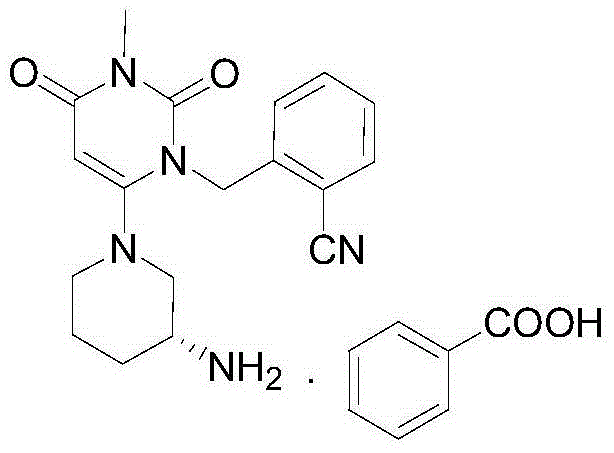
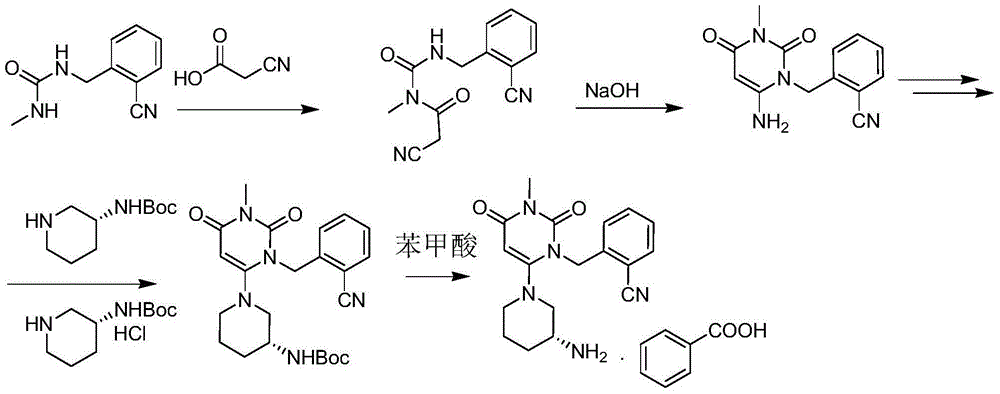
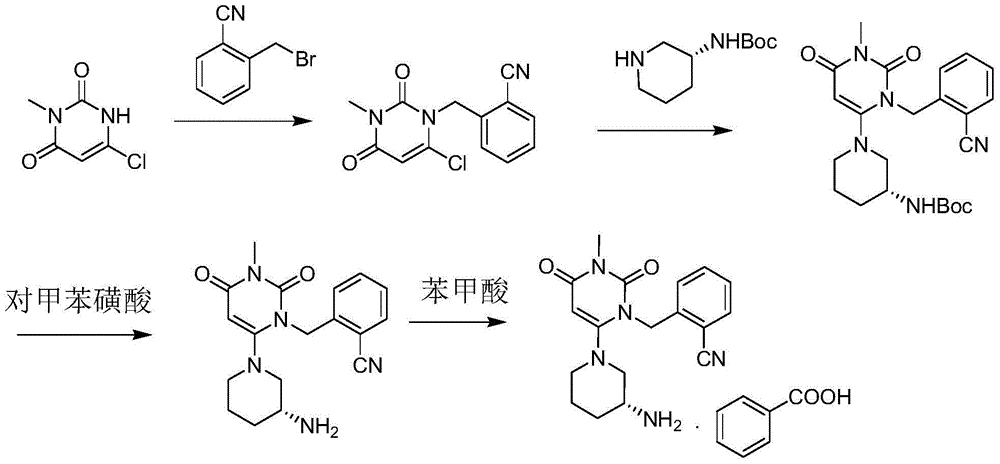
Method for synthesizing alogliptin intermediate
A technology for intermediates and compounds, applied in the field of intermediates for the production of dipeptidyl peptidase inhibitor alogliptin, can solve problems such as troublesome handling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]
Embodiment 1-1
[0042] Add 6-chloro-3-methyluracil (30.2g, 0.19mol) and (R)-3-tert-butoxycarbonylaminopiperidine (37.4g, 0.19mol) into a 500ml reaction flask, add 180ml of acetonitrile, 36.0 g (0.28 mol) of N,N-diisopropylethylamine was stirred and heated to 78-82°C, and reacted for 10 hours. Stop heating, cool to 15-25°C, filter under reduced pressure, rinse the filter cake with 100ml of acetonitrile and drain to obtain 55.1g of off-white solid, yield: 90.8%. 1 HNMR(DMSO)δ: 10.57(br,1H), 6.87(d,1H), 4.79(s,1H), 3.59(dd,1H), 3.02(s,3H), 2.84~2.69(br,2H), 1.76~1.63(br,2H), 1.57~1.41(br,2H), 1.36(s,9H); 1.22~1.25(br,2H); MS + = 325.2.
Embodiment 1-2~1-12
[0044] Referring to Example 1-1, change the substituent R 2 , alkali, solvent and alkali and the mol ratio of I, yield result is as shown in table 1:
[0045] Table 1
[0046]
[0047]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com