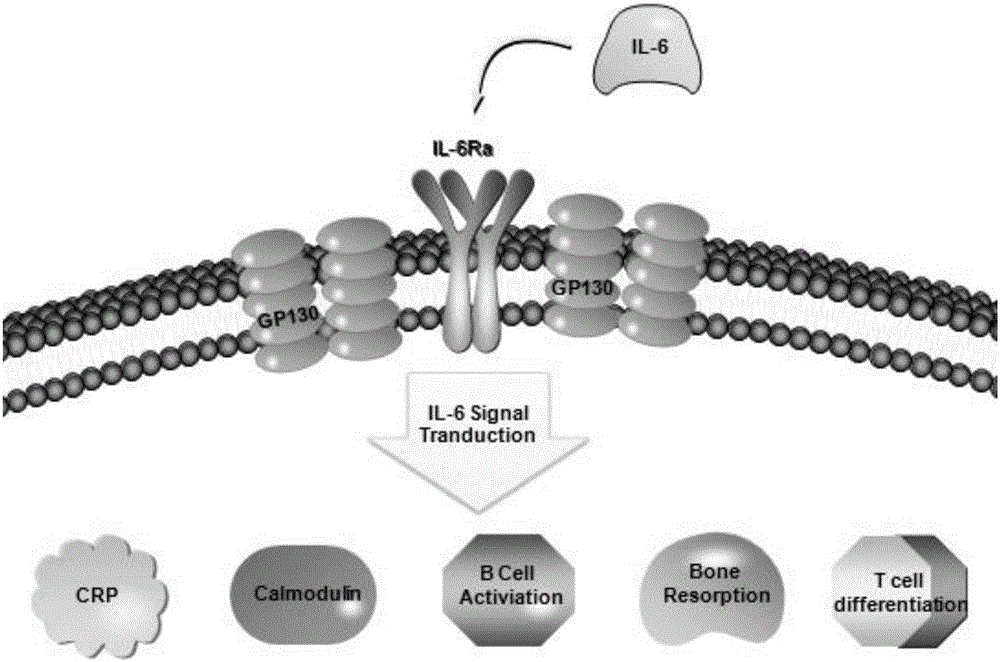
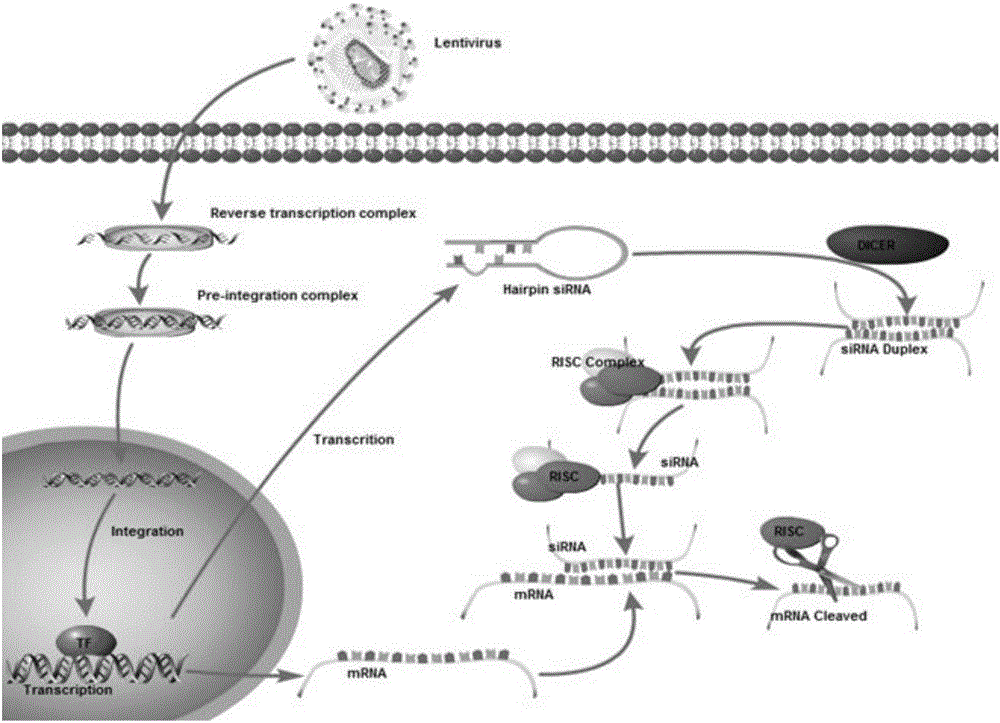
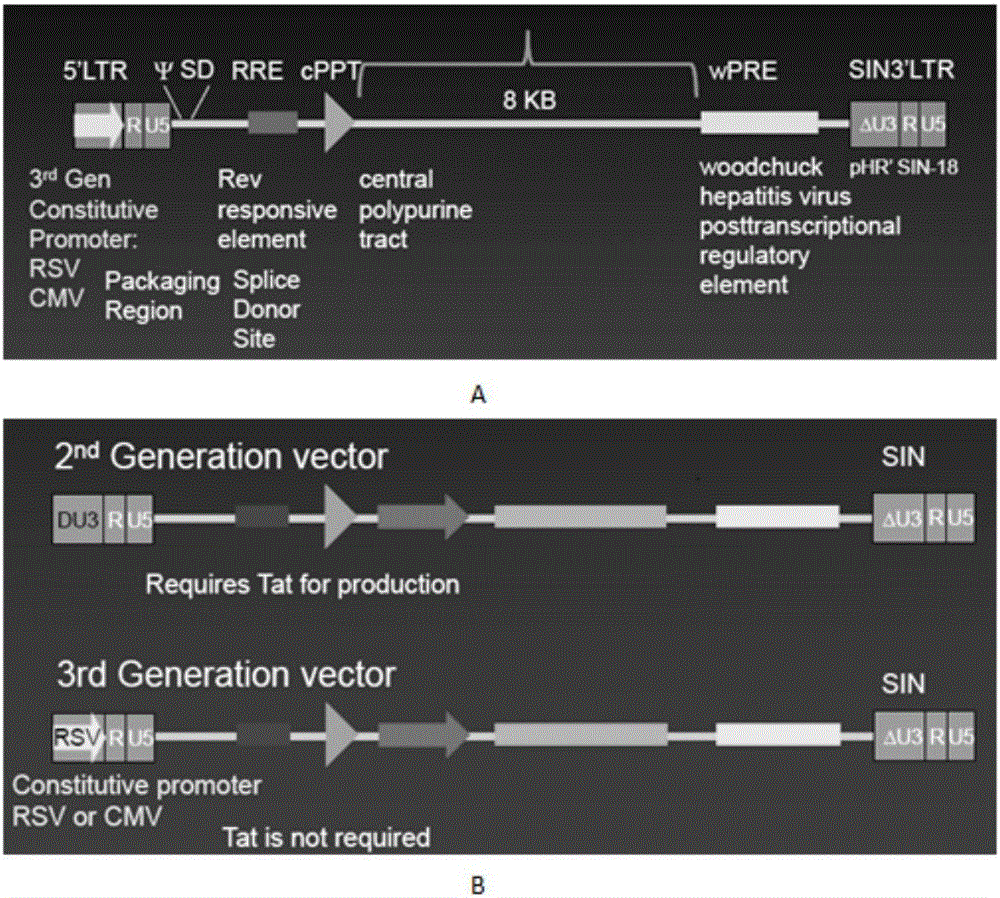
SiRNA of humanized interleukin 6, recombination expression carrier CAR-T and construction method and application of recombination expression carrier CAR-T
An interleukin and expression vector technology, applied in the field of tumor immunotherapy, can solve the problems of high cost, unbearable for ordinary families, and easy infection of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Example 1 Construction of recombinant lentiviral vector
[0083] 1. Materials
[0084] 1. Lentiviral backbone plasmid pLenti-3G silencer, lentiviral packaging plasmids pPac-GP, pPac-R and membrane protein plasmid pEnv-G, HEK293T / 17 cells, homologous recombination enzyme, Oligo Annealing Buffer are provided by Shiao (Shanghai) Bio Provided by Medical Technology Co., Ltd.;
[0085] 2. Primers: Design the primers required for amplifying DNA fragments and target sites according to the primer design principles, specifically:
[0086] EF1α-F: 5'-ATTCAAAATTTTATCGATGCTCCGGTGCCCGTCAGT-3' (SEQ ID NO.25)
[0087] EF1α-R: 5'-TCACGACACCTGAAATGGAAGA-3' (SEQ ID NO.26)
[0088] CD8leader-F: 5'-GGTGTCGTGAGGATCCGCCACCATGGCCTTACCAGTGACCGC-3' (SEQ ID NO.27)
[0089] CD8leader-R: 5'-GTGTCATCTGGATGTCCGGCCTGGCGGCGTG-3' (SEQ ID NO.28)
[0090] VL-F: 5'-CACGCCGCCAGGCCGGACATCCAGATGACACAGACTACATC-3' (SEQ ID NO.29)
[0091] VL-R: 5'-TGTGATCTCCAGCTTGGTCC-3' (SEQ ID NO.30)
[0092] OLC-VH-F...
Embodiment 2
[0196] Example 2 Concentration and detection of recombinant lentiviral vector
[0197] 1. Purification of recombinant lentiviral vectors by ion exchange chromatography (such as Figure 8 shown);
[0198] (1) Use a Thermo vacuum pump to filter the collected supernatant through a 0.22 μm-0.8 μm PES filter to remove impurities;
[0199] (2) Add 1.5M NaCl 250mM Tris-HCl (pH 6-8) to the supernatant at a ratio of 1:1 to 1:10;
[0200] (3) Place two ion exchange columns in series, and pass through the columns sequentially with 4ml 1M NaOH, 4ml 1M NaCl, 5ml 0.15M NaCl25mM Tris-HCl (pH 6-8);
[0201] (4) The solution obtained in step 2 is loaded on the ion exchange column with a speed of 1-10ml / min by a peristaltic pump;
[0202] (5) After passing all the supernatant through the column, wash it once with 10ml 0.15M NaCl 25mM Tris-HCl (pH 6-8) solution;
[0203] (6) Use 1-5ml 1.5M NaCl 25mM Tris-HCl (pH 6-8) for elution according to the loading amount, and collect the eluate;
[02...
Embodiment 3
[0255] Example 3 Functional detection of recombinant lentiviral vectors lvCAR19-1761-lvCAR19-1769.
[0256] 1. Cell-level expression detection of CAR gene:
[0257] (1) After the recombinant lentiviral vector lvCAR19-1761~lvCAR19-1769 and the control virus MOCK infected PBMC cells, the collected cells were detected by RT-PCR for CAR mRNA transcription level to verify the expression of CAR gene. If the CAR mRNA transcription level increased, then It shows that the transcription level expression of CAR gene is successful;
[0258] (2) After the recombinant lentiviral vector lvCAR19-1761~lvCAR19-1769 and the control virus MOCK infected PBMC cells, the cells were collected to detect the expression level of CAR protein by western blot to verify the expression of CAR gene. If the expression level of CAR protein increased, then It shows that the translation level expression of CAR gene is successful;
[0259] (3) Cells were infected with lvCAR19-1761~lvCAR19-1769 at MOI=15 and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com