Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
228results about How to "High chiral purity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparing method of phosphodiesterase 5 inhibitor tadalafil
ActiveCN103980275AGood removal effectHigh chiral purityOrganic chemistryPhosphodiesterase 5 inhibitorTadalafil
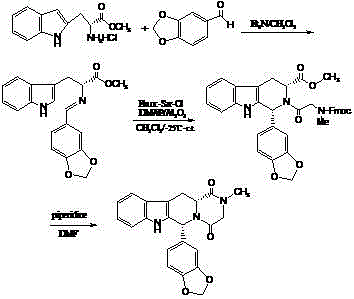
The invention relates to a preparing method of a phosphodiesterase 5 inhibitor tadalafil. D-methyl tryptophanate hydrochloride is adopted as an initial raw material, and is subjected to cyclization with heliotropin, N-acylation, aminolysis-cyclization, and other reactions to obtain a tadalafil crude product. The tadalafil crude product is recrystallized to obtain a tadalafil finished product. The method has characteristics of mild reaction conditions, short reaction time, high yield, good product stability and convenience for industrial production.
Owner:湖北省医药工业研究院有限公司
Method for preparing brivaracetam
ActiveCN106588741AHigh chiral puritySimple processing capacityOrganic chemistryPurification methodsOrganic solvent
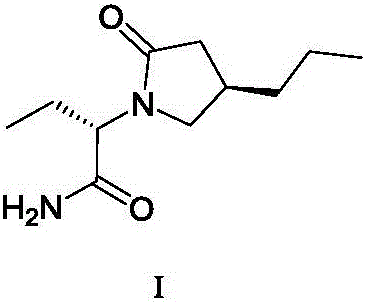
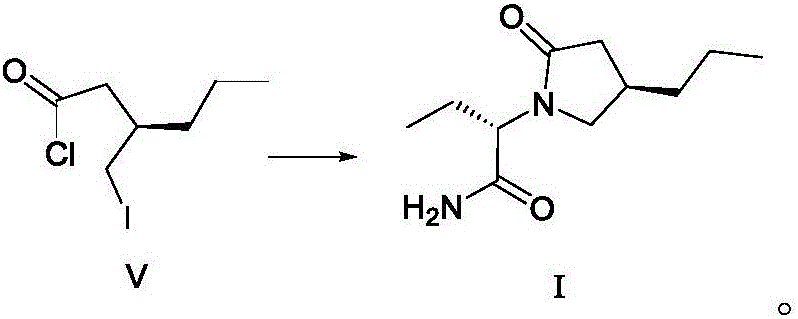
The invention discloses a method for preparing brivaracetam. The method for preparing brivaracetam comprises the following step that in an organic solvent, under the condition of anhydrous and inert gas shielding, a compound V and L-2-aminobutanamide are subjected to a condensation reaction to obtain brivaracetam I. According to the method for preparing brivaracetam, brivaracetam is prepared through only four steps of reacting, the reaction steps are short, the total yield is high, aftertreatment steps and purifying methods are simple, a product with the de value larger than 99.80% can be prepared only through recrystallization, the grade API is reached, the production cost is low, and the method is suitable for industrial production. The formula is shown in the description.
Owner:SHANGHAI BOCIMED PHARMA CO LTD
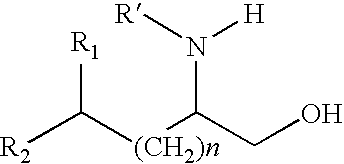
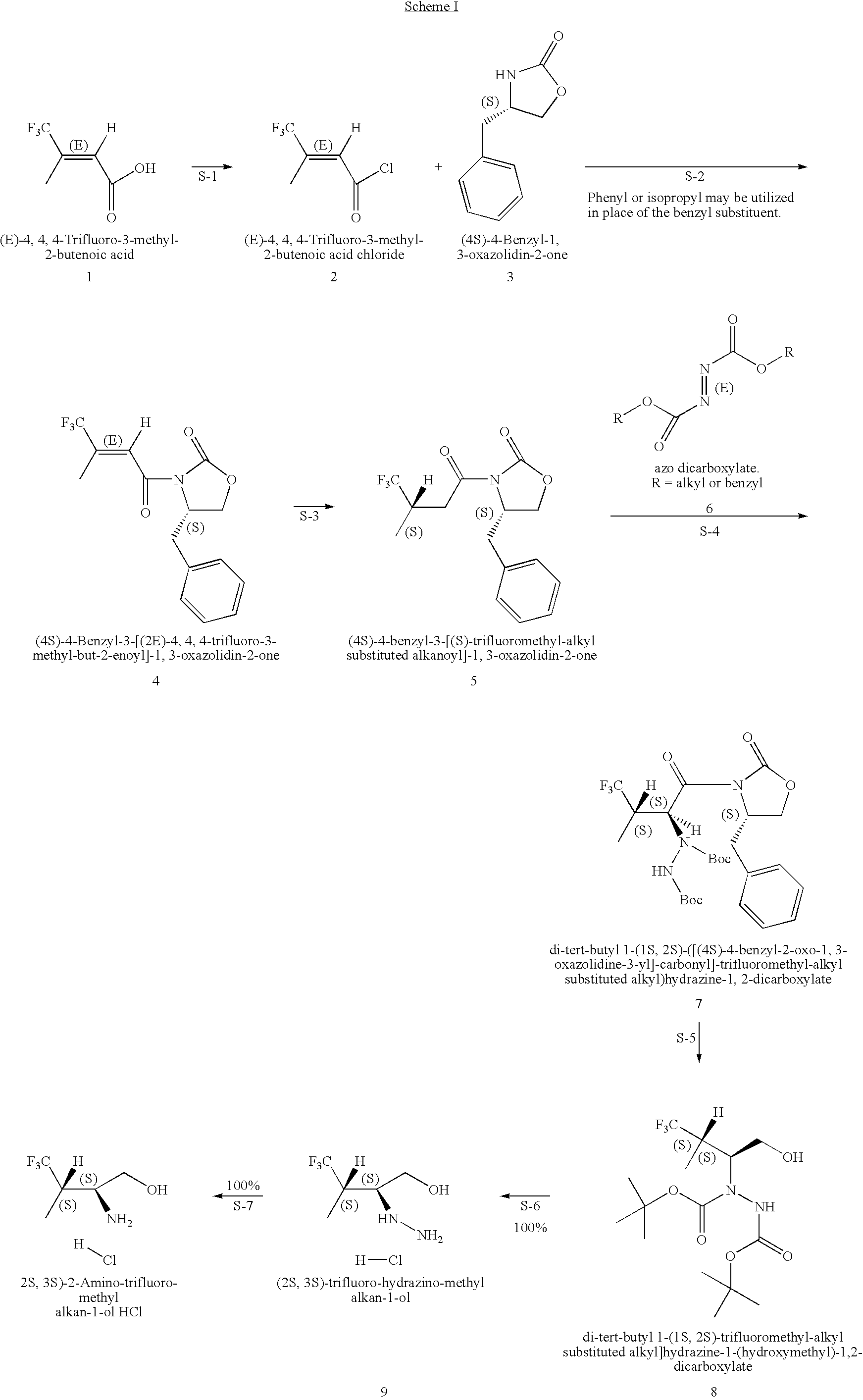
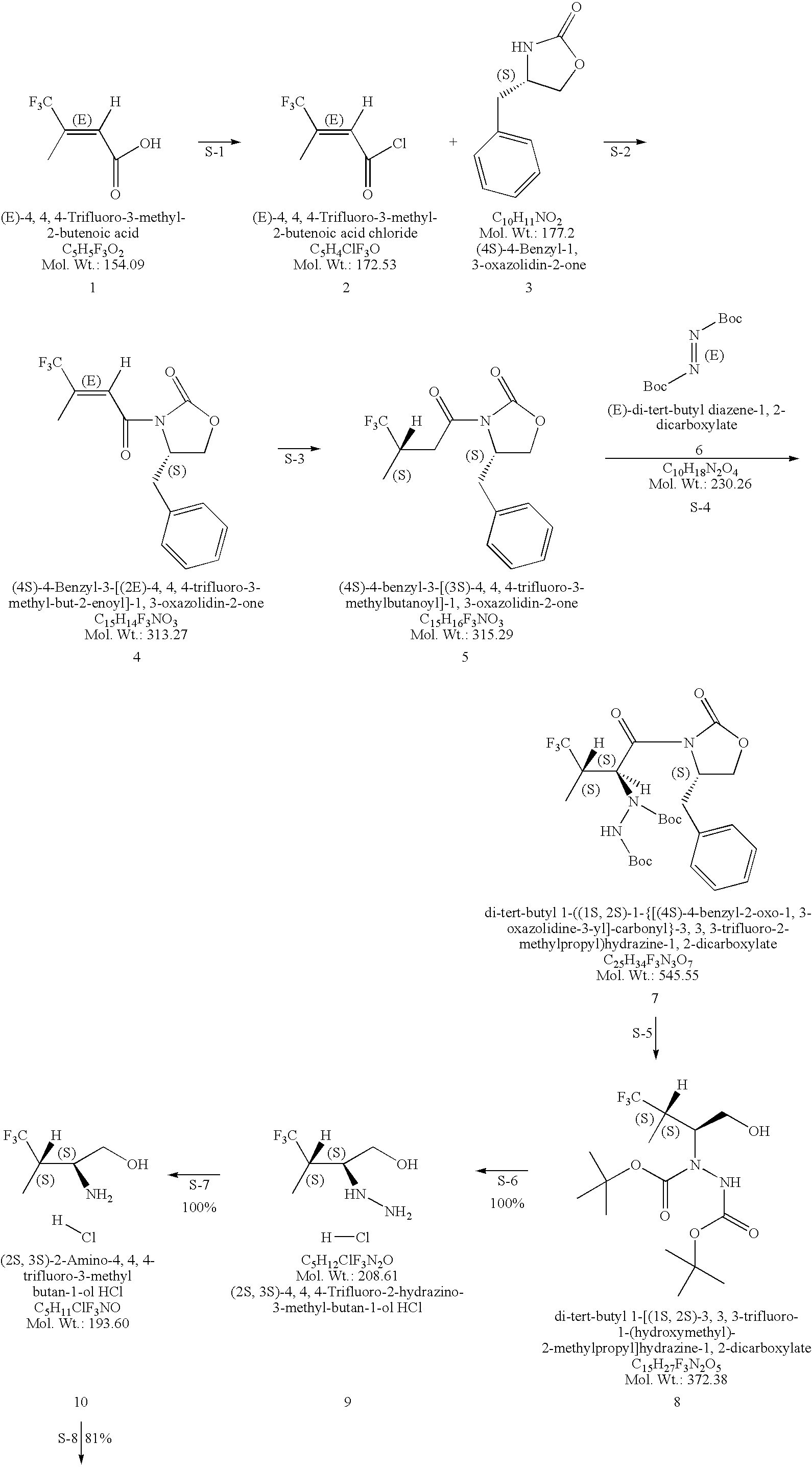
Production of chirally pure amino alcohol intermediates, derivatives thereof, and uses thereof
InactiveUS20070249869A1High chiral purityHigh chemical purityOrganic compound preparationOrganic chemistry methodsArylAlcohol
A method of selectively preparing a chiral 2S-amino alcohol useful in preparation of an amide sulfonated or acylated with alkyl, substituted aryl or substituted heteroaryl is described. The method involves reacting a di-tert-butyl diazene-1,2-dicarboxylate with a (4S)-4-benzyl-3-[(S)-trifluoromethyl-alkyl substituted alkanoyl]-1,3-oxazolidin-2-one to afford a di-tert-butyl 1-(1S,2S)-([(4S)-4-benzyl-2-oxo-1,3-oxazolidine-3-yl]-carbonyl}-trifluoromethyl-alkyl substituted alkyl)hydrazine-1,2-dicarboxylate. This dicarboxylate is then reduced to yield di-tert-butyl 1-(1S,2S)-[trifluoromethyl-alkyl substituted alkyl]hydrazine-1-(hydroxymethyl)-1,2-dicarboxylate. The resulting product is deblocked with an acid to yield the acid addition salt of (2S,3S)-trifluoro-hydrazino-methyl alkan-1-ol. The acid addition salt of (2S,3S)-trifluor-2-hydrazino-methyl alkan-1-ol is hydrogenated in the presence of a suitable metal catalyst to yield the amino alcohol (2S,3S)-2-amino-trifluoro-methyl alkan-1-ol HCl.
Owner:WYETH LLC
Method for producing L-2-aminobutyric acid by virtue of biological catalysis
ActiveCN104774881AImprove protectionHigh yieldFermentationL-2-Aminobutyric AcidL-alpha-Aminobutyric Acid
The invention discloses a new method for synthesising L-alpha-aminobutyric acid with a high optical activity. The new method comprehensively applies alcohol dehydrogenase, threonine aldolase, threonine deaminase and L-amino acid dehydrogenase, and ethanol and glycine raw material are directly synthesised into L-alpha-aminobutyric acid by a 'one-pot' method. According to the method disclosed by the invention, L-alpha-aminobutyric acid is synthesised in one pot by virtue of the simple and easily available raw materials; the method has the advantages of being few in operation steps, environment-friendly, good in bio-safety and simple in equipment, and is beneficial to industrial large-scale production for L-alpha-aminobutyric acid with a high optical activity and L-alpha-aminobutyric acid series products.
Owner:HUNAN FLAG BIOTECHNOLOGY CO LTD
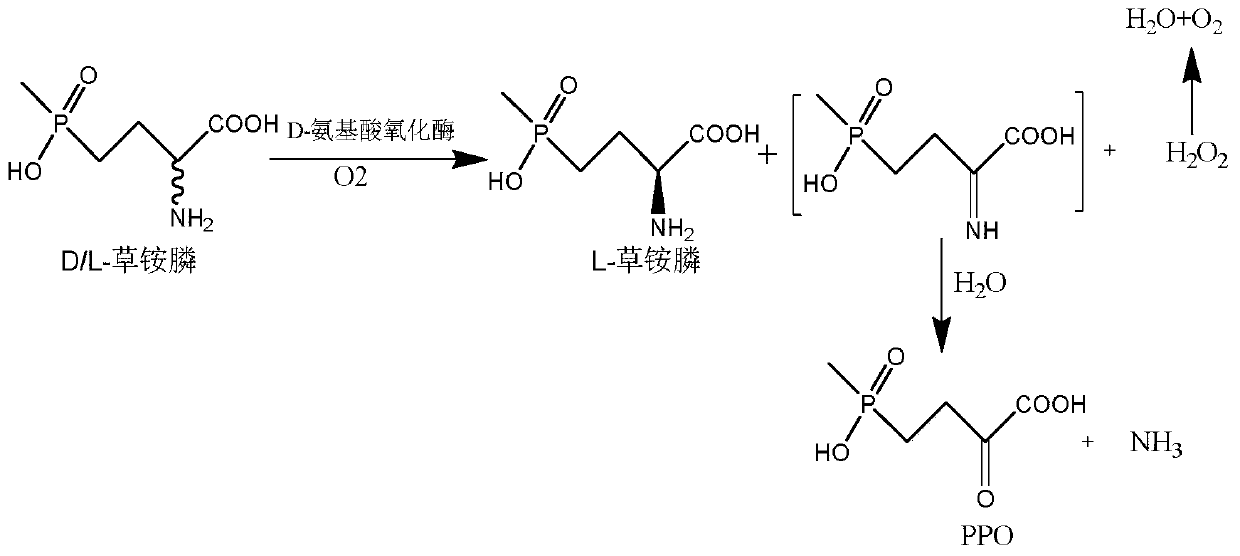
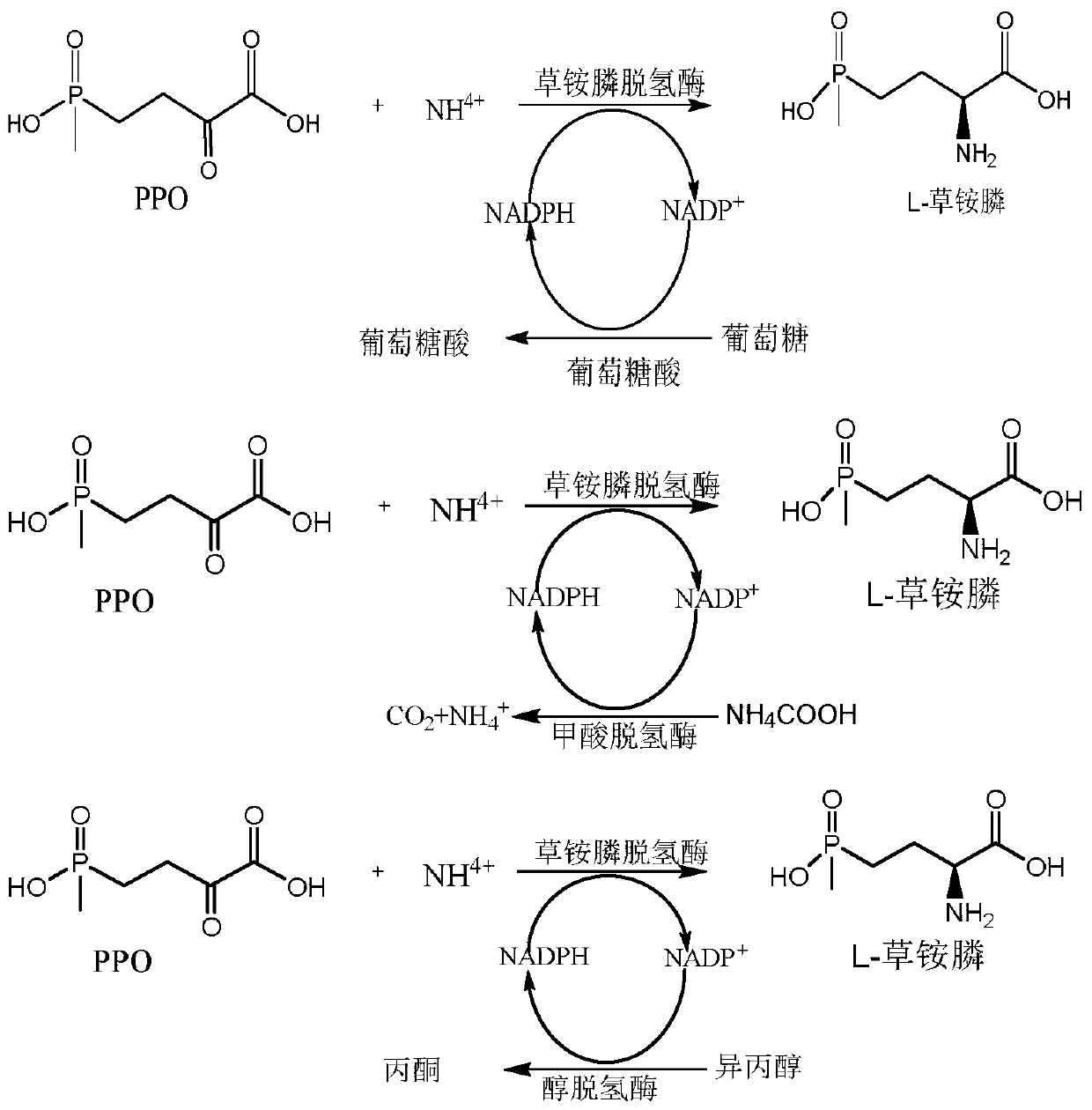
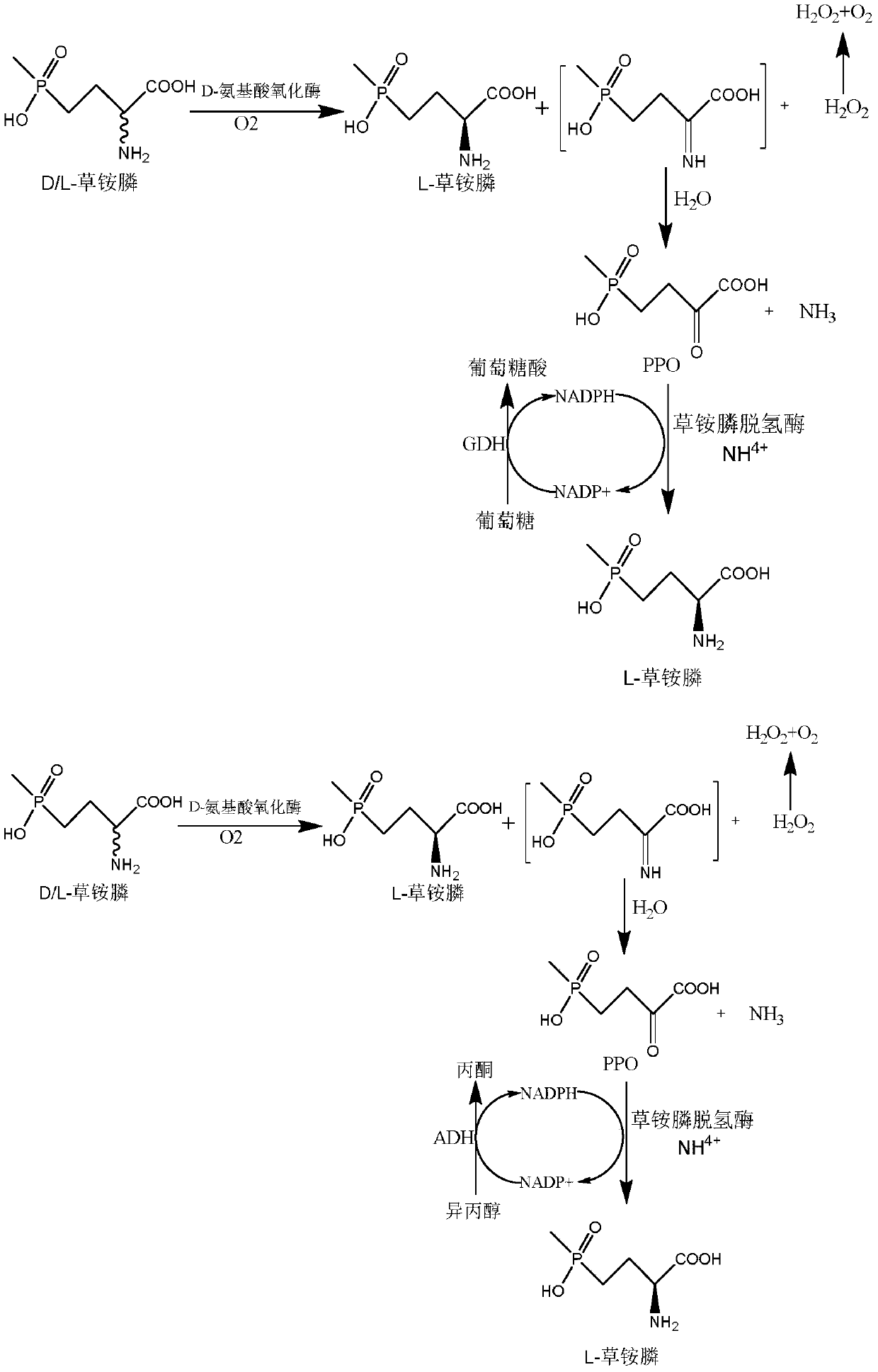
Glufosinate-ammonium dehydrogenase mutant and application thereof to production of L-glufosinate-ammonium through oxidation-reduction of multienzymes
InactiveCN110592036AHigh catalytic activityHigh substrate concentrationBacteriaMicroorganism based processesMutantSubstrate concentration
The invention discloses a glufosinate-ammonium dehydrogenase mutant and an application thereof to production of L-glufosinate-ammonium through oxidation-reduction of multienzymes. According to the method, a method for preparing an L-glufosinate-ammonium precursor through D-amino acid oxidase is utilized, D, L-glufosinate-ammonium or D-glufosinate-ammonium is used as substrate, and in aerobic environment or under the situation that catalase exists, through excised D-amino acid oxidase or in vitro expression of cells of D-amino acid oxidase, the D-glufosinate-ammonium is catalyzed to obtain 4-(hydroxymethylphosphinyl)-2-oxobutanoic; and under the action of glufosinate-ammonium dehydrogenase, the 4-(hydroxymethylphosphinyl)-2-oxobutanoic is catalyzed to generate the L-glufosinate-ammonium, the activity of a catalyst is improved by about 10 times, and the concentration of the substrate is increased by 5 times. The method is high in raw material conversion rate and high in yield, and products are easy to separate and purify.
Owner:ZHEJIANG UNIV OF TECH
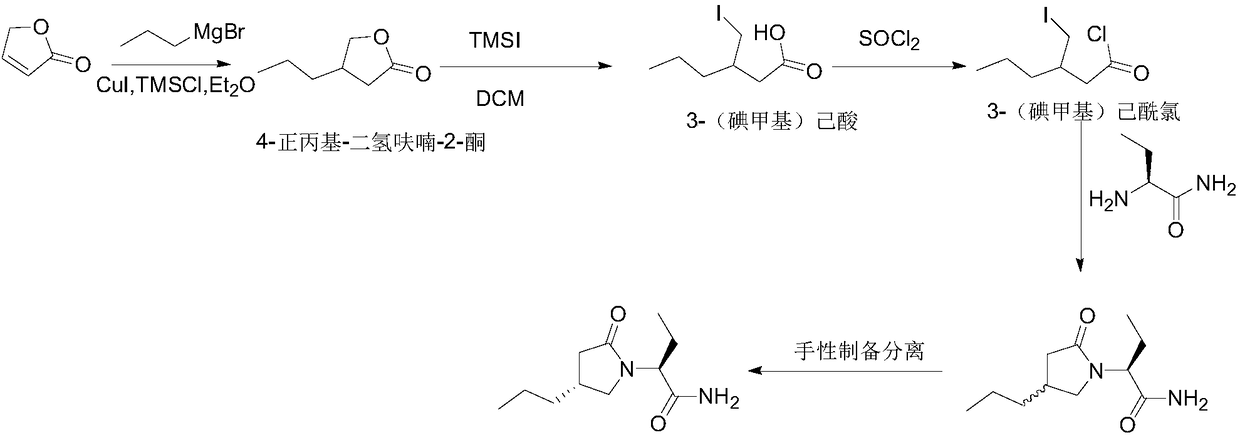
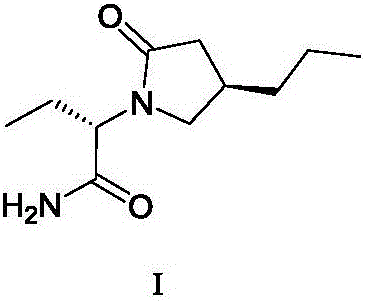
New preparation method of brivaracetam
ActiveCN108503573AControl amountEffect of racemizationOrganic chemistry methodsChemical synthesisBrivaracetam
The invention relates to a new preparation method of brivaracetam, and belongs to the field of chemical synthesis. According to the present invention, optically pure (R)-4-n-propyl-dihydrofuran-2(3H)-one is used as a raw material, and ring opening, halogenation, condensation, ring closure and other steps are performed to obtain the high-purity brivaracetam; and the preparation method has advantages of easily available raw materials, low cost, high total yield, high optical purity of the obtained product, simple reaction conditions and simple operation process.
Owner:BEIJING ABLEPHARMTECH CO LTD
Preparation method of R-3-aminobutyric acid
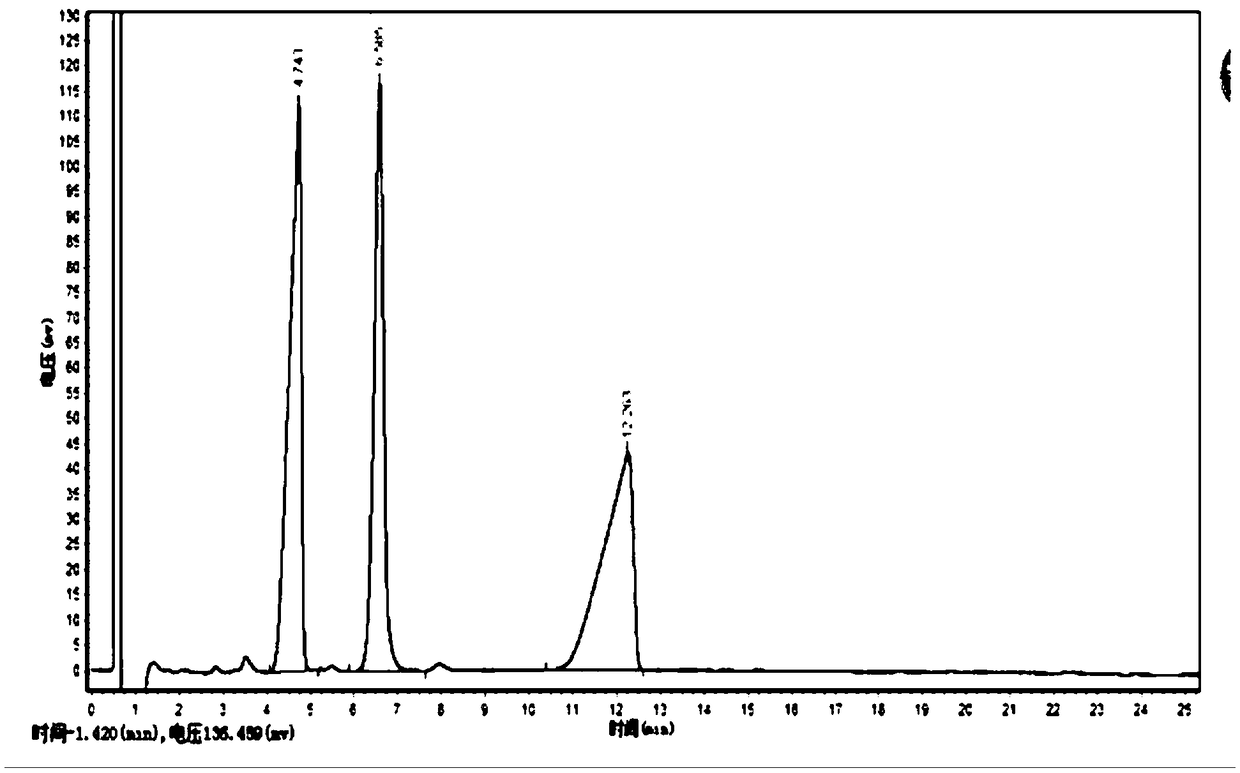
The invention discloses a preparation method of R-3-aminobutyric acid. The method comprises the following steps: taking crotonic acid and ammonium salt as substrates; adding salt containing magnesiumions and regulating the pH (Potential of Hydrogen) by utilizing ammonia water; then adding recombinant aspartase as a biological enzyme catalyst; reacting at proper temperature and under an alkaline condition; after reacting, separating, purifying and crystallizing to obtain the R-3-aminobutyric acid. According to the method disclosed by the invention, ammonium ions of a reaction system are controlled, and reverse reaction is controlled and generation of byproducts is reduced; impurities including bacterial sludge, zymoprotein, sulfate ions, pigments and the like are intercepted by utilizing microfiltration and nanofiltration; high-quality products with high chiral purity and high impurity purity are obtained through continuous concentration and crystallization.
Owner:CHANGXING PHARMA
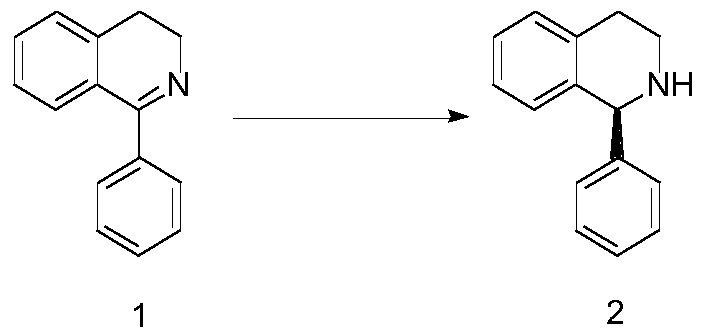
(S)1-phenyl-1,2,3,4-tetrahydroisoquinoline synthesis method
ActiveCN110683986AMild reaction conditionsProcess stabilityOrganic chemistry methodsPhenyl groupQuinoline
The invention relates to a (S)1-phenyl-1,2,3,4-tetrahydroisoquinoline synthesis method, wherein the reaction route is defined in the specification. The synthesis method comprises: 1) dissolving a rawmaterial 1 in a solvent, and adding an alkali and a catalyst; and (2) carrying out gas replacement by using hydrogen to form a hydrogen atmosphere, and carrying out an pressurization reaction to obtain (S)1-phenyl-1,2,3,4-tetrahydroisoquinoline 2, wherein the catalyst is a BIAMH system catalyst, a D-BIMAH system catalyst or a P-BIMAH system catalyst.
Owner:ENANTIOTECH CORP +1
High performance liquid chromatography detection analysis method for controlling ticagrelor isomer
The invention discloses a high performance liquid chromatography detection analysis method for controlling a ticagrelor isomer, which employing a cellulose filler a chiral chromatographic column and takes lower paraffin hydrocarbon-lower alcohol as mobile phases, and the method can rapidly separate isomer impurity of ticagrelor.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Intermediate for preparing bictegravir and preparation method thereof
ActiveCN109020911AHigh stereoselectivityHigh chiral purityCarbamic acid derivatives preparationOrganic compound preparationDouble bondStereochemistry
The invention relates to the technical field of a drug, and concretely relates to an intermediate for preparing bictegravir and a preparation method thereof. The present invention provides two novel types of compounds and three routes for preparation of a compound (VI). Through substrate induction, chiral catalysis or synergistic effect of substrate induction and chiral catalysis, the stereoselectivity of a Diels-Alder reaction can be greatly improved, and a high chiral purity of a common intermediate (III) can be obtained; cut-out of N-O bond and reduction the double bond use catalytic hydrogenation, which can be environmentally friendly; the reaction conditions are mild, the yield is higher than the existing preparation method, the method is economic and effective, and is adapted to large-scale industrial production.
Owner:CHANGZHOU PHARMA FACTORY
Azide and preparation method thereof
ActiveCN103304535AEasy to prepareThe reaction conditions are mild and easy to controlOrganic chemistryAbsolute configurationAzide
The invention discloses an azide and a preparation method thereof. The azide (I) can be used as a key raw material for preparing medicines with a 1,2,3-triazole structure, such as ticagrelor. The preparation method for the azide comprises the following step of: performing azidation reaction on an amino compound (II) and an azidation reagent (III) to obtain the azide (I). The preparation method is moderate in conditions, safe and environmentally-friendly, high in chemical yield, and especially capable of keeping the original absolute configuration and chiral purity.
Owner:徐州飞云泡沫制品有限责任公司
Method for producing optical homochiral amygdalic acid
This invention discloses a method for producing optical pure chiral mandelic acid. Use methyl benzoyl formate or the derivative of phenylglyoxylic acid as the bottom thing, the microorganism's cell of yeasts or mould whitlying etc. as catalyst, alternatively reduces the carbonyl in the bottom thing to the hydroxyl to get the chiral mandelic acid relying mainly on a kind of type (usually as R a type). This invention adopts chemistry-biology combination craft , with low cost, the chiral purity is high, easy to realize production for industrialization, it is easy to popularize and apply.
Owner:SHANDONG UNIV
Method for synthesizing upadacitinib intermediate and the intermediate
The invention discloses a method for synthesizing upadacitinib intermediate. The method includes performing a ring-forming reaction with 2-pentynoate and N-(methoxymethyl)-N-(trimethylsilylmethyl)benzylamine under a catalyst A to prepare a compound (3), by which the compound (7) or the compound (8) can be obtained in various manners. The method especially the first and the third technical schemes,is high in yield and purity and is high in overall yield. The method is simple in post-treatment and is suitable for industrial large-scale production.
Owner:ZHEJIANG NORMAL UNIVERSITY
Method for preparing furanone compounds
ActiveCN106588831AHigh chiral purityShort synthetic routeOrganic compound preparationPreparation from carboxylic acid esters/lactonesInorganic saltsSolvent
The invention discloses a method for preparing furanone compounds, and provides a method for preparing a furanone compound III. The method includes the following steps that in a solvent, in the presence of inorganic salt, a compound II and a reducing agent are subjected to a reduction reaction, and the furanone compound III is obtained; the solvent is a fatty alcohol solvent or a mixed solvent of a fatty alcohol solvent and water. The brivaracetam can be prepared with the furanone compound III only with the three steps, and the synthetic route is short; the ee value of the compound II is larger than 99.0%, racemization does not occur in the reaction process, and the de value of a brivaracetam I crude product is larger than 99.0%; the brivaracetam I crude product is further purified through a crystal instead of a chirality high-pressure-liquid-phase preparing column, and the chirality purity of brivaracetam I can be further increased to be the de value of 99.80% or above; meanwhile, the content of other individual impurities of the brivaracetam I is smaller than 0.1%, and reaches the API level, and the method is suitable for industrial production. The formula is defined in the description.
Owner:上海云晟研新生物科技有限公司
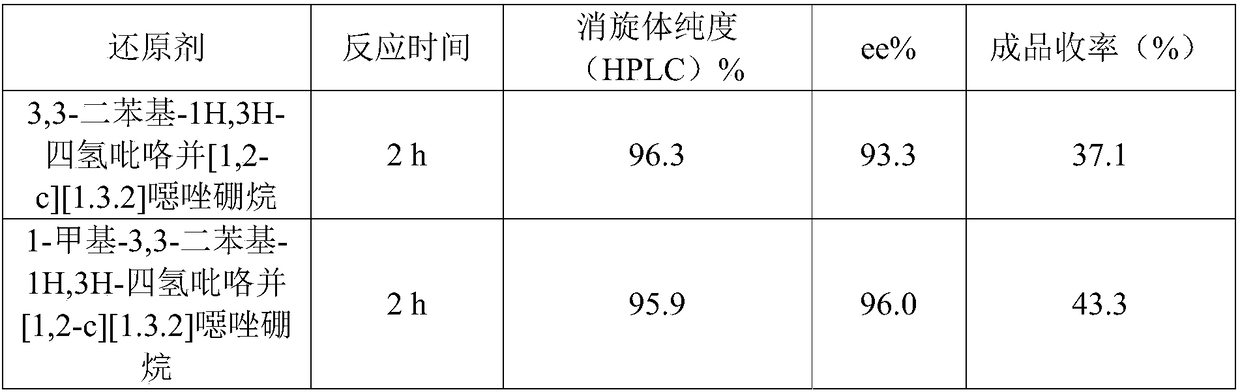
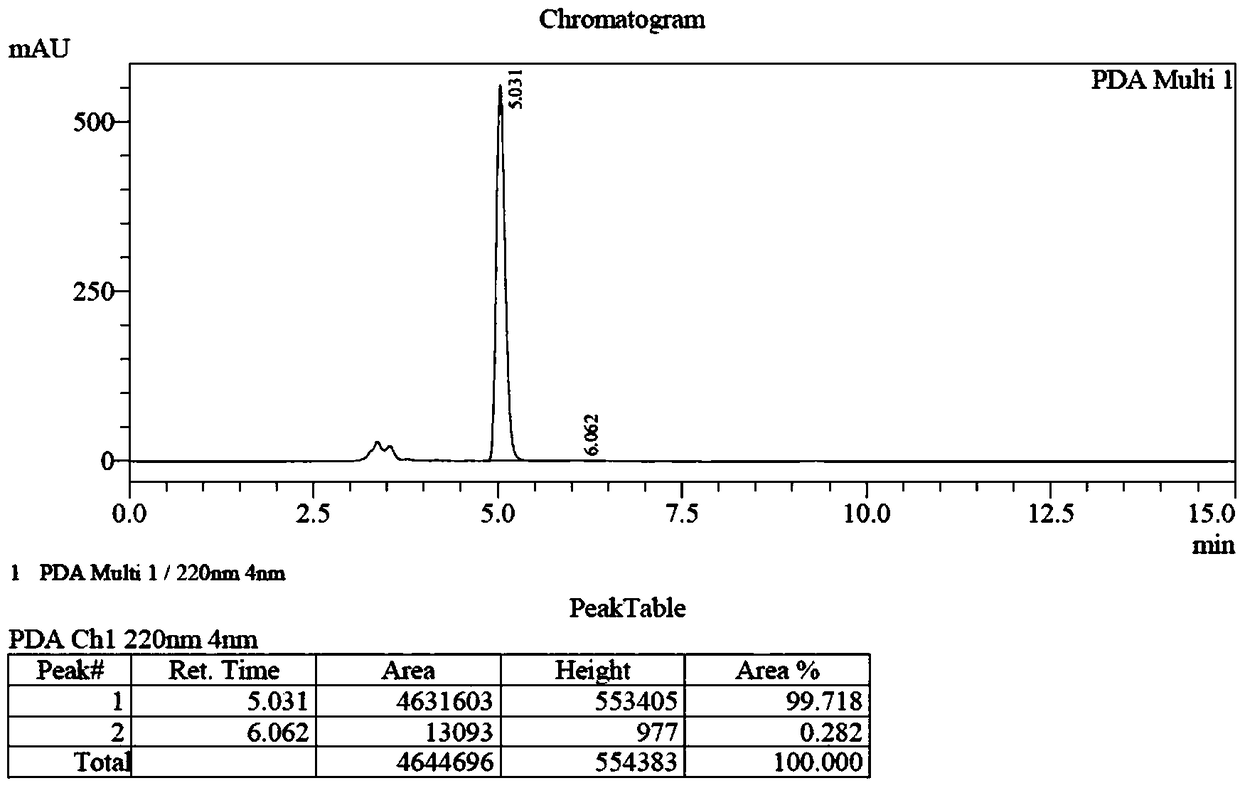
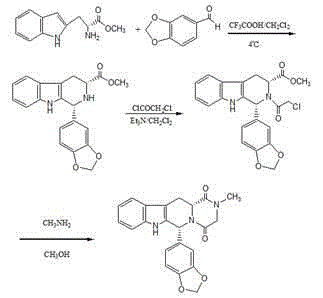
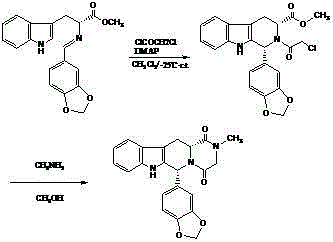
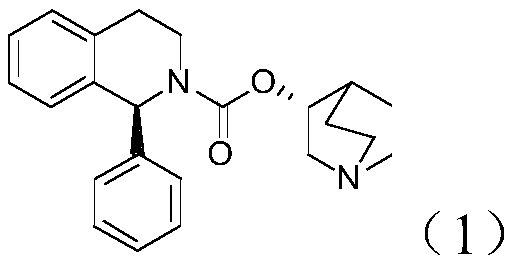
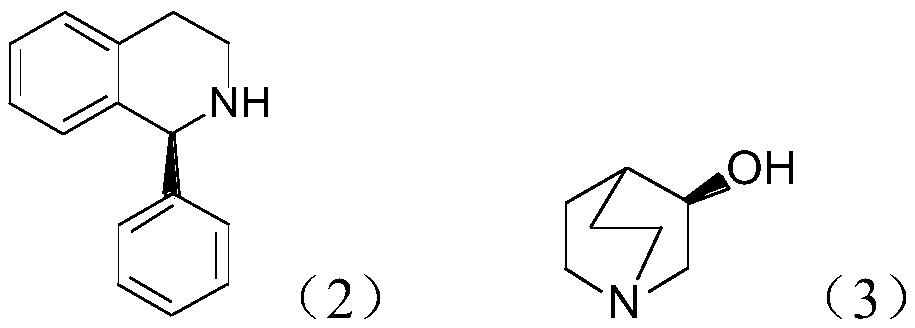
Preparation method of (S, S)-2, 8-diazabicyclo[4, 3, 0]nonane
The invention discloses a preparation method of (S, S)-2, 8-diazabicyclo[4, 3, 0]nonane and belongs to the field of the preparation method of a moxifloxacin intermediate. The preparation method comprises eight processes. A resolving process is carried out in initial of the preparation method, in the third process, an ester is resolved to form a chiral intermediate and then through a simple chemical reaction, the (S, S)-2, 8-diazabicyclo[4, 3, 0]nonane is prepared. The invention provides the preparation method utilizing an effective and economic synthesis route to prepare the high-chiral purity (S, S)-2, 8-diazabicyclo[4, 3, 0]nonane. The preparation method is free of an expensive resolving agent and greatly reduces a process cost. In the whole technology, the intermediate is not purified and the crude product can be directly used. The preparation method has simple processes and a high overall yield and can produce the product with chiral purity of 99%.
Owner:HEADING NANJING PHARMTECH CO LTD
Method for coproducing D-arginine and gamatine through biotransformation
InactiveCN104152478ALow costLow priceMicroorganism based processesFermentationMolecular biologyTransformation systems
The invention discloses a method for coproducing D-arginine and gamatine through a biological method. The method comprises the following steps: synthesizing arginine racemase genes argR according to a designed primer, establishing an expression vector pET24a-argR and transferring the expression vector into an Escherichia coli strain, thus obtaining genetically engineered bacteria of the arginine racemase; establishing an expression vector pET24a-speA through a speA gene segment amplified by PCR, transferring the expression vector pET24a-speA into Escherichia coli, thus obtaining L-arginine decarboxylase genetically engineered bacteria; stirring and reacting a transformation system containing L-arginine and a wet vector for expressing the genetically engineered bacteria of the arginine racemase, thus obtaining an enantiomer of L-arginine and D-arginine; removing the arginine racemase, supplementing a wet vector for expressing L-arginine decarboxylase, and continuously reacting until the L-arginine is completely consumed; and finally, separating and purifying the D-arginine and gamatine in the reaction system. The catalyzing enzyme activity is high, the separation and purification operations are simple, the raw materials are cheap, the total production cost is low; and the method has high conversion rate, can realize large-scale production and is suitable for industrial application.
Owner:洛阳华荣生物技术有限公司
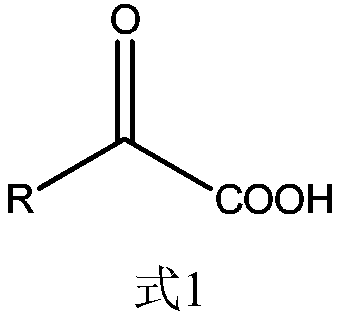
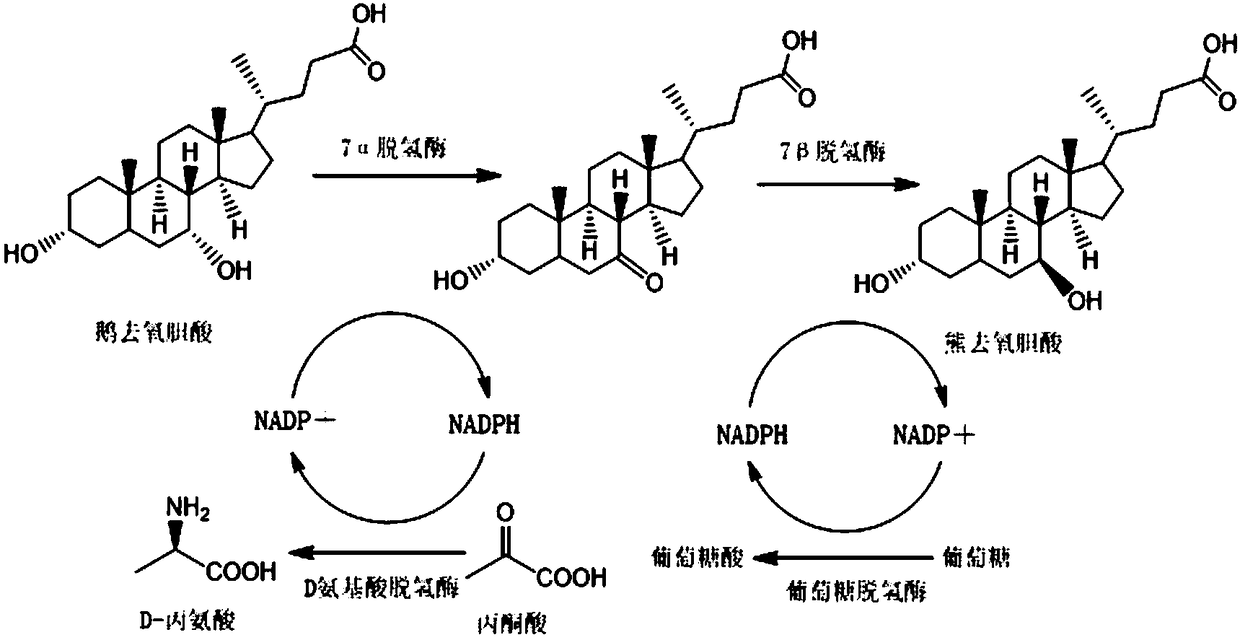
Method for synthesizing ursodeoxycholic acid and high-chiral-purity D-amino acid based on enzyme-method coupling technology
The invention discloses a method for synthesizing ursodeoxycholic acid (UDCA) and high-chiral-purity D-amino acid based on an enzyme-method coupling technology. The method comprises the following steps: putting chenodeoxycholic acid and alpha-ketonic acid into a solution system containing 7alpha-HSDH (Homoserine Dehydrogenase), DAADH and NADP (Nicotinamide Adenine Dinucleotide Phosphate) and carrying out enzyme catalysis reaction; separating a reaction solution by adopting an ultra-filtration membrane to obtain a concentrated mixed enzyme solution; regulating the pH (Potential of Hydrogen) ofa dialysis solution and crystallizing; filtering and separating to obtain 7-KLCA wet powder and filtrate; carrying out chromatographic treatment on the filtrate to obtain the D-amino acid; putting the7-KLCA wet powder into a solution system containing glucose, the NADP, the 7alpha-HSDH and GDH (Glutamate Dehydrogenase) and carrying out enzyme catalysis reaction; separating the reaction solution by adopting the ultra-filtration membrane to obtain the concentrated mixed enzyme solution; crystallizing, filtering and separating the dialysis solution, so as to obtain ursodeoxycholic acid. By adopting the method provided by the invention, UDCA and the high-chiral-purity D-amino acid can be obtained at the same time, the enzyme utilization rate is high, synthesis steps are simple and the cost isreduced; meanwhile, a metal reduction reagent and an organic solvent do not need to be added in a reaction process and conditions are mild; the method is environmentally friendly and is suitable forindustrial production.
Owner:HUNAN BAOLISHI BIOTECH
Preparation method of retegravir intermediate
The invention relates to the technical field of medical intermediates, in particular to a preparation method of a retegravir intermediate. The chemical name of the compound is (2R, 3R, 4R, 5R)-2-(4-aminopyrrole [2, 1-f] [1, 2, 4] triazine-7-yl)-3, 4, 5, 6-tetracarboxylic acid. The preparation method comprises the following steps: (1) in a solvent, carrying out asymmetric addition on 2, 3, 5-tribenzyloxy-D-ribose-1, 4-lactone to form (2R, 3R, 4R, 5R)-3, 4-bis (benzyloxy)-5-(((benzyloxy) methyl) tetrahydrofuran-2-nitrile; and 2) in a solvent, enabling (2R, 3R, 4R, 5R)-3, 4-bis (benzyloxy)-5-((benzyloxy) methyl)-2-hydroxytetrahydrofuran-2-nitrile to react with a hydroxyl activator and then react with 7-iodopyrrole[2, 1-f][1, 2, 4]triazine-4-amine to form (2R, 3R, 4R, 5R)-2-(4-aminopyrrole [2,1-f][1, 2, 4]triazine-7-yl)-3, 4-bis (benzyloxy)-5-((benzyloxy)methyl)tetrahydrofuran-2-nitrile.
Owner:JIANGSU ALPHA PHARM CO LTD
Preparation method of intermediate of rosuvastatin calcium side chain
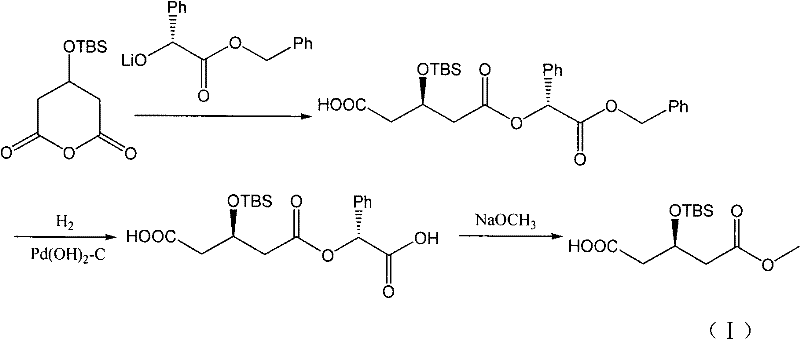
The invention discloses a preparation method of an intermediate of a rosuvastatin calcium side chain, which comprises the following steps: firstly allowing (R)-4-cyano-3- methyl hydroxybutyrate to react with tert-butyl-dimethylchlorosilane, performing nitrile group hydrolysis in water phase under the action of an enzyme with the obtained (R)-4-cyano-3-tert-butyl dimethylsiloxyl methyl butyrate (II) as a substrate to generate a target product with a structure (I). The invention has the characteristics of being mild in reaction, high in yield, environment friendly, low in cost, and high in chiral purity of the prepared product.
Owner:JIANGSU ALPHA PHARM CO LTD
Preparation method of 1S,4R-1-methyl-4-(1-methylvinyl)-2-cyclohexene-1-ol
InactiveCN110143847AEase of industrial productionHigh yieldOrganic compound preparationHydroxy compound preparationAlcoholCyclohexene
The invention relates to a novel synthesis route of 1S,4R-1-methyl-4-(1-methylvinyl)-2-cyclohexene-1-ol. According to the preparation method, limonene is taken as the raw material, 1S,4R-1-methyl-4-(1-methylvinyl)-2-cyclohexene-1-ol is synthesized after four steps: epoxidation reactions, addition reactions, oxidation reactions, and elimination reactions, and the yield is high. The provided 1S,4R-1-methyl-4-(1-methylvinyl)-2-cyclohexene-1-ol preparation method has the advantages of high yield, high chiral purity product, low cost, environmental friendliness, and easy operation, and is suitablefor industrialization.
Owner:南京焕然生物科技有限公司
Synthesis process of trans-menthyl-2, 8-diene-1-ol
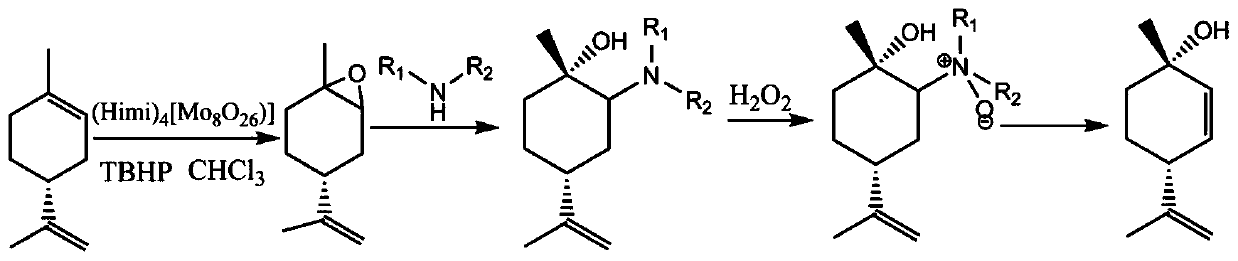
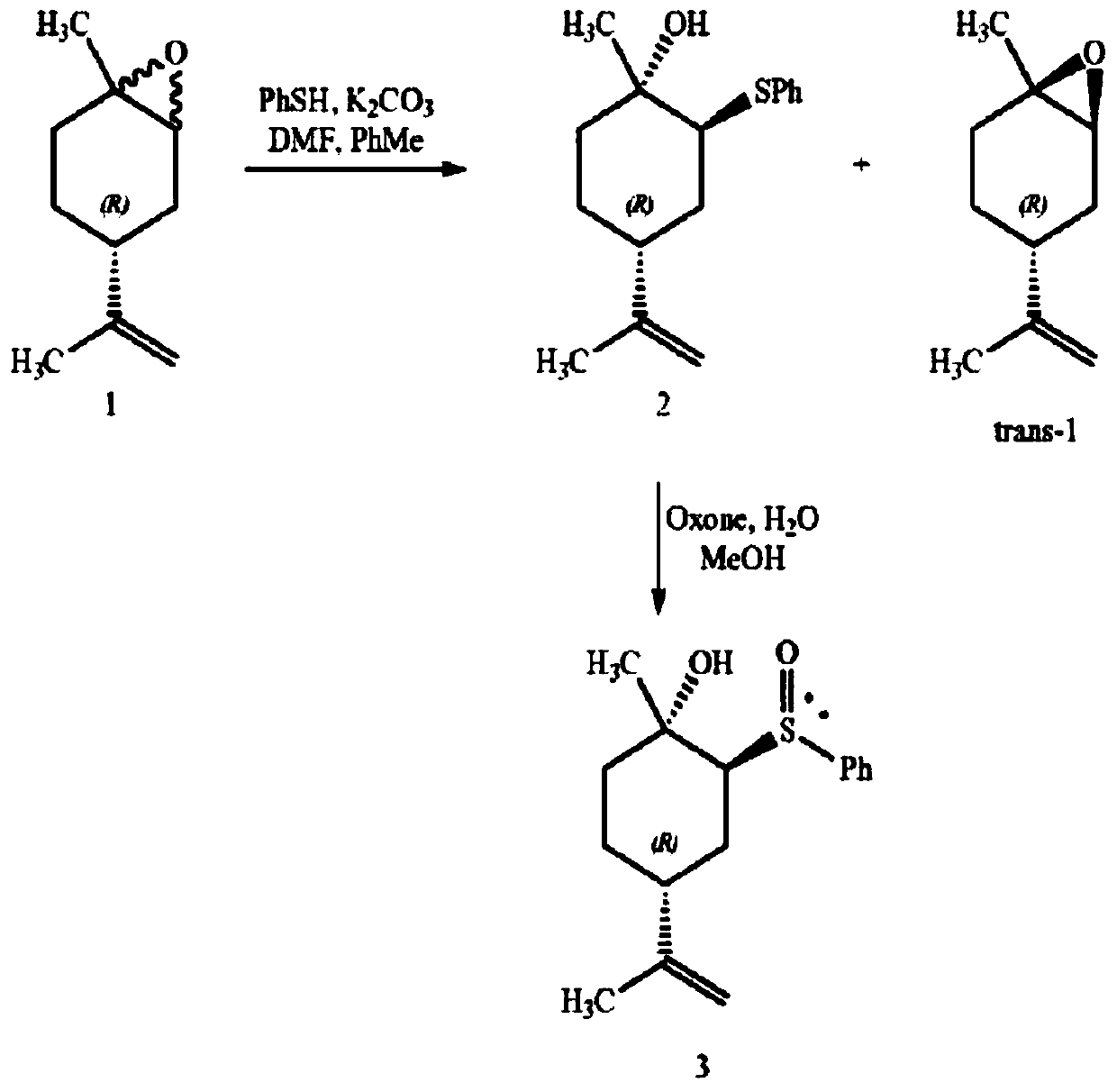
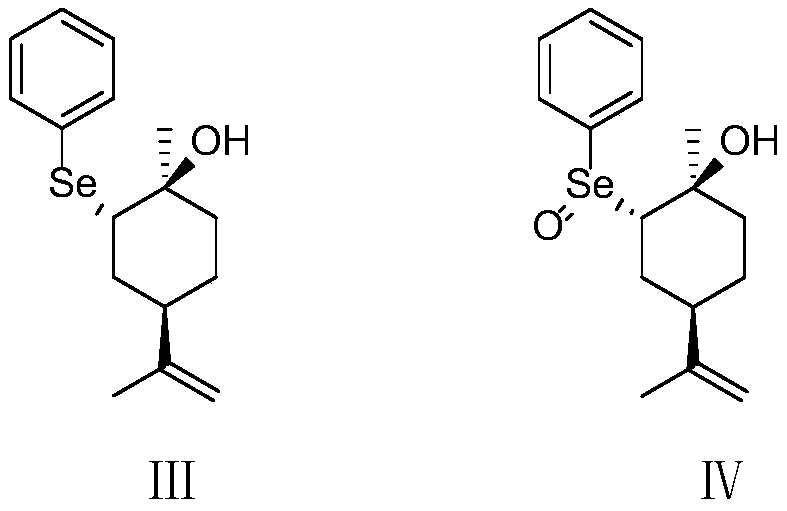
ActiveCN109734554AHigh chiral purityHigh yieldOrganic compound preparationHydroxy compound preparationEpoxyChiral selectivity
The invention belongs to the technical field of preparation of trans-menthyl-2, 8-diene-1-ol, and particularly relates to a synthesis process of trans-menthyl-2, 8-diene-1-ol. The synthesis process comprises the following steps: (1) taking limonene as a raw material, and carrying out lipase catalytic oxidation to prepare 1, 2-epoxy limonene; (2) ring-opening the 1, 2-epoxy limonene in the presenceof sodium borohydride and diphenyl diselenide to form limonene selenide; (3) forming selenium oxide under the action of an oxidizing agent by the limonene selenide and then carrying out elimination reaction to prepare the trans-menthyl-2, 8-diene-1-ol. According to the invention, the 1, 2-epoxy limonene is firstly prepared by a lipase catalysis method with high chiral selectivity, the purity of areaction intermediate is improved without a complex purification process, and the chiral purity of the final product trans-menthyl-2, 8-diene-1-ol is further improved.
Owner:JIANGSU JIMING PHARMA TECH
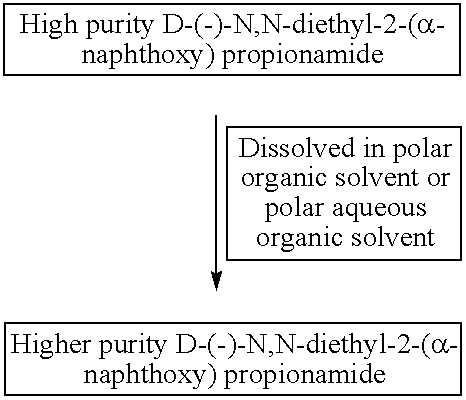
Process for manufacture of high purity D-(−)-N,N-diethyl-2-(α-naphthoxy) propionamide
ActiveUS8309765B2High chiral purityAcceptable high yieldBiocideOrganic compound preparationEthyl groupCombinatorial chemistry
According to one aspect of the present invention there is provided a substantially high purity D-(−)-N,N-diethyl-2-(α-naphthoxy)propionamide and a process for the manufacture of substantially higher purity D-(−)-N,N-diethyl-2-(α-naphthoxy)propionamide having chemical purity near about or above 95%, and chiral purity near about or more than 97%. According to another aspect of the invention is to provide an agrochemical compositions containing highly pure optically active D-(−)-N,N-diethyl-2-(α-naphthoxy)propionamide.
Owner:UNITED PHOSPHORUS LTD
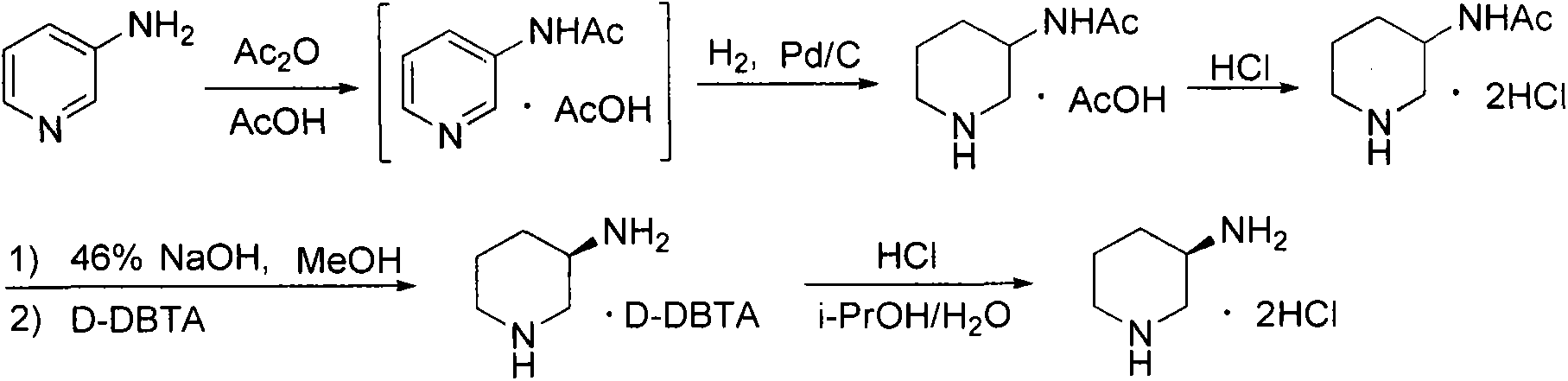
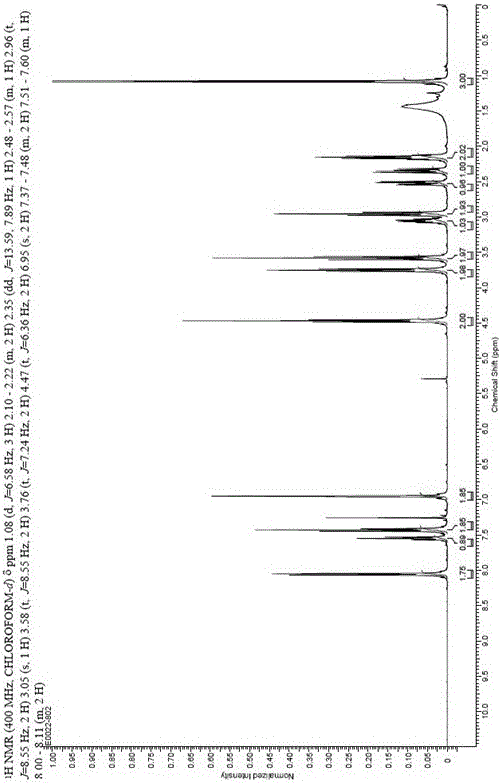
Preparation method for alogliptin intermediate R-3-aminopiperidine dihydrochloride
ActiveCN103319399AHigh chiral purityReduce stepsOptically-active compound separationOrganic racemisationFluoboric acidChirality
The invention aims to provide a preparation method for an alogliptin intermediate R-3-aminopiperidine dihydrochloride. The method has a brief route and is environmentally friendly and low-cost. A racemic compound 3- piperidine carboxamide which is cheap and easy to get is taken as a raw material, and is subjected to Hoffmann rearrangement reaction under the action of 1- fluoronaphthalene, hydrogen peroxide and fluoboric acid, which is similar to acid amides, to obtain 3-aminopiperdine which has one less carbon than the substrate. The method has simple reaction conditions, can be implemented at the room temperature, has simple operations in process, and is easy to monitor. The solvents are an ethanol-water mixture, and are low-cost and environmentally friendly. The carbon-lessened product 3-aminopiperidine is acidized and salified by concentrated hydrochloric acid. The hydrochloride is separated and salified by D-tartaric acid to obtain the target product R-3-aminopiperidine dihydrochloride. The chirality purity is high. The ee value is more than 99.5%. The reaction overall yield can reach 89%-93%. The cost is low. The method is suitable for industrial mass production.
Owner:迪嘉药业集团股份有限公司
Preparation method of indoline derivative for synthesizing silodosin
ActiveCN106380438AHigh optical purityChemical cost reductionOrganic chemistryBenzoic acidPalladium on carbon
The invention provides a preparation method of an indoline derivative for synthesizing silodosin. Indoline is taken as a starting raw material and reacts with benzoic acid and 1-chloro-3-bromopropane to prepare a compound (1); a reaction is carried out in a Vilsmeier agent, and a formyl group in introduced to the 5th position to prepare a compound (2); the compound (2) and nitroethane undergoes an asymmetric Henry reaction in the catalysis of quinidine-copper acetate to prepare a compound (3); the compound (3) is subjected to acetylation through acetic anhydride to prepare a compound (4); a cyano group is introduced to the 7th position to prepare a compound (6); and two functional groups are reduced through palladium-on-carbon hydrogenation in one step to obtain a high-chiral-purity target compound: (R)-1-[1-(3-benzoyloxypropyl)-5-(2-aminopropyl)-7-cyano]indoline. In the early stage of the method, cheap quinidine is used to perform an asymmetric Henry reaction for introduction of chiral centers, thereby avoiding resolution in the latter stage. The method is reasonable in design, simple to operate, effectively improved in yield and reduced in cost, and therefore is suitable for massive production.
Owner:江苏宇田医药有限公司
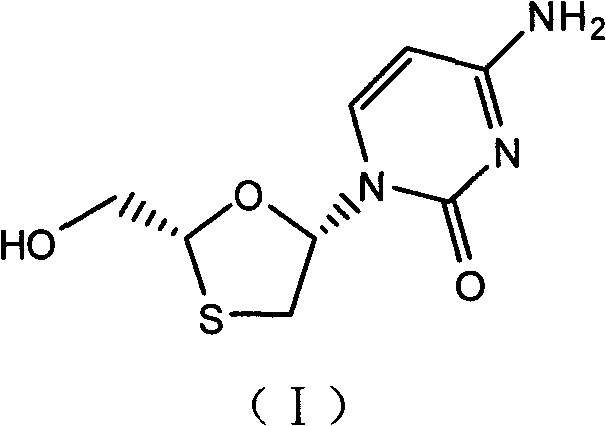
Industrial preparation method for lamivudine
The invention discloses an industrial preparation method for lamivudine (3TC). The method is characterized in that: 5-hydroxyl-1,3-oxathiolane-2-carboxylic acid [(1<,>R, 2<,>S, 5<,>R)-5<,>-methyl-2<,>-(1-methylethyl) cyclohexyl] ester reacts with a acylating agent to obtain a acylate; the acylate and monosilylated acyl cytosine or monosilylated cytosine schiff's base are subjected to a glycosylation through a catalysis of lewis acid to obtain a 3TC intermediate, followed by reducing and deacylating or hydrolyzing and reducing to obtain the 3TC. The method provided by the present invention has advantages of simple, available and cheap raw materials, simple operation, safe production, high yield, less waste pollution, and is applicable for industrial production.
Owner:吉斯凯(苏州)制药有限公司
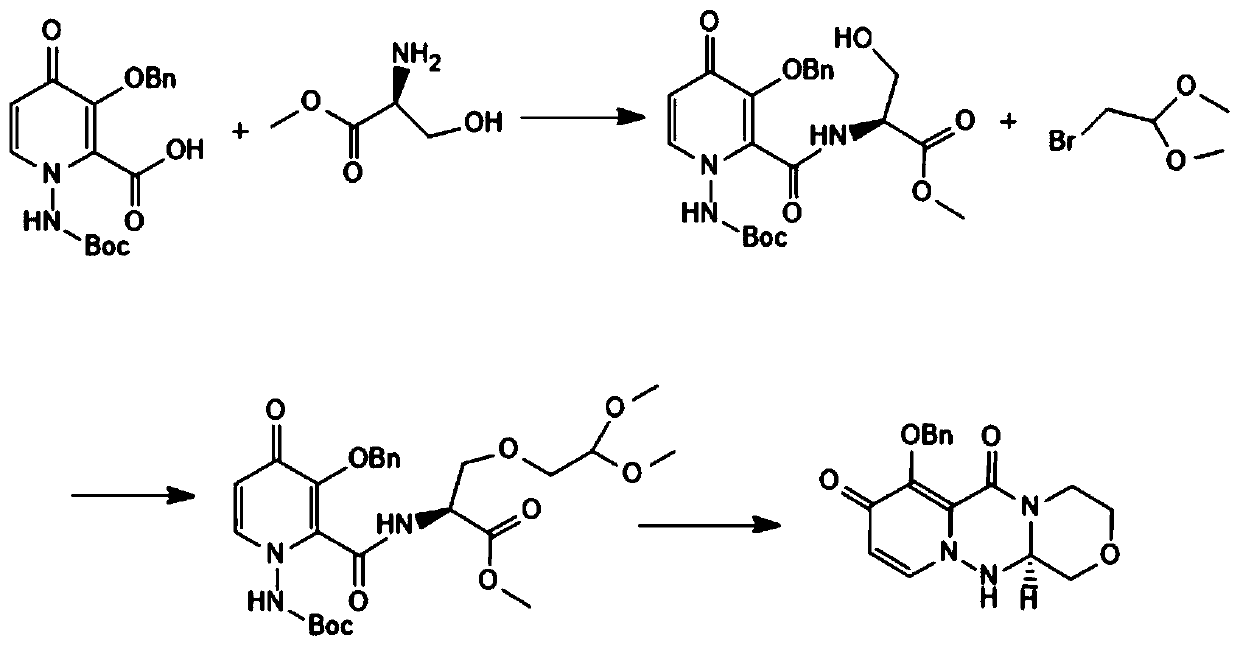
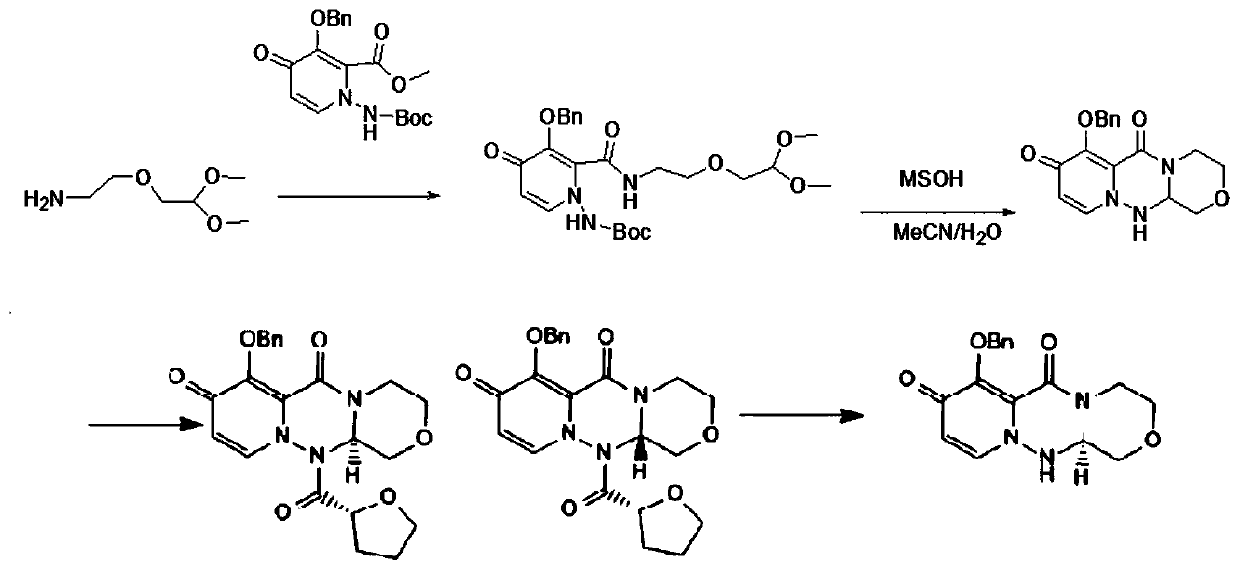
Preparation method of balosavir intermediate
ActiveCN111205304AHigh yieldHigh chiral purityOrganic chemistryCarboxylic acidPharmaceutical Substances
The invention discloses a preparation method of a baloxavir intermediate (R)-7-(benzyloxy)-3, 4, 12, 12a-tetrahydro-1H-[1, 4]oxazine[3, 4-c]pyrido[2, 1-f][1, 2, 4]-triazine-6, 8-diketone, and belongsto the field of drugs. According to the method, 3-benzyloxy-1-Boc amino-4-carbonyl-1, 4-dihydropyridine-2-carboxylic acid methyl ester and a chiral substrate L-serine ester are taken as raw materialsto carry out condensation reaction, substitution reaction and cyclization decarboxylation reaction to synthesize a baloxavir key intermediate (R)-7-(benzyloxy)-3, 4, 12, 12a-tetrahydro-1H-[1, 4]oxazine[3, 4-c]pyrido[2, 1-f][1, 2, 4]-triazine-6, 8-diketone and creatively develop the synthetic route for synthesizing the baloxavir intermediate (R)-7-(benzyloxy)-3, 4, 12, 12a-tetrahydro-1H-[1, 4]oxazine[3, 4-c]pyrido[2, 1-f][1, 2, 4]-triazine-6, 8-diketone. Compared to the traditional route, the preparation method has the advantages of the high yield, low cost, no need of resolution, the high chiral purity, convenience in industrialization and the like.
Owner:南京法恩化学有限公司
Preparing method for caproic acid derivative
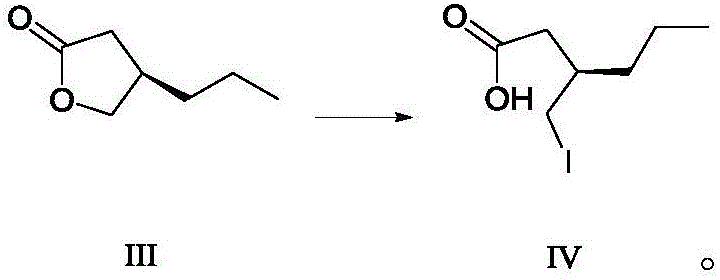
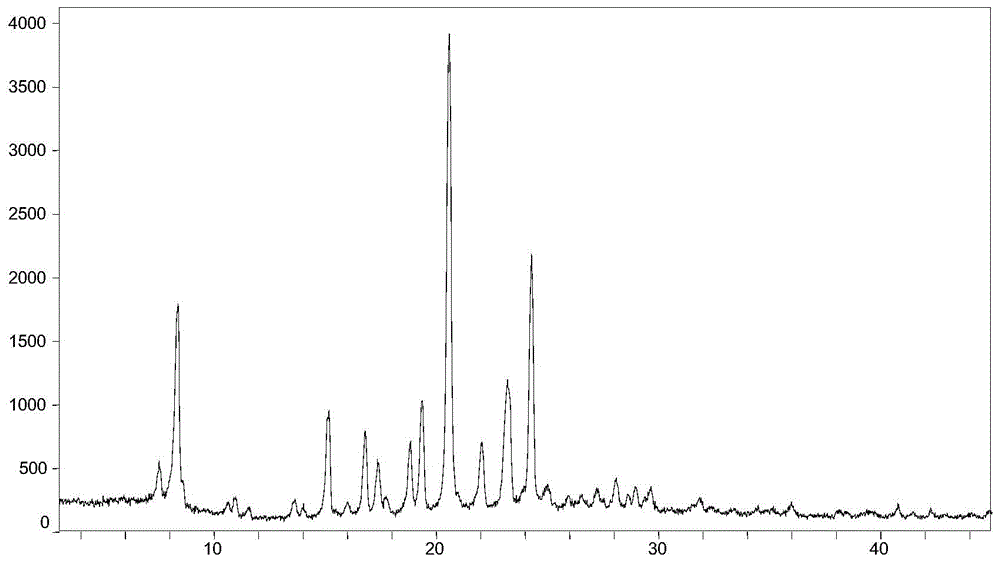
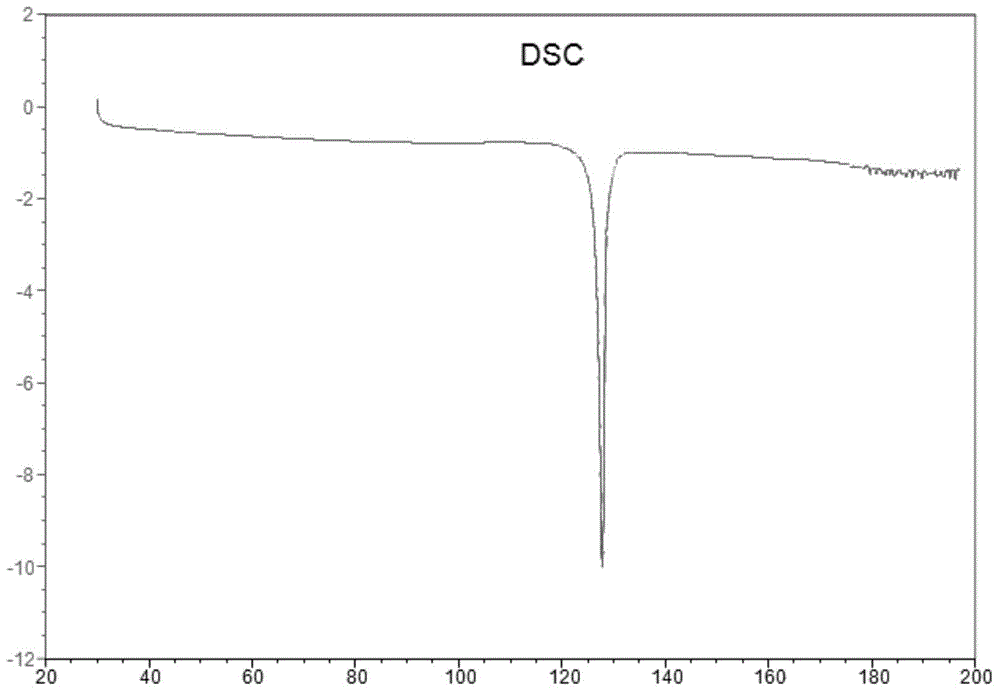
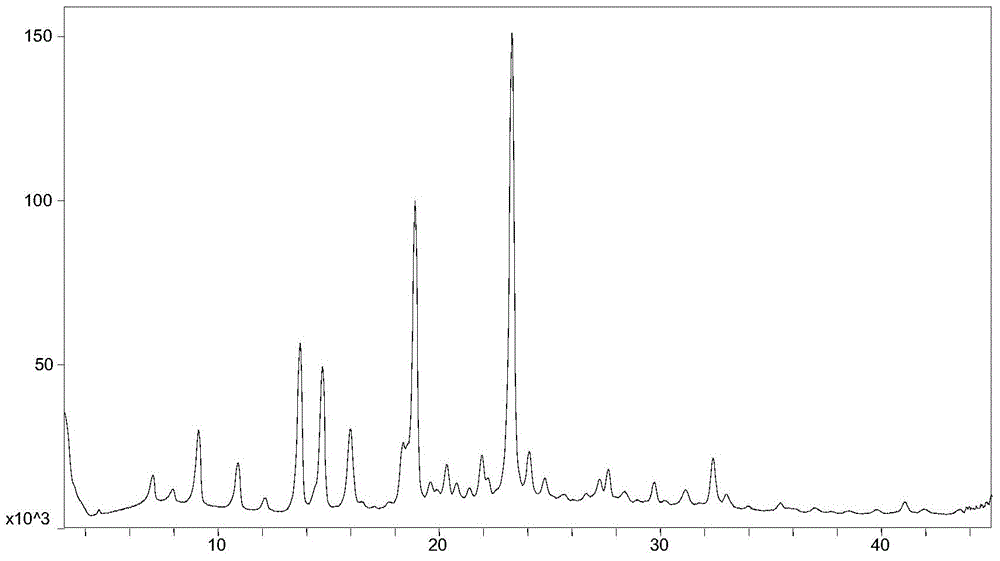
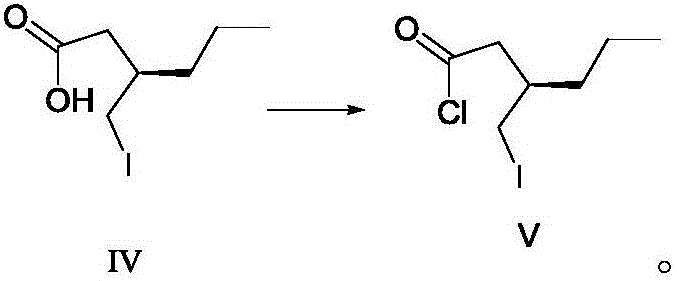
ActiveCN106588740AHigh chiral purityShort synthetic routeOrganic compound preparationPreparation from carboxylic acid esters/lactonesTwo stepHigh pressure
The invention discloses a preparing method for a caproic acid derivative and provides a preparing method for a caproic acid derivative IV. The method comprises the following steps that in a polar nonprotic organic solvent, a compound III and an iodization reagent are subjected to a nucleophilic substitution reaction under inert gas shielding, and the caproic acid derivative IV is obtained. Brivaracetam can be prepared from the caproic acid derivative IV only through two steps, the synthetic route is short, the reaction condition is mild, posttreatment is simple, the reaction yield is high, and the production cost is low. Racemization does not happen in the reaction process, further purification is conducted through crystallization instead of a chiral high-pressure liquid phase preparing column, the chiral purity of the brivaracetam I can be improved till the de value is larger than 99.80%, meanwhile, the content of other single impurities of the brivaracetam I is smaller than 0.1%, the API level is reached, and the method is suitable for industrial production. The expression is shown in the description.
Owner:上海云晟研新生物科技有限公司
AHU-377alpha-phenethylamine salt polycrystalline type and preparation method and application thereof
InactiveCN105367438AEasy to separateHigh chiral purityOrganic active ingredientsSenses disorderOperabilityCrystallinity
The invention discloses AHU-377alpha-phenethylamine salt polycrystalline type and a preparation method and application thereof and particularly discloses the AHU-377alpha-phenethylamine salt polycrystalline type. An X-radial powder diffraction pattern comprises peaks located at the diffraction angles 2 theta, namely 20.58 + / -0.2 degrees, 24.28 + / -0.2 degrees, 8.38 + / - 0.2 degrees and 23.20 + / -0.2 degrees or peaks located at the diffraction angles 2 theta, namely 23.28+ / -0.2 degrees, 18.9+ / -0.2 degrees, 13.7+ / -0.2 degrees and 14.72+ / -0.2 degrees. The AHU-377 is prepared into the phenethylamine salt to change the physicochemical properties of the AHU-377, such as crystallinity, solubleness and hygroscopicity. The AHU-377alpha-phenethylamine salt polycrystalline type and the preparation method and application thereof are mature in process and high in operability, the obtained product is high in quality, homogeneous and stable, chemical stability is achieved, storage is facilitated, and wide application prospect is achieved.
Owner:SHANGHAI HANSOH BIOMEDICAL +1
Method for synthesizing noradrenaline
ActiveCN108069863AReduce costs and safety hazardsHigh chiral purityOrganic compound preparationOrganic chemistry methodsEpinephrineReducing agent
The invention discloses a method for synthesizing noradrenaline. The method comprises the steps that (-)-diisopinocampheyl chloroborane serves as a catalyst, a reducing agent is utilized in an ether solvent at a low temperature, particularly (-)-diisopinocampheyl chloroborane directly reduces noradrenaline to generate levarterenol, and a solution containing noradrenaline is obtained. According tothe method, levarterenol can be directly obtained by using (-)-diisopinocampheyl chloroborane as the reducing agent, resolution is not needed, and the cost and potential safety hazards are reduced simultaneously.
Owner:WUHAN WUYAO PHARMA
Polybasic nitrogen heterocyclic non-natural chiral amino acid and synthesis method thereof
ActiveCN108752253ASimple processSave raw materialsOrganic chemistry methodsBulk chemical productionAlkaneSynthesis methods
The invention relates to a polybasic nitrogen heterocyclic non-natural chiral amino acid and a synthesis method thereof. The amino acid can be applied to molecule building for antibiotic synthesis. According to the synthesis method, 2-aminodiethyl malonate and halogenated alkanes carry out substitution reactions, cyclization reactions, and decarboxylation reactions, and the reaction products are split to obtain the polybasic nitrogen heterocyclic non-natural chiral amino acid. The provided novel synthesis method has the advantages of simple synthesis route, low cost, convenient operation, andeasiness for commercial production, the chiral purity of obtained products is high, and the application prospect is good.
Owner:广西茵诺圣药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com















![Preparation method of (S, S)-2, 8-diazabicyclo[4, 3, 0]nonane Preparation method of (S, S)-2, 8-diazabicyclo[4, 3, 0]nonane](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/02391988-7d43-4f83-9dc9-af83912b5570/HDA0000583691930000011.PNG)
![Preparation method of (S, S)-2, 8-diazabicyclo[4, 3, 0]nonane Preparation method of (S, S)-2, 8-diazabicyclo[4, 3, 0]nonane](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/02391988-7d43-4f83-9dc9-af83912b5570/HDA0000583691930000012.PNG)
![Preparation method of (S, S)-2, 8-diazabicyclo[4, 3, 0]nonane Preparation method of (S, S)-2, 8-diazabicyclo[4, 3, 0]nonane](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/02391988-7d43-4f83-9dc9-af83912b5570/HDA0000583691930000021.PNG)