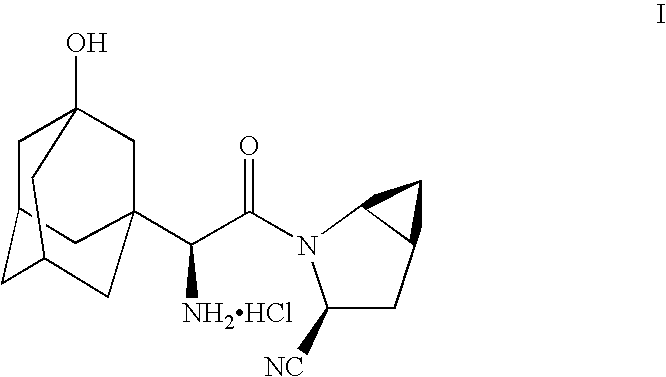
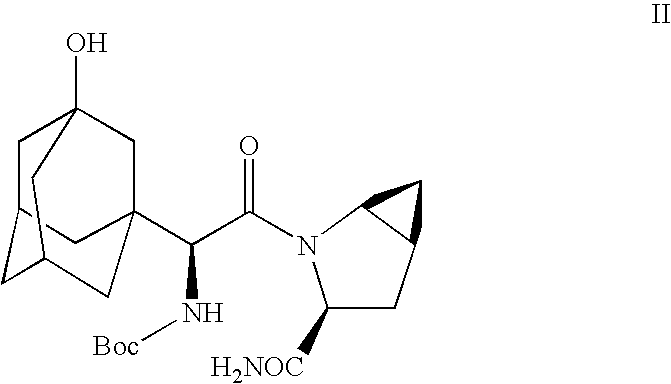
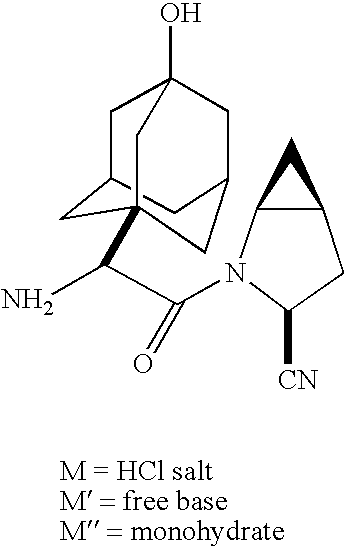
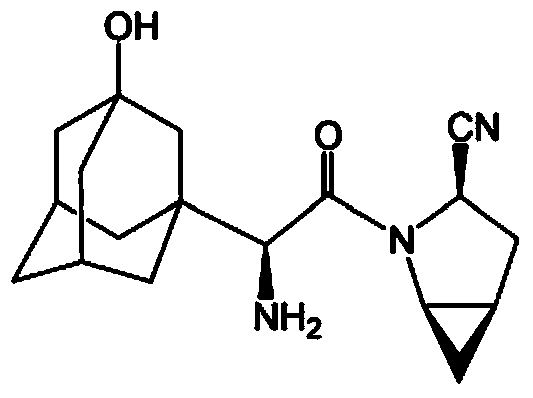
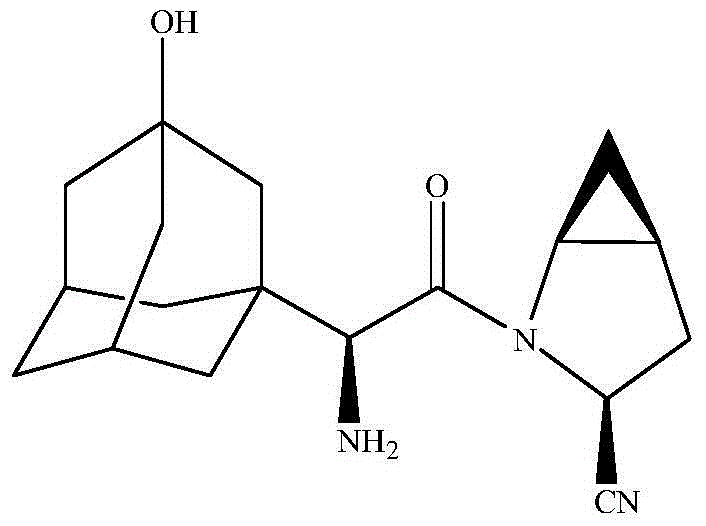
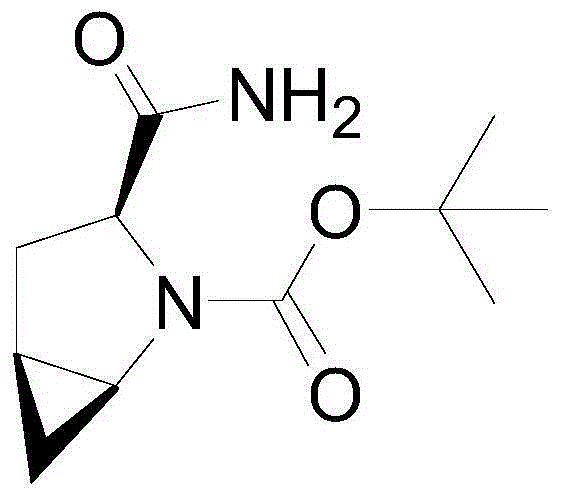
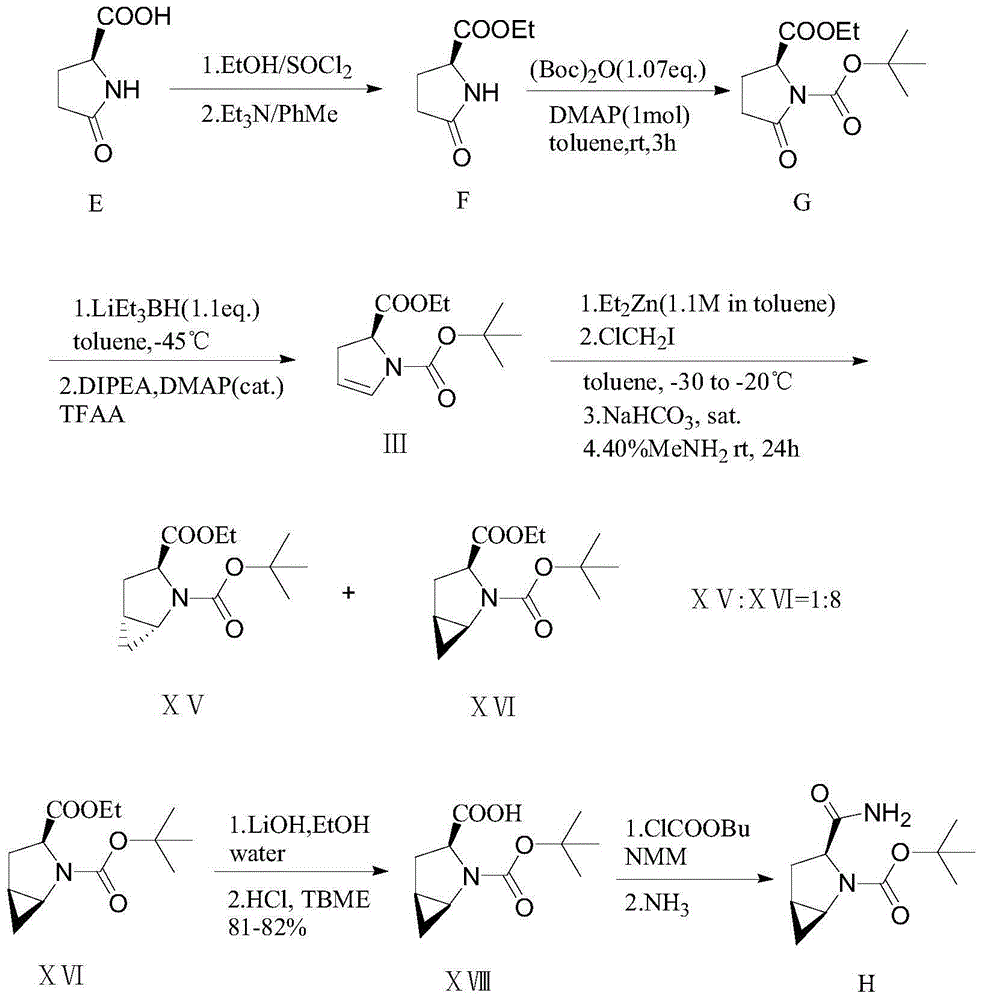
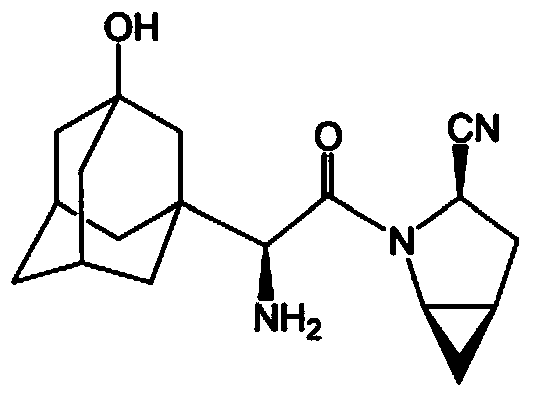
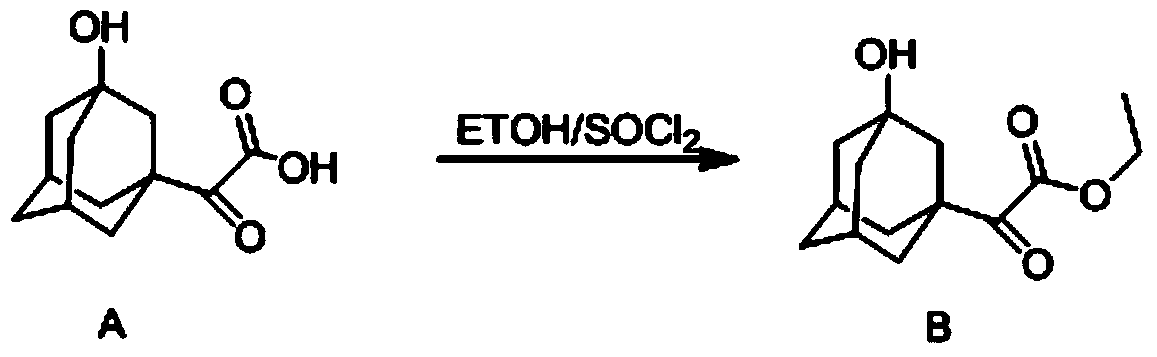
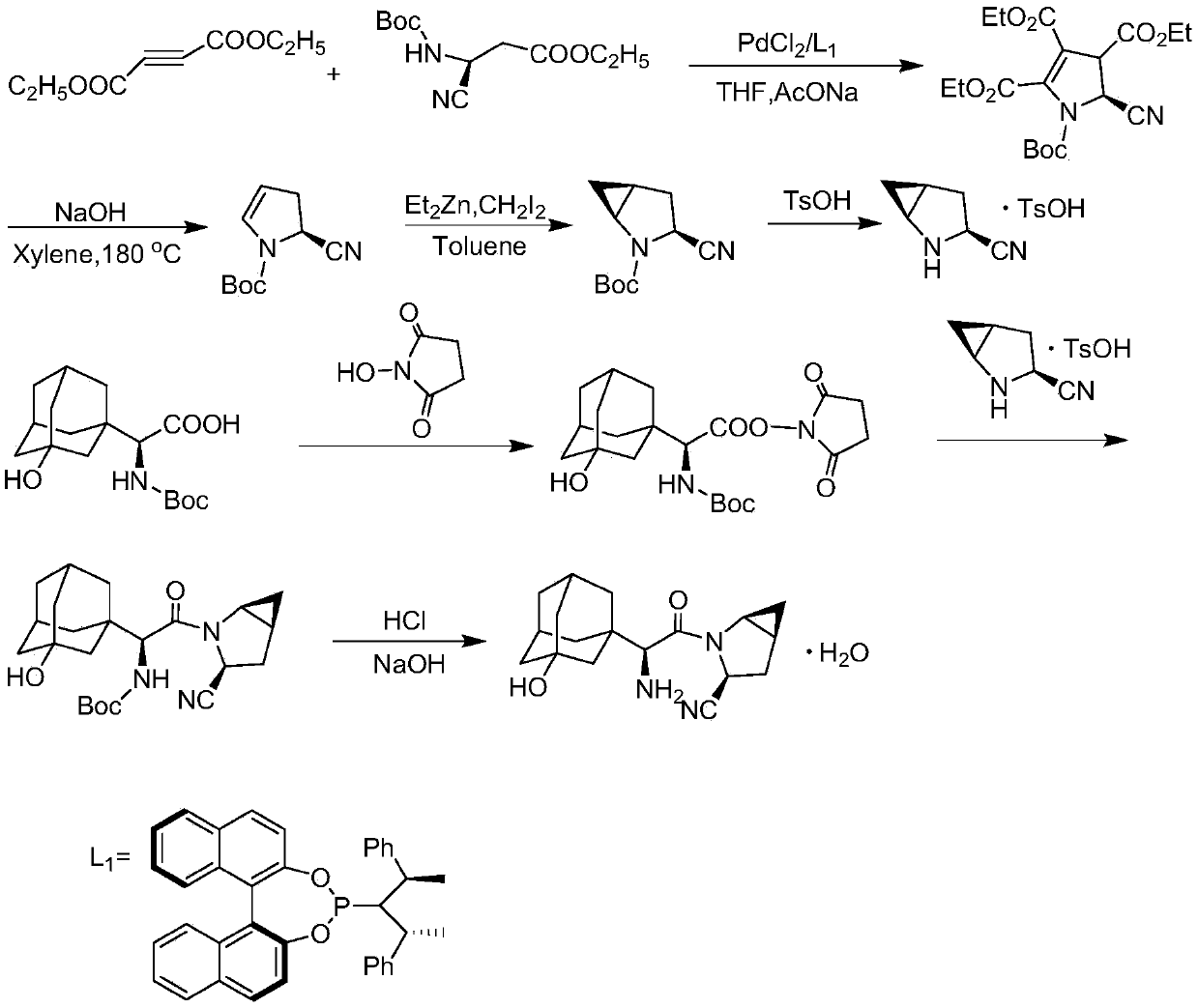
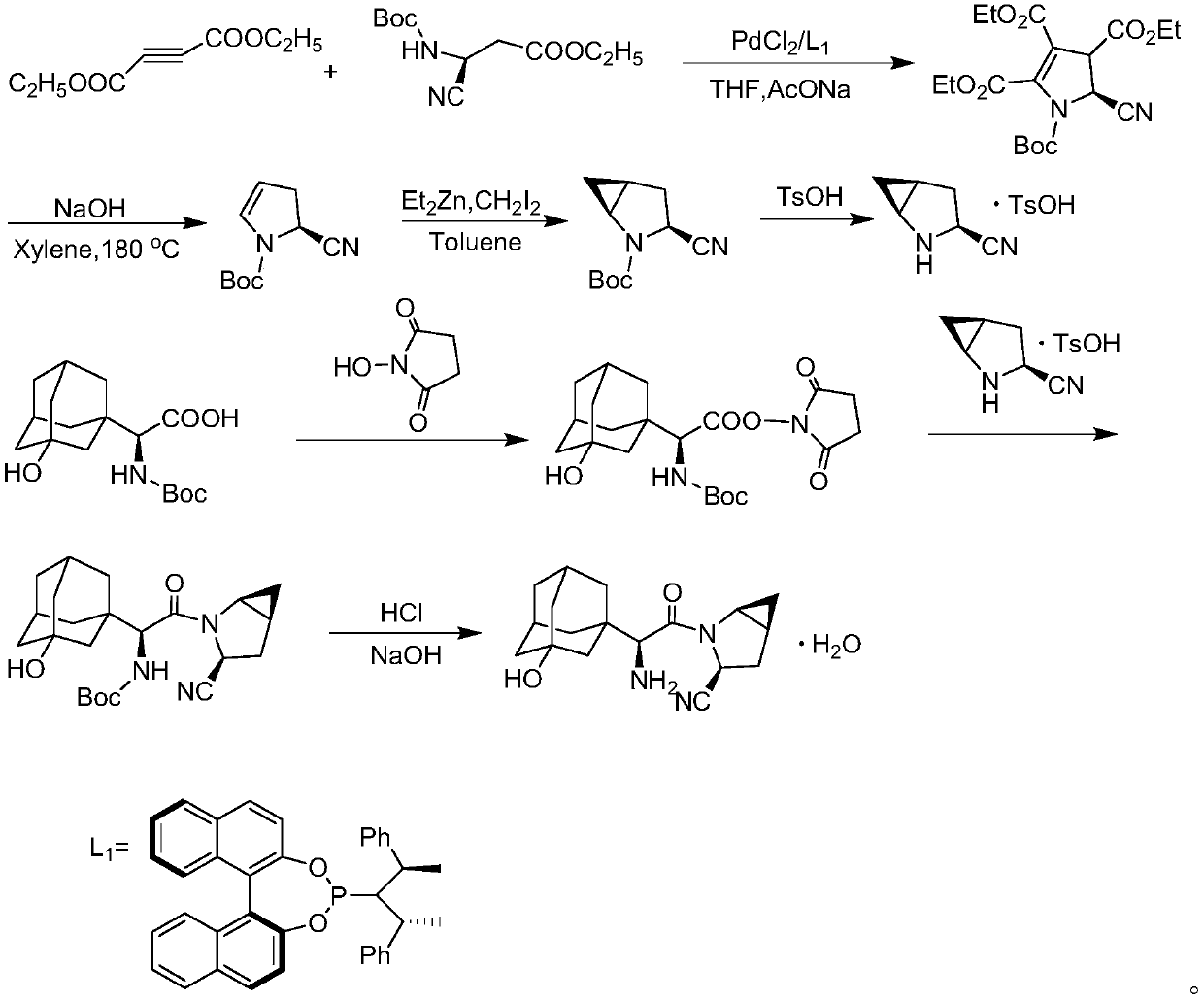
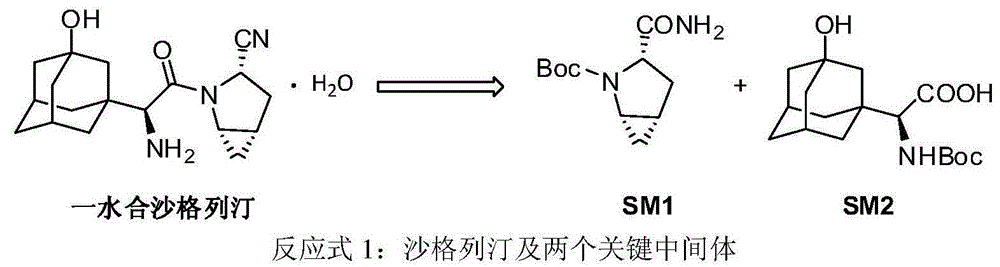
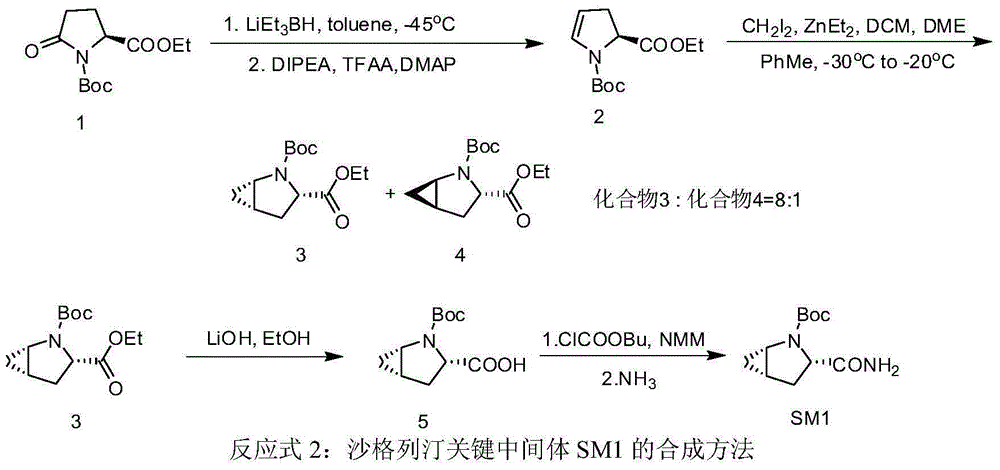
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
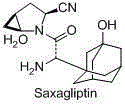
97 results about "Saxagliptin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Saxagliptin is used with a proper diet and exercise program to control high blood sugar in people with type 2 diabetes.
Process for producing a dipeptidyl peptidase IV inhibitor
Owner:BRISTOL MYERS SQUIBB CO
Stimulators of incretin hormones secretion, method for preparation and use thereof
InactiveUS20140100216A1Improve treatment efficiencySimplifies direct careOrganic active ingredientsBiocideDiseaseTreatment effect
The invention relates to the area of medicinal chemistry, pharmacology and medicine and includes description of pharmaceutical compositions and combined medicaments on the base of secretion stimulators and protectors of incretin hormones for treatment of metabolic diseases (among them, diabetes, obesity, metabolic syndrome and the like). The invention consists in that that pharmaceutical composition or combined medicament comprises a derivative of tetrahydrobenzo[f][1,4]oxazepine—either nonsteroidal agonist of bile aids receptor TGR5, or one of endogenous bile acids which stimulate incretin hormones secretion, and also one of the known inhibitors of DPP-IV proteinase. In this case administration of TGR5 agonists is carried out peroral, and administration of endogenous bile acids is exercised rectal in the form of suppository or gel. As proteinase DPP-IV inhibitors could be used Vildagliptin, Saxagliptin, Sitagliptin, Teneligliptin, Linagliptin, Dutogliptin, Alogliptin, Gemigliptin, Carmegliptin and the like. Besides, the invention includes description of novel tetrahydrobenzo[f][1,4]oxazepine derivatives—nonsteroidal agonist of bile aids receptors TGR5, and also methods for their preparation. The invention provides enhancement of therapy effectiveness owing to synergetic action of the components, thus making possible simultaneous treatment of diabetes, and obesity, other metabolic diseases and their cardiovascular and renal complications.
Owner:SAVCHUK NIKOLAY FILIPPOVICH +2
Synthesis method of saxagliptin chiral intermediate
ActiveCN103555683AOrganic chemistryBacteriaPhenylalanine dehydrogenaseTert-Butyloxycarbonyl protecting group
The invention provides a phenylalanine dehydrogenase (PDH) mutant derived from geobacillus, which is high in enzyme activity and thermal stability compared to wild PDH. Furthermore, the invention provides a method for catalytic synthesis of a saxagliptin chiral intermediate, namely (S)-N-t-butyloxycarbonyl-3-hydroxy-1-adamantyl-D-glycine (Boc-HAG) through the PDH mutant. According to the method provided by the invention, Boc-HAG can be directly prepared through a reaction in two steps, and e.e. (enantiomeric excess) value exceeds 99.9%; generation of side products can be reduced, yield of 95% can be achieved within 12 hours, catalysis time is greatly shortened, energy consumption is reduced and a post-treatment process is simplified.
Owner:弈柯莱(台州)药业有限公司
Blood sugar reducing compound, preparation method of blood sugar reducing compound, medicine composition including blood sugar reducing compound and application of medicine composition
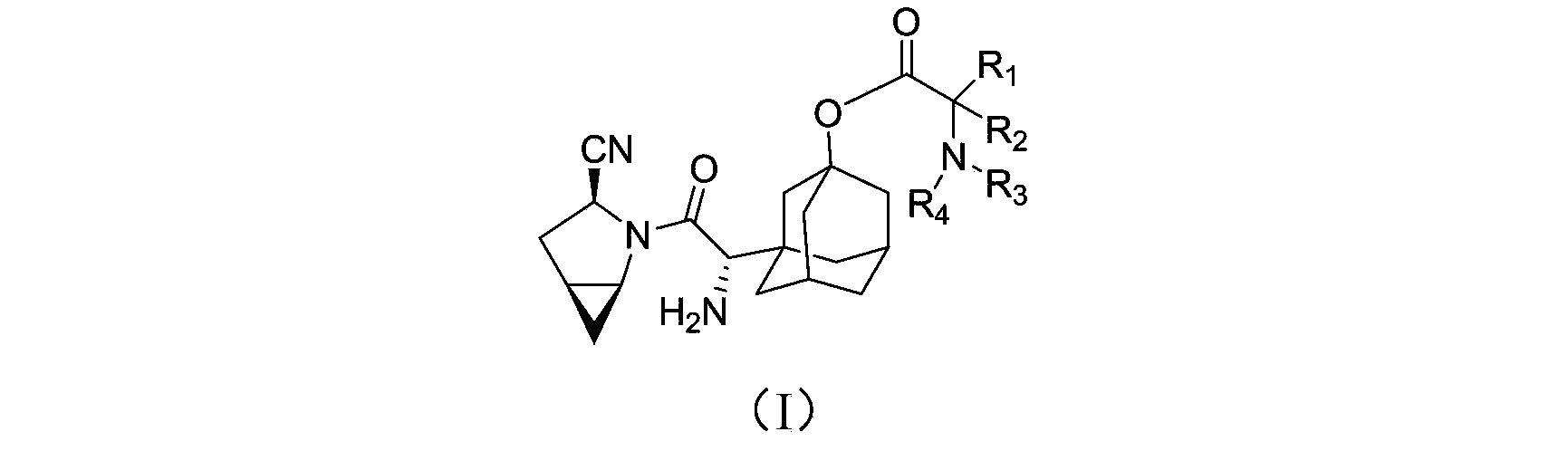
The invention provides a blood sugar reducing compound, a preparation method of the blood sugar reducing compound, a medicine composition including the blood sugar reducing compound and an application of the medicine composition. The compound is a compound shown in a general formula (I) or a pharmaceutically acceptable salt, a solvate, a polymorphism body, an enantiomer or a racemic mixture thereof, the general formula is described in the specification, wherein R1, R2, R3 and R4 are independently H, optionally substituted alkyl, optionally substituted naphthenic base, optionally substituted alkoxy, optionally substituted aryl, optionally substituted aralkyl, or optionally substituted naphthenic base formed by R1 and R2, or optionally substituted heterocyclic radical formed by R2 and R3, or optionally substituted heterocyclic radical formed by R3 and R4. The compound is actively transported by a PepT1 transporter to be absorbed by the gastrointestinal tract, and decomposed in the intestinal tract and the liver to slowly generate saxagliptin, thus the blood concentration of the saxagliptin is increased, and the retention time of the saxagliptin in blood is prolonged. The blood sugar reducing treatment effect is favorably increased and the side reaction is favorably reduced due to stable blood concentration.
Owner:BEIJING LABSOLUTIONS PHARMA
Amino-protected 3-hydroxy adamantane glycine benzothiazole-2-thiol active ester as well as preparation method and application thereof
ActiveCN106349185AIncrease structural diversityCheap and easy to getOrganic chemistryChemical recyclingSaxagliptinChemical structure
The invention relates to an amino-protected 3-hydroxy adamantane glycine benzothiazole-2-thiol active ester as well as a preparation method and application thereof. The thiol active ester is prepared by virtue of reaction between amino-protected 3-hydroxy adamantane glycine and dibenzothiazyl disulfide. The invention further discloses application of the compound in the preparation of a saxagliptin intermediate and saxagliptin. The invention provides a brand new chemical structure. The preparation method is simple and low in cost; the compound is applicable to the preparation of saxagliptin, so that the preparation process can be effectively simplified; and the reaction is mild, and the compound has a wide generalization prospect.
Owner:艾博仕医药科技石家庄有限公司
Preparation method for saxagliptin
InactiveCN104098505AReduce manufacturing costDifficult to solveSulfonic acid amide preparationSaxagliptinChemical synthesis
The invention discloses a preparation method for saxagliptin. The preparation method for the saxagliptin uses a chemical synthesis method to replace saxagliptin intermediate preparation in a traditional technique and uses chiral inducer in the reaction to perform chiral synthesis. The preparatoin method for the saxagliptin is easy and convenient to operate, is high in yield, enables the intermediate production cost to be lowered and solves the technical problem of difficulty in biological enzyme preparing and storing due to the fact that the traditional technique needs to use biological enzyme to perform resolution.
Owner:天津民祥生物医药股份有限公司
Preparation method of saxagliptin intermediate
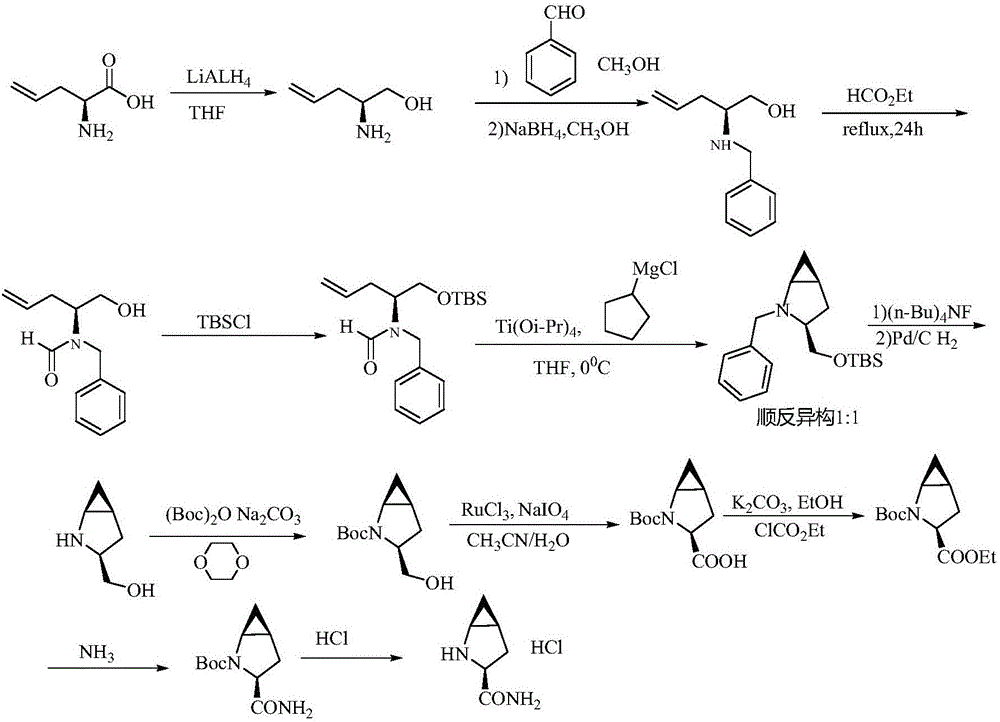
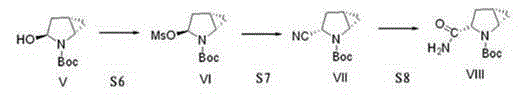
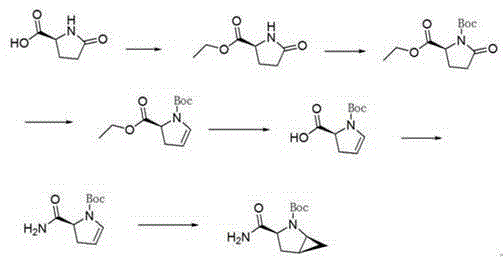
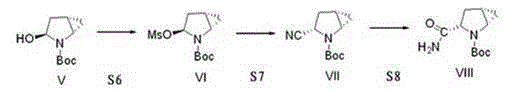
The invention provides a preparation method of a saxagliptin intermediate (1S, 3S, 5S)-3-(amino carbonyl)-2-azabicyalo[3.1.0]hexane-2-tert-butyl formate. According to the preparation method, L-pyroglutamic acid is taken as a raw material, and an esterification reaction, Boc protection, reduction, elimination, cyclopropylation, resolution, hydrolyzation, ammonolysis, Boc deprotection and ammonolysis are performed on L-pyroglutamic acid, and finally, the compound (1S, 3S, 5S)-3-(amino carbonyl)-2-azabicyalo[3.1.0]hexane-2-tert-Butyl formate is obtained. The preparation method of the saxagliptin intermediate is cheap and easily available in raw materials, reasonable in process, simple and convenient to operate, high in enantiomer selectivity and high in yield.
Owner:SHANGHAI INST OF TECH
Saxagliptin metformin double-layer tablet and preparation method thereof
InactiveCN109432030AStable efficacyImprove securityOrganic active ingredientsMetabolism disorderCarboxymethyl celluloseSaxagliptin
The invention provides a saxagliptin metformin double-layer tablet and a preparation method thereof. The saxagliptin metformin double-layer tablet mainly comprises metformin particles and saxagliptinparticles; the metformin particles mainly comprise the following components in parts by mass: 500-1000 parts of metformin hydrochloride, 25-50 parts of carboxymethyl cellulose, 3.5-7 parts of silicondioxide, 190-390 parts of hydroxypropyl methylcellulose, and 5-10 parts of magnesium stearate; and the saxagliptin particles mainly comprise the following components in parts by mass: 2.5-5.4 parts ofsaxagliptin monohydrate, 138-142 parts of microcrystalline cellulose, 30-60 parts of lactose, 3-6 parts of hydroxypropyl methylcellulose, and 0.5-2 parts of magnesium stearate. According to the saxagliptin metformin double-layer tablet disclosed by the invention, raw materials and auxiliary materials are effectively compounded, so that the double-layer tablet prepared after compounding has the advantages of stable drug effect, high safety and low cost.
Owner:瀚晖制药有限公司
Saxagliptin medicinal composition and preparation method thereof
InactiveCN104644591AMature technologyEasy to operateOrganic active ingredientsMetabolism disorderSaxagliptinOral medication
The invention relates to a preparation method of a saxagliptin medicinal composition. The form of the above preparation can be safely applied to patients, can improve the stability of saxagliptin, and also can improve the gastrointestinal tract absorption. The invention concretely relates to a preparation method of a medicinal composition containing amorphous saxagliptin and beta-cyclodextrin. The preparation method comprises the following steps: grinding saxagliptin and beta-cyclodextrin under an alkaline condition, drying, crushing to obtain 150-250 mesh fine powder, mixing the powder with an appropriate auxiliary material to prepare granules, tabletting, and dressing to obtain the composition. The saxagliptin composition greatly improves the in vitro release in order to improve the bioavailability, can effectively treat type II diabetes, and has the advantages of convenient oral administration, covering of bad taste, fast disintegration, fast absorption and convenient carrying.
Owner:TIANJIN HANKANG PHARMA BIOTECH
A kind of metformin crystal and its pharmaceutical composition with saxagliptin and preparation method
ActiveCN102266325AReduced preparation processWell mixedOrganic active ingredientsMetabolism disorderSolubilitySaxagliptin
The invention discloses a melbine crystal and a medicinal composition of melbine and saxagliptin and a preparation method thereof. The medicinal composition consists of active ingredients of medicines and a pharmaceutic adjuvant, wherein the active ingredients of the medicines comprise the following components in part by weight: 5 to 30 parts of saxagliptin and 200 to 800 parts of melbine, and the melbine is the melbine crystal; and the pharmaceutic adjuvant comprises a filling agent, a disintegrating agent, an adhesive and a lubricating agent. The melbine crystal used in the medicinal composition has the characteristic of low solubility, so the sustained-release effect of the melbine is realized by using ordinary matrix materials in the medicinal composition prepared from the melbine andthe saxagliptin, the administration safety is improved; and the synergistic effect of the melbine and the saxagliptin is good, so the curative effect is improved. In addition, due to the adoption of few matrix materials, the stability of the medicines is improved.
Owner:HAINAN JINRUI PHARMA
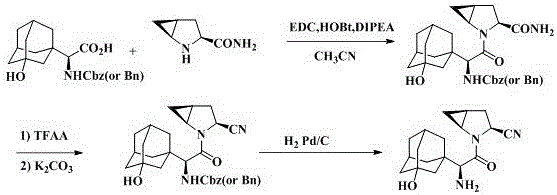
Method for preparing N-tert-butyloxycarbonyl-3-hydroxy-1-adamantyl-d-glycine
InactiveCN104098487AReduce manufacturing costSimple and fast operationCarbamic acid derivatives preparationOrganic compound preparationSaxagliptinGlycine
The invention discloses a method for preparing N-tert-butyloxycarbonyl-3-hydroxy-1-adamantyl-d-glycine. A chemical synthesis method is adopted to replace preparation of a saxagliptin intermediate in the prior art, a chiral inducer participates in a reaction to perform chiral synthesis, and the preparation method is simple and convenient to operate and high in yield, reduces production cost of the intermediate and solves the technical problem of difficulty in biological enzyme preparation and storage caused by the fact that resolution needs to be performed through biological enzymes in the prior art.
Owner:天津民祥生物医药股份有限公司
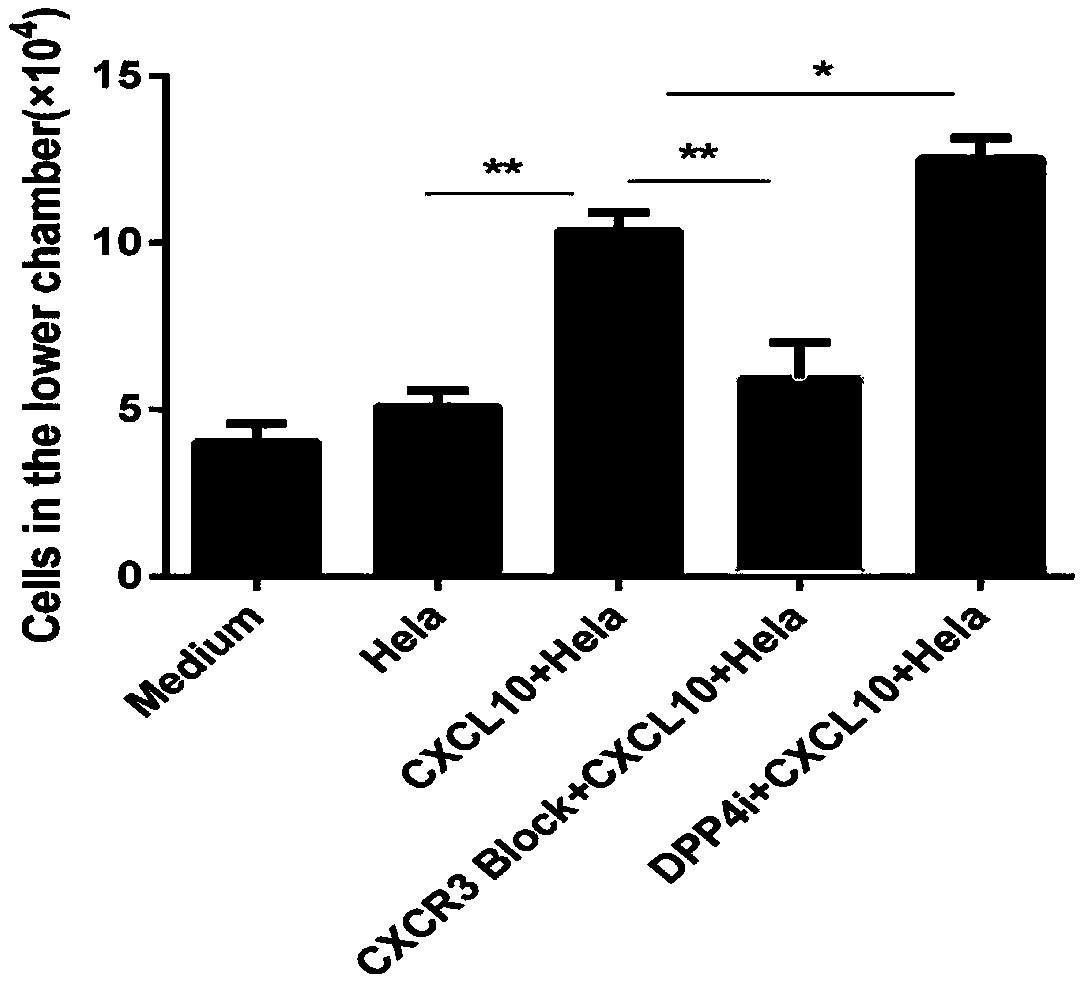
Reagent for enhancing capacity of homing CAR-T cell to solid tumor tissue
PendingCN109593726AEnhanced tumor homingEnhanced tumor enrichment capacityPolypeptide with localisation/targeting motifOrganic active ingredientsAntigen receptorsTherapeutic effect
The invention belongs to the technical field of the biological medicine, and specifically relates to a CAR-T cell, a reagent for enhancing capacity of homing CAR-T cell to solid tumor tissue, and application thereof. The reagent is a DPP4 inhibitor, includes but not limited to sitagliptin, vildagliptin, saxagliptin, alogliptin and linagliptin. The CAR-T cell expression includes ScFv, a hinge structure and a transmembrane structure, and a chimeric antigen receptor (CAR) of an intracellular activation signal domain; an amino acid sequence of the hinge structure is as shown in SEQ ID NO.1 or SEQID NO.2 or SEQ ID NO.3 or SEQ ID NO.4 or SEQ ID NO.5. The invention provides a new application of the DPP4 inhibitor for participating tumor treatment. The reagent can enhance the tumor homing capacity and tumor enrichment capacity of the CAR-T cell, and a therapeutic effect on the solid tumor by the CAR-T therapy is improved.
Owner:CHONGQING PRECISION BIOTECH CO LTD
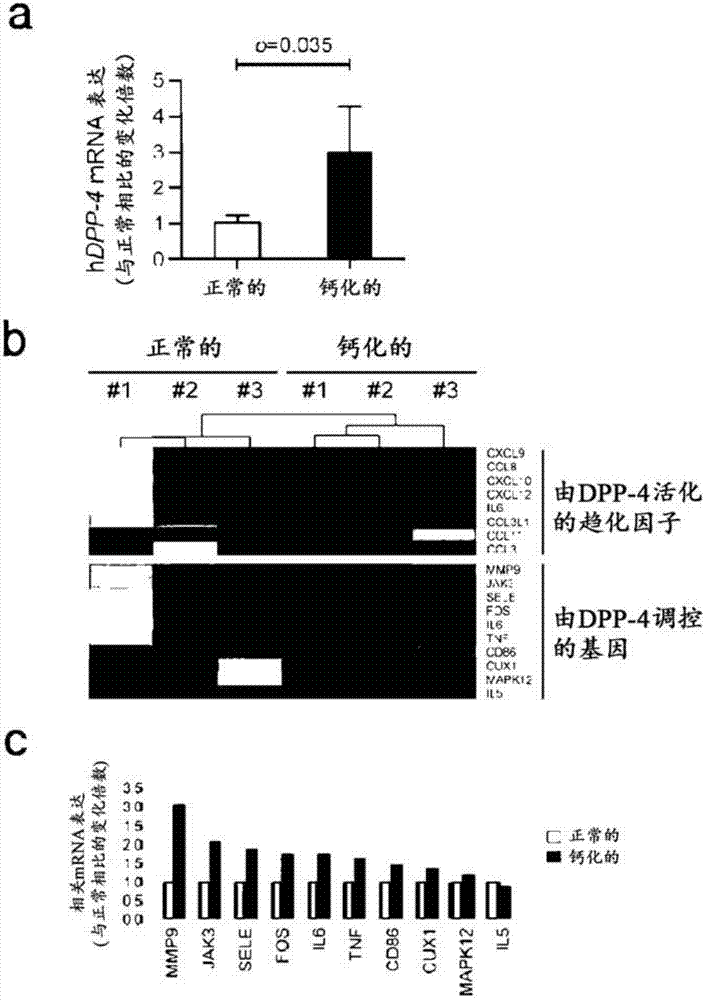
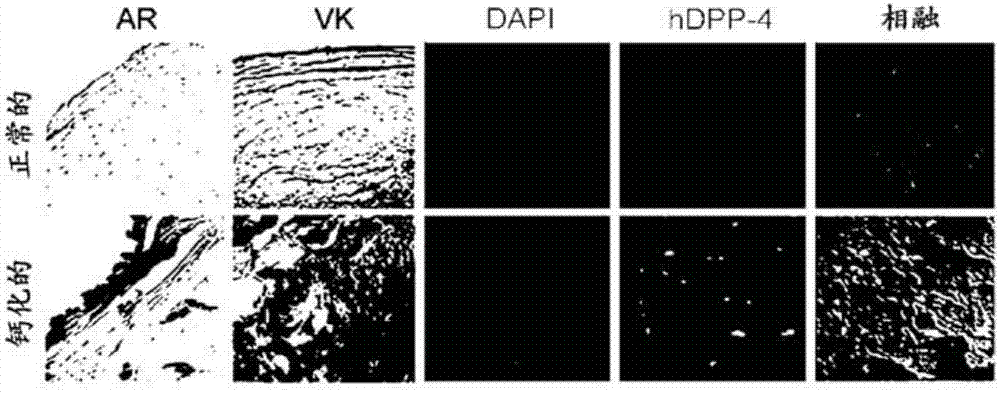
Composition for preventing or treating valve calcification, containing dpp-4 inhibitor
The present invention relates to a composition for preventing or treating valve calcification, containing a dipeptidyl peptidase-4 (DPP-4) inhibitor. The DPP-4 inhibitor according to the present invention may include all of what can inhibit the expression of DPP-4 nucleotides or the activity of DPP-4 proteins, wherein: DPP-4 antibodies, sitagliptin, vildagliptin, saxagliptin, linagliptin, dutogliptin, gemigliptin, alogliptin, Anagliptin, evogliptin, Berberine, Diprotin, or Lupeol; DPP-4 mRNA anti-sense nucleotides, aptamers, small interfering RNA (siRNA), short hairpin RNA (shRNA), and microRNA (miRNA), or RNA interference (RNAi); or the like can be used.
Owner:THE ASAN FOUND +1
Saxagliptin intermediate, its salt, preparation method and application
ActiveCN103965065AImprove conversion rateLower control costsOrganic compound preparationAmino-carboxyl compound preparationOrganic acidSaxagliptin
The invention discloses a saxagliptin intermediate, its salt, preparation method and application. The preparation method for the compound 1 or its salt comprises the step of: subjecting an intermediate compound 2 to reduction reaction at a reaction temperature ranging from -15DEG C to 45DEG C in a solvent under the effects of a reducing agent and an organic acid to obtain an intermediate compound 1 or its salt, with the reducing agent being a borohydride of an alkali metal. The preparation method for the compound 2 comprises the step of: under the protection of an inert gas and the action of the organic acid, reacting a compound B with a compound F in a solvent. The invention also discloses application of the intermediate compound or its salt in preparation of saxagliptin. The saxagliptin intermediate involved in the invention has the advantages of easy preparation, simple operation, mild condition, low cost and environmental friendliness, and can meet the requirements of industrial production. (formula 1 and formula 2).
Owner:SHANGHAI SHYNDEC PHARMA CO LTD +1
Combination product containing limonin compound and DPP-4 inhibitors
The invention relates to a combination product of a limonin compound, pharmaceutically-acceptable derivatives, esters, stereoisomers, salts or prodrugs thereof and dipeptidyl peptidase-4(DPP-4) inhibitors such as sitagliptin, saxagliptin, vildagliptin, linagliptin, alogliptin, trelagliptin and omarigliptin. The invention further relates to application of the combination product in treating and / orpreventing diseases related to diabetes.
Owner:NATURAL MEDICINE INST OF ZHEJIANG YANGSHENGTANG
Method for producing saxagliptin
The invention provides a method for producing saxagliptin. The method comprises the following steps of: 1, providing a compound d; and 2, reacting the compound d, isopropanol and concentrated hydrochloric acid at the temperature of between 50 and 80 DEG C, and collecting the product to obtain the saxagliptin. Compared with the prior art, the method has the advantages that the compound d is directly converted into the target product, so that the reaction rate is greatly improved, the reaction yield is improved, the post treatment method is simplified, and industrial wastewater is greatly reduced.
Owner:SHANGHAI TWISUN BIO PHARM
Preparation method of saxagliptin intermediate
ActiveCN104098481ALow priceMild reaction conditionsOrganic compound preparationCarboxylic acid amides preparationSaxagliptinHydrogen
The invention elates to the medicine synthesis field, and concretely relates to a preparation method of a saxagliptin intermediate. A technical scheme adopted in the invention is characterized in that a compound with the structure represented by formula (I) shown in the specification; and in the formula (I), M is hydrogen or various ether hydroxy protection groups, R1 and R2 are H or CH3 respectively independently, and at least one of R1 and R2 is H.
Owner:浙江四维医药科技有限公司
Preparation method of medication saxagliptin for treating diabetes
ActiveCN109761876AHigh purityShort reaction pathOrganic chemistryTert-Butyloxycarbonyl protecting groupReaction rate
The invention provides a preparation method of saxagliptin with a shorter synthetic route. (S)-3-amino-3-cyano-propionic ether protected by diethyl acetylenedicarboxylate and an amino group is used asa starting material in the presence of an organic phosphine ligand and a palladium catalyst, and is subjected to cyclization, decarboxylation and cyclopropanation to obtain an intermediate product (1S, 3S, 5S)-2-azabicyclo[3.1.0]hexane-3-carbonitrile p-toluenesulfonate, and then the intermediate product is reacted with carboxyl-activated (S)-N-t-butyloxycarboryl-(3-hydroxyadamantane-1-yl)glycineto increase the reaction rate and obtain a saxagliptin product with a high yield and high purity, the reaction route is greatly shortened, the yield in each step is high, the reaction time is short, the production cost is reduced, and industrial production is facilitated.
Owner:THE SECOND PEOPLES HOSPITAL OF SHENZHEN
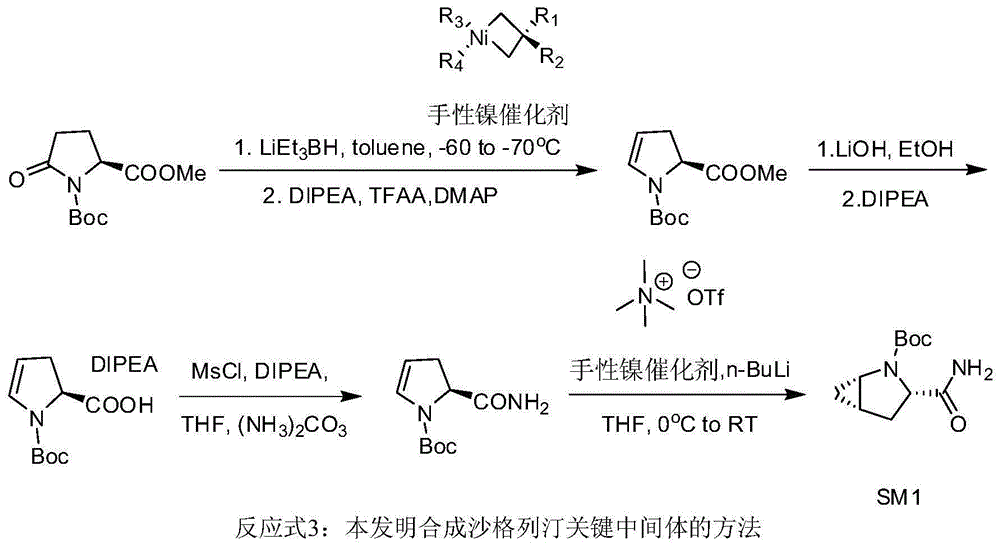
Preparation method of key intermediate of saxagliptin
InactiveCN106554301AImprove conversion rateReduce manufacturing costOrganic chemistryTert-Butyloxycarbonyl protecting groupTrifluoroacetic anhydride
The invention relates to a preparation method of (1S, 3S, 5S)-3-(amidogen carbonyl)-2- azabicyalo [3.1.0] hexane-2-tert-butyl formate. The preparation method comprises the following steps that (1), Boc-L-pyroglutamic acid methyl ester is restored through lithium triethylborohydride and then dewatered through trifluoroacetic anhydride to obtain (S)-1-N-tert-butyloxycarbonyl-2,3-dihydro-2-pyrrole ethyl formate; (2), DIPEA is added in the hydrolysis reaction of (S)-1-N-tert-butyloxycarbonyl-2,3-dihydro-2-pyrrole ethyl formate under a alkaline condition, then salifying is conducted, and (S)-1-N-tert-butyloxycarbonyl-2,3-dihydro-2-pyrrole formic acid N, N-diisopropyl ethylamine salt is obtained; (3), (S)-1-N-tert-butyloxycarbonyl-2,3-dihydro-2-pyrrole formic acid N and N-diisopropyl ethylamine salt obtained in the step (2) are subjected to amidating, and (S)-1-N- tert-butyloxycarbonyl-2,3-dihydro-2-pyrrole formamide is obtained; and (4), (S)-1-N-tert-butyloxycarbonyl-2,3-dihydro-2-pyrrole formamide is catalyzed through a chirality nickel catalyst and subjected to a ciprofloxacin reaction, and a target material with single configuration, namely (1S, 3S, 5S)-3-(amidogen carbonyl)-2-azabicyalo [3.1.0] hexane-2-tert-butyl formate (SM1), is obtained.
Owner:HYBIO PHARMA
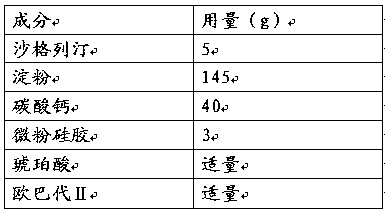
Saxagliptin medicinal preparation
ActiveCN105497023AOvercome instabilityReduce degradationOrganic active ingredientsMetabolism disorderSaxagliptinCurative effect
The invention discloses a novel saxagliptin medicinal preparation. The novel saxagliptin medicinal preparation comprises saxagliptin, acidic granules and lubricants. The acidic granules are made of fillers and acid. The novel saxagliptin medicinal preparation has the advantages that the shortcoming of instable properties of the saxagliptin can be effectively overcome by the aid of the novel saxagliptin medicinal preparation, degradation of the saxagliptin which is an active constituent can be reduced, and accordingly curative effects can be guaranteed.
Owner:BEIJING WINSUNNY PHARMA CO LTD
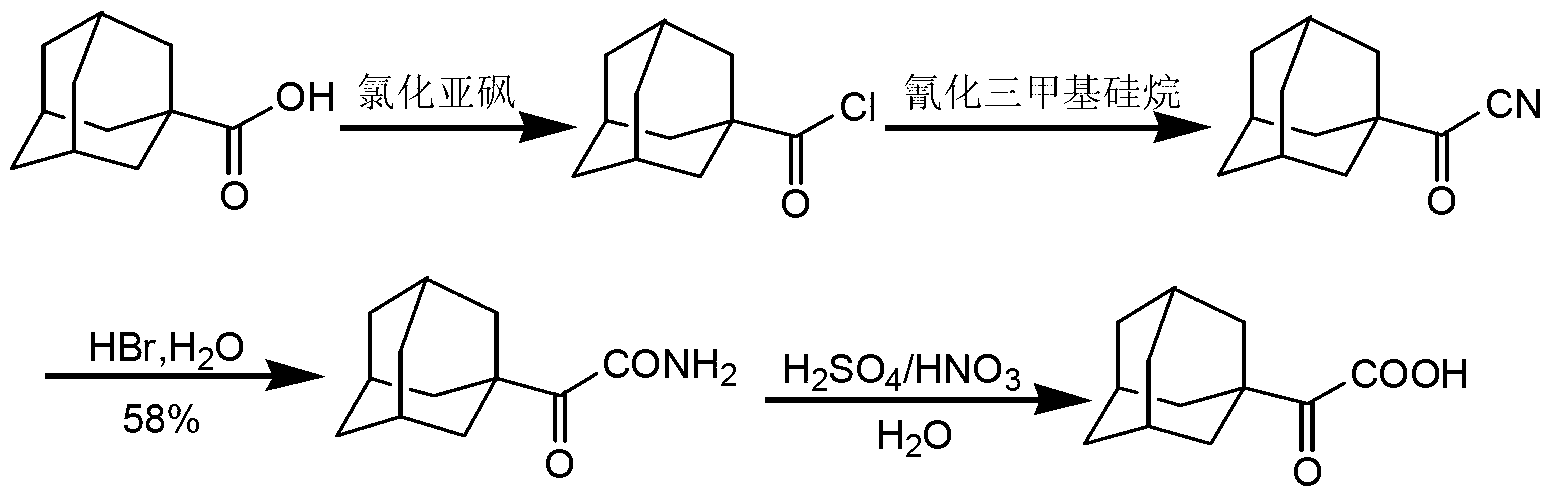
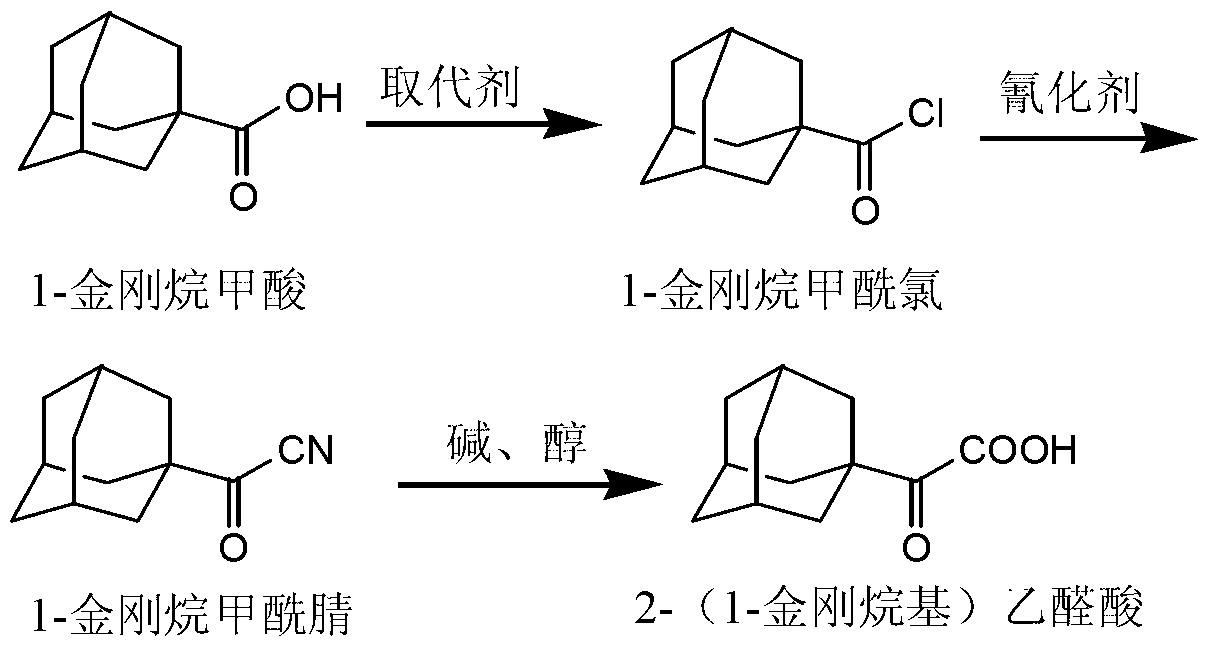
Preparation method of 2-(1-adamantly) glyoxylic acid
InactiveCN103304406AEasy to useAchieve recyclingPreparation from nitrilesChemical recyclingGlyoxylic acidSaxagliptin
The invention relates to a preparation method of saxagliptin intermediate 2-(1-adamantly) glyoxylic acid, which belongs to the technical field of pharmaceutical synthesis and is used for solving the problems that the reaction steps of the preparation method of the 2-(1-adamantly) glyoxylic acid in the prior art are tedious, dangerous reagent is used in the reaction, the yield is low and the like. The preparation method comprises the following steps of: substitution reaction, wherein substitution reaction is carried out on 1- adamantly to obtain 1- adamantly formyl chloride; cyaniding reaction, wherein cyaniding reaction is carried out on 1- adamantly formyl chloride under the action of cyaniding agent to obtain 1- adamantly formyl nitrile; and cyano-group hydrolysis reaction, wherein the cyano-group hydrolysis reaction is carried out on the 1- adamantly formyl nitrile under the alkaline system to obtain 2-(1-adamantly) glyoxylic acid. According to the preparation method of the 2-(1-adamantly) glyoxylic acid disclosed by the invention, the using reagent is cheap and easily available, green and environment-friendly; the reaction conditions are gentle; the reaction time is short; the post-treatment operation is simple; the conversation rate, the yield and the purity are high; the product quality is good.
Owner:SUZHOU UUGENE BIOPHARMA
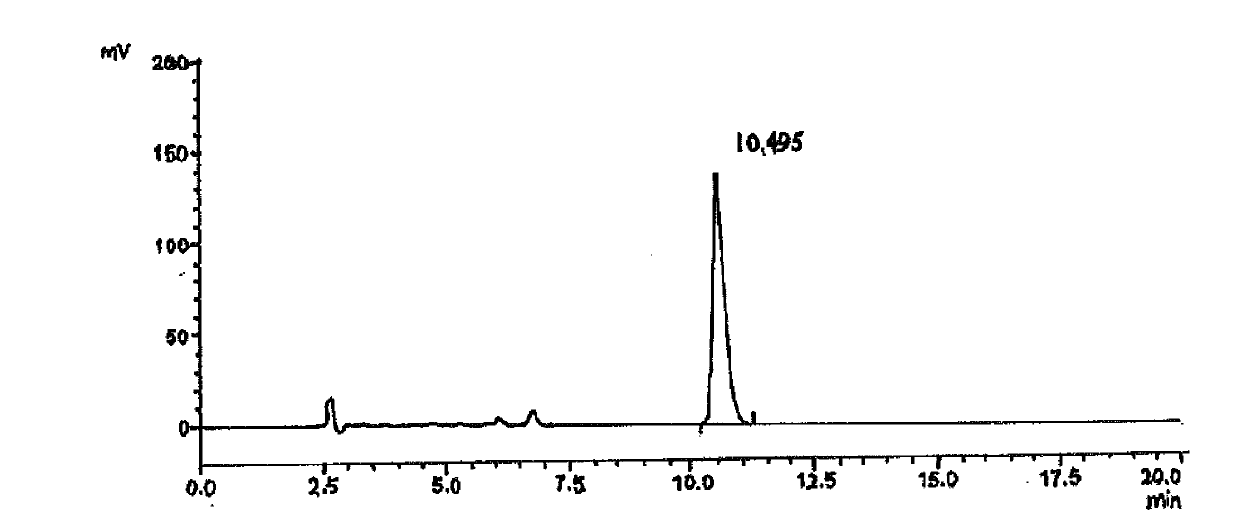
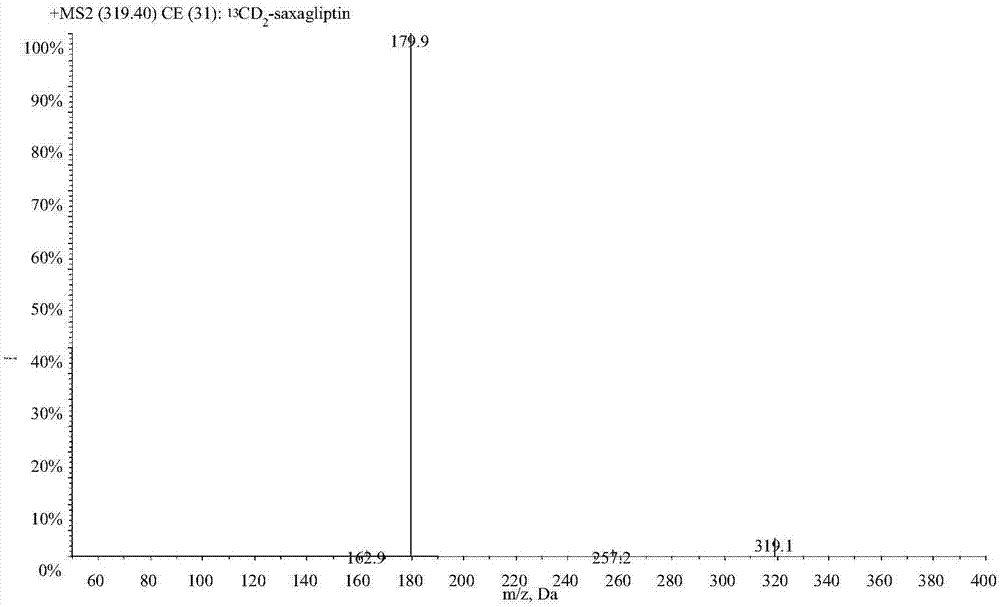
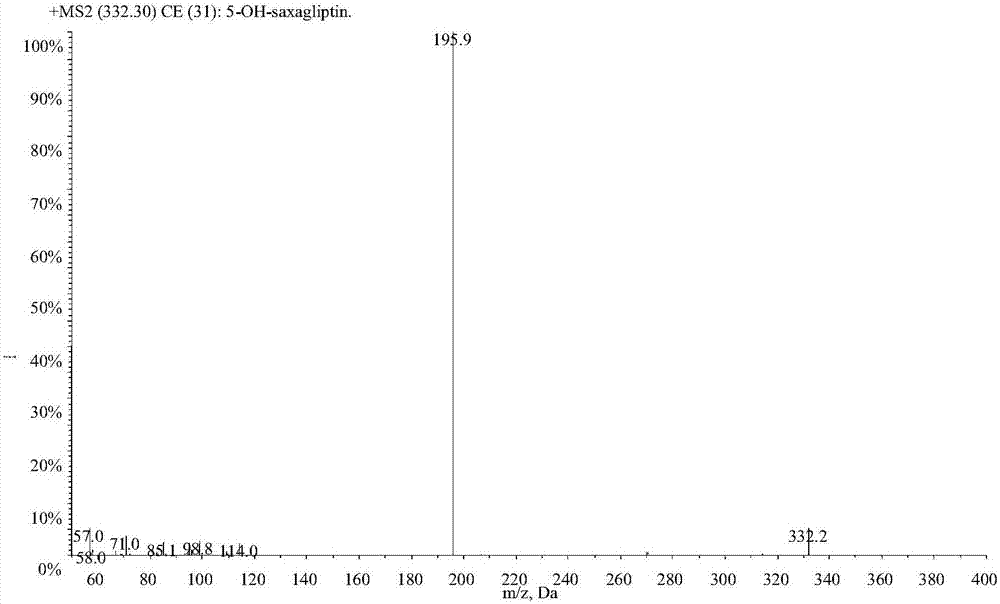
LC-MS/MS high-throughput detection method of saxagliptin and 5-hydroxysaxagliptin in human blood plasma
The invention relates to an LC-MS / MS high-throughput detection method of saxagliptin and 5-hydroxysaxagliptin in human blood plasma. The method comprises the following steps: taking a blood plasma sample to be detected, adding an internal standard solution, adding an aqueous solution of CHAPS, carrying out eddy current heating, adding acetonitrile, centrifuging the obtained solution, taking the obtained supernatant, blowing the supernatant with nitrogen until the supernatant is dry, adding a redissolving solution to redissolve the dried supernatant in order to obtain a sample to be detected, and carrying out LC-MS / MS analysis, wherein the internal standard solution is an acetonitrile and water (50:50, v / v) mixed solution with the isotopic label <13>CD2-saxagliptin concentration being 5.00 ng / mL and the <13>CD2-hydroxysaxagliptin concentration being 10.0 ng / mL, and the redissolving solution is an acetonitrile: water: formic acid mixed solution with the volume ratio being 95:5:0.1. The method satisfies the demands of clinical large-scale sample analysis; and compared with the prior art, the method has the advantages of simplicity in operation, short extraction time, and suitableness for high-throughput sample pretreatment.
Owner:苏州海科医药技术有限公司
Stable pharmaceutical composition of saxagliptin
Disclosed herein is a stable pharmaceutical composition comprising a substrate having deposited on its surface a layer comprising saxagliptin or pharmaceutically acceptable salts thereof, wherein a seal coat is not present between the substrate and the saxagliptin layer.
Owner:GLENMARK GENERRICS LTD
Method for preparing saxagliptin intermediate
ActiveCN106748965AMild reaction conditionsHigh yieldOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsChemical synthesisSaxagliptin
The invention discloses a method for preparing a saxagliptin intermediate, namely a method for preparing (1S, 3S, 5S)-3-(amino carbonyl)-2-azabicyalo [3.1.0] hexane-2-formic acid-tert-butyl ester, and belongs to the field of chemical synthesis. The method comprises the following steps: in a dichloromethane solvent, under the action of a chiral catalyst, performing Simmons-Smith cyclopropanation preparation on (S)-1-N-tert-butyloxycarboryl-2,3-dihydro-2-pyrrole formamide, thereby obtaining the (1S, 3S, 5S)-3-(amino carbonyl)-2-azabicyalo [3.1.0] hexane-2-formic acid-tert-butyl ester, wherein the reaction temperature is minus 30-40 DEG C, and the reaction time is 1-48 hours. The method has the characteristics of being short in route, simple and convenient to operate, free of pollution and easy in industrial production, and is a very economic, simple and convenient method.
Owner:JIAXING UNIV
Method for preparing saxagliptin intermediate
ActiveCN109970620AVolume controlHigh yieldOrganic chemistryBulk chemical productionSaxagliptinChemistry
The invention provides a method for preparing a saxagliptin intermediate 2 as shown in the specification. An existing process is improved by using the method, so that the yield and purity of a productare greatly increased, in addition, it is convenient realize aftertreatment, and the method is particularly suitable for industrial production.
Owner:JIANGSU VCARE PHARMATECH
Preparation method of saxagliptin intermediate
InactiveCN103724254ALow costEasy temperature controlOrganic chemistrySodium bicarbonatePtru catalyst
The invention discloses a preparation method of a saxagliptin intermediate methyl (1S, 3S, 5S)-tertiary butyl-3-formamyl-2-azabicyclo[3.1.0] hexane-2-carboxylate. The preparation method comprises the following steps: firstly, with iodine as a catalyst, adding cuprous bromide, Zn powder, an organic solvent and diiodomethane, and stirring to obtain a carbene reagent A; then dissolving tert-butyl (S)-tertiary butyl-2-formamyl-2, 3-dihydro-1H-pyrrole-1-formate in an organic solvent to obtain a substrate solution B; finally, mixing the carbene reagent A and the substrate solution B to react to obtain a reaction solution, adding a saturated sodium bicarbonate water solution to the reaction solution, and then sequentially carrying out filtration, extraction, drying and spinning drying to obtain the saxagliptin intermediate methyl (1S, 3S, 5S)-tertiary butyl-3-formamyl-2-azabicyclo[3.1.0] hexane-2-carboxylate. The preparation method disclosed by the invention is simple to operate and mild in reaction conditions, and the cost is reduced while relatively high yield is achieved.
Owner:SHANGHAI INST OF TECH
Saxagliptin midbody preparing method
The invention relates to the technical field of medicine midbody preparing and particularly discloses a saxagliptin midbody preparing method. The method includes the following steps that (R)-5-hydroxylpyrrole-2-ketone serves as a raw material, benzyl alcohol is used as a solvent, and 5-benzylhydroxylpyrrole-2-ketone is obtained through dehydration under the temperature of 70 DEG C to 80 DEG C; Boc is introduced for protection, reduction, dehydration and kulinkovich are conducted, IV compound is generated, debenzylation is conducted, methylsulfonyl is introduced, cyanogroup is introduced, and finally (1S,3S,5S)-3-(amino-carbonyl)-2-azabicyclo[3.1.0]hexane-2-tert-butyl formate is obtained through hydrolysis. According to the saxagliptin midbody preparing method, raw materials are easy and convenient to prepare, operation conditions are easy to control, the reaction yield is high, no pollution is generated, the selectivity of enantiomer is high, and the method is suitable for industrial production.
Owner:CANGZHOU SENARY CHEM SCI TEC
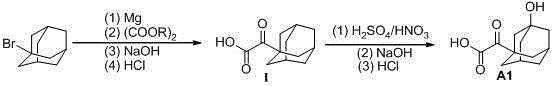
Preparation method of saxagliptin intermediate (A1)
InactiveCN105461540ALow costEasy to operateOxygen-containing compound preparationOrganic compound preparationSaxagliptinBiochemical engineering
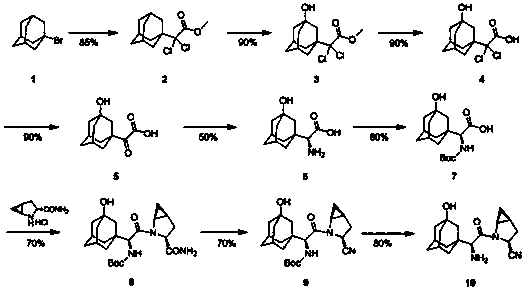
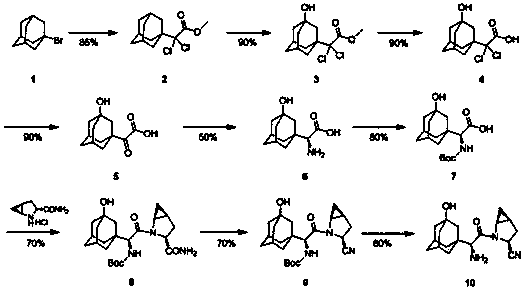
The invention discloses a preparation method of a saxagliptin intermediate (A1), and belongs to the field of chemical pharmacy. Adamantyloxoacetic acid is obtained through a Grignard reaction, hydrolysis and a mixed acid reaction with bromoadamantane as an initial raw material. The method has the advantages of simple and easy operation, high yield, small pollution, and suitableness for industrial production.
Owner:南京克瑞特科技信息咨询有限公司
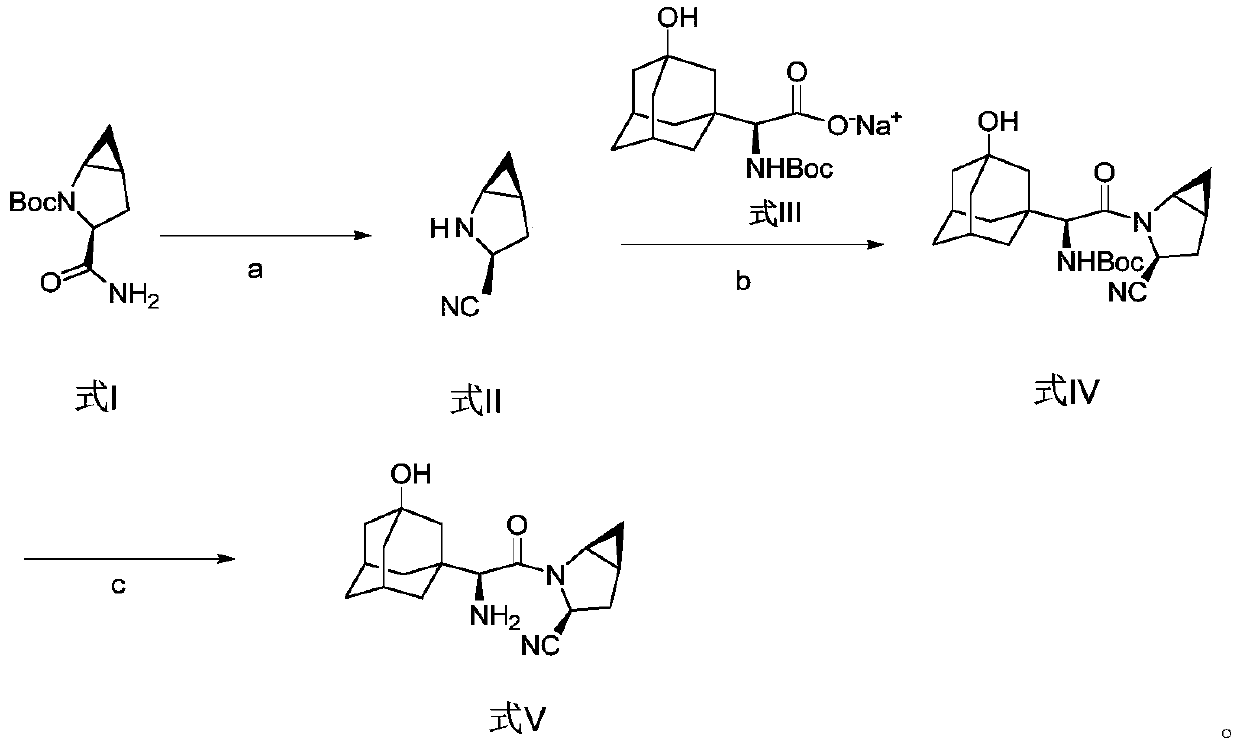
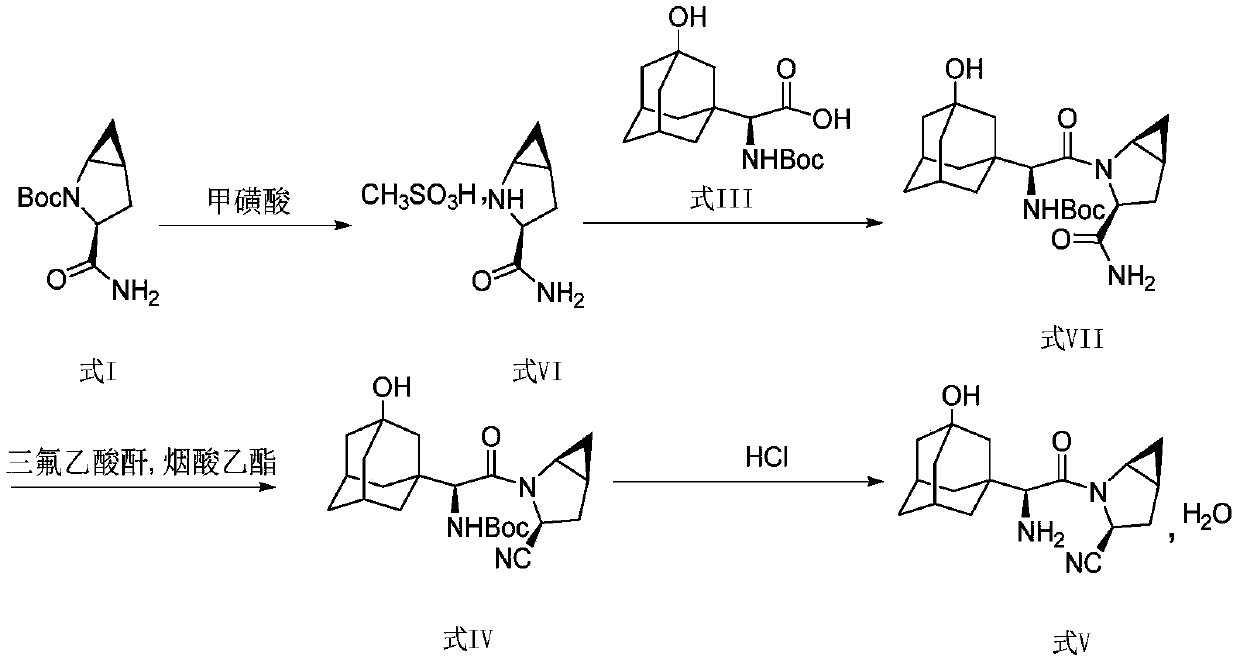
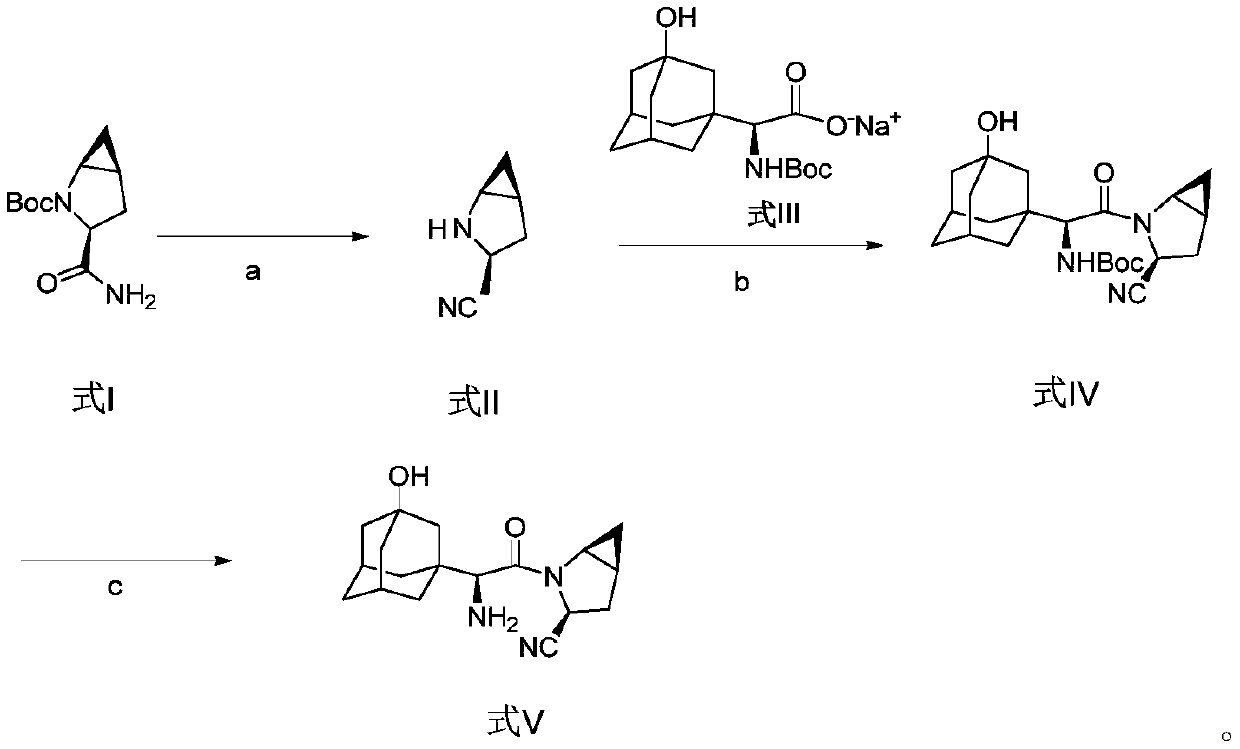
Method for preparing saxagliptin
The invention discloses a method for preparing saxagliptin, and belongs to the technical field of drug synthesis. The method comprises the following steps: (a) converting amide on a compound shown ina formula I into cyano and removing Boc groups to obtain a compound shown in a formula II; (b) condensing the compound shown in the formula II and a compound shown in a formula III to obtain a compound shown in a formula IV; and (c) removing Boc groups on the compound shown in the formula IV to obtain a compound shown in a formula V. According to the method, different raw materials and reaction reagents are selected, and a process route is changed, so that four procedures of salifying, condensing, cyanating and de-protecting are shortened into three procedures of cyanating, condensing and de-protecting; meanwhile, cyanating and condensation reaction are realized at the room temperature, the requirements on reaction conditions are reduced, and industrial production can be realized.
Owner:连云港恒运药业有限公司
Saxagliptin pharmaceutical composition and preparation method thereof
InactiveCN105078971ASimple processEasy to operate and controlOrganic active ingredientsMetabolism disorderSaxagliptinSide effect
The invention relates to saxagliptin pharmaceutical composition and a preparation method thereof. The saxagliptin pharmaceutical composition is freeze-dried orally-disintegrating preparation which comprises saxagliptin, filler, water-soluble or water-dispersable binder, pH adjusting agent, corrigent and / or flavoring agent and preservatives. The freeze-dried orally-disintegrating preparation refers to fast-disintegrating oral tablets or fast-disintegrating oral films obtained through freeze drying. The invention further provides the preparation method of the saxagliptin pharmaceutical composition. The saxagliptin pharmaceutical composition has the advantages that the saxagliptin pharmaceutical composition is simple in component, high in auxiliary material safety and simple and controllable in preparation process, a user can take the saxagliptin pharmaceutical composition without water and chewing, the total disintegrating time of the saxagliptin pharmaceutical composition in the oral cavity is less than 2 seconds, the saxagliptin pharmaceutical composition is fast in release and absorption, high in bioavailability, good in stability and low in toxic and side effects, and beneficial and unexpected technical effects are achieved.
Owner:陈跃坚
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com