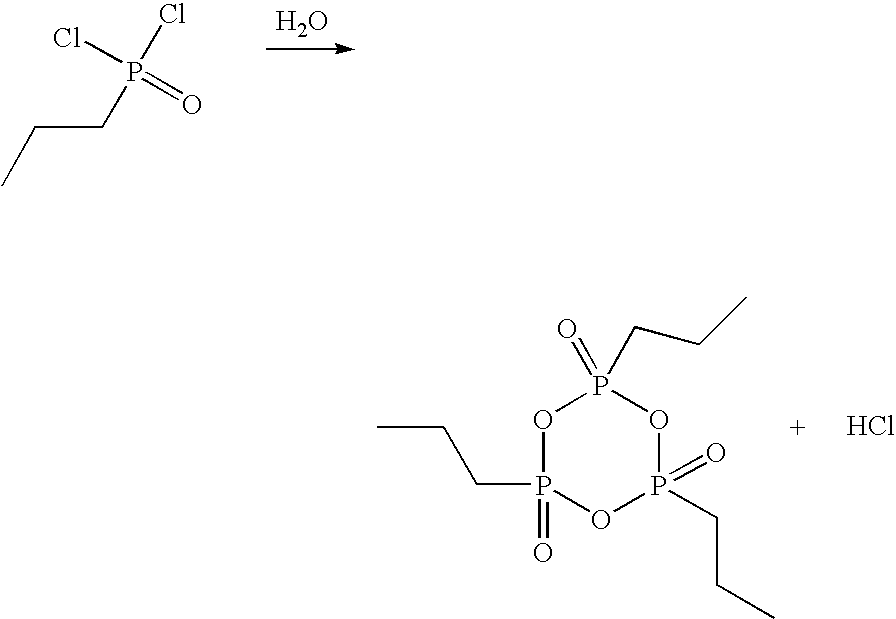
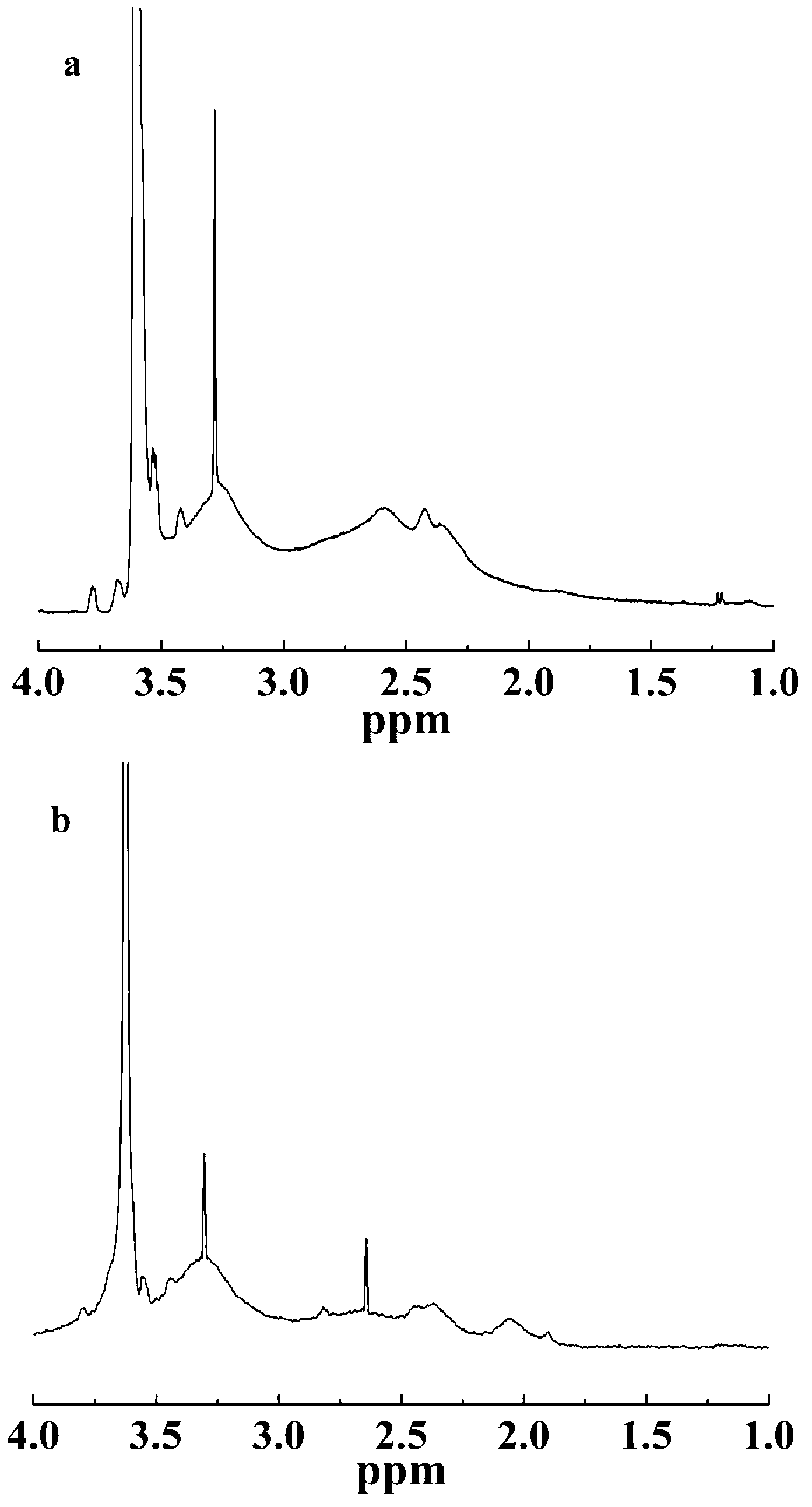
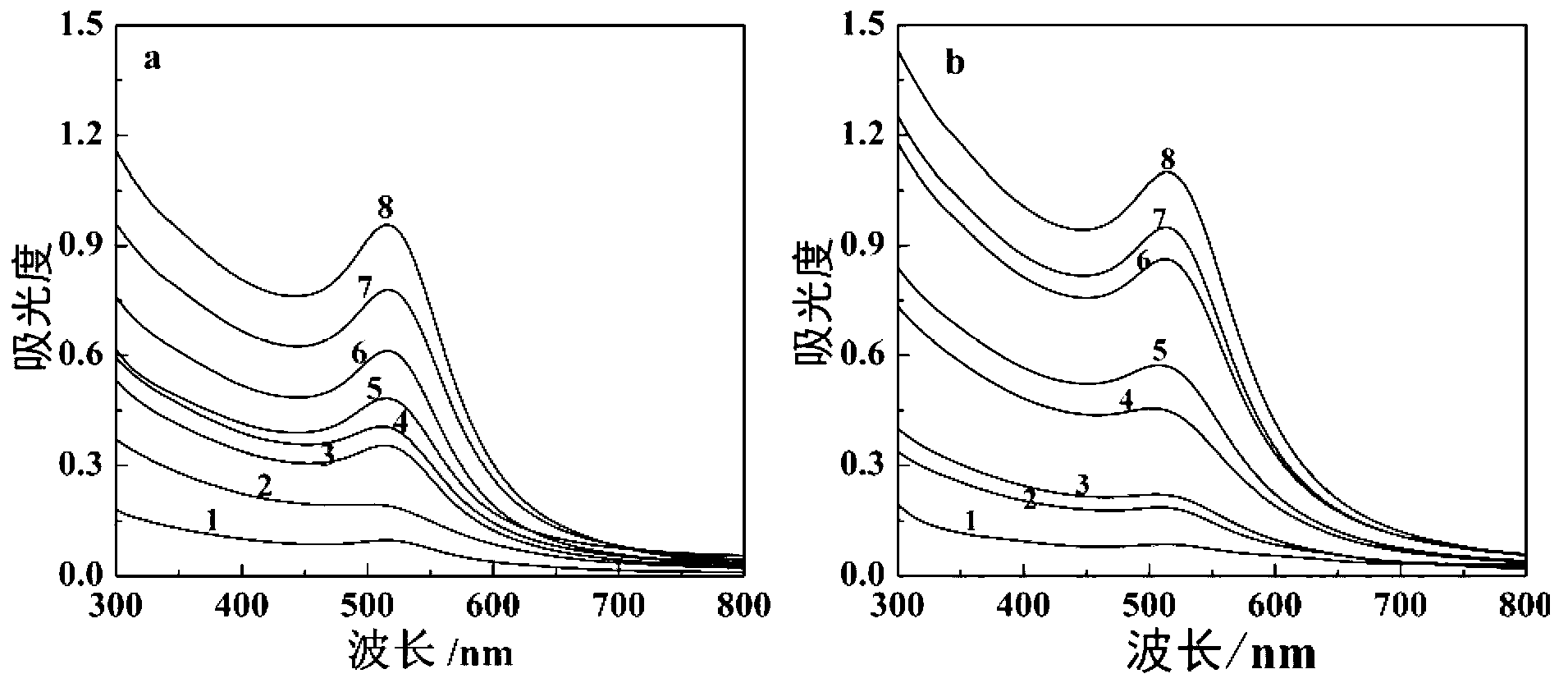
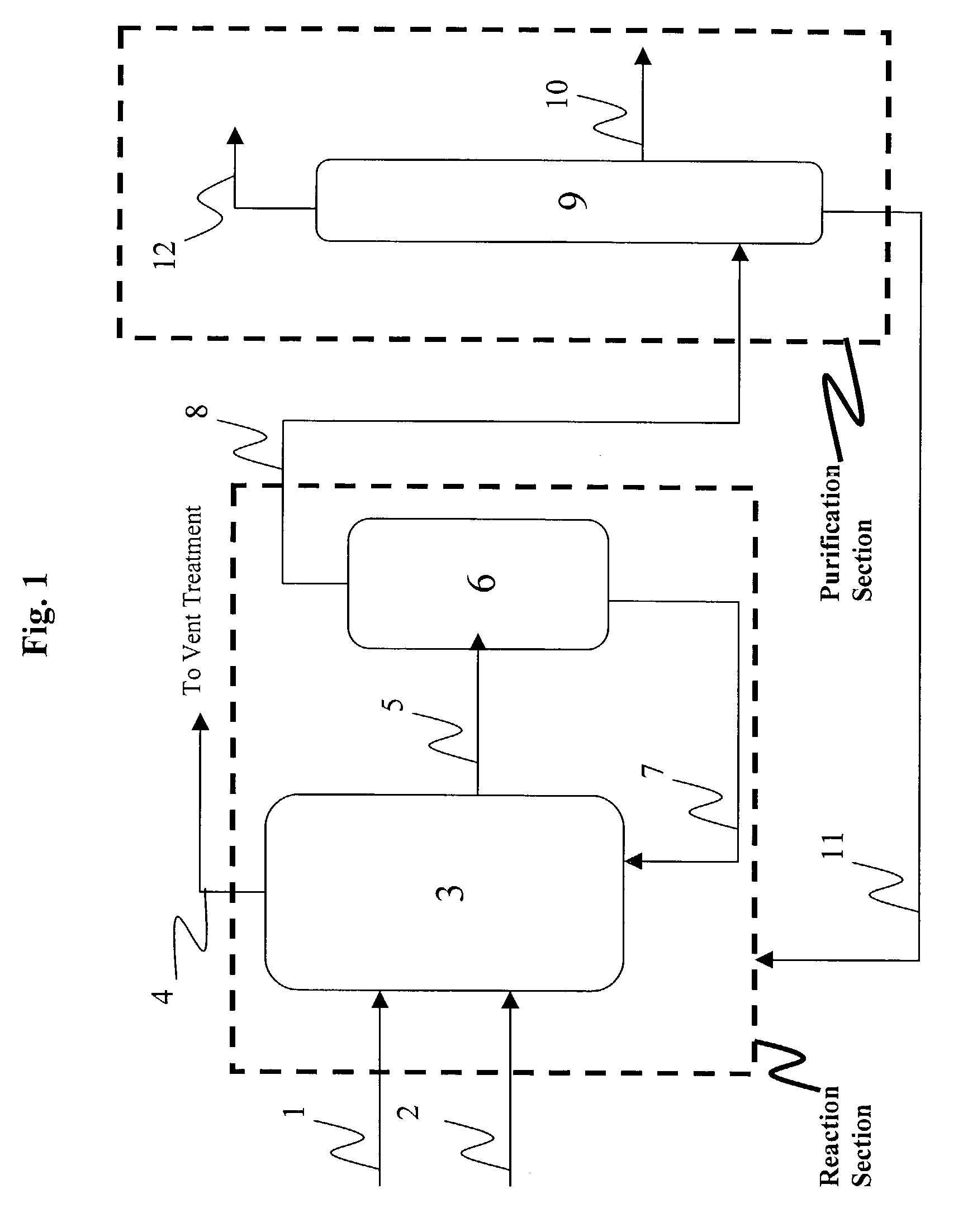
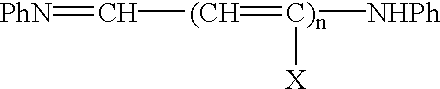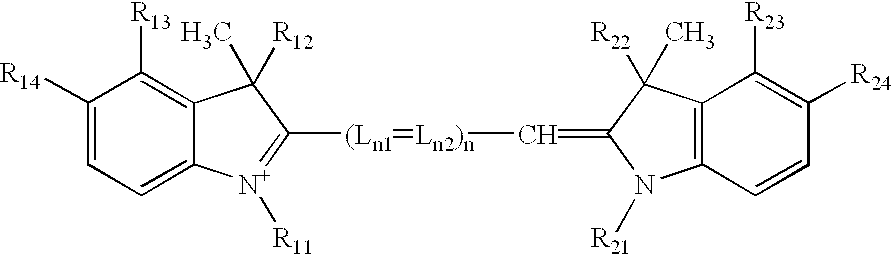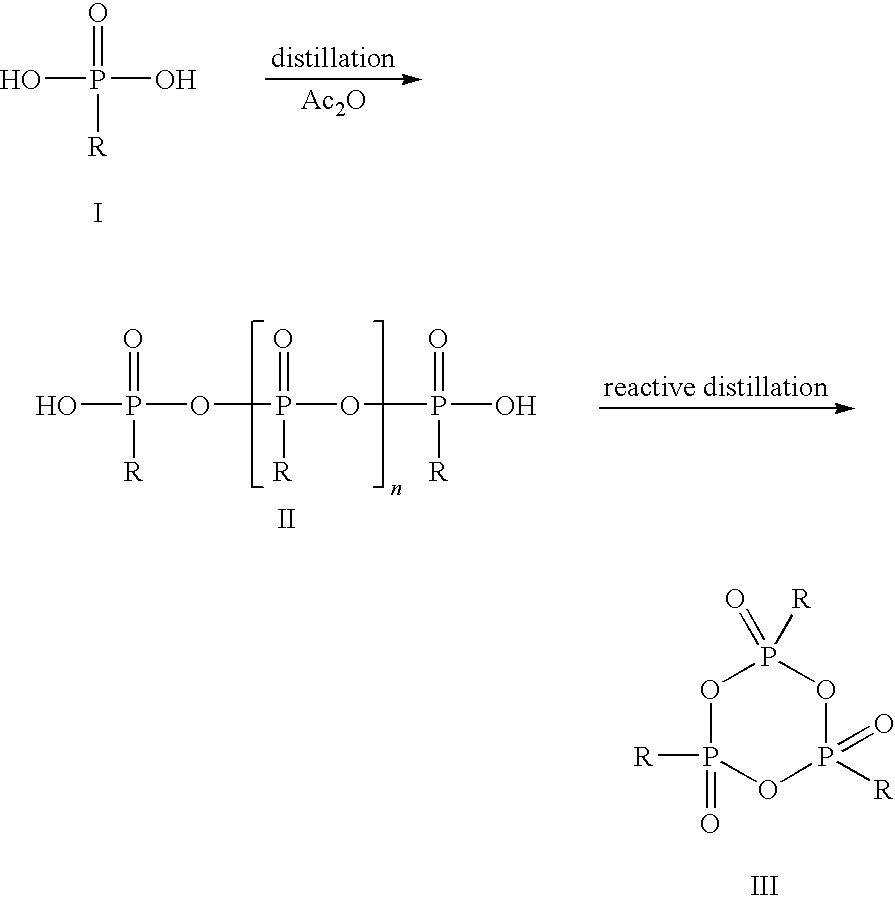Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
3218 results about "Acetic anhydride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula (CH₃CO)₂O. Commonly abbreviated Ac₂O, it is the simplest isolable anhydride of a carboxylic acid and is widely used as a reagent in organic synthesis. It is a colorless liquid that smells strongly of acetic acid, which is formed by its reaction with moisture in the air.
Low water methanol carbonylation process for high acetic acid production and for water balance control
ActiveUS7005541B2High acetic acid production rateIncrease chanceOrganic compound preparationCarboxylic preparation from carbon monoxide reactionWater methanolAcetic anhydride
The invention relates to a process for the production of acetic acid by carbonylation of methanol, and reactive derivatives thereof, in a reaction mixture using a rhodium-based catalyst in low water conditions. The process is used to achieve reaction rates of at least 15 g mol / l / hr. The high rate reactions proceed at water concentrations of less than 2.0 wt. %. Under certain conditions, the water concentration in the reaction mixture of the process is maintained at a desired concentration by at least one process step including adding a compound such as methyl acetate, dimethyl ether, acetic anhydride, or mixtures of these compounds to the reaction system. The process step of adding the components to the reaction mixture may be combined with other process steps for controlling water concentrations in reaction mixtures for the carbonylation of methanol.
Owner:CELANESE INT CORP
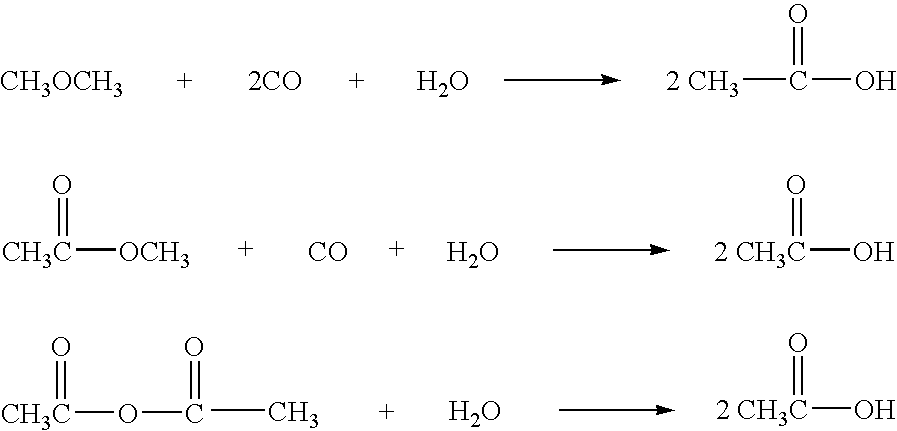
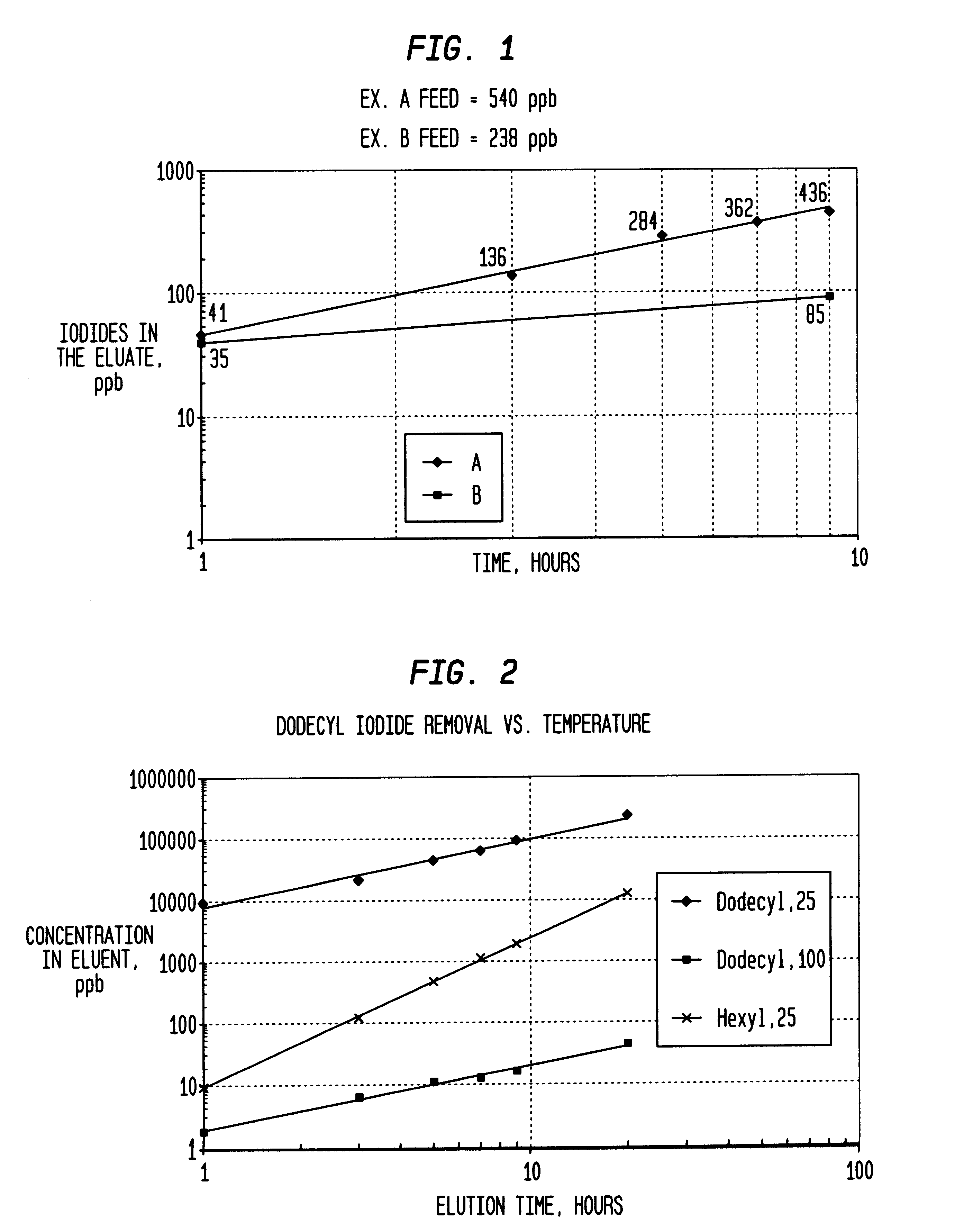
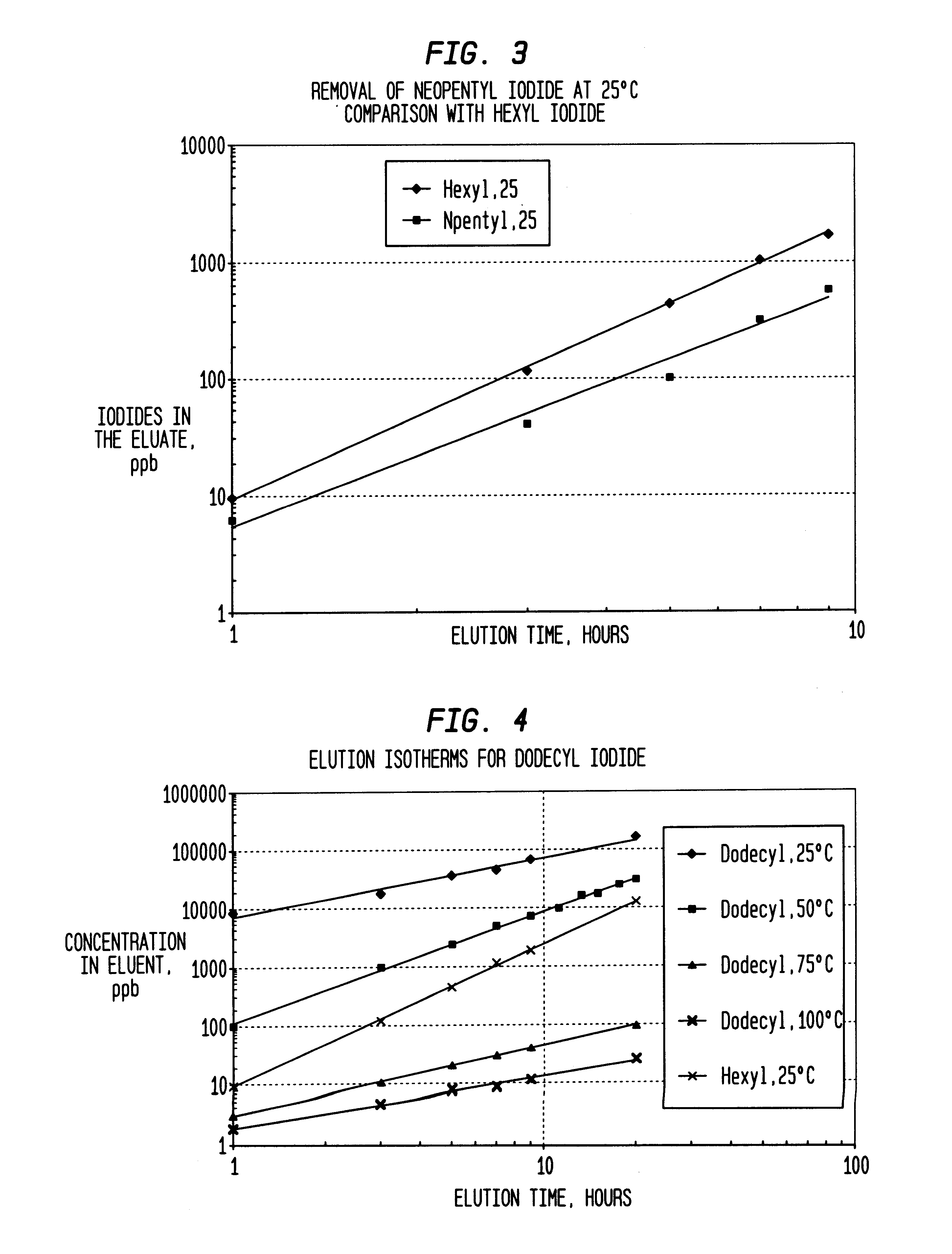
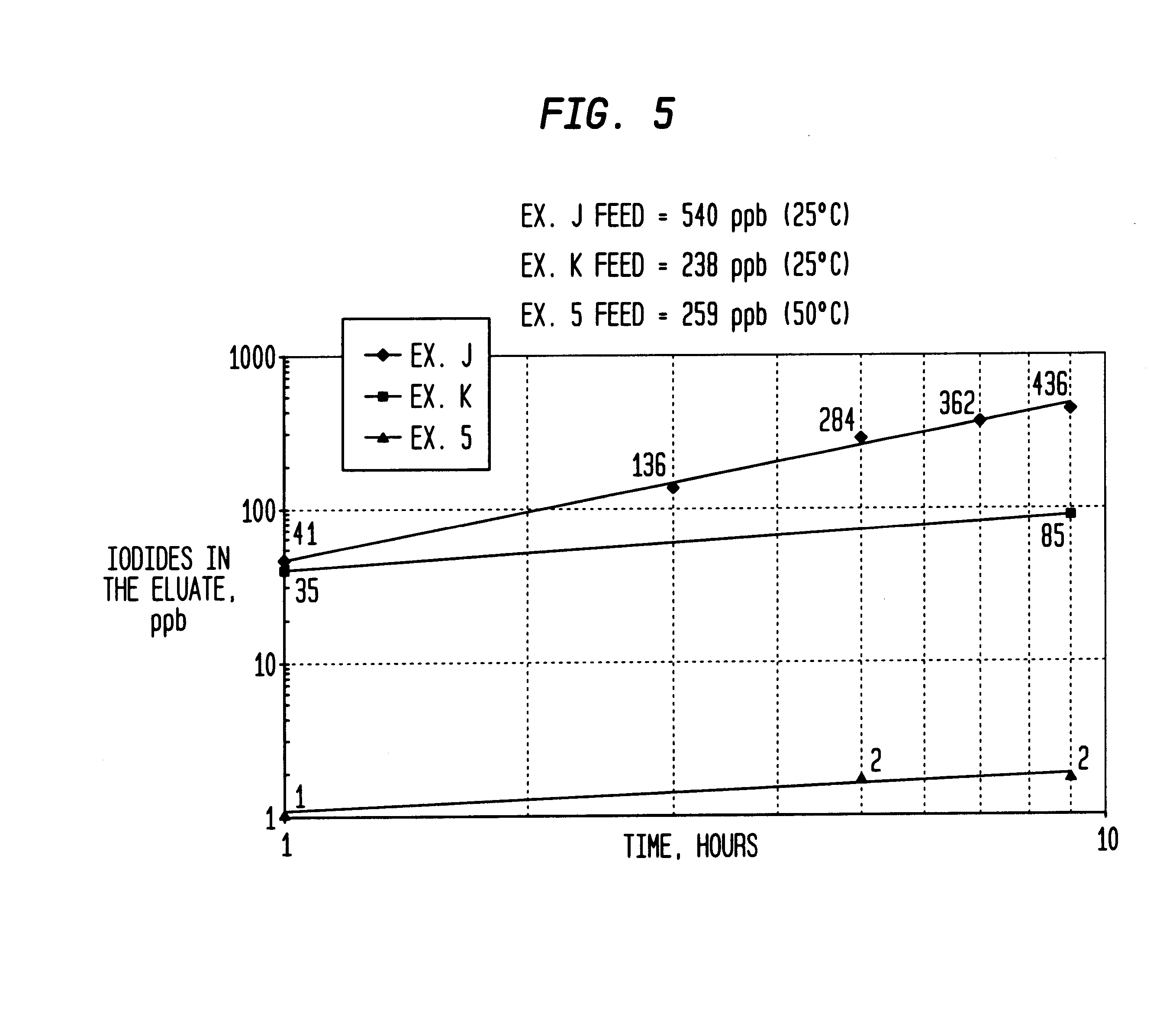
Method of removing organic iodides from organic media
InactiveUS6225498B1Efficient removalCation exchanger materialsOrganic compound preparationAcetic acidAcetic anhydride
Owner:CELANESE INT CORP
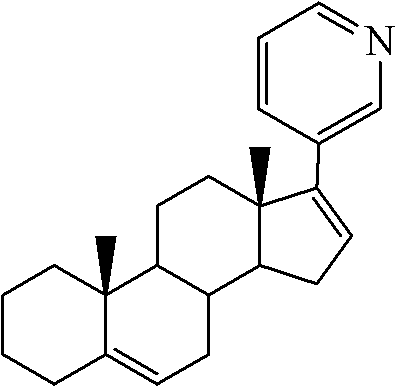
Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process
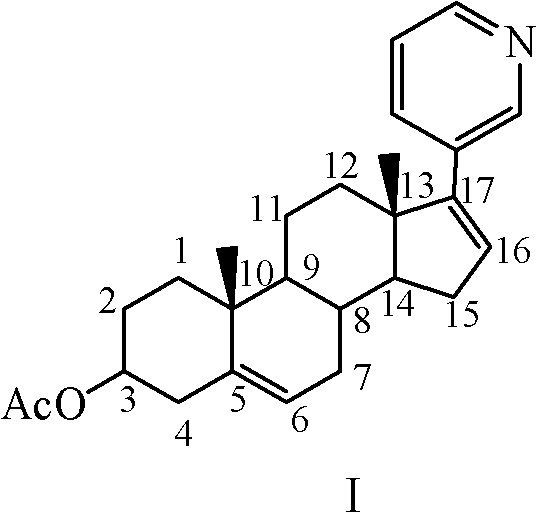
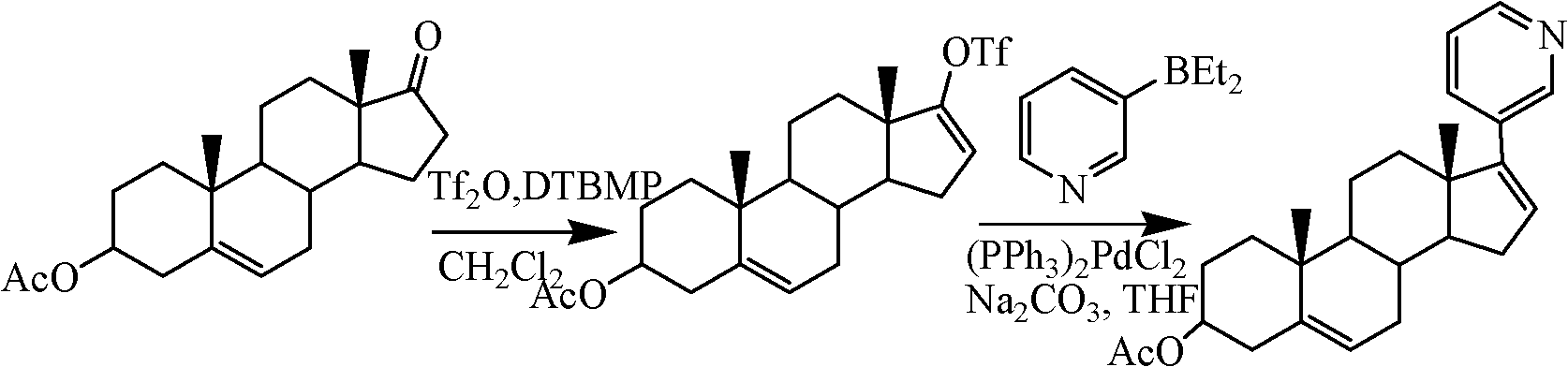
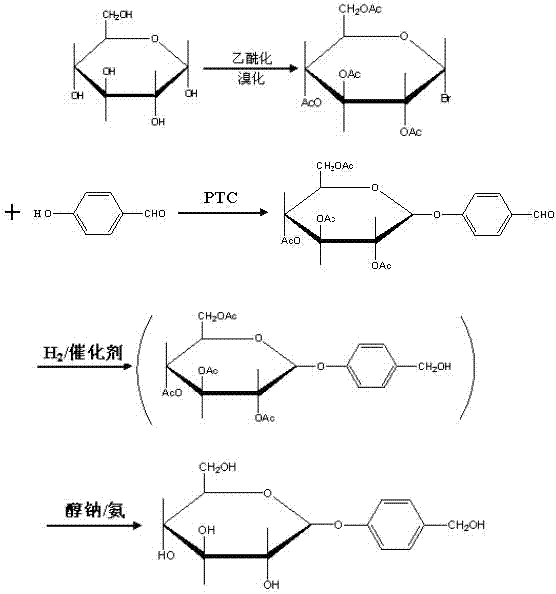
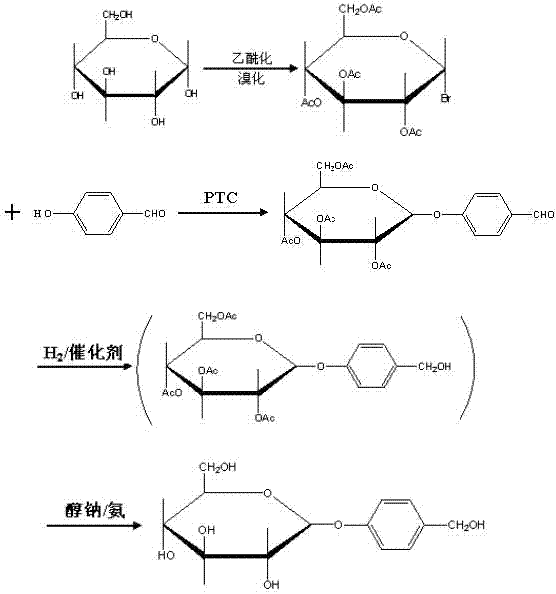
The present invention relates to a new industrial process for the synthesis of solvate- free 17a-acetoxy-11ss-[4-(N,N-dimethyl-amino)-phenyl]-19-norpregna-4,9-diene-3,20-dione [CDB -2914] of formula (I) which is a strong antiprogestogene and antiglucocorticoid agent. The invention also relates to compounds of formula (VII) and (VIII) used as intermediates in the process. The process according to the invention is the following: i) 3-(ethylene-dioxy)-estra-5(10),9(11)-diene-17-one of formula (X) is reacted with potassium acetilyde formed in situ in dry tetrahydrofuran by known method, ii) the obtained 3-(ethylene-dioxy)-17a-ethynyl-17ss-hydroxy-estra-5(10),9(11)-diene of formula (IX) is reacted with phenylsulfenyl chloride in dichloromethane in the presence of triethylamine and acetic acid, iii) the obtained isomeric mixture of 3-(ethylene-dioxy)-21-(phenyl-sulfinyl)-19-norpregna-5(10),9(11),17(20),20-tetraene of formula (VIII) is reacted first with sodium methoxide in methanol, then with trimethyl phosphite, iv) the obtained 3-(ethylene-dioxy)-17a-hydroxy-20-methoxy-19-norpregna-5(10),9(11),20-triene of formula (VII) is reacted with hydrogen chloride in methanol, then v) the obtained 3-(ethylene-dioxy)-17a-hydroxy-19-norpregna-5(10),9(11l); -diene-20- one of formula (VI) is reacted with ethylene glycol hi dichloromethane in the presence of trimethyl orthoformate and p-toluenesulfonic acid by known method, vi) the obtained 3,3,20,20-bis(ethylene-dioxy)-17a-hydroxy-19-norpregna- 5(10),9(11)-diene of formula (V) is reacted with hydrogen peroxide in a mixture of pyridine and dichloromethane in the presence of hexachloroacetone by known method, vii) the obtained 3,3,20,20-bis(ethylene-dioxy)-17a-hydroxy-5,10-epoxy-19-norpregn-9(11)-ene of formula (IV), containing approximately a 1:1 mixture of 5a,10a- and 5ss,10ss-epoxides, is isolated from the solution and reacted with a Grignard reagent obtained from 4-bromo-N,N-dimethyl-aniline in tetrahydrofuran.
Owner:RICHTER GEDEON NYRT
Process and method for the preparation of asymmetric monofunctionalized indocyanine labelling reagents and obtained compounds
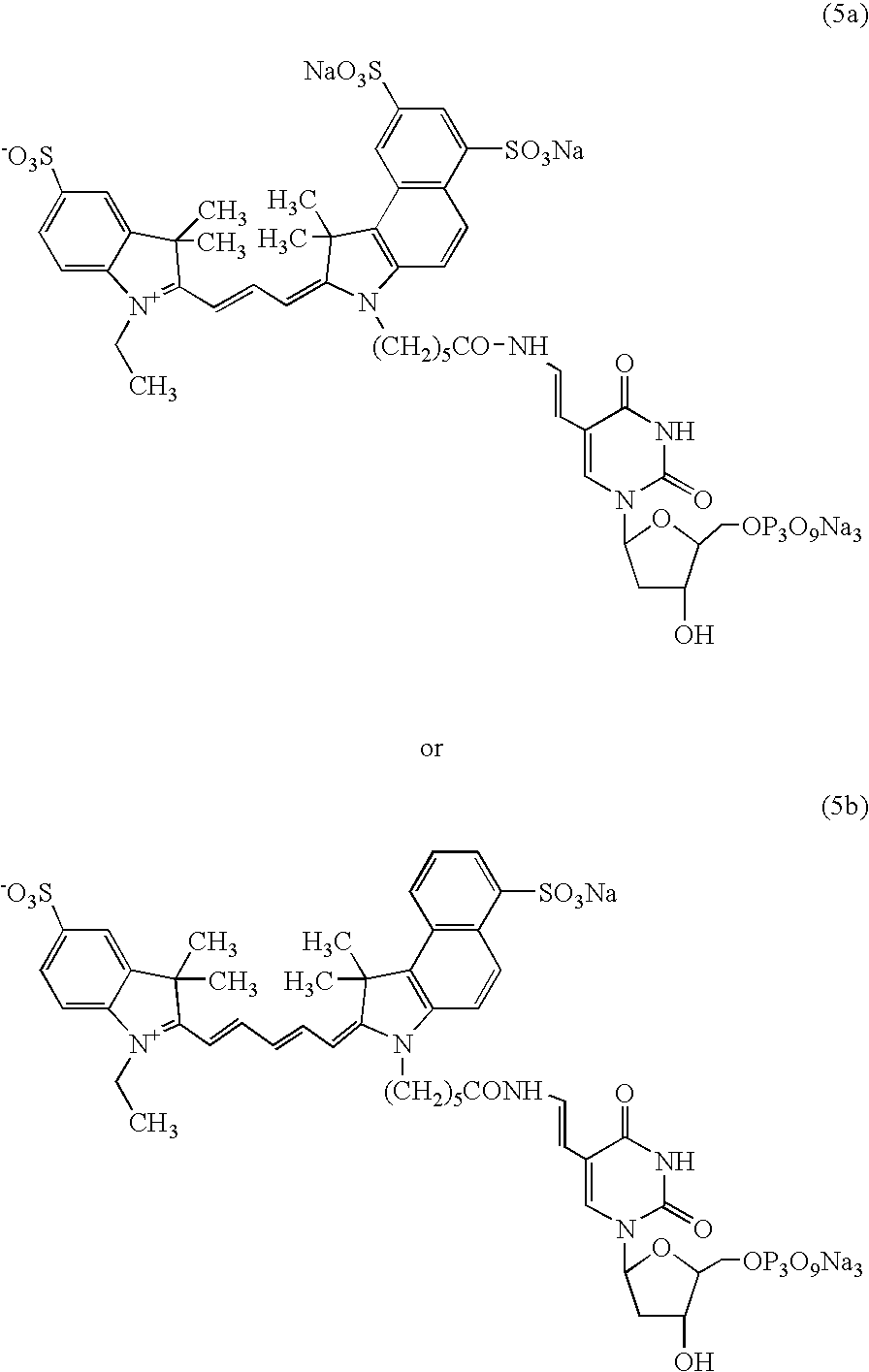
A process for preparing an asymmetrical indocyanine dye comprising the steps of:a) reacting a first quaternised indolenine or substituted indolenine with a compound of the formula (II)or hydrochloride thereof, wherein n is 0 or 1 Ph is phenyl or substituted phenyl X is hydrogen, halogen or alkyl, preferably chlorine, in a solvent selected from the group consisting of acetic acid, acetic anhydride and mixtures thereof in the presence of acetyl chloride, to obtain an intermediate hemicyanine, andb) further reacting said intermediate hemicyanine with a second quaternised indolenine or substituted indolenine different from said first indolenine.
Owner:VISEN MEDICAL INC
Modifying method for wood elements
InactiveUS6632326B1Good dimensional stabilityReduce processing timeCellulosic pulp after-treatmentLiquid surface applicatorsManufacturing cost reductionAcetic acid
The present invention's modifying method for wood elements includes a step for soaking wood elements in one or a mixture of acetic anhydride, acetic acid, or chloroacetic acid; and a step for acetylating the impregnated wood elements in a gaseous phase. This method makes it possible to reduce the time required for the step of acetylating the wood elements, simplifies the process, reduces fabrication costs, and enables fabrication of a wood fiberboard having high dimensional stability.
Owner:YAMAHA CORP
Hydrogenated pyridine derivative and method for preparing salt thereof
InactiveCN101177430AMild reaction conditionsEasy to scale up productionOrganic active ingredientsOrganic chemistryPyridiniumHalogen
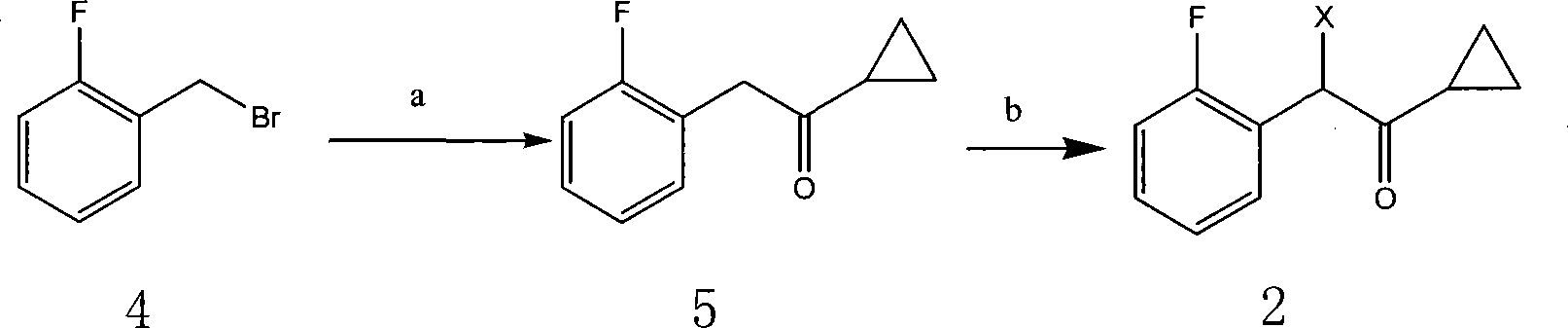
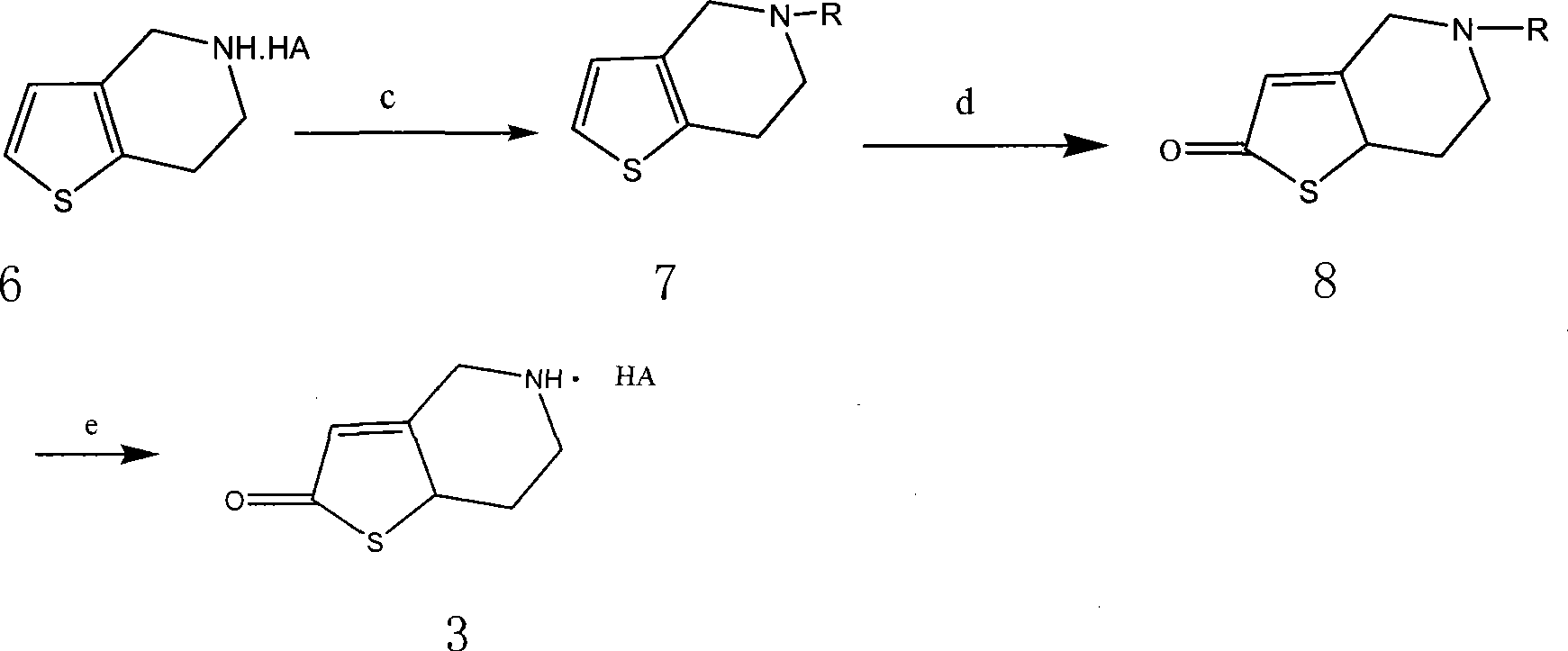
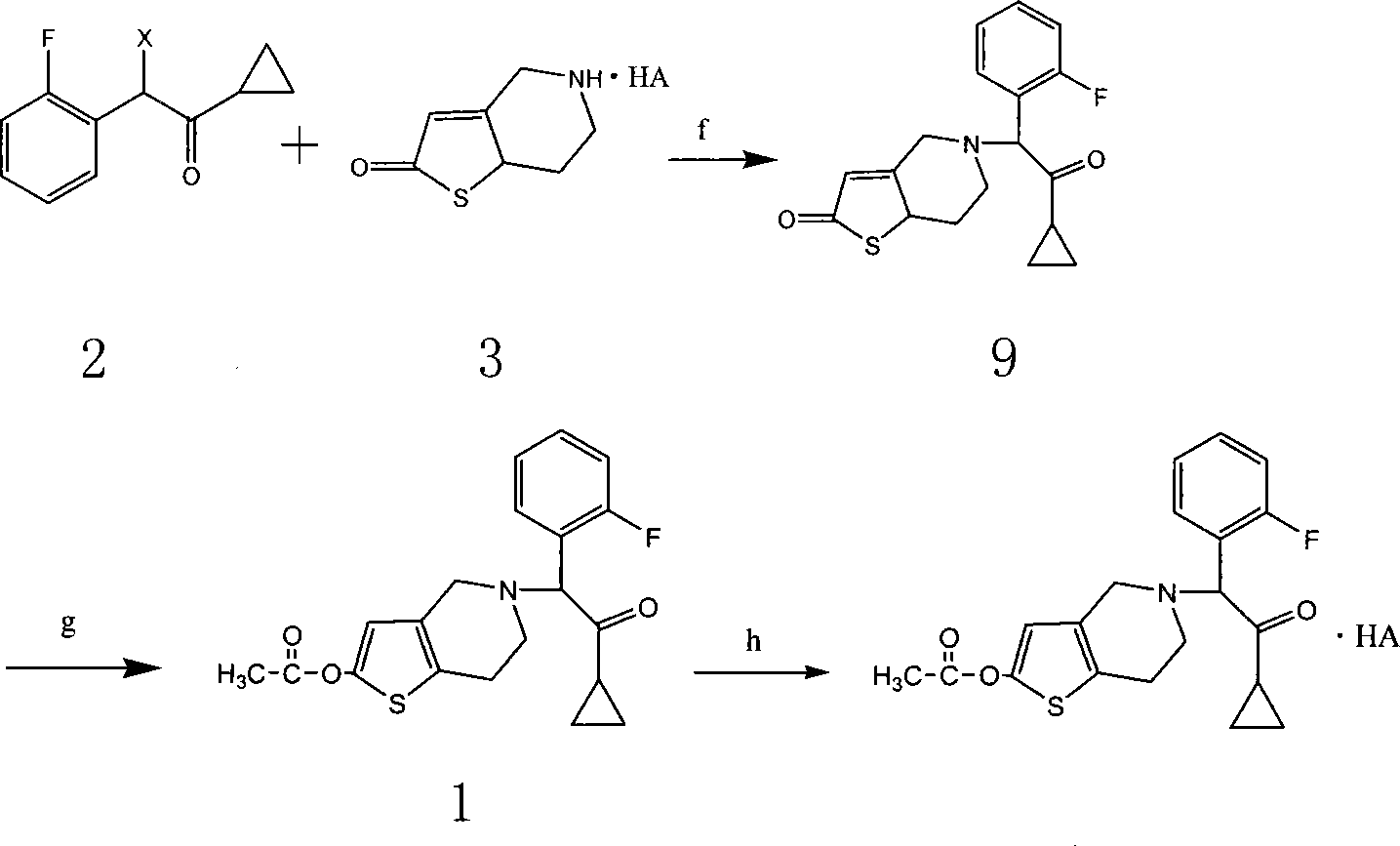
The invention relates to the hydropyridine derivatives of 2-acetoxy-5- (Alpha-cyclopropyl carbonyl-2-fluorobenzyl) -4, 5, 6, 7-tetrahydrothiophene plus [3, 2-C] pyridine and salts of the hydropyridine derivative as well as the preparation method. The invention comprises following steps: preparing two main intermediates: Alpha-cyclopropyl carbonyl-2 fluorobenzyl halogen 2 (wherein, X=F, CL, Br, I) and 2-oxygen-2, 4, 5, 6, 7, 7 Alpha-hexahydrothiophene plus [3, 2-C] pyridinium salt 3 (wherein, HA=HCL, H2SO4, HBr, HI); esterifying the product obtained through condension of the two main intermediates using acetic anhydride to get target product. The target product and the needed acid are combined to be salts via addition reaction; during the process of crystallization, necessary crystal seed is added to achieve single-morphic crystals.
Owner:LUNAN PHARMA GROUP CORPORATION
Preparation method of abiraterone acetate
ActiveCN102627681AReduce usageAvoid separation and purificationSteroidsAcetic anhydrideEthyl Chloride
The invention relates to a preparation method of abiraterone acetate. The method comprises: taking dehydroepiandrosterone as the raw material, which successively reacts with hydrazine hydrate and idoine so as to obtain 17-iodo-adrost-5, 16-diene-3beta-ol, which then undergoes a Negishi coupling reaction with 3-pyridine zinc halide to obtain abiraterone, and finally conducting esterification with acetyl chloride or acetic anhydride, thus obtaining the target product abiraterone acetate.
Owner:SHANDONG NEWTIME PHARMA
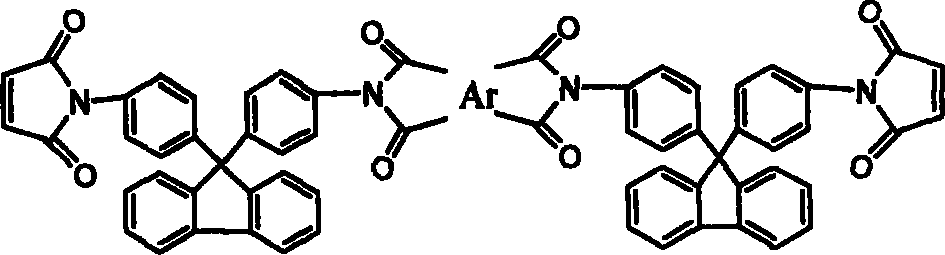
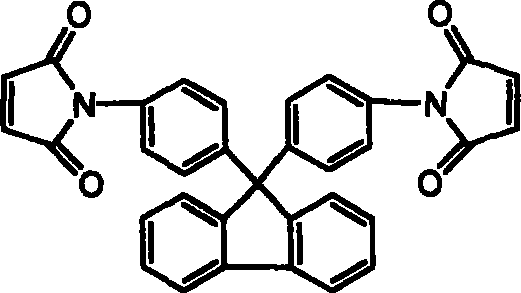
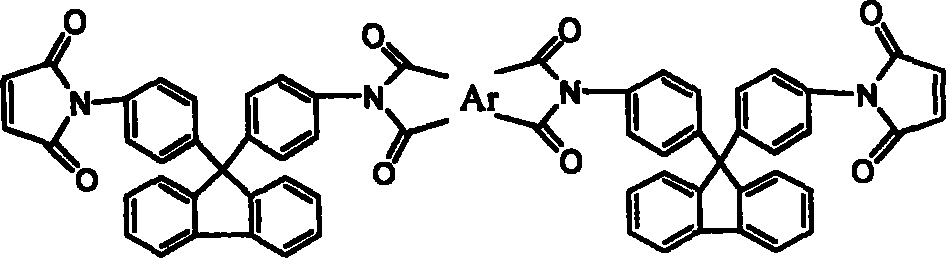
Chain-prolonged type fluorenyl bimaleimide and its preparation method
The invention discloses a making method of new chain-extending typed fluorenyl dimaleimide, which comprises the following steps: dissolving 9, 9-di(4-amino phenyl) fluorene into N, N-dimethyl acetamide; stirring; dripping maleic anhydride of N, N-dimethyl acetamide with the same molar weight to react for 2h; adding dianhydride directly with the same molar weight; stirring 3h; adding the compound of acetic anhydride and potassium acetate; stirring under indoor temperature to react for 4h; sedimenting in the water; obtaining the product with receiving rate over 95%.
Owner:FUDAN UNIV
Process for the preparation of dronedarone
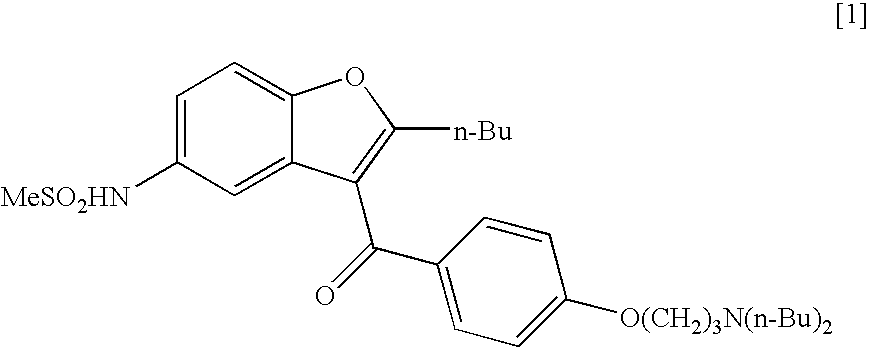
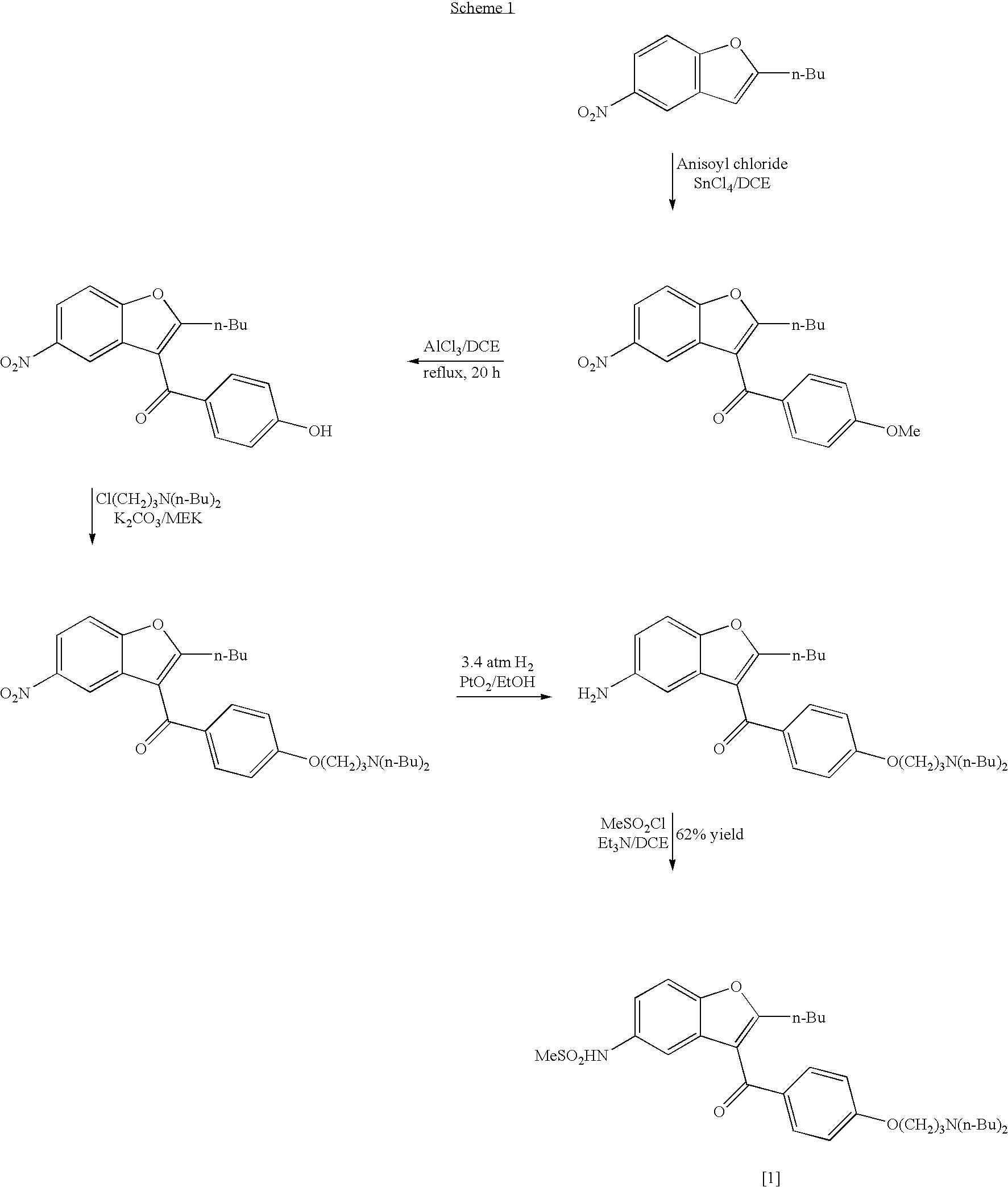
InactiveUS20050049302A1High yieldLow costBiocideCarbamic acid derivatives preparationAluminium chlorideDronedarone
The present invention provides, according to an aspect thereof, a novel process for the preparation of dronedarone [1] and pharmaceutically acceptable salts thereof. According to a preferred embodiment, the process comprises N-acetylating of p-anisidine or p-phenetidine with acetic anhydride, reacting of the obtained N-(4-alkoxyphenyl)acetamide with 2-bromohexanoyl chloride or bromide in the presence of aluminum chloride or bromide to obtain N-[3-(2-bromohexanoyl)-4-hydroxyphenyl]acetamide [6a], converting the compound [6a] into 2-butyl-5-benzofuranamine hydrochloride [12a] and subsequently converting [12a] into [1] or pharmaceutically acceptable salts thereof. In accordance with another aspect of this invention, there are provided novel intermediates, inter alia the novel compounds [6a] and [12a]. The novel intermediates of the present invention are stable, solid compounds, obtainable in high yields, which can be easily purified by crystallization and stored for long periods of time.
Owner:ISP INVESTMENTS LLC
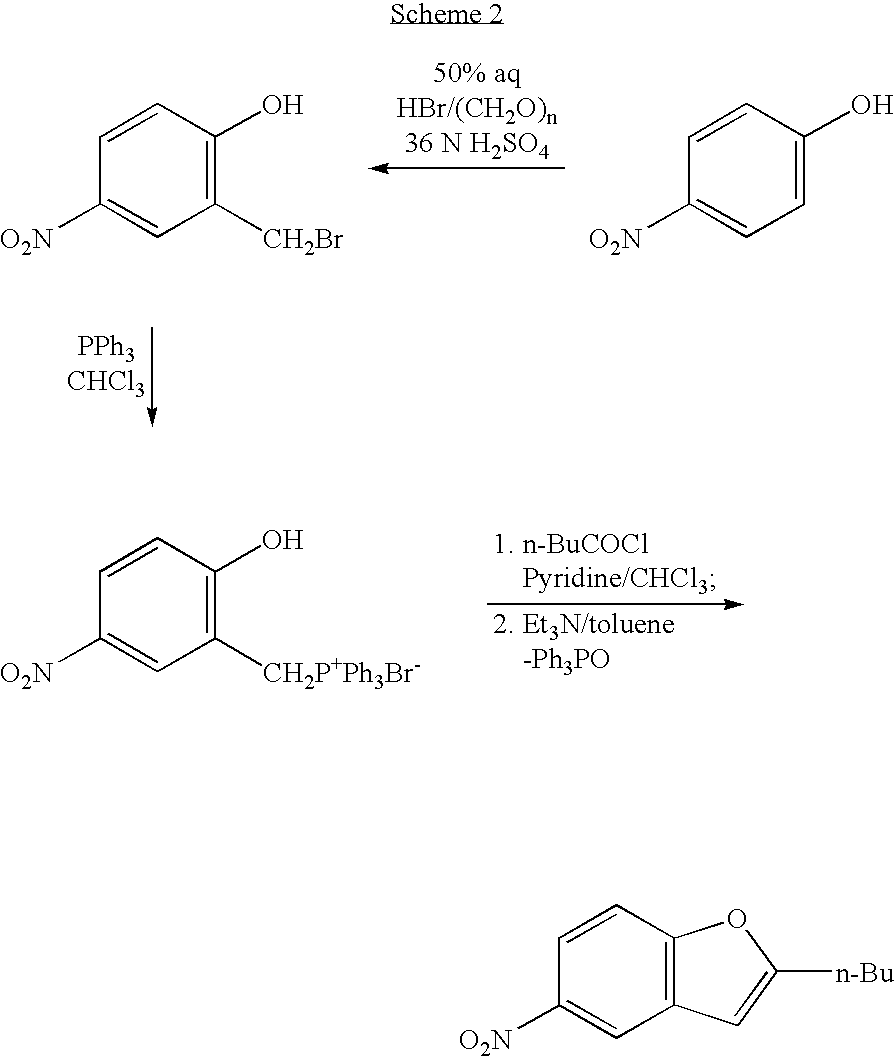
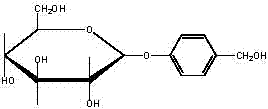
Gastrodin synthesizing method
InactiveCN102516329AReduce pollutionSuitable for industrial productionSugar derivativesSugar derivatives preparationKetone solventsPressure reduction
The invention relates to a gastrodin synthesizing method, which can effectively solve the preparation problem of gastrodin to meet the requirements of the gastrodin in pharmaceuticals. The method comprises the steps of adding catalyst perchloric acid, acetylating anhydrous dextrose by using acetic anhydride to produce per-acetyl dextrose, feeding hydrogen bromide to bromizing hemiacetal hydroxyl of the per-acetyl dextrose to produce bromo-tetraacethyl glucose, further and dropwise adding a bromo-tetraacethyl glucose solution into chloroform and tetrabutyl ammonium bromide, carbonate and para hydroxybenzene in water to obtain 4-formyl benzene-2', 3', 4', 6'- tetraacetyl-beta-D-glucopyranose, performing re-crystallization with ethanol, adding raney nickel or palladium and carbon, feeding hydrogen and pressurizing to hydrogenate, performing filtering, adding sodium alcoholate or ammonia in to filtrate to perform protecting group removal until the reaction is finished completely, performing pressure reduction and concentration to obtain crude gastrodin, and re-crystallizing the crude gastrodin by using alcohol or an alcohol and ester solvent or an alcohol and ketone solvent to obtain the gastrodin. The gastrodin synthesizing method is abundant and cheap in raw materials, simple in process, recycled in solvent, small in pollution and high in quality.
Owner:SHANGHAI MODERN HASEN SHANGQIU PHARMA
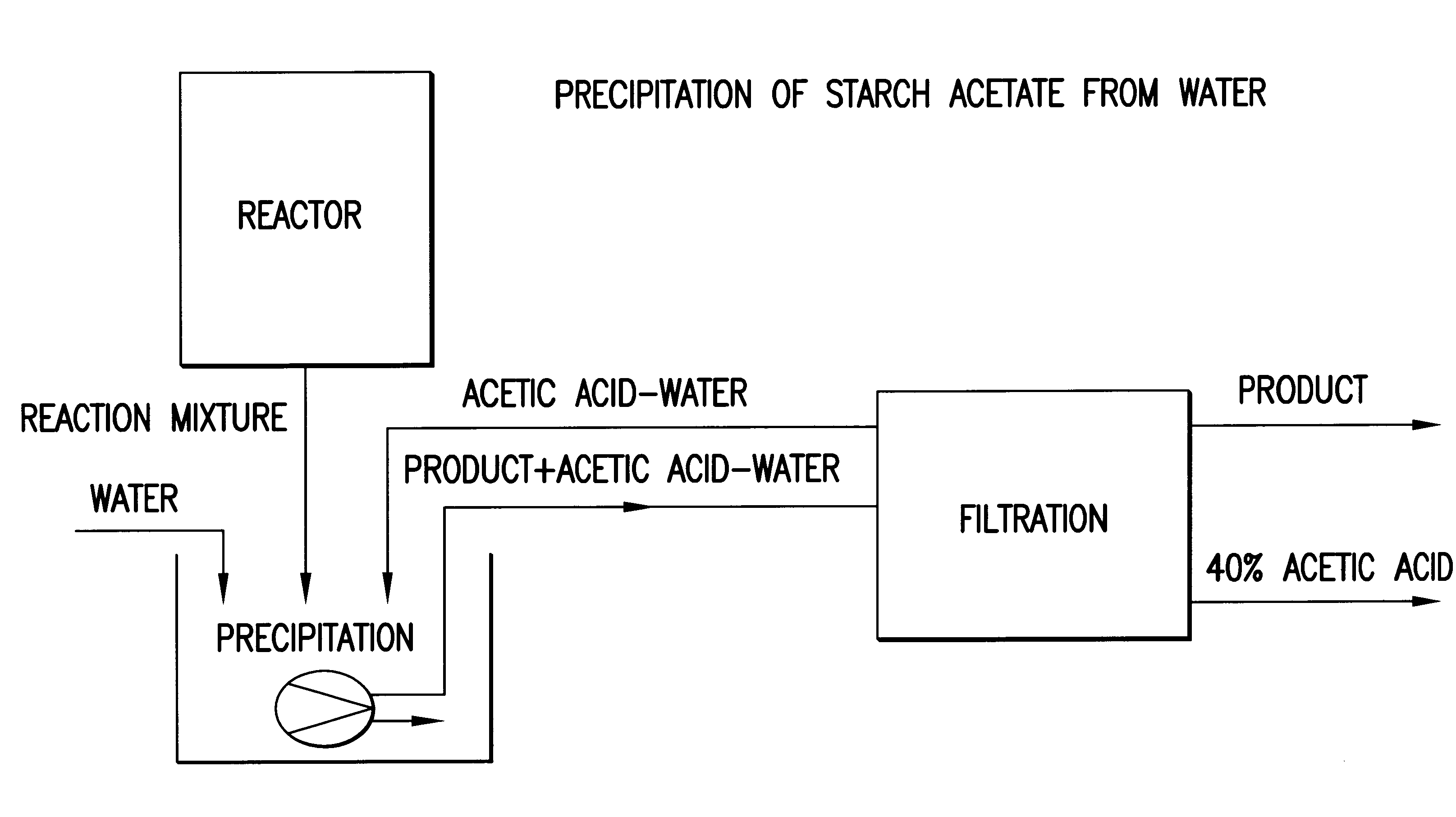
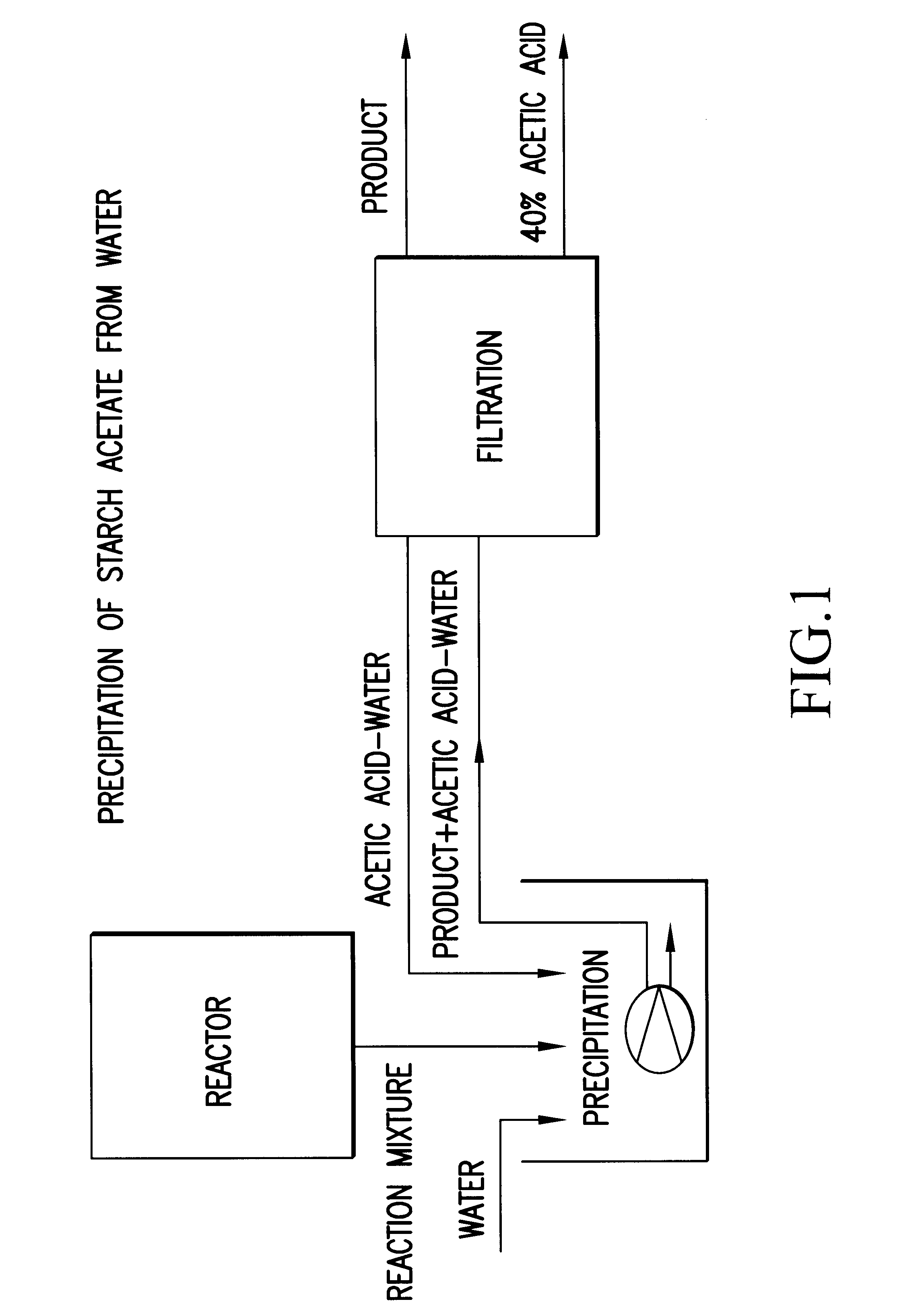
Process for the preparation of a starch ester
InactiveUS6605715B1Easy to controlDelayed reaction timeEsterified saccharide compoundsSugar derivativesSodium acetateAcetic acid
Process for the preparation of a starch ester, in particular a starch acetate, wherein a starch-based feedstock is reacted with an organic carboxylic acid anhydride in the presence of a catalyst. The reaction of the starch-based feedstock and the anhydride is performed at an excess pressure of about 0.1 to 50 bar in an essentially anhydrous medium containing 10% by weight of water at the most. As catalyst, sodium acetate, sodium hydroxide or sulphuric acid is used, and as the reaction medium, acetic acid or excess acetanhydride is used. By means of the invention, the duration of the esterification reaction can be significantly shortened, the separation of the product can be facilitated, and the recirculation of the waste solutions can be rendered more efficient.
Owner:VALTION TEKNILLINEN TUTKIMUSKESKUS
Preparation method of thermotropic liquid crystal polyarylester fiber
InactiveCN104389045AEasy to operateReaction condition controlArtificial filament heat treatmentMonocomponent polyesters artificial filamentFiberAcetic anhydride
The invention discloses a preparation method of a thermotropic liquid crystal polyarylester fiber, relates to a preparation method of thermotropic liquid crystal polyarylester, and aims to solve the problems that the existing method for preparing the thermotropic liquid crystal polyarylester is complex and the polyarylester fiber is low in tensile strength. The preparation method of the thermotropic liquid crystal polyarylester fiber comprises the following steps: step one, adding p-hydroxybenzonic acid, 2-(4-hydroxyphenyl)-5-carboxylbenzimidazole, acetic anhydride, 4-diethylaminopyridine and an antioxidant into a polymerization kettle for carrying out melt condensation polymerization to prepare a pre-polymer of thermotropic liquid crystal polyarylester; step two, putting the pre-polymer in a rotary kiln under the condition with nitrogen protection for carrying out solid phase polycondensation reaction to obtain high-molecular-weight polymer powder; step three, mixing the high-molecular-weight polymer powder, then cooling and drawing to prepare primary polyarylester fiber; and step four, carrying out heat treatment on the primary polyarylester fiber. The preparation method of the thermotropic liquid crystal polyarylester fiber is simple to operate; the tensile strength of the polyarylester fiber which is finally obtained can reach 4.0-4.5GPa.
Owner:HEILONGJIANG JUXIANG TECH DEV CO LTD
Method for extracting ketene dimer by rectifying residues and pumped liquid
The invention relates to a method for extracting ketene dimer by rectifying residues and pumped liquid. The method comprises the following steps that firstly, hydrolysis is carried out, wherein primary residues and the pumped liquid are mixed in the proportion of 2:1 and heated to 65-75 DEG C through a membrane type evaporator, ketene dimer is evaporated out, crude ketene dimer obtained through cooling of a primary condenser and a secondary condenser enters a high-order storage tank and is mixed with crude ketene dimer generated in a polymerization section, and then mixed crude ketene dimer enters a rectifying section; secondly, secondary residues formed in the membrane type evaporator and acetic anhydride in the condensers enter a hydrolysis section for hydrolysis, and acetone, dilute acetic acid and solid waste residues are obtained; thirdly, tail gas at the hydrolysis section is fed to a water scrubber to be absorbed. The method has the advantages that crude ketene dimer is recycled through heating of the membrane evaporator, the yield of crude ketene dimer is increased inside the system, consumption of glacial acetic acid is reduced, waste gas and residues generated in the production process are digested and absorbed, solid waste is greatly reduced, and zero waste water emission is achieved.
Owner:ANHUI JINGHE IND
Method for preparing water soluble chitosan
InactiveCN1554267AHigh yieldLow costSugar derivativesSugar derivatives preparationAcetic anhydrideWater soluble chitosan
The process of preparing water soluble chitosan includes adding chitosan into 2-8 wt% concentration acetic acid aqua to compound acetic acid solution of chitosan of 2-10 wt% concentration, adding enzyme or oxidant to degrade chitosan to polymerization degree below 1000, cooling the solution to 0-10 deg.c, adding ethanol or methanol solution of acetic anhydride, stirring first at normal temperature for 2-24 hr and then at 20-40 deg.c for another 20-40 deg.c, adding alkali to regulate pH to over 8 under stirring, adding ethanol to deposit, centrifugation to obtain solid product, soaking in alcohol solution of KOH, centrifugation, alcohol washing, and drying to obtain water soluble chitosan of acetyl degree of 45-55%. The present invention has simple preparation process, and the product is used as material for tumor resisting and immunity raising medicine and health product and as additive for cosmetics and food.
Owner:孝感学院
Novel process for the preparation of linezolid and related compounds
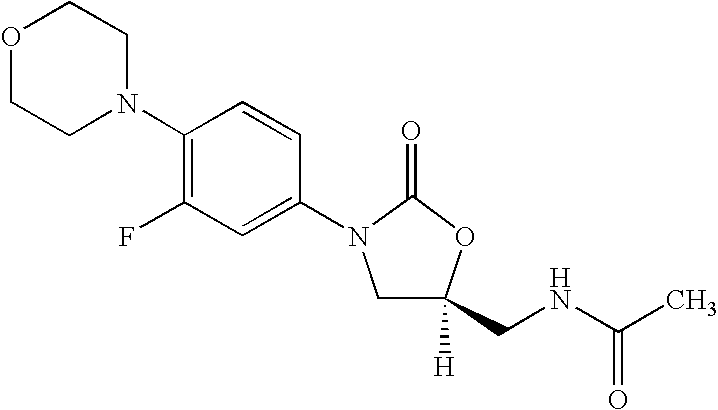
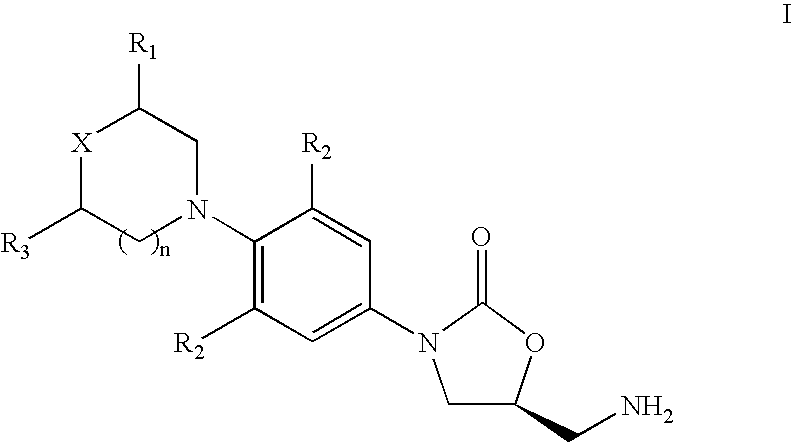
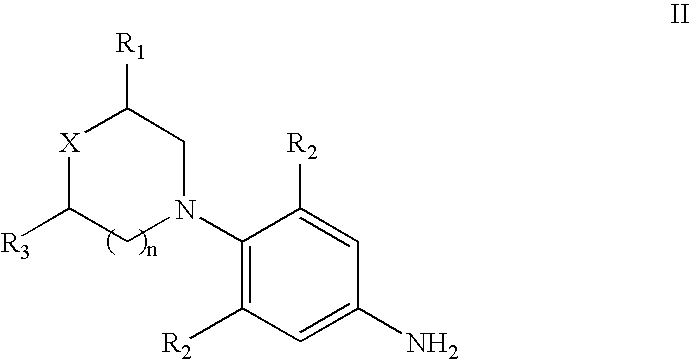
The present invention provides a novel process for preparation of 5-aminomethyl substituted oxazolidinones, key intermediates for oxazolidinone antibacterials including linezolid. Thus linezolid is prepared by a) reacting 3-fluoro-4-morpholinyl aniline with R-epichlorohydrin; b) subjecting N-[3-Chloro-2-(R)-hydroxypropyl]-3-fluoro-4-morpholinyl aniline produced above to carbonylation; c) reacting (5R)-5-(chloromethyl)-3-[3-fluoro-4-(4-morpholinyl)phenyl]-2-oxazolidinone produced above with potassium phthalinide; d) reacting (S)-N-[[3-[3-Fluoro-4-[4-morpholinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]phthalimide produced above with hydrazine hydrate; and e) reacting S-N-[[3-[3-Fluoro-4-[4-morpholinyl]phenyl]-2-oxo-5-oxazo-lidinyl]methyl]amine produced above with acetic anhydride to produce linezolid.
Owner:HETERO USA INC
Solid-phase synthetic process for degarelix
ActiveCN102329373AAdvantages of solid phase synthesis processReduce usagePeptide preparation methodsBulk chemical productionAcetic anhydrideSide chain
The invention relates to a solid-phase synthetic method for degarelix. The method comprises the following steps of: 1) reacting resin with Fmoc-D-Ala-OH to obtain Fmoc-D-Ala-resin, wherein the resin is amino resin; 2) sequentially connecting according to the amino acid sequence of the degarelix by adopting an Fmoc solid-phase synthetic strategy; 3) removing Fmoc from a N terminal, and acetylating by using acetic anhydride and pyridine; 4) removing a protective group X on the 6th amino acid residue -4Aph(X) from a C terminal; 5) connecting L-4,5-dihydrooroticacid to a side-chain amino group of the 6th amino acid residue -4Aph at the C terminal; 6) cutting peptide resin by using a cracking reagent, precipitating by using anhydrous ether, and centrifuging to obtain crude peptide; and 7) purifying and separating to obtain the degarelix. The method is easy to operate and slightly damages human bodies and environments; and by the process, the content of impurities is effectively reduced, and the large-scale production can be performed.
Owner:HYBIO PHARMA
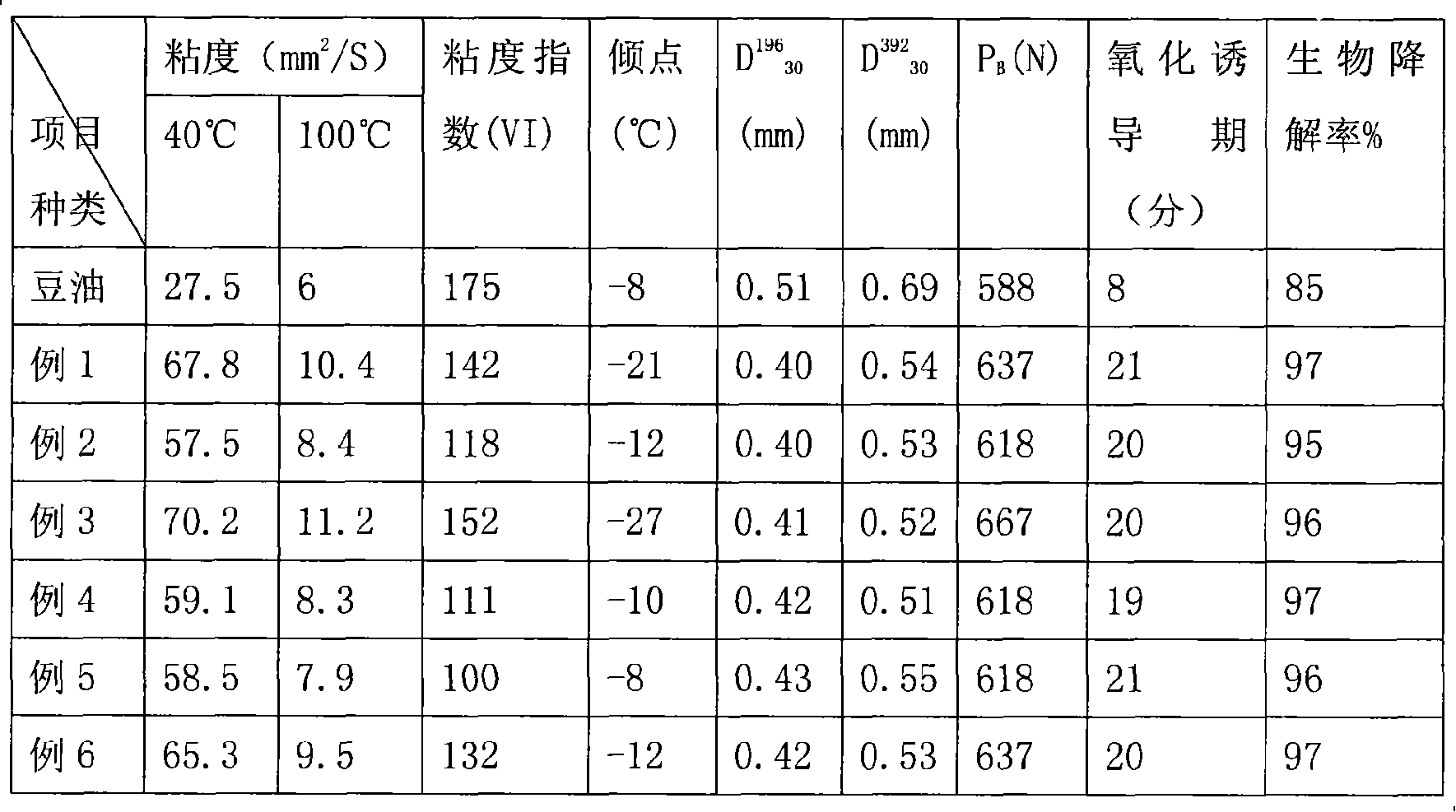
Preparation method for lubricating oil
InactiveCN101121907ALow pour pointReduced rheological propertiesFatty-oils/fats refiningBase-materialsPolyesterAcetic anhydride
The invention discloses a lubricant preparing method, particularly relates to a method for preparing an environment-friendly polyester lubricating-oil basic oil. The invention takes use of chemical modification of vegetable oil to improve the rheologic behavior and oxidation stability. Firstly, the vegetable oil is mixed with alcohol for esterification reaction to achieve vegetable-oil monoester and introduce branched chain into the molecular structure. Subsequently, hydrogen peroxide is used to turn the double-bonds in the vegetable-oil basic oil into saturated epoxy-bonds; then, the epoxy-bonds are opened by fatty acid to achieve vegetable-oil monoether containing hydroxy groups. And finally, acetic anhydride is used to esterify the generated hydroxy groups and achieve polyether vegetable-oil ether. Compared with vegetable oil, the modified polyester vegetable-oil ether is largely improved in frictional behavior, rheologic behavior and oxidation stability.
Owner:TSINGHUA UNIV
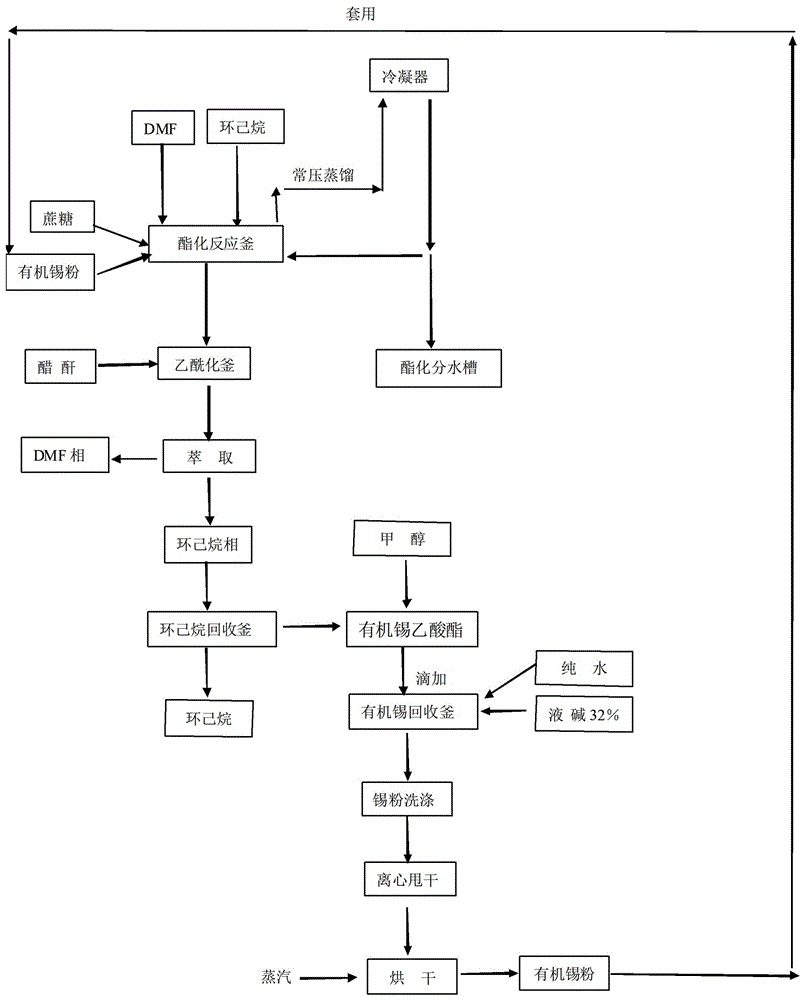
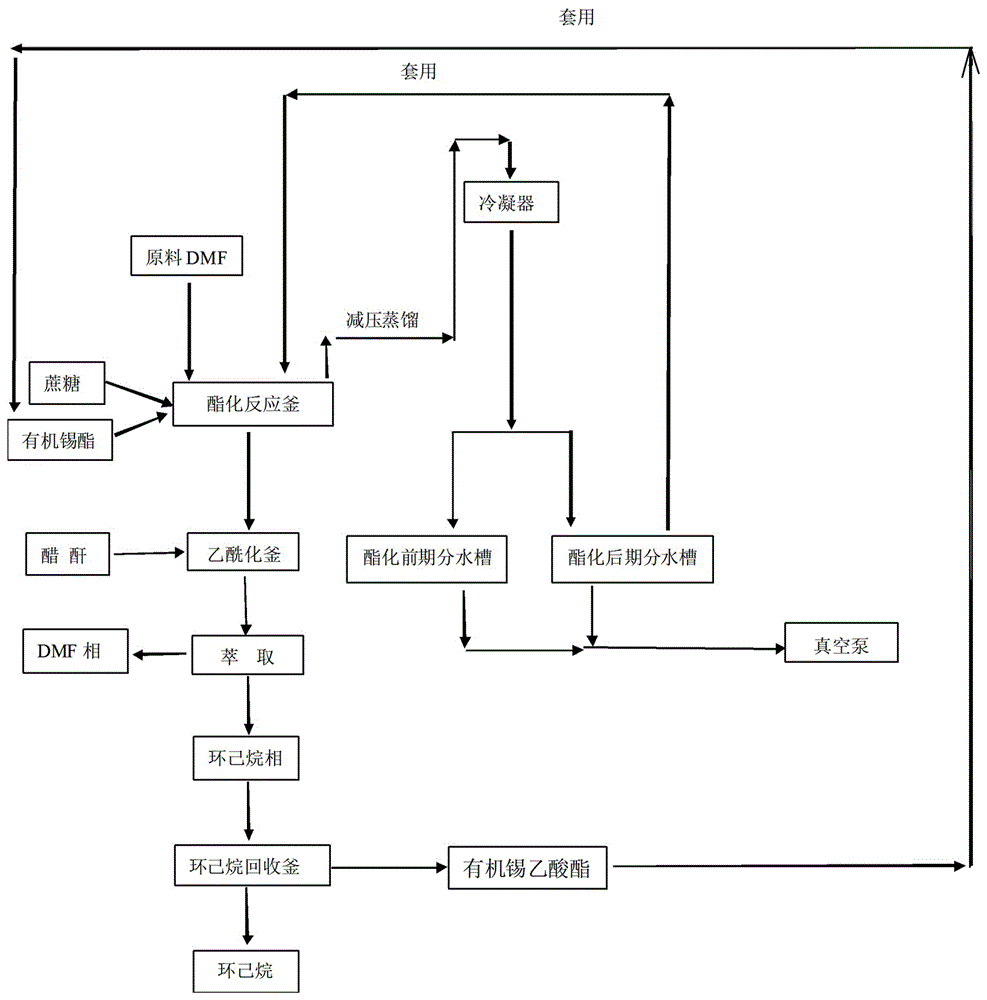
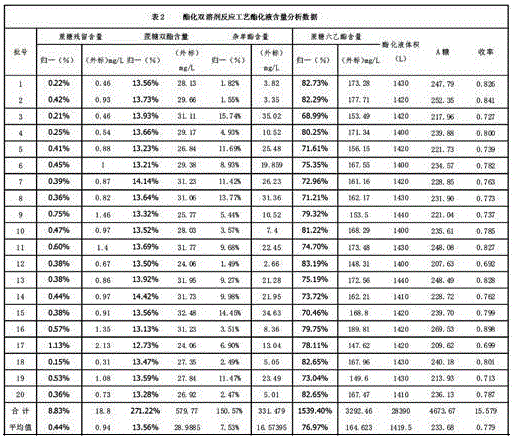
Method for reaction of sucralose esterified single solvent
ActiveCN106349300AEliminate recyclingReduce consumptionEsterified saccharide compoundsTin organic compoundsAcetic anhydrideSucrose
The invention relates to a method for the reaction of sucralose esterified single solvent, characterized by: 1) DMF, sucrose and organotin acetate are subjected to negative pressure esterification in an esterification kettle while DMF is recovered to the bypass channels of earlier stage by negative pressure distillation and condensing, and the recovered DMF whose water content is larger than 0.9-1.0% ppm is to be delivered into the recovery system; 2) Negative pressure recovery of DMF continues until the water content is less than 0.6% ppm, then which is returned to the esterification kettle for the next batch of esterification reaction; 3) The esterification reaction material is added into the esterification kettle, and acetic anhydride is added dropwisely in; water is added after adding dropwisely, and sucrose 6 ester and organotin acetate are extracted and separated with cyclohexane; organic tin acetate present in the cyclohexane phase, which is produced through evaporating of the cyclohexane solvent. The advantages of the method for the reaction of sucralose esterified single solvent is shortening the production cycle, eliminating the original tin recovery process, reducing the consumption of raw materials and energy and maximizing the savings in all costs.
Owner:ANHUI JINGHE IND
Synthesis method of amino-protecting glycine dipeptidase derivant
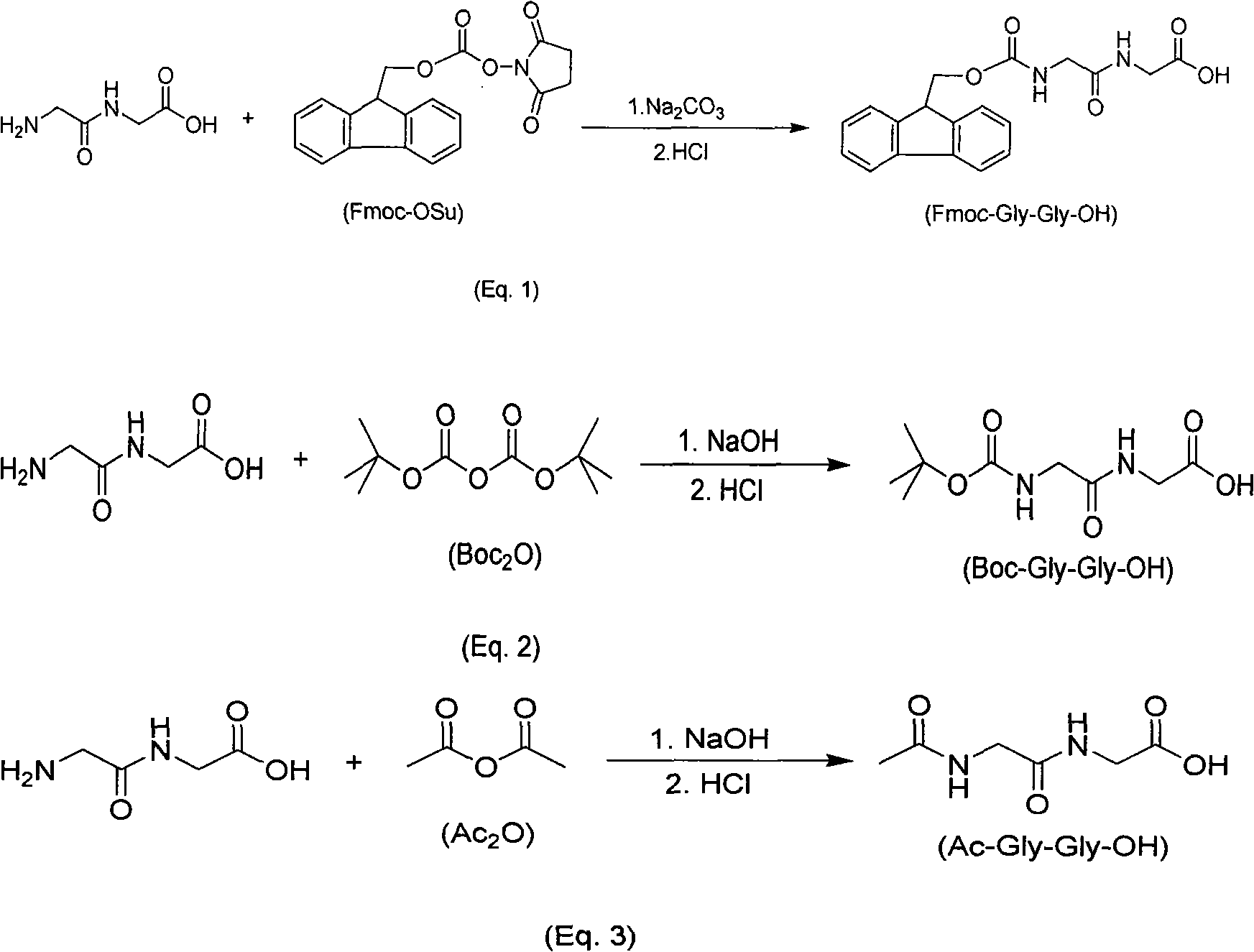
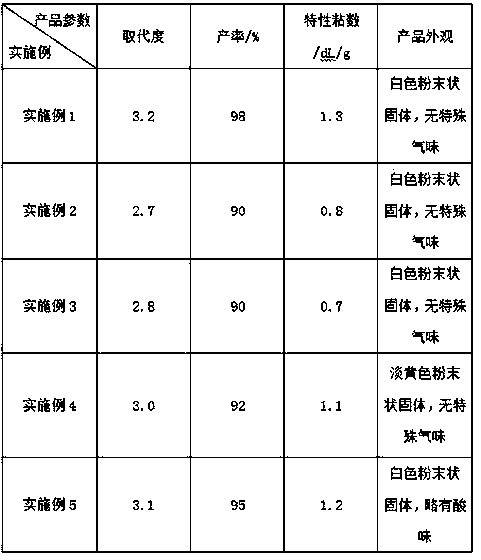
The invention relates to a synthesis method of amino-protecting glycine dipeptidase derivant. The amino-protecting glycine dipeptidase derivant is N-9-Fmoc glycine dipeptidase, N-Boc glycine dipeptidase and N-acetyl glycine dipeptidase. The invention has the technology that glycine dipeptidase is dissolved in 1-20% of inorganic aqueous alkali, and the organic solution of Fmoc-OSu or Boc2O or Ac2Ois dropwise added; reaction is finished within 1-12 hours, the yield of the product Fmoc-Gly-Gly-OH, Boc-Gly-Gly-OH and Ac-Gly-Gly-OH is between 80-95%, and the content of the product is above 99%. The invention has simple technology, easy industrialization and low cost. The product of the invention is the important raw material for synthesizing polypeptide compound.
Owner:上海力智生化科技有限公司
Preparation method and application of acetylated sodium hyaluronate
ActiveCN109206537AThe production process is simpleShorten the production cycleAcetic acidAcetic anhydride
The invention provides a preparation method of acetylated sodium hyaluronate. The preparation method comprises following steps: adding hyaluronic acid or hyaluronate into a mixed solvent of acetic acid and acetic anhydride; carrying out acylation reactions under the catalytic action of concentrated sulfuric acid, after reactions, adding the reaction liquid into water to carrying out precipitation,filtering, washing the precipitate to obtain acetylated hyaluronic acid, adding alkali to adjust the pH value until the solution becomes neutral; filtering to remove the impurities, and carrying outspray drying to obtain acetylated sodium hyaluronate. The preparation time is largely shortened, precipitation and washing do not need a large amount of organic solvents, the environment is protected,the cost is reduced, and the preparation method is especially suitable for industrial large scale production.
Owner:BLOOMAGE BIOTECHNOLOGY CORP LTD +1
Preparation method of 4,4'-(Hexafluoroisopropylidene) diphthalic anhydride
ActiveCN101696199AOvercoming the Factors Affecting Industrial Safety ProductionHigh yieldOrganic chemistryAlkyl transferO-Xylene
The invention discloses a preparation method of 4,4'-(Hexafluoroisopropylidene) diphthalic anhydride (6-FDA), belonging to the technical field of liquid crystal materials. The main technical scheme is achieved as follows: o-xylene and 2,2-dichloro-hexafluoropropane undergo alkylation reaction in an ionic liquid under the catalytic action of Lewis acids (AlCl3, ZnCl2) to obtain 4,4'-(Hexafluoroisopropylidene) di(2-xylene); then the 4,4'-(Hexafluoroisopropylidene) di(2-xylene) is oxidized by permanganic acid TEBA triethylbenzylammonium salts to obtain 4,4'-(Hexafluoroisopropylidene) diphthalandione; and finally, the 4,4'-(Hexafluoroisopropylidene) diphthalandione is dehydrated by acetic oxide to obtain the 4,4'-(Hexafluoroisopropylidene) diphthalicanhydride (6-FDA). The preparation method has the advantages of mild reaction condition, less three-waste emission and high yield.
Owner:天津众泰材料科技有限公司
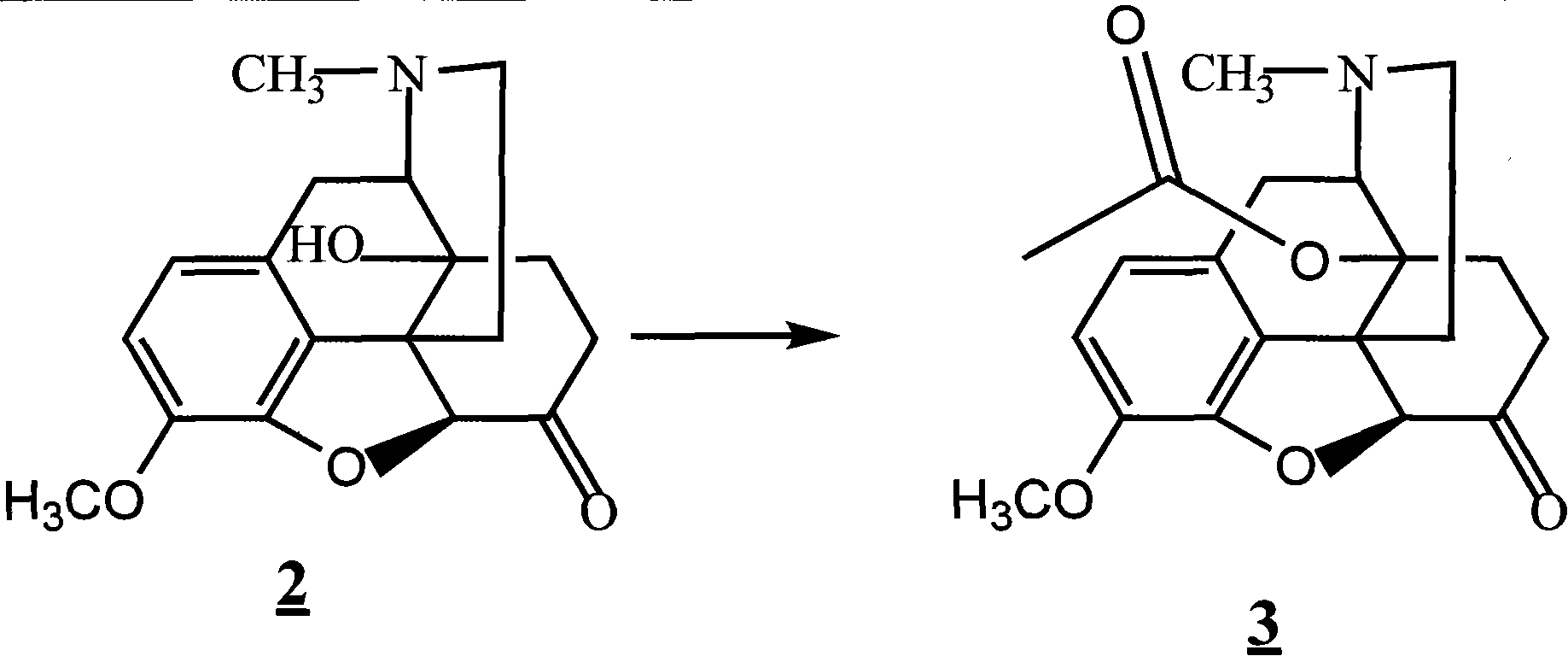
Method for synthesizing naloxone or naltrexone
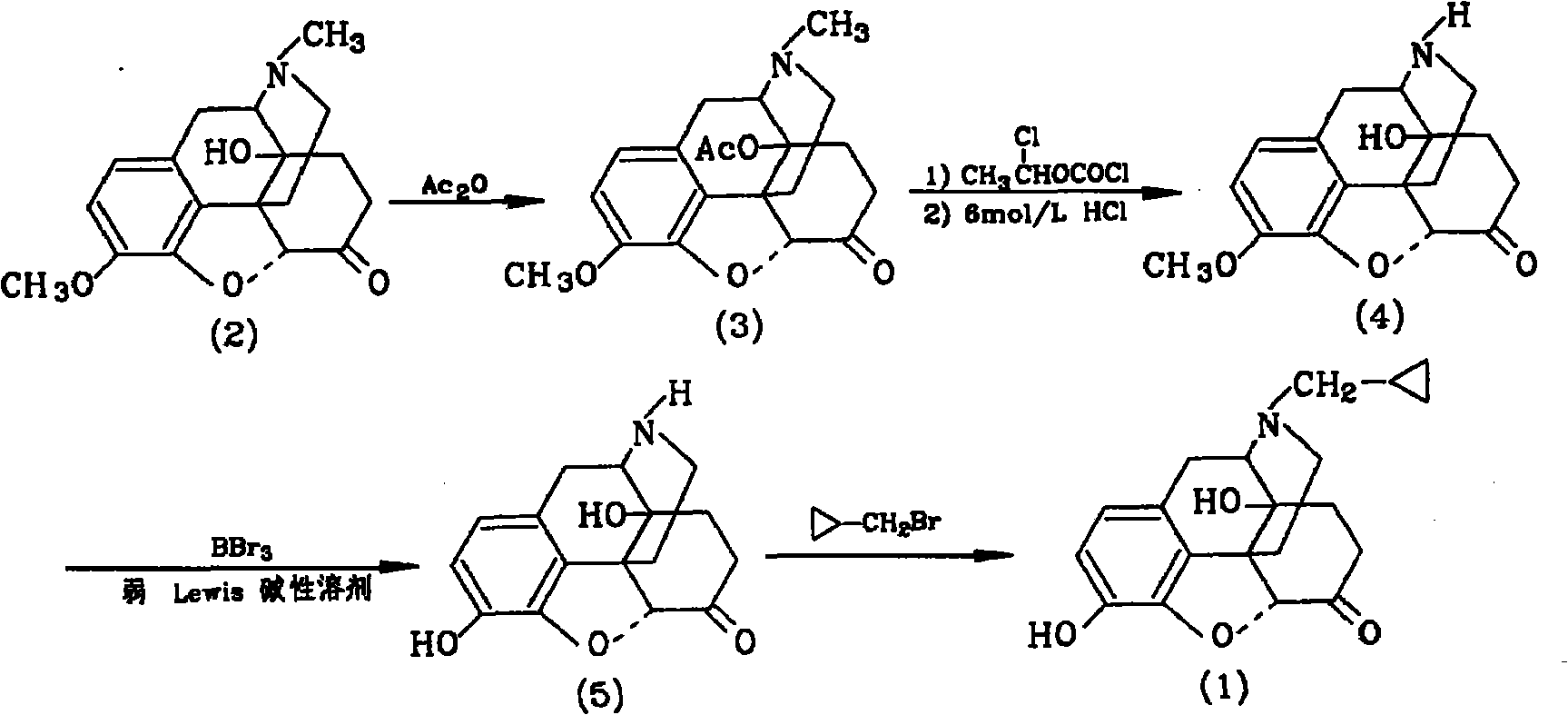
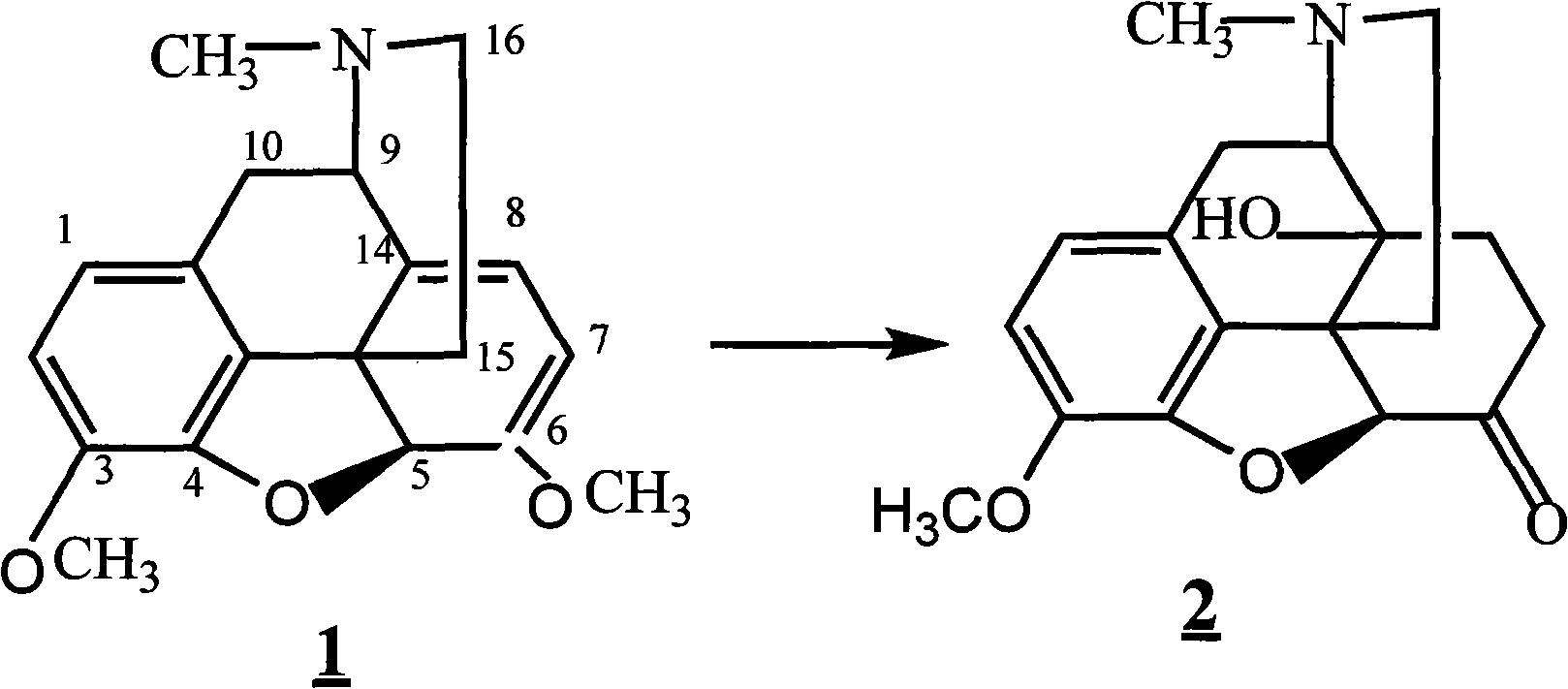
The invention provides a method for synthesizing naloxone or naltrexone, which comprises the following steps of: dissolving thebaine in formic acid, uniformly stirring, dripping an oxidant, keeping the temperature of between 20 and 40 DEG C for 3 to 7 hours, displacing gas in a reaction vessel by inert gas serving as protective gas for 3 to 5 times, adding a metallic framework catalyst, displacing the gas by hydrogen for 3 to 5 times, keeping the temperature of between 25 and 45 DEG C and stabilizing a system for 7 to 13 hours to obtain a compound 2; reacting the compound 2 with acetic anhydride at the temperature of between 60 and 100 DEG C for 1 to 2 hours to obtain a compound 3; taking the inert gas as the protective gas, adding toluene, chloroformic acid-1-chloroethyl ester and potassium bicarbonate into the compound 3, heating to the temperature of between 75 and 100 DEG C and reacting for 20 to 40 hours, concentrating under reduced pressure until the system is fully dry, adding 10 percent hydrochloric acid, and heating and refluxing for 2 to 6 hours to obtain a compound 4; dissolving the compound 4 and at least one alkylation reagent in an organic solvent 1 and reacting with alkali at the temperature of between 50 and 100 DEG C to obtain a compound 5; and reacting the compound 5 with boron tribromide in an organic solvent 2 at the temperature of between -10 and 40 DEG C for 2 to 4 hours to obtain a compound 6, namely the naloxone or naltrexone.
Owner:甘肃普安制药股份有限公司
Oil absorption material using cellulose as base and preparation method thereof
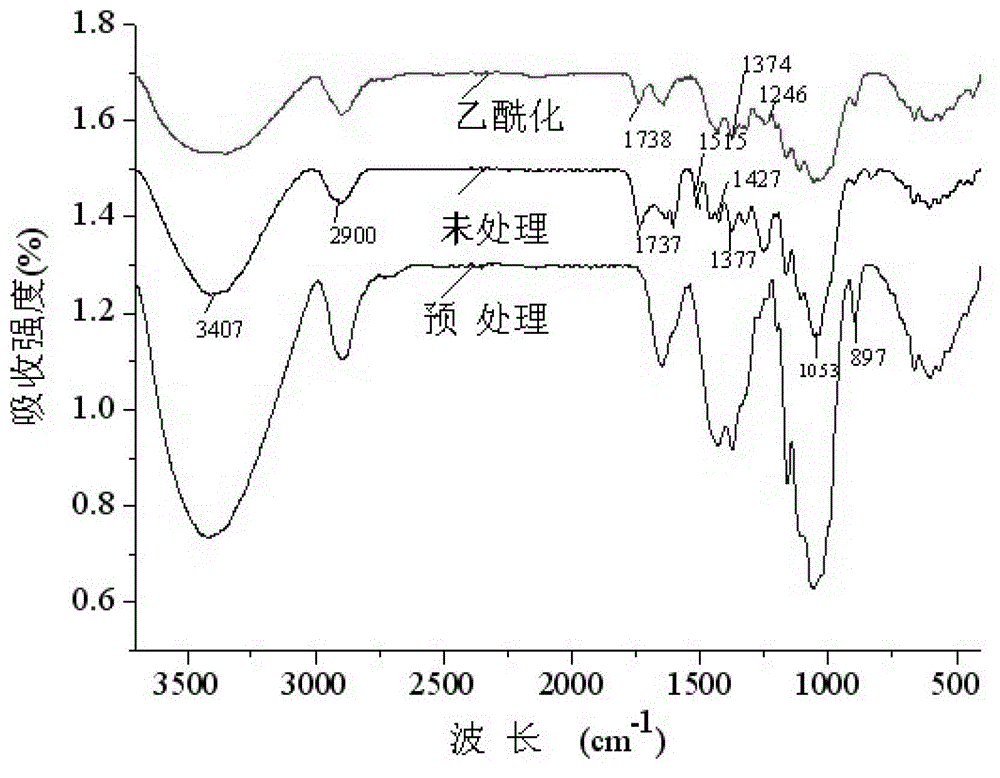
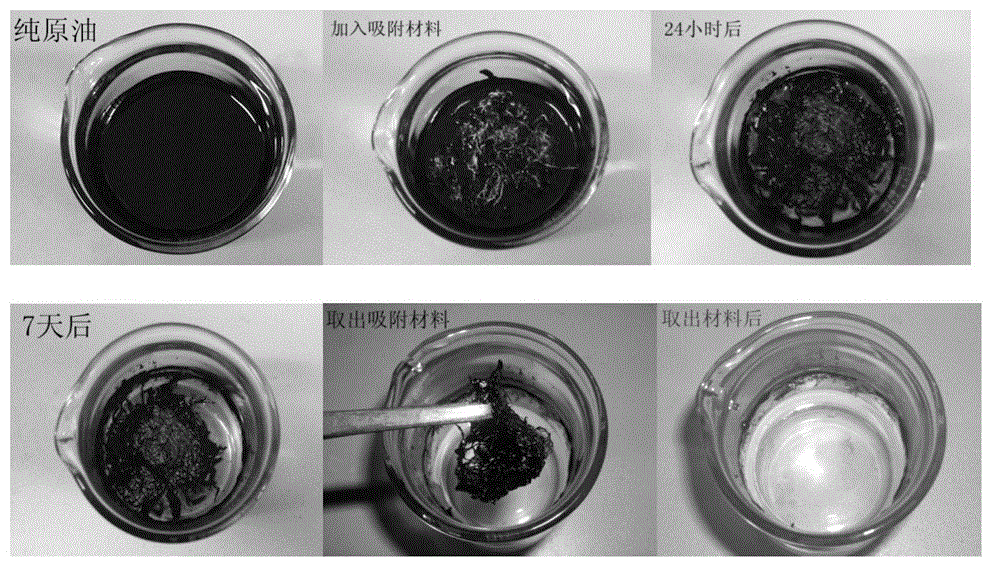
InactiveCN103143326AHigh oil absorptionAbundant resourcesFatty/oily/floating substances removal devicesOther chemical processesCelluloseAcetic anhydride
The invention discloses an oil absorption material using cellulose as a base and a preparation method thereof. The preparation method comprises the following steps: pretreating the raw material corn straws with sodium hydroxide and sodium hypochlorite to extract cellulose, and carrying out acetylation modification on the cellulose with acetic anhydride to obtain the natural high-efficiency oil absorption material. The technical scheme disclosed by the invention has efficient adsorption effect on marine oil spill; the oil absorption rate can reach more than 60 times; and the preparation method has the advantages of simple process and easy recovery.
Owner:TIANJIN UNIV
Preparation of CT/MRI dual-modality imaging contrast agent based on dendrimer
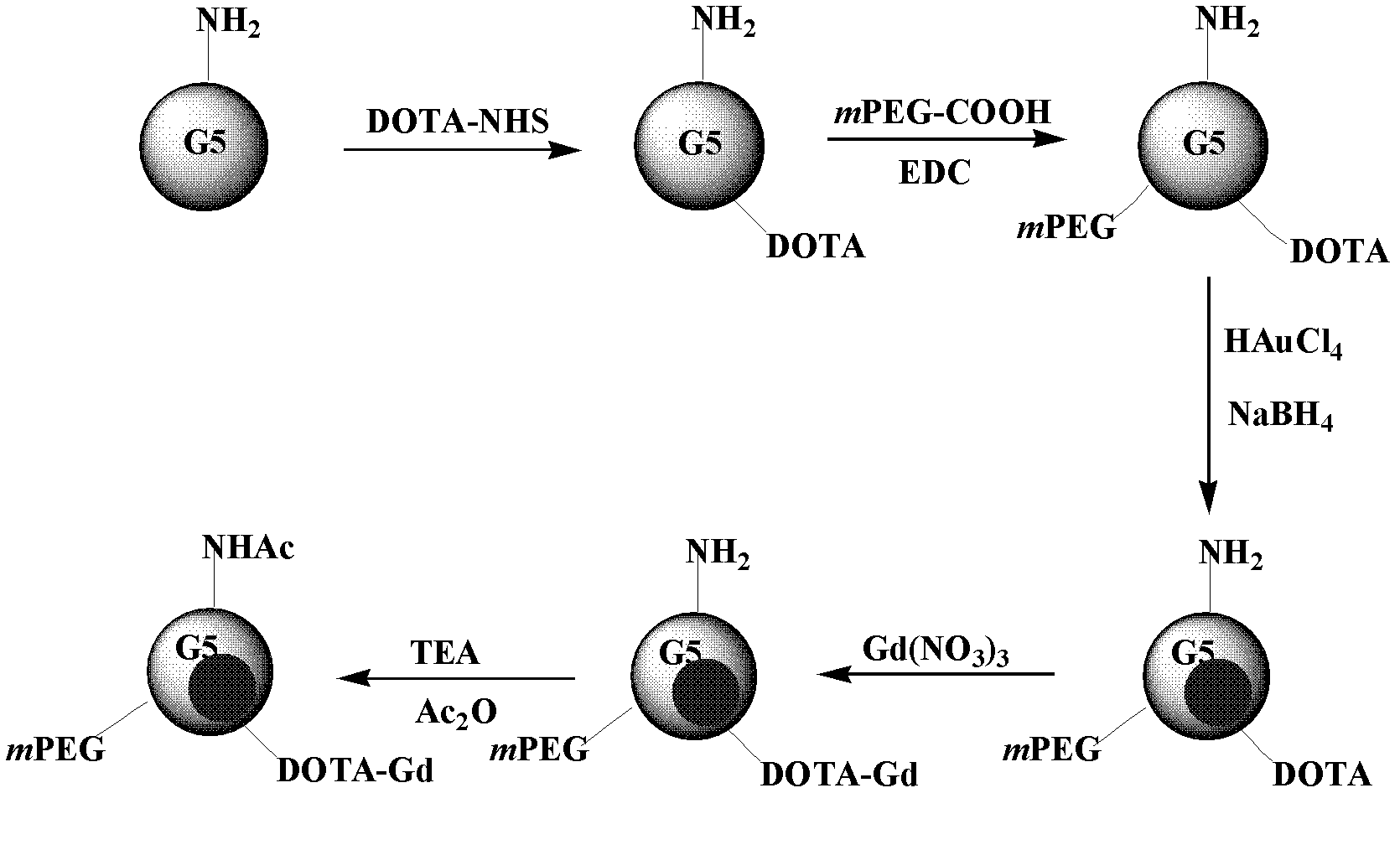
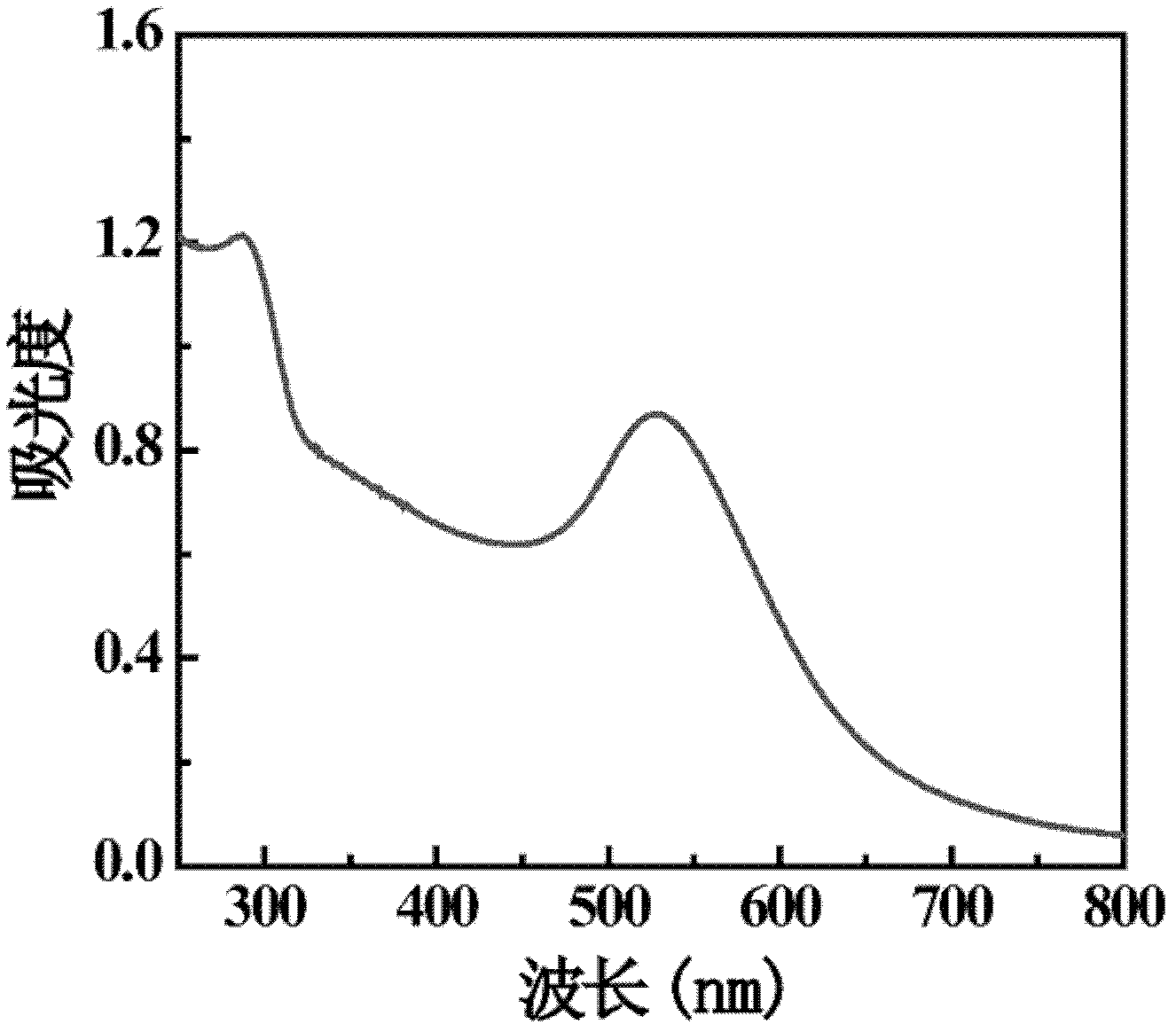
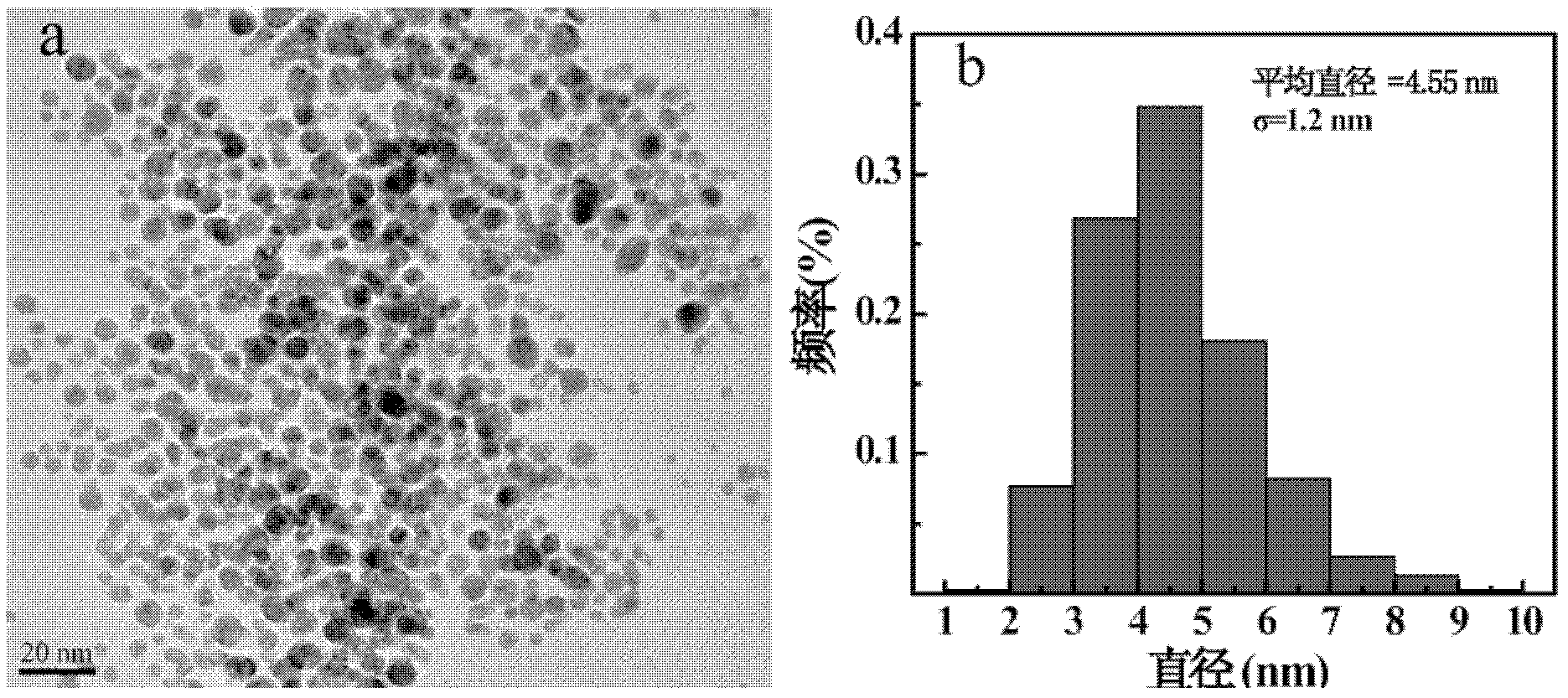
InactiveCN102294038AShorten the relaxation timeNarrow size distributionX-ray constrast preparationsDiagnostic Radiology ModalityAcetic anhydride
The invention relates to a method for preparing a computed tomography (CT) / magnatic resonance imaging (MRI) bimodal imaging contrast agent based on dendrimers. The method comprises the following steps of: (1) adding DOTA-N-hydroxy succinimide (NHS) solution into the fifth generation of poly(amidoamine) (PAMAM) dendrimer solution, adding methoxy poly(ethylene glycol) (mPEG)-COOH solution which is subjected to 1-ethy 1-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC) activation, reacting with stirring, and thus obtaining functionalized dendrimer solution; (2) adding chloroauric acid solution and sodium borohydride solution into the functionalized dendrimer solution, reacting with stirring, adding gadolinium nitrate solution, stirring, adding triethylamine and acetic anhydride, andreacting with stirring for 8 to 24 hours; and (3) dialyzing the solution obtained in the step (2), performing freeze drying treatment, and thus obtaining the contrast agent. The method has a simple preparation process, and experimental conditions are realized at normal temperature and under normal pressure; and the CT / MRI bimodal imaging contrast agent prepared by the method has a good CT / MRI effect, and a favorable foundation is laid for the development of a novel multifunctional contrast agent.
Owner:DONGHUA UNIV
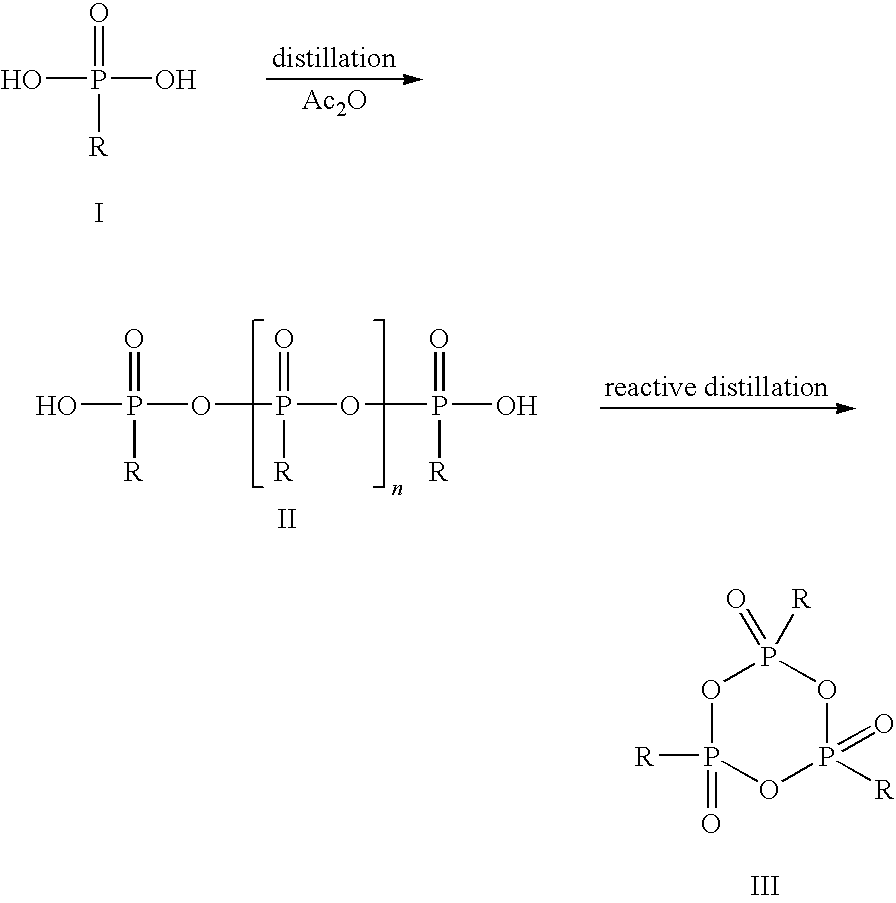
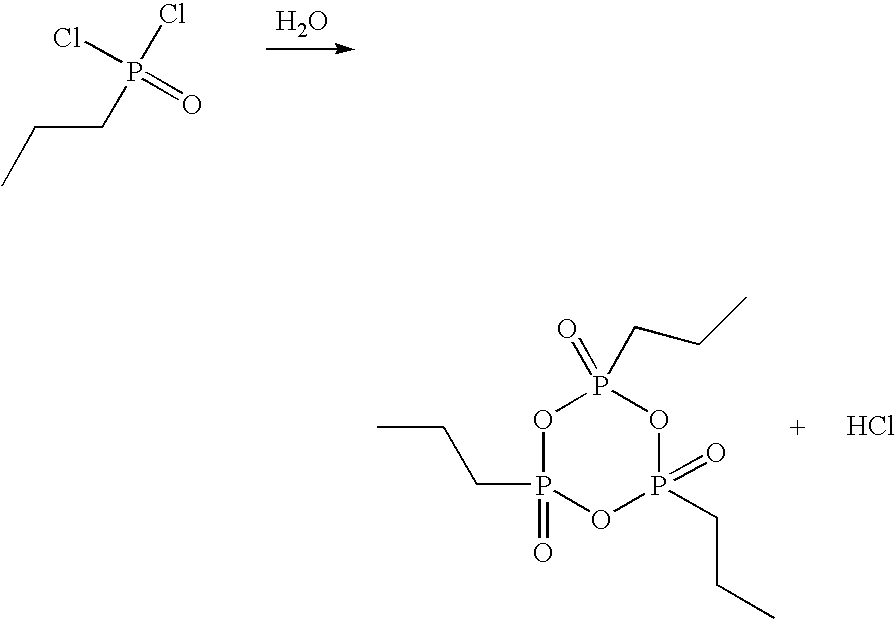
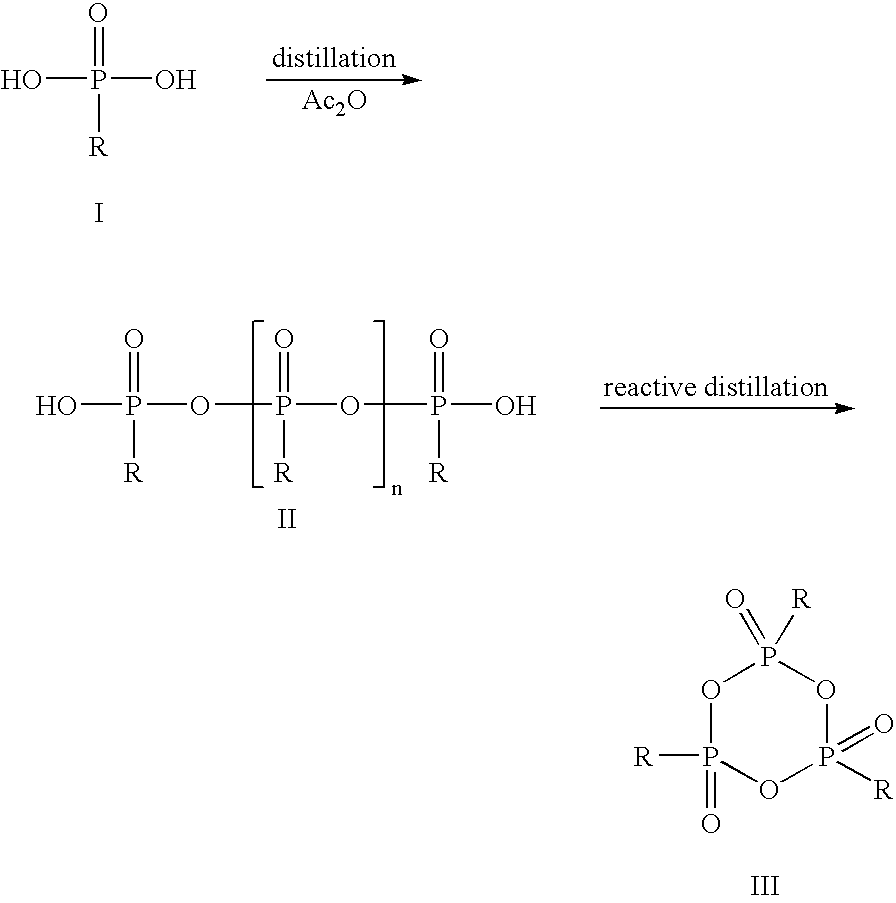
Method for the production of cyclic phosphonic acid anhydrides
Method of performing condensation reactions, acylations or of preparing heterocycles comprising forming cyclic phosphonic anhydride of the formula (III) by a) reacting phosphonic acid derivatives of formula (I) with acetic anhydride at a temperature ranging between 30 and 150° C. while separating a mixture of ethanoic acid and acetic anhydride by means of distillation, b) then reactively distilling the oligomeric phosphonic acid anhydrides of formula (II) obtained in step a) and transforming the same into the corresponding cyclic trimeric phosphonic acid anhydrides of formula (III), wherein n represents a number between 0 and 300 while R represents allyl, aryl, or open-chain, cyclic, or branched C1 to C8 alkyl radicals, aryloxy, allyloxy, or alkoxy comprising open-chain, cyclic, or branched C1 to C8 alkyl radicals. Preferably the cyclic trimeric phosphonic acid anhydrides formed in step b) are immediately dissolved in an organic solvent that exhibits an inert behavior relative thereto.
Owner:EUTICALS
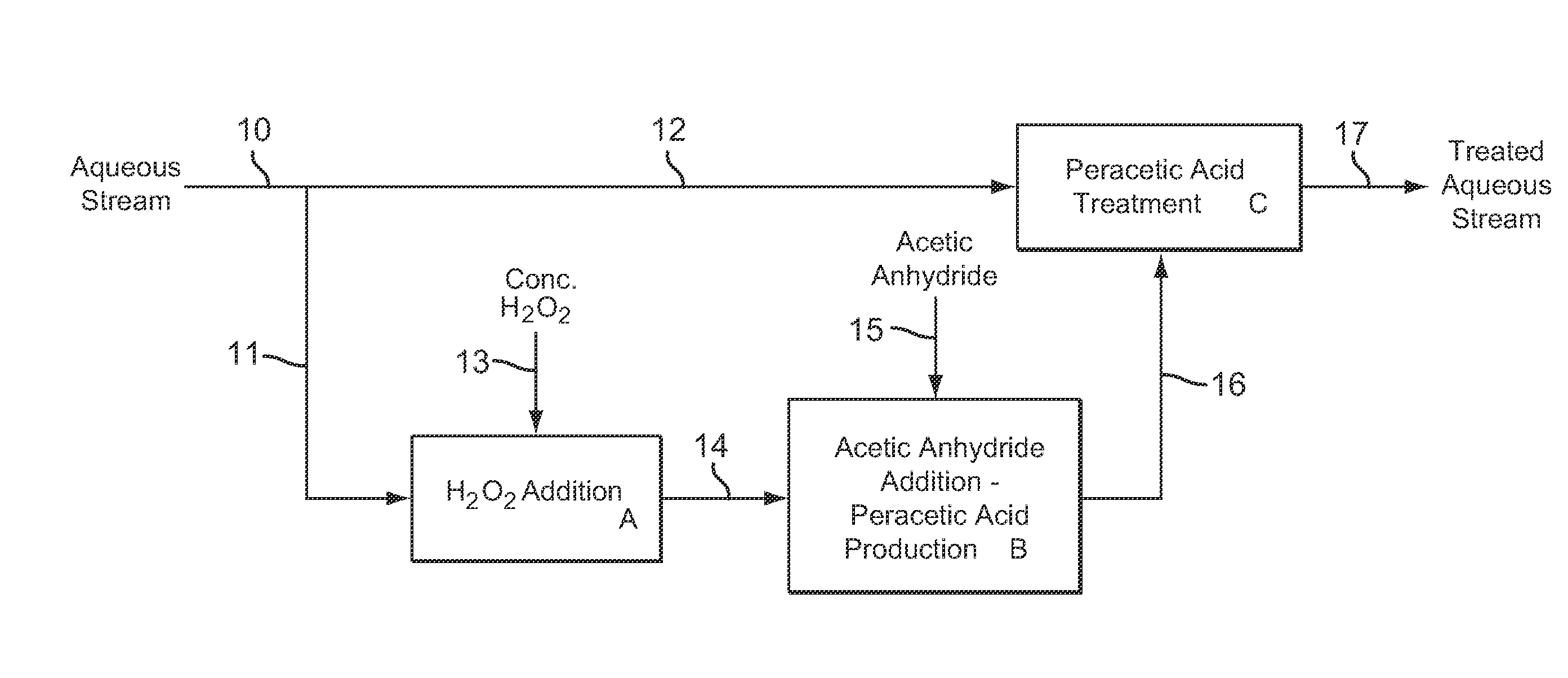
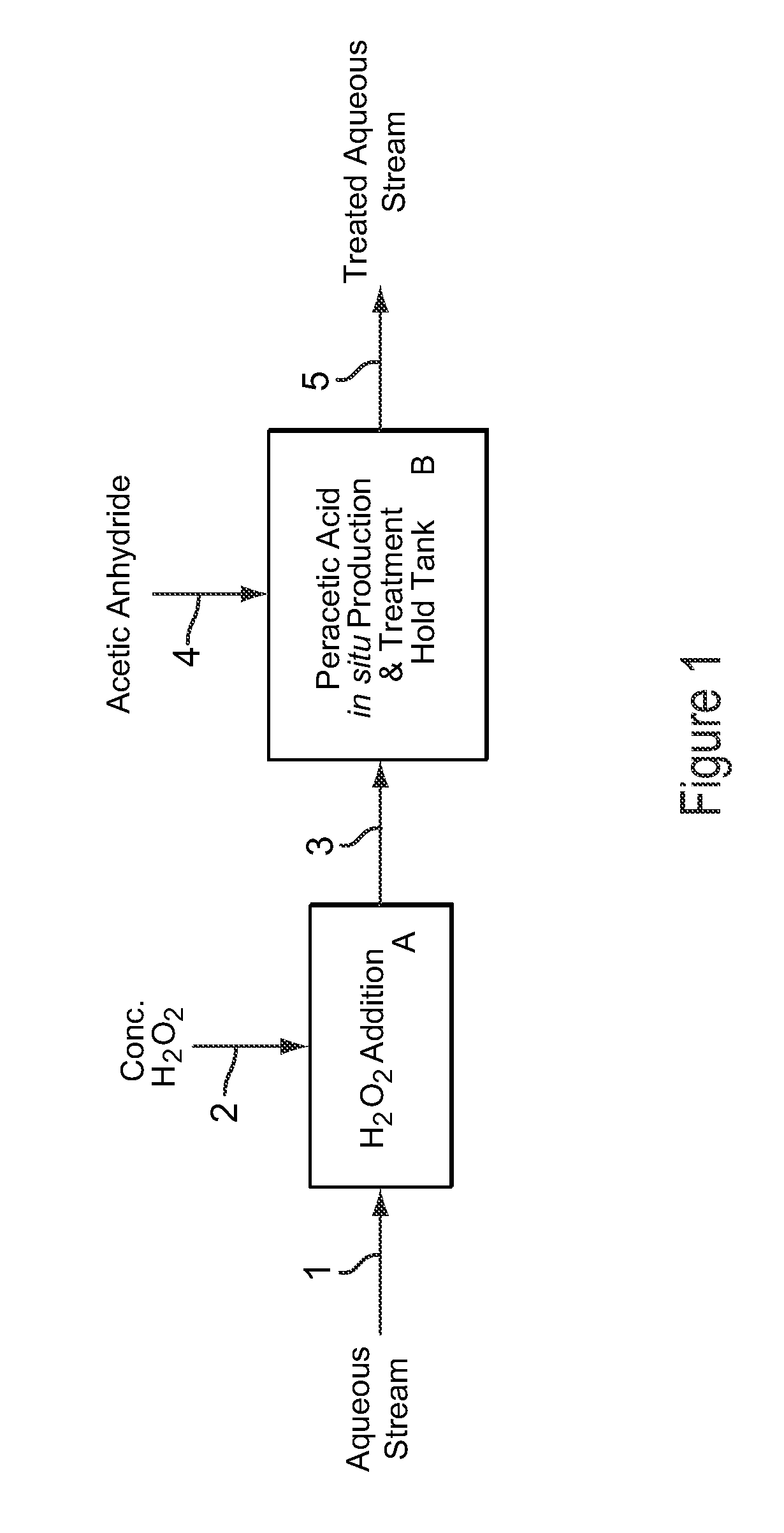
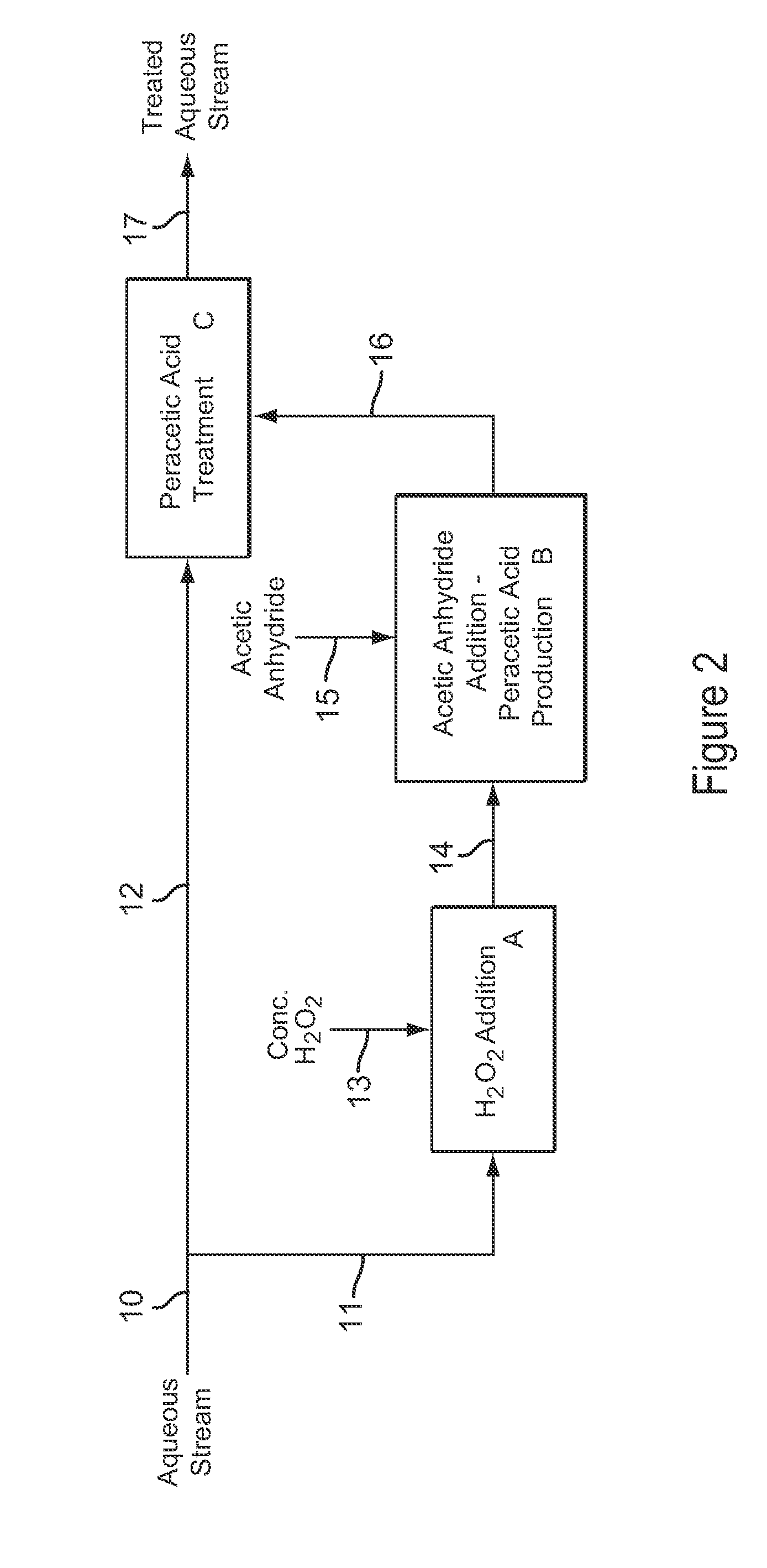
Dilute Stabilized Peracetic Acid Production and Treatment Process
InactiveUS20090043123A1Improve stabilityMinimizes decompositionBiocideOrganic compound preparationAcetic acidAcetic anhydride
A process for the rapid production and stabilization of dilute aqueous peracetic acid in which acetic anhydride and hydrogen peroxide are reacted in an aqueous medium with a stoichiometric excess of hydrogen peroxide and the aqueous medium containing the peracetic acid reaction product is adjusted, as necessary, to a pH of less than about 8 to provide a stabilized dilute peracetic acid solution. The dilute peracetic acid may be produced on site or in situ for treatment of an aqueous medium requiring disinfecting, biocidal, antimicrobial or bleaching treatment.
Owner:FMC CORP
Method for preparing polyimide fibers
InactiveCN104928790AHigh crystallinityImprove mechanical propertiesMonocomponent synthetic polymer artificial filamentFiberImide
A method for preparing polyimide fibers belongs to the field of high-performance organic fibers. The method comprises the following steps: preparing a copolymerized polyamide acid spinning solution through condensation polymerization of dianhydride and diamine; adding acetic anhydride and a catalyst in the polyamide acid spinning solution to obtain partly imidized polyamide acid spinning solution; spinning by adopting a wet or dry-wet spinning process, and solidifying and washing to obtain partly imidized polyimide fibers; conducting thermal imidization on the fibers at gradient temperature, and preparing the polyimide fibers with high strength, high modulus and high mechanical performance. The method provided by the invention is used for preparing the polyimide fibers by combination of chemical imidization and thermal imidization, and can be used for further improving the mechanical performance of the polyimide fibers, thereby meeting requirements of various applications.
Owner:BEIJING UNIV OF CHEM TECH
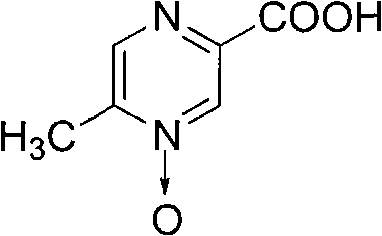
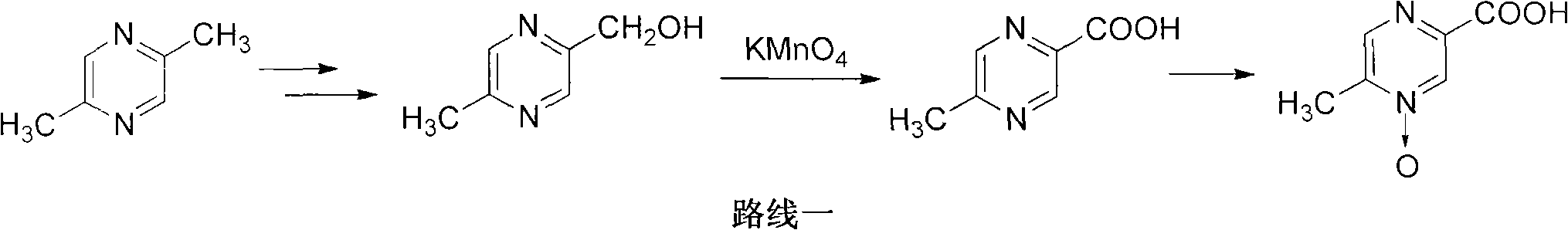
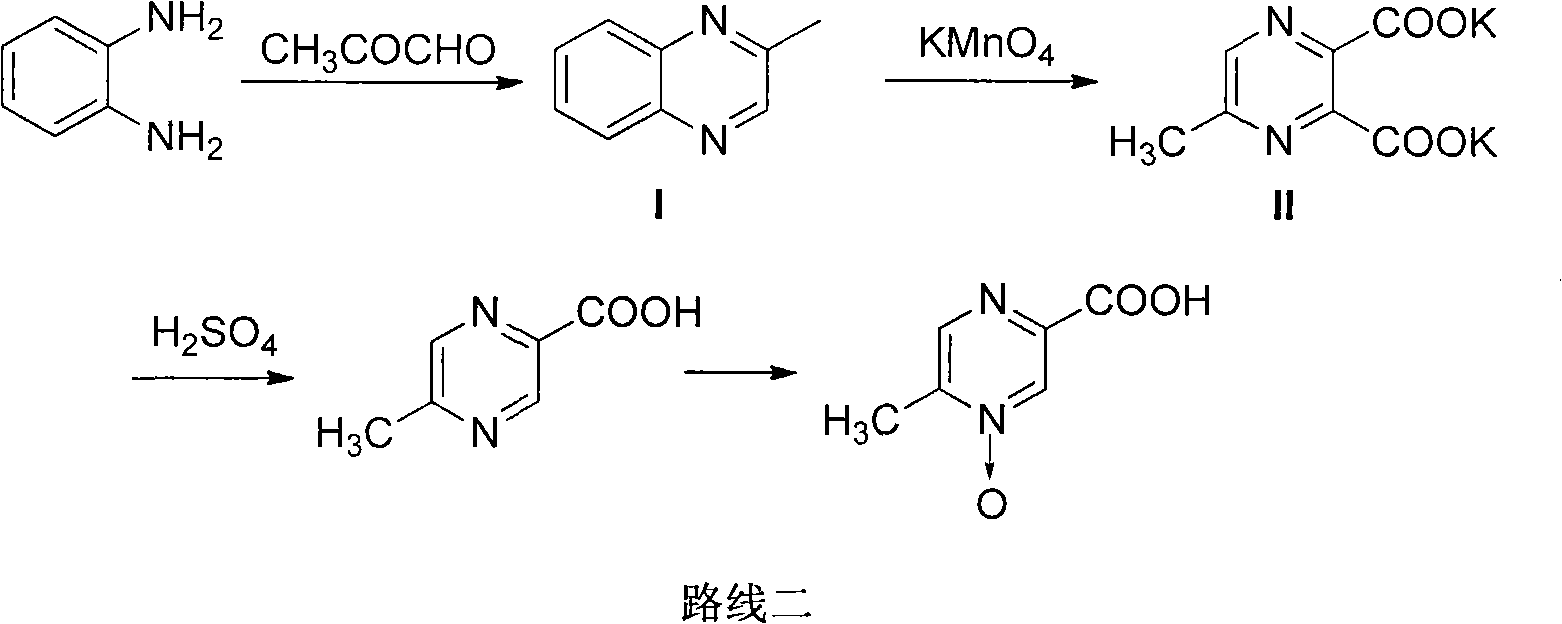
Preparation method of acipimox
InactiveCN103508963AReduce usageEasy to get materialOrganic chemistryAcetic anhydrideCarboxylic acid
The invention relates to a preparation method of acipimox for treating hyperlipidemia, belonging to the field of medicines. The invention provides an acipimox synthesis process which is simple and easy to operate. The preparation method comprises steps of with 2, 5-dimethylpyrazine as a raw material, carrying out nitrogen oxidation, reaction with acetic anhydride and alkaline hydrolysis to obtain 2-hydroxymethyl-5-methylpyrazine; directly oxidizing a post-treatment fluid by using 2,2,6,6-tetramethyl-1-piperidinyloxy / sodium hypochlorite / potassium bromide to obtain 5-methylpyrazine-2-carboxylic acid, wherein the post-treatment fluid is not needed to be dried and concentrated; oxidizing 5-methylpyrazine-2-carboxylic acid by using hydrogen peroxide / sodium tungstate to obtain acipimox.
Owner:WEIHAI WEITAI PHARMA TECH DEV
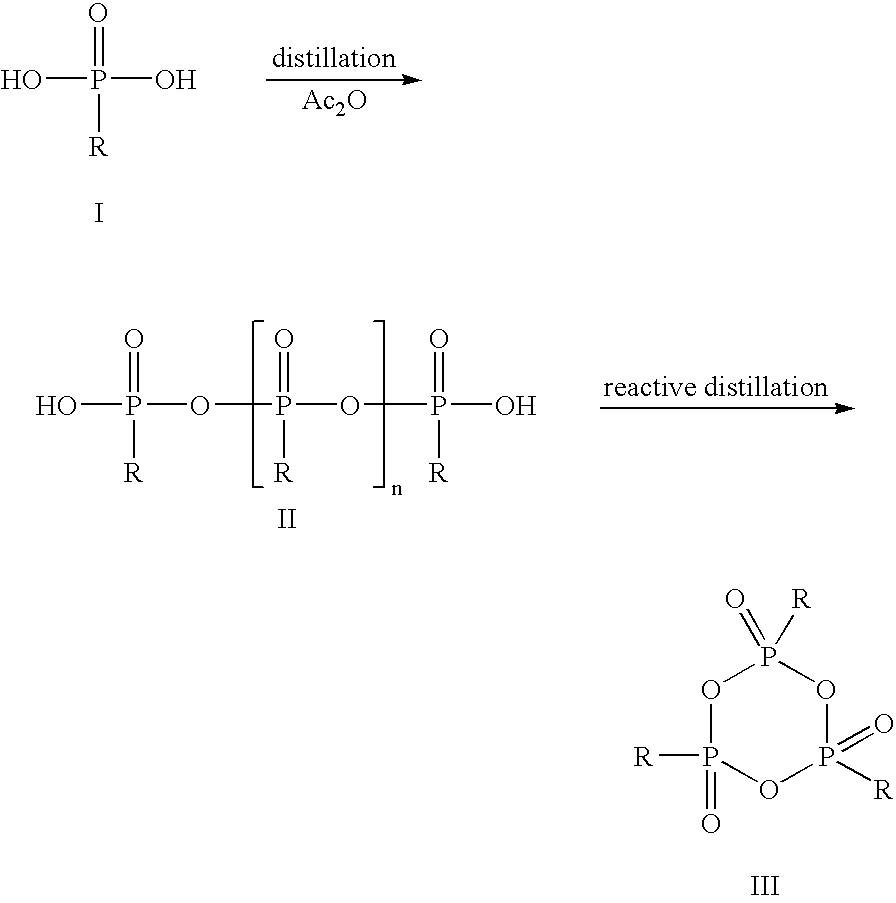
Method for the Production of Cyclic Phosphonic Acid Anhydrides
Method of performing condensation reactions, acylations or of preparing heterocycles comprising forming cyclic phosphonic anhydride of the formula (III) by a) reacting phosphonic acid derivatives of formula (I) with acetic anhydride at a temperature ranging between 30 and 150° C. while separating a mixture of ethanoic acid and acetic anhydride by means of distillation, b) then reactively distilling the oligomeric phosphonic acid anhydrides of formula (II) obtained in step a) and transforming the same into the corresponding cyclic trimeric phosphonic acid anhydrides of formula (III), wherein n represents a number between 0 and 300 while R represents allyl, aryl, or open-chain, cyclic, or branched C1 to C8 alkyl radicals, aryloxy, allyloxy, or alkoxy comprising open-chain, cyclic, or branched C1 to C8 alkyl radicals. Preferably the cyclic trimeric phosphonic acid anhydrides formed in step b) are immediately dissolved in an organic solvent that exhibits an inert behavior relative thereto.
Owner:EUTICALS
Preparation method of pegylation modified hyperbranched poly(ethylene imine) coated nano-gold particles
InactiveCN103239738AEasy to prepareMild reaction conditionsX-ray constrast preparationsGranular deliveryAcetic anhydrideFreeze-drying
The invention relates to a preparation method of pegylation modified hyperbranched poly(ethylene imine) (PEI) coated nano-gold particles, which comprises the following steps: modifying PEI by using mPEG (polyethylene glycol)-COOH, and sequentially carrying out dialysis and freeze-drying on the obtained product so as to obtain PEI-mPEG; taking the solid, dissolving the solid by using water, adding a HAuCl4 solution into the dissolved solid, stirring the obtained product, adding a NaBH4 solution into the obtained product, and carrying out reaction on the obtained mixture at room temperature; and adding triethylamine and acetic anhydride into the obtained object, and after the reaction is completed, carrying out dialysis and freezing on the obtained product so as to obtain pegylation modified hyperbranched polymine coated nano-gold particles. According to the invention, the cheap and easily-obtained PEI is taken as a carrier, so that the cost of materials is reduced; the surface of PEI is modified by using mPEG-COOH, so that the biocompatibility of materials and the colloidal stability of nano-gold particles are improved, and the nano-gold particles are successfully applied to vivo CT (computed tomography) imaging. The method disclosed by the invention is simple in design, mild in reaction conditions and easy to operate, and has an industrialized implementation prospect.
Owner:DONGHUA UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00221.PNG)
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00231.PNG)
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00232.PNG)