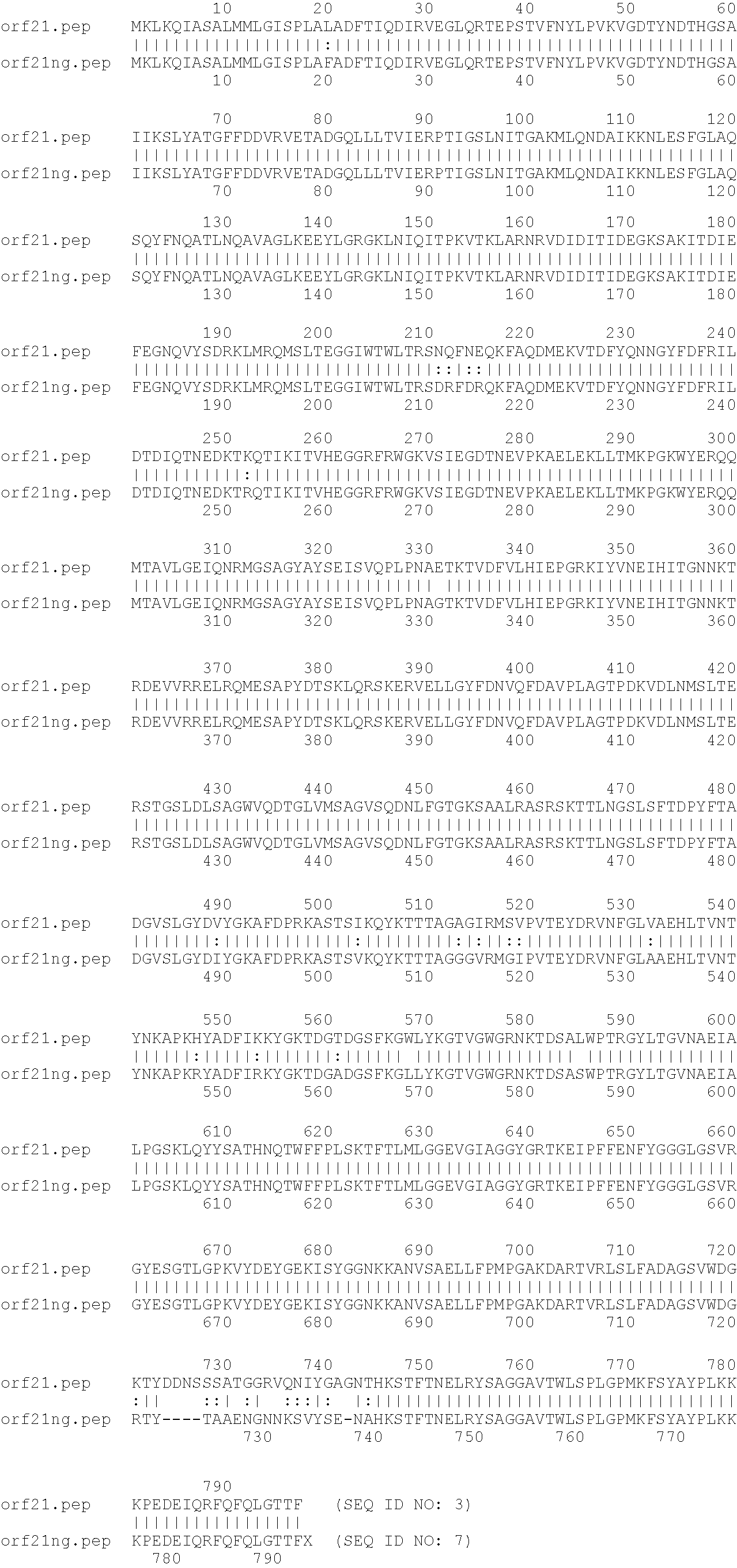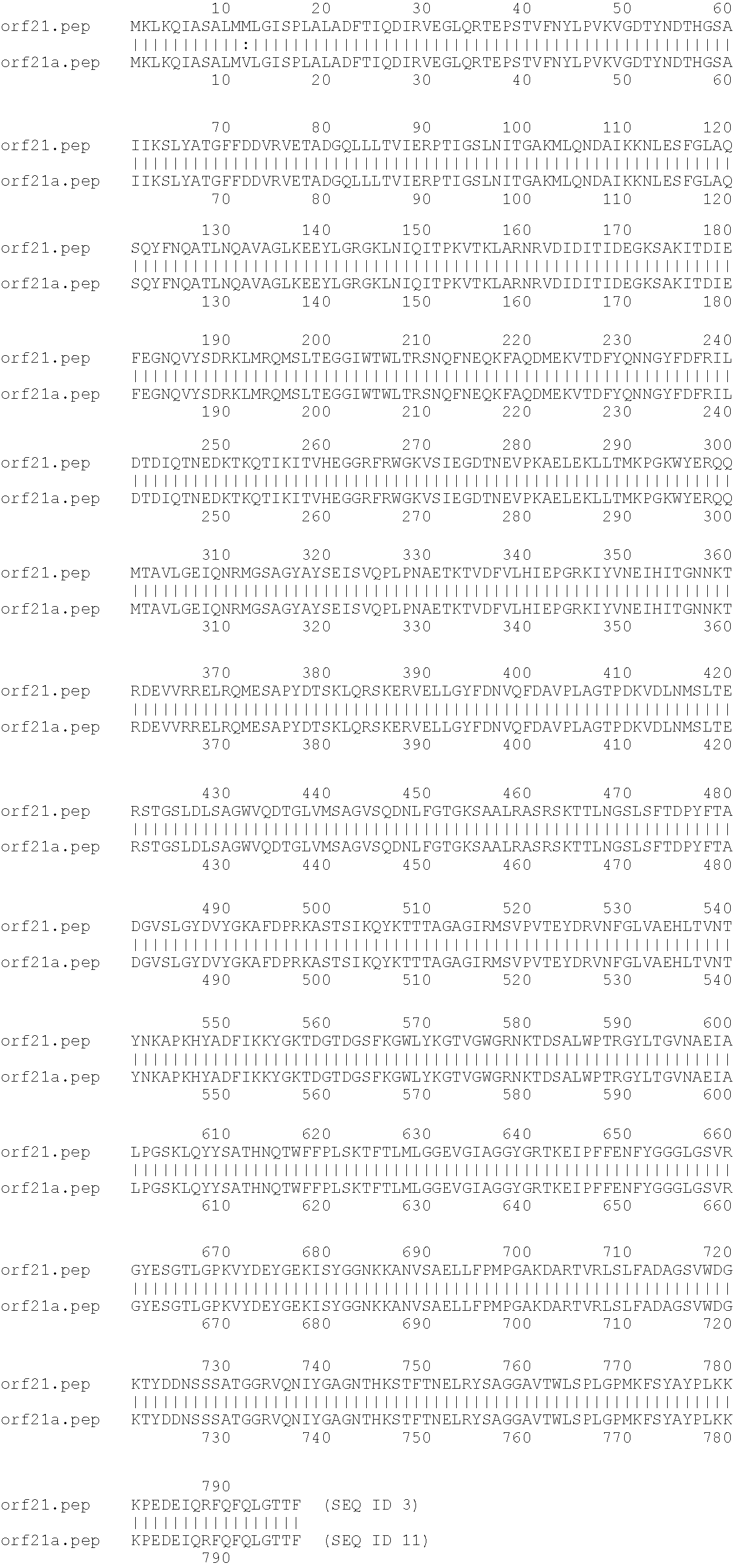Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
45 results about "Serogroup c" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Outer membrane vesicle (OMV) vaccine comprising N. meningitidis serogroup B outer membrane proteins
ActiveUS8273360B2Broadens their efficacyAntibacterial agentsNervous disorderProtective antigenNeisseria weaveri
A composition comprising (a) Neisseria meningitidis serogroup B outer membrane vesicles (OMVs), and (b) an immunogenic component selected from other Neisseria proteins, or immunogenic fragments thereof. Component (b) preferably includes a protein from a different NmB strain from that from which the OMV of component (a) is derived. The OMVs are preferably obtained by deoxycholate extraction. Optionally, the composition may also comprise a protective antigen against other pathogens.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Mucosal meningococcal vaccines
InactiveUS20070207090A1Improve securityReduced dosAntibacterial agentsBacterial antigen ingredientsMucosal Immune ResponsesAdjuvant
The invention provides immunogenic compositions for mucosal delivery comprising capsular saccharides from at least two of serogroups A, C, W135 and Y of N. meningitidis. It is preferred that the capsular saccharides in the compositions of the invention are conjugated to carrier protein(s) and / or are oligosaccharides. Conjugated oligosaccharide antigens are particularly preferred. The invention also provides immunogenic compositions comprising (a) a capsular saccharide antigen from serogroup C of N. meningitidis, and (b) a chitosan adjuvant. The composition preferably comprises (c) one or more further antigens and / or (d) one or more further adjuvants. The compositions are particularly suitable for mucosal delivery, including intranasal delivery. The use of chitosan and / or detoxified ADP-ribosylating toxin adjuvants enhances anti-meningococcal mucosal immune responses and can shift the Th1 / Th2 bias of the responses.
Owner:NOVARTIS AG
Neisseria meningitidis compositions and methods thereof
ActiveUS20130243807A1Antibacterial agentsBacterial antigen ingredientsSalmonella serotype typhiMeningococcal carriage
In one aspect, the invention relates to an isolated polypeptide comprising an amino acid sequence that is at least 95% identical to SEQ ID NO: 71. In another aspect, the invention relates to an immunogenic composition including an isolated non-lipidated, non-pyruvylated ORF2086 polypeptide from Neisseria meningitidis serogroup B, and at least one conjugated capsular saccharide from a meningococcal serogroup.
Owner:PFIZER INC
GNA1870-based vesicle vaccines for broad spectrum protection against diseases caused by Neisseria meningitidis
ActiveUS8968748B2Broad protectionReduce expressionAntibacterial agentsBiocideTGE VACCINENeisseria meningitidis
Owner:CHILDREN S HOSPITAL &RES CENT AT OAKLAN
Conjugate Vaccines
InactiveUS20080305127A1Improving immunogenicityReduce chain lengthAntibacterial agentsBacterial antigen ingredientsImmunologyHuman patient
The invention provides vaccines against Neisseria meningitidis, pneumococcus and DTPa / w. In particular, it provides vaccines based on conjugated capsular saccharides from multiple meningococcal and / or pneumococcal serogroups. It further provides vaccine administration schemes for the immunisation of human patients with two or more of these vaccines.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Multivalent meningococcal derivatized polysaccharide-protein conjugates and vaccine
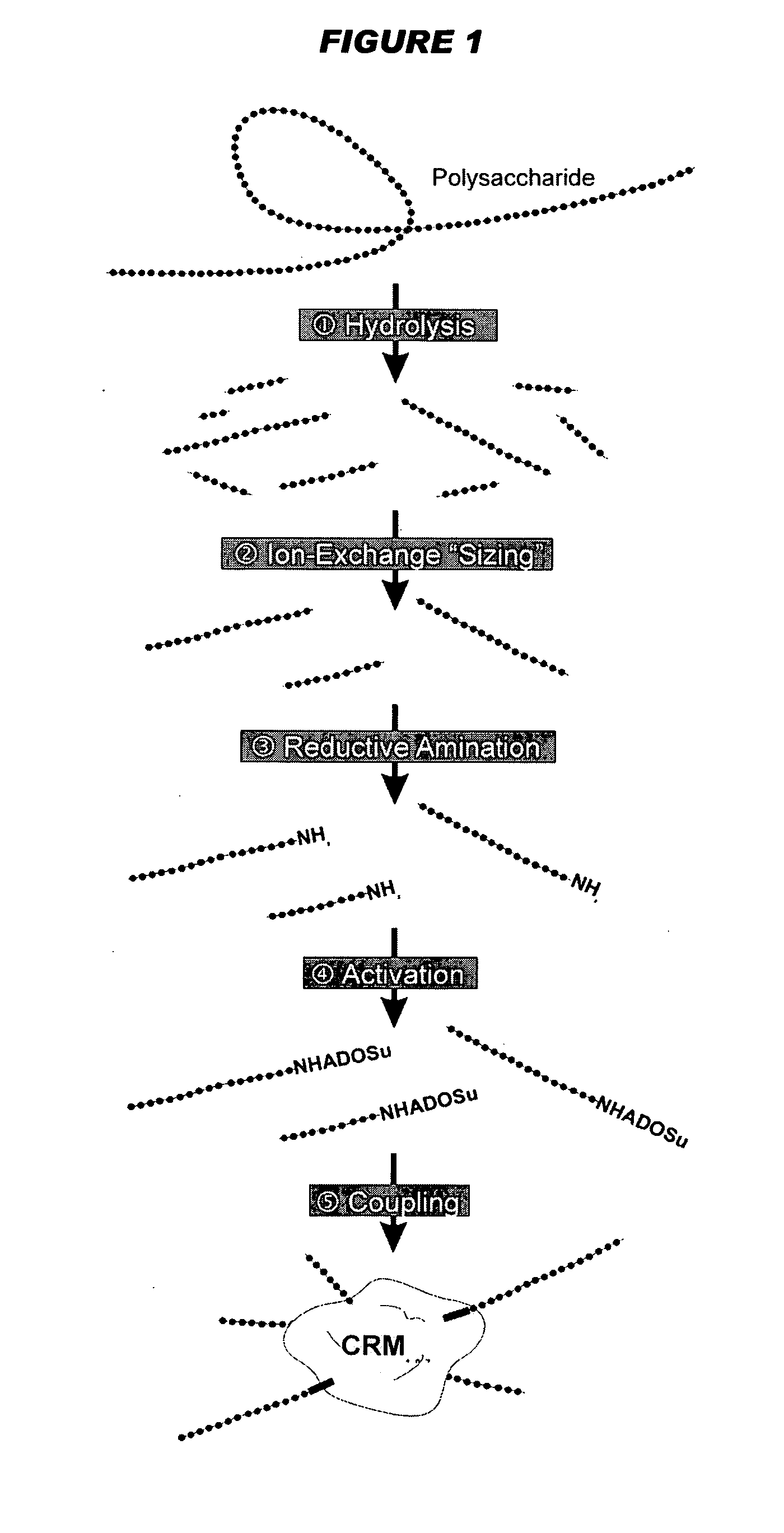
The present invention describes derivatized polysaccharide-protein conjugates, a composition comprising one or more of such derivatized polysaccharide-protein conjugates and methods of immunizing human patients with the same. The derivatized polysaccharide-protein conjugates are purified capsular polysaccharides from Neisseria meningitidis serogroups A, C, W-135, and Y, derivatized chemically activated and selectively attached to a carrier protein by means of a covalent chemical bond, forming polysaccharide-protein conjugates capable of eliciting long-lasting immunity to a variety of N. meningitidis strains.
Owner:AVENTIS PASTEUR INC
Neisseria meningitidis serogroup a capsular polysaccharide acetyltransferase, methods and compositions
InactiveUS20060073168A1Easy to optimizeComplete acetylationAntibacterial agentsBacteriaSalmonella serotype typhiAcetyltransferase
Provided are recombinant DNA molecules that do not occur in nature encoding an O-acetyltransferase, vectors that direct expression of an O-acetyltransferase, recombinant host cells which express an O-acetyltransferase, methods for recombinant production of an O-acetyltransferase, methods for acetylating capsular polysaccharides, especially those of a Serogroup A Neisseria meningitidis using a recombinant O-acetyltransferase, and immunogenic compositions comprising the acetylated capsular polysaccharide.
Owner:EMORY UNIVERSITY +1
Multiple vaccination including serogroup C meningococcus
Various improvements to vaccines that include a serogroup C meningococcal conjugate antigen, including: (a) co-administration with acellular B. pertussis antigen; (b) co-administration with an inactivated poliovirus antigen; (c) supply in a kit together with a separate pneumococcal conjugate component, which may be in a liquid form; and (d) use in combination with a pneumococcal conjugate antigen but without an aluminum phosphate adjuvant. A kit may have: (a) a first immunogenic component that comprises an aqueous formulation of a conjugated capsular saccharide from Streptococcus pneumoniae; (b) a second immunogenic component that comprises a conjugated capsular saccharide from Neisseria meningitidis serogroup C.
Owner:GSK VACCINES GMBH
Neisseria meningitidis compositions and methods thereof
ActiveUS20180214532A1Elicit immune responseAntibacterial agentsOrganic active ingredientsNeisseria meningitidisPolysaccharide
In one aspect, the invention relates to a composition including a factor H binding protein (fHBP) and a Neisseria meningitidis non-serogroup B capsular polysaccharide. The invention further relates to uses of a composition that includes fHBP, such as, for example, uses to elicit an immune response against N. meningitidis serogroup B strains and non-serogroup B strains. The compositions and methods described herein are directed to administration in humans, including adults, adolescents, toddlers, and infants.
Owner:PFIZER INC
Multiple vaccination including Serogroup C Meningococcus
ActiveUS20100034847A1Accurate measurementAvoid potential suppression effectAntibacterial agentsBacterial antigen ingredientsVaccinationAdjuvant
Various improvements to vaccines that include a serogroup C meningococcal conjugate antigen, including: (a) co-administration with acellular B. pertussis antigen; (b) co-administration with an inactivated poliovirus antigen; (c) supply in a kit together with a separate pneumococcal conjugate component, which may be in a liquid form; and (d) use in combination with a pneumococcal conjugate antigen but without an aluminium phosphate adjuvant. A kit may have: (a) a first immunogenic component that comprises an aqueous formulation of a conjugated capsular saccharide from Streptococcus pneumoniae; (b) a second immunogenic component that comprises a conjugated capsular saccharide from Neisseria meningitidis serogroup C.
Owner:GSK VACCINES GMBH
Mucosal vaccines with chitosan adjuvant and meningococcal antigens
InactiveUS20060051378A1Improve securityReduced dosAntibacterial agentsOrganic active ingredientsSalmonella serotype typhiCarrier protein
The invention provides immunogenic compositions comprising (a) a capsular saccharide antigen from serogroup C of N. meningitidis, and (b) a chitosan adjuvant. The composition preferably comprises (c) one or more further antigens and / or (d) one or more further adjuvants. The compositions are particularly suitable for mucosal delivery, including intranasal delivery. The invention also provides immunogenic compositions for mucosal delivery comprising capsular saccharides from at least two of serogroups A, C, W135 and Y of N. meningitidis. It is preferred that the capsular saccharides in the compositions of the invention are conjugated to carrier protein(s) and / or are oligosaccharides. Conjugated oligosaccharide antigens are particularly preferred.
Owner:NOVARTIS AG +1
Combination meningitidis B/C vaccines
InactiveUS8007815B1Antibacterial agentsBacterial antigen ingredientsLiposome VesicleNeisseria meningitidis
Combination vaccines for treating or preventing Neisseria meningitidis infection are described. The vaccines include Neisseria meningitidis serogroup B proteoliposomic vesicles and Neissera meningitidis serogroup C conjugated oligosaccharides.
Owner:NOVARTIS AG +2
Neisseria meningitidis compositions and methods thereof
ActiveUS20190231861A1Elicit immune responseAntibacterial agentsOrganic active ingredientsNeisseria meningitidisPolysaccharide
In one aspect, the invention relates to a composition including a factor H binding protein (fHBP) and a Neisseria meningitidis non-serogroup B capsular polysaccharide. The invention further relates to uses of a composition that includes fHBP, such as, for example, uses to elicit an immune response against N. meningitidis serogroup B strains and non-serogroup B strains. The compositions and methods described herein are directed to administration in humans, including adults, adolescents, toddlers, and infants.
Owner:PFIZER INC
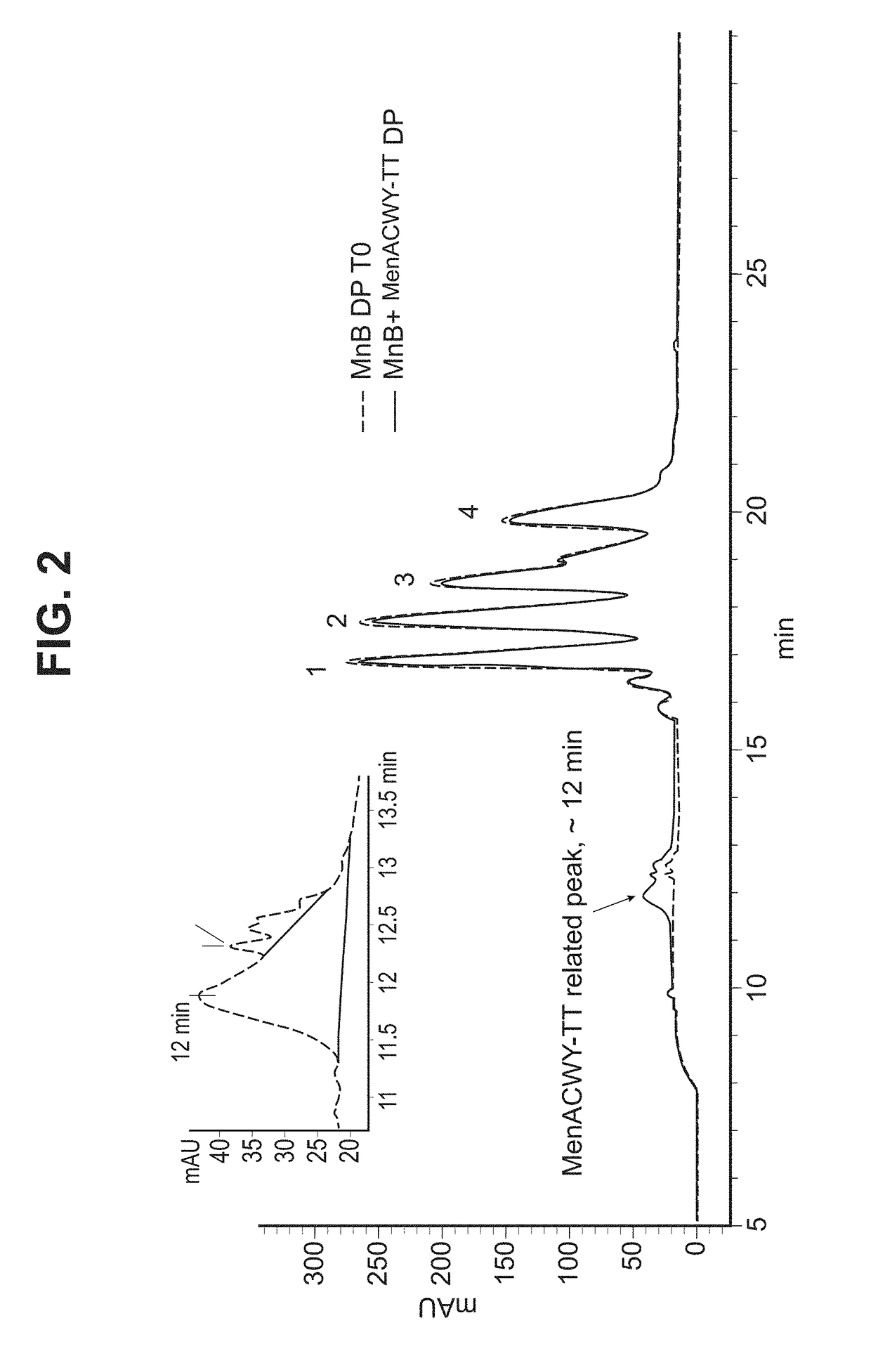
Analysis of Saccharide Vaccines Without Interference
InactiveUS20080286300A1Improve quality controlQuicker and easy to performAntibacterial agentsRadiation pyrometryConjugate vaccineSialic acid
The invention is based on methods that allow analysis of mixed meningococcal saccharides from multiple serogroups even though they share monosaccharide units. With a combination of saccharides from serogroups C, W135 and Y, the invention analyses sialic acid, glucose and galactose content. The glucose and galactose results are used to directly quantify saccharides from serogroups Y and W135, respectively, and the combined glucose and galactose content is subtracted from the sialic acid content to quantify saccharides from serogroup C. The three serogroups can thus be resolved even though their monosaccharide contents overlap. The three different monosaccharide analyses can be performed on the same material, without interference between the monosaccharides and without interference from any other saccharide materials in the composition (e.g. lyophilisation stabilisers). The method can be used to analyse total and free saccharide in conjugate vaccines and simplifies quality control of vaccines containing capsular saccharides from multiple serogroups.
Owner:NOVARTIS AG
Compositions comprising neisseria meningitidis antigens from serogroups b and c
ActiveUS20050074450A1Easy to prepareEasy to manageAntibacterial agentsBacterial antigen ingredientsProtective antigenImmunogenicity
International patent application WO99 / 61053 discloses immunogenic compositions that comprise N. meningitidis serogroup C oligosaccharide conjugated to a carrier, in combination with N. meningitidis serogroup B outer membrane protein. These are disclosed in the present application in combination with further Neisserial proteins and / or protective antigens against other pathogenic organisms (e.g. Haemophilus influenzae, DTP, HBV, etc.).
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Combination vaccines with serogroup B meningococcus and D/T/P
InactiveUS9526776B2Low amountEnhance immune responseAntibacterial agentsImmunological disordersAdjuvantImmunity response
Serogroup B meningococcus antigens can successfully be combined with diphtheria, tetanus and pertussis toxoids (“DTP”) to provide effective combination vaccines for protecting against multiple pathogens. These combinations are effective with a range of different adjuvants, and with both pediatric-type and booster-type DTP ratios. The adjuvant can improve the immune response which the composition elicits; alternatively, by including an adjuvant it is possible for the compositions to have a relatively lower amount of antigen while nevertheless having immunogenicity which is comparable to unadjuvanted combination vaccines.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
85kDa neisserial antigen
An 85 kDa antigen from Neisseria meningitidis and Neisseria gonorrhoeae has been cloned, sequenced and expressed. The antigen is common to diverse strains, serogroups and serotypes of N. meningitidis, and also to N. gonorrhoeae, N. polysaccharia and N. lactamica. The protein sequences of N. meningitidis (serogroups A and B) and N. gonorrhoeae are highly homologous.
Owner:STATENS INST FOR FOLKEHELSE +1
Adjuvanting meningococcal factor h binding protein
InactiveUS20120070458A1Reduce chain lengthOptimum chain lengthAntibacterial agentsBacterial antigen ingredientsPoint of zero chargeAntigen
Factor H binding protein (fHBP) has been proposed for use in immunising against serogroup B meningococcus (‘MenB’). This antigen can be efficiently adsorbed to an aluminium hydroxyphosphate adjuvant by (i) ensuring that adsorption takes place at a pH which is equal to or below the adjuvant's point of zero charge (PZC), and / or (ii) selecting a fHBP and adjuvant with an isoelectric point / PZC within the range of 5.0 to 7, and / or (iii) selecting a fHBP with an isoelectric point above the adjuvant's PZC and using a buffer to bring the pH to within 1.2 pH units of the PZC. The adsorption is particularly useful for compositions which include multiple fHBP variants, and also in situations where an aluminium hydroxide adjuvant should be avoided. Buffered pharmaceutical compositions can include at least two different meningococcal fHBP antigens, both of which are at least 85% adsorbed to aluminium hydroxyphosphate adjuvant.
Owner:NOVARTIS AG
Real-time fluorescent quantitative RT-PCR detection primers for serotype of Palyam serogroup virus (PALV), probes and detection kit
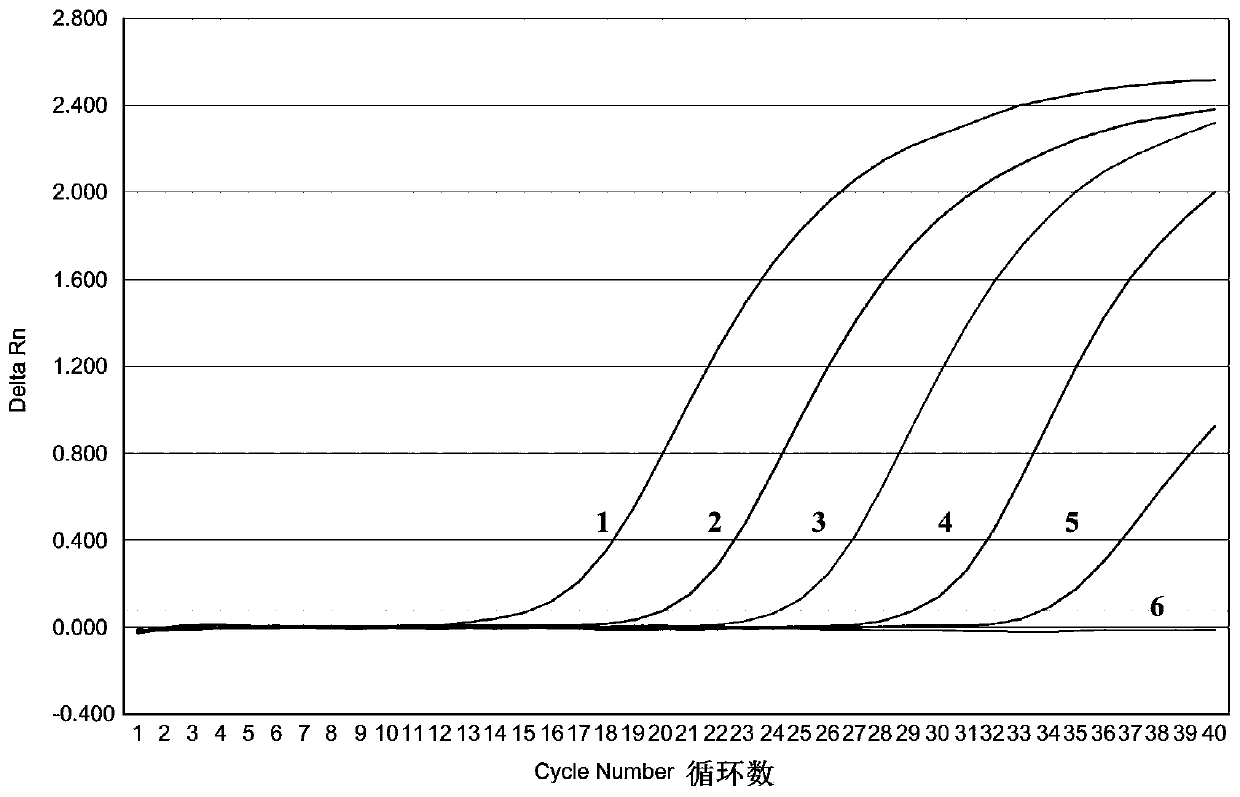
ActiveCN110669870AStrong specificityShorten the timeMicrobiological testing/measurementMicroorganism based processesAnimal virusSingle strand
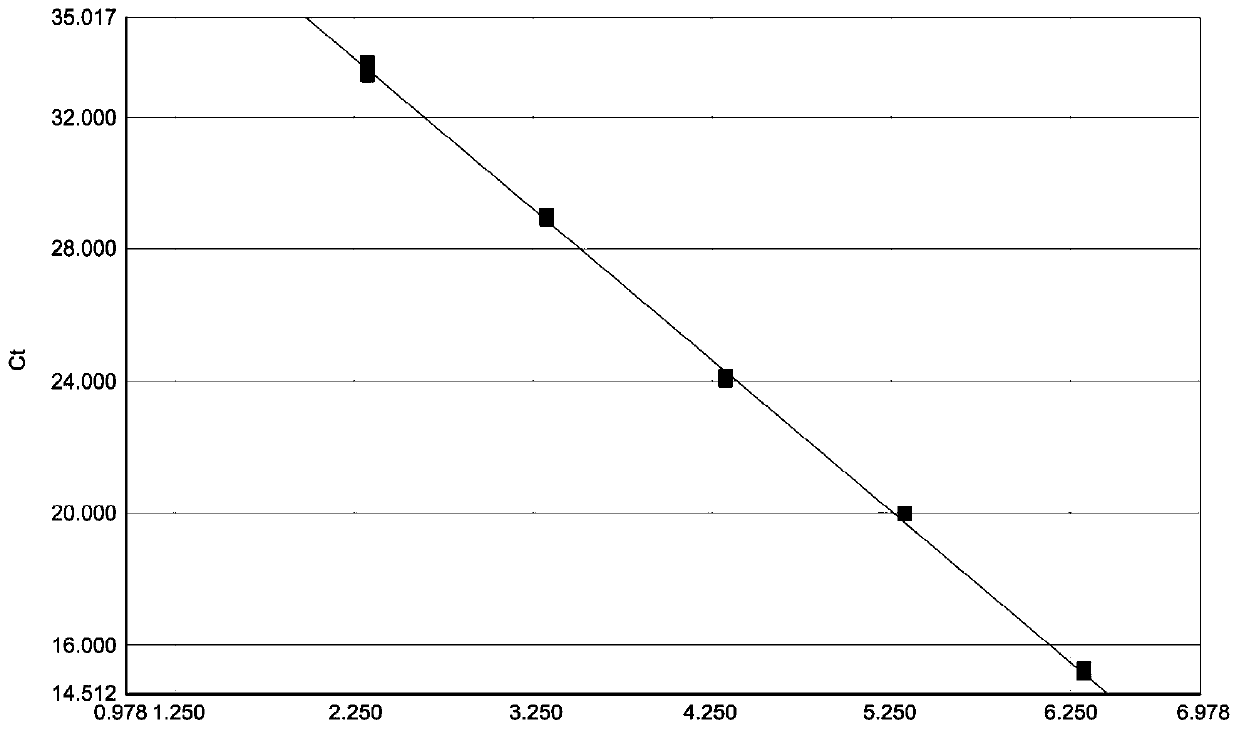
The invention relates to real-time fluorescent quantitative RT-PCR detection primers for a serotype of a Palyam serogroup virus (PALV), probes and a detection kit and belongs to the technical field ofmolecular biological detection on animal viruses. The kit comprises 3 pairs of primers and further comprises three probes, a negative control template, positive control templates, standard substancetemplates and a PCR amplification reagent. RNase-free water serves as the negative control template; the positive control templates are CHUV, BCV and DAV inactivated viruses separately; and the standard substance templates are gene segment 2 single-stranded RNAs of CHUV, BCV and DAV. The kit disclosed by the invention can be used for detecting three kinds of serotype PALVs popular in China and hasthe advantages of specificity, sensitivity, rapidness, high efficiency and the like; and a standard curve established by utilizing the standard substance templates in a matched manner can be used forcarrying out quantitative detection on RNAs of all PALV serotype strains in clinical samples, and thus, the working efficiency is increased greatly.
Owner:YUNNAN ANIMAL SCI & VETERINARY INST
Methods for isolating molecular mimetics of unique Neisseria meningitidis serogroup B epitopes
InactiveUS7063949B2Determine autoreactivityMethod securityAntibacterial agentsPeptide librariesEscherichia coliSalmonella serotype typhi
Novel bactericidal antibodies against Neisseria meningitidis serogroup B (“MenB”) are disclosed. The antibodies either do not cross-react or minimally cross-react with host tissue polysialic acid and hence pose minimal risk of autoimmune activity. The antibodies are used to identify molecular mimetics of unique epitopes found on MenB or E. coli K1. Examples of such peptide mimetics are described that elicit serum antibody capable of activating complement-mediated bacteriolysis of MenB. Vaccine compositions containing such mimetics can be used to prevent MenB or E. coli K1 disease without the risk of evoking autoantibody.
Owner:CHILDREN S HOSPITAL &RES CENT AT OAKLAN
Novel synthetic oligomers of neisseria meningitis serogroup x and process of preparing them
InactiveUS20170066794A1Short time durationMinimizeEsterified saccharide compoundsAntibacterial agentsBacterial meningitisChemical synthesis
The present invention relates to synthesis of novel higher oligomers and process of preparing the same. In particular the present invention relates to the chemical synthesis of oligomers of Neisseria meningitidis serogroup X (‘hereinafter Men-X), more particularly tetramer. The present invention provides Men-X capsular oligomers obtained from synthetic pathway using purified saccharides of specific chain length and provides said novel oligomers as candidates for the development of conjugate vaccine against bacterial meningitis caused due to Men-X infections.
Owner:MSD WELLCOME TRUST HILLEMAN LAB PVT LTD
Outer membrane vesicle vaccine against disease caused by neisseria meningitidis serogroup a and process for the production thereof
InactiveUS20040131642A1Increase productionEfficient killingAntibacterial agentsBacterial antigen ingredientsSerum igeDisease
Owner:STATENS INST FOR FOLKEHELSE
Mucosal vaccines with chitosan adjuvant and meningococcal antigens
InactiveUS8926992B2Protective efficacyImproving immunogenicityAntibacterial agentsBiocideSalmonella serotype typhiCarrier protein
The invention provides immunogenic compositions comprising (a) a capsular saccharide antigen from serogroup C of N. meningitidis, and (b) a chitosan adjuvant. The composition preferably comprises (c) one or more further antigens and / or (d) one or more further adjuvants. The compositions are particularly suitable for mucosal delivery, including intranasal delivery. The invention also provides immunogenic compositions for mucosal delivery comprising capsular saccharides from at least two of serogroups A, C, W135 and Y of N. meningitidis. It is preferred that the capsular saccharides in the compositions of the invention are conjugated to carrier protein(s) and / or are oligosaccharides. Conjugated oligosaccharide antigens are particularly preferred.
Owner:NOVARTIS AG +1
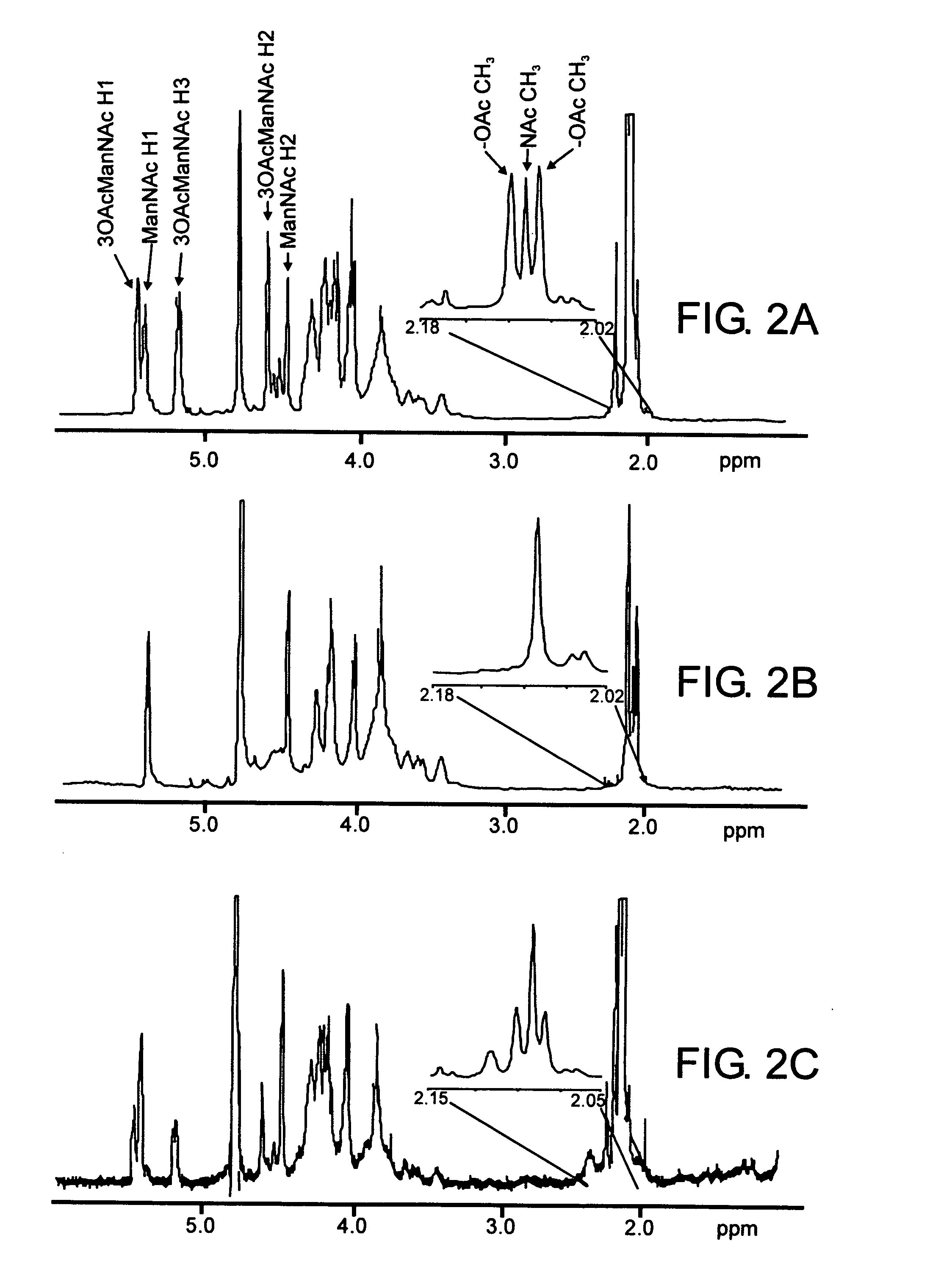
Analysis of Mannosamine-containing Capsular Saccharides
InactiveUS20100022015A1High resolution separationEnhanced mass transferBiological testingImmunoassaysPhosphateHydrolysate
Analysis of compositions that include saccharides having mannosamine residues, such as the capsular saccharide of N. meningitidis serogroup A, is facilitated by a method comprising the steps of: (i) hydrolysing polysaccharide in the sample, to give a hydrolysate; (ii) subjecting the hydrolysate to liquid chromatography; and (iii) detecting any mannosamine-6-phosphate separated in step (ii).
Owner:NOVARTIS AG
Composition and method for controlling intestinal pathogenic organisms
ActiveUS7935355B2Easily and effectively administeredReducing of to detectPeptide/protein ingredientsSnake antigen ingredientsAntigenEscherichia coli
An antigen composition for stimulating an immune response in an inoculated avian species to at least one intestinal pathogenic organism includes naturally-occurring wild Salmonella enterica subspecies in O-serogroups B, C3 and D. Subspecies in O-serogroup B can include Salmonella typhimurium and / or Salmonella agona. Subspecies in O-serogroup C3 can include Salmonella Kentucky. Subspecies in O-serogroup D can include Salmonella enteritidis. The antigen composition can be used alone or in combination with a Marek's Disease vaccine to reduce shedding of E. coli and / or Salmonella bacteria.
Owner:NUTRITIONAL HEALTH INST LAB
Real-time fluorescent quantitative RT-PCR detection primer, probe and kit for Palyamserogroup virus
PendingCN110643740AStrong specificitySensitiveMicrobiological testing/measurementDNA/RNA fragmentationAnimal virusGel electrophoresis
The invention relates to a real-time fluorescent quantitative RT-PCR detection primers, probe and kit for Palyamserogroup virus, and belongs to the technical field of animal virus molecular biologicaldetection. The kit comprises an upstream primer, a downstream primer, the probe matched with the primers for use, a negative control template, a positive control template, a standard template and a PCR amplification reagent. The kit is adopted for detection, the reaction speed is high, and the whole amplification process is less than 1 hour; only virus RNA needs to be extracted, reverse transcription is not needed, operation steps are few, easy and convenient, and RNA degradation and pollution can be effectively avoided; meanwhile, after qRT-PCR amplification is completed, whether PALV RNA exists in a sample to be detected or not can be directly judged without agarose gel electrophoresis; and in addition, a standard curve established with the help of the standard template can be used forquantitatively detecting the PALV RNA in the clinical sample, so that the working efficiency is greatly improved, and the detection cost is reduced.
Owner:YUNNAN ANIMAL SCI & VETERINARY INST
Meningococcal and pneumococcal conjugate vaccine and method of using same
ActiveUS8003112B2Efficient infectionLow costAntibacterial agentsBacterial antigen ingredientsCoccidiaMeningococcal carriage
This disclosure relates to vaccine formulations comprising an immunogenic composition for inducing antibodies to both S. pneumoniae and N. meningitides in a subject. In a preferred aspect, the immunogenic composition comprises covalently conjugated recombinant PsaA (“rPsaA”) from S. pneumoniae and capsular polysaccharide from N. meningitidis serogroup C. This disclosure further relates to methods for producing the immunogenic composition as well as methods for their use.
Owner:HOWARD UNIVERSITY +1
Downstream process for purifying polysaccharides
InactiveUS10011662B2Easy to handleReduce impurityMicroorganismsUltrafiltrationConjugate vaccineBacterial polysaccharide
Owner:MSD WELLCOME TRUST HILLEMAN LAB PVT LTD
Rapid high yielding purification process for neisseria meningitidis serogroup X capsular polysaccharide
PendingCN110892076AReduce recyclingHigh yieldBacteriaBiological material analysisMicrobiologyTGE VACCINE
The present invention discloses a rapid high yielding purification process for Neisseria meningitidis serogroup X capsular polysaccharide. The capsular polysaccharide of present invention is capable of being used in the production of economical monovalent capsular polysaccharide or polysaccharide protein conjugate vaccine or multivalent combination vaccines as well as a diagnostic tool against meningococcal serogroup X infections. The process employs simple salts and lesser quantity of ethanol. The process is rapid, economic and scalable with high yield of purified polysaccharide of Neisseriameningitidis serogroup X.
Owner:MSD WELLCOME TRUST HILLEMAN LAB PVT LTD
85kDa neisserial antigen
An 85kDa antigen from Neisseria meningitidis and Neisseria gonorrhoeae has been cloned, sequenced and expressed. The antigen is common to diverse strains, serogroups and serotypes of N. meningitidis, and also to N. gonorrhoeae, N. polysaccharia and N. lactamica. The protein sequences of N. meningitidis (serogroups A and B) and N. gonorrhoeae are highly homologous.
Owner:STATENS INST FOR FOLKEHELSE +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com