Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33results about How to "Efficiently depleted" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
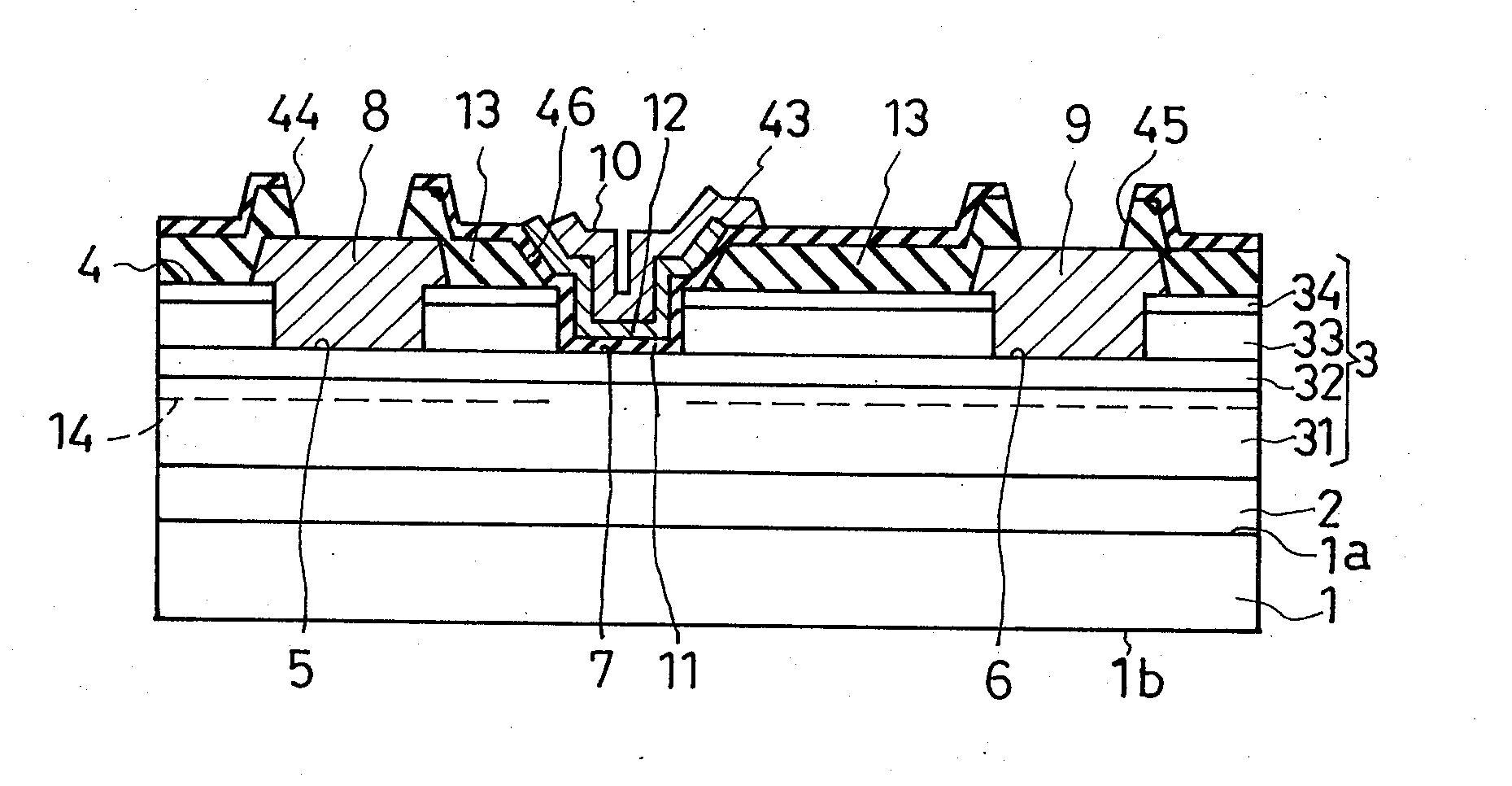
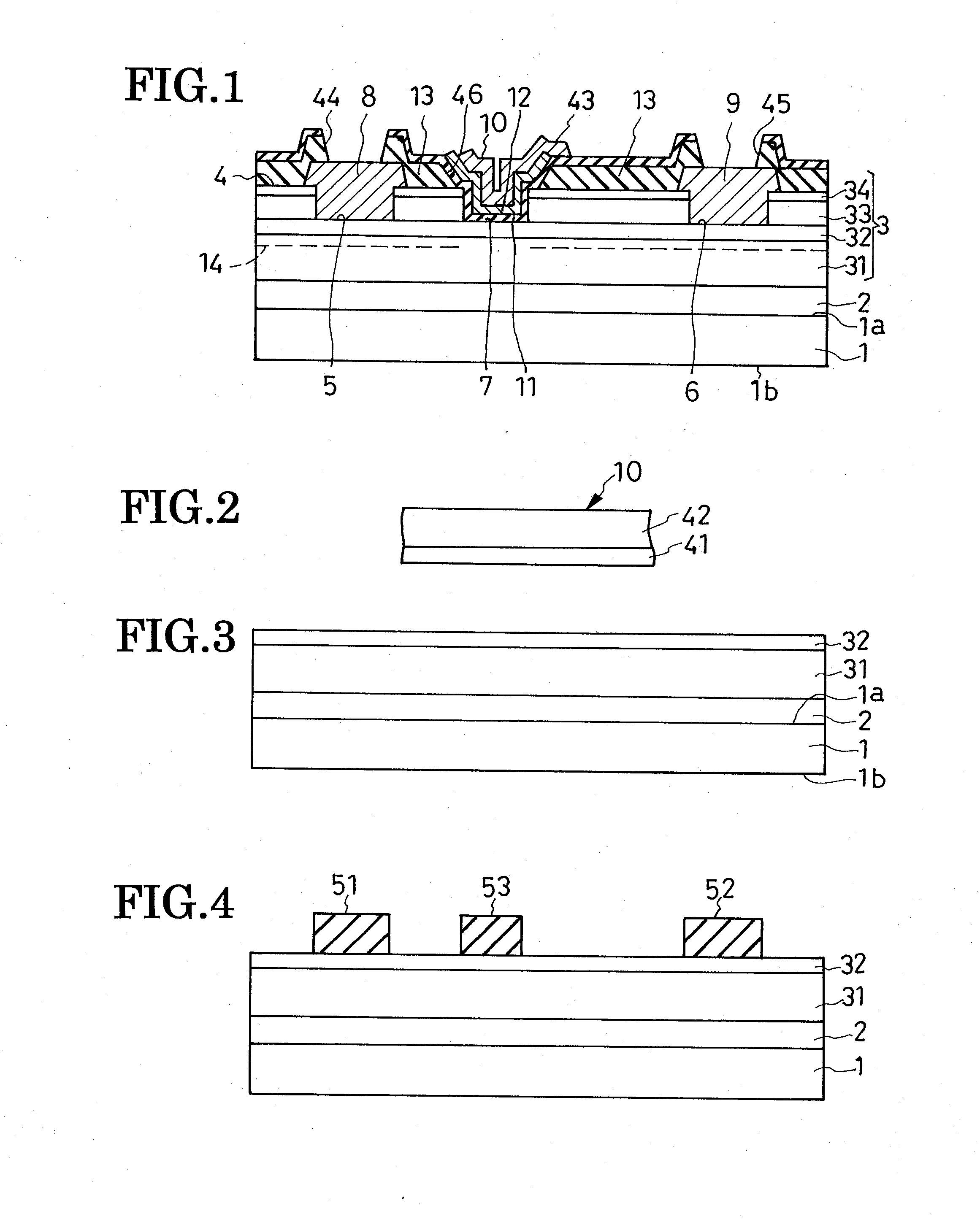
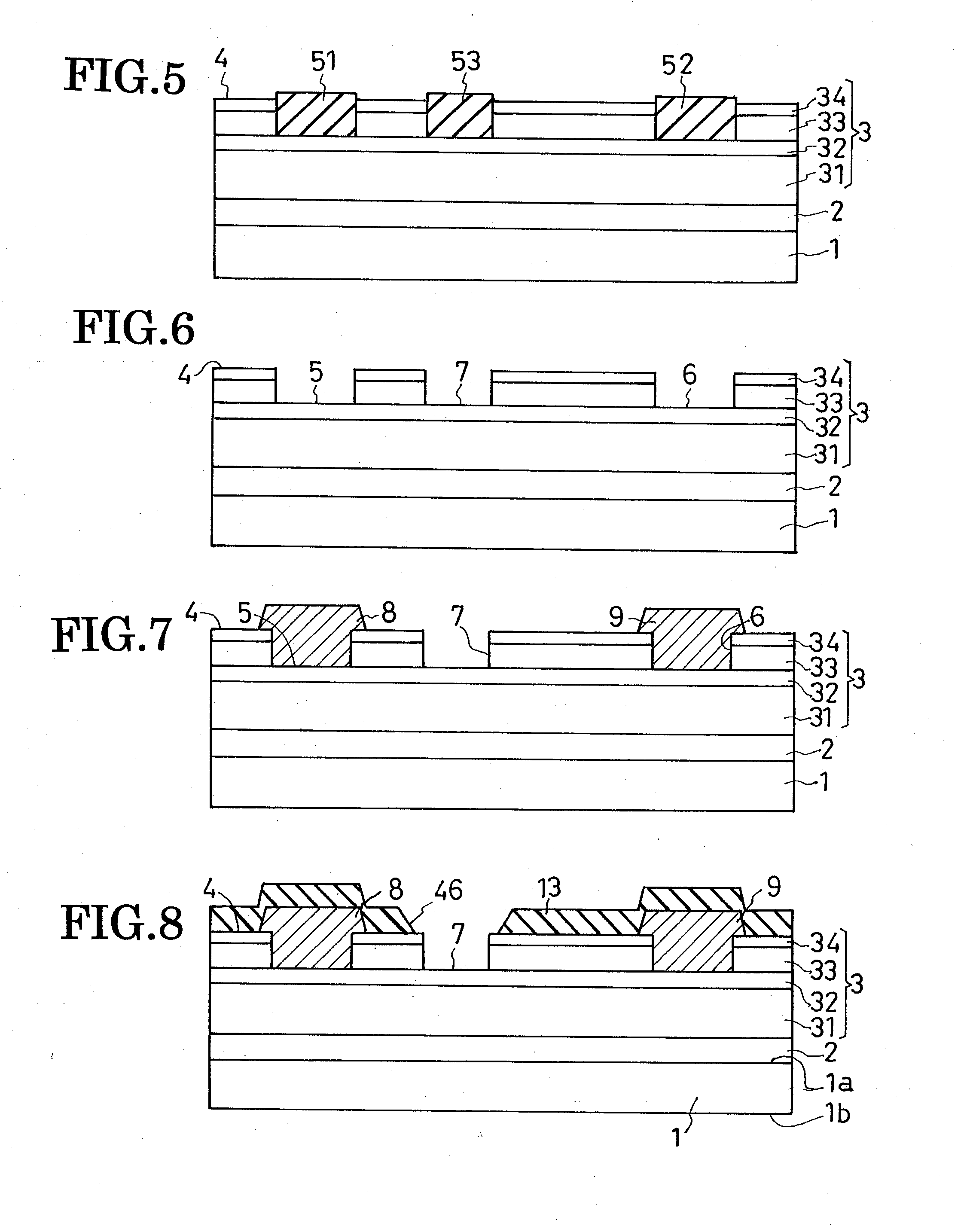
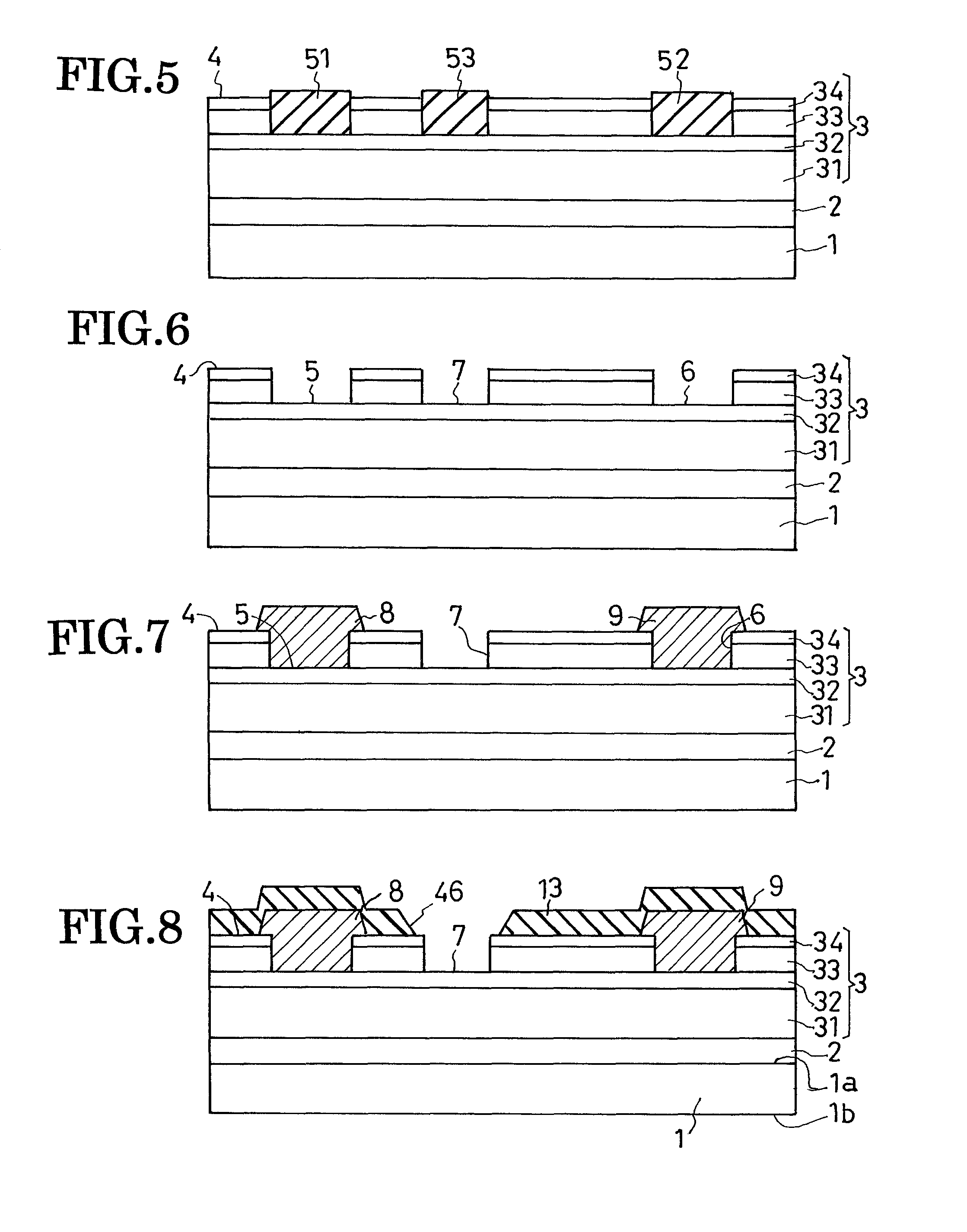
Field-effect semiconductor device, and method of fabrication
ActiveUS20100155720A1Leakage currentLittle and no physical impairmentSemiconductor/solid-state device manufacturingSemiconductor devicesHeterojunctionField effect
A heterojunction field-effect semiconductor device has a main semiconductor region comprising two layers of dissimilar materials such that a two-dimensional electron gas layer is generated along the heterojunction between the two layers. A source and a drain electrode are placed in spaced positions on a major surface of the main semiconductor region and electrically coupled to the 2DEG layer. Between these electrodes, a gate electrode is received in a recess in the major surface of the main semiconductor region via a p-type metal oxide semiconductor film and insulating film, whereby a depletion zone is normally created in the 2DEG layer, making the device normally off. The p-type metal oxide semiconductor film of high hole concentration serves for the normally-off performance of the device with low gate leak current, and the insulating film for further reduction of gate leak current.
Owner:SANKEN ELECTRIC CO LTD
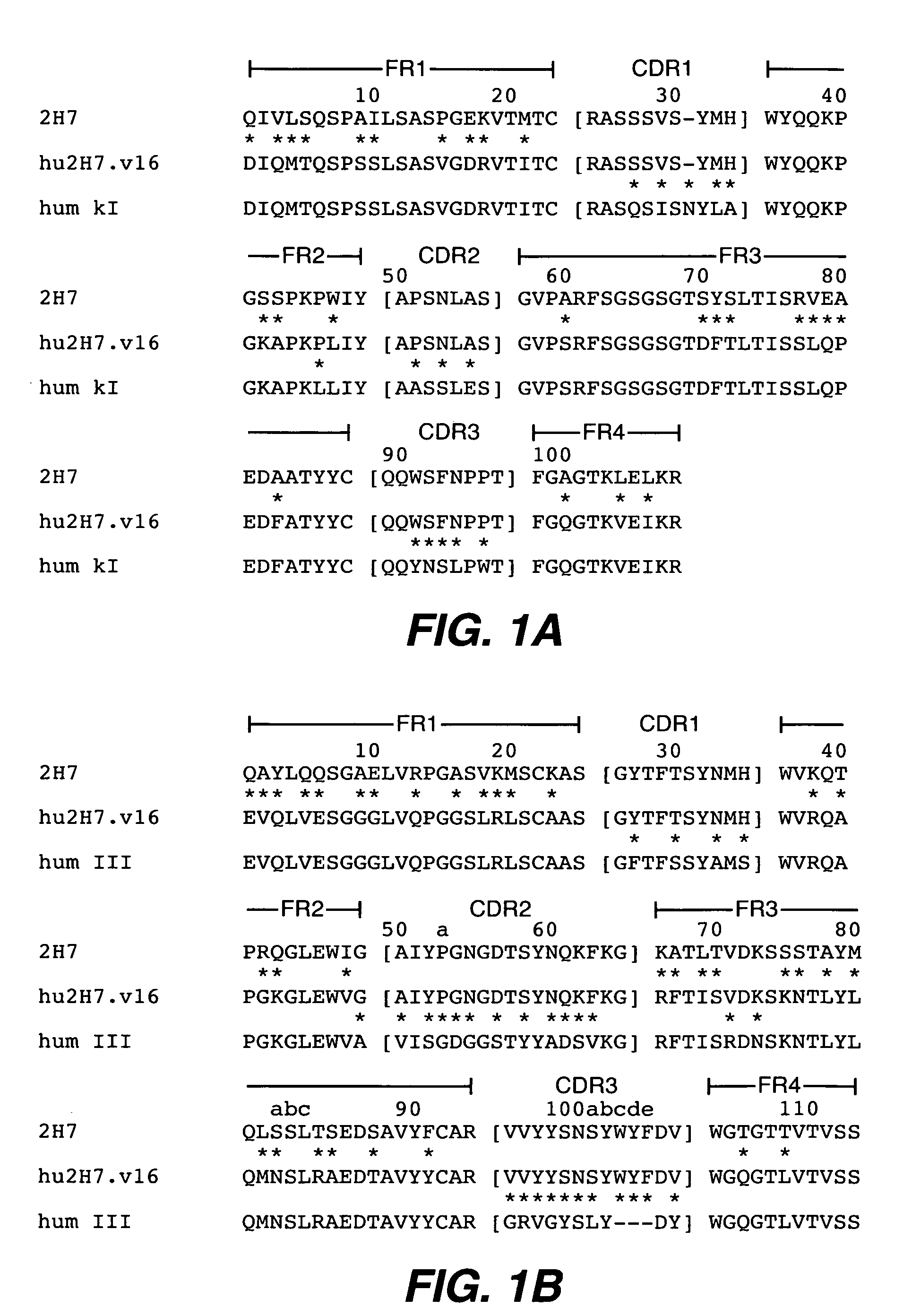
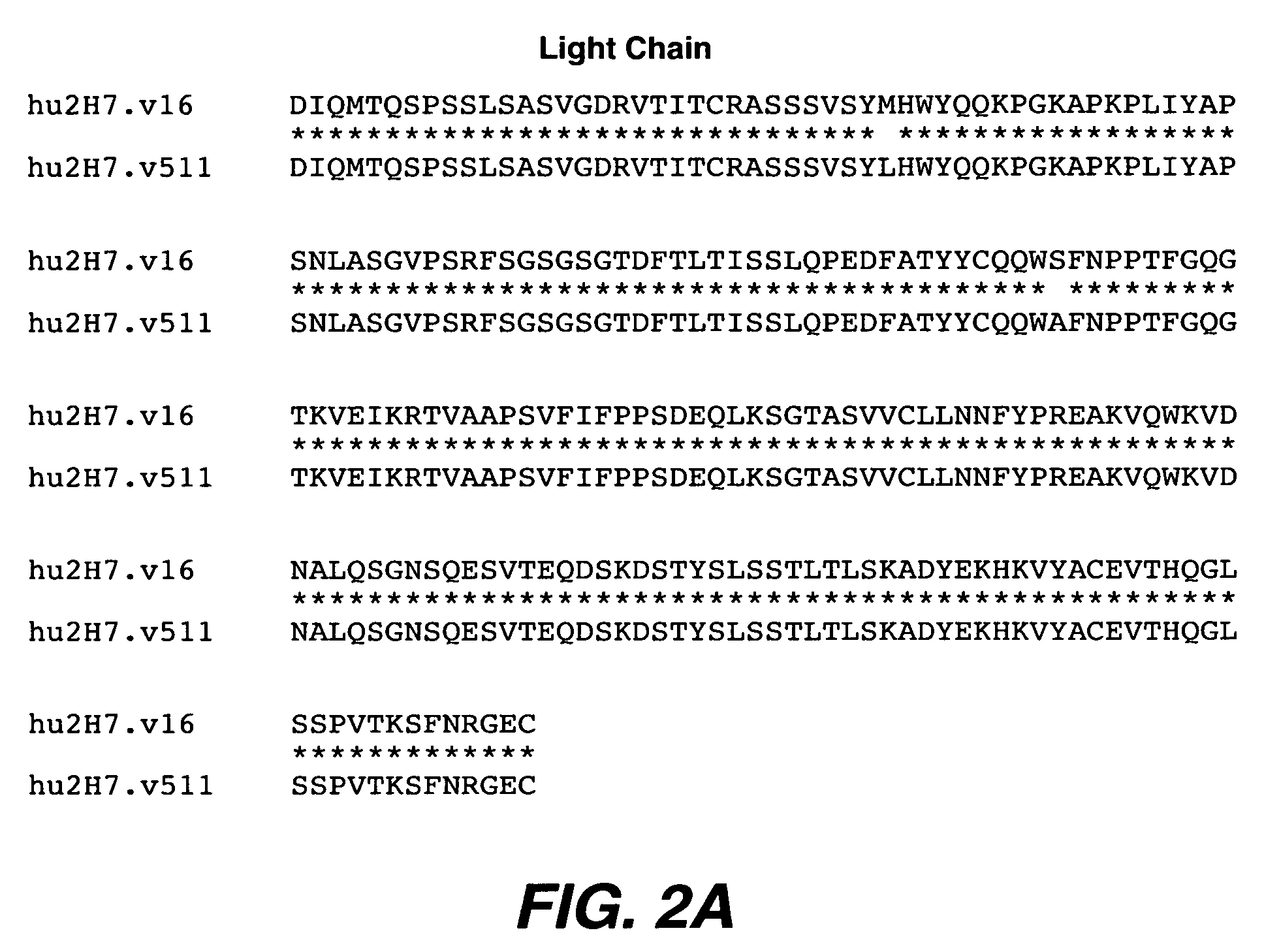
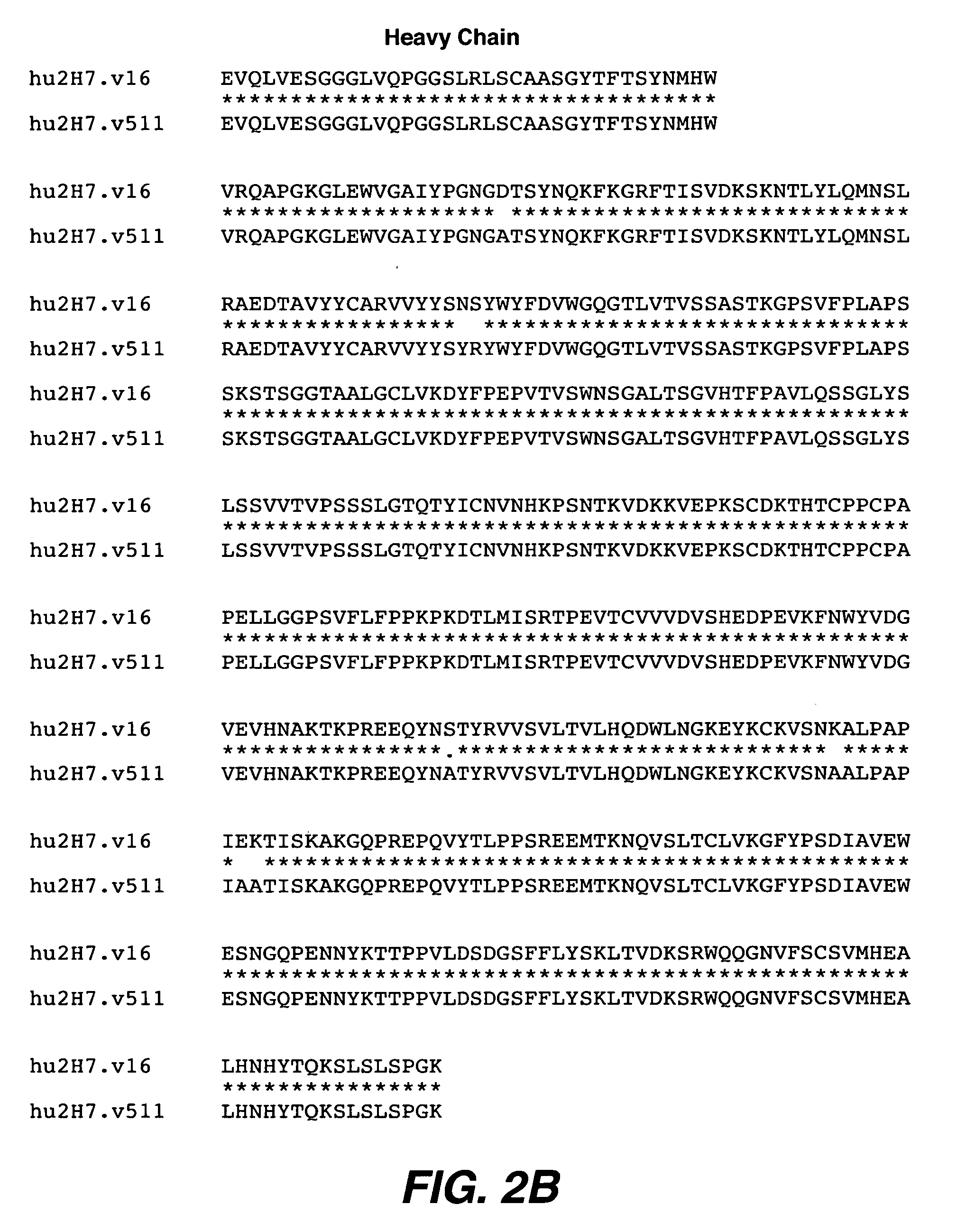
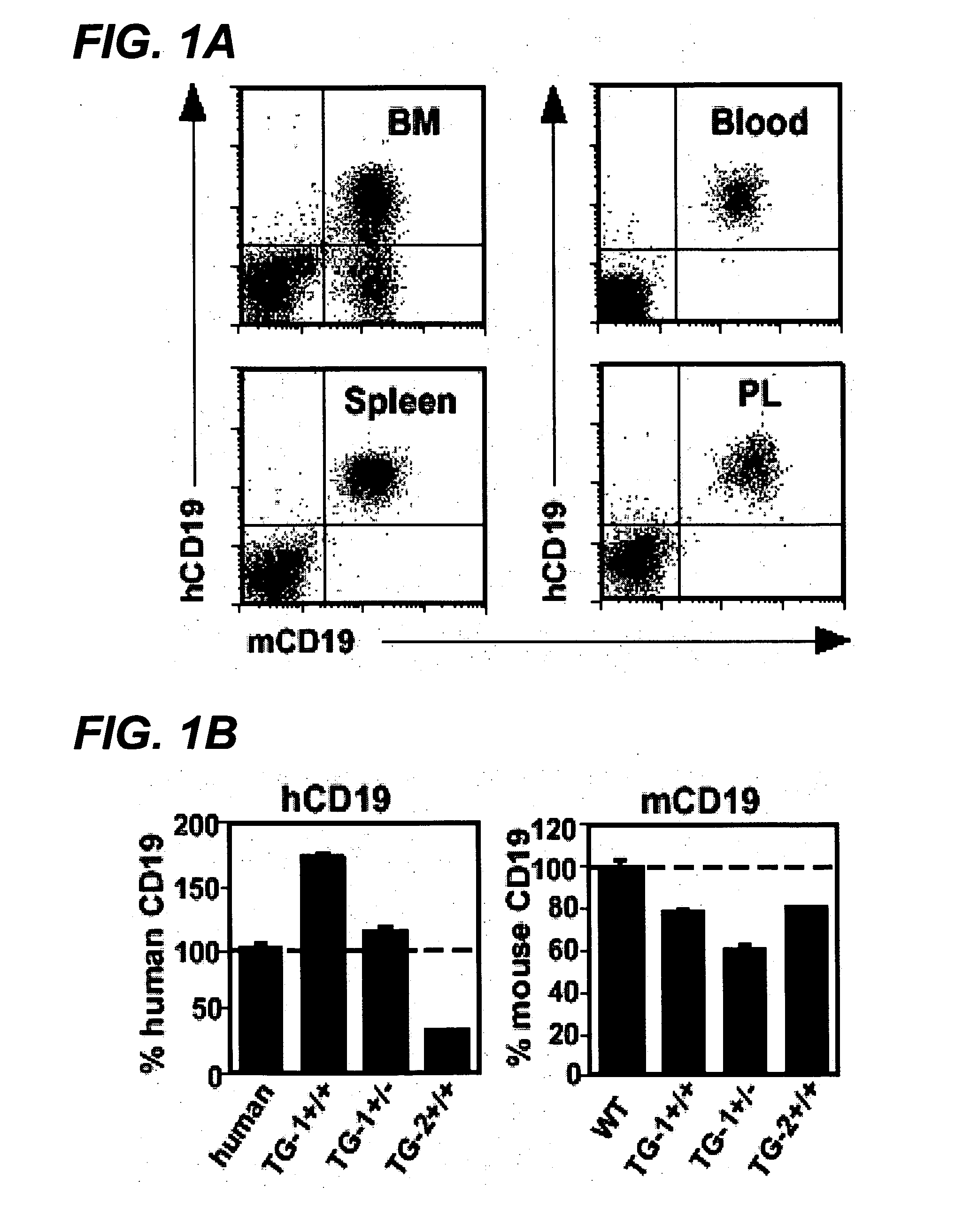
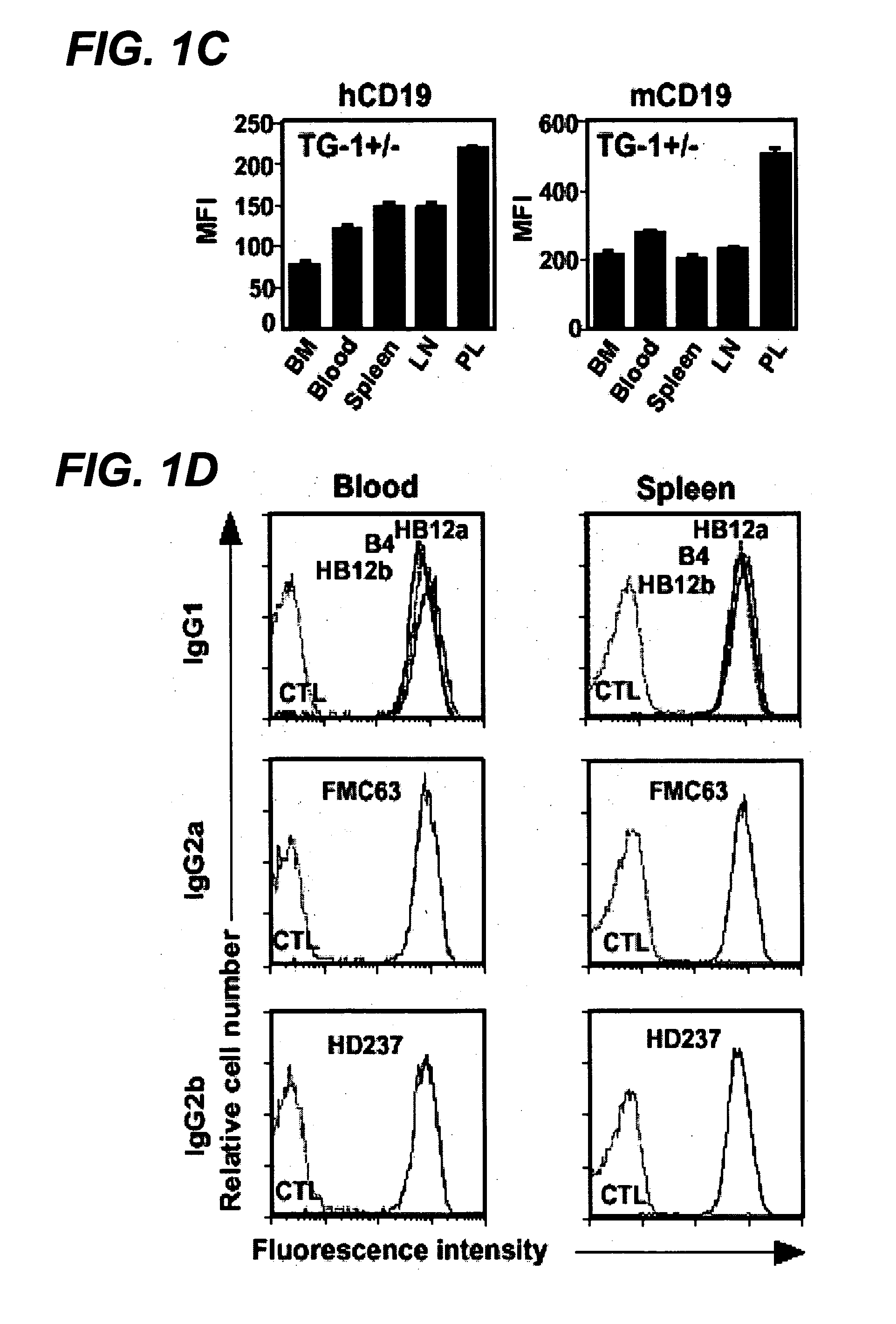
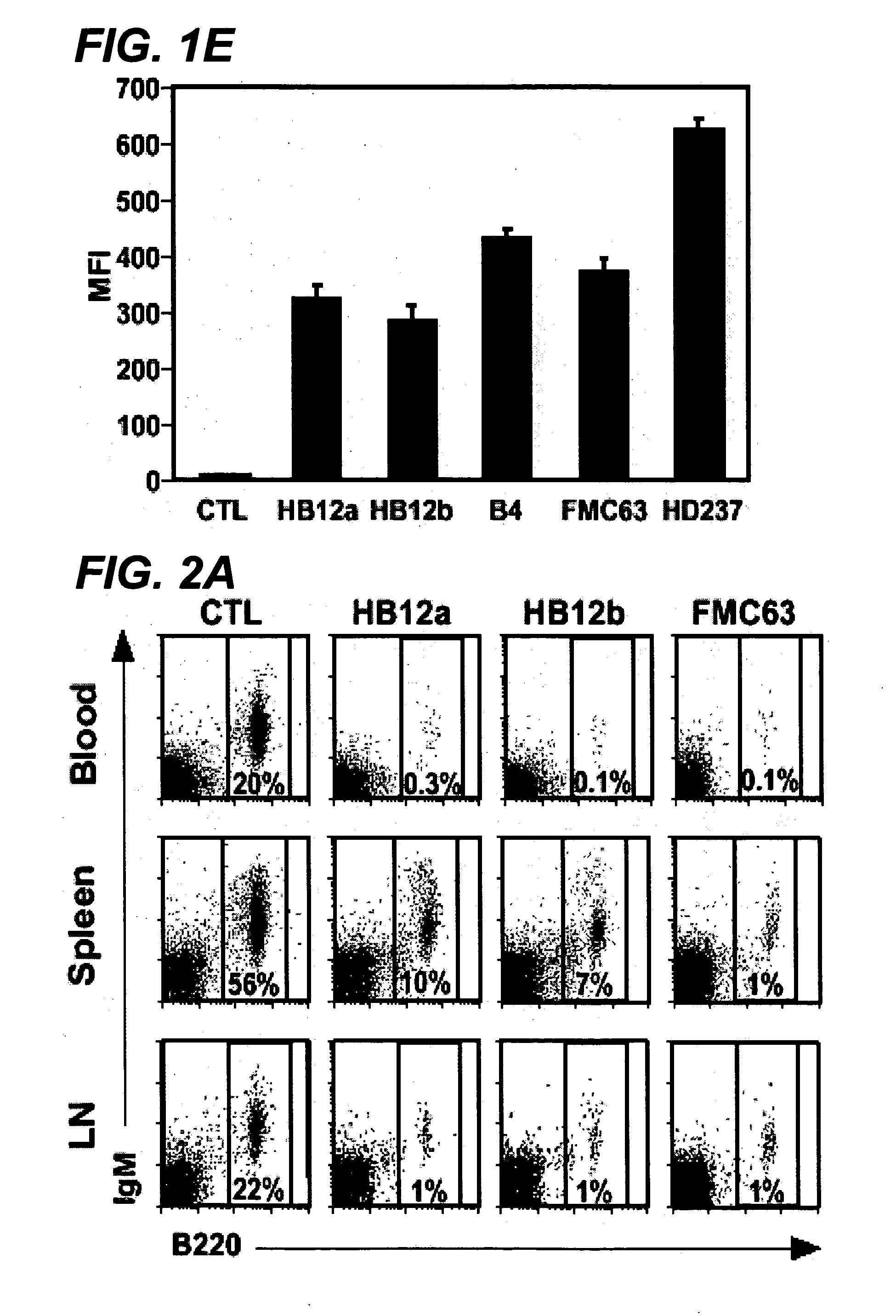
Antibody variants and uses thereof
The invention provides improved humanized CD20 binding antibodies for treatment of B cell malignancies and autoimmune diseases.
Owner:GENENTECH INC
Anti-CD19 antibody therapy for transplantation
InactiveUS20060280738A1Efficient productionEfficiently depletedBiocidePhosphorous compound active ingredientsAntigenDisease
The invention relates to immunotherapeutic compositions and methods for the treatment and prevention of GVHD, humoral rejection, and post-transplantation lymphoproliferative disorder in human subjects using therapeutic antibodies that bind to the human CD19 antigen and that preferably mediate human ADCC. The present invention relates to pharmaceutical compositions comprising human or humanized anti-CD 19 antibodies of the IgG1 or IgG3 human isotype. The present invention relates to pharmaceutical compositions comprising human or humanized anti-CD19 antibodies of the IgG2 or IgG4 human isotype that preferably mediate human ADCC. The present invention also relates to pharmaceutical compositions comprising chimerized anti-CD19 antibodies of the IgG1, IgG2, IgG3, or IgG4 isotype that mediate human ADCC. In preferred embodiments, the present invention relates to pharmaceutical compositions comprising monoclonal human, humanized, or chimeric anti-CD19 antibodies.
Owner:DUKE UNIV
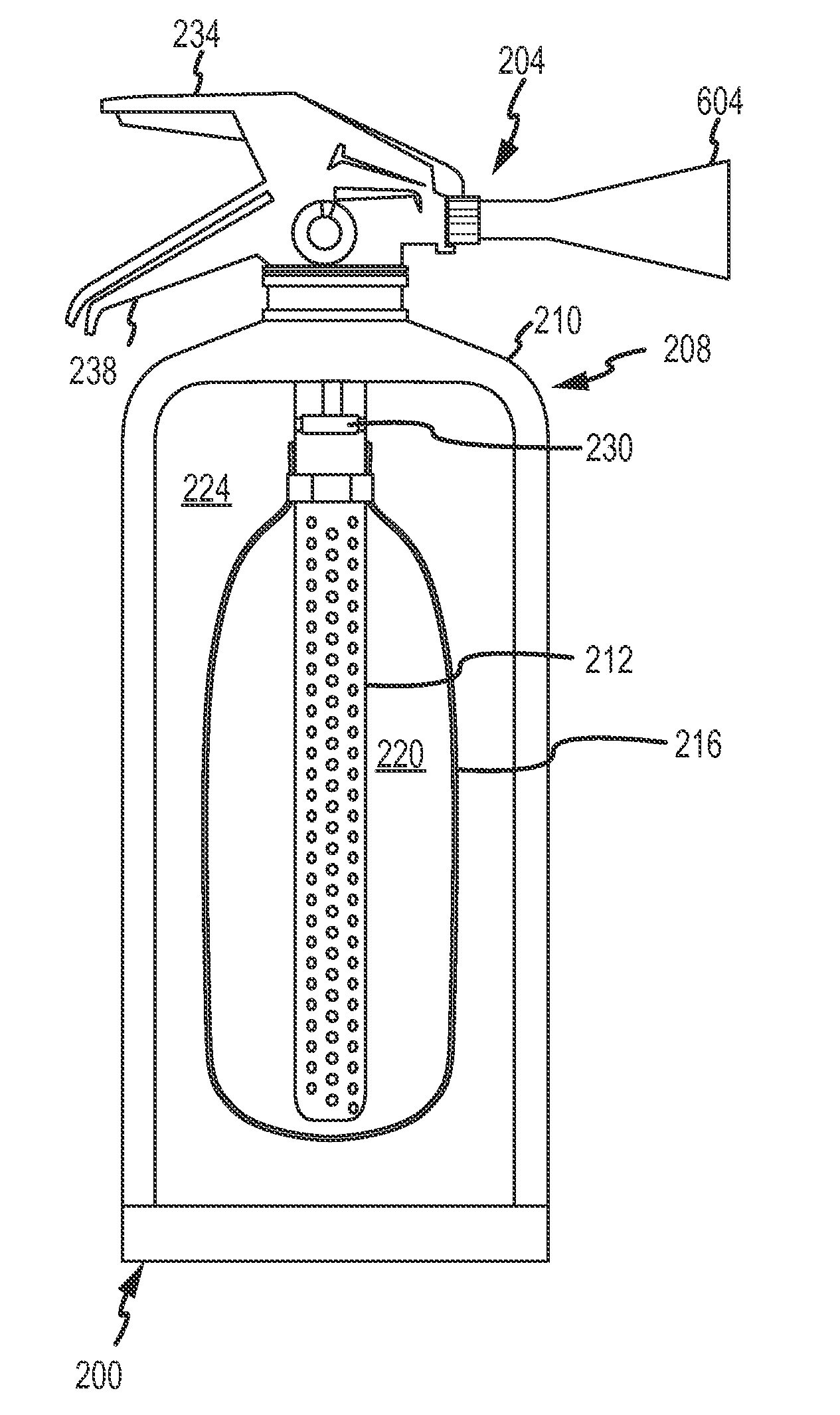
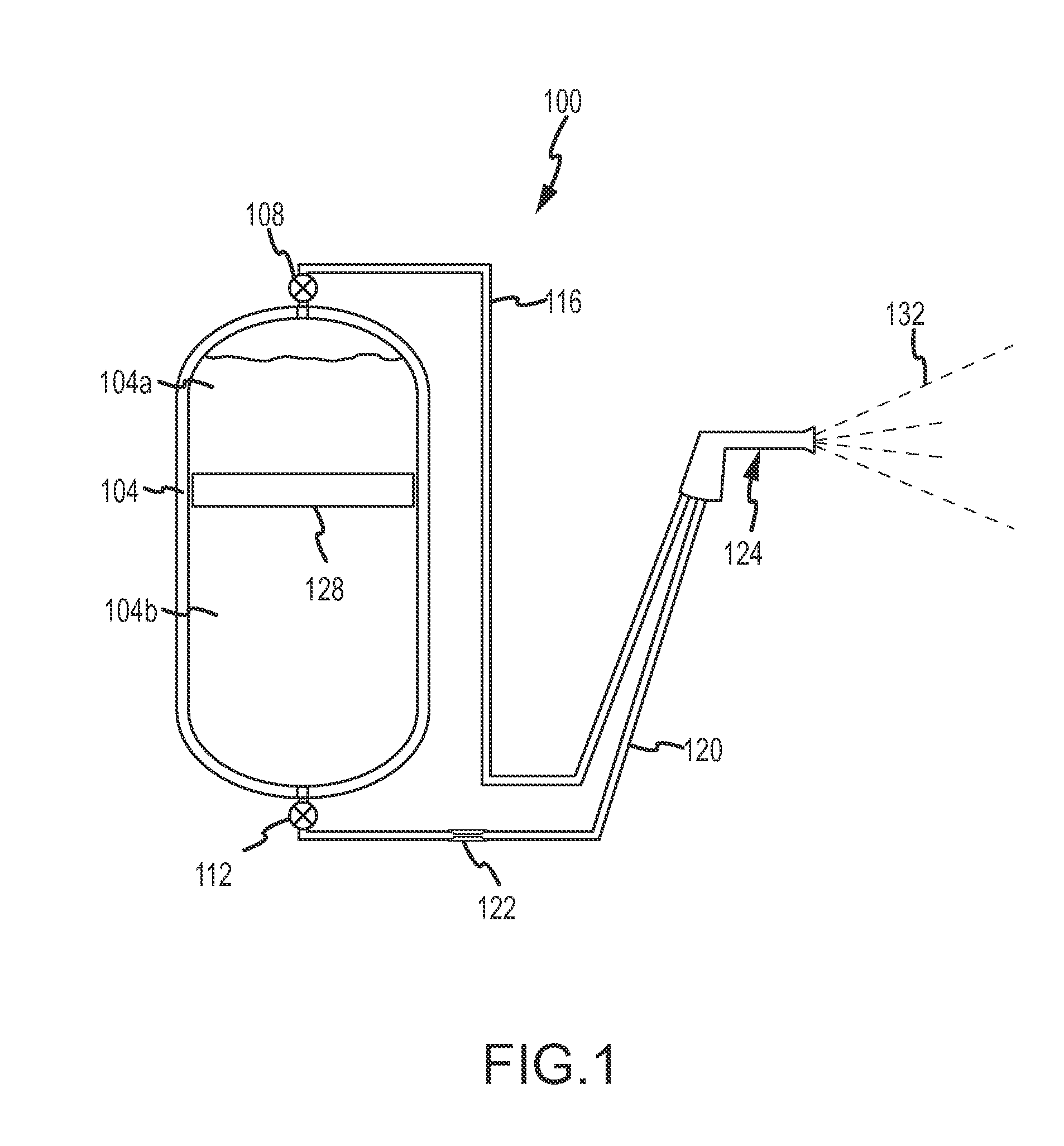
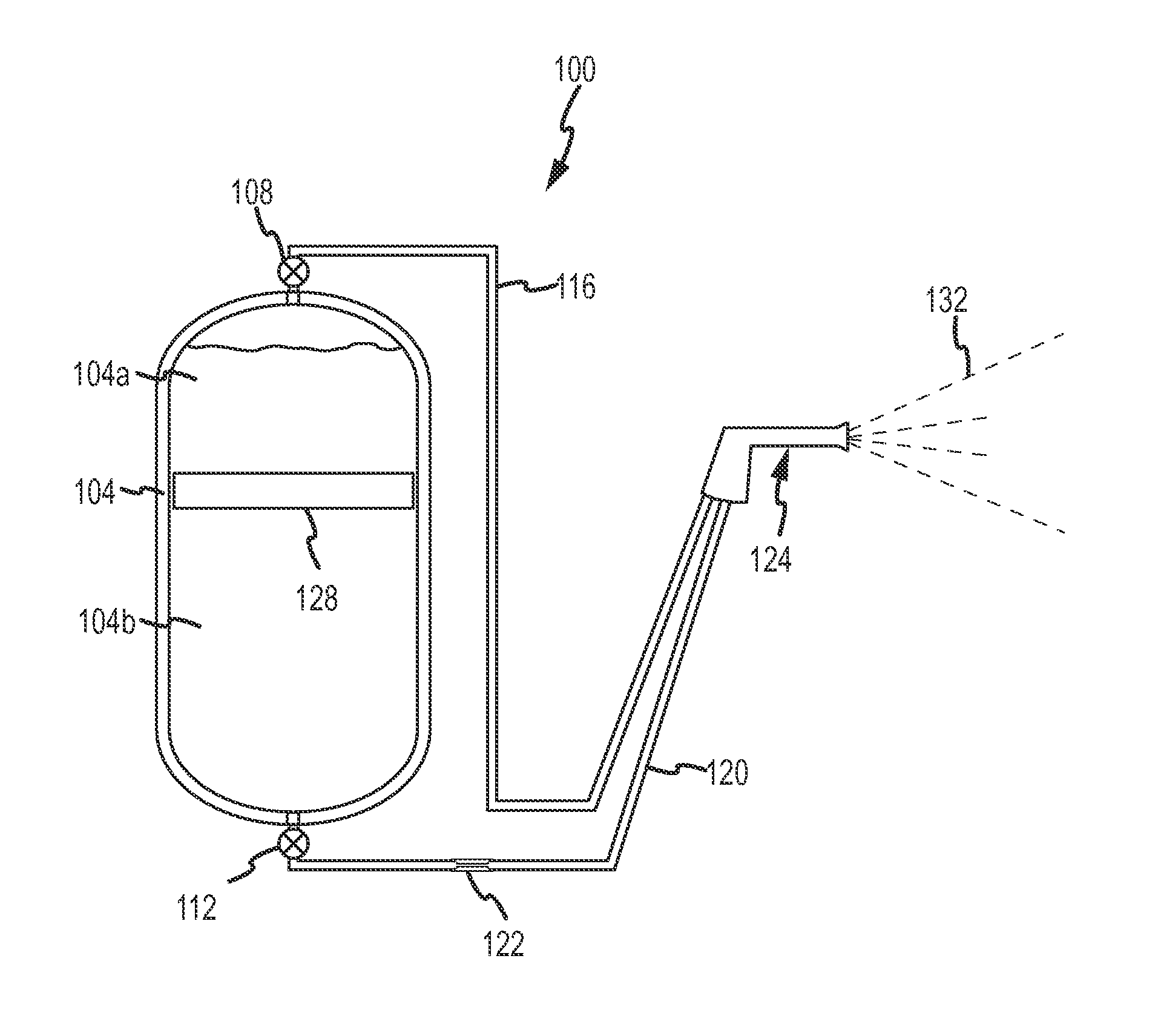
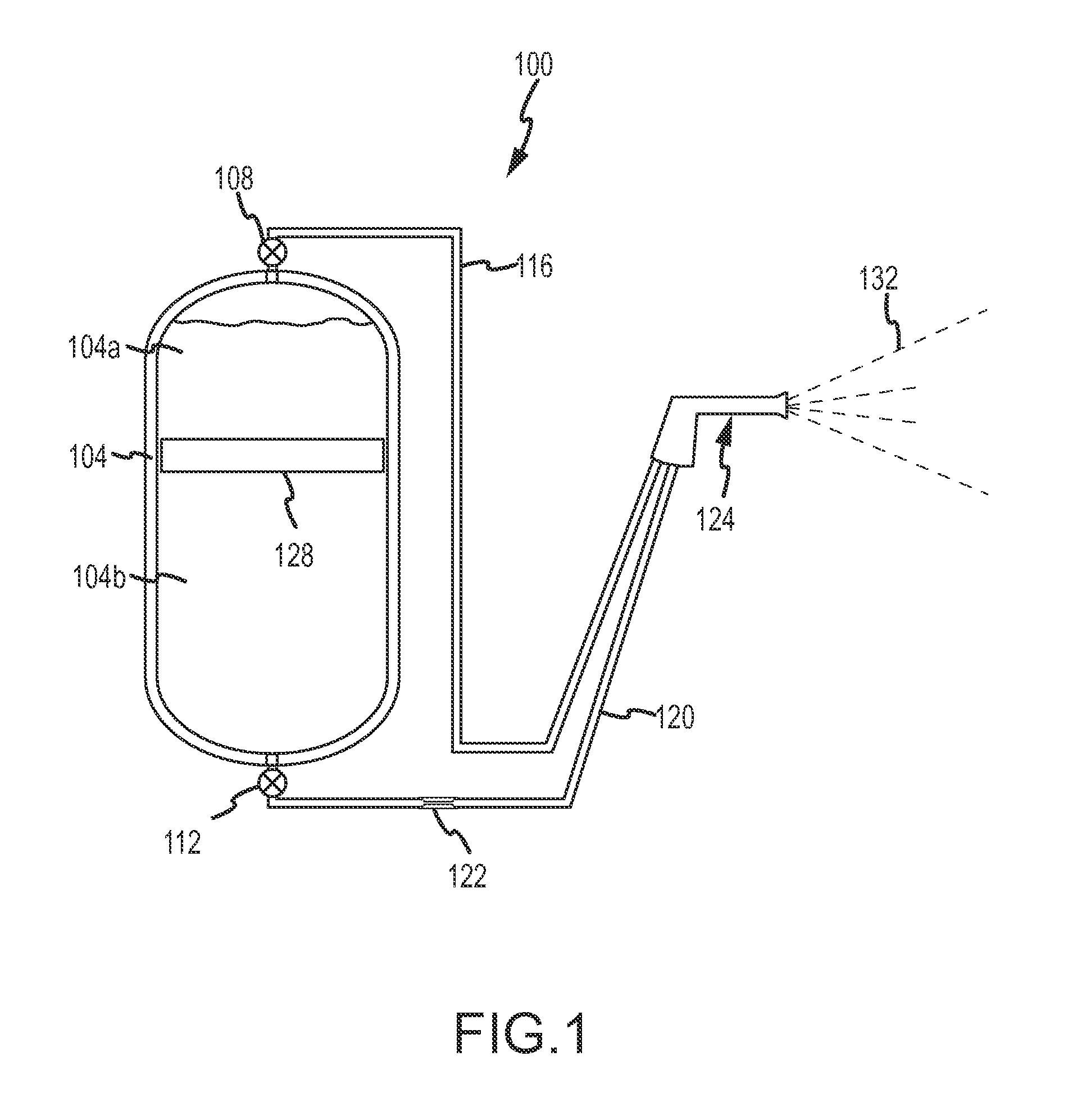
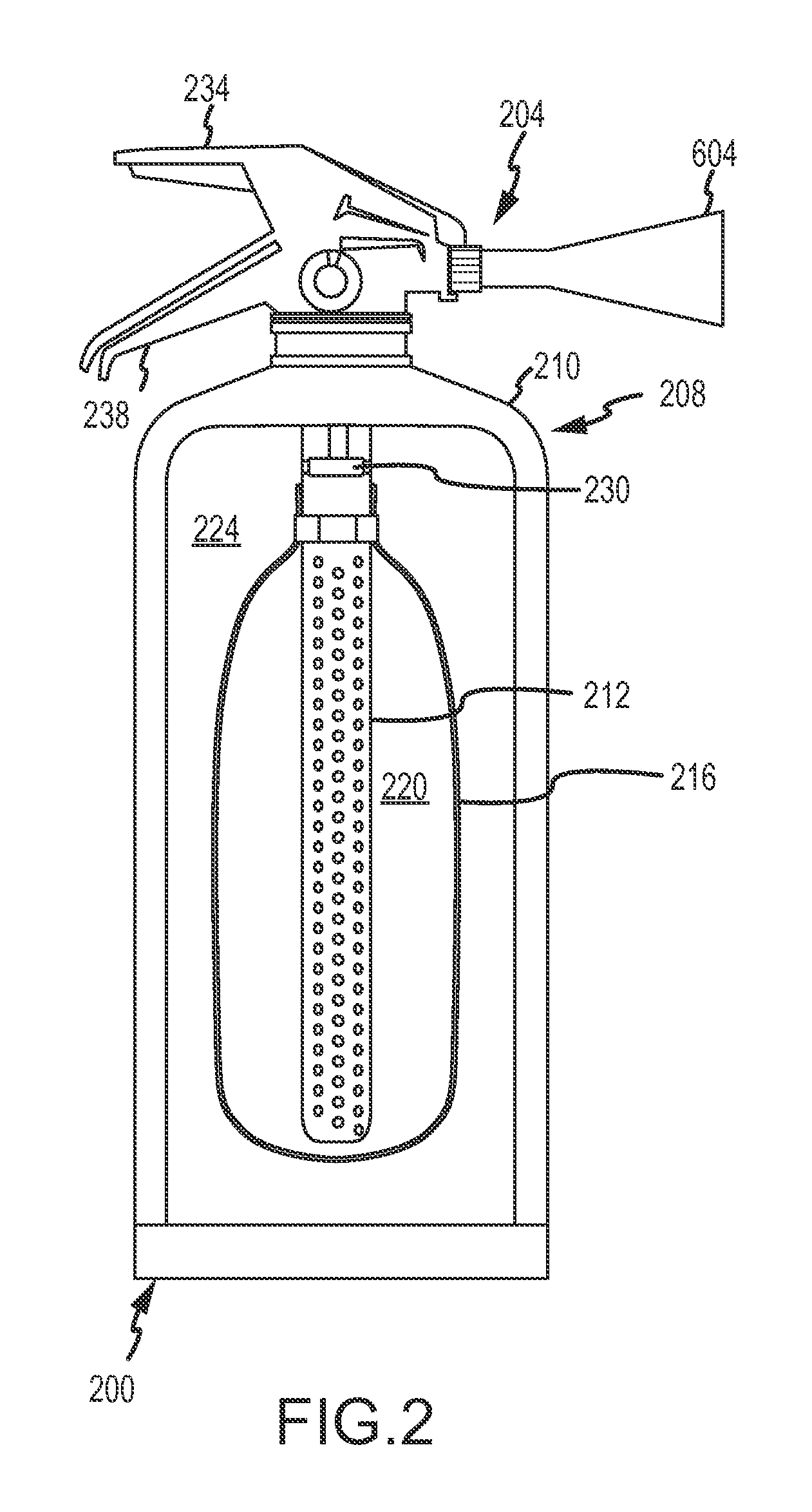
Fine water mist multiple orientation discharge fire extinguisher
ActiveUS20080128145A1Promote generationReduce weightFire rescueBoring toolsShape changeFire extinguisher
The present invention is directed to a suppression system in which a carrier gas and suppression liquid are contained in a common containment vessel and separated by a separation member. The separation is one or more of movable, deformable, or shape changing in response to pressure exerted by the stored gas.
Owner:ADA TECH +1
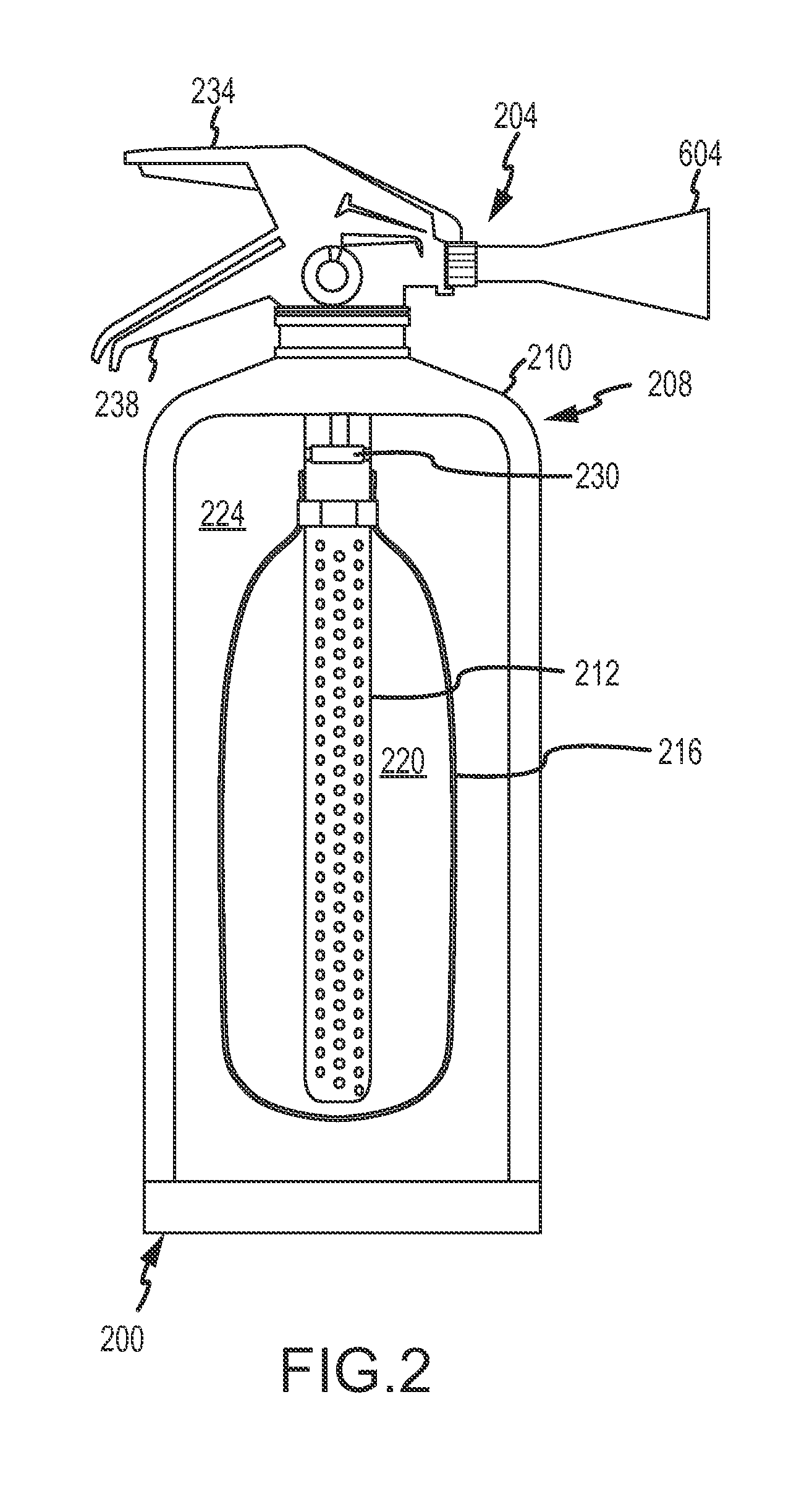
Fine water mist multiple orientation discharge fire extinguisher
ActiveUS8746357B2Reduce weightPromote generationFire rescueBoring toolsShape changeFire extinguisher
The present invention is directed to a suppression system in which a carrier gas and suppression liquid are contained in a common containment vessel and separated by a separation member. The separation is one or more of movable, deformable, or shape changing in response to pressure exerted by the stored gas.
Owner:ADA TECH +1
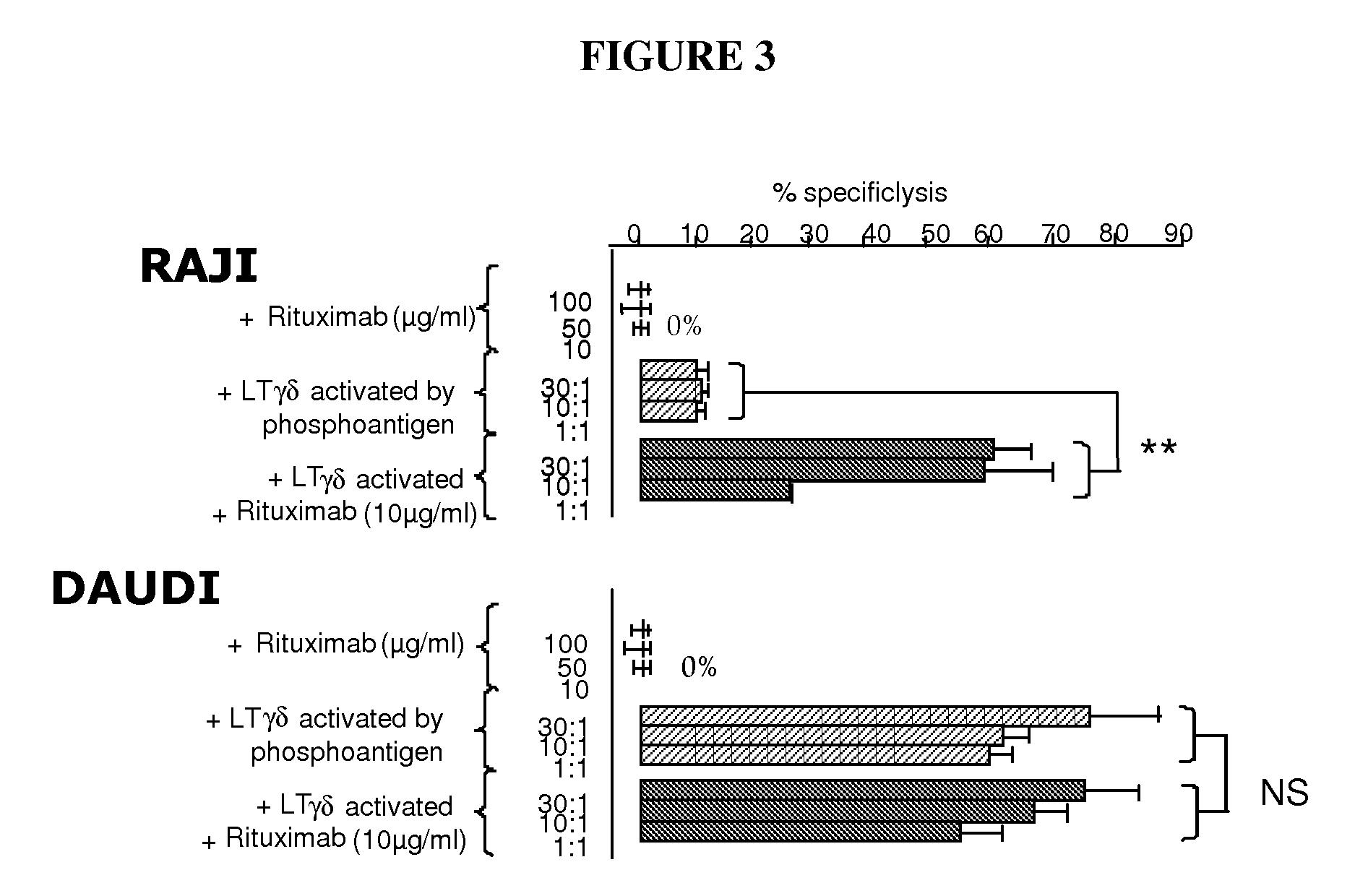
Methods and Compositions for Increasing the Efficiency of Therapeutic Antibodies Using Gamma Delta T Cell Activators
InactiveUS20090304688A1Good curative effectGreater target cell lysisOrganic active ingredientsAntiviralsTherapeutic antibodyT cell
The present invention relates to methods and compositions for increasing the efficiency of therapeutic antibodies. More particularly, the invention relates to the use of a therapeutic antibody in combination with a γδ T cell activating compound or activated γδ T cells. thereby allowing a potentiation of γδ T cell cytotoxicity in mammalian subjects in order to enhance the efficiency of the treatment in human subjects, particularly through an increase of the depletion of targeted cells.
Owner:INNATE PHARMA SA +1
Electrochemical sensor
InactiveUS20060237313A1Easy to useGood mannersWeather/light/corrosion resistanceVolume/mass flow measurementEngineeringAuxiliary electrode
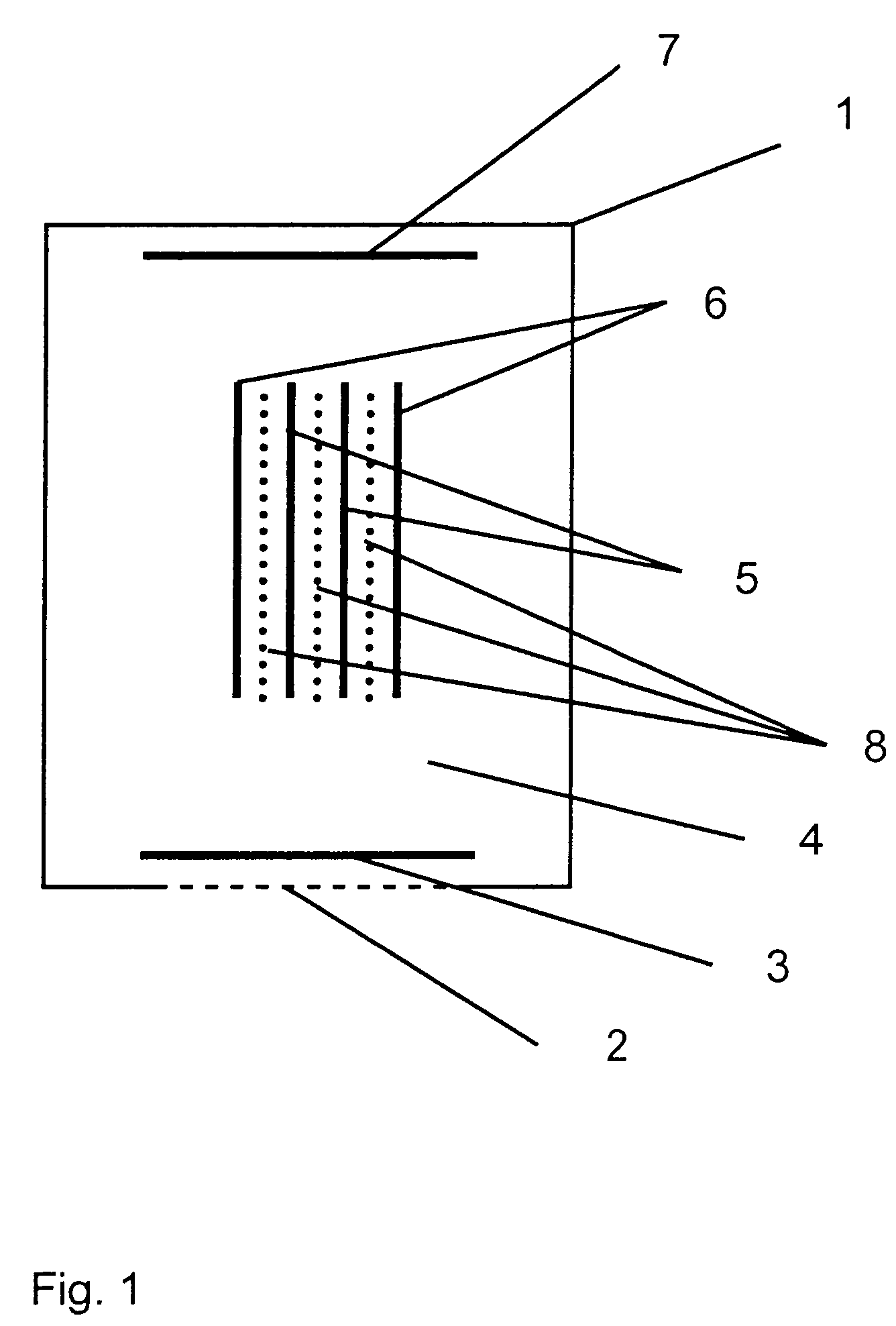
An electrochemical sensor with at least one measuring electrode (3), at least one auxiliary electrode (7) and at least one reference electrode (5), wherein a protective electrode (6), which ensures at the reference electrode (5) the at least partial shielding of the reference electrode (5) against substances that would lead to a change in the reference potential when reaching the reference electrode (5), is arranged in the vicinity of the reference electrode (5). A highly stable reference potential can be obtained with the present invention.
Owner:DRAGER SAFETY
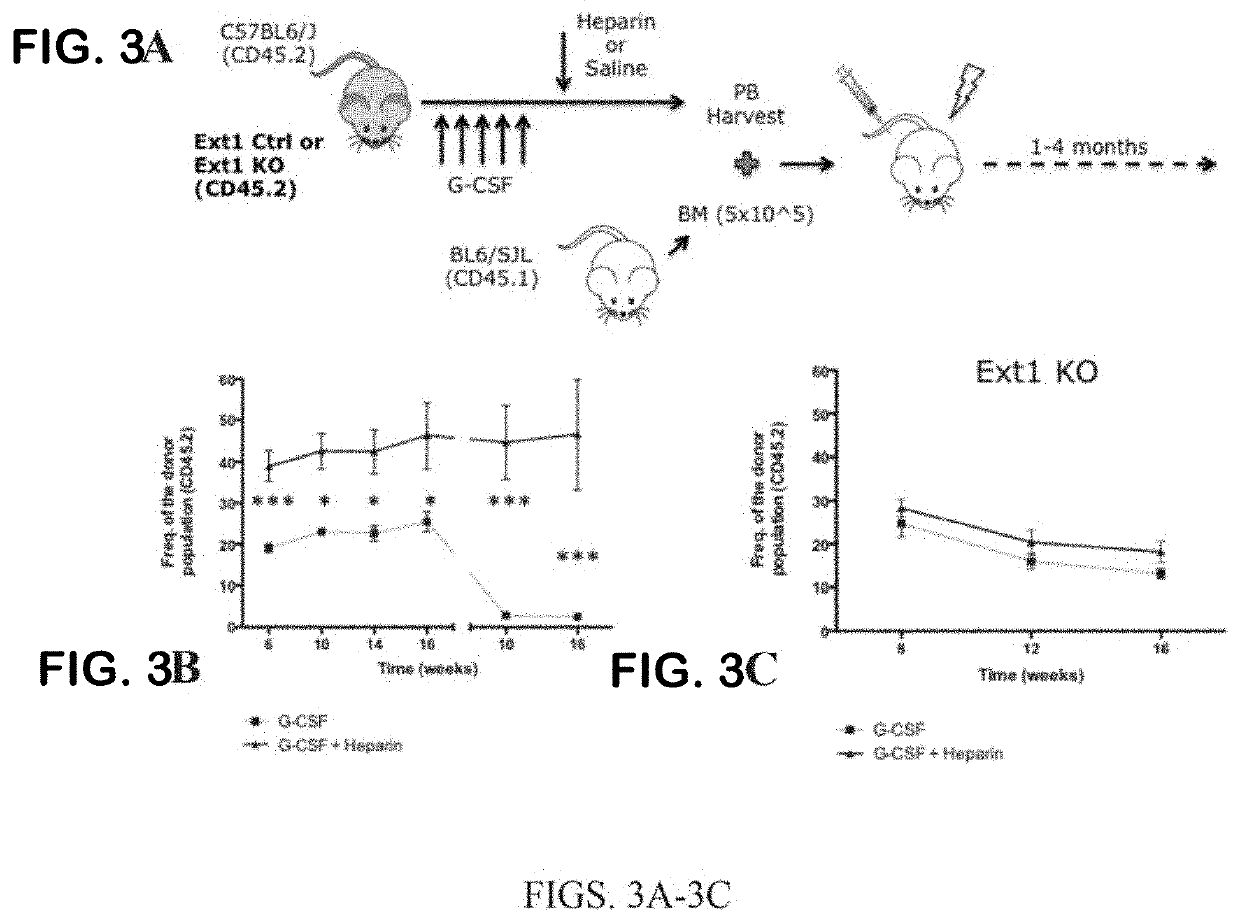
Methods and compositions for mobilizing stem cells
ActiveUS20160120947A1Efficiently depletedRapid mobilizationOrganic active ingredientsPeptide/protein ingredientsProgenitor cellPbsc mobilization
The present invention relates to methods and compositions for mobilizing hematopoietic stem cells and / or progenitor cells, and related methods of conditioning for engraftment of transplanted hematopoietic stem cells and / or progenitor cells, and methods of treating diseases requiring hematopoietic stem cell and / or progenitor cell transplantation.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +1
Field-effect semiconductor device
ActiveUS8125004B2Little and no physical impairmentEasy to makeSemiconductor/solid-state device manufacturingSemiconductor devicesHeterojunctionField effect
Owner:SANKEN ELECTRIC CO LTD
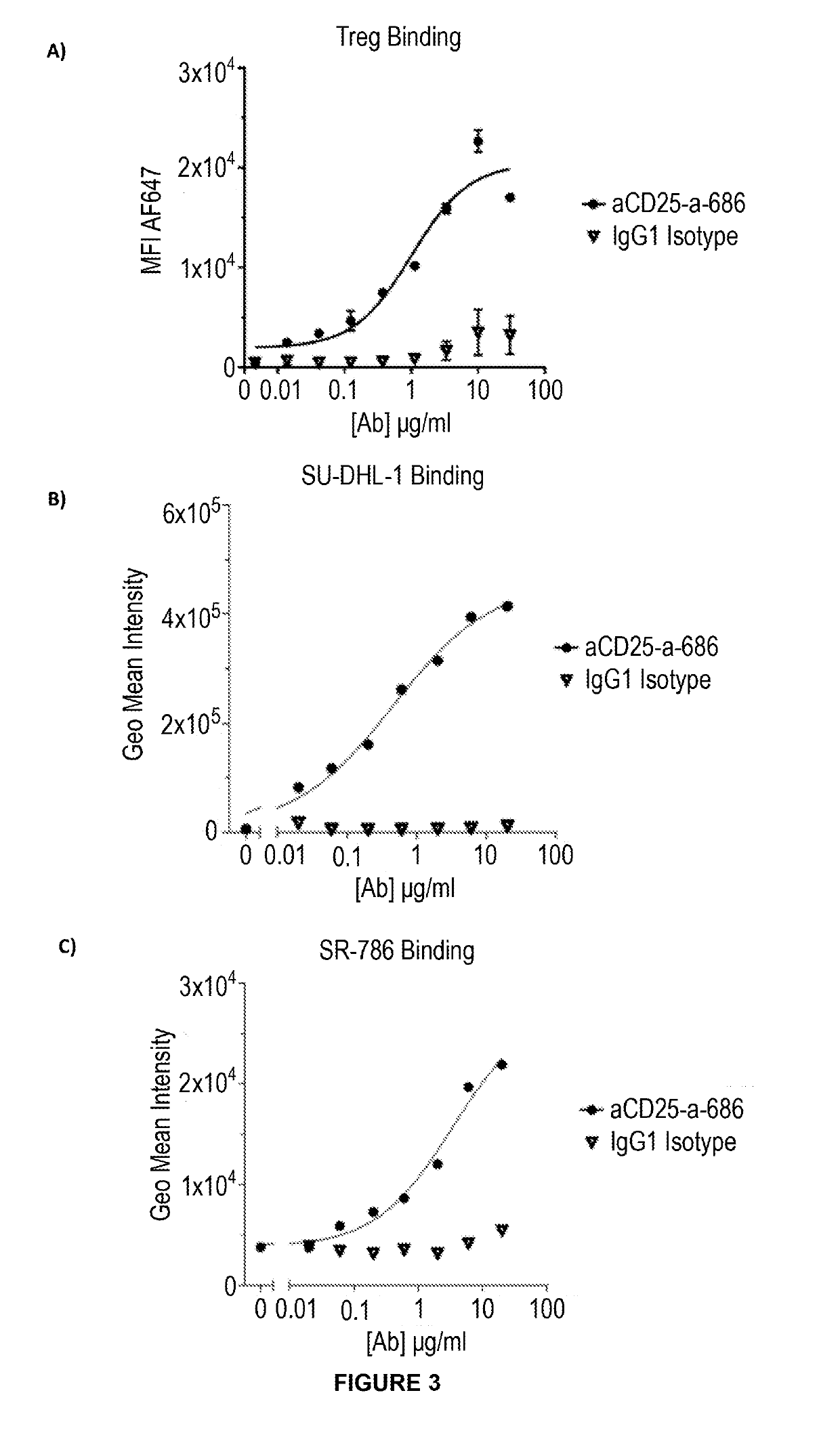
Anti-cd25 antibody agents
ActiveUS20190284287A1Good effectRaise the ratioImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigen bindingCancer
The present disclosure provides antibody sequences found in antibodies that bind to human CD25. In particular, the present disclosure provides sequences of anti-human CD25 antibodies, which do not block the binding of CD25 to IL-2 or IL-2 signalling. Antibodies and antigen-binding portions thereof including such sequences can be used in pharmaceutical composition and methods of treatment, in particular for treating cancer.
Owner:TUSK THERAPEUTICS LTD +1
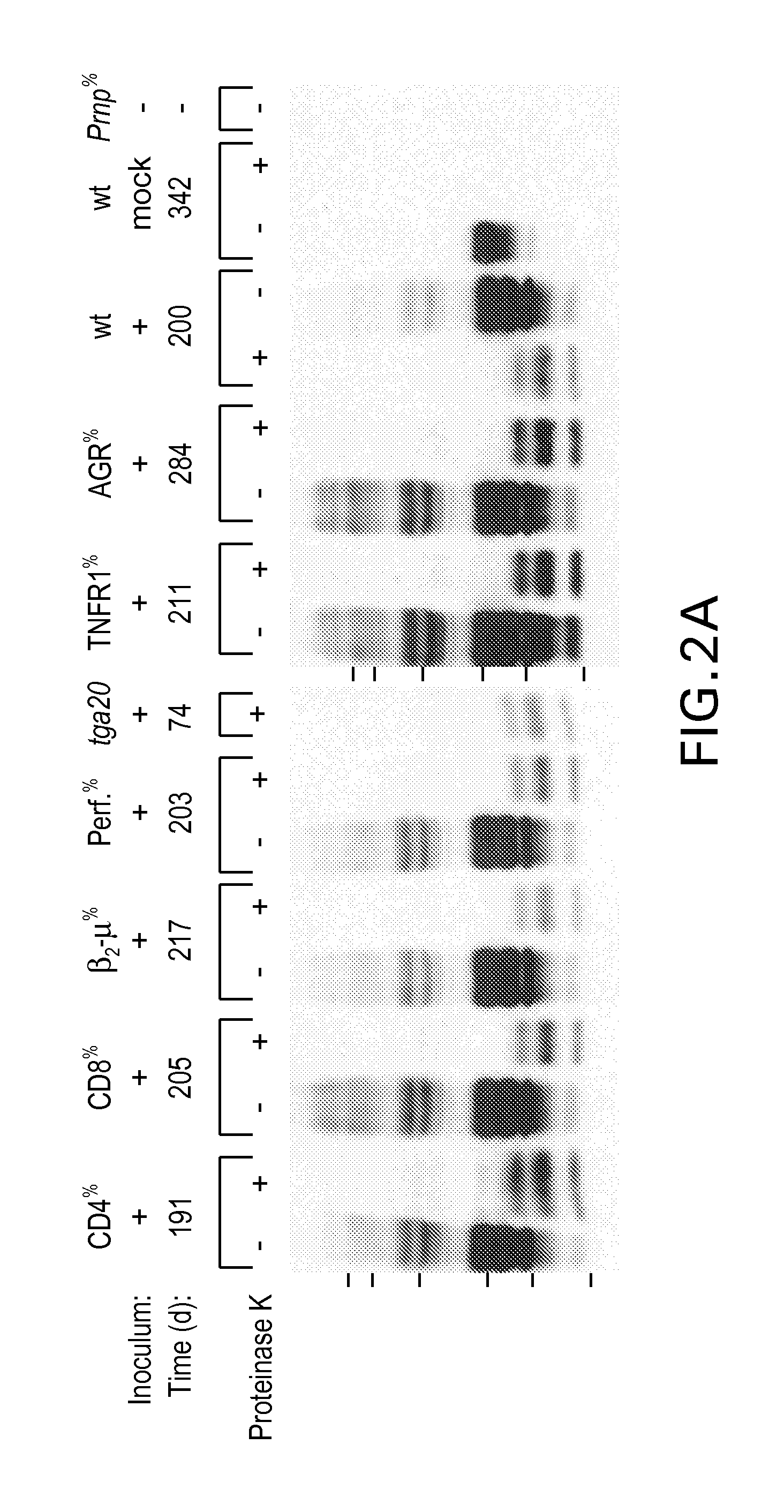
Diagnostics And Therapeutics For Transmissible Spongiform Encephalopathy And Methods For The Manufacture Of Non-Infective Blood Products And Tissued Derived Products
InactiveUS20080063600A1Efficiently depletedOrganic active ingredientsNervous disorderBiological bodyWhole blood product
B-cells have been identified as being the crucial carriers of infectivity in the spread of transmissible spongiform encephalopathy within an infected organism. In a second step, B-cells may infect further components_of the immune system, e.g. T-cells. Accordingly, the present invention provides B-cell and T-cell specific ligands for the use in diagnostics and therapeutics for transmissible spongiform encephalopathy and provides methods for the manufacture of non-infective blood products and tissue derived products. Thus, the present invention provides medicaments comprising B-cell and / or T-cell depletants, for the treatment of pathologies where the depletion of B-cells and / or T-cells, and more particularly of tse-infected B-cells and / or T-cells is therapeutically effective.
Owner:ABBOTT LAB INC
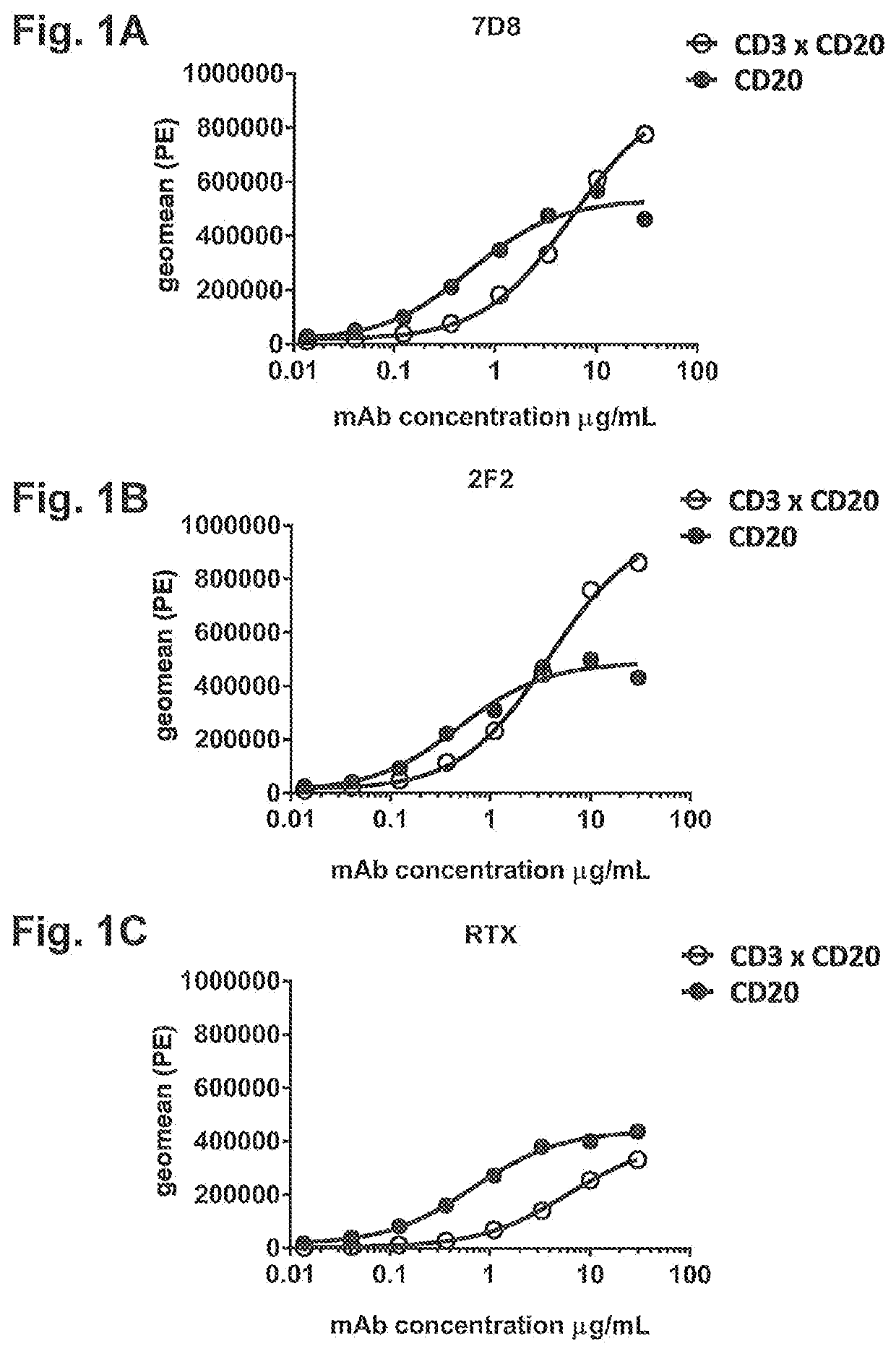
Bispecific antibodies against cd3 and cd20
PendingUS20200199231A1High killing efficiencyPotent in eradicatingImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCD20Disease
Bispecific antibodies directed to CD3 and CD20 and uses of such bispecific antibodies, in particular use thereof in the treatment of diseases in which specific targeting and T cell-mediated killing of cells that express CD20 is desired.
Owner:GENMAB AS
Alpha olefin production
InactiveUS20040122278A1Less brittleHigh impact strengthHydrocarbon by isomerisationMolecular sieve catalystIsomerizationCatalytic distillation
A method for making alpha olefins from internal olefins using catalytic distillation techniques and an olefin double bond isomerization catalyst, and separately recovering said alpha olefins.
Owner:EQUSR CHEM LP
Electrochemical sensor
InactiveUS7615139B2Stabilizing reference potentialEfficiently depletedWeather/light/corrosion resistanceVolume/mass flow measurementAuxiliary electrodeEngineering
An electrochemical sensor with at least one measuring electrode (3), at least one auxiliary electrode (7) and at least one reference electrode (5), wherein a protective electrode (6), which ensures at the reference electrode (5) the at least partial shielding of the reference electrode (5) against substances that would lead to a change in the reference potential when reaching the reference electrode (5), is arranged in the vicinity of the reference electrode (5). A highly stable reference potential can be obtained with the present invention.
Owner:DRAGER SAFETY
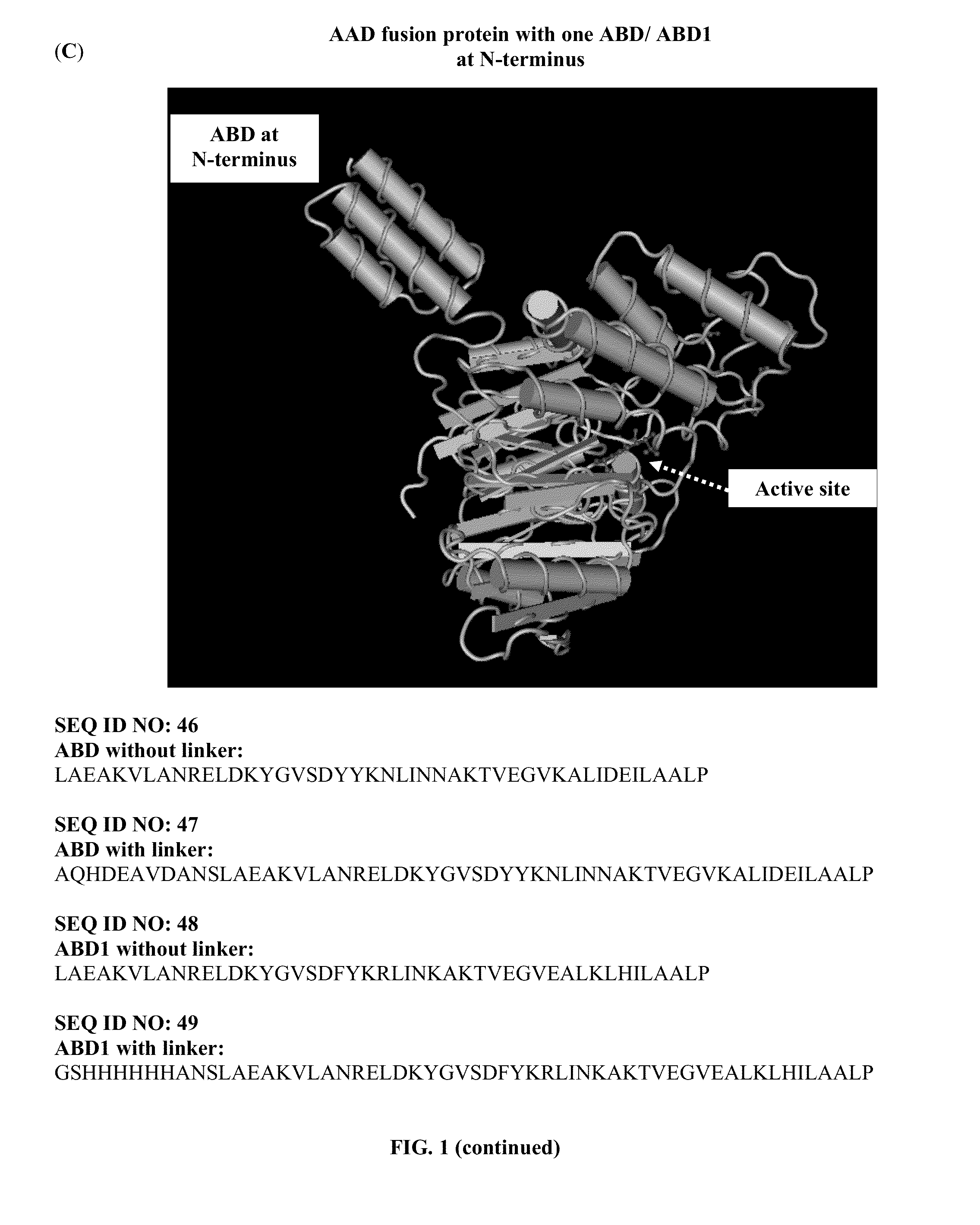
Pharmaceutical composition comprising albumin-binding arginine deiminase for cancer targeting treatment
ActiveUS20140255377A1Efficiently depletedHigh activityHydrolasesPeptide/protein ingredientsDiseaseHalf-life
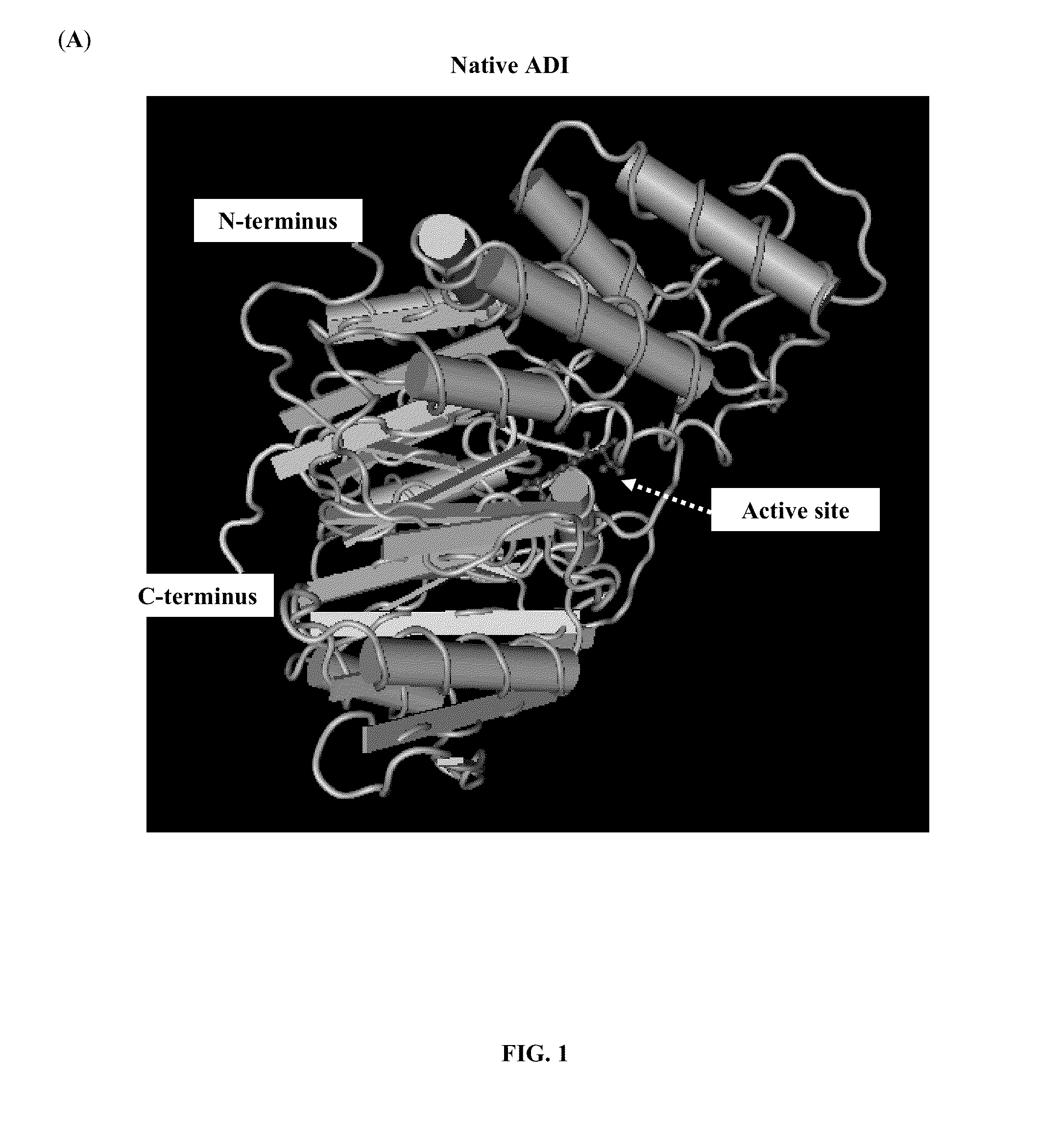
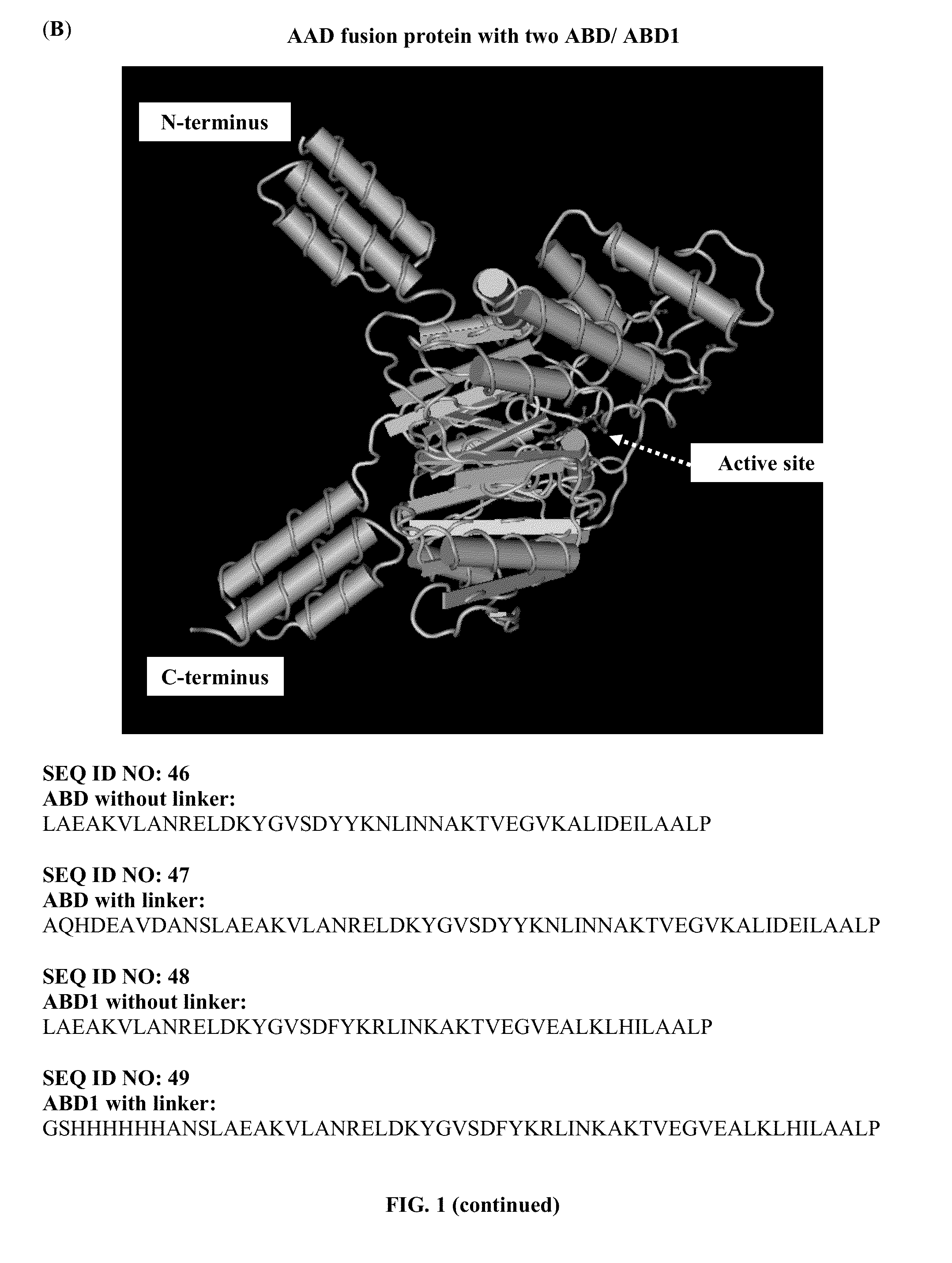
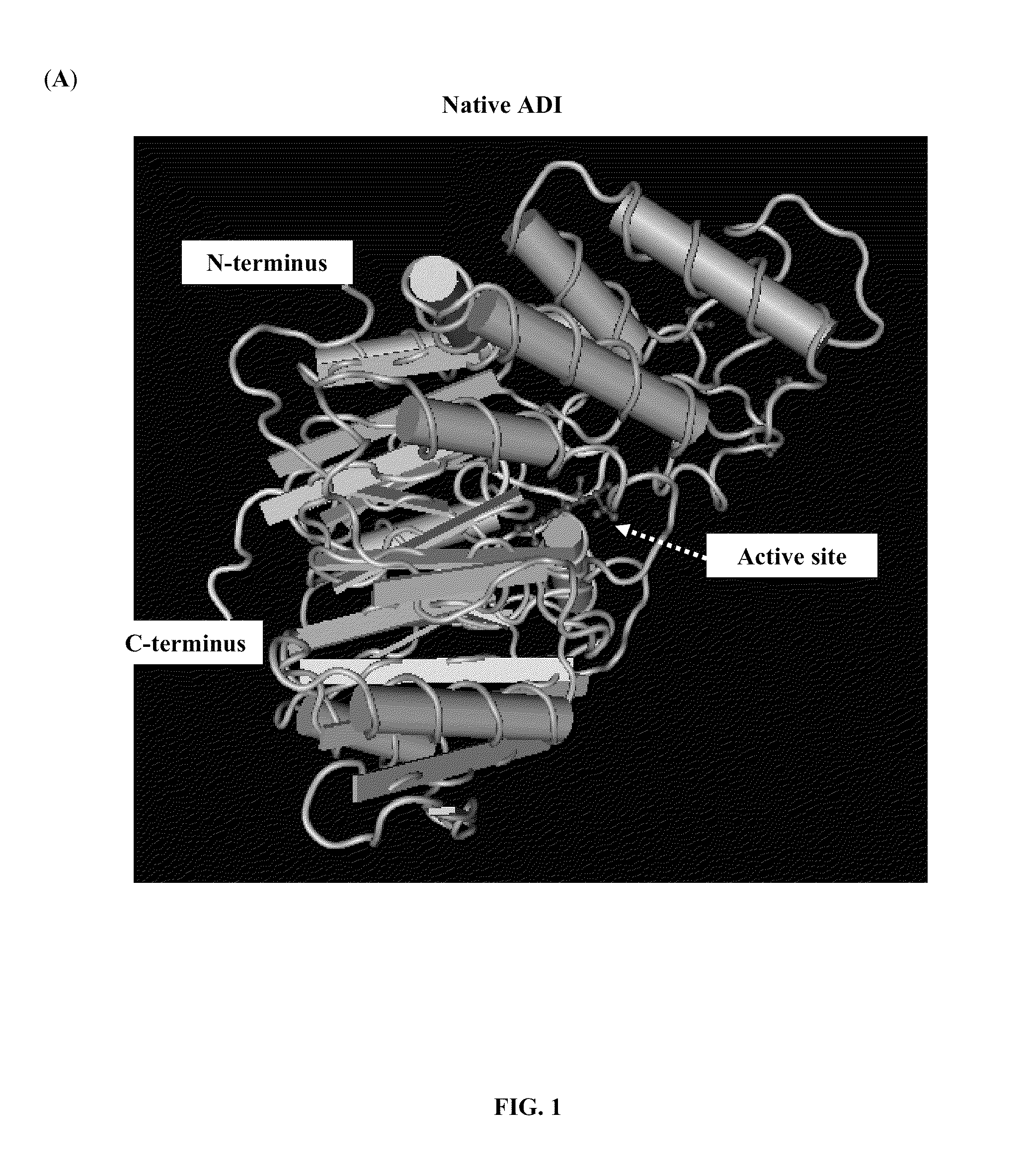
The present invention provides a pharmaceutical composition containing albumin-binding arginine deiminase fusion protein (AAD) for treating cancer or other arginine-dependent diseases. The AAD fusion protein can be purified from both soluble and insoluble fractions of crude proteins, it binds to human serum albumin (HSA) and has its high activity with longer half life for efficient depletion of arginine in cancer cells. The specific activities of wild-type ADI and AAD in the present invention are 8.4 and 9.2 U / mg (at physiological pH 7.4), respectively. The AAD used in the present invention can be used in the treatment of various cancers (e.g. pancreatic cancer, leukemia, head and neck cancer, colorectal cancer, lung cancer, breast cancer, liver cancer, nasopharyngeal cancer, esophageal cancer, prostate cancer, stomach cancer & brain cancer) and curing arginine-dependent diseases. The composition can be used alone or in combination with at least one chemotherapeutic agent to give a synergistic effect on cancer treatment and / or inhibiting metastasis.
Owner:VISION GLOBAL HLDG
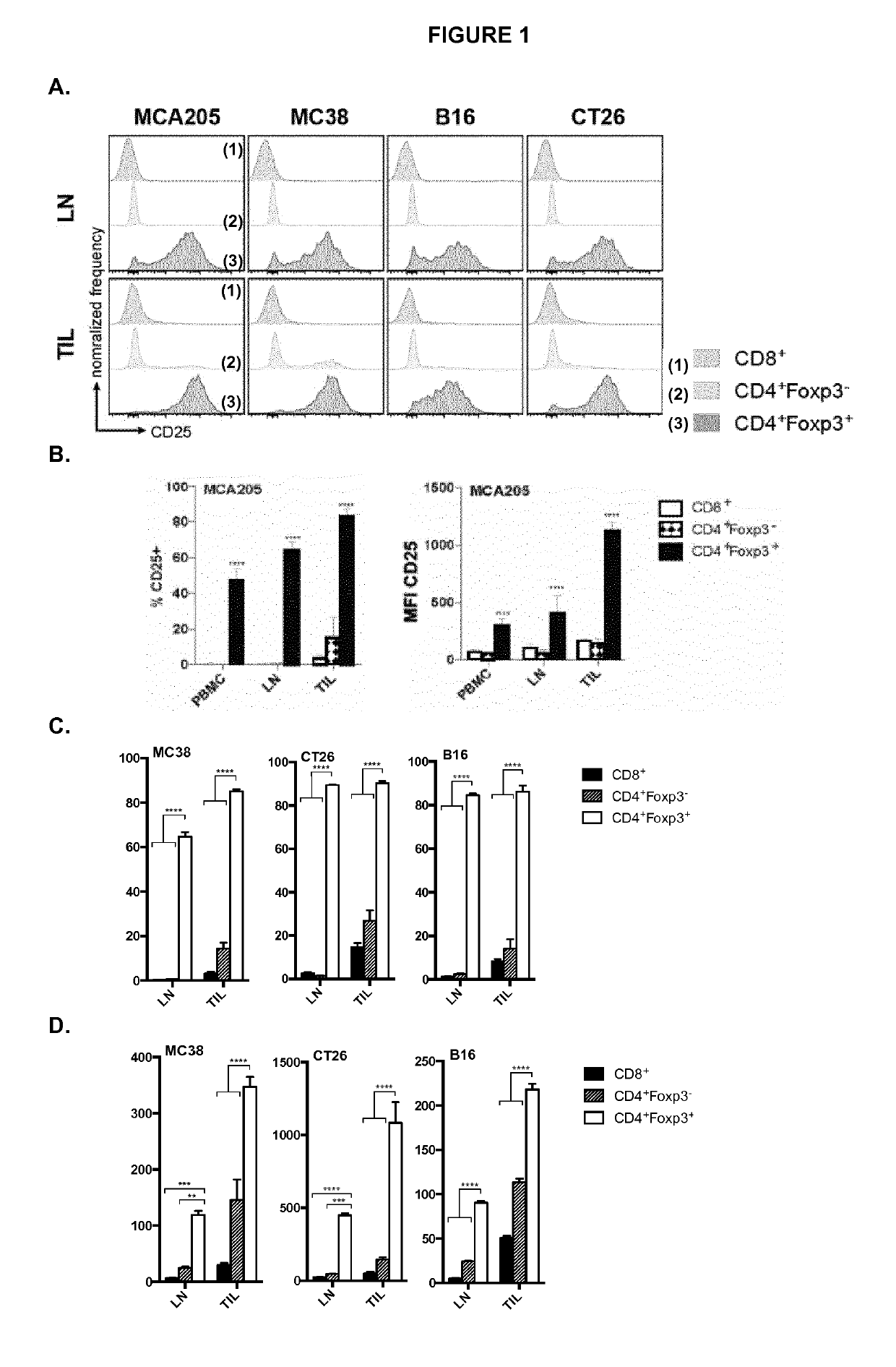
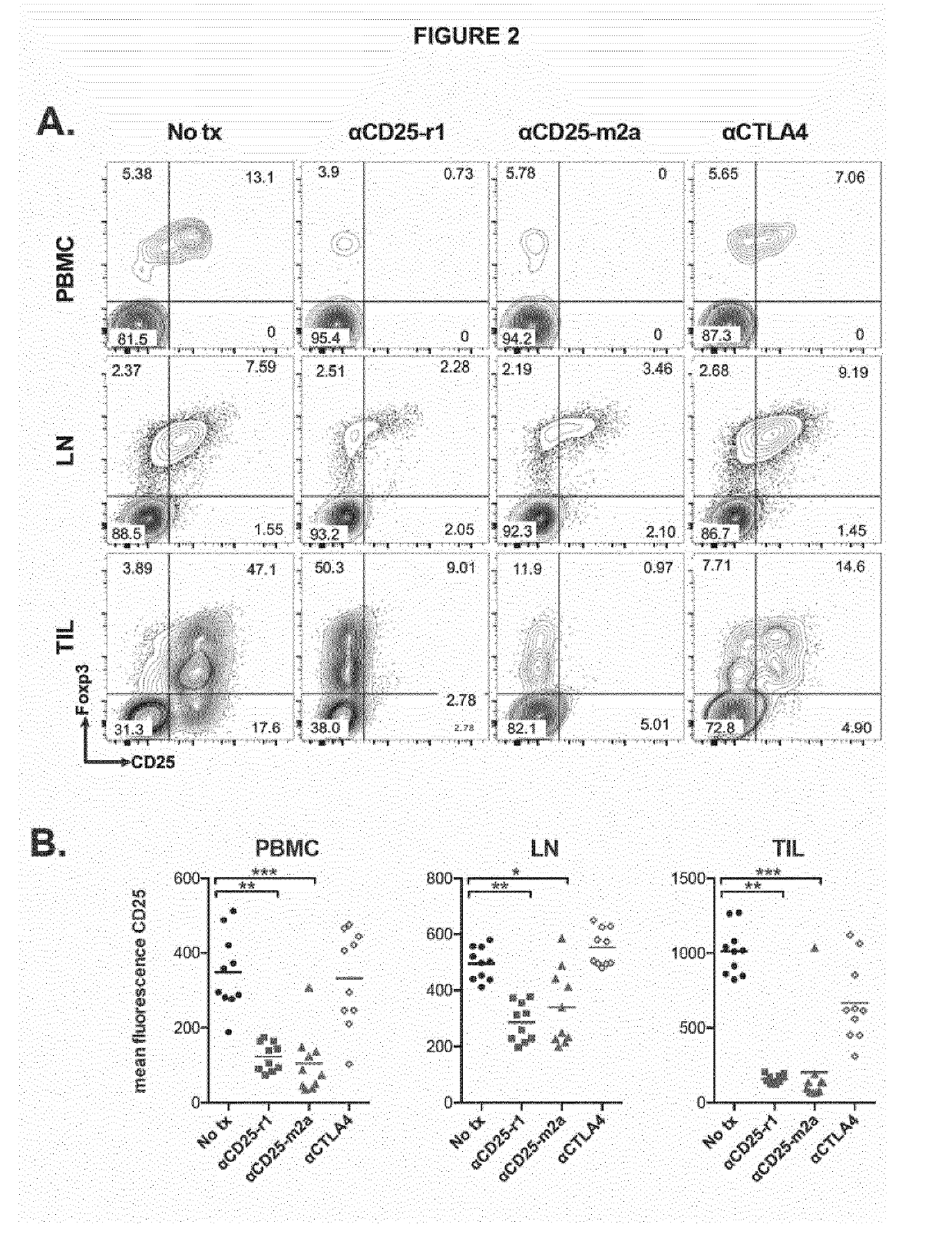
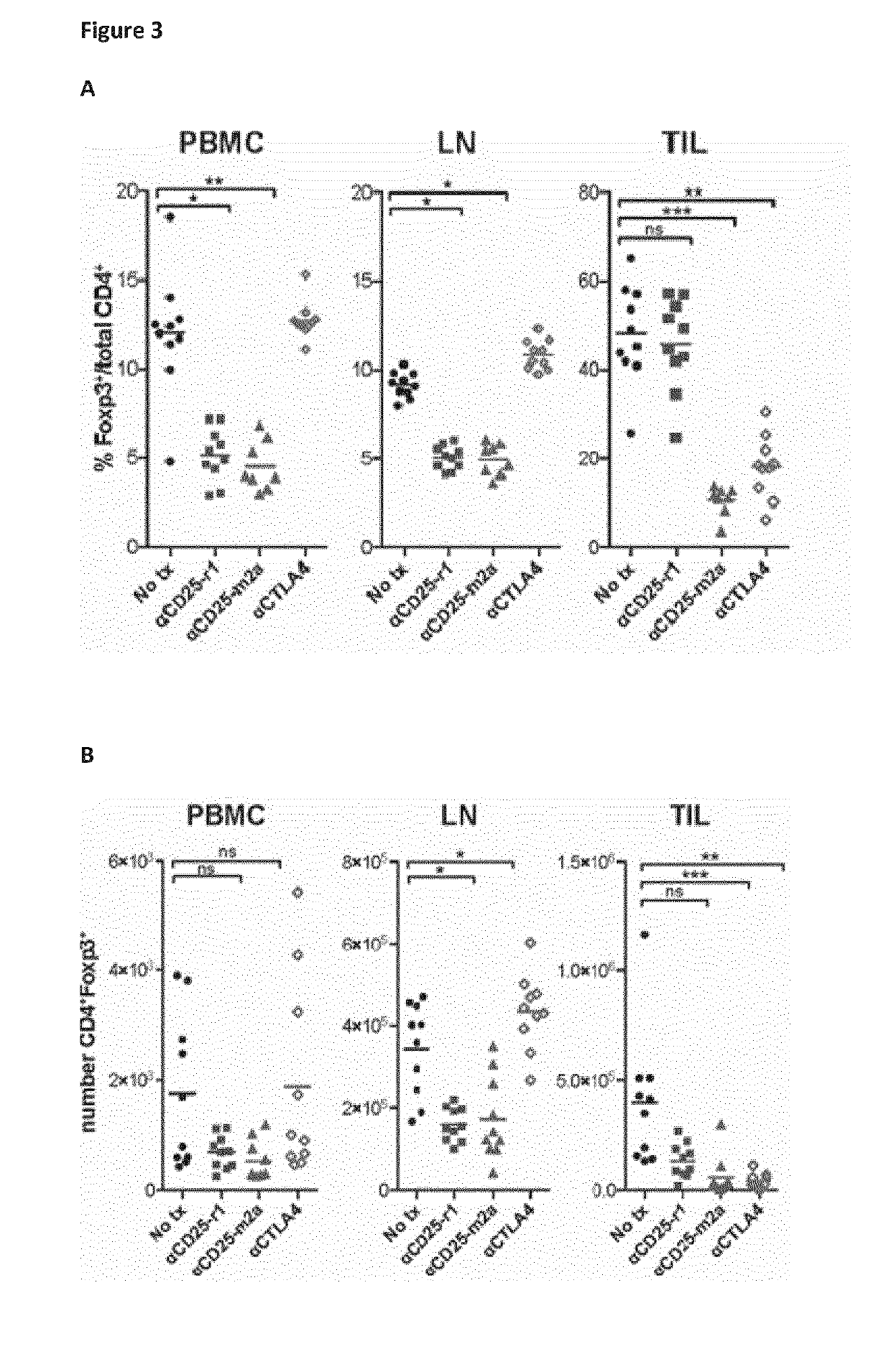
Anti cd25 fc gamma receptor bispecific antibodies for tumor specific cell depletion
InactiveUS20190135925A1Efficiently depletedAdd featureHybrid immunoglobulinsAntibody mimetics/scaffoldsFc receptorRegulatory T cell
The present disclosure relates to a method of treating a solid tumour, wherein said method involves the use of an antibody to CD25. In particular, the antibody to CD25 is optimized for depletion of regulatory T cells (Treg) within tumours. The present invention also provides novel anti-CD25 antibodies and their combination with other anti-cancer drugs, such as immune checkpoint inhibitors, compounds that target cancer antigens or the inhibitory Fc receptor FcyR11b (CD32b).
Owner:CANCER RES TECH LTD
Methods and compositions for mobilizing stem cells
InactiveUS20200268850A1Efficiently depletedRapid mobilizationOrganic active ingredientsNervous disorderProgenitor cellPbsc mobilization
The present invention relates to methods and compositions for mobilizing hematopoietic stem cells and / or progenitor cells, and related methods of conditioning for engraftment of transplanted hematopoietic stem cells and / or progenitor cells, and methods of treating diseases requiring hematopoietic stem cell and / or progenitor cell transplantation.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +1
Novel uses
InactiveUS20080274078A1Efficiently depletedPeptide/protein ingredientsAntibody ingredientsAntiendomysial antibodiesAnti antibody
The present invention relates generally to the use of human IL-18 combinations in the treatment of cancers. In particular, the present invention relates to combination of human IL-18 and an anti-CD20 antibody.
Owner:HASKOVA ZDENKA +4
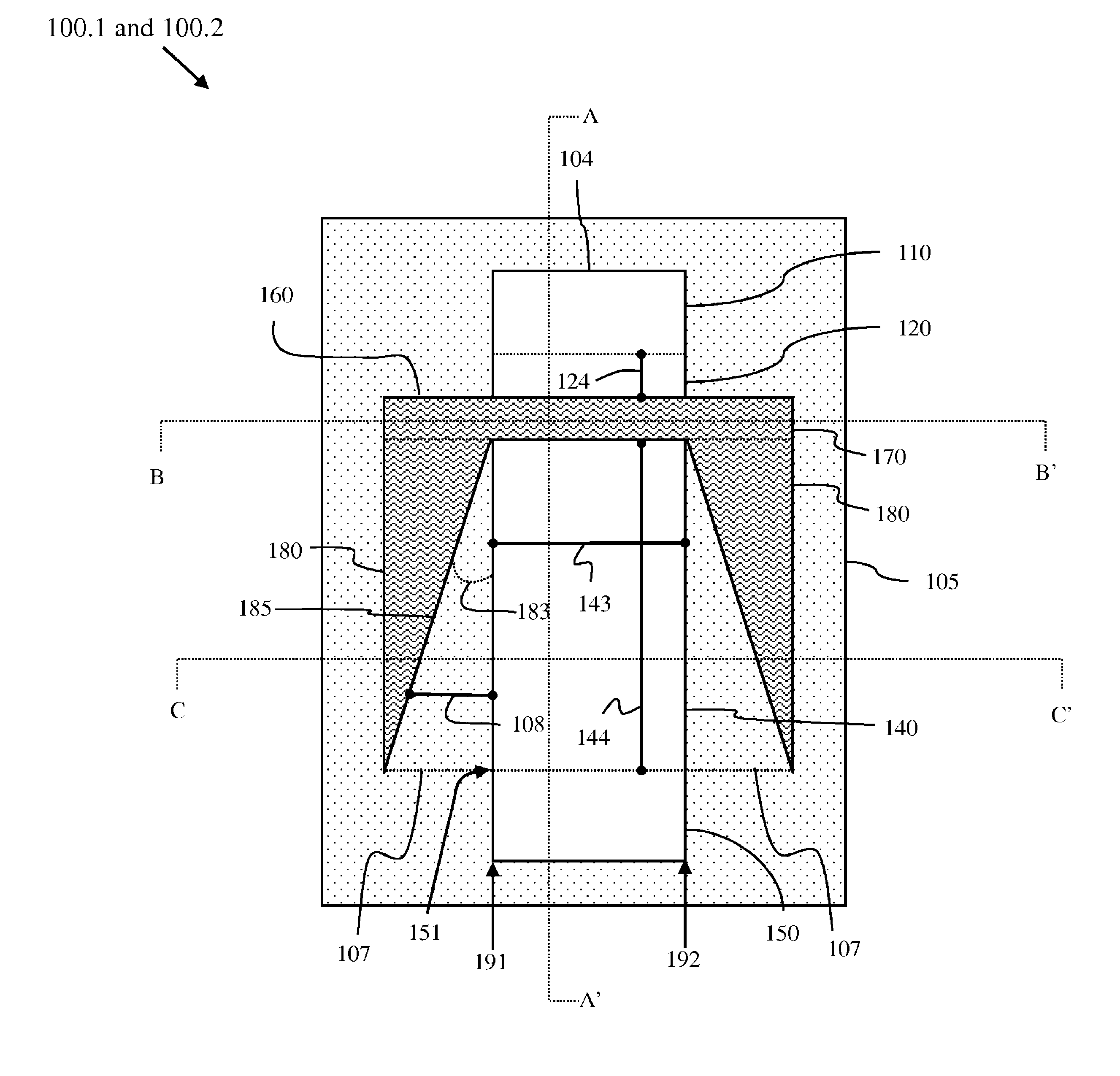
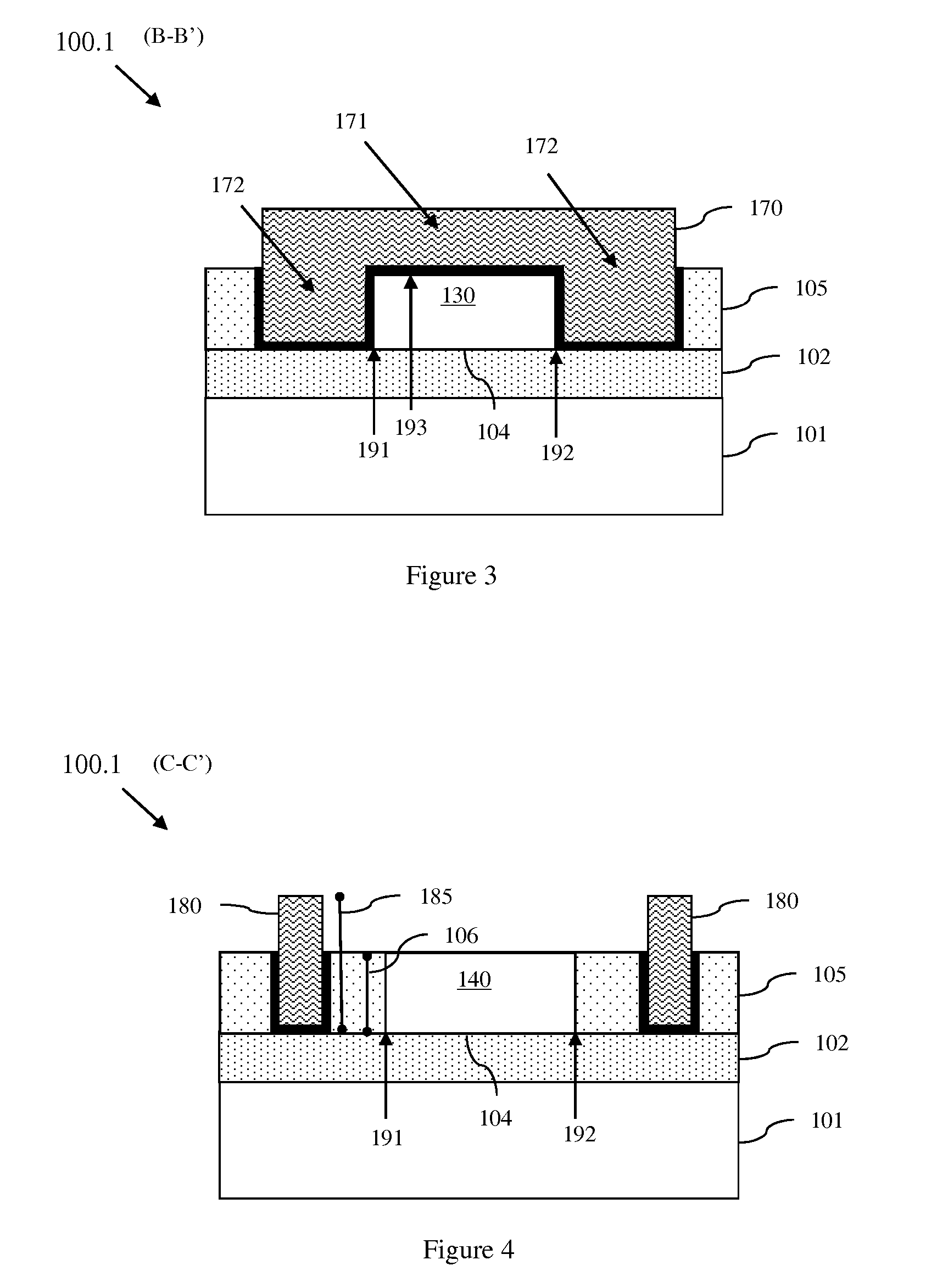
Lateral extended drain metal oxide semiconductor field effect transistor (LEDMOSFET) with tapered dielectric plates
InactiveUS8299547B2Efficiently depletedImprove breakdown voltageSemiconductor/solid-state device manufacturingCAD circuit designDielectric plateEngineering
A lateral, extended drain, metal oxide semiconductor, field effect transistor (LEDMOSFET) with a high drain-to-body breakdown voltage (Vb) incorporates gate structure extensions on opposing sides of a drain drift region. The extensions are tapered such that a distance between each extension and the drift region increases linearly from one end adjacent to the channel region to another end adjacent to the drain region. In one embodiment, these extensions can extend vertically through the isolation region that surrounds the LEDMOSFET. In another embodiment, the extensions can sit atop the isolation region. In either case, the extensions create a strong essentially uniform horizontal electric field profile within the drain drift. Also disclosed are a method for forming the LEDMOSFET with a specific Vb by defining the dimensions of the extensions and a program storage device for designing the LEDMOSFET to have a specific Vb.
Owner:GLOBALFOUNDRIES INC
Treating cancer
ActiveUS20180038860A1Reducing level of circulatingAvoid enteringOther blood circulation devicesDisease diagnosisHIV receptorChemokine receptor CCR5
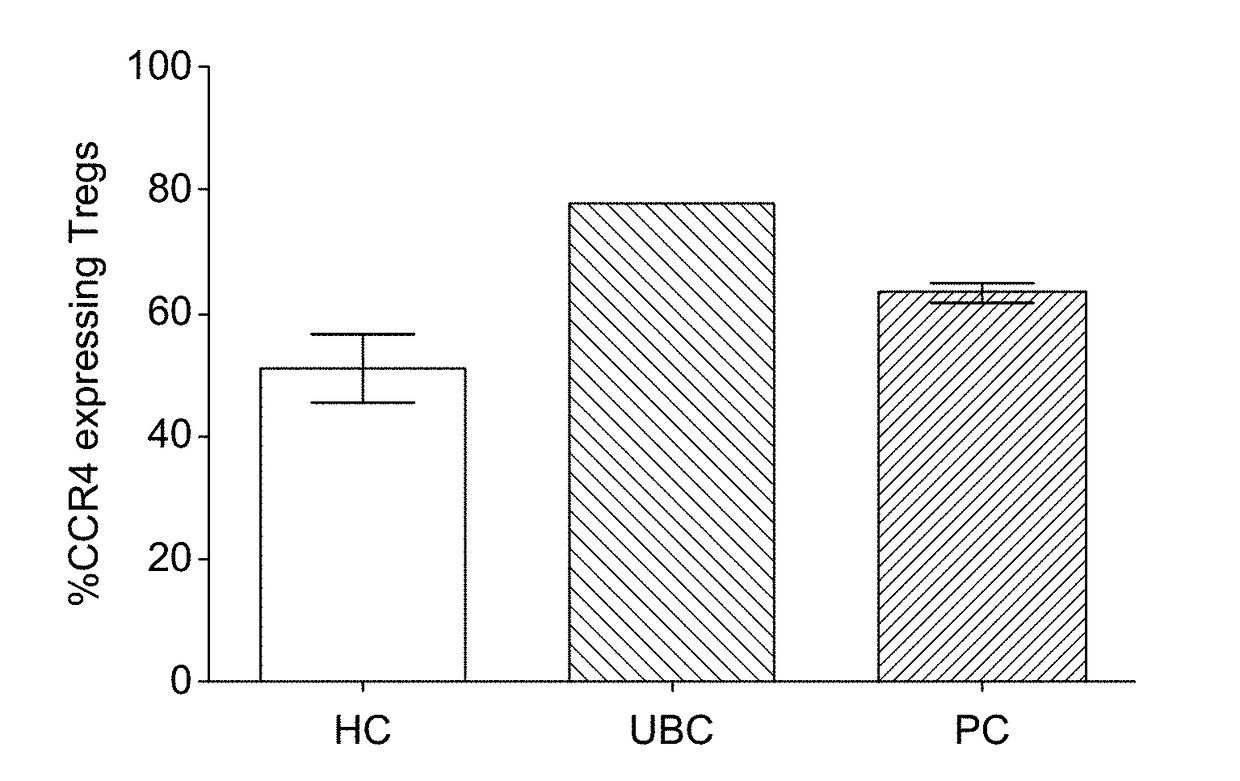
A method for treating cancer comprising applying peripheral blood from a patient or subject to an apheresis column loaded with a solid support comprising one or more binding reagents capable of specifically binding to a chemokine receptor, optionally the chemokine receptor CCR7, CCR5, CCR6, CCR8, CXCR4, CXCR7, CCR4, CCR9, CCR10, CXCR3 or CXCR5 or to a Treg receptor immobilized directly or indirectly on the support thus removing one or more chemokine receptor, optionally CCR7, CCR5, CCR6, CCR8, CXCR4, CXCR7, CCR4, CCR9, CCR10, CXCR3 or CXCR5 or Treg receptor expressing cells from the peripheral blood of the patient or subject. Various companion diagnostic methods and useful binding reagents are also described.
Owner:TLA TARGETED IMMUNOTHERAPIES AB
Albumin-binding arginine deminase and the use thereof
ActiveUS9255262B2Efficiently depletedHigh activityHydrolasesPeptide/protein ingredientsDiseaseLymphatic Spread
The present invention provides a pharmaceutical composition containing albumin-binding arginine deiminase fusion protein (AAD) for treating cancer or other arginine-dependent diseases. The AAD fusion protein can be purified from both soluble and insoluble fractions of crude proteins, it binds to human serum albumin (HSA) and has its high activity with longer half life for efficient depletion of arginine in cancer cells. The specific activities of wild-type ADI and AAD in the present invention are 8.4 and 9.2 U / mg (at physiological pH 7.4), respectively. The AAD used in the present invention can be used in the treatment of various cancers (e.g. pancreatic cancer, leukemia, head and neck cancer, colorectal cancer, lung cancer, breast cancer, liver cancer, nasopharyngeal cancer, esophageal cancer, prostate cancer, stomach cancer & brain cancer) and curing arginine-dependent diseases. The composition can be used alone or in combination with at least one chemotherapeutic agent to give a synergistic effect on cancer treatment and / or inhibiting metastasis.
Owner:VISION GLOBAL HLDG
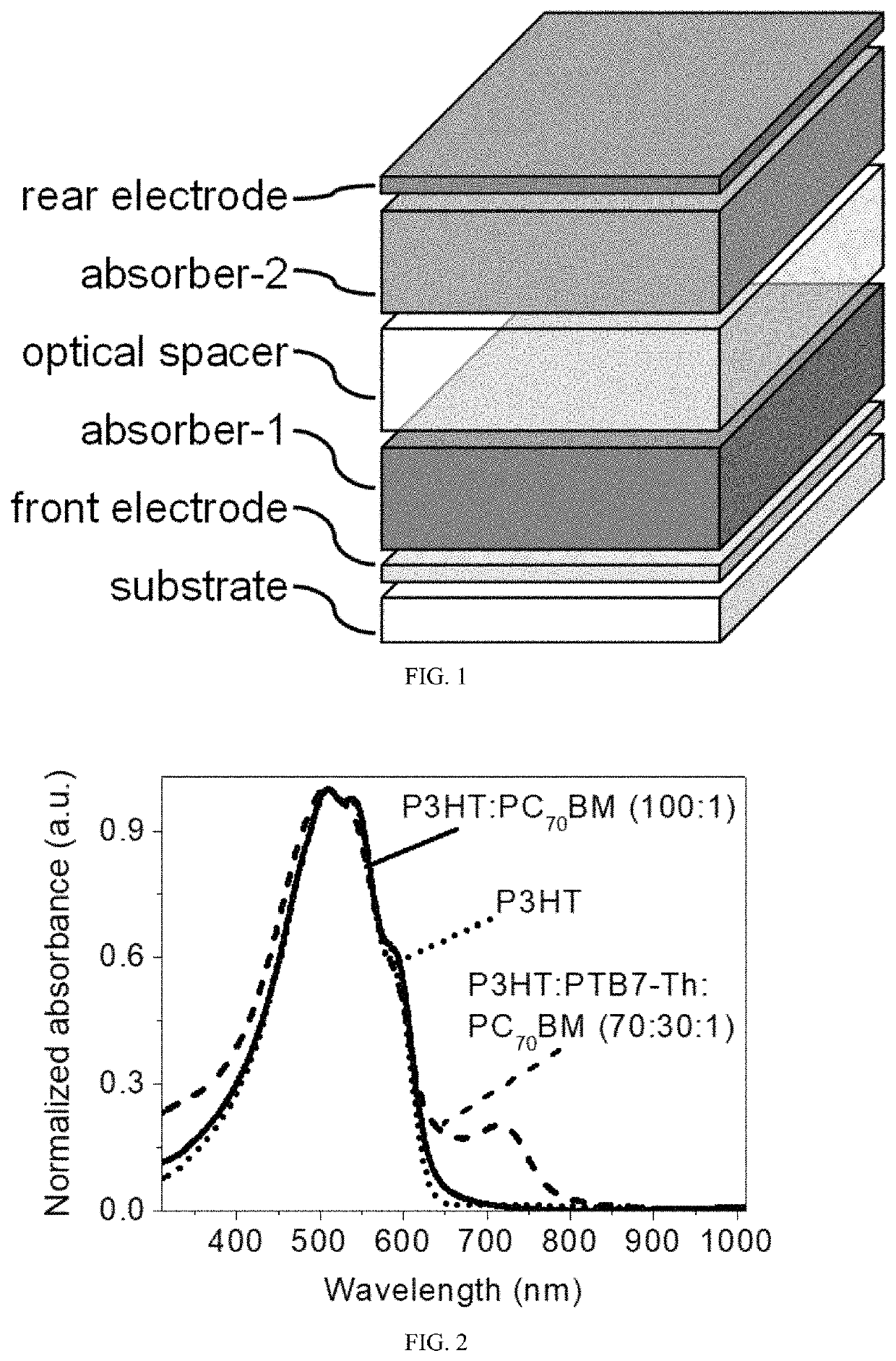
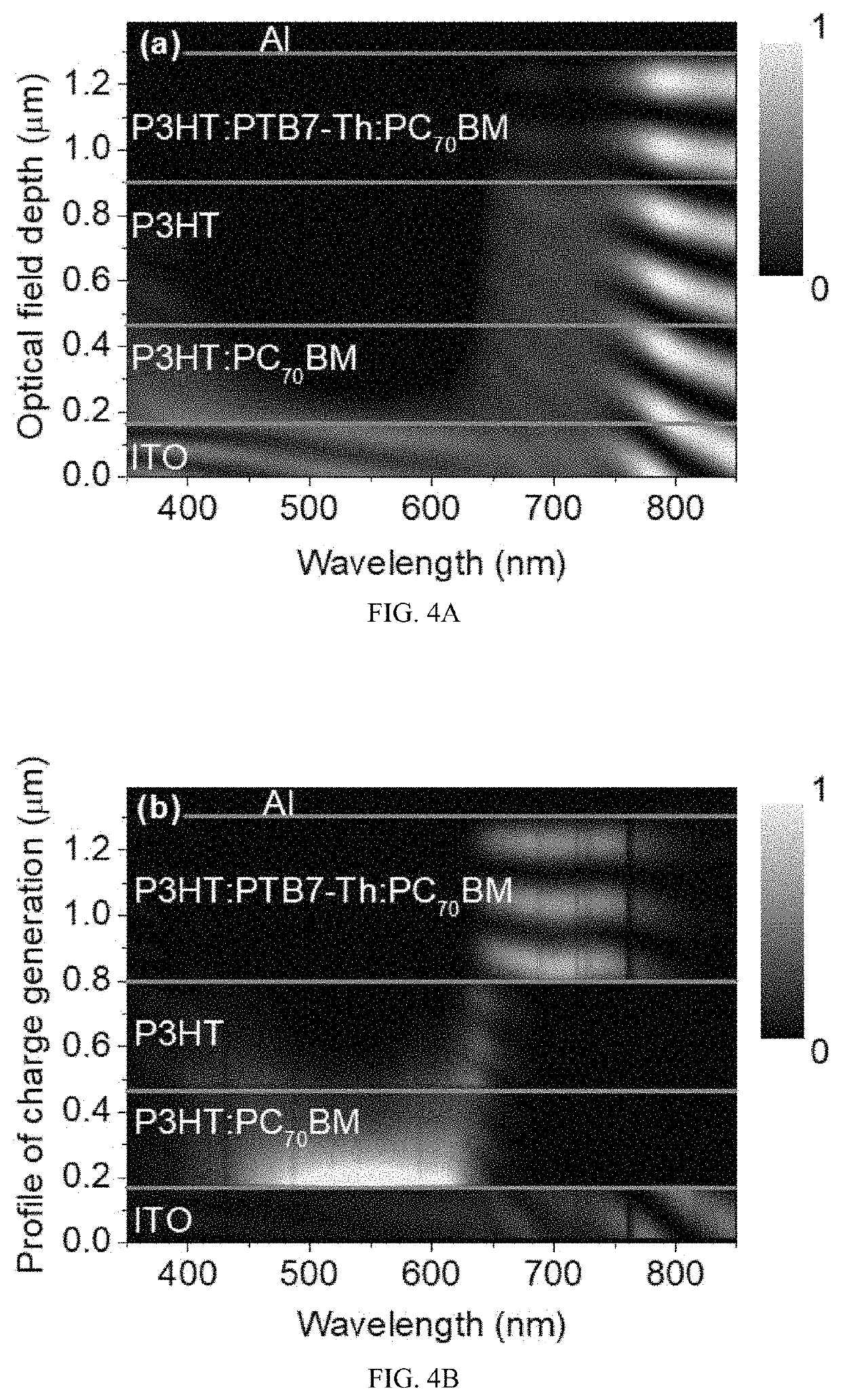
Multi-mode photodetectors and methods of fabricating the same
ActiveUS20200111980A1Efficiently depletedSolid-state devicesSemiconductor/solid-state device manufacturingSpectral responsePhotodetector
The present invention relates to a bias-switchable spectral response high performance PD with multi-mode detection, e.g., dual-mode photoresponses in NIR and visible light ranges. The dual-mode PD has the absorber / spacer type components in its active layer, e.g., a tri-layer configuration of absorber-1 (absorber-1 absorbs the electromagnetic wave of the first wavelength comprising visible light) / optical spacer / absorber-2 (absorber-2 absorbs the electromagnetic wave of the second wavelength comprising IR light). In the presence of IR light, photocurrent generates in the IR light absorbing layer under a reverse bias. In the presence of visible light, photocurrent generates in the visible light absorbing layer under a forward bias. A bias-switchable spectral response PD offers an attractive option for applications in environmental pollution, bio, medical, agricultural, automotive, fishery, food, wellness and security monitoring, detection and imaging in two or different or multiple distinct bands.
Owner:HONG KONG BAPTIST UNIV
Prodrug of 1,1′-(1,6-dioxo-1,6-hexanediyl)bis-D-proline
ActiveUS9737505B2Efficiently depletedGood physiochemical propertyOrganic active ingredientsOrganic chemistryDiseaseCarboxylic acid
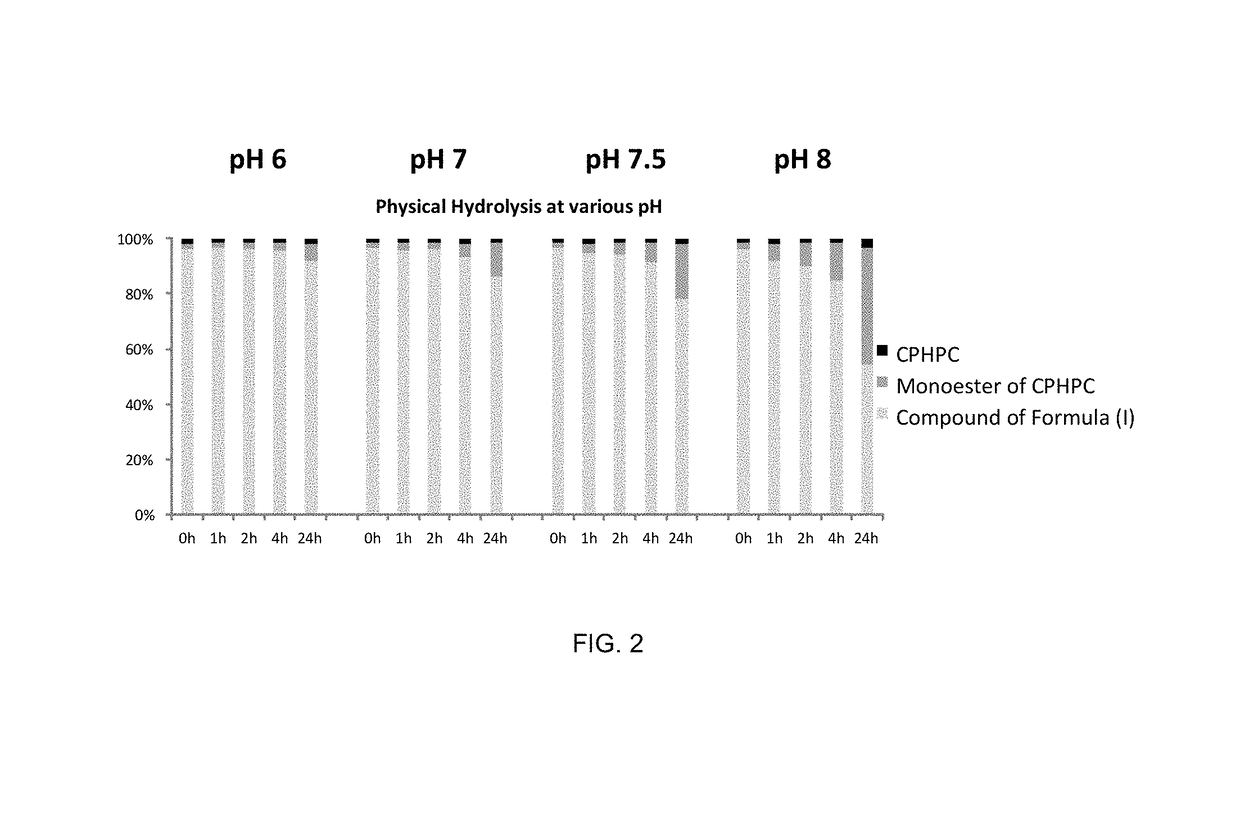
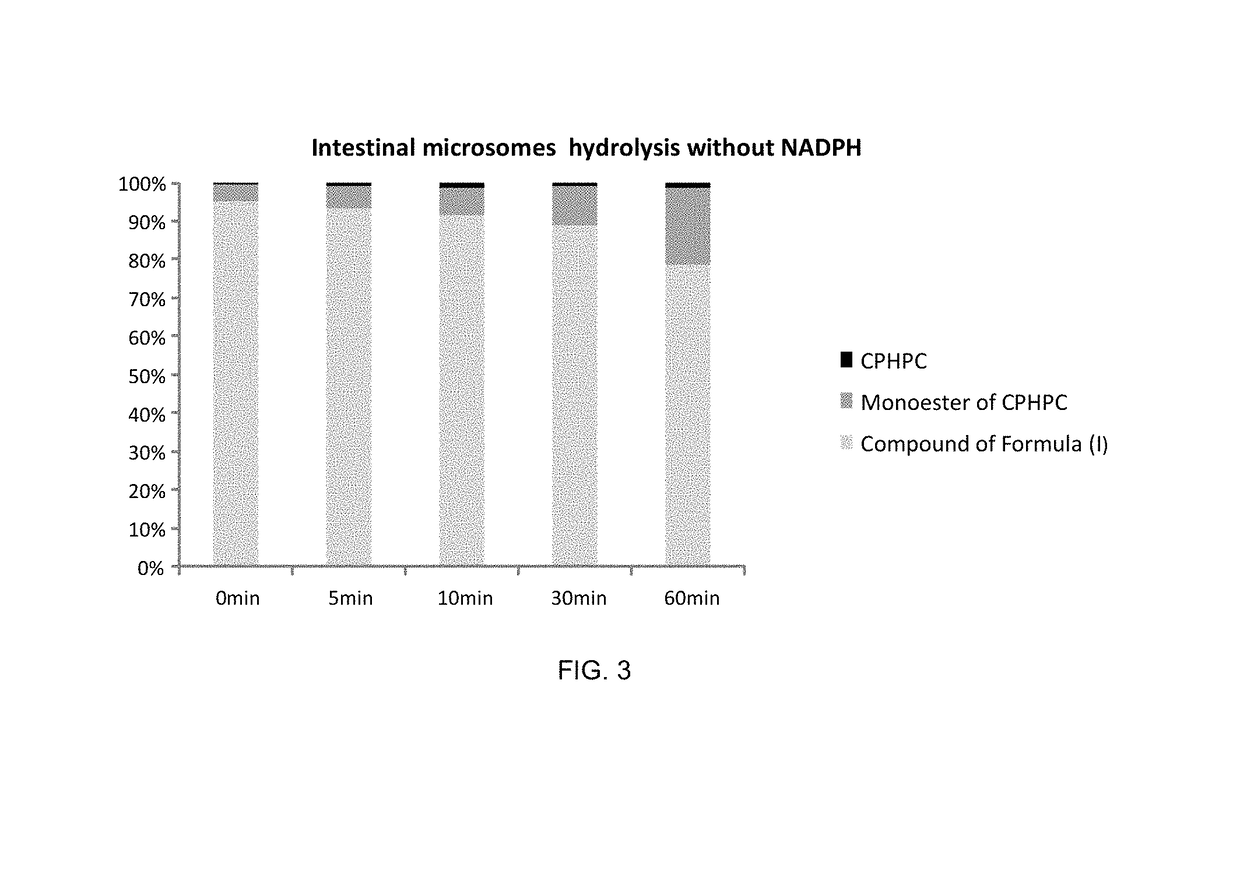
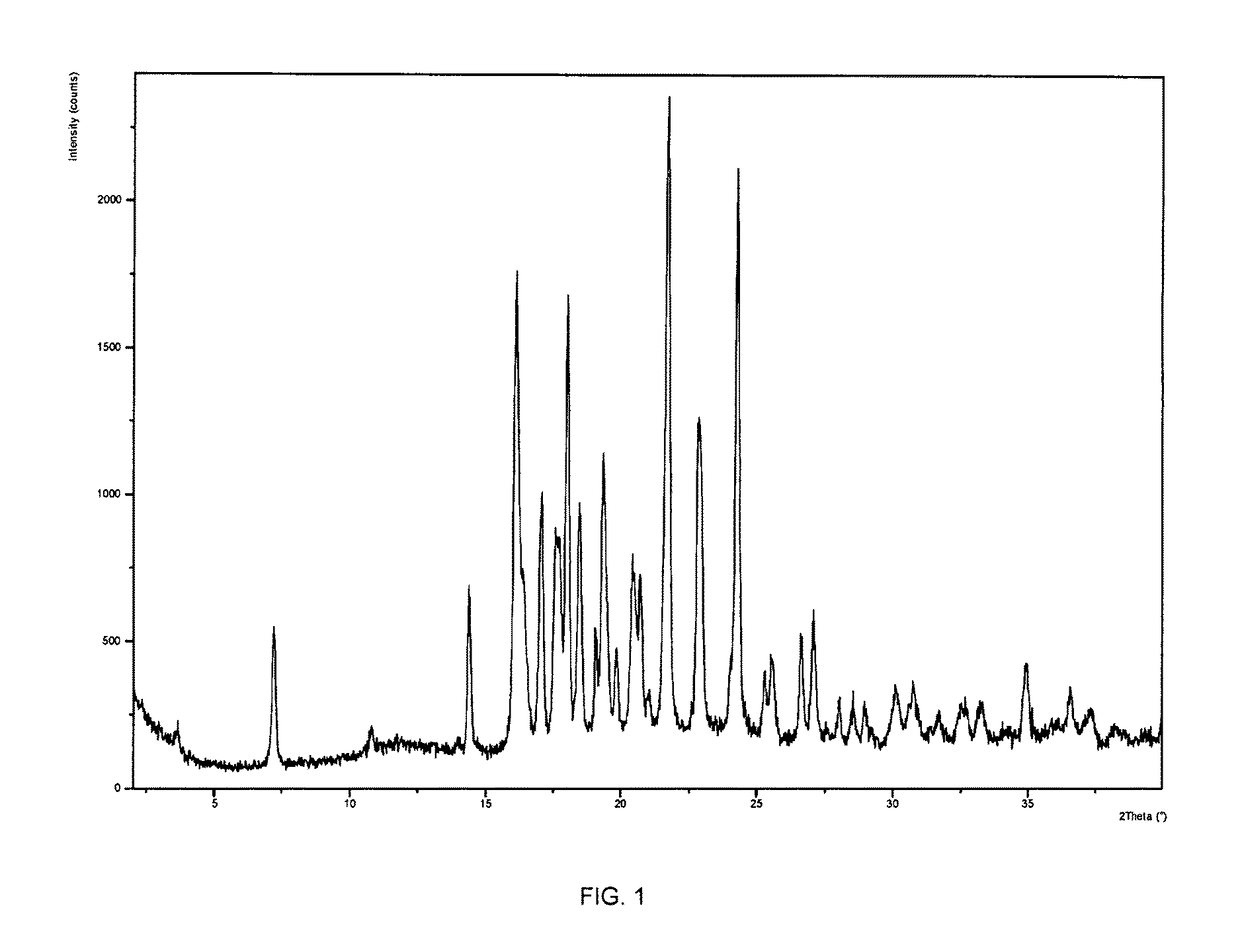
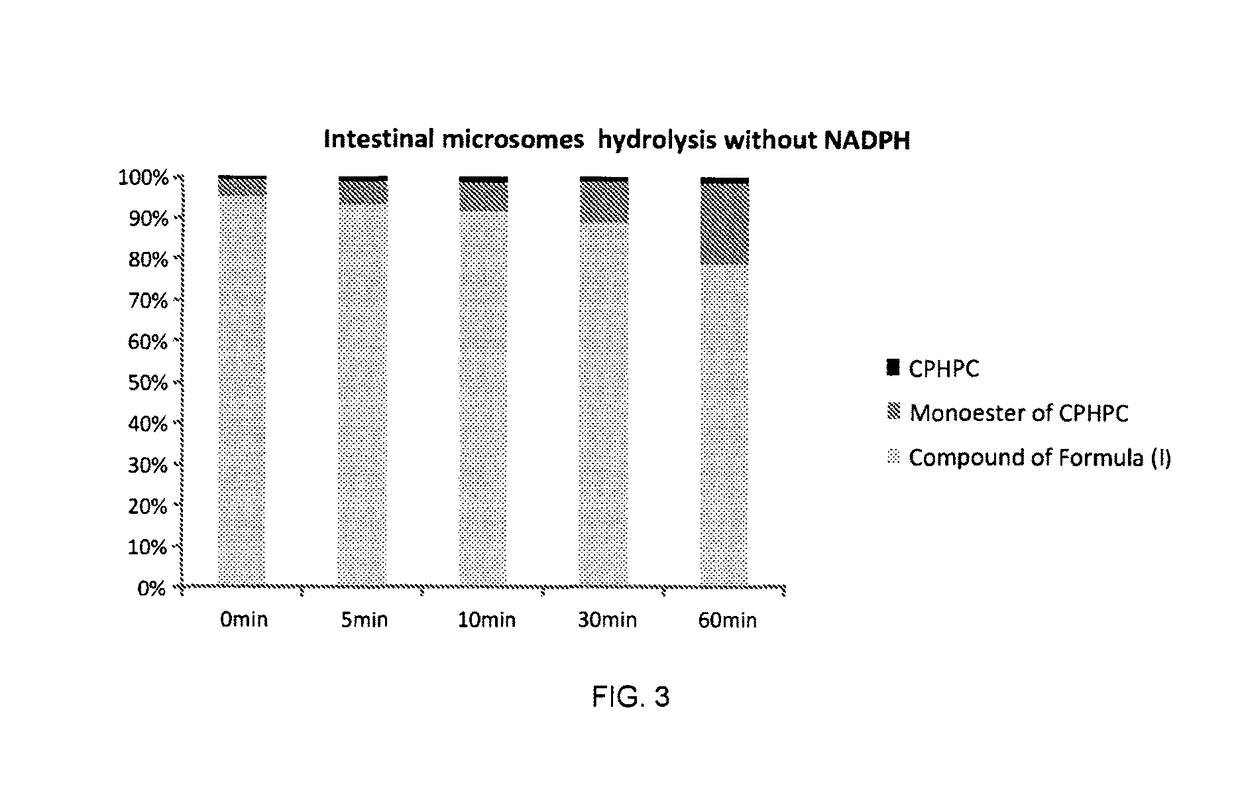
The compound (2R,2′R)-bis(((((tetrahydro-2H-pyran-4-yl)oxy)carbonyl)oxy)methyl) 1,1′-adipoylbis(pyrrolidine-2-carboxylate) possesses physicochemical properties suitable for pharmaceutical development and is capable of generating (R)-1-[6-[(R)-2-carboxy-pyrrolidin-1-yl]-6-oxo-hexanoyl]pyrrolidine-2-carboxylic acid (CPHPC) in quantities capable of depleting serum amyloid P component (SAP) efficiently following oral administration, making it useful in the treatment of amyloidosis, Alzheimer's disease, type 2 diabetes mellitus and osteoarthritis.
Owner:GLAXOSMITHKLINE INTPROP DEV LTD
Prodrug of 1,1′-(1,6-dioxo-1,6-hexanediyl)bis-D-proline
ActiveUS9701668B2Efficiently depletedGood physiochemical propertyOrganic active ingredientsNervous disorderCarboxylic acidSerum amyloid P component
The present invention relates to the compound (2R,2′R)-bis(((((tetrahydro-2H-pyran-4-yl)oxy)carbonyl)oxy)methyl) 1,1′-adipoylbis(pyrrolidine-2-carboxylate) of formula (I), pharmaceutical compositions comprising the same and the use of the same for treatment of diseases or disorders wherein depletion of serum amyloid P component (SAP) would be beneficial, including amyloidosis, Alzheimer's disease, type 2 diabetes mellitus and osteoarthritis.
Owner:GLAXOSMITHKLINE INTPROP DEV LTD
Method for the detection of gaseous impurities in materials
InactiveUS8113035B2Easy to monitorSmall regionPreparing sample for investigationUsing mechanical meansHydrogenNiobium
Disclosed is a method for detecting hydrogen in steel. According to the method, hydrogen contained in steel is transferred into and preferably concentrated in at least one second material. Representative second materials include metals such as vanadium, niobium, tantalum, and their alloys. Upon transfer to the second material, the hydrogen is detected and preferably quantitatively determined. The data obtained with the method enables conclusions to be drawn about the presence of hydrogen in steel. Preferably, the concentration of hydrogen in steel is quantitatively determined from information obtained about the presence of hydrogen in the second material.
Owner:KIRCHHEIM REINER +2
Bioresponsive Particles
InactiveUS20190365869A1Valid conversionEfficiently depletedPowder deliveryCarbon-sulfur lyasesSilicon dioxideDrug biological activity
Shielding enzymes are made by modifying the enzyme surface with silica precursors and then depositing silica to a desired thickness while retaining biological activity of the enzyme.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Humanized anti-CD22 antibodies and their use in treatment of oncology, transplantation and autoimmune disease
InactiveUS20130028888A1Efficient productionEfficiently depletedSugar derivativesImmunoglobulins against animals/humansAutoimmune conditionAutoimmune disease
The present invention provides chimeric and humanized versions of anti-CD22 mouse monoclonal antibody, HB22.7. The anti-CD22 antibodies of the invention comprise four human or humanized framework regions of the immunoglobulin heavy chain variable region (“VH”) and four human or humanized framework regions of the immunoglobulin light chain variable region (“VK”). The invention further comprises heavy and / or light chain FW regions that contain one or more backmutations in which a human FW residue is exchanged for the corresponding residue present in the parental mouse heavy or light chain. Human or humanized VH framework regions of antibodies of the invention may comprise one or more of the following residues: a valine (V) at position 24 of framework region 1, a glycine (G) at position 49 of framework region 2, and an asparagine (N) at position 73 of framework region 3, numbered according to Kabat. The invention further relates to pharmaceutical compositions, immunotherapeutic compositions, and methods using therapeutic antibodies that bind to the human CD22 antigen and that preferably mediate human ADCC, CDC, and / or apoptosis for: the treatment of B cell diseases and disorders in human subjects, such as, but not limited to, B cell malignancies, for the treatment and prevention of autoimmune disease, and for the treatment and prevention of graft-versus-host disease (GVHD), humoral rejection, and post-transplantation lymphoproliferative disorder in human transplant recipients.
Owner:AERES BIOMEDICAL
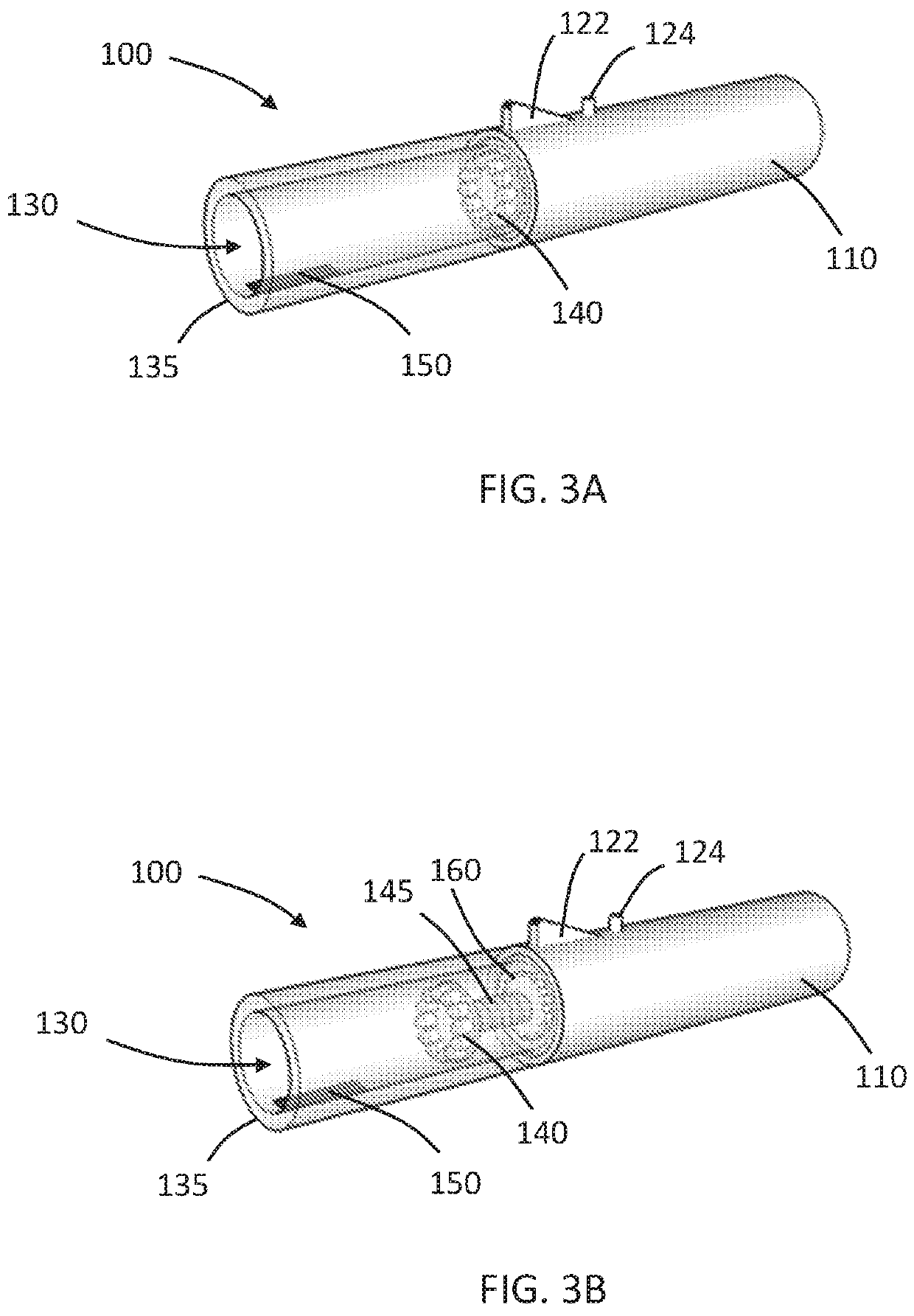
Aerosol generating device with spiral movement for heating
ActiveUS11478590B2SmallEnergy efficiency efficientCigar manufactureMedical devicesEngineeringPhysics
An aerosol generating device includes a housing having an open end and forms a cavity in communication with the open end for receiving an aerosol generating article. The device further includes a rotatable capture element disposed in the cavity. The rotatable capture element is operable to retain the aerosol generating article within the cavity. The rotatable capture element is longitudinally movable within the cavity. The device further includes a heating element in communication with the cavity. The heating element is operable to heat the aerosol generating article retained by the capture element within the cavity. Combined rotation and longitudinal movement of the capture element causes the aerosol generating element to spiral relative to the heating element.
Owner:PHILIP MORRIS PROD SA
Multi-mode photodetectors and methods of fabricating the same
ActiveUS11329239B2Efficiently depletedSolid-state devicesSemiconductor/solid-state device manufacturingSpectral responsePhotodetector
The present invention relates to a bias-switchable spectral response high performance PD with multi-mode detection, e.g., dual-mode photoresponses in NIR and visible light ranges. The dual-mode PD has the absorber / spacer type components in its active layer, e.g., a tri-layer configuration of absorber-1 (absorber-1 absorbs the electromagnetic wave of the first wavelength comprising visible light) / optical spacer / absorber-2 (absorber-2 absorbs the electromagnetic wave of the second wavelength comprising IR light). In the presence of IR light, photocurrent generates in the IR light absorbing layer under a reverse bias. In the presence of visible light, photocurrent generates in the visible light absorbing layer under a forward bias. A bias-switchable spectral response PD offers an attractive option for applications in environmental pollution, bio, medical, agricultural, automotive, fishery, food, wellness and security monitoring, detection and imaging in two or different or multiple distinct bands.
Owner:HONG KONG BAPTIST UNIV
Bioresponsive Particles
ActiveUS20190367902A1Efficiently depletedExtended functional lifePowder deliveryPeptide/protein ingredientsSilicon dioxideEnzyme
Shielding enzymes are made by modifying the enzyme surface with silica precursors and then depositing silica to a desired thickness while retaining biological activity of the enzyme.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com