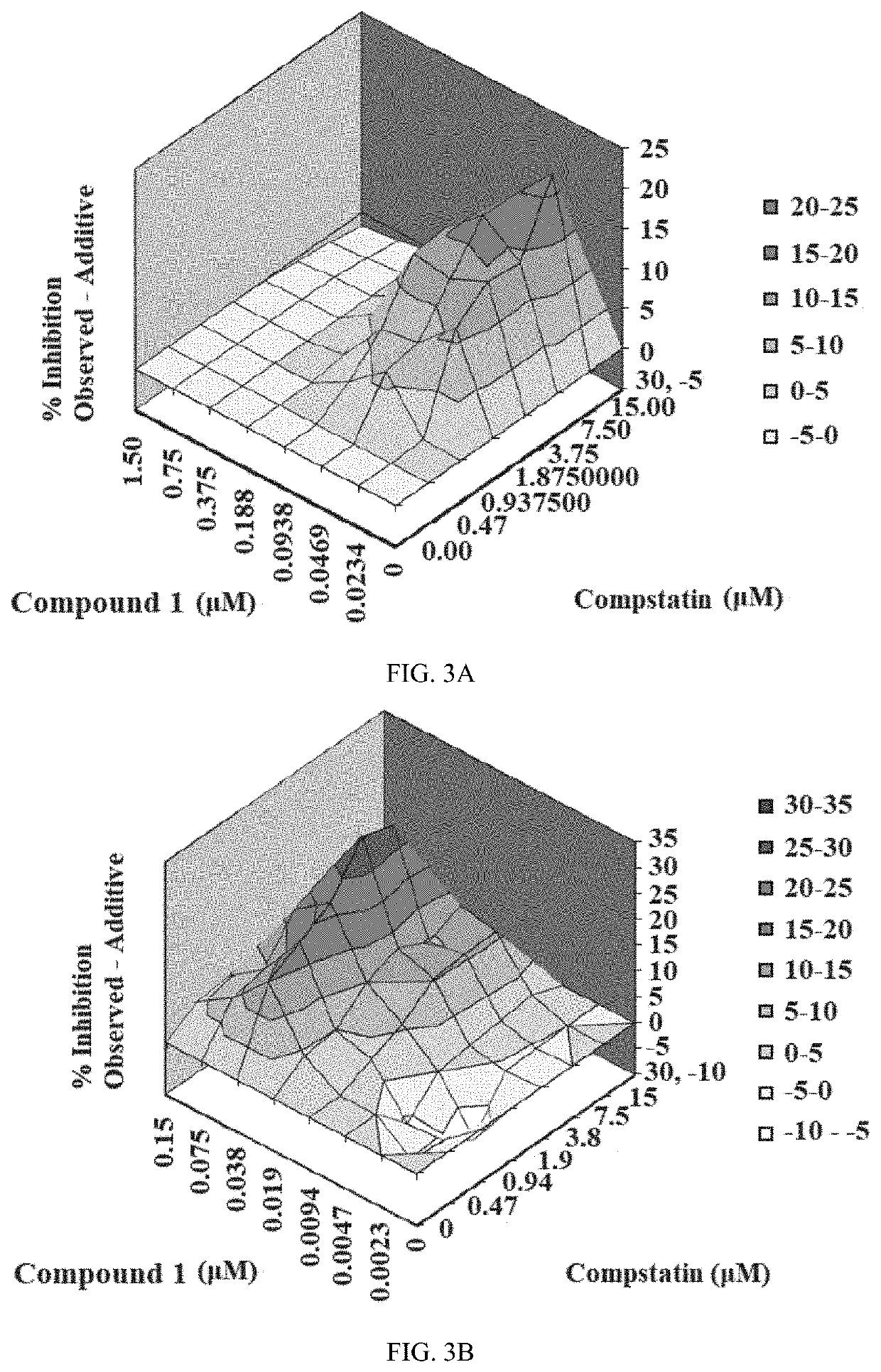
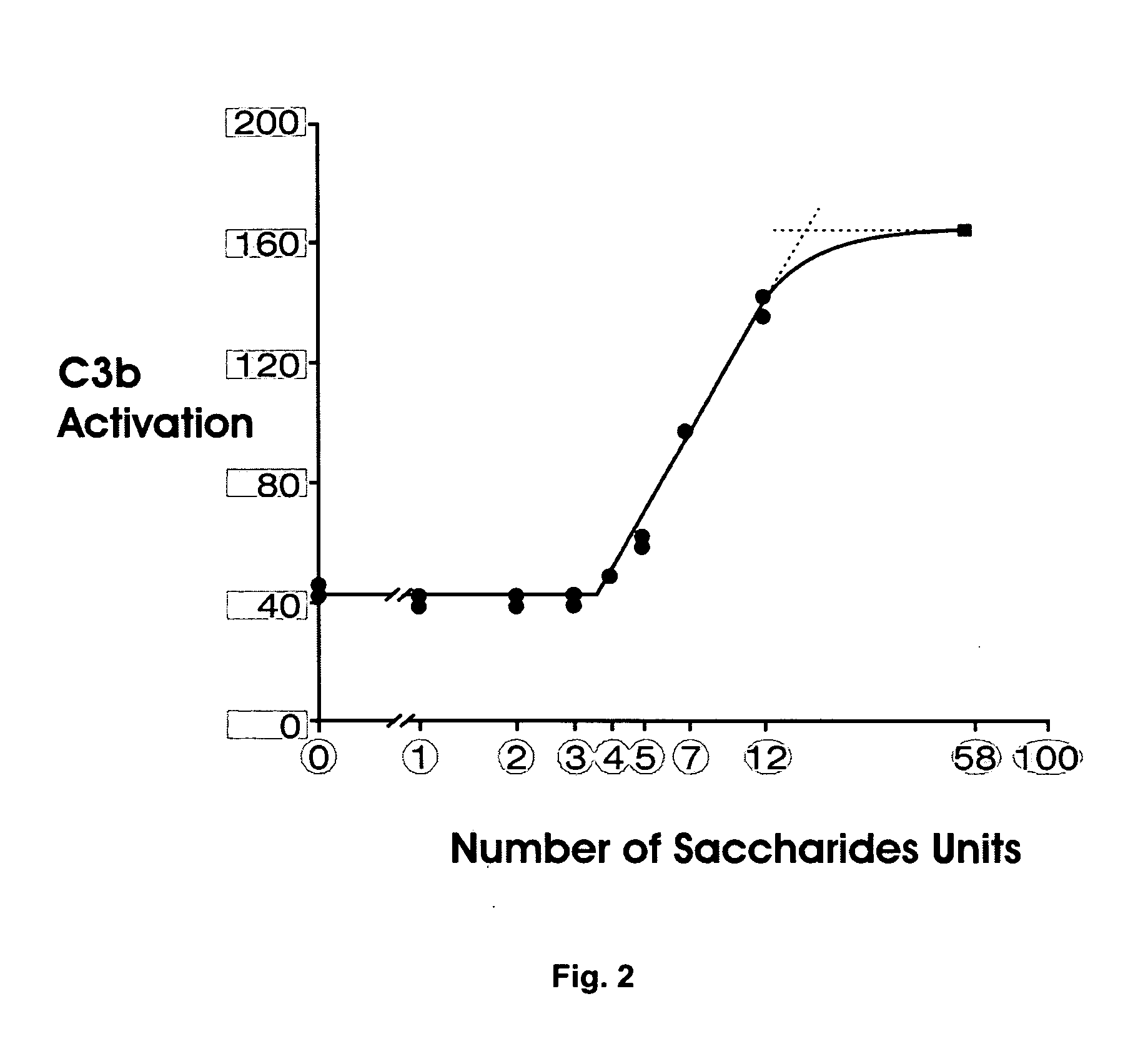
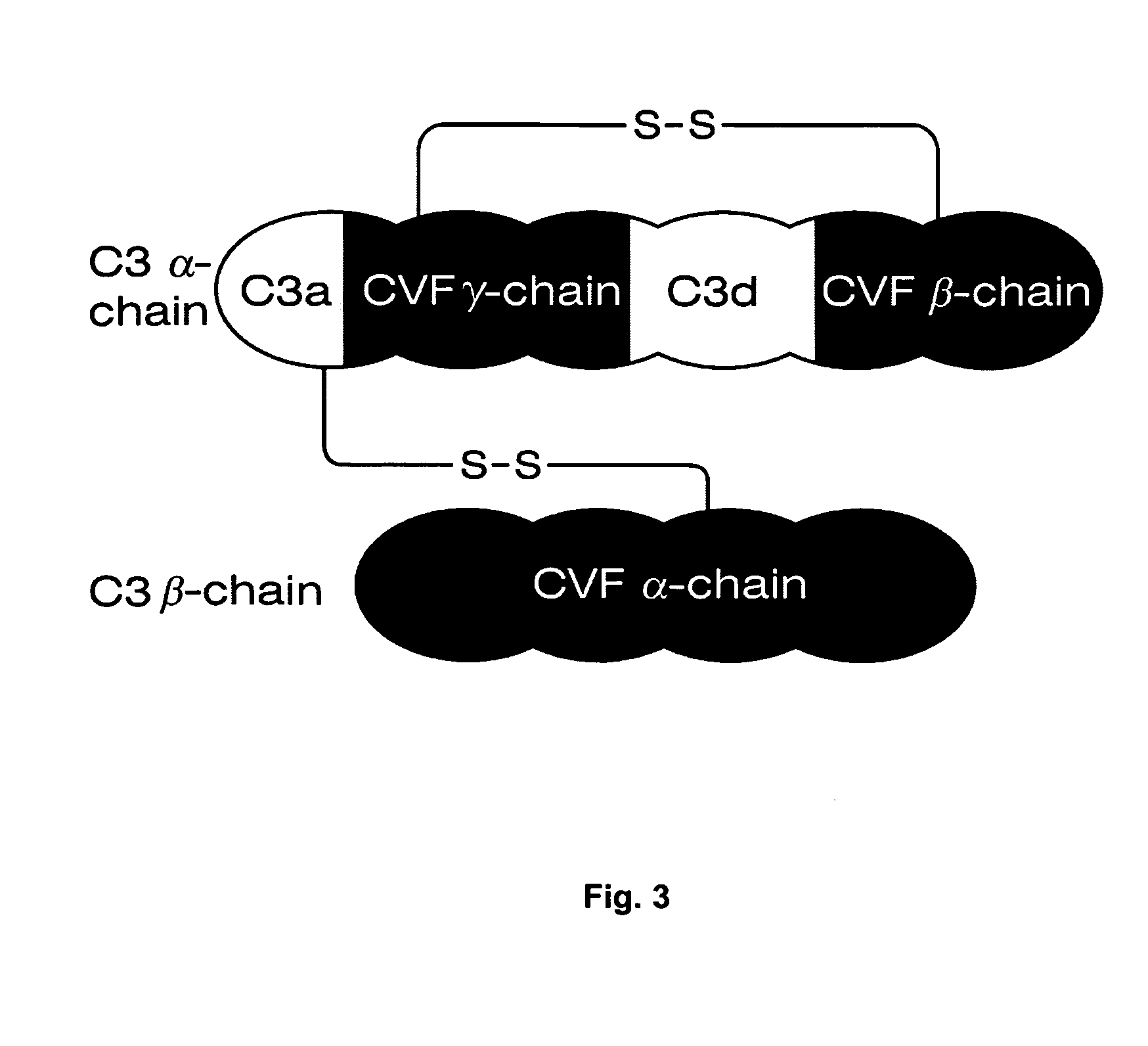
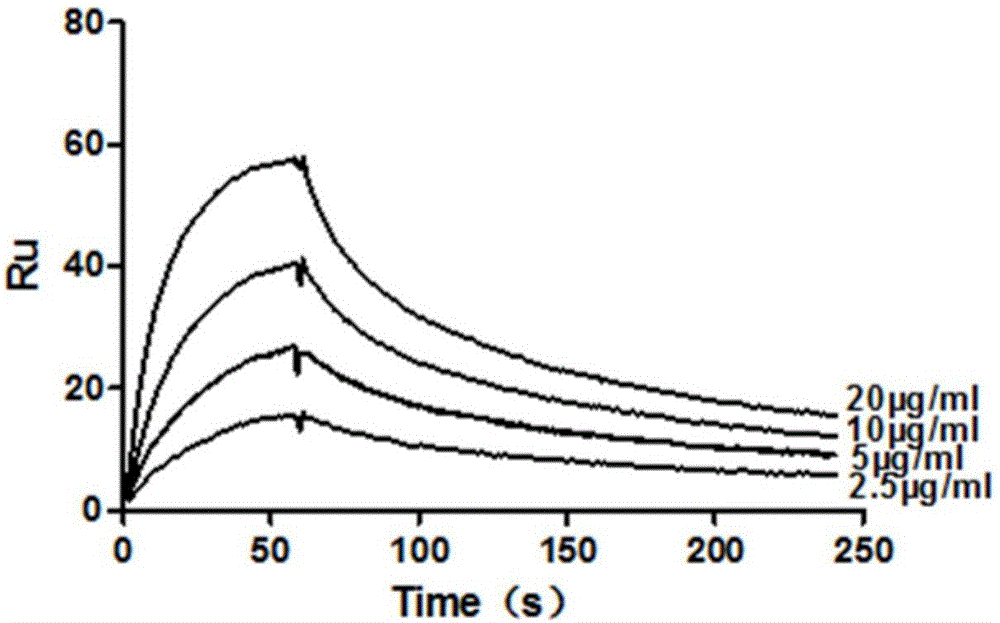
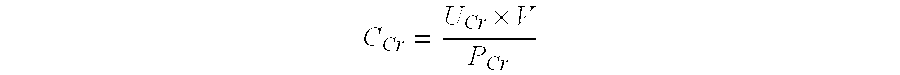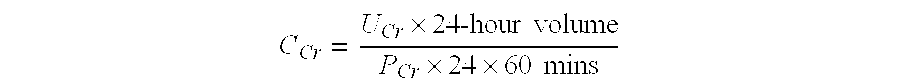Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
81 results about "Complement factor I" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
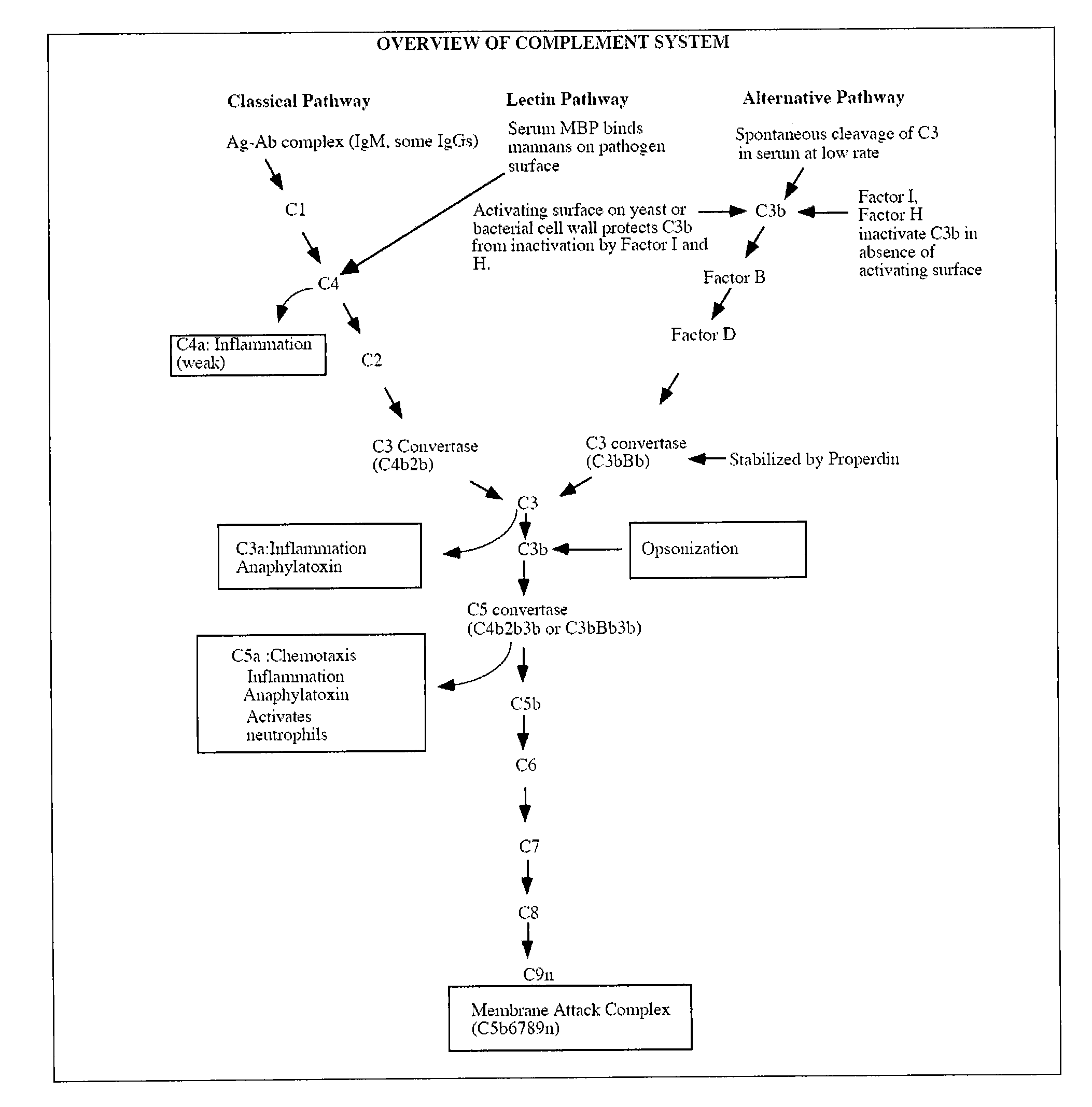
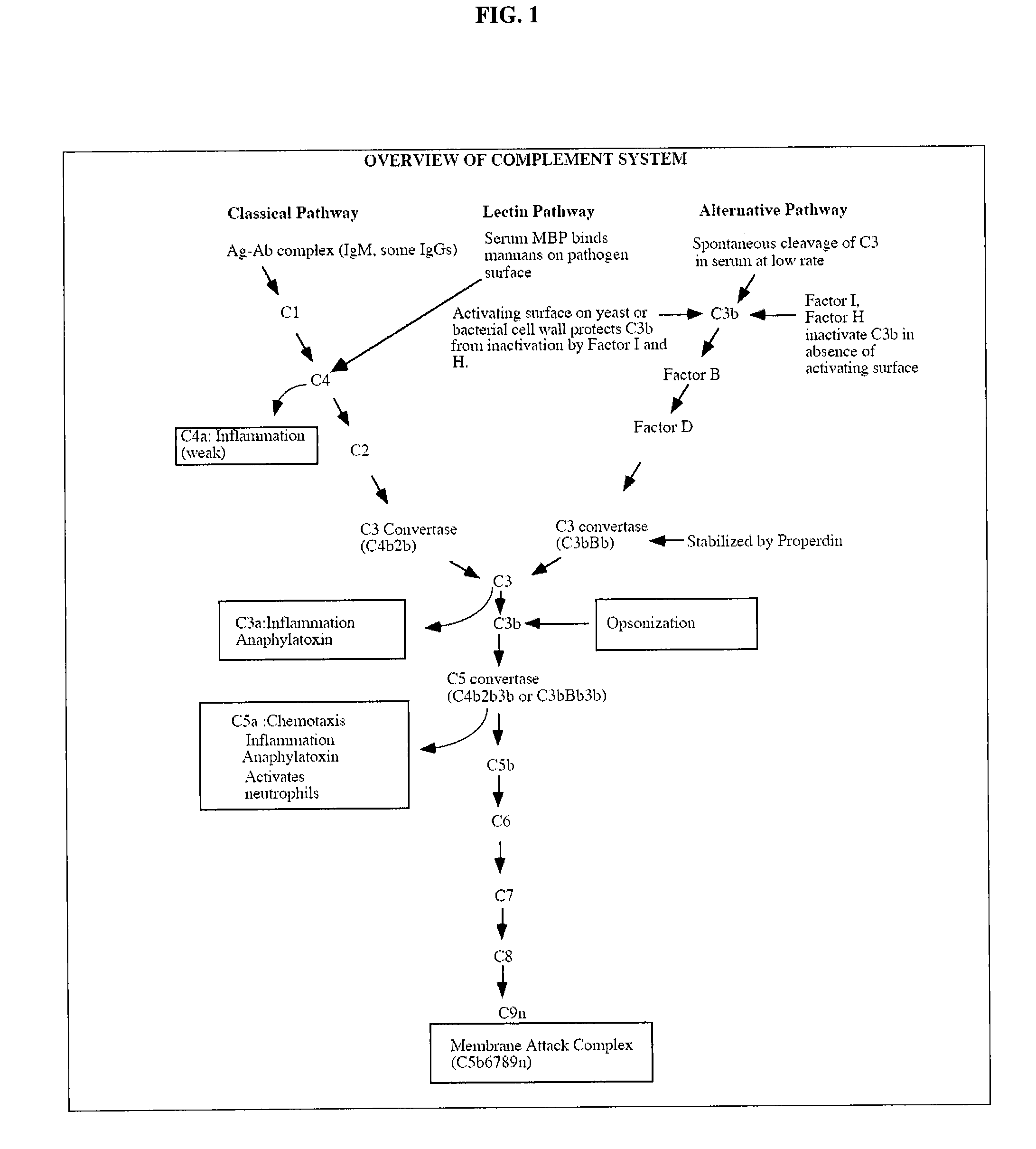
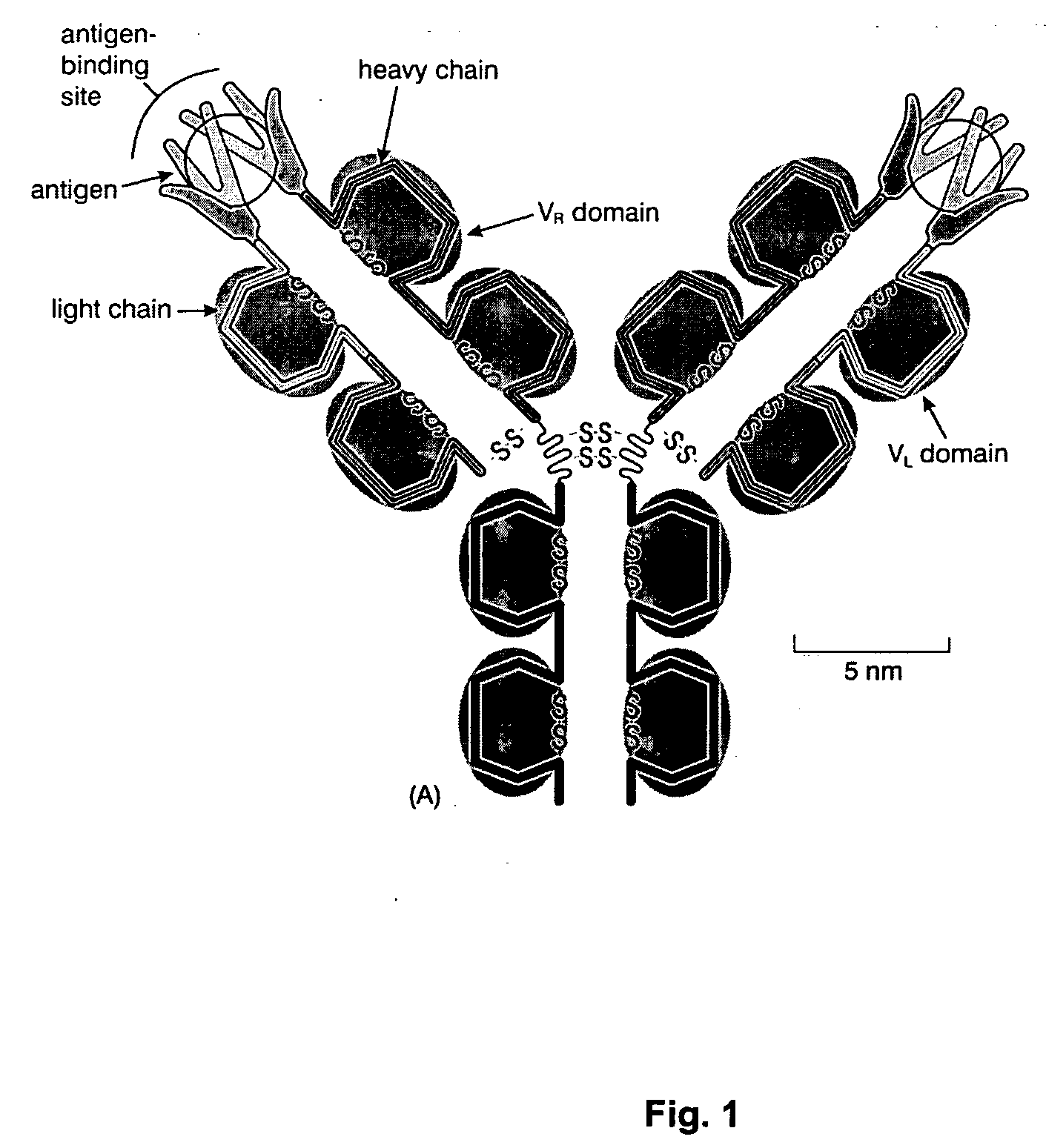
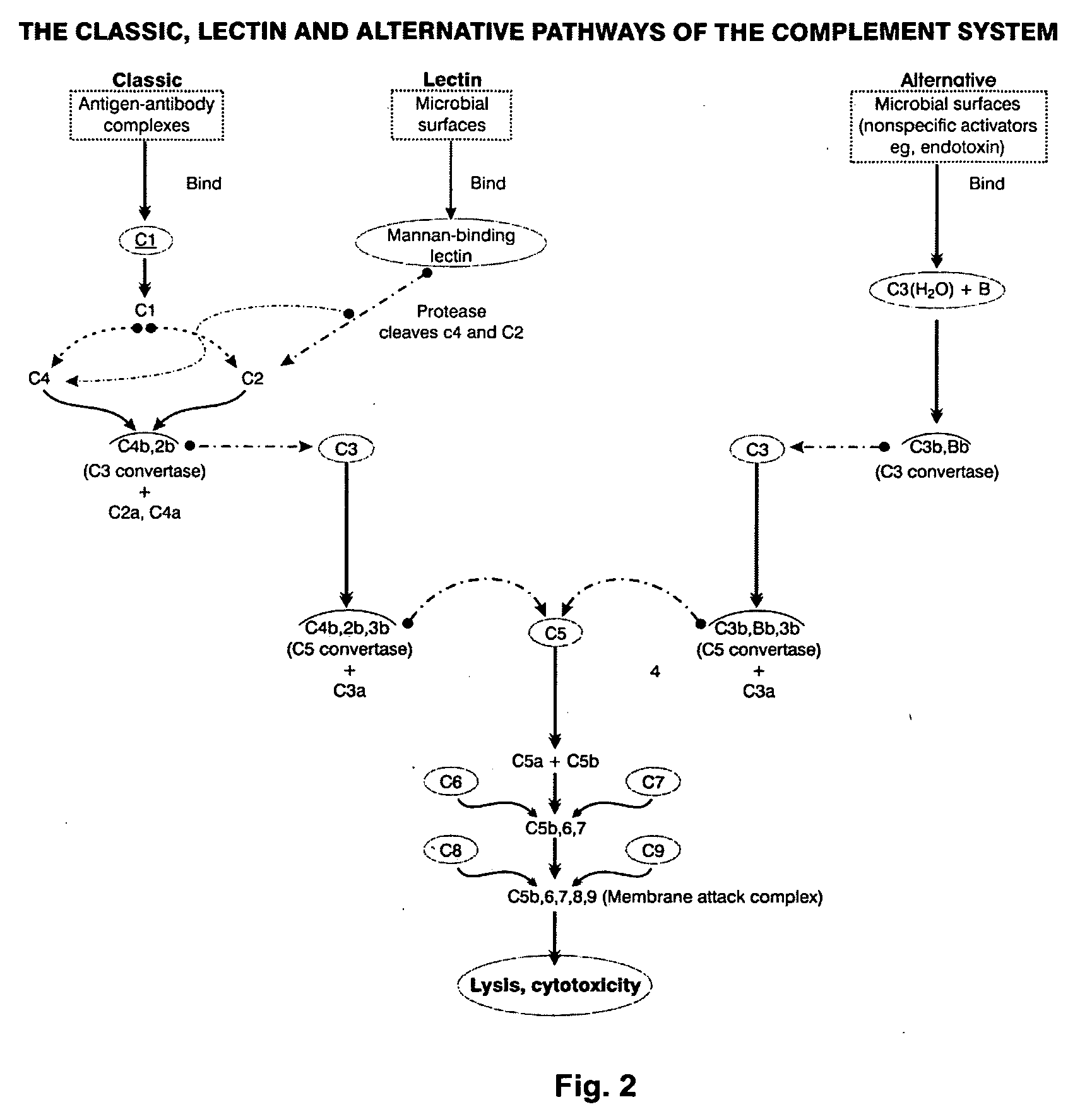
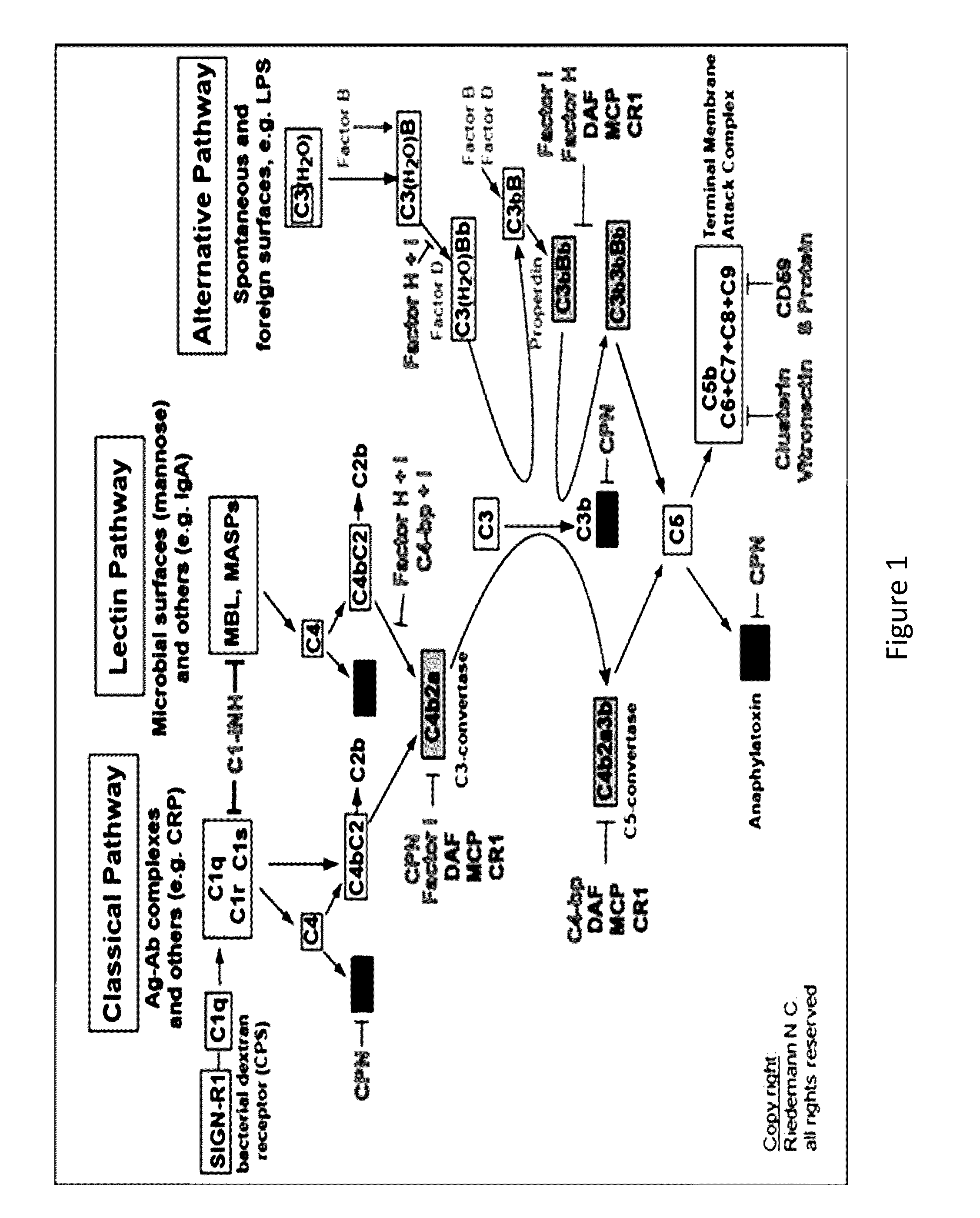
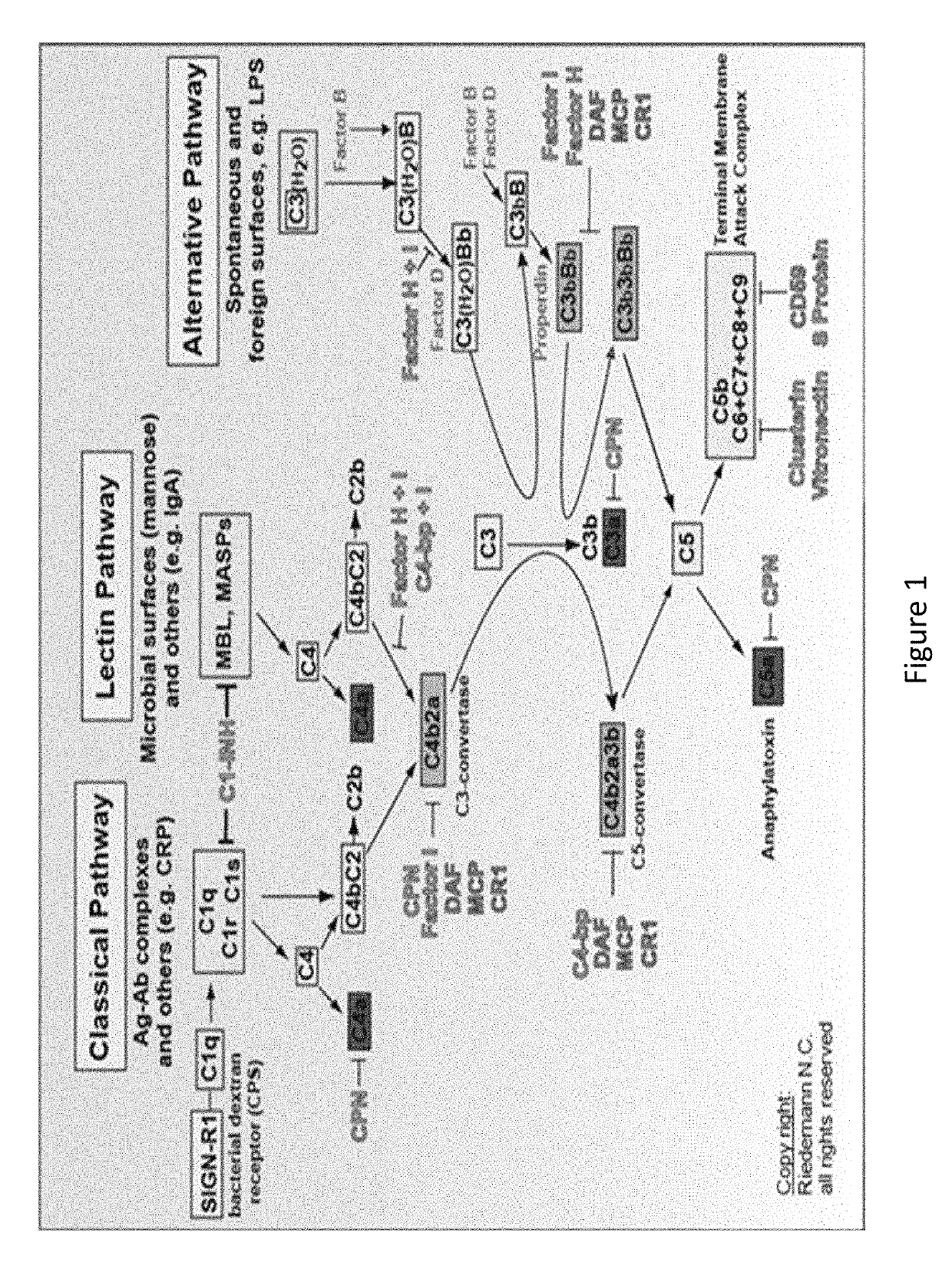
Complement factor I, also known as C3b/C4b inactivator, is a protein that in humans is encoded by the CFI gene. Complement factor I (factor I) is a protein of the complement system, first isolated in 1966 in guinea pig serum, that regulates complement activation by cleaving cell-bound or fluid phase C3b and C4b. It is a soluble glycoprotein that circulates in human blood at an average concentration of 35 μg/mL.
Treatment of age-related macular degeneration using inhibitors of complement factor d
InactiveUS20080269318A1Avoid developmentInhibit the loss of visual acuityBiocideSenses disorderComplement Factor H GeneFactor ii
The present invention provides methods for identifying a patient at risk for developing AMD by identifying the presence of the Y402H polymorphism or other at risk variants in the complement factor H gene. The present invention further provides methods for treating persons having AMD or at risk for developing AMD as a result of having the Y402H polymorphism or other at risk variants in the complement factor H gene.
Owner:ALCON RES LTD
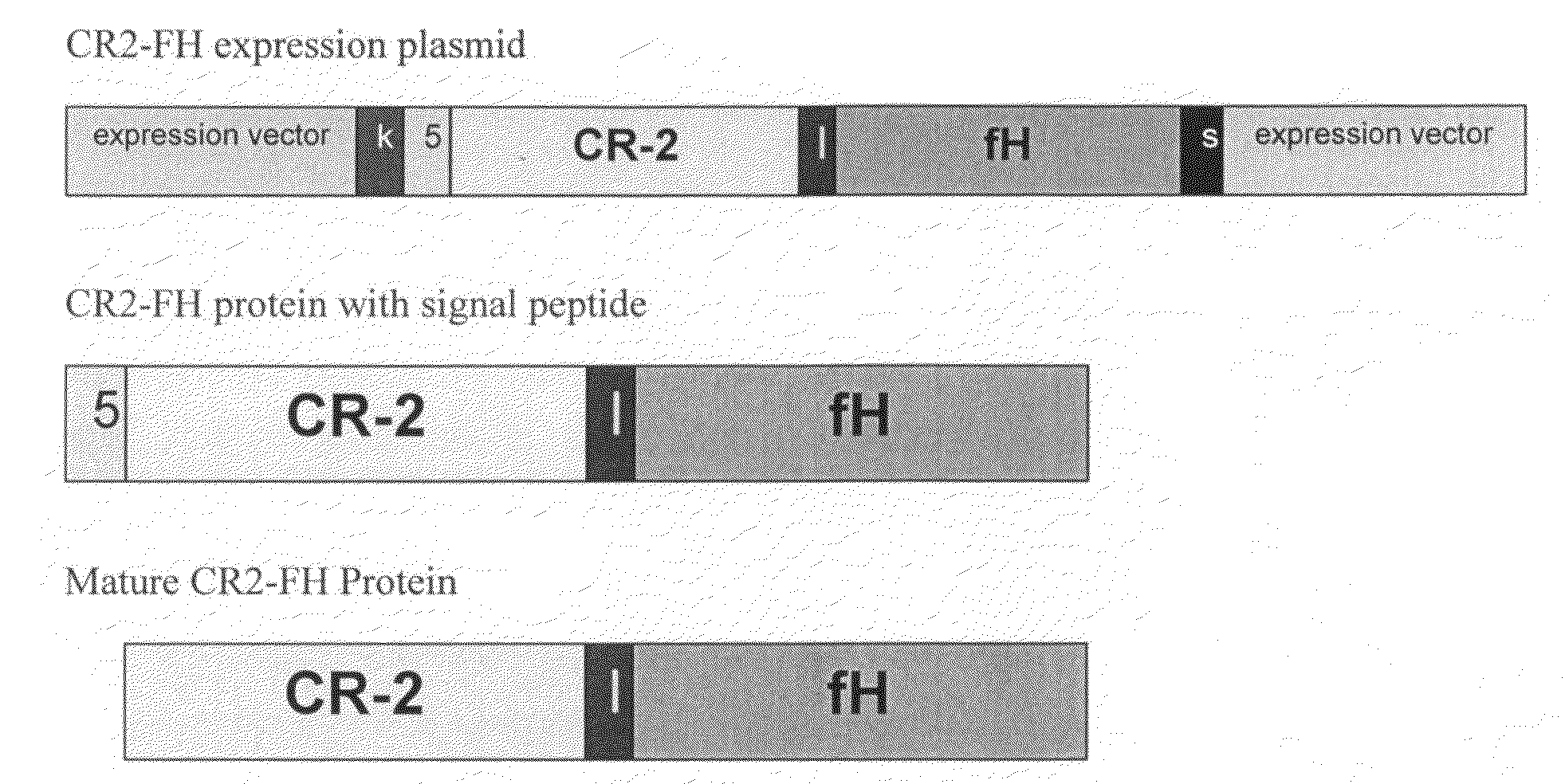
Targeting complement factor H for treatment of diseases
ActiveUS20080221011A1Improve survivalSenses disorderPeptide/protein ingredientsFactor iiComplement factor I
The invention provides a CR2-FH molecule comprising a CR2 portion comprising CR2 protein or a fragment thereof and a FH portion comprising a factor H protein or a fragment thereof, and pharmaceutical compositions comprising a CR2-FH molecule. Also provided are methods of using the compositions for treatment diseases in which the alternative complement pathway is implicated, such as age-related macular degeneration, rheumatoid arthritis, and ischemia reperfusion.
Owner:MUSC FOUND FOR RES DEV +1
Immunogenic composition and method of developing a vaccine based on factor H binding sites
InactiveUS20050112139A1Rapid decrease in viremiaEasy to copyVirus peptidesPharmaceutical delivery mechanismBinding siteAutoimmune responses
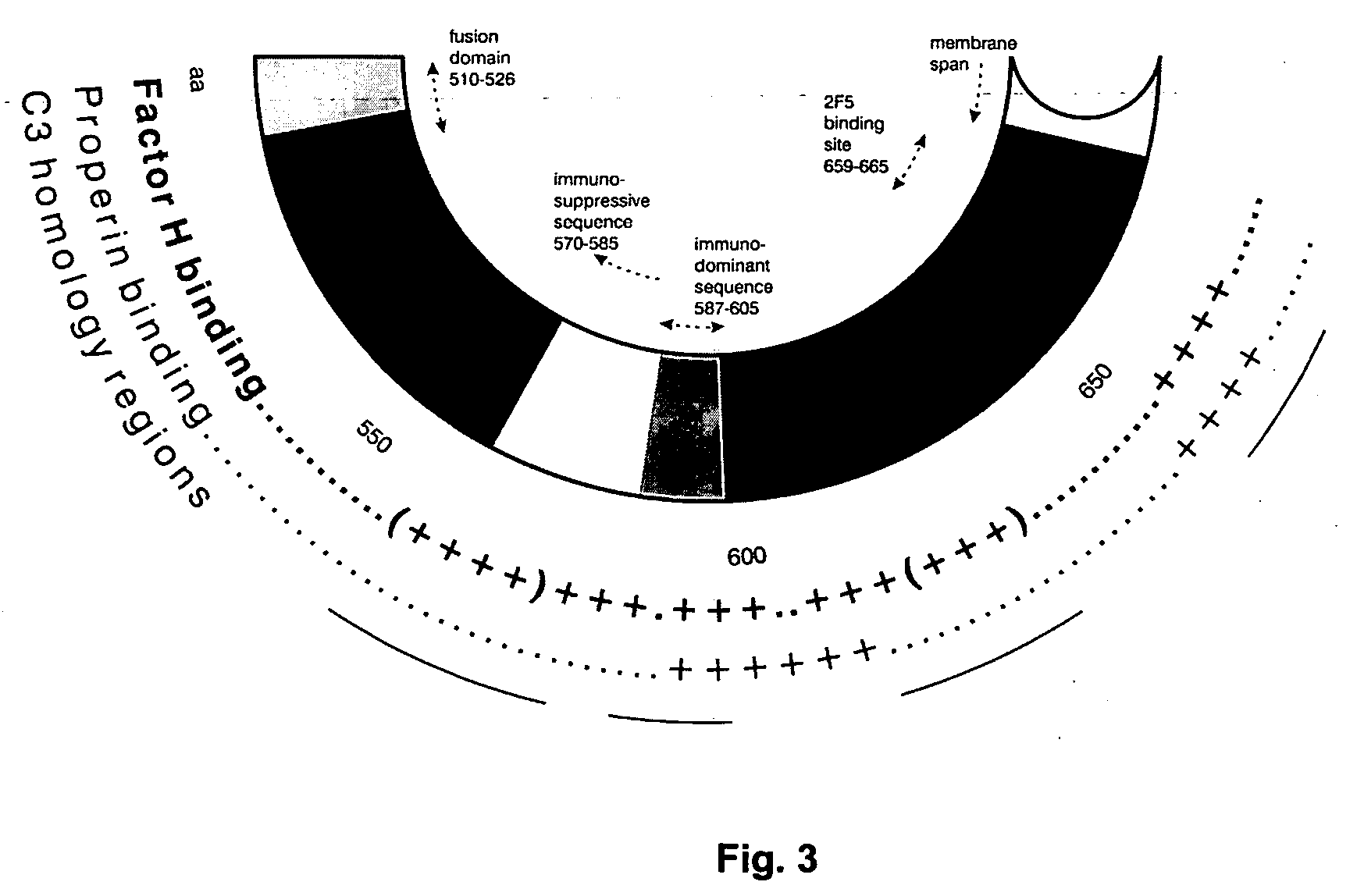
An immunogenic composition able to provide an immune response to Human Complement Factor H binding on one or more HIV glycoproteins is disclosed, which is characterized by at least one binding site epitope of the glycoproteins. Such immunogenic composition wherein the glycoprotein comprises gp120, gp41, or both glycoproteins gp120 and gp41 is hereby disclosed. Sialic acid is removed to enhance immune recognition of the composition and to impair Factor H binding. A medication having an inhibitive action on autoimmune response by specific inhibition of the cleavage of C3b by Factor H into inactive cell fragments.
Owner:KARP NELSON M
Protein markers for cardiovascular events
InactiveCN101889205AUse to reduce or preventReduce or prevent the risk of cardiovascular eventsDisease diagnosisBiological testingCathepsin SMacrophage migration inhibitory factor
The present invention relates to a method for predicting the risk of a subject developing a cardiovascular event comprising detecting at least one biomarker in (a sample of) the cardiovascular system from said subject, wherein said biomarker comprises at least one protein selected from the group consisting of Tumor Necrosis Factor Alpha Precursor; Lysosomal-associated Membrane Protein (1); Interleukin-5 Precursor; Interleukin-6 Precursor; C-C Motif Chemokine (2) Precursor; C-C Motif Chemokine (5) Precursor RANTES; Cathepsin L1 Precursor; Adenylate Kinase (1); Leukotriene B4 Receptor (1); Complement Factor D; Secreted Phosphoprotein (1); Small Inducible Cytokine A17 Precursor; C-X-C Motif Chemokine (10) Precursor; Tumor Necrosis Factor Ligand Superfamily Member (11)(RANKL); C-C Motif Chemokine (18) Precursor; 72 kDa Type IVCollagenase Precursor; Neutrophil Collagenase Precursor; fatty acid binding protein (4); calpain (2), (m / II) large subunit; Macrophage Migration Inhibitory Factor; Cathepsin S Precursor; Interleukin (13) Precursor; and soluble ICAM-1.
Owner:卡瓦迪斯有限责任公司
Targeting complement factor H for treatment of diseases
ActiveUS7759304B2Improve survivalSenses disorderAntibody mimetics/scaffoldsComplement factor IDisease cause
The invention provides a CR2-FH molecule comprising a CR2 portion comprising CR2 protein or a fragment thereof and a FH portion comprising a factor H protein or a fragment thereof, and pharmaceutical compositions comprising a CR2-FH molecule. Also provided are methods of using the compositions for treatment diseases in which the alternative complement pathway is implicated, such as age-related macular degeneration, rheumatoid arthritis, and ischemia reperfusion.
Owner:MUSC FOUND FOR RES DEV +1
Diagnostic and therapeutic target for macular degeneration
InactiveUS20070037183A1Reduce usageReduce dosageMicrobiological testing/measurementDiseaseComplement factor I
The present invention is based on the discovery of genetic polymorphisms that are associated with ocular diseases and disorders, such as age-related macular degeneration (AMD). In particular, the present invention relates to methods for determining an individuals susceptibility to ocular disorders such as AMD by screening for mutations and / or polymorphisms in the human complement factor H (CFH) gene or gene product that confer susceptibility to such disorders. Also encompassed in the present invention are nucleic acid molecules containing the polymorphisms, variant proteins encoded by such nucleic acid molecules, reagents for detecting the polymorphic nucleic acid molecules and proteins, and methods of treatment following detection of susceptibility.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
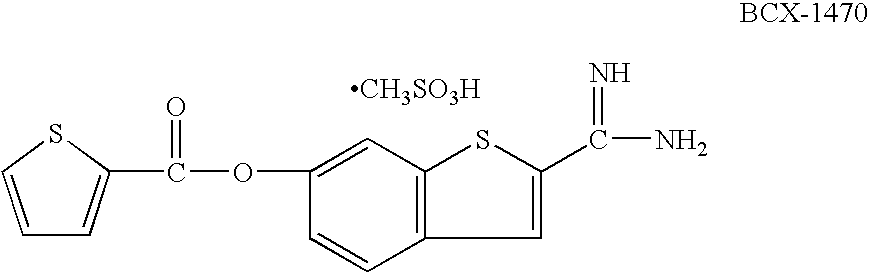
Quinazoline and indole compounds to treat medical disorders
Owner:ACHILLION PHARMA INC
Aryi, Heteroaryl, and Heterocyclic Pharmaceutical Compounds for Treatment of Medical Disorders
ActiveUS20200071301A1Reduce the possibilityMinimal hydrolysisOrganic active ingredientsGroup 5/15 element organic compoundsDiseaseAryl
Complement Factor D inhibitors, pharmaceutical compositions, and uses thereof, as well as processes for their manufacture are provided. The compounds provided include Formula I, Formula II, Formula III, Formula IV, and Formula V, or a pharmaceutically acceptable salt, prodrug, isotopic analog, N-oxide, or isolated isomer thereof, optionally in a pharmaceutically acceptable composition. The inhibitors described herein target Factor D and inhibit or regulate the complement cascade.
Owner:ACHILLION PHARMA INC
COMPLEMENT FACTOR Bb ANTIBODIES
Owner:ALLERGAN INC
Humanized anti-complement factor C1Q antibodies
Owner:ANNEXON
Extracorporeal devices and methods of treating complement factor related diseases
PendingUS20190247560A1Improve the quality of lifeBeneficially influenceOther chemical processesOther blood circulation devicesDiseaseComplement factor I
The present disclosure relates to devices for the extracorporeal treatment of a patient having a complement factor related disease. The devices are adapted to remove said complement factors from the blood or blood plasma of a patient in need. The disclosure further relates to extracorporeal circuits comprising such devices and methods for the treatment of a patient suffering from a complement factor related disease.
Owner:GAMBRO LUNDIA AB
Aryl, heteroaryl, and heterocyclic pharmaceutical compounds for treatment of medical disorders
ActiveUS11084800B2Decrease potential for formationReduce the possibilityOrganic active ingredientsGroup 5/15 element organic compoundsDiseaseAryl
Owner:ACHILLION PHARMA INC
Methods and Compositions For Diagnosis of Age-Related Macular Degeneration
InactiveUS20110117557A1Microbiological testing/measurementDisease diagnosisMacular degenerationPathology
The invention generally concerns methods and compositions for screening individuals for susceptibility to age-related macular degeneration (AMD). In particular, association with the various markers including complement factor H, LOC387717 / ARMS2, C2 / CFB, C3 and VEGF, indicates that a subject is at risk of AMD.
Owner:UNIV OF MIAMI +1
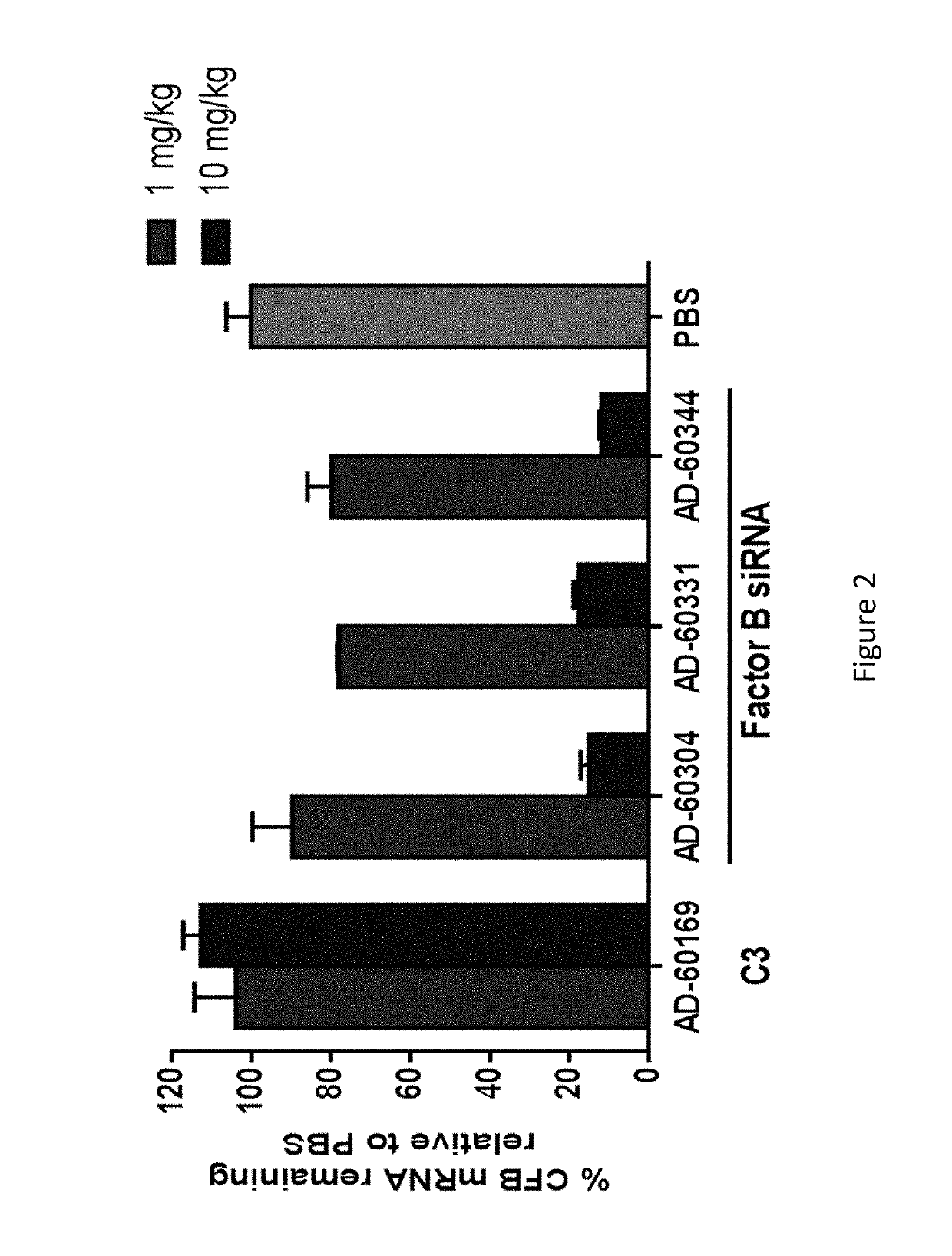
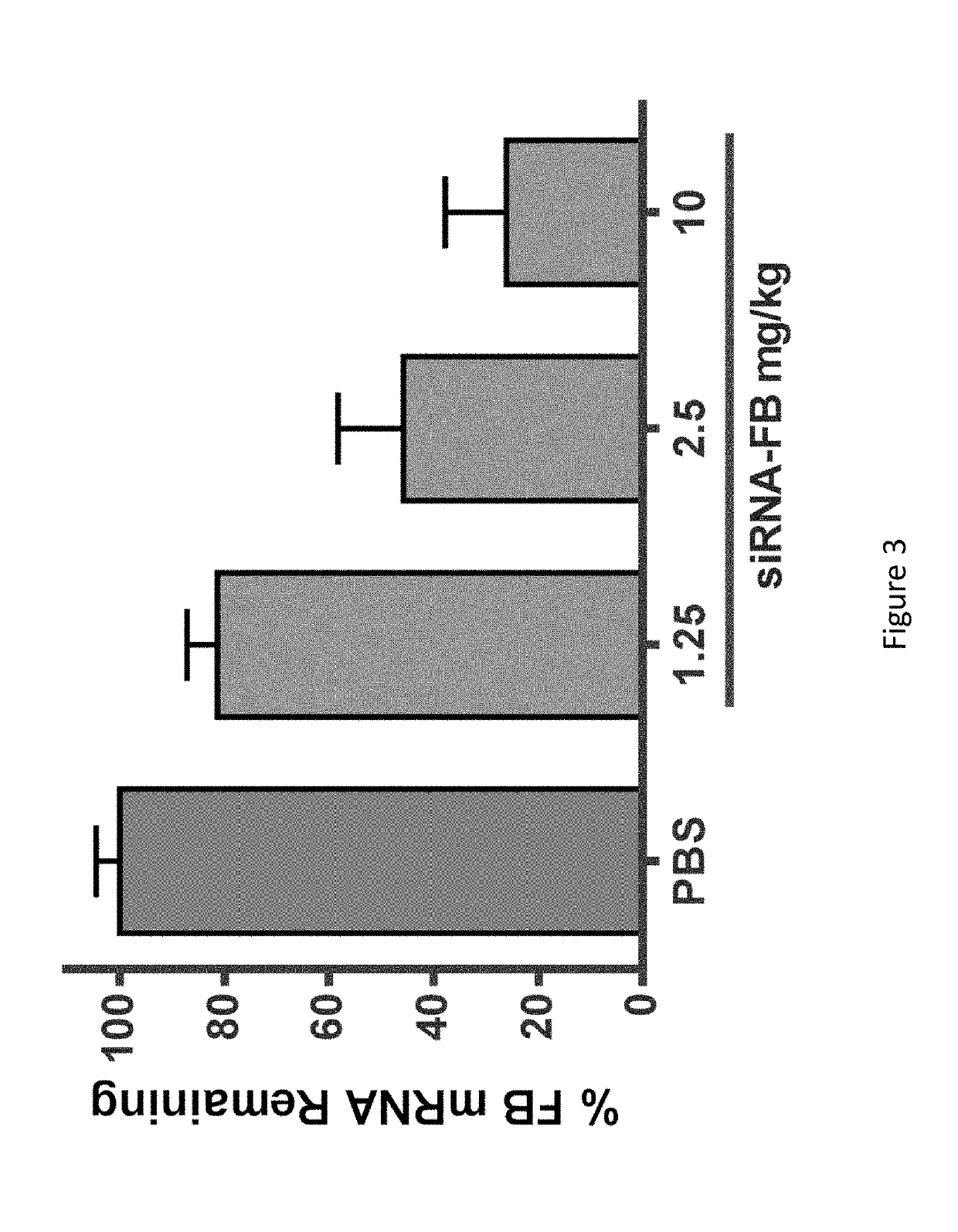
COMPLEMENT COMPONENT iRNA COMPOSITIONS AND METHODS OF USE THEREOF
ActiveUS20160298124A1Avoid symptomsReduce expressionAntibacterial agentsOrganic active ingredientsParoxysmal AFDisease
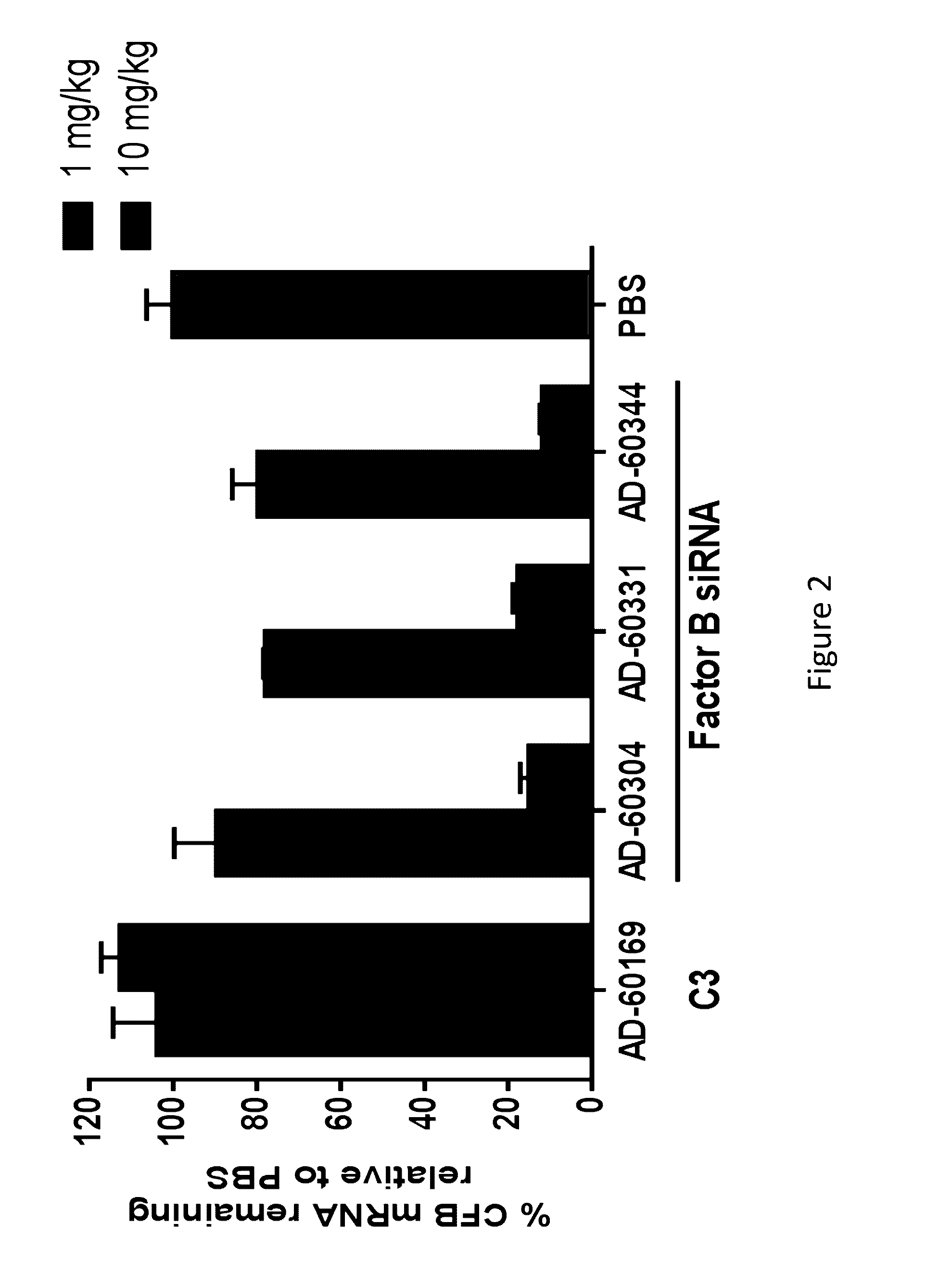
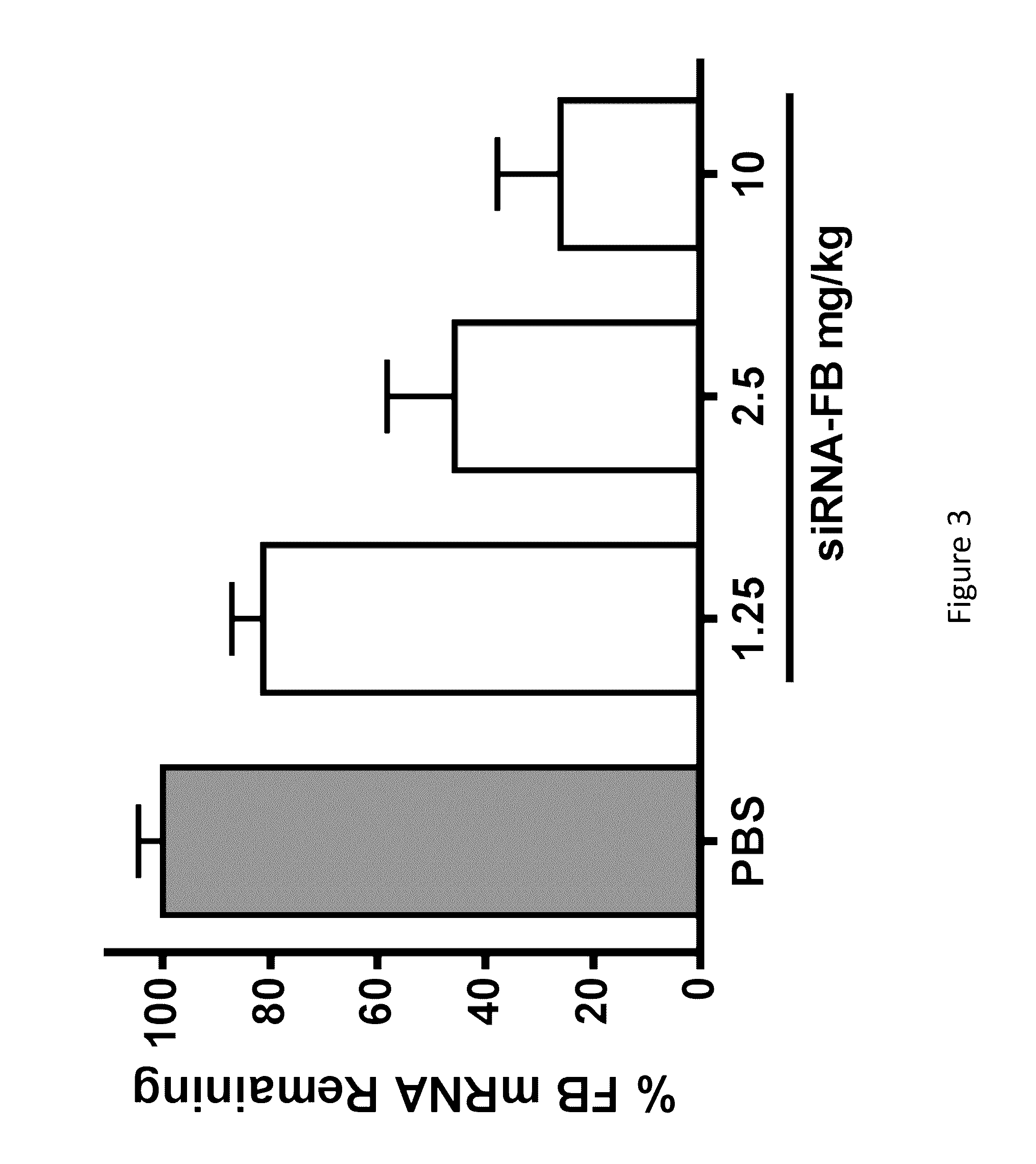
The invention relates to iRNA, e.g., double-stranded ribonucleic acid (dsRNA), compositions targeting the complement factor B (CFB) gene, the complement component C3 gene, and the complement component C9 gene and methods of using such iRNA, e.g., dsRNA, compositions to inhibit expression of CFB, C9 and / or C3 and to treat subjects having a complement component-associated disease, e.g., paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome.
Owner:ALNYLAM PHARM INC
Complement factor B inhibitors and uses there of
The present invention provides a compound of formula (I): wherein X is N or CH, Y is NH, O or S, methods for manufacturing these compounds, and their uses as Factor B inhibitors for the treatment of conditions and diseases associated with complement alternative pathway activation such as age-related macular degeneration, diabetic retinopathy and related ophthalmic diseases. The present invention further provides pharmaceutical compositions and combinations of pharmacologically active agents.
Owner:NOVARTIS AG
Novel biomarker and uses thereof in diagnosis, treatment of autism
InactiveUS20130267441A1High sensitivityHigh selectivityNervous disorderPeptide/protein ingredientsSide chainArginine
A new biomarker, a peptide having sequence SSKITHRIHWESASLLR*, wherein the side chain of the C-terminal arginine denoted with the asterisk is lacking the NH2-C═NH moiety normally present in the side chain. Usefulness of the biomarker in the diagnosis of neurological and / or neuropsychiatric disorders (in particular autism) is disclosed, as well as are methods for determining the concentration of the new biomarker and antibodies directed to the new biomarker. Treatment of autism, comprising administering a complement factor I inhibitor to the subject.
Owner:AUTISM BIOTECH
Complement component iRNA compositions and methods of use thereof
ActiveUS10465194B2Avoid symptomsReduce expressionAntibacterial agentsOrganic active ingredientsParoxysmal AFDisease
The invention relates to iRNA, e.g., double-stranded ribonucleic acid (dsRNA), compositions targeting the complement factor B (CFB) gene, the complement component C3 gene, and the complement component C9 gene and methods of using such iRNA, e.g., dsRNA, compositions to inhibit expression of CFB, C9 and / or C3 and to treat subjects having a complement component-associated disease, e.g., paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome.
Owner:ALNYLAM PHARMA INC
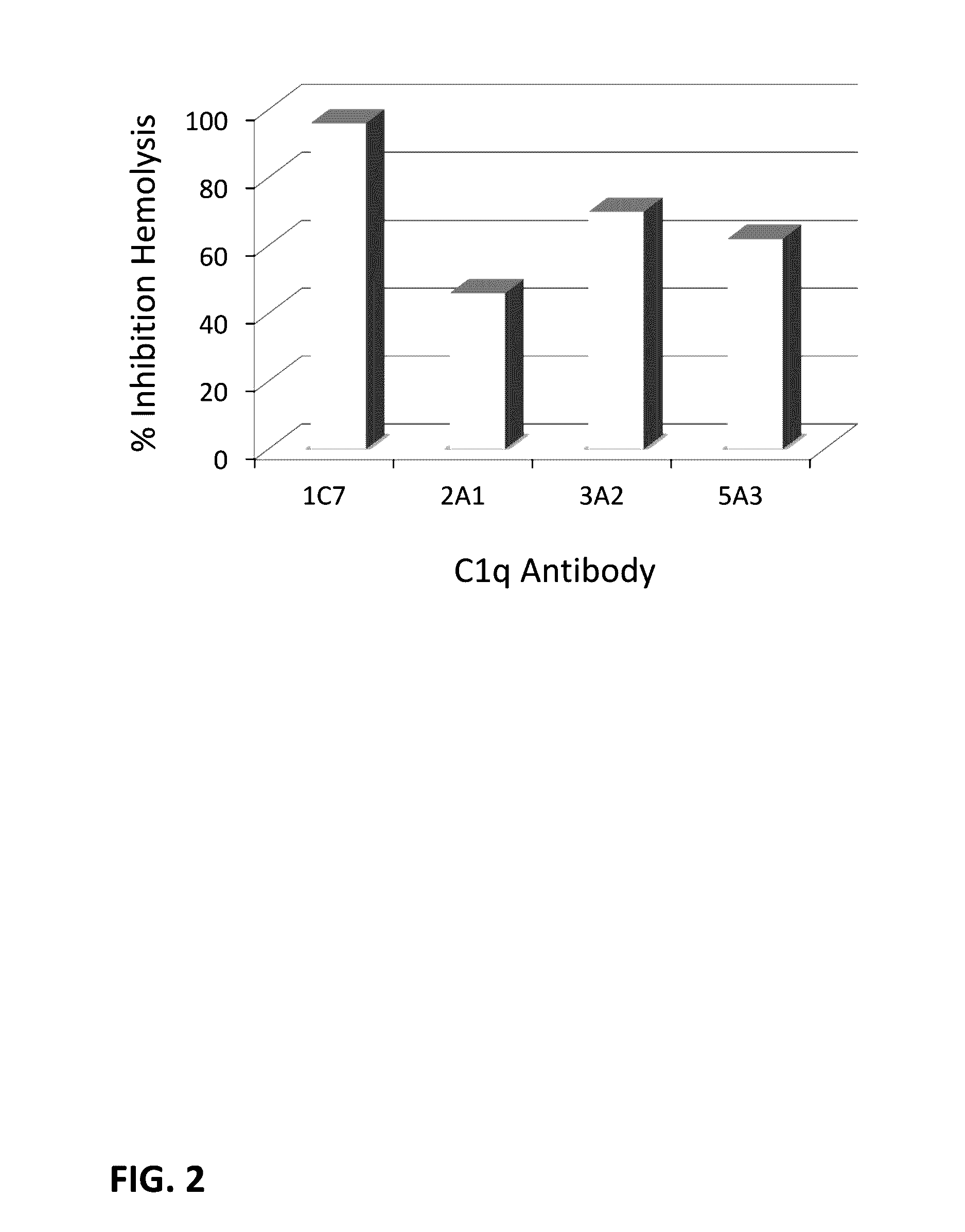
Anti-complement factor c1q antibodies and uses thereof
ActiveUS20160368973A1Compounds screening/testingSenses disorderComplement factor IComplement factor B
Owner:ANNEXON
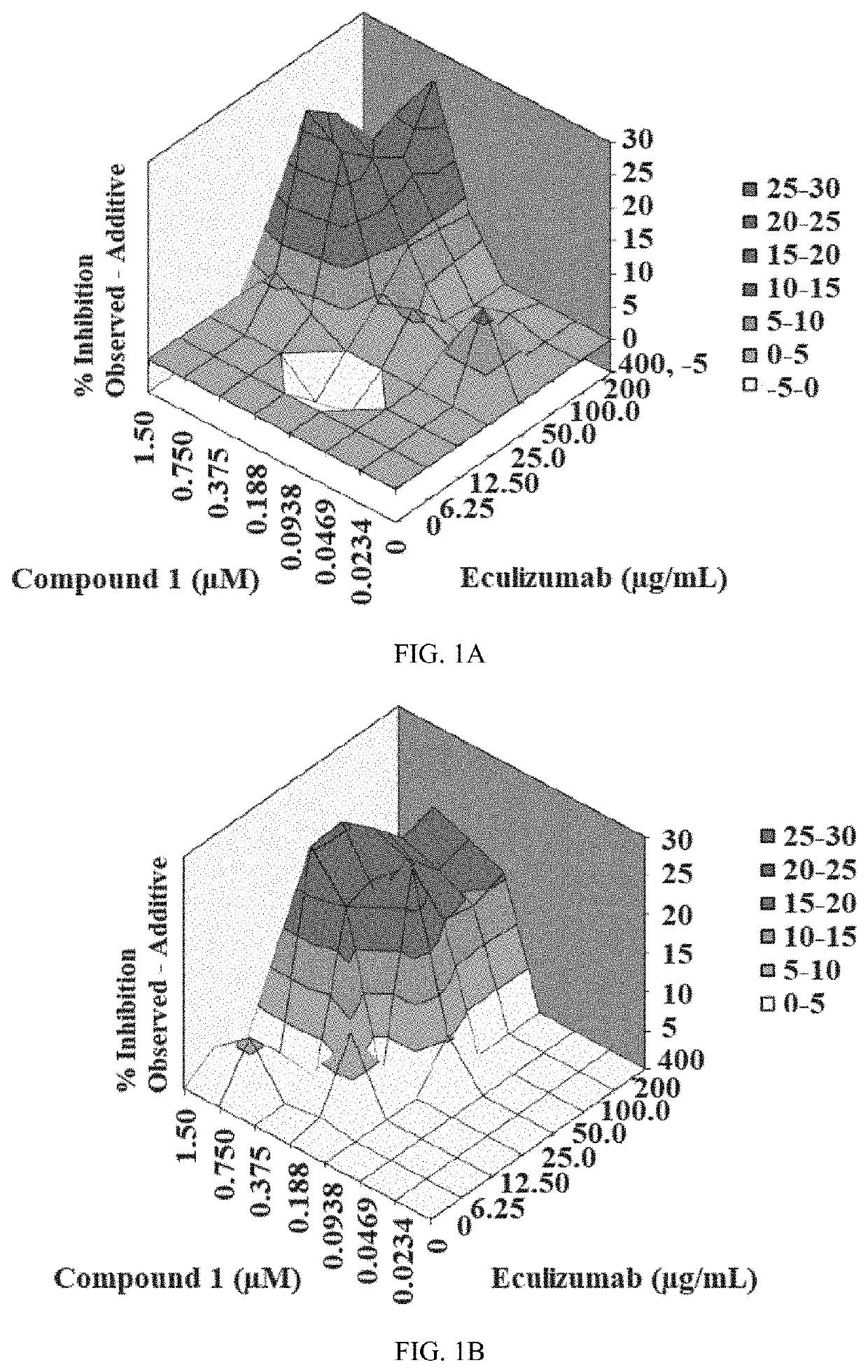
Therapeutic regimens for treatment of paroxysmal nocturnal hemoglobinuria
PendingUS20200101071A1Growth inhibitionIncrease depositionOrganic active ingredientsImmunoglobulins against animals/humansParoxysmal AFRegimen
Provided herein are methods for treating a subject with PNH comprising administering to a subject a therapeutically effective amount of complement component C5 (C5) inhibitor, complement component C3 (C3) inhibitor, or complement factor B (CFB) inhibitor in combination with a therapeutically effective amount of small molecule complement factor D (CFD) inhibitor of Formula I or Formula II, or a pharmaceutically acceptable salt thereof.
Owner:ACHILLION PHARMA INC
Bladder cancer detection kit and method and application of human complement factor H related protein therein
ActiveCN105785044AHigh sensitivityStrong specificityBiological material analysisBiological testingBladder cancerPatient compliance
The invention relates to a bladder cancer detection kit and method. A monoclonal antibody and a polyclonal antibody which are formed through the human complement factor H are involved. The invention further relates to application of human complement factor H related protein. BTA is selected as a marker for detecting bladder cancer, three different monoclonal antibodies obtained from polypeptide immune are mixed to be used as a coating antibody, the sensitivity is high, and specificity is high. The coating antibody and a detection antibody are obtained from antigen immune of different sources, and then detection accuracy is improved. The bladder cancer detection kit and method are high in detection precision, good in specificity, high in patient compliance and capable of achieving detection conveniently.
Owner:HEBEI TEWENTE BIOTECH DEV CO LTD
Aryl, heteroaryl, and heterocyclic compounds for treatment of medical disorders
Compounds, methods of use, and processes for making inhibitors of complement Factor D comprising Formula I, or a pharmaceutically acceptable salt or composition thereof wherein R12 or R13 on the A group is an aryl, heteroaryl or heterocycle (R32) are provided. The inhibitors of Factor D described herein reduce the excessive activation of complement.
Owner:ACHILLION PHARMA INC
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
InactiveUS20130316370A1Eliminate needEasy to adaptDisease diagnosisBiological testingWhite blood cellPancreatic hormone
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using assays that detect one or more biomarkers selected from the group consisting of Beta-nerve growth factor, Interleukin-17A, Follitropin subunit beta, Collagenase 3, Follistatin, Vitamin D Binding Protein, Islet amyloid polypeptide, Insulin C-peptide, Complement Factor H, Gastric inhibitory polypeptide, Glucagon-like peptide 1, Glucagon, Involucrin, Type II cytoskeletal Keratin-1 / Keratin-10, Type II cytoskeletal Keratin-6A / 6B / 6C, Osteocalcin, Lipopolysaccharide, Pancreatic prohormone, Peptide YY, Agouti-related protein, Ciliary neurotrophic factor, Appetite-regulating hormone, Transthyretin, Insulin receptor substrate 1, and NF-kappa-B inhibitor alpha as diagnostic and prognostic biomarker assays in renal injuries.
Owner:ASTUTE MEDICAL
Method of developing an immunogenic composition and HIV vaccine
InactiveUS20050112143A1Broad antigenic responseEasy to produceVirus peptidesPharmaceutical delivery mechanismImmune recognitionComplement factor I
An antigenic and immunogenic composition of predetermined inactivated strains of human immunodeficiency virus (HIV) is disclosed. Inactivation is through psoralen and ultraviolet radiation; the composition is rendered more effective by the removal of structural features of HIV that interfere with immune response. In particular, sialic acid is removed to enhance immune recognition of the composition and to impair Complement Factor H binding. CD55 and CD59 are also removed to prevent the binding of Complement Factor H. Determination of strains for inactivation may be though immunotherapeutic genotyping or probabilistic assessment of risk of exposure.
Owner:KARP NELSON M M D
Anti-complement factor c1s antibodies and uses thereof
The present invention relates generally to the generation and characterization of neutralizing anti-C1s monoclonal antibodies. The invention further relates to the use of such anti-C1s antibodies in the detection of complement factors of the classical complement activation pathway, such as C1s. Additionally, the antibodies of this disclosure are useful for the diagnosis and treatment of disorders associated with an increased activation of the classical complement pathway, in particular autoimmune disorders and neurodegenerative diseases, including neurodegenerative diseases with synapse loss, such as Alzheimer's Disease. Methods of treatment of autoimmune and neurodegenerative diseases are also provided.
Owner:ANNEXON
Complement factor h-based assays for serum bactericidal activity against neisseria meningitidis
InactiveCN102203276AHigh bactericidal potencyReduced bactericidal potencyMicrobiological testing/measurementImmunoassaysSerum igeComplement factor I
Assays for detection of bactericidal anti-Neisserial antibodies using a factor H polypeptide having a human amino acid sequence that mediates binding to Neisserial factor H binding protein (fhBp) are provided, as well as non-human animal models of Neisserial infection.
Owner:CHILDREN S HOSPITAL &RES CENT AT OAKLAN +2
Complement factor H inhibitor and application related to same
ActiveCN106519030APrevent relapseReduce severityImmunoglobulins against animals/humansBiological material analysisMonoclonal antibodyCancer therapy
The invention discloses a CFH inhibitor for treating cancer. The CFH inhibitor is a CFH specific antibody. More particularly, the CFH inhibitor is a CFH specific monoclonal antibody. The CFH inhibitor stops combination of CFH and C3b to improve deposition of C3b on tumor cells and promote compliment mediated cytotoxic effect. On that basis, the invention provides a method for treating cancer.
Owner:GUANGZHOU TAINUODI BIOTECH +2
Pharmaceutical compounds for treatment of medical disorders
ActiveUS11447465B2Decreases potential for formationReduce the possibilityGroup 5/15 element organic compoundsDiseaseMedical disorder
Complement Factor D inhibitors, pharmaceutical compositions, and uses thereof, as well as processes for their manufacture are provided. The compounds provided include Formula I, Formula II, Formula III, and Formula IV or a pharmaceutically acceptable salt, prodrug, isotopic analog, N-oxide, or isolated isomer thereof, optionally in a pharmaceutically acceptable composition. The inhibitors described herein target Factor D and inhibit or regulate the complement cascade.
Owner:ACHILLION PHARMA INC
Amide compounds for treatment of complement mediated disorders
InactiveCN106413707ALess quantityIncreased detectable levelsSenses disorderNervous disorderDepressantPharmaceutical medicine
Compounds, methods of use, and processes for making inhibitors of complement factor D comprising Formula I, or a pharmaceutically acceptable salt or composition thereof, wherein R12 or R13 on the A group is an amide substituent (R32) are provided. The inhibitors described herein target factor D and inhibit or regulate the complement cascade at an early and essential point in the alternative complement pathway, and reduce factor D's ability to modulate the classical and lectin complement pathways. The inhibitors of factor D described herein are capable of reducing the excessive activation of complement, which has been linked to certain autoimmune, inflammatory, and neurodegenerative diseases, as well as ischemia-reperfusion injury and cancer.
Owner:ACHILLION PHARMA INC
Plasma protein marker, detection reagent or reagent tool for diagnosing severe change of new coronal virus pneumonia from mild symptom
The invention relates to the technical field of biology, in particular to a plasma protein marker, a detection reagent or a reagent tool for diagnosing severe change of new coronal virus pneumonia from mild symptoms. The plasma protein marker comprises zinc-alpha2-glycoprotein, mucoid 1, mucoid 2 and a complement factor I. Results show that the area (AUC) value under a subject working characteristic curve (ROC) for predicting the severe illness of a patient suffering from mild symptoms through combination of the four proteins is 1, the true positive rate for predicting the severe symptoms of the patient suffering from mild symptoms is 100%, and the false positive rate for predicting the severe symptoms of the patient suffering from mild symptoms is 0. In addition, enzyme-linked immunosorbent assay (ELISA) experiments find that the concentrations of the four proteins have significant differences in blood plasma of patients with mild symptoms and critical patients, so that the accuracy of predicting the severe case of mild symptoms by using the combination is further determined.
Owner:武汉市金银潭医院
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com