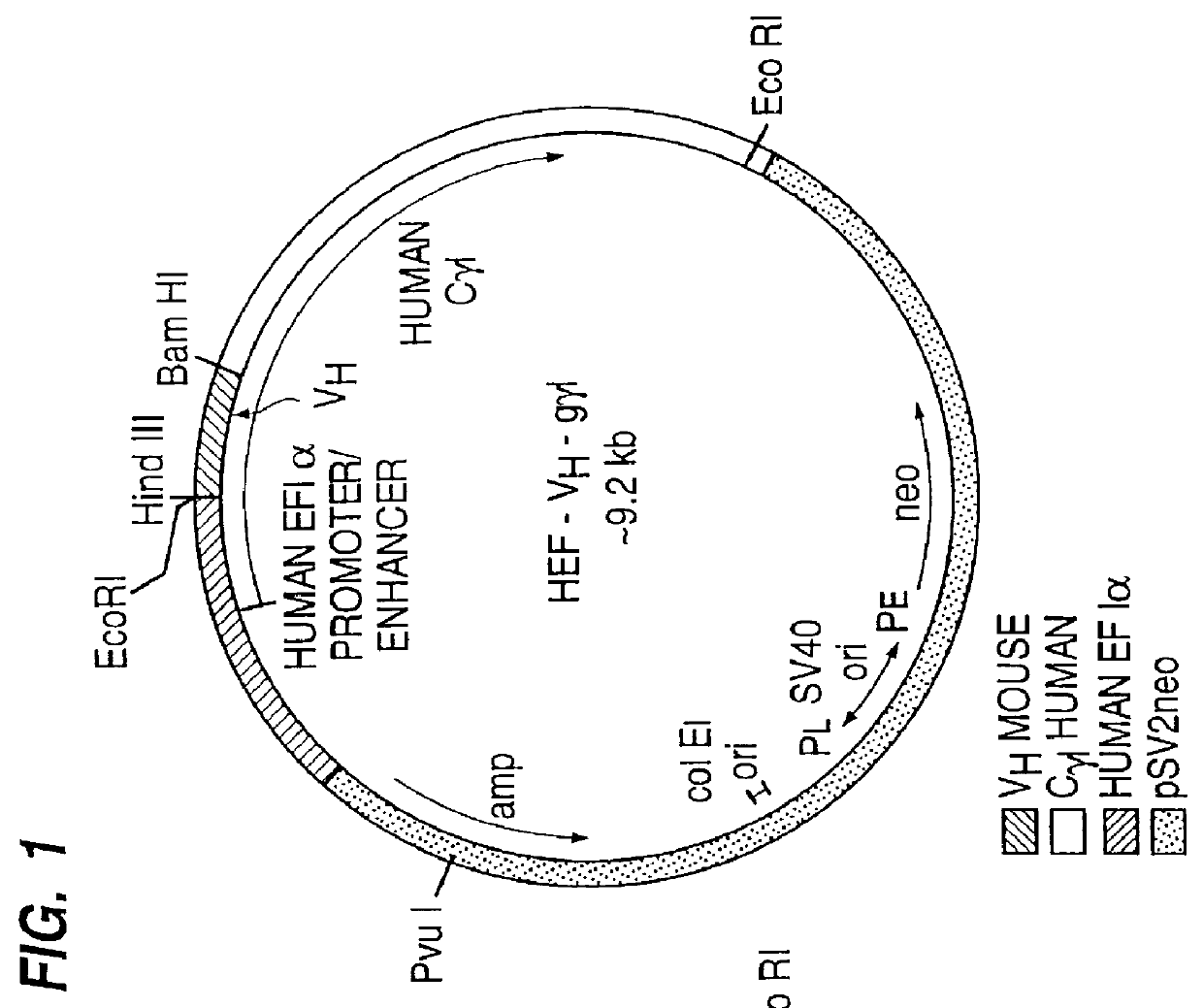
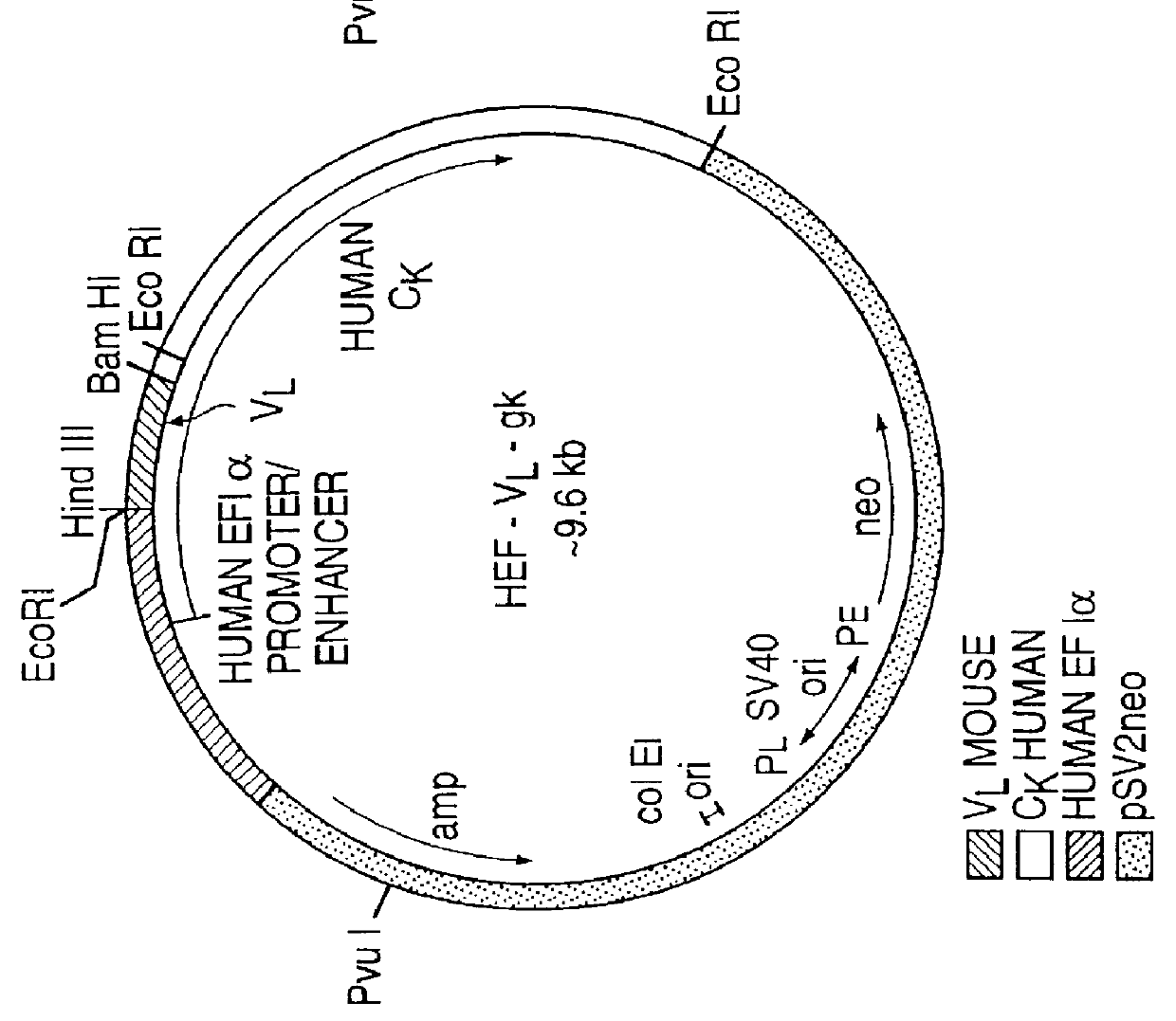
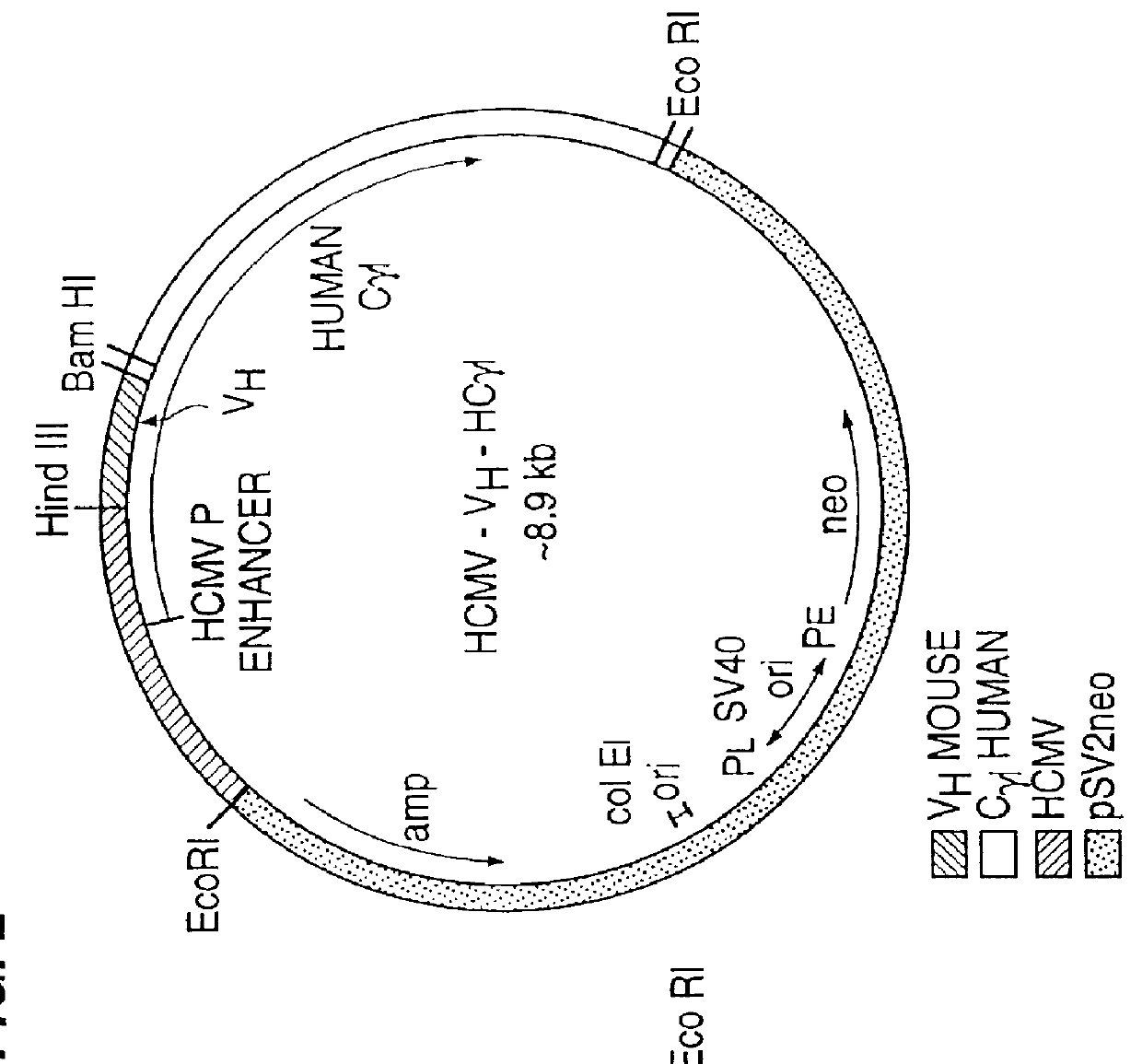
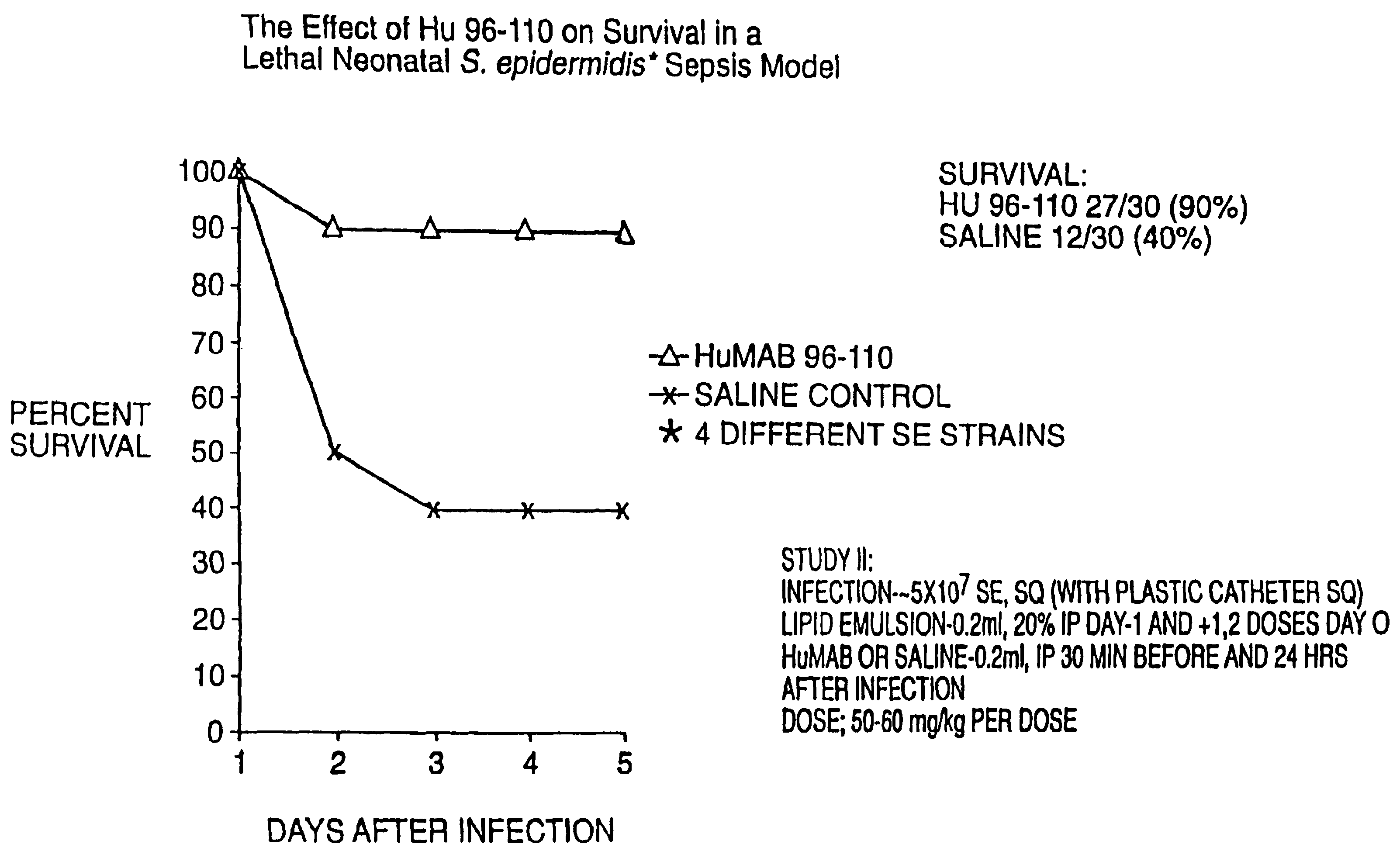
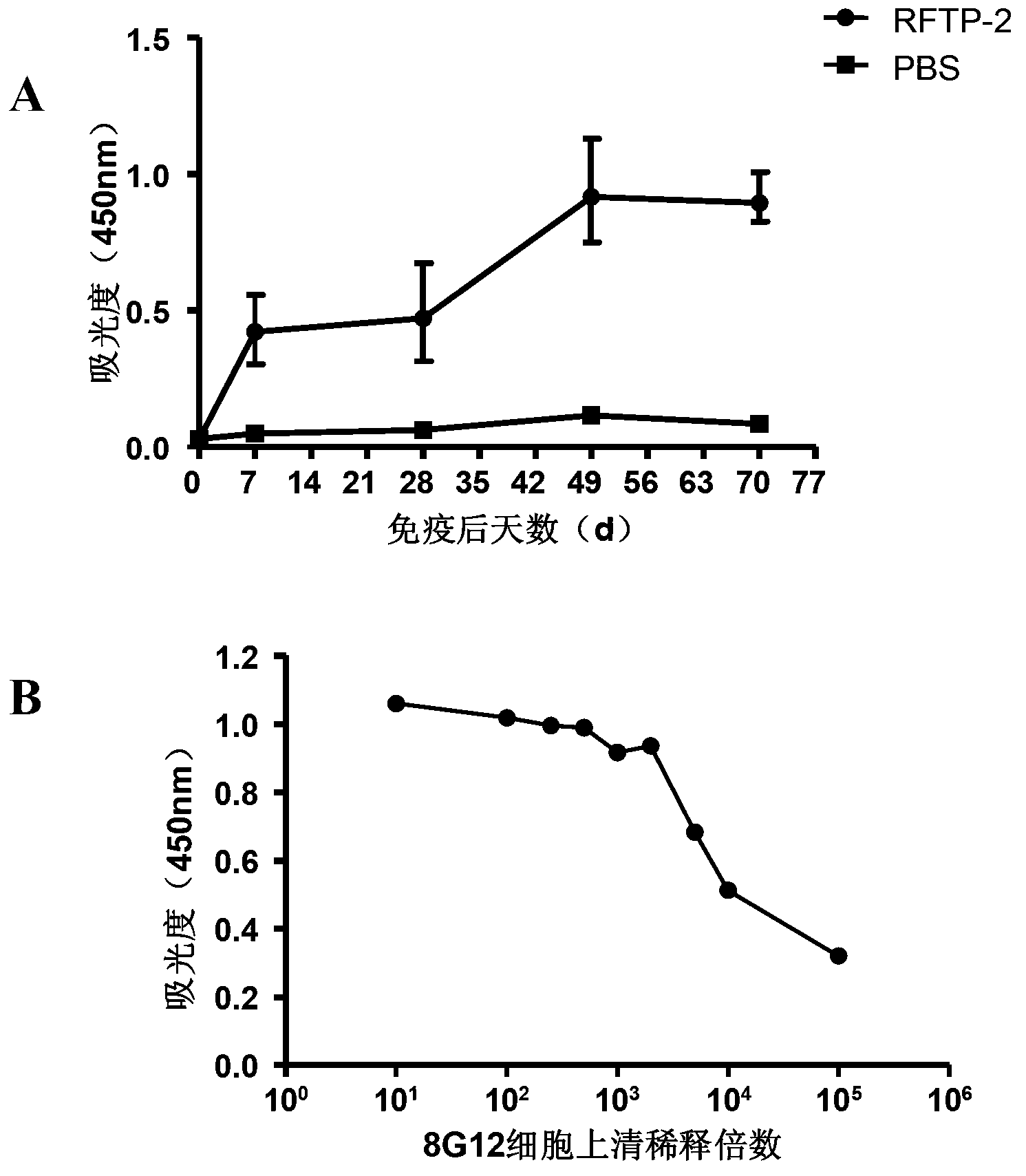
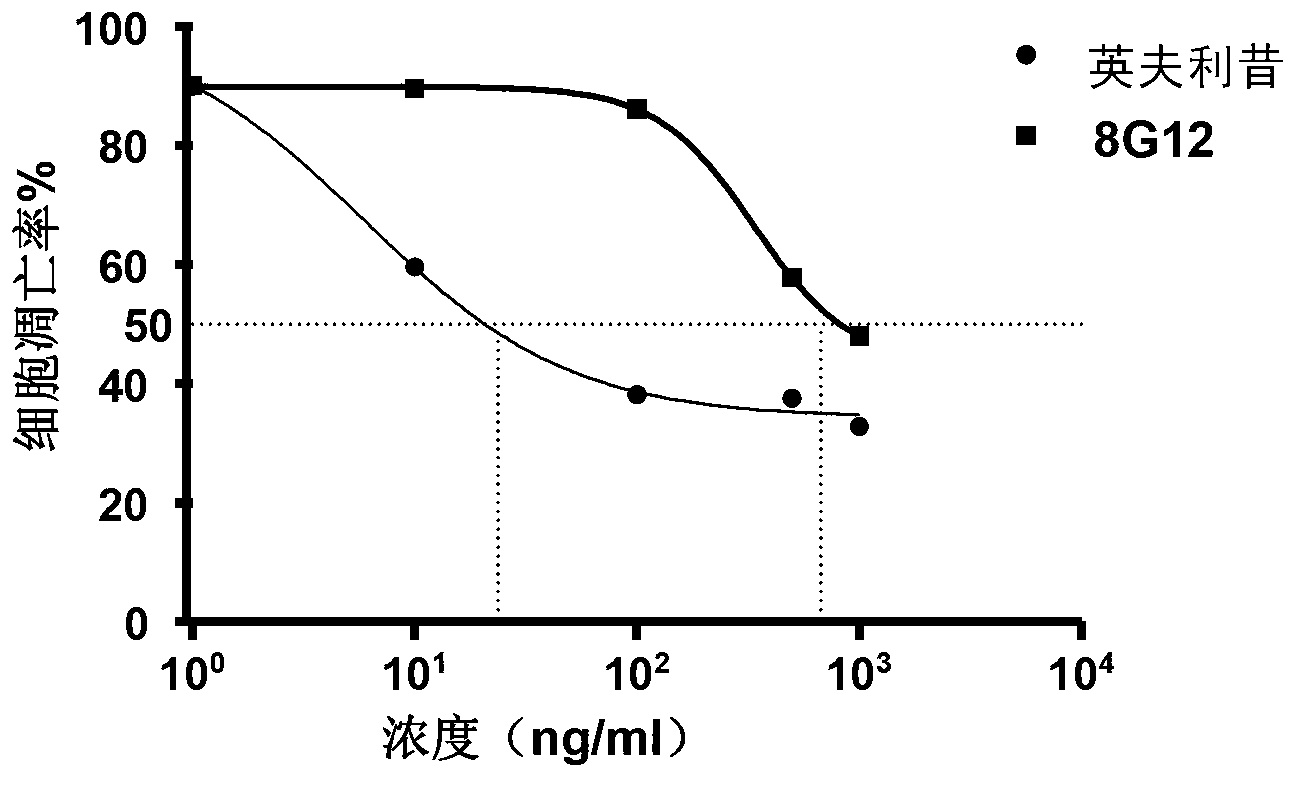
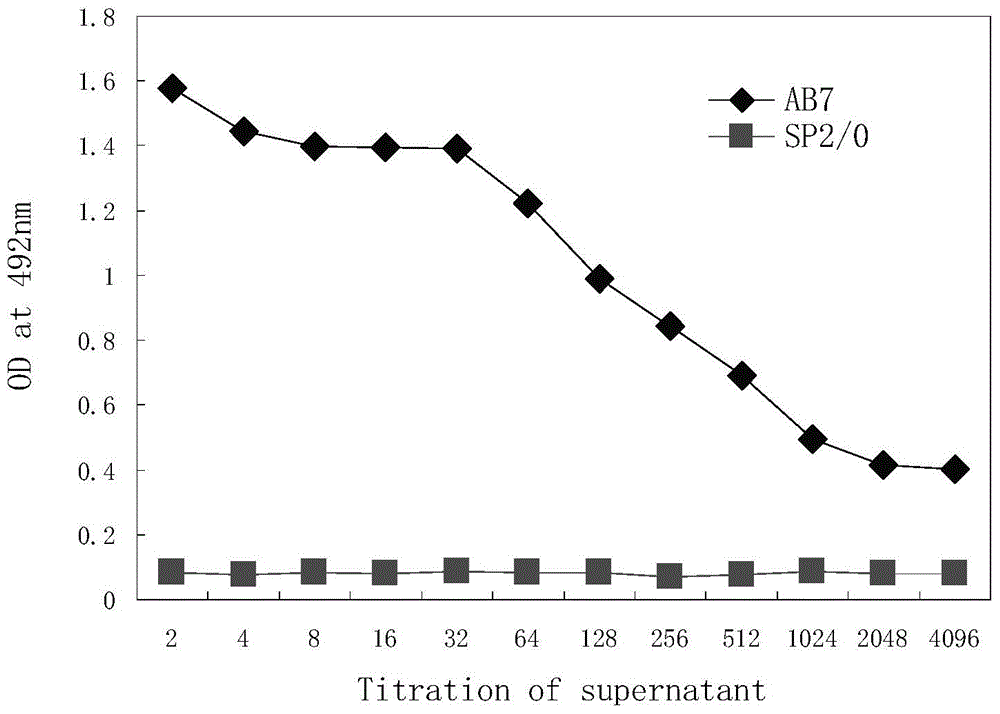
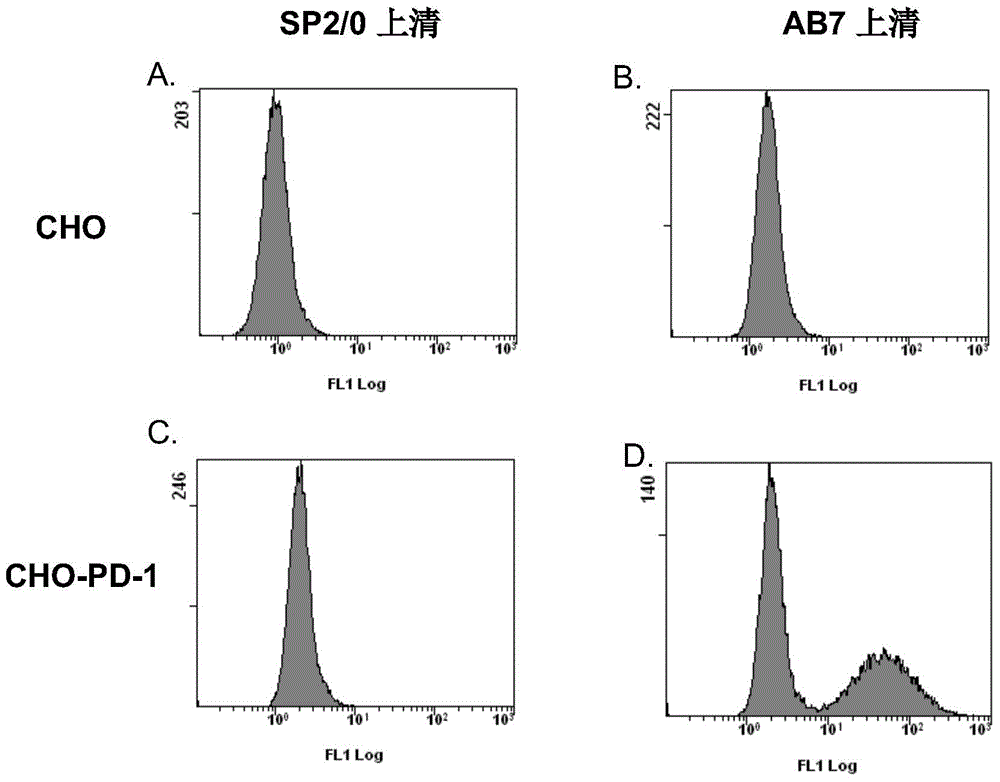
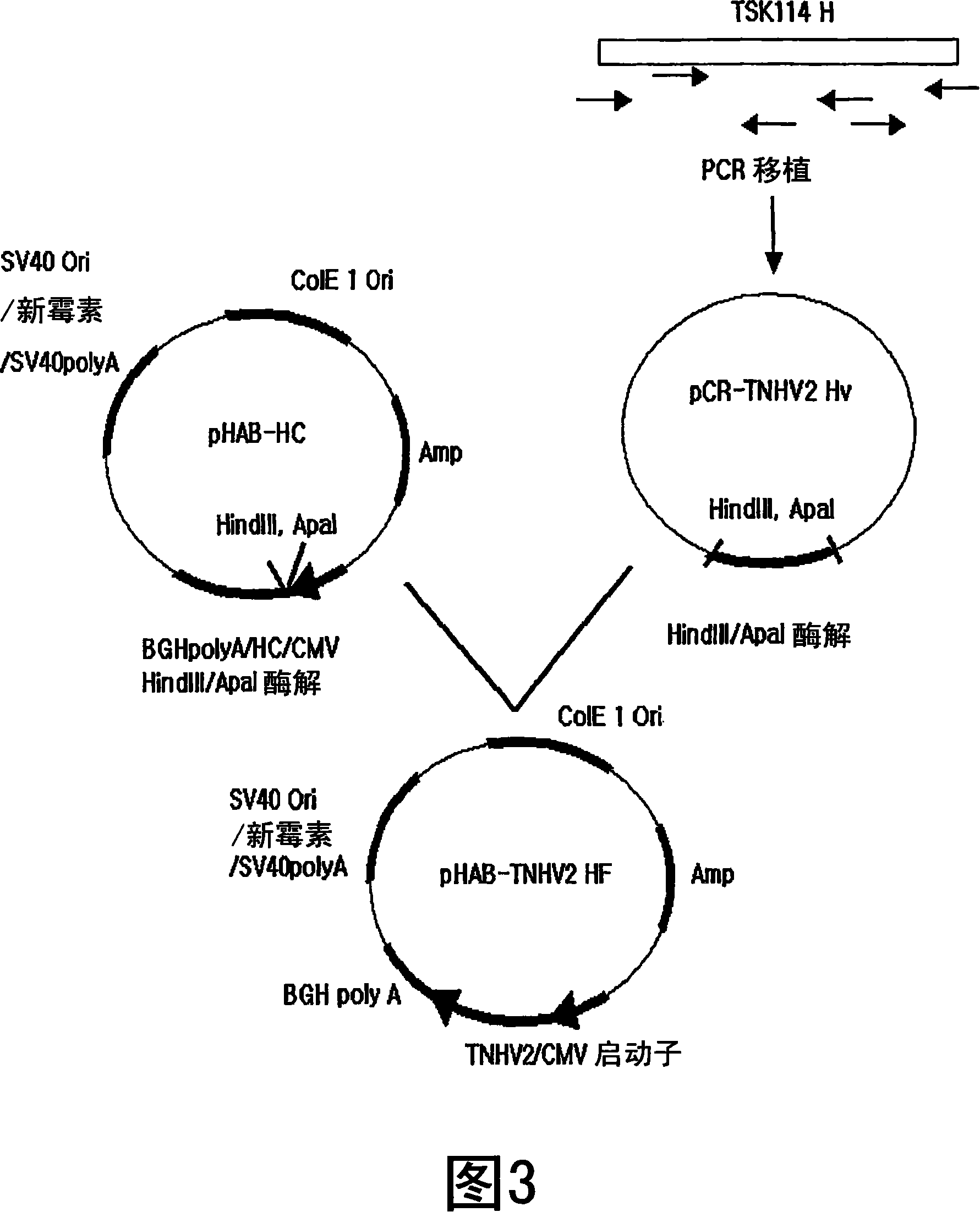
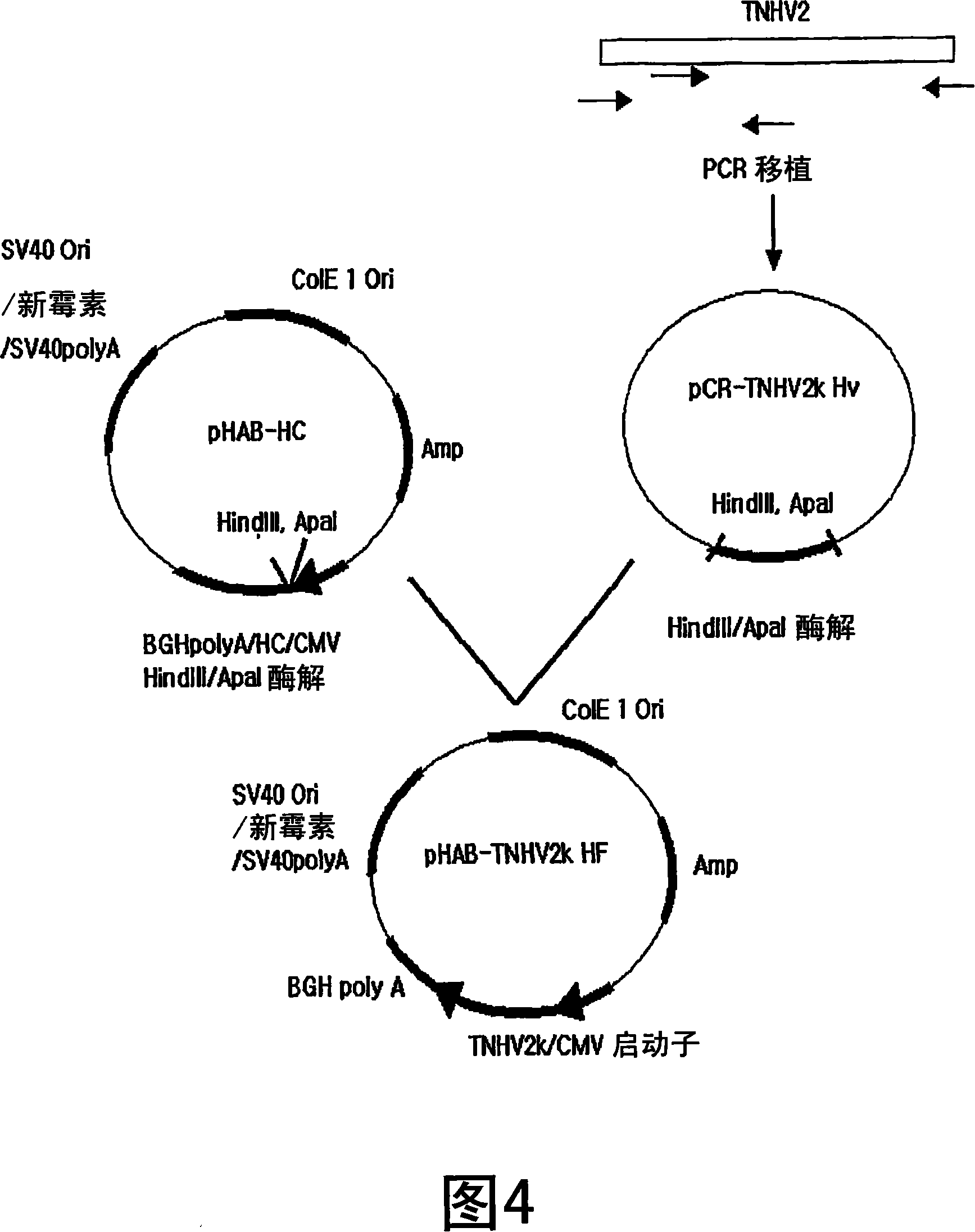
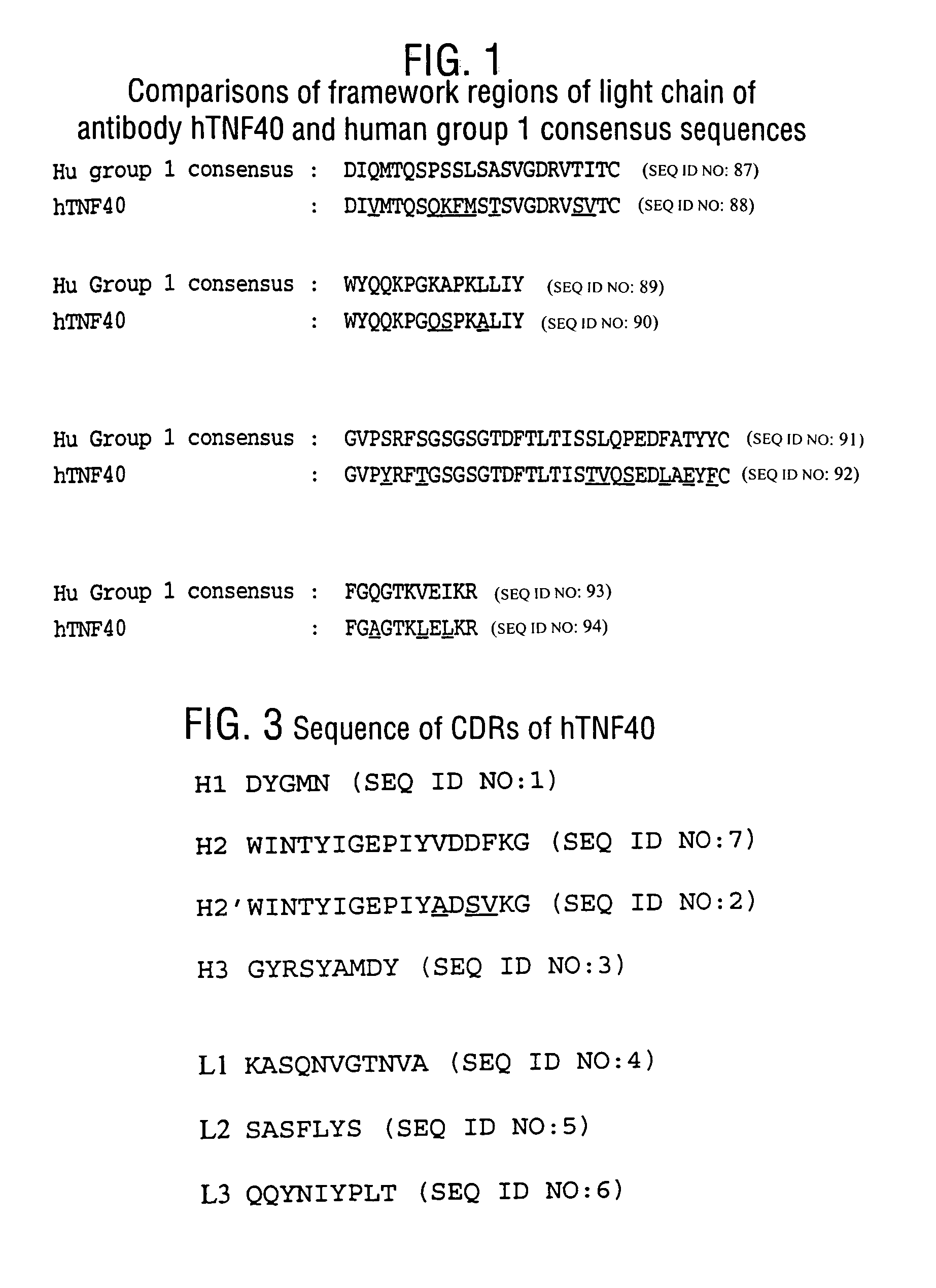
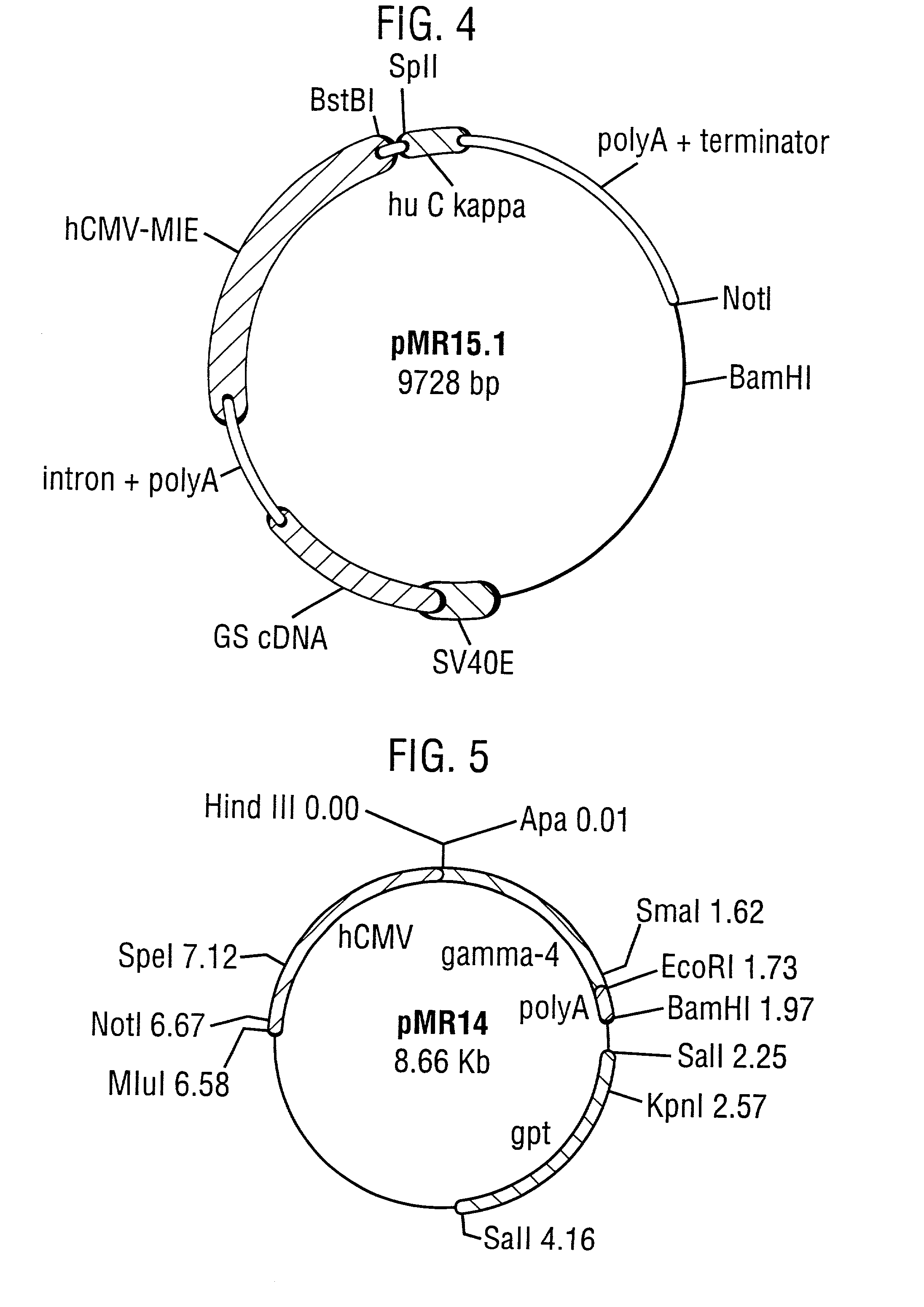
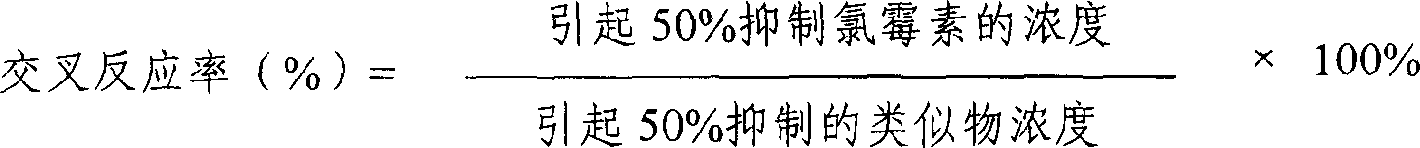Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
223 results about "Mouse monoclonal antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
ABclonal is a leader in the Research and Development of custom mouse monoclonal antibody services. Our team strives to deliver successful monoclonal antibody production to customers every time. We work with each customer to develop a solution for both the simplest and most complicated mouse monoclonal development.
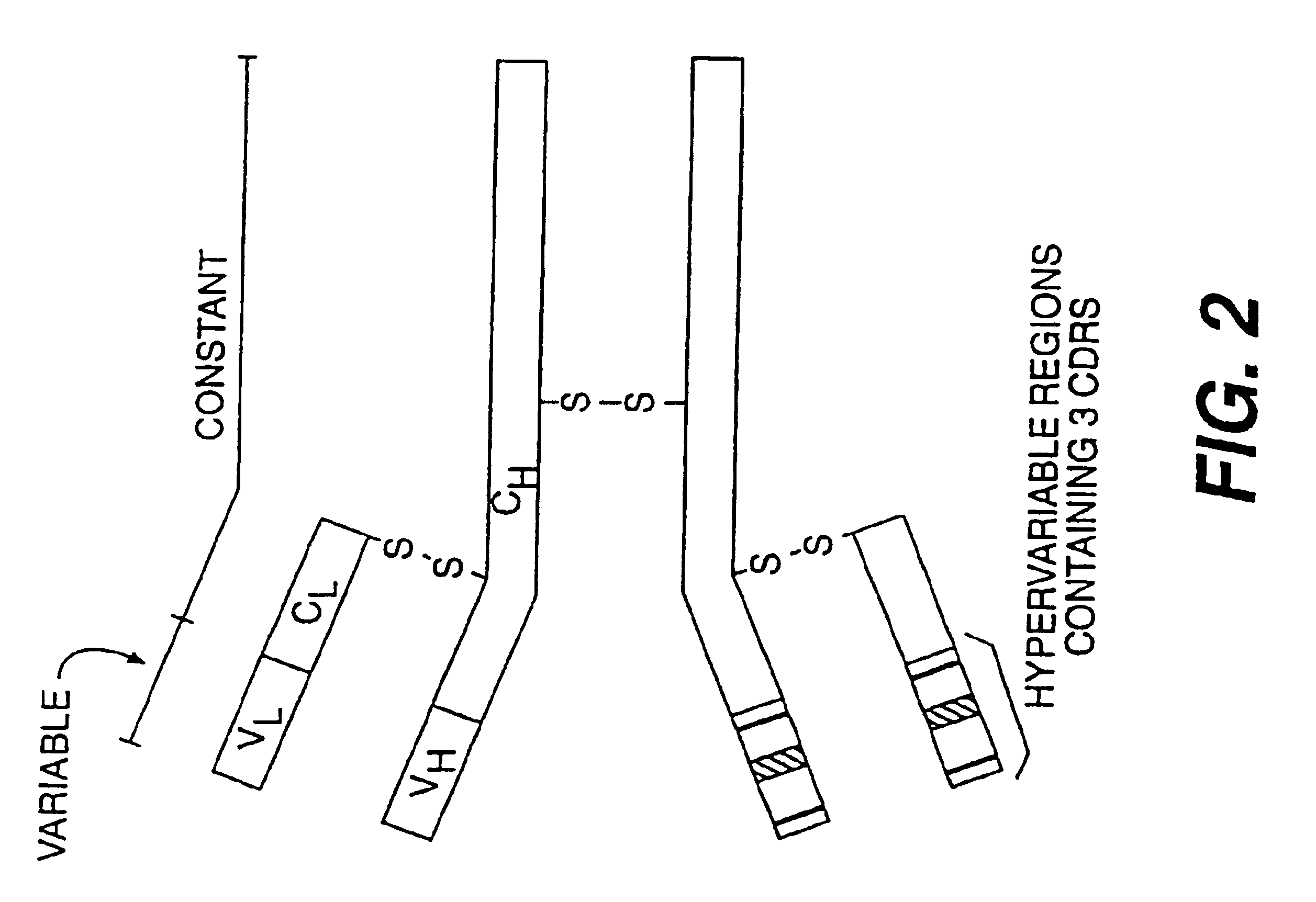
Reshaped human antibody to human interleukin-6
InactiveUS6121423AUseful purposeAntibody mimetics/scaffoldsImmunoglobulins against cytokines/lymphokines/interferonsDiseaseV region
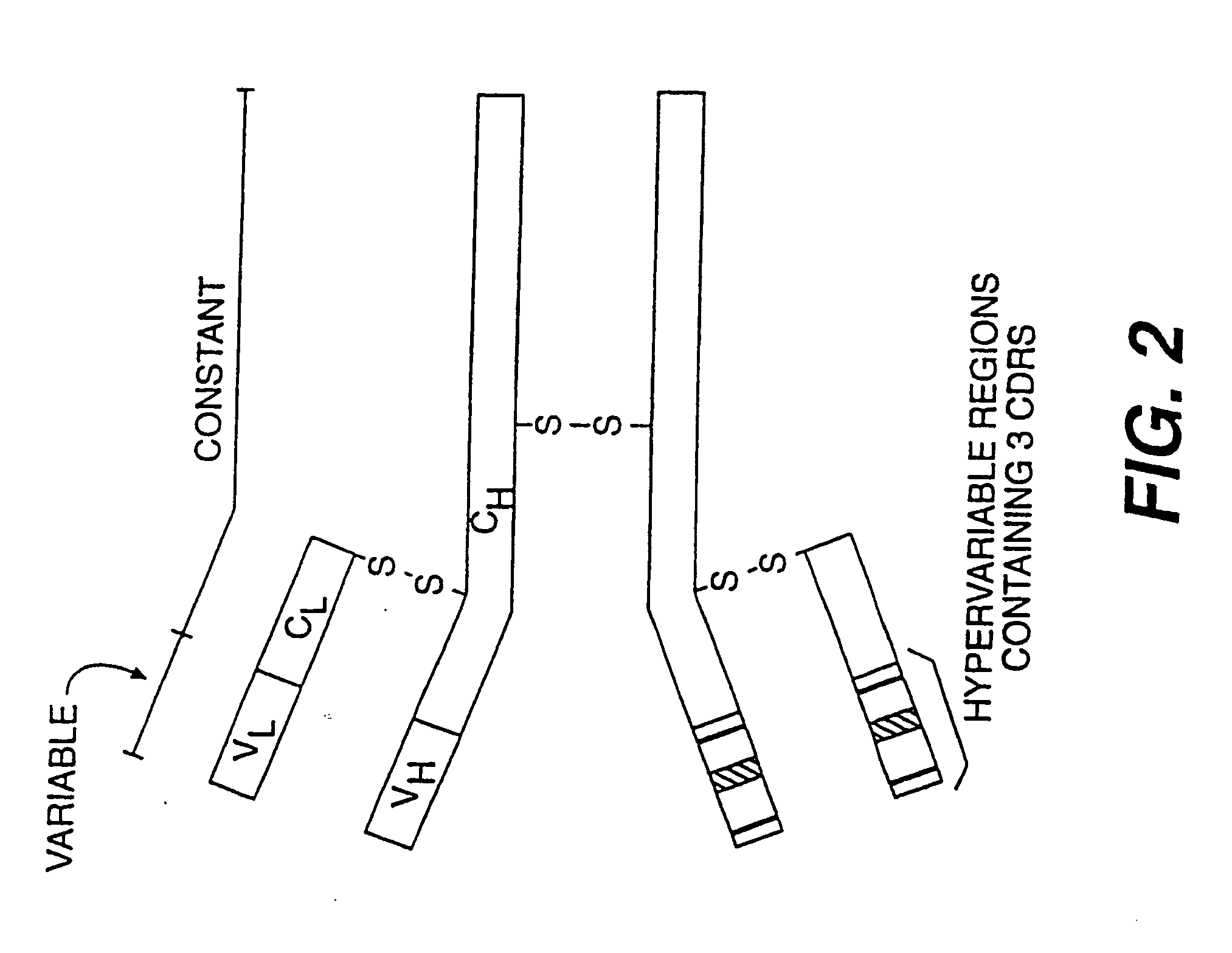
A reshaped antibody comprising: (A) L chains comprising: (1) a human C region, and (2) an L chain V region comprising human L chain FRs and L chain CDRs of a mouse monoclonal antibody; and (B) H chains comprising: (1) a human H chain C region, and (2) an H chain V region comprising human H chain FRs, and H chain CDRs of a mouse monoclonal antibody to human IL-6. Since the major portions of the reshaped human antibody are derived from human, and the mouse CDRs are less immunogenic, then the present reshaped human antibody is less immunogenic, and therefore inhibits information transfer by IL-6, and is promising as a therapeutic agent for diseases caused by IL-6.
Owner:CHUGAI PHARMA CO LTD
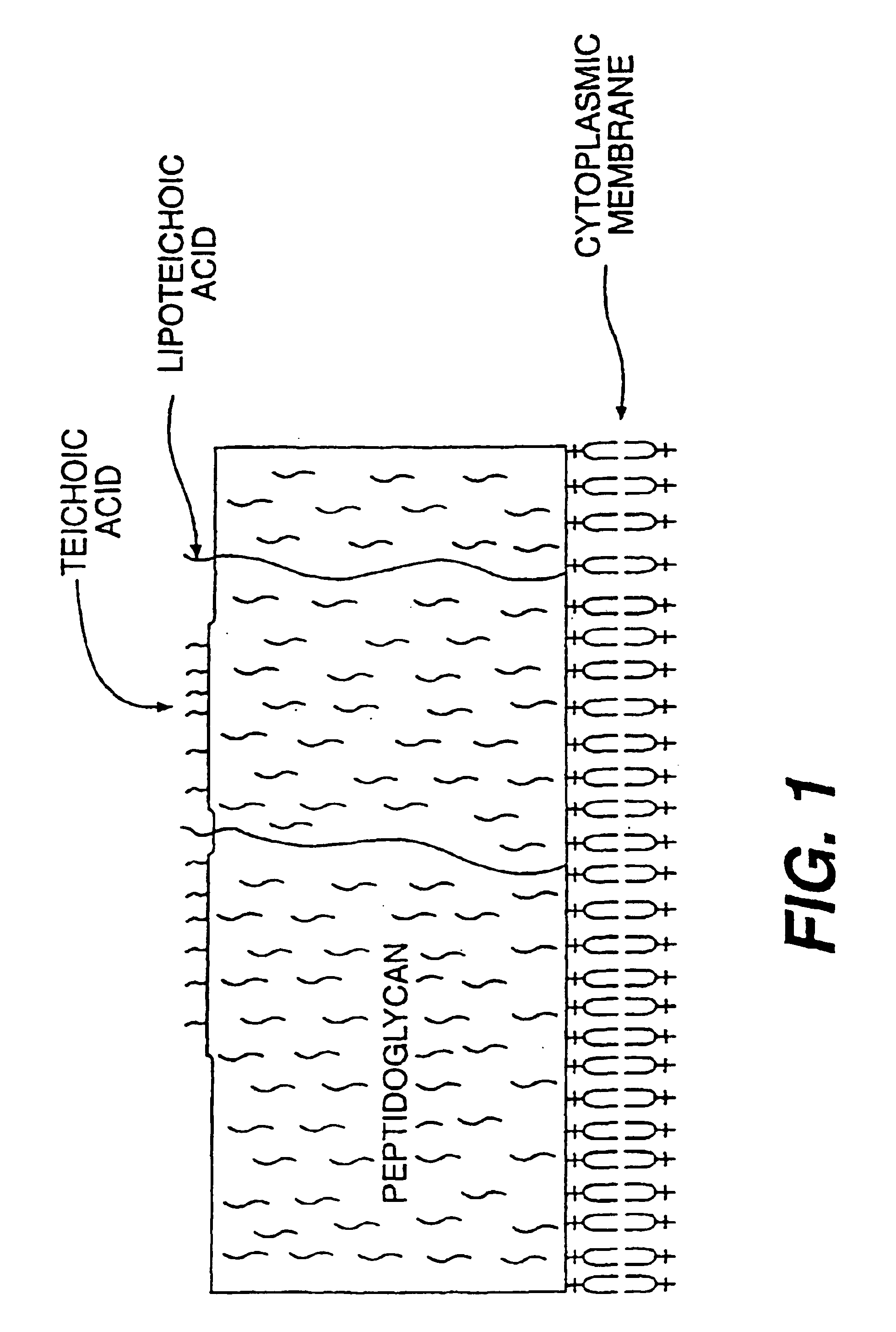
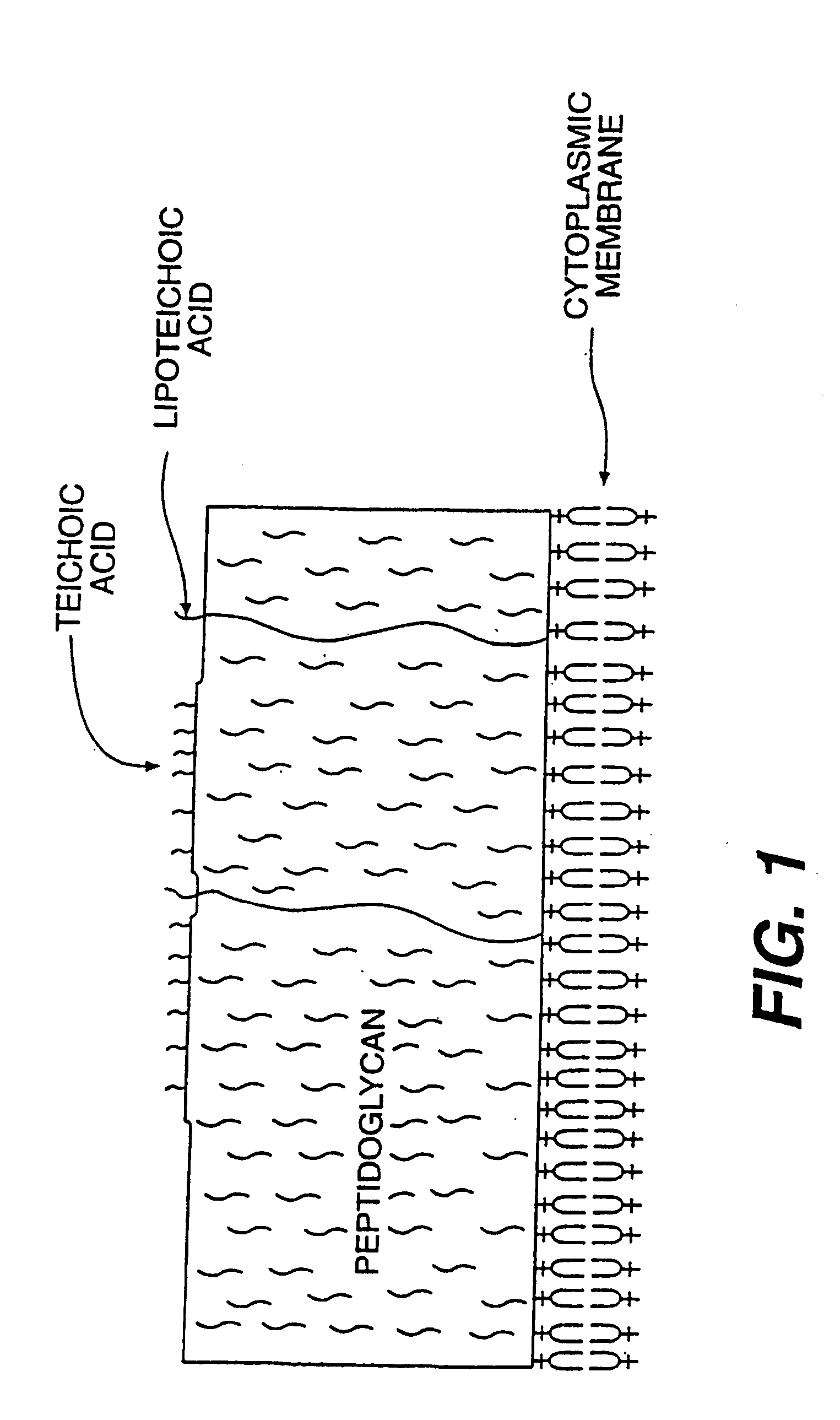
Opsonic and protective monoclonal and chimeric antibodies specific for lipoteichoic acid of gram positive bacteria
InactiveUS6939543B2Enhance phagocytosis and killing of the bacteriaIncrease infectionAntibacterial agentsHybrid immunoglobulinsBacteroidesBinding site
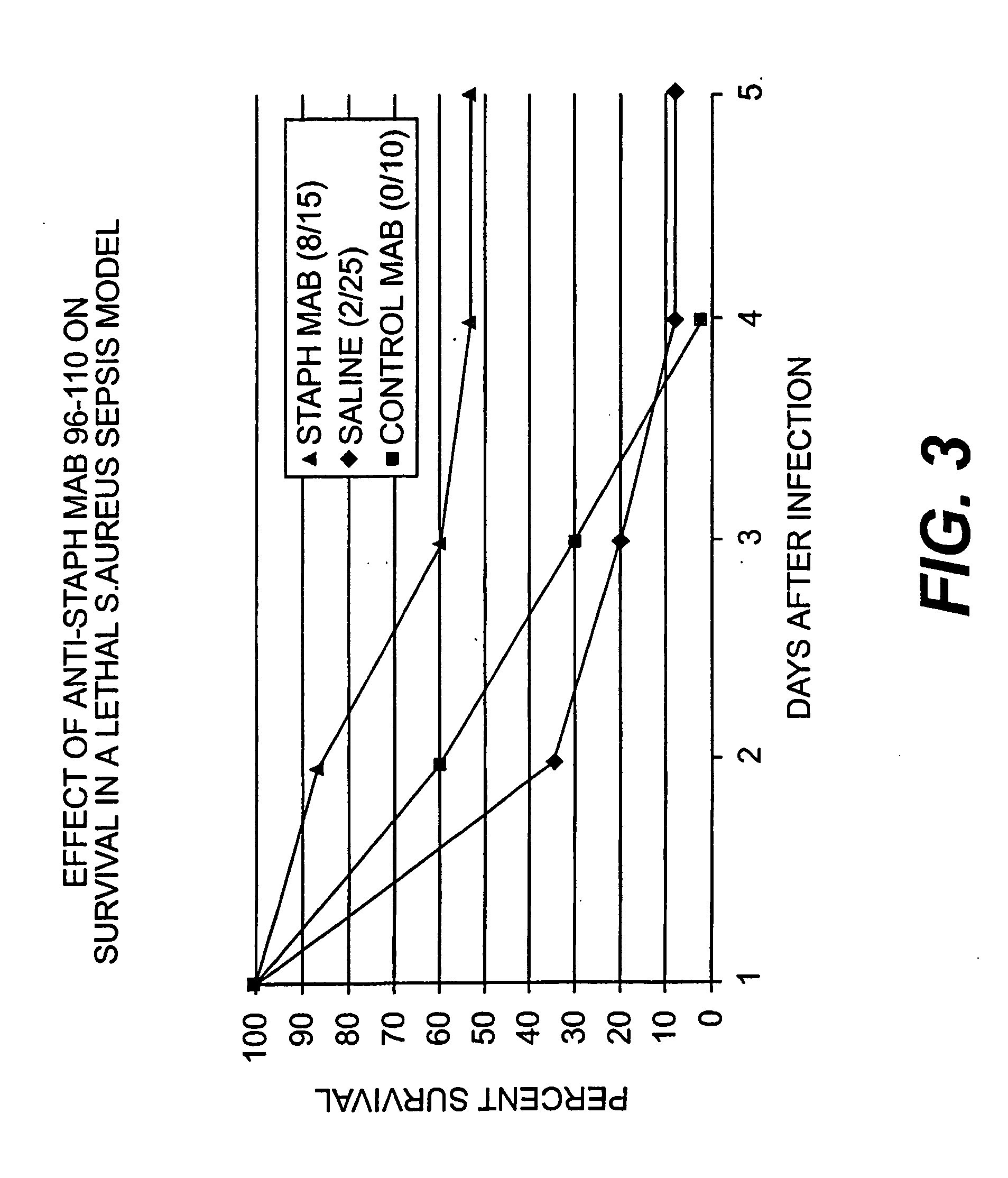
The present invention encompasses monoclonal and chimeric antibodies that bind to lipoteichoic acid of Gram positive bacteria. The antibodies also bind to whole bacteria and enhance phagocytosis and killing of the bacteria in vitro and enhance protection from lethal infection in vivo. The mouse monoclonal antibody has been humanized and the resulting chimeric antibody provides a previously unknown means to diagnose, prevent and / or treat infections caused by gram positive bacteria bearing lipoteichoic acid. This invention also encompasses a peptide mimic of the lipoteichoic acid epitope binding site defined by the monoclonal antibody. This epitope or epitope peptide mimic identifies other antibodies that may bind to the lipoteichoic acid epitope. Moreover, the epitope or epitope peptide mimic provides a valuable substrate for the generation of vaccines or other therapeutics.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC +1
Humanized anti-CD22 antibodies and their use in treatment of oncology, transplantation and autoimmune disease
InactiveUS7829086B2Sugar derivativesImmunoglobulins against animals/humansAntigenImmunoglobulin Heavy Chain Variable Region
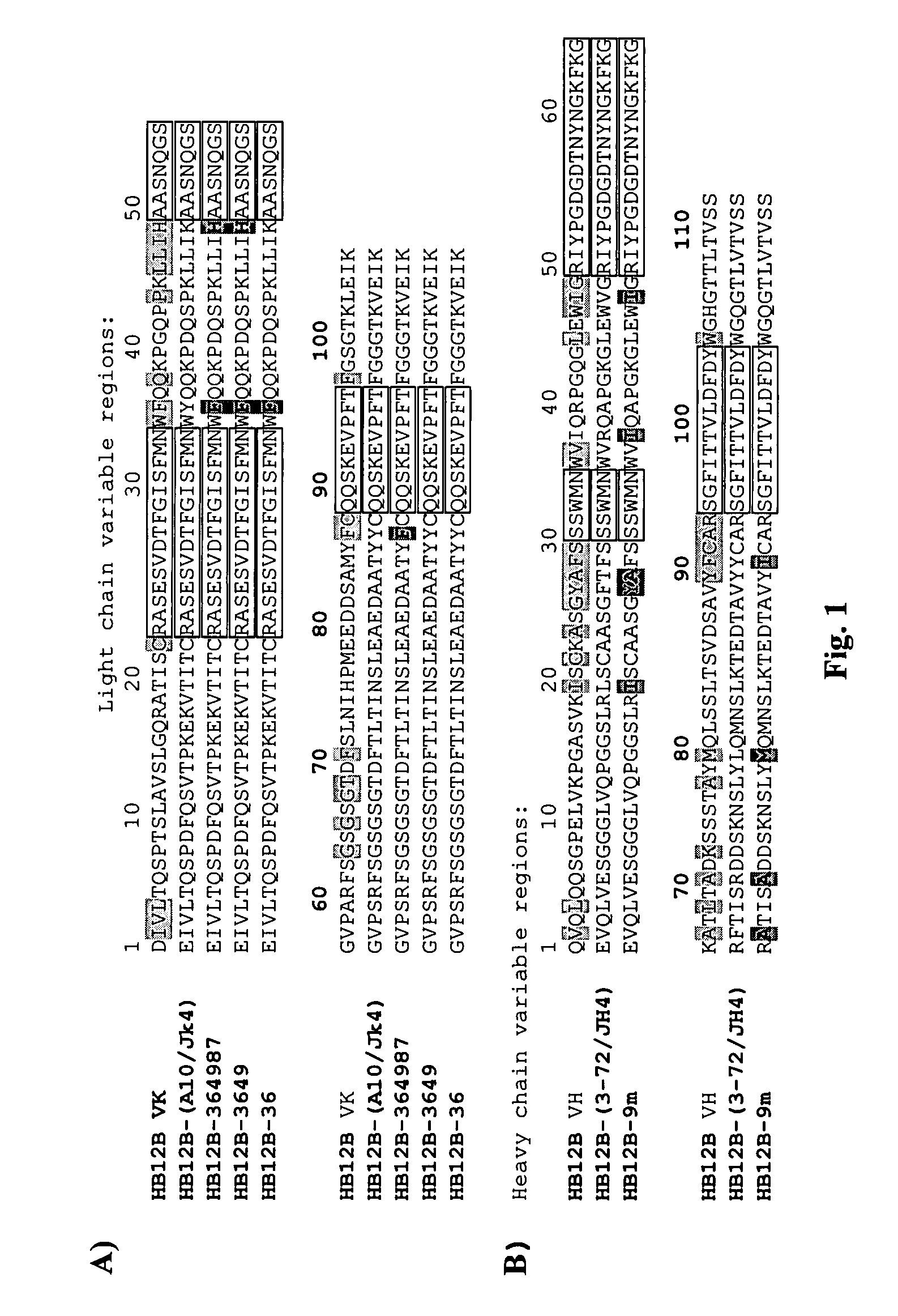
The present invention provides chimeric and humanized versions of anti-CD22 mouse monoclonal antibody, HB22.7. The anti-CD22 antibodies of the invention comprise four human or humanized framework regions of the immunoglobulin heavy chain variable region (“VH”) and four human or humanized framework regions of the immunoglobulin light chain variable region (“VK”). The invention further comprises heavy and / or light chain FW regions that contain one or more backmutations in which a human FW residue is exchanged for the corresponding residue present in the parental mouse heavy or light chain. Human or humanized VH framework regions of antibodies of the invention may comprise one or more of the following residues: a valine (V) at position 24 of framework region 1, a glycine (G) at position 49 of framework region 2, and an asparagine (N) at position 73 of framework region 3, numbered according to Kabat. The invention further relates to pharmaceutical compositions, immunotherapeutic compositions, and methods using therapeutic antibodies that bind to the human CD22 antigen and that preferably mediate human ADCC, CDC, and / or apoptosis for: the treatment of B cell diseases and disorders in human subjects, such as, but not limited to, B cell malignancies, for the treatment and prevention of autoimmune disease, and for the treatment and prevention of graft-versus-host disease (GVHD), humoral rejection, and post-transplantation lymphoproliferative disorder in human transplant recipients.
Owner:MEDIMMUNE LLC +1
Humanized anti-CD22 antibodies and their use in treatment of oncology, transplantation and autoimmune disease
InactiveUS20070258981A1Efficient productionEfficiently depletedSugar derivativesImmunoglobulins against animals/humansAntigenAutoimmune condition
Owner:MEDIMMUNE LLC +1
Monoclonal antibody for anti-human CEA, a composition containing same and application thereof
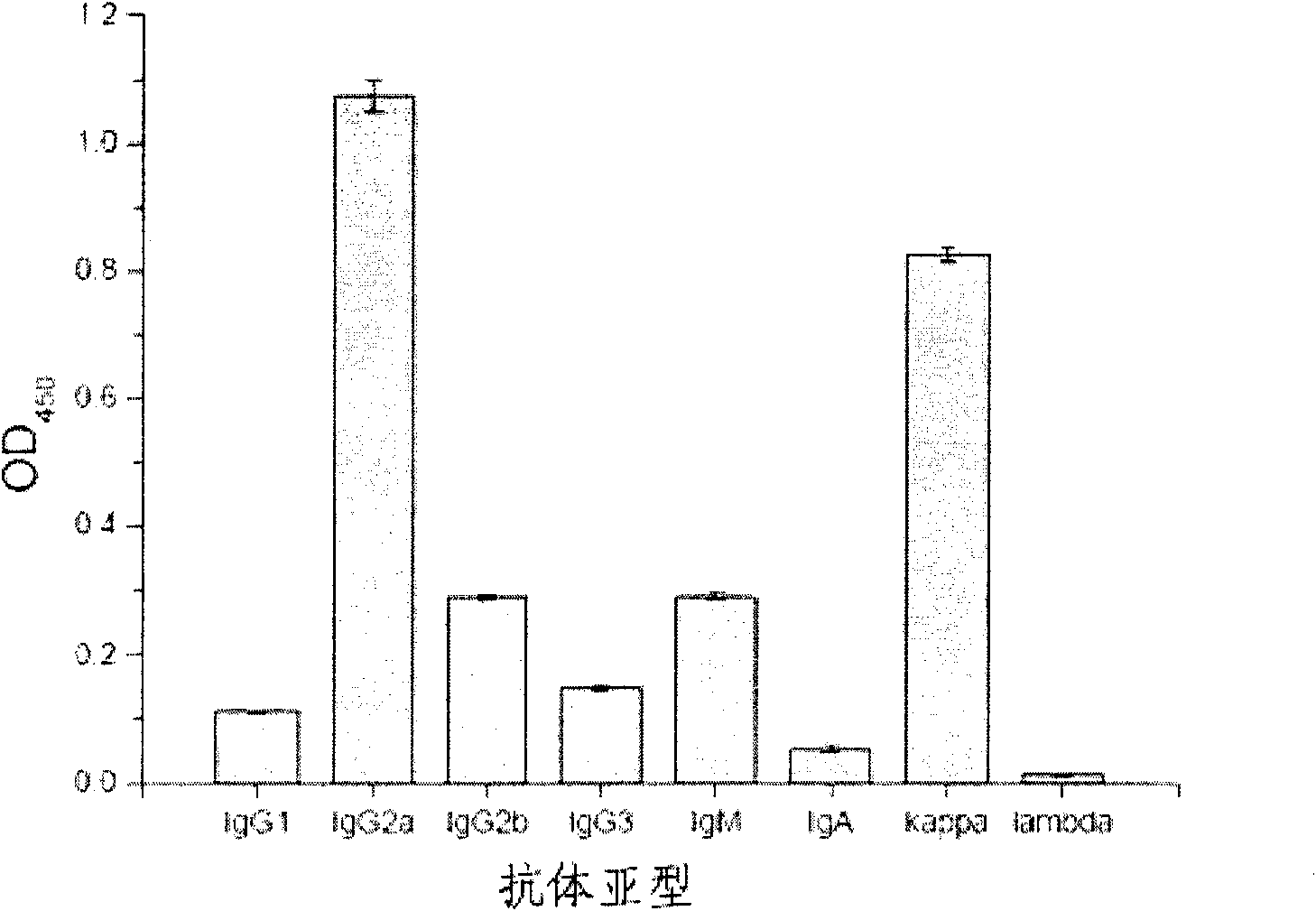
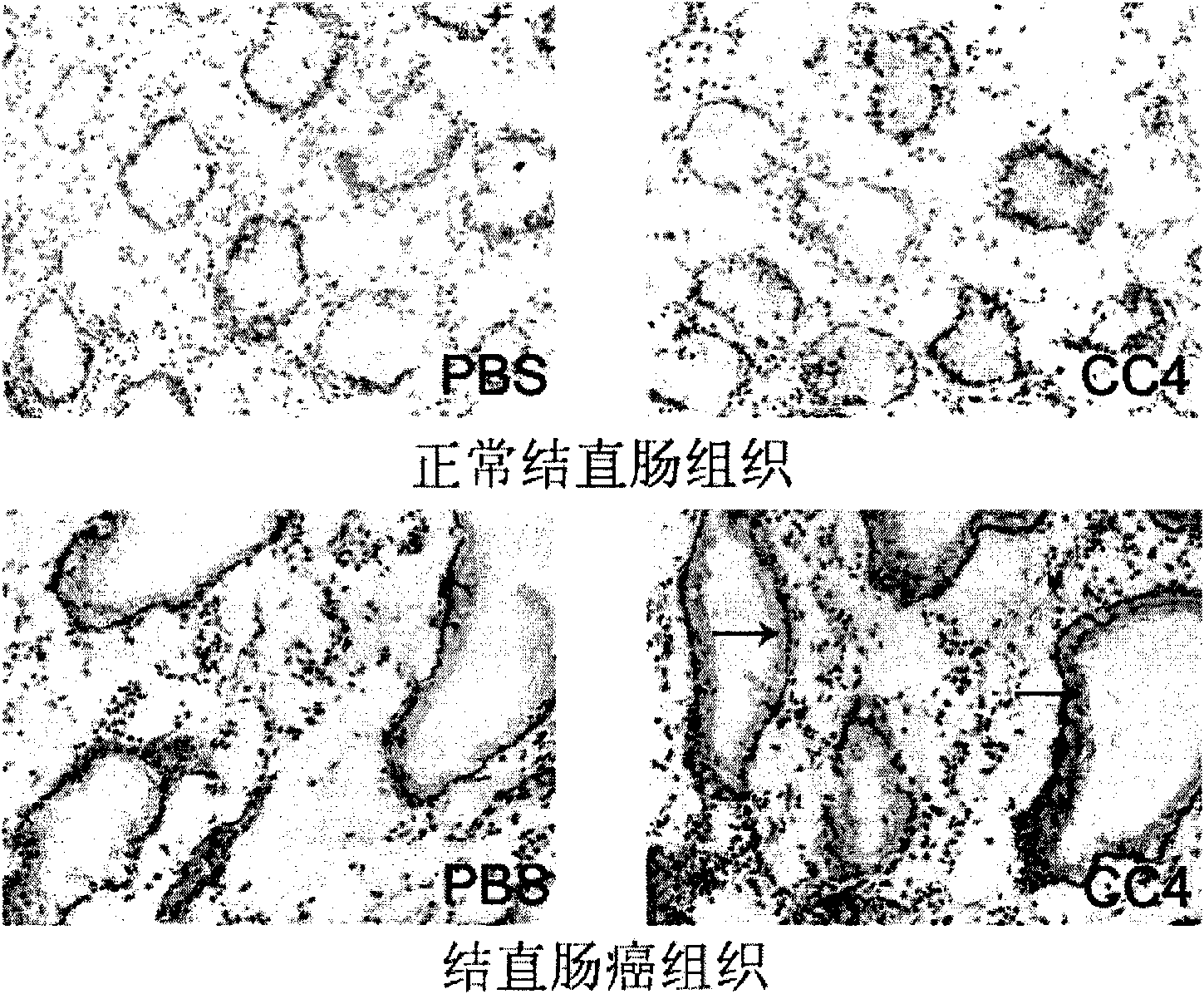
The invention relates to a mouse monoclonal antibody of anti-human carcino-embryonic antigen (CEA) resisting colorectal neoplasm activity, prepared by a biological technology. The method comprise a method for detecting CEA family protein by utilizing the antigen-combining capacity specificity of the mouse monoclonal antibody CC4 of anti-human CEA and a neoplasm treatment method by utilizing the colorectal neoplasm activity. The antibody can be used for specifically recognizing the human CEA at molecular, cellular and texture levels and recognizing a special antigen epiposition widely distributed in the CEA family protein. The CC4 is specifically combined with colorectal neoplasm texture at the texture level and presents stronger capabilities of restraining neoplasm growth, neoplasm metastasis, neoplasm invasion in an animal model and at the cellular level. The antibody and detection and treatment methods based on the antibody can become an effective neoplasm diagnosis and treatment tool in basal research or clinical application.
Owner:INSITUTE OF BIOPHYSICS CHINESE ACADEMY OF SCIENCES
Vaccines, methods, and antibodies specific for lipoteichoic acid of gram positive bacteria
InactiveUS20060002939A1Enhance phagocytosis and killing of the bacteriaIncrease infectionAntibacterial agentsHybrid immunoglobulinsBacteroidesBinding site
Owner:ALTOR BIOSCIENCE CORP +1
Humanized antibody specific for surface antigen pre-S1 of HBV and preparation method thereof
InactiveUS7115723B1High affinityLow immunogenicitySugar derivativesDigestive systemAntigenHumanized antibody
The present invention relates to humanized antibodies specific for HBV surface antigen pre-S1, which show binding affinity similar to mouse monoclonal antibody and which show remarkably reduced immunogenicity since they have less mouse-derived amino acid residues. Thus, the humanized antibodies of the present invention may be useful for the prevention of HBV infection and for the treatment of hepatitis B.
Owner:KOREA INST OF SCI & TECH +1
Humanized antibody specific for human 4-1BB and pharmaceutical compsn comprising same
The present invention is directed to humanized antibodies that specifically bind the protein 4-1BB. The antibodies can be made by grafting of the complementarity determining regions (CDR's) of mouse monoclonal antibody to human 4-1BB to the remaining portions of a human antibody and by making further amino acid replacements. In addition, a pharmaceutical composition that includes the humanized antibody can be made and can be used to treat autoimmune diseases to suppress an immune response. The humanized antibody of the invention has high affinity for human 4-1BB, and exhibits sequence similarity to human antibody. As a result, the pharmaceutical composition of the present invention can be used to treat autoimmune disease and act as an immunosuppressant in humans without much side-effect.
Owner:LG CHEM LTD
Monoclonal antibody for antagonizing and inhibiting binding of vascular endothelial cell growth factor and its receptor, and coding sequence and use thereof
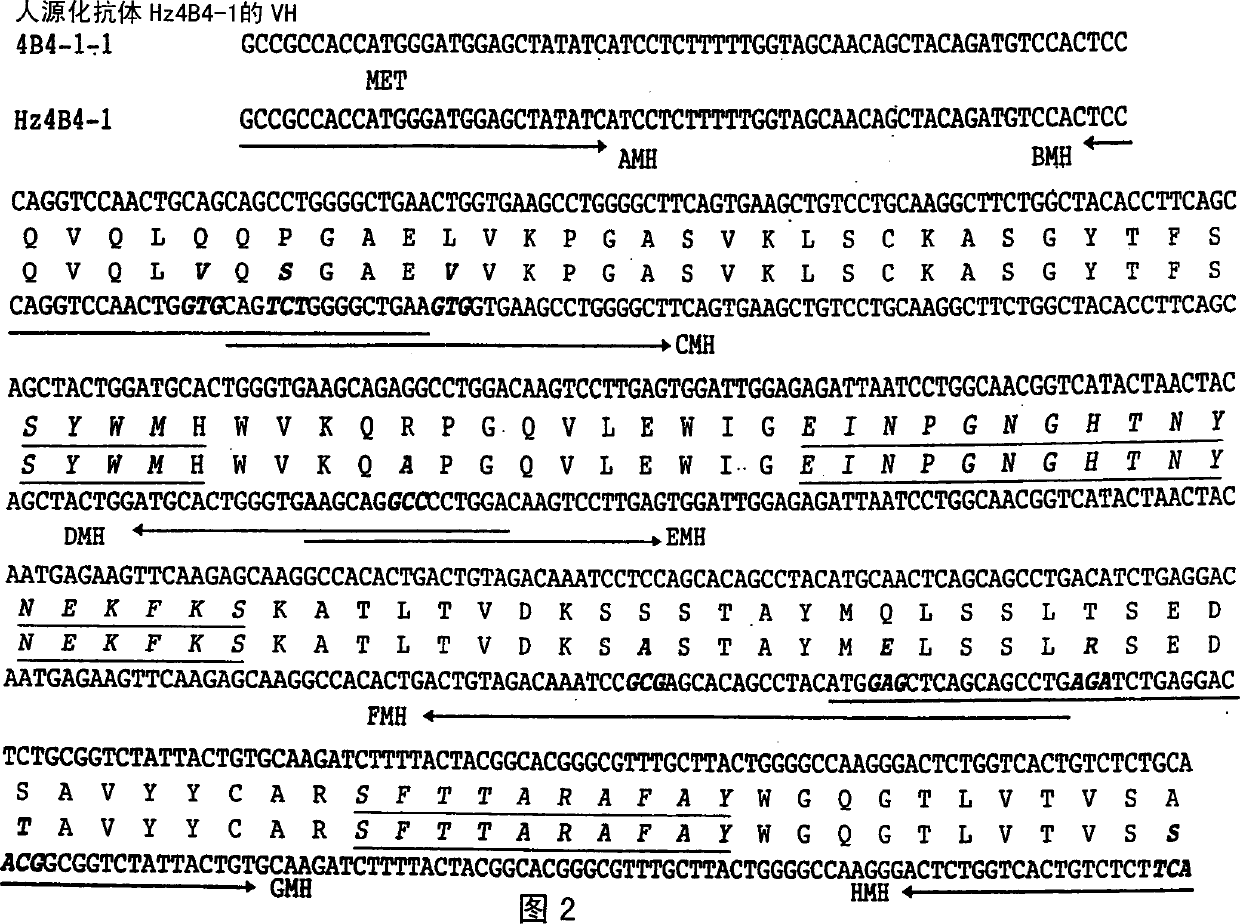
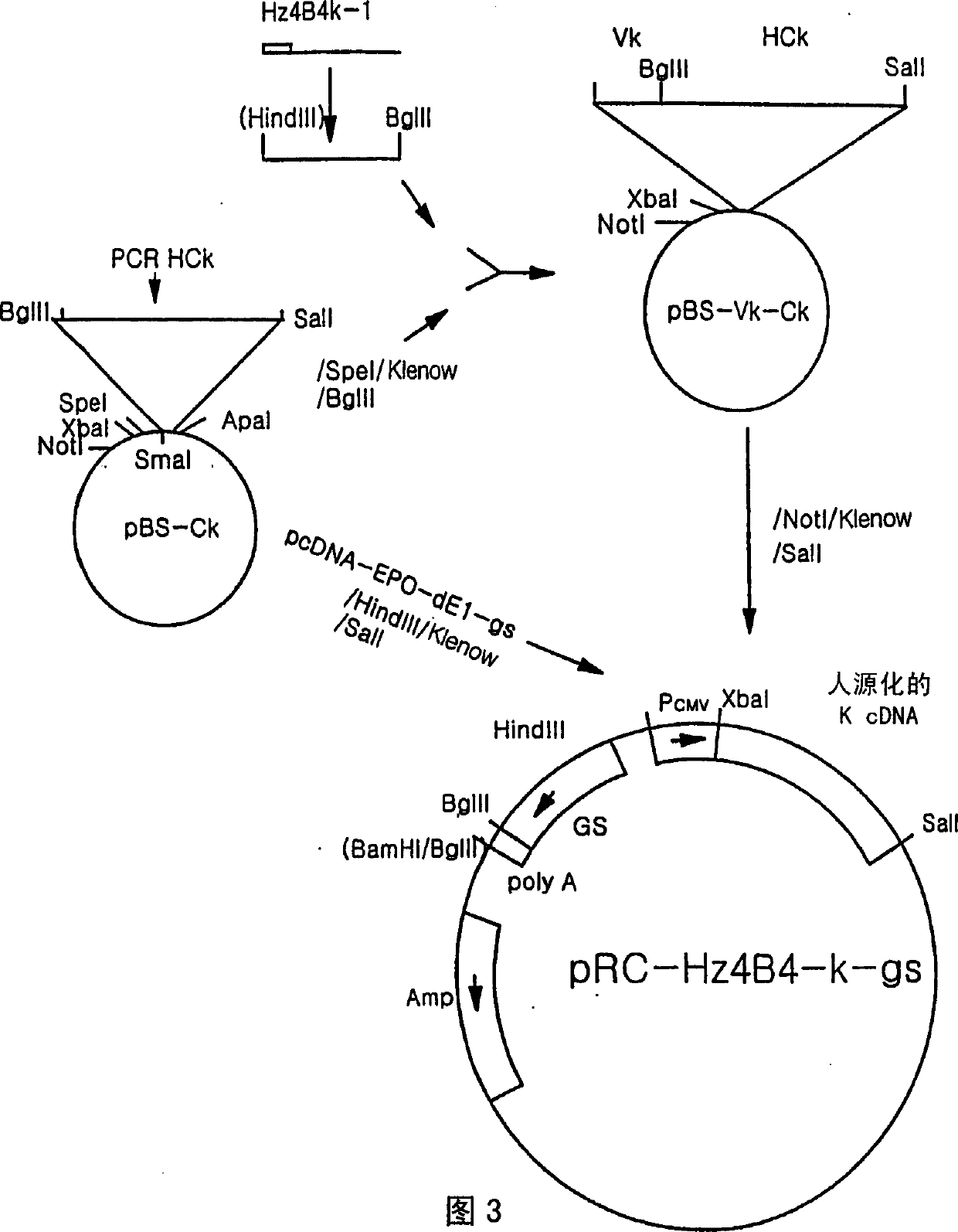
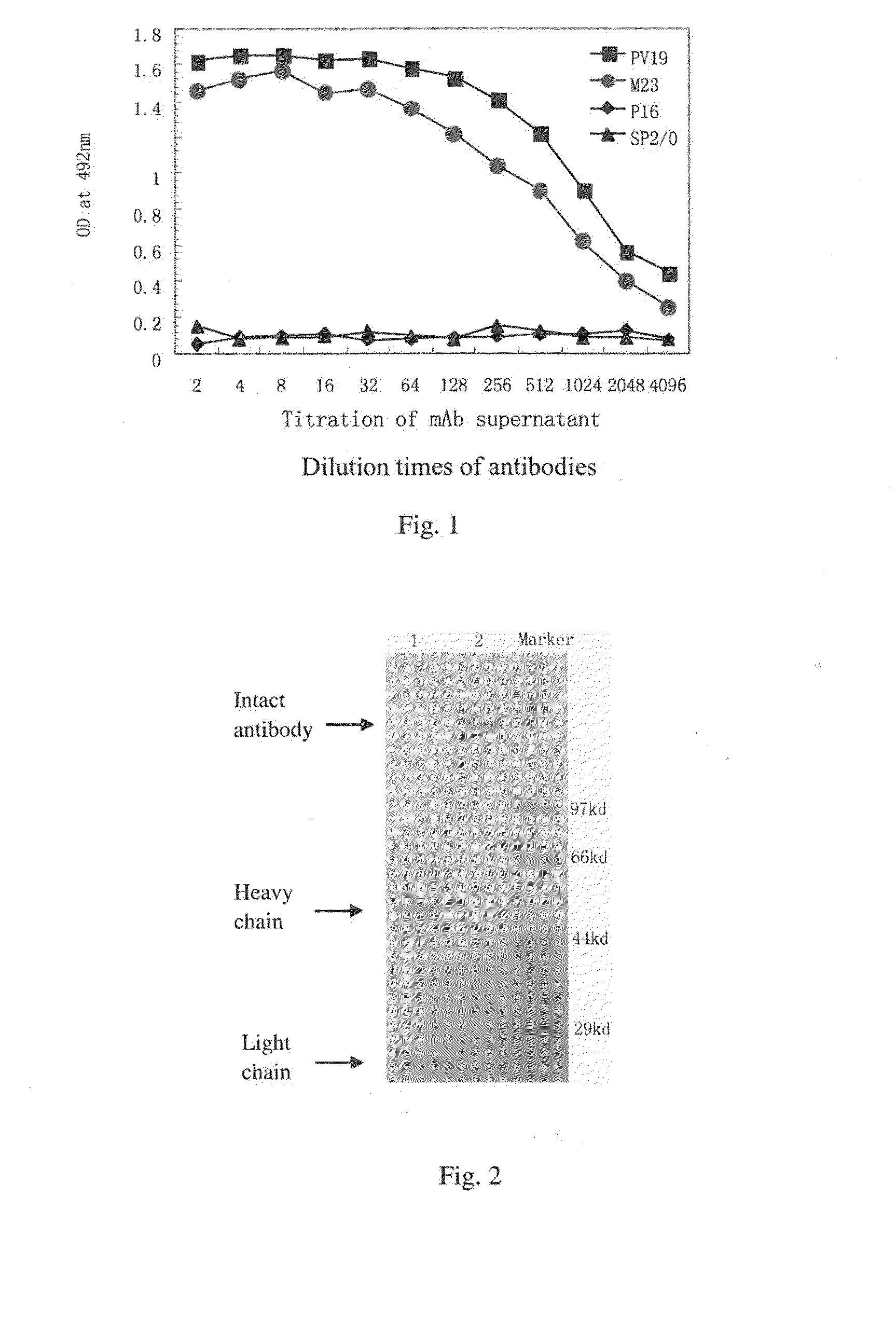
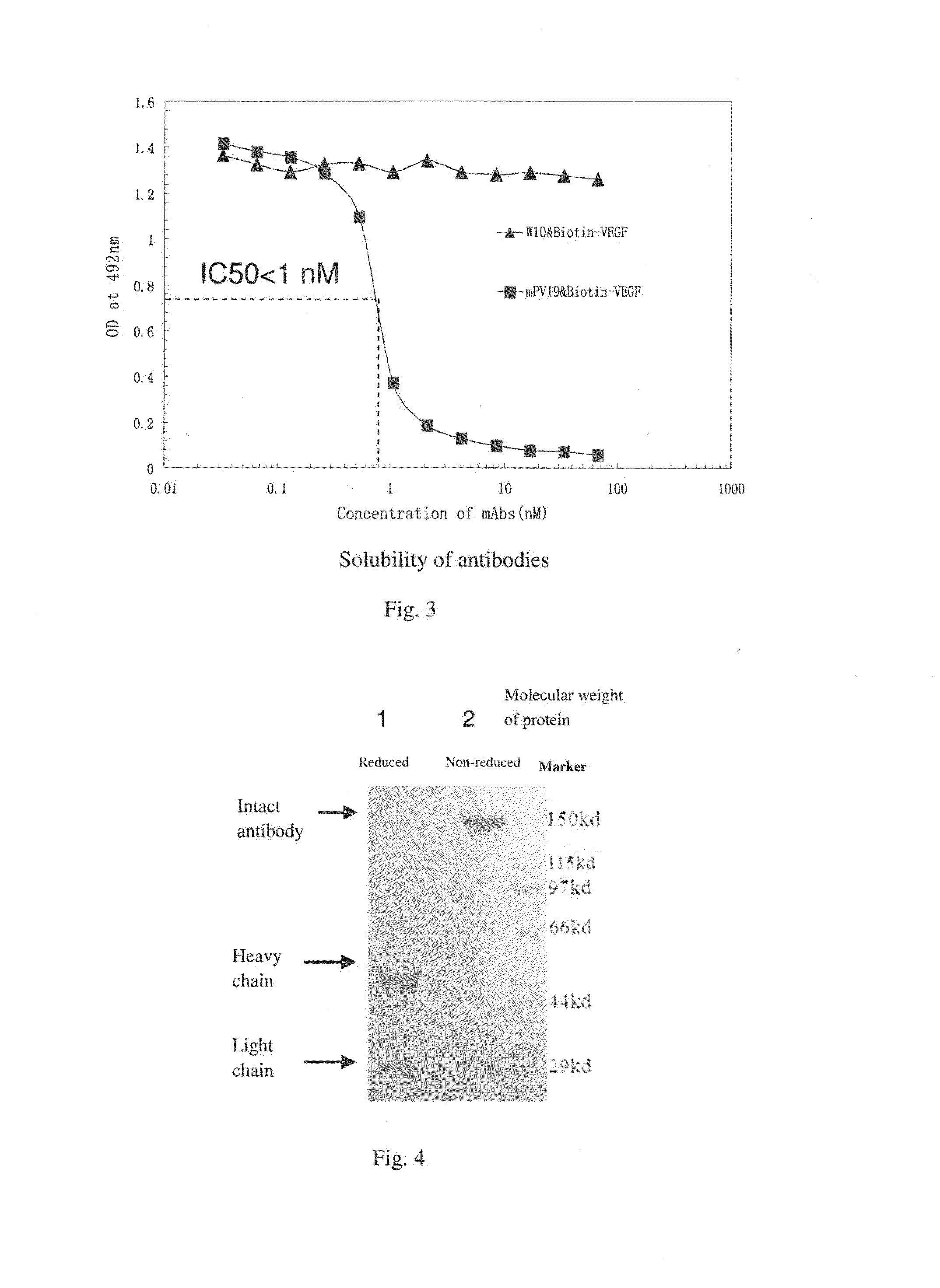
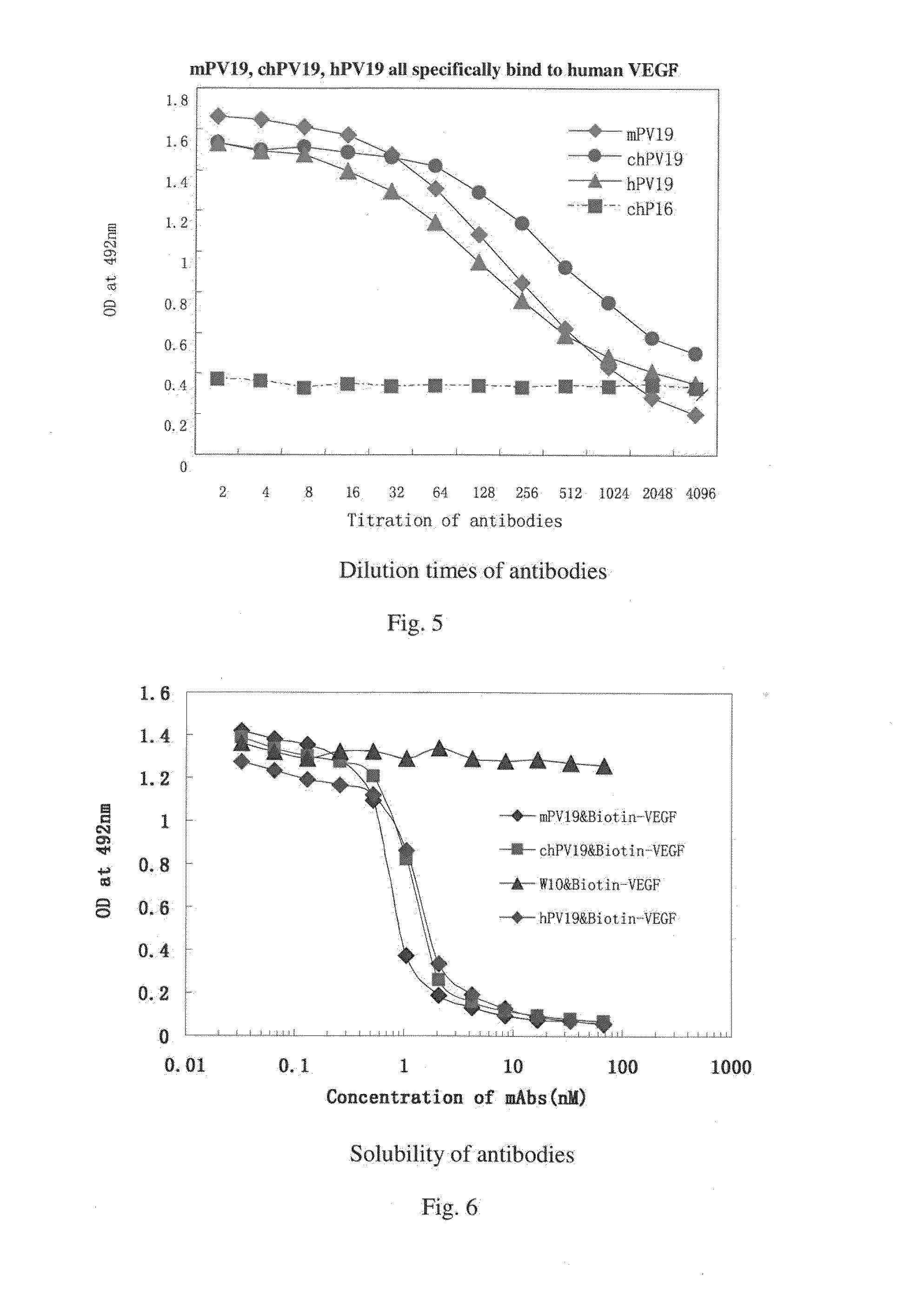
ActiveUS20160039921A1Reduce the impactAnimal cellsSugar derivativesHumanized antibodyMouse monoclonal antibody
A mouse monoclonal antibody for antagonizing and inhibiting binding of a vascular endothelial cell growth factor (VEGF) and its receptor (VEGF-R), and a heavy chain variable region and light chain variable region amino acid sequence thereof. Also disclosed are a humanized preparation process of the antibody and a heavy chain variable region and light chain variable region amino acid sequence of the humanized antibody. The humanized antibody or its derivative can act as an ingredient of a pharmaceutical composition or be prepared into a suitable pharmaceutical preparation, is administered alone or in combination with a chemotherapy drug or other treatment means, and is used in broad-spectrum treatment of various solid tumors such as colon cancer, breast cancer and rhabdomyosarcoma.
Antibodies specific for fullerenes
This invention provides a hybridoma produced by the fusion of a mouse antibody-producing cell and a mouse myeloma which is designated 1-10F-8A and deposited with the ATCC under Accession Number PTA-279, said hybridoma producing a monoclonal antibody which binds to fullerene C60. This invention provides a mouse monoclonal antibody specific for a fullerene-C60 and produced by the mouse monoclonal antibody-producing hybridoma designated 1-10F-8A. The invention provides the amino acid and encoding nucleic acid sequences of the heavy and light chains of the 1-10F-8A monoclonal antibody. This invention also provides methods of determining a serum concentration of a fullerene in a subject and of purifying a fullerene from a sample.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Anti-HMFG antibodies and processes for their production
InactiveUS6506881B1Improve featuresPeptide/protein ingredientsAntibody mimetics/scaffoldsComplementarity determining regionBinding site
Humanized antibody molecules (HAMs) are described having specificity for human milk fat globule and having an antigen binding site wherein at least one of the complementarity determining regions (CDRs) of the variable domains is derived from the mouse monoclonal antibody CTMO1 and the remaining immunoglobulin-derived parts of the HAM are derived from a human immunoglobulin. The HAMs may be chimeric humanized antibodies or CDR-grafted humanized antibodies and are preferably produced by recombinant DNA techniques. The HAMs are useful for in vivo diagnosis and therapy.
Owner:CELLTECH R & D LTD
Antihuman CD146 monoclone antibody, composition containing the same, and method for testing dissolubility CD146
ActiveCN101245101AHigh sensitivityCell receptors/surface-antigens/surface-determinantsImmunoglobulins against cell receptors/antigens/surface-determinantsSolubilityDisease
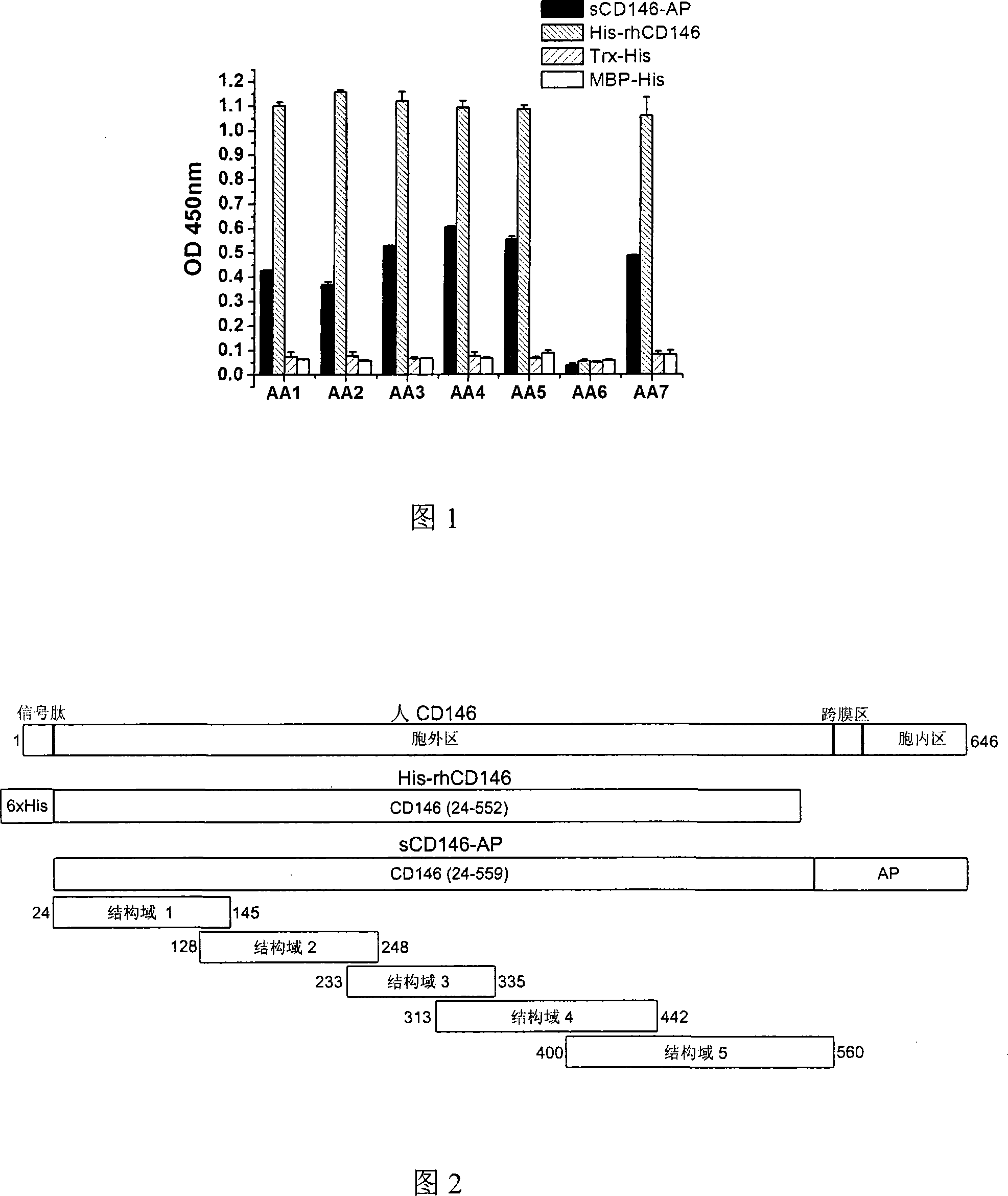
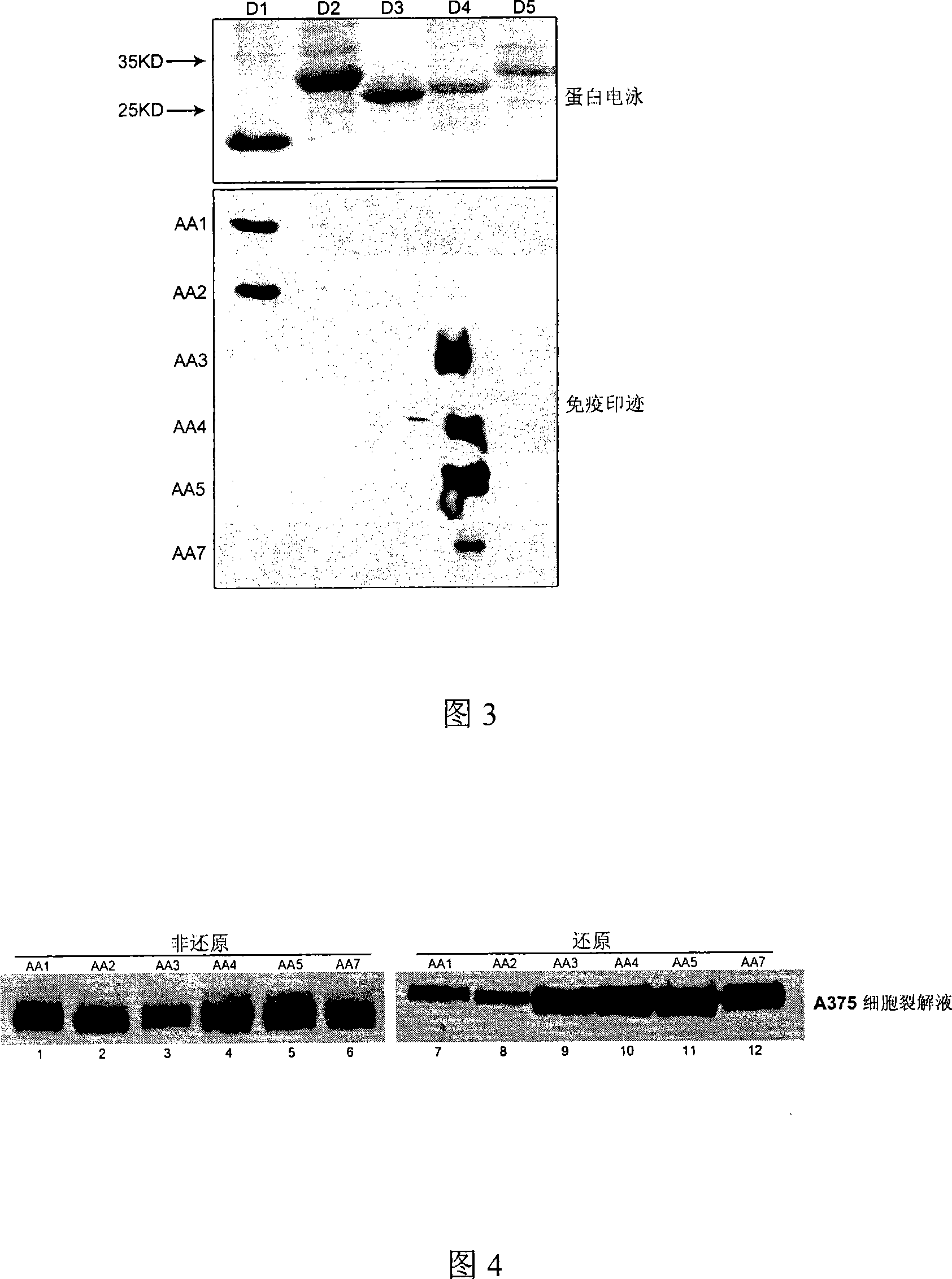
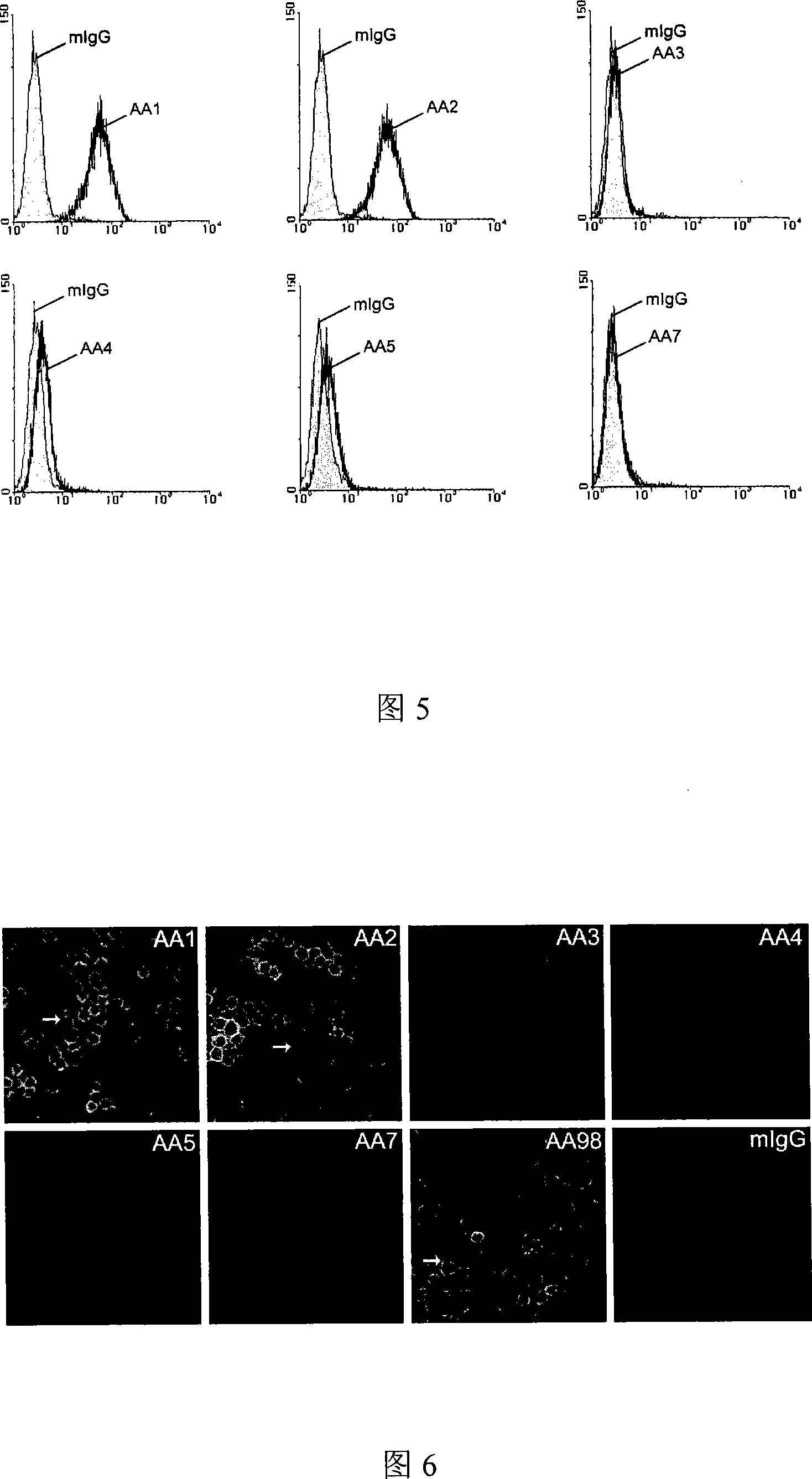
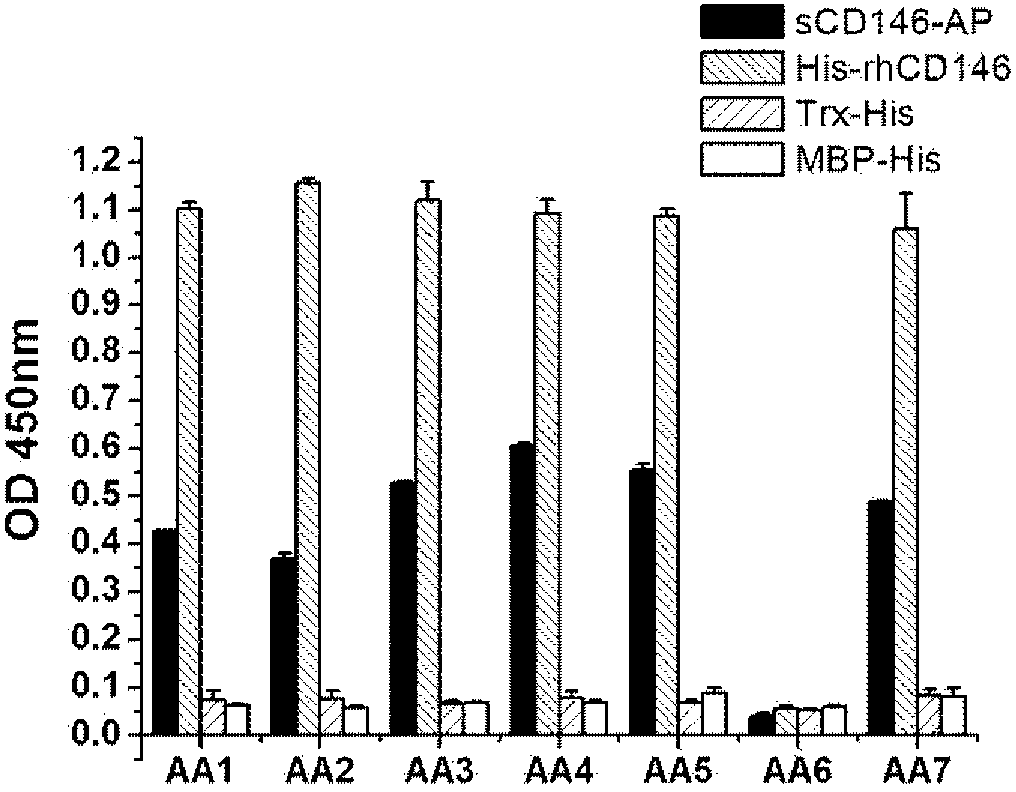
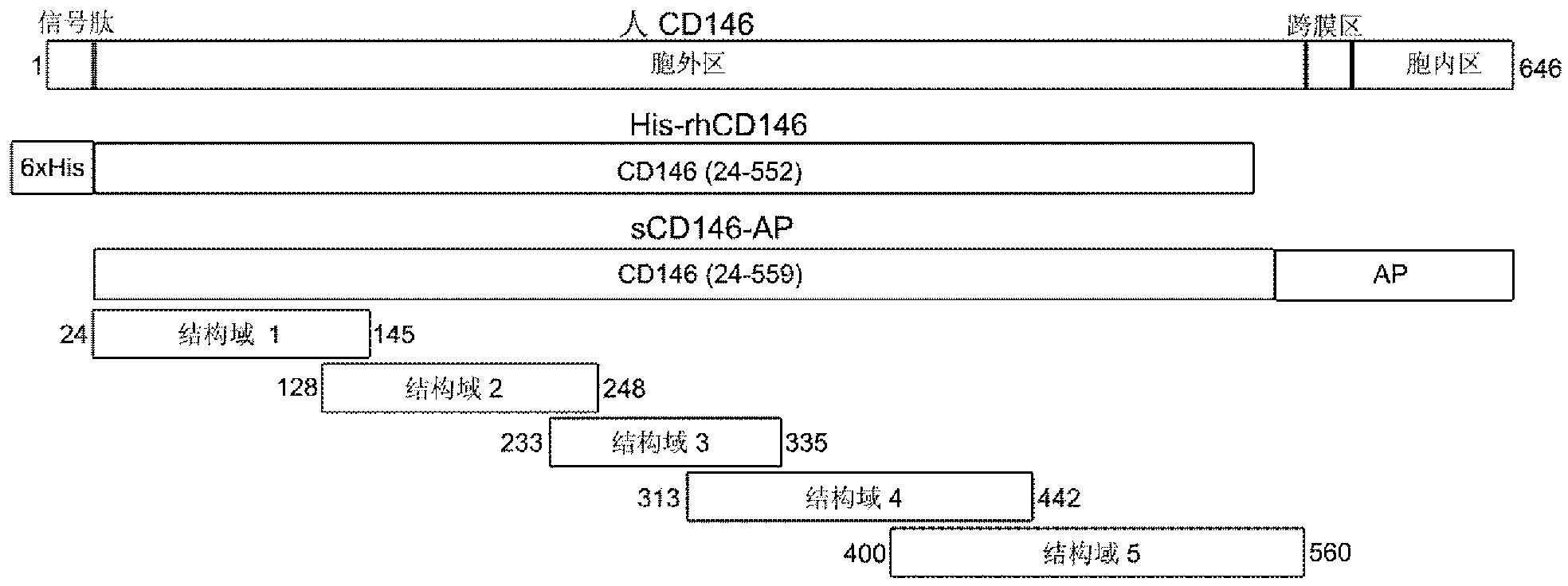
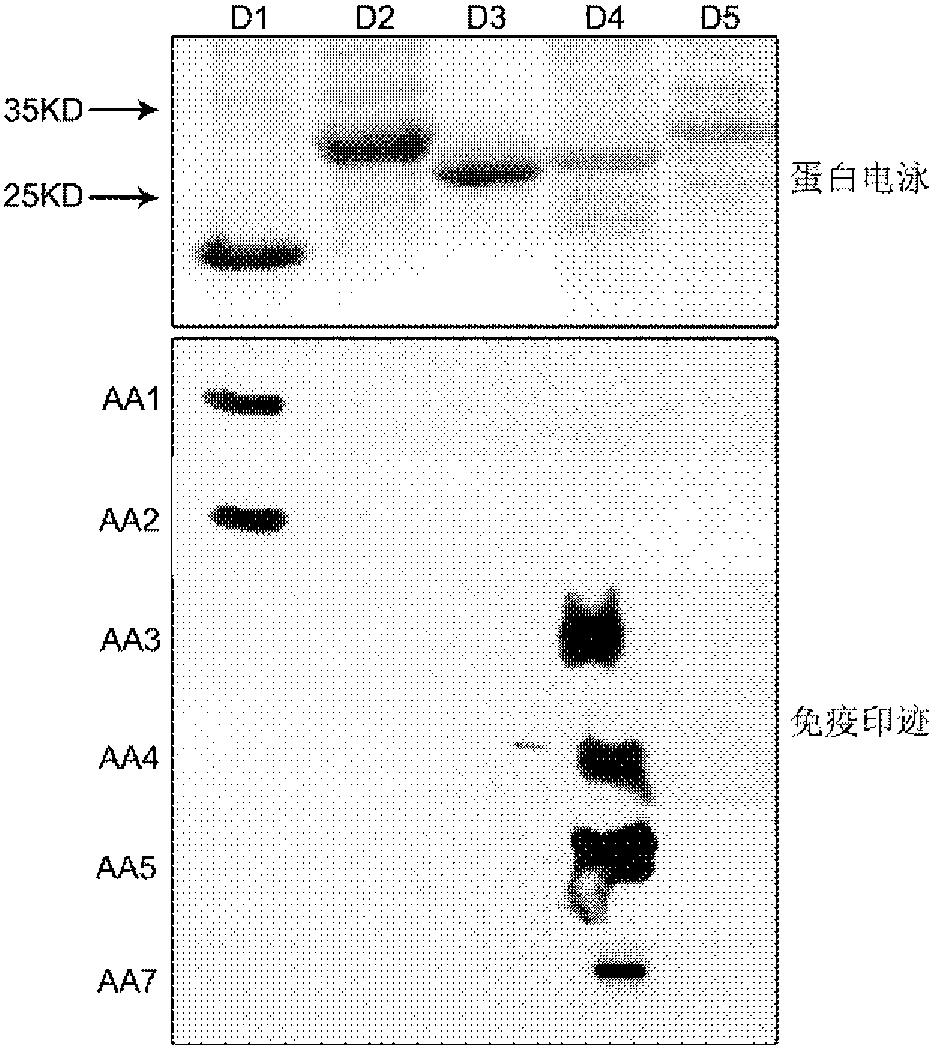
The invention relates to a group of anti-human CD146 molecules which are developed by utilizing the biological technology and an established high-sensitive sandwich ELISA method for detecting the solubility CD146. The invention includes anti-human CD146 mouse monoclonal antibodies of AA1, AA2, AA3, AA4, AA5 and AA7, and the method of utilizing the combination of the antibodies and the sandwich ELISA specificity for detecting the solubility CD146. The group of antibodies can identify the human source CD146 on the molecular, the cellular and the tissue levels, which can be divided into two categories based on the identified different epitopes. The sandwich ELISA method which is combined by the antibody AA1 and another strain of anti-human CD146 mouse monoclonal antibody AA98 can detect the solubility CD146 at each milliliter nano-gram level. The antibodies and the detection means can become the effective detection or diagnosis tools and method in the basic research or the clinical application and provide good carriers for the targeted therapy of the CD146-related diseases at the same time.
Owner:INSITUTE OF BIOPHYSICS CHINESE ACADEMY OF SCIENCES
Humanized anti-CD19 antibodies and their use in treatment of oncology, transplantation and autoimmune disease
ActiveUS8323653B2Alleviation and mitigation and decreaseSenses disorderNervous disorderAntigenApoptosis
Owner:VIELA BIO INC
ELISA kit for detecting ractopamine in animal derived food
The invention discloses an enzyme immune agent box for detecting lake drugs of animal foodstuff; it also provides a method for using the agent to detect the lake drugs residue. The agent box comprises: enzyme mark plate which coats lake drugs antigen or antibody, lake drugs mouse monoclonal antibody or polyclonal antibody working solution, enzyme mark antibody or enzyme mark lake drugs antigen, lake drugs standard solution, base material color developing solution, compression cleaning liquid, ending solution and compression twin solution. The invention also discloses a method for applying the detecting method, which comprises: first doing sample front process, then using the agent box to detect, at last analyzing the detected result.
Owner:BEIJING WANGER BIOTECH
Humanized Anti-cd 19 antibody formulations
InactiveUS20120148576A1Enhanced effector functionImprove stabilityPharmaceutical delivery mechanismAntibody ingredientsDiseaseApoptosis
The present invention provides stable liquid formulations comprising chimeric and humanized versions of anti-CD 19 mouse monoclonal antibodies that may mediate ADCC, CDC, and / or apoptosis for the treatment of B cell diseases and disorders.
Owner:MEDIMMUNE LLC
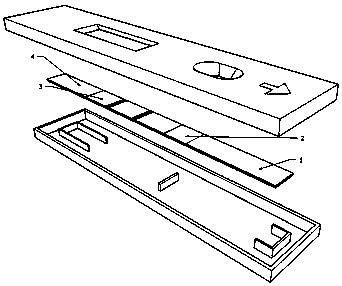
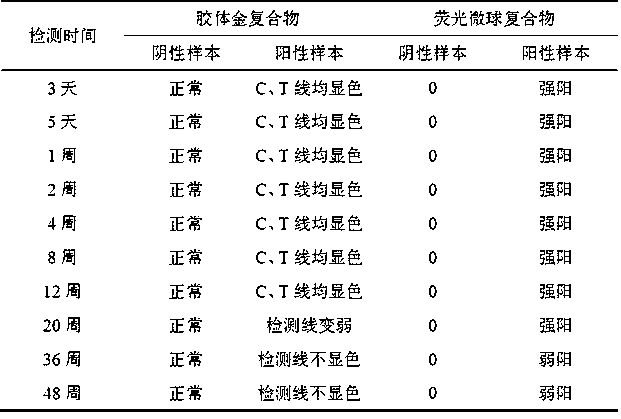
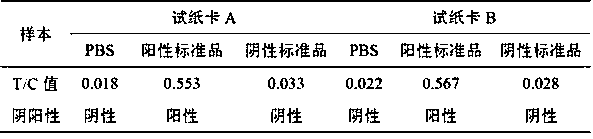
Test paper card for detecting swine transmissible gastroenteritis virus antibody and preparation and detection method of test paper card
InactiveCN107942061AImprove stabilityHigh sensitivityMaterial analysisCelluloseTransmissible gastroenteritis virus Antibody
The invention relates to a test paper card for detecting porcine transmissible gastroenteritis virus antibody, preparation and detection method thereof, and belongs to the field of immunological detection. The test paper card includes a jammed case and a test strip, and the test strip includes a bottom plate and sequentially lapped and pasted on the Absorbent pad, detection pad, binding pad and sample pad on the base plate; the detection pad is a nitrocellulose membrane with a quality control line C and a detection line T, the quality control line C is coated with goat anti-mouse monoclonal antibody, and the detection line T is Coated with inactivated porcine transmissible gastroenteritis virus; the binding pad is a glass cellulose membrane embedded with time-resolved fluorescent microsphere-labeled anti-E2 protein monoclonal antibody; the sample pad is dried glass after soaking in the sample treatment solution Cellulose film. The test paper card prepared by the invention has better stability and higher sensitivity, and can achieve the purpose of semi-quantitative detection through fluorescence signal analysis.
Owner:洛阳现代生物技术研究院有限公司
RANKL-TNF sample region mouse monoclonal antibody and its preparation method and use
The invention relates to a RANKL-TNF sample region mouse monoclonal antibody and its preparation method and use. The RANKL-TNF sample region mouse monoclonal antibody is extracted from a RANKL-TNF sample region mouse monoclonal antibody hybridoma cell strain and has the preservation number of CGMCC NO.6853. The RANKL-TNF sample region mouse monoclonal antibody and its related reagents can effectively neutralize TNF-alpha in vitro thereby inhibiting cell apoptosis caused by TNF-alpha, and neutralize RANKL in vitro thereby inhibiting osteoclast generation. The RANKL-TNF sample region mouse monoclonal antibody can effectively relieve an acute inflammation degree, improve bone mass of a mouse suffering from osteoporosis, improve a cancellous bone trabecula microstructure, and effectively improve osteoporosis symptoms.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Monoclonal antibody capable of antagonizing and inhibiting bonding of programmed death-1 receptor (PD-1) and ligand thereof as well as encoding sequence and use of monoclonal antibody
ActiveCN104558177AAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsProtein detectionHeavy chain
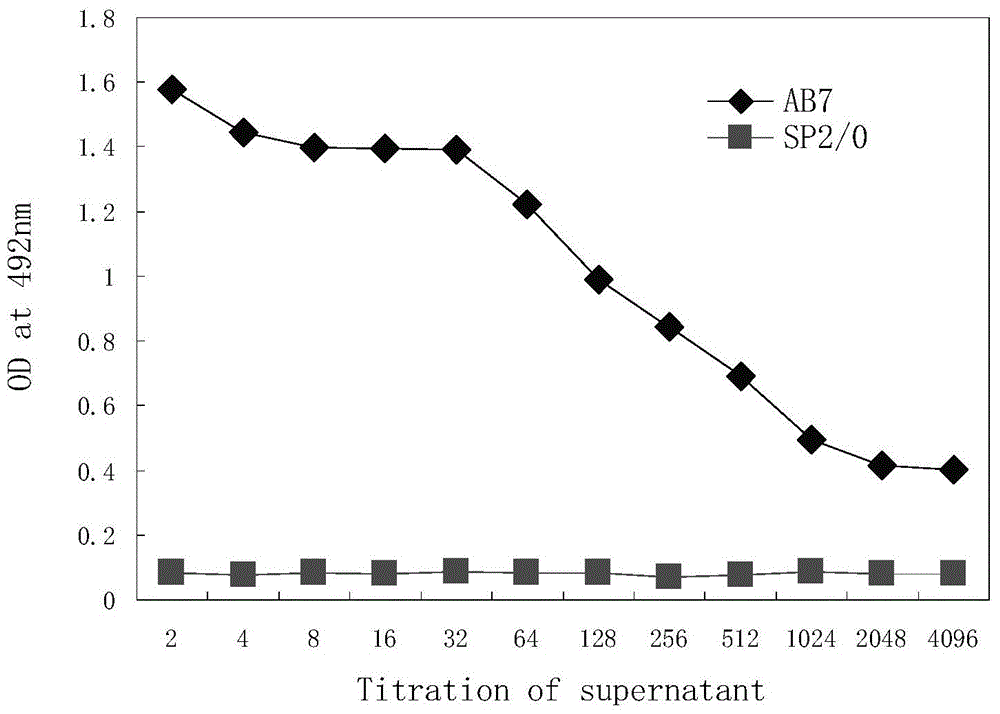
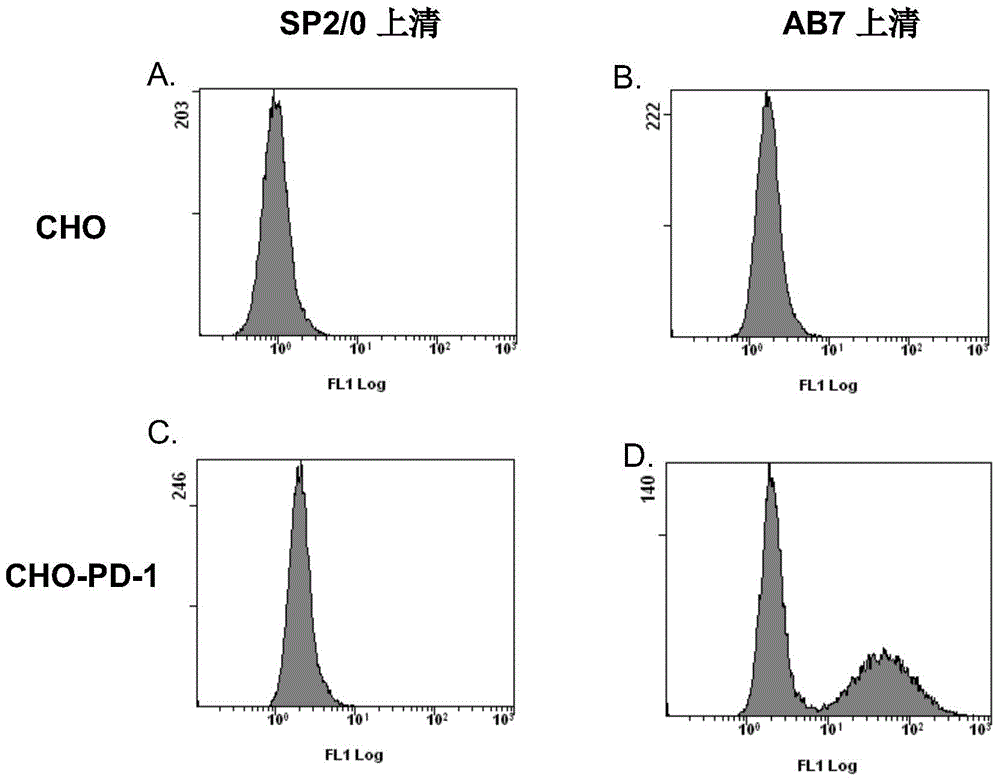
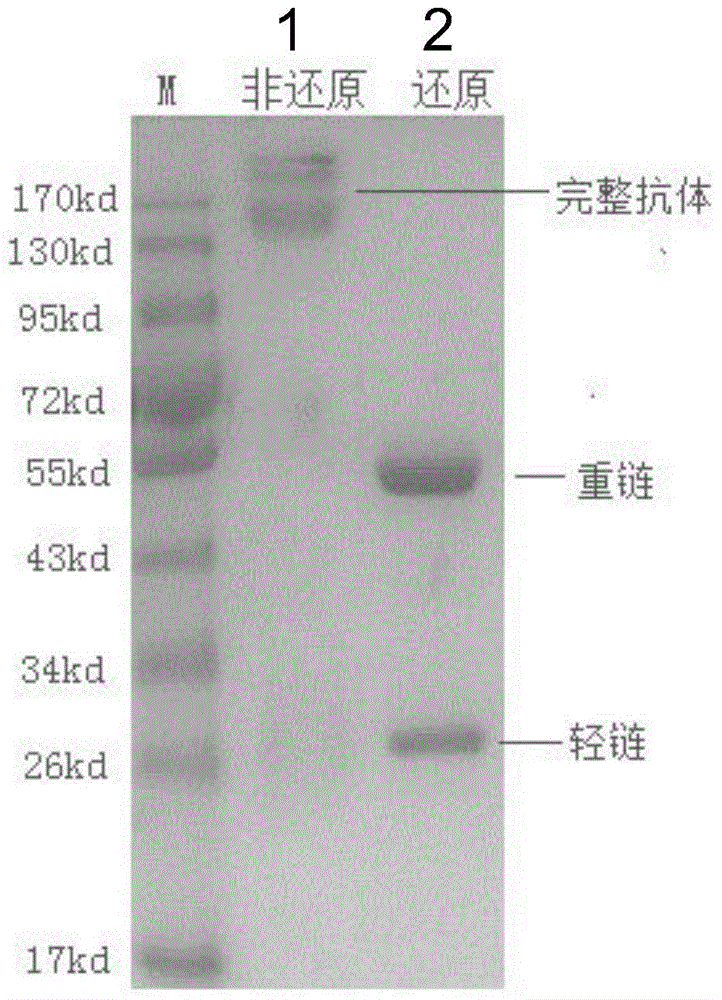
Disclosed in the present invention are a mouse monoclonal antibody for antagonizing and inhibiting the binding of a programmed death-1 (PD-1) to the ligand thereof, and the heavy chain variable region and light chain variable region amino acid sequences thereof. Also disclosed in the present invention is a DNA molecule nucleotide sequence encoding the heavy chain variable region and light chain variable region of the antibody. Also disclosed in the present invention are a method for preparing a human-mouse chimeric antibody of the antibody and derivatives thereof, and the use thereof in PD-1 protein detection.
Owner:ACROIMMUNE BIOTECH CO LTD
Humanized antibody against S-surface antigen of hepatitis B virus
A humanized antibody of the present invention shows similar antigen-binding affinity to a mouse monoclonal antibody and significantly low immunogenecity. Therefore, the humanized antibody of the present invention can be effectively used for treating chronic hepatitis B and preventing HBV infection of a patient received liver transplantation and vertical transmission from a mother infected with HBV to a fetus.
Owner:YUHAN
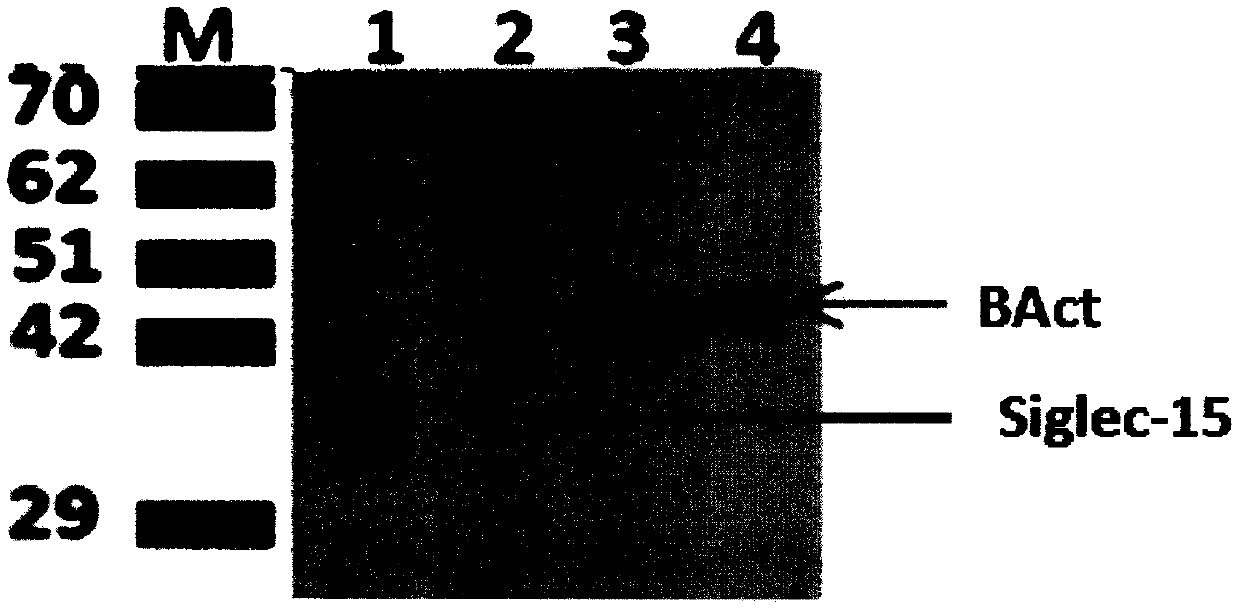
Preparation and application of mouse monoclonal antibody against human Siglec-15 protein
InactiveCN110386982AImprove featuresImprove reliabilityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsPD-L1Wilms' tumor
The invention discloses a mouse monoclonal antibody against a human Siglec-15 protein. The specificity is high, the performance is stable and the titer is high. The invention further relates to application of the monoclonal antibody to preparation of immunohistochemical detection tools for the Siglec-15 protein. According to the mouse monoclonal antibody against the human Siglec-15 protein, an immunohistochemistry method is used for detecting expression of Siglec-15 on the tumor cell surface for selection, the curative effect and the prognosis of therapeutic schedules of malignant tumors. Theinvention further relates to the application of the monoclonal antibody to preparation of antibody drugs for immunotherapy of the malignant tumors. The important application value is achieved. The adopted antibody drugs can be full-length or partial sections (Fab, F(ab)'2 or ScFv) of an Siglec-15 monoclonal antibody or combined use of the Siglec-15 monoclonal antibody and a PD-L1 monoclonal antibody. The malignant tumors mainly include colorectal cancer, endometrial cancer, thyroid cancer, bladder cancer, kidney cancer, lung cancer, liver cancer and the like.
Owner:广东三九脑科医院 +1
Anti-human CD146 monoclonal antibodies, compositions containing anti-human CD146 monoclonal antibodies, and soluble CD146 detection method
The present invention relates to a group of anti-human CD146 molecules developed by using a biological technology and an established high sensitivity sandwich ELISA method for soluble CD146 detection. The present invention provides anti-human CD146 mouse monoclonal antibodies (AA1, AA2, AA3, AA4, AA5 and AA7), and a method for specially detecting soluble CD146 by using antibody combinations and sandwich ELISA, wherein the antibodies can specifically recognize human source CD146 at a molecular level, a cellular level and a tissue level, and can be divided into two classes based on recognition of different epitopes. With an antibody AA1 and anti-human CD146 mouse monoclonal antibody AA98 combined sandwich ELISA method, nanogram-scale soluble CD146 in per ml of the solution can be detected. The antibodies and the detection method can become effective detection or diagnosis tools and methods in basic researches or clinical applications, and can further provide good carriers for targeted therapies of CD146-related diseases.
Owner:INSITUTE OF BIOPHYSICS CHINESE ACADEMY OF SCIENCES
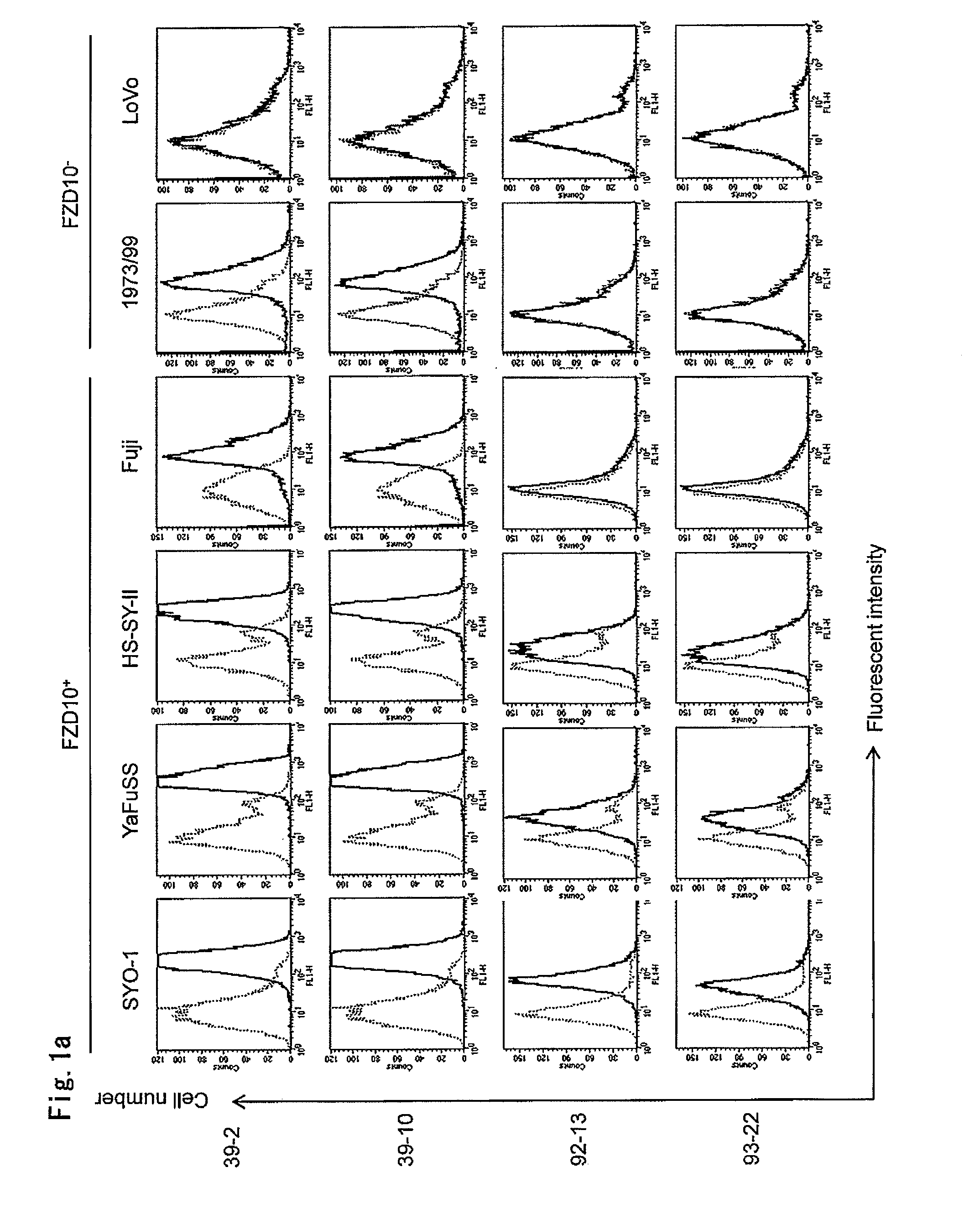
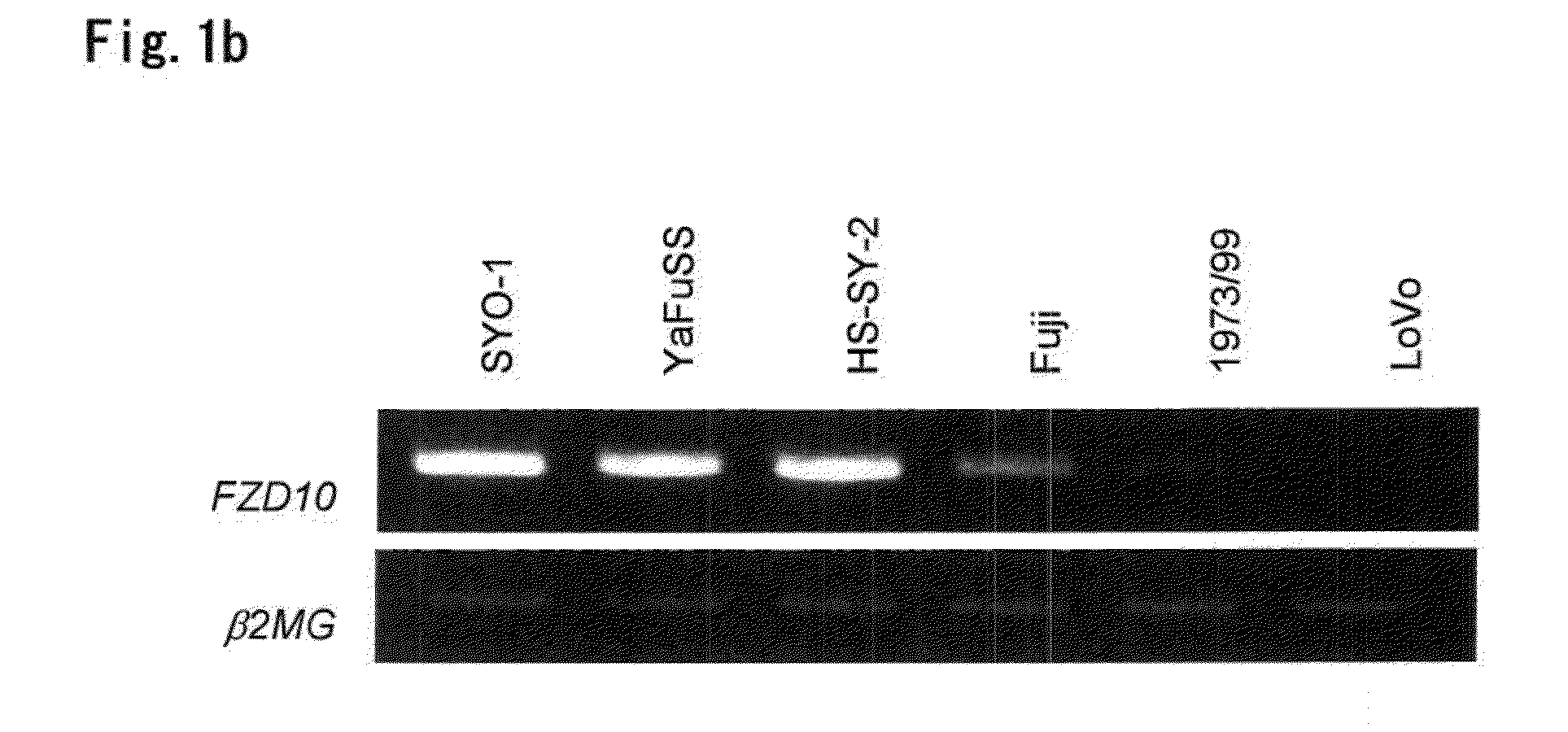
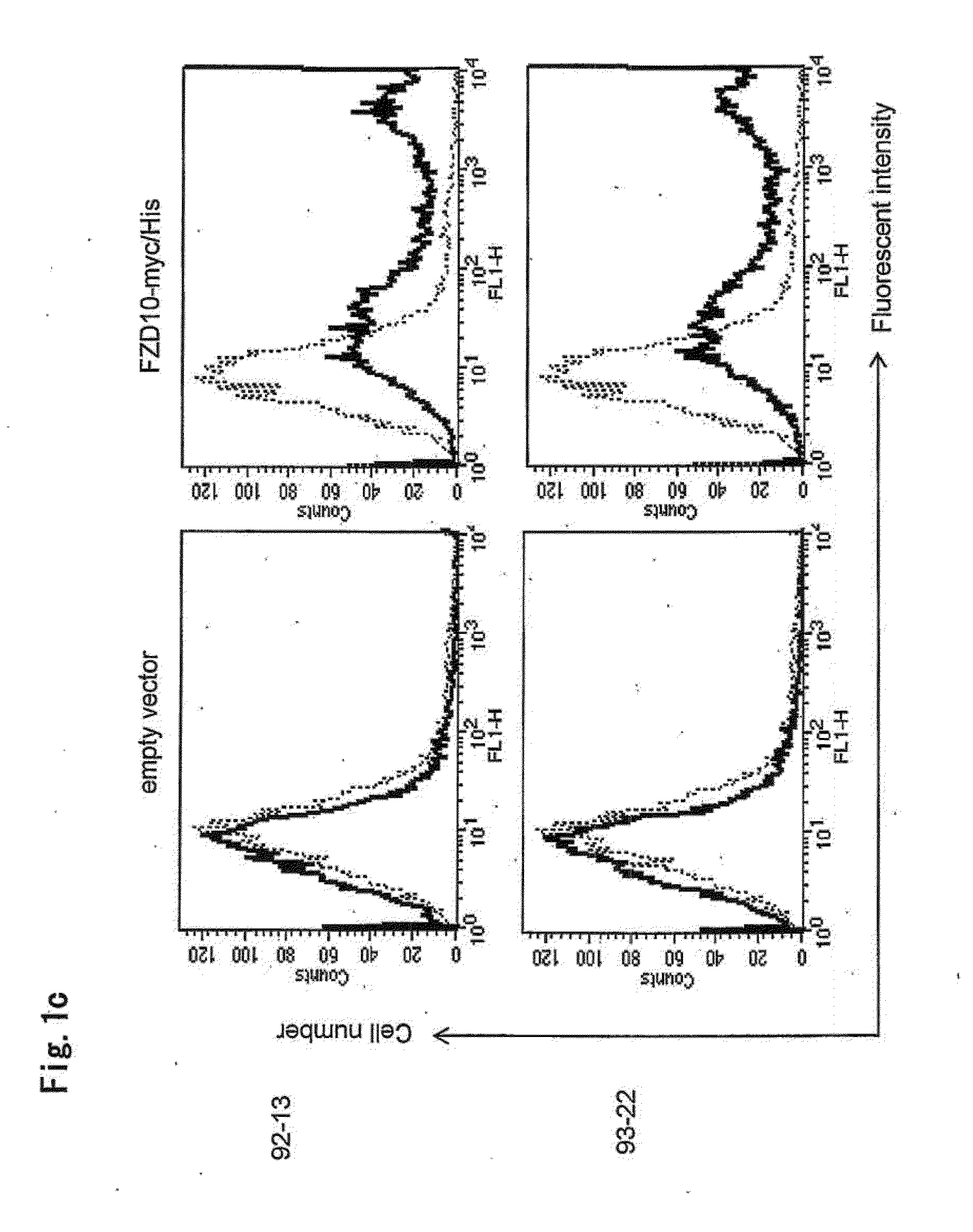
Tumor-Targeting Monoclonal Antibodies to FZD10 and Uses Thereof
ActiveUS20130230521A1In-vivo radioactive preparationsGeneral/multifunctional contrast agentsTumor targetTumor targeting
The present invention relates to an antibody or a fragment thereof which is capable of binding to a Frizzled homologue 10 (FZD10) protein, such as a mouse monoclonal antibody, a chimeric antibody and a humanized antibody. Also, the present invention relates to a method for treating and / or preventing FZD10-associated disease; a method for diagnosis or prognosis of FZD10-associated disease; and a method for in vivo imaging of FZD10 in a subject.
Owner:ONCOTHERAPY SCI INC
ELISA kit for detecting chloramphenicols in animal derived food
ActiveCN1766629AQuick checkLow pre-processing requirementsBiological testingSite monitoringElisa kit
The invention provides an enzyme immune agent box for detecting chloromycetin drugs of animal foodstuff which comprises: enzyme mark plate which coats chloromycetin drugs antigen or antibody, chloromycetin drugs mouse monoclonal antibody compression solution, chloromycetin drugs standard solution, enzyme mark material, base material color developing solution, compression cleaning liquid, ending solution and compression twin solution. The invention also discloses a method for applying the detecting method, which comprises: first doing sample front process, then using the agent box to detect, at last analyzing the detected result.
Owner:BEIJING WANGER BIOTECH
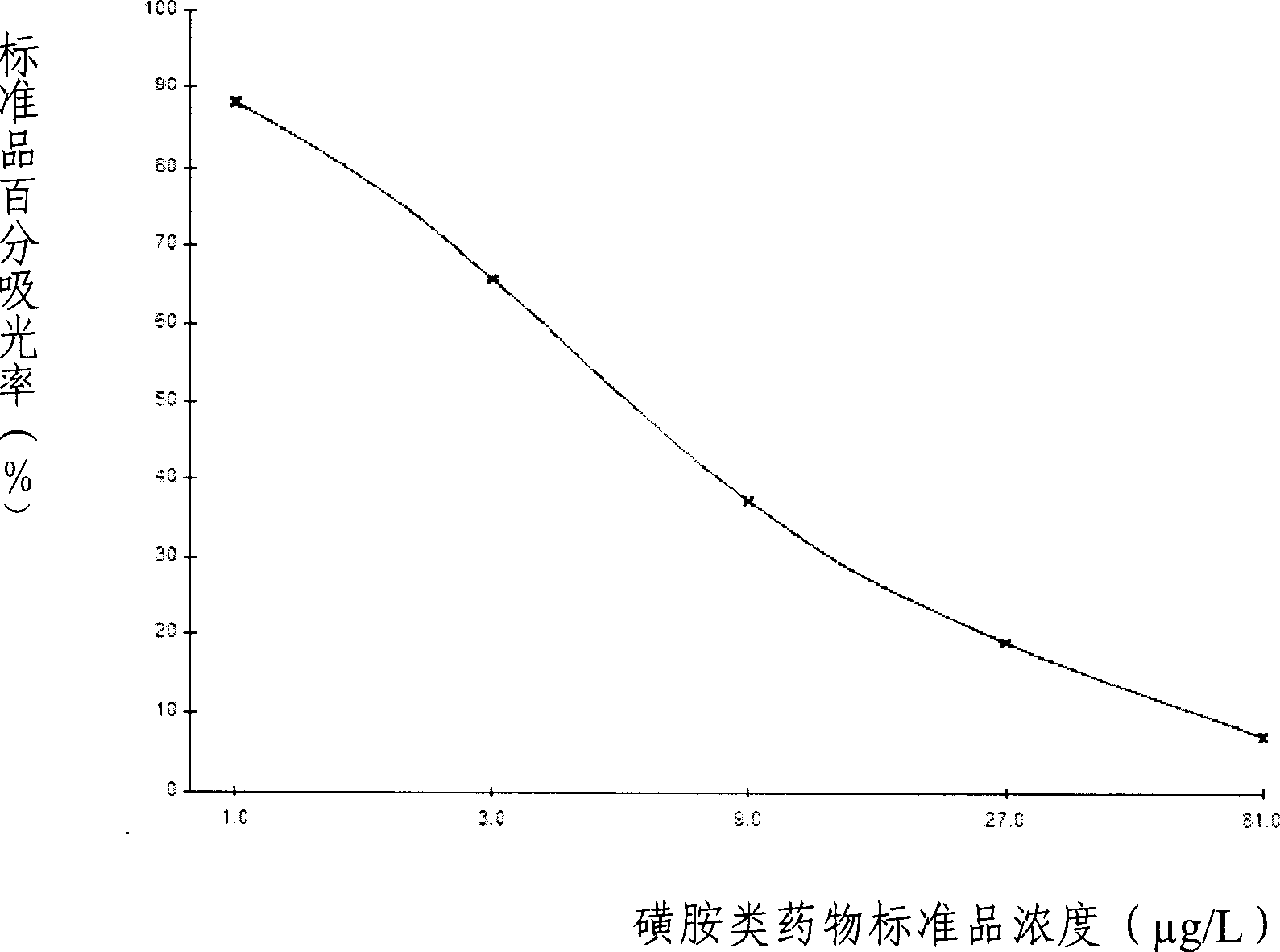
ELISA kit for detecting sulfanilamides residue in animal derived food
The invention provides an enzyme immune agent box for detecting sulpha drugs of animal organization, which uses enzyme immune method to detect the preprocessed animal organization, honey, urine, milk. The enzyme immune agent box comprises: enzyme mark plate which coats sulpha drugs antigen or antibody, sulpha drugs mouse monoclonal antibody working solution, enzyme mark antibody or sulpha drugs antigen solution, sulpha drugs standard solution, base material color developing solution, compression cleaning liquid, ending solution and compression extracting solution. The invention also discloses a method for applying the detecting method, which comprises: first doing sample front process, then using the agent box to detect, at last analyzing the detected result.
Owner:BEIJING WANGER BIOTECH
Antibody capable of recognizing HLA class i
InactiveUS20120244142A1Inhibit cell growthProlong lifeImmunoglobulins against animals/humansAntibody ingredientsSpleen cellCell Aggregations
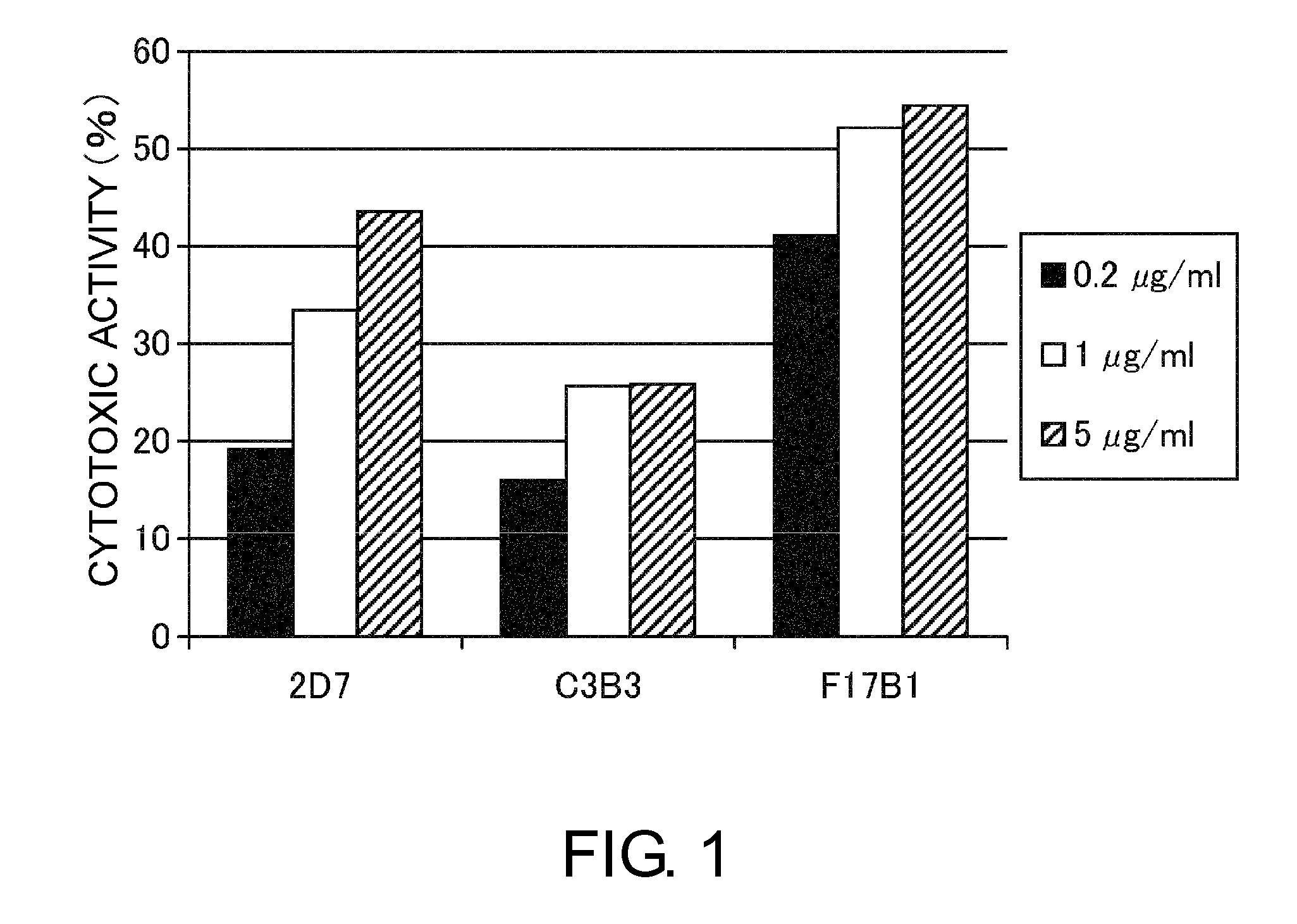
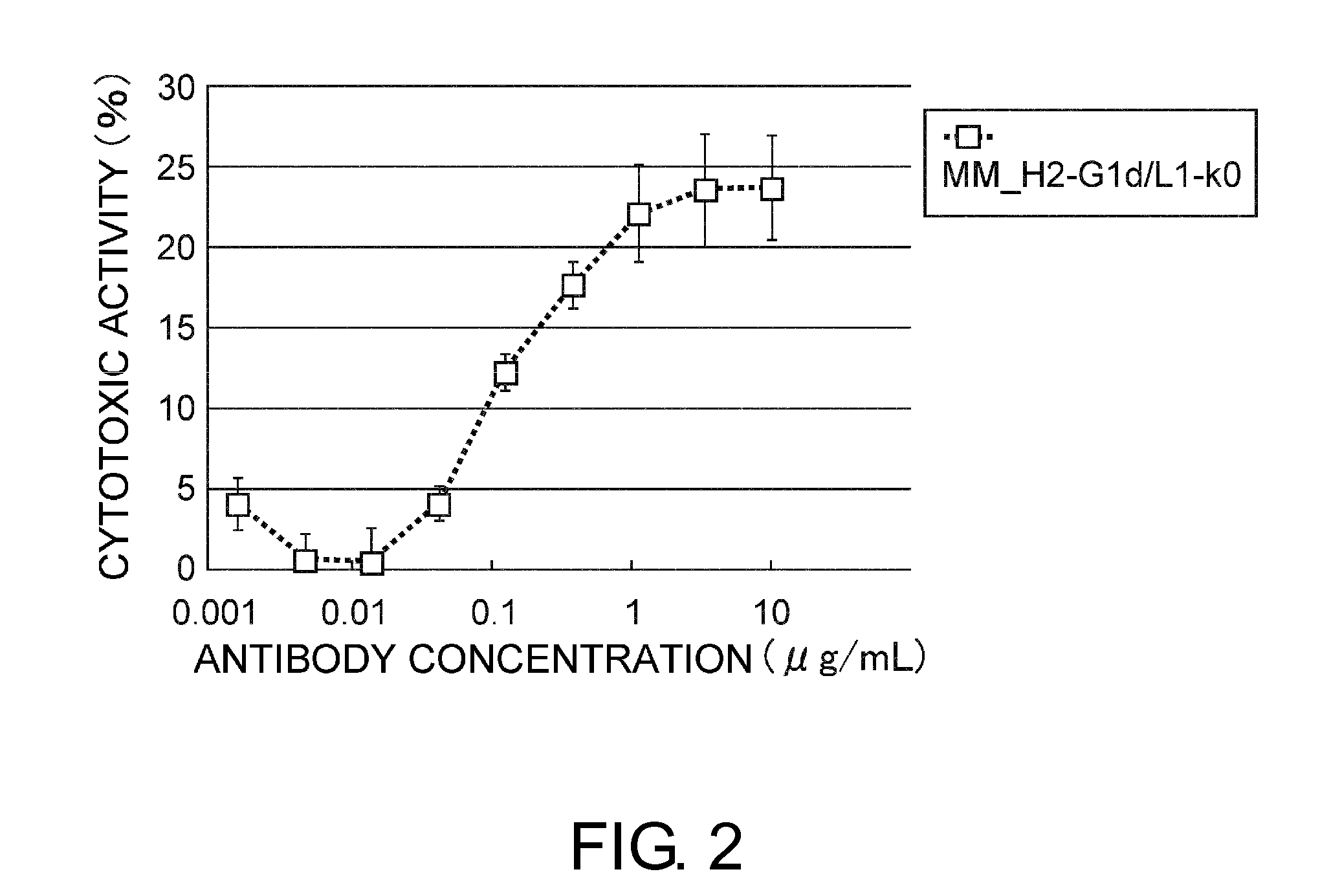
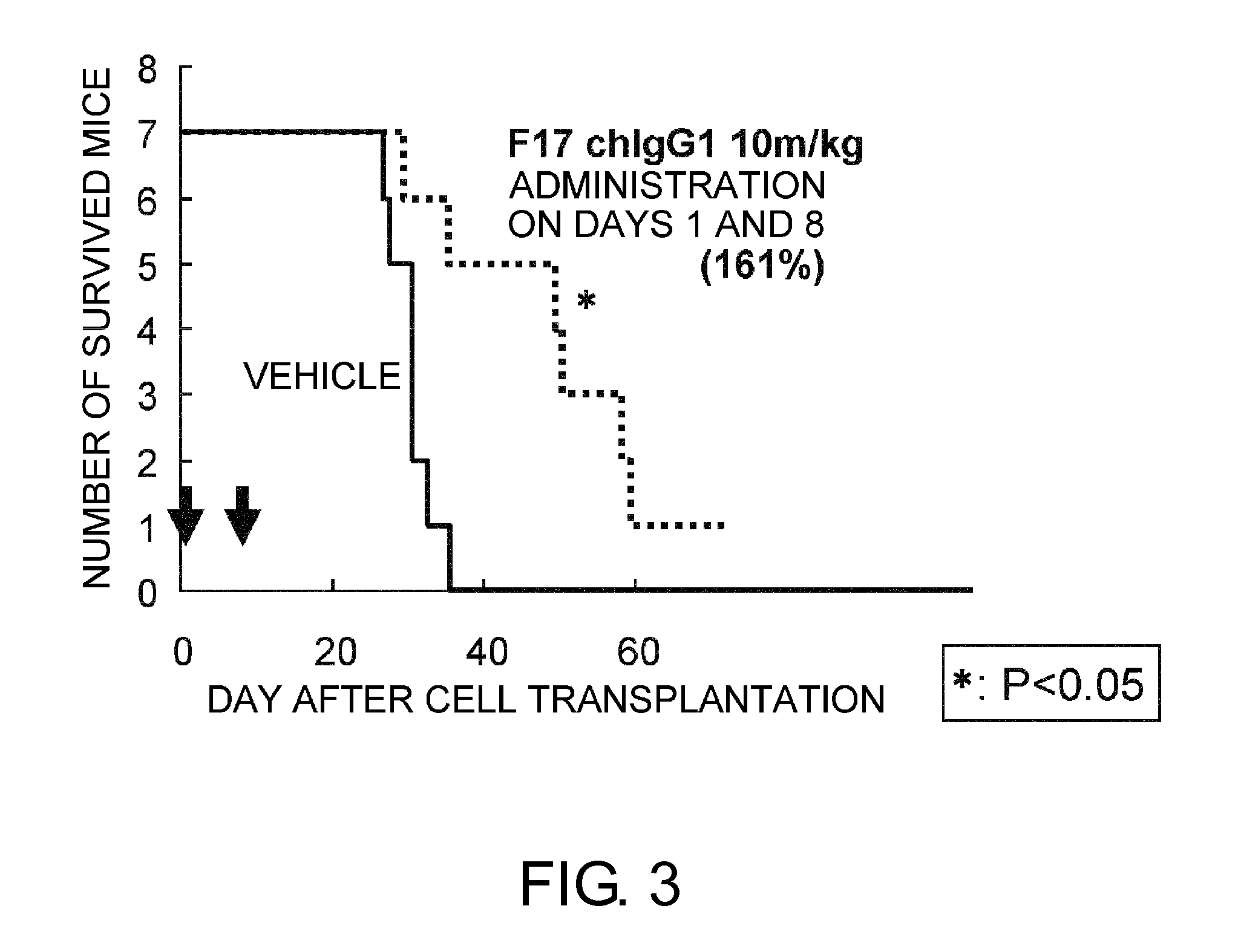
An objective of the present invention is to provide antibodies that recognize HLA class I and have significant cell death-inducing activity. To achieve the above objective, first, the present inventors immunized mice with soluble HLA-A to which a FLAG tag is attached. Four days after the final immunization, spleen cells from the mice were fused with mouse myeloma cells. Then, hybridoma screening was carried out using the cell aggregation-inducing activity and cell death-inducing activity as an index. Thus, the monoclonal antibody F17B1 that has strong cell death-inducing activity was selected. The sequences of the H chain and L chain variable regions of the mouse monoclonal antibody F17B1 were determined to humanize this antibody. Then, the humanized anti-HLA class I antibody H2-G1d_L1-k0 was assessed for cell death induction. The result showed that H2-G1d_L1-k0 suppressed the growth of the IM9 cell line in a concentration dependent manner.
Owner:CHUGAI PHARMA CO LTD
Preparation and application of mouse monoclonal antibody against noroviruses GII.4
ActiveCN106188281AImmunoglobulins against virusesAntiviralsVirus-like particleMouse monoclonal antibody
The invention discloses preparation and application of a mouse monoclonal antibody against noroviruses GII.4. Experimental results indicate that the monoclonal antibody has high neutralizing activity for the noroviruses GII.4, does not generate a cross reaction with GI.1 virus-like particles, and can specifically recognize the GII.4 virus-like particles.
Owner:中国科学院上海免疫与感染研究所
Production of humanised antibodies to TNFalpha
There is disclosed antibody molecules containing at least one CDR derived from a mouse monoclonal antibody having specificity for human TNFα. There is also disclosed a CDR grafted antibody wherein at least one of the CDRs is a hybrid CDR. Further disclosed are DNA sequences encoding the chains of the antibody molecules, vectors, transformed host cells and uses of the antibody molecules in the treatment of diseases mediated by TNFα.
Owner:UCB PHARMA SA +2
Method for constructing humanized Ph chromosome positive acute lymphocytic leukemia mouse model
InactiveCN103740639AImprove efficiencyGood repeatabilitySkeletal/connective tissue cellsAnimal husbandryAcute lymphoblastic leukemiasMouse monoclonal antibody
The invention discloses a method for constructing humanized Ph chromosome positive acute lymphocytic leukemia mouse model, and the method comprises the following steps: acquisition of leukemia cells, pretreatment of NOD / SCID mouse and implantation of leukemia cells, and implantation of leukemia cells. Specifically, the method comprises: 60Co irradiation of sub-lethal dose combined with a pretreatment with rat anti-mouse CD122 monoclonal antibody, and injection of Ph+ALL bone marrow mononuclear cells from knee joint marrow cavity of NOD / SCID mouse, thereby a humanized Ph+ALL mouse model is established with high molding efficiency and good repeatability; cell sorting is not needed, and the molding process is easy to be operated; the method provides an experiment platform for carrying out mankind Ph+ALL research on the level of living animals, and will promote deep research of domestic Ph+ALL.
Owner:PEOPLES HOSPITAL PEKING UNIV
Monoclonal antibodies for antagonizing and inhibiting combination of programmed death-1 (PD-1) and ligands of PD-1, hybridoma cell line secreting monoclonal antibodies and application of monoclonal antibodies
InactiveCN104560884ABinding block inhibitionImmunoglobulins against cell receptors/antigens/surface-determinantsTissue cultureProgrammed deathMouse monoclonal antibody
The invention discloses mice monoclonal antibodies for antagonizing and inhibiting combination of programmed death-1 (PD-1) and ligands of PD-1 and a hybridoma cell line secreting the monoclonal antibodies. The preservation number of the hybridoma cell line is CGMCC No. 8351. The invention further discloses an application of the monoclonal antibodies to detection of PD-1 protein.
Owner:SUZHOU STAINWEI BIOTECH INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com