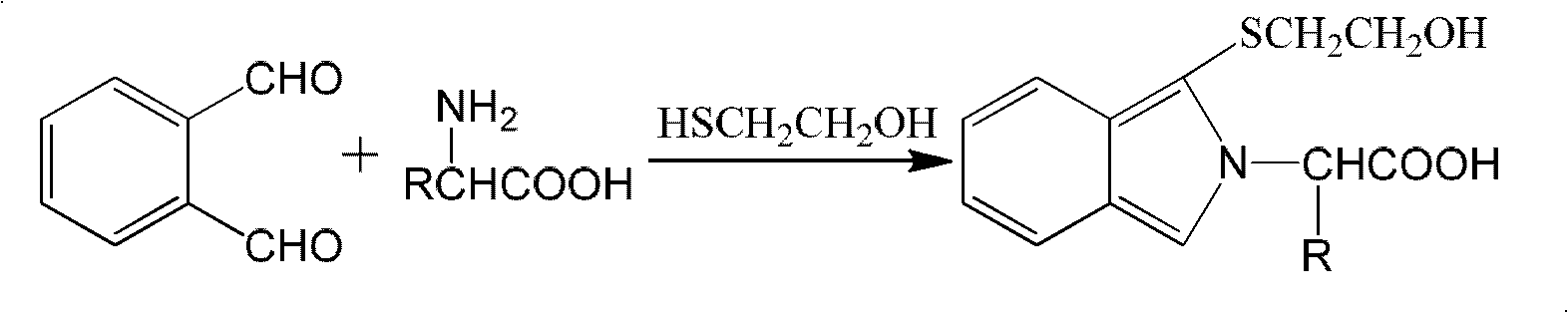Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
995 results about "Asparagine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Asparagine (symbol Asn or N), is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH⁺₃ form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO⁻ form under biological conditions), and a side chain carboxamide, classifying it as a polar (at physiological pH), aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it. It is encoded by the codons AAU and AAC.
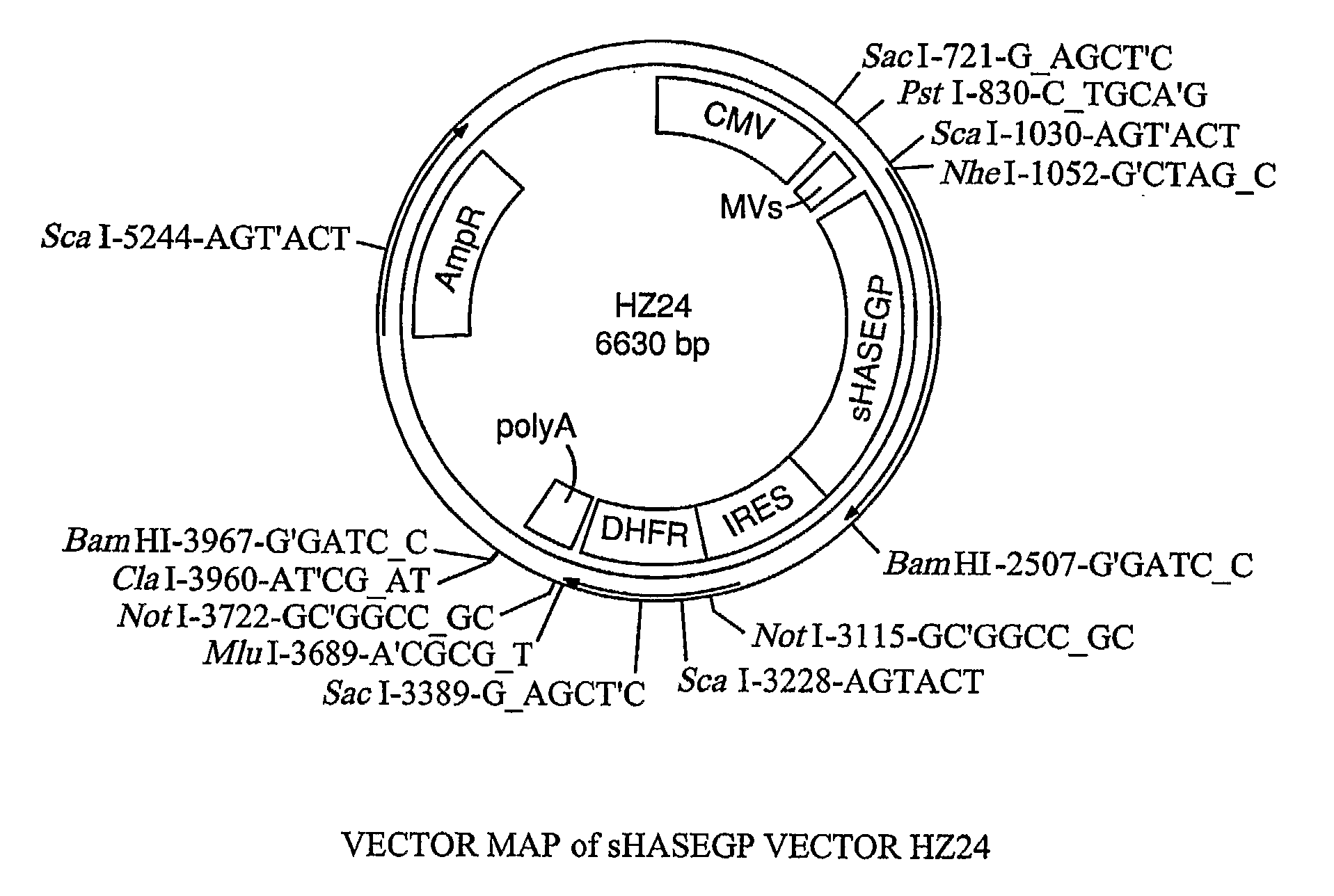
Soluble glycosaminoglycanases and methods of preparing and using soluble glycosaminogly ycanases
PendingUS20060104968A1Improve extentIncrease ratingsSenses disorderNervous disorderHyaluronidaseRecombinant glycoprotein
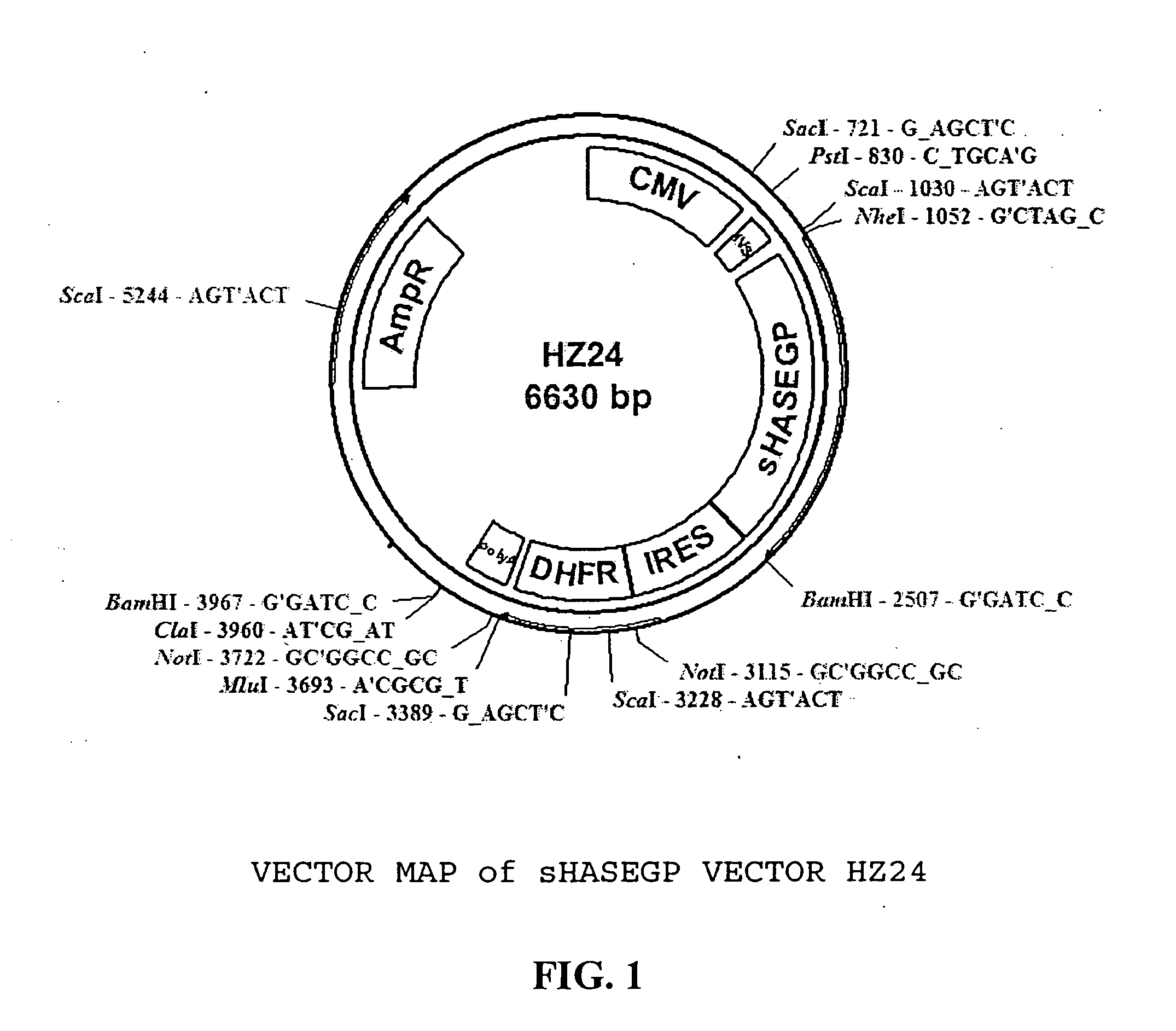
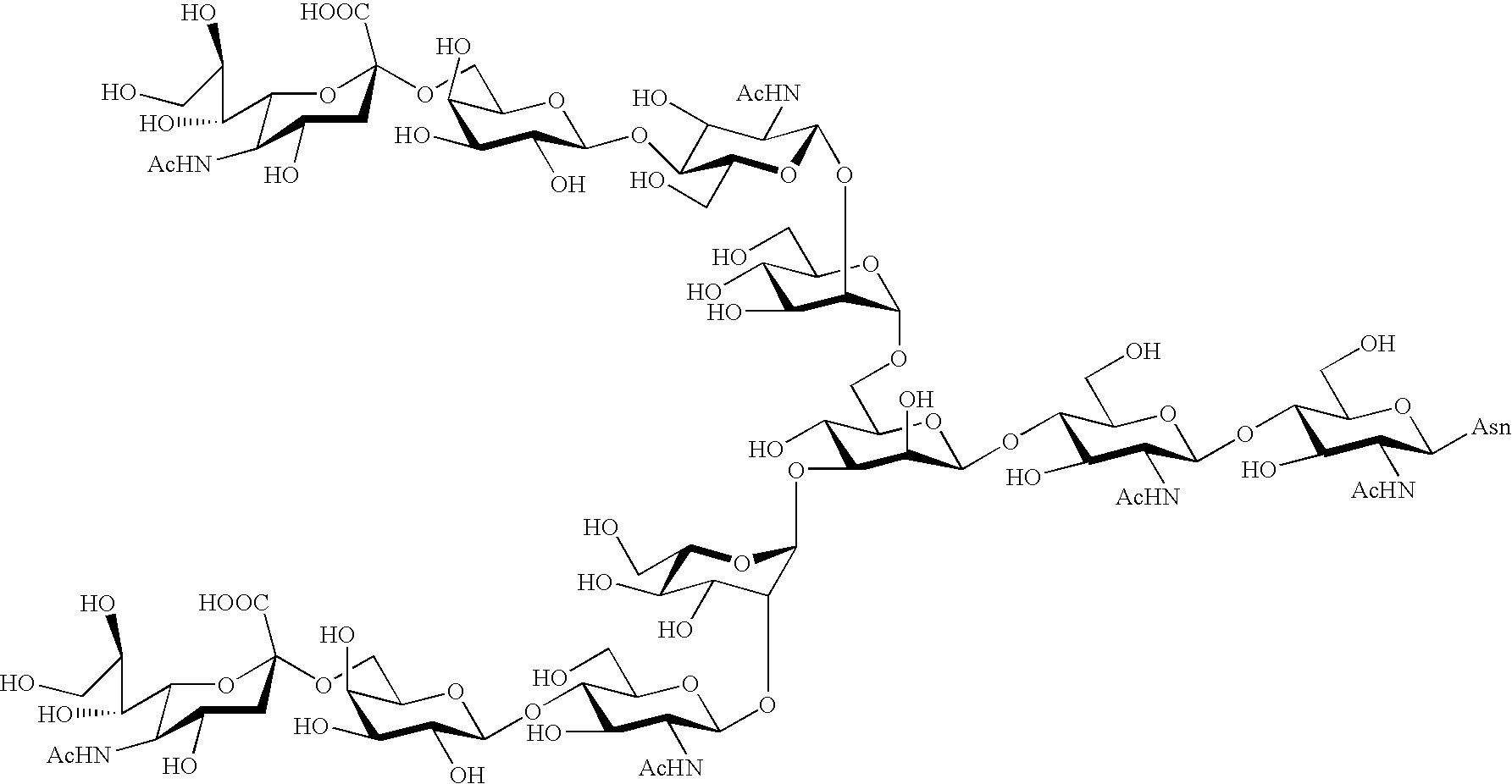
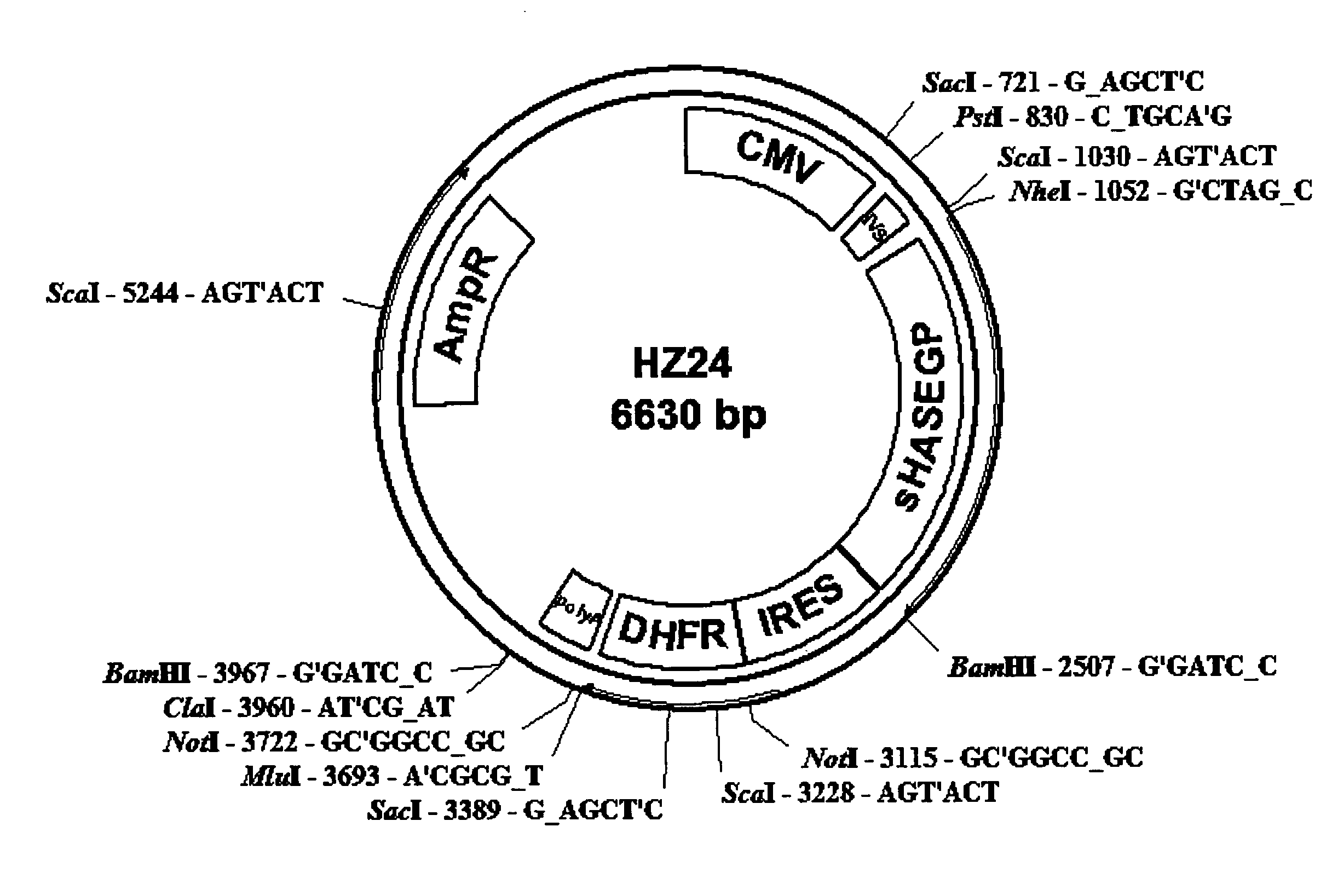
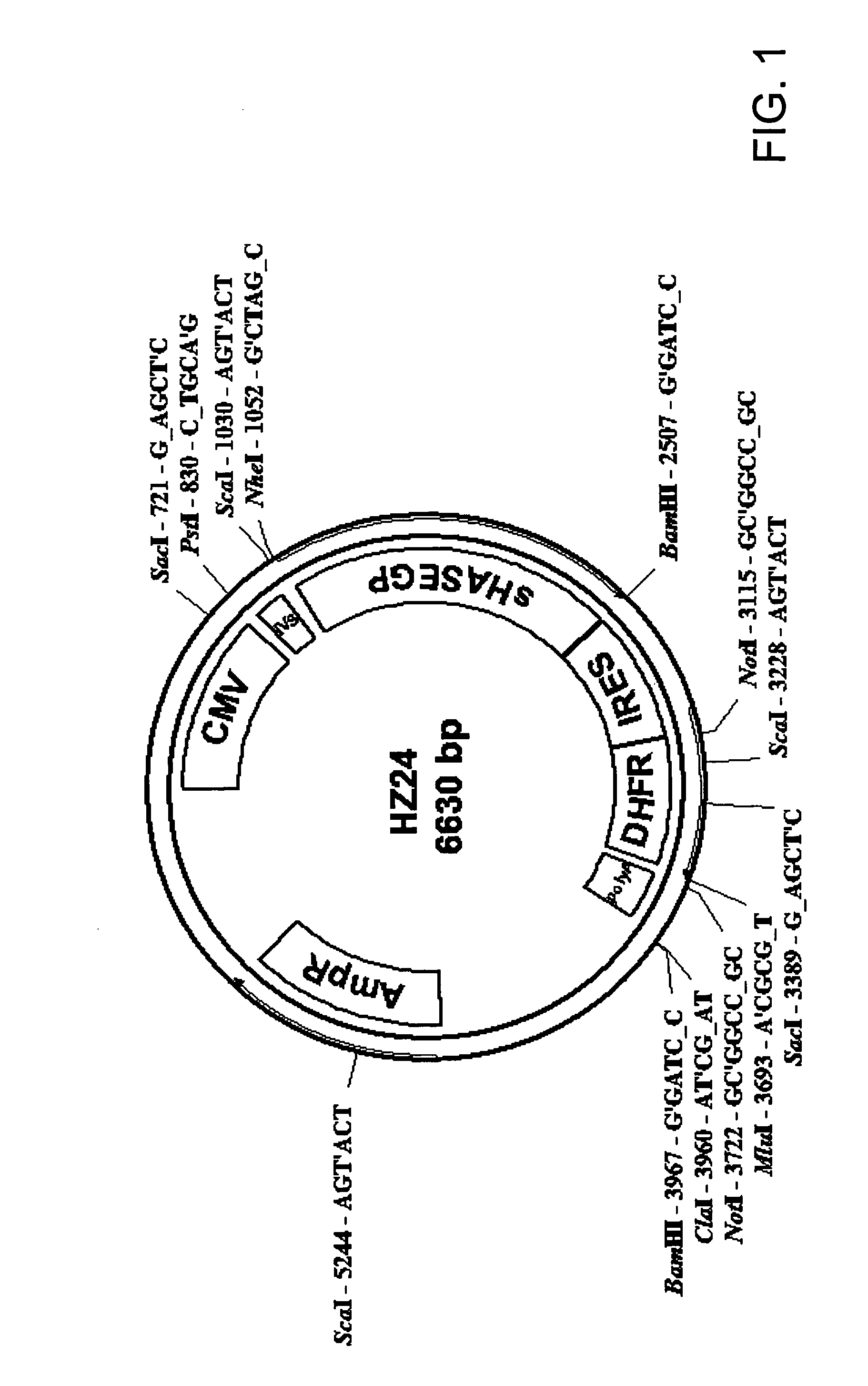
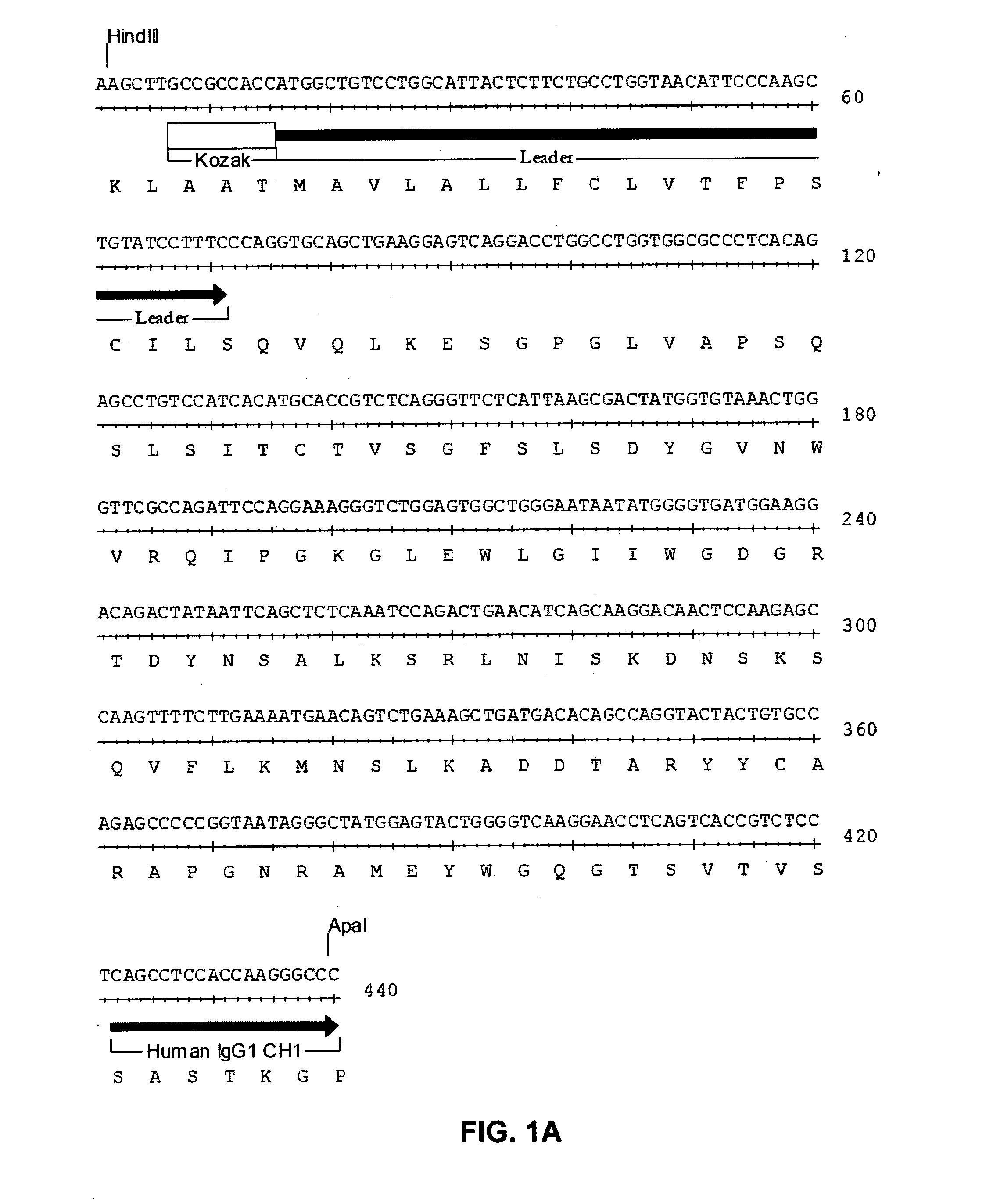
The invention relates to the discovery of novel soluble neutral active Hyaluronidase Glycoproteins (sHASEGPs), methods of manufacture, and their use to facilitate administration of other molecules or to alleviate glycosaminoglycan associated pathologies. Minimally active polypeptide domains of the soluble, neutral active sHASEGP domains are described that include asparagine-linked sugar moieties required for a functional neutral active hyaluronidase domain. Included are modified amino-terminal leader peptides that enhance secretion of sHASEGP. The invention further comprises sialated and pegylated forms of a recombinant sHASEGP to enhance stability and serum pharmacokinetics over naturally occurring slaughterhouse enzymes. Further described are suitable formulations of a substantially purified recombinant sHASEGP glycoprotein derived from a eukaryotic cell that generate the proper glycosylation required for its optimal activity.
Owner:HALOZYME
Thermal treatment process for tobacco materials
ActiveUS20100300463A1Alter natureAlter characterTobacco preparationTobacco treatmentArgininePhenylalanine
A method of thermally processing a tobacco material is provided, the method including the steps of (i) mixing a tobacco material, water, and an additive selected from the group consisting of lysine, glycine, histidine, alanine, methionine, glutamic acid, aspartic acid, proline, phenylalanine, valine, arginine, di- and trivalent cations, asparaginase, saccharides, phenolic compounds, reducing agents, compounds having a free thiol group, oxidizing agents, oxidation catalysts, plant extracts, and combinations thereof, to form a moist tobacco mixture; (ii) heating the moist tobacco mixture at a temperature of at least about 60° C. to form a heat-treated tobacco mixture; and (iii) incorporating the heat-treated tobacco mixture into a tobacco product. Heat-treated tobacco composition prepared according to the method are also provided, such as heat-treated smokeless tobacco composition comprising a tobacco material, water, flavorant, binder, and filler, the heat-treated smokeless tobacco composition having an acrylamide content of less than about 2000 ppb.
Owner:R J REYNOLDS TOBACCO COMPANY
Intein-mediated protein ligation of expressed proteins
InactiveUS6849428B1Eliminate needBacteriaFusion with post-translational modification motifProtein targetIntein
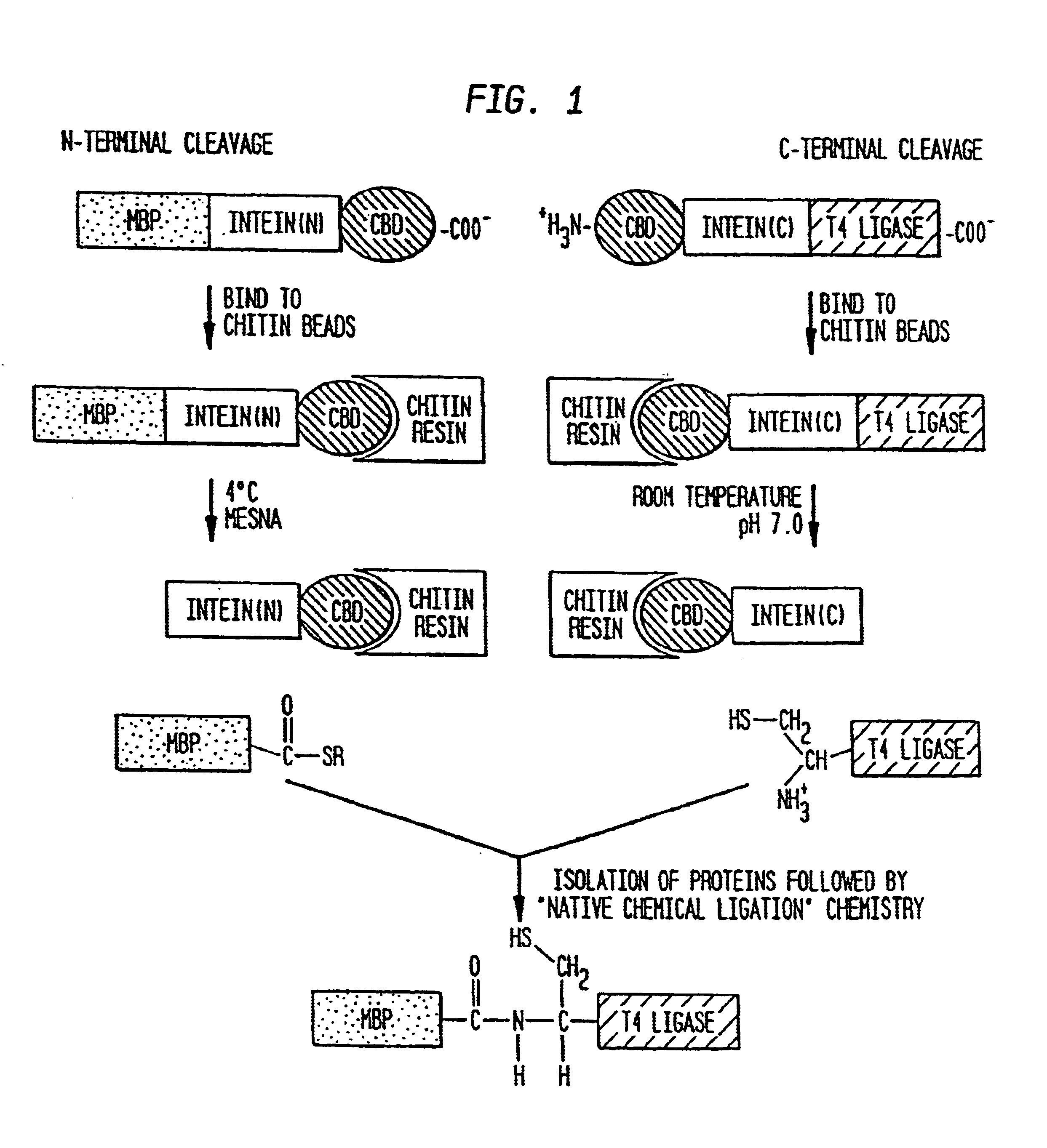
A method for the ligation of expressed proteins which utilizes inteins, for example the RIR1 intein from Methanobacterium thermotrophicum, is provided. Constructs of the Mth RIR1 intein in which either the C-terminal asparagine or N-terminal cysteine of the intein are replaced with alanine enable the facile isolation of a protein with a specified N-terminal, for example, cysteine for use in the fusion of two or more expressed proteins. The method involves the steps of generating a C-terminal thioester-tagged target protein and a second target protein having a specified N-terminal via inteins, such as the modified Mth RIR1 intein, and ligating these proteins. A similar method for producing a cyclic or polymerized protein is provided. Modified inteins engineered to cleave at their C-terminus or N-terminus, respectively, and DNA and plasmids encoding these modified inteins are also provided.
Owner:NEW ENGLAND BIOLABS
Nutrition noodles containing Chinese medicine components and preparation method thereof
The invention provides nutrition noodles containing Chinese medicine components and a preparation method thereof; the nutrition noodles are prepared from the raw materials which comprise flour and the following Chinese medicine components, wherein every 5kg of flour is mixed with the Chinese medicine components by weight: 4 to 10g of ginseng, 3 to 6g of radix codonopsitis, 3 to 6g of astragalus, 4 to 8g of polygonatum, 2 to 5g of atractylode, 10 to 20g of yam, 3 to 6g of rehmannia, 4 to 8g of polygonum, 3 to 8g of angelica, 4 to 10g of donkey-hide gelatin, 5 to 10g of medlar, 4 to 6g of root of herbaceous peony, 4 to 8g of radix, 3 to 6g of asparagine, 4 to 8g of polygonatum, 4 to 8g of root of straight ladybell, 10 to 15g of lily, 4 to 8g of ligustrum lucidum, 5 to 8g of psoralen, 4 to 8g of cistanche, 3 to 8g of morinda, 4 to 7g of dodder, 5 to 10g of cornus, 3 to 7g of schisandra, 3 to 7g of poria, 5 to 8g of coix seed, 3 to 6g of clove, 4 to 8g of nutmeg, 3 to 6g of fructus tsaoko, 4 to 8g of rhizoma gastrodiae, 4 to 8g of kidney tonifying nut, 3 to 6g of fructus momordicae, 10 to 20g of lotus seeds and 3 to 7g of notoginseng. The nutrition noodles have various health care effects.
Owner:杨占良
Novel oral forms of a phosphonic acid derivative
InactiveUS20120190647A1Improved aqueous solubilityIncrease ratingsBiocideOrganic active ingredientsEnprofyllineArginine
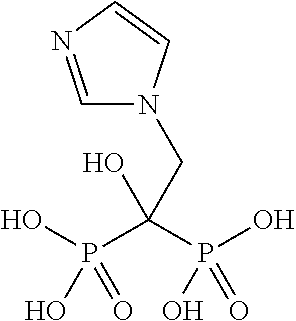
Novel solution complexes of zoledronic acid are described which give rise to improved properties of zoledronic acid. The invention includes aqueous solution and molecular complexes of zoledronic acid with and optical isomers of asparagine, histidine, arginine and proline as well as pharmaceutical complexes containing them and methods of treatment using them.
Owner:THAR PHARMA
Mutant protein
InactiveUS20060194950A1Improve stabilityIncreased pH-valuesBacteriaSerum immunoglobulinsMutated proteinComplementarity determining region
Owner:GE HEALTHCARE BIO SCI CORP
Mutated immunoglobulin-binding protein
ActiveUS20050143566A1Improve stabilityIncreased pH-valuesSerum immunoglobulinsComponent separationComplementarity determining regionChemical stability
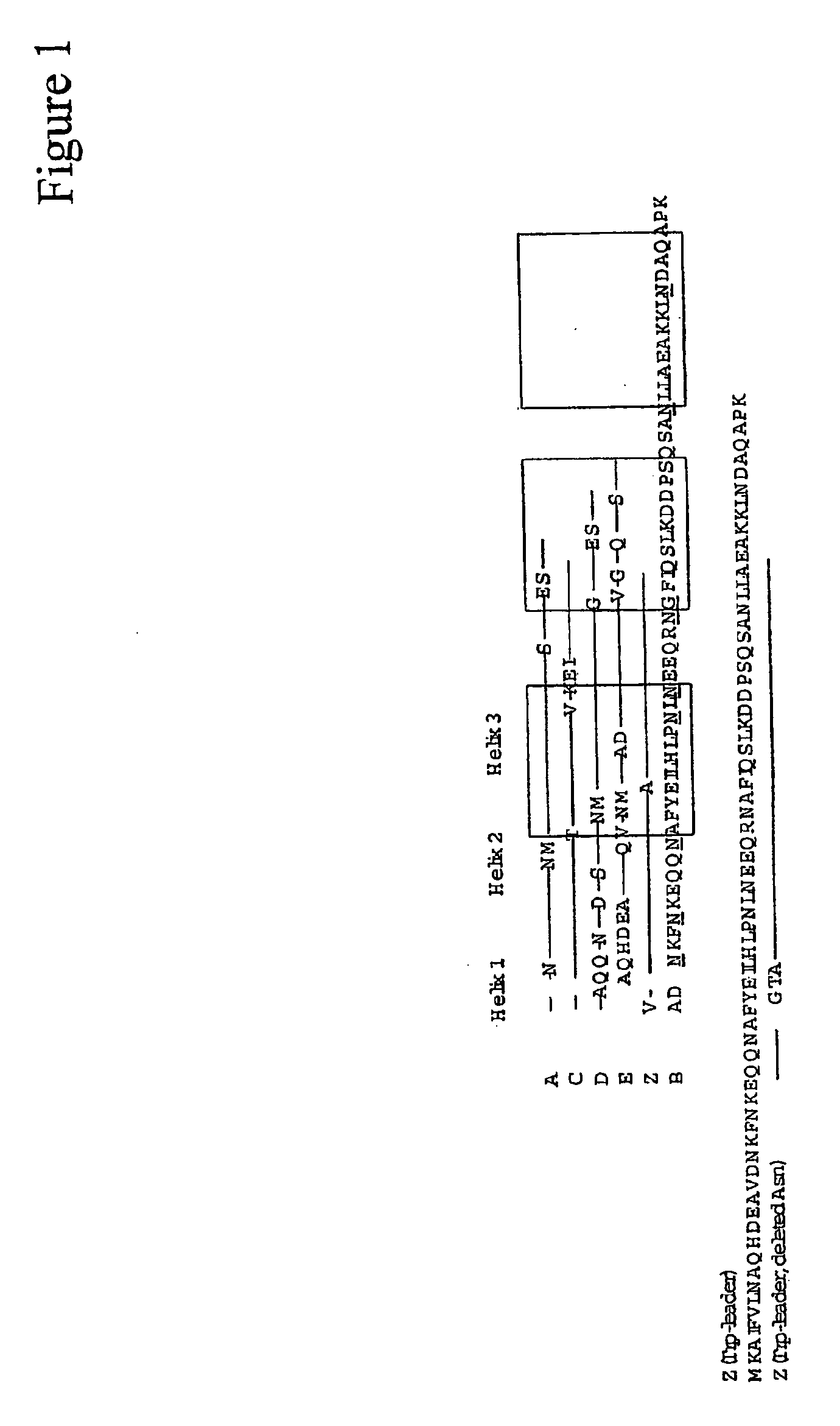
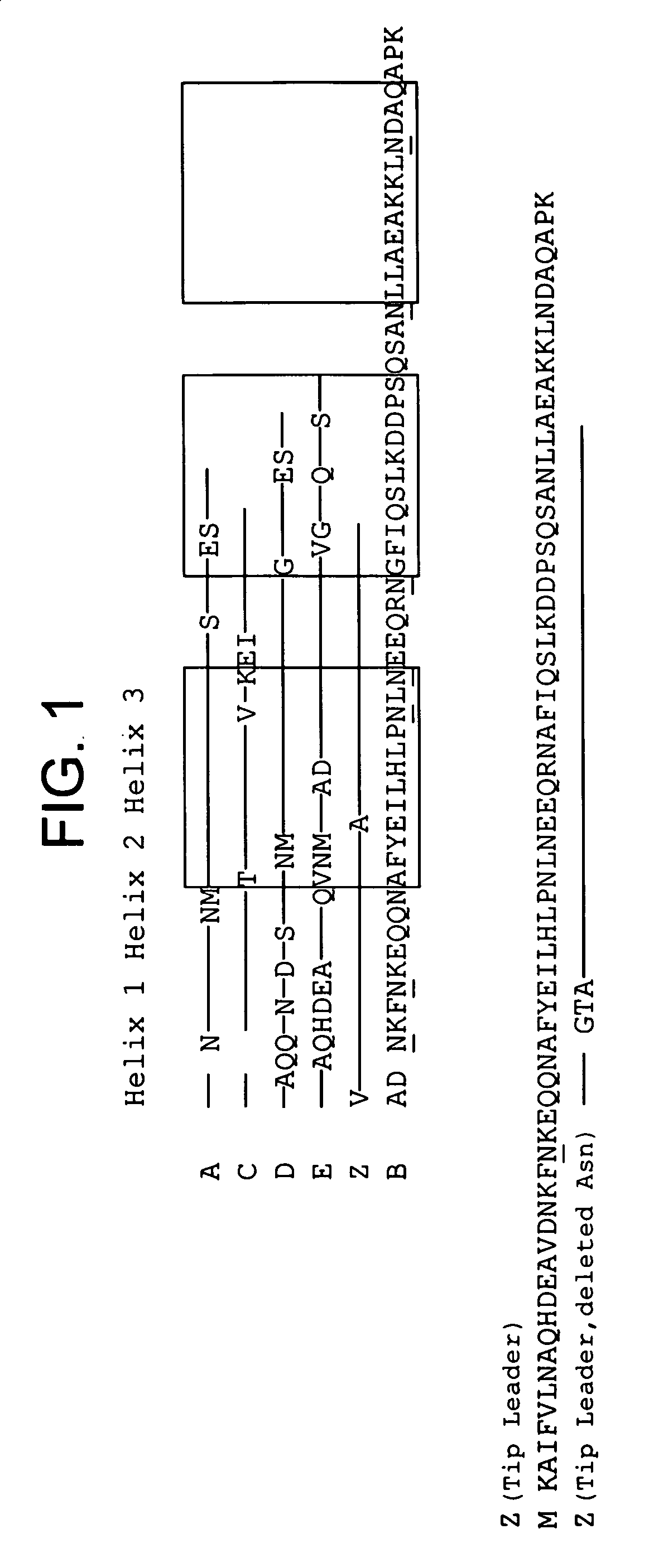
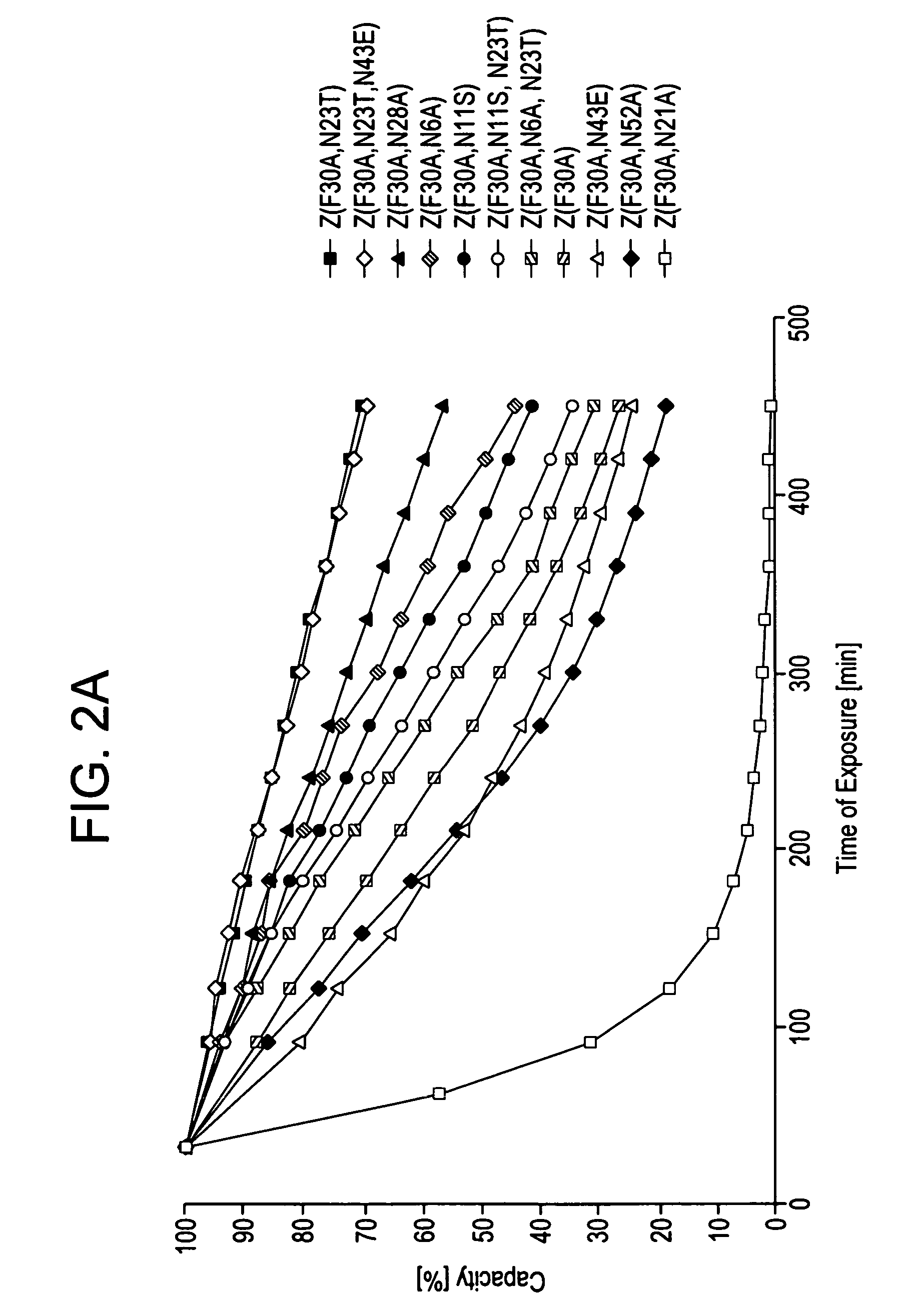
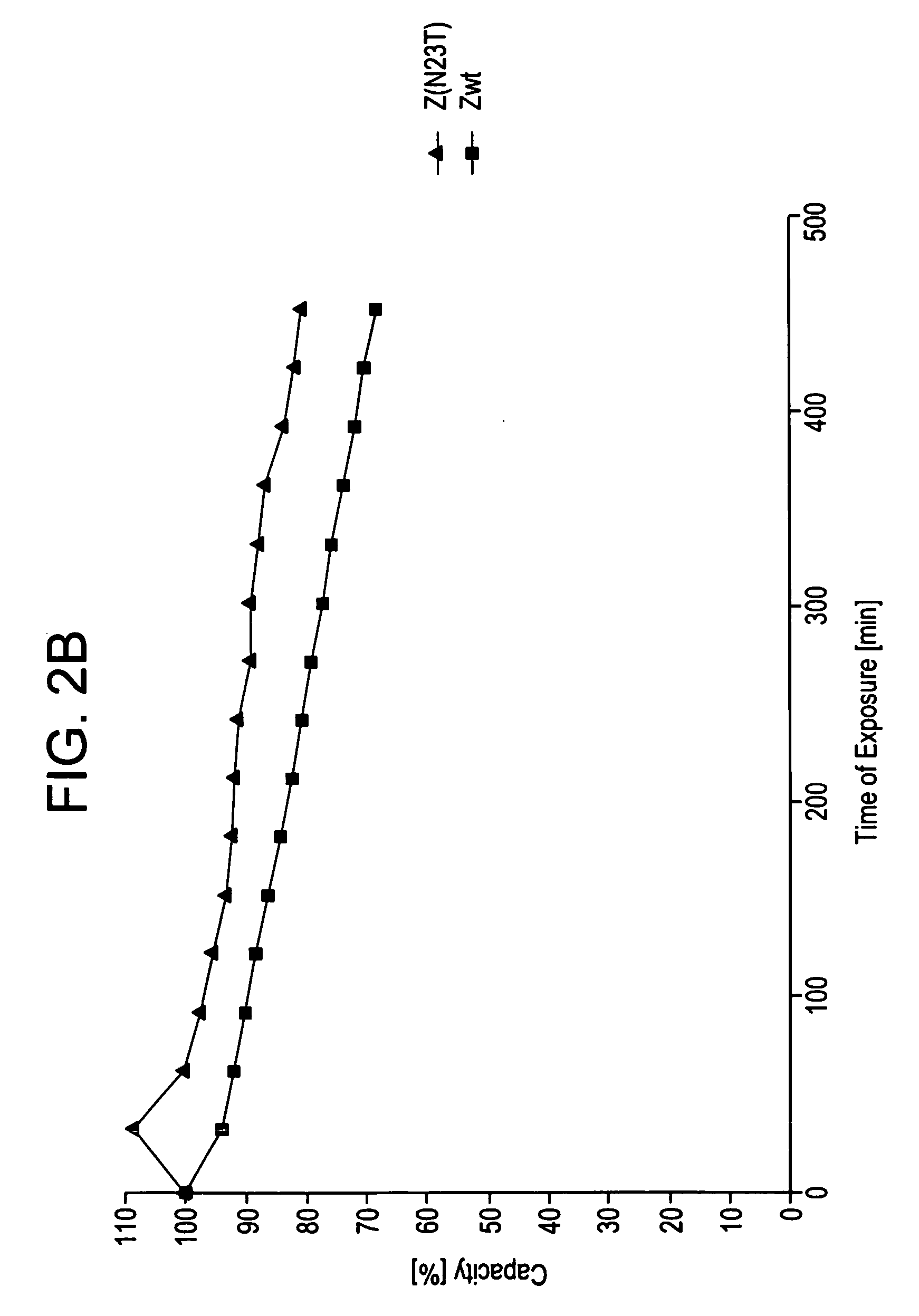
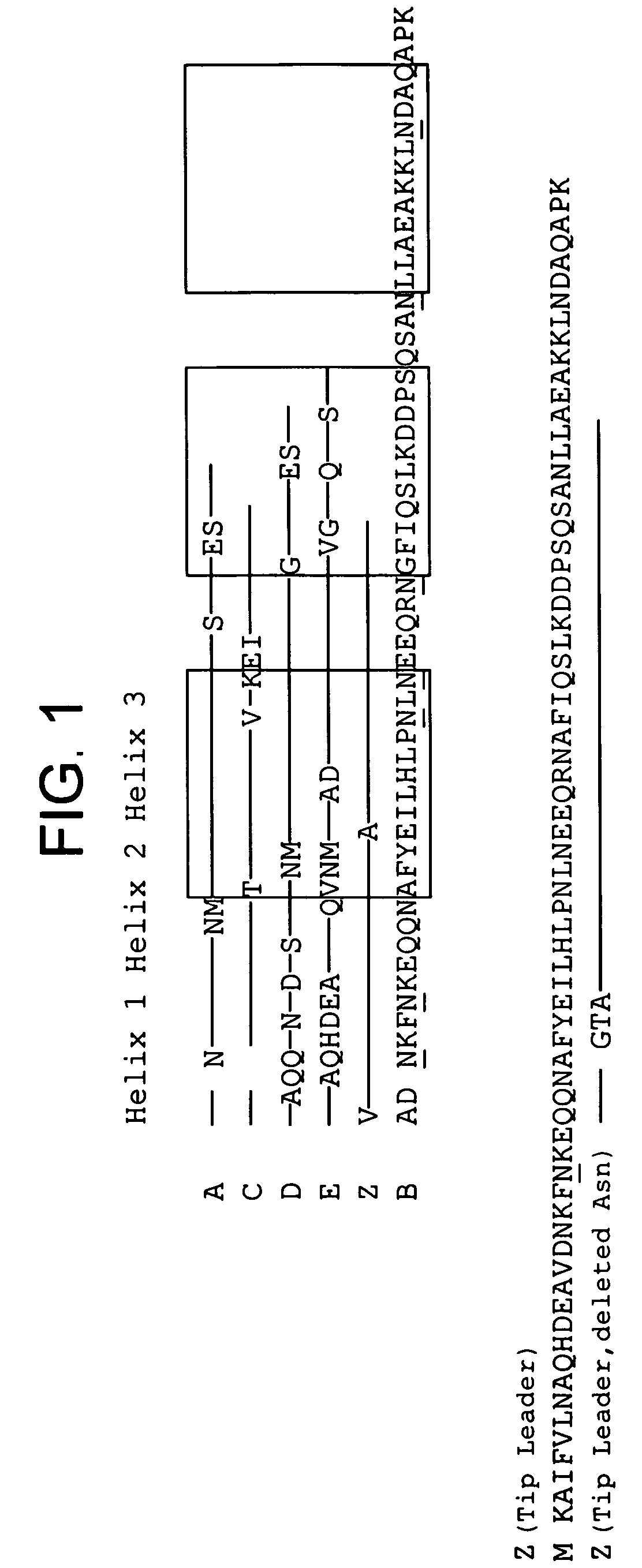
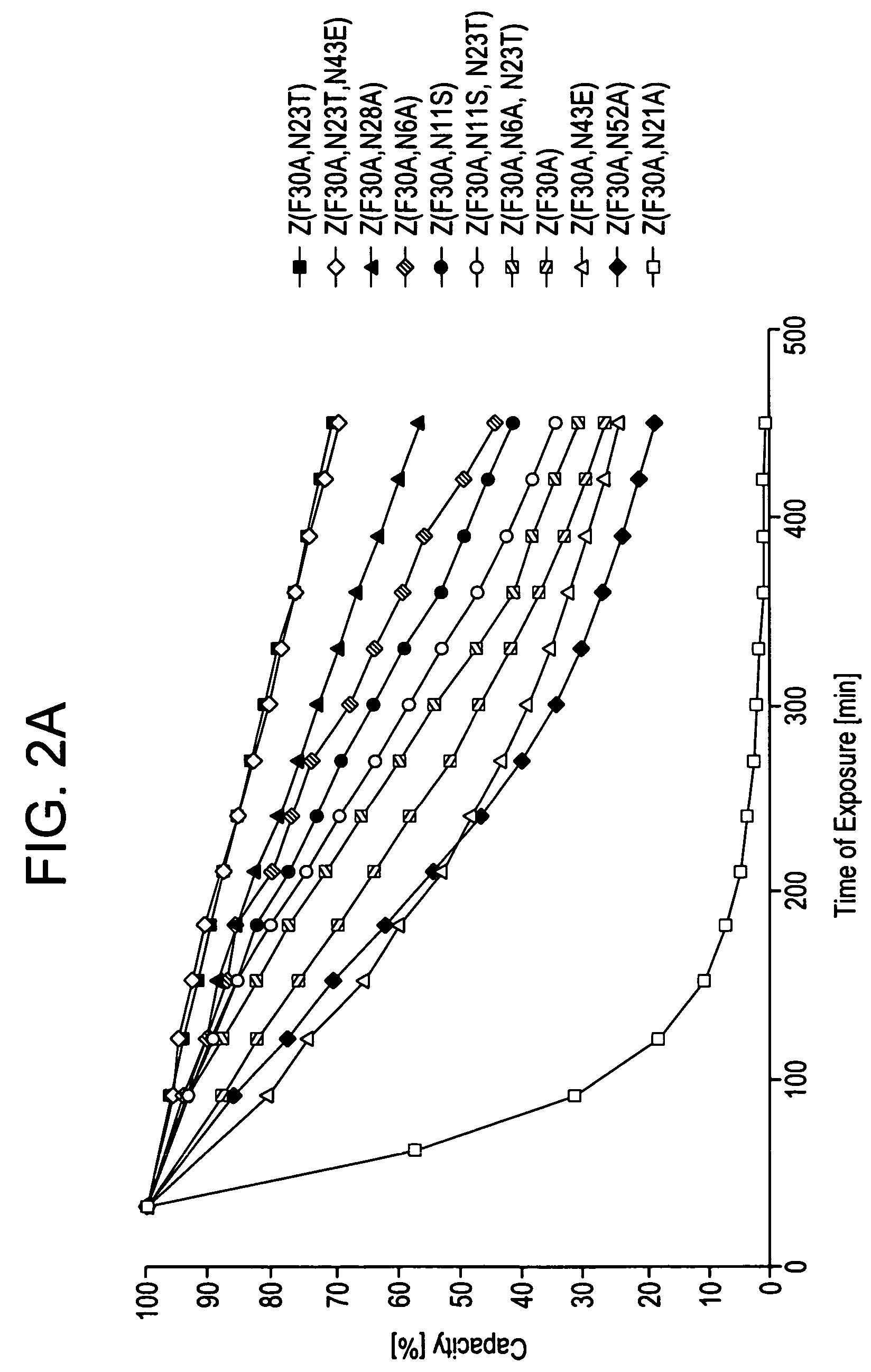
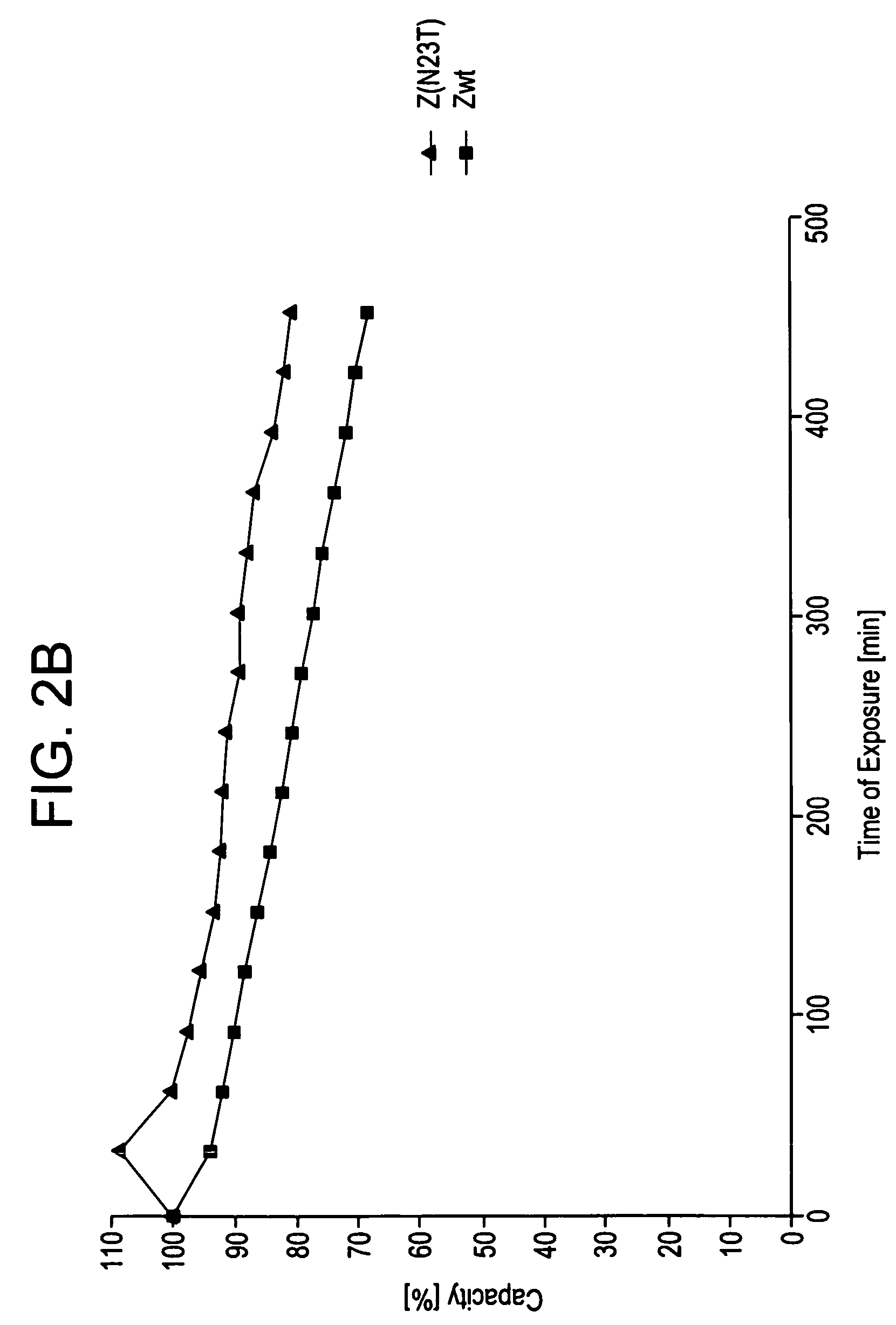
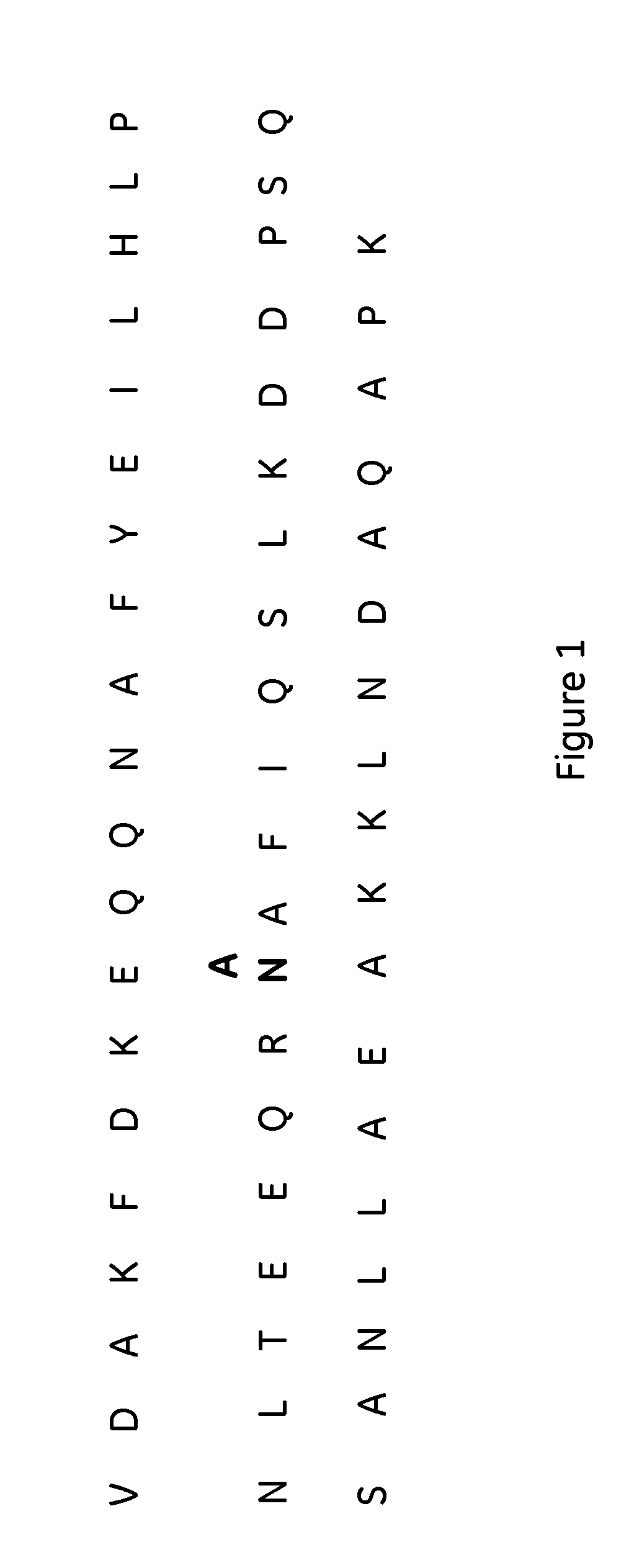
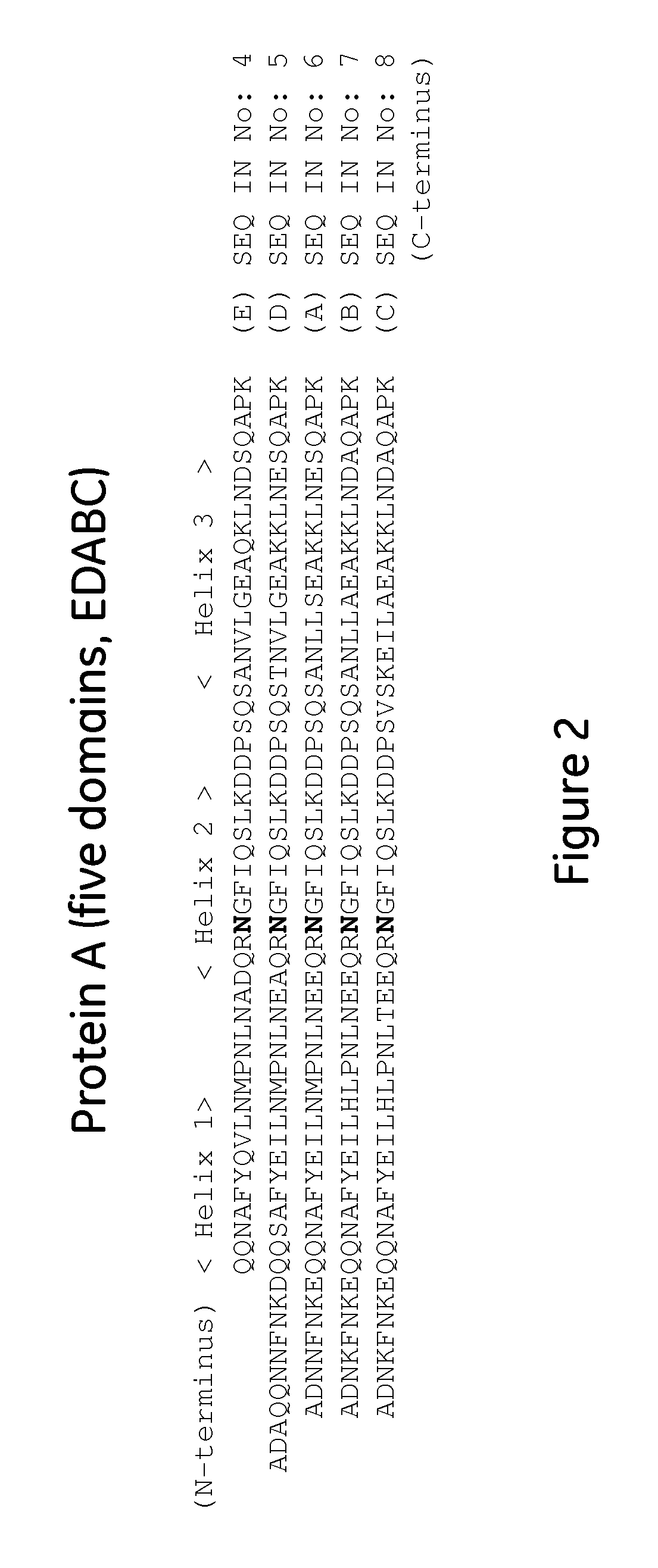
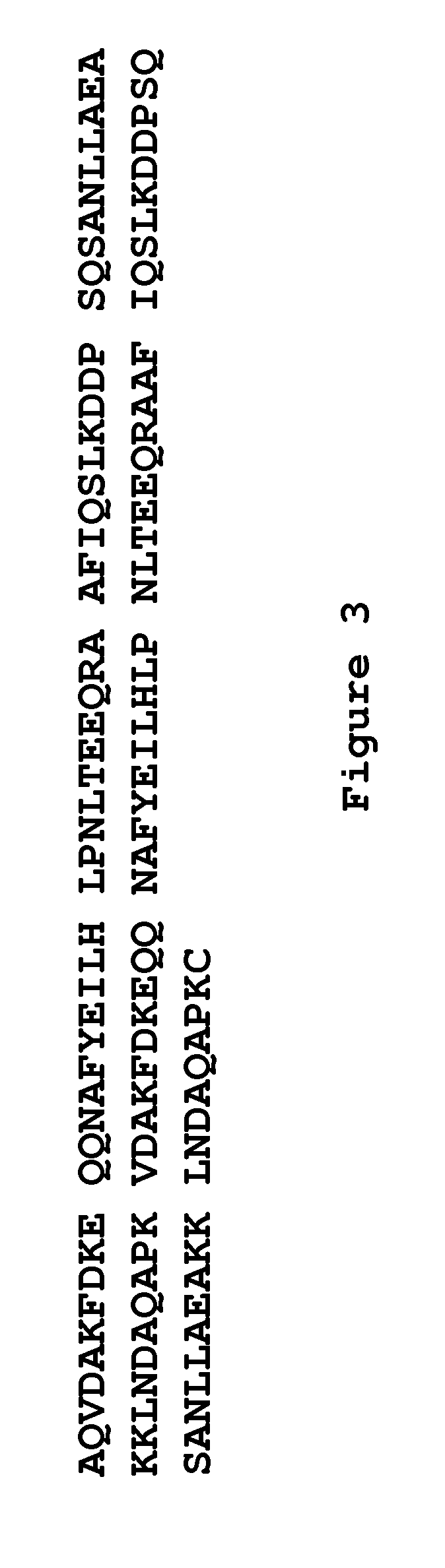
The present invention relates to an immunoglobulin-binding protein, wherein at least one asparagine residue has been mutated to an amino acid other than glutamine or aspartic acid, which mutation confers an increased chemical stability at pH-values of up to about 13-14 compared to the parental molecule. The protein can for example be derived from a protein capable of binding to other regions of the immunoglobulin molecule than the complementarity determining regions (CDR), such as protein A, and preferably the B-domain of Staphylococcal protein A. The invention also relates to a matrix for affinity separation, which comprises an immunoglobulin-binding protein as ligand coupled to a solid support, in which protein ligand at least one asparagine residue has been mutated to an amino acid other than glutamine.
Owner:CYTIVA BIOPROCESS R&D AB
Glycoconjugation of polypeptides using oligosaccharyltransferases
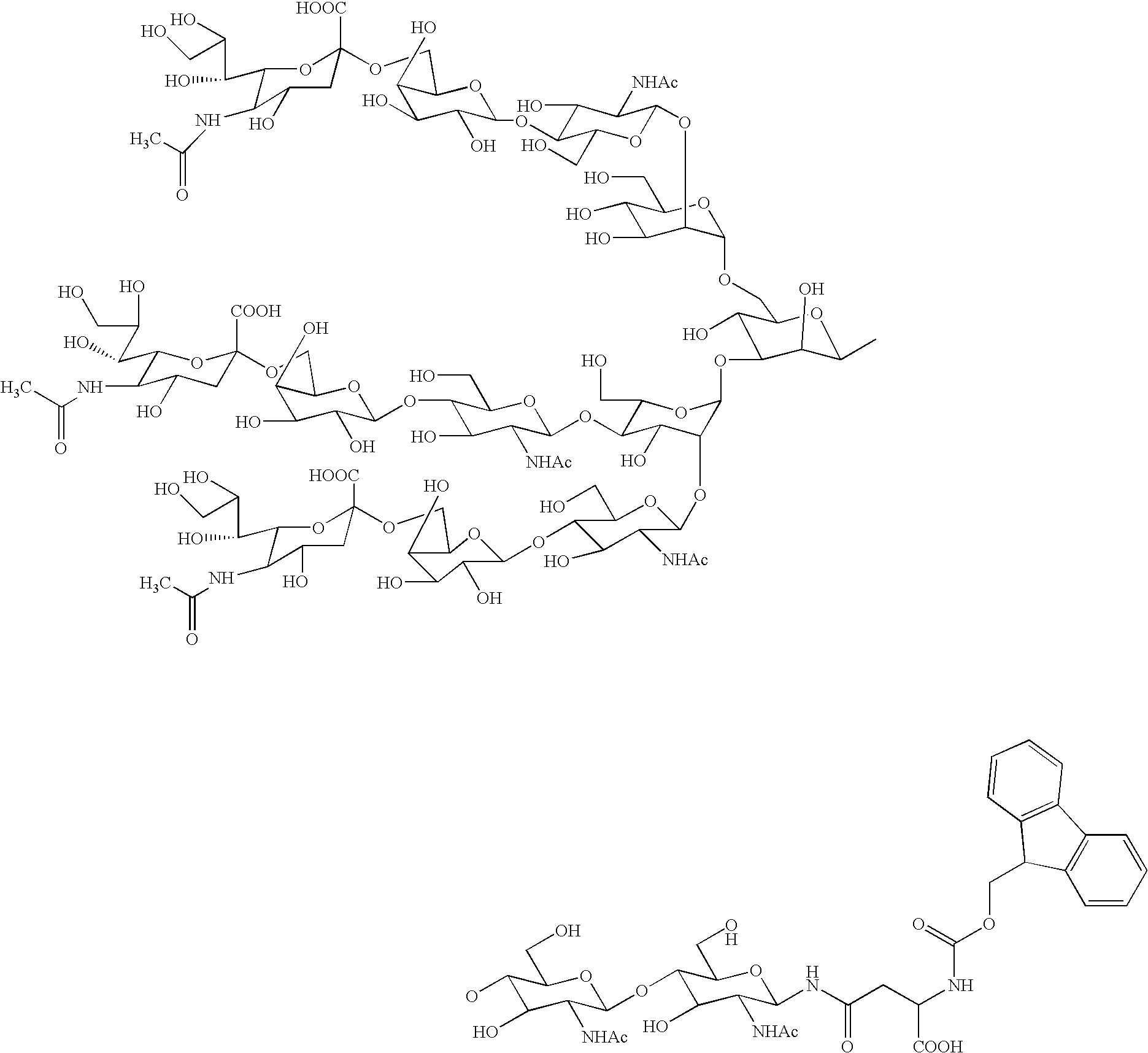
The current invention provides polypeptides and polypeptide conjugates that include an exogenous N-linked glycosylation sequence. The N-linked glycosylation sequence is preferably a substrate for an oligosaccharyltransferase (e.g., bacterial PgIB), which can catalyze the transfer of a glycosyl moiety from a lipid-bound glycosyl donor molecule (e.g., a lipid-pyrophosphate-linked glycosyl moiety) to an asparagine (N) residue of the glycosylation sequence. In one example, the asparagine residue is part of an exogenous N-linked glycosylation sequence of the invention. The invention further provides methods of making the polypeptide conjugates that include contacting a polypeptide having an N-linked glycosylation sequence of the invention and a lipid-pyrophosphate-linked glycosyl moiety (or phospholipid-linked glycosyl moiety) in the presence of an oligosaccharyltransferase under conditions sufficient for the enzyme to transfer the glycosyl moiety to an asparagine residue of the N-linked glycosylation sequence. Exemplary glycosyl moieties that can be conjugated to the glycosylation sequence include GlcNAc, GlcNH, bacillosamine, 6-hydroybacillosamine, GalNAc, GaINH, GlcNAc-GlcNAc, GlcNAc-GlcNH, GlcNAc-Gal, GlcNAc-GlcNAc-Gal-Sia, GlcNAc-Gal-Sia, GlcNAc-GlcNAc-Man, and GlcNAc-GlcNAc-Man(Man)2. The transferred glycosyl moiety is optionally modified with a modifying group, such as a polymer (e.g., PEG). In one example, the modified glycosyl moiety is a GIcNAc or a sialic acid moiety.
Owner:RATIOPHARM GMBH +1
Method for treating plants with probiotics
InactiveUS20130269719A1Reduction in asparagine contentReduce acrylamide contentTobacco preparationTobacco treatmentNicotiana tabacumTobacco product
Owner:R J REYNOLDS TOBACCO COMPANY
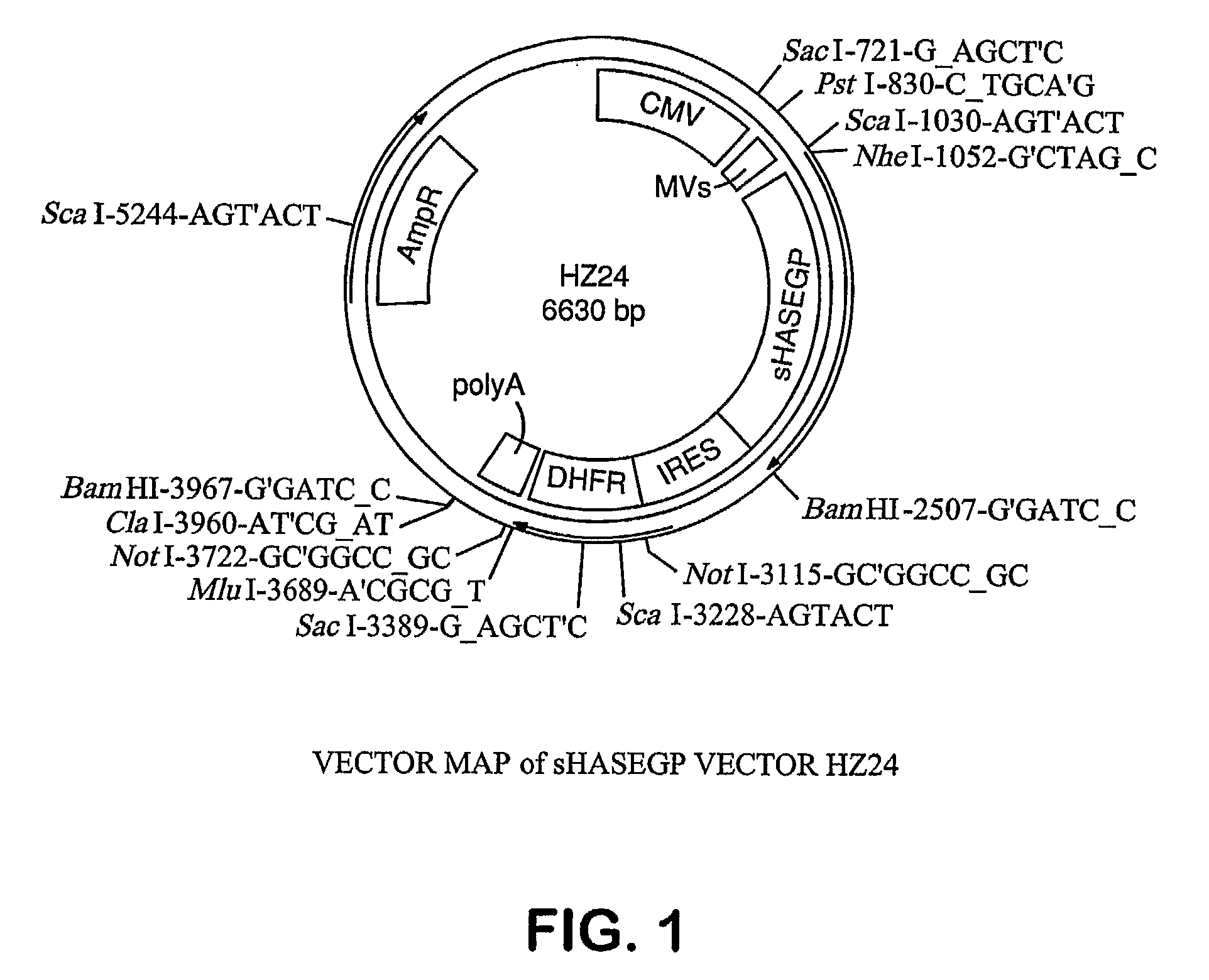
Soluble Glycosaminoglycanases and Methods of Preparing and Using Soluble Glycosaminoglycanases
InactiveUS20090123367A1Facilitated DiffusionEnhance convective transportBacterial antigen ingredientsPeptide/protein ingredientsHyaluronidaseNuclear chemistry
The invention relates to the discovery of novel soluble neutral active Hyaluronidase Glycoproteins (sHASEGPs), methods of manufacture, and their use to facilitate administration of other molecules or to alleviate glycosaminoglycan associated pathologies. Minimally active polypeptide domains of the soluble, neutral active sHASEGP domains are described that include asparagine-linked sugar moieties required for a functional neutral active hyaluronidase domain. Included are modified amino-terminal leader peptides that enhance secretion of sHASEGP. The invention further comprises sialated and pegylated forms of a recombinant sHASEGP to enhance stability and serum pharmacokinetics over naturally occurring slaughterhouse enzymes. Further described are suitable formulations of a substantially purified recombinant sHASEGP glycoprotein derived from a eukaryotic cell that generate the proper glycosylation required for its optimal activity.
Owner:HALOZYME +6
Protein ligands
ActiveUS20060194955A1Retention characteristicReduce leakageImmunoglobulins against animals/humansBiological testingComplementarity determining regionStaphylococcus
The present invention relates to the use of an alkali-stable protein, wherein at least one asparagine residue has been mutated to an amino acid other than glutamine or aspartic acid, which mutation confers an increased chemical stability at pH-values of up to about 13-14 compared to the parental molecule. The protein can for example be derived from a protein capable of binding to other regions of the immunoglobulin molecule than the complementarity determining regions (CDR), such as protein A, and preferably the B-domain of Staphylococcal protein A. The invention also relates to a matrix for affinity separation, which comprises an immunoglobulin-binding protein as ligand coupled to a solid support, in which protein ligand at least one asparagine residue has been mutated to an amino acid other than glutamine.
Owner:CYTIVA BIOPROCESS R&D AB
Method of affinity separation and ligands for use therein
Methods of affinity separation wherein the affinity ligand is an immobilized proteinaceous ligand wherein one or more of its asparagine (Asn) residues has been modified. Methods of making a stabilized combinatorial protein by a) modification of Asn residues within a protein molecule to increase stability of the protein in alkaline conditions, and b) randomization of a protein molecule to modify its binding characteristics, and combinatorial proteins wherein in a step separate from the randomization step, the stability of the protein in alkaline conditions has been increased by modifying one or more of its Asn residues.
Owner:AFFIBODY TECH AB
Production of polypeptides
ActiveUS20060121568A1Cell receptors/surface-antigens/surface-determinantsGenetically modified cellsTotal amino acidsInorganic ions
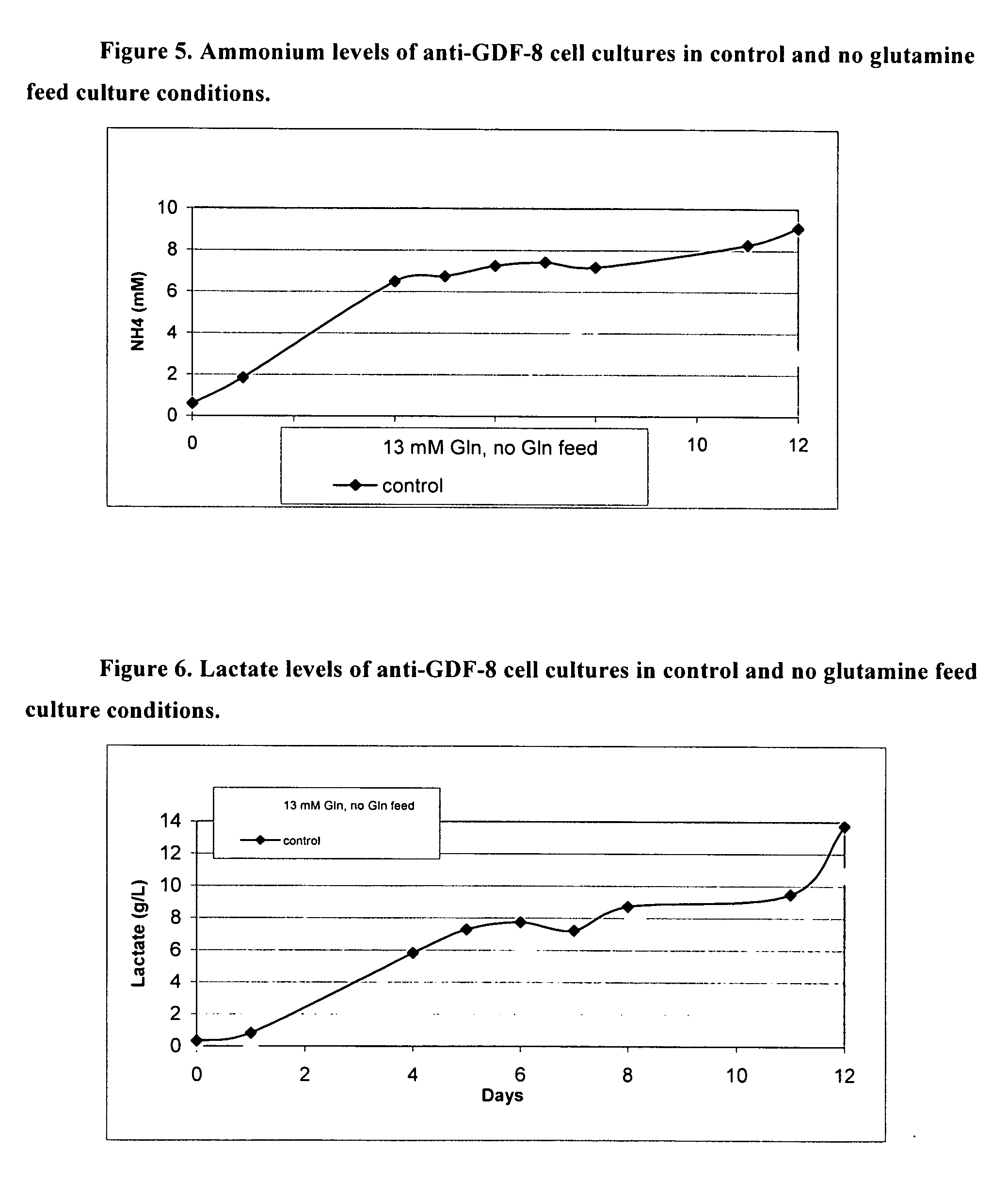
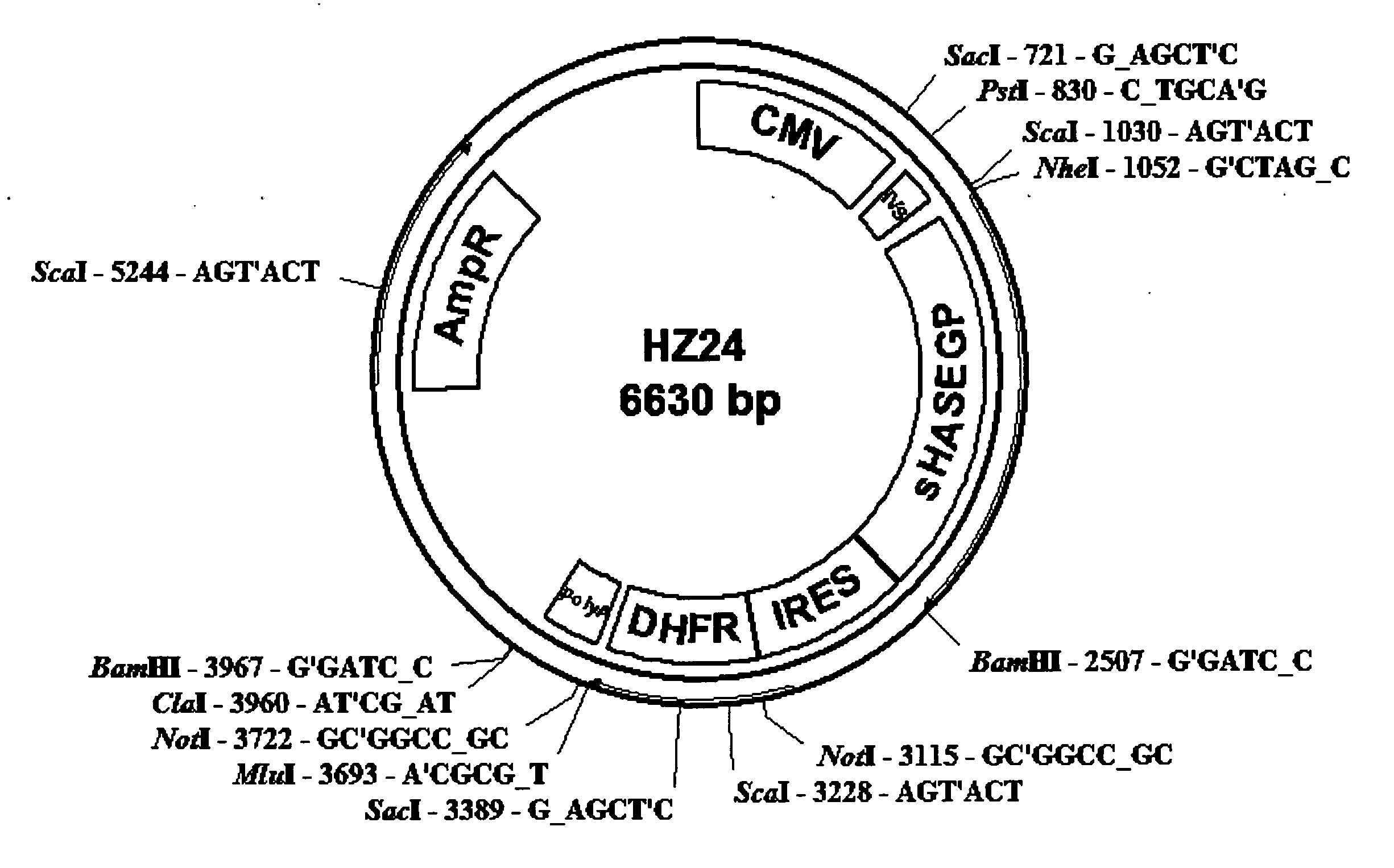
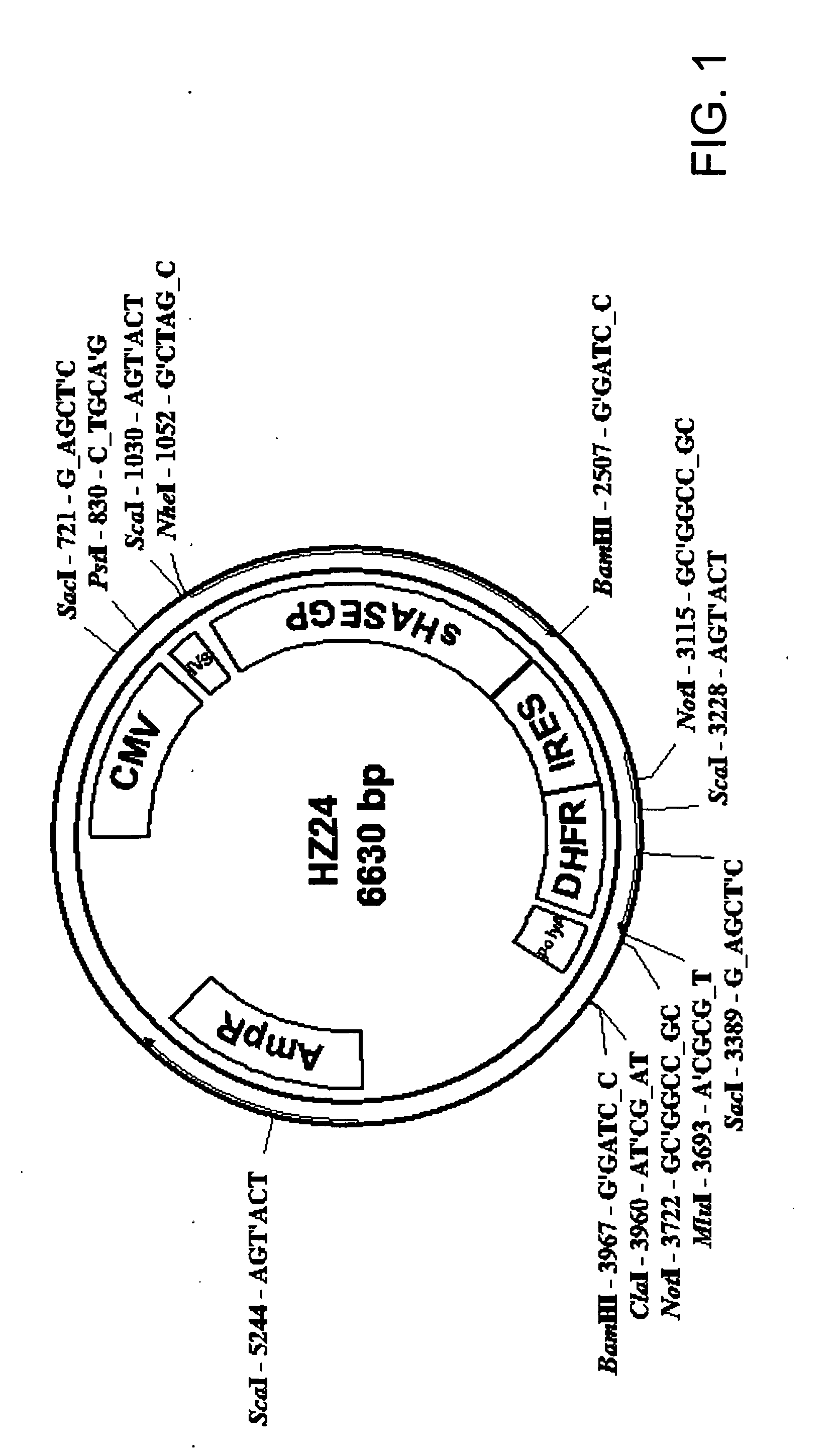
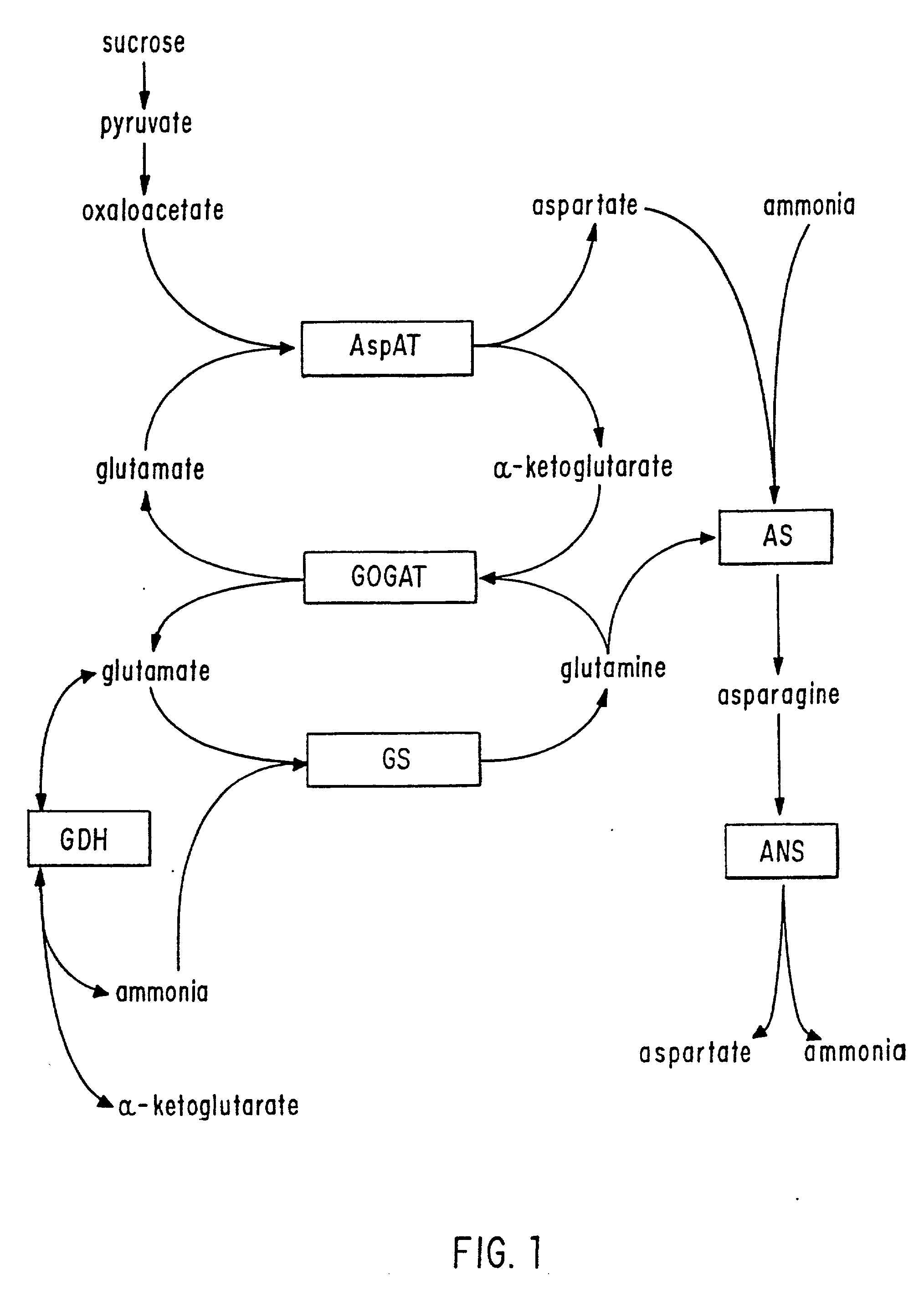
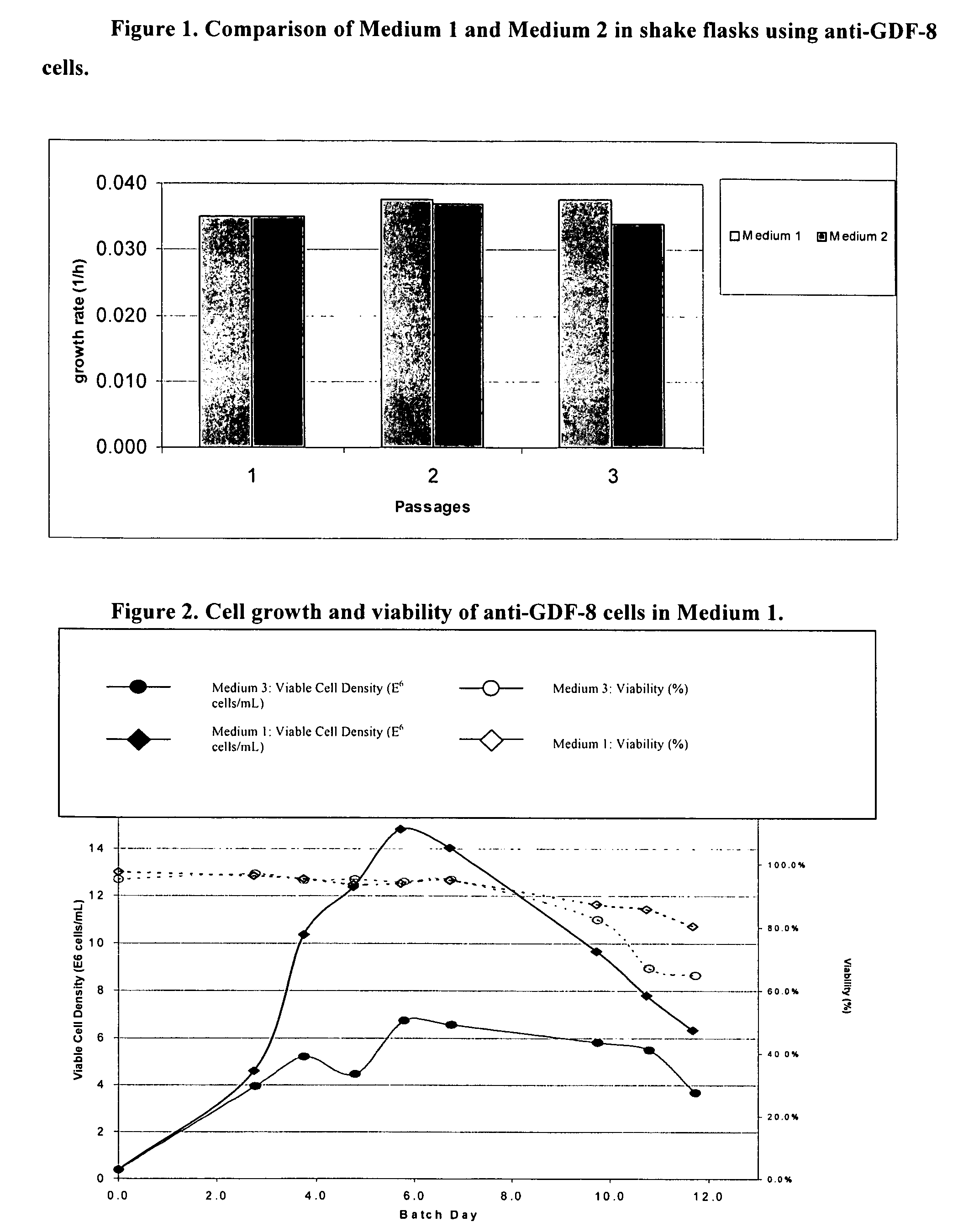
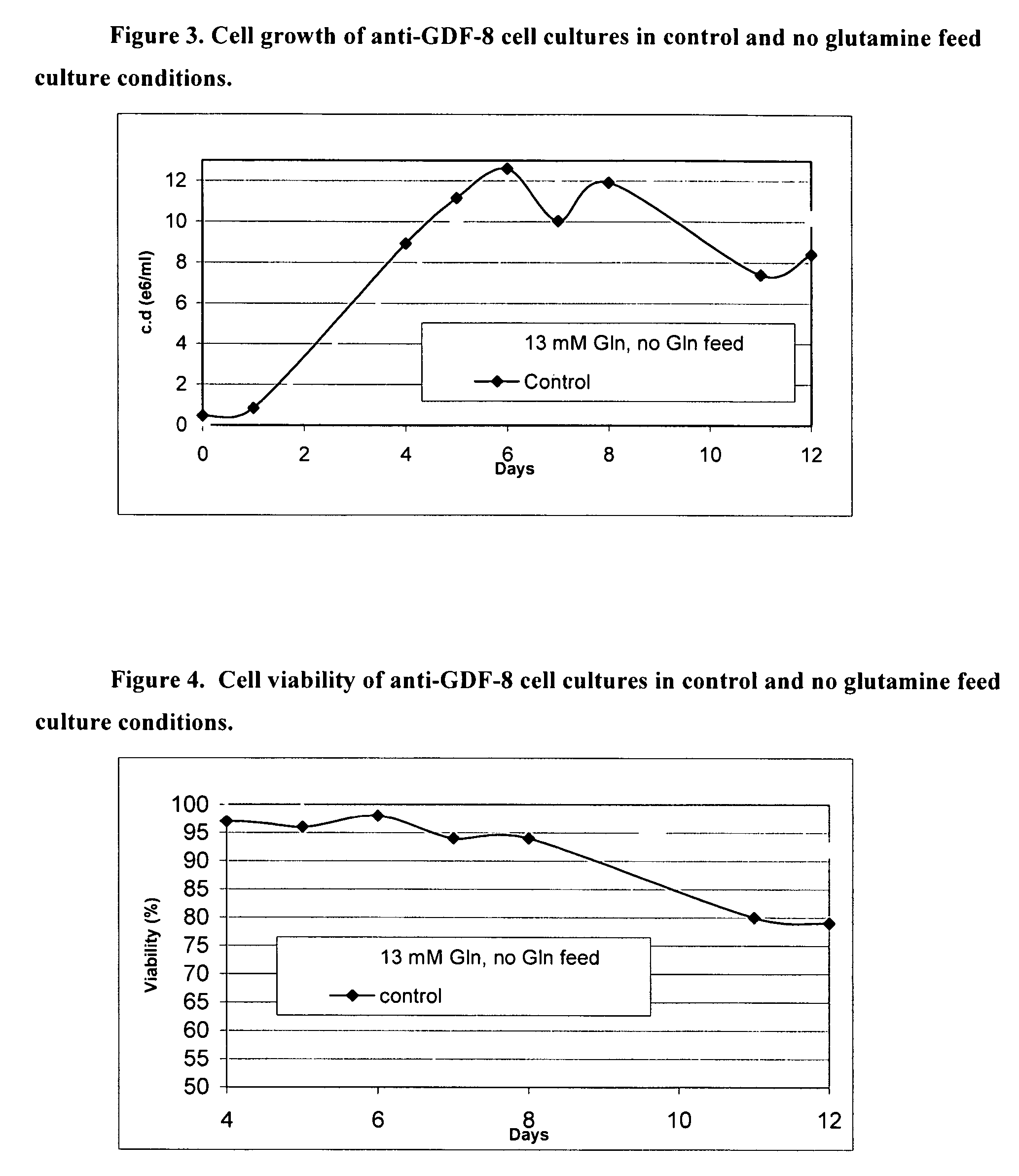
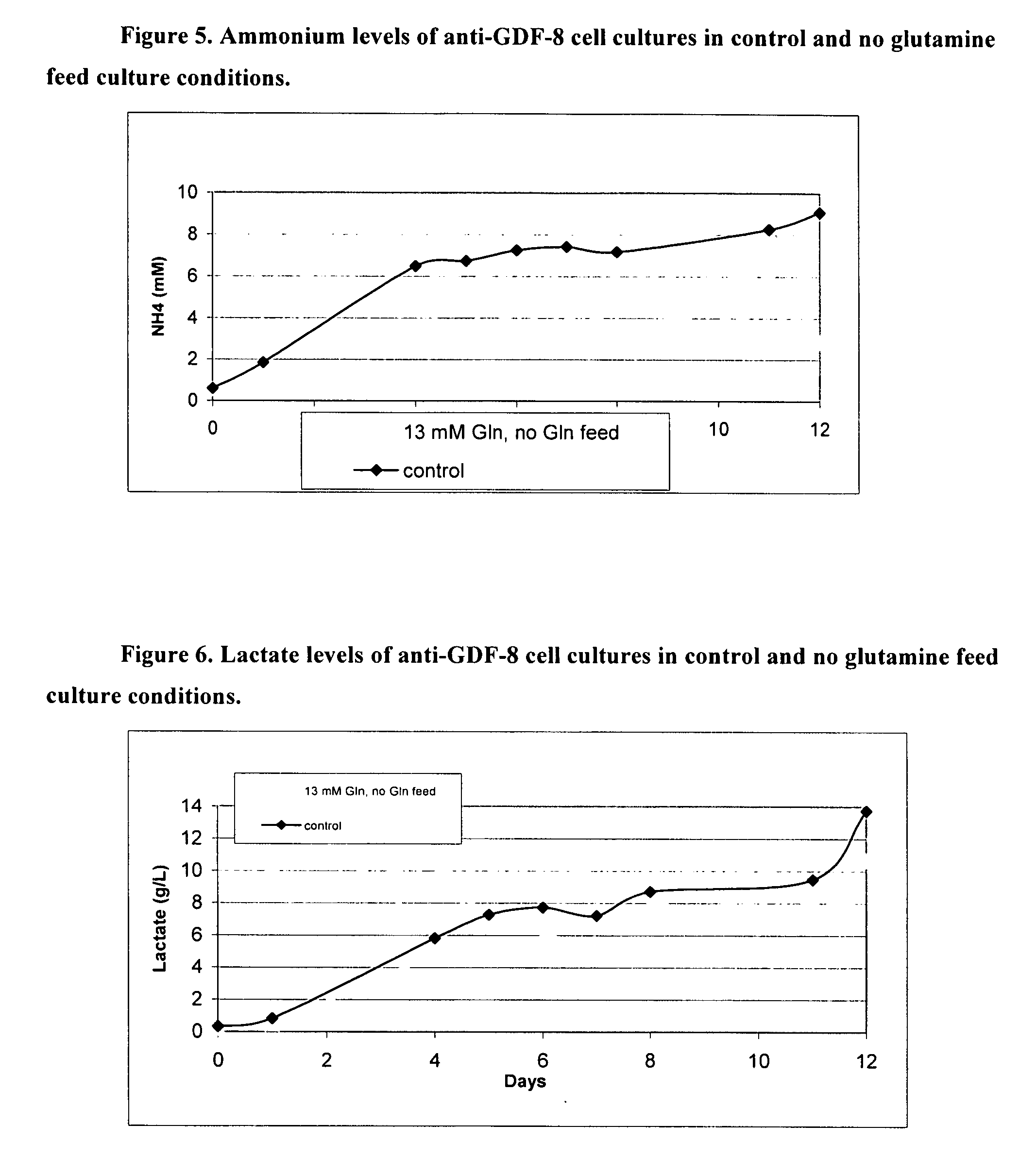
An improved system for large scale production of proteins and / or polypeptides in cell culture, particularly in media characterized by one or more of: i) a cumulative amino acid concentration greater than about 70 mM; ii) a molar cumulative glutamine to cumulative asparagine ratio of less than about 2; iii) a molar cumulative glutamine to cumulative total amino acid ratio of less than about 0.2; iv) a molar cumulative inorganic ion to cumulative total amino acid ratio between about 0.4 to 1; or v) a combined cumulative glutamine and cumulative asparagine concentration between about 16 and 36 mM, is provided. The use of such a system allows high levels of protein production and lessens accumulation of certain undesirable factors such as ammonium and / or lactate. Additionally, culture methods including a temperature shift, typically including a decrease in temperature when the culture has reached about 20-80% of it maximal cell density, are provided. Alternatively or additionally, the present invention provides methods such that, after reaching a peak, lactate and / or ammonium levels in the culture decrease over time.
Owner:PFIZER IRELAND PHARM CORP
Soluble hyaluronidase glycoprotein (sHASEGP), process for preparing the same, uses and pharmaceutical compositions comprising thereof
ActiveUS20090214505A1Extended half-lifeOptimize allocationAntibacterial agentsOrganic active ingredientsHyaluronidasePathology diagnosis
Provided are soluble neutral active Hyaluronidase Glycoproteins (sHASEGP's), methods of manufacture, and their use to facilitate administration of other molecules or to alleviate glycosaminoglycan associated pathologies. Minimally active polypeptide domains of the soluble, neutral active sHASEGP domains are described that include asparagine-linked sugar moieties required for a functional neutral active hyaluronidase domain. Included are modified amino-terminal leader peptides that enhance secretion of sHASEGP. Sialated and pegylated forms of the sHASEGPs also are provided. Methods of treatment by administering sHASEGPs and modified forms thereof also are provided.
Owner:HALOZYME
Transgenic plants that exhibit enhanced nitrogen assimilation
InactiveUS20020069430A1Improve growth characteristicsImproved vegetativeClimate change adaptationOther foreign material introduction processesPlant cellThreonine
Transgenic plants containing free amino acids, particularly at least one amino acid selected from among glutamic acid, asparagine, aspartic acid, serine, threonine, alanine and histidine accumulated in a large amount, in edible parts thereof, and a method of producing them are provided. In this method, glutamate dehydrogenase (GDH) gene is introduced into a plant together with a regulator sequence suitable for over expressing the sequence encoding GDH gene in plant cells.
Owner:AJINOMOTO CO INC
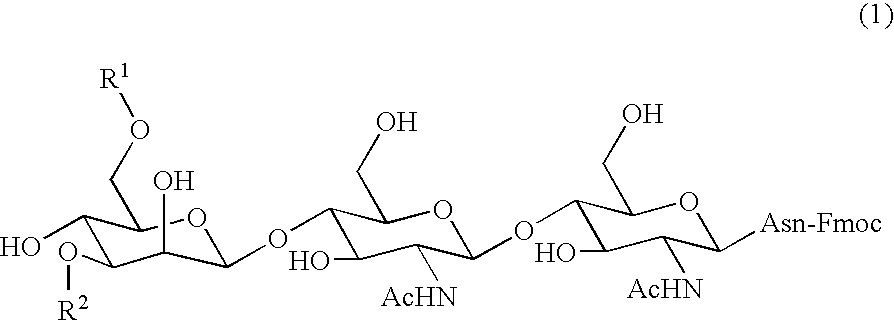
Process for producing sugar chain asparagine derivative
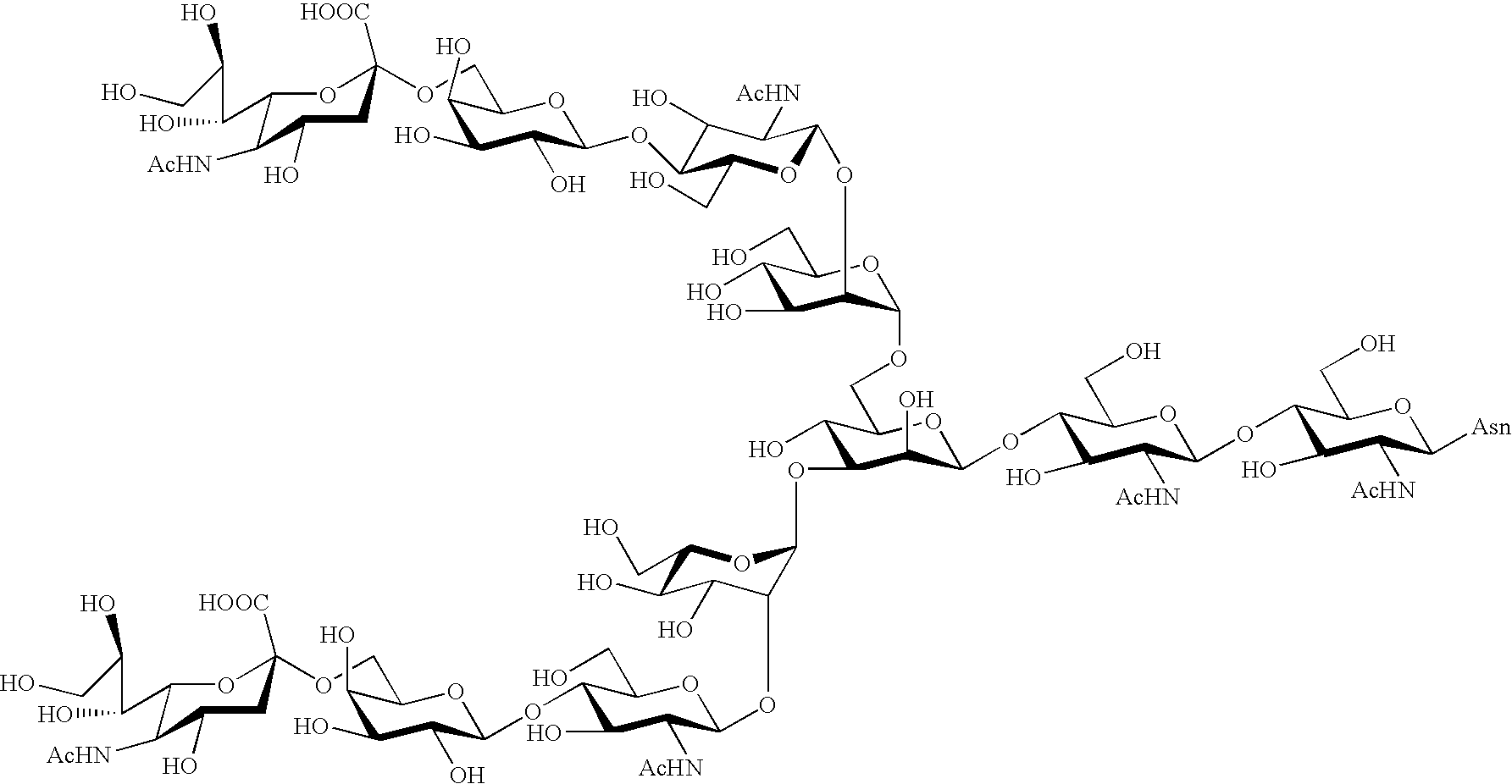
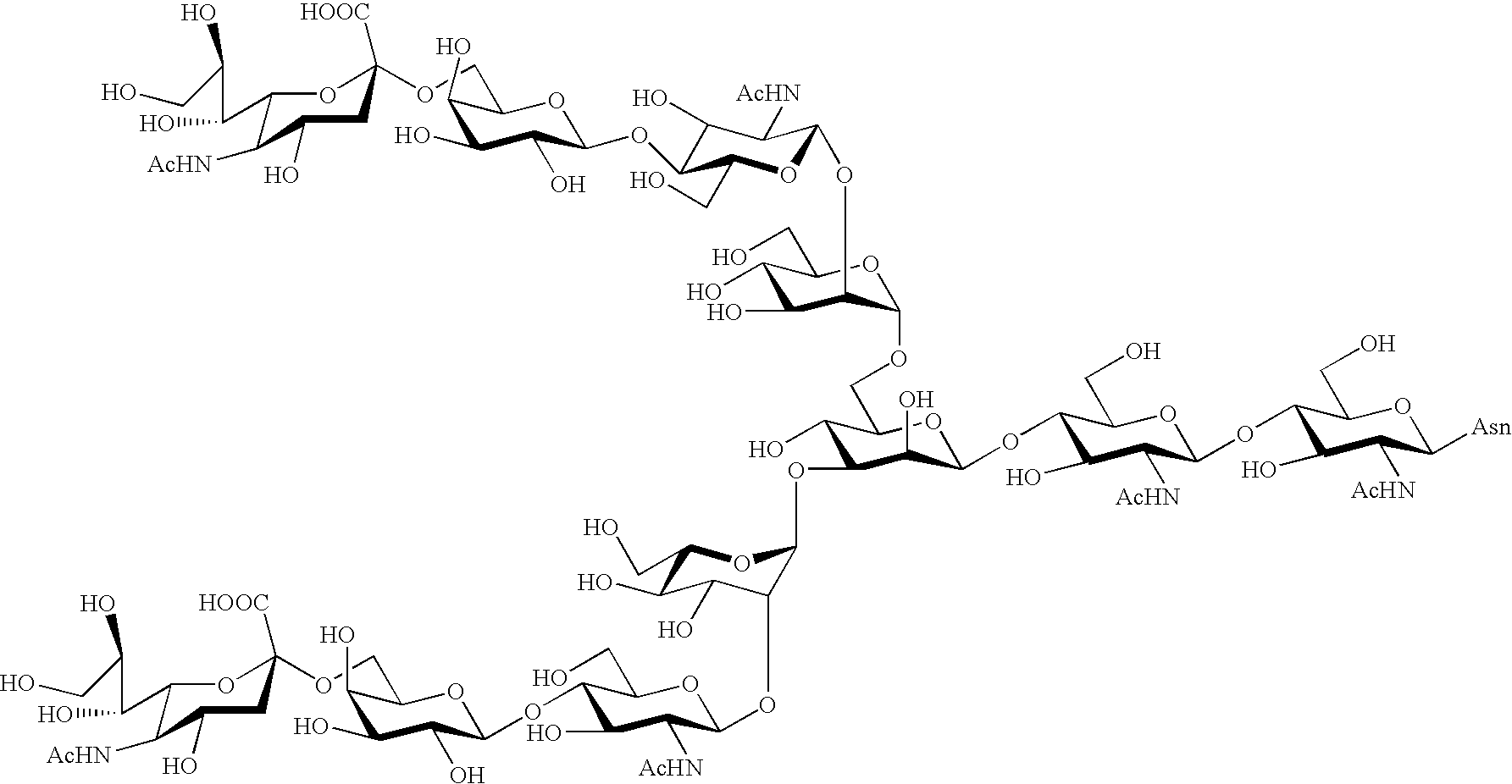
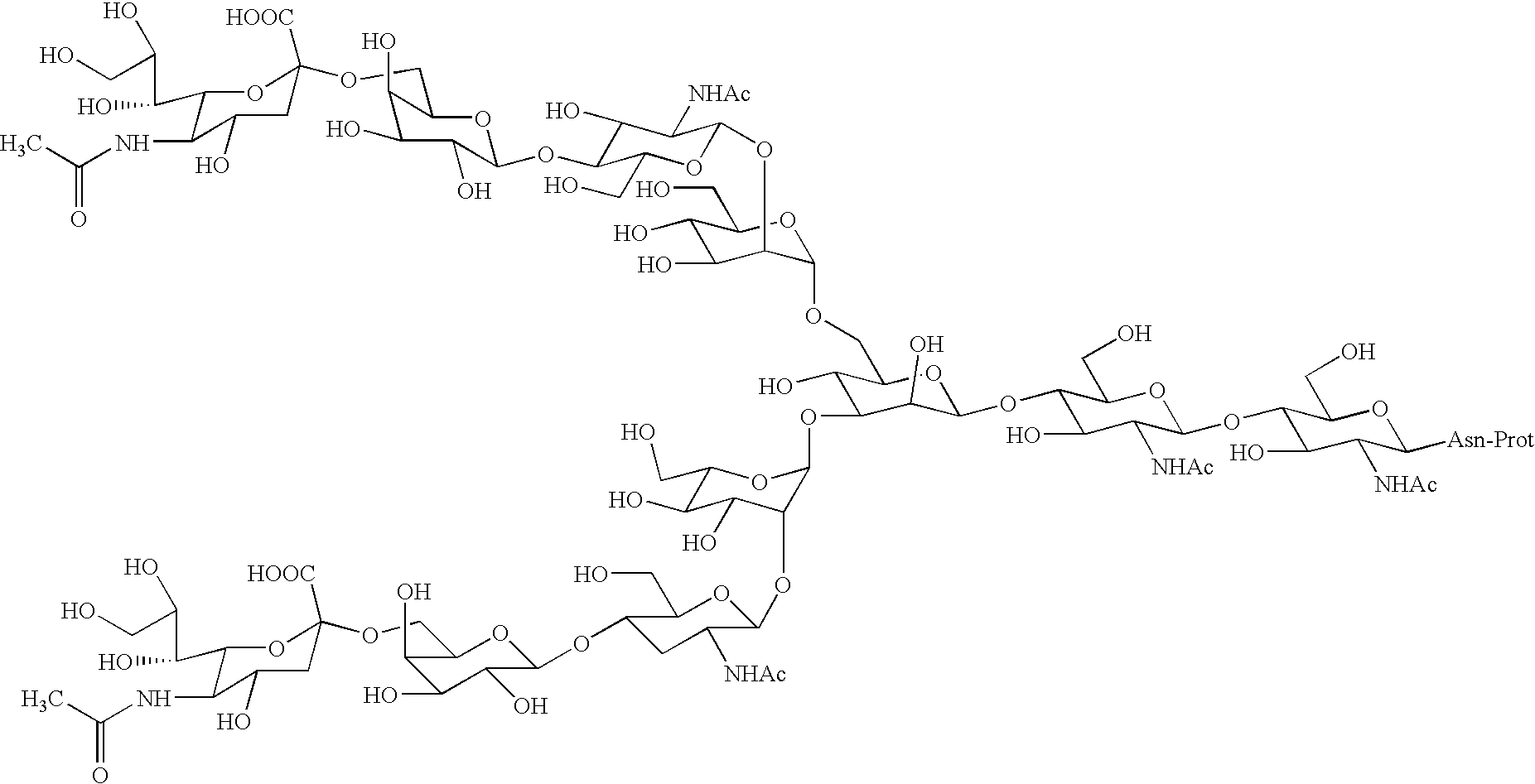
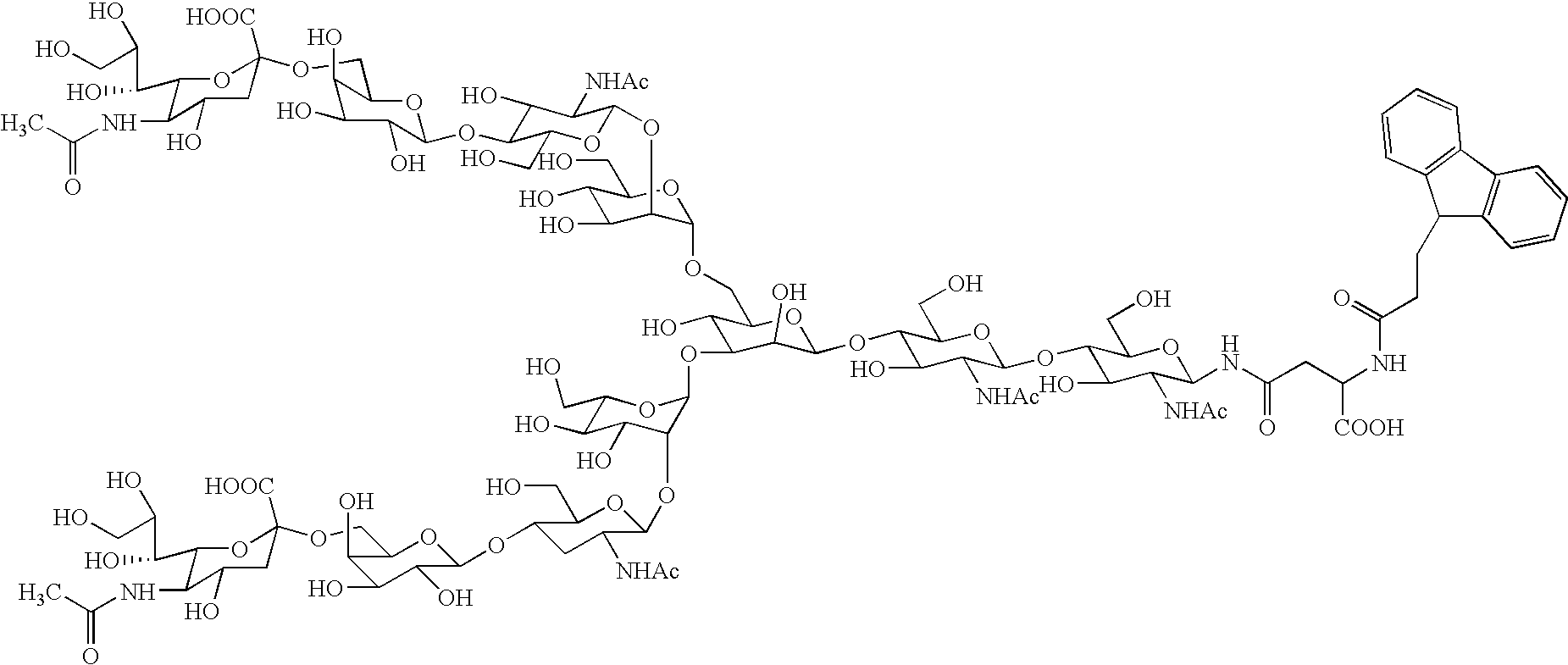
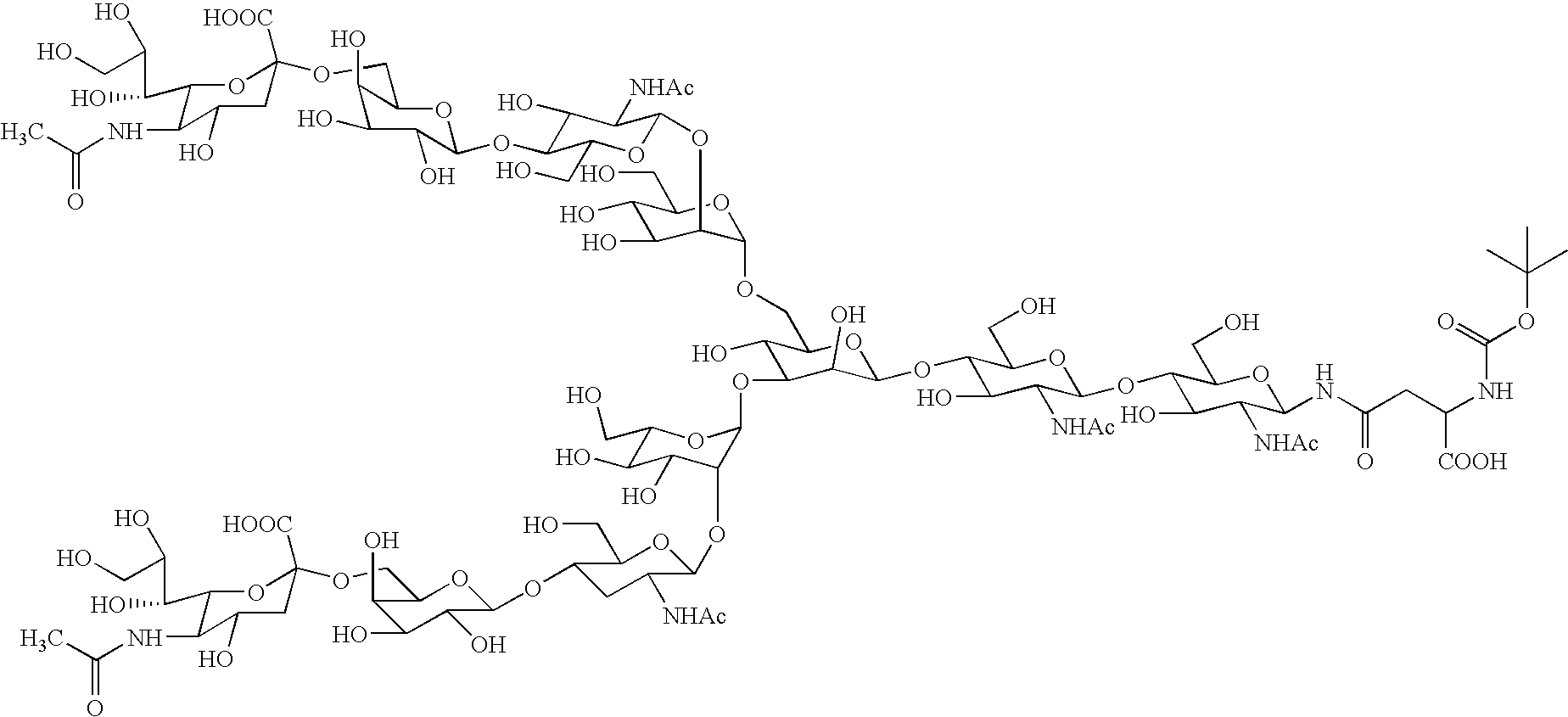
InactiveUS20040181054A1Conveniently obtainEsterified saccharide compoundsSugar derivativesSugarMedicinal chemistry
The present invention provides a process for preparing a sugar chain asparagine derivative. According to the process, various isolated sugar chain asparagine derivatives useful in the field of the development of pharmaceuticals and the like can be conveniently obtained in a large amount as compared to that of the prior art. The present invention also provides a process for preparing a sugar chain asparagine and a process for preparing a sugar chain via a step for preparing a sugar chain asparagine derivative. The present invention further provides a novel sugar chain asparagine derivative, a sugar chain asparagine and a sugar chain.
Owner:YASUHIRO KAJIHARA +1
Soluble hyaluronidase glycoprotein (sHASEGP), process for preparing the same, uses and pharmaceutical compositions comprising thereof
ActiveUS20090181013A1Reduce deliveryImprove usabilityAntibacterial agentsOrganic active ingredientsHyaluronidasePathology diagnosis
The invention relates to the discovery of novel soluble neutral active Hyaluronidase Glycoproteins (sHASEGP's), methods of manufacture, and their use to facilitate administration of other molecules or to alleviate glycosaminoglycan associated pathologies. Minimally active polypeptide domains of the soluble, neutral active sHASEGP domains are described that include asparagine-linked sugar moieties required for a functional neutral active hyaluronidase domain. Included are modified amino-terminal leader peptides that enhance secretion of sHASEGP. The invention further comprises sialated and pegylated forms of a recombinant sHASEGP to enhance stability and serum pharmacokinetics over naturally occurring slaughterhouse enzymes. Further described are suitable formulations of a substantially purified recombinant sHASEGP glycoprotein derived from a eukaryotic cell that generate the proper glycosylation required for its optimal activity.
Owner:HALOZYME
Production of anti-abeta
InactiveUS7335491B2Antibody mimetics/scaffoldsGenetically modified cellsBiotechnologyTotal amino acids
Owner:PFIZER IRELAND PHARM CORP
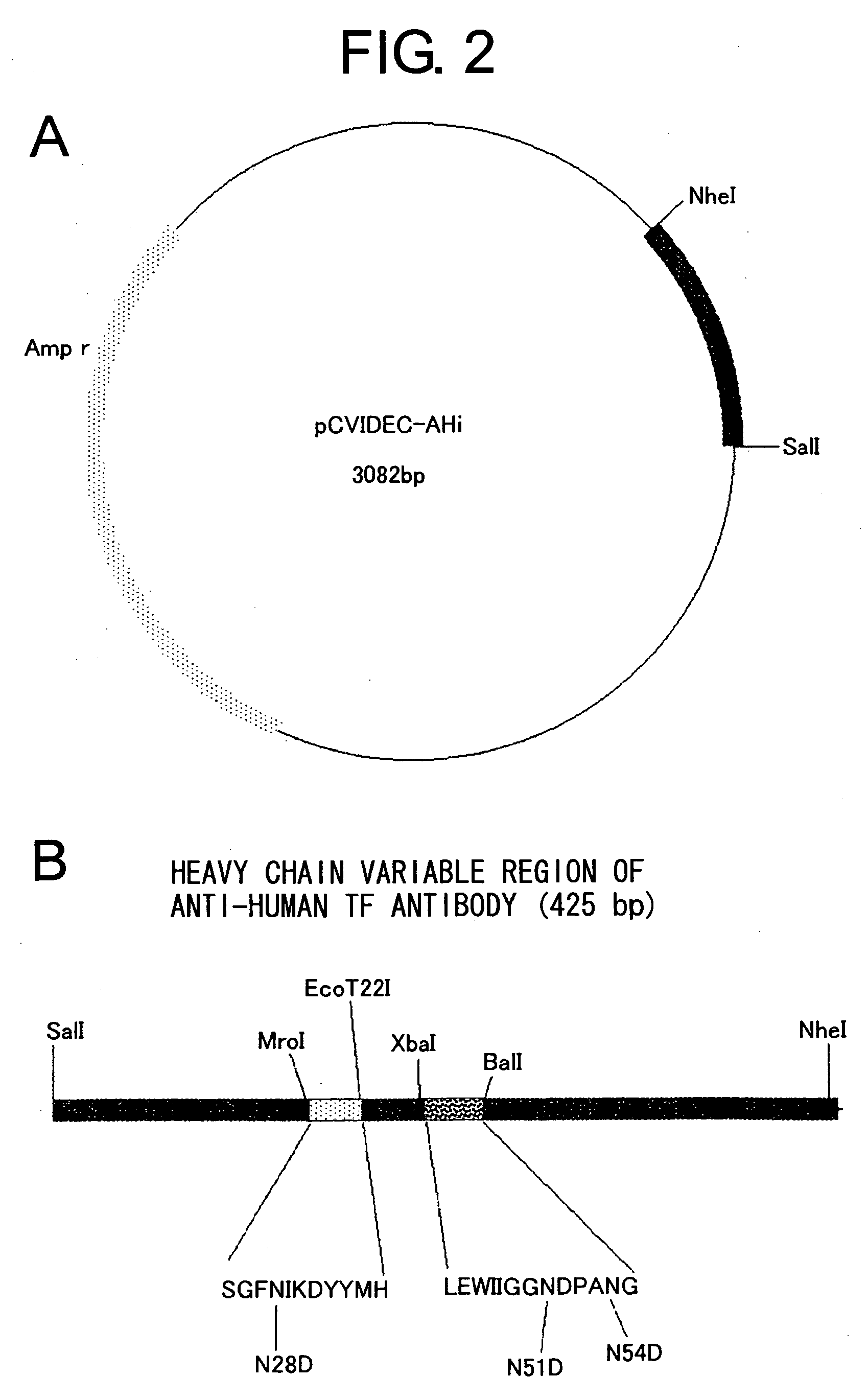
Humanized anti-CD22 antibodies and their use in treatment of oncology, transplantation and autoimmune disease
InactiveUS7829086B2Sugar derivativesImmunoglobulins against animals/humansAntigenImmunoglobulin Heavy Chain Variable Region
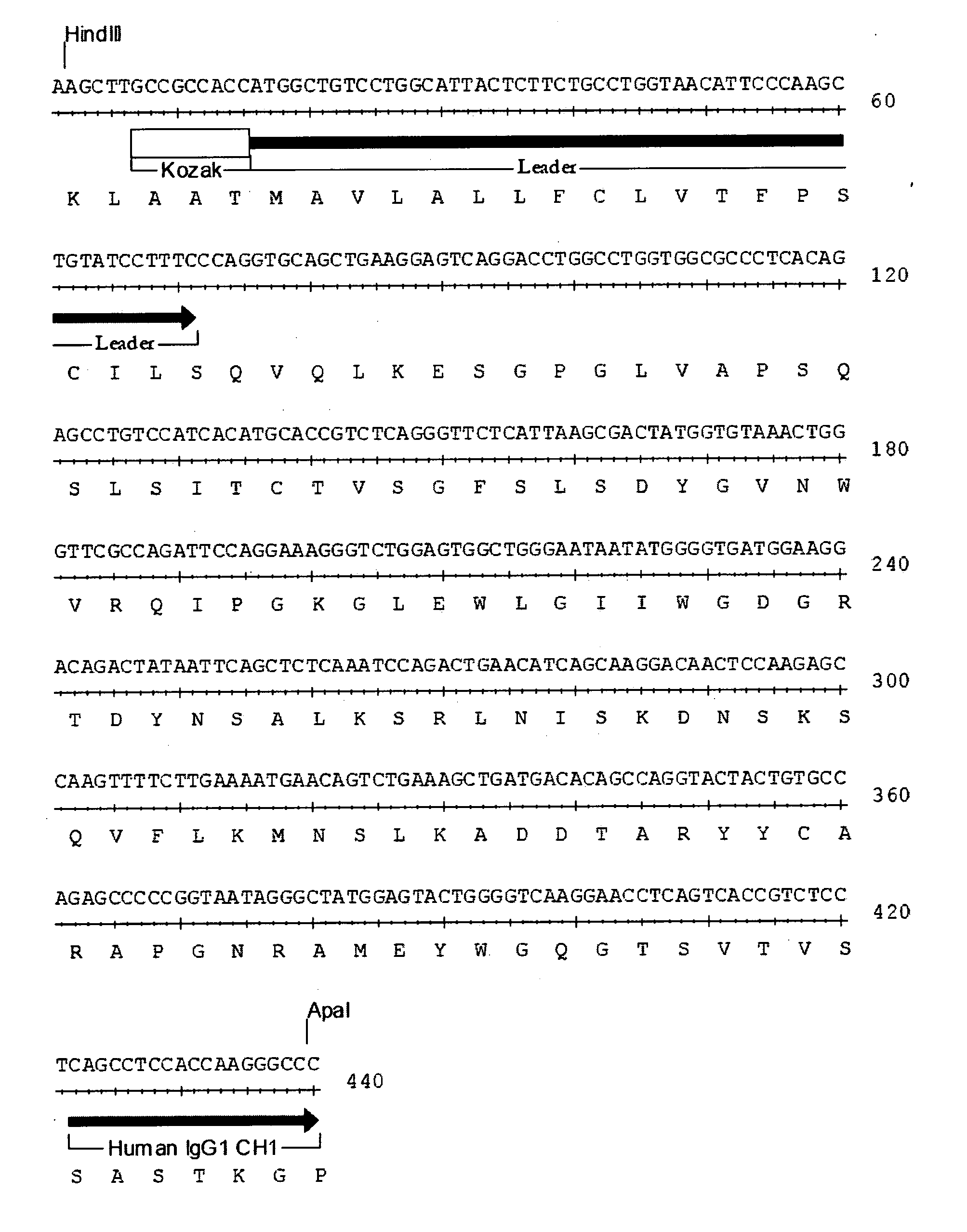
The present invention provides chimeric and humanized versions of anti-CD22 mouse monoclonal antibody, HB22.7. The anti-CD22 antibodies of the invention comprise four human or humanized framework regions of the immunoglobulin heavy chain variable region (“VH”) and four human or humanized framework regions of the immunoglobulin light chain variable region (“VK”). The invention further comprises heavy and / or light chain FW regions that contain one or more backmutations in which a human FW residue is exchanged for the corresponding residue present in the parental mouse heavy or light chain. Human or humanized VH framework regions of antibodies of the invention may comprise one or more of the following residues: a valine (V) at position 24 of framework region 1, a glycine (G) at position 49 of framework region 2, and an asparagine (N) at position 73 of framework region 3, numbered according to Kabat. The invention further relates to pharmaceutical compositions, immunotherapeutic compositions, and methods using therapeutic antibodies that bind to the human CD22 antigen and that preferably mediate human ADCC, CDC, and / or apoptosis for: the treatment of B cell diseases and disorders in human subjects, such as, but not limited to, B cell malignancies, for the treatment and prevention of autoimmune disease, and for the treatment and prevention of graft-versus-host disease (GVHD), humoral rejection, and post-transplantation lymphoproliferative disorder in human transplant recipients.
Owner:MEDIMMUNE LLC +1
Human chondroitinase glycoprotein (CHASEGP), process for preparing the same, and pharmaceutical compositions comprising thereof
InactiveUS7544499B2BacteriaSugar derivativesChondroitinase activityChondroitin Sulfate Proteoglycans
Owner:HALOZYME
Protein ligands
ActiveUS7709209B2Retention characteristicReduce leakageImmunoglobulins against animals/humansBiological testingComplementarity determining regionChemical stability
Owner:CYTIVA BIOPROCESS R&D AB
Humanized anti-CD22 antibodies and their use in treatment of oncology, transplantation and autoimmune disease
InactiveUS20070258981A1Efficient productionEfficiently depletedSugar derivativesImmunoglobulins against animals/humansAntigenAutoimmune condition
Owner:MEDIMMUNE LLC +1
Process for producing sugar chain asparagine derivative
ActiveUS20060205039A1Low costEfficiently and in large amountFermentationBulk chemical productionYolkPerylene derivatives
A process for preparing asparagine-linked oligosaccharide derivatives including the steps of: (a) treating a delipidated egg yolk with a protease to obtain a mixture of peptide-linked oligosaccharides, (b) treating the mixture of peptide-linked oligosaccharides with a peptidase to obtain a mixture of asparagine-linked oligosaccharides, (c) introducing a lipophilic protective group into the asparagine-linked oligosaccharides in the mixture to obtain a mixture of asparagine-linked oligosaccharide derivatives, and (d) subjecting the mixture of asparagine-linked oligosaccharide derivatives to chromatography to separate the mixture into individual asparagine-linked oligosaccharide derivatives.
Owner:GLYTECH
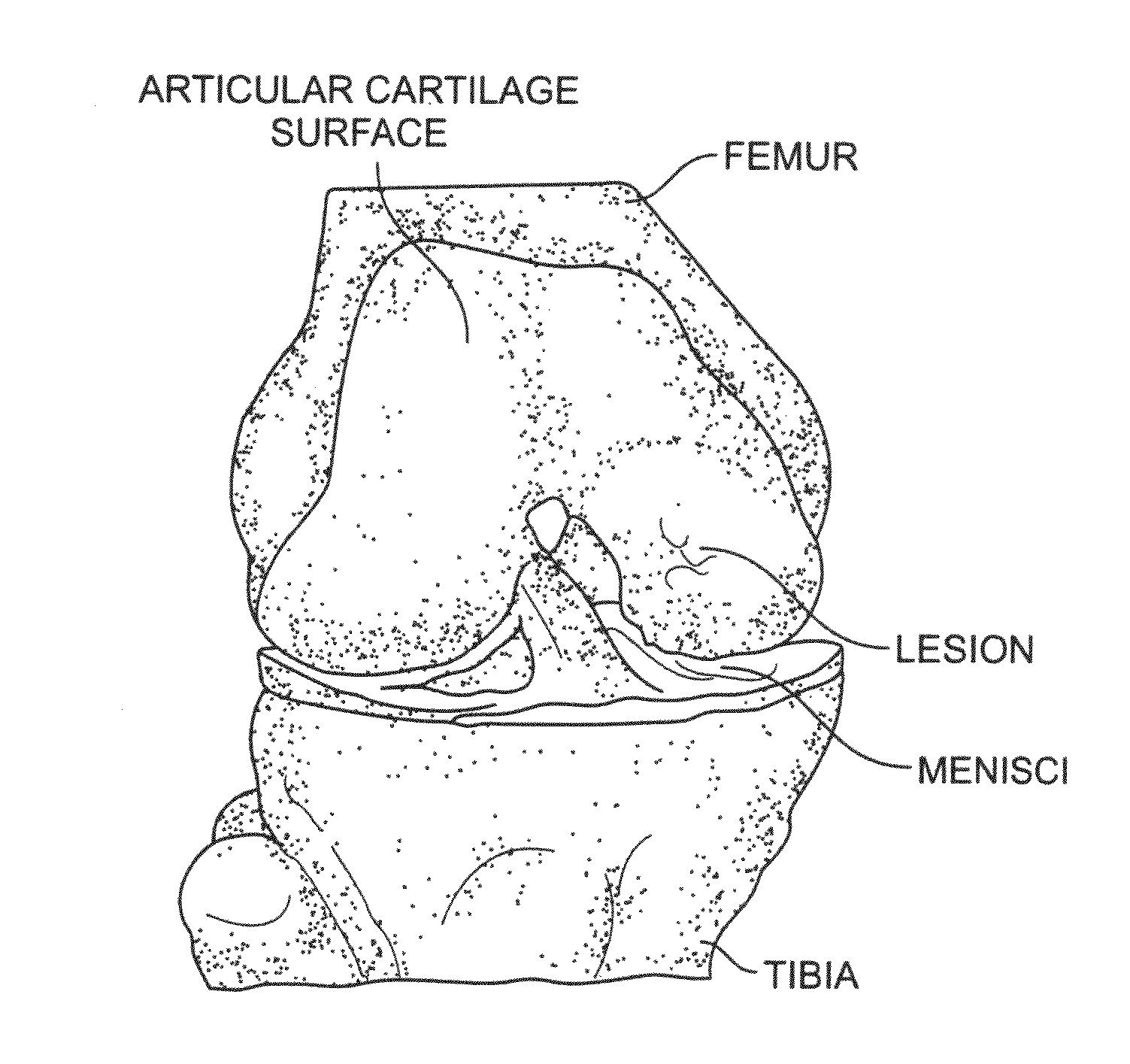
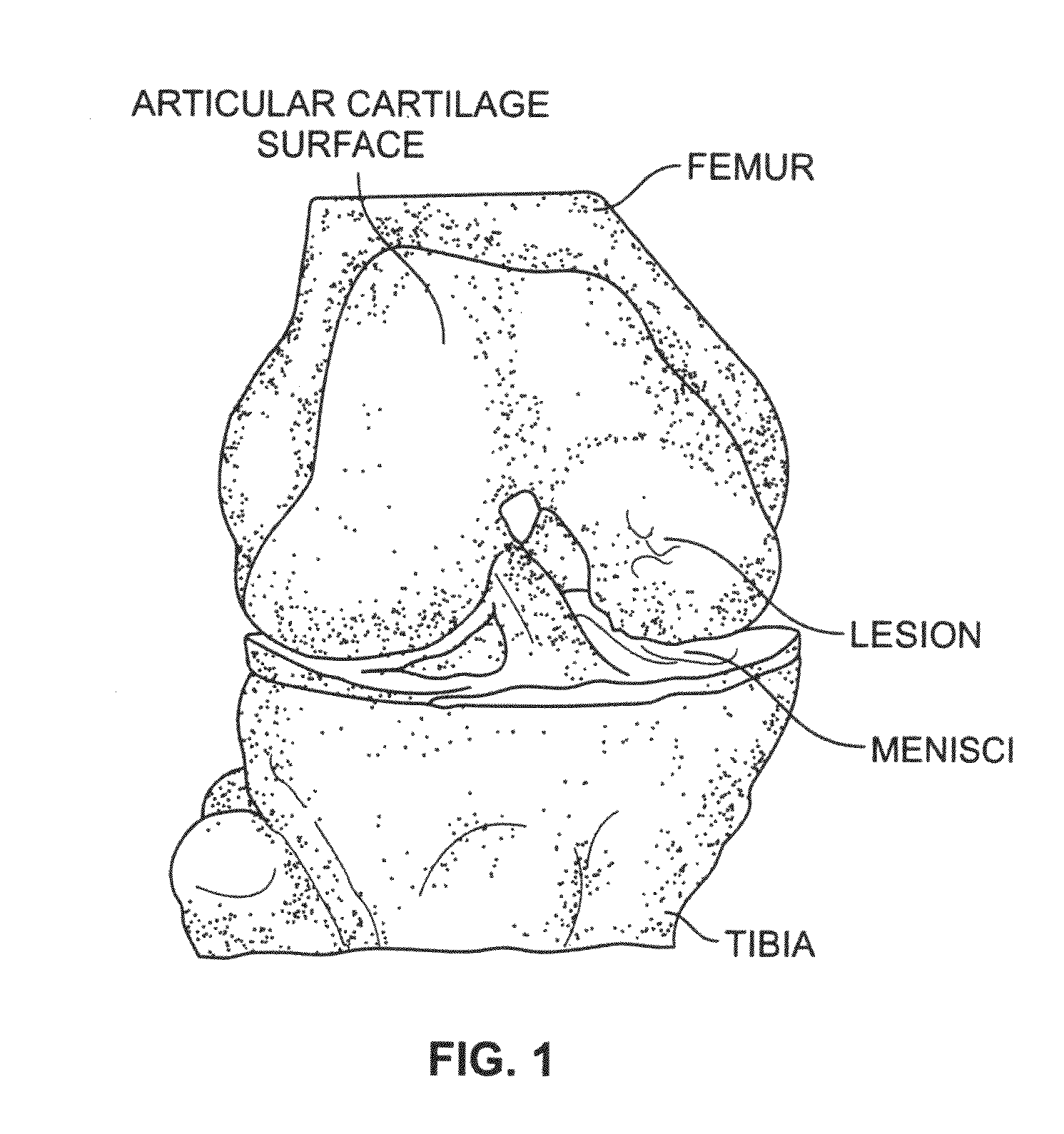
Cartilage particle tissue mixtures optionally combined with a cancellous construct
Mixtures, such as gels or pastes, comprising freeze-milled cartilage particles and exogenous growth factors are used for repairing chondral defects. Such mixtures may be applied to constructs comprising cancellous bone for implantation at the defect site. Suitable growth factors include variants of FGF-2, particularly variants that include a sole amino acid substitution for asparagine at amino acid 111 of the β8-β9 loop of the FGF-2 peptide. Such FGF-2 variants are released slowly and continuously at a constant rate from cartilage pastes. In other embodiments, the amino acid substituted for asparigine is glycine. Other variants that may be used include FGF-9 variants having truncated chains and a sole amino acid substitution in the β8-β9 loop of the FGF-9 peptide either for tryptophan at amino acid 144 or for asparagine at amino acid 143.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC +1
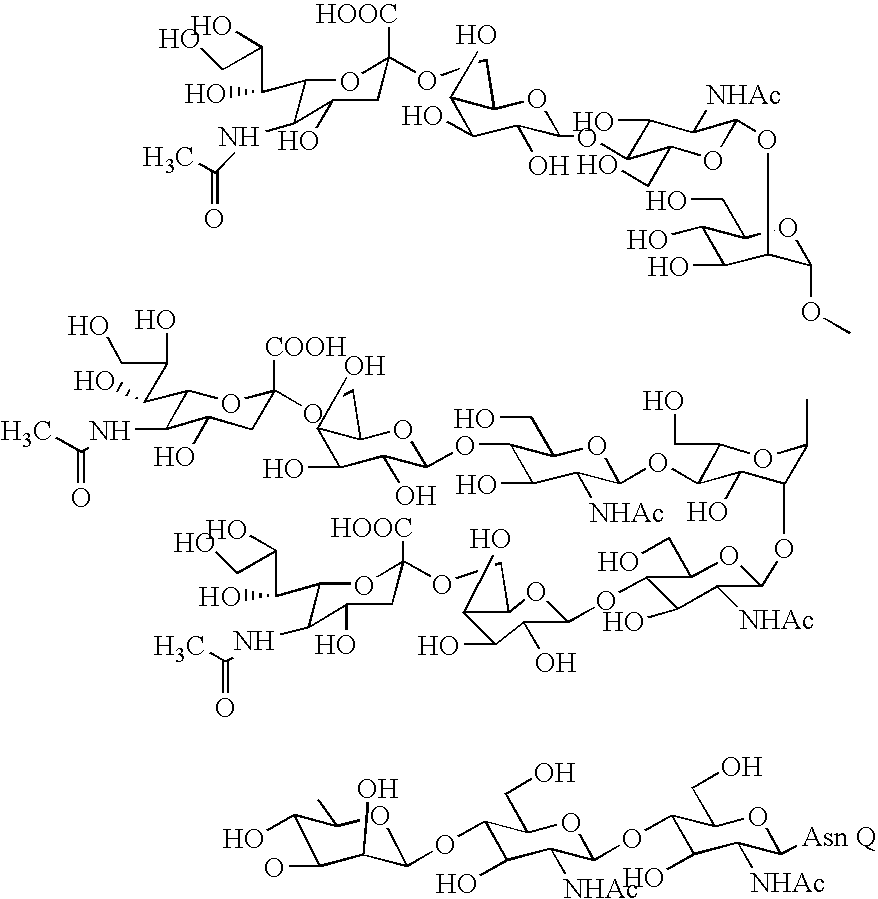
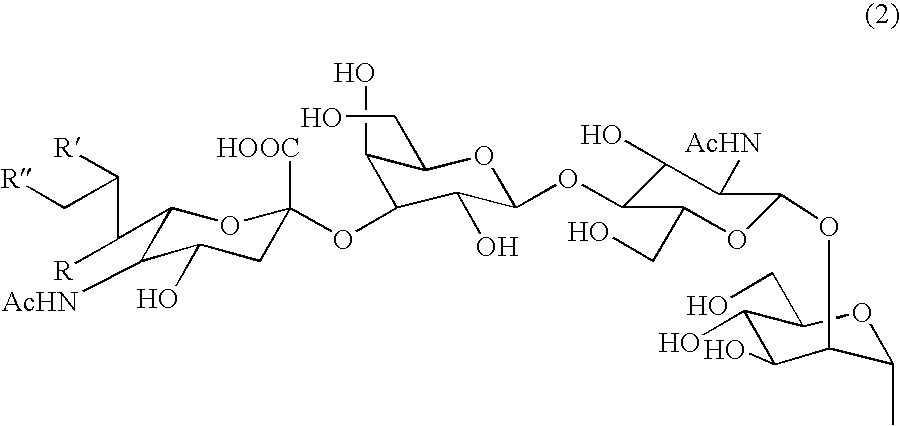
Three-branched sugar-chain asparagine derivatives, the sugar-chain asparagines, the sugar chains, and processes for producing these
A 3-branched asparagine-linked oligosaccharide derivative of the formula (1) wherein the nitrogen of amino group of asparagine is modified with a lipophilic protective group, biotin group or FITC group; a 3-branched asparagine-linked oligosaccharide derivative which contains at least one fucose in N-acetylglucosamine on the nonreducing terminal side of the asparagine-linked oligosaccharide of the derivative; asparagine-linked oligosaccharides and oligosaccharides thereof wherein Q is a lipophilic protective group, biotin group or FITC group.
Owner:GLYTECH
Affinity chromatography matrix
ActiveUS20130274451A1Increased elution pHGuaranteed cost-effective operationSolid sorbent liquid separationPeptide preparation methodsElutionA domain
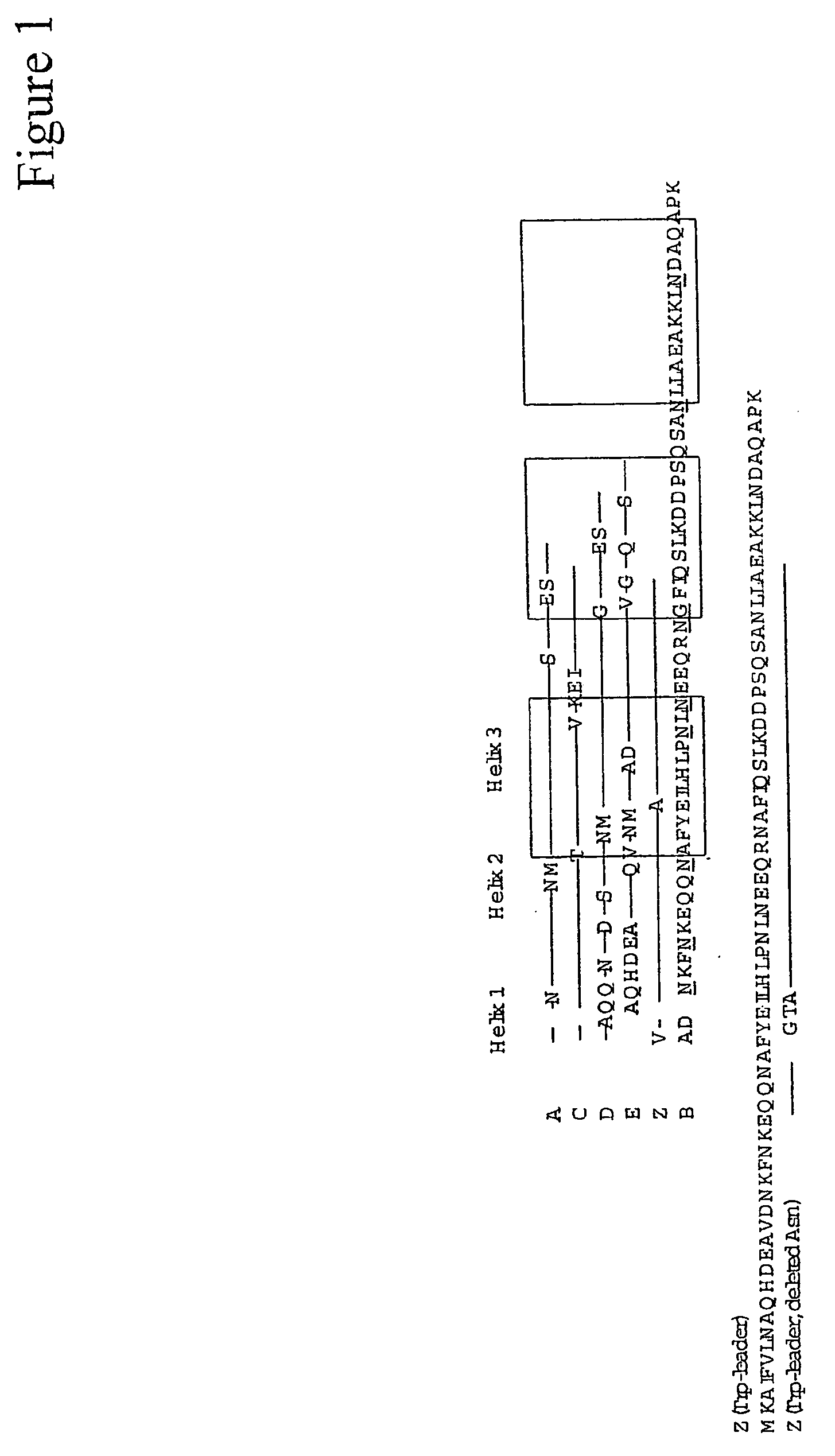
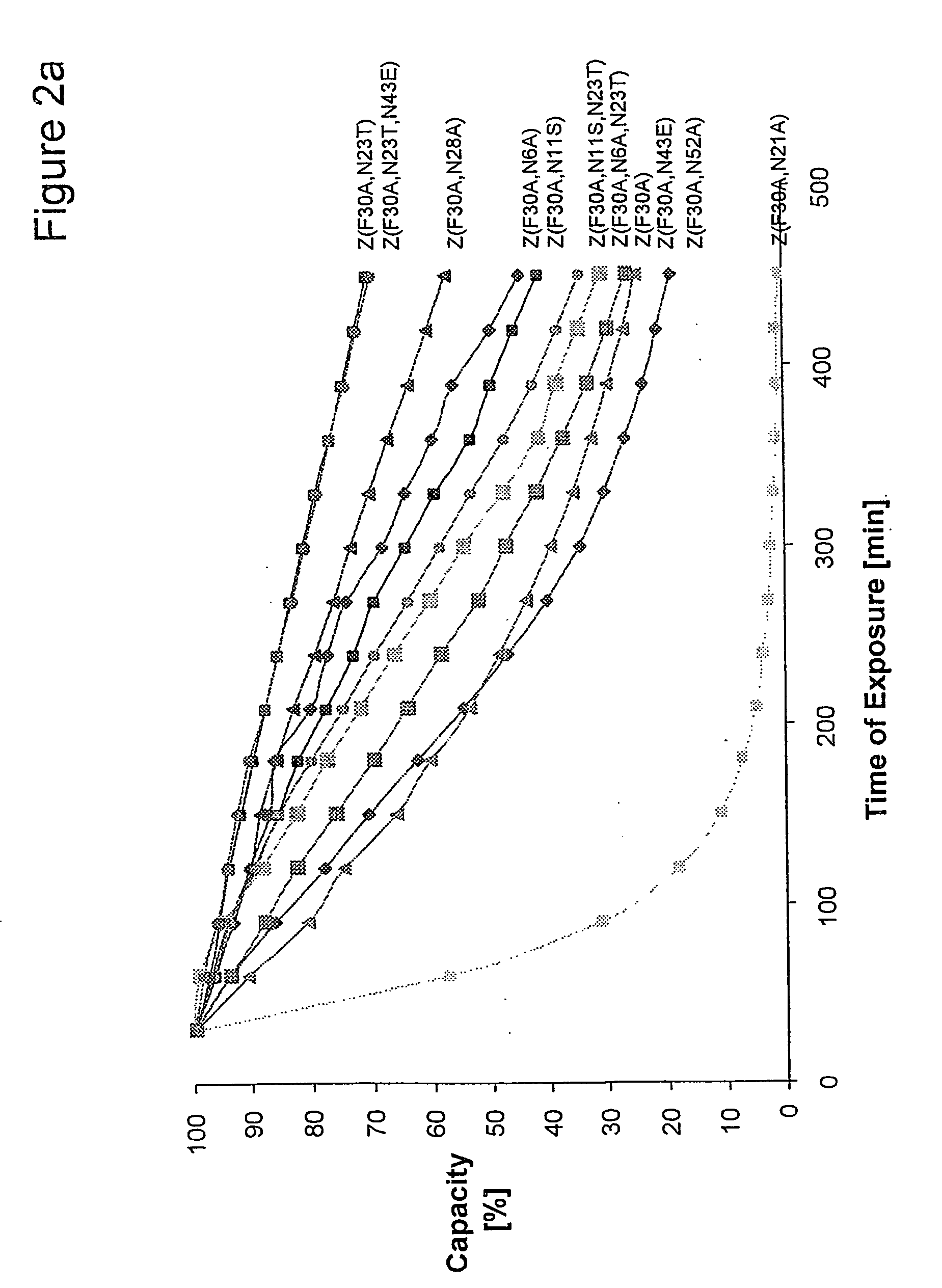
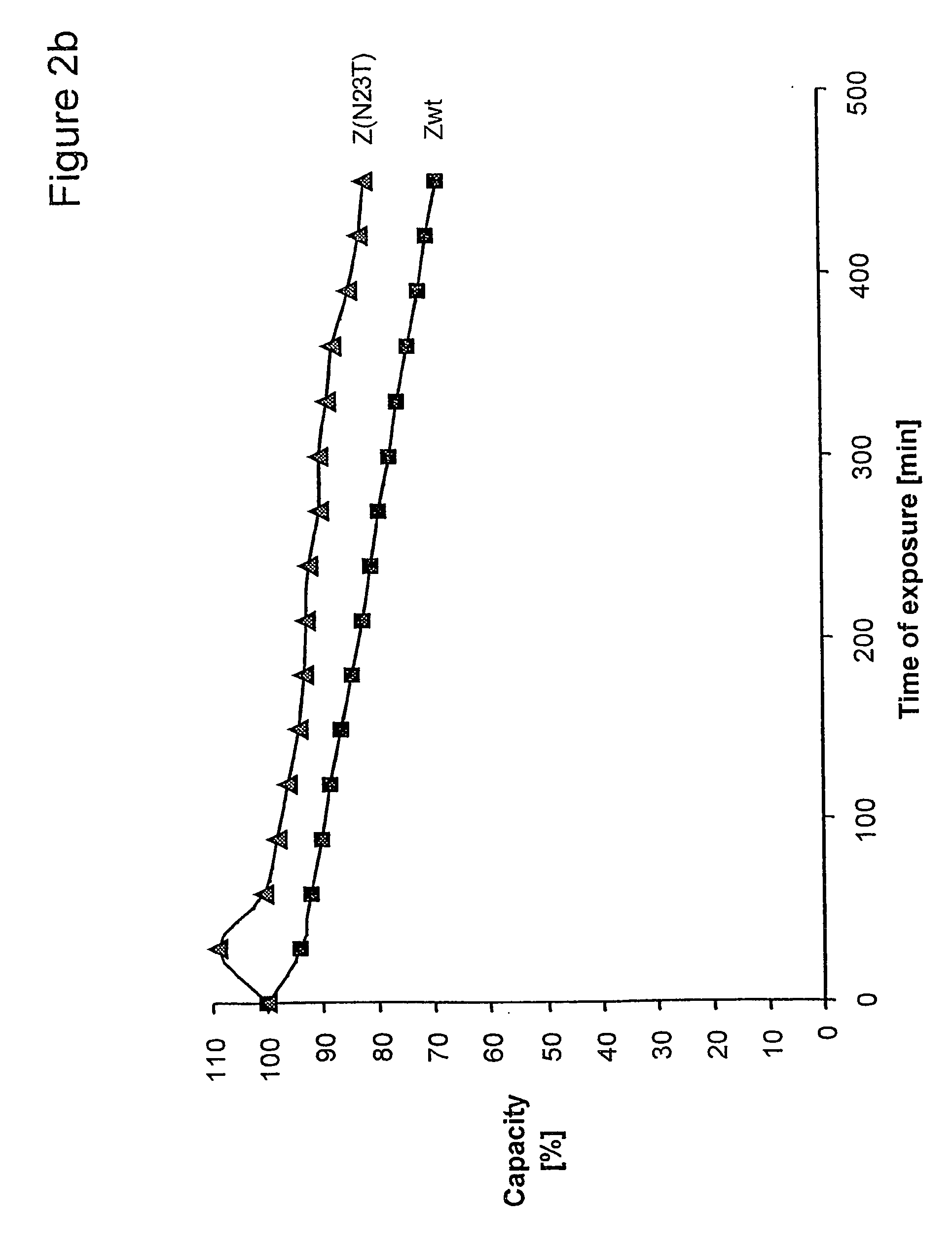
The present invention relates to a method of separating one or more immunoglobulin containing proteins from a liquid. The method includes first contacting the liquid with a separation matrix comprising ligands immobilised to a support; allowing the immunoglobulin containing proteins to adsorb to the matrix by interaction with the ligands; followed by an optional step of washing the matrix containing the immunoglobulin containing proteins adsorbed thereon; and recovering said immunoglobulin containing proteins by contacting the matrix with an eluent which releases the proteins. The method improves upon previous separation methods in that each of the ligands comprises one or more of a protein A domain (E, D, A, B, C), or protein Z, or a functional variant thereof, with at least one of the monomers having a substitution of the Asparagine at the position corresponding to N28 of B domain of Protein A or Protein Z, and wherein the ligand provides an increase in elution pH compared to non-substituted ligand.
Owner:CYTIVA BIOPROCESS R&D AB
Thermal treatment process for tobacco materials
A method of thermally processing a tobacco material is provided, the method including the steps of (i) mixing a tobacco material, water, and an additive selected from the group consisting of lysine, glycine, histidine, alanine, methionine, glutamic acid, aspartic acid, proline, phenylalanine, valine, arginine, di- and trivalent cations, asparaginase, saccharides, phenolic compounds, reducing agents, compounds having a free thiol group, oxidizing agents, oxidation catalysts, plant extracts, and combinations thereof, to form a moist tobacco mixture; (ii) heating the moist tobacco mixture at a temperature of at least about 60° C. to form a heat-treated tobacco mixture; and (iii) incorporating the heat-treated tobacco mixture into a tobacco product. Heat-treated tobacco composition prepared according to the method are also provided, such as heat-treated smokeless tobacco composition comprising a tobacco material, water, flavorant, binder, and filler, the heat-treated smokeless tobacco composition having an acrylamide content of less than about 2000 ppb.
Owner:R J REYNOLDS TOBACCO COMPANY
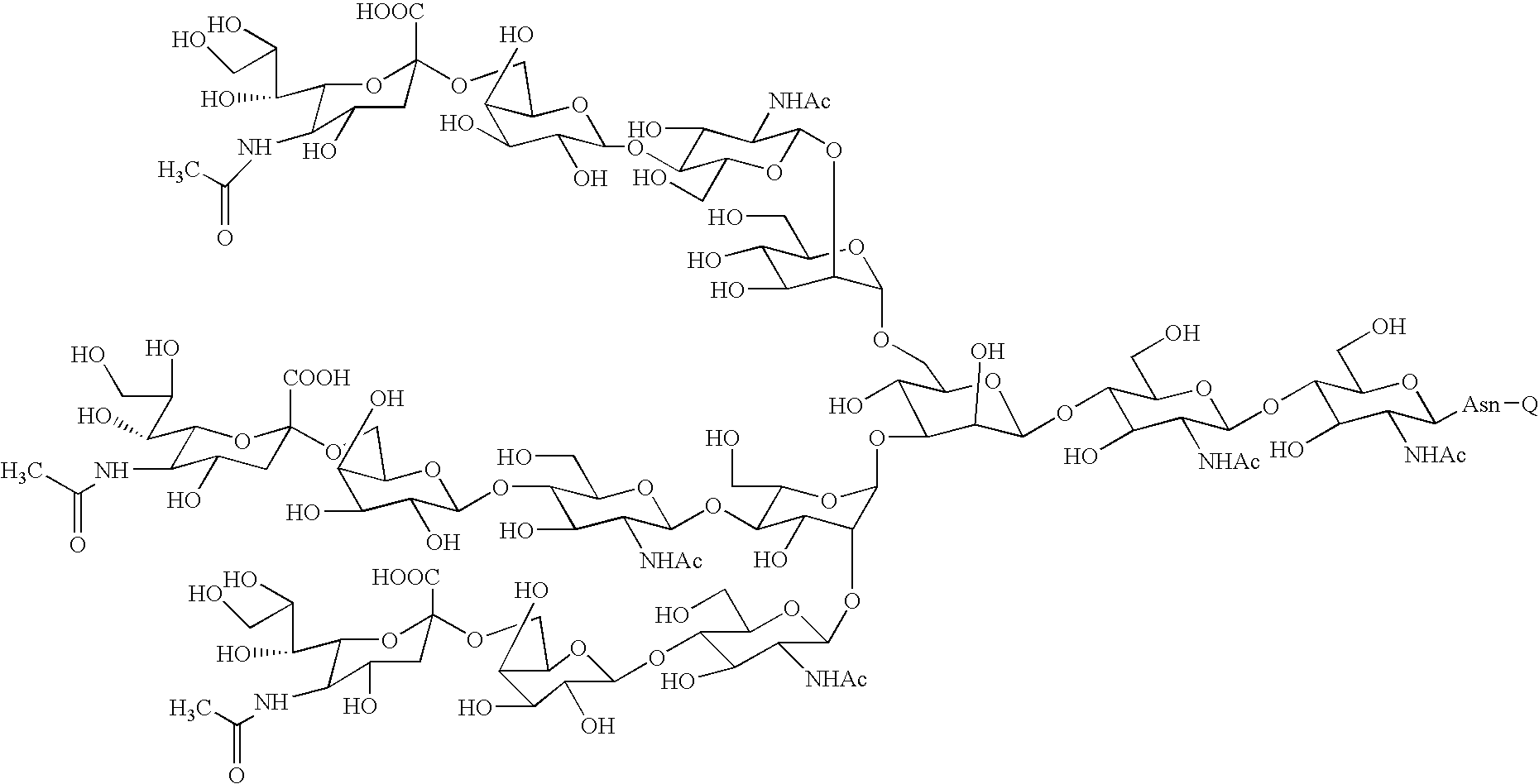
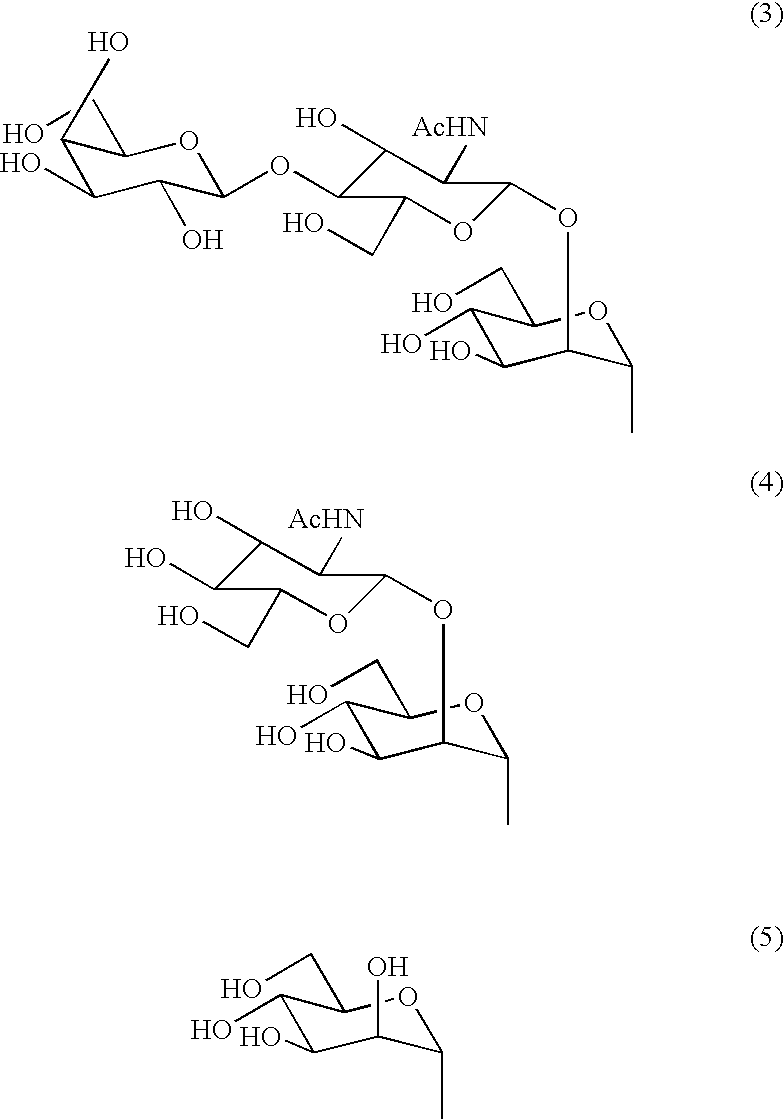
Sugar chain asparagine derivatives, sugar chain asparagine, sugar chain, and processes for producing these
ActiveUS20060228784A1Easy to prepareEfficiently and in large amountOrganic active ingredientsBiocideN-AcetylglucosamineSugar
An asparagine-linked α2,3-oligosaccharide having undeca- to hepta-saccharides, an asparagine-linked α2,6-oligosaccharide having undeca- to hepta-saccharides and containing fluorine and an asparagine-linked oligosaccharide derivative containing at least one fucose in N-acetylglucosamine on the nonreducing terminal side of an asparagine-linked oligosaccharide wherein the asparagine has amino group protected with a lipophilic protective group, and a process for producing these compounds.
Owner:GLYTECH
Detergent composition containing amino acid component for washing fruits, vegetables and dishes
InactiveCN102492571APromote degradationMaintain stain releaseNon-ionic surface-active compoundsOrganic detergent compounding agentsAntioxidantArginine
The present invention relates to a detergent composition containing an amino acid component for washing fruits, vegetables and dishes, which comprises the following components of an anionic surfactant, a non-ionic surfactant, an amphoteric surfactant, amino acid, a viscosity modifier, a chelating agent, an antiseptic, a light stabilizer, an antioxidant, a flavor and deionized water. The amino acid composition is one or a mixture with more than two of alanine, arginine, asparagine, aspartic acid, cystine, glutamine, glutamic acid, leucine, lysine, methionine, phenylalanine, prolinol acid, serine, threonine, glycine, histidine, isoleucine, tryptophan, tyrosine and valine. The detergent of the invention can maintain good decontamination capability, improve the feeling when the detergent composition is used, reduce the hands skin irritation and enhance the hand skin moisturizing ability to improve dry feeling.
Owner:GUANGZHOU LIBY
Method of stabilizing protein
InactiveUS20050171339A1Increase valueImprove and qualityImmunoglobulins against blood coagulation factorsImmunoglobulin superfamilyGlycineAsparagine
The present inventors revealed that deamidation of an antibody can be suppressed without influencing its activity by substituting a glycine that is located adjacent to an asparagine with another amino acid.
Owner:CHUGAI PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com