MRNA vaccine and synthesis method thereof and kit
A technology of vaccines and receptor binding domains, applied in the field of biopharmaceuticals, can solve problems such as undiscovered, and achieve the effects of easy transportation, short production cycle and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
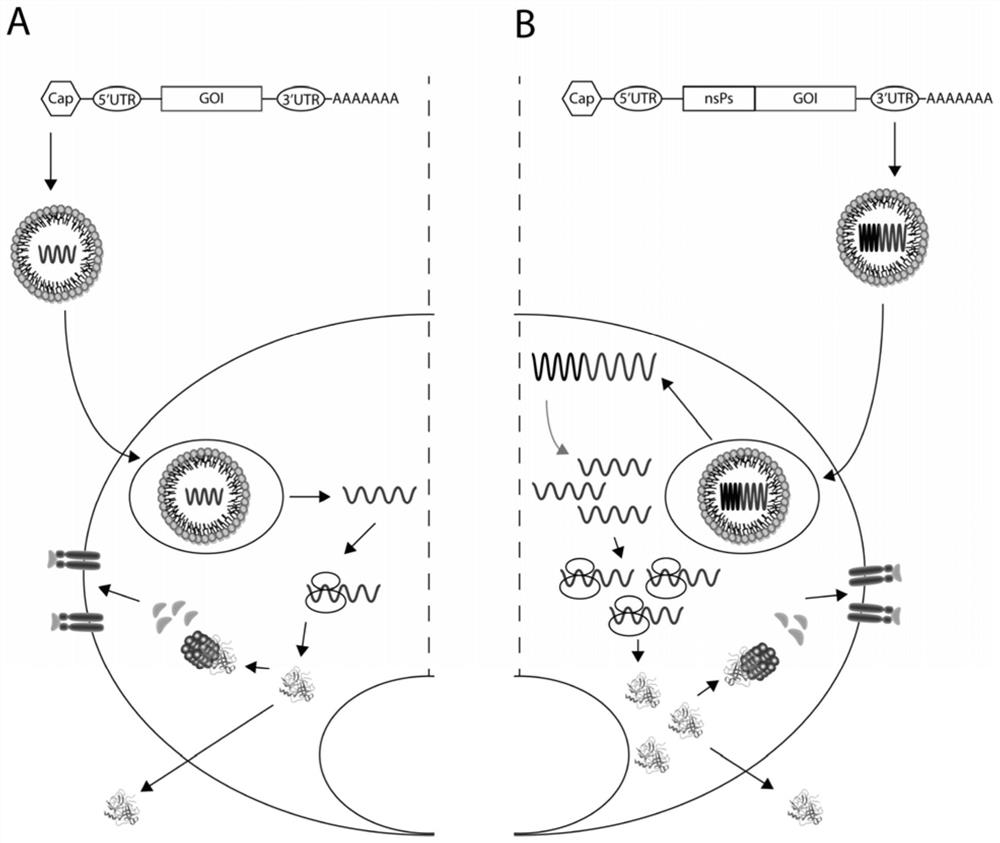
[0062] Example 1 Antigens of conventional mRNA vaccines and long-acting mRNA vaccines
[0063] In this example, conventional mRNA vaccines encoding different antigen fragments and long-acting mRNA vaccines capable of self-replication were transfected into 293T cells. After 12 hours, the expression results of the antigens encoded by the vaccines were detected by Western blot.
[0064] 1) Preparation of transfection reagent. Mix 100ng mRNA with the transfection reagent TransIT thoroughly, add 50uL serum-free medium, and let stand at room temperature for 5 minutes.
[0065] 2) Cell transfection. Remove the medium in the culture plate, wash once with PBS or serum-free medium, add 200 uL of fresh medium; then add the transfection reagent prepared in step 1) evenly into the cell culture medium.
[0066] 3) Western blot experiment. After 24 hours, the cells in step 2) were lysed and the lysate was collected for western blotting. According to conventional molecular biology experimental met...
Embodiment 2
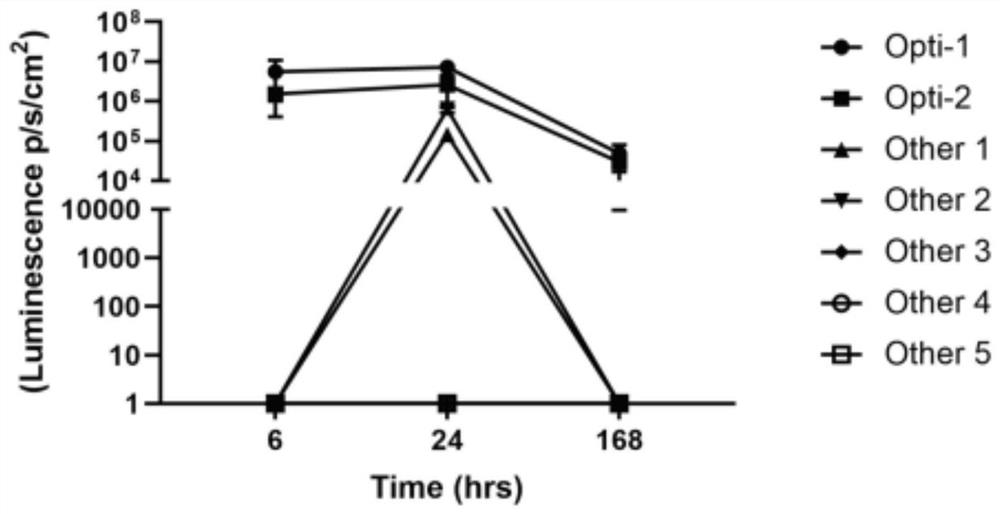
[0068] Example 2 Expression and yield of mRNA vaccine in cells
[0069] 1. According to Table 1, connect SEQ ID: 1, SEQ ID: 3, SEQ ID: 5, SEQ ID: 7 and SEQ ID: 9 into a sequence Opti-1 through a connecting peptide, and connect SEQ ID: 2, SEQ ID :4, SEQ ID: 6, SEQ ID: 8 and SEQ ID: 10 are linked to form a sequence Opti-2 by connecting peptides to synthesize DNA fragments encoding S protein, M protein, E protein, N protein and RBD, and combine all The DNA fragment and the fragment of the DNA sequence encoding the luciferase are cloned into a DNA plasmid vector at the same time, so that the antigen and the luciferase form a fusion protein. After the DNA plasmid vector is linearized, the mRNA vaccine is synthesized by in vitro transcription. At the same time, a control group was set up to look for optimized programs (Other 1 to 5) for the new coronavirus mRNAs on the market to synthesize control mRNA vaccines. And injected the mRNA vaccine prepared above into mice to obtain Figure ...
Embodiment 3
[0071] Example 3 Immune response of mRNA vaccine
[0072] 1. Vaccination. Mice were vaccinated with the new coronavirus 2019-nCoV mRNA vaccine (specifically, the conventional mRNA vaccine prepared in Example 1 (see Image 6 ) And long-acting mRNA vaccines (see Figure 7 )), to stain the intracellular cytokines to quantify the mRNA vaccine prepared from different antigen mRNA sequences in CD4 (left) and CD8 (right) to stimulate mouse T cells to produce IFN-g (interferon gamma) or TNF- a (Tumor Necrosis Factor) Percentage of activated T cells. Detection of activated T cells of mouse T cells immunized with luciferase mRNA (n=5 per group) or mRNA vaccine (n=5 per group) at 0, 1, and 2 weeks Percentage, get as Image 6 Middle A and Figure 7 Data results shown in A.
[0073] 2. Antibody response induced by mRNA vaccine. The end-point dilution ELISA titer of the novel coronavirus 2019-nCoV antibody in the serum of immunized mice was determined by optical density, and the results were ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com