Application of sakuranetin and derivatives thereof in preparation of anti-H5N1 virus medicament
The technology of a derivative, sakuratin, is applied in the application field of sakuratin and its derivatives in the preparation of anti-H5N1 virus drugs, can solve the problems such as the undiscovered anti-H5N1 virus of sakuratin and its derivatives, and achieves strong pharmacological effects, The effect of high yield and rich source of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1, cherry blossom element extraction and purification
[0031] Hydrangea (Galium aparine L) whole herb 60kg, cold soaked with 480kg of 60% ethanol and extracted 3 times (3 days / time), the extracts were combined, the ethanol was recovered until there was no alcohol smell, and petroleum ether, chloroform, ethyl acetate were used successively Ester, n-butanol extraction, decompression recovery solvent to obtain n-butanol extract 210g, n-butanol extract 1.2L deionized water dissolved and filtered, through macroporous resin AB-8 type column chromatography, followed by water, 25% ethanol , 50% ethanol, 95% ethanol for elution, and the eluents of the above parts were concentrated under reduced pressure respectively to obtain 80 g of water eluate, 22 g of 25% ethanol eluate, 67 g of 50% ethanol eluate, and 95% ethanol eluate. Desiccant 35g. Wherein 25% ethanol eluate (22g) is carried out silica gel column chromatography separation, separates 14 components through th...
Embodiment 2
[0032] Embodiment 2, synthesis and purification of sakurain
[0033]
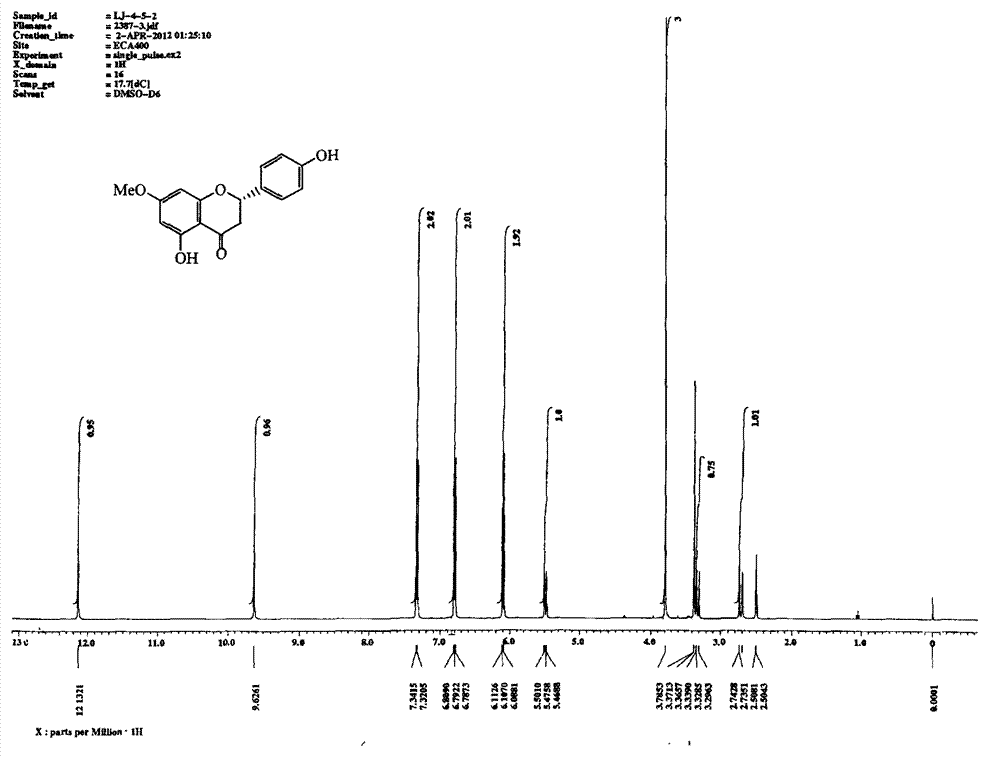
[0034] Dissolve 20.0g naringenin in 200mL acetone, add K 2 CO 3 15.0g, add (CH 3 ) 2 SO 4 10.0g, heated to reflux for 24h. Filter and spin dry. Purified by 200-300 mesh silica gel column chromatography, eluting with petroleum ether: ethyl acetate = 5:1, to obtain 19.2 g of sakurain with a yield of 91%. See attached for hydrogen spectrum figure 1 .
Embodiment 3
[0035] Synthesis and purification of embodiment 3,7-benzylnaringenin
[0036]
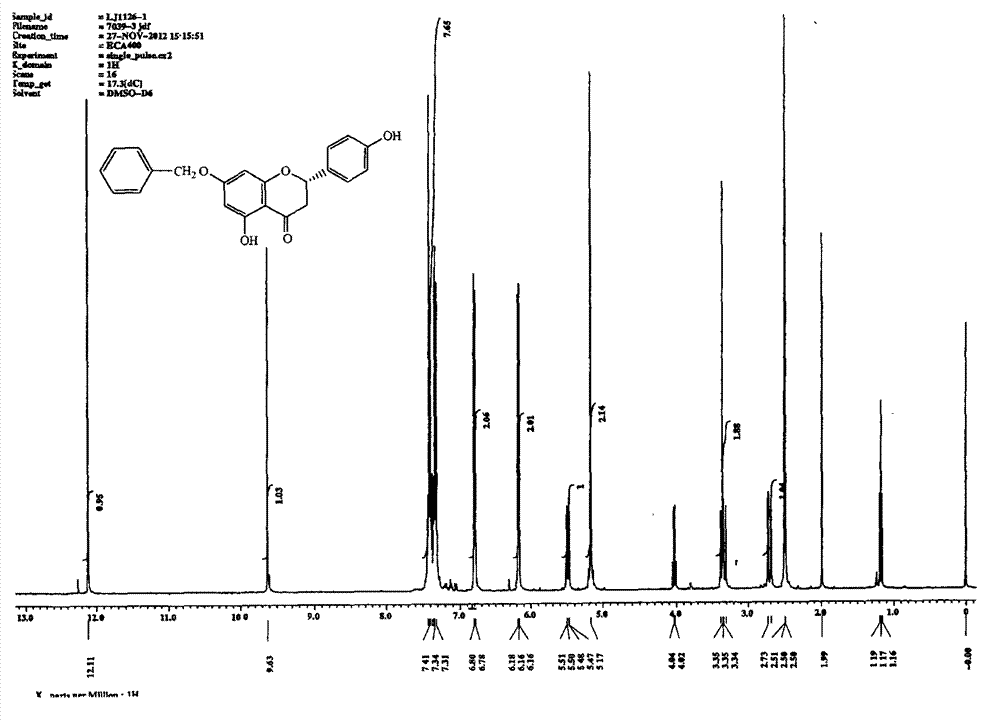
[0037] Dissolve 25.0g naringenin in 500mL acetone, add K 2 CO 3 35.0g, plus 31.0g benzyl bromide, heated to reflux for 24h. Filter and spin dry. Purified by 200-300 mesh silica gel column chromatography, eluting with petroleum ether: ethyl acetate = 2:1, to obtain 23.4 g of sakurain with a yield of 69%. See attached for hydrogen spectrum figure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com