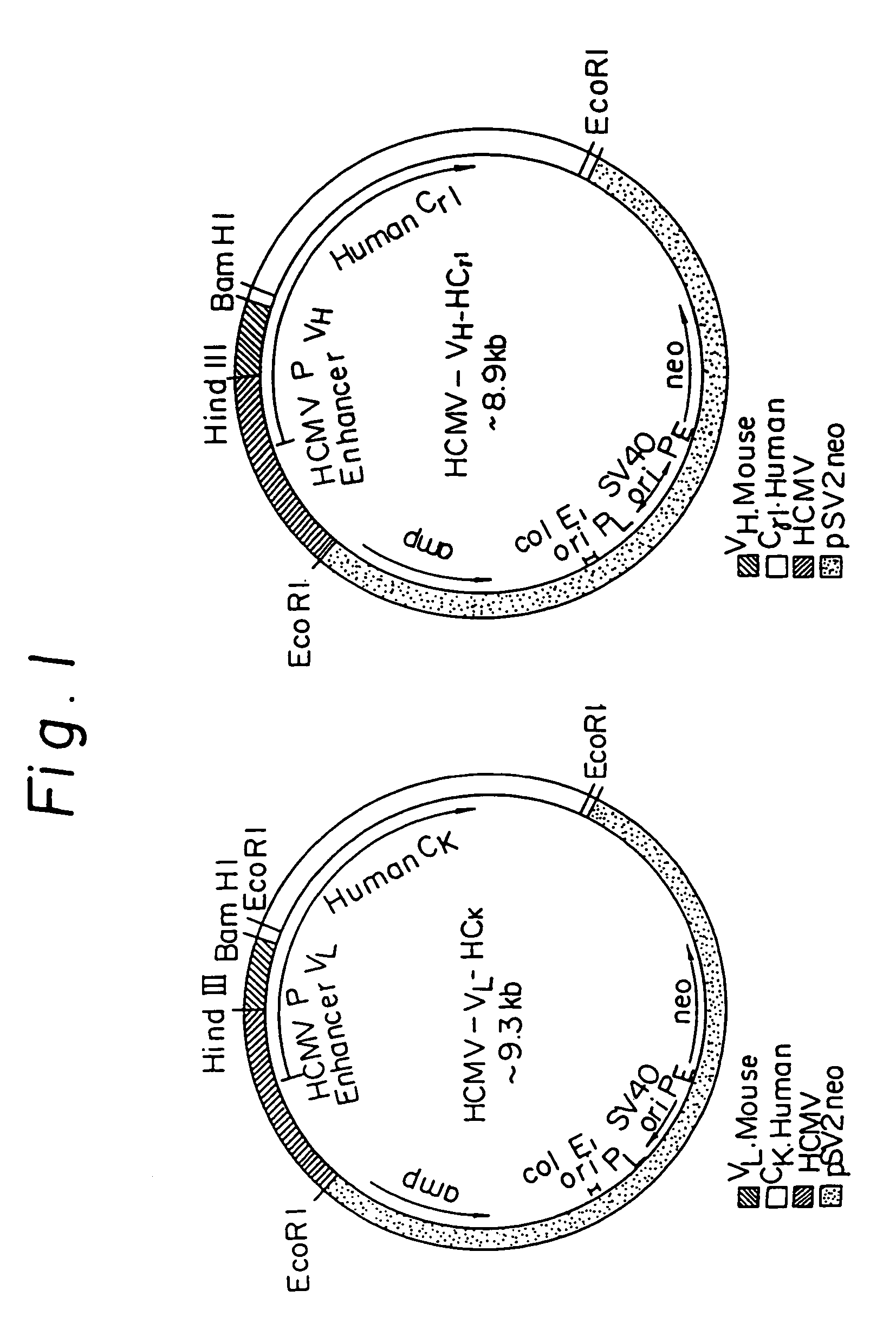
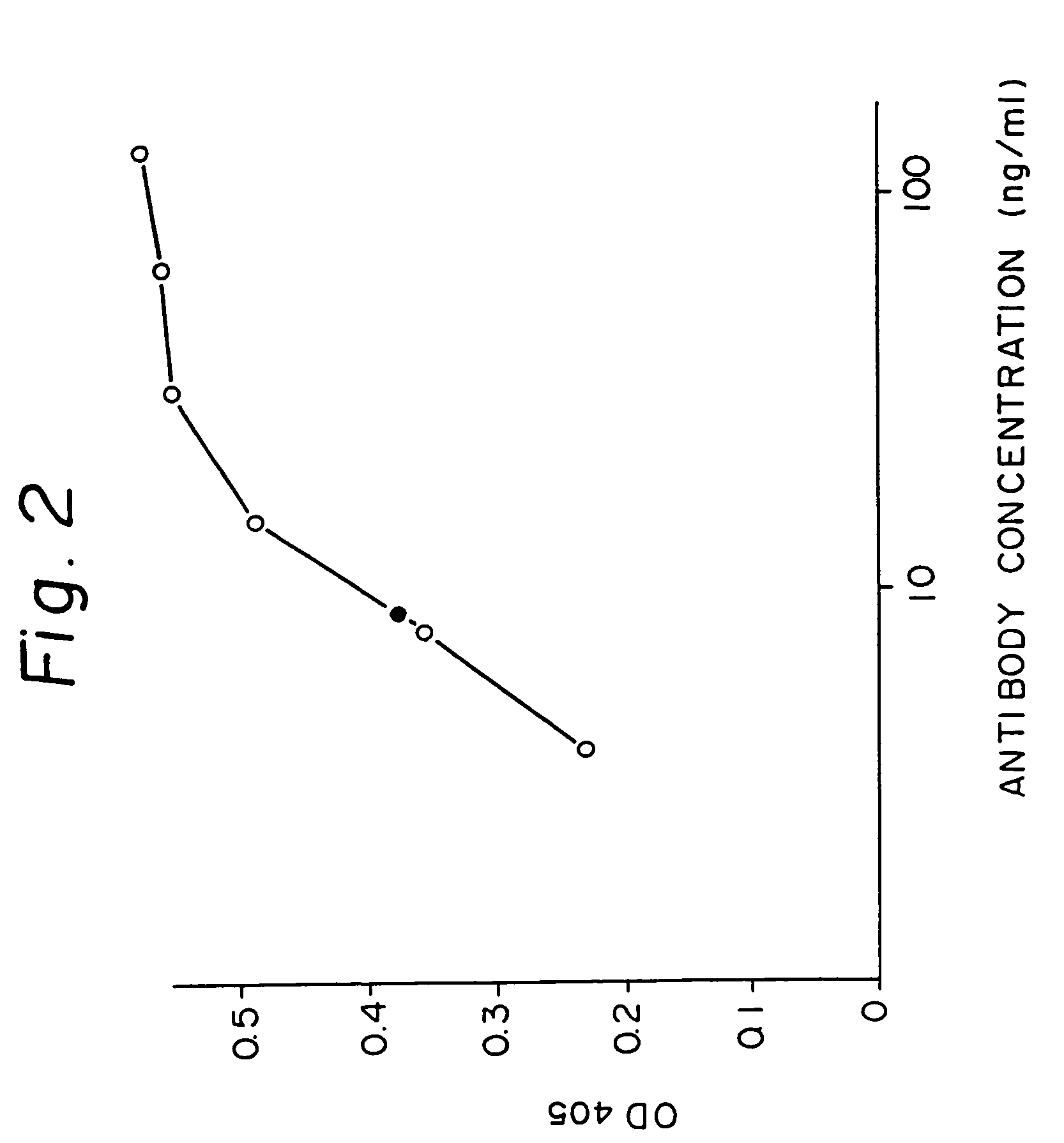
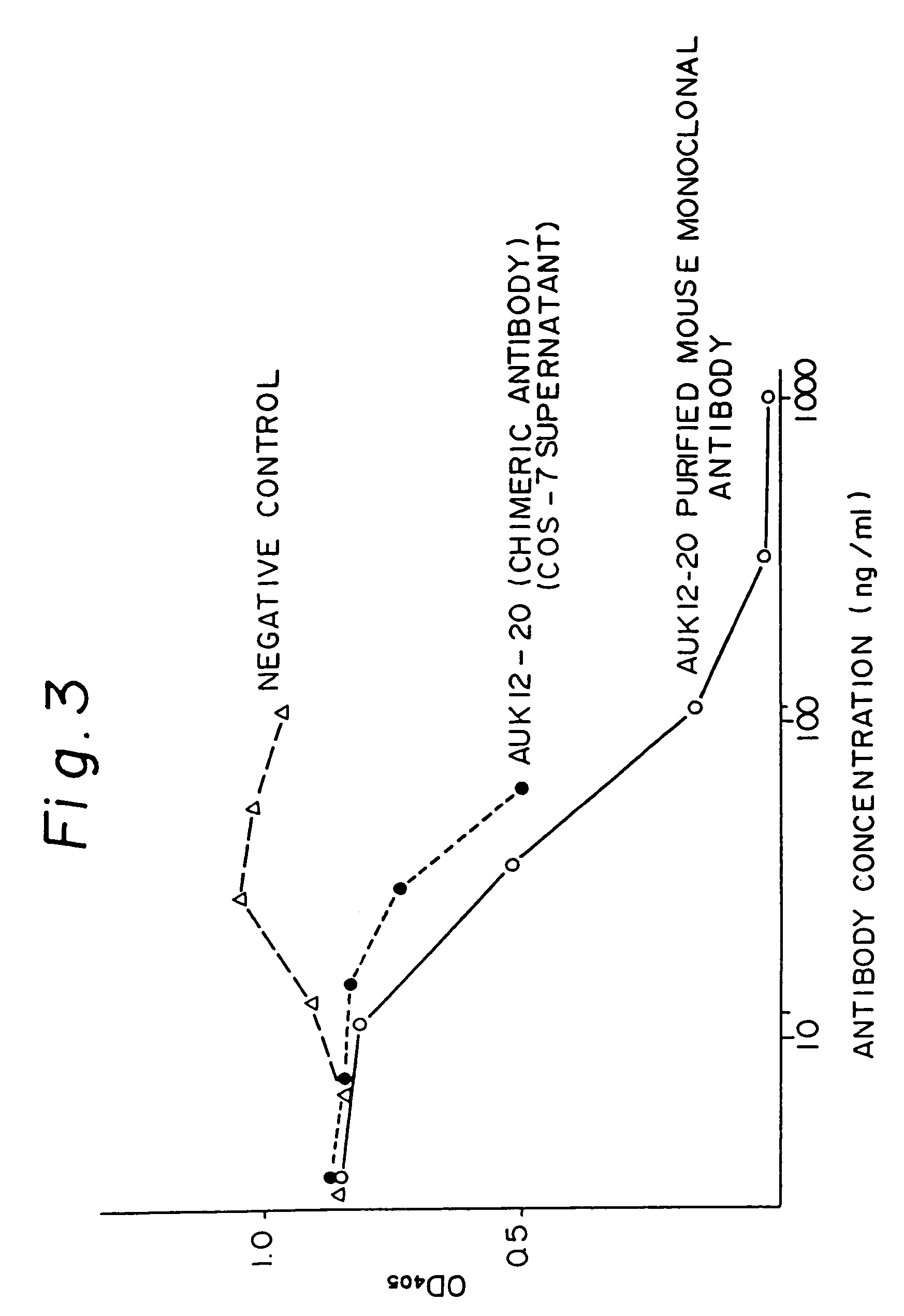
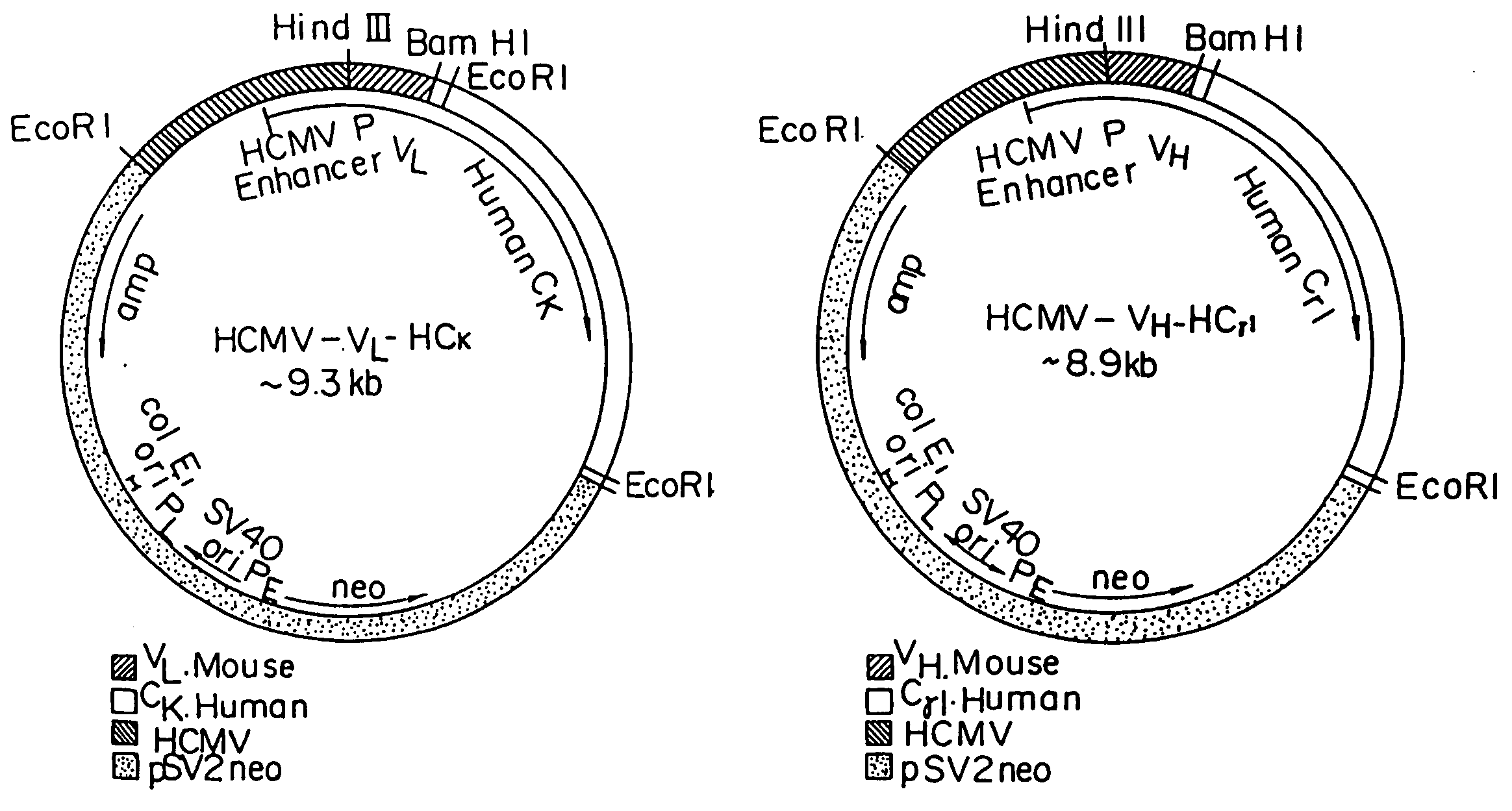
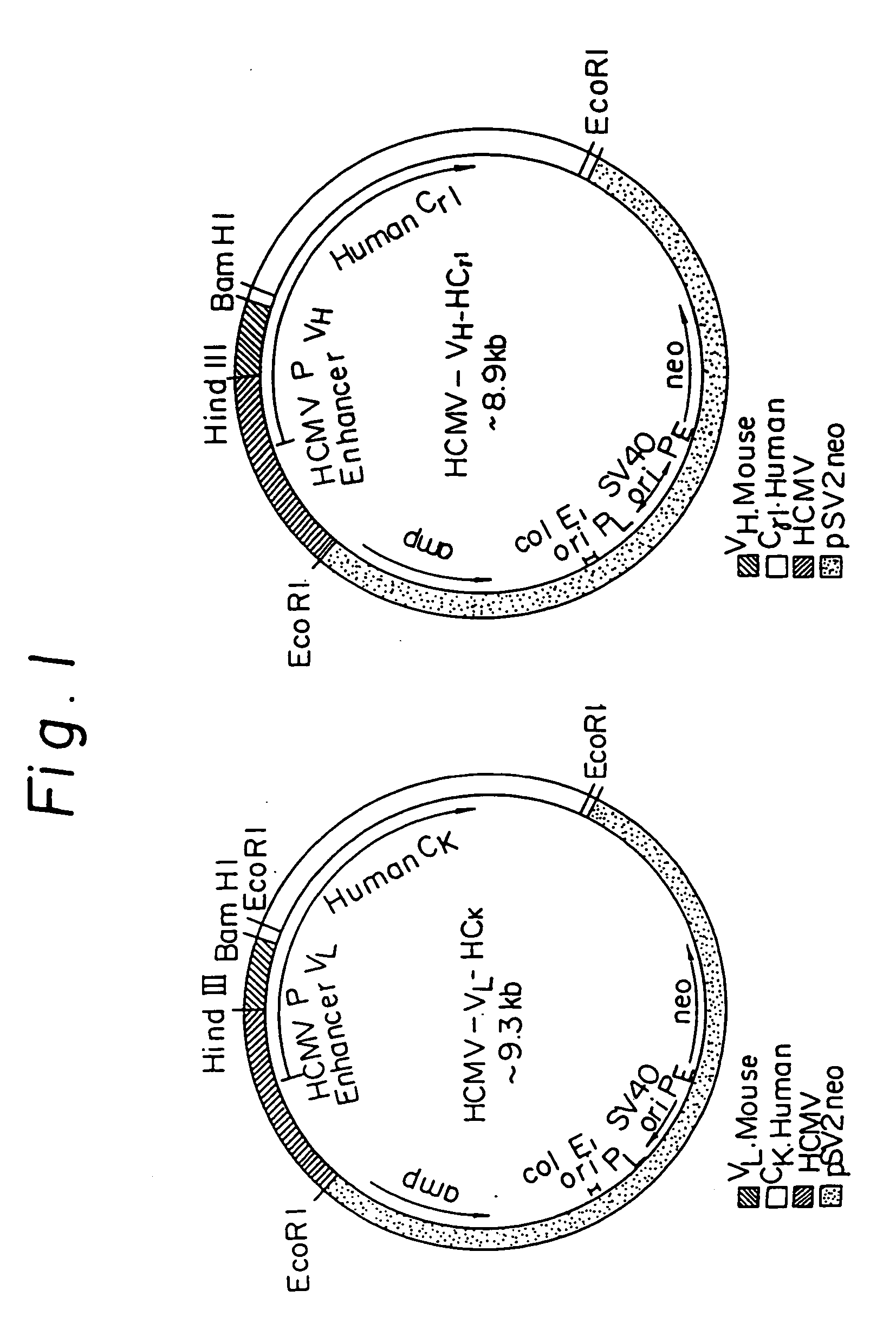
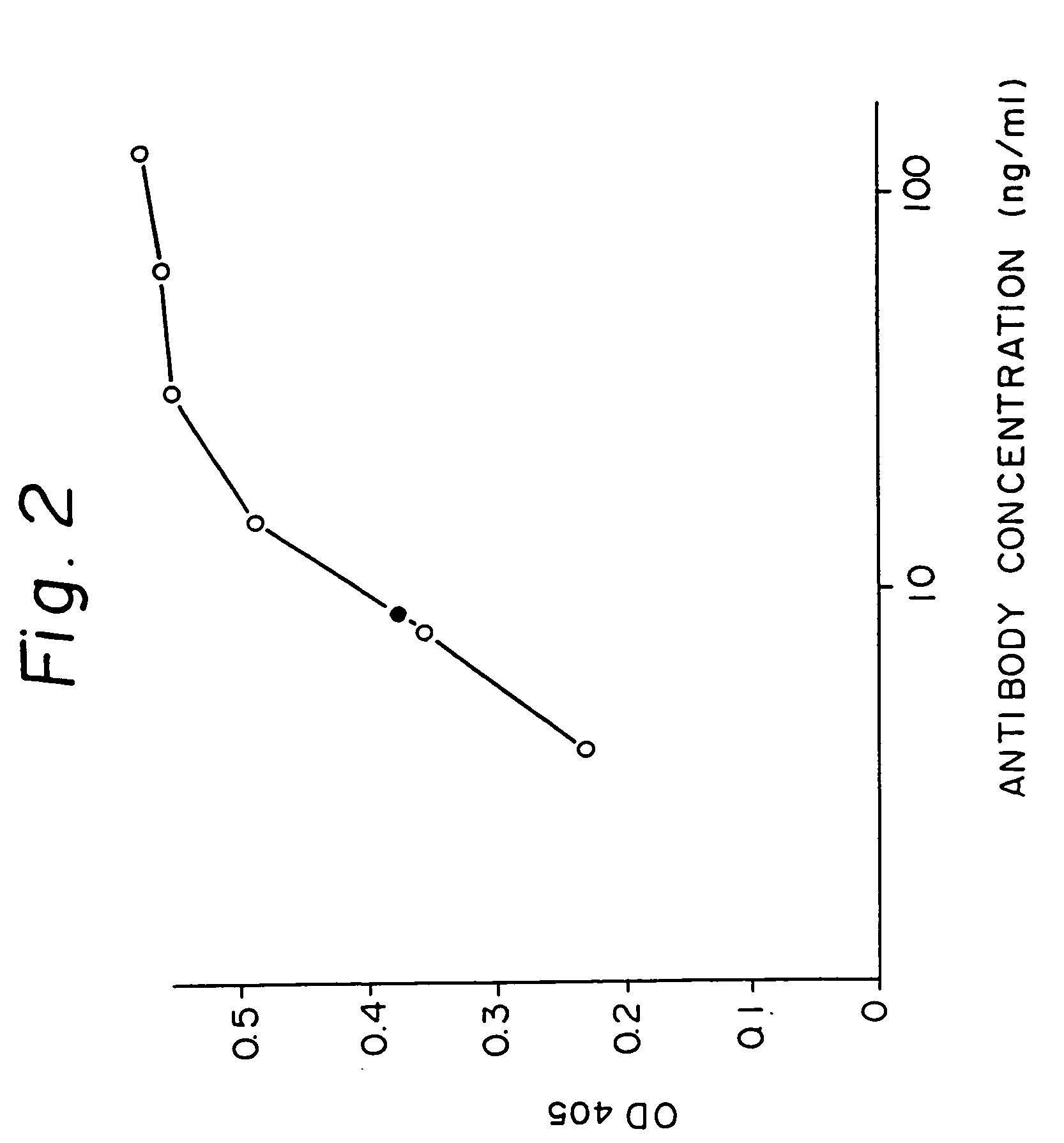
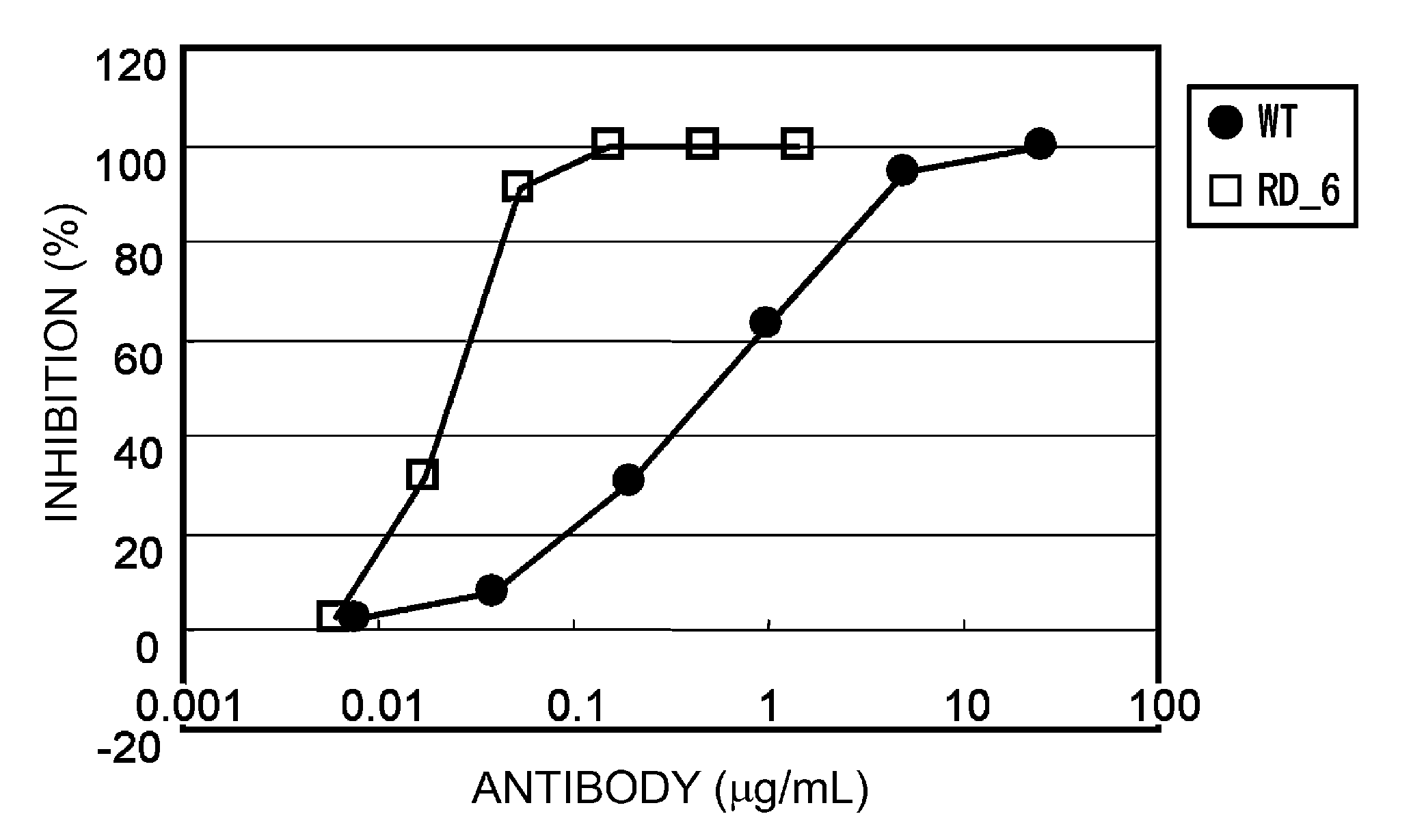
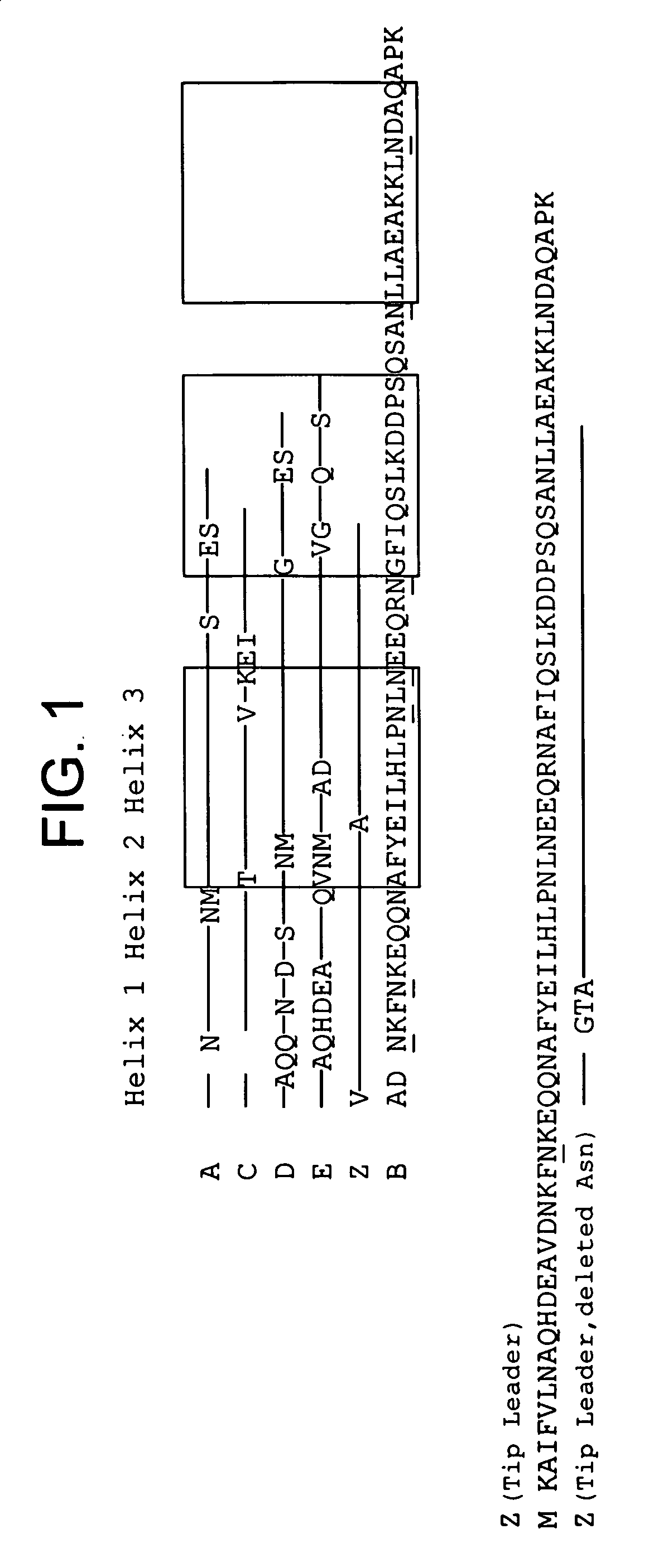
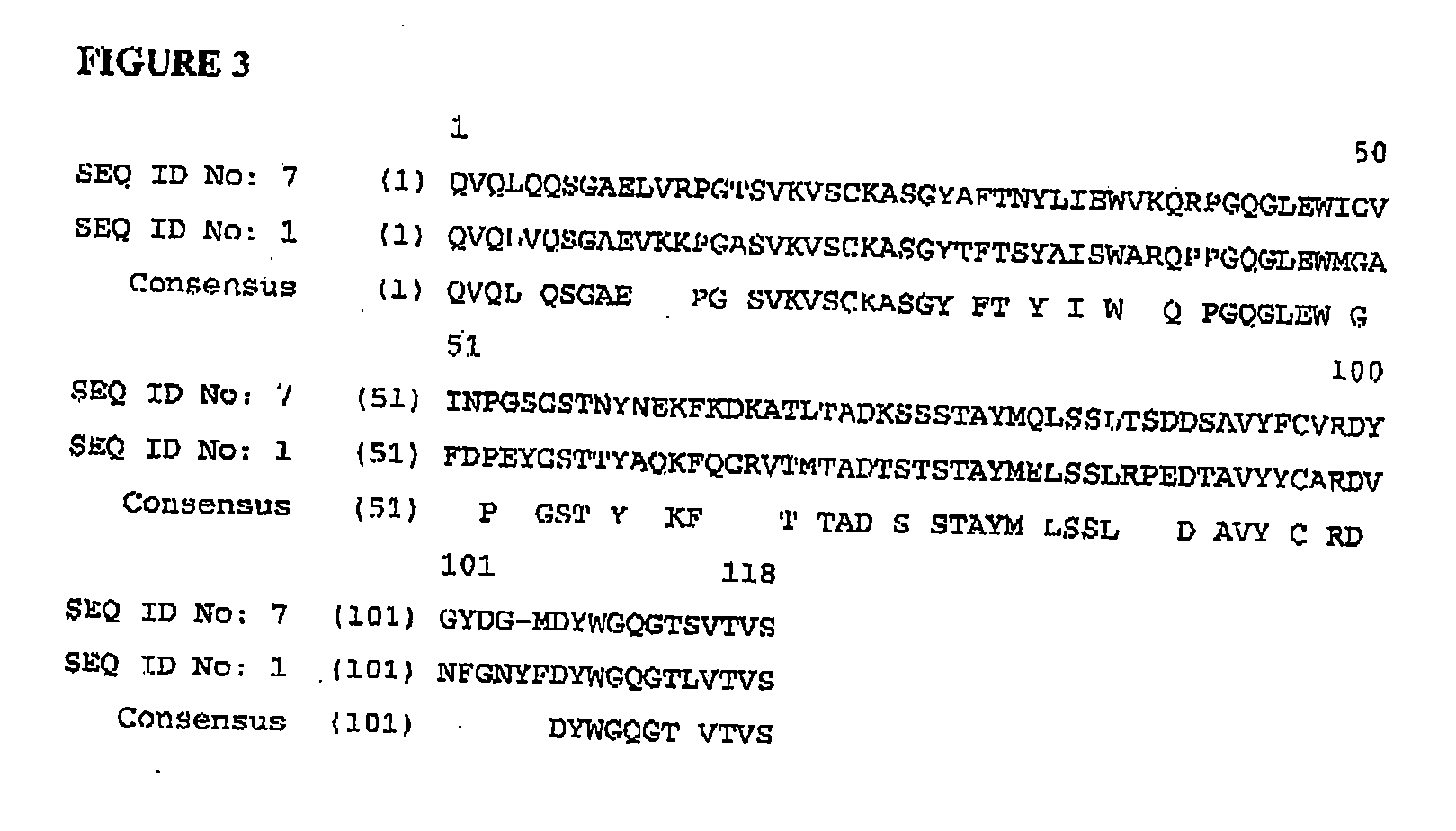
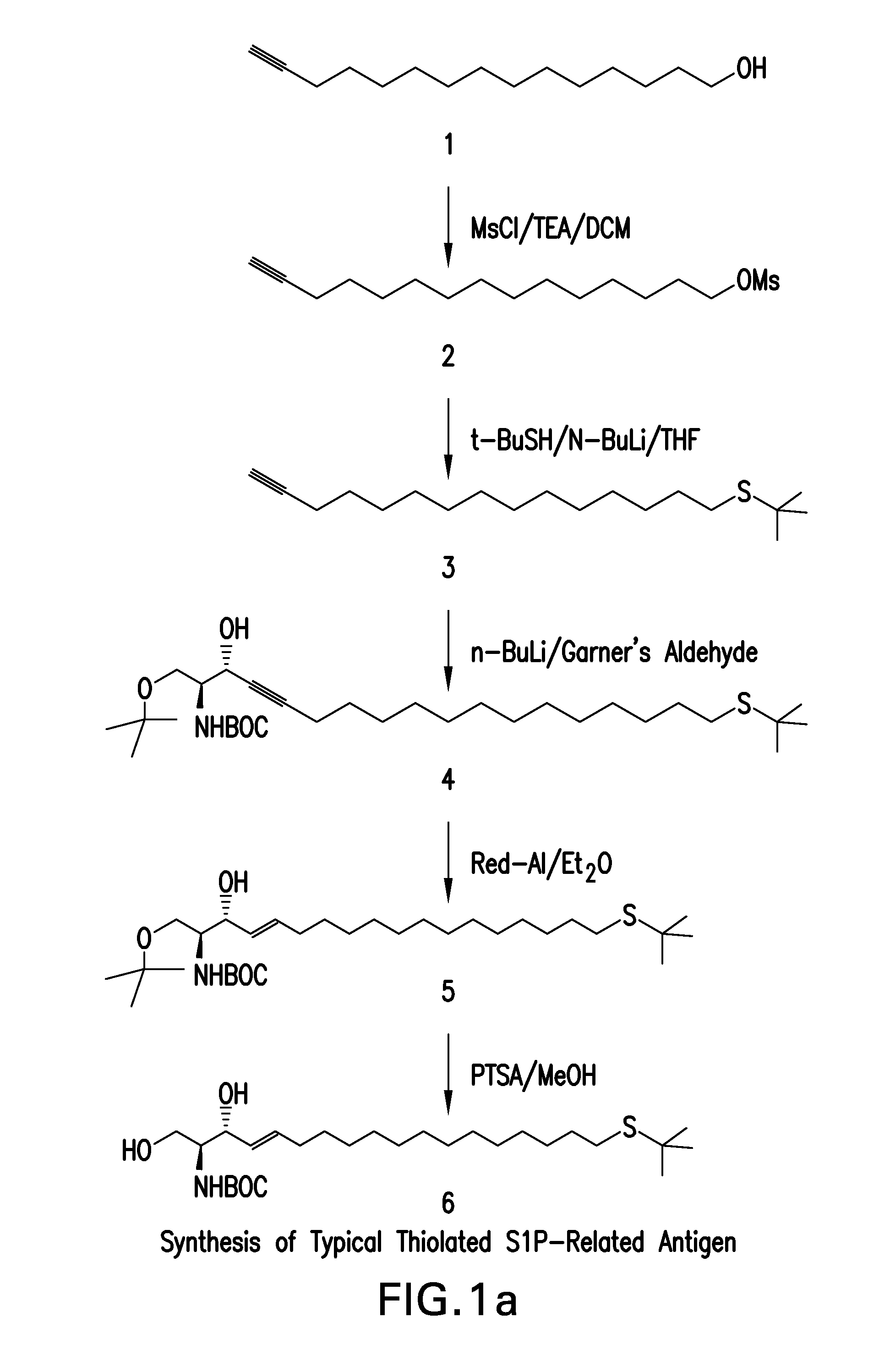
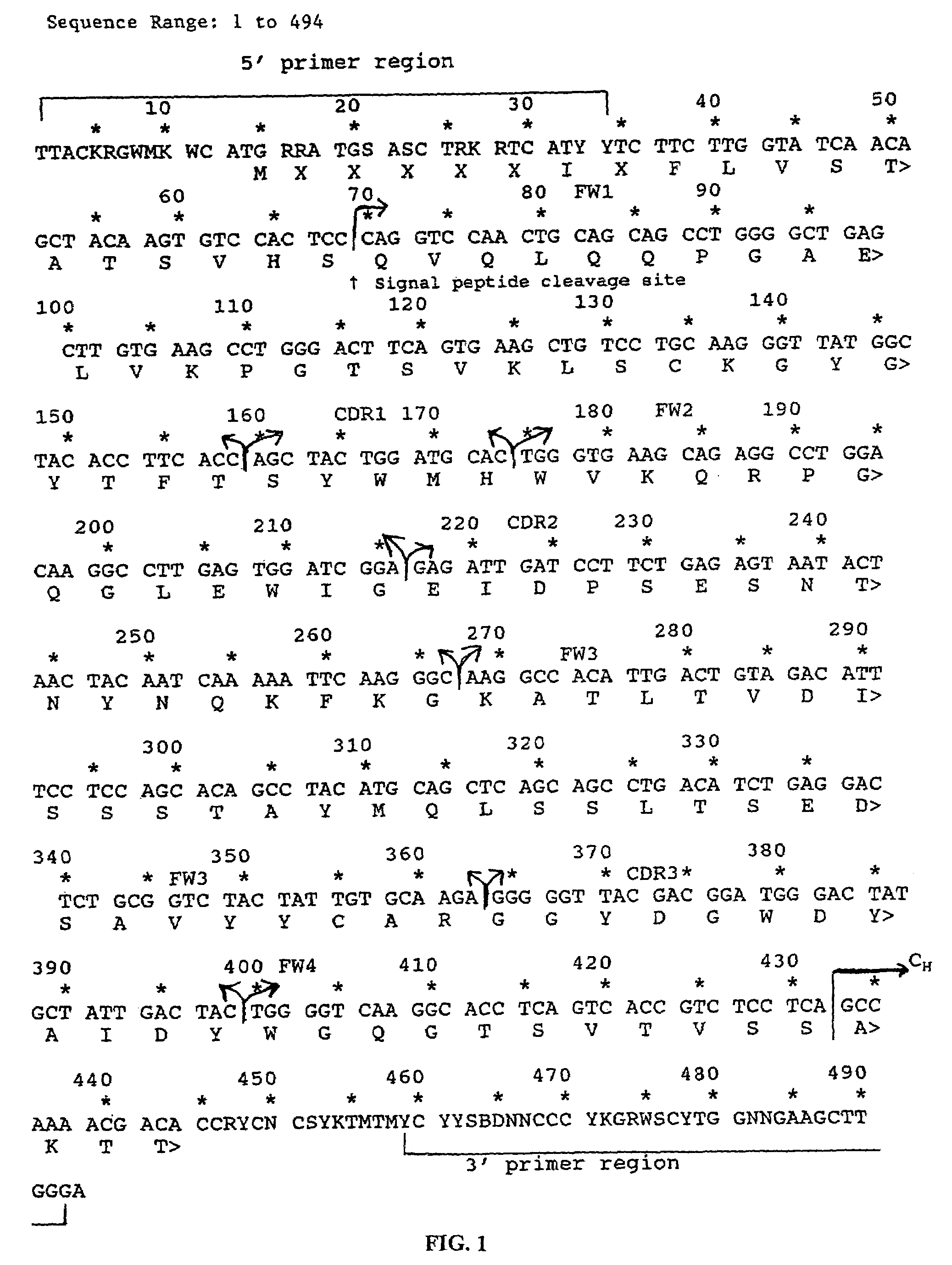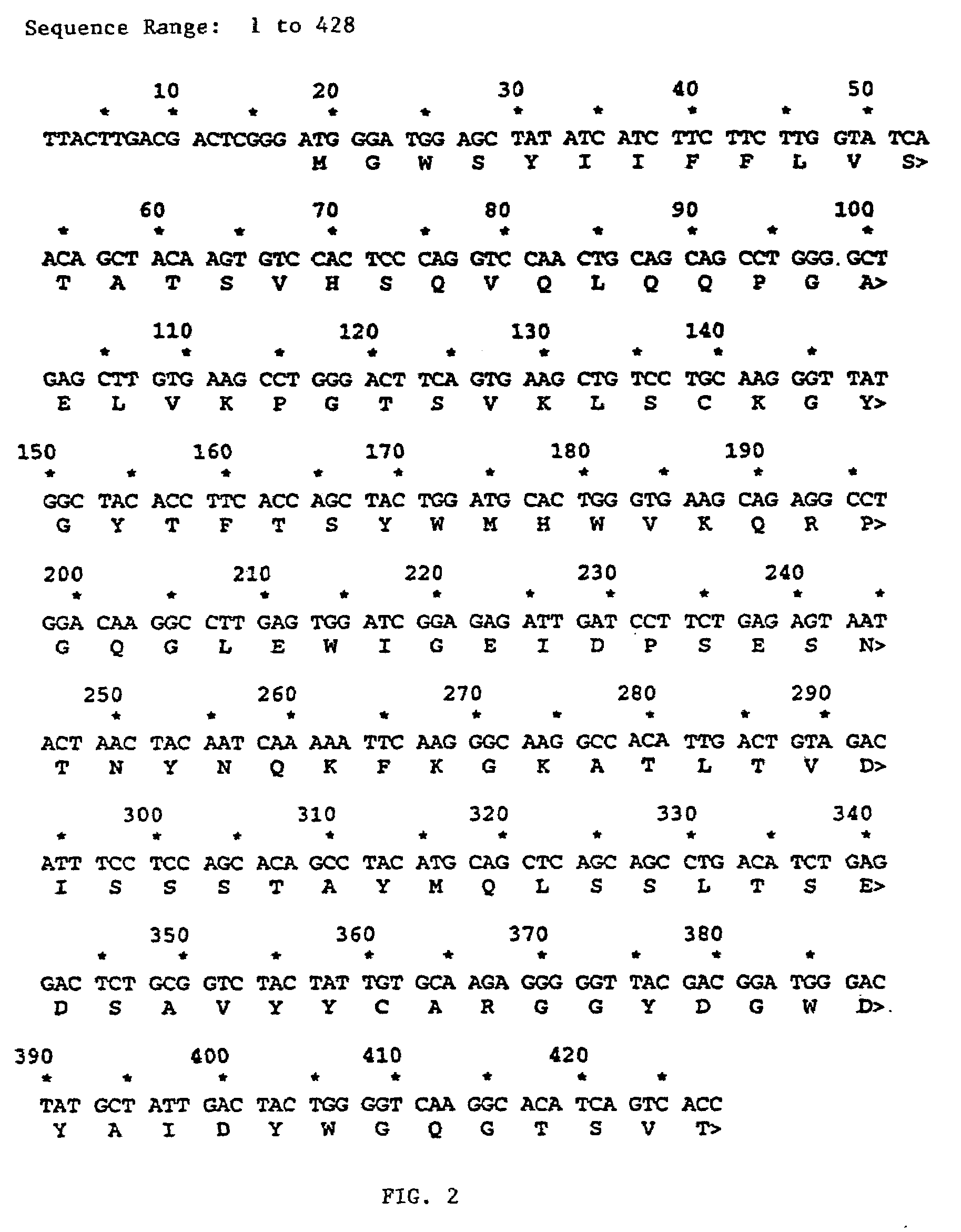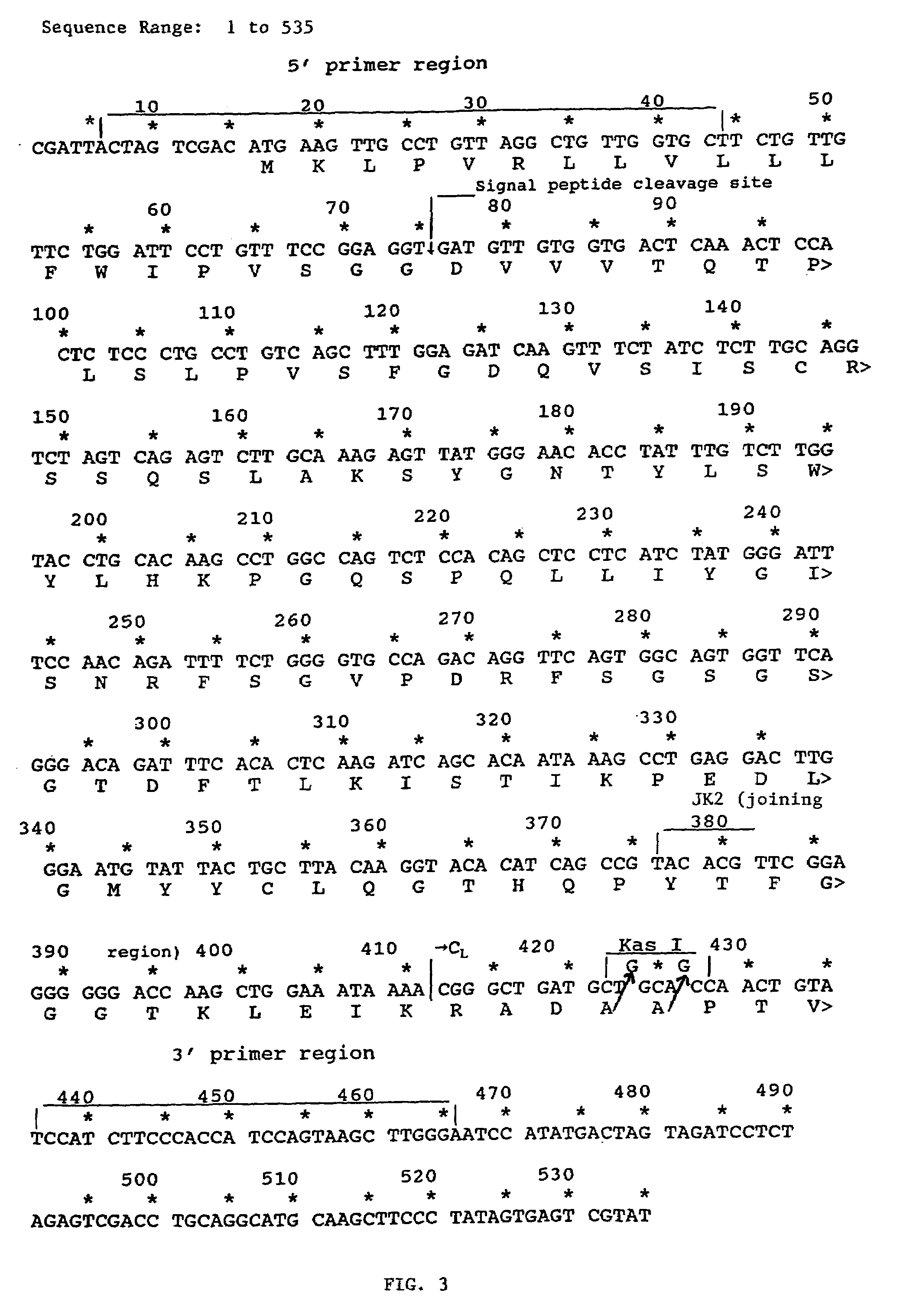Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
677 results about "Complementarity determining region" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
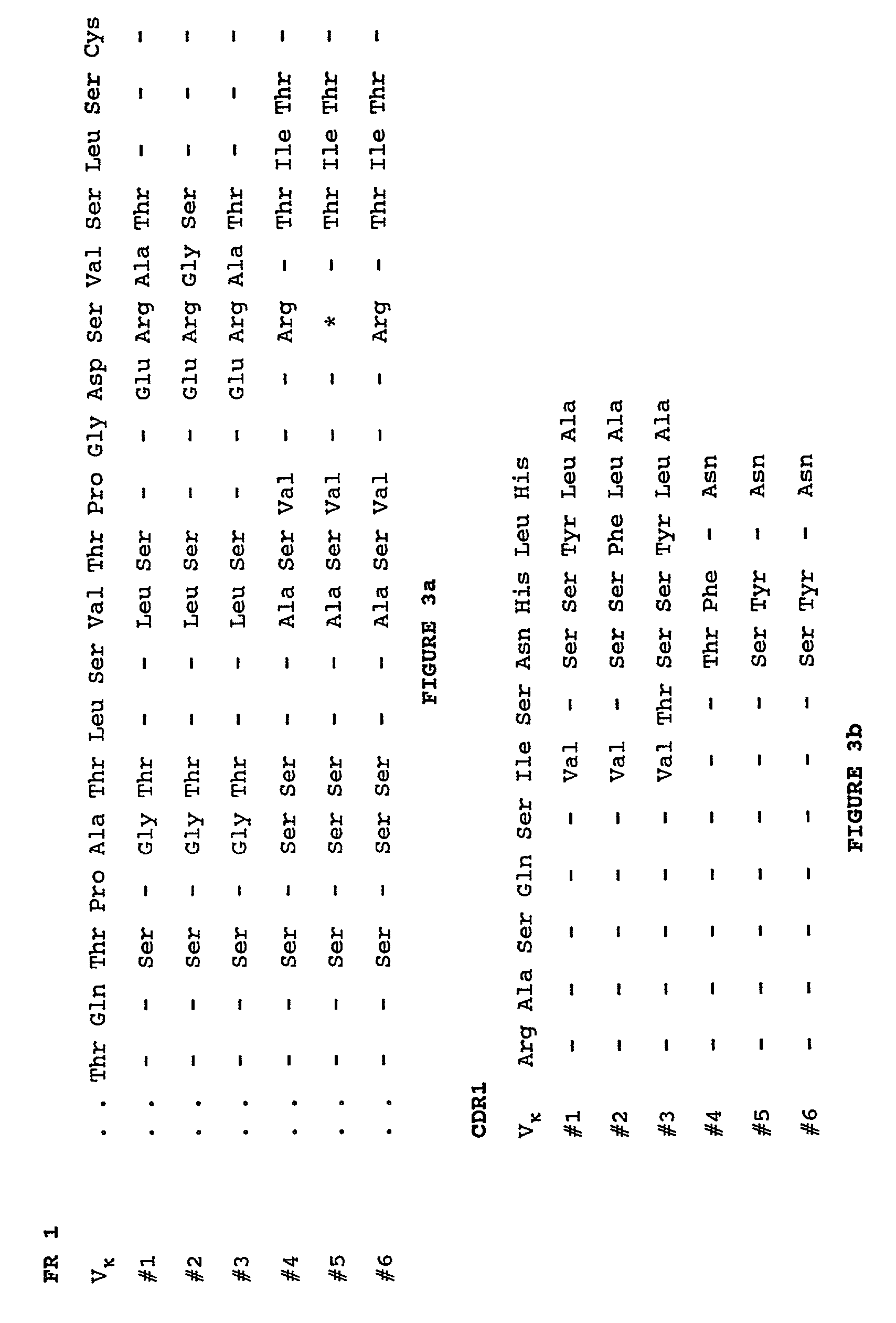
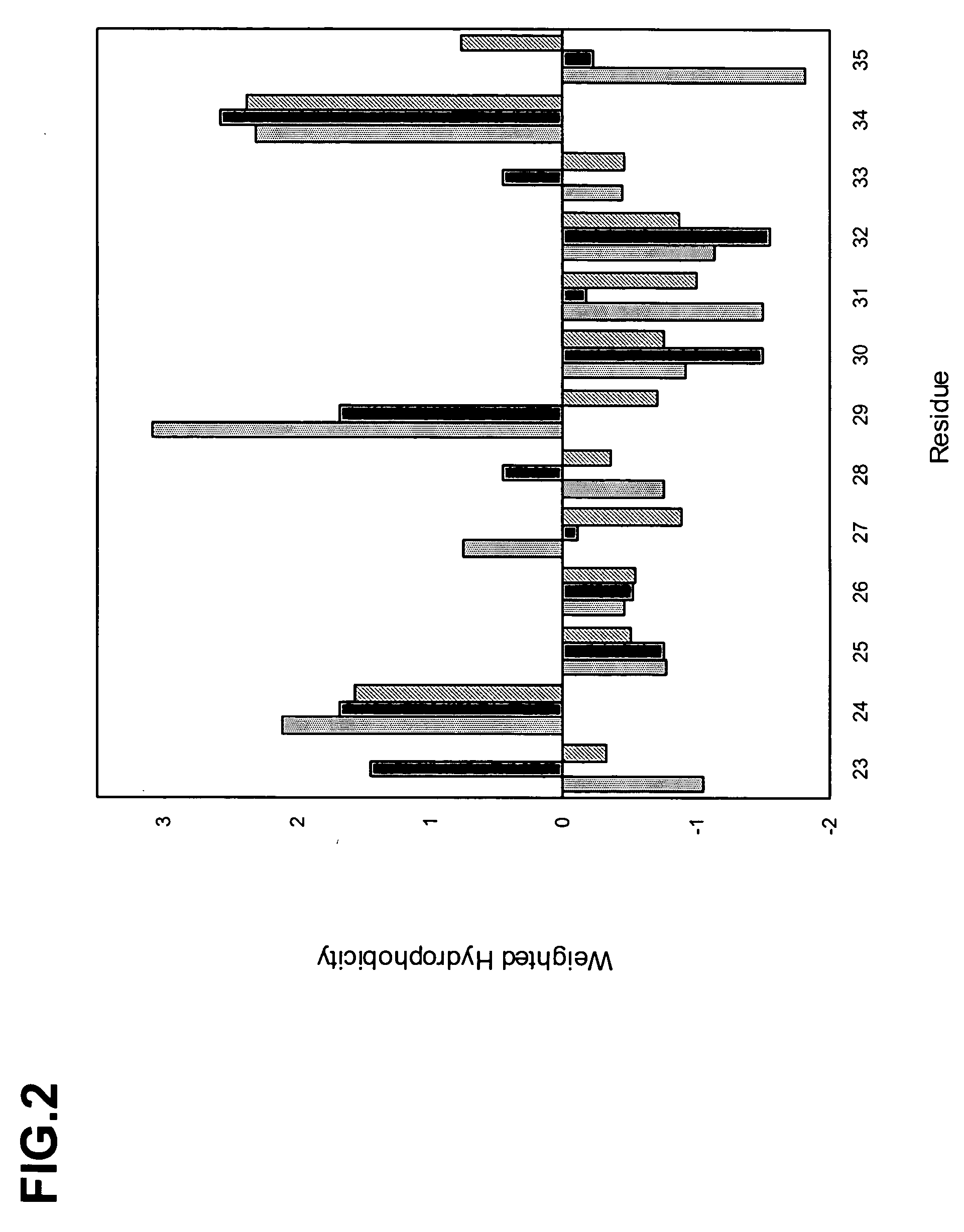
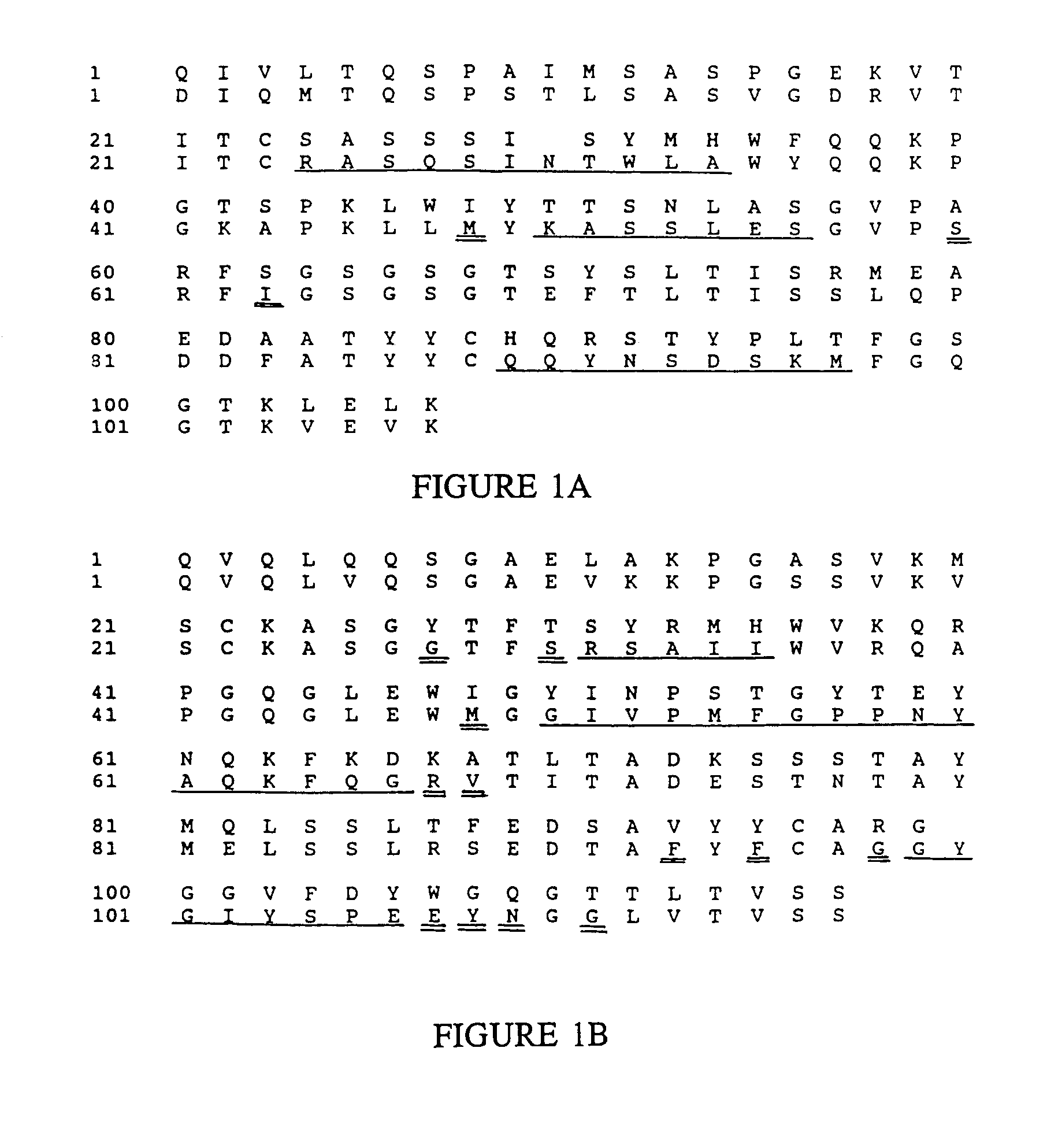
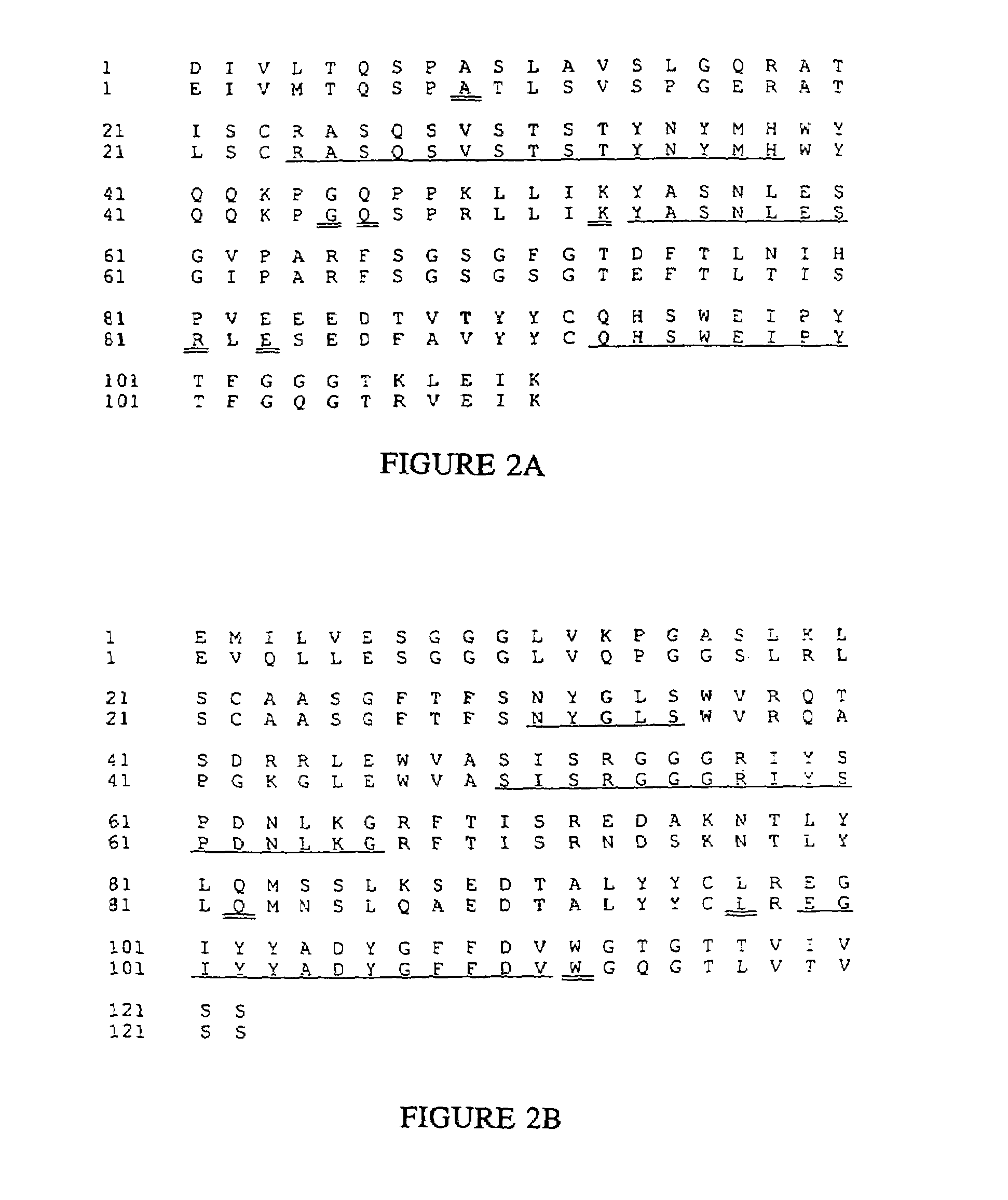
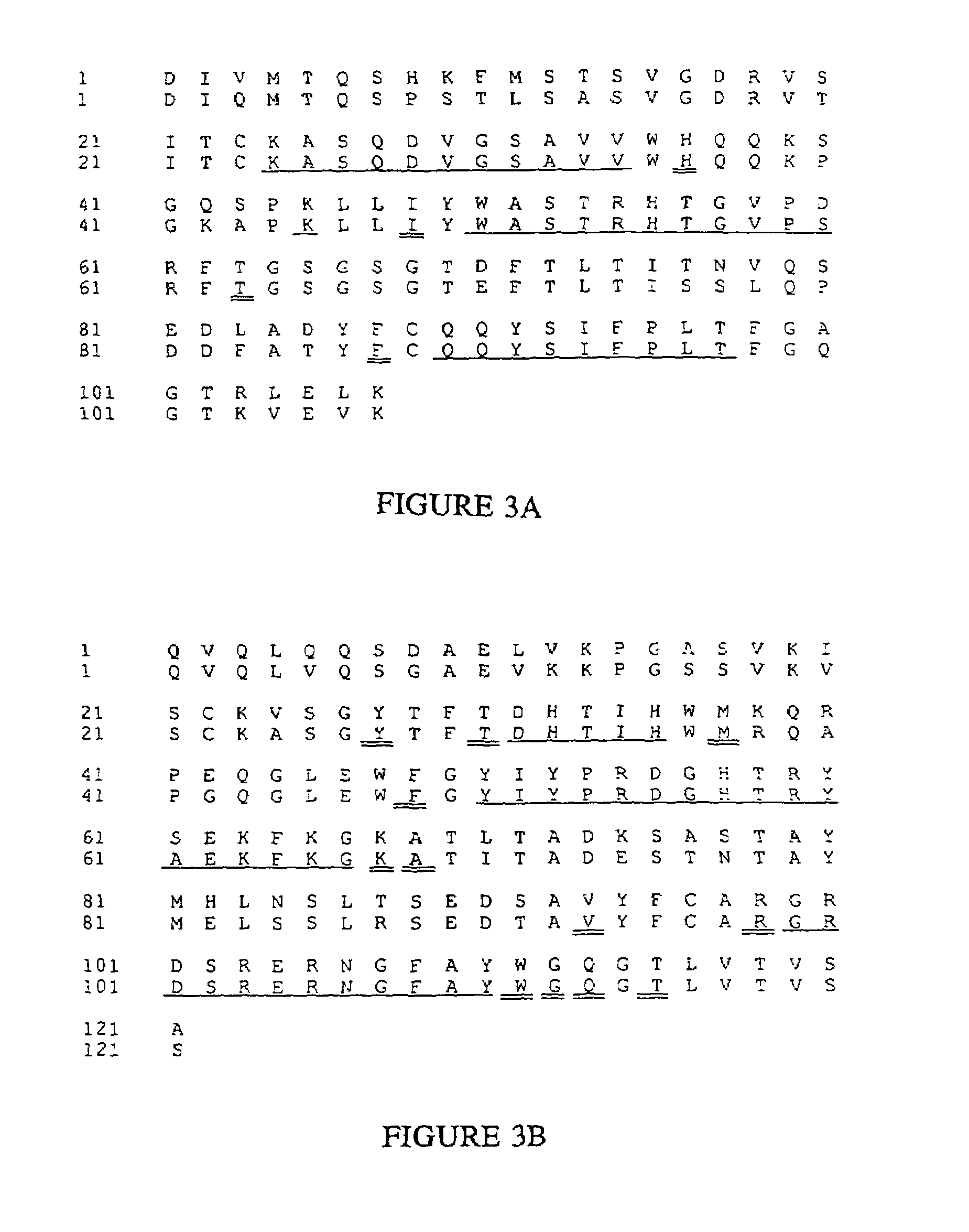
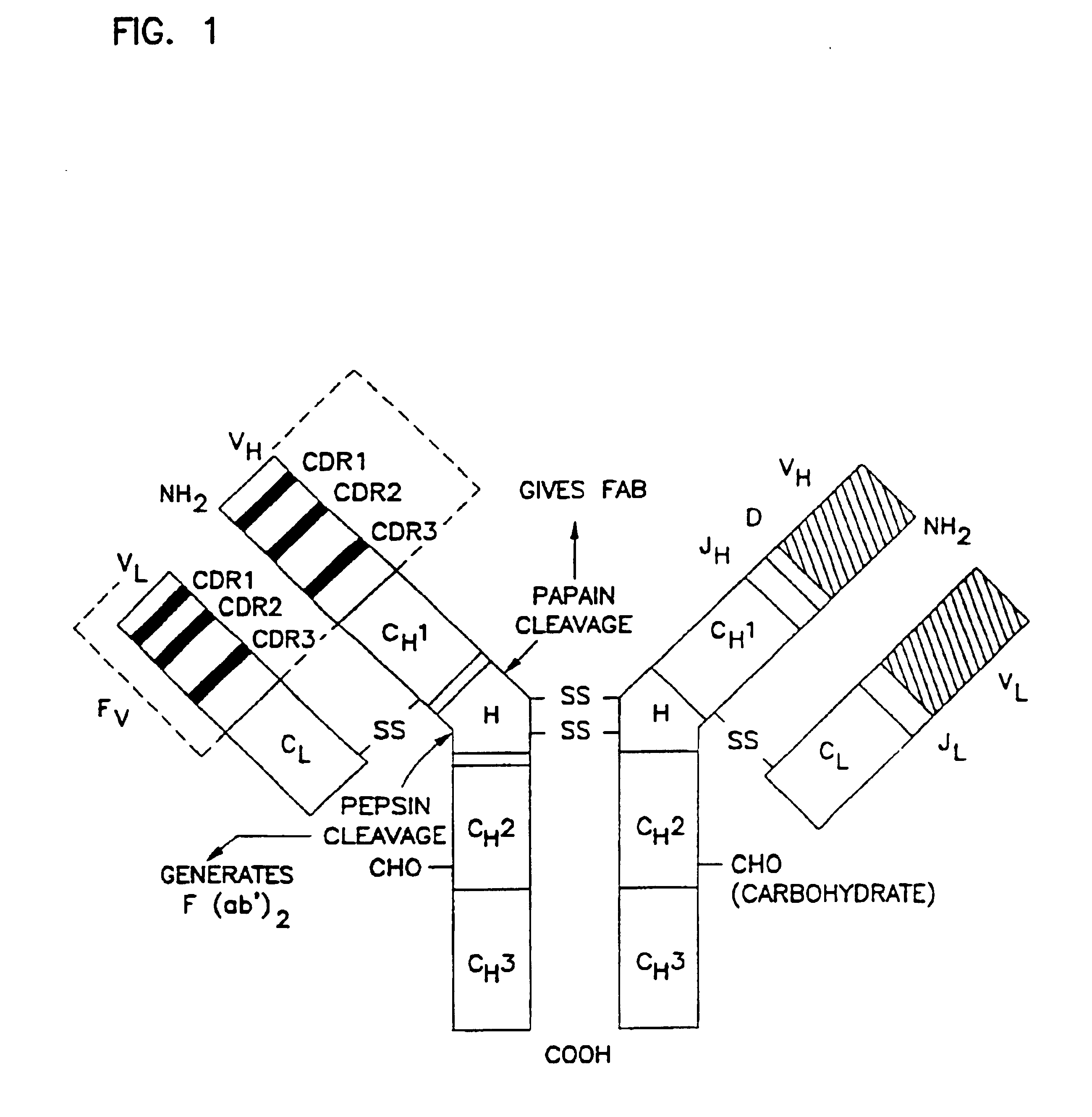
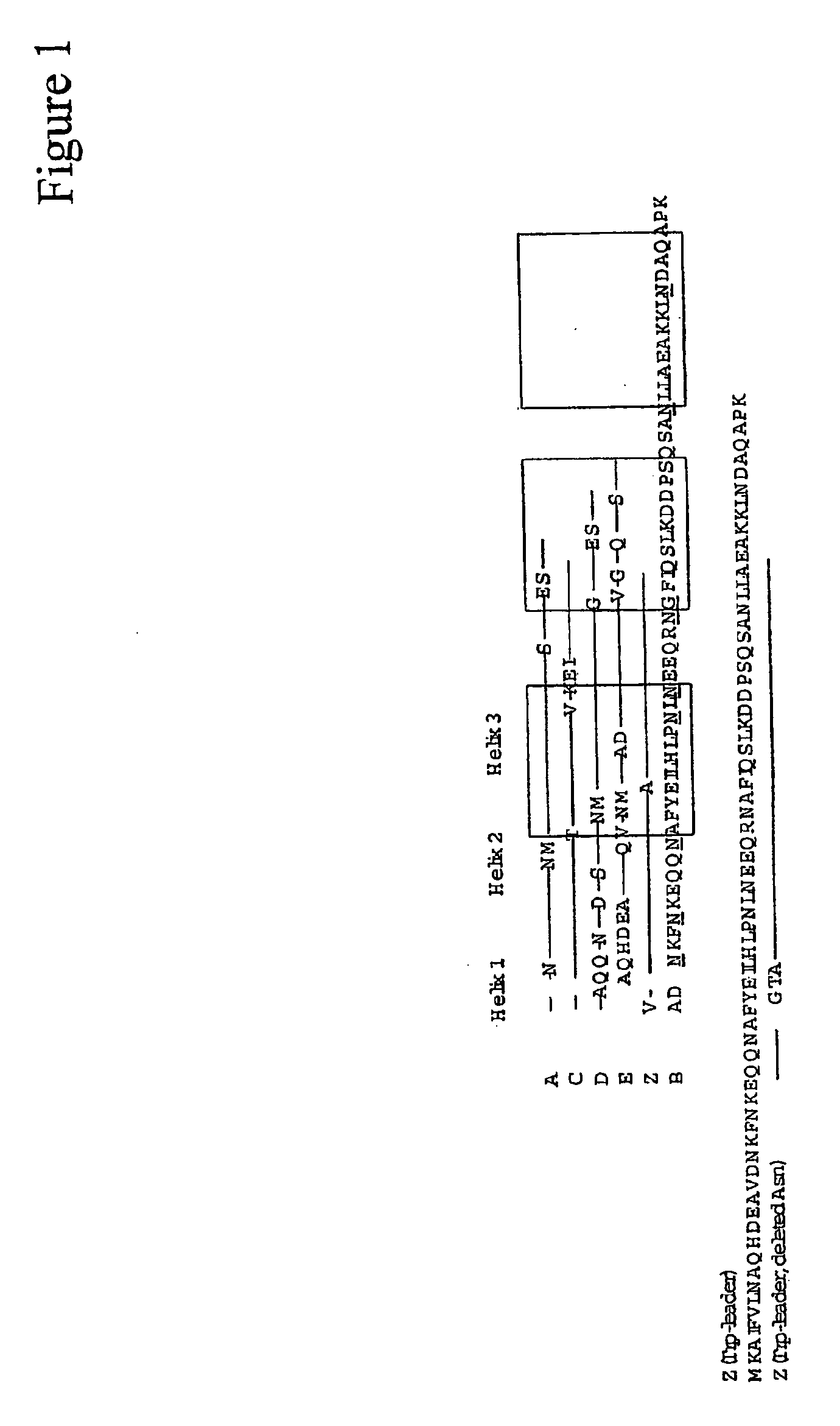
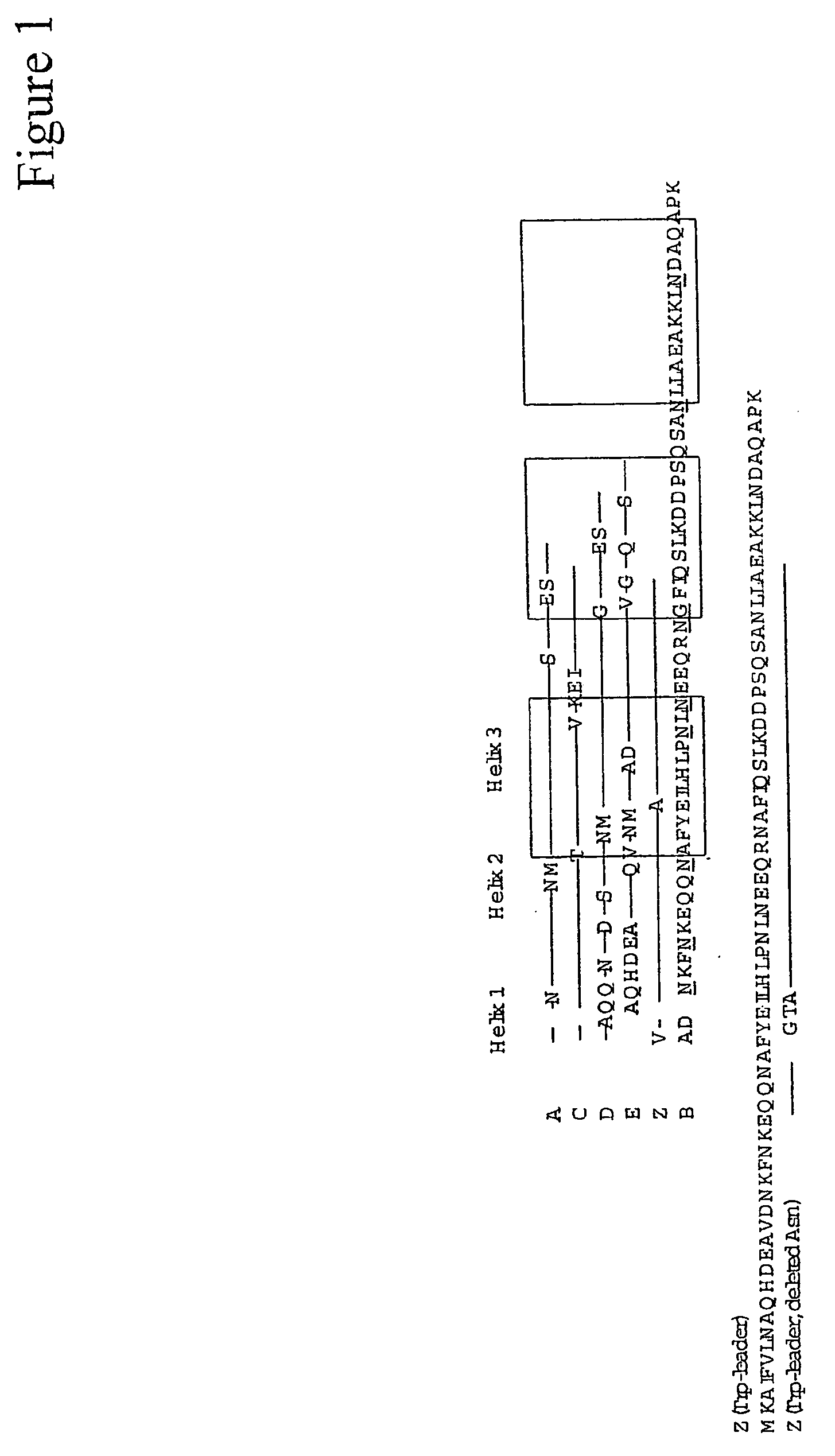
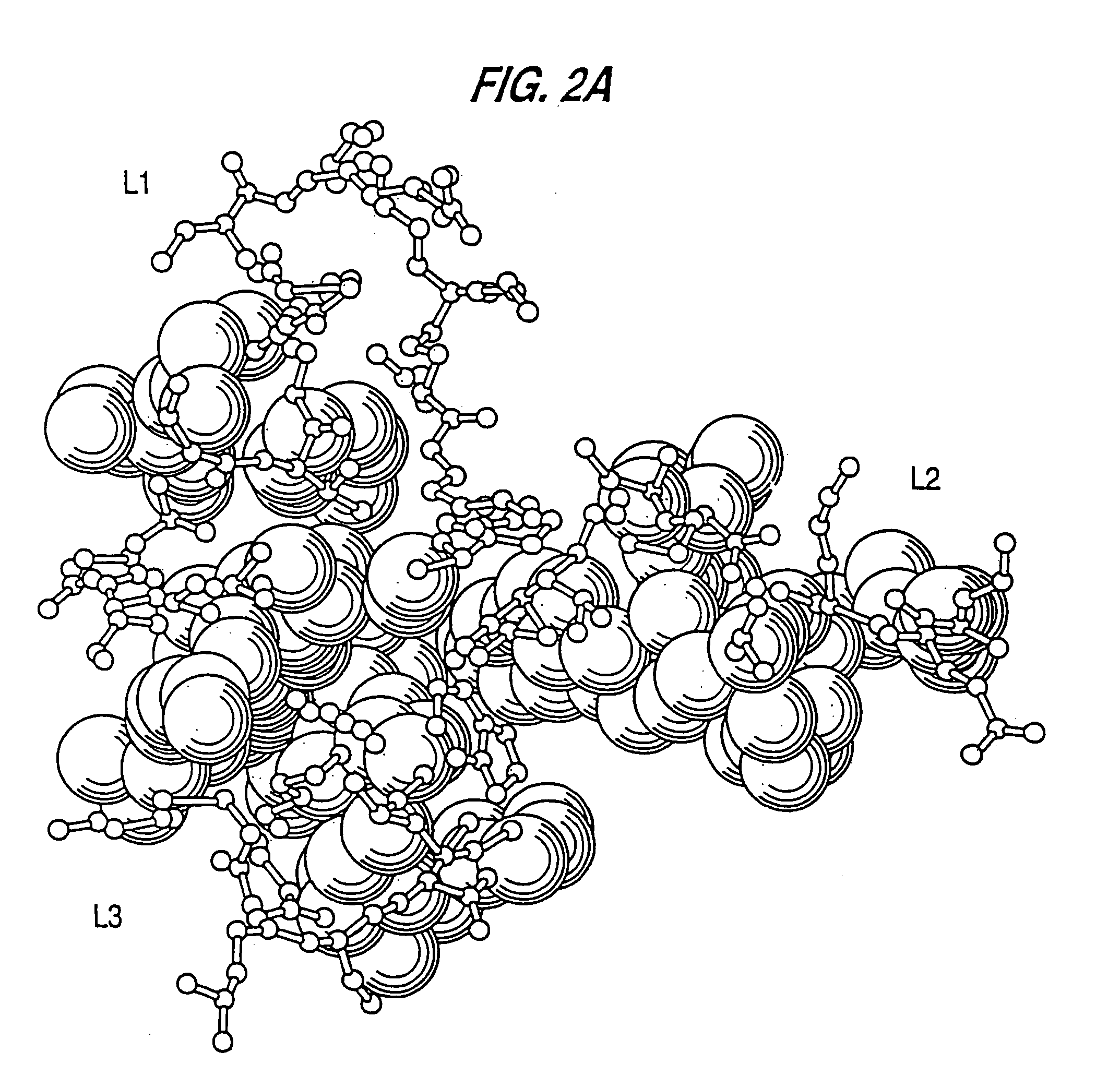
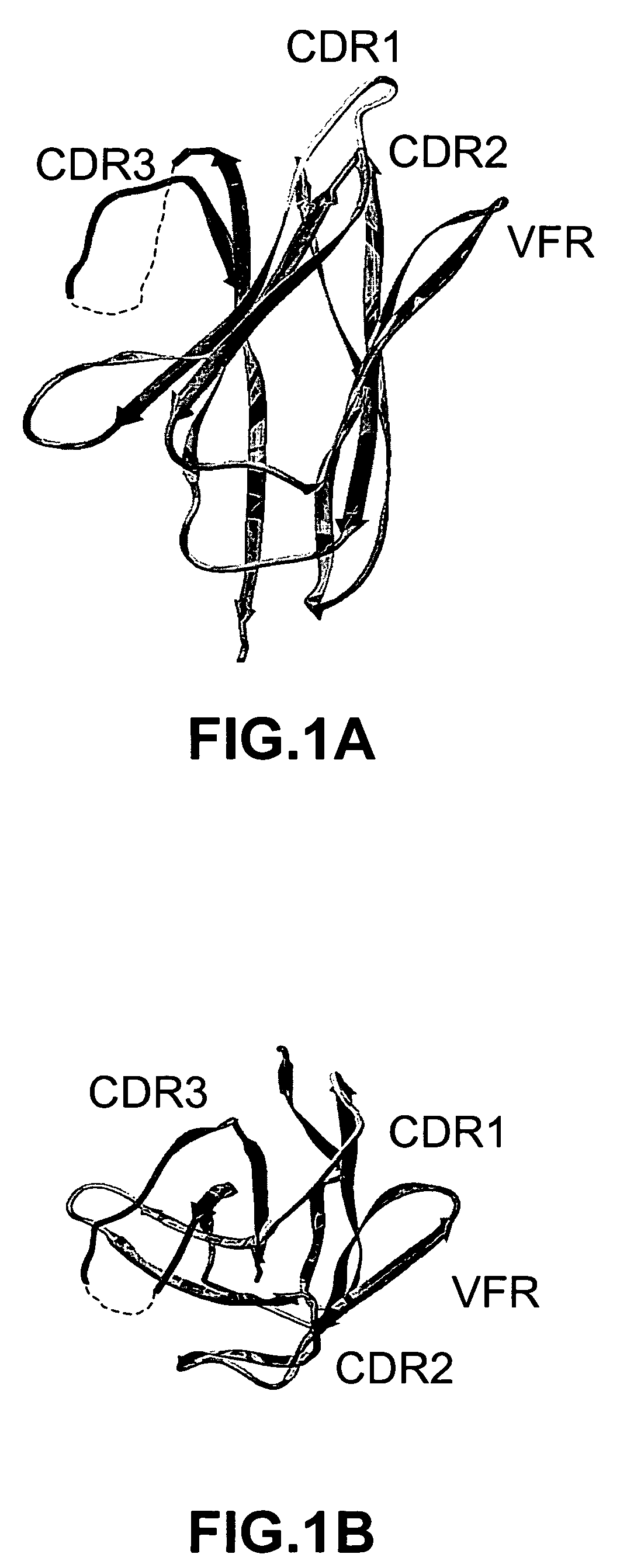
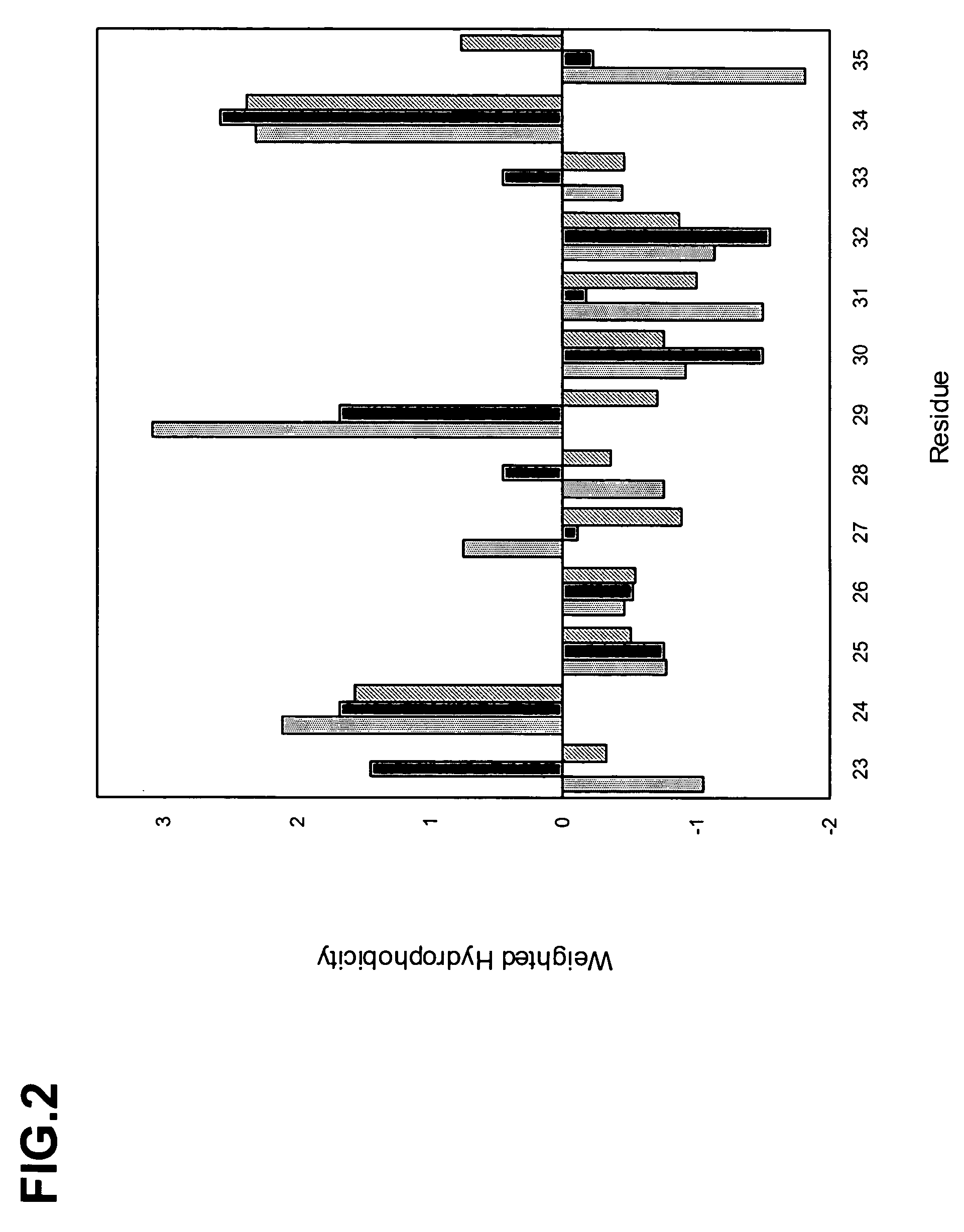
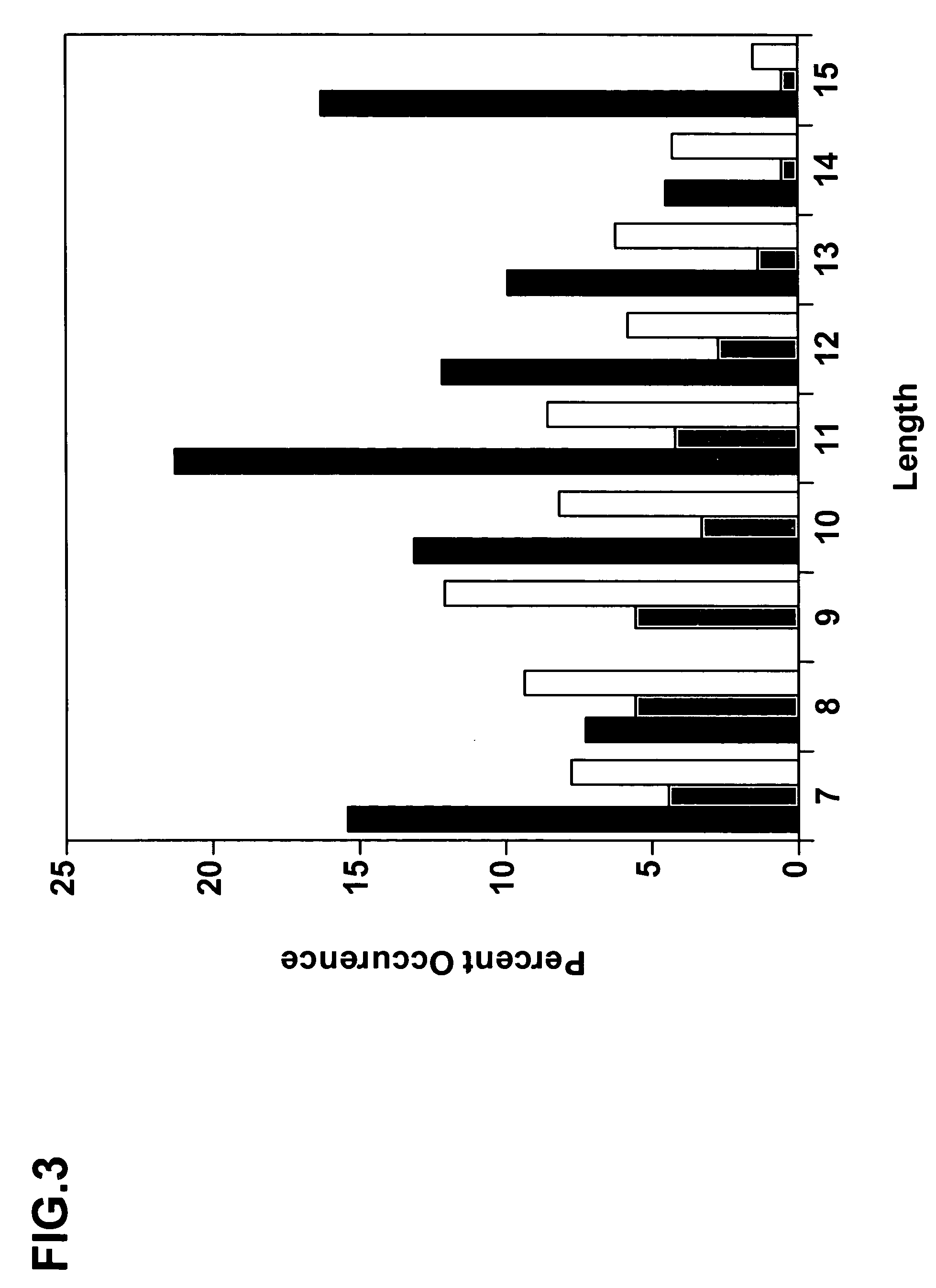
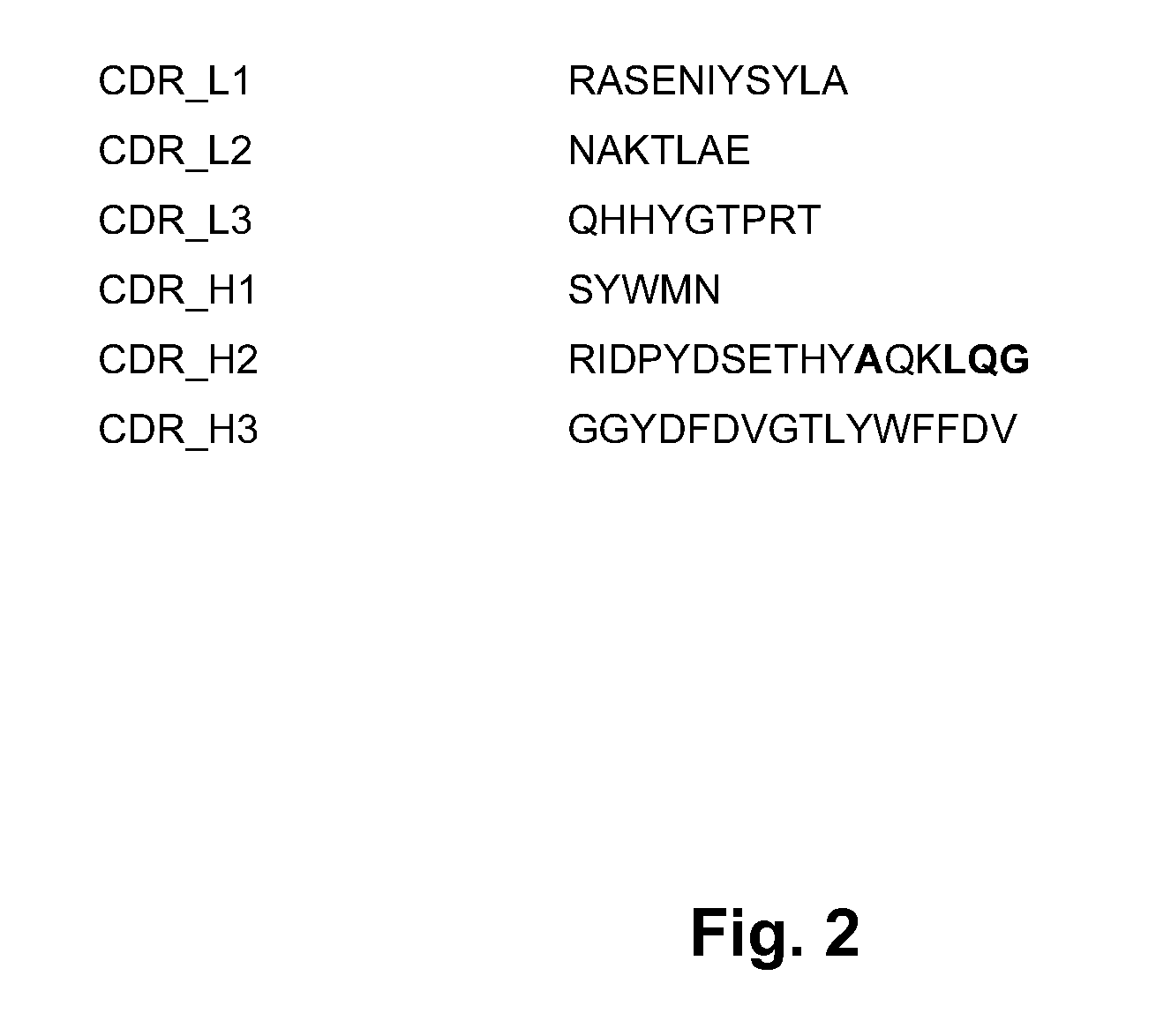
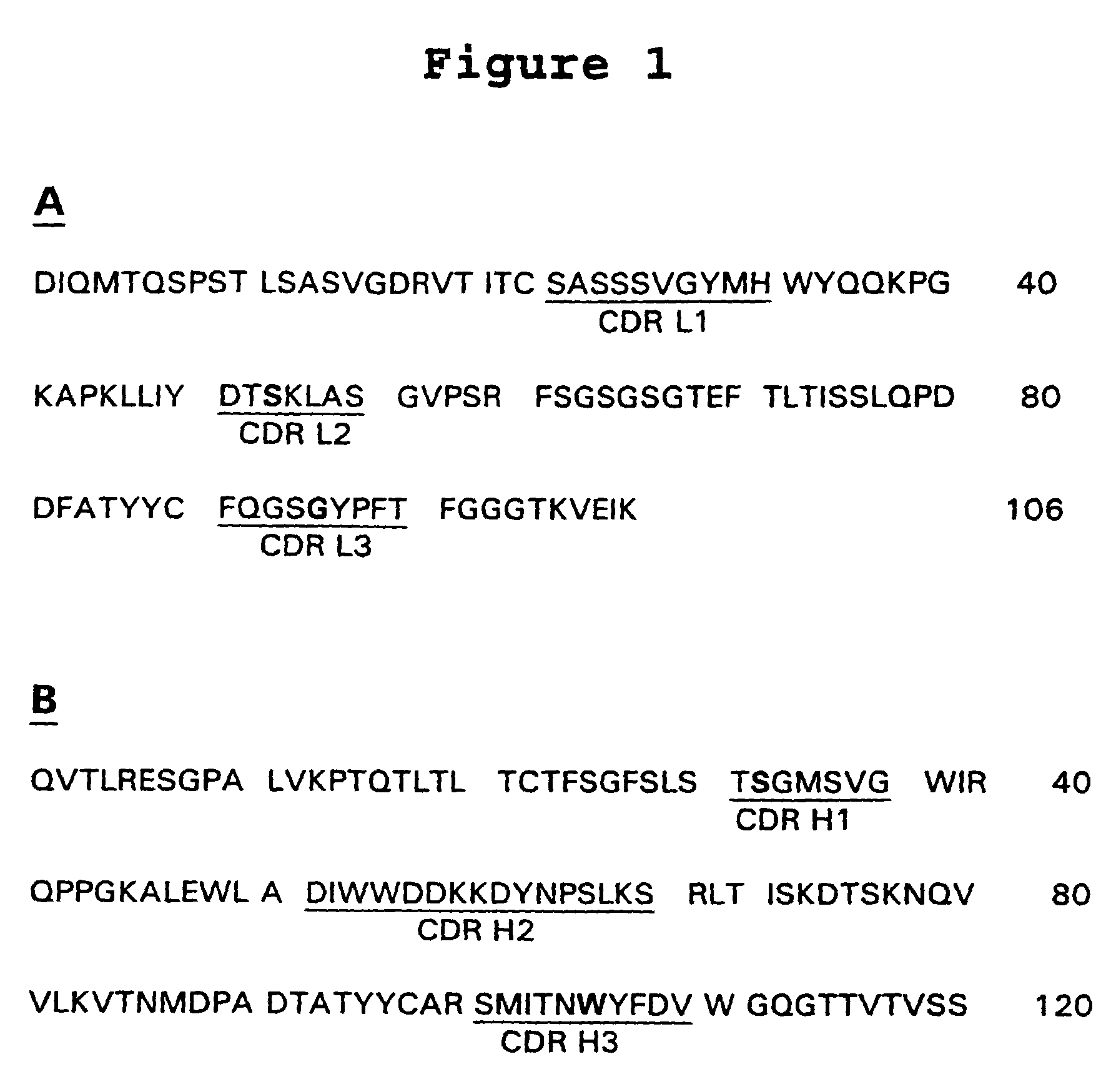
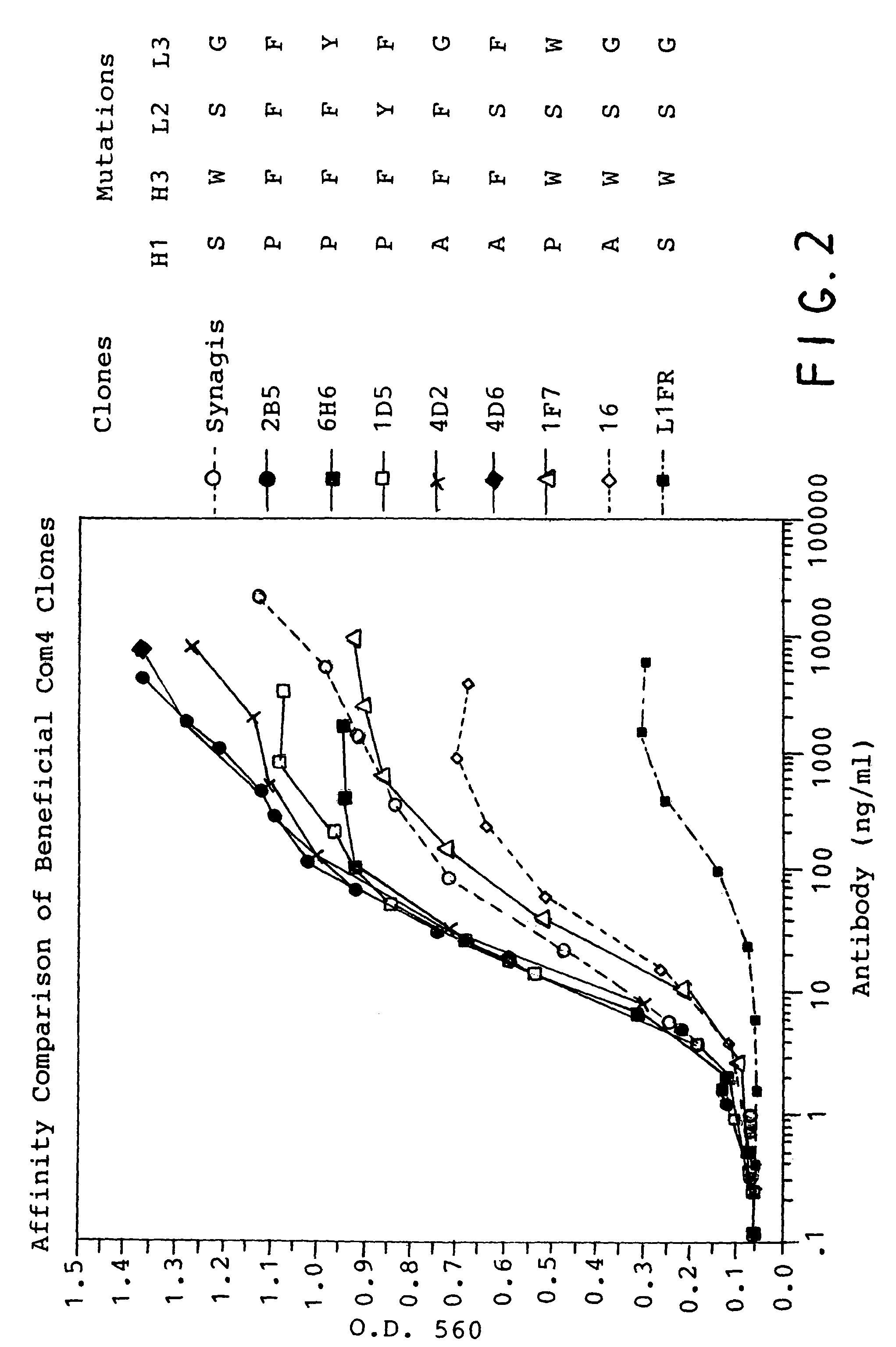
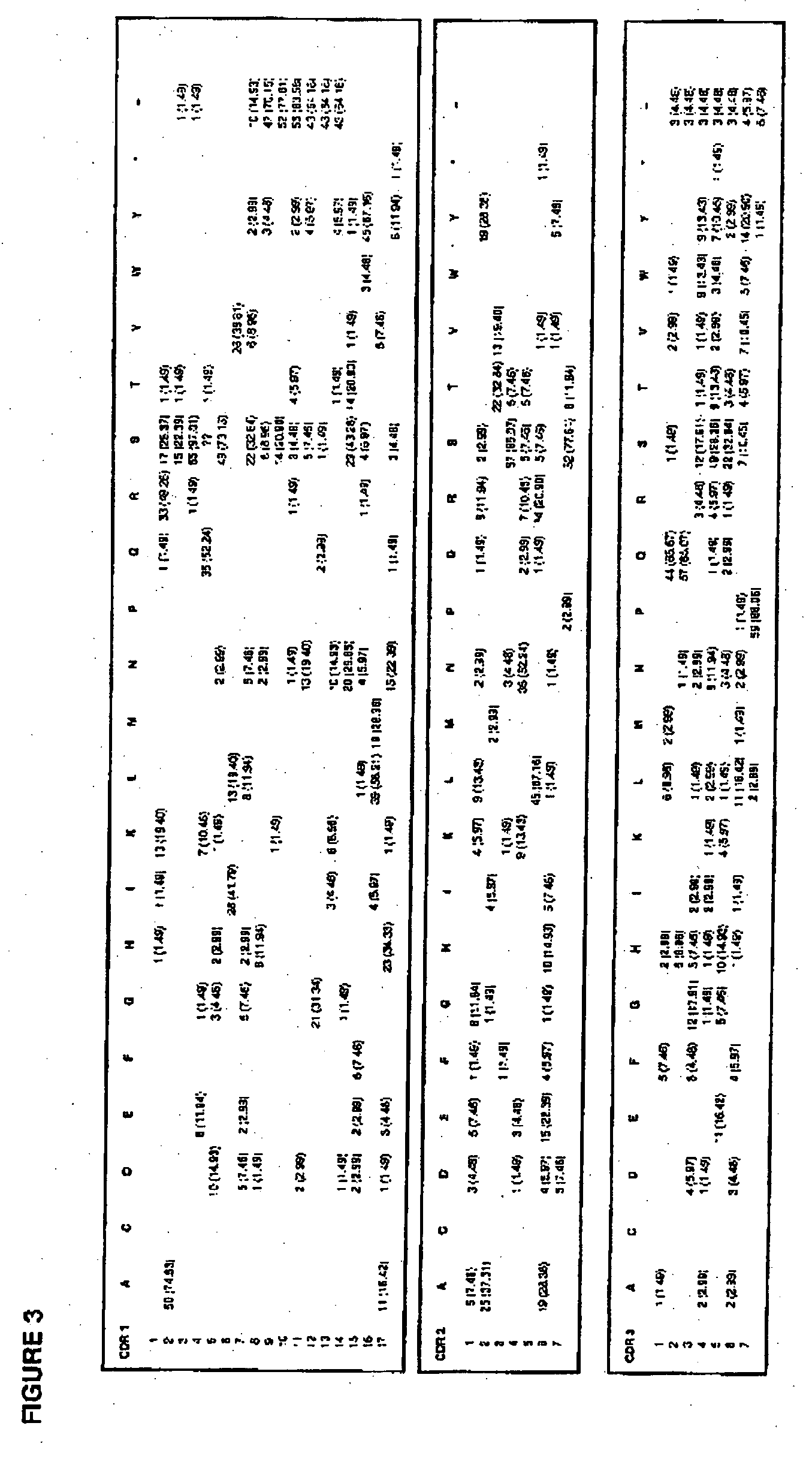
Complementarity-determining regions (CDRs) are part of the variable chains in immunoglobulins (antibodies) and T cell receptors, generated by B-cells and T-cells respectively, where these molecules bind to their specific antigen. A set of CDRs constitutes a paratope. As the most variable parts of the molecules, CDRs are crucial to the diversity of antigen specificities generated by lymphocytes.
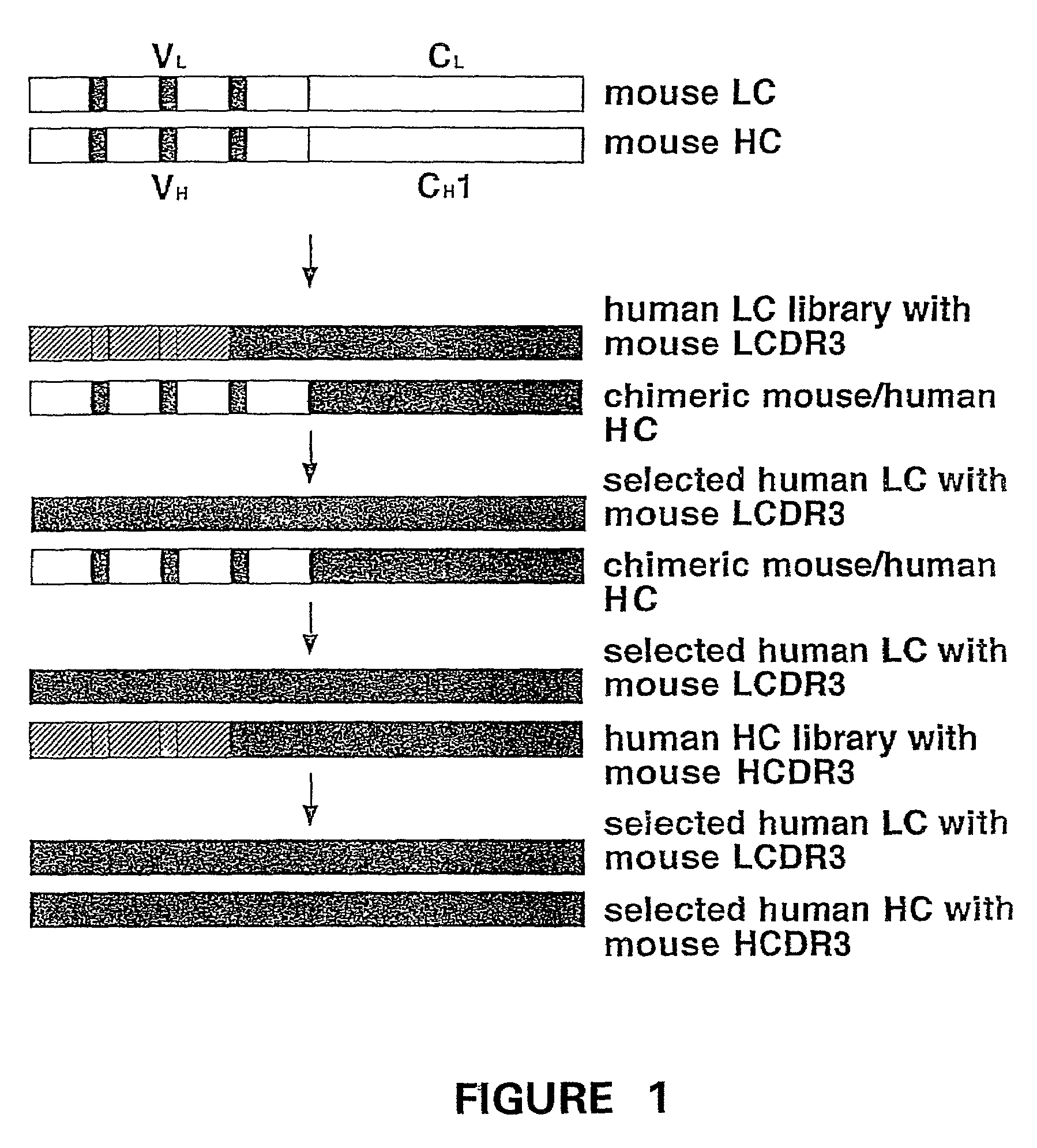
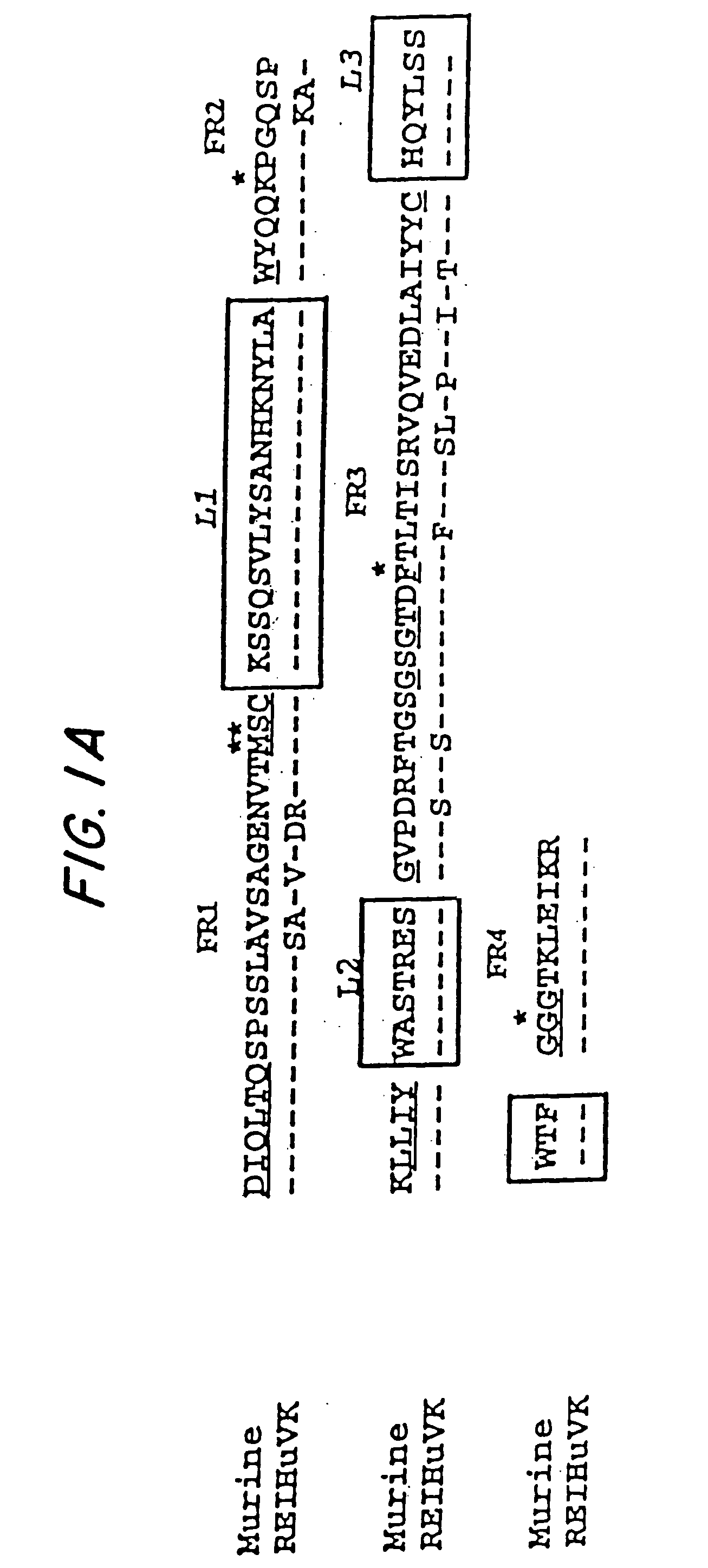
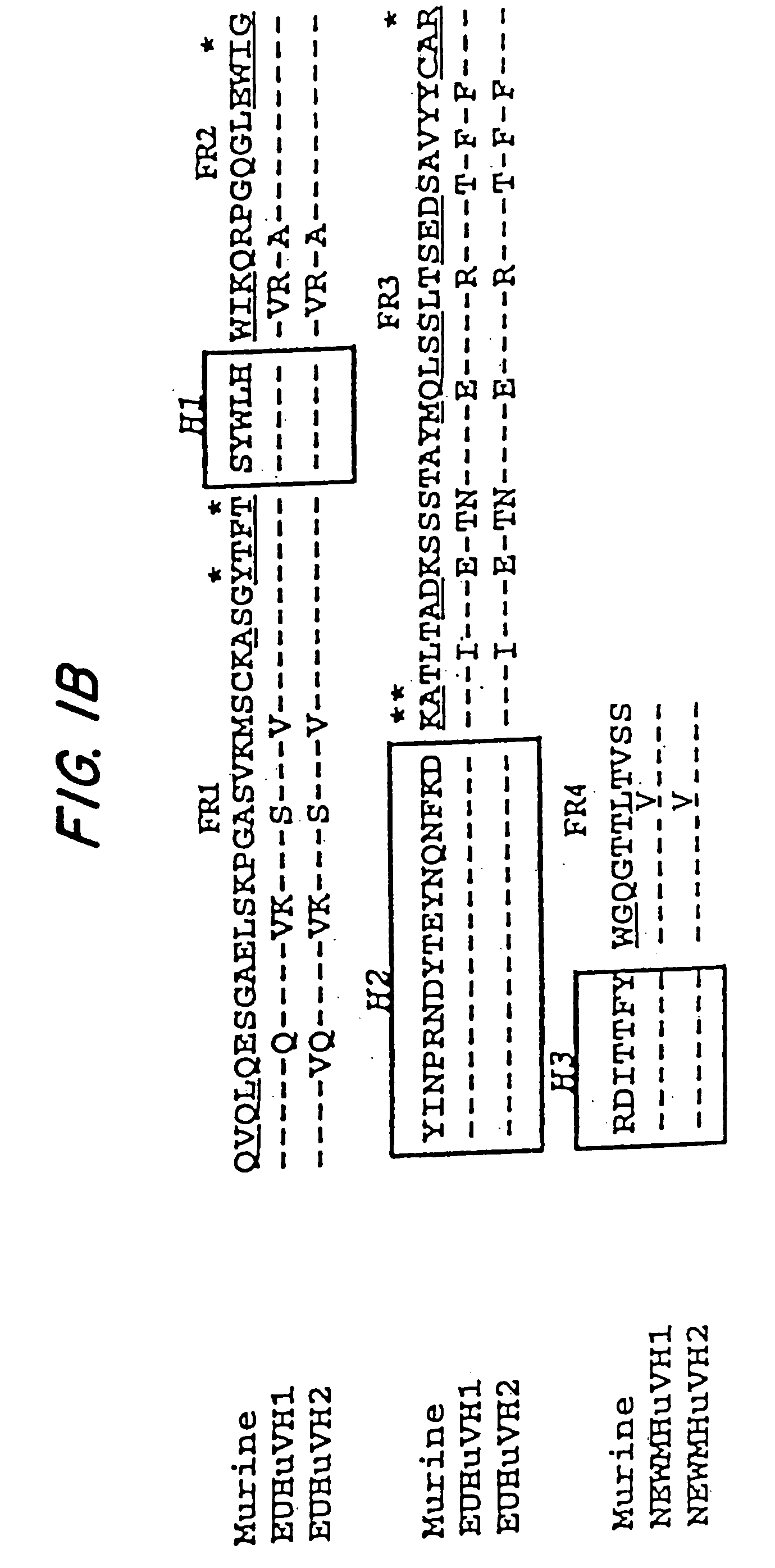
Humanization of murine antibody
InactiveUS7087409B2Hybrid immunoglobulinsSugar derivativesComplementarity determining regionHeavy chain
A humanized murine antibody is provided. The amino acid sequences of a light chain complementarity determining region from a mouse antibody are grafted onto a human light chain, and a heavy chain complementarity determining region from a mouse antibody are grafted onto a human antibody heavy chain to produce libraries from which a humanized murine antibody having the desired specificity is selected.
Owner:THE SCRIPPS RES INST
Variable domain library and uses
ActiveUS20050266000A1Generate efficientlyQuality improvementImmunoglobulins against growth factorsImmunoglobulins against cell receptors/antigens/surface-determinantsComplementarity determining regionAntigen binding
The invention provides polypeptides comprising a variant heavy chain variable framework domain (VFR). In some embodiments, the amino acids defining the VFR form a loop of an antigen binding pocket. In an embodiment, the polypeptide is a variable domain of a monobody and has a variant VFR. The polypeptide may optionally comprise one or more complementary determining regions (CDRs) of antibody variable domains. In an embodiment, the polypeptide is a variable domain of a monobody and has a variant VFR and one or more variant CDRs. Libraries of polypeptides that include a plurality of different antibody variable domains generated by creating diversity in a VFR, and optionally, one or more CDRs are provided and may be used as a source for identifying novel antigen binding polypeptides that can be used therapeutically or as reagents. The invention also provides fusion polypeptides, compositions, and methods for generating and using the polypeptides and libraries.
Owner:GENENTECH INC
Humanized immunoglobulins
Novel methods for producing, and compositions of, humanized immunoglobulins having one or more complementarity determining regions (CDR's) and possible additional amino acids from a donor immunoglobulin and a framework region from an accepting human immunoglobulin are provided. Each humanized immunoglobulin chain will usually comprise, in addition to the CDR's, amino acids from the donor immunoglobulin framework that are, e.g., capable of interacting with the CDR's to effect binding affinity, such as one or more amino acids which are immediately adjacent to a CDR in the donor immunoglobulin or those within about about 3Å as predicted by molecular modeling. The heavy and light chains may each be designed by using any one or all of various position criteria. When combined into an intact antibody, the humanized immunoglobulins of the present invention will be substantially non-immunogenic in humans and retain substantially the same affinity as the donor immunoglobulin to the antigen, such as a protein or other compound containing an epitope.
Owner:PDL BIOPHARMA INCORPORATED
Reshaped human antibody to human interleukin-6 receptor
InactiveUS7479543B2Peptide/protein ingredientsAntibody mimetics/scaffoldsComplementarity determining regionV region
A reshaped human antibody to the human IL-6R, comprising:(A) an L chain comprising,(1) a human L chain C region, and(2) an L chain V region comprising human L chain framework regions (FRs), and mouse L chain complementary determination regions (CDRs) of a momoclonal antibody to the IL-6 receptor (IL-6R); and(B) an H chain comprising,(1) a human H chain C region, and(2) an H chain V region comprising human H chain FRS, and mouse H chain CDRs of a monoclonal antibody to the IL-6R.Since major portion of the reshaped human antibody is derived from a human antibody and the mouse CDRs which are less immunogenic, the present reshaped human antibody is less immunogenic to human, and therefor is promised for therapeutic uses.
Owner:CHUGAI PHARMA CO LTD
Reshaped human antibody to human interleukin-6 receptor
InactiveUS20050142635A1Less immunogenicPeptide/protein ingredientsHydrolasesComplementarity determining regionV region
A reshaped human antibody to the human IL-6R, comprising: (A) an L chain comprising, (1) a human L chain C region, and (2) an L chain V region comprising human L chain framework regions (FRs), and mouse L chain complementary determination regions (CDRs) of a momoclonal antibody to the IL-6 receptor (IL-6R); and (B) an H chain comprising, (1) a human H chain C region, and (2) an H chain V region comprising human H chain FRS, and mouse H chain CDRs of a monoclonal antibody to the IL-6R. Since major portion of the reshaped human antibody is derived from a human antibody and the mouse CDRs which are less immunogenic, the present reshaped human antibody is less immunogenic to human, and therefor is promised for therapeutic uses.
Owner:CHUGAI PHARMA CO LTD
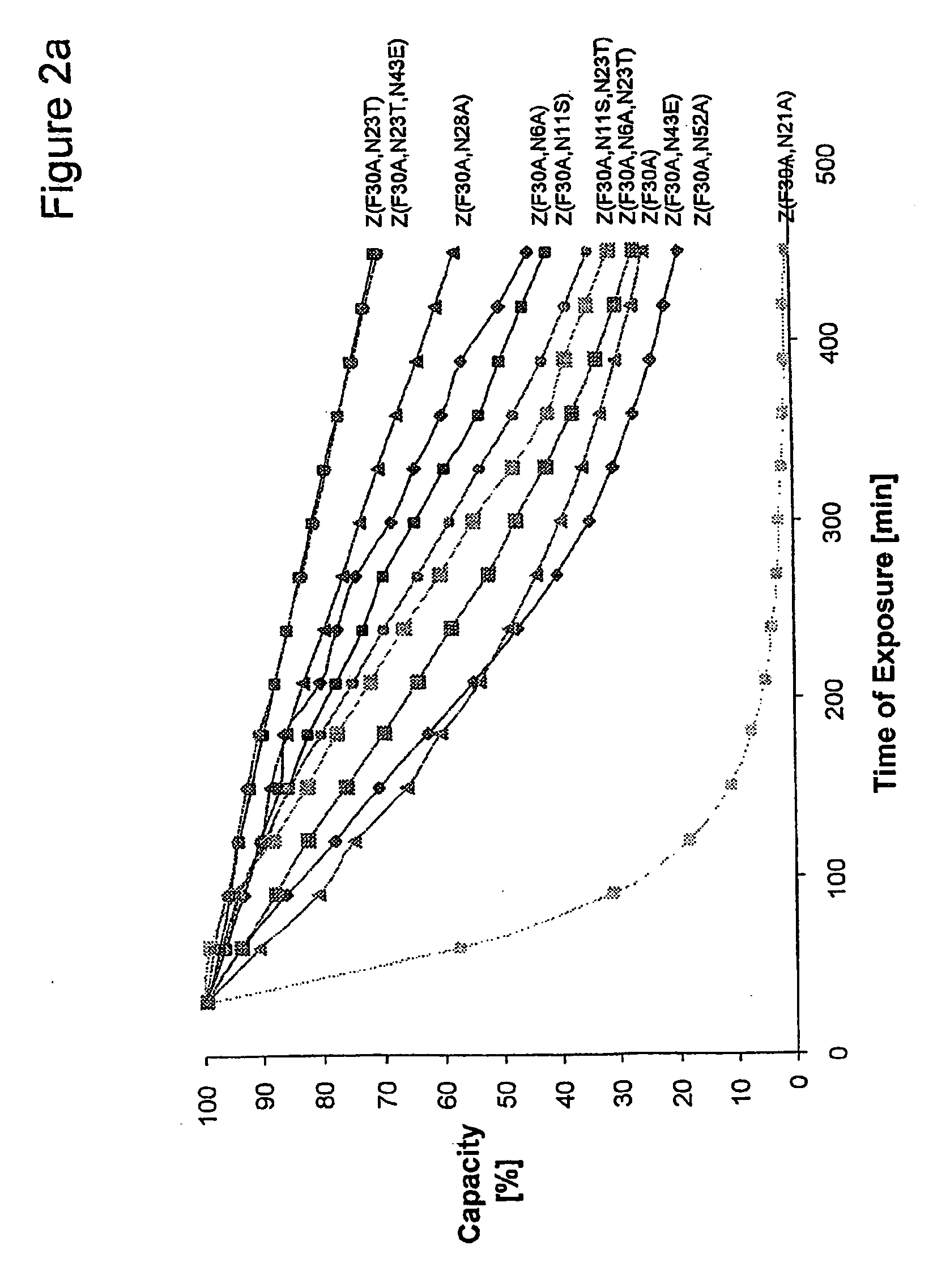
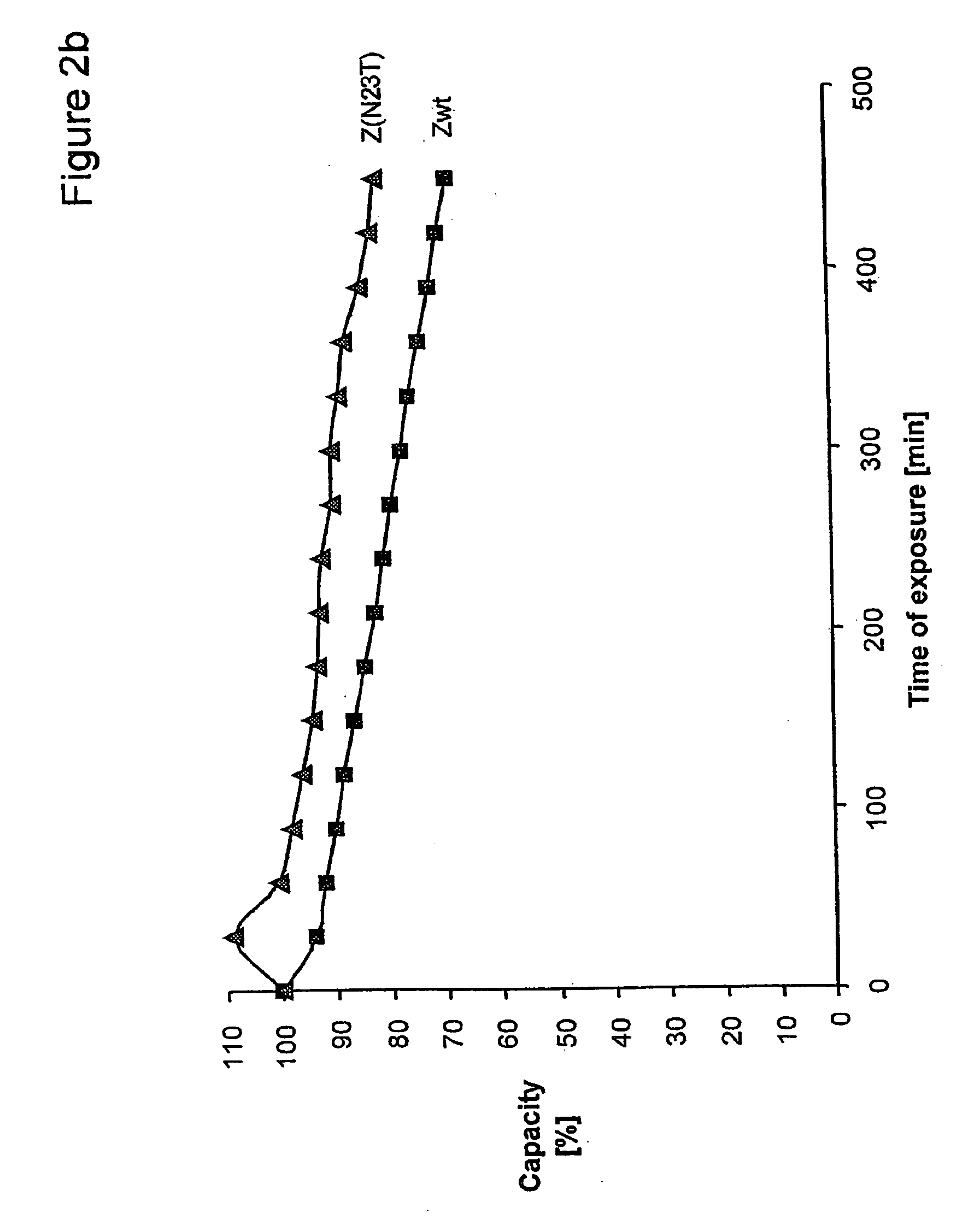
Method of Modifying Isoelectric Point of Antibody Via Amino Acid Substitution in CDR
ActiveUS20110076275A1Enhanced antigen-neutralizing activityImprove retentionSugar derivativesImmunoglobulins against cell receptors/antigens/surface-determinantsComplementarity determining regionAmino acid substitution
The present inventors provide methods for modifying the isoelectric point of an antibody while retaining its antigen-binding activity, comprising modifying the charge of at least one exposable amino acid residue on the surface of the complementarity determining region (CDR). The present invention also provides methods for purifying multispecific antibodies, comprising modifying isoelectric point, and methods for improving the plasma pharmacokinetics of antibodies, comprising modifying isoelectric point. The present invention further provides antibodies with a modified isoelectric point, pharmaceutical compositions comprising the antibodies as an active ingredient, and methods for producing the antibodies and compositions.
Owner:CHUGAI PHARMA CO LTD
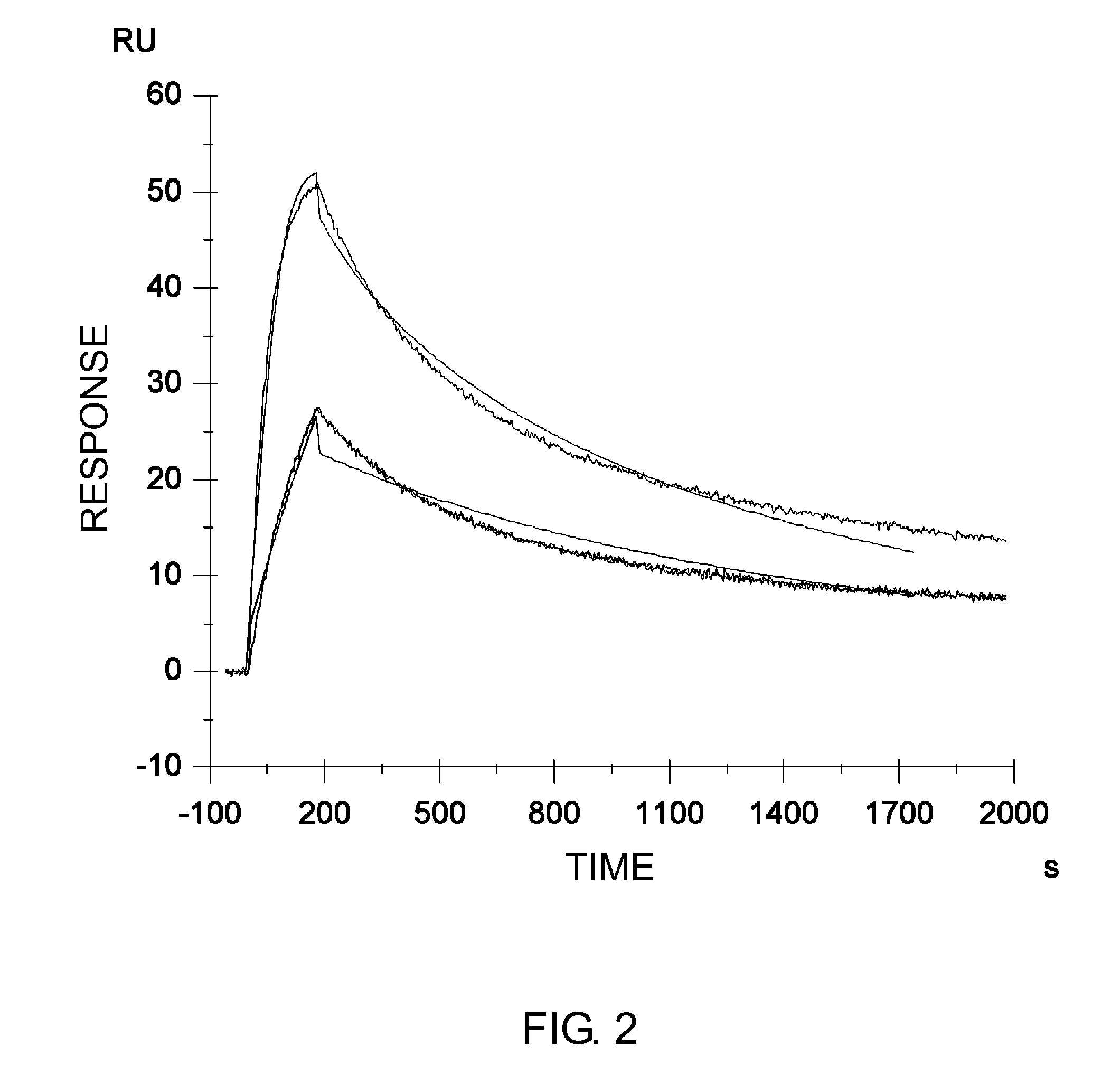
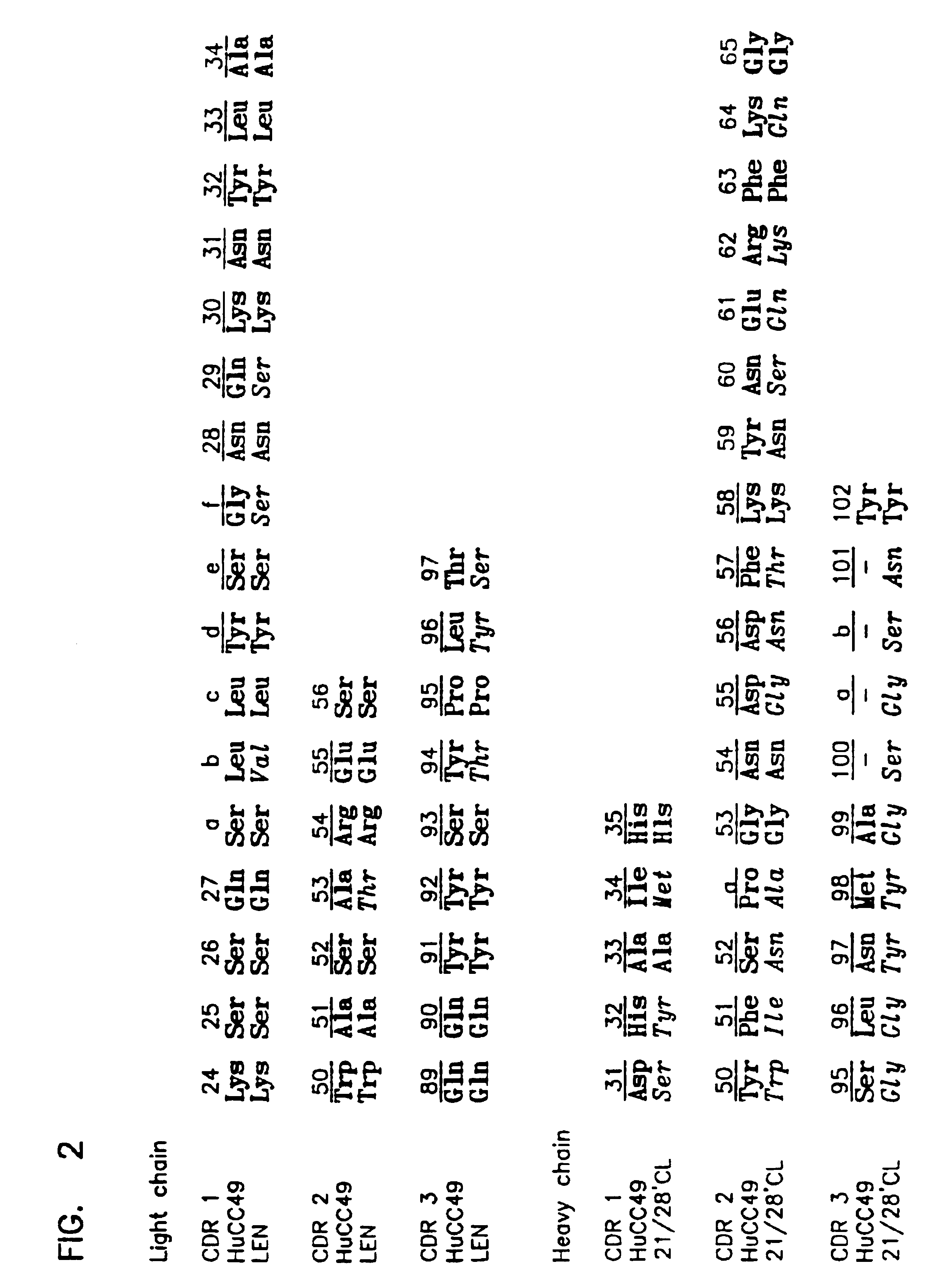
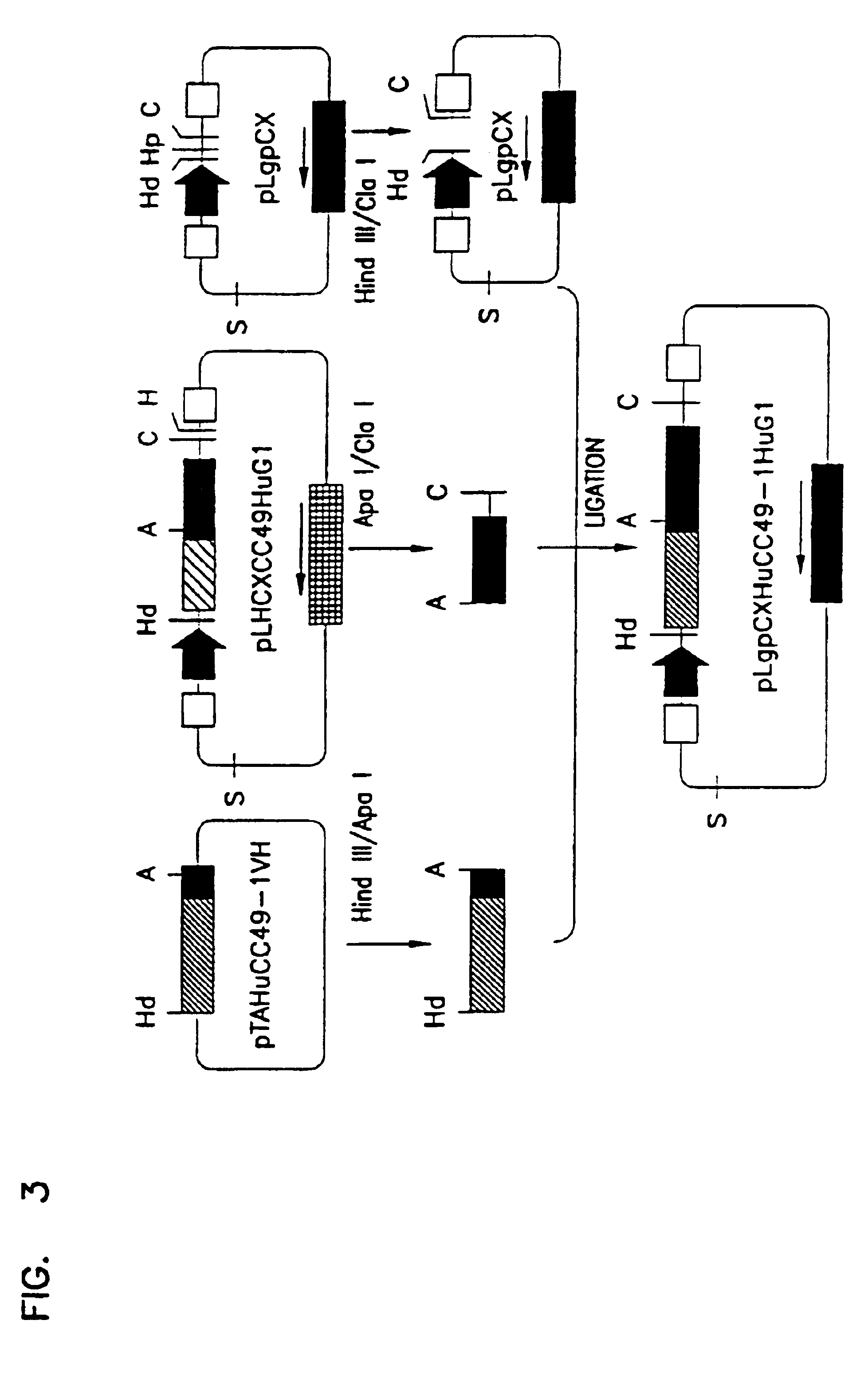
Variants of humanized anti carcinoma monoclonal antibody cc49
InactiveUS6818749B1Elicit adverse responseVirusesPeptide/protein ingredientsComplementarity determining regionHeavy chain
The invention is directed towards mouse-human chimeric variants of CC49 monoclonal antibodies with minimal murine content. A first aspect of the invention provides CDR variants of humanized monoclonal antibody (HuCC49) in which less than all six (three heavy chain and three light chain) Complementarity Determining Regions (CDRs) of CC49 are present. A second aspect of the invention provides SDR variants of humanized monoclonal antibody (HuCC49) in which only Specificity Determining Regions (SDRs) of at least one CDR from CC49 are present. The invention is also directed towards biotechnological methods of making the variants and therapeutic methods of using the variants.
Owner:UNITED STATES OF AMERICA
Mutant protein
InactiveUS20060194950A1Improve stabilityIncreased pH-valuesBacteriaSerum immunoglobulinsMutated proteinComplementarity determining region
Owner:GE HEALTHCARE BIO SCI CORP
Mutated immunoglobulin-binding protein
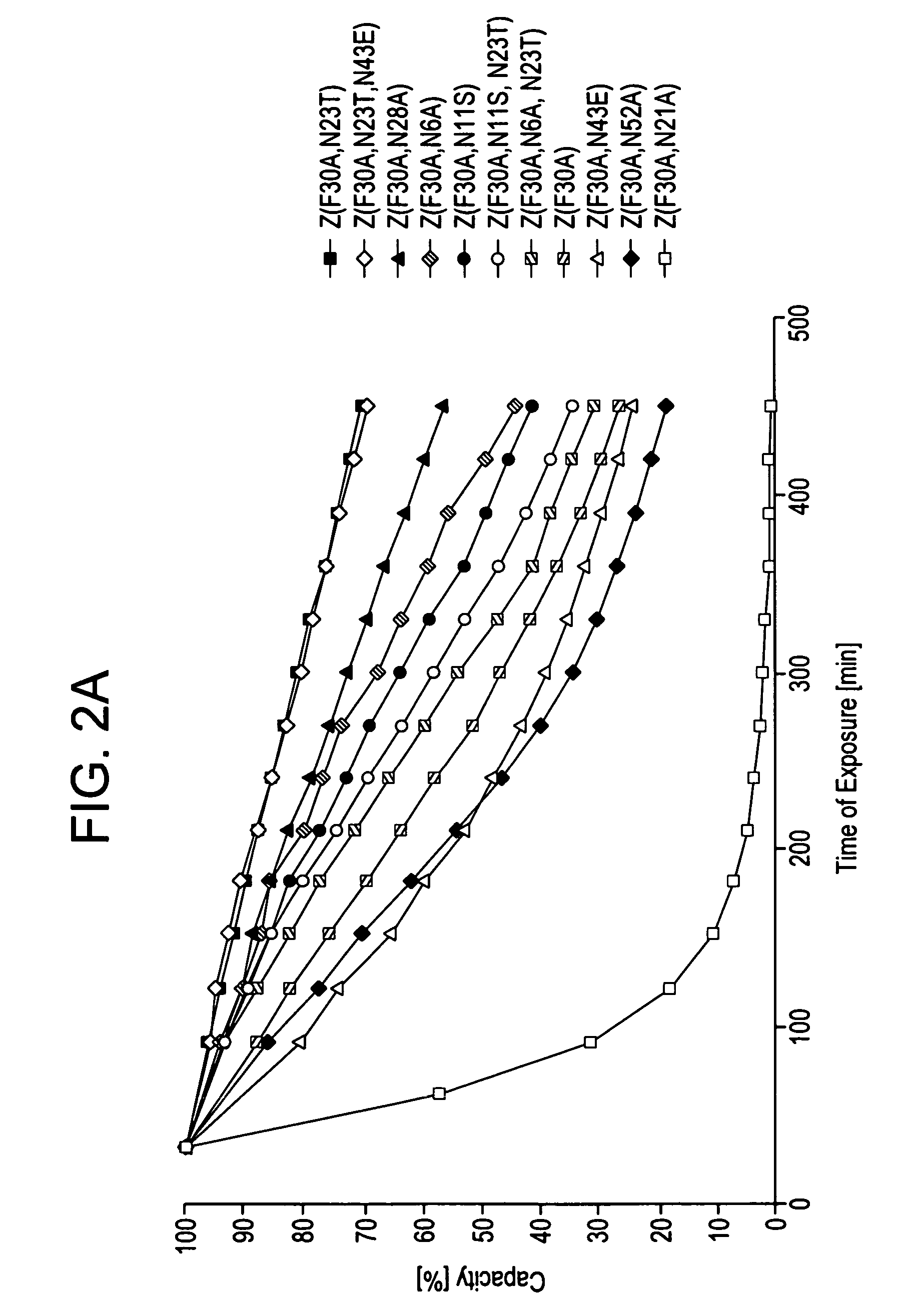
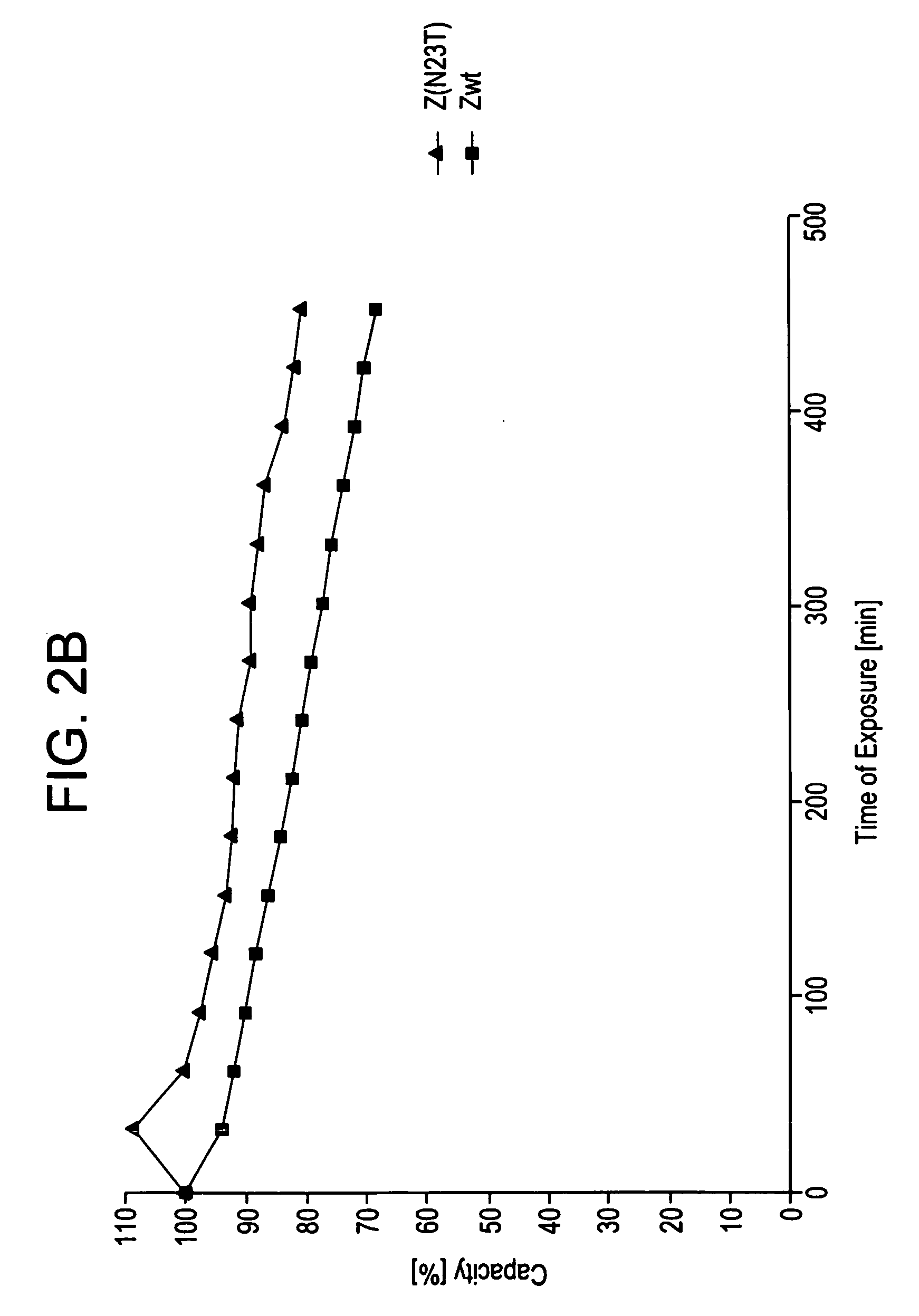
ActiveUS20050143566A1Improve stabilityIncreased pH-valuesSerum immunoglobulinsComponent separationComplementarity determining regionChemical stability
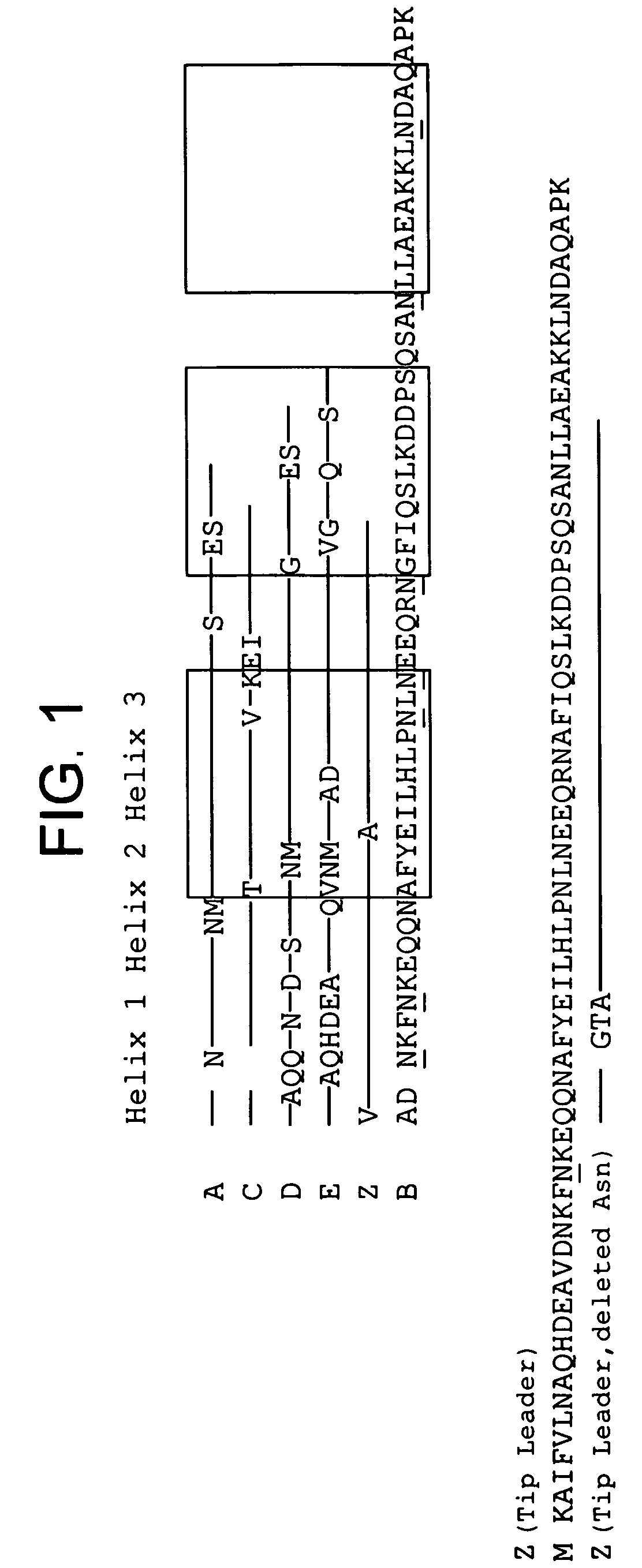
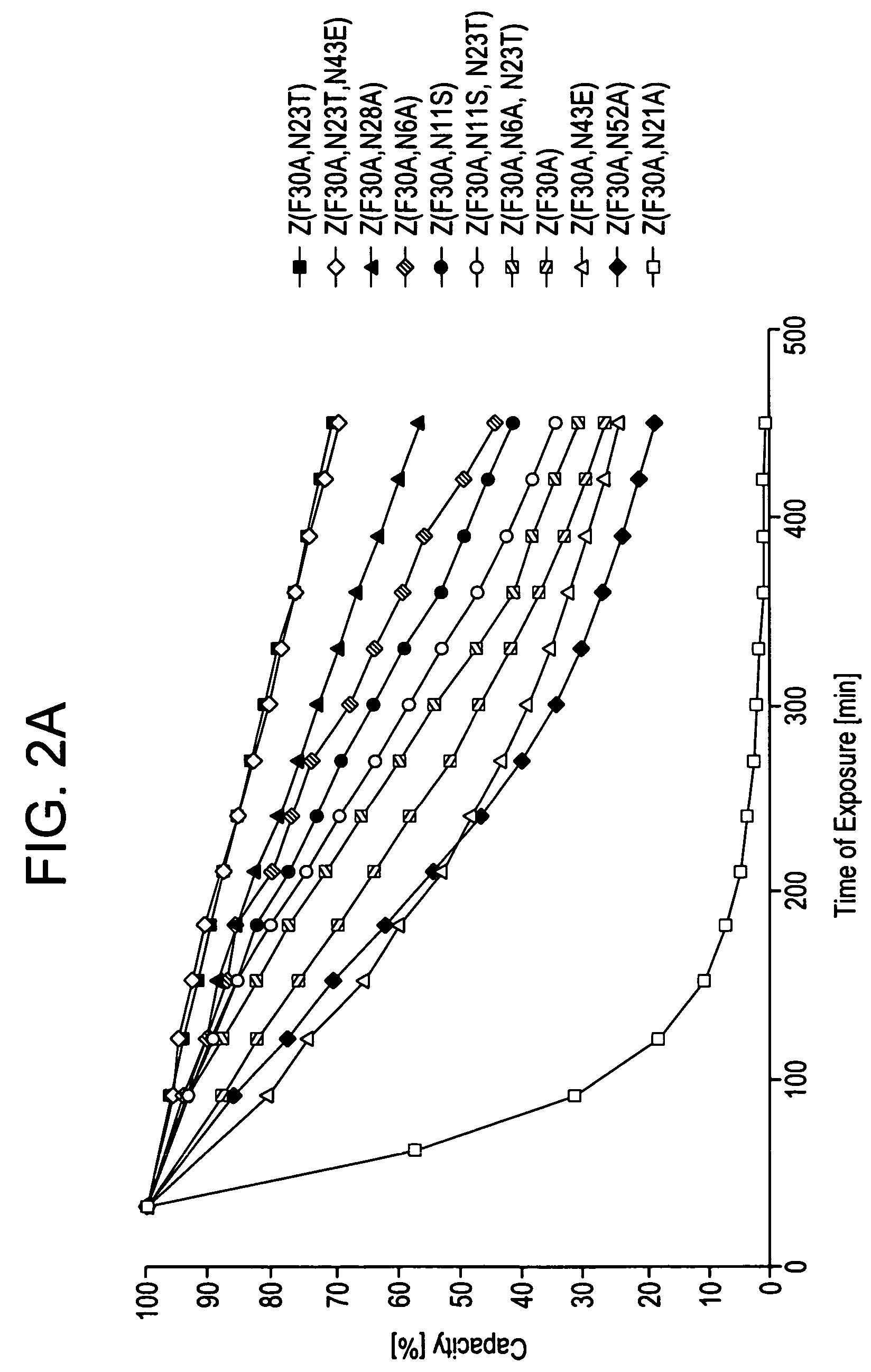
The present invention relates to an immunoglobulin-binding protein, wherein at least one asparagine residue has been mutated to an amino acid other than glutamine or aspartic acid, which mutation confers an increased chemical stability at pH-values of up to about 13-14 compared to the parental molecule. The protein can for example be derived from a protein capable of binding to other regions of the immunoglobulin molecule than the complementarity determining regions (CDR), such as protein A, and preferably the B-domain of Staphylococcal protein A. The invention also relates to a matrix for affinity separation, which comprises an immunoglobulin-binding protein as ligand coupled to a solid support, in which protein ligand at least one asparagine residue has been mutated to an amino acid other than glutamine.
Owner:CYTIVA BIOPROCESS R&D AB
Immunoconjugates and humanized antibodies specific for B-cell lymphoma and leukemia cells
InactiveUS20050106108A1Lowered HAMA reactionReduced responsePeptide/protein ingredientsAntibody mimetics/scaffoldsHuman antimouse AntibodyComplementarity determining region
A chimeric LL2 monoclonal antibody is described in which the complementarity determining regions (CDRs) of the light and heavy chains of the murine LL2 anti-B-lymphoma, anti-leukemia cell monoclona lantibody has been recombinantly joined to the human kappa and IgG1 constant region domains, respectively, which retains the immunospecificity and B-cell lymphoma and leukemia cell internalization capacity of the parental murine LL2 monoclonal antibody, and which has the potential of exhibiting reduced human anti-mouse antibody production activity. A humanized LL2 monoclonal antibody is described in which the CDRs of the light and heavy chains have been recombinantly joined to a framework sequence of human light and heavy chains variable regions, respectively, and subsequently linked to human kappa and IgG1 constant region domains, respectively, which retains the immunospecificity and B-lymphoma and leukemia cell internalization capacities of the parental murine and chimeric LL2 monoclonal antibodies, and which has the potential for exhibiting reduced human anti-mouse antibody production activity. Vectors for producing recombinant chimeric and humanized chimeric monoclonal antibodies are provided. Isolated DNAs encoding the amino acid sequences of the LL2 variable light and heavy chain and CDR framework regions are described. Conjugates of chimeric and humanized chimeric LL2 antibodies with cytotoxic agents or labels find use in therapy and diagnosis of B-cell lymphomas and leukemias.
Owner:IMMUNOMEDICS INC
Variable domain library and uses
ActiveUS7785903B2Generate efficientlyQuality improvementImmunoglobulins against growth factorsImmunoglobulins against cell receptors/antigens/surface-determinantsComplementarity determining regionAntigen binding
The invention provides polypeptides comprising a variant heavy chain variable framework domain (VFR). In some embodiments, the amino acids defining the VFR form a loop of an antigen binding pocket. In an embodiment, the polypeptide is a variable domain of a monobody and has a variant VFR. The polypeptide may optionally comprise one or more complementary determining regions (CDRs) of antibody variable domains. In an embodiment, the polypeptide is a variable domain of a monobody and has a variant VFR and one or more variant CDRs. Libraries of polypeptides that include a plurality of different antibody variable domains generated by creating diversity in a VFR, and optionally, one or more CDRs are provided and may be used as a source for identifying novel antigen binding polypeptides that can be used therapeutically or as reagents. The invention also provides fusion polypeptides, compositions, and methods for generating and using the polypeptides and libraries.
Owner:GENENTECH INC
Humanized immunoglobulin reactive with α4β7 integrin
InactiveUS7147851B1Reduce inflammationLess immunogenicFungiBacteriaComplementarity determining regionAntigen binding
The present invention relates to humanized immunoglobulins having binding specificity for α4β7 integrin, comprising an antigen binding region of nonhuman origin (e.g., rodent) and at least a portion of an immunoglobulin of human origin (e.g., a human framework region, a human constant region). In one embodiment, the humanized immunoglobulin can compete with murine Act-1 for binding to human α4β7 integrin. In a preferred embodiment, the antigen binding region of the humanized immunoglobulin comprises each of the complementarity determining regions of the light and heavy chains of the murine Act-1 antibody.
Owner:MILLENNIUM PHARMA INC
Protein ligands
ActiveUS20060194955A1Retention characteristicReduce leakageImmunoglobulins against animals/humansBiological testingComplementarity determining regionStaphylococcus
The present invention relates to the use of an alkali-stable protein, wherein at least one asparagine residue has been mutated to an amino acid other than glutamine or aspartic acid, which mutation confers an increased chemical stability at pH-values of up to about 13-14 compared to the parental molecule. The protein can for example be derived from a protein capable of binding to other regions of the immunoglobulin molecule than the complementarity determining regions (CDR), such as protein A, and preferably the B-domain of Staphylococcal protein A. The invention also relates to a matrix for affinity separation, which comprises an immunoglobulin-binding protein as ligand coupled to a solid support, in which protein ligand at least one asparagine residue has been mutated to an amino acid other than glutamine.
Owner:CYTIVA BIOPROCESS R&D AB
Anti-properdin antibodies, and methods for making and using same
The present invention is related to antibodies directed to the antigen properdin and uses of such antibodies. In particular, in accordance with the present invention, there are provided fully human monoclonal antibodies directed to the antigen properdin. Nucleotide sequences encoding, and polypeptides comprising, heavy and light chain immunoglobulin molecules, particularly sequences corresponding to contiguous heavy and light chain sequences spanning the framework regions and / or complementarity determining regions (CDR's), specifically from FR1 through FR4 or CDR1 through CDR3, are provided. Hybridomas or other cell lines expressing such immunoglobulin molecules and monoclonal antibodies are also provided.
Owner:AMGEN FREMONT INC
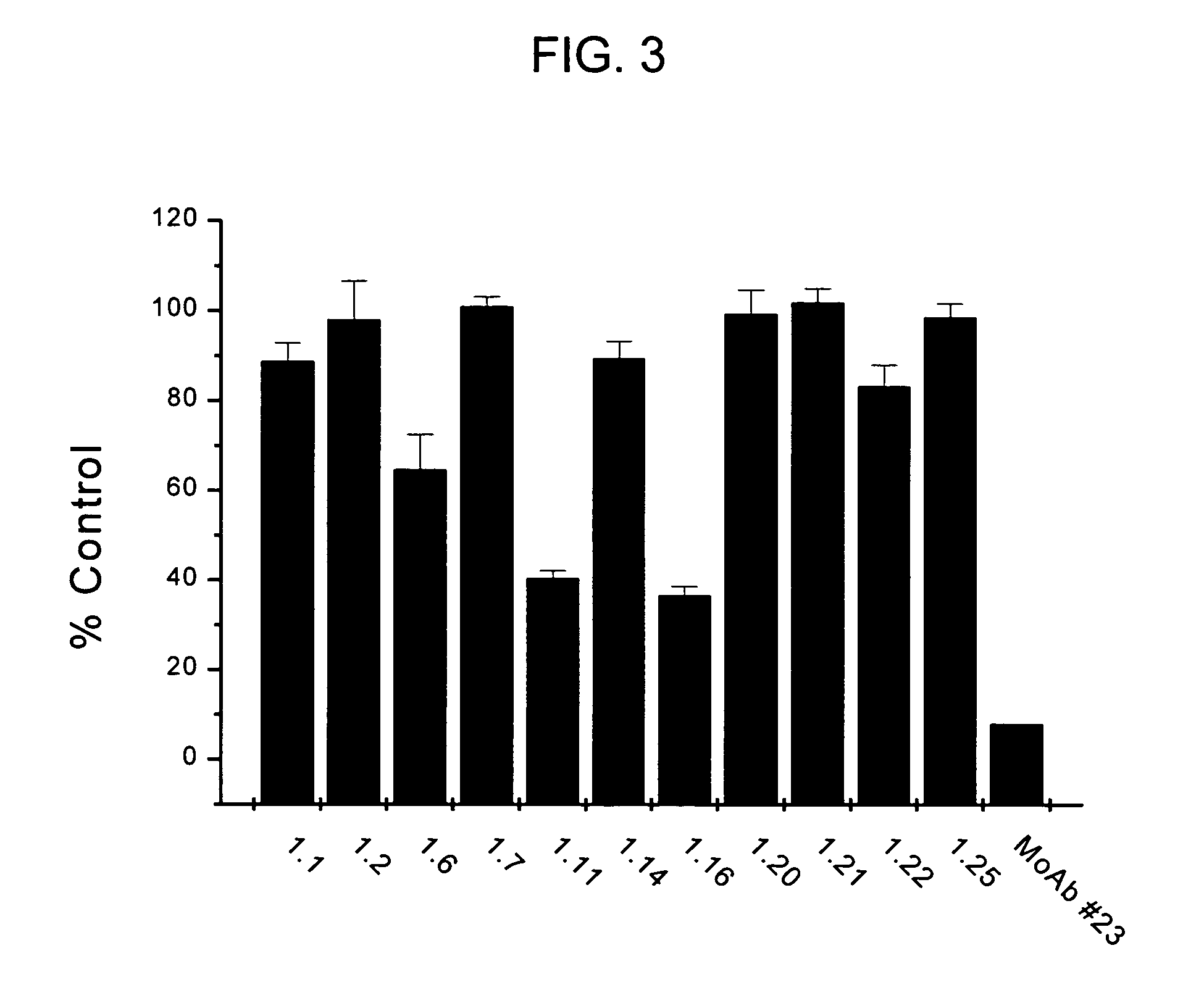
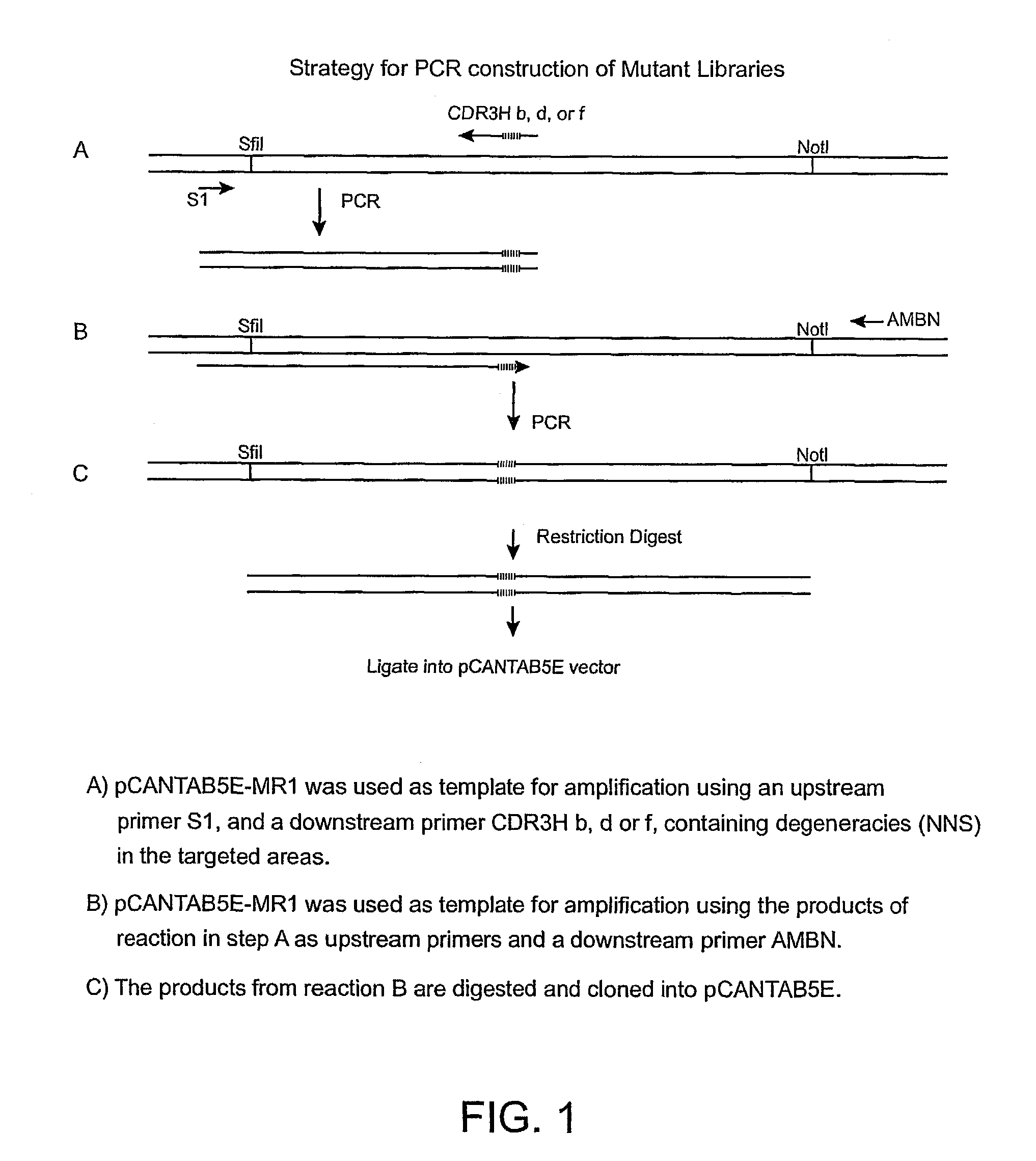
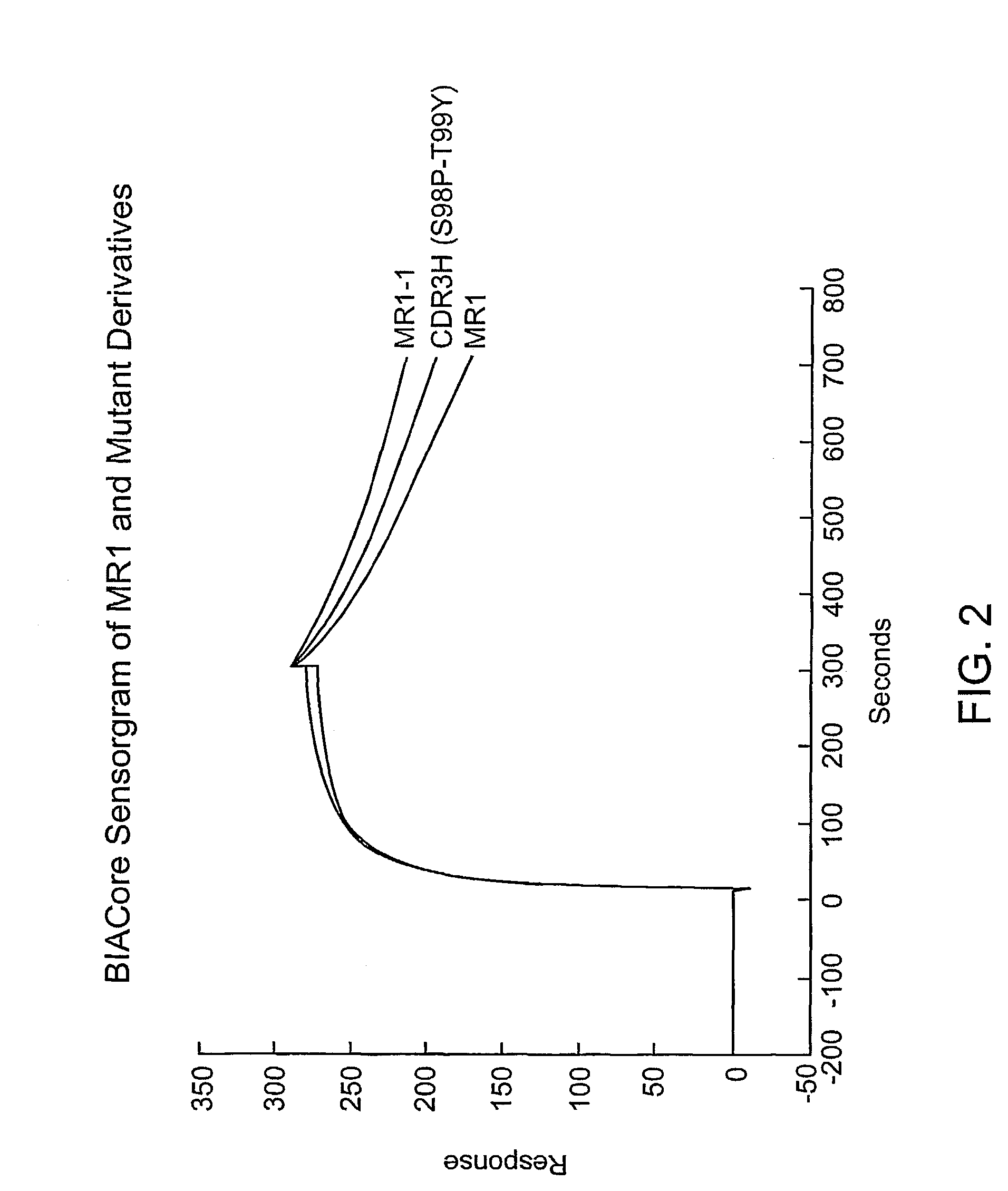
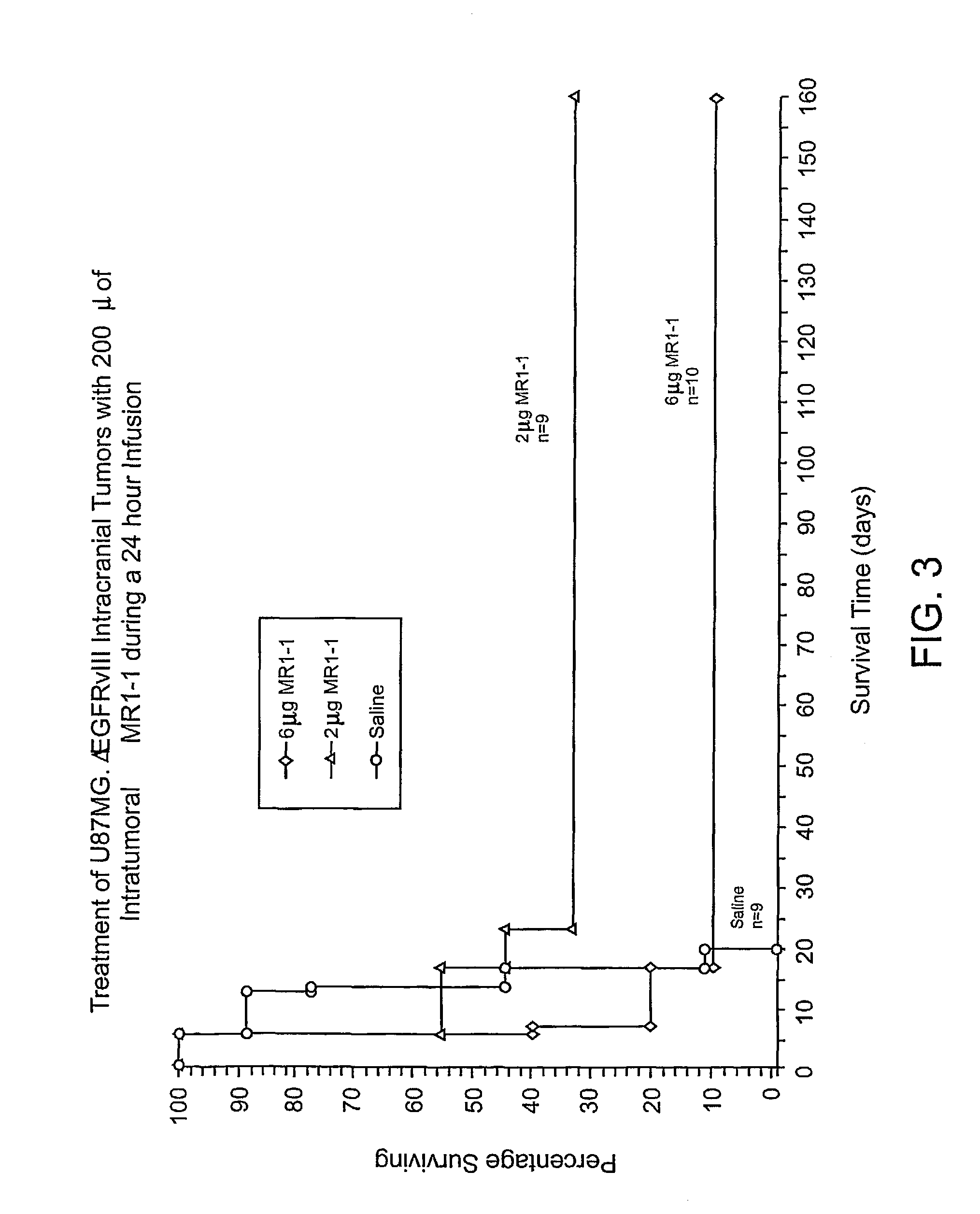
Anti-EGFRvIII scFvs with improved cytotoxicity and yield, immunotoxins based thereon, and methods of use thereof
InactiveUS7129332B2Improve bindingPeptide/protein ingredientsAntibody mimetics/scaffoldsComplementarity determining regionCytotoxicity
The invention provides antibodies to a mutant form of the epidermal growth factor receptor known as EGFRvIII found only or primarily on the surface of glioblastoma cells, and on cells of breast, ovarian and non-small cell lung carcinomas. The antibodies provided by the invention have the complementarity determining regions (“CDRs”) of the scFv designated MR1, but with mutations at positions 98 and 99 in the CDR3 of the heavy chain variable region and, optionally, in other CDRs. In particular, the invention provides an antibody, designated MR1-1, which mutates MR1 in the CDR3 of the VH and VL chains. The invention provides additional antibodies in which MR1 is mutated in the CDR1 and 2 of VH or VL, or both.
Owner:UNITED STATES OF AMERICA +2
Method of modifying isoelectric point of antibody via amino acid substitution in CDR
ActiveUS9096651B2Enhanced antigen-neutralizing activityGood treatment effectImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsComplementarity determining regionAmino acid substitution
Methods are described for modifying the isoelectric point of an antibody while retaining its antigen-binding activity, comprising modifying the charge of at least one exposable amino acid residue on the surface of the complementarity determining region (CDR). The disclosure also provides methods for purifying multispecific antibodies, comprising modifying isoelectric point, and methods for improving the plasma pharmacokinetics of antibodies with a modified isoelectric point. The disclosure further provides antibodies with a modified isoelectric point, pharmaceutical compositions comprising the antibodies as an active ingredient, and methods for producing the antibodies and compositions.
Owner:CHUGAI PHARMA CO LTD
Anti-NKG2A antibodies and uses thereof
ActiveUS8206709B2Improved propertyLow immunogenicityHybrid immunoglobulinsAntipyreticComplementarity determining regionAmino acid
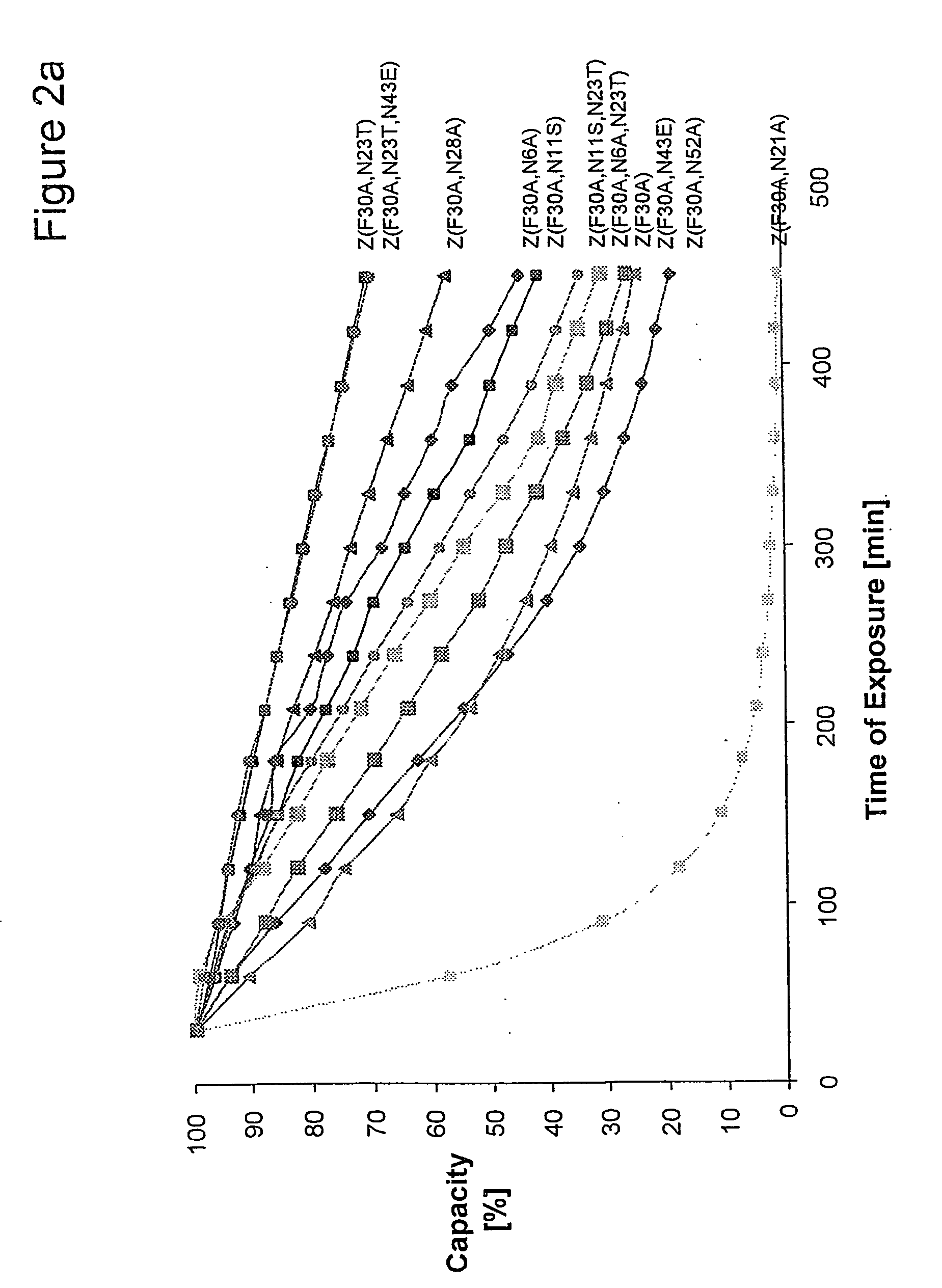
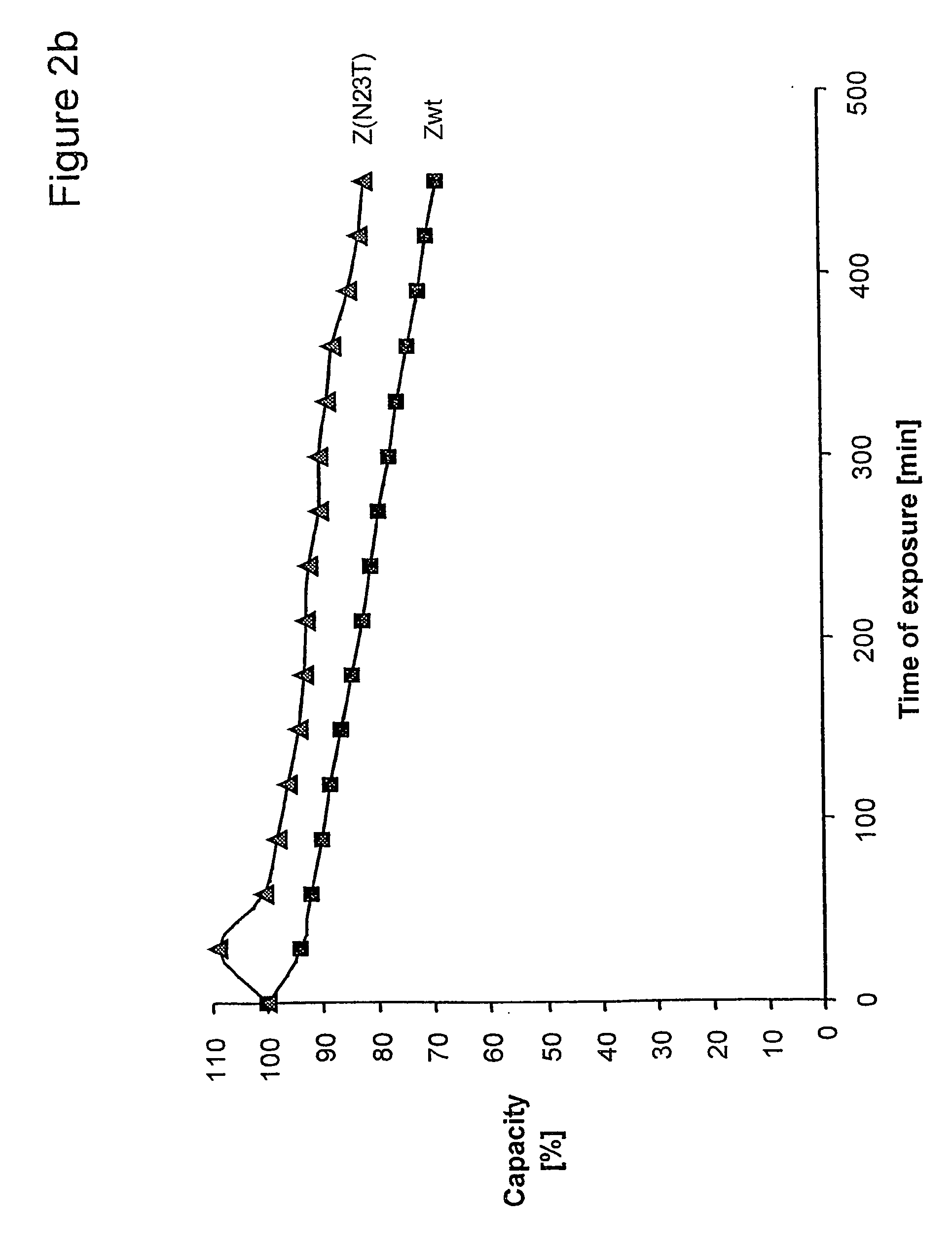
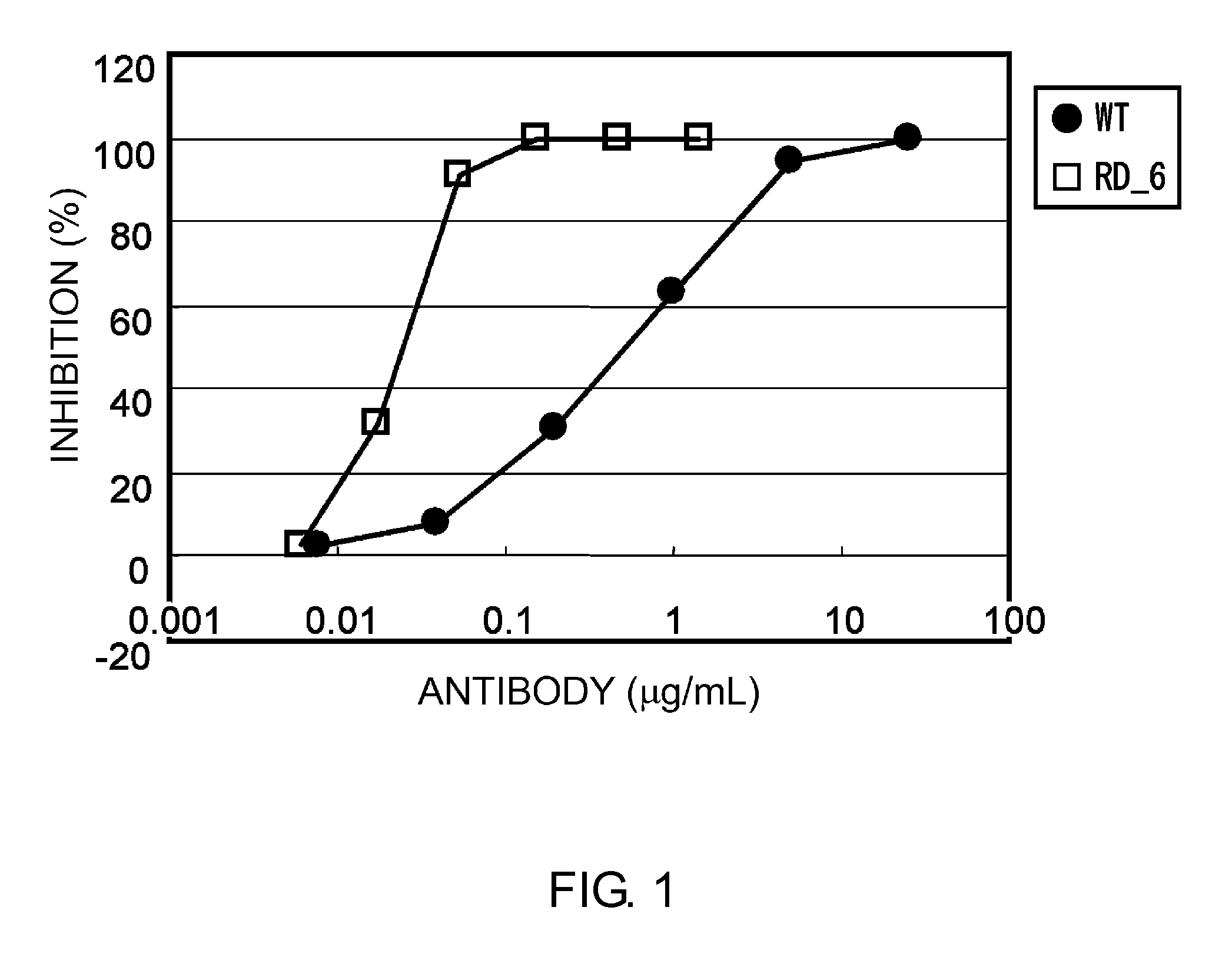
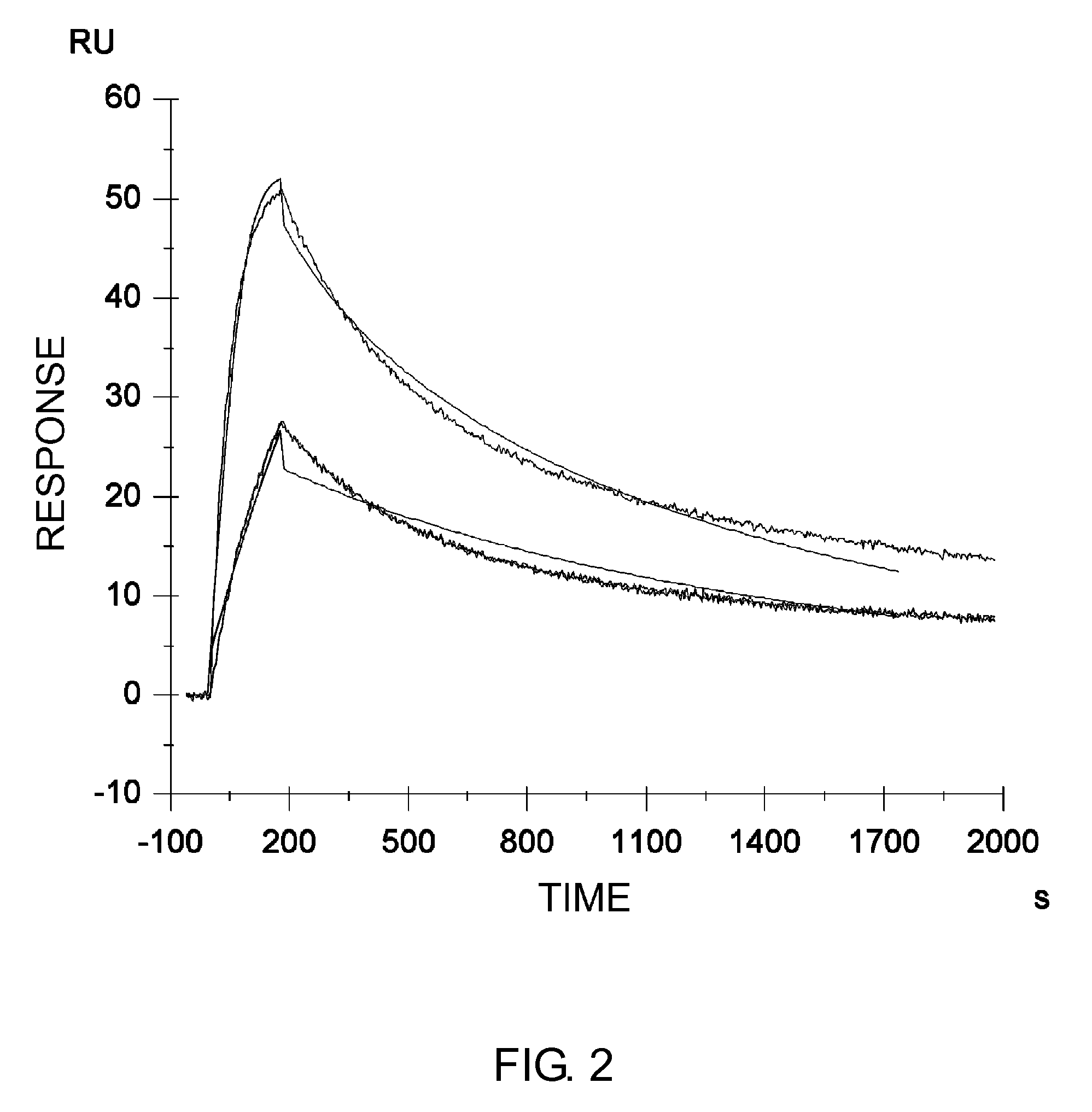
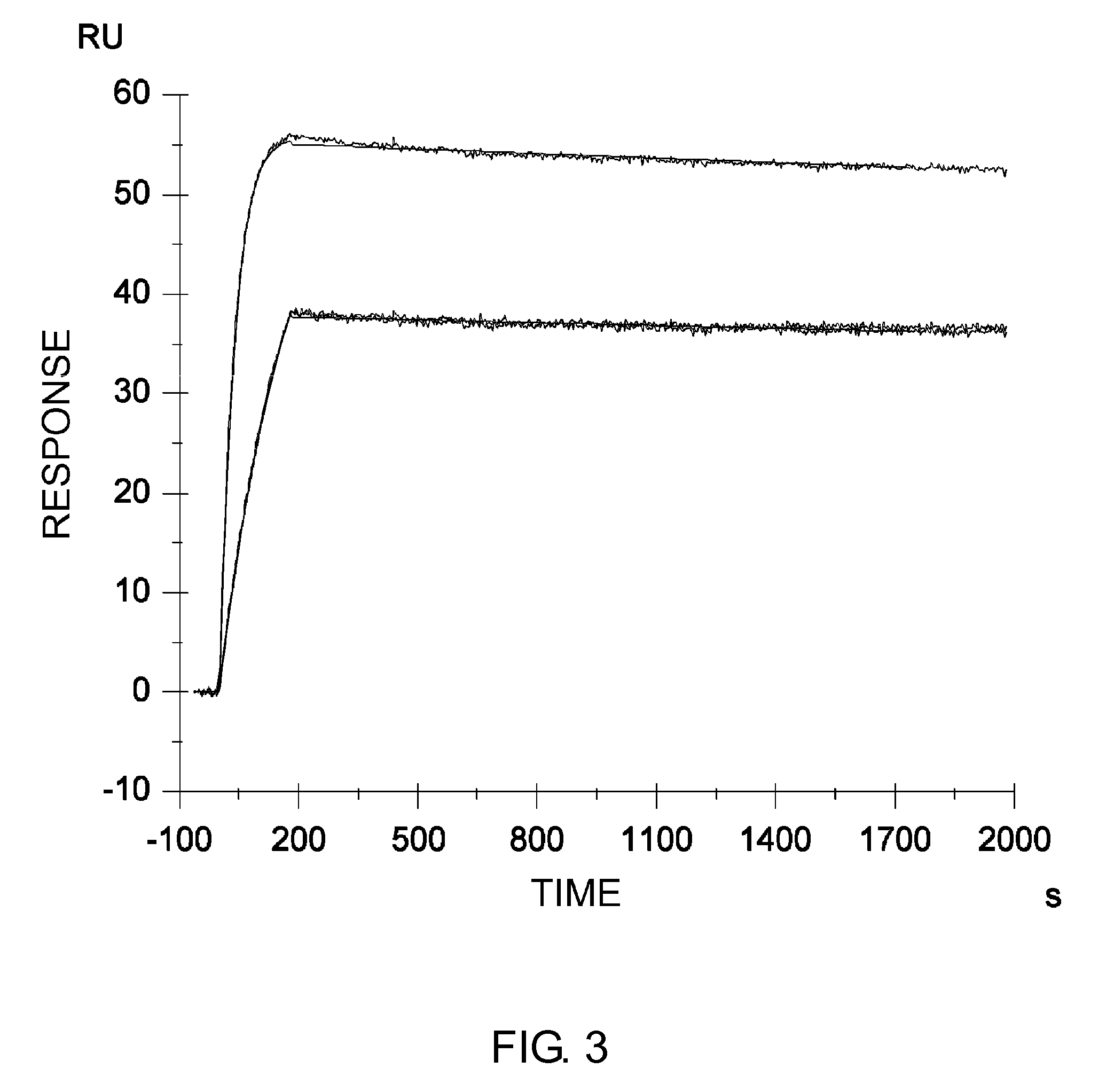
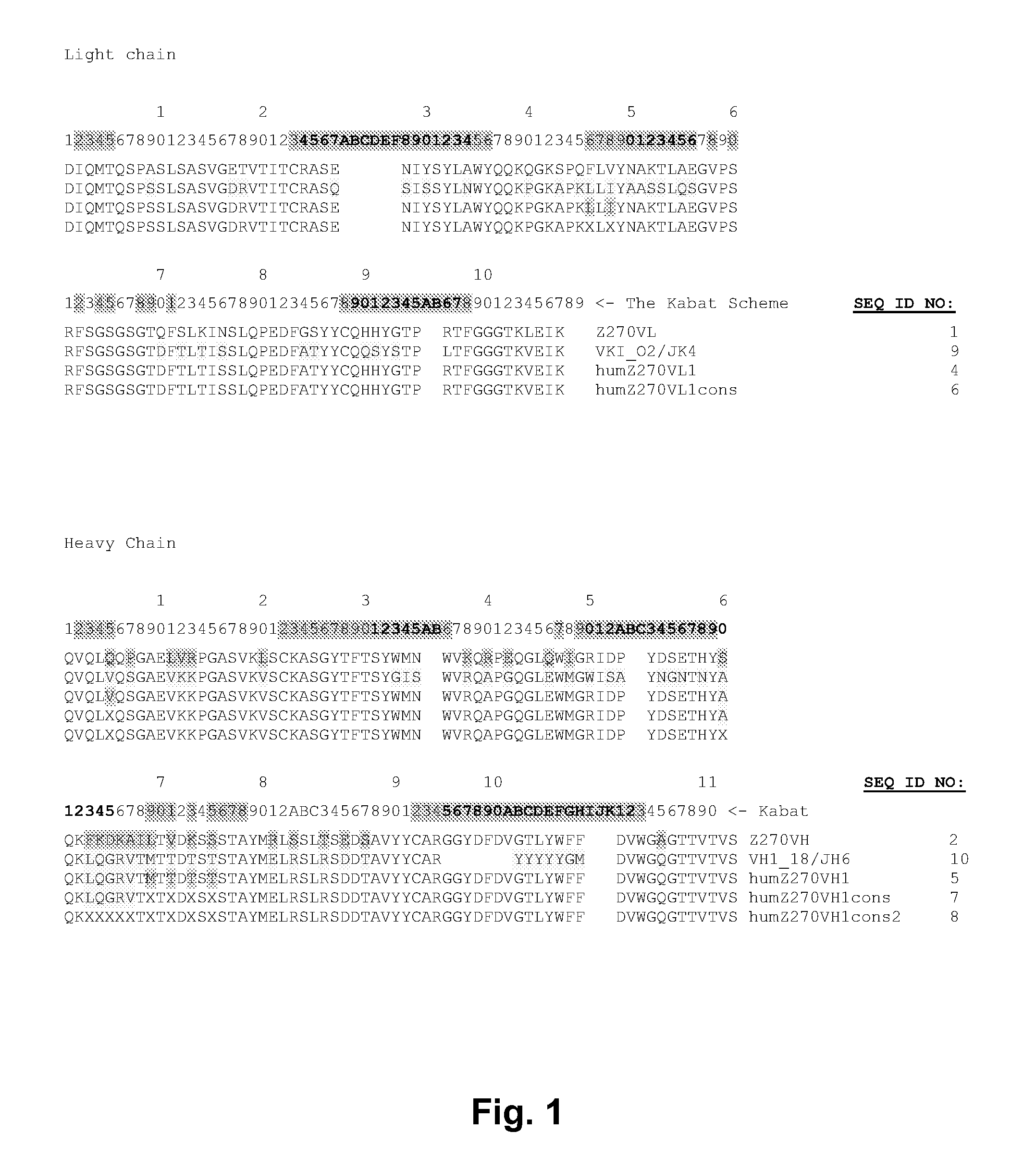
Described herein are anti-NKG2A antibodies suitable for human therapy, including humanized versions of murine anti-NKG2A antibody Z270, as well as related methods and materials for producing and using such antibodies. Exemplary complementarity-determining regions (CDRs) sequences and sites for optional amino acid back-substitutions in framework region (FR) and / or CDRs of such antibodies are also described.
Owner:NOVO NORDISK AS
Anti-properdin antibodies, and methods for making and using same
InactiveUS20060093599A1Antibacterial agentsNervous disorderComplementarity determining regionHeavy chain
The present invention is related to antibodies directed to the antigen properdin and uses of such antibodies. In particular, in accordance with the present invention, there are provided fully human monoclonal antibodies directed to the antigen properdin. Nucleotide sequences encoding, and polypeptides comprising, heavy and light chain immunoglobulin molecules, particularly sequences corresponding to contiguous heavy and light chain sequences spanning the framework regions and / or complementarity determining regions (CDR's), specifically from FR1 through FR4 or CDR1 through CDR3, are provided. Hybridomas or other cell lines expressing such immunoglobulin molecules and monoclonal antibodies are also provided.
Owner:AMGEN FREMONT INC
Humanized immunoglobulin reactive with alpha4beta7 integrin
The present invention relates to a humanized immunoglobulin that has binding specificity for α4β7 integrin and comprises the complementarity determining regions (CDRs) of mouse Act-1 antibody, and to the humanized light chain of the humanized immunoglobulin. The present invention further relates to a humanized immunoglobulin light chain. The invention also relates to isolated nucleic acids, recombinant vectors and host cells that comprise a sequence which encodes a humanized immunoglobulin or immunoglobulin light chain, and to a method of preparing a humanized immunoglobulin. The humanized immunoglobulins can be used in therapeutic applications, for example to control lymphocyte infiltration to mucosal tissue or to treat inflammatory bowel disease.
Owner:MILLENNIUM PHARMA INC
Anti human ovarian cancer-anti CD3 bispecific antibody
InactiveUS7262276B2Low toxicityHigh efficiency and cost-effectivenessImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsComplementarity determining regionAntiendomysial antibodies
The present invention provides an anti-ovarian cancer bispecific antibody. Said antibody includes two polypeptide domains connected by a polypeptide linker, one is anti-ovarian cancer antibody, or its Fab fragment, single complementarity determining region (CDR) antibody or single chain Fv (scFv) and the other is anti-CD3 antibody, or its Fab fragment, single CDR antibody or scFv. The present invention also provides DNA sequences encoding said antibody, an expression vector containing said DNA sequence, and a host cell containing said expression vector.
Owner:DONGGUAN HAOFA BIOTECH DEVAL +2
Complementarity determining region-grafted antibody against ganglioside GD3 and derivative of antibody against ganglioside GD3
InactiveUS7253263B1Promote absorptionHigh activityAnimal cellsSugar derivativesComplementarity determining regionGanglioside GD3
Owner:KYOWA HAKKO KIRIN CO LTD
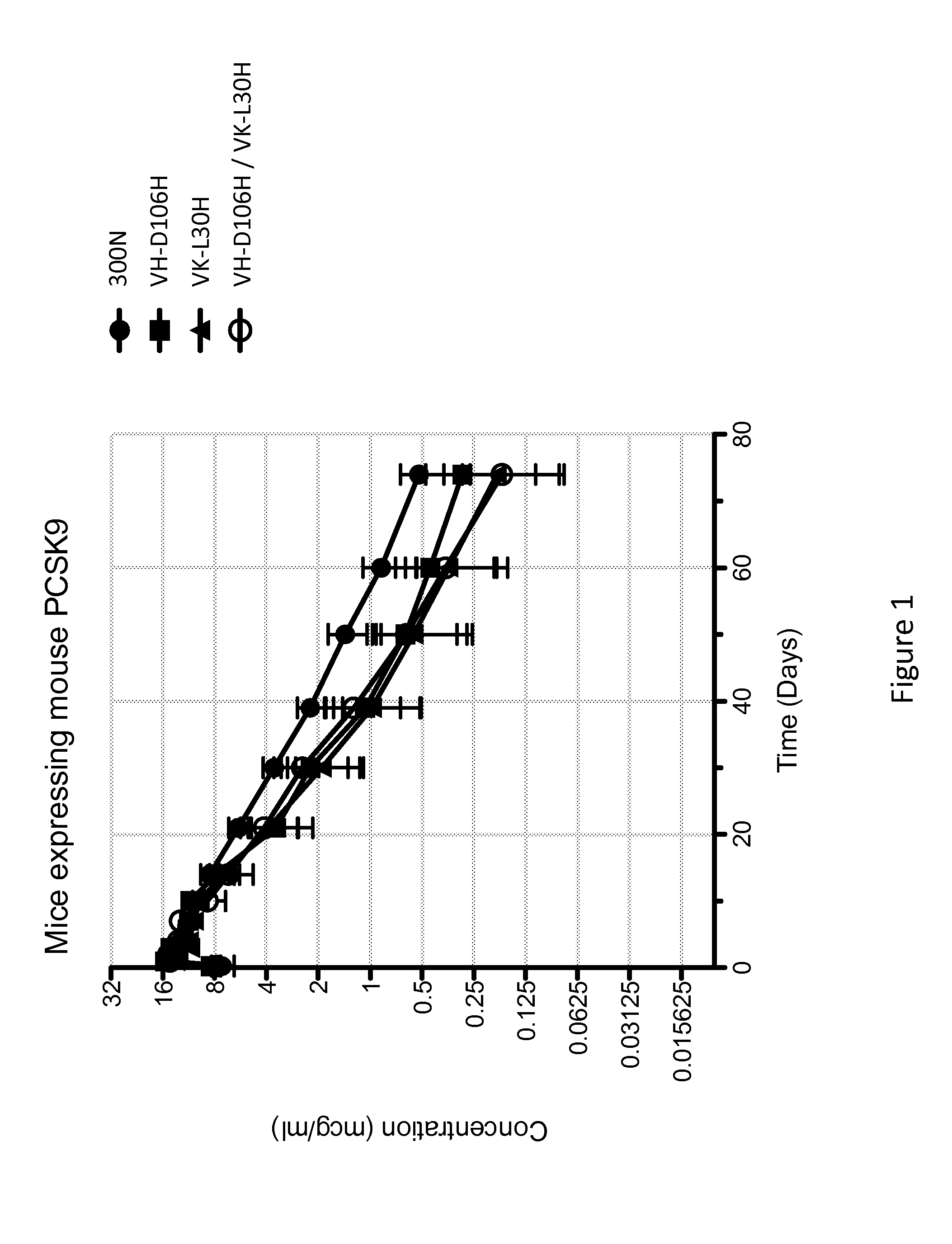
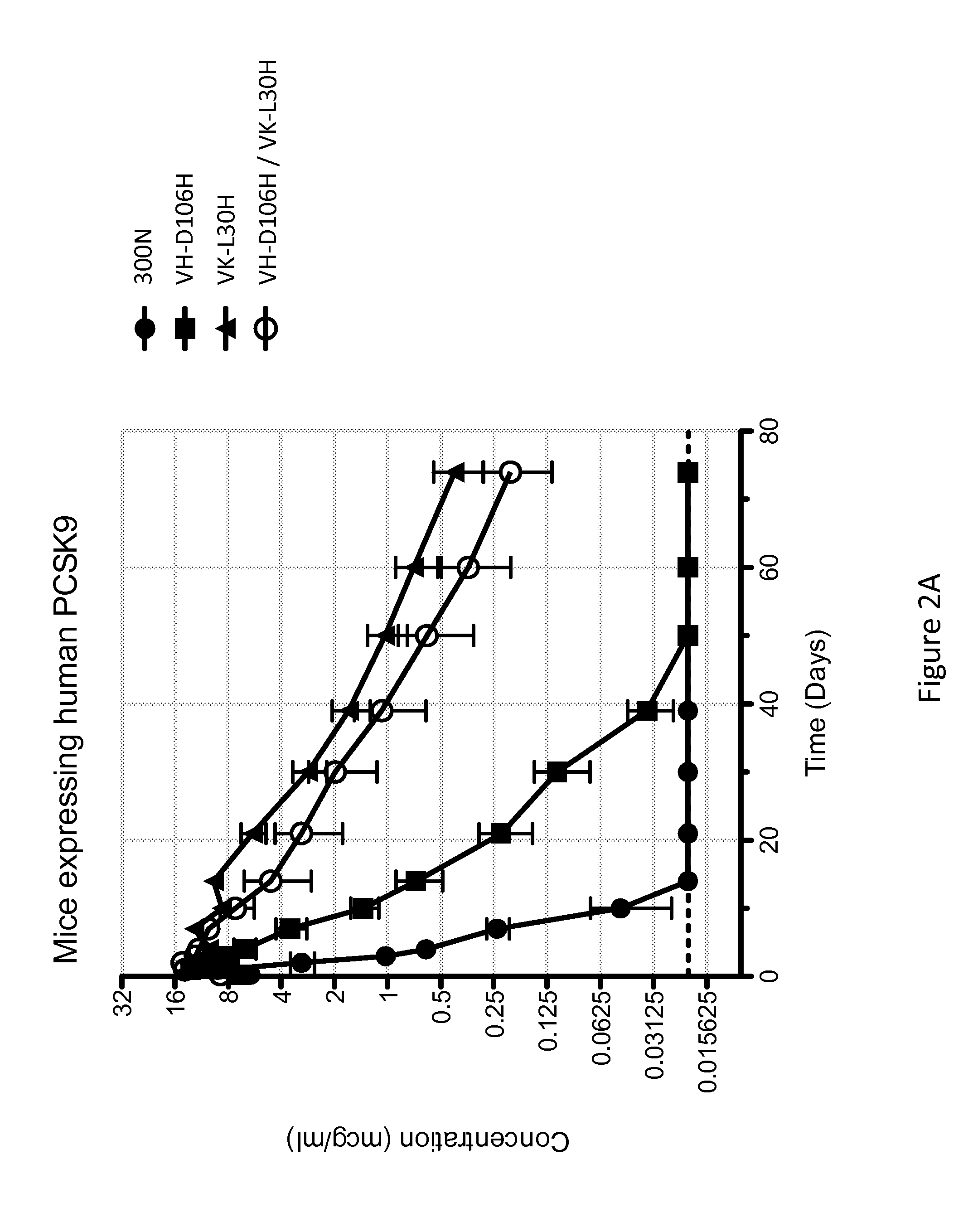
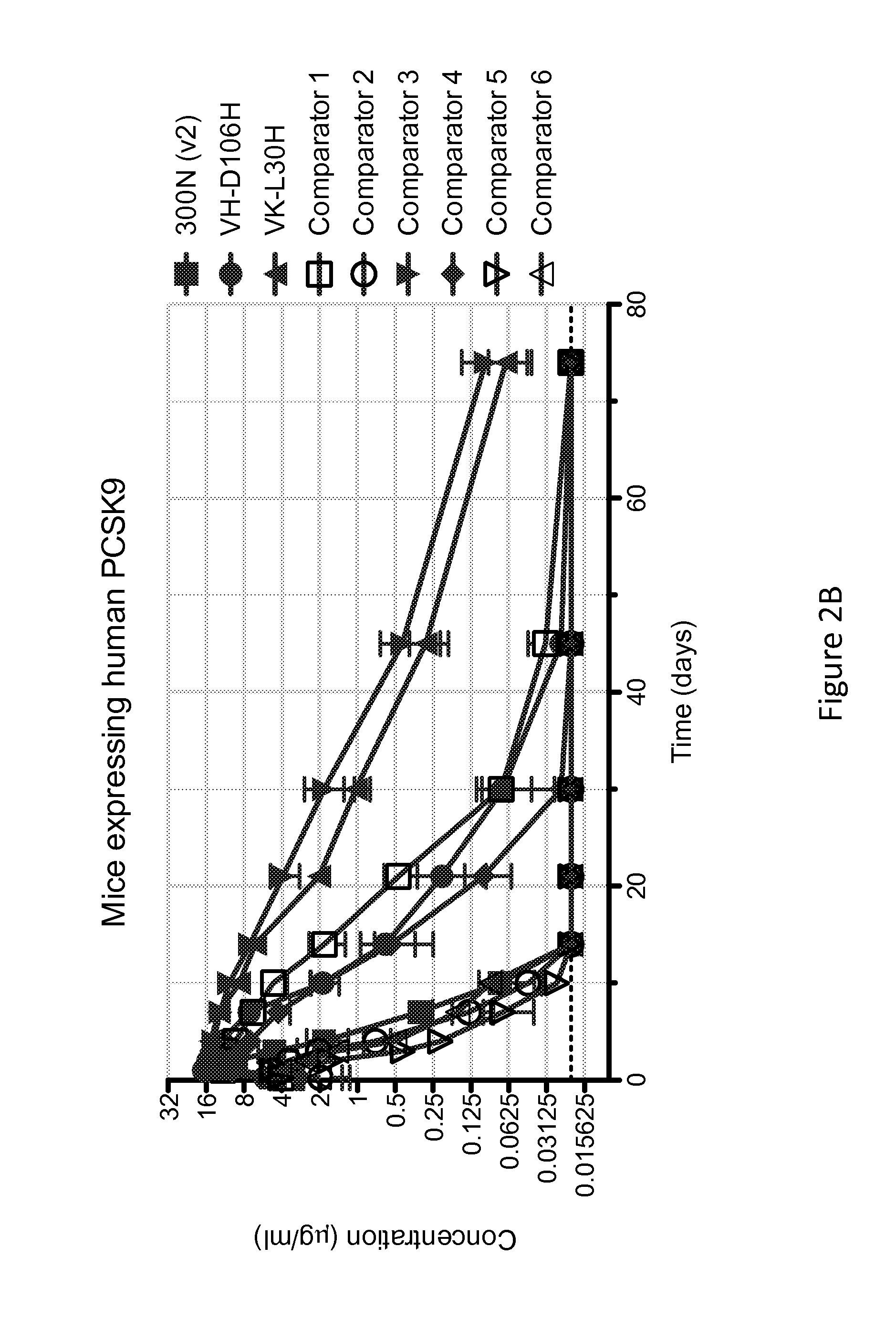
ANTI-PCSK9 ANTIBODIES WITH pH-DEPENDENT BINDING CHARACTERISTICS
ActiveUS20140044730A1High affinityReduced binding affinityMetabolism disorderAntibody ingredientsDiseaseKexin
The present invention provides antibodies and antigen-binding fragments thereof that specifically bind proprotein convertase subtilisin / kexin-9 (PCSK9) with greater affinity at neutral pH than at acidic pH. The antibodies of the invention may possess one or more amino acid changes as compared to antibodies that do not exhibit pH-dependent binding properties. For example, the present invention includes anti-PCSK9 antibodies which possess one or more histidine substitutions in one or more complementarity determining regions. The antibodies of the invention, with pH-dependent binding properties, remain in circulation and exhibit cholesterol lowering activity for prolonged periods of time in animal subjects as compared to anti-PCSK9 antibodies that do not exhibit pH-dependent binding properties. The antibodies of the invention are therefore useful for treating diseases and disorders related to elevated HDL cholesterol, wherein the antibodies of the invention can be administered to a patient at a lower dose and / or with less frequent dosing as compared to antibodies that do not exhibit pH-dependent binding properties.
Owner:REGENERON PHARM INC
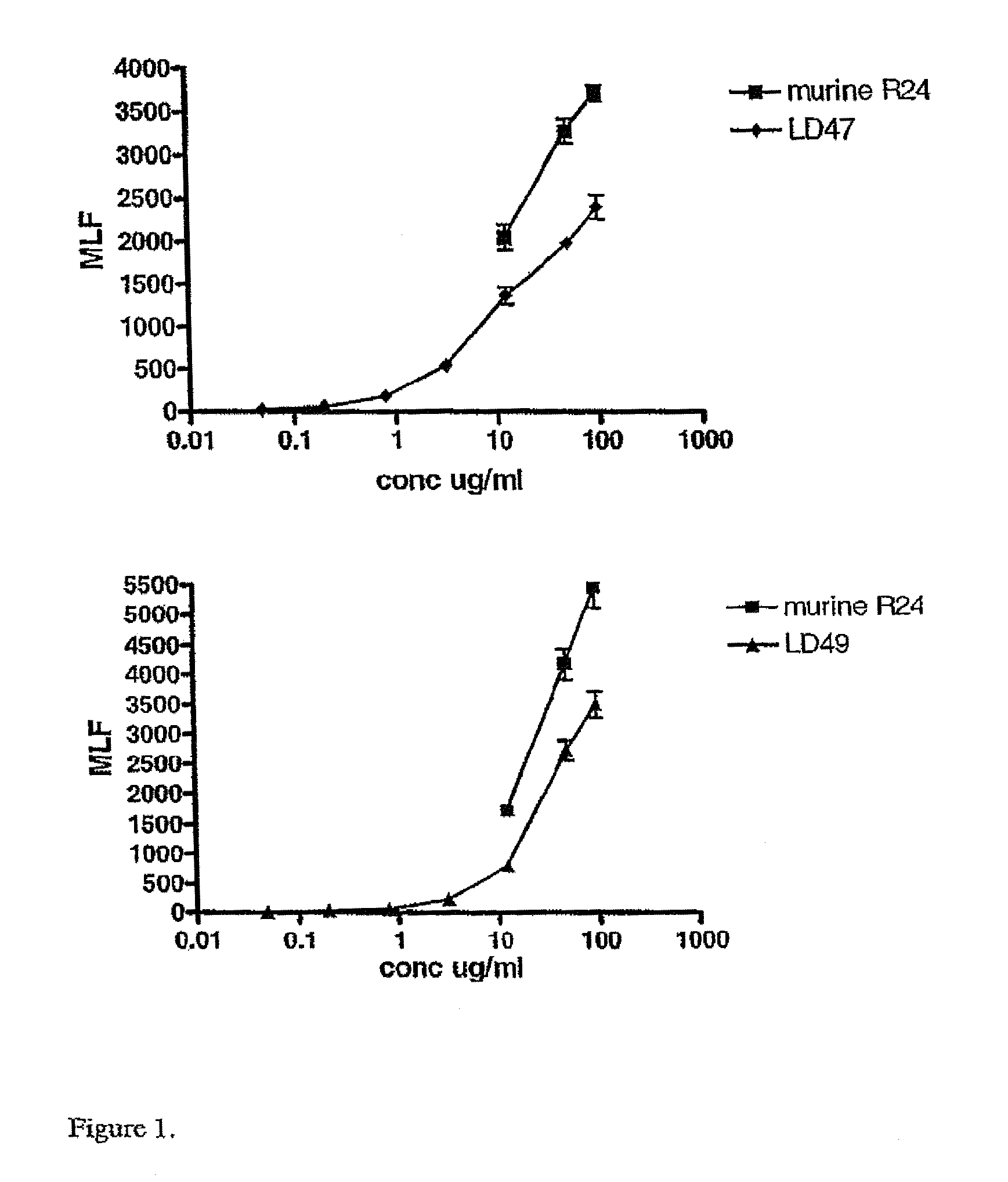
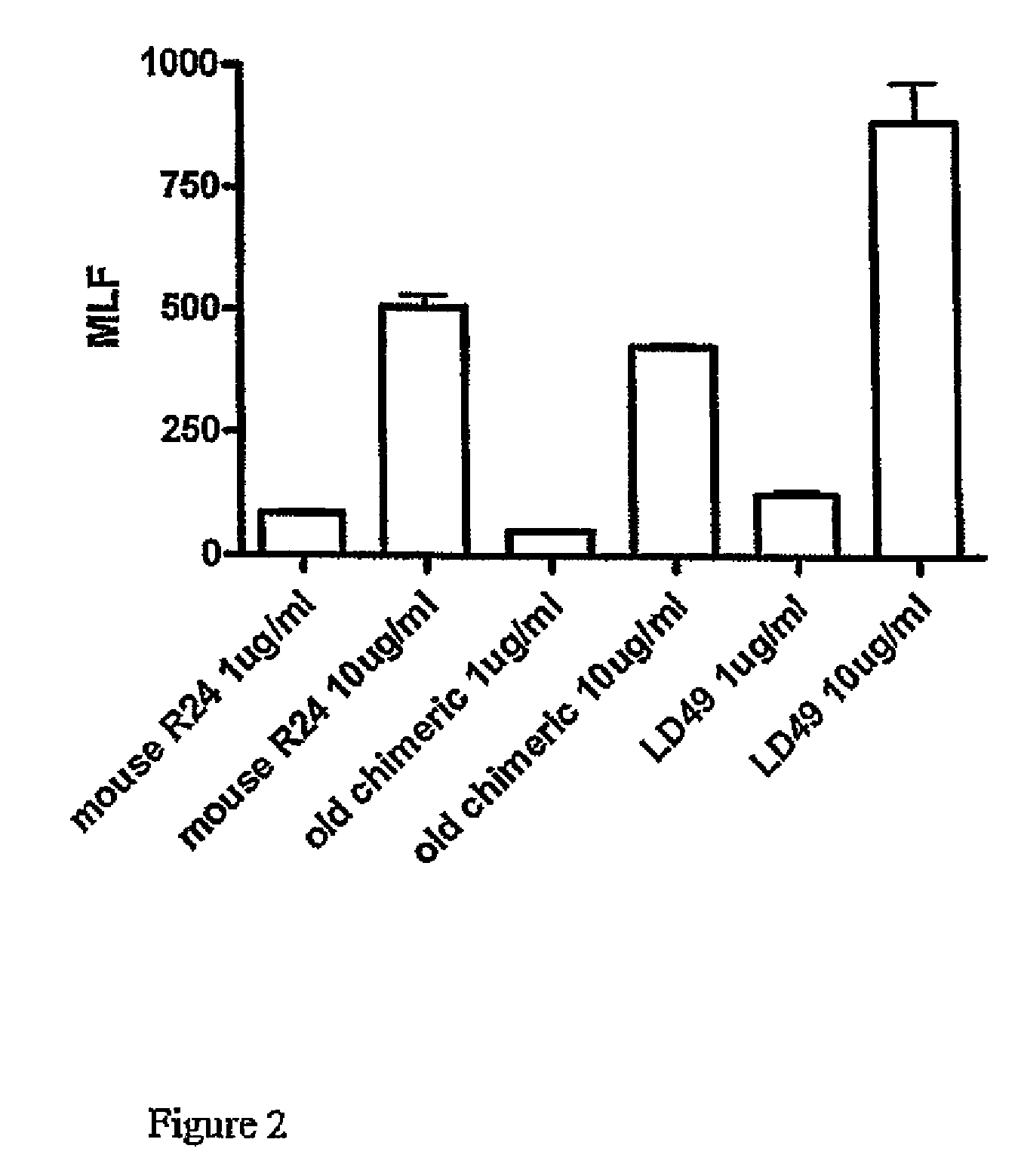
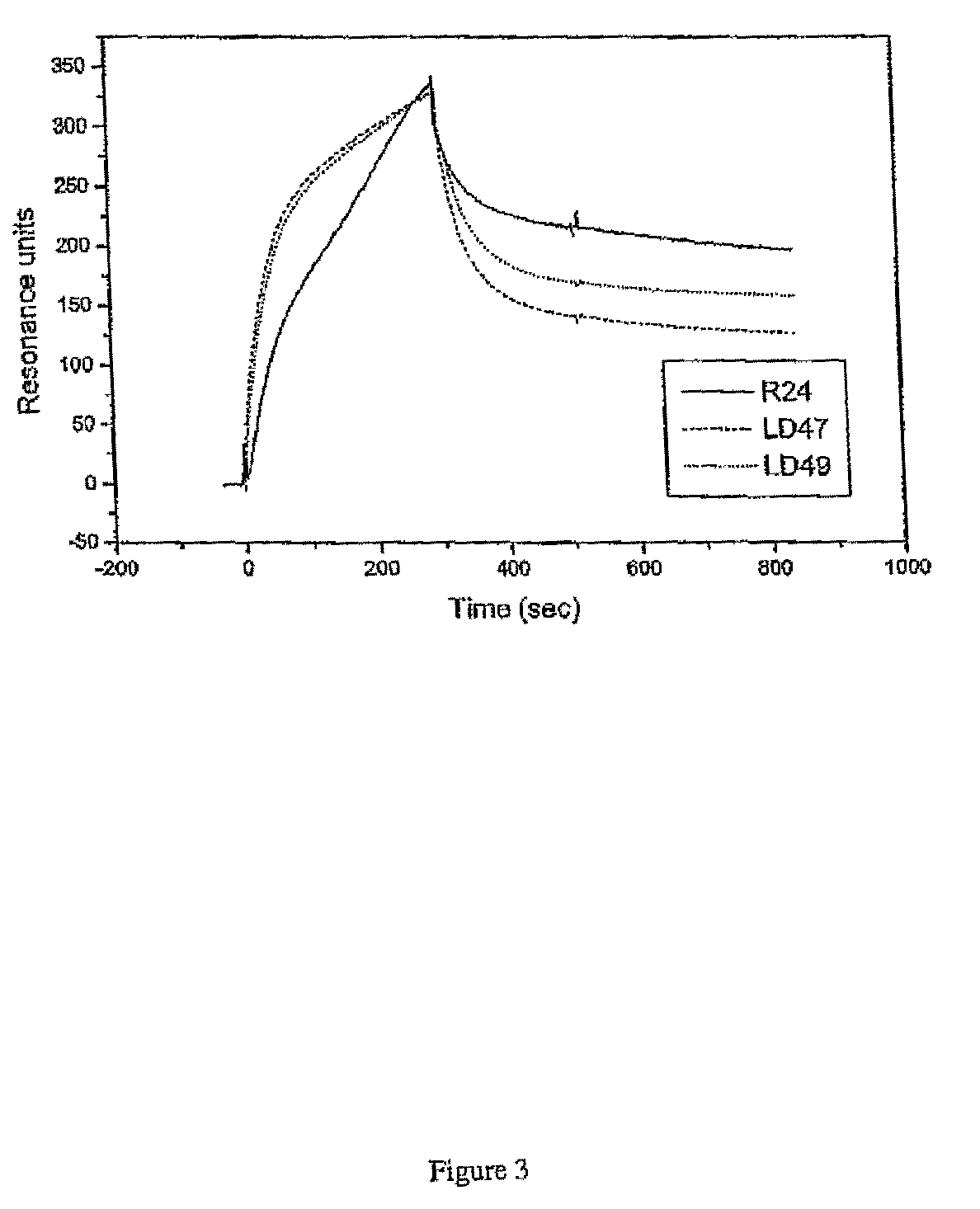
Anti ganglioside GD3 antibodies and uses thereof
The present invention is related to complementarity determining region (cdr)-grafted humanized r24 antibodies that bind to the gd3 ganglioside antigen. The humanized antibodies disclosed herein have characteristics that are comparable or superior to the murine r24 antibody, and the humanized antibodies are useful in treating cancer (e.g. Melanoma).
Owner:SLOAN KETTERING INST FOR CANCER RES
Ultra high affinity neutralizing antibodies
InactiveUS7740851B2Low costReduce efficacyHybrid immunoglobulinsAntibody mimetics/scaffoldsDiseaseComplementarity determining region
Ultra high affinity antibodies with binding affinities in the range of 1010 M−1, and even 1011 M−1 are disclosed. Such antibodies include antibodies having novel high affinity complementarity determining regions (CDRs), especially those with framework and constant regions derived from either humans or mice. Methods of preparing and screening such antibodies, as well as methods of using them to prevent and / or treat disease, especially virus-induced diseases, are also disclosed.
Owner:MEDIMMUNE LLC
Protein ligands
ActiveUS7709209B2Retention characteristicReduce leakageImmunoglobulins against animals/humansBiological testingComplementarity determining regionChemical stability
Owner:CYTIVA BIOPROCESS R&D AB
Intrabodies with defined framework that is stable in a reducing environment and applications thereof
InactiveUS7258986B2Stable environmentRaise the possibilityAntibody mimetics/scaffoldsMicroorganismsYeastComplementarity determining region
A method for the isolation of CDRs in a defined framework that is stable and soluble in reducing environment is described as well as thus obtainable scFv. Starting from such scFv with defined framework a scFv library can be generated wherein the framework is conserved while at least one complementary determining region (CDR) is randomized. Such library, e.g. in yeast cells, is suitable for screening for antibody / CDR-interactions or for screening for antibodies.
Owner:ESBATECH
Engineered antibodies with new world primate framework regions
InactiveUS20080095767A1Low immunogenicityAntibacterial agentsNervous disorderPrimateComplementarity determining region
The present invention provides an antibody or antigen-binding portion thereof having a variable region comprising at least two complementarity determining regions (CDRs) and at least three framework regions. The the framework regions are, or are derived from New World primate framework regions, and at least one of the CDRs is a non-New World primate CDR.
Owner:CEPHALON AUSTRALIA
Compositions and methods for binding lysophosphatidic acid
ActiveUS20100034814A1Low effective concentrationSugar derivativesMicrobiological testing/measurementComplementarity determining regionVariable domain
Compositions and methods for making and using anti-LPA agents, for example, monoclonal antibodies, are described. Variable domain and complementarity determining region amino acid sequences of several monoclonal antibodies against LPA are disclosed, as is a consensus anti-LPA monoclonal antibody variable domain sequence.
Owner:APOLLO ENDOSURGERY INC
Antibodies against non functional p2x7 receptor
InactiveUS20100036101A1Immunoglobulins against cell receptors/antigens/surface-determinantsFermentationComplementarity determining regionBinding site
A recombinant or synthetic antibody or fragment thereof, said antibody or fragment thereof including three complementarity determining regions (CDR1L or H, CDR2L or H and CDR3L or H) for forming an antigen binding site that is capable of binding to a non functional P2X7 receptor but not capable of binding to a functional P2X7 receptor.
Owner:BIOSCEPTRE INT
Pd-1 antibody, antigen-binding fragment thereof, and medical application thereof
ActiveUS20160376367A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigenDisease
The present invention provides a human PD-1 antibody, an antigen-binding fragment thereof, and medical use thereof, and further provides a chimeric antibody and humanized antibodies comprising a complementarity-determining region (CDR) of the antibody, a pharmaceutical composition comprising the human PD-1 antibody and the antigen-binding fragment thereof, and use of the antibody in preparing medicines for treating diseases or disorders.
Owner:SUZHOU SUNCADIA BIOPHARM CO LTD +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com