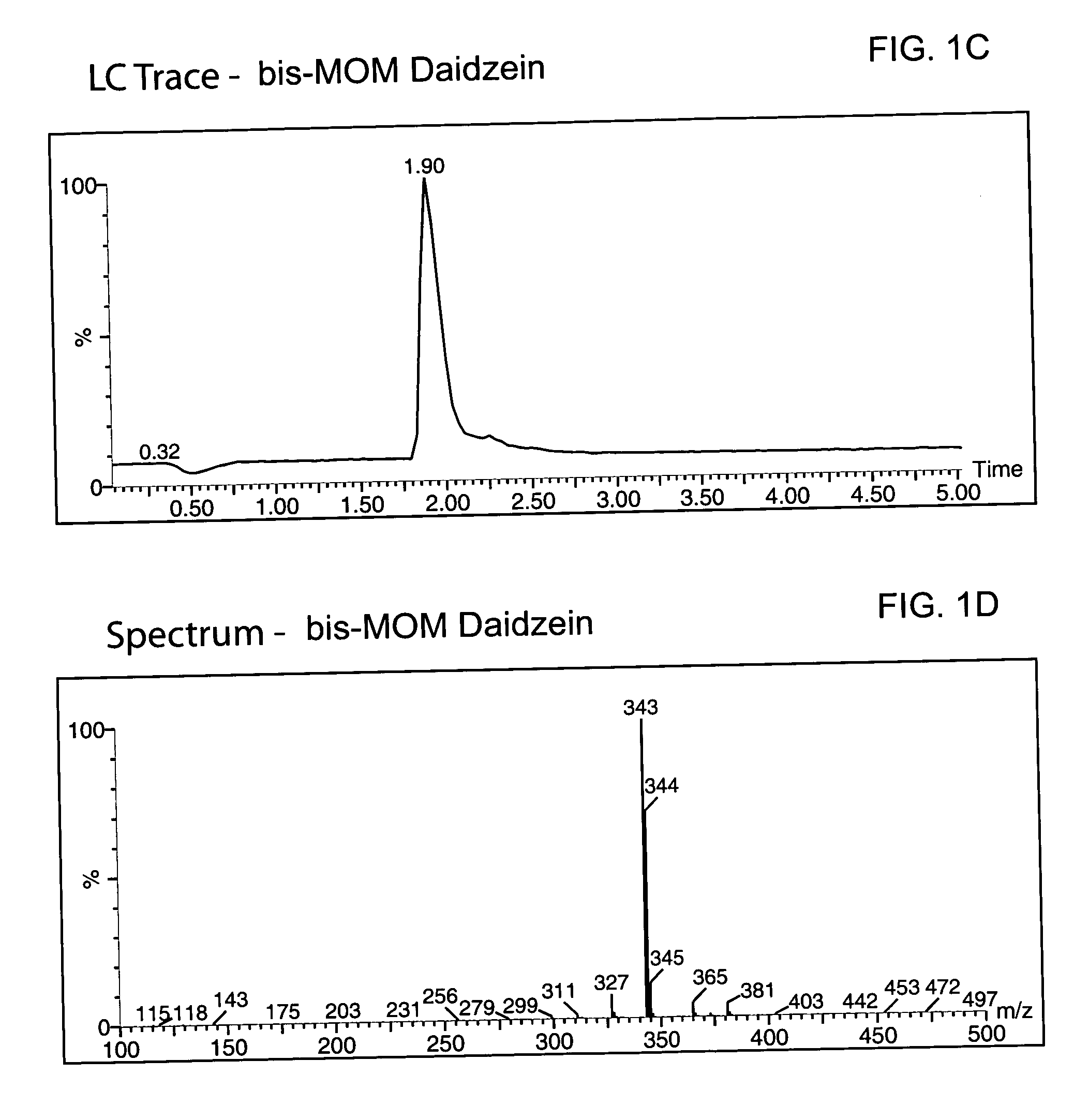
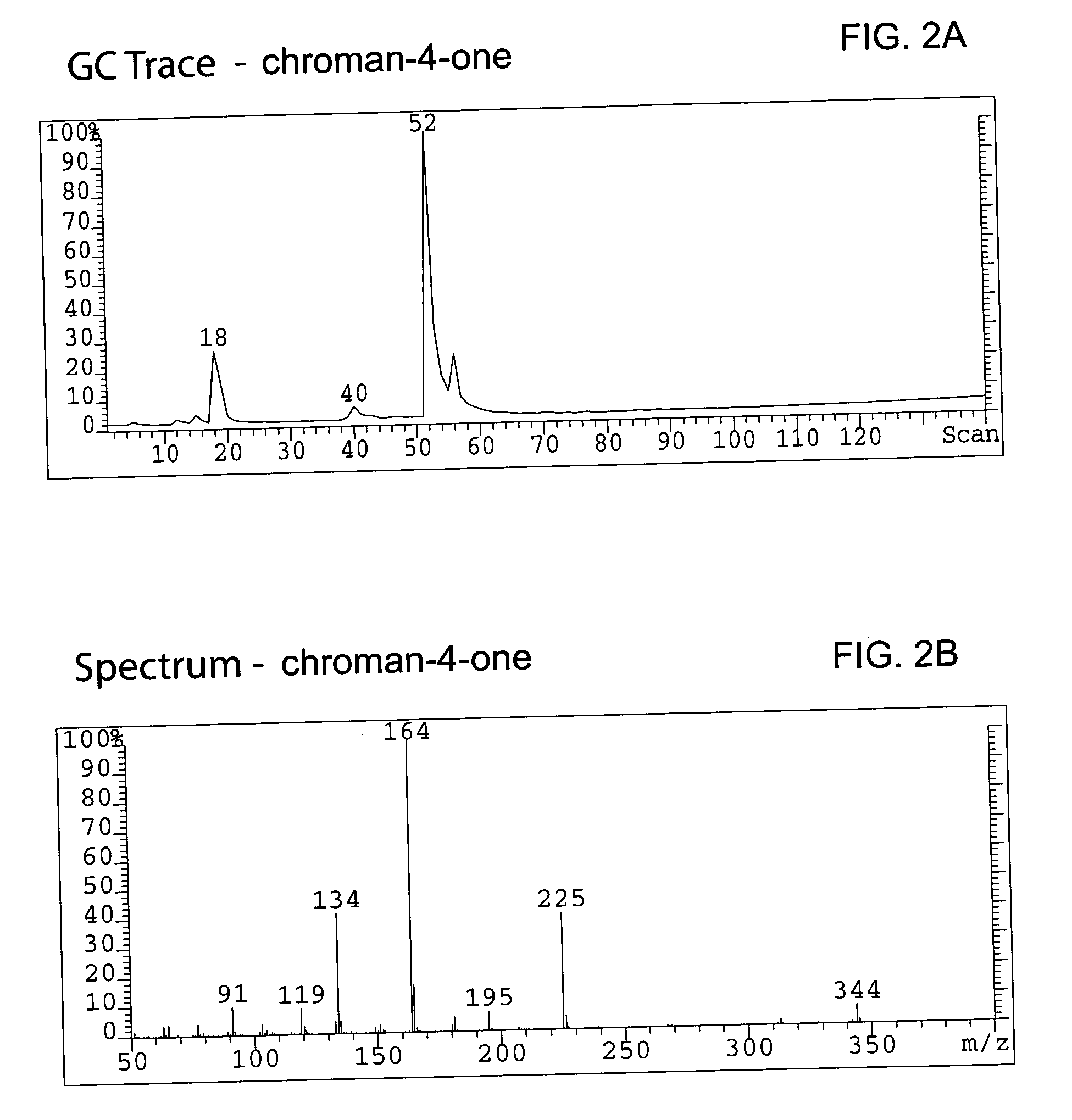
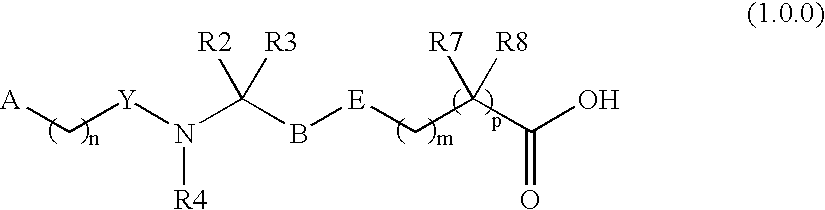
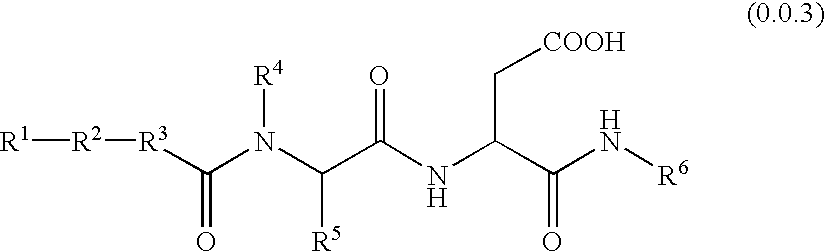
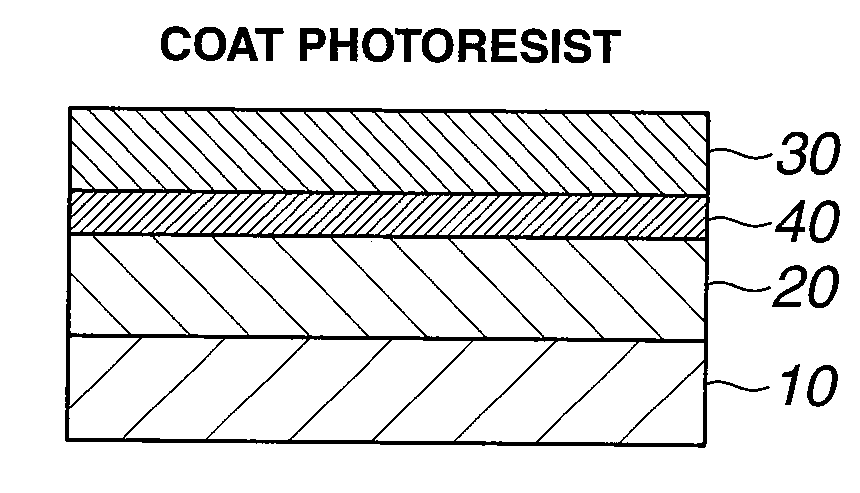
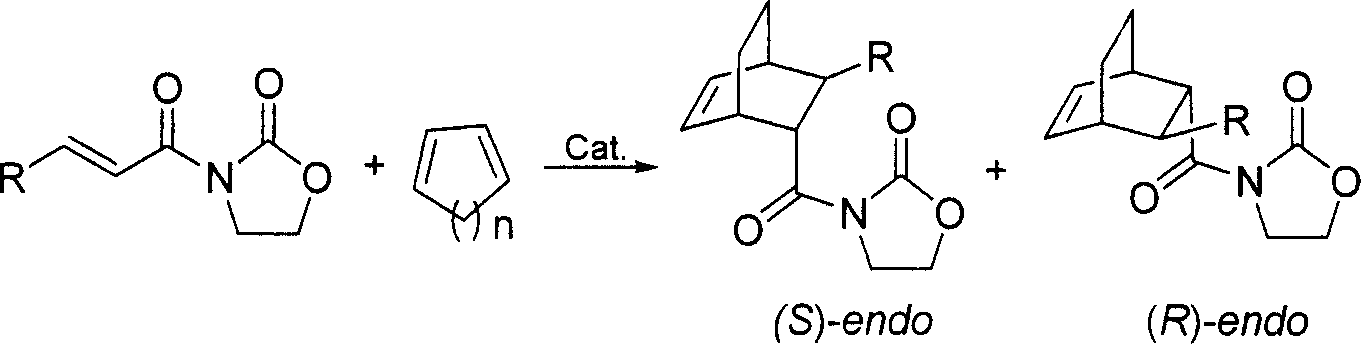
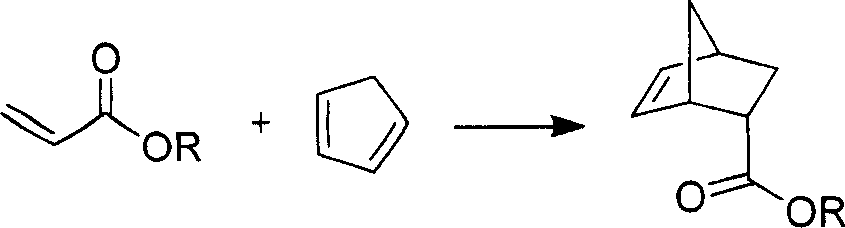
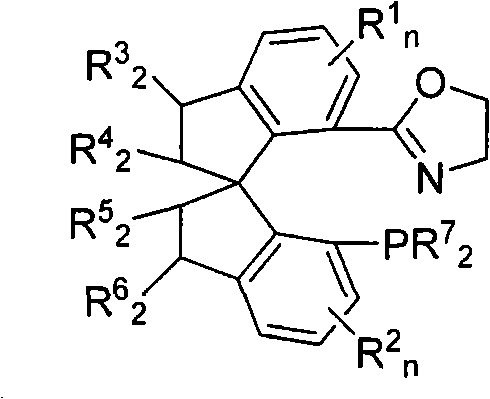
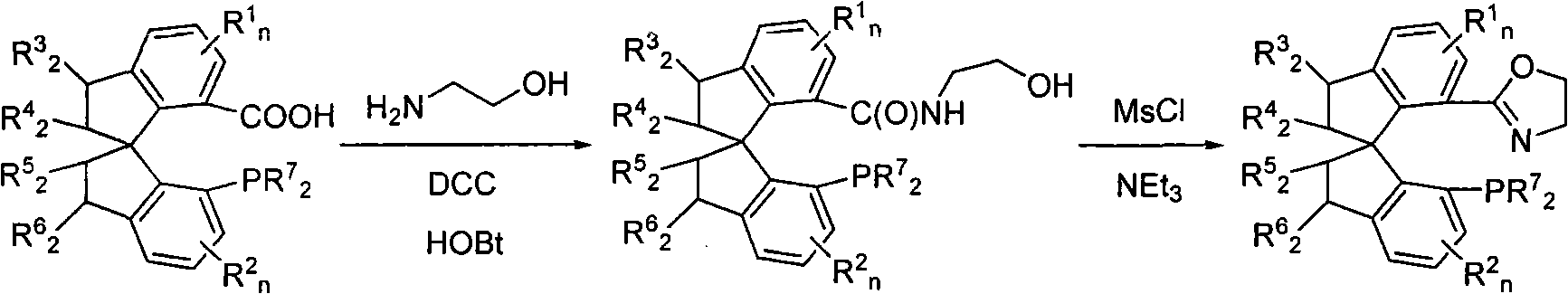
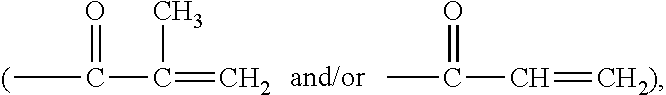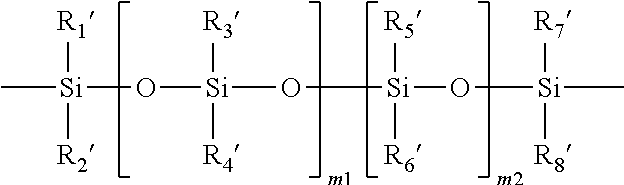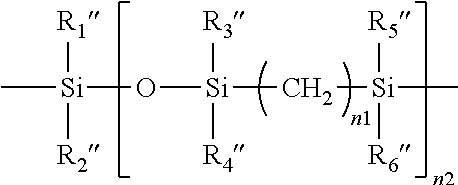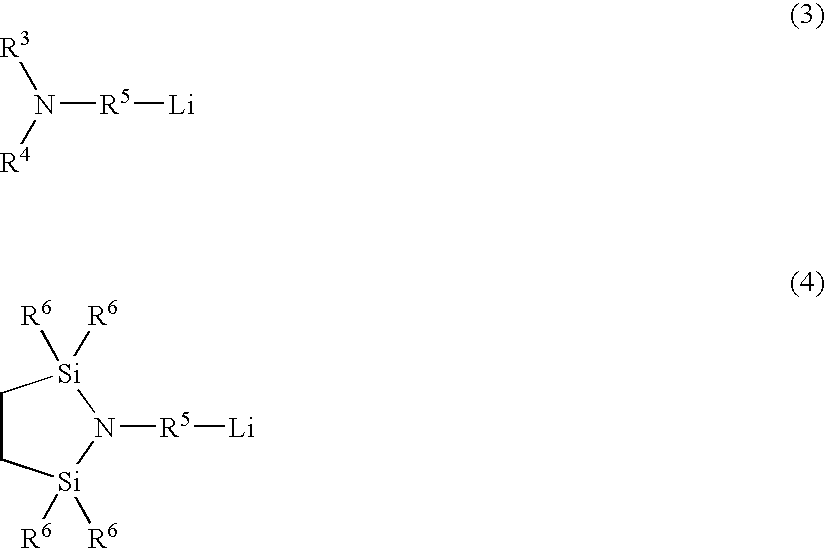Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
624 results about "Oxazoline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Oxazoline is a five-membered heterocyclic chemical compound containing one atom each of oxygen and nitrogen. It was likely first synthesized in 1884 but it was not until 5 years later that Siegmund Gabriel correctly assigned the structure. It was named in-line with the Hantzsch–Widman nomenclature and is part of a family of heterocyclic compounds, where it exists between oxazole and oxazolidine in terms of saturation.
Acid sensitive ARC and method of use
InactiveUS6110653APhotosensitive materialsSemiconductor/solid-state device manufacturingVinyl etherCross-link
A composition used to form an acid sensitive +E,uns a+EE nti+E,uns r+EE eflective +E,uns c+EE oating (ARC) includes a water soluble resin and a cross-linker. Radiation adsorptive components may be provided as part of the resin or, more preferably, as a separate dye. The composition may be applied on a substrate as a radiation adsorbing layer and additionally cross-linked to form an acid sensitive, water insoluble ARC on which a +E,uns p+EE hoto+E,uns p+EE atterning +E,uns r+EE esist (PPR) layer may be formed. Being acid sensitive, selected portions of an ARC formed from the composition may be removed by a suitable reversal of the cross-linking followed by a develop step, preferably with an aqueous developer, more preferably de-ionized water. The water soluble resin is preferably hydroxystyrene-sulfonated styrene copolymer, poly(2-isopropenyl-2-oxazoline), or poly(acrylic acid), the cross-linker is preferably an acetal diacid or a water soluble divinyl ether, and the dye is preferably 9-anthracene methanol or a squaric acid derivative. If a suitable +E,uns p+EE hoto+E,uns a+EE cid +E,uns g+EE enerator (PAG) is included then an ARC formed from such components may exhibit a photosensitivity similar to or even lower than that of the overlying PPR. The photosensitivity is preferably less than about 900 mJ / cm2, more preferably 100 mJ / cm2 or less.
Owner:IBM CORP
Poly(oxazoline-co-ethyleneimine)-epichlorohydrin copolymers and uses thereof
The invention is related to poly(2-oxazoline-co-ethyleneimine)-epichlorohydrin copolymers and chemically-modified derivatives thereof as well as their uses in formation of non-silicone hydrogel coatings on silicone hydrogel contact lenses.
Owner:ALCON INC
Synthesis of clasto-lactacystin .beta.-lactone and analogs thereof
InactiveUS6849743B2High activity in inhibitionPrevent and reduce sizeBiocideOrganic compound preparationCombinatorial chemistryFormamide
The present invention is directed to an improved synthesis of clasto-lactacystin-β-lactone, and analogs thereof, that proceeds in fewer steps and in much greater overall yield than syntheses described in the prior art. The synthetic pathway relies upon a novel stereospecific synthesis of an oxazoline intermediate and a unique stereoselective addition of a formyl amide to the oxazoline. Also described are novel clasto-lactacystin-β-lactones, and analogs thereof and their use as proteasome inhibitors.
Owner:MILLENNIUM PHARMA INC
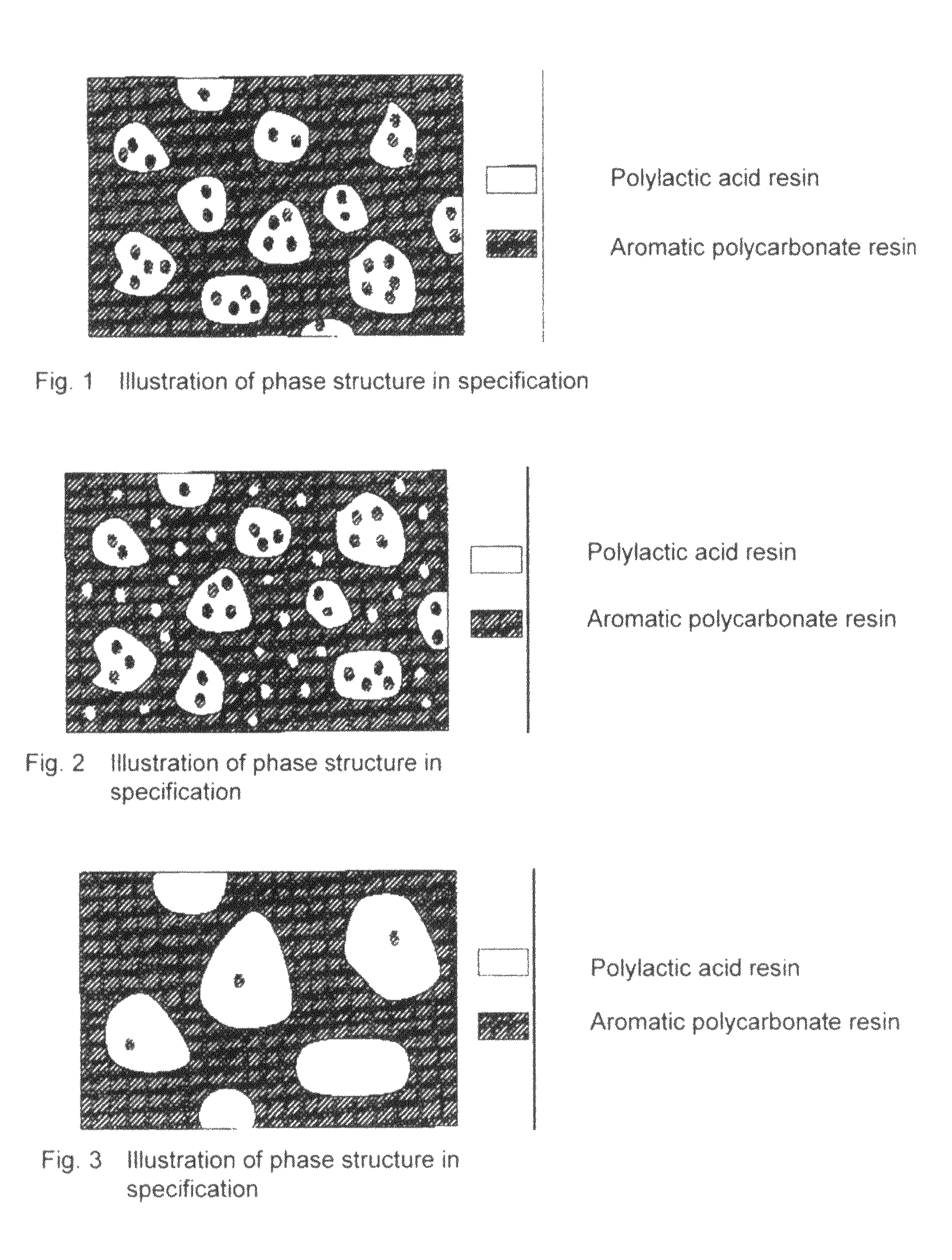
Resin Composition and Molded Article Comprising the Same
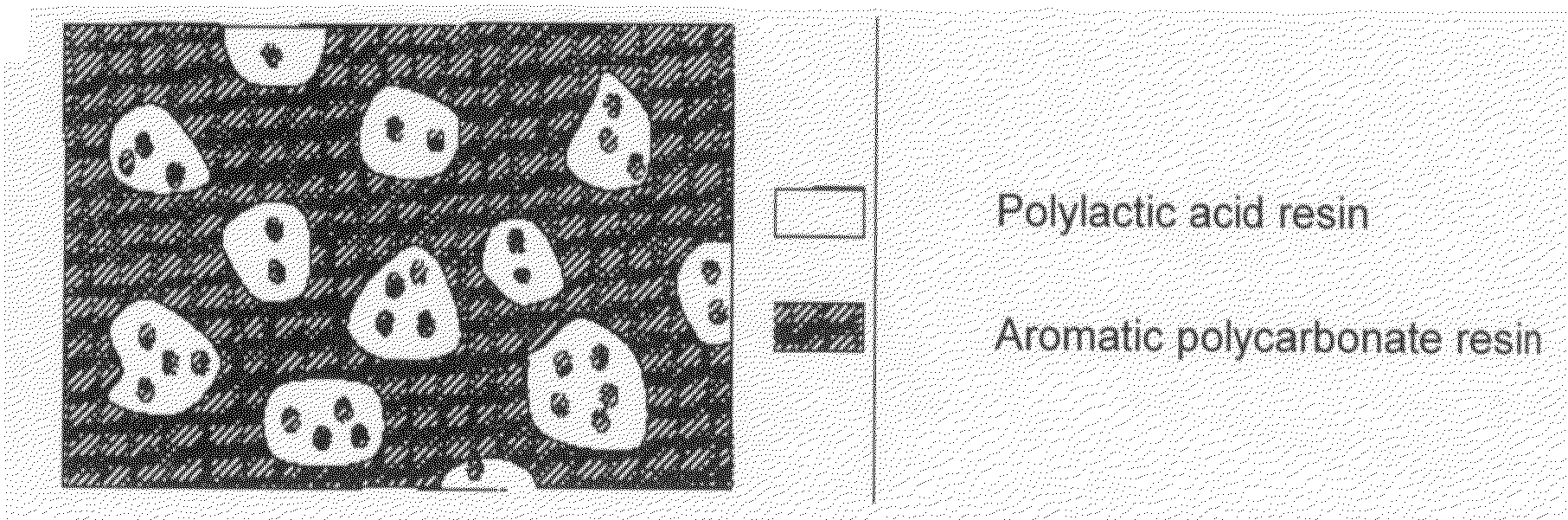
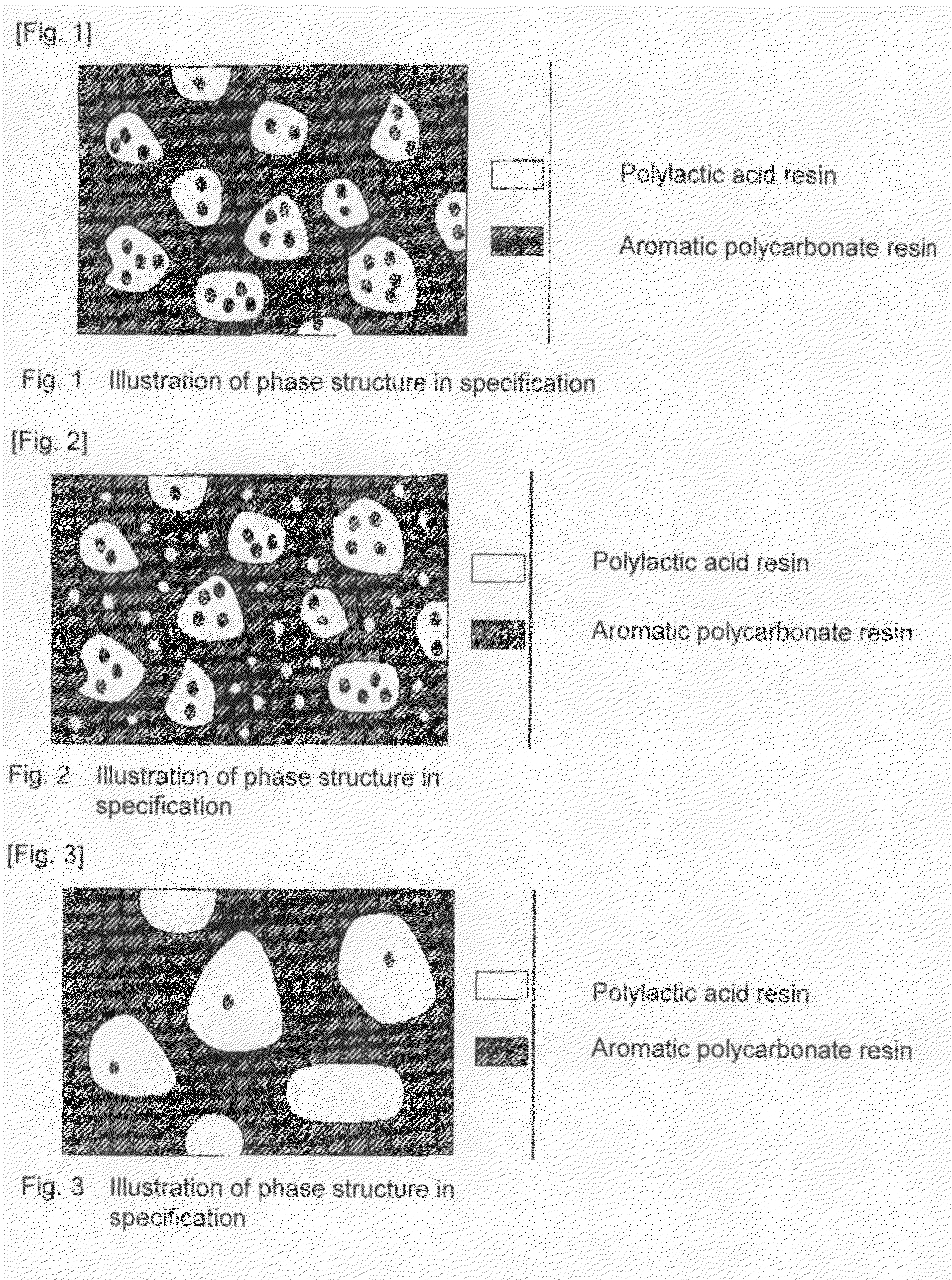
A resin composition in which a polylactic acid resin (A) 95-5 wt %, an aromatic polycarbonate resin (B) 5-95 wt %, and, with respect to 100 wt parts of the total of the (A) and the (B), at least one compatibilizer selected from a polymer compound containing an acrylic resin or styrene resin unit as a graft (C), a polymer compound to which a glycidyl compound or an acid anhydride is grafted or copolymerized (D) and an oxazoline compound, an oxazine compound and a carbodiimide compound (E) are compounded.
Owner:TORAY IND INC
Ink-jet recording medium with an opaque or semi-opaque layer coated thereon, method for recording an image, and a recorded medium with at least one layer rendered clear or semi-opaque
InactiveUS20040109958A1Extended drying timeAvoid excessive adhesionLayered productsCoatingsAziridineCross linker
The present invention features a multi-layer ink-jet recording medium, suitable for recording images with dye and pigmented inks and thereby providing light-emitting, reflective, glossy, metallic-looking or holographic images, comprising a substrate coated with at least two layers comprising: (a) a first transparent ink-receptive layer comprising a polymeric binder and a cross-linker and optionally having a plasticizer and pigment particles such as alumina and silica coated over the substrate, wherein the cross-linker comprises and azetidinium polymer or a salt thereof, and / or a polyfunctinal aziridine or a salt thereof or a polyfunctional oxazoline or a salt thereof; and (b) a second ink-receptive layer comprising an opaque or semi-opaque coating composition, wherein the opaque or semi-opaque coating composition is capable of accepting a printed image and thereby becoming semi transparent or clearly transparent from application of ink-jet printing ink or similar inks, while presenting a light-emitting, reflective, glossy, metallic-looking or holographic image of high clarity and quality, wherein said first layer is located between said second layer and the substrate in said recording medium and the first and second layers are chemically coupled.
Owner:PIXTERRA
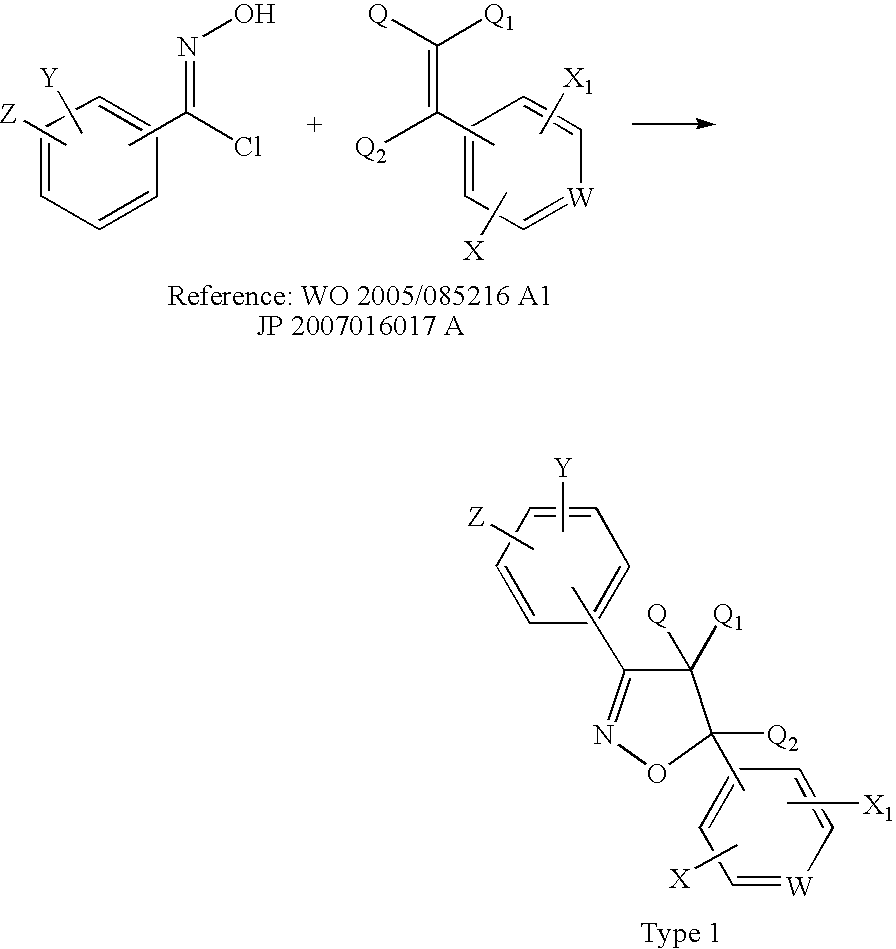
Diarylisoxazolines
Owner:DOW AGROSCIENCES LLC
Resin composition and molded article comprising the same
Owner:TORAY IND INC
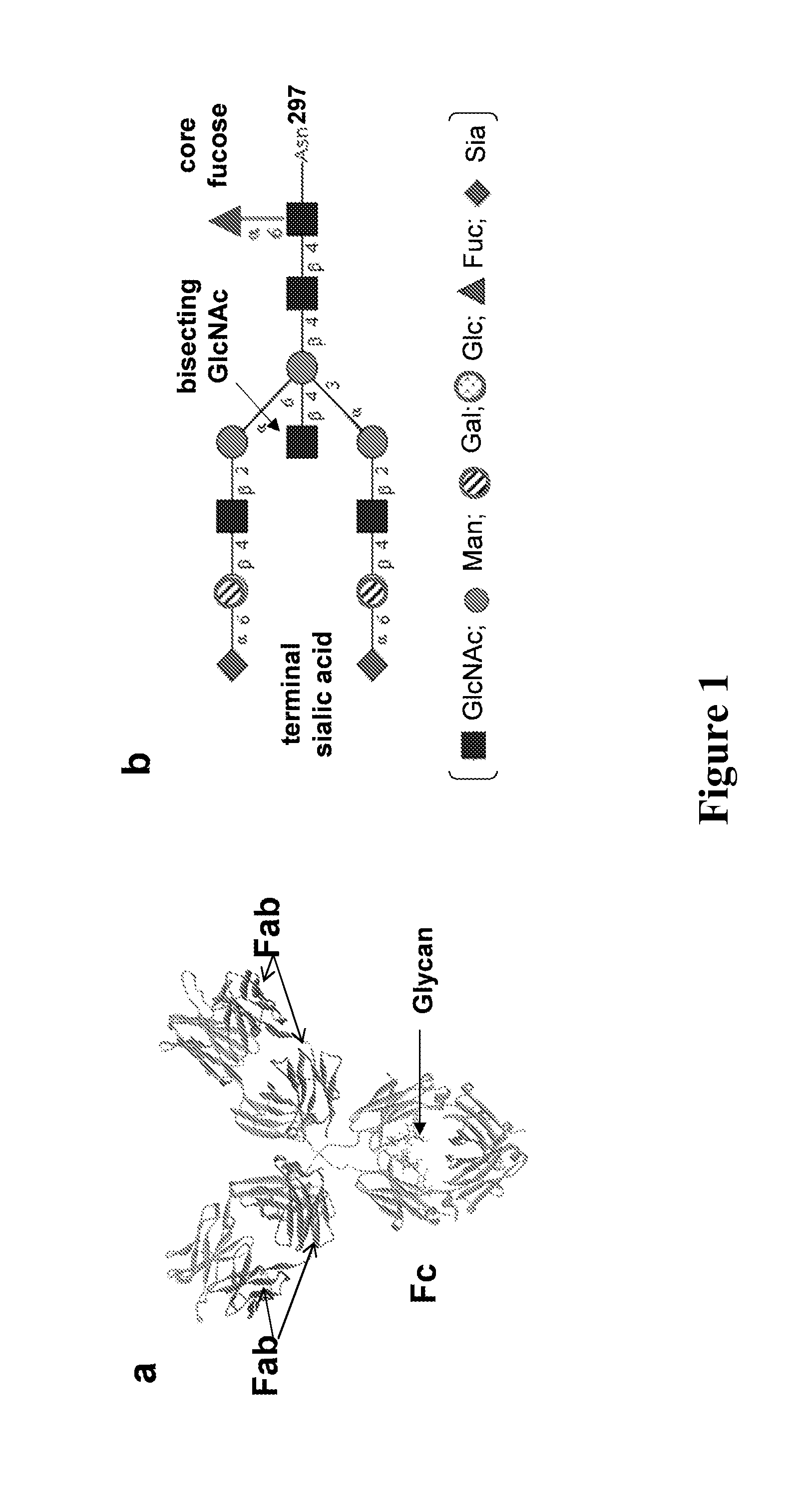
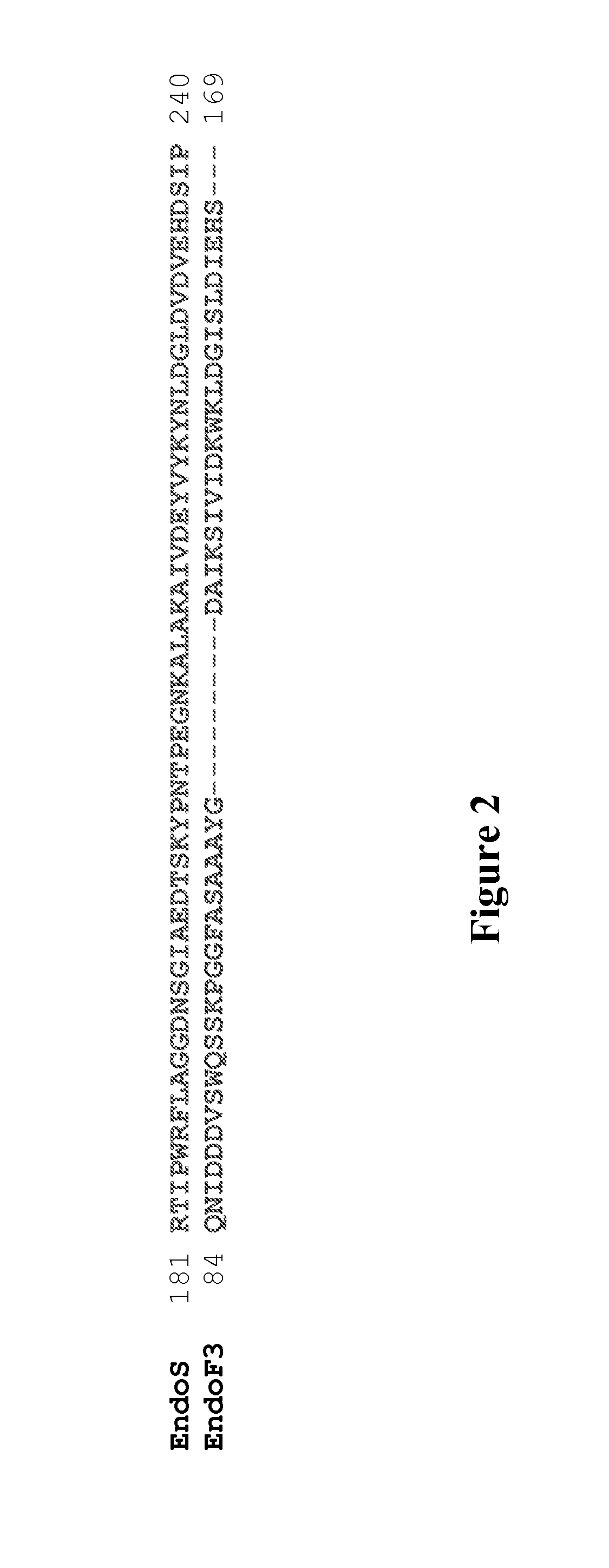
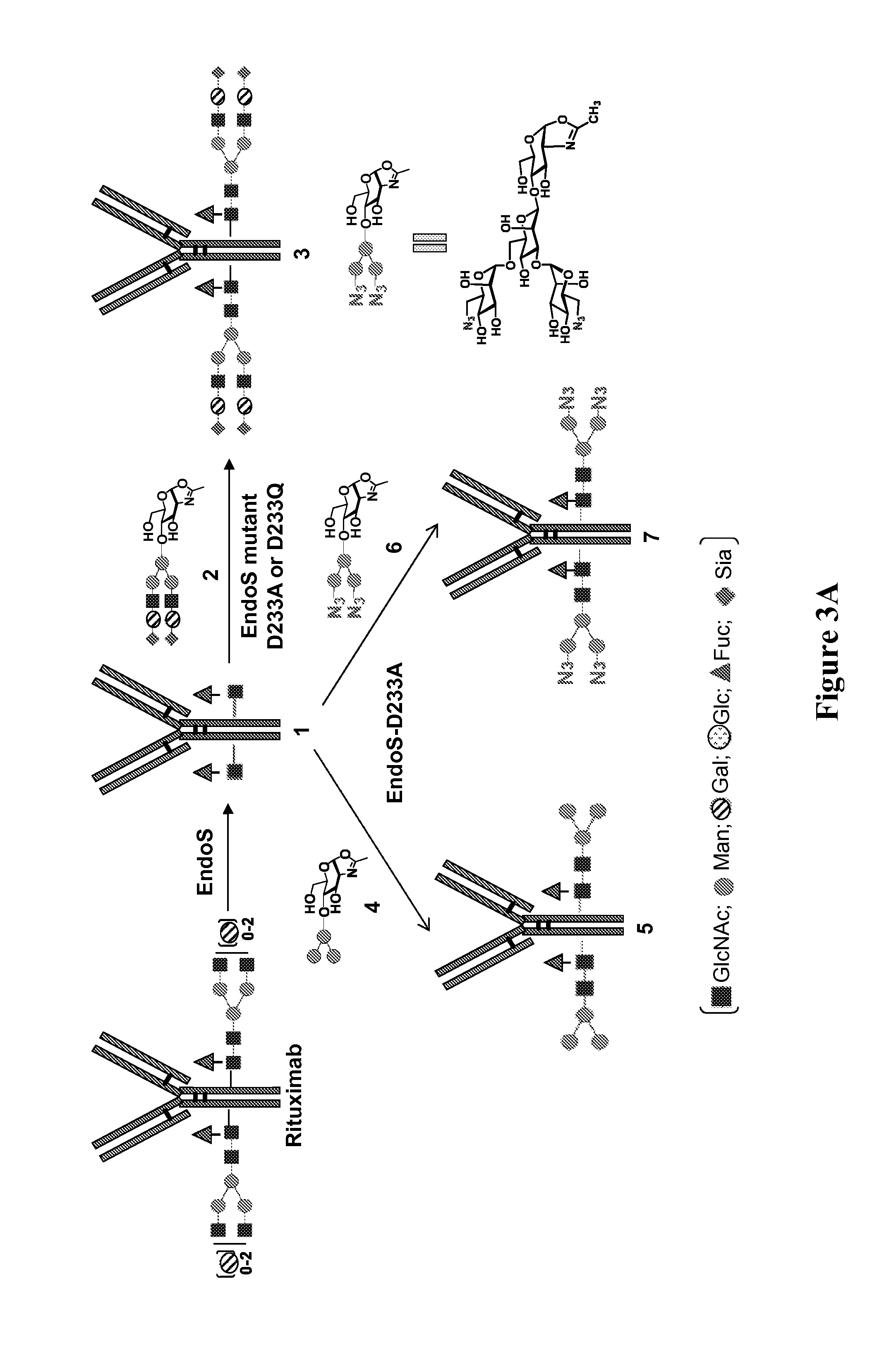
Core fucosylated glycopeptides and glycoproteins: chemoenzymatic synthesis and uses thereof
ActiveUS20120226024A1Prolonged half-life-timeLess immunogenicityImmunoglobulins against animals/humansEnzymesFucosylationEndorhamnosidase
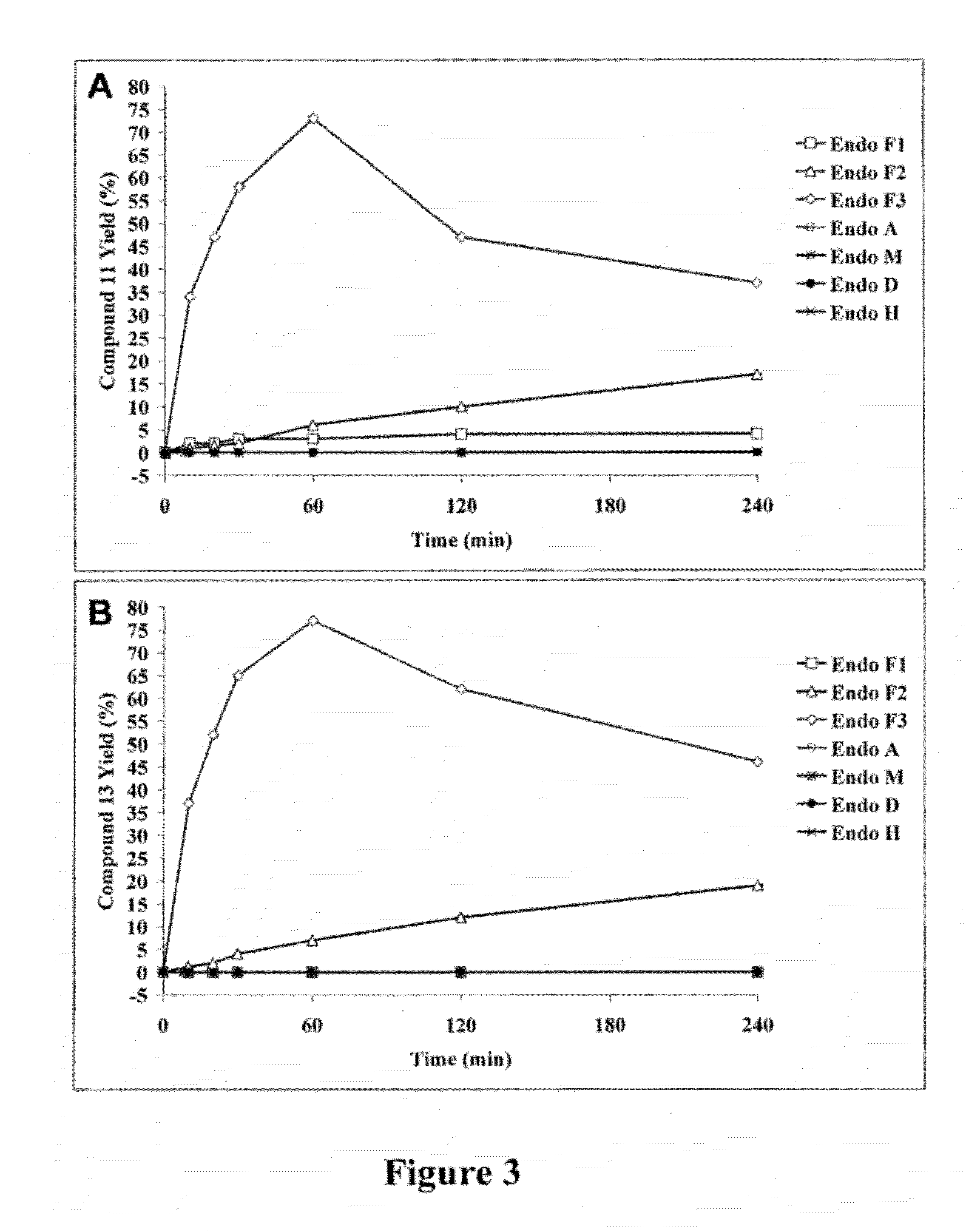
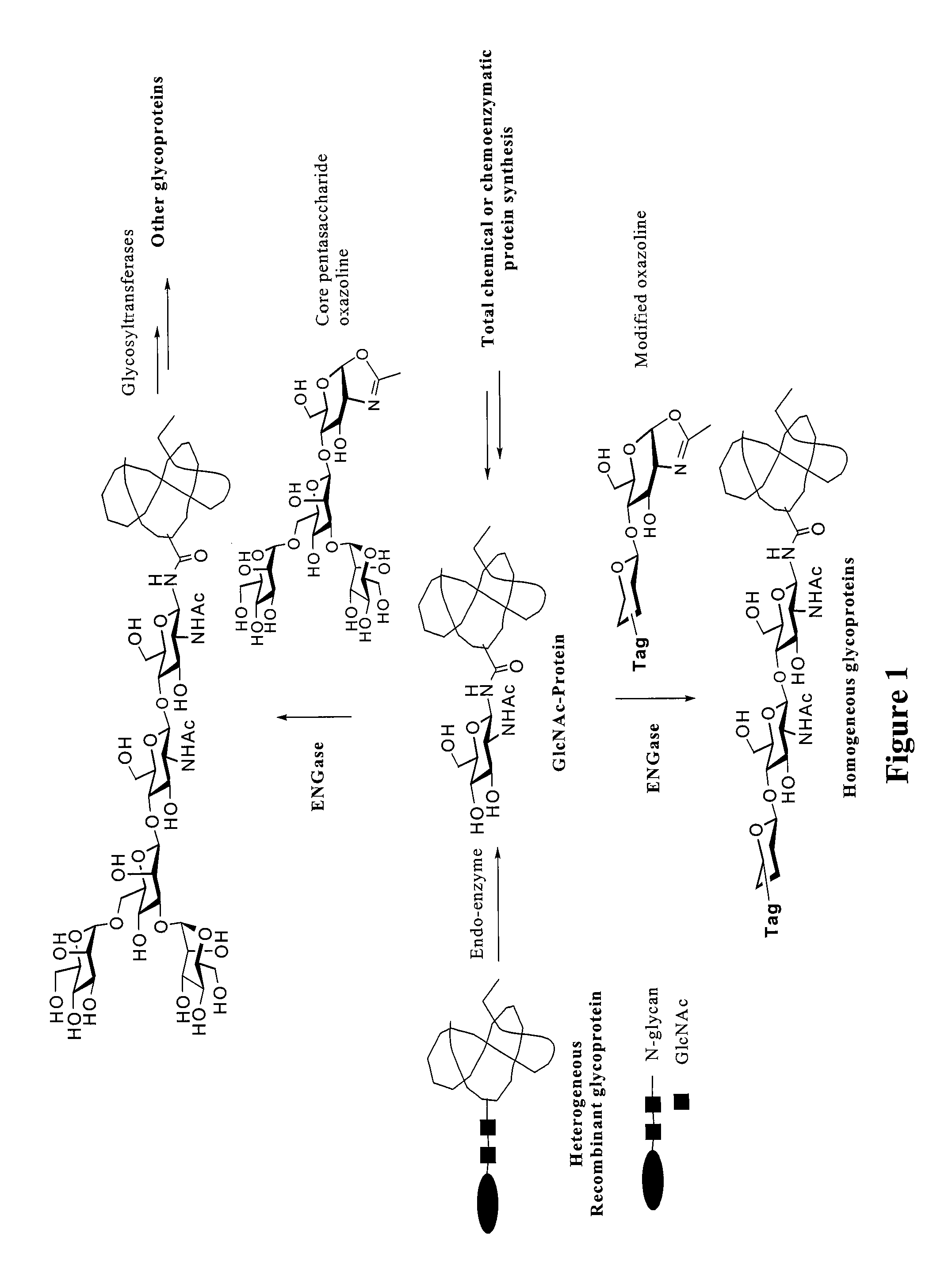
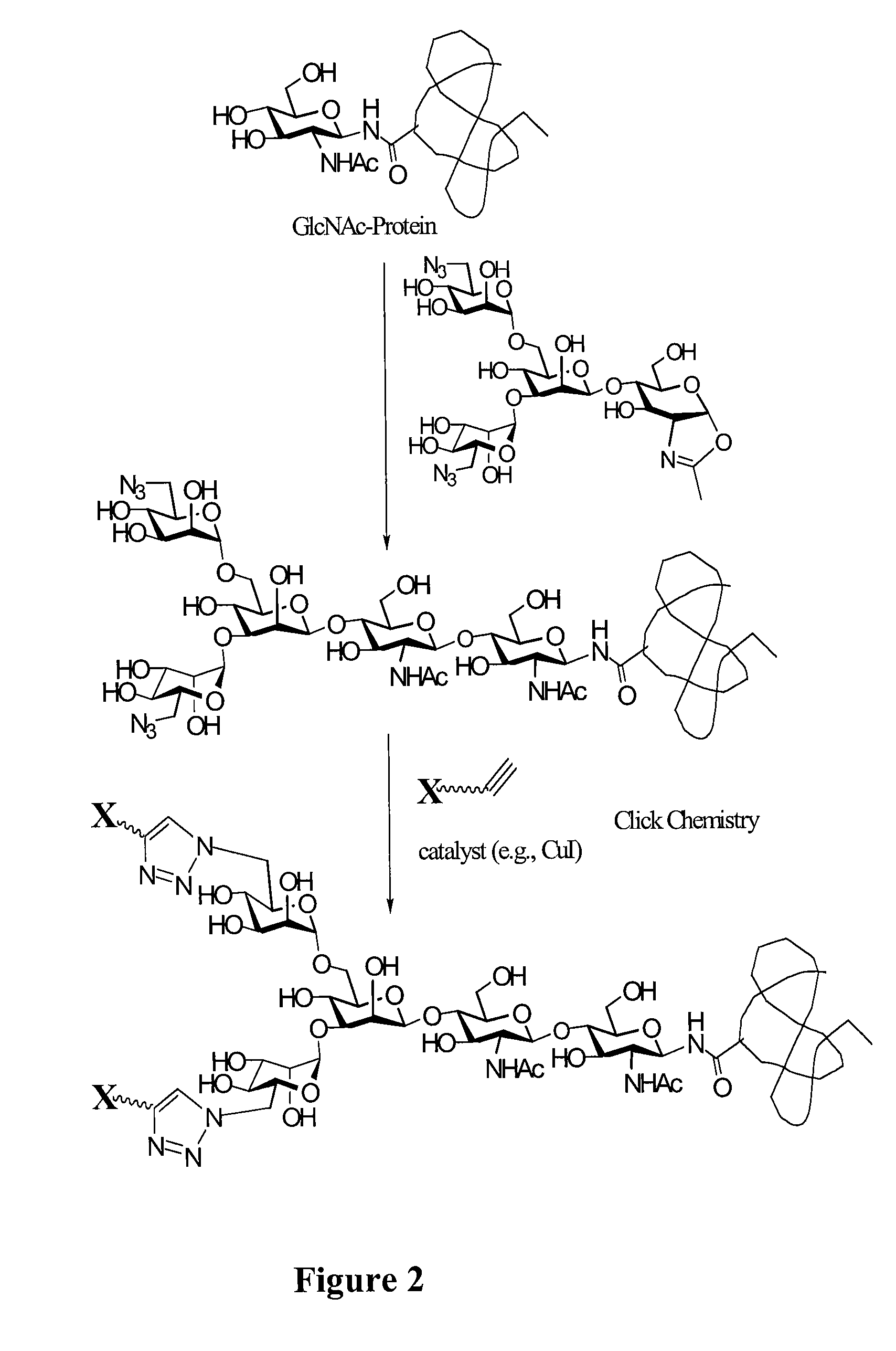
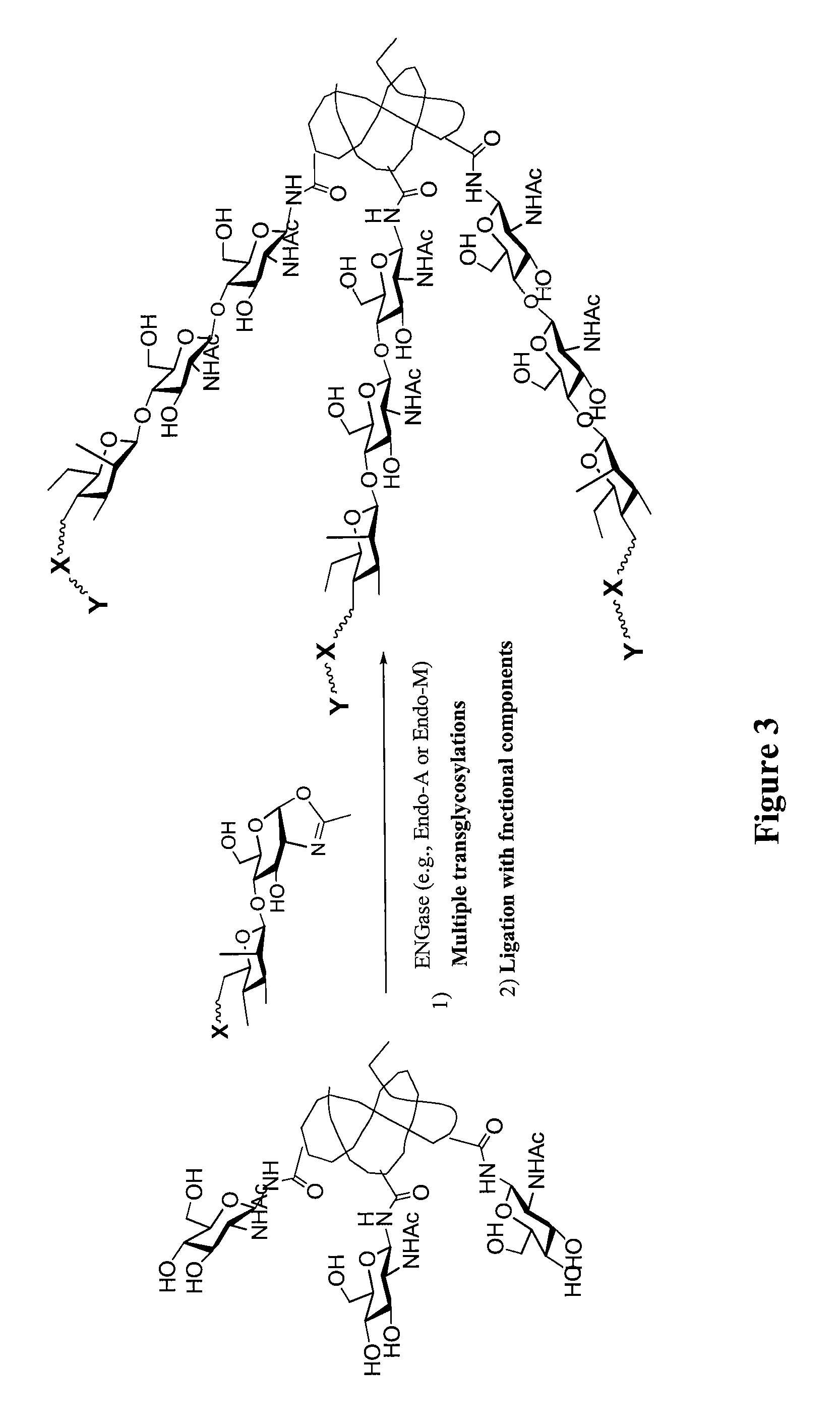
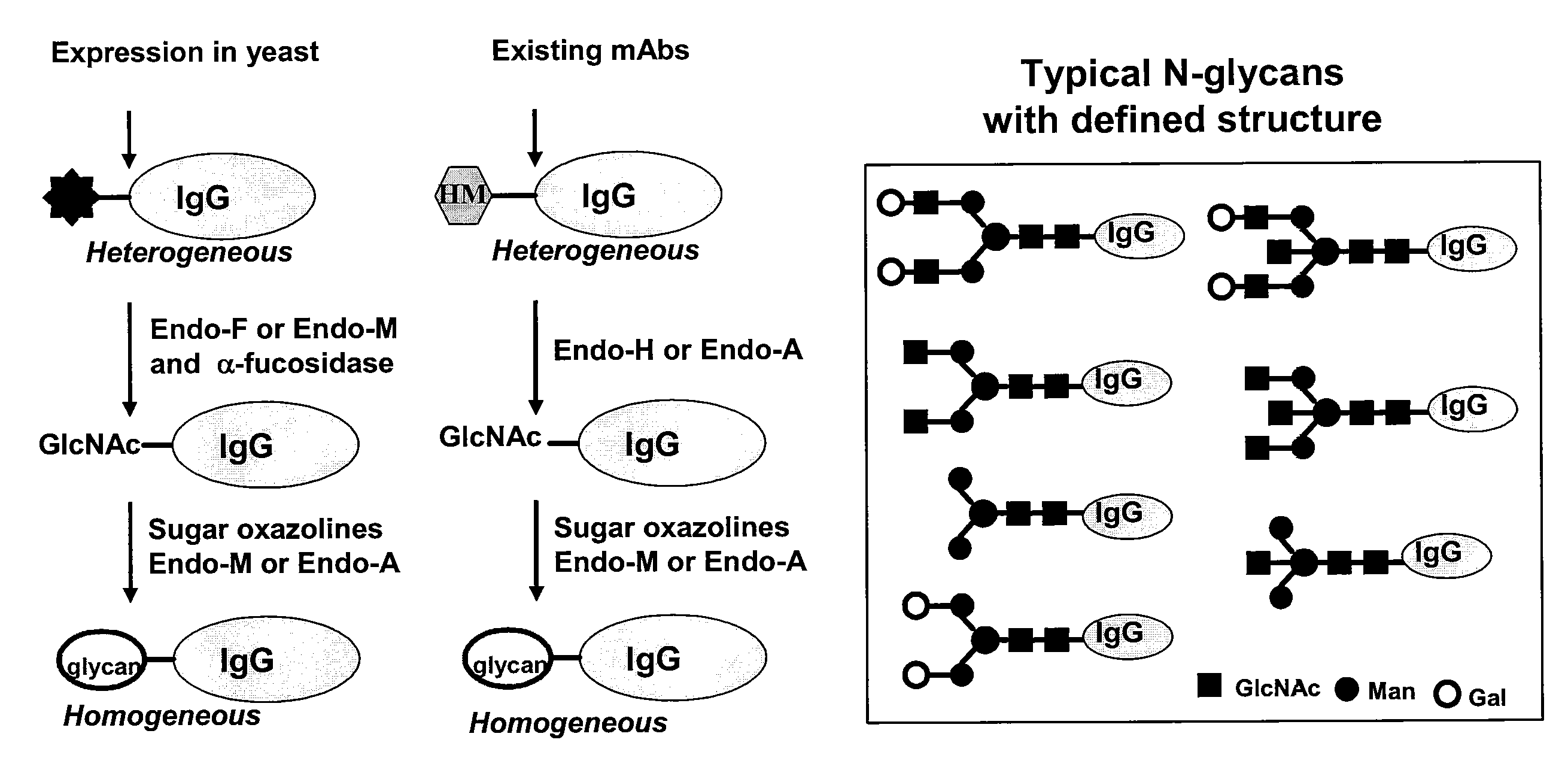
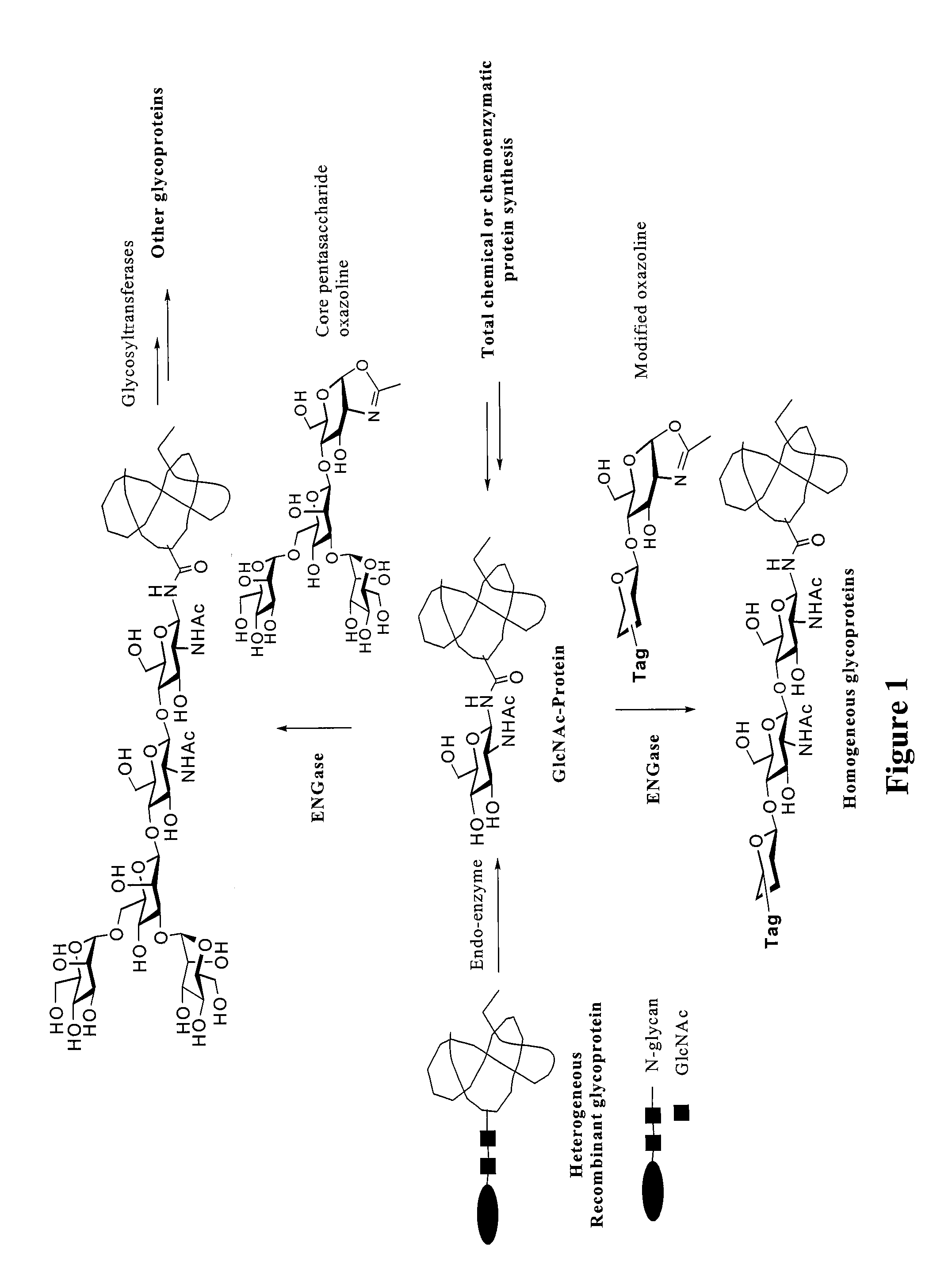
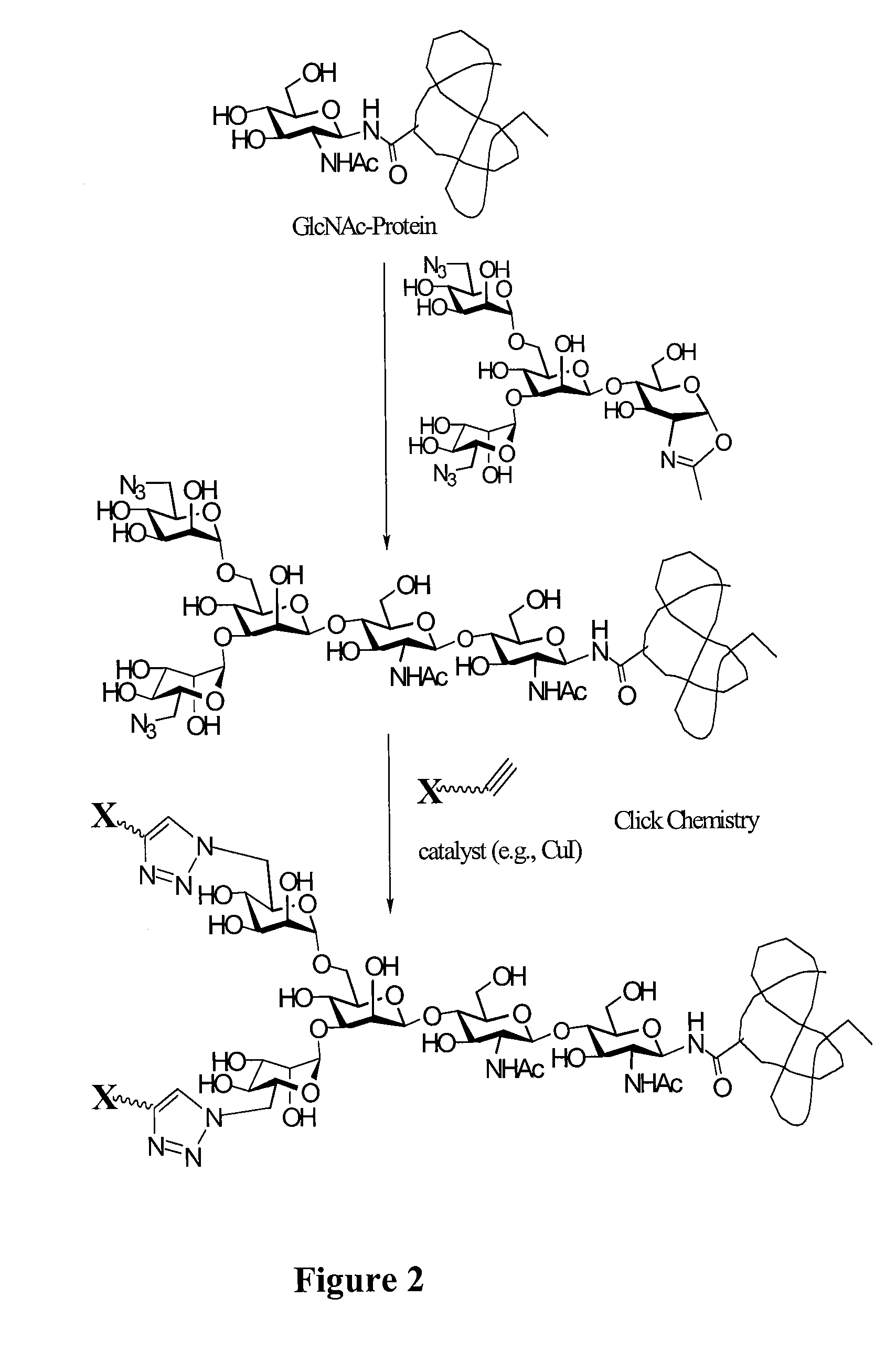
A chemoenzymatic method for the preparation of a core-fucoslyated glycoprotein or glycopeptide, including (a) providing an acceptor selected from the group consisting of a fucosylated GlcNAc-protein and fucosylated GlcNAc-peptide; and (b) reacting the acceptor with a donor substrate including an activated oligosaccharide moiety, in the presence of an endoglycosidase (ENGase) selected from Endo;F1, Endo-F2, Endo-F3, Endo-D and related glycosynthase mutants to transfer the oligosaccharide moiety to the acceptor and yield the structure defined core-fucosylated glycoprotein or glycopeptide. The donor substrate includes, in a specific implementation, a synthetic oligosaccharide oxazoline. A related method of fucosylated glycoprotein or fucosylated glycopeptide remodeling with a predetermined natural N-glycan or a tailor-made oligosaccharide moiety, and a method of remodeling an antibody to include a predetermined sugar chain to replace a heterogeneous sugar chain, are also described.
Owner:UNIV OF MARYLAND BALTIMORE
Adhesive composition, adhesive optical film and image display device
ActiveUS20060188712A1Improve adhesionImprove heat resistanceBlast furnace componentsSynthetic resin layered productsPolymer scienceMeth-
To provide an adhesive composition which shows high adhesion with a glass substrate and has excellent heat resistance, moisture resistance and moist heat resistance, an adhesive optical film comprising an adhesive layer made of the adhesive composition, which shows high adhesion between the adhesive layer and an optical film and effectively suppresses the occurrence of adhesive residue and lack of adhesive and also has excellent heat resistance, moisture resistance and moist heat resistance, and an image display device comprising the adhesive optical film, the adhesive optical film comprises an optical film 1, an adhesive layer 3 made of a water dispersible adhesive composition comprising, as raw monomers, an alkyl(meth)acrylate ester whose alkyl group has 4 to 18 carbon atoms, a carboxyl group-containing vinyl monomer, a phosphoric acid group-containing vinyl monomer, and a copolymerizable vinyl monomer which is optionally copolymerizable with the above monomers, wherein a carboxyl group concentration is from 0.05 to 1.50 mmol / g and a phosphoric acid group concentration is from 0.01 to 0.45 mmol / g in the raw monomers, an under coat layer 2 containing an oxazoline group-containing polymer, and being interposed between the optical film 1 and the adhesive layer 3, and the adhesive optical film is applied onto an image display device.
Owner:NITTO DENKO CORP
Hydrolysis-resistant, transparent, biaxially oriented film made from a crystallizable thermoplastic, and process for its production
InactiveUS6855758B2Maintain good propertiesLow shrinkageAluminium compoundsOrganic chemistryPolyesterThermoplastic
The invention relates to transparent, biaxially oriented and heat-set films having one or more layers and compris, as main constituent, at least one crystallizable thermoplastic, in particular a polyester, and also comprise at least one hydrolysis stabilizer. The hydrolysis stabilizer is preferably a phenolic compound, an oxazoline, and / or a monomeric or polymeric carbodiimide, where appropriate combined with an organic phosphite. It is preferably added in the form of a masterbatch. The film exhibits low longitudinal and transverse shrinkage. On exposure to moisture and heat it shows practically no embrittlement and retains its ultimate tensile strength. The additional functionality is preferably that the film has been made UV-resistant, or flame-retardant, or on one side or on both sides has been coated, or is sealable, and / or has been corona- or flame-treated. The film is generally produced by extrusion or coextrusion, the hydrolysis stabilizer being added in the form of a predried or precrystallized masterbatch.
Owner:MITSUBISHI POLYESTER FILM
Chemoenzymatic glycoengineering of antibodies and fc fragments thereof
ActiveUS20150087814A1Reduced hydrolysis activityIncreased transglycosylation activityBacteriaMicroorganism based processesFucosylationFc domain
The present invention provides for recombinant Endo-S mutants that exhibit reduced hydrolysis activity and increased transglycosylation activity for the synthesis of glycoproteins wherein a desired sialylated oxazoline or synthetic oligosaccharide oxazoline is added to a core fucosylated or nonfucosylated GlcNAc-protein acceptor. Such recombinant Endo-S mutants are useful for efficient glycosylation remodeling of IgGl-Fc domain to provide different antibody glycoforms carrying structurally well-defined Fc N-glycans.
Owner:UNIV OF MARYLAND
Acid sensitive ARC and method of use
InactiveUS6319651B1Photosensitive materialsPhotosensitive materials for photomechanical apparatusVinyl etherCross linker
A composition used to form an acid sensitive antireflective coating (ARC) includes a water soluble resin and a cross-linker. Radiation adsorptive components may be provided as part of the resin or, more preferably, as a separate dye. Being acid sensitive, selected portions of an ARC formed from the composition may be removed by a suitable reversal of the cross-linking followed by a develop step, preferably with an aqueous developer, more preferably de-ionized water. The water soluble resin is preferably hydroxystyrene-sulfonated styrene copolymer, poly(2-isopropenyl-2-oxazoline), or poly(acrylic acid), the cross-inker is preferably an acetal diacid or a water soluble divinyl ether, and the dye is preferably 9-anthracene methanol or a squaric acid derivative.
Owner:INT BUSINESS MASCH CORP
Transition metal catalyst of polymerized ethene agent, prepartion method and application
ActiveCN1795988ALarge space for decorationEasy to implementOrganic-compounds/hydrides/coordination-complexes catalystsHydrocarbonsOligomerDimmer
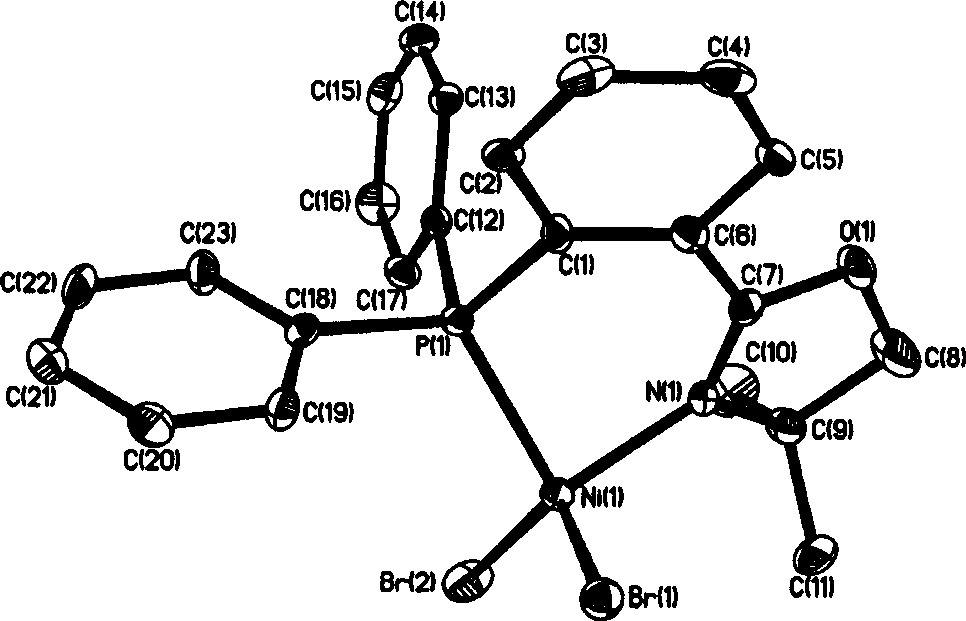
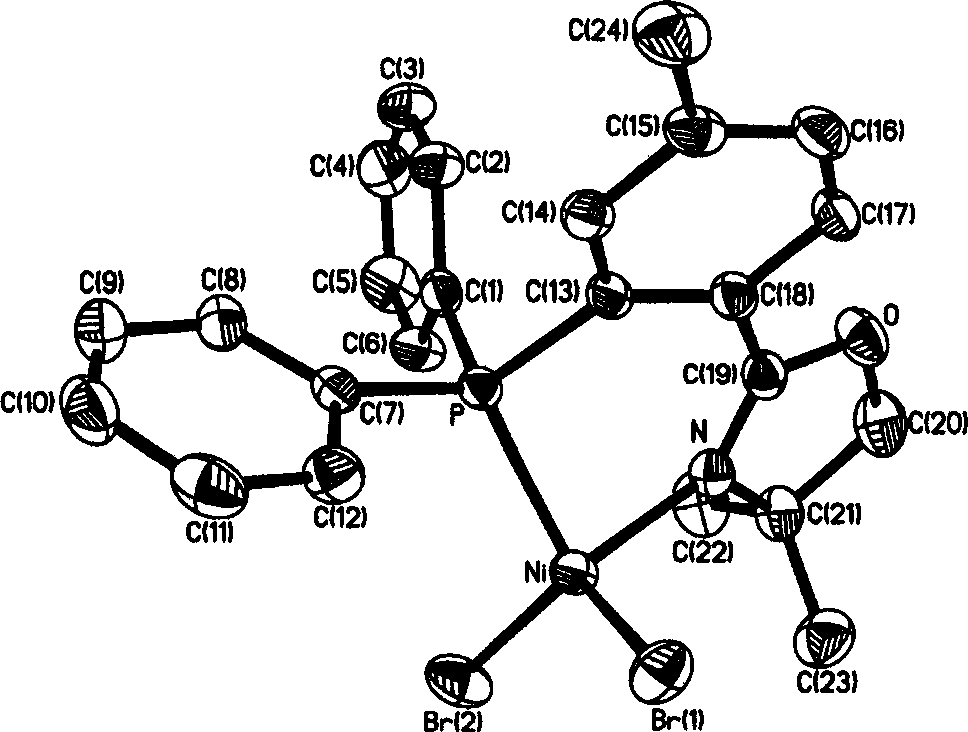
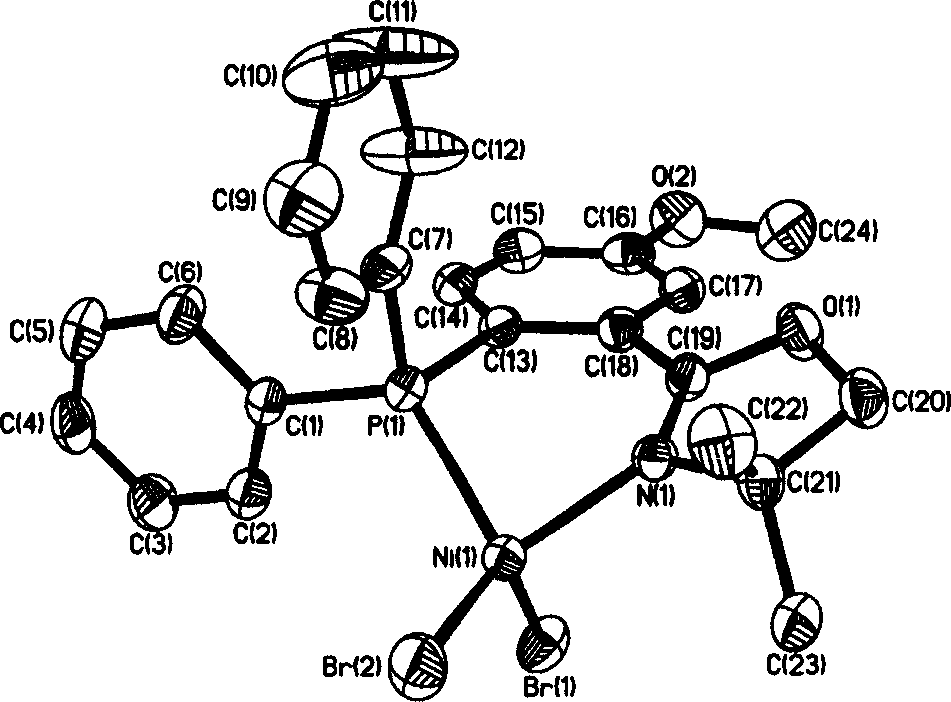
The invention discloses a 2-(2-(diphenyl phosphine) phenyl) oxazoline nickel halogenide series complex. Said invention provides the structure formula of said complex and its preparation method. Said invention also provides catalyst composition containing said complex and its application in ethylene oligopolymerization. Said catalyst composition has higher ethylene oligopolymerization activity, and the obtained oligomer takes ethylene dimmer and trimer as main components.
Owner:CHINA PETROLEUM & CHEM CORP
Glycoprotein synthesis and remodeling by enzymatic transglycosylation
ActiveUS7807405B2Prolonged half-life-timeLess immunogenicVirus peptidesPeptide preparation methodsHalf-lifeGlycopeptide
A chemoenzymatic method for the preparation of a homogeneous glycoprotein or glycopeptide, including (a) providing an acceptor selected from the group consisting of GlcNAc-protein and GlcNAc-peptide; and (b) reacting the acceptor with a donor substrate including an activated oligosaccharide moiety, in the presence of a catalyst comprising endoglycosidase (ENGase), to transfer the oligosaccharide moiety to the acceptor and yield the homogeneous glycoprotein or glycopeptide. The donor substrate includes, in a specific implementation, a synthetic oligosaccharide oxazoline. A related method of glycoprotein or glycopeptide remodeling with a predetermined natural N-glycan or a tailor-made oligosaccharide moiety, and a method of remodeling an antibody including a heterogeneous sugar chain, are also described. The disclosed methodology enables glycoprotein drugs to be modified for prolonged half-life in vivo, reduced immunogenicity, and enhanced in vivo activity, and for targeting and drug delivery.
Owner:UNIV OF MARYLAND
Polyphenylene sulfide alloy composition
The present invention relates to polymer compositions containing polyphenylene sulfide, a polymeric grafting agent and an ethylene copolymer. The polymeric grafting agent is a copolymer of at least about 50% by weight ethylene, about 0.5% to about 15% by weight of a first reactive moiety selected from the group consisting of: (i) an unsaturated epoxide of 4-11 carbon atoms, (ii) an unsaturated isocyanate of 2-11 carbon atoms, (iii) an alkoxy or alkyl silane wherein the alkyl group is from 1-12 carbon atoms, and (iv) an oxazoline; and about 0 to about 49% by weight of a second moiety selected from at least one of an alkyl acrylate, alkyl methacrylate, vinyl ether, carbon monoxide, and sulfur dioxide, where the alkyl and ether groups are of 1-12 carbon atoms. The ethylene copolymer comprises about 1% to about 20% by weight of an ethylene copolymer which comprises at least about 50% by weight ethylene, about 1% to about 35% by weight of an acid-containing unsaturated mono-carboxylic acid, and about 0 to about 49% by weight of a moiety selected from at least one of alkyl acrylate, alkyl methacrylate, vinyl ether, carbon monoxide, and sulfur dioxide, and further wherein the acid groups are neutralized from 0-100% by a metal ion. The molar ratio of polymeric grafting agent to ethylene copolymer preferably ranges from about 1.0 to about 5.5.
Owner:DUPONT POLYMERS INC
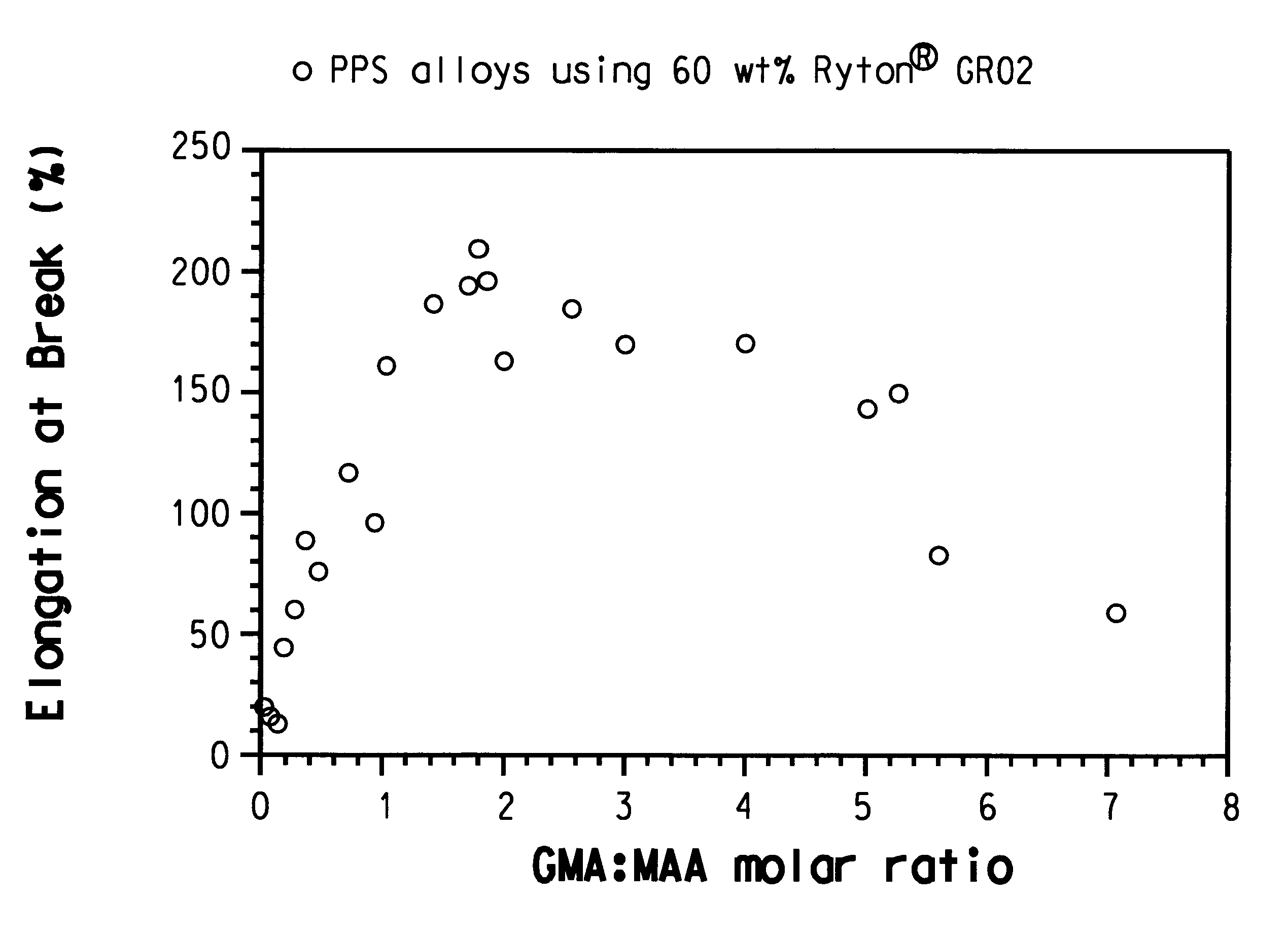
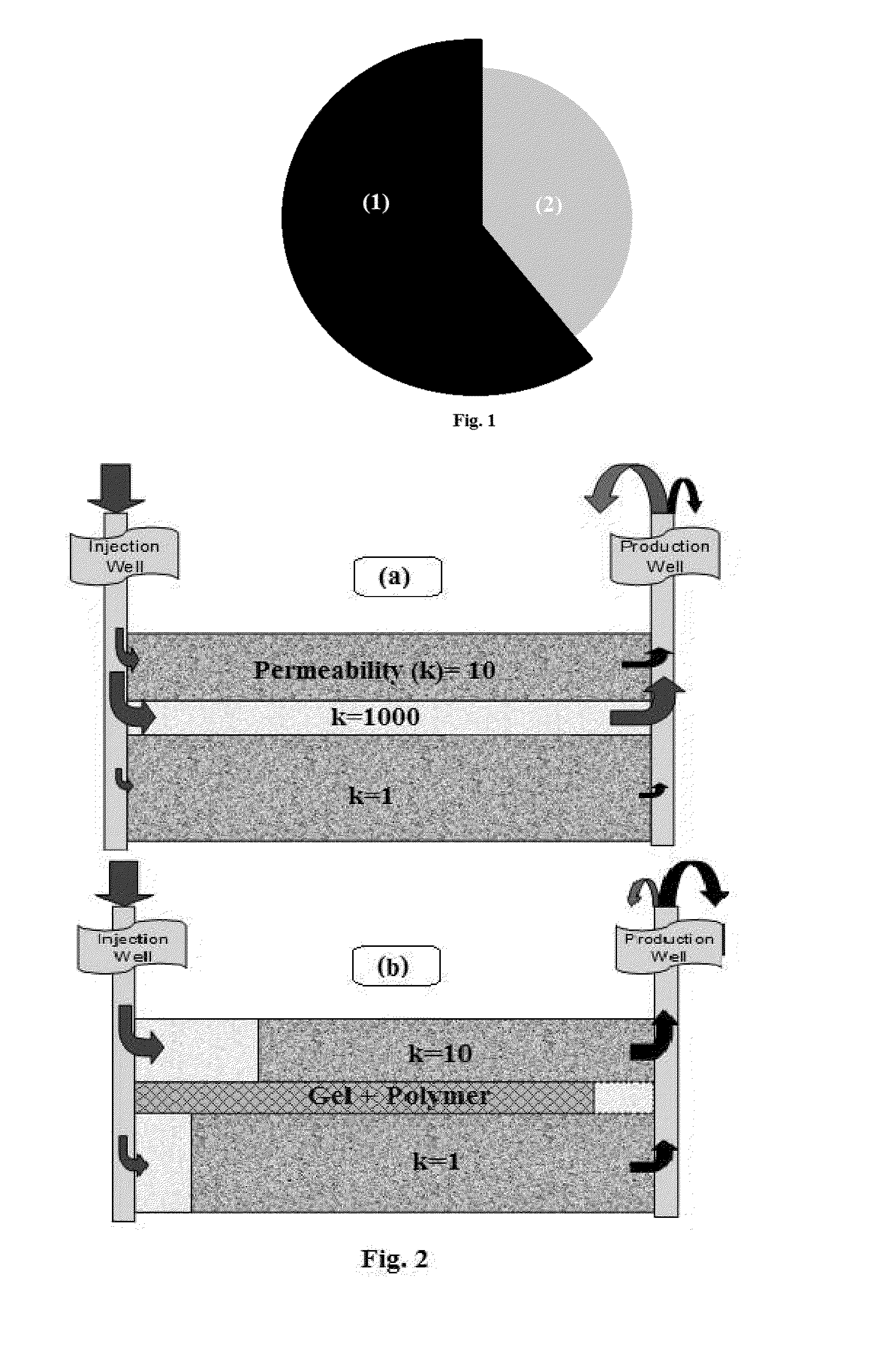
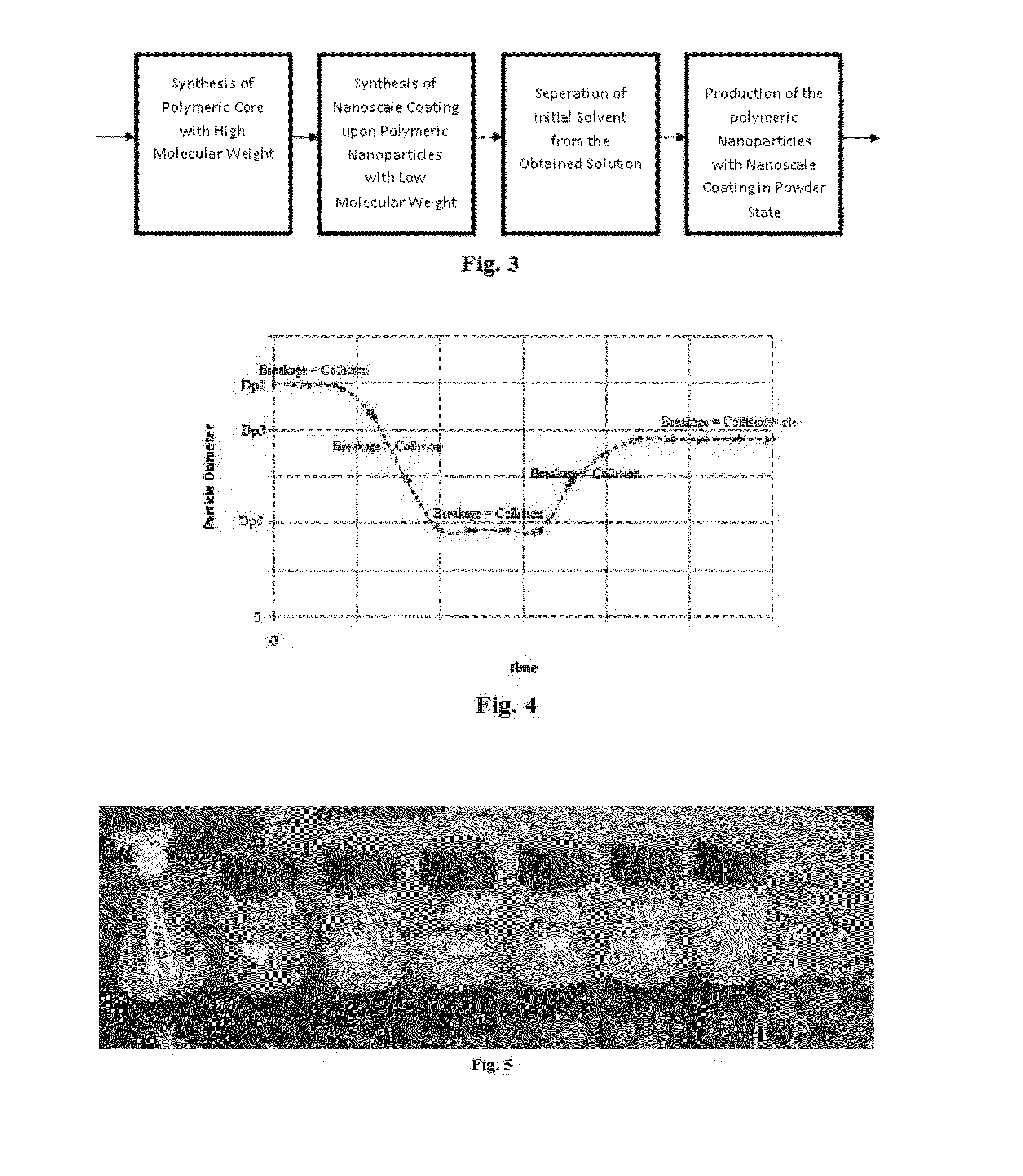
Producing Nanostructure of Polymeric Core-Shell to Intelligent Control solubility of Hidrophilic Polymer during Polymer Flooding Process
Hydrophilic polymer particles have been obtained using polyacrylamide, xanthane, maleic anhydride polymers, allylamine, ethyleneimine, and oxazoline as core polymers. Then, hydrophobic polymers shells have been produced on the core-side using styrene, styrene copolymers, polyvinyl state, polysolfune, polymethyl methacrylate, and polycyclohxyl methacrylate by in-situ polymerization of monomer as method one and inverse emulsion process as method two. These particles can release hydrophilic polymers at oil-water interface at the reservoir temperature where the water flooding should have the maximum viscosity. So, active materials cause to decrease the mobility ratio of water to oil in the reservoirs and on the other hand, plug the swept porosities and prevent to act the water fingering process.
Owner:TAMSILIAN YOUSEF +1
Method for enantioselective hydrogenation of chromenes
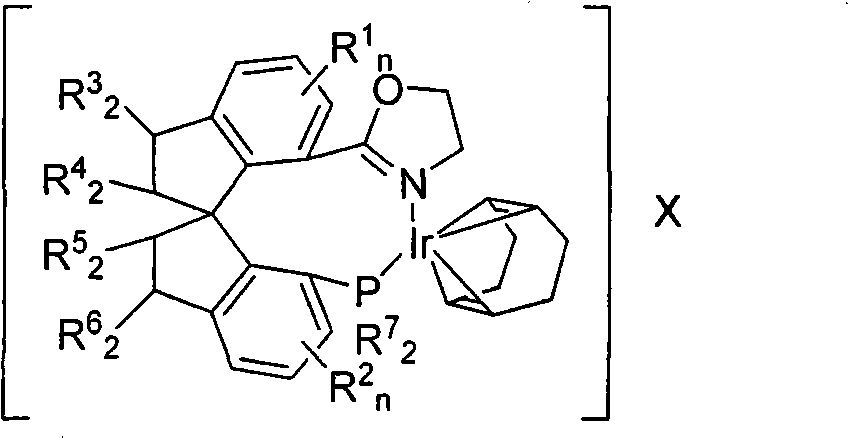
A method for preparing an enantiomeric chromane, by asymmetrically hydrogenating a chromene compound in the presence of an Ir catalyst having a chiral ligand. The method includes the enantioselective preparation of enantiomeric equol. A preferred Ir catalyst has a chiral phosphineoxazoline ligand. Enantiomeric chromanes of high stereoselective purity can be obtained.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI +1
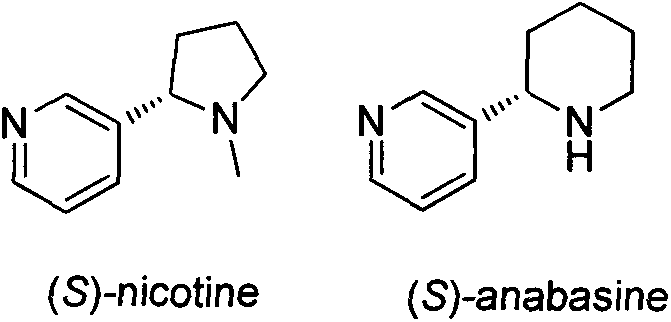
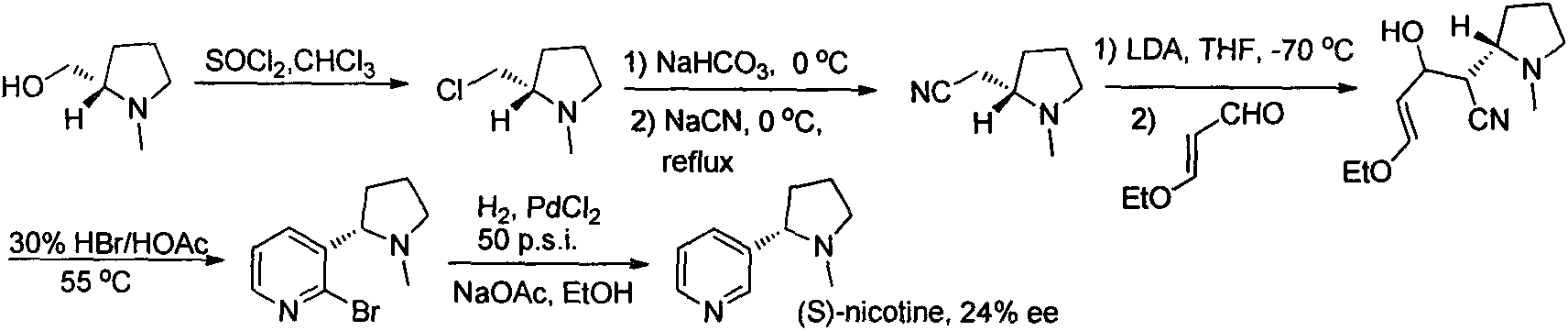
Asymmetric synthesis method for botanical pesticide nicotine and anabasine
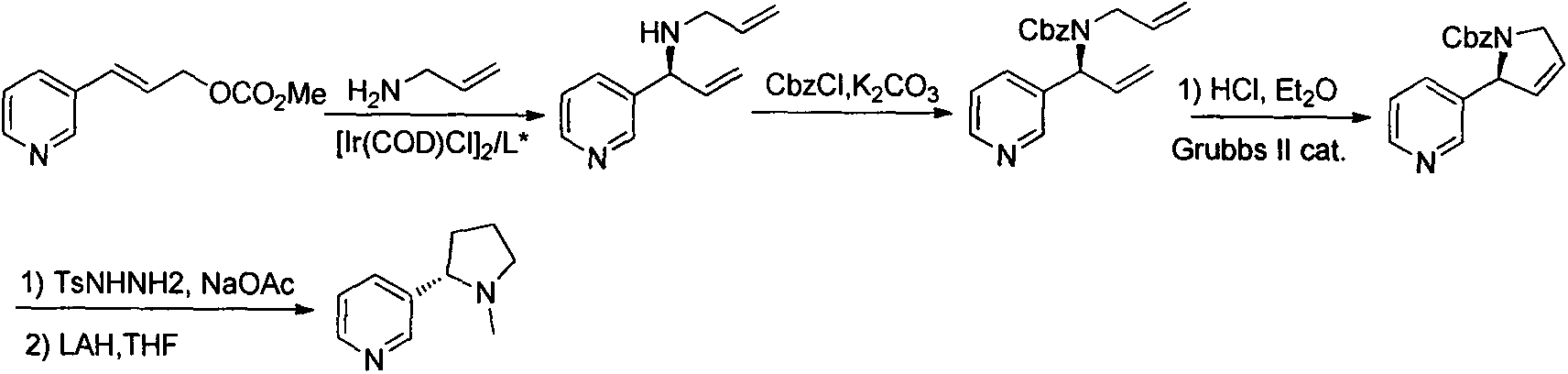
ActiveCN104341390AHigh purityEasy to operateOrganic chemistryBulk chemical productionIridiumSynthesis methods
The invention relates to an asymmetric synthesis method for botanical pesticide nicotine and anabasine. The low-cost and easily acquired 2,5-dibromopyridine is taken as an initial raw material and is processed in two steps, so that the hydrogenation precursor annular imine is acquired; under the induction of the chiral catalyst, iridium-phosphine oxazoline, an important hydrogenated product intermediate is acquired through high enantioselectivity; the intermediate is processed in two steps, so that L-nicotine is acquired; the intermediate is converted into L-anabasine in one step. The asymmetric hydrogenation of the annular imine containing pyridine gene is taken as the key step of the method. According to the invention, the chiral catalyst, iridium-phosphine oxazoline, is used for catalyzing the asymmetric hydrogenation and the key intermediate with ultrahigh ee value is acquired, and then the methylation and reduction bromine-removing two-step reaction is performed for converting, so that the target products, natural nicotine and anabasine, are acquired. According to the invention, the operation is stable, the purity is high and the cost is low.
Owner:NANKAI UNIV
Glycoprotein synthesis and remodeling by enzymatic transglycosylation
ActiveUS20080138855A1Prolonged half-life-timeLess immunogenicImmunoglobulins against animals/humansDepsipeptidesHalf-lifeGlycopeptide
A chemoenzymatic method for the preparation of a homogeneous glycoprotein or glycopeptide, including (a) providing an acceptor selected from the group consisting of GlcNAc-protein and GlcNAc-peptide; and (b) reacting the acceptor with a donor substrate including an activated oligosaccharide moiety, in the presence of a catalyst comprising endoglycosidase (ENGase), to transfer the oligosaccharide moiety to the acceptor and yield the homogeneous glycoprotein or glycopeptide. The donor substrate includes, in a specific implementation, a synthetic oligosaccharide oxazoline. A related method of glycoprotein or glycopeptide remodeling with a predetermined natural N-glycan or a tailor-made oligosaccharide moiety, and a method of remodeling an antibody including a heterogeneous sugar chain, are also described. The disclosed methodology enables glycoprotein drugs to be modified for prolonged half-life in vivo, reduced immunogenicity, and enhanced in vivo activity, and for targeting and drug delivery.
Owner:UNIV OF MARYLAND BALTIMORE
Resin composition and molded product thereof
A resin composition includes: a resin component comprising 50 to 100 parts by mass of (i-1) a polylactic acid and 50 to 0 part by mass of (i-2) a polyolefin [the total of (i-1) and (i-2) is 100 parts by mass], and 1 to 100 parts by mass, per 100 parts by mass of the resin component, of (ii) a functional group-containing, hydrogenated, diene-based polymer containing at least one kind of functional group selected from the group consisting of carboxyl group, acid anhydride group, epoxy group, (meth)acryl group, amino group, alkoxysilyl group, hydroxyl group, isocyanate group and oxazoline group; and a molded article thereof. The resin composition and the molded article are superior in balance between tensile property or stiffness and impact resistance as well as in appearance when molded and, moreover, have biodegradability (disintegratability); and, therefore, can be used in various applications such as packaging materials, industrial materials, industrial products, containers, medical tools and the like.
Owner:JSR CORPORATIOON
Non-peptidyl inhibitors of VLA-4 dependent cell binding useful in treating inflammatory, autoimmune, and respiratory diseases
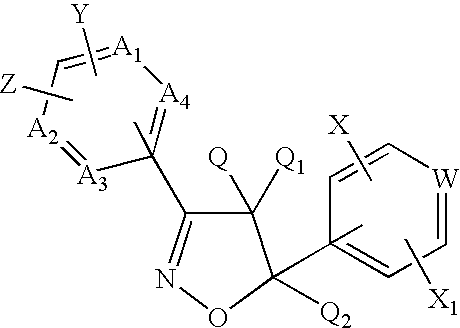
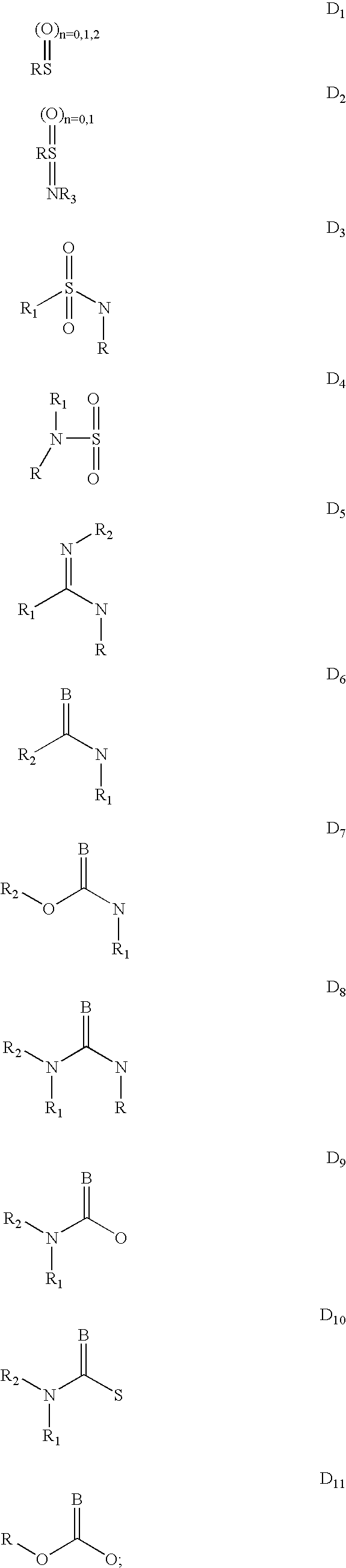
There is disclosed a genus of non-peptidyl compounds, wherein said compounds are VLA-4 inhibitors useful in treating inflammatory, autoimmune, and respiratory diseases, and wherein said compounds comprise a compound of Formula (1.0.0):and pharmaceutically acceptable salts and other prodrug derivatives thereof, wherein: A is (C1-C6) alkyl, cycloalkyl, aryl, heteroaryl or heterocyclyl as defined herein; where said alkyl, cycloalkyl, aryl, heteroaryl or heterocyclyl is optionally substituted with 0 to 3 R<9>; or is a member selected from the group consisiting of the following radicals: A<1>-NHC(=O)NH-A<2>-, A<1>-NHC(=O)O-A<2>-, A<1>-OC(=O)NH-A<2>-, A<1>-NHSO2NH-A<2>-, A<1>-NHC(=O)-A<2>-, A<1>-C(=O)NH-A<2>-, A<1>-NHSO2-A<2>-, A<1>-SO2NH-A<2>-, A<1>-(CH2)rO-A<2>-, A<1>-O(CH2)r-A<2>-, A<1>-(CH2)r-A<2>-, where A<1 >is aryl and A<2 >is aryl or pyridyl; where said aryl or pyridyl group is substituted with 0 to 3 R<9>; -B is oxazolinyl or isoxazolinyl; wherein said oxazolinyl and said isoxazolinyl may be optionally substituted with R<9>; R<2 >is hydrogen or methyl; R<3 >is taken together with R<4 >and the carbon and nitrogen atoms to which it is attached to form a pyrrolidinyl group substituted with 0 to 3 R<13>;and the remaining variables are as defined in the specification.
Owner:PFIZER INC
Multilayer film
ActiveUS20130260144A1Excellent gas barrier performanceHigh laminate strengthSynthetic resin layered productsRecord information storageAcrylic resinPeak intensity
The invention provides a multilayer film comprising a substrate film and a coating layer arranged on at least one surface of the substrate film, wherein the coating layer contains an oxazoline group and comprises an acrylic resin, the coating layer has a thickness (D) of 5-150 nm, and the ratio (P1 / P2) of the peak intensity (P1) of a peak that has an absorption maximum in a region of 1655±10 cm−1 to the peak intensity (P2) of a peak that has an absorption maximum in a range of 1580±10 cm−1 in the total reflection infrared absorption spectrum of the coating layer and the thickness (D) of the coating layer fulfill the relationship represented by the formula: 0.03≦(P1 / P2) / D≦0.15.
Owner:TOYO TOYOBO CO LTD
Patterning process and resist composition
InactiveUS20100159392A1Reduce spacingPhotosensitive materialsPhotomechanical exposure apparatusPolymer scienceAlcohol
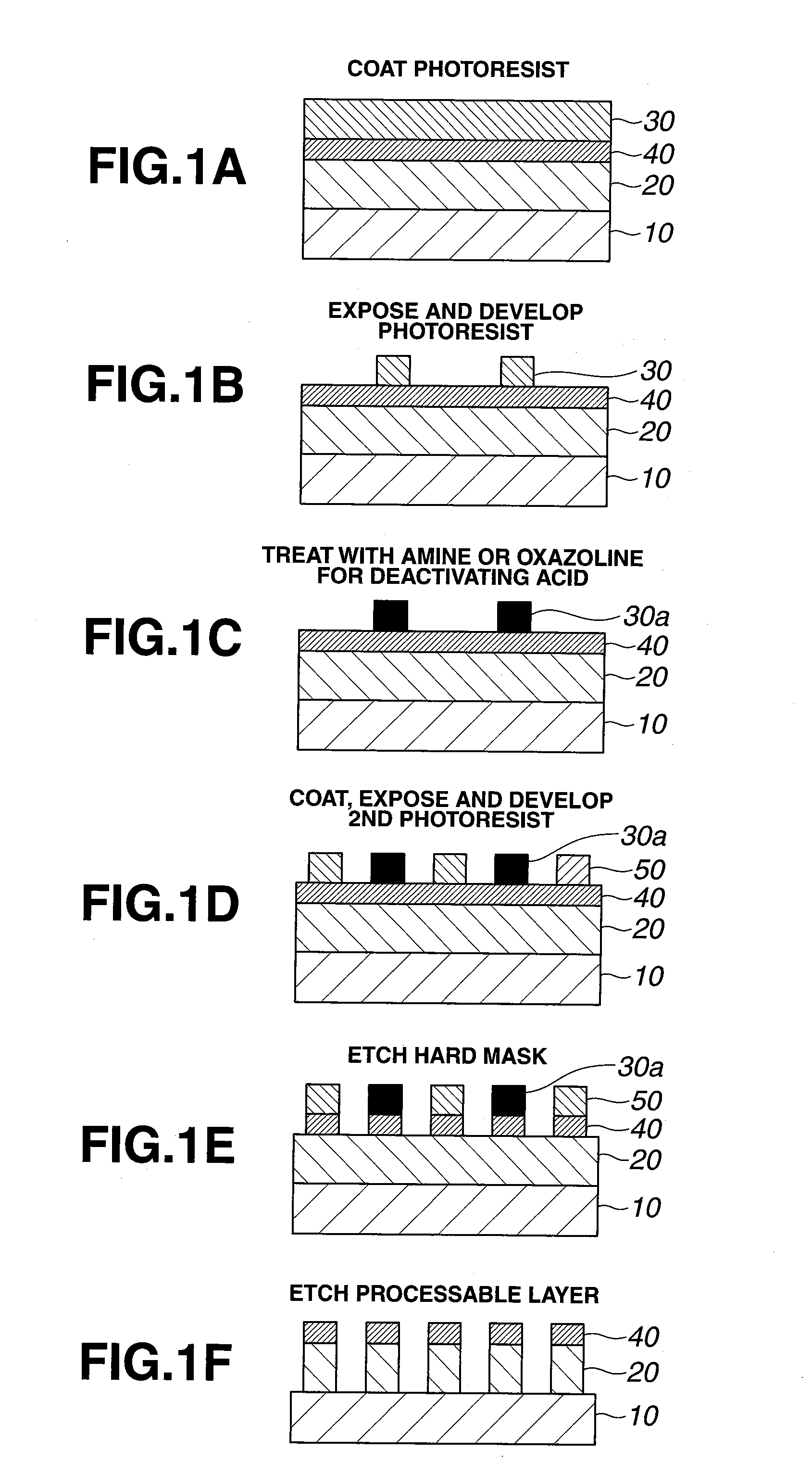
A pattern is formed by coating a first positive resist composition comprising a copolymer comprising lactone-containing recurring units and acid labile group-containing recurring units onto a substrate to form a first resist film, patternwise exposure, PEB, and development to form a first resist pattern, applying an amine or oxazoline compound to the first resist pattern for inactivation, coating a second positive resist composition comprising a C3-C8 alcohol and an optional C6-C12 ether onto the first resist pattern-bearing substrate to form a second resist film, patternwise exposure, PEB, and development to form a second resist pattern.
Owner:SHIN ETSU CHEM IND CO LTD
Chiral tridentate PNN ligand and application of same in asymmetric hydrogenation
ActiveCN105153229AHigh electronegativityGood chiral environmentOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsIridiumKetone
The invention discloses a chiral tridentate PNN ligand and application of the same in asymmetric hydrogenation and similar reactions. The tridentate nitrogen phosphine ligand disclosed in the invention has been the first reported nitrogen phosphine ligand containing chiral oxazoline so far and has been successfully applied to high-efficiency and high-selectivity hydrogenation and similar reactions of ketone and imine salts. Compared with other ligand, the ligand provided by the invention is simpler in synthetic route, higher in yield and environment-friendlier; moreover, the metal complex of the ligand shows better selectivity and a higher turn-over number in asymmetric hydrogenation. An iridium complex of the chiral tridentate PNN ligand has successfully realized asymmetric reduction of beta-ketone ester into beta-alcohol ester (which is a raw material for synthesis of molecular drugs duloxetine and atomoxetine), asymmetric hydrogenation of alpha-hydroxyacetophenone into alpha-hydroxyphenylethyl alcohol and asymmetric hydrogenation of acetophenone into phenylethyl alcohol, which is of important significance to production of the medical industry.
Owner:SHENZHEN CATALYS SCI & TECH CO LTD +2
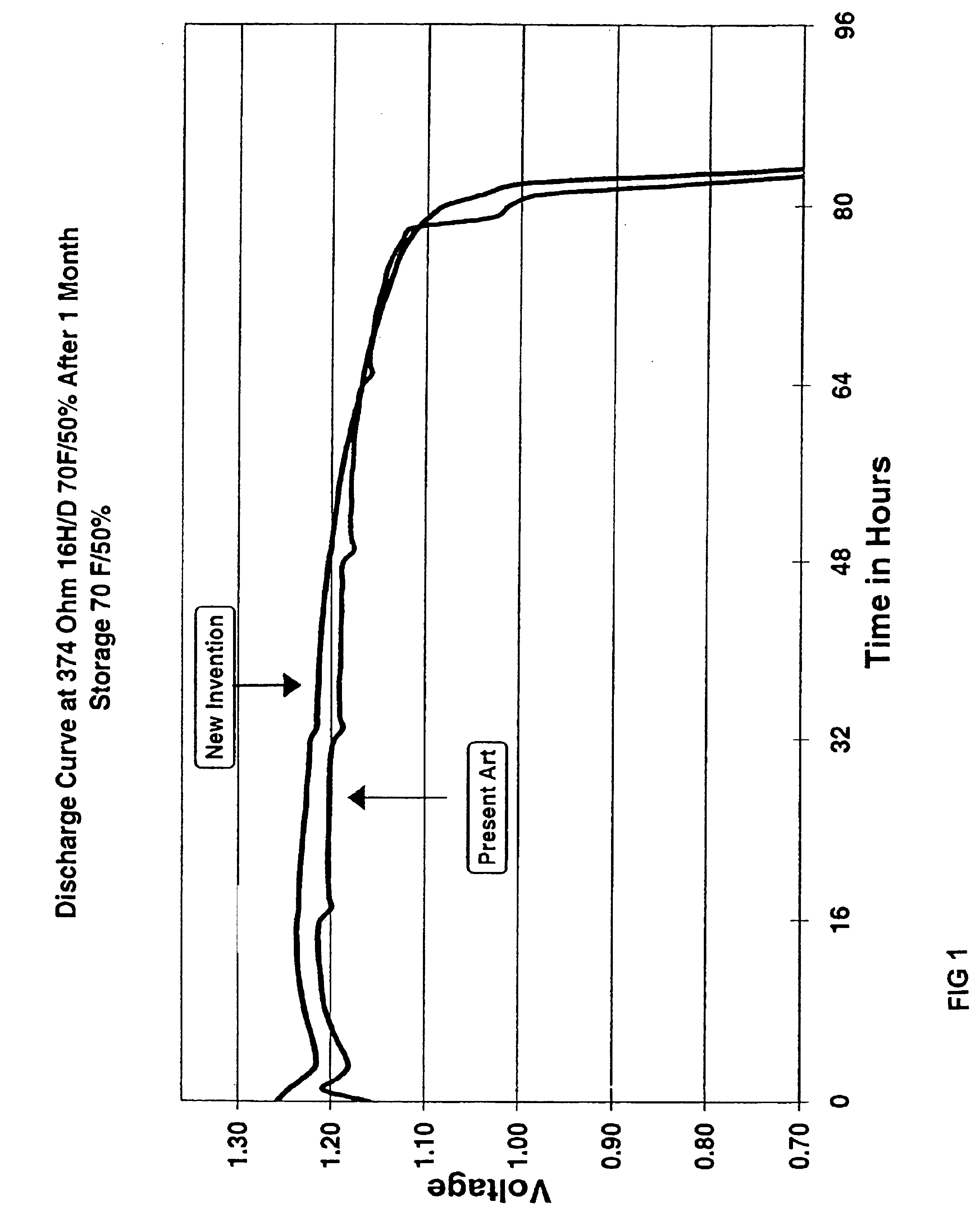
Oxazoline surfactant anode additive for alkaline electrochemical cells
InactiveUS6927000B2Improve discharge efficiencyIncrease working voltageFuel and primary cellsAlkaline accumulatorsHigh ratePhysical chemistry
According to the present invention an alkaline electrochemical cell can contain an anode having an anode active material, an alkaline electrolyte, a gelling agent and an oxazoline surfactant additive. The invention relates to an anode mix, to an anode containing the mix, and to an electrochemical cell containing the anode and to methods for making the anode mix, the anode and the cell. Performance improvements can be realized when the oxazoline surfactant is provided in the anode, which can include increased operating voltage, good high rate pulse capability, elimination of initial potential dip, good shelf life and reduced sensitivity to open circuit rest.
Owner:ENERGIZER BRANDS +1
Preparation method and application for phosphine-oxazoline ligand, and ionic metal complex, enantiomer or racemate thereof
InactiveCN102875601AThe synthesis method is simpleSynthetic method is economicalOrganic compound preparationGroup 5/15 element organic compoundsPlanar chiralityStructural formula
The invention discloses a preparation method and an application for phosphine-oxazoline ligand, and ionic metal complex, enantiomer or racemate thereof. The ligand and the ionic metal complex thereof have the following structural formulas. The phosphine ligand related by the invention employs biphenyl as a skeleton, and realizes completely transmission from planar chirality to axial chirality through an asymmetric desymmerization. The synthetic method is simple and economic, omits a common and complex chiral separation process in the preparation of the chiral ligand. The obtained chiral ligand has the advantages of high reactive activity, good enantiomorphous selectivity and the like in a model reaction.
Owner:SUN YAT SEN UNIV
Use of chiral oxazoline
InactiveCN101099936AOrganic-compounds/hydrides/coordination-complexes catalystsPreparation by hydrogen cyanide additionEthyl groupChirality
Owner:HEFEI UNIV OF TECH
Chirality pyridine double-oxazoline catalyzer and method for preparing the same and application thereof
InactiveCN101116828AHigh activityHigh enantioselectivityOrganic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationOxazolidoneLanthanide
A catalyst of chiral bis-oxazolinylpyridine is provided, which comprises two parts including a 4-z-2, 6-bis [4'(s)-R oxazoline-2'-] pyridine as the chiral ligand of the bis-oxazolinylpyridine, and a Scandate, Yttrium and Lanthanide metal salt. The preparation method is to mix the metal salt, the ligand of bis-oxazolinylpyridin with the equal molar quantity, and a 4-molecular sieve together, the mixture is added with the needed solvent, and then stirred under the temperature of -78 to 25 DEG C to obtain the catalyst prepared in situ. The usual dosage of the catalyst used for catalyzing Diels-Alder reaction is 1percent to 5percent of the dosage of the substrate. The catalyst can catalyze Diels-Alder reaction of both alpha, beta-unsaturated N-acetyl oxazolidone and alpha, beta-unsaturated ester with high efficiency in high selectivity. Under condition of the normal pressure with the temperature of 0 to 25 DEG C, the catalyst can accomplish the conversion rate as high as 100 percent and the enantiomeric selectivity of 96 percent.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Spiro phosphine-oxazoline and preparation method and application thereof
ActiveCN101565434AHigh activityHigh enantioselectivityRuthenium organic compoundsIndium organic compoundsIridiumIon exchange
The invention belongs to spirophosphine-oxazoline and a preparation method and an application thereof, in particular to novel spirophosphine-oxazoline and a preparation method of iridium complex thereof. The novel spirophosphine-oxazoline is synthesized through two reactions by using substitutional 7-diarylphosphino-7'-carboxyl-1, 1'-spirobiindane as starting raw materials. Complexation and ion exchange are performed for the novel spirophosphine-oxazoline and iridium precursor, so as to obtain iridium / spirophosphine-oxazoline complex compound containing different anions. The invention overcomes the defects of the prior art, synthesizes the novel spirophosphine-oxazoline without substitutional group on oxazoline 4- position by using cheap aminoethanol as raw materials, can catalyze asymmetric hydrogenation reaction of Alpha-substituting acrylic acid, expresses higher activity and enantioselectivity and has higher research value and industrial prospect.
Owner:ZHEJIANG JIUZHOU PHARM CO LTD
Chiral compound comprising iminopyridyl oxazoline and preparation method thereof
ActiveCN104447725AHigh chemical conversionOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAsymmetric hydrogenationDouble bond
The invention discloses a synthetic compound comprising iminopyridyl oxazoline, a preparation method thereof, a metal complex of the compound and application of a prochiral organic compound which contains at least one carbon / carbon or carbon / heteroatomic double bond in hydroboration. According to the compound, the total yield of two steps of a high-efficiency synthetic route can reach 50%, and the metal complex is an excellent catalyst or a catalyst precursor which is used for asymmetric synthesis (such as asymmetric hydrogenation of prochiral, unsaturated and organic compounds). The invention also provides application of the metal complex as a homogeneous catalyst. The catalyst is used for preparing a chiral organic compound by being subjected to the hydroboration and hydrosilation asymmetric addition on the carbon or carbon heteroatomic double bond of the prochiral organic compound, and an ee value can be more than 90%.
Owner:ZHEJIANG UNIV
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com