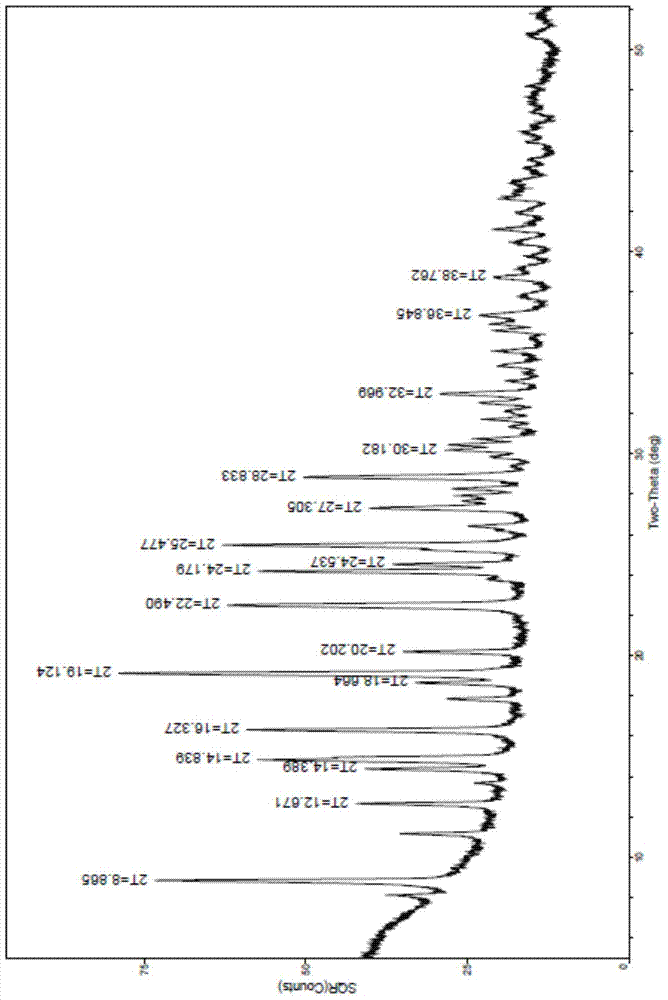
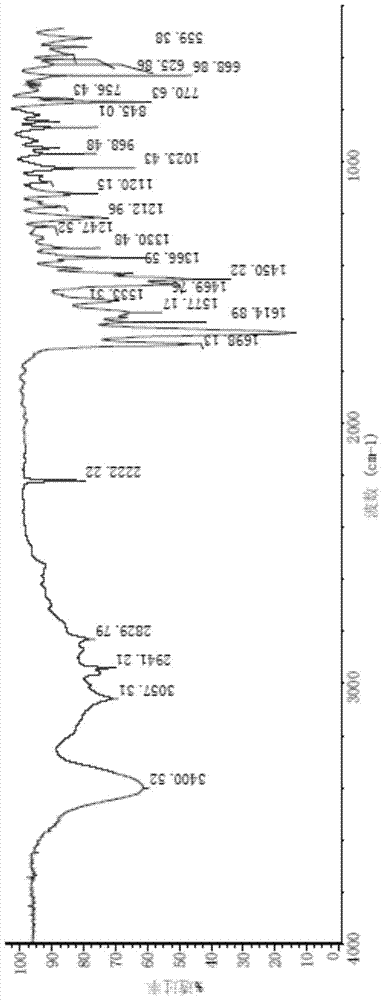
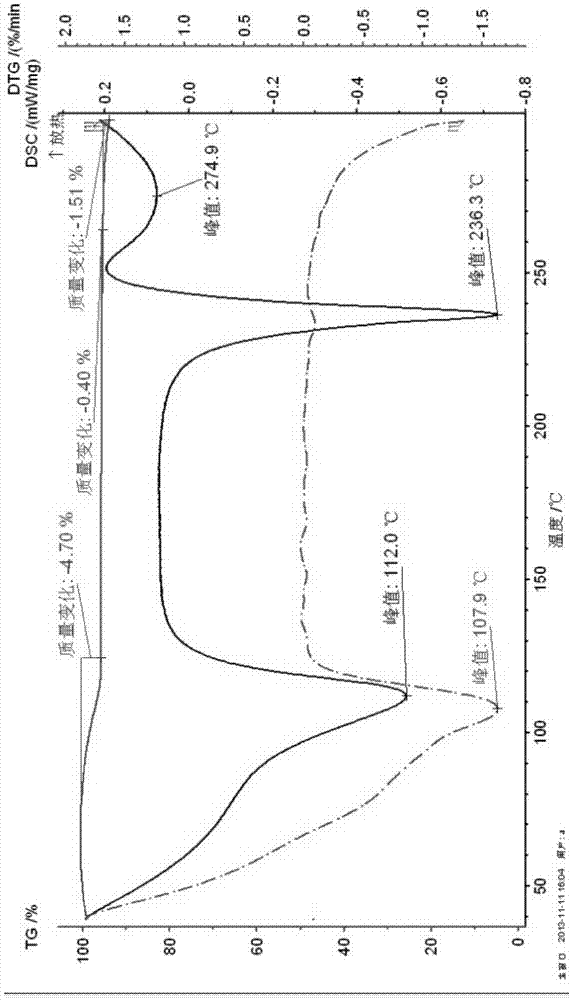
Polycrystalline B-type crystal of alogliptin hydrochloride, preparation method and production purpose thereof
A hydrochloride, crystal technology, applied in the field of crystals of pharmaceutical compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1 Alogliptin reaction solution preparation
[0052]Add 400ml of absolute ethanol into a 1L three-necked reaction flask, stir, and then add 80.5g of 2-(6-chloro-3-methyl-2,4-dioxo-3,4-dihydro-2-H-pyrimidine- 1-ylmethyl)benzonitrile, 53.1g of (R)-3-aminopiperidine dihydrochloride and 68.1g of sodium carbonate, the system was heated up to 75°C for reaction, and the direct raw material reaction was completed.
[0053] After the reaction, the system was cooled to below 40° C., the inorganic salts were removed by filtration, and the filter cake was washed twice with 80 ml of absolute ethanol. Collect the mother liquor for later use (see the attachment for HPLC test results) Figure 4 : 3.8% of the dimerized by-product percentage of general formula (A) is arranged in the mother liquor, and 19.5min is the peak time of the dimerized by-product of general formula (A).
Embodiment 2
[0054] Example 2 Preparation of Polymorph B of Alogliptin Hydrochloride
[0055] Concentrate 112ml of the mother liquor of Example 1 to remove the solvent, add 80.6ml of absolute ethanol to dissolve it, transfer it to a 250ml third-product reaction bottle, stir it magnetically, cool the system to 0-5°C, add 1.5 equivalents of hydrochloric acid dropwise, and control the temperature at 0-5°C. 10°C. After dropping, keep warm at 0-10°C for 1h. Suction filtration, the filter cake was washed twice with 10ml of absolute ethanol, and vacuum-dried at 50-60°C to obtain 17.6g of the polycrystalline type B product of alogliptin hydrochloride (see the attached Figure 5 : In the product, the percentage of dimerized by-products of general formula (A) is ≤0.1%, and 19.5min is the peak time of the dimerized by-products of general formula (A).
Embodiment 3
[0056] Example 3 Preparation of Crystal Form B of Alogliptin Hydrochloride
[0057] Concentrate 56ml of the mother liquor of Example 1 to remove the solvent, add 40.3ml of absolute ethanol to dissolve it, transfer it to a 100ml three-product reaction bottle, stir it magnetically, cool the system to 0-5°C, add 1 equivalent of hydrochloric acid dropwise, and control the temperature at -5 ~0°C. After dropping, keep warm at -5~0℃ for 1h. Suction filtration, the filter cake was washed twice with 10ml of absolute ethanol, and vacuum-dried at 50-60°C to obtain 8.7g of polycrystalline type B product of alogliptin hydrochloride (HPLC test result: there is general formula (A) in the product The percentage of dimerization by-products ≤ 0.1%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com