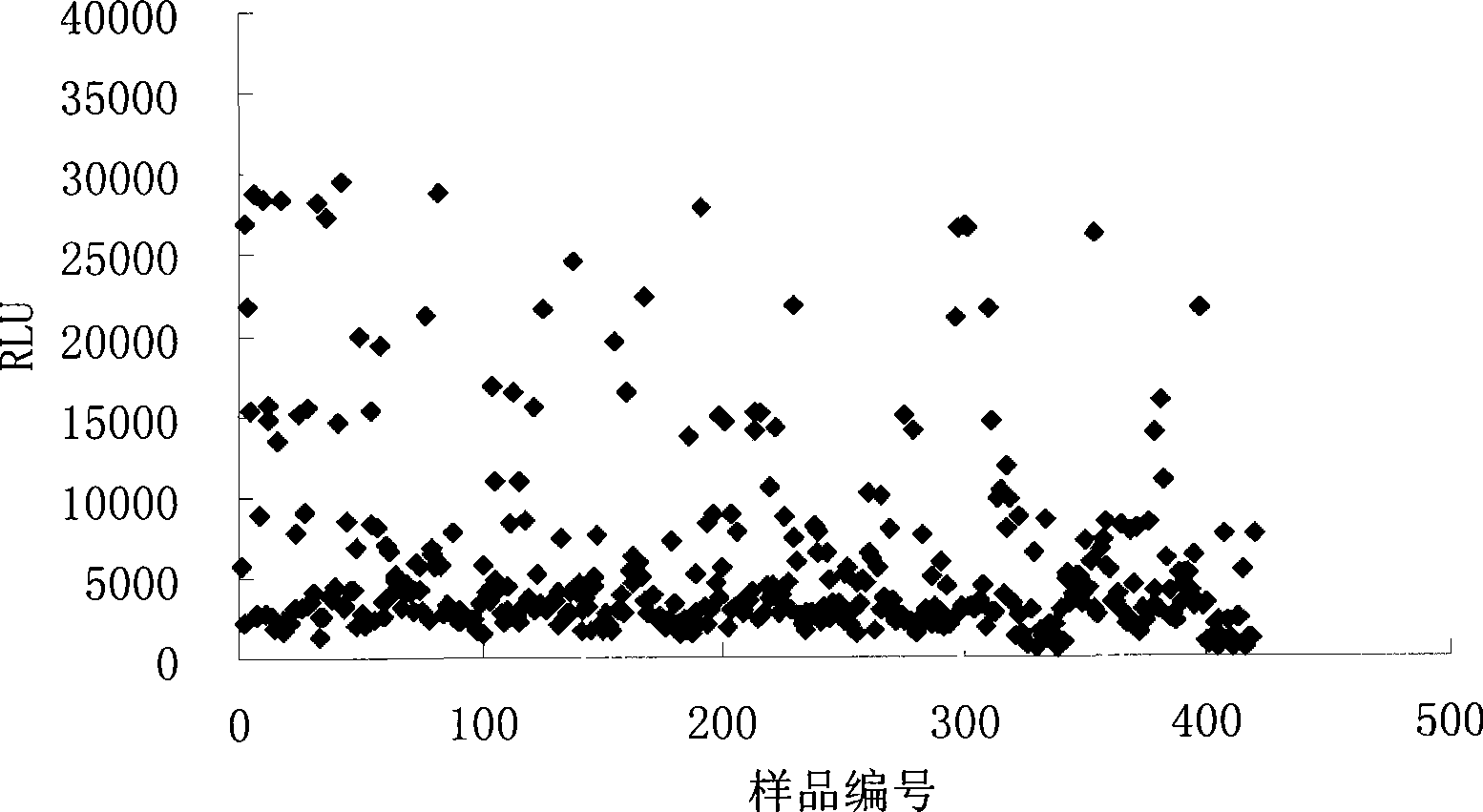
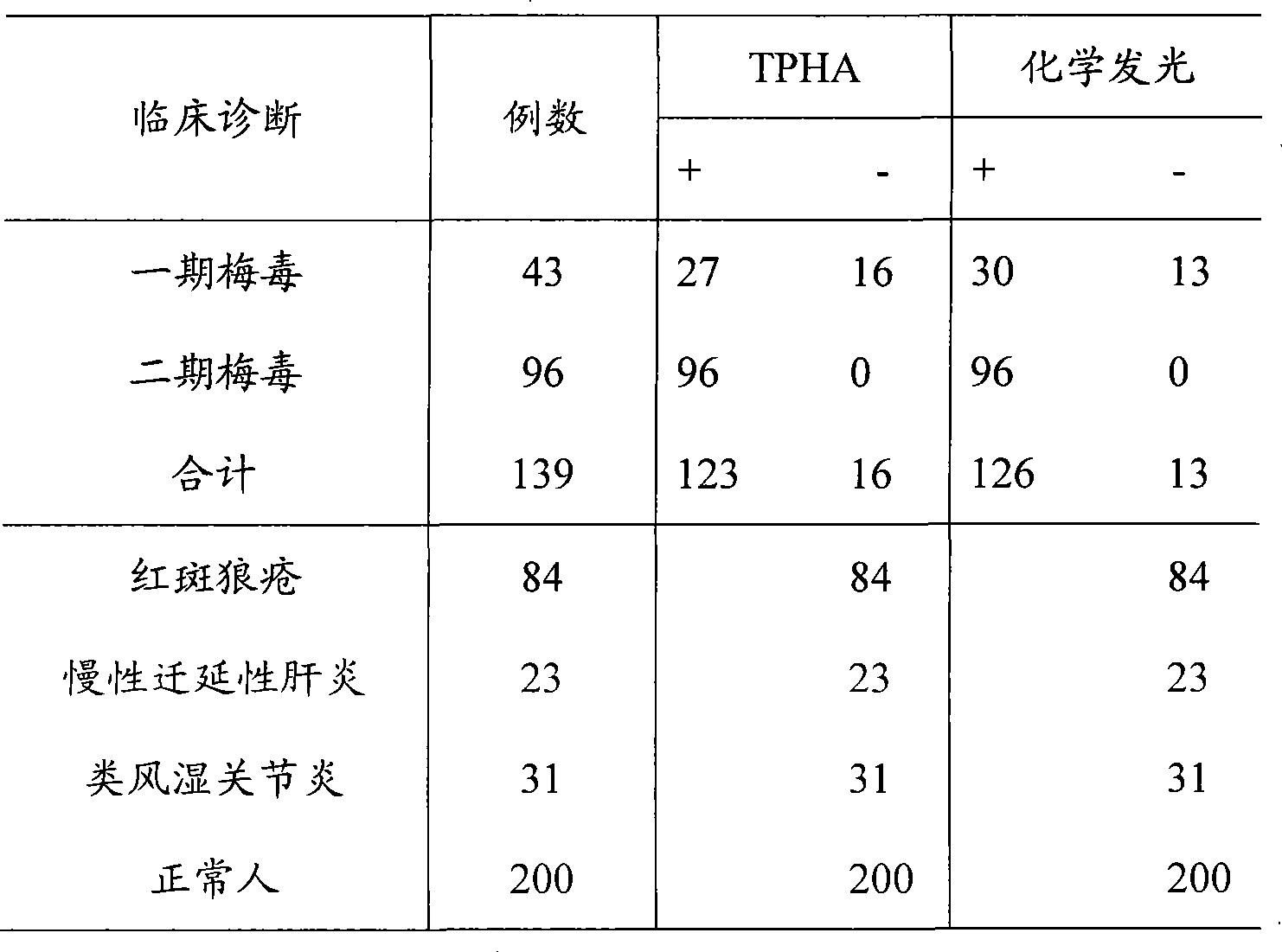
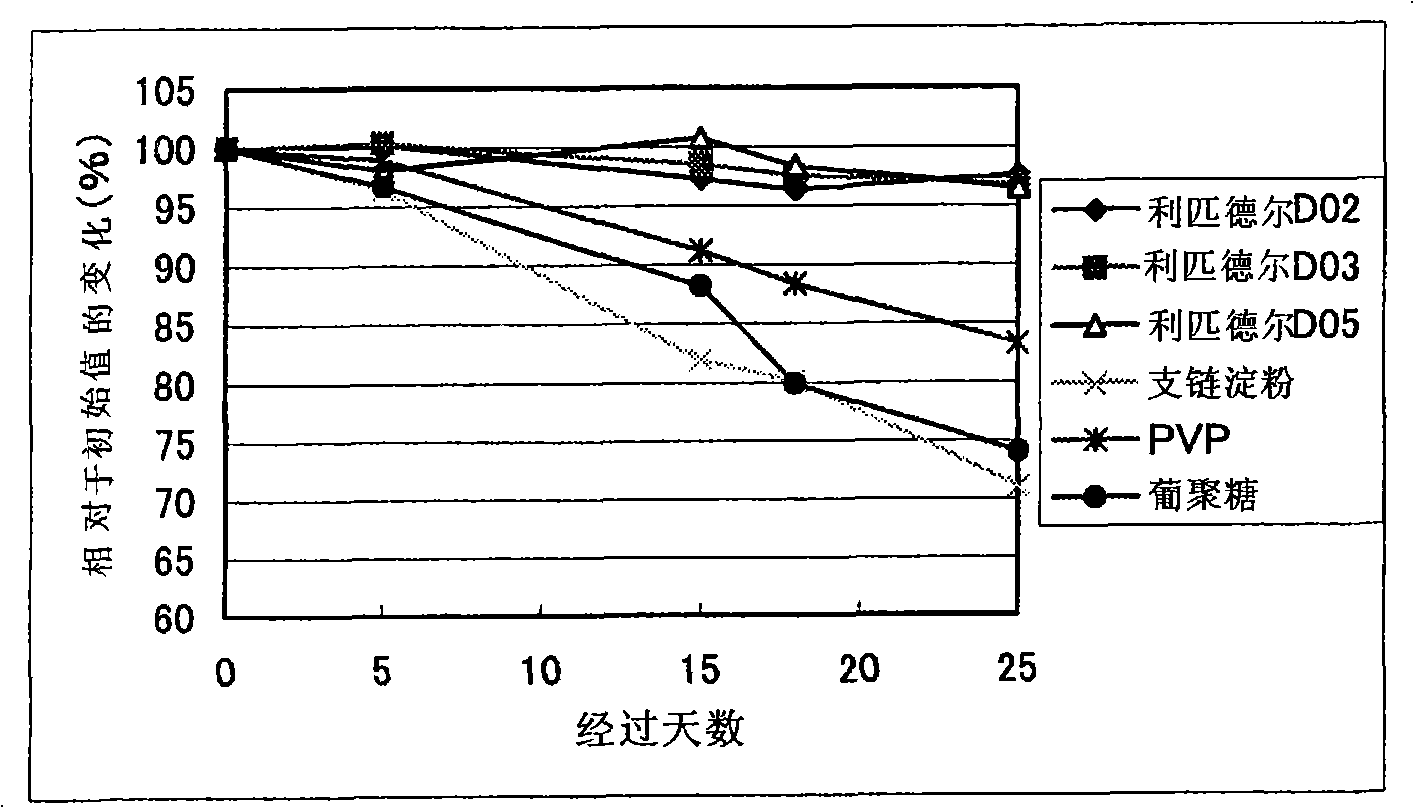
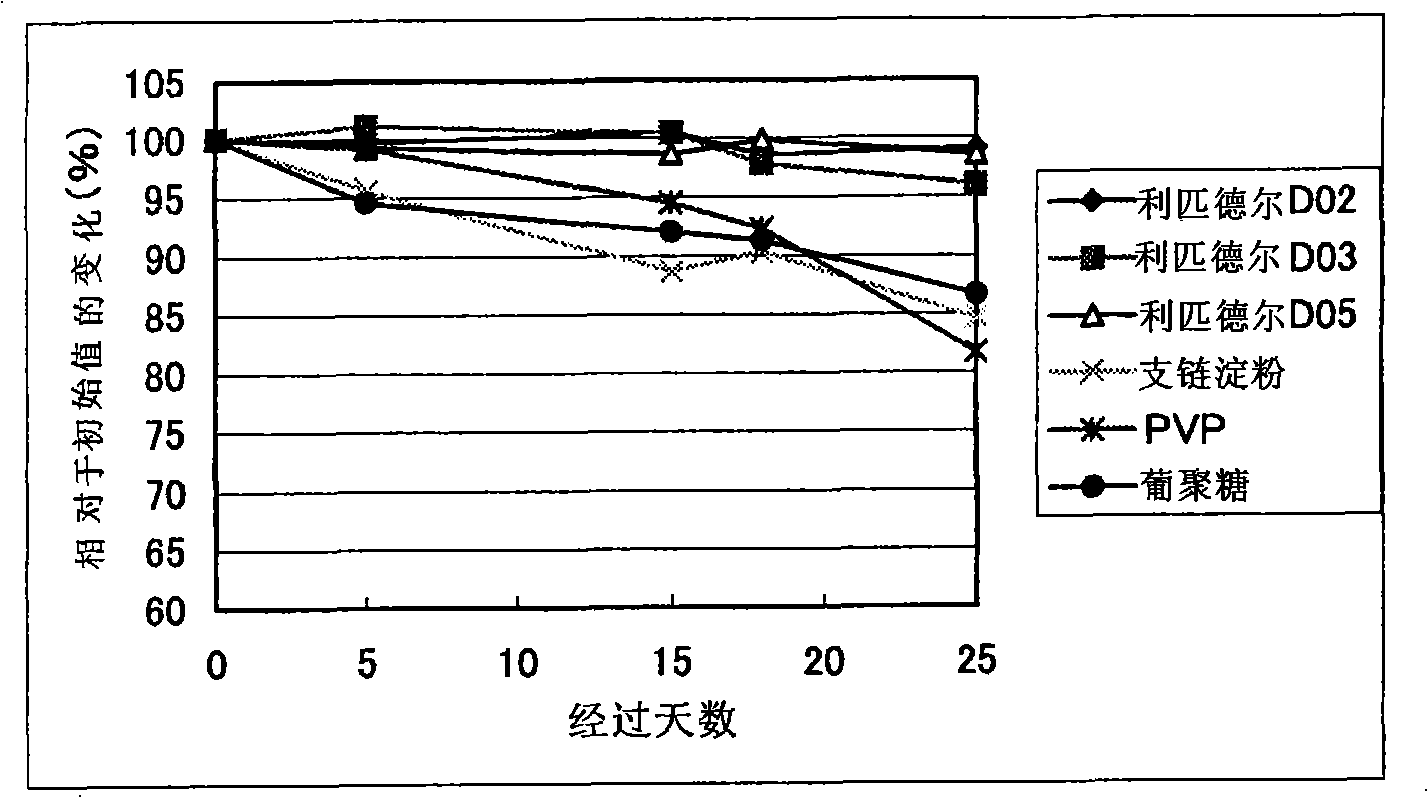
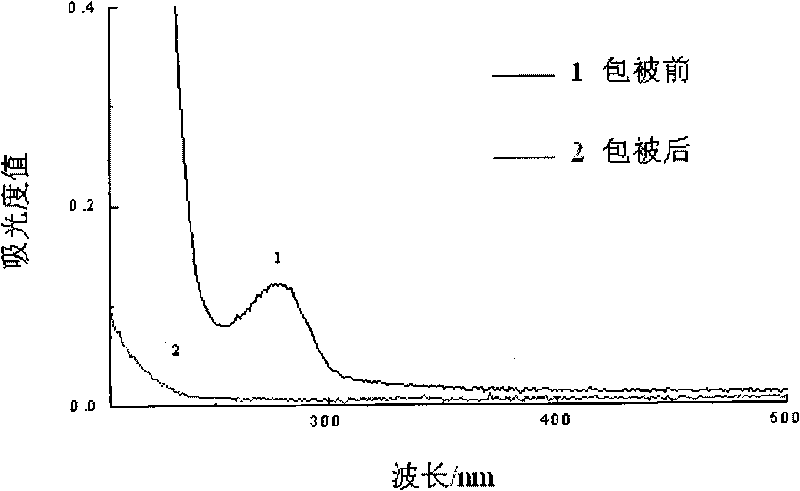
Syphilis helicoid antibody chemiluminescence immune assay determination kit and method for preparing same
InactiveCN101363860AEfficient use ofGuaranteed SensitivityChemiluminescene/bioluminescencePositive controlNon toxicity
The invention discloses a kit of chemiluminescent immunological analysis measurement of a Treponema pallidum antibody, and preparation method thereof, which belongs to the technical field of immunological analysis medical diagnosis. The kit comprises (1) a carrier coated with a treponema pallidum specific recombination protein antigen; (2) treponema pallidum antibody negative and positive reference substances; (3) an enzyme labeled treponema pallidum specific recombination protein antigen; and (4) an enzyme acted chemiluminescent primer. Furthermore, the preparation method of the kit based on the invention comprises the following steps of (1) preparing TP antibody negative and positive reference substances; (2) labeling the TP specific recombination protein antigen with an enzyme; (3) coating with the carrier; (4) sub-packaging; and (5) assembling. The kit of the detection of the Treponema pallidum antibody has the advantages of easy and simple operation, easy popularization, high sensitivity, strong specificity, good repeatability, safety, non toxicity, and no pollution.
Owner:北京科美东雅生物技术有限公司
Reagent for assaying antiphospholipid antibody and reagent for assaying anti-treponema pallidum antibody
The invention aims to provide a reagent for assaying an antiphospholipid antibody which is excellent in long-term storage stability and capable of diagnosing syphilis infection or the like and a reagent for assaying an anti-Treponema pallidum antibody which is capable of diagnosing syphilis infection with high accuracy by preventing the occurrence of serum interference. The invention is directed to the reagent for assaying an antiphospholipid antibody to be used for diagnosing syphilis infection comprising an insoluble carrier carrying an antiphospholipid antigen and a copolymer with a segment derived from 2-methacryloyloxyethyl phosphorylcholine and a segment derived from a hydrophilic monomer.
Owner:SEKISUI MEDICAL CO LTD +1
Treponema pallidum antibody diagnostic kit and preparation method thereof
The invention belongs to the technical field of immunologic diagnosis, in particular to a treponema pallidum antibody diagnostic kit by a chemiluminescence method and a preparation method thereof. The kit comprises an anti-TP test reaction plate, an anti-TP test enzyme complex, chemiluminescence substrate liquid, concentrated washing liquor, a negative contrast and a positive contrast. The invention also discloses a preparation method of the diagnostic kit, which adopts a chemiluminescence immunoassay technology; compared with ELISA (enzyme-linked immuno sorbent assay), the method has higher sensitivity and specificity, is suitable for the auxiliary diagnosis of clinical syphilis and screening of blood donors and fills a blank of the production of a treponema pallidum antibody diagnostic reagent detected by the domestic chemiluminescence method.
Owner:威海威高生物科技有限公司
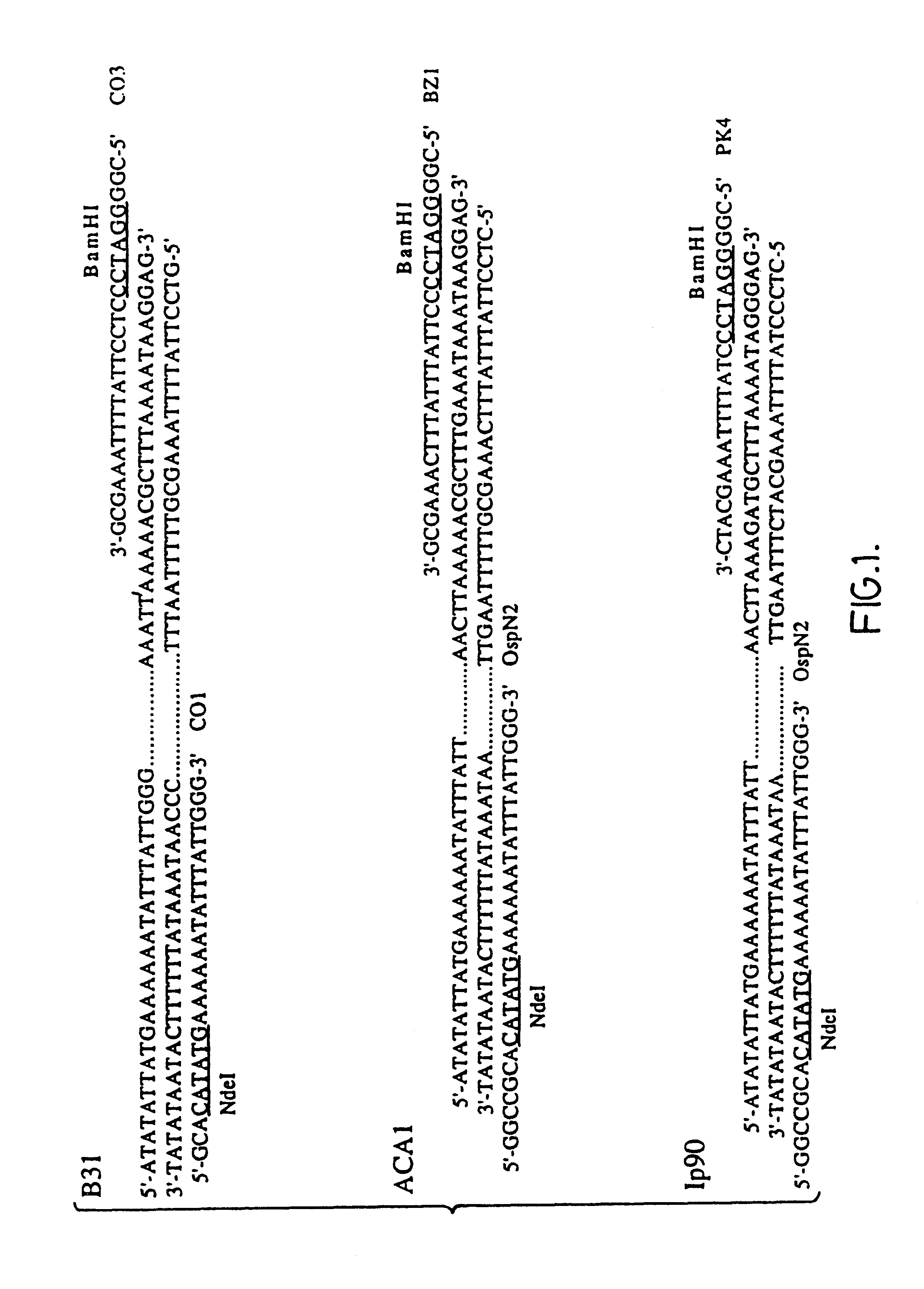
66 kDa antigen from Borrelia
InactiveUS6054296AReduce sensitivityHigh selectivityAntibacterial agentsAntibody mimetics/scaffoldsProtozoaAntigen
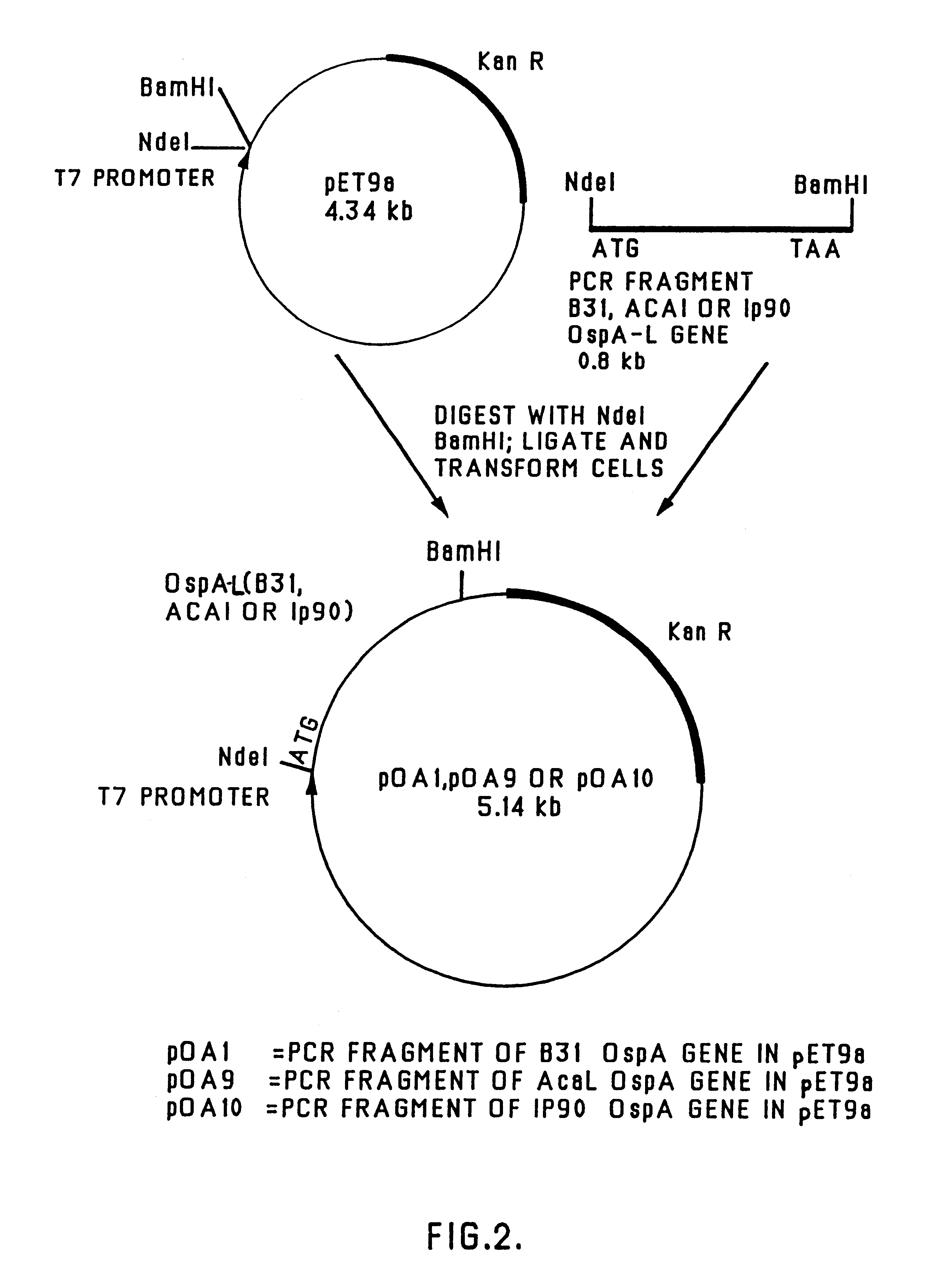
The present invention relates to nucleic acid molecules, polypeptides encoded by the same, antibodies directed thereto and a method of preparing such polypeptides including: (a) inserting an isolated DNA molecule coding for a polypeptide which is immunoreactive with a 66 kDa polypeptide derived from Borrelia garinii IP90 into an expression vector; (b) transforming a host organism or cell with the vector; (c) culturing the transformed host cell under suitable conditions; and (d) harvesting the polypeptide. The isolated DNA molecule is preferably at least 10 nucleotides in length, and the method may optionally include subjecting the polypeptide to post-translational modification. The host cell can be a bacterium, a yeast, a protozoan, or a cell derived from a multicellular organism such as a fungus, an insect cell, a plant cell, or a mammalian cell.
Owner:SYMBICOM
66 kDa antigen from Borrelia
InactiveUS6068842AReduce sensitivityHigh selectivityAntibacterial agentsAntibody mimetics/scaffoldsAntigenProtozoa
The present invention relates to nucleic acid molecules, polypeptides encoded by the same, antibodies directed thereto and a method of preparing such polypeptides including: (a) inserting an isolated DNA molecule coding for a polypeptide which is immunoreactive with a 66 kDa polypeptide derived from Borrelia garinii IP90 into an expression vector; (b) transforming a host organism or cell with the vector; (c) culturing the transformed host cell under suitable conditions; and (d) harvesting the polypeptide. The isolated DNA molecule is preferably at least 10 nucleotides in length, and the method may optionally include subjecting the polypeptide to post-translational modification. The host cell can be a bacterium, a yeast, a protozoan, or a cell derived from a multicellular organism such as a fungus, an insect cell, a plant cell, or a mammalian cell.
Owner:SYMBICOM
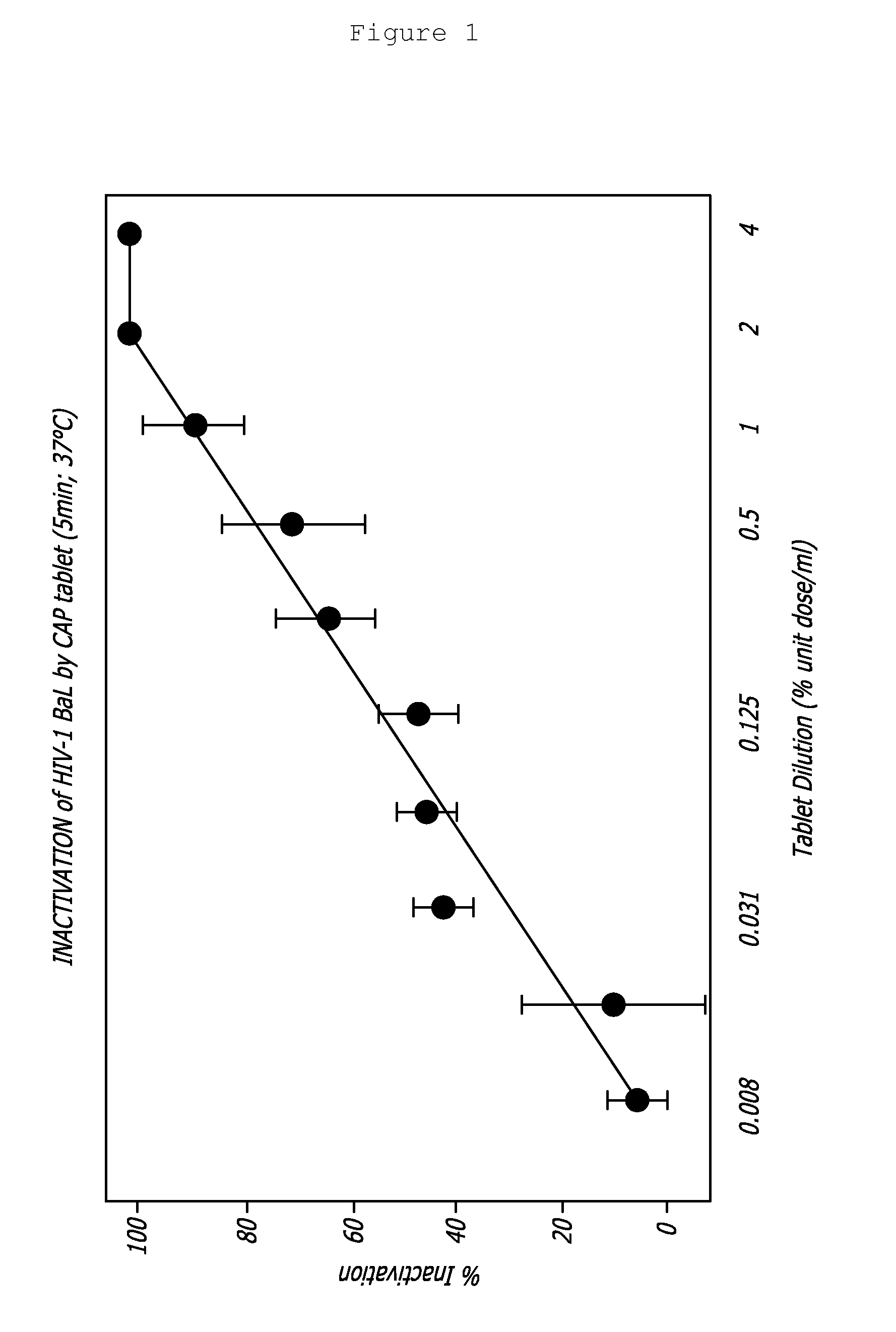
Rapidly dispersible vaginal tablet that provides a bioadhesive gel
InactiveUS20110159091A1Safe and relatively inexpensive methodAvoid spreadingAntibacterial agentsBiocideBacterial vaginosisSpiroplasma
A tablet for insertion into a vagina including 0.01 to 500 mg of a vaginal medication, such as a microbicide, such as cellulose acetate 1,2-benzenedicarboxylate (CAP); 100 to 500 mg of mannitol powder; 50 to 300 mg of inert microcrystalline cellulose; 10 to 80 mg of hydroxypropyl methylcellulose; 50 to 250 mg of glycerol and optionally 2 to 4 mg of at least one preservative which protects against microbicidal contamination and discourages the growth of yeast in the vagina. The tablet which includes CAP as the vaginal medication is vaginally administered before coitus in methods for preventing the sexual transmission of HIV-1, HIV-2, herpesvirus, or an infection caused by Neisseria gonorrhoeae, Chlamydia trachomatis, Trichomonas vaginalis, Haemophilus ducreyi or Treponema pallidum. The tablet which includes CAP as the vaginal medication is vaginally administered to prevent or treat bacterial vaginosis.
Owner:NEW YORK BLOOD CENT
Gene chip of main pathogenic microorganism in drinking water and testing kit
InactiveCN101748192ALow costImprove throughputMicrobiological testing/measurementAgainst vector-borne diseasesEscherichia coliBacteroides
The invention provides a gene chip of main pathogenic microorganism in drinking water and a testing kit, which mainly aims at 11 kinds of bacteria of colibacillus / Shigella, salmonella, vibrio cholera, vibrio parahaemolyticus, staphylococcus aureus, enterococcus faecails, pseudomonas aeruginosa, legionella pneumophilia, pneumobacillus, yersinia enterocolitica and the like, and L.interrogans. The gene chip comprises a solid phase carrier and a oligonucleotide probe fixed on the solid phase carrier, wherein the oligonucleotide probe contains gyrB gene with tremendous evolutionary advantage, ITS gene and DNA segment selected from 16srRNA gene or complementary DNA segment. The gene chip and the testing kit of the invention can test the main pathogenic microorganism in drinking water, and has the characteristics of simple operation, high throughput, high accuracy, strong repeatability and the like, and can be used for clinical test for the water quality monitoring department.
Owner:NANKAI UNIV
Diagnostic test for borrelia infection
InactiveUS6617441B1Inhibit digestionSugar derivativesAntibody mimetics/scaffoldsDiseaseLone star ticks
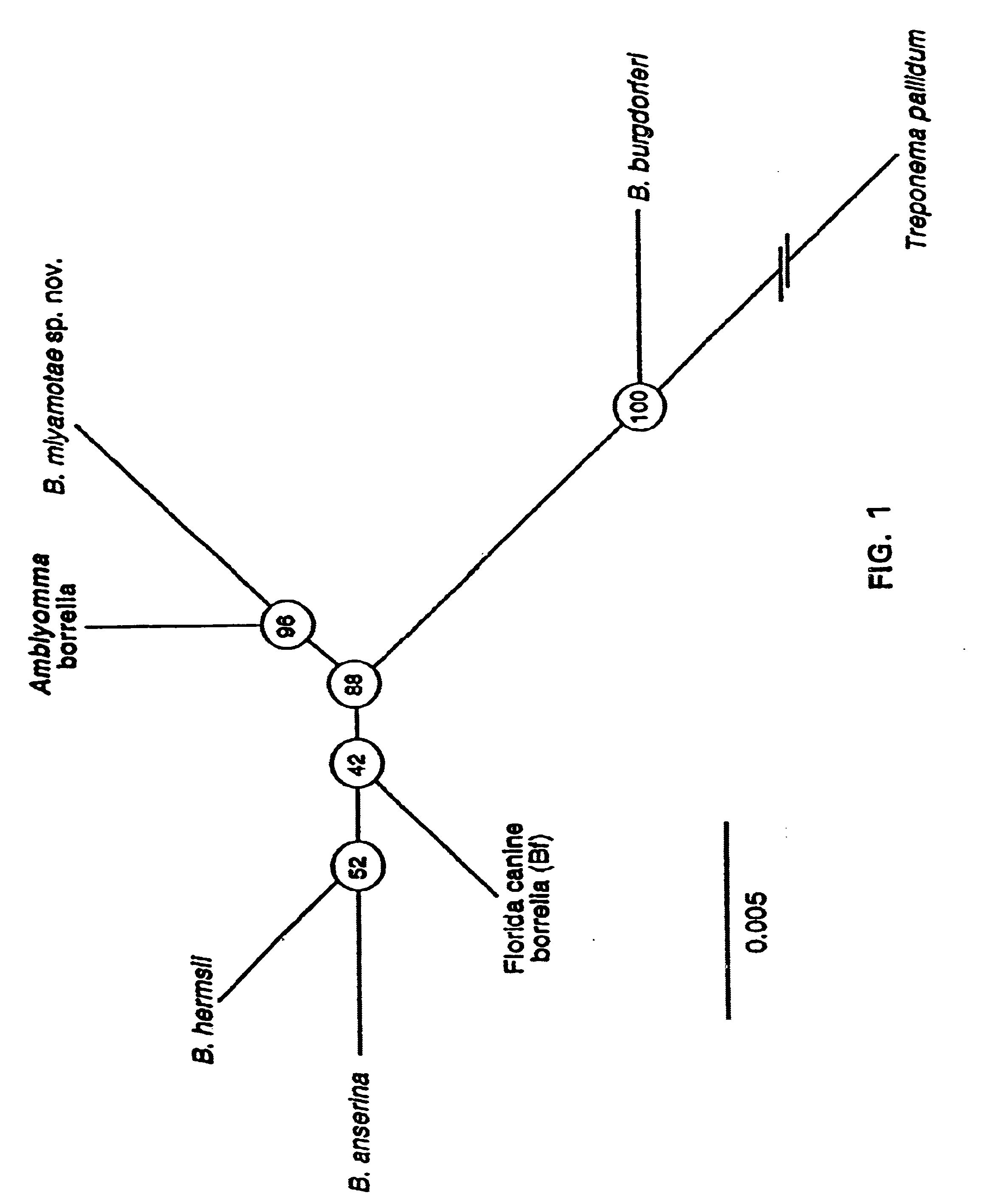
Bites from Amblyomma americanum, a hard tick, have been associated with a Lyme disease-like illness in the southeastern and south-central United States. Present in 2% of ticks collected in four states were uncultivable spirochetes. Through use of the polymerase chain reaction, partial sequences of the flagellin and 16s rRNA genes of microorganisms from Texas and New Jersey were obtained. The sequences showed that the spirochete was a Borrelia sp. but distinct from other known members of this genus, including B. burgdorferi, the agent of Lyme disease. Species-specific differences in the sequences of the flagellin protein, the flagellin gene and the 16s rRNA gene between the new Borrelia species and previously known species provide compositions and methods for assay for determining the presence of this new spirochete, or for providing evidence of past or present infection by this spirochete in animal reservoirs and humans.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Method for magnetic antibody immunoassay chemiluminescence detection of treponema pallidum antibodies
The invention belongs to the technical field of immunoassay and diagnosis and relates to a method for magnetic antibody immunoassay chemiluminescence detection of treponema pallidum antibodies. The method comprises the following steps of: using GoldMag particles as carriers, coating one or more antigens of specific recombinant proteins of treponema pallidum antibodies, TP15, TP17, TP47 and TP44.5, then adding a sample to be detected and enzyme labeled antigens, and finally adding luminescence substrates for luminescence detection. The GoldMag particle surface has a larger coupling capacity to the antigen and has characteristics of high specificity and no pollution of the enzyme linked immunoassay as well as high sensitivity of chemiluminescence, and therefore, the method has the advantages of high detection sensitivity, good specificity, wide linear range, no radioactive pollution and the like.
Owner:XIAN GOLDMAG NANOBIOTECH
Treponema pallidum antibody test kit and preparation method and detection method thereof
The invention relates to a treponema pallidum (TP) antibody test kit based on the flow microspheric carrier technology and a preparation method and detection method thereof, belonging to the technical field of immunoassay medical diagnosis. The preparation method comprises the following steps: using TP recombinant antigen to coat heavy polymer microsphere, using bovine serum albumin to seal empty binding site, preparing specific TP probe-heavy polymer microsphere; performing coculture with a sample to be tested to capture TP antibody, washing and centrifuging to remove the unbound TP antibody, then adding fluorescently-labeled anti-human IgG or IgM antibody; and using a flow cytometry to detect the fluorescence intensity of the microsphere, and performing qualitative or quantitative analysis to the tested antibody. The method has the advantages of high sensitivity and specificity and good stability and can be used to perform microanalysis or multivalent analysis to the sample.
Owner:NANJING UNIV OF TECH
Syphilis spirochete membrane antigen with shorten expression and uses thereof
InactiveCN101293919ASignificant antigen reactivityAntigen reactivity verificationBacteriaDepsipeptidesAntigenSpiroplasma
The invention discloses a DNA sequence expressing a truncated treponema pallidum membrane antigen and an amino acid sequence The membrane antigen is removed of the part having high homology with human fibronectin, so as to avoid false positive and improve the specificity of the serological test for diagnosis of treponema pallidum infection. The invention also discloses the application of the membrane antigen in preparing diagnostic reagents for detecting treponema pallidum infection.
Owner:ARMY MEDICAL UNIV
Herbal disinfectant for space spray disinfection
InactiveCN102792976AEasy to usePrevent the spread of epidemicsBiocideFungicidesMedicinal herbsSpiroplasma
The invention discloses a herbal disinfectant for space spray disinfection. The herbal disinfectant is composed of fourteen pharmaceuticals including rhizoma atractylodis, rhubarb, coptidis rhizoma, herba artemisiae scopariae, radix angelicae dahuricae, cyrtomium rhizome, sweet wormwood herb, betel nuts, cloves, costustoot, borneol, mint, chrysanthemum, and agastache. The medicinal materials selected for the herbal disinfectant for space spray disinfection of the present invention have the functions of clearing heat and removing toxicity, removing miasmic toxin and dispelling filth, restoring consciousness and opening the orifices, supporting body healthy qi, and fending off evil. The volatile oils, alkaloids and other active ingredients contained in the medicinal materials have different levels of direct inhibition or killing function for a variety of bacteria, viruses, fungi, and spirochetes, mycoplasma, etc. The present invention utilizes a scientific method to extract total extract from the medicinal materials for preparing the space spray medicament, and has a good disinfection effect.
Owner:张宜东
Oral vaccine for Borrelia
The present invention relates to vaccines for control of Borrelia infections in animal and human populations. In particular, the present invention provides compositions and methods comprising recombinant bacteria engineered to express one or more Borrelia burgdorferi antigens for use as Lyme disease vaccines. In some embodiments, the recombinant bacteria are freeze-dried.
Owner:US BIOLOGIC INC
General trivalent gene recombination vaccine for preventing infection of leptospira interrogans of different sero-groups and preparation method thereof
InactiveCN101874898ASolve the disadvantages of no cross protectionSolve the disadvantages of large side effectsAntibacterial agentsBacterial antigen ingredientsSpiroplasmaNucleotide
The invention relates to a trivalent gene recombination vaccine for preventing leptospirosis and a preparation method thereof and aims to effectively prevent infection of leptospira interrogans of different sero-groups with low manufacturing cost. The technical scheme is that: the trivalent gene recombination vaccine for preventing the infection of leptospira interrogans of different sero-groups consists of a lipL32 / 1 gene, a lipL21 gene and an ompL1 / 2 gene cloned from a leptospira icterohemorrhagiae genome; a fusion gene lipL32 / 1-lipL21-ompL1 / 2 prepared by a technique for gene engineering has a nucleotide sequence and an amino acid sequence shown in a sequence 5; and the lipL32 / 1 gene is connected with the lipL21 gene, and the lipL21 gene is connected with the ompL1 / 2 gene through two same flexible peptides shown in a sequence 4.
Owner:严杰
Isothermal chain multiple detection card of pathogen nucleic acid
ActiveCN102230032AAvoid cross contaminationAvoid false positivesMicrobiological testing/measurementSpiroplasmaQuarantine
The invention discloses an isothermal chain multiple displacement detection card of pathogen nucleic acid. The isothermal chain multiple displacement detection card of the pathogen nucleic acid utilizes the detection card which is prepared through a nucleic acid isothermal chain displacement method and a colloidal gold detection technology to carry out multiple detection on HBV-DNA, HCV-RNA, HIV-RNA, and TP-DNA. The isothermal chain multiple displacement detection card of the pathogen nucleic acid has the characteristics of simple and rapid operation, high sensitivity, and no need of professional equipment. The pathogen nucleic acid to be detected is not amplified in the detection process, so the detection card has the advantages of preventing amplified matter cross contamination in laboratories and preventing false positivity, can be widely used in high sensitivity pathogen nucleic acid detection in the fields of clinical detection, inspection and quarantine, infectious disease control, biological technology and the like, and has a wide application prospect.
Owner:武汉中科志康生物科技有限公司
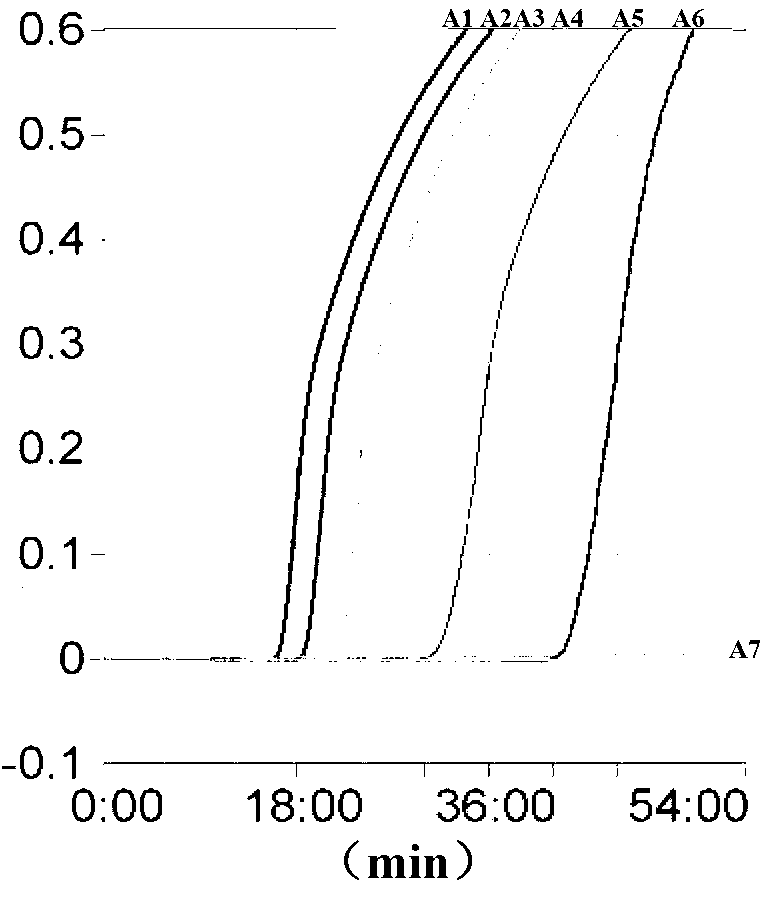
Loop-mediated isothermal amplification method for detecting lyme disease spirochete
ActiveCN102703587AImprove featuresHigh sensitivityMicrobiological testing/measurementAgainst vector-borne diseasesSerum igeSpiroplasma
The invention provides a loop-mediated isothermal amplification method for detecting lyme disease spirochete. DNA (deoxyribonucleic acid) of a sample to be detected is subjected to constant temperature amplification reaction by adopting a FIP (forward inner primer) and a BIP (backward inner primer) and F3 and B3 (shown in Seq ID No.1-4). According to the loop-mediated isothermal amplification method, a LAMP (loop-mediated isothermal amplification) primer constant-temperature amplification technique is used for fast detecting the lyme disease spirochete, and the lyme disease spirochete can be accurately detected from suspected patient serum. The specificity and sensitivity of the method are higher than those of the common PCR (polymerase chain reaction). Different virulence genes of lyme disease spirochetes can be detected, and the method has significance in the aspects of early diagnosis and early treatment of the lyme disease and the like. Investment on an expensive apparatus can be avoided and the method is convenient for grass-root popularization and use.
Owner:ICDC CHINA CDC
Lyme disease spirochaete detection RPA primer and probe and detection method thereof
InactiveCN105524989AImprove featuresHigh sensitivityMicrobiological testing/measurementAgainst vector-borne diseasesSerum igeSpiroplasma
The present invention provides a lyme disease spirochaete detection RPA primer and probe, the RPA primer is designed on the basis of lyme disease spirochaete recA gene, and primer sequences are as shown in Seq ID No: 1 and 2. The probe is designed on the basis of the RPA primer, and has a size of 46-52bp, 5 'end of the probe is marked with FAM, 3' end of the probe is modified by C3Spacer, and dSpacer or THF modification is performed at a position 30bp apart from the 5 'end of the probe. The present invention further provides a kit comprising the RPA primer and the probe. The present invention further provides a lyme disease spirochaete detection RPA method baed on the RPA primer and the probe. By combined use of RPA primer isothermal amplification technology and lateral flow chromatography test paper, lyme disease spirochaetes can be quickly and accurately detected from serum of suspicious patients. The specificity and sensitivity of the method are higher than that of conventional PCR, the method can be used for detecting lyme disease spirochaetes of different pathogenic genotypes, has important significance for lyme disease early diagnosis and treatment and other aspects of the lyme disease, can eliminates excessively high instrument cost, and is easy to promote and use in grassroots.
Owner:ICDC CHINA CDC
Compositions and methods for administering Borrelia burgdorferi antigens
InactiveUS7094391B1Induce immune responseOrganic active ingredientsPeptide/protein ingredientsOral medicationSpiroplasma
Mucosal administration of OspA and compositions therefor are disclosed and claimed. More particularly, oral administration of OspA and compositions therefor for eliciting an immunological response against Borrelia burgdorferi, such as a protective response preventive of Lyme disease are disclosed and claimed. Thus, oral Lyme disease vaccines or immunological compositions and methods of use are disclosed and claimed.
Owner:THE UNIV OF TEXAS SYST
Quick detection reagent for syphilis leptospira antigen and preparation method thereof
The related fast detection agent for syphilis spirochete antibody comprises a fast detection paper prepared by cellulose nitrate covered by pure syphilis spirochete monoclonal antibody and dried gold-mark monoclonal antibody. Wherein, with colloid gold immune chromatography technology and double antigen sandwich way, detecting syphilis spirochete and its fragment in humor, secretion and culture. This invention has more clinic meaning, high specificity, well sensitivity, and fit to wide spread.
Owner:吴学记
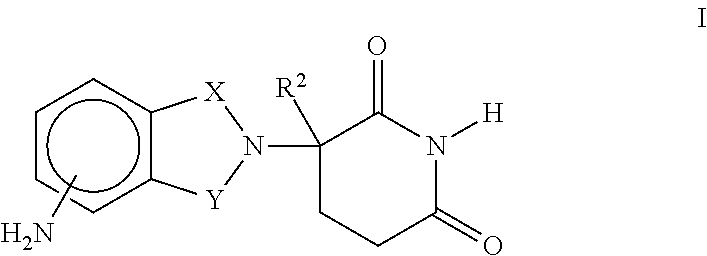
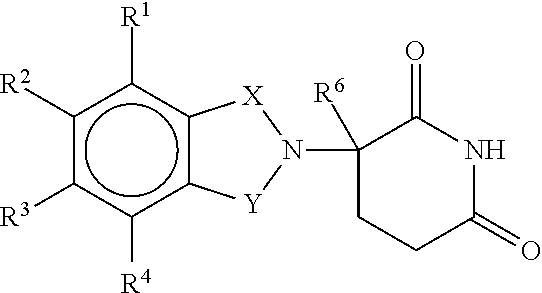
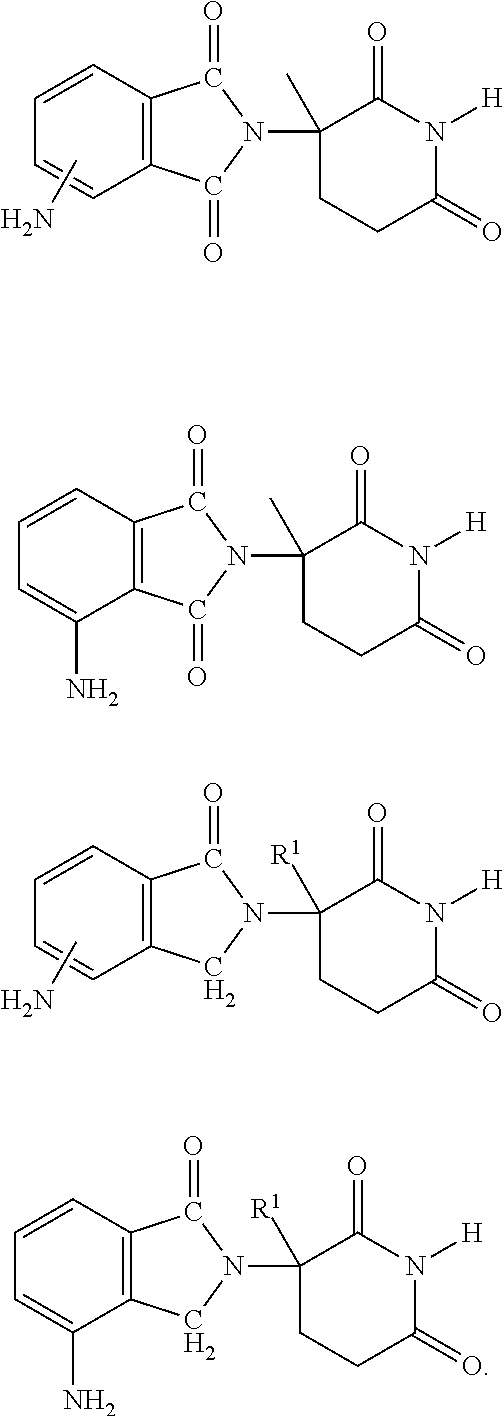
Methods and Compositions Using Immunomodulatory Compounds for the Treatment and Management of Spirochete and Other Obligate Intracellular Bacterial Diseases
Methods of treating, preventing and / or managing a spirochete and / or other obligate intracellular bacterial disease or disorder are disclosed. Specific methods encompass the administration of an immunomodulatory compound alone or in combination with a second active agent.
Owner:CELGENE CORP
Production of OspA for Lyme Disease Control
InactiveUS20110117131A1Avoid developmentAntibacterial agentsBacterial antigen ingredientsBiotechnologyOral medication
The present invention relates, generally, to the production of one or more OspA proteins in plant cells. Heterologous DNA comprising genes encoding one or more desired OspA protein(s) are introduced into plant cells. The one or more OspA protein(s) can be recombinantly-produced in the plant cells, optionally purified from the plant cells, and used as an oral vaccine to prevent the transmission of Lyme disease, particularly by animal vectors. The recombinantly-produced OspA protein(s) can be provided in oral and parenteral formulations. The present invention also relates to oral administration of OspA protein(s) to vaccinate against Lyme disease. The OspA protein(s) may be provided in a dosage form that is suitable for oral administration as a vaccine to prevent an animal from developing Lyme disease after exposure to a source of Borrelia burgdorferi.
Owner:VENTRIA BIOSCIENCE
Spiroplasma pathogenic microorganism PCR fast checking technique
InactiveCN1733936AQuick checkIncreased sensitivityMicrobiological testing/measurementMaterial analysis by electric/magnetic meansPathogenic microorganismSpiroplasma
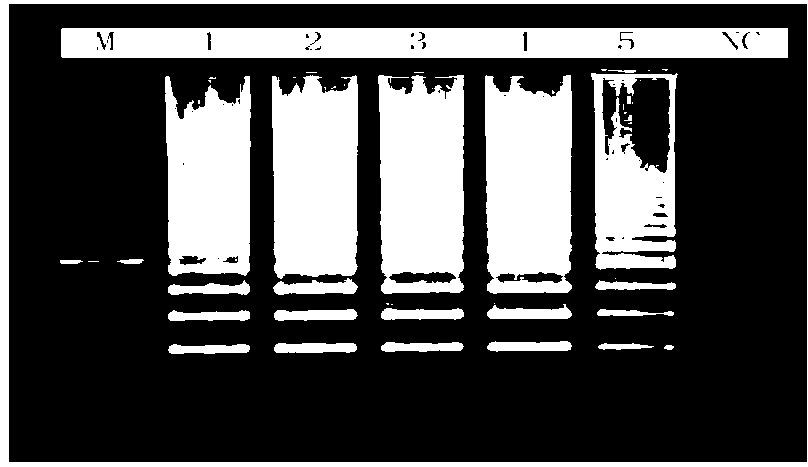
Disclosed is a PCR fast detection technique for Spiroplasma pathogen microorganisms comprising the following steps: (1) extracting template DNA in the detected sample with Chelex-100 method, (2) proceeding polymerase chain reaction with the 16S rDNA specific primer of the Spiroplasma, (3) subjecting the PCR product to electrophoresis detection, wherein the existence of Spiroplasma can be determined by the emergence of destination electrophoresis strip. The destination electrophoresis strip is at the position of 271bp, it can product certain diagnosis within 2-4 hours, and can carry out further semi-quantitative determination.
Owner:NANJING NORMAL UNIVERSITY
Method for detecting Lyme disease pathogen in tick bodies and kit
ActiveCN101886113AIncreased sensitivityMicrobiological testing/measurementAgainst vector-borne diseasesSpiroplasmaElectrophoresis
The invention relates to a loop-mediated isotheral amplification method (LAMP) for detecting Lyme disease pathogen in tick bodies, which is characterized by taking 16S rRNA gene as the target gene, using special software to design the LAMP for detecting the primers of the Lyme disease pathogen-Borrelia burgdorferi in the tick bodies, then selecting the primer of the specific fragment of the Lyme disease pathogen from the primers, extracting DNA after piercing the ticks to be detected, uniformly mixing the obtained DNA and the reaction buffer, the reaction mixture of the selected primer of the specific fragment and DNA polymerase to carry out amplification, adding loading buffer after activating the amplified product, then placing the mixture into agarose gel containing ethidium bromide to carry out electrophoresis detection and detecting whether the detected ticks carry the Lyme disease pathogen according to whether the specific band occurs after electrophoresis.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Diagnosis reagent for syphilis spirochete antibody
This invention discloses a diagnostic reagent of syphilis spirochete antibody enzyme labeling for diagnosing syphilis spirochete infection, containing synthetic peptide antigen or genetic engineering expressed antigen: antigen P15, P17, P47, 0.1-10 mg / ml coated on the enzyme labeling, the enzyme labeling two antigens (IgG, IgM): containing 1:20-1:100K their diluents solution and color-developing agent combination and solubilizer in which the said color-developing agent in the combination is TMB. The said combination can include combination of TMB with hydrogen peroxide cumon ir its derivatives or TMB with hydroxyurea. On the bases of applied synthetic peptide antigen and genetic engineering expressing antigen, a TMB saline is used in the diagnostic reagent with excellent water solubility and stable performance, matched with proper solubilizers, stabilizers to get much better stable performance.
Owner:肖洪武
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com