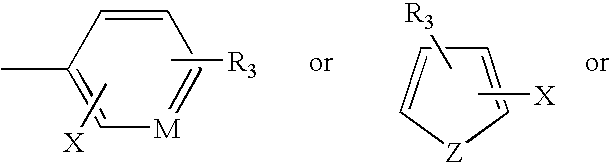Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
155 results about "Oxazolidine E" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pro-fragrances
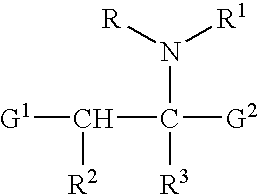
InactiveUS6861402B1Enhanced perfume longevityCosmetic preparationsOrganic chemistryFlavorAdditive ingredient
The present invention relates to fragrance delivery systems which comprise: A) from about 0.01% by weight of a pro-fragrance component which comprises pro-fragrances or pro-accords selected from at least two of the following: i) aldehyde and ketone releasing pro-fragrances, preferably an oxazolidine pro-fragrance; ii) β-amino pro-fragrances; and iii) orthoester pro-accords; and B) the balance carries and others adjunct ingredients.
Owner:THE PROCTER & GAMBLE COMPANY
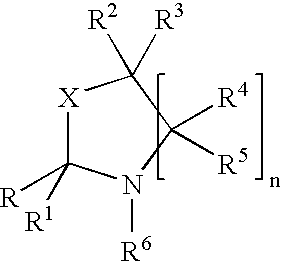
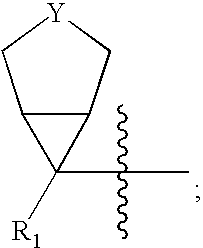
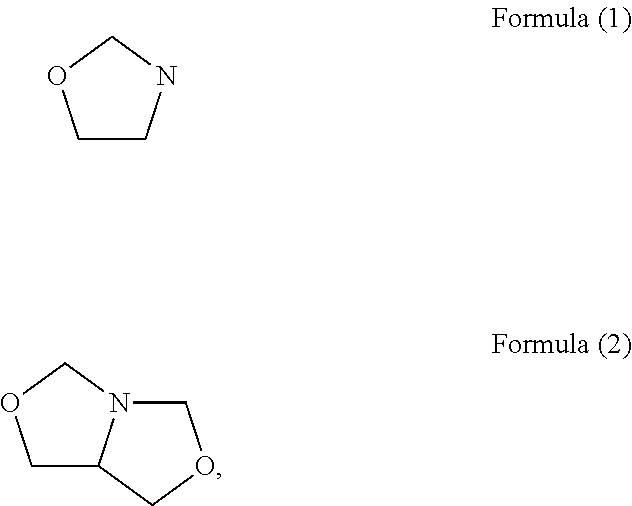
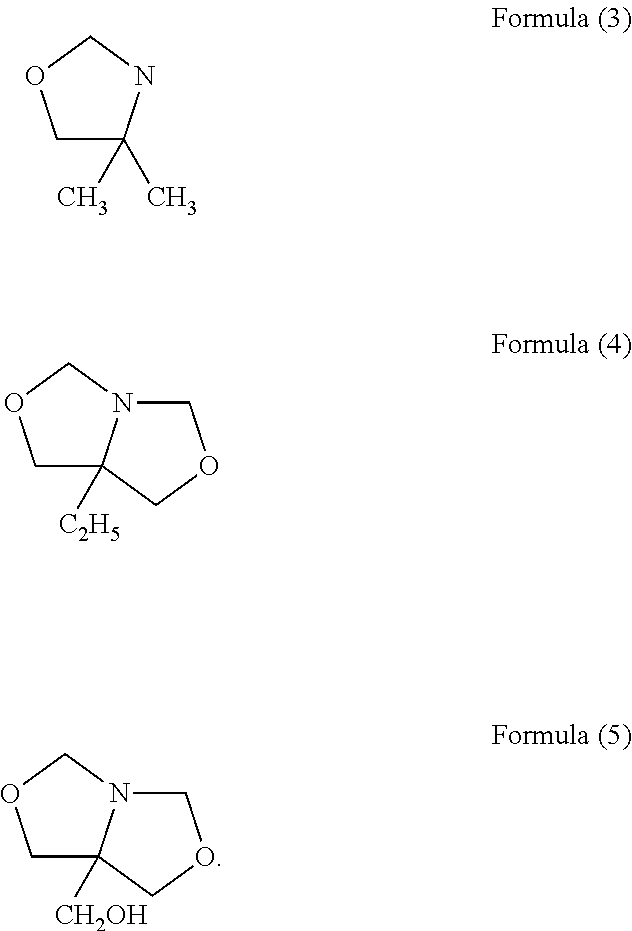
Fragrance pro-accords and aldehyde and ketone fragrance libraries
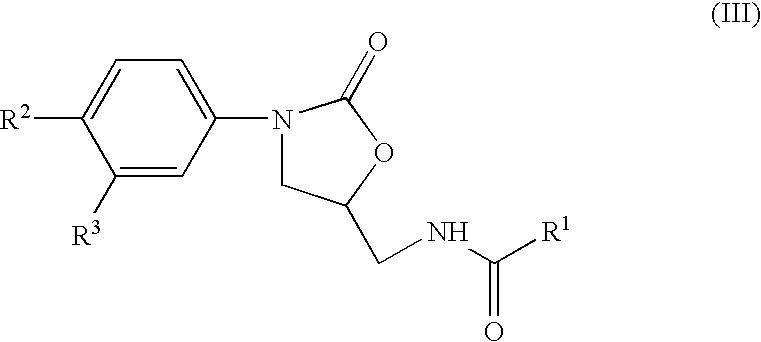
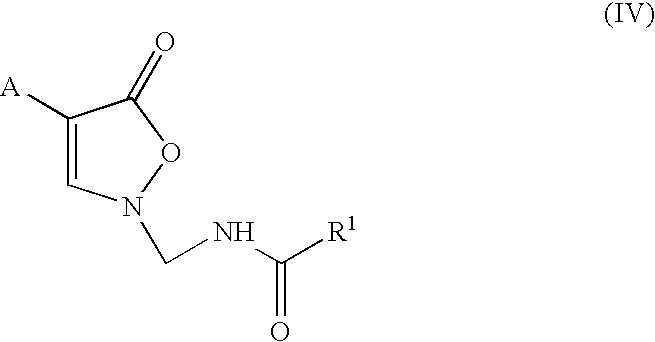
InactiveUS7018978B2Extend your lifeEasy to prepareCosmetic preparationsOrganic chemistryFlavorOxazolidine E
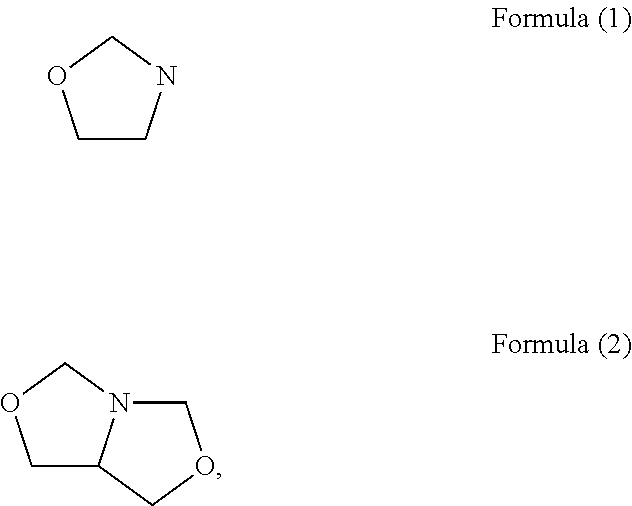
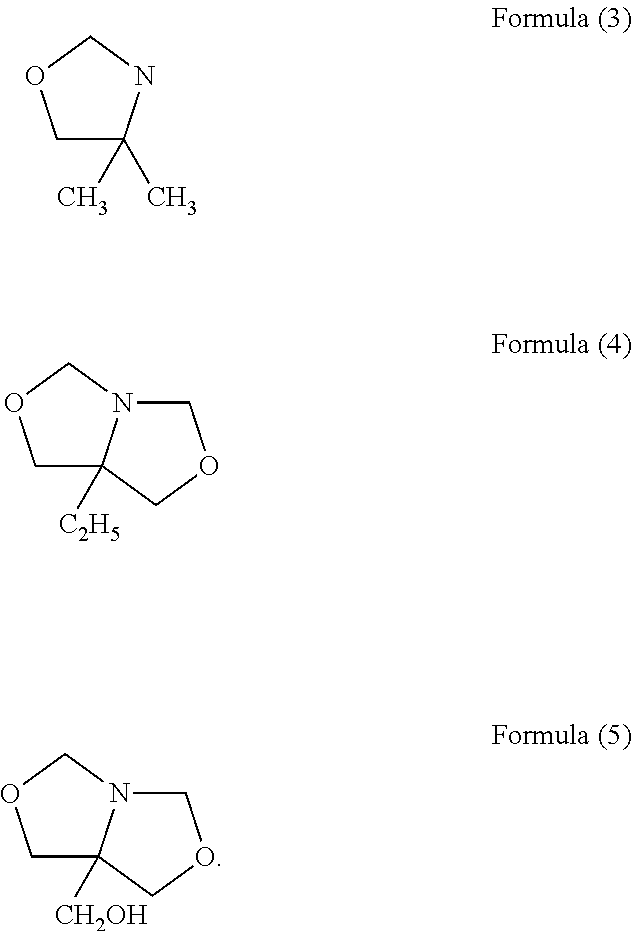
The present invention relates to novel heterocyclic pro-fragrances, preferably oxazolidines, tertahydro-1,3-oxazines, thiazolidines, or tetrahydro-1,3-thiazines, more preferably oxazolidines, or tertahydro-1,3-oxazines, most preferably oxazolidines, which are capable of sustained release of fragrance raw material ketones and aldehydes and to fragrance delivery systems which comprise said pro-fragrances.
Owner:THE PROCTER & GAMBLE COMPANY
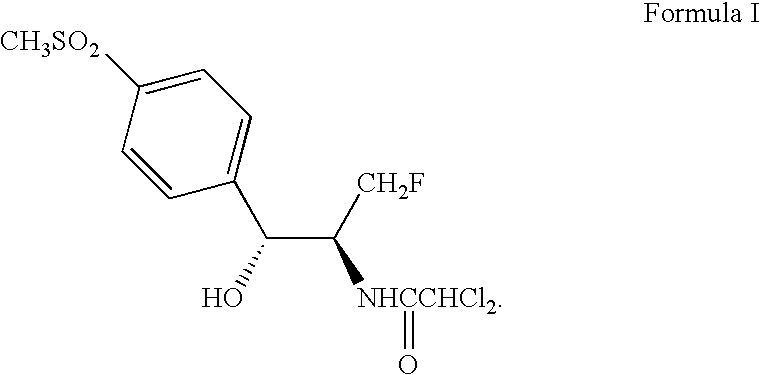
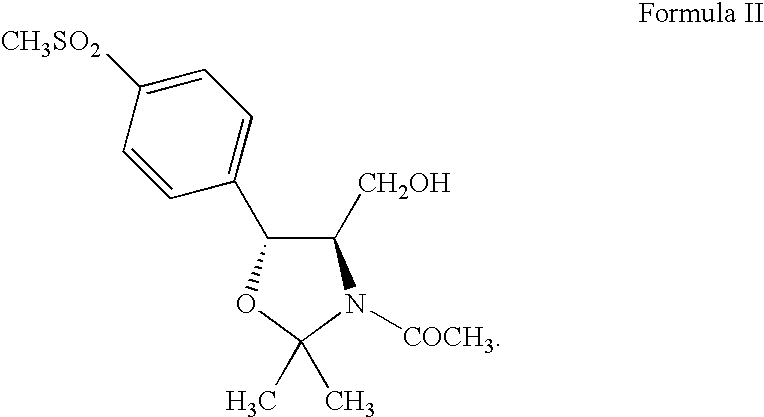
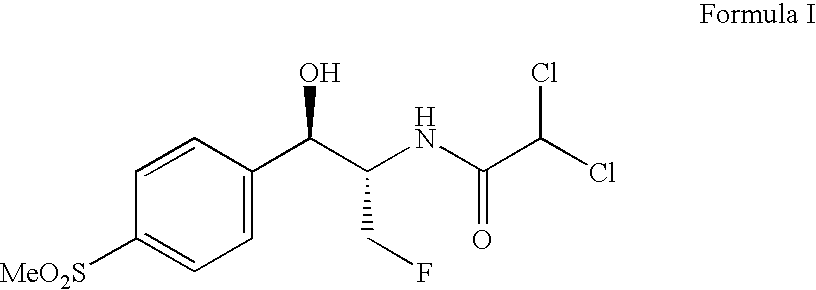
Process for preparing florfenicol
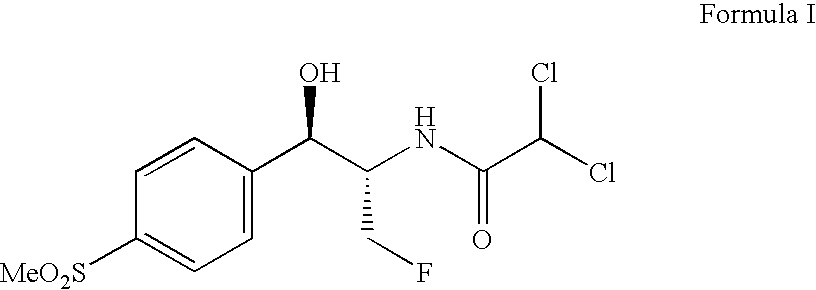
The present invention is directed to a new process of preparing highly pure Florfenicol. The invention is further directed to new oxazolidine derivatives useful in making Florfenicol and processes of making these derivatives. Examples of such intermediates include (4R,5R)-3-acetyl-2,2-dimethyl-4-hydroxymethyl-5-[4-(methylsulfonyl)phenyl]-1,3-oxazolidine; and (4S,5R)-3-acetyl-2,2-dimethyl-4-fluoromethyl-5-[4-(methylsulfonyl)phenyl]-1,3-oxazolidine.
Owner:AUROBINDO PHARMA LTD
Substituted oxazolidinones and their use in the field of blood coagulation
InactiveUS20080090815A1Reduce bleeding riskEasy to adjustBiocideNervous disorderDiseaseOxazolidine E
Owner:BAYER INTELLECTUAL PROPERTY GMBH
One component glass primer including oxazoladine
Improved long open time one component primer compositions for bonding substrates, methods and the articles made therefrom. The primer compositions include an ingredient including an oxazolidine ring or derivative or analog thereof.
Owner:DOW GLOBAL TECH LLC
Preparation method for rivaroxaban intermediate and rivaroxaban
InactiveCN102250076AMild reaction conditionsSimple and fast operationOrganic chemistryOxazolidine EMorpholine
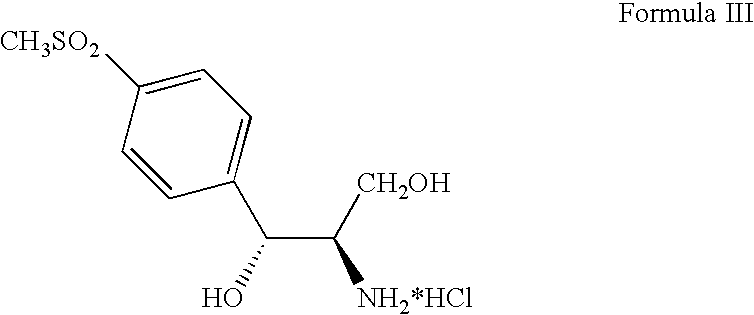
The invention discloses a rivaroxaban intermediate, a structural formula of the rivaroxaban intermediate is represented by the following formula (III). The invention further discloses a preparation method for rivaroxaban. The method is characterized by: carrying out a cyclization reaction for 4-(4-isocyanatophenyl)-3-morpholinone and (R)-epichlorohydrin under a catalytic action of magnesium halide to generate (S)-4- morpholinonephenyloxazolidinone, followed by carrying out functional group conversion to obtain 4-{4-[(5S)-5-(aminomethyl)-2-oxo-1,3-oxazolidine-3-yl] phenyl}-morpholine-3-ketone, then reacting with 5-chlorothiophene-2-formyl chloride to prepare the rivaroxaban. The preparation method provided by the present invention has advantages of mild reaction conditions, simple operation, high reaction yield and the like. In addition, the use of toxic and harmful reagents is avoided during the synthesis process, such that environmental pollution is reduced, and the method is applicable for the industrial production.
Owner:HENGDIAN GRP JIAYUAN CHEM
Reactive hot melt adhesive
ActiveUS20100152394A1Low volatile monomer contentImprove mechanical propertiesOrganic chemistryPolyureas/polyurethane adhesivesPolymer scienceOxazolidine E
Solvent free, moisture curable reactive hot melt adhesives are prepared using an oxazolidine functional prepolymer and a polyfunctional isocyanate.
Owner:HENKEL KGAA
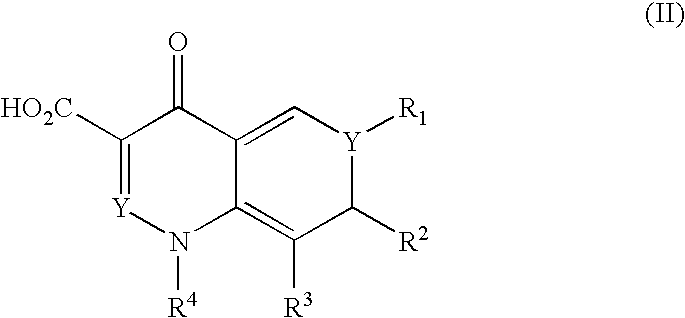
Method for preparing oxazolidinone compound and intermediate thereof
InactiveCN104496979AHigh yieldSignificant raw materialOrganic compound preparationGroup 5/15 element organic compoundsBiochemical engineeringOxazolidine E
The invention relates to a method for preparing an oxazolidinone compound and an intermediate thereof. Specifically, two fragments are coupled to prepare the oxazolidinone compound by virtue of Suzuki reaction. The method is simple in process, short in reaction time and high in yield and is suitable for industrial production. Furthermore, the method for preparing the intermediate of the oxazolidinone compound has the advantages of simplicity in operation, high yield and low cost.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD +1
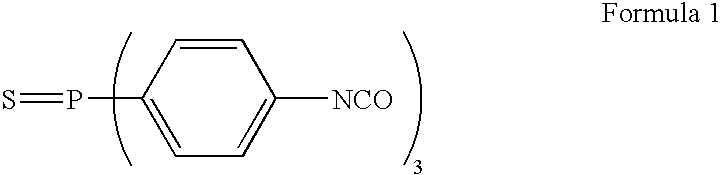
Antimicrobial quinolone derivatives and use of the same to treat bacterial infections
Substituted quinolone derivatives in which an oxazolidinone, isoxazolinone, or isoxazoline is covalently bonded to a quinolone, methods of using the quinolone derivatives, and pharmaceutical compositions containing the quinolone derivatives are disclosed. Methods of synthesizing these substituted quinolone derivatives are also disclosed, and in particular a method of manufacturing a 7-(2-oxo-1,3-oxazolidin-3-yl)aryl-3-quinolinecarboxylic acid by condensing a 4-(2-oxo-1,3-oxazolidin-5-yl)aryl boronic acid with a 7-halo-quinolone derivative. The quinolone derivatives possess antibacterial activity, and are effective against a number of human and veterinary pathogens in the treatment of bacterial diseases.
Owner:PHARMACIA & UPJOHN CO
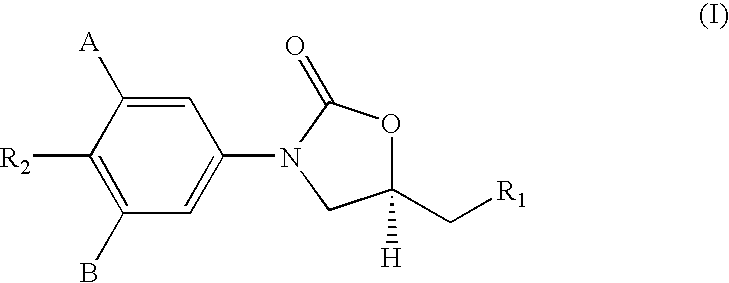
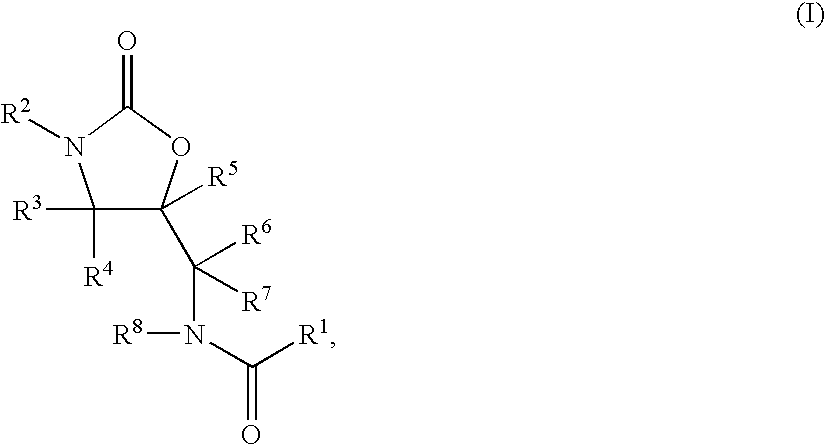
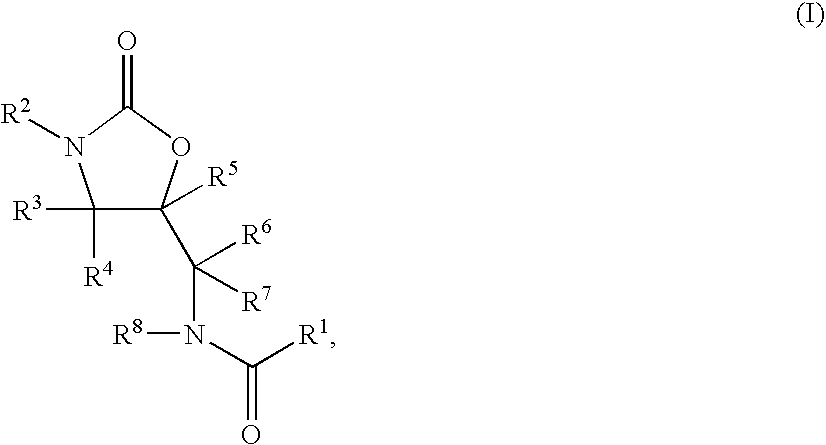
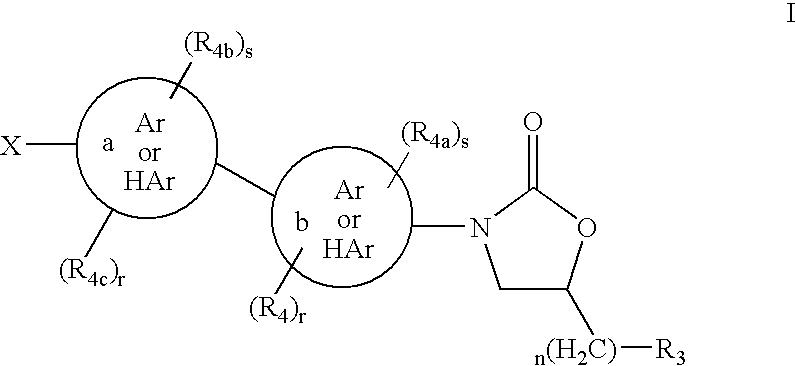
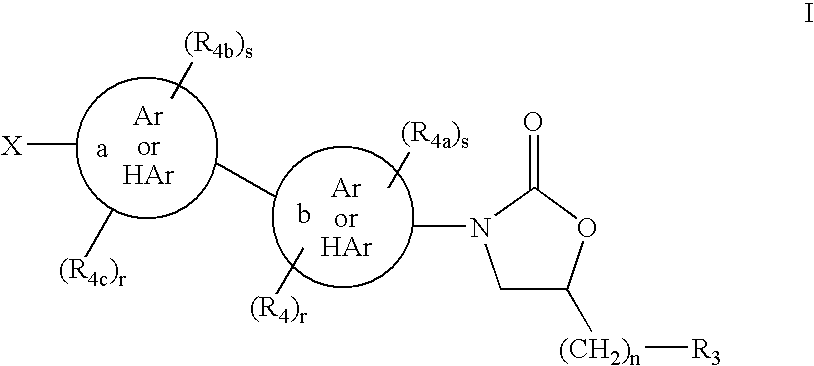
1,3-Oxazolidin-2-One Derivatives Useful as Cetp Inhibitors
Compounds having the structure of Formula I, including pharmaceutically acceptable salts of the compounds, are CETP inhibitors, and are useful for raising HDL-cholesterol, reducing LDL-cholesterol, and for treating or preventing atherosclerosis. The compounds have 3 cyclic groups connected by single bonds, as for example triphenyl, which are attached directly to the ring of formula I or attached at the position B.
Owner:MERCK SHARP & DOHME LLC
Benzoxazepin oxazolidinone compounds and methods of use
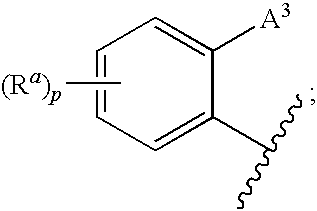
Described herein are benzoxazepin oxazolidinone compounds with phosphoinositide-3 kinase (PI3K) modulation activity or function having the Formula I structure:or stereoisomers, tautomers, or pharmaceutically acceptable salts thereof, and with the substituents and structural features described herein. Also described are pharmaceutical compositions and medicaments that include the Formula I compounds, as well as methods of using such PI3K modulators, alone and in combination with other therapeutic agents, for treating diseases or conditions that are mediated or dependent upon PI3K dysregulation.
Owner:GENENTECH INC
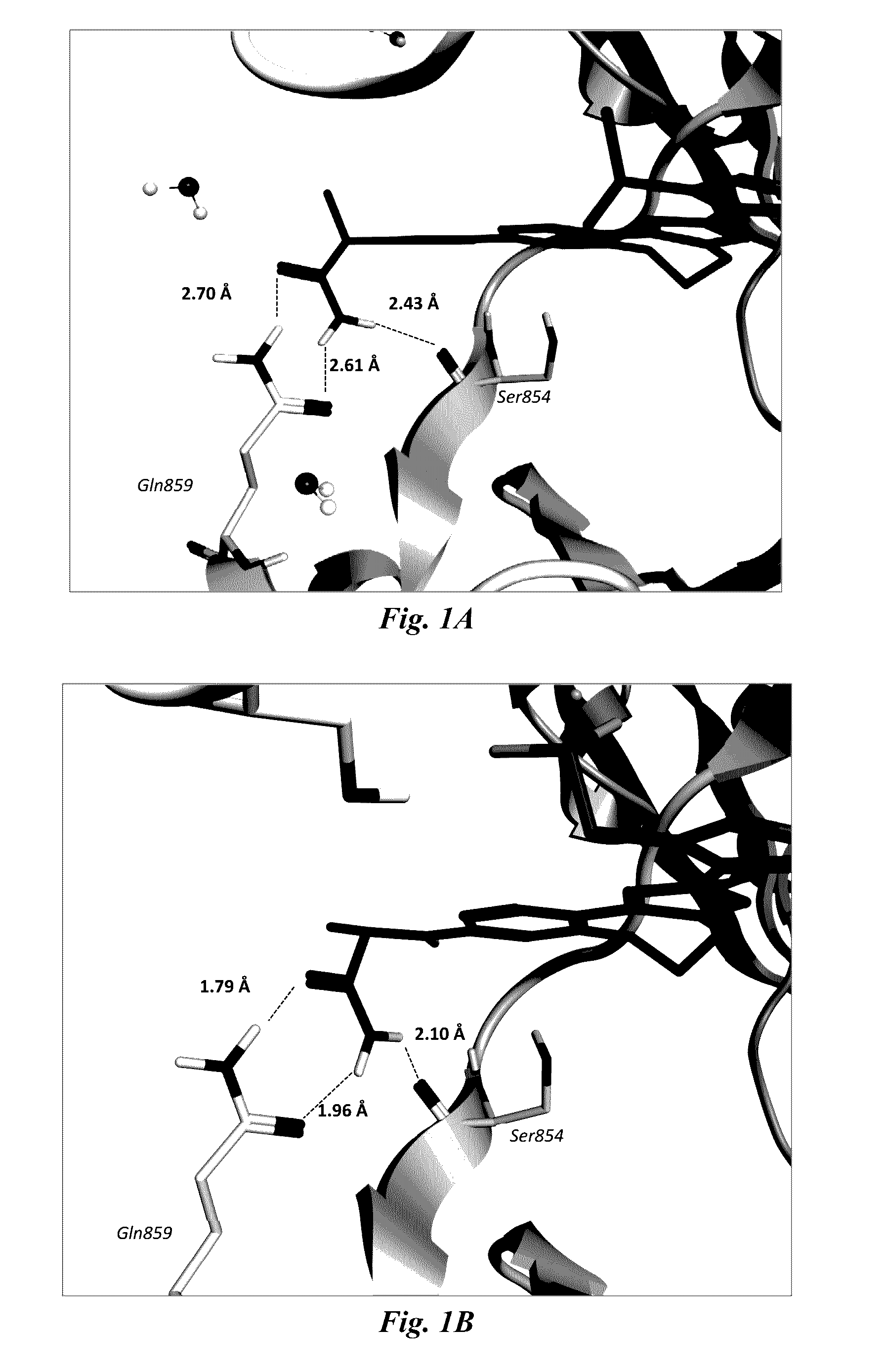
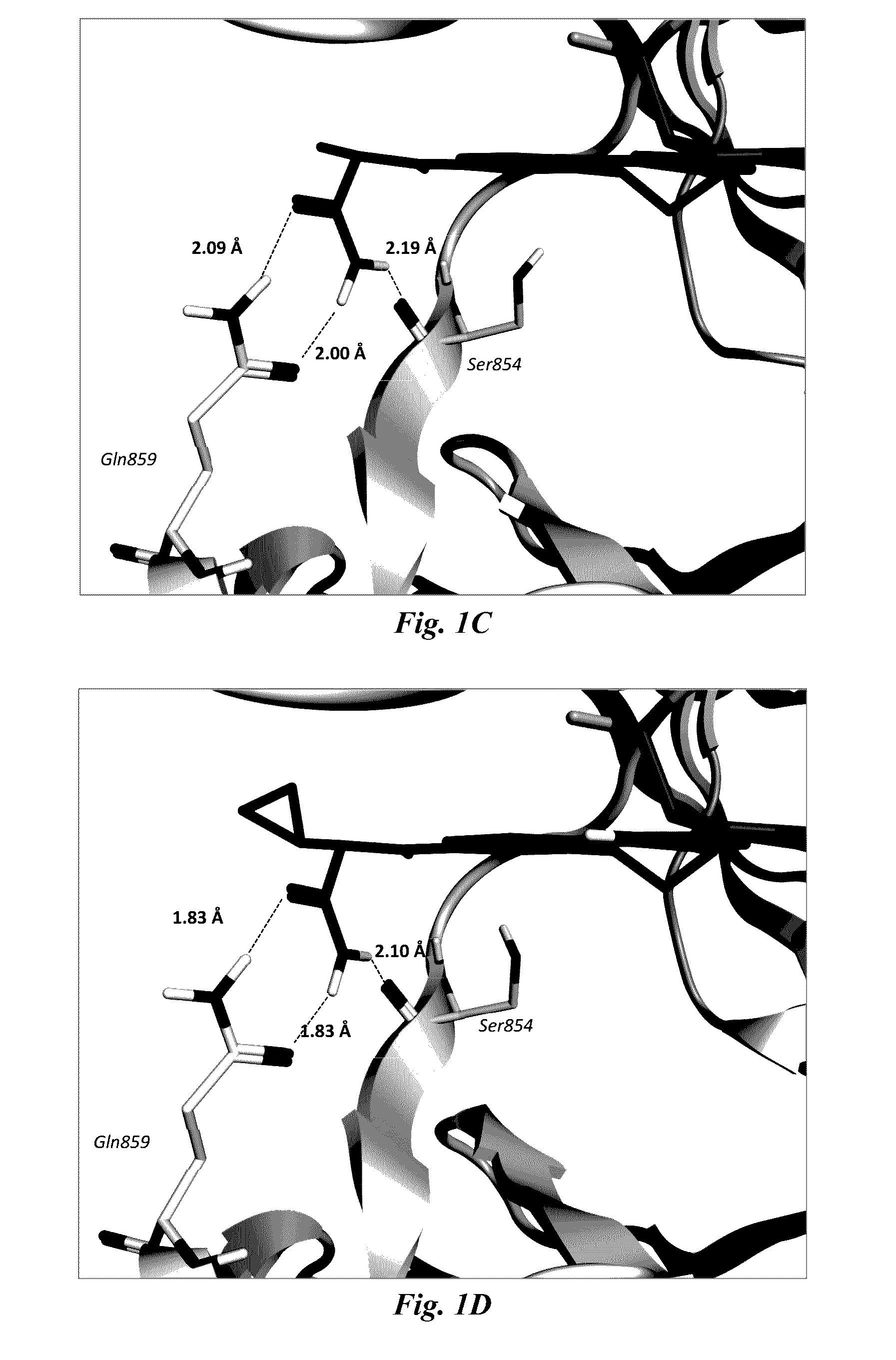
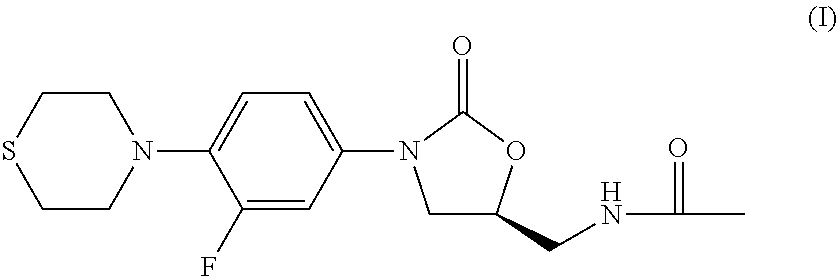
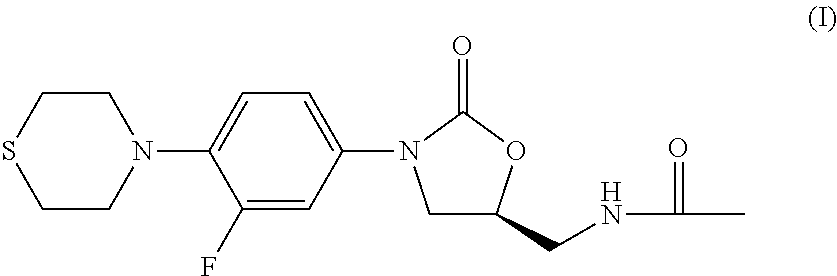
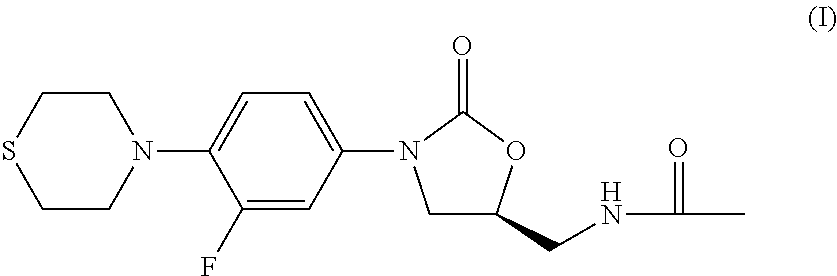
Combination Therapy for Tuberculosis
The present invention relates to methods of treating tuberculosis, including multi-drug resistant varieties and latent tuberculosis. More particularly, the present invention relates to a method of treating tuberculosis in a mammal comprising administering to said mammal in need thereof an effective amount of a compound of formula (I), (S)—N-[[3-[3-fluoro-4-(4-thiomorpholinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]acetamide, or a pharmaceutically acceptable salt thereof in combination with at least two agents useful in the treatment of tuberculosis. The present invention also relates to a pharmaceutical composition comprising a therapeutically effective amount of a compound of formula (I) or a pharmaceutically acceptable salt or solvate thereof, (ii) a therapeutically effective amount of at least one agent useful in the treatment of tuberculosis and (iii) one or more pharmaceutically acceptable carriers or vehicles.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
Process for reaction of CO2 on heterocyclic compound under co-catalysis of transistion metal complex and organic alkali
InactiveCN1369489AMild conditionsReduce energy consumptionOrganic chemistryOrganic synthesisCarbonate ester
A process for preparing annular carbonate or oxazolidinone compound features that reaction of epoxy propane compound or ternary acacyclic compound on CO2 under the co-catalysis of dinaphthylamine-transition metal complex and organic alkali to generate said product, which can be used to prepare amidocarbonate and its unsaturated derivatives, used as furnace, dihydrofuranone, etc. Its advantages are high output rate, less consumption of catalyst, and no poison and pollution.
Owner:SHANGHAI INST OF ORGANIC CHEMISTRY - CHINESE ACAD OF SCI
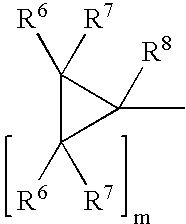
Cyclopropyl group substituted oxazolidinone antibiotics and derivatives thereof
This invention relates to new oxazolidinones having a cyclopropyl moiety, which are effective against aerobic and anerobic pathogens such as multi-resistant staphylococci, streptococci and enterococci, Bacteroides spp., Clostridia spp. species, as well as acid-fast organisms such as Mycobacterium tuberculosis and other mycobacterial species. The compounds are represented by structural formula I: its enantiomer, diastereomer, or pharmaceutically acceptable salt or ester thereof.
Owner:MERCK SHARP & DOHME CORP
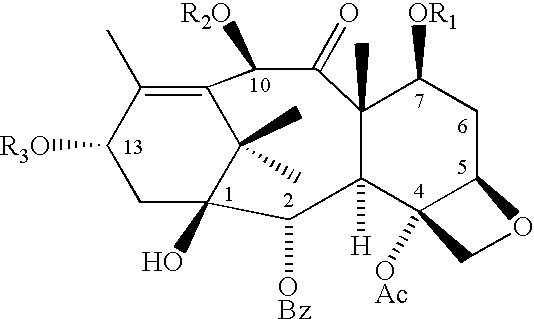
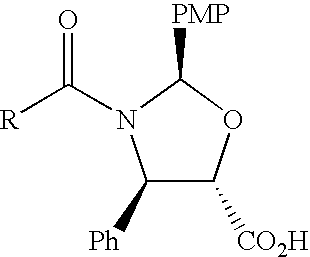
Anticancer taxanes such as paclitaxel, docetaxel and their structural analogs, and a method for the preparation thereof
A process for the preparation of taxanes comprising wherein R is a tert. butoxycarbonyl or benzoyl group; PMP is p-methoxyphenyl group; G1 is acetyl group; G2 is haloacetyl group comprisinga) protecting the C-7 hydroxyl group of 10-deacetylbaccatin III with haloacetyl chlorides and then acetylating the C-10 hydroxyl group with acetyl chloride to obtain a protected 10-deacetylbaccatin III (1);b) subjecting the protected 10-deacetylbaccatin III (1) to coupling with an oxazolidine-5-caboxylic acid of formula 2 wherein R is tert. butoxycarbonyl or benzoyl; PMP is p-methoxyphenyl group in the presence of a condensation agent and an activating agent to obtain C-13 esters of formula 3;c) treating the coupled products 3 with weak acidic medium to open the oxazolidine ring to obtain intermediates of formula 4; wherein R is a tert. butoxycarbonyl or benzoyl group; G1 is acetyl group; G2 is haloacetyl groupd) subjecting the intermediates of compound 4 to selective deprotection of haloacyl group in the presence of acetyl group under mild alkaline condition at −20 to +40° C. for 6-24 h in the presence of ammonia or aliphatic amine or aromatic amines or their combination to obtain paclitaxel or docetaxel.
Owner:DABUR PHARM LTD
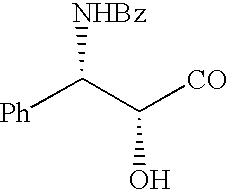
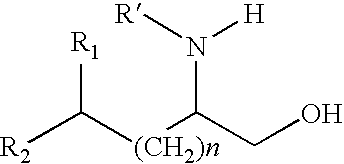
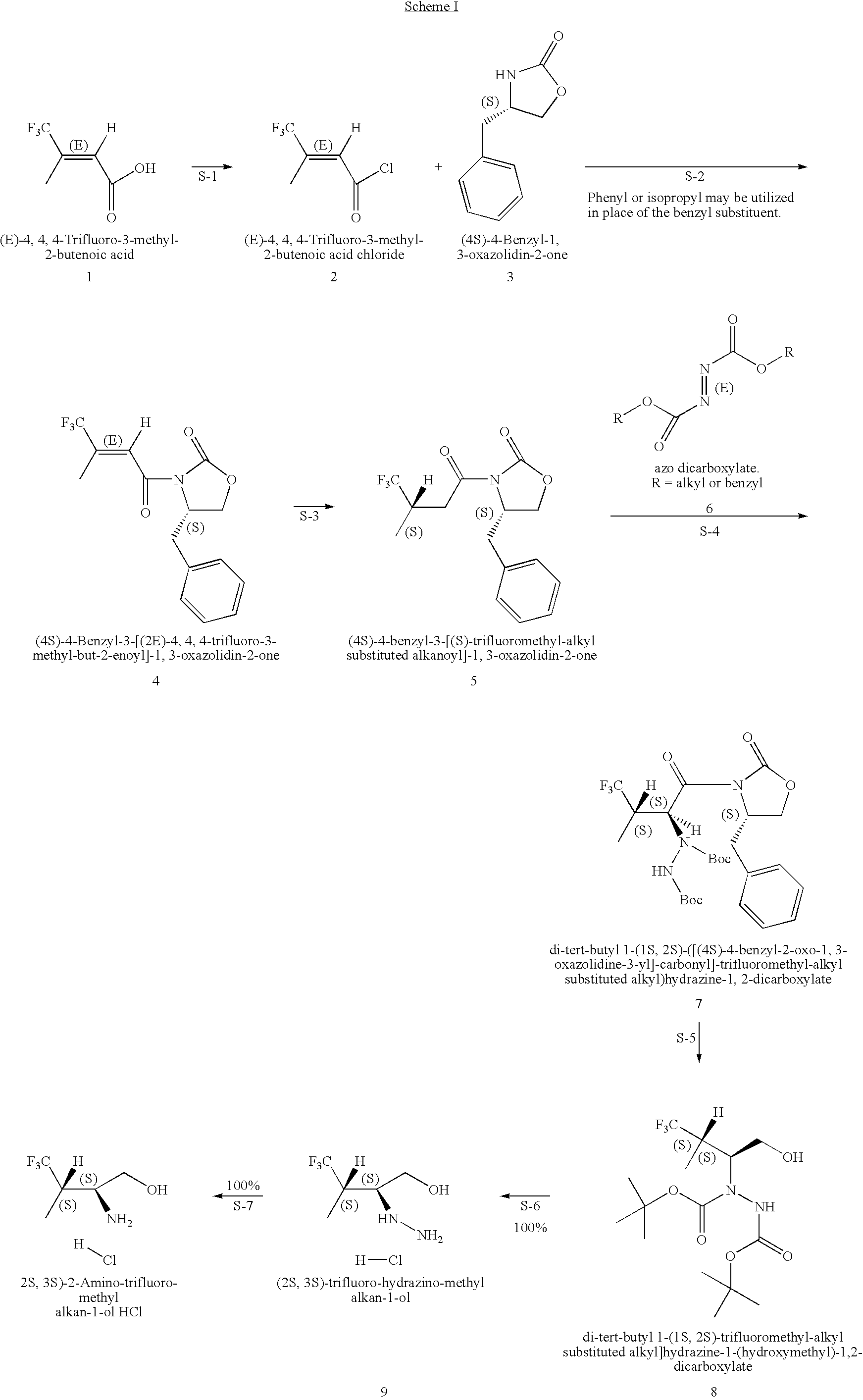
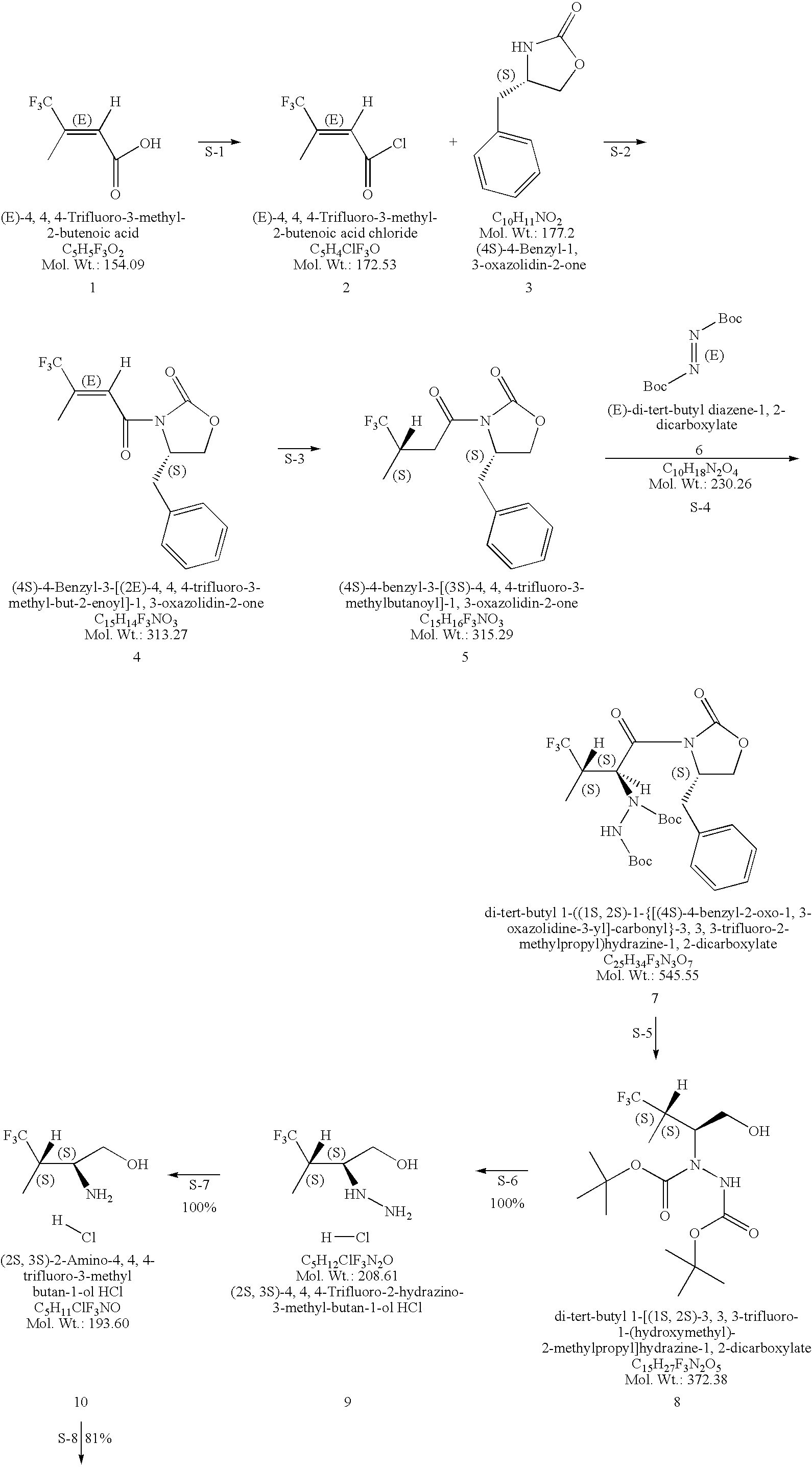
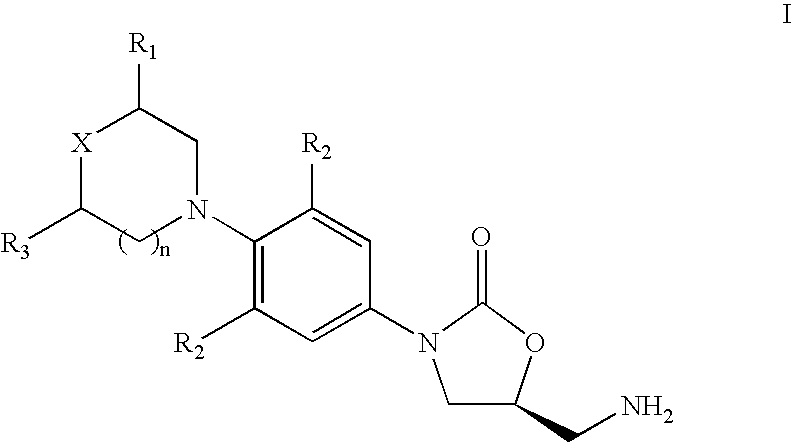
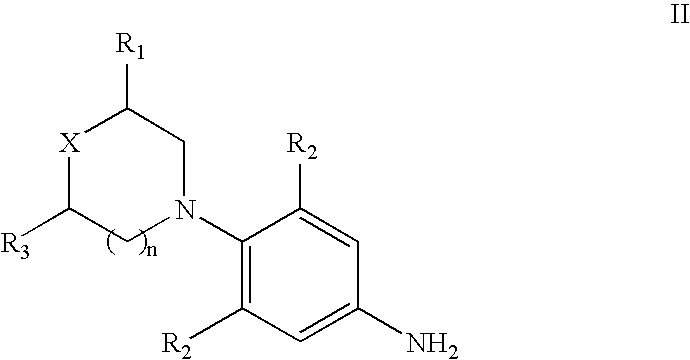
Production of chirally pure amino alcohol intermediates, derivatives thereof, and uses thereof
InactiveUS20070249869A1High chiral purityHigh chemical purityOrganic compound preparationOrganic chemistry methodsArylAlcohol
A method of selectively preparing a chiral 2S-amino alcohol useful in preparation of an amide sulfonated or acylated with alkyl, substituted aryl or substituted heteroaryl is described. The method involves reacting a di-tert-butyl diazene-1,2-dicarboxylate with a (4S)-4-benzyl-3-[(S)-trifluoromethyl-alkyl substituted alkanoyl]-1,3-oxazolidin-2-one to afford a di-tert-butyl 1-(1S,2S)-([(4S)-4-benzyl-2-oxo-1,3-oxazolidine-3-yl]-carbonyl}-trifluoromethyl-alkyl substituted alkyl)hydrazine-1,2-dicarboxylate. This dicarboxylate is then reduced to yield di-tert-butyl 1-(1S,2S)-[trifluoromethyl-alkyl substituted alkyl]hydrazine-1-(hydroxymethyl)-1,2-dicarboxylate. The resulting product is deblocked with an acid to yield the acid addition salt of (2S,3S)-trifluoro-hydrazino-methyl alkan-1-ol. The acid addition salt of (2S,3S)-trifluor-2-hydrazino-methyl alkan-1-ol is hydrogenated in the presence of a suitable metal catalyst to yield the amino alcohol (2S,3S)-2-amino-trifluoro-methyl alkan-1-ol HCl.
Owner:WYETH LLC
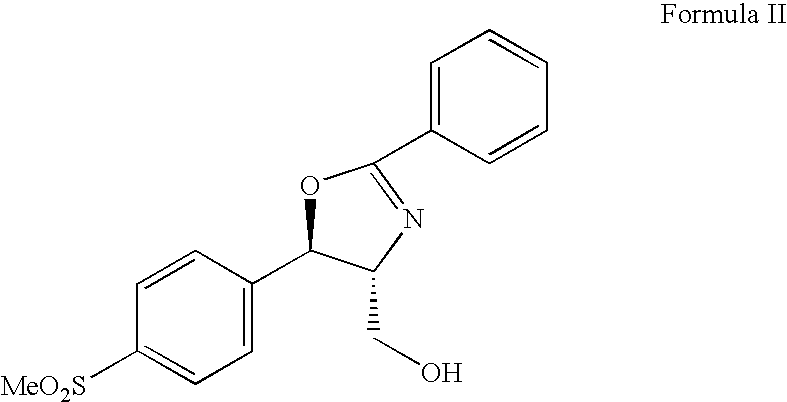
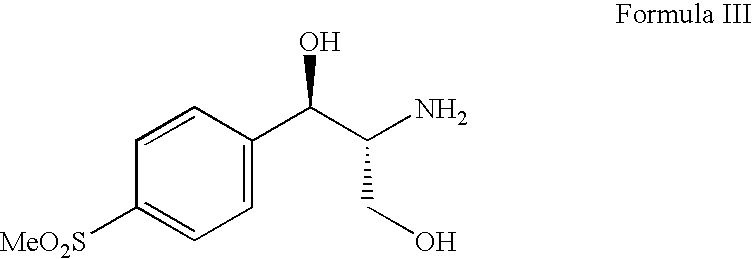
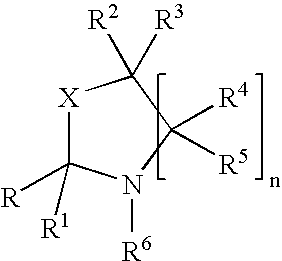
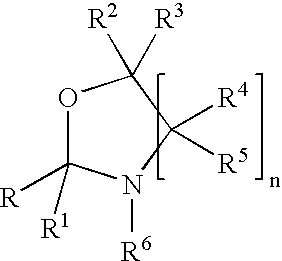
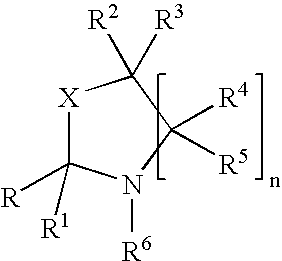
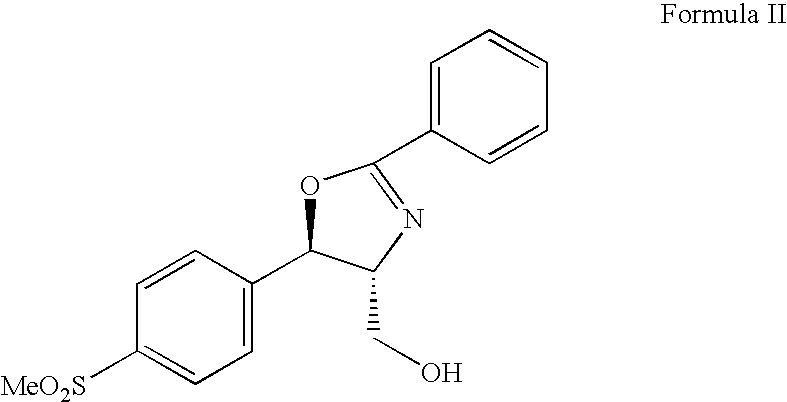
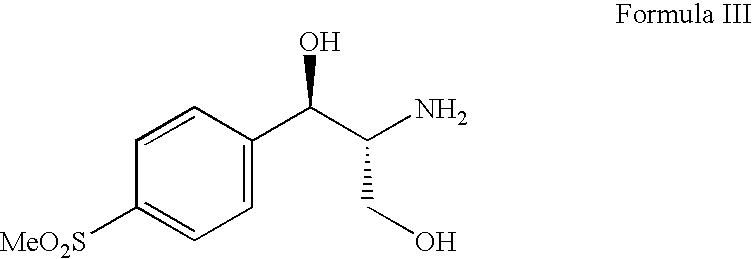
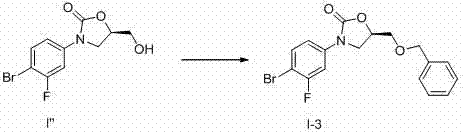
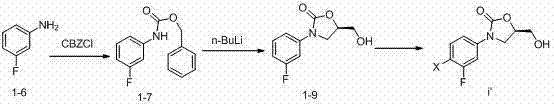
Process for preparing oxazolidine protected aminodiol compounds useful as intermediates to Florfenicol
InactiveUS20070055067A1Low costIncrease productionOrganic chemistryOrganic compound preparationOxazolidine ECombinatorial chemistry
An improved method of preparing oxazolidine protected aminodiol compounds is disclosed. These compounds are useful intermediates in processes for making Florfenicol.
Owner:SCHERING PLOUGH ANIMAL HEALTH +2
Process for the preparation of linezolid and related compounds
The present invention provides a novel process for preparation of 5-aminomethyl substituted oxazolidinones, key intermediates for oxazolidinone antibacterials including linezolid. Thus linezolid is prepared by a) reacting 3-fluoro-4-morpholinyl aniline with R-epichlorohydrin; b) subjecting N-[3-Chloro-2-(R)-hydroxypropyl]-3-fluoro-4-morpholinyl aniline produced above to carbonylation; c) reacting (5R)-5-(chloromethyl)-3-[3-fluoro-4-(4-morpholinyl)phenyl]-2-oxazolidinone produced above with potassium phthalinide; d) reacting (S)-N-[[3-[3-Fluoro-4-[4-morpholinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]phthalimide produced above with hydrazine hydrate; and e) reacting S-N-[[3-[3-Fluoro-4-[4-morpholinyl]phenyl]-2-oxo-5-oxazo-lidinyl]methyl]amine produced above with acetic anhydride to produce linezolid.
Owner:HETERO USA INC
Method for treating an animal substrate
ActiveUS20170240980A1Need be addressLeather manufacturingTanning treatmentChromium freeParticulate material
A method for tanning an animal substrate comprising the steps: i) agitating the animal substrate with a chromium-free tanning agent; and ii) agitating the animal substrate with a tanning agent having an oxazolidine group; wherein at least some of the agitation is performed in the presence of a solid particulate material having an average particle size of from 1 to 500 mm.
Owner:XEROS LTD
Certain (2S)-n-[(1S)-1-cyano-2-phenylethyl]-1,4-oxazepane-2-carboxamides for treating anca associated vasculitides
The present disclosure relates to methods for treating an ANCA associated vasculitis, for example, granulomatosis with polyangiitis (GPA), with compositions comprising an effective amount of certain (2S)—N-[(1S)-1-cyano-2-phenylethyl]-1,4-oxazepane-2-carboxamide compounds of Formula (I), including pharmaceutically acceptable salts thereof, Formula (I) that inhibit dipeptidyl peptidase 1 (DPP1) activity. In one embodiment, the compound of Formula (I) is (2S)—N-{(1S)-1-cyano-2-{4-(3-methyl-2-oxo-2,3-dihydro-1,3-benzoxazol-5-yl)phenyl]ethyl}-1,4-oxazepane-2-carboxamide (INS1007).
Owner:INSMED INC
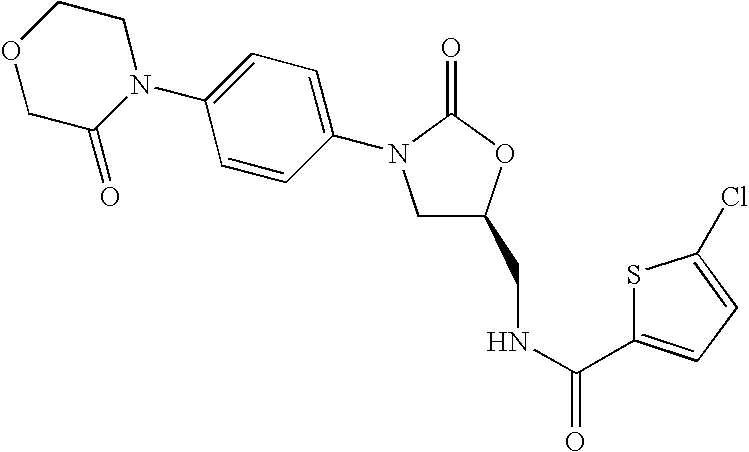
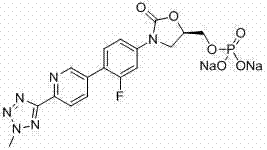
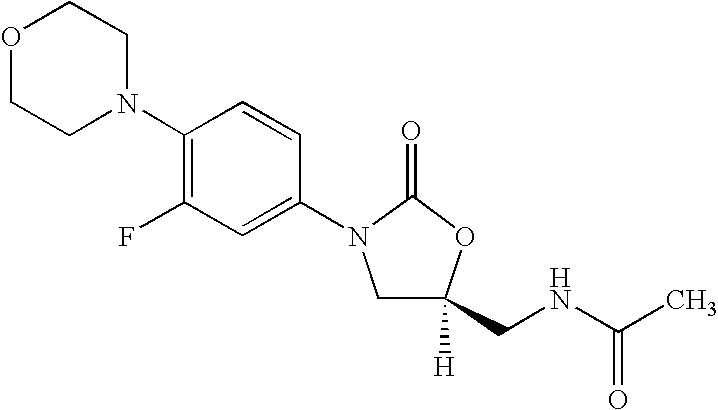
New method for synthesizing rivaroxaban intermediate 4-{4-[(5S)-5-(aminomethyl)-2-oxo-1,3-oxazolidine-3-yl]phenyl}morpholine-3-ketone
The invention discloses a new method for synthesizing rivaroxaban intermediate 4-{4-[(5S)-5-(aminomethyl)-2-oxo-1,3-oxazolidine-3-yl]phenyl}morpholine-3-ketone. The method comprises the following steps: reacting a compound 1 and a compound 2 in an inert solvent in the presence of lithium to get a compound 3; and carrying out an ester group removing reaction under the effect of hydrochloric acid or hydrogen chloride to get a compound 4, that is, 4-{4-[(5S)-5-(aminomethyl)-2-oxo-1,3-oxazolidine-3-yl]phenyl}morpholine-3-ketone. The new method provided by the invention has advantages of mild reaction condition, simple operation, easy purification and low production cost, and is environmentally friendly and suitable for industrial production.
Owner:SHANGHAI SYNCORES TECH INC
Process for synthesizing annular carbonate or oxazolidinone compounds
InactiveCN1369488ANo reduction in catalytic efficiencyGood value for industrial productionOrganic chemistryCompound aOrganic synthesis
A process for synthesizing annular carbonate or oxazolidione compound features that reaction of epoxy propane compounds or ternary acacyclic compounds on CO2 under the co-catalysis of phenol compound, schiff base and organic alkali to generate said product which can be used to prepare amidocarbonate and its unsaturated derivatives. Its advantages are cyclnc use of catalyst, high efficiency, and no poison and pollution.
Owner:SHANGHAI INST OF ORGANIC CHEMISTRY - CHINESE ACAD OF SCI
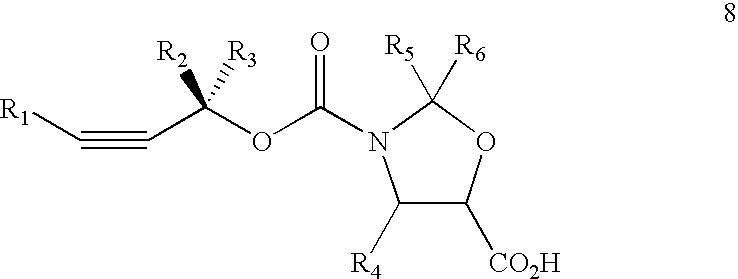
Method of preparation of paclitaxel (taxol) using 3-(alk-2-ynyloxy) carbonyl-5-oxazolidine carboxylic acid
3-(alk-2-ynyloxy)carbonyl-5-oxazolidine carboxylic acid and its analogs having a formulaand wherein R1 is hydrogen, aryl, heteroaryl, alkyl, alkenyl, alkynyl, R2 and R3 are independently selected from hydrogen, alkyl, alkenyl, alkynyl, aryl, heteroaryl, R4 is hydrogen, alkyl, alkenyl, alkynyl, aryl, substituted aryl, heteroaryl, R5 and R6 independently selected from hydrogen, alkyl, alkenyl,alkynyl, arly, heteroaryl, alkoxy, alkeyloxy, alkynyloxy, aryloxy, heteroaryloxy.
Owner:DABUR PHARM LTD
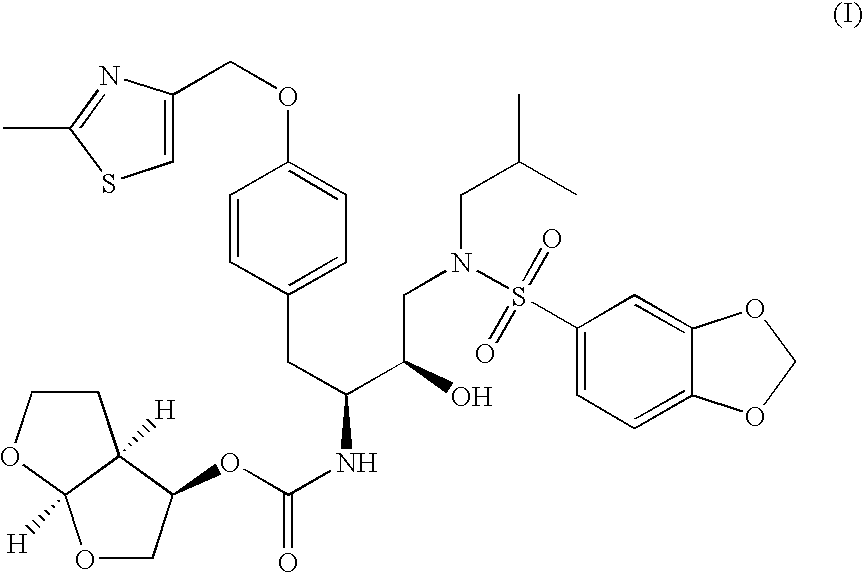
Preparation of chemical compounds
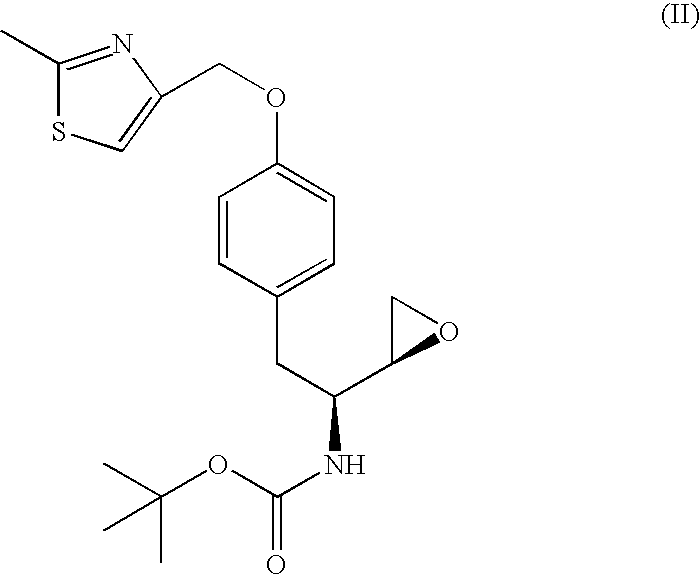
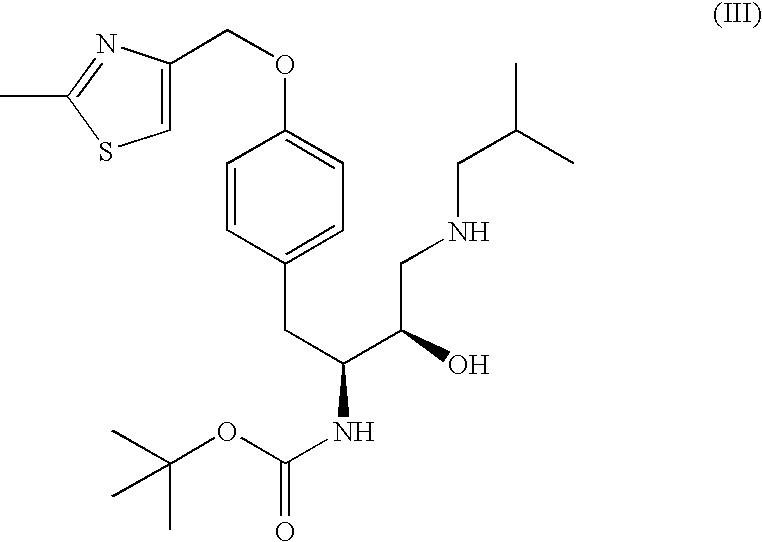
The present invention is directed to processes for the preparation of N-(3R, 3aS, 6aR)-hexahydrofuro[2,3-b]furan-3-yl-oxycarbonyl-, (4S,5R)-4-[4-(2-methylthiazolo-4-methyloxy)-benzyl]-5-i-butyl-[(3,4-methylenedioxyphenyl)sulfonyl]-aminomethyl-2,2-dimethyl-oxazolidine.
Owner:MARTIN MICHAEL TOLAR +2
Process for preparing oxazolidine protected aminodiol compounds useful as intermediates to florfenicol
InactiveUS20070055066A1Increase productionLow costOrganic chemistryOrganic compound preparationOxazolidine ECombinatorial chemistry
An improved method of preparing oxazolidine protected aminodiol compounds is disclosed. These compounds are useful intermediates in processes for making Florfenicol.
Owner:SCHERING PLOUGH ANIMAL HEALTH +2
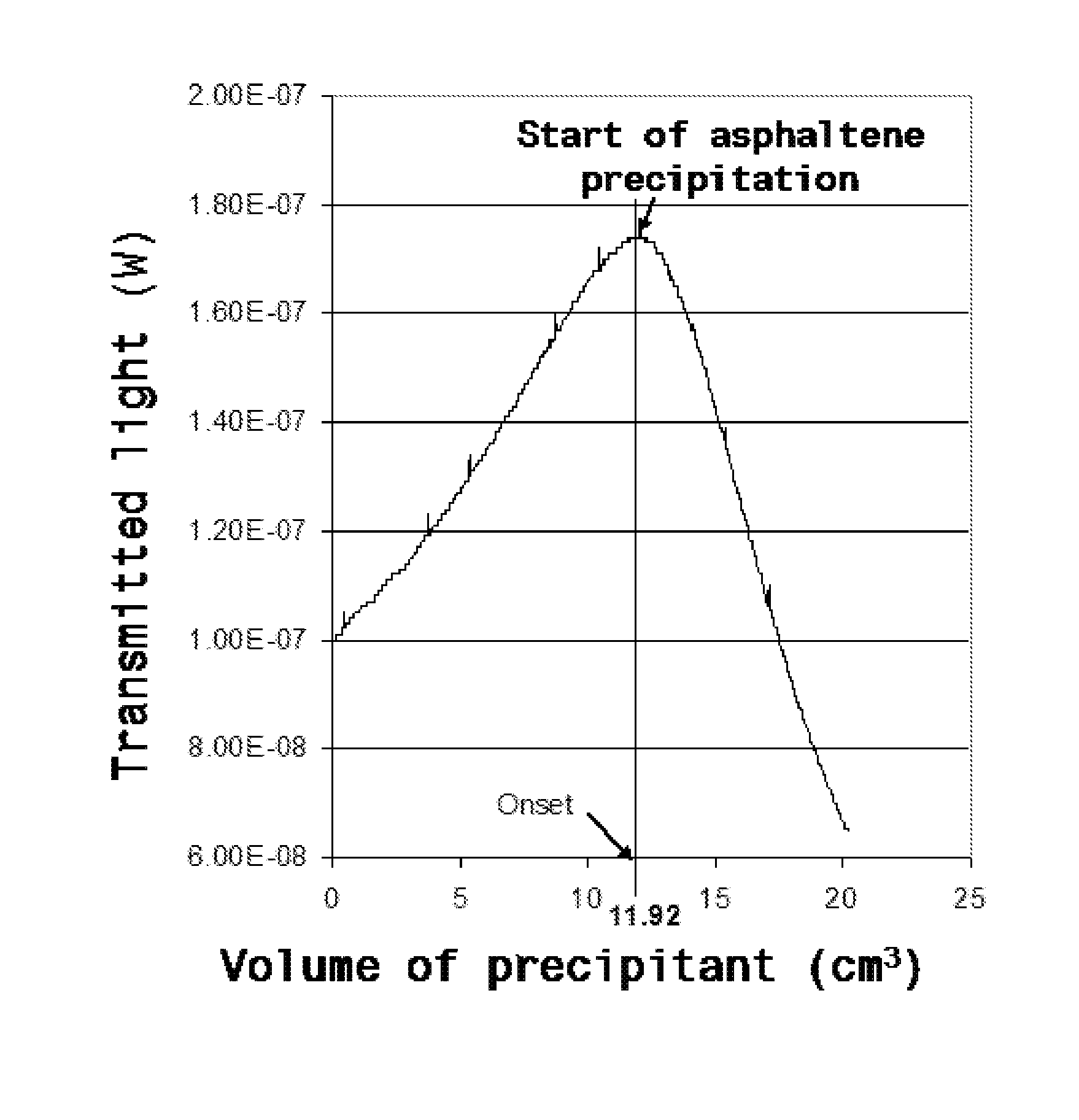
Asphaltene-dispersing/inhibiting additive based on oxazolidines derived from polyalkyl or polyalkenyl N-hydroxyalkyl succinimides
The present invention relates to formulations of asphaltenes' inhibitor-dispersant additives based on oxazolidine derived from polyalkyl or polyalkenyl N-hydroxyalkyl succinimides. Said formulations can contain inert organic solvents, preferably including: toluene, mixtures of xylene, o-xylene, p-xylene, kerosene, turbo-fuel; or inert hydrocarbon solvents having boiling points within the range of gasoline and diesel; or inert hydrocarbon or organic solvents having a boiling point within a range from 75 to 300° C. The ratio in weight of inert organic solvents to additive that prevents and controls the precipitation and deposition of asphaltenes ranges from 1:9 to 9:1, preferably from 1:3 to 3:1.
Owner:INST MEXICANO DEL GASOLINEEO
Method for treating an animal substrate
A method for tanning an animal substrate comprising the steps: i) agitating the animal substrate with a chromium-free tanning agent; and ii) agitating the animal substrate with a tanning agent having an oxazolidine group; wherein at least some of the agitation is performed in the presence of a solid particulate material having an average particle size of from 1 to 500 mm.
Owner:XEROS LTD
Process for preparing florfenicol
The present invention is directed to a new process of preparing highly pure Florfenicol. The invention is further directed to new oxazolidine derivatives useful in making Florfenicol and processes of making these derivatives. Examples of such intermediates include (4R,5R)-3-acetyl-2,2-dimethyl-4-hydroxymethyl-5-[4-(methylsulfonyl)phenyl]-1,3-oxazolidine; and (4S,5R)-3-acetyl-2,2-dimethyl-4-fluoromethyl-5-[4-(methylsulfonyl)phenyl]-1,3-oxazolidine.
Owner:AUROBINDO PHARMA LTD
Process for preparing paclitaxel
InactiveUS6130336ASpeed up the processEasy to disassembleOrganic chemistryAntineoplastic agentsOxazolidine EBenzoyl chloride
PCT No. PCT / KR97 / 00157 Sec. 371 Date Feb. 23, 1999 Sec. 102(e) Date Feb. 23, 1999 PCT Filed Aug. 25, 1997 PCT Pub. No. WO98 / 08832 PCT Pub. Date Mar. 5, 1998The present invention elates to a process for preparing paclitaxel represented by formula (1) characterized in that: (a) an oxazolidine derivative represented by formula (2) or its salt in which X represents halogen, is coupled with a 7-trihaloacetyl-baccatin III represented by formula (3) in which R1 represents trihaloacetyl, in a solvent in the presence of a condensing agent to produce an oxazolidine substituent-containing taxane represented by formula (4) in which X and R1 are each as previously defined; (b) the oxazolidine ring is opened in a solvent in the presence of an acid, and the product thus obtained is reacted with benzoyl chloride in the presence of a base to produce a protected paclitaxel wherein the hydroxy group at 7-position is protected with trihaloacetyl group represented by formula (5) in which R1 is as previously defined; (c) then the protecting group at 7-position is removed by ammonia or a salt of ammonia with a weak acid in a solvent.
Owner:HANMI PHARMA
Novel antimycobacterial compounds
Novel compounds belonging to the class of oxazolidinones possessing potent antimycobacterial properties especially useful in the treatment of acid fast organisms such as Mycobacterium tuberculosis, Mycobacterium avium-intracellular complex, M. fortuitum and M. kansai. The compound and its pharmaceutically acceptable salts thereof act as antibacterial agents. Also disclosed is a method for inhibiting growth of mycobacterial cells as well as a method of treating mycobacterial conditions such as Mycobacterium tuberculosis, drug resistant Mycobacterium tuberculosis, Mycobacterium avium-intracellular complex, M. fortuitum and M. kansai, comprising administering an antimycobacterially effective amount of the said compound and / or pharmaceutically acceptable salts thereof. There is also disclosed a process for the manufacture of the said compound or its pharmaceutically acceptable salts.
Owner:LUPIN LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

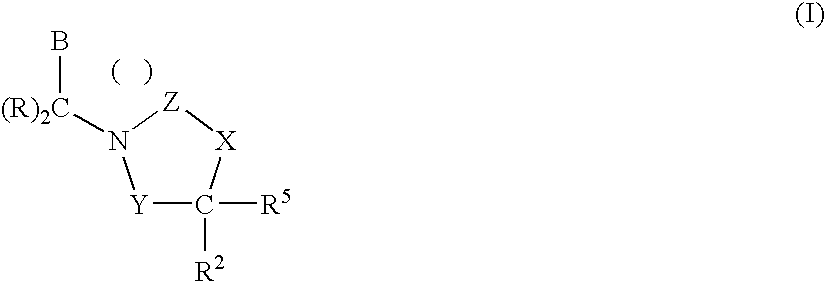
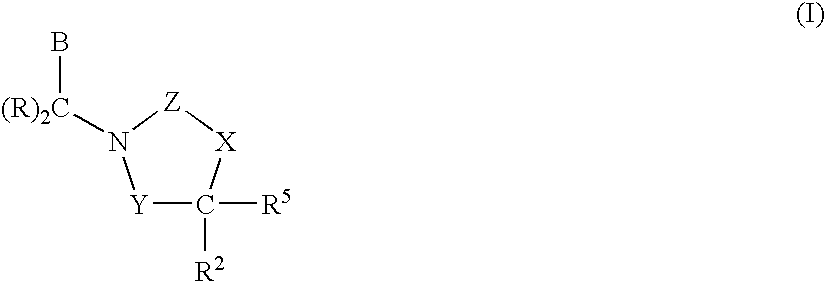



![Certain (2S)-n-[(1S)-1-cyano-2-phenylethyl]-1,4-oxazepane-2-carboxamides for treating anca associated vasculitides Certain (2S)-n-[(1S)-1-cyano-2-phenylethyl]-1,4-oxazepane-2-carboxamides for treating anca associated vasculitides](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/892b2928-275b-46a0-9287-cc5a28f65cb3/US20200390781A1-D00001.png)
![Certain (2S)-n-[(1S)-1-cyano-2-phenylethyl]-1,4-oxazepane-2-carboxamides for treating anca associated vasculitides Certain (2S)-n-[(1S)-1-cyano-2-phenylethyl]-1,4-oxazepane-2-carboxamides for treating anca associated vasculitides](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/892b2928-275b-46a0-9287-cc5a28f65cb3/US20200390781A1-D00002.png)
![Certain (2S)-n-[(1S)-1-cyano-2-phenylethyl]-1,4-oxazepane-2-carboxamides for treating anca associated vasculitides Certain (2S)-n-[(1S)-1-cyano-2-phenylethyl]-1,4-oxazepane-2-carboxamides for treating anca associated vasculitides](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/892b2928-275b-46a0-9287-cc5a28f65cb3/US20200390781A1-D00003.png)
![New method for synthesizing rivaroxaban intermediate 4-{4-[(5S)-5-(aminomethyl)-2-oxo-1,3-oxazolidine-3-yl]phenyl}morpholine-3-ketone New method for synthesizing rivaroxaban intermediate 4-{4-[(5S)-5-(aminomethyl)-2-oxo-1,3-oxazolidine-3-yl]phenyl}morpholine-3-ketone](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f9293812-1233-416c-8f94-176d6c578af2/350714DEST_PATH_IMAGE004.PNG)
![New method for synthesizing rivaroxaban intermediate 4-{4-[(5S)-5-(aminomethyl)-2-oxo-1,3-oxazolidine-3-yl]phenyl}morpholine-3-ketone New method for synthesizing rivaroxaban intermediate 4-{4-[(5S)-5-(aminomethyl)-2-oxo-1,3-oxazolidine-3-yl]phenyl}morpholine-3-ketone](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f9293812-1233-416c-8f94-176d6c578af2/380987DEST_PATH_IMAGE001.PNG)
![New method for synthesizing rivaroxaban intermediate 4-{4-[(5S)-5-(aminomethyl)-2-oxo-1,3-oxazolidine-3-yl]phenyl}morpholine-3-ketone New method for synthesizing rivaroxaban intermediate 4-{4-[(5S)-5-(aminomethyl)-2-oxo-1,3-oxazolidine-3-yl]phenyl}morpholine-3-ketone](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f9293812-1233-416c-8f94-176d6c578af2/528251DEST_PATH_IMAGE003.PNG)