Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
202 results about "Positive contrast" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Positive contrast is an increase in the rate of responding in one setting as a result of a decrease in reinforcement (or an increase in punishment) in another setting.
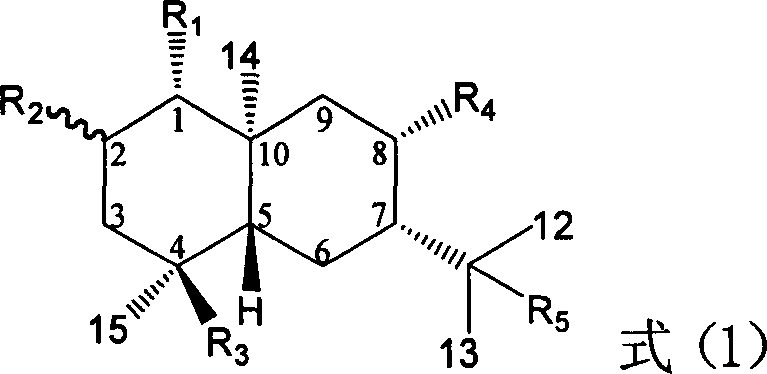
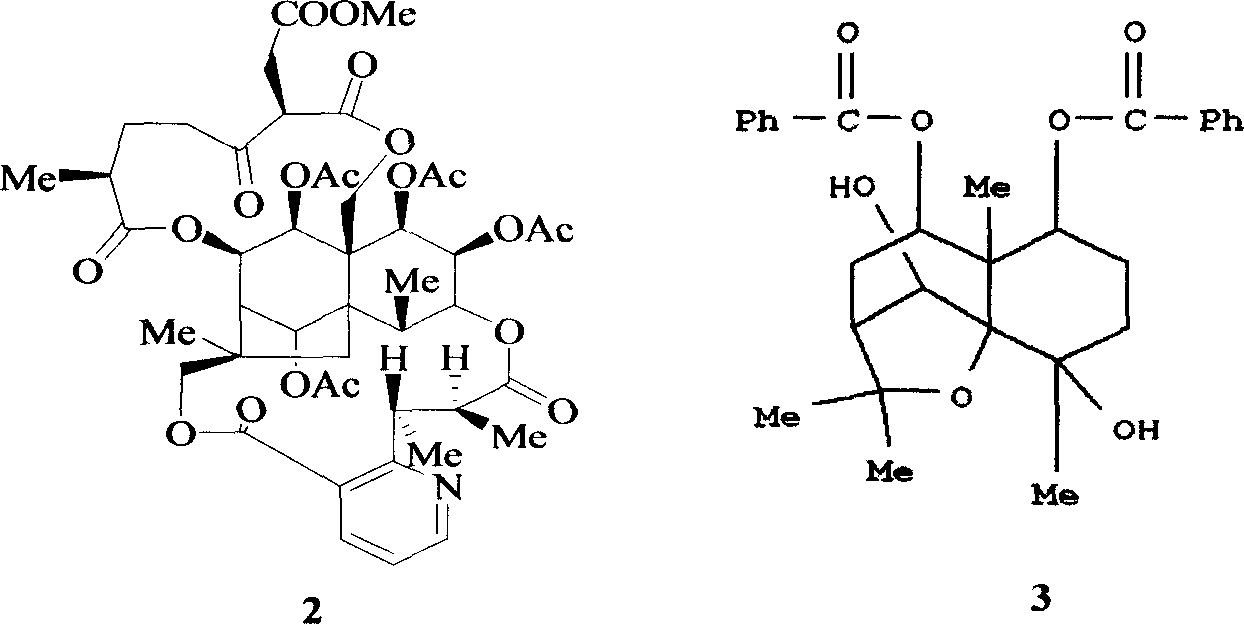
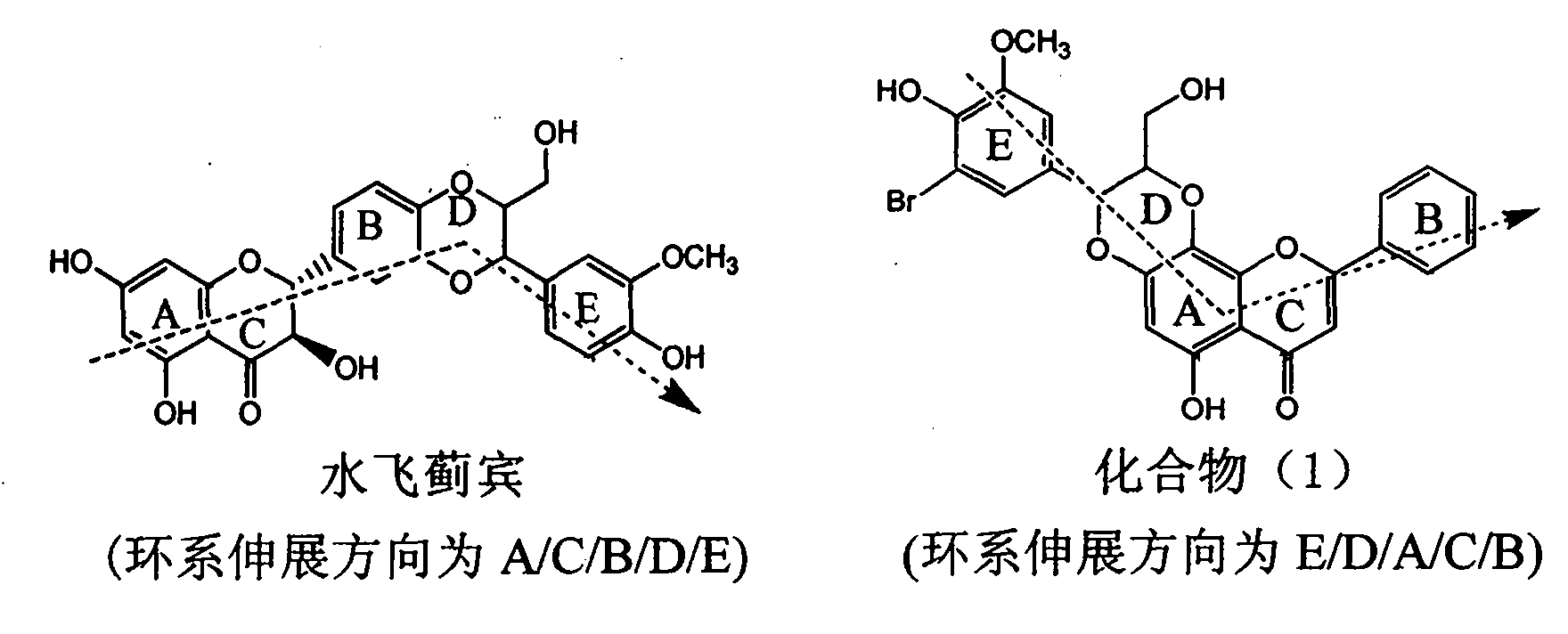
Pharmaceutical use of ent-eudesmane alcohol type sesquiterpene for inhibiting hepatitis virus
InactiveCN1935762APrevention and treatment of viral hepatitis BHBsAg reductionSugar derivativesHydroxy compound active ingredientsDiseaseSolvent
The invention relates to an enantiomorphic amine alkyl sesquiterpene alcohol and glucoside and the medicated salt or solvent thereof, as well as the effect and activity of the composed medicine combination, mainly relating to the medical use in reducing HBV-DNA replication activity. And it has considerably strong inhibiting effect on HBsAG screted by HepG2.2.15 and HBV-DNA replication as compared with positive contrast Lamivudine; and it has obvious inhibition activity to HBV-DNA replication at large dosage (100 mug / mL) and medium dosage(20 mug / mL) as contrasted with Lamivudine, and can be expected to apply to preparing medicines for curing HB virus infection disease.
Owner:赵昱
Medical use of 2 alpha, 3 beta-dihydroxy-5, 11(13)- diallyl eudesmane-12 acid for inhibiting hepatitis B virus
InactiveCN1923188APrevention and treatment of viral hepatitis BHBsAg reductionOrganic active ingredientsDigestive systemDiseaseDrug compound
The invention relates to a sesterterpane as formula (1) and relative medical drug or solvent, and relative drug compound, which can reduce hepatitis B surface antigen and restrain hepatitis B HBVDNA copy activity. Wherein, said invention has strong restrain on hepatitis B surface antigen (HBsAg) generated by HepG2.2.15 cell and the copy of hepatitis B deoxyribonucleic acid (HBV-DNA), while it restrain ability is higher than positive contrast difuradin; and the copy restrain activity at large amount (100 mug / mL) and middle amount (20 mug / mL) on the hepatitis B HBV-DNA are both higher then difuradin.
Owner:赵昱
Male multi-tumor marker detection protein chip and kit thereof
InactiveCN101603966AChemiluminescene/bioluminescenceBiological testingProtein markersProstate cancer
The invention discloses a male multi-tumor marker detection protein chip and a kit thereof. The chip comprises a substrate, protein markers distributed in an array type and point coatings of contrasts, wherein the substrate is a glass substrate or a film substrate; and the tumor markers and the point coatings of the contrasts are seven protein markers of AFP, CEA, NSE, CYFRA21-1, CA19-9, tPSA and SCC-ag, a positive contrast and a negative contrast which are uniformly distributed and latticed on the substrate. A reaction result of various indexes can be obtained only through one reaction by utilizing the protein chip, and the following tumors can be simultaneously screened: primary liver cancer, prostatic cancer, pancreatic cancer, lung cancer, esophageal cancer, gastric cancer and colorectal cancer. The invention is particularly suitable for the general examination of malignant tumors of male asymptomatic groups and high risk groups.
Owner:上海裕隆生物科技有限公司
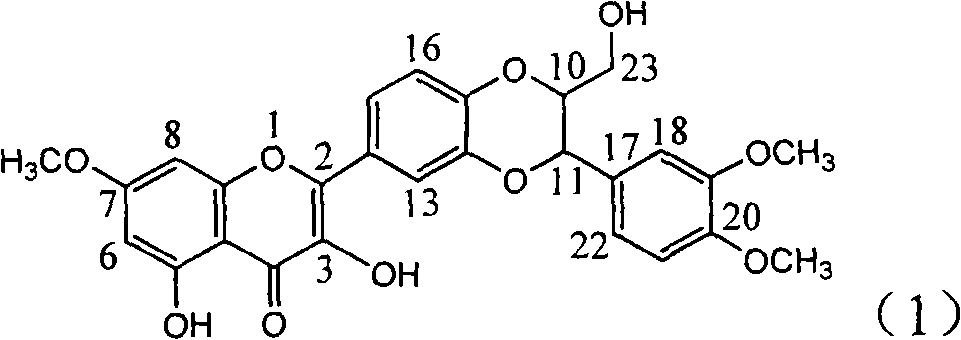
Use of acetamide dehydrogenation silibinin as medicament for treating viral hepatitis B
InactiveCN101829091APowerful removalInhibitory activityOrganic active ingredientsDigestive systemAntigenDisease
The invention relates to the use of acetamide dehydrogenation silibinin as a medicament for treating viral hepatitis B, in particular to the use of dehydrogenation silibinin esters flavonoid lignanoid replaced by A ring methoxy formyl amine or pharmaceutically acceptable salt as the medicament for eliminating HBsAg (hepatitis B surface antigen) and HBeAg (hepatitis Be antigen) and restraining copy of HBV DNA. The cetamide dehydrogenation silibinin can obviously restrain the HBsAg and HBeAg activity, and the strengths for eliminating the HBsAg and HBeAg are 90.5% and 63.6% at the concentration of 20 microgramme / milliter and are 5.6 times and 3.8 times more than positive contrast medicament alpha-interferon. Meanwhile, the restraining rate to the HBV DNA is 90.4% at the concentration, is 12% higher than lamivudine, and is 2.4 times more than a- interferon. Therefore, the flavonoid lignanoid or the pharmaceutically acceptable salt can be expected for treating hepatitis B virus infection as the non-nucleoside medicament.
Owner:DALI UNIV
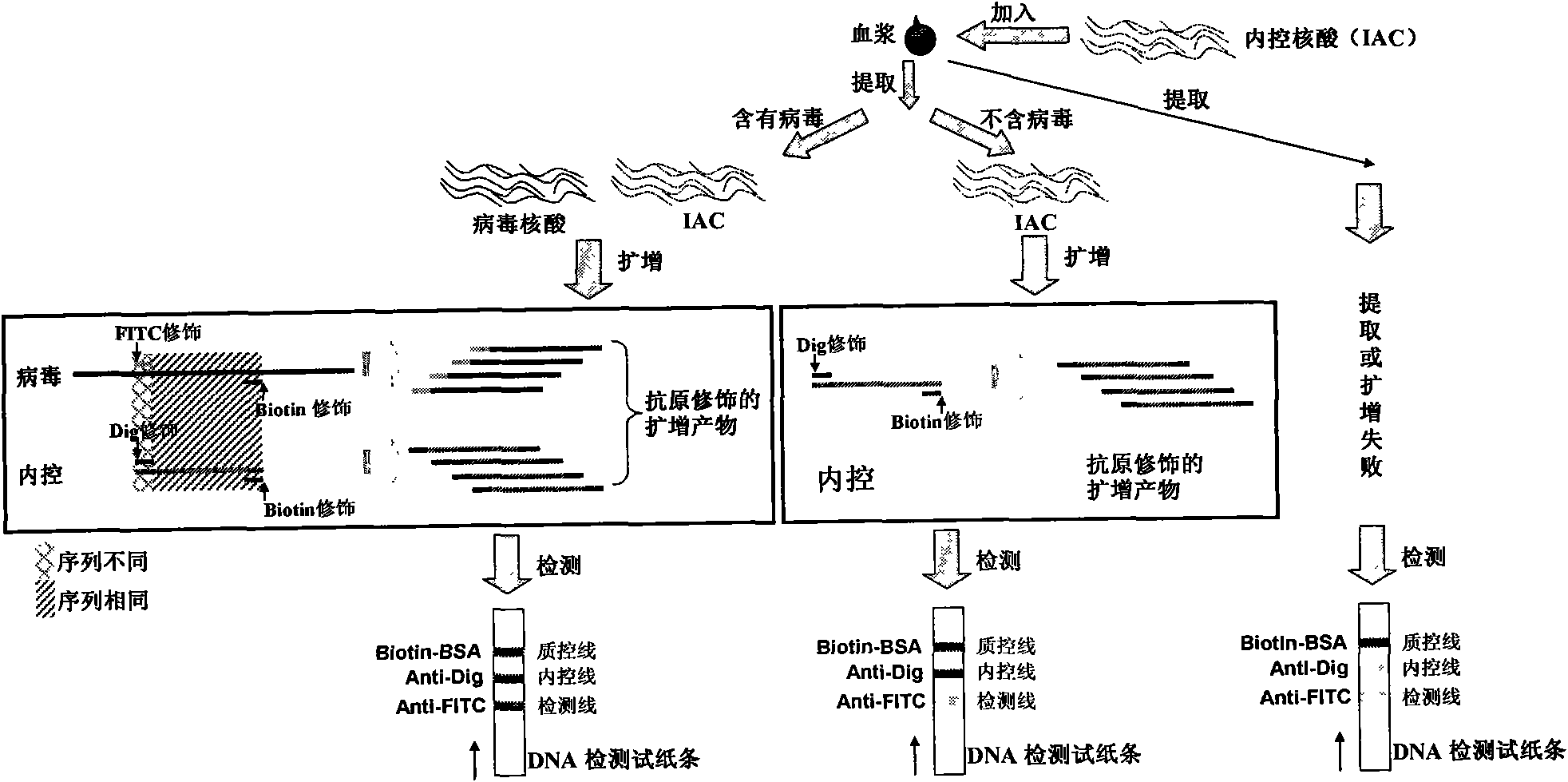
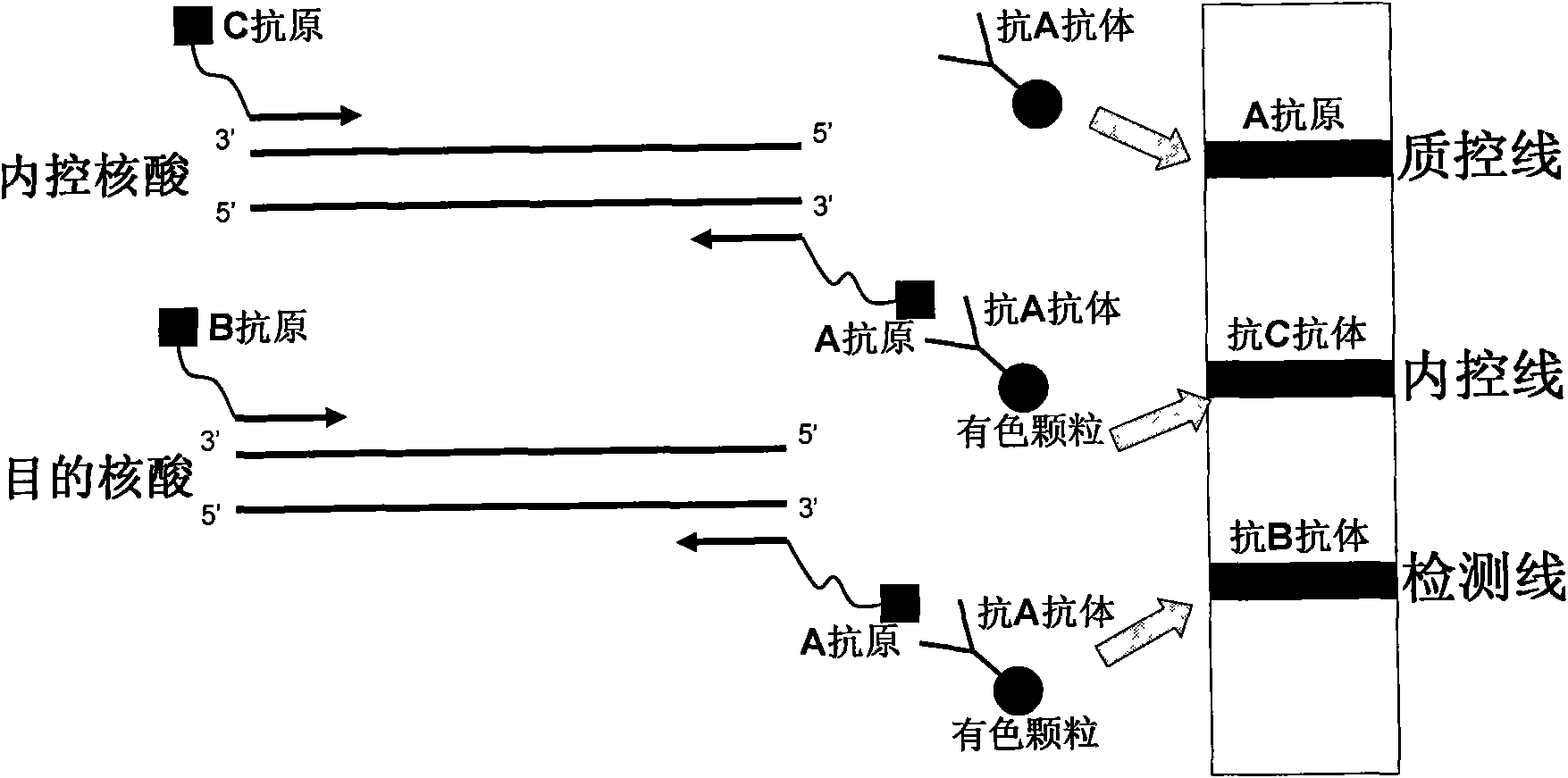
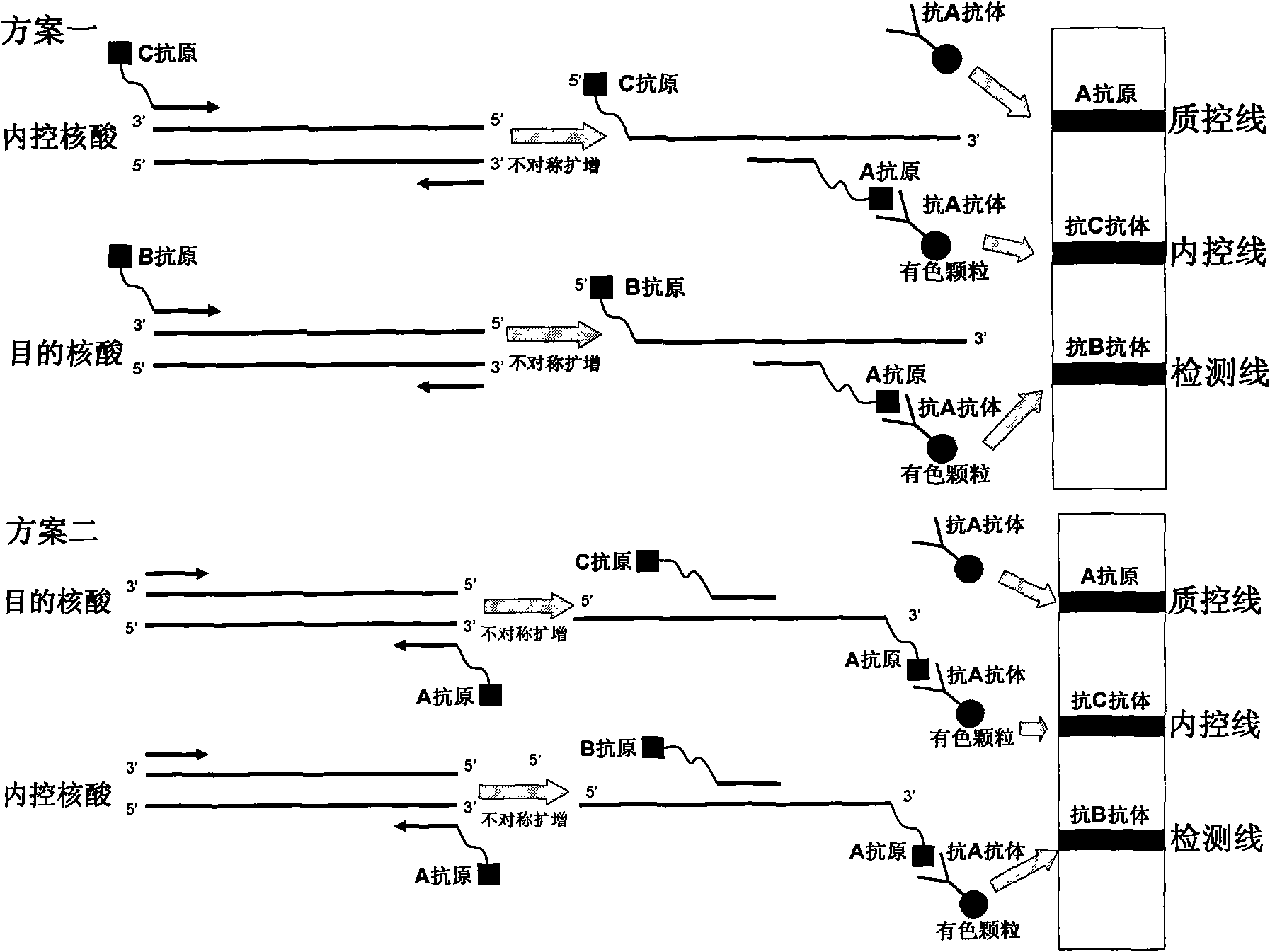
Method for semi-quantitatively detecting pathogenic nucleic acid by adding internal control nucleic acid
InactiveCN101957373AAvoid diagnostic problems that are prone to false negativesAvoid problems prone to false negativesMicrobiological testing/measurementMaterial analysisTest sampleQuality control
The invention belongs to the field of nucleic acid detection and discloses a method for semi-quantitatively detecting pathogenic nucleic acid by adding internal control nucleic acid. Corresponding internal control is added in the whole process of extracting and amplifying target nucleic acid and testing by using a test paper, so that the internal control and a target segment are parallelly operated, and the semi-quantitative detection is performed finally through color development and intensity contrast of three strips, namely a detection line, an internal control line and a quality control line on the test paper. In the method, in the whole process of processing the target nucleic acid, the corresponding internal control is taken as a positive contrast, and false negative results due to links such as extraction, amplification or sample application errors are avoided in the processing of detecting by using the test paper. Meanwhile, by comparing color development intensity of the internal control line and a sample line and introducing the semi-quantitative function on the basis of the qualitative function of the immunochromatographic test paper to estimate the copy number of tested samples, the detection results are more detailed, accurate and reliable. The method has the advantages of convenient and quick operation and capacity of meeting the actual clinical requirement.
Owner:HUADONG RES INST FOR MEDICINE & BIOTECHNICS
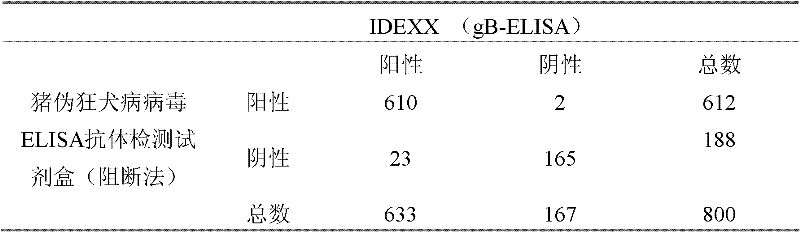
Kit for detecting pig pseudorabies virus antibodies and block enzyme-linked immuno sorbent assay (ELISA) method
The invention discloses a kit for detecting pig pseudorabies virus antibodies and a block enzyme-linked immuno sorbent assay (ELISA) method. The kit for detecting pig pseudorabies virus antibodies comprises pig pseudorabies virus monoclonal antibodies which are labelled by horseradish peroxidase, wherein the pig pseudorabies virus monoclonal antibodies are monoclonal antibodies obtained by pig pseudorabies viruses as immunogens and the pig pseudorabies viruses are pseudorabies virus strain Ea. The kit for detecting pig pseudorabies virus antibodies also comprises an enzyme label plate, a sample diluent, negative and positive contrasts, a coloured solution, a washing solution, and a stopping solution. The block ELISA method comprises the following steps of 1, taking out a detection plate pre-coated with virus antigens from the kit for detecting pig pseudorabies virus antibodies, adding diluted blood serum needing to be detected into the detection plate pre-coated with the virus antigens, and simultaneously, setting negative and positive contrast apertures, 2, shaking up the diluted blood serum in the negative and the positive contrast apertures, shaking off a solution in the negative and the positive contrast apertures, and washing the detection plate by the washing solution, and 3, adding the pig pseudorabies virus monoclonal antibodies labelled by horseradish peroxidase into the negative and the positive contrast apertures, washing, adding the colored solution into the negative and the positive contrast apertures to carry out room-temperature coloration in the dark, adding the stopping solution into the negative and the positive contrast apertures, and determining OD630nm values of the negative and the positive contrast apertures by an ELISA apparatus. The block ELISA method has the advantages of good singularity, high sensitivity, short detection time, and high accuracy because of utilization of an S / N ratio method in result determination.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
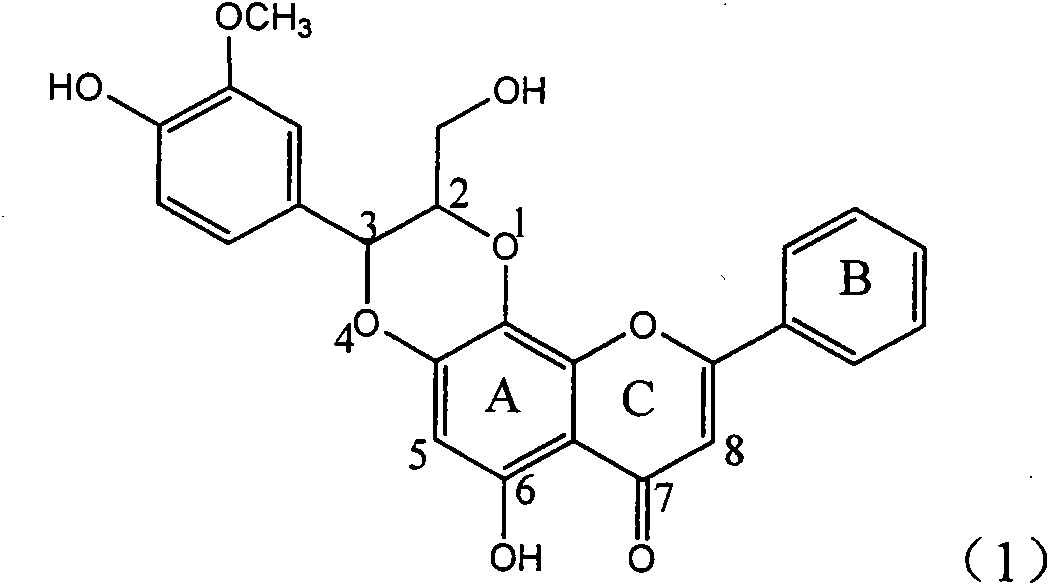
Application of flavone lignan (+/-) Scutellaprostin A in preparing medicaments for treating viral hepatitis type B
InactiveCN101953827AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseaseLignan
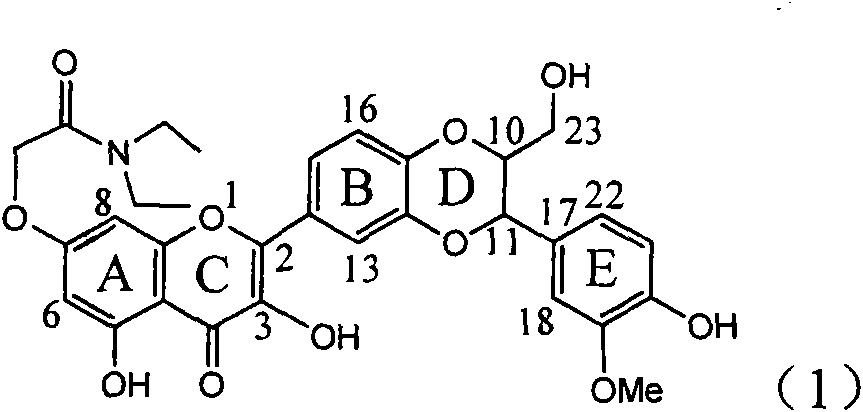
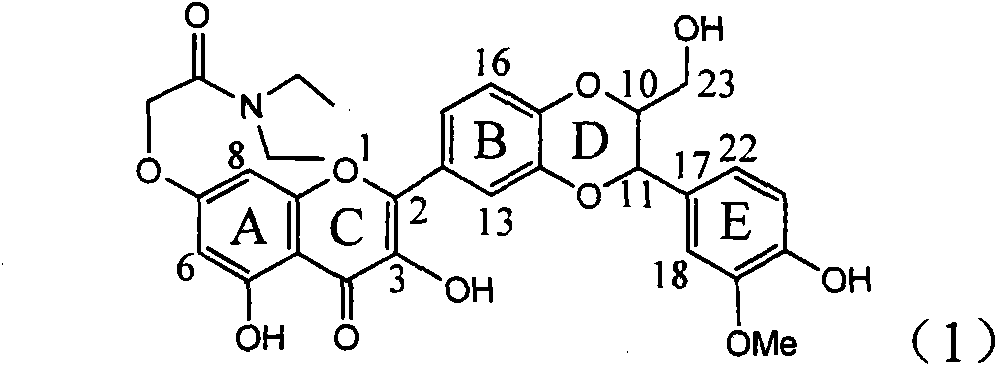
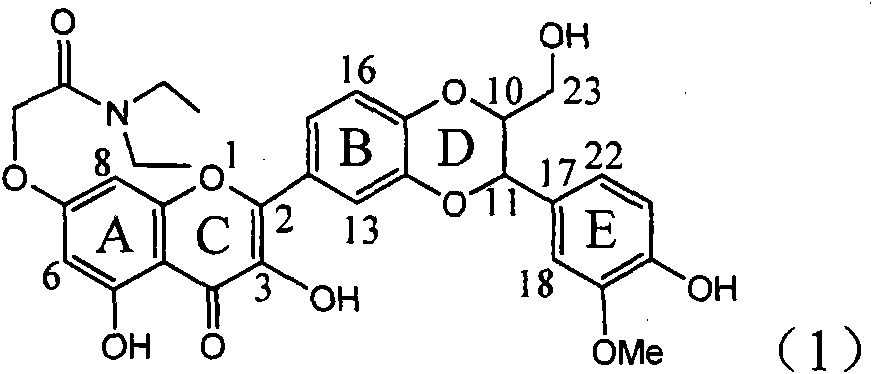
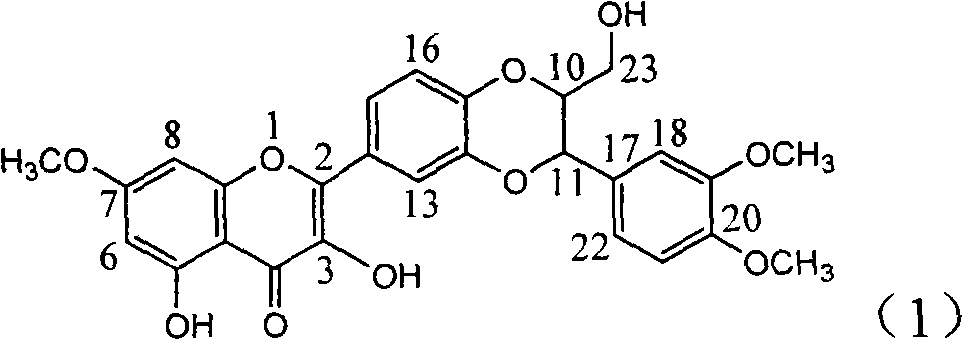
The invention relates to application of flavone lignan (+ / -) Scutellaprostin A in preparing medicaments for treating viral hepatitis type B, in particular to a compound with the formula (1) or pharmaceutically-acceptable salts thereof for preparing medicaments for clearing HBsAg and HBeAg and suppressing HBV (Hepatitis B Virus) DNA replication. In the invention, the intensities of the compound for clearing the HBsAg and the HBeAg under the concentration of 20 micrograms / milliliter respectively reach 81.8 percent and 81.9 percent, which are respectively 5.1 times and 4.8 times as high as the corresponding activity of alpha-interferon used as a positive contrast medicament; and what is more exciting, when the compound has the concentration, the compound performs a suppression ratio higher than 81 percent, and the value is also higher than that of both lamivudine and alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically-acceptable salts can be expectably used for preparing nucleoside medicaments for clearing the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infected diseases.
Owner:DALI UNIV
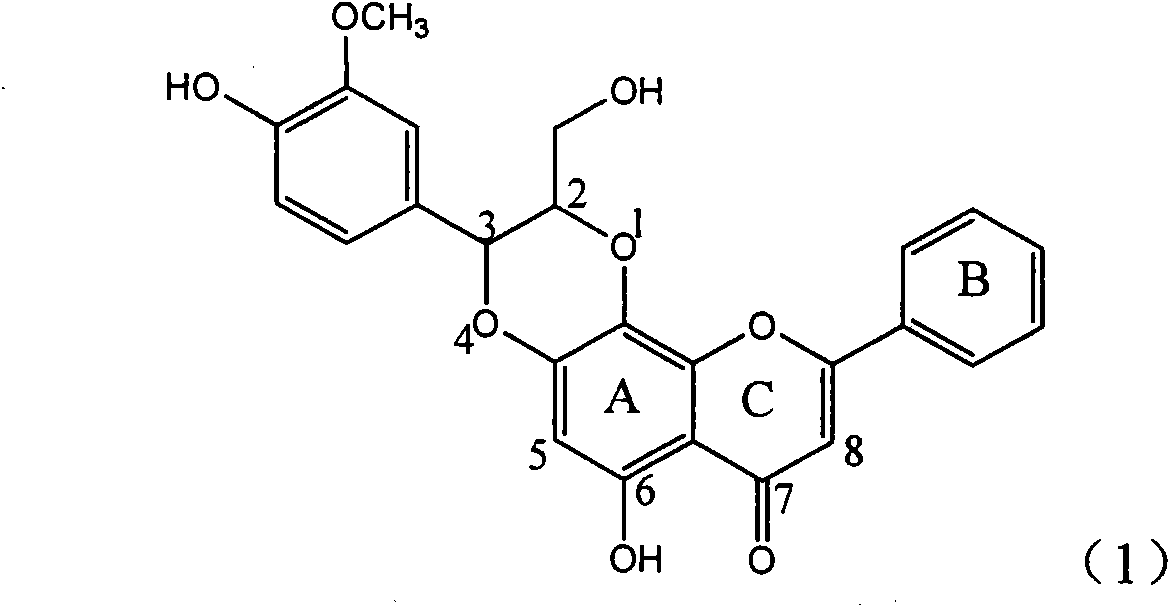
Application of dimethyl dehydrated silybin in preparing medicaments for treating virus hepatitis B
InactiveCN101912385AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseaseNucleoside Reverse Transcriptase Inhibitor
The invention relates to application of dimethyl dehydrated silybin in preparing medicaments for treating virus hepatitis B, in particular to the application of 7 and 20-position methyl substituted dehydrated silybin or pharmaceutically acceptable salts thereof in preparing medicaments for removing HBsAG and HBsAg and medicaments for inhibiting HBV DNA replication. The dehydrated silybin has remarkably HBsAg and HBeAg inhibiting activity, wherein the strength for removing the HBsAg and the HBeAg at the concentration of 20 milligram / milliliter is 88.9 percent and 84.1 percent respectively, which are 5.5 times and 5.0 times that of a positive contrast medicament. More importantly, the dehydrated silybin shows the HBV DNA inhibition ratio of about 99.6 percent at the concentration of 20 milligram / milliliter, the activity exceeds lamivudine by 23 percent, which is 2.6 times that of interferon. Therefore, favonolignan or pharmaceutically acceptable salts thereof can be predictably used for preparing the non-nucleoside reverse transcriptase inhibitor medicaments for removing the HBsAg and HBeAg, inhibiting HBV DNA replication and treating hepatitis B virus infection diseases.
Owner:DALI UNIV
Device for displaying detection result of specimen using visual sign
ActiveCN1828301ABioreactor/fermenter combinationsBiological substance pretreatmentsReagent stripAnalyte
The device comprises: a sample application zone, a reagent zone, a detection zone with positive and negative contrast zones and the combination zone for detected target, and a reagent strip. Wherein, the positive contrast zone comprises one or more material to show the first color on dried condition and the second color when on wet condition. If there is detected target in sample, the interactive action of the combination zone and contrast zone forms the legible symbols.
Owner:雅培快速诊断国际无限公司
Rapid diagnostic kit based on loop-mediated isothermal amplification technique for hepatitis A virus genes and detection method thereof
ActiveCN101660005AHigh sensitivityEasy to identifyMicrobiological testing/measurementMicroorganism based processesHepatitis A virusesReverse transcriptase
The invention discloses a rapid diagnostic kit based on a loop-mediated isothermal amplification technique for hepatitis A virus genes and a detection method thereof. The kit comprises two pairs of primers, Bst DNA polymerase, revertase, an RNase inhibitor, a stabilizing solution, a reaction solution, a chromogenic solution and a positive contrast solution, wherein the nucleotide sequences of thetwo pairs of primers are shown in SEQ ID NO: 1-4; and the eight solutions are respectively contained in containers. The kit and the detection method can detect the hepatitis A virus with high efficiency and high specificity, are based on the loop-mediated isothermal amplification technique, apply six segments, four primers and one constant temperature to complete an amplification reaction within less than one hour, and have the advantages of low detection cost, short time consumption, high yield, high specificity, significant chromogenic difference between a positive result and a negative result, high authentication rate, distinctness and reliability.
Owner:GUANGZHOU HUAFENG BIOTECH
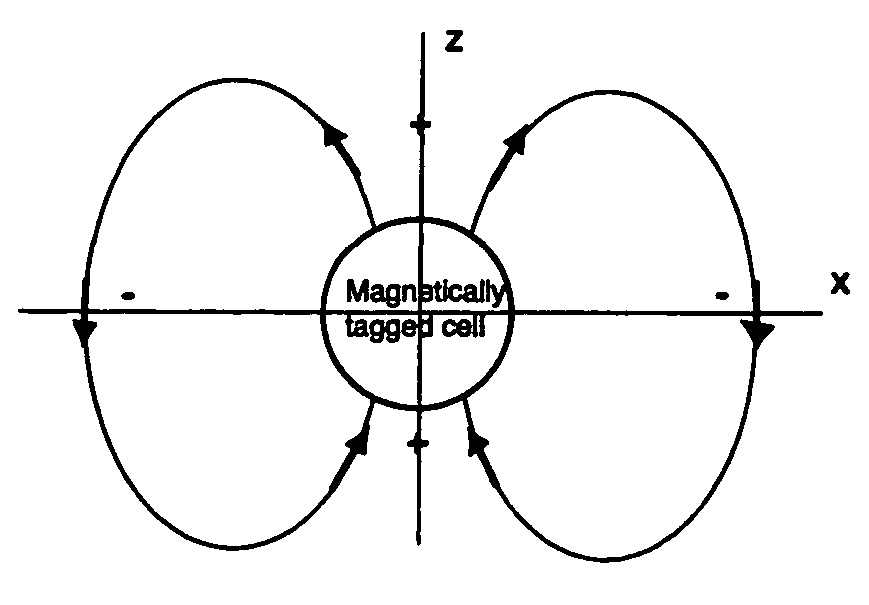
Positive contrast MRI of magnetically tagged cells, objects, tissues
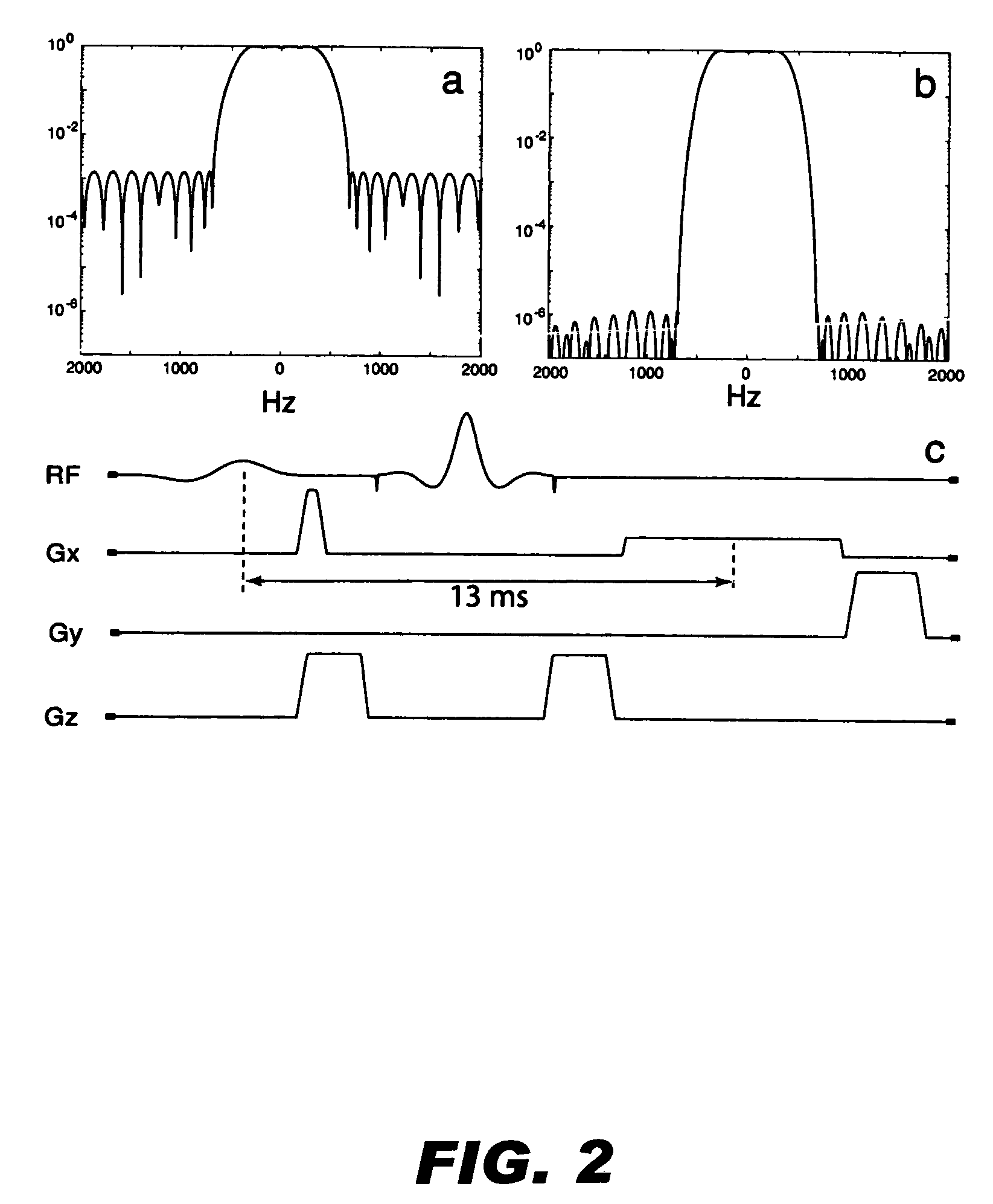
ActiveUS20050261575A1Positive contrastReadily apparentDiagnostic recording/measuringSensorsNegative Contrast AgentMagnetic marker
Contrast agents incorporating super-paramagnetic iron-oxide (SPIO) nanoparticles have shown promise as a means to visualize labeled cells using MRI. Labeled cells cause significant signal dephasing due to the magnetic field inhomogeneity induced in water molecules near the cell. With the resulting signal void as the means for detection, the particles are behaving as a negative contrast agent, which can suffer from partial-volume effects. Disclosed is a new method for imaging labeled cells with positive contrast. Spectrally-selective RF pulses are used to excite and refocus the off-resonance water surrounding the labeled cells so that only the fluid and tissue immediately adjacent to the labeled cells are visible in the image. Phantom, in vitro, and in vivo experiments show the feasibility of the new method. A significant linear correlation (r=0.87, p<0.005) between the estimated number of cells and the signal has been observed.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Treponema pallidum antibody diagnostic kit and preparation method thereof
The invention belongs to the technical field of immunologic diagnosis, in particular to a treponema pallidum antibody diagnostic kit by a chemiluminescence method and a preparation method thereof. The kit comprises an anti-TP test reaction plate, an anti-TP test enzyme complex, chemiluminescence substrate liquid, concentrated washing liquor, a negative contrast and a positive contrast. The invention also discloses a preparation method of the diagnostic kit, which adopts a chemiluminescence immunoassay technology; compared with ELISA (enzyme-linked immuno sorbent assay), the method has higher sensitivity and specificity, is suitable for the auxiliary diagnosis of clinical syphilis and screening of blood donors and fills a blank of the production of a treponema pallidum antibody diagnostic reagent detected by the domestic chemiluminescence method.
Owner:威海威高生物科技有限公司
Immunologic diagnosis kit for detecting type II dengue virus NS1 antigen
ActiveCN101226196AAccurate detectionQuick checkMaterial analysisAgainst vector-borne diseasesSerotypeElisa test
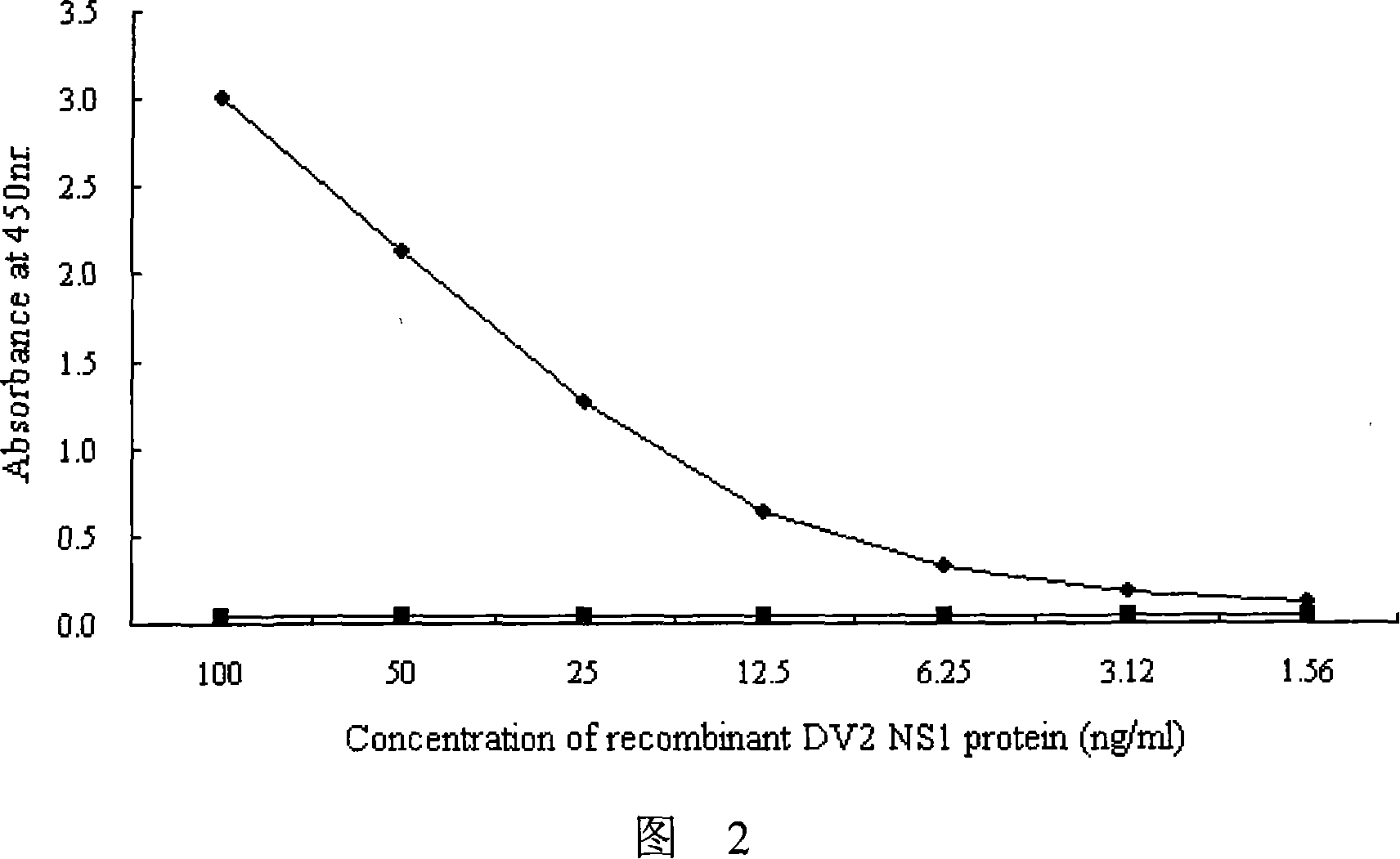
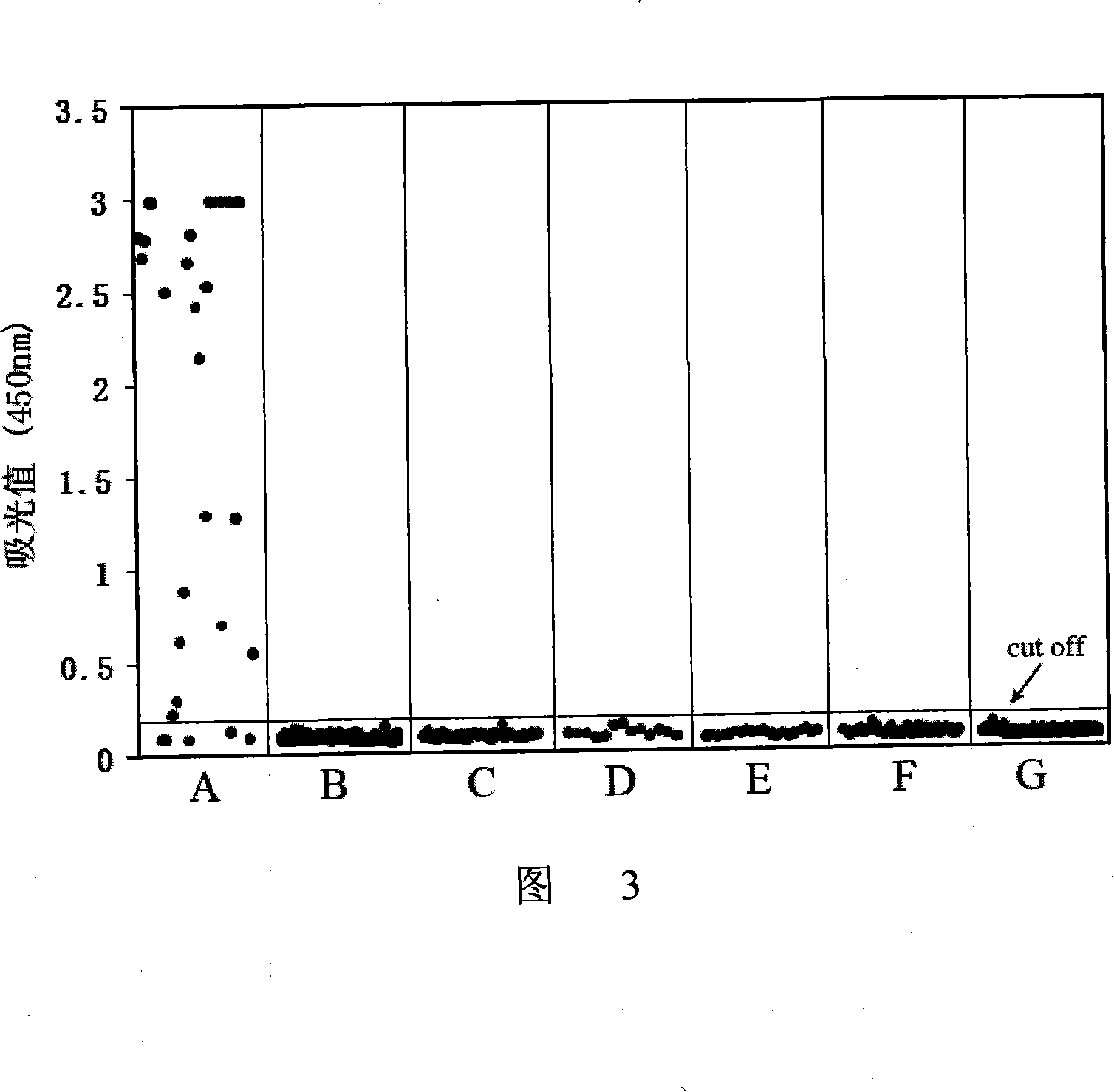
The invention provides an immunity diagnosis test kit for detecting II-type dengue virus antigen, which comprises a porous reaction plate covering monoclonal antibody DV2-M6, a sample treatment liquid, a monoclonal antibody DV2-M15 marked with a label, a positive contrast, a negative contrast, a concentration washing liquid, a develop liquid and a termination liquid, wherein the monoclonal antibodies DV2-M6 and DV2-M14 of the test kit can be specifically combined with NS1 protein of II-type dengue virus, without cross reaction with other three kinds of serotype dengue viruses NS1 and respectively combined with different antigen points of NS1, while the check sensitivity of NS1 protein of II-type dengue virus can reach 3ng / ml and the check sensitivity of culture supernatant of II-type dengue virus infection cell is 8 power of Pan-E dengue early elisa test kit, thereby improving the sensitivity of clinical serum sample check.
Owner:SOUTHERN MEDICAL UNIVERSITY
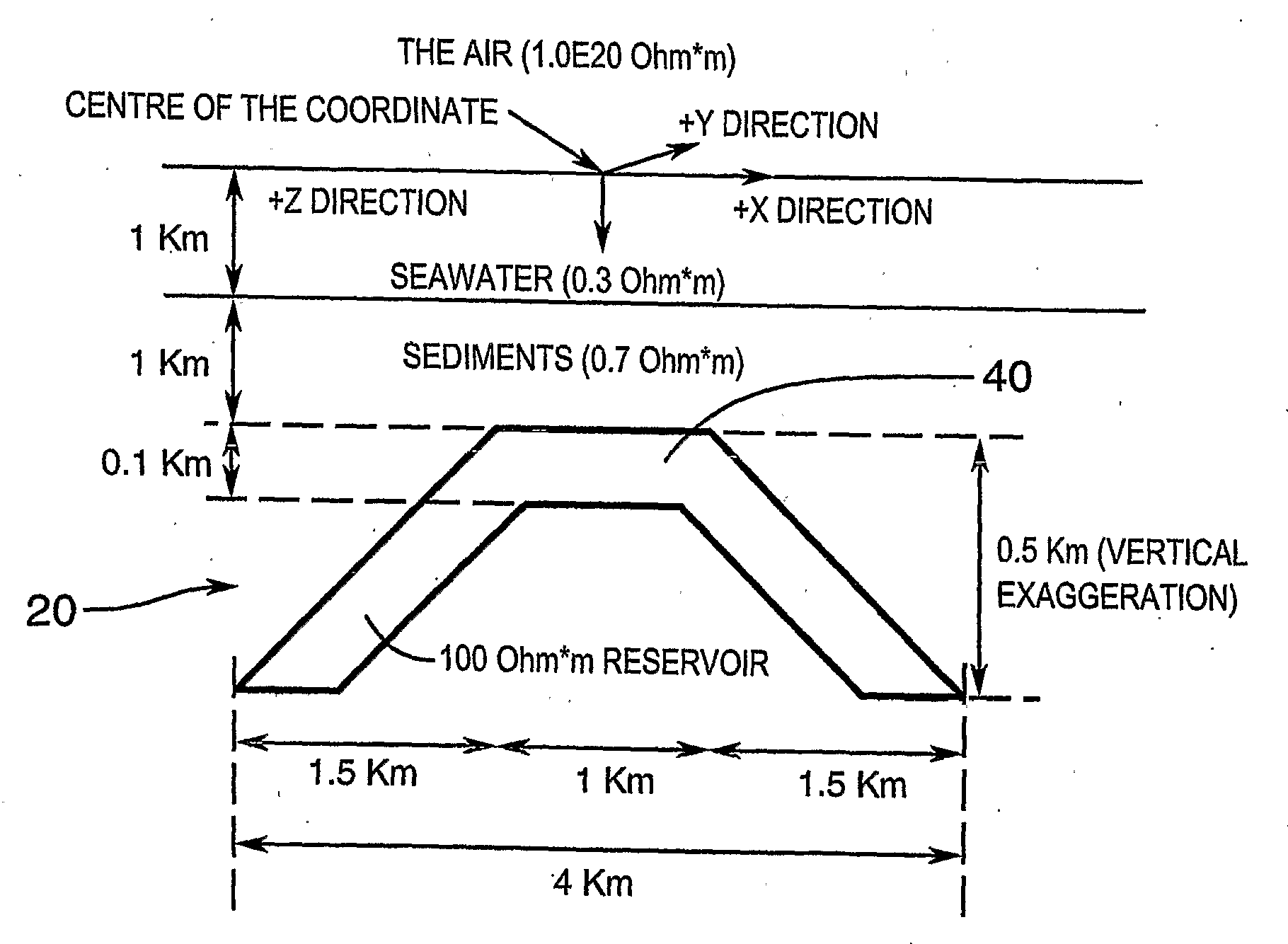
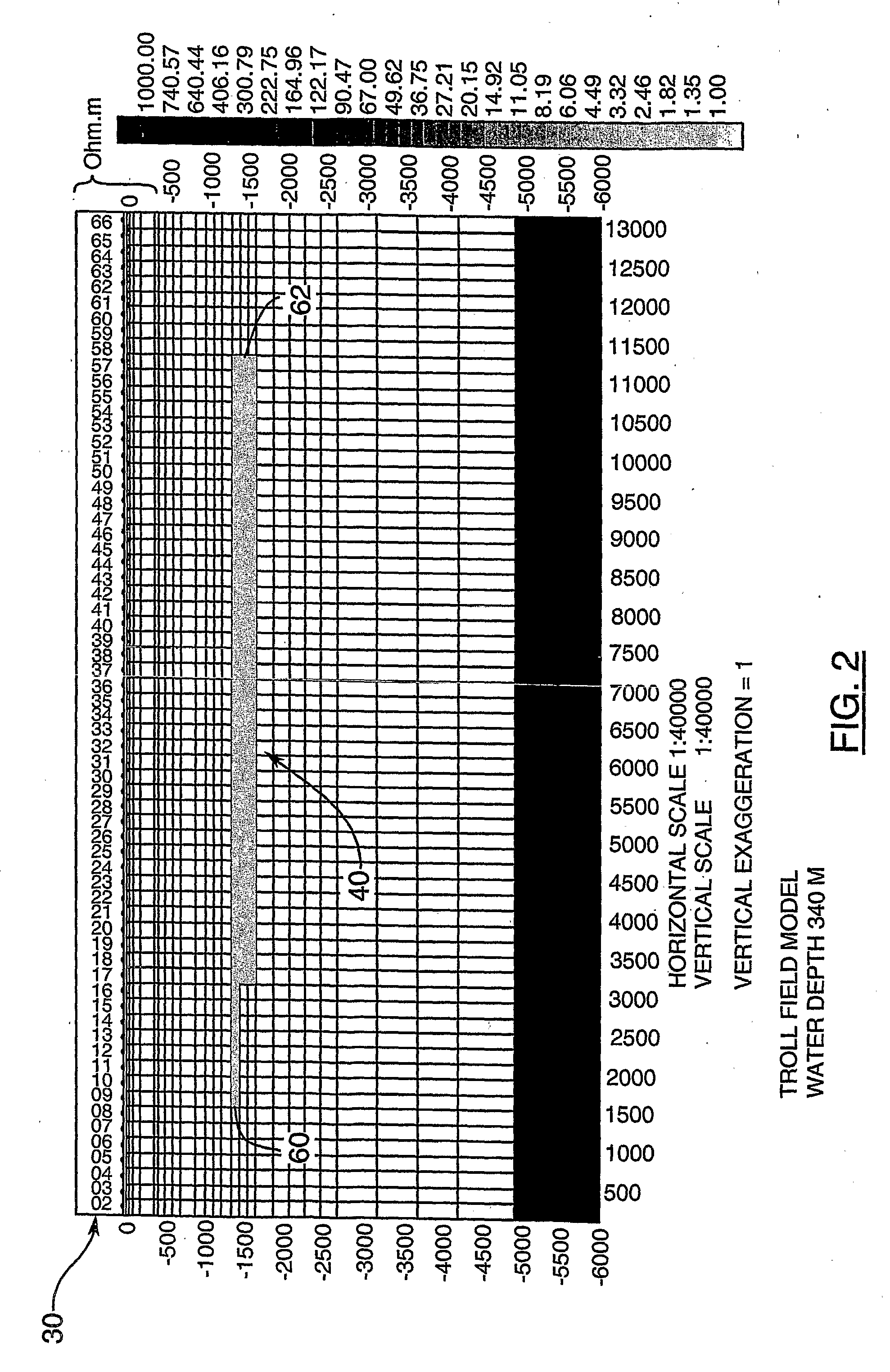
Detection of Resistivity of Offshore Seismic Structures Mainly Using Vertical Magnetic Component of Earth's Naturally Varying Electromagnetic Field
InactiveUS20090315563A1Understand clearlyImprove productivityResistance/reactance/impedenceElectric/magnetic detectionNatural sourceElectrical resistance and conductance
The invention measures the vertical component Hz of a magnetic field arising from natural sources (MT) simultaneously at a plurality of points (70) on the sea floor to determine places having a non-zero vertical component Hz indicative of an edge of a resistive body (structure) (40), in order to determine whether or not a sub-bottom geologic structure (20), known from marine seismic measurements, exhibits a resistivity contrast with the surrounding rocks, a positive contrast being interpreted as indicating hydrocarbon charge within the structure.
Owner:FOX ANTHONY C L +1
Korean novel duck hepatitis viral antibody ELISA (Enzyme-Linked Immunosorbent Assay) detection kit
InactiveCN102127531ASolve the difficulty of purificationImprove securityRecombinant DNA-technologyFermentationDuck hepatitis A virusViral antibody
The invention discloses a Korean novel duck hepatitis viral antibody ELISA (Enzyme-Linked Immunosorbent Assay) detection kit. The detection kit contains an ELISA board coated by Korean novel duck hepatitis VP1 (Phenotypic Variance1) recombination protein, a sample diluent, concentrated washing liquid, an enzyme conjugate working solution, a chromogenic reagent (A), a chromogenic reagent (B), a stopping solution, a positive contrast solution and a negative contrast solution. The VP1 recombination protein is obtained by using the following method: using Korean novel duck hepatitis viruses as a material, augmenting and cloning the VP1 gene through an RT-PCR (Reverse Transcriptase-Polymerase Chain Reaction) method to obtain recombinant expression plasmid pMD (physical medium dependent)-VP1; then, directionally inserting to an expression vector pET-32a (+) and screening to obtain recombinant expression plasmid pET-32a(+)-VP1; and inducing, expressing and purifying by ITPG (Isopropyl beta-D-Thiogalactopyranoside) to obtain VP1 recombination protein. The detection kit is used for detecting the Korean novel duck hepatitis and has strong specificity, high sensitivity, simplicity of operation, easiness of popularization and application in a large-area range and wide market prospects.
Owner:POULTRY INST SHANDONG ACADEMY OF AGRI SCI
Composite kit for detecting avian influenzas and Newcastle disease viruses and detection method
InactiveCN101798602AQuality improvementEasy to operateMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceBiology
The invention relates to a composite kit for detecting avian influenzas and Newcastle disease viruses and a detection method, characterized in that the kit comprises a lysis solution, an RT-PCR buffer solution, primers and a probe mixture solution, particularly a primer and a probe sequenc for detecting various subtype avian influenzas viruses, a primer and a probe sequenc for detecting Newcastle medium and strong viruses, enzyme mixture, DEPC water and negative and positive contrasts. The detection method comprises the following steps of: carrying out an RT-PCR reaction and judging a quality control standard and results. The invention has the advantages of high sensitivity, good specificity and simple operation. The process from processing samples to getting a result only needs 4 hours. The invention overcomes the defects of single fluorescence and realizes the aim of simultaneously detecting two viruses by one real-time fluorescent quantification PCR (Polymerase Chain Reaction) reaction.
Owner:INSPECTION & QUARANTINE TECH CENT SHANDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Positive contrast MRI of magnetically tagged cells, objects, tissues
Contrast agents incorporating super-paramagnetic iron-oxide (SPIO) nanoparticles have shown promise as a means to visualize labeled cells using MRI. Labeled cells cause significant signal dephasing due to the magnetic field inhomogeneity induced in water molecules near the cell. With the resulting signal void as the means for detection, the particles are behaving as a negative contrast agent, which can suffer from partial-volume effects. Disclosed is a new method for imaging labeled cells with positive contrast. Spectrally-selective RF pulses are used to excite and refocus the off-resonance water surrounding the labeled cells so that only the fluid and tissue immediately adjacent to the labeled cells are visible in the image. Phantom, in vitro, and in vivo experiments show the feasibility of the new method. A significant linear correlation (r=0.87, p<0.005) between the estimated number of cells and the signal has been observed.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
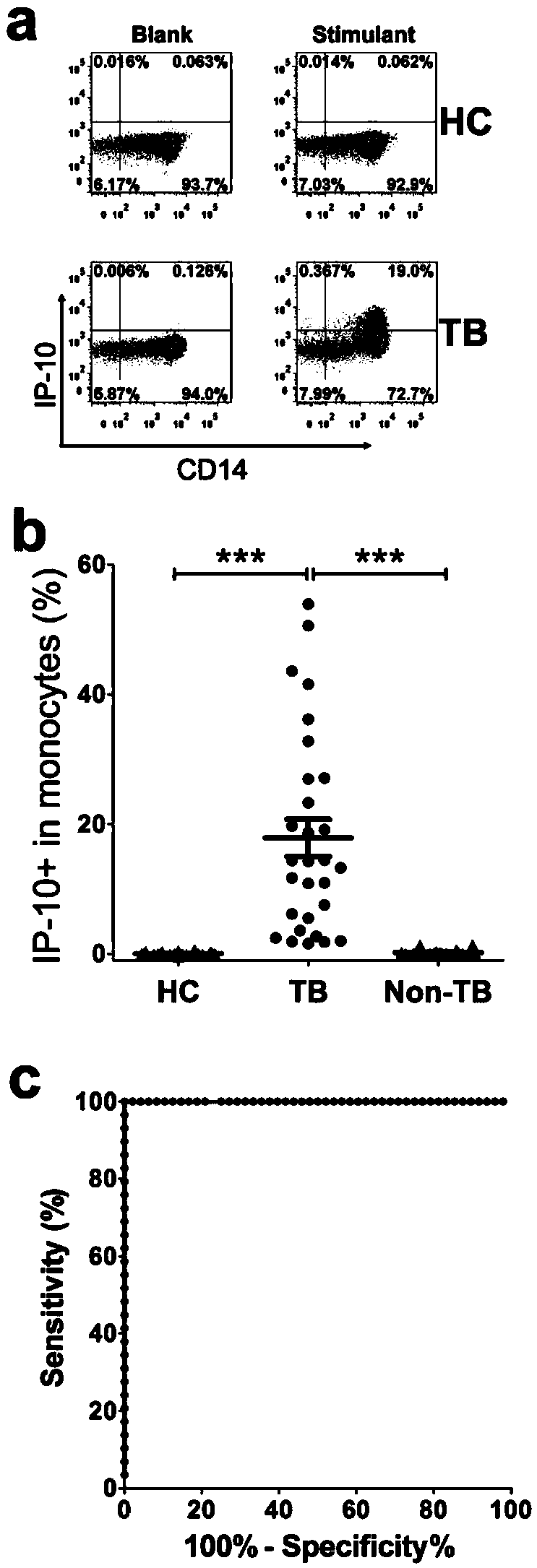
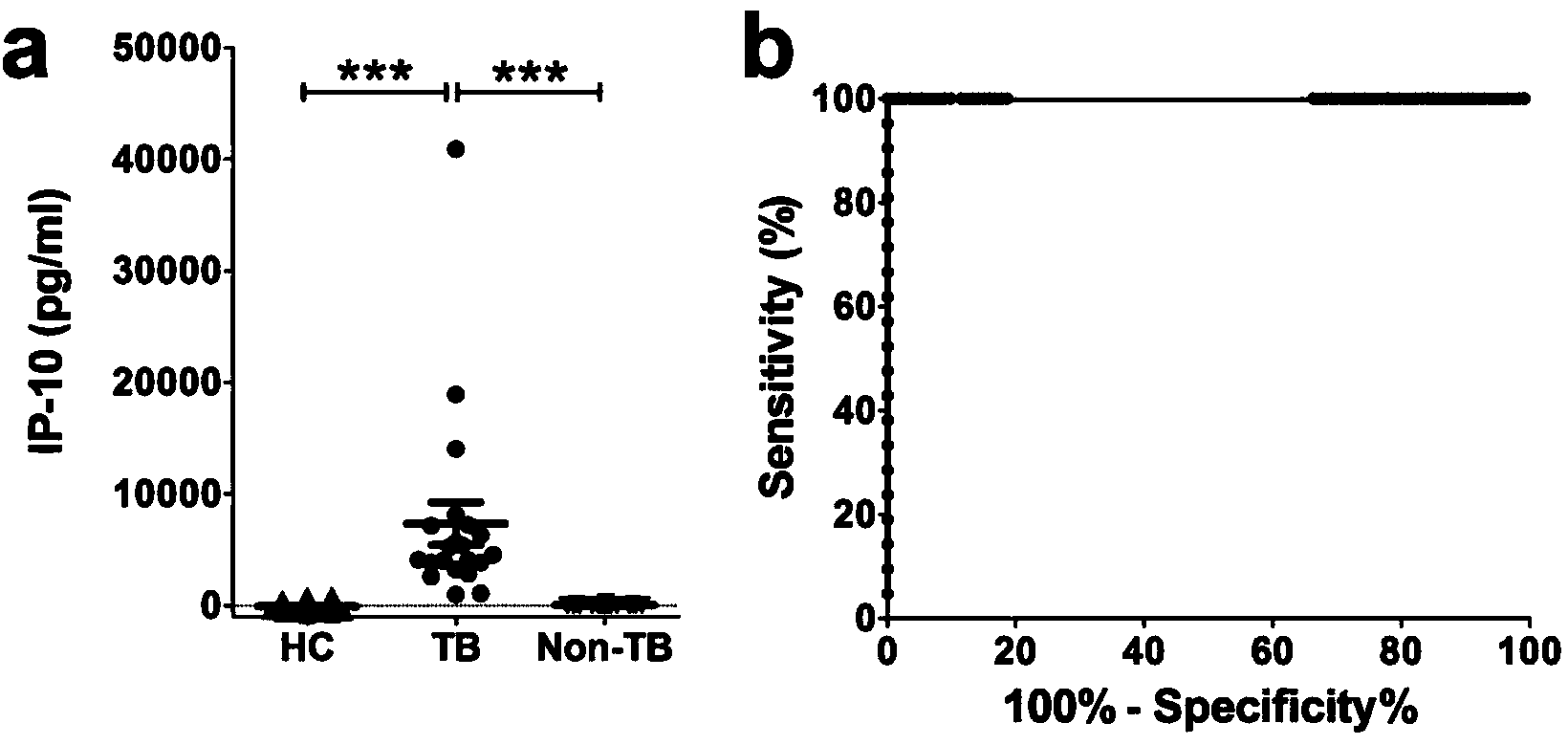
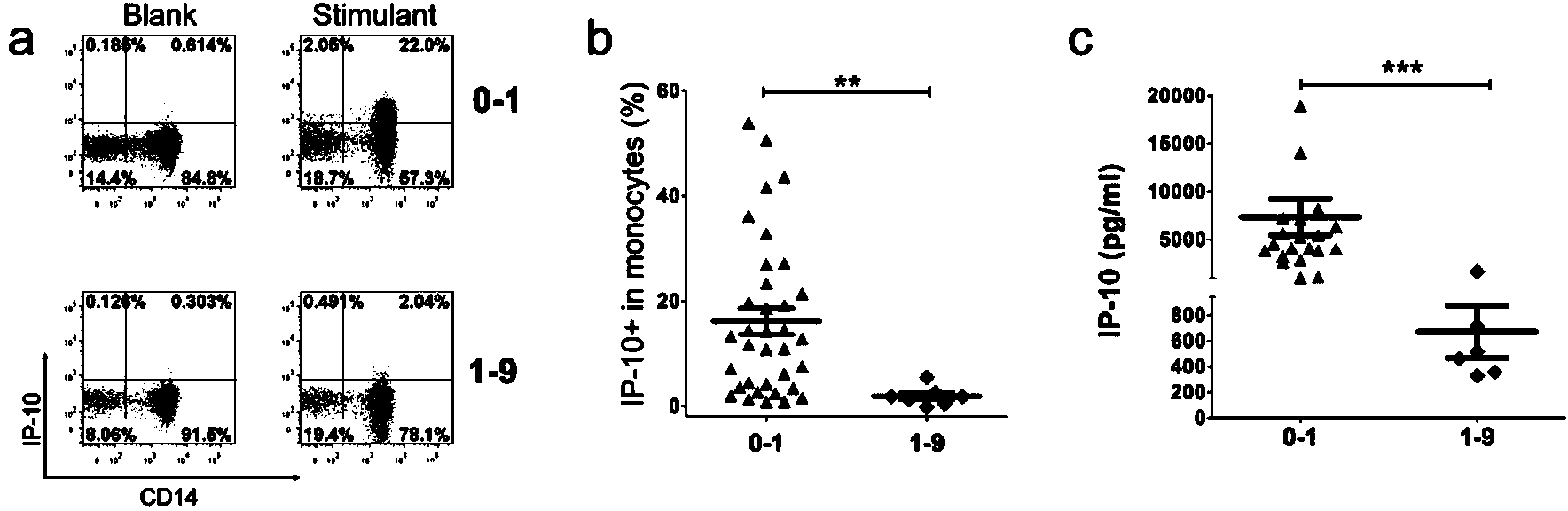
Kit for detecting mycobacterium tuberculosis infection and monitoring clinical treatment effect and application of kit
ActiveCN104020297AIncreased sensitivityImprove featuresDisease diagnosisBiological testingTherapeutic effectSpecific antibody
The invention discloses a kit for detecting the mycobacterium tuberculosis infection and monitoring a clinical treatment effect. The kit provided by the invention comprises a specific antibody, namely an IP-10 antibody and / or CD14 antibody, an antigen irritant and a positive contrast irritant. The kit disclosed by the invention can be used for diagnosing an active tuberculosis patient or a tuberculosis latent infection patient and is not affected by BCG (bacillus calmette-guerin) inoculation. The sensitivity and the specificity of the kit for diagnosing the active tuberculosis patient are higher than those of a commercial T-SPOT.TB kit and can be up to 100 percent. After the antituberculosis therapy is performed for 1 month, the detection rates of over 80 percent of clinical tuberculosis patients are converted to be negative, so that the kit can be used for detecting the clinical antituberculosis treatment effect.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
Nucleic acid detection kit for synchronously identifying and diagnosing newcastle disease virus and avian influenza virus
InactiveCN101240352AAvoid potential risksEasy to operateMicrobiological testing/measurementHighly pathogenicFluorescence
The invention belongs to the field of inspection and quarantine technology. Specifically, the invention is a nucleic acid detection reagent kit for synchronous discriminating and diagnosing avian influenza virus and newcastle disease virus. The detection reagents of the reagent kit include extraction reagent for extracting virus by silicon gel absorption column method, detection amplification reagent for detecting nucleic acid by RT-PCR Taq Man fluorescent probe method, and pretreatment liquid for solid tissue specimen for extracting virus RNA. Further, the invention employs in vitro transcription RNA as a positive contrast of the reagent kit. The reagent kit can rapidly and synchronously discriminate and diagnose avian influenza virus and newcastle disease virus which are highly infectious among avian plagues and have similar symptom, determine current major prevalent subtypes, such as H5, H7, H9, etc., and discriminate whether a infection source is an avian influenza having high pathogenicity, non-pathogenic avian influenza or mildly pathogenic avian influenza to human. The reagent kit is suitable for livestock and veterinarian station, import and export inspection and quarantine bureau, as well as other laboratories, and can be used for large-scale detection of influenza and epidemic surveillance.
Owner:SHANGHAI KEHUA BIO ENG
Hepatitis c virus antigen-antibody joint detection reagent box and preparation method thereof
ActiveCN104237520AThe "window period" is shortenedHigh detection specificityMaterial analysisAntigenPolyethylene glycol
A hepatitis c virus antigen-antibody joint detection reagent box is characterized by comprising a calibrator(1), a double-marker enzyme conjugate (2), a negative and positive contrast (3), light-emitting liquid (4) and a micropore coated plate (5), wherein the light-emitting liquid contains light-emitting liquid 1 and light-emitting liquid 2, the light-emitting liquid 1 contains luminal 0.7 g / L, cinnamic acid 0.9 g / L, 4-iodophenylboronic acid 0.2 g / L, iodobiphenol 0.25 g / L, dimethylformamide 25 ml / L, polyving akohol 5 g / L, polyvinylpyrrolidone 8 g / L, polyethylene glycol 600 3 g / L, ethylenediamine tetraacetic acid 4 g / L, gentamicin sulfate 1600 thousands / L, urea peroxide 0.4 g / L, and pH 9.0 Tris buffer solution 0.1 mol / L. The light-emitting liquid 2 contains acridinium ester derivative 0.1 mg / ml, polyethylene glycol 600 3 g / L and 0.1 mol / L of pH 9.0 Tris buffer solution containing 0.1% of TWEEN-20. The invention further discloses a preparation method and a using method of the reagent box. The hepatitis c virus antigen-antibody joint detection reagent box has the advantages of being quick in reaction and low in cost.
Owner:BIOSCIENCE (TIANJIN) DIAGNOSTIC TECH CO LTD
Positive contrast roadway lighting system
A luminaire assembly generally includes a reflector having a center of symmetry and a lamp supported within the reflector, wherein the lamp is offset from the reflector center of symmetry, whereby light emitted from the lamp is reflected by the reflector in an asymmetric pattern. In a method of illuminating a roadway having traffic moving in a flow direction, a luminaire having a reflector and a lamp is mounted at a side of the roadway and light emitted from the lamp is reflected with the reflector whereby a greater portion of the reflected light is directed in the traffic flow direction than is directed against the traffic flow direction.
Owner:WALKER CHRIS
Colour development protein chip using BCIP/NBT as substrate as well as application in detection of autoantibody thereof
InactiveCN101226192AIncreased sensitivityImprove featuresMaterial analysis by observing effect on chemical indicatorBiological testingAntigenAutoantibody production
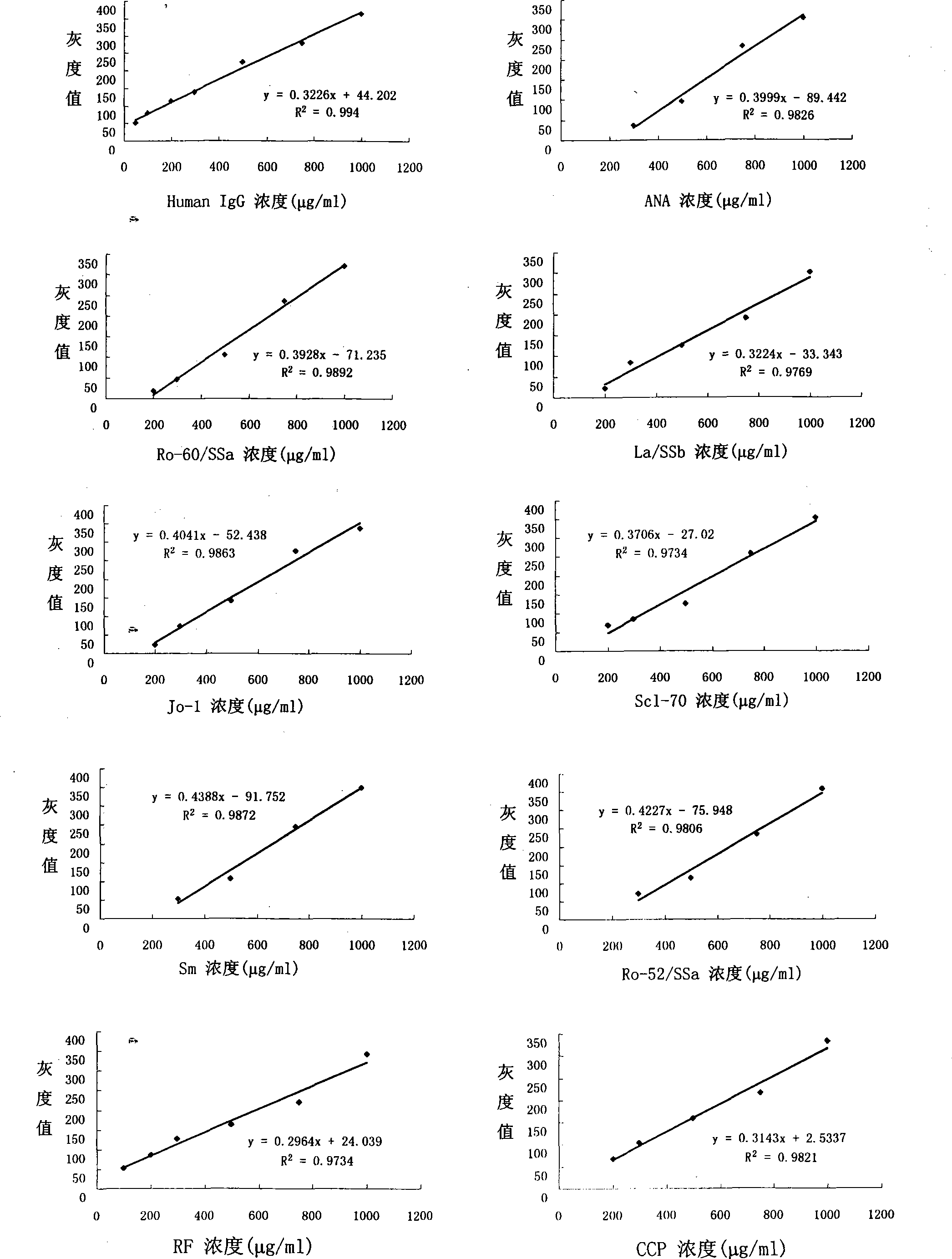
The invention discloses a color protein chip which substrate is BCIP / NBT. The invention is composed of a substrate, an array, 12 antigens coated on the array, negative contrast, positive contrast and blank contrast, wherein the substrate is a glass slide decorated by APES, the kinds and point sample densities of the 12 antigens are 520ug / ml ANA, 465ug / ml Ro-60, 530ug / ml La / SSb, 530ug / ml Jo-1, 525ug / ml Scl-70, 520ug / ml Sm, 615ug / ml Ro-52 / SSa, 340ug / ml RF, 465ug / ml CCP, 410ug / ml u1RNP, 490ug / ml CENP-B, 580ug / ml dsDNA. The invention uses the alkali phosphatase coloring method whose substrate is BCIP / NBT as the final check signal of protein chip to be widely used for self antibody check, while the check sensitivity has higher coincidence rate than prior art, with reduced check cost and time. The invention can be used in clinical check, sanitary supervision, regular sanitary supervision and scientific research or the like.
Owner:SHANDONG MEDICAL BIO TECH RES CENT
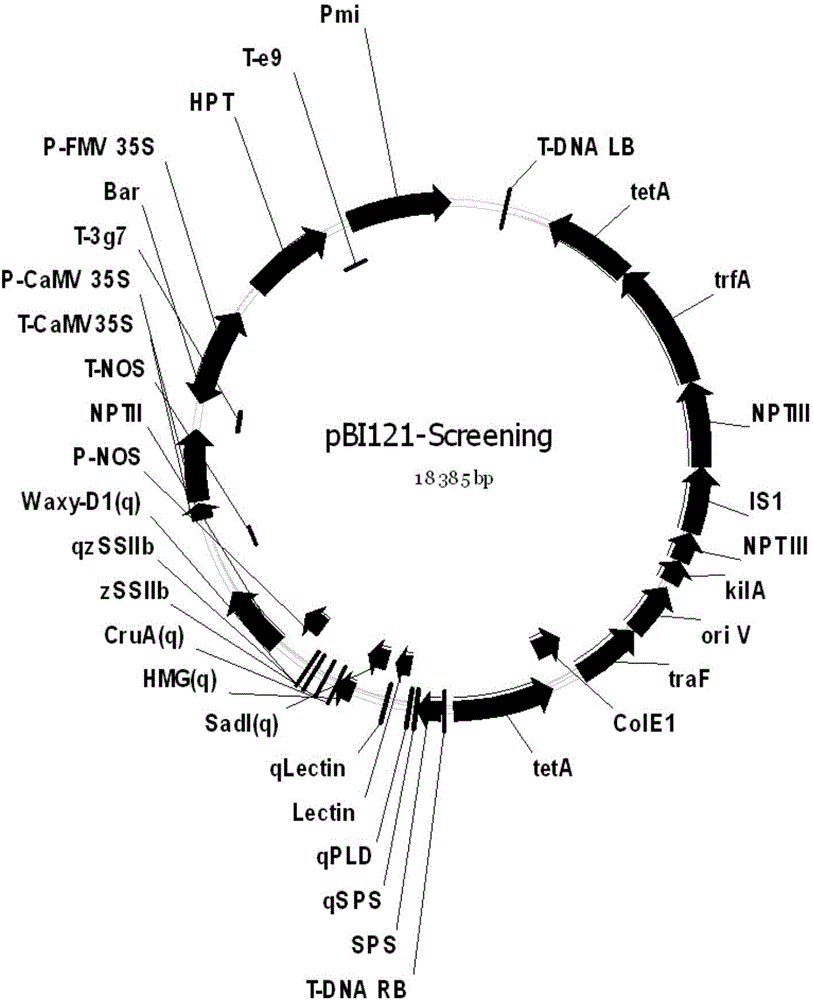
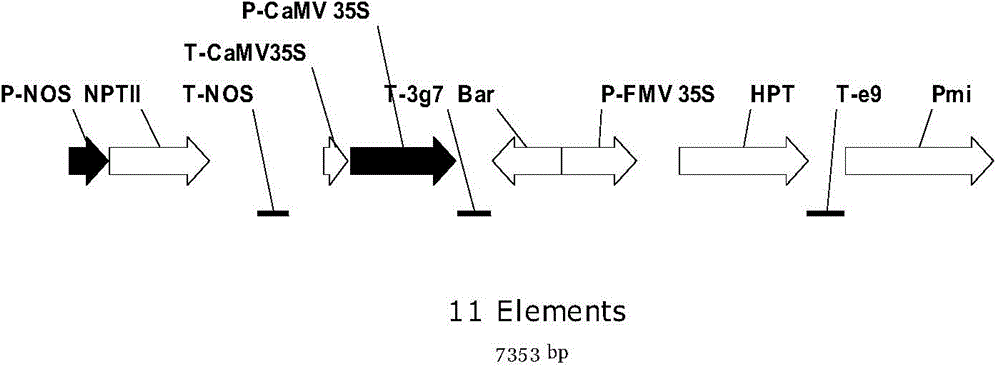
Positive plasmid molecule pBI121-Screening and application thereof
InactiveCN104651511AAvoid the problem of setting up multiple positive controlsReduce labor costsMicrobiological testing/measurementVector-based foreign material introductionFluorescenceGenetics
The invention discloses a positive plasmid molecule pBI121-Screening and application thereof and relates to the technical field of safety supervision and screening detection of transgenic crops in the transgenic safety field. The base sequence of the pBI121-Screening is as shown in SEQ NO.1. serving as both a positive contrast and a quality control sample, pBI121-Screening disclosed by the invention can be respectively used in general PCR and real-time fluorescence PCR screening detection of transgenic components of six transgenic crop samples, namely paddy rice, rapes, soybeans, corns, cotton and wheat. The pBI121-Screening disclosed by the invention can be used for solving the technical problem that the lack of a positive contrast or a standard sample in the screening detection of the transgenic crops, and prevents the problem that a plurality of positive contrasts are arranged for a plurality of detection targets in the screening detection, thus reducing the labor cost and economic cost for preparing the plurality of positive contrasts.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
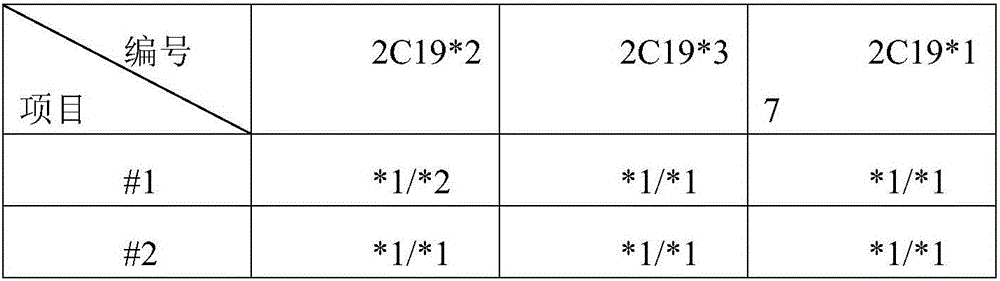
Detection probe for SNP (Single Nucleotide Polymorphism) of human CYP2C19 gene and application of detection probe
InactiveCN106702019AIncreased sensitivityHigh Primer Match RateMicrobiological testing/measurementDNA/RNA fragmentationFluoProbesMedicine
The invention provides a detection probe for an SNP (Single Nucleotide Polymorphism) of a human CYP2C19 gene. A detection primer consists of a specific upstream and downstream primer pair of the gene and a specific Taqman double fluorescent probe which are respectively used for correspondingly detecting SNPs at CYP2C19*2, CYP2C19*3 and CYP2C19*17 sites. The detection probe has the characteristics of quick detection and high specificity; furthermore, the detection method is simple; positive contrast and negative contrast which are necessary to conventional fluorescent quantitation PCR are eliminated, so that the operation steps and the experimental cost are reduced; the subsequent clinical treatment strategy can be guided more quickly and better.
Owner:北京一立科技发展有限公司
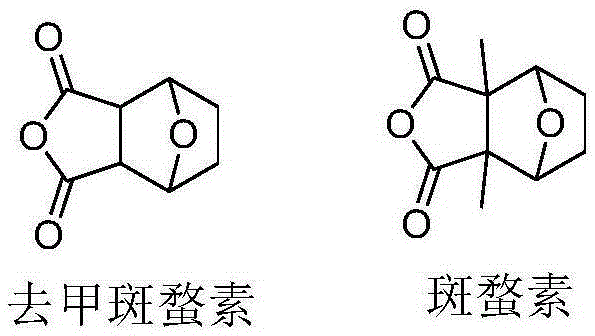
Acid-sensitive camptothecin-site 20 norcantharidate derivative and antineoplastic application thereof
InactiveCN105399757AImprove anti-tumor effectHigh inhibition rate in vitroOrganic chemistryAntineoplastic agentsSolubilityCantharidin
The invention provides a camptothecin-site 20 norcantharidate derivative with a structural formula as shown in I which is described in the specification, and a preparation method and antineoplastic application thereof. Results of activity test prove that the camptothecin-site 20 norcantharidate derivative as shown in I is an appropriate candidate antineoplastic drug, especially a candidate drug for resisting liver cancers, stomach cancers and colorectal cancers. Compared with positive contrast drugs, i.e., camptothecin and cantharidin, the camptothecin-site 20 norcantharidate derivative has improved water solubility and stability; and the camptothecin-site 20 norcantharidate derivative is sensitive to acid and can be easily hydrolyzed. Moreover, the preparation method for the camptothecin-site 20 norcantharidate derivative employs easily available raw materials, has high yield and is easy to operate and implement.
Owner:ZUNYI MEDICAL UNIVERSITY
Oligonucleotide chip capable of detecting five enteroviruses simultaneously and application thereof
InactiveCN101654713AStrong specificityHigh compliance rateNucleotide librariesMicrobiological testing/measurementFluorescenceOligonucleotide chip
The invention discloses an oligonucleotide chip capable of detecting five enteroviruses simultaneously and an application thereof. The chip comprises a substrate, probes of pathogens of five enteroviruses coated on the substrate, negative contrast, positive contrast and blank contrast, wherein the probes of pathogens are five 54-70mer of HAV-P, ROV-P, NOV-P, ASV-P and ADV-PV1 probes. The chip of the invention adopts multiple PCR to amplify a plurality of target sequences of pathogens simultaneously and uses the downstream primer Tamara labeling method to perform fluorescence labeling to PCR product, the labeled PCR product is hybridized with the chip to realize the accurate detection of pathogens, the detection sensitivity is equal to that of PCR and the specificity is high. The detectionefficiency and accuracy are obviously shortened. The technology system of the invention is applicable to fields such as sea water and marine life specimens monitoring, food hygiene surveillance, customs quarantine, related clinical detection and the like.
Owner:SHANDONG MEDICAL BIO TECH RES CENT +1
Enzyme-linked detection kit and preparation method thereof
An enzyme-linked detection kit for detecting VEGF. A kit body contains an enzyme-linked reaction plate coated by a VEGF acceptor and a monoclonal antibody, an enzyme conjugate labelled by HRP and configured by antibody and a protective agent, a freeze-drying powder of the VEGF protein as a positive contrast, a protein freeze-drying powder as a negative contrast, a sample diluent, a PBST concentrated lotion, a chromogenic substrate configured by 3,3',5,5'-tetramethyl benzidine, 2MH2SO4 as a stopping solution and a seal plate gummed paper. The kit can be used in diagnosis and prognosis of cancer, angiopathy, diabetes, retinopathy and rheumatoid arthritis, and has advantages of accuracy, specificity, sensitivity, stability and convenience, etc.
Owner:北京健平金星医疗器械有限公司
Method for detecting canine rabies virus antibody and detection kit
The invention provides a method for detecting canine rabies virus antibody (IgG) and a detection kit. The kit is composed of a coated plate and a reagent reaction system, and comprises a rabies virus antigen-coated reaction plate, a standard substance, positive contrast serum, a washing lotion (20X), a sample weak solution, an enzyme-marked combination substance, a developer A, a developer B and a stopping solution. The method is characterized in that the method uses an ELISA method to determine that the content of the canine rabies virus antibody (IgG) reaches a absorbance corresponding to a protective level by detecting the standard substance of the kit, and by compared the absorbance of the sample to be detected with the absorbance of the standard substance, the content of the canine rabies virus antibody (IgG) contained in the sample to be detected is judged whether to reach an immunization protective level. The method and the kit can simultaneously detect a lot of samples, and a detection result is remarkably with a result by a neutralization experiment, has high accuracy degree, is suitable for monitoring an immunization inoculation effect and determining an individual immunization state, and can be used for investigating animal eqpidemic diseases.
Owner:ZHENGZHOU ZHONGDAO BIOTECHNOLOGY CO LTD
CpG insular methylation test reagent kit and its application
InactiveCN1357636AGood curative effectHigh sensitivityMicrobiological testing/measurementWilms' tumorBiology
The present invention relates to gene determining technology and is especially CpG insular methlation test reagent kit and its application for diagnosing tumor and screening demethylation medicine. The reagent kit includes primer, medicine for extracting template DNA, medicine for modifying genome, medicine for purifying DNA, specific MSP-PCR reaction system and negative and positive contrast samples. The present invention utilizes human gene sequences and designs primer based on base mismatch principle, and different gene primer is selected to constitute different reagent kit for detecting different diseases. The present invention may be used widely in the diagnosis of cancer and the screening of relevant medicine.
Owner:JINAN UNIVERSITY +2
Multi-PCR detection kit for poultry salmonella and non-diagnostic detection method of poultry salmonella
ActiveCN105648055AHigh sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesBiologyMultiplex pcrs
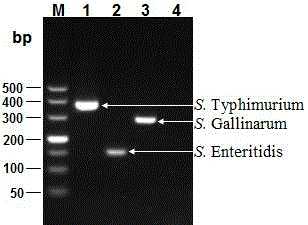
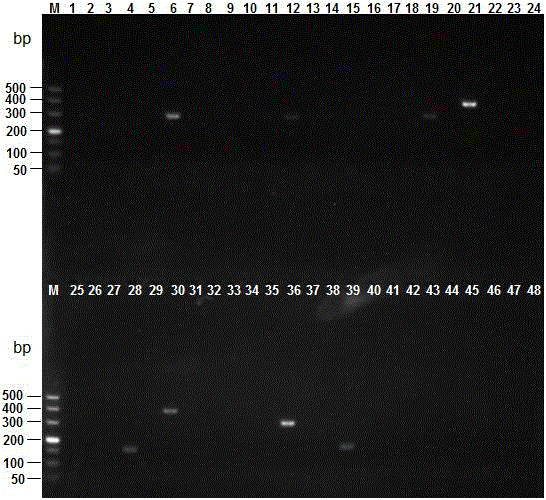
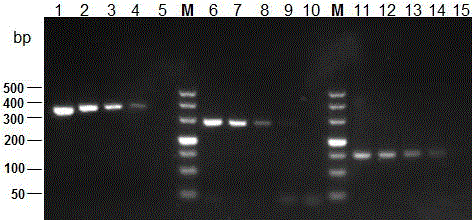
The invention discloses a multi-PCR detection kit for poultry salmonella and a non-diagnostic detection method of the poultry salmonella. The kit comprises 10*PCR buffering liquid, 2.5 U / mu l Taq DNA polymerase, 10 Mm dNTPs, a multi-PCR detection primer group, a positive contrast material and a negative contrast material. The positive contrast material comprises salmonella enteritidis ATCC13076 genome DNA, salmonella typhimurium ATCC14028 genome DNA and salmonella gallinarum ATCC 9184 genome DNA; the negative contrast material is sterilized double distilled water. The invention further discloses a multi-PCR method for detecting the poultry salmonella by applying the kit. The method has the advantages of being rapid, simple, high in specificity and high in sensitivity, three types of salmonella can be detected and classified rapidly through a one-time PCR reaction, compared with traditional serological typing and ordinary PCR detection, great advantages are achieved on the aspect of detection time and detection cost, and the multi-PCR detection kit and the detection method are suitable for batch detection.
Owner:JIANGSU INST OF POULTRY SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com