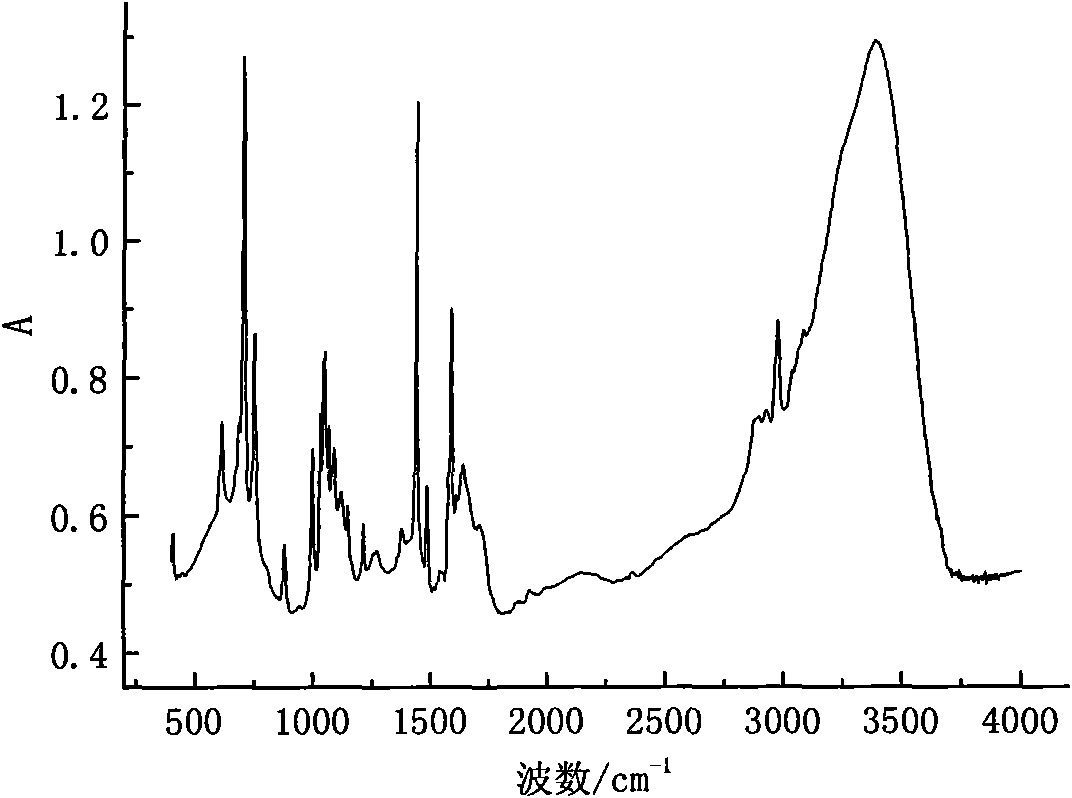
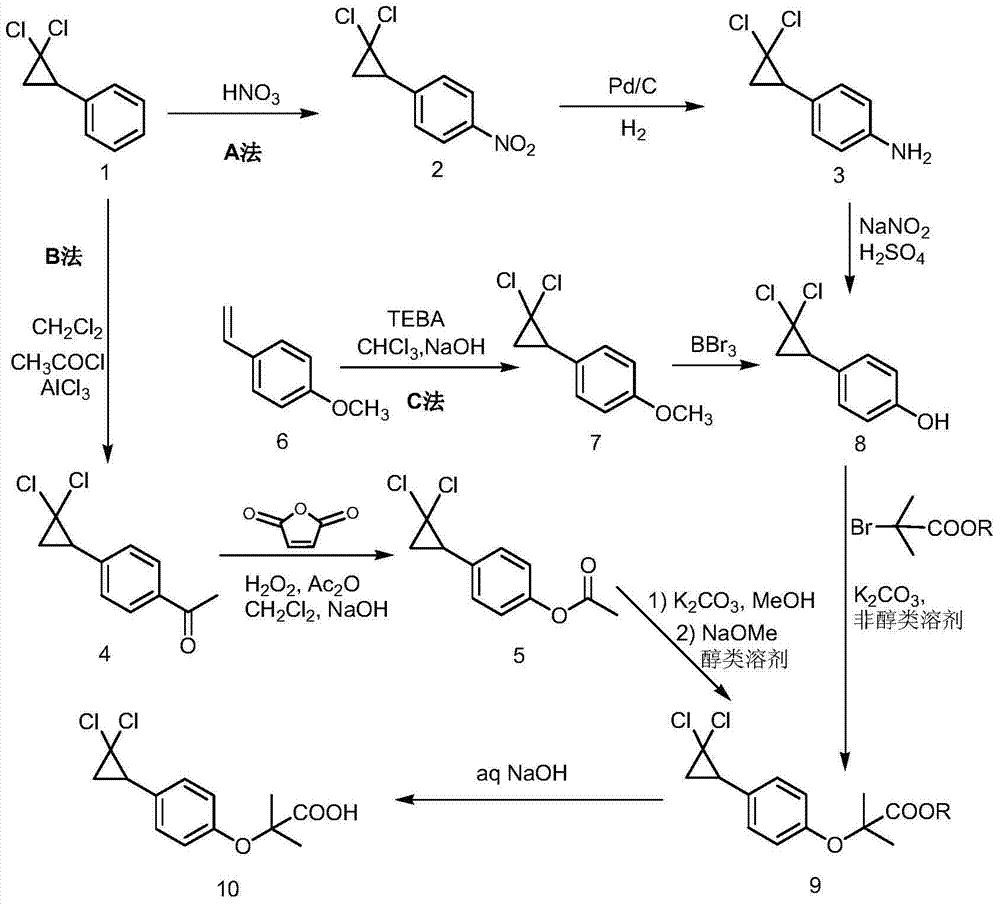
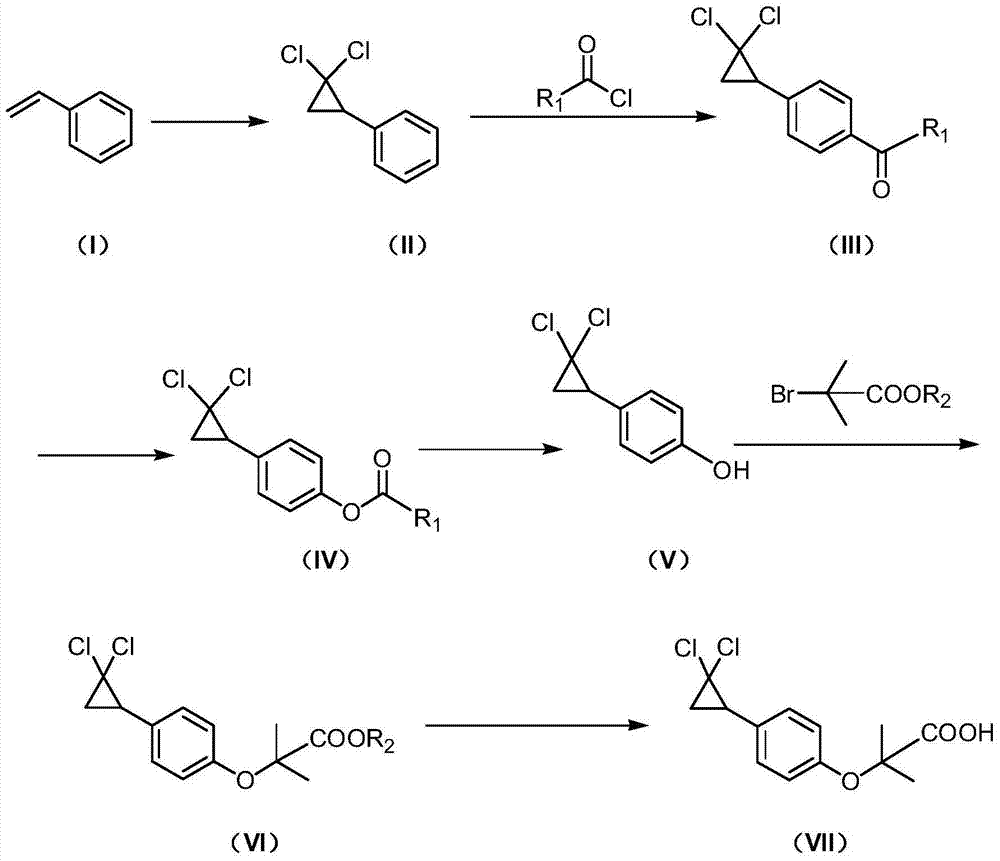
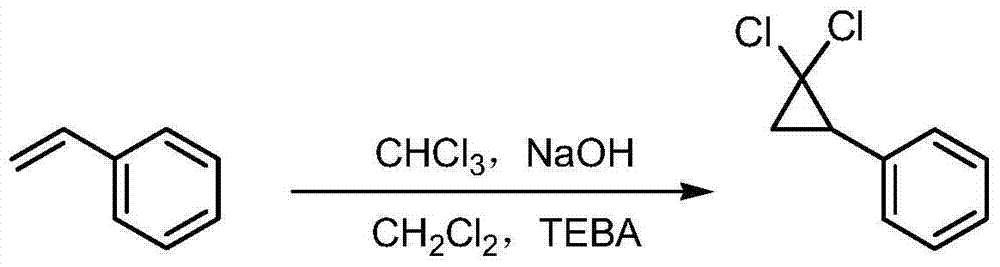
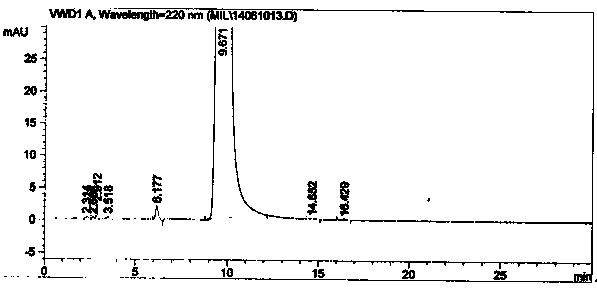
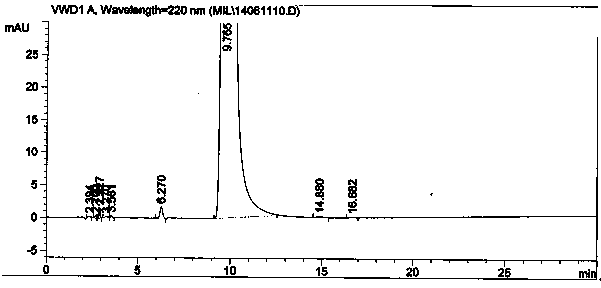
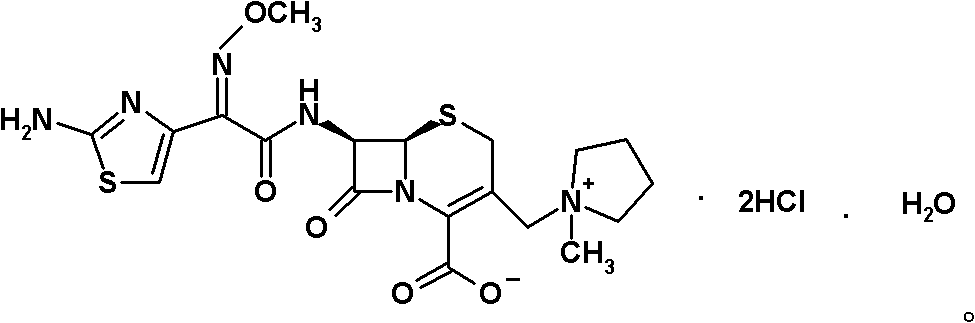
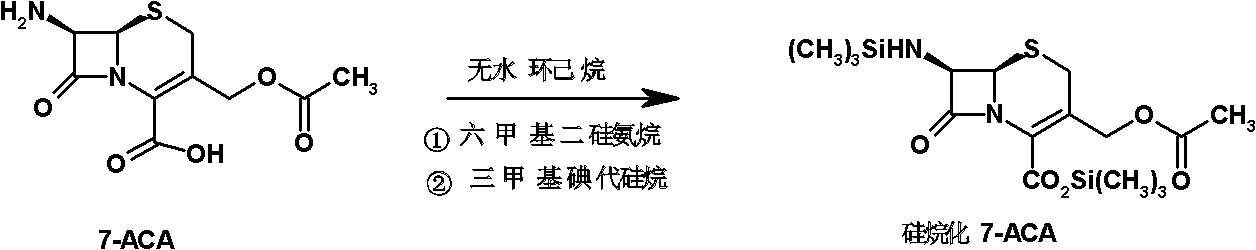
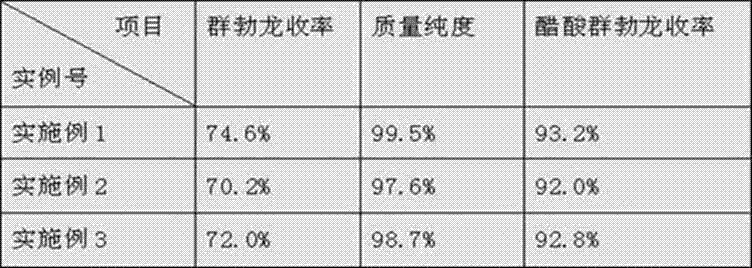
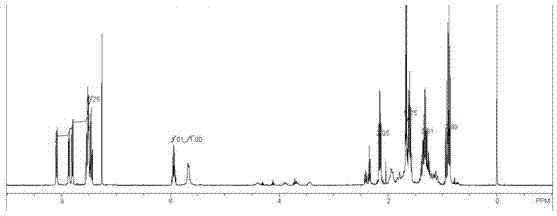
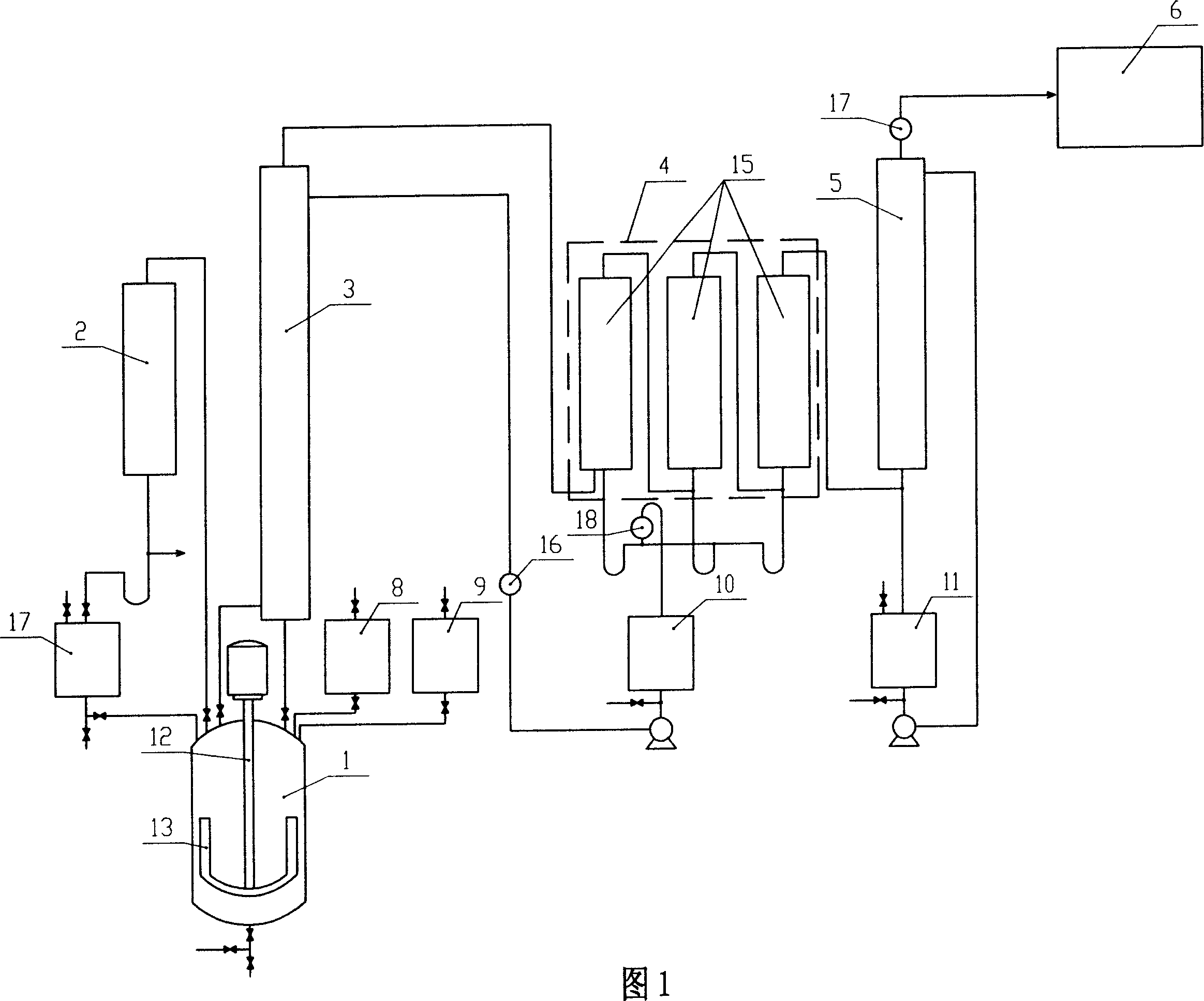
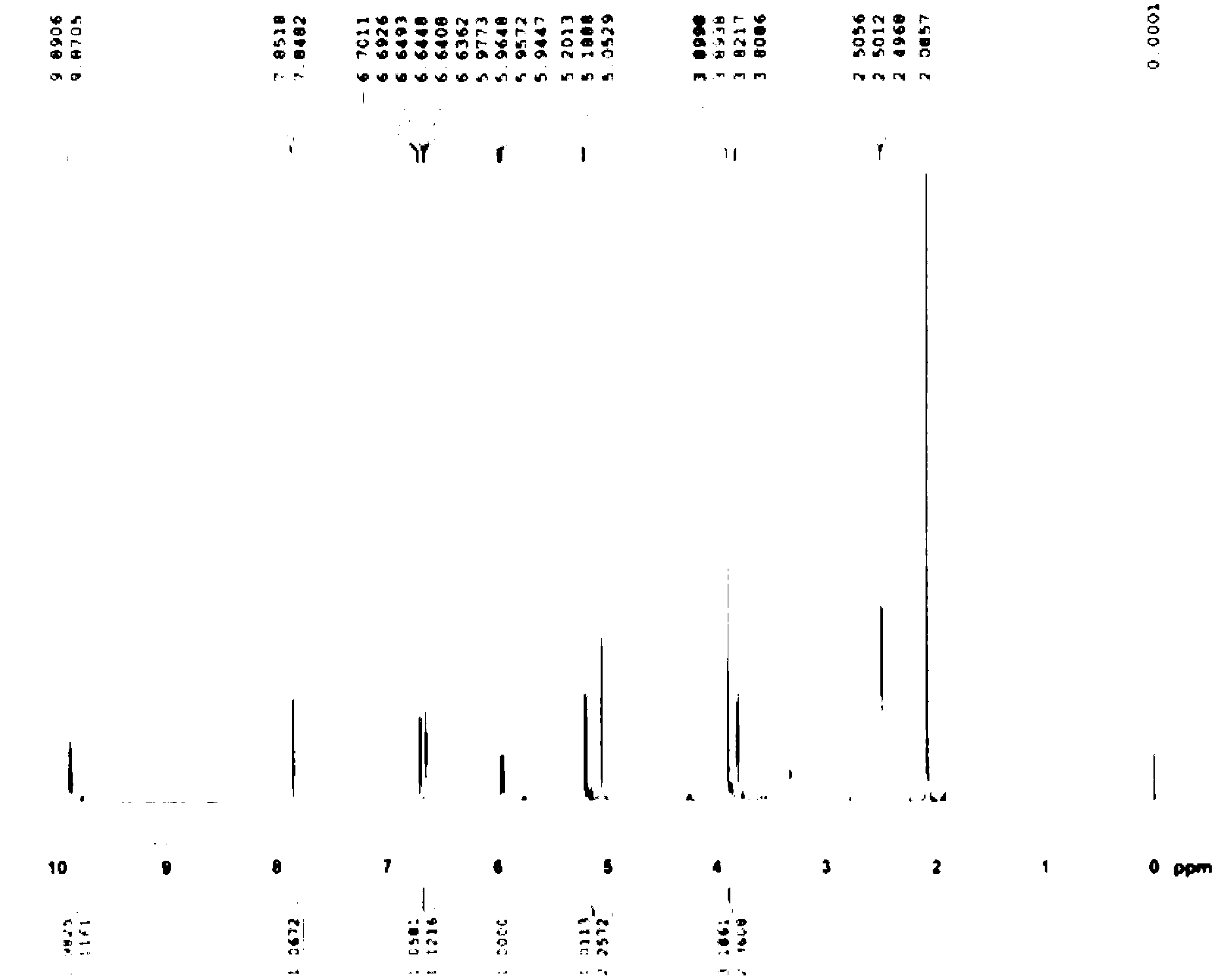
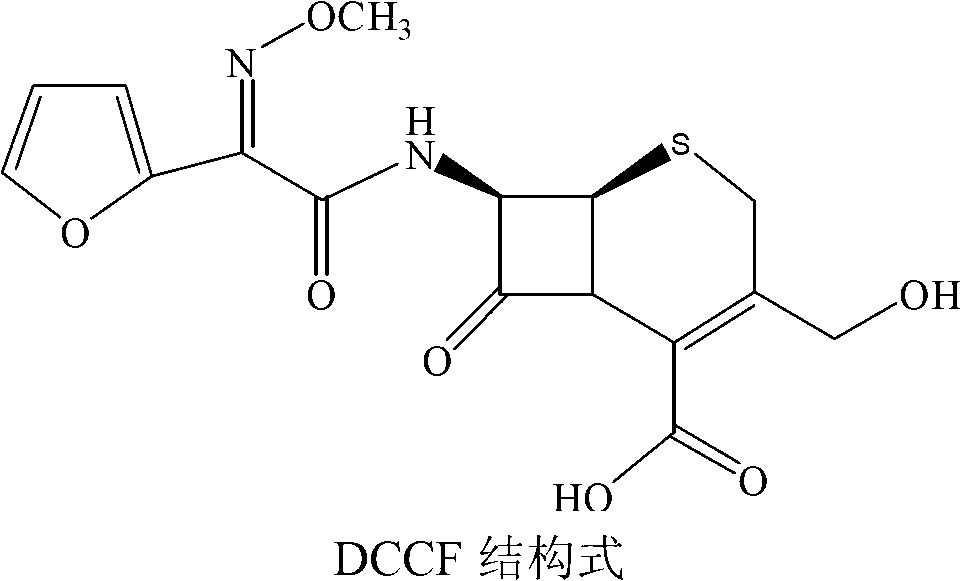
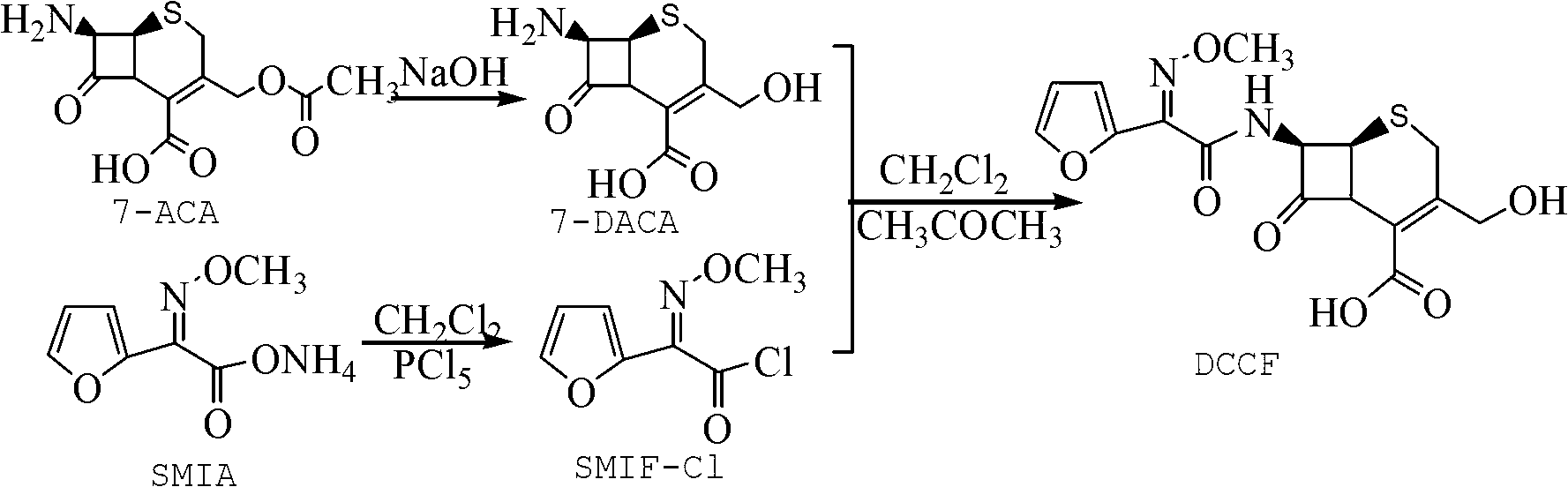
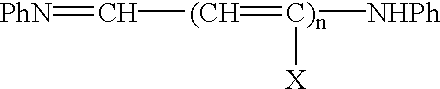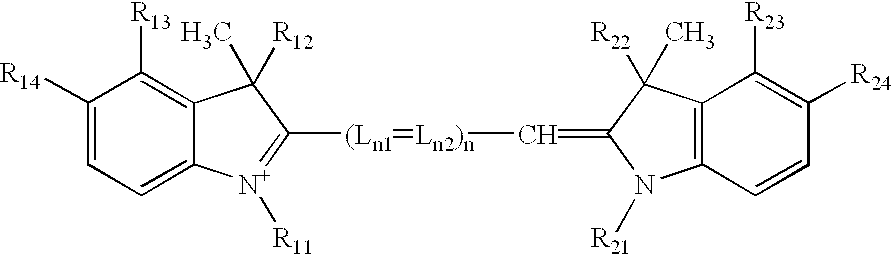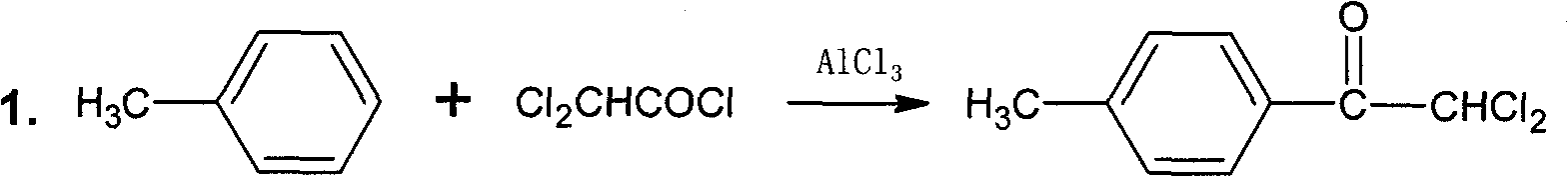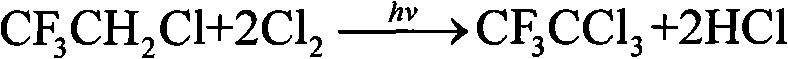Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
873 results about "Acetyl chloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Acetyl chloride (CH₃COCl) is an acyl chloride derived from acetic acid. It belongs to the class of organic compounds called acyl halides. It is a colorless, corrosive, volatile liquid.
Process and method for the preparation of asymmetric monofunctionalized indocyanine labelling reagents and obtained compounds
A process for preparing an asymmetrical indocyanine dye comprising the steps of:a) reacting a first quaternised indolenine or substituted indolenine with a compound of the formula (II)or hydrochloride thereof, wherein n is 0 or 1 Ph is phenyl or substituted phenyl X is hydrogen, halogen or alkyl, preferably chlorine, in a solvent selected from the group consisting of acetic acid, acetic anhydride and mixtures thereof in the presence of acetyl chloride, to obtain an intermediate hemicyanine, andb) further reacting said intermediate hemicyanine with a second quaternised indolenine or substituted indolenine different from said first indolenine.
Owner:VISEN MEDICAL INC
Preparation method of abiraterone acetate
ActiveCN102627681AReduce usageAvoid separation and purificationSteroidsAcetic anhydrideEthyl Chloride
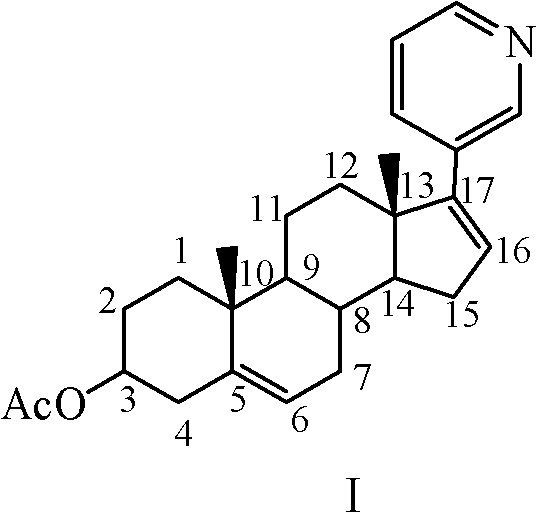
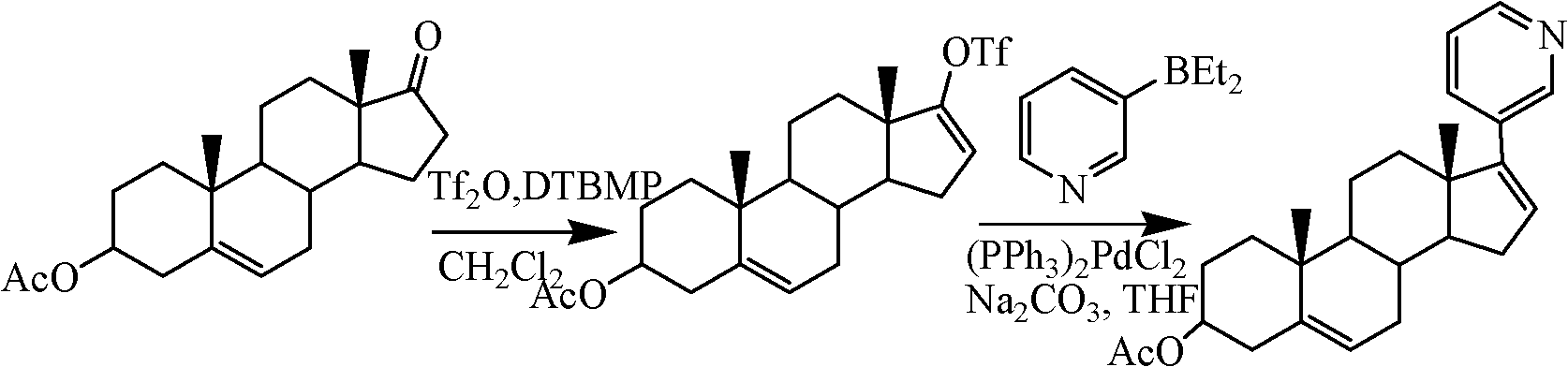
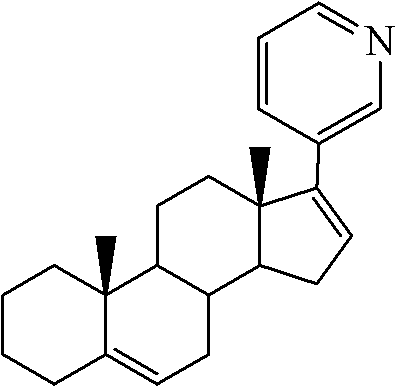
The invention relates to a preparation method of abiraterone acetate. The method comprises: taking dehydroepiandrosterone as the raw material, which successively reacts with hydrazine hydrate and idoine so as to obtain 17-iodo-adrost-5, 16-diene-3beta-ol, which then undergoes a Negishi coupling reaction with 3-pyridine zinc halide to obtain abiraterone, and finally conducting esterification with acetyl chloride or acetic anhydride, thus obtaining the target product abiraterone acetate.
Owner:SHANDONG NEWTIME PHARMA
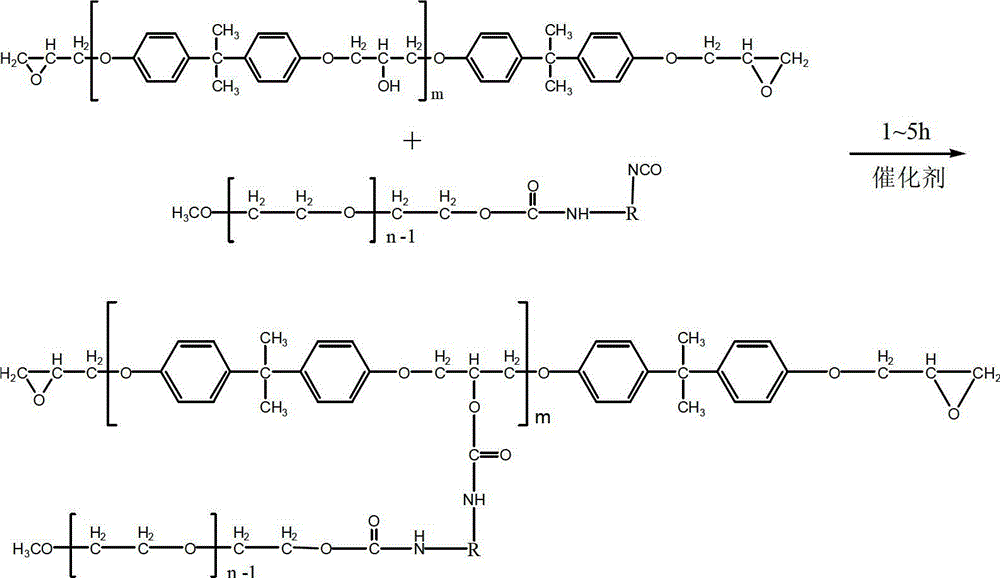
Nonionic self-emulsifying waterborne epoxy resin and preparation method and application thereof
The invention relates to nonionic self-emulsifying waterborne epoxy resin and a preparation method and application thereof. The nonionic self-emulsifying waterborne epoxy resin is prepared by the following steps of: mixing 15 to 55 parts of diisocyanate, 70 to 150 parts of polyethylene glycol monoethylether and 4 to 10 parts of an acetyl chloride acetone solution together in parts by mass, and then reacting at 35 to 65 DEG C for 1 to 5 hours; and adding 100 to 300 parts of epoxy resin into the mixed system, adding 0.5 to 5 parts of a catalyst into the mixed system, and reacting at 40 to 85 DEG C for 1 to 5 hours after mixing. The invention also provides a method for preparing the waterborne epoxy resin into a waterborne epoxy varnish. According to the nonionic self-emulsifying waterborne epoxy resin, after a polyethylene glycol monomethyl ether chain is introduced into a segment, alkaline water or volatile organic weak monoacid is not needed to be added, so that the application range of an emulsion is wide, and the emulsion is easy to prepare; and after crosslinking curing of the emulsion, the network chain molecular weight is improved, the crosslinking density is reduced, and a formed coating film is toughened.
Owner:XI AN JIAOTONG UNIV
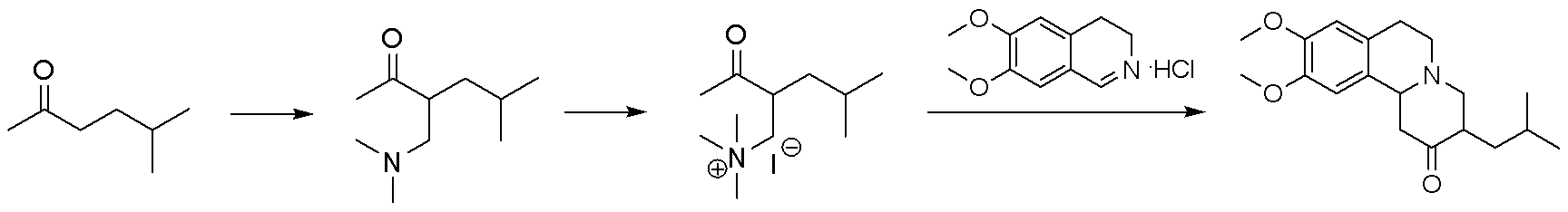
Method for synthesizing tetrabenazine
The invention discloses a method for synthesizing tetrabenazine. The method comprises: step one, using a dimethylamine aqueous solution and a formaldehyde aqueous solution as initial materials to be reacted1 to obtain tetramethyl methane diamine; step two, dissolving the tetramethyl methane diamine obtained in the step one in an organic solvent, dropwise adding acetyl chloride and 5- methyl-2-hexanone, and performing amine methylation to obtain an intermediate 3-[(dimethyl amino) methyl]-5-methyl-2-hexanone; and step three, reacting the 3-[(dimethyl amino) methyl]-5-methyl-2-hexanone obtained in the step two with 6,7-dimethoxy-3,4-dihydroisoquinoline hydrochloride to obtain the tetrabenazine. According to the method for synthesizing tetrabenazine, the cheap and accessible materials are used as the initial materials, imine salts serve as amine methylation reagents to perform amine methylation reaction on the 5- methyl-2-hexanone, and accordingly, the region-selectivity of chemical reactions is improved; and water serves as a reaction solvent to prepare the tetrabenazine, so that the operation is simple and convenient, no complex post-processing process exists, and good industrial application prospects are provided.
Owner:JIANGSU JIMING PHARMA TECH
Process for extracting, preparing and purifying gamma methyllinolenate from algae
InactiveCN1448383AHigh purityEconomical and reasonable processCarboxylic acid esters separation/purificationBulk chemical productionSolventNitrogen gas
Algae powder is added into methanol as entainer, the mixture is homogenated and supercritical extracted with CO2 as solvent and general ester is obtained after concentration. The general ester is dissolved and esterified in methanol containing acetyl chloride to obtain general fatty methyl ester, which is distilled with the distillate being collected. The distillate is mixed with acetone, crystallized in 4 deg.c, -10 deg.c and -30 deg.c successively, filtered to eliminate separated matter, the filtrate is concentrated and mixed with urea and methanol, and the mixture is reflux for 1 hr under the protection of nitrogen, cooled, set, filtered to eliminate crystal, mixed with water, petroleum ether extracted to obtain gamma-methyl linolenate after petroleum ether is recovered via distillation. The obtained gamma-methyl linolenate is purified via high vacuum distillation at 100-150 deg.c.
Owner:NANJING UNIV
Cefuroxime sodium and preparation method thereof
The invention provides cefuroxime sodium and a preparation method thereof. The preparation method comprises the following steps that: (1) 7-aminocephalosporanic acid reacts with 2-(furan-2-base)-2-(methoxyimino) acetyl chloride to produce 3-deacety-7-aminocephalosporanic acid; (2) crystallization is conducted after the 3-deacety-7-aminocephalosporanic acid reacts with chlorosulfonyl isocyanate to produce cefuroxime acid; and (3) the cefuroxime acid is salified to obtain the cefuroxime sodium, wherein solvent for crystallization is selected from one or more of petroleum ether, normal hexane, cyclohexane, solvent oil and tetrahydrofuran. Since the preparation method adopts solvents such as petroleum ether for crystallization in the process of the preparation of the cefuroxime acid, the invention has the advantages that the yield of the product is effectively improved, the product purity is further improved, the impurity content is reduced, the product quality is compliant with Chinese Pharmacopoeia of version 2005, the operation of the method is simple, the raw materials can be easily obtained, the cost is relatively low and the industrial production can be realized easily.
Owner:LIVZON PHARM GRP INC
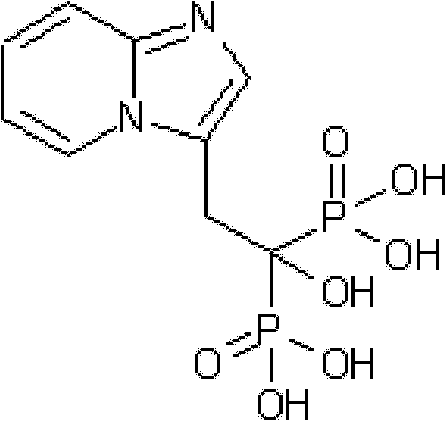
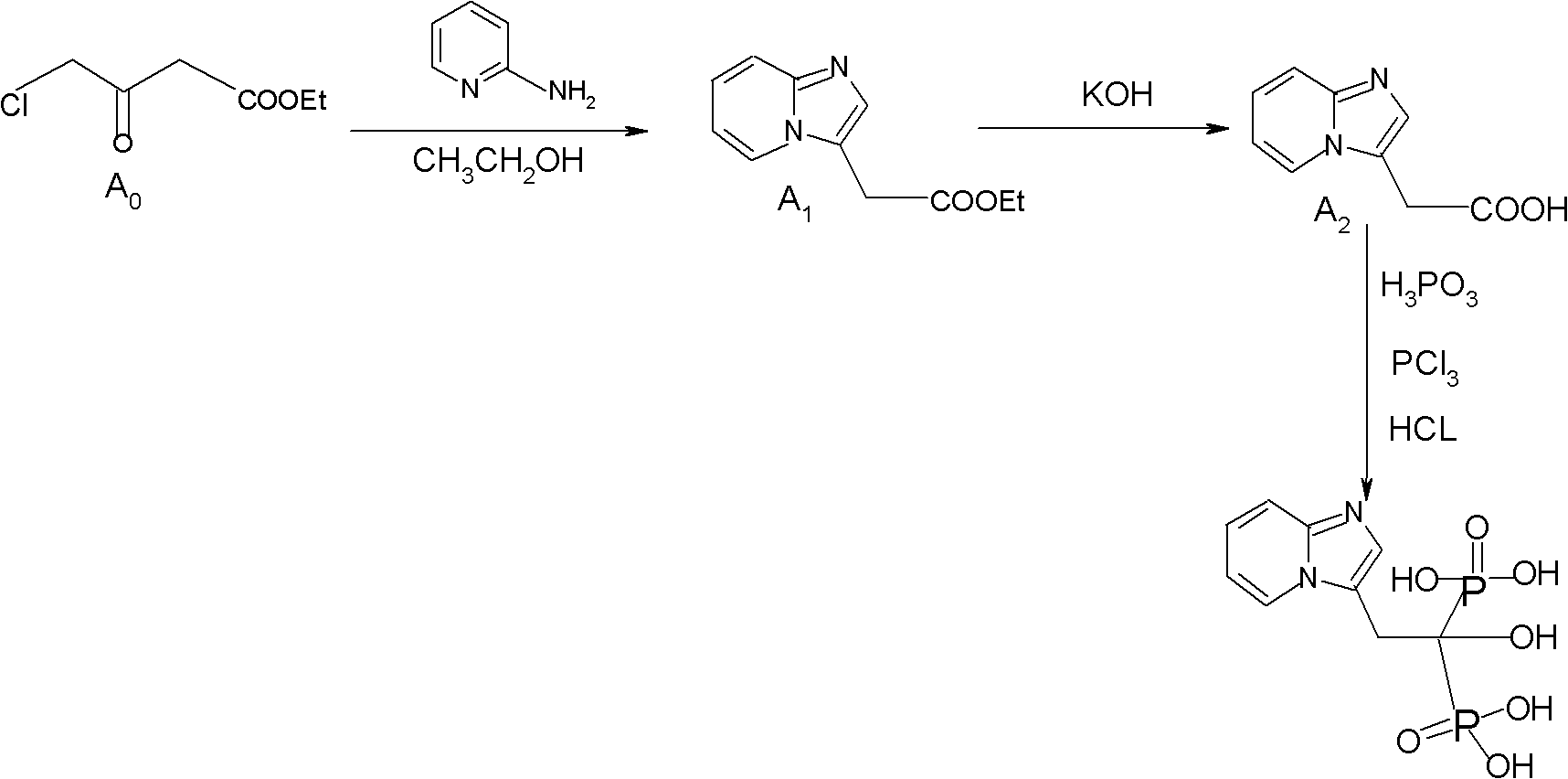
Synthesis method of minodronate midbody and synthesis of minodronate
ActiveCN102153585AAvoid pollutionImprove securityGroup 5/15 element organic compoundsSynthesis methodsFiltration
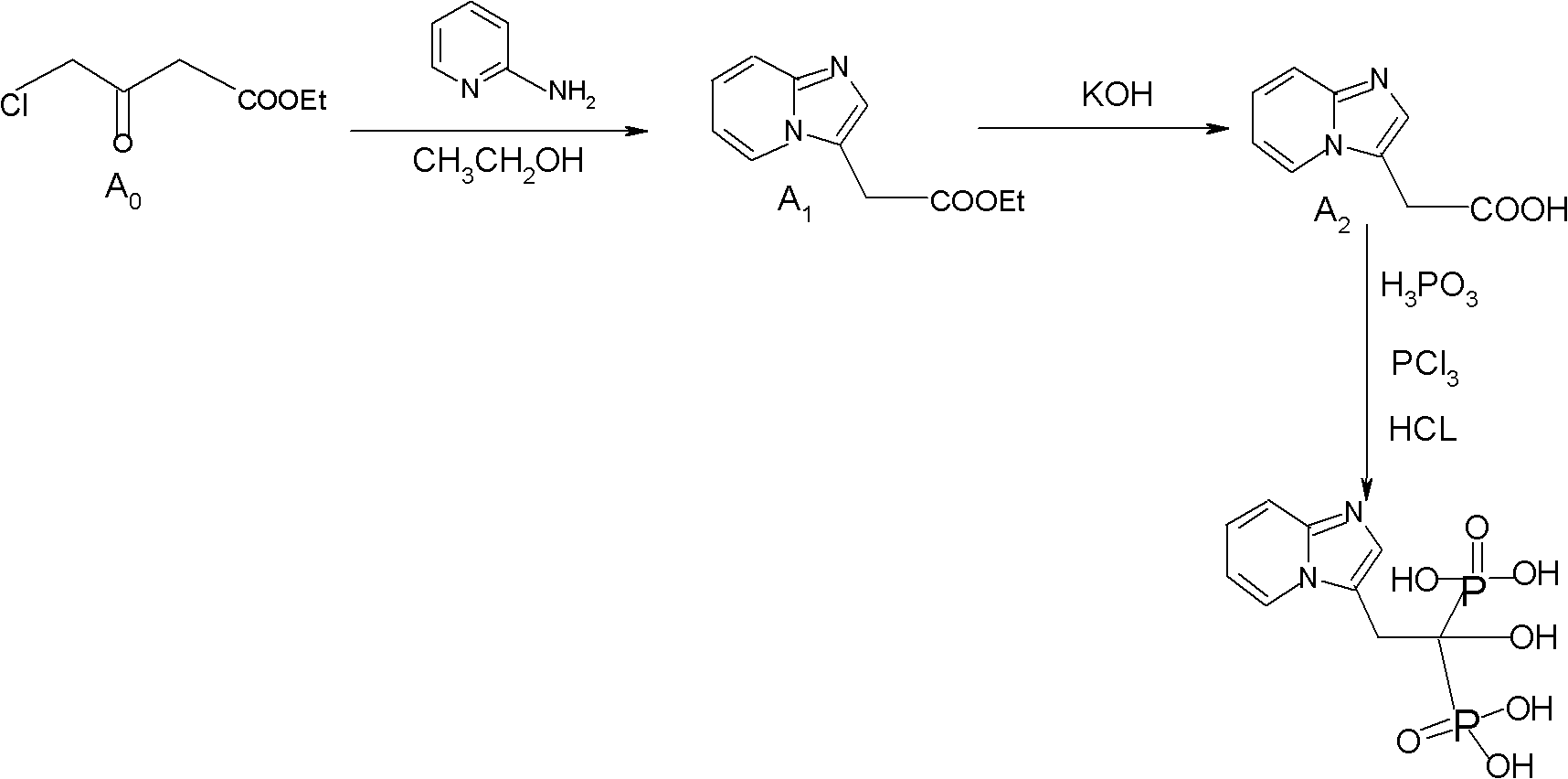
The invention relates to the field of pharmaceutical chemistry, in particular to a synthesis method of a minodronate midbody and synthesis of minodronate. The preparation method of minodronate includes the following steps: using organic solvent to dissolve 2-aminopyridine, adding 4-acetyl chloride ethyl acetoacetate for reaction, monitoring the reaction solution by TLC(Thin-Layer Chromatography) until spots of 4-acetyl chloride ethyl acetoacetate disappear, concentrating to a dry state, dissolving concentrate in water, washing a water layer to remove impurities, extracting the water layer with the organic solvent, washing extract liquor, separating out an organic layer, conducting filtration, and concentrating the concentrate to be in a dry state, thereby obtaining A1.
Owner:福建太平洋制药有限公司
Method for synthesizing 4-chloro-2-(trifluoroacetyl)aniline hydrochloride hydrate intermediate
ActiveCN101844990AGood reaction safetyLow costOrganic chemistryOrganic compound preparationAcetyl chlorideOrganic solvent
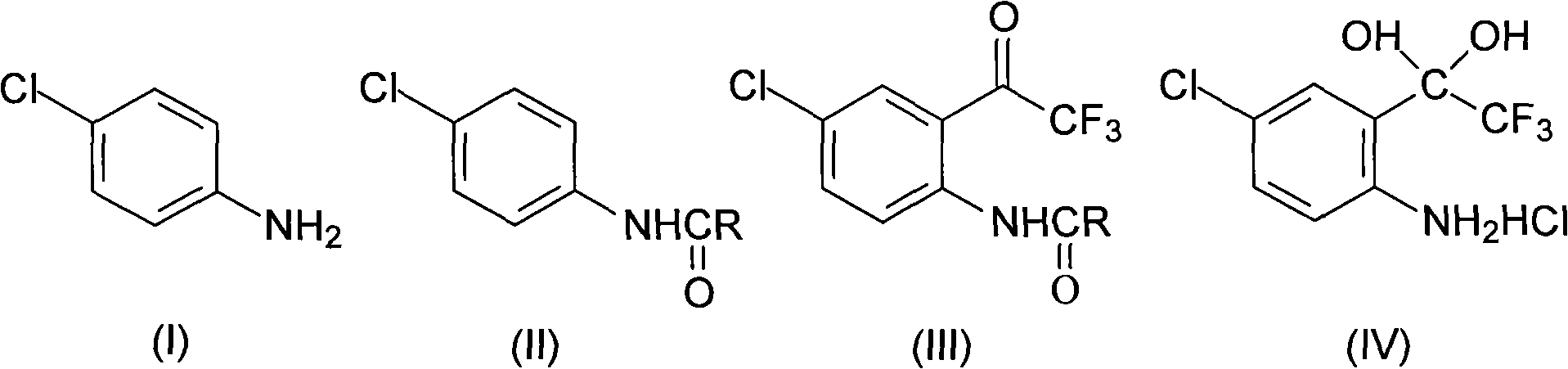
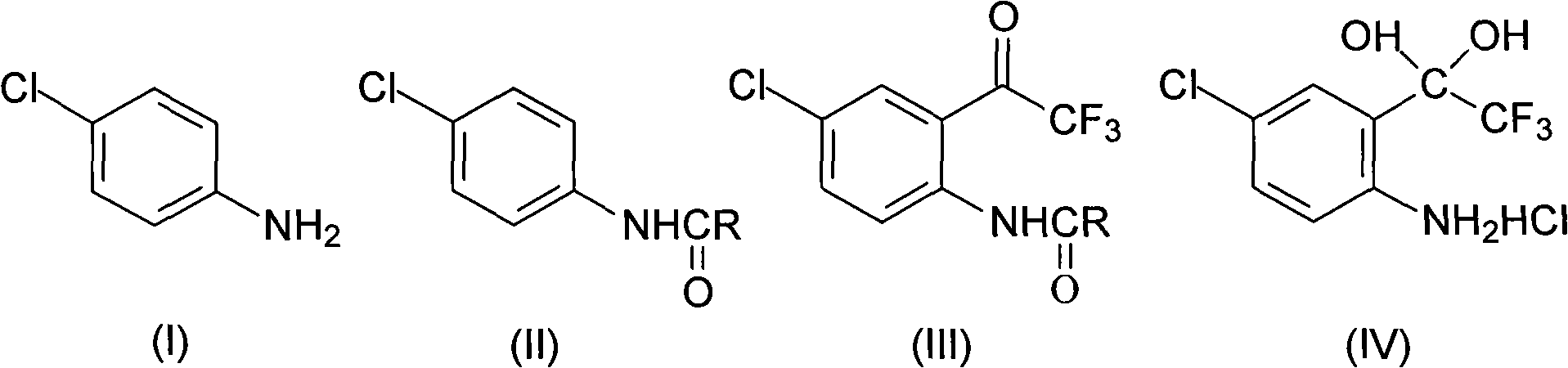
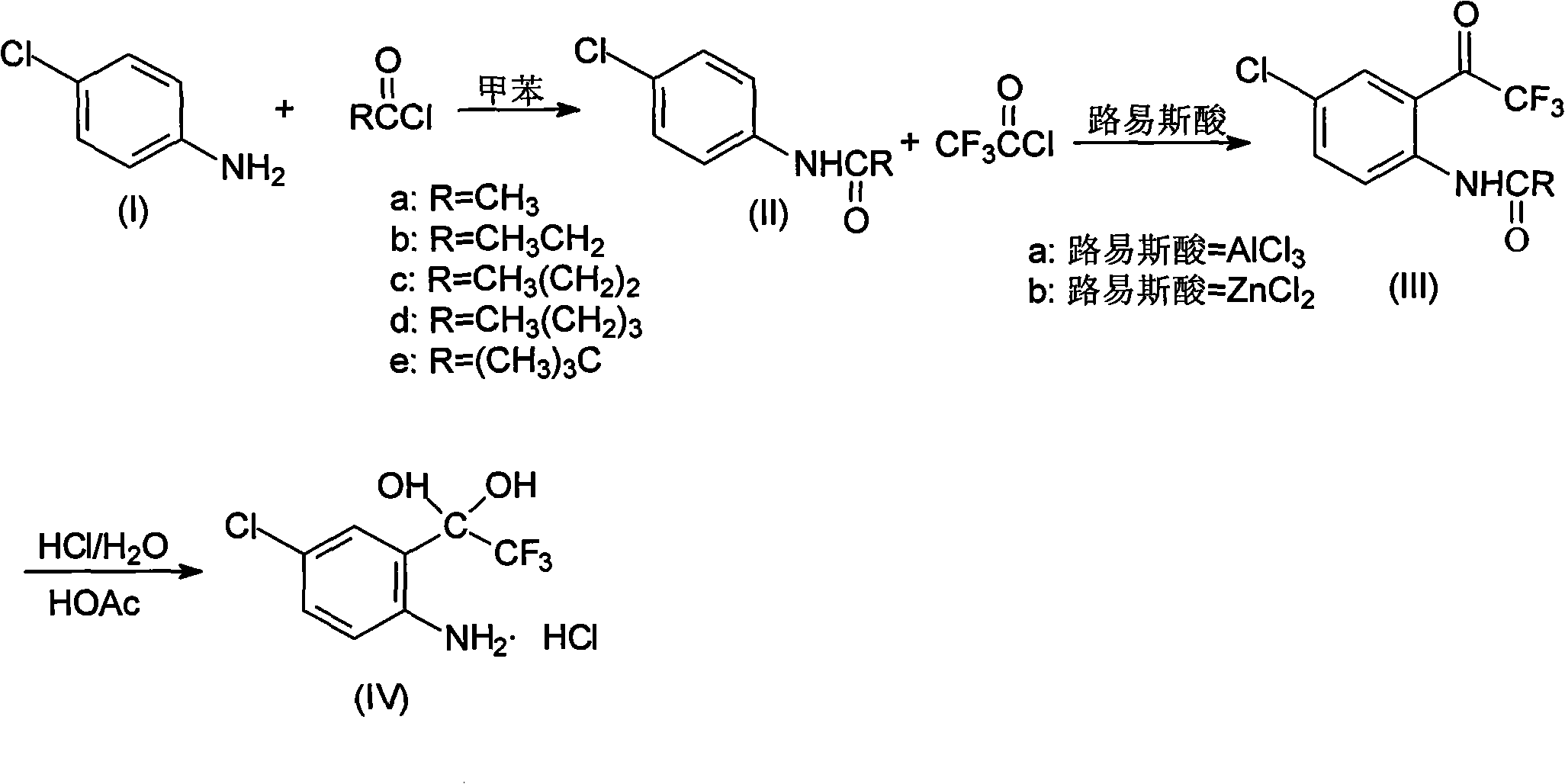
The invention discloses a method for synthesizing a 4-chloro-2-(trifluoroacetyl)aniline hydrochloride hydrate intermediate, which belongs to the technical field of compound preparation methods. The method comprises the following steps of: 1) reacting parachloroaniline with acyl chloride in the presence of a methylbenzene solvent to obtain an intermediate (II); 2) dissolving the intermediate (II) in organic solvent to obtain solution, introducing trifluoro-acetyl chloride into a flask which is filled with the mixed liquor of the organic solvent and lewis acid and slowly filling the solution ofthe intermediate (II) into the flask dropwise to perform a Friedel-Crafts reaction to obtain an acylate intermediate (III), wherein a charging molar ratio of the trifluoro-acetyl chloride to the intermediate (II) is 1: 1; and 3) hydrolyzing the intermediate (II) to remove protective groups to form a salt under acid condition to obtain the 4-chloro-2-(trifluoroacetyl)aniline hydrochloride hydrate intermediate(IV). The method has the advantages of high safety, low cost, simple operation, less pollution, total yield of over 78.1 percent and content of more than or equal to 99.0 percent.
Owner:浙江沙星科技股份有限公司
Alkyl acyloxy silane mixture and preparation method thereof
ActiveCN101531775AEasy to prepareReduce manufacturing costGroup 4/14 element organic compoundsLiquid productAcetic anhydride
The invention relates to an alkyl acyloxy silane mixture and a preparation method thereof, belonging to the technical field of preparation of alkyl acyloxy silane mixture. In the mixture, the content of methyltriacetoxysilane is 75% to 85%, and the content of alkyltriacetoxysilane is 15% to 25%; alkyl is ethyl, ethylene, n-propyl, isopropyl or allyl, the content of active principle is greater than 98%, and the appearance is water-white transparent liquid. The preparation method of the mixture has low production cost and environment-protection benefit with excessive raw material acetic anhydride and generated by-product acetyl chloride recovered by rectification. The invention solves the difficulties of production, package transportation and application as the high-purity alkyl acyloxy silane mixture product is solid crystal at room temperature at present as well as the existing problems of low content of active principle, color, unstable quality as the mixture is not condensed into liquid product at room temperature so that the product can only be applied to low-end single-compound room temperature sulphurated siliastic. The preparation method can be applied to high-end single-compound room temperature sulphurated siliastic.
Owner:JINGZHOU JIANGHAN FINE CHEM
Synthesis method of 4-chloro-2-cyano-1-dimethylamino-sulfonyl-5-(4-methylphenyl)imidazo
ActiveCN102424671AEmission reductionShort reaction timeOrganic chemistrySodium dithioniteN-Chlorosuccinimide
The invention discloses a synthesis method of 4-chloro-2-cyano-1-dimethylamino-sulfonyl-5-(4-methylphenyl)imidazo, which aims to solve the technical problems of long reaction time, low yield and environment pollution of the prior art. The method is used for preparing 2,2-dichloro-4'-methyl acetophenone as an intermediate through an acylation reaction of methylbenzene and dchloroethanoyl chloride and preparing 4(5)-chloro-2-cyano-5(4)-(4-methylphenyl)imidazo as an intermediate by taking ethyl acetate and the like as solvent, N-succinchlorimide as chlorinating agent and sodium dithionite and the like as reductant. The synthesis method has short reaction time, high yield and total yield up to 61.7% and is mainly used for preparing the 4-chloro-2-cyano-1-dimethylamino-sulfonyl-5-(4-methylphenyl)imidazo.
Owner:XIAN MODERN CHEM RES INST
Preparation method of 2,5-dichlorophenol
InactiveCN104591973AThe synthesis process is simpleShort routeOrganic compound preparationCarboxylic acid esters preparationHydrolysisPeroxide
The invention discloses a preparation method of 2,5-dichlorophenol and relates to the technical field of pesticide intermediate synthesis. The preparation method comprises the following steps: with p-dichlorobenzene as a start raw material, performing a Friedel-Crafts acylation reaction between the p-dichlorobenzene and acetyl chloride in the presence of aluminum trichloride to obtain 2,5-dichloroacetophenone; performing a Baeyer-Villiger oxidation reaction between the 2,5-dichloroacetophenone and a peroxide in the presence of a catalyst at room temperature to obtain 2,5-dichlorobenzene acetate; and performing a hydrolysis reaction between the 2,5-dichlorobenzene acetate and inorganic aqueous alkali in a reflux condition to obtain 2,5-dichlorophenol. The preparation method disclosed by the invention has the characteristics of simple synthesis process, short line, low production cost and high yield; and moreover, with less quantity of generated three wastes and high environmental protection property, the preparation method is more suitable for large-scale industrial production.
Owner:ANHUI XUELANG BIOTECHNOLOGY CO LTD
Alkynyl thiophene ketone compound and preparation method and applications thereof
InactiveCN101565416ALow toxicityAvoid Persistent ResiduesBiocideOrganic chemistryMicroorganismBiological body
The invention discloses an alkynyl thienone ketone compound, and a preparation method and applications thereof. The alkynyl thienone ketone compound contains a structure shown as a general formula (I): in an inert solvent and under the action of catalyst, substituted 2-aromatic acetylenyl thiophene and substituted aromatic acetyl chloride are reacted to prepare the alkynyl thienone ketone compound. The alkynyl thienone ketone compound has toxicity or lower toxicity in self, can generate free radical for killing the toxicity under the action of light and oxygen, attack more biomacromolecules in an organism and by a free radical chain reaction and shows high efficiency; the compound exerts toxic effect and simultaneously also occurs the degradation and avoids the persistent residues in the environment, thereby further improving the security; the compound has insecticidal activity, weeding activity and the activities to microbes, such as epiphyte, bacteria, virus, and the like and has simple and portable preparation method as well as lower cost.
Owner:SOUTH CHINA AGRI UNIV
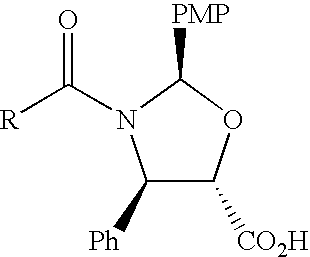
Anticancer taxanes such as paclitaxel, docetaxel and their structural analogs, and a method for the preparation thereof
A process for the preparation of taxanes comprising wherein R is a tert. butoxycarbonyl or benzoyl group; PMP is p-methoxyphenyl group; G1 is acetyl group; G2 is haloacetyl group comprisinga) protecting the C-7 hydroxyl group of 10-deacetylbaccatin III with haloacetyl chlorides and then acetylating the C-10 hydroxyl group with acetyl chloride to obtain a protected 10-deacetylbaccatin III (1);b) subjecting the protected 10-deacetylbaccatin III (1) to coupling with an oxazolidine-5-caboxylic acid of formula 2 wherein R is tert. butoxycarbonyl or benzoyl; PMP is p-methoxyphenyl group in the presence of a condensation agent and an activating agent to obtain C-13 esters of formula 3;c) treating the coupled products 3 with weak acidic medium to open the oxazolidine ring to obtain intermediates of formula 4; wherein R is a tert. butoxycarbonyl or benzoyl group; G1 is acetyl group; G2 is haloacetyl groupd) subjecting the intermediates of compound 4 to selective deprotection of haloacyl group in the presence of acetyl group under mild alkaline condition at −20 to +40° C. for 6-24 h in the presence of ammonia or aliphatic amine or aromatic amines or their combination to obtain paclitaxel or docetaxel.
Owner:DABUR PHARM LTD
Method for preparing lidocaine
ActiveCN102070483AImprove responseHigh purityOrganic compound preparationCarboxylic acid amides preparationDimethylaniline N-oxideAcetyl chloride
The invention provides a method for preparing lidocaine. The method comprises the following steps: using 2,6-dimethylaniline and chloroacetic chloride as raw materials to prepare an intermediate, namely acetyl chloride-2,6-dimethylaniline, and using the prepared intermediate and diethylamine to react and obtain lidocaine, wherein acetone is used as solvent and carbonate is used as catalyst in thereaction process. The method of the invention has simple synthetic technology and does not require the complicated step that the intermediate is washed with acid firstly and washed with base secondlyin the post-treatment, thus avoiding unnecessary loss. Therefore, the yields of the intermediate and lidocaine prepared by the method are higher; and the prepared lidocaine has high purity which is more than 99%, and good industrial application prospect. In addition, the method of the invention uses acetone as solvent, thus the solvent is non-toxic basically and environmentally friendly, has no stimulation and can be recycled.
Owner:BENGBU BBCA MEDICINE SCI DEV
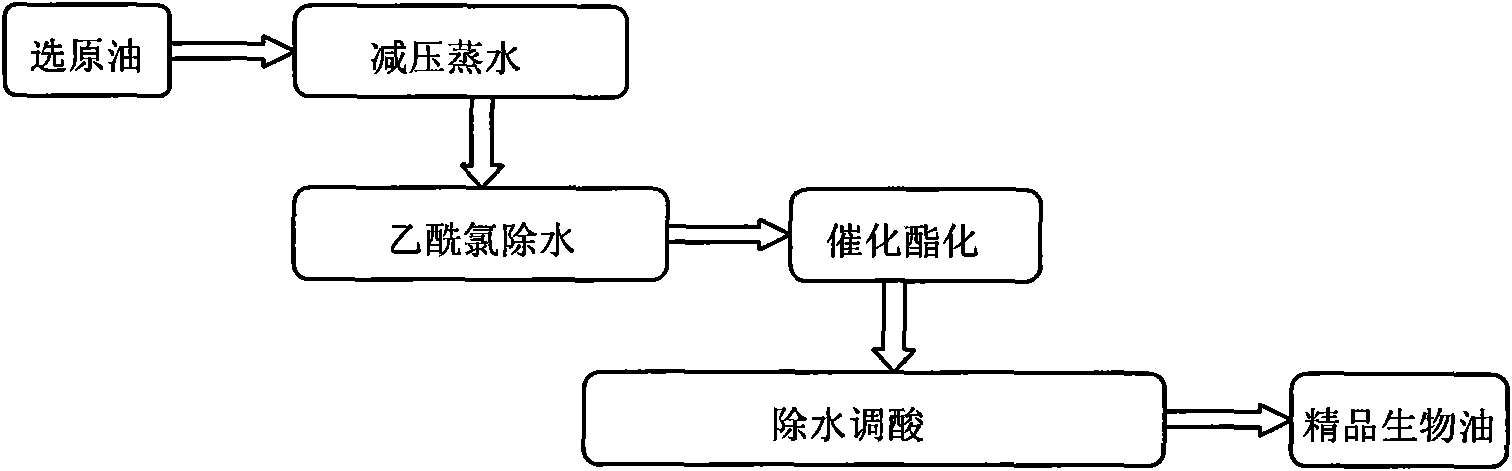
Biomass pyrolysis oil refining method
InactiveCN101899334AWith surfactantRaise the pHLiquid carbonaceous fuelsHydrocarbon oils treatmentWater bathsMass ratio
The invention belongs to the technical field of biomass energy conversion, and relates to a biomass pyrolysis oil refining method. The method comprises the following steps: mixing biological oil stock solution and isooctyl alcohol in the volume ratio of 5:1, adding 2-10 zeolite grains, keeping the low vacuum of 0.02 Mpa, and distilling under reduced pressure in a 80 DEG C water bath until no water can be distilled off, thereby obtaining low-water biological oil; during magnetic stirring, adding equal volume of acetyl chloride to react until no bubbles burst out, thereby obtaining anhydrous biological oil; calculating the generation amount of acetic acid according to the amount of the anhydrous biological oil, adding ethanol to acetic acid in the mol ratio of 2.8:1, mixing catalyst and theanhydrous biological oil in the mass ratio of 1:35, adding 2-10 zeolite grains, stirring, heating for back flow at 80 DEG C, and esterifying to obtain the esterified biological oil; adding anhydrous magnesium sulfate to esterified biological oil in the mass ratio of 1:5 to dehydrate and regulate the acidity; and filtering under reduced pressure to remove solid, thereby obtaining the refined biological oil. The invention has the advantages of simple process route, favorable dehydration effect and high product yield.
Owner:QINGDAO UNIV
Method for producing 2,4-dichloroacetophenone by using solid waste chlorobenzene tar as raw material
InactiveCN101898947ASolve your worriesAlleviate supply and demand pressureCarbonyl compound preparation by condensationAcetyl chlorideM-dichlorobenzene
The invention discloses a method for producing 2,4-dichloroacetophenone by using solid waste chlorobenzene tar as a raw material. The method mainly comprises the following steps of: extracting m-dichlorobenzene from the solid waste chlorobenzene tar, and then performing five steps of acylation, washing, distillation, crystallization and packing in the 2,4-dichloroacetophenone production process. The method is characterized in that: the acylation reaction comprises that: the m-dichlorobenzene and acetyl chloride undergo the acylation reaction under the action of anhydrous aluminum trichloride, the dripping temperature of the acetyl chloride is kept between 50 and 60 DEG C during reaction, the temperature is gradually raised to between 90 and 95 DEG C after the dripping is finished, and reflux stirring reaction is performed for about 4 hours to obtain the 2,4-dichloroacetophenone. The method has the advantages that: the m-dichlorobenzene is reclaimed from the solid waste chlorobenzene tar during production, and the reclaimed m-dichlorobenzene is used as the raw material for producing the 2,4-dichloroacetophenone, so by the method, the 2,4-dichloroacetophenone is produced by using the m-dichlorobenzene as the raw material while extra worries of m-dichlorobenzene and o-dichlorobenzene production enterprises are solved, the supply and demand pressure of chlorobenzene markets is relieved and extended products are further developed and utilized.
Owner:JIANGSU LONGCHANG CHEM
Synthetic method of ciprofibrate
ActiveCN103613498AAvoid generatingHigh yieldOxygen-containing compound preparationOrganic compound preparationAcetic anhydrideOrtho position
The invention discloses a synthetic method of ciprofibrate. The method comprises the following steps: taking styrene as a starting material; obtaining the ciprofibrate by the processes of cyclization, acylation, Baeyer-Villiger oxidation, alcoholysis, alkylation and hydrolysis, wherein an acylation reagent adopted in the acylation process is R1COCl; the R1 is C4-C12 alkyl. Fatty acyl chloride of which a hydrocarbyl structure is a long chain is adopted in acylation reaction, so that the steric hindrance is increased, the reaction activity of acyl chloride is reduced, the content of the produced ortho-position isomer is smaller than 0.2% and superior to that of acetyl chloride reaction (the content of the ortho-position isomer is 0.5-1%), the yield and the purity of the product are improved, and meanwhile, a room-temperature reaction condition is adopted, so that the energy consumption of production is reduced. Urea peroxide instead of hydrogen peroxide is used as an oxidant in the Baeyer-Villiger oxidation, and acetic acid is used instead of acetic anhydride, so that the reaction condition is milder, and the operation is more controllable.
Owner:ZHEJIANG SANMEN HYGECON PHARMA CO LTD
Method for preparing 2, 4-dichloroacetophenone
InactiveCN102675073AImprove securityThe operation process is safe and controllableCarbonyl compound preparation by condensationAcetic anhydrideDistillation
The invention discloses a method for producing 2, 4-dichloroacetophenone. The method mainly includes acylation, hydrolysis, washing, distillation and crystallization, and m-dichlorobenzene and acetic anhydride are in acylation reaction under the effects of anhydrous aluminum trichloride. The method has the advantages that the m-dichlorobenzene is used as a raw material, the acetic anhydride is used as an acylating agent, A1C13 is used as a catalyst to prepare the 2, 4-dichloroacetophenone by means of reaction, and yield of obtained products is higher than that of products prepared by using acetyl chloride as an acylating agent; as the acetic anhydride is used as the acylating agent, the A1C13 is used as the catalyst and the m-dichlorobenzene which is extracted from chlorinated aromatics waste is comprehensively used as the raw material to prepare the 2, 4-dichloroacetophenone, by-products in a benzene chlorination process are disposed, reusing rate of chlorinated aromatics is increased, and the method has high economical and economical benefits.
Owner:JIANGSU LONGCHANG CHEM
Method for preparing acetsalicylamide
InactiveCN101386581AReduce pollutionImprove conversion rateOrganic compound preparationCarboxylic acid amides preparationNitrogen gasSolvent
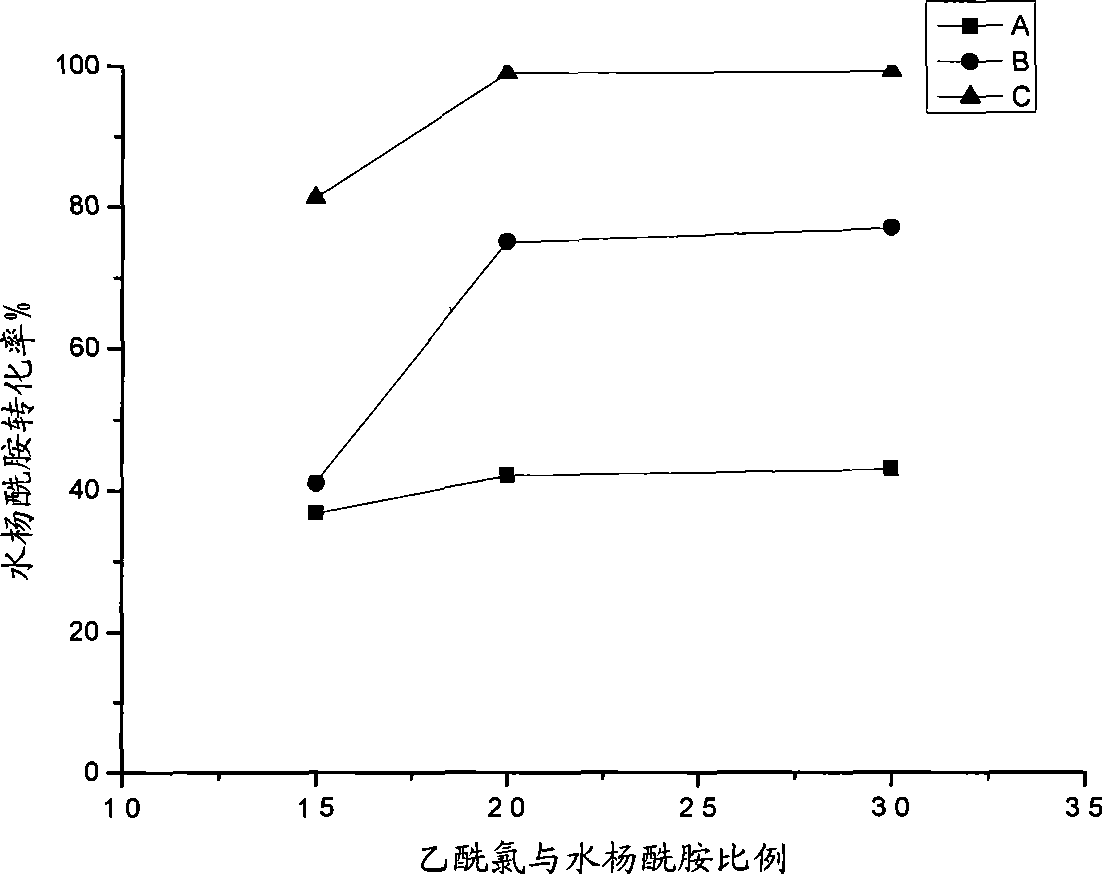
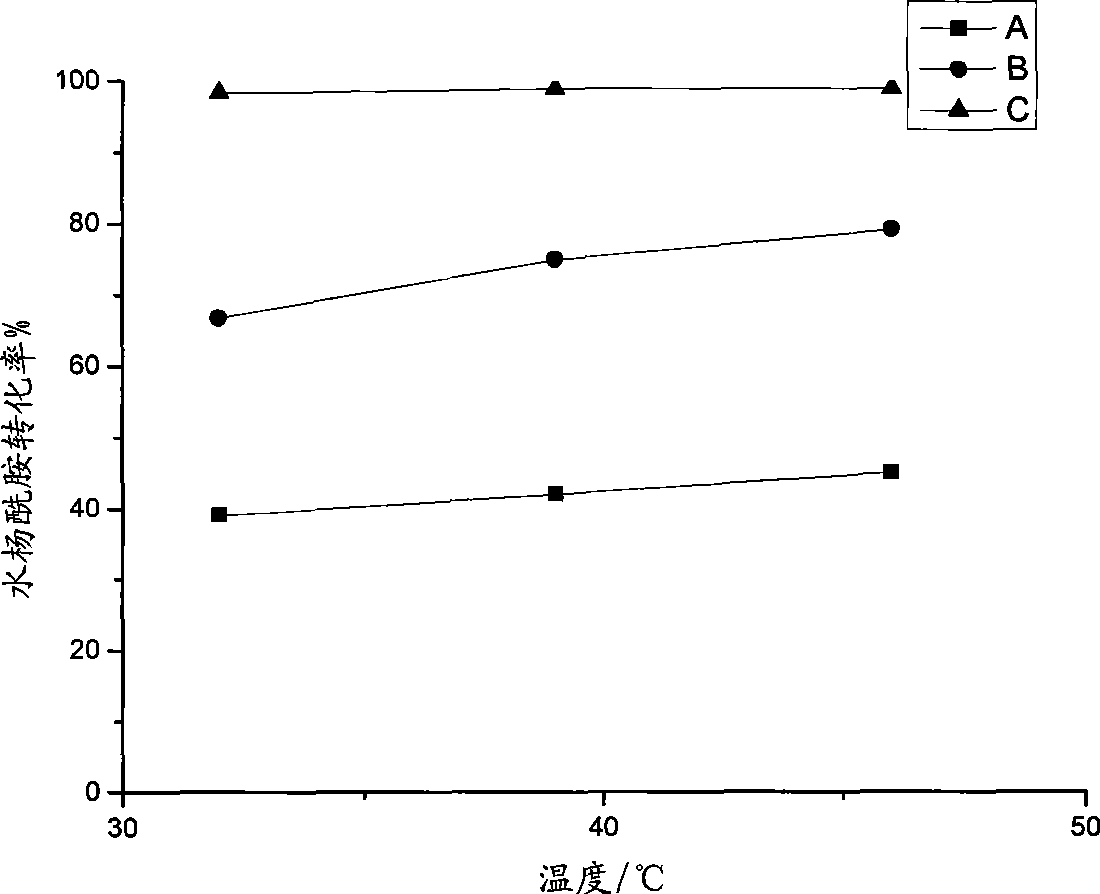
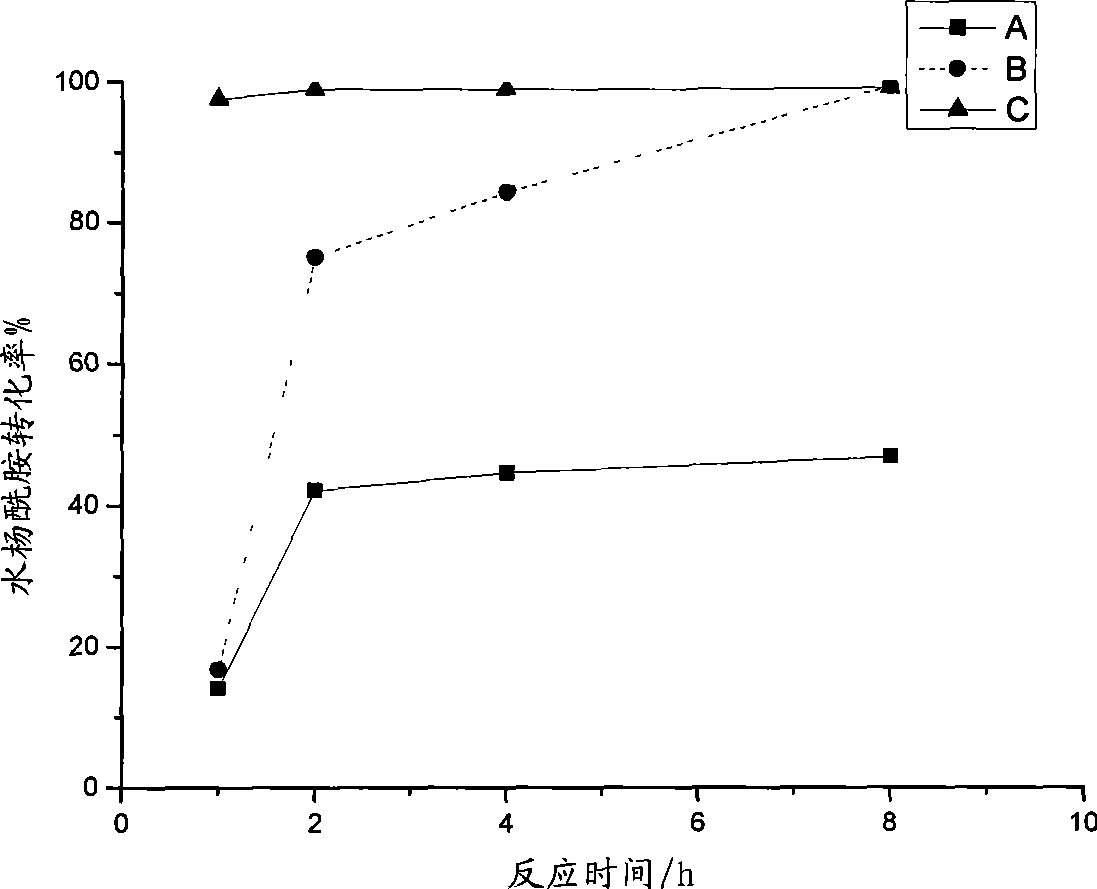
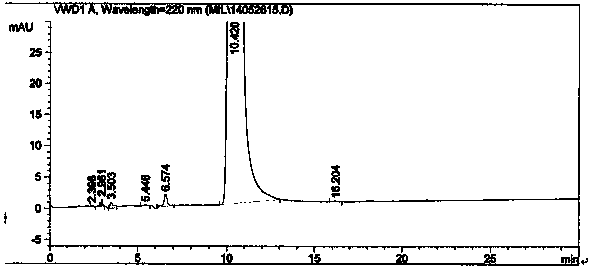
The present invention relates to a method for preparing actylamide, in particular to a novel acylation method for preparing actylamide by using triethyl ammonium-alchlor (C2H5)3NHCl-nAL3 (n is equal to 2.0 minus 2.5) ion liquid as a catalyst and a solvent to catalyze salicylamide. The method is characterized in that in the presence of nitrogen, acetyl chloride is added to undergo synthesis reaction when the temperature of the salicylamide reactant in the ion liquid rises to between 32 and 46 DEG C. The method has the advantages that the obtained actylamide is high in conversion rate, good in selectivity, and high in purity, the reaction time of the process is short, the method is economic in resources, low in risk and toxicity, low in contamination, easy to control reaction conditions, and easy and simple in the process, and the like.
Owner:JIANGSU UNIV
Preparation method for high-purity milrinone
ActiveCN104387320AQuality improvementImprove crystal effectOrganic chemistryAcetic anhydrideSingle crystal
The invention discloses a preparation method for high-purity milrinone (shown as a formula (I), 1,6-dihydro-2-methyl-6-oxo-3,4-bipyridine-5-carbonitrile), and belongs to the field of chemical medicines. The method comprises: employing 4-methylpyridine as a raw material and acetylating with acetyl chloride, and hydrolyzing after the reaction is finished, so as to obtain a compound of a formula (III); mixing the compound of the formula (III) with glacial acetic acid, acetic anhydride and triethyl orthoformate, and reacting at 35 DEG C-45 DEG C, so as to obtain a compound of a formula (IV); performing cyclization on the compound of the formula (IV) and alpha-cyanoacetamide, so as to obtain a crude product of a compound of the formula (I); and refining the crude product of the formula (I) compound through an ethanol-water system, so as to obtain a high-purity refined product with the maximum interplanar spacing d of 8.39 + / - 0.02 Angstrom. The technology is relatively mild in reaction conditions and relatively simple in operation, and is capable of preparing the milrinone product with high purity and a single crystal form. The obtained milrinone crystal form is relatively excellent in solubility in normal saline or glucose, and is beneficial for improvement of the preparation quality.
Owner:HUZHOU ZHANWANG PHARMA
Preparation method of cefepime hydrochloride
ActiveCN101935325ASimple processAvoid the phenomenon of inhomogeneous crystal form and poor fluidityOrganic chemistryCefepime hydrochlorideBetaine
The invention discloses a preparation method of cefepime hydrochloride, comprising the following steps of: reacting oxalyl chloride with 2-methoxyimino-2-(2-aminothiazole-4-yl) acetic acid hydrochloride to obtain a midbody I, i.e. 2-methoxyimino-2-(2-aminothiazole-4-yl) acetyl chloride hydrochloride; mixing silanized 7-aminoce-phalosporanic acid and silanized N-methylpyrrolidine, and reacting to obtain a midbody II, i.e. hydriodic acidification (6R, 7R)-7-amino-3-[(1-methyl-1-tetrahydro pyrrolidine) methyl]-3-cephem-4-formic betaine, in the presence of trimethyl idodine silicon hydride, isopropanol and an aqueous solution of hydrogen iodide; dissolving the midbody II into dichloromethane, sequentially adding trimethylchlorosilane and hexamethyldisilazane for reaction, and then adding the midbody I and triethylamine to react to prepare the cefepime hydrochloride. The cefepime hydrochloride prepared by the method has the advantages of uniform crystal form, good flowability and simple process and is suitable for industrialized production.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
Rubber with fluorescence response to pH value and preparation method thereof
InactiveCN102174131AOzone resistantUV resistantFluorescence/phosphorescenceEthylenediamineChemical reaction
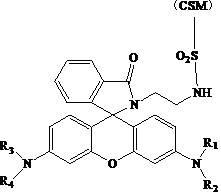
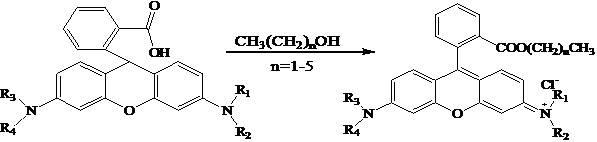
The invention relates to rubber with fluorescence response to a pH value and a preparation method thereof. The method comprises the following steps of: measuring acetyl chloride, adding into rhodamine B-containing alcohol solution dropwise, preserving heat for reaction, evaporating to remove the alcohol solution under reduced pressure, and washing by using ethyl acetate to obtain rhodamine B ester; dissolving the rhodamine B ester in hot methanol, adding ethylenediamine, performing refluxing reaction, cooling to room temperature, removing the methanol, adding an organic solvent 1 and water, and washing and drying an organic layer to obtain ethylenediamine substituted rhodamine B; and weighing chlorosulfonated polyethylene rubber, swelling in an organic solvent 2, adding the ethylenediamine substituted rhodamine B, reacting, filtering off a solid, washing, and drying to obtain the rubber with the fluorescence response to the pH value. The rubber prepared by the method is functional rubber with the fluorescence response to the pH value, and can detect and analyze the pH value of a biological system, a chemical reaction system, industrial wastewater, a river, a lake or an ocean.
Owner:FUJIAN NORMAL UNIV
Improved tadalafil preparation method
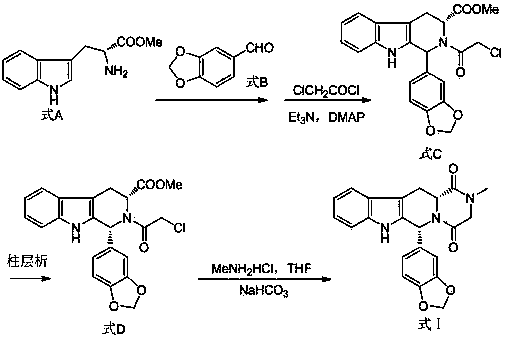
The invention belongs to the field of preparation of chemical raw medicaments, and more in particular relates to an improved preparation method for a phosphodiesterase 5 inhibitor tadalafil. A specific synthesis route is shown in the specification. The method comprises the following steps of performing Pictet-Spengler cyclization reaction and chloroacetyl chloride acylation on starting reactants (D-tryptophan methyl ester hydrochloride and piperonal) to obtain an intermediate product, directly performing subsequent reaction on the intermediate product without purification, preparing an intermediate 1-(1,3-benzodioxol-5-yl)-2-(chloracetyl)-2,3,4,9-tetrahydro-1H-pyridino-[3,4,-B]indol-3-thiophenate methyl by using a one-pot reaction method, performing column chromatography purification to obtain a single cis-isomer, and finally reacting the single cis-isomer with methylamine hydrochloride in the presence of an inorganic base to obtain the tadalafil.
Owner:ANHUI WANBANG MEDICAL TECH
Preparation method of 2-bromo-2,2-difluoroacetyl chloride and 2-bromo-2,2-difluoro acetate and recycling method of waste difluoro trichloroethane
InactiveCN104761446AEnable recyclingIncrease added valuePreparation by hydrogen halide split-offPreparation from carboxylic acid halidesBromoethanePhenol
The invention relates to a preparation method of 2-bromo-2,2-difluoroacetyl chloride and 2-bromo-2,2-difluoro acetate and a recycling method of waste difluoro trichloroethane. The preparation method comprises the following steps: with waste difluoro trichloroethane produced in the production process of dichlorotrifluoroethane as a raw material, carrying out dehydrochlorination to obtain difluoro dichloroethylene, carrying out addition reaction on difluoro dichloroethylene and bromine to obtain difluoro dichlone dibromoethane; reacting difluoro dichlone dibromoethane with sulfur trioxide to obtain 2-bromo-2,2-difluoroacetyl chloride; and reacting 2-bromo-2,2-difluoroacetyl chloride with alcohol or phenol to obtain 2-bromo-2,2-difluoro acetate series products. According to the preparation method and the recycling method, recycling of waste difluoro trichloroethane is realized; 2-bromo-2,2-difluoroacetyl chloride and 2-bromo-2,2-difluoro acetate are prepared by a temperature oscillation method, so that the production cost is reduced; and meanwhile, the preparation method is an environment-friendly technique for producing products.
Owner:JIANGXI SUNWAY CHEM CO LTD
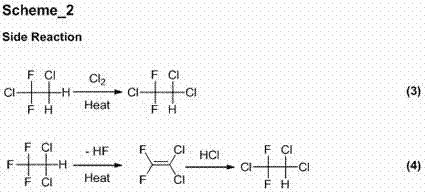
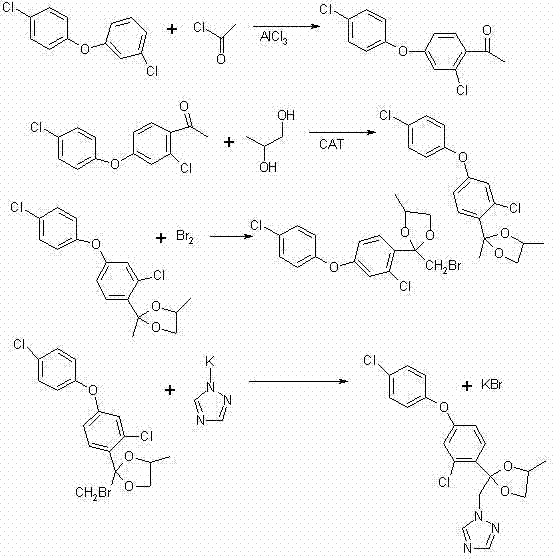
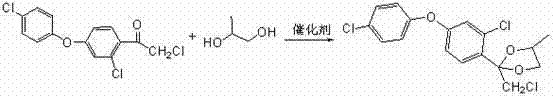
Preparation method of difenoconazole
InactiveCN102250072ASave bromination reaction stepHigh cost of solutionOrganic chemistrySulfolaneChlorobenzene
The invention relates to a preparation method of difenoconazole, comprising the steps of: (1) in the presence of aluminium trichloride, subjecting 3, 4'-dichloro diphenyl ether and chloroacetyl chloride to a Friedel-Crafts reaction so as to generate chlorobenzene ether ketone; (2) in the presence of a catalyst, conducting a cyclisation reaction to chlorobenzene ether ketone and 1, 2-propylene glycol, thus obtaining cis, trans-3-chlorine-4-(4-methyl-2-chloromethyl-1, 3-dioxolane-2-yl) phenyl-4'-chlorophenyl ether; (3) in the presence of sulfolane, carrying out a condensation reaction at a temperature of 190-230DEG C to cis, trans-3-chlorine-4-(4-methyl-2-chloromethyl-1, 3-dioxolane-2-yl) phenyl-4'-chlorophenyl ether and 1, 2, 4-triazole, thus obtaining cis, trans-3-chlorine-4-[4-methyl-2-1H-1, 2, 4-triazole-1-ylmethyl]-1, 3-dioxapentane-2-yl) phenyl 4-chlorophenyl ether, then performing filtration and exsolution when the reaction is over, thus obtaining a crude product of difenoconazole; (4) implementing rectification to the crude product, then carrying out crystallization with a solvent and performing centrifugation, thus obtaining the product of difenoconazole. The method provided in the invention has the advantages of short production period, low production cost and good production security.
Owner:JIANGSU SEVENCONTINENT GREEN CHEM CO LTD
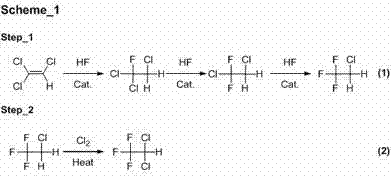
Method for preparation of trifluoro acetyl chloride with trifluoroethane chlorinated mixture
ActiveCN101747176AReduce consumptionThe synthetic route is simpleCarboxylic acid halides preparationAcetyl chlorideGas phase
The invention discloses a method for preparation of trifluoro acetyl chloride with trifluoroethane chlorinated mixture, which comprises the following steps: 1) adding trifluoroethane chlorinated mixture into a reactor, continuously introducing oxygen and chlorine under stirring, irradiating with a mercury lamp for photochemical oxidation, continuously discharging reactants in the form of gas phase from the upper part of the reactor, and continuously supplementing the trifluoroethane chlorinated mixture to maintain the level in the reactor constant; and 2) condensing the gasified trifluoroethane chlorinated mixture in the reactor through a condensation and separation tower and making the condensed trifluoroethane chlorinated mixture return to the reactor to continuously react, discharging the product trifluoro acetyl chloride from the top of the condensation and separation tower and collecting the trifluoro acetyl chloride after compression and condensation. Compared with the existing preparation method, the invention has the advantages that: the trifluoroethane chlorinated mixture can be directly used for preparation of the trifluoro acetyl chloride without separation, the processis advanced and reasonable, the synthesis route is simplified, the time is reduced, the production efficiency is greatly enhanced, and the equipment investment and energy consumption are reduced.
Owner:福建舜跃科技股份有限公司
High-yield synthesis method of trenbolone acetate
The invention relates to a synthesis method of trenbolone acetate, which comprises the following steps: 1. etherification reaction: adding methanol, acetyl chloride and oestrogenic steroid-4,9-diene-3,17-dione into a reaction tank, stirring, regulating the pH value, carrying out vacuum filtration, washing with water, and centrifuging; 2. reduction reaction: adding potassium borohydride, stirring, regulating the pH value, washing with water, and concentrating; 3. hydrolysis reaction: adding methanol and hydrochloric acid, dotting the plate, regulating the pH value, concentrating, adding dichloromethane to be dissolved, washing with water, and dehydrating; 4. dehydrogenation reaction: rinsing with dichloromethane, adding DDQ (2,3-dichloro-5,6-dicyano-1,4-benzoquinone), dotting the plate, carrying out vacuum filtration, washing with water, concentrating, cooling, and drying to obtain trenbolone; and 5. acylation reaction: sucking in benzene and pyridine, adding the trenbolone and 4-DMAP (4-dimethylaminopyridine), dropwisely adding acetyl chloride, keeping the temperature, dotting the plate, washing with water, and concentrating. The method provided by the invention enhances the yield of the trenbolone acetate from 88-90% to 92-93.5%, and can lower the cost by 200 yuan or so per kilogram.
Owner:YICHENG GOTO PHARMA
Dynamic kinetic resolution method of 1-(1-naphthyl) ethylamine
InactiveCN102392065AIncrease reaction rateReduce the temperatureFermentationPhenethyl alcoholAcetophenone
The invention discloses a dynamic kinetic resolution method for 1-(1-naphthyl) ethylamine, which comprises the following steps of: firstly, adding parachlorophenol, organic acid, dicyclohexylcarbodiimide and 4-dimethylaminopyridine, carrying out agitating reaction and filtering, drying, concentrating and carrying out column chromatography to obtain an acyl donor; or adding acetophenone and sodium borohydride, carrying out agitating reaction, depressurizing and distilling, washing, extracting, drying and concentrating to obtain phenethyl alcohol, adding the phenethyl alcohol, acetyl chloride and triethylamine, carrying out agitating reaction and washing, drying, concentrating and carrying out column chromatography to obtain an acyl donor; secondly, adding palladium salt and a carrier, heating, adding formaldehyde and sodium hydroxide, heating and centrifuging, washing and vacuum-drying to obtain a racemic catalyst for standby; and thirdly, adding the 1-(1-naphthyl) ethylamine in methyl benzene, the acyl donor, lipase and the racemic catalyst and carrying out hydrogenation reaction to obtain amide. The dynamic kinetic resolution method has high reaction speed, lower temperature, high conversion rate and product optical purity and great application value.
Owner:ZHEJIANG UNIV
Preparation method and device of acetyl chloride and hydroxy ethylene diphosphonic acid coproduction
InactiveCN1948258AImprove qualityImprove quality indicatorsOrganic compound preparationGroup 5/15 element organic compoundsAcetic acidAcetyl chloride
The present invention belongs to the field of chemical technology, in the concrete, it relates to a preparation method for simultaneously producing acetyl chloride and hydroxyethylidene diphosphonate and its equipment. Said method includes the following steps: adding glacial acetic acid and phosphorus trichloride according to the mole ratio of 3.0-3.2:1 in a reaction still, adopting continuous three-stage heating mode: 40-55 deg.C, 48-65 deg.C and 55-100 deg.C to make them produce reaction in the still so as to form hydroxyethylidene diphosphonate acetate and produce acetyl chloride vapor, making said acetyl chloride vapor be passed through rectification device and condensation device so as to obtain the liquid acetyl chloride whose content is greater than 99%.
Owner:常州晓舟科贸有限公司
Method for preparing 3-descarbamoyl-cefuroxime acid
The invention relates to a method for preparing 3-descarbamoyl-cefuroxime acid. The method comprises the following steps of: (1) dissolving 7-aminocephalosporanic acid (7-ACA) in water or a methanol solution to prepare a 7-ACA solution, and performing hydrolysis reaction to obtain a 3-deacetyl-7-aminocephalosporanic acid (7-DACA) solution; (2) dissolving an acylchlorination reagent in a solvent, adding a cosolvent, adding (Z)-2-methoxyimino-2-(furyl-2-yl)acetic acid ammonium salt (SIMA), reacting, filtering, adding water or performing rotary evaporation to remove the excessive acylchlorination reagent, performing vacuum rotary evaporation, adding a homogenization reagent, and dissolving to obtain a (Z)-2-(furyl-2-yl)-2-(methoxyimino)-acetyl chloride (SMIF-Cl) solution; (3) adding the homogenization reagent into the 7-DACA solution, dripping the SMIF-Cl solution, regulating the pH value, preserving heat and reacting to obtain a reaction solution; and (4) decolorizing the reaction solution, regulating the pH value, adding purified water, growing a crystal, filtering, and performing vacuum drying. Reactants form a homogeneous system by adding the homogenization reagent, so that the contact area of the reactants is expanded, the reaction rate is increased, and reaction time is shortened.
Owner:SHANDONG UNIV
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com