Saxagliptin midbody preparing method
A technology of intermediates and compounds, applied in the field of preparation of pharmaceutical intermediates, can solve the problems of cumbersome post-processing, poor chiral selectivity, large amount of solvent, etc., and achieve the effects of easy control of operating conditions, stable activity, and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
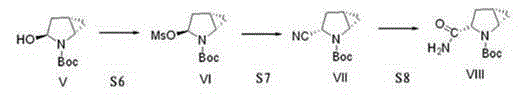
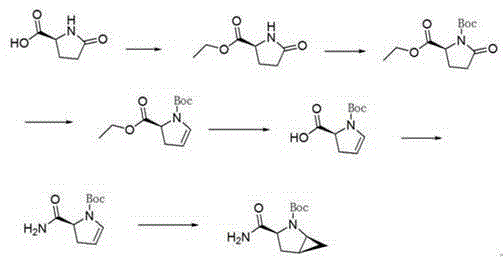
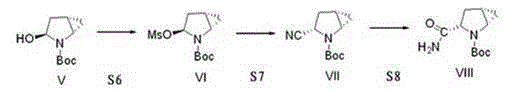
[0038] The invention discloses a preparation method of a saxagliptin intermediate, which relates to the technical field of preparation of heterocyclic compounds with 1 heterocycle, 3 chiral centers, and amide groups directly connected to ring carbon atoms. Compound of formula VIII. The method comprises the following steps: using (R)-5-hydroxypyrrole-2-one as a raw material, and using benzyl alcohol as a solvent, dehydrating at 70-80°C to obtain 5-benzylhydroxypyrrole-2-one; , reduction, dehydration, cyclopropanation, to generate a compound of formula IV, followed by debenzylation, methylsulfonyl, cyano, and finally hydrolysis to obtain (1S, 3S, 5S)-3-(aminocarbonyl)-2-aza tert-butyl bicyclo[3.1.0]hexane-2-carboxylate.
[0039] The reaction formula is as follows:
[0040]
[0041] The raw materials used in the method of the invention are easy to prepare, the operating conditions are easy to control, the reaction yield is high, there is no pollution, the corresponding isomer ...
Embodiment 1
[0046] Put 30g (R)-5-hydroxypyrrol-2-one, 312g benzyl alcohol, and 2ml hydrochloric acid into the reaction bottle, heat to 70-80°C, keep stirring for 8 hours, check that the reaction of raw materials is complete, cool down to 15-20°C, and filter , the filter cake was rinsed with 10ml of ethyl acetate, and dried to obtain 49.9g of compound I, with a yield of 88% and a melting point of 80-82°C.
Embodiment 2
[0048] Put 10g (R)-5-hydroxypyrrol-2-one, 134g benzyl alcohol, and 0.8ml hydrochloric acid into the reaction bottle, heat to 50-60°C, keep stirring for 10h, check that the reaction of raw materials is complete, and cool down to 15-20°C, After filtering, the filter cake was rinsed with 5ml of ethyl acetate, and dried to obtain 19.5g of compound I, with a yield of 86% and a melting point of 80-82°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com