Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
42 results about "Pathogenic avian influenza" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Avian influenza—known informally as avian flu or bird flu is a variety of influenza caused by viruses adapted to birds. The type with the greatest risk is highly pathogenic avian influenza (HPAI).
Nucleic acid detection kit for synchronously identifying and diagnosing newcastle disease virus and avian influenza virus
InactiveCN101240352AAvoid potential risksEasy to operateMicrobiological testing/measurementHighly pathogenicFluorescence
The invention belongs to the field of inspection and quarantine technology. Specifically, the invention is a nucleic acid detection reagent kit for synchronous discriminating and diagnosing avian influenza virus and newcastle disease virus. The detection reagents of the reagent kit include extraction reagent for extracting virus by silicon gel absorption column method, detection amplification reagent for detecting nucleic acid by RT-PCR Taq Man fluorescent probe method, and pretreatment liquid for solid tissue specimen for extracting virus RNA. Further, the invention employs in vitro transcription RNA as a positive contrast of the reagent kit. The reagent kit can rapidly and synchronously discriminate and diagnose avian influenza virus and newcastle disease virus which are highly infectious among avian plagues and have similar symptom, determine current major prevalent subtypes, such as H5, H7, H9, etc., and discriminate whether a infection source is an avian influenza having high pathogenicity, non-pathogenic avian influenza or mildly pathogenic avian influenza to human. The reagent kit is suitable for livestock and veterinarian station, import and export inspection and quarantine bureau, as well as other laboratories, and can be used for large-scale detection of influenza and epidemic surveillance.
Owner:SHANGHAI KEHUA BIO ENG
Kit for detecting highly pathogenic avian influenza virus subtype h5n1
InactiveUS20120028246A1Reduce pathogenicityMicrobiological testing/measurementBiological material analysisH5N1 virusWater soluble
Disclosed by the invention are an immunoassay kit and an immunoassay method for detecting highly pathogenic avian influenza virus subtype H5N1 rapidly, conveniently and specifically. Also disclosed are an immunochromatographic detection kit and an immunochromatographic detection method for detecting the virus subtype H5N1 rapidly, conveniently and specifically. It is found that a monoclonal antibody 4G6 produced by using the virus subtype H5N1 as an immunogen does not react with the subtype H5N2 virus or a subtype H5N3 virus and reacts only with a subtype H5N1 virus specifically. It is also found that only an avian influenza virus subtype H5N1 can be detected specifically by an immunoassay utilizing the monoclonal antibody 4G6. It is further found that the sensitivity of the detection of immunochromatography can be increased by adding a nonionic surface and a water-soluble vinyl polymer having a polar group containing an oxygen atom and a nitrogen atom to a developing solution to be used in the immunochromatography.
Owner:OSAKA UNIV +1
Anthropogenic H5N1-resisting hemagglutinin protein broad-spectrum neutralising antibody and application thereof
The invention provides an anthropogenic H5N1-resisting hemagglutinin protein broad-spectrum neutralising antibody. The antibody is obtained by screening through a phage display technology. The sequence of the hypervariable region (CDRs) determining the specificities of the antibody is represented by figure 1. The antibody of the invention specifically identifies H5N1 highly pathogenic avian influenza virus particle antigen, has obvious fluorescence immune reaction and enzyme-coupled immune reaction with the avian influenza virus H5N1 aiming at avian influenza virus hemagglutinin protein HA, and has the neutralising activity function of resisting the avian influenza virus H5N1. The antibody of the invention can be made into specific antibody medicines clinically used for preventing and treating human avian influenza that is caused by the H5N1 avian influenza virus, so as to clinically prevent or treat human avian influenza that is caused by the H5N1 avian influenza virus.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
ScFv antibody for resisting H5N1 type highly-pathogenic avian influenza and application thereof
The invention relates to an scFv antibody for resisting H5N1 type highly-pathogenic avian influenza and an application thereof. A light chain amino acid sequence is shown as SEQIDNo: 1; a heavy chain amino acid sequence is shown as SEQIDNo: 2; a light chain nucleotide sequence is shown as SEQIDNo: 3; and a heavy chain nucleotide sequence is shown as SEQIDNo: 4. The scFv antibody for resisting the H5N1 type highly-pathogenic avian influenza is applied to the preparation of a medicine for resisting the H5N1 type avian influenza virus. Proven by protection tests of embryonated eggs, the 4F5 antibody has a 100% protective rate on prevention of the avian source and human source H5N1 type avian influenza virus infection, and the 4F5 antibody has a 100% protective rate on treatment of the avian source H5N1 type avian influenza virus infection. The highest protective rate on treatment of the human source H5N1 type highly-pathogenic avian influenza virus infection is 62.5%.
Owner:NANJING MEDICAL UNIV +1
Recombinant H7N9 subtype avian influenza virus-like particle, and preparation method and application thereof
ActiveCN112079904AGood cross reactivityMild diseaseSsRNA viruses negative-senseViral antigen ingredientsAdjuvantImmunogenicity
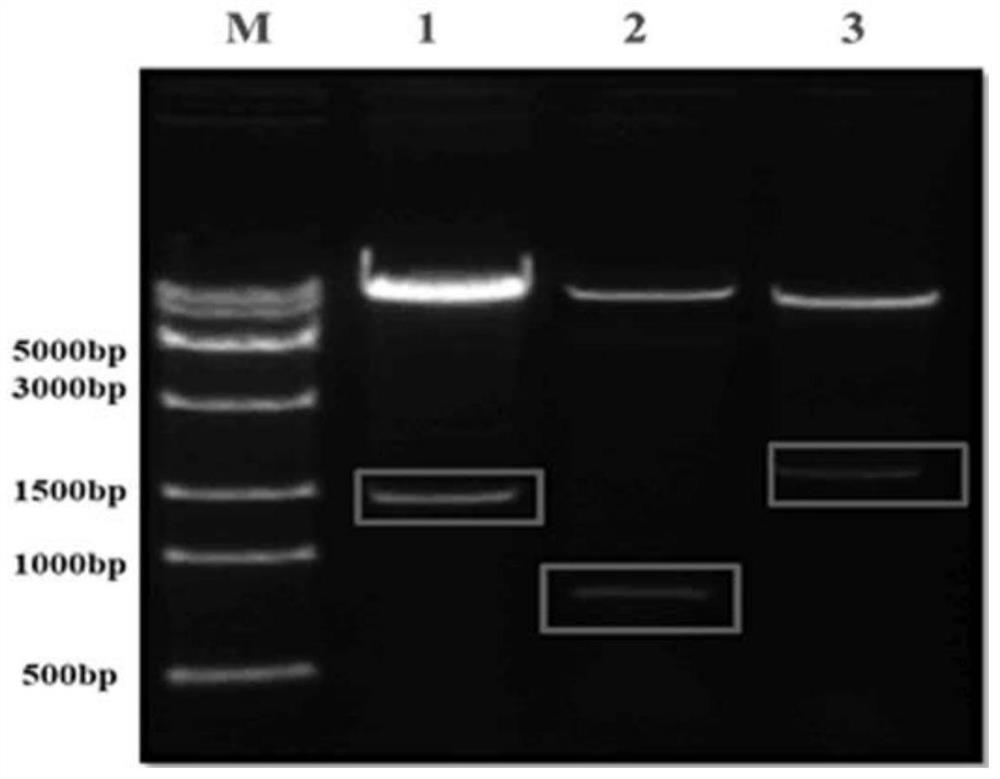
The invention discloses a recombinant H7N9 subtype avian influenza virus-like particle, and a preparation method and application thereof. Recombinant baculoviruses for expressing HA protein, NA protein and M1 protein of an H7N9 highly pathogenic avian influenza virus are respectively constructed on the basis of a recombinant baculovirus insect cell culture system. The three recombinant baculovirusstrains are used for co-infecting suspended insect cells, so that the H7N9 subtype avian influenza virus-like particle capable of being self-assembled in the cells can be obtained. After the concentration and purification with cane sugar with different gradient concentrations through an ultrafiltration tube, the H7N9 subtype avian influenza virus-like particle is mixed and emulsified with an adjuvant to prepare a vaccine. When a chicken is immunized by the prepared vaccine, the body can be induced to generate a specific antibody; and the advantages of high immunogenicity, good safety, high hereditary stability and the like are realized. After the attack by a lethal dose of H7N9 subtype highly pathogenic avian influenza viruses, the complete clinical protection can be provided, and virus expelling of the chicken is obviously inhibited. The invention provides a novel method for preventing H7N9 subtype avian influenza virus infection, and also lays a foundation for the development of a novel influenza virus vaccine.
Owner:YANGZHOU UNIV
Polypeptide vaccine for treating diseased induced by high pathogenic avian influenza virus H5N1
InactiveCN101380466AEasy to store and transportFacilitate automated mass productionAntiviralsAntibody medical ingredientsDiseaseSide effect
The invention relates to a polypeptide vaccine for treating diseases caused by highly pathogenic avian influenza virus H5N1, which is characterize in that: the amino acid sequence is as follows: amino terminal-QIGNISIWV-carboxyl terminal; and lysine is adopted as a linker to connect a plurality of amino acid sequences to form a multi-copy series structure. The invention has the advantages of convenient transportation and preservation and automated mass production, and can activate specific cytotoxic T lymphocyte (CTL) which can effectively kill and lyses target cells with the N1 protein of the highly pathogenic avian influenza virus H5N1 without toxicity and side effects.
Owner:CHONGQING UNIV
Method of biochip for simultaneous testing avian influenza infection of human and fowls
A biochip for detecting avain fluenza virus of both human and fowls is prepared as providing activated chip substrate, providing various fluenza virus subset sections to be sample-set, providing quality control protein, carrying out arrangement pattern design of point measurement, making sample-sets of quality control protein and various avain fluenza virus subset sections according to prepared pattern for detecting out antibodies generated by various subsets simultaneously.
Owner:深圳市赛尔生物技术有限公司
Kit for detecting multiple influenza viruses by polymerase chain reaction (PCR) microarray
ActiveCN102337352AEasy to operateShort timeMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceInfluenza A (H1N1) virus
The invention relates to a kit for detecting multiple influenza viruses by a polymerase chain reaction (PCR) microarray. The kit comprises mainly a PCR microarray reaction plate, a PCR reaction solution, and a packaging box for centralized and partitioned packaging of reagent bottles or reagent tubes. Through the combination of a real-time fluorescence PCR technology and a microarray technology, on the single PCR microarray reaction plate, the kit can detect eight influenza viruses comprising influenza A virus, influenza B virus, seasonal influenza H1 subtype virus, seasonal influenza H3 subtype virus, influenza A H1N1 virus, highly pathogenic avian influenza H5 subtype virus, highly pathogenic avian influenza H7 subtype virus, and highly pathogenic avian influenza H9 subtype virus simultaneously. A result of detection adopting the kit can be utilized for differential diagnosis and monitoring of pathogens capable of causing influenza.
Owner:DAAN GENE CO LTD
Recognition method of highly pathogenic avian influenza virus hemagglutinin protein
InactiveCN101308526ALarge amount of informationEnhanced Representational CapabilitiesSpecial data processing applicationsSupport vector machineCovariance method
The invention discloses a highly-pathogenic avian influenza virus hemagglutinin protein identification method, which can be used for highly-pathogenic avian influenza virus hemagglutinin protein identification and provide a reference for understanding the structural characteristics of the highly-pathogenic avian influenza virus hemagglutinin protein; through the identification of the hemagglutinin protein, the method can indirectly distinguish the degree of the pathogenicity of the avian influenza virus strain to which the hemagglutinin protein belongs. The method comprises the following steps: a), to establish an amino acid holographic topological structure score vector, based on principal component analysis; b), to apply the amino acid holographic topological structure score vector to characterize the structure of the influenza virus hemagglutinin protein; c), to carry out normalized processing to the characteristic variable of each influenza virus hemagglutinin protein through a self-cross-covariance method; d), to establish an avian influenza virus hemagglutinin protein identification model through an RBF kernel-based support vector machine; e), to establish a highly-pathogenic avian influenza virus hemagglutinin protein identification model through the RBF kernel-based support vector machine.
Owner:CHONGQING UNIV
Recombinational bacillus subtilis of expressing highly pathogenic avian influenza H5N1 hemagglutinin HA protein
ActiveCN106434728AImprove developmentBoosts specific immune response levelsSsRNA viruses negative-senseBacteriaHemagglutininWestern blot
The invention relates to recombinational bacillus subtilis(B.S.-HA) of expressing highly pathogenic avian influenza(AIV) HSN1 hemagglutinin HA protein, and belongs to the field of biotechnology genetic engineering. The recombinational bacillus subtilis(B.S.-HA) of expressing the highly pathogenic avian influenza(AIV) HSN1 hemagglutinin HA protein successfully establishes recombinational bacillus subtilis expression plasmid pHT43 of the highly pathogenic avian influenza hemagglutinin HA protein, and successfully conducts elec-transformation of the recombinational bacillus subtilis expression plasmid pHT43 of the highly pathogenic avian influenza hemagglutinin HA protein into bacillus subtilis WB800N bacterial strain (B.S.). As verified by Western-blot, the highly pathogenic avian influenza hemagglutinin HA protein can be successfully expressed in the recombinational bacillus subtilis. Oral immunization can stimulate chicken body of 10-day old chicken to generate an effective AIV specific immune response level. The recombinational bacillus subtilis(B.S.-HA) is hopeful to be developed to genetic engineering oral vaccine in preventing the highly pathogenic avian influenza.
Owner:NANJING AGRICULTURAL UNIVERSITY
Polypeptide medicament for treating highly pathogenic bird flu virus H5N1induced disease
InactiveCN101411866AEasy to store and transportFacilitate automated mass productionPeptide/protein ingredientsAntiviralsDiseaseBird flu
The invention provides a polypeptide drug for treating diseases initiated by highly pathogenic bird flu virus H5N1. The polypeptide drug is characterized in that the amino acid sequence of the polypeptide drug is amino terminal-FLNVEWSYI-carboxyl terminal; a plurality of the amino acid sequences form a multicopy serial structure by using lysine as a joint. The polypeptide drug has the advantages of convenient transportation and storage and automatic batch production, and has the capability of activating specificity cytotoxicity T lymphocyte (CTL). The specificity CTL has a target cell which kills and dissolves H5 albumen with the highly pathogenic bird flu virus H5N1 with obvious effect without toxic side effect.
Owner:ARMY MEDICAL UNIV
Method for detecting H5-subtype and H7N9-subtype highly pathogenic avian influenza viruses as well as H9-subtype avian influenza virus
ActiveCN109722492AHigh sensitivityShort detection timeMicrobiological testing/measurementMicroorganism based processesFluorescenceReaction tube
The invention provides a rapid detection method capable of synchronously detecting and differentiating H5-subtype and H7N9-subtype highly pathogenic avian influenza viruses as well as H9-subtype avianinfluenza virus. By analyzing nucleotide sequences of HA genes of an H5-subtype highly pathogenic avian influenza virus, an H7N9-subtype highly pathogenic avian influenza virus and an H9 subtype avian influenza virus isolated in recent years, as well as designing primers and probes in conservative regions, a rapid real-time fluorescence quantitative RT-PCR nucleic acid detection method for the H5-subtype and H7N9-subtype highly pathogenic avian influenza viruses as well as the H9-subtype avian influenza virus is established. The rapid real-time fluorescence quantitative RT-PCR nucleic acid detection method for the H5-subtype and H7N9-subtype highly pathogenic avian influenza viruses as well as the H9-subtype avian influenza virus provided by the invention has the advantages of being highin sensitivity, short in detection time, and free of open links such as electrophoresis and the like; moreover, only one fluorescent PCR instrument is required for accomplishing detection on the threesubtypes of the avian influenza viruses in a closed reaction tube. In addition, quick judgment on results is allowed for real-time viewing of response curve is feasible during the detection process.
Owner:CHINA ANIMAL HEALTH & EPIDEMIOLOGY CENT
Method and device for identifying pathogenic influence of concurrent bacteria infection against poultry influenza virus infection
InactiveCN1442485AHighly pathogenicAchieve infectionMicrobiological testing/measurementTissue/virus culture apparatusBacteroidesBird flu
A method for determining the pathogenic affection of concurrent bacterial infection to avian influenza virus includes such steps as inoculating the bacterium able to secrete subtilisinoid and / or the bacterium able to secrete tryptase and the low-pathogenicity avian influenza virus in culture medium of CEF in a particular mode, culturing, and observing if CPE presents. Its cell culture equipment is composed of cell culture bottle and bacteria culture chamber. Its advantages are simple method, short time, high correctness and low cost.
Owner:SUN YAT SEN UNIV
H9 subtype lowly pathogenic avian influenza virus and application thereof
ActiveCN107557346AImprove featuresImproving immunogenicityMicroorganism based processesAntiviralsH5N2 Avian Influenza VirusFowl
The invention discloses a H9 subtype lowly pathogenic avian influenza virus which is classified and named as H9 subtype lowly pathogenic avian influenza virus X-13 strain which is preserved in the China Center for Type Culture Collection; the preservation constitute is called as CCTCC for short; the address of the preservation constitute is Wuhan University, Wuhan, China; the preservation date isJuly 26, 2017; and the preservation number is CCTCC NO:V201733. The H9 subtype lowly pathogenic avian influenza virus disclosed by the invention is good in specificity and immunogenicity, chicken immunized with the strain can endure infection of the H9 subtype lowly pathogenic avian influenza virus. As a vaccine strain for the novel H9 subtype lowly pathogenic avian influenza virus, the strain iscapable of preventing H9 subtype lowly pathogenic avian influenza of livestock, and can be also applied to virus identification and epidemiological investigation.
Owner:HENAN AGRICULTURAL UNIVERSITY
Liquid-phase chip for detecting flu and H5N1 avian influenza virus and specific detection primer and preparation method thereof
InactiveCN101392298BQuick checkHigh sensitivityMicrobiological testing/measurementInfluenza Viruses Type AAvian virus
The invention relates to a method for detecting influenza viruses, in particular to a method for detecting influenza viruses and H5N1 subtype avian influenza virus by a liquid-phase chip, belonging to the technical field of medical monitoring. The method is a fast and highly efficient one which can identify FluA and FluB influenza viruses including the H5N1 subtype avian influenza virus by types.With the utilization of a liquid-phase chip technology and the application of bioinformatics knowledge and related bioinformatics software, the liquid-phase chip which can specifically distinguish H5and N1 gene fragments, NP gene fragment of the FluA influenza virus and HA gene fragment of the FluB influenza virus is manufactured. The method has the advantages that fast detecting and early-stagediagnosis of the FluA and the FluB influenza viruses including the H5N1 subtype highly pathogenic avian influenza virus can be carried out; and a basis for developing other fast and highly effective detecting methods for detecting various subtype influenza viruses is provided. The method is characterized by detecting multiple types of viruses, short response time and high sensitivity, thus determining that the method has wide application prospect.
Owner:JIANGSU PROVINCIAL CENT FOR DISEASE PREVENTION & CONTROL
A kind of recombinant h7n9 subtype avian influenza virus-like particle and its preparation method and application
ActiveCN112079904BGood cross reactivityMild diseaseSsRNA viruses negative-senseViral antigen ingredientsAdjuvantImmunogenicity
The invention discloses a recombinant H7N9 subtype avian influenza virus-like particle and its preparation method and application. Based on the recombinant baculovirus insect cell culture system, the expression of H7N9 highly pathogenic avian influenza virus HA protein and NA protein is respectively constructed. , M1 protein recombinant baculovirus. Three strains of recombinant baculoviruses were co-infected in suspended insect cells, and H7N9 subtype avian influenza virus-like particles self-assembled in the cells could be harvested. After being concentrated and purified by ultrafiltration tubes and different gradient concentrations of sucrose, it is mixed with adjuvant and emulsified to prepare the vaccine. The prepared vaccine immunized chickens can induce the body to produce specific antibodies, and has the advantages of strong immunogenicity, good safety, high genetic stability and the like. After challenge with a lethal dose of highly pathogenic avian influenza virus of H7N9 subtype, it can provide complete clinical protection and significantly inhibit the shedding of chickens. The invention provides a new method for preventing H7N9 subtype avian influenza virus infection, and also lays a foundation for the development of new influenza virus vaccines.
Owner:YANGZHOU UNIV
Recombinant bacillus subtilis for expressing avian influenza virus HA antigen fragment and PB1-F2 protein in tandem
The invention relates to recombinant bacillus subtilis for expressing avian influenza virus HA antigen fragment and PB1-F2 protein in tandem, and belongs to the field of biotechnical genetic engineering. The invention successfully establishes a recombinant bacillus subtilis integrated expression plasmid plnHAPB1-F2 for expressing the avian influenza virus HA-PB1-F2 protein, and successfully achieves chemical conversion and electric shock conversion into bacillus subtilis WB600. Western-blot verification shows that the avian influenza virus HA antigen fragment and the PB1-F2 protein which are expressed in tandem can be successfully expressed in the recombinant bacillus subtilis. After one-day-old SPF chick is immunized through oral administration, a chick body can be stimulated to generate an effective HA-PB1-F2 specific immune response level, and nonspecific immunity is significantly enhanced. The recombinant bacillus subtilis is expected to be successfully developed into a genetic engineering oral vaccine for preventing the highly pathogenic avian influenza of poultry.
Owner:NANJING AGRICULTURAL UNIVERSITY
Polypeptide drug for treating diseases caused by highly pathogenic avian influenza virus H5N1
InactiveCN102058873AEasy to store and transportFacilitate automated mass productionPeptide/protein ingredientsAntiviralsDiseaseSide effect
The invention relates to a polypeptide drug for treating diseases caused by highly pathogenic avian influenza virus H5N1, in particular to a polypeptide drug about diseases caused by highly pathogenic avian influenza virus H5N1, belonging to the field of biomedicine. The polypeptide drug is characterized in that the amino acid sequence of the polypeptide drug is amido-FLNVEWSYI-carboxyl, which is a special connection mode formed by using the characteristic that lysine has two amidoes and one carboxyl and using the lysine as a joint. The invention has the advantages of convenience for transportation and preservation and automated batch production; the polypeptide drug has the capacity of activating specific cytotoxicity T lymphocyte (CTL); and the specific CTL cells have target cells for killing and dissolving H5 protein having the highly pathogenic avian influenza virus H5N1, with obvious effects but without toxic or side effects.
Owner:CHONGQING UNIV
Antibody for specific binding with highly-pathogenic avian influenza virus
The invention relates to a broad spectrum humanized monoclonal antibody, which can specifically bind with highly-pathogenic avian influenza virus H5N1 and is obtained through following one or more mutation of an antibody 37 hAb, which is obtained by humanization of a monoclonal antibody 13D4, which is produced by a hybridoma cell strain with a preservation number of CCTCC-C200721; wherein the mutations is as follows: the 100th F position of the heavy chain CDR3 can mutate into L, S, Y, N, or T, the 102nd position V can mutate into E, Q, K, D, or T; the 51st position I of the heavy chain CDR2 can mutate into M, V, or S, the 54th position S can mutate into T, A, P, or V, the 57th position I can mutate into S, W, or D, the 59th position Y can mutate into A, F, C, or E; and applications of these antibodies on preventing and curing of highly-pathogenic avian influenza virus diseases.
Owner:XIAMEN UNIV +1
Polypeptide vaccine for treating diseases caused by highly pathogenic avian influenza viruses H5N1
InactiveCN102085362AEasy to store and transportFacilitate automated mass productionPeptide/protein ingredientsAntiviralsDiseaseSide effect
The invention discloses a polypeptide vaccine for treating diseases caused by highly pathogenic avian influenza viruses H5N1 and relates to the field of biomedicine, in particular to a polypeptide vaccine for treating diseases caused by the highly pathogenic avian influenza viruses H5N1. The polypeptide vaccine is characterized in that the amino acid sequence of the polypeptide vaccine is amino-QIGNISWV-carboxyl; and the polypeptide vaccine forms a special connecting mode by utilizing the characteristics that the lysine has two amino groups and a carboxyl group and using the lysine as a joint. The polypeptide vaccine has the advantages of convenience for transportation and storage and automatic batch production and the capacity of activating specific cytotoxic T lymphocyte (CTL), wherein the specific CTL has the capacities of killing and dissolving target cells with the highly pathogenic avian influenza virus H5N1 protein, and the polypeptide vaccine has obvious effect and no toxic and side effects.
Owner:CHONGQING UNIV
Application of sakuranetin and derivatives thereof in preparation of anti-H5N1 virus medicament
InactiveCN103191098AActivity hasEnhance pharmacological effectsOrganic active ingredientsAntiviralsDiseaseAnti virus
The invention relates to a new application of sakuranetin and derivatives thereof in preparation of an anti-highly pathogenic avian influenza (HPAI) H5N1 virus medicament. Experiments prove that the sakuranetin and the derivatives thereof have a good inhibiting and inactivating effect. The anti-H5N1 virus medicament can be prepared by using the compounds as an active ingredient. The anti-virus medicament can be used for clinically preventing and treating influenza viruses, particularly diseases caused by HPAI H5N1 viruses. The compounds are safe, low in toxicity and strong in pharmacologic action; and the raw material sources are rich, wide and cheap, the preparation cost is low, the process is simple, and the yield is high. In order to prevent and treat the influenza viruses, particularly the HPAI H5N1 viruses, the invention provides a new medicament source, which has great practical significance and a broad application prospect in the fields of medicine and bio-pharmaceuticals, particularly in the field of preparation of anti-H5N1 virus medicaments.
Owner:中国人民解放军防化学院
A highly pathogenic h7n9 avian influenza virus antigen with low receptor binding activity and preparation method thereof
InactiveCN110106193BDecreased receptor binding affinityHigh biosecuritySsRNA viruses negative-senseVirus peptidesAntigenArginine
The invention discloses a highly pathogenic H7N9 avian influenza virus antigen with low receptor binding activity and a preparation method thereof, comprising the following steps: preparing a mutant HA gene; preparing the full-length highly pathogenic H7N9 avian influenza virus after mutation The gene sequence of the HA protein is to obtain the R220G mutant HA gene; wherein the R220G mutant HA gene sequence is shown in SEQ ID NO.2; virus rescue: the R220G mutant HA gene is used to rescue the recombinant influenza virus. In the recombinant highly pathogenic H7N9 avian influenza virus prepared by the method, the arginine at position 220 in the receptor binding region is mutated into glycine to reduce the binding affinity of the HA protein receptor.
Owner:GUANGZHOU MEDICAL UNIV
A highly pathogenic h7n9 avian influenza virus antigen with low receptor binding activity and preparation method thereof
InactiveCN110106192BDecreased receptor binding affinityImprove the accuracy of experimentsSsRNA viruses negative-senseVirus peptidesAntigenArginine
The invention discloses a highly pathogenic H7N9 avian influenza virus antigen with low receptor binding activity and a preparation method thereof, comprising the following steps: preparing a mutant HA gene; preparing the full-length highly pathogenic H7N9 avian influenza virus after mutation The gene sequence of the HA protein is to obtain the R229I mutant HA gene; wherein the R229I mutant HA gene sequence is shown in SEQ ID NO.2; virus rescue: the R229I mutant HA gene is used to rescue the recombinant influenza virus. In the recombinant highly pathogenic H7N9 avian influenza virus prepared by the method, the arginine at position 229 in the receptor binding region is mutated into isoleucine to reduce the receptor binding affinity of the HA protein.
Owner:GUANGZHOU MEDICAL UNIV
Construction and application of recombinant turkey herpes virus expressing h7n9 subtype highly pathogenic avian influenza virus ha protein
ActiveCN110218706BThoroughly purifiedShorten the timeSsRNA viruses negative-senseViral antigen ingredientsNucleotideTGE VACCINE
The invention discloses construction and application of a recombinant turkey herpesvirus expressing H7N9 subtype highly pathogenic avian influenza virus HA protein. The recombinant turkey herpesvirusis obtained by inserting an exogenous gene expression box between the HVT-065 and HVT-066 genes in a non-essential replication area of a coding sequence of a turkey herpesvirus (HVT), wherein the exogenous gene expression box is formed by connecting an MCMV promoter, an exogenous gene and SV40 poly A in sequence; the exogenous gene is the H7HA gene, and the nucleotide sequence of the exogenous gene is shown as SEQ ID NO:1. The construction method of the recombinant turkey herpesvirus is also provided. An ultrasonic cracking and breaking method is adopted for purification, the screening time isshortened, and the obtained recombinant turkey herpesvirus rHVT-H7HA provides good protection for the highly pathogenic H7N9 subtype avian influenza virus and can be used for subsequent development of new avian influenza virus vaccines.
Owner:SOUTH CHINA AGRI UNIV
Recombinant Bacillus subtilis expressing highly pathogenic avian influenza h5n1 hemagglutinin ha protein
ActiveCN106434728BBoosts specific immune response levelsPromote mucosal immunitySsRNA viruses negative-senseBacteriaBiotechnologyHemagglutinin
The invention relates to recombinational bacillus subtilis(B.S.-HA) of expressing highly pathogenic avian influenza(AIV) HSN1 hemagglutinin HA protein, and belongs to the field of biotechnology genetic engineering. The recombinational bacillus subtilis(B.S.-HA) of expressing the highly pathogenic avian influenza(AIV) HSN1 hemagglutinin HA protein successfully establishes recombinational bacillus subtilis expression plasmid pHT43 of the highly pathogenic avian influenza hemagglutinin HA protein, and successfully conducts elec-transformation of the recombinational bacillus subtilis expression plasmid pHT43 of the highly pathogenic avian influenza hemagglutinin HA protein into bacillus subtilis WB800N bacterial strain (B.S.). As verified by Western-blot, the highly pathogenic avian influenza hemagglutinin HA protein can be successfully expressed in the recombinational bacillus subtilis. Oral immunization can stimulate chicken body of 10-day old chicken to generate an effective AIV specific immune response level. The recombinational bacillus subtilis(B.S.-HA) is hopeful to be developed to genetic engineering oral vaccine in preventing the highly pathogenic avian influenza.
Owner:NANJING AGRICULTURAL UNIVERSITY
Neutralization molecule of high-pathogenicity avian influenza and preparation method thereof
Provided is a binding molecule of highly pathogenic avian influenza virus, and a preparation method therefor. The binding molecule has a good neutralizing effect on avian influenza virus. Also disclosed is a binding site where the binding molecule is bound on hemagglutinin of the avian influenza virus.
Owner:INST PASTEUR OF SHANGHAI CHINESE ACADEMY OF SCI +1
Recognition method of highly pathogenic avian influenza virus hemagglutinin protein
InactiveCN101308526BLarge amount of informationEnhanced Representational CapabilitiesSpecial data processing applicationsSupport vector machineCovariance method
Owner:CHONGQING UNIV
A kind of preparation method of highly pathogenic h7n9 avian influenza virus, vaccine, detection reagent and virus, vaccine
InactiveCN110172452BHigh biosecurityDoes not affect immunogenicitySsRNA viruses negative-senseViral antigen ingredientsImmunogenicityTGE VACCINE
The invention discloses a preparation method of a highly pathogenic H7N9 avian influenza virus, which can improve the immunogenicity of the virus, comprising the following steps: preparing a Q226L mutant HA gene, and preparing a highly pathogenic H7N9 avian influenza virus with a Q226L mutation The gene sequence of the full-length HA protein is obtained to obtain the Q226L mutant HA gene; the virus is rescued, and the recombinant influenza virus is rescued by using the Q226L mutant HA gene. The invention also discloses the highly pathogenic H7N9 avian influenza virus prepared by the method, the vaccine prepared by using the highly pathogenic H7N9 avian influenza virus and its preparation method, and the detection method prepared by using the highly pathogenic H7N9 avian influenza virus reagent. The preparation method of the highly pathogenic H7N9 avian influenza virus of the invention improves the biological safety and immunogenicity of the recombinant virus.
Owner:GUANGZHOU MEDICAL UNIV
Avian influenza virus-like particle antigen, vaccine, preparation method of avian influenza virus-like particle antigen and application of avian influenza virus-like particle antigen or vaccine
ActiveCN112079905AGood cross reactivityInhibition of replicationSsRNA viruses negative-senseAntibody mimetics/scaffoldsEmbryoNeutralizing antibody
The invention discloses an avian influenza virus-like particle antigen, a vaccine, a preparation method of the avian influenza virus-like particle antigen and application of the avian influenza virus-like particle antigen or the vaccine. A strain of recombinant baculovirus based on tandem expression of HA, NA and M1 genes of an H7N9 subtype avian influenza virus transfects insect cells and performs self-assembly to form the avian influenza virus-like particle antigen; and the virus-like particle antigen prepared according to the invention is used for preparing the vaccine. After a chicken group is immunized by the vaccine prepared according to the invention, HI antibodies, neutralizing antibodies and IgY antibodies with the higher titer can be generated, 100-percent clinical protection onthe H7N9 subtype highly pathogenic avian influenza virus can be provided, and virus expelling can be effectively inhibited. The rely on the chicken embryos is avoided; the production cost is low; theperiod is short; and higher protection capability can be induced only by once low-dose immunization. A foundation is laid for industrial production of VLP vaccines.
Owner:YANGZHOU UNIV
Method and device for identifying pathogenic influence of concurrent bacteria infection against poultry influenza virus infection
InactiveCN1204264CHighly pathogenicAchieve infectionMicrobiological testing/measurementTissue/virus culture apparatusBacteroidesSubtilisin
The invention relates to a method for identifying the influence of concurrent bacterial infection on the pathogenicity of avian influenza virus infection and a device used therefor. Co-inoculate bacteria that secrete subtilisin-like protease or / and bacteria that secrete tryptase and low-pathogenic avian influenza virus in the CEF culture, so that the bacteria cannot directly contact CPE but the enzymes they secrete can enter the culture medium, continue Cultivate and observe the presence or absence of CPE; according to the appearance of CPE, the cause of a large number of chicken deaths can be identified. The cell in vitro homogeneous culture device used in the method is composed of a cell culture bottle and a bacteria culture room, and the bacteria culture room is a closed structure with a microporous filter membrane with a pore size not greater than 0.22 microns that can communicate with the cell culture bottle. The method of the invention is simple and easy to operate, the identification time is short, the result is accurate and reliable, and the device used is low in price, and has important significance for the detection and prevention of bird flu.
Owner:SUN YAT SEN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com