Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
467 results about "Membrane protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
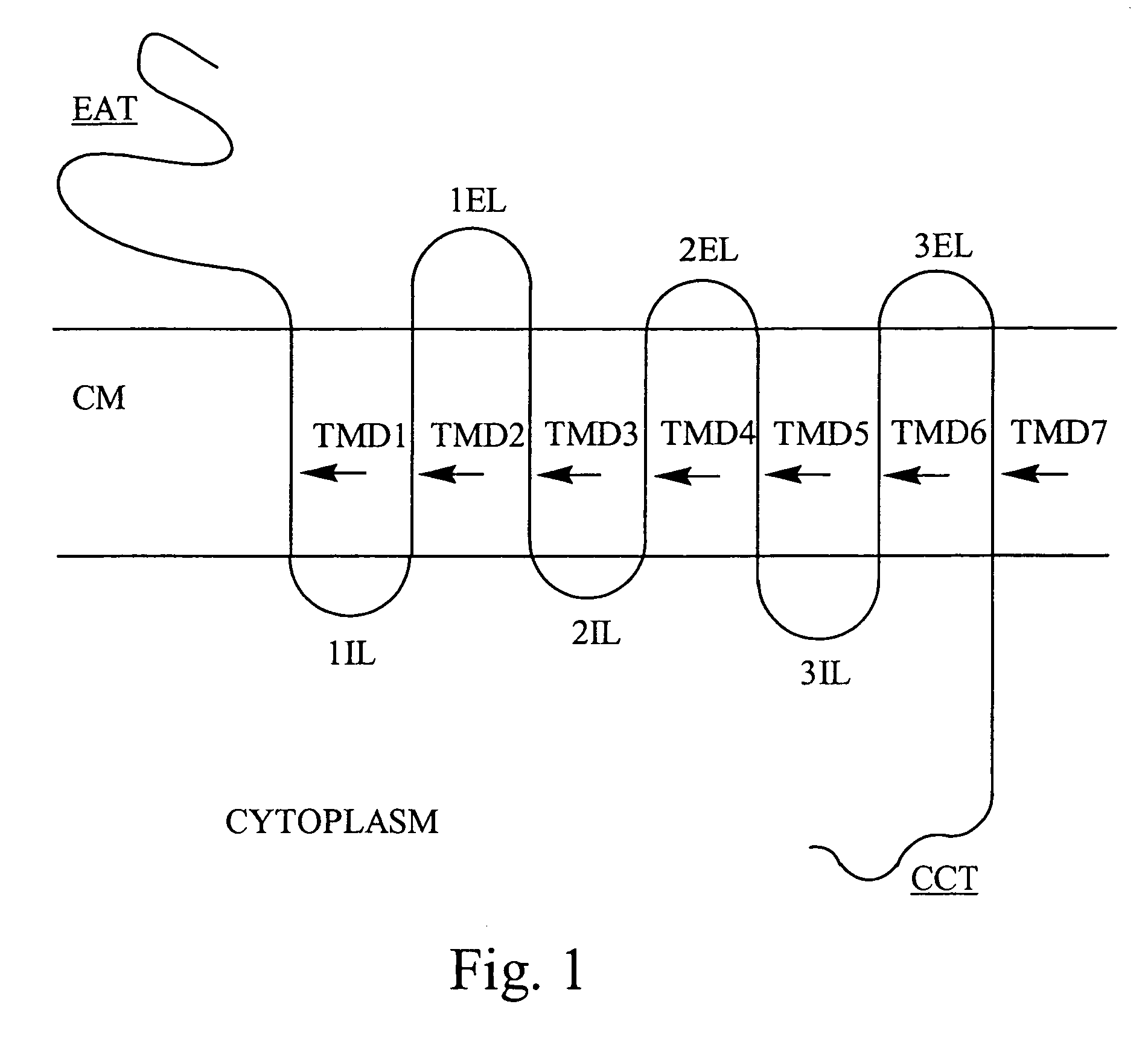
Membrane proteins are common proteins that are part of, or interact with, biological membranes. Membrane proteins fall into several broad categories depending on their location. Integral membrane proteins are a permanent part of a cell membrane and can either penetrate the membrane (transmembrane) or associate with one or the other side of a membrane (integral monotopic). Peripheral membrane proteins are transiently associated with the cell membrane.
Outer membrane vesicle (OMV) vaccine comprising N. meningitidis serogroup B outer membrane proteins
ActiveUS8273360B2Broadens their efficacyAntibacterial agentsNervous disorderProtective antigenNeisseria weaveri
A composition comprising (a) Neisseria meningitidis serogroup B outer membrane vesicles (OMVs), and (b) an immunogenic component selected from other Neisseria proteins, or immunogenic fragments thereof. Component (b) preferably includes a protein from a different NmB strain from that from which the OMV of component (a) is derived. The OMVs are preferably obtained by deoxycholate extraction. Optionally, the composition may also comprise a protective antigen against other pathogens.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Novel proteosome-liposaccharide vaccine adjuvant
InactiveUS20030044425A1Increase secretionUniform processSsRNA viruses negative-senseBiocideImmunotherapeutic agentCytokine
An adjuvant complex composed of bacterial outer membrane protein proteosomes complexed to bacterial liposaccharide is prepared to contain the component parts under a variety of conditions. The complex can be formulated with antigenic material to form immunogenic compositions, vaccines and immunotherapeutics. An induced immune response includes protective antibodies and / or type 1 cytokines is shown for a variety of protocols.
Owner:ID BIOMEDICAL CORP LAVAL
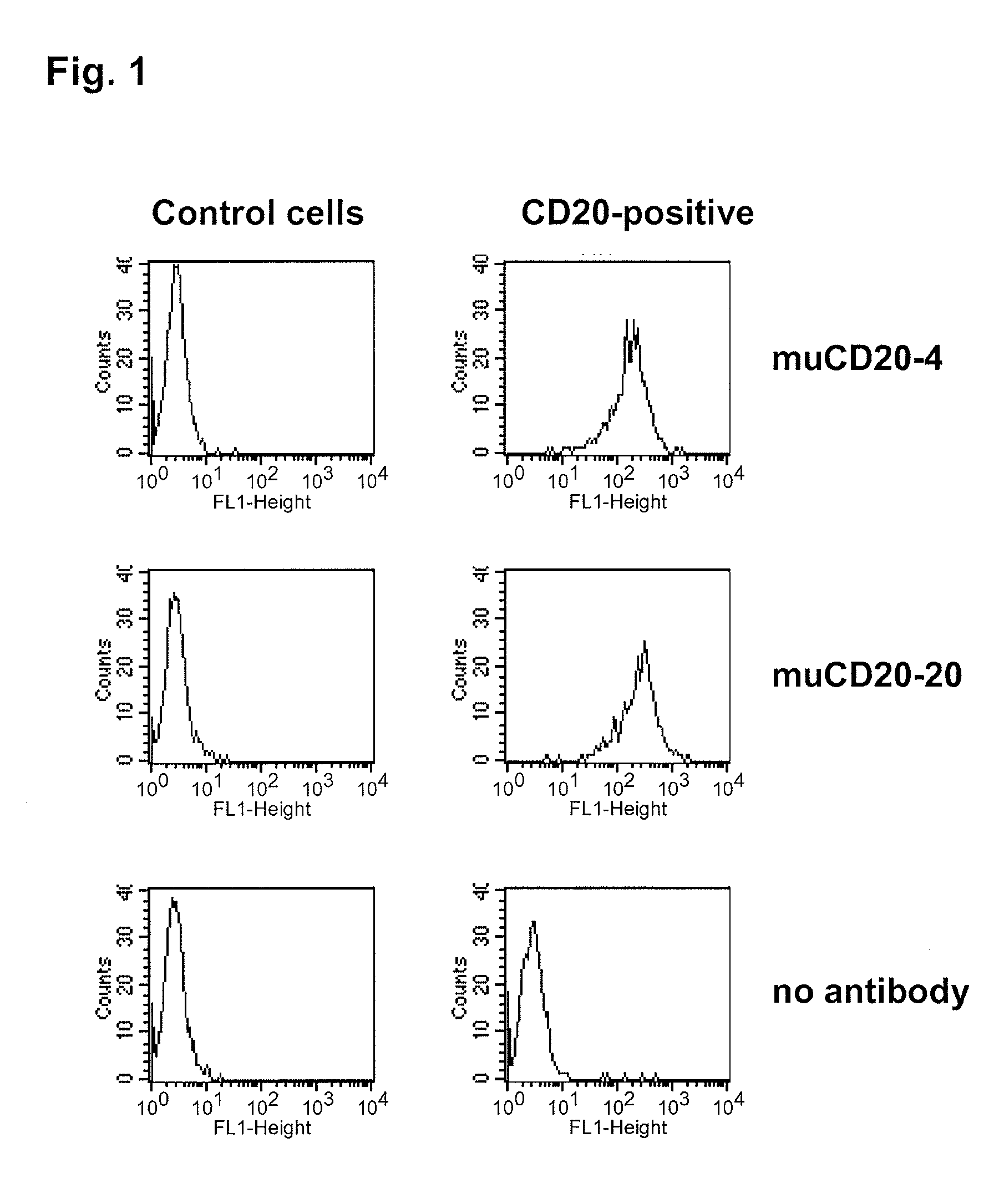
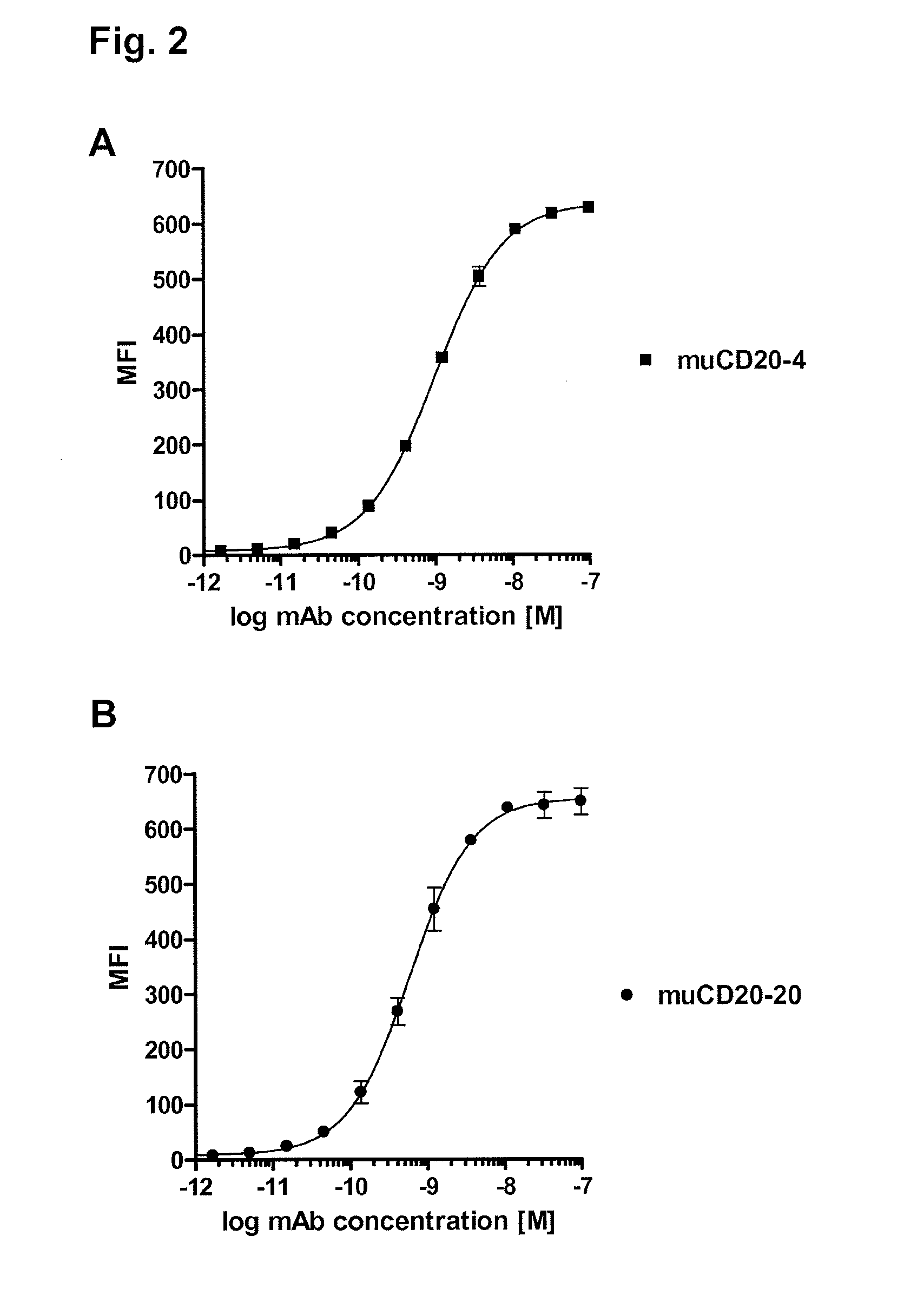
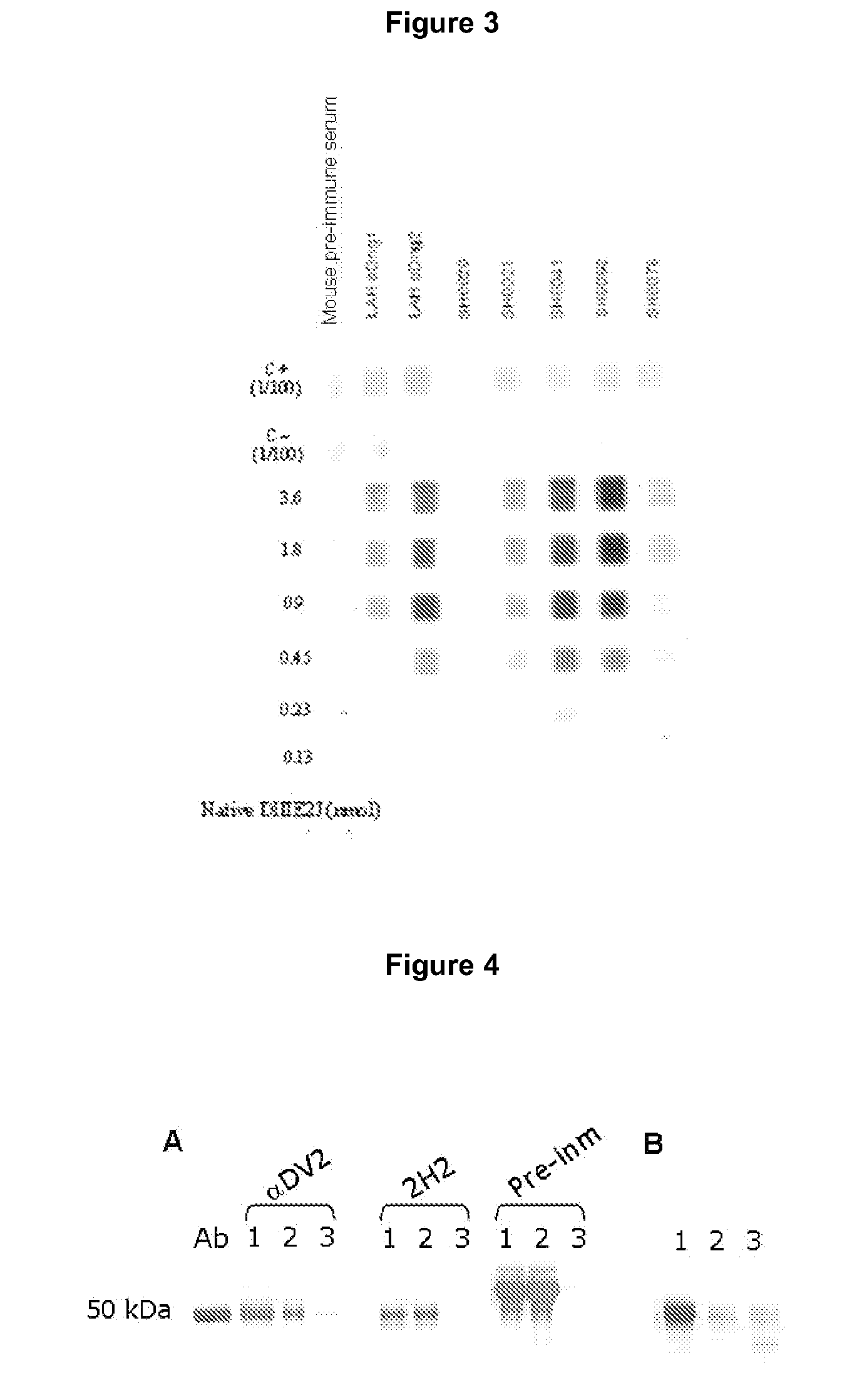
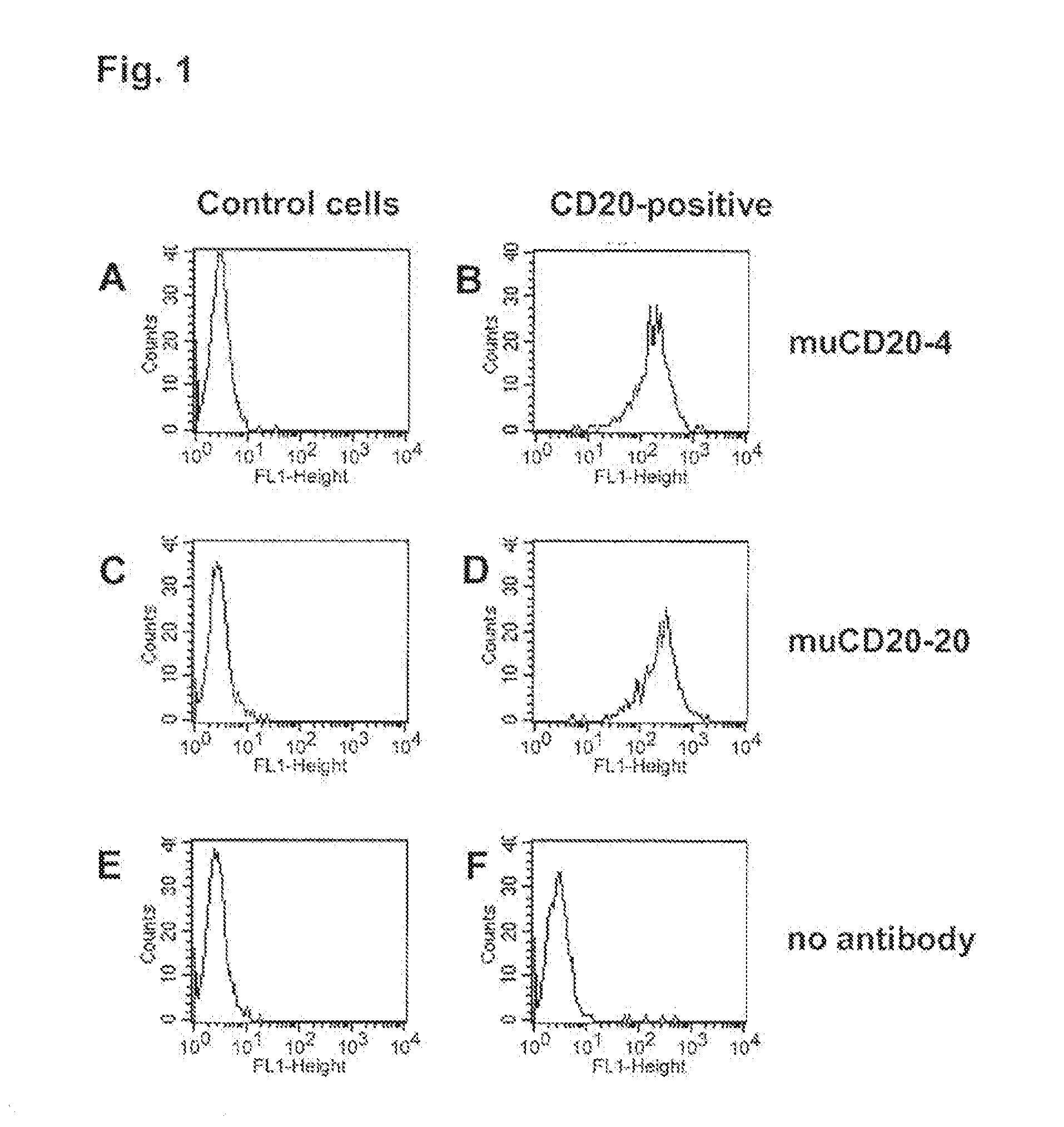
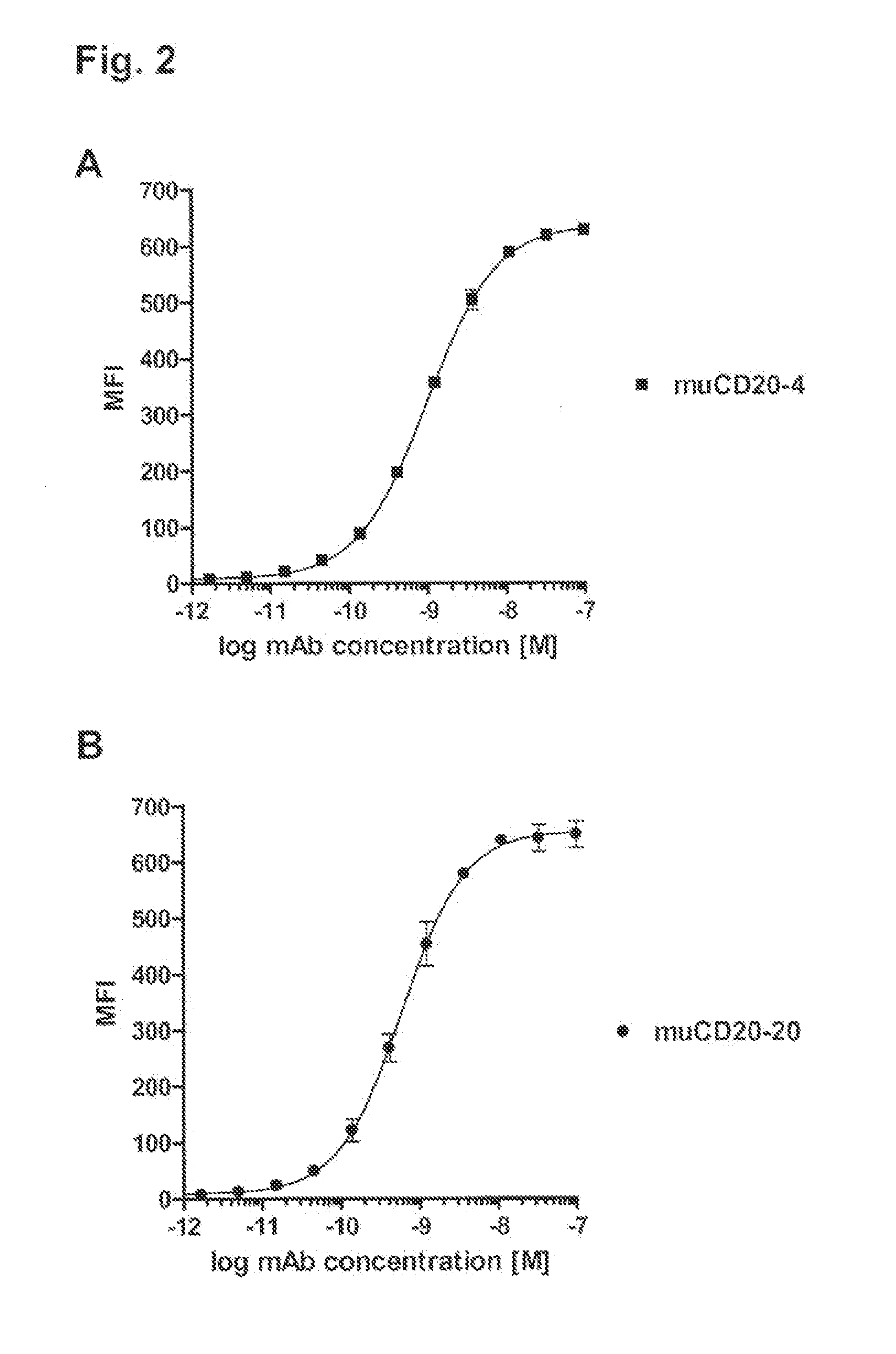
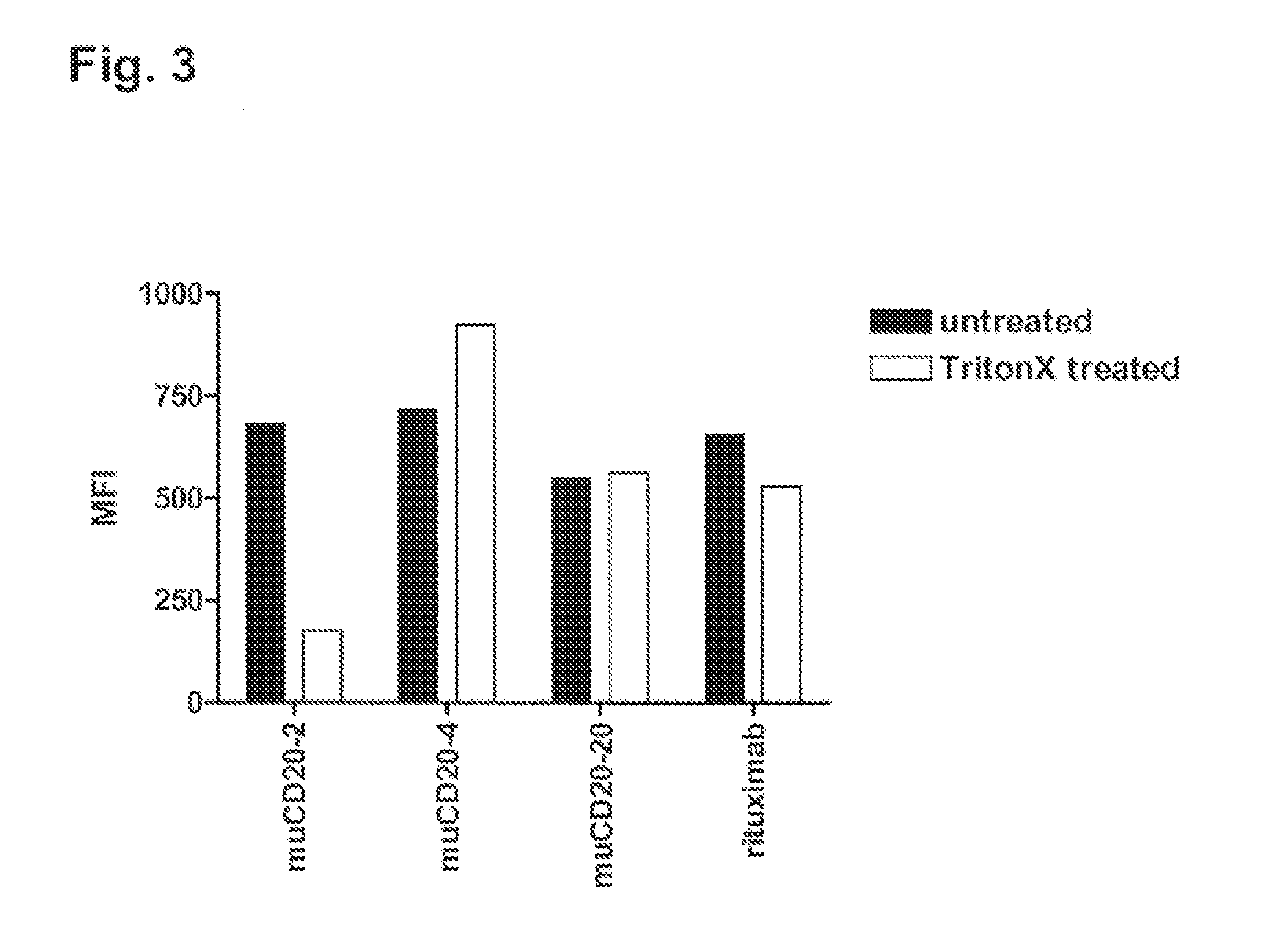
Cd20 antibodies and uses thereof
CD20 is a transmembrane protein of the tetra-spanin family expressed on the surface of B-cells and has been found on B-cells from peripheral blood as well as lymphoid tissues. CD20 expression persists from the early pre-B cell stage until the plasma cell differentiation stage. Conversely, it is not found on hematopoietic stem cells, pro-B cells, differentiated plasma cells or non-lymphoid tissues. In addition to expression in normal B-cells, CD20 is expressed in B-cell derived malignancies such as non-Hodgkin's lymphoma (NHL) and B-cell chronic lymphocytic leukemia (CLL). CD20 expressing cells are known to play a role in other diseases and disorders, including inflammation. The present invention includes anti-CD20 antibodies, forms and fragments, having superior physical and functional properties; immunoconjugates, compositions, diagnostic reagents, methods for inhibiting growth, therapeutic methods, improved antibodies and cell lines; and polynucleotides, vectors and genetic constructs encoding same.
Owner:IMMUNOGEN INC
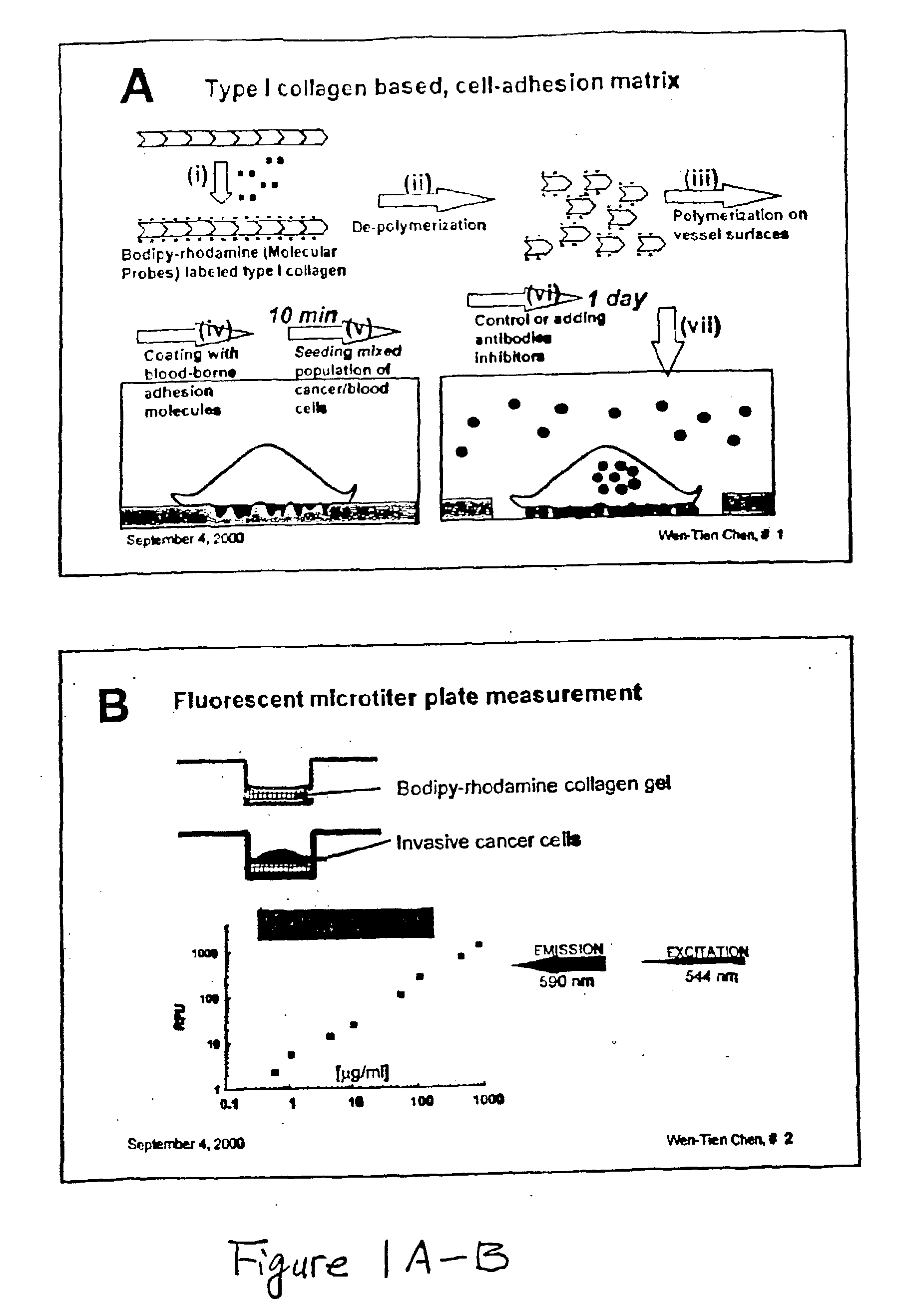
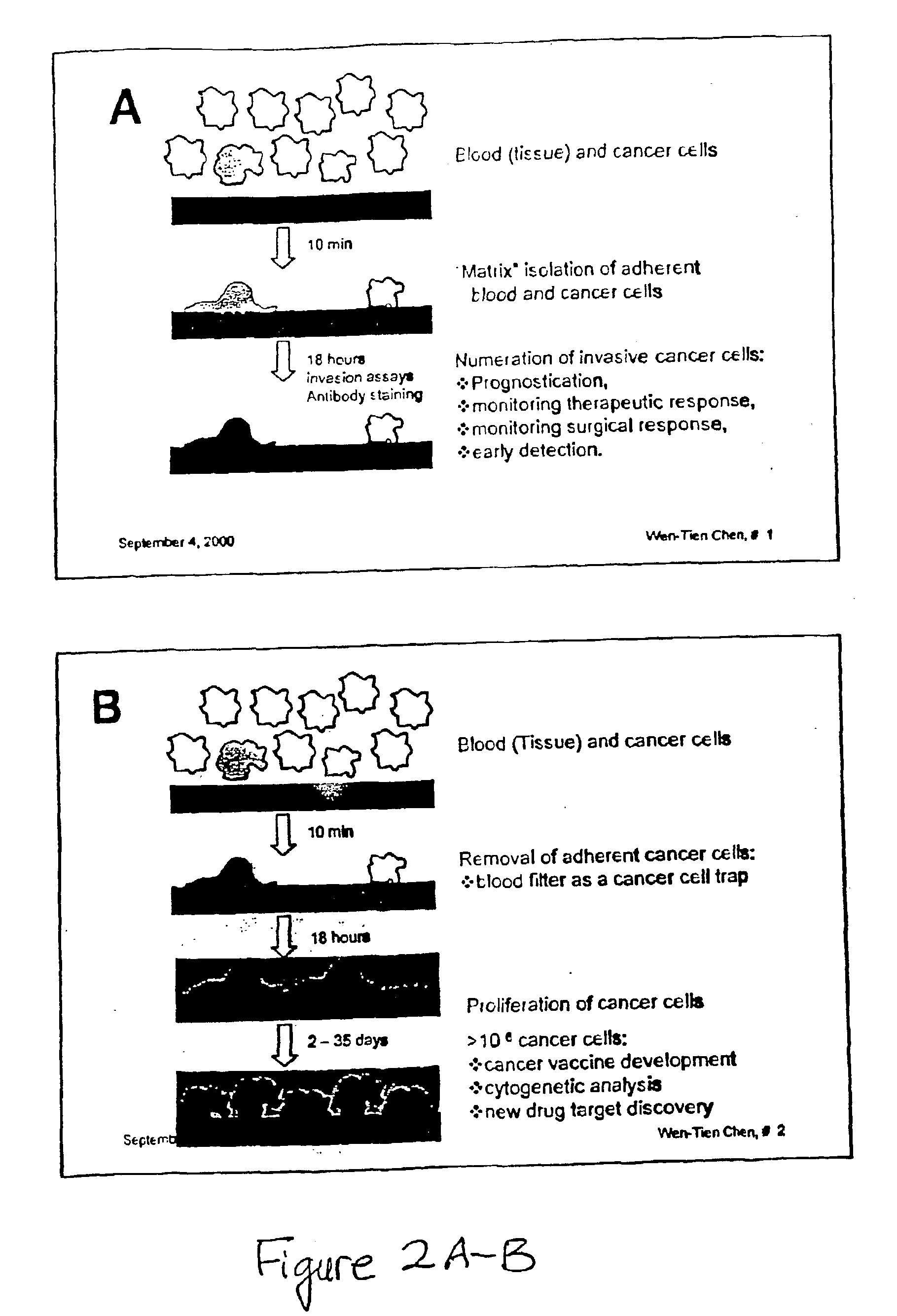
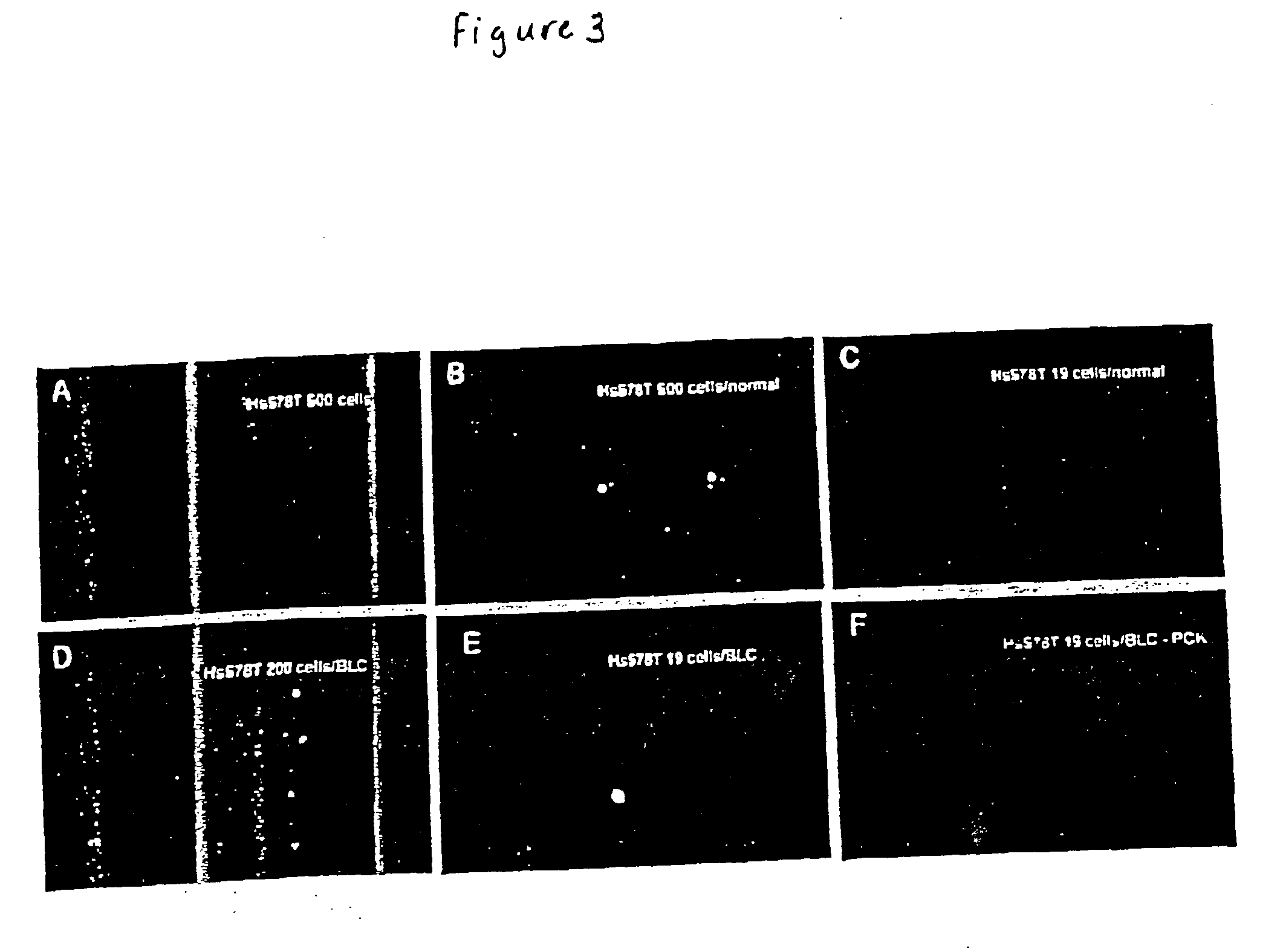
Method and compositions for isolating metastatic cancer cells, and use in measuring metastatic potentatial of a cancer thereof
InactiveUS20030206901A1Quick checkRapid isolationSolvent extractionOther blood circulation devicesCancer cellProteinase activity
The present invention relates to novel methods and compositions for detection and isolation of cancer cells with metastatic potential. The invention further relates to assays for measuring the metastatic potential of such cancer cells and drug screening assays for the identification of agents having anti-metastatic potential. The present invention further provides methods and compositions for inhibiting the metastatic potential of cancer cells by modulating the activity of serine integral membrane proteases [(SIMP) consisting of seprase and dipedidyl peptidase IV (DPPIV)]expressed on the surface of metastasizing cancer cells.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
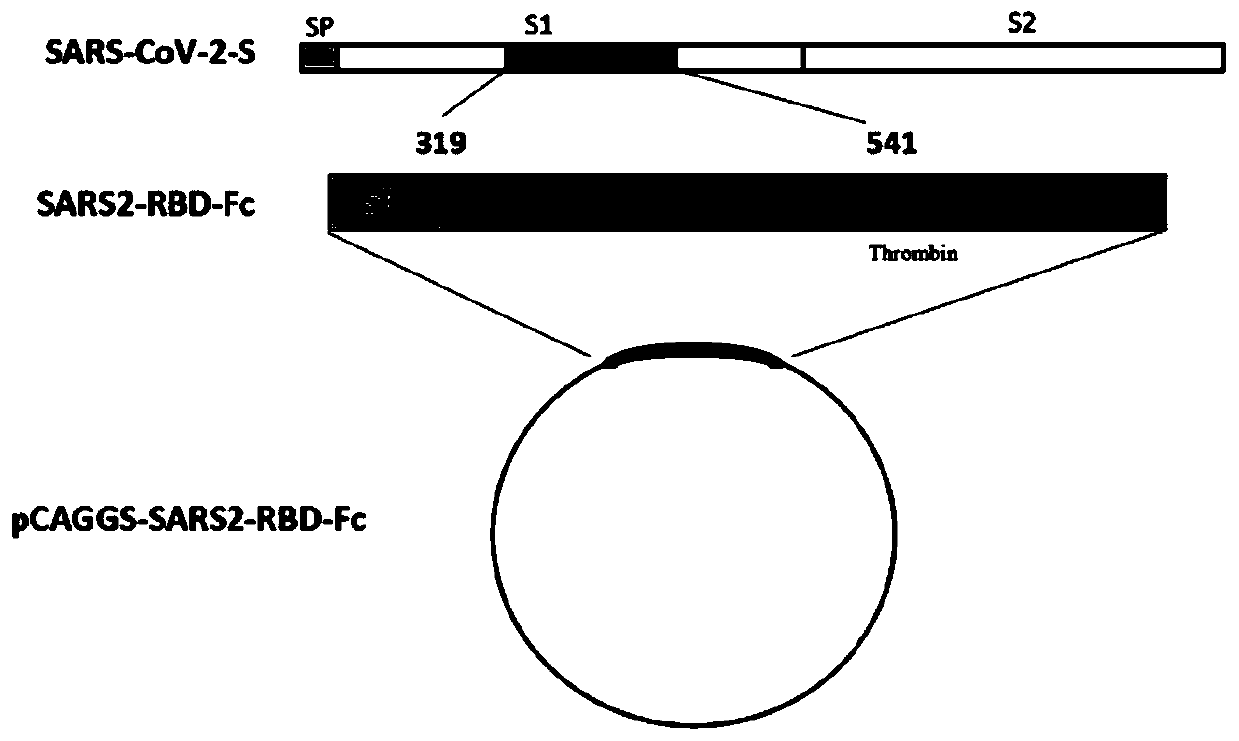
Subunit vaccine for novel coronavirus and application of subunit vaccine
InactiveCN111533809AImproving immunogenicityImprove stabilitySsRNA viruses positive-senseViral antigen ingredientsAntibody fragmentsTGE VACCINE
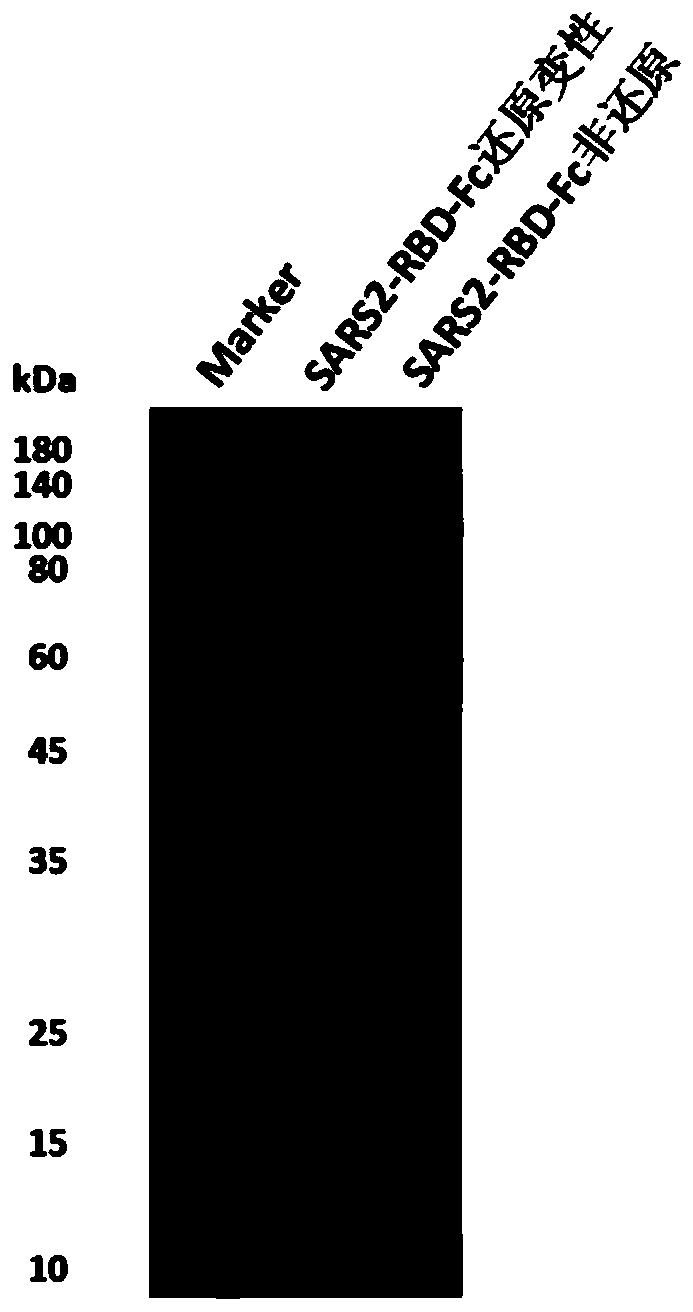
The invention discloses a fusion protein of a novel coronavirus envelope protein and an application of the fusion protein. The fusion protein (SARS2-RBD-Fc) is obtained by fusing an RBD structural domain of a novel coronavirus envelope protein S with an antibody Fc fragment; and as a subunit vaccine, the fusion protein can induce an organism to generate an efficient neutralizing antibody through nasal drip immunization and intramuscular injection. It indicates that the SARS2-RBD-Fc can be used as a candidate vaccine for preventing and treating new coronavirus infection.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Methods of enhancing translocation of charged analytes through transmembrane protein pores
ActiveCN102317310AMicrobiological testing/measurementBiological material analysisAnalyteProtein translocation
The invention relates to enhancing translocation of a charged analyte through a transmembrane protein pore. Translocation is enhanced by increasing the net opposing charge of the barrel or channel and / or entrance of the pore. The invention also relates to pores enhanced in accordance with the invention.
Owner:OXFORD UNIV INNOVATION LTD
Plasma membrane vesicles comprising functional transmembrane proteins
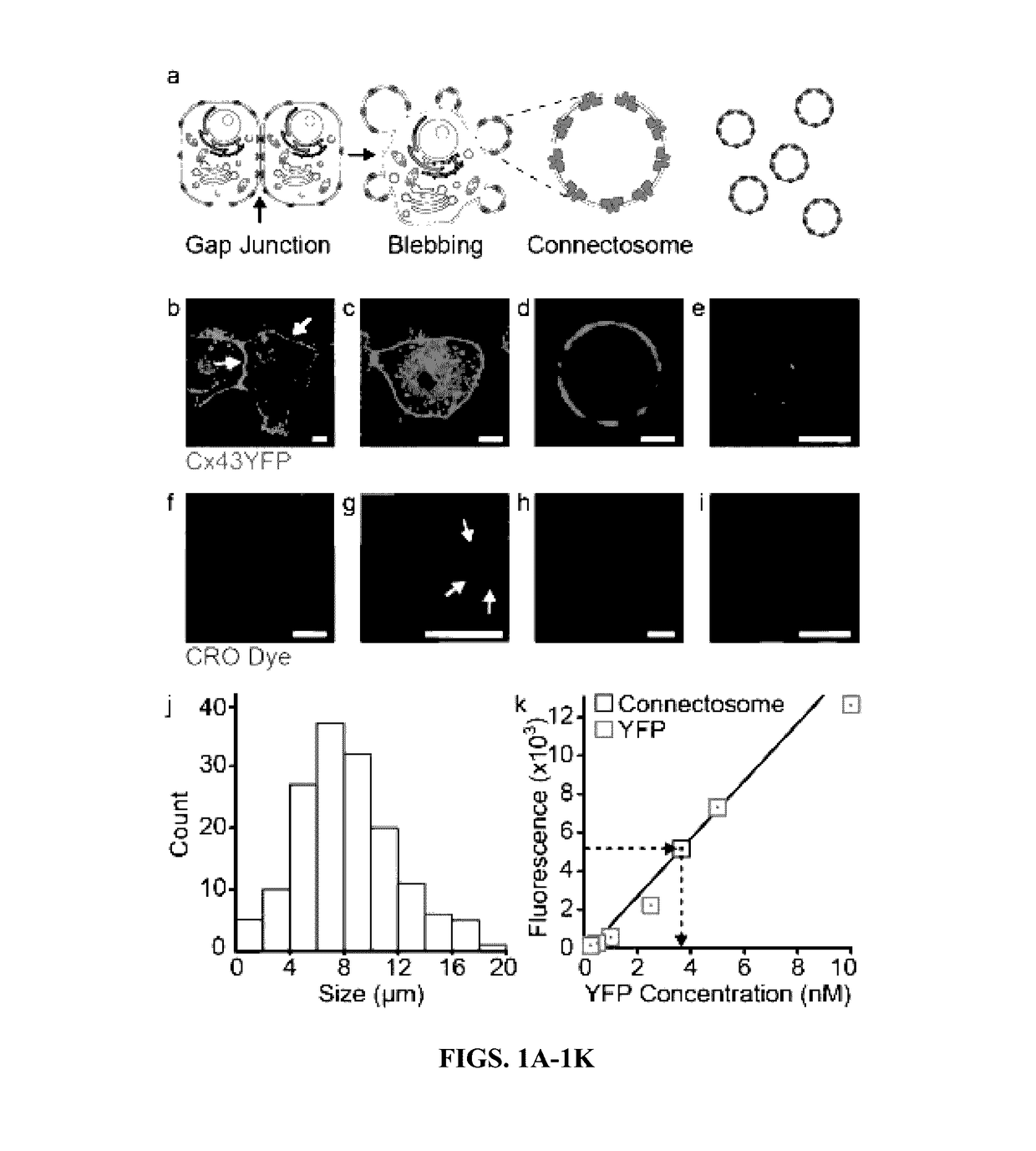
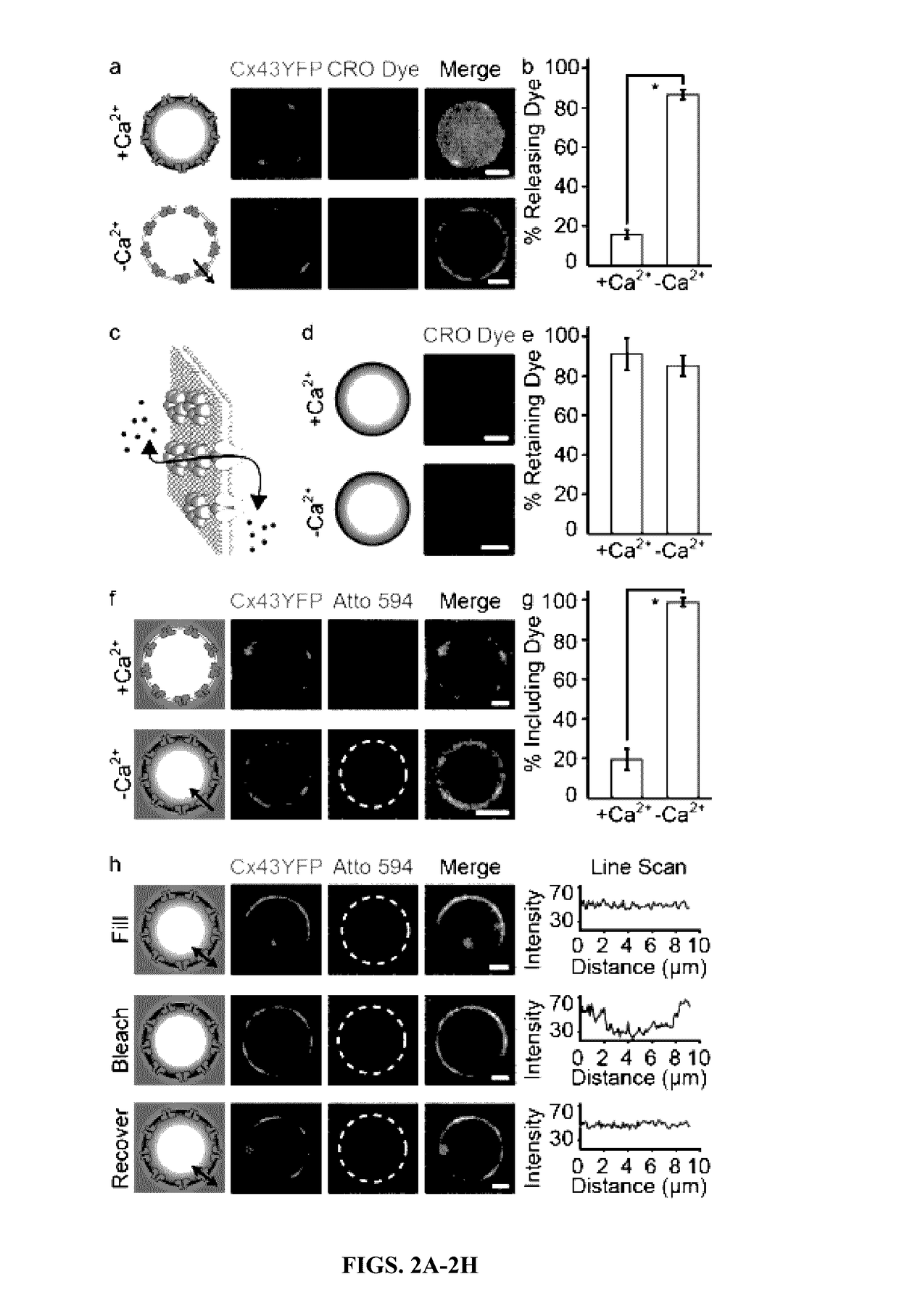
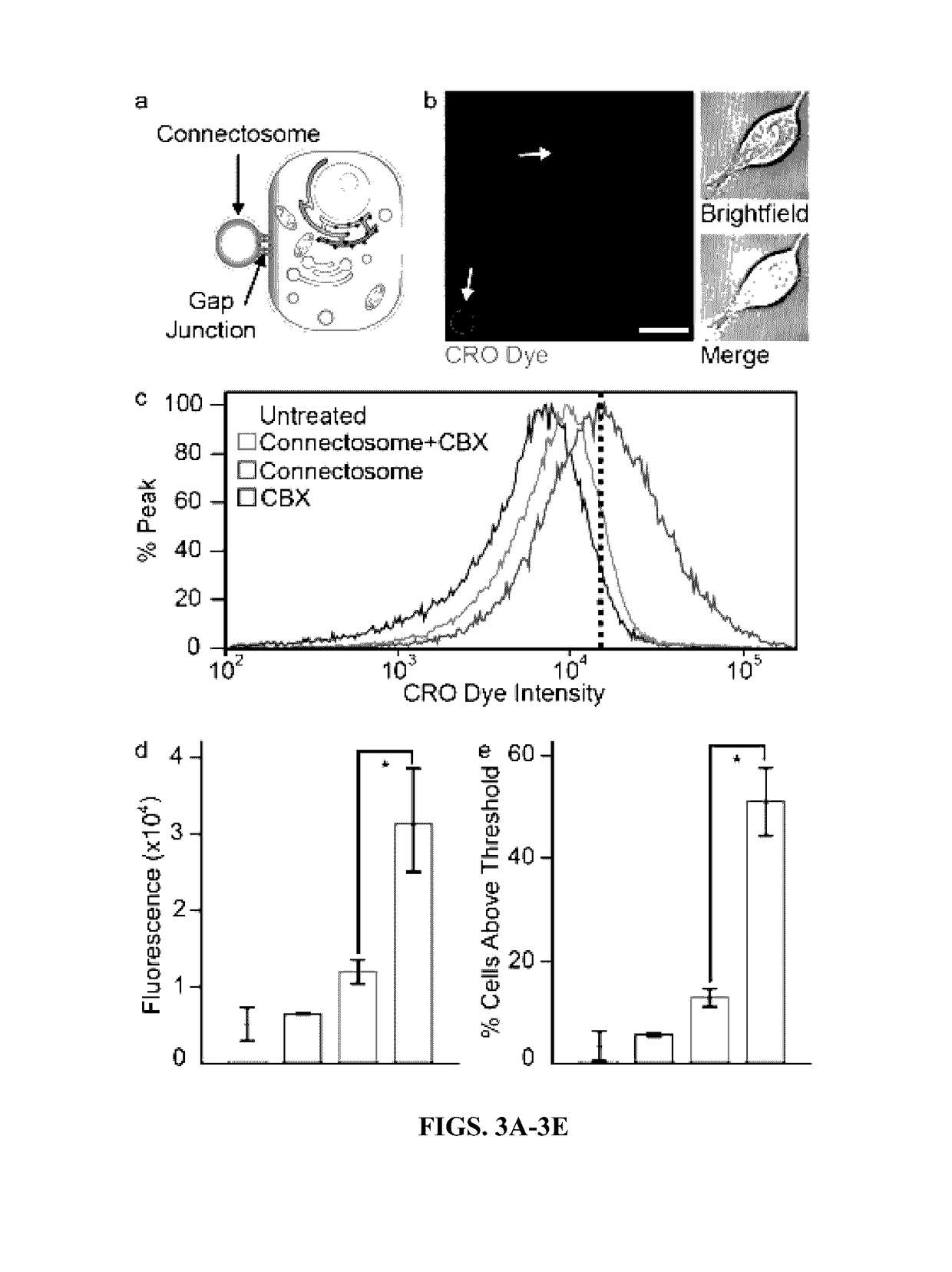
InactiveUS20170112773A1Enhances and restores cellular gap junction communicationEnhances and restores endogenous CFTR functionOrganic active ingredientsPharmaceutical non-active ingredientsMedicineVesicle/vacuole
Provided herein are methods and compositions for treating a subject in need thereof comprising administering an effective amount of vesicles with functional transmembrane proteins embedded in the plasma membrane. Also provided herein are methods of restoring gap junctional communication comprising the administration of an effective amount of vesicles.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Stabilized viral envelope proteins and uses thereof
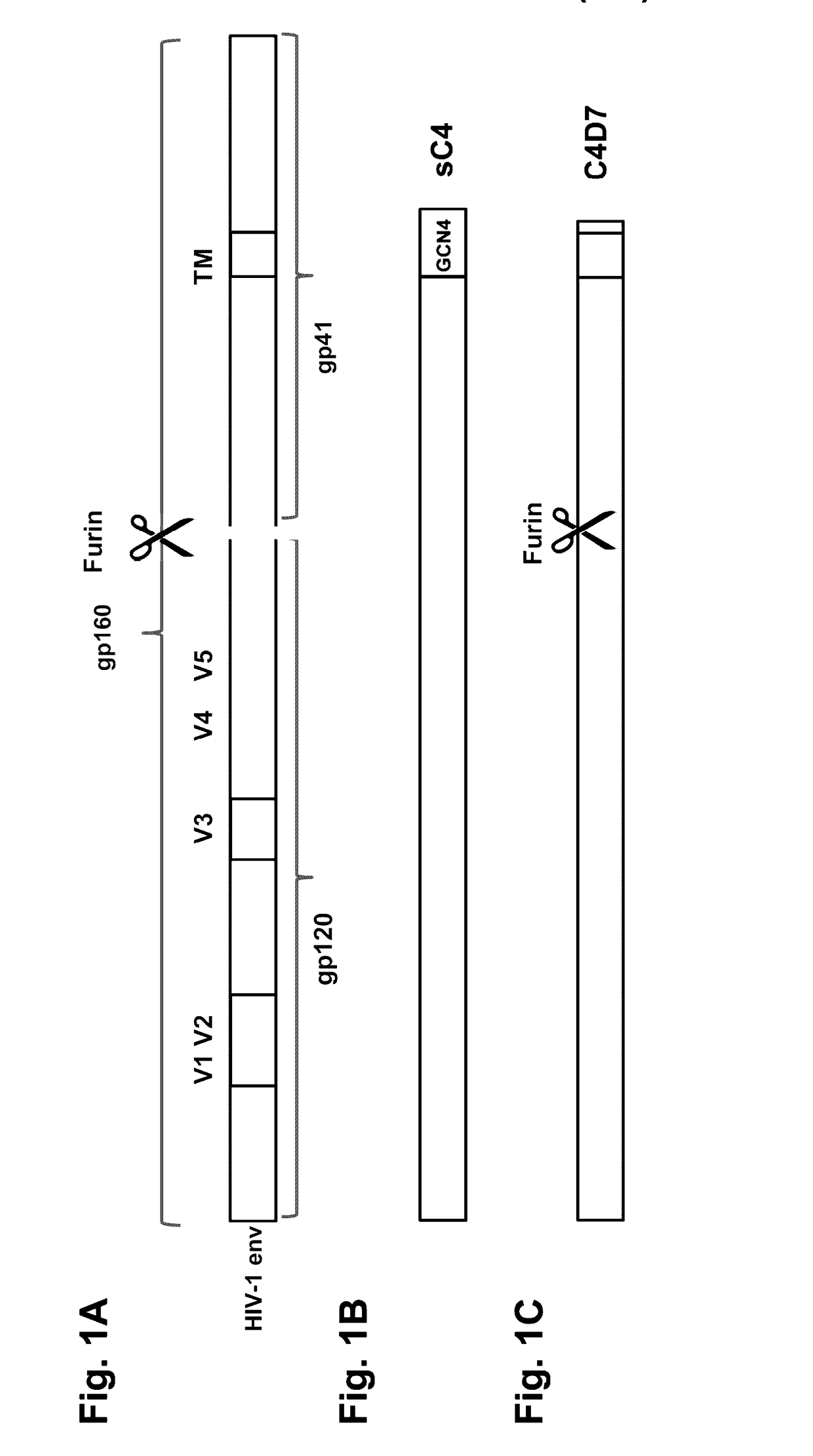
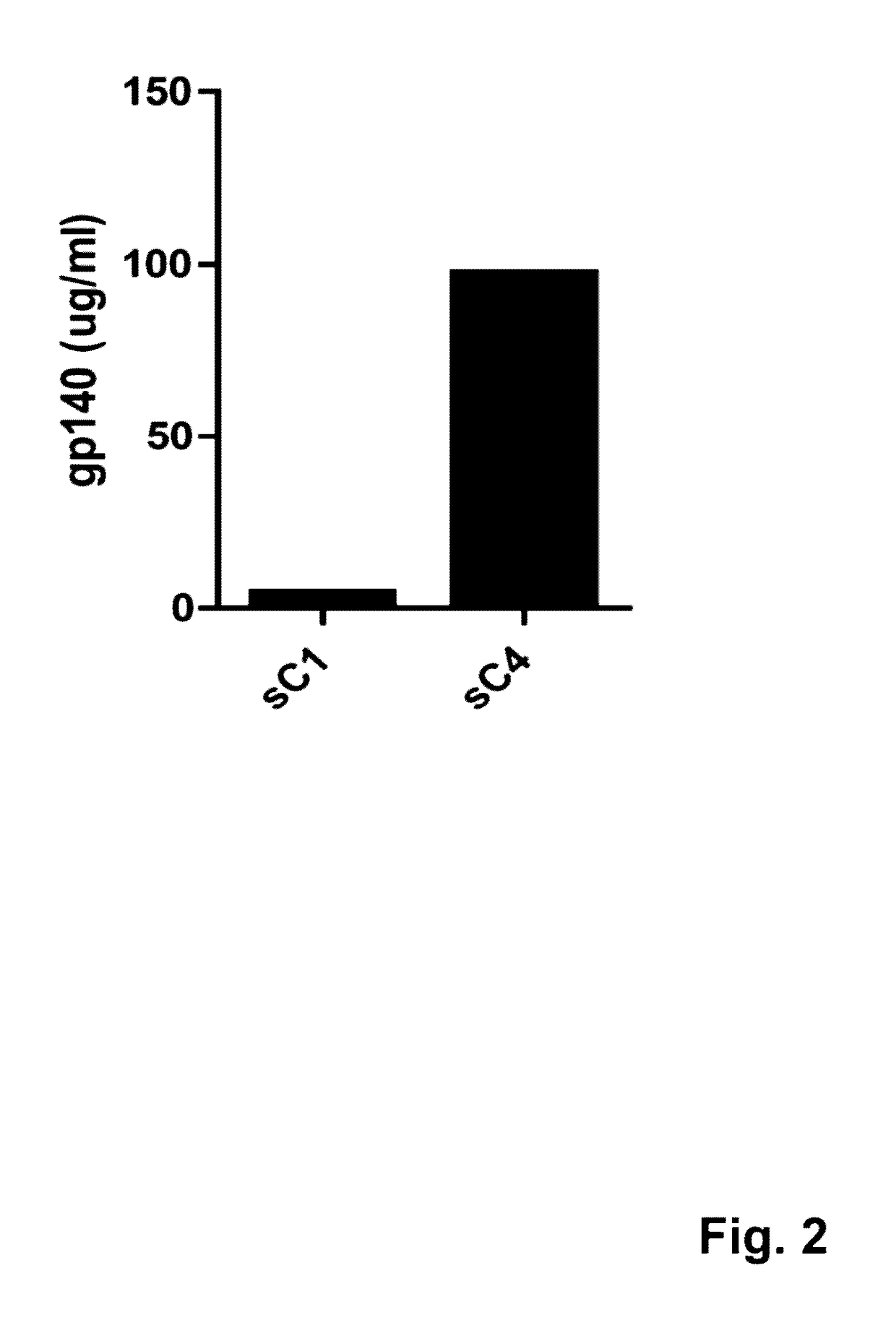
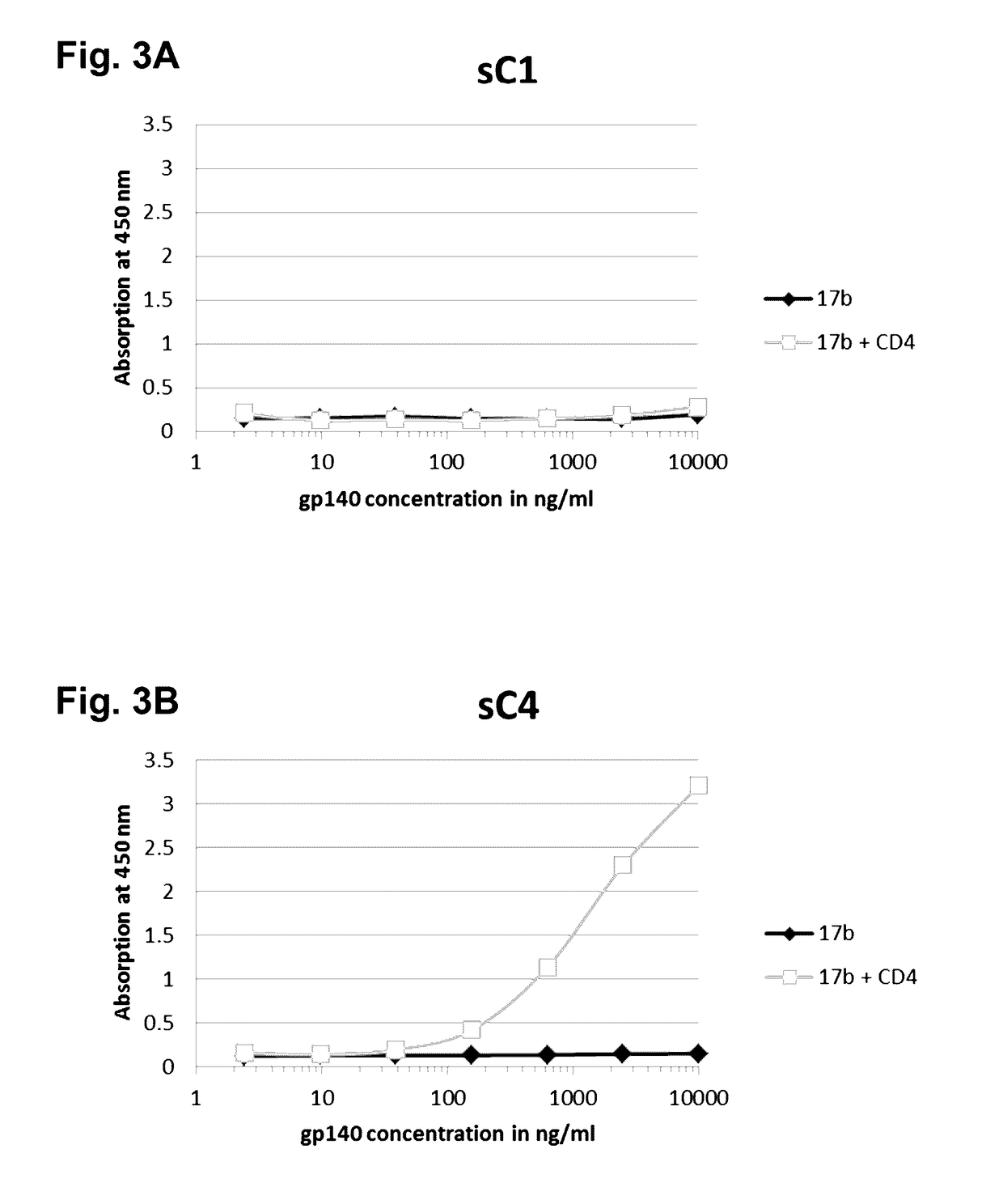
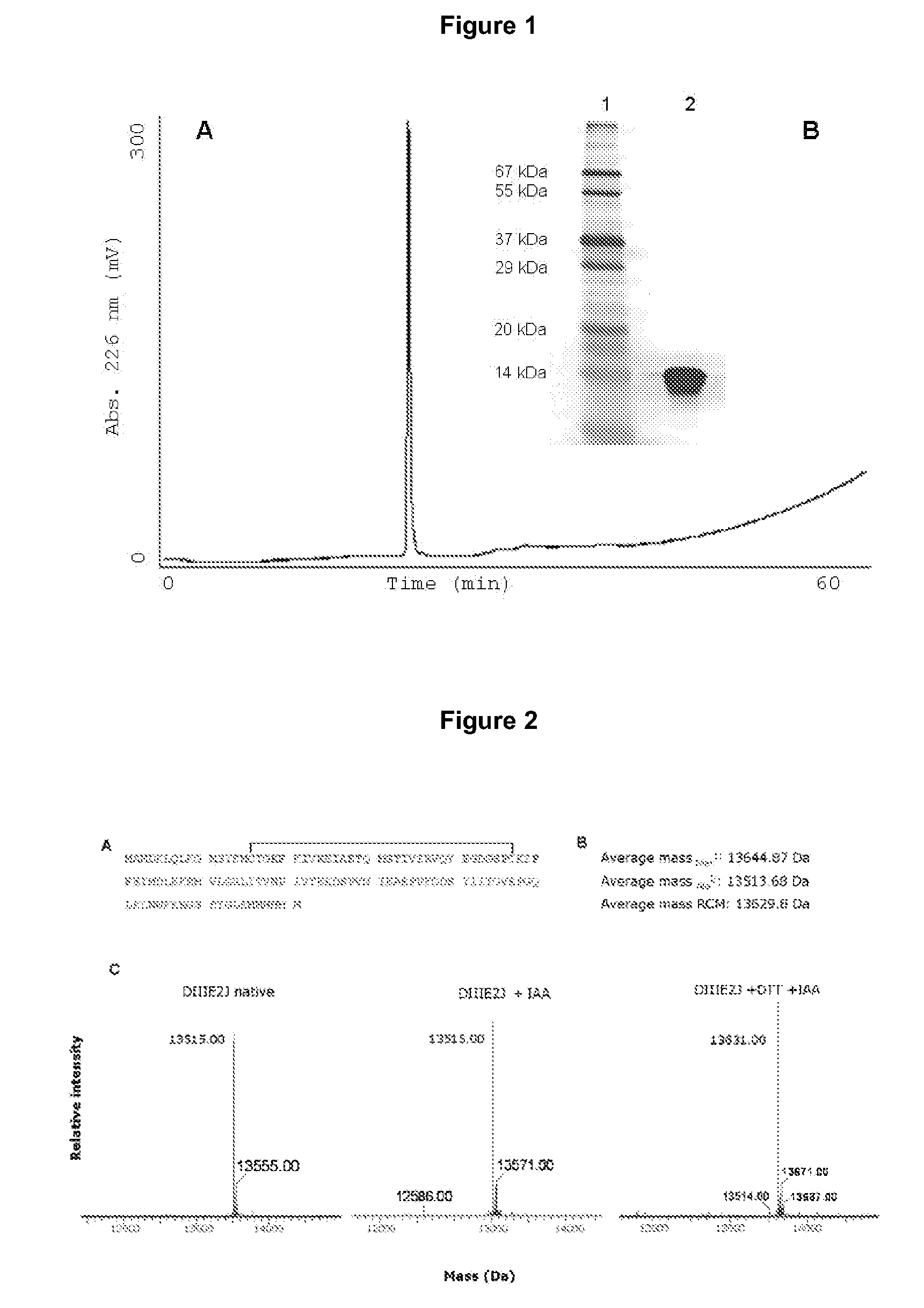
This invention provides an isolated nucleic acid which comprises a nucleotide segment having a sequence encoding a viral envelope protein comprising a viral surface protein and a corresponding viral transmembrane protein wherein the viral envelope protein contains one or more mutations in amino acid sequence that enhance the stability of the complex formed between the viral surface protein and transmembrane protein. This invention also provides a viral envelope protein comprising a viral surface protein and a corresponding viral transmembrane protein wherein the viral envelope protein contains one or more mutations in amino acid sequence that enhance the stability of the complex formed between the viral surface protein and transmembrane protein. This invention further provides methods of treating HIV-1 infection.
Owner:PROGENICS PHARMA INC
Nutrition intensified vegetable paper and manufacturing method thereof
The invention discloses a nutrition intensified vegetable paper and manufacturing method, wherein the preparing process comprises choosing fresh vegetables, cleaning, chopping, blanching, charging thickening agent, plasticizing agent, membrane forming protein, flavoring agent and nutrescin, homogenizing into vegetable pulp, drying, removing film, cutting and packaging.
Owner:JINAN UNIVERSITY
Chlamydia antigens and corresponding DNA fragments and uses thereof
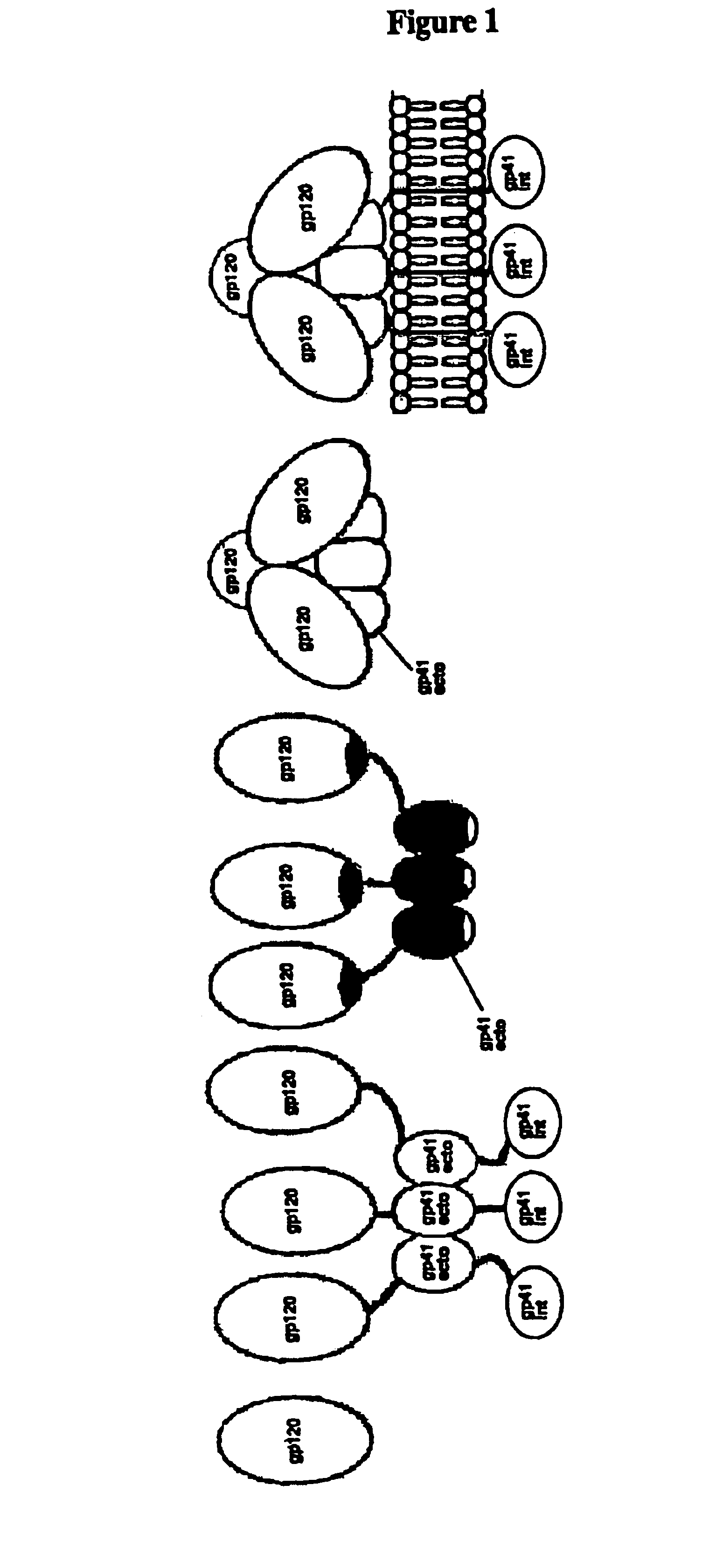
The present invention provides nucleic acids, proteins and vectors for a method of nucleic acid, including DNA, immunization of a host, including humans, against disease caused by infection by a strain of Chlamydia, specifically C. pneumoniae. The method employs a vector containing a nucleotide sequence encoding a transmembrane protein of a strain of Chlamydia pneumoniae and a promoter to effect expression of the transmembrane protein gene product in the host. Modifications are possible within the scope of this invention.
Owner:AVENTIS PASTEUR LTD
O-type foot-and-mouth disease virus multi-epitope mucous membrane immunization vaccine and use
This invention relates to a fusion protein used for preventing aftosa, its preparation method and application. This fusion protein contains O type foot-and-mouth disease virus main cytomembrane protein VP1 epitope, colibacillus thermolability toxin B subunit, thymus derived cell epitope and purification label.
Owner:GUANGZHOU PUTAI BIOTECH
Paramyxovirus-derived RNP
InactiveUS20070009949A1Small genome sizeLow costSsRNA viruses negative-senseMicrobiological testing/measurementNegative strandLipofectamine
A functional RNP containing negative-strand single-stranded RNA derived from Sendai virus, which has been modified so as not to express at least one envelope protein, has been successfully prepared. An RNP comprising a foreign gene is prepared and inserted into a cell with the use of a cationic liposome, thereby successfully expressing the foreign gene.
Owner:KITAZATO KAIO +9
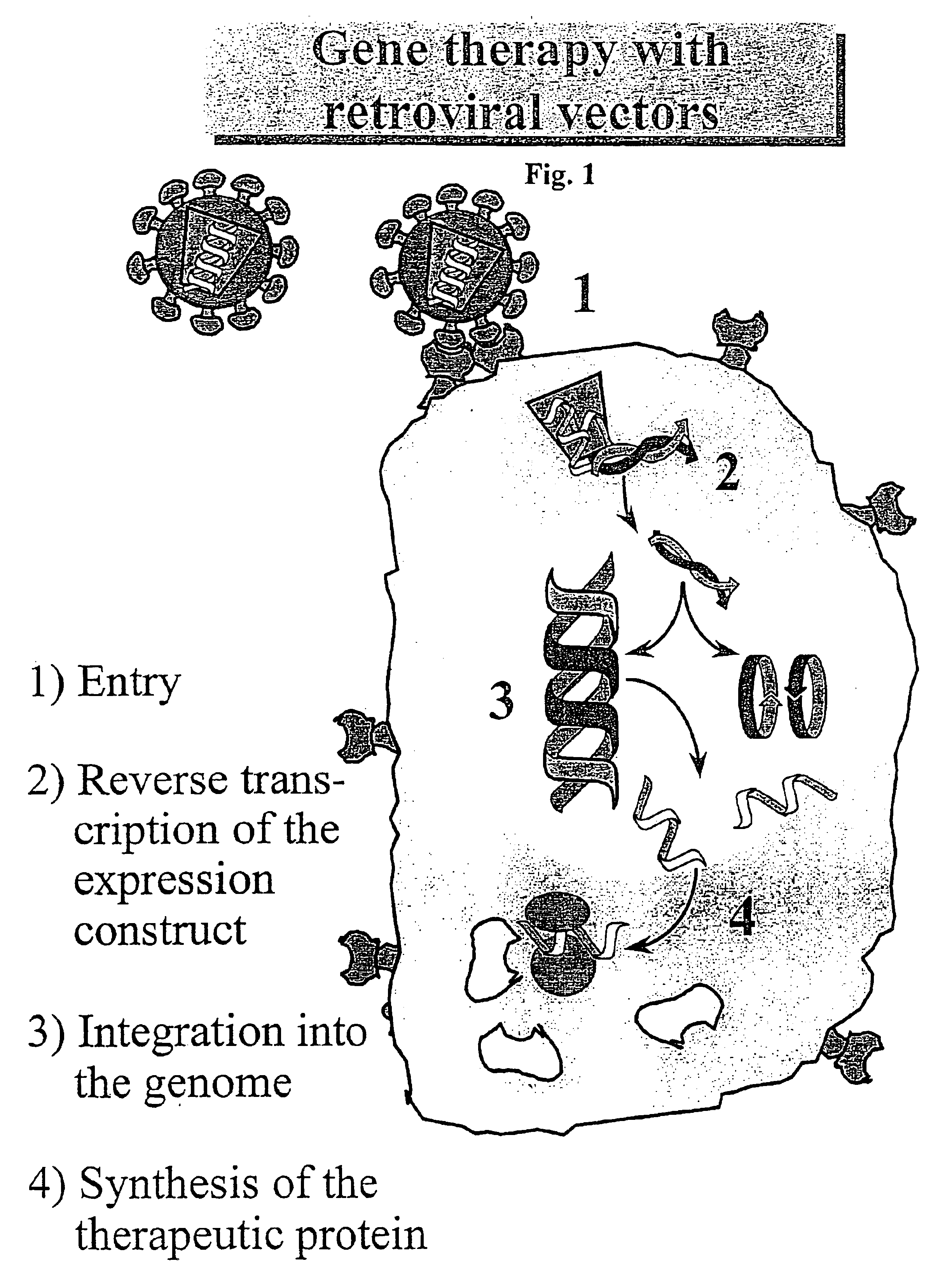
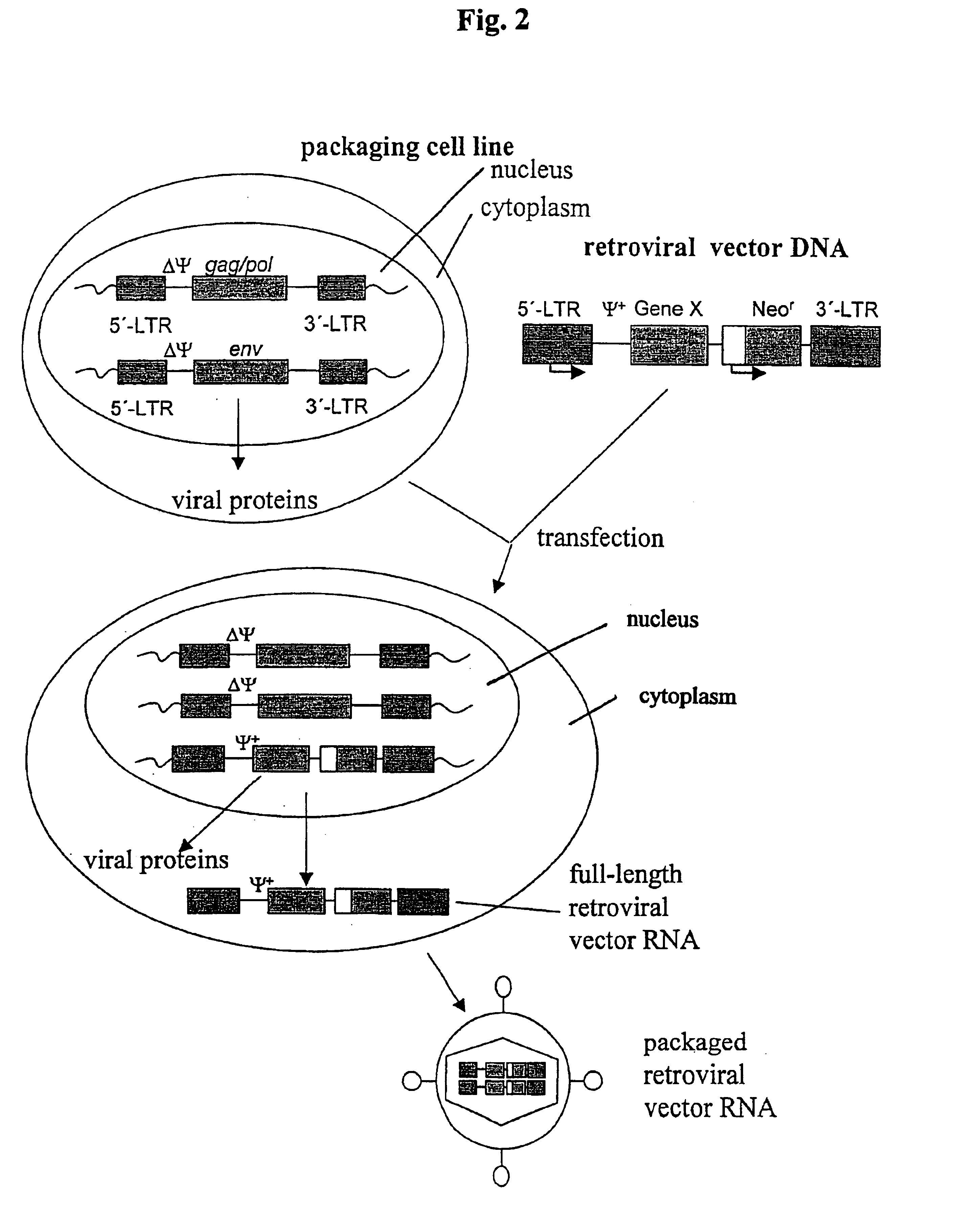
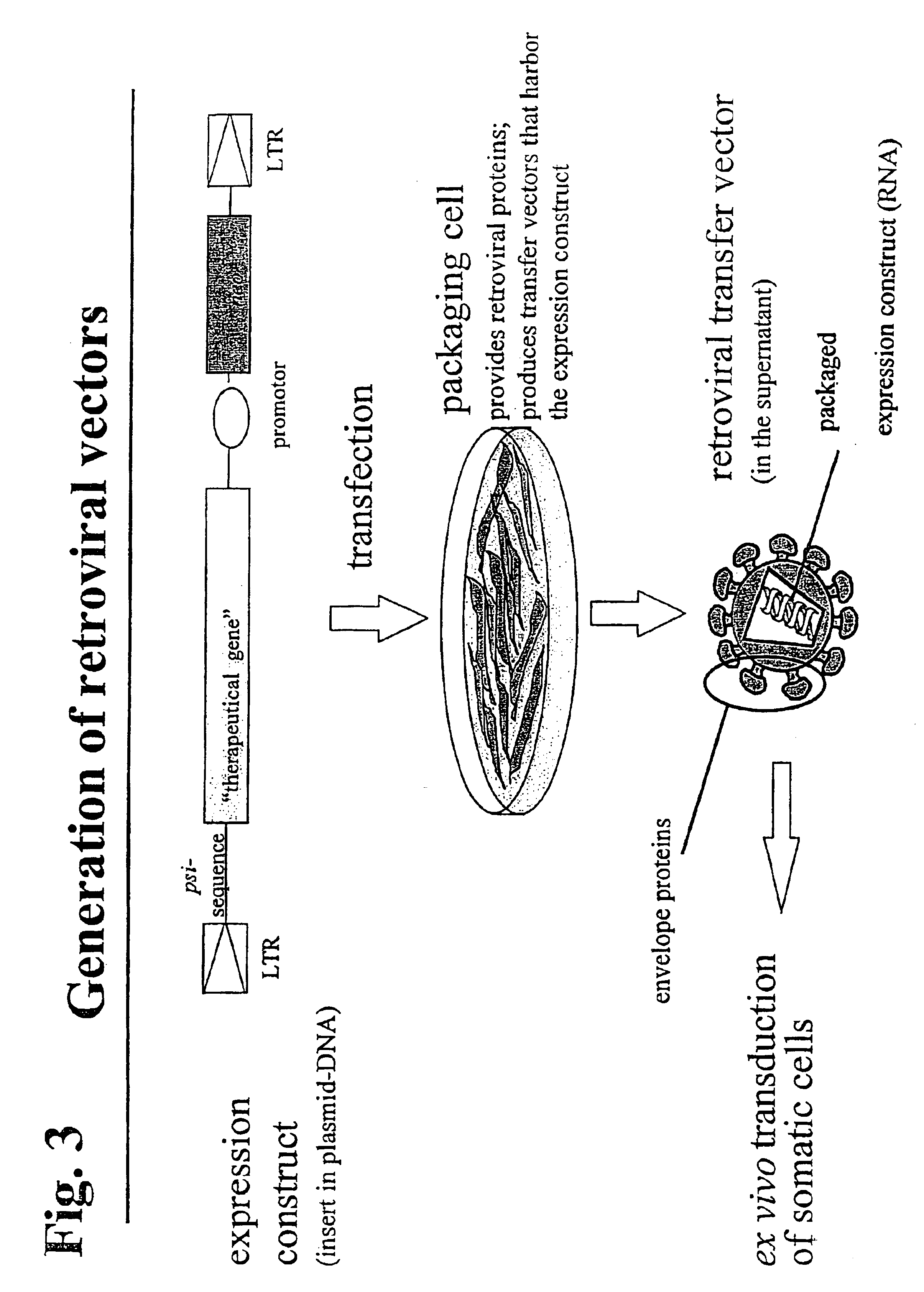
Retroviral vectors, methods for their preparation and their use for gene transfer into CD4-positive cells
InactiveUS6902929B1Improve efficiencyEasy constructionBiocideGenetic material ingredientsCell specificImmunodeficiency virus
The invention relates to the production and use of retroviral vectors for cell specific gene transfer, specially to a production method of retroviral vectors containing capsid particles of murine leukemia virus (MLV) and envelope proteins of human immunodeficiency vises (HIV) or simian immunodeficiency viruses (SIV). Said vectors can be used for gene transfer in selected cell types, specially in CD4-positive mammal cells.
Owner:BUNDESREPUBLIK DEUTLAND LETZTVERTRETEN DURCH DEN PRASIDENTEN DES PAUL EHRLICH INSTITUTS
Duck flavivirus, and vaccine and kit thereof
The invention belongs to the field of bioengineering and discloses duck flavivirus. The amino acid sequence of an envelope protein E is shown as SEQ ID NO:4 or the homology of the amino acid sequence which is shown as the SEQ ID NO:4 is over 98 percent. The invention also discloses vaccine for preventing duck flavivirus, and a kit for detecting duck flavivirus; a new virus of duck flavivirus disease which causes output reduction and death of laying duck are separated and identified by various detection means, a method for diagnosing duck flavivirus disease is established, the vaccine for duck flavivirus disease is developed, important basis and means are provided for preventing duck flavivirus, and relative duck flavivirus diseases which cause the output reduction and death of laying duck can be prevented and diagnosed by using the vaccine and a diagnostic reagent which are developed by using the virus.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
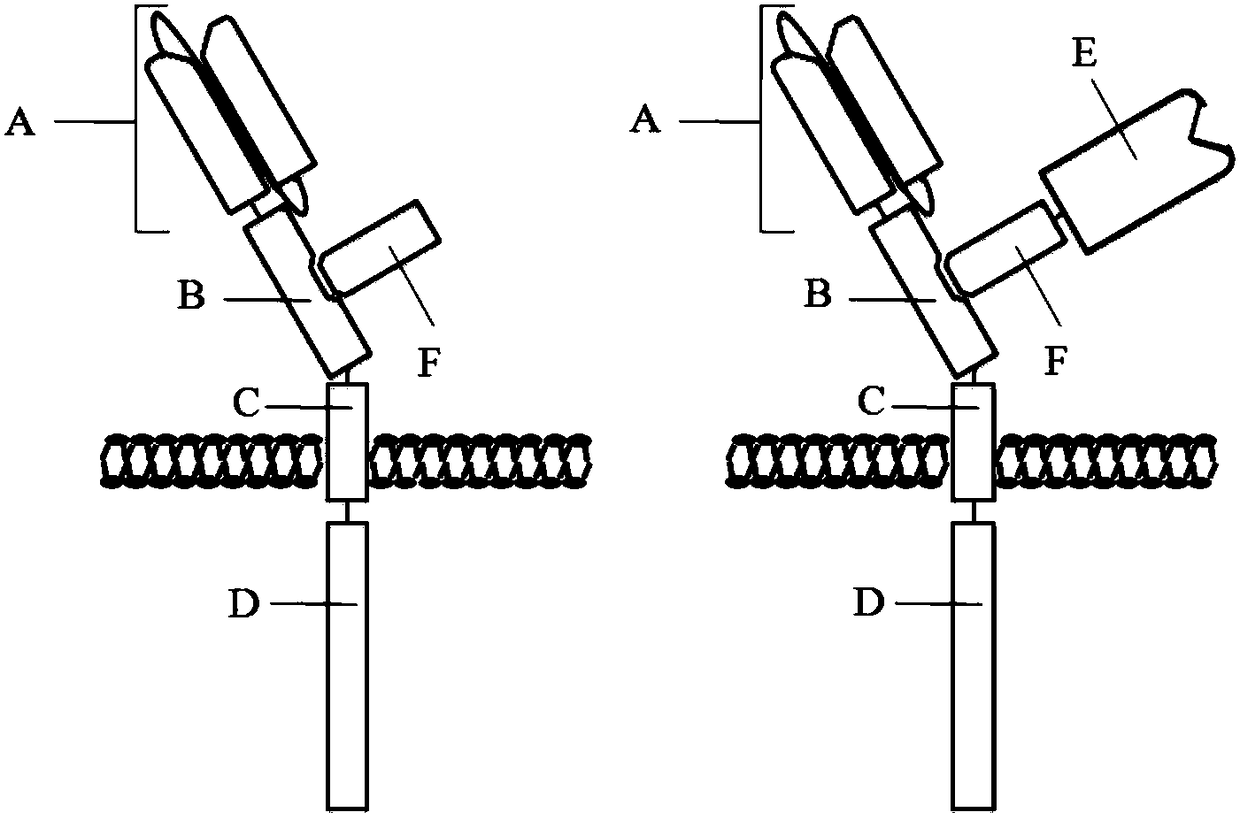
Multi-target chimeric antigen receptor
InactiveCN108250301AEliminate dependenciesReduced activityPolypeptide with localisation/targeting motifImmunoglobulin superfamilyTransmembrane domainChimeric antigen receptor
The invention discloses a multi-target chimeric antigen receptor. The multi-target chimeric antigen receptor provided by the invention consists of a main peptide chain and an assistant peptide chain.The main peptide chain includes an antigen binding domain A, an assistant peptide chain connection domain B, a transmembrane domain C and an intracellular signal conduction domain D. The assistant peptide chain includes a main peptide chain connection domain F. The antigen binding domain A is a polypeptide with antigen-bonding function. The assistant peptide chain connection domain B and the mainpeptide chain connection domain F are mutually combined. The transmembrane domain C is the transmembrane domain of arbitrary membrane binding protein or the transmembrane domain of transmembrane protein. The intracellular signal conduction domain D includes a primary signal conduction region. The multi-target chimeric antigen receptor provided by the invention can mediate specific cell killing bycombining two antigen binding domains with different antigens. Also a cytokine and cytokine receptor compound are introduced into the multi-target chimeric antigen receptor provided by the invention and can play the role of cytokines.
Owner:TIMMUNE BIOTECH INC
Anti-flavivirus envelope E protein monoclonal antibody and application thereof
ActiveCN101891806AGood broad-spectrum anti-flavivirus effectInfection fromFungiBacteriaCell strainProtein.monoclonal
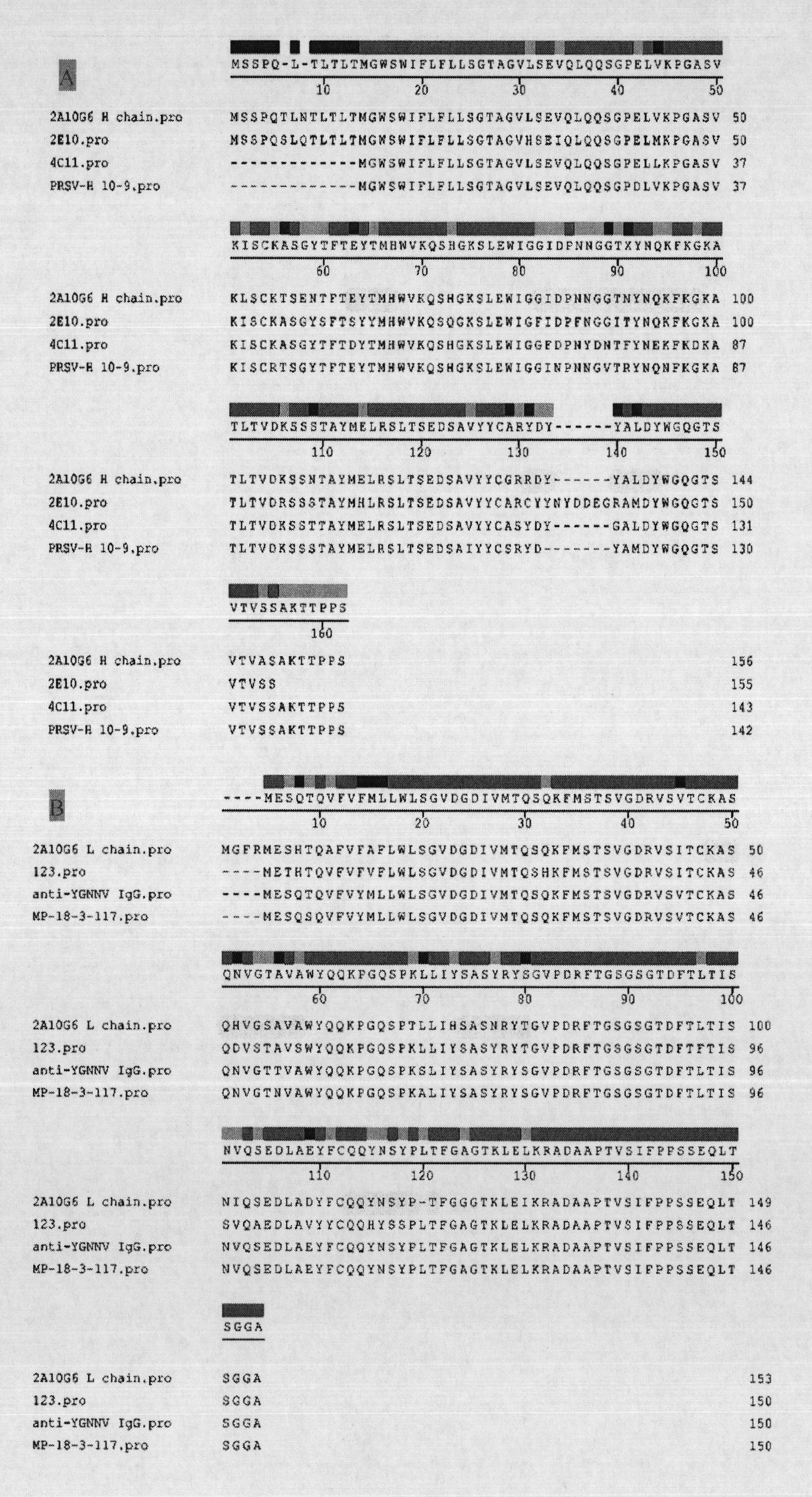
The invention discloses an anti-flavivirus envelope E protein monoclonal antibody and application thereof. The anti-flavivirus envelope E protein monoclonal antibody of the invention is secreted by a mouse source hybrid tumour cell strain D2-2A10G6 with the preservation number of CGMCC No. 3292. The anti-flavivirus monoclonal antibody of the invention can be specifically combined with the function epitope of flavivirus envelope E protein. The amino acid sequence of the functional epitope of envelope E protein is SEQ ID NO: 17. The monoclonal antibody of the invention is subject to screening by indirect immunofluorescent method, and indirect enzyme-linked immunization is used for evaluating the specificity and affinity when being combined with antigen. By adopting the monoclonal antibody or immunoconjugate of the invention, flavivirus infection cell can be blocked and suckling mouse can be protected from virus attack, thus achieving the effect of inhibiting virus infection. The monoclonal antibody or immunoconjugate of the invention also can be used for flavivirus envelope E protein detection.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
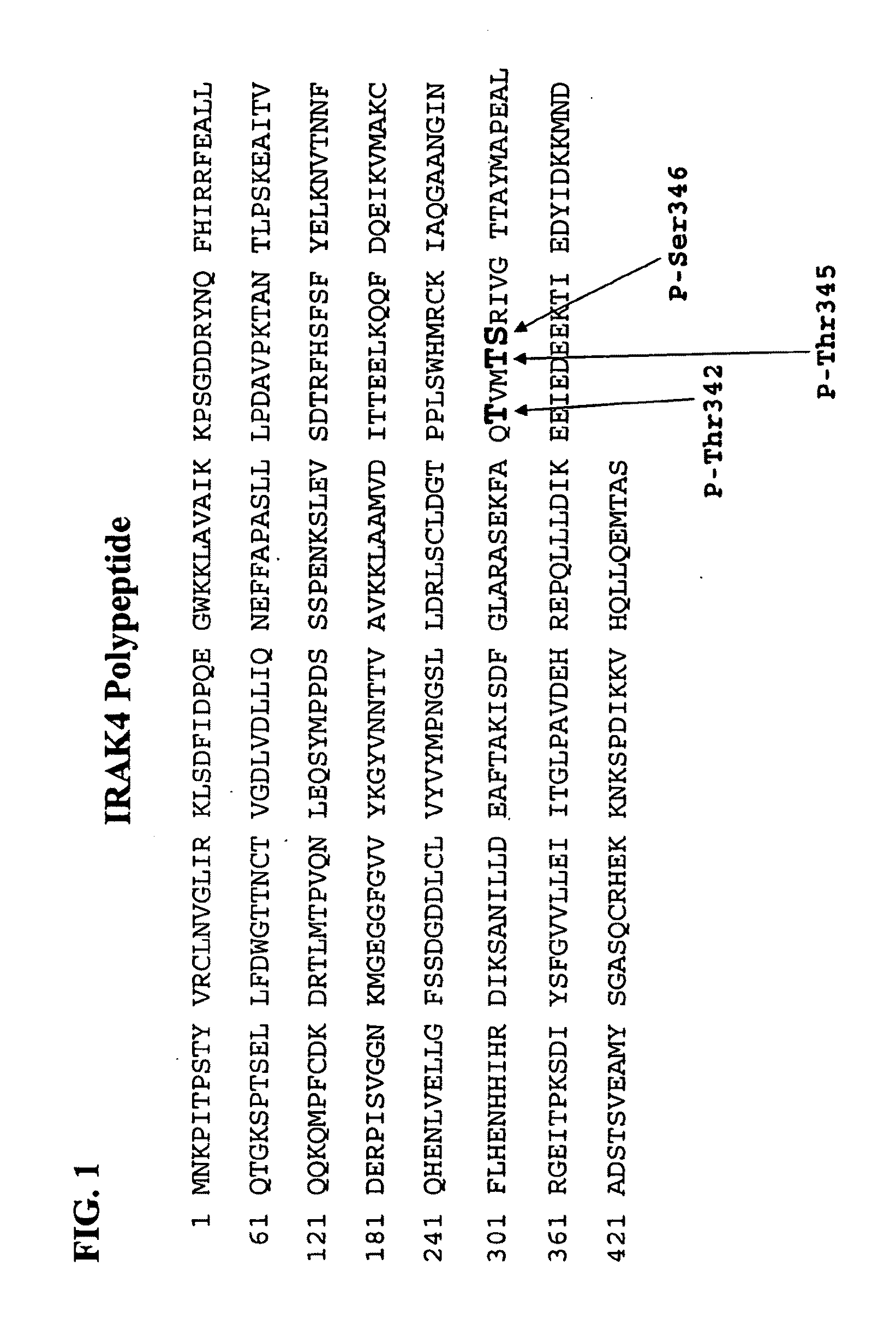
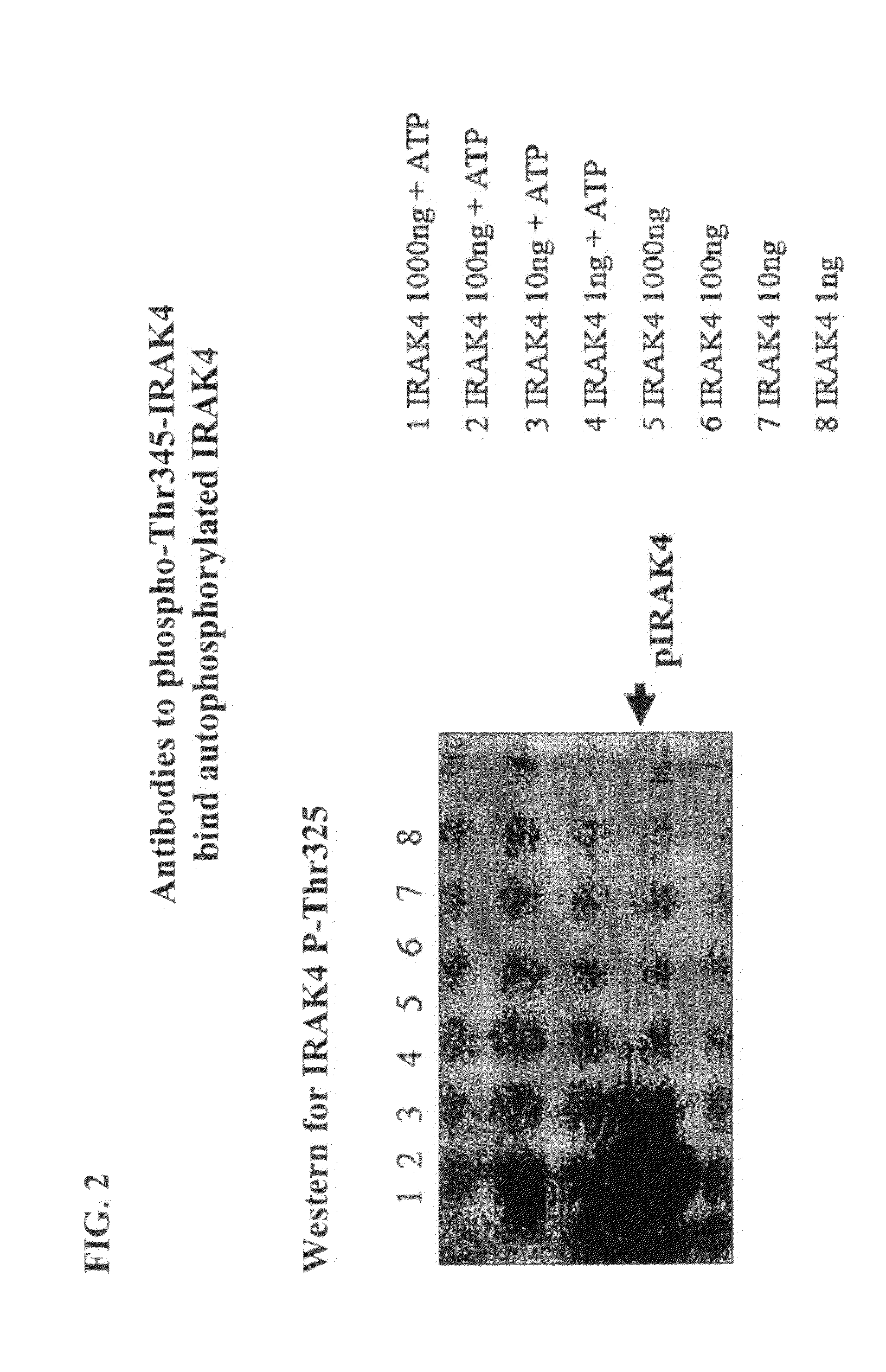
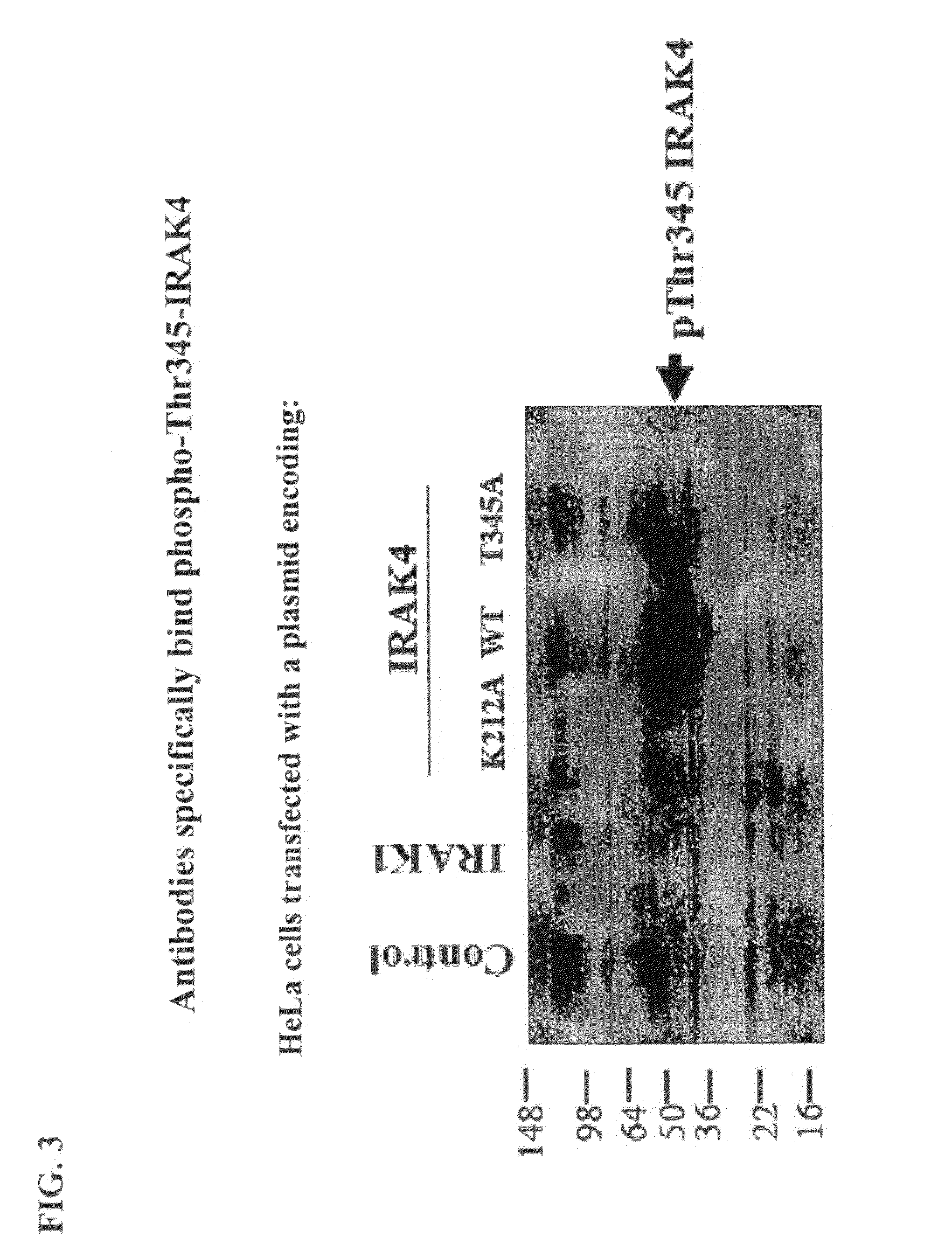
Antibodies to Phosphorylated IRAK4
InactiveUS20100279316A1Convenient bindingCompound screeningApoptosis detectionPhosphorylationThreonine
The present invention relates to antibodies that bind phosphorylated forms of IRAK4, methods of using such antibodies to detect IRAK4 biological activity, and methods for the detection, diagnosis, and prognostication of pathological conditions related to IRAK4 biological activity. ERAK4 is one member of a small family of highly conserved cytoplasmic signal transduction proteins characterized by the presence of an N-terminal “death domain” and a C-terminal serine-threonine kinase domain. IRAK4 functions in cytoplasmic signal transduction pathways by interacting with membrane spanning proteins which play, inter alia, critical roles in vertebrate immune system function.
Owner:GORELIK LEONID +5
Methods and composition related to in vivo imaging of gene expression
InactiveCN101171337AVectorsCell receptors/surface-antigens/surface-determinantsMethod of imagesIn vivo
Disclosed are nucleic acid sequences encoding a recombinant seven transmembrane G-protein associated receptor (GPCR) amino acid sequence operatively coupled to a tissue-selective promoter sequence. Also disclosed are methods of imaging or treating cells in a subject that involve introducing a nucleic acid encoding a reporter detectable in vivo using non-invasive methods operatively coupled to a tissue-selective promoter or amplified tissue specific promoter to the subject, and subjecting the subject to a non-invasive imaging technique or a therapeutic that selectively interacts with the reporter or gene of interest. For example, the subject may be a subject with cancer, and the therapeutic may be an anti-cancer agent. In particular, the hTERT promoter or amplified hTERT promoter drives expression of a reporter such hSSTR2 and / or hSSTR2 derivative) and these may be linked to a gene of interest. Imaging may be performed by a variety of methods including nuclear medicine.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
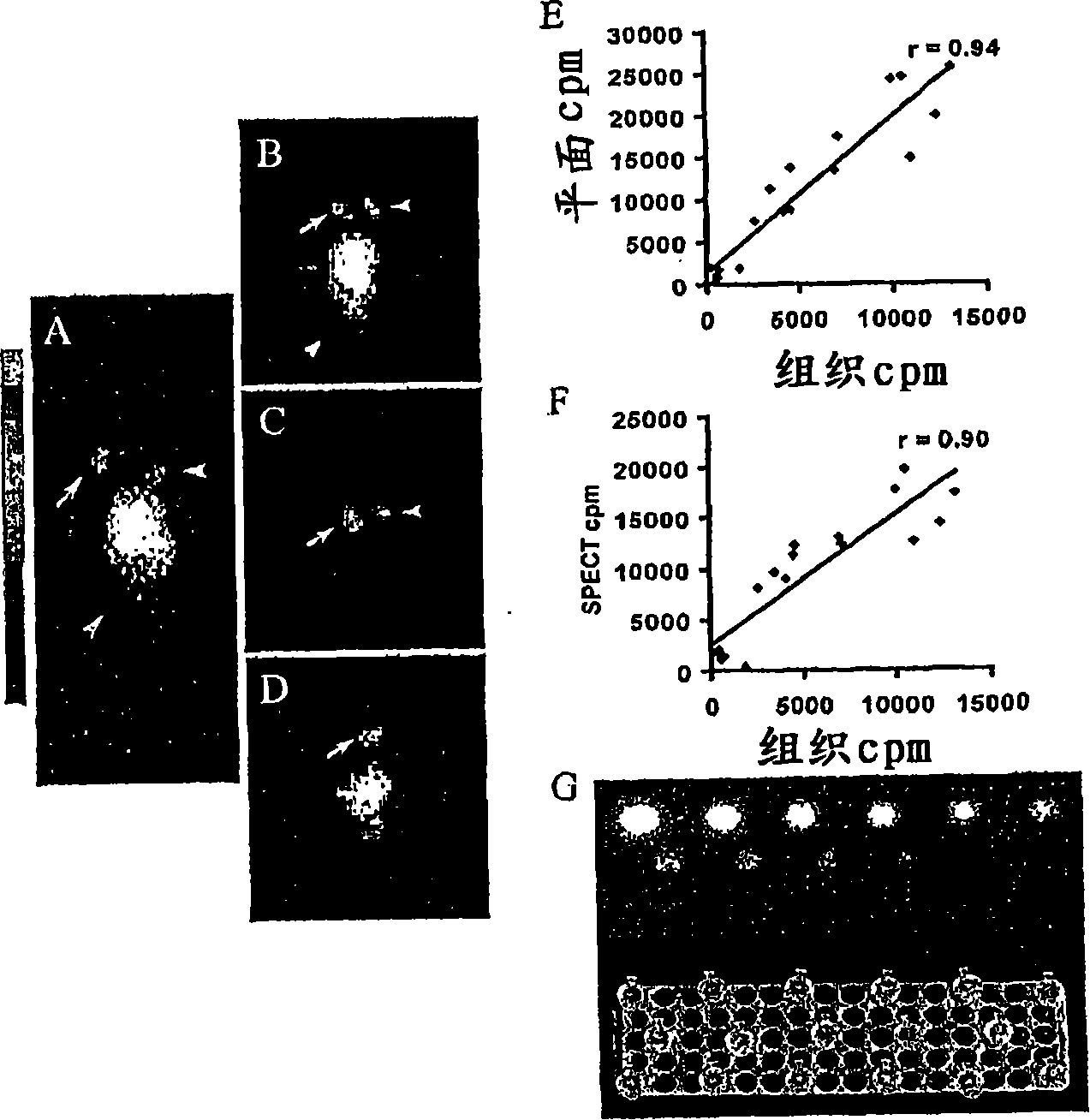
Construction of hepatitis B virus siRNA expression vector and its application in antivirus treatment
InactiveCN1523113AInhibit expressionInhibition of replicationGenetic material ingredientsGene therapyChemical synthesisConserved sequence
The present invention relates to construction of hepatitis B virus siRNA expression vector and its antiviral therapeutic application. According to the design principle said construction includes the following steps: respectively selecting nucleotide sequence of HBV envelope protein (MS) coding region 69nt-87nt, polymerase(p) coding region 708nt-726nt and core protein (c) coding region 2052 nt-2070nt, designing correspondent siRNA sequence, using BLAST software to analyze homogenously to confirm that it is HBV highly conservative sequence, then chemically synthesizing single-stranded oligonucleotide, external annearing to form double-stranded DNA, connecting it with eukaryotic expression vector pTZU6+1, enzyme excision identification and sequence determination and analysis show that siRNA extression vectors of three target hepatitis B virus (HBV) gene sequences are constructed. The tests show that HBV siRNA has the strong action of inhibiting virus replication and expression.
Owner:CHONGQING MEDICAL UNIVERSITY
Human immunodeficiency virus antigens, vectors, compositions, and methods of use thereof
ActiveUS20170165355A1Improved cell surface expressionImproved expression stabilityViral antigen ingredientsVirus peptidesAntigenHiv envelope
Synthetic HIV envelope proteins, vectors and compositions thereof, and methods for inducing protective immunity against human immunodeficiency virus (HIV) infection are described. Viral expression vectors encoding the synthetic HIV envelope proteins can be used in vaccines to provide improved protective immunity against HIV.
Owner:JANSSEN VACCINES & PREVENTION BV
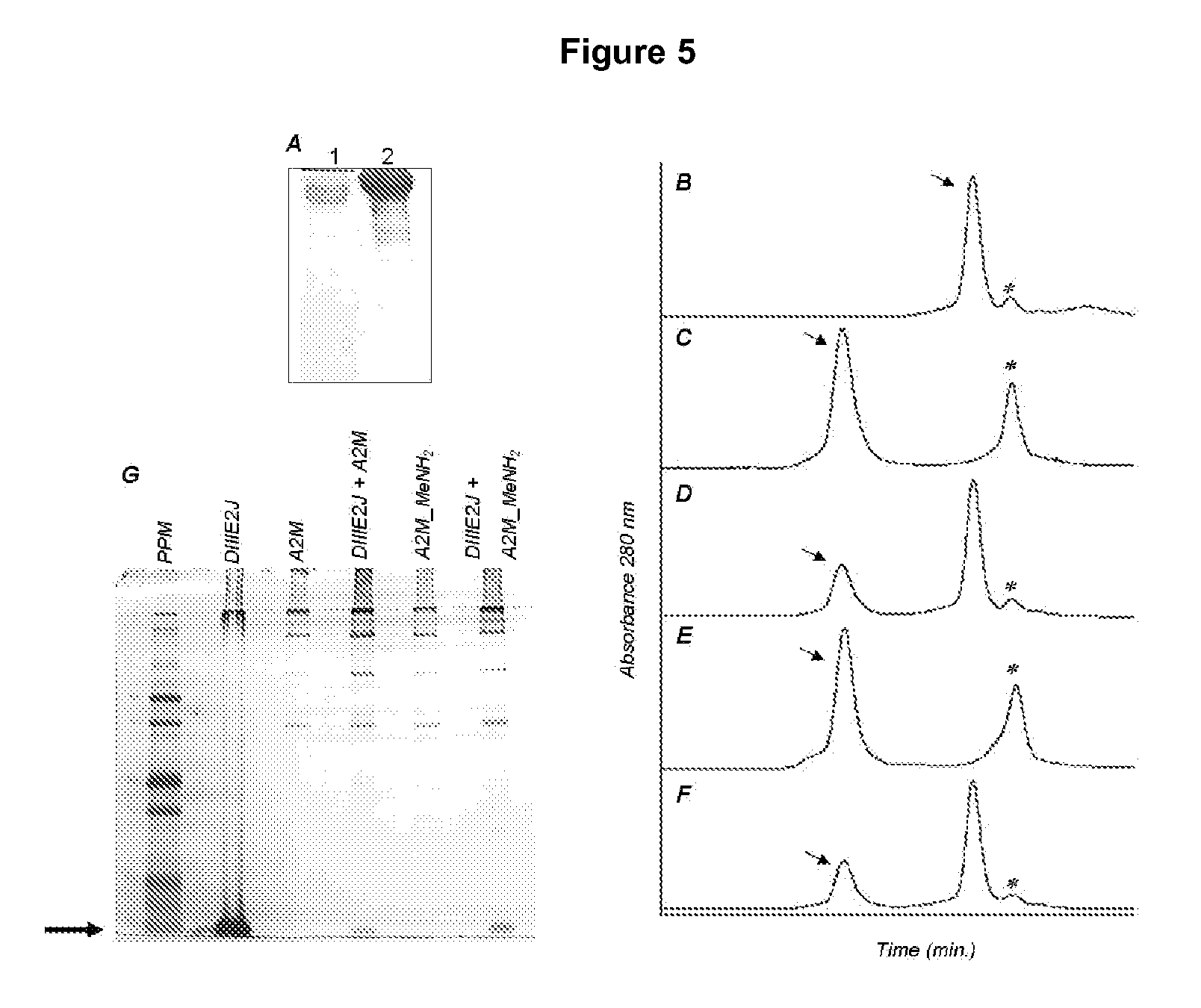
Method to block the infection by flaviviruses, molecules and uses
ActiveUS20110212105A1Modulate the DV infectionReducing and increasing interactionSsRNA viruses positive-sensePeptide/protein ingredientsMolecular biologyCarrier protein
The present invention is related to a method for blocking the infection of cells by dengue virus, based on interfering the direct interaction of the viral envelope protein with a cellular receptor or its indirect interaction with said cellular receptor through a carrier protein, as well as related uses; wherein said cellular receptor is the alpha-2 macroglobulin receptor, also known as the low density receptor-related protein or as CD91, and said carrier protein is human alpha-2 macroglobulin.
Owner:CENT DE ING GENETICA & BIOTECNOLOGIA
Bivalent binding molecules of 7 transmembrane G protein-coupled receptors
Described herein are methods for identifying and preparing bivalent binding molecules to 7 transmembrane G protein-coupled receptors. The methods disclosed herein are based on the SELEX method for generating high affinity nucleic acid ligands. SELEX is an acronym for Systematic Evolution of Ligands by EXponential enrichment. The methods of this invention combine two or more binding domains to two or more different epitopes of the same 7 transmembrane G protein-coupled receptor. These bivalent binding molecules are useful as therapeutic and diagnostic agents.
Owner:GILEAD SCI INC
CD20 Antibodies and Uses Thereof
CD20 is a transmembrane protein of the tetra-spanin family expressed on the surface of B-cells from peripheral blood as well as lymphoid tissues. CD20 expression persists from the early pre-B cell stage until the plasma cell differentiation stage. In addition to expression in normal B-cells, CD20 is expressed in B-cell derived malignancies such as non-Hodgkin's lymphoma (NHL) and B-cell chronic lymphocytic leukemia (CLL). The present invention includes anti-CD20 antibodies and antigen-binding fragments thereof comprising a light chain variable region and a heavy chain variable region, wherein the CDR-L1, CDR-L2, and CDR-L3 of said light chain variable region comprise the amino acid sequences of SEQ ID NOs: 23-25, respectively, and wherein the CDR-H1, CDR-H2, and CDR-H3 of said heavy chain variable region comprise the amino acid sequences of SEQ ID NOs: 26-28, respectively.
Owner:IMMUNOGEN INC
MRNA vaccine and synthesis method thereof and kit
ActiveCN111821433AIncreased immunogenicityFlexible designSsRNA viruses positive-senseViral antigen ingredientsReceptorGenetic engineering
The invention discloses an mRNA vaccine and a synthesis method and a kit. The mRNA vaccine is prepared from an epitope antigen gene sequence of trimerical spike glycoprotein S, and / or an epitope antigen gene sequence of a transmembrane protein-envelope E, and / or, an epitope antigen gene sequence of membrane glycoprotein M, and / or, an epitope antigen gene sequence of nucleocapsid N, and / or, an epitope antigen gene sequence of a receptor binding domain (RBD) in the trimerical spike glycoprotein. According to the technical scheme of the invention, the mRNA vaccine is designed through a genetic engineering method so as to achieve immunity to the novel coronavirus.
Owner:深圳瑞吉生物科技有限公司
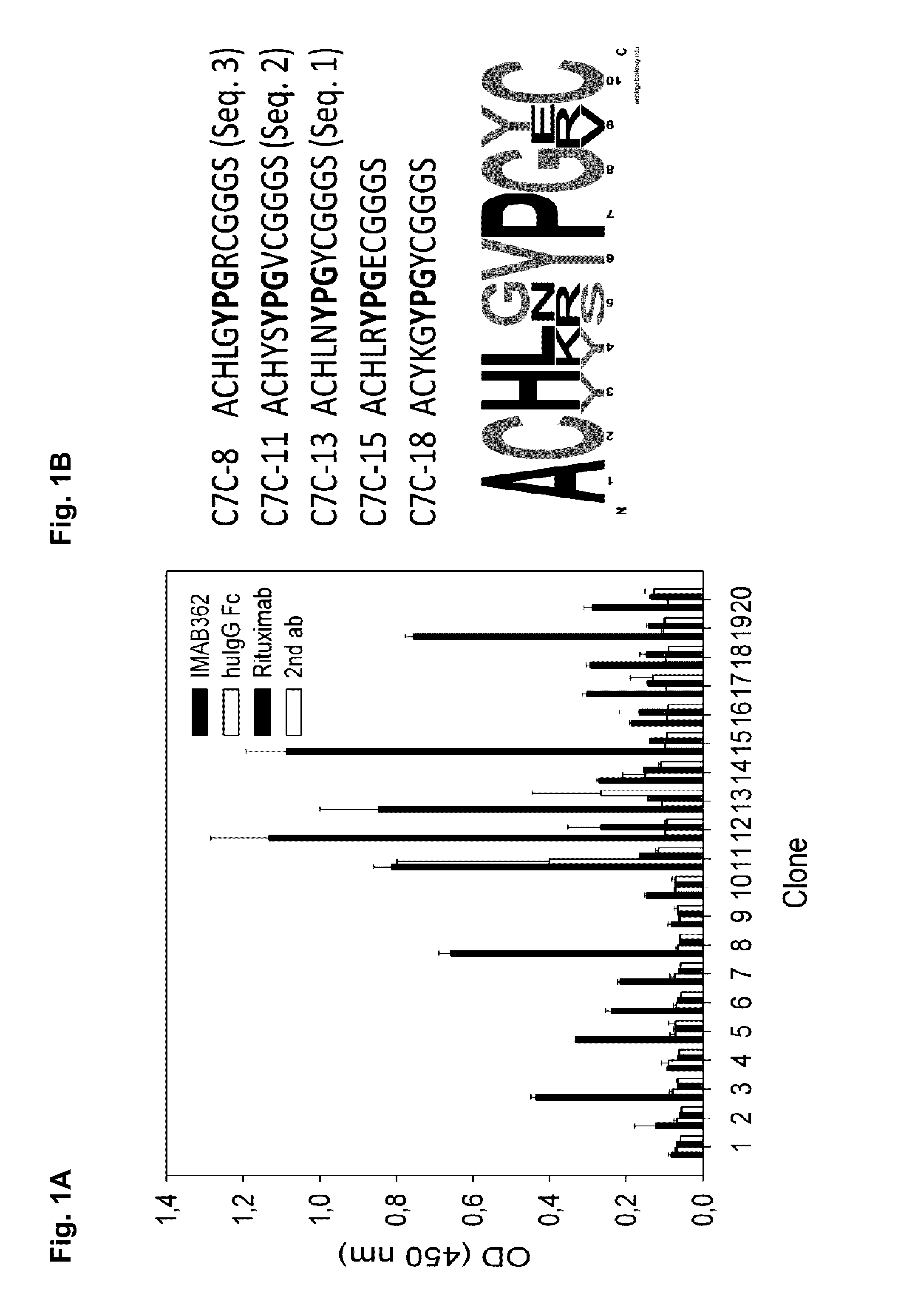
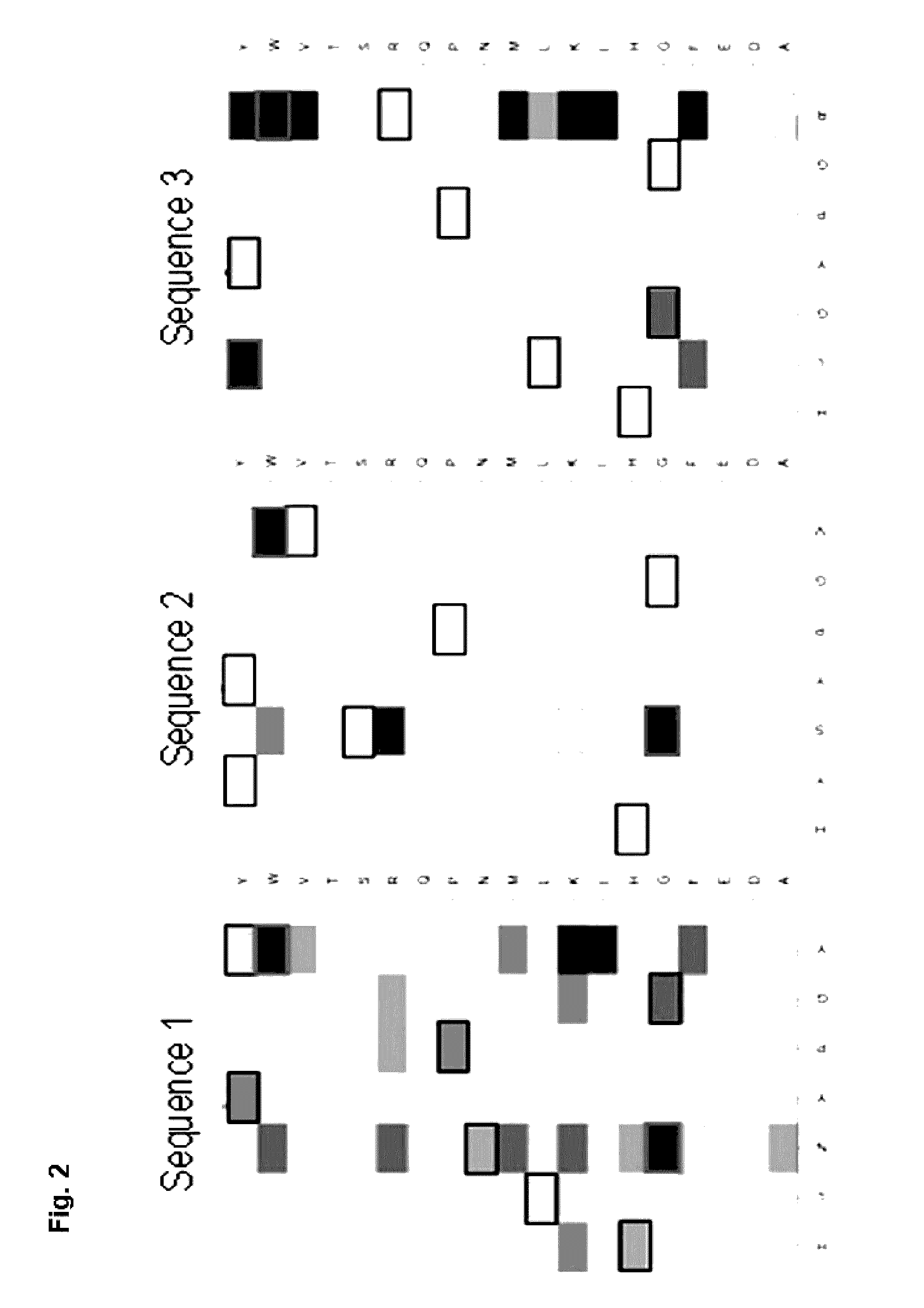
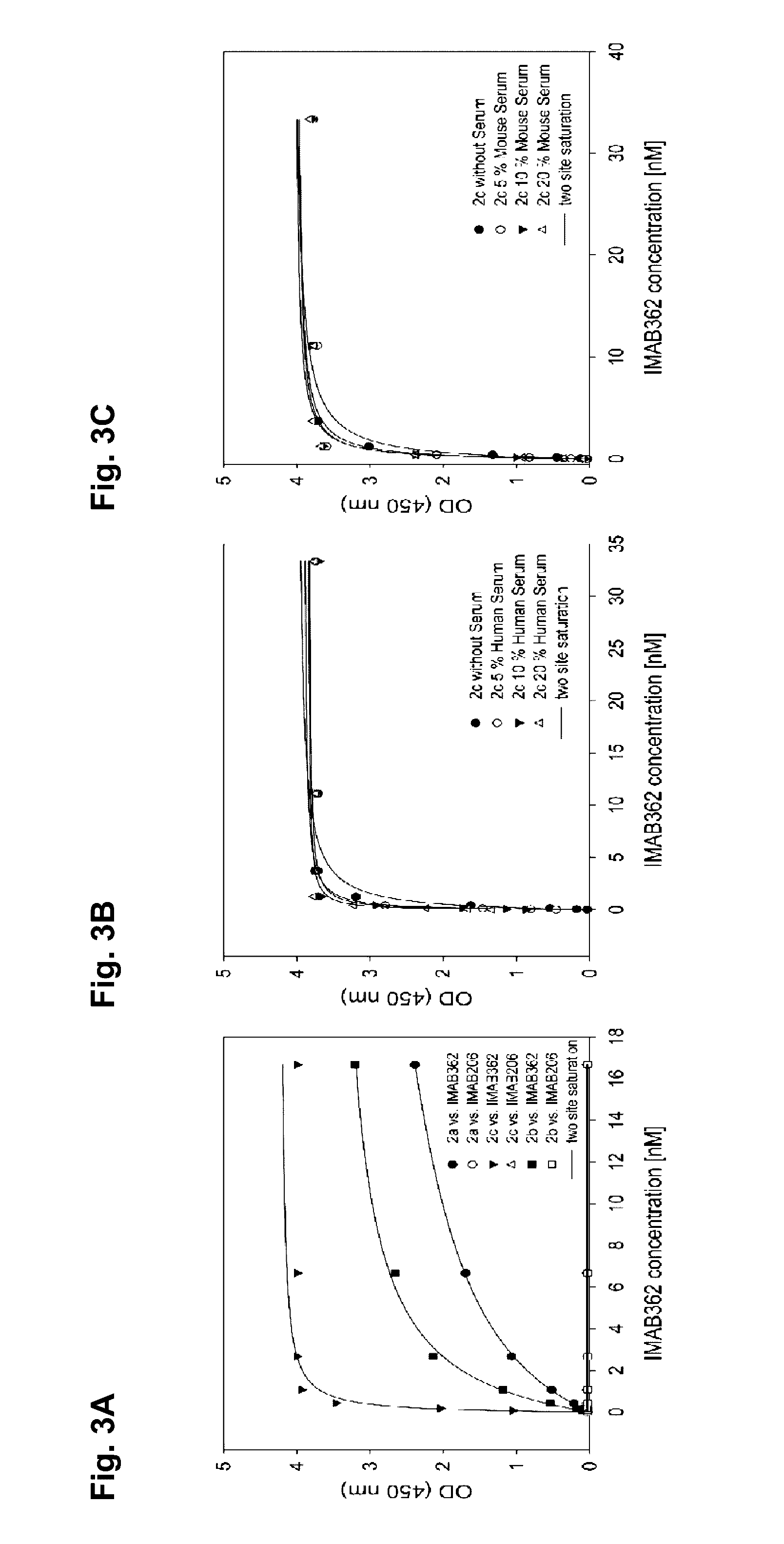
Peptide mimotopes of claudin 18.2 and uses thereof
ActiveUS20160347815A1Optimization of binding propertyCompound screeningCell receptors/surface-antigens/surface-determinantsAntigenBinding domain
The present invention provides molecules that mimic antigenic determinants of the integral transmembrane protein claudin 18.2 (CLDN18.2). These molecules compete with CLDN18.2 for binding to a CLDN18.2 binding domain, e.g. a CLDN18.2 binding domain of an antibody, and are capable of detecting antibodies against CLDN18.2. The mimotopes of the invention may be used to generate or inhibit immune responses in animals and preferably humans. Furthermore, they can be used for purposes of detecting agents comprising a CLDN18.2 binding domain in biological samples as well as for purifying agents comprising a CLDN18.2 binding domain.
Owner:JPT PEPTIDE TECH +1
Amino acid sequences directed against envelope proteins of a virus and polypeptides comprising the same for the treatment of viral diseases
InactiveCN102112155AAntibody mimetics/scaffoldsImmunoglobulins against virusesViral diseaseBioinformatics
The present invention relates in part to amino acid sequences that are directed against and / or that can specifically bind to an envelope protein of a virus, as well as to compounds or constructs, and in particular proteins and polypeptides, that comprise or essentially consist of one or more such amino acid sequences.
Owner:ABLYNX NV
HPV vaccines and methods for using the same
ActiveUS8168769B2SsRNA viruses negative-senseSsRNA viruses positive-senseHPV vaccinesRecombinant vaccines
Improved anti-HIV immunogens and nucleic acid molecules that encode them are disclosed, Immunogens disclosed include those having consensus sequences for HIV Subtype A Envelope protein, those having consensus sequences for HIV Subtype B Envelope protein, those having consensus sequences for HIV Subtype C Envelope protein, those having consensus sequences for HIV Subtype D Envelope protein, those having consensus sequences for HIV Subtype B consensus Nef-Rev protein, and those having consensus sequences form HIV Gag protein subtypes A, B, C and D. Improved anti-HPV immunogens and nucleic acid molecules that encode them; improved anti-HCV immunogens and nucleic acid molecules that encode them; improved hTERT immunogens and nucleic acid molecules that encode them; and improved anti-Influenza immunogens and nucleic acid molecules that encode them are disclosed. Pharmaceutical composition, recombinant vaccines comprising and live attenuated pathogens are disclosed as well methods of inducing an immune response in an individual against HIV, HPV, HCV, hTERT and Influenza are disclosed.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Double receptor identified serial cell-penetrating peptide modified tumor targeted nano drug delivery system
The invention provides a serial cell-penetrating peptide modified tumor targeted and tumor penetrated nano drug delivery system capable of simultaneously identifying an integrin receptor and a transmembrane protein receptor, wherein the serial cell-penetrating peptide is mainly formed by connecting a DGR polypeptide fragment covalently at the C-tail end of polyarginine of the cell-penetrating peptide. The polypeptide not only can selectively identify two highly expressed receptors on the surfaces of the tumor cells, but also can be efficiently mediated into the cells. The nano drug delivery system mainly consists of a nano carrier, a drug and a lipid-polyethylene glycol-serial cell-penetrating peptide ligand interstitial compound and is a potential tumor targeted treatment carrier.
Owner:SICHUAN UNIV
Pseudo-virus and packaging method thereof and drug evaluation system
ActiveCN111662884AAvoid the risk of secondary infectionImprove infection abilitySsRNA viruses positive-senseMicrobiological testing/measurementDiseaseReceptor
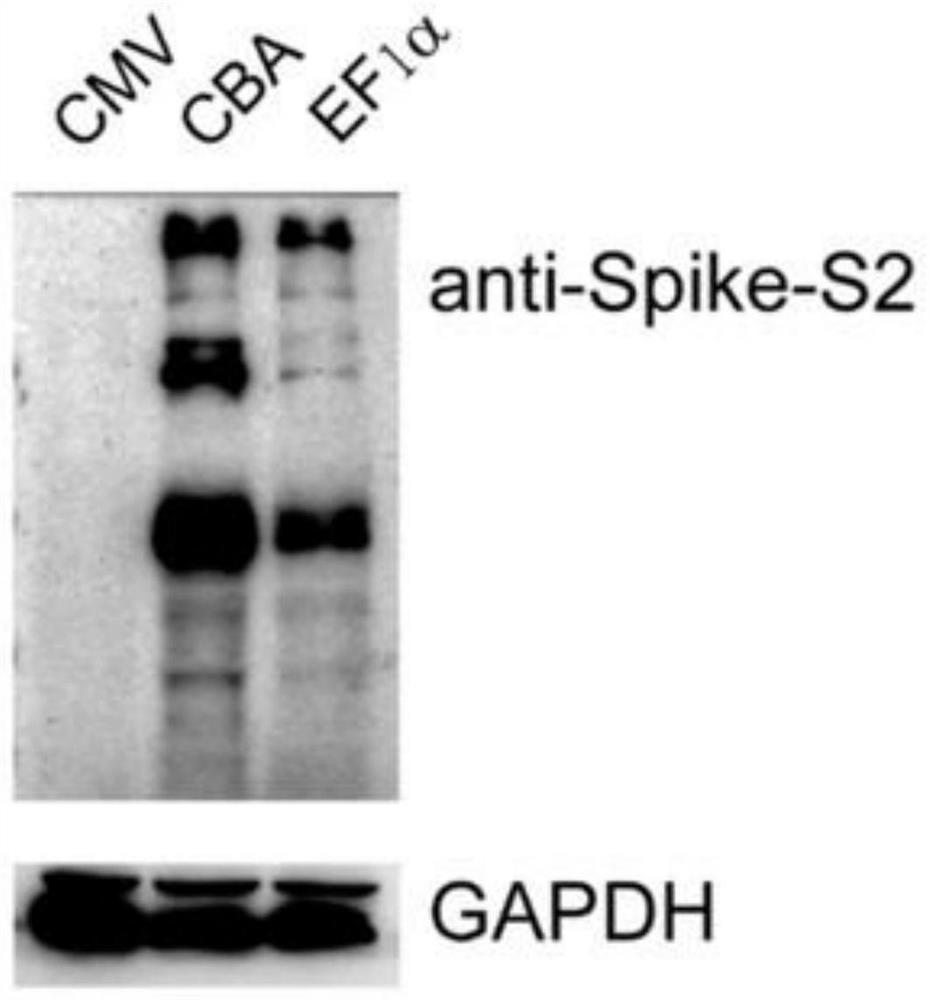
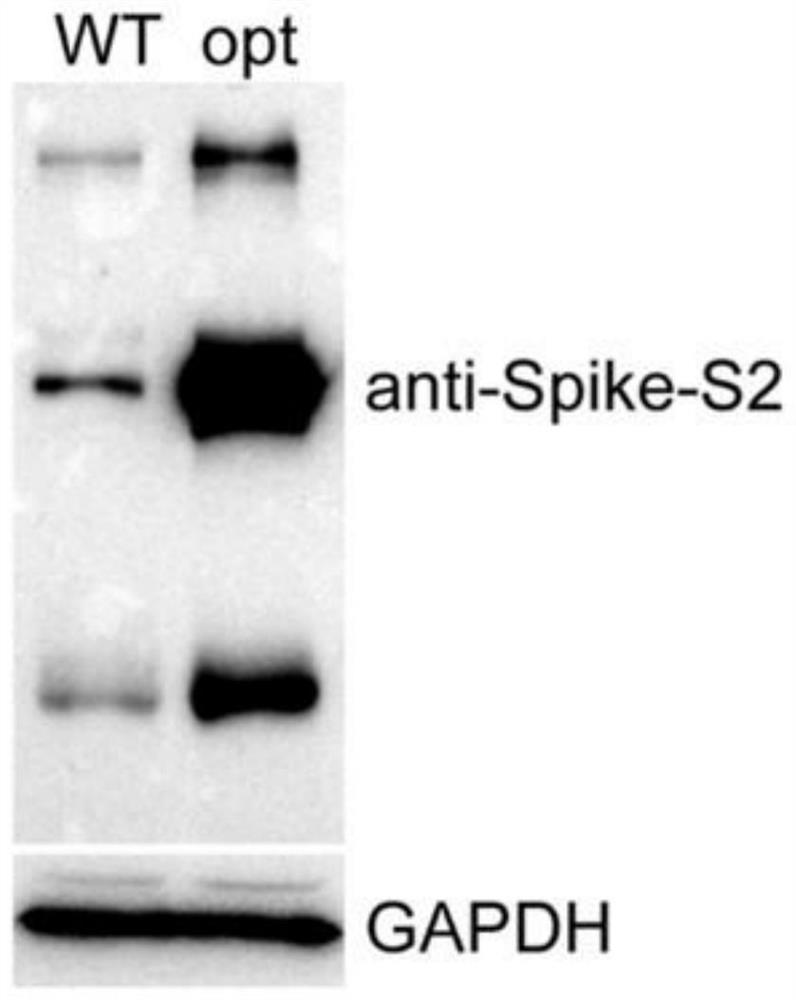
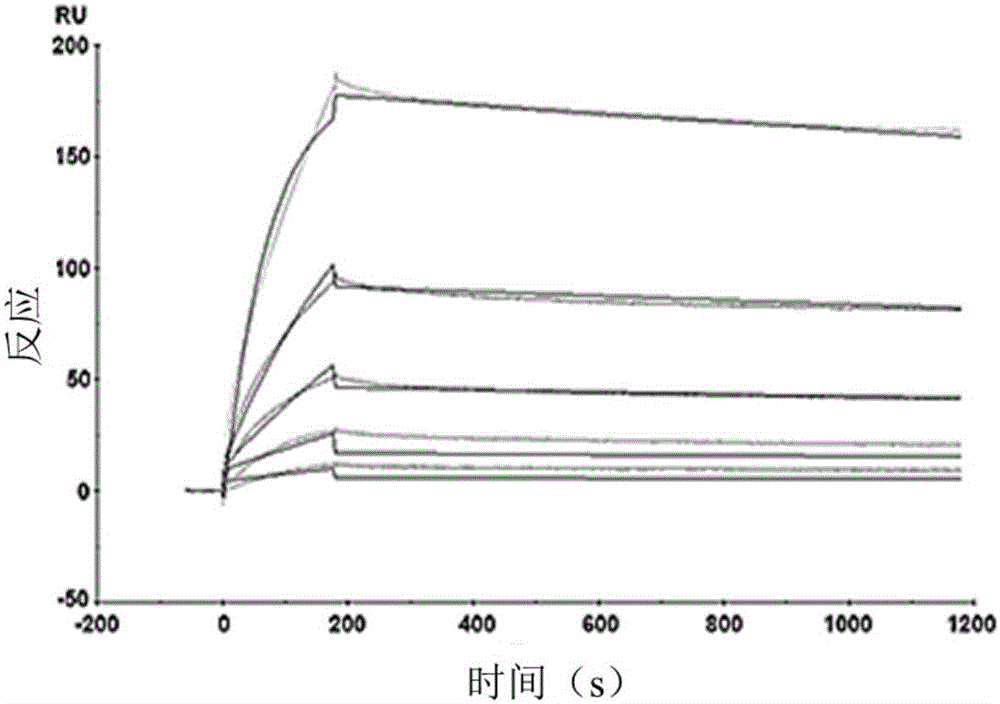
The invention relates to pseudo-virus. The pseudo-virus comprises a plasmid for expressing envelope protein Spike, a reporter gene plasmid and a packaging helper plasmid, wherein the plasmid for expressing the envelope protein Spike contains a CBA promoter and a human optimized Spike expression sequence, and is used for driving the expression of the envelope protein Spike. The pseudo-virus disclosed by the invention can simulate the process of infecting cells by wild novel coronavirus, can be used for researching the relationship between virus and host cells, cloning a receptor of virus and replacing a traditional neutralization test, and is used for researching differential diagnosis of diseases, evaluation and screening of antiviral drugs, evaluation of vaccine immune effect and the like. The invention also relates to a packaging method of the pseudo-virus and a novel coronavirus virus drug evaluation system containing the pseudo-virus, wherein the pseudo-virus is used for infectingthe 293T monoclonal cell which overexpresses the human ACE2 gene under the condition of drug interference, and by detecting the expression quantity of a reporter gene in the pseudo-virus (such as fluorescence intensity detection), the effectiveness of the drug is preliminarily evaluated.
Owner:中吉当康(北京)基因技术有限公司
Fully-human-derived anti-HCV (hepatitis C virus) neutralizing antibody-TRN1001
ActiveCN106749644AHigh potencyHigh cross activityImmunoglobulins against virusesAntiviralsSide effectMonoclonal antibody
The invention discloses a fully-human-derived monoclonal antibody for neutralizing HCV (hepatitis C virus) and an application thereof and particularly discloses an antibody capable of recognizing and combining HCV envelope protein E2, a coding gene thereof, an expression vector and an application. The monoclonal antibody can stop the HCV from infecting susceptible cells and is fully-human-derived, and the antibody has greatly reduced immunogenicity, good affinity, good treatment effect and low side effects as compared with other animal-derived anti-HCV molecules.
Owner:JINAN UNIVERSITY +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com