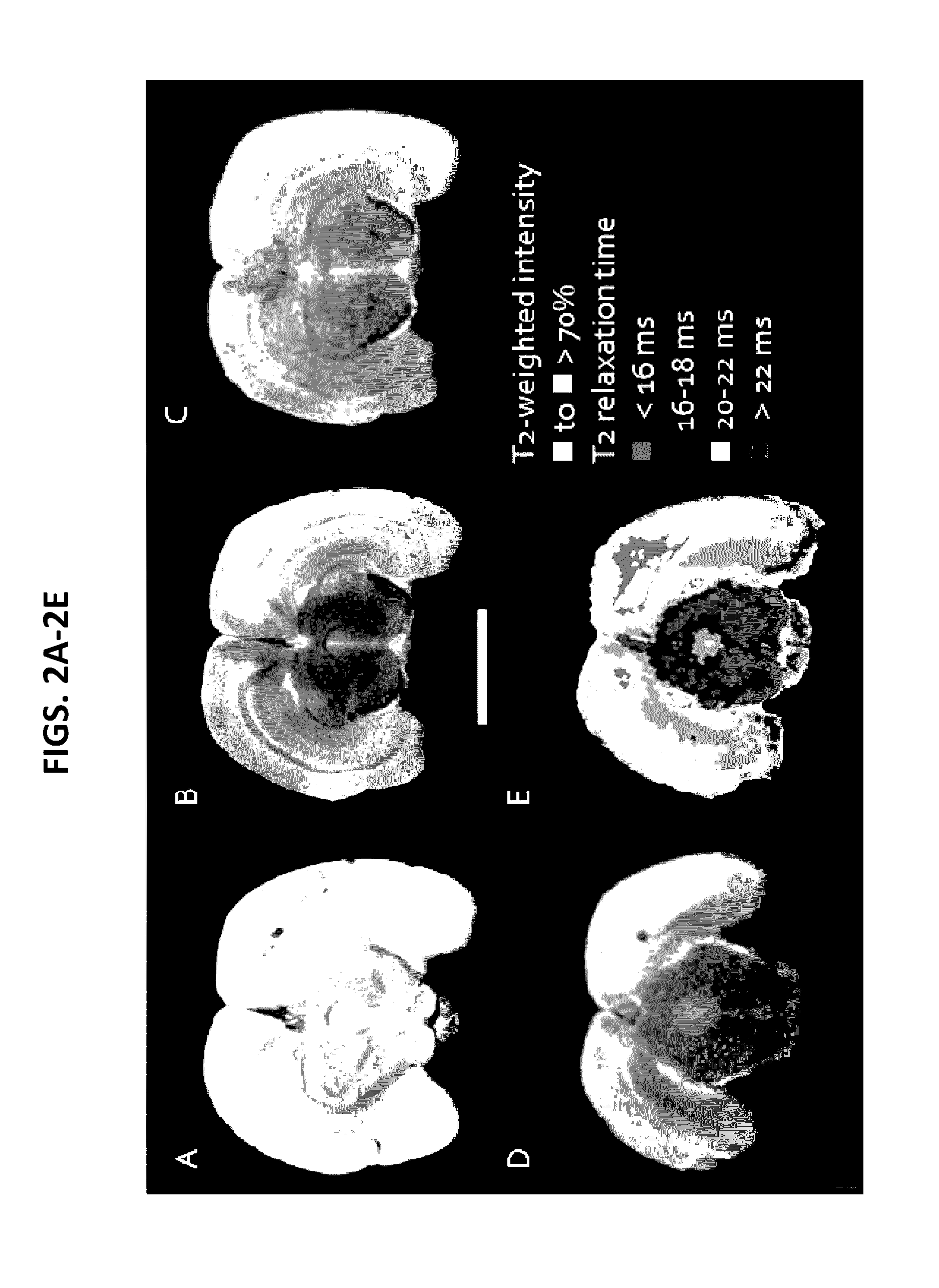
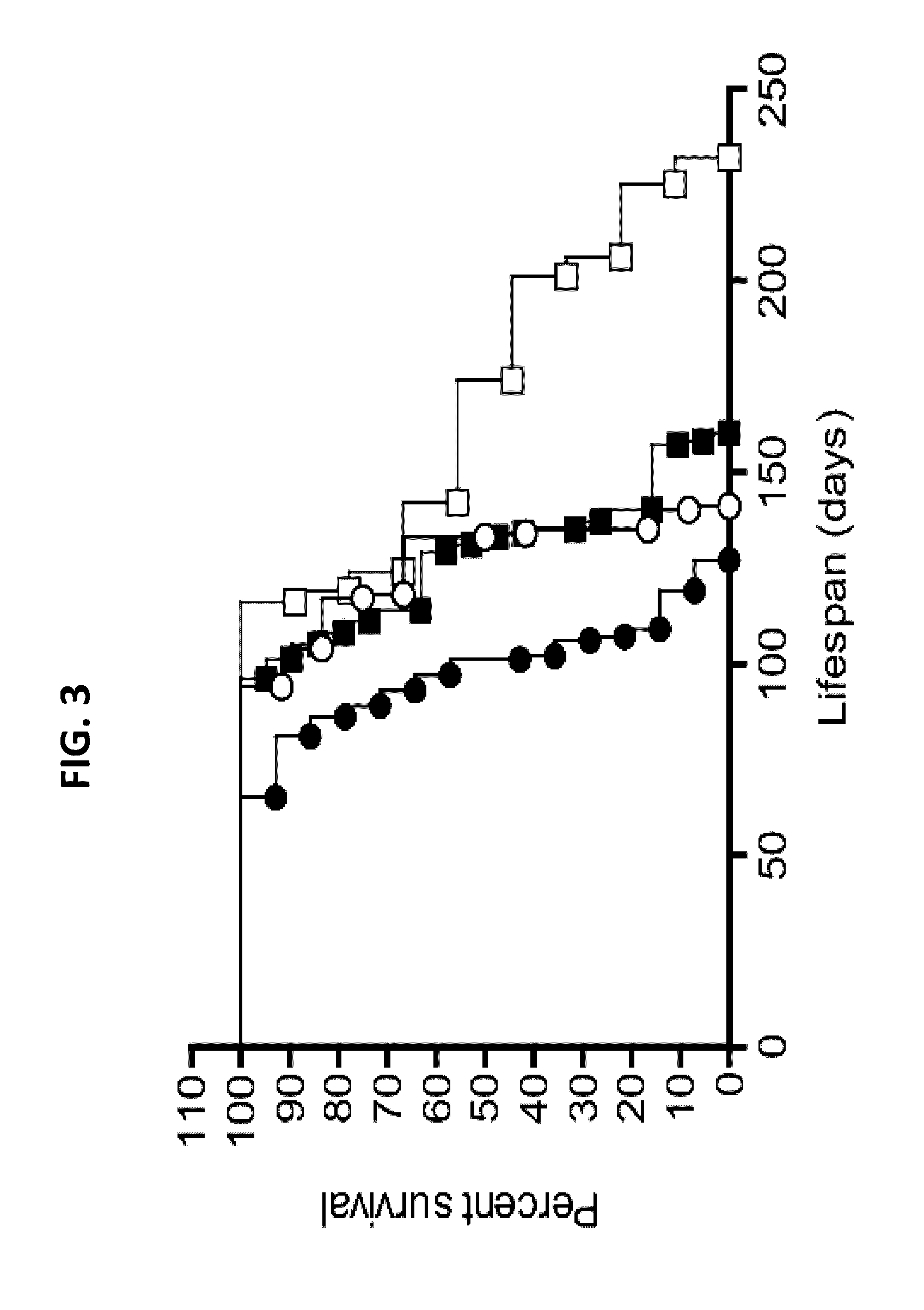
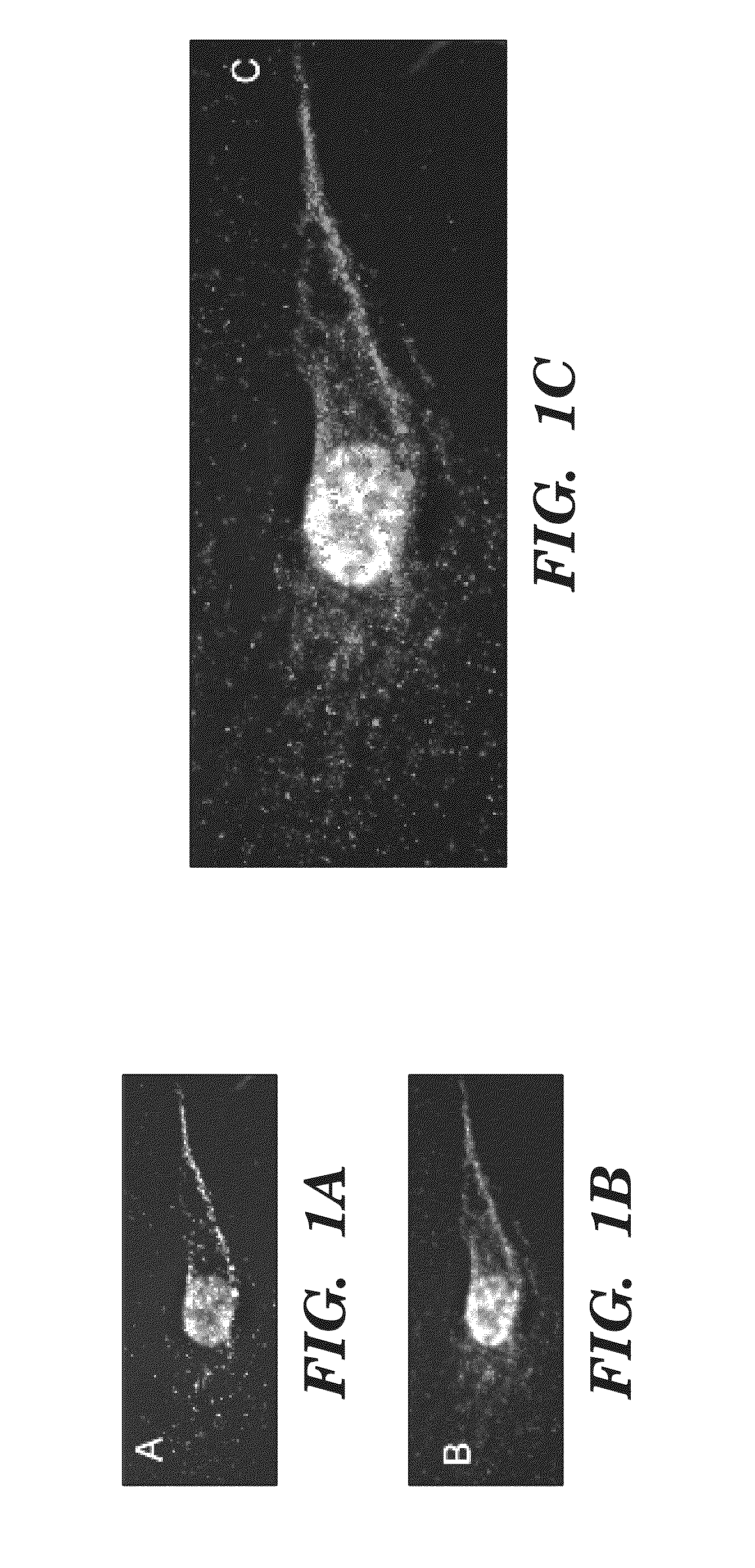
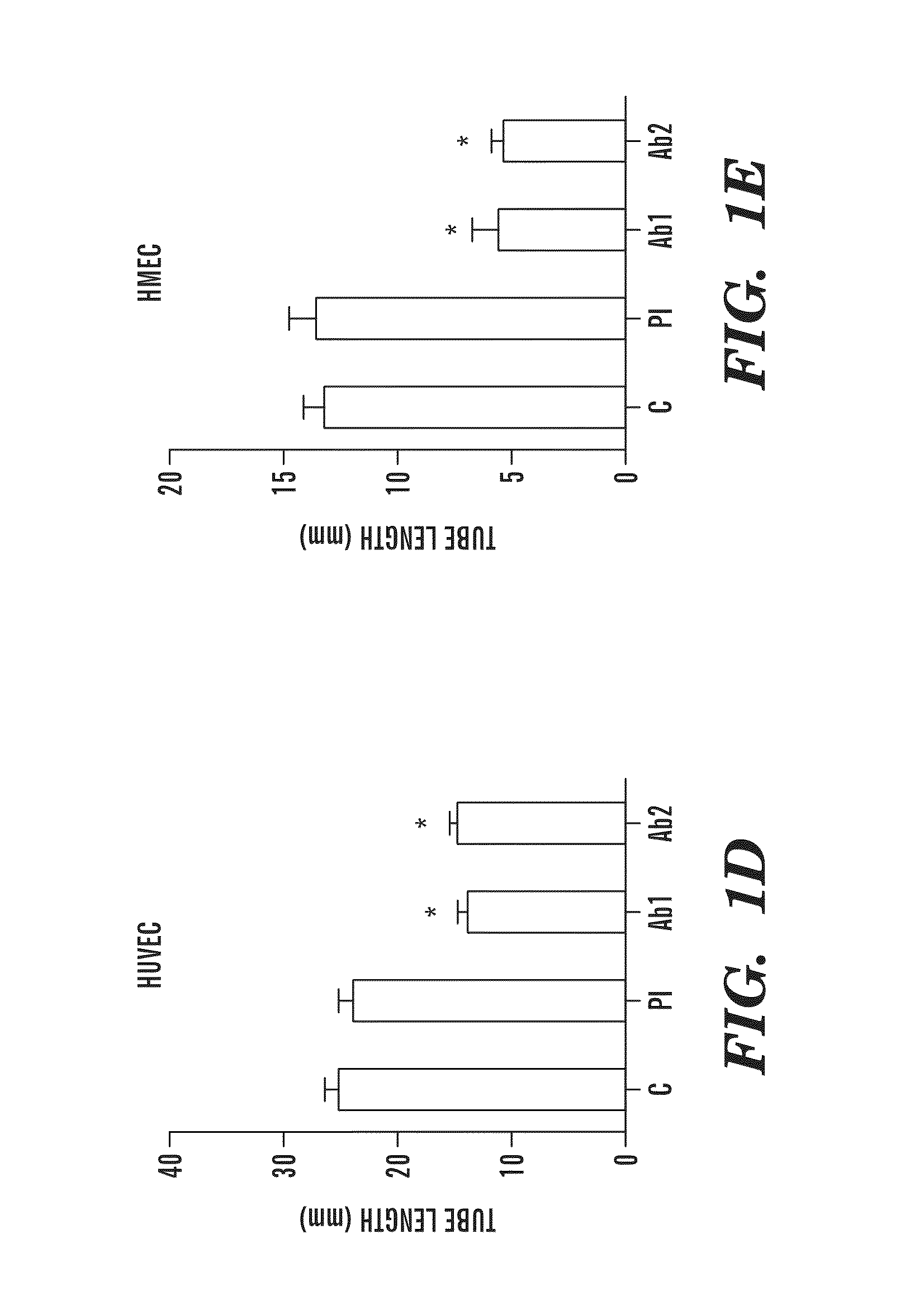
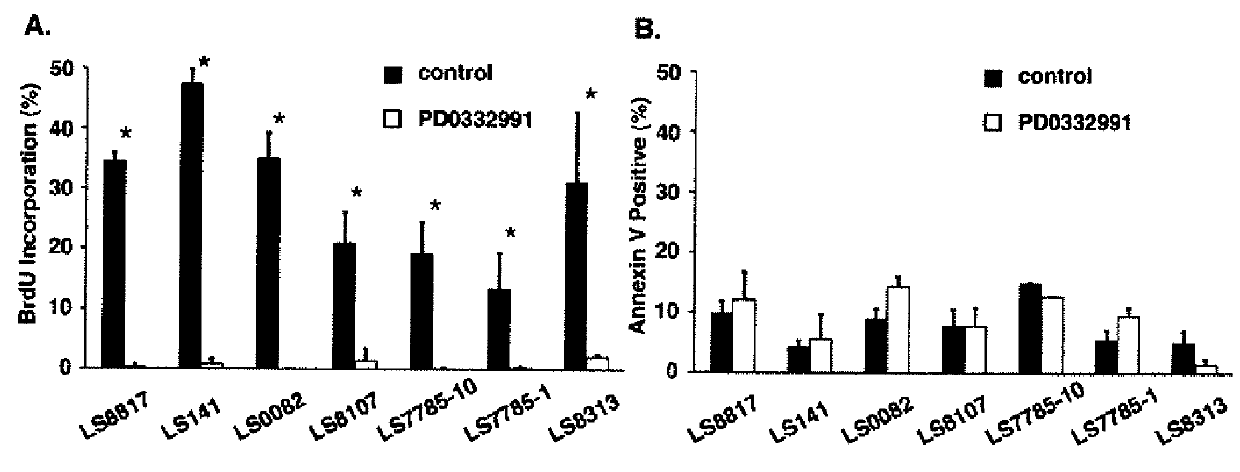
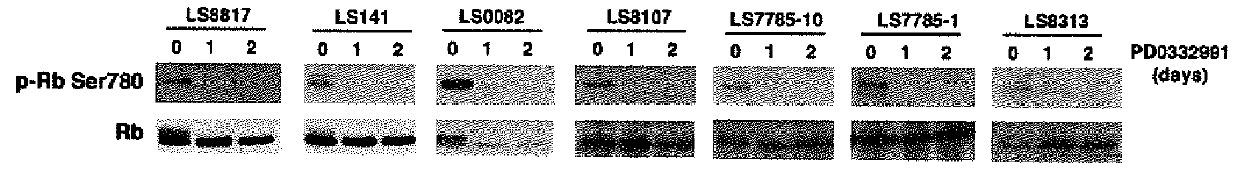
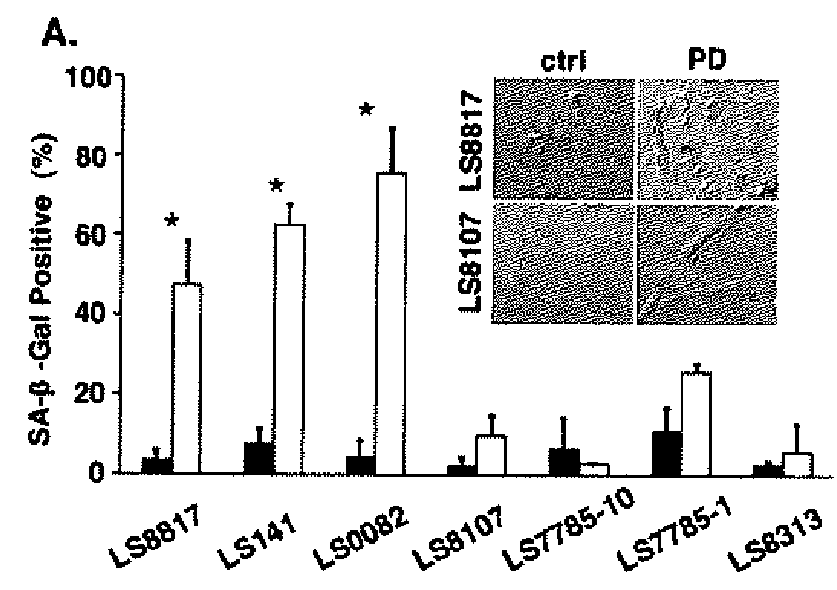
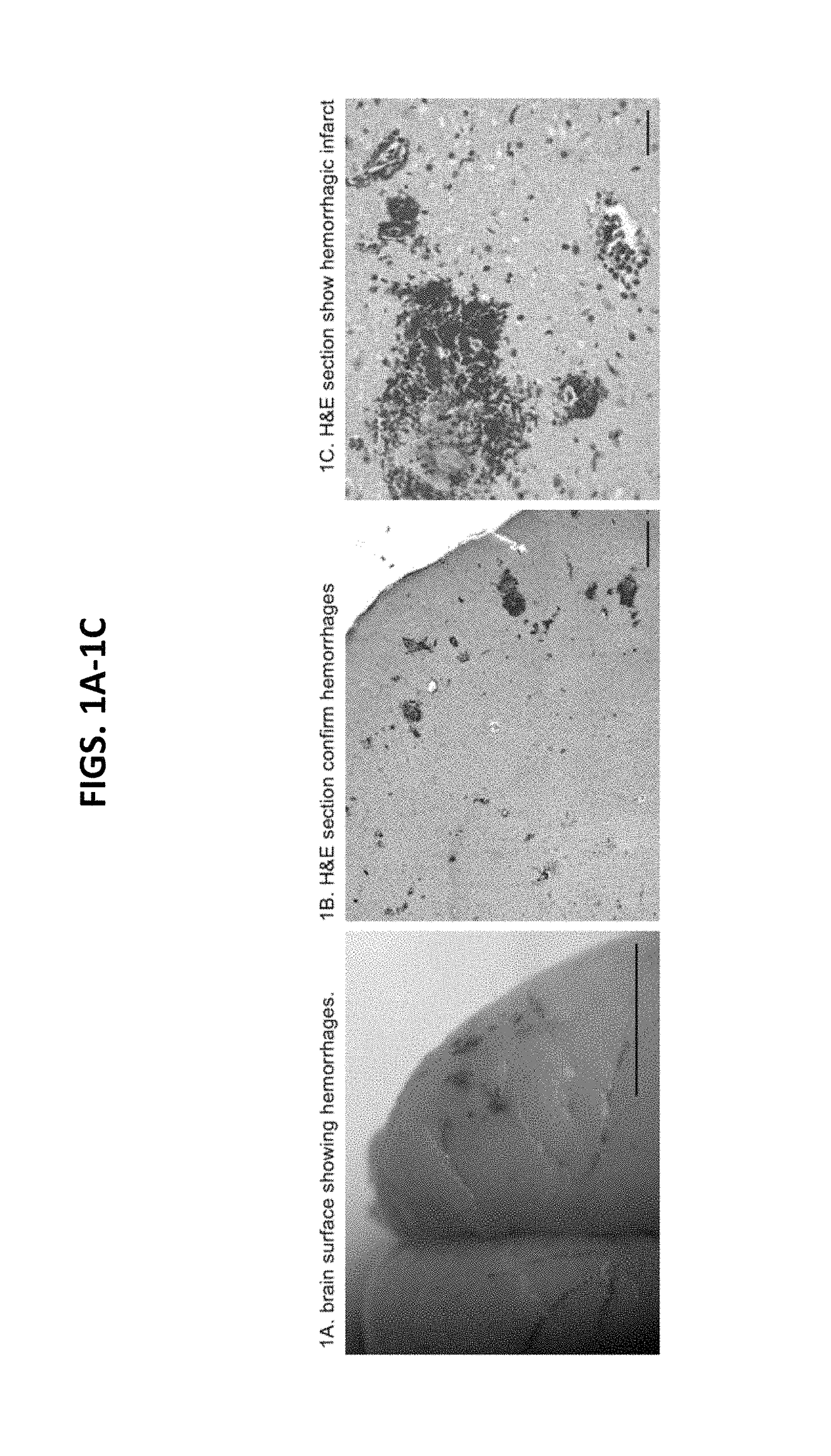
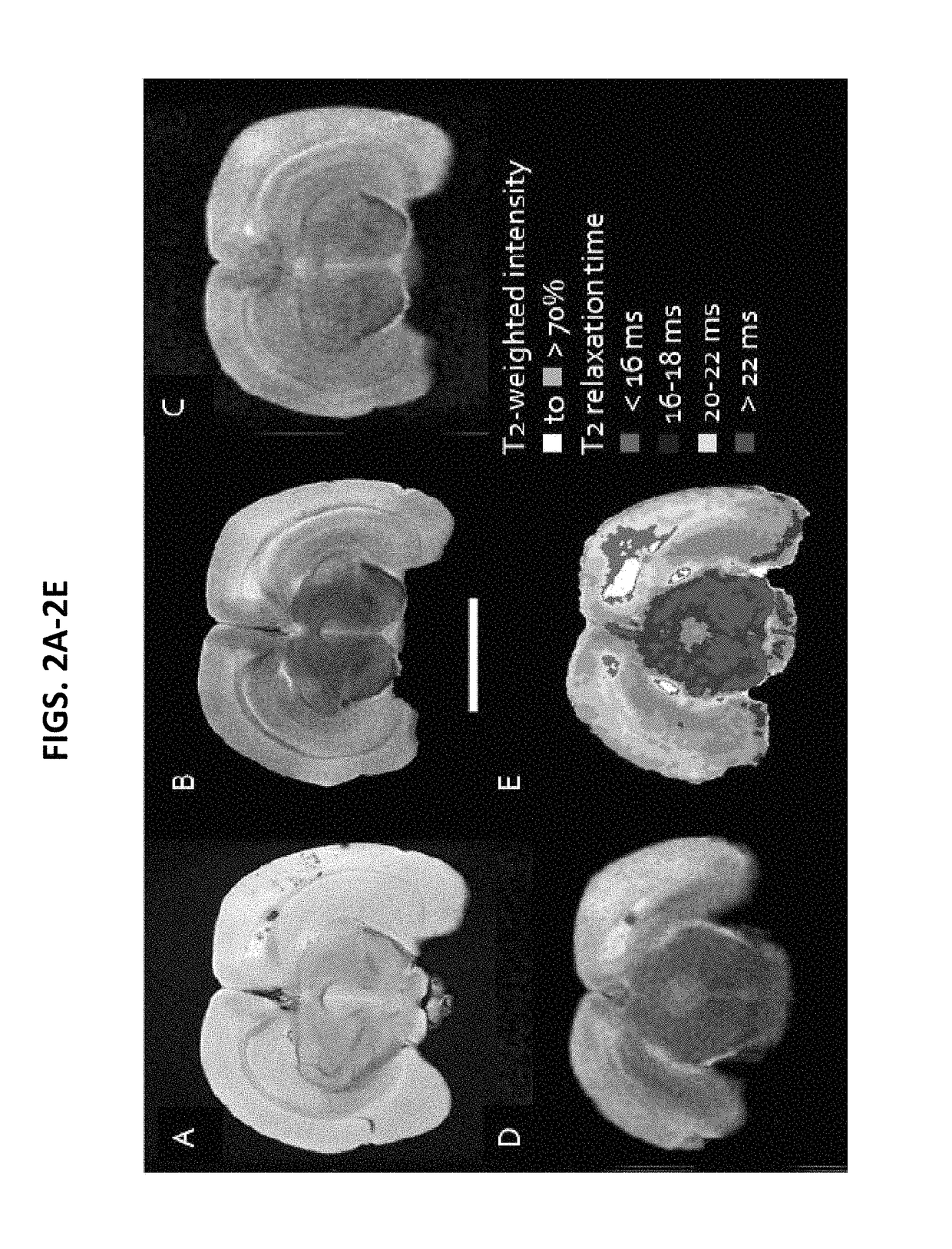
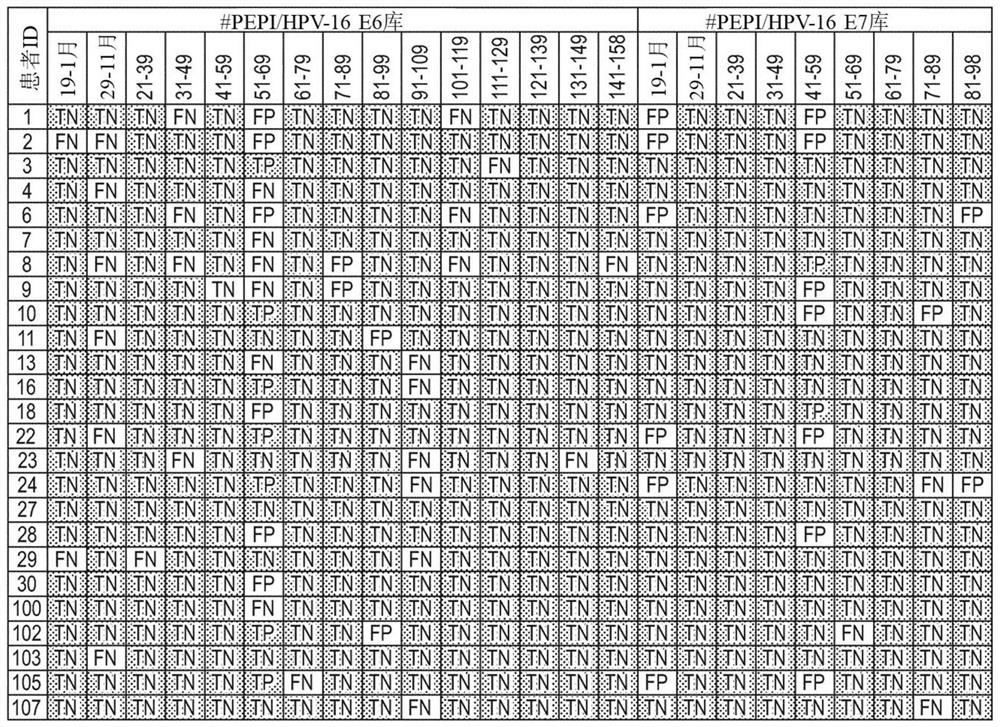
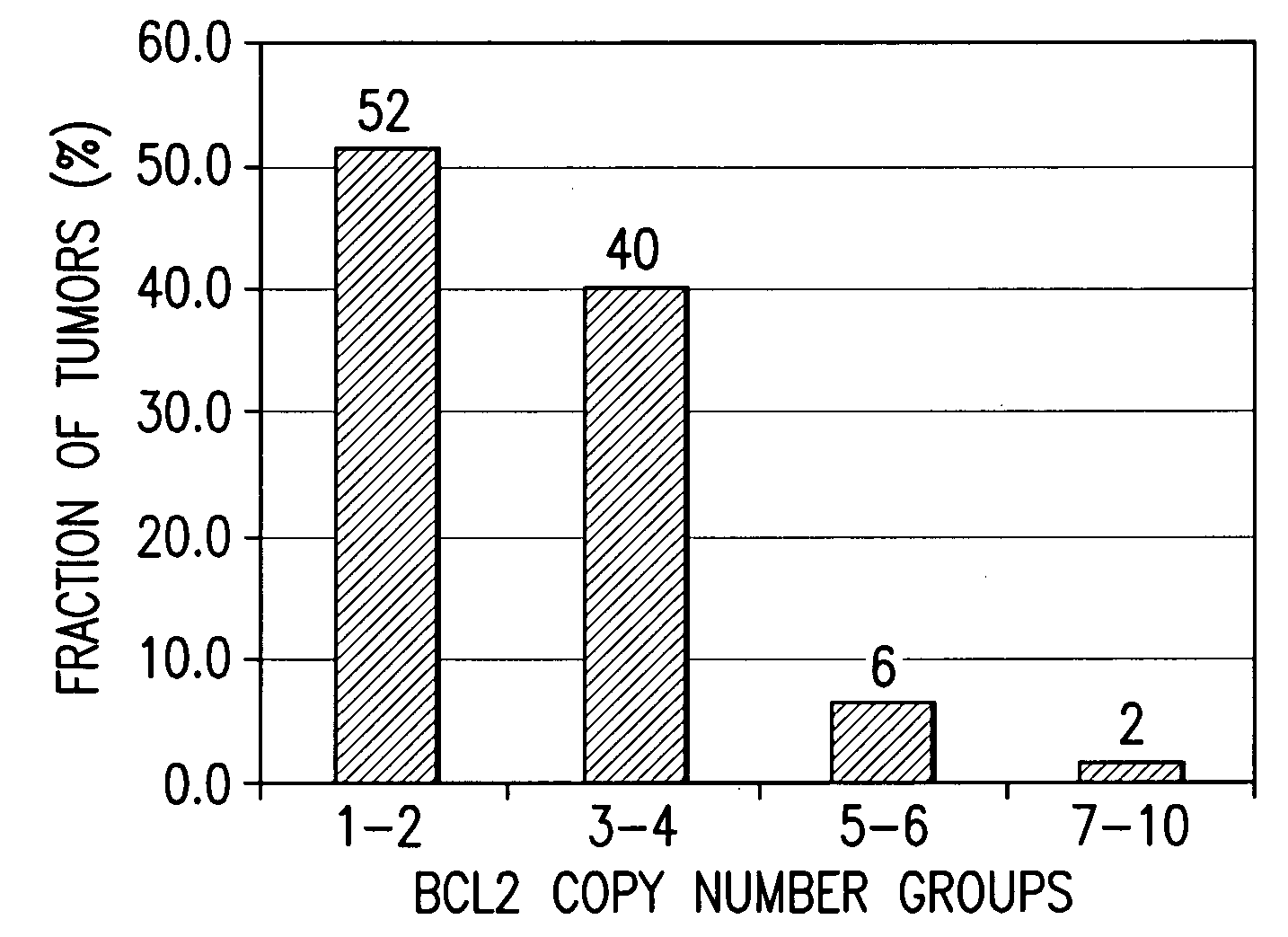Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
82 results about "Companion diagnostic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A companion diagnostic (CDx) is a diagnostic test used as a companion to a therapeutic drug to determine its applicability to a specific person. Companion diagnostics are co-developed with drugs to aid in selecting or excluding patient groups for treatment with that particular drug on the basis of their biological characteristics that determine responders and non-responders to the therapy.
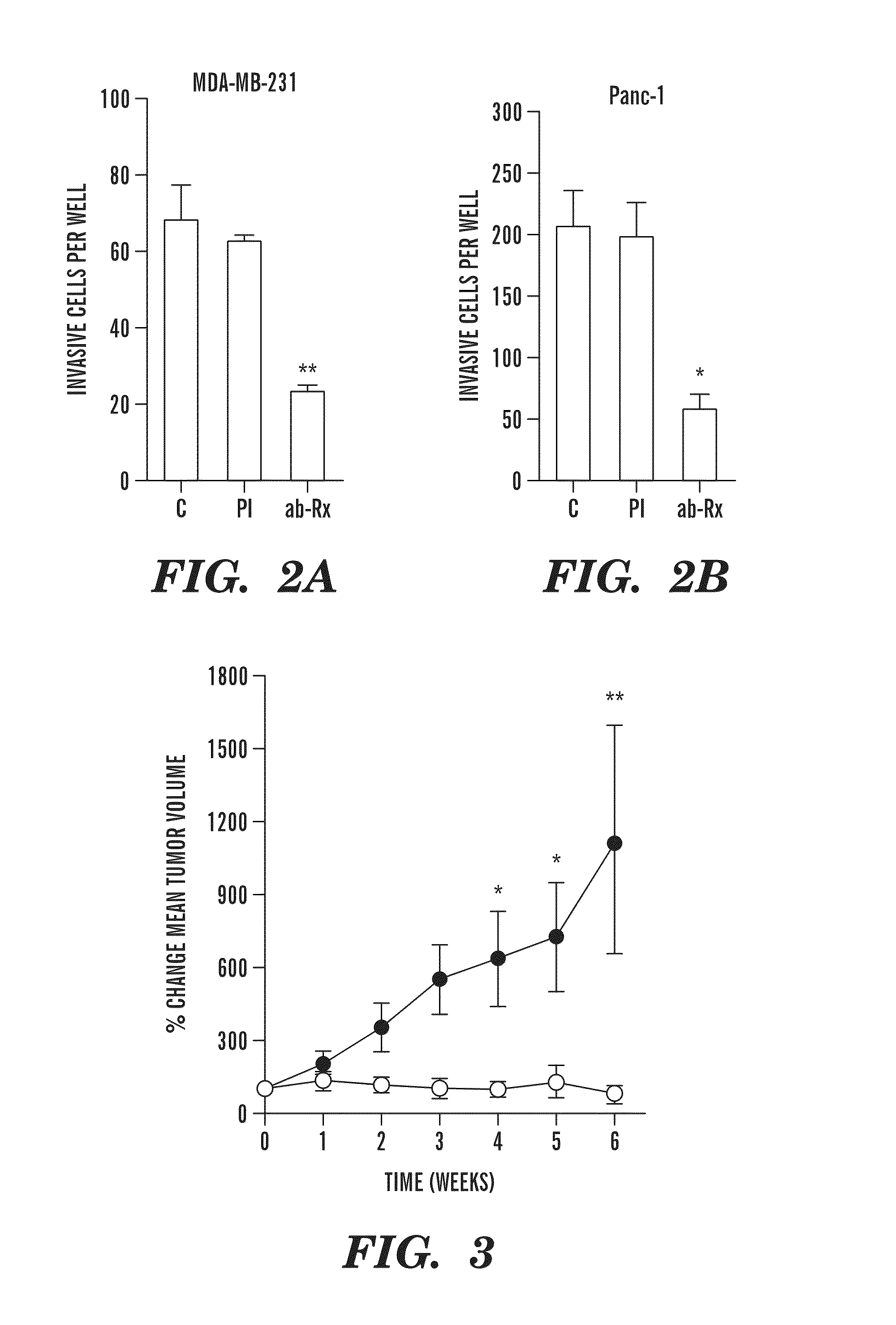
Companion diagnostic assays for cancer therapy
InactiveUS20080193943A1Promote stratificationParticular utilitySugar derivativesMicrobiological testing/measurementPhenacylOncology
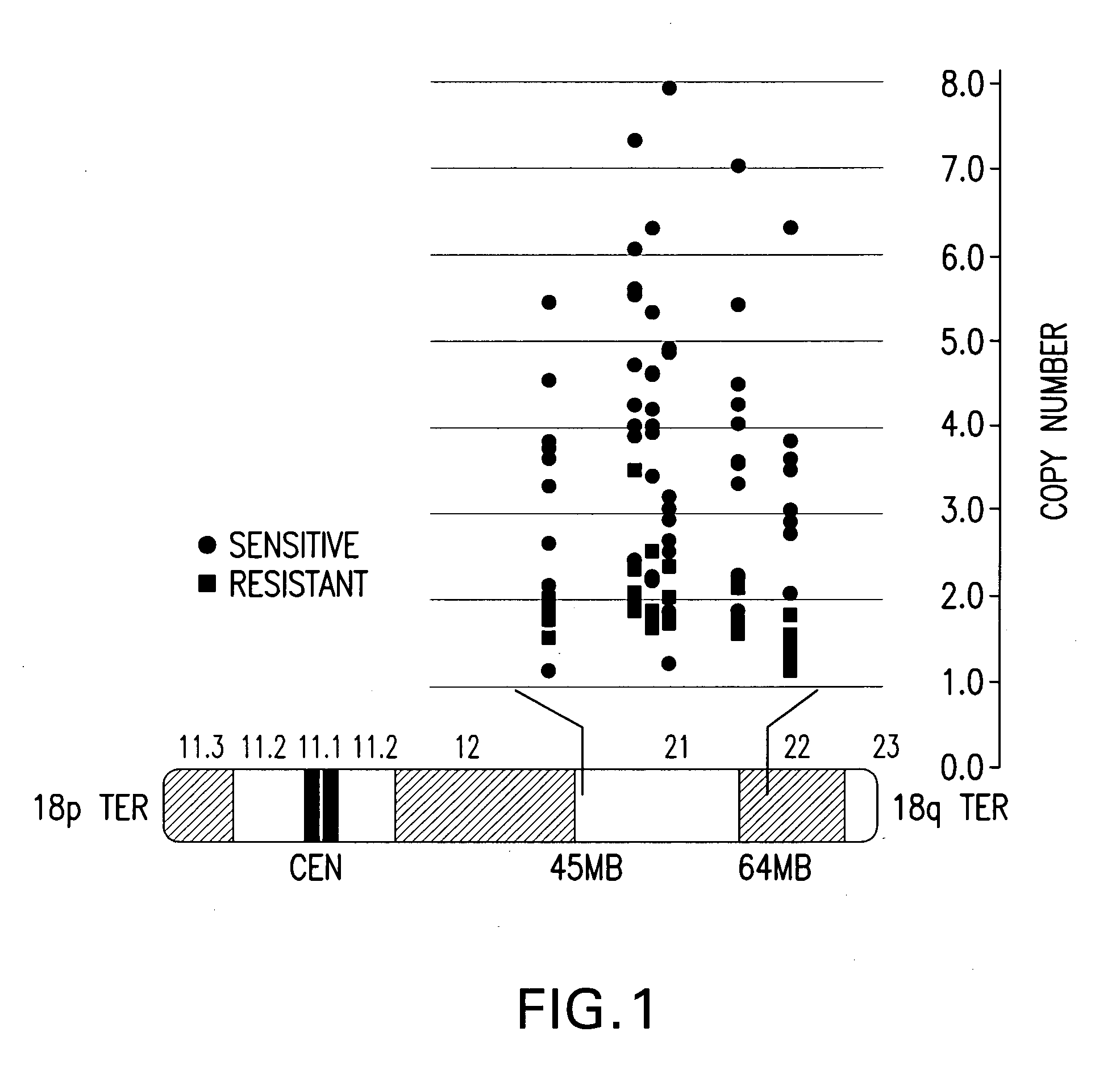
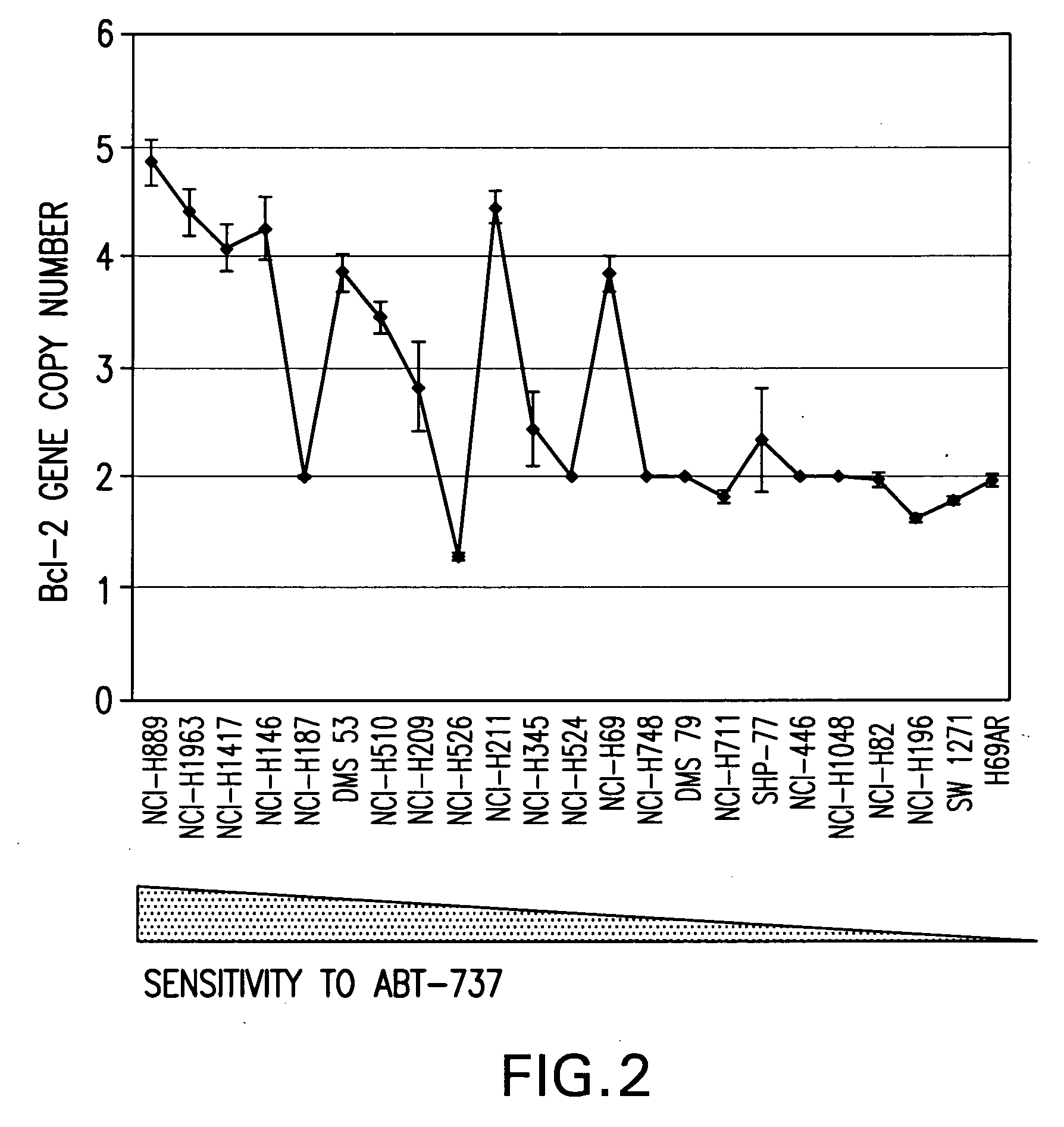
A method for classifying cancer patients as eligible to receive cancer therapy with a small molecule inhibitor of Bcl-2 comprising determination of the presence or absence in a patient tissue sample of chromosomal copy number status at the chromosomal locus 13q14 comprising the microRNA's miR-15a and miR-16-1 or at the chromosomal locus 11q23.1 comprising the microRNA miR-34c. The classification of cancer patients based upon the presence or absence of 13q14 loss or gain allows better selection of patients to receive chemotherapy with a small molecule Bcl-2 inhibitor such as N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-1-cyclohex-1-en-1-yl) methyl)piperazin-1-yl)benzoyl)-4-(((1R)-3-(morpholin-4-yl)-1-((phenylsulfanyl) methyl)propyl)amino)-3-((trifluoromethyl)sulfonyl)benzenesulfonamide, and for monitoring patient response to this therapy.
Owner:ABBOTT LAB INC
ANTI-DEspR MONOCLONAL ANTIBODY TARGETED THERAPY AND IMAGING FOR CANCER AND STROKE
ActiveUS20170058036A1Low toxicitySensitive and increased discriminationImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntiendomysial antibodiesIn vivo
Provided herein are novel compositions comprising anti-DEspR antibodies and fragments thereof derived from 6G8G7 and 7C5B2 anti-DEspR variant antibodies, including fully human, composite engineered human, humanized, monoclonal, and polyclonal anti-DEspR antibodies and fragments thereof, and methods of their use in a variety of therapeutic applications. The compositions comprising the anti-DEspR antibodies and fragments thereof described herein are useful in diagnostic and imaging methods, such as DEspR-targeted molecular imaging of angiogenesis, and for companion diagnostic and / or in vivo non-invasive imaging and / or assessments.
Owner:TRUSTEES OF BOSTON UNIV
DEspR ANTAGONISTS AND AGONISTS AS THERAPEUTICS
InactiveUS20130022551A1Shrink tumor sizeReducing tumor tumor metastasisCompounds screening/testingUltrasonic/sonic/infrasonic diagnosticsMolecular imagingAngiogenesis growth factor
Provided herein are novel compositions comprising DEspR-specific antagonists and agonists, and methods of their use in a variety of therapeutic applications. The compositions comprising the DEspR-specific anatgonists and agonists described herein are useful in therapeutic, diagnostic and imaging methods, such as DEspR-targeted molecular imaging of angiogenesis, and for companion diagnostic and / or in vivo-non invasive imaging and / or assessments.
Owner:TRUSTEES OF BOSTON UNIV
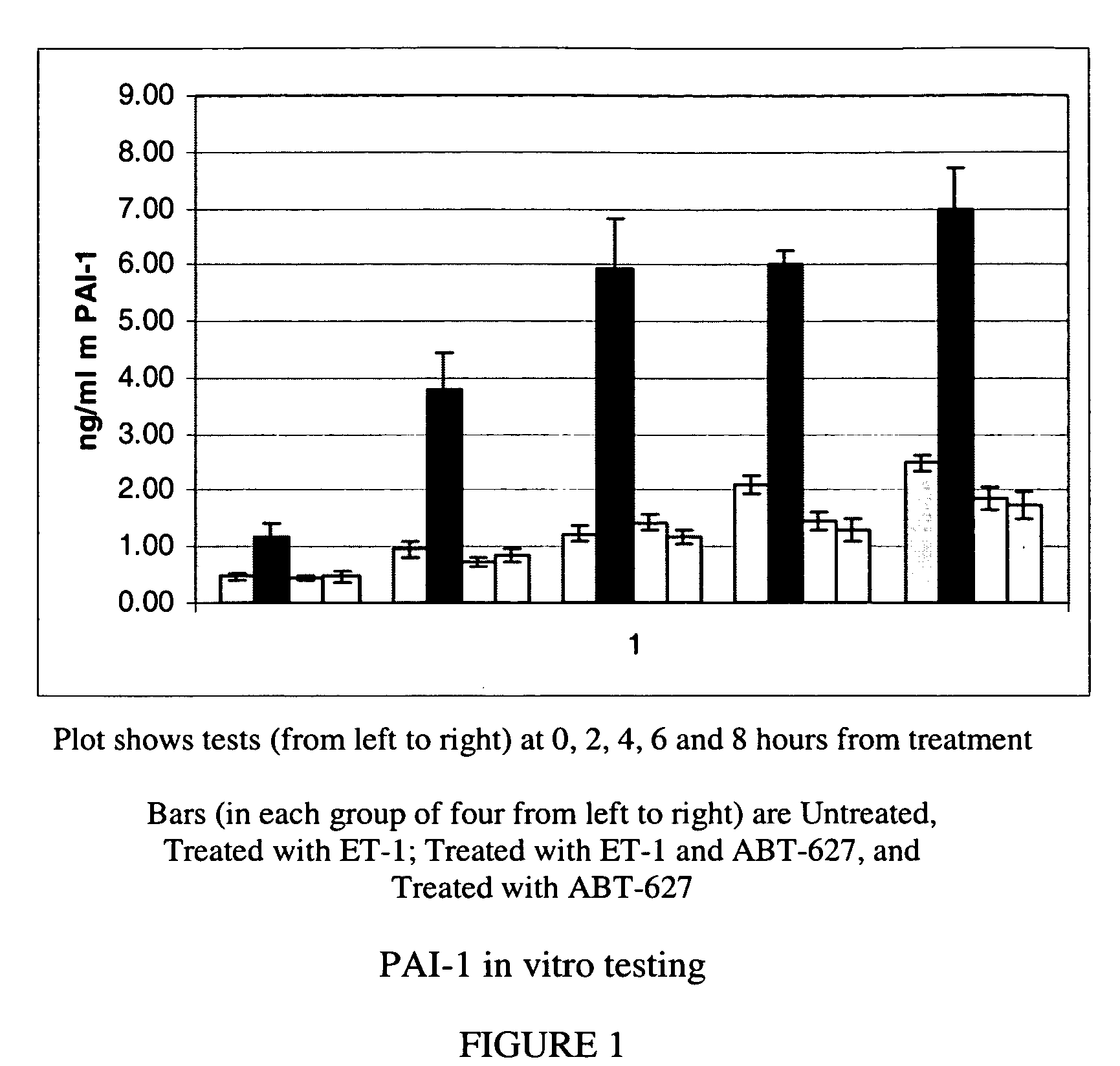
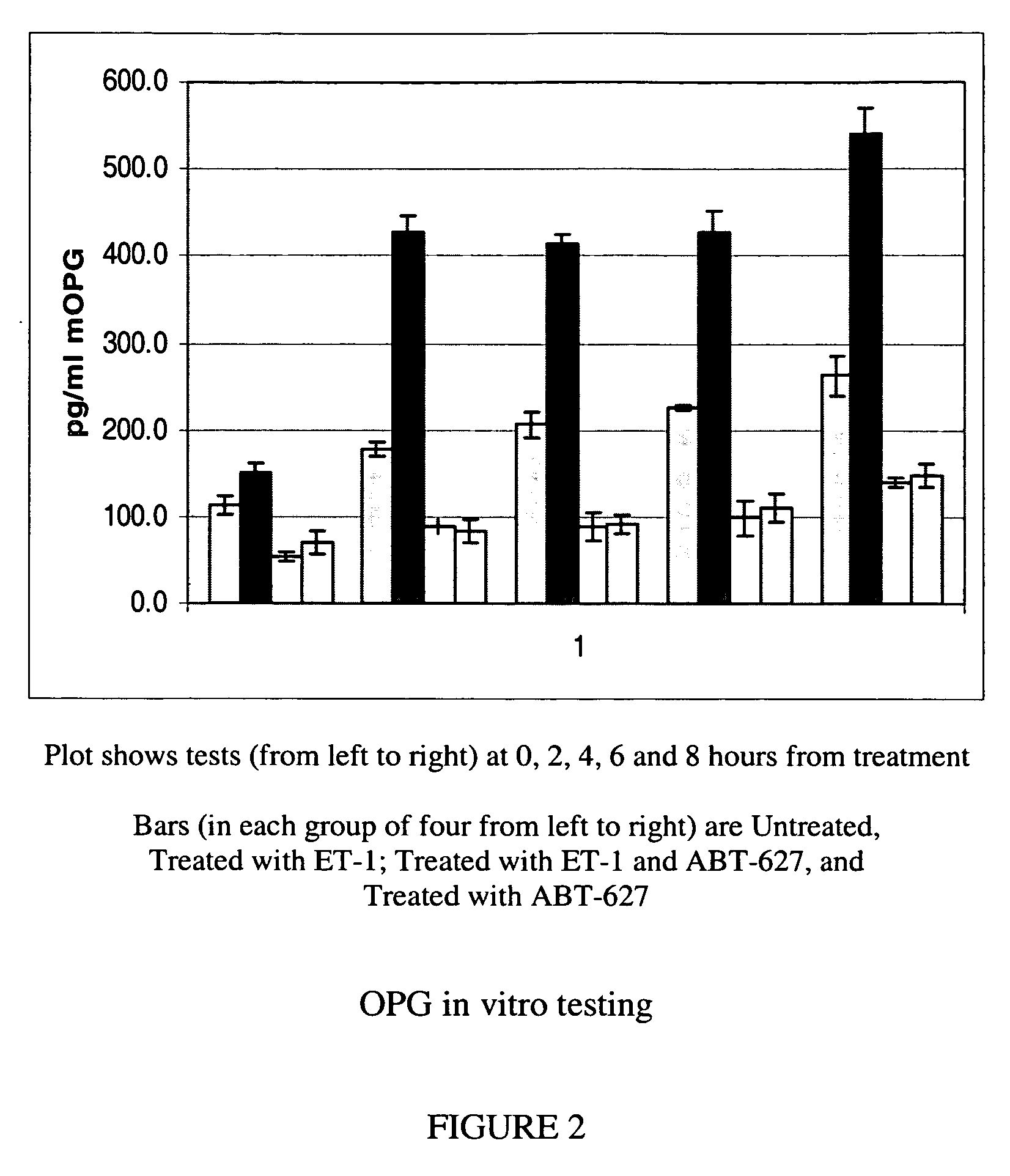
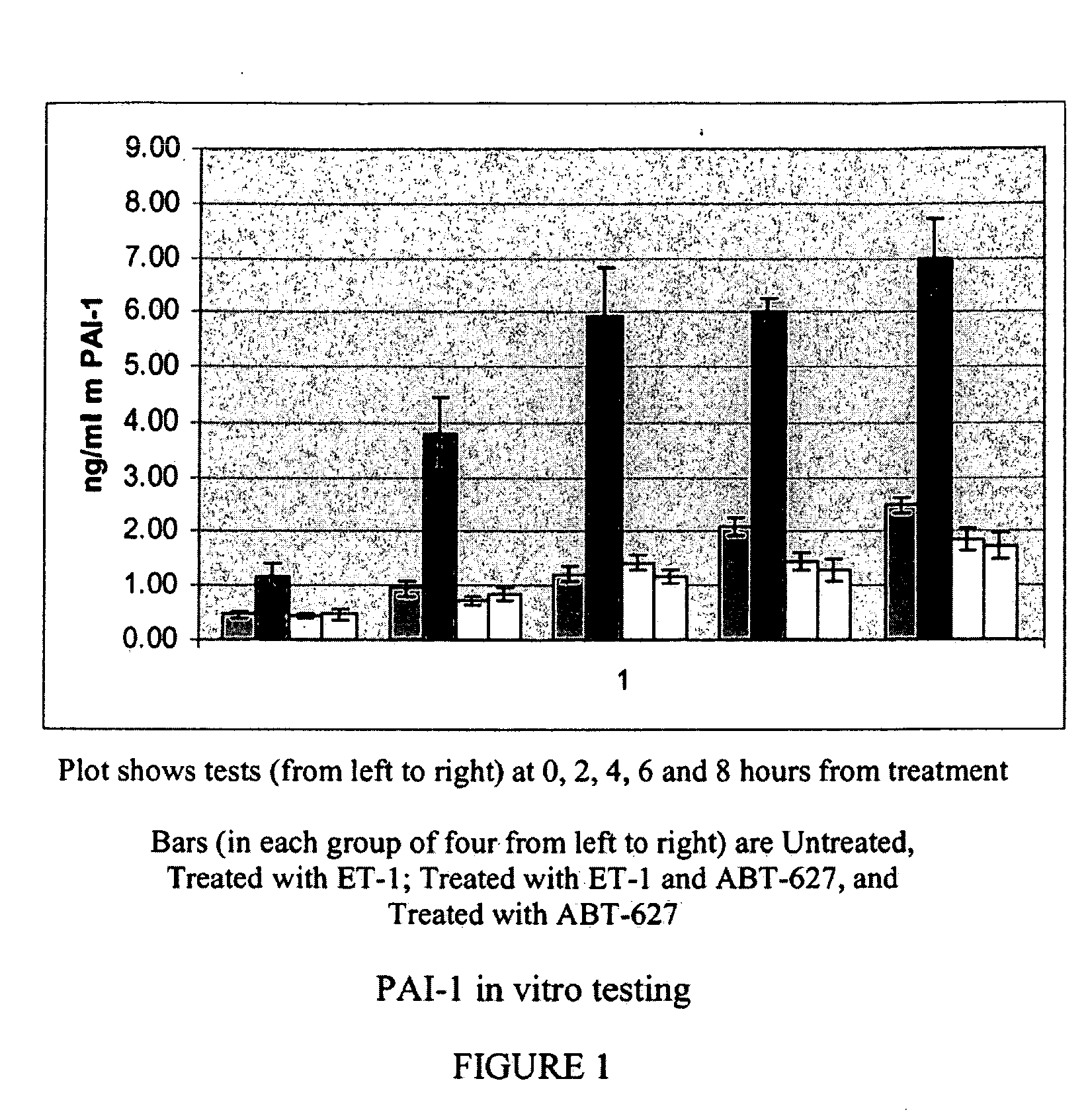
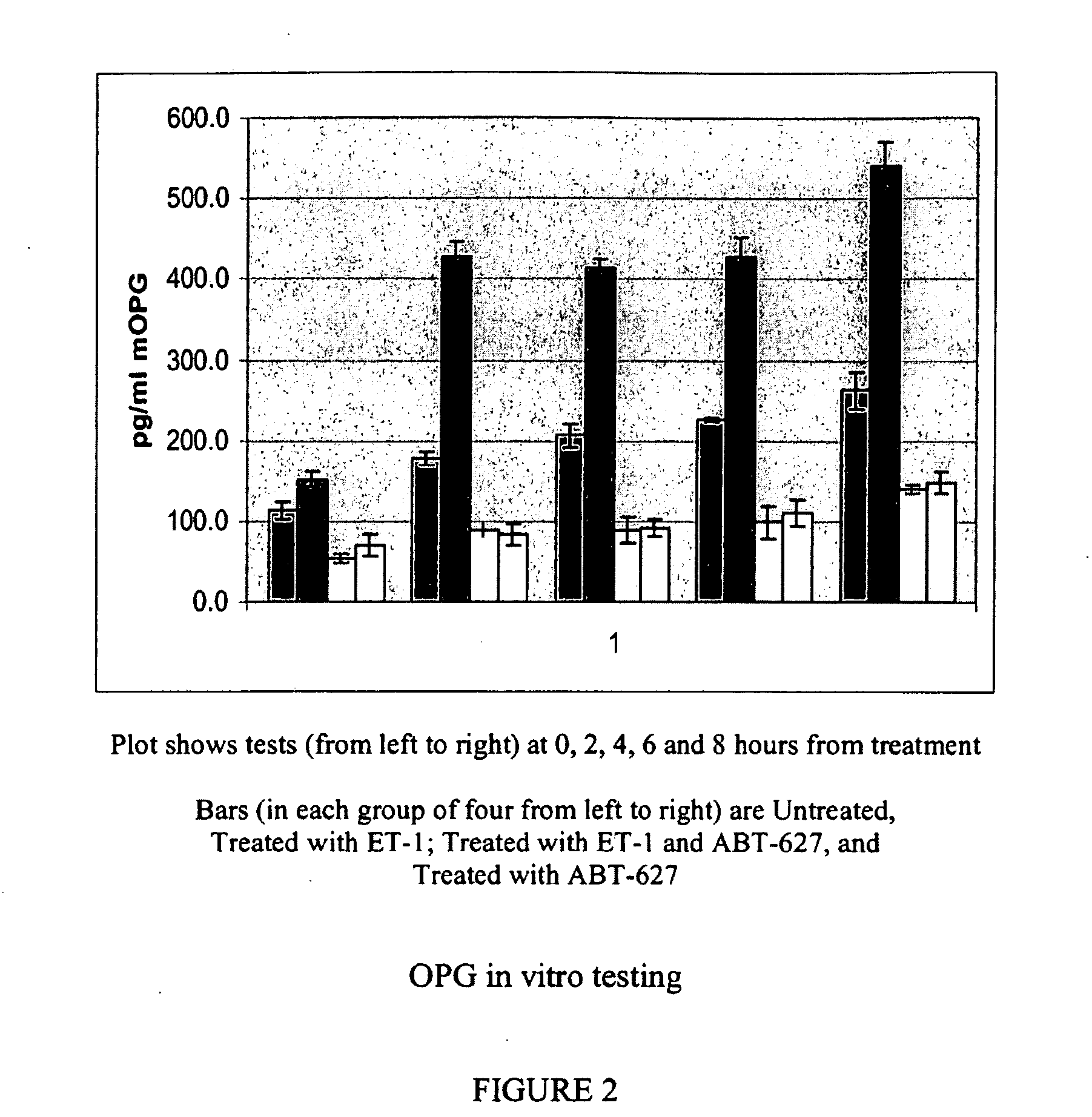
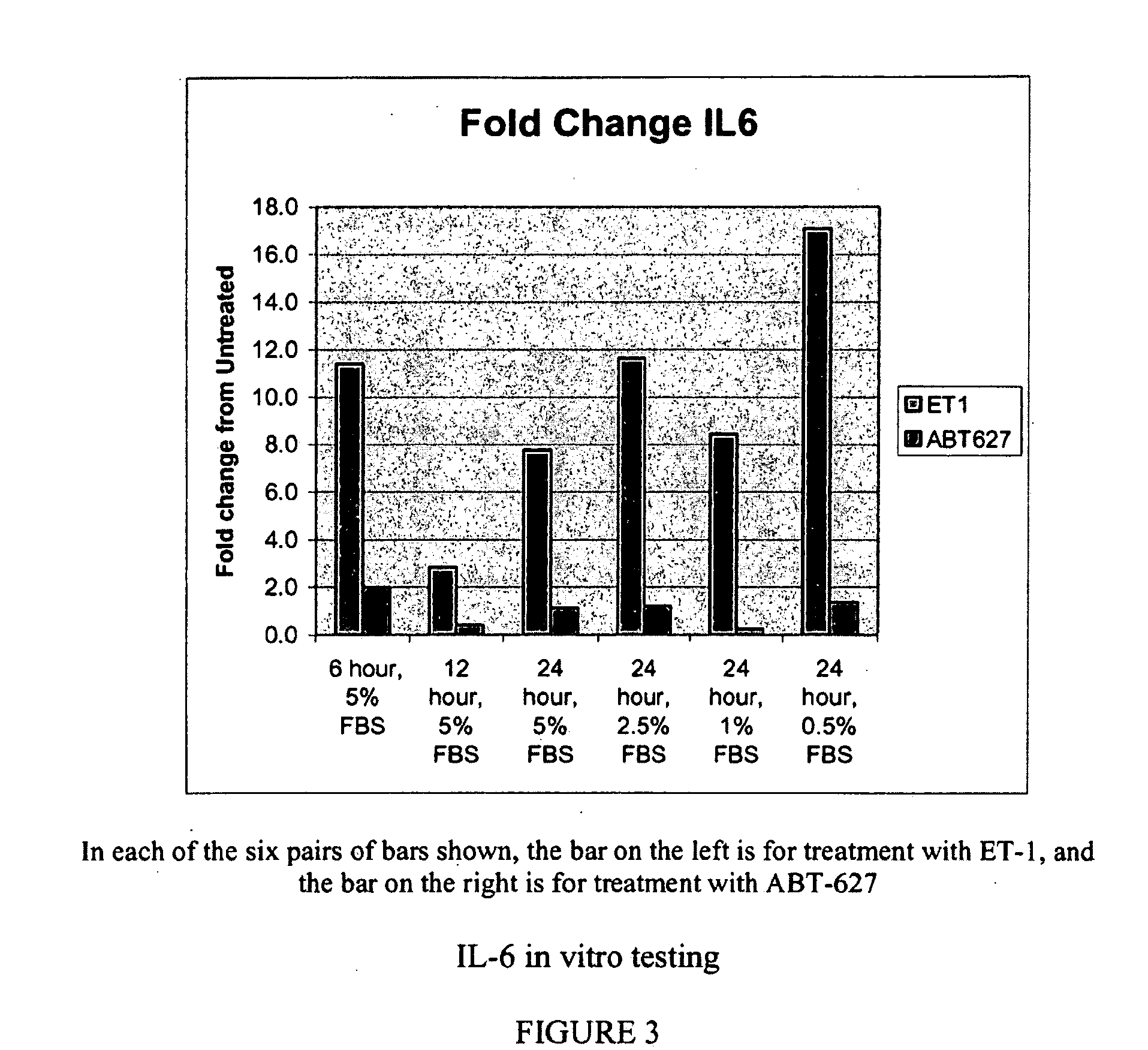
Companion diagnostic assays for endothelin receptor antagonists
InactiveUS20080102451A1Increase choiceMicrobiological testing/measurementBiological testingMedicineTissue sample
Methods for identifying cancer patients eligible to receive endothelin receptor antagonist therapy and for monitoring patient response to endothelin receptor antagonist therapy comprise assessment of the expression levels of at least one of PAI-1, uPA, TGFbeta2, IL-6, IL-8 and OPG in a patient tissue sample. The methods of the invention allow more effective identification of patients to receive endothelin receptor antagonist therapy and of determination of patient response to the therapy.
Owner:ABBOTT LAB INC
Determining cancer agressiveness, prognosis and responsiveness to treatment
The invention provides methods of determining the aggressiveness, prognosis and response to therapy for particular cancers, which include comparing the expression levels of one or a plurality of differentially expressed genes from one or more 5 functional metagenes, including a carbohydrate / lipid metabolism metagene, a cell signalling metagene, a cellular development metagene, a cellular growth metagene, a chromosome segregation metagene, a DNA replication / recombination metagene, an immune system metagene, a metabolic disease metagene, a nucleic acid metabolism metagene, a post-translational modification metagene, a protein 10 synthesis / modification metagene and a multiple networks metagene. The method disclosed herein may be particularly suitable as a companion diagnostic for cancer therapies.
Owner:COUNCIL OF THE QUEENSLAND INST OF MEDICAL RES
Compositions and methods for treating heart failure
InactiveUS20140364366A1Increase differentiationSimple structurePeptide/protein ingredientsNeuregulinsNeuregulinCardiac failure therapy
The present invention provides methods for treating chronic heart failure patients using the medication comprising neuregulin. The methods comprise first performing a companion diagnostic test of each patient before treatment; and then providing a suitable treatment to the patient according to the results of the companion diagnostic test. When the result of the test is within a favorite treatment zone, the patient is suitable for heart failure treatment by administering an effective amount of neuregulin.
Owner:ZENSUN (SHANGHAI) SCI & TECH CO LTD
Companion Diagnostic Assays For Endothelin Receptor Antagonists
InactiveUS20100184026A1Increase choiceMicrobiological testing/measurementDisease diagnosisMedicineEndothelin receptor antagonist
Methods for identifying cancer patients eligible to receive endothelin receptor antagonist therapy and for monitoring patient response to endothelin receptor antagonist therapy comprise assessment of the expression levels of at least one of PAI-1, uPA, TGFbeta2, IL-6, IL-8 and OPG in a patient tissue sample. The methods of the invention allow more effective identification of patients to receive endothelin receptor antagonist therapy and of determination of patient response to the therapy.
Owner:ABBOTT MOLECULAR INC
ANTI-DEspR INHIBITORS AS THERAPEUTICS FOR INHIBITION OF PATHOLOGICAL ANGIOGENESIS AND TUMOR CELL INVASIVENESS AND FOR MOLECULAR IMAGING AND TARGETED DELIVERY
ActiveUS20160108124A1Low toxicityIncreasing in vivo serum half-lifeSenses disorderNervous disorderAntiendomysial antibodiesIn vivo
Owner:TRUSTEES OF BOSTON UNIV
Method for discovering pharmacogenomic biomarkers
InactiveUS20140031242A1Quality improvementPredict likelihood of successSugar derivativesMicrobiological testing/measurementPharmacogenomicsDrugs response
The present invention relates to a method of discovering pharmacogenomic biomarkers that are correlated with varied individual responses (efficacy, adverse effect, and other end points) to therapeutic agents. The present invention provides a mean to utilize archived clinical samples to perform genome-wide association study in order to identify novel pharmacogenomic biomarkers. The newly discovered biomarkers can then be developed into companion diagnostic tests which can help to predict drug responses and apply drugs only to those who will be benefited, or exclude those who might have adverse effects, by the treatment.
Owner:DENOVO BIOPHARMA HANGZHOU LTD
Companion diagnostic for cdk4 inhibitors
ActiveUS20160030433A1Lower protein levelEffective treatmentOrganic active ingredientsPeptide librariesCancer cellMdm2 Protein
The present invention relates to the use of one or more biomarkers to evaluate the likelihood that a CDK4 inhibitor would produce an anti-cancer effect in a subject. It is based, at least in part, on the discovery that cancer treatment with a CDK4 inhibitor is more effective where treated cancer cells undergo cellular senescence rather than a transient cell cycle arrest, where cellular senescence is associated with decreased MDM2 protein level. Accordingly, in non-limiting embodiments, the present invention provides for methods, compositions, and kits for a companion diagnostic for CDK4 inhibitors, and in particular, to the use of MDM2 expression as a biomarker for the likelihood that a cancer can be successfully treated by CDK4 inhibition.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Anti-DEspR monoclonal antibody targeted therapy and imaging for cancer and stroke
ActiveUS10202457B2Low toxicitySensitive and increased discriminationPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsMolecular imagingMonoclonal antibody
Provided herein are novel compositions comprising anti-DEspR antibodies and fragments thereof derived from 6G8G7 and 7C5B2 anti-DEspR variant antibodies, including fully human, composite engineered human, humanized, monoclonal, and polyclonal anti-DEspR antibodies and fragments thereof, and methods of their use in a variety of therapeutic applications. The compositions comprising the anti-DEspR antibodies and fragments thereof described herein are useful in diagnostic and imaging methods, such as DEspR-targeted molecular imaging of angiogenesis, and for companion diagnostic and / or in vivo non-invasive imaging and / or assessments.
Owner:TRUSTEES OF BOSTON UNIV
Vaccine
The disclosure relates to polypeptides and pharmaceutical compositions comprising polypeptides that find use in the prevention or treatment of cancer, in particular breast cancer, ovarian cancer and colorectal cancer. The disclosure also relates to methods of inducing a cytotoxic T cell response in a subject or treating cancer by administering pharmaceutical compositions comprising the peptides, and companion diagnostic methods of identifying subjects for treatment. The peptides comprise T cell epitopes that are immunogenic in a high percentage of patients.
Owner:TREOS BIO LTD
Companion diagnostic for CDK4 inhibitors
ActiveUS9889135B2Organic active ingredientsMicrobiological testing/measurementCancer cellBiomarker (petroleum)
The present invention relates to the use of one or more biomarkers to evaluate the likelihood that a CDK4 inhibitor would produce an anti-cancer effect in a subject. It is based, at least in part, on the discovery that cancer treatment with a CDK4 inhibitor is more effective where treated cancer cells undergo cellular senescence rather than a transient cell cycle arrest, where cellular senescence is associated with decreased MDM2 protein level. Accordingly, in non-limiting embodiments, the present invention provides for methods, compositions, and kits for a companion diagnostic for CDK4 inhibitors, and in particular, to the use of MDM2 expression as a biomarker for the likelihood that a cancer can be successfully treated by CDK4 inhibition.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Companion diagnostic assays for cancer therapy
InactiveUS20080269067A1Promote stratificationParticular utilityMicrobiological testing/measurementLibrary screeningTissue sampleCopy number gain
A method for classifying cancer patients as eligible to receive cancer therapy comprising determination of the presence or absence in a patient tissue sample of chromosomal copy number gain at chromosomal locus 18q21-q22. The classification of cancer patients based upon the presence or absence of 18q21-q22 gain allows selection of patients to receive chemotherapy, such as therapy with a Bcl-2 family inhibitor, and for monitoring patient response to therapy.
Owner:ABBOTT LAB INC
Methods and compositions of predicting activity of retinoid x receptor modulator
ActiveUS20150368720A1Chemical property predictionCompound screeningGenomic BiomarkerGenetic linkage disequilibrium
The present invention describes genomic biomarkers that have been discovered to correlate with varied individual responses (efficacy, adverse effect, and other end points) to therapeutic retinoid X receptor modulator, such as bexarotene, in treating diseases such as, non small cell lung cancer. The newly discovered biomarkers and others in linkage disequilibrium with them can be used in companion diagnostic tests which can help to predict drug responses and apply drugs only to those who will be benefited, or exclude those who might have adverse effects, by the treatment.
Owner:DENOVO BIOPHARMA HANGZHOU LTD
Compositions and methods for treating heart failure
ActiveUS20170326204A1Increase differentiationSimple structureNeuregulinsPeptide/protein ingredientsNeuregulinCardiac failure therapy
The present invention provides methods for treating chronic heart failure patients using the medication comprising neureplin. The methods comprise first performing a companion diagnostic test of each patient before treatment; and then providing a suitable treatment to the patient according to the results of the companion diagnostic test. When the result of the test is within a favorite treatment zone, the patient is suitable for heart failure treatment by administering an effective amount of neuregulin.
Owner:ZENSUN (SHANGHAI) SCI & TECH CO LTD
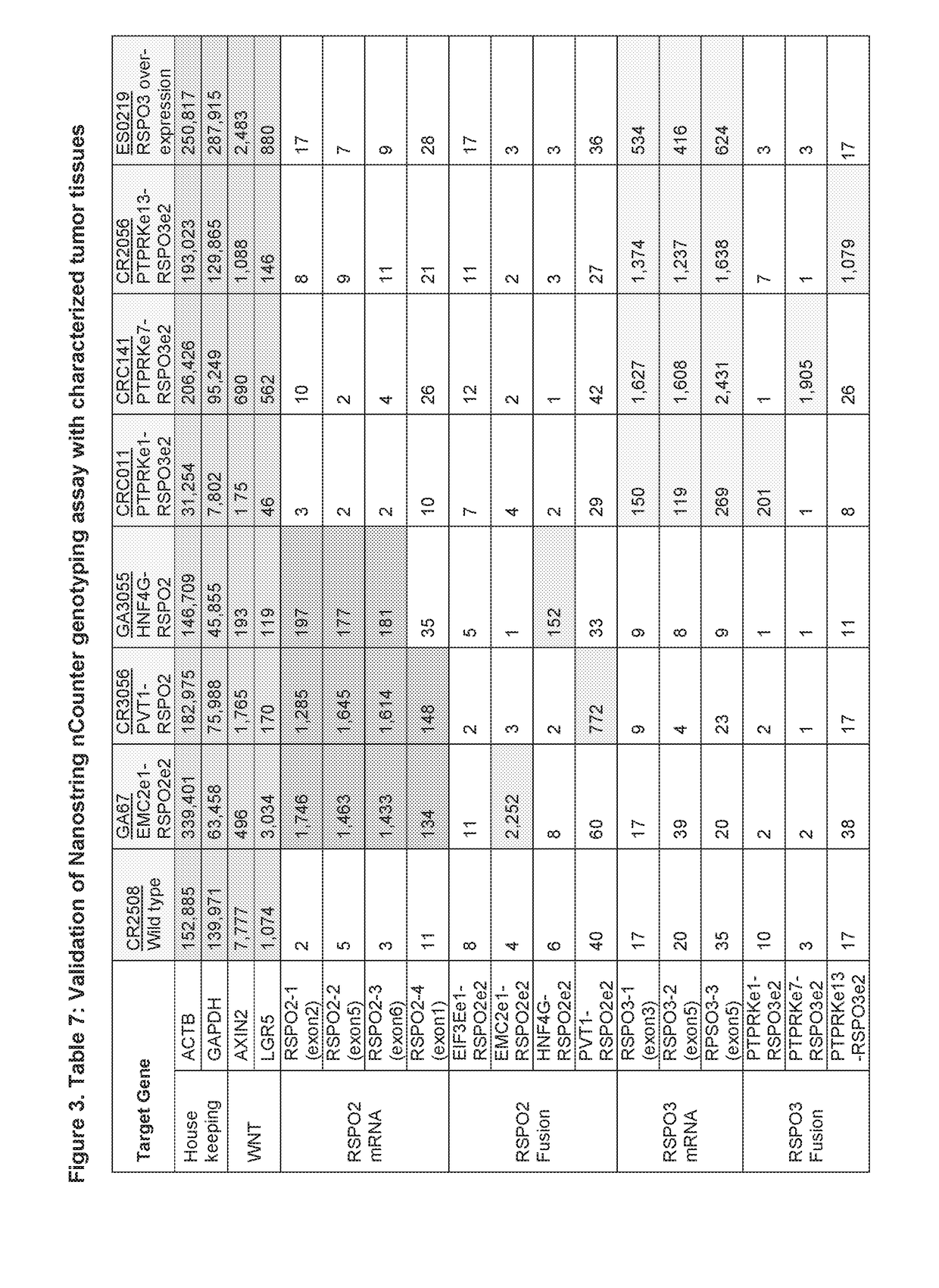
Method for identifying or detecting genomic rearrangements in a biological sample
ActiveUS9133514B2Improves structural and functional analysisHigh resolutionMicrobiological testing/measurementDiseaseImage resolution
Owner:GENOMIC VISION
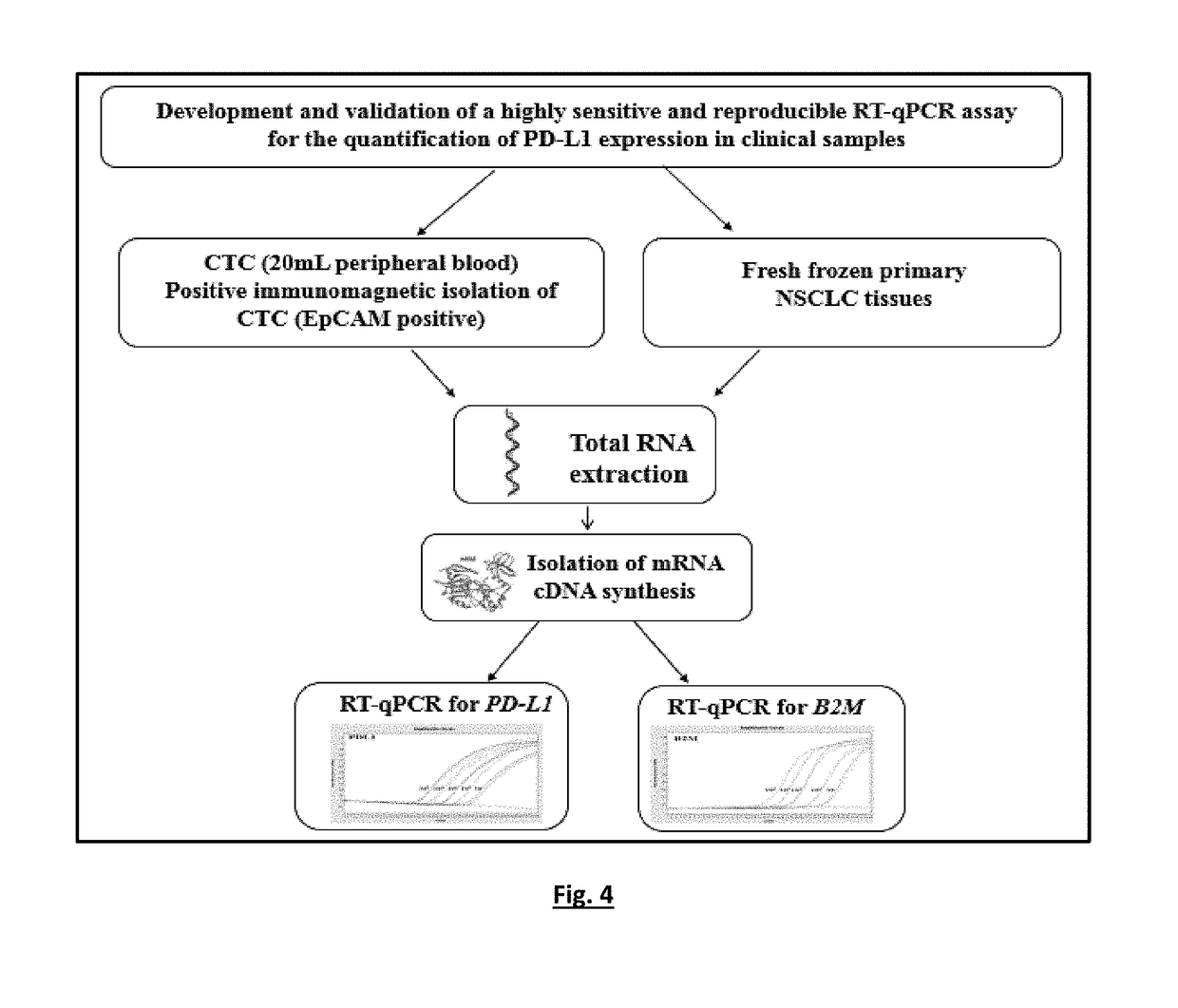
Method for the quantification of pd-l1 expression
InactiveUS20190085401A1Avoid false negative resultsMicrobiological testing/measurementOncologyTumor cells
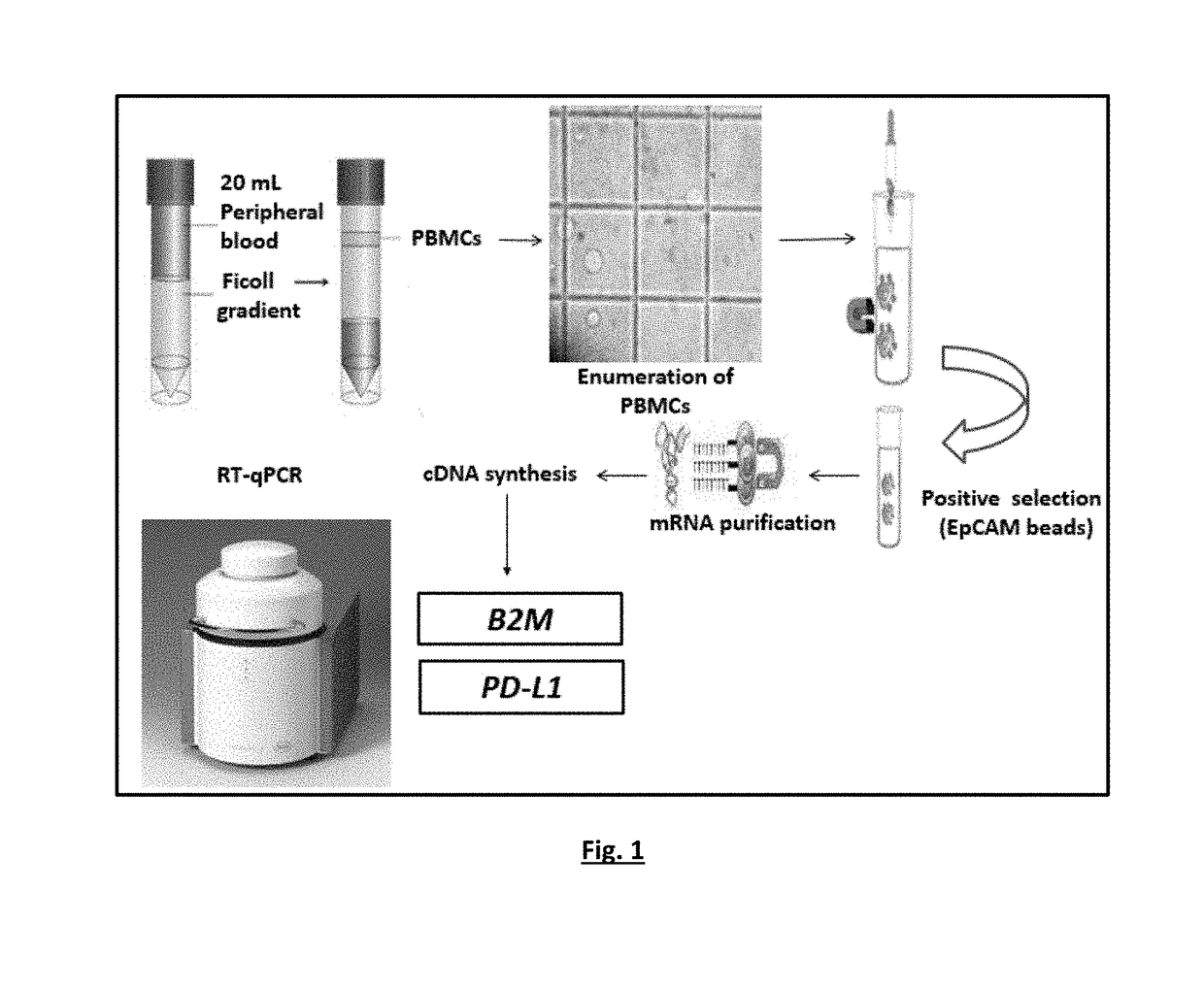
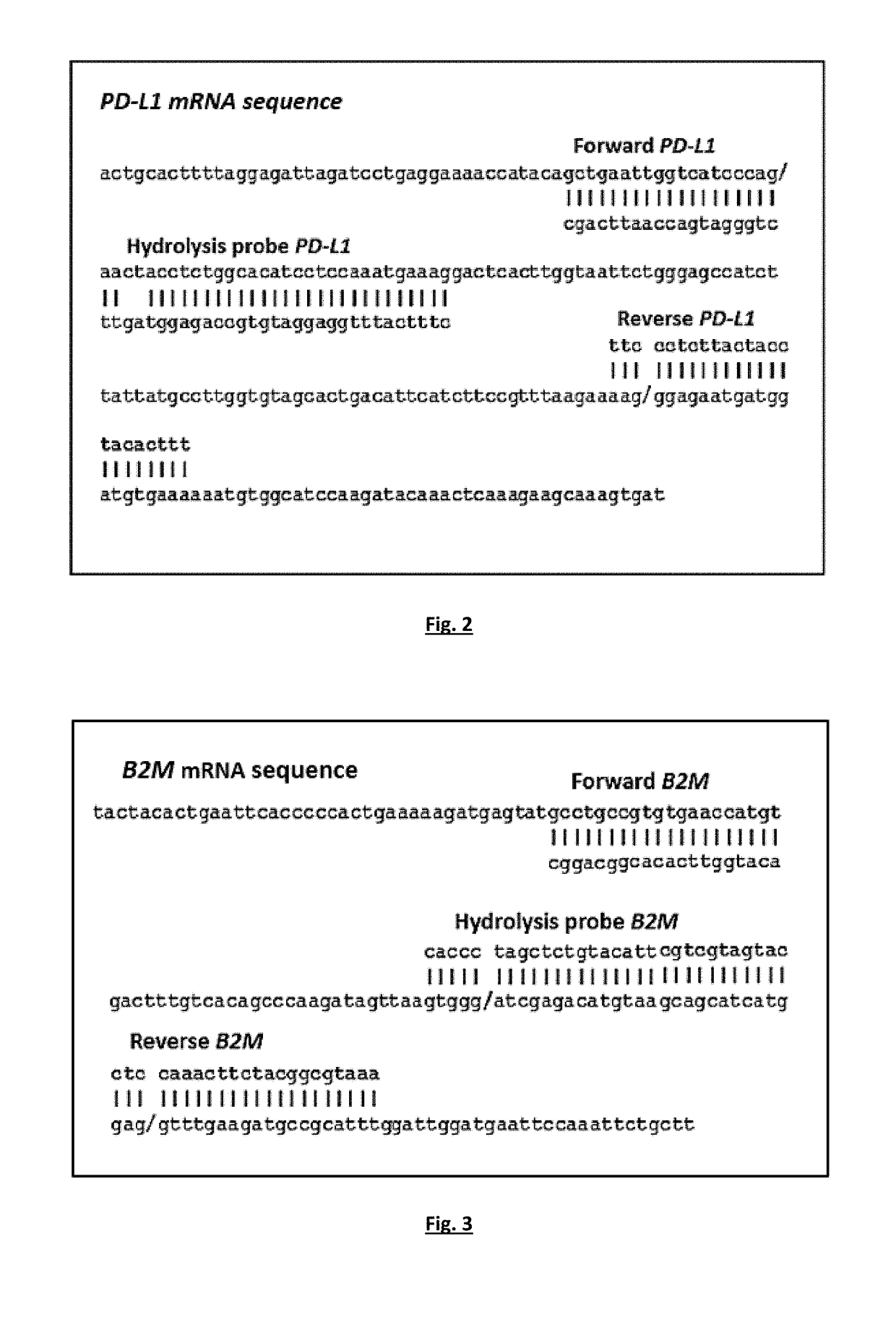
A highly sensitive for determining the expression of PD-L1 that is based on a RT-qPCR in a RNA sample of, for example, Circulating Tumor Cells (CTC) or fresh frozen primary tumor tissues. In particular, to a method for the detection of PD-L1 mRNA positive CTCs or primary tumor tissues (fresh frozen) based on the quantitative determination of the molecular marker (PD-L1) in biological samples of patients suffering from cancer. By using the method, detection can take place before, during or after the immune therapy or any other treatment in order to provide significant information concerning guiding or monitoring of the anti-PD-L1 agents effectiveness. This RT-qPCR assay could comprise a promising companion diagnostic test in order to evaluate the PD-L1 expressional status on CTC or tumor tissue, providing clinical applications, which could have an important impact on therapeutic interventions since the expression of PD-L1 is associated with response to immunotherapy.
Owner:PHARMASSIST
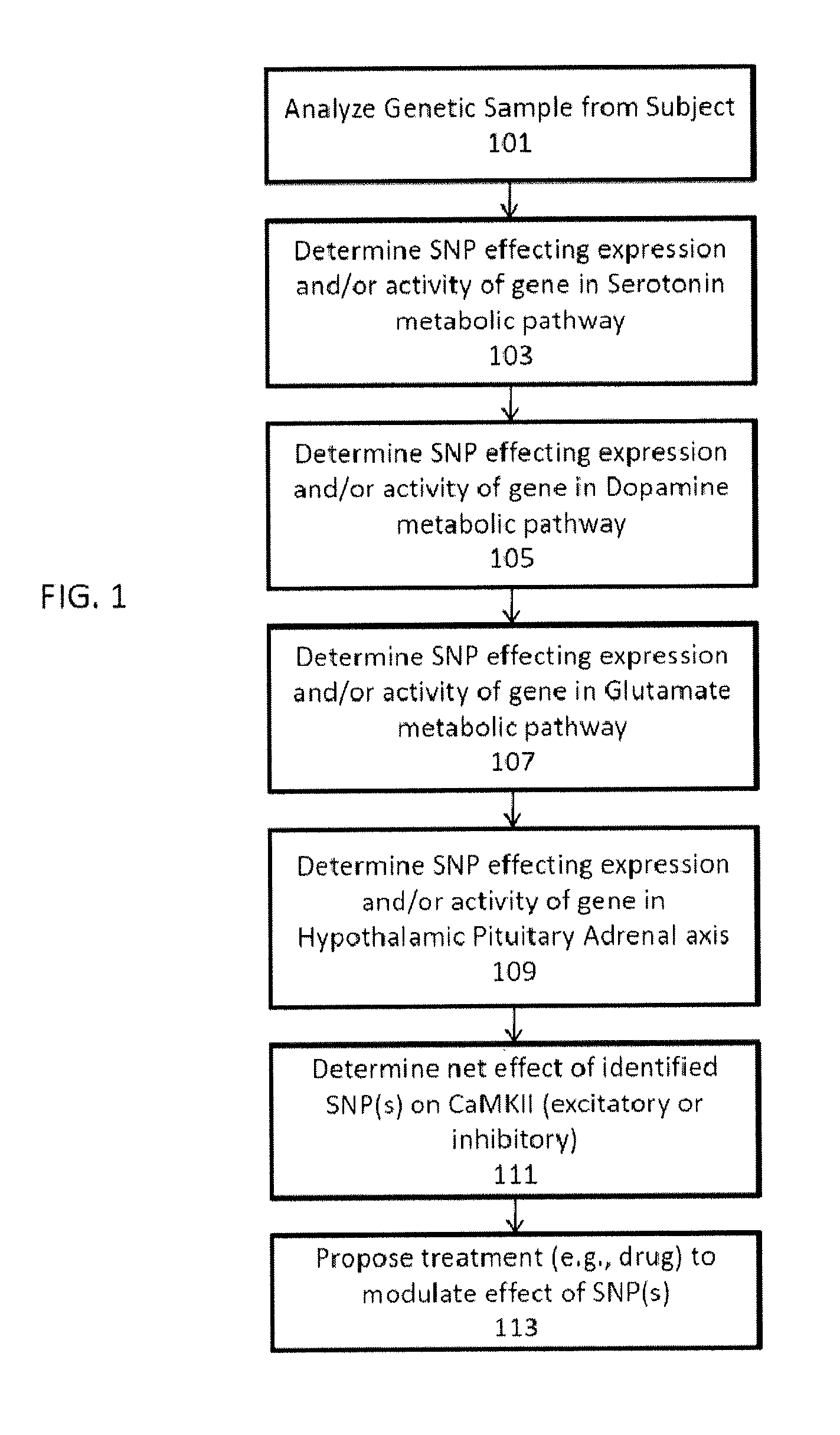
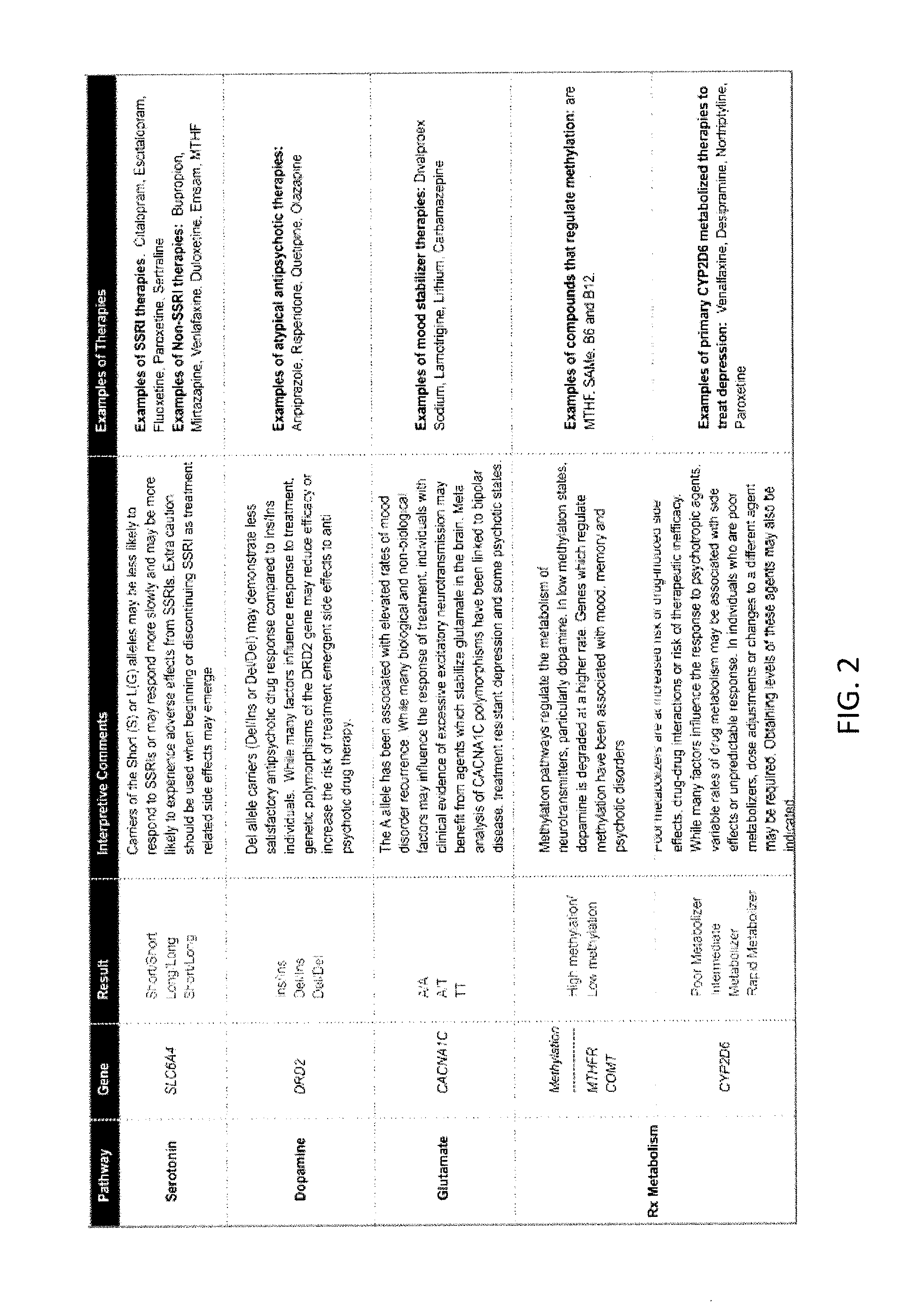
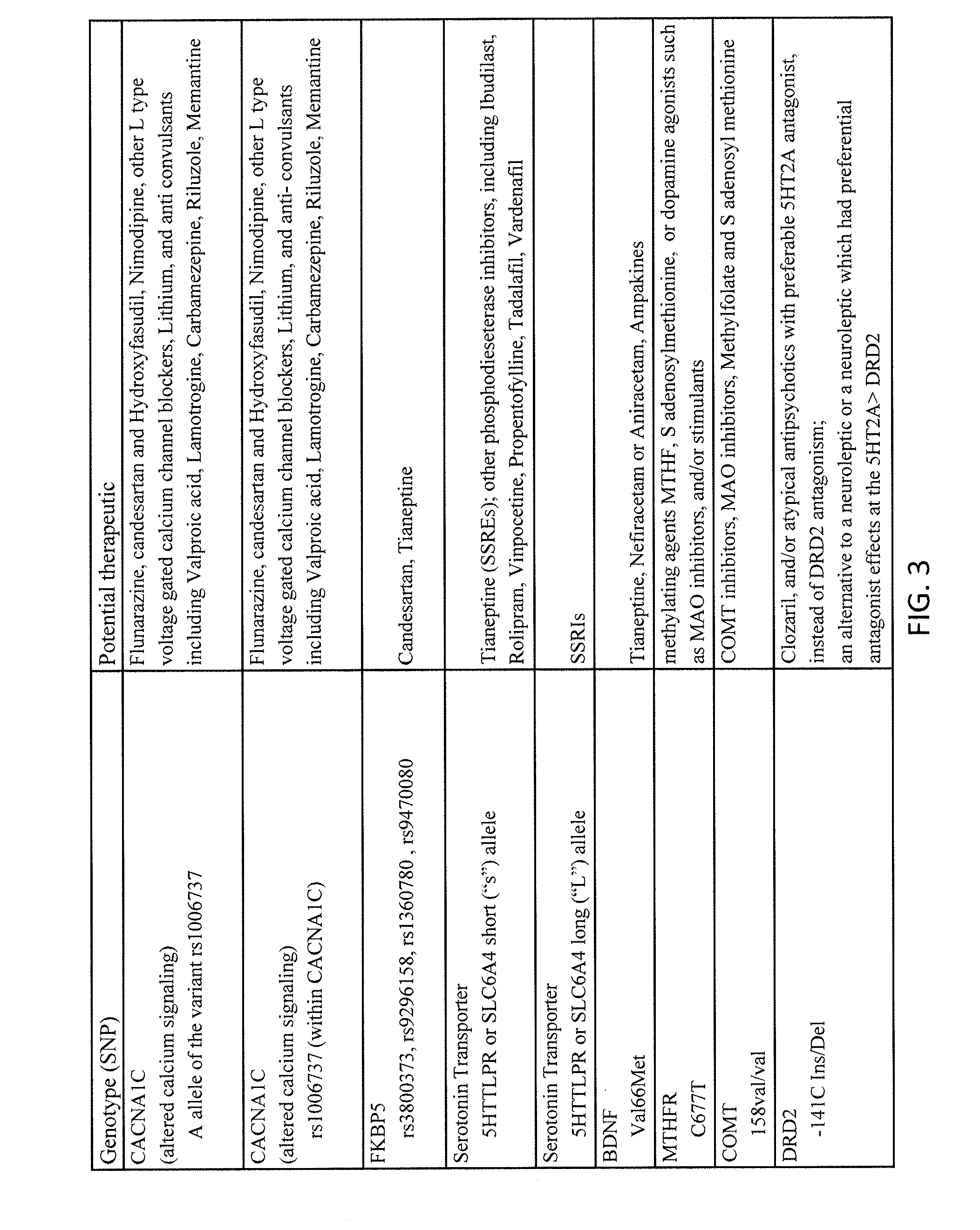
Methods for assessment and treatment of mood disorders via single nucleotide polymorphisms analysis
Described herein are assays, kits and methods for treating mood disorders by testing for one or more polymorphisms in a specific group of genes and for analyzing the results of polymorphism testing; the genes included may converge in one or more signaling pathways, and may be epigenetic. The genes are included based on the relationships of the proteins encoded by the genes in the context of particular signaling pathways and provide a diagnostically relevant nexus. Also described herein are methods of presenting the data collected by the screen, including methods of delivering interpretive comments and / or treatment guidance based on the results of the genetic screening either individually or based on the genetic composition of particular clusters of genes which may be related to each other. Importantly, drugs which modulate these genetic disturbances are described for targeted therapeutic use based upon companion diagnostic method.
Owner:GENOMIND
Method of identifying diagnostic reagents
Disclosed herein are methods for identifying diagnostic reagents (e.g., antigen-binding molecules, such as antibodies) that are useful, for instance, as primary diagnostic, prognostic, and / or predictive (e.g., companion diagnostic) reagents in a disease state, such as cancer.
Owner:VENTANA MEDICAL SYST INC
Vaccine
The disclosure relates to polypeptides and pharmaceutical compositions comprising polypeptides that find use in the prevention or treatment of cancer, in particular breast cancer, ovarian cancer and colorectal cancer. The disclosure also relates to methods of inducing a cytotoxic T cell response in a subject or treating cancer by administering pharmaceutical compositions comprising the peptides, and companion diagnostic methods of identifying subjects for treatment. The peptides comprise T cell epitopes that are immunogenic in a high percentage of patients.
Owner:TREOS BIO LTD
Immuno imaging agent for use with antibody-drug conjugate therapy
ActiveUS20140256916A1Easy to stratifyFacilitates early evaluationFermentationPlant genotype modificationMedicineImaging agent
The invention relates to a companion diagnostic antibody-like binding protein based on the humanized monoclonal antibody, DS6, to be used as diagnostic tool for in vivo detection and quantification of the tumor-associated MUC1-sialoglycotope, CA6.
Owner:SANOFI SA
Companion diagnostic assays for cancer therapy
InactiveUS20080233567A1Promote stratificationParticular utilitySugar derivativesMicrobiological testing/measurementOncologyPatient Base
Owner:ABBOTT LAB INC
Tumor biomarkers and use thereof
InactiveUS20180112273A1Organic active ingredientsMicrobiological testing/measurementTumor BiomarkersOncology
Disclosed herein are biomarkers related to WNT signal transduction pathway, as well as methods and kits comprising the same. Further, the present disclosure relates to the use of the biomarkers in patient selection, companion diagnostics, and treatment of cancer.
Owner:CUREGENIX CORP
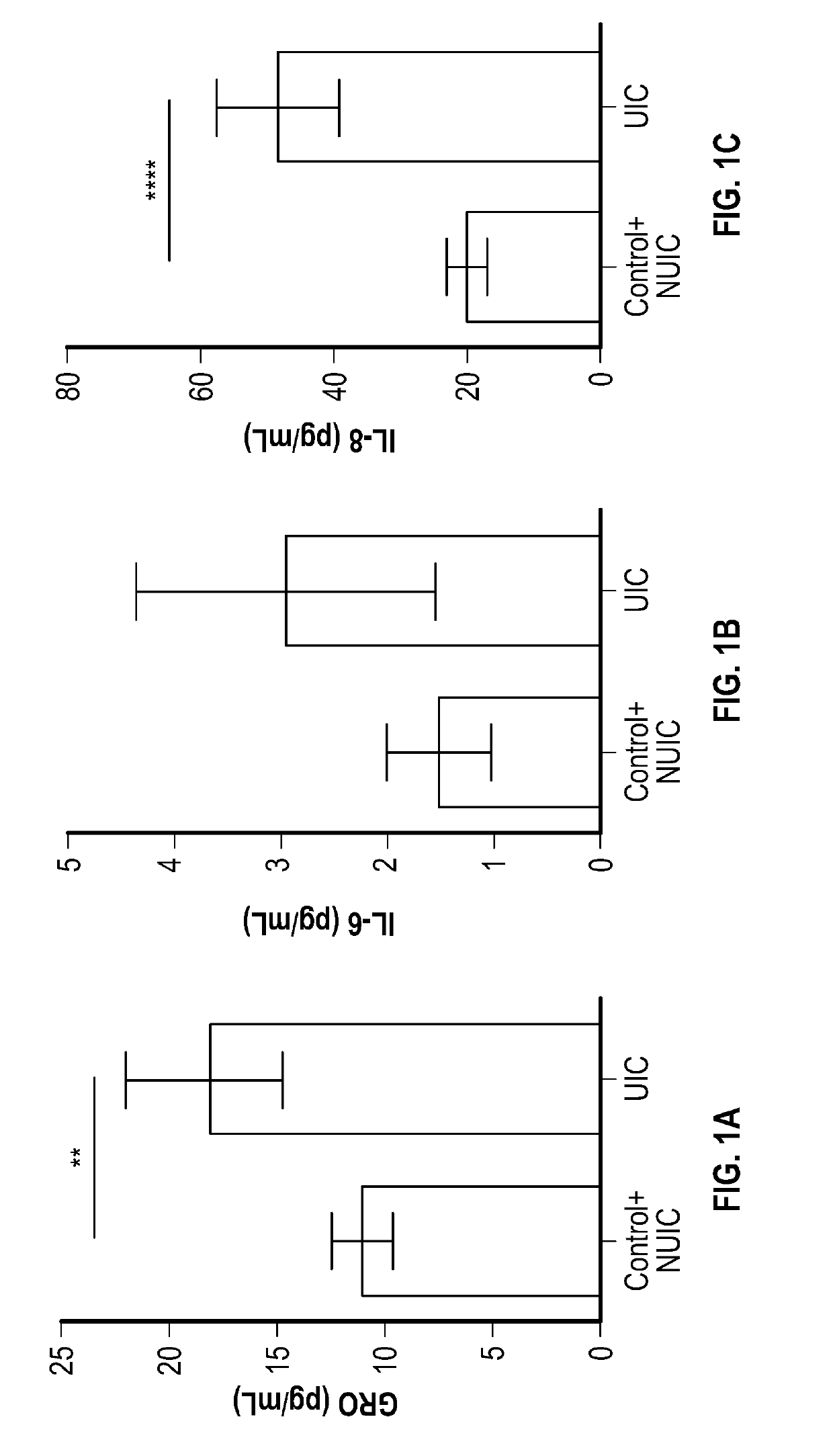
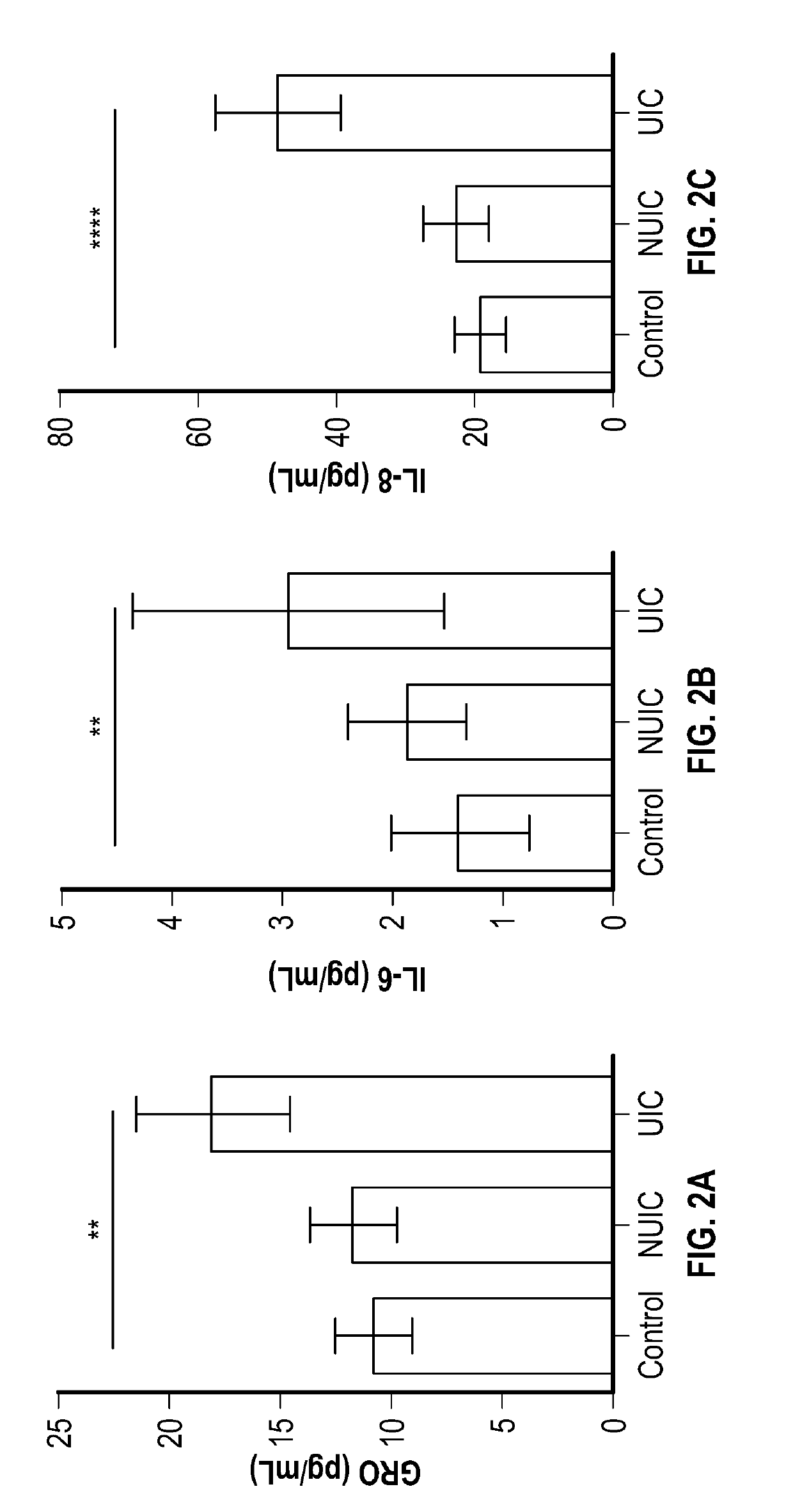
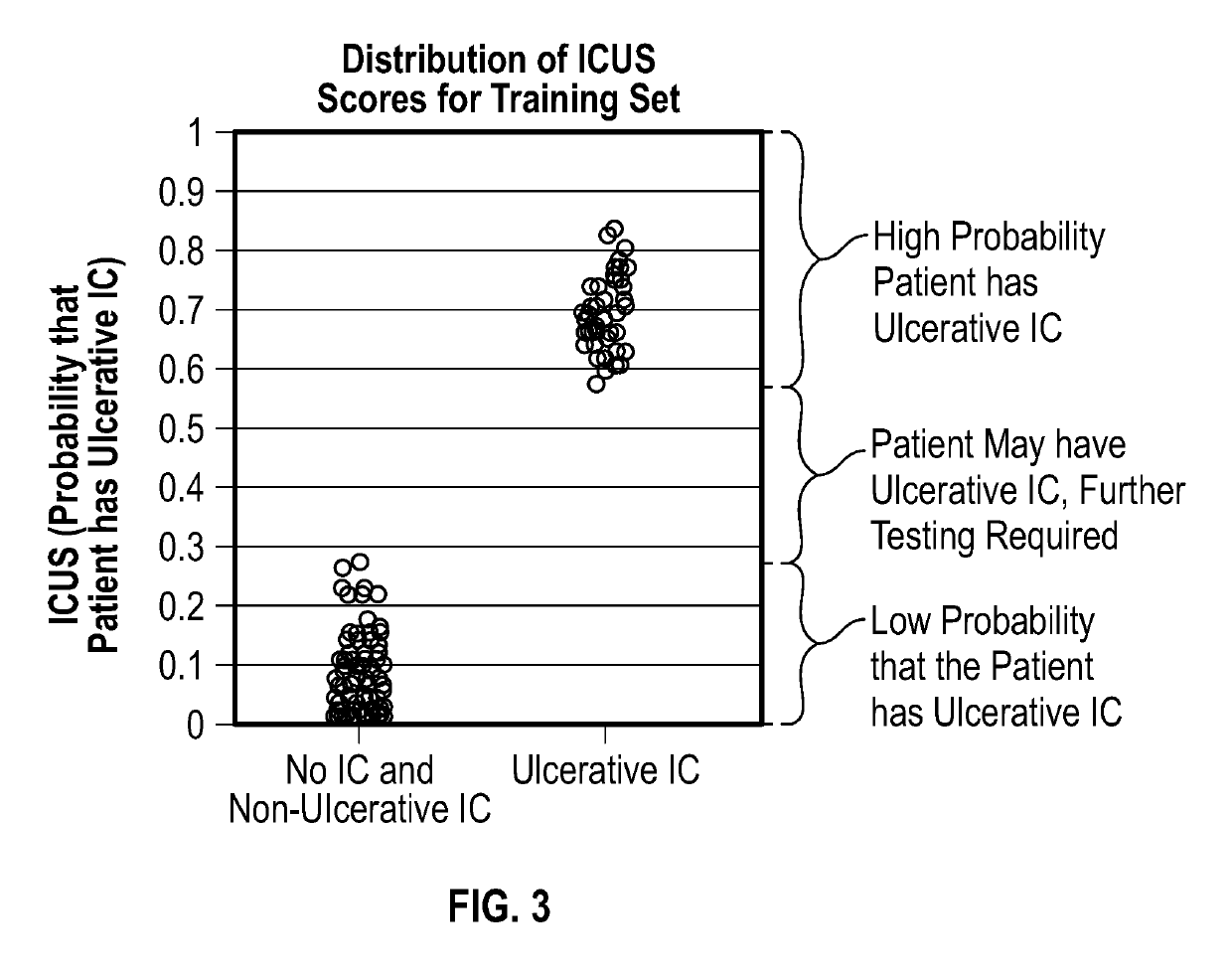
Methods for detecting, diagnosing and treating ulcerative interstitial cystitis
The present invention relates to methods for detecting, diagnosing and / or treating ulcerative interstitial cystitis (UIC) by detecting in a urine sample from a patient the levels of each of the proteins IL-6, IL-8 and GRO [also known as CXCL 1 (chemokine C-X-C motif ligand 1]. In some embodiments, the method also includes diagnosing the patient with UIC when each of the proteins IL-6, IL-8 and GRO in the urine sample is at a different level than a statistically validated threshold for the respective proteins. In some embodiments a companion diagnostic, e.g., a cystoscopy, is used in conjunction with the protein biomarker diagnostic. In some embodiments, once UIC is diagnosed, the patient is treated for the UIC.
Owner:WILLIAM BEAUMONT HOSPITAL
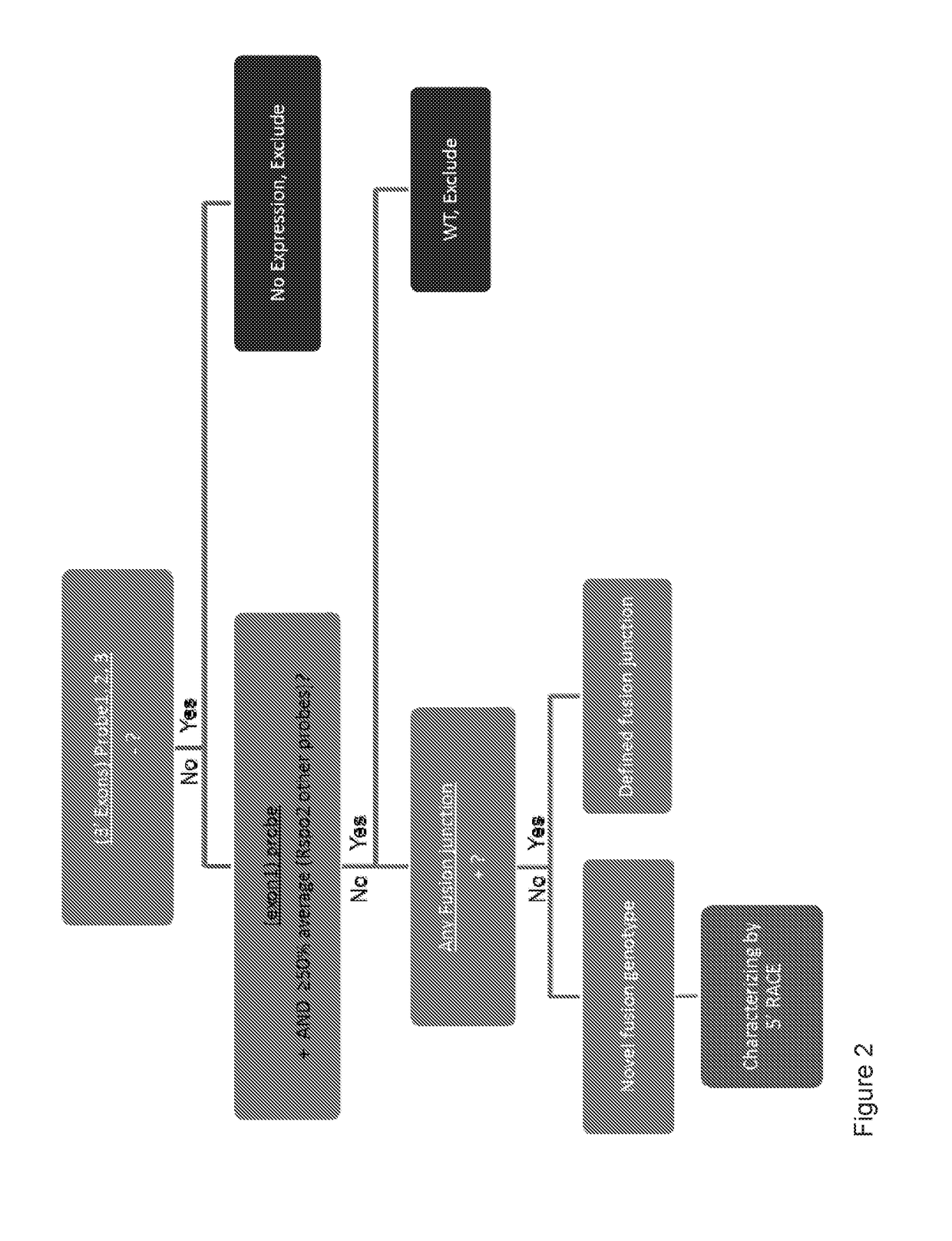
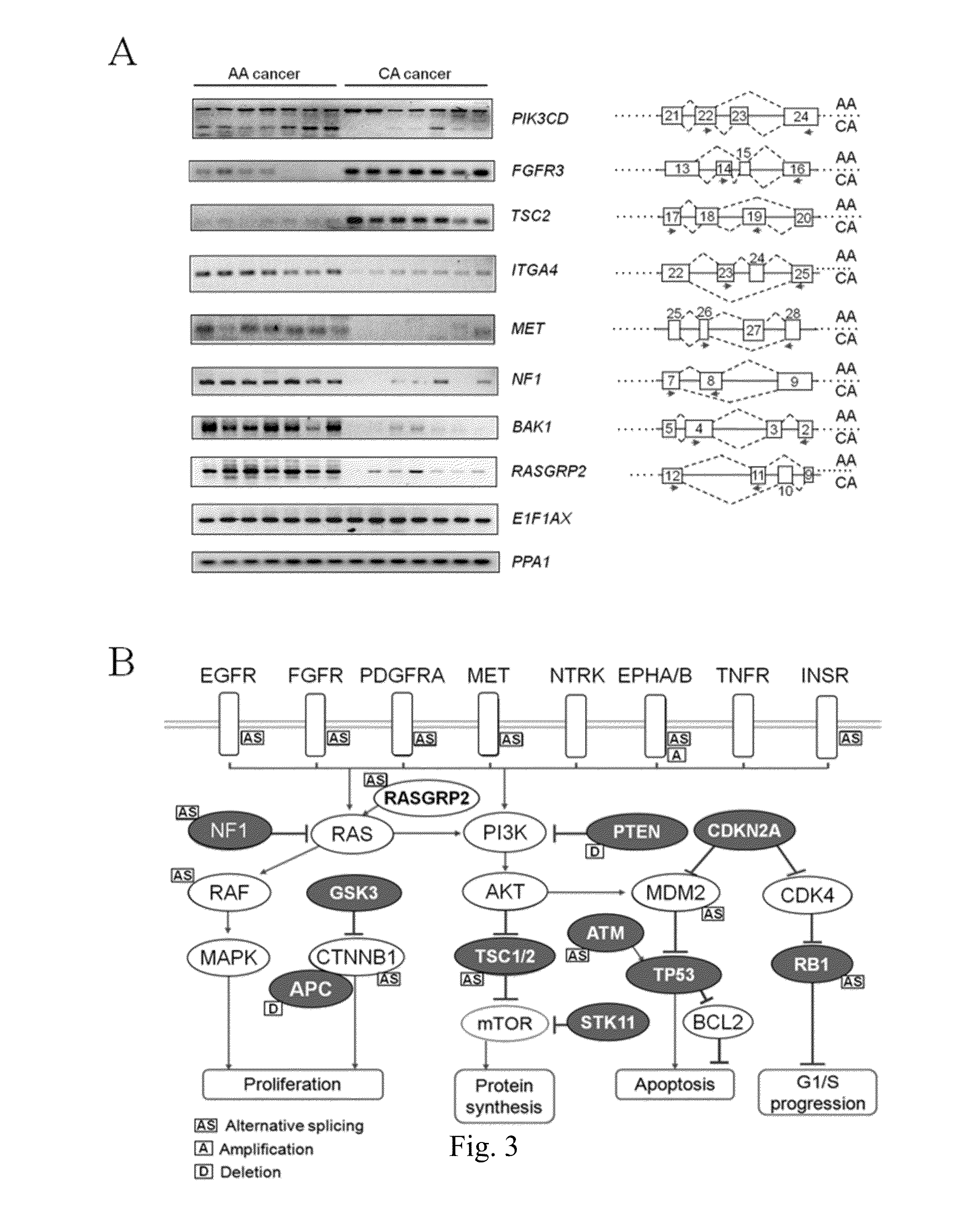
Companion diagnostics for cancer and screening methods to identify companion diagnostics for cancer based on splicing variants
InactiveUS20150252432A1High incidenceHigh mortality rateCompound screeningApoptosis detectionScreening cancerScreening method
A method of classifying a patient for eligibility for cancer therapy based on the presence or absence of splicing variants in a sample of the patient's cancer tissue. Also, a method of screening cancer therapies for efficacy against splicing variants. More specifically, the methods relate to novel splicing variants of genes associated with cancer risk and survival, particularly splicing variants of PIK3CD, FGFR3, TSC2, RASGRP2, ITGA4, MET, NF1 and BAK1. Also more specifically, the methods relate to classifying a patient for eligibility for cancer therapy involving the use of GS-1101.
Owner:GEORGE WASHINGTON UNIVERSITY
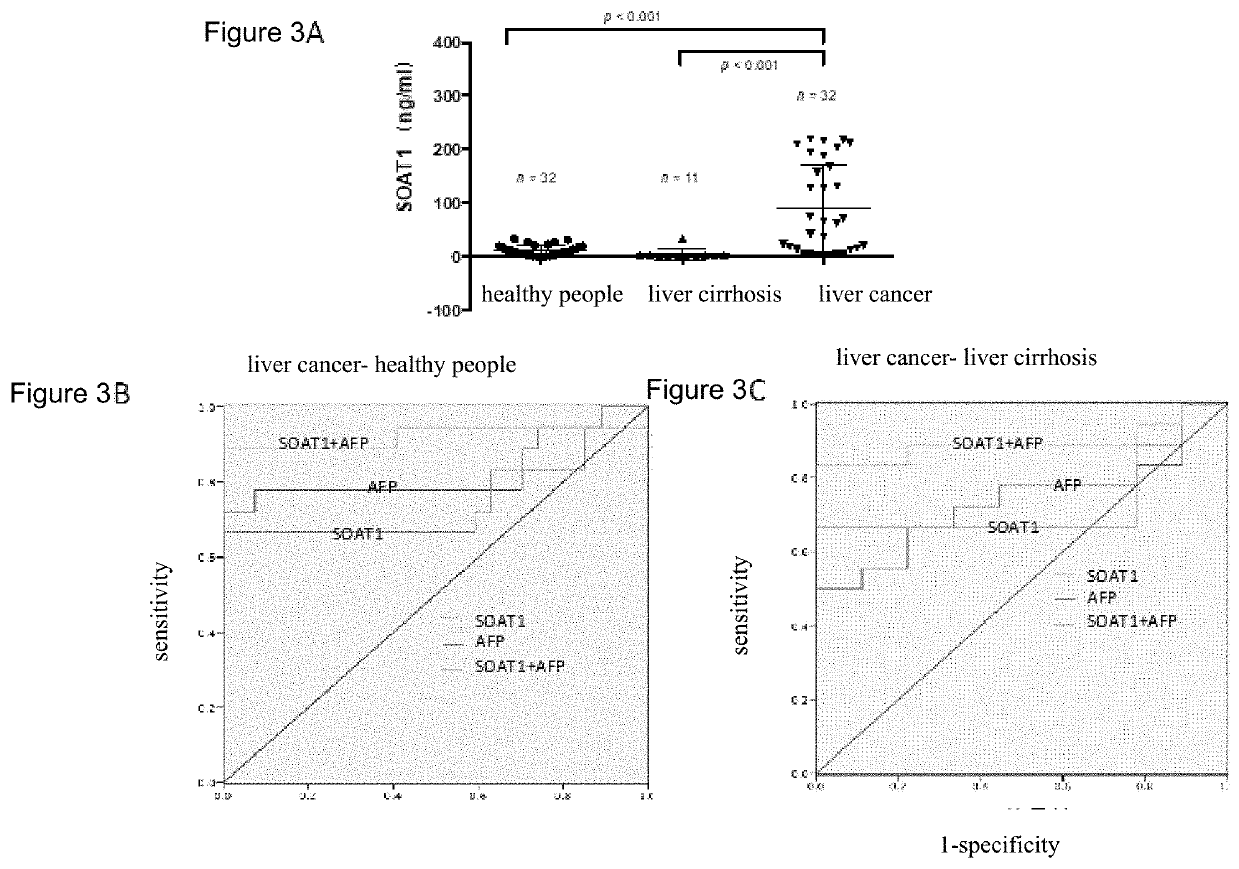
Use of acyl coenzyme a: cholesterol acyltransferase-1 in diagnosis and treatment of liver cancer
PendingUS20220034891A1Improve accuracyStrong complementarityOrganic active ingredientsMicrobiological testing/measurementCholesterolApoptosis
A use of a substance for inhibiting SOAT1 gene expression and / or protein activity. The use is selected from at least one of: (a) Preparation of kits for liver cancer diagnosis; (b) Preparation of kits for liver cancer prognosis; (c) Preparation of companion diagnostic kits for treatment of liver cancer; (d) For the preparation of drugs for the prevention and / or treatment of cancer; (e) For the preparation of drugs for the prevention and / treatment of cancer spread and metastasis; (f) For the preparation of drugs that promote the apoptosis of cancer cells; (g) For the preparation of drugs for inhibiting cancer cell formation; (h) For the preparation of drugs that inhibit the proliferation and growth of cancer cells in vitro. Experiments have shown that SOAT1 is highly expressed in liver cancer tissues and serum, and its high abundance indicates poor prognosis of liver cancer patients.
Owner:BEIJING PROTEOME RES CENT
Immuno imaging agent for use with antibody-drug conjugate therapy
ActiveUS20140255305A1Facilitates stratification and early evaluationShort exposure timeHybrid immunoglobulinsRadioactive preparation carriersMedicineImaging agent
The invention relates to a companion diagnostic antibody-like binding protein based on the humanized monoclonal antibody, DS6, to be used as diagnostic tool for in vivo detection and quantification of the tumor-associated MUC1-sialoglycotope, CA6.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
Process for preparing vaccine compositions
PendingCN113383009ATumor rejection antigen precursorsMicrobiological testing/measurementInducer CellsTGE VACCINE
The disclosure relates to polypeptides, polynucleic acids and pharmaceutical compositions comprising polypeptides that find use in the prevention or treatment of cancer. The disclosure also relates to methods of inducing a cytotoxic T cell response in a subject or treating cancer by administering pharmaceutical compositions comprising the peptides, and companion diagnostic methods. The disclosure also relates to a method of preparing a peptide or polynucleic acid for use in a method of inducing a T cell response against a target polypeptide, wherein the method comprises identifying epitopes in the antigen that bind to multiple HLA alleles of the highest proportion of subjects in a target population.
Owner:特雷斯生物有限公司
A companion diagnostic method for use in the treatment of irritable bowel syndrome with dietary interventions or faecal microbiota transplant
The present invention provides a diagnostic method which may be used to determine the likelihood that a subject with Irritable Bowel Syndrome (IBS) will respond to treatment with an IBS intervention diet or faecal microbiota transplant (FMT). In particular, the method may be used to predict, or determine the likelihood of, a positive response of the subject with IBS to treatment with an IBS intervention diet or FMT, especially to determine the likelihood that the dietary intervention or FMT may have a positive (i.e. beneficial) effect on the subject's Gl tract, specifically the Gl tract microbiota, or other symptoms or complications of IBS (e.g. reducing severity thereof). The method of the present invention is based on analysing the abundance of certain bacteria in Gl tract samples, e.g.by nucleic acid analysis.
Owner:GENETIC ANALYSIS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com