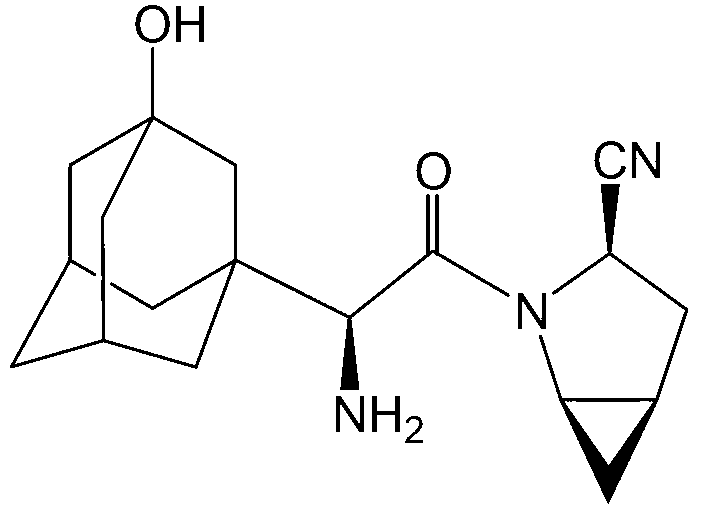
Method for producing saxagliptin
A technology for products and compounds, applied in the direction of organic chemistry, etc., can solve the problems of reduced total yield and cumbersome operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] refer to figure 1 , the method for synthesizing saxagliptin in the present embodiment is as follows:
[0039] Compound d was synthesized by referring to the method of J.Med.Chem, 2005, 48:5025-5037.
[0040] Compound d (384g), isopropanol (384ml), water (384ml) and 36% hydrochloric acid (126ml) were put into the reaction bottle, heated to an internal temperature of 65°C, and stirred for 1.5h; TLC tracking confirmed that the reaction was complete.
[0041] Concentrate the reaction solution in a 65°C water bath; add water (500ml) and methylene chloride (3.0L) to the concentrate, adjust the pH value of the upper layer to 9-10 with 20% potassium carbonate solution under stirring, add sodium chloride (480g ), stirred for 30 min; allowed to stand, the upper layer was extracted with dichloromethane (3.0L), and discarded after TLC showed no product; the lower layers were combined, washed with water (3.0L), and filtered to remove mechanical impurities.
[0042] Concentrate to ...
Embodiment 2
[0044] Provides compound d.
[0045] Compound d (350g), isopropanol (385ml), water (385ml) and 36% hydrochloric acid (125ml) were put into the reaction flask, heated to an internal temperature of 65°C, and stirred for 1.5h; TLC tracking confirmed that the reaction was complete.
[0046] Concentrate the reaction solution in a 65°C water bath; add water (500ml) and methylene chloride (3.0L) to the concentrate, adjust the pH value of the upper layer to 9-10 with 20% potassium carbonate solution under stirring, add sodium chloride (480g ), stirred for 30 min; the upper layer was extracted with dichloromethane; the lower layers were combined, washed with water (3.0 L), and filtered to remove mechanical impurities.
[0047]Concentrate to about 500ml, add ethyl acetate (1.2L), stir slowly; after 0.5h, add 3ml of water, stir for 0.5h, add 3ml of water again, stir at room temperature for 0.5h; remove residual dichloromethane in the system; stir at room temperature for 15h ; Filter, wa...
Embodiment 3
[0049] refer to figure 2 , the method for synthesizing saxagliptin in the present embodiment is as follows:
[0050] Preparation of compound a
[0051] In a four-necked flask, compound a-1 (150g, 0.667mol, (1S,3S,5S)-3-(aminocarbonyl)-2-azabicyclo[3.1.0], CAS: 361440-67-7), Isopropanol Isopropanol (1.5L), methanesulfonic acid (83.2g, 0.866mol), heated to an internal temperature of 65°C under stirring, kept stirring for 3h, cooled the reaction solution to 0-5°C, kept stirring for 1h, A large amount of solid precipitated, filtered, washed with 200ml of isopropanol, and dried at 55°C for 8h to obtain off-white solid a (142.5g).
[0052] Preparation of compound c
[0053] At room temperature, put a (142.5g), b (204.5g, N-tert-butoxycarbonyl-3-hydroxy-1-adamantyl-D-glycine, CAS: 361442-00-4), HOBT into the reaction bottle (86.6g, CAS: 80029-43-2), EDC·HCl (131.3g, CAS: 25952-53-8), DIPEA (170.5g, CAS: 7087-68-5) with acetonitrile (520ml), ethyl acetate The ester (500ml) w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com