Antibody for specific binding with highly-pathogenic avian influenza virus
An avian influenza virus, highly pathogenic technology, applied in the direction of antibodies, antiviral agents, antiviral immunoglobulins, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Preparation of HAO fusion protein used as antigen
[0037] Construction of expression recombinant vector and transfection expression
[0038] The HA0 gene of influenza virus H5N1 subtype A / Ck / HK / YU777 / 02 was retrieved by our laboratory, and primers were designed, using the HA0 gene of A / Ck / HK / YU777 / 02 as a template, and the signal peptide and its preceding base were removed The base sequence (48bp), plus the signal peptide of TPA (105bp); remove the transmembrane region (108bp) at the tail of HA0, plus a sequence (135bp) with 6 His (the bacteriophage T4 fibritin foldon trimerization sequence), the construction see Figure 6 . It was inserted into the expression vector PTT5, and then the transfection density was 8×10 6 cells / ml CHO-S cells for expression.
[0039] Table 1. HA0 gene cloning primers
[0040]
[0041] HA0 purification
[0042] Because a HIS tag was added to the end of the HAO gene when the recombinant vector was constructed, the HIS tag ...
Embodiment 2
[0045] Example 2: CDR replacement experiment of humanized antibody 37 hAb
[0046] In the previous research, we have obtained the broad-spectrum neutralizing antibody 13D4 of highly pathogenic avian influenza virus (produced by the hybridoma cell line with the deposit number CCTCC-C200721), and then humanized it to obtain human Antibody 37 hAb, whose heavy chain variable region amino acid sequence is SEQ ID NO: 13, and light chain variable region amino acid sequence is SEQ ID NO: 14, still retains the broad-spectrum neutralizing activity of 13D4 mouse monoclonal antibody, and in animals Experiments have shown good therapeutic effects. Because in the process of antigen-antibody binding, the site that directly interacts with the antigen is mainly the CDR region. Many studies have shown that not all of the six CDRs of the heavy chain and light chain are key regions for antigen-antibody binding. Several CDR regions are key regions, so the CDR replacement method can be used to pre...
Embodiment 3
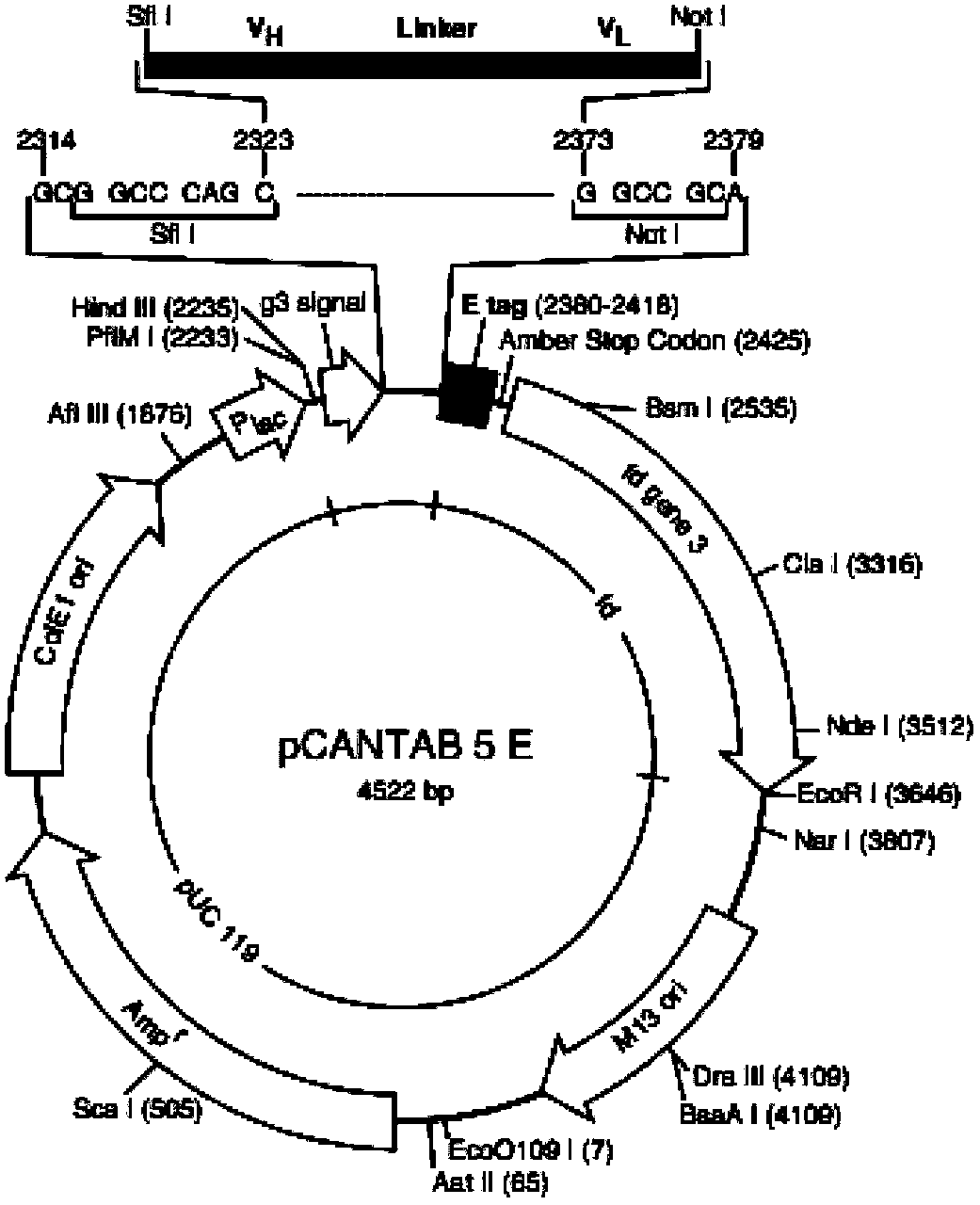
[0066] Example 3: Construction of humanized antibody 37 phage ScFv
[0067] After obtaining the sequences of the 37hAb heavy chain variable region and light chain variable region, primers were designed and constructed on the phage vector pCANTAB 5E to form a phage-ScFv recombinant vector. Wherein ScFv is constructed into a kind of VH-(G 4 S) 3 - In the VL form, there is a connecting peptide consisting of 15 amino acids between the heavy chain variable region sequence and the light chain variable region sequence, and the existence of the connecting peptide will not affect the correct folding of the ScFv.
[0068] Construction of 37 phage recombinant vectors
[0069] After obtaining the sequences of the variable region of the heavy chain and the variable region of the light chain of 37hAb, the upstream and downstream primers 37VHF, 37VHR and 37VKF, 37VKR at both ends were designed respectively for the sequence of the variable region of the heavy chain and the sequence of the v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com