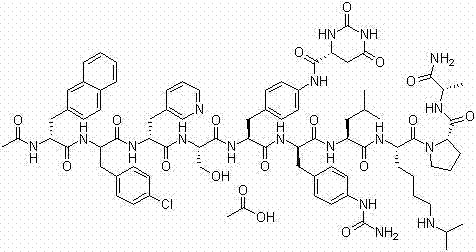
Solid-phase synthetic process for degarelix
A technology of solid-phase synthesis and degarelix, applied in the field of solid-phase synthesis of degarelix, can solve the problems of easy isomerization, increase the possibility of isomerization, complicated operation, etc., and achieve simple operation. , reducing the likelihood, the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: the synthesis of Fmoc-D-Ala-Sieber resin
[0041]Put Sieber resin (10mmol, substitution degree 0.3mmol / g) into the solid-phase reaction column, wash it twice with DMF, add 500ml DMF to swell for 30min. Add 500ml 20% DBLK for deprotection for 10min, then add 500ml 20% DBLK for deprotection for 15min, and wash with DMF for 6 times. Dissolve 9.34g Fmoc-D-Ala-OH and 4.26g HOBt in 60ml DMF, ice bath for 10 minutes, add 4.9ml DIC, pre-activate for 2~5min, add the activated solution to the solid phase reaction column, stir for 2h, ninhydrin The test was negative. Drained, washed 6 times with DMF, washed 3 times with DCM, shrunk MeOH three times (5min, 5min and 10min respectively), and dried to obtain Fmoc-D-Ala-Sieber resin. The degree of substitution was 0.298mmol / g.
Embodiment 2
[0042] Embodiment 2: the synthesis of Fmoc-D-Ala-Sieber resin
[0043] Put Sieber resin (10mmol, substitution degree 0.5mmol / g) into the solid-phase reaction column, wash it twice with DMF, add 500ml DMF to swell for 30min. Add 500ml 20% DBLK for deprotection for 10min, then add 500ml 20% DBLK for deprotection for 15min, and wash with DMF for 6 times. Dissolve 9.34g Fmoc-D-Ala-OH and 4.26g HOBt in 60ml DMF, ice bath for 10 minutes, add 4.9ml DIC, pre-activate for 2~5min, add the activated solution to the solid phase reaction column, stir for 2h, ninhydrin The test was negative. Drain, wash 6 times with DMF, wash 3 times with DCM, shrink MeOH three times (the time is 5min, 5min and 10min respectively), and after drying, Fmoc-D-Ala-Sieber resin is obtained, and the substitution degree is 0.495mmol / g.
Embodiment 3
[0044] Embodiment 3: the synthesis of Fmoc-D-Ala-Sieber resin
[0045] Sieber resin (10mmol, substitution degree 0.8mmol / g) was loaded into the solid-phase reaction column, washed twice with DMF, and 500ml DMF was added to swell for 30min. Add 500ml 20% DBLK for deprotection for 10min, then add 500ml 20% DBLK for deprotection for 15min, and wash with DMF for 6 times. Dissolve 9.34g Fmoc-D-Ala-OH and 4.26g HOBt in 60ml DMF, ice bath for 10 minutes, add 4.9ml DIC, pre-activate for 2~5min, add the activated solution to the solid phase reaction column, stir for 2h, ninhydrin The test was negative. Drain, wash 6 times with DMF, wash 3 times with DCM, shrink MeOH three times (5min, 5min and 10min respectively). After drying, Fmoc-D-Ala-Sieber resin is obtained, and the substitution degree is 0.799mmol / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com