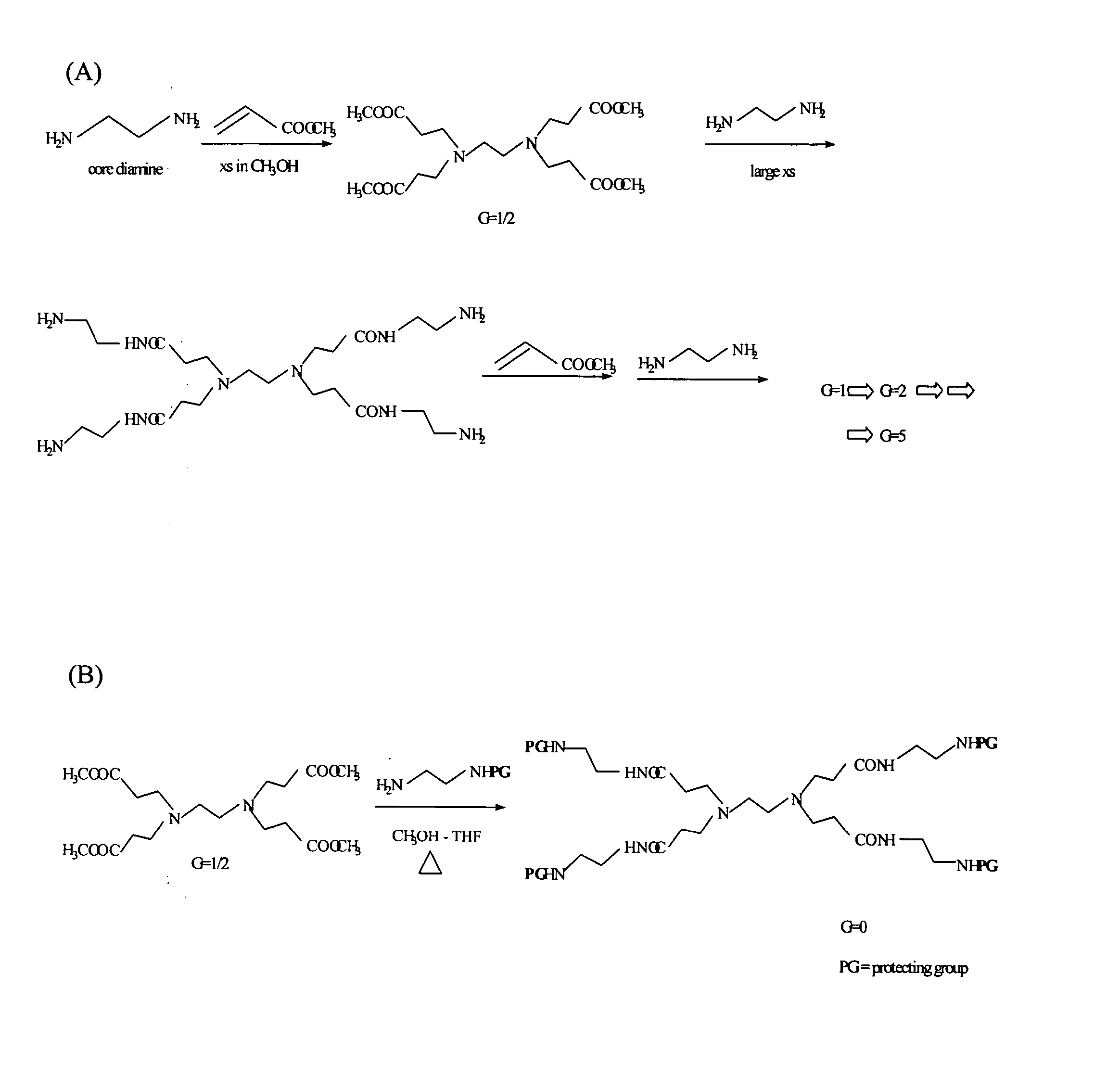
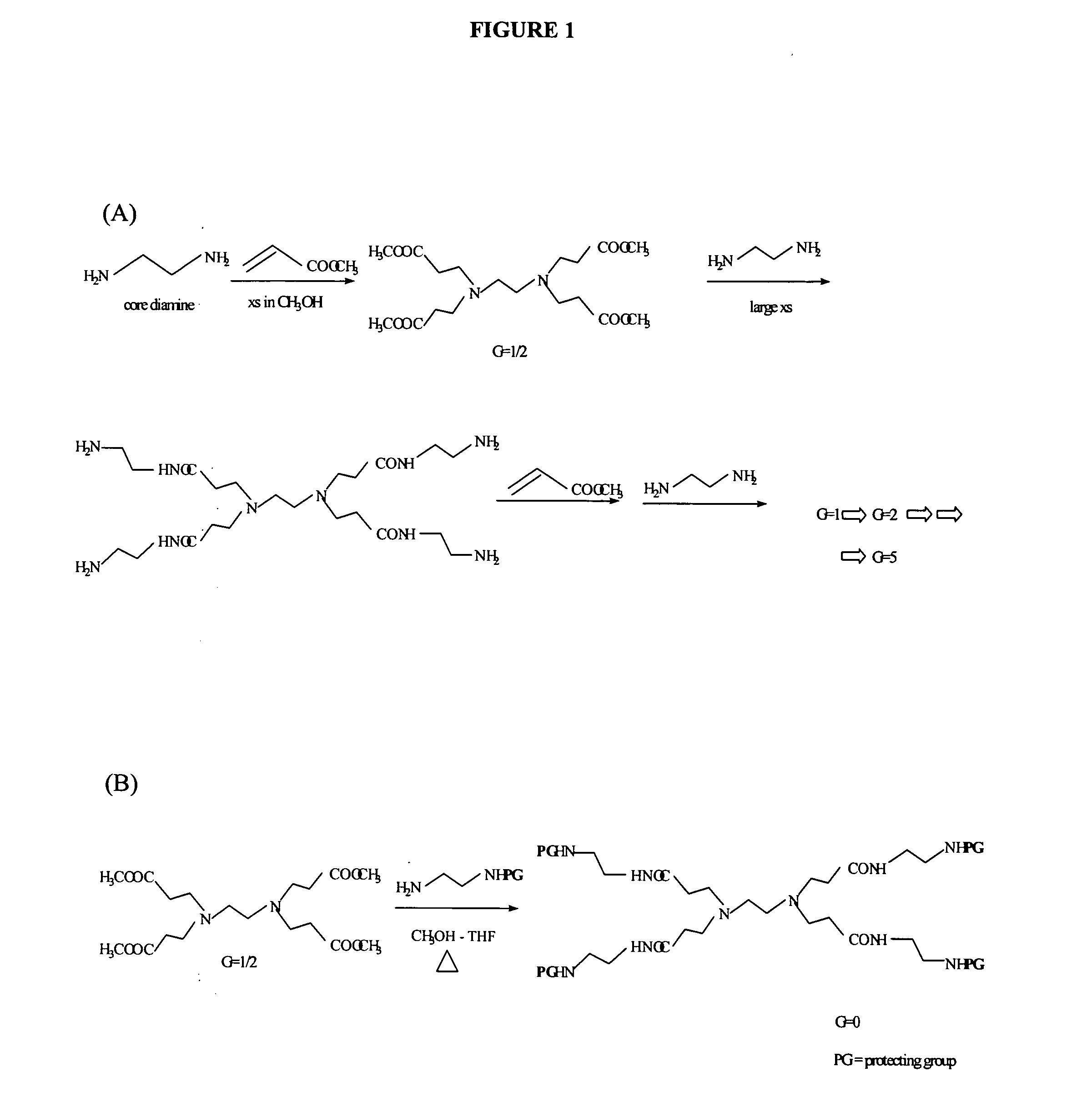
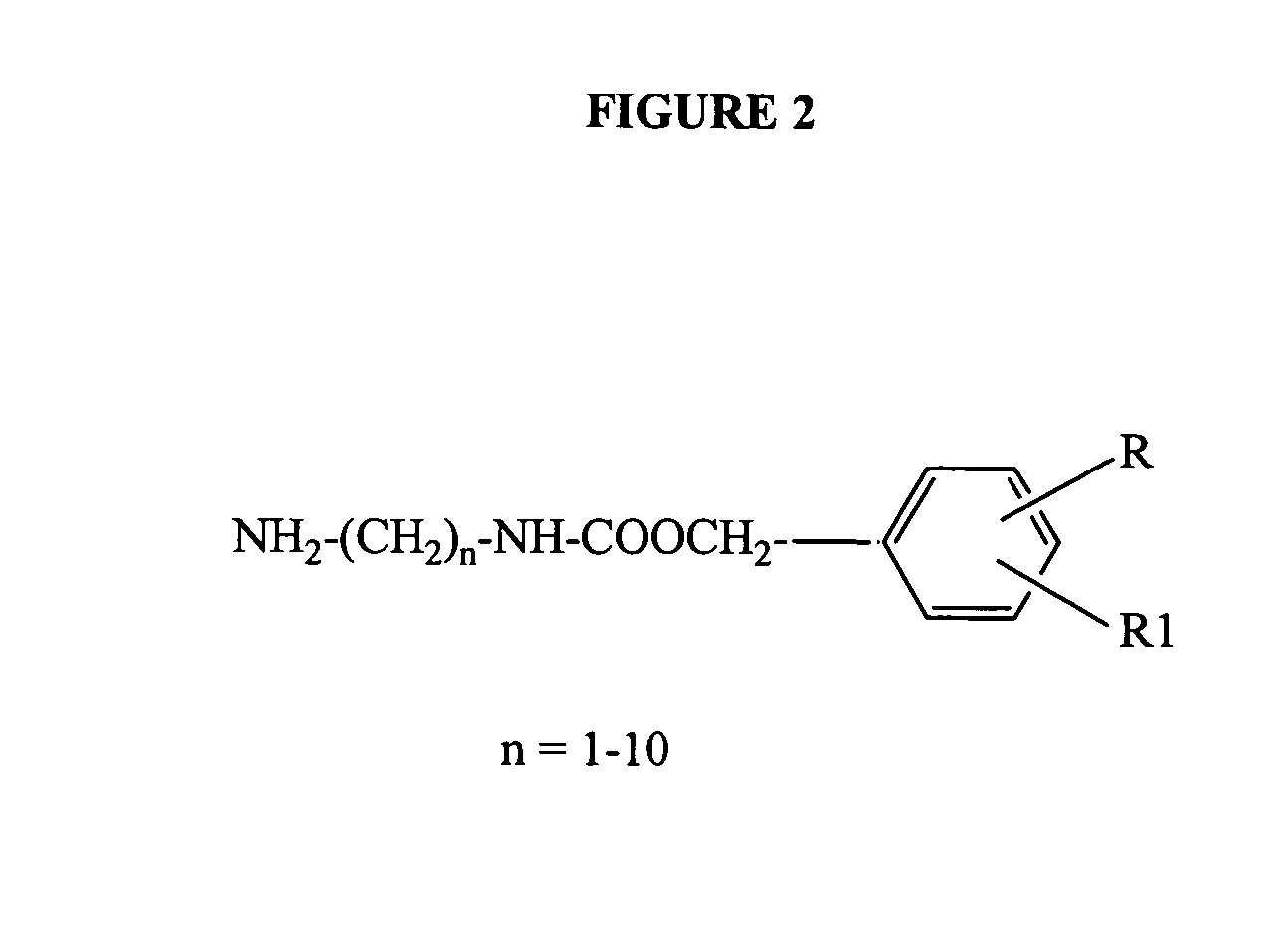
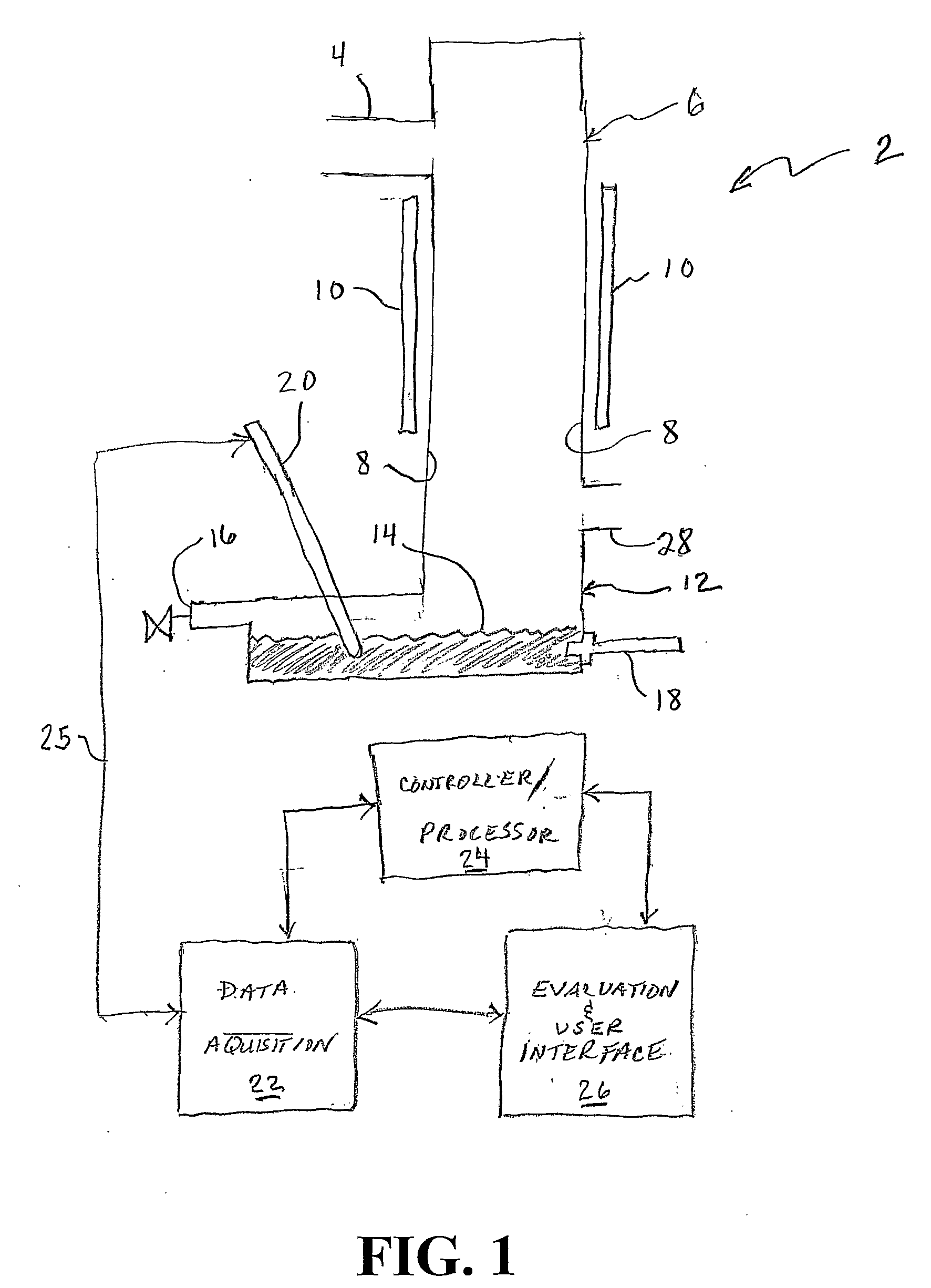
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
217 results about "Response to therapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
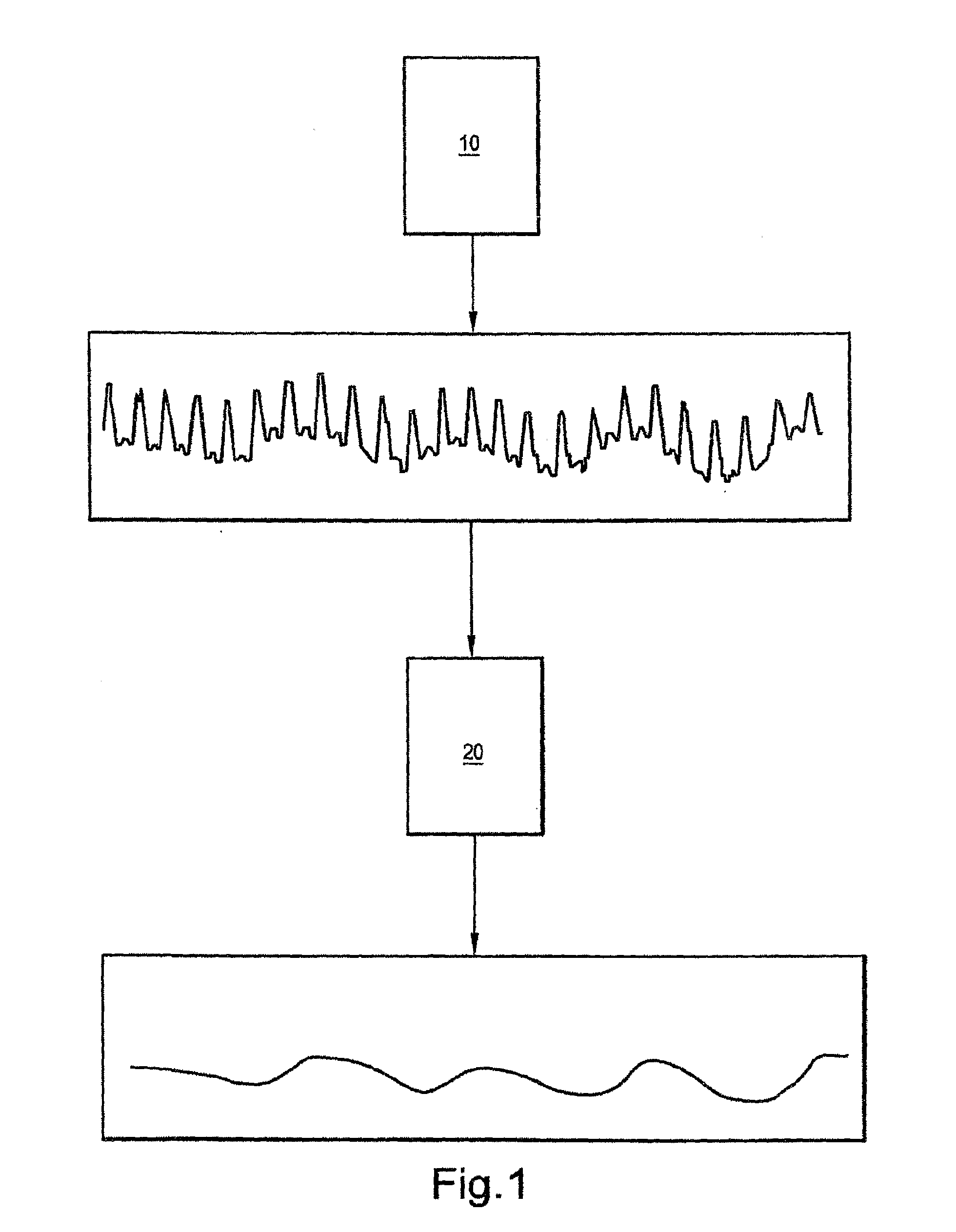
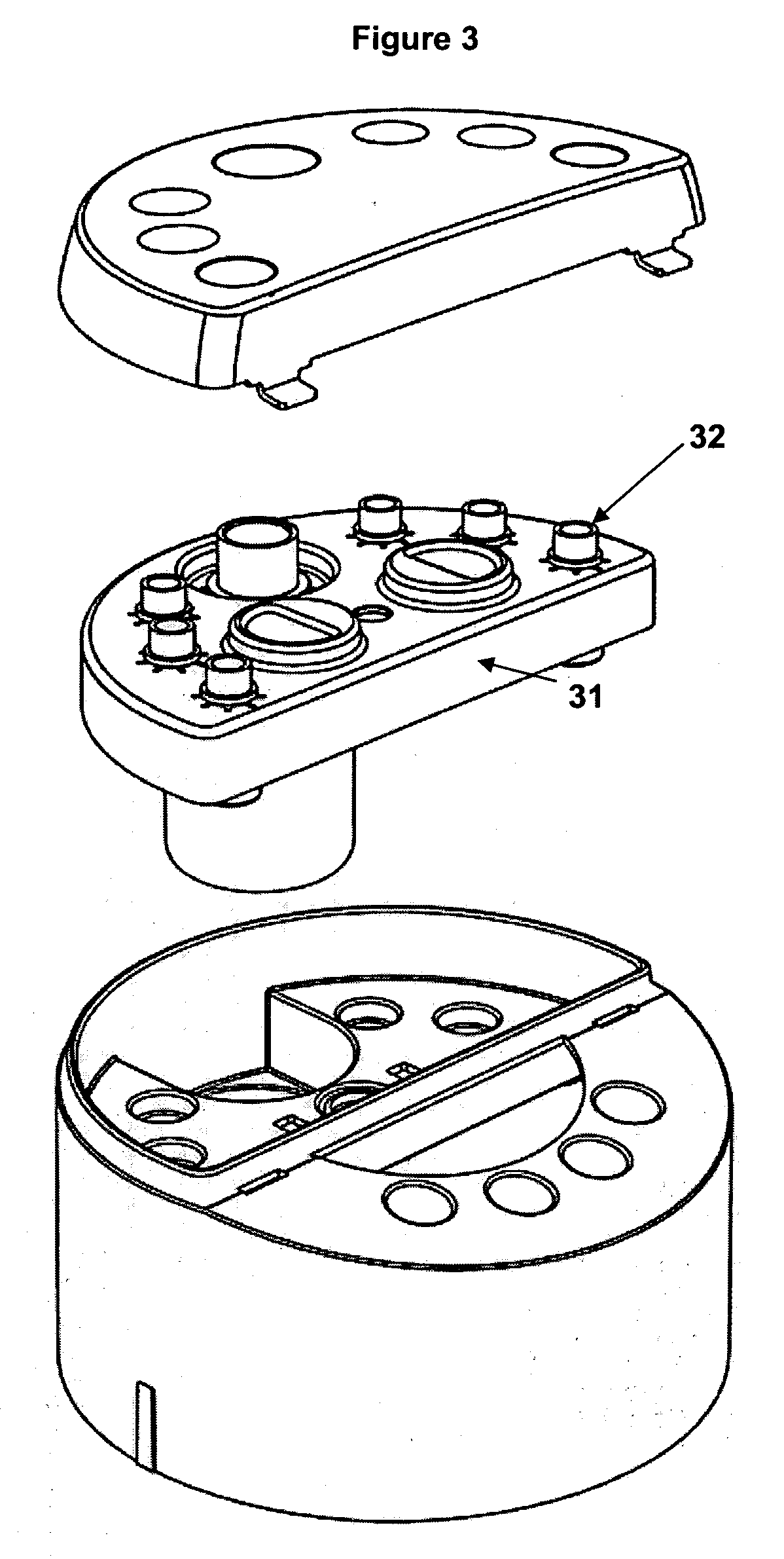
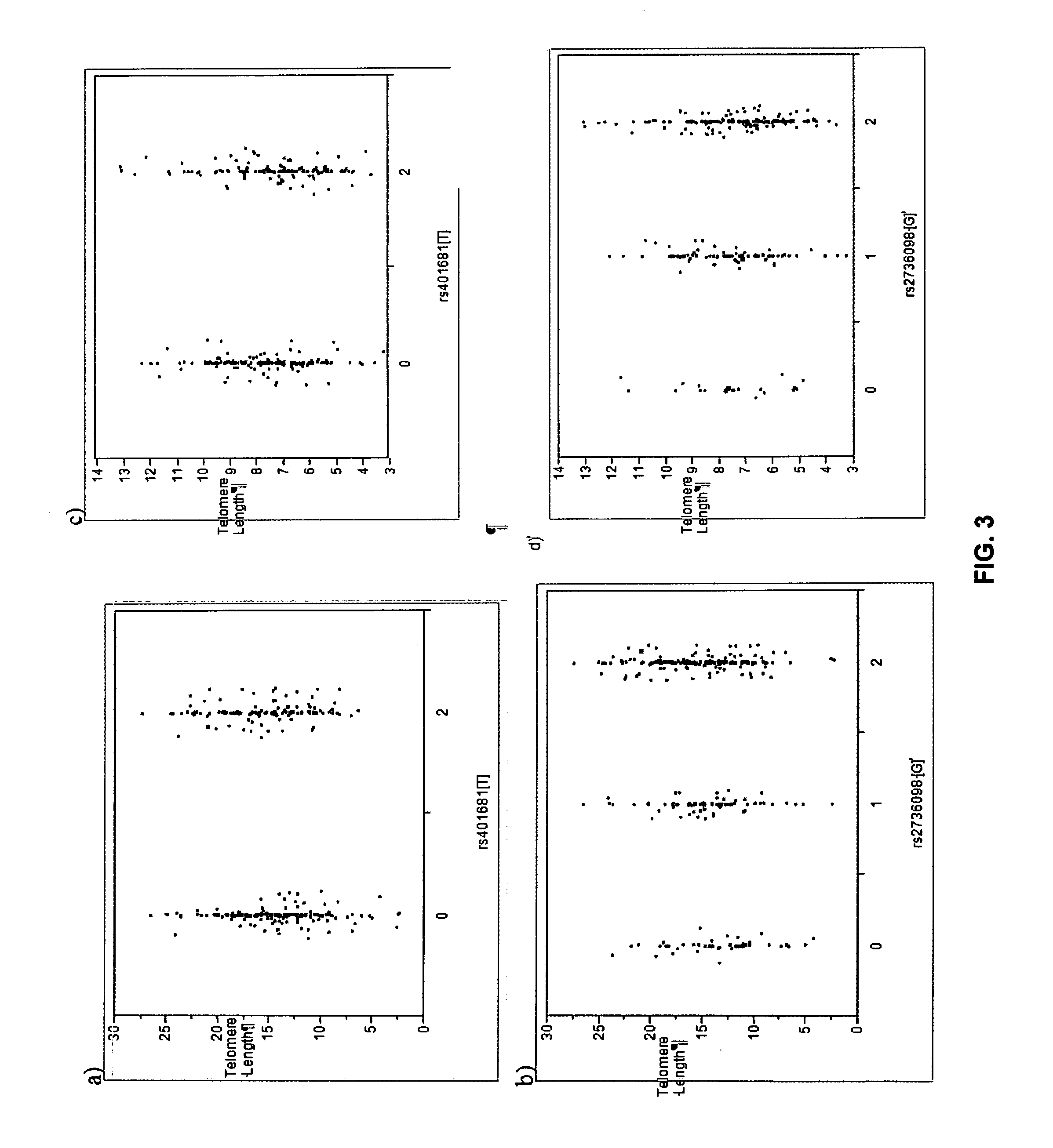
Systems and methods for displaying changes in biological responses to therapy
InactiveUS7720306B2Better convey information to the viewerImprove visibilityDrawing from basic elementsPerson identificationCorrelation functionDisplay device
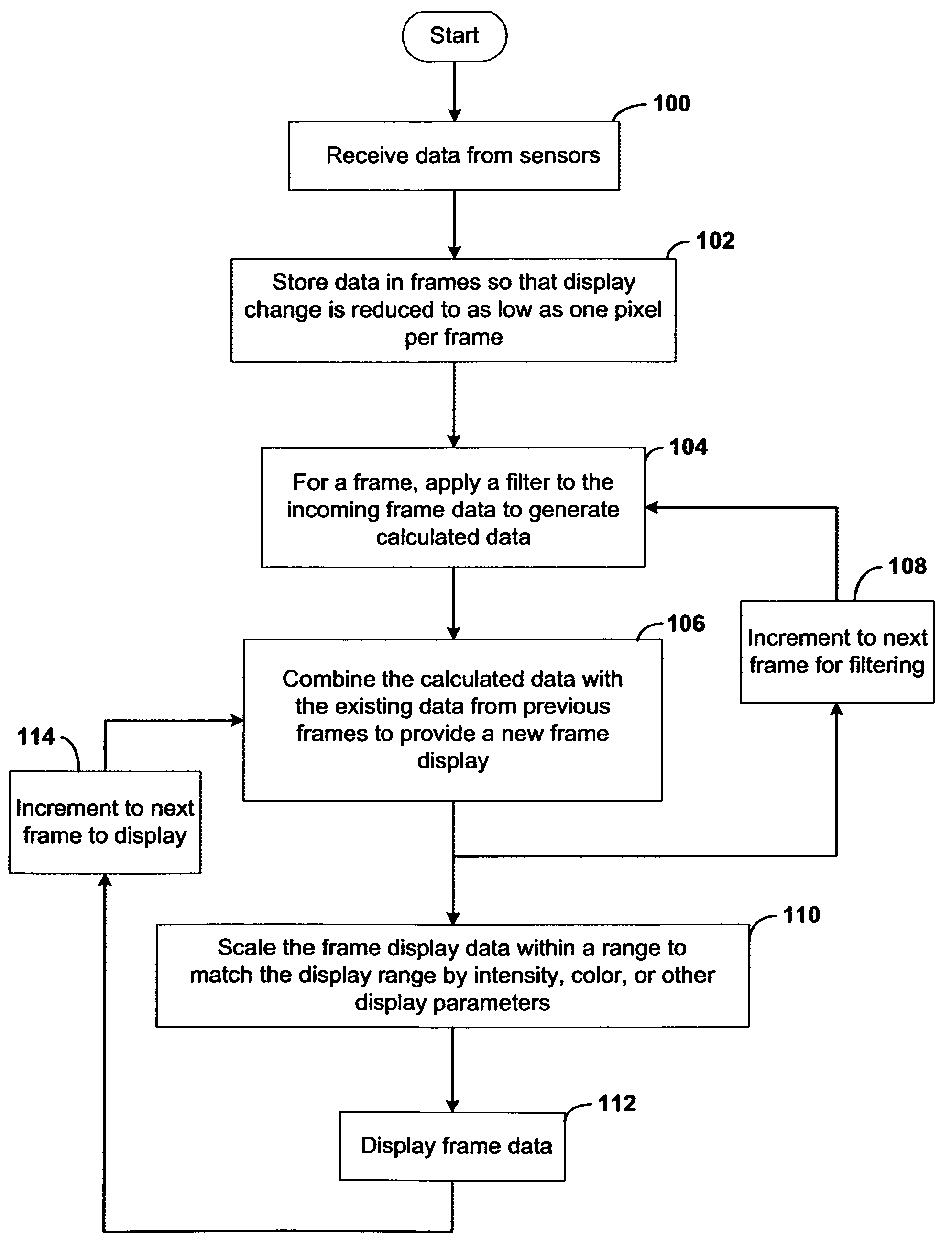
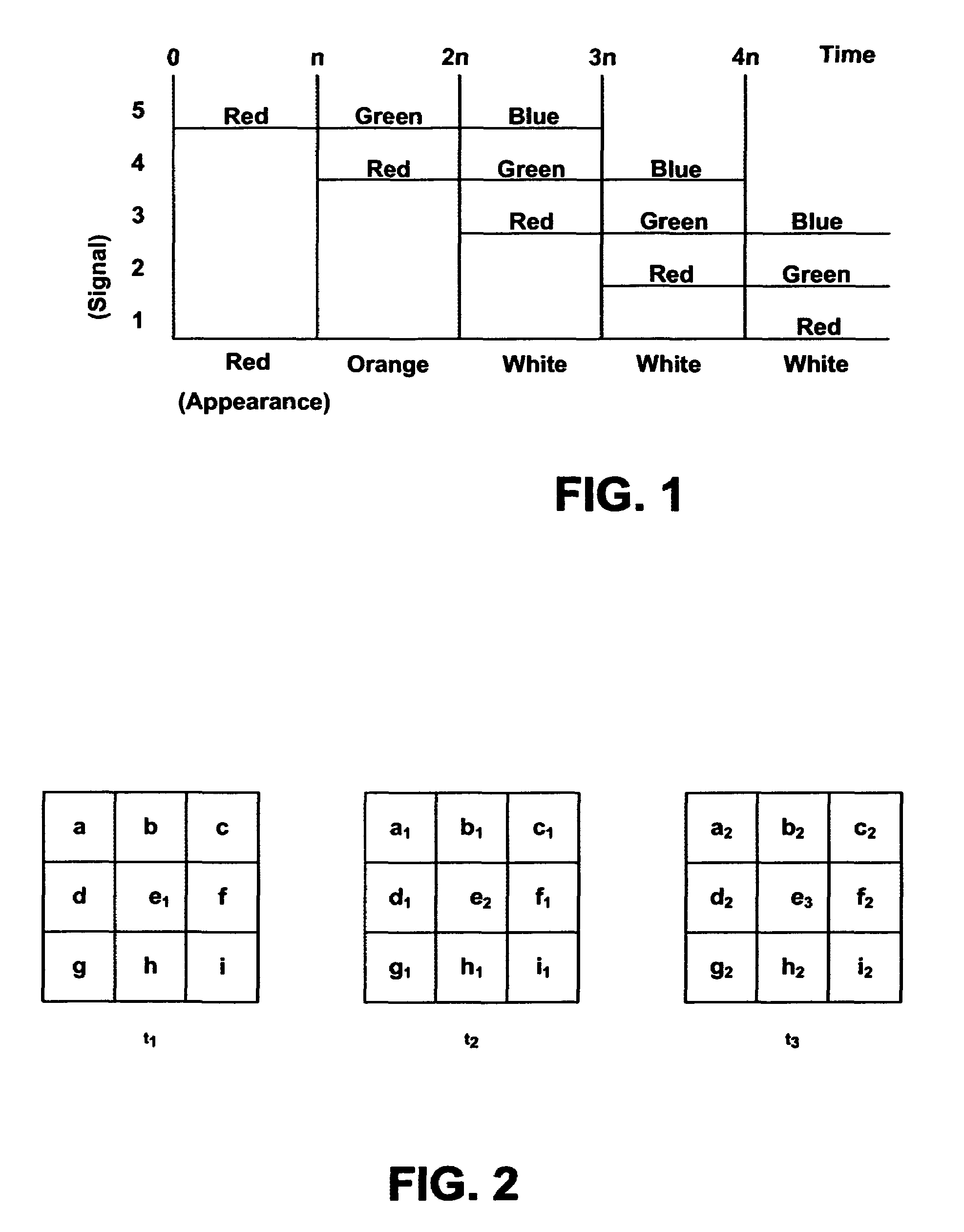
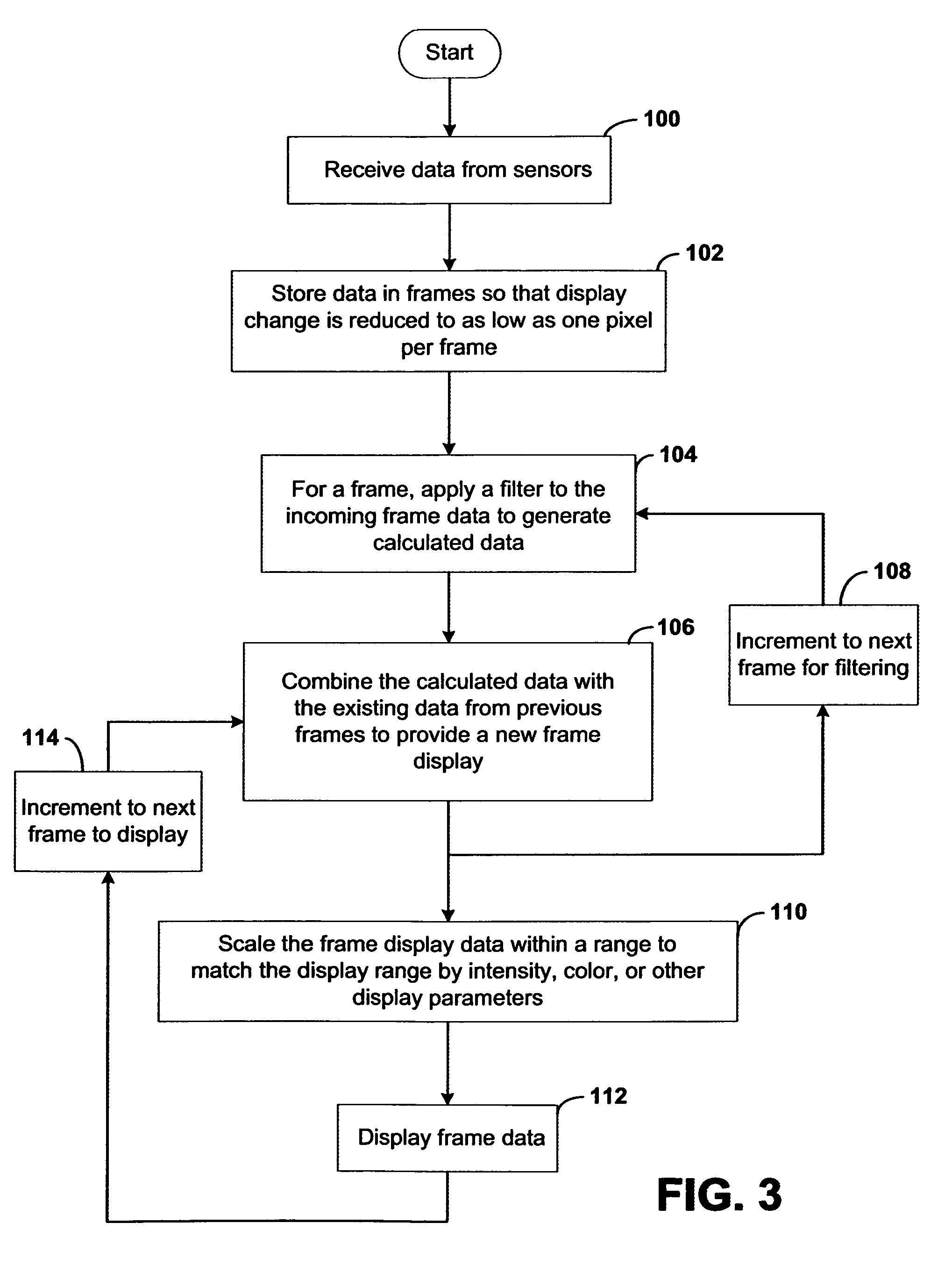
Systems and methods of this invention display data using pixels with information indicated by color and intensity changes, particularly used for monitoring of physiological variables in real time. For certain methods, physiological data can be acquired by sensors, acquired data can be stored in data frames, data frames can be processed using computer-implemented methods, and processed data frames can be scaled to a display frame for display on a display device. Using such methods, a spot made up of a group of pixels can be updated during a time frame, or cycle using a computer-implemented method, such as addition, subtraction, multiplication, division or a time dependent function. Newly received data can be combined with prior received data to indicate time-dependent changes. In this way, each spot contains a cumulative history of data starting at some initial time. In other embodiments, visual contrast can be enhanced between desired data and other data. In further embodiments, two or more different types of data can be plotted together to indicate relationships between variables. Real time monitoring of signals during therapeutic treatment using light, electricity or other nerve or muscle stimuli can allow a user to monitor physiological responses during stimulation and to make rapid decisions about medical treatment.
Owner:PHOTOMED TECH
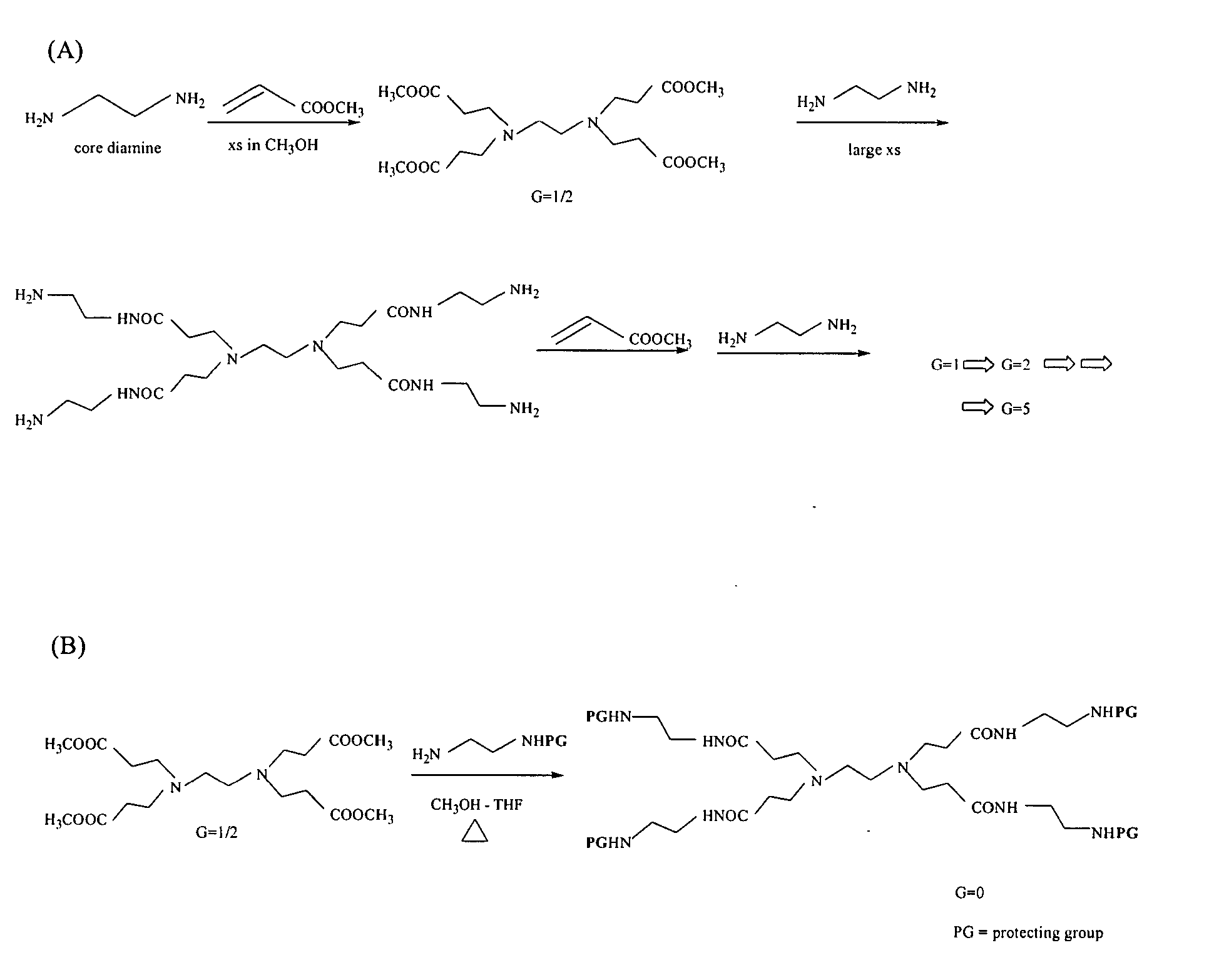
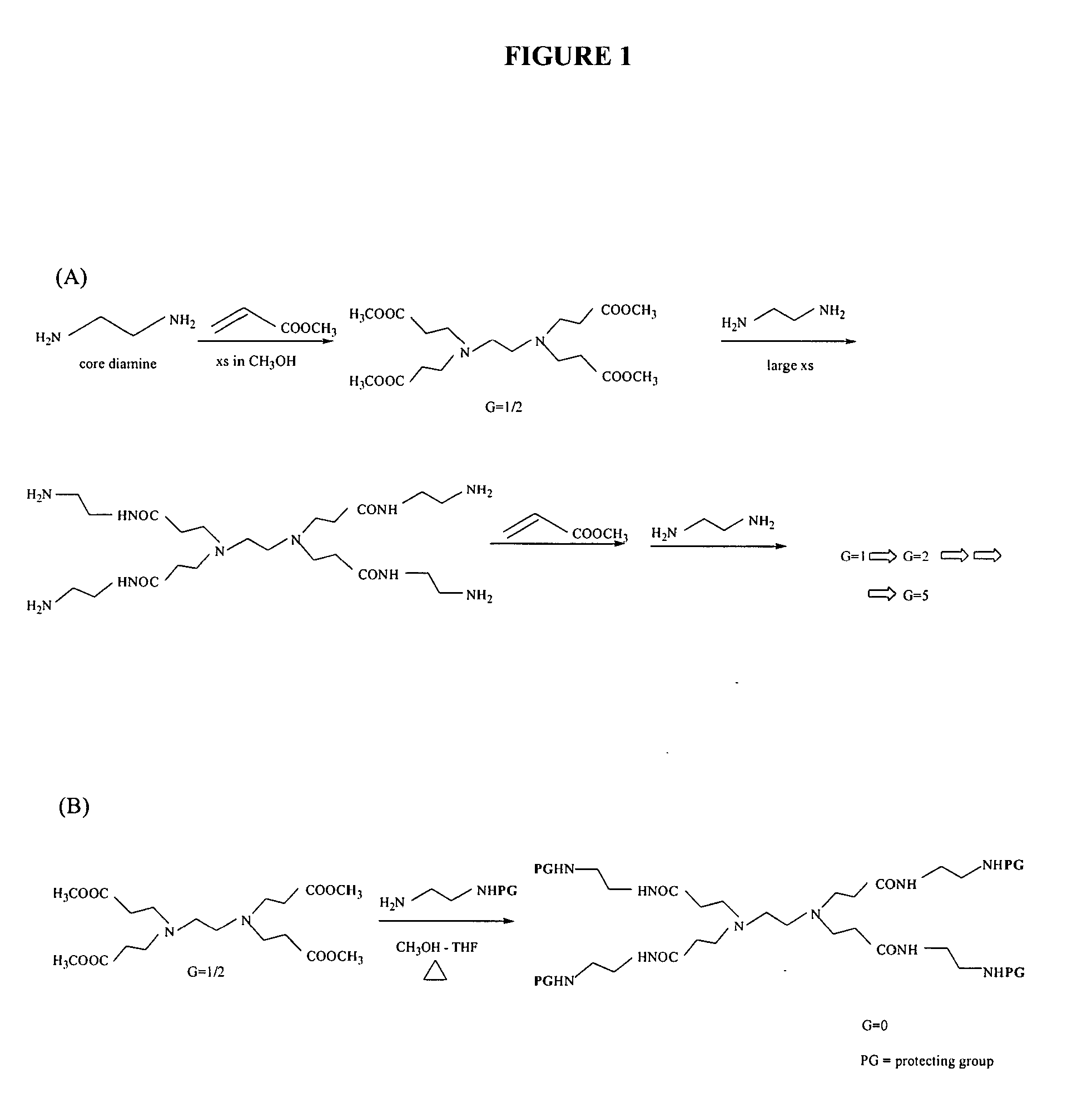
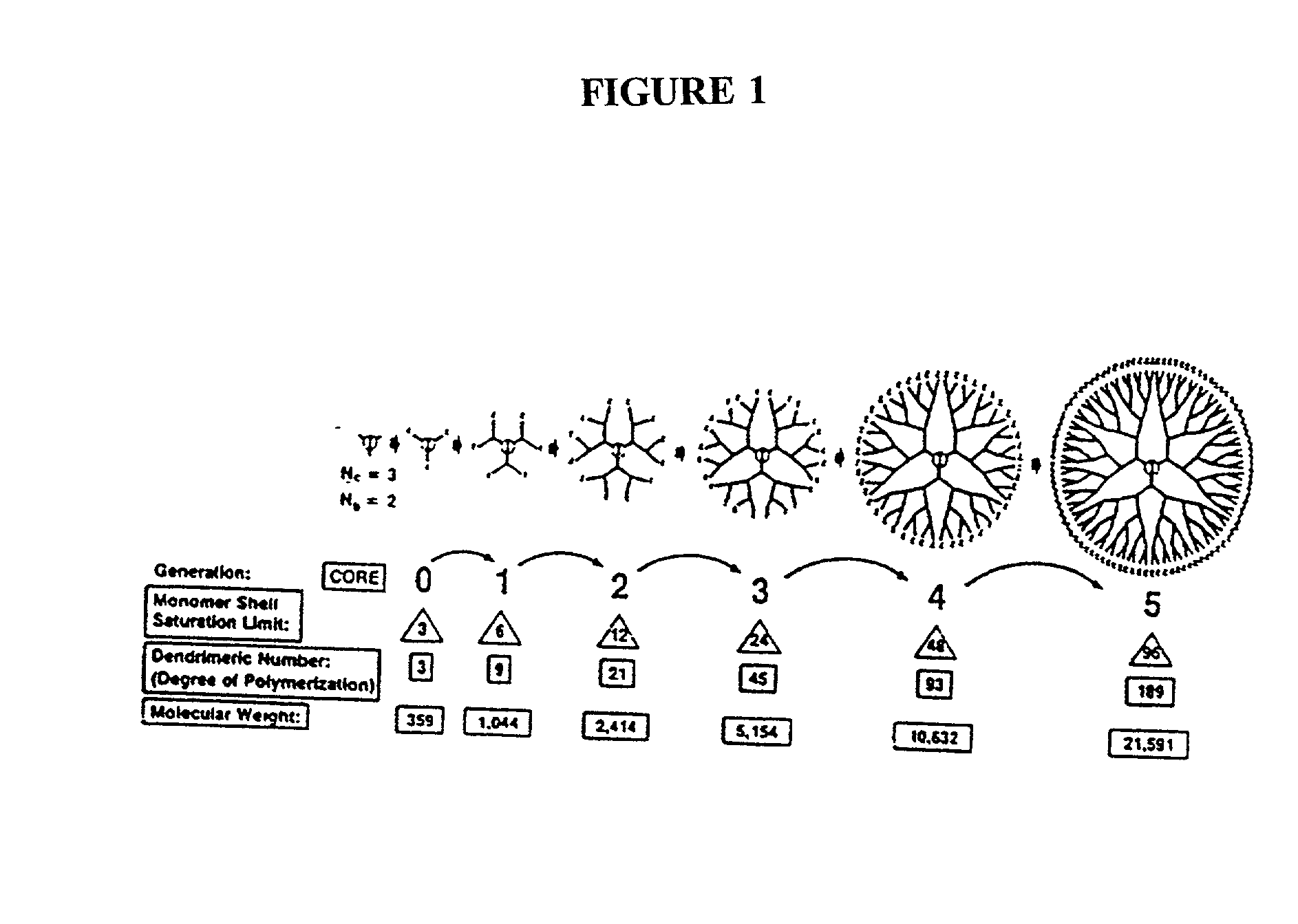
Multifunctional nanodevice platform
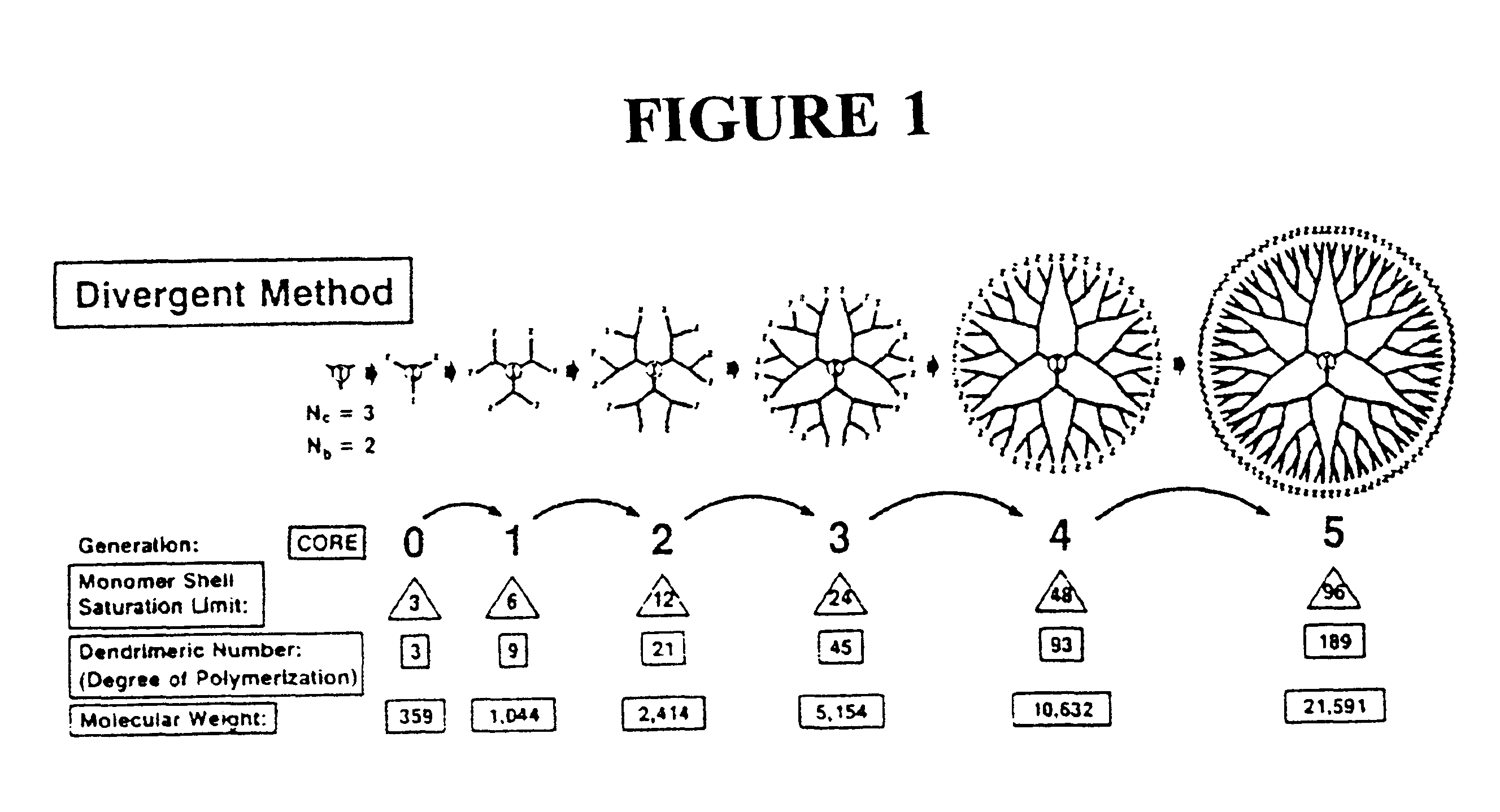
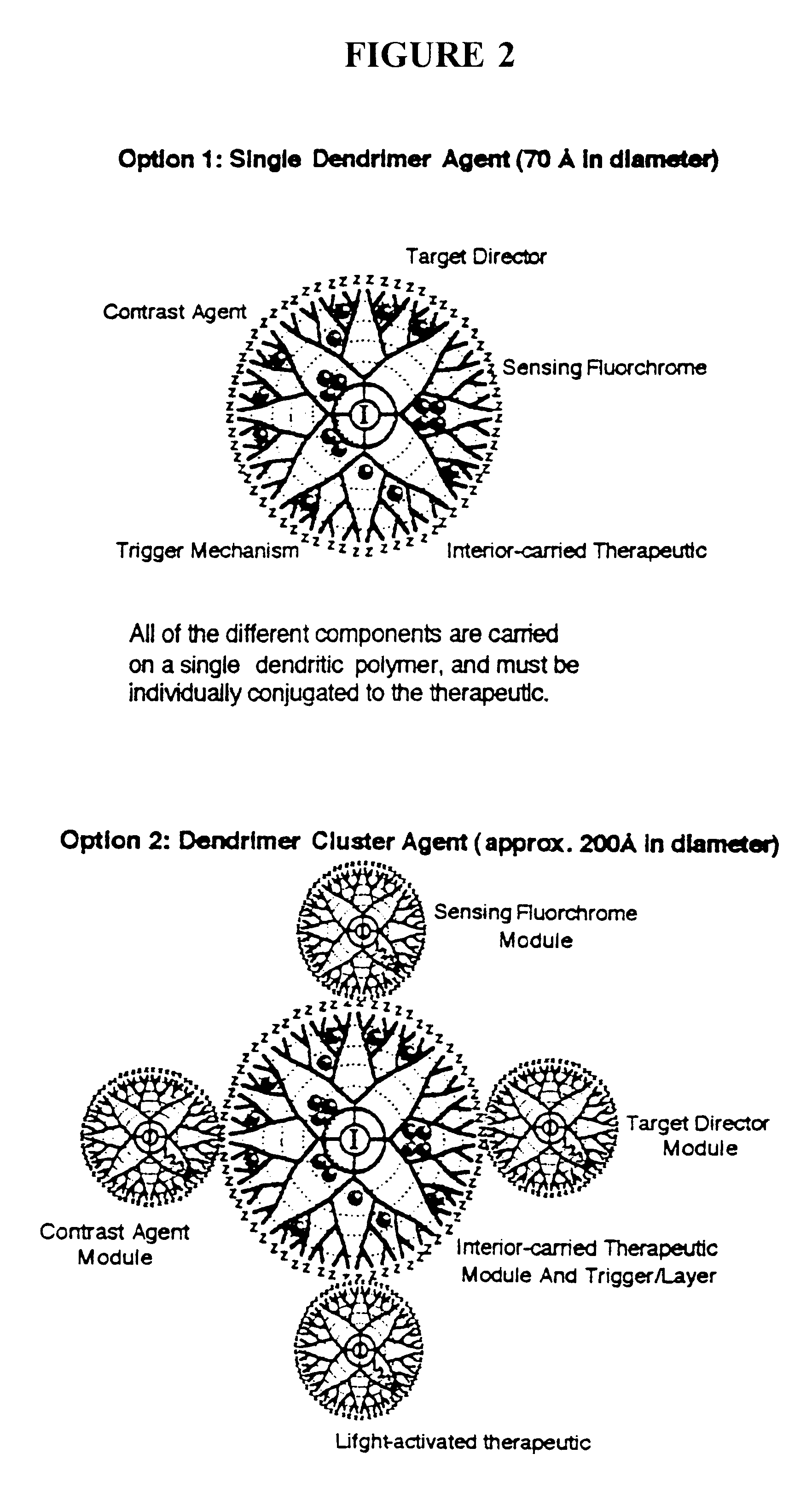
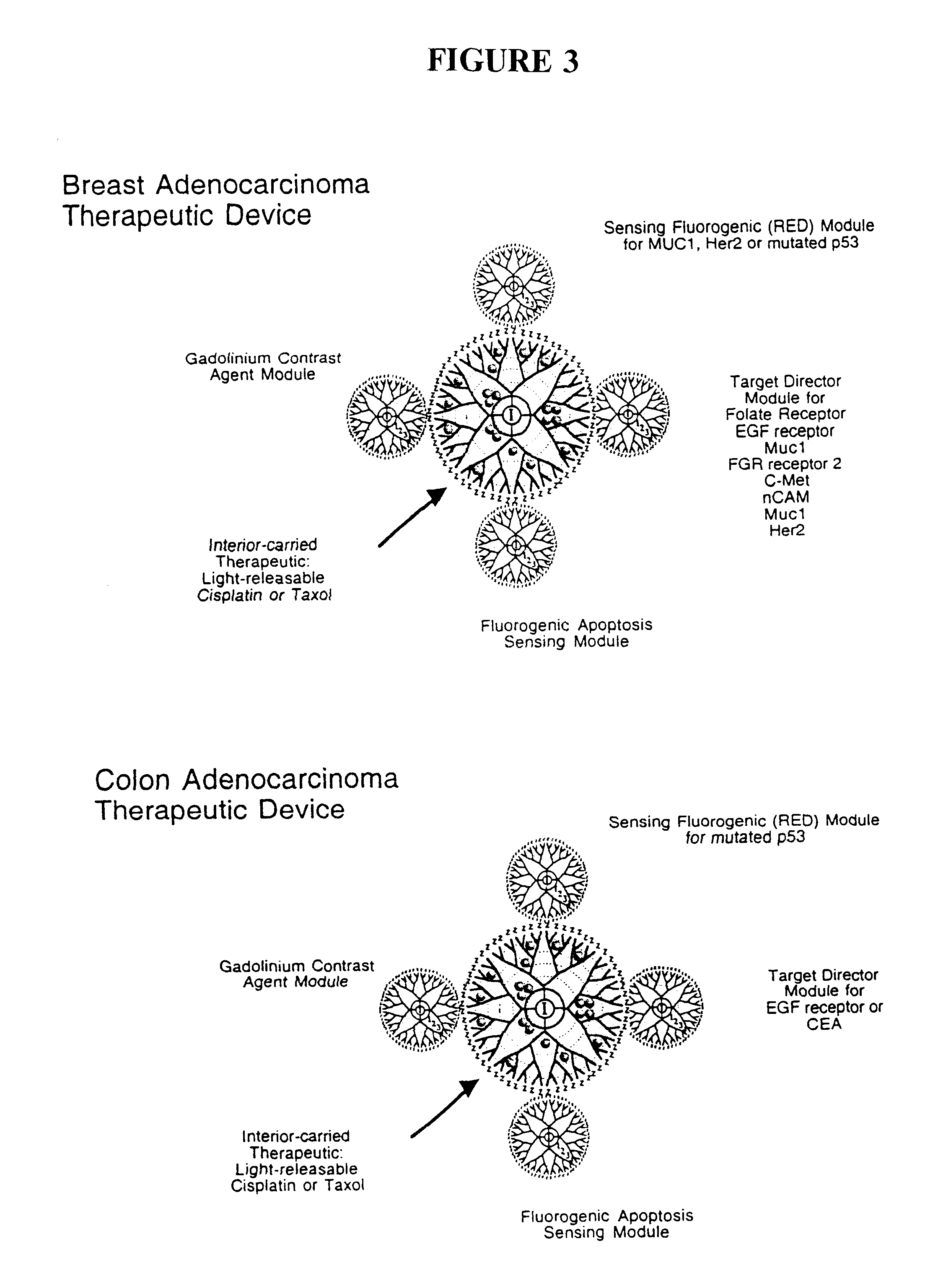
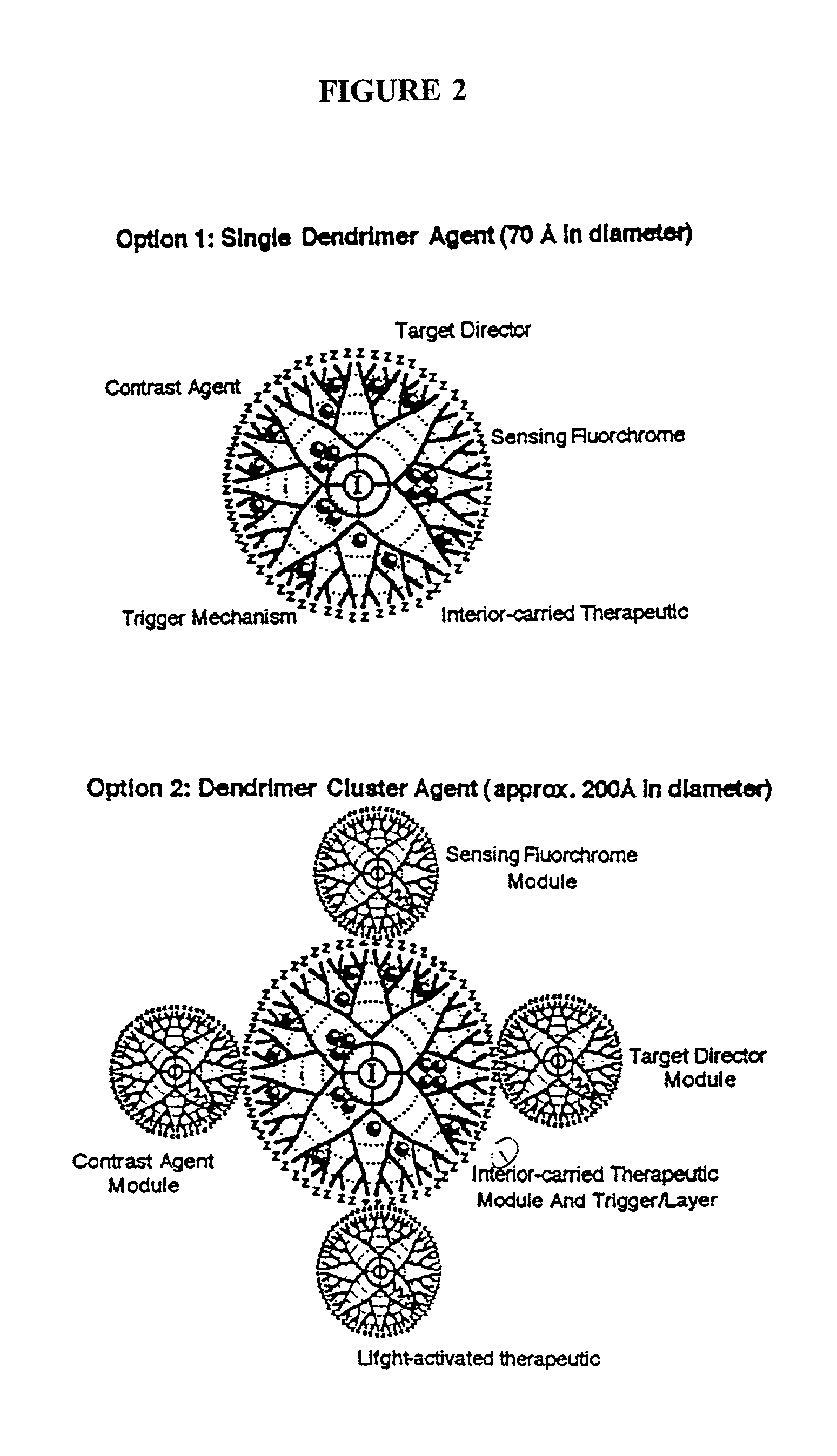
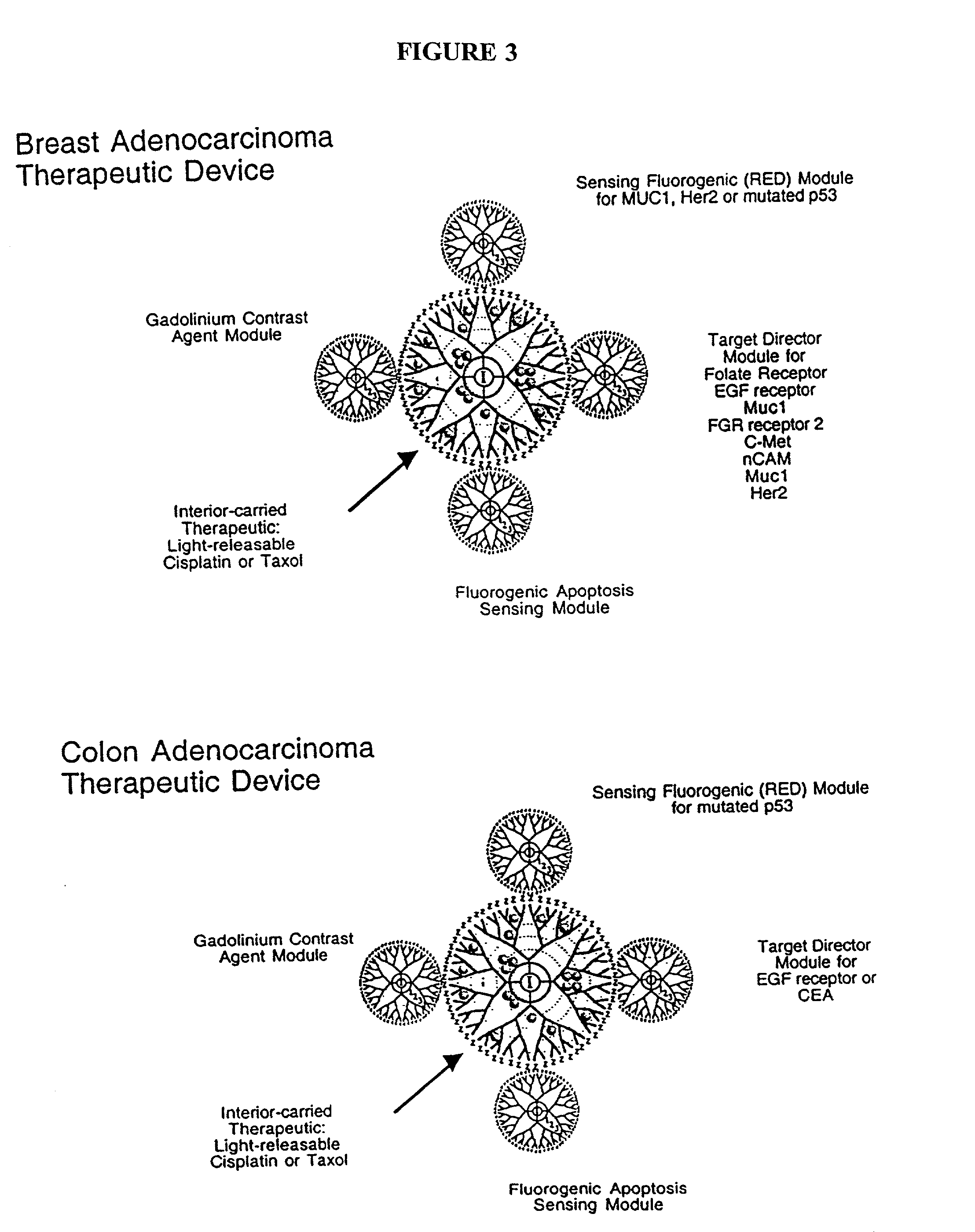
The present invention relates to novel therapeutic and diagnostic arrays. More particularly, the present invention is directed to dendrimer based multifunctional compositions and systems for use in disease diagnosis and therapy (e.g., cancer diagnosis and therapy). The compositions and systems generally comprise two or more separate components for targeting, imaging, sensing, and / or triggering release of a therapeutic or diagnostic material and monitoring the response to therapy of a cell or tissue (e.g., a tumor).
Owner:MICHIGAN UNIV OF RGT THE
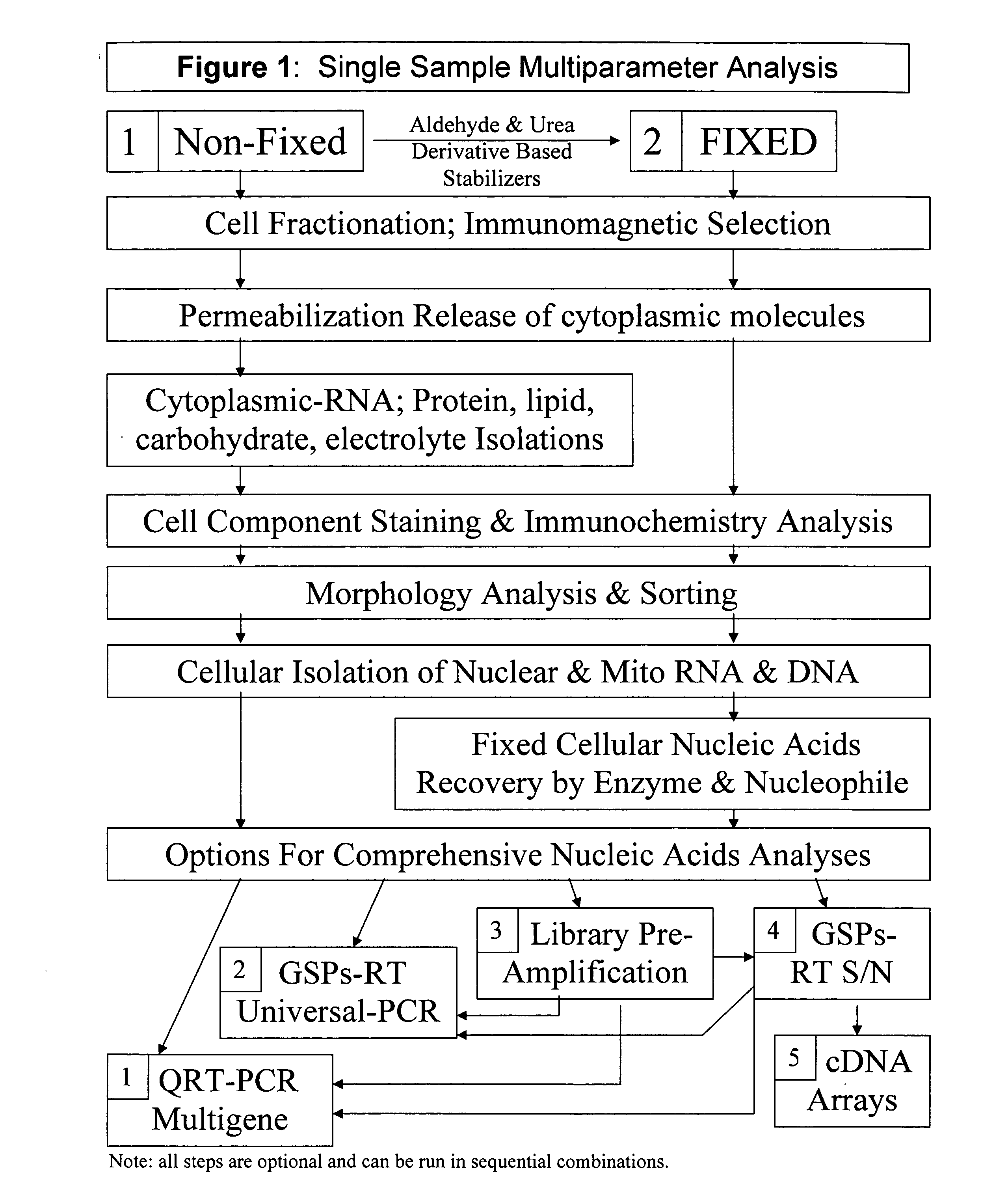
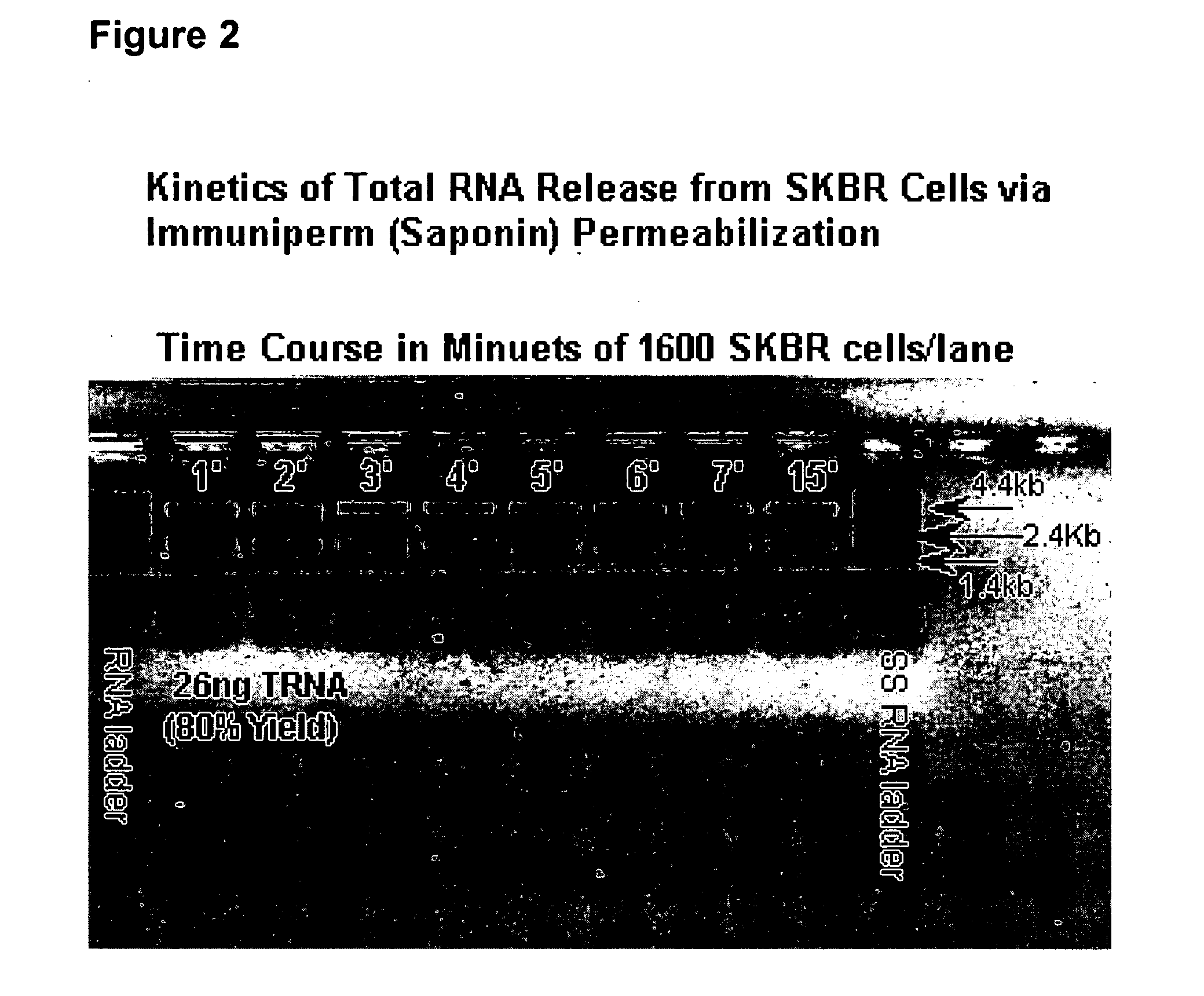
Multiparameter analysis of comprehensive nucleic acids and morphological features on the same sample
InactiveUS20060008807A1Microbiological testing/measurementMicroorganism lysisProtein profilingMass spectrometry
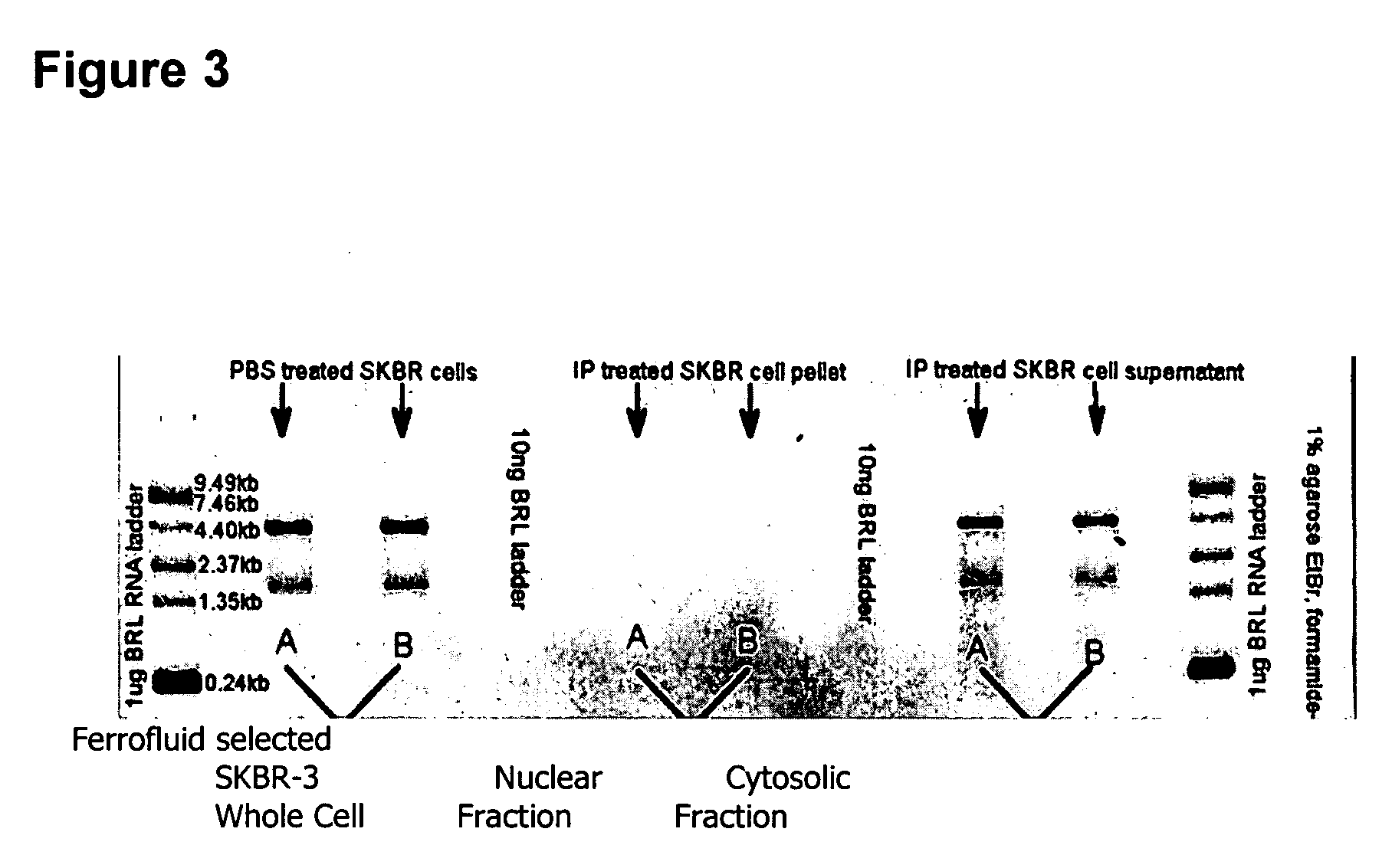
A highly sensitive assay is disclosed which utilizes a method for gene specific primed amplification of mRNA libraries from rare cells and rare transcripts found in blood. The assay allows detection of rare mRNA (10 copies / cell) found in 1 to 10 cells isolated through immunomagnetic enrichment. The assay is an improvement over multiplex PCR and allows efficient detection of rare coding sequences for circulating carcinoma cells in the blood. The methods are useful in profiling of cells isolated from tissues or body fluids and serves as an adjunct to clinical diagnosis of diverse carcinomas including early stage detection and classification of circulating tumor cells. Monitoring of nucleic acid and protein profiles of cells either in conventional or microarray formats, facilitates management of therapeutic intervention including staging, monitoring response to therapy, confirmation of remission and detection of regression.
Owner:VERIDEX LCC
Antibody profiling for determination of patient responsiveness
InactiveUS20080026485A1Increase probabilityConvenient careDisease diagnosisDiseaseAutoimmune disease
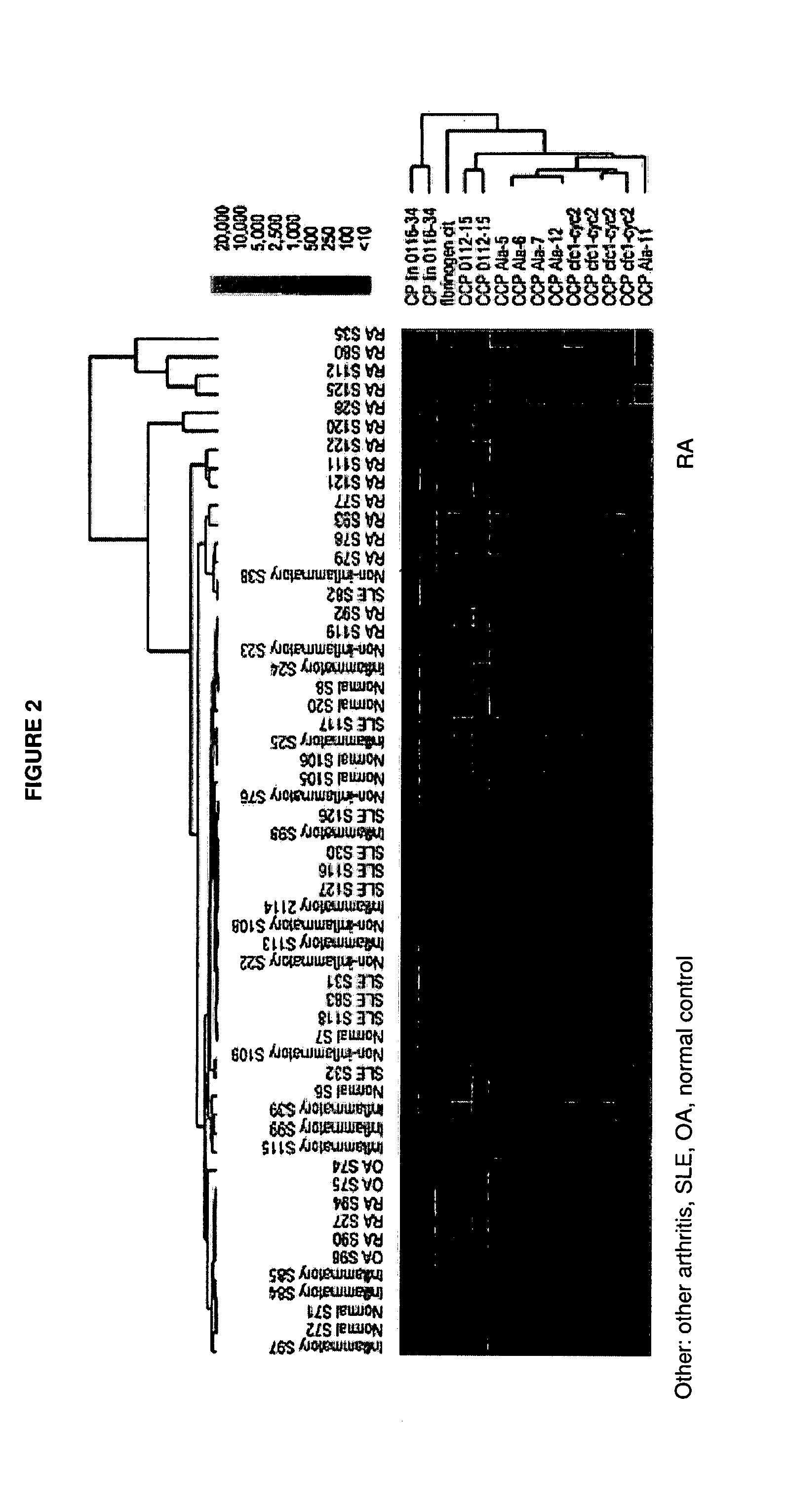
Compositions and methods are provided for prognostic classification of autoimmune disease patients into subtypes, which subtypes are informative of the patient's need for therapy and responsiveness to a therapy of interest. The patterns of circulating blood levels of serum autoantibodies and / or cytokines provides for a signature pattern that can identify patients likely to benefit from therapeutic intervention as well as discriminate patients that have a high probability of responsiveness to a therapy from those that have a low probability of responsiveness. Additionally, serum autoantibody and / or cytokine signature patterns can be utilized to monitor responses to therapy. Assessment of this signature pattern of autoantibodies and / or cytokines in a patient thus allows improved methods of care. In one embodiment of the invention, the autoimmune disease is rheumatoid arthritis.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
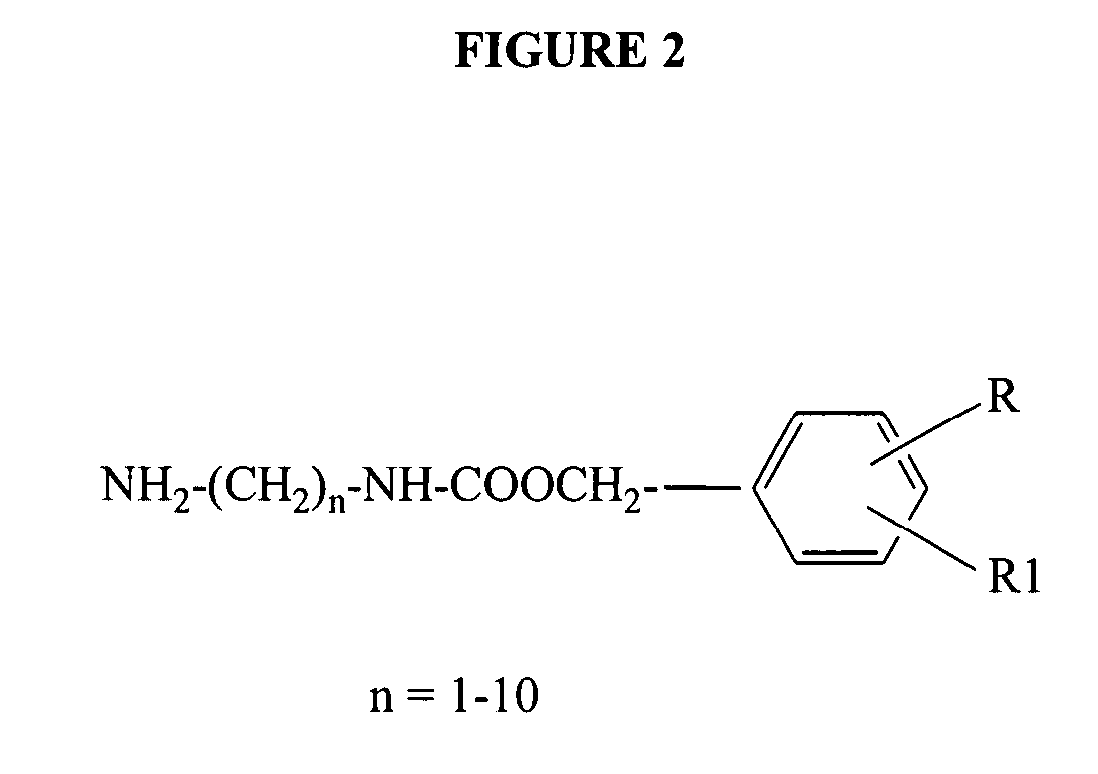
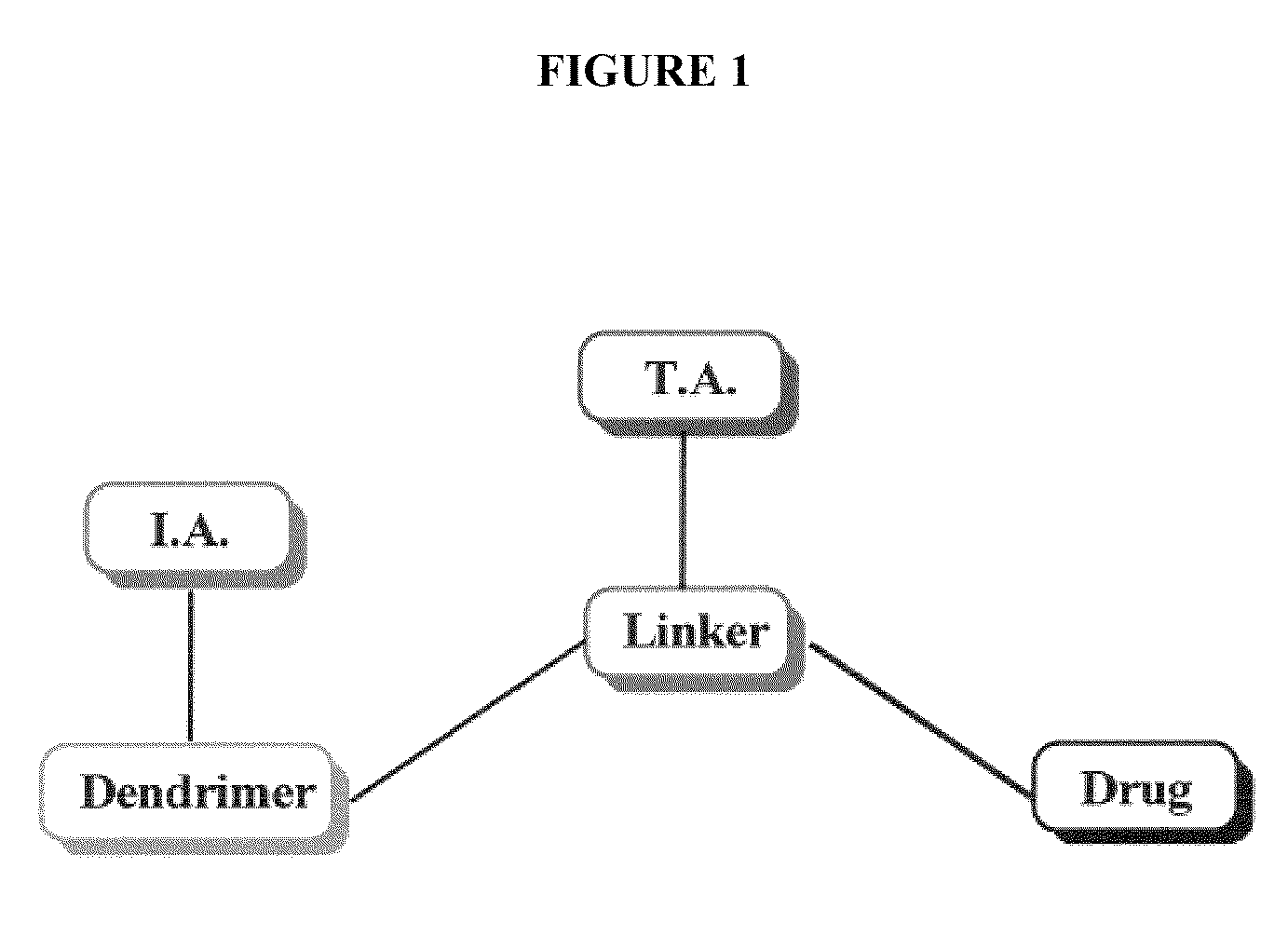
Dendrimer based compositions and methods of using the same
The present invention relates to novel therapeutic and diagnostic dendrimers. In particular, the present invention is directed to dendrimer based compositions and systems for use in disease diagnosis and therapy (e.g., cancer diagnosis and therapy). The compositions and systems comprise one or more components for targeting, imaging, sensing, and / or providing a therapeutic or diagnostic material and monitoring the response to therapy of a cell or tissue (e.g., a tumor).
Owner:RGT UNIV OF MICHIGAN
Exhaled Breath Condensate Collection and Assay System and Method
InactiveUS20080214947A1Easy to determineDetermining the predictive ability of these testsRespiratory organ evaluationSensorsTime courseAssay
A method and system that provides for minute-to-minute EBC pH monitoring (or for other EBC characteristic monitoring) of a subject that greatly assists in determining the time-course of airway pH changes (or other characteristic changes) in evolving disease processes, and may assist in determining the predictive ability of these tests, as well as determining response to therapy.
Owner:UNIV OF VIRGINIA
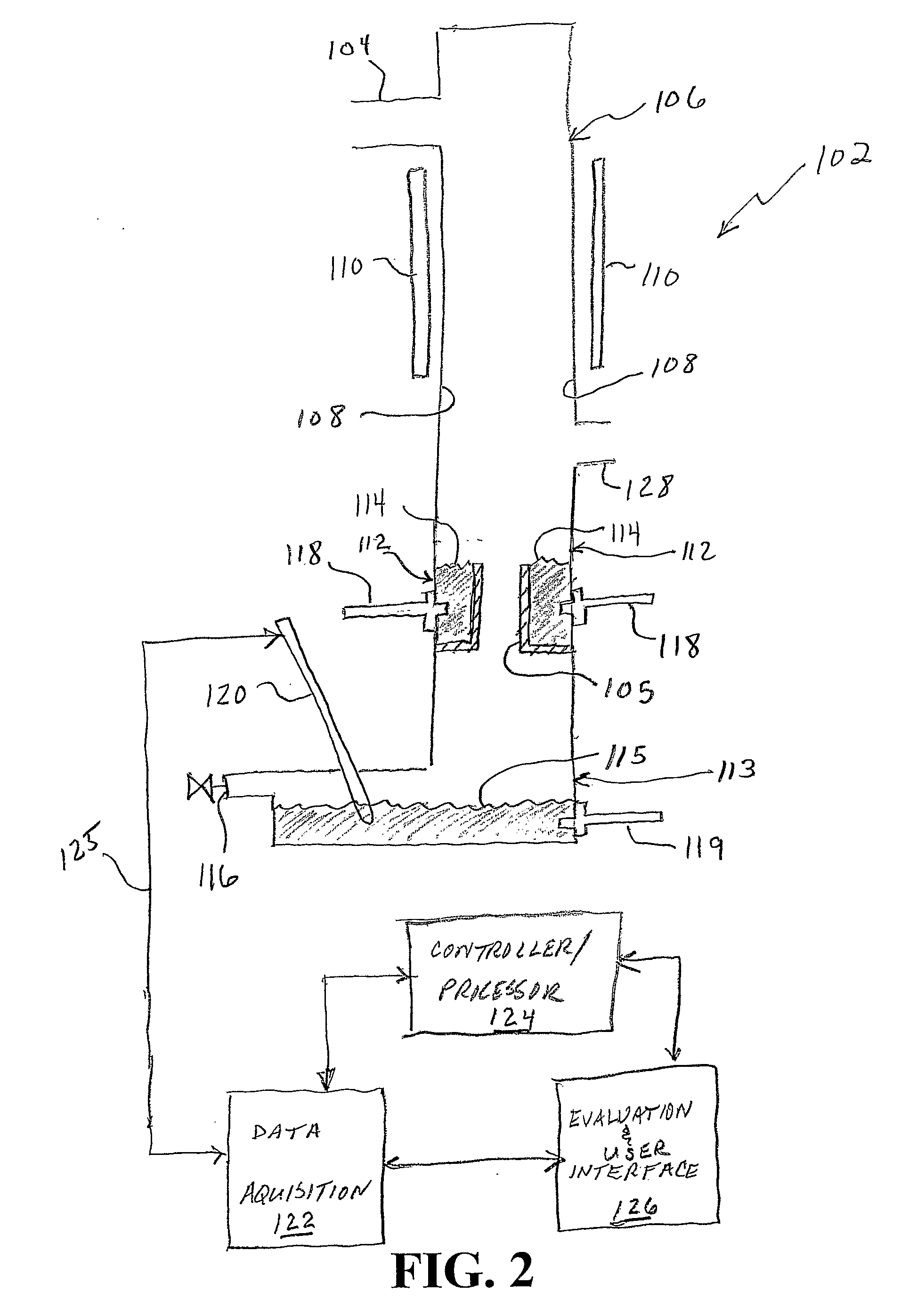
System and method for monitoring disease progression or response to therapy using multi-modal visualization
Owner:SIEMENS HEALTHCARE GMBH
Systems and methods for the physiological assessment of brain health and the remote quality control of eeg systems
InactiveUS20150038869A1Verify performanceElectroencephalographyAutomatic recalibrationPortable EEGData acquisition
A system for calibrating and / or verifying system performance of a remote portable EEG system having at least one EEG sensor. Embodiments of the invention can provide various reference signals to calibrate and quality control the remote performance of the data acquisition EEG system. In addition a calibration cable connects a reference signal source to the EEG sensors to enable remote calibration and quality control assessment. Further, a diagnostic biomarker is included to assess the state or function of a subject's brain and enables the classification, prognosis, diagnosis, monitoring of treatment, or response to therapy applied to the brain by measuring any one of a list of candidate features extracted from a given cognitive or sensory task, and measuring changes in the EEG feature and task combination over time, among multiple states, or compared to a normative database.
Owner:CERORA
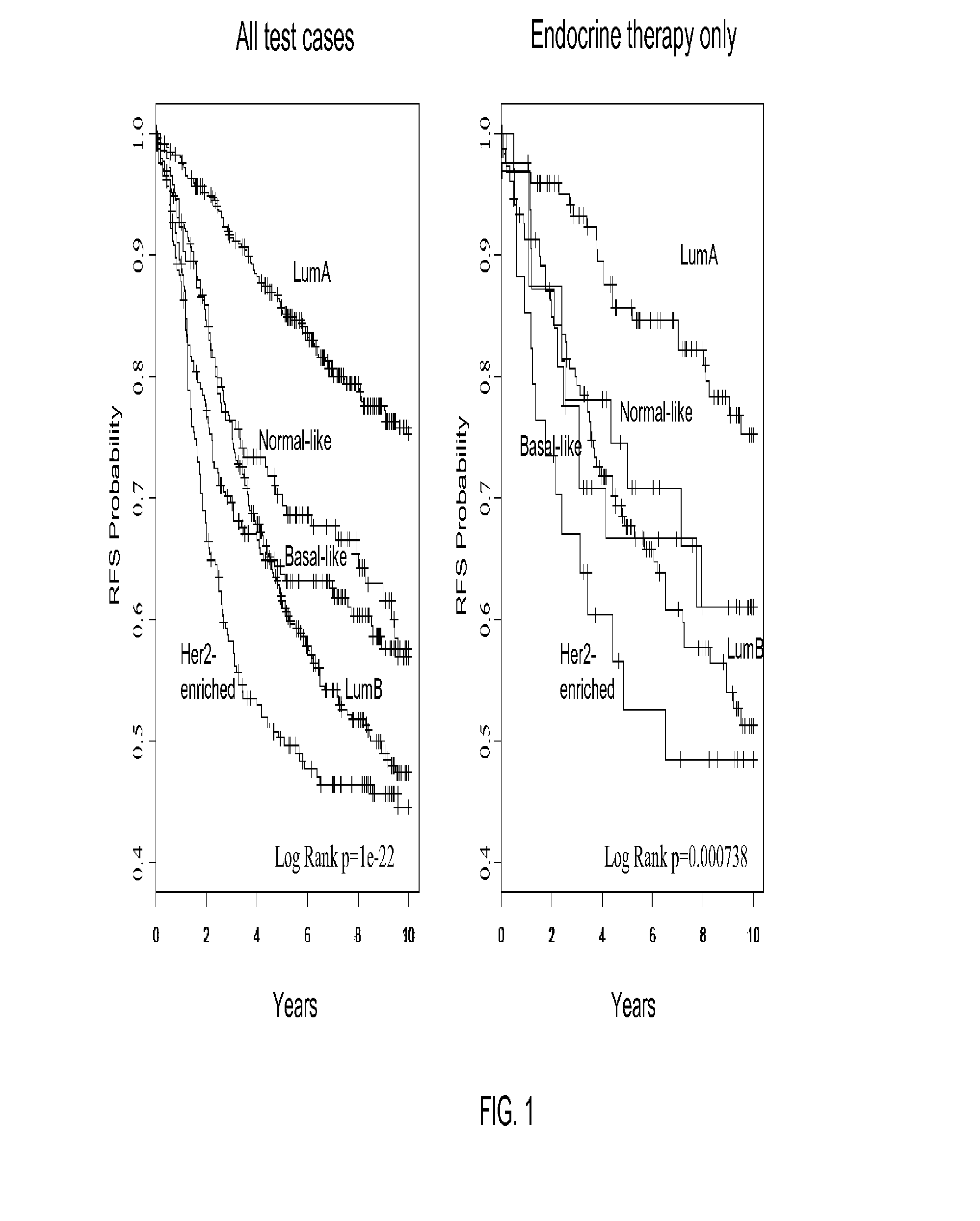
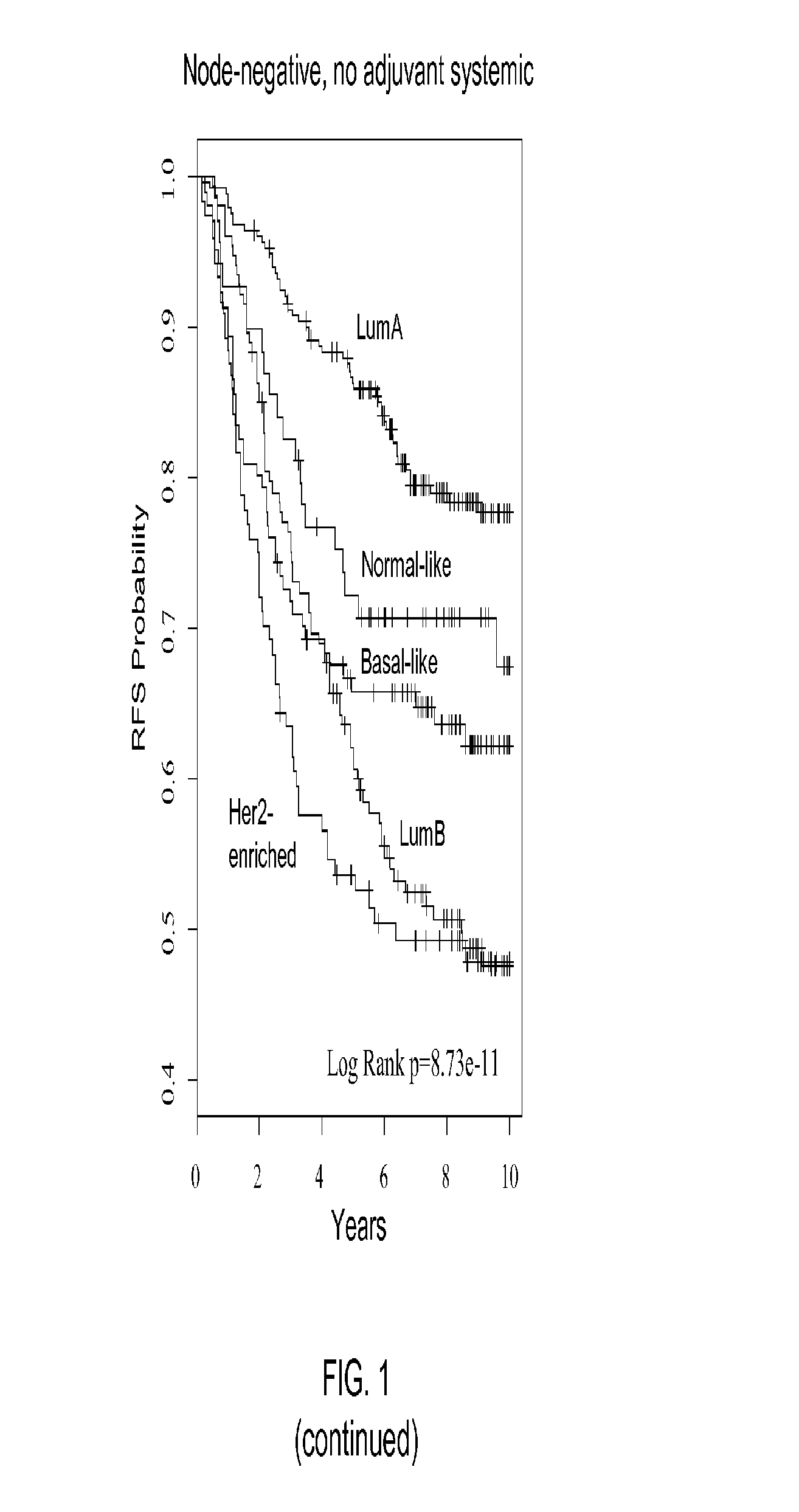
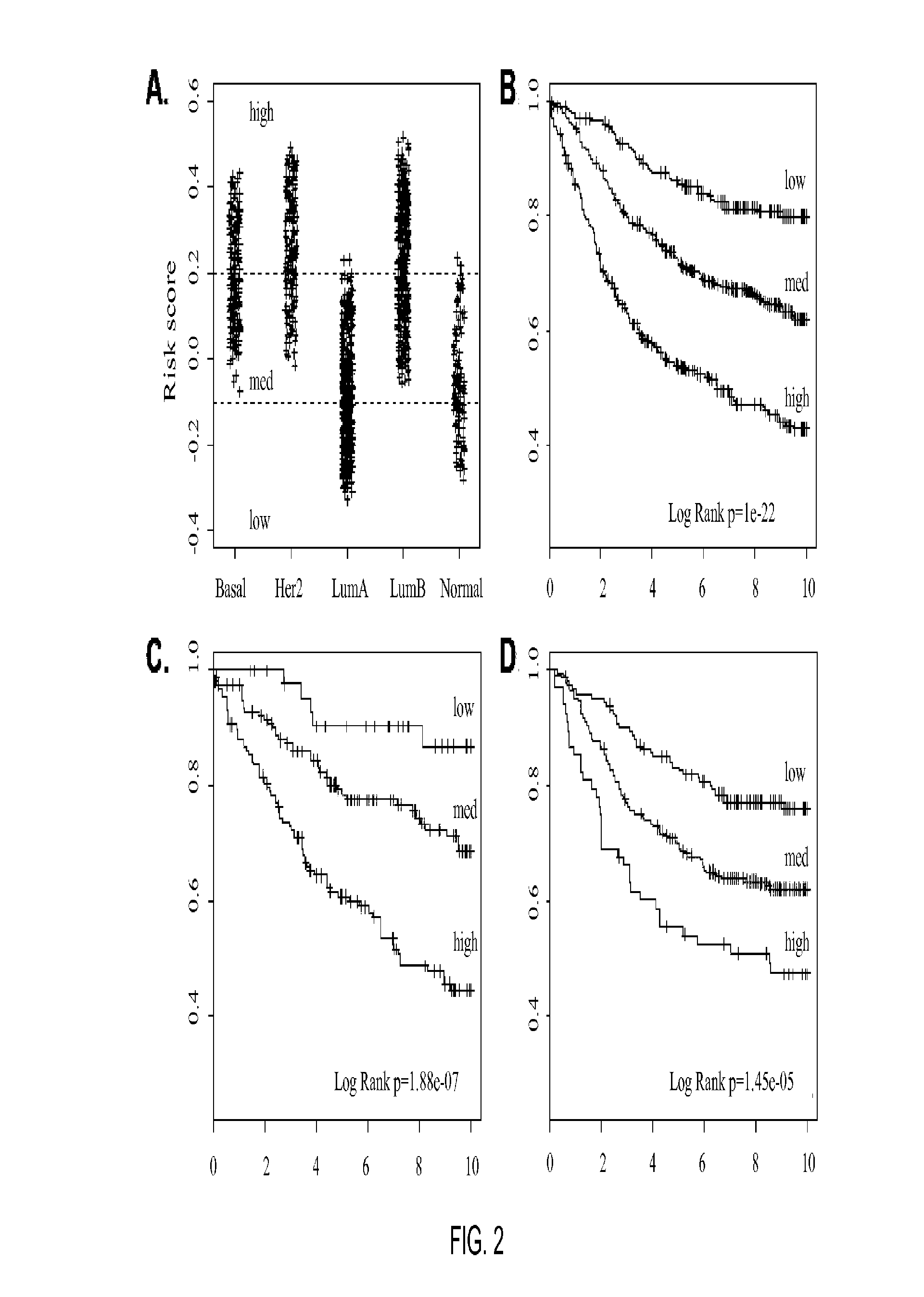
Gene expression profiles to predict breast cancer outcomes
ActiveUS20110145176A1Evaluating prognosisEvaluating treatmentMicrobiological testing/measurementDigital computer detailsTreatment optionsIntrinsics
Methods for classifying and for evaluating the prognosis of a subject having breast cancer are provided. The methods include prediction of breast cancer subtype using a supervised algorithm trained to stratify subjects on the basis of breast cancer intrinsic subtype. The prediction model is based on the gene expression profile of the intrinsic genes listed in Table 1. This prediction model can be used to accurately predict the intrinsic subtype of a subject diagnosed with or suspected of having breast cancer. Further provided are compositions and methods for predicting outcome or response to therapy of a subject diagnosed with or suspected of having breast cancer. These methods are useful for guiding or determining treatment options for a subject afflicted with breast cancer. Methods of the invention further include means for evaluating gene expression profiles, including microarrays and quantitative polymerase chain reaction assays, as well as kits comprising reagents for practicing the methods of the invention.
Owner:BRITISH COLUMBIA CANCER AGENCY BRANCH +3
Method for cancer detection, diagnosis and prognosis
ActiveUS20110053152A1Rapidly and efficiently captureImprove capture efficiencyBioreactor/fermenter combinationsBiological substance pretreatmentsTelomeraseParylene
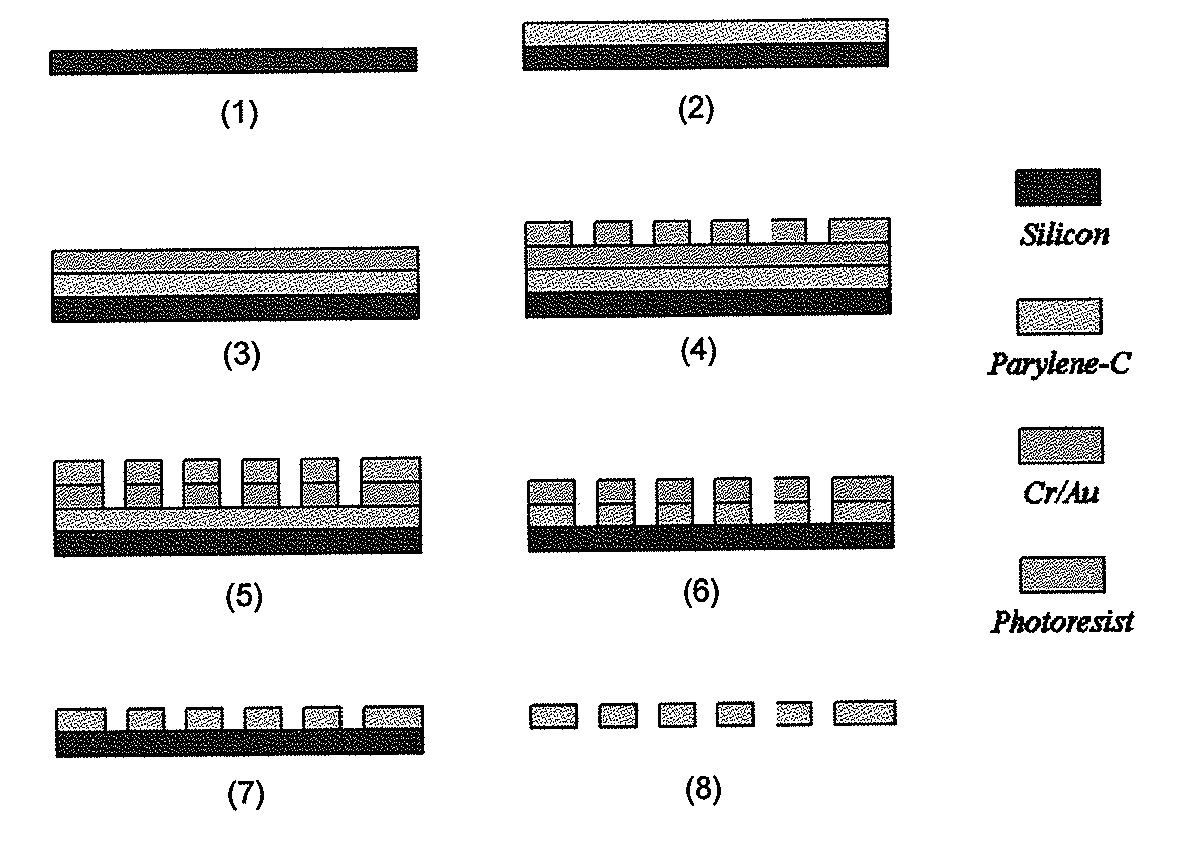
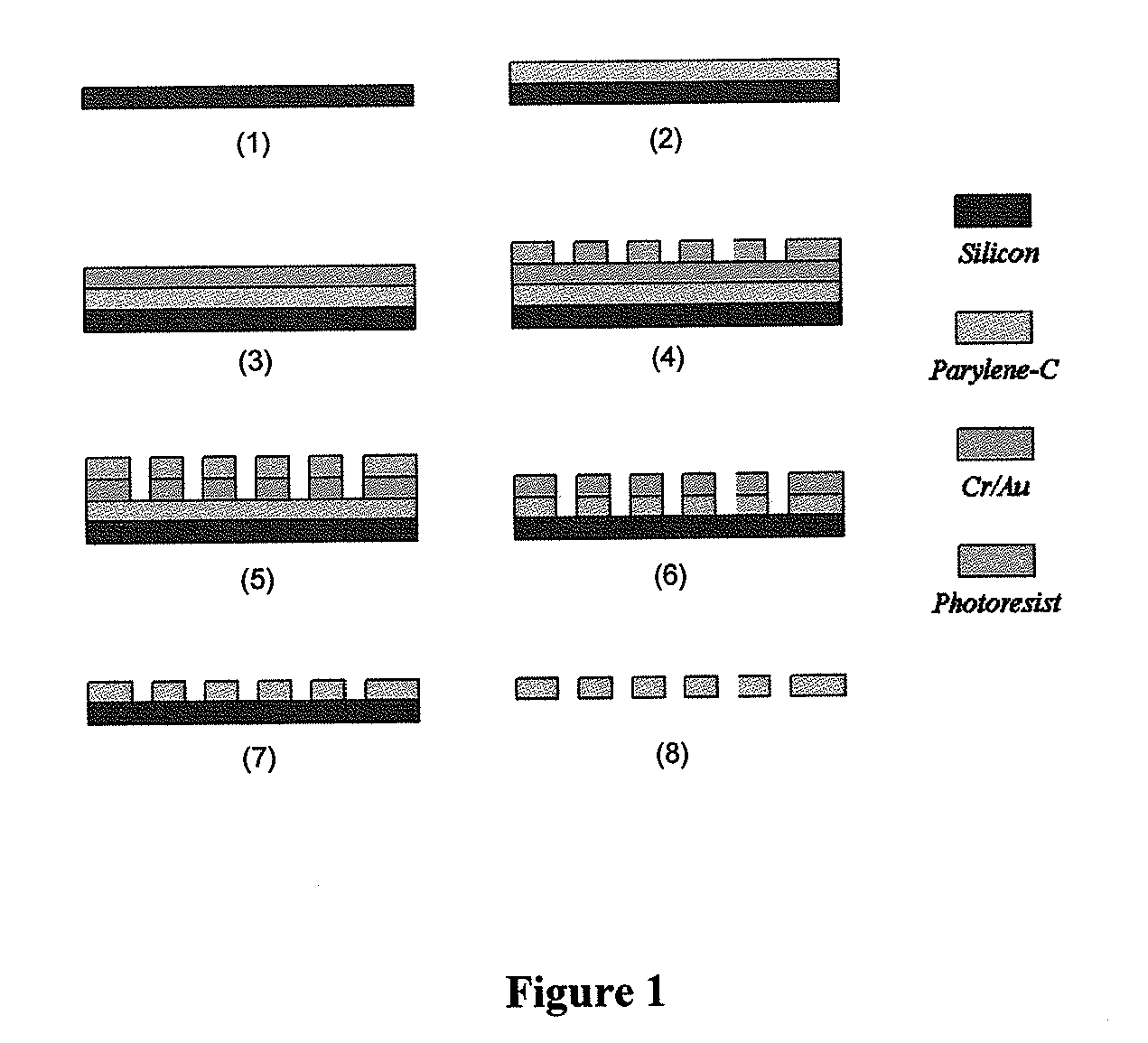
The present invention provides a method for diagnosing cancer, predicting a disease outcome or response to therapy in a patient sample. The method involves isolating a circulating tumor cell (CTC), for example, a viable CTC, from a sample using a parylene microfilter device comprising a membrane filter having or consisting of a parylene substrate, which has an array of holes with a predetermined shape and size; and detecting and quantifying telomerase activity in blood circulating tumor cells. The invention further provides methods of using cells live-captured in various applications.
Owner:UNIV OF SOUTHERN CALIFORNIA +1
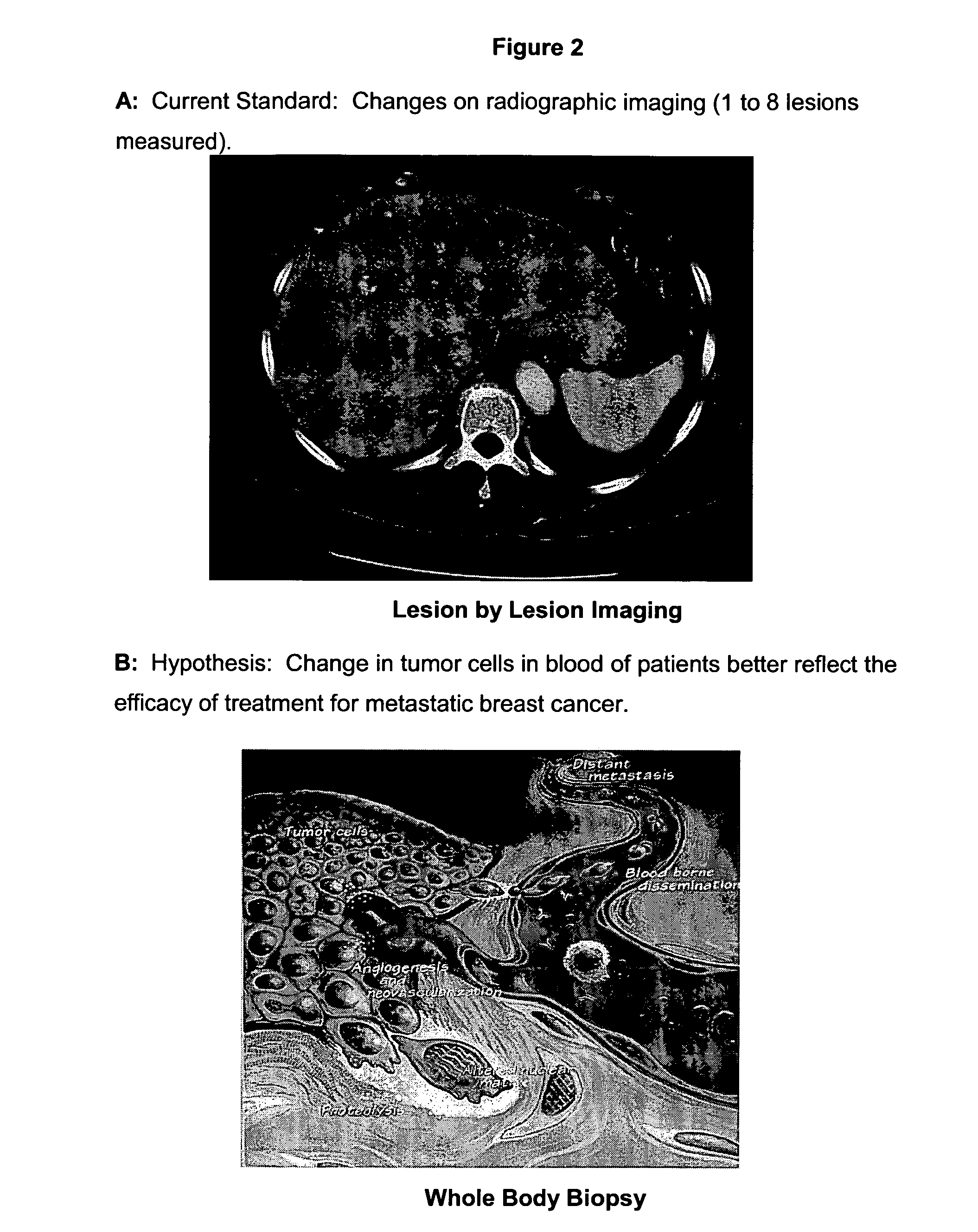
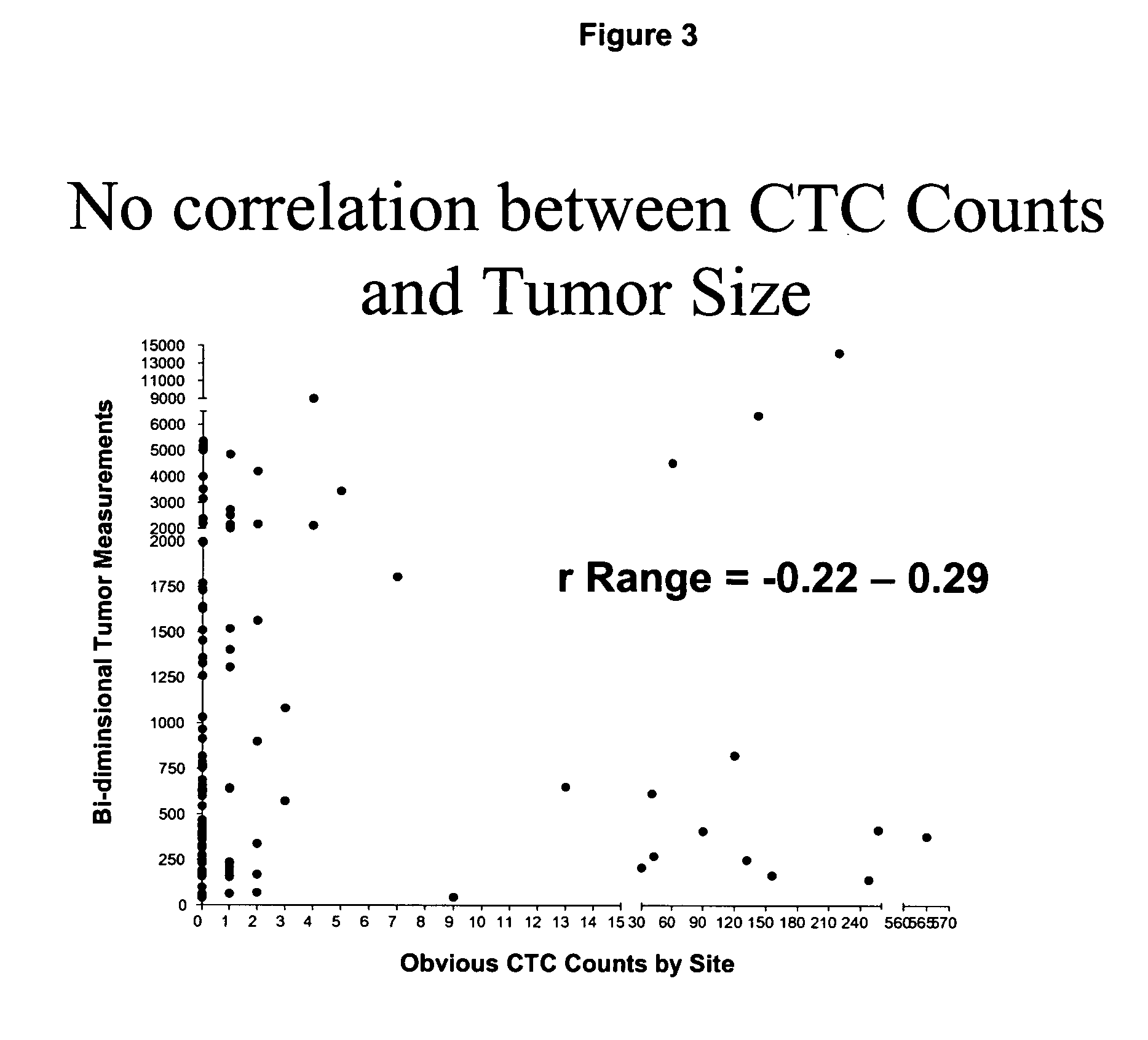
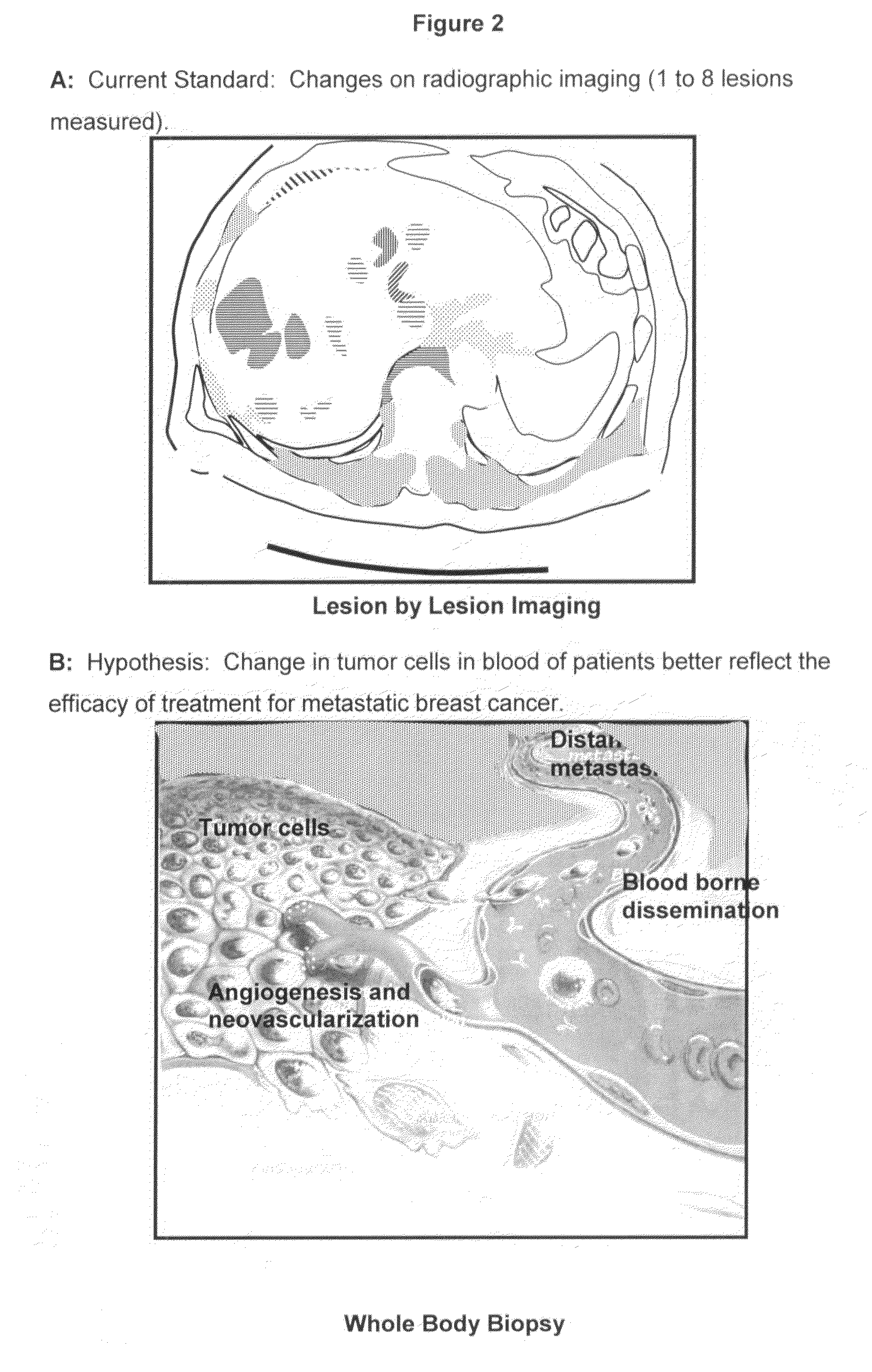
Circulating tumor cells (CTC's): early assessment of time to progression, survival and response to therapy in metastatic cancer patients
InactiveUS20070037173A1Less side effectsImprove the quality of lifeMicrobiological testing/measurementBiological testingClinical trialOncology
A cancer test having prognostic utility in predicting time to disease progression, overall survival, and response to therapy in patients with MBC based upon the presence and number of CTC's. The Cell Spotter® System is used to enumerate CTC's in blood. The system immunomagnetically concentrates epithelial cells, fluorescently labels the cells and identifies and quantifies CTC's. The absolute number of CTC's detected in the peripheral blood tumor load is, in part, a factor in prediction of survival, time to progression, and response to therapy. The mean time to survival of patients depended upon a threshold number of 5 CTC's per 7.5 ml of blood. Detection of CTC's in metastatic cancer represents a novel prognostic factor in patients with metastatic cancers, suggests a biological role for the presence of tumor cells in the blood, and indicates that the detection of CTC's could be considered an appropriate surrogate marker for prospective therapeutic clinical trials.
Owner:JANSSEN DIAGNOSTICS LLC
Dendrimer based compositions and methods of using the same
The present invention relates to novel therapeutic and diagnostic dendrimers. In particular, the present invention is directed to dendrimer based compositions and systems for use in disease diagnosis and therapy (e.g., cancer diagnosis and therapy). The compositions and systems comprise one or more components for targeting, imaging, sensing, and / or providing a therapeutic or diagnostic material and monitoring the response to therapy of a cell or tissue (e.g., a tumor).
Owner:RGT UNIV OF MICHIGAN
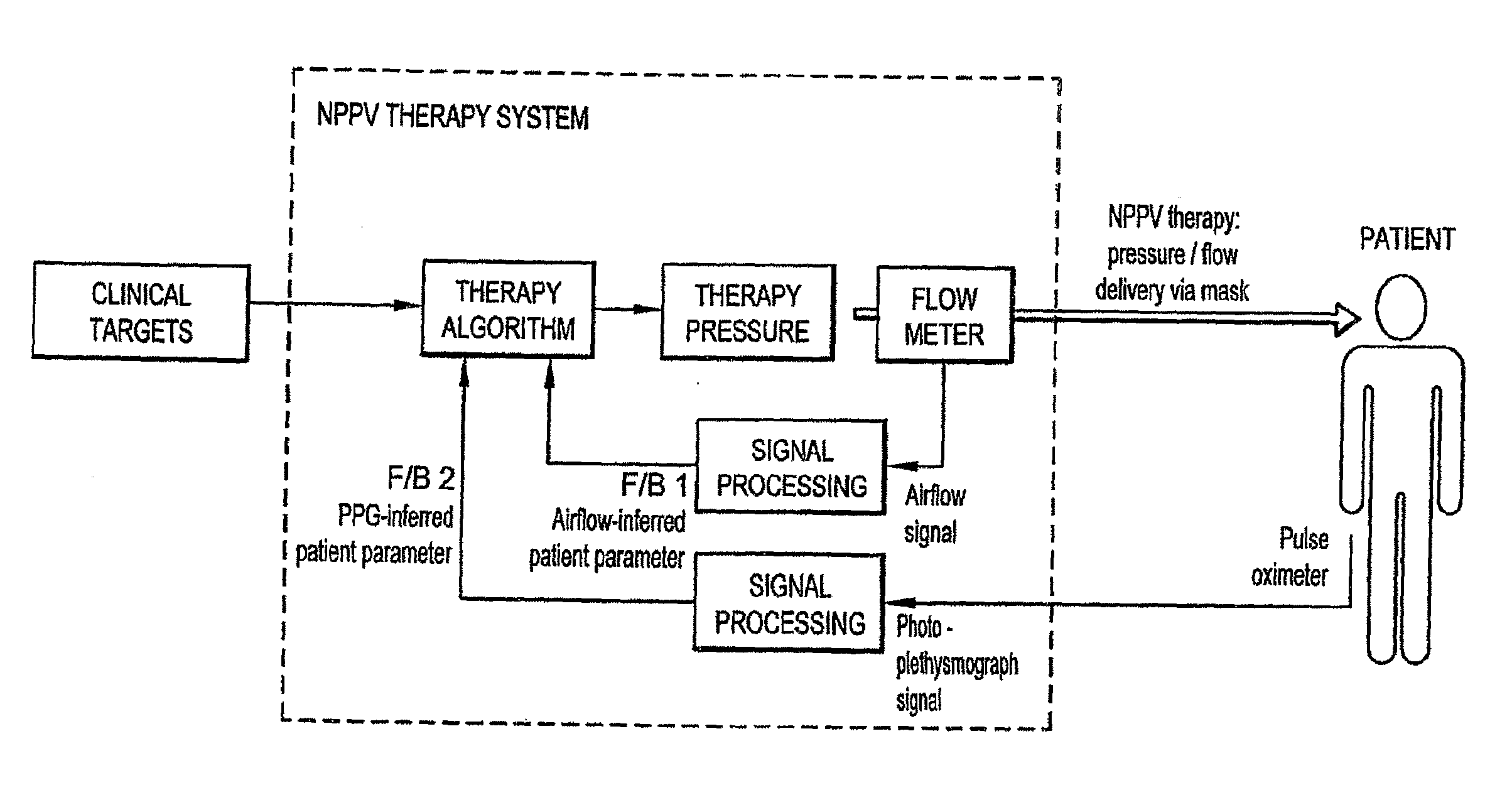
Systems, Methods, and/or Apparatuses for Non-Invasive Monitoring of Respiratory Parameters in Sleep Disordered Breathing
ActiveUS20100016694A1Eliminate side effectsIncrease pressureRespiratorsOperating means/releasing devices for valvesPulse oximetersSleep disordered breathing
In certain example embodiments, an air delivery system includes a controllable flow generator operable to generate a supply of pressurized breathable gas to be provided to a patient for treatment and a pulse oximeter. In certain example embodiments, the pulse oximeter is configured to determine, for example, a measure of patient effort during a treatment period and provide a patient effort signal for input to control operation of the flow generator. Oximeter plethysmogram data may be used, for example, to determine estimated breath phase; sleep structure information; autonomic improvement in response to therapy; information relating to relative breathing effort, breathing frequency, and / or breathing phase; vasoconstrictive response, etc. Such data may be useful in diagnostic systems.
Owner:RESMED LTD
Multifunctional nanodevice platform
InactiveUS20020165179A1Reduce the amount requiredEnhanced signalAntibacterial agentsHeavy metal active ingredientsDendrimerDisease
The present invention relates to novel therapeutic and diagnostic arrays. More particularly, the present invention is directed to dendrimer based multifunctional compositions and systems for use in disease diagnosis and therapy (e.g., cancer diagnosis and therapy). The compositions and systems generally comprise two or more separate components for targeting, imaging, sensing, and / or triggering release of a therapeutic or diagnostic material and monitoring the response to therapy of a cell or tissue (e.g., a tumor).
Owner:RGT UNIV OF MICHIGAN
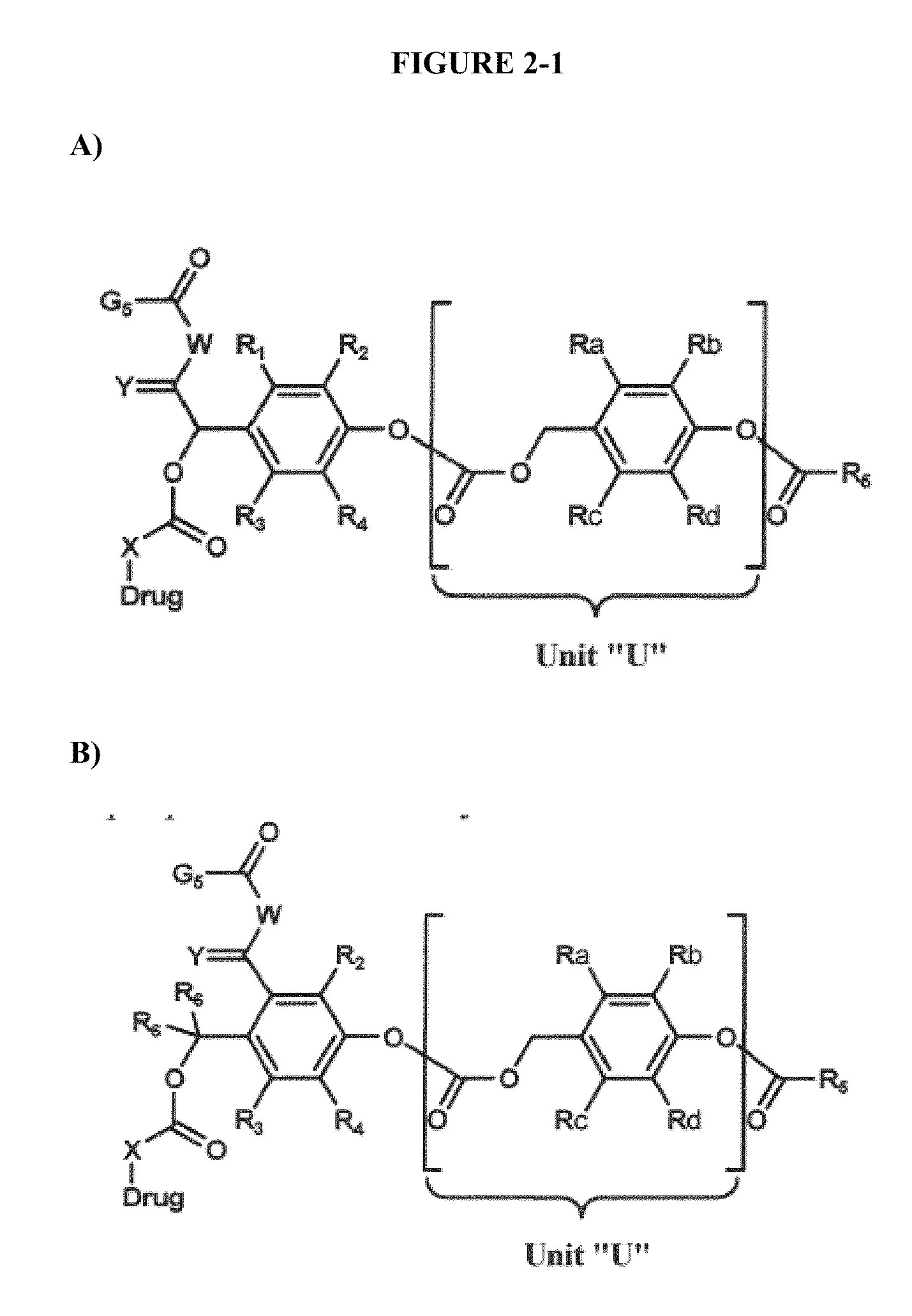
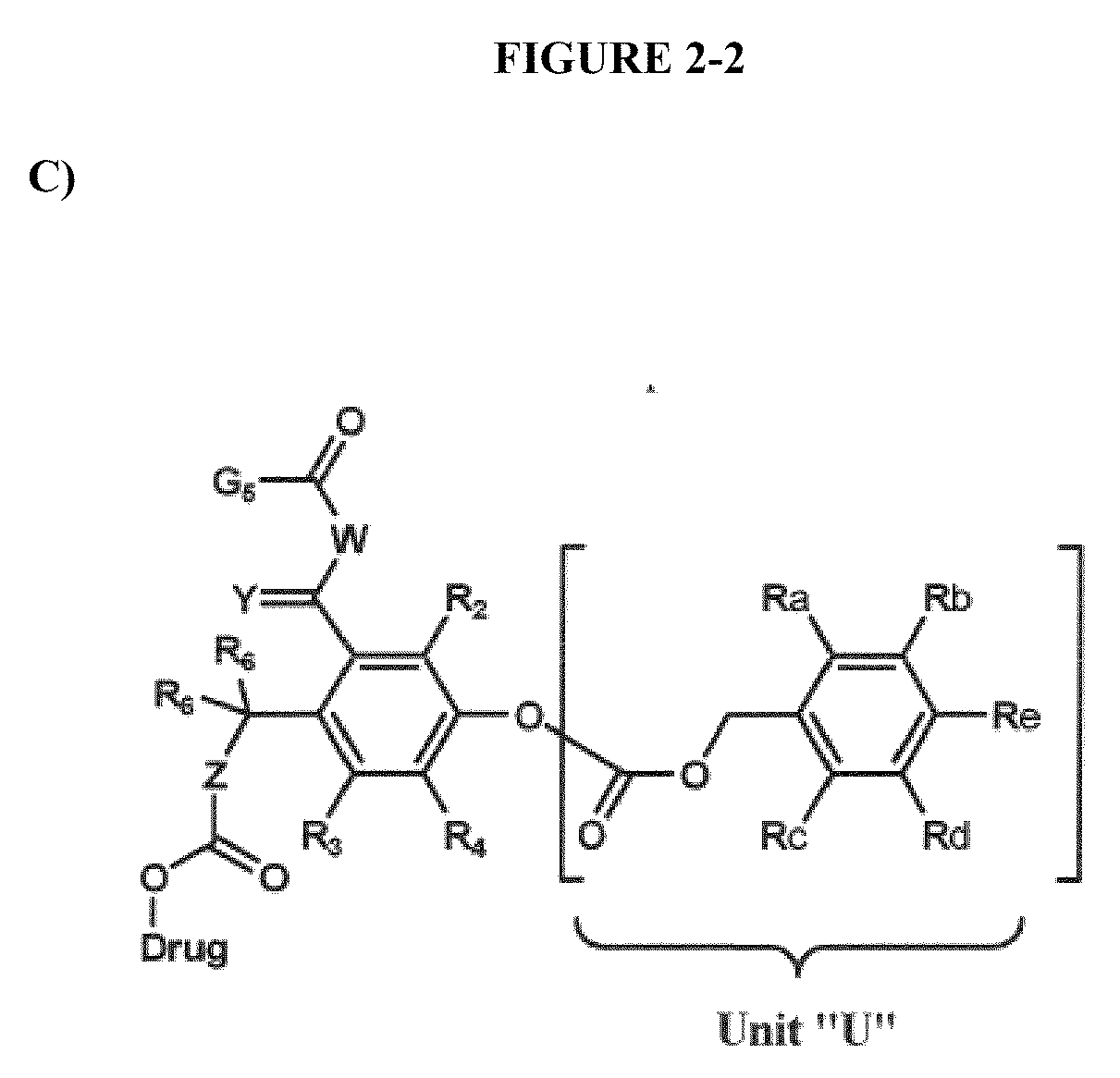
Dendrimer conjugates
InactiveUS20100160299A1Prevent adverse side effectsPreventing respiratory depressionBiocideOrganic chemistryDiseaseDendrimer
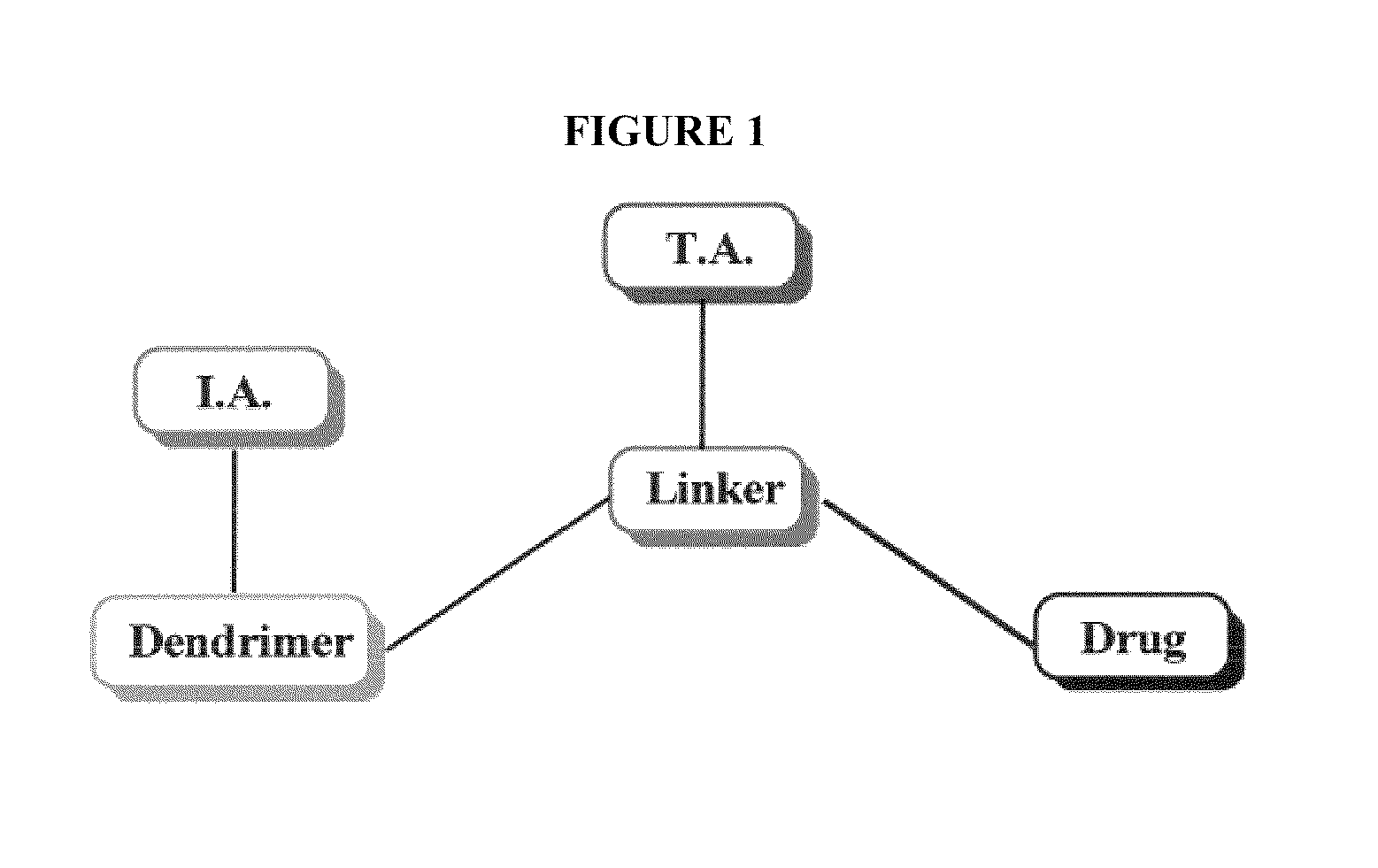
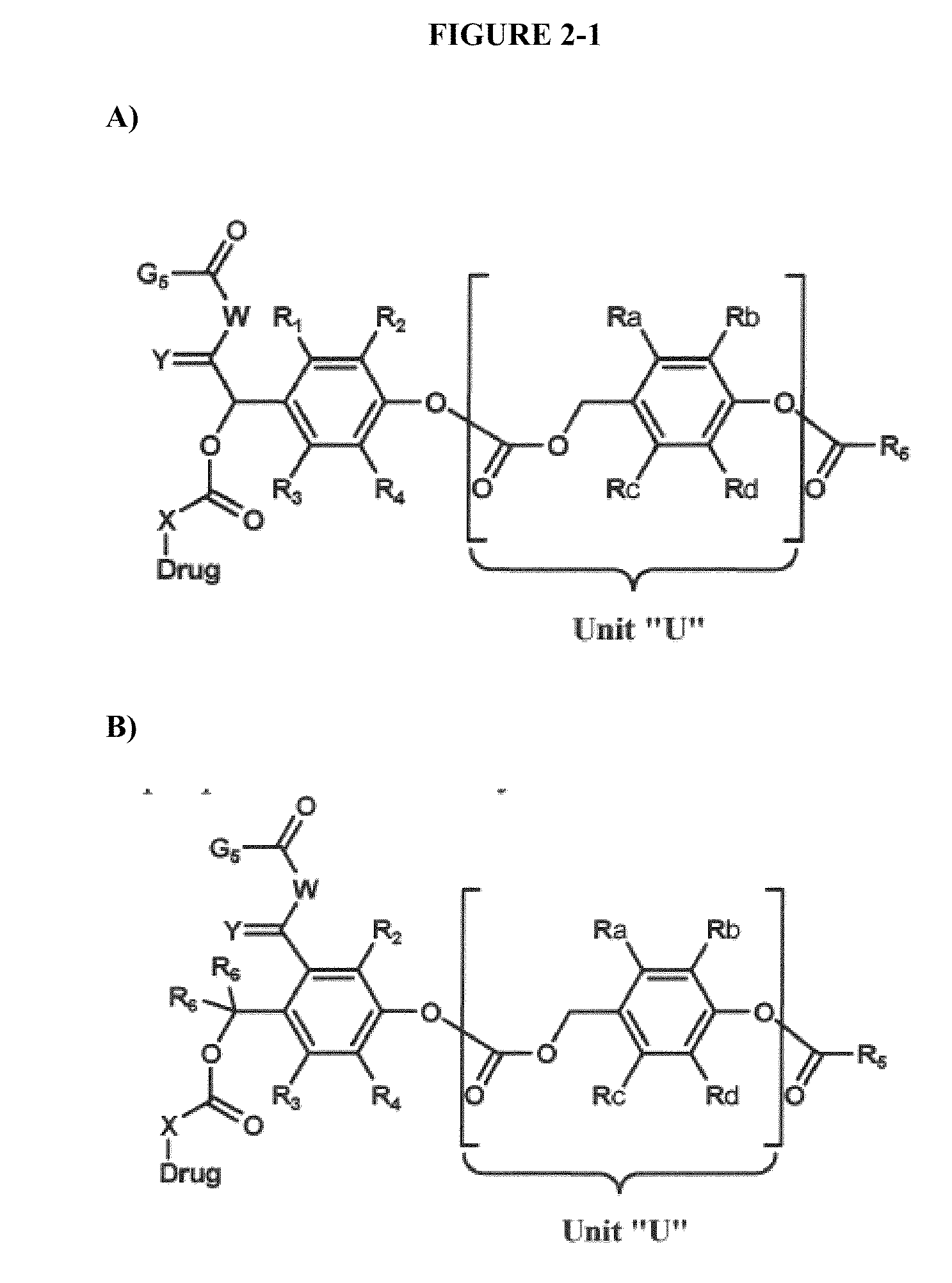
The present invention relates to novel therapeutic and diagnostic dendrimers. In particular, the present invention is directed to dendrimer-linker conjugates, methods of synthesizing the same, compositions comprising the conjugates, as well as systems and methods utilizing the conjugates (e.g., in diagnostic and / or therapeutic settings (e.g., for the delivery of therapeutics, imaging, and / or targeting agents (e.g., in disease (e.g., cancer) diagnosis and / or therapy, pain therapy, etc.)). Accordingly, dendrimer-linker conjugates of the present invention may further comprise one or more components for targeting, imaging, sensing, and / or providing a therapeutic or diagnostic material and / or monitoring response to therapy.
Owner:RGT UNIV OF MICHIGAN
Tape stripping methods for analysis of skin disease and pathological skin state
ActiveUS7183057B2Less traumaticEfficient methodBioreactor/fermenter combinationsBiological substance pretreatmentsCuticleAdhesive
The present invention provides non-invasive methods for detecting, monitoring, and diagnosing skin disease and pathological skin states such as irritated skin and psoriasis. The methods include using tape stripping to analyze expression in epidermal samples, of one or more skin markers. In illustrative examples, the tape stripping is performed using pliable tape that has a rubber adhesive. Furthermore, the present invention provides methods for predicting and monitoring response to therapy for a skin disease, such as psoriasis or dermatitis. Finally, the methods can include the use of a microarray.
Owner:DERMTECH INT
SERUM MARKERS PREDICTING CLINICAL RESPONSE TO ANTI-TNFa ANTIBODIES IN PATIENTS WITH ANKYLOSING SPONDYLITIS
InactiveUS20110251099A1Sustained responseBioreactor/fermenter combinationsBiological substance pretreatmentsSerum markersAnkylosing spondylitis
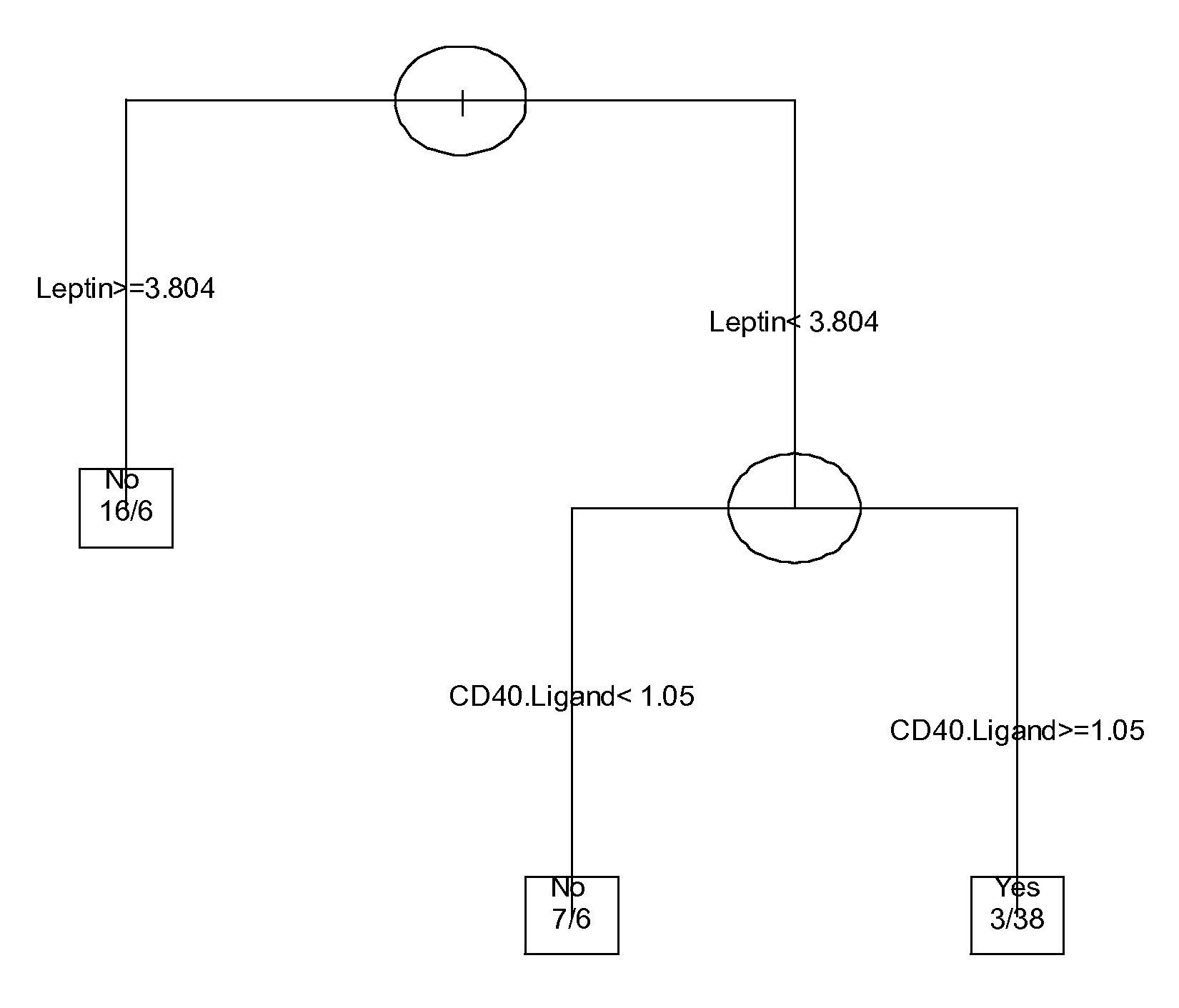
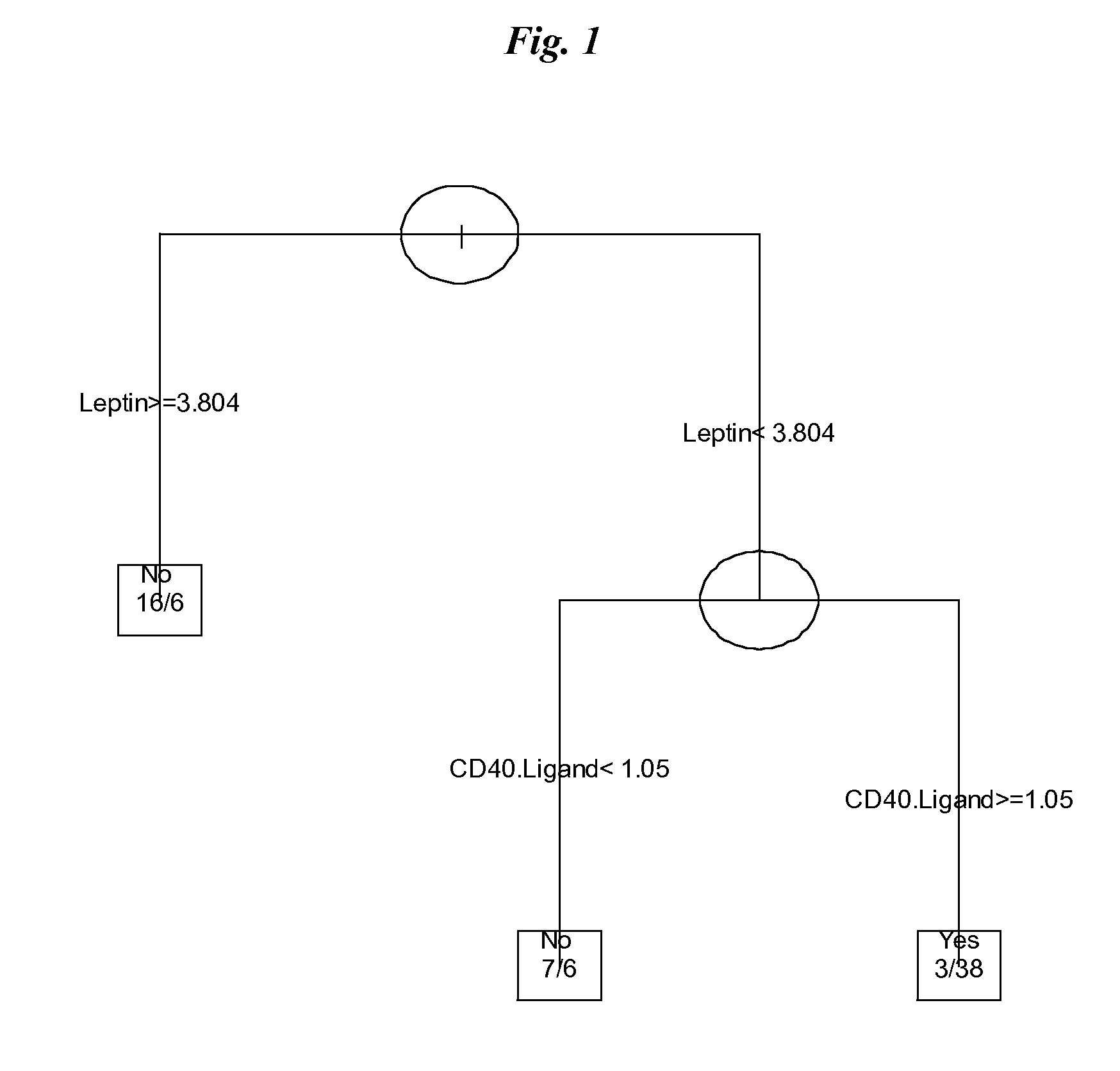
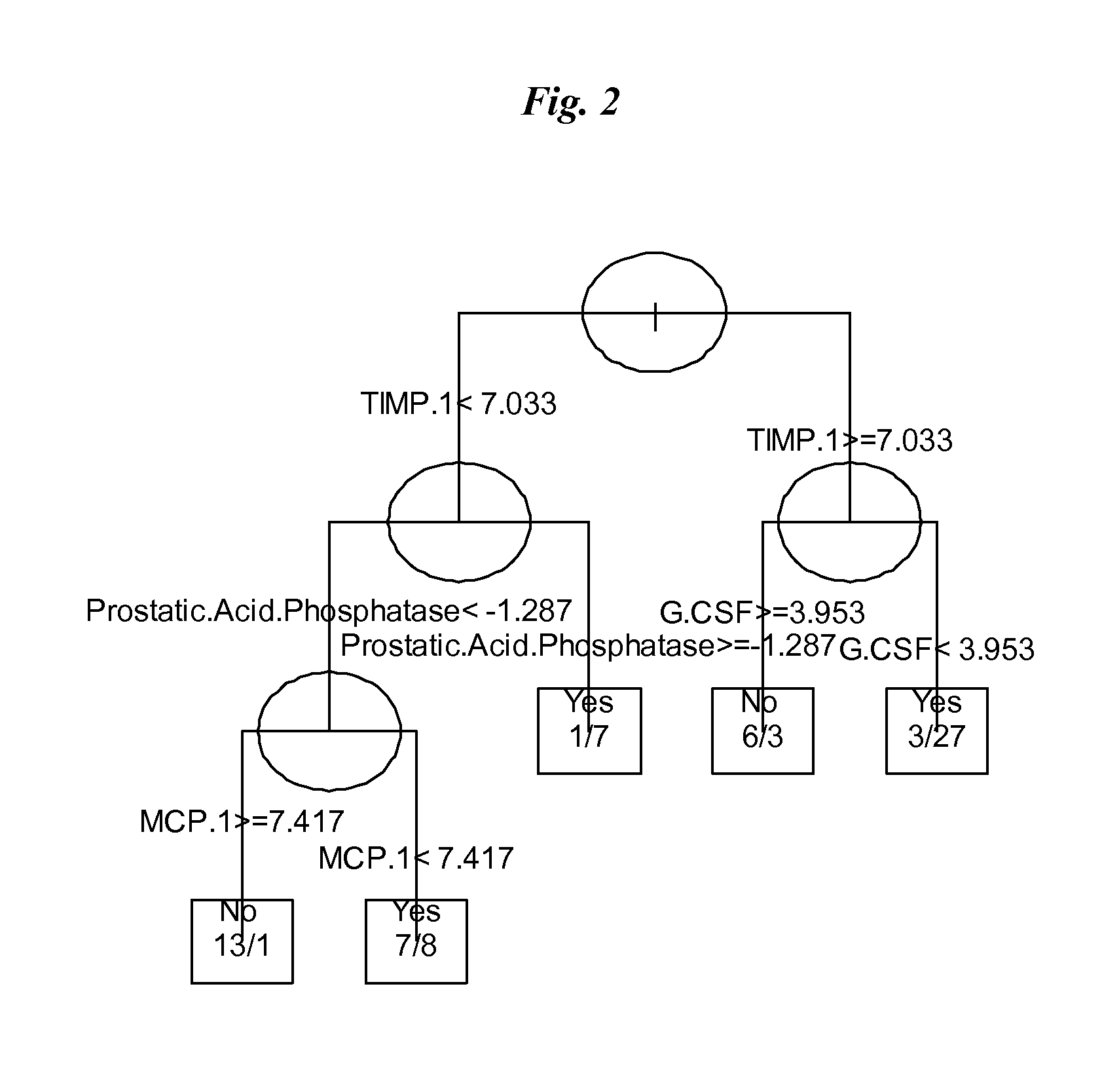
The invention provides tools for management of patients diagnosed with ankylosing spondylitis and prior to the initiation of therapy with an anti-TNFalpha agent. The tools are specific markers and algorithms of predicting response to therapy based on standard clinical primary and secondary end-points using serum marker concentrations. In one embodiment the baseline level of leptin or osteocalcin is used to predict the response at Week 14 after the initiation of therapy. In another embodiment, the change in a serum protein biomarker after 4 weeks of therapy is used such as complement component 3.
Owner:JANSSEN BIOTECH INC
Tape stripping methods for analysis of skin disease and pathological skin state
ActiveUS20050221334A1Less traumaticEfficient methodBioreactor/fermenter combinationsBiological substance pretreatmentsAdhesiveNon invasive
The present invention provides non-invasive methods for detecting, monitoring, and diagnosing skin disease and pathological skin states such as irritated skin and psoriasis. The methods include using tape stripping to analyze expression in epidermal samples, of one or more skin markers. In illustrative examples, the tape stripping is performed using pliable tape that has a rubber adhesive. Furthermore, the present invention provides methods for predicting and monitoring response to therapy for a skin disease, such as psoriasis or dermatitis. Finally, the methods can include the use of a micro array.
Owner:DERMTECH INT
Operator independent programmable sample preparation and analysis system
ActiveUS20070212698A1Minimal operator interactionImprove processing efficiencyBioreactor/fermenter combinationsBiological substance pretreatmentsRegimenHandling system
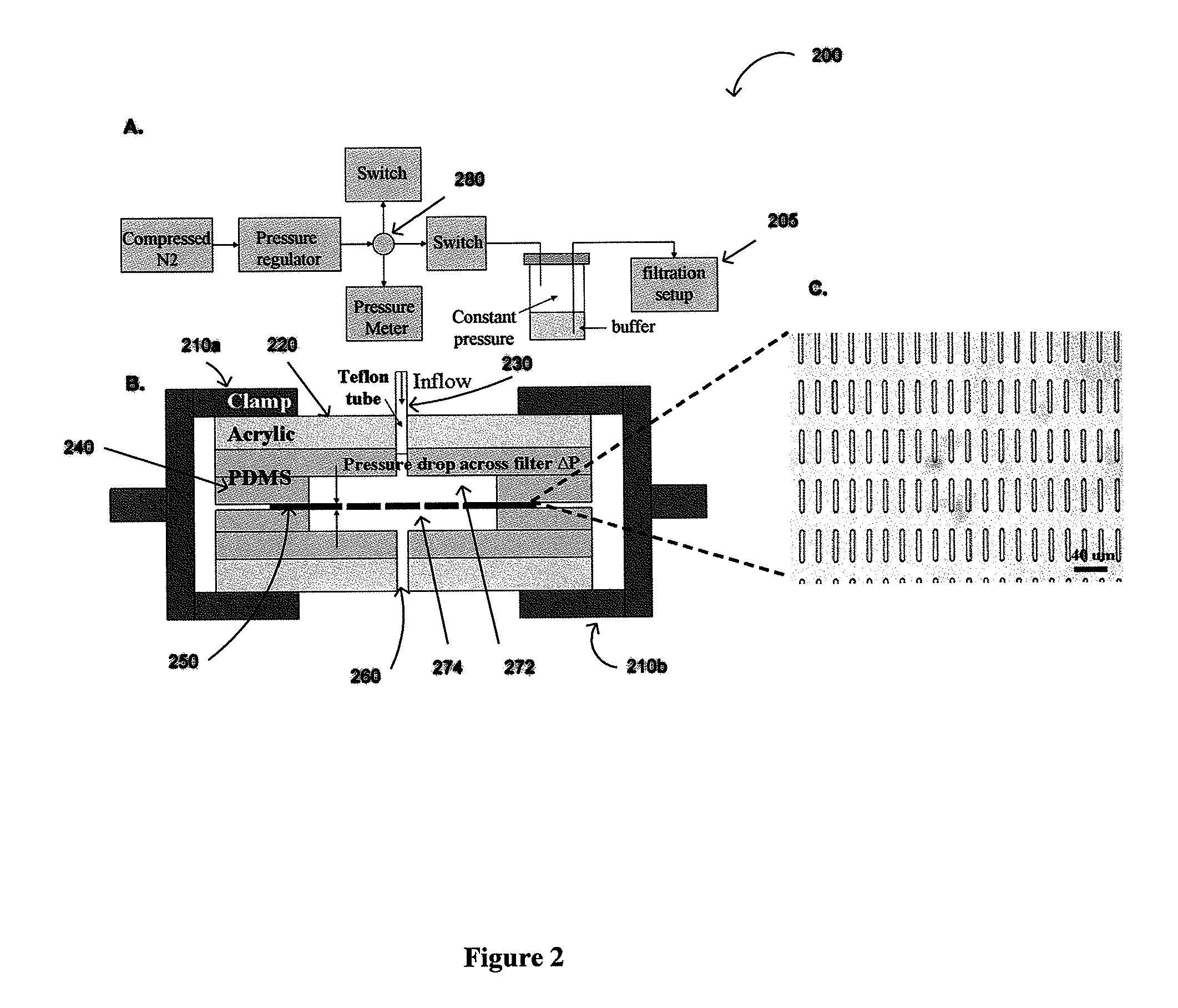
The present invention provides a protocol and apparatus for enriching circulating tumor cells and other rare cells from blood, including debris and other components, from samples with high precision and at high throughput rates. This invention discloses an improved processing system from previously described semi-automated sample processing. The system further reduces operator intervention and hands-on time from prior systems. While this system has general utility in processing diverse materials, the system is configured for sample processing of biological specimens to provide an enriched fraction suitable for detection, enumeration and identification of target cells by appropriate analytical methodologies. The presence and quantities of such target cells in a sample specimen can utilized for screening and detection in disease such as cancer, assessing early stage pre-metastatic cancer, monitoring for disease remission in response to therapy and selection of more effective dose regimens or alternative therapies in case of relapse.
Owner:MENARINI SILICON BIOSYSTEMS SPA
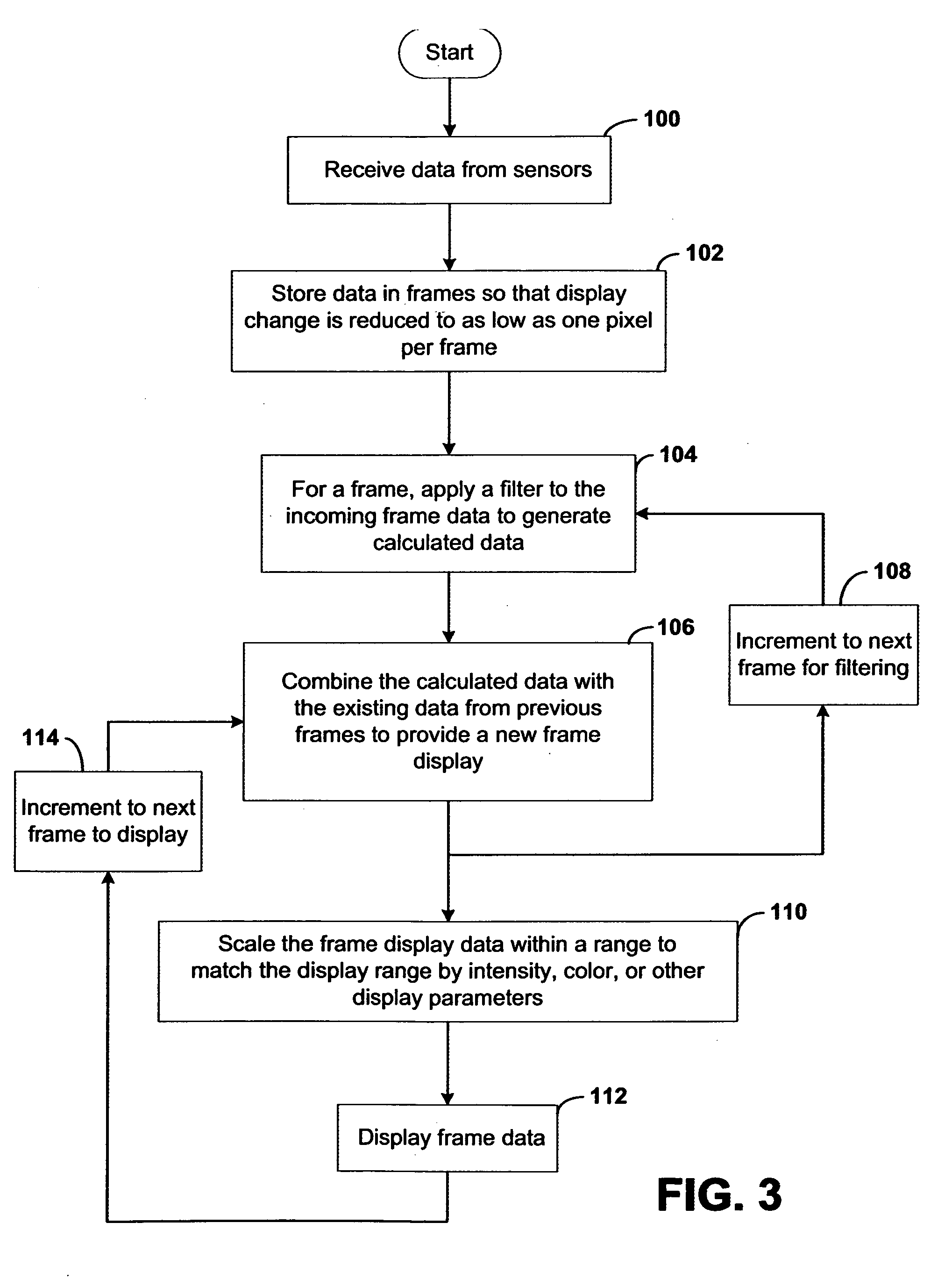
Systems and methods for displaying changes in biological responses to therapy
InactiveUS20070091091A1Better convey information to the viewerImprove visibilityDrawing from basic elementsPerson identificationDisplay deviceTherapeutic treatment
Systems and methods of this invention display data using pixels with information indicated by color and intensity changes, particularly used for monitoring of physiological variables in real time. For certain methods, physiological data can be acquired by sensors, acquired data can be stored in data frames, data frames can be processed using computer-implemented methods, and processed data frames can be scaled to a display frame for display on a display device. Using such methods, a spot made up of a group of pixels can be updated during a time frame, or cycle using a computer-implemented method, such as addition, subtraction, multiplication, division or a time dependent function. Newly received data can be combined with prior received data to indicate time-dependent changes. In this way, each spot contains a cumulative history of data starting at some initial time. In other embodiments, visual contrast can be enhanced between desired data and other data. In further embodiments, two or more different types of data can be plotted together to indicate relationships between variables. Real time monitoring of signals during therapeutic treatment using light, electricity or other nerve or muscle stimuli can allow a user to monitor physiological responses during stimulation and to make rapid decisions about medical treatment.
Owner:PHOTOMED TECH
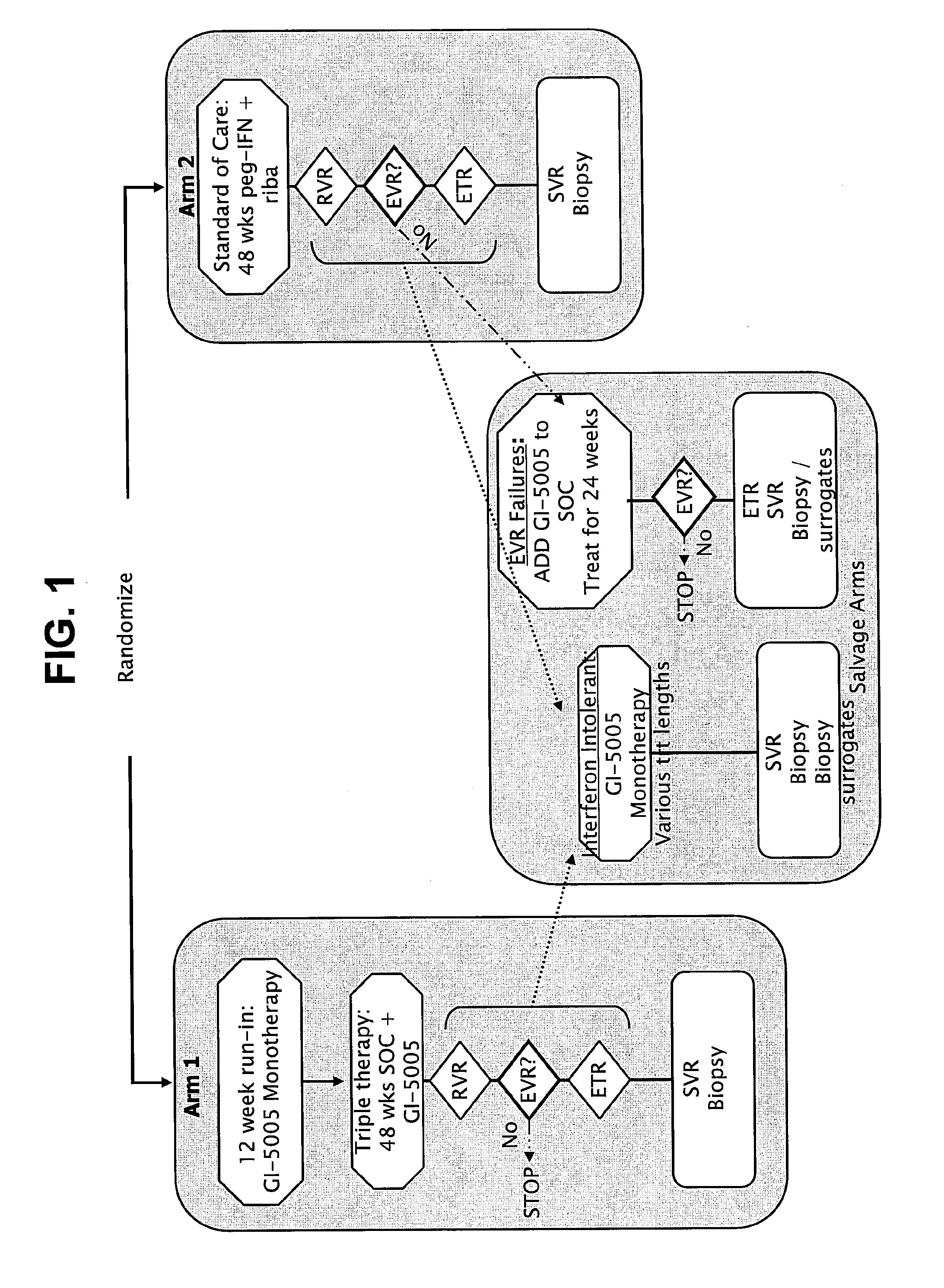
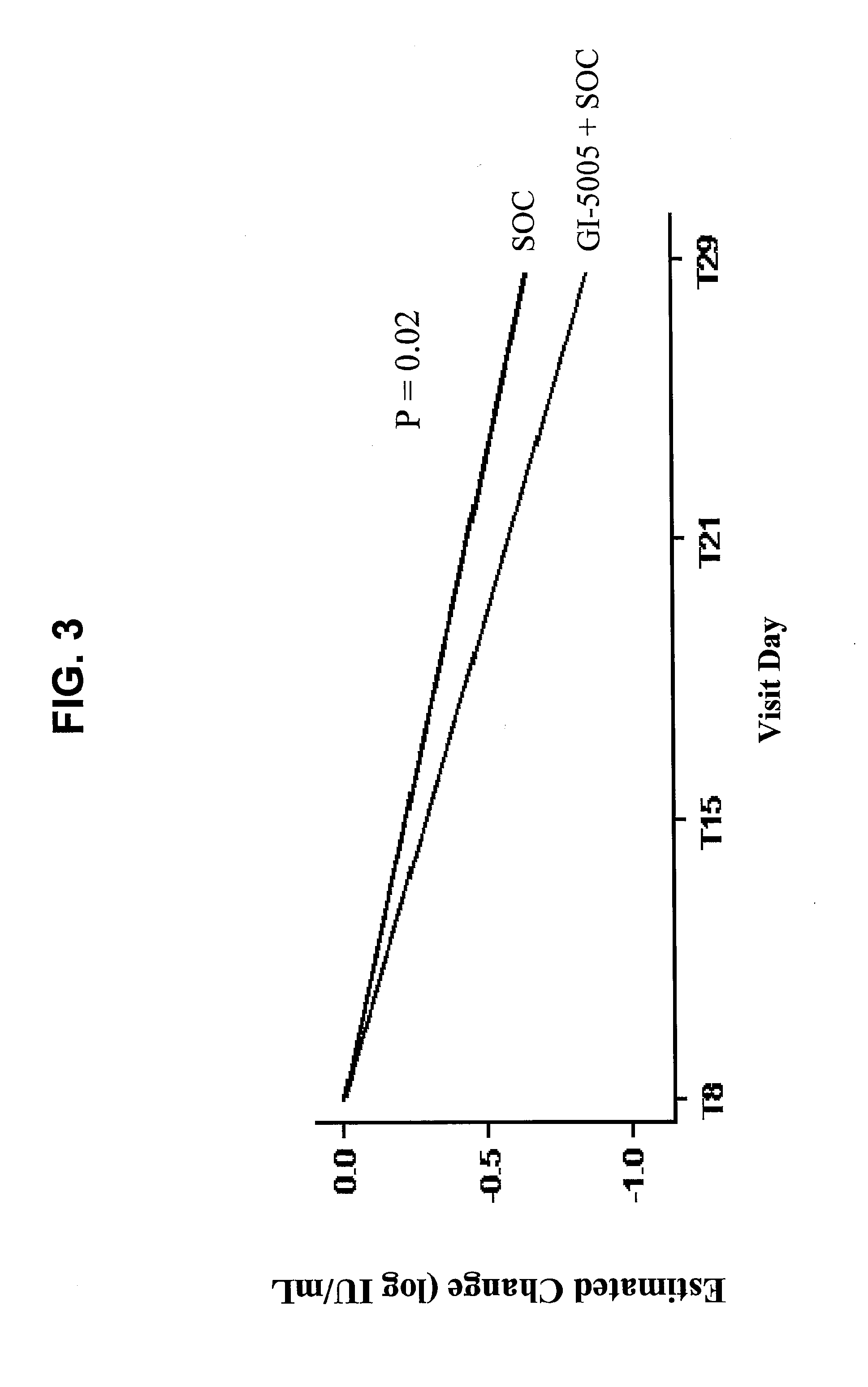
Immunotherapy for chronic hepatitis c virus infection
InactiveUS20110256098A1Increase the number ofReduce in quantitySsRNA viruses positive-sensePeptide/protein ingredientsChronic viral hepatitis CInterferon therapy
Disclosed are uses of immunotherapeutic compositions in combination with Standard of Care (SOC), or interferon therapy combined with anti-viral therapy, for the improved treatment of chronic hepatitis C virus (HCV) infection and related conditions, including liver function. The compositions, kits and uses of the invention, as compared to the use of SOC therapy alone: improves the rate of early response to therapy as measured by early virologic markers (e.g., RVR and EVR), enlarges the pool of patients who will have sustained responses to therapy over the long term, offers shortened courses of therapy for certain patients, enables “rescue” of patients who are non-responders or intolerant to SOC therapy, improves liver function and / or reduces liver damage in patients, and enables the personalization of HCV therapy for a patient, which can result in dose sparing, improved patient compliance, reduced side effects, and improved long term therapeutic outcomes.
Owner:GLOBE IMMUNE INC
Methods, systems and products for predicting response of tumor cells to a therapeutic agent and treating a patient according to the predicted response
InactiveUS20110027291A1Microbiological testing/measurementAntibody ingredientsResponse treatmentsStatistical classification
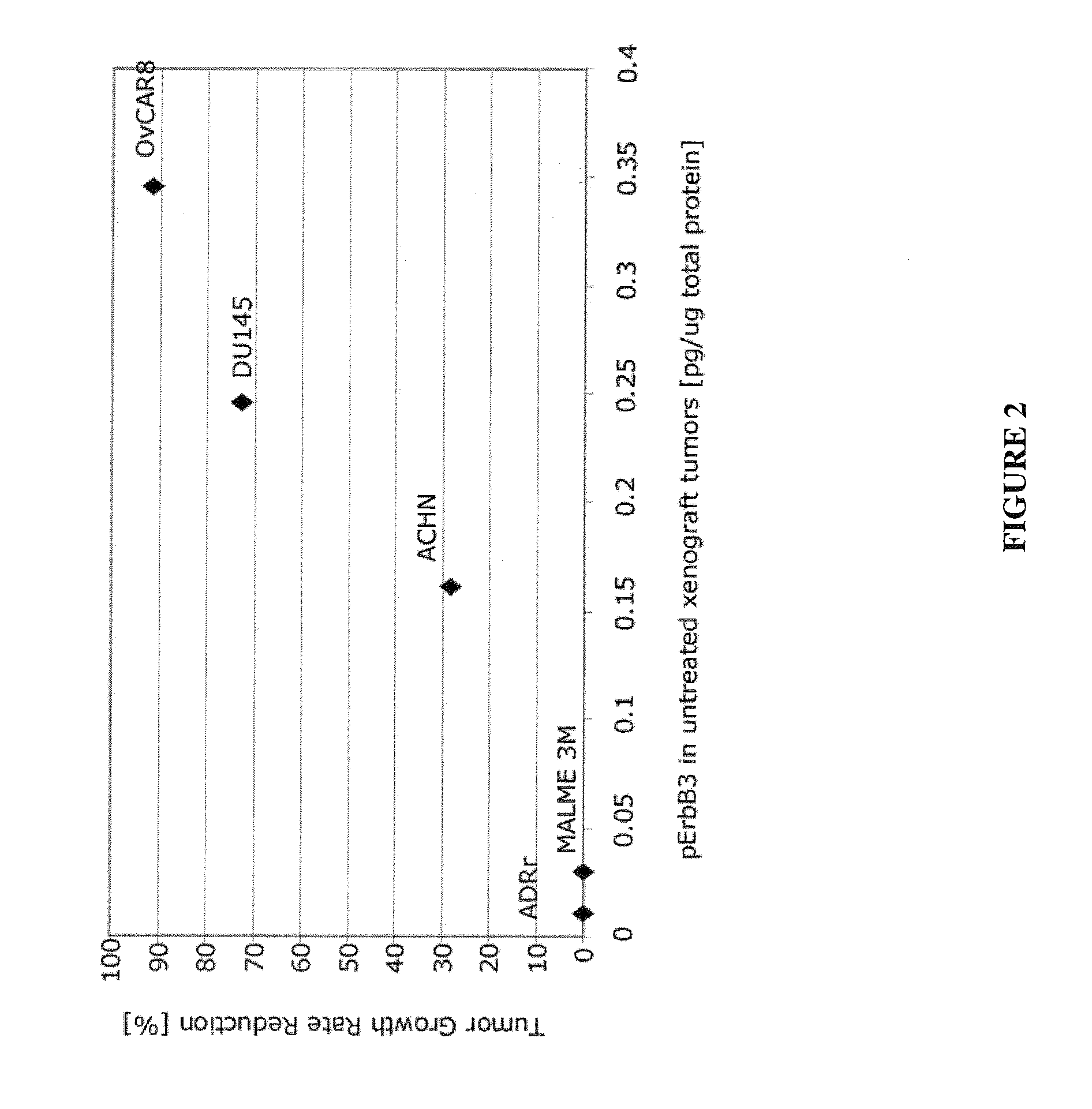
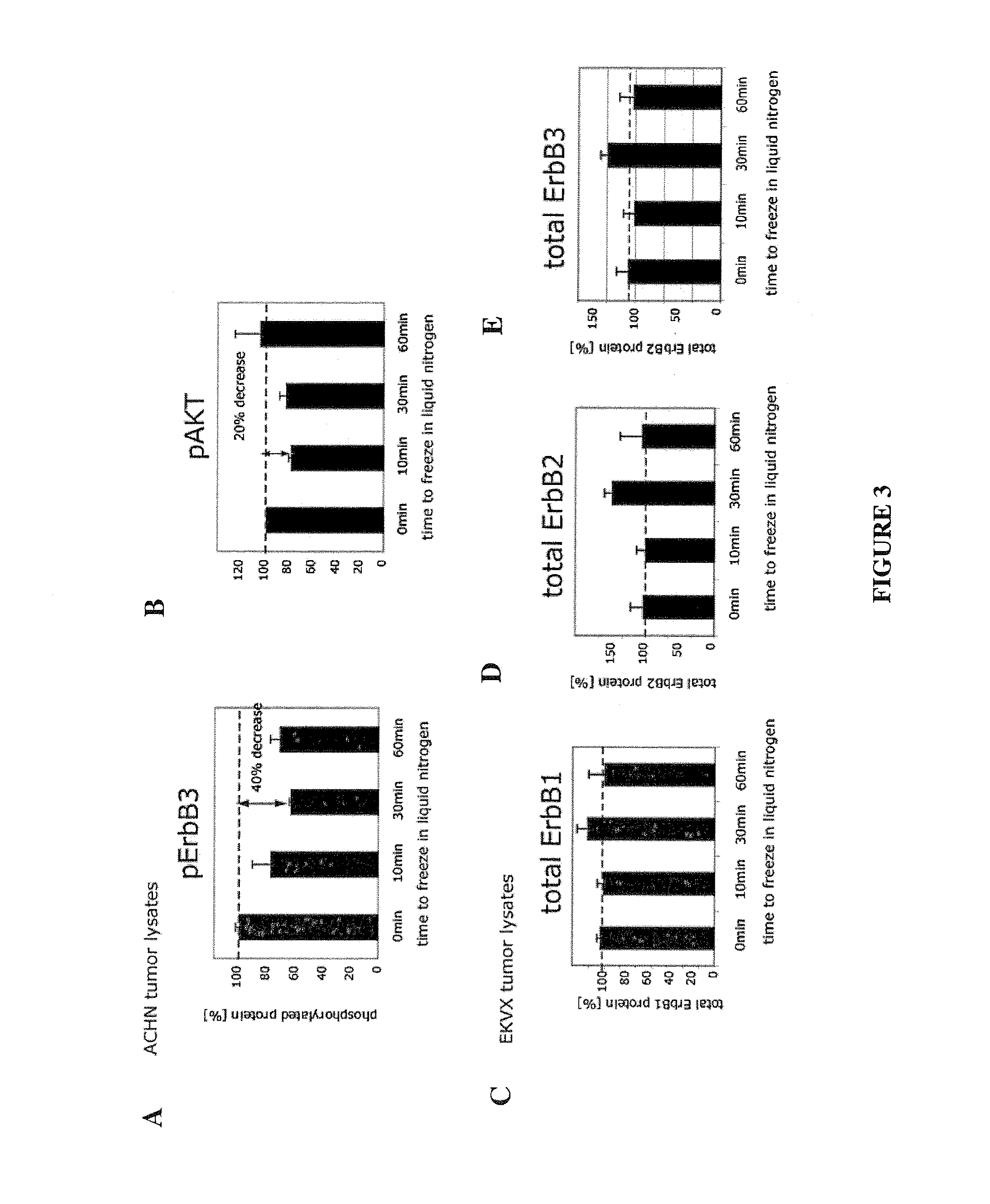
The invention provides methods for treating patients which methods comprise methods for predicting responses of cells, such as tumor cells, to treatment with therapeutic agents. These methods involve measuring, in a sample of the cells, levels of one or more components of a cellular network and then computing a Network Activation State (NAS) or a Network Inhibition State (NIS) for the cells using a computational model of the cellular network. The response of the cells to treatment is then predicted based on the NAS or NIS value that has been computed. The invention also comprises predictive methods for cellular responsiveness in which computation of a NAS or NIS value for the cells (e.g., tumor cells) is combined with use of a statistical classification algorithm. Biomarkers for predicting responsiveness to treatment with a therapeutic agent that targets a component within the ErbB signaling pathway are also provided.
Owner:MERRIMACK PHARMACEUTICALS INC
Automated Enumeration and Characterization of Circulating Melanoma Cells in Blood
InactiveUS20090136946A1Microbiological testing/measurementBiological material analysisTumor LoadMelanoma
The CellTracks® System provides a system to enumerate circulating melanoma cells in blood. The system immunomagnetically concentrates epithelial cells, fluorescently labels the cells and identifies and quantifies circulating melanoma cells. The absolute number of circulating melanoma cells detected in the peripheral blood tumor load is, in part, a factor in prediction of survival, time to progression, and response to therapy. Diagnosis and monitoring of melanoma has been limited by the inability to monitor circulating melanoma cells. The present invention provides a method to enumerate circulating melanoma cells in blood samples. Accordingly, this technology provides a means and device for monitoring disease progression in patients with melanoma.
Owner:JANSSEN DIAGNOSTICS LLC
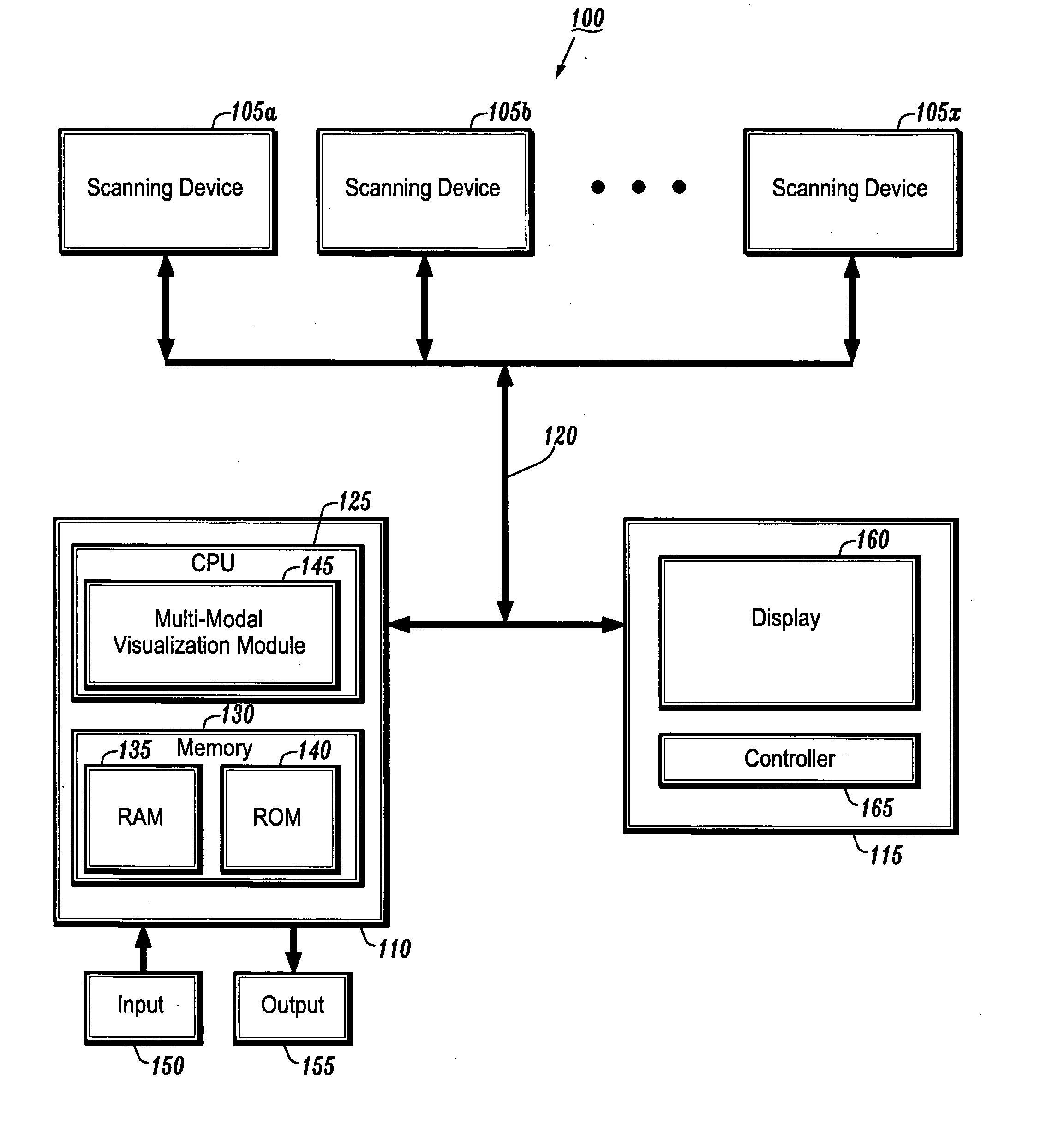
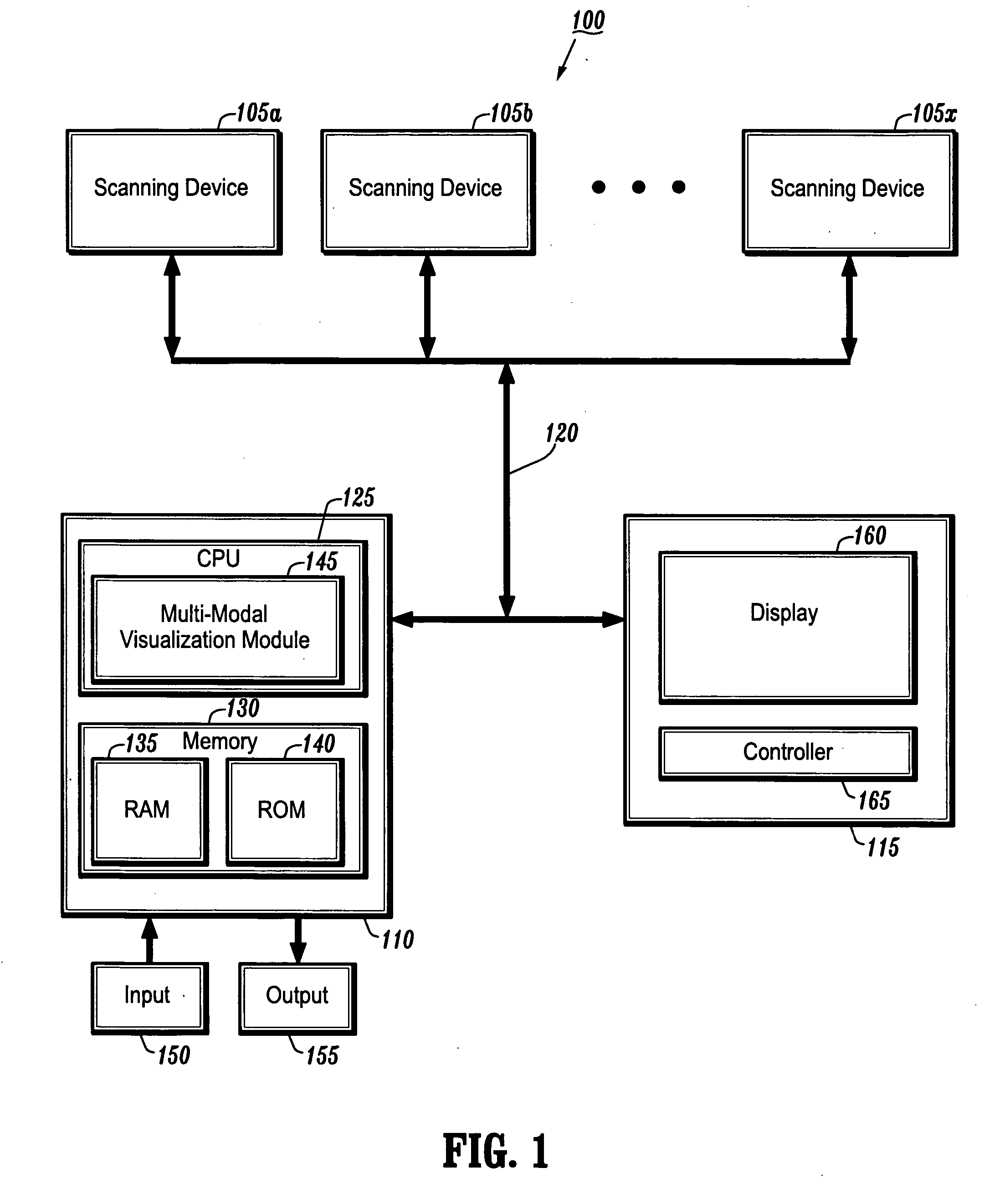
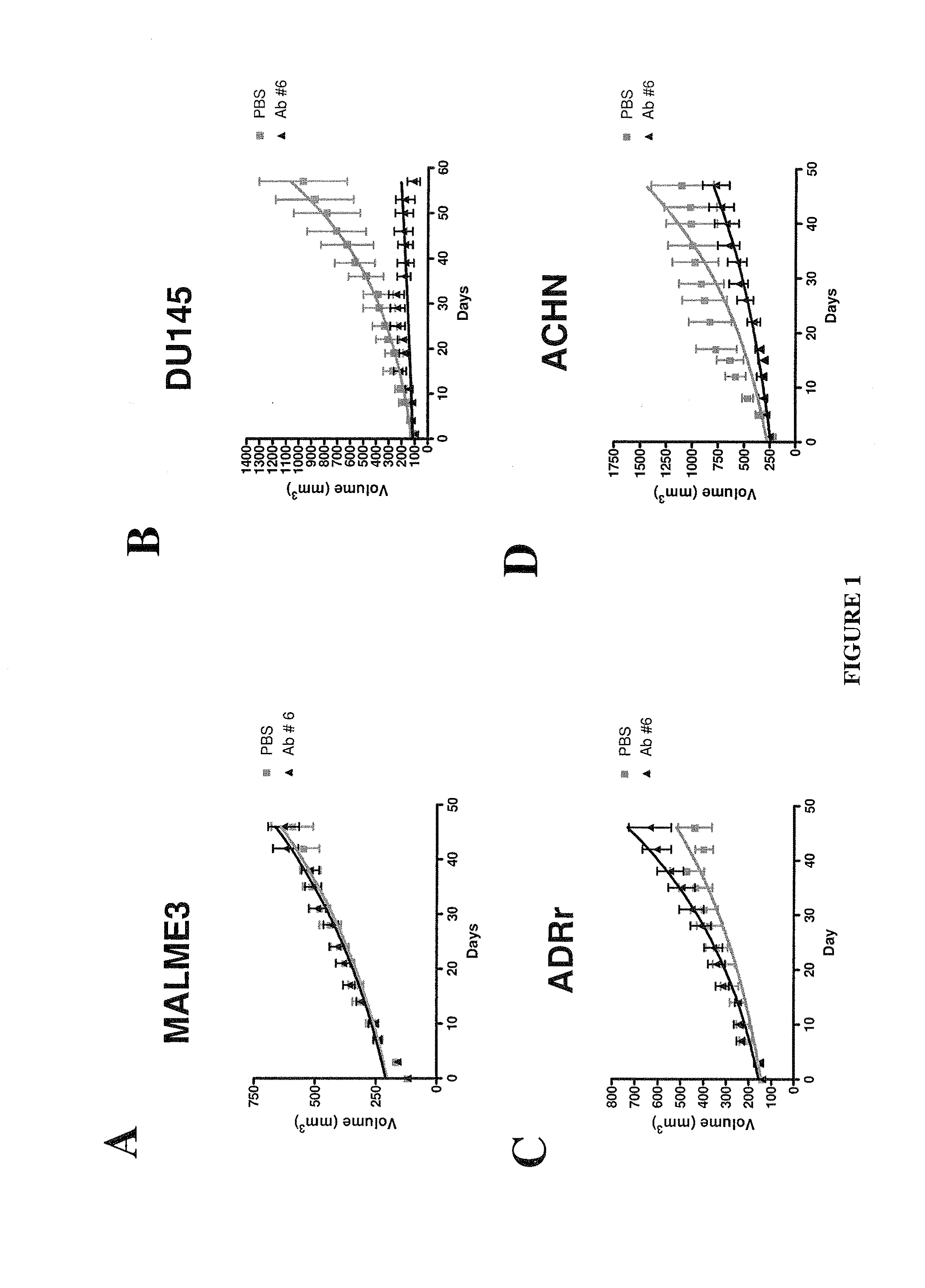
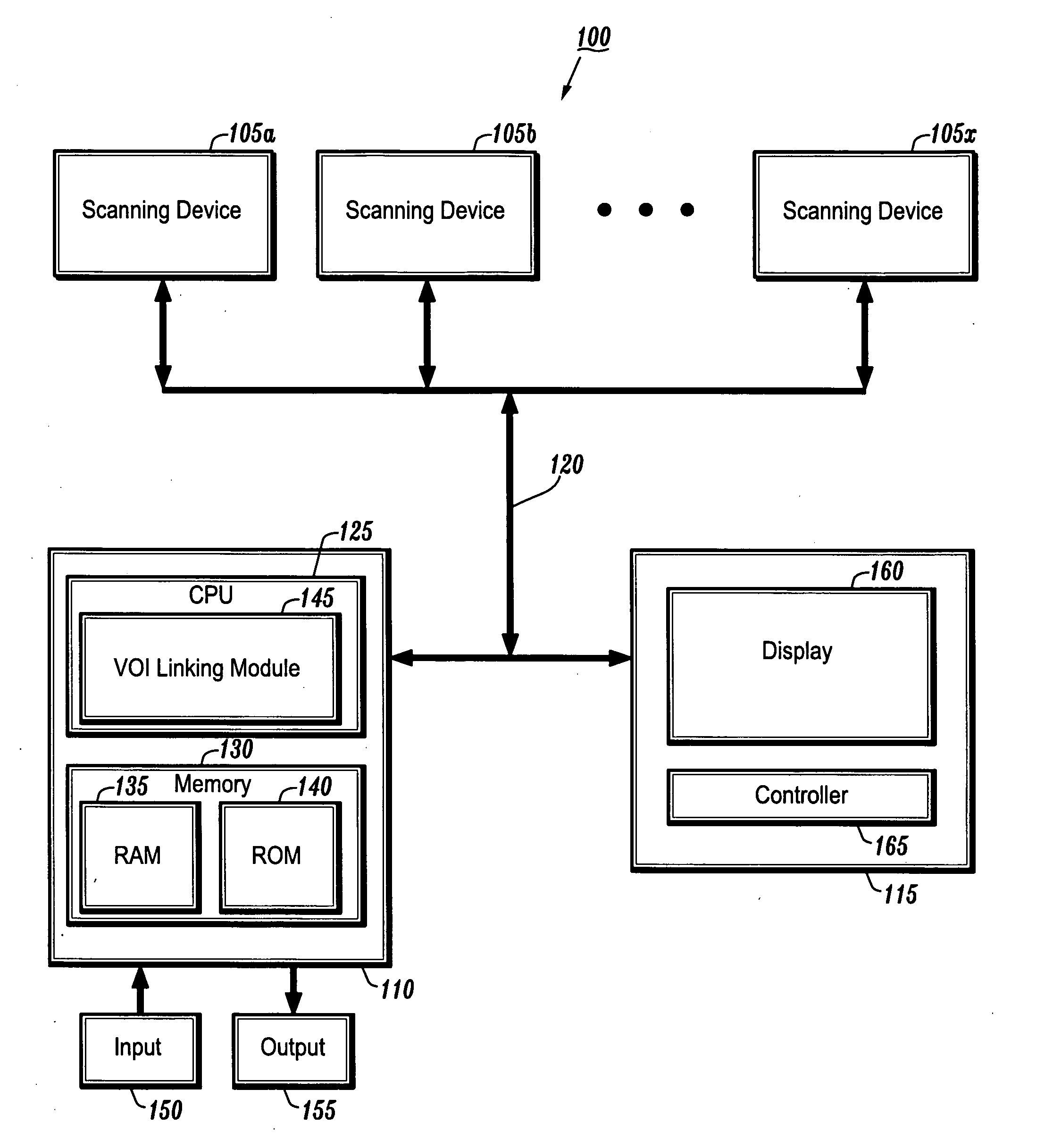
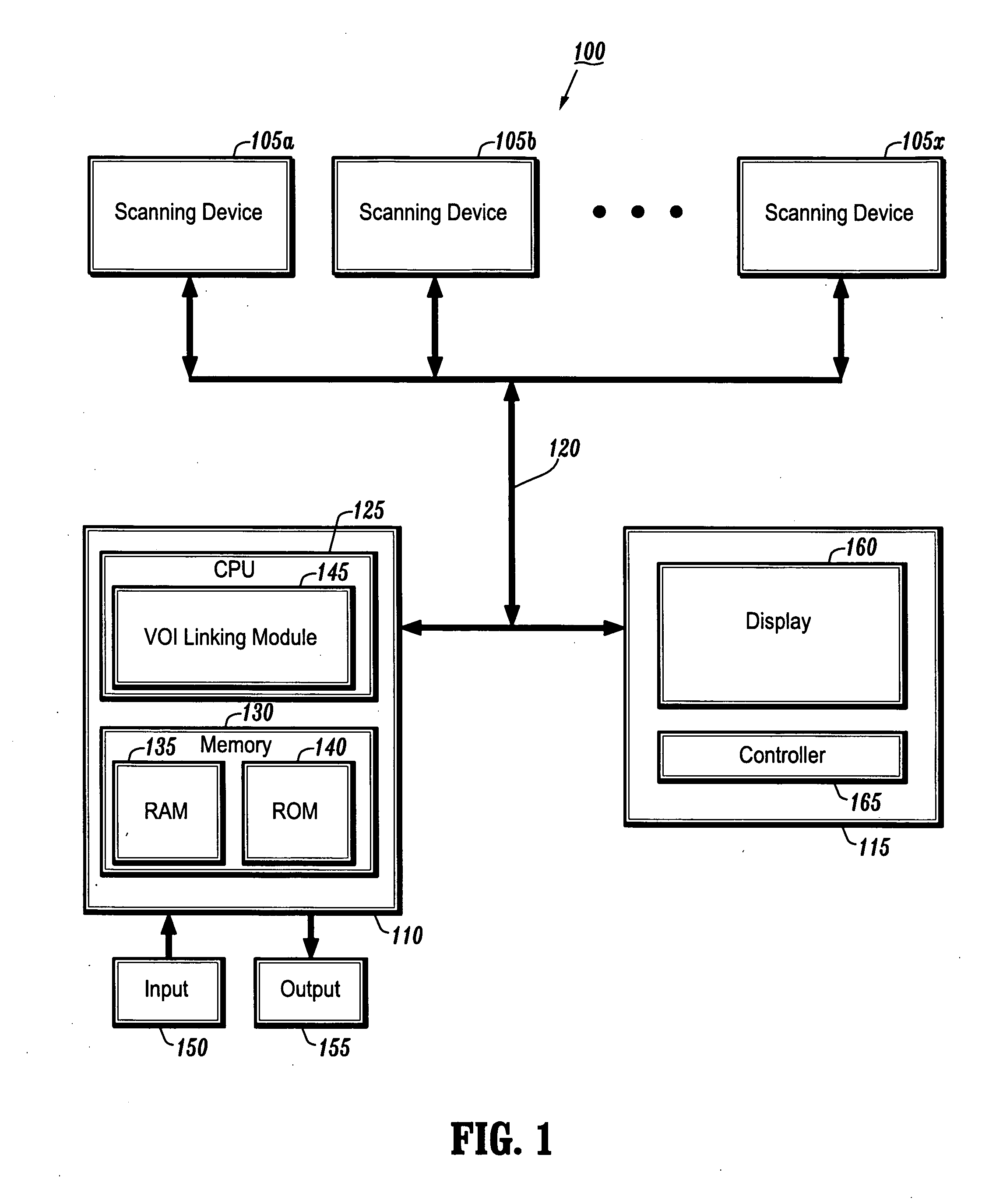
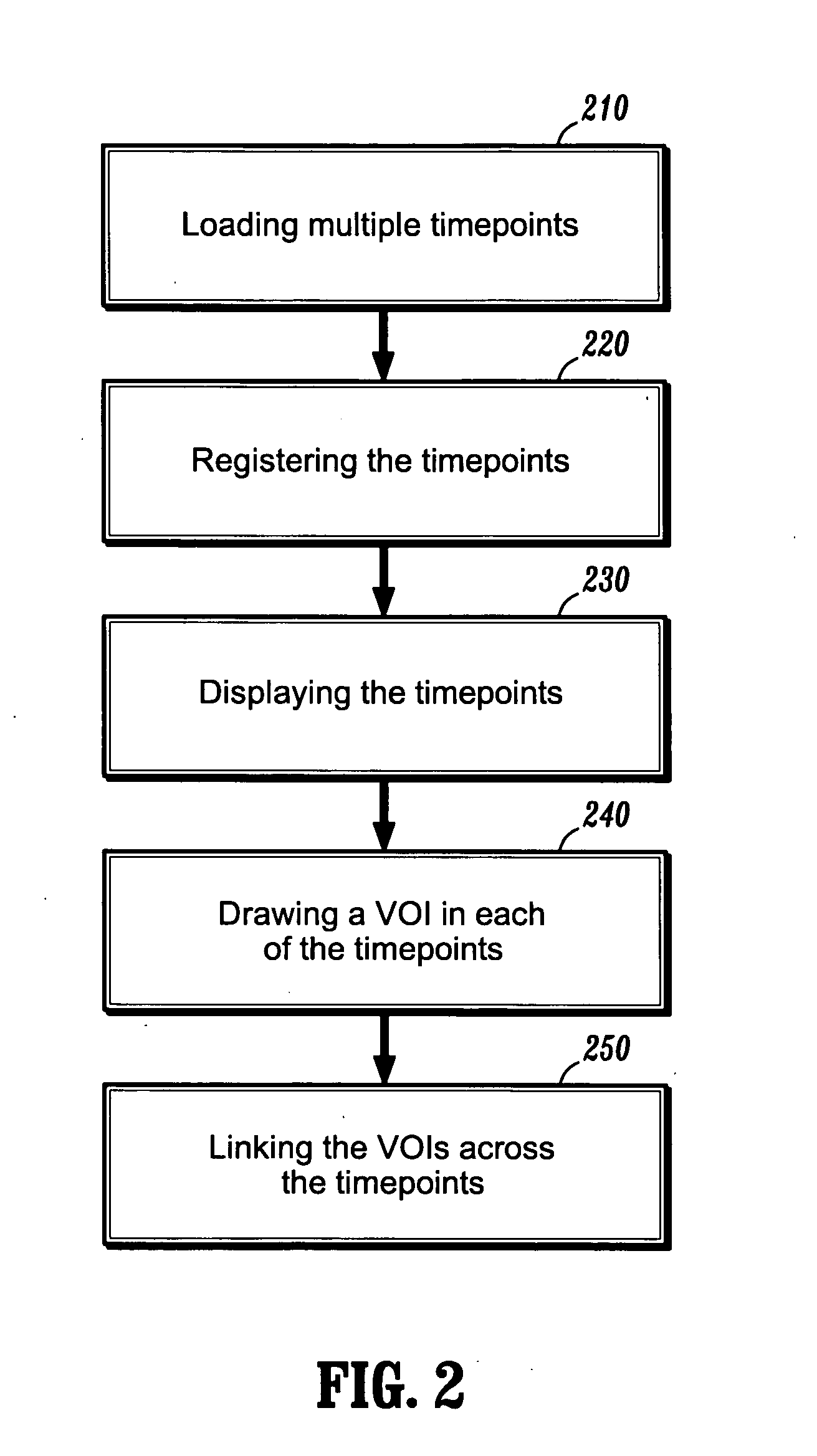
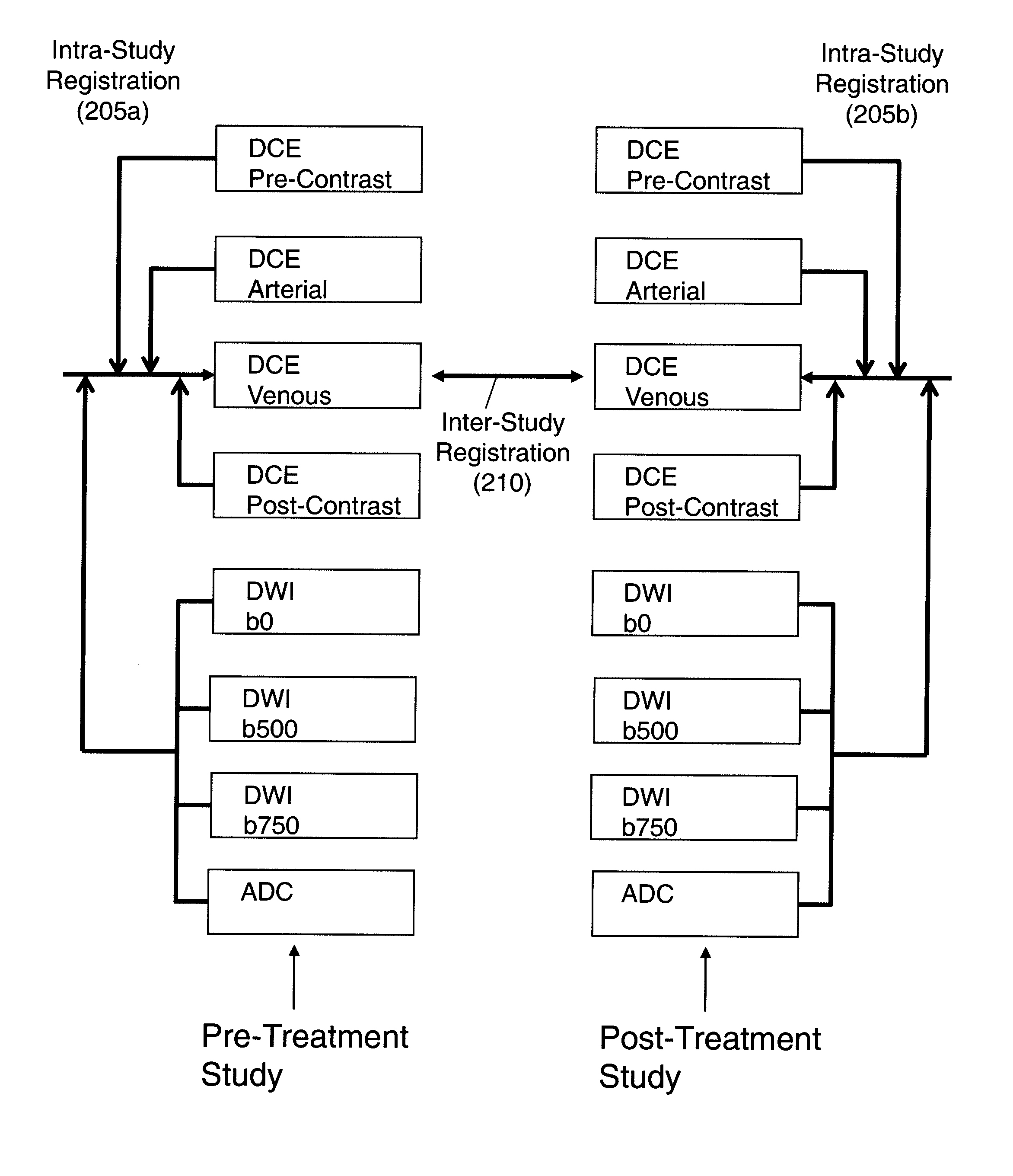
System and method for linking VOIs across timepoints for analysis of disease progression or response to therapy
A system and method for linking volumes of interest (VOIs) across timepoints are provided. The method comprises: loading an image dataset of a first timepoint and an image dataset of a second timepoint; registering the image dataset of the first timepoint and the image dataset of the second timepoint; displaying the image dataset of the first timepoint and the image dataset of the second timepoint; selecting a VOI in the image dataset of the first timepoint and the image dataset of the second timepoint; and linking the VOIs in the image dataset of the first timepoint and the image dataset of the second timepoint.
Owner:SIEMENS HEATHCARE GMBH
Method for predicting progression free and overall survival at each follow-up time point during therapy of metastatic breast cancer patients using circulating tumor cells
InactiveUS20090061456A1Less side effectsImprove the quality of lifeDisease diagnosisBiological testingOncologyDisease progression
A cancer test having prognostic utility in predicting time to disease progression, overall survival, and response to therapy in patients with MBC based upon the presence and number of CTC's. The Cell Spotter® System is used to enumerate CTC's in blood. The system immunomagnetically concentrates epithelial cells, fluorescently labels the cells and identifies and quantifies CTC's. The absolute number of CTC's detected in the peripheral blood tumor load is, in part, a factor in prediction of survival, time to progression, and response to therapy. The mean time to survival of patients depended upon a threshold number of 5 CTC's per 7.5 ml of blood. Detection of CTC's in metastatic cancer represents a novel prognostic factor in patients with metastatic cancers, suggests a biological role for the presence of tumor cells in the blood, and indicates that the detection of CTC's could be considered an appropriate surrogate marker for prospective therapeutic clinical trials.
Owner:VERIDEX LCC
Dendrimer conjugates
The present invention relates to novel therapeutic and diagnostic dendrimers. In particular, the present invention is directed to dendrimer-linker conjugates, methods of synthesizing the same, compositions comprising the conjugates, as well as systems and methods utilizing the conjugates (e.g., in diagnostic and / or therapeutic settings (e.g., for the delivery of therapeutics, imaging, and / or targeting agents (e.g., in disease (e.g., cancer) diagnosis and / or therapy, pain therapy, etc.)). Accordingly, dendrimer-linker conjugates of the present invention may further comprise one or more components for targeting, imaging, sensing, and / or providing a therapeutic or diagnostic material and / or monitoring response to therapy.
Owner:RGT UNIV OF MICHIGAN
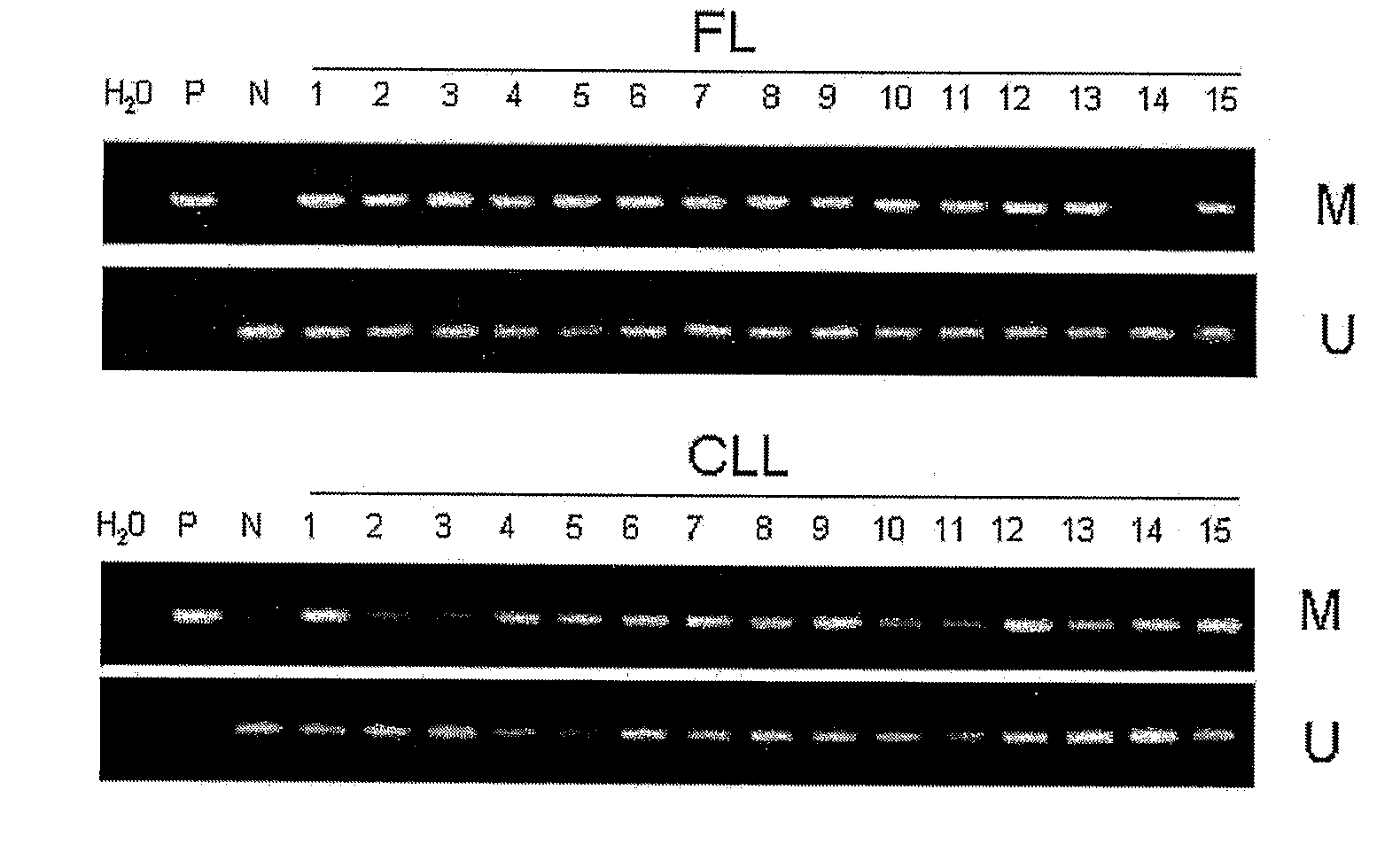
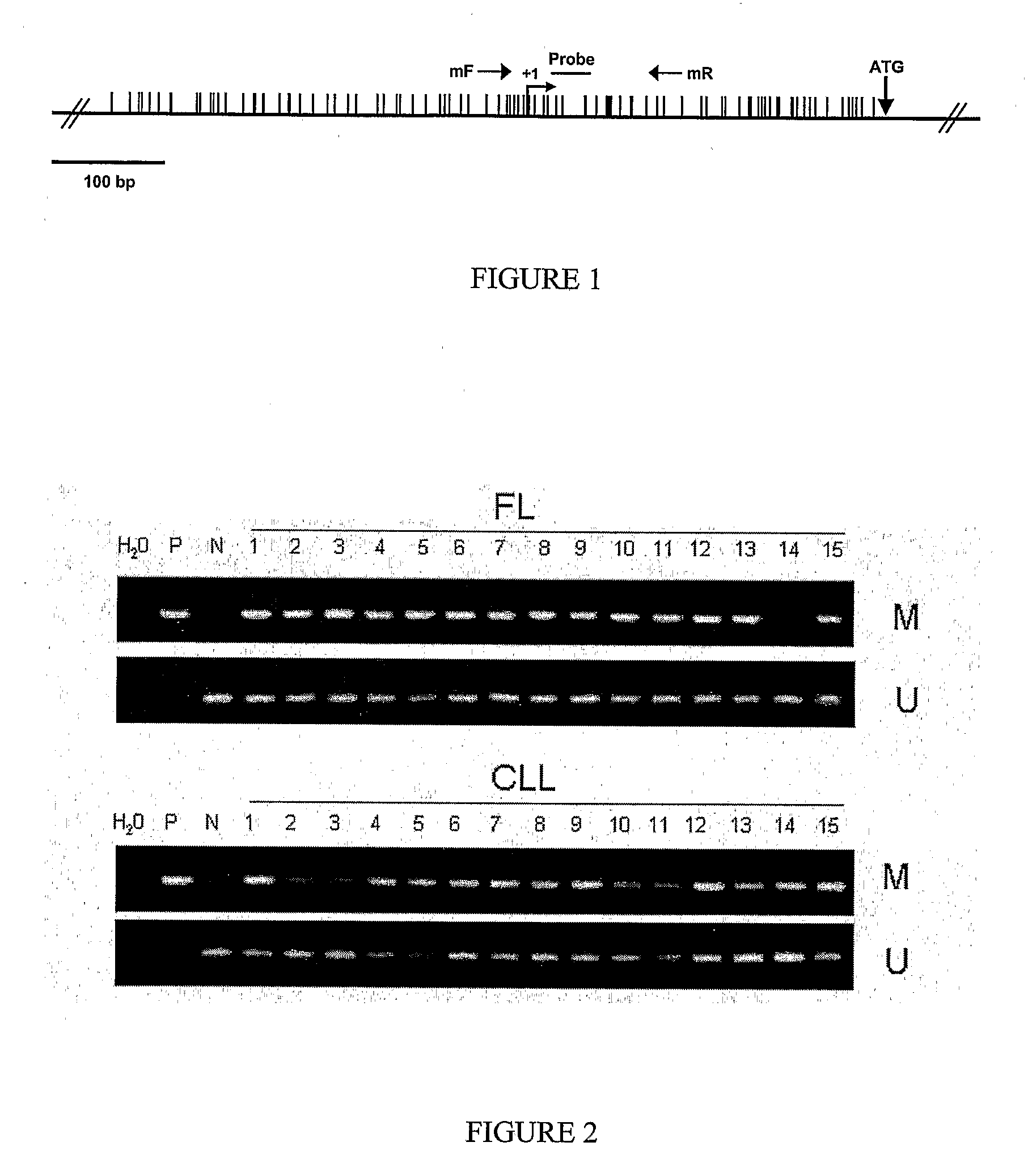
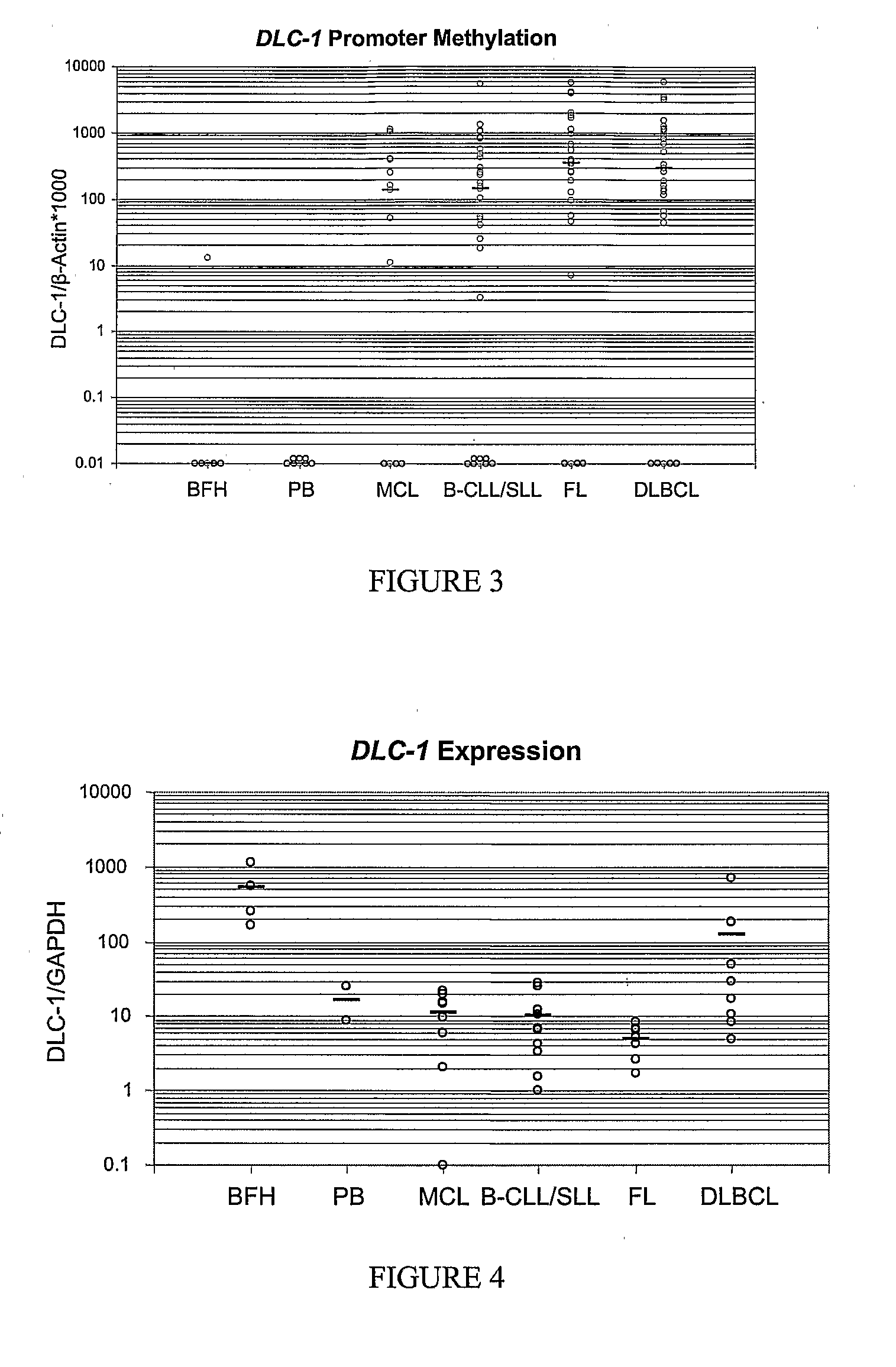
DNA methylation biomarkers in lymphoid and hematopoietic malignancies
InactiveUS20090264306A1Guaranteed maximum utilizationImprove the detection rateMicrobiological testing/measurementLibrary screeningDNA methylationLymphocytic cell
Differential Methylation Hybridization (DMH) was used to identify novel methylation markers and methylation profiles for hematopoieetic malignancies, leukemia, lymphomas, etc. (e.g., non-Hodgkin's lymphomas (NHL), small B-cell lymphomas (SBCL), diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), mantle cell lymphoma (MCL), B-cell chronic lymphocytic leukemia / small lymphocytic lymphoma (B-CLL / SLL), chronic lymphocytic leukemia (CLL), multiple myeloma (MM), acute myelogenous leukemia (AML), acute lymphoblastic leukemia (ALL), etc.). Particular aspects provide novel biomarkers for NHL and subtypes thereof (e.g., MCL, B-CLL / SLL, FL, DLBCL, etc.), AML, ALL and MM, and further provide non-invasive tests (e.g. blood tests) for lymphomas and leukemias. Additional aspects provide markers for diagnosis, prognosis, monitoring responses to therapies, relapse, etc., and further provide targets and methods for therapeutic demethylating treatments. Further aspects provide cancer staging markers, and expression assays and approaches comprising idealized methylation and / or patterns” (IMP and / or IEP) and fusion of gene rankings.
Owner:UNIVERSITY OF MISSOURI
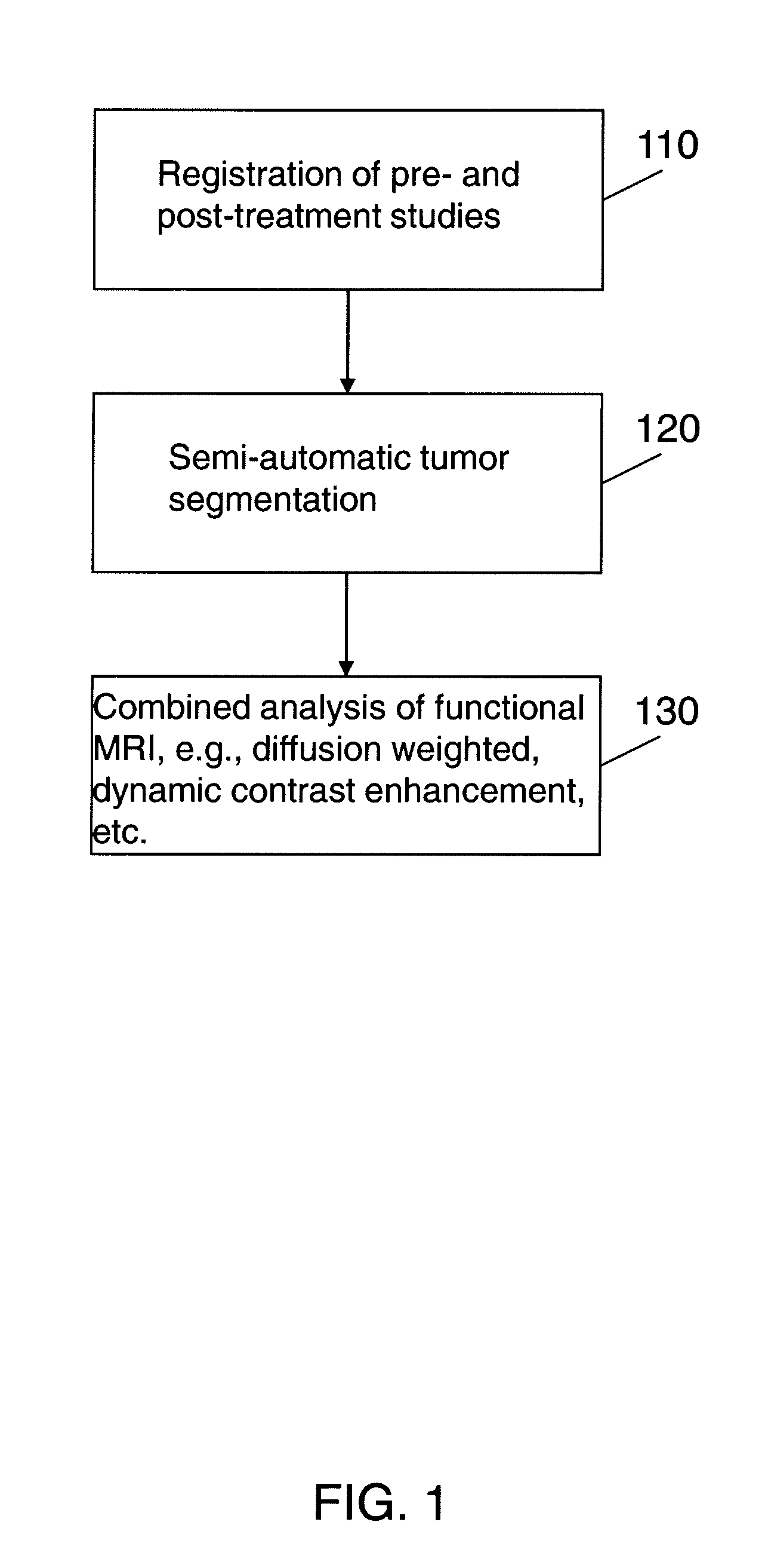
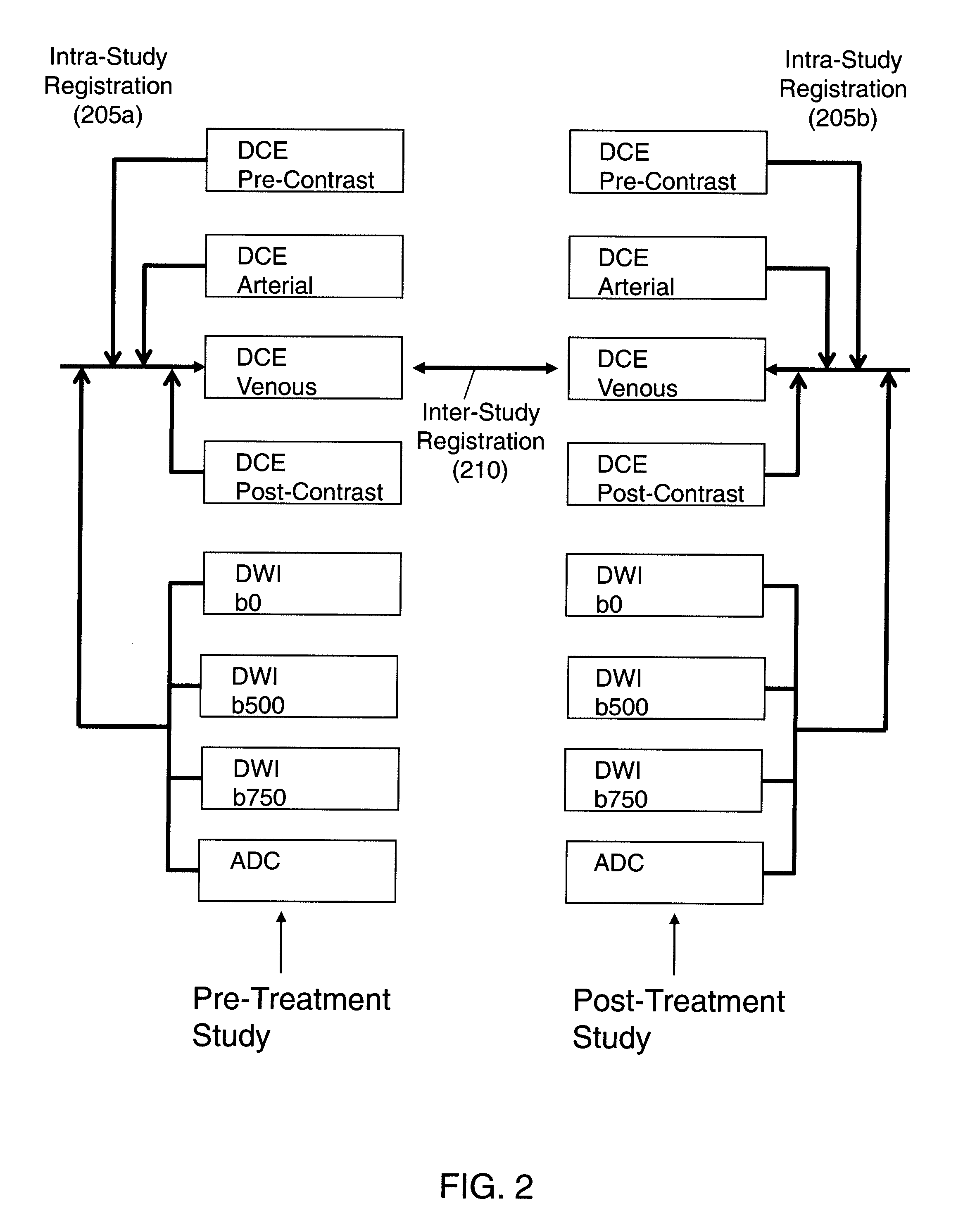
Automated method for assessment of tumor response to therapy with multi-parametric MRI
A method for assessing a tumor's response to therapy, includes providing images of a first study of a patient and images of a second study of the patient, the second study occurring after the first study and after the patient undergoes therapy to treat a tumor, each study comprising first and second types of functional magnetic resonance (fMR) images, performing a first registration in which the images within each study are registered, performing a second registration in which reference images from both studies are co-registered, segmenting the tumor in an image of each of the second registered studies; and determining that first and second fMR measure differences exist between the segmented tumor's of the first and second studies, the first fMR measure difference being obtained from the first type of fMR images, the second fMR measure difference being obtained from the second type of fMR images.
Owner:SIEMENS AG
Genetic Variants Predictive of Cancer Risk
InactiveUS20110212855A1Increased susceptibilityHigh riskMicrobiological testing/measurementLibrary screeningDiseaseGenetic variants
The invention discloses genetic variants that have been determined to be susceptibility variants of cancer. Methods of disease management, including determining increased susceptibility to cancer, methods of predicting response to therapy and methods of predicting prognosis of cancer using such variants are described. The invention further relates to kits useful in the methods of the invention.
Owner:DECODE GENETICS EHF
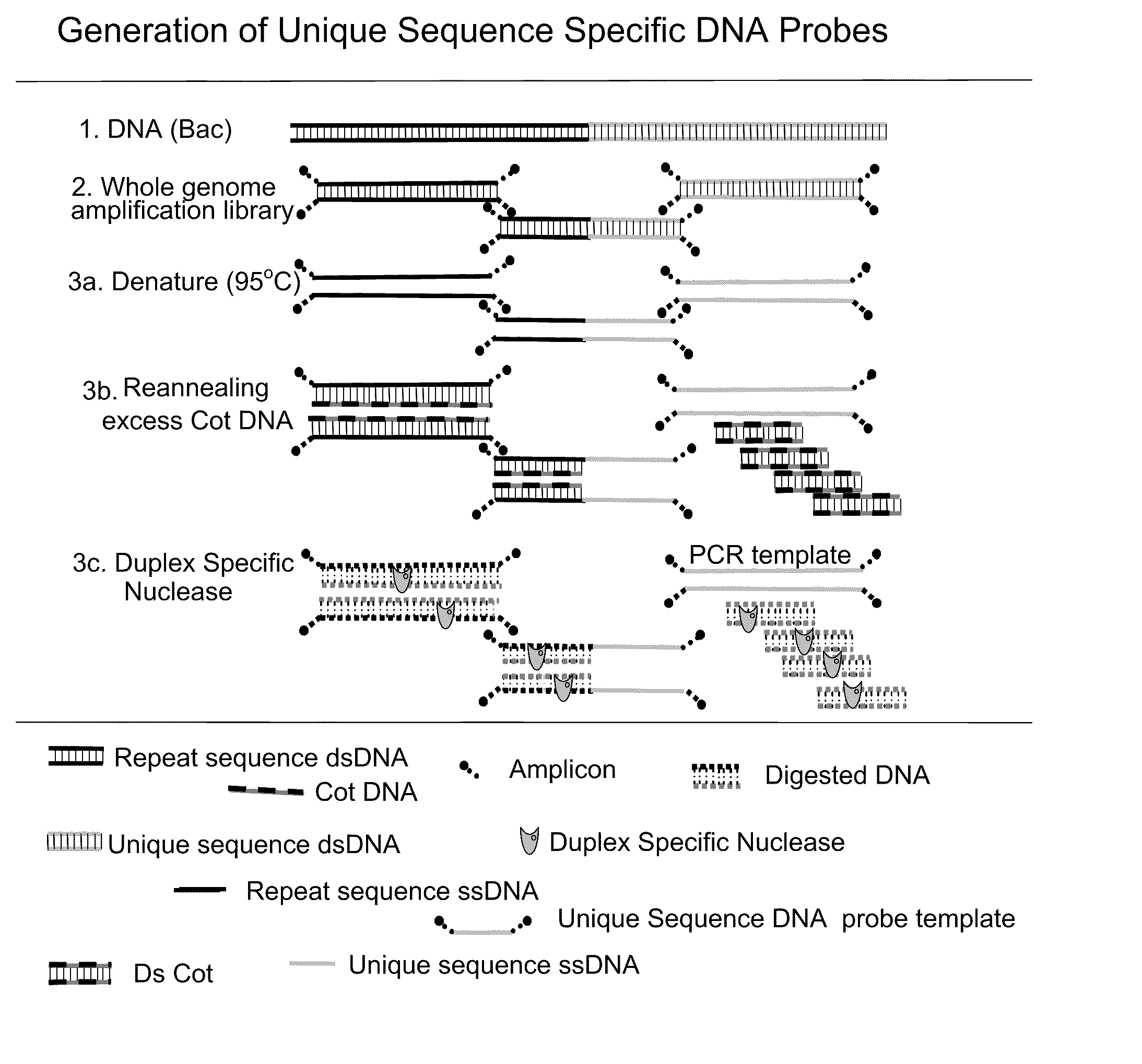
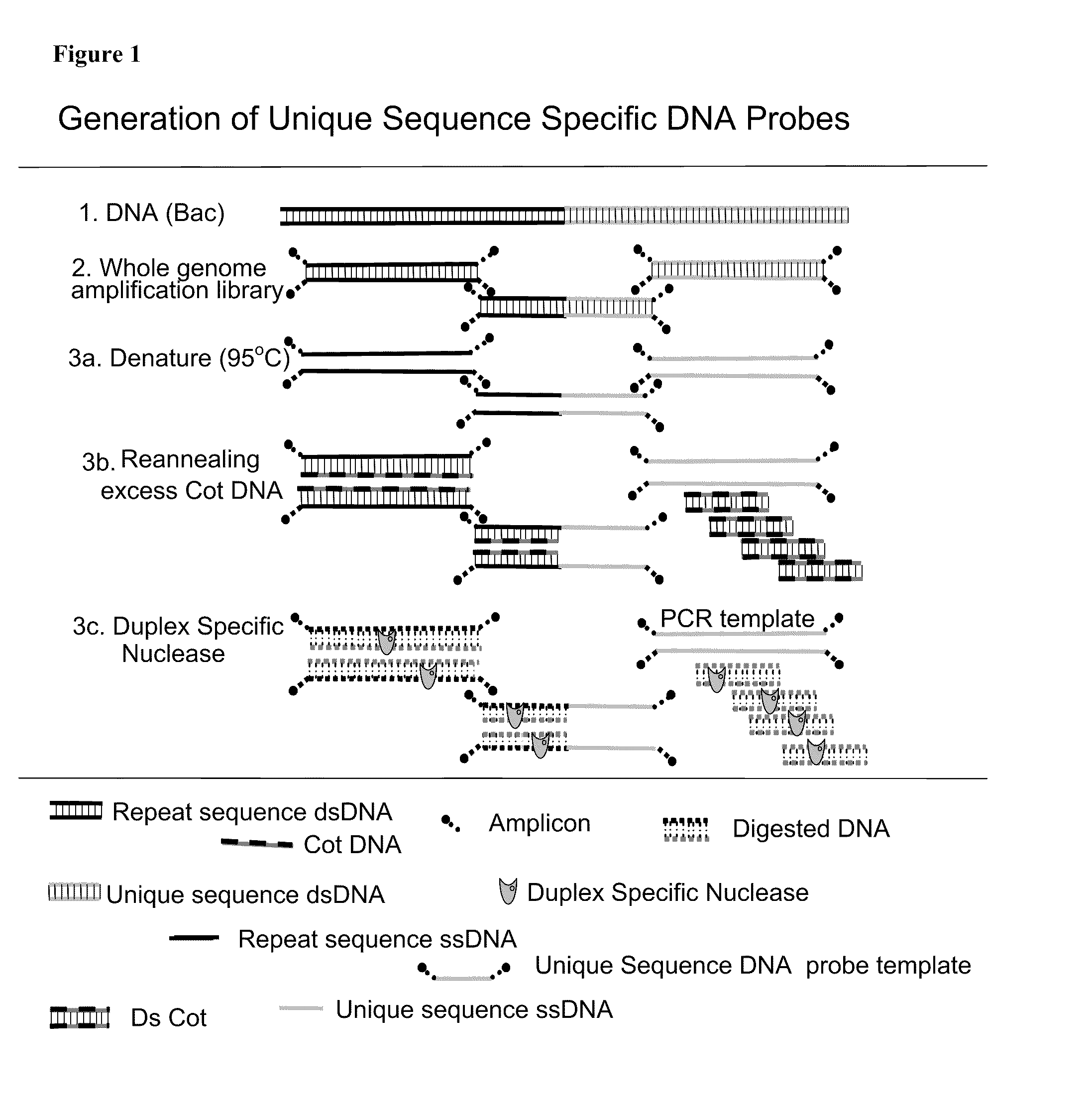
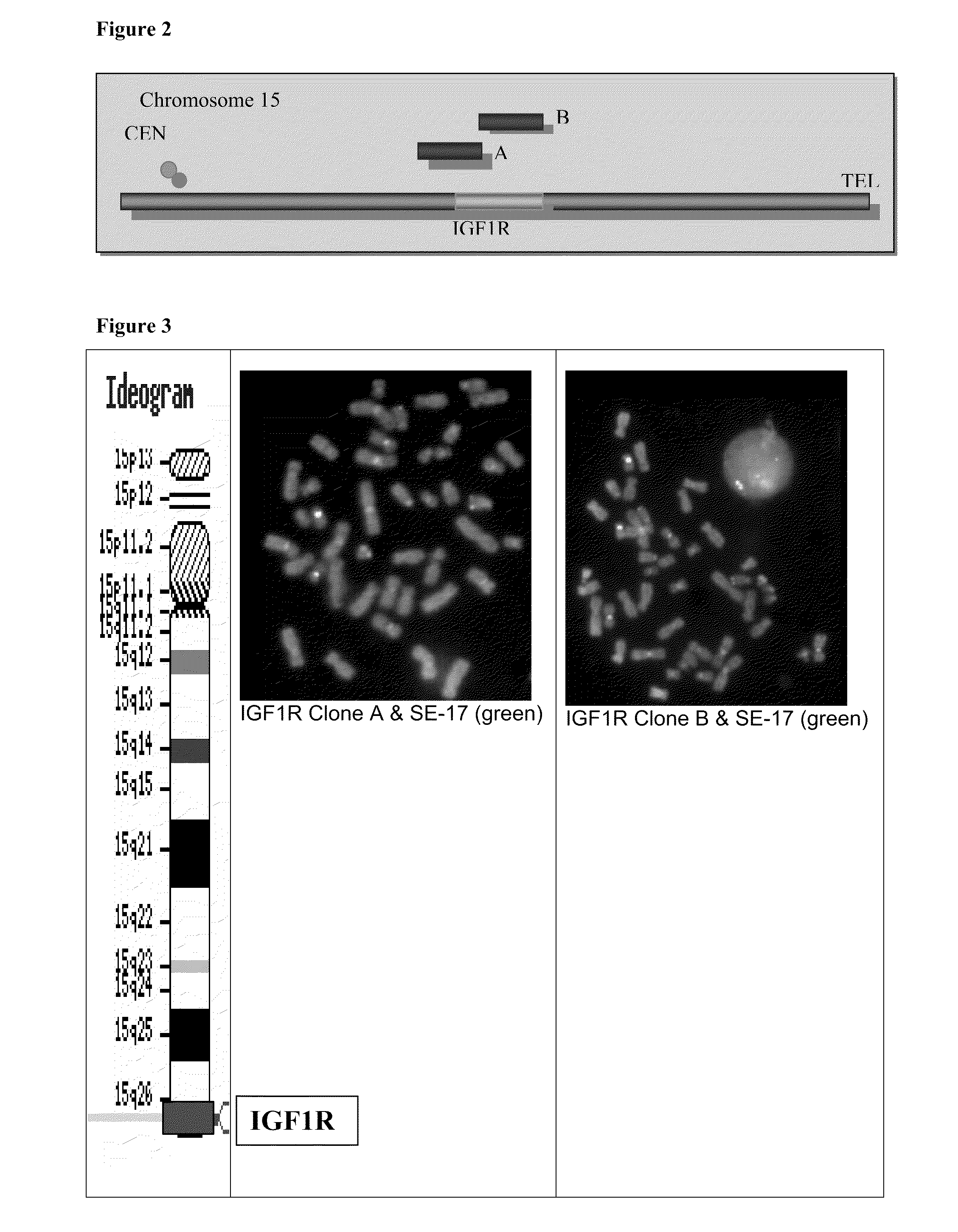
METHOD FOR DETECTING IGF1R/Chr 15 in CIRCULATING TUMOR CELLS USING FISH
InactiveUS20090258365A1Microbiological testing/measurementBiological material analysisFluorescenceCirculating cancer cell
The present invention describes methods and probe composition for an automated FISH assay of a blood sample containing circulating tumor cells expressing the IGF-1R gene. The assay provides genetic analysis of suspect circulating tumor cells that have been identified after immunomagnetic selection and fluorescent labeling. Using unique, repeat-free probes to the IGF-1R locus and a chromosome 15 reference probe, cell lines expressing an aberrant number of IGF-1R and Chr 15 signals were detected, including one cell line with a low level of IGF-1R amplification. The ability to directly examine the genetic profile of IGF-1R on circulating tumor cells may provide an automated means for assessing disease and patient response to therapy.
Owner:VERIDEX LCC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com