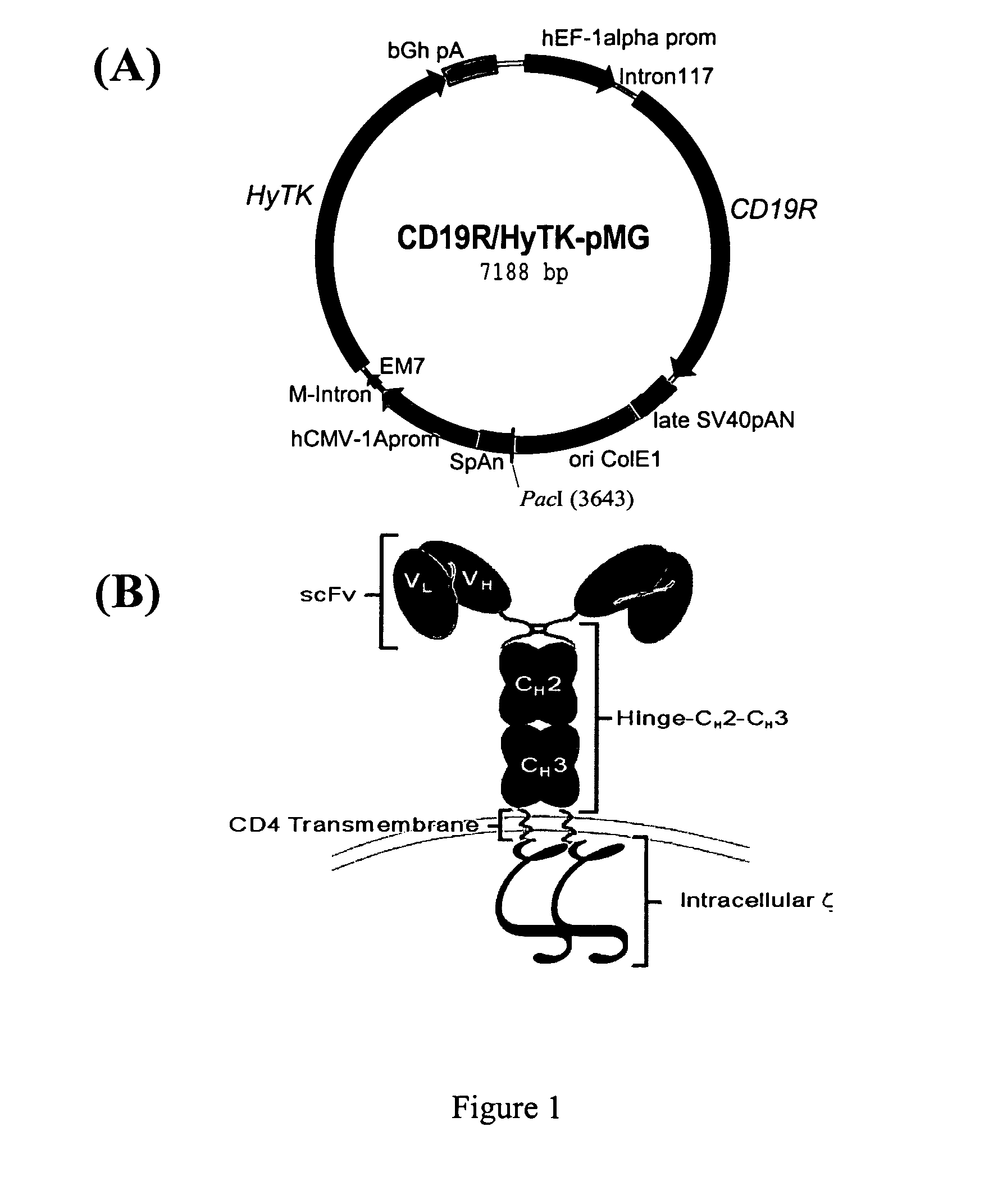
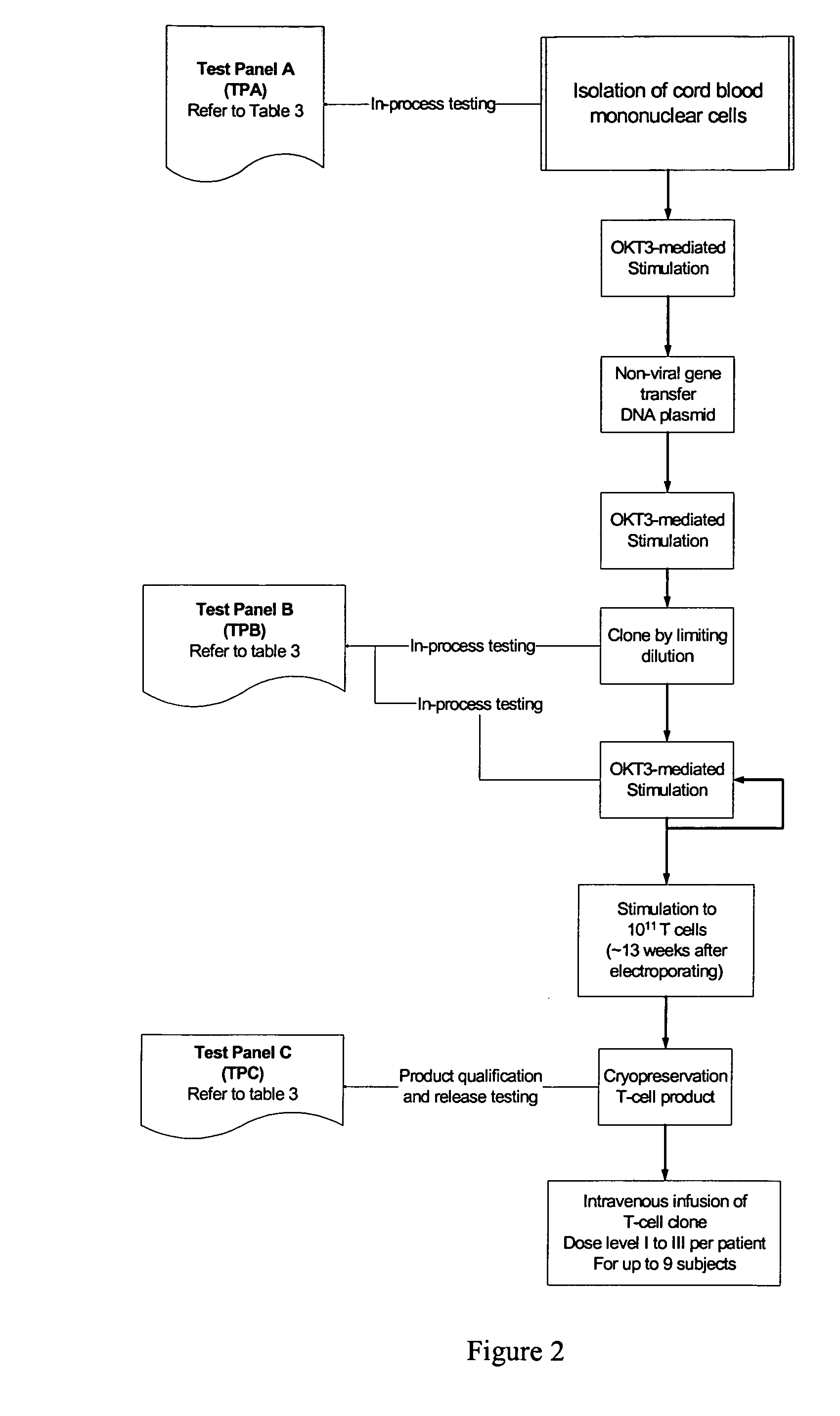
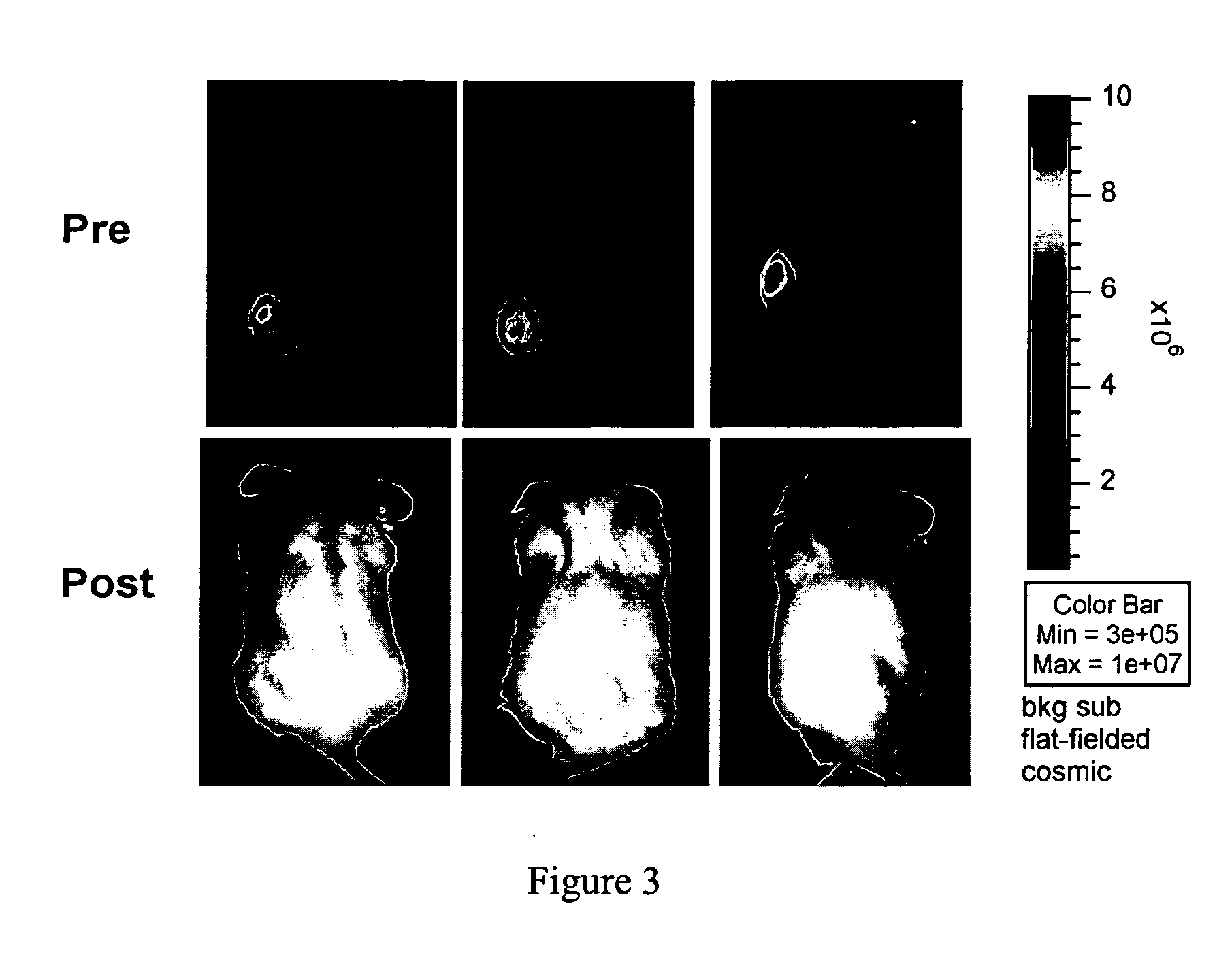
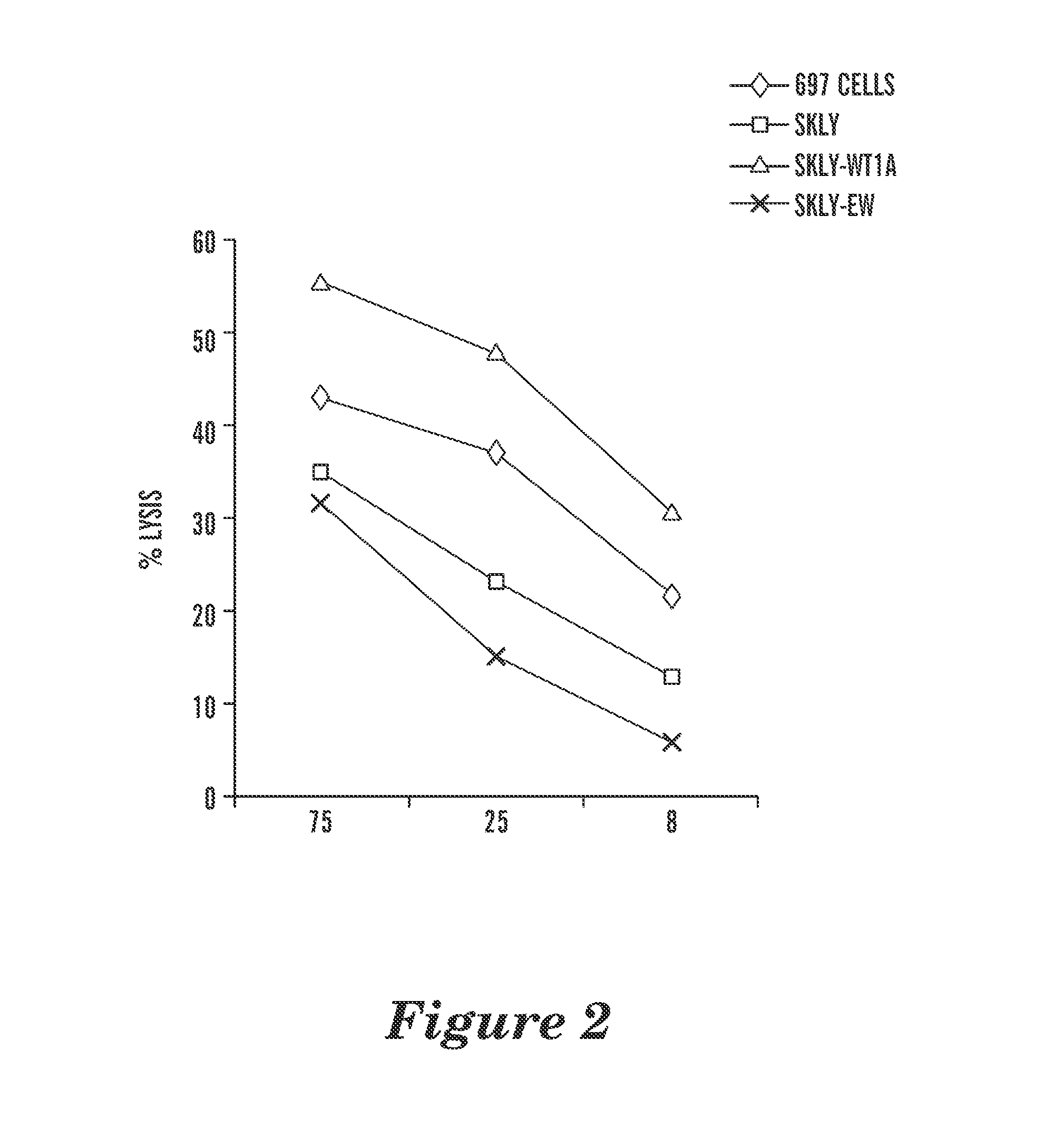
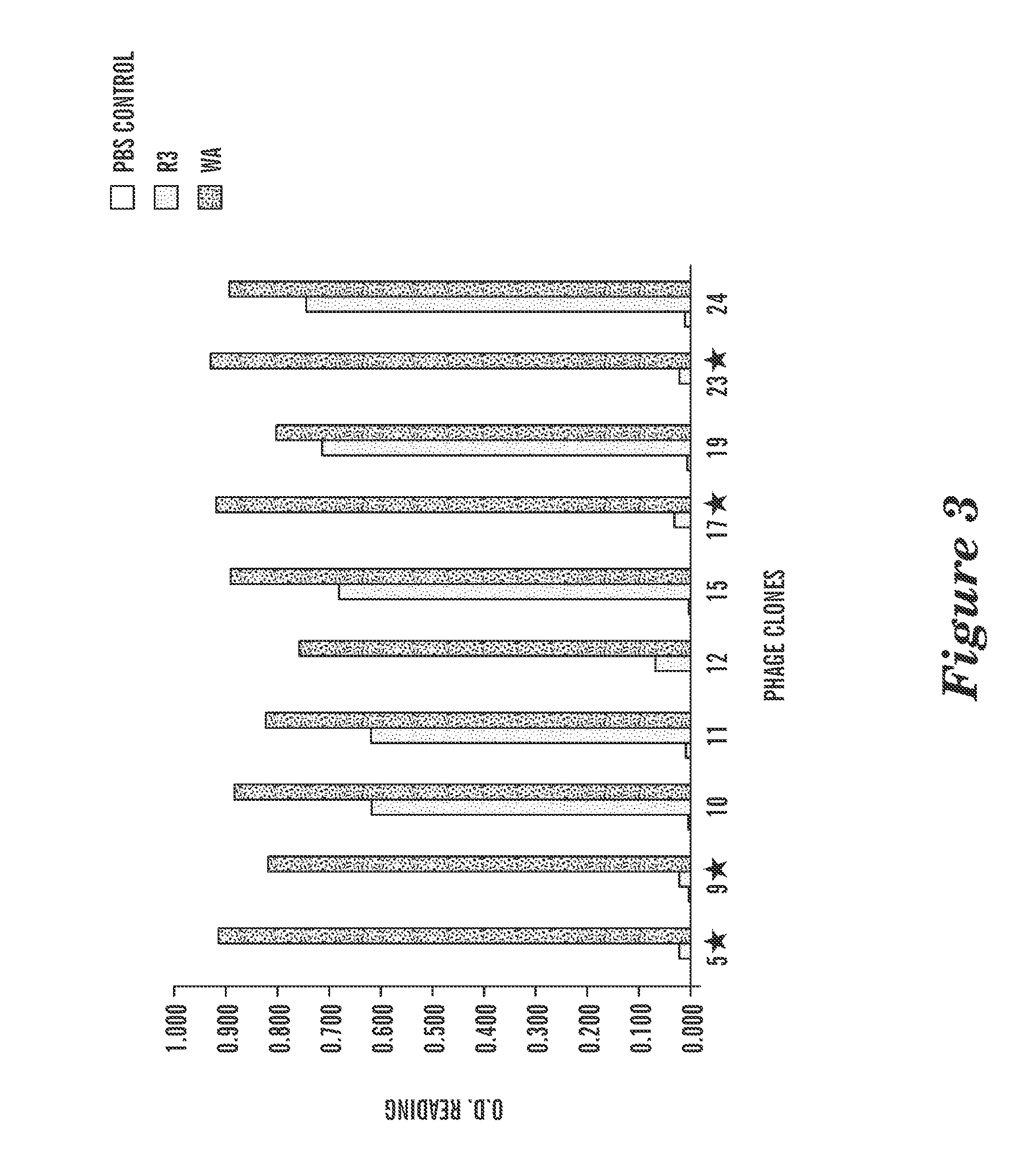
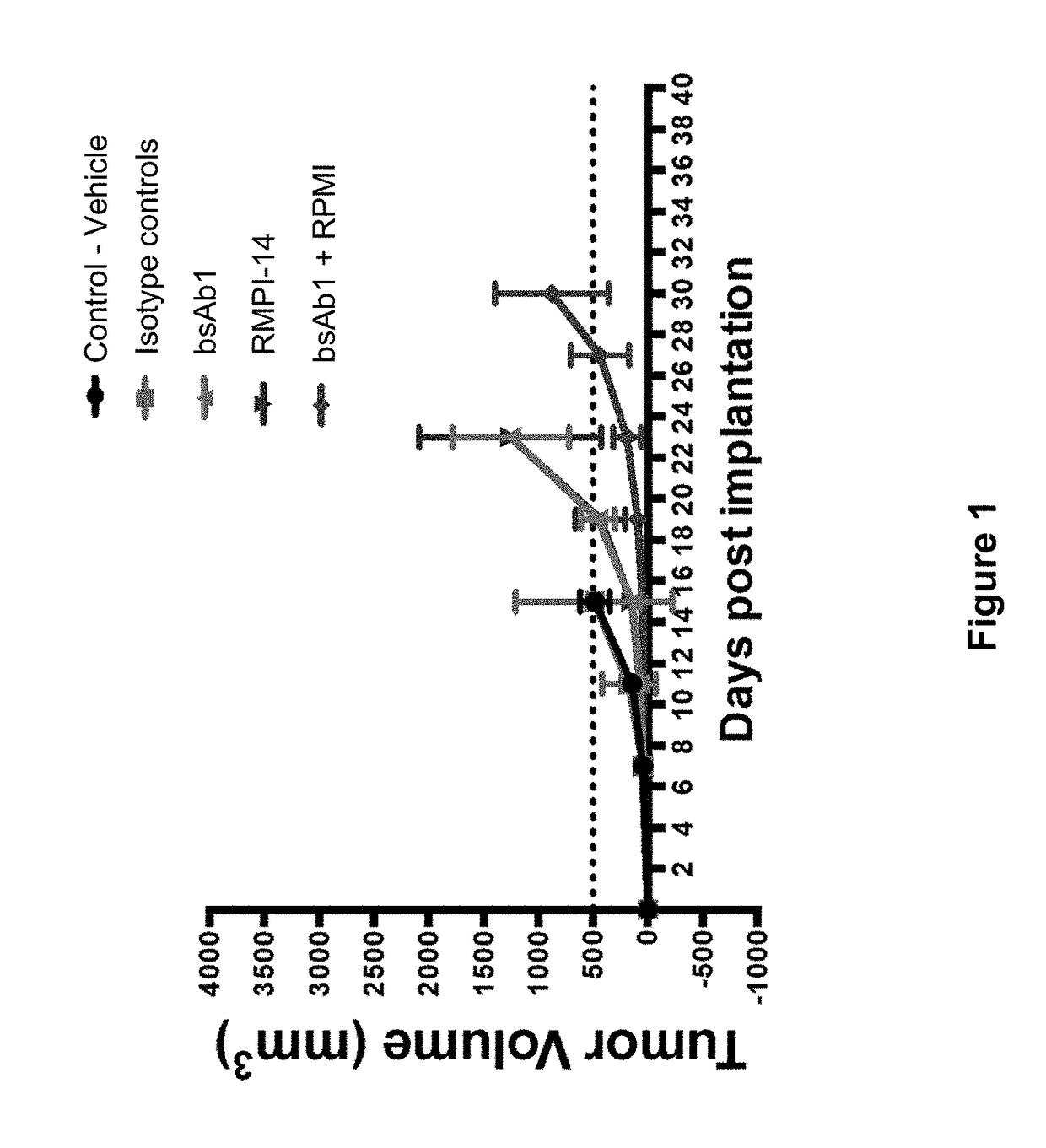
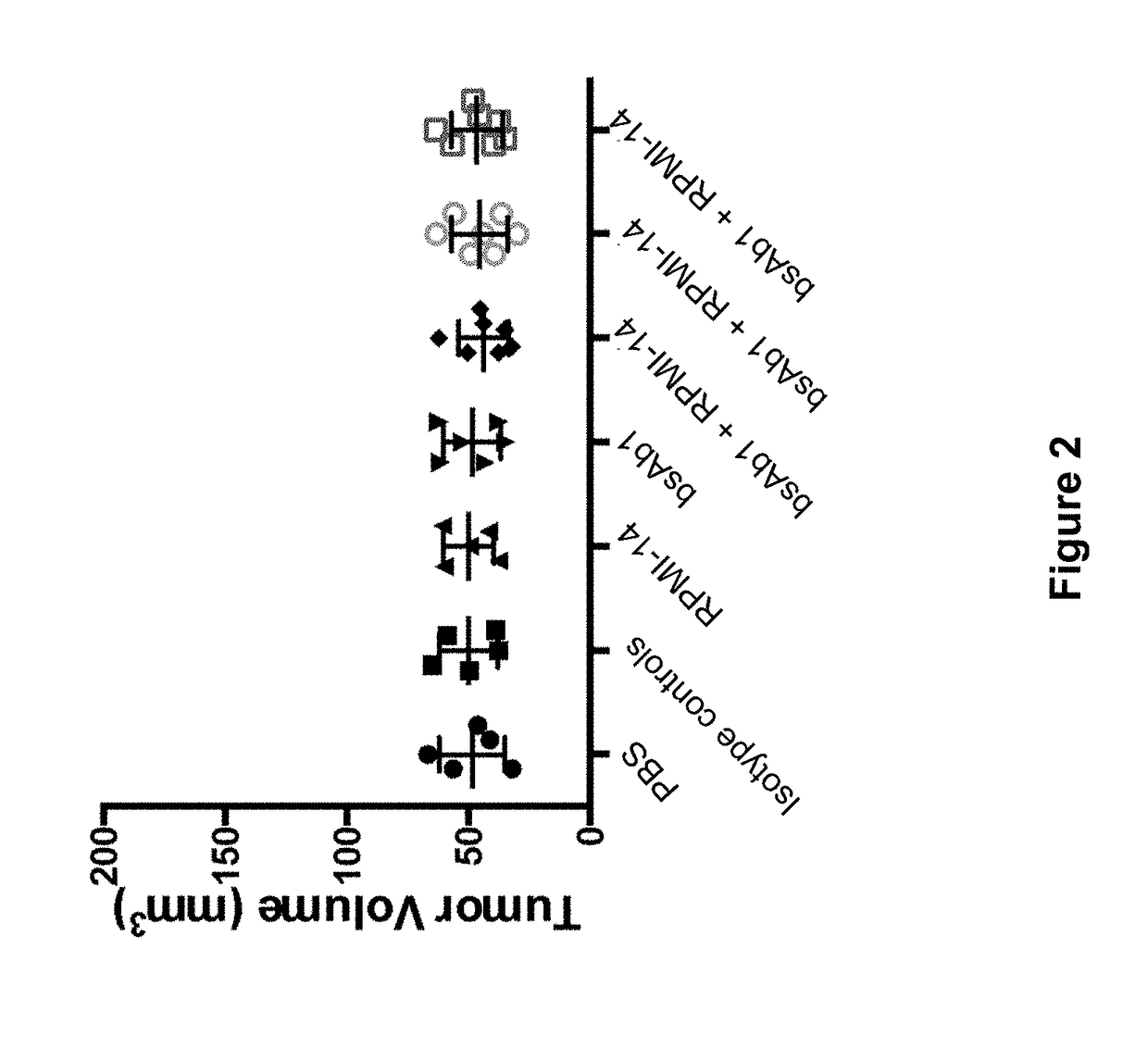
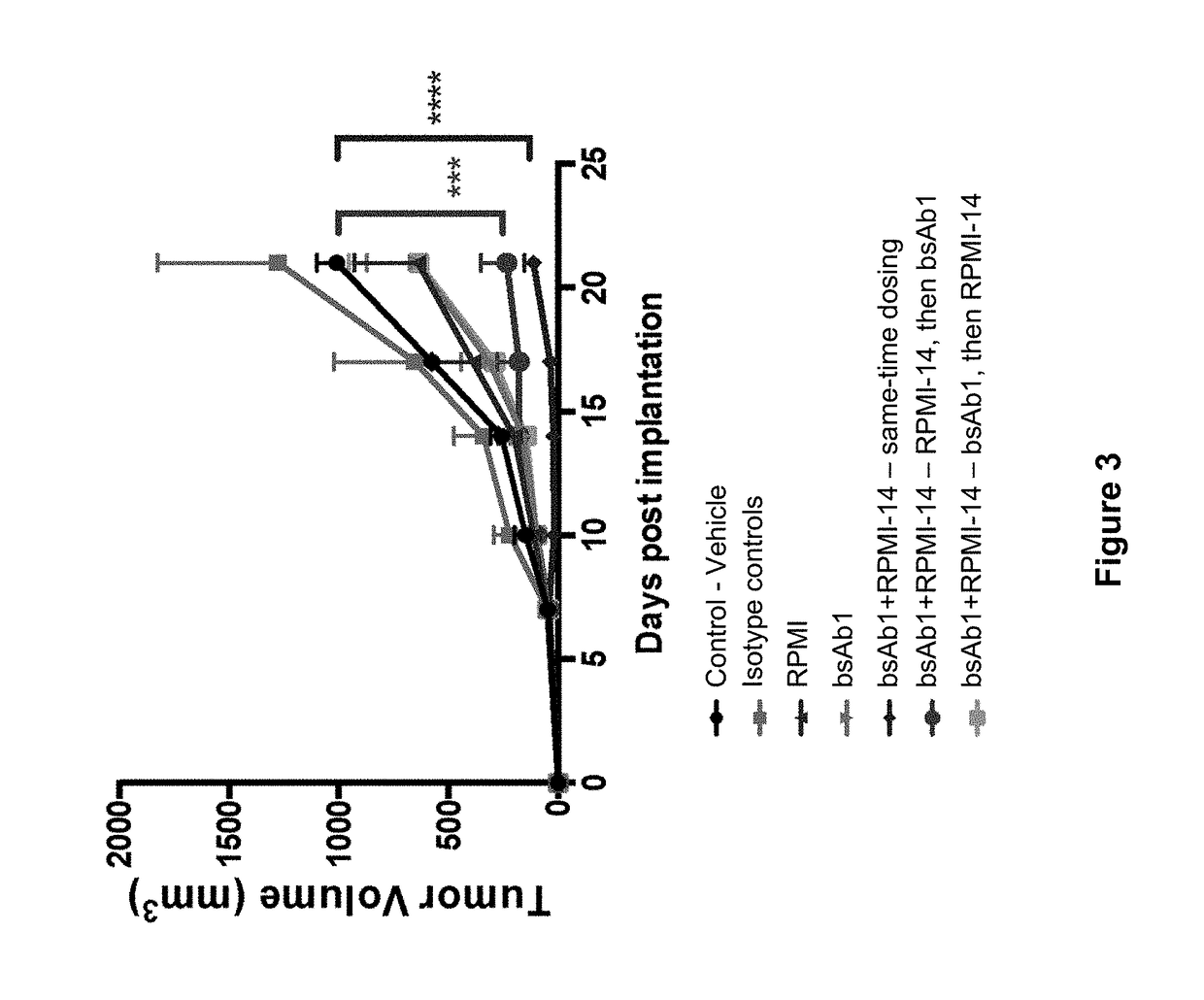
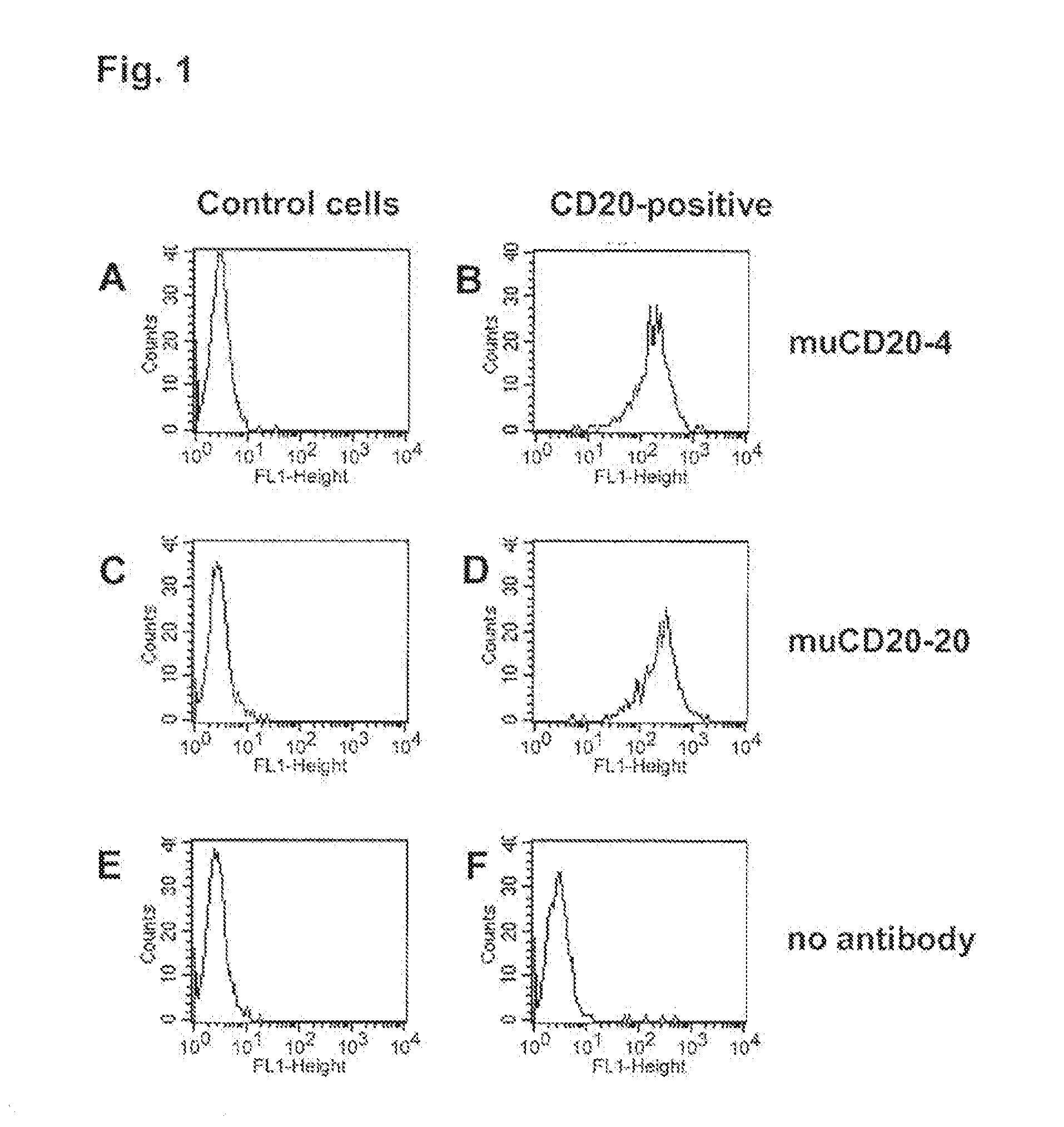
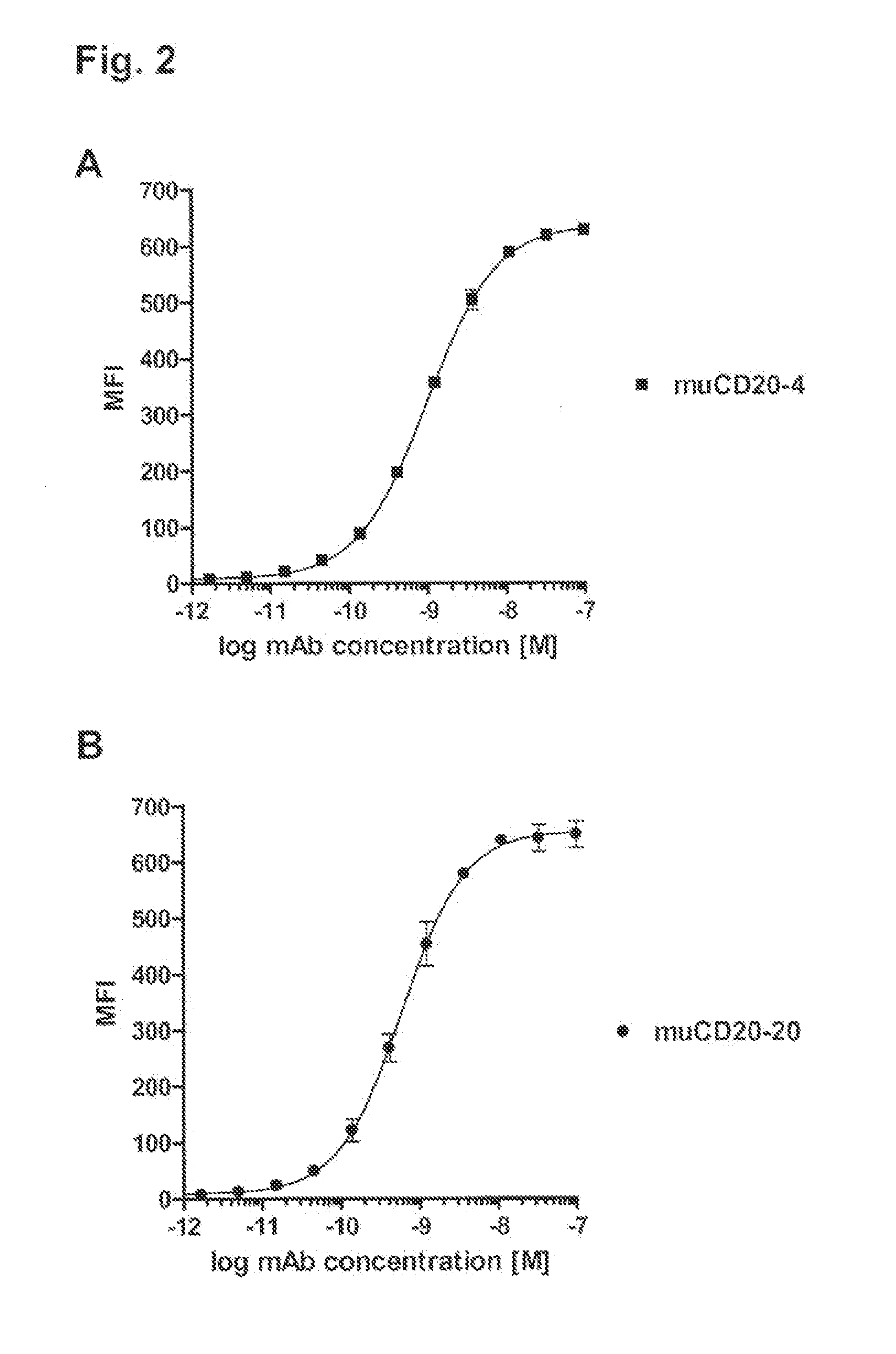
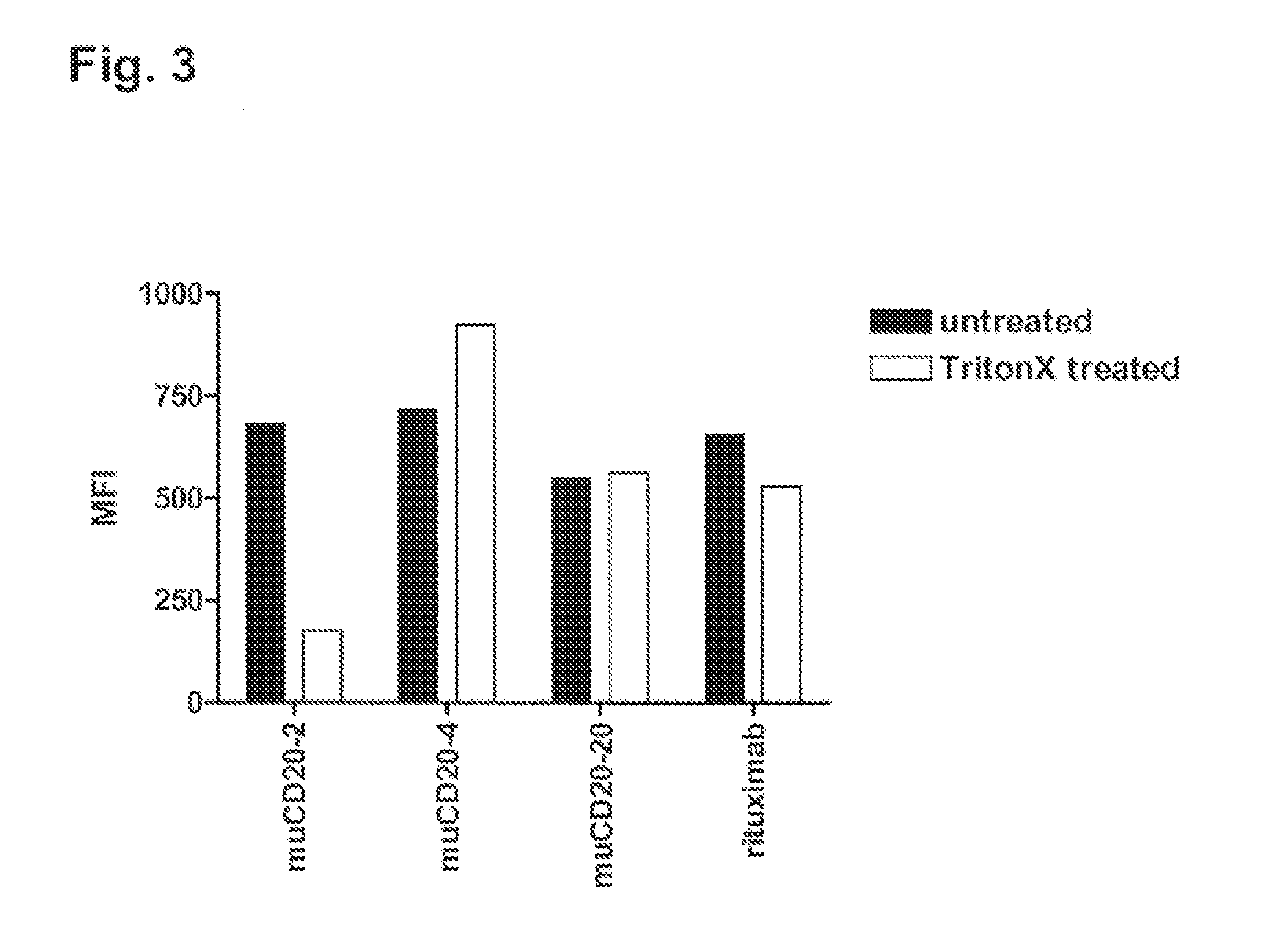
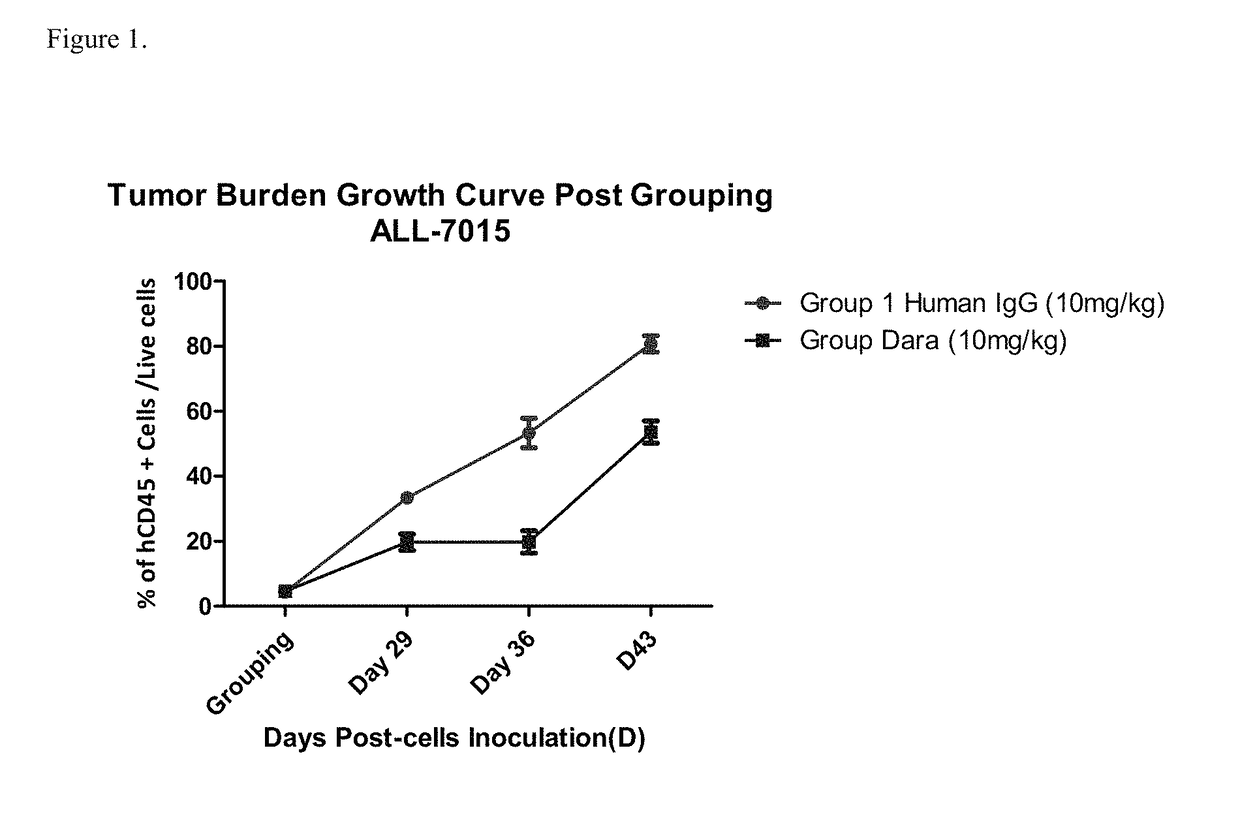
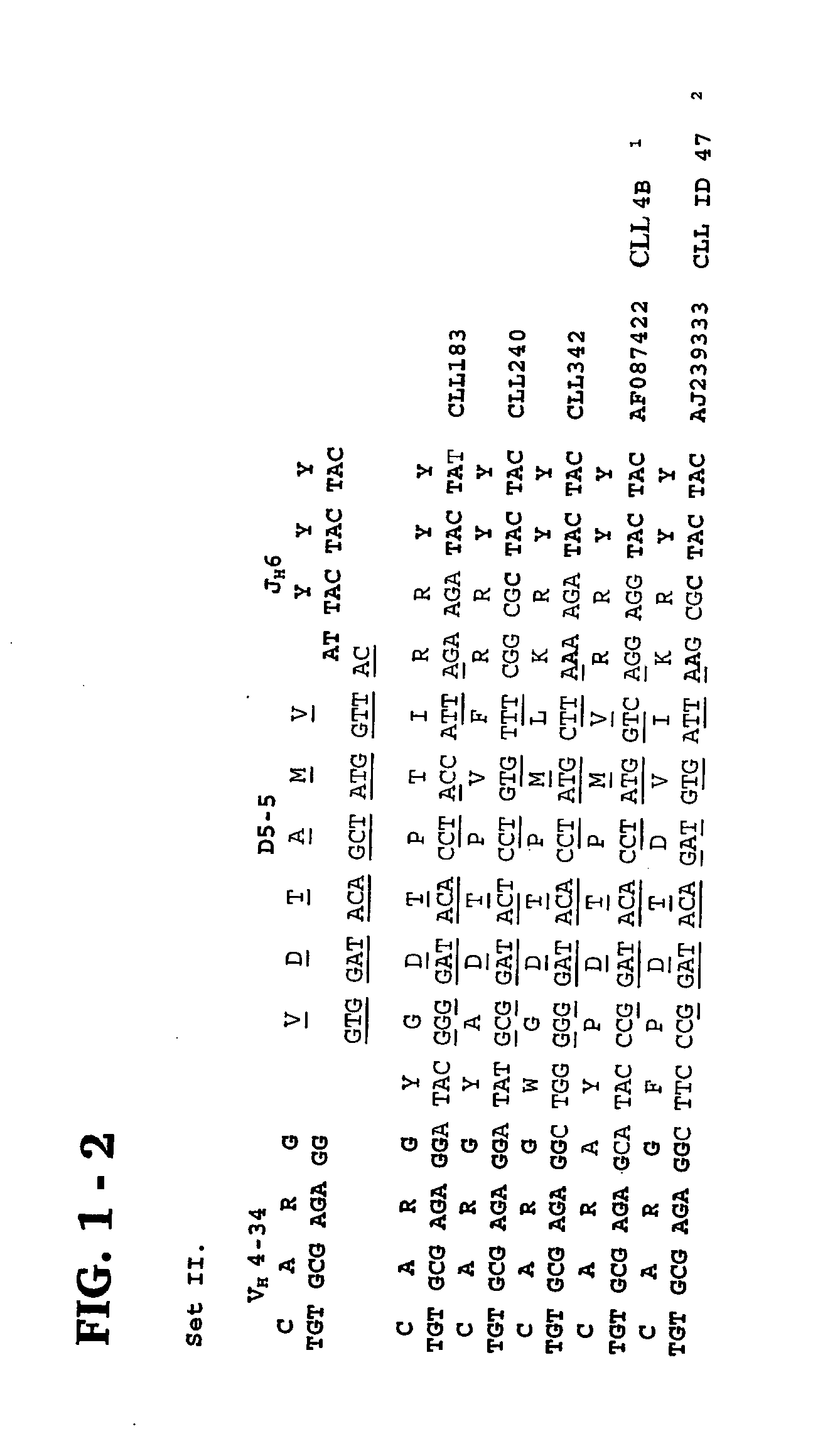
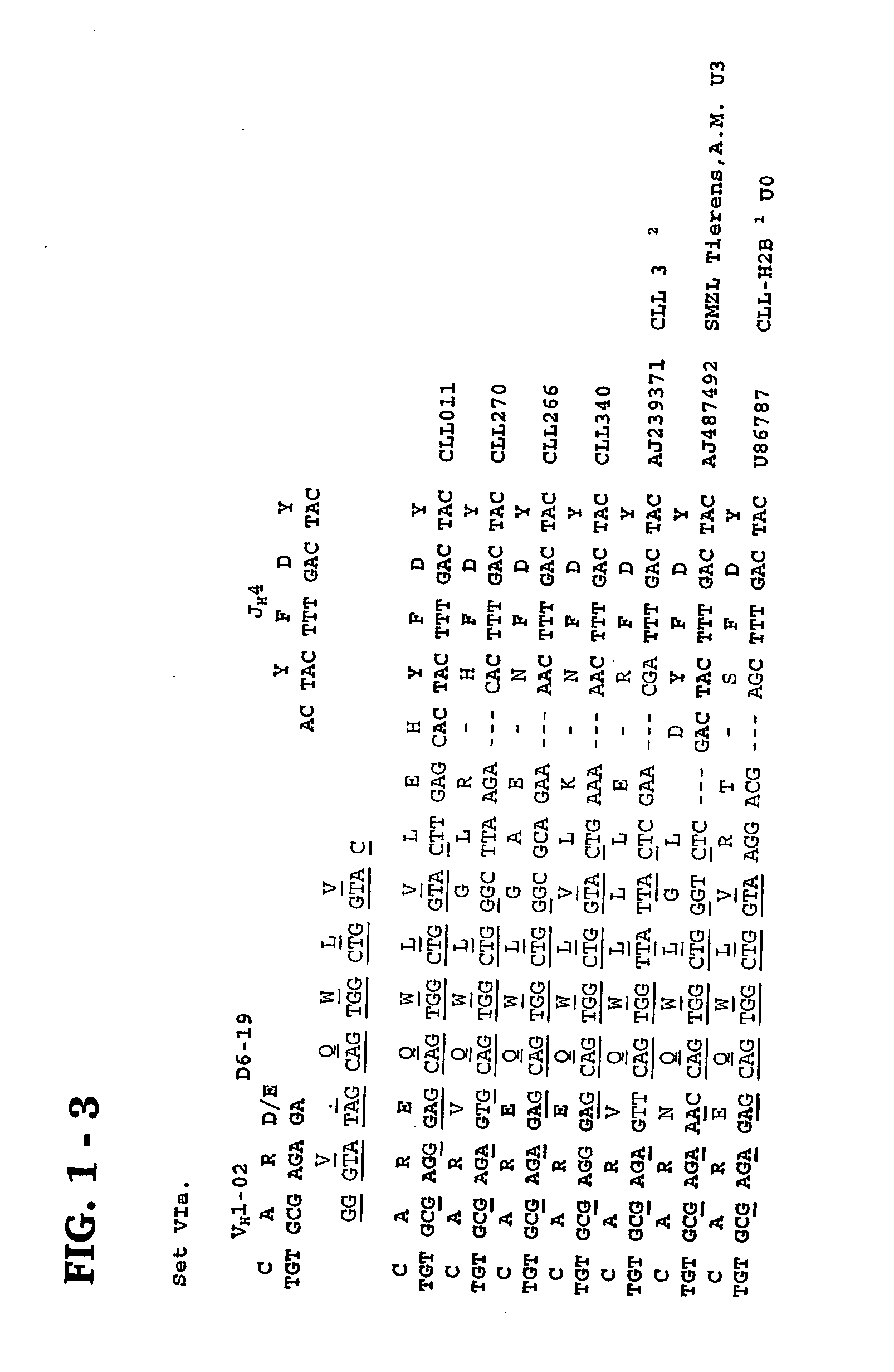
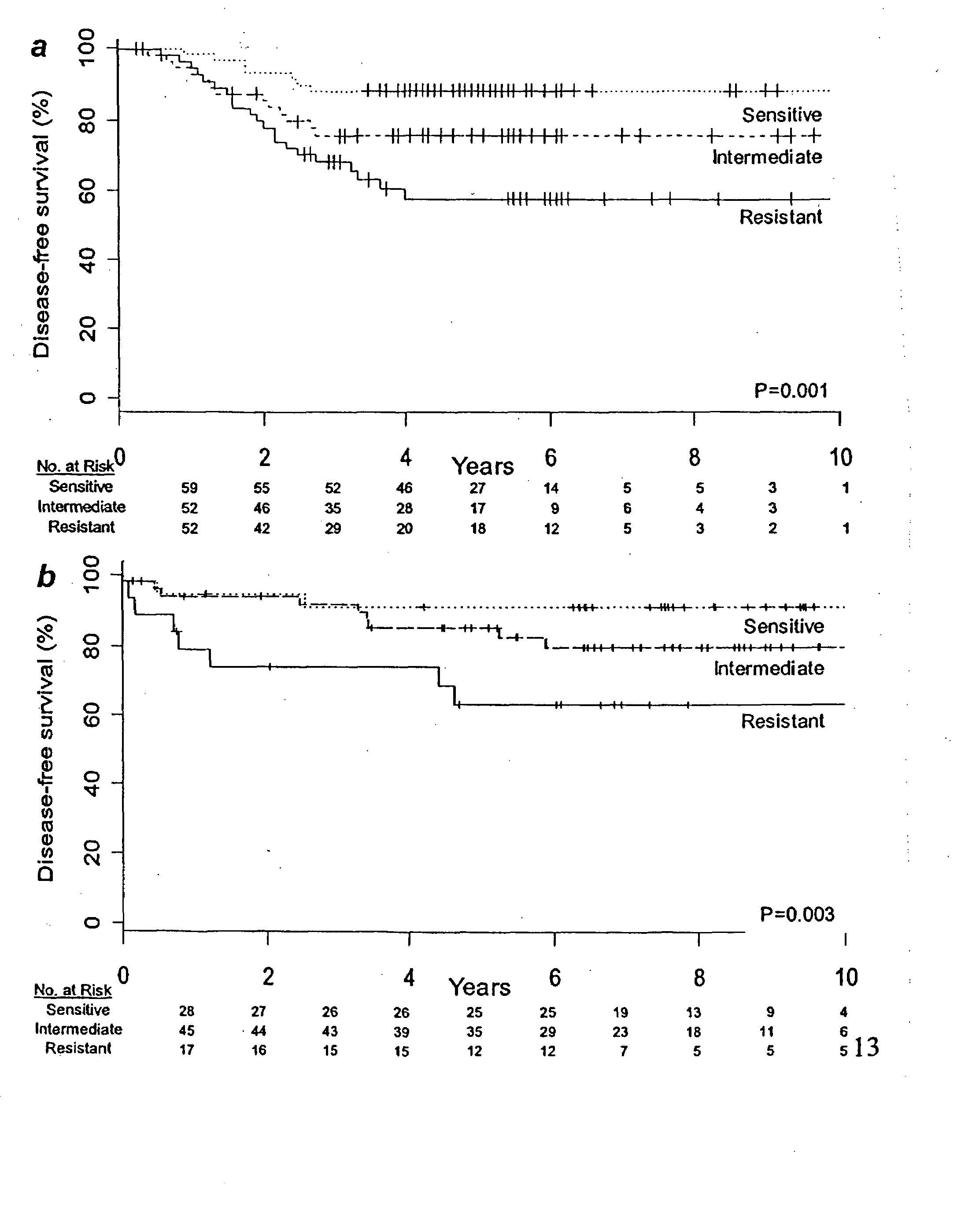
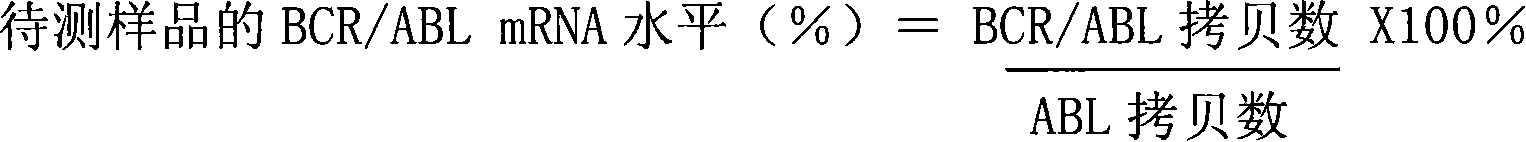Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
243 results about "Lymphoblastic Leukemia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Generation and application of universal T cells for B-ALL
InactiveUS20070036773A1Hinder recognitionEnhanced siRNA effectBiocideGenetic material ingredientsAntigenNatural Killer Cell Inhibitory Receptors
The present invention is directed to universal T cells and their use in treating diseases and other physiological conditions. More specifically, the present invention is directed to universal T cells and their use in treating treating B-lineage acute lymphoblastic leukemia (B-ALL) in particular and malignancy in general. The universal T cells contain (i) nucleic acid encoding a chimeric antigen receptor (CAR) to redirect their antigen specificity and effector function and (ii) nucleic acids encoding shRNA and / or siRNA molecules to down-regulate cell-surface expression of T cell classical HLA class I and / or II genes to avoid recognition by recipient T cells. The universal T cells may also contain a nucleic acid encoding a non-classical HLA gene, such as an HLA E gene to enforce expression of HLA E genes and / or an HLA G gene to enforce expression of HLA G genes, to avoid recognition by recipient NK cells. The universal T cells may further contain a nucleic acid encoding a selection-suicide gene.
Owner:CITY OF HOPE
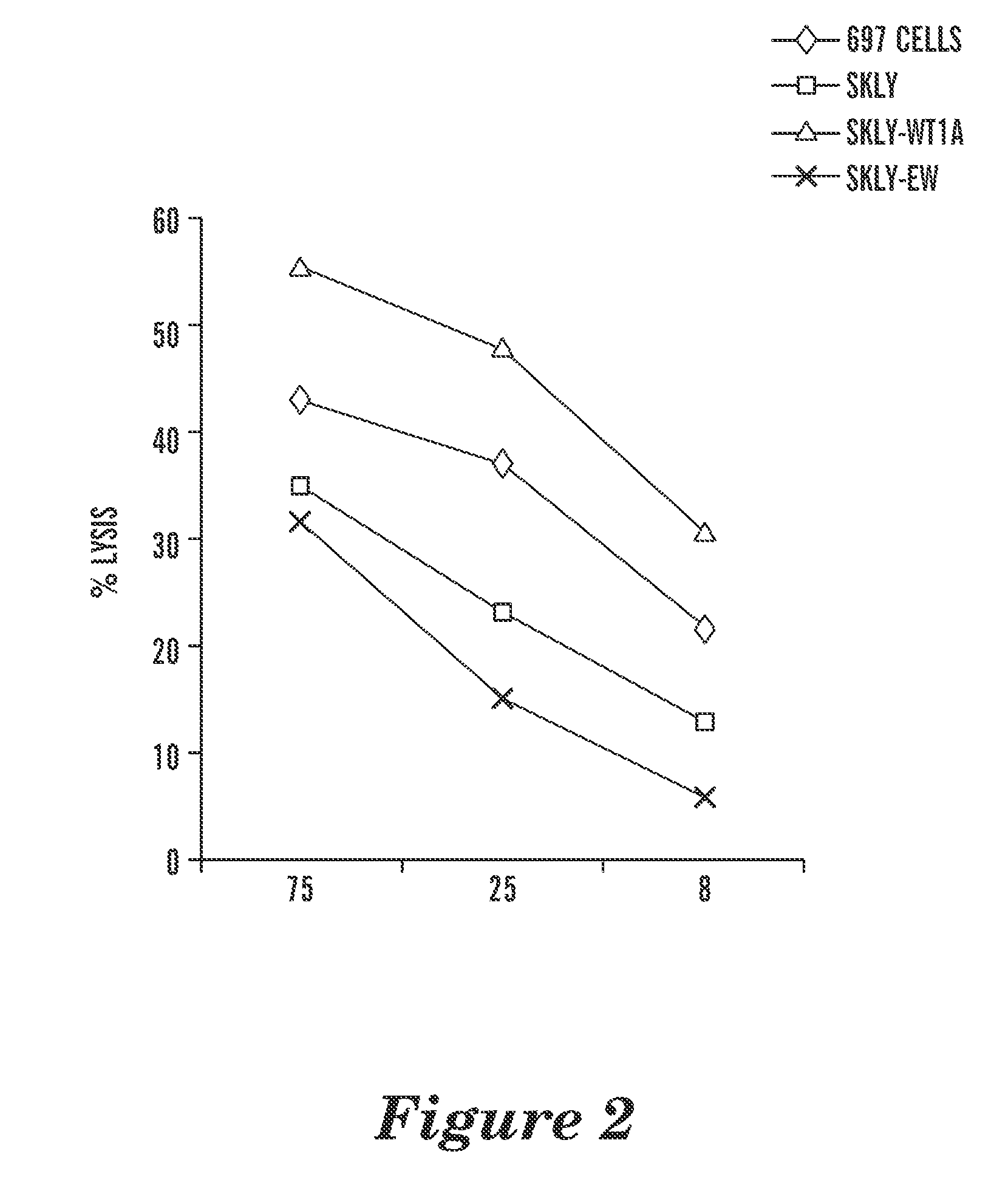
T cell receptor-like antibodies specific for a wti peptide presented by hla-a2
The present invention provides antigen binding proteins that specifically bind to Wilms' tumor protein (WT1), including humanized, chimeric and fully human antibodies against WT1, antibody fragments, chimeric antigen receptors (CARs), fusion proteins, and conjugates thereof. The antigen binding proteins and antibodies bind to HLA-A0201-restricted WT1 peptide. Such antibodies, fragments, fusion proteins and conjugates thereof are useful for the treatment of WT1 associated cancers, including for example, breast cancer, ovarian cancer, prostate cancer, chronic myelocytic leukemia, multiple myeloma, acute lymphoblastic leukemia (ALL), acute myeloid / myelogenous leukemia (AML) and myelodysplastic syndrome (MDS). In more particular embodiments, the anti-WT1 / A antibodies may comprise one or more framework region amino acid substitutions designed to improve protein stability, antibody binding and / or expression levels.
Owner:EUREKA THERAPEUTICS INC +1
Combination of Anti-PD-1 Antibodies and Anti-CD20/Anti-CD3 Antibodies to Treat Cancer
InactiveUS20170174779A1Growth inhibitionImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigenCD20
The present invention provides methods for treating, reducing the severity, or inhibiting the growth of cancer (e.g., a B-cell cancer such as Hodgkin's lymphoma or acute lymphoblastic leukemia). The methods of the present invention comprise administering to a subject in need thereof a therapeutically effective amount of an antibody or antigen-binding fragment thereof that specifically binds to programmed death 1 (PD-1) receptor in combination with a therapeutically effective amount of a bispecific antibody that specifically binds to CD20 and CD3.
Owner:REGENERON PHARM INC
Fc.gamma.RIIB-Specific Antibodies and Methods of Use Thereof
ActiveUS20080044429A1Good curative effectEnhanced effector functionSugar derivativesPeptide/protein ingredientsTreatment effectAntigen Binding Fragment
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA. The present invention also provides the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, as a single agent therapy for the treatment, prevention, management, or amelioration of a cancer, preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention.
Owner:MACROGENICS INC
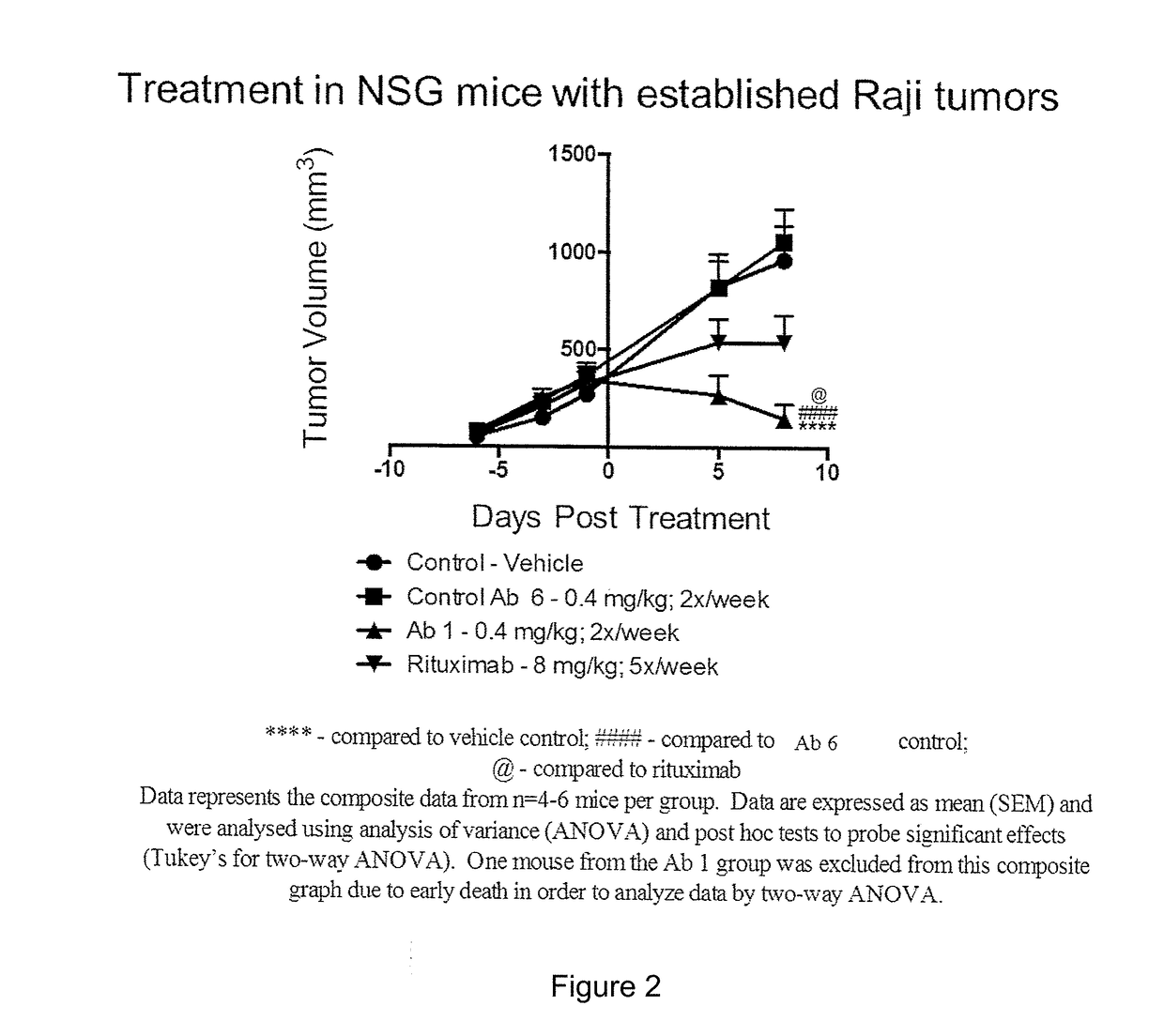
Bispecific Anti-CD20/Anti-CD3 Antibodies to Treat Acute Lymphoblastic Leukemia
The present invention provides methods for treating, reducing the severity, or inhibiting the growth of acute lymphoblastic leukemia. The methods of the present invention comprise administering to a subject in need thereof a therapeutically effective amount of a bispecific antibody that specifically binds to CD20 and CD3.
Owner:REGENERON PHARM INC
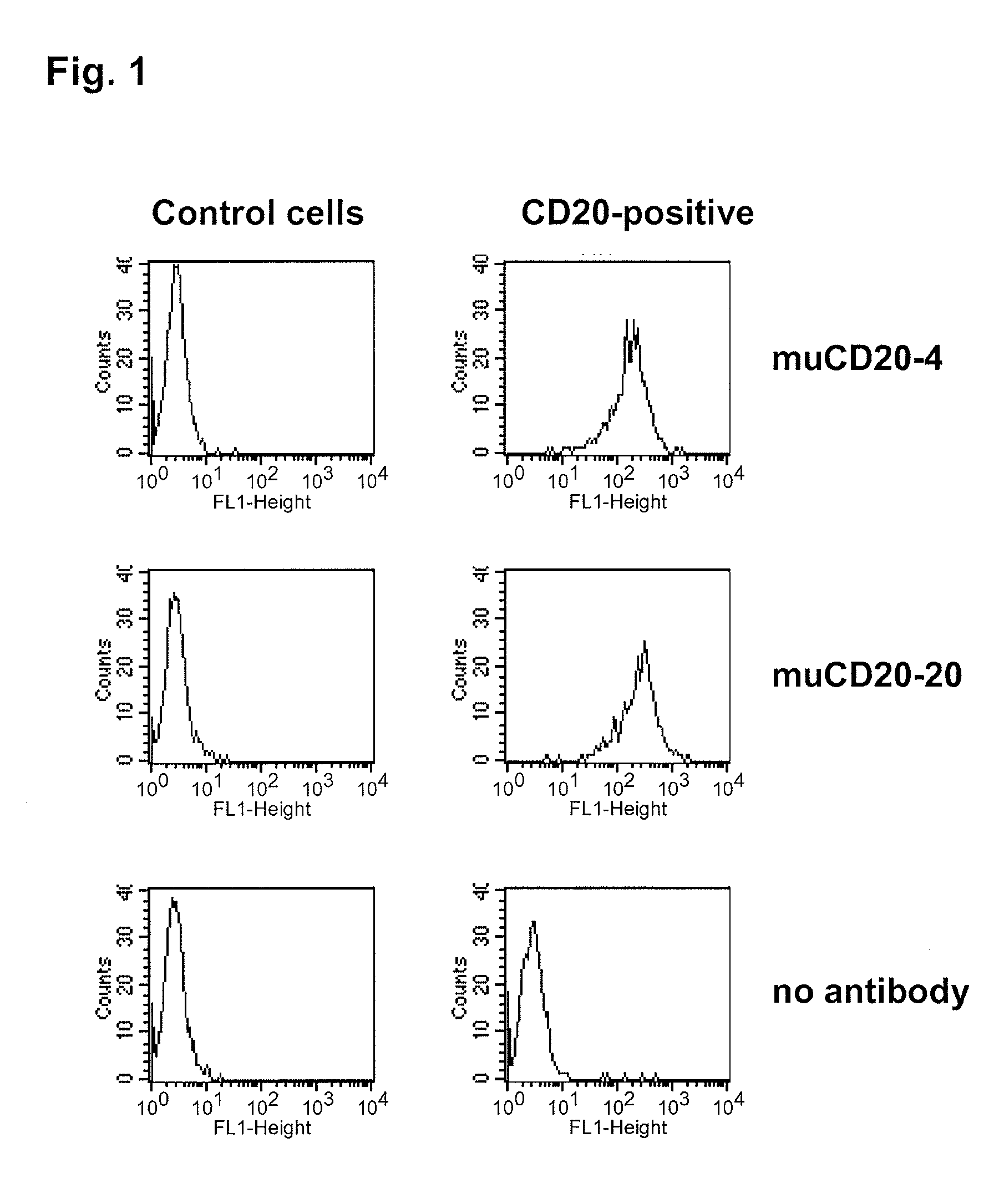
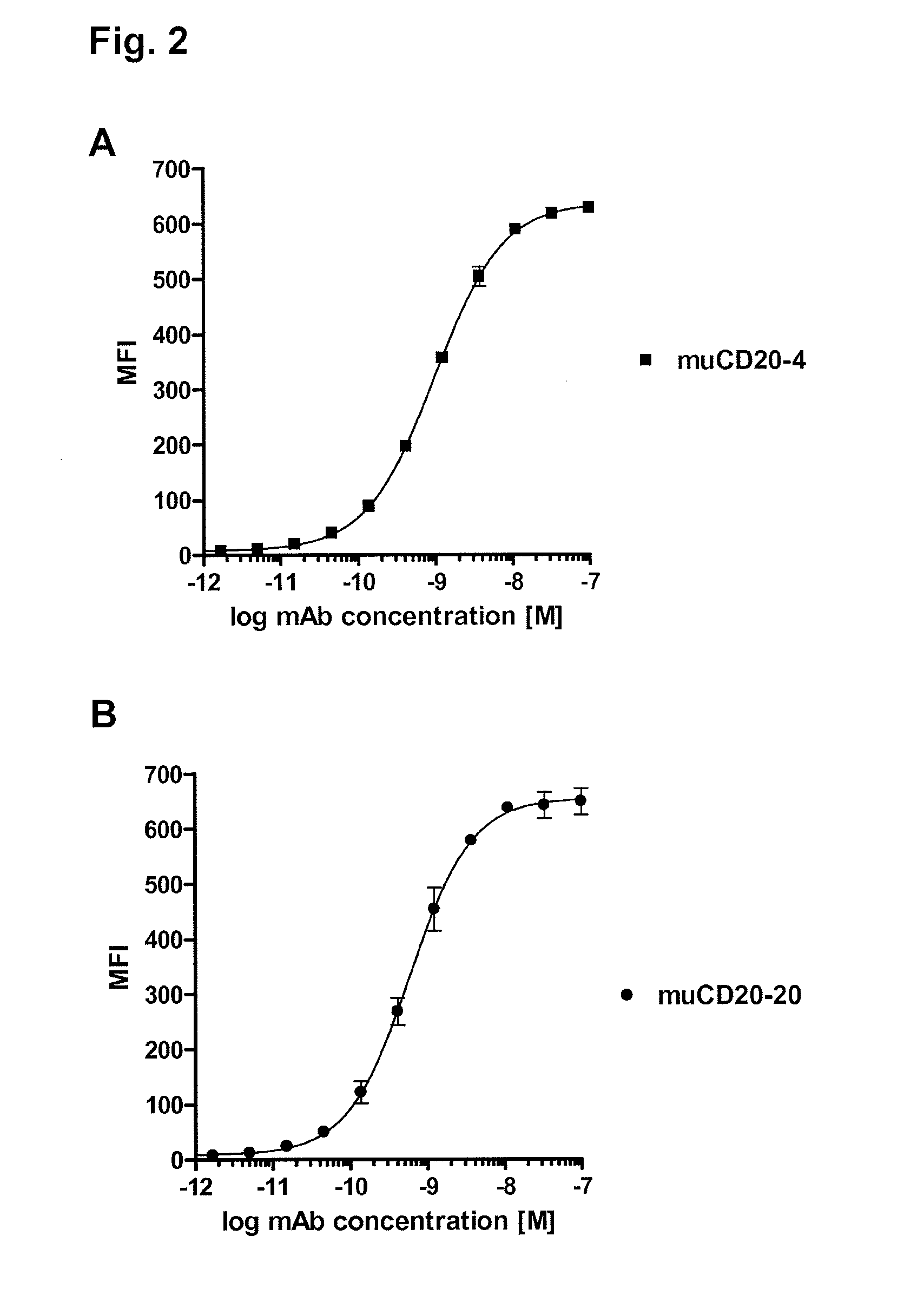
Cd20 antibodies and uses thereof
CD20 is a transmembrane protein of the tetra-spanin family expressed on the surface of B-cells and has been found on B-cells from peripheral blood as well as lymphoid tissues. CD20 expression persists from the early pre-B cell stage until the plasma cell differentiation stage. Conversely, it is not found on hematopoietic stem cells, pro-B cells, differentiated plasma cells or non-lymphoid tissues. In addition to expression in normal B-cells, CD20 is expressed in B-cell derived malignancies such as non-Hodgkin's lymphoma (NHL) and B-cell chronic lymphocytic leukemia (CLL). CD20 expressing cells are known to play a role in other diseases and disorders, including inflammation. The present invention includes anti-CD20 antibodies, forms and fragments, having superior physical and functional properties; immunoconjugates, compositions, diagnostic reagents, methods for inhibiting growth, therapeutic methods, improved antibodies and cell lines; and polynucleotides, vectors and genetic constructs encoding same.
Owner:IMMUNOGEN INC
Biomarkers for Diagnosis and Treatment of Chronic Lymphocytic Leukemia
InactiveUS20110190157A1Improve clinical outcomesMicrobiological testing/measurementLibrary screeningMolecular classificationHer Disease
A molecular classification procedure based on activity levels of modules in protein networks, wherein the proteins are biomarkers for chronic lymphocytic leukemia (CLL), and method for use of the subnetworks to distinguish between patients at low or high risk of progression of their disease.
Owner:RGT UNIV OF CALIFORNIA
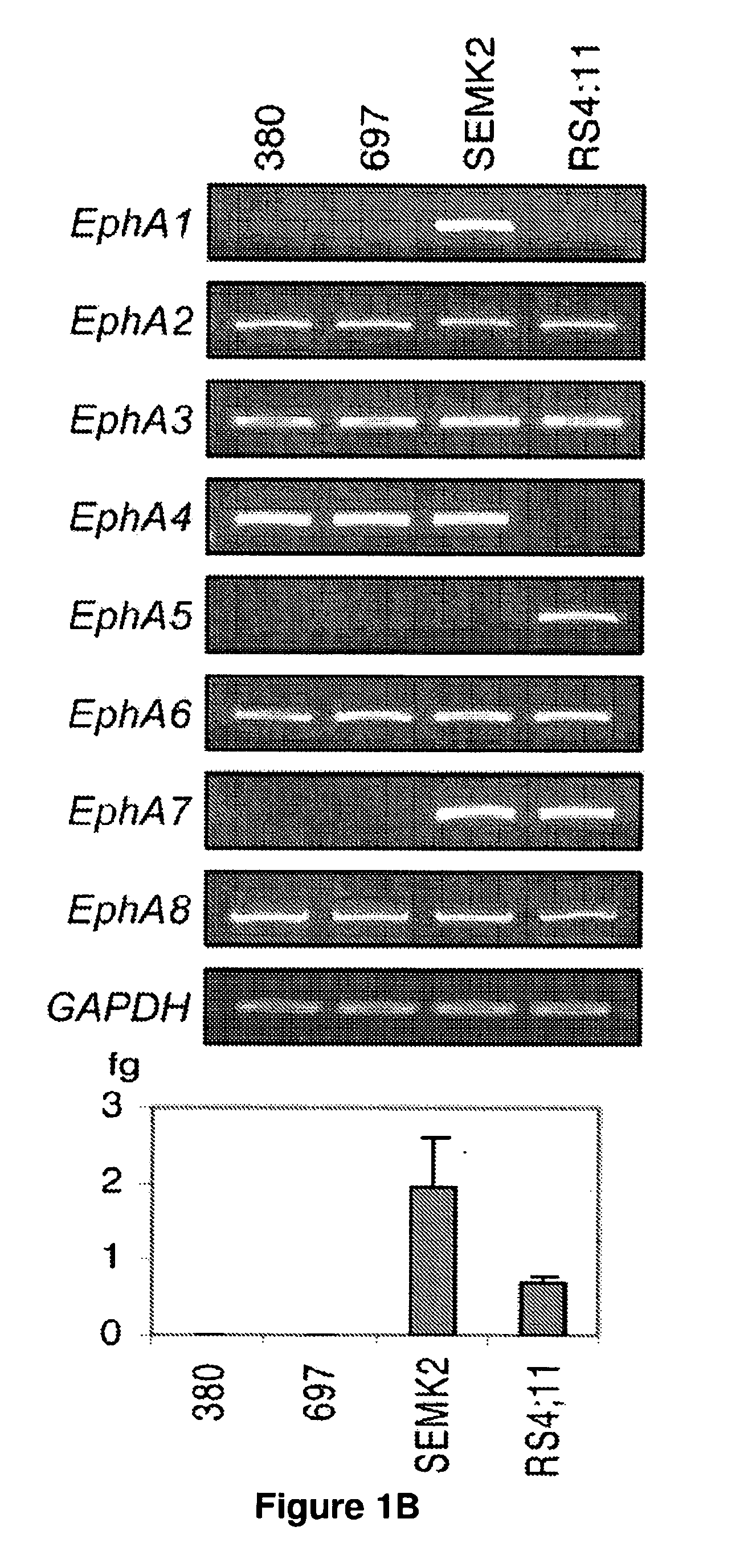
Methods and Compositions for Inducing Deregulation of EPHA7 and ERK Phosphorylation in Human Acute Leukemias
InactiveUS20100317610A1Sufficient amountReduce severityBiocideDisease diagnosisAcute leukemiaLymphoblastic Leukemia
Methods for assessing a pathological condition in a subject include measuring one or more markers where a difference is indicative of acute lymphoblastic leukemia (ALL) or a predisposition to ALL, uses and compositions are disclosed.
Owner:THE OHIO STATE UNIV RES FOUND
T cell receptor-like antibodies specific for a WT1 peptide presented by HLA-A2
The present invention provides antigen binding proteins that specifically bind to Wilms' tumor protein (WT1), including humanized, chimeric and fully human antibodies against WT1, antibody fragments, chimeric antigen receptors (CARs), fusion proteins, and conjugates thereof. The antigen binding proteins and antibodies bind to HLA-A0201-restricted WT1 peptide. Such antibodies, fragments, fusion proteins and conjugates thereof are useful for the treatment of WT1 associated cancers, including for example, breast cancer, ovarian cancer, prostate cancer, chronic myelocytic leukemia, multiple myeloma, acute lymphoblastic leukemia (ALL), acute myeloid / myelogenous leukemia (AML) and myelodysplastic syndrome (MDS). In more particular embodiments, the anti-WT1 / A antibodies may comprise one or more framework region amino acid substitutions designed to improve protein stability, antibody binding and / or expression levels.
Owner:EUREKA THERAPEUTICS INC +1
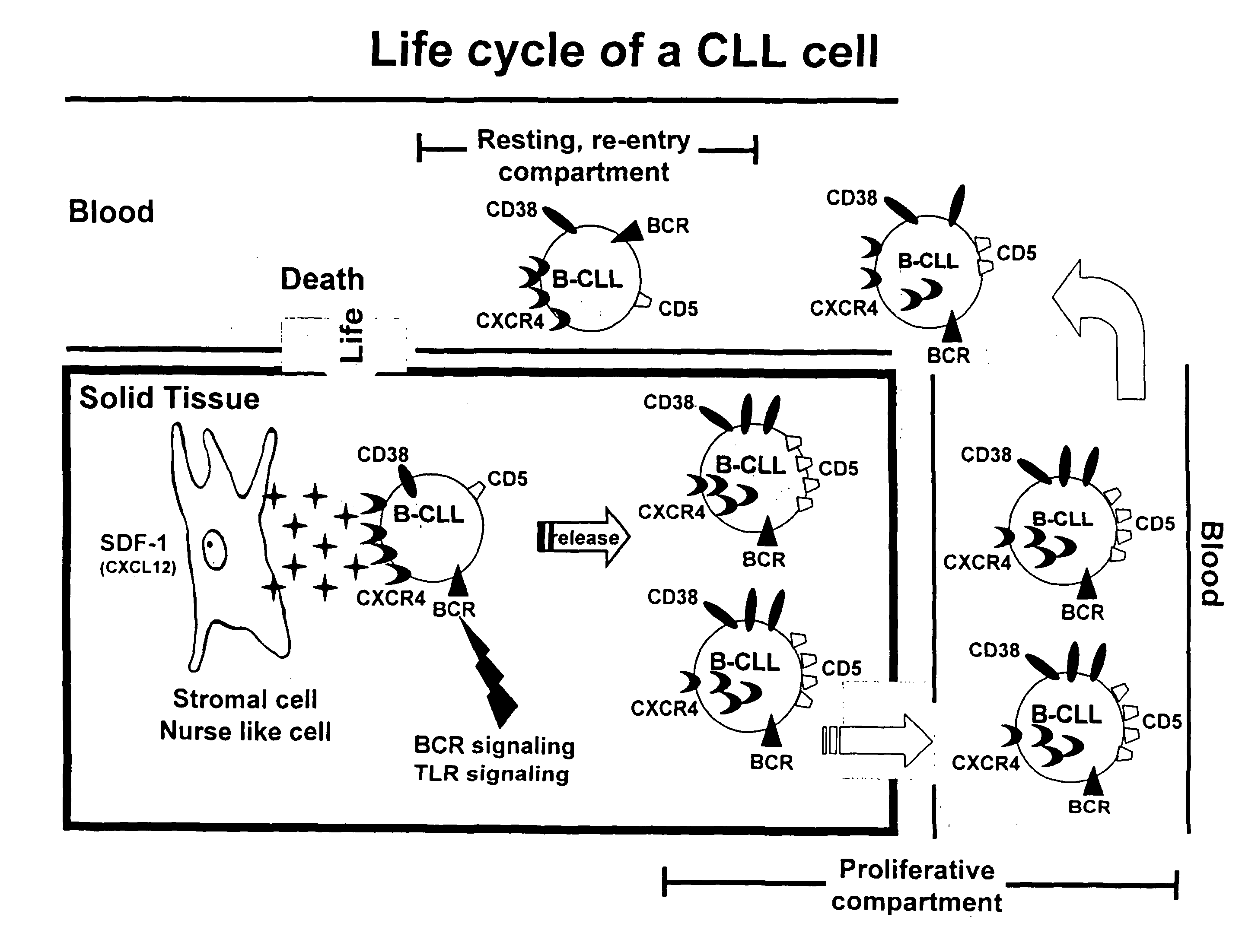
Method for treating chronic lymphocytic leukemia
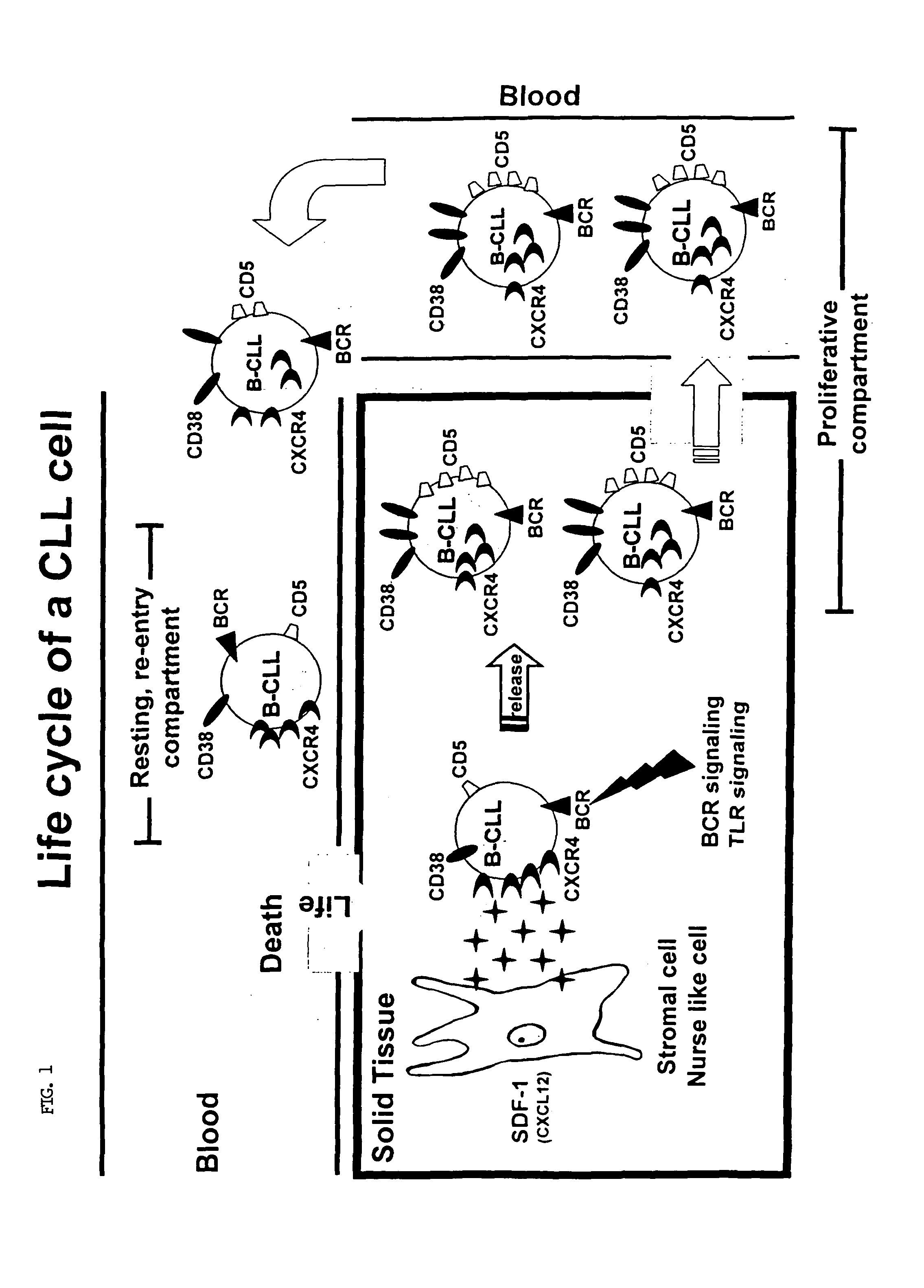
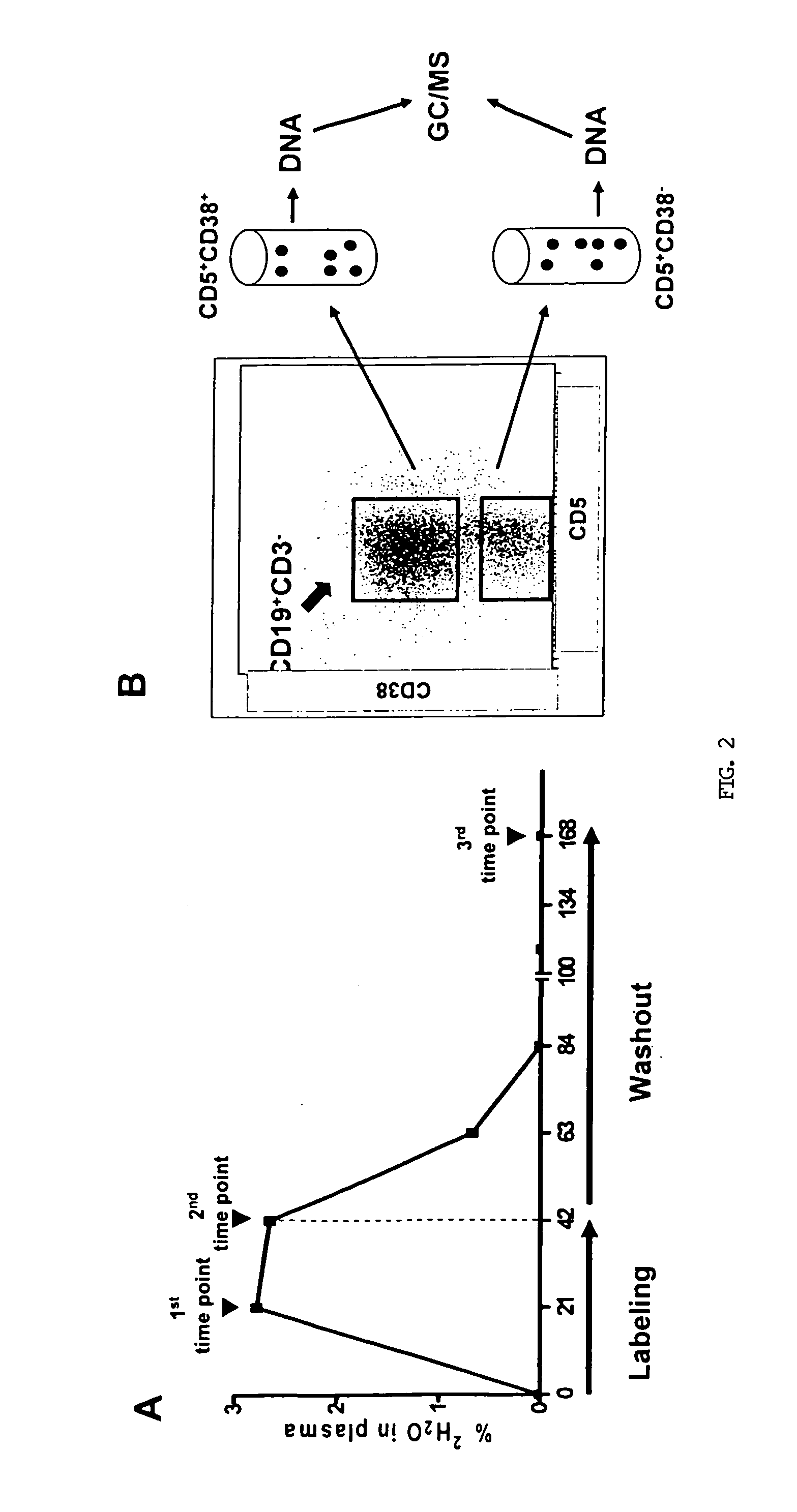
The present invention provides a method for treating a subject having chronic lymphocytic leukemia (CLL) comprising administering to the subject one or more agents that target cell surface membrane antigens expressed preferentially on cells of the proliferative compartment of a CLL clone of the subject to treat chronic lymphocytic leukemia in the subject. The present invention also provides a method for treating a subject having chronic lymphocytic leukemia comprising administering to the subject one or more agents that target cell surface membrane antigens expressed preferentially on cells of the “resting re-entry compartment” to treat chronic lymphocytic leukemia in the subject.
Owner:THE FEINSTEIN INST FOR MEDICAL RES
DNA methylation biomarkers in lymphoid and hematopoietic malignancies
InactiveUS20090264306A1Guaranteed maximum utilizationImprove the detection rateMicrobiological testing/measurementLibrary screeningDNA methylationLymphocytic cell
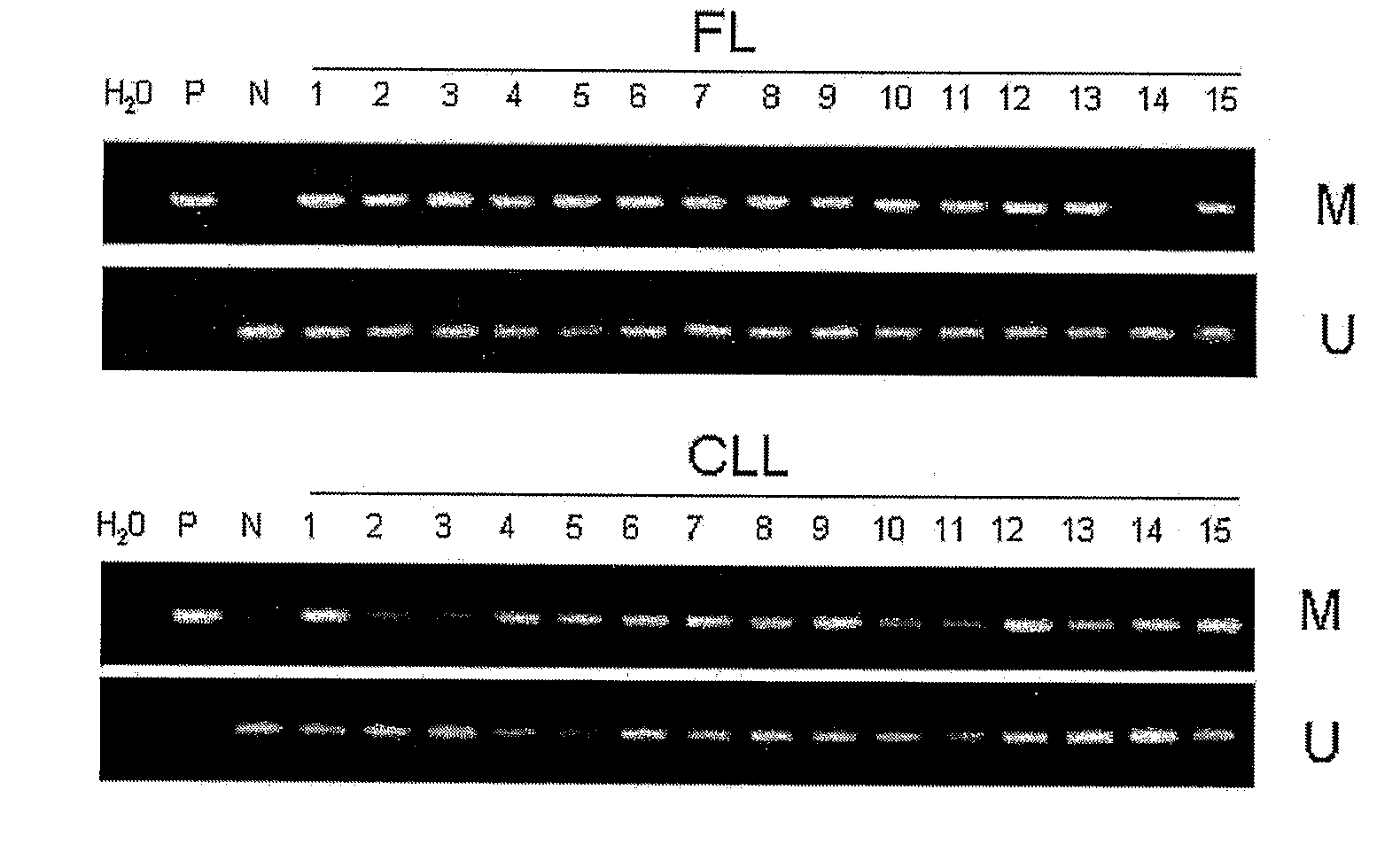
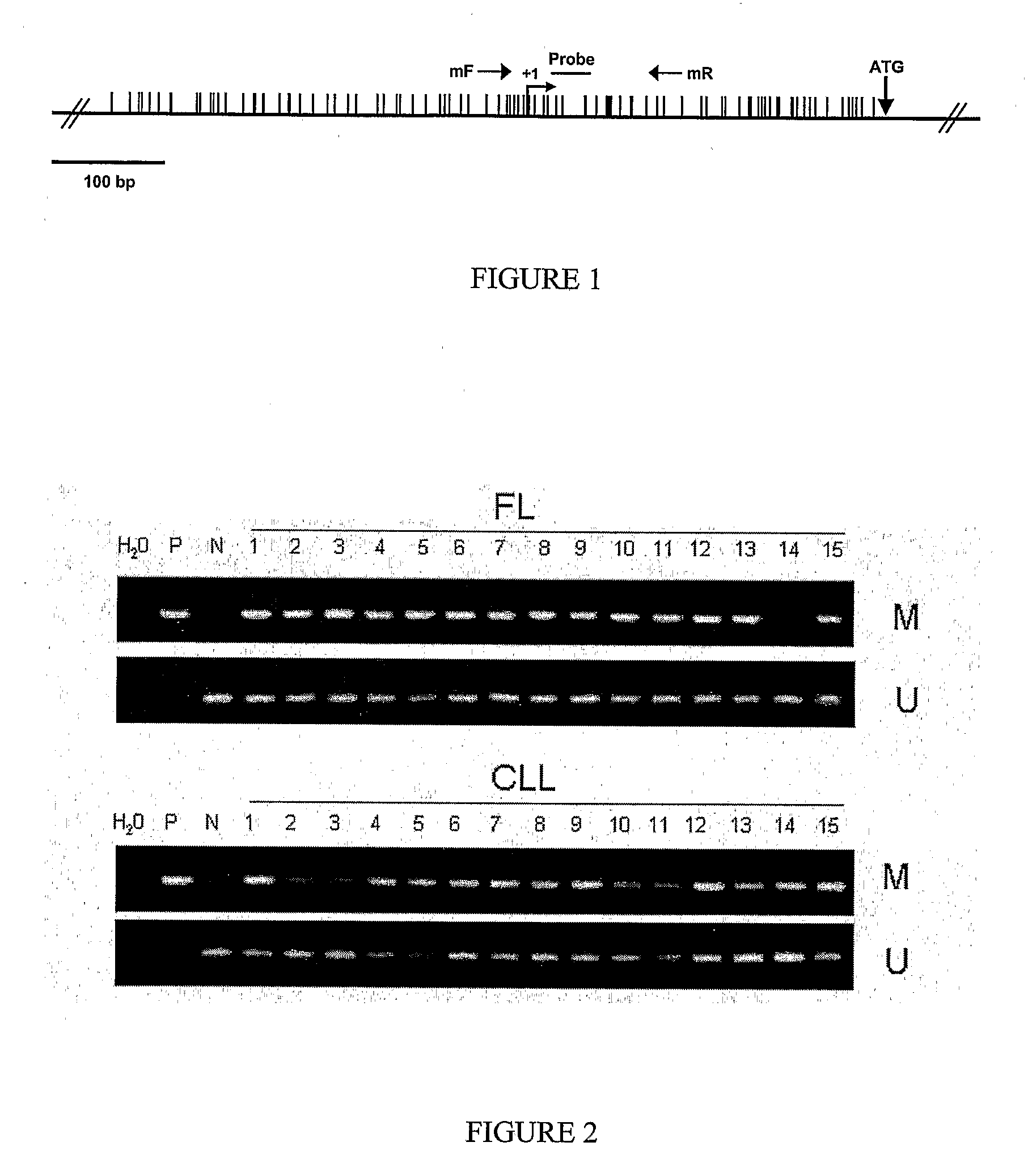
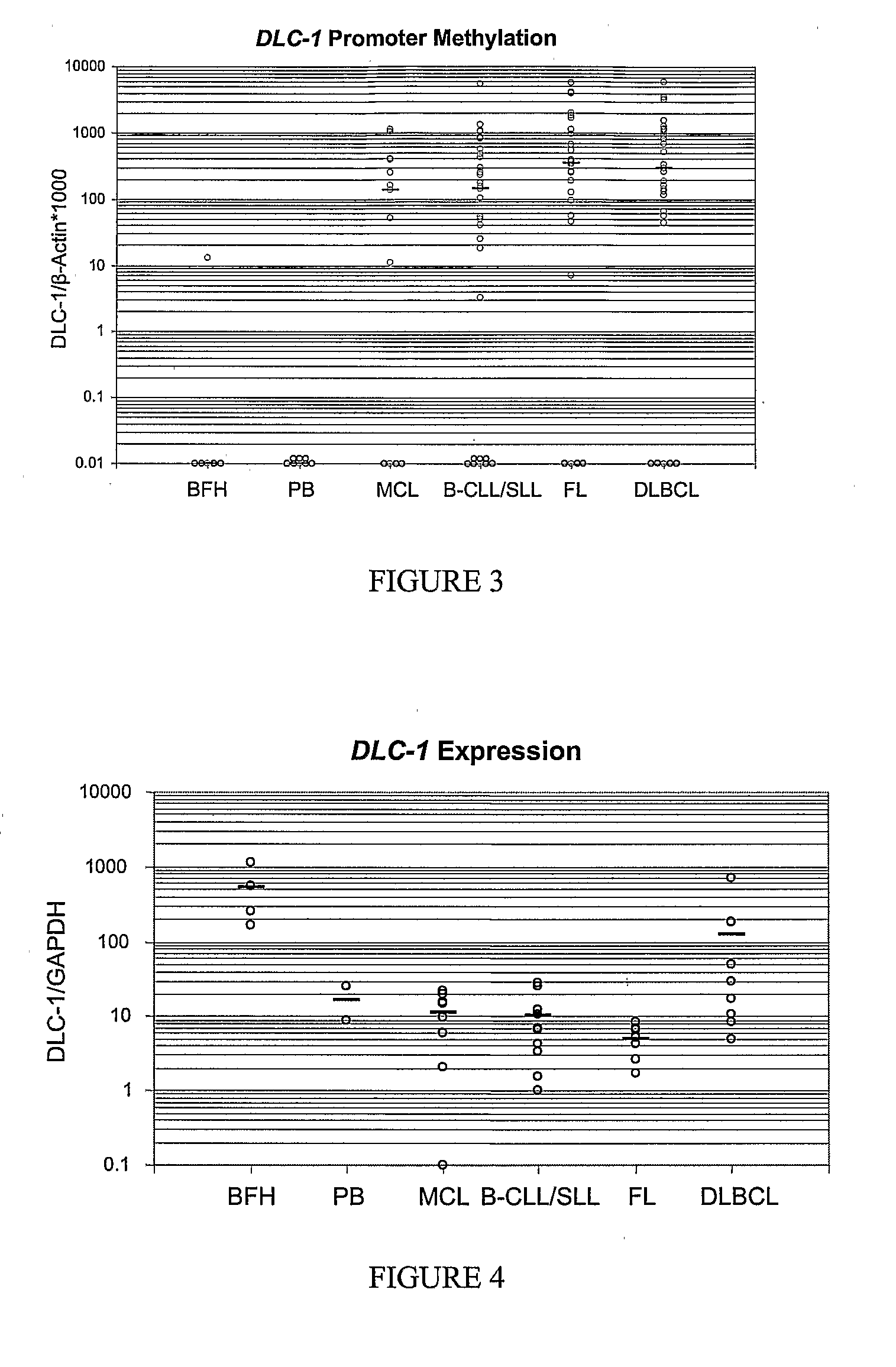
Differential Methylation Hybridization (DMH) was used to identify novel methylation markers and methylation profiles for hematopoieetic malignancies, leukemia, lymphomas, etc. (e.g., non-Hodgkin's lymphomas (NHL), small B-cell lymphomas (SBCL), diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), mantle cell lymphoma (MCL), B-cell chronic lymphocytic leukemia / small lymphocytic lymphoma (B-CLL / SLL), chronic lymphocytic leukemia (CLL), multiple myeloma (MM), acute myelogenous leukemia (AML), acute lymphoblastic leukemia (ALL), etc.). Particular aspects provide novel biomarkers for NHL and subtypes thereof (e.g., MCL, B-CLL / SLL, FL, DLBCL, etc.), AML, ALL and MM, and further provide non-invasive tests (e.g. blood tests) for lymphomas and leukemias. Additional aspects provide markers for diagnosis, prognosis, monitoring responses to therapies, relapse, etc., and further provide targets and methods for therapeutic demethylating treatments. Further aspects provide cancer staging markers, and expression assays and approaches comprising idealized methylation and / or patterns” (IMP and / or IEP) and fusion of gene rankings.
Owner:UNIVERSITY OF MISSOURI
Methods for treating hematological disorders through inhibition of DNA methylation and histone deacetylase
InactiveUS20050159347A1Address bad outcomesReduce dosageAntibacterial agentsBiocideCyclic peptideHydroxamic acid
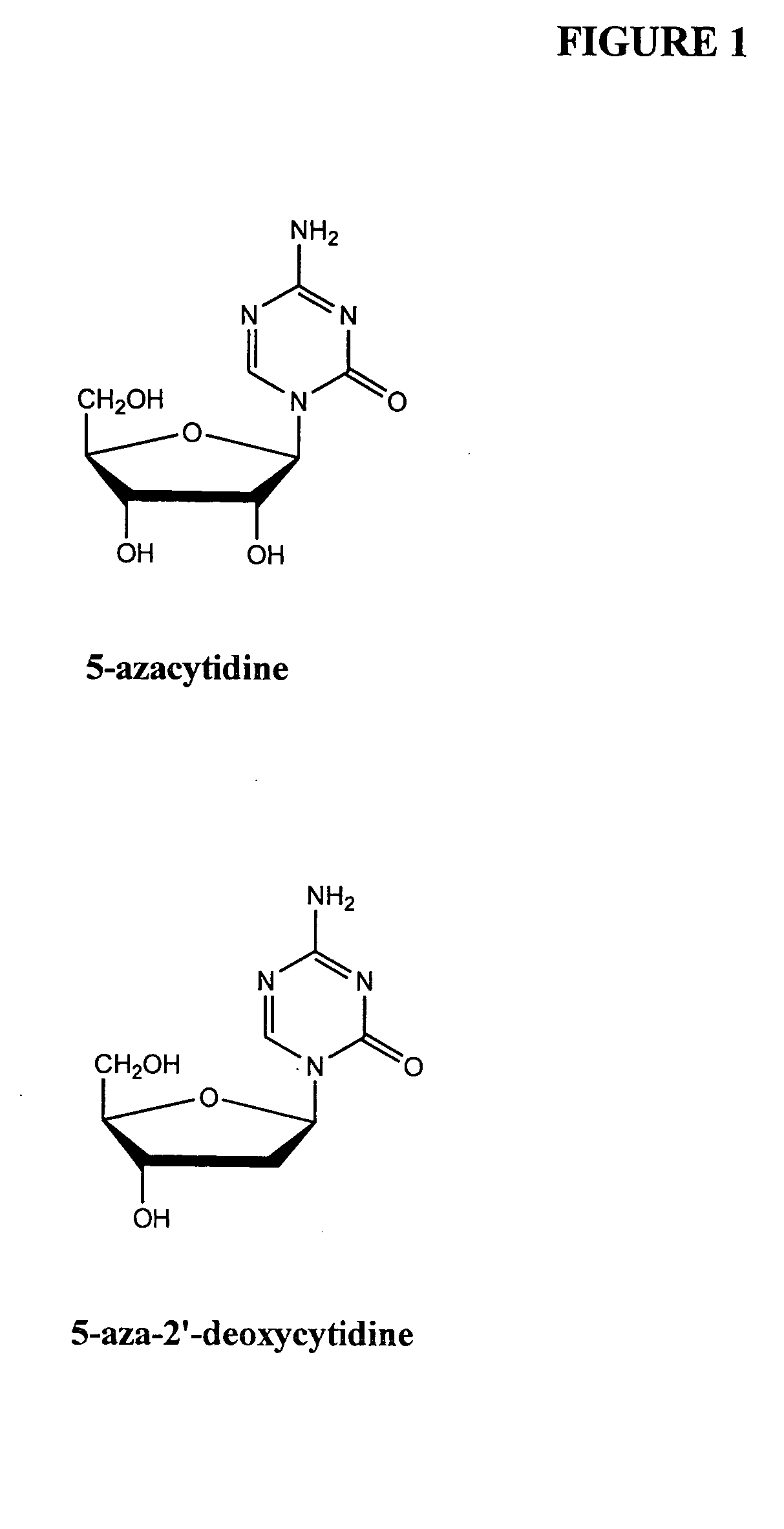
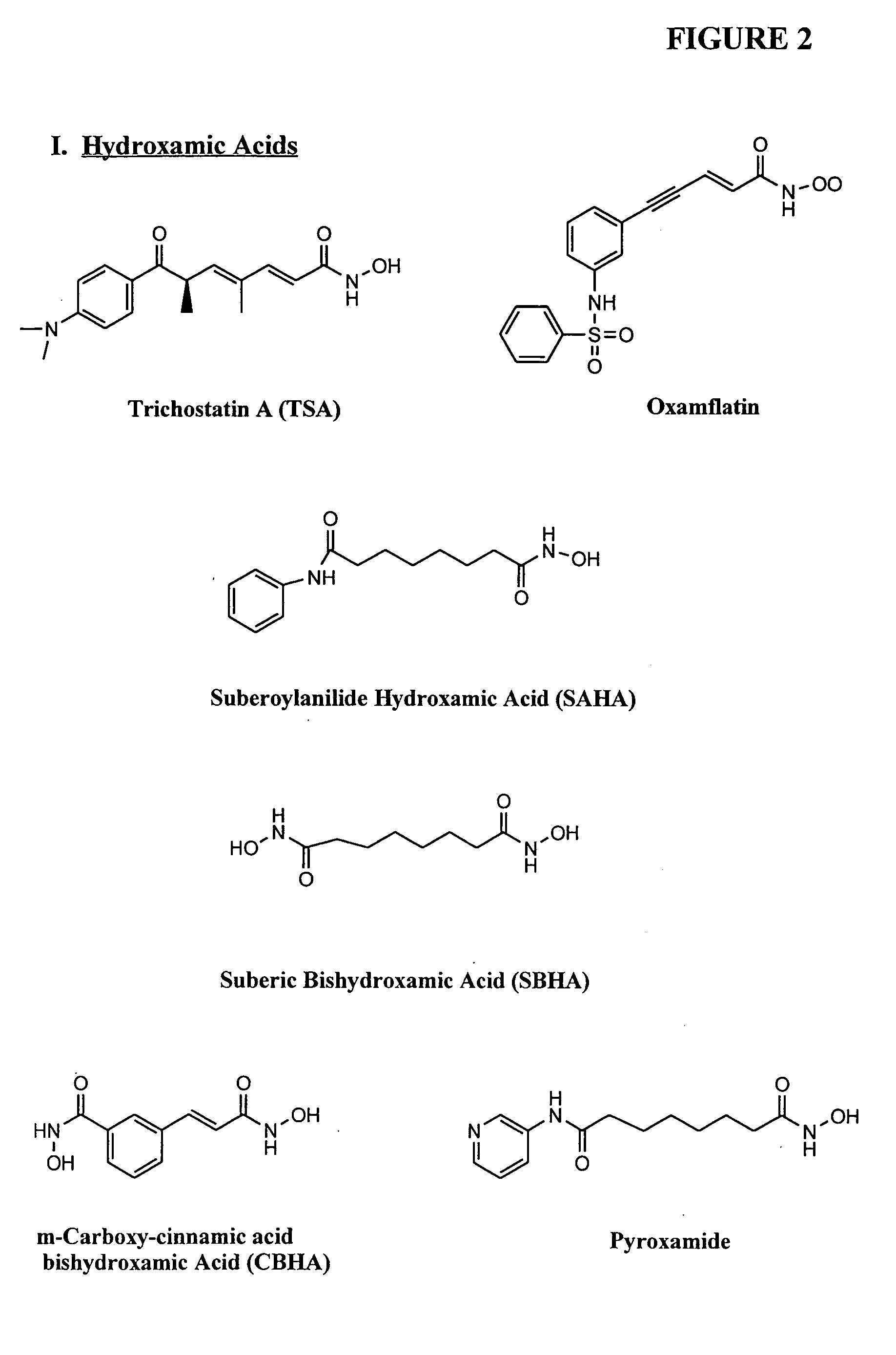
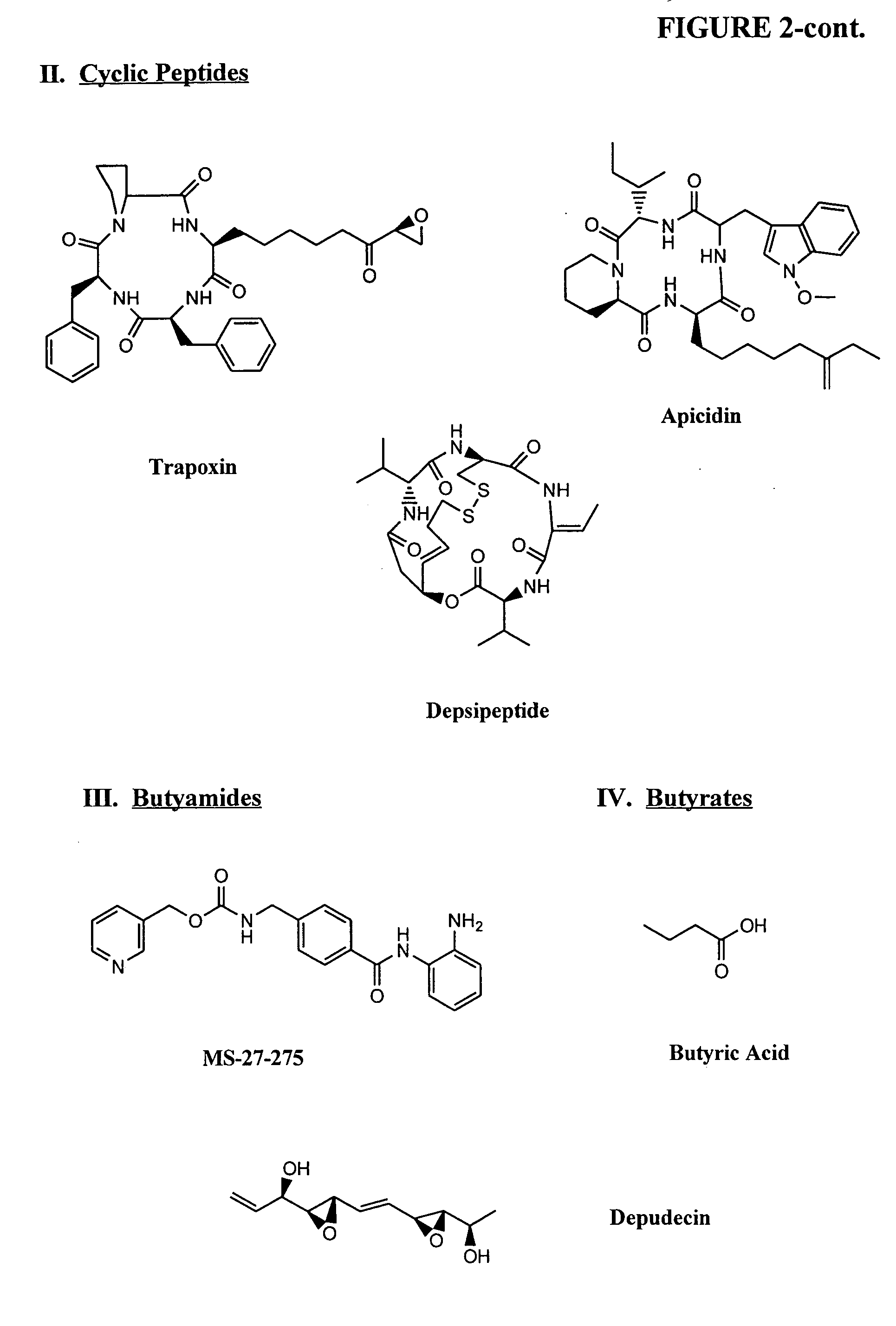
Methods are provided for treating hematological disorders by inhibition of DNA hypomethylation and histone deacetylase. Such disorders include, for example, acute promyelocytic leukemia, acute lymphoblastic leukemia, chronic myelogenous leukemia, myelodysplastic syndromes, and sickle cell anemia. The methods comprise: administering to a patient suffering from the disease a therapeutically effective amount of a DNA methylation inhibitor such as a cysteine analog such as decitabine, in combination with an effective amount of histone deacetylase inhibitor such as hydroxamic acid, cyclic peptide, benzamide, butyrate, and depudecin.
Owner:SUPERGEN
Compositions and Methods for Differential Diagnosis of Chronic Lymphocytic Leukemia
InactiveUS20080280297A1Microbiological testing/measurementImmunoglobulinsReference genesLymphocytic cell
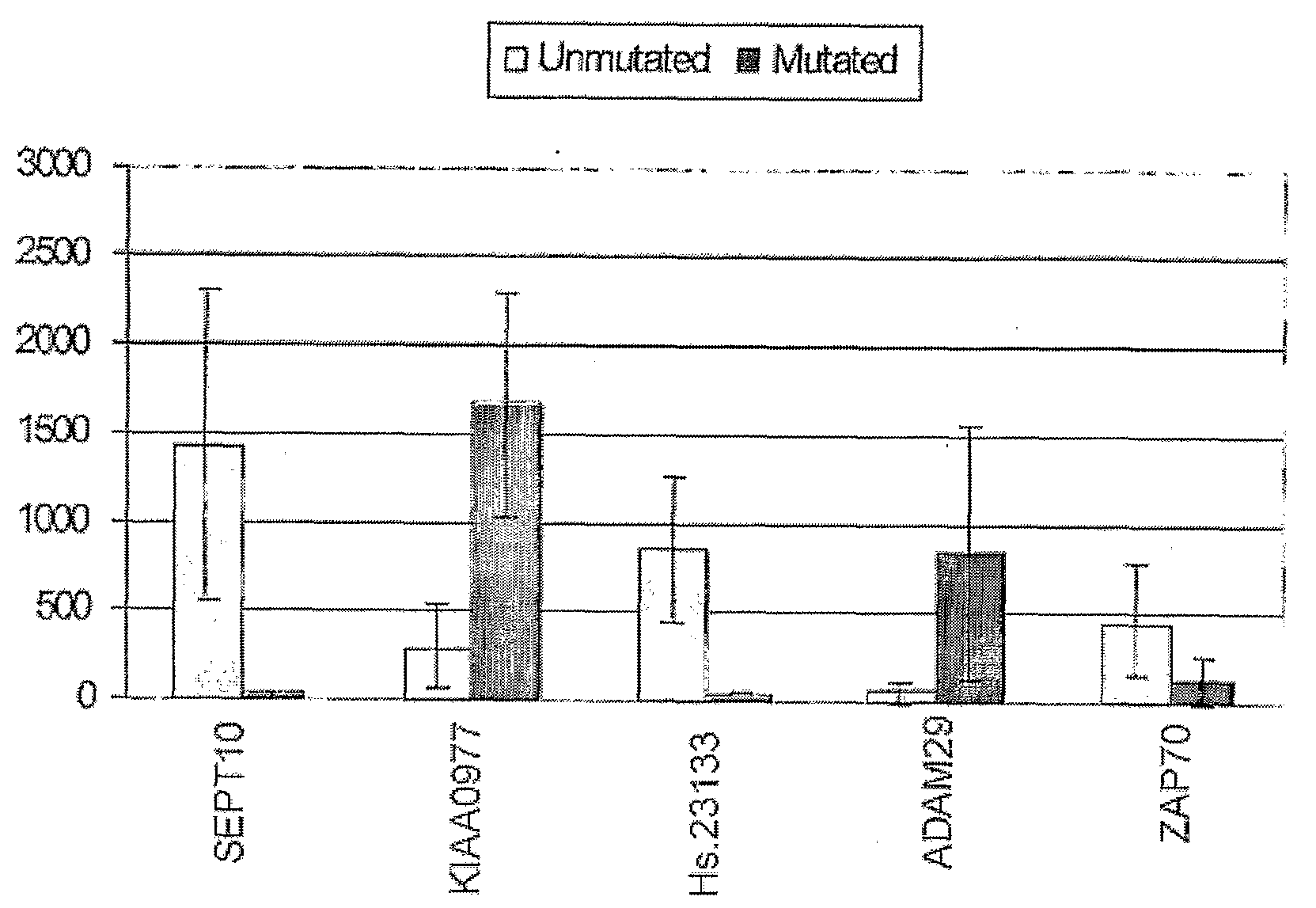
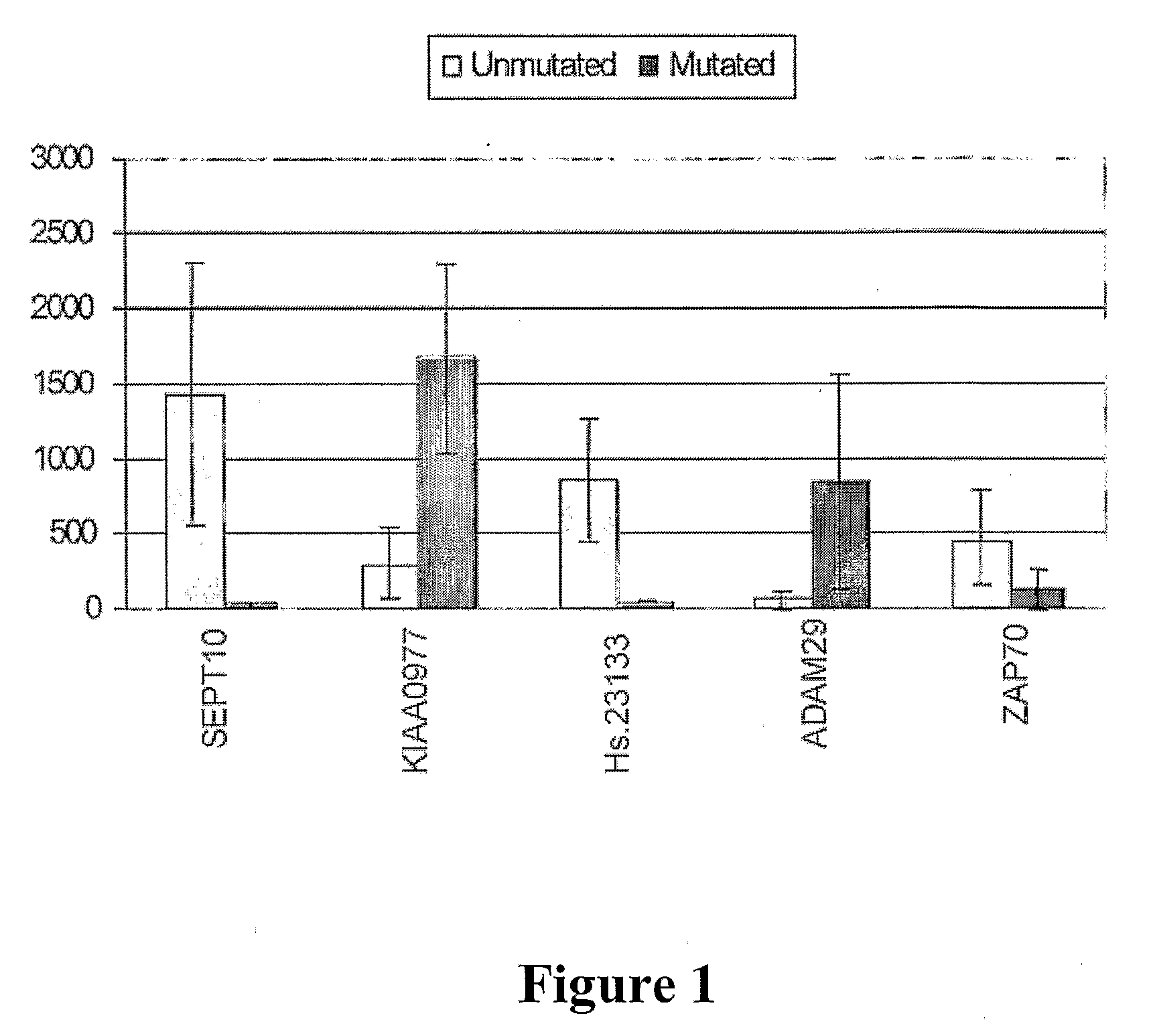
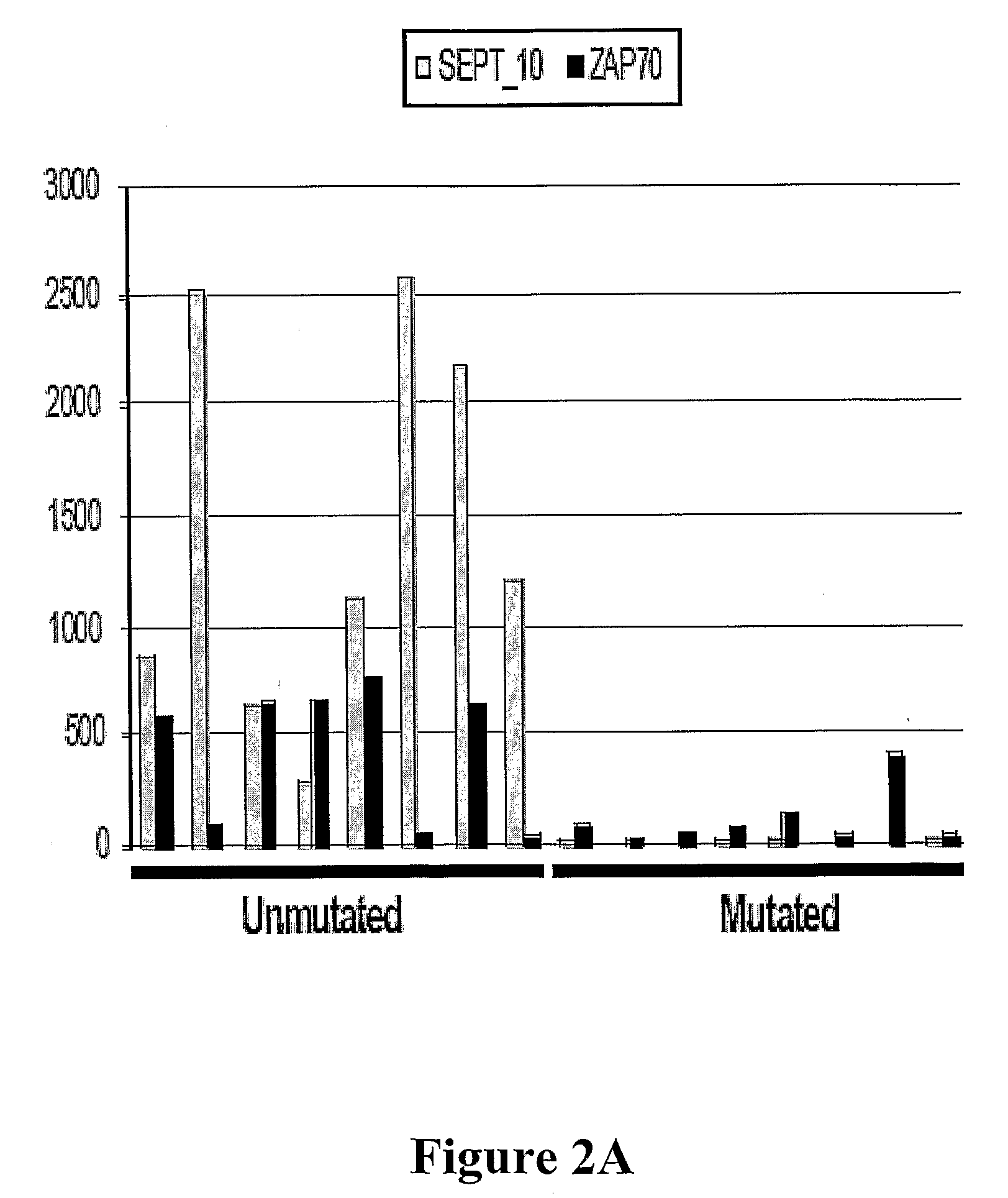
The invention provides compositions and methods for determining a prognosis of a B cell chronic lymphocytic leukemia (CLL) in a subject based on the level of expression of at least one marker gene. Marker genes provided by the invention are SEPTlO, KIAA0799, Hs.23133, and ADAM29. The marker genes can be used to differentially diagnose CLL in a subject based on relative gene expression levels in the subject compared to reference gene expression levels established from a clinically characterized population of patients. The invention also provides diagnostic reagents and compositions and kits based on the marker genes.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
MLL translocations specify a distinct gene expression profile, distinguishing a unique leukemia
InactiveUS7011947B2Easy diagnosisConvenient treatmentMicrobiological testing/measurementDisease diagnosisChronic myelogenous leukemiaFhit gene
The present invention relates to the diagnosis of mixed lineage leukemia (MLL), acute lymphoblastic leukemia (ALL), and acute myelogenous leukemia (AML) according to the gene expression profile of a sample from an individual, as well as to methods of therapy and screening that utilize the genes identified herein as targets.
Owner:WHITEHEAD INST FOR BIOMEDICAL RES +1
Methods and compositions for identifying minimal residual disease in acute lymphoblastic leukemia
This invention provides methods and kits for diagnosing, ascertaining the clinical course of minimal residual disease associated with acute lymphoblastic leukemia (ALL). Specifically the invention provides methods and kits useful in the diagnosis and determination of clinical parameters associated with diseases associated with ALL based on patterns of surface marker expression unique to ALL.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
Methods for treatment of acute lymphocytic leukemia
InactiveUS7026330B2Improved prognosisAvoid seizuresBiocideAntibody ingredientsChronic lymphocytic leukemiaAcute lymphocytic leukemia
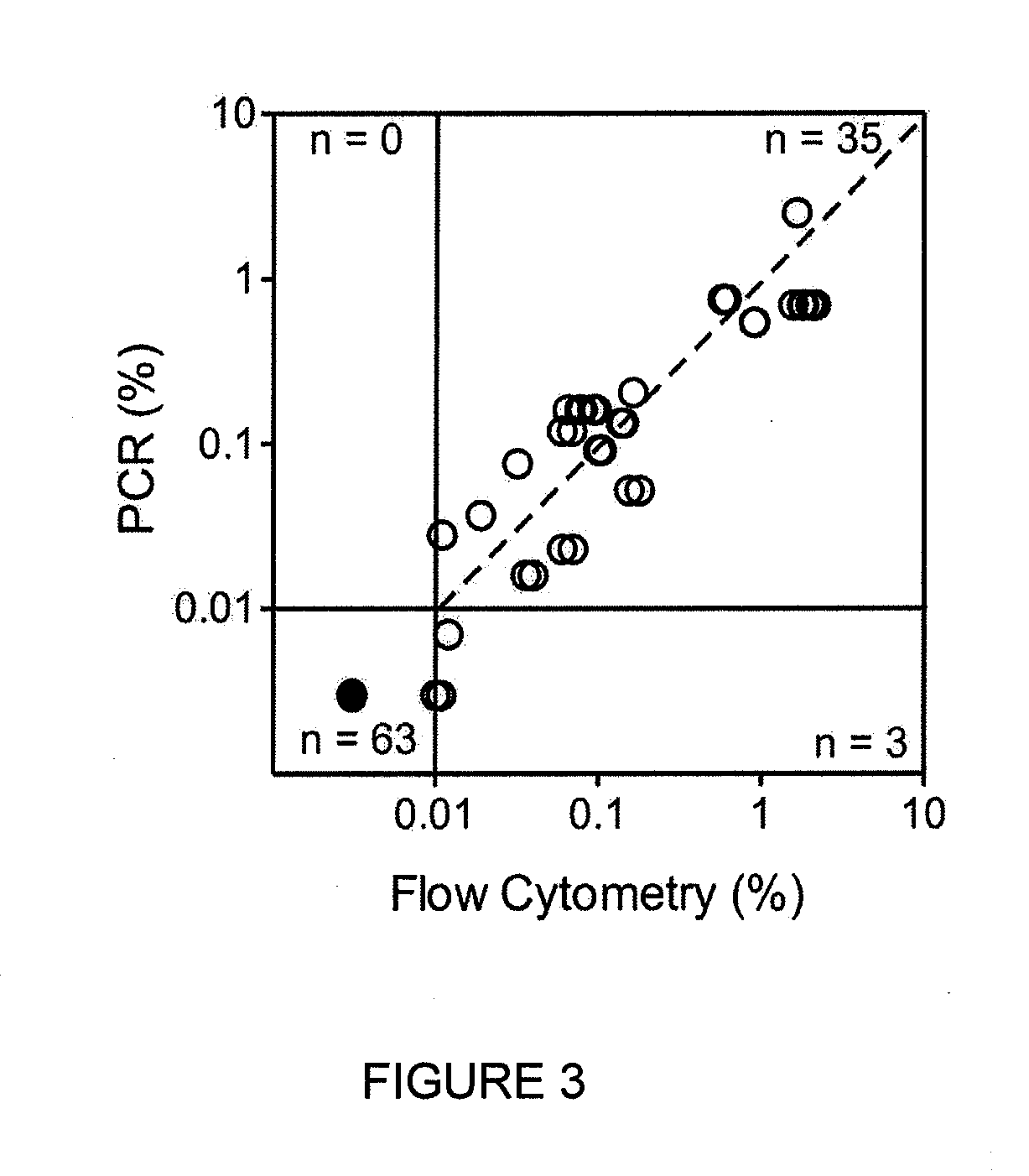
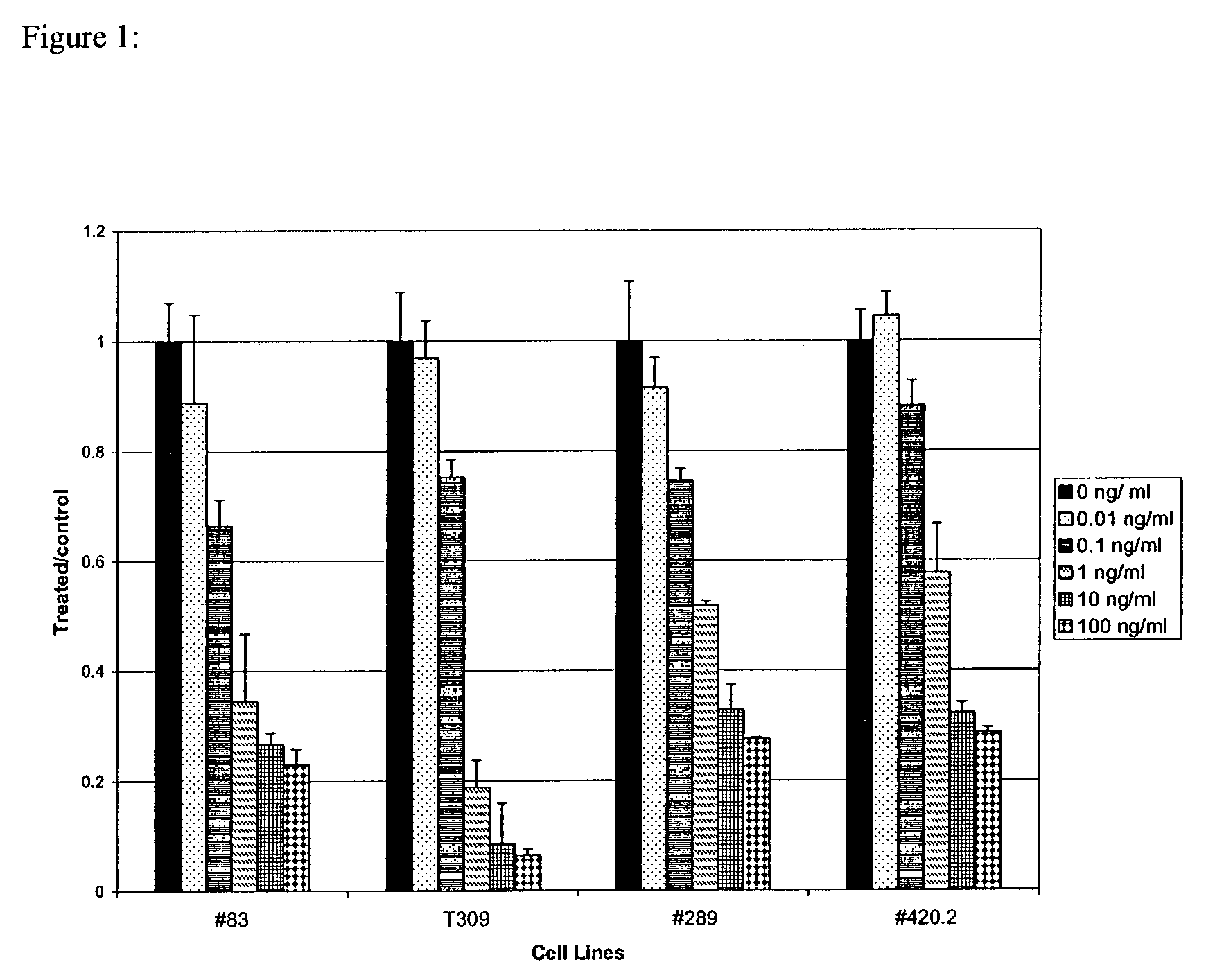
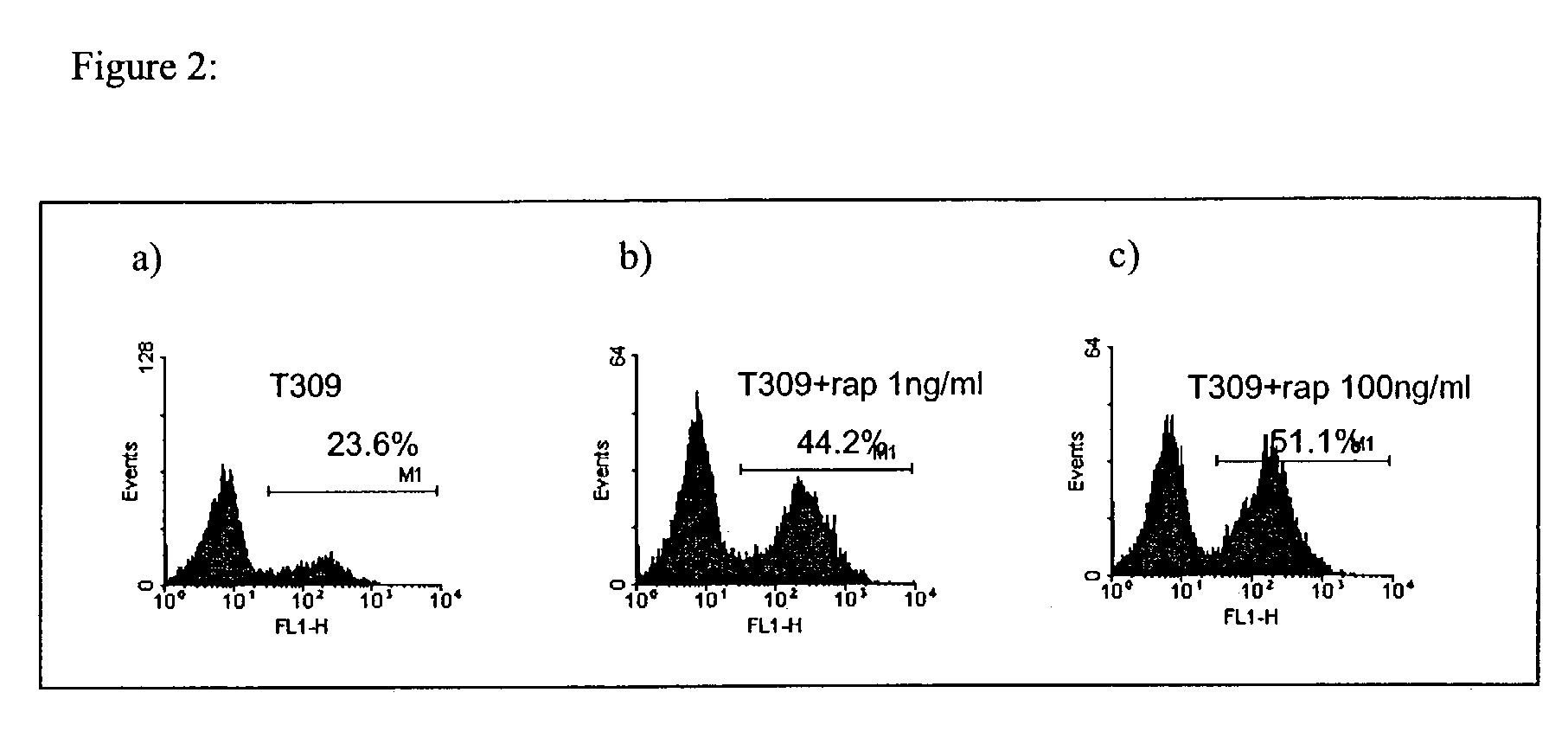
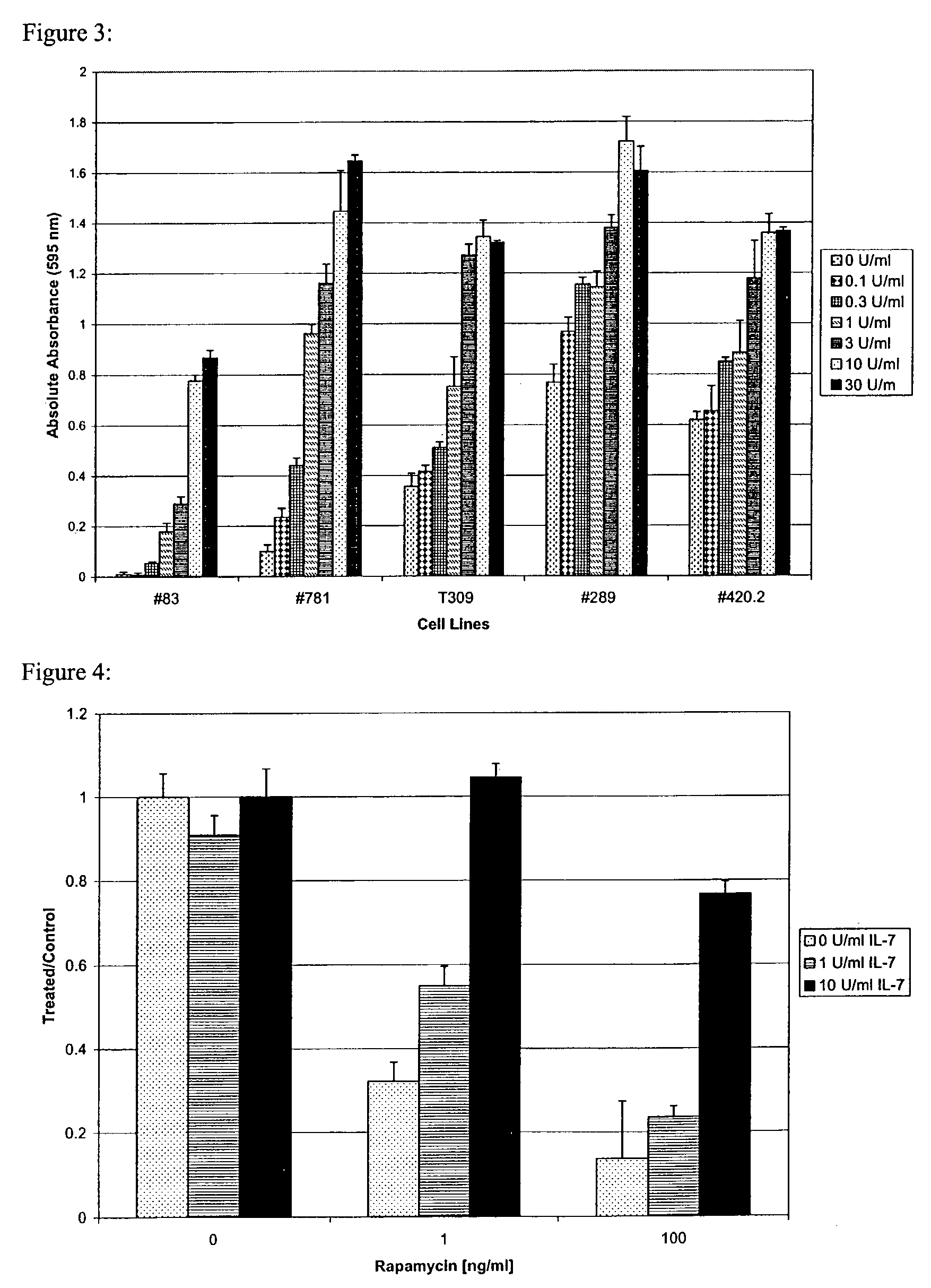
Methods for treating patients having an early B cell derived acute lymphoblastic leukemia with rapamycin or a derivative thereof are provided. Also provided are methods for treating patients having an early B cell derived acute lymphoblastic leukemia with rapamycin or a derivative thereof in combination with an IL-7 inhibitor. Finally methods for preventing GVHD in ALL patients following a bone marrow transplant are disclosed.
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA
Self-assembled nucleic-acid aptamer/protein composite nano probe, preparation method, kit and application thereof
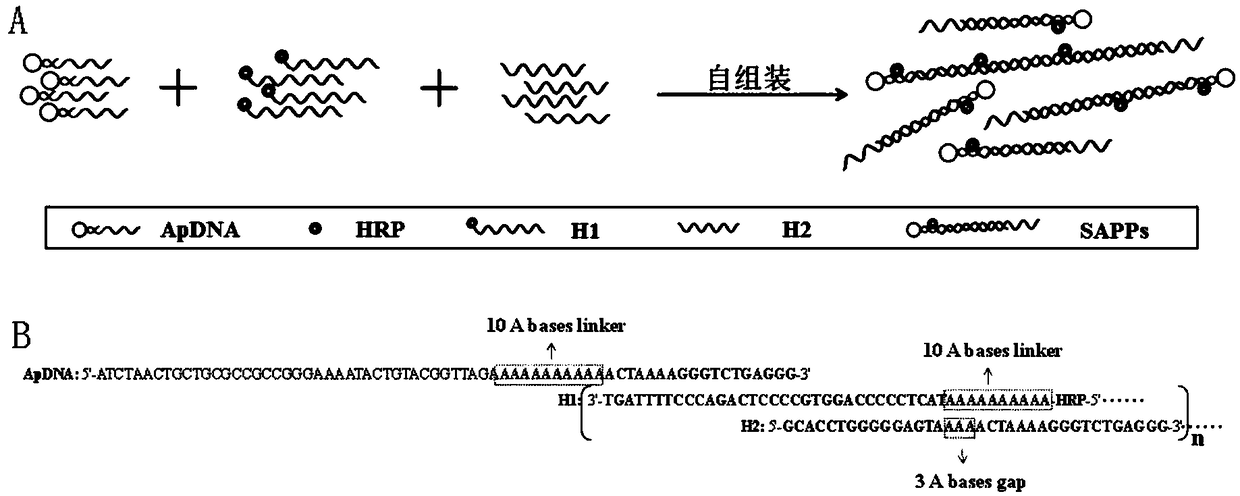
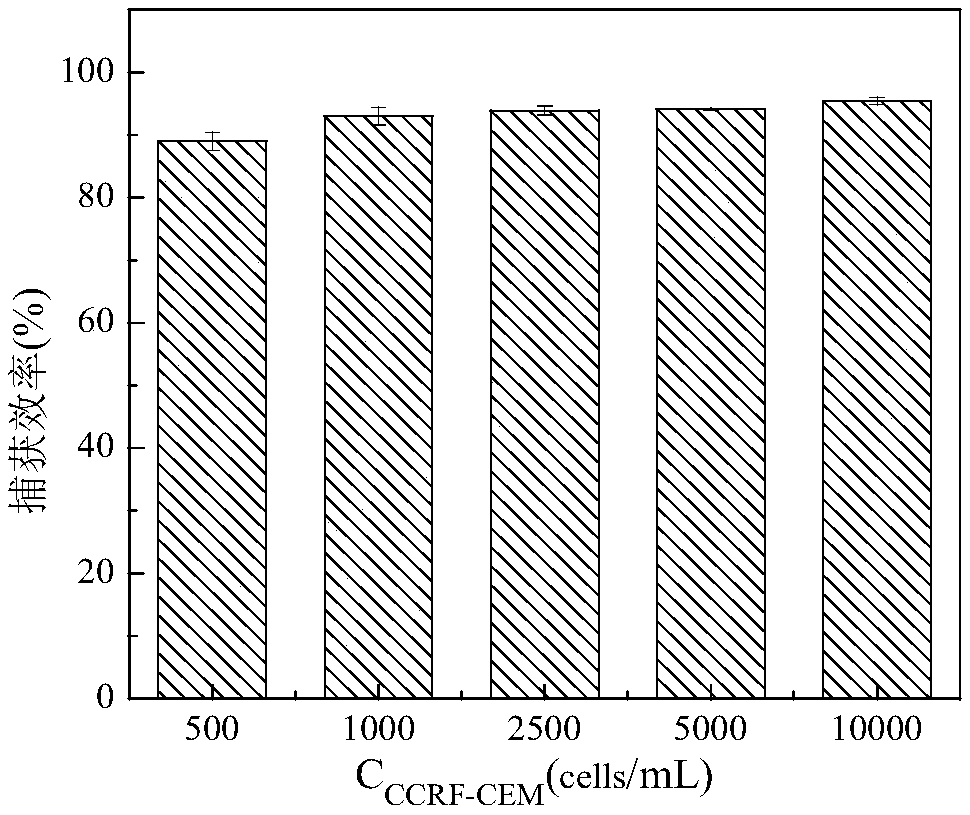
The invention discloses a self-assembled nucleic-acid aptamer / protein composite nano probe, a preparation method, a kit and application thereof. According to the invention, three DNA probes, one of which is a DNA probe containing a nucleic-acid aptamer sequence and another is marked with horseradish peroxidase, are hybridized to prepare a self-assembled nucleic-acid aptamer / protein composite nanoprobe; and magnetic nano-particles functionalized by a nucleic-acid aptamer are used as magnetic separation solid-phase carriers for specific capture of human acute lymphoblastic leukemia T lymphocytes (CCRF-CEM) in blood, to improve the capture efficiency of target substances. When the magnetic nano-particles are used in combination with the self-assembled nucleic-acid aptamer / protein composite nano probe, the detection of CCRF-CEM has a wide linear range, a low detection limit, and high precision and accuracy, and high-sensitivity, high-specificity and rapid detection of CCRF-CEM in peripheral blood samples can be realized, which has great significance for auxiliary diagnosis, curative effect evaluation and prognosis judgment of leukemia.
Owner:ZHENGZHOU UNIV
Kit for quantitatively detecting BCR/ABL mRNA level
InactiveCN101624621AWide coverageImprove positive detection rateMicrobiological testing/measurementMRD NegativeQuantitative determination
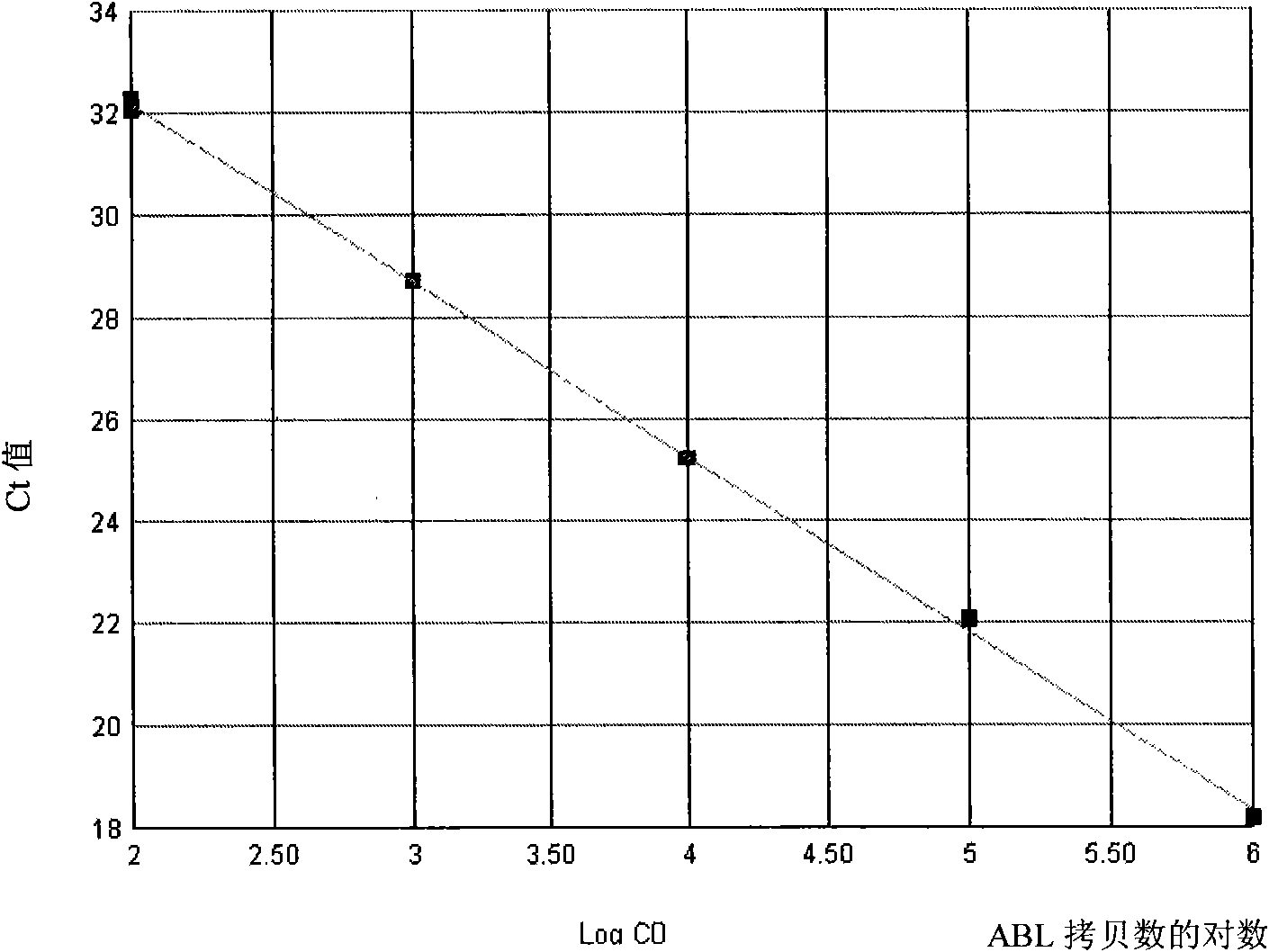
The invention discloses a kit for quantitatively detecting a BCR / ABL mRNA level. The kit comprises a standard product which is used for manufacturing a standard curve, an inner reference gene real-time quantitative PCR system and at least one of the following three real-time quantitative PCR systems: an M-type BCR / ABL real-time quantitative PCR system, m-type BCR / ABL real-time quantitative PCR system and a mu-type BCR / ABL real-time quantitative PCR system. The kit can accurately, quickly and quantitatively detect various BCR / ABL mRNA levels, is used for diagnosing chronic myelogenous leukemia and acute lymphoblastic leukemia expressed by BCR / ABL and monitoring minimal residual diseases in a treatment process, and provides an important molecular basis for accurate diagnosis of clinical diseases, determination of a treatment proposal, curative effect evaluation and prognosis.
Owner:PEOPLES HOSPITAL PEKING UNIV
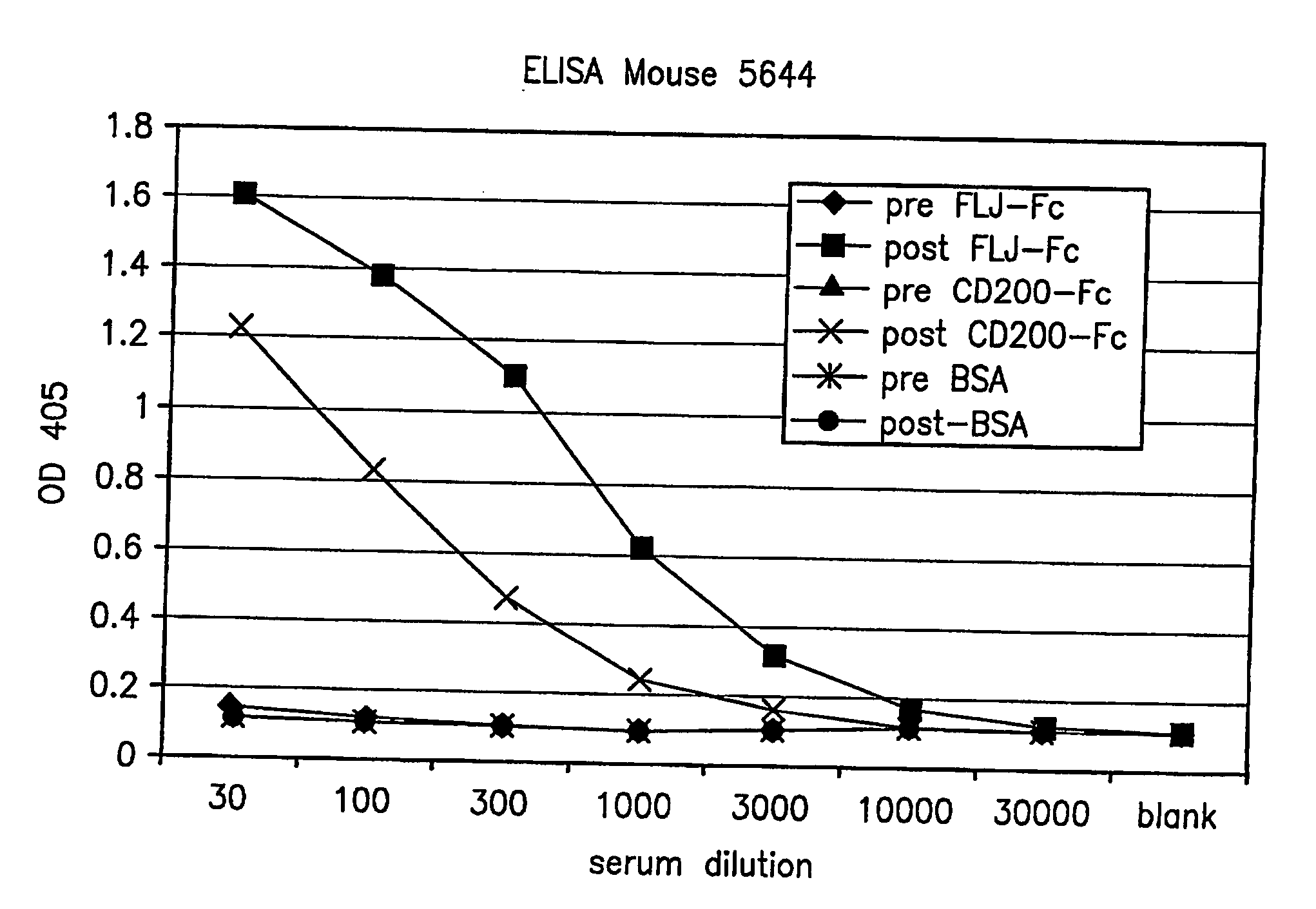
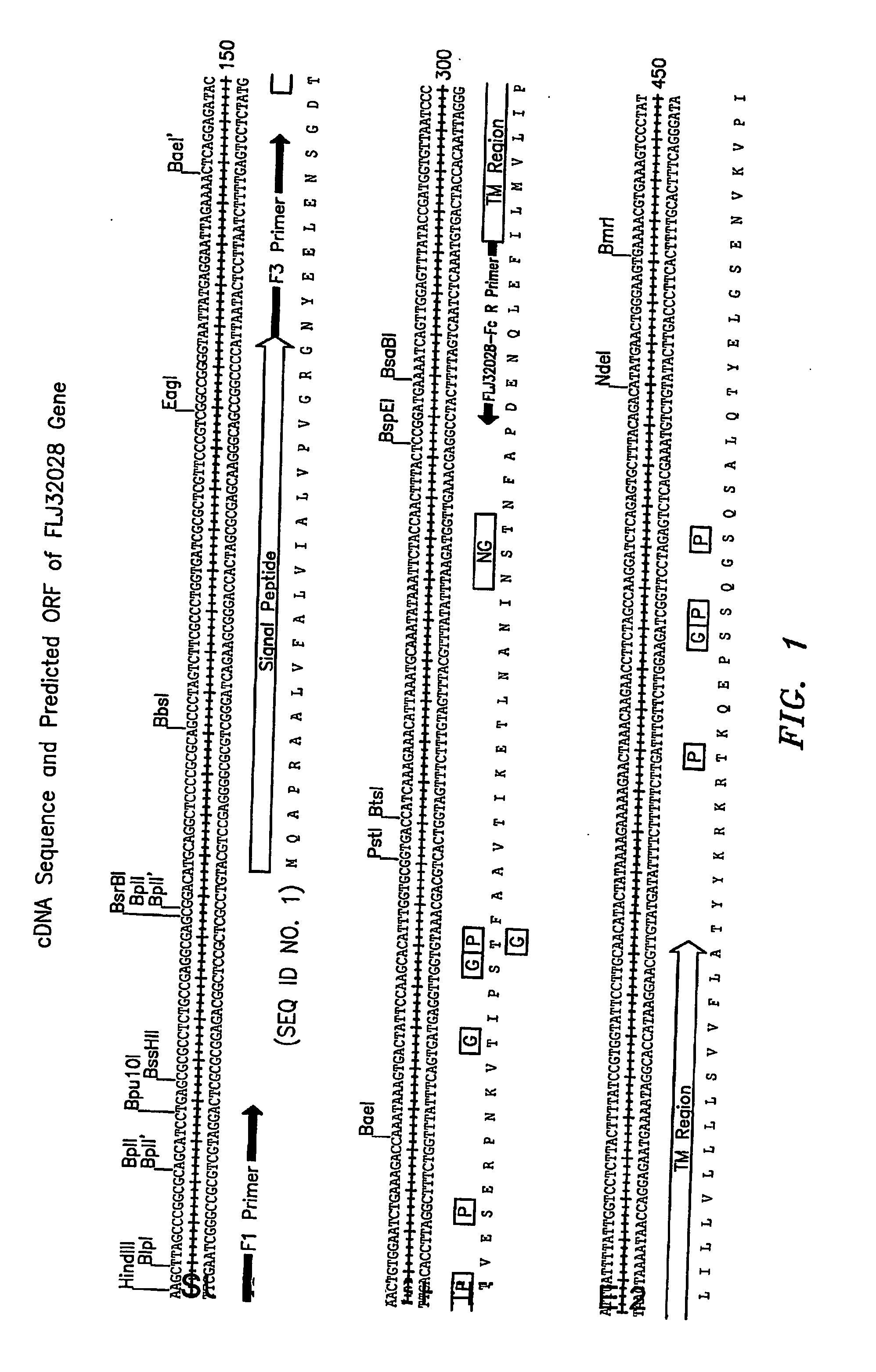
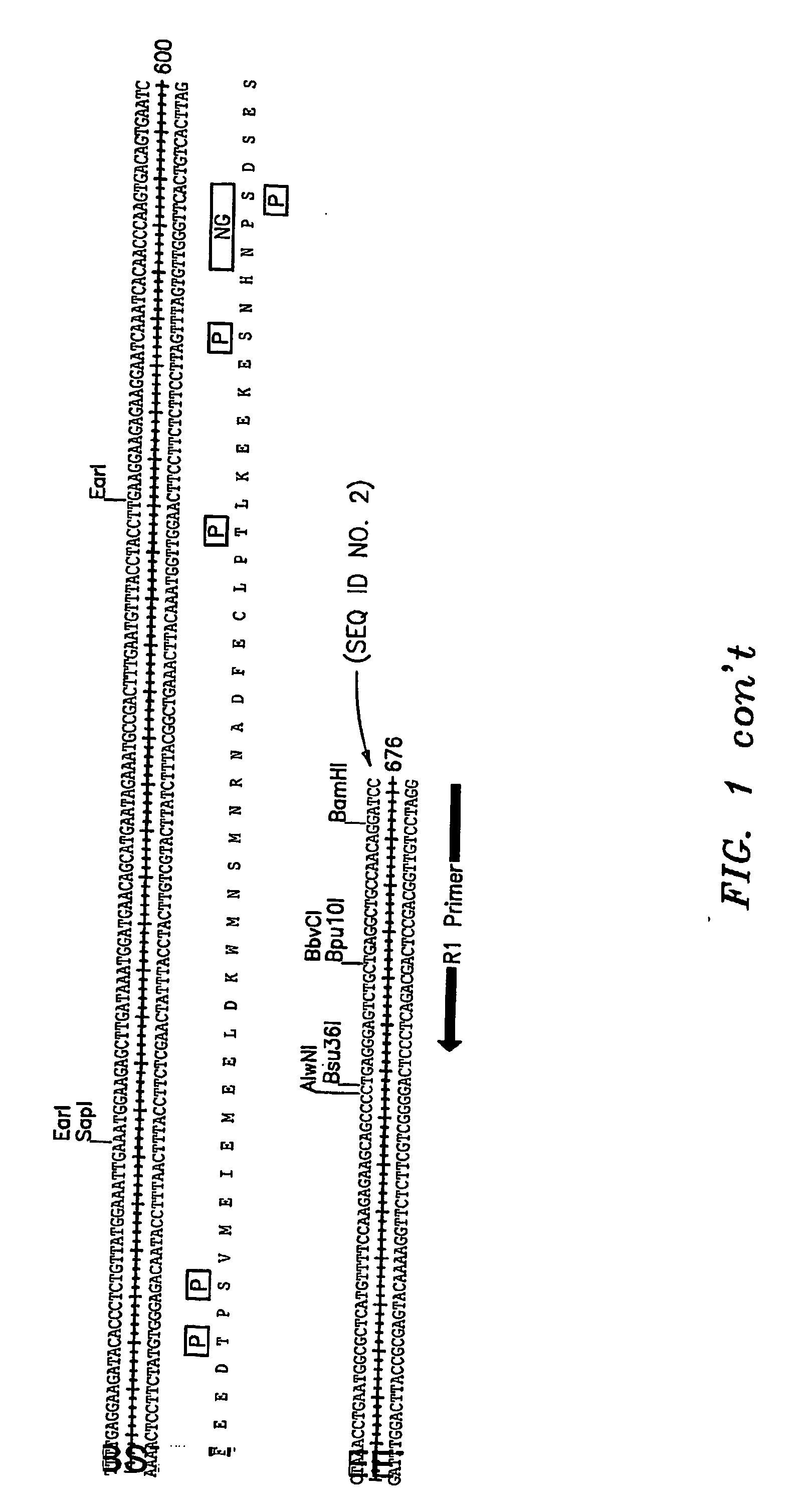
Cell Surface Protein Associated with Human Chronic Lymphocytic Leukemia
InactiveUS20080057519A1Sugar derivativesAntibody mimetics/scaffoldsChronic lymphoblastic leukemiaDiagnostic marker
Protein (referred to herein as “FLJ32028”) that is associated with B-cell chronic lymphocytic leukemia is isolated. Isolated nucleic acid encoding the protein, the generation of monoclonal antibodies recognizing at least a portion of this protein, and the use of this protein or antibodies thereto as a diagnostic marker or therapeutic target for B-CLL are also disclosed.
Owner:ALEXION PHARM INC
CD20 Antibodies and Uses Thereof
CD20 is a transmembrane protein of the tetra-spanin family expressed on the surface of B-cells from peripheral blood as well as lymphoid tissues. CD20 expression persists from the early pre-B cell stage until the plasma cell differentiation stage. In addition to expression in normal B-cells, CD20 is expressed in B-cell derived malignancies such as non-Hodgkin's lymphoma (NHL) and B-cell chronic lymphocytic leukemia (CLL). The present invention includes anti-CD20 antibodies and antigen-binding fragments thereof comprising a light chain variable region and a heavy chain variable region, wherein the CDR-L1, CDR-L2, and CDR-L3 of said light chain variable region comprise the amino acid sequences of SEQ ID NOs: 23-25, respectively, and wherein the CDR-H1, CDR-H2, and CDR-H3 of said heavy chain variable region comprise the amino acid sequences of SEQ ID NOs: 26-28, respectively.
Owner:IMMUNOGEN INC
Anti CD19 engineered antibody for target conjugated lymphocyte, leuco cyte and its use
InactiveCN1775808AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsTriturationGenetic engineering
The invention discloses an anti-CD19 engineering antibody and its application which is used in target direction combining lymphocytic leukemia cell. It lays a foundation for the next trituration genetic engineering medicine of the target direction therapy leukemia. It relates to anti-CD19 monoclonal antibody HI19a heavy and light chain variable region gene, and application of the gene code polypeptide, the gene carrier, and using the gene and polypeptide to make leukemia therapy medicine. The heavy and light chain variable region gene is come from the anti-CD19 monoclonal antibody HI19a. The invention successfully adopts gene engineering technique to make anti-CD19 gene engineering antibody, and lays a foundation for the target direction therapy of the leukemia.
Owner:INST OF HEMATOLOGY & BLOOD HOSPITAL CHINESE ACAD OF MEDICAL SCI
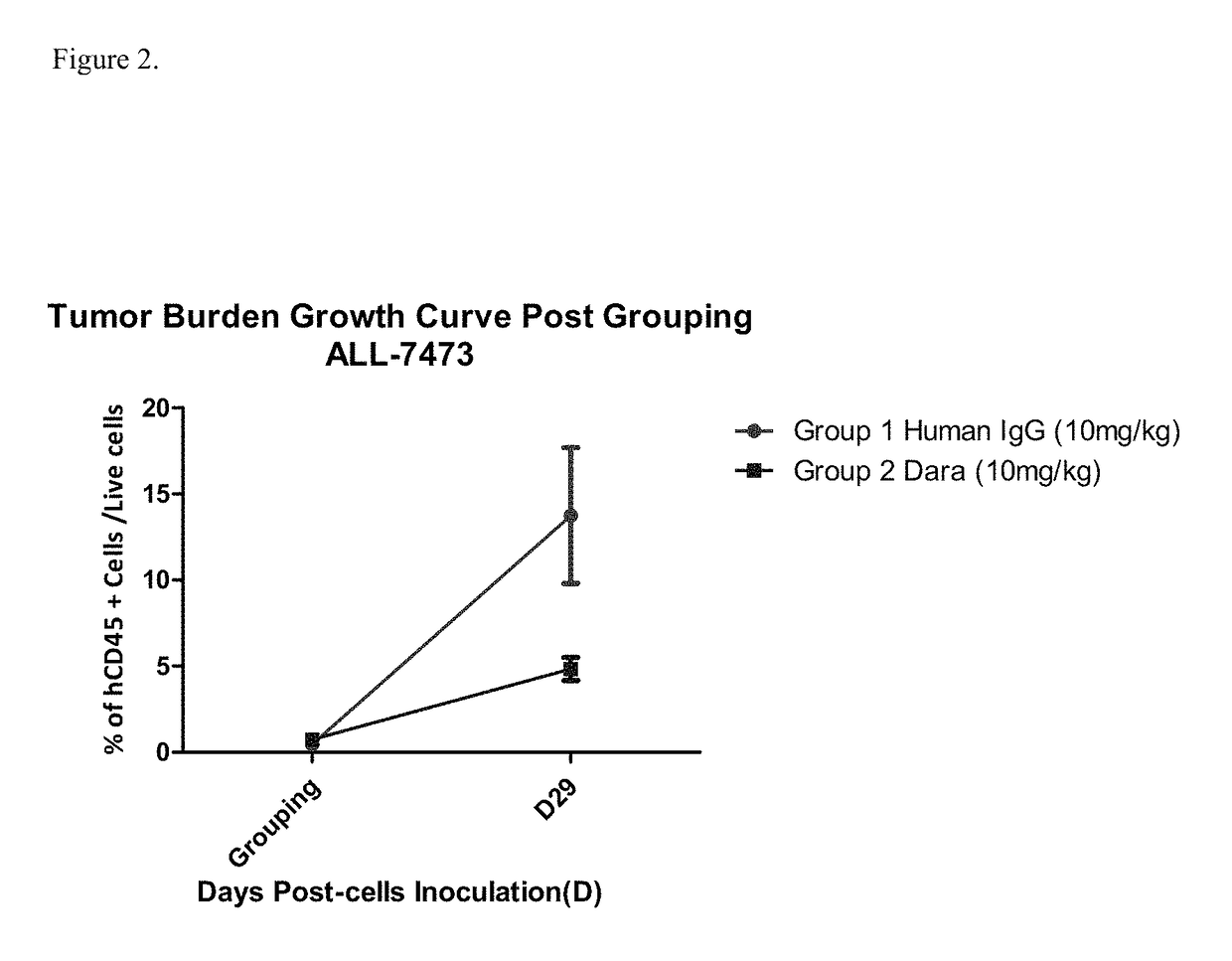
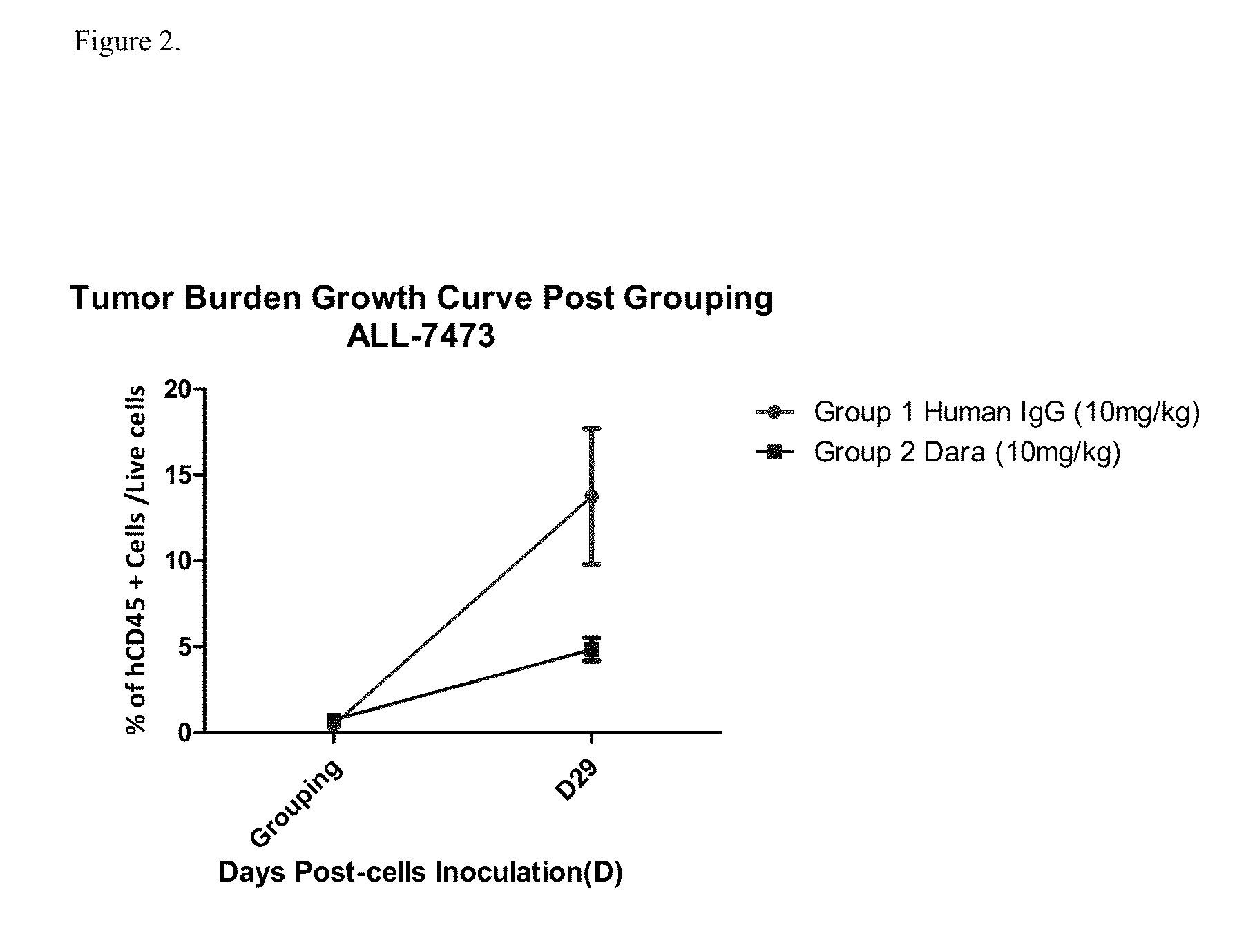
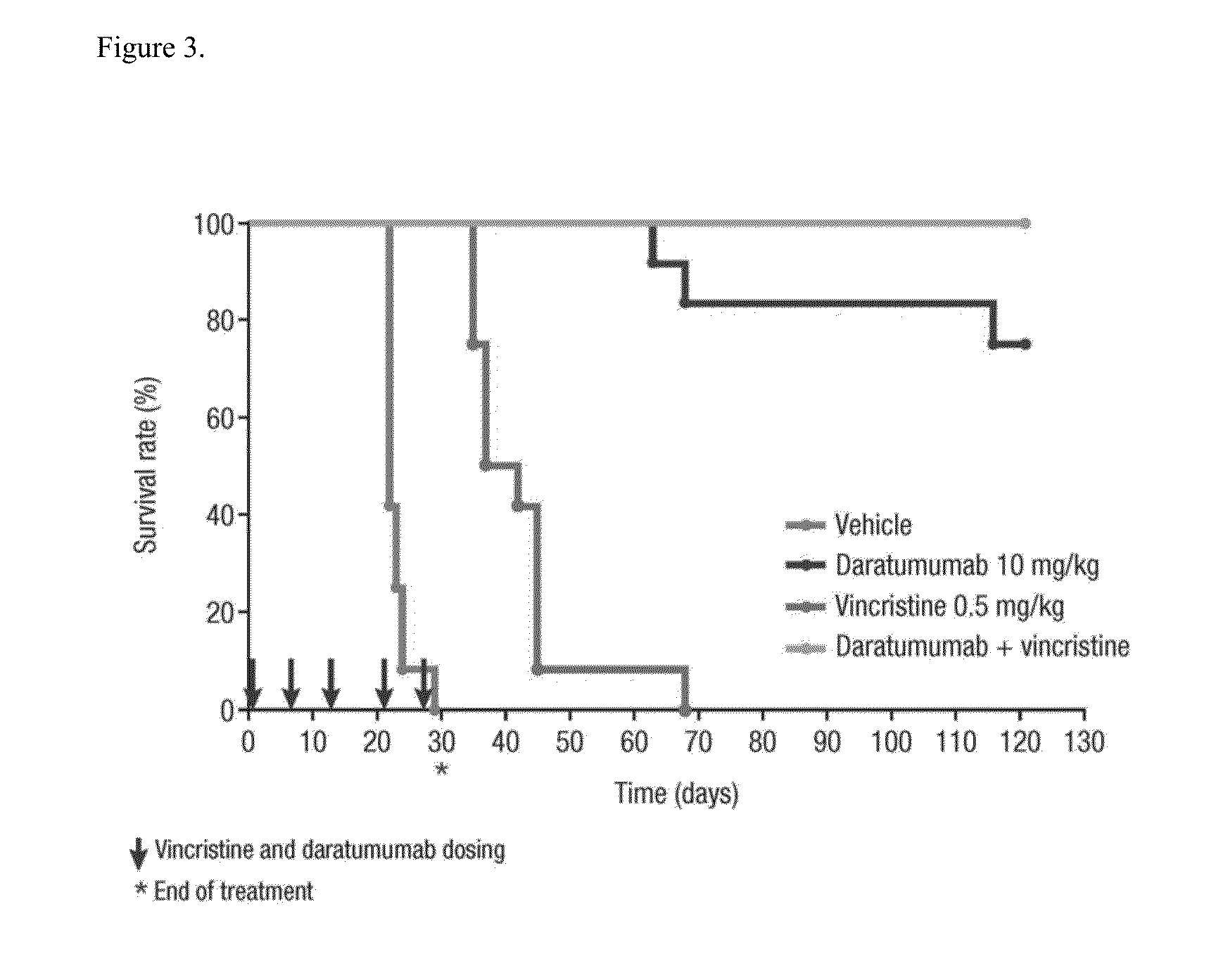
Anti-CD38 antibodies for treatment of acute lymphoblastic leukemia
ActiveUS9732154B2Organic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAcute lymphocytic leukemiaCombination therapy
Owner:JANSSEN BIOTECH INC
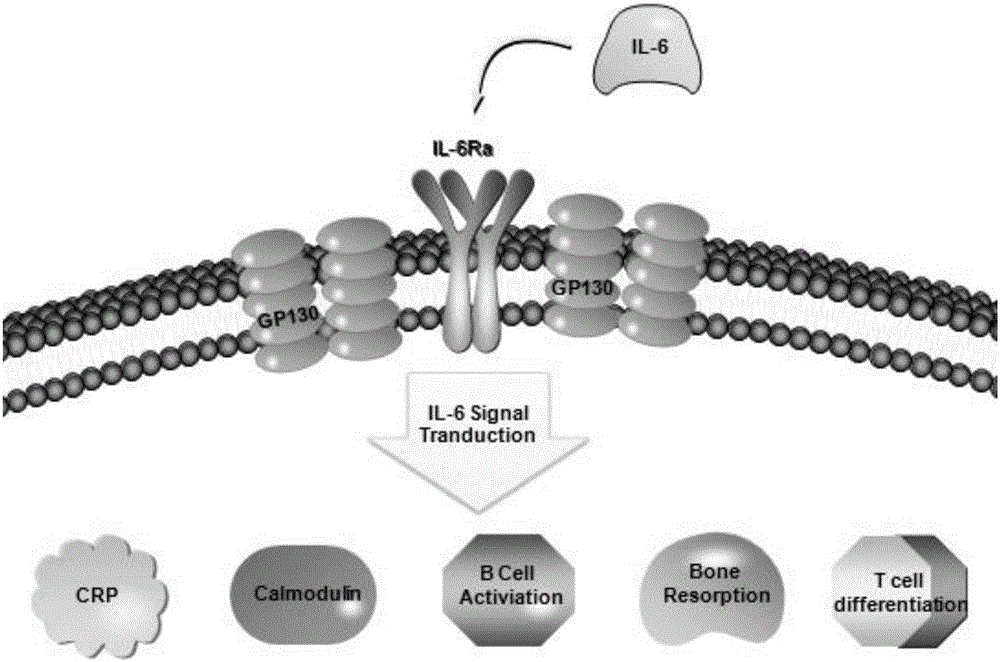
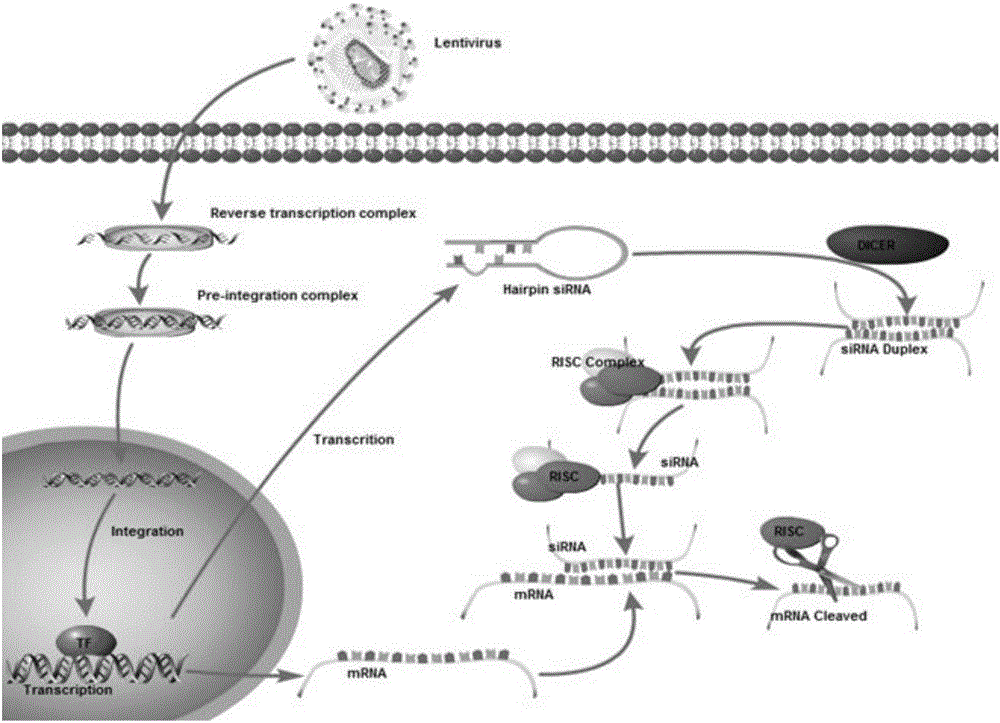
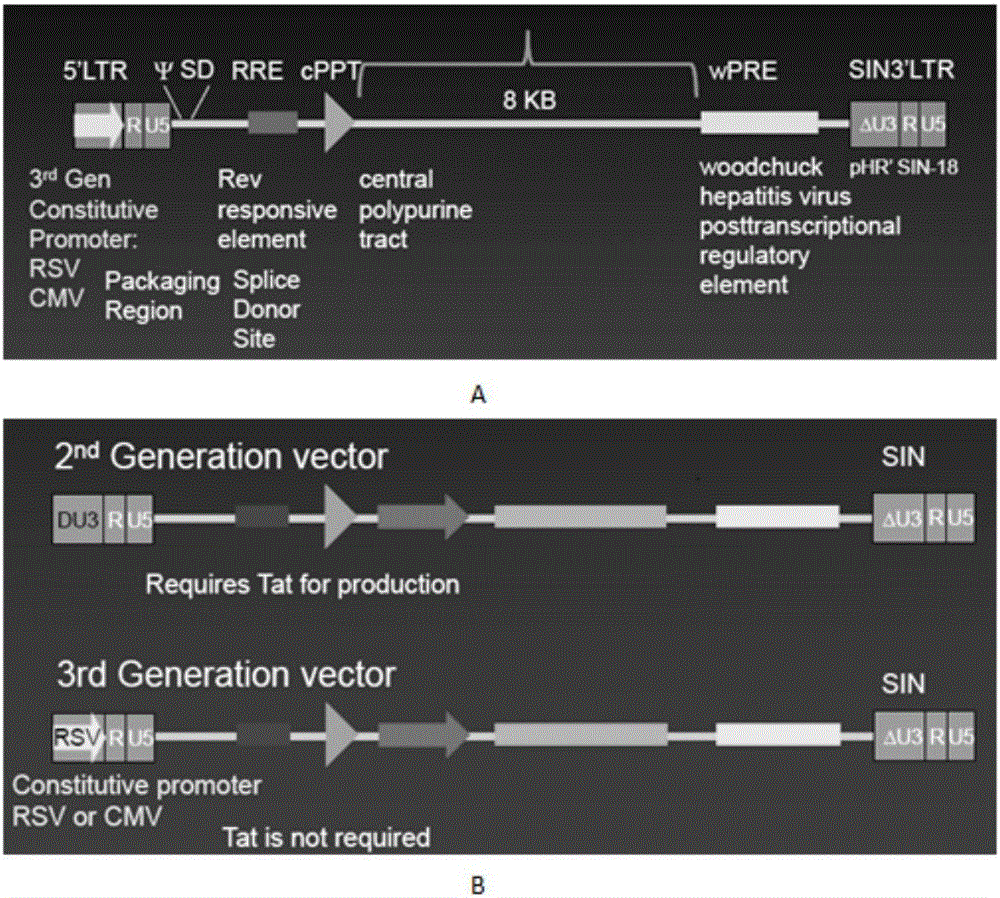
SiRNA of humanized interleukin 6, recombination expression carrier CAR-T and construction method and application of recombination expression carrier CAR-T
ActiveCN106636090ARelieve painReduce the risk of CRSOrganic active ingredientsGenetic material ingredientsAbnormal tissue growthInterleukin 6
The invention discloses an siRNA of humanized interleukin 6, a recombination expression carrier CAR-T and a construction method and application of the recombination expression carrier CAR-T. An IL-6 knock-down siRNA expression cassette and an siRNA expression product not only can be applied to eliminating or alleviating of CRS symptoms in treatment of B lineage acute lymphoblastic leukemia (ALL) through CAR19-T, but also can be applied to alleviating of CRS symptoms caused in treatment of all types of tumors like B lymphoma, pancreatic cancer, brain glioma and myeloma through CAR-T, and is even applied to alleviating of CRS caused by other types of treatment.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
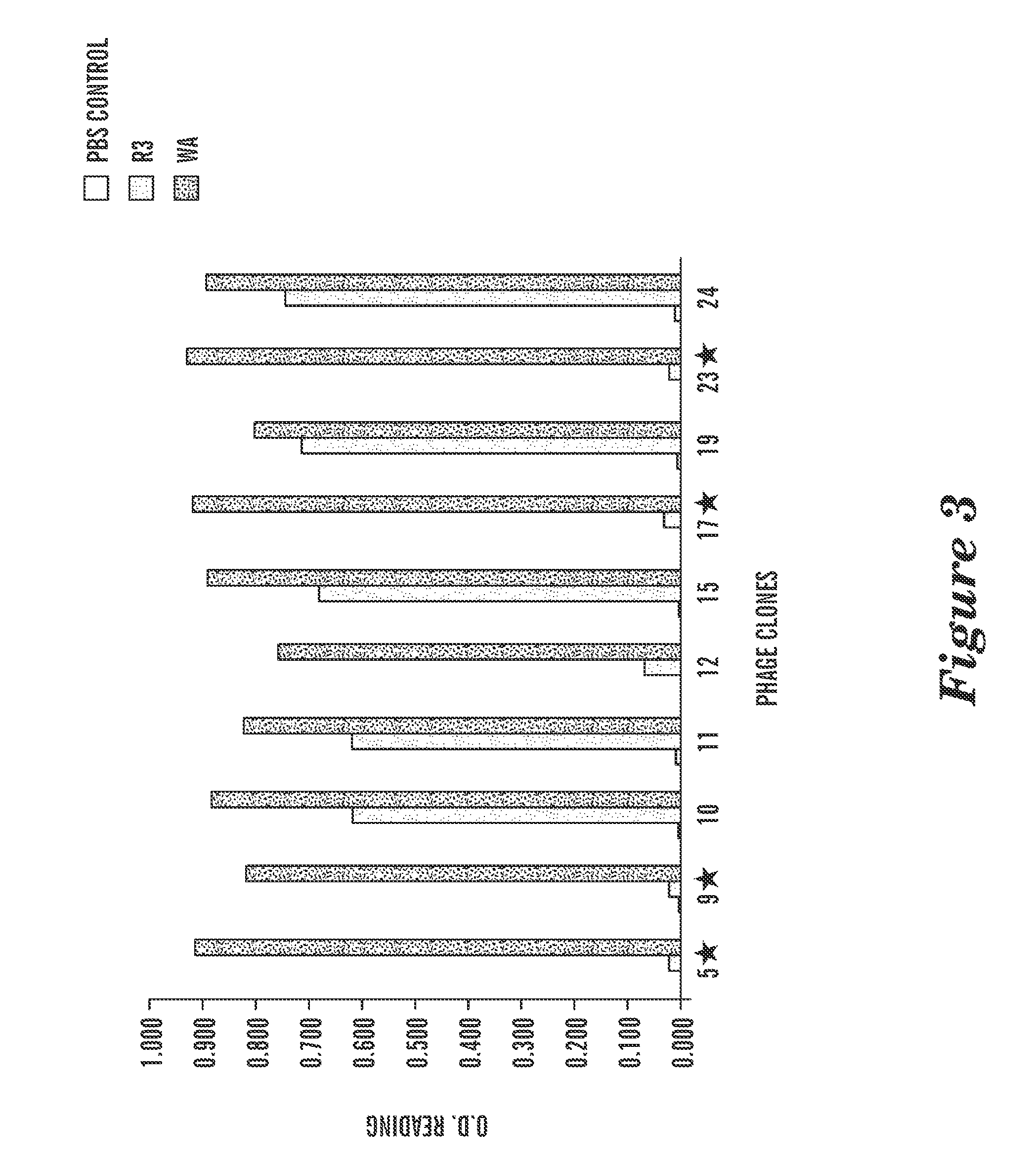
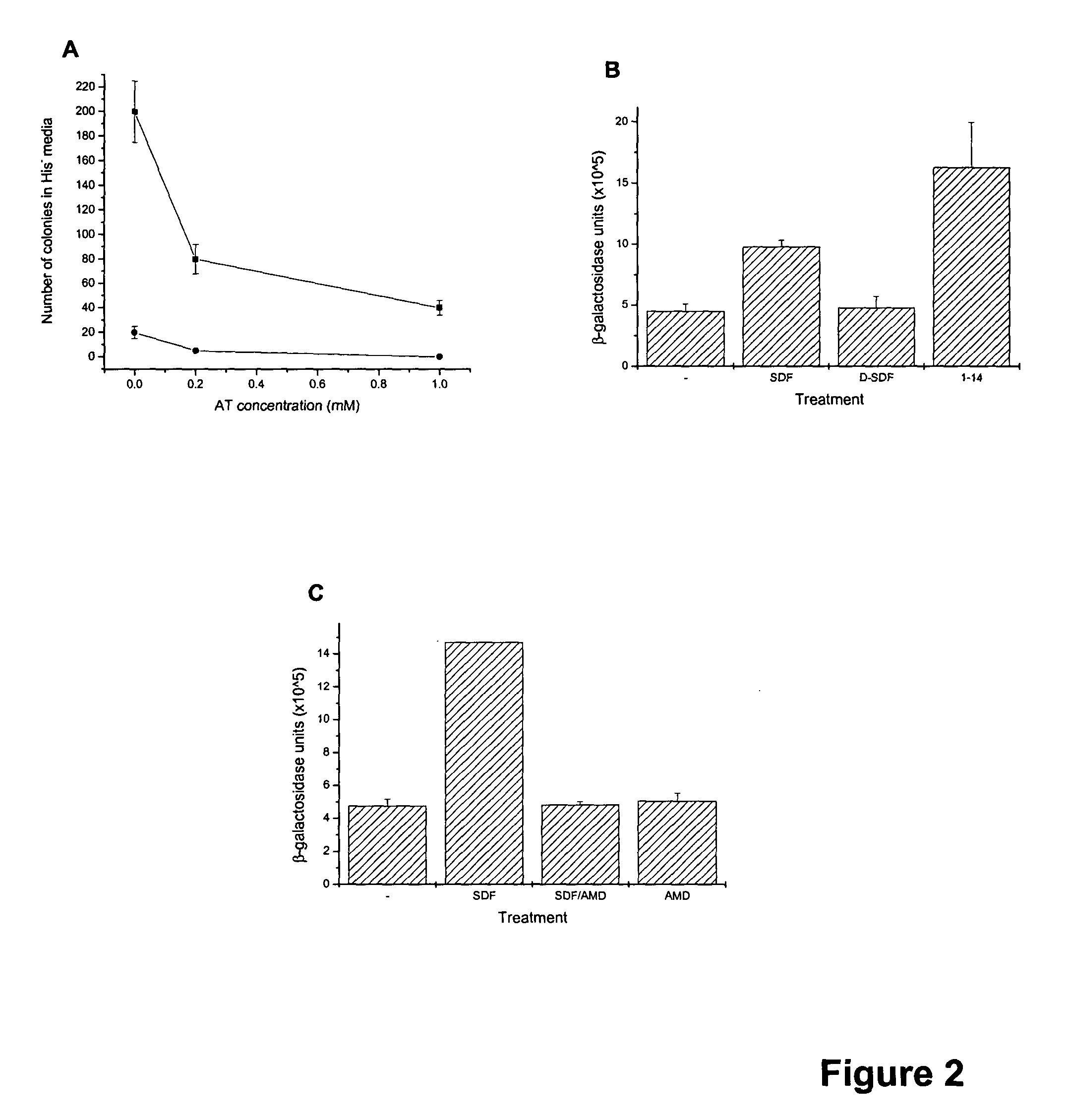
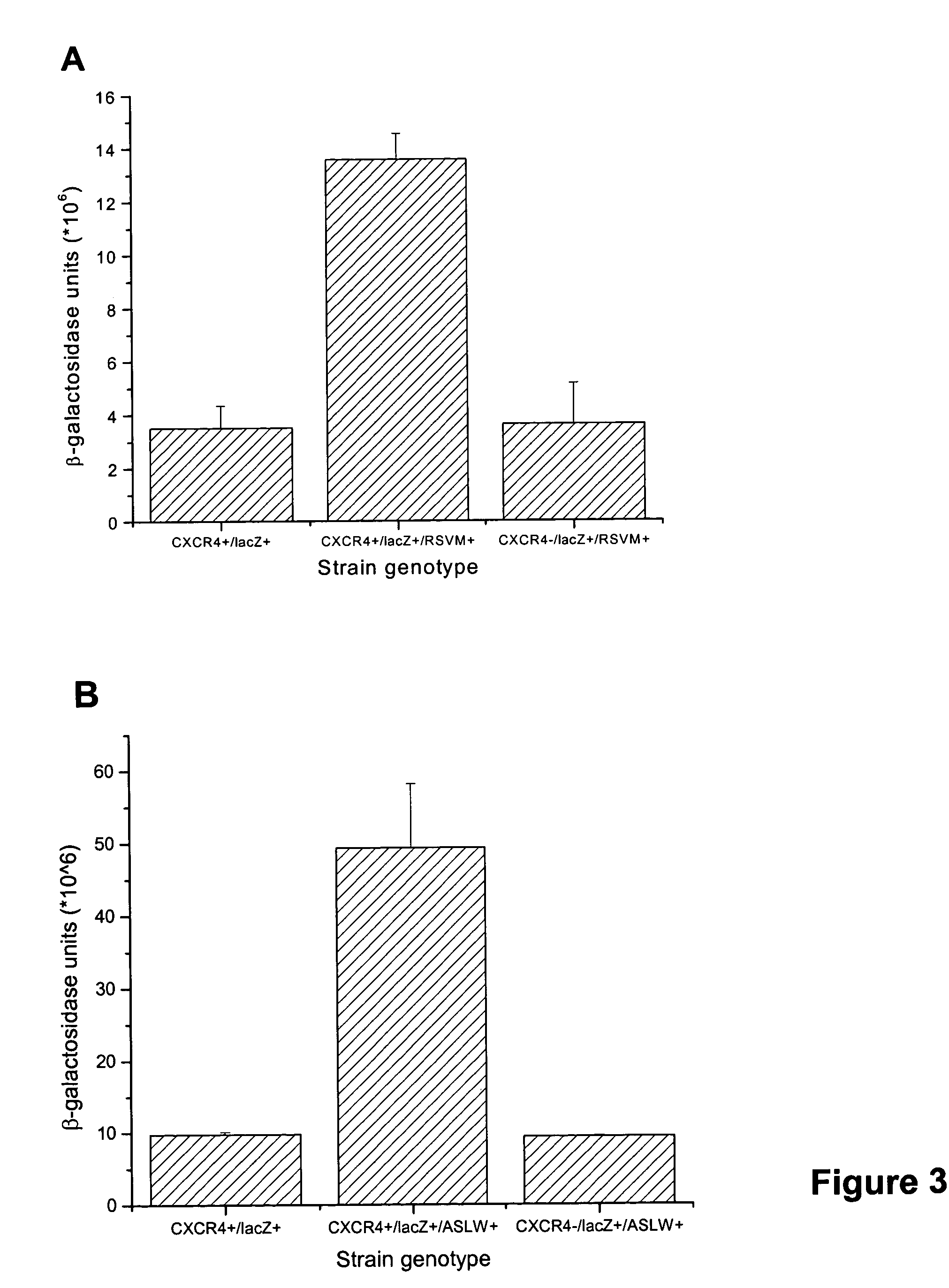
Identification of allosteric peptide agonists of CXCR4
The chemokine receptor CXCR4 is a co-receptor for T-tropic strains of HIV-1. A number of small molecule antagonists of CXCR4 are in development, but all are likely to lead to adverse effects due to the physiological function of CXCR4. To prevent these complications, allosteric agonists may be therapeutically useful as adjuvant therapy in combination with small molecule antagonists. A synthetic cDNA library coding for 160,000 different SDF-based peptides was screened for CXCR4 agonist activity in a yeast strain expressing functional receptor. Peptides that activated CXCR4 in an autocrine manner induced colony formation. Two peptides, designated RSVM and ASLW, were identified as novel agonists that are insensitive to the CXCR4 antagonist AMD3100. In chemotaxis assays using the acute lymphoblastic leukemia cell line CCRF-CEM, RSVM behaves as a partial agonist and ASLW as a superagonist. The superagonist activity of ASLW may be related to its inability to induce receptor internalization. In CCRF-CEM cells, the two peptides are also not inhibited by another CXCR4 antagonist, T140, or the neutralizing monoclonal antibodies 12G5 and 44717.111. These results suggest that alternative agonist binding sites are present on CXCR4 that could be screened to develop molecules for therapeutic use.
Owner:LOLIS ELIAS +3
B7. 1-CD19scFv fusion gene engineering albumen for treating B lymphocyte leukemia and lymph tumour and use thereof
InactiveCN1919871AProlong lifePromote clonal proliferationHybrid immunoglobulinsAntibody ingredientsB lymphoblastic leukemiaFhit gene
The invention discloses a B7.1-CD19scFv merge gene engineering protein and usage to treat B lymphocyte leukosis, lymph tumor, which comprises the following parts: human B7.1 external cell area, antihuman CD19 monoclonal antibody heavy chain and light chain variable area gene.
Owner:INST OF HEMATOLOGY & BLOOD HOSPITAL CHINESE ACAD OF MEDICAL SCI
Methods and compositions for diagnosis and treatment of b cell chronic lymphocytic leukemia
Provided are isolated and purified preparations of a combination of a light chain antibody gene and a heavy chain antibody gene, where the light chain and heavy chain antibody genes are the same among more than one patient with B cell chronic lymphocytic leukemia (B-CLL). Vectors comprising those genes and cells comprising those vectors are also provided, as are isolated and purified antibodies encoded by the antibody genes. Anti-idiotype antibodies, peptides, and aptamers that bind to the antigen-binding region of an antibody encoded by the antibody genes are additionally provided, as are multimeric molecules comprising multiple binding sites that bind to the antigen-binding region of an antibody encoded by the antibody genes. Methods of determining whether a patient with B cell chronic lymphocytic leukemia (B-CLL) has a form of B-CLL that is susceptible to treatment directed to eliminating idiotype specific B cell receptor-bearing B-CLL cells are also provided, as are methods of following the progression of treatment of B-CLL in the patient. Additionally, methods of treating a patient having B-CLL are provided, as are methods of identifying a therapeutic agent for B-CLL.
Owner:THE FEINSTEIN INST FOR MEDICAL RES
Diagnosis and Treatment of Drug Resistant Leukemia
The present invention encompasses methods and compositions useful in the diagnosis and treatment of drug resistant leukemia. The invention provides a number of genes that are differentially expressed between drug resistant and drug sensitive acute lymphoblastic leukemia (ALL). These genes act as biomarkers for drug resistant leukemia, and further serve as molecular targets for drugs useful in treating drug resistant leukemia. Accordingly, the invention provides methods of diagnosing drug resistant leukemia and methods of selecting a therapy for subjects affected by drug-resistant leukemia. The invention also provides methods for screening for compounds for treating drug-resistant leukemia, and improved methods for treating drug-resistant leukemia. Compositions of the invention include arrays, computer readable media, and kits for use in the methods of the invention.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
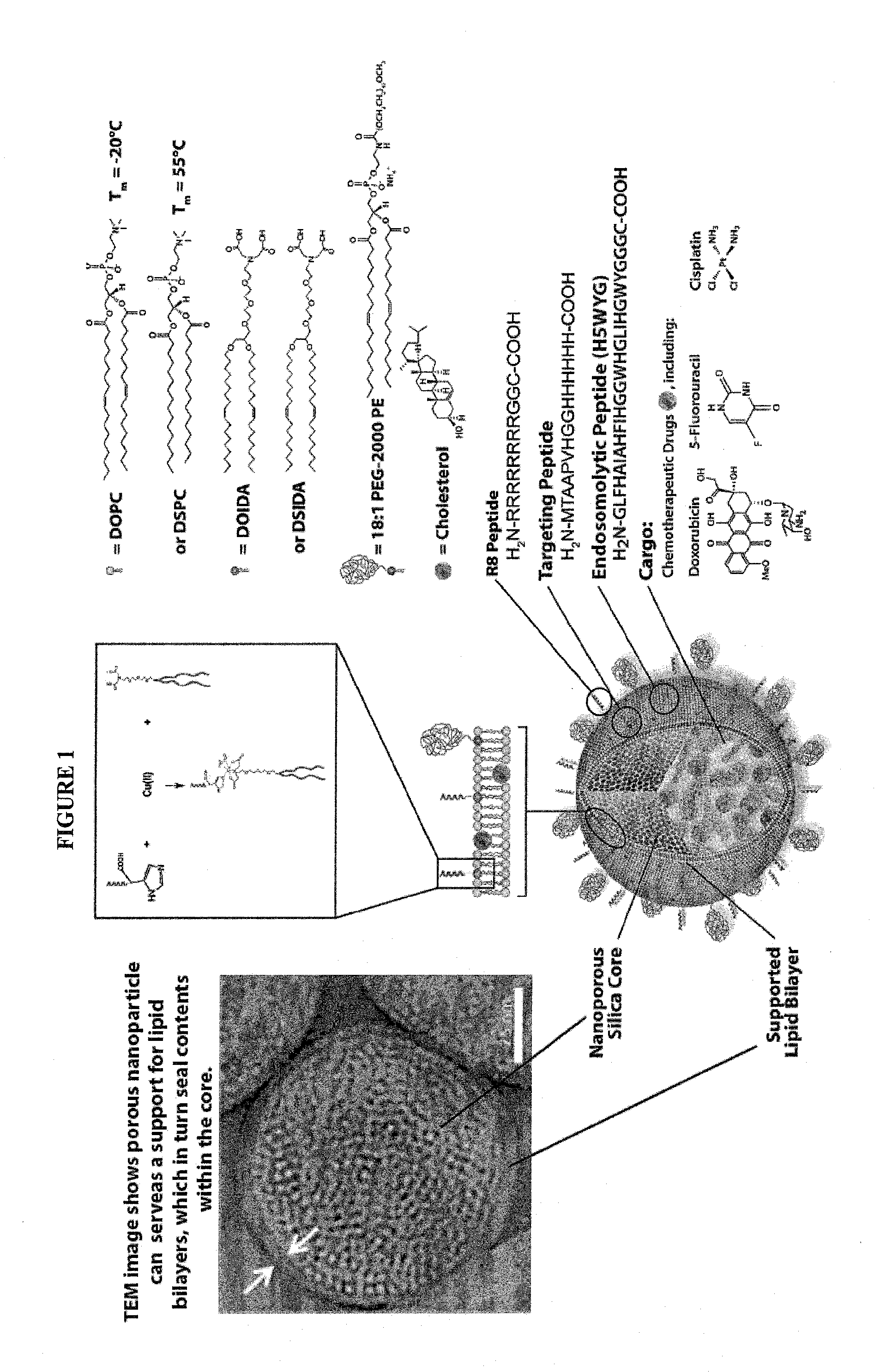
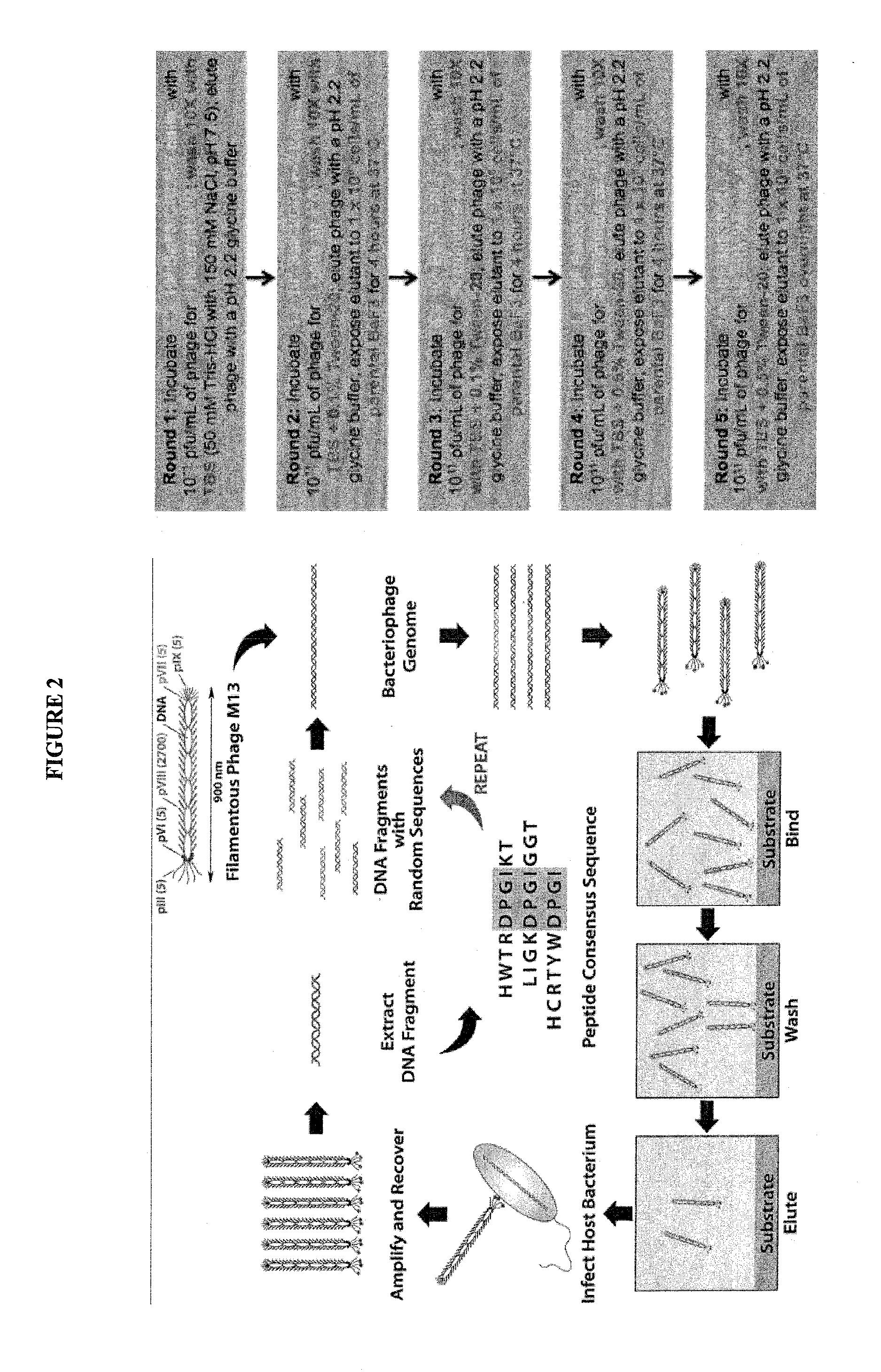
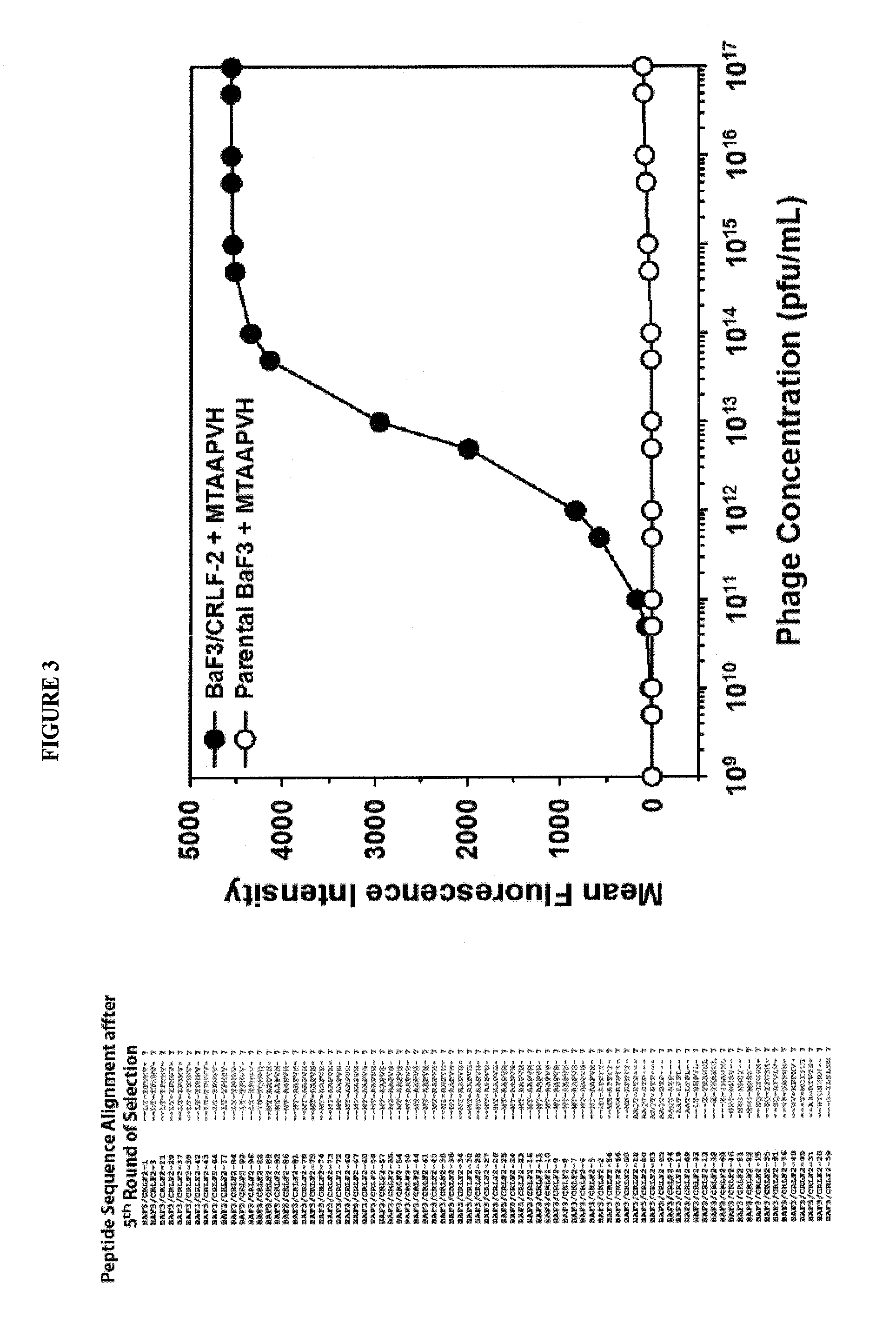
Crlf-2 binding peptides, protocells and viral-like particles useful in the treatment of cancer, including acute lymphoblastic leukemia (ALL)
InactiveUS20150010475A1High-capacity encapsulationLarge capacityUltrasonic/sonic/infrasonic diagnosticsBiocideBinding peptidePhospholipid
The present invention relates to the use of which are attached or anchored phospholipid biolayers further modified by CRLF-2 and CD 19 binding peptides which may be used for delivering pharmaceutical cargos, to cells expressing CRLF-2 and CD 19, thereby treating cancer, in particular, acute lymphoblastic leukemia (ALL), including (B-precursor acute lymphoblastic leukemia (B-ALL). Novel CRLF-2 binding peptides and CLRF-2 and CD19-binding viral-like particles (VLPs) useful in the treatment of cancer, including ALL are also provided.
Owner:STC UNM +1
Anti-CD38 Antibodies for Treatment of Acute Lymphoblastic Leukemia
ActiveUS20150246975A1Organic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAcute lymphocytic leukemiaCombination therapy
Owner:JANSSEN BIOTECH INC
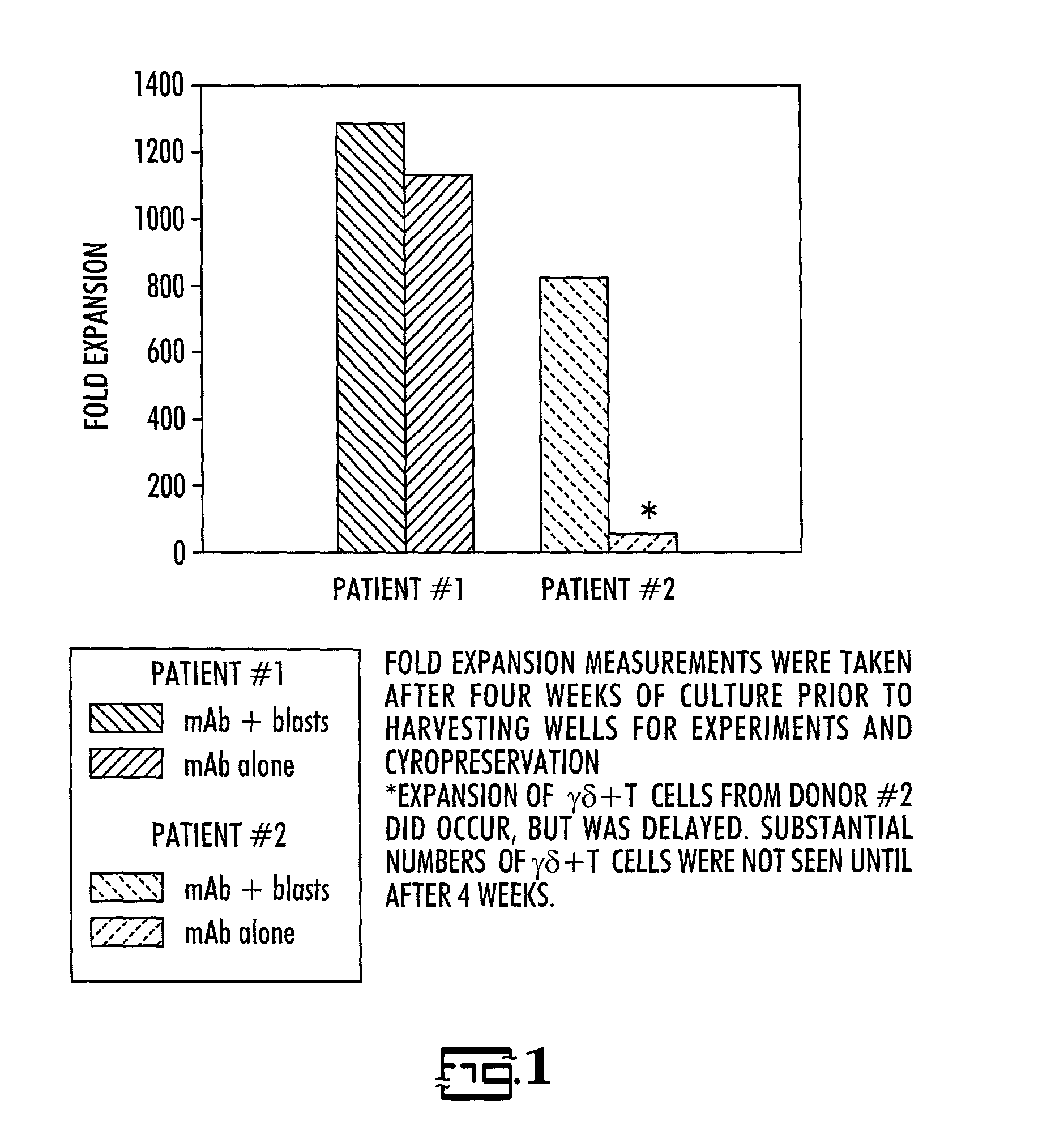
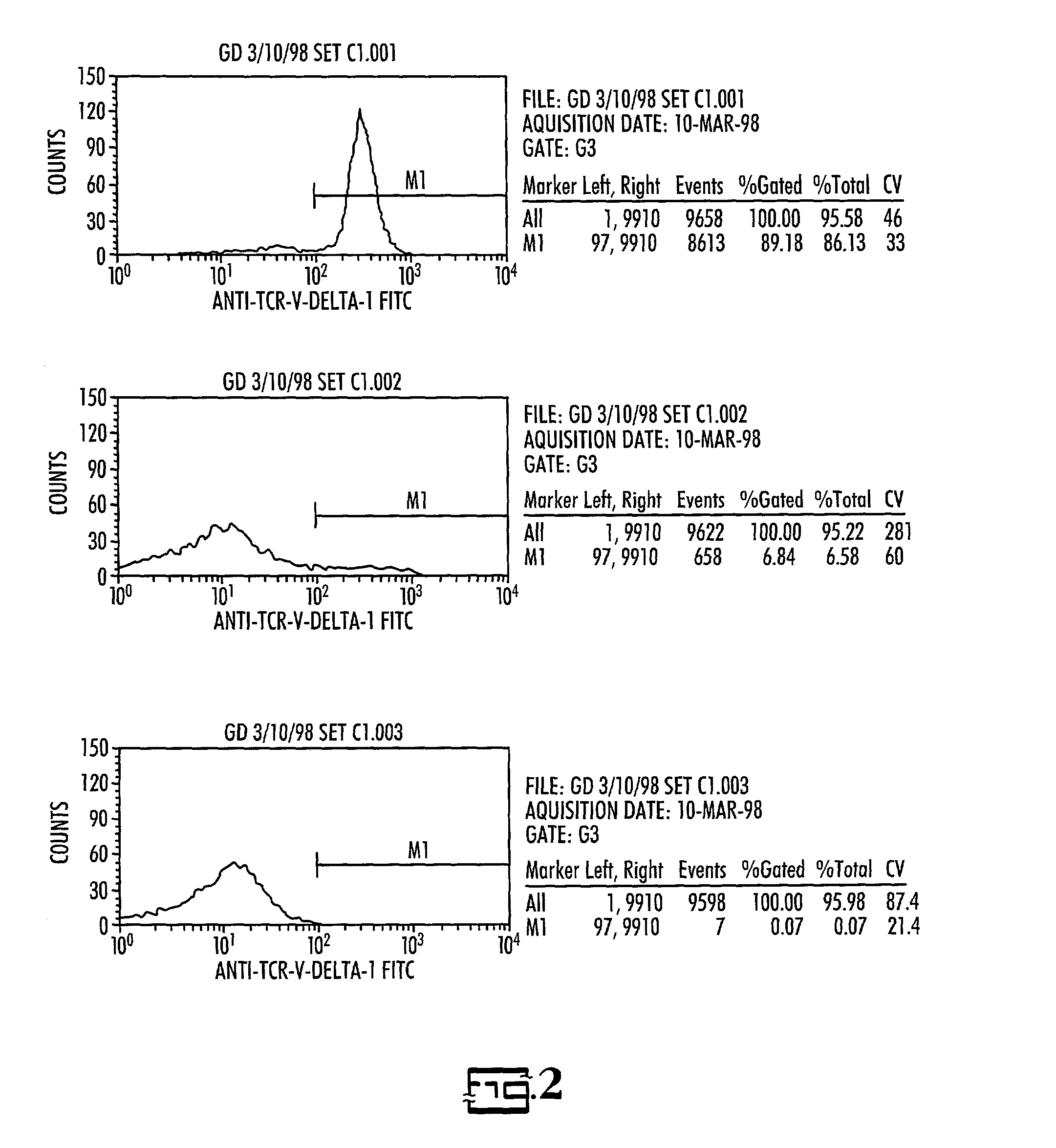
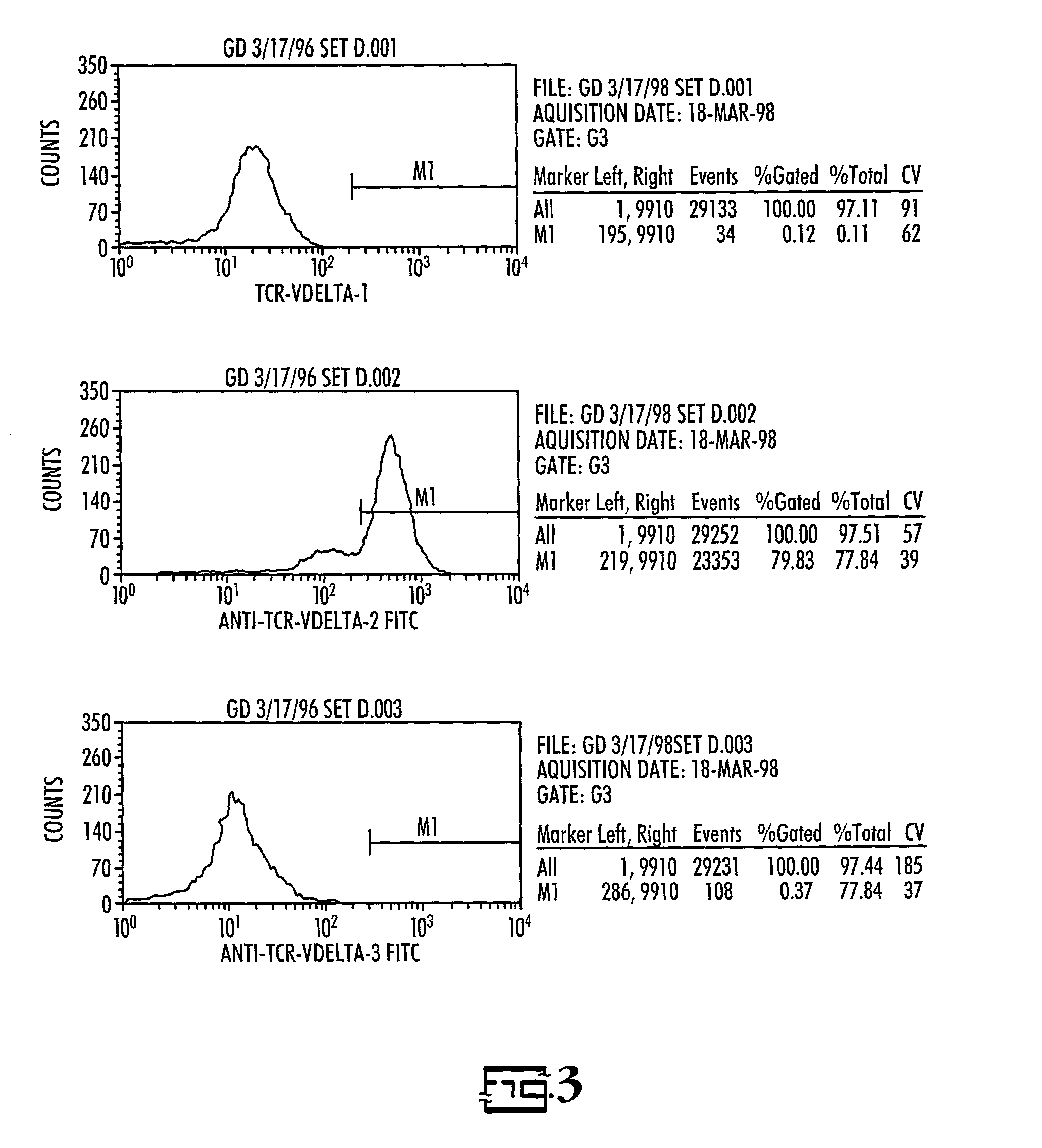
In vitro activated gamma delta lymphocytes
Owner:PALMETTO HEALTH ALLIANCE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com