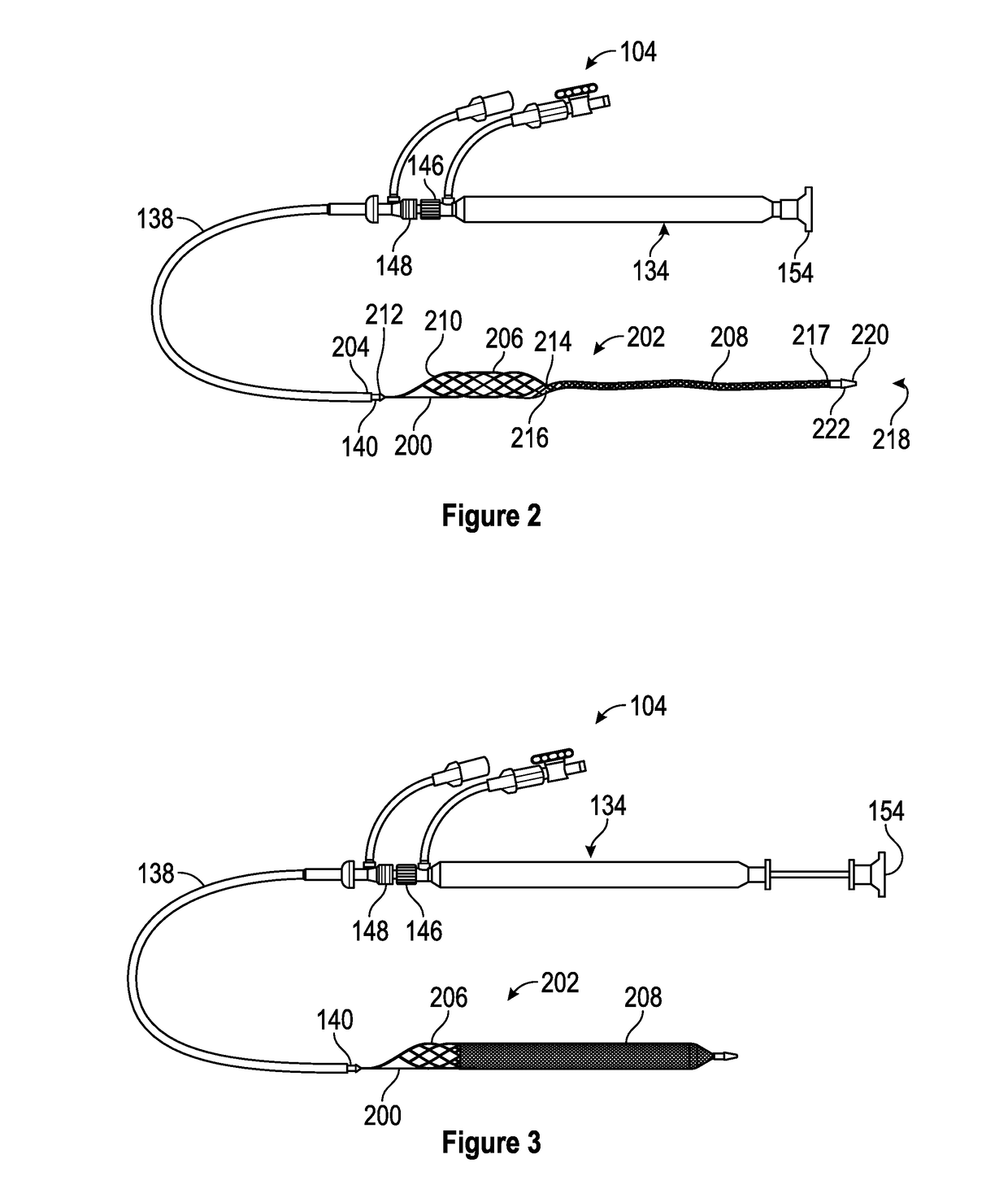
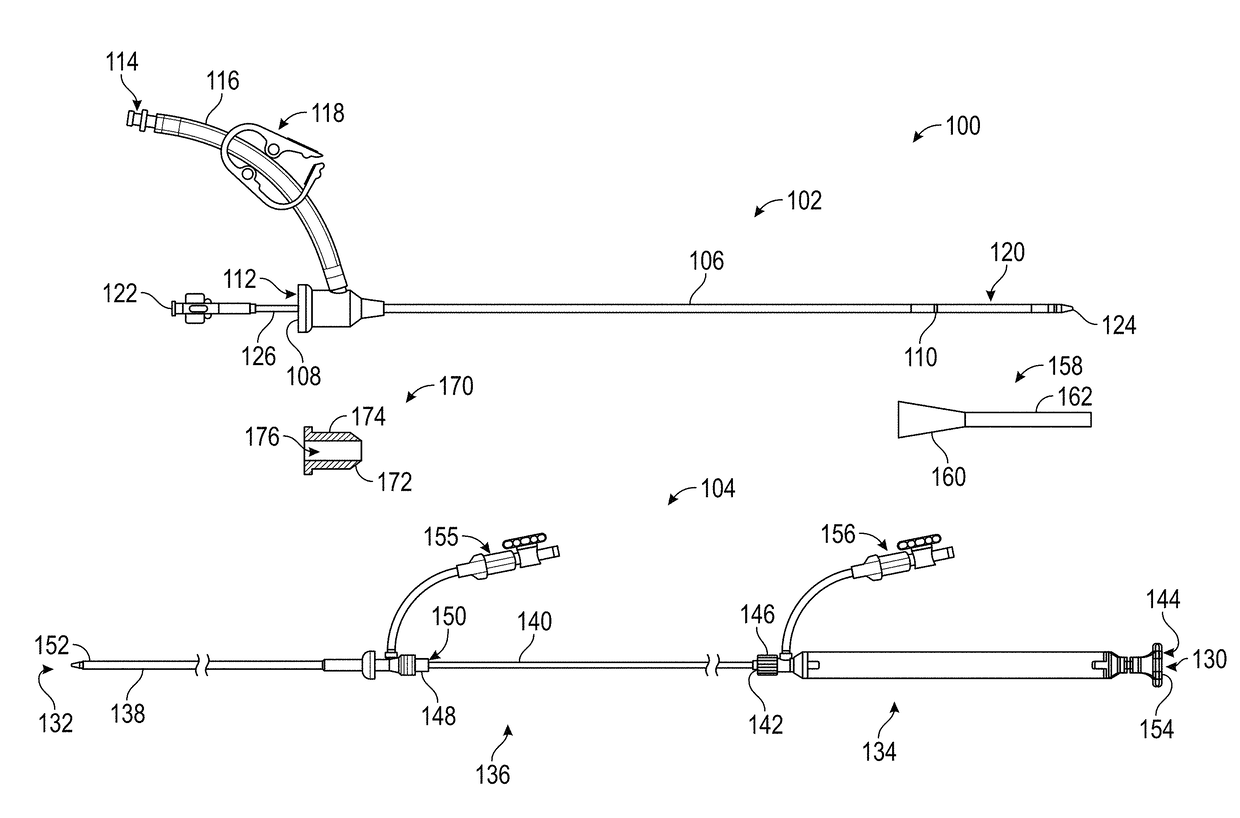
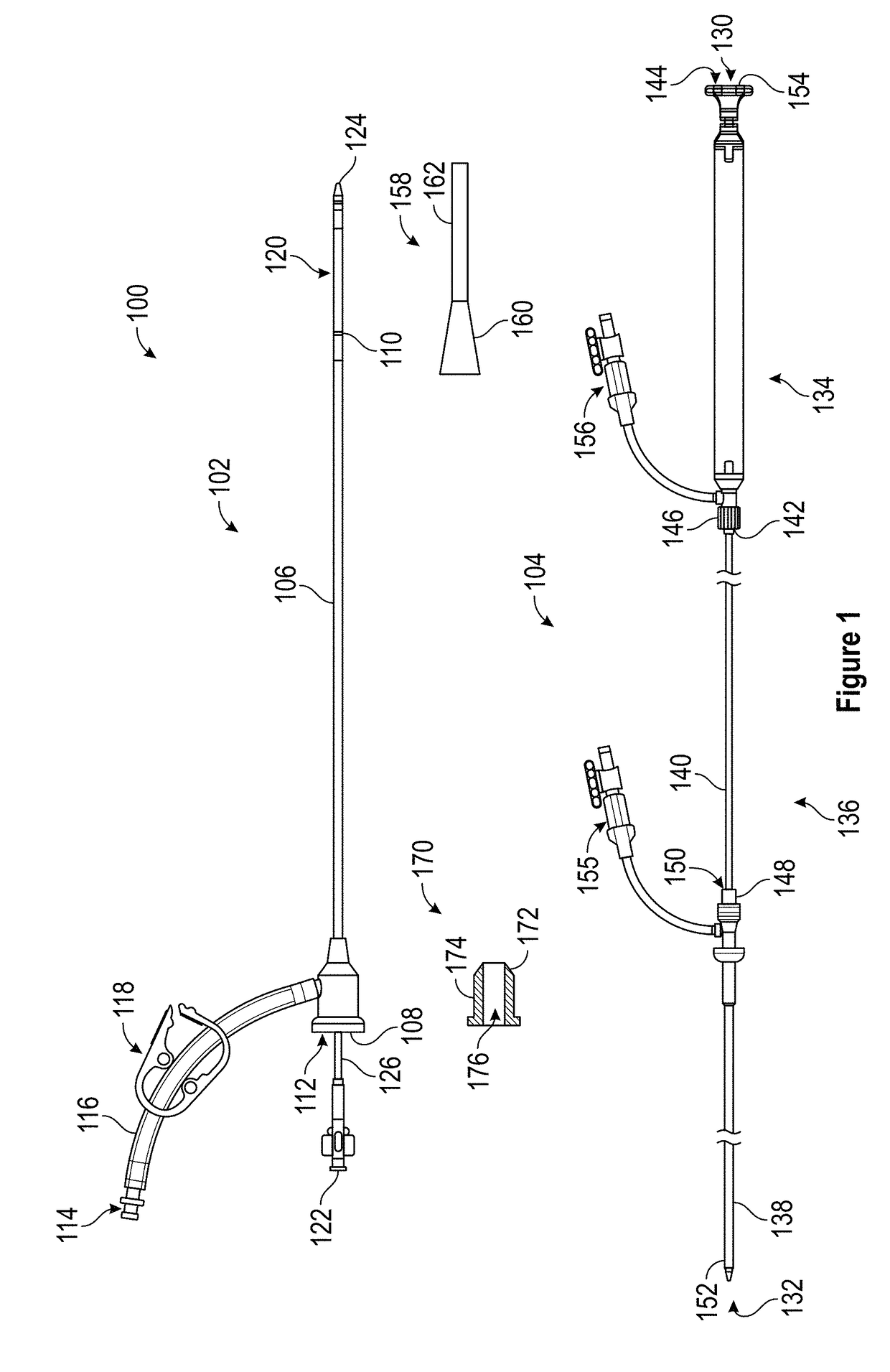
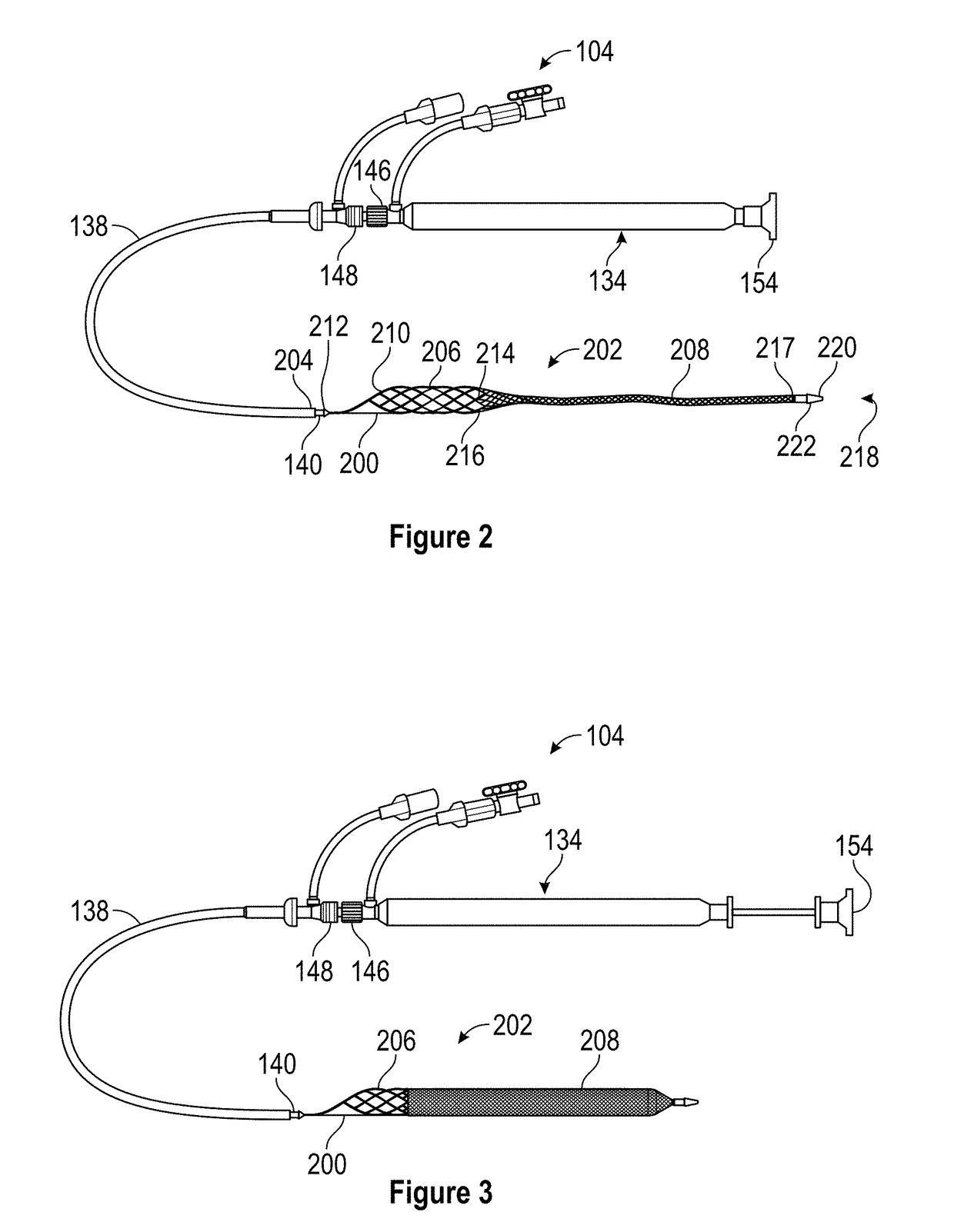
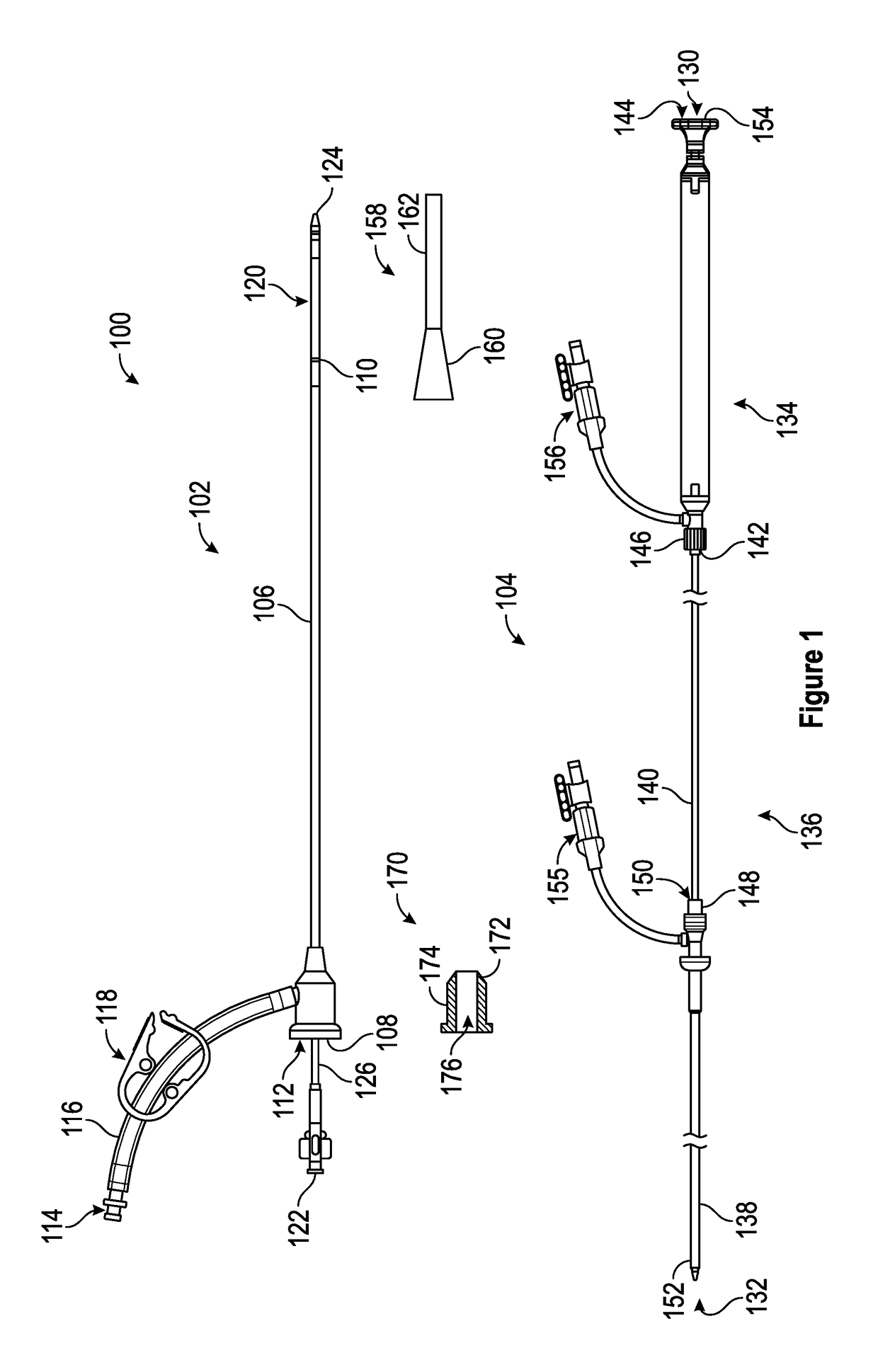
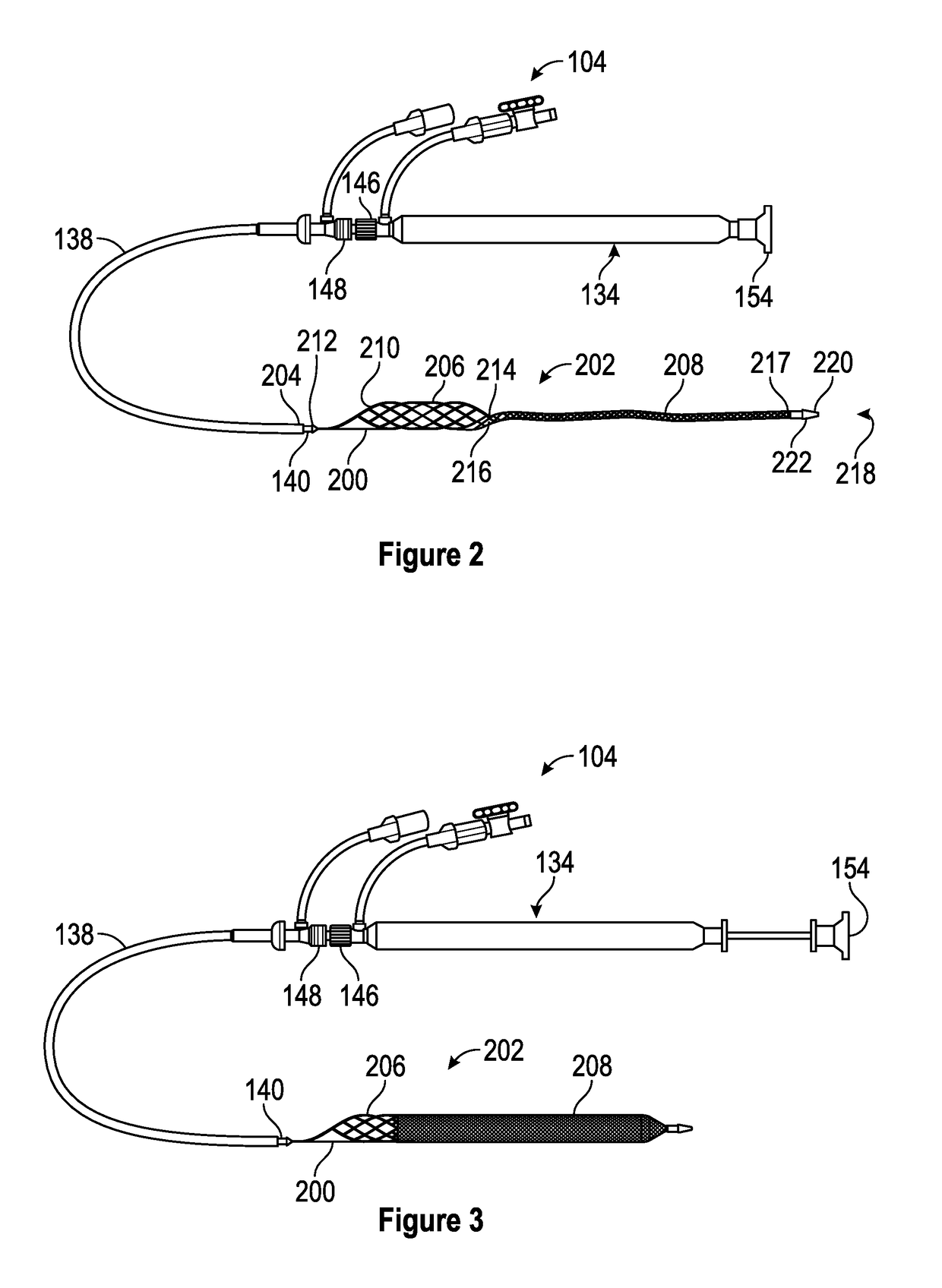
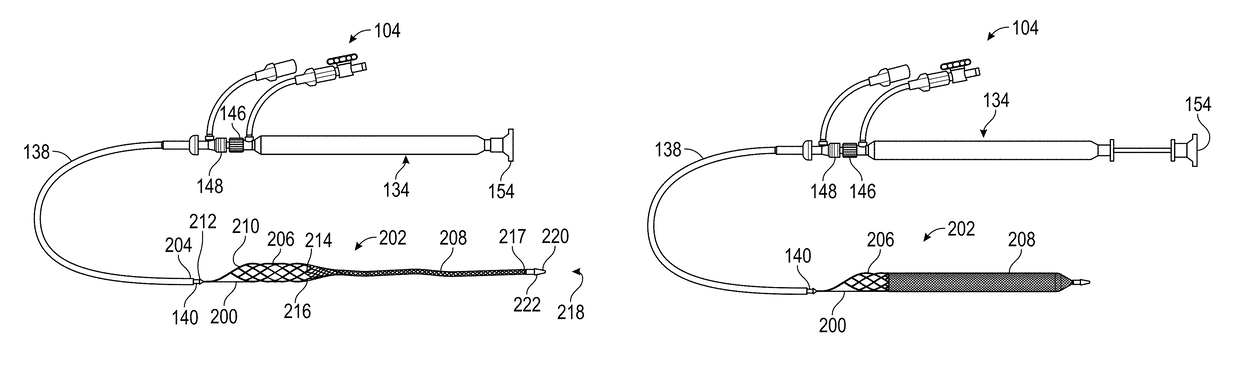
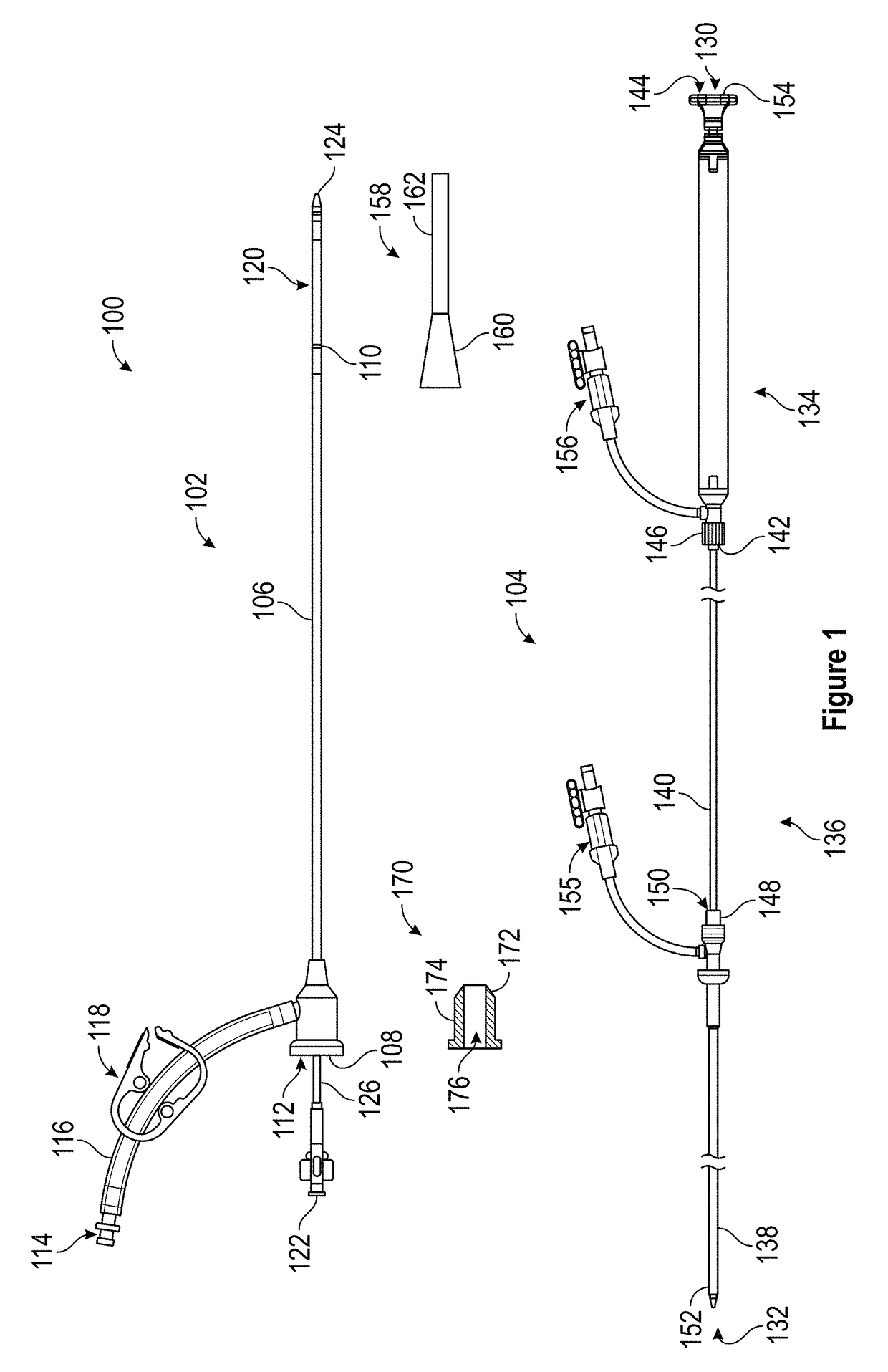
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
202results about How to "Reduce bleeding risk" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
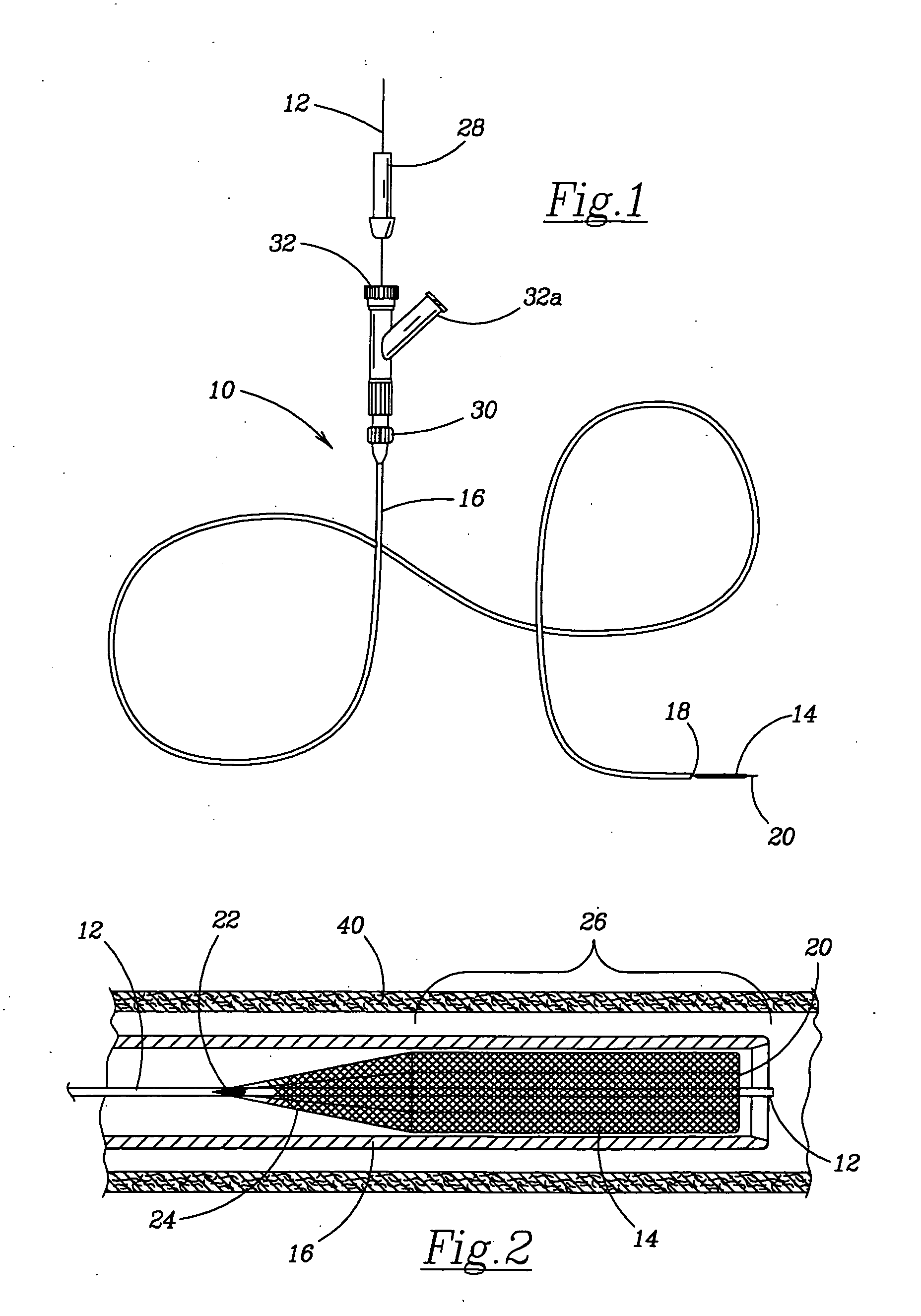
Bipolar surgical instruments having focused electrical fields
InactiveUS6162220AReduce capacityLess of a tendency to cause bleedingSurgical needlesSurgical instruments for heatingElectricityEngineering
A bipolar surgical device includes a pair of actuable jaws. A first electrode member which optionally includes a line of electrically coupled tissue-penetrating elements is formed on one of the jaws, and a second electrode member which optionally includes a line of electrically coupled tissue-penetrating elements is formed on the same or the other jaw. The electrode members are laterally spaced-apart and arranged in a parallel, usually linear manner so that the lateral distance therebetween remains generally constant. In operation, tissue may be grasped between the jaws so that the electrode members contact and / or the tissue-penetrating elements enter into the tissue. By energizing the electrode members at opposite polarities using a high frequency energy source, tissue between the jaws will be heated, coagulated, and / or necrosed, while heating of tissue outside of the lines will be minimized.
Owner:PERFECT SURGICAL TECHN
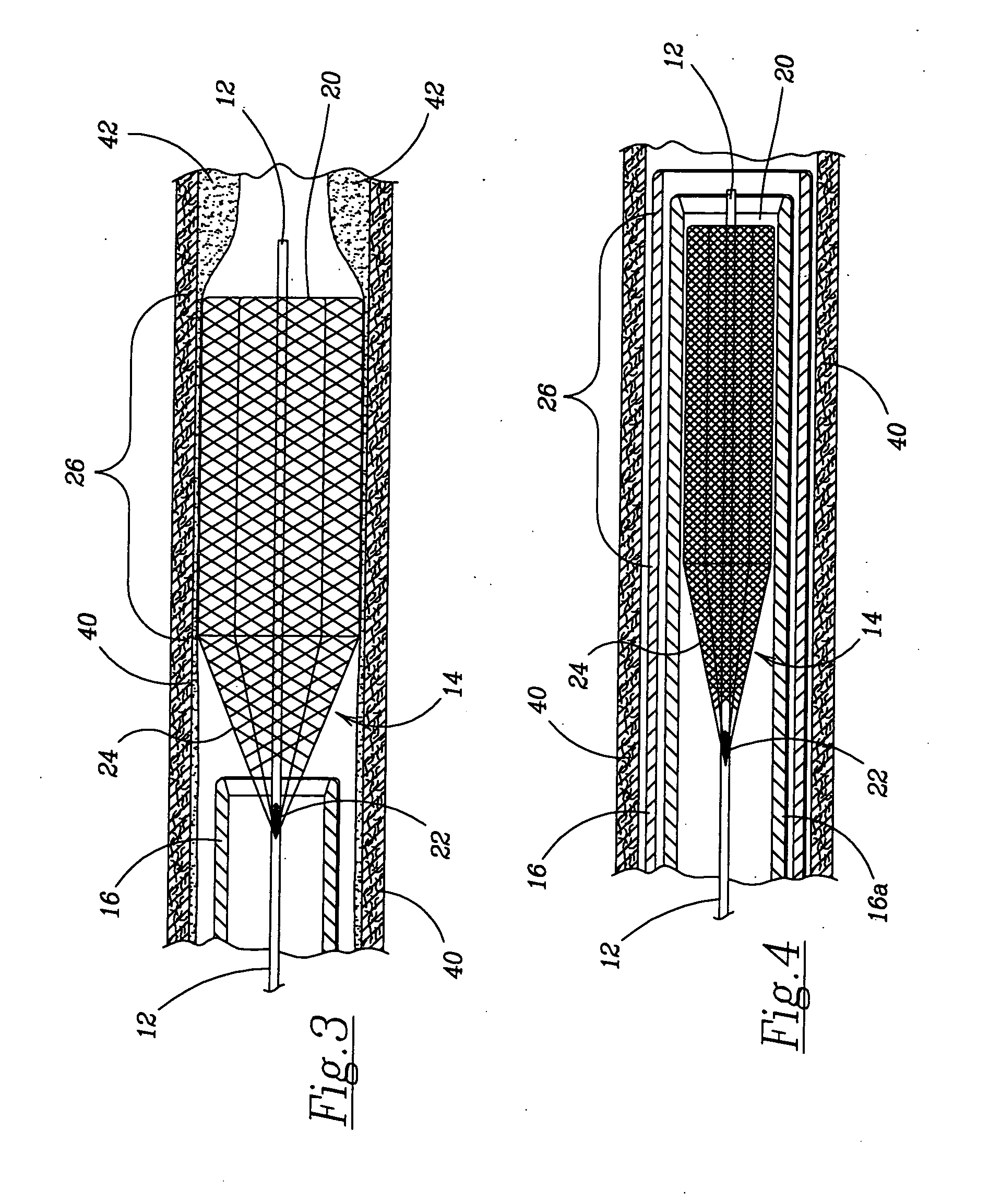
Clot retrieval device for removing occlusive clot from a blood vessel
ActiveUS20140371779A1Reduce radial forceMinimize compressionDilatorsExcision instrumentsBiomedical engineeringBlood vessel
Owner:NEURAVI
Clot retrieval device for removing occlusive clot from a blood vessel
ActiveUS20150164523A1Prevent distal egressReduce radial forceDilatorsExcision instrumentsDistal portionBlood vessel spasm
Owner:NEURAVI
Devices and methods for treating vascular occlusion
ActiveUS20190046219A1Reduce needEffectively core and separateExcision instrumentsBlood vessel filtersThrombusCatheter
Systems and methods for removal of thrombus from a blood vessel in a body of a patient are disclosed herein. The method can include: providing a thrombus extraction device including a proximal self-expanding member formed of a fenestrated structure, a substantially cylindrical portion formed of a net-like filament mesh structure having a proximal end coupled to a distal end of the fenestrated structure; advancing a catheter constraining the thrombus extraction device through a vascular thrombus, deploying the thrombus extraction device by stacking a portion of the net-like filament mesh structure outside of the catheter by distally advancing the self-expanding member until the self-expanding member is beyond a distal end of the catheter; retracting the self-expanding member to unstack the portion of the net-like filament mesh structure and to capture the portion of the thrombus; and withdrawing the thrombus extraction device from the body.
Owner:INARI MEDICAL INC
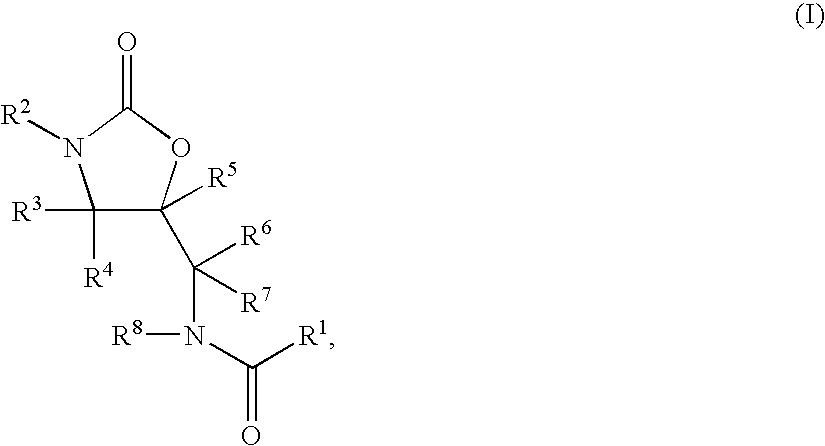
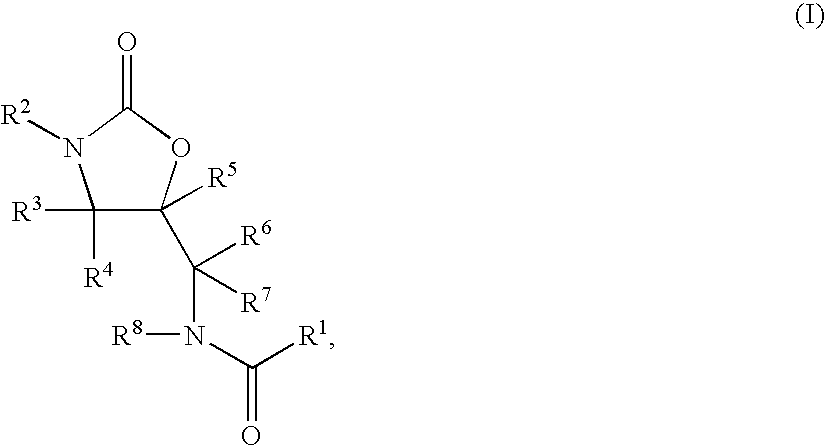
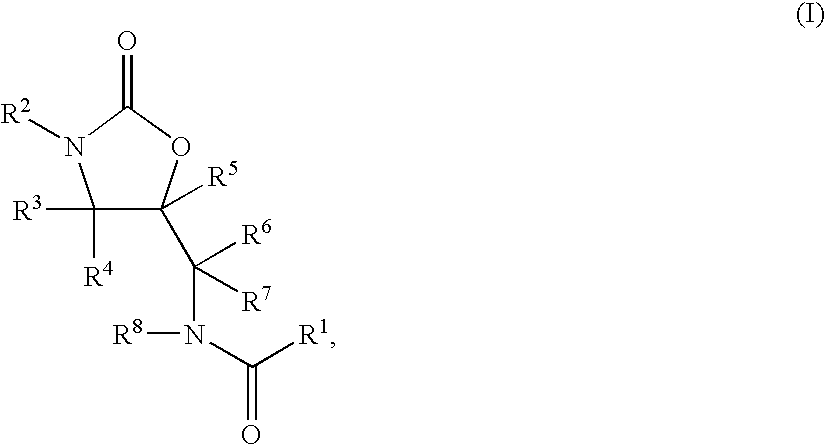
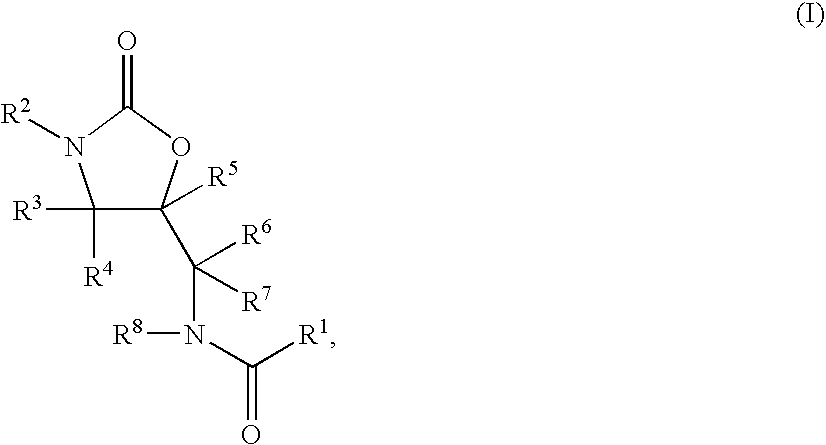
Substituted Oxazolidinones and Their Use in the Field of Blood Coagulation
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Intravascular treatment of vascular occlusion and associated devices, systems, and methods
Systems and methods for removal of thrombus from a blood vessel in a body of a patient are disclosed herein. The method can include: providing a thrombus extraction device including a proximal self-expanding member formed of a unitary fenestrated structure, a distal substantially cylindrical portion formed of a net-like filament mesh structure, and an inner shaft member connected to a distal end of the net-like filament mesh structure; advancing a catheter constraining the thrombus extraction device through a vascular thrombus, deploying the thrombus extraction; retracting the thrombus extraction device to separate a portion of the thrombus from the vessel wall and to capture the portion of the thrombus within the net-like filament mesh structure; and withdrawing the thrombus extraction device from the body to remove thrombus from the patient.
Owner:INARI MEDICAL INC
Intravascular treatment of vascular occlusion and associated devices, systems, and methods
ActiveUS20170265878A1Reduce needEffectively core and separateExcision instrumentsBlood vessel filtersVeinVenous vessel
Systems and methods for treating thrombosis and or emboli in a peripheral vasculature of a patient are disclosed herein. The method can include providing a thrombus extraction device including a proximal self-expanding coring portion formed of a unitary fenestrated structure and a distal expandable tubular portion formed of a braided filament mesh structure; advancing a catheter constraining the thrombus extraction device through a vascular thrombus in a vessel; deploying the thrombus extraction device from the catheter from a constrained configuration to an expanded configuration; retracting the thrombus extraction device proximally so that the coring portion cores and separates a portion of the vascular thrombus from the venous vessel wall while the mesh structure captures the vascular thrombus portion; and withdrawing the thrombus extraction device from the patient to remove the vascular thrombus portion from the vessel.
Owner:INARI MEDICAL INC
Devices and methods for treating vascular occlusion
ActiveUS20180193043A1Reduce risk of bleedCost reductionExcision instrumentsBlood vessel filtersThrombusVascular occlusion
Systems and methods for removal of thrombus from a blood vessel in a body of a patient are disclosed herein. The method can include: providing a thrombus extraction device including a proximal self-expanding member formed of a fenestrated structure, a substantially cylindrical portion formed of a net-like filament mesh structure having a proximal end coupled to a distal end of the fenestrated structure; advancing a catheter constraining the thrombus extraction device through a vascular thrombus, deploying the thrombus extraction device by stacking a portion of the net-like filament mesh structure outside of the catheter by distally advancing the self-expanding member until the self-expanding member is beyond a distal end of the catheter; retracting the self-expanding member to unstack the portion of the net-like filament mesh structure and to capture the portion of the thrombus; and withdrawing the thrombus extraction device from the body.
Owner:INARI MEDICAL INC
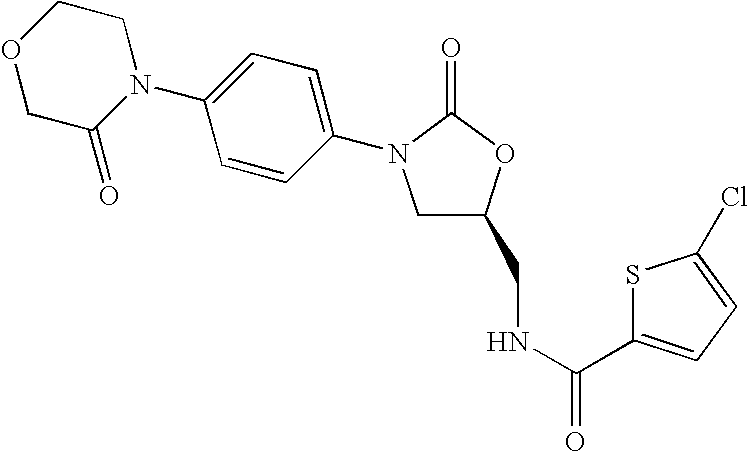
Substituted oxazolidinones and their use in the field of blood coagulation
InactiveUS20080090815A1Reduce bleeding riskEasy to adjustBiocideNervous disorderDiseaseOxazolidine E
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Intravascular treatment of vascular occlusion and associated devices, systems, and methods
ActiveUS9700332B2Reduce needEffectively core and separateExcision instrumentsBlood vessel filtersVeinVenous vessel
Systems and methods for treating thrombosis and or emboli in a peripheral vasculature of a patient are disclosed herein. The method can include providing a thrombus extraction device including a proximal self-expanding coring portion formed of a unitary fenestrated structure and a distal expandable tubular portion formed of a braided filament mesh structure; advancing a catheter constraining the thrombus extraction device through a vascular thrombus in a vessel; deploying the thrombus extraction device from the catheter from a constrained configuration to an expanded configuration; retracting the thrombus extraction device proximally so that the coring portion cores and separates a portion of the vascular thrombus from the venous vessel wall while the mesh structure captures the vascular thrombus portion; and withdrawing the thrombus extraction device from the patient to remove the vascular thrombus portion from the vessel.
Owner:INARI MEDICAL INC
Devices and methods for treating vascular occlusion
ActiveUS10098651B2Reduce needEffectively core and separateExcision instrumentsBlood vessel filtersThrombusBlood vessel
Systems and methods for removal of thrombus from a blood vessel in a body of a patient are disclosed herein. The method can include: providing a thrombus extraction device including a proximal self-expanding member formed of a fenestrated structure, a substantially cylindrical portion formed of a net-like filament mesh structure having a proximal end coupled to a distal end of the fenestrated structure; advancing a catheter constraining the thrombus extraction device through a vascular thrombus, deploying the thrombus extraction device by stacking a portion of the net-like filament mesh structure outside of the catheter by distally advancing the self-expanding member until the self-expanding member is beyond a distal end of the catheter; retracting the self-expanding member to unstack the portion of the net-like filament mesh structure and to capture the portion of the thrombus; and withdrawing the thrombus extraction device from the body.
Owner:INARI MEDICAL INC
Intravascular treatment of vascular occlusion and associated devices, systems, and methods
ActiveUS10342571B2Reduce needEffectively core and separateSurgical needlesCatheterThrombusBlood vessel
Systems and methods for removal of thrombus from a blood vessel in a body of a patient are disclosed herein. The method can include: providing a thrombus extraction device including a proximal self-expanding member formed of a unitary fenestrated structure, a distal substantially cylindrical portion formed of a net-like filament mesh structure, and an inner shaft member connected to a distal end of the net-like filament mesh structure; advancing a catheter constraining the thrombus extraction device through a vascular thrombus, deploying the thrombus extraction; retracting the thrombus extraction device to separate a portion of the thrombus from the vessel wall and to capture the portion of the thrombus within the net-like filament mesh structure; and withdrawing the thrombus extraction device from the body to remove thrombus from the patient.
Owner:INARI MEDICAL INC
Ligands for use in therapeutic compositions for the treatment of hemostasis disorders
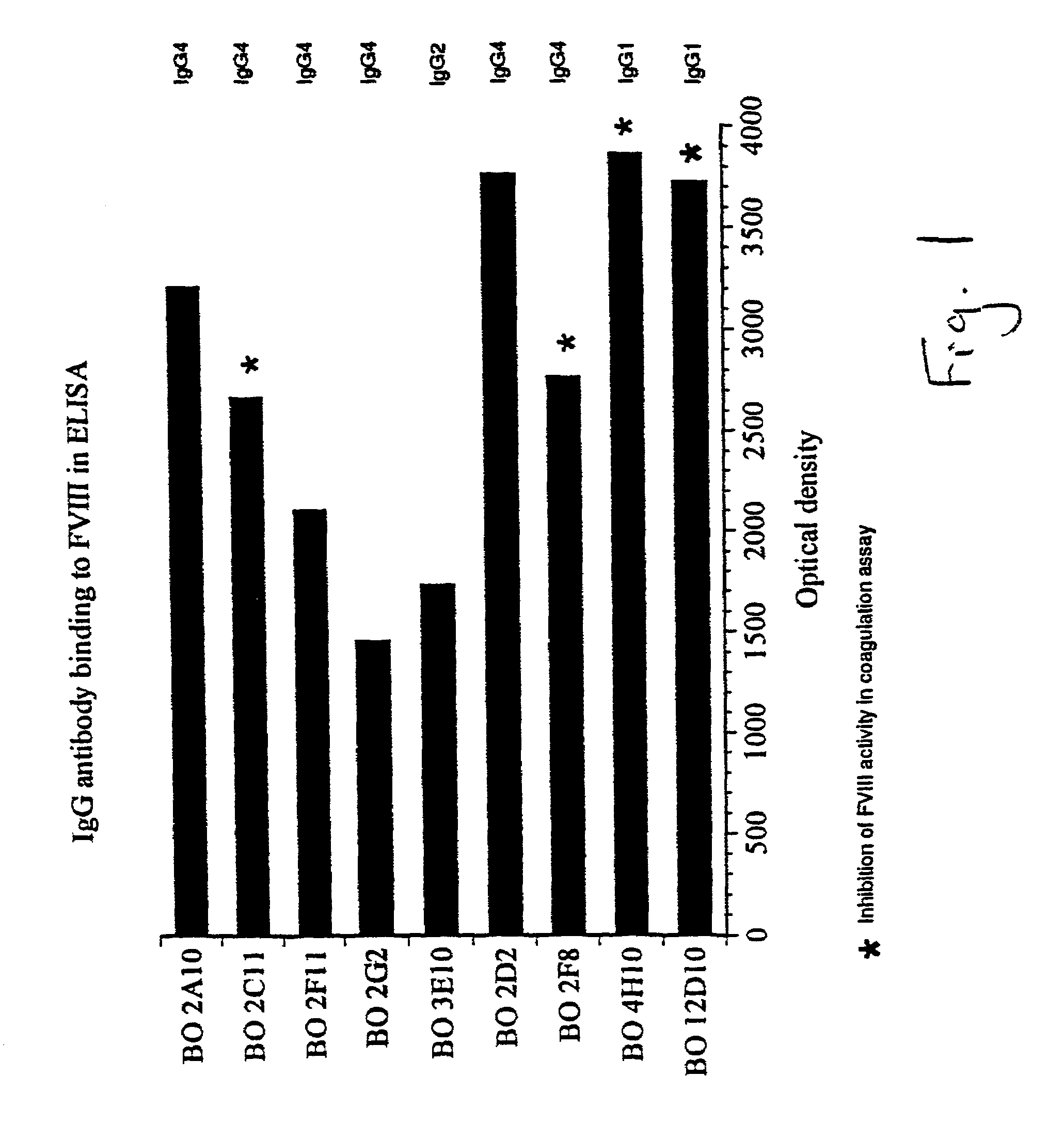
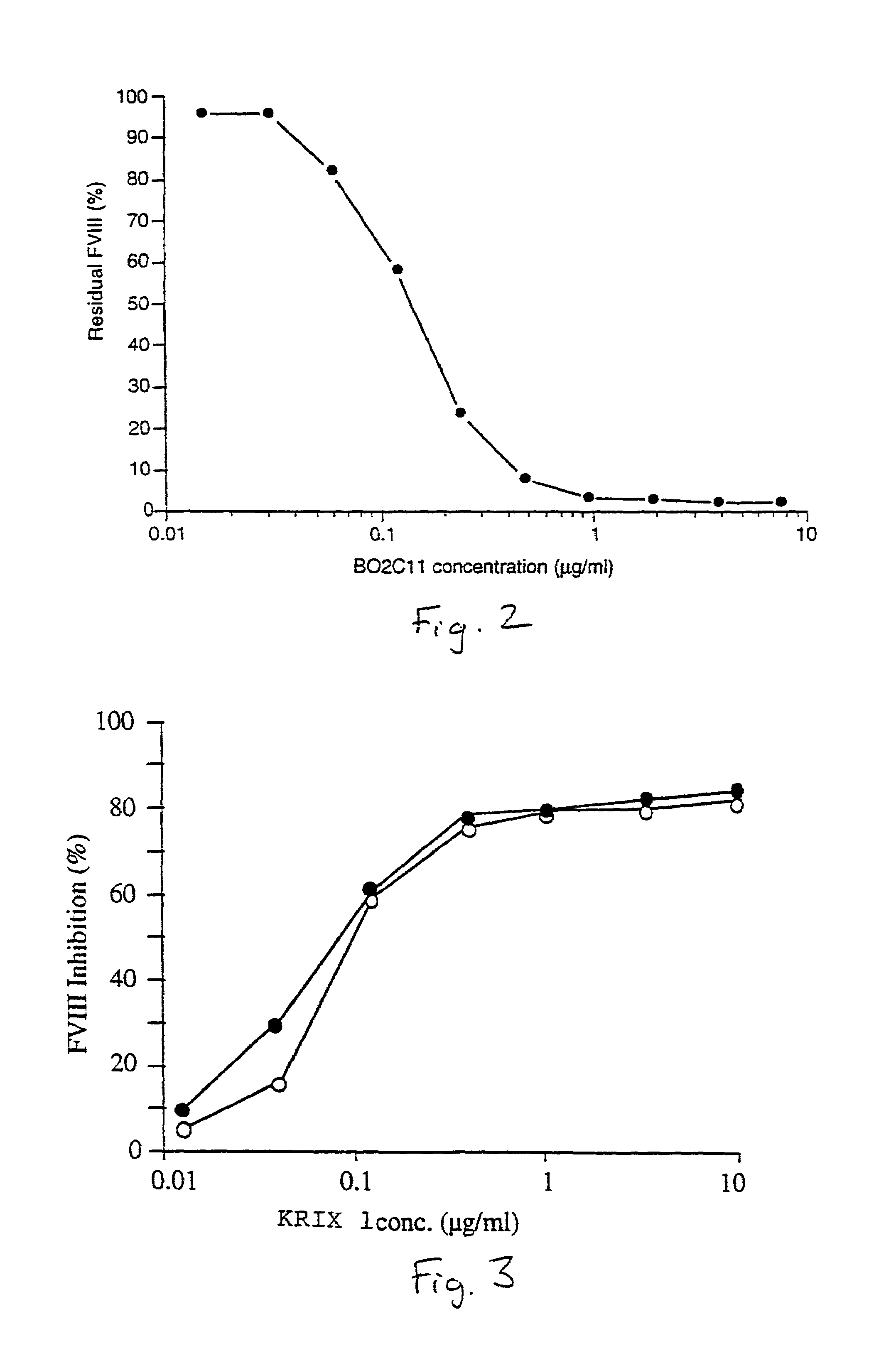
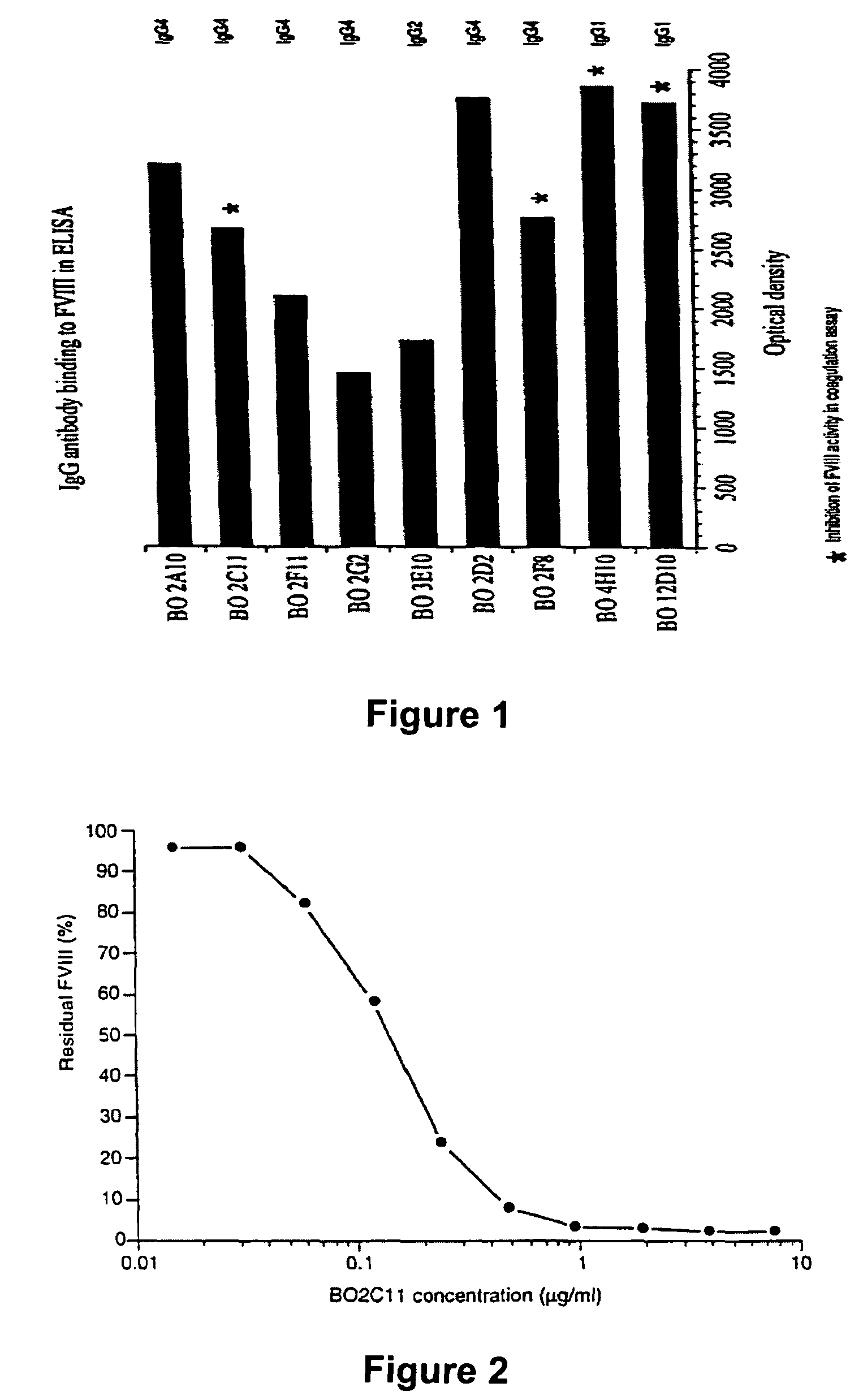
InactiveUS7067313B1Reduce bleeding riskSafe and effective therapyImmunoglobulins against blood coagulation factorsAnimal cellsAnti-factor VIIIPhysiological function
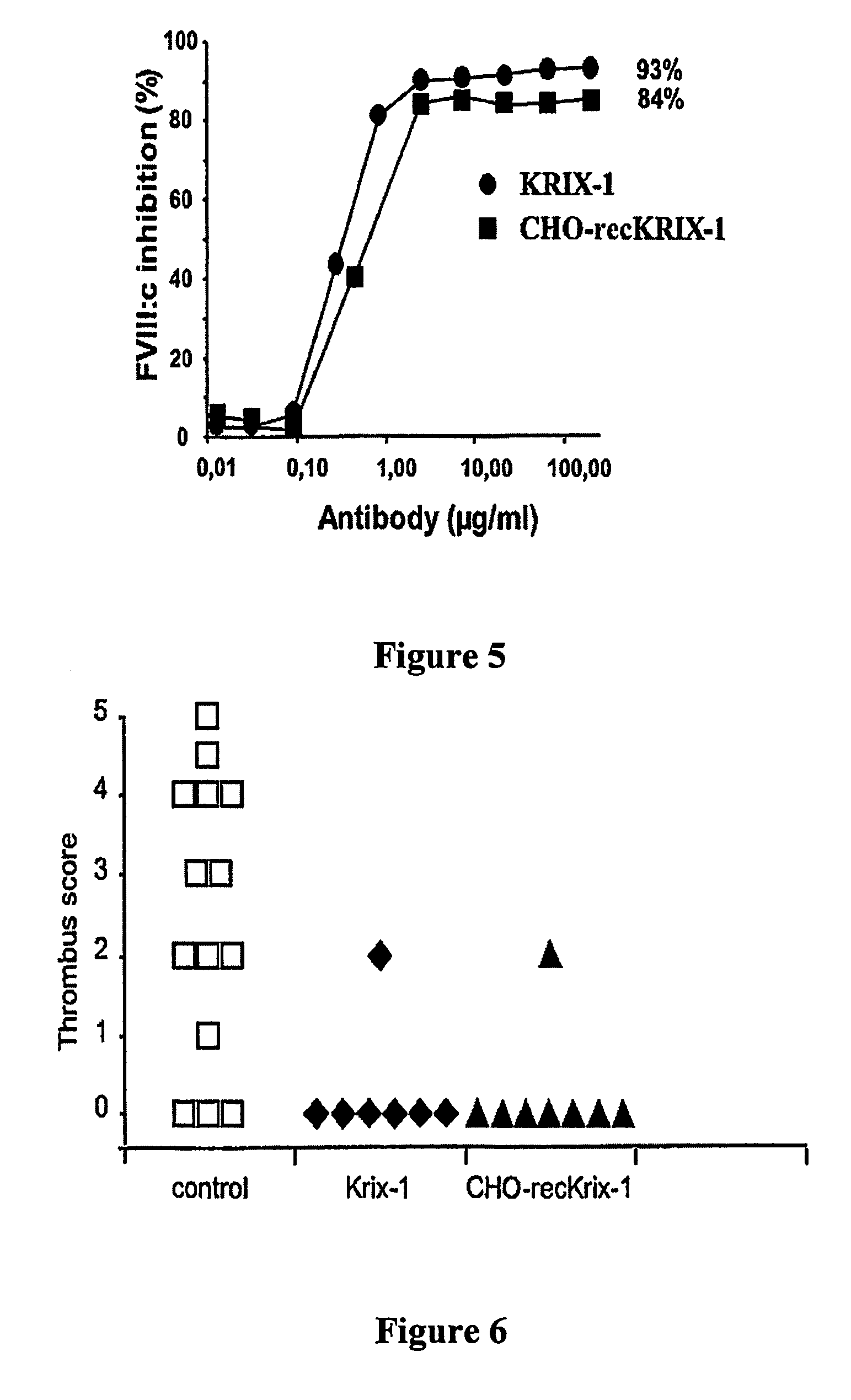
The present invention comprises ligands and methods of manufacture thereof as well as pharmaceutical preparations including the ligands. The ligands may be human or humanized monoclonal antibodies and fragments, derivatives and homologs thereof. These may exhibit an unforeseen “plateau effect”, i.e. the achievement of only a partial inactivation of a factor involved in hemostasis, in particular in the coagulation cascade, either individually or in combination even in molar excess. The ligands may bind to a factor or a complex of factors resulting in only partially impairing the function of a physiologically functional site of the said factor or factor complex even in molar excess. This makes the ligands particularly suitable for treating coagulation disorders and resulting thrombotic pathologic conditions while minimizing the risk of bleeding. Particularly useful is a property of ligands in accordance with the present invention to allow some physiological function of the affected site even when the ligand is in molar excess. The ligands may be anti-factor VIII antibodies or antibodies against a factor VIII complex, in particular human or human hybrid monoclonal antibodies which bind to factor VIII or a factor VIII complex and at least partially inhibit the activity of factor VIII.
Owner:LIFE SCI RES PARTNERS VZW
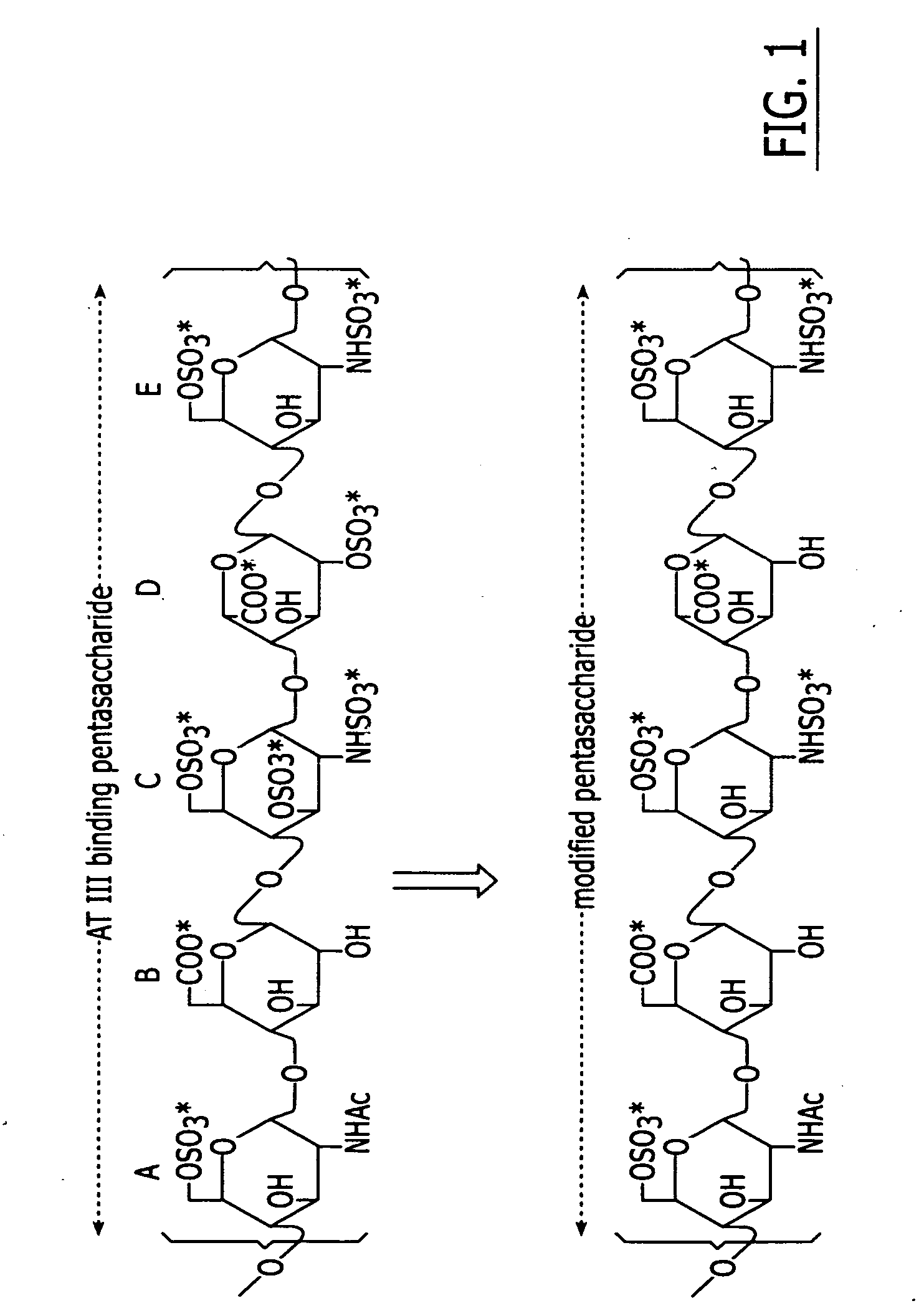
Glycosaminoglycans derived from K5 polysaccharide having high anticoagulant and antithrombotic activities and process for their preparation
InactiveUS8227449B2High activityReduce bleeding riskBiocideOrganic active ingredientsAntithrombotic AgentSulfation
Glycosaminoglycans derived from K5 polysaccharide having high anticoagulant and antithrombotic activity and useful for the control of coagulation and as antithrombotic agents are obtained starting from an optionally purified K5 polysaccharide by a process comprising the steps of N-deacetylation / N-sulfation, C5 epimerization, O-oversulfation, selective O-desulfation, 6-O-sulfation, N-sulfation, and optional depolymerization, in which said epimerization is performed with the use of the enzyme glucoronosyl C5 epimerase in solution or in immobilized form in the presence of divalent cations. New, particularly interesting antithrombin compounds are obtained by controlling the reaction time in the selective O-desulfation step and submitting the product obtained at the end of the final N-sulfation step to depolymerization.
Owner:GLYCORES 2000 SRL
Protection-sleeve-carrying paclitaxel drug balloon and preparation method thereof
The invention relates to a protection-sleeve-carrying paclitaxel drug balloon and a preparation method thereof. The protection-sleeve-carrying paclitaxel drug balloon comprises a drug balloon and a paclitaxel-containing degradable drug coating which is coated on a balloon work section. The drug balloon can be used as a pre-expansion balloon, a conveying balloon and a post-expansion balloon and the drug balloon comprises a HUB protection-sleeve handle, a near-end push rod, a transition section, a far-end push rod and a head end. The coating, which is configured by the paclitaxel and a contrast agent, or which includes the paclitaxel, a degradable polymer and a solvent of trichloroethane, acetone or tetrahydrofuran, can be layeredly coated on the balloon work section. Through the arrangement of a protection sleeve, the drug balloon is, during conveying processes from entering blood vessels to reaching diseased regions, in a protection state, so that drugs on the drug balloon may not be scoured, ineffective loss is avoided, initial drug loading capacity of the drug balloon can be reduced, the treatment effect can be meet and at the same time safety of the drug balloon is greatly improved.
Owner:BIOVAS (WUHAN) MEDICAL TECH CO LTD
Microwave radiating antenna of direct puncture for treating tumour
InactiveCN1676176AAvoid burnsImprove burnsMicrowave therapyAbnormal tissue growthElectrical conductor
The present invention relates to a microwave radiation antenna capable of making percutaneous puncture to cure tumor of several regions of human body. Said invention is formed from antenna connector, hard coaxial line, ceramic tube, water inlet tube, water outlet tube, temperature-measuring system, stainless steel sleeve and handle. Its output power can be up to above 100w, and is therapeutic effect is reliable and safe.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
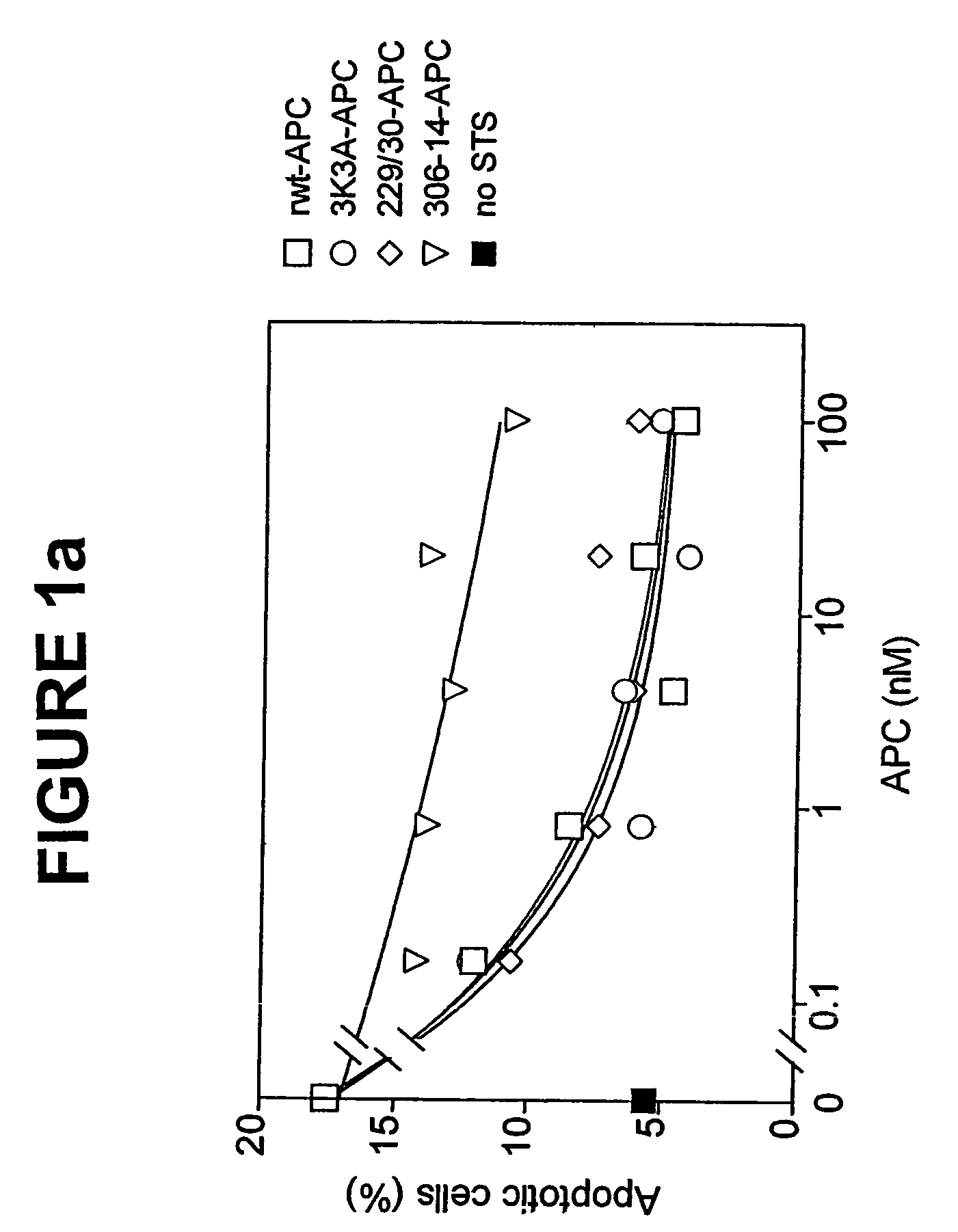
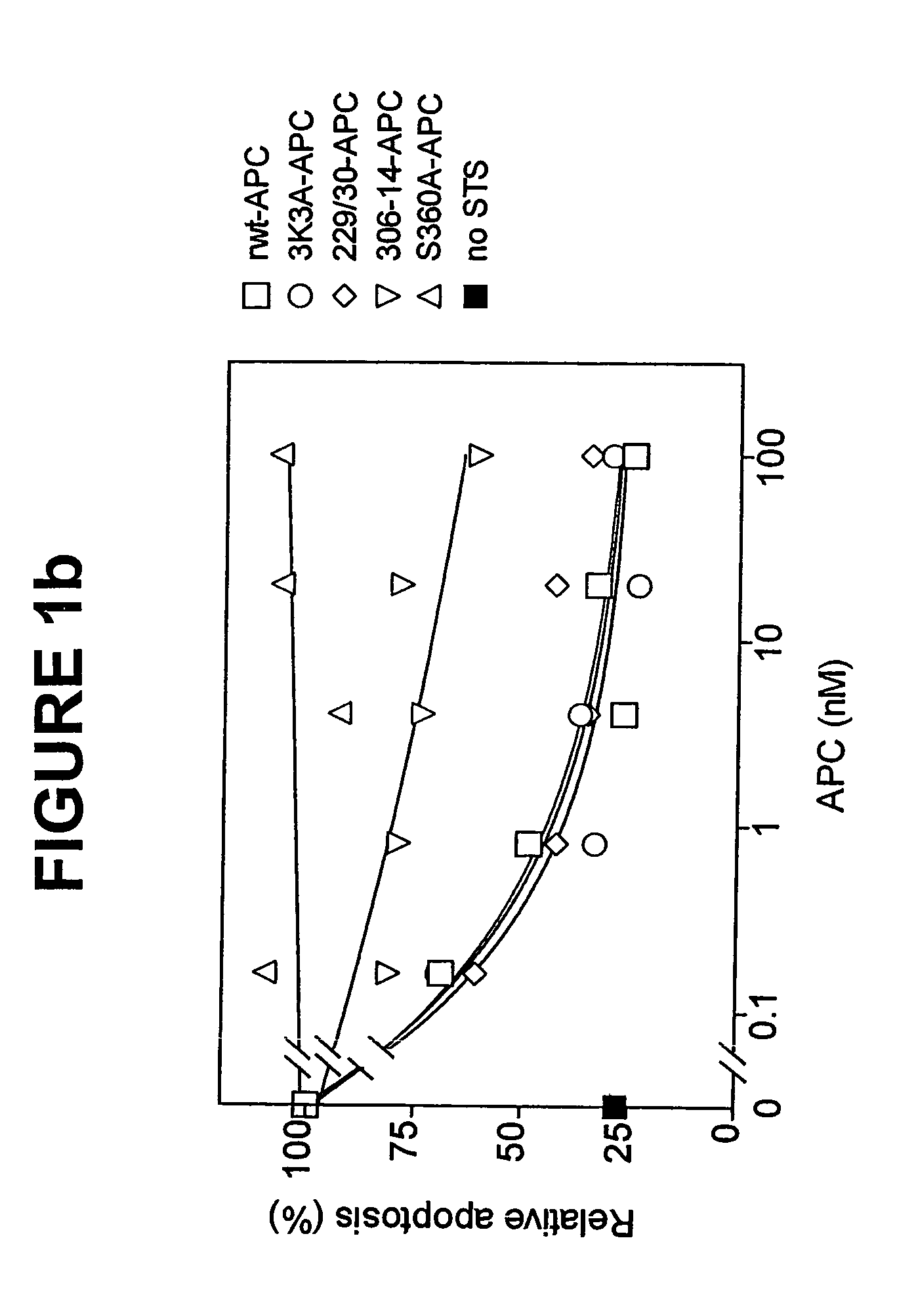
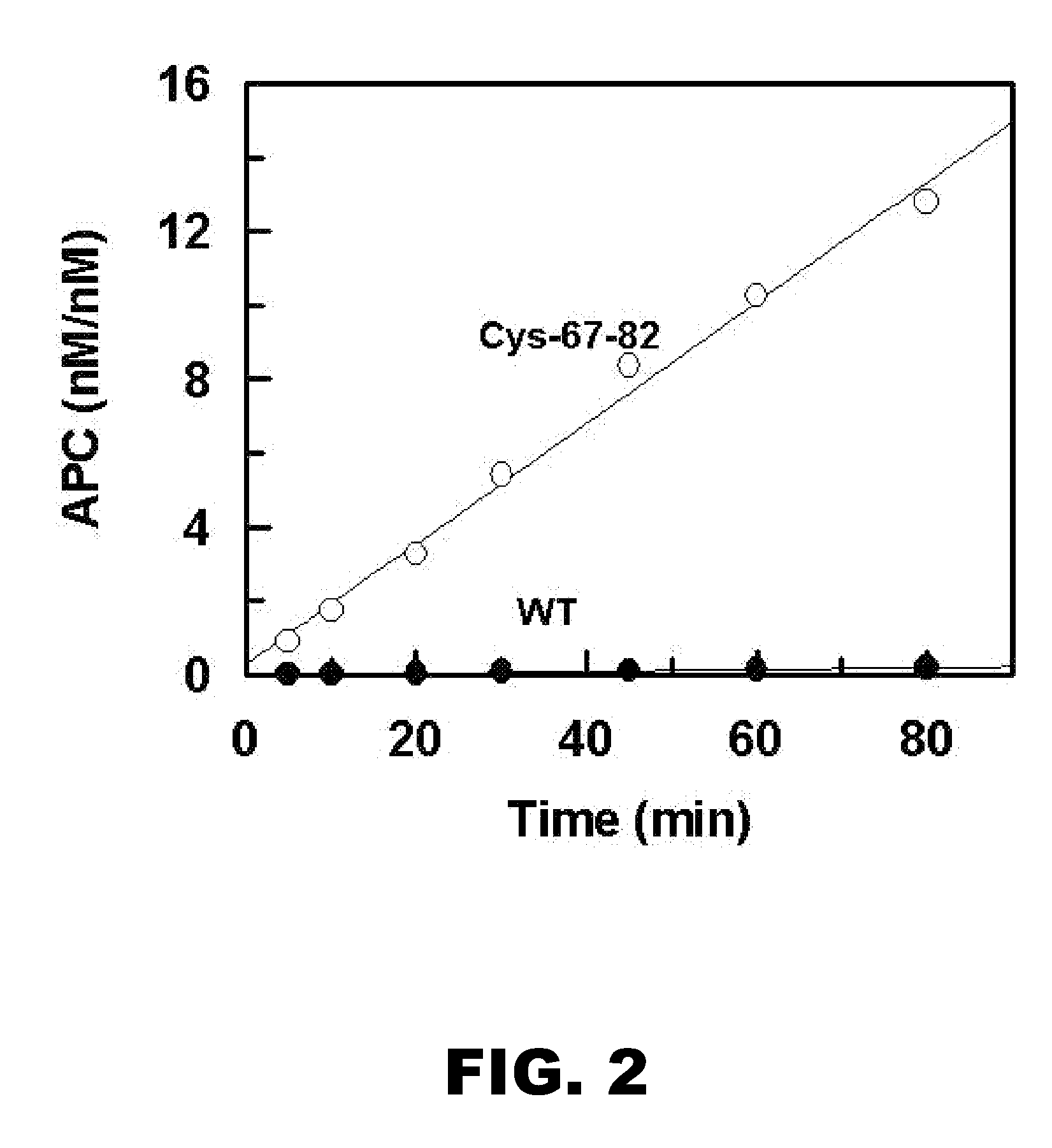
Activated protein C variants with normal cytoprotective activity but reduced anticoagulant activity
ActiveUS7498305B2Reduced activityReduce bleeding riskAntibacterial agentsNervous disorderReperfusion injuryApoptosis
Variants (mutants) of recombinant activated protein C (APC) or recombinant protein C (prodrug, capable of being converted to APC) that have substantial reductions in anticoagulant activity but that retain normal levels of anti-apoptotic activity are provided. Two examples of such recombinant APC mutants are KKK191-193AAA-APC and RR229 / 230M-APC. APC variants and prodrugs of the invention have the desirable property of being cytoprotective (anti-apoptotic effects), while having significantly reduced risk of bleeding. The invention also provides a method of using the APC variants or prodrugs of the invention to treat subjects who will benefit from APC's cytoprotective activities that are independent of APC's anticoagulant activity. These subjects include patients at risk of damage to blood vessels or tissue in various organs caused, at least in part, by apoptosis. At risk patients include, for example, those suffering (severe) sepsis, ischemia / reperfusion injury, ischemic stroke, acute myocardial infarction, acute or chronic neurodegenerative diseases, or those undergoing organ transplantation or chemotherapy, among other conditions. Methods of screening for variants of recombinant protein C or APC that are useful in accordance with the invention are also provided.
Owner:THE SCRIPPS RES INST
Glycosaminoglycans derived from k5 polysaccharide having high anticoagulant and antithrombotic activities and process for their preparation
InactiveUS20110281820A1High activityReduce bleeding riskOrganic active ingredientsBiocideSulfationAntithrombotic Agent
Glycosaminoglycans derived from K5 polysaccharide having high anticoagulant and antithrombotic activity and useful for the control of coagulation and as antithrombotic agents are obtained starting from an optionally purified K5 polysaccharide by a process comprising the steps of N-deacetylation / N-sulfation, C5 epimerization, O-oversulfation, selective O-desulfation, 6-O-sulfation, N-sulfation, and optional depolymerization, in which said epimerization is performed with the use of the enzyme glucoronosyl C5 epimerase in solution or in immobilized form in the presence of divalent cations. New, particularly interesting antithrombin compounds are obtained by controlling the reaction time in the selective O-desulfation step and submitting the product obtained at the end of the final N-sulfation step to depolymerization.
Owner:GLYCORES 2000 SRL
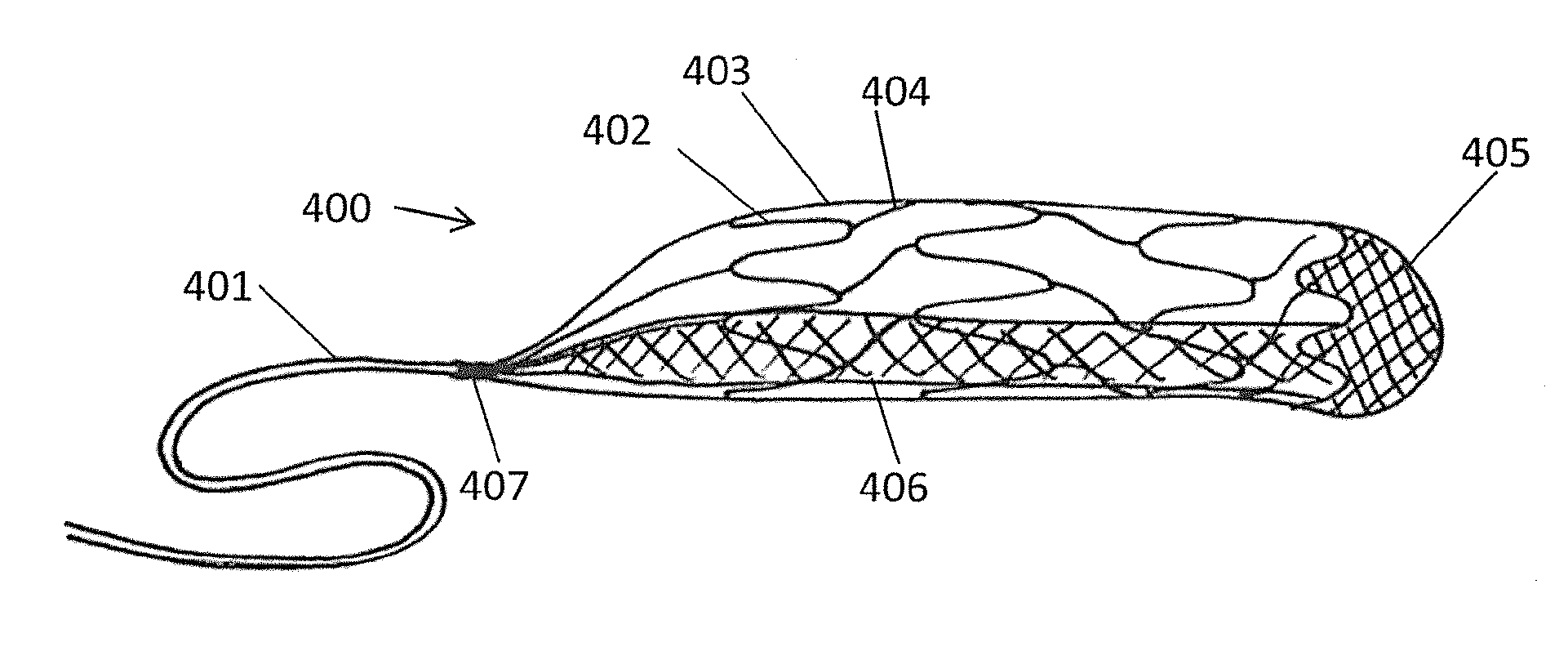
Devices to Support, Measure and Characterize Luminal Structures
InactiveUS20140296706A1Minimize traumaReducing and eliminating damageHeart valvesSurgical needlesThree dimensional shapeIliac artery
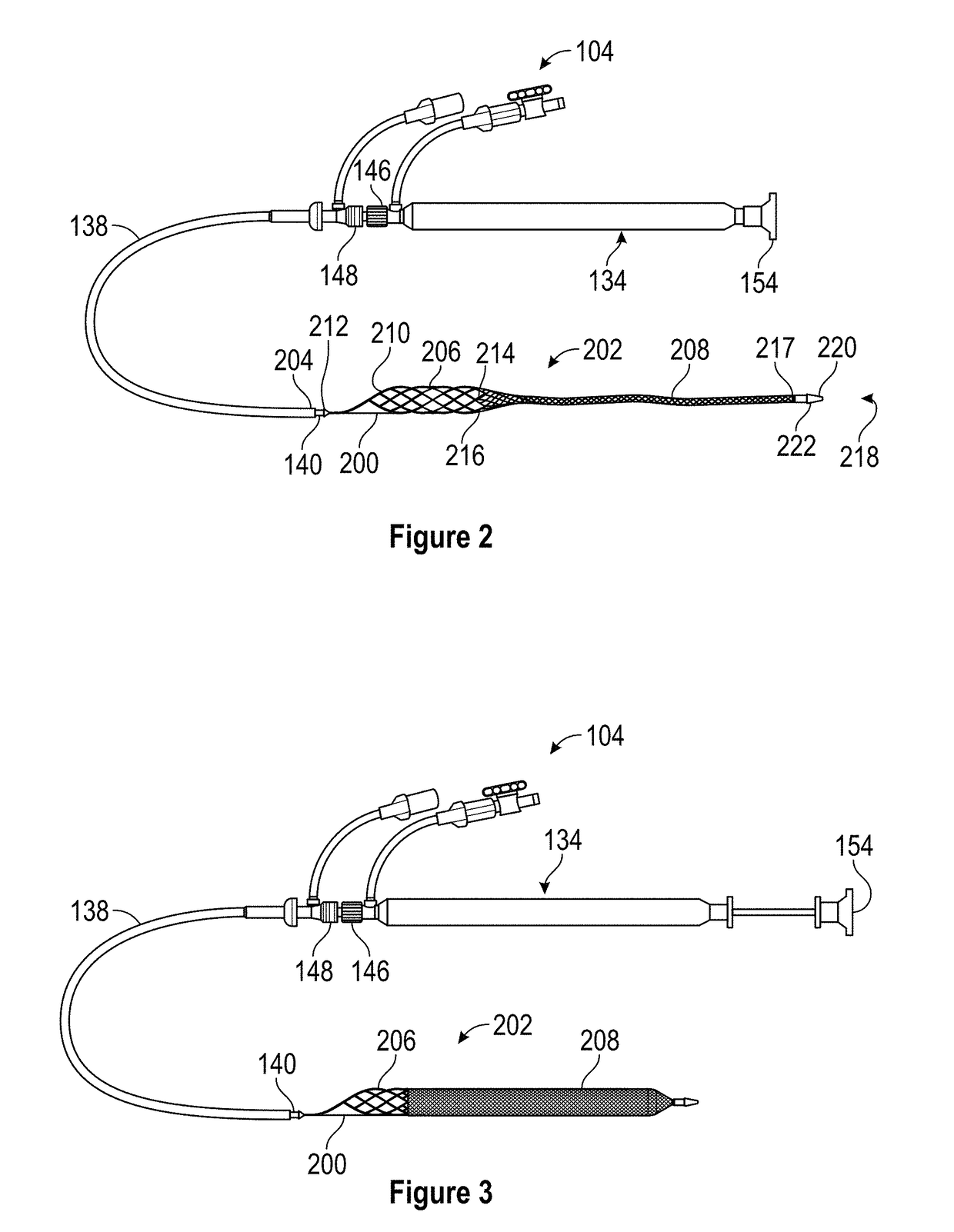
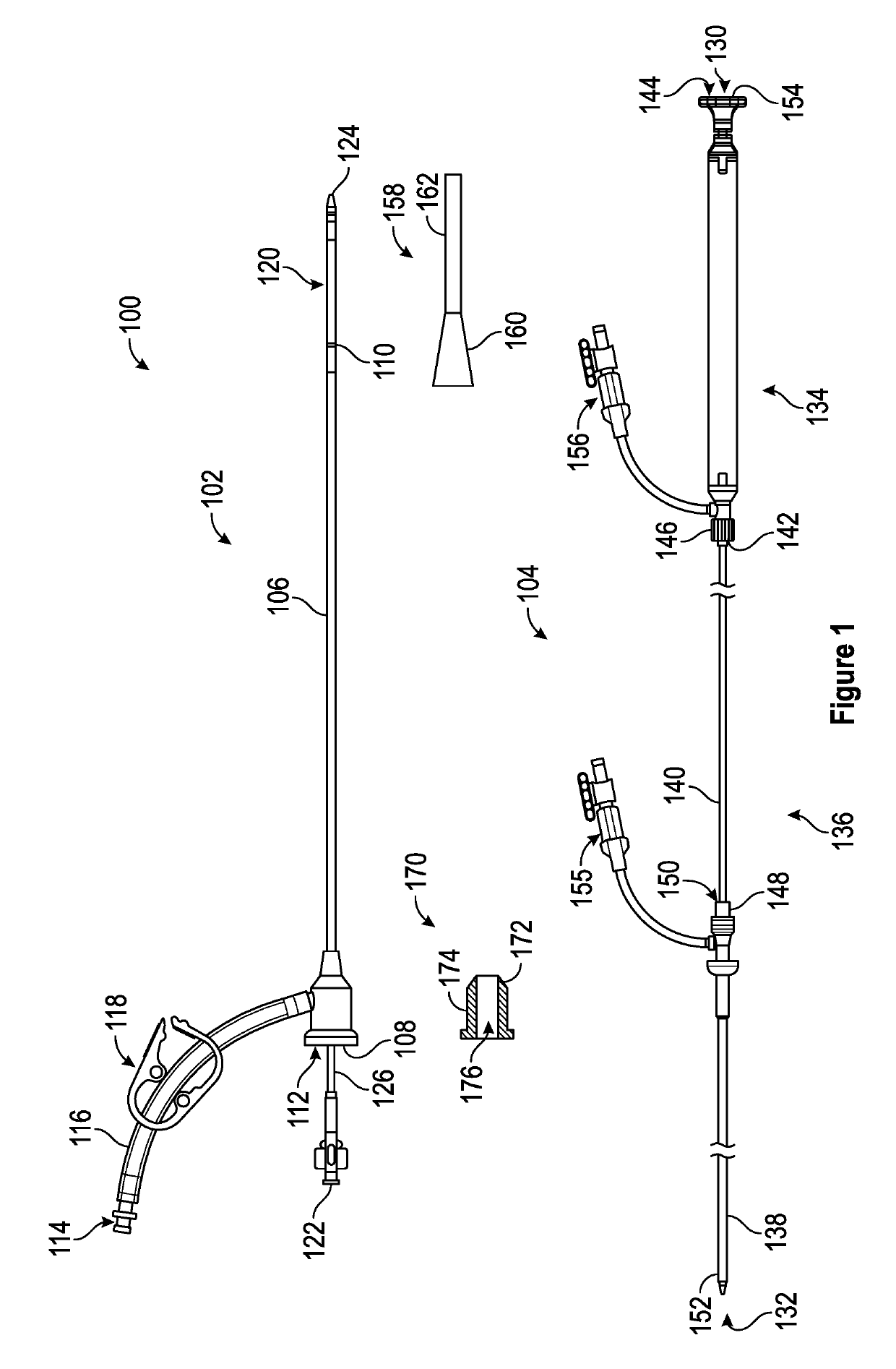
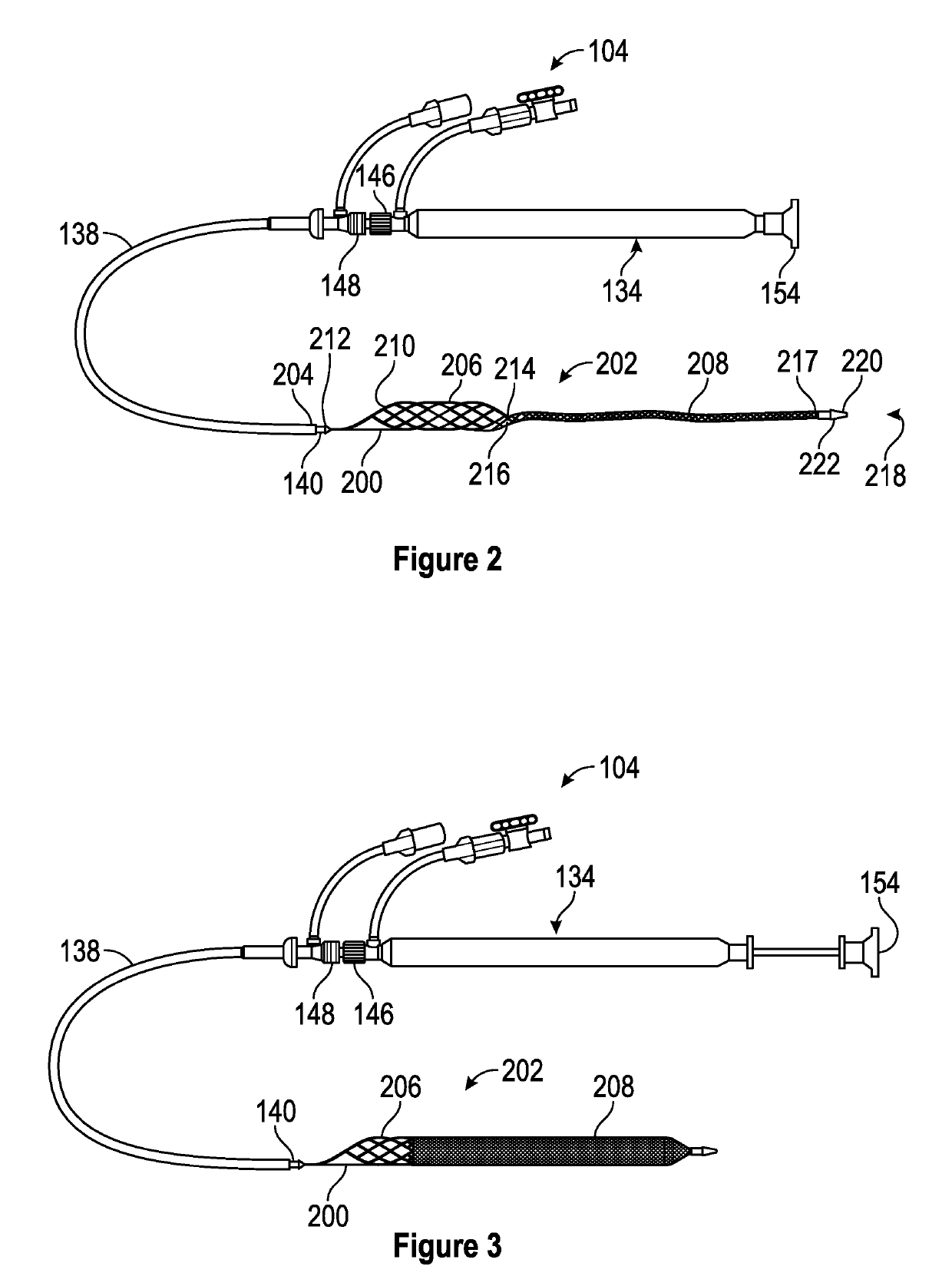
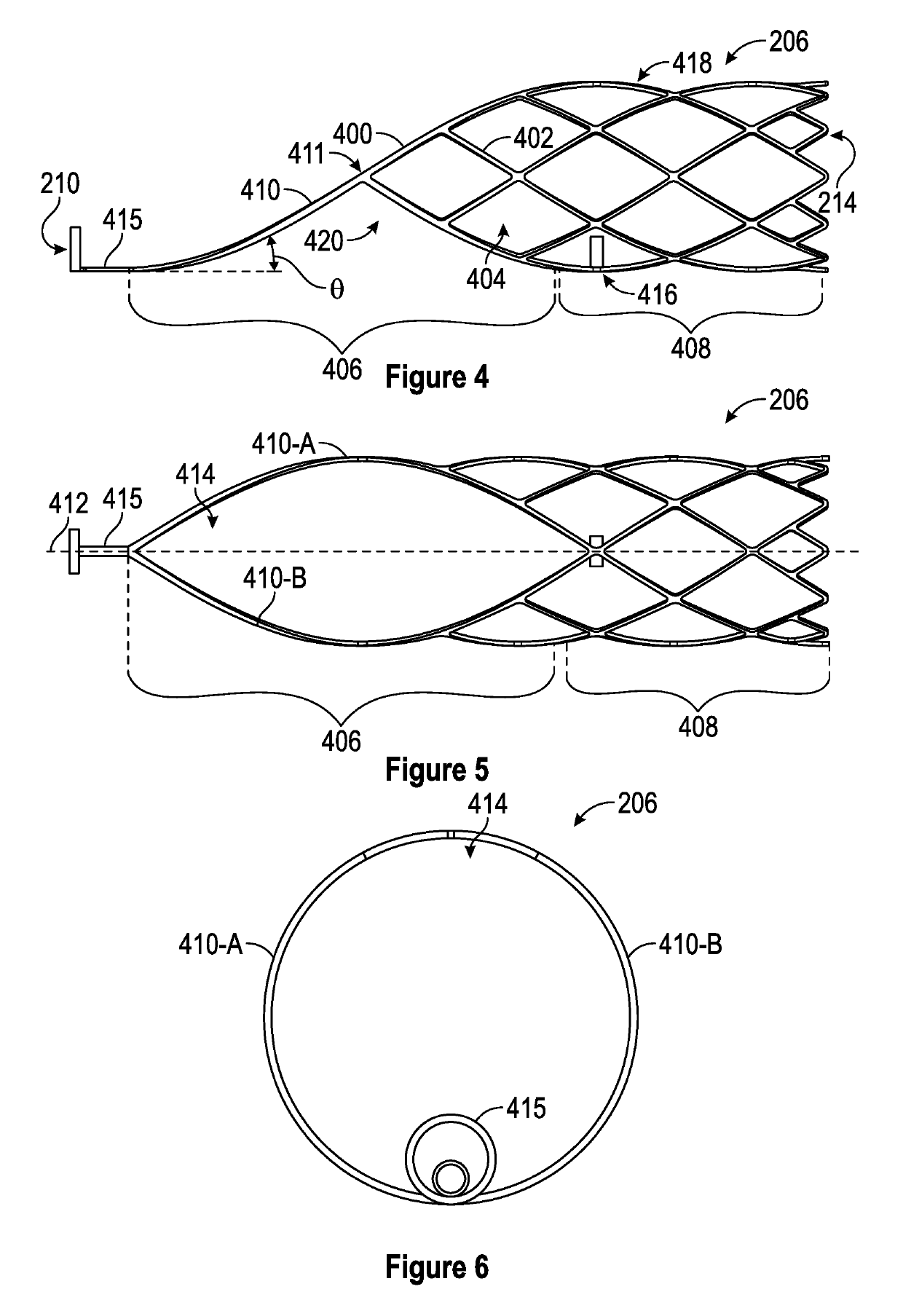
A device for characterizing a luminal dimension, such as the aortic annulus, has also been developed. These include a balloon or basket with sensing and transmitting elements for assessing two or three dimensional shape of lumens using a guide wire and catheter. Sheath introducer devices were developed for percutaneous delivery of bioprosthetic valves during various percutaneous procedures, such as TAVI. A marker needle dispenser for pre-marking anatomical features that are either desirable to target or desirable to avoid has been developed. The needle contains a central passage or lumen for loading marker and spacer material. These are characterized by specific spacing, color, shape or diagnostic imaging criteria to facilitate passage through and placement within the vasculature. Hemostatic stents or balloons are used to prevent bleeding and facilitate closure at sites for entry of catheters or introducer sheaths into luminal structures, especially for procedures such as TAVI through the subclavian artery.
Owner:INTERPERC TECH
PAR-1 (protease-activated receptor-1) antagonists for treating thrombotic diseases, as well as preparation and application thereof
ActiveCN102442965AReduce bleeding riskOrganic active ingredientsOrganic chemistryDiseasePharmaceutical medicine
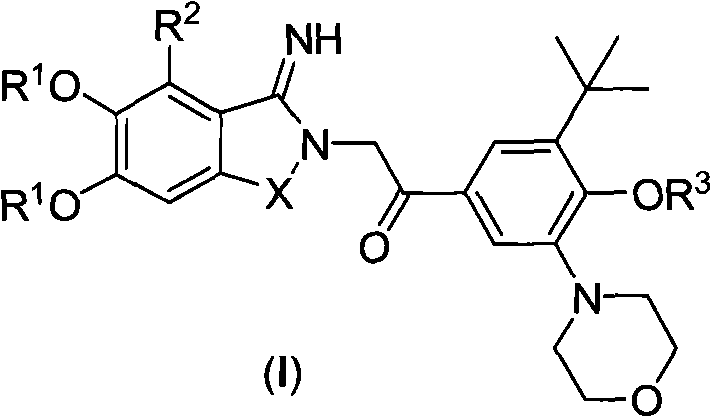
The invention relates to PAR-1 (protease-activated receptor-1) antagonists for treating thrombotic diseases, as well as a preparation and application thereof. The antagonists are compounds shown in formula (I) or pharmaceutically acceptable salts thereof, wherein R<1> is C1-C3 alkyl, R<2> is H, F or Cl, R<3> is C1-C3 alkyl, and X is O, S, C(CH3)2, CH2CH2, CH=CH or NCH3. The invention also provides a preparation method of the compounds, a medicinal composition containing the compounds, and application of the compounds. The compounds provided by the invention can prevent and / or treat thrombotic diseases.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Intravascular treatment of vascular occlusion and associated devices, systems, and methods
PendingUS20220015798A1Reduce needEffectively core and separateSurgical needlesCatheterThrombusEndovascular therapy
Systems and methods for removal of thrombus from a blood vessel in a body of a patient are disclosed herein. The method can include: providing a thrombus extraction device including a proximal self-expanding member formed of a unitary fenestrated structure, a distal substantially cylindrical portion formed of a net-like filament mesh structure, and an inner shaft member connected to a distal end of the net-like filament mesh structure; advancing a catheter constraining the thrombus extraction device through a vascular thrombus, deploying the thrombus extraction; retracting the thrombus extraction device to separate a portion of the thrombus from the vessel wall and to capture the portion of the thrombus within the net-like filament mesh structure; and withdrawing the thrombus extraction device from the body to remove thrombus from the patient.
Owner:INARI MEDICAL INC
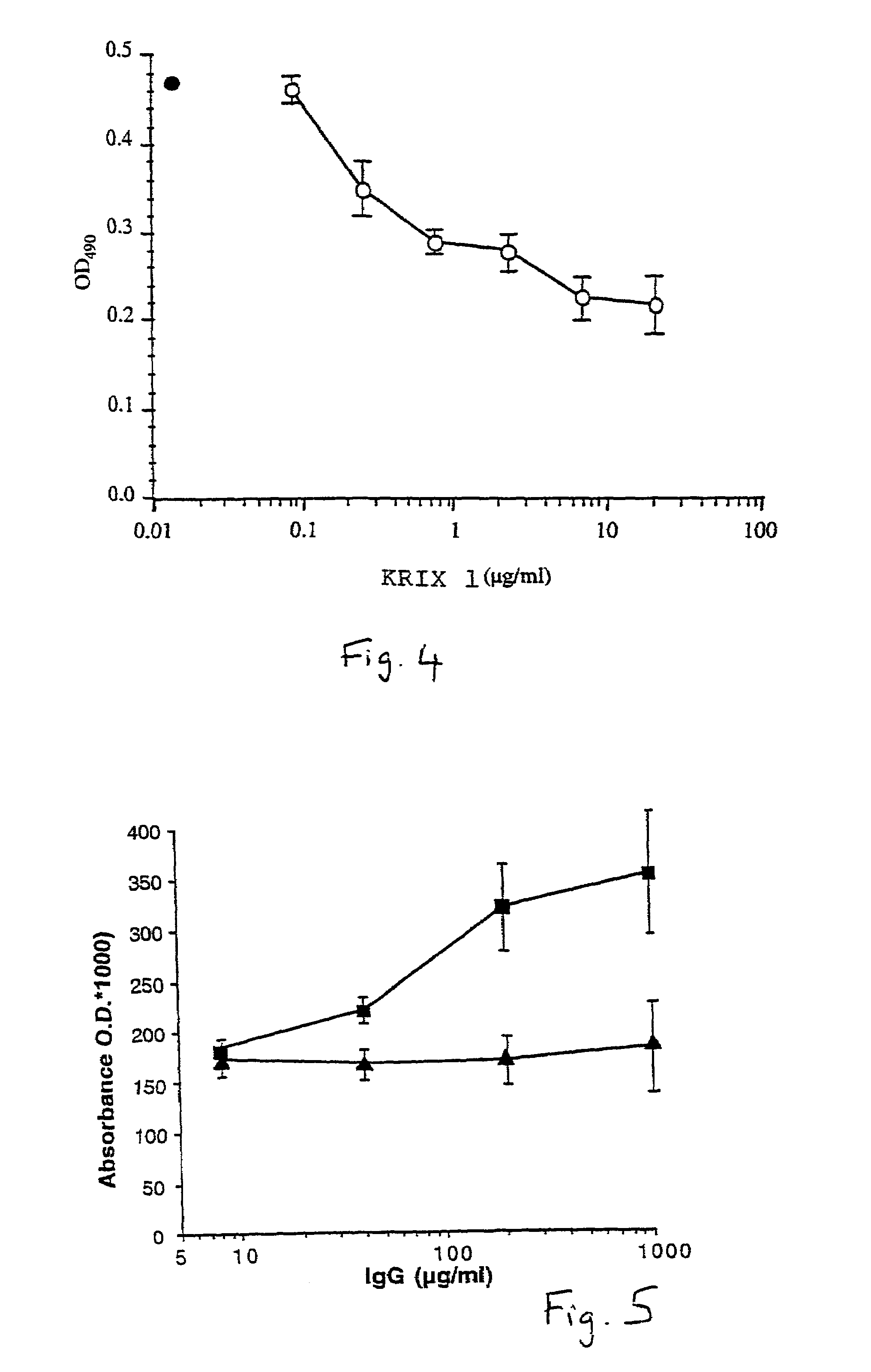
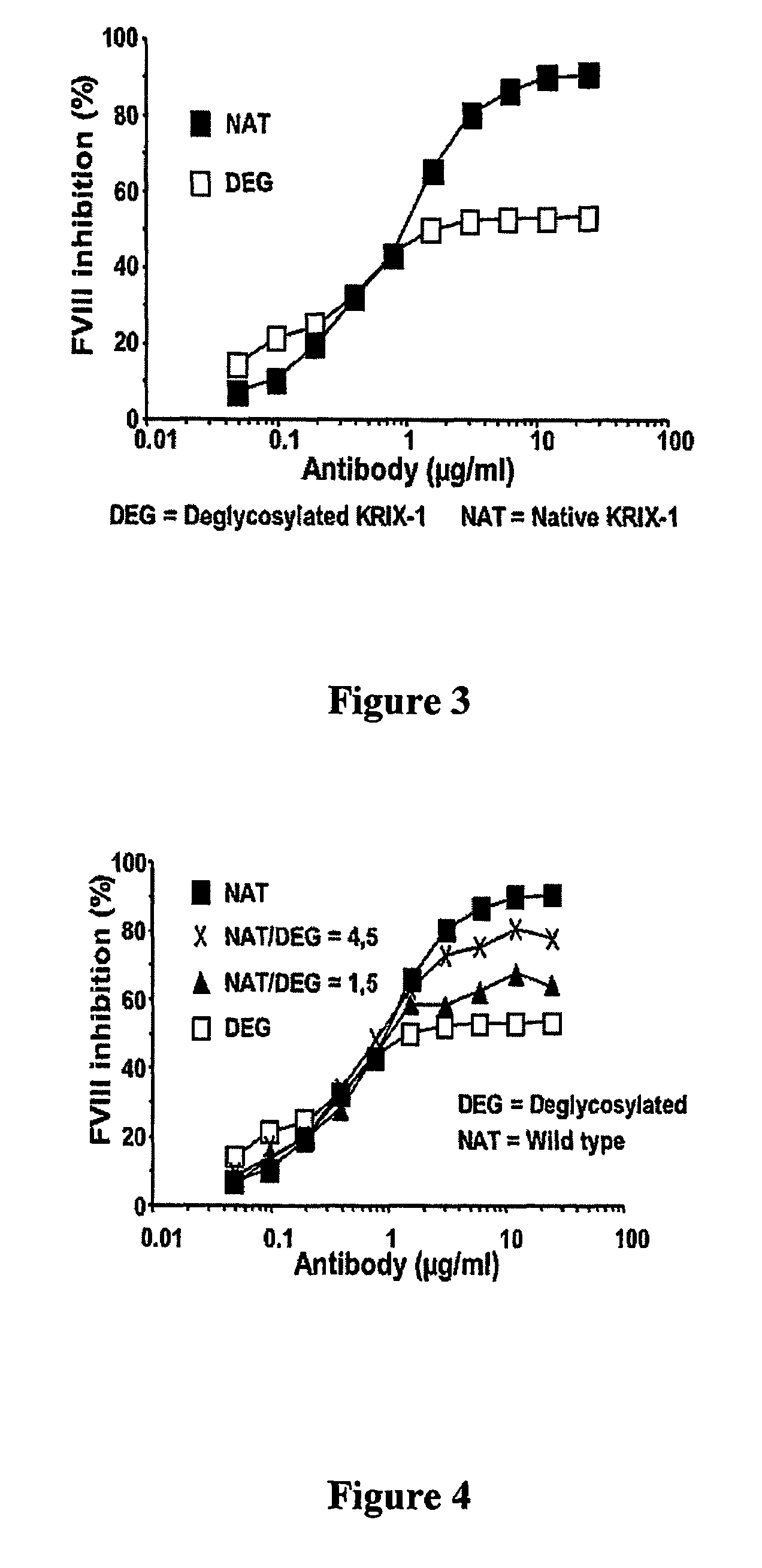
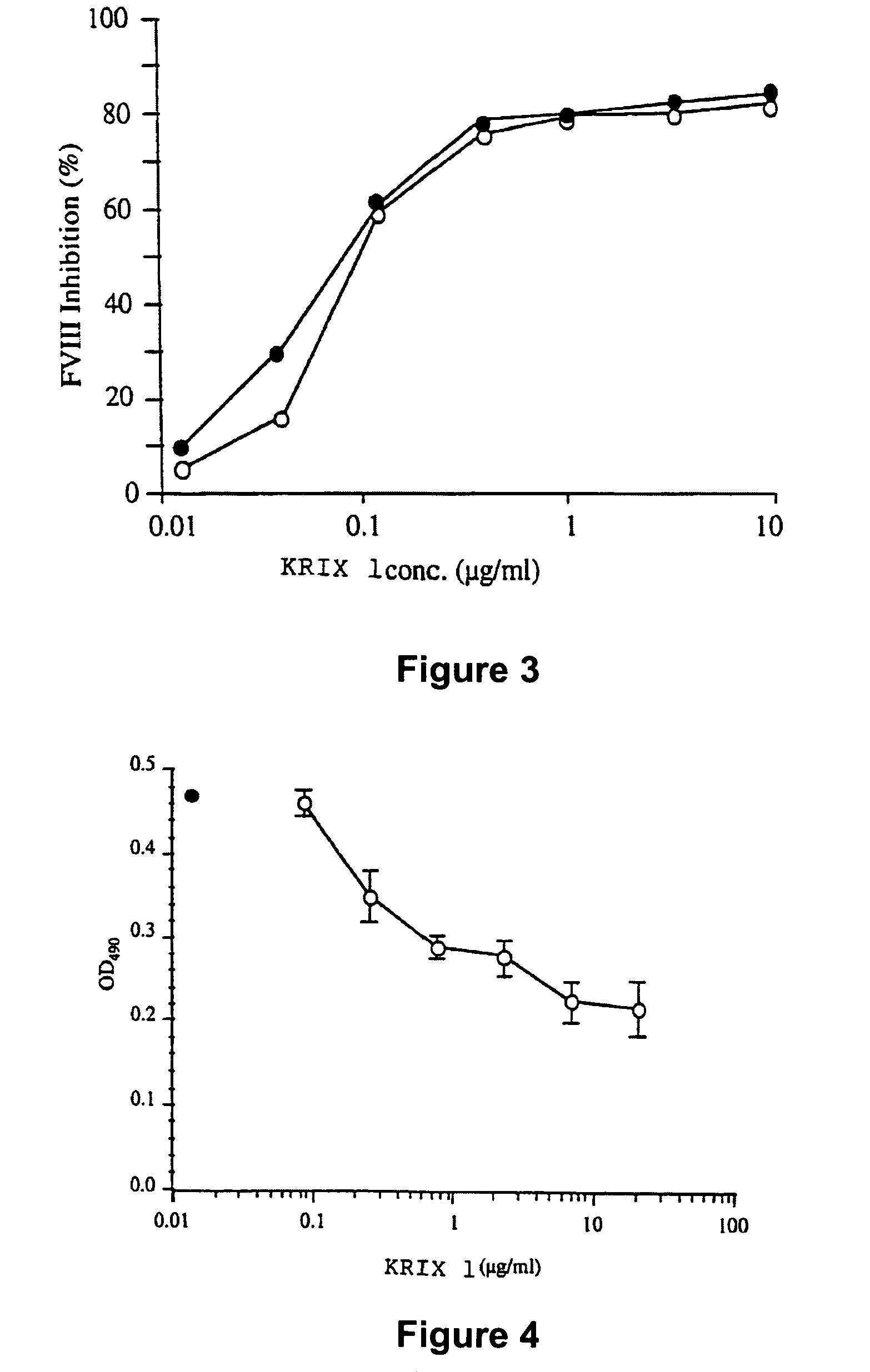
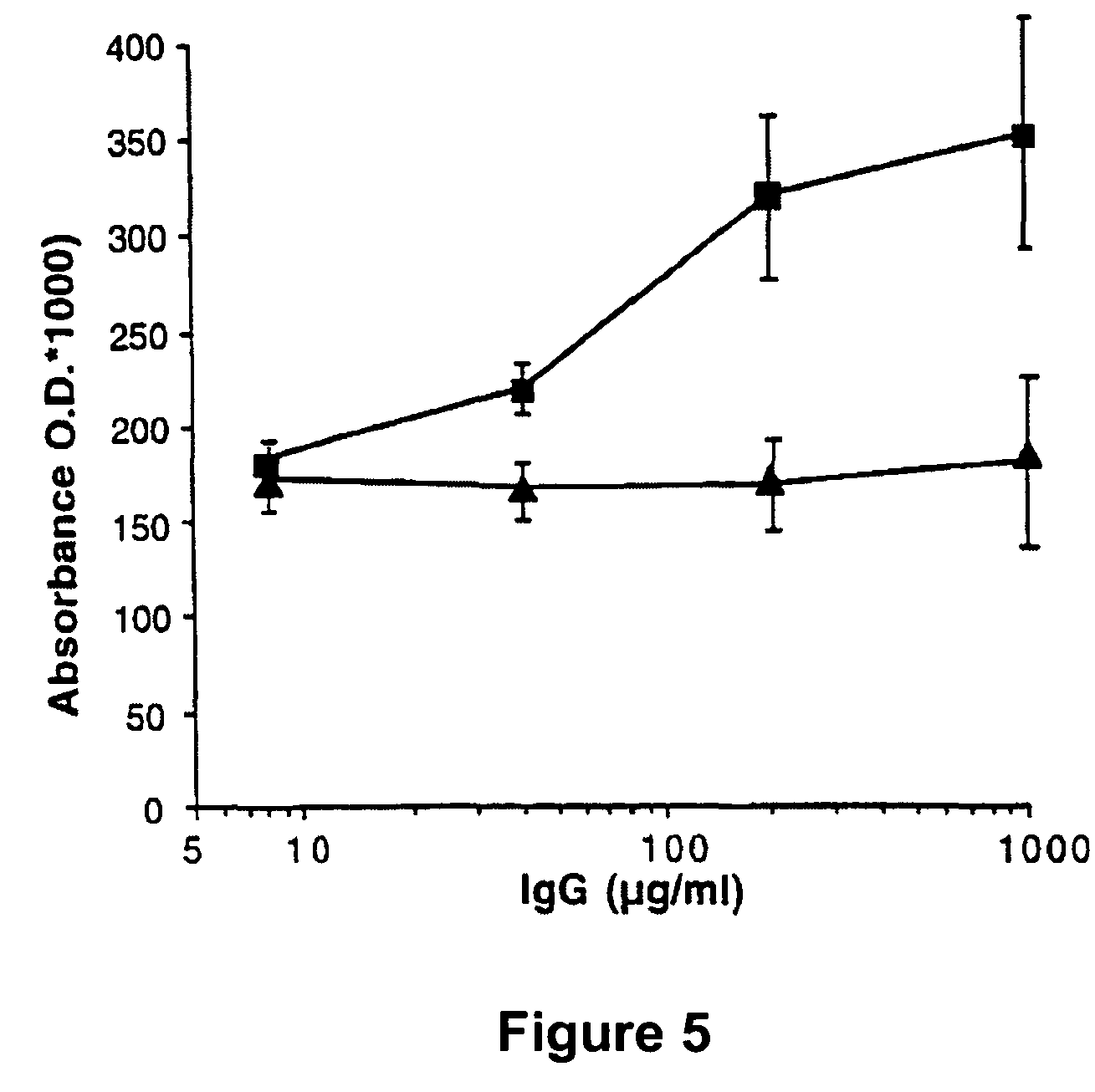
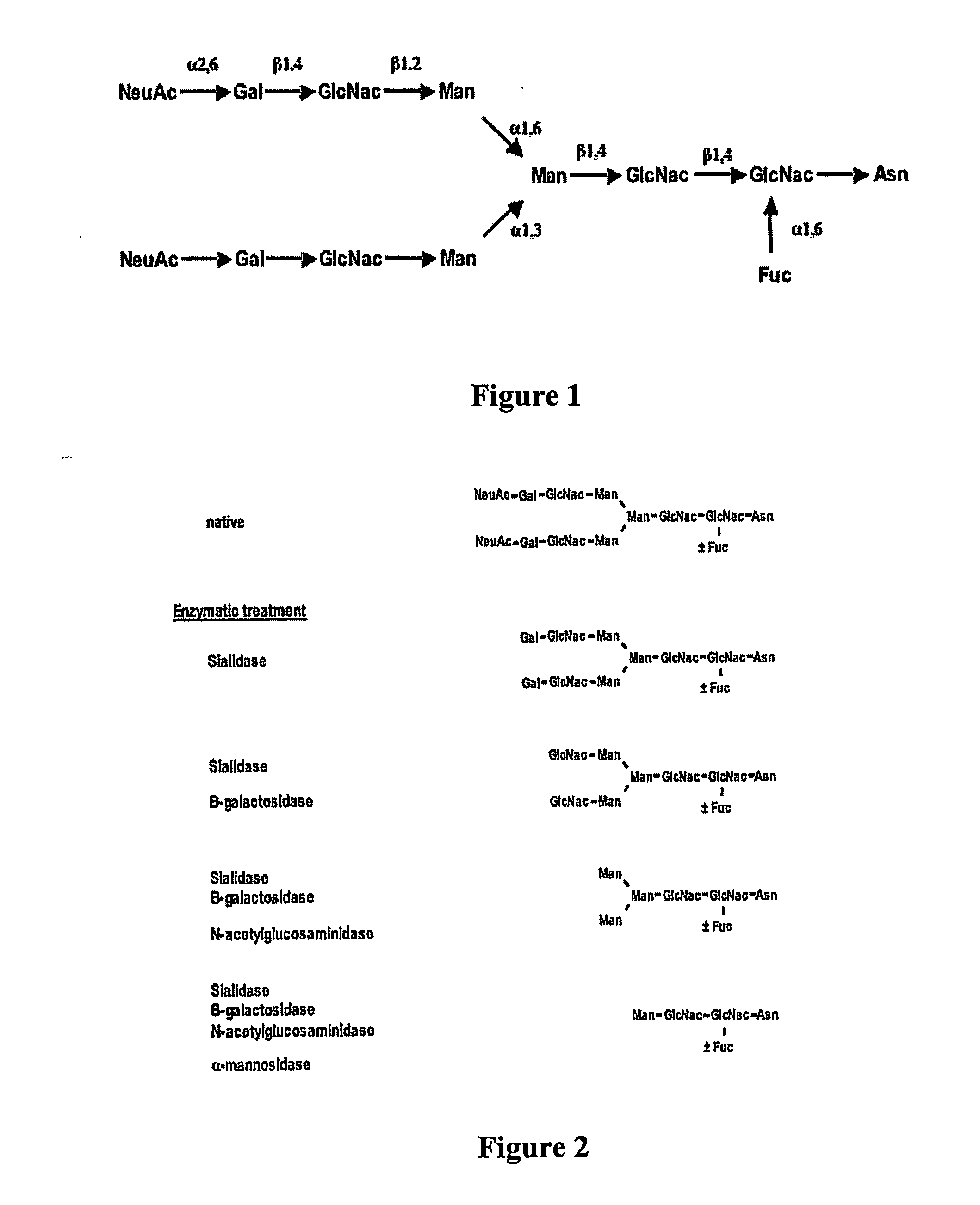
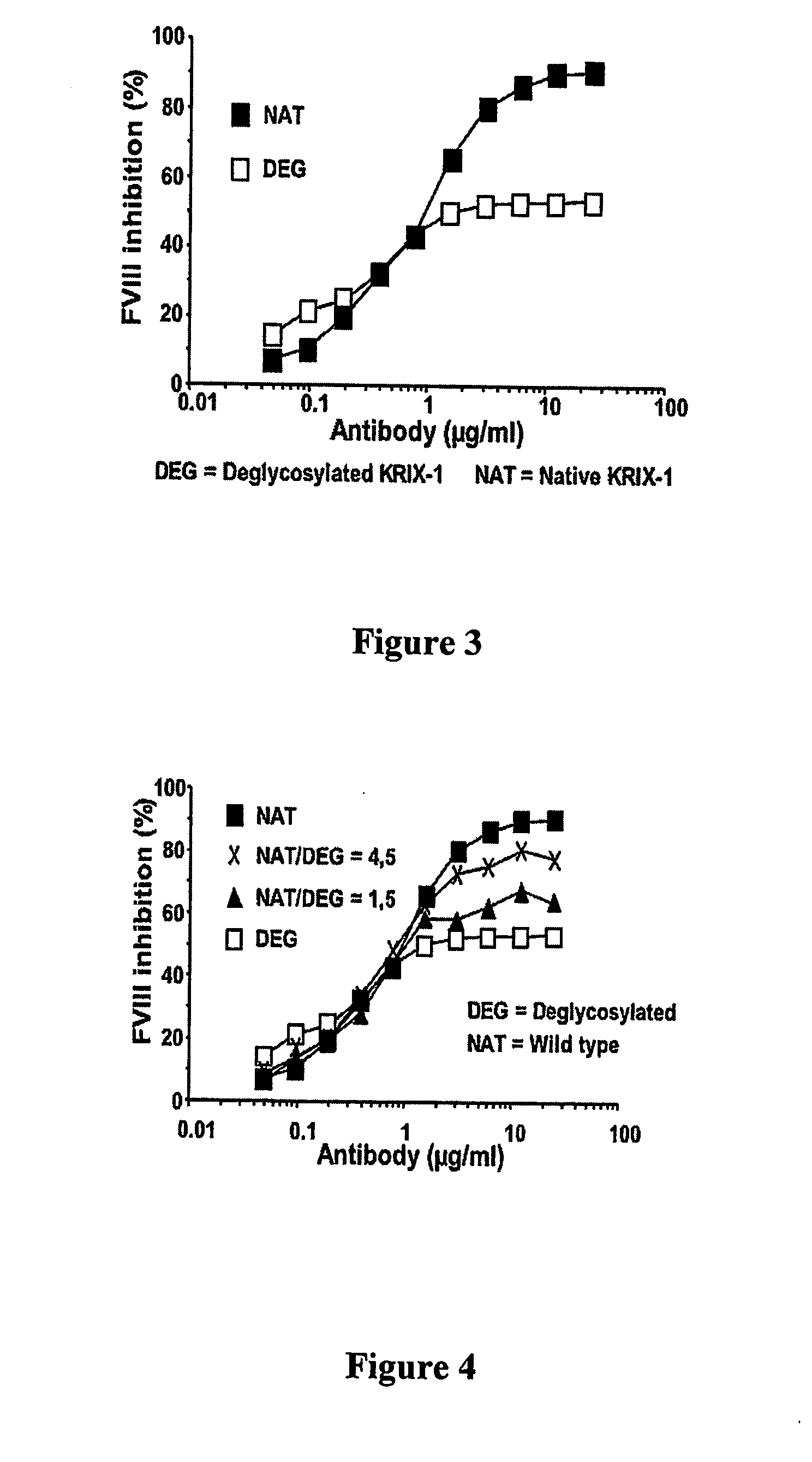
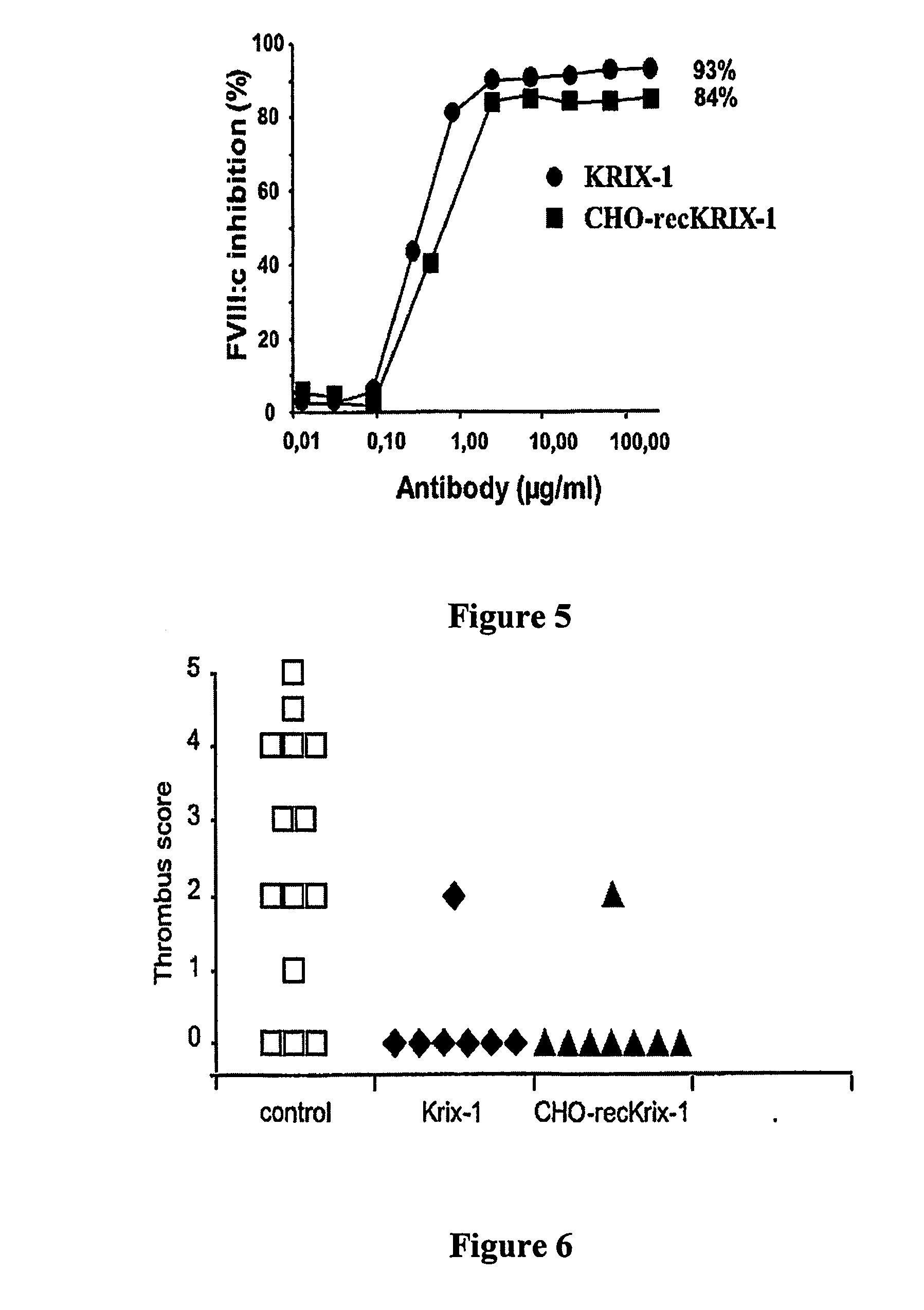
Factor VIII inhibitory antibodies with reduced glycosylation
InactiveUS7785594B2Reduce bleeding riskEffective anti-thromboticImmunoglobulins against blood coagulation factorsAntibacterial agentsFactor iiSite-directed mutagenesis
The present invention discloses inhibitory antibodies against Factor VIII with modified glycosylation, either by enzymatic deglycosylation or by site directed mutagenesis. Said antibodies with modified glycosylation have equal affinity for FVIII but show different inhibiting properties. The use of one or a mixture of said antibodies allow modulation of the inhibition of factor VII to levels between 40 and 95%. The present invention further disclosed pharmaceutical compositions comprising inhibitory antibodies against Factor VIII with modified glycosylation, combinations of these antibodies and methods for treating haemostasis disorders using said antibodies and antibody mixtures.
Owner:LIFE SCI RES PARTNERS VZW
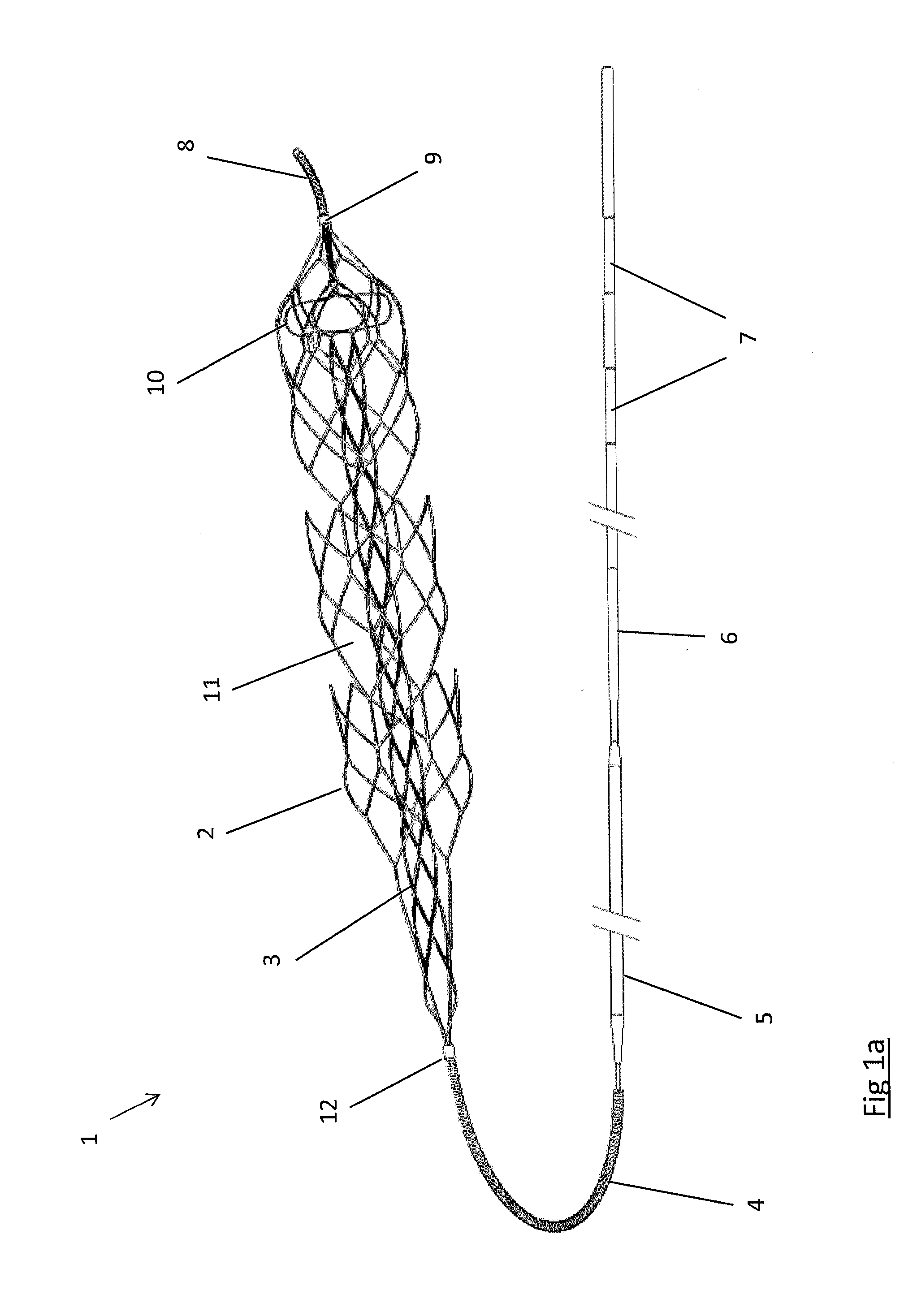
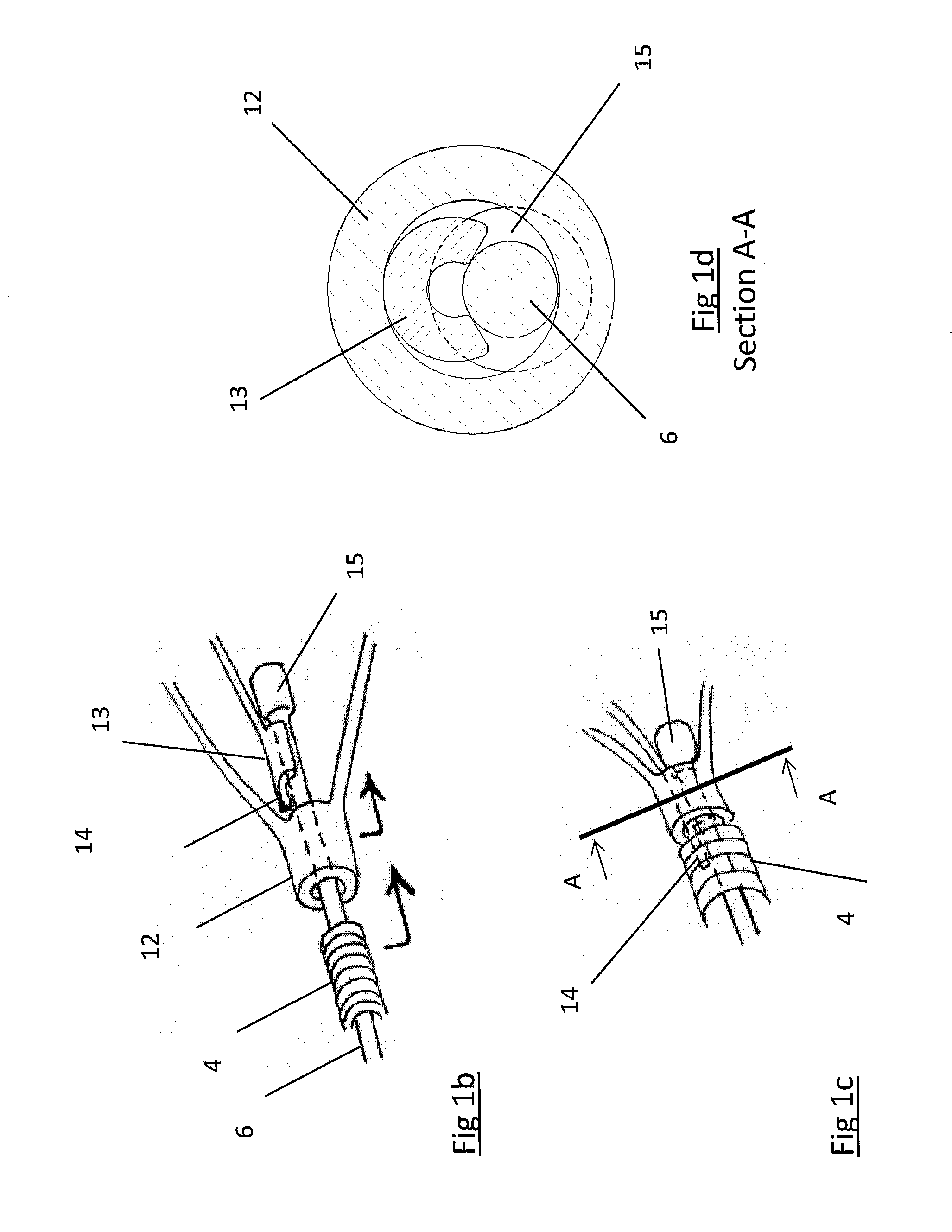
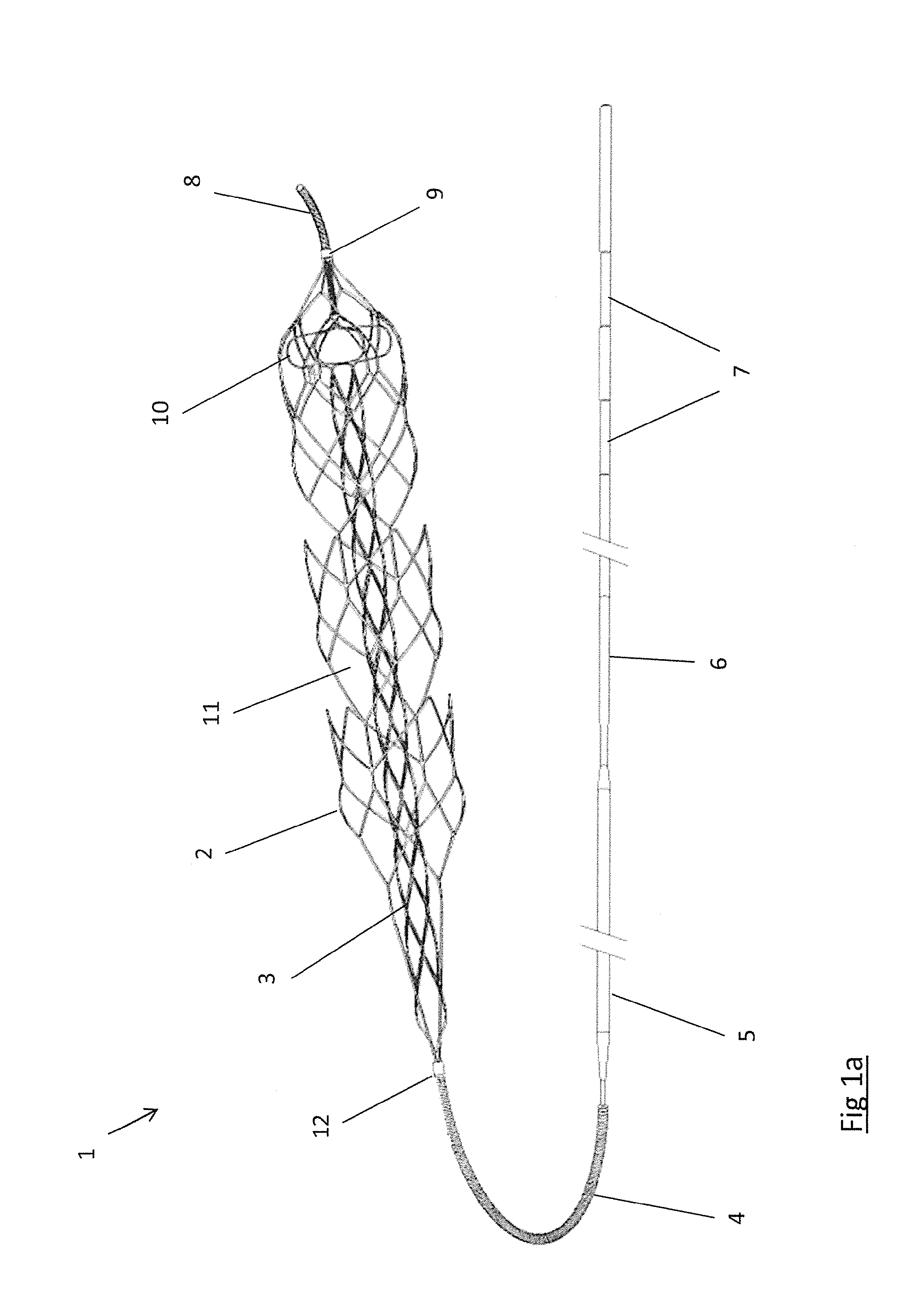
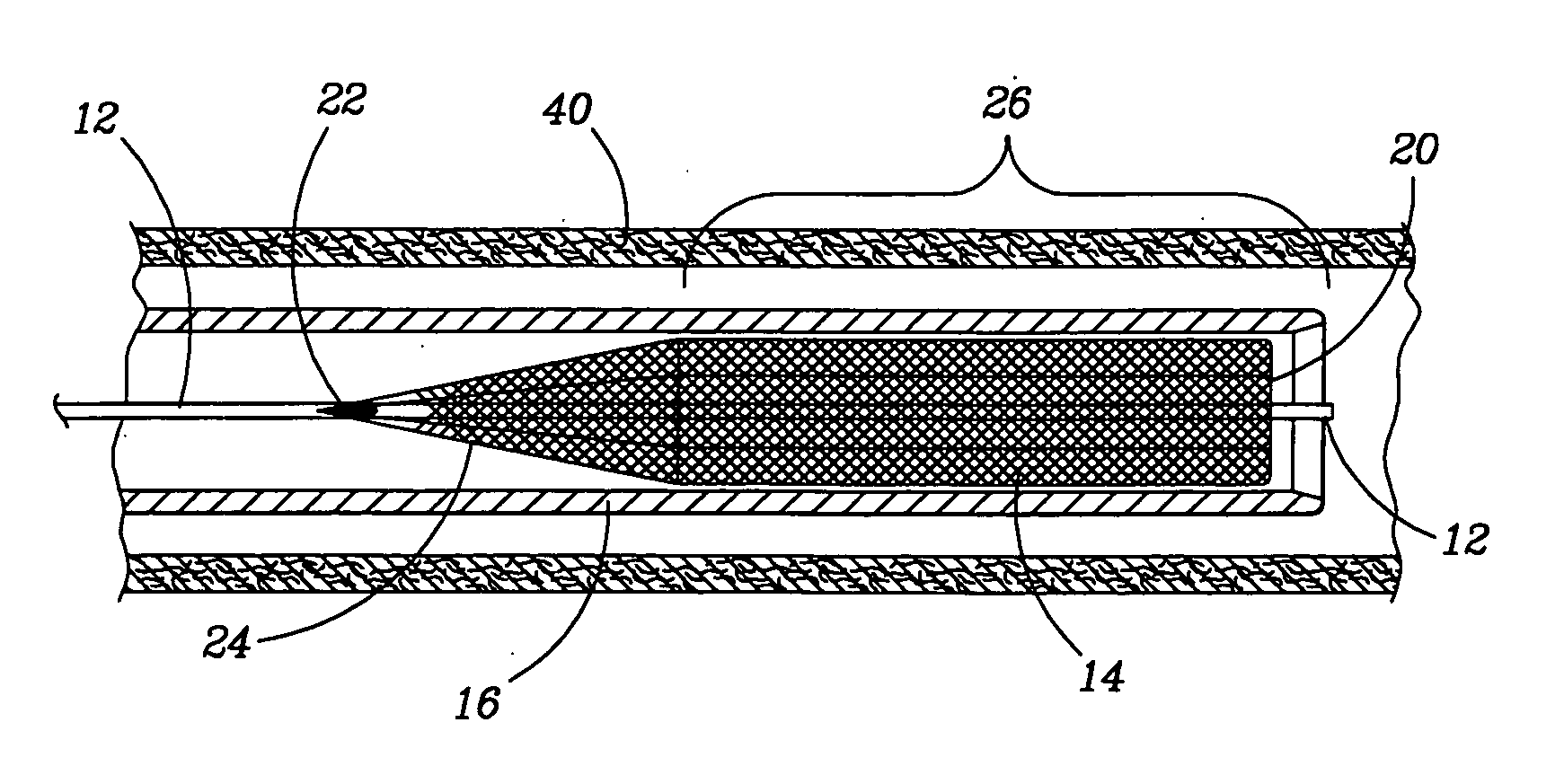
Retractable Flow Maintaining Stent
A self-expanding stent is delivered to an affected site within the human body with a guide wire and hand-manipulable control apparatus. The stent is capable of full expansion along a pre-determined length of its body with the remainder of the overall length conically tapering to a fixed connection point with the guide wire with a flexible joint between the sections. A covering sheath delivers the stent to the affected area with a tip capable of penetrating a blockage or obstruction in a vessel such that the stent can be exposed to begin its expansion. Once the obstruction is opened, the stent can be recaptured or retrieved by pulling it back into the sheath, collapsing the expanded stent with the assistance of the conical portion and flexible joint, and withdrawing the stent and wire completely from the vessel. A rotating motion will cause the conical and cylindrical sections of the stent to separate at the flexible joint.
Owner:KLUCK BRYAN W
Methods of treating hemostasis disorders using antibodies binding the C1 domain of factor VIII
InactiveUS7829085B2Reduce bleeding riskTreatment safetyImmunoglobulins against blood coagulation factorsAntibody ingredientsFactor iiThrombus
The invention, in general, features a method of treatment and / or prevention of a thrombotic pathological condition, in a mammal, which includes administering to the mammal in need of such treatment a therapeutically effective amount of a composition including an antibody directed against the C1 domain of Factor VIII, which is a partially inhibitory antibody of Factor VIII.
Owner:LIFE SCI RES PARTNERS VZW
Retractable flow maintaining stent wire
InactiveUS20120101560A1Reduce bleeding riskFurther treatmentStentsGuide wiresDamages tissueAffected site
The present invention is a self-expanding stent delivered to the affected site within the human body with a guide wire and hand-manipulable control apparatus. The stent is capable of full expansion only along a pre-determined length of its body with the remainder of the overall length tapering (in substantially conical form) to a fixed connection point with the guide wire. The stent is delivered to the affected area in a covering sheath that has a tip that is capable of penetrating a blockage or obstruction in a vessel such that the stent can be exposed to begin its expansion. Once the obstruction has been opened, the stent can be recaptured or retrieved by pulling it back into the sheath, collapsing the expanded stent, and withdrawing the stent and wire completely from the vessel. The present invention may be coated with a variety of therapeutic agents to aid in the treatment of the damaged tissue.
Owner:KLUCK BRYAN W
Methods and compositions for activated protein c with reduced Anti-coagulant properties
InactiveUS20080058265A1Promote activationReduce bleeding riskNervous disorderPeptide/protein ingredientsProtein activationWild type
This invention relates to a novel form of protein C or activated protein C. More specifically, the invention is directed to a variant of protein C that is activated at a higher rate than wild-type or other variants and produces an activated protein C with reduced anticoagulant properties while retaining the protective anti-inflammatory and anti-apoptotic properties of wild-type activated protein C. This novel APC variant will be beneficial for treating inflammatory and apoptotic disorders with a reduced risk for bleeding.
Owner:SAINT LOUIS UNIVERSITY
Anticoagulant and antithrombotic LMW-glycosaminoglycans derived from K5 polysaccharide and process for their preparation
InactiveUS20050027117A1Improved and selective antithrombin activityReduce bleeding riskDepolymerizationSulfate
Low molecular weight glycosaminoglycans derived from K5 polysaccharide having an activity of the same order of magnitude as that of low molecular weight heparin on the coagulation parameters and a lower hemorrhagic risk are obtained starting from an optionally purified K5 polysaccharide by a process comprising the sequential steps of N-deacetylation / N-sulfation, C5-epimerization, depolymerization of the obtained epiK5-N-sulfate, O-oversulfation of the LMW-epiK5-N-sulfate, selective O-desulfation, 6-O-sulfation, N-sulfation.
Owner:MARIETTI GILSON E TRUPIANO
Variable antibodies
InactiveUS20060292149A1Reduce bleeding riskEffective anti-thromboticAntibacterial agentsImmunoglobulins against blood coagulation factorsSite-directed mutagenesisChemistry
The present invention discloses inhibitory antibodies against Factor VIII with modified glycosylation, either by enzymatic deglycosylation or by site directed mutagenesis. Said antibodies with modified glycosylation have equal affinity for FVIII but show different inhibiting properties. The use of one or a mixture of said antibodies allow modulation of the inhibition of factor VII to levels between 40 and 95%. The present invention further disclosed pharmaceutical compositions comprising inhibitory antibodies against Factor VIII with modified glycosylation, combinations of these antibodies and methods for treating haemostasis disorders using said antibodies and antibody mixtures.
Owner:LIFE SCI RES PARTNERS VZW
Method and medicament for sulfated polysaccharide treatment of heparin-induced thrombocytopenia (HIT) syndrome
ActiveUS20070123489A1Reduced activityReduced anticoagulant activityOrganic active ingredientsBiocideHeparin antibodySulfated polysaccharides
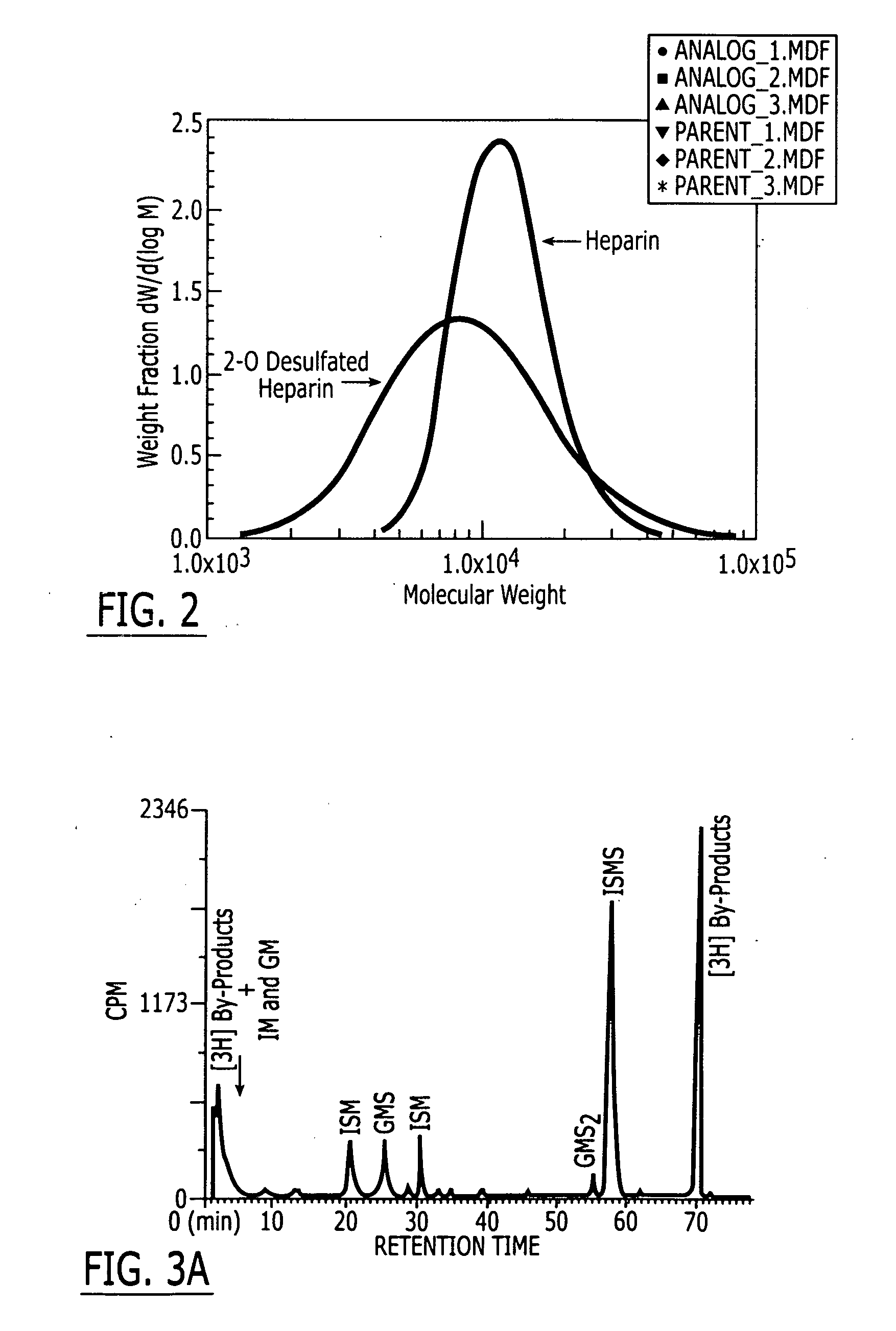
A method and medicament for treating and preventing platelet activation or thrombosis in the presence of heparin- and platelet factor 4-complex reactive antibodies using a 2-O desulfated heparin with an average degree of sulfation of 0.6 sulfate groups per monosaccharide or greater and an average molecular weight or 2.4 kD or greater. The medicament preferably is administered intravenously, by aerosolization or orally. Preferably, the 2-O desulfated heparin medicament includes a physiologically acceptable carrier which may be selected from the group consisting of physiologically buffered saline, normal saline, and distilled water. Additionally provided is a method of synthesizing 2-O desulfated heparin.
Owner:CANTEX PHARMA
Marine fair-faced concrete containing limestone powder and preparation method of concrete
The invention discloses marine fair-faced concrete containing limestone powder. The marine fair-faced concrete is prepared from components as follows: a concrete additive, a mixed cementing material, aggregate and water, wherein the mixed cementing material is prepared from cement and a concrete admixture; the concrete admixture is prepared from the limestone powder and granulated blast-furnace slag powder, the added limestone powder accounts for 5%-20% of the total mass of the concrete, and the added granulated blast-furnace slag powder accounts for 15%-40% of the total mass of the concrete; the consumption of the cementing material is 320-450 kg / m<3>, and the ratio of water to the cementing material is 0.30-0.42. According to the fair-faced concrete, fly ash is replaced with the limestone powder, the problem of raw material shortage due to adoption of fly ash is solved, defects of the fair-faced concrete adopting the fly ash are also avoided, the homogeneity and the workability of the concrete are improved simultaneously, the concrete bleeding risk is reduced, the requirement for durability is met, the appearance quality of the concrete structure is stable, and the requirement for the appearance quality of the fair-faced concrete is met.
Owner:CCCC WUHAN HARBOR ENG DESIGN & RES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com