Humanized antibody specific for tumor necrosis factor-alpha
一种肿瘤坏死因子、人源化抗体的技术,应用在抗体、抗肿瘤药、抗细胞因子/淋巴因子/干扰素免疫球蛋白等方向,能够解决降低体内免疫应答、丧失原始小鼠抗体高度选择性和反应性等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Construction of genes encoding humanized antibody heavy chain variable regions
[0052] Use the gene encoding the heavy chain variable region of the TNF-α-specific mouse monoclonal antibody TSK114 (deposit number: KCTC10514BP and KCTC 10515BP) as a template and the primer pair of SEQ ID NO: 1 and 14 to carry out PCR to amplify the leader sequence Gene A encoding the heavy chain variable region of the humanized antibody. The amplified PCR product was subjected to electrophoresis on an agarose gel and recovered from the gel using a QIAgel recovery kit (Qiagen, USA).
[0053]The gene A thus obtained was subjected to PCR using the primer pair of SEQ ID NO: 1 and 7 or SEQ ID NO: 6 and 14 to amplify the DNA fragment, and the resulting DNA fragment and the primer pair of SEQ ID NO: 1 and 14 were used for PCR Overlap PCR. The amplified PCR product was cloned by incubating the amplified PCR product with TOPO vector (TOPO TA cloning kit, Invitrogen lnc., USA) at roo...
Embodiment 2
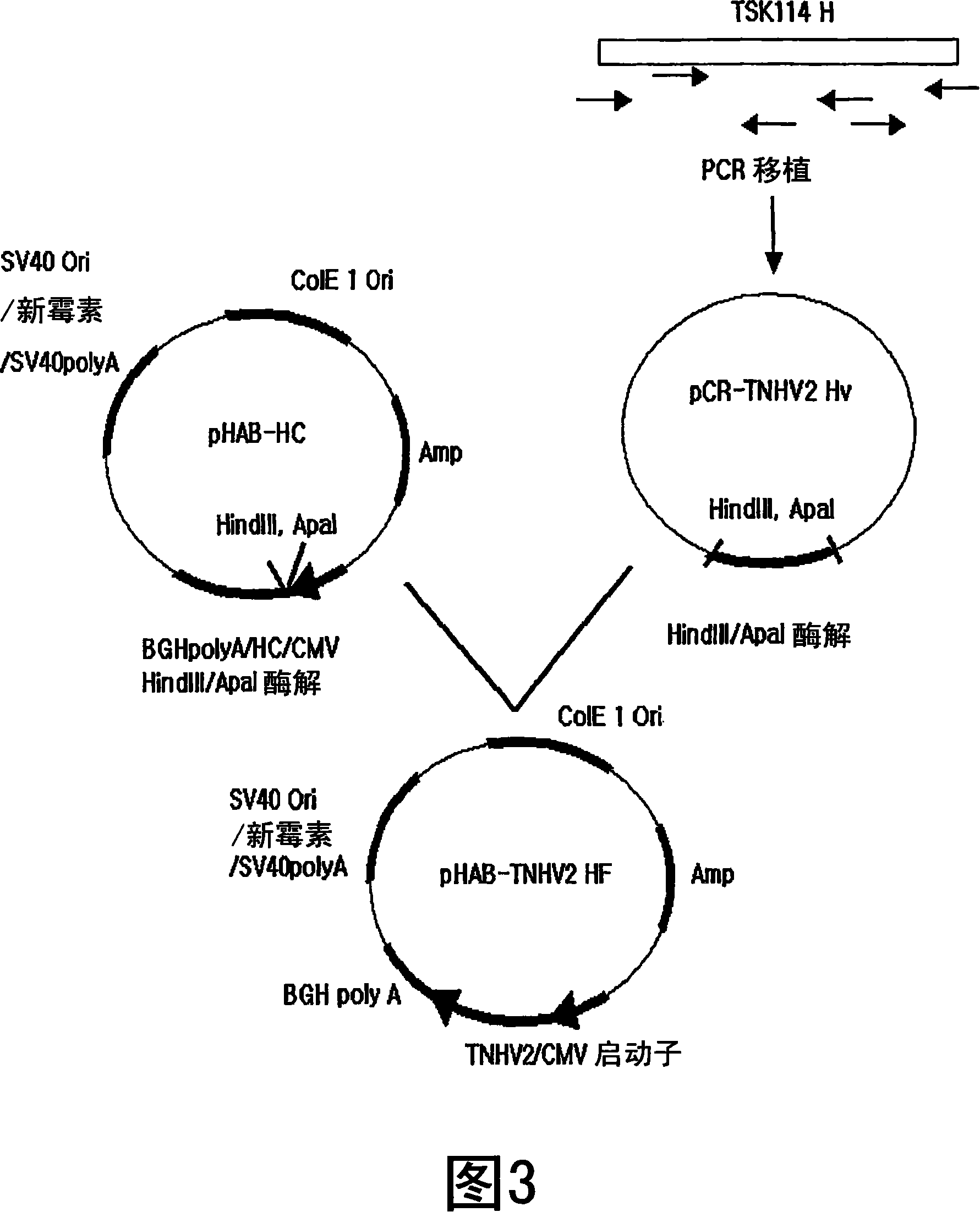
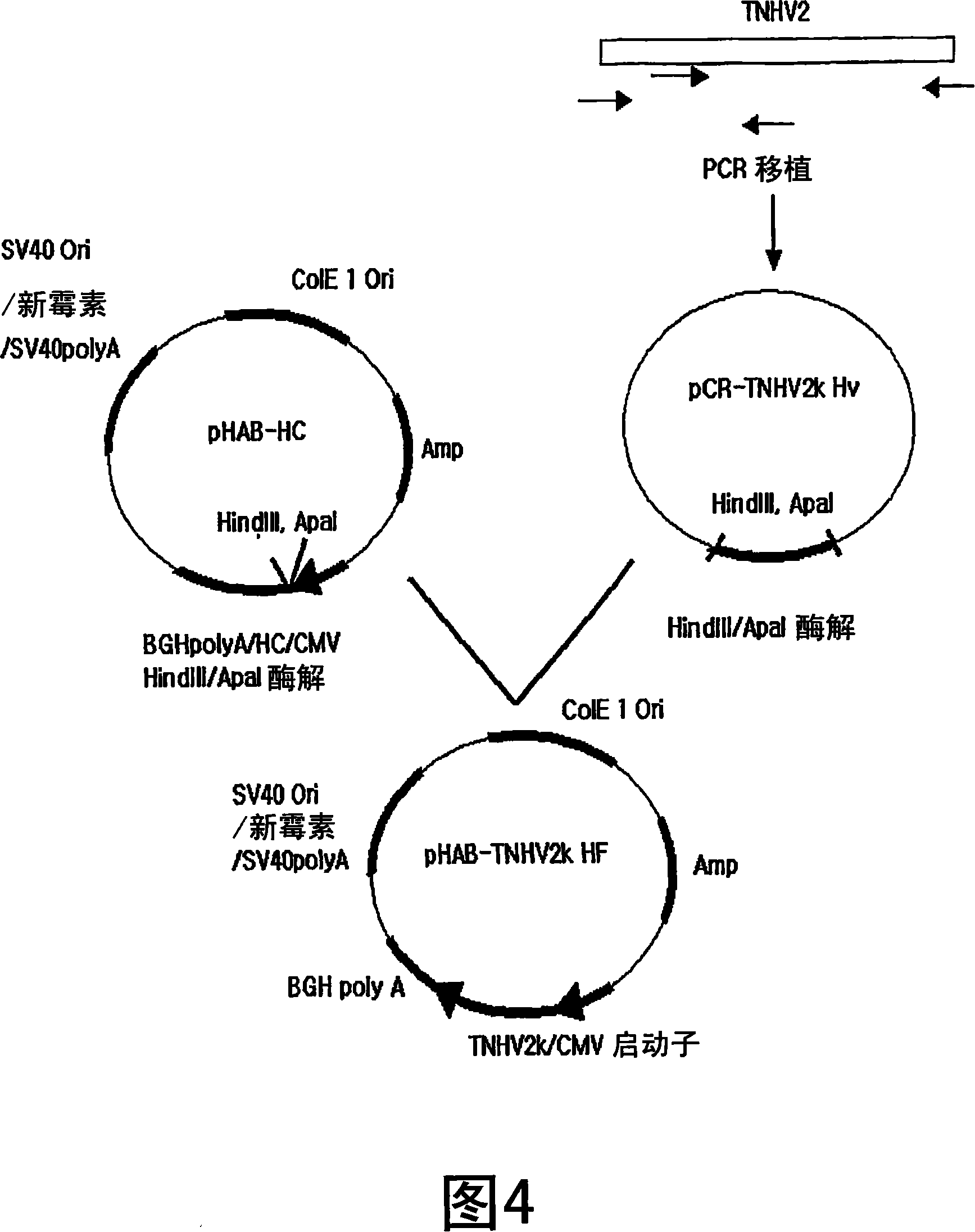
[0062] Example 2: Construction of the full-length heavy chain gene of a humanized antibody and its expression vector
[0063] To construct a gene encoding a humanized full-length heavy chain using the gene encoding a heavy chain variable region inserted into a TNHV2 Hv vector prepared in Example 1, pHAB-HC containing a gene encoding a heavy chain constant region of a human antibody was used (KCTC 10229BP, Korean Patent Publication No. 2004-12266) carrier.
[0064] First, in order to insert the gene TNHV2 encoding the heavy chain variable region of the humanized antibody into the expression vector pHAB-HC, pCR-TNHV2 Hv was treated with Hind III / ApaI (BioLabs, Inc., USA) at 37°C for 2 hours, Electrophoresis was performed on 1.5% agarose gel and stained with ethidium bromide (EtBr), and a gene fragment of about 450 bp was observed.
[0065] And, Hind III / ApaI treatment, electrophoresis and staining were performed on pHAB-HC, and an enzymatic vector fragment of about 6.5 kb was...
Embodiment 3
[0068] Example 3: Construction of genes encoding light chain variable regions of humanized antibodies
[0069] Use the gene encoding the light chain variable region of the mouse monoclonal antibody TSK114 that specifically recognizes TNF-α as a template and the primer pair of SEQ ID NO: 16 and 30 to carry out PCR to amplify the coding light chain containing the leader sequence. Gene A of the variable region. Gene A was subjected to agarose gel electrophoresis and EtBr staining, and recovered from the gel using a QIAgel recovery kit.
[0070] The gene A thus purified was subjected to PCR using the primer pair of SEQ ID NO: 16 and 25 or SEQ ID NO: 24 and 30 to amplify the DNA fragment, and the resulting DNA fragment and the primers of SEQ ID NO: 16 and 30 were used Overlap PCR was performed on . The amplified PCR product was incubated at room temperature for 45 minutes using TOPO vector (TOPO TA cloning kit, Invitrogen lnc., USA), and the cloned vector was transformed into E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com