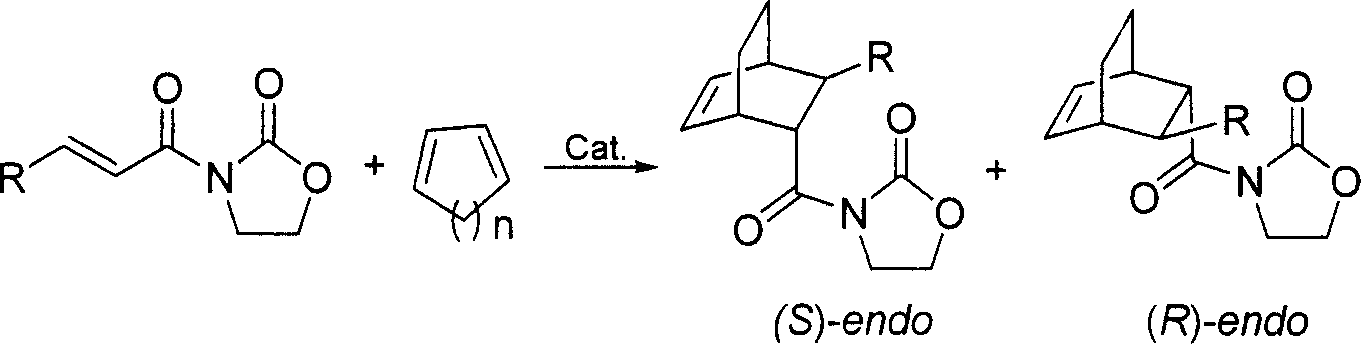
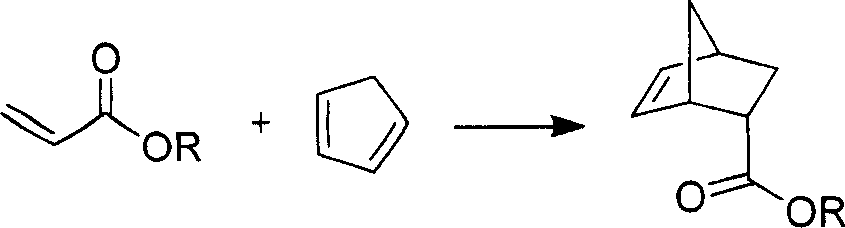
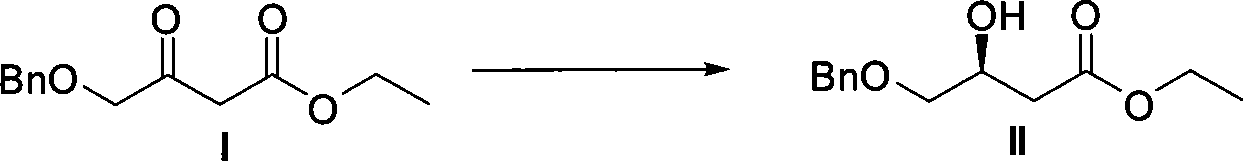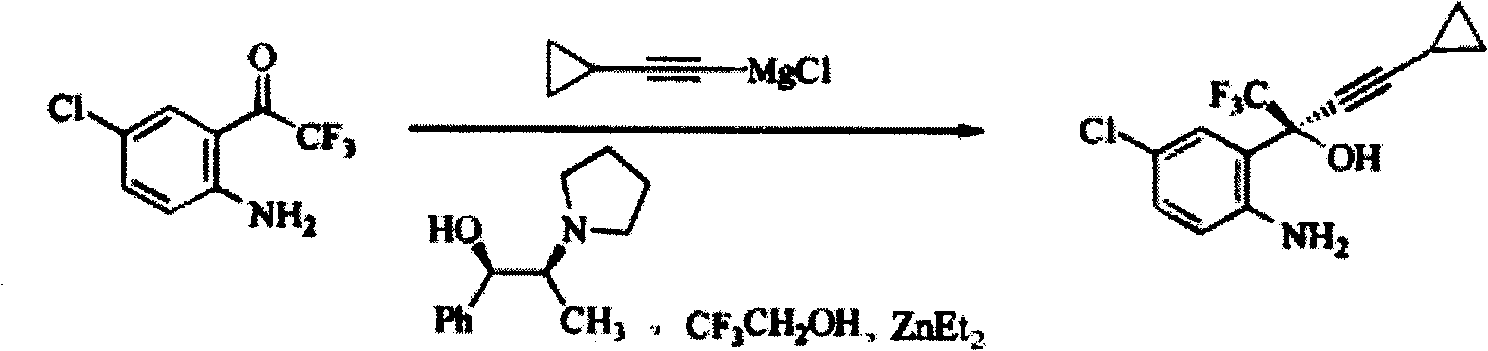Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
466 results about "Chirality" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
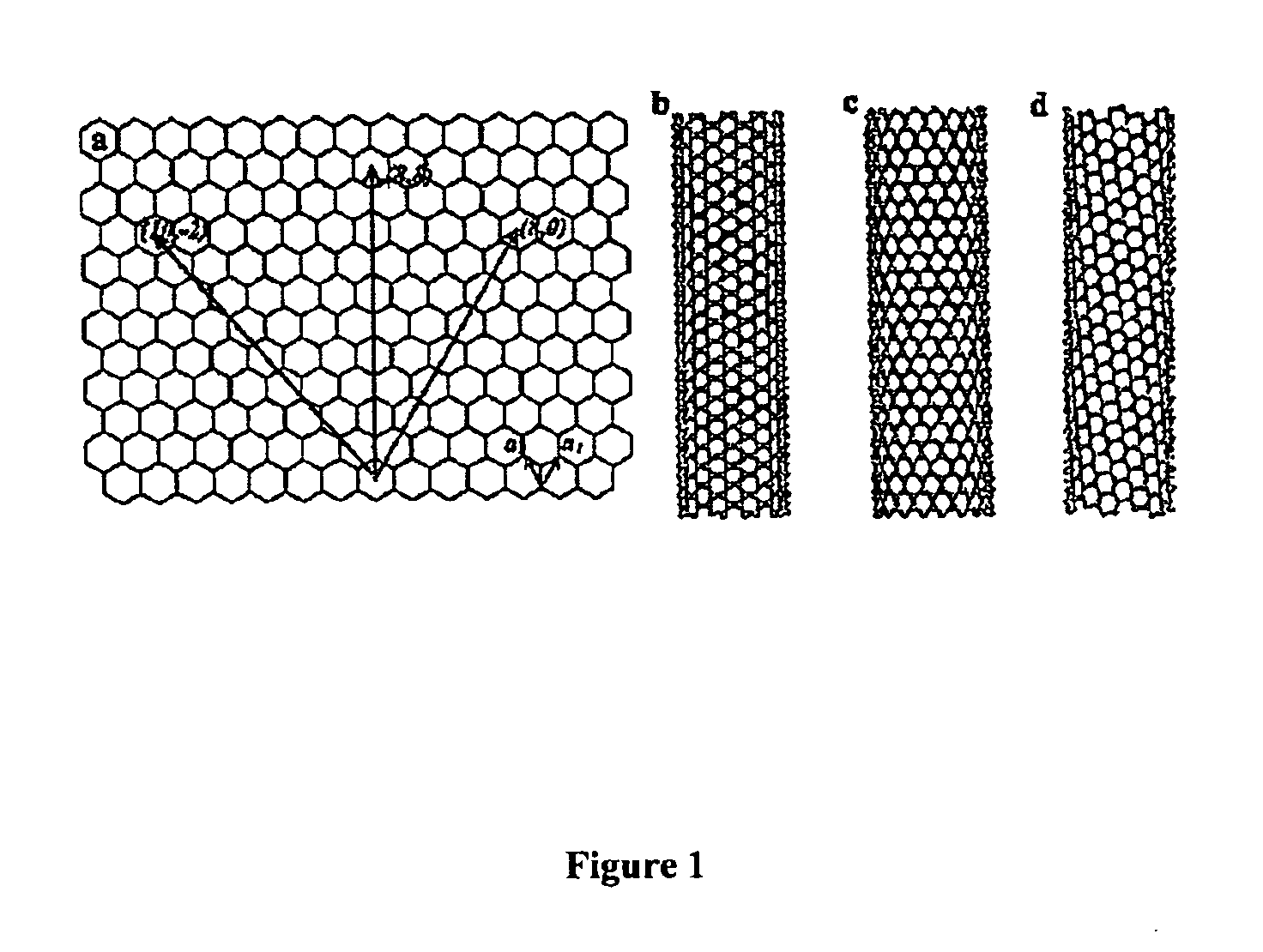
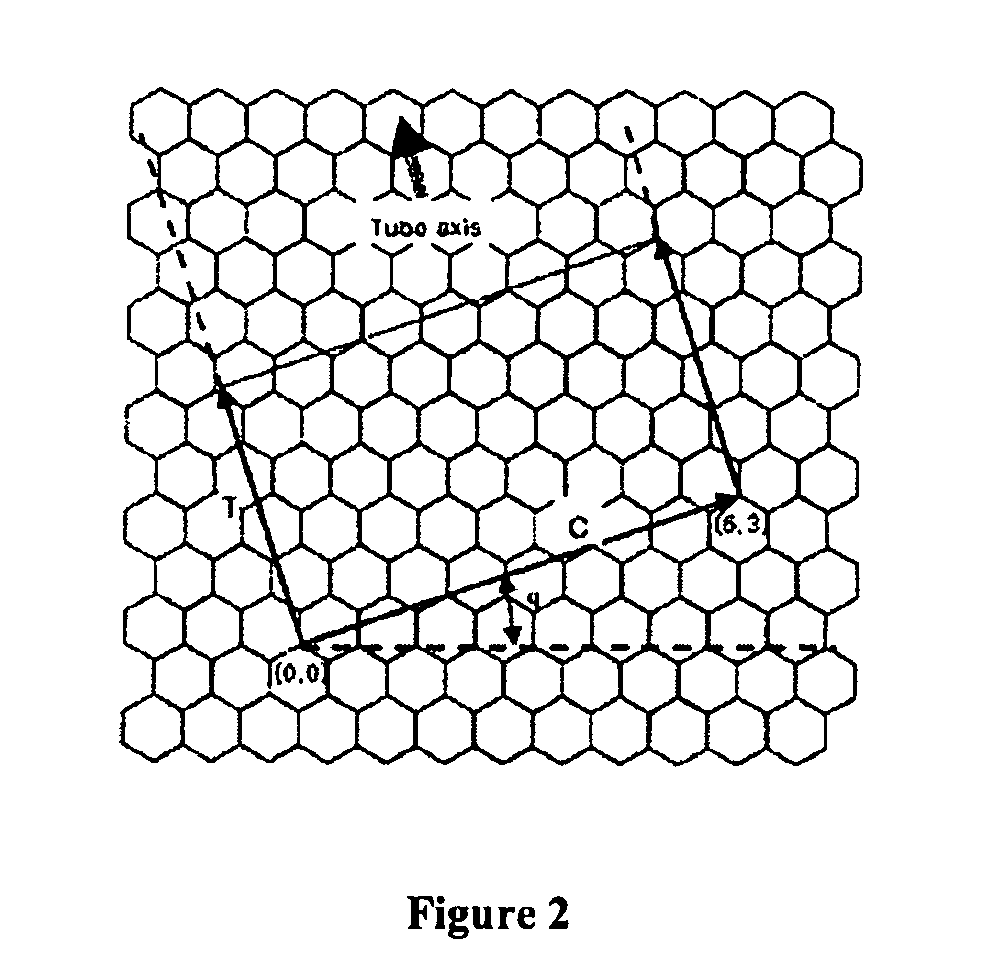
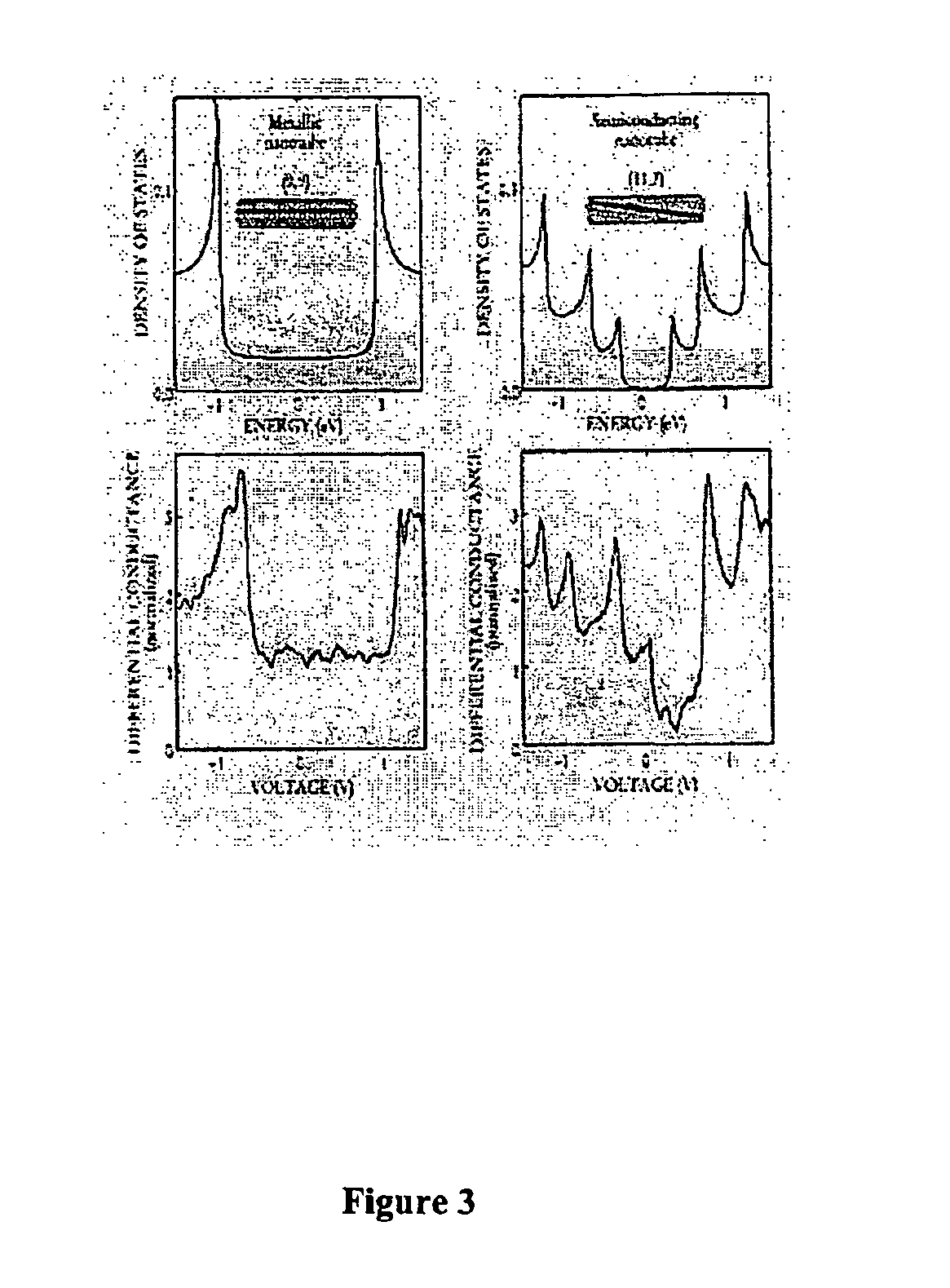
Chirality /kaɪˈrælɪtiː/ is a property of asymmetry important in several branches of science. The word chirality is derived from the Greek χειρ (kheir), "hand," a familiar chiral object. An object or a system is chiral if it is distinguishable from its mirror image; that is, it cannot be superposed onto it. Conversely, a mirror image of an achiral object, such as a sphere, cannot be distinguished from the object. A chiral object and its mirror image are called enantiomorphs (Greek, "opposite forms") or, when referring to molecules, enantiomers. A non-chiral object is called achiral (sometimes also amphichiral) and can be superposed on its mirror image.
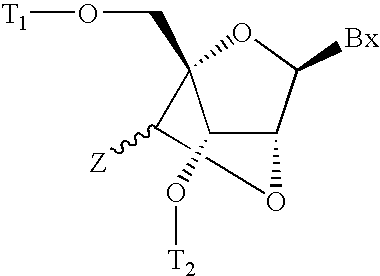
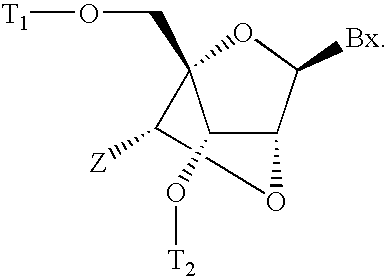
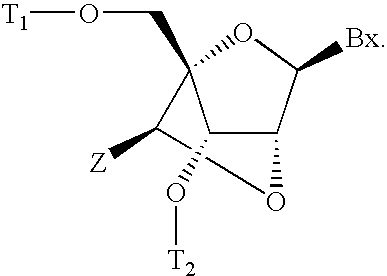
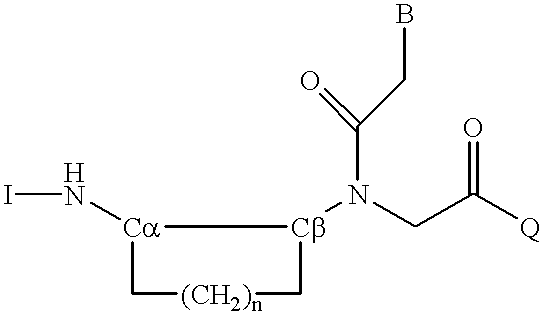
6-modified bicyclic nucleic acid analogs
The present invention provides 6-modified bicyclic nucleoside analogs and oligomeric compounds comprising these nucleoside analogs. In preferred embodiments the nucleoside analogs have either (R) or (S)-chirality at the 6-position. These bicyclicnucleoside analogs are useful for enhancing properties of oligomeric compounds including nuclease resistance.
Owner:IONIS PHARMA INC
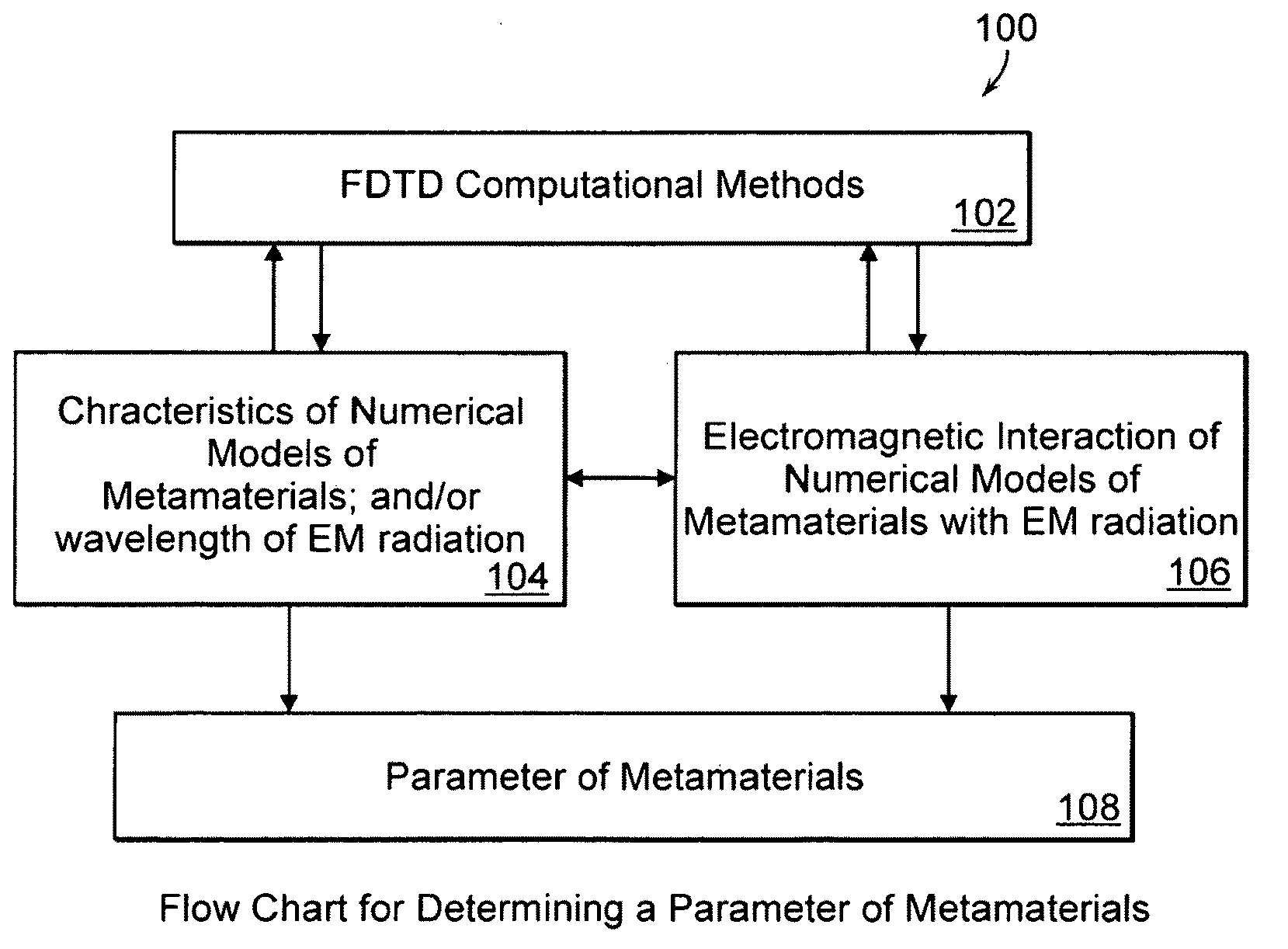
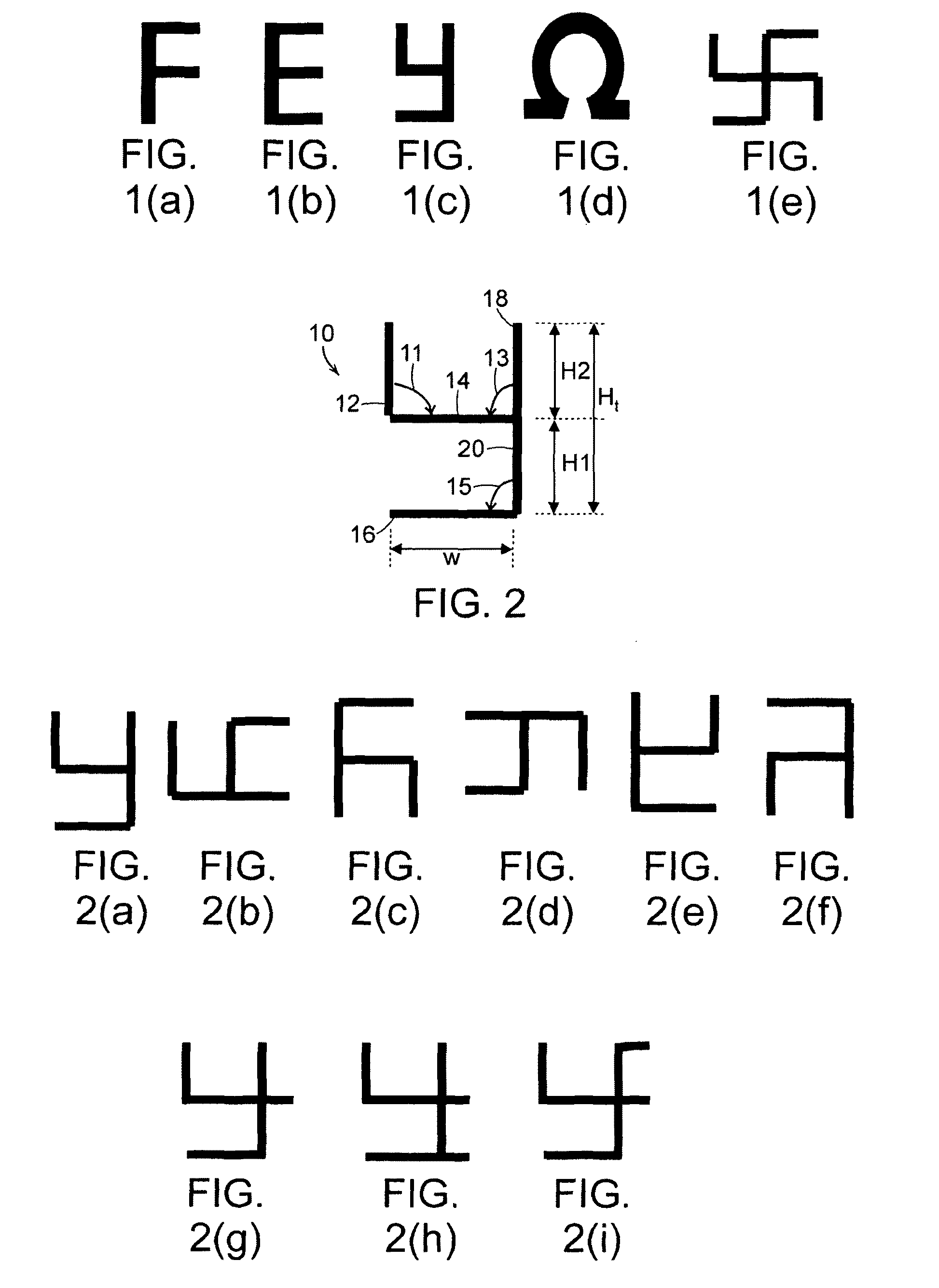
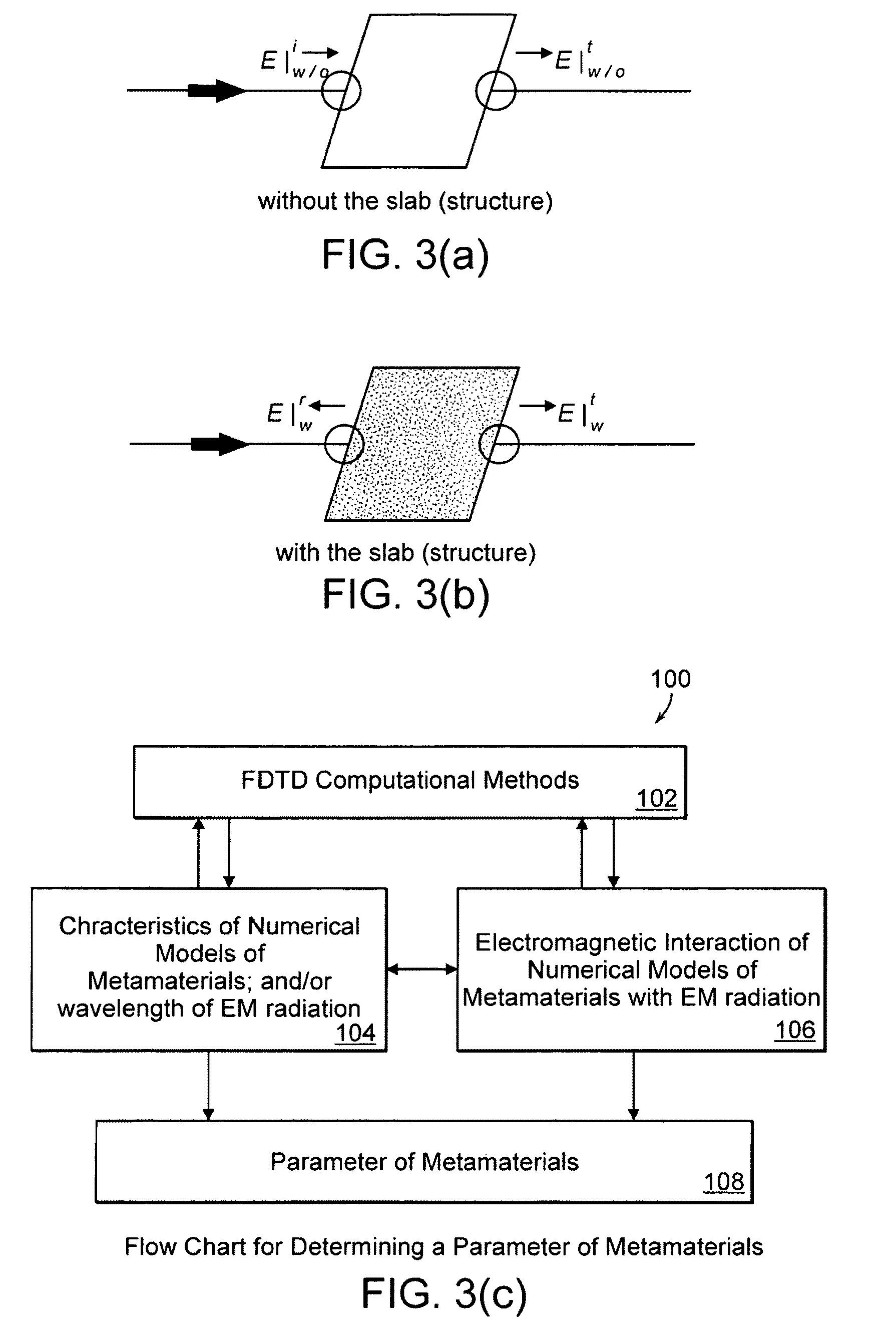
Chiral Metamaterials
InactiveUS20100141358A1Effective controlEnhance the imagePrinted circuit assemblingMultiple-port networksDielectric substrateLength wave
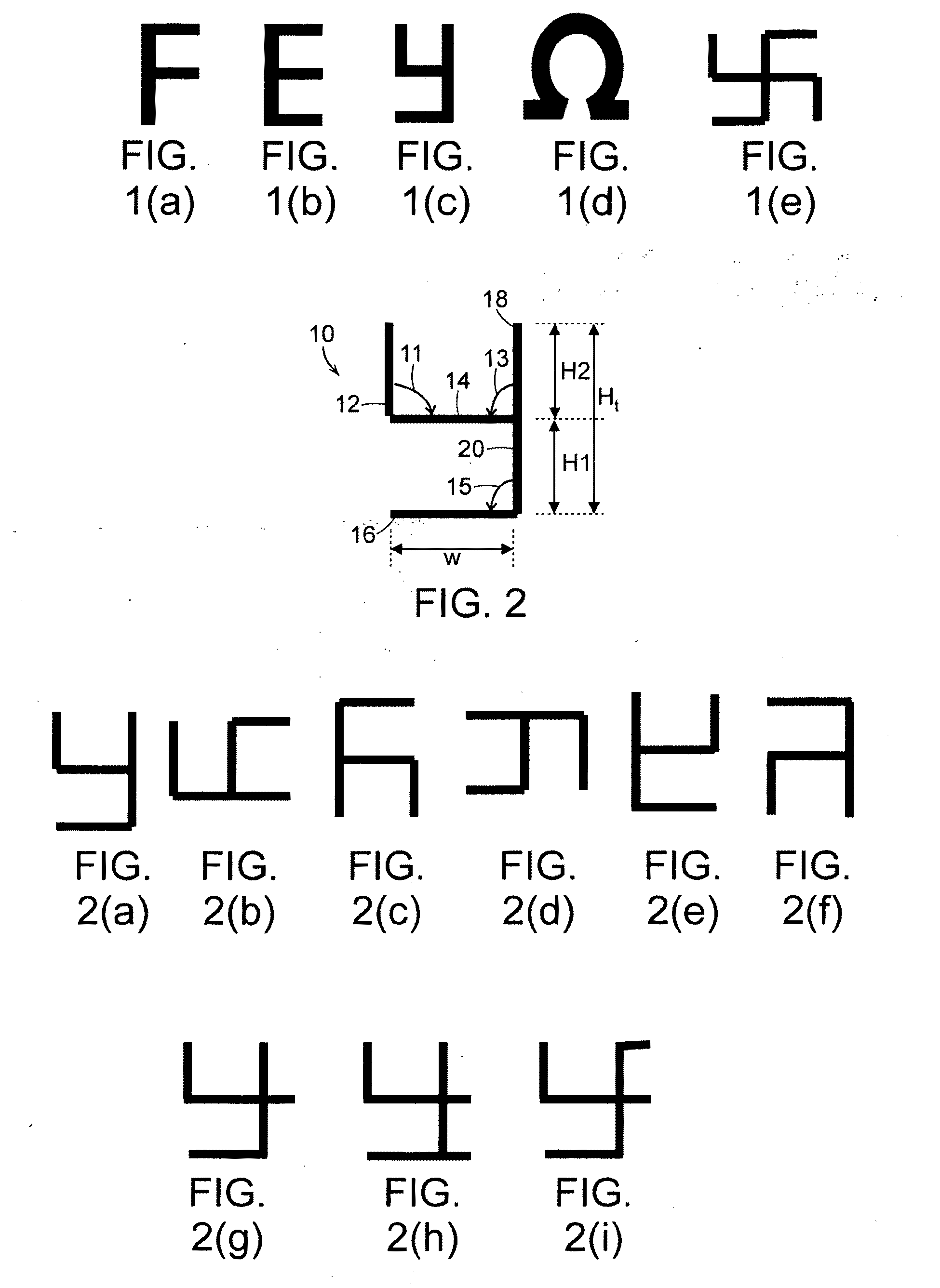
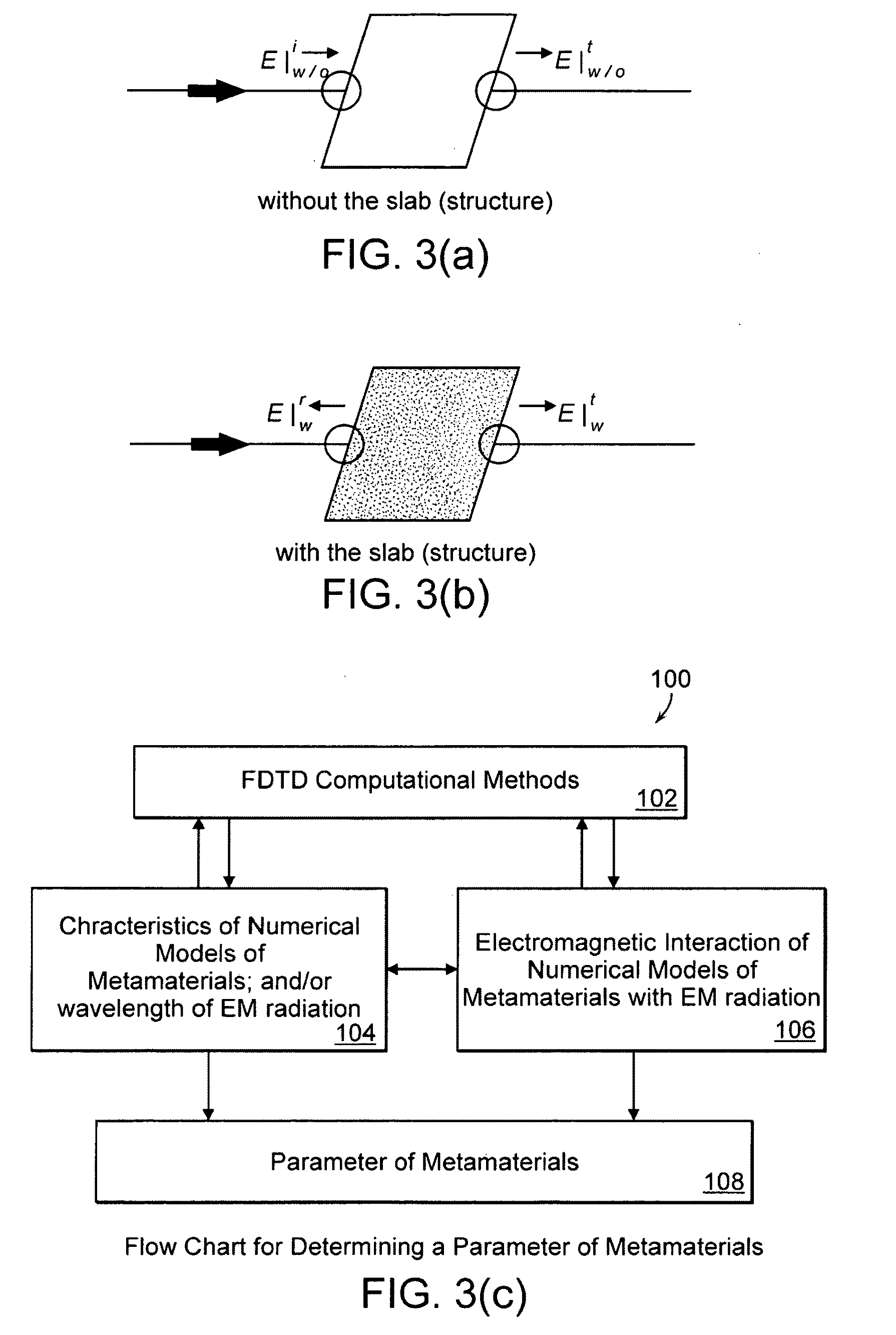
A metamaterial includes a dielectric substrate and an array of discrete resonators at the dielectric substrate, wherein each of the discrete resonators has a shape that is independently selected from: an F-type shape; an E-type shape; or a y-type shape. A parameter of a chiral metamaterial is determined and a chiral metamaterial having such a parameter is prepared by the use of a model of the chiral metamaterial. The metamaterial model includes an array of discrete resonators. In one embodiment, each of the discrete resonators has a shape that is independently selected from the group consisting of: an F-type shape; an E-type shape; and a y-type shape. To the metamaterial model, electromagnetic (EM) radiation, preferably plane-polarized EM radiation in a visible, ultraviolet or near-infrared region, having at least one wavelength that is larger than the largest dimension of at least resonator of the metamaterial model, is applied. Varying at least one characteristic of the metamaterial model and / or at least one wavelength of the applied EM radiation modulates EM interaction of the applied EM radiation with the metamaterial model, thereby determining a parameter of the chiral metamaterial. By the use of a model of the chiral metamaterial, a number of discrete resonators of a chiral metamaterial that are arrayed in a direction perpendicular to a propagation axis of EM radiation is also determined.
Owner:UNIVERSITY OF MASSACHUSETTS LOWELL
Peptide nucleic acid incorporating a chiral backbone
A novel class of peptide nucleic acid monomers are synthesized having chirality in the backbone. Peptide nucleic acid oligomers are synthesized to incorporate these chiral monomers.
Owner:PETER E NIELSEN
Monodisperse single-walled carbon nanotube populations and related methods for providing same
InactiveUS20080217588A1Material nanotechnologyIndividual molecule manipulationCarbon nanotubeChirality
The present teachings provide methods for providing populations of single-walled carbon nanotubes that are substantially monodisperse in terms of diameter, electronic type, and / or chirality. Also provided are single-walled carbon nanotube populations provided thereby and articles of manufacture including such populations.
Owner:NORTHWESTERN UNIV
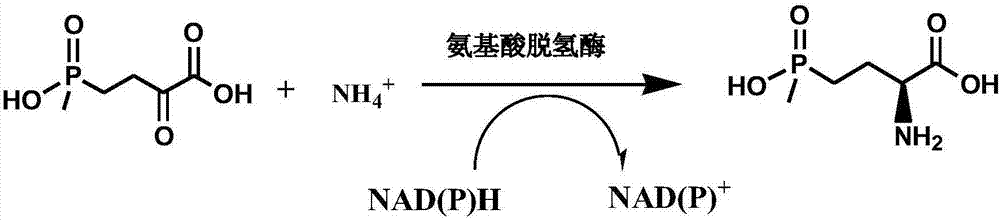
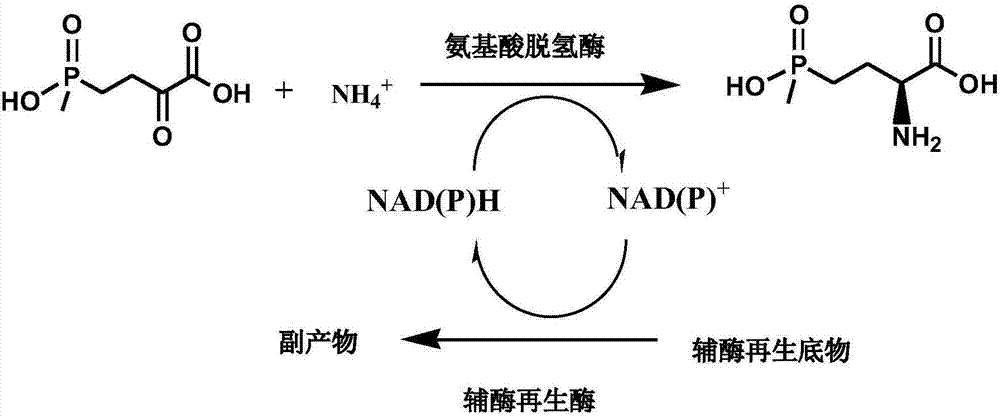
Method for preparing L-glufosinate-ammonium by use of amino acid dehydrogenase
The invention discloses a method for preparing L-glufosinate-ammonium by use of amino acid dehydrogenase. The method comprises the following steps: taking 2-carbonyl-4-(hydroxymethylphosphonyl)butyric acid or a salt thereof as a substrate, taking a cell of the isolated glutamate dehydrogenase or in vitro expression glutamate dehydrogenase as a catalyst to perform reductive amination reaction under the condition of the existence of inorganic amino donor and reduced coenzyme, thereby acquiring the L-glufosinate-ammonium. The method disclosed by the invention is high in raw material conversion rate and high in yield, the product is easy to separate and purify, and the chirality purity is high; compared with the transaminase and like catalysis technology, the process is relatively simple, and the raw material conversion rate reaches up to 100%.
Owner:ZHEJIANG UNIV
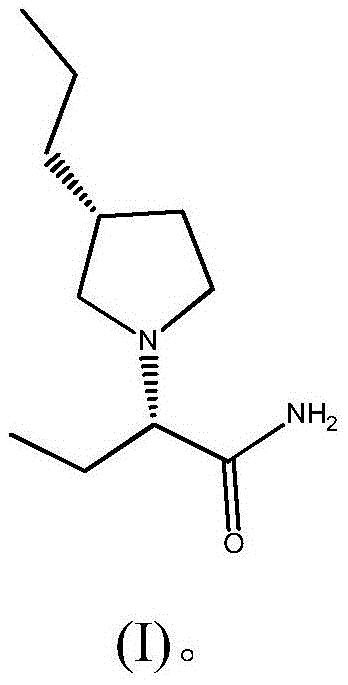
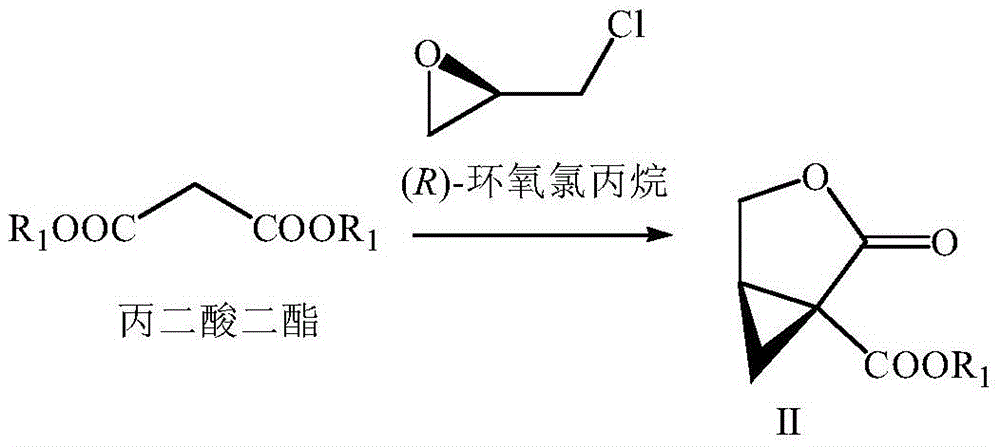
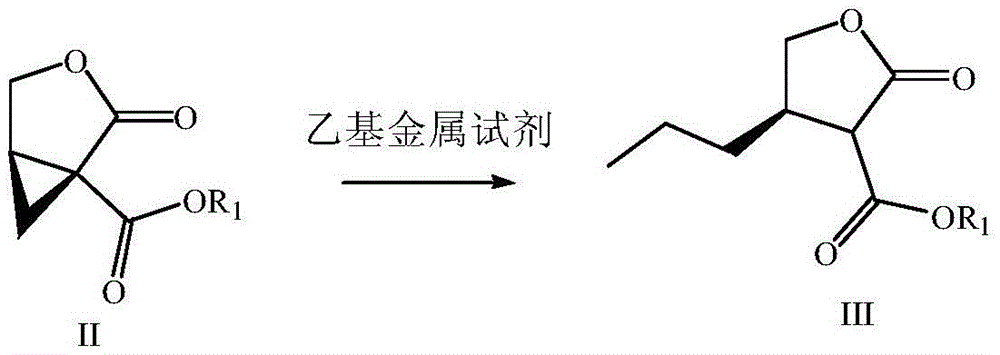
Preparation method of brivaracetam
The invention provides a preparation method of brivaracetam. The preparation method of brivaracetam comprises the following steps of in an alkaline reagent, reacting malonate ester and (R)-epichlorohydrin to obtain lactone (II), reacting the lactone (II) and an ethyl metal-based reagent to obtain an intermediate (III), removing carboxylase to obtain (R)-4-propyl-dihydrofuran-2-ketone (IV), performing ring-opening reaction under the action of a halogenated ring-opening reagent to obtain (R)-3-halogenarated methyl hexanoate or (R)-3- halogenarated methyl hexyl acetate (V), and reacting with (S)-2-aminobutanamide or an acceptable salt, so as to obtain the brivaracetam. The preparation method has the advantages that the high-purity brivaracetam (HPLC (high performance liquid chromatography: greater than 96%) and stereo rotary brivaracetam (chirality HPLC: greater than 98%) can be directly prepared; the silicagel column separation and purification or the chirality preparation column separation and purification is not used, so that the complicated separation and purification step is not performed, the cost is saved, and the preparation method is more suitable for industrial production.
Owner:佛山市隆信医药科技有限公司
Data Storage Device and Method
InactiveUS20080278998A1Improve scalabilityImprove storage densityNanotechSemiconductor/solid-state device manufacturingMass storageNanowire
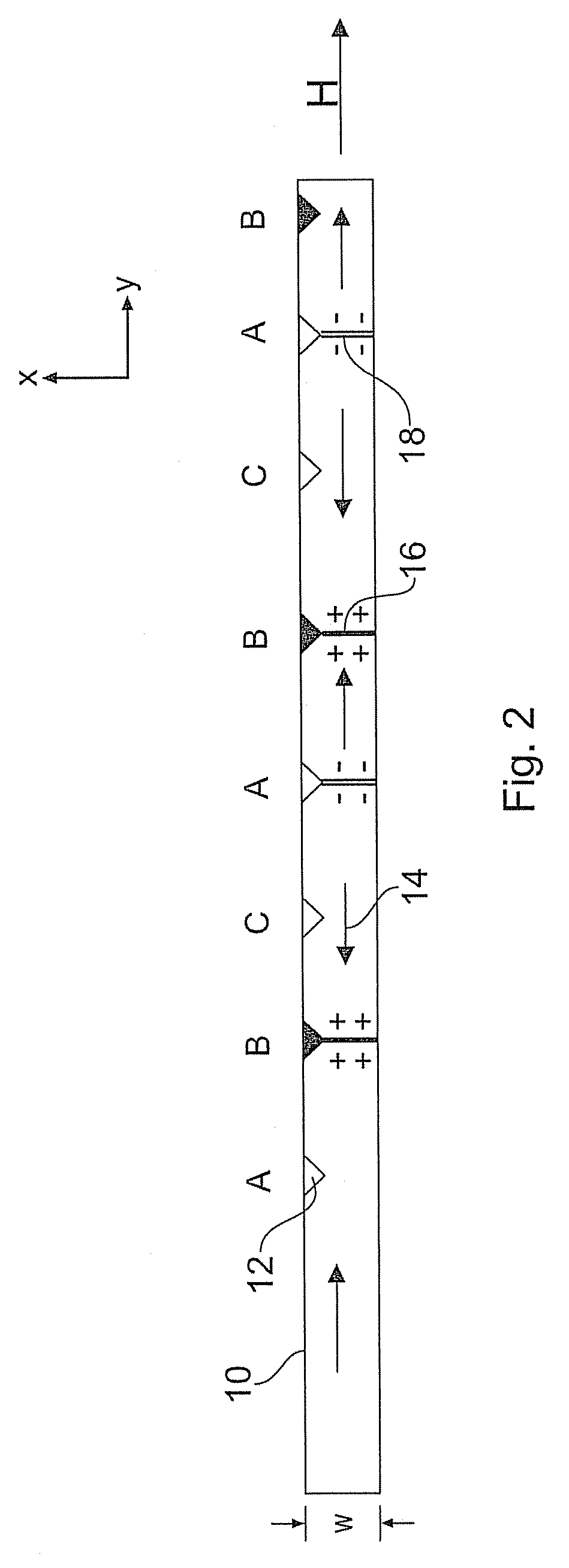
A serial magnetic mass storage device and associated data storage method is provided based on magnetic nanowires that support single magnetic domains separated by domain walls. Each data-storing nanowire has a plurality of crossing nanowires along its length, forming cross junctions that constitute domain wall pinning sites. Data is fed through each data-storing nanowire by moving the magnetic domains under the action of a field that alternates between alignment and anti-alignment with the crossing nanowires. The data is encoded in the chirality of the domain walls, with up and down chirality transverse domain walls being used to encode 0's and 1's. Data is clocked into each nanowire with suitable nucleation generators capable of nucleating domains with domain walls of pre-defined chirality. Data is clocked out of each nanowire with suitable magnetic field sensors that sense the chirality.
Owner:INGENIA HLDG LTD
Carbon nanotubes, process for their production, and catalyst for production of carbon nanotubes
ActiveUS7311889B2Efficient productionGrowth inhibitionMaterial nanotechnologyFibre chemical featuresMetal catalystCarbon nanotube
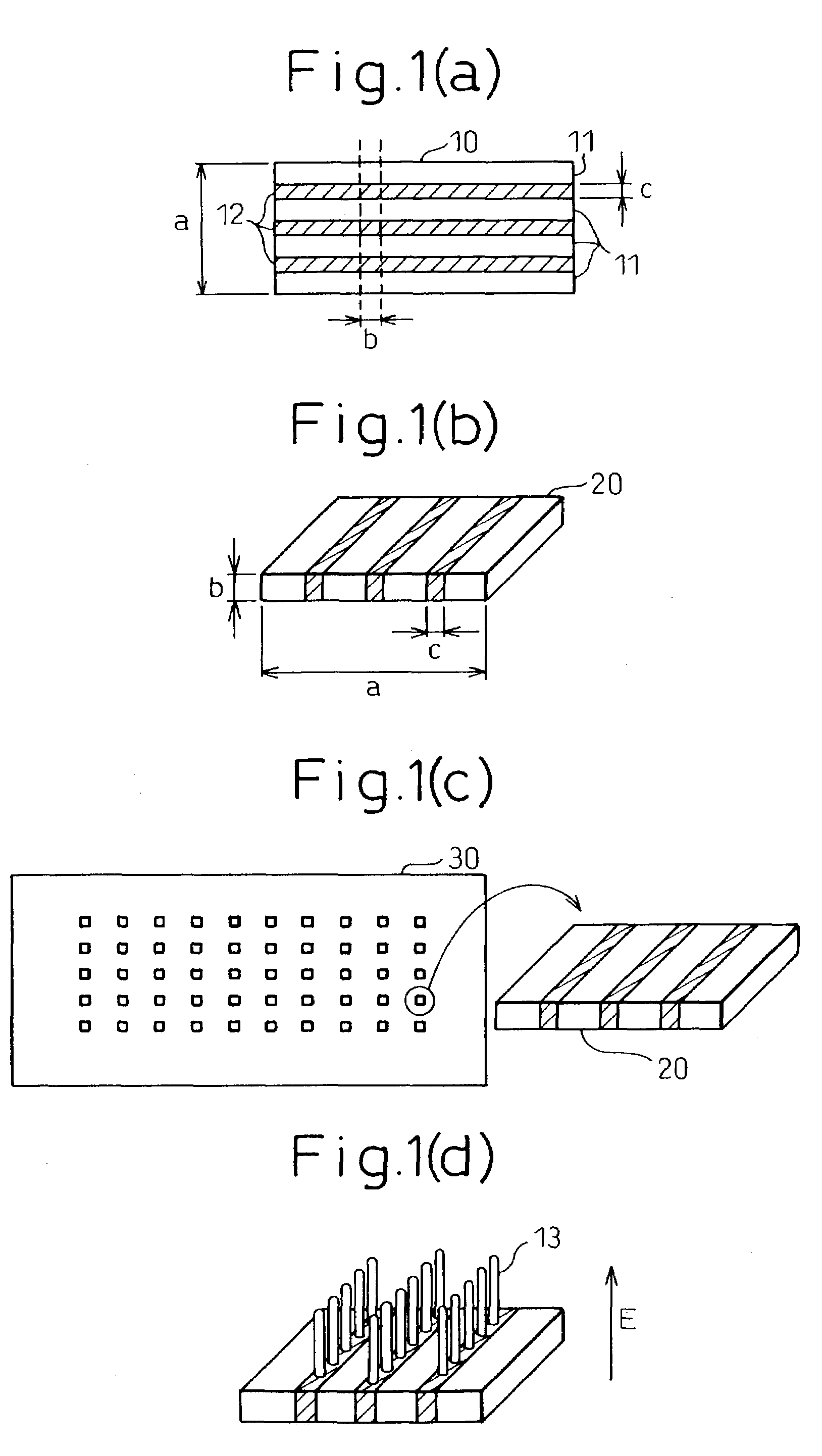
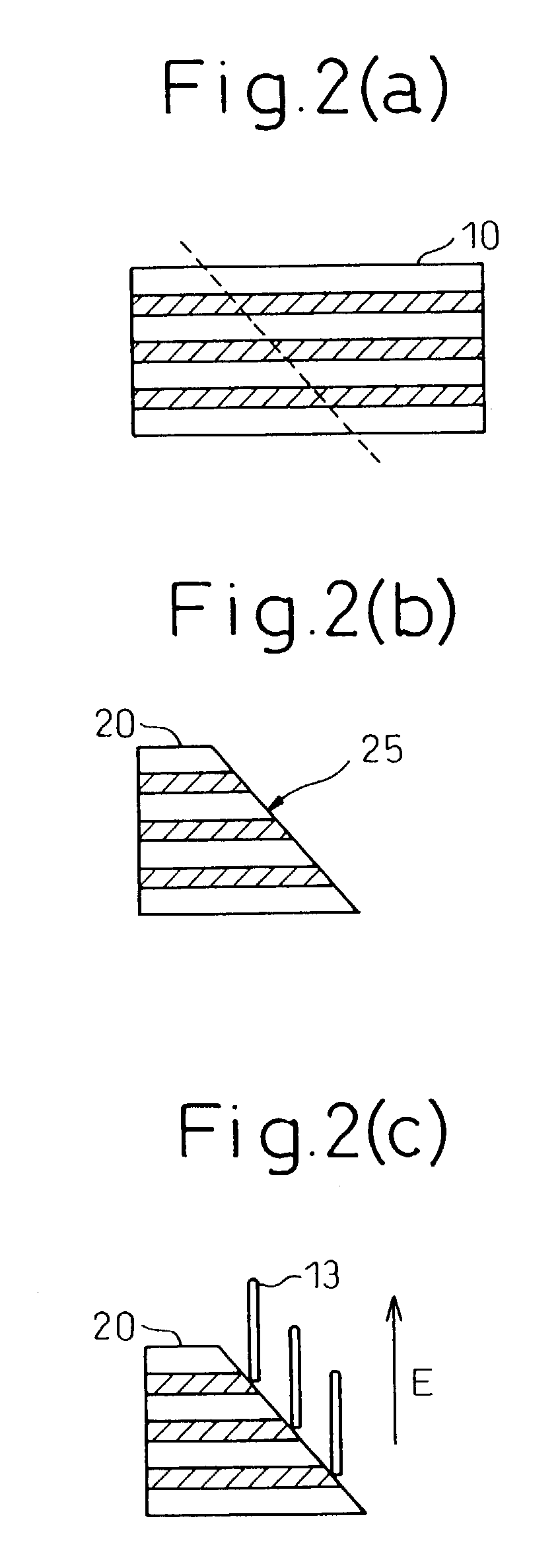
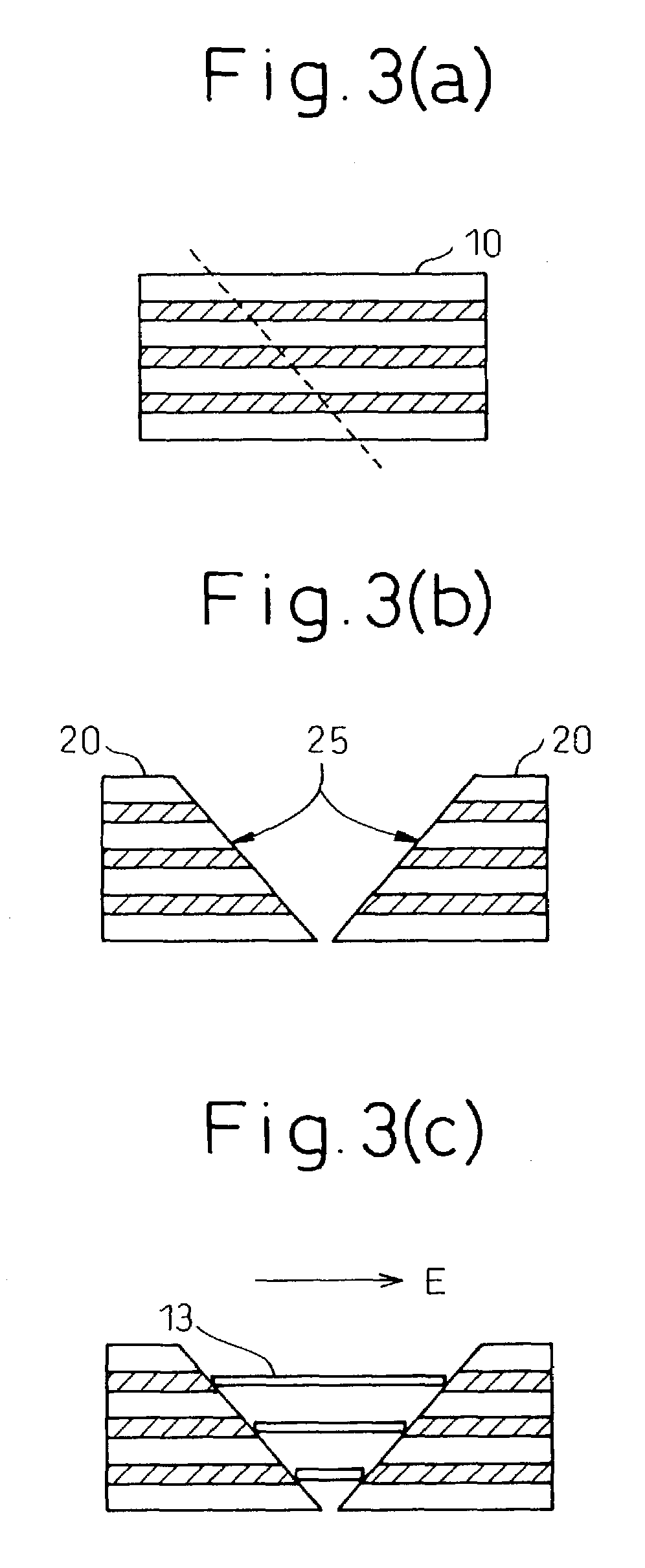
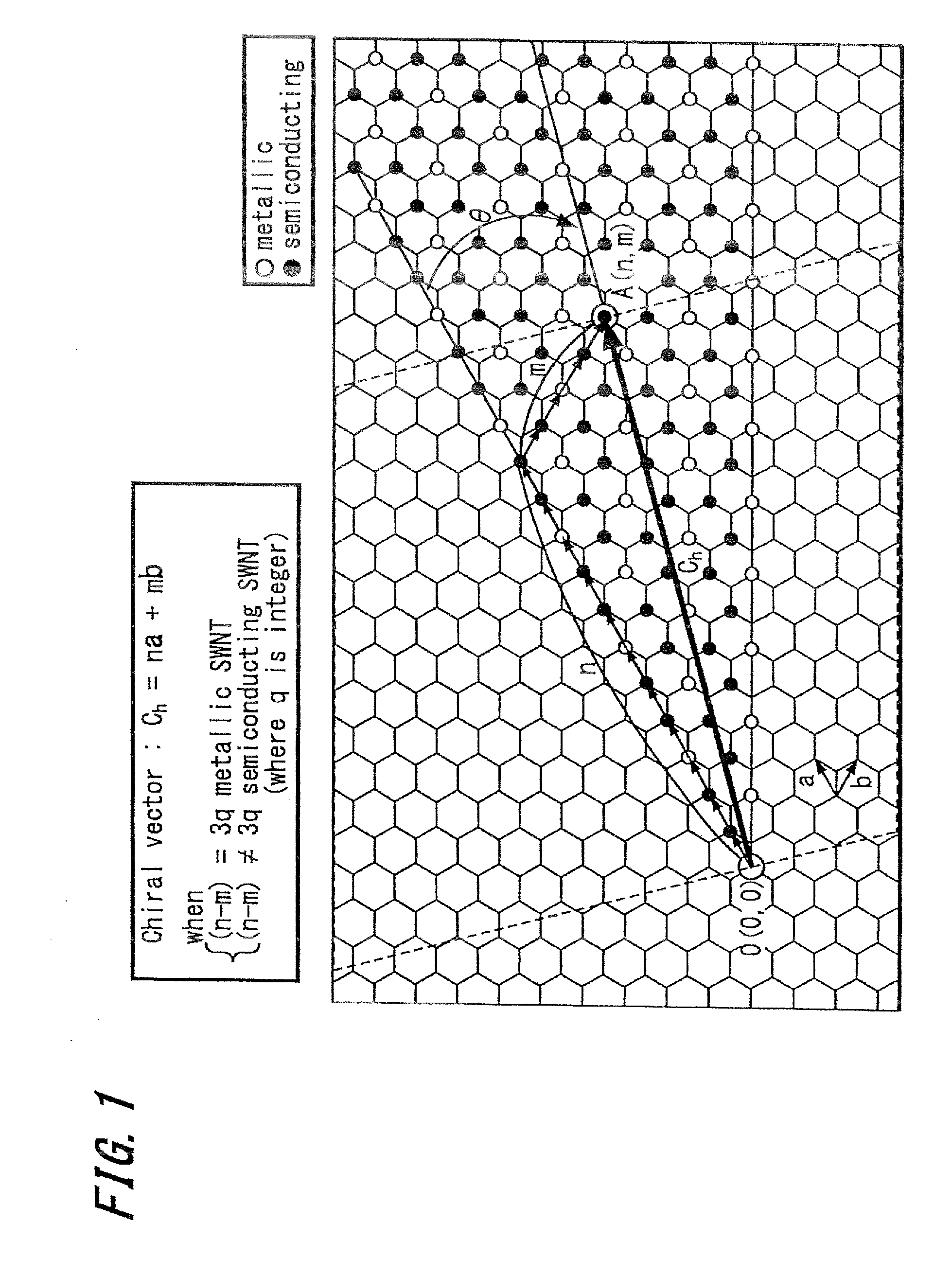
The invention provides a process for production of carbon nanotubes whereby a laminate prepared by alternating lamination of a metal catalyst and a material other than the metal catalyst is cut to expose the laminated structure, and carbon nanotubes are grown on the metal catalyst at the cut surface of the laminate. The process results in high-quality carbon nanotubes, with minimized bundle growth, which are each individually and independently arranged in a highly precise manner at prescribed locations.The invention also provides a carbon nanotube production process comprising a step of preparing a substrate which is inclined in one or two dimensions from a specific highly symmetrical crystal orientation and vapor depositing a metal catalyst along the atomic steps appearing on the surface of the substrate, and a step of growing the carbon nanotubes by chemical vapor deposition (CVD) using the metal catalyst as nuclei. It is thus possible to control the growth locations, diameters, orientation and chirality of carbon nanotubes.
Owner:FUJITSU LTD
Pure-chirality carbon nanotubes and methods
A method of providing bulk products of pure-chirality single walled nanotubes having substantially one chirality, and bulk products of pure-chirality nanotubes having at least 50% one chirality. By providing bulk products of pure-chirality nanotubes, the electrical conductivity of the nanotubes can be predetermined and can be made more electrically conductive or more semi-conductive, as desired. Also provided are methods of purifying bulk products of multiple chirality nanotubes into pure-chirality nanotube bulk products, as well as methods of identifying chiralities of bulk product nanotubes. Moreover, fluorocarbon surfactant systems capable of solubilizing nanotubes in perfluorocarbon solvents and facilitating purification and processing are also provided.
Owner:LUNA INNOVATIONS
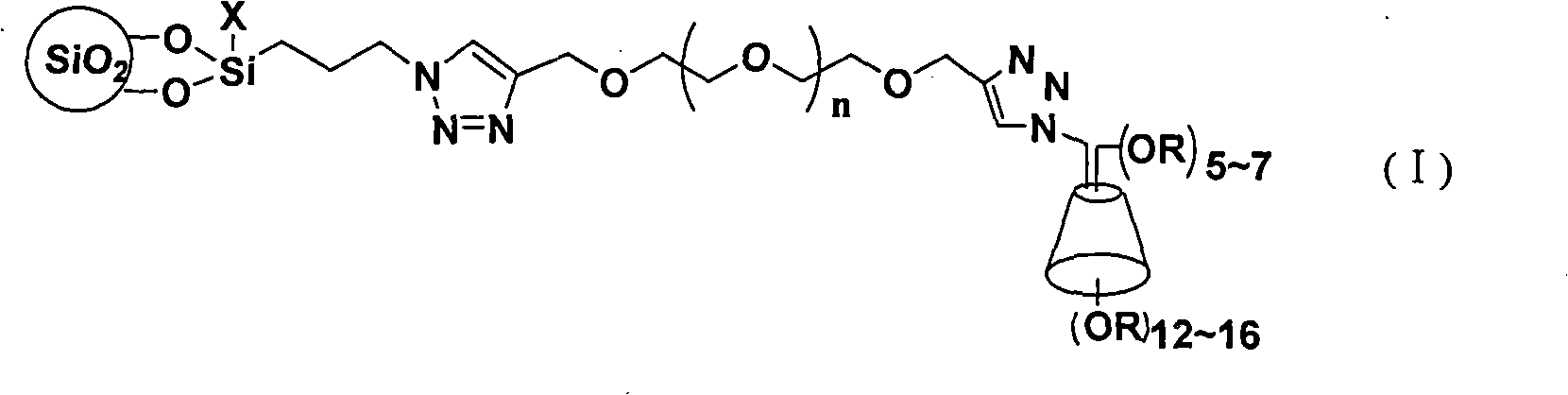
Cyclodextrin chiral chromatogram fixed phase and preparation method thereof
InactiveCN101306354AHigh column efficiencyHigh selectivityOther chemical processesAzirineChemical reaction
The invention discloses a cyclodextrin chiral stationary phase, the structure of which is shown in the general formula (I), wherein X is -OCH3 or -OCH2CH3, n is equal to 1-7, and R is -H, -CH3, -COCH3, -COC6H5 and -CONHC6H5. The preparation method of the stationary phase comprises the following steps: a silane coupling agent, sodium azide and a catalyst are added into an organic solvent, then spheroidal silicon is added for preparing azide silica gel derivant; oligomeric ethylene glycol, sodium hydride and propargyl bromide are added into tetrahydrofuran for preparing bialkynyl oligomeric ethylene glycol; monosubstituted nascent and derivative cyclodextrin containing azid groups is prepared; finally, the click chemistry reaction method is used for bonding the cyclodextrin. The cyclodextrin chiral stationary phase has the advantages that the selectivity of the bonding reaction is high, and the surface bonded amount is large; the chiral separation ability is strong, thereby being especially suitable for the chiral separation of a high efficiency liquid chromatography in the reversed-phase mode; the preparation method is simple and has less steps, the bonding reaction is the click chemistry reaction, the reaction condition is mild, and the reaction is carried out in the water solution.
Owner:EAST CHINA UNIV OF SCI & TECH
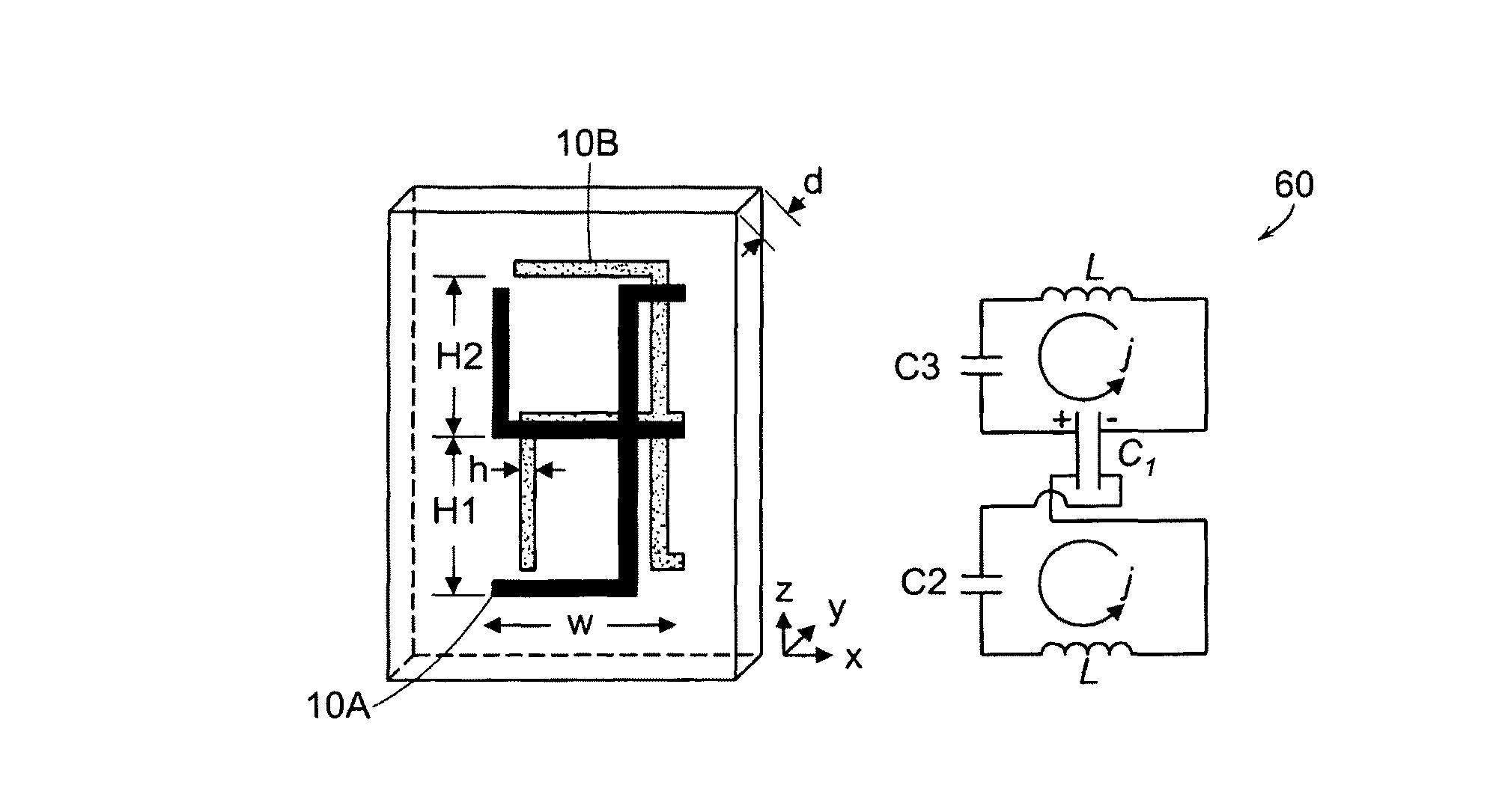
Chiral metamaterials
InactiveUS8271241B2Effective controlEnhance the imagePrinted circuit assemblingMultiple-port networksDielectric substrateLength wave
A metamaterial includes a dielectric substrate and an array of discrete resonators at the dielectric substrate, wherein each of the discrete resonators has a shape that is independently selected from: an F-type shape; an E-type shape; or a y-type shape. A parameter of a chiral metamaterial is determined and a chiral metamaterial having such a parameter is prepared by the use of a model of the chiral metamaterial. The metamaterial model includes an array of discrete resonators. In one embodiment, each of the discrete resonators has a shape that is independently selected from the group consisting of: an F-type shape; an E-type shape; and a y-type shape. To the metamaterial model, electromagnetic (EM) radiation, preferably plane-polarized EM radiation in a visible, ultraviolet or near-infrared region, having at least one wavelength that is larger than the largest dimension of at least resonator of the metamaterial model, is applied. Varying at least one characteristic of the metamaterial model and / or at least one wavelength of the applied EM radiation modulates EM interaction of the applied EM radiation with the metamaterial model, thereby determining a parameter of the chiral metamaterial. By the use of a model of the chiral metamaterial, a number of discrete resonators of a chiral metamaterial that are arrayed in a direction perpendicular to a propagation axis of EM radiation is also determined.
Owner:UNIVERSITY OF MASSACHUSETTS LOWELL
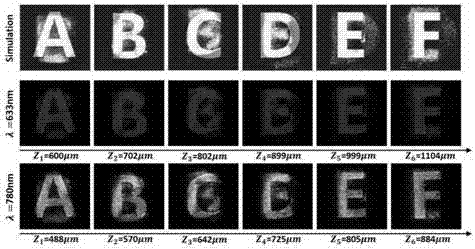
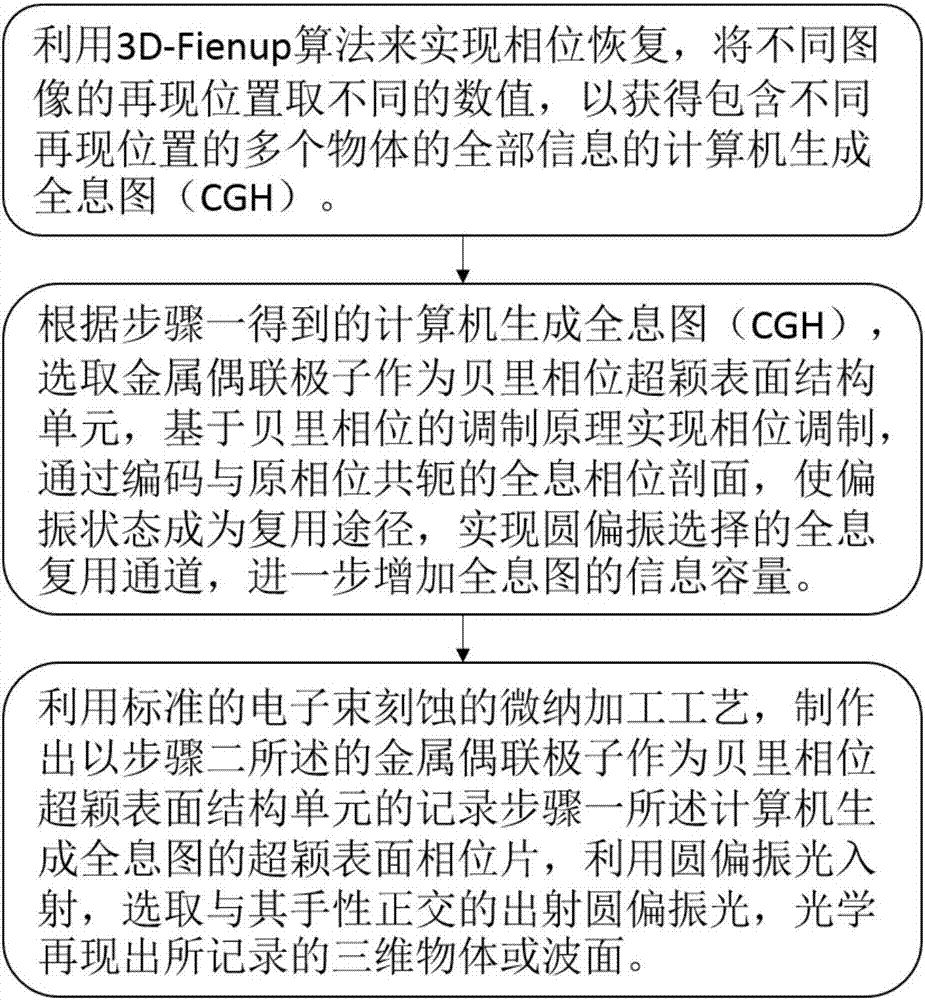
Berry phase metasurface-based multi-plane holographic multiplexing method
ActiveCN107065490AIncrease information capacityHigh reproduction qualityInstrumentsWavelengthChirality
The invention discloses a berry phase metasurface-based multi-plane holographic multiplexing method, and belongs to the field of micronano optical and holographic multiplexing application. The berry phase metasurface-based multi-plane holographic multiplexing method comprises the steps of achieving phase recovery by a 3D-Fienup algorithm, taking different numerical values of reproduction positions of different images, and acquiring a computer generation holographic diagram containing all information; selecting a metal coupling polaron as a berry phase metasurface structure unit, achieving phase modulation based on a berry phase modulation principle, and enabling a polarization state to become a multiplexing path by coding a holographic phase profile conjugated with an original phase to achieve a holographic multiplexing passage selected by circular polarization; making a metasurface phase sheet for recording the computer generation holographic diagram, selecting an emergent circular polarization light orthogonal to chirality of the polarization stat, and optically reproducing a three-dimensional object or a wave surface which is recorded. The holographic multiplexing method with sub-wavelength pixel of visible light and near-infrared bands, ultrathinness, large visual angle and large capacity is provided by the invention; and moreover, the crosstalk can be effectively reduced, and the holographic multiplexing of circular polarization selectivity is achieved.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
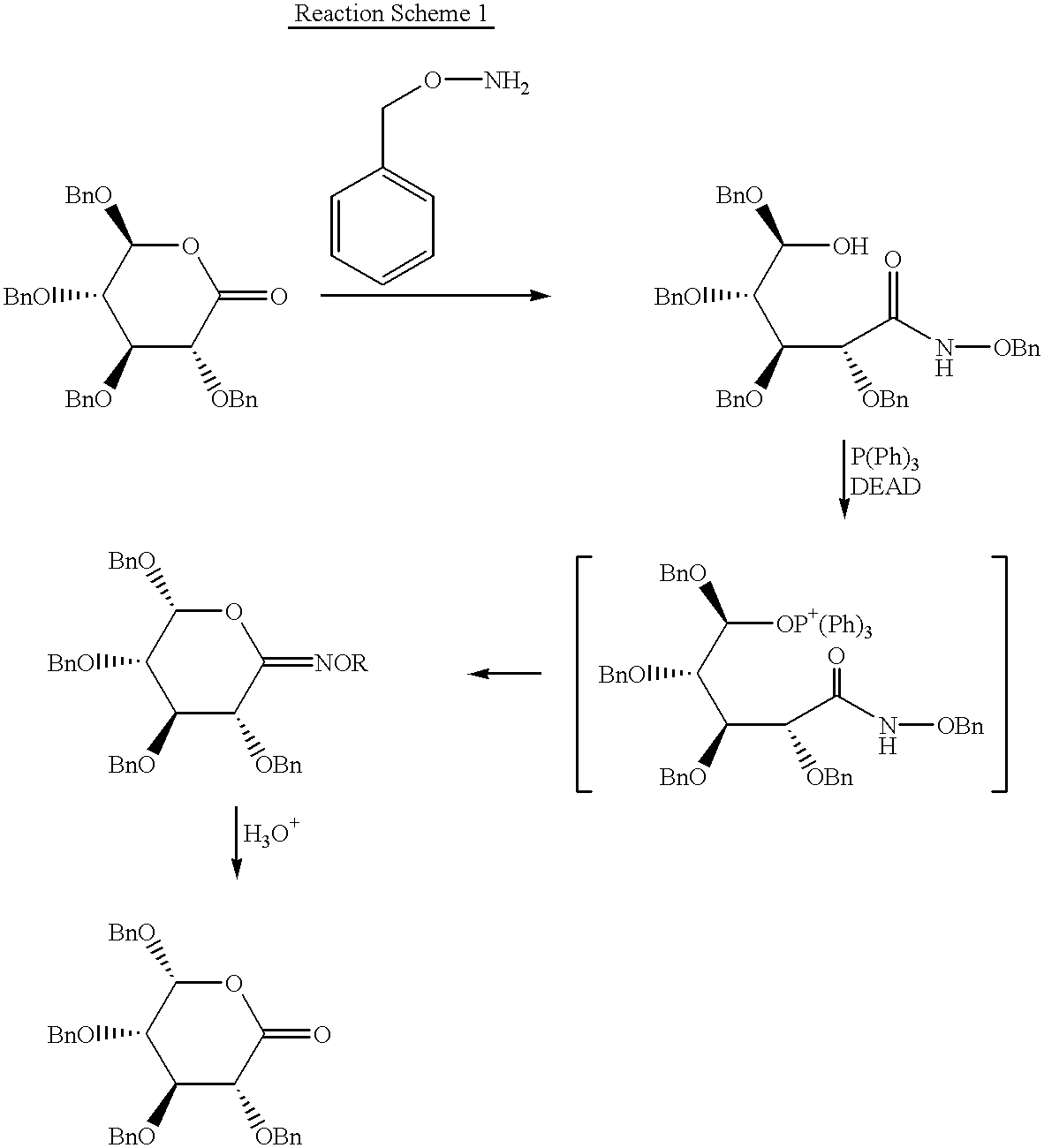
Chirality conversion method in lactone sugar compounds
InactiveUS6448415B1Process stabilityHigh reaction yieldSugar derivativesSugar derivatives preparationRiboseSugar
The present invention relates to a new process for effectively converting the chirality of 4- or 5-position carbon of a 1,4-lactone- or 1,5-lactone sugar compound which comprises reacting the lactone compound with secondary amine and sulfonyl group-containing compound. The compound of which chirality is converted according to the present invention can be advantageously used for preparing such expensive rare sugar compounds as L-ribose, D-talose, etc.
Owner:HANCHEM
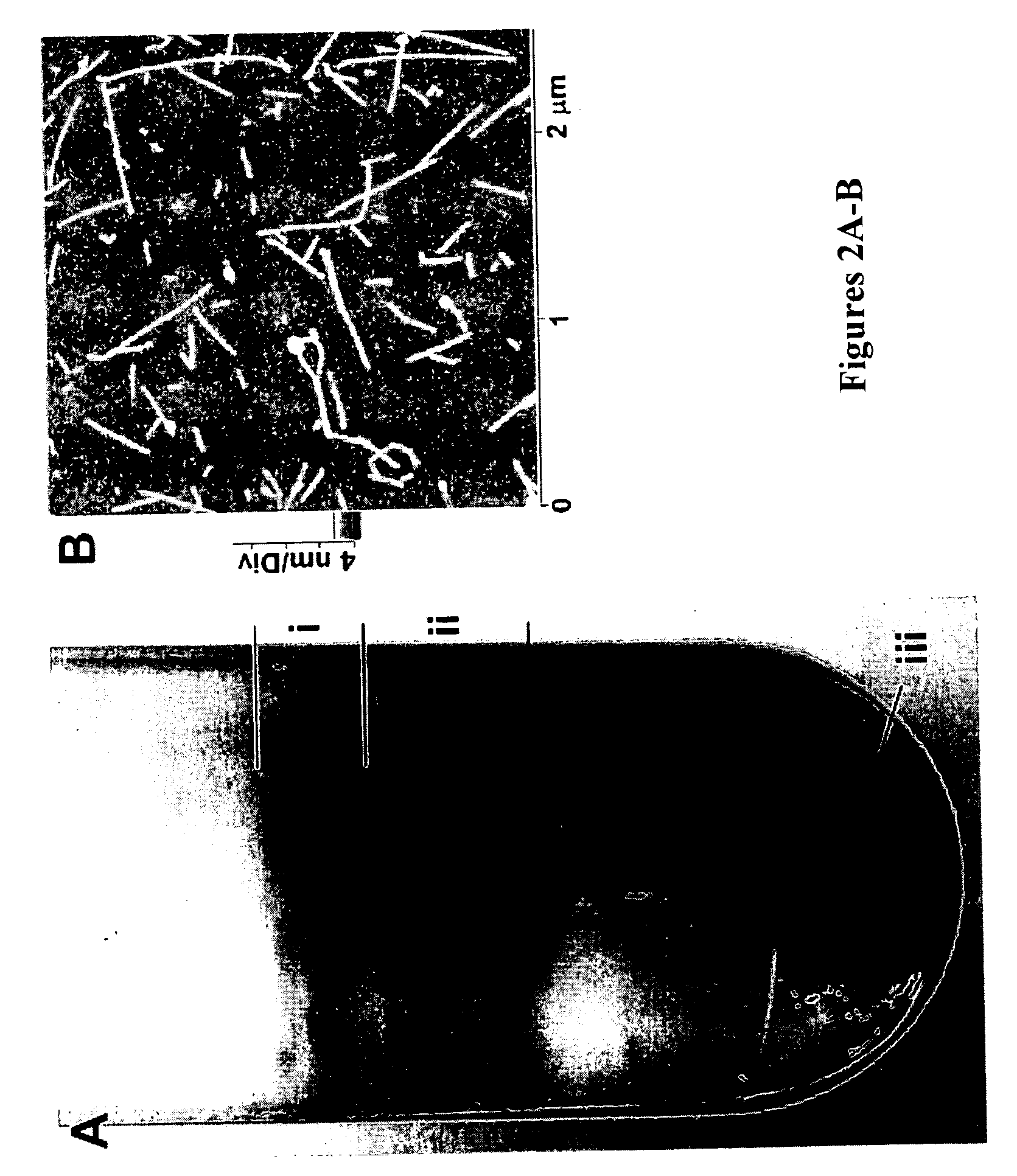
Directed flow method and system for bulk separation of single-walled tubular fullerenes based on helicity
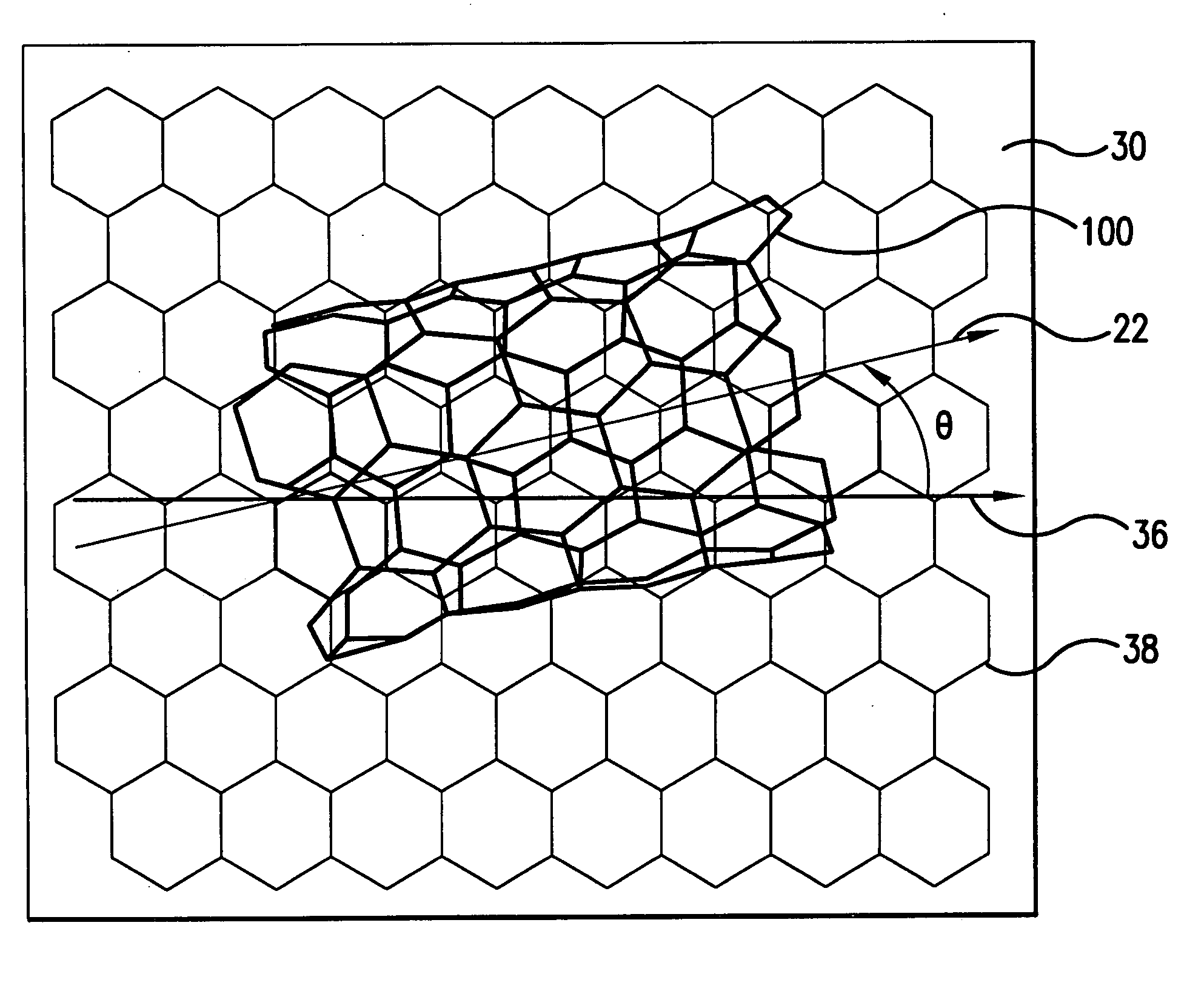
A method for bulk separation of single-walled tubular fullerenes (100) based on helicity is provided wherein a solution or suspension of the single-walled tubular fullerenes (100) is flowed onto a crystalline or highly oriented substrate (30). The single-walled tubular fullerenes (100) that flow onto the substrate (30) have a respective longitudinal axis that is aligned with the flow direction (105). The direction of flow (105) is oriented at a predetermined angle with respect to a lattice axis (24) of the substrate (30) for energetically favoring adsorption of a respective plurality of single-walled fullerenes (100) having a tubular contour and a selected helicity. Subsequently, the adsorbed single-walled tubular fullerenes (100) of the selected chirality are removed from the substrate (30).
Owner:ETHICON INC +2
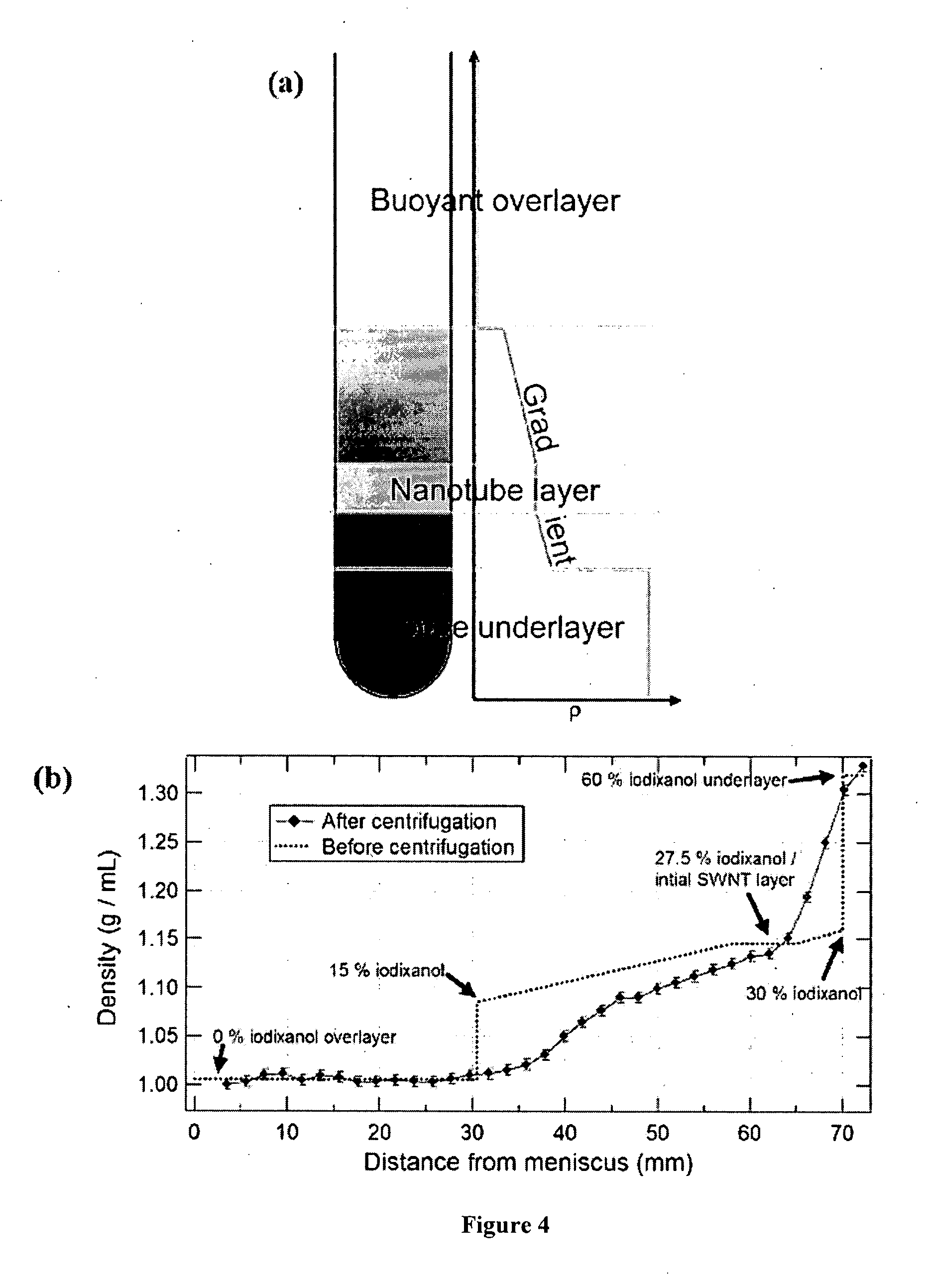
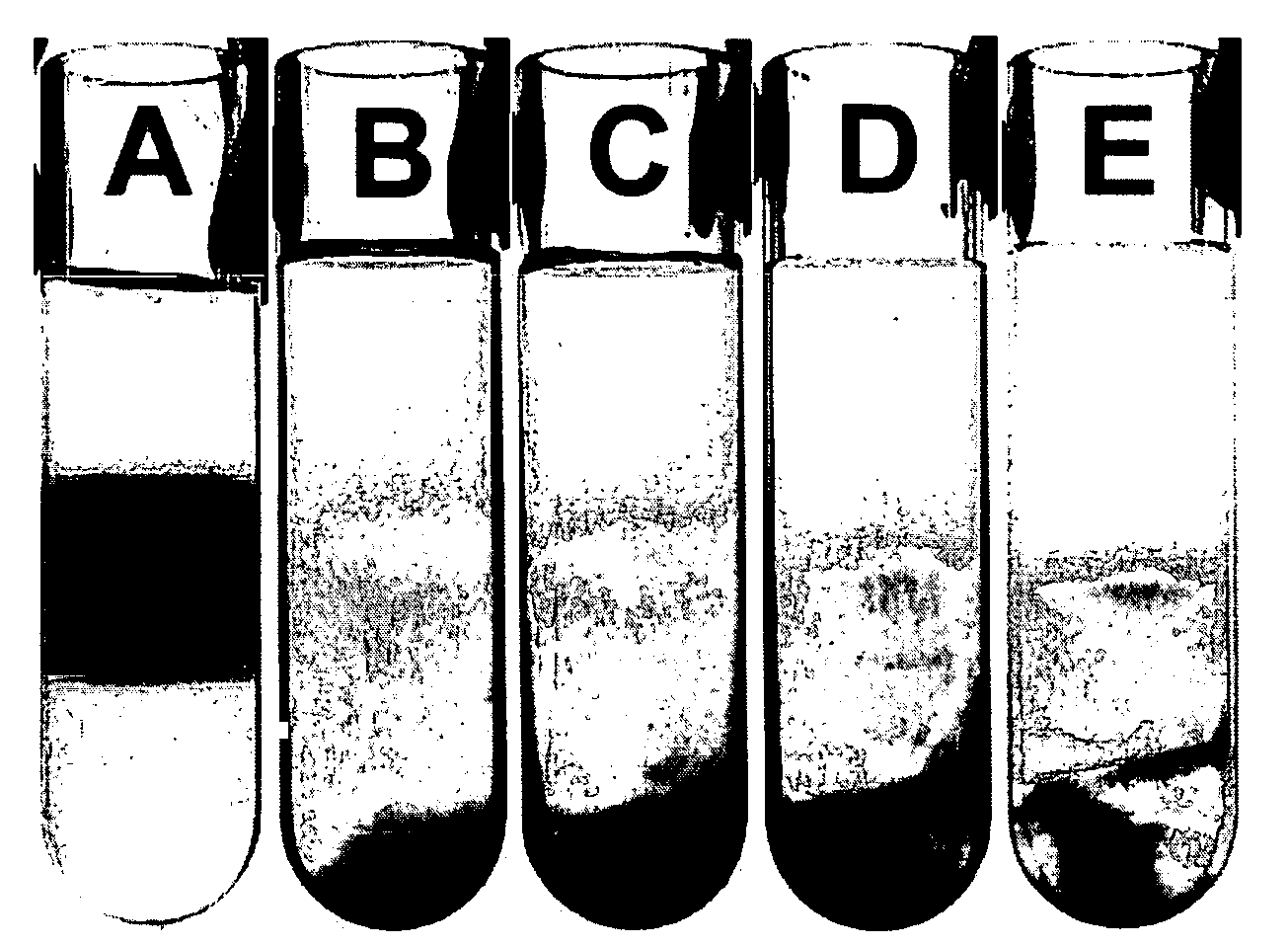
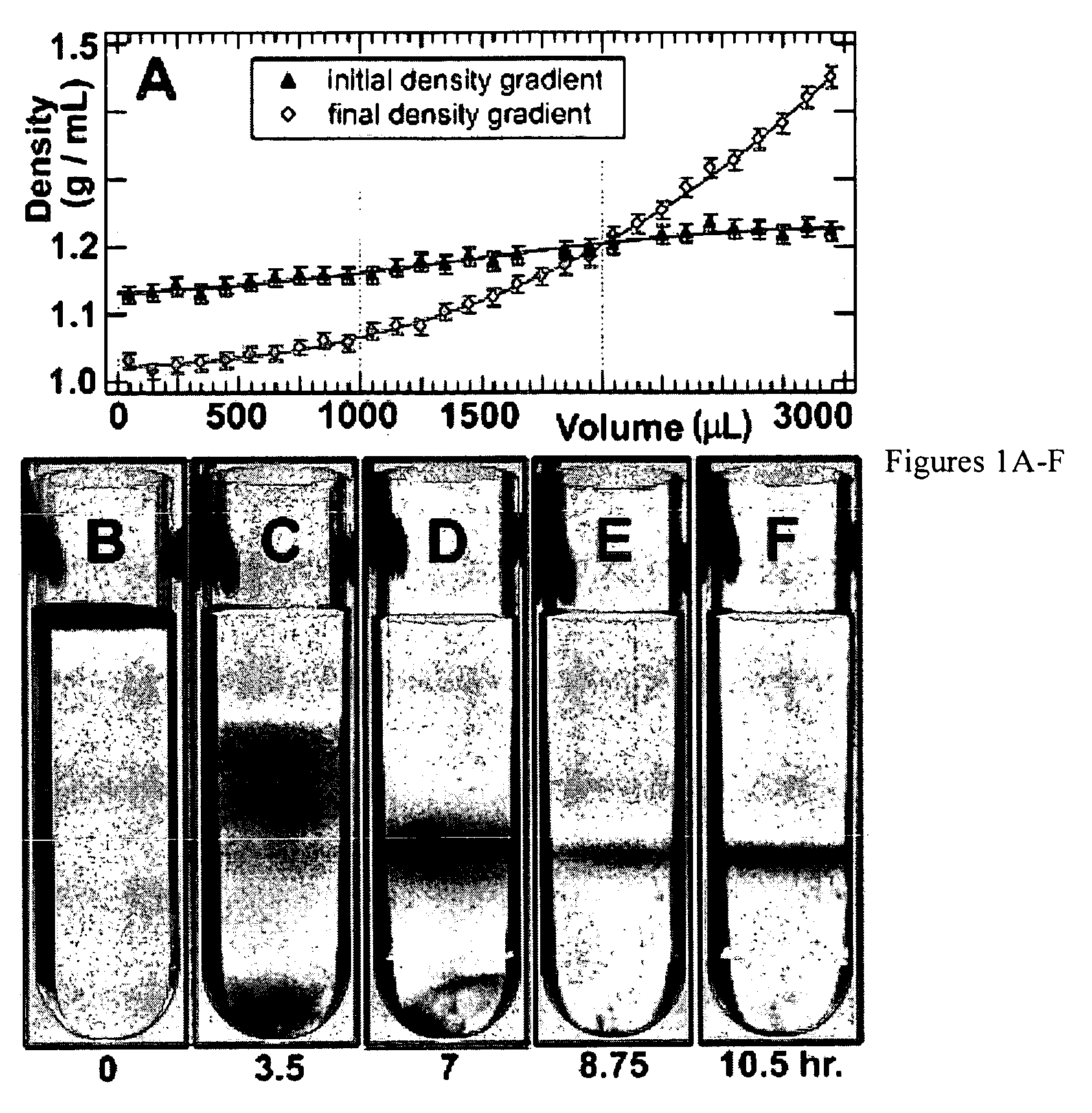
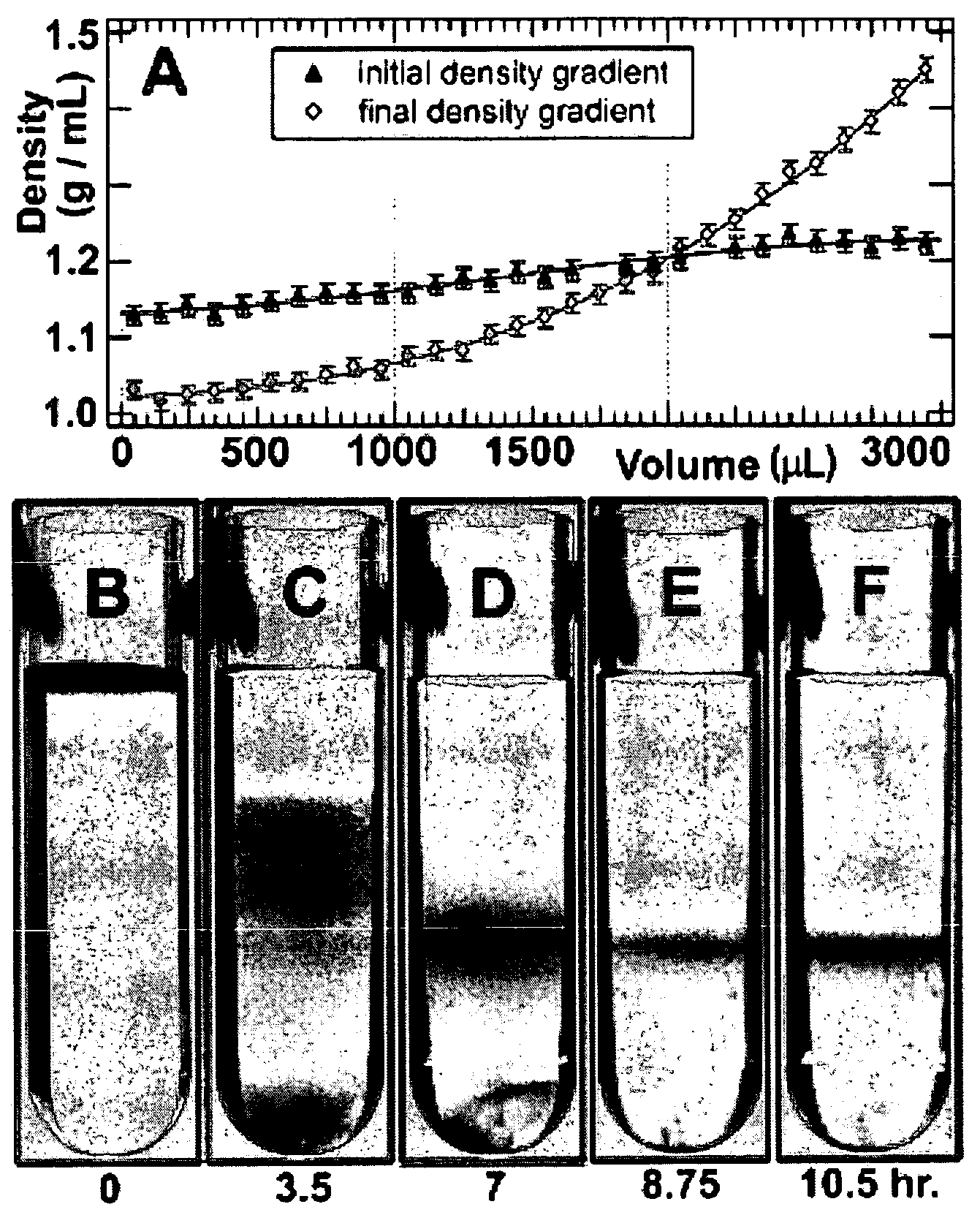
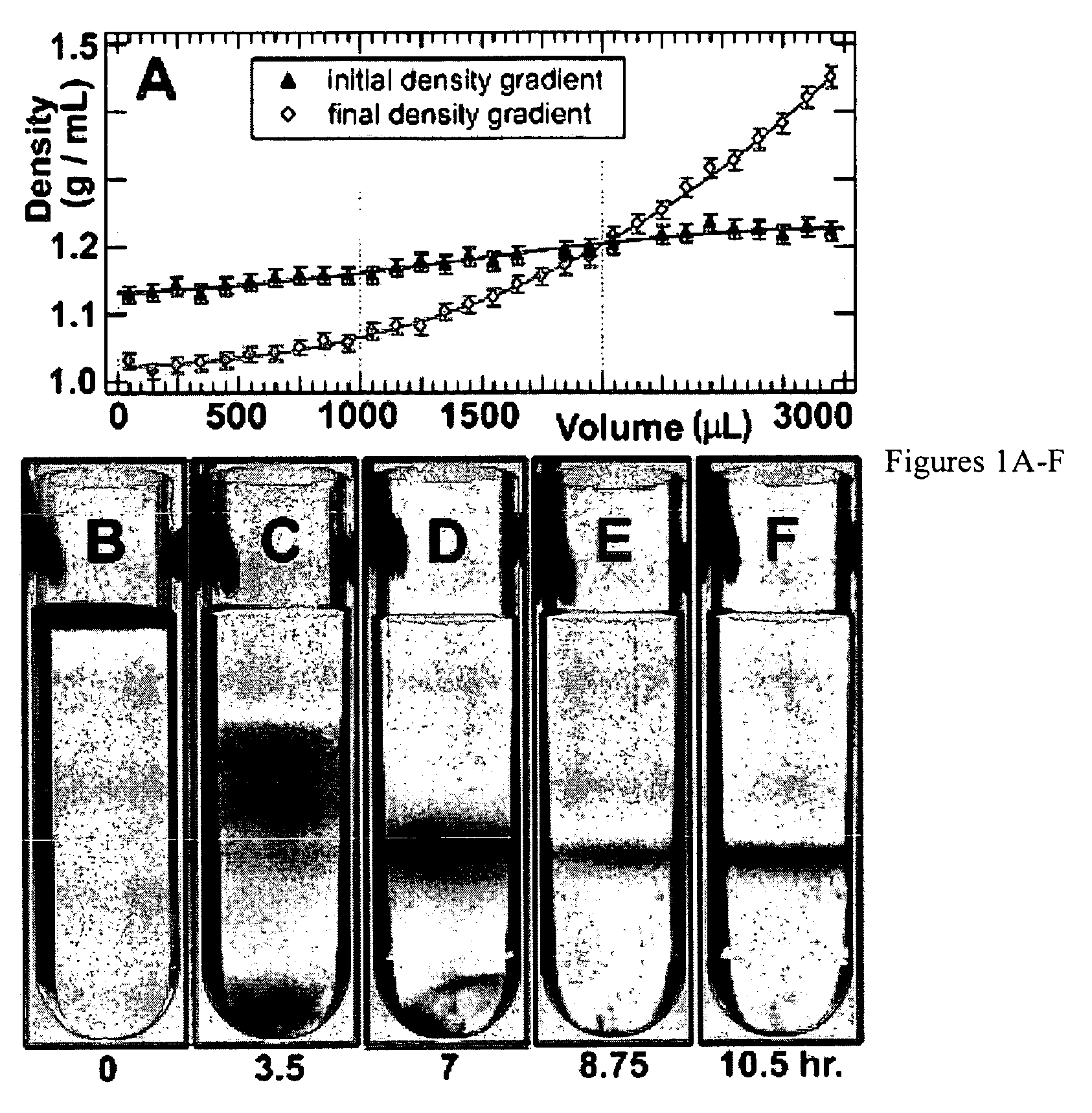
Separation of carbon nanotubes in density gradients
ActiveUS20090173918A1Scalable productionOptical radiation measurementMaterial nanotechnologyCentrifugationActive component
The separation of single-walled carbon nanotubes (SWNTs), by chirality and / or diameter, using centrifugation of compositions of SWNTs in and surface active components in density gradient media.
Owner:NORTHWESTERN UNIV
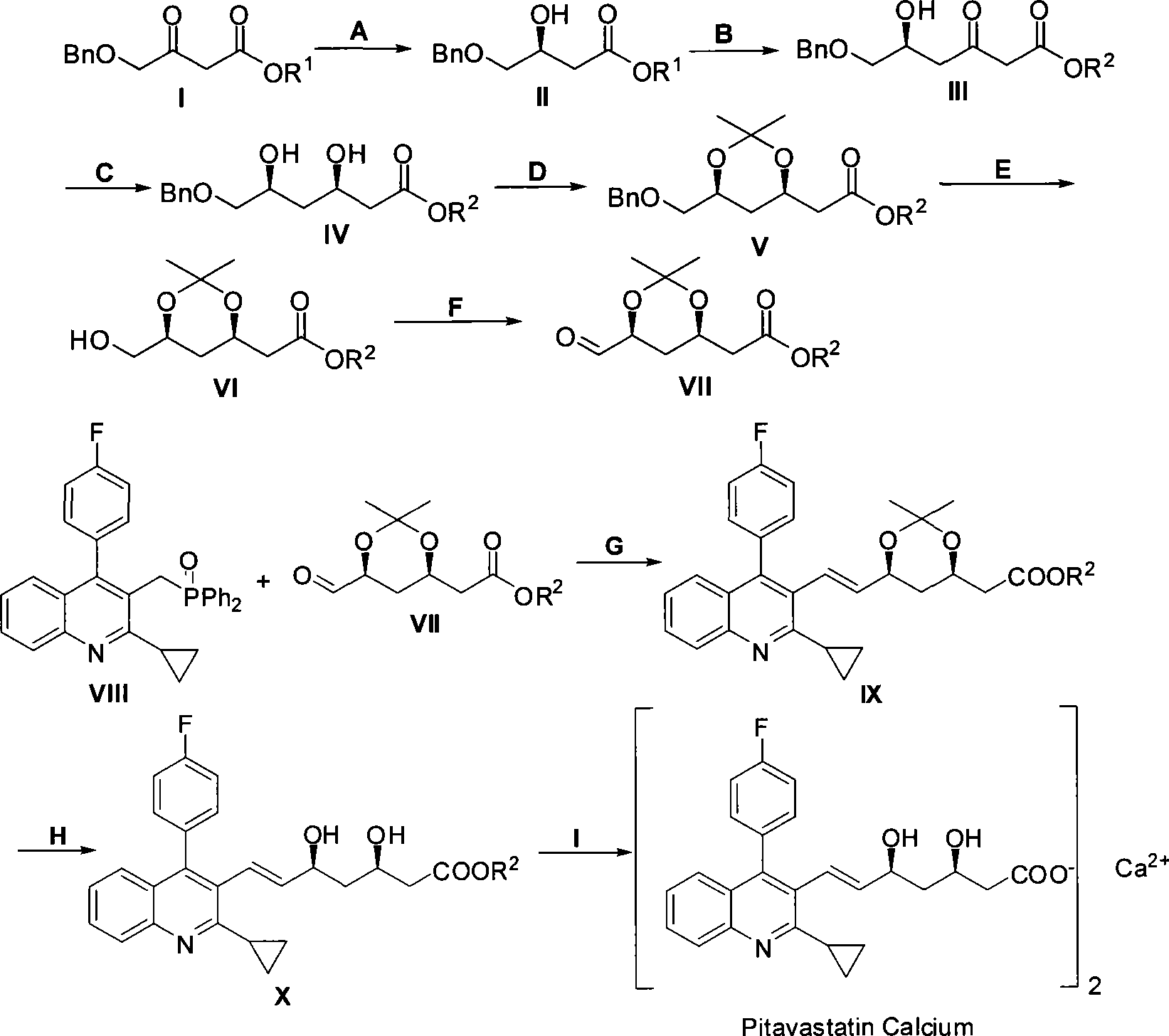
Method for preparing pitavastatin calcium raw material medicine using asymmetric hydrogenation
ActiveCN101386592ASolve the problem of expensive chiral side chainsMetabolism disorderAsymmetric synthesesClaisen condensationEthyl butyrate
The invention relates to a method for preparing a pitavastatin calcium raw material by asymmetric hydrogenation in the technical field of medicinal chemistry. The method comprises the following steps: performing catalytic hydrogenation on 4-benzyloxy-ethyl-acetoacetate I by using a chiral catalyst to prepare (S)-4-benzyloxy-3-hydroxy-ethyl-butyrate, and then obtaining the pitavastatin calcium through a series of reactions such as Claisen condensation, reduction, hydroxyl protection, removal of benzyl, oxidation and the like. In the invention, the highly-efficient chirality added value is realized by the use of catalytic amount of the chiral catalyst (wherein the ratio of substrate to the catalyst can reach 3, 000 to 1), and by performing the asymmetric hydrogenation and the reduction on non-chiral ketones to generate chiral alcohols compounds directly (wherein the enantioselectivity can be up to 94.3 percent).
Owner:JIANGSU WANBANG BIOPHARMLS +1
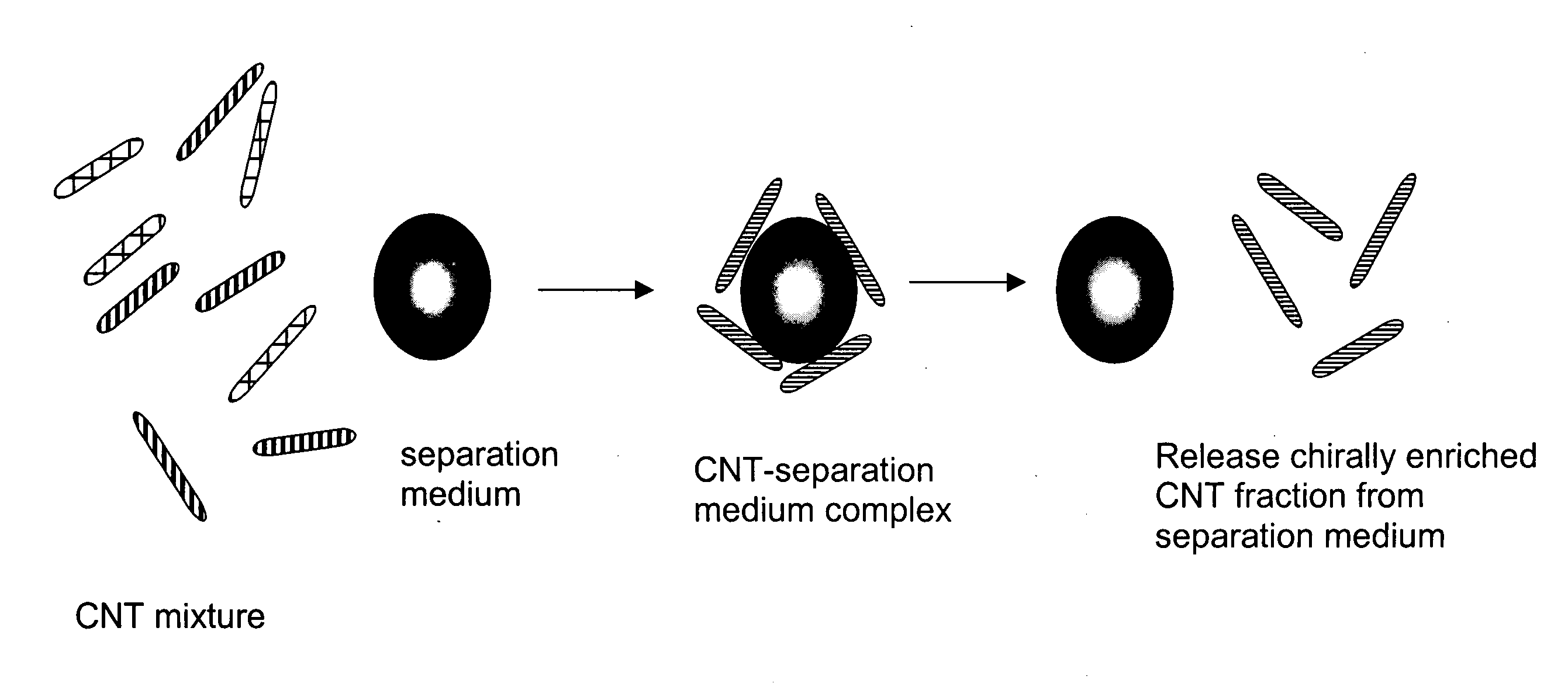
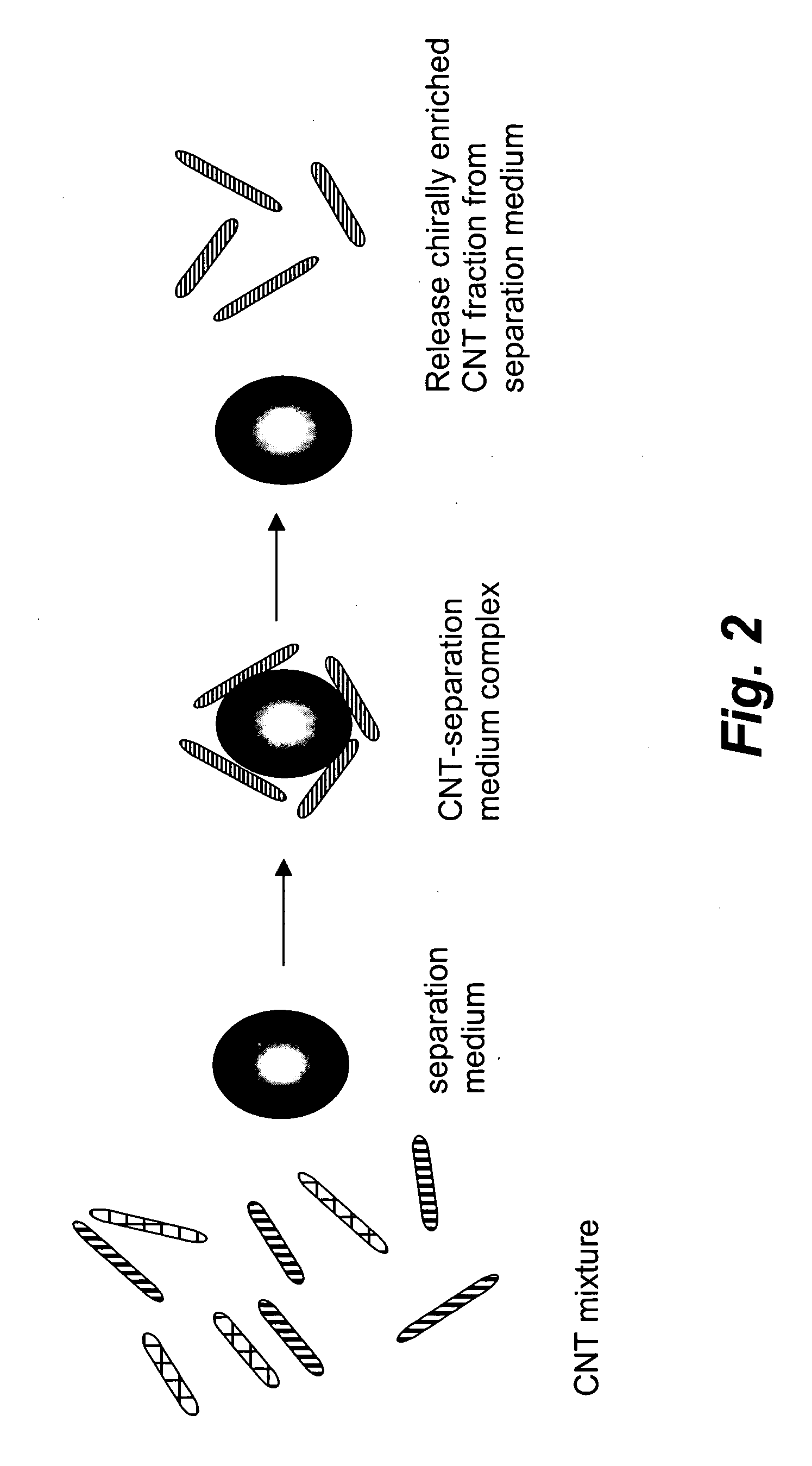
Chirality-based separation of carbon nanotubes
A mixture of carbon nanotubes is separated into fractions that are enriched with a desired chirality by exposing a solution or suspension of the carbon nanotubes to a separation medium. A portion of the mixture forms complexes with, and becomes attached to, the separation medium. Exposure to other reagents results in dissociation of the complexes and release of the nanotubes from the separation medium.
Owner:LOS ALAMOS NATIONAL SECURITY
Monodisperse Single-Walled Carbon Nanotube Populations and Related Methods for Providing Same
ActiveUS20110155964A1Material nanotechnologyIndividual molecule manipulationCarbon nanotubeChirality
The present teachings provide methods for providing populations of single-walled carbon nanotubes that are substantially monodisperse in terms of diameter, electronic type, and / or chirality. Also provided are single-walled carbon nanotube populations provided thereby and articles of manufacture including such populations.
Owner:NORTHWESTERN UNIV
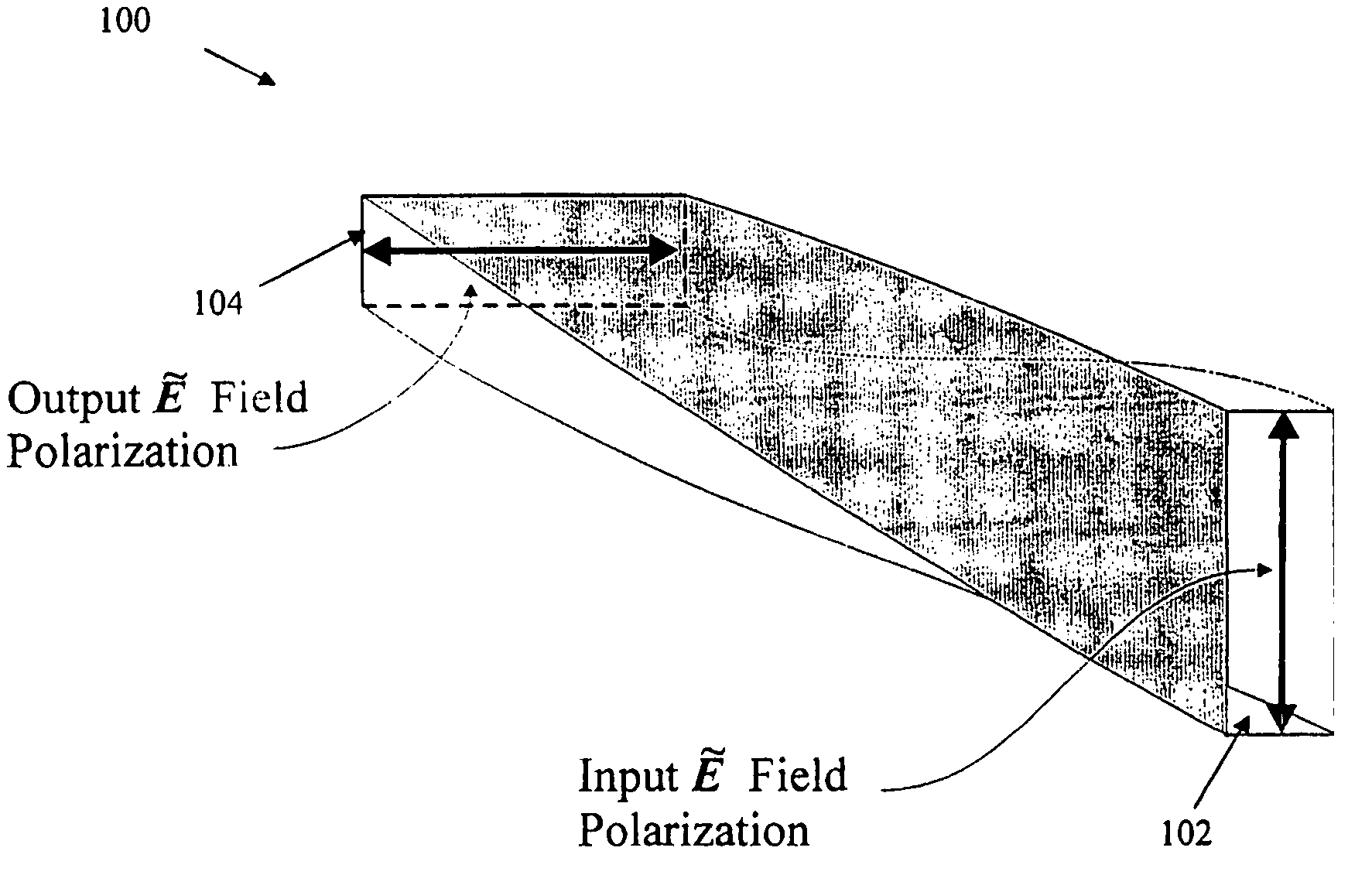
Chiral boardband tuning apparatus and method
ActiveUS7009679B2Laser using scattering effectsOptical fibre with graded refractive index core/claddingSpectral responsePhotonics
A chiral structure having an expanded adjustable reflection band to provide broadband tunability is provided. In the preferred embodiment, the chiral structure is implemented as a chiral fiber structure and comprises two or more sequential chiral fiber elements of different pitches, each having a tunable chiral defect generator. The pitches are selected such that the individual photonic band gaps of the elements are formed into one expanded reflection band such that at least one defect state can be formed and moved within the expanded reflection band by selectively activating and adjusting one or more of the tunable chiral defect generator. The tunable chiral defect generators may generate and control defect state(s) in the structure's spectral response by introducing chiral twists and / or spacing between the chiral elements, with the length of the spacings and angles of chiral twists being proportional to the position of the defect state(s) within the reflection band of the structure.
Owner:CHIRAL PHOTOINICS
Dual functions ligand compound of chirality dioxazoline, preparation and application
InactiveCN1626524AEasy to getEasy to manufactureOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsChemical compoundSubstitution reaction
A bifunctional chiral bioxazoline ligand used as the catalyst for asymmetrical reactions, such as cyanosiliconizing reaction, is prepared from propylbinitrile or its derivative through substitution reaction and cyclizing reaction on chiral aminoalcohol, or from the bioxazoline methane through substitution reaction.
Owner:PEKING UNIV
Separation of carbon nanotubes in density gradients
ActiveUS7662298B2Scalable productionOptical radiation measurementMaterial nanotechnologyActive componentCentrifugation
The separation of single-walled carbon nanotubes (SWNTs), by chirality and / or diameter, using centrifugation of compositions of SWNTs in and surface active components in density gradient media.
Owner:NORTHWESTERN UNIV
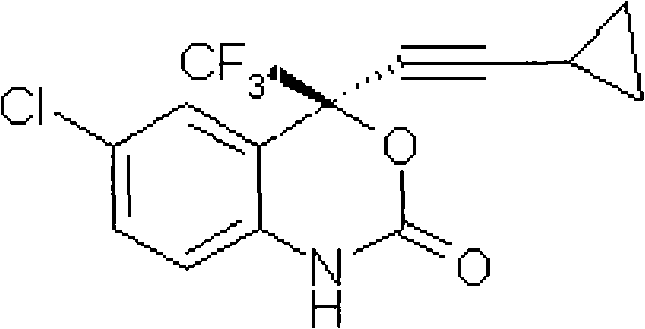
Method for preparing chiral cylopropyl acetenyl tertiary alcohol compound
InactiveCN101786959ALow costSuitable for industrial productionOrganic compound preparationAmino-hyroxy compound preparationTrifluorideButyl lithium
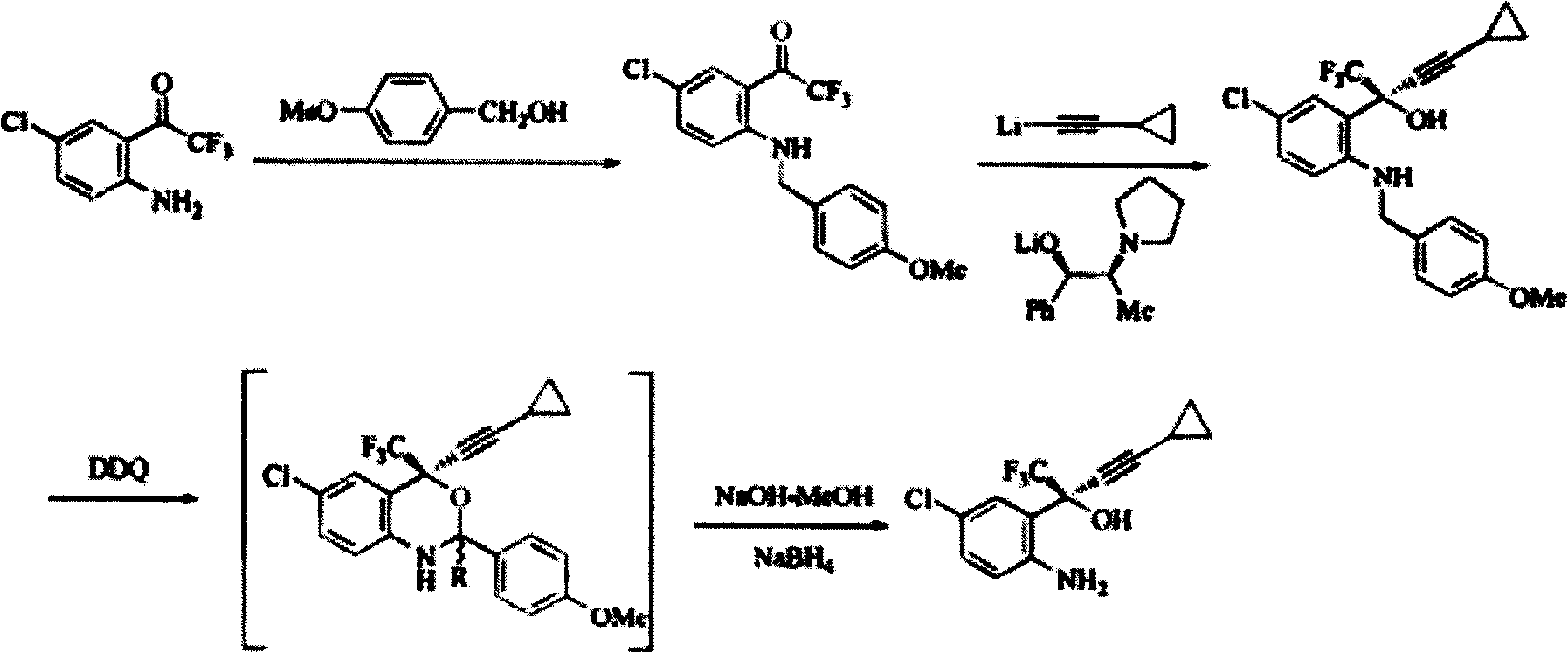
The invention discloses a method for preparing a chiral cylopropyl acetenyl tertiary alcohol compound.The steps of the method are as follows: (a) cylopropyl ethylnen or other derivatives react with a chiral induction reagent, a chirality auxiliary reagent and zinc halide in an organic solvent under the action of an alkaline reagent so as to obtain a zinc complex; (b) addition reaction is carried out between the obtained zinc complex and 5-chlorin-2-amino trifluoro-benzophenone; (c) (S)-2-amino-5-chlorin-alpha-cylopropyl ethylnen-alpha-benzyl alcohol trifluoride is collected from reaction liquid. Compared with butyl lithium and diethylzinc, the alkaline reagent adopted in the invention is safer; in addition, the cheap zinc halide is used as a ligand, which greatly reduces the cost. Therefore, the method is suitable for industrial production.
Owner:SHANGHAI ACEBRIGHT PHARMA GRP +1
Field effect transistor chiral sensor and manufacture method thereof
InactiveCN101923065AReduce volumeNo consumptionMaterial analysis by electric/magnetic meansSemiconductor/solid-state device manufacturingQuantum dotField-effect transistor
The invention discloses a field effect transistor chiral sensor and a manufacture method thereof. By utilizing the structure of a field effect transistor, the chiral sensor comprises a substrate, a gate electrode, a gate insulating layer, an active layer, a source electrode and a drain electrode. The chiral sensor is characterized in that the active layer is made of a quantum dot material with chiral recognizing and detecting function. The quantum dot material of the active layer is a semiconductor nano microcrystal which is modified by chiral molecules and has the size of smaller than 100nm.The invention sensor capable of detecting a chiral substance by using a quantum dot film with the chiral recognizing and detecting function as the active layer of the field effect transistor on the basis of the quantum dot field effect transistor and the manufacture method thereof. The fluorescent chiral molecule modified quantum dot can be also made into a film by the chiral sensor for being used as the field effect transistor active layer, thereby overcoming the defect of chiral molecule detection by the traditional homogeneous phase fluorescent sensor and realizing a more stable detecting signal.
Owner:SUZHOU INST OF NANO TECH & NANO BIONICS CHINESE ACEDEMY OF SCI
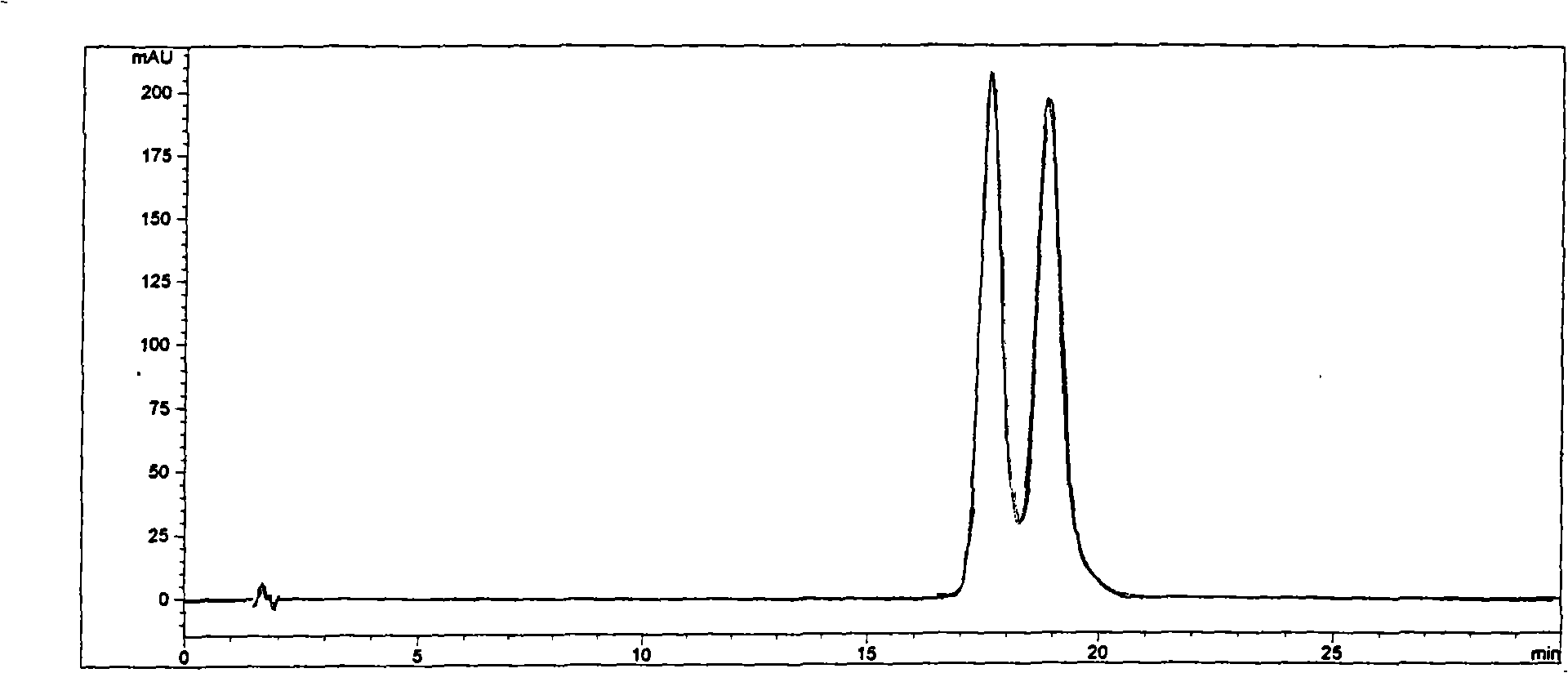
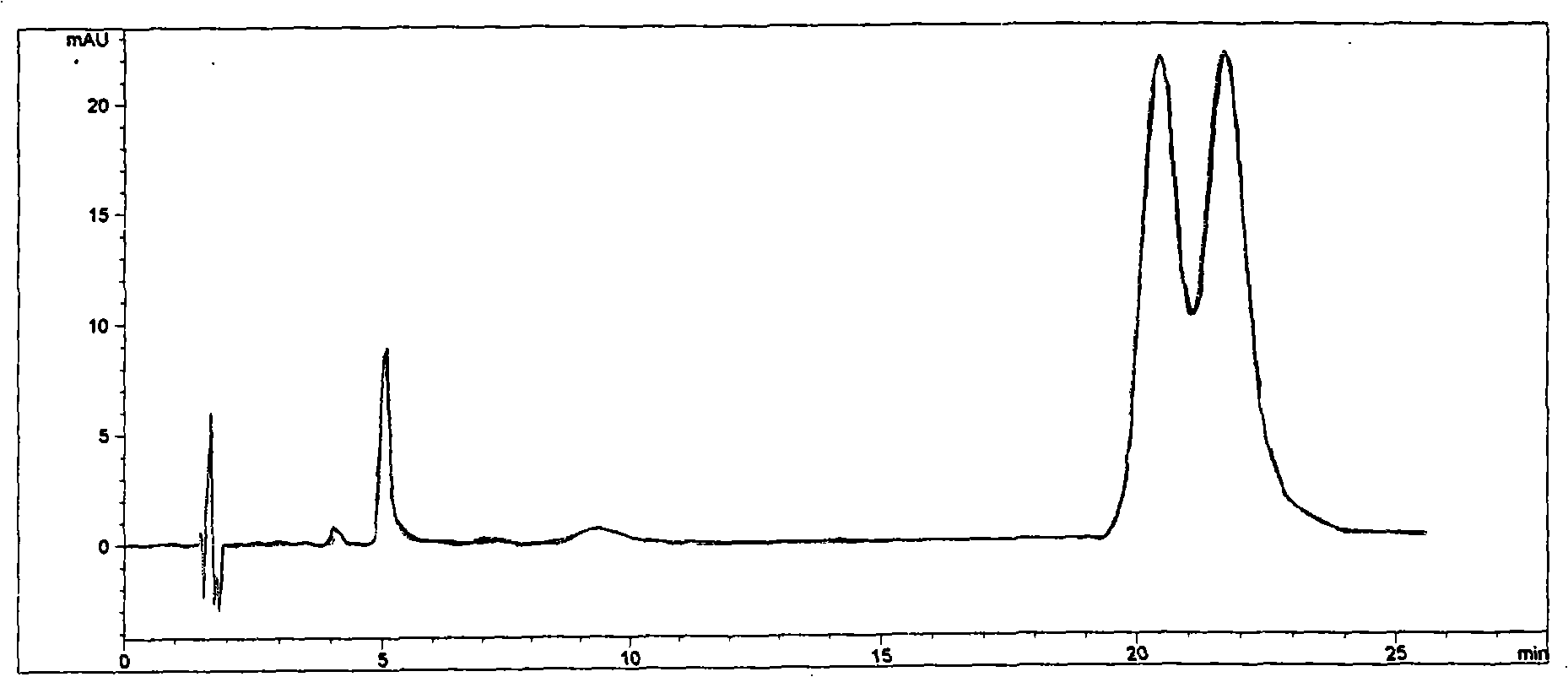
Method for separating and measuring ticagrelor and optical isomer of ticagrelor
The invention belongs to the field of analytical chemistry and in particular relates to a method for separating and measuring ticagrelor and a diastereoisomer of the ticagrelor. The method is characterized in that a chiral HPLC (High Performance Liquid Chromatography) column taking polysaccharide derivative as a filler is used and a mixed solution of lower paraffin hydrocarbon and low alcohol is used as a moving phase; according to the separating and detecting method, the ticagrelor and the diastereoisomer of the ticagrelor are effectively separated, and the mass of the ticagrelor can be effectively controlled. The method can be used for separating and detecting the ticagrelor and the diastereoisomer of the ticagrelor simply, rapidly and accurately.
Owner:SUNSHINE LAKE PHARM CO LTD
Use of chiral oxazoline
InactiveCN101099936AOrganic-compounds/hydrides/coordination-complexes catalystsPreparation by hydrogen cyanide additionEthyl groupChirality
Owner:HEFEI UNIV OF TECH
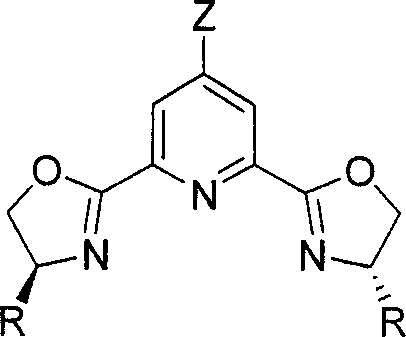
Chirality pyridine double-oxazoline catalyzer and method for preparing the same and application thereof
InactiveCN101116828AHigh activityHigh enantioselectivityOrganic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationOxazolidoneLanthanide
A catalyst of chiral bis-oxazolinylpyridine is provided, which comprises two parts including a 4-z-2, 6-bis [4'(s)-R oxazoline-2'-] pyridine as the chiral ligand of the bis-oxazolinylpyridine, and a Scandate, Yttrium and Lanthanide metal salt. The preparation method is to mix the metal salt, the ligand of bis-oxazolinylpyridin with the equal molar quantity, and a 4-molecular sieve together, the mixture is added with the needed solvent, and then stirred under the temperature of -78 to 25 DEG C to obtain the catalyst prepared in situ. The usual dosage of the catalyst used for catalyzing Diels-Alder reaction is 1percent to 5percent of the dosage of the substrate. The catalyst can catalyze Diels-Alder reaction of both alpha, beta-unsaturated N-acetyl oxazolidone and alpha, beta-unsaturated ester with high efficiency in high selectivity. Under condition of the normal pressure with the temperature of 0 to 25 DEG C, the catalyst can accomplish the conversion rate as high as 100 percent and the enantiomeric selectivity of 96 percent.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Beta-cyclodextrin derivative capillary gas chromatography chiral fixed phase and preparing method thereof
InactiveCN101015789AImprove stabilityCapable of chiral recognitionOther chemical processesCapillary gas chromatographyGas phase
The invention relates to a novel beta-cyclodextrin derivant capillary gas spectrum chiral fixed phase and relative preparation. The invention is characterized in that the invention leads allyl group with two keys into 2 and 3 positions of beta-cyclodextrin molecule, and leads valeryl group, enanthoyl group or caprylyl group into 6 position of beta-cyclodextrin molecule, to obtain novel beta-cyclodextrin detrivant. The inventive detrivant can be used as chiral fixed phase of capillary gas spectrum, with some chiral recognize ability to separate antimer of chiral compound.
Owner:CHINA AGRI UNIV
Carbon nanotube composition, method for manufacturing the same, array, and electronic device
InactiveUS20090253590A1High selectivityMaterial nanotechnologyElectrolysis componentsCarbon nanotubeChirality
The present invention attempts to establish a method for surface-fixing single-walled carbon nanotubes having a desired chirality highly selected from among the single-walled carbon nanotubes having various chiralities, and utilizes the method to provide an array of the carbon nanotubes for electronic devices. The present invention attempts also to provide a carbon nanotube composition including carbon nanotubes having a single chiral vector (n, m) at a purity of more than 50% based on the unit of number wherein n and m are integers, and a method for manufacturing the same.
Owner:HOKKAIDO UNIVERSITY
Chiral sensing element and equipment, chiral representation method as well as concentration representation method
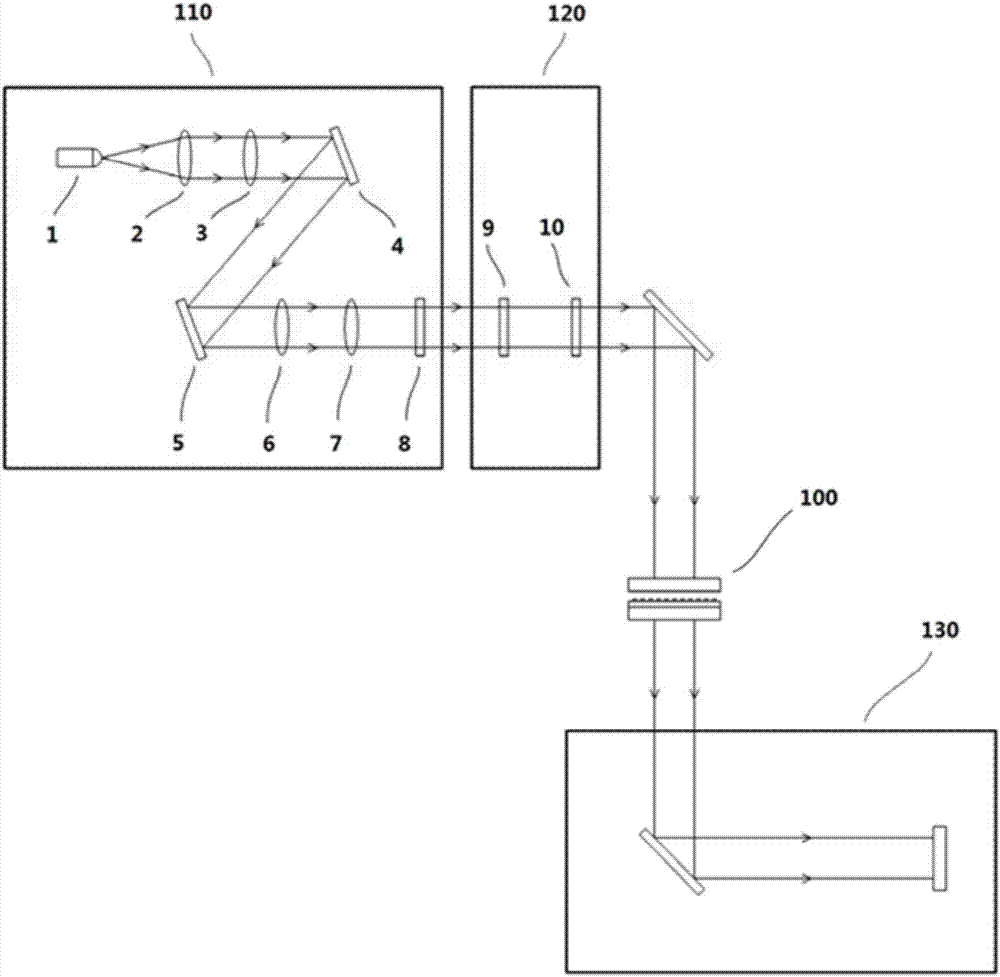
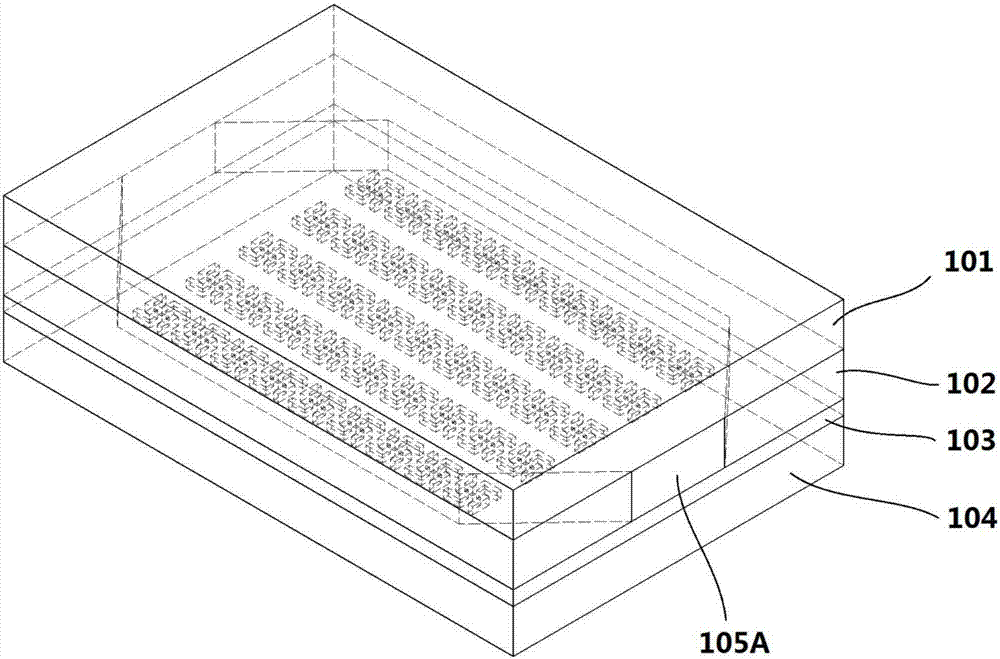
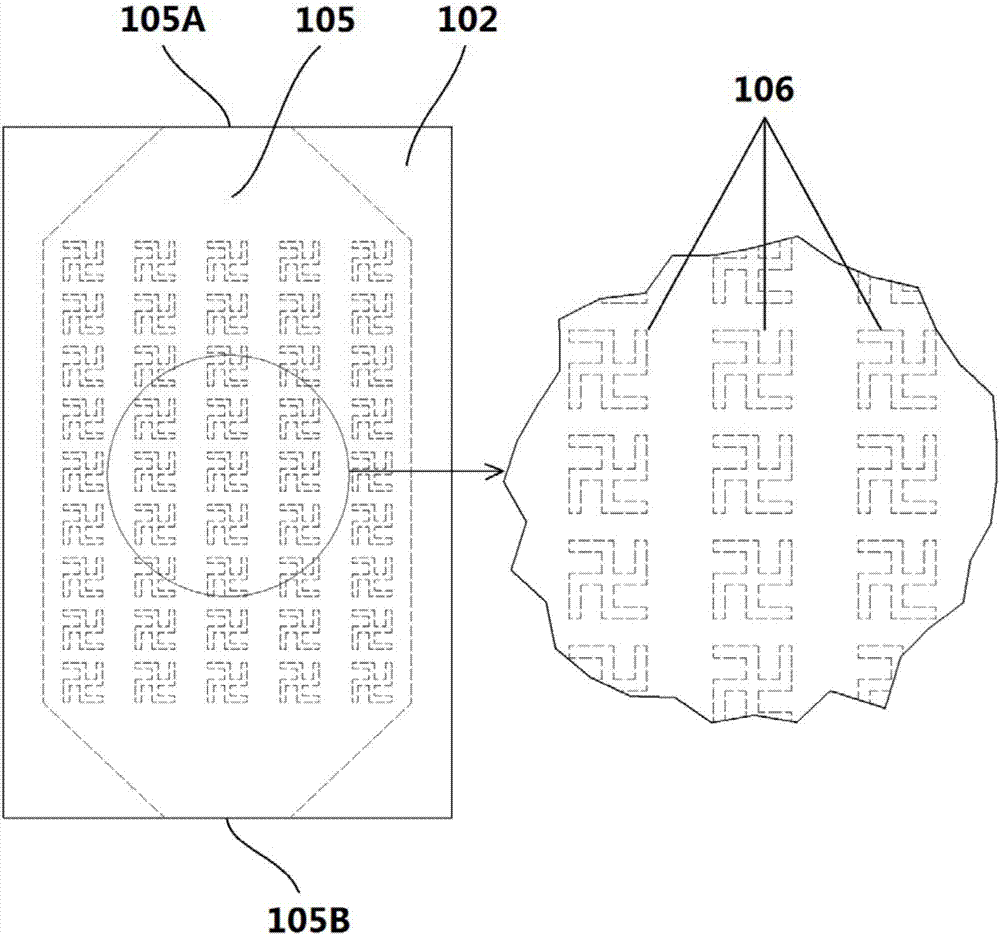
InactiveCN107036971ARealize highly sensitive detectionHigh detection sensitivityPolarisation-affecting propertiesMicrofluidic channelNanostructure
The invention relates to the field of biological detection and provides a chiral sensing element and equipment, a chiral representation method as well as a concentration representation method, so that the detection sensitivity of molecular chirality is improved; the equipment and detection cost is reduced, and the detection efficiency and accuracy are improved; meanwhile, high-sensitivity representation of concentration of biological molecules in a solution is realized. The chiral sensing equipment comprises the chiral sensing element, the chiral sensing element comprises an upper covering layer, a middle covering layer and a lower covering layer which have light transmission, as well as a microfluidic channel formed by the upper covering layer, the middle covering layer and the lower covering layer; a chiral nanostructure array is arranged on the lower covering layer in the microfluidic channel. The invention applies to detection of weak circular-dichroism signals.
Owner:SICHUAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com