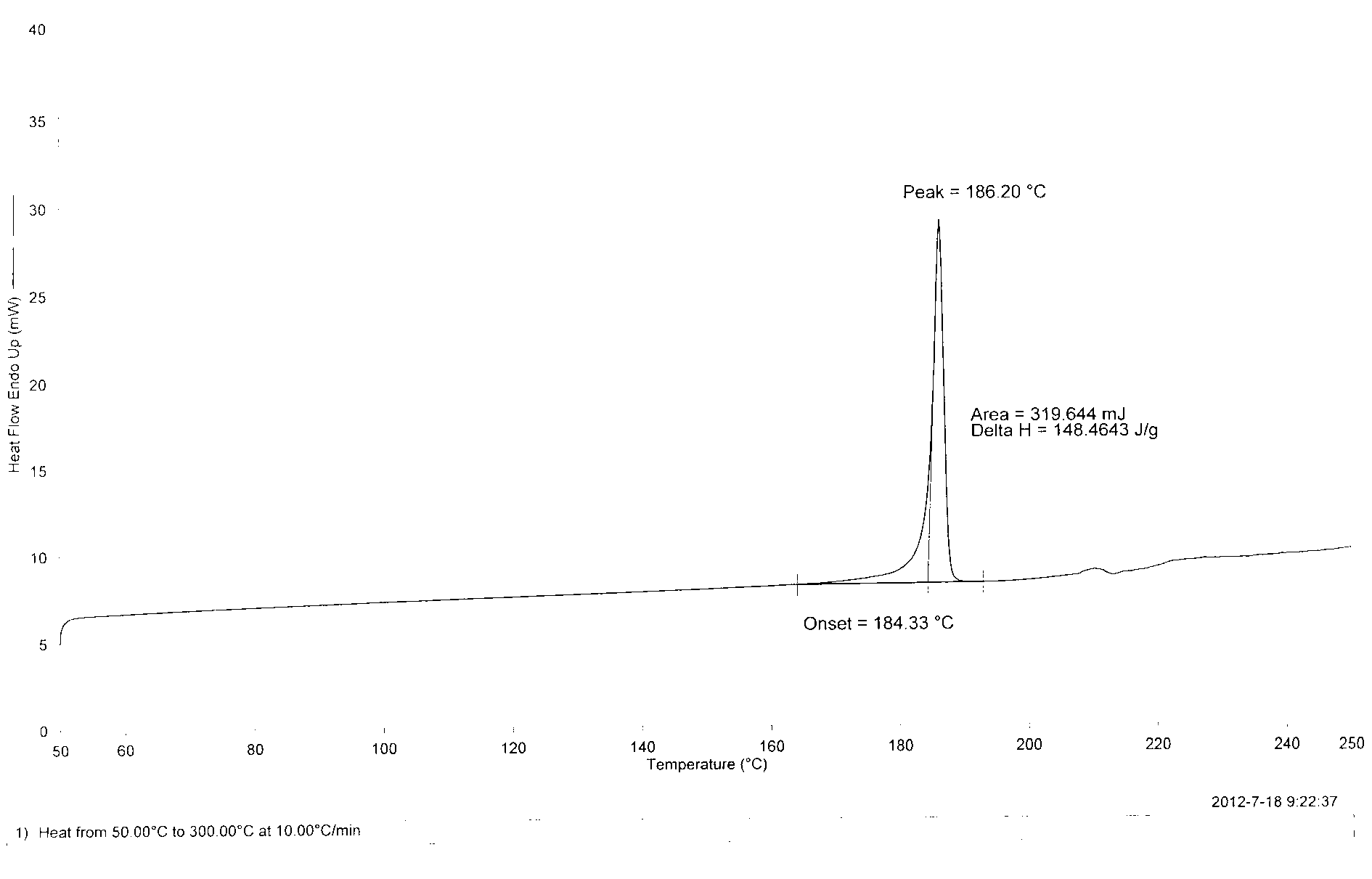
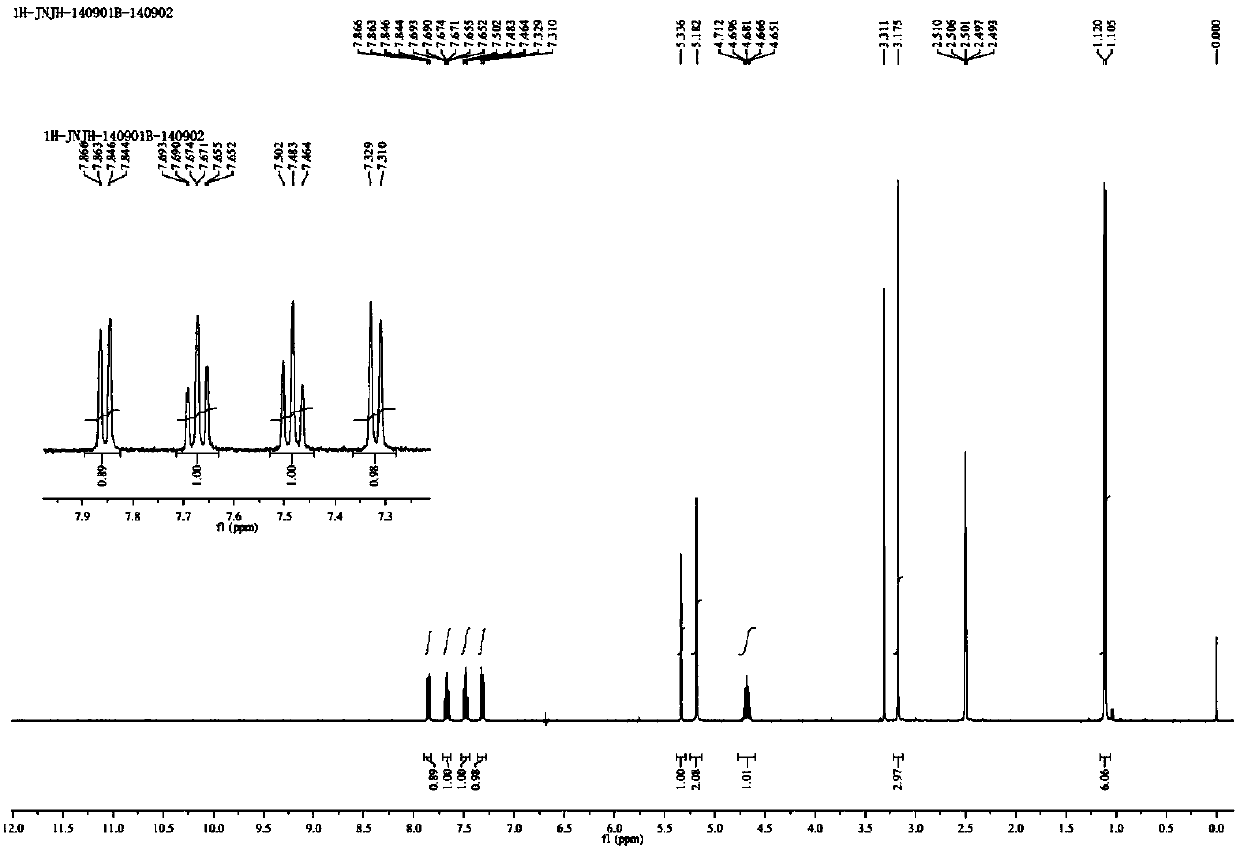
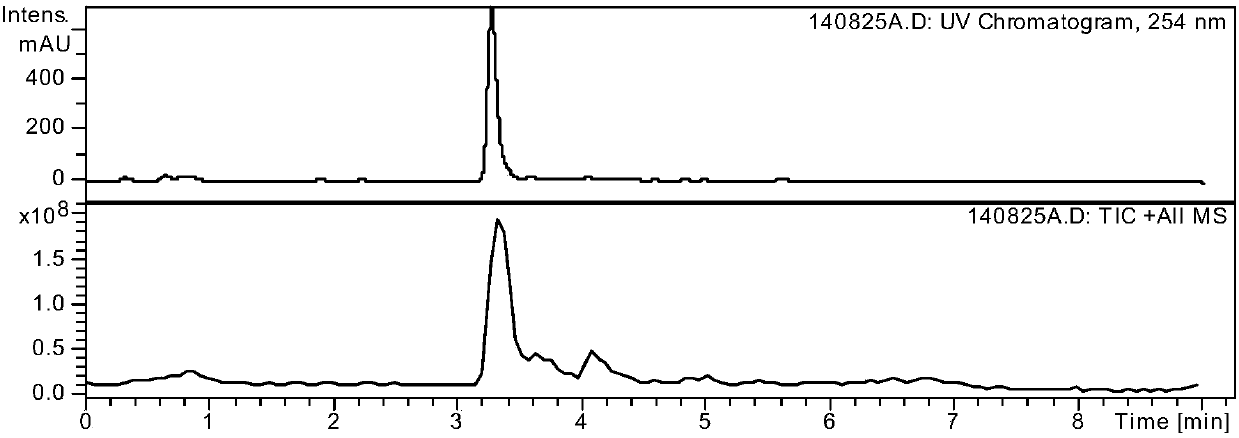
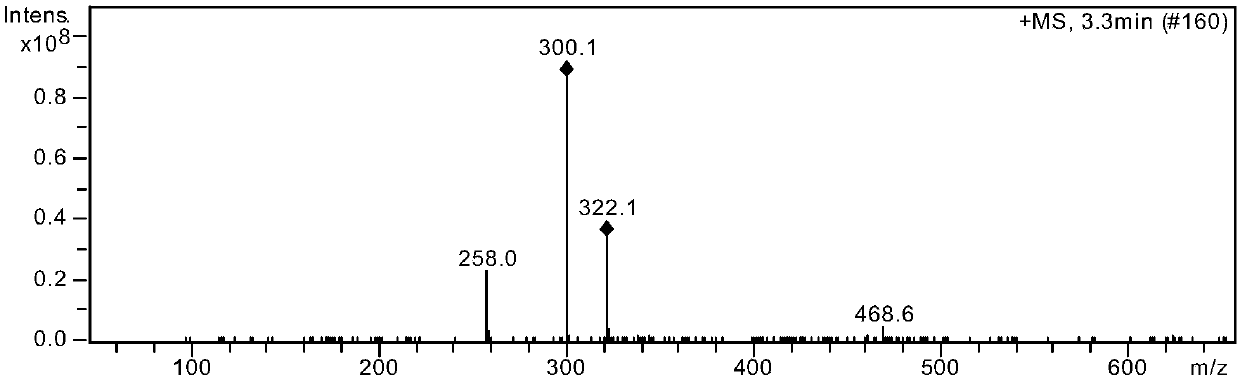
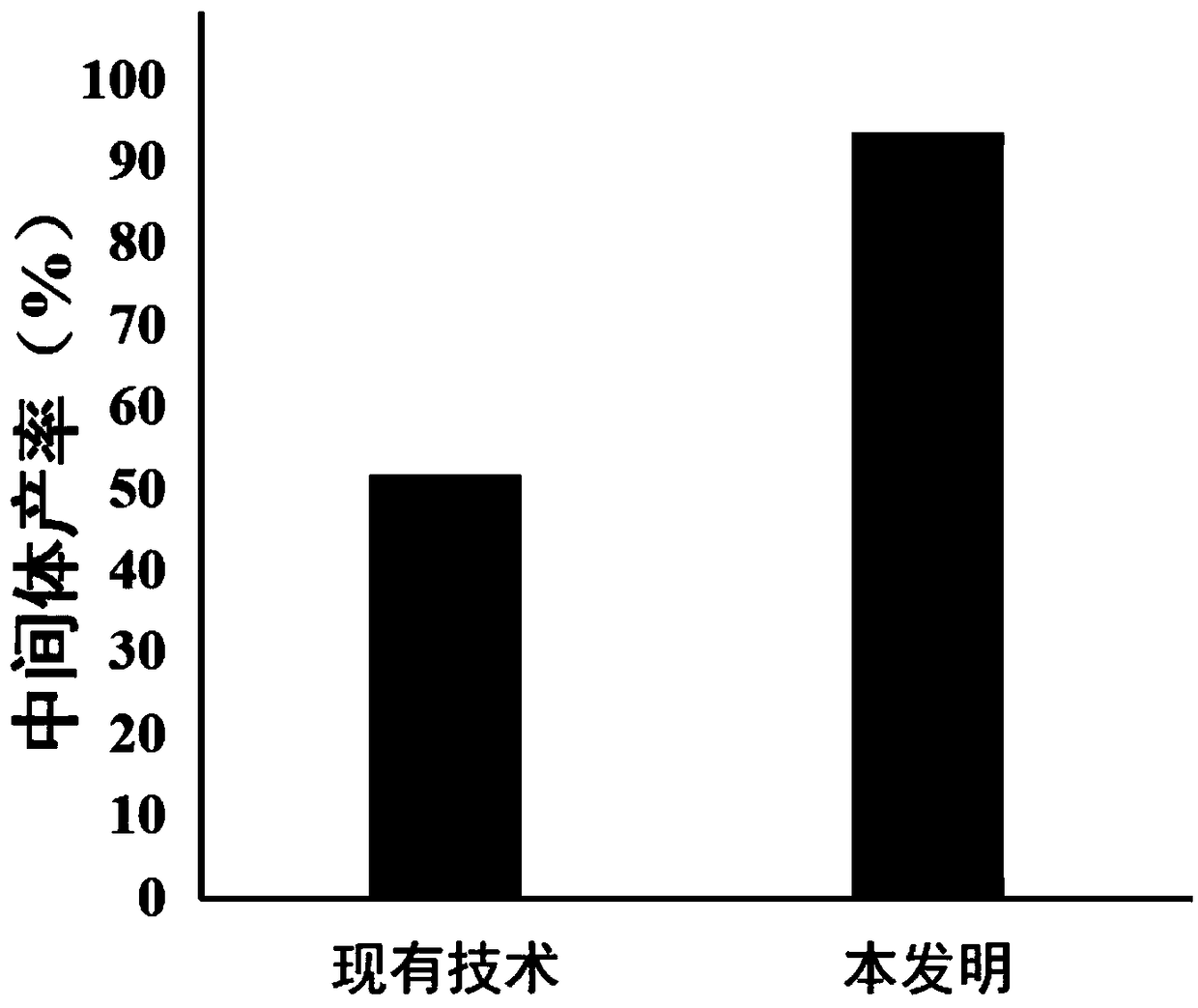
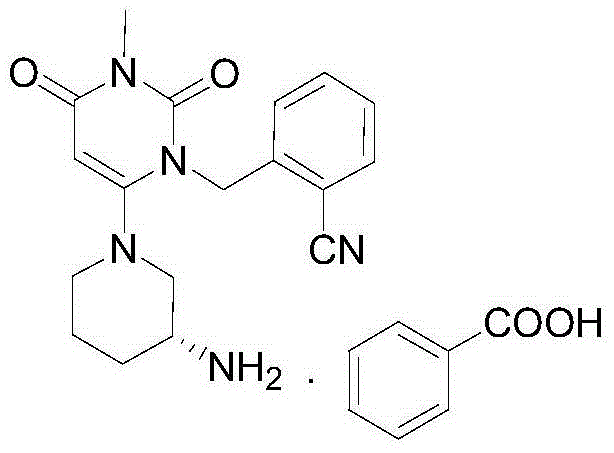
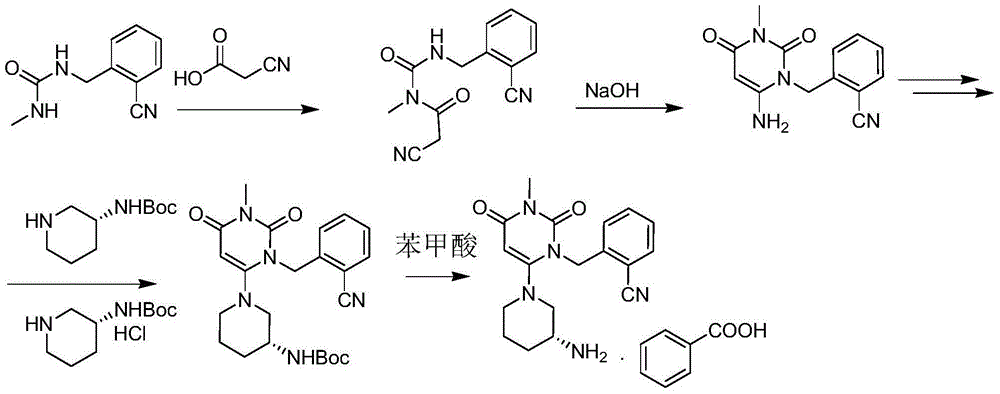
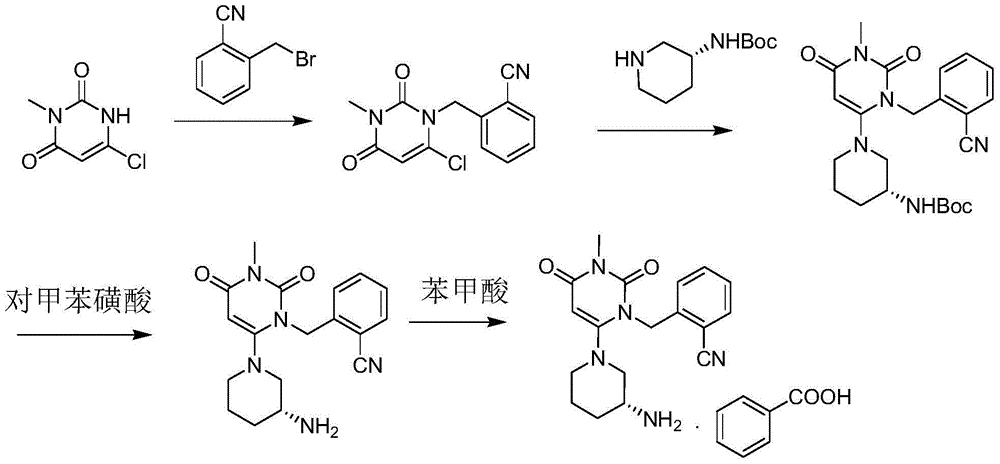
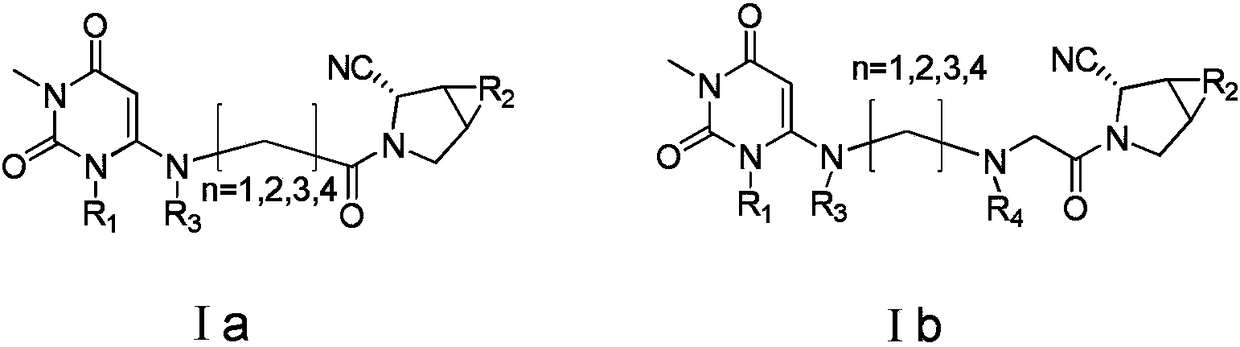
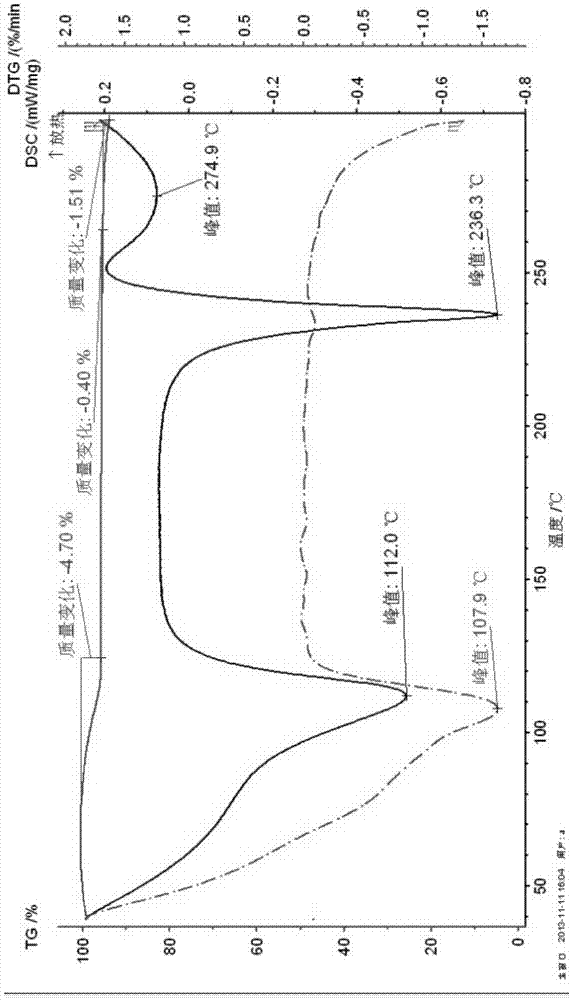
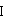Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
84 results about "Alogliptin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Alogliptin is used with a proper diet and exercise program to control high blood sugar in people with type 2 diabetes.
Solid preparation comprising alogliptin and pioglitazone
ActiveUS20100092551A1High dissolution rateReduce adverse effectsBiocideOrganic active ingredientsDiabetes mellitusAlogliptin
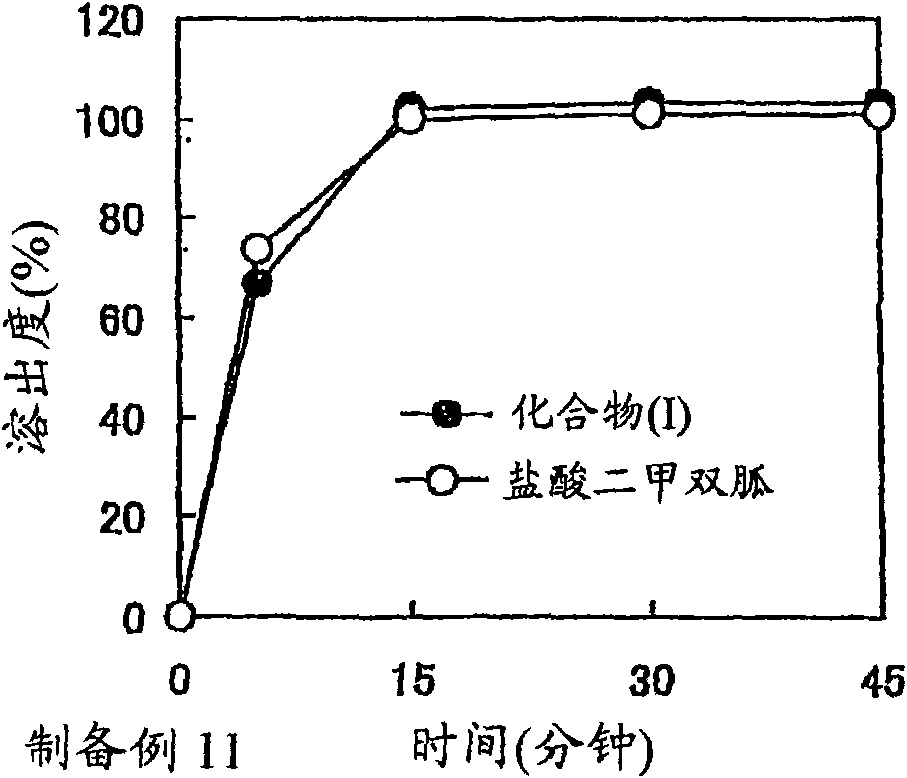
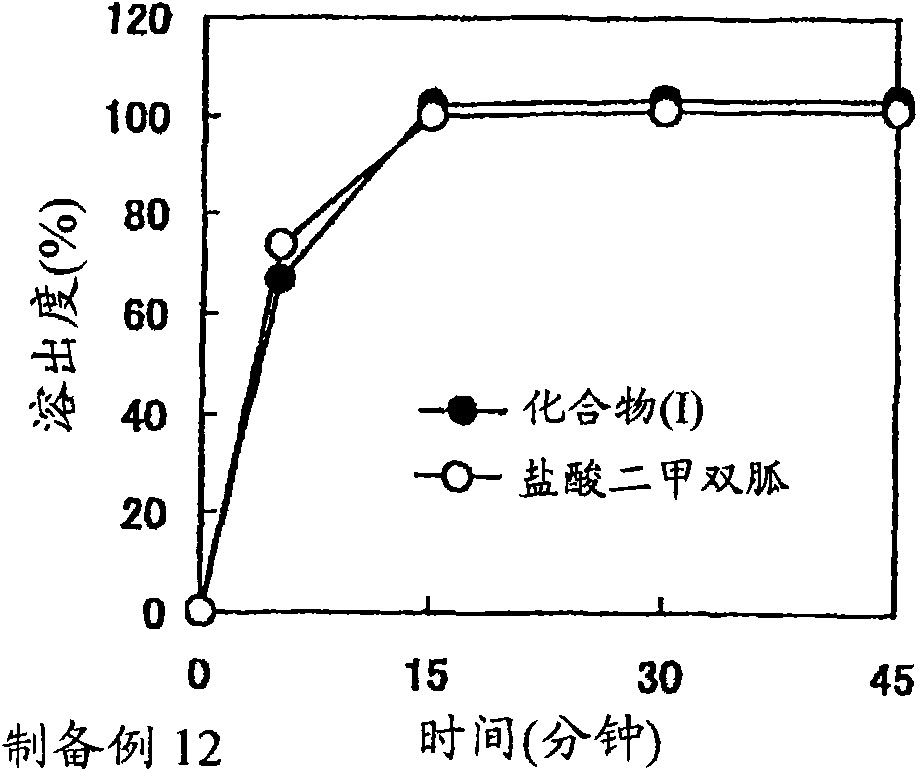
A solid preparation containing compound (I), wherein the definition of compound (I) is as defined in the description, and pioglitazone, which is useful as a therapeutic drug for diabetes and the like and superior in the dissolution property, chemical stability and dissolution stability, is provided. A solid preparation containing the following first and second parts:(1) the first part containing compound (I) or a salt thereof and, as the first excipient, sugar or sugar alcohol; and(2) the second part containing pioglitazone or a salt thereof and, as the second excipient, sugar or sugar alcohol.
Owner:TAKEDA PHARMA CO LTD
Process for the preparation of alogliptin
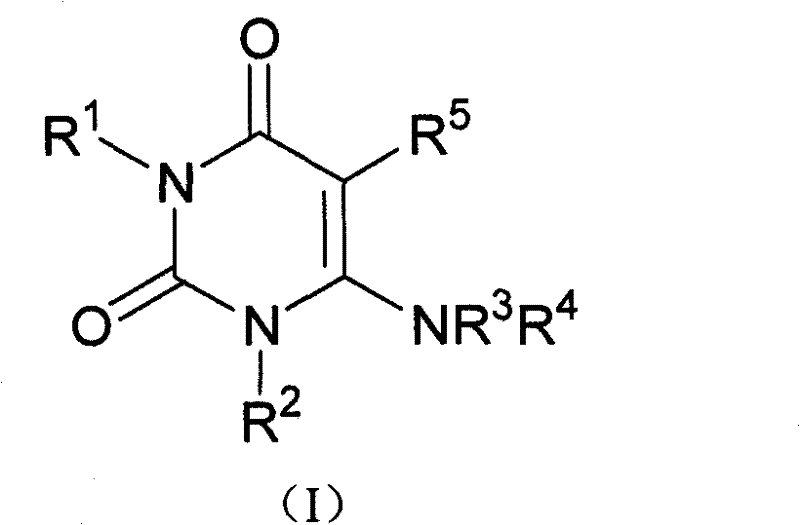
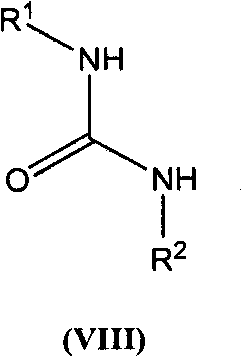
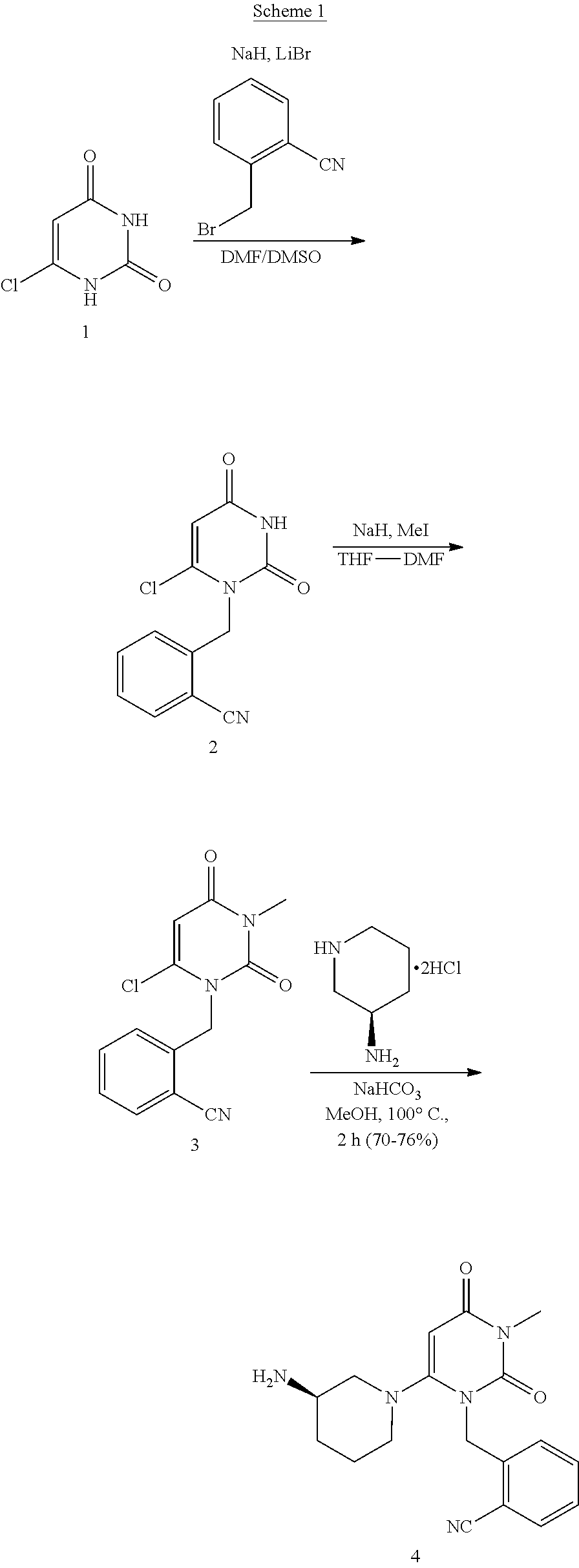
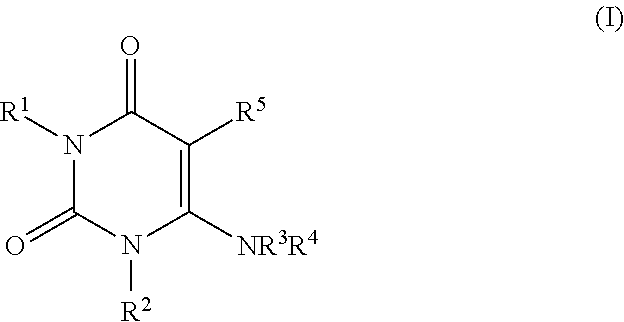
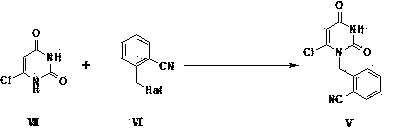
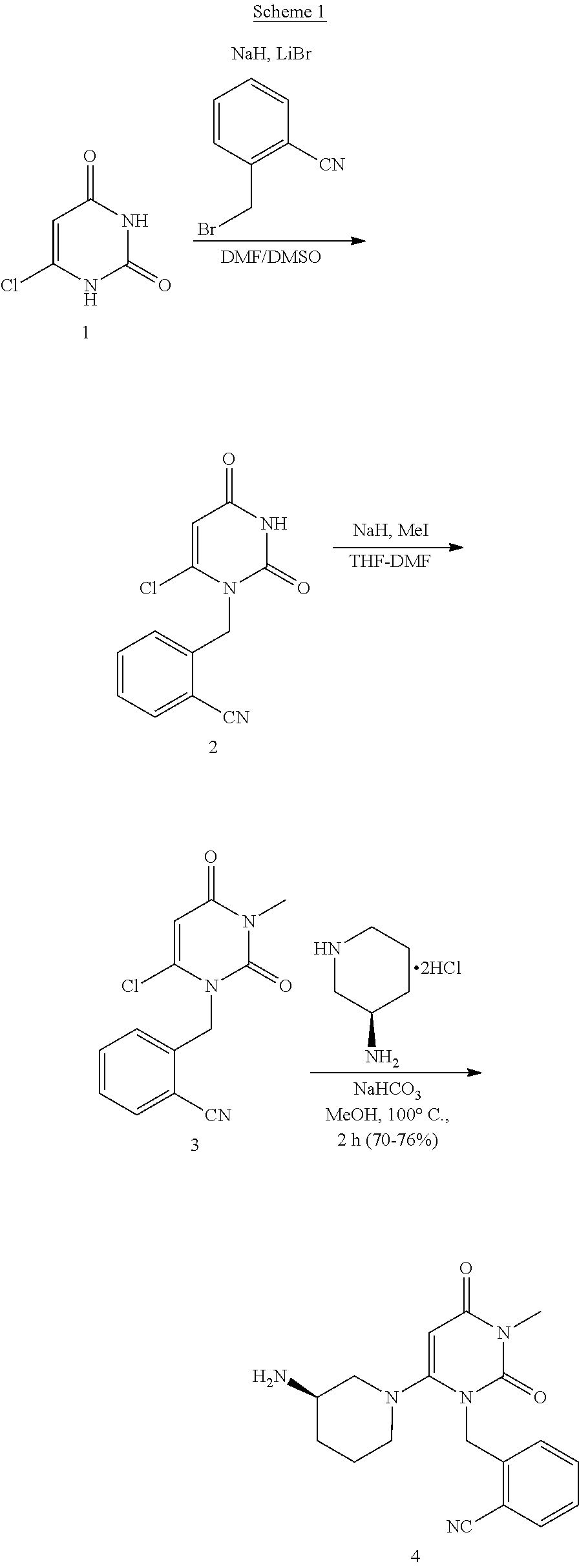
The present invention is based on the discovery of a process for preparing pyrimidin- dione compounds, especially alogliptin and its derivatives, which comprises the reaction of a urea derivative of formula (VIII) with a malonic acid or its derivatives to form intermediates of formulae (VII) or (VII-A), which are subsequently converted to a compound of formula (II) upon introduction of a leaving group X. Compound (II) then reacts with an amine to form compound (I), which is optionally converted to its salts of formula (IV).
Owner:MAPI PHARMA
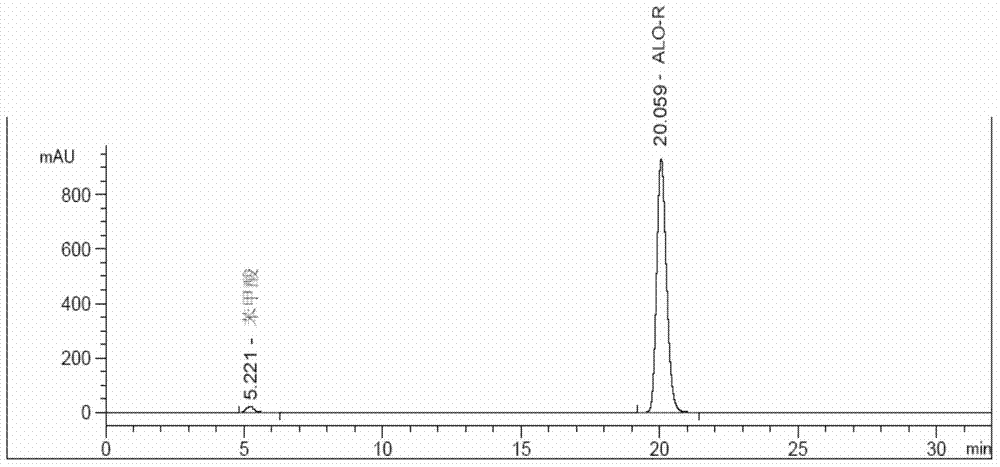
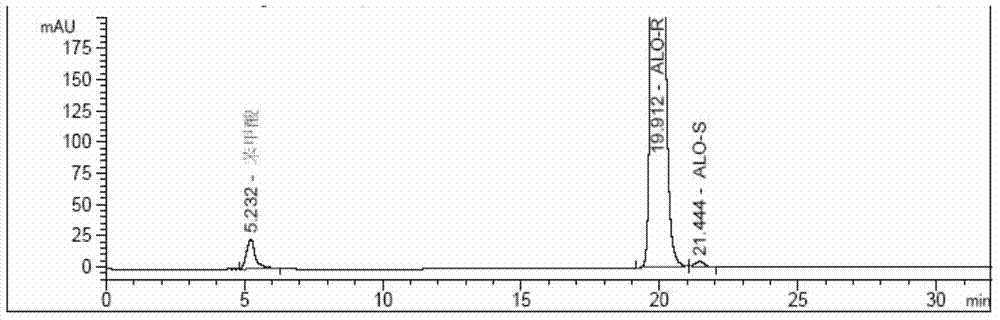
Method for determining enantiomer impurity in alogliptin crude drug and preparation by virtue of HPLC
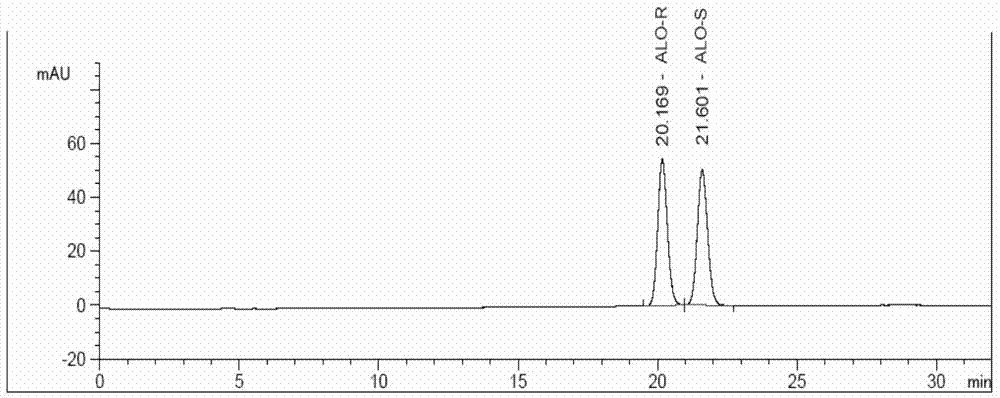
The invention discloses a method for determining an enantiomer impurity in an alogliptin crude drug and a preparation by virtue of HPLC. The method comprises the step of carrying out gradient elution by selecting a silica gel coated with amylose-tri((S)-alpha-phenethyl carbamate on the surface as a stationary phase, and taking a mixed solvent of n-hexane and ethanol containing triethylamine or diethylamine respectively as a mobile phase. With the adoption of the method, alogliptin or the salt thereof can be separated from the enantiomer of alogliptin, and the masses of the alogliptin crude drug and the preparation thereof can be effectively controlled. According to the detection method, the specificity is high, the precision and the accuracy are high, the tolerance is good, the operation is convenient, and medicine quality can be effectively controlled.
Owner:WATERSTONE PHARMA WUHAN
Preparation method of alogliptin
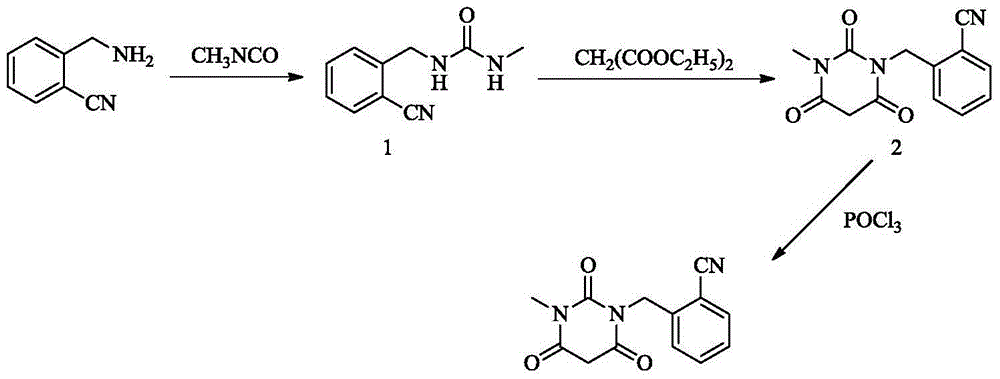
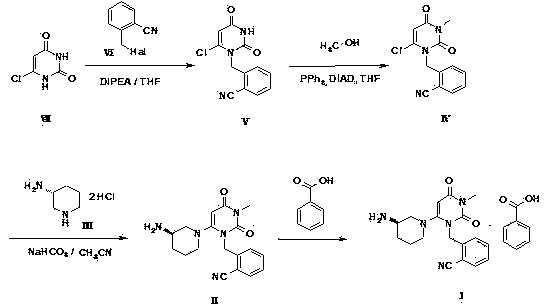
The invention relates to a preparation method of alogliptin. The preparation method comprises the following steps: performing cyclization by taking N-methylurea which is low in price and easy to obtain as a raw material to obtain a compound shown in the formula III, introducing a leaving group to obtain a compound shown in the formula IV, reacting with a compound shown in the formula V to obtain a compound shown in the formula VI, then performing reaction on the compound shown in the formula VI and (R)-3-Boc aminopiperidine to obtain a compound shown in the formula VII, and performing deprotection on the compound shown in the formula VII to form a salt, thereby obtaining alogliptin or the salt thereof. Compared with a traditional method, the method has the advantages of low cost, easiness in control of reaction, simple post-treatment operation and the like, and is suitable for industrial production of alogliptin or the salt thereof.
Owner:SHANGHAI TIANCI BIOLOGICAL VALLEY BIOLOGICAL ENG
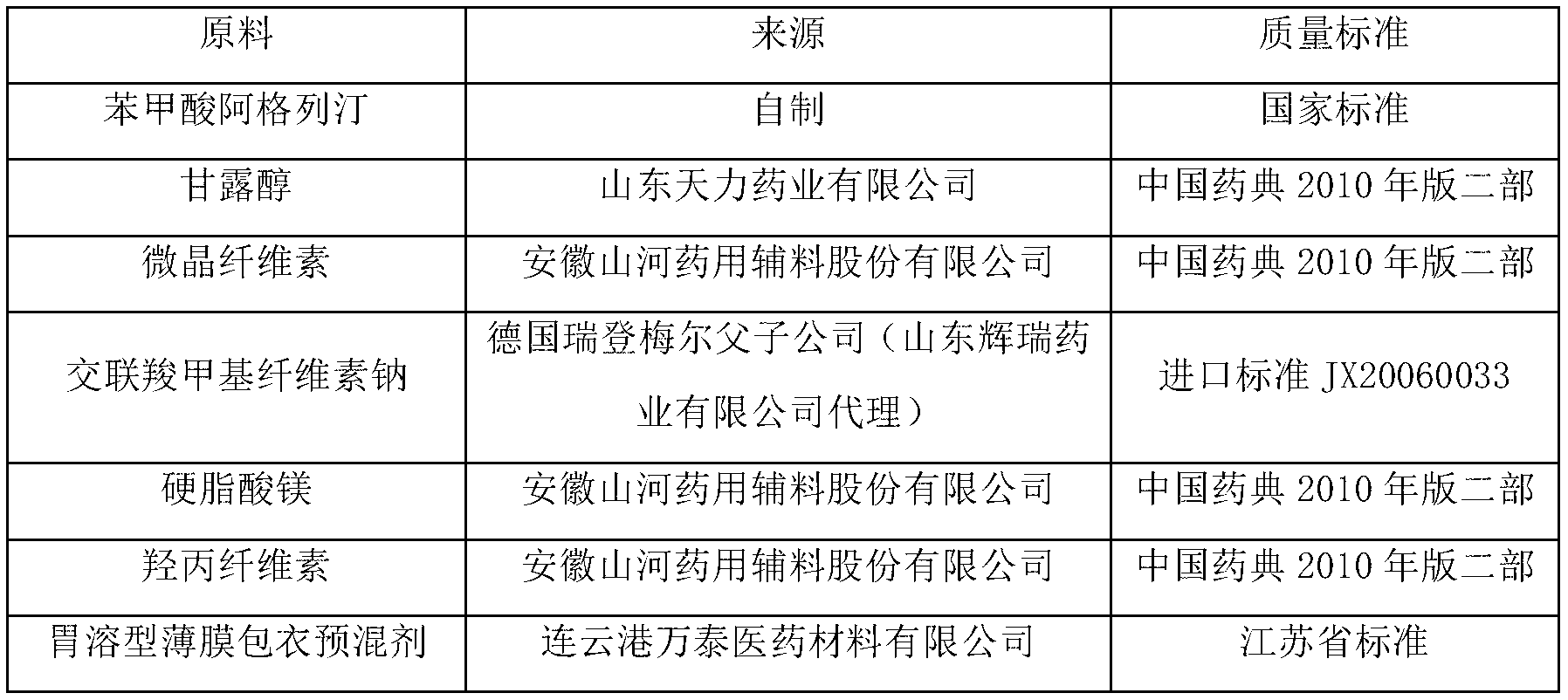
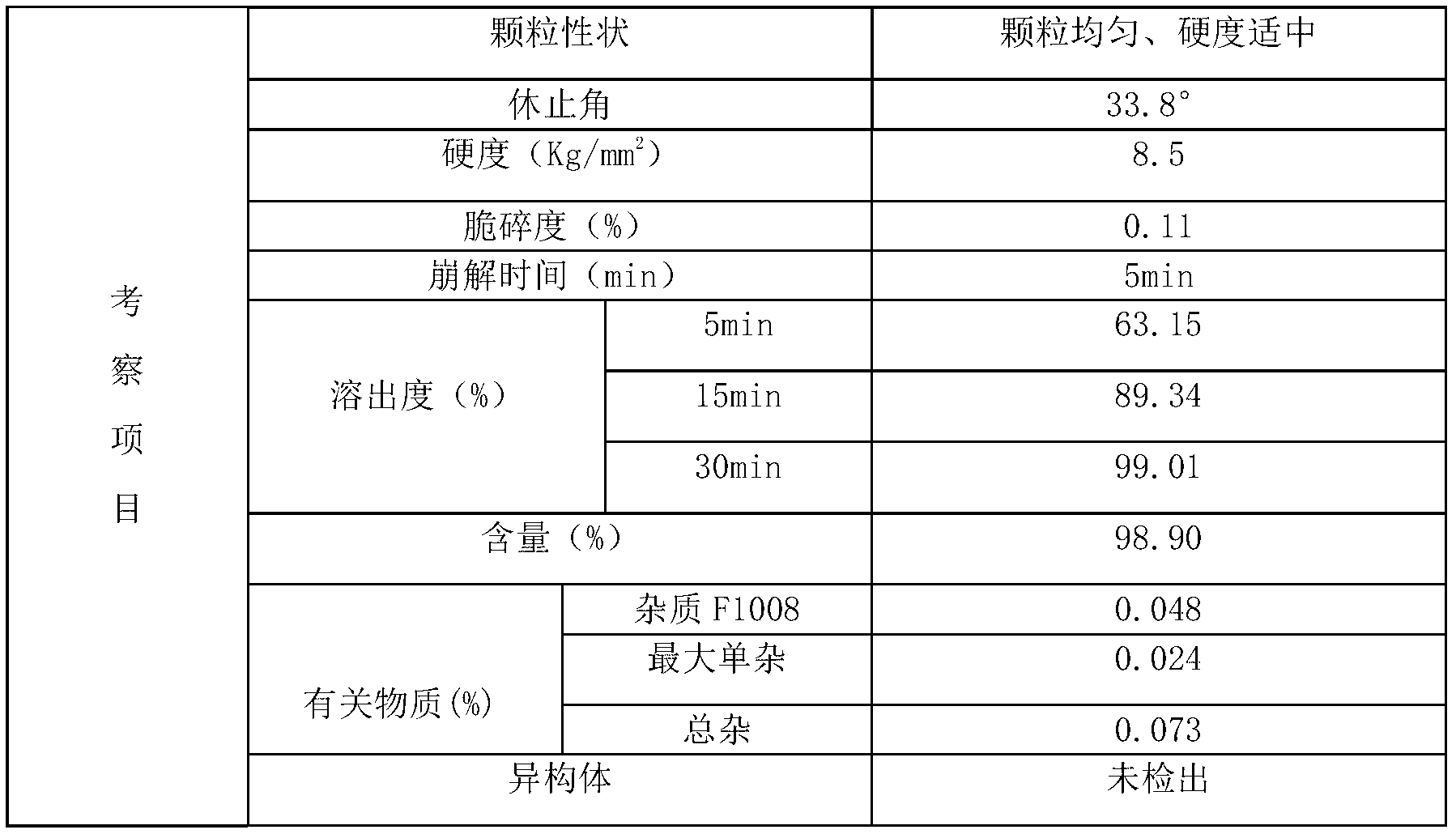
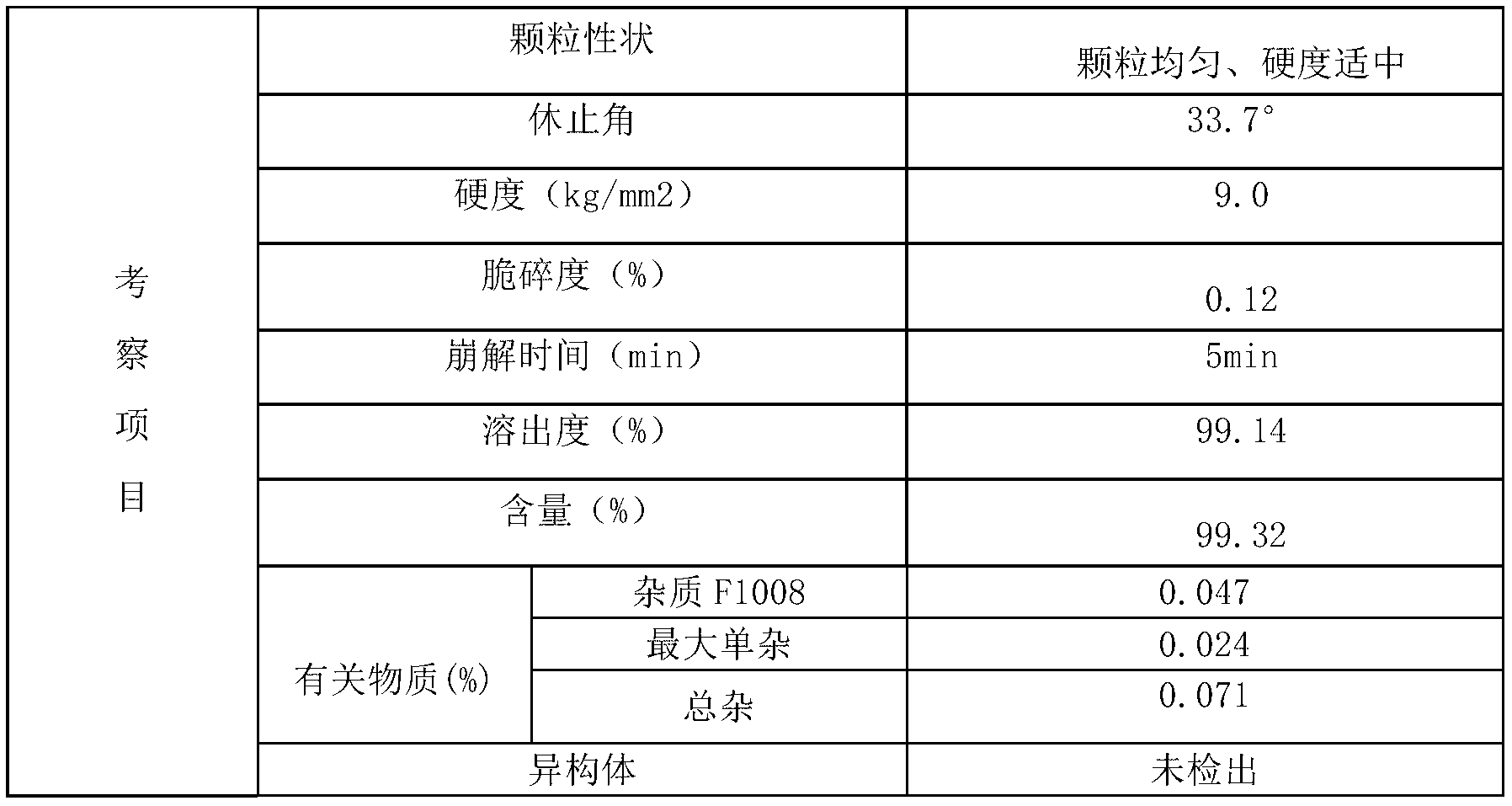
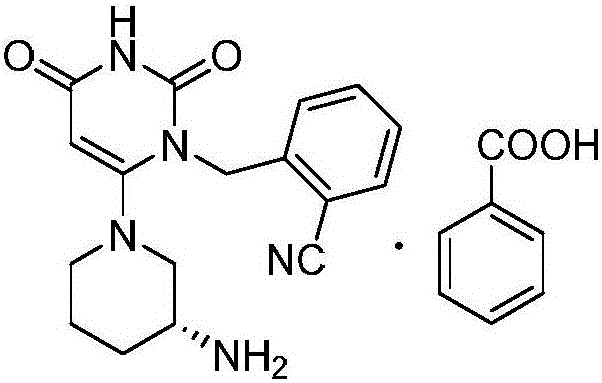
Benzoic acid alogliptin composition troche and preparation method thereof
InactiveCN103156819AReduced stabilityImprove stabilityOrganic active ingredientsMetabolism disorderBenzoic acidMANNITOL/SORBITOL
The invention relates to the field of pharmacy, and discloses a benzoic acid alogliptin composition troche and a preparation method thereof. The effective components of the benzoic acid alogliptin composition troche comprise benzoic acid alogliptin, mannitol, microcrystalline cellulose, croscarmellose sodium, magnesium stearate and 5% hydroxy propyl cellulose aqueous liquor. The synthetic materials are easy to obtain and low in cost. The troche is quick in disintegration speed, high in dissolution rate, good in stability, accurate in dosage and convenient to take and carry. The benzoic acid alogliptin composition troche is simple in step, short in production period, low in production cost and suitable for industrialized application, and the technical process is easy to control,.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Stimulators of incretin hormones secretion, method for preparation and use thereof
InactiveUS20140100216A1Improve treatment efficiencySimplifies direct careOrganic active ingredientsBiocideDiseaseTreatment effect
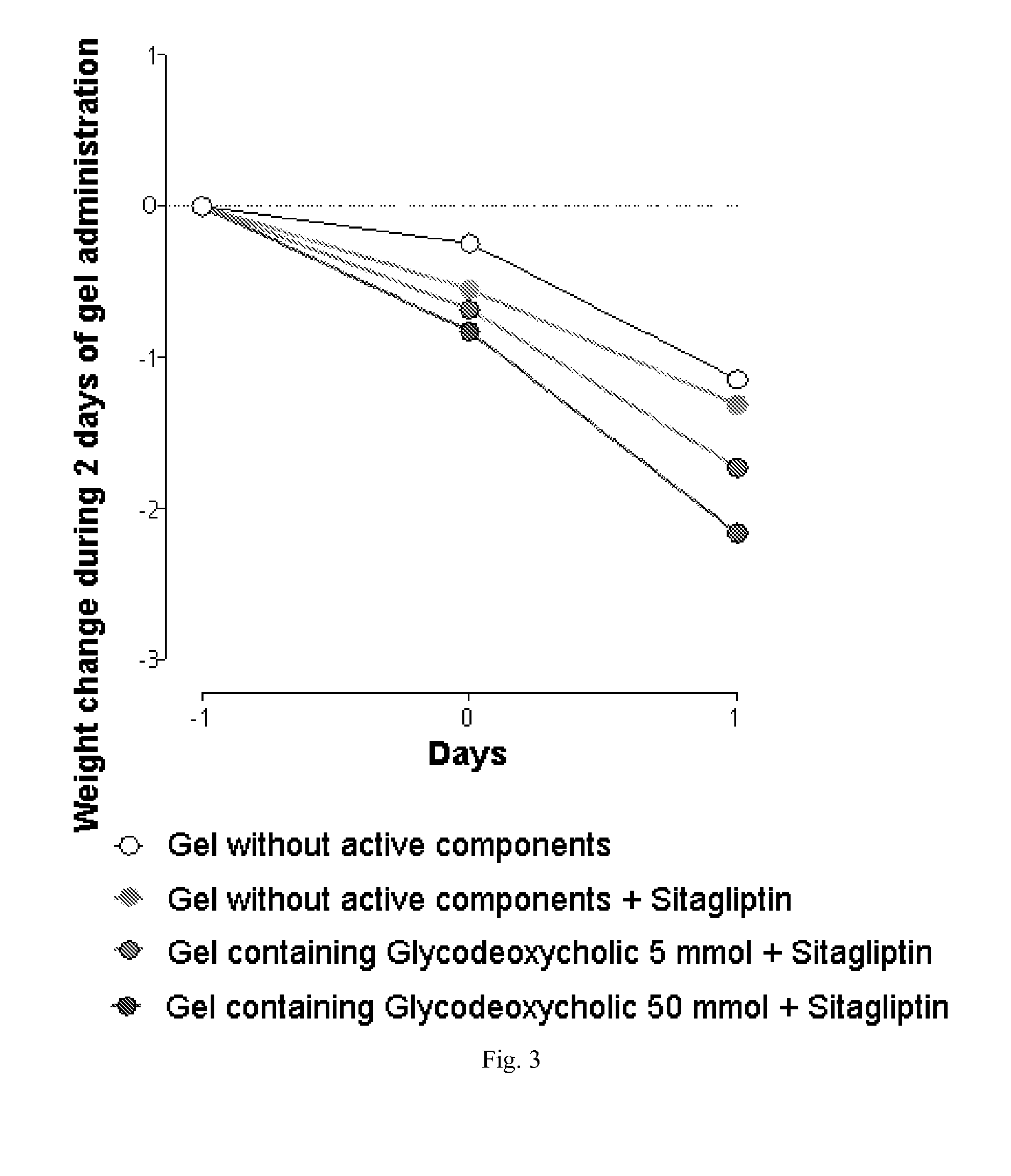
The invention relates to the area of medicinal chemistry, pharmacology and medicine and includes description of pharmaceutical compositions and combined medicaments on the base of secretion stimulators and protectors of incretin hormones for treatment of metabolic diseases (among them, diabetes, obesity, metabolic syndrome and the like). The invention consists in that that pharmaceutical composition or combined medicament comprises a derivative of tetrahydrobenzo[f][1,4]oxazepine—either nonsteroidal agonist of bile aids receptor TGR5, or one of endogenous bile acids which stimulate incretin hormones secretion, and also one of the known inhibitors of DPP-IV proteinase. In this case administration of TGR5 agonists is carried out peroral, and administration of endogenous bile acids is exercised rectal in the form of suppository or gel. As proteinase DPP-IV inhibitors could be used Vildagliptin, Saxagliptin, Sitagliptin, Teneligliptin, Linagliptin, Dutogliptin, Alogliptin, Gemigliptin, Carmegliptin and the like. Besides, the invention includes description of novel tetrahydrobenzo[f][1,4]oxazepine derivatives—nonsteroidal agonist of bile aids receptors TGR5, and also methods for their preparation. The invention provides enhancement of therapy effectiveness owing to synergetic action of the components, thus making possible simultaneous treatment of diabetes, and obesity, other metabolic diseases and their cardiovascular and renal complications.
Owner:SAVCHUK NIKOLAY FILIPPOVICH +2
Solid preparation comprising alogliptin and metformin hydrochloride
ActiveCN101801351AGood storage stabilityImprove featuresOrganic active ingredientsMetabolism disorderDiabetes mellitusAlogliptin
The present invention provides a solid preparation containing compound (I) [compound (I) is as defined in the specification] or a salt thereof, and metformin hydrochloride, which is useful as a therapeutic drug for diabetes and the like, and superior in the preservation stability. A solid preparation having a first part and a second part:a first part: a part containing compound (I) or a salt thereof and substantially free of metformin hydrochloride a second part: a part containing metformin hydrochloride and substantially free of compound (I) and a salt thereof.
Owner:TAKEDA PHARMA CO LTD
Industrial production method of Alogliptin benzoate raw material medicine
InactiveCN104803976AShorten production timeReduce production energy consumptionOrganic chemistryBiotechnologyBenzoic acid
The invention discloses an industrial production method of Alogliptin benzoate raw material medicine. According to the method, ethanol / water mixed solvents are used as crystallization solvents; the ethanol / water mixed solvents and Alogliptin benzoate crude products are heated and flow back to a state that the solution is clear; the temperature is lowered, and crystal seeds are added; the gradient temperature reduction crystal separation is carried out; centrifugation and drying are carried out, and the Alogliptin benzoate raw material medicine is obtained. The adopted mixed solvents have low toxicity, and a better environment-friendly effect can be achieved. Compared with the method in the prior art, the industrial production method provided by the invention has the advantages that the solvent use type is reduced, the process is simple, the yield is high, and the production cost is reduced; through the method, the purity of the obtained Alogliptin benzoate finished product is at least 99.8 percent, the single impurity content is lower than or equal to 0.05 percent, the ethanol residue is low (lower than or equal to 0.1 percent) and is much lower than the limit of 0.5 percent of medical raw material medicine; impurities X generated by the reaction between phthalic acid and Alogliptin can be removed.
Owner:SUZHOU YABAO PHARMA R&D CO LTD
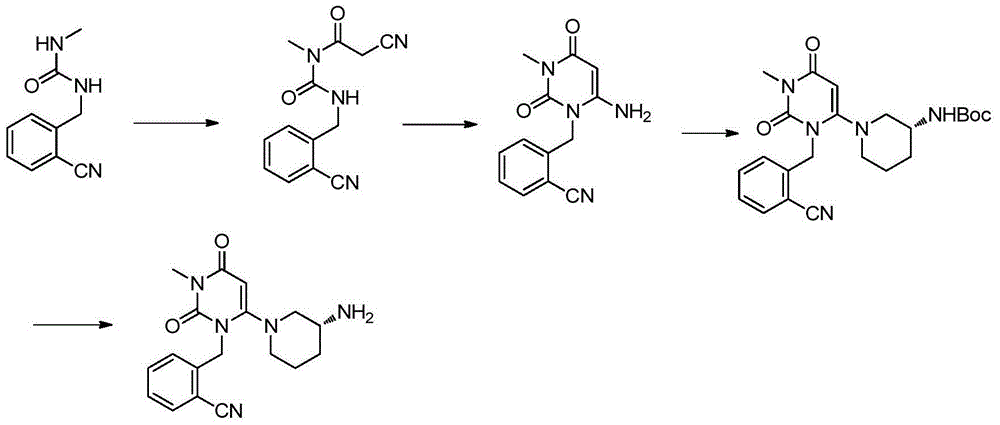
Synthesis method of Alogliptin intermediate
InactiveCN104151253ARaw materials are cheap and easy to getLow costOrganic chemistryMalonic acidAlogliptin
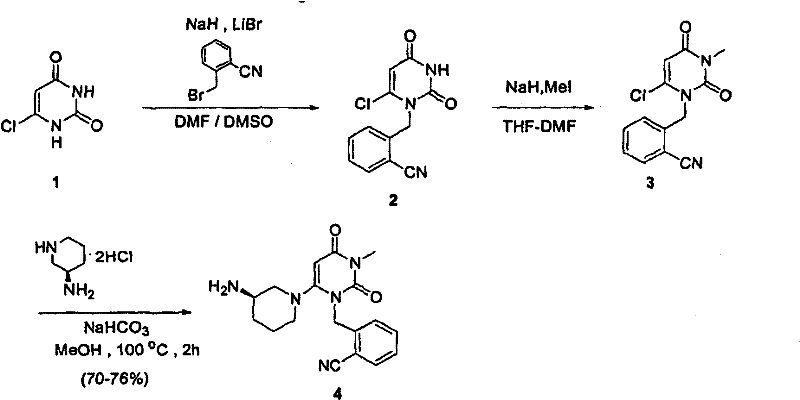
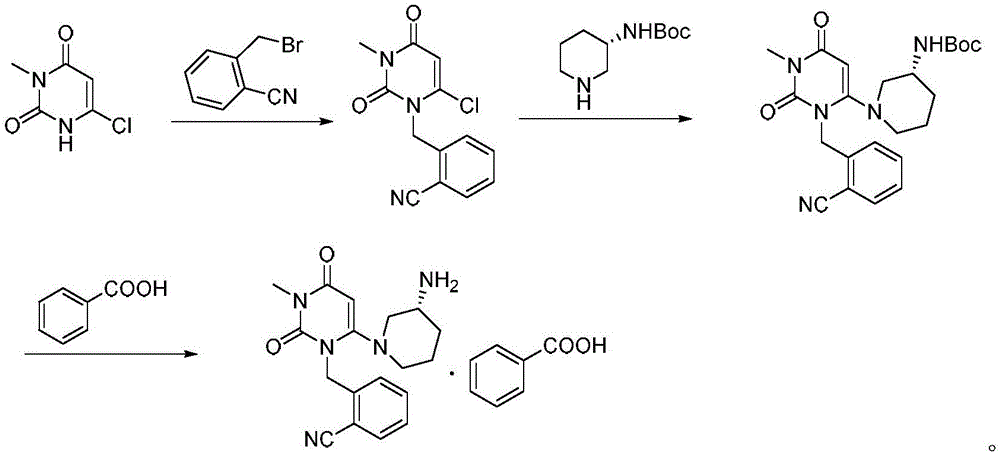
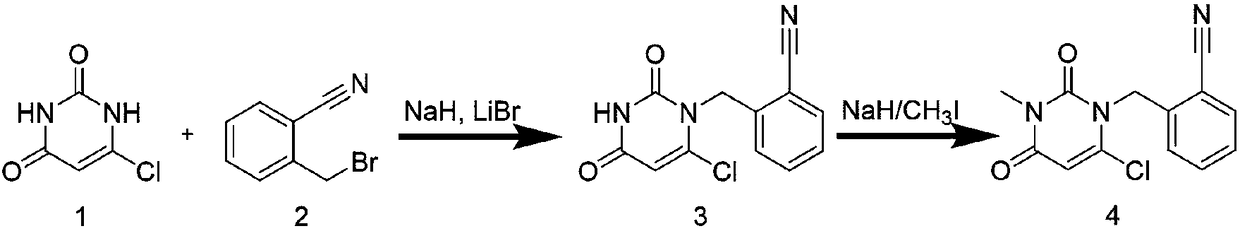
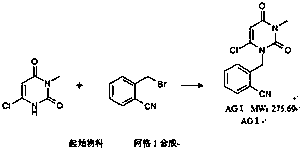
The invention provides a method for synthesizing Alogliptin intermediate 2-[(6-chlorine-3,4-dihydro-3-methyl-2,4-dioxo-1(2H)-pyrimidyl) methyl] cyanobenzene, and belongs to the field of preparation methods of chemical drug intermediates. The preparation method comprises the following steps: (1) obtaining 1-methyl barbituric acid through reaction of methylurea with malonic acid; (2) obtaining 3-methyl-6-chlorouracil through chlorination reaction of 1-methyl barbituric acid and phosphorus oxychloride; (3) obtaining Alogliptin intermediate 2-[(6-chlorine-3,4-dihydro-3-methyl-2,4-dioxo-1(2H)-pyrimidyl) methyl] cyanobenzene through reaction of 3-methyl-6-chlorouracil with 2-cyanobenzyl bromide. The synthesis method of Alogiptin intermediate adopts safe reagents and achieves good convenience in postprocessing and high yield rate of product preparation, thereby being suitable for industrial production.
Owner:SICHUAN TONGSHENG BIOTECH
Preparation method for alogliptin intermediate R-3-aminopiperidine dihydrochloride
ActiveCN103319399AHigh chiral purityReduce stepsOptically-active compound separationOrganic racemisationFluoboric acidChirality
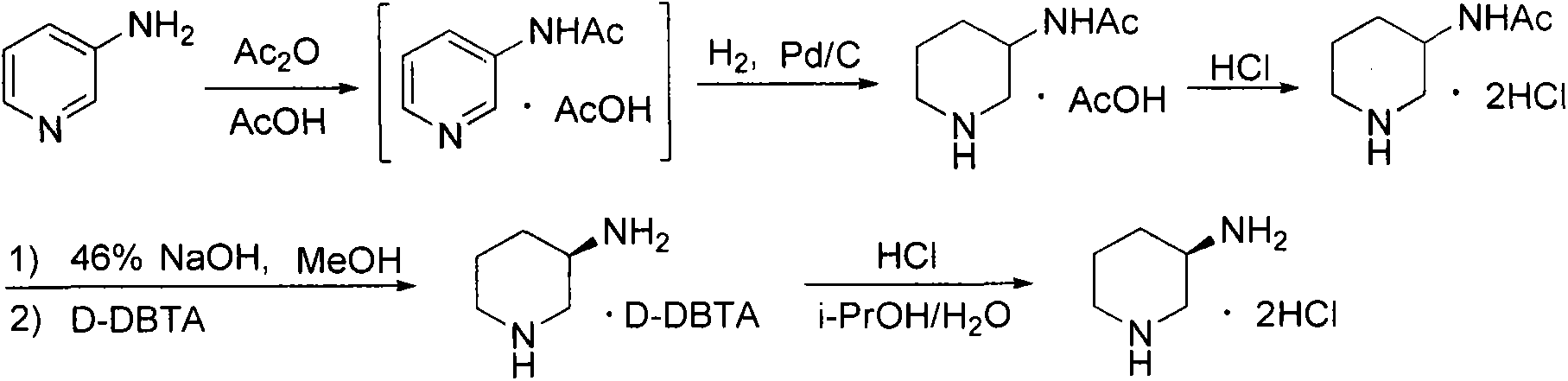
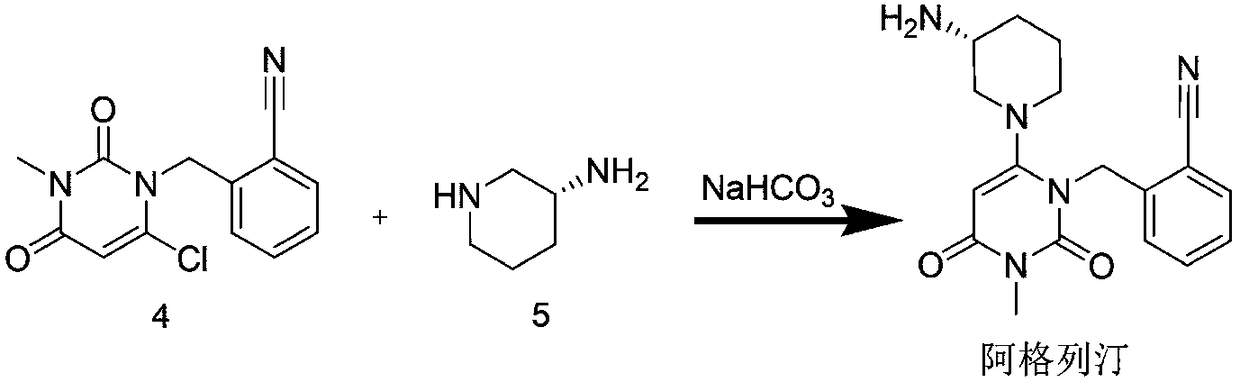
The invention aims to provide a preparation method for an alogliptin intermediate R-3-aminopiperidine dihydrochloride. The method has a brief route and is environmentally friendly and low-cost. A racemic compound 3- piperidine carboxamide which is cheap and easy to get is taken as a raw material, and is subjected to Hoffmann rearrangement reaction under the action of 1- fluoronaphthalene, hydrogen peroxide and fluoboric acid, which is similar to acid amides, to obtain 3-aminopiperdine which has one less carbon than the substrate. The method has simple reaction conditions, can be implemented at the room temperature, has simple operations in process, and is easy to monitor. The solvents are an ethanol-water mixture, and are low-cost and environmentally friendly. The carbon-lessened product 3-aminopiperidine is acidized and salified by concentrated hydrochloric acid. The hydrochloride is separated and salified by D-tartaric acid to obtain the target product R-3-aminopiperidine dihydrochloride. The chirality purity is high. The ee value is more than 99.5%. The reaction overall yield can reach 89%-93%. The cost is low. The method is suitable for industrial mass production.
Owner:迪嘉药业集团股份有限公司
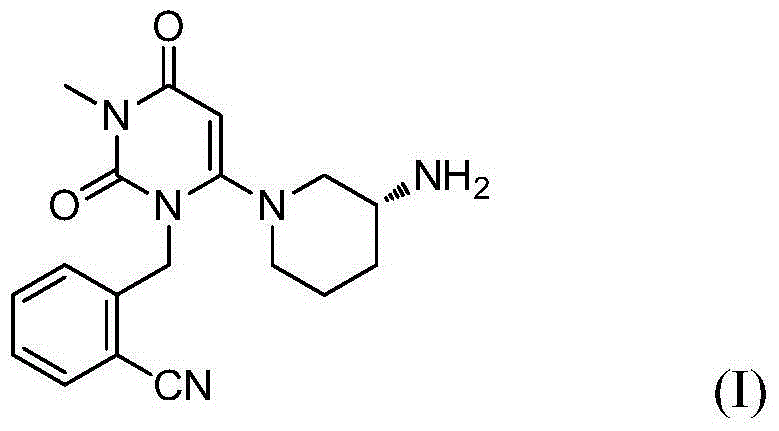
An alogliptin purifying method
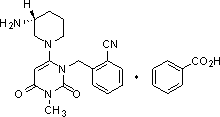
The invention relates to an alogliptin purifying method through separating an alogliptin dimer impurity, and an alogliptin preparing method including the purifying method. The purifying method includes (1) providing an ethanol solution of 2-[6-[(3R)-aminopiperidin-1-yl]-3-methyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-1-yl-methyl]benzonitrile, (2) adsorbing the alogliptin dimer impurity through adsorption, and filtering, and (3) crystallizing the filtrate and separating to obtain crystals. The purifying method is characterized by being simple, convenient and efficient in operation, low in cost, environmental friendly, and outstanding in effects, and is suitable for industrial large-scale production.
Owner:瀚晖制药有限公司 +1
Mass production process of polycrystalline high-content benzoic acid alogliptin
The invention relates to a mass production process of polycrystalline high-content benzoic acid alogliptin. According to the process, the products do not need to be refined and the qualified products conforming to the pharmaceutical production demand can be obtained directly. The process comprises the following steps: putting 5kg of the benzoic acid into a 200L reaction kettle; adding 100L of pure water; stirring, heating and refluxing for 1 hour; centrifuging after the reaction liquid is decreased to 50 to 60 DEG C; filtering into a filter cake in a pressing manner by using a plate-and-frame filter press and performing the forced air drying at the temperature of 60 to 65 DEG C for 10 to 12 hours, thereby obtaining 2.41kg of a white needle-shaped crystal benzoic acid refined product; putting 3.11kg of alogliptin and 1.13kg of the white needle-shaped crystal benzoic acid refined product into the reaction kettle; adding 15L of a solvent; heating and dissolving; heating until refluxing for 2 to 3 hours; stopping the heating until separating out white solids; naturally cooling to the room temperature; stirring and separating out a crystal for 10 to 12 hours; centrifuging; washing the obtained solid by using 1L of the solvent; and performing the forced air drying for 8 to 10 hours at the temperature of 40 to 45 DEG C, thereby obtaining 3.82kg of the finished polycrystalline high-content benzoic acid alogliptin product.
Owner:合肥拓锐生物科技有限公司
Reagent for enhancing capacity of homing CAR-T cell to solid tumor tissue
PendingCN109593726AEnhanced tumor homingEnhanced tumor enrichment capacityPolypeptide with localisation/targeting motifOrganic active ingredientsAntigen receptorsTherapeutic effect
The invention belongs to the technical field of the biological medicine, and specifically relates to a CAR-T cell, a reagent for enhancing capacity of homing CAR-T cell to solid tumor tissue, and application thereof. The reagent is a DPP4 inhibitor, includes but not limited to sitagliptin, vildagliptin, saxagliptin, alogliptin and linagliptin. The CAR-T cell expression includes ScFv, a hinge structure and a transmembrane structure, and a chimeric antigen receptor (CAR) of an intracellular activation signal domain; an amino acid sequence of the hinge structure is as shown in SEQ ID NO.1 or SEQID NO.2 or SEQ ID NO.3 or SEQ ID NO.4 or SEQ ID NO.5. The invention provides a new application of the DPP4 inhibitor for participating tumor treatment. The reagent can enhance the tumor homing capacity and tumor enrichment capacity of the CAR-T cell, and a therapeutic effect on the solid tumor by the CAR-T therapy is improved.
Owner:CHONGQING PRECISION BIOTECH CO LTD
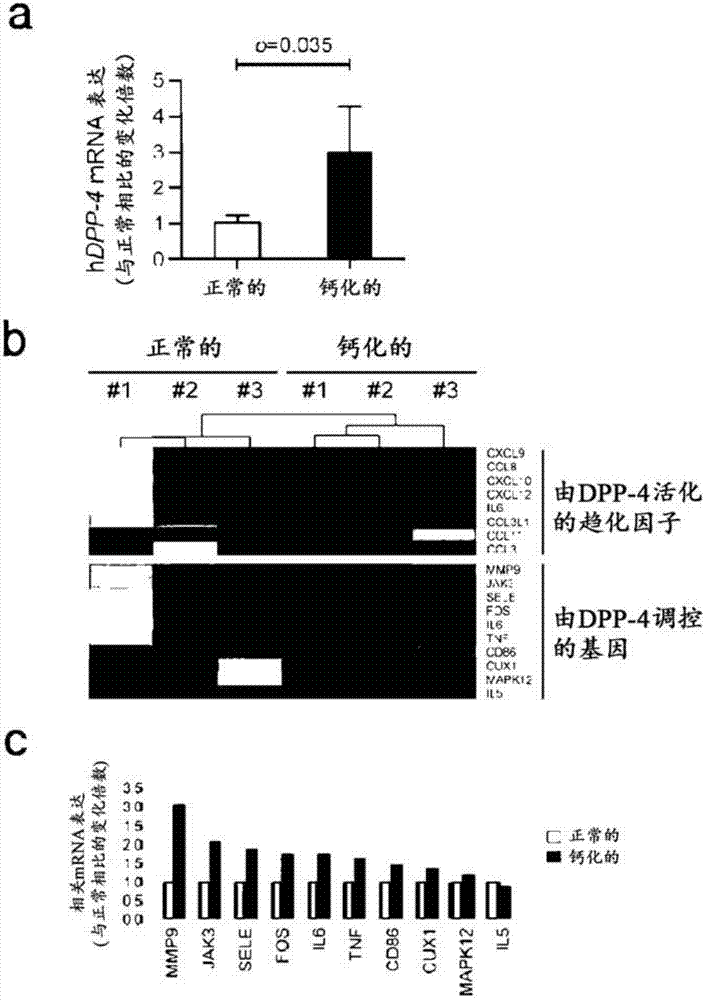
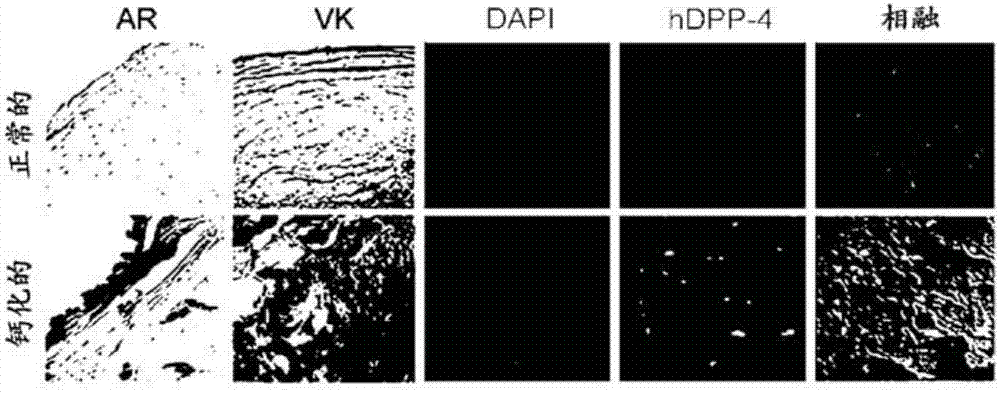
Composition for preventing or treating valve calcification, containing dpp-4 inhibitor
The present invention relates to a composition for preventing or treating valve calcification, containing a dipeptidyl peptidase-4 (DPP-4) inhibitor. The DPP-4 inhibitor according to the present invention may include all of what can inhibit the expression of DPP-4 nucleotides or the activity of DPP-4 proteins, wherein: DPP-4 antibodies, sitagliptin, vildagliptin, saxagliptin, linagliptin, dutogliptin, gemigliptin, alogliptin, Anagliptin, evogliptin, Berberine, Diprotin, or Lupeol; DPP-4 mRNA anti-sense nucleotides, aptamers, small interfering RNA (siRNA), short hairpin RNA (shRNA), and microRNA (miRNA), or RNA interference (RNAi); or the like can be used.
Owner:THE ASAN FOUND +1
Composition of anti-diabetic drugs
InactiveCN105030724AAvoid contactTo avoidOrganic active ingredientsMetabolism disorderFiller ExcipientAdhesive
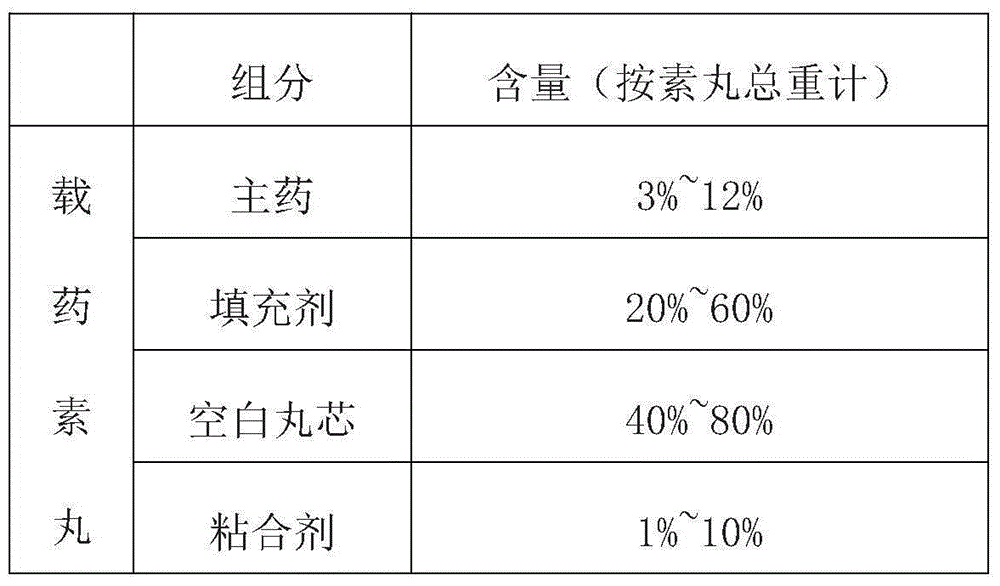
The invention provides a composition of anti-diabetic drugs. The composition comprises alogliptin coated pellets, pioglitazone coated pellets and a gelatin capsule shell and is used for treating type II diabetes, wherein blank pellets comprise main drugs, filling agents and adhesives; in the total blank pellets, the main drugs account for 3-12wt%, the filling agents account for 20-60wt%, blank pellet cores account for 40-80wt% and the adhesives account for 1-10wt%; the weights gained by coating layers are about 2-10% of the weights of the drug-loaded blank pellets. Alogliptin and pioglitazone pellets with different coating colors are preferentially prepared. The composition has the advantages of simple and reliable production process, low loss, good product stability and few by-products, so that the composition is beneficial to large-scale industrial production.
Owner:杭州成邦医药科技有限公司
Preparation method for benzoic acid alogliptin polycrystalline type crystal
ActiveCN104151291AReduce pollutionMild reaction conditionsOrganic chemistry methodsBenzoic acidAlcohol
The invention relates to a preparation method for a benzoic acid alogliptin polycrystalline type crystal and belongs to the technical field of medicinal chemistry. The preparation method comprises the following steps: methyl alcohol is added into a reaction still and stirred, benzoic acid alogliptin crude product obtained after synthesis is added, heating reflux is carried out for 15-30 minutes, the benzoic acid alogliptin crude product is filter-pressed into a refining-drying-packing crystallization kettle and stirred, methyl tertiary butyl ether is pressed into the crystallization kettle, the mixture is subjected to natural cooling to the room temperature, stirring and crystallization for 3-4 hours and centrifugation to obtain a solid, and the solid is washed with methyl alcohol and dried at 45-55 DEG C through blasting to obtain the benzoic acid alogliptin polycrystalline type crystal. The obtained benzoic acid alogliptin polycrystalline type crystal is low in impurity content and higher in medicine quality.
Owner:JIANGSU DEYUAN PHARMA
Process for the preparation of alogliptin
The present invention is based on the discovery of a process for preparing pyrimidin-dione compounds, especially alogliptin and its derivatives, which comprises the reaction of a urea derivative of formula (VIII) with a malonic acid or its derivatives to form intermediates of formulae (VII) or (VII-A), which are subsequently converted to a compound of formula (II) upon introduction of a leaving group X. Compound (II) then reacts with an amine to form compound (I), which is optionally converted to its salts of formula (IV).
Owner:MAPI PHARMA
Method for preparing drug Alogliptin for treating diabetes type II
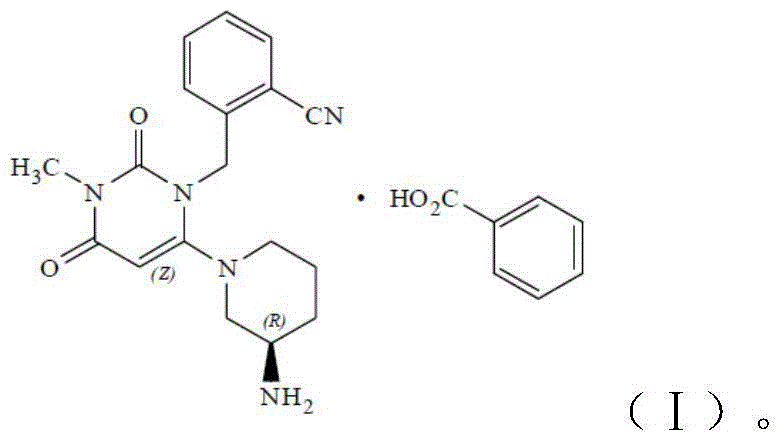
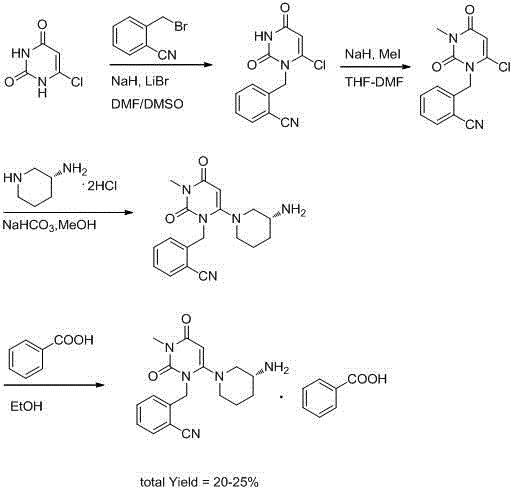
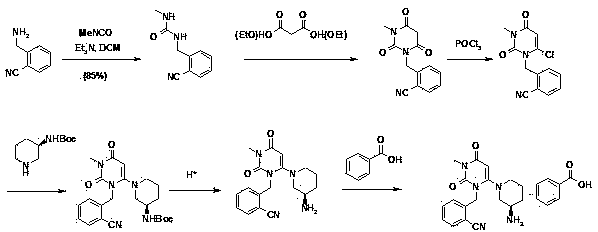
The invention discloses a method for preparing alogliptin, a medicine for treating type II diabetes. The method comprises: 1) in the presence of copper acetate and triethylamine, 6-chloro-3-methyluracil and 2-cyano Benzyl bromide is carried out contact reaction in acetonitrile, and after reaction finishes, reaction solution is poured into water, filters, washes with water, dries to obtain 6-chloro-1-(2-isocyanobenzyl)-3-methylpyrimidine-2, 4-(1H,3H)-dione; 2) 6-chloro-1-(2-isocyanobenzyl)-3-methylpyrimidine-2,4-(1H,3H)-dione, (R )‑3‑tert-butyloxycarbonylaminopiperidine and potassium carbonate are mixed in DMF. After the reaction, add water, then extract with dichloromethane and concentrate to obtain (R)‑tert-butyl‑1‑(3‑ (2‑isocyanobenzyl)‑1‑methyl‑2,6‑dioxo‑1,2,3,6‑tetrahydropyrimidin‑4‑yl)piperidin‑3‑ylcarbamate; 3 ) dissolving the formate ester obtained in step 2) in ethanol and reacting with benzoic acid at 65-70°C, then cooling to -5-5°C for crystallization, filtering and drying to obtain alogliptin. The invention has simple steps, high yield and faster reaction.
Owner:QINGDAO CHENDA BIOLOGICAL SCI & TECH
Preparation method of alogliptin benzoate impurity
InactiveCN107556249AEasy to operateThe reaction conditions are mild and controllableOrganic chemistryBenzoic acidDiketone
The invention relates to a preparation method of alogliptin benzoate impurity and belongs to the technical field of pharmaceutical chemicals. The preparation method of alogliptin benzoate impurity provided by the invention comprises the following steps: (1) preparing TM1 2-((6-chloro-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)methyl)cyanophenyl by taking 6-chloro-3-methylpyrimidin-2,4(1H,3H)-diketone as a raw material which reacts with 2-bromomethyl cyanophenyl; (2) preparing alogliptin benzoate impurity BP1 through a reaction between the TM1 and monohydric alcohol in an alkaline condition, wherein the structural formula of the BP1 is shown in the description. The preparation method provided by the invention is convenient to operate, the reaction conditions are mild and controllable,the side reactions are reduced, and therefore, the target product alogliptin benzoate is easy to separate and purify and has high purity.
Owner:山东淄博新达制药有限公司
Pharmaceutical composition of alogliptin and metformin
ActiveUS20180235966A1Improve blood sugar controlComposition is stableOrganic active ingredientsPill deliveryAlogliptinGlycemic
Present invention relates to a stable pharmaceutical composition comprising intimate mixture of alogliptin and metformin, and suitable pharmaceutically acceptable excipient / s; wherein metformin is present in about 3.3 parts or more parts by weight relative to 100 parts by weight of the total weight of part comprising alogliptin. Invention also encompasses various processes of preparing said pharmaceutical composition and its use in improving glycemic control in adults with type 2 diabetes mellitus.
Owner:TORRENT PHARMA LTD
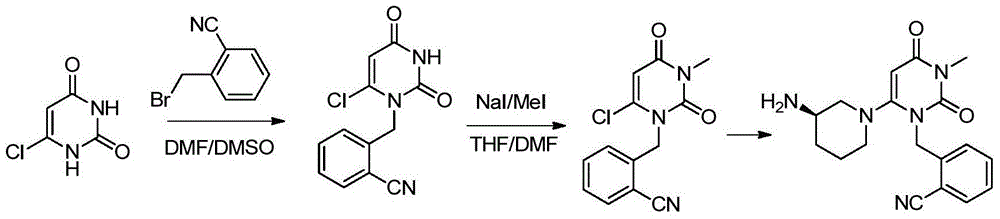
Preparation method of alogliptin intermediate
The invention discloses a preparation method of an alogliptin intermediate. The method comprises the following steps of adding 10.0g of raw material compound into 100ml of mixed solvent of DMSO (dimethyl sulfoxide) / DMF (dimethyl formamide) in a volume ratio of 1 to 1, stirring at normal temperature to dissolve, after dissolving, adding 2.5g of sodium hydride, 2.5g of lithium bromide and 5g of composite oxide; dissolving 13.4g of raw material compound 2 in 30ml of mixed solvent of DMSO / DMF in a volume ratio of 1 to 1, adding the solution of the compound 2 slowly into a mixed solution of the compound 1 in a stirring condition, and stirring to react for 12 hours; filtrating a reaction solution, and concentrating to obtain a crude product; purifying by using silica gel column chromatography, and then obtaining an intermediate compound 3. The composite oxide can be used for effectively catalyzing and synthesizing the alogliptin intermediate. The preparation method provided by the inventioneffectively improves the yield of the alogliptin intermediate.
Owner:张正光
Industrial production method of Alogliptin benzoate
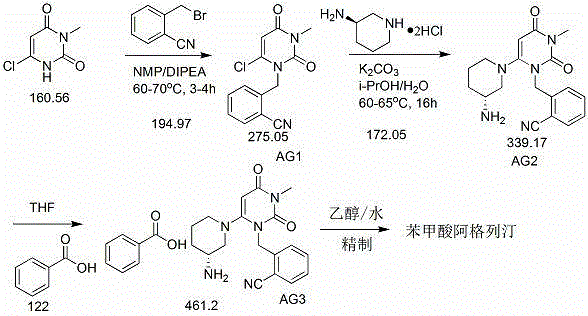
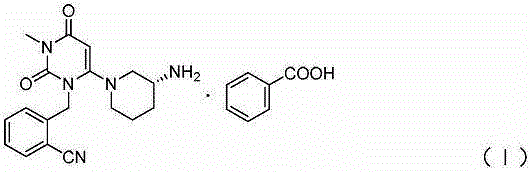
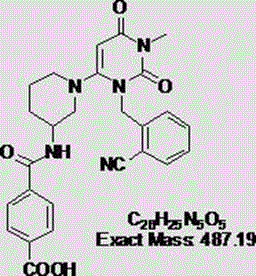
InactiveCN109232532AReduce pollutionAppropriate reaction conditionsCarboxylic acid salt preparationBenzoic acidAlogliptin
The invention relates to an industrial production method of Alogliptin benzoate, and belongs to the technical field of industrial pharmacy. The method comprises three steps of 1, preparing an Alogliptin intermediate AG I; 2, preparing an Alogliptin intermediate AG II; 3, preparing the Alogliptin benzoate. The industrial production method has the beneficial effects that the reaction conditions areproper; the operation is simple and convenient; the realization is easy; only the Alogliptin intermediate AG II is prepared; then, the AG II and benzoic acid are subjected to tetrahydrofuran synthesis; the Alogliptin benzoate can be obtained; the process is saved; the cost is reduced; used solvents have small environment pollution; the operation is safe; under the large-scale industrial productioncondition, the existing average yield is only about 30 percent; under the condition that the final yield reaches 19.32kg, the finial product yield reaches 94.77 percent; the total yield reaches 60.11percent; high quality and purity can be maintained; the production efficiency is greatly improved.
Owner:NANHAI PHARMA CHONGQING
Method of preparing Alogliptin
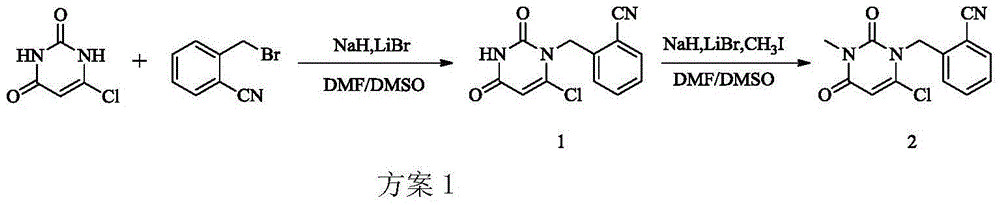
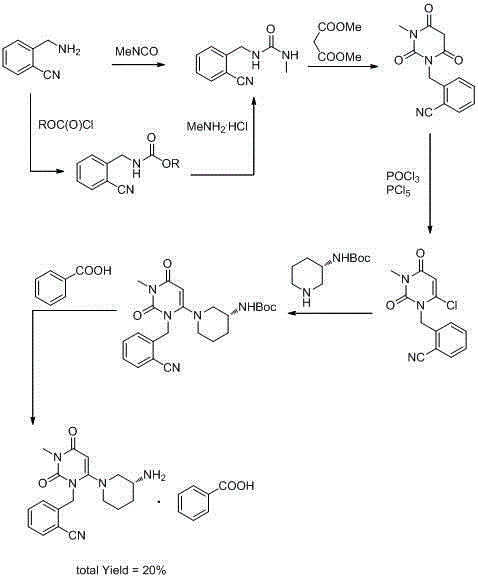
The invention relates to Alogliptin, benzoate thereof and a preparation method of an intermediate of the Alogliptin. The method comprises the following steps: alkylating 6-chlorouracil by using 2-(bromomethyl)benzonitrile on the basis of a tetrahydrofuran solvent and the catalysis of alkali to generate N-benzyluracil derivatives, and further alkylating by using methanol through a Mitsunobu reaction to obtain 1,3-disubstituted uracil (intermediate). The method does not need a NaH risk agent and highly toxic agent methyl iodide in the prior art, and is safe and environment-friendly.
Owner:CHONGQING PHARMA RES INST
Method for synthesizing alogliptin intermediate
The present invention provides a method for synthesizing an intermediate V. The method comprises: (a) allowing a compound I and a compound II to react while being heated in a solvent under an alkaline condition to obtain an intermediate III; and (b) the intermediate III reacting with a compound IV in the solvent under the alkaline condition to obtain the intermediate V. The intermediate V can be used to synthesize alogliptin.
Owner:SHANGHAI SYNCORES TECH INC
DPP-4 inhibitor with dual action mechanisms and application of DPP-4 inhibitor
InactiveCN108558836AInhibits DPP-4 enzyme activityOrganic active ingredientsNervous disorderAlogliptinDual action
The invention discloses a DPP-4 inhibitor with dual action mechanisms and application of the DPP-4 inhibitor, relates to the field of dipeptidyl peptidase IV inhibitor medicines and in particular relates to a DPP-4 inhibitor, a preparation method of a compound of formula I and medicinal application of the compound as a novel DPP-4 inhibitor, and particularly application of the inhibitor in preparing anti-diabetic medicines. In-vivo and in-vitro pharmacodynamic experiment results show that the compound of formula I has a remarkable inhibition function on DPP-4, has an excellent blood sugar reduction effect, and in addition has a relatively good action effect when being compared with similar medicines alogliptin and vildagliptin. Meanwhile, the compound disclosed by the invention is simple and short in preparation route, easy in raw material obtaining, simple in process, applicable to industrial large-scale production and very great in development prospect.
Owner:SOUTHEAST UNIV
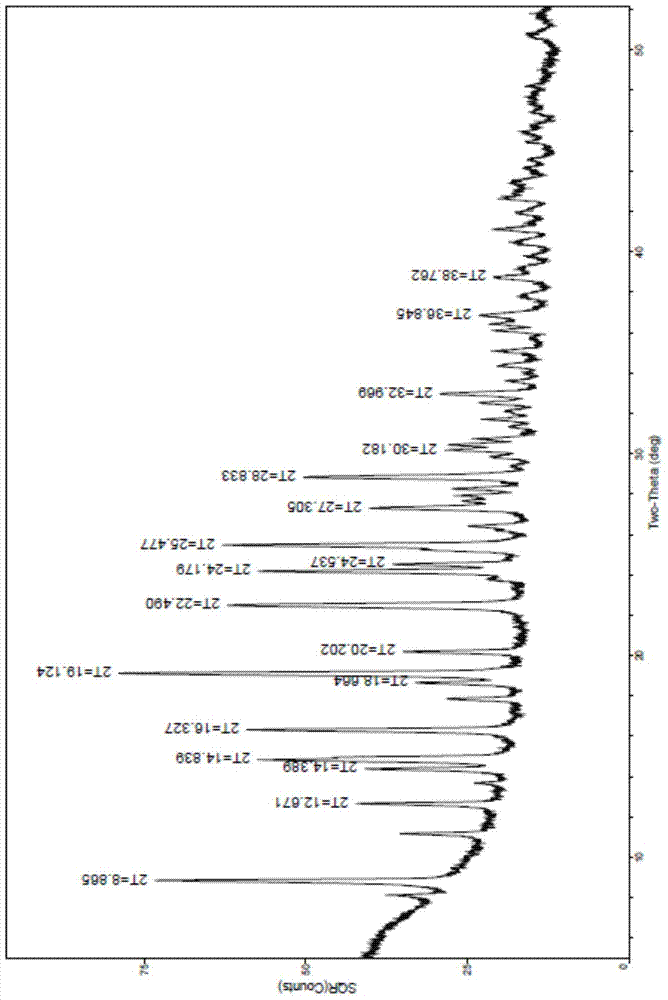
Polycrystalline B-type crystal of alogliptin hydrochloride, preparation method and production purpose thereof
The invention discloses a polycrystalline B-type crystal of alogliptin hydrochloride and a preparation method thereof. The polycrystalline B-type crystal is prepared by an x-ray powder diffraction method of Cu-K alpha radiation, and the x-ray powder diffraction comprises peaks with 2 theta being 8.87+ / -0.2 degrees, 12.67+ / -0.2 degrees, 14.39+ / -0.2 degrees, 14.84+ / -0.2 degrees, 16.33+ / -0.2 degrees, 18.66+ / -0.2 degrees, 19.12+ / -0.2 degrees, 20.20+ / -0.2 degrees, 22.49+ / -0.2 degrees, 24.18+ / -0.2 degrees, 24.54+ / -0.2 degrees, 25.48+ / -0.2 degrees, 27.31+ / -0.2 degrees, 28.83+ / -0.2 degrees, 30.18+ / -0.2 degrees, 32.97+ / -0.2 degrees, 36.85+ / -0.2 degrees and 38.76+ / -0.2 degrees. The high-purity crystal obtained can be precipitated out of a system, a dimer by-product in the reaction can be effectively removed, production efficiency is raised, and production cost is reduced.
Owner:湖北华世通生物医药科技有限公司
Novel capsule filled with alogliptin solid preparation and pioglitazone solid preparation
InactiveCN103356621AMeet the safety rangeWill not happenOrganic active ingredientsMetabolism disorderAlogliptinHard Capsule
The invention relates to an oral-taking hard capsule, and in particular relates to a tablet capsule filled with an alogliptin tablet and a pioglitazone tablet. The tablet capsule comprises a capsule and tablets in the capsule, wherein the capsule contains an upper capsule body and a lower capsule body; at least one tablet taking alogliptin as an active component and at least one tablet taking pioglitazone as an active component are filled in the capsule. As the active components in the capsule are not in contact with each other, chemical compatibility among various components does not occur and a byproduct is not produced, so that the stability of the product is improved, especially a degradation product produced through the contact of alogliptin and pioglitazone is reduced, and related substances of the tablet capsule conform to the safety range of a human body.
Owner:HEILONGJIANG FUHE HUAXING PHARMA GROUP
Process for the preparation of alogliptin
The present invention is based on the discovery of a process for preparing pyrimidin-dione compounds, especially alogliptin and its derivatives, which comprises the reaction of a urea derivative of formula (VIII) with a malonic acid or its derivatives to form intermediates of formulae (VII) or (VII-A), which are subsequently converted to a compound of formula (II) upon introduction of a leaving group X. Compound (II) then reacts with an amine to form compound (I), which is optionally converted to its salts of formula (IV).
Owner:MAPI PHARMA
Alogliptin-metformin sustained-release tablet and preparation method thereof
InactiveCN105287581AExcellent sustained slow release effectSteady slow release effectOrganic active ingredientsMetabolism disorderSustained Release TabletImmediate release
The invention provides an alogliptin-metformin sustained-release tablet consisting of a metformin hydrochloride sustained-release portion and an alogliptin benzoate immediate-release portion. The alogliptin-metformin sustained-release tablet comprises the following ingredients by weight percentage as shown in the description. Through series of experiments, the alogliptin-metformin sustained-release tablet provides favorable conditions for rapid absorption of alogliptin benzoate in vivo, and metformin hydrochloride achieves more sustained and stable sustained-release effect. Compared with common preparations, the alogliptin-metformin sustained-release tablet has the advantages that the alogliptin-metformin sustained-release tablet is rapider and more stable in release and can enable a diabetes patient to keep stable blood sugar concentration for the whole day. The alogliptin-metformin sustained-release tablet is taken once per day conveniently and has the combination medication advantage and high clinical application value. A preparation method of the alogliptin-metformin sustained-release tablet is good in reproducibility and stability and suitable for industrial production.
Owner:SHANGHAI SUNTECH PHARMA
DDP-4 inhibitor drug orally disintegrating tablets and preparing method
InactiveCN106913535AGood content uniformityStable in natureOrganic active ingredientsMetabolism disorderDrug contentOrally disintegrating tablet
The invention relates to DDP-4 inhibitor drug orally disintegrating tablets and a preparing method. The DDP-4 inhibitor drug orally disintegrating tablets comprise a DDP-4 inhibitor and pharmaceutic adjuvants. The pharmaceutic adjuvants include a diluent, a disintegrant, a corrigent, a lubricant and a glidant, but do not include a binder. By the adoption of the technical scheme, the problems of drugs like Alogliptin prepared through wet granulation that drug content is not uniform and more impurities are generated during long-term storage due to migration of soluble medicine composition particles during drying of wet particles are effectively solved, and an idea is provided for drugs with similar properties.
Owner:JIANGSU WANBANG BIOPHARMLS +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com