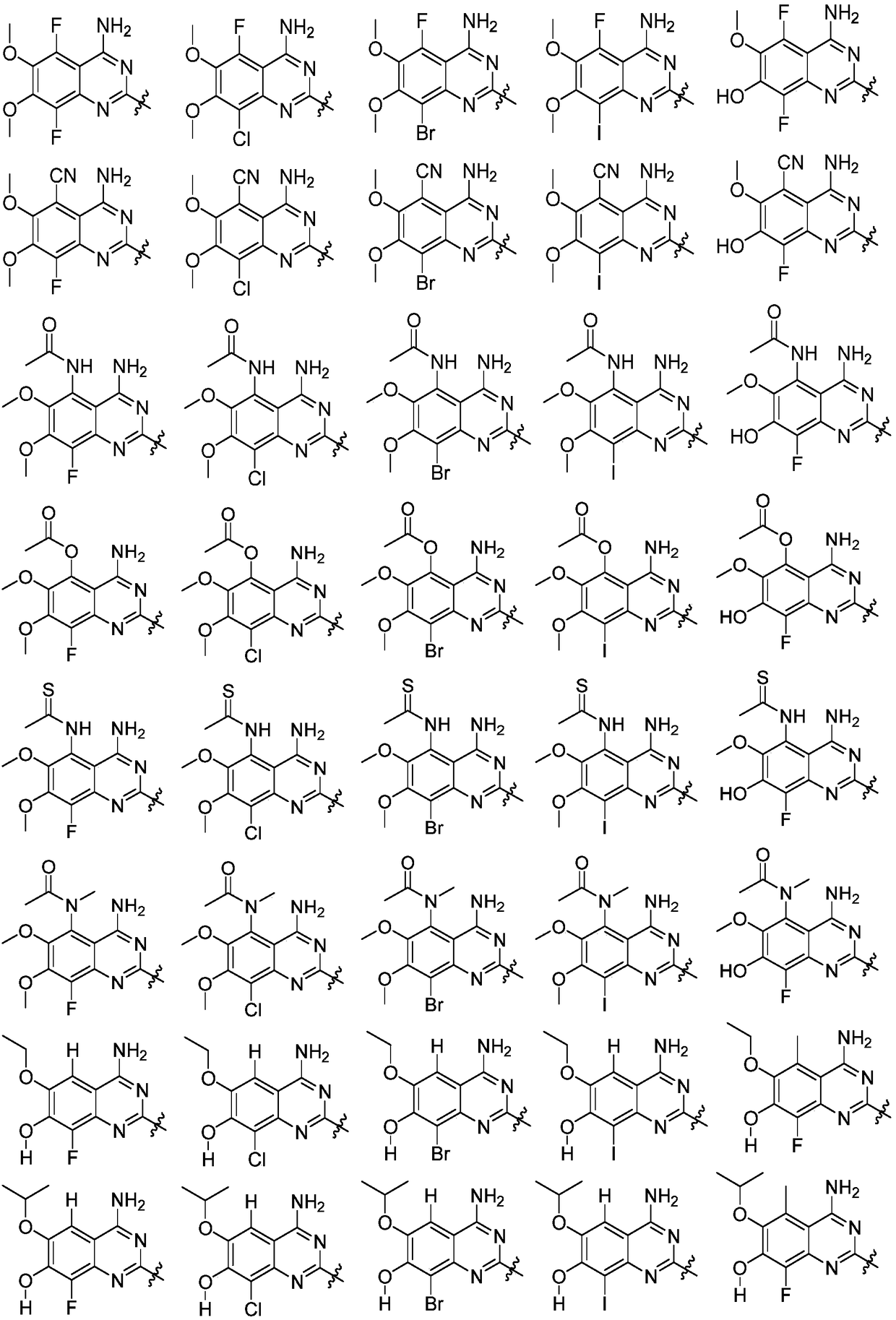
Quinazoline and indole compounds to treat medical disorders
A compound and pharmacy technology, applied in the fields of compounds, chemical instruments and methods, drug combinations, etc. of Group 5/15 elements of the periodic table, can solve the problem of no small molecule factor B inhibitors, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0576] The preparation method of the compound of the present invention
[0577] abbreviation
[0578] ACN acetonitrile
[0579] Acetyl
[0580] Ac 2 O acetic anhydride
[0581] AcOEt ethyl acetate
[0582] AcOH acetic acid
[0583] Boc 2 O di-tert-butyl dicarbonate
[0584] Butyl
[0585] CBz benzyloxycarbonyl
[0586] CH 3 OH, MeOH Methanol
[0587] DCM,CH 2 Cl 2 Dichloromethane
[0588] DMA N,N-Dimethylacetamide
[0589] DMAP 4-Dimethylaminopyridine
[0590] DMF N,N-Dimethylformamide
[0591] DMS Dimethyl Sulfide
[0592] DMSO Dimethyl Sulfoxide
[0593] Et ethyl
[0594] Et 3 N,TEA Triethylamine
[0595] EtOAc ethyl acetate
[0596] EtOH ethanol
[0597] HBTU N,N,N′,N′-tetramethyl-O-(1H-benzotriazol-1-yl)urea hexafluorophosphate
[0598] HCl hydrochloric acid
[0599] HMTA Hexamethylenetetramine
[0600] iBu, i-Bu, isoBu isobutyl
[0601] iPr, i-Pr, isoPr isopropyl
[0602] i PR 2 NEt N,N-Diisopropylethylamine
[0603] K 2 CO 3 potassium c...
Embodiment 1
[0659] Embodiment 1. General synthetic route
[0660] Scheme 1-1
[0661]
[0662] Scheme 1-1: Compounds of the present invention can be prepared from, for example, central core moieties. In Step 1, the central core is coupled with an appropriately substituted quinazoline known in the art to provide species of formula A-C. In step 2, species A-C are coupled with an appropriately substituted linker with the B moiety to provide a compound of formula I.
[0663] Scheme 1-2
[0664]
[0665] Scheme 1-2: Compounds of the invention can be prepared from eg protected piperazines. In Step 1, piperazine is coupled with an appropriately substituted quinazoline known in the art to provide species of formula A-C. In step 2, piperazine is deprotected as known in the art to provide the nucleophilic amine. In step 3, piperazine is subjected to appropriately substituted acid chlorides to provide compounds of formula I.
[0666]
[0667] Schemes 1-3: Compounds of the invention ca...
Embodiment 2
[0680] Embodiment 2. The synthesis of quinazoline (A) part
[0681] Scheme 2-1
[0682]
[0683] Scheme 2-1: Ring A of the present invention can be prepared from, for example, aniline. In step 1, an appropriately substituted aniline is converted to a urea as known in the art. In step 2, an appropriately substituted urea is cyclized by nucleophilic attack of the carbonyl moiety, followed by loss of R to provide a heterocycle. In step 3, an appropriately substituted heterocycle is arylated as known in the art to provide a halogenated quinazoline which can optionally be further modified in steps 4 and 5. In step 4, an appropriately substituted quinazoline is reduced as known in the art to the halogenated species, which can be used as described in Example 1. In step 5, the appropriately substituted dihalide is instead further derivatized as known in the art to provide the halogenated species, which can be used as described in Example 1.
[0684] Scheme 2-2
[0685]
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com