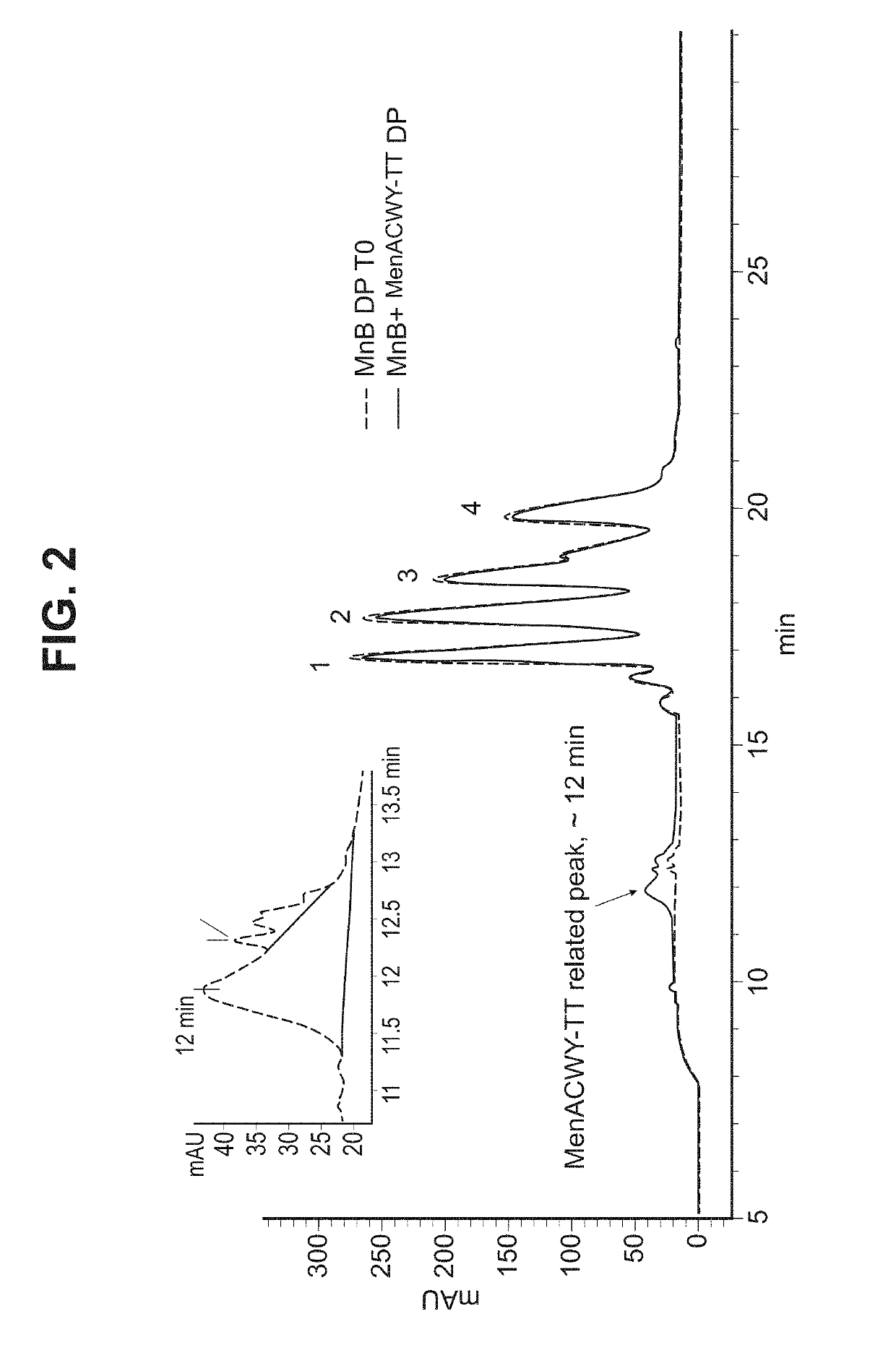
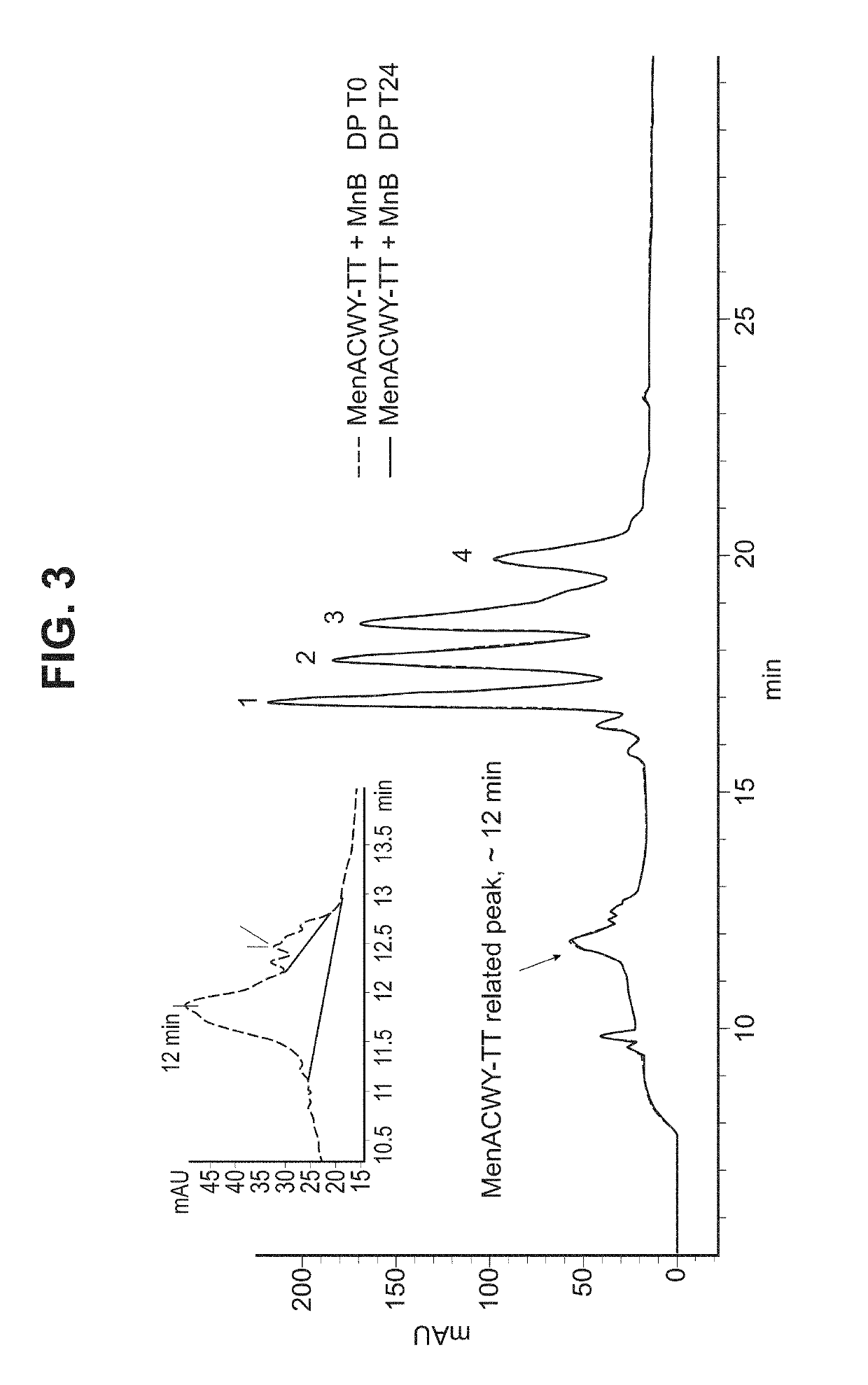
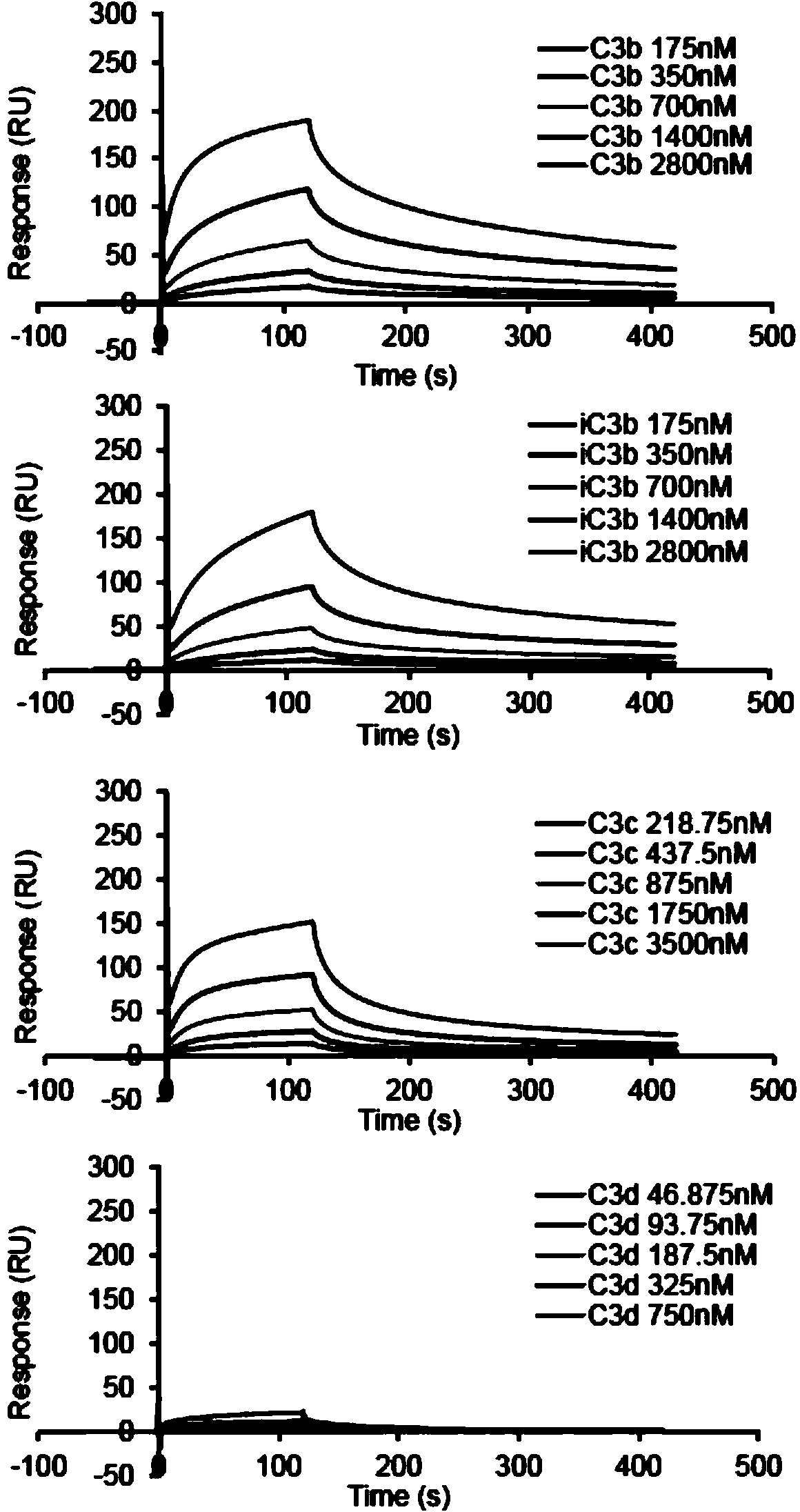
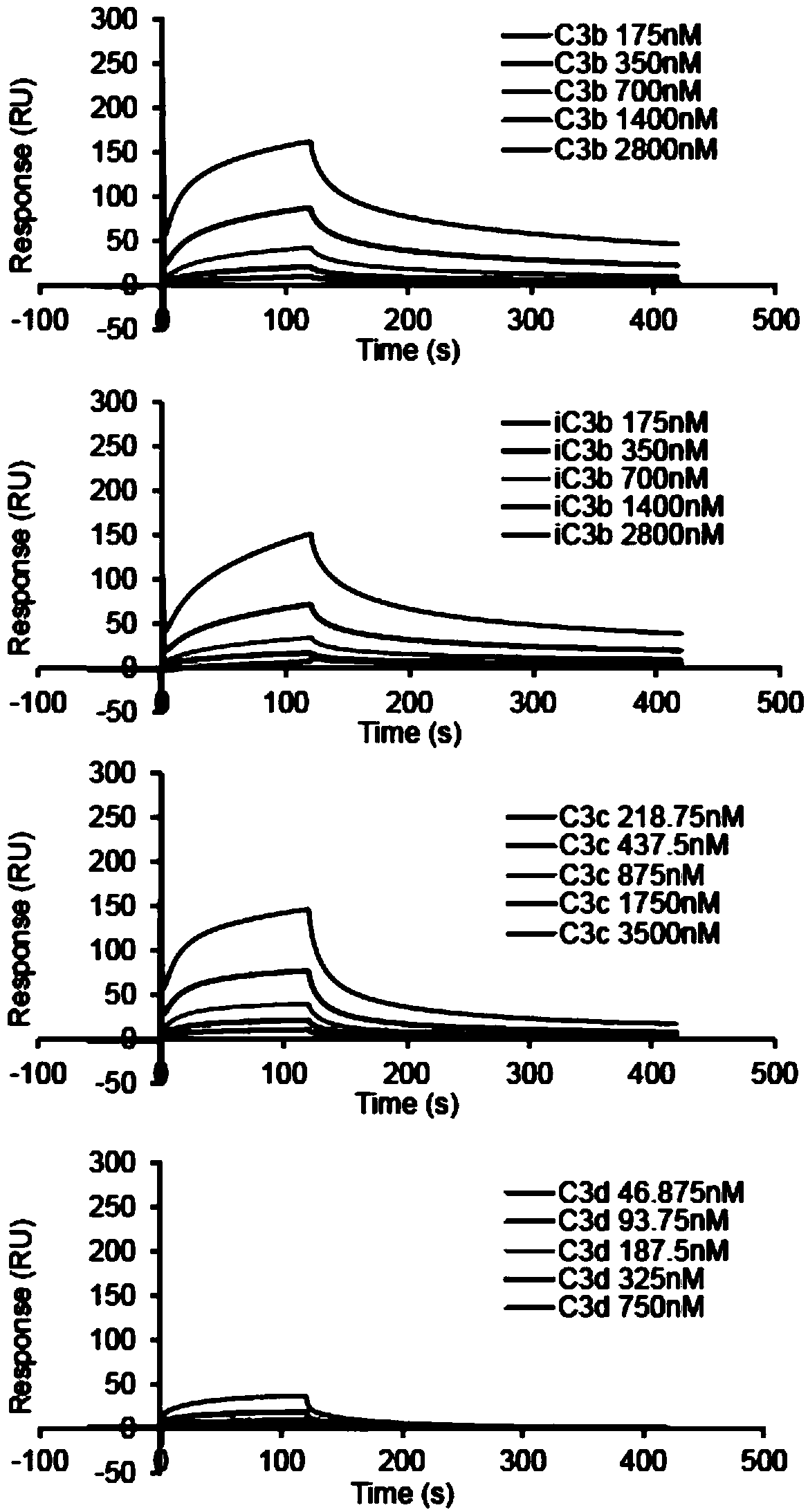
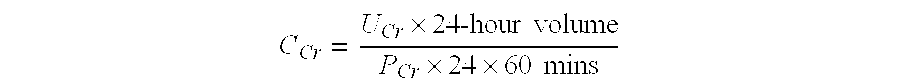Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
121 results about "Factor H" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
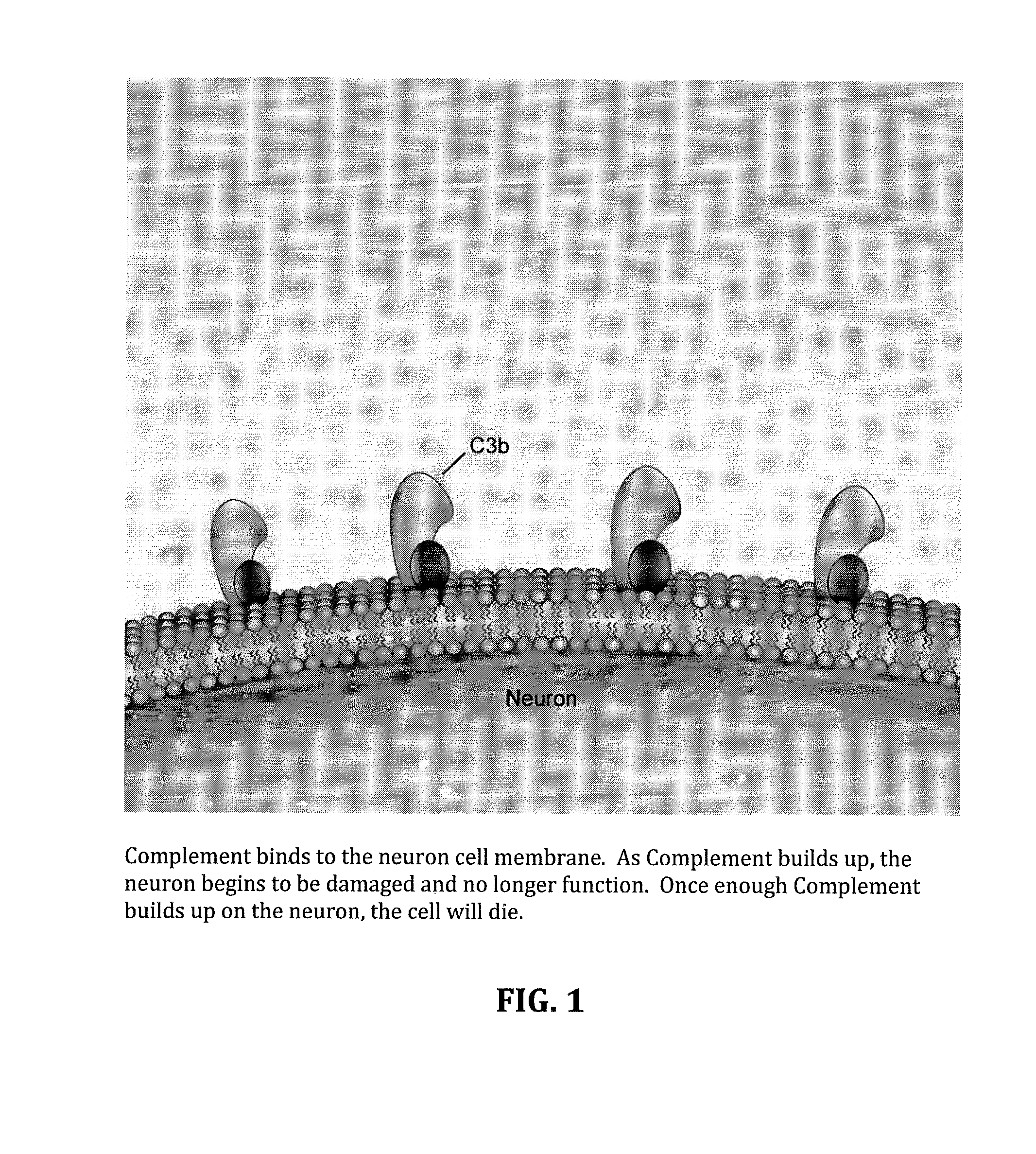
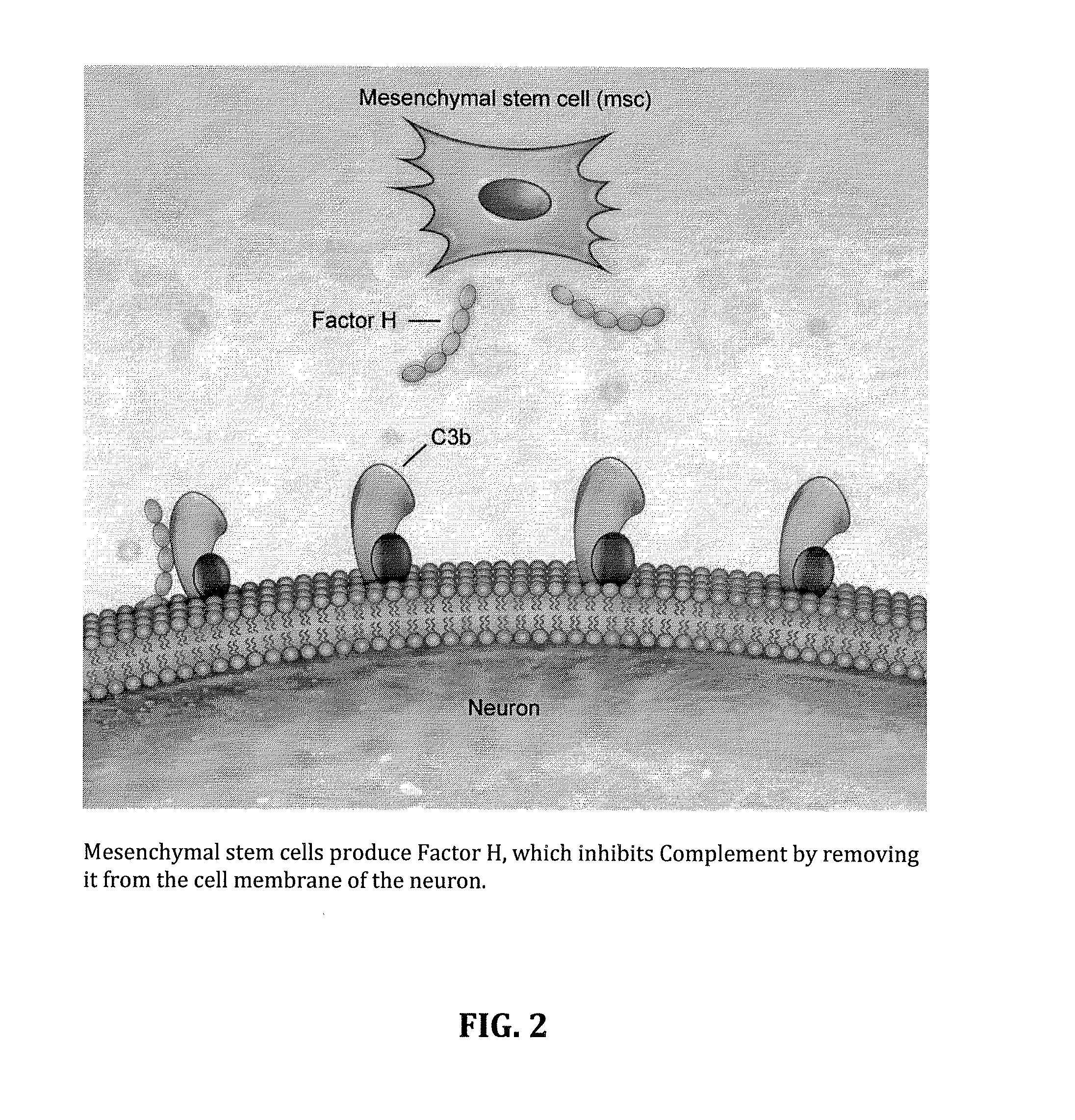
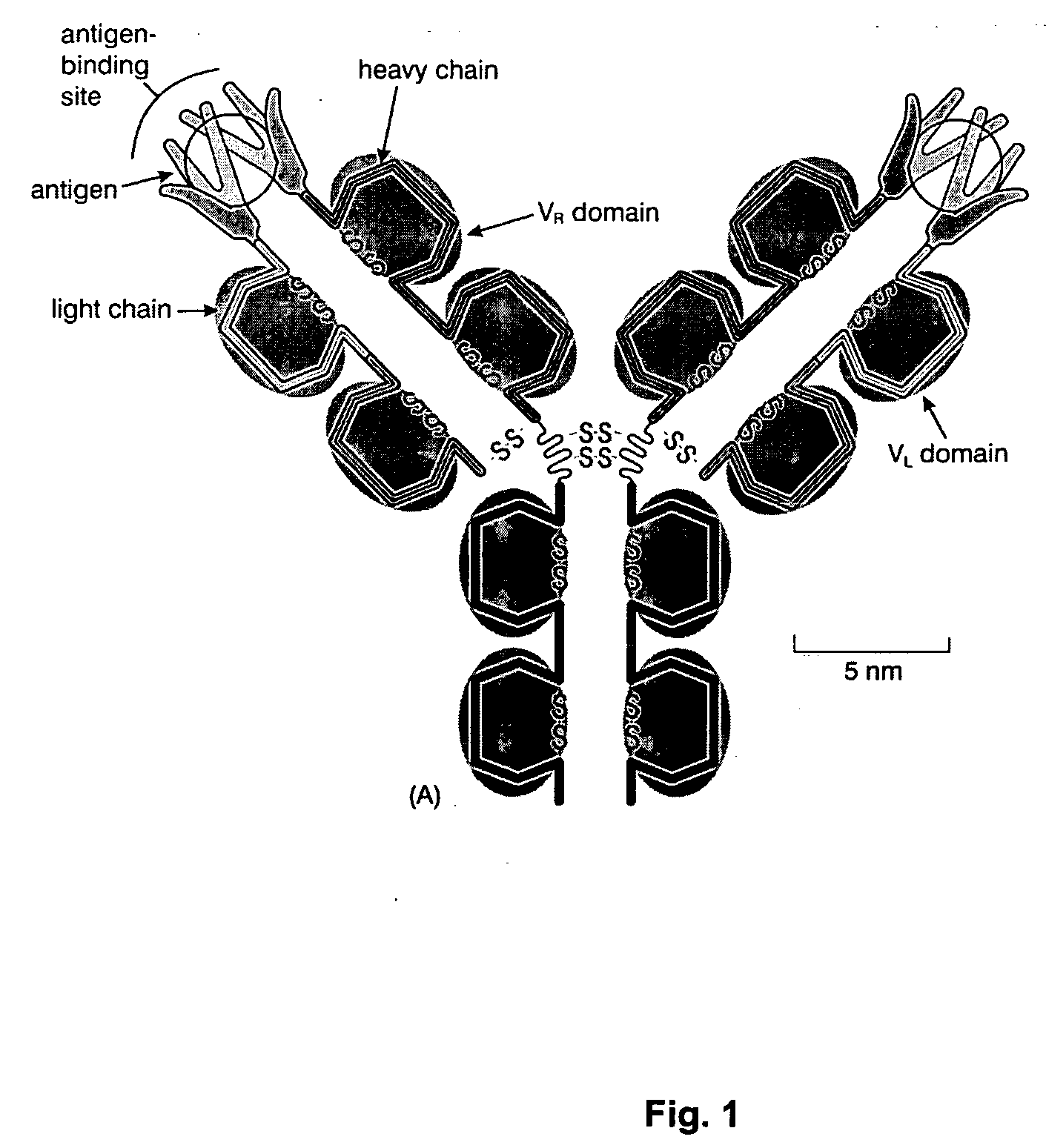
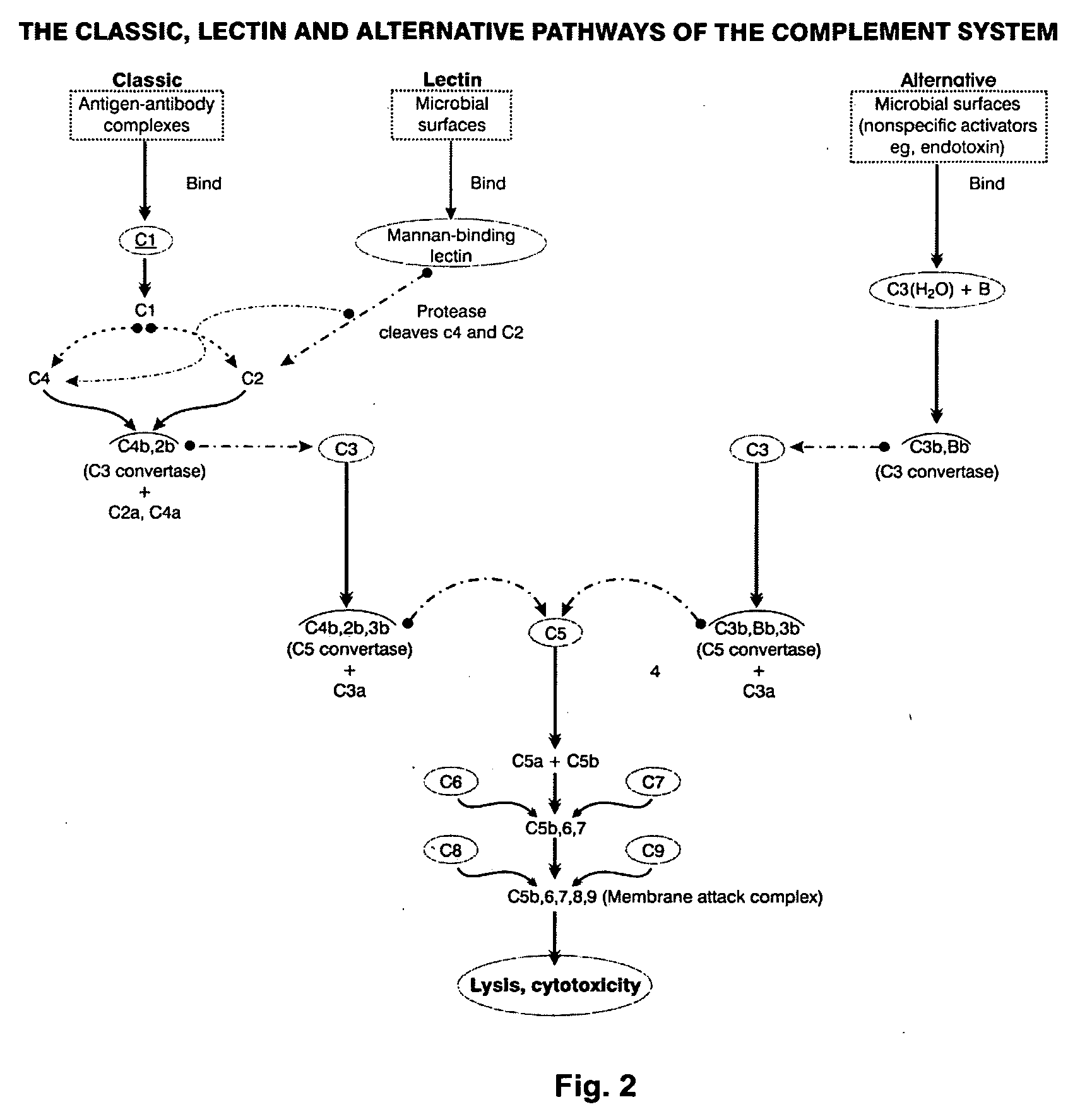
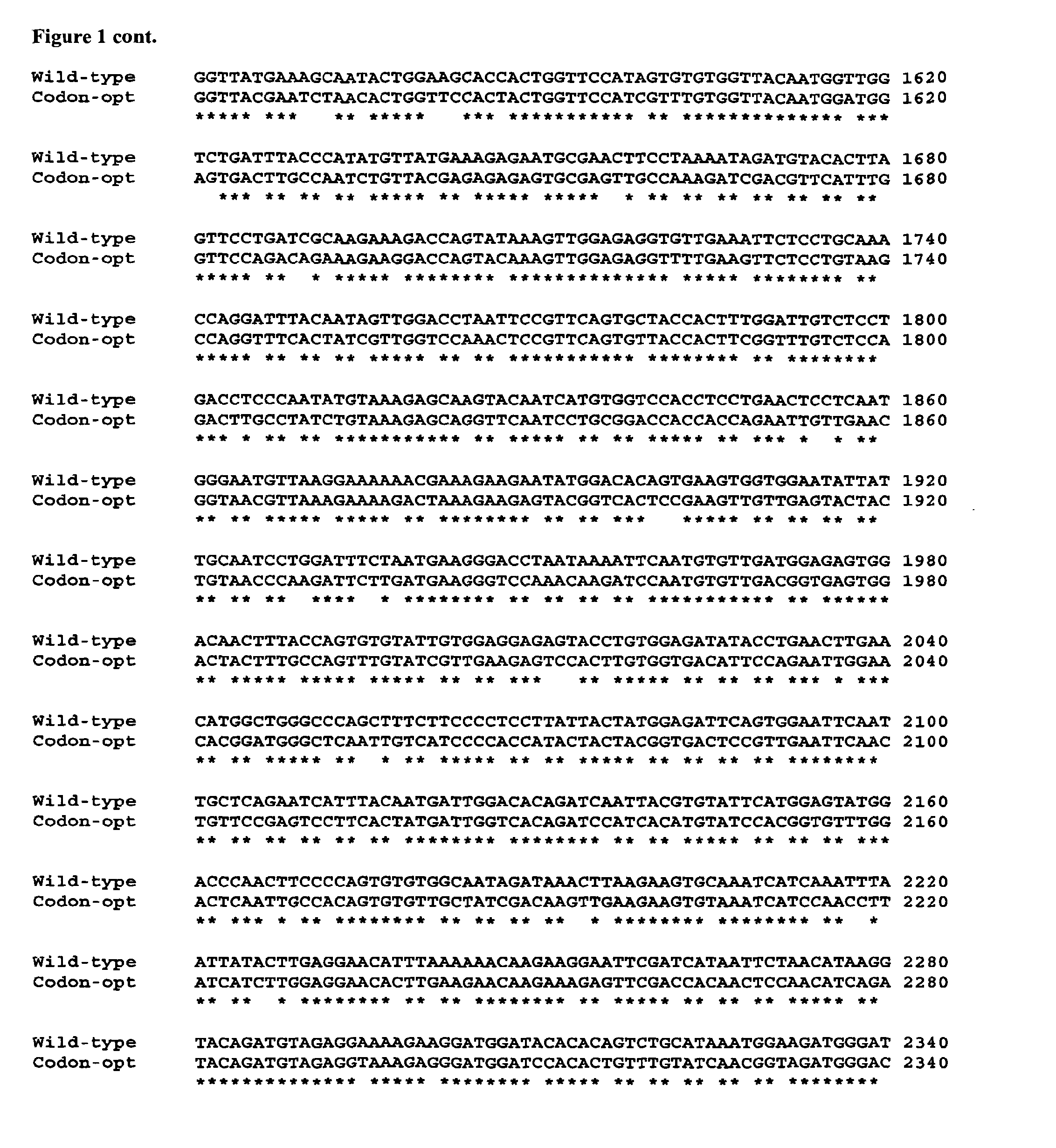
Factor H is a member of the regulators of complement activation family and is a complement control protein. It is a large (155 kilodaltons), soluble glycoprotein that circulates in human plasma (at typical concentrations of 200–300 micrograms per milliliter). Its principal function is to regulate the alternative pathway of the complement system, ensuring that the complement system is directed towards pathogens or other dangerous material and does not damage host tissue. Factor H regulates complement activation on self cells and surfaces by possessing both cofactor activity for the Factor I mediated C3b cleavage, and decay accelerating activity against the alternative pathway C3-convertase, C3bBb. Factor H exerts its protective action on self cells and self surfaces but not on the surfaces of bacteria or viruses. This is thought to be the result of Factor H having the ability to adopt conformations with lower or higher activities as a cofactor for C3 cleavage or decay accelerating activity. The lower activity conformation is the predominant form in solution and is sufficient to control fluid phase amplification. The more active conformation is thought to be induced when Factor H binds to glycosaminoglycans (GAGs) and or sialic acids that are generally present on host cells but not, normally, on pathogen surfaces ensuring that self surfaces are protected whilst complement proceeds unabated on foreign surfaces.
Use of complement inhibitors to treat ocular diseases
Owner:GENENTECH INC
Combinations of meningococcal factor h binding proteins
ActiveUS20130189295A1Reduce chain lengthOptimum chain lengthAntibacterial agentsBacterial antigen ingredientsMeningococcal carriageBioinformatics
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
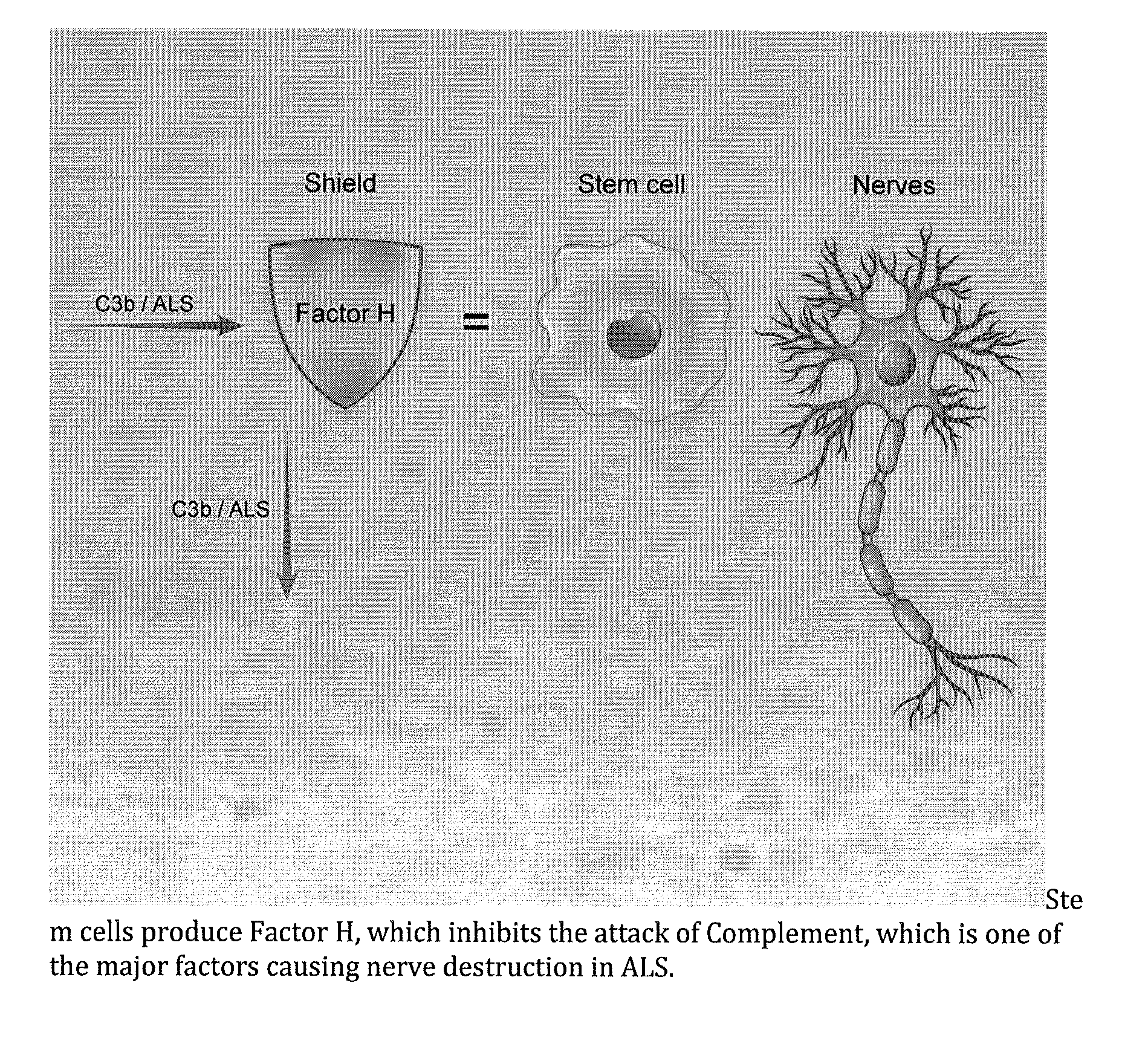
Method for treating ALS via the increased production of factor h
InactiveUS20140234275A1Improve satisfactionInhibition effectBiocideOrganic active ingredientsWhole bodyPrimary motor neuron
Methods and systems for the treatment for ALS incorporating stem cells harvested from the subject to be treated. These stem cells may be genetically altered with the addition of several genes of interest. Then, the patient will receive systemic gene therapy for the muscles and directed specifically at motor neurons. In this multi-pronged treatment approach, the stem cells provide immune regulation and the regeneration of motor neurons. And, the new motor neurons carry the added genes, which are protective against motor neuron death from ALS. The systemic therapy increases the amount of genes, which further reduces the effects of ALS. Additional gene therapy administered in the muscle will be further protective of the axon, while maintaining muscle mass and function.
Owner:BIOVIVA USA
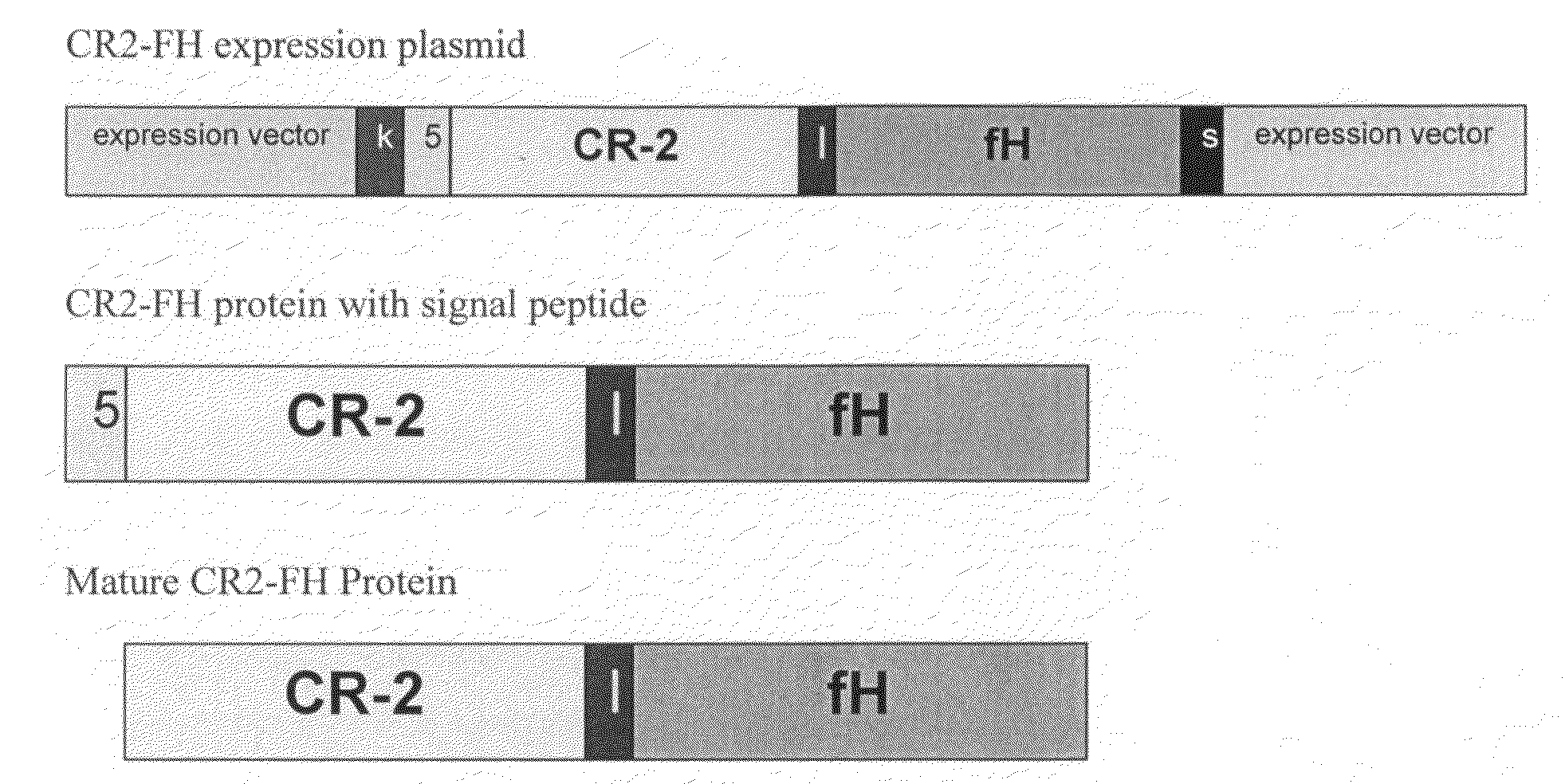
Targeting complement factor H for treatment of diseases
ActiveUS20080221011A1Improve survivalSenses disorderPeptide/protein ingredientsFactor iiComplement factor I
The invention provides a CR2-FH molecule comprising a CR2 portion comprising CR2 protein or a fragment thereof and a FH portion comprising a factor H protein or a fragment thereof, and pharmaceutical compositions comprising a CR2-FH molecule. Also provided are methods of using the compositions for treatment diseases in which the alternative complement pathway is implicated, such as age-related macular degeneration, rheumatoid arthritis, and ischemia reperfusion.
Owner:MUSC FOUND FOR RES DEV +1
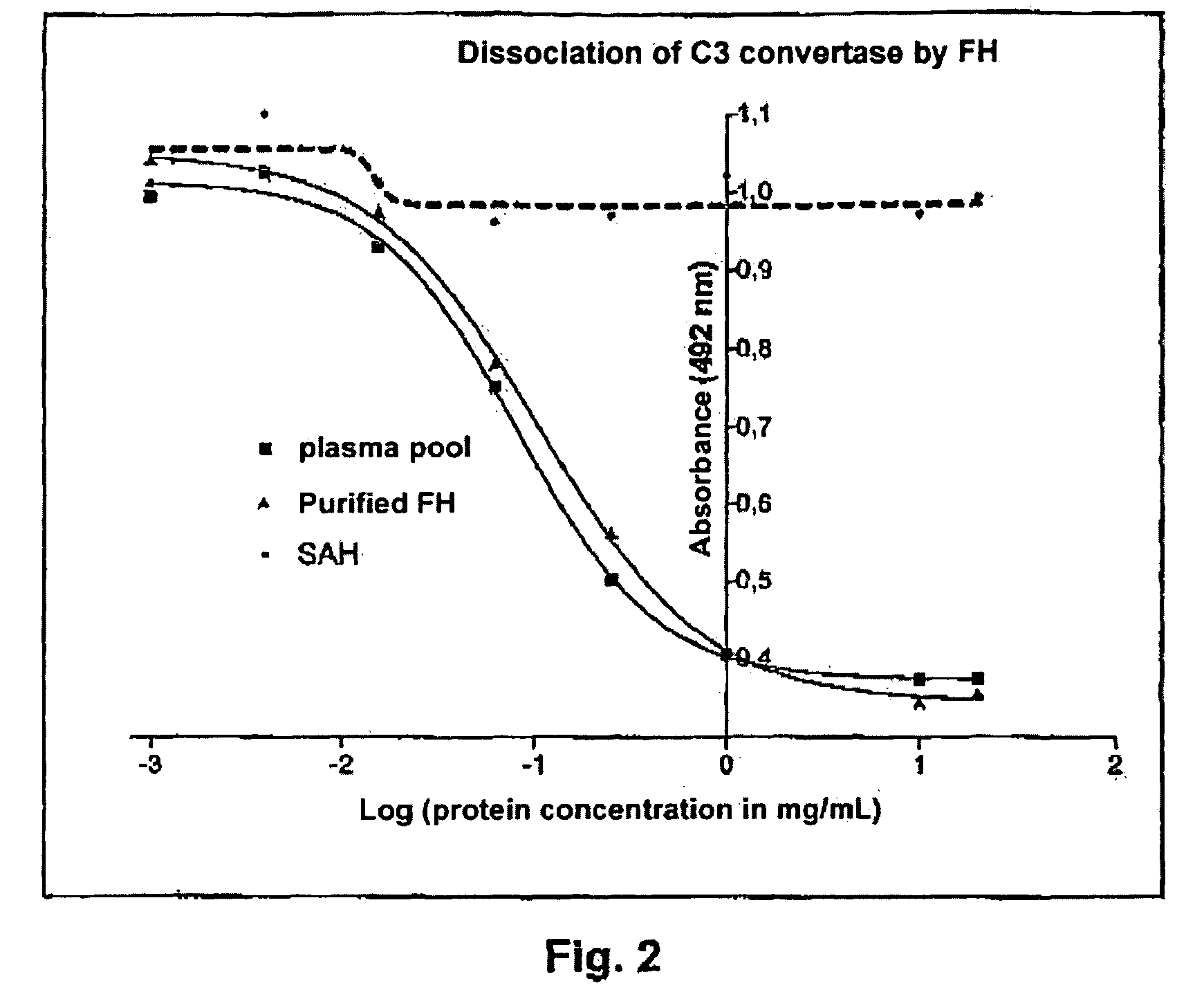
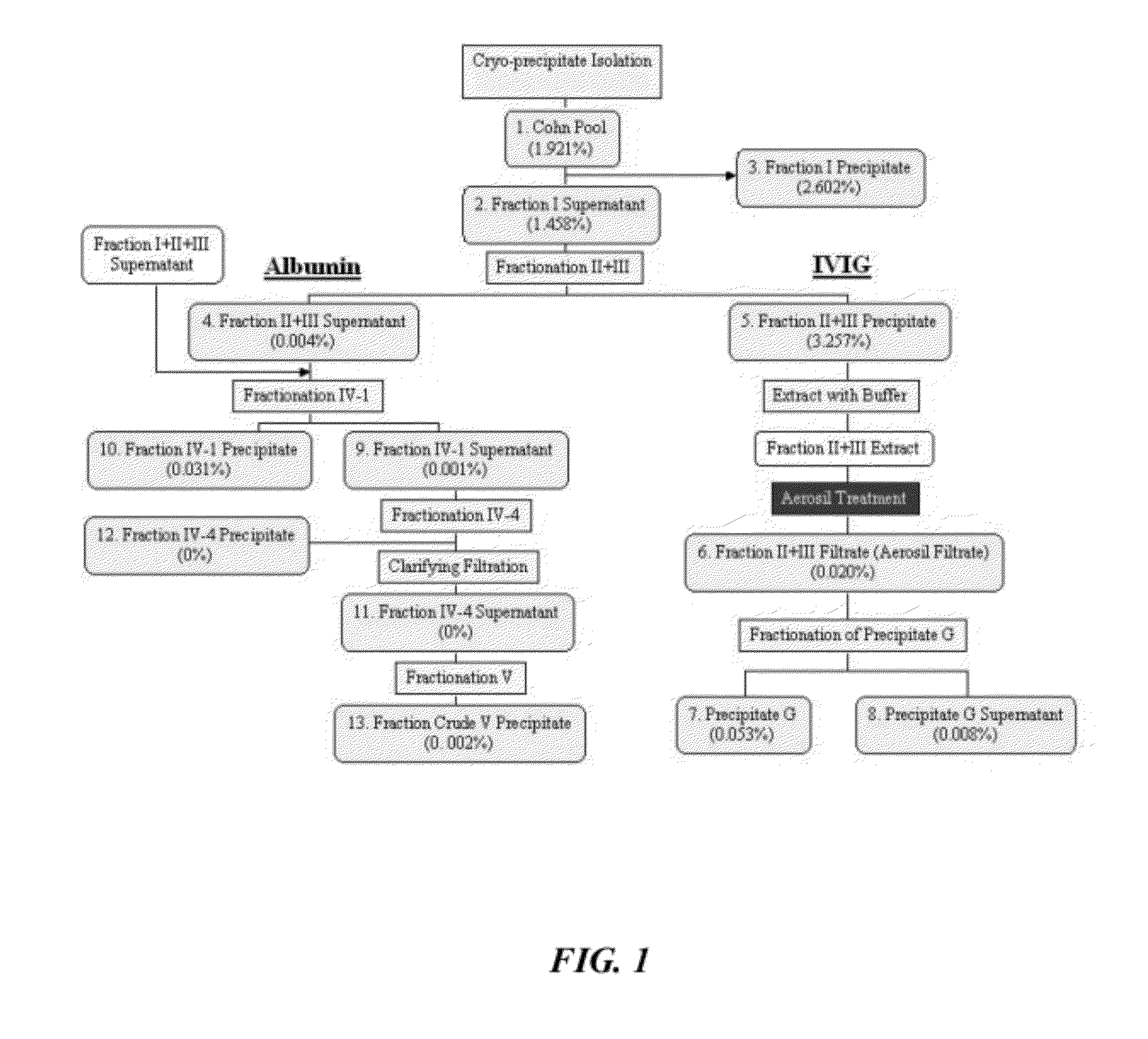
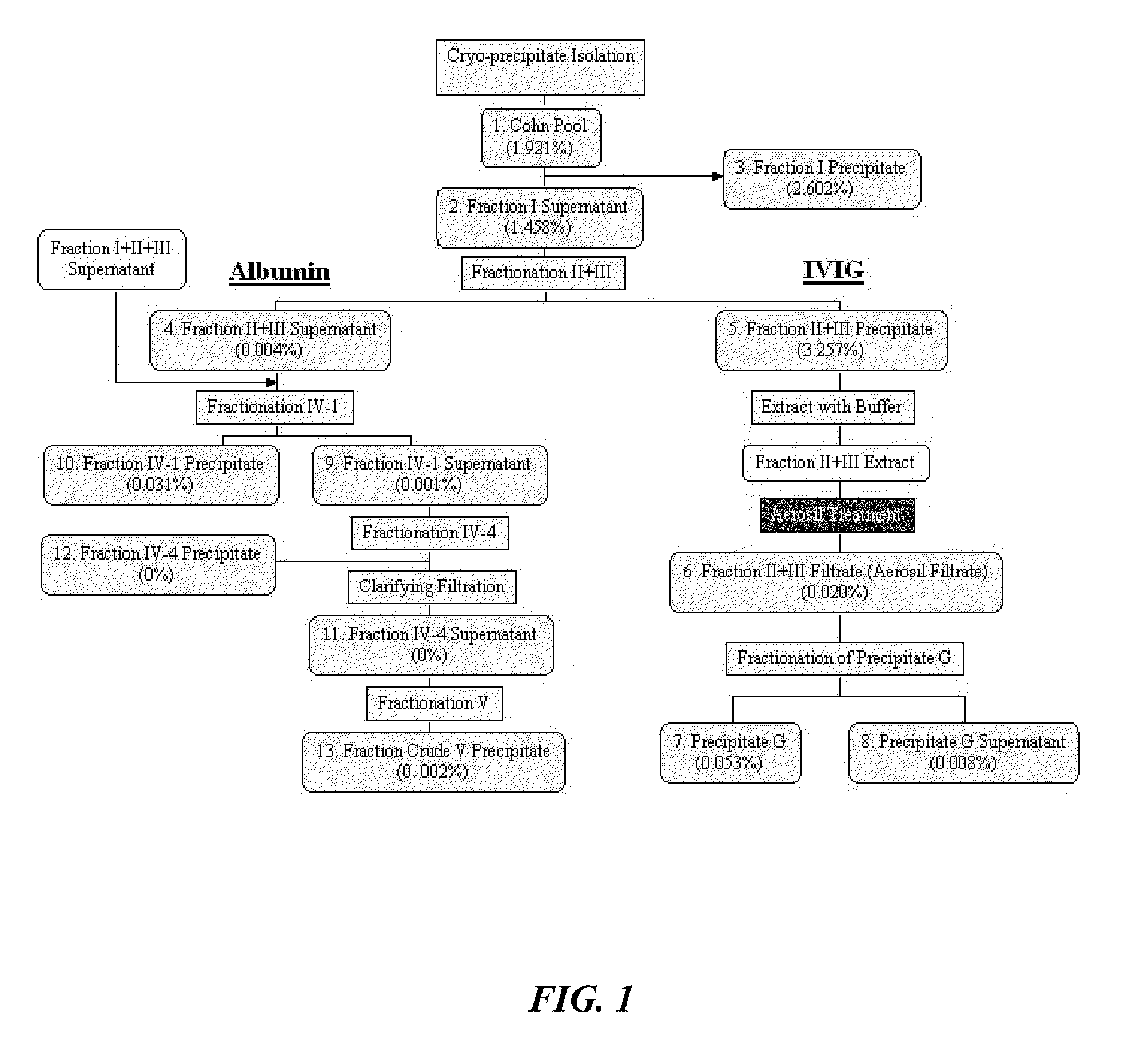
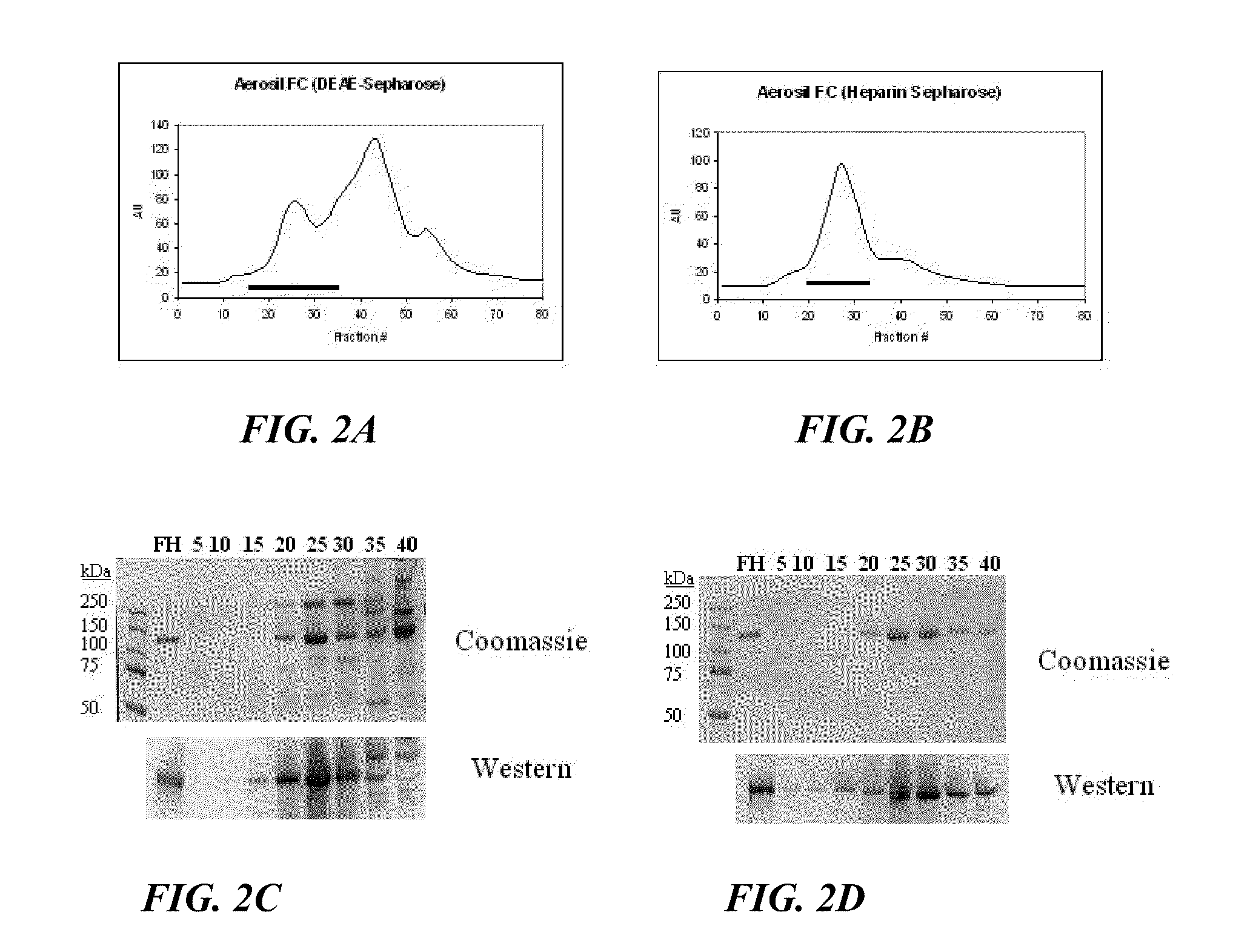
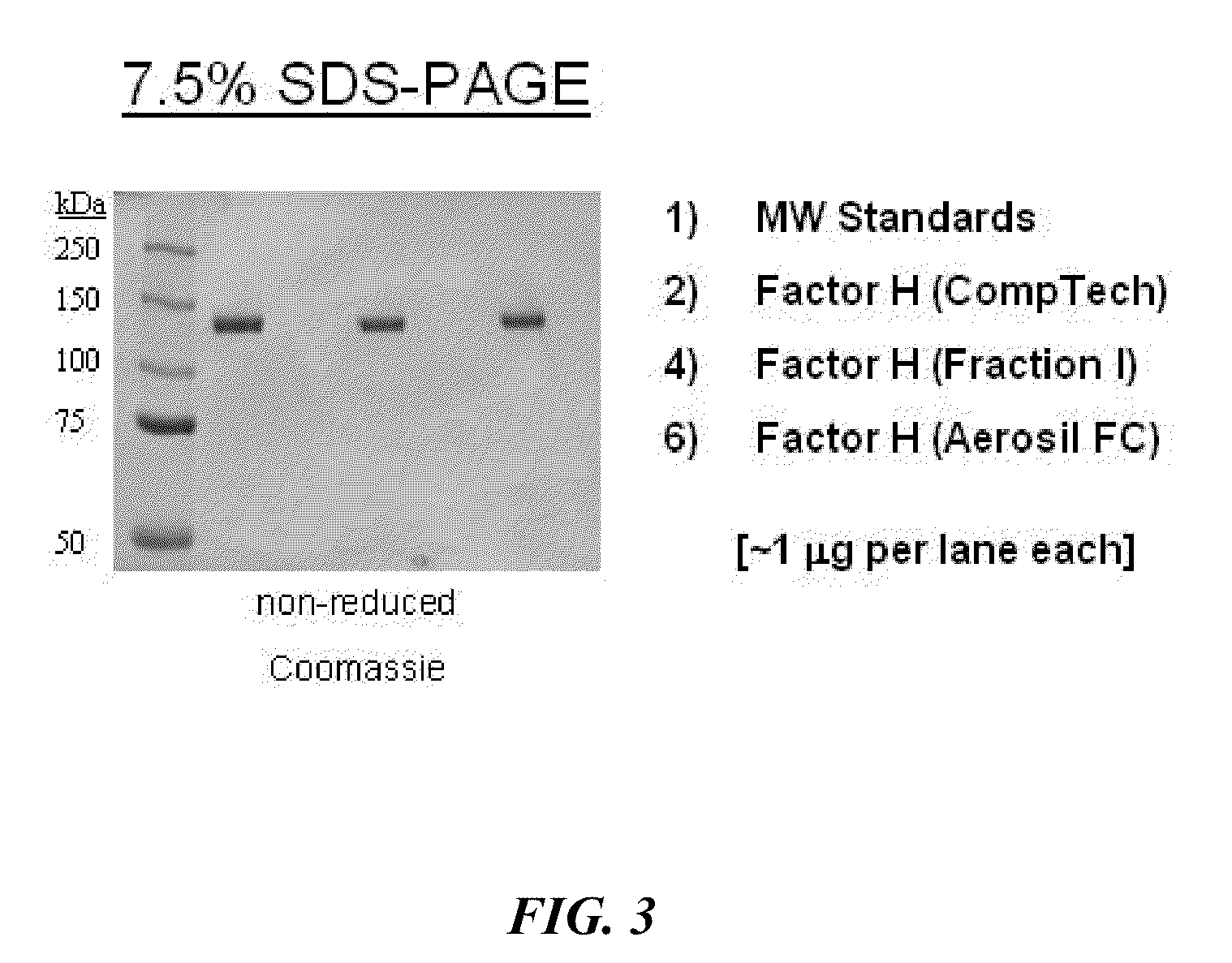
Method For Preparing a Factor H Concentrate and the Use Thereof in the Form of a Drug
InactiveUS20080318841A1Restore deficiencySafe and stable and effective productPeptide/protein ingredientsAntiviralsMedicineFactor H
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
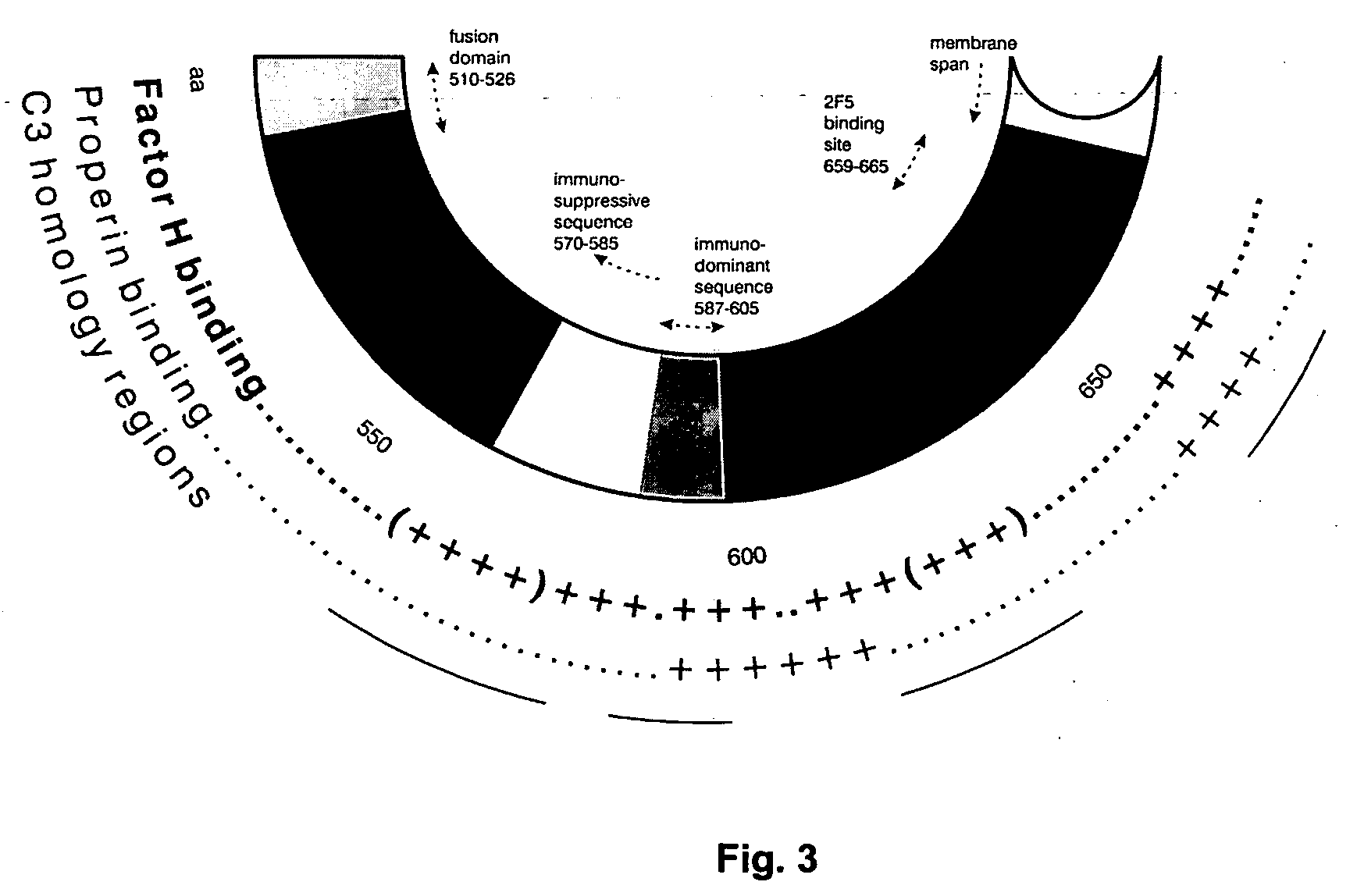
Immunogenic composition and method of developing a vaccine based on factor H binding sites
InactiveUS20050112139A1Rapid decrease in viremiaEasy to copyVirus peptidesPharmaceutical delivery mechanismBinding siteAutoimmune responses
An immunogenic composition able to provide an immune response to Human Complement Factor H binding on one or more HIV glycoproteins is disclosed, which is characterized by at least one binding site epitope of the glycoproteins. Such immunogenic composition wherein the glycoprotein comprises gp120, gp41, or both glycoproteins gp120 and gp41 is hereby disclosed. Sialic acid is removed to enhance immune recognition of the composition and to impair Factor H binding. A medication having an inhibitive action on autoimmune response by specific inhibition of the cleavage of C3b by Factor H into inactive cell fragments.
Owner:KARP NELSON M
Targeting complement factor H for treatment of diseases
ActiveUS7759304B2Improve survivalSenses disorderAntibody mimetics/scaffoldsComplement factor IDisease cause
The invention provides a CR2-FH molecule comprising a CR2 portion comprising CR2 protein or a fragment thereof and a FH portion comprising a factor H protein or a fragment thereof, and pharmaceutical compositions comprising a CR2-FH molecule. Also provided are methods of using the compositions for treatment diseases in which the alternative complement pathway is implicated, such as age-related macular degeneration, rheumatoid arthritis, and ischemia reperfusion.
Owner:MUSC FOUND FOR RES DEV +1
Diagnostic and therapeutic target for macular degeneration
InactiveUS20070037183A1Reduce usageReduce dosageMicrobiological testing/measurementDiseaseComplement factor I
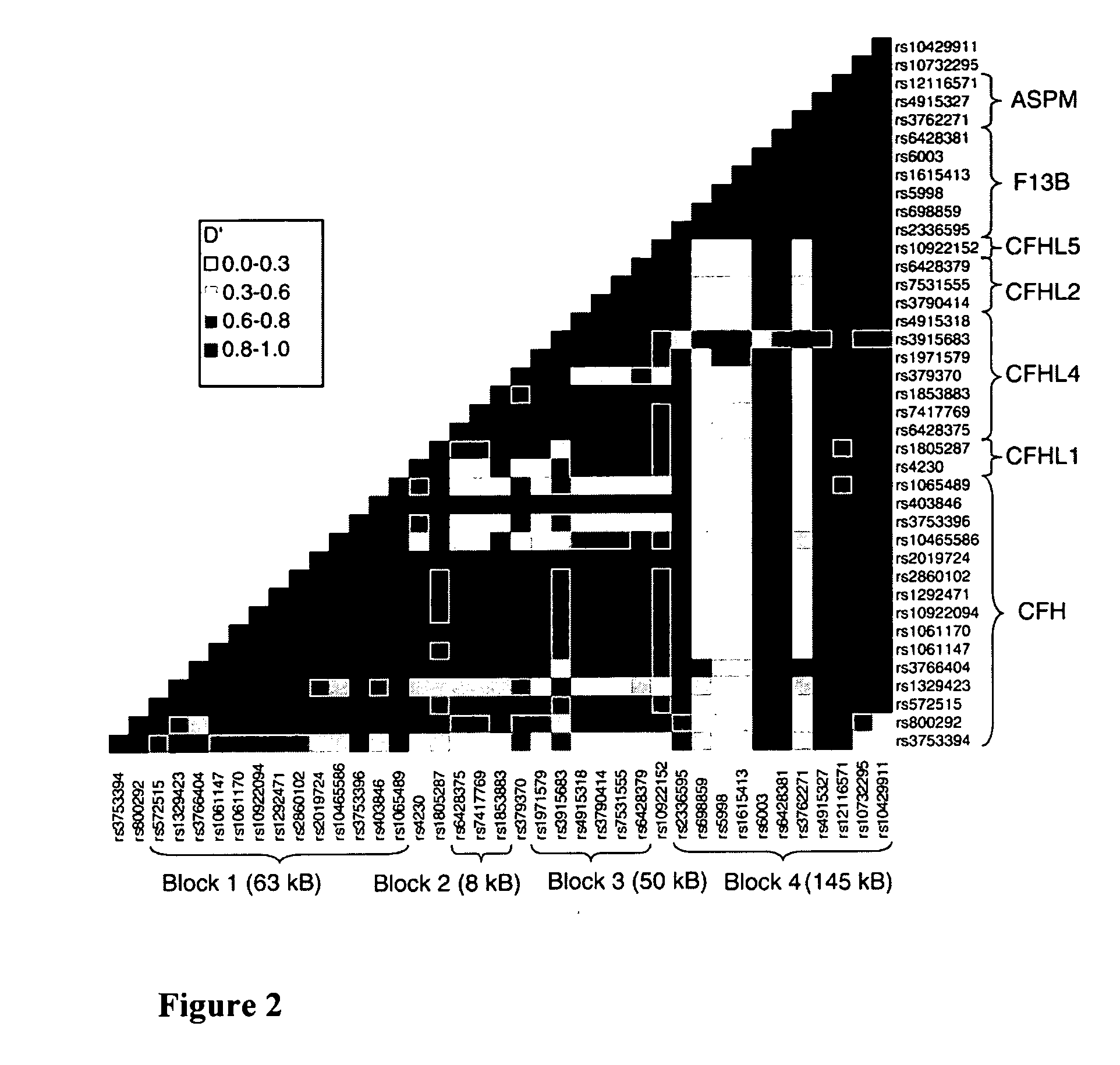
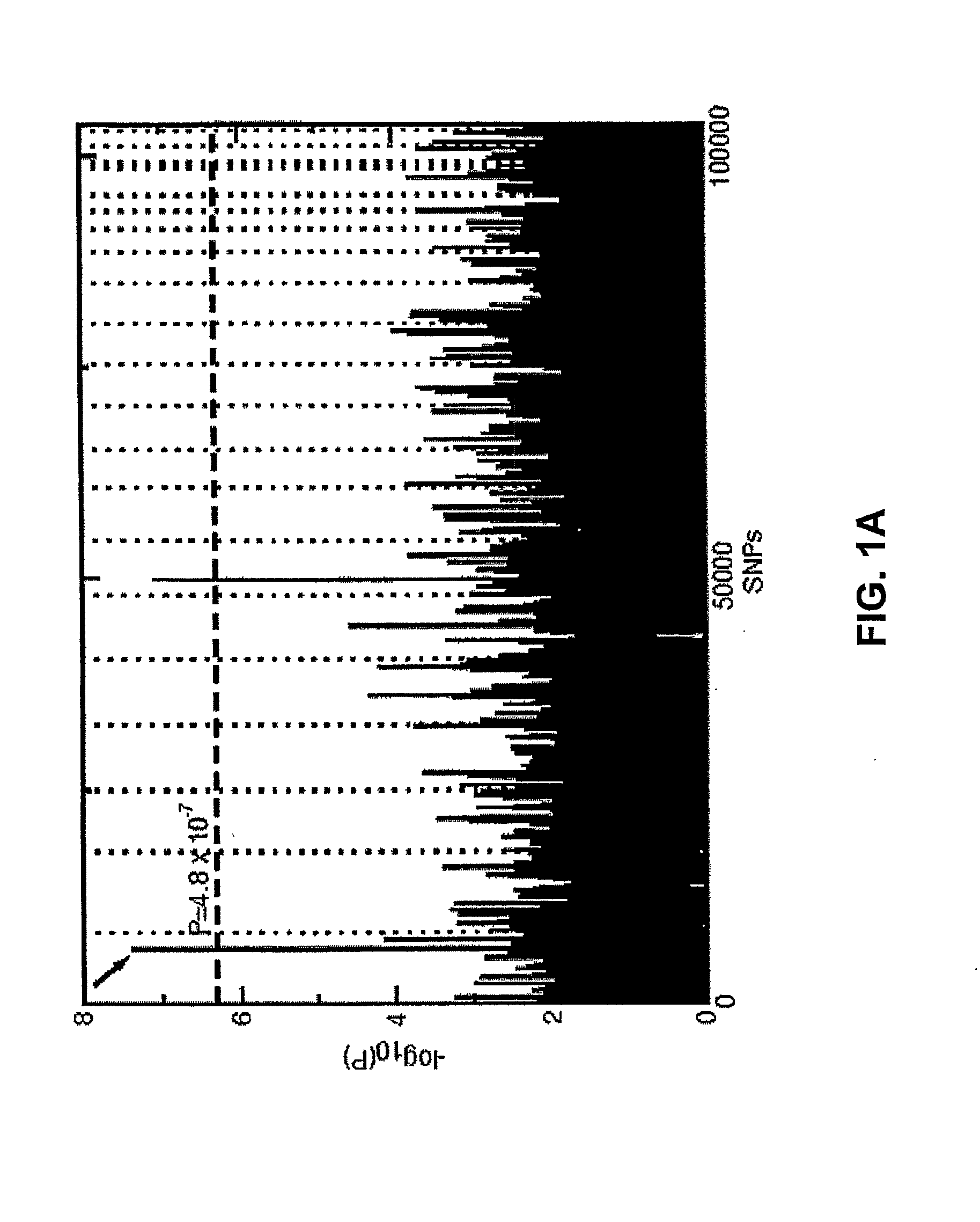
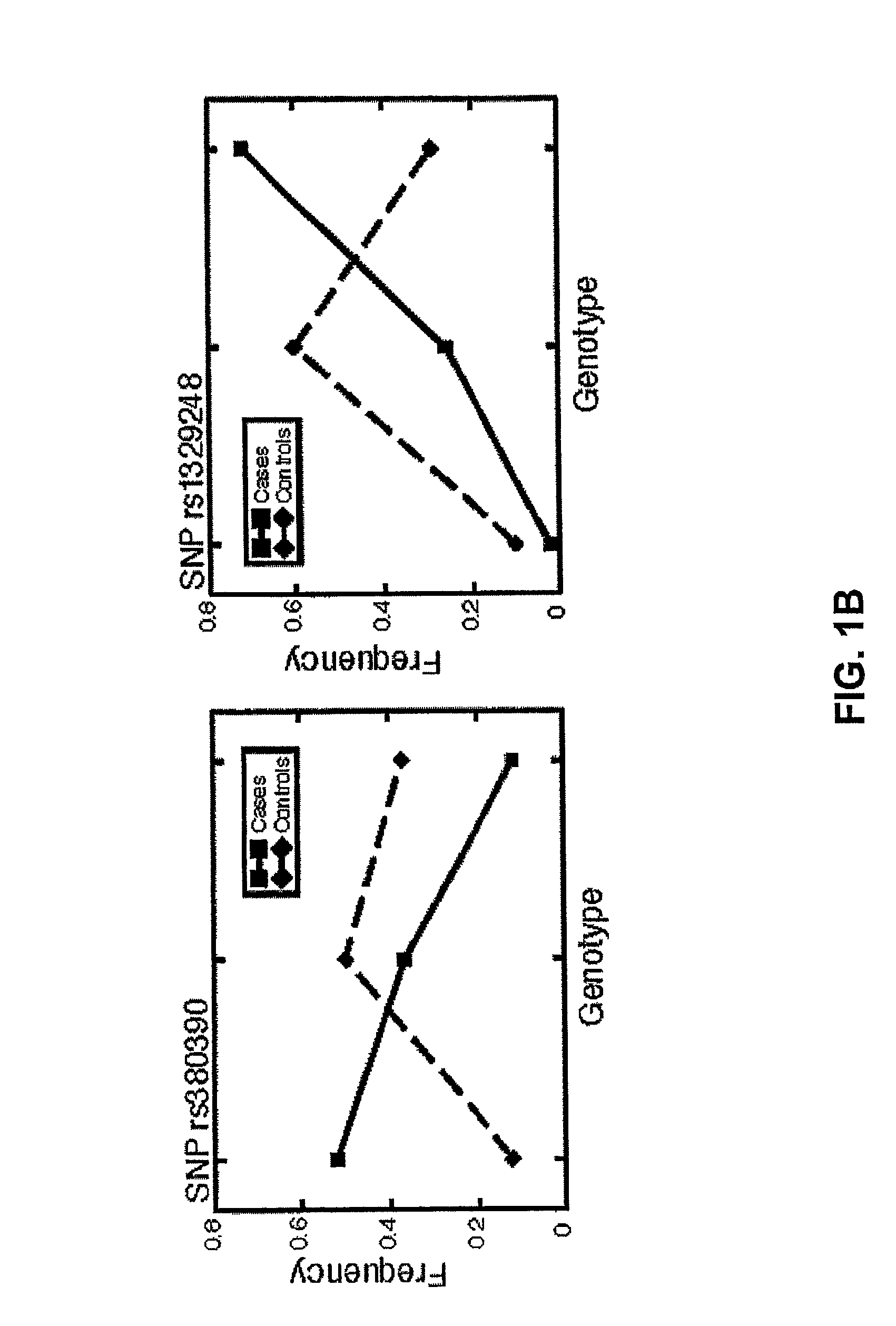
The present invention is based on the discovery of genetic polymorphisms that are associated with ocular diseases and disorders, such as age-related macular degeneration (AMD). In particular, the present invention relates to methods for determining an individuals susceptibility to ocular disorders such as AMD by screening for mutations and / or polymorphisms in the human complement factor H (CFH) gene or gene product that confer susceptibility to such disorders. Also encompassed in the present invention are nucleic acid molecules containing the polymorphisms, variant proteins encoded by such nucleic acid molecules, reagents for detecting the polymorphic nucleic acid molecules and proteins, and methods of treatment following detection of susceptibility.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
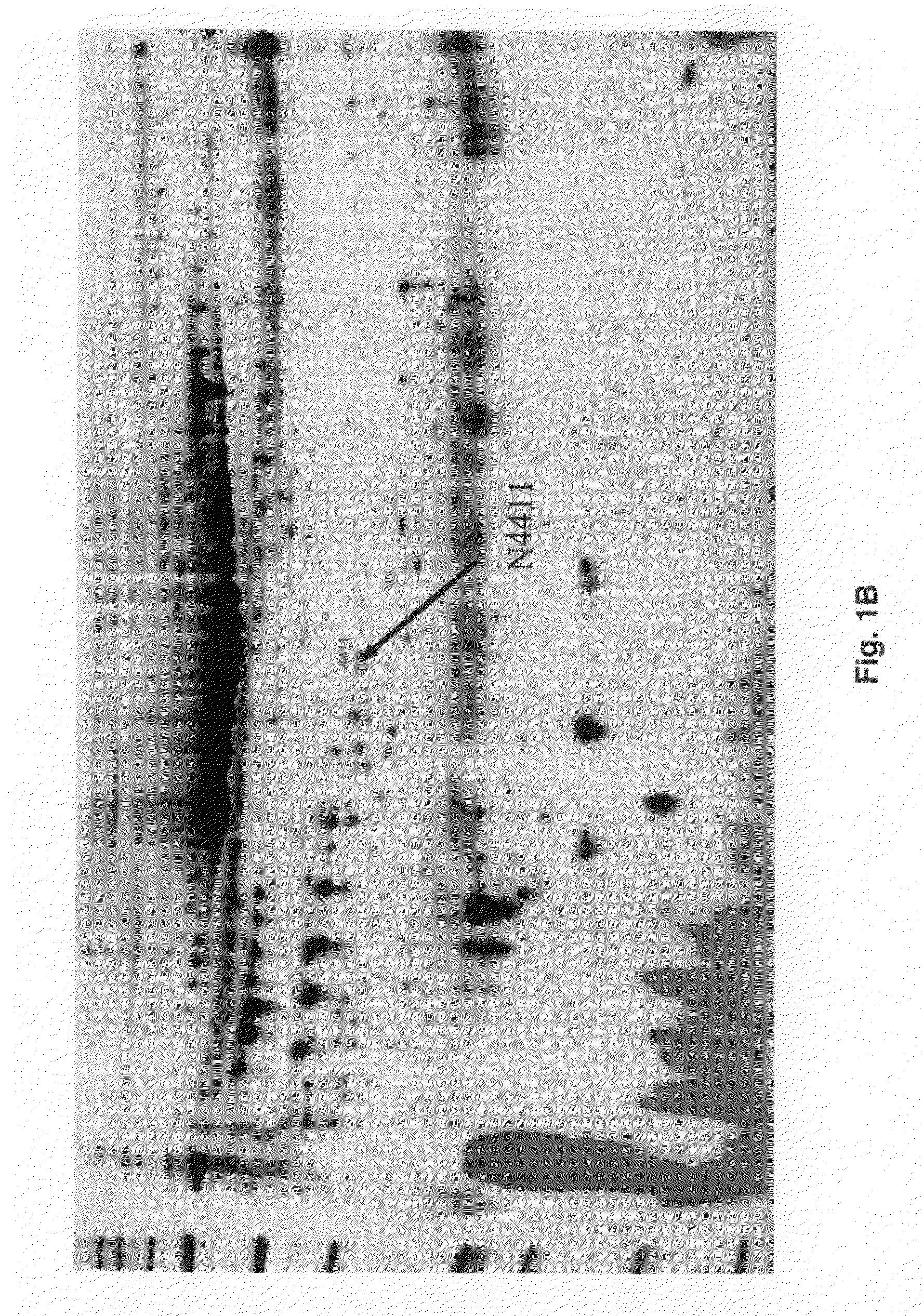
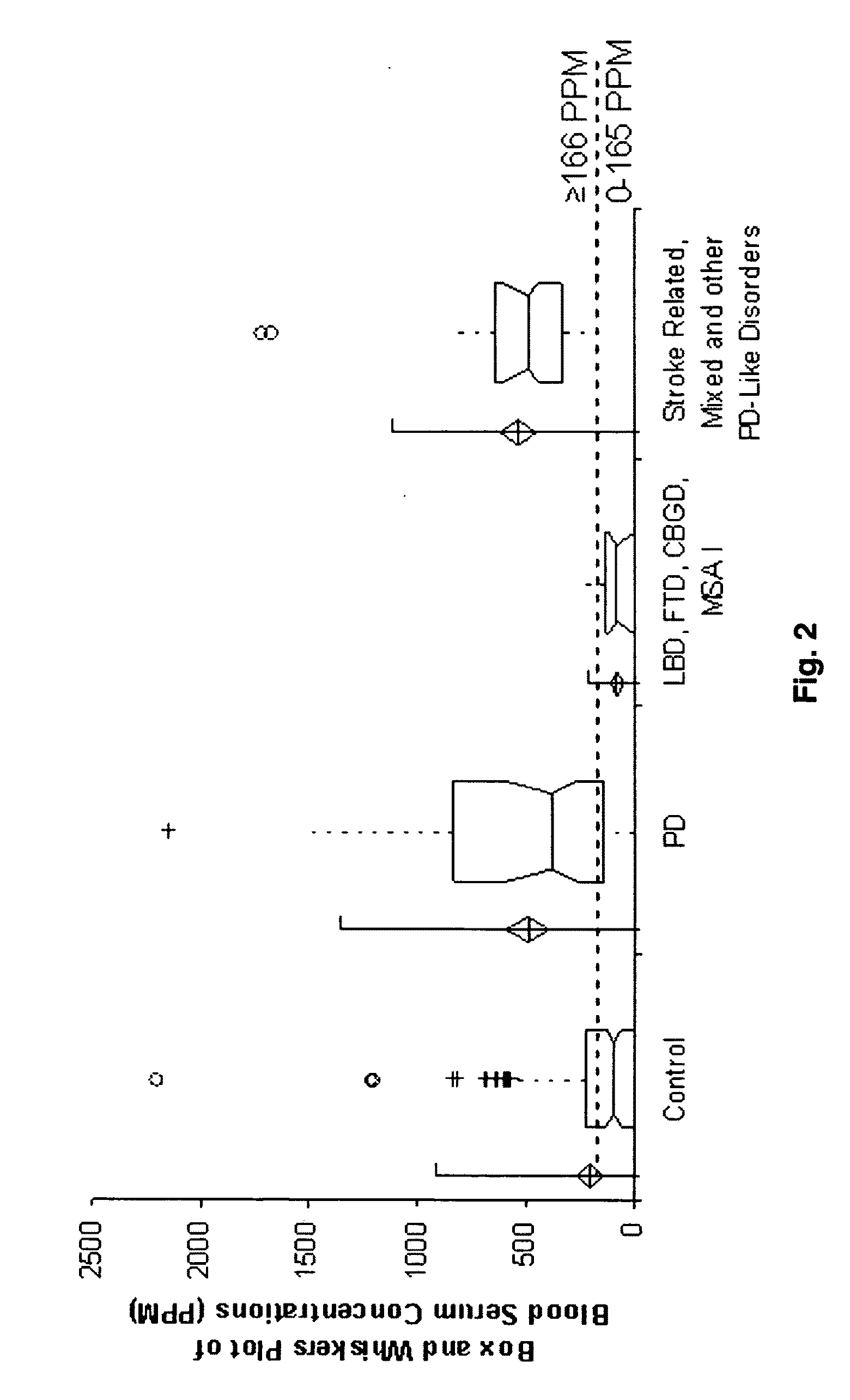
Complement factor H protein as a biomarker of Parkinson's disease
InactiveUS20090275046A1Increase concentrationPeptide/protein ingredientsMammal material medical ingredientsBiologyParkinson's disease
The present invention relates to a Complement Factor H protein as a biomarker for neurodegenerative disease, including Parkinson's disease, and the related diseases. More specifically, the present invention relates to the identification of a Complement Factor H protein, useful for the screening, diagnosis, and differentiation between neurodegenerative diseases.
Owner:NEOGENOMICS INC
MENINGOCOCCAL fHBP POLYPEPTIDES
InactiveUS20130022633A1Improved antigenHigh affinity bindingAntibacterial agentsBacteriaMeningococcal carriageAmino acid
The factor H binding activity of meningococcal fHBP can be uncoupled from its bactericidal sensitivity. NMR studies have identified various amino acid residues involved in the fHBP / fH interaction and one or more of these residues is modified in a fHBP to reduce or eliminate its ability to bind to fH.
Owner:UNIVERSITY OF FLORENCE +1
Methods and Compositions for Treating Ocular Disorders
InactiveUS20090017029A1Reduced activityAltered splicingAntibacterial agentsBiocideOcular diseaseGene
The present invention relates to identification of a human gene, Complement Factor H (CFH), associated with the occurrence for developing age related macular degeneration (AMD), which is useful for identifying or aiding in identifying individuals at risk for developing AMD, as well as for diagnosing or aiding in the diagnosis or AMD.
Owner:THE ROCKEFELLER UNIV +1
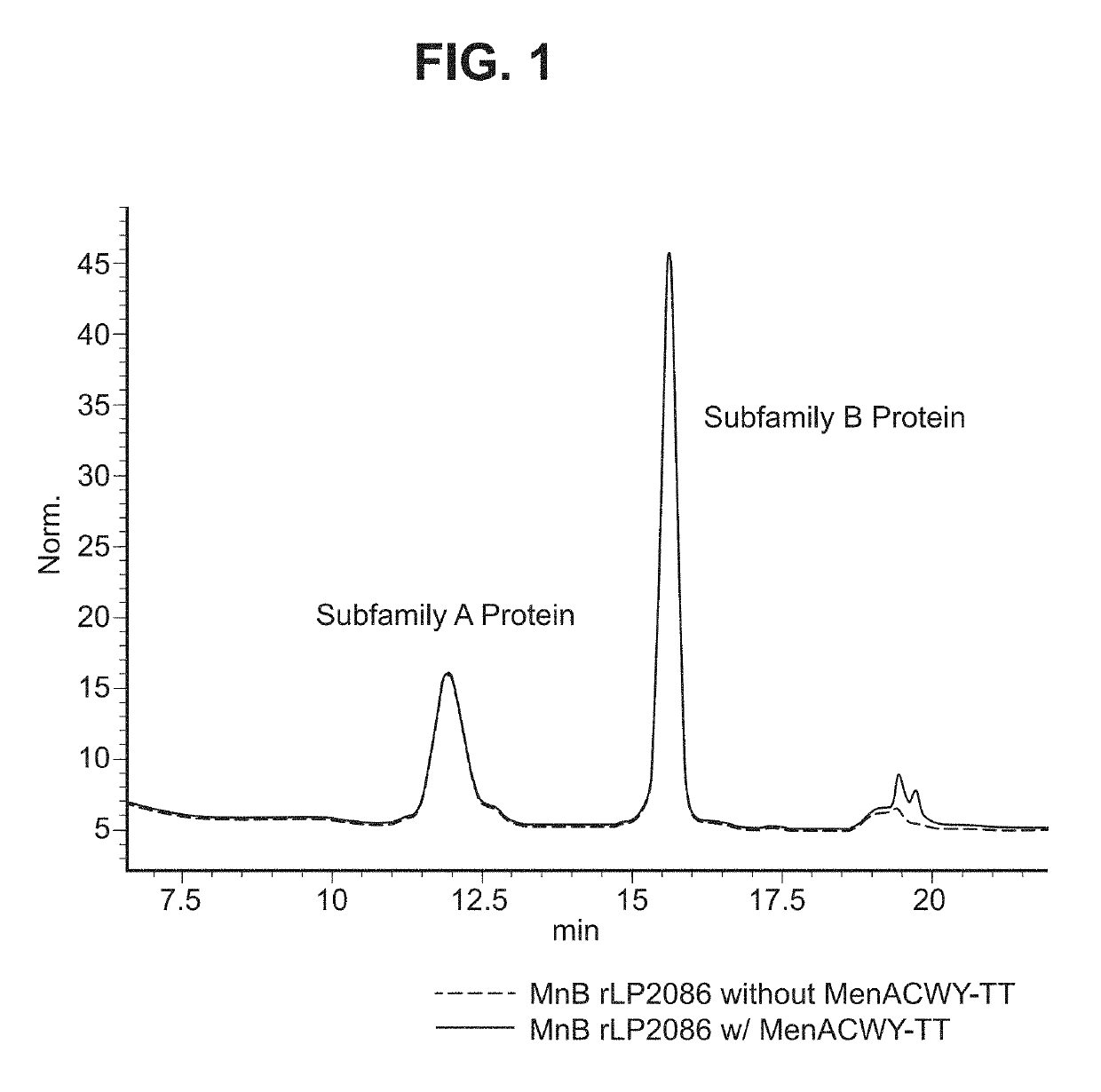
Neisseria meningitidis compositions and methods thereof
ActiveUS20180214532A1Elicit immune responseAntibacterial agentsOrganic active ingredientsNeisseria meningitidisPolysaccharide
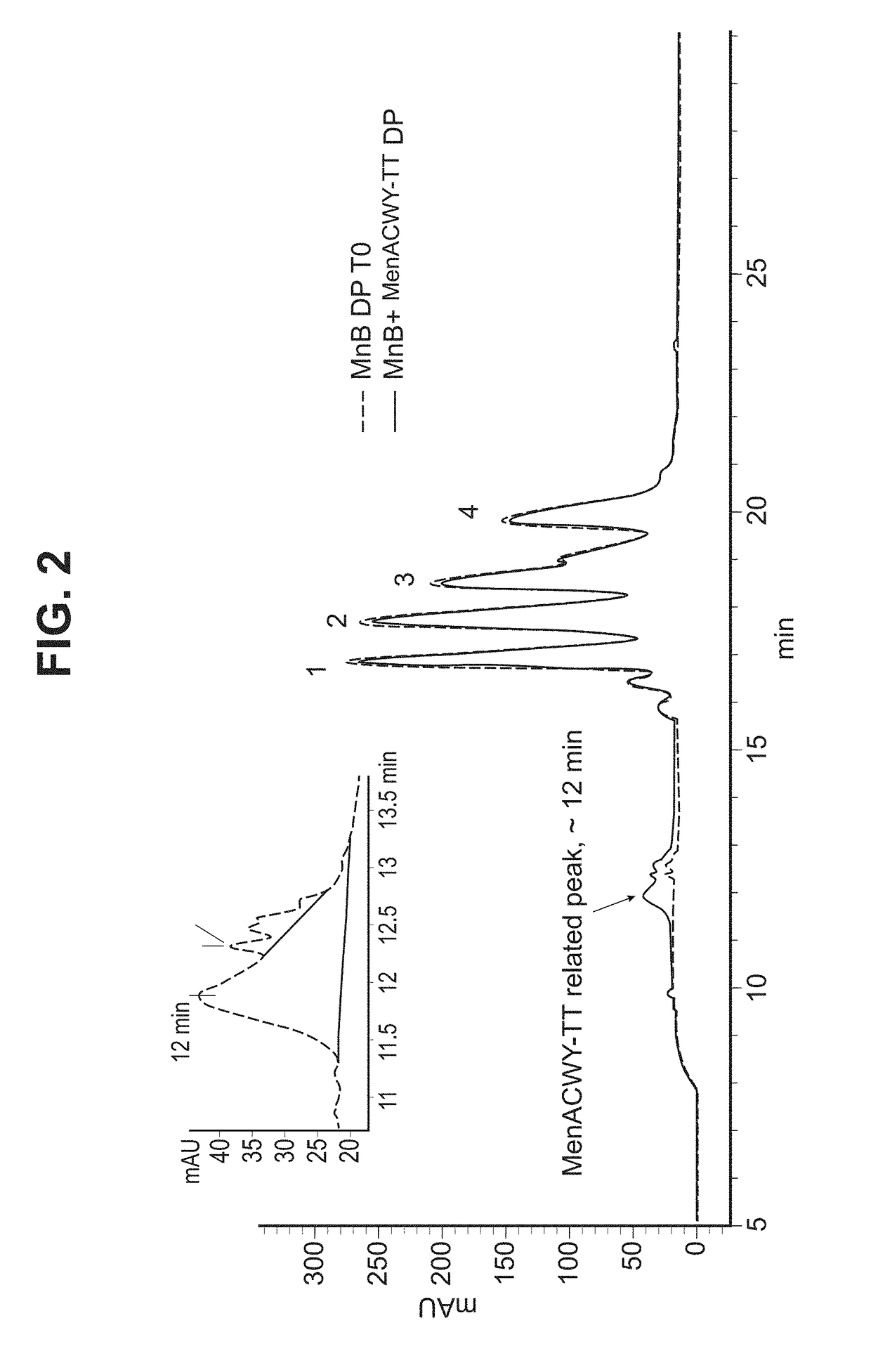
In one aspect, the invention relates to a composition including a factor H binding protein (fHBP) and a Neisseria meningitidis non-serogroup B capsular polysaccharide. The invention further relates to uses of a composition that includes fHBP, such as, for example, uses to elicit an immune response against N. meningitidis serogroup B strains and non-serogroup B strains. The compositions and methods described herein are directed to administration in humans, including adults, adolescents, toddlers, and infants.
Owner:PFIZER INC
Modulating the Alternative Complement Pathway
ActiveUS20110229497A1Modulate activityPeptide/protein ingredientsAntibody mimetics/scaffoldsAlternative complement pathwayProtein C
Provided herein are compositions, including pharmaceutical compositions, and methods for modulating, i.e., stimulating or inhibiting, activity of the alternative complement pathway, and methods of identifying factor H-binding proteins.
Owner:UNIV OF COLORADO THE REGENTS OF
Liver disease markers
InactiveUS20130225428A1Quantitative precisionPeptide librariesNucleotide librariesDiseaseLiver fibrosis
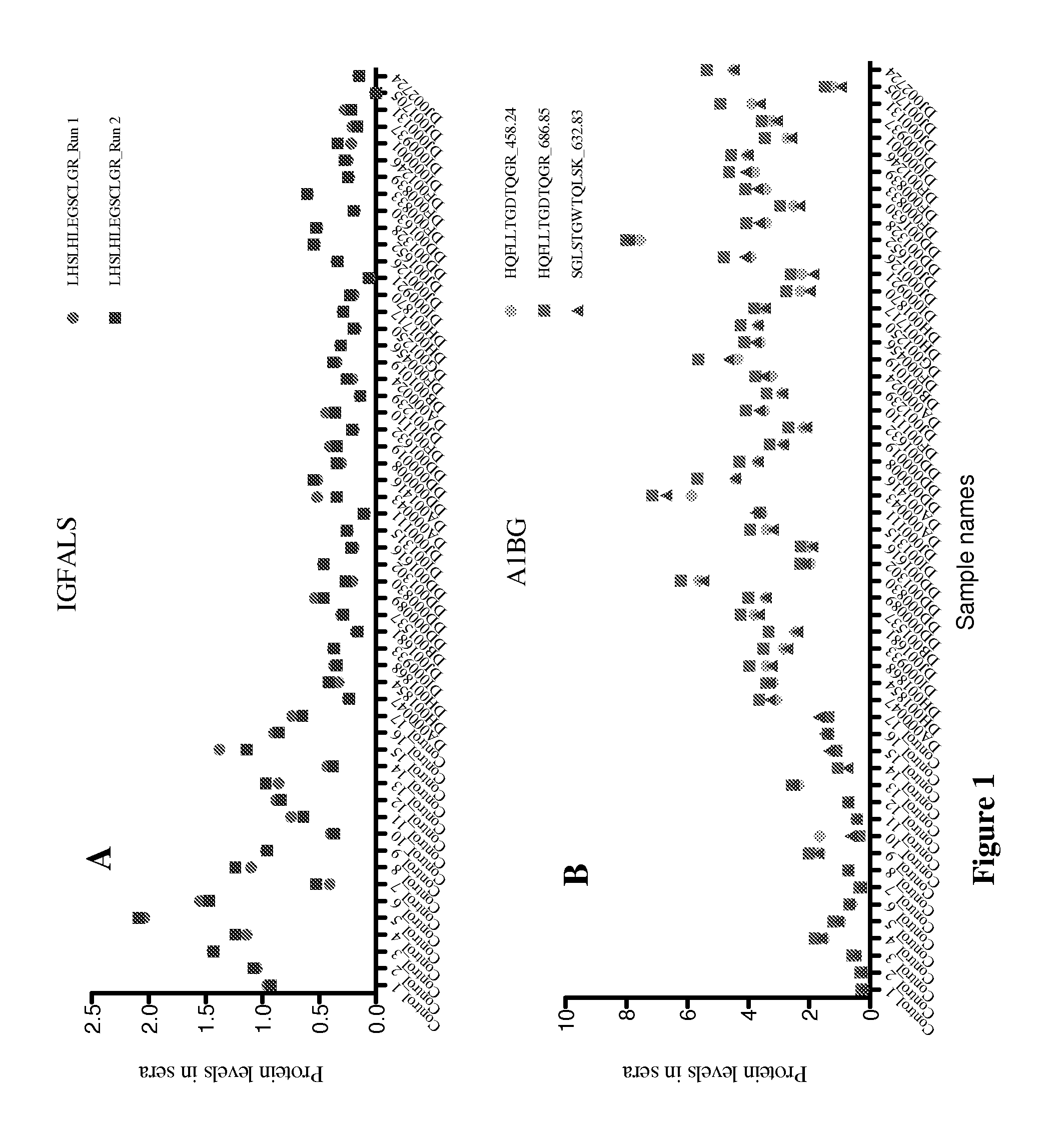
New markers for diseased liver conditions have been identified. A combination of protein C (PROC) and retinol binding protein 4 (RBP4) in blood are biomarkers to distinguish patients at different stages of hepatic fibrosis due, for example, to chronic infection with hepatitis C virus (HCV). Also, alpha-1-B glycoprotein (A1BG), complement factor H (CFH) and insulin-like growth factor binding protein acid labile subunit (IGFALS) distinguish subjects with chronic or acute liver conditions such as hepatitis infection or liver fibrosis from healthy controls.
Owner:INSTITUTE FOR SYSTEMS BIOLOGY
Determination of AM-binding proteins and the association of adrenomedullin (AM) therewith
InactiveUS20070025915A1Sufficient amountInhibitory activityCompound screeningHybrid immunoglobulinsDiabetes mellitusAdrenomedullin binding
The present invention provides methods for the isolation, identification, and purification of adrenomedullin (AM)-binding proteins. Also, provided are methods for utilizing the purified AM-binding proteins, or functional portions thereof, to diagnose, treat, and monitor AM-related diseases, for example, diseases or disorders associated with abnormally elevated AM levels. In addition, the present invention provides a newly identified complex between AM and a specific AM-binding protein 1 (AMBP-1); which has been isolated and identified herein as factor H (fH). The invention also provides AM / AMBP complexes, particularly AM / FH complexes, and antibodies specifically reactive with this complexes. Further provided are methods for identifying and purifying complexes of AM and an AM binding protein using anti-AM / fH antibodies, and methods for treating conditions such as cancer or diabetes utilizing compositions comprising these antibodies. The present invention additionally provides methods for identifying antagonists agents that inhibit the function of AM, factor H, or the AM / factor H complex. The invention also provides methods for treating conditions such as cancer or diabetes using these antagonist agents.
Owner:UNITED STATES OF AMERICA
Neisseria meningitidis compositions and methods thereof
ActiveUS10183070B2Elicit immune responseAntibacterial agentsOrganic active ingredientsSalmonella serotype typhiNeisseria meningitidis
In one aspect, the invention relates to a composition including a factor H binding protein (fHBP) and a Neisseria meningitidis non-serogroup B capsular polysaccharide. The invention further relates to uses of a composition that includes fHBP, such as, for example, uses to elicit an immune response against N. meningitidis serogroup B strains and non-serogroup B strains. The compositions and methods described herein are directed to administration in humans, including adults, adolescents, toddlers, and infants.
Owner:PFIZER INC
Clinical diagnosis of hepatic fibrosis using a novel panel of human serum protein biomarkers
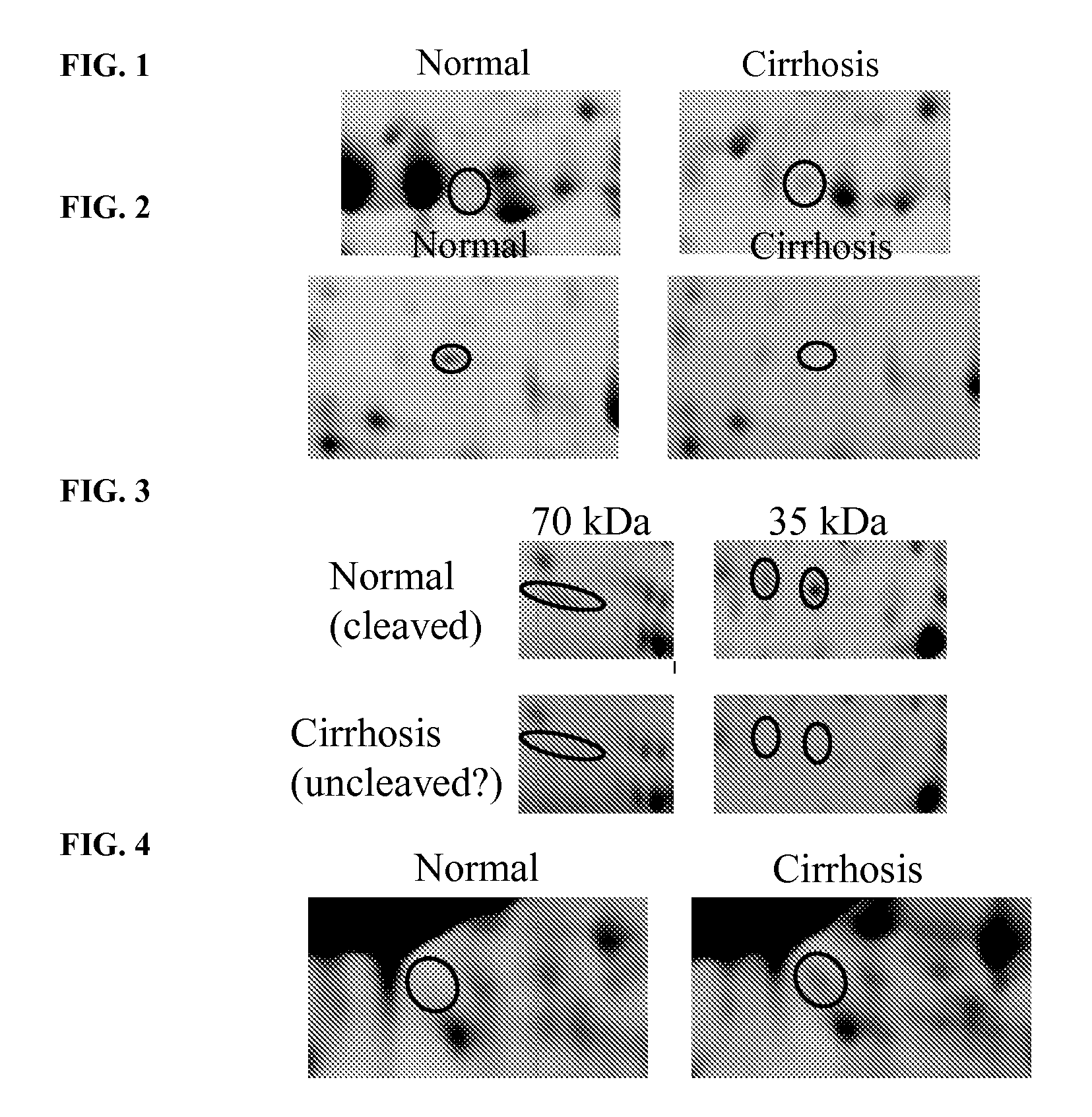
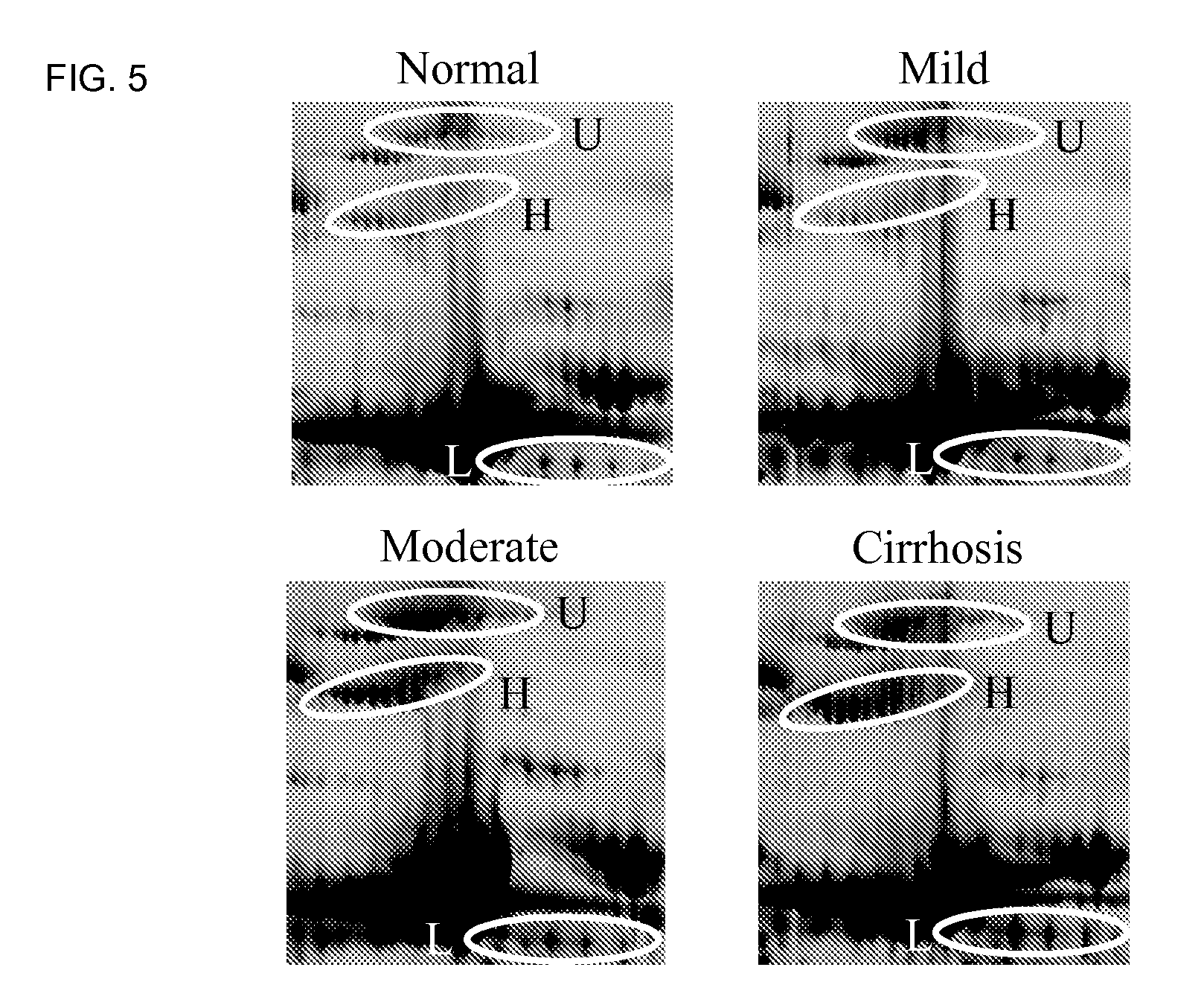
The inventors have proposed a novel panel of human serum protein biomarkers for diagnosing hepatic fibrosis and cirrhosis. Presently there is no reliable non-invasive way of assessing liver fibrosis. A 2D-PAGE based proteomics study was used to identify potential fibrosis biomarkers. Serum from patients with varying degrees of hepatic scarring induced by infection with the hepatitis C virus (HCV) was analysed. Several proteins associated with liver scarring and / or viral infection were identified. These proteins include the inter-α-trypsin inhibitor heavy chain H4 fragments, complement factor H-related protein 1, CD5L, Apo L1, and β2GPI. Increased and decreased thiolester cleavage of a2M and Complement C3, respectively, was also detected. The concentrations of these novel biomarkers can be determined using an immunoassay where the concentrations would reflect the extent of fibrosis. A fibrosis scoring scale for each of the novel biomarkers is proposed. The additive result from the scores of all the novel biomarkers would give a more reliable indication of the degree of fibrosis rather than examining individual biomarkers.
Owner:UNIV OF OXFORD
Recombinant proteins having factor h activity
InactiveUS20170190753A1Increase dissociationIncrease secretionPolypeptide with localisation/targeting motifPolypeptide with His-tagChemistryFactor H
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
Neisseria meningitidis compositions and methods thereof
ActiveUS20190231861A1Elicit immune responseAntibacterial agentsOrganic active ingredientsNeisseria meningitidisPolysaccharide
In one aspect, the invention relates to a composition including a factor H binding protein (fHBP) and a Neisseria meningitidis non-serogroup B capsular polysaccharide. The invention further relates to uses of a composition that includes fHBP, such as, for example, uses to elicit an immune response against N. meningitidis serogroup B strains and non-serogroup B strains. The compositions and methods described herein are directed to administration in humans, including adults, adolescents, toddlers, and infants.
Owner:PFIZER INC
Methods and Compositions For Diagnosis of Age-Related Macular Degeneration
InactiveUS20110117557A1Microbiological testing/measurementDisease diagnosisMacular degenerationPathology
The invention generally concerns methods and compositions for screening individuals for susceptibility to age-related macular degeneration (AMD). In particular, association with the various markers including complement factor H, LOC387717 / ARMS2, C2 / CFB, C3 and VEGF, indicates that a subject is at risk of AMD.
Owner:UNIV OF MIAMI +1
Targeting specificity complement system inhibitor and preparation method and application thereof
ActiveCN104231085AComplement inhibition is achievedNervous disorderPeptide/protein ingredientsPharmacyMedicine
The invention belongs to the field of biological pharmacy, and particularly relates to a targeting specificity complement system inhibitor and the design, preparation and clinical application of the targeting specificity complement system inhibitor. CRIg which can be combined with fragments C3b and / or iC3b which are formed after a complement component C3 is activated to generate targeting effect is directly connected with another component with a complement inhibiting effect, for example factor H or is indirectly connected by virtue of a flexible peptide fragment (Gly4Ser)3 and the like to prepare a fusion protein by methods such as genetic engineering, thus achieving the effect of inhibiting complement activation in a targeting manner. The medicine can be applied to treatment and prevention of a plurality of human diseases which are related to abnormal complement activation.
Owner:SHANGHAI COMGEN BIO PHARMA CO LTD
Bladder cancer detection kit and method and application of human complement factor H related protein therein
ActiveCN105785044AHigh sensitivityStrong specificityBiological material analysisBiological testingBladder cancerPatient compliance
The invention relates to a bladder cancer detection kit and method. A monoclonal antibody and a polyclonal antibody which are formed through the human complement factor H are involved. The invention further relates to application of human complement factor H related protein. BTA is selected as a marker for detecting bladder cancer, three different monoclonal antibodies obtained from polypeptide immune are mixed to be used as a coating antibody, the sensitivity is high, and specificity is high. The coating antibody and a detection antibody are obtained from antigen immune of different sources, and then detection accuracy is improved. The bladder cancer detection kit and method are high in detection precision, good in specificity, high in patient compliance and capable of achieving detection conveniently.
Owner:HEBEI TEWENTE BIOTECH DEV CO LTD
Recombinant factor h and variants and conjugates thereof
InactiveUS20150139975A1Inhibit aggregationMinimise proteolysisAntibacterial agentsSenses disorderChemistryFactor H
The present invention relates to recombinant factor H and variants and conjugates thereof and methods of their production, as well as uses and methods of treatment involving the materials.
Owner:THE UNIV COURT OF THE UNIV OF EDINBURGH
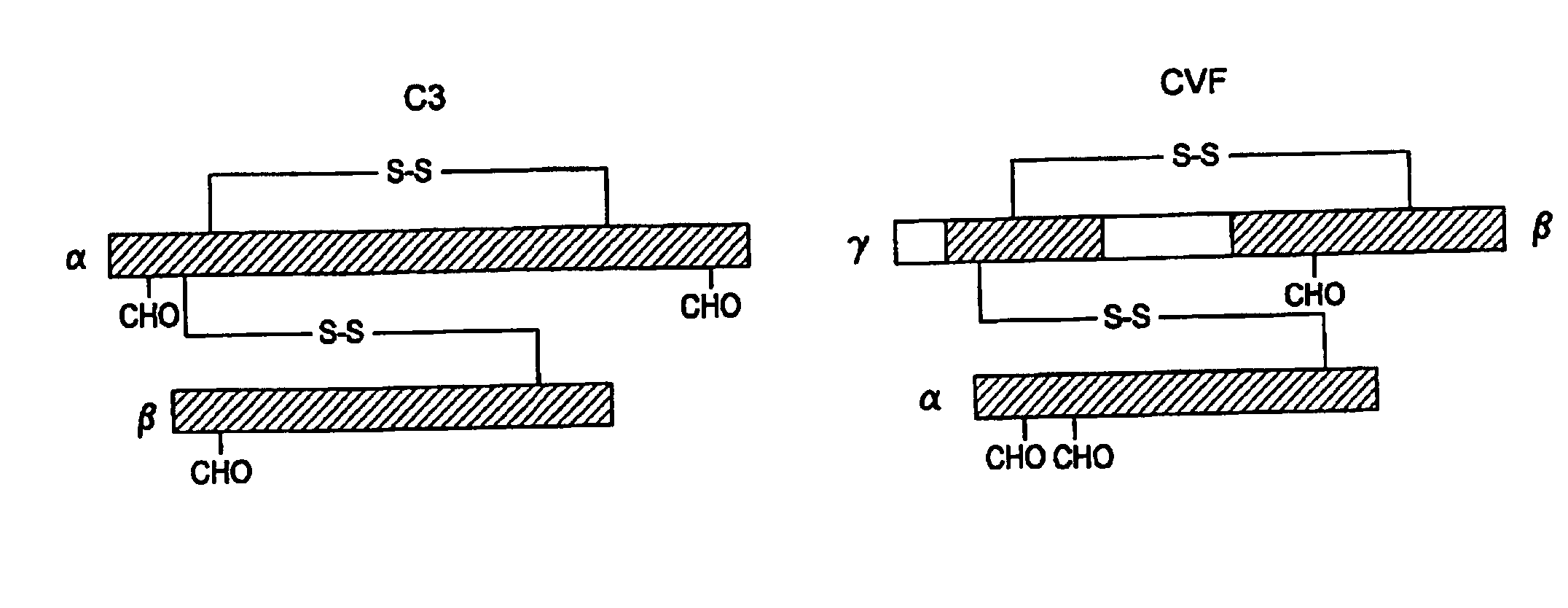
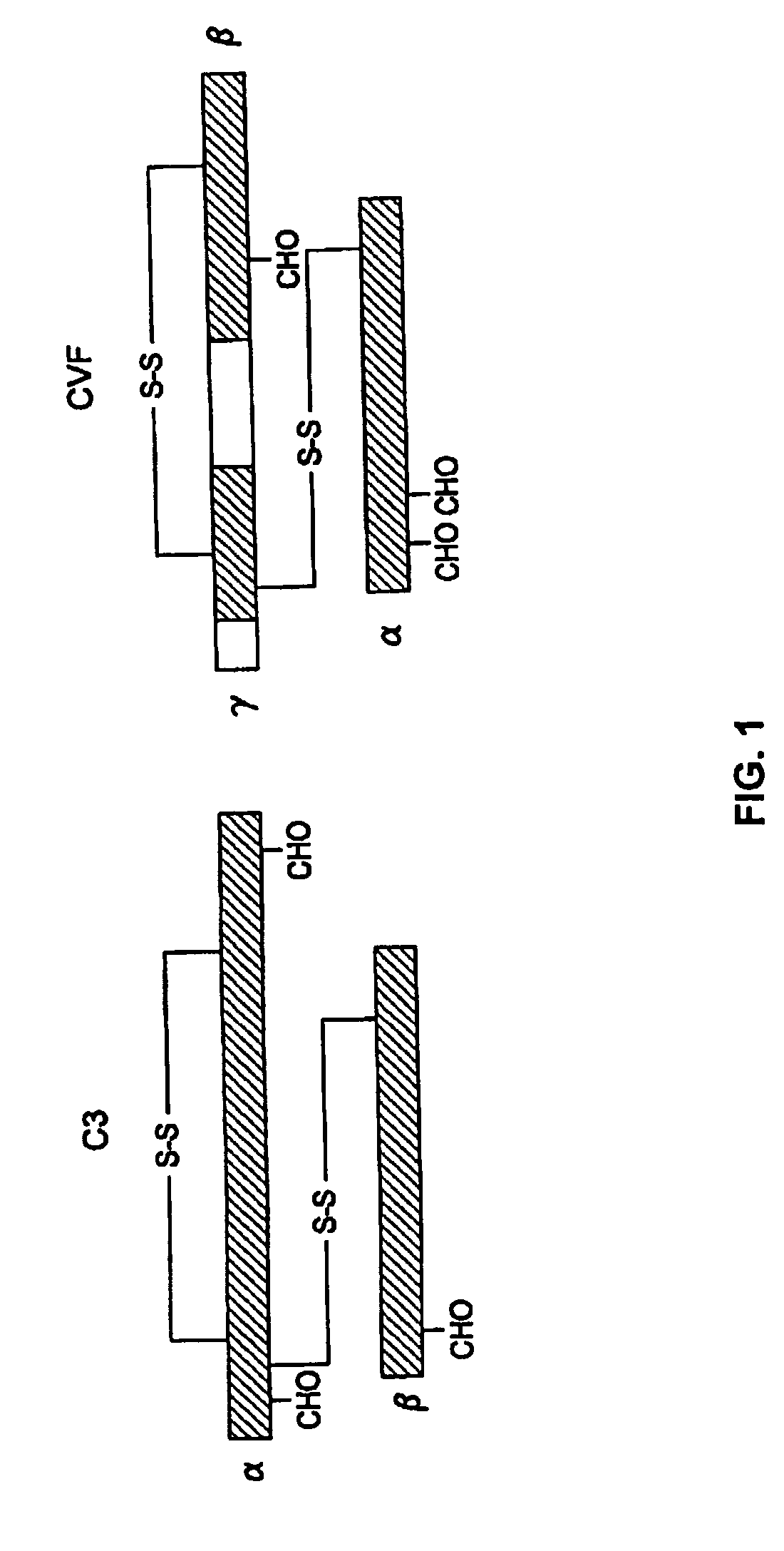
Human Complement C3 Derivates with Cobra Venom Factor-Like Function
ActiveUS20080311132A1Avoiding and ameliorating reperfusion injuryDeplete complementAntibacterial agentsNervous disorderDiseaseReperfusion injury
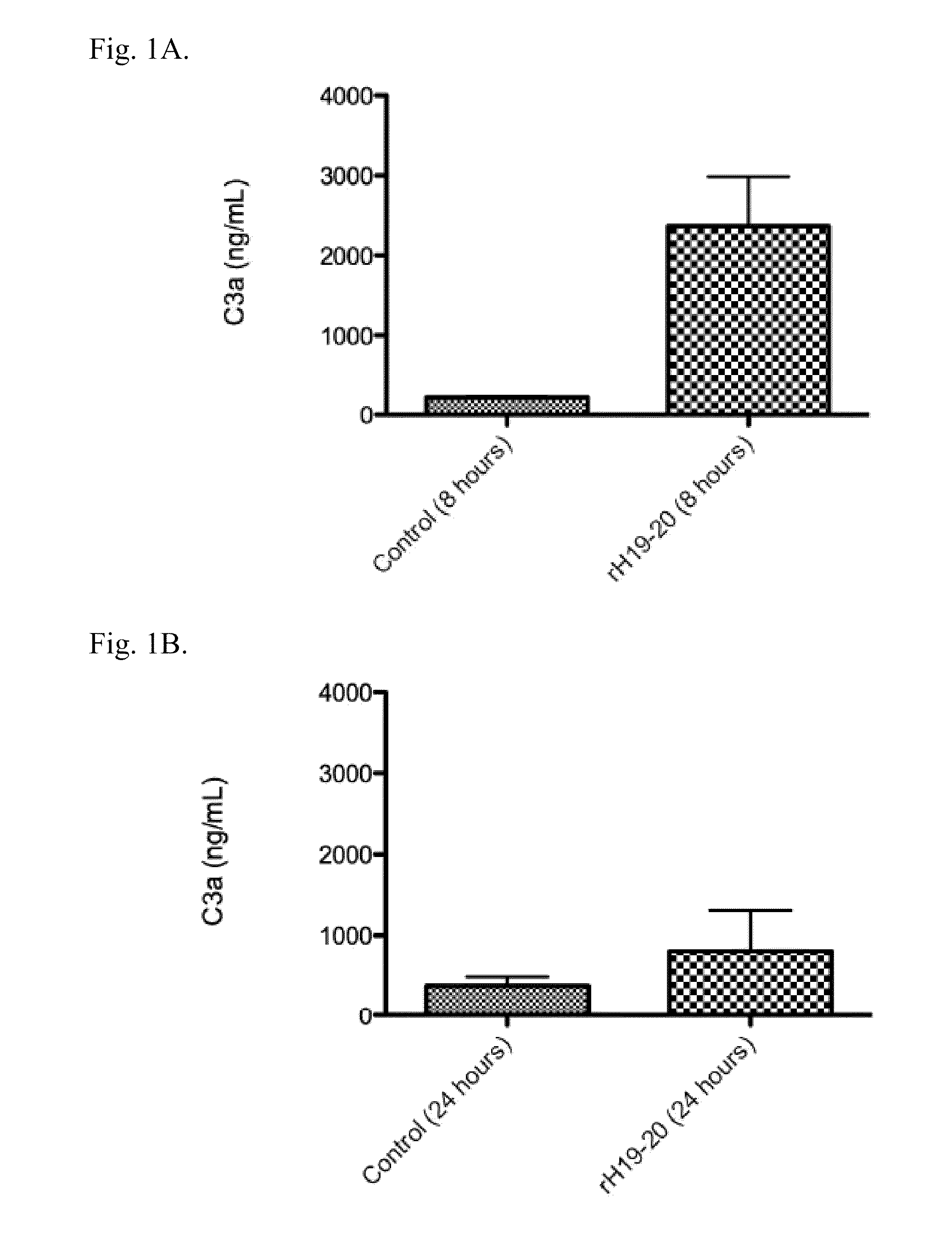
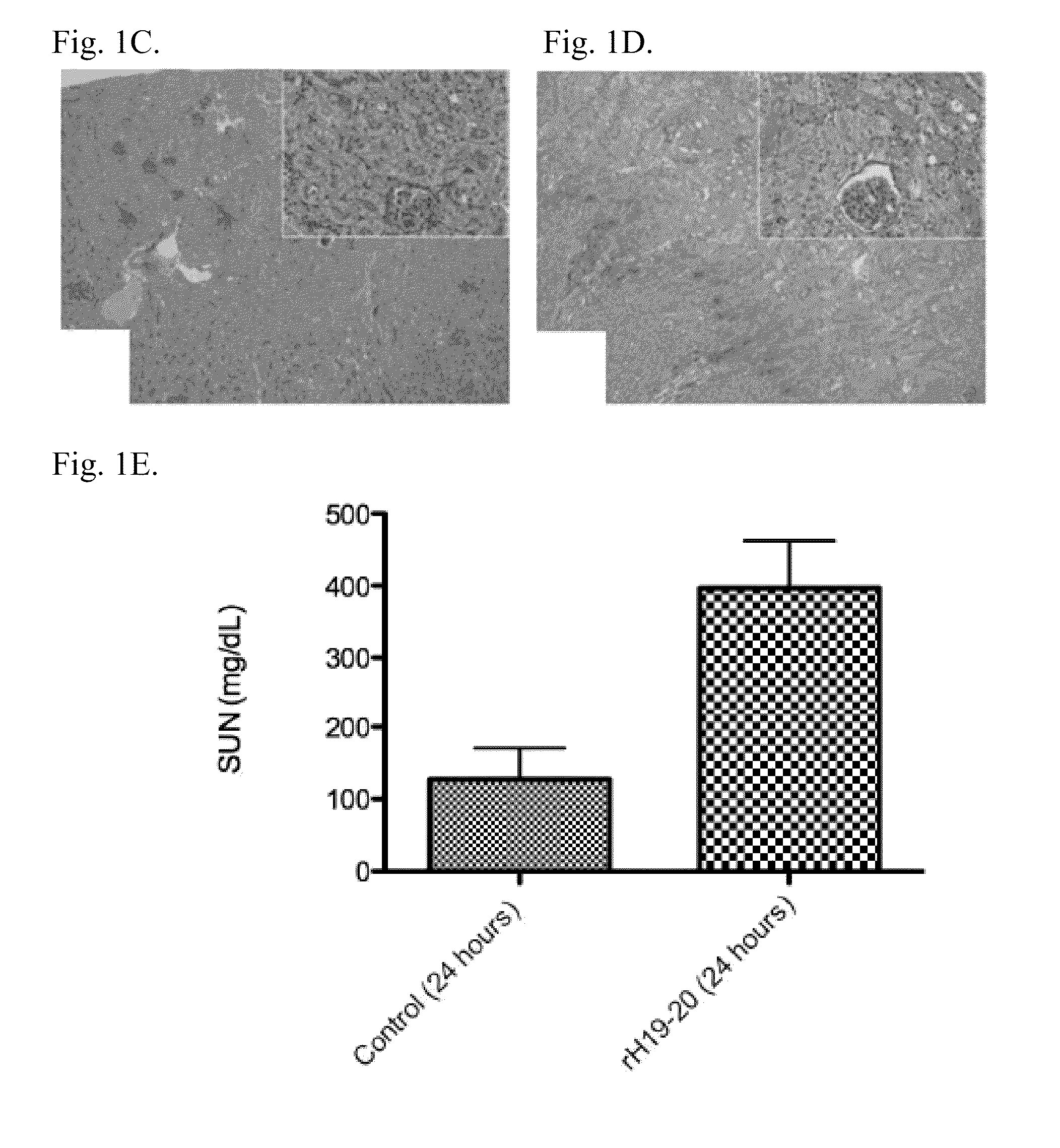
A modified human complement C3 protein (C3) is disclosed comprising a substitution of a portion of a human C3 protein, with a corresponding portion of a Cobra Venom Factor protein (CVF) which results in a human C3 protein with CVF functions, but with substantially reduced immunogenicity. Advantageously, the C3 protein can be manipulated to contain at least one of the following CVF functions: increased stability of the C3 convertase and increased resistance to the actions of factors H and / or I. A large number of hybrid C3 proteins containing substitutions in the C-terminal portion of the alpha chain of C3 are presented and tested for the above functions. Methods of treatment of diseases such as reperfusion injury, autoimmune diseases, and other diseases of increased complement activation are presented as well as methods of increasing the effectiveness of gene therapeutics and other therapeutics.
Owner:UNIV OF HAWAII
Blocking interaction between pathogen factors and factor h to inhibit hemorrhagic syndromes
InactiveUS20100150912A1Antibacterial agentsPeptide/protein ingredientsInhibitory effectActive immunisation
If pathogen factors such as meningococcal NMB 1870 or flaviviral NS1 are able to sequester factor H in the blood then its inhibitory effect on complement may be disturbed, thereby permitting C3 to initiate a dramatic attack on host endothelial tissue. In combination with a strong inflammatory response, this attack can result in sever damage to the endothelium, with resulting hemorrhagic syndrome. Blocking the interaction between pathogen factors and factor H may thus be used to treat and / or prevent these pathogen-induced hemorrhagic syndromes. The interaction may, for instance, be blocked by antibodies, either delivered endogenously (passive immunisation) or produced by a patient's immune system (active immunisation).
Owner:NOVARTIS AG
Adjuvanting meningococcal factor h binding protein
InactiveUS20120070458A1Reduce chain lengthOptimum chain lengthAntibacterial agentsBacterial antigen ingredientsPoint of zero chargeAntigen
Factor H binding protein (fHBP) has been proposed for use in immunising against serogroup B meningococcus (‘MenB’). This antigen can be efficiently adsorbed to an aluminium hydroxyphosphate adjuvant by (i) ensuring that adsorption takes place at a pH which is equal to or below the adjuvant's point of zero charge (PZC), and / or (ii) selecting a fHBP and adjuvant with an isoelectric point / PZC within the range of 5.0 to 7, and / or (iii) selecting a fHBP with an isoelectric point above the adjuvant's PZC and using a buffer to bring the pH to within 1.2 pH units of the PZC. The adsorption is particularly useful for compositions which include multiple fHBP variants, and also in situations where an aluminium hydroxide adjuvant should be avoided. Buffered pharmaceutical compositions can include at least two different meningococcal fHBP antigens, both of which are at least 85% adsorbed to aluminium hydroxyphosphate adjuvant.
Owner:NOVARTIS AG
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
InactiveUS20130316370A1Eliminate needEasy to adaptDisease diagnosisBiological testingWhite blood cellPancreatic hormone
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using assays that detect one or more biomarkers selected from the group consisting of Beta-nerve growth factor, Interleukin-17A, Follitropin subunit beta, Collagenase 3, Follistatin, Vitamin D Binding Protein, Islet amyloid polypeptide, Insulin C-peptide, Complement Factor H, Gastric inhibitory polypeptide, Glucagon-like peptide 1, Glucagon, Involucrin, Type II cytoskeletal Keratin-1 / Keratin-10, Type II cytoskeletal Keratin-6A / 6B / 6C, Osteocalcin, Lipopolysaccharide, Pancreatic prohormone, Peptide YY, Agouti-related protein, Ciliary neurotrophic factor, Appetite-regulating hormone, Transthyretin, Insulin receptor substrate 1, and NF-kappa-B inhibitor alpha as diagnostic and prognostic biomarker assays in renal injuries.
Owner:ASTUTE MEDICAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com