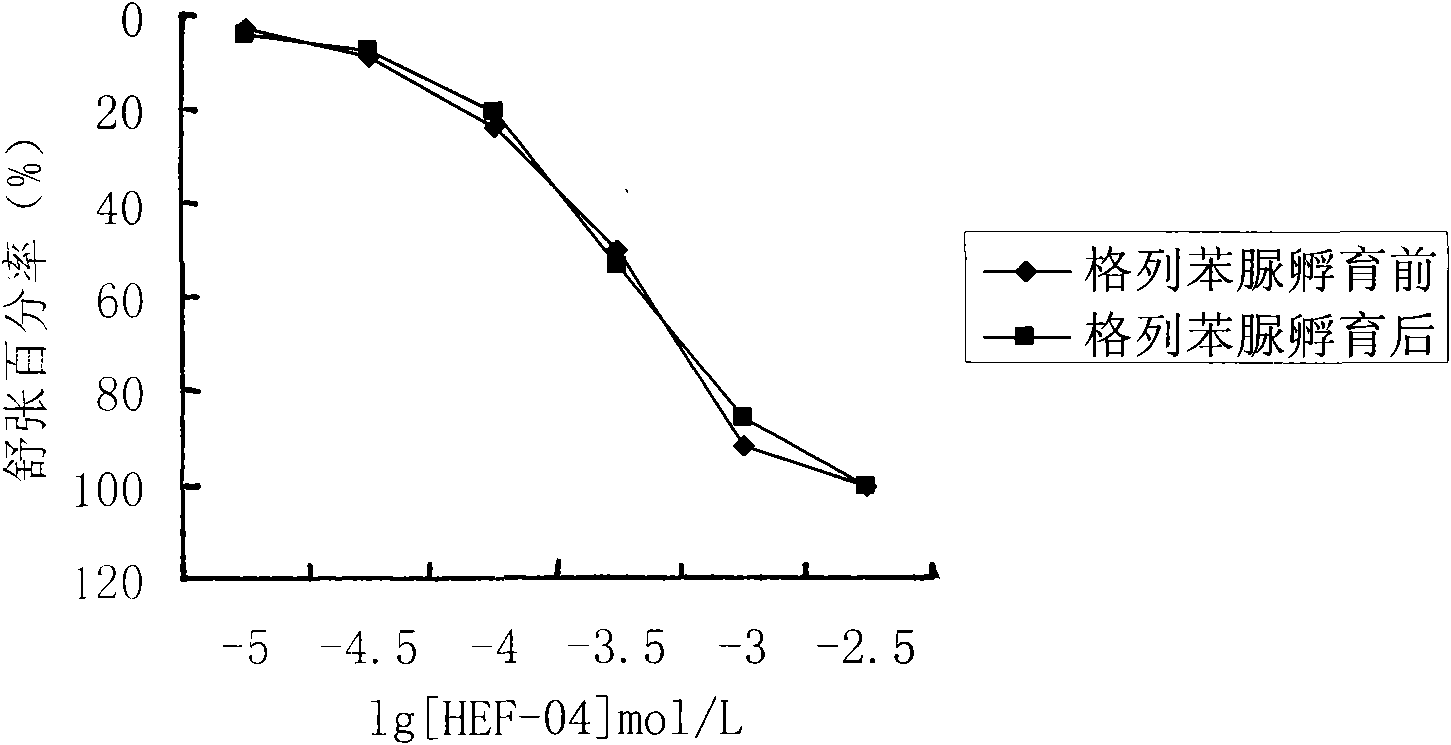Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
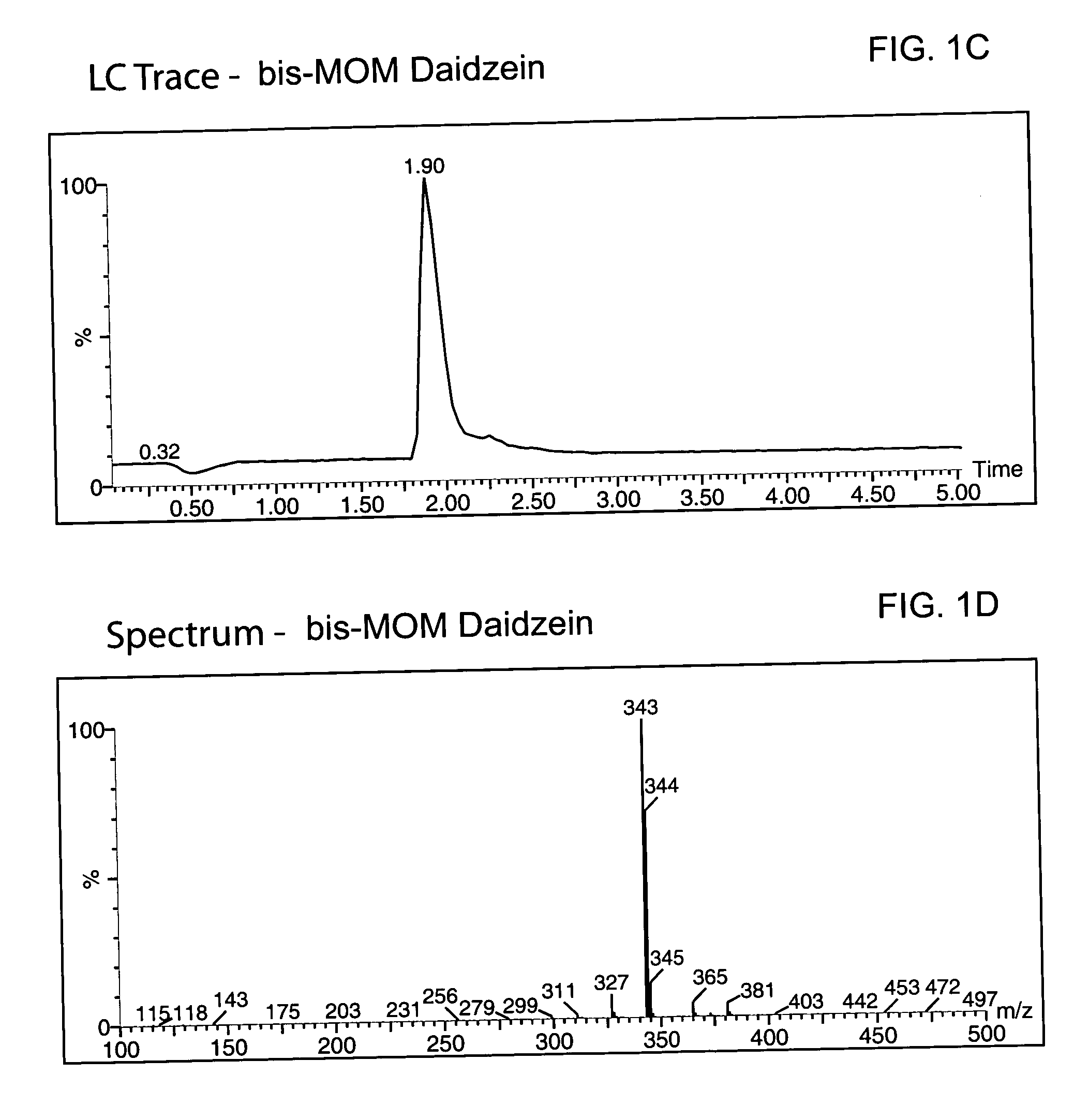
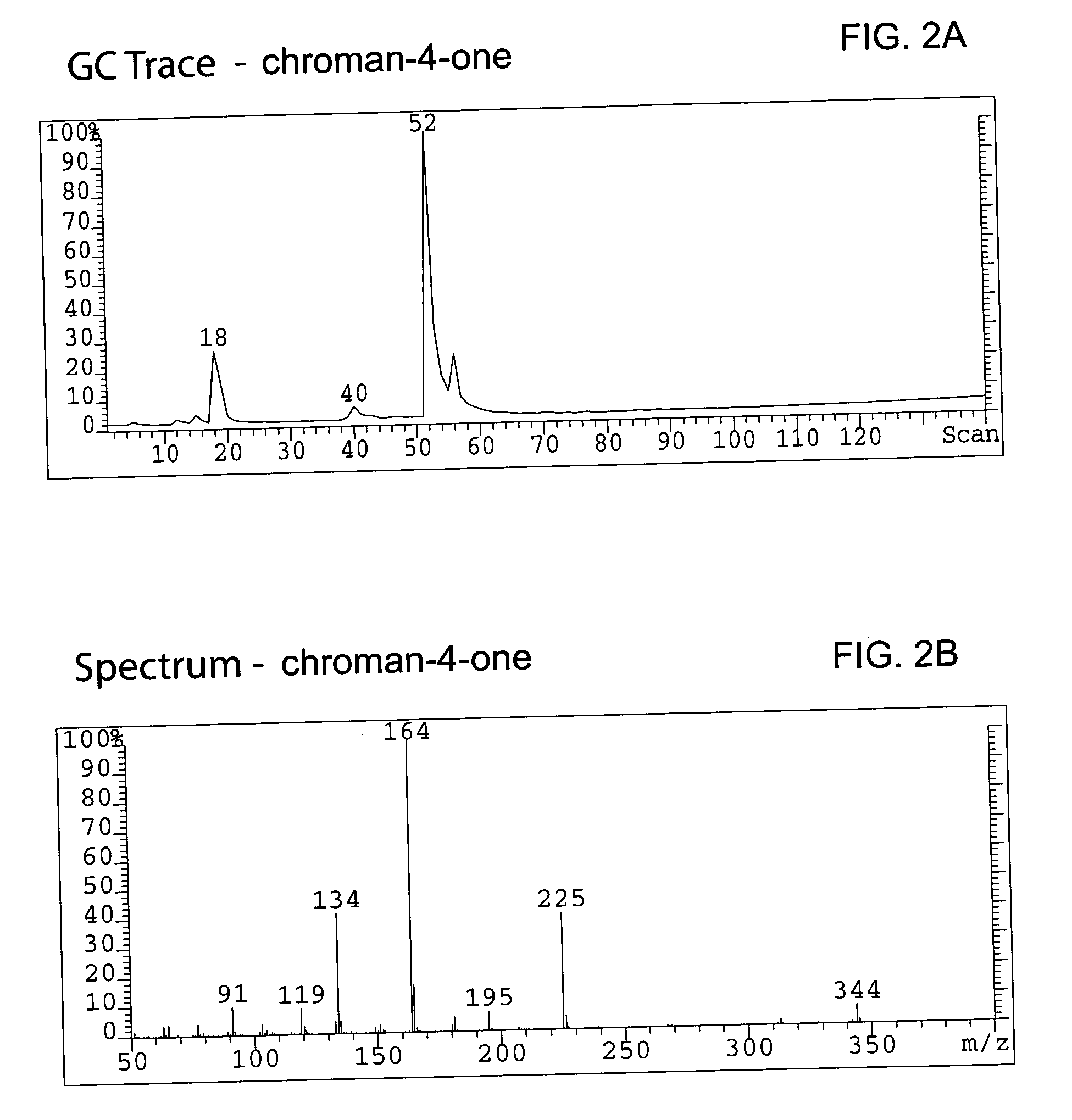
52 results about "Chromane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
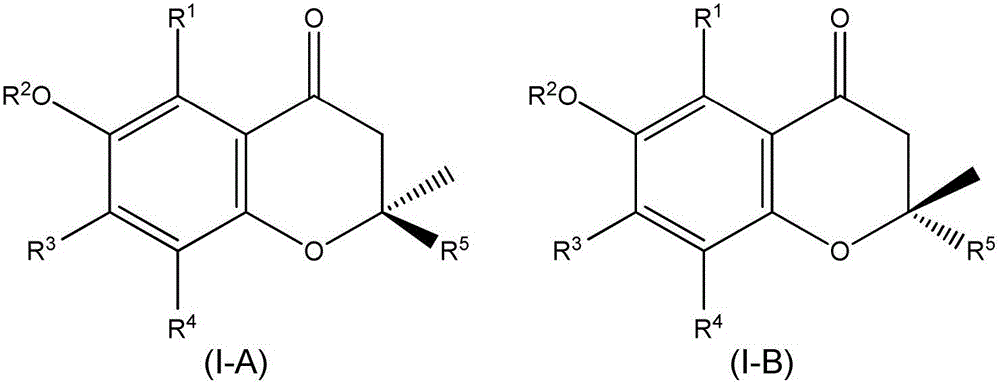
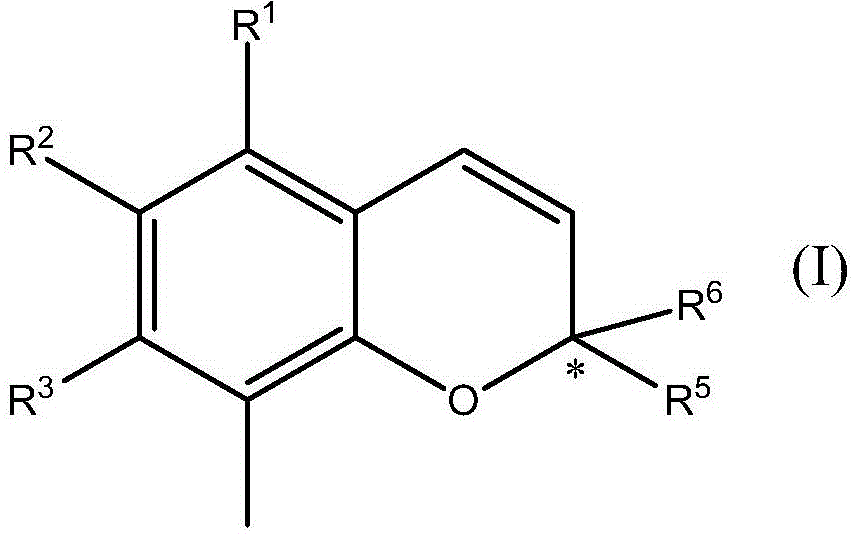
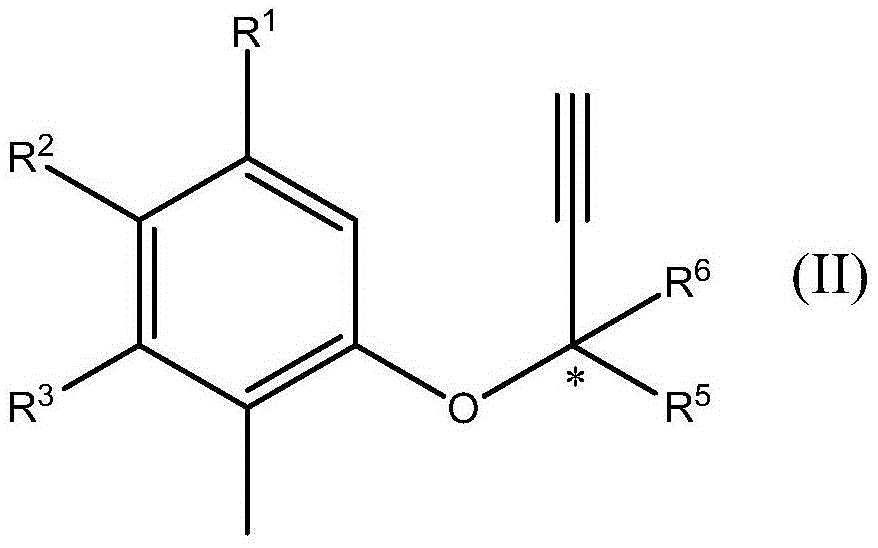
Chromane (benzodihydropyran) is a heterocyclic chemical compound with the chemical formula C₉H₁₀O. Chromane is a structural feature of more complex compounds including E vitamins (tocopherols and tocotrienols), Dianin's compound, and the pharmaceutical drugs troglitazone, ormeloxifene, and nebivolol. Such compounds are sometimes described as chromans.
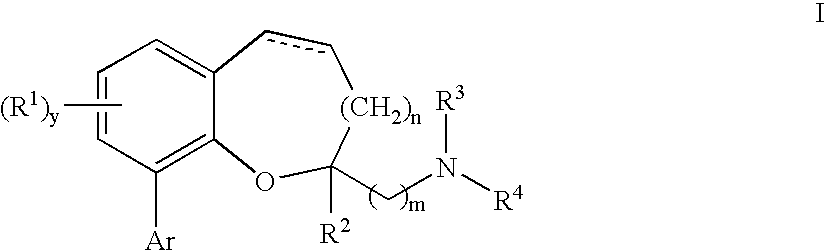
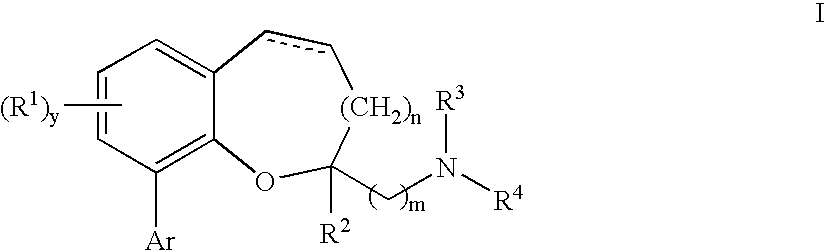
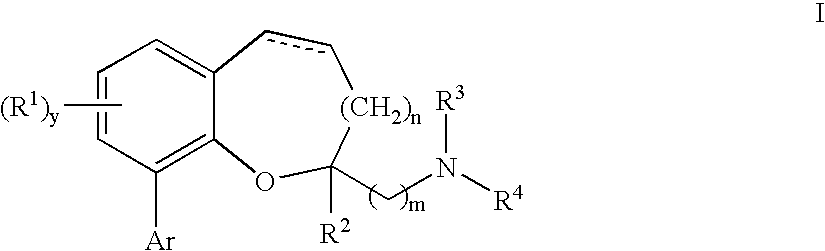
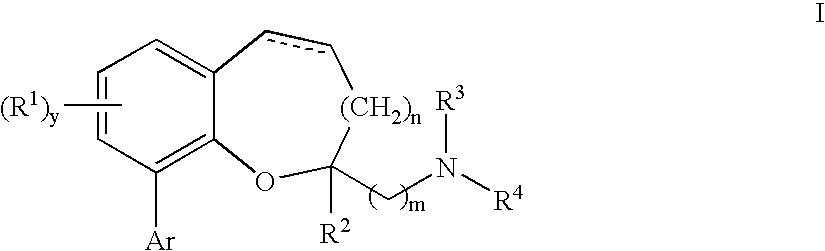
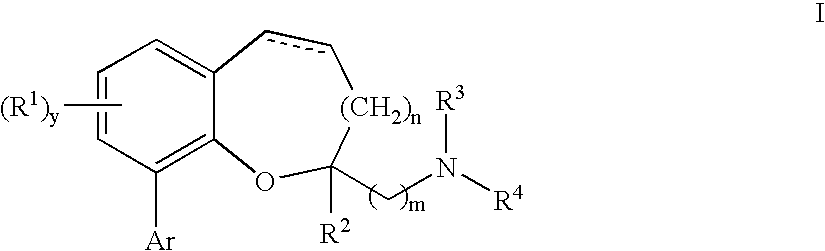
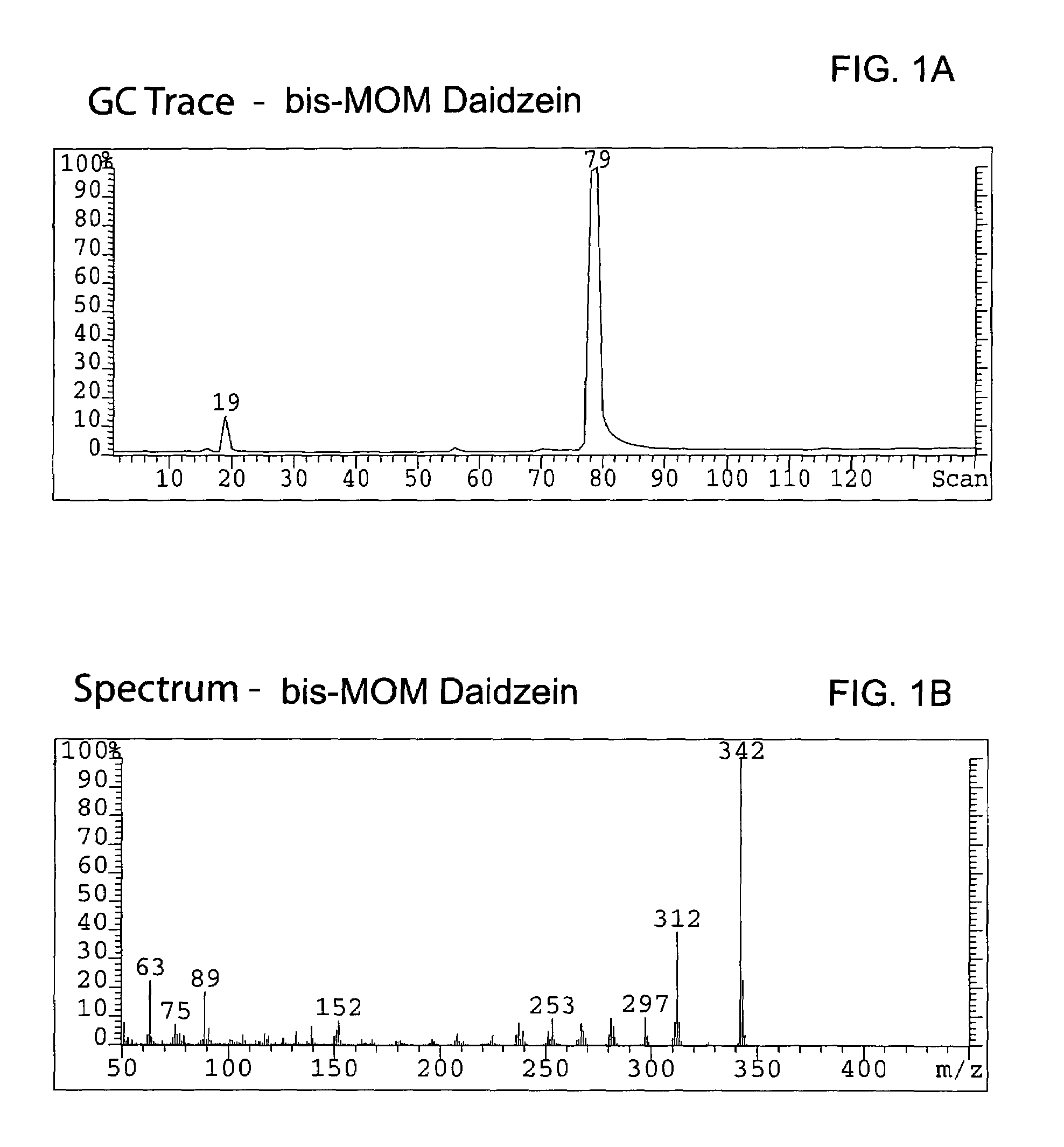
Chromane and chromene derivatives and uses thereof
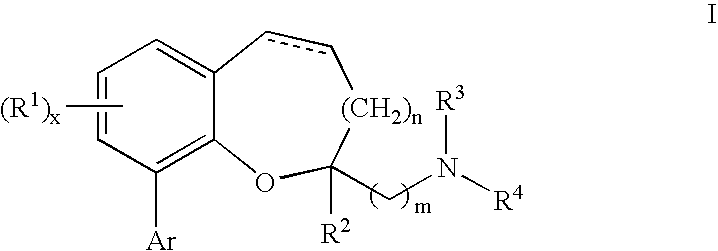
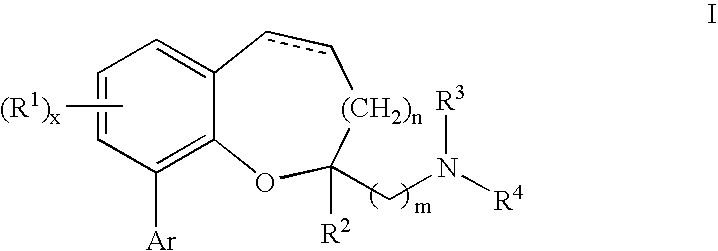
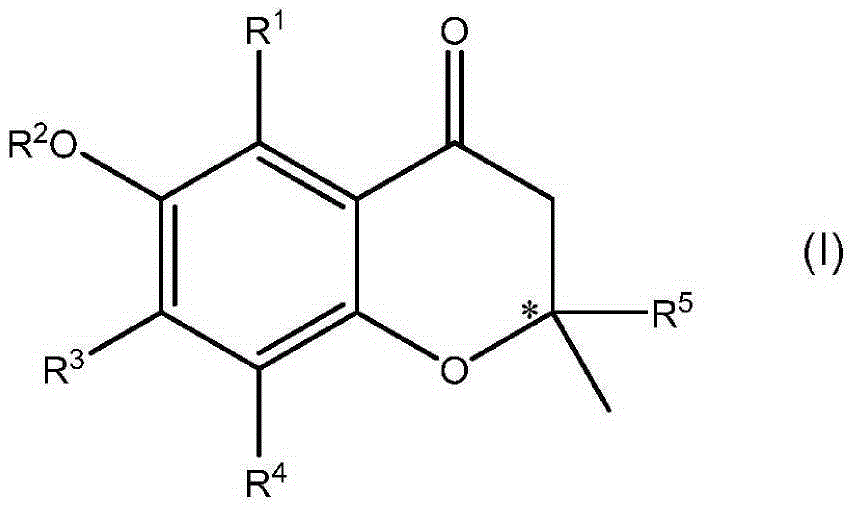
Compounds of formula I or pharmaceutically acceptable salts thereof are provided: wherein each of R1, R2, R3, R4, y, m, n, and Ar are as defined, and described in classes and subclasses herein, which are agonists or partial agonists of the 2C subtype of brain serotonin receptors. The compounds, and compositions containing the compounds, can be used to treat a variety of central nervous system disorders such as schizophrenia.
Owner:WYETH
Method for enantioselective hydrogenation of chromenes
A method for preparing an enantiomeric chromane, by asymmetrically hydrogenating a chromene compound in the presence of an Ir catalyst having a chiral ligand. The method includes the enantioselective preparation of enantiomeric equol. A preferred Ir catalyst has a chiral phosphineoxazoline ligand. Enantiomeric chromanes of high stereoselective purity can be obtained.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI +1
Ppar alpha selective compounds for the treatment of dyslipidemia and other lipid disorders
InactiveUS20060089404A1Efficacious in amelioratingEfficacious in treatingBiocideSenses disorderDyslipidemiaLipid disorder
A class of chromane and chromene compounds having the structure shown below and pharmaceutically acceptable salts thereof are useful as therapeutic compounds, particularly in the treatment and control of hyperlipidemia, hypercholesterolemia, dyslipidemia, and other lipid disorders, and in delaying the onset of or reducing the risk of conditions and sequelae that are associated with these diseases, such as atherosclerosis.
Owner:MERCK SHARP & DOHME CORP
Method for enantioselective hydrogenation of chromenes
A method for preparing an enantiomeric chromane, by asymmetrically hydrogenating a chromene compound in the presence of an Ir catalyst having a chiral ligand. The method includes the enantioselective preparation of enantiomeric equol. A preferred Ir catalyst has a chiral phosphineoxazoline ligand. Enantiomeric chromanes of high stereoselective purity can be obtained.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI +1
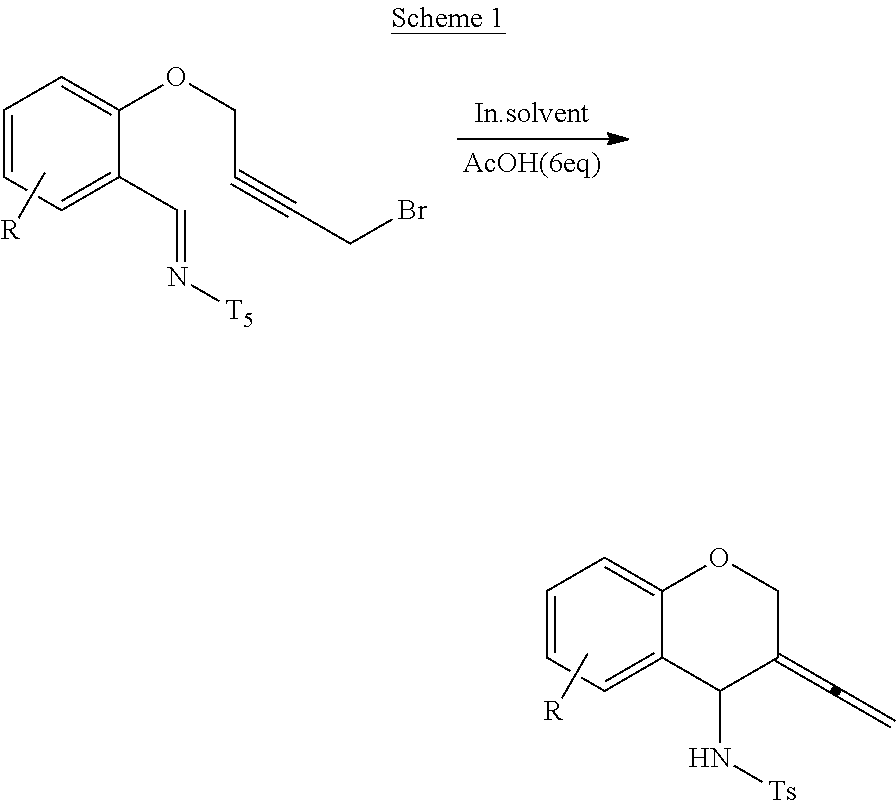
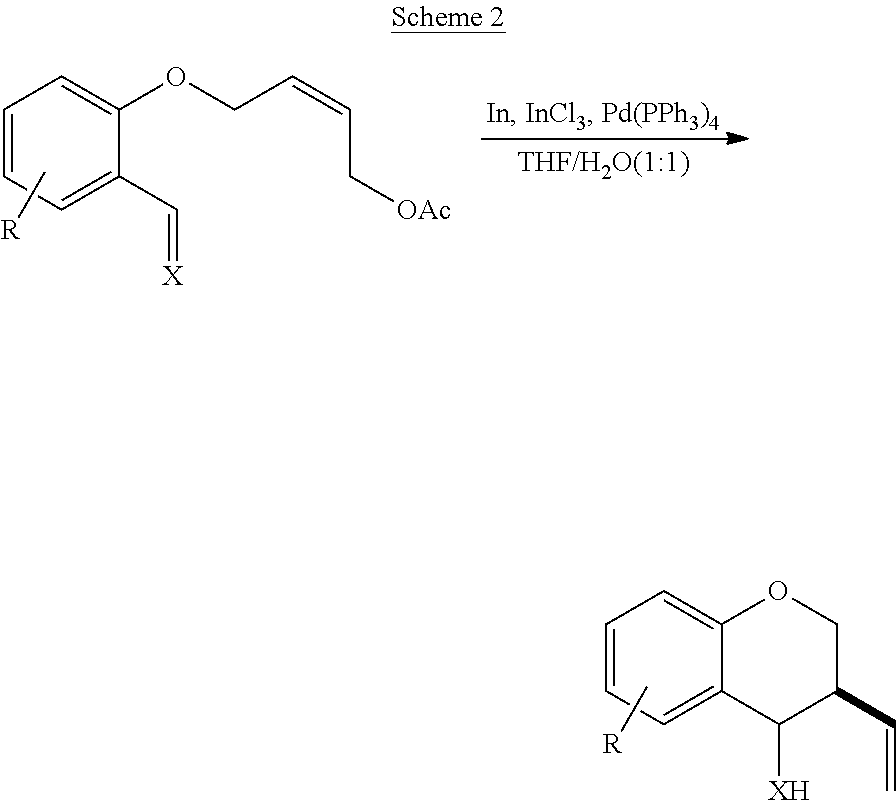
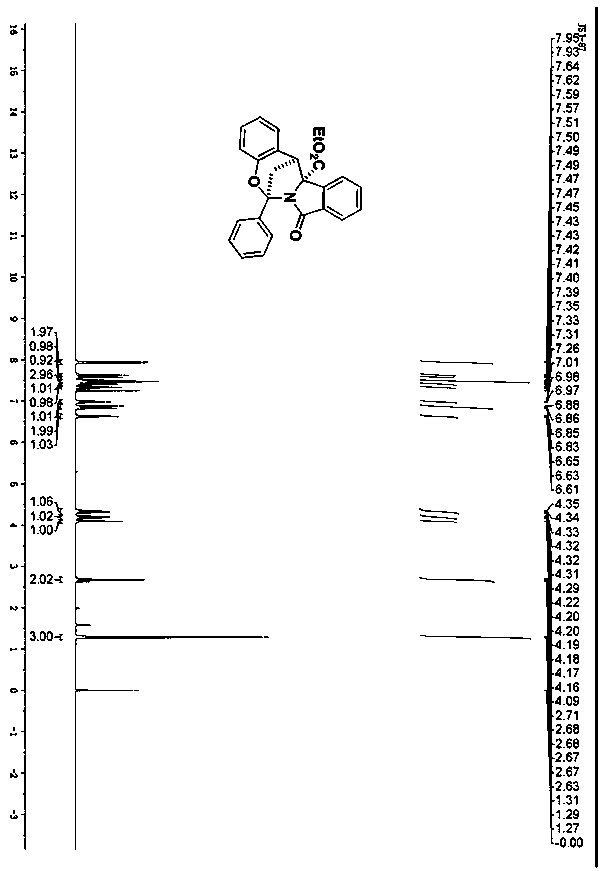
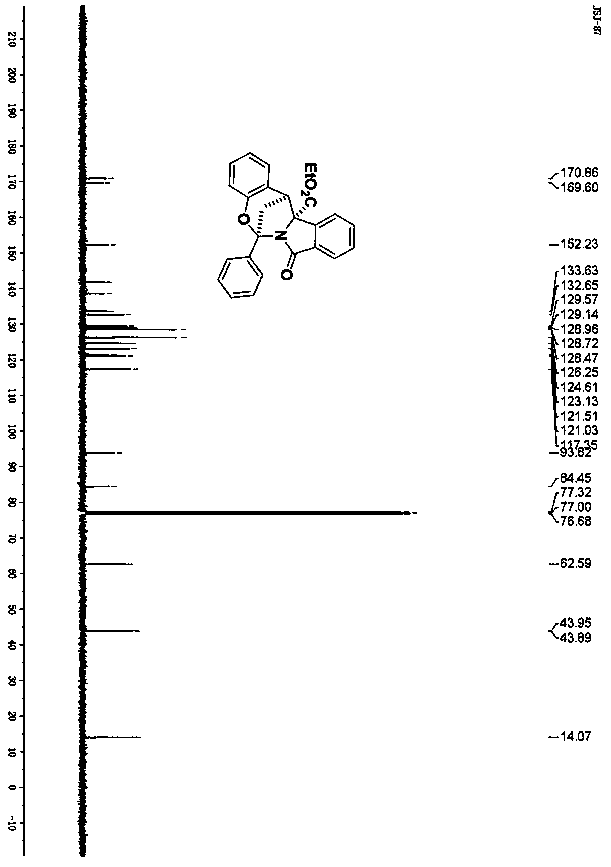
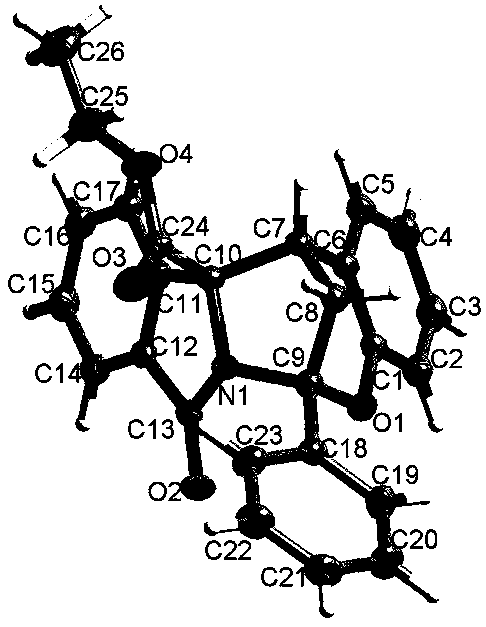
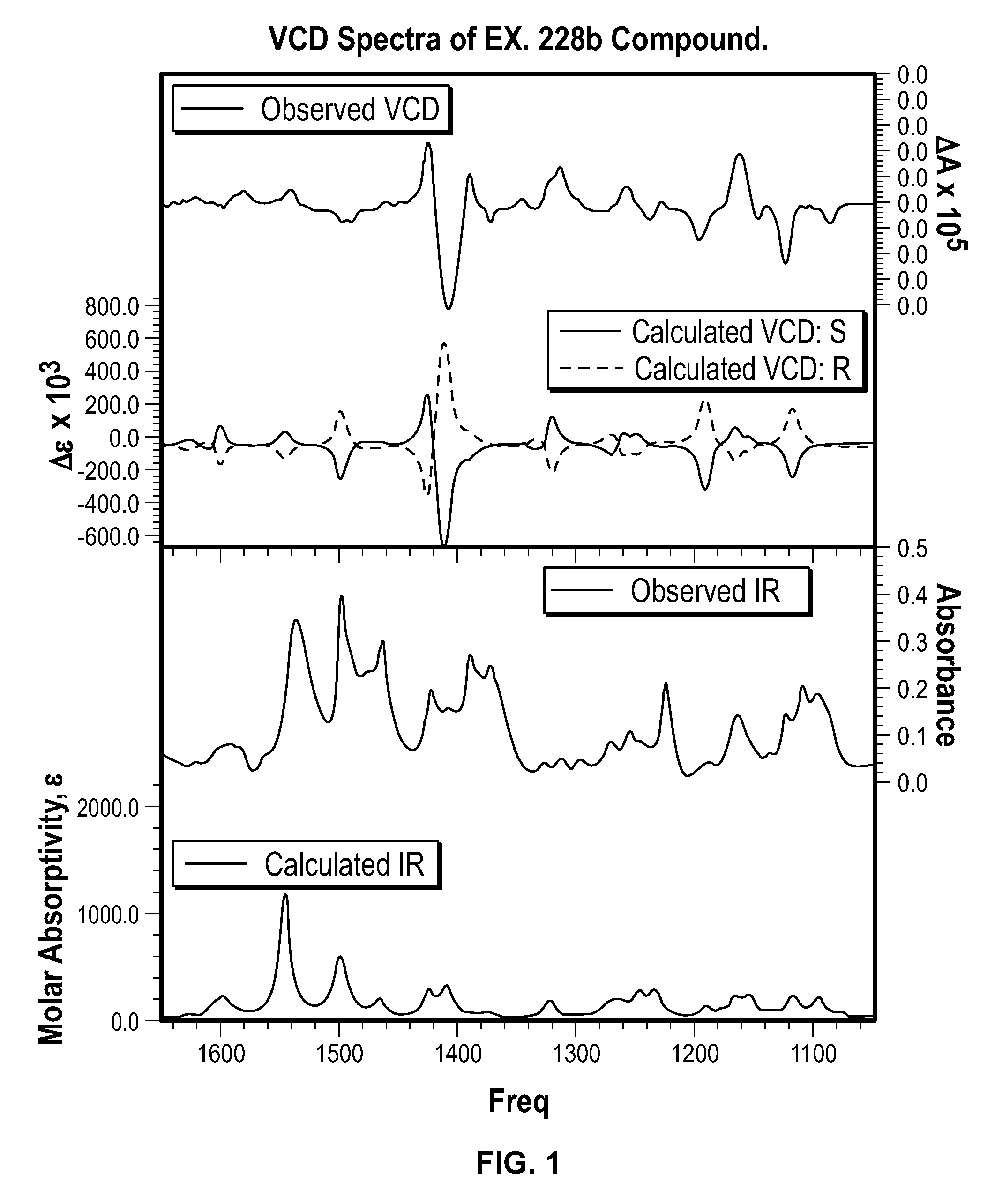
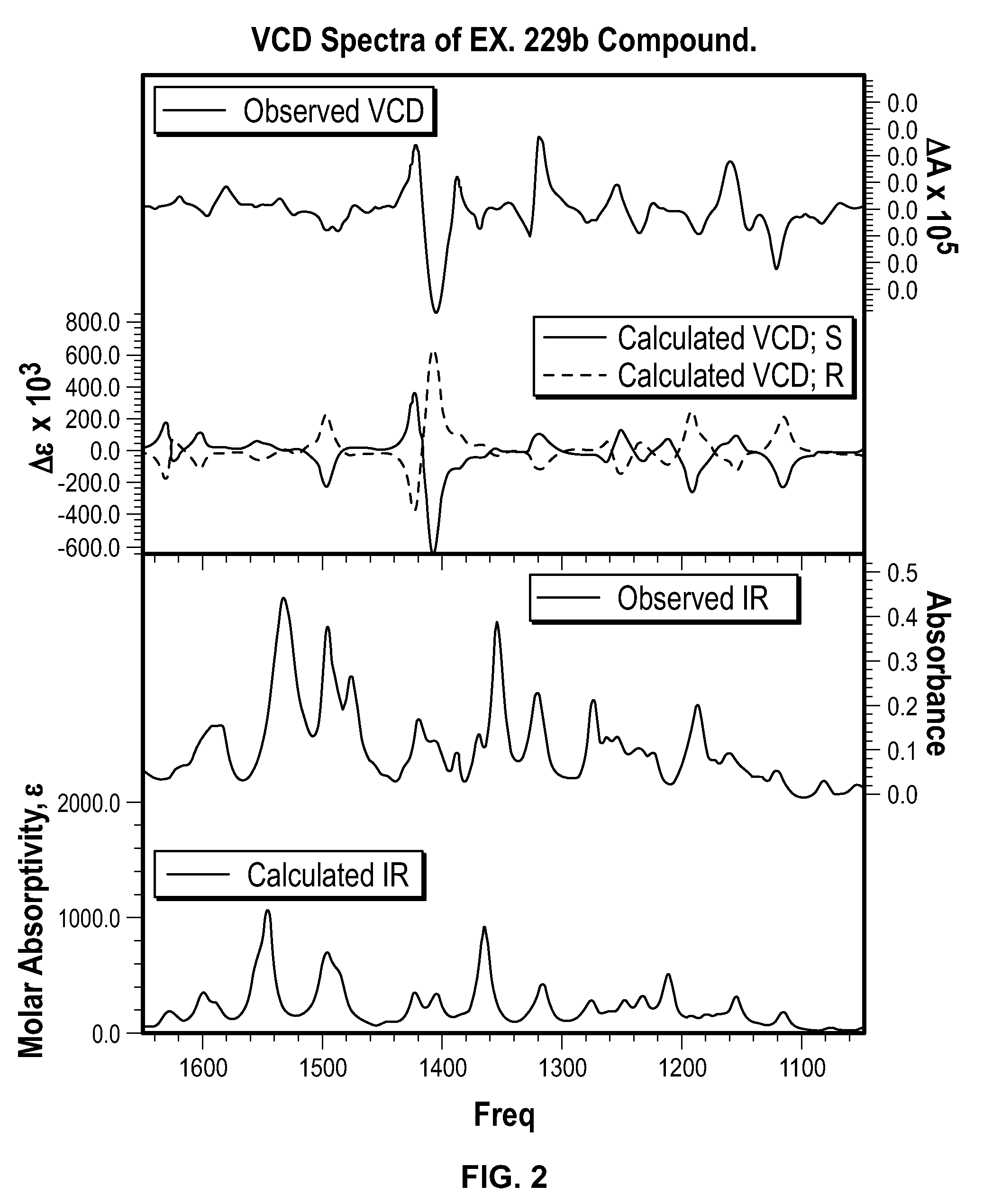
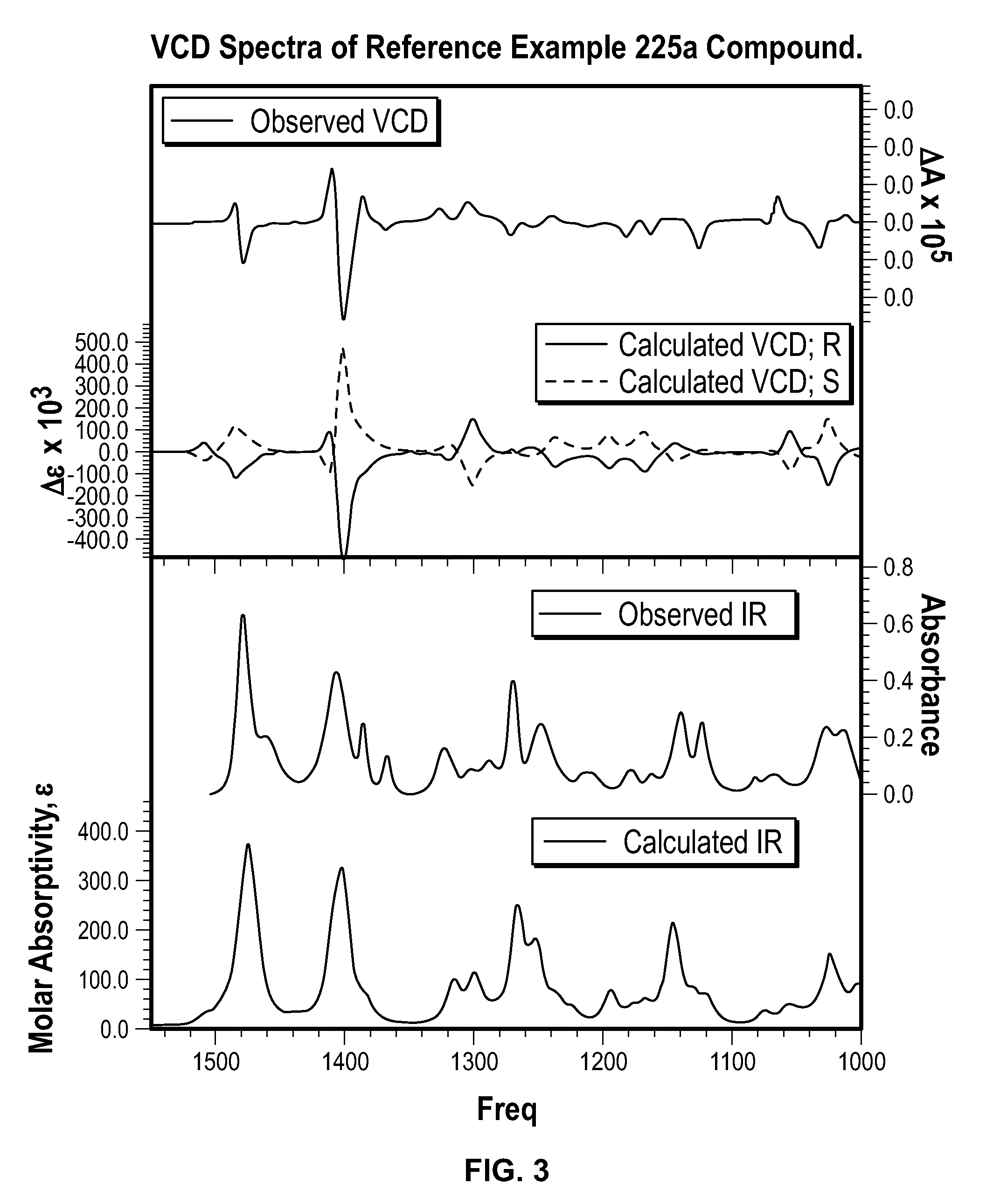
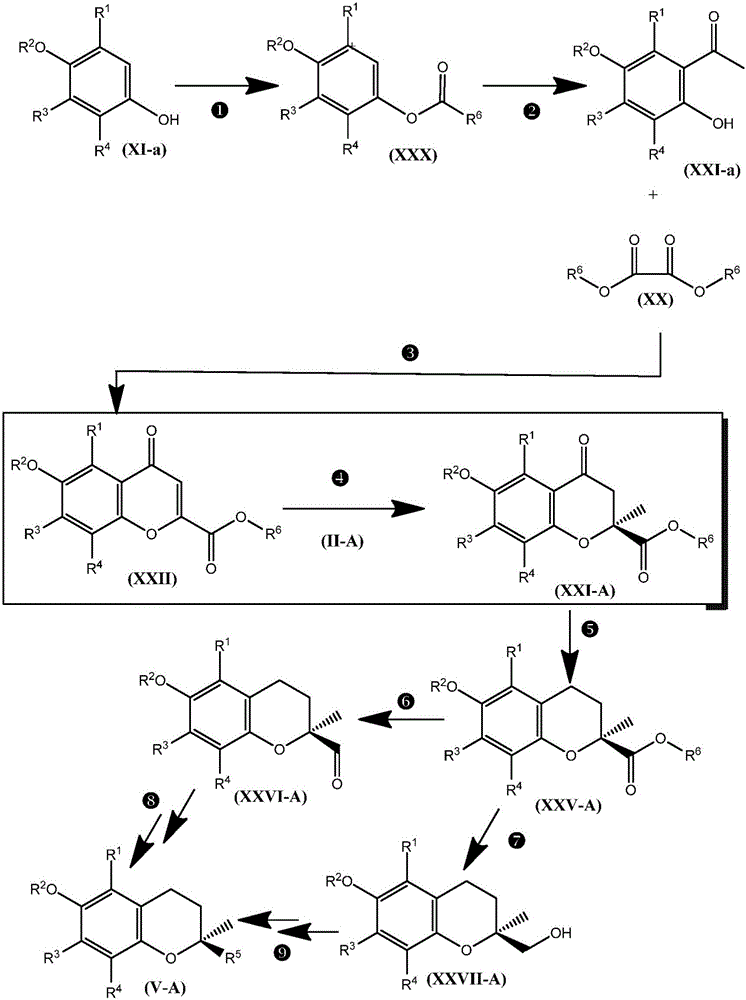
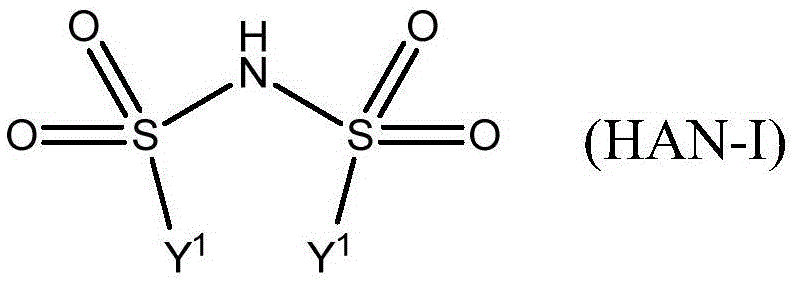
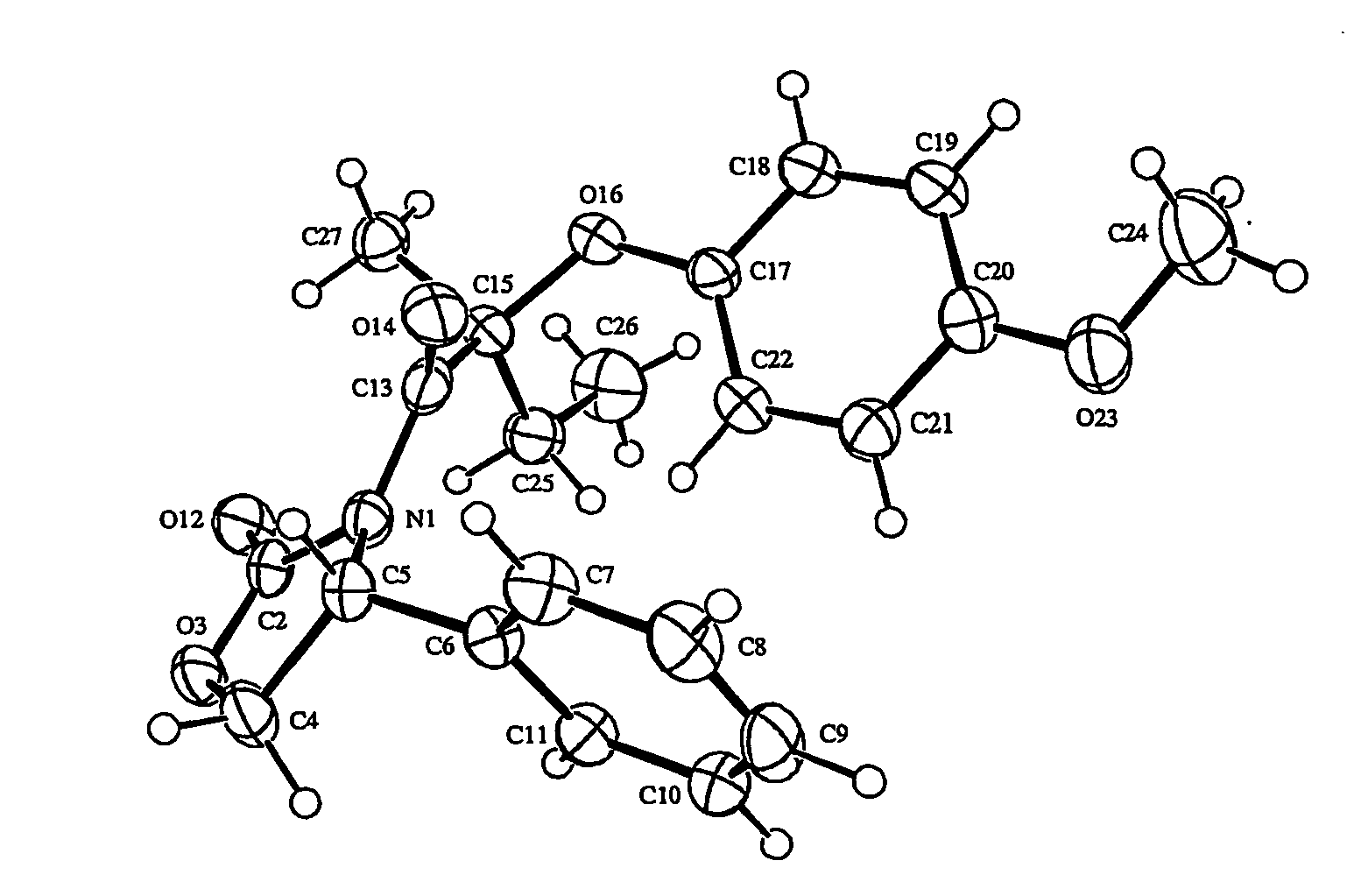
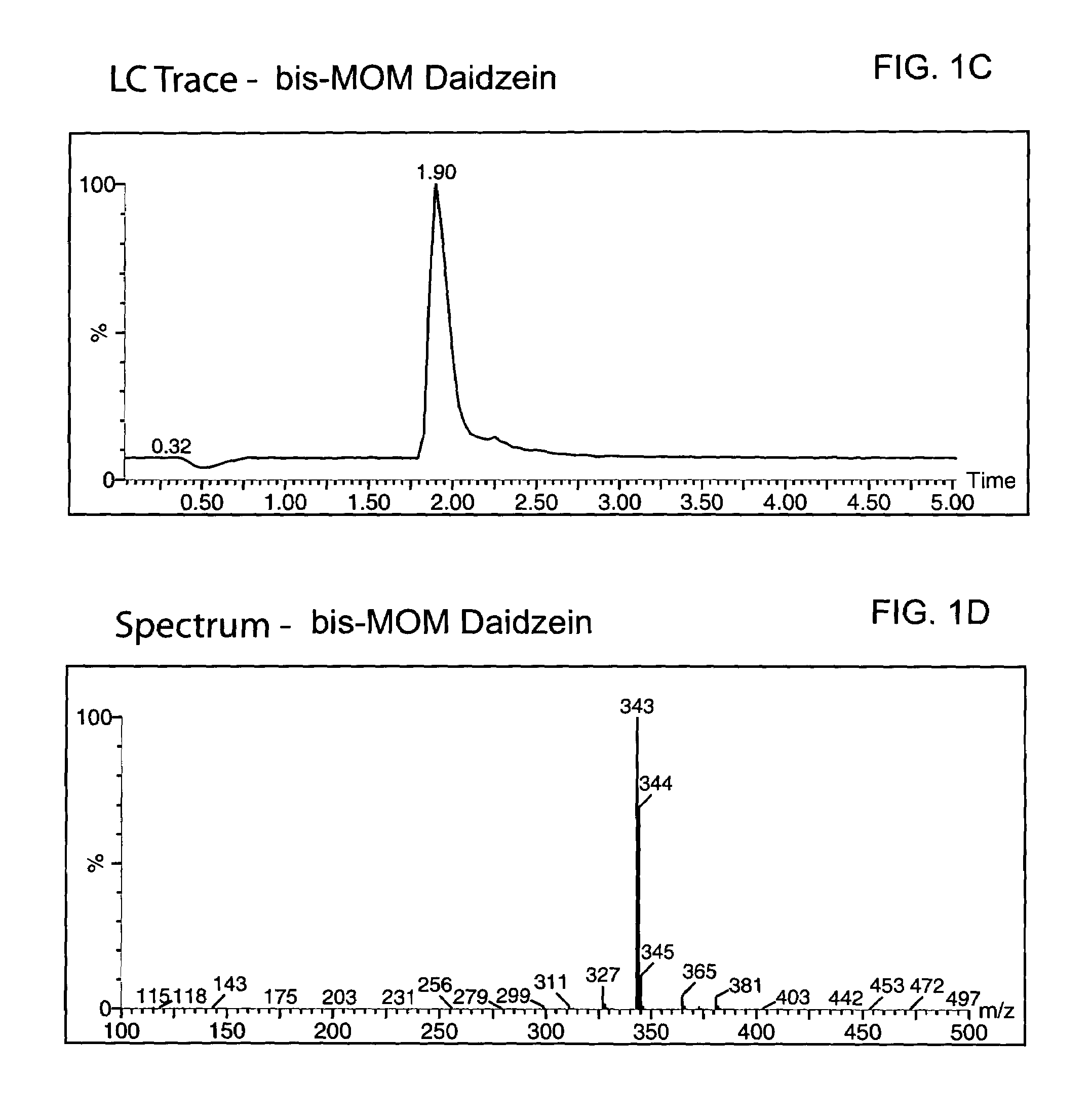
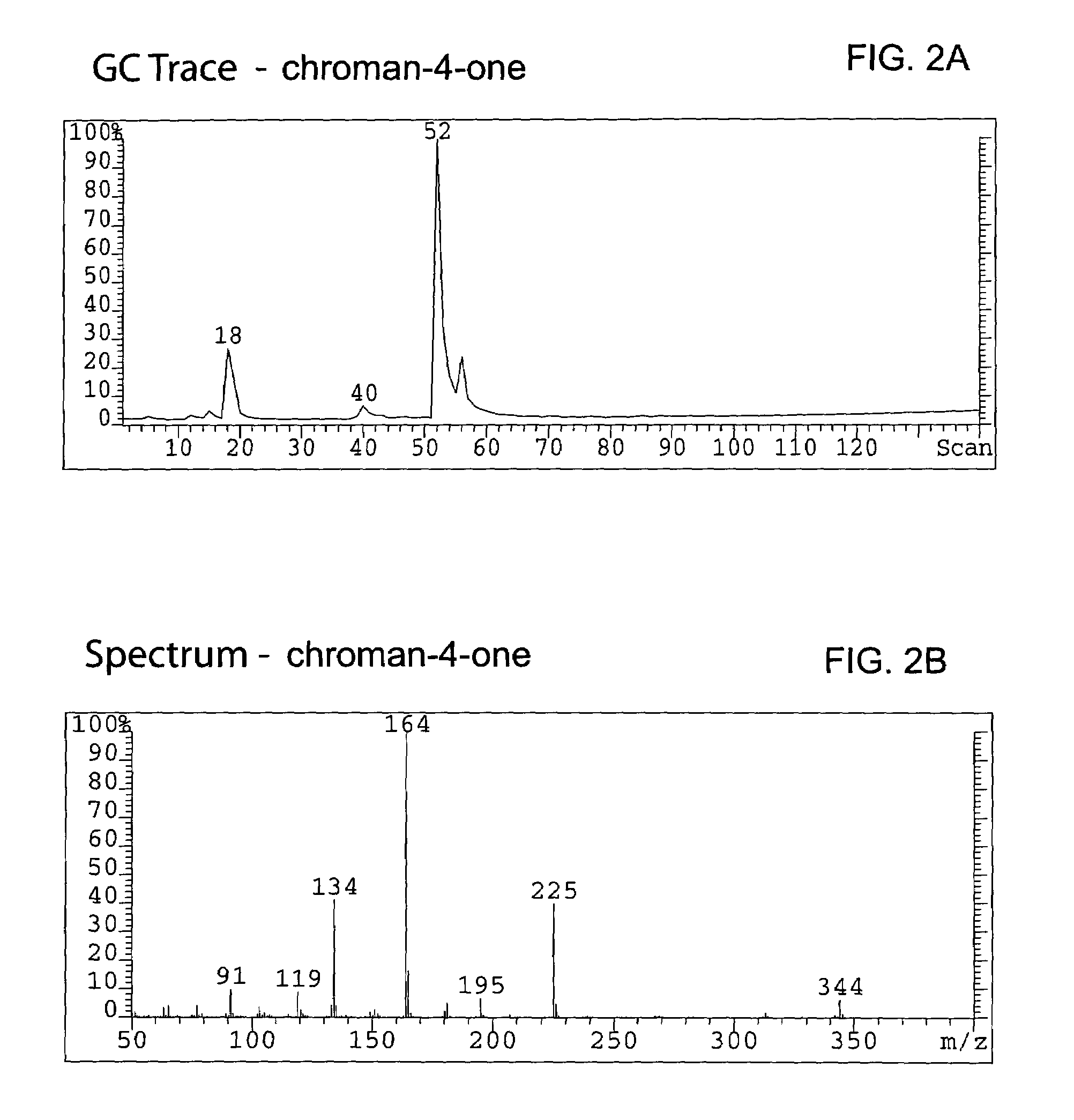
Spiro[chromane-oxindole] derivatives, synthesis method and application thereof
InactiveCN107955011AReliable responseEasy to operateOrganic chemistryAntineoplastic agentsSynthesis methodsKetone
Owner:EAST CHINA NORMAL UNIV
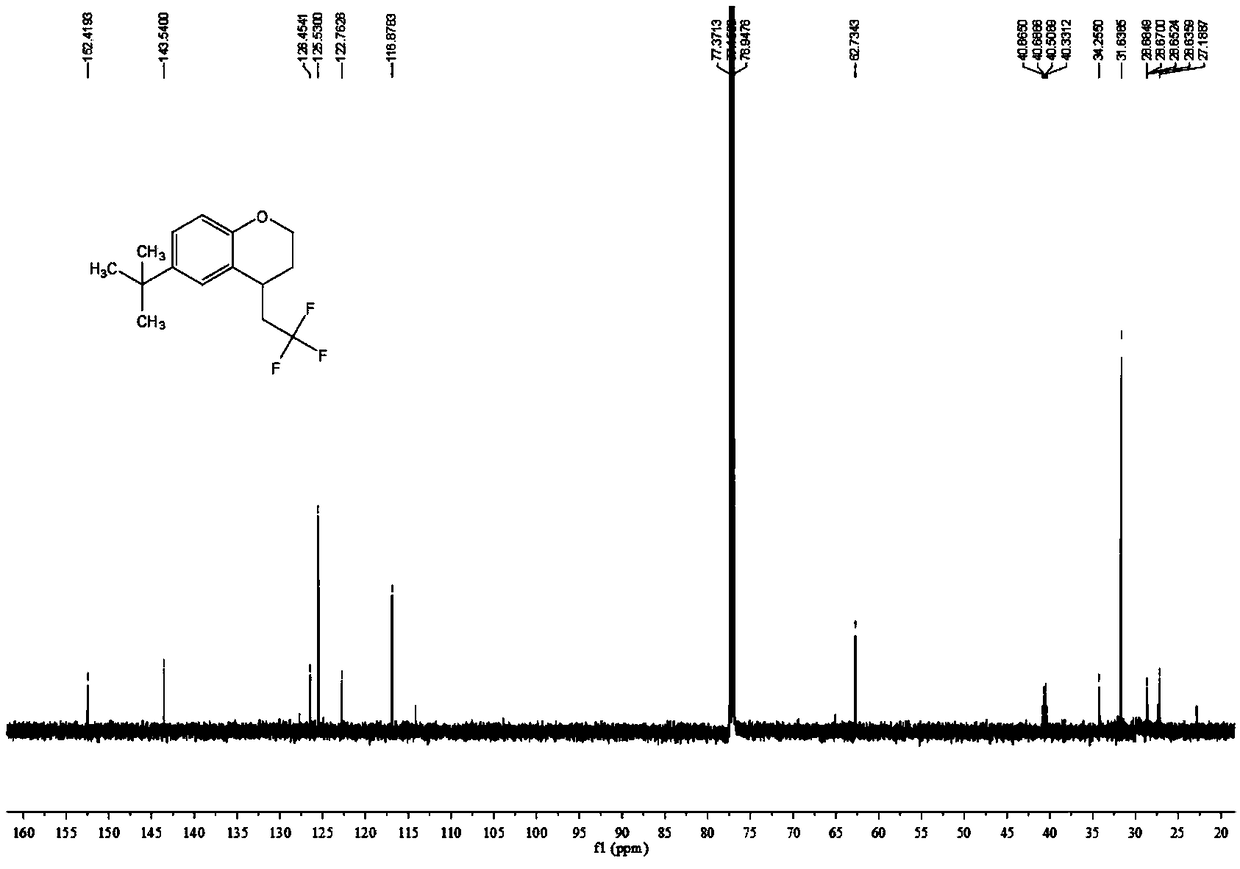
Chromane compound and preparation method thereof
InactiveCN109320489AMild reaction conditionsEasy to operateOrganic chemistryTrifluoromethylationSolvent
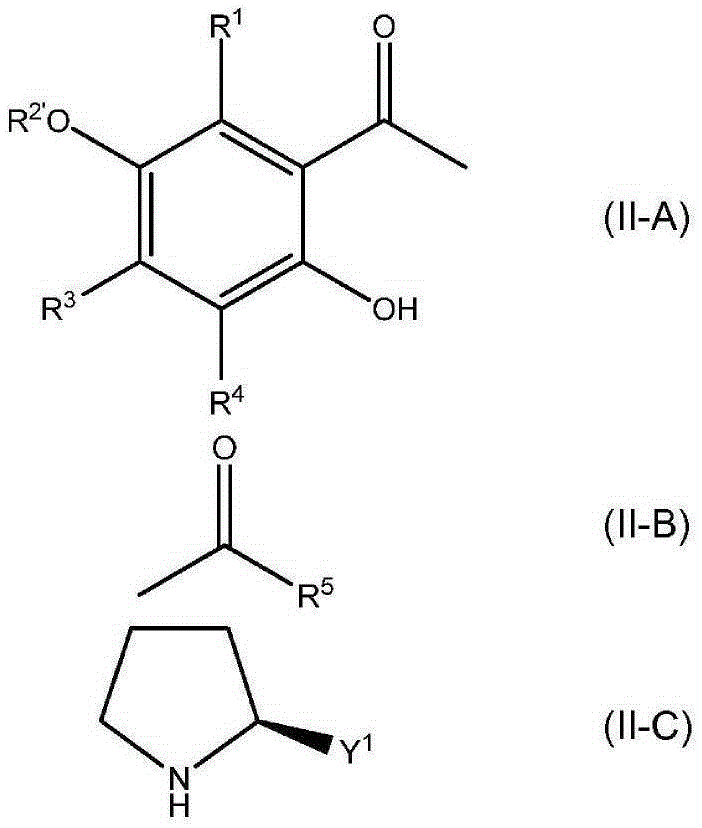
The invention discloses a chromane compound and a preparation method thereof. The preparation method comprises the following specific steps: dispersing olefin shown as a structure (I), a trifluoromethyl reagent shown as a structure (II) and an oxidizing agent in a solvent; heating and stirring the mixture to obtain the chromane compound shown as a structure (III), wherein the structure (III) is shown in the description. The invention further provides a novel method for building a trifluoromethylation chromane compound by taking an olefin compound (I) as a starting raw material of a reaction and sodium trifluoromethanesulfonate (II) as a trifluoromethyl source, and persulfate as an oxidizing agent, and performing free radical addition, free radical arylation cyclization and oxidization on trifluoromethyl and the olefin. The method has the advantages of mild reaction conditions, easiness in operation, diverse products, and the capability of realizing scale production.
Owner:XINYANG NORMAL UNIVERSITY
Lupin total extract consisting of a lupin sugar extract and a lupin peptide extract, method for the production and use thereof
The invention relates to a composition comprising a lupin total extract consisting of a lupin sugar extract, comprising at least 50 % galactooligosacharides by weight, in relation to the weight of dry matter, and a lupin peptide extract. The composition can also contain a chromane derivative or a chromene derivative. The invention also relates to a pharmaceutical and / or cosmetic composition comprising said lupin total extract, used advantageously as an anti-inflammatory agent, which repairs the cutaneous barrier and heals, used particularly in the prevention and / or treatment of erythemas.
Owner:LAB EXPANSCIENCE
Protective agent for retinal nerve or optic nerve
InactiveCN101594884AGood effectLong-term administrationSenses disorderNervous disorderOptic nerveOxygen
To provide a protective agent for a retinal nerve or an optic nerve, which exhibits its efficacy through a mechanism that is different from that for a conventional therapeutic agent and can be ingested over a long period. [MEANS FOR SOLVING PROBLEMS] Disclosed is protective agent for a retinal nerve or an optic nerve, which comprises a compound having an inhibitory activity on an aldose reductase, such as a compound represented by the general formula below, as an active ingredient. Preferably, the compound is (2S,4S)-6- fluoro-2',5'-dioxospiro[chromane-4,4'-imidazolidine]-2- carboxamide. wherein X represents a halogen atom ora hydrogen atom; R and R independently represent a hydrogen atom or a C1-6 alkyl group which may be substituted, or R and R together with a nitrogen atom located at the base of R and R or additionally together with other nitrogen atom or an oxygen atom may form a 5- to 6-membered heterocyclic ring.
Owner:SANWAKAGUKU KENKYUSHO CO LTD
Method of producing s-(-)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid and product obtained by the method
InactiveUS20100063305A1High chemical purityHigh optical purityAsymmetric synthesesArylCarboxylic acid
The present invention provides an industrially available method for efficiently producing high-purity S-(−)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid excellent in solid-liquid separability from an S-(−)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid ester, and also provides products obtained by the method.Under a temperature condition of 50-80° C. in an aqueous solvent, (A) an S-(−)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid ester represented by the general formula (1) is hydrolyzed under a basic condition for 1-3 hours; then (B) the insoluble matters contained in the reaction solution resulting from the hydrolysis are removed; and (C) an acid is added to the resulting solution to effect crystallization; provided that R in the general formula (1) represents an alkyl or aryl group.
Owner:MITSUBISHI GAS CHEM CO INC
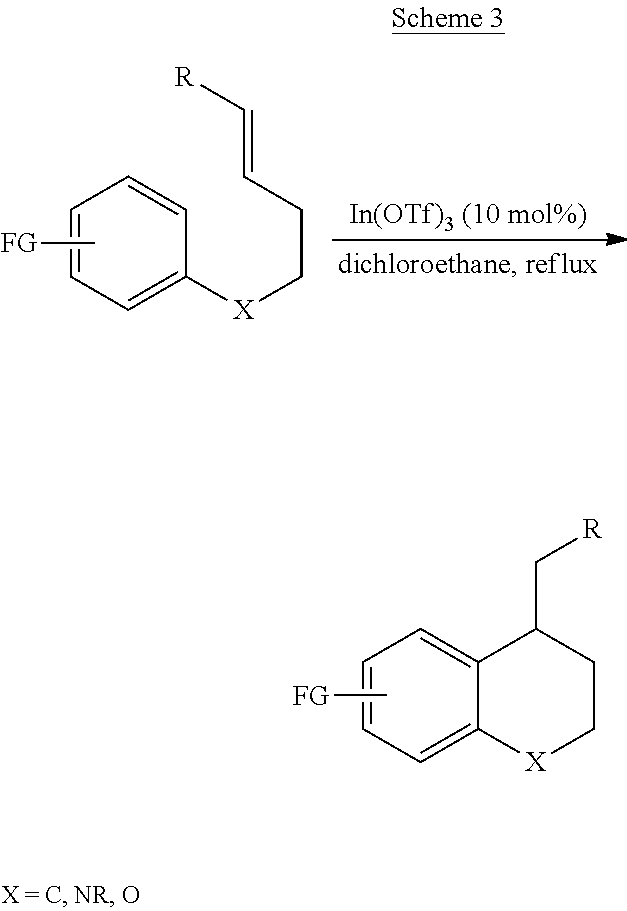
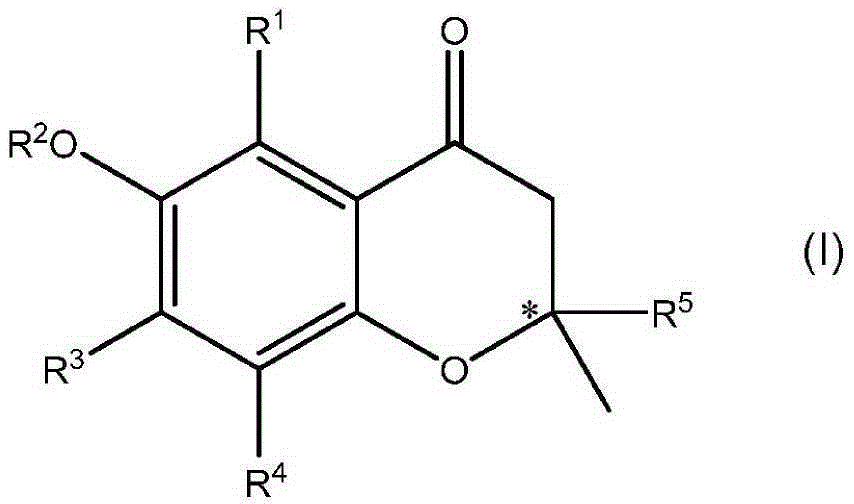
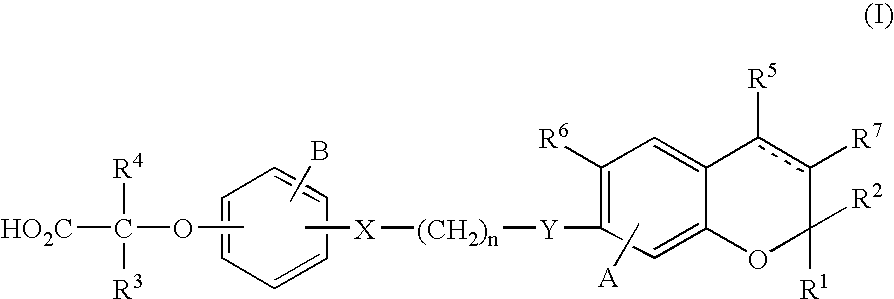
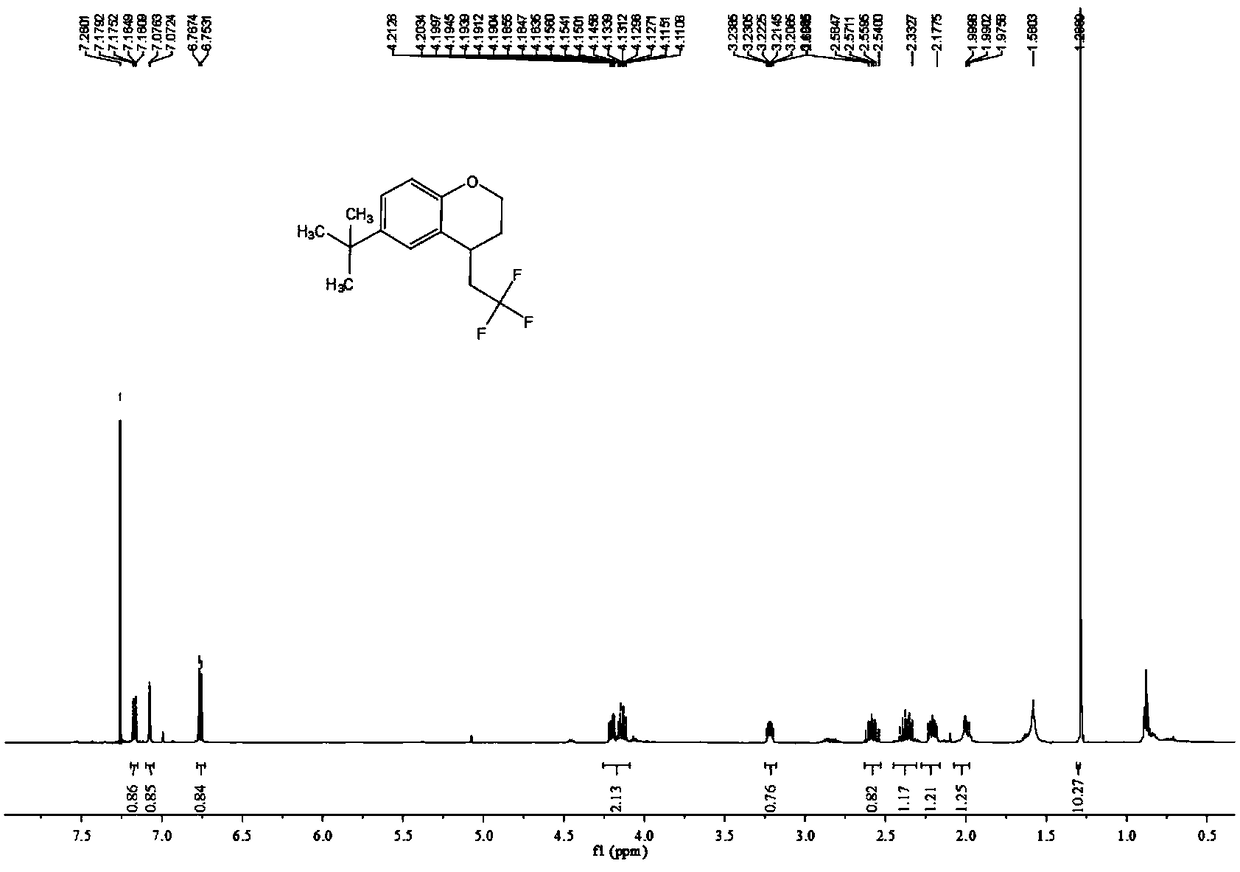
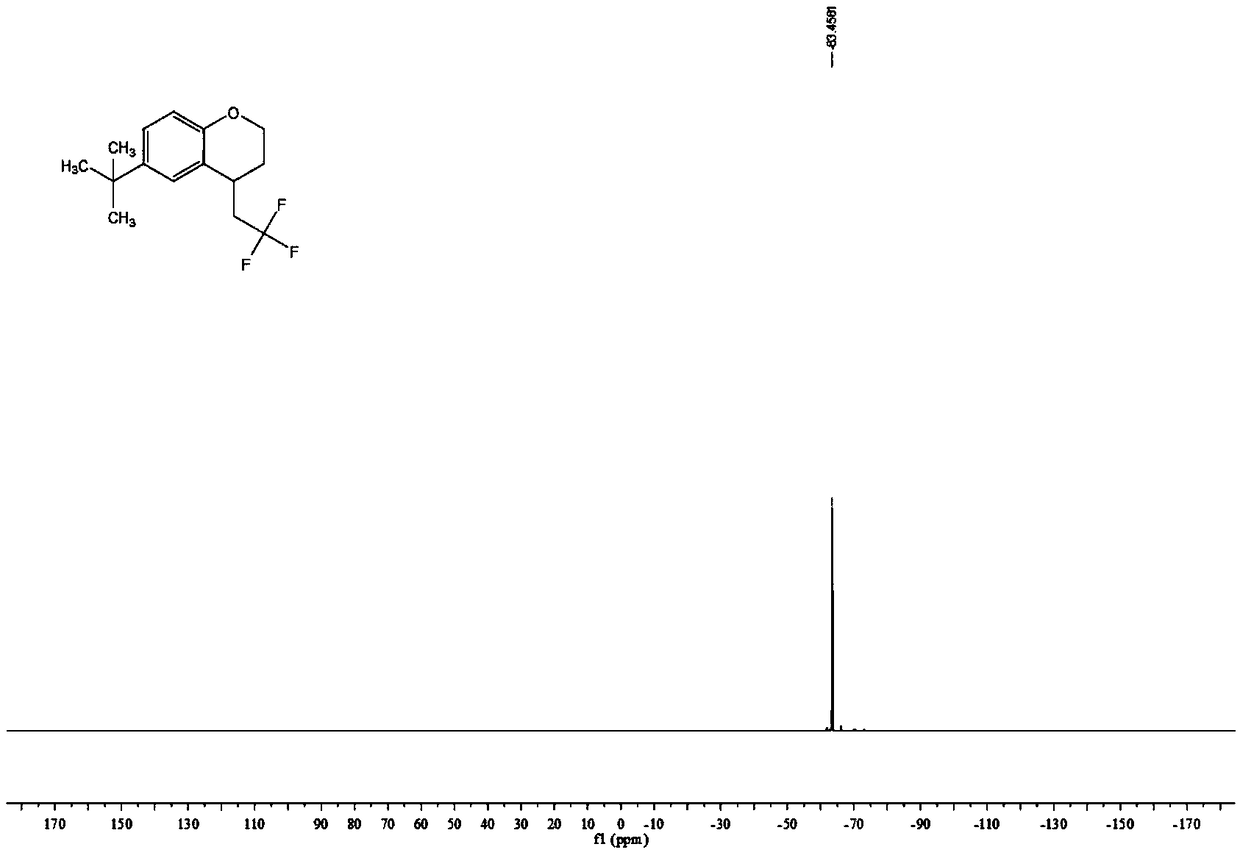
Preparation method of 2,3-dihydro-1H-benzo[f] chromane-2-amine derivative
ActiveCN110240586ANew ideasHigh yieldOrganic chemistry methodsBulk chemical productionAmine derivativesChromane
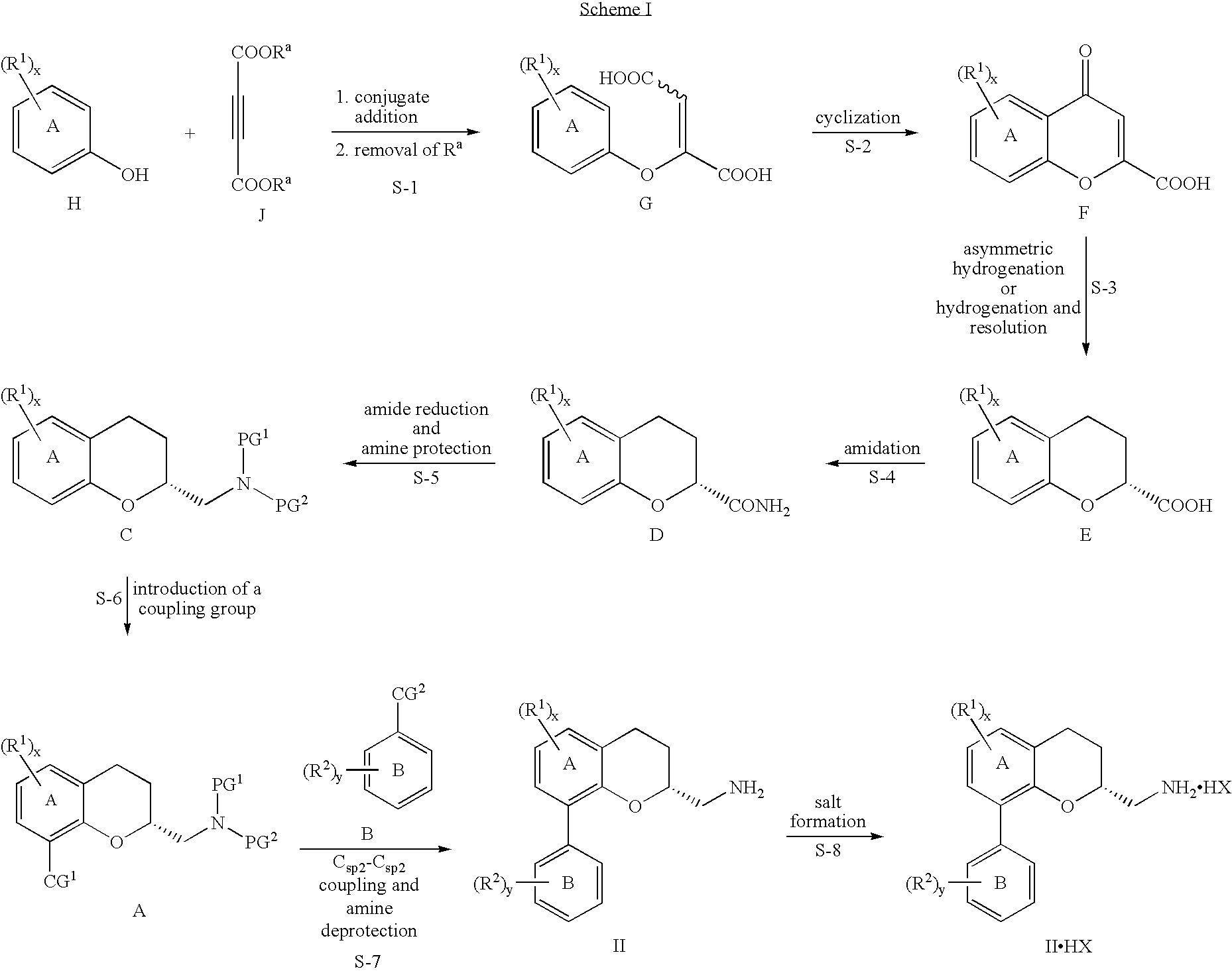
The invention provides a preparation method of a (2S, 3R)-2-amidogen-3-(2,4,5-trifluorophenyl)-9-methoxyl-2,3-dihydro-1H-benzo[f] chromane-8 bit substitutive derivative shown as a formula I shown in the accompanying drawing, wherein R is selected from H, OCH3, CN, SO2CH3 and NHSO2CH3. The method has the advantages that the yield is high; the purification is convenient; the cost is low, and the like.
Owner:SHANDONG BIOPOLAR DICHANG PHARM CO LTD
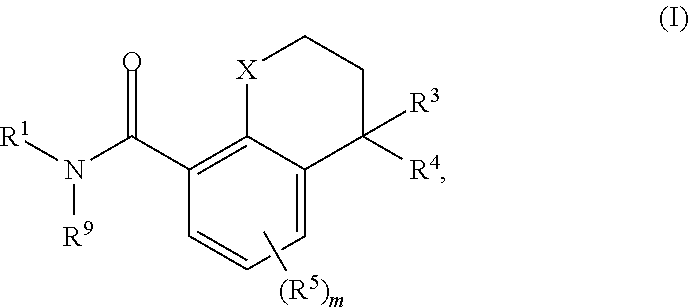
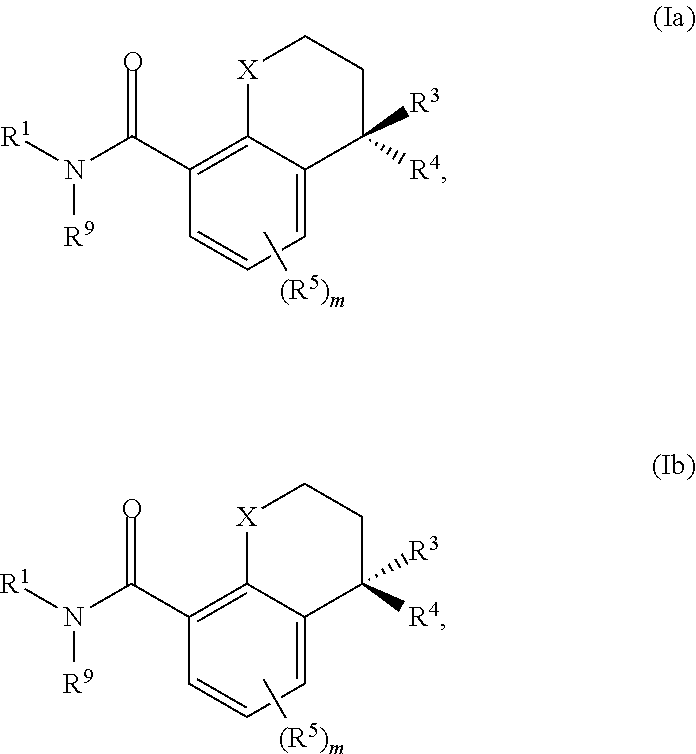
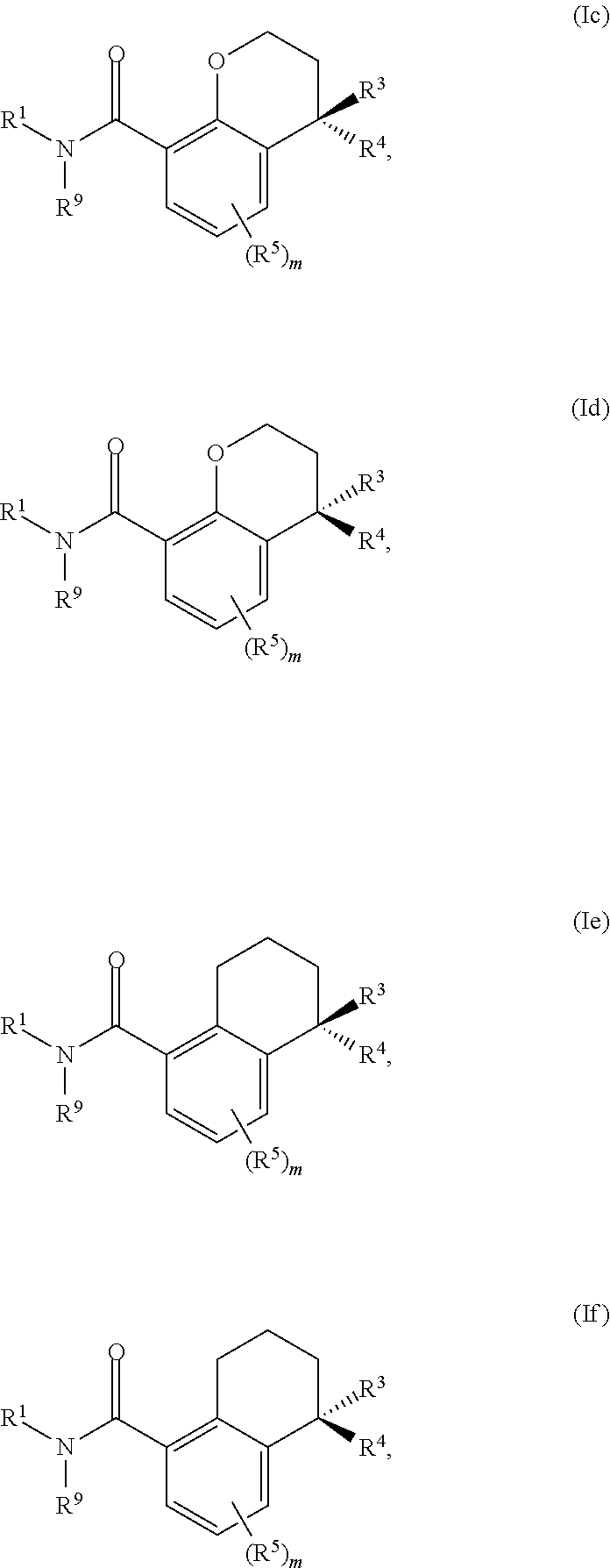
Substituted chromane-8-carboxamide compounds and analogues thereof, and methods using same
The present invention includes novel substituted bicyclic (such as 4-substituted-chromane-8-carboxamide compounds), and compositions comprising the same, that can be used to treat or prevent hepatitis B virus (HBV) infections in a patient. In certain embodiments, the compounds and compositions of the invention are capsid inhibitors.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
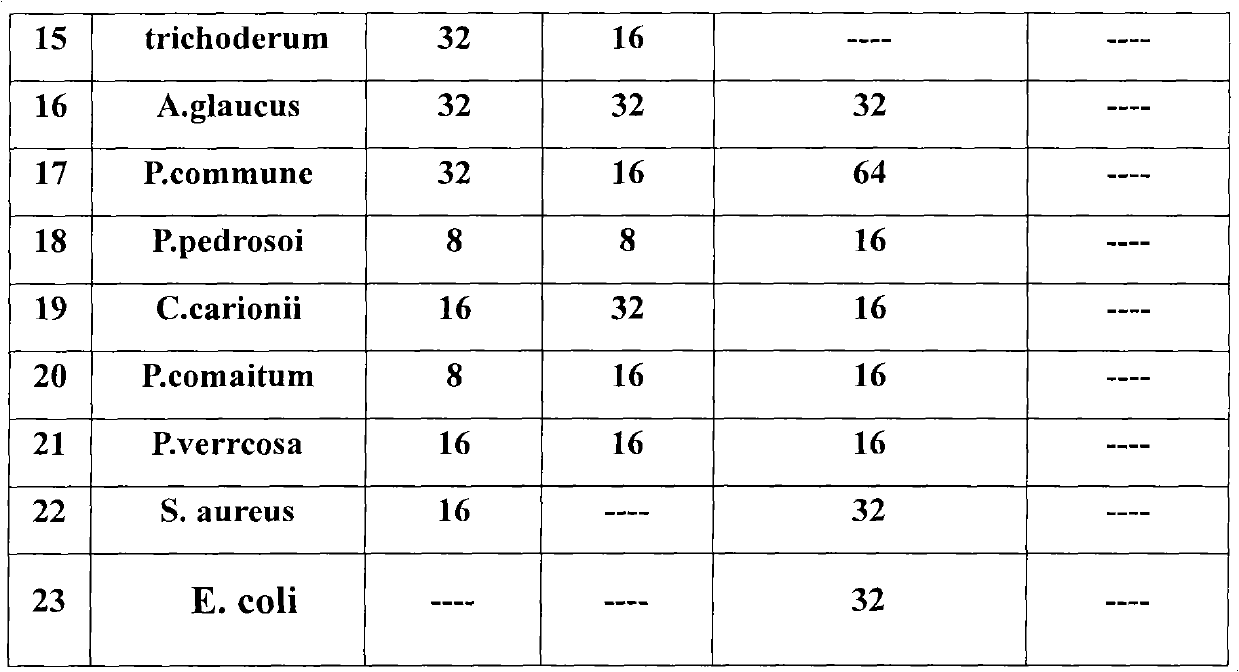
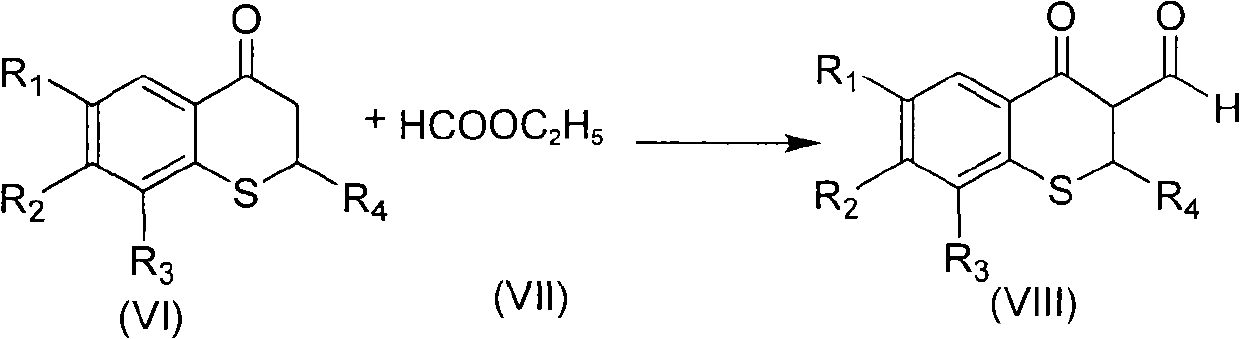
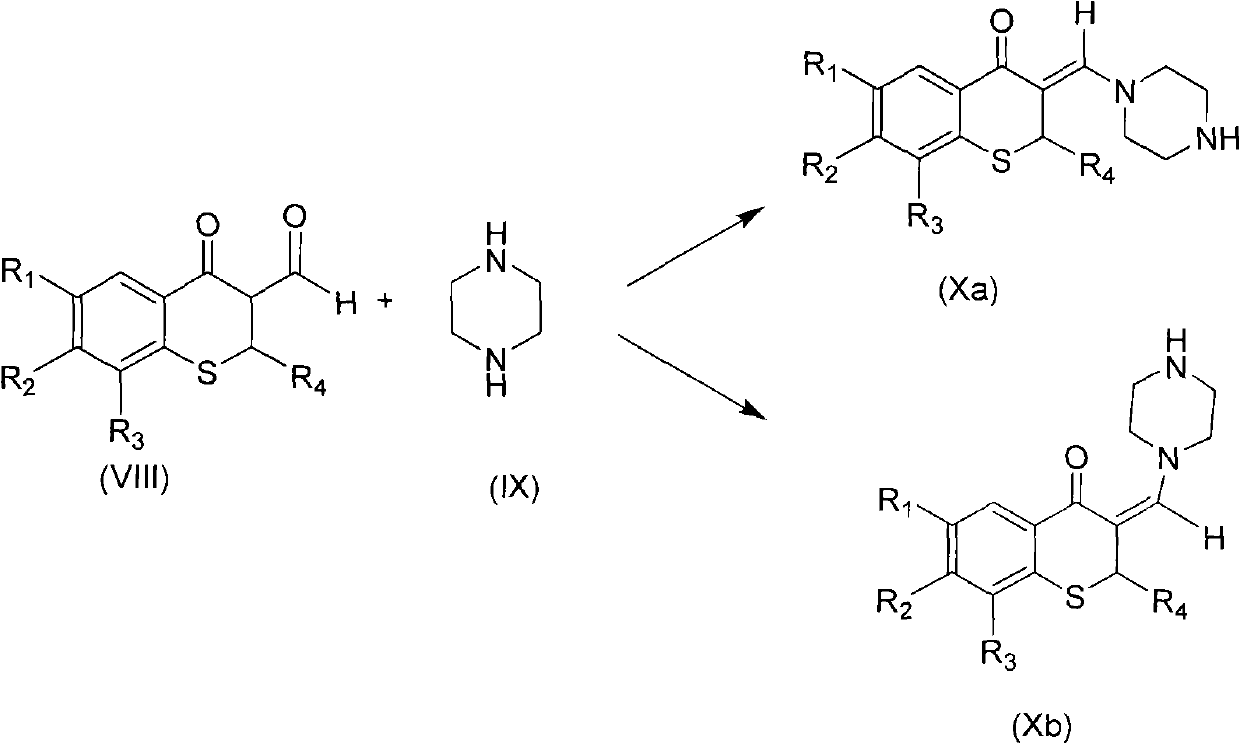
3-(1-halogenated methylene) sulphur chromane-4-ketone compound as well as preparation method and application thereof
The invention discloses a 3-(1-halogenated methylene) sulphur chromane-4-ketone compound shown as a formula (I) as well as a stereoisomer, a racemic or non-racemic mixture of the stereoisomer or a pharmaceutically acceptable salt or solvate, a preparation method, a pharmaceutical composition and antibacterial and anticancer application thereof.
Owner:杨更亮
Chromane and chromene derivatives and uses thereof
Compounds of formula I or pharmaceutically acceptable salts thereof are provided:wherein each of R1, R2, R3, R4, y, m, n, and Ar are as defined, and described in classes and subclasses herein, which are agonists or partial agonists of the 2C subtype of brain serotonin receptors. The compounds, and compositions containing the compounds, can be used to treat a variety of central nervous system disorders such as schizophrenia.
Owner:WYETH LLC
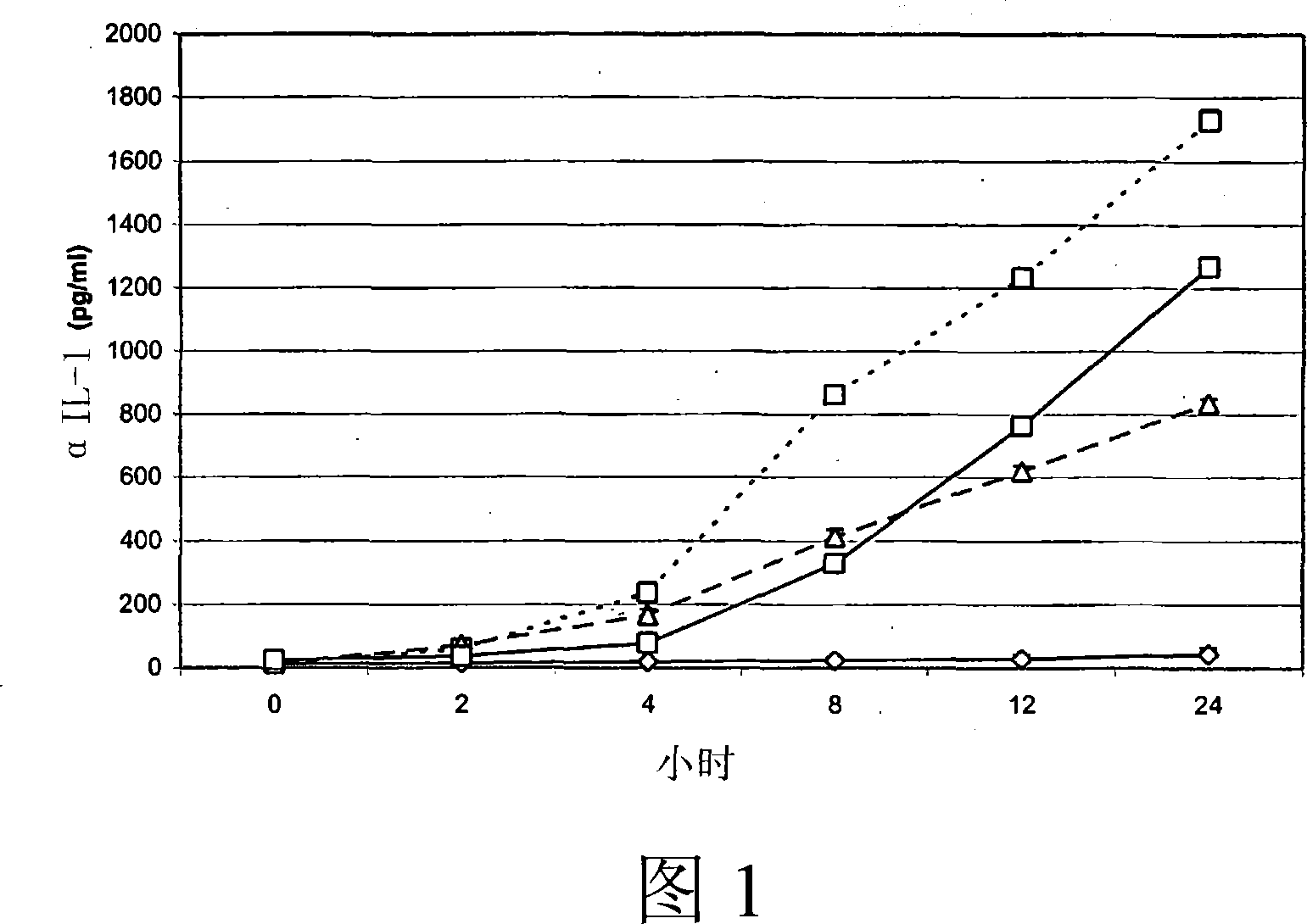
Application of chromane compound HEF-04 in relaxing blood vessel smooth muscle
InactiveCN101618041AReduce tensionOrganic active ingredientsUrinary disorderVascular endotheliumPotassium
The invention relates to the technical field of medicine and provides an application of chromane compound HEF-04 in relaxing blood vessel smooth muscle. In animal in vitro experiment, HEF-04 can relax rabbit in vitro blood vessel smooth muscle shrunk by barium chloride and potassium chloride; the vasodilation effect of chromane compound HEF-04 is not inhibited by the incubation of methylene blue, on the contrary, the blood vessel dose-effect curve shifts left due to higher level of potassium in spasm-induced agent; EC50 value has significant difference before and after the incubation. Documents report that the existence of NO and PGI2 can weaken the generation of EDHF; after NO and PGI2 are inhibited, the effect of endothelium-dependent vascular relaxation caused by EDHF is more prominent, showing that the vascular relaxation effect of HEF-04 is possibly related to the increase of EDHF releasing or caused by the interactions of vascular endothelium relaxing factors.
Owner:SHENYANG PHARMA UNIVERSITY
Preparation method of nebivolol intermediate, intermediate for preparing nebivolol intermediate, and preparation method of intermediate
ActiveCN108997297ARaw materials are easy to obtainSuitable for industrial applicationsOrganic chemistry methodsLithiumCarboxylic salt
The invention relates to a preparation method of alpha-haloketone of a nebivolol intermediate of a formula II, an intermediate compound I for preparing the alpha-haloketone, and a preparation method of the intermediate compound I. The preparation method disclosed by the invention comprises the following steps: taking chromane-2-carboxylic acid as a raw material, carrying out a reaction with dihalide or disulfonate, so as to obtain chromane-2-carboxylate I; further carrying out a reaction with dihalomethane under the effect of organo-lithium, so as to obtain the nebivolol intermediate alpha-haloketone II. According to the method disclosed by the invention, the defect that the intermediate for producing the alpha-haloketone is high in cost and low in purity is overcome. Moreover, the methoddisclosed by the invention has the advantages of being simple in operation, environmentally friendly, convenient for industrial production and the like.
Owner:ZHEJIANG HISOAR PHARMA +1
Process for the production of 4-substituted chromanes via gold catalysis
Disclosed herein is single step process for the synthesis of 4-aryl substituted chromanes of compound of formula 2 comprising subjecting 3-aryloxy-1-phenylpropan-1-ol of formula 1 to gold(III) chloride-catalyzed intramolecular Friedel-Crafts reaction to obtain 4-aryl substituted chromanes. The invention further discloses novel 4-substituted Chromane compounds.
Owner:COUNCIL OF SCI & IND RES
Chromane bridged-ring isoindolinone and preparation method thereof
The invention discloses a chromane bridged-ring isoindolinone and a preparation method thereof, and belongs to the technical field of organic synthesis. The preparation method comprises the followingsteps: adding isoindolinone and o-hydroxychalone to acetonitrile solvent, and carrying out a Michael addition / condensation / cyclization tandem reaction with p-toluene sulfonic acid as a catalyst at 35DEG C to prepare the chromane bridged-ring isoindolinone. According to the preparation method, intermediate separation is avoided, and pure products can be obtained through filtration or column chromatography. The preparation method has the advantages of mild reaction conditions, simple operation, wide substrate range and the like.
Owner:HENAN UNIVERSITY
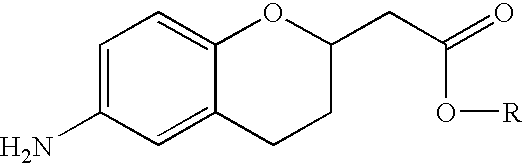
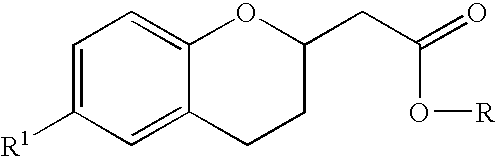
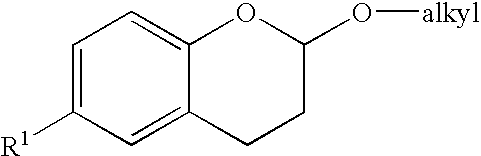
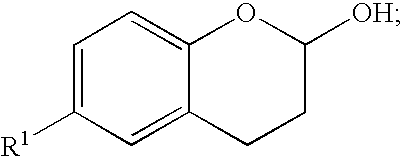
Methods for producing amino substituted chromanes and intermediates therefor
Disclosed are process steps and processes for producing chromane compounds, preferably 2-(6-amino-chroman-2yl) acetic acid esters which are intermediates for producing platelet aggregation inhibitors and / or are themselves potent platelet aggregation inhibitors.
Owner:MILLENNIUM PHARMA INC
Preparation process of ethyl chromane-4-formate
The invention relates to a preparation process of ethyl chromane-4-formate. The preparation process is characterized by comprising the following steps of: carrying out Friedel-Crafts acylation reaction and cyclization, addition reaction, hydrolysis reaction and esterification reaction, wherein, in the Friedel-Crafts acylation reaction and cyclization, the Friedel-Crafts acylation reaction is carried out in the presence of anhydrous aluminium trichloride by using a compound of formula 1 and 3-chloropropionylchloride as initial raw materials, then carrying out cyclization on an obtained product and 10% NaOH to obtain a compound of formula 2; in the addition reaction, the addition reaction is carried out on the compound of formula 2 and trimethylsilyl cyanide in the presence of zinc iodide to obtain a compound of formula 3; in the hydrolysis reaction, the compound of formula 3 is hydrolyzed under the conditions of stannous chloride dihydrate, concentrated hydrochloric acid and acetic acid to obtain a compound of formula 4; and in the esterification reaction, the compound of formula 4 is esterified in the presence of concentrated sulfuric acid to obtain a compound of formula 5. According to the preparation process, expensive trifluoromethanesulfonic acid is replaced with cheap anhydrous aluminium trichloride in the Friedel-Crafts acylation step, and the yield is improved, so that the cost of preparing the compound is greatly reduced, and the high-quality product is obtained; and compared with the traditional method, the preparation process has obvious advantages.
Owner:苏州莱克施德药业有限公司
Chromane compounds
The present invention provides a compound which is useful as an active ingredient of a pharmaceutical composition, in particular, a pharmaceutical composition for preventing or treating diseases or conditions associated with and / or mediated by β-secretase activity, hydrolysis of a β-secretase site of a β-amyloid precursor protein, and / or β-amyloid protein accumulation, including a pharmaceutical composition for preventing or treating diseases including, but not limited to, Glaucoma, MCI (Mild cognitive impairment) or Alzheimer's disease, especially, Alzheimer's disease.
Owner:COMENTIS INC
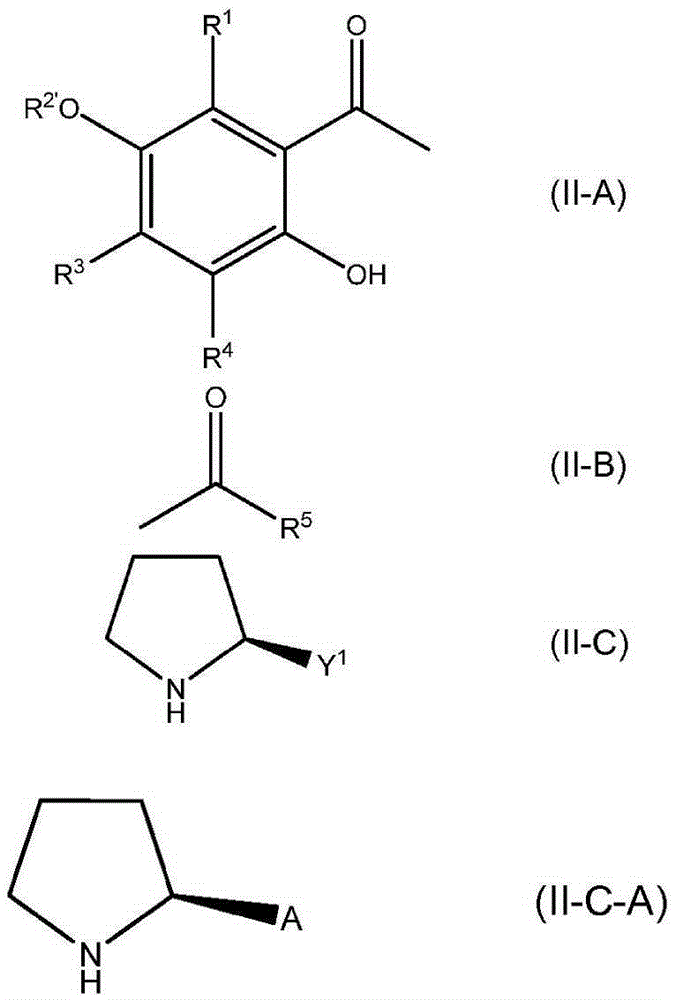
Formation of chiral 4-chromanones using chiral pyrrolidines in the presence of acids
The present invention relates to a synthesis of chromanones or chromanes in a stereospecific matter in view of the 2-position in the chromanone or chromane ring. It has been found that this synthesis is particularly possible in the presence of a chiral compound of a specific type and of at least one Bransted acid or in the presence a specific chiral compound having a Bransted acid functional group in the molecule.
Owner:DSM IP ASSETS BV
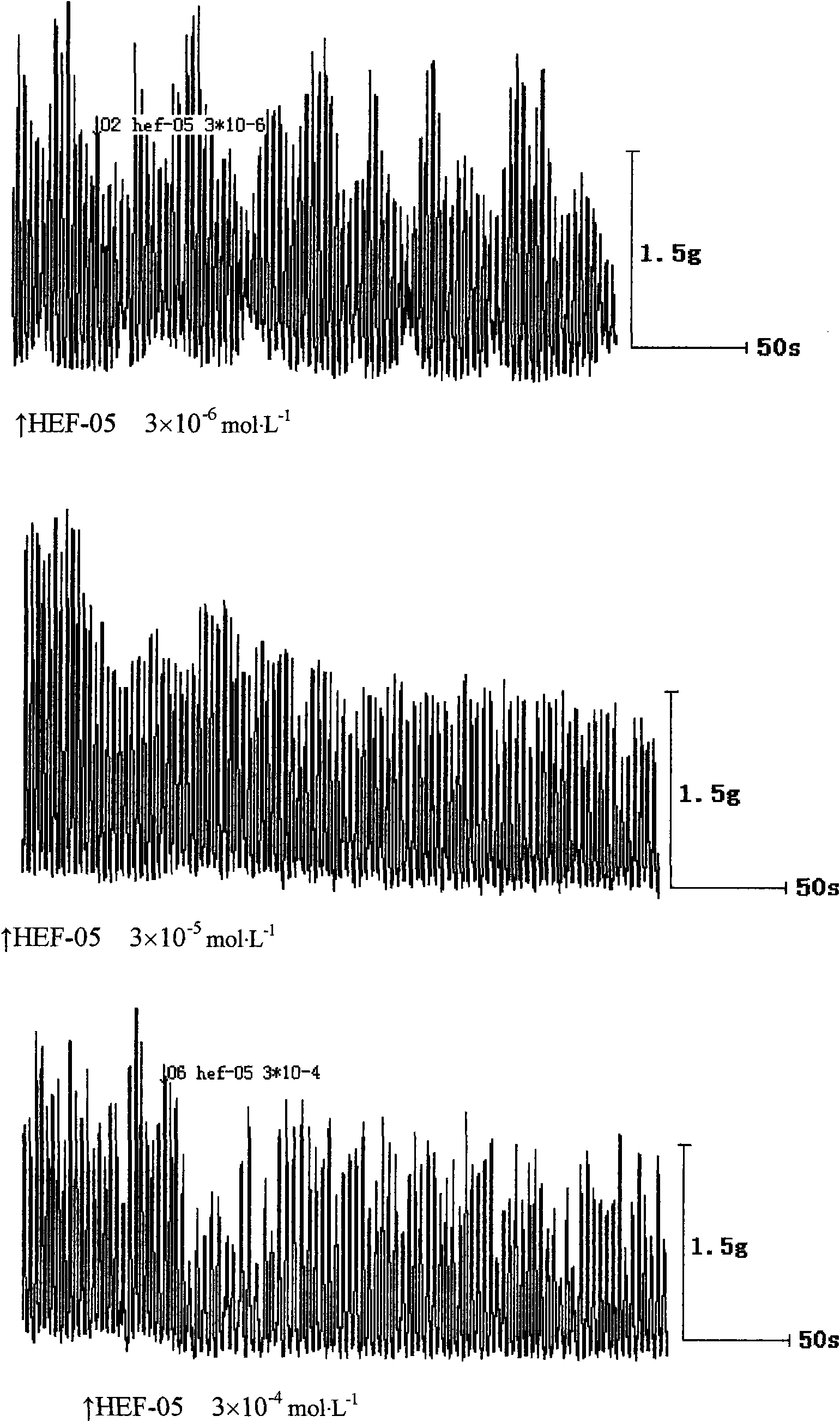
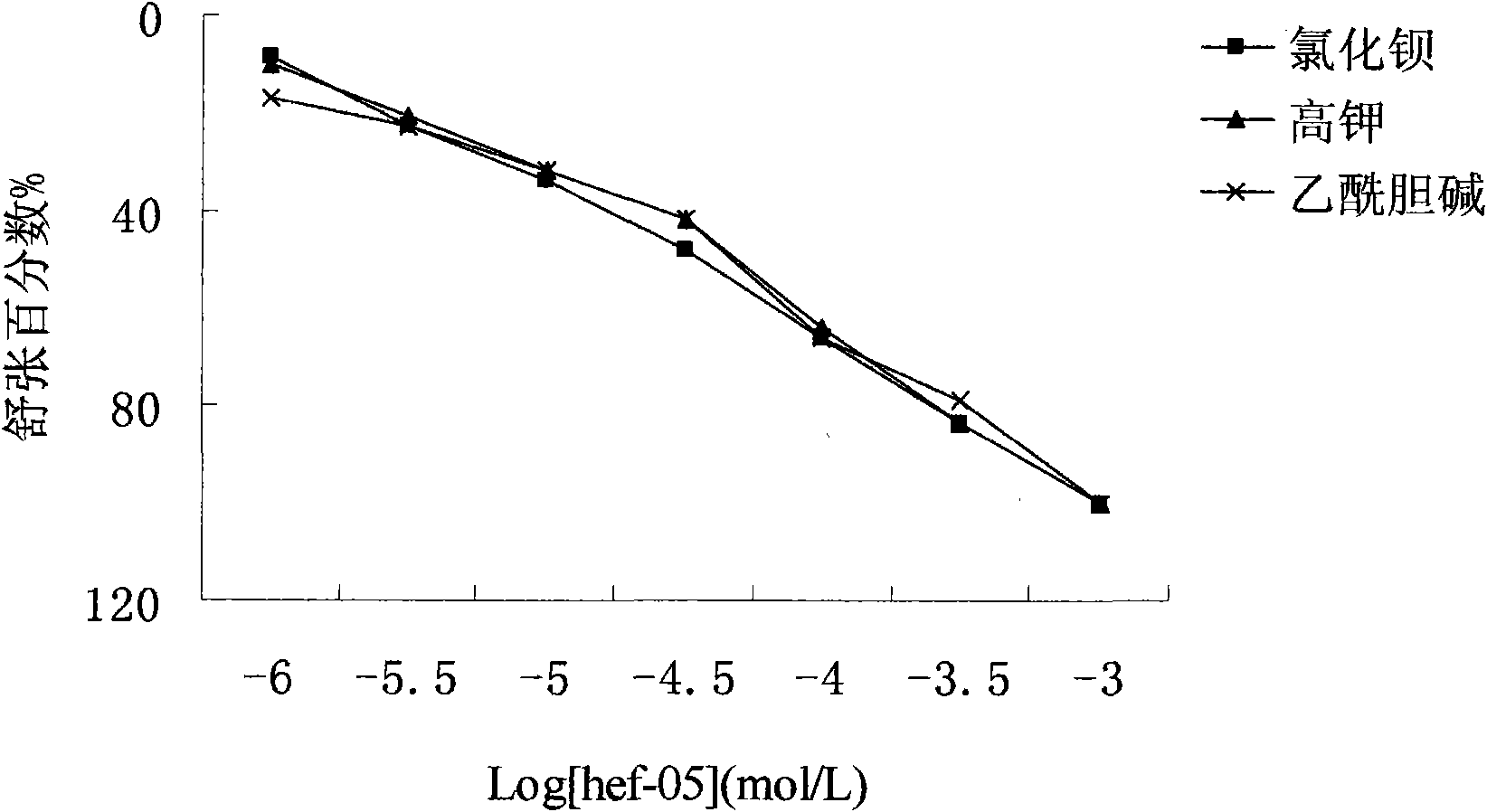
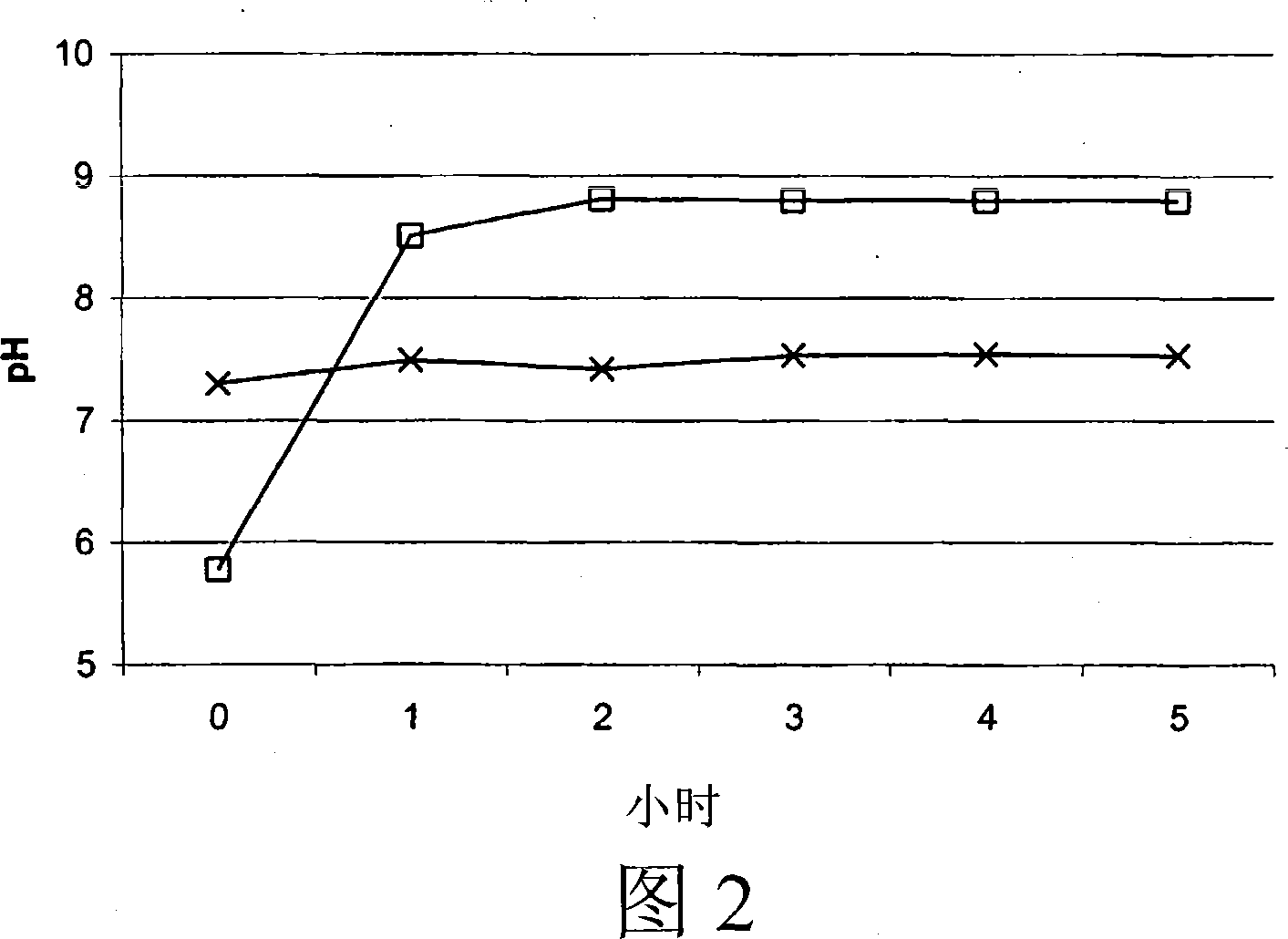
New medical application of chromane compound HEF-05
InactiveCN101618033ADiastolic inhibitionReduce tensionOrganic active ingredientsOrganic chemistrySmooth muscle spasmATP-sensitive potassium channel
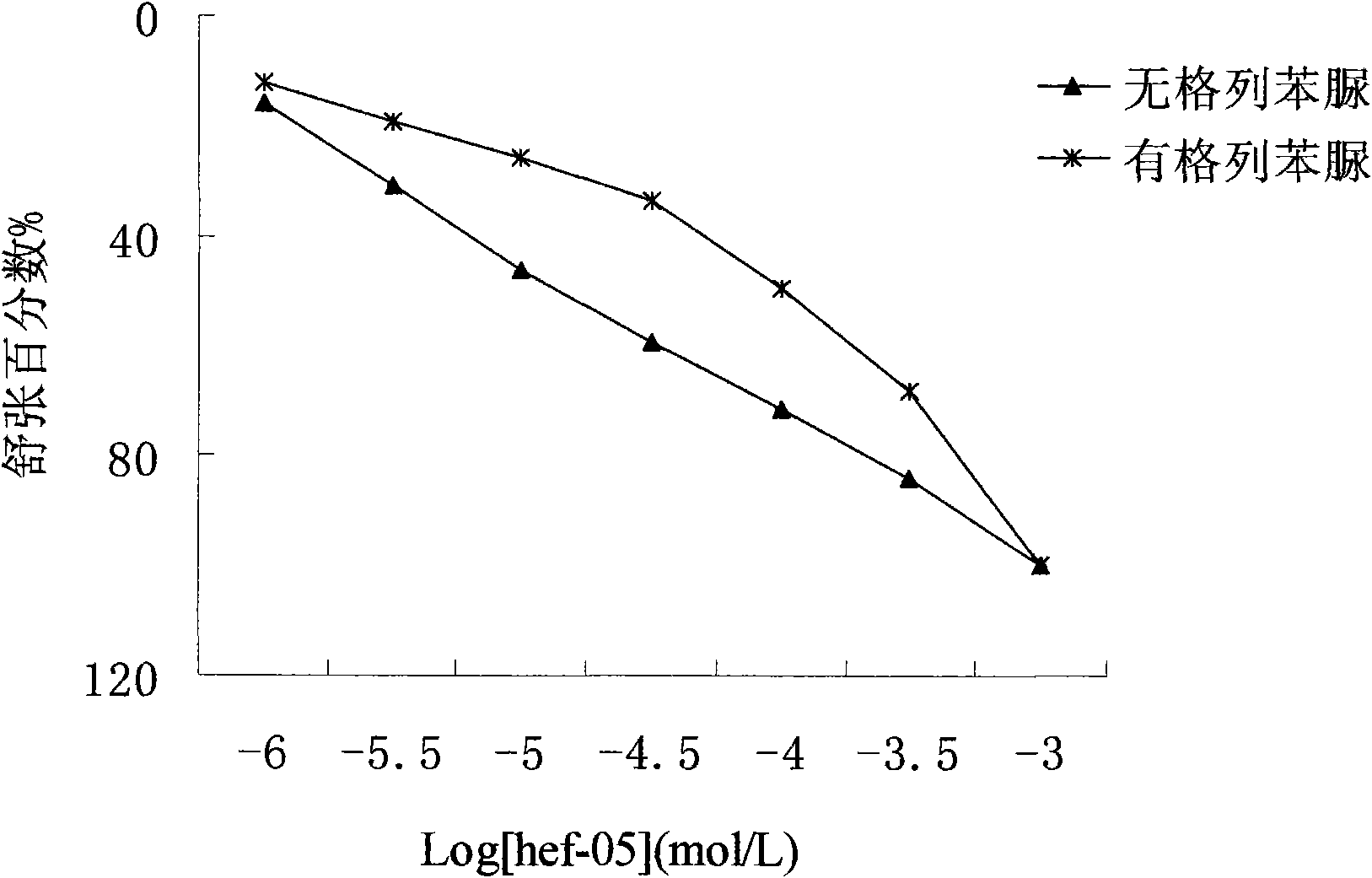
The invention relates to the technical field of medicine, mainly relating to the applications of chromane compound HEF-05 in preparing intestinal canal smooth muscle spasmolytic and in serving as potassium ion channel opener; the invention further comprises a preparation method of the inspected object HEF-05 in the invention. In animal in vitro experiment, HEF-05 can not only relax the amplitude and the tension of spontaneous activities of rabbit in vitro intestinal canal smooth muscle, but also relax the shrinkage of small intestine smooth muscle caused by acetylcholine chloride, barium chloride and potassium chloride. In addition, preliminary study on the relaxation mechanism of chromane compound HEF-05 proves that the relaxation effect of chromane compound HEF-05 is inhibited after the small intestine smooth muscle is incubated by ATP-sensitive potassium channel blocking agent Glibenclamide, therefore, HEF-05 can be used for preparing spasmolytic which is used for treating intestinal canal smooth muscle spasm, and can also be served as potassium ion channel opener.
Owner:SHENYANG PHARMA UNIVERSITY
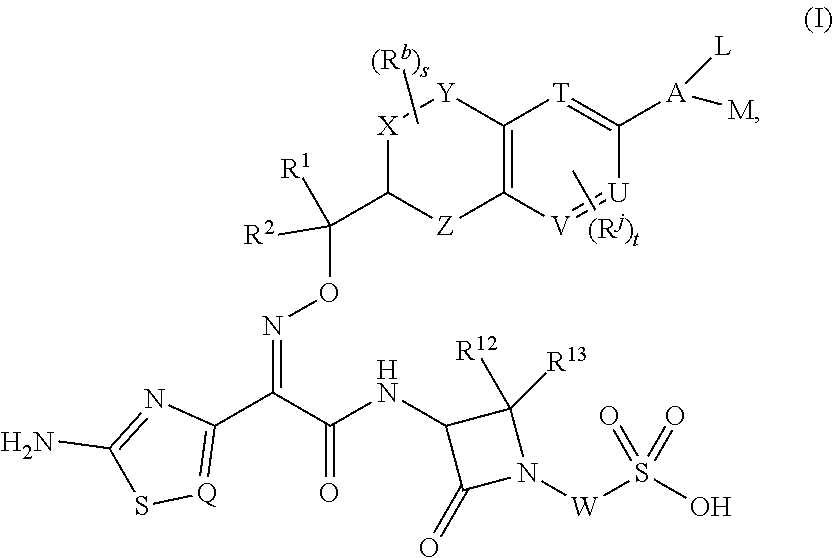
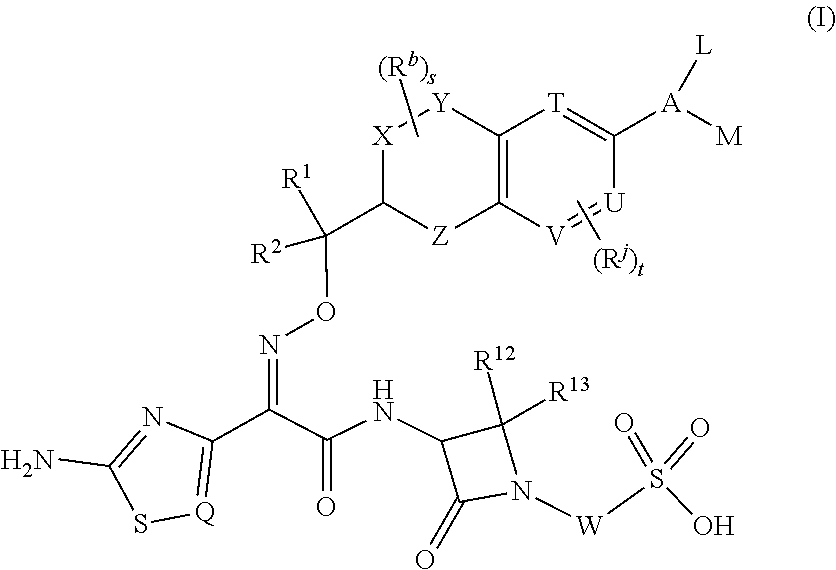
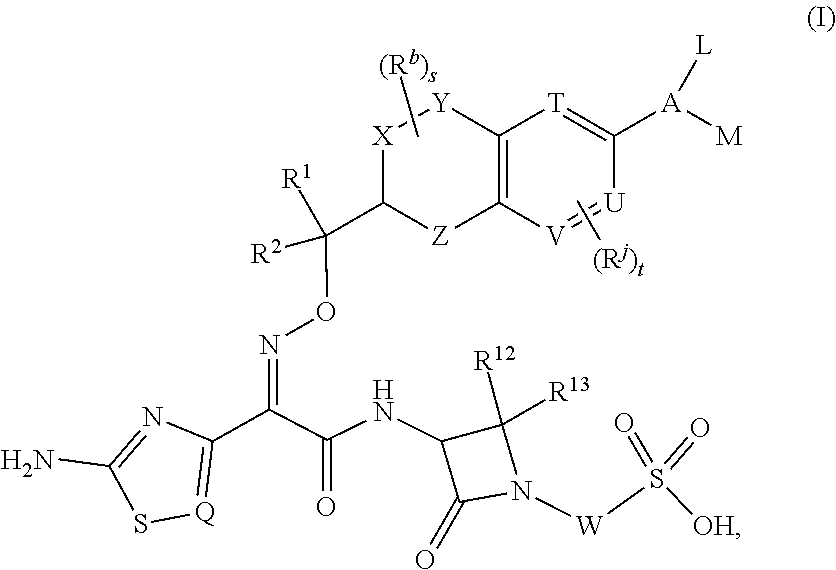
Chromane monobactam compounds for the treatment of bacterial infections
Owner:MERCK SHARP & DOHME LLC
Chromane and chromene derivatives and uses thereof
Methods of preparing compounds of formula I or pharmaceutically acceptable salts thereof are provided: wherein each of R1, R2, R3, R4, x, m, n, and Ar are as defined, and described in classes and subclasses herein, which are agonists or partial agonists of the 2C subtype of brain serotonin receptors. The compounds, and compositions containing the compounds, can be used to treat a variety of central nervous system disorders such as schizophrenia.
Owner:WYETH LLC
Synthesis of chromane compounds and their derivatives by a copper-catalyzed conjugate addition reaction
The present invention relates to the preparation of chromane compounds and their derivatives by a copper-catalyzed conjugate addition reaction. It has been observed that in highly stereoselective manner a methyl group can be introduced to the 2 position of chromane ring by reaction of a chromenone with Al(CH3)3 in the presence of a Cu(I) salt and specific chiral phosphoramidites. The compounds obtained are important intermediates particularly for the synthesis of chiral tocopherols.
Owner:DSM IP ASSETS BV
Formation of chromanes and chromenes by using silver(i) or gold(i) salts or complexes
The present invention relates to the method of preparing chiral chromanes and chromenes in high yields by intramolecular hydroarylation of chiral aryl alkynes catalysed by either a silver(I) or gold(I) salt or complex in combination of a specific acid or by a silver(I) or gold(I) metal salt or complex having specific anion. By the hydroarylation a chromene is formed which has a chiral centre in the 2 position. The corresponding chromane is obtained by hydrogenation of the chromenes.
Owner:DSM IP ASSETS BV
Methods for producing amino substituted chromanes and intermediates thereof
Disclosed are processes for producing chromane compounds, preferably chroman-2-yl acetic acid compounds and 6-amino-chroman-2-yl acetic acid esters which are intermediates for producing platelet aggregation inhibitors and / or are themselves potent platelet aggregation inhibitors.
Owner:MILLENNIUM PHARMA INC
Formation of chiral 4-chromanone using chiral pyrrolidine in the presence of phenol or thiophenol
The present invention relates to a synthesis of chromanone or chromane in a stereo-specific matter in view of the 2-position in the chromanone or chromane ring. It has been found that this synthesis is particularly possible in the presence of a chiral compound of formula of a specific type and of at least one phenol or thiophenol.
Owner:DSM IP ASSETS BV
Chromanone compound, and preparation method and application thereof
The invention discloses a chromanone compound, and a preparation method and application thereof. The chromanone compound is Tobchromanone A and is separated from aromatic tobacco; the molecular formula is C14H16O4; and the structure is shown in the specification. The preparation method of the chromanone compound comprises the following steps: crushing an aromatic tobacco sample, performing ultrasonic extraction with 95% ethanol for 3-5 times, merging the extracting solutions, and concentrating under reduced pressure to obtain an extract; and performing primary separation on the extract through silica gel column chromatography, and then performing further separation through high pressure liquid chromatography to obtain the required compound, namely the Tobchromanone A. The compound has a better cytotoxic effect on human neuroblastoma cells (SHSY5Y) with the IC50 value up to 2.8 mu M, and indicates a medium cytotoxic effect on other measured cell strains.
Owner:YUNNAN MINZU UNIV
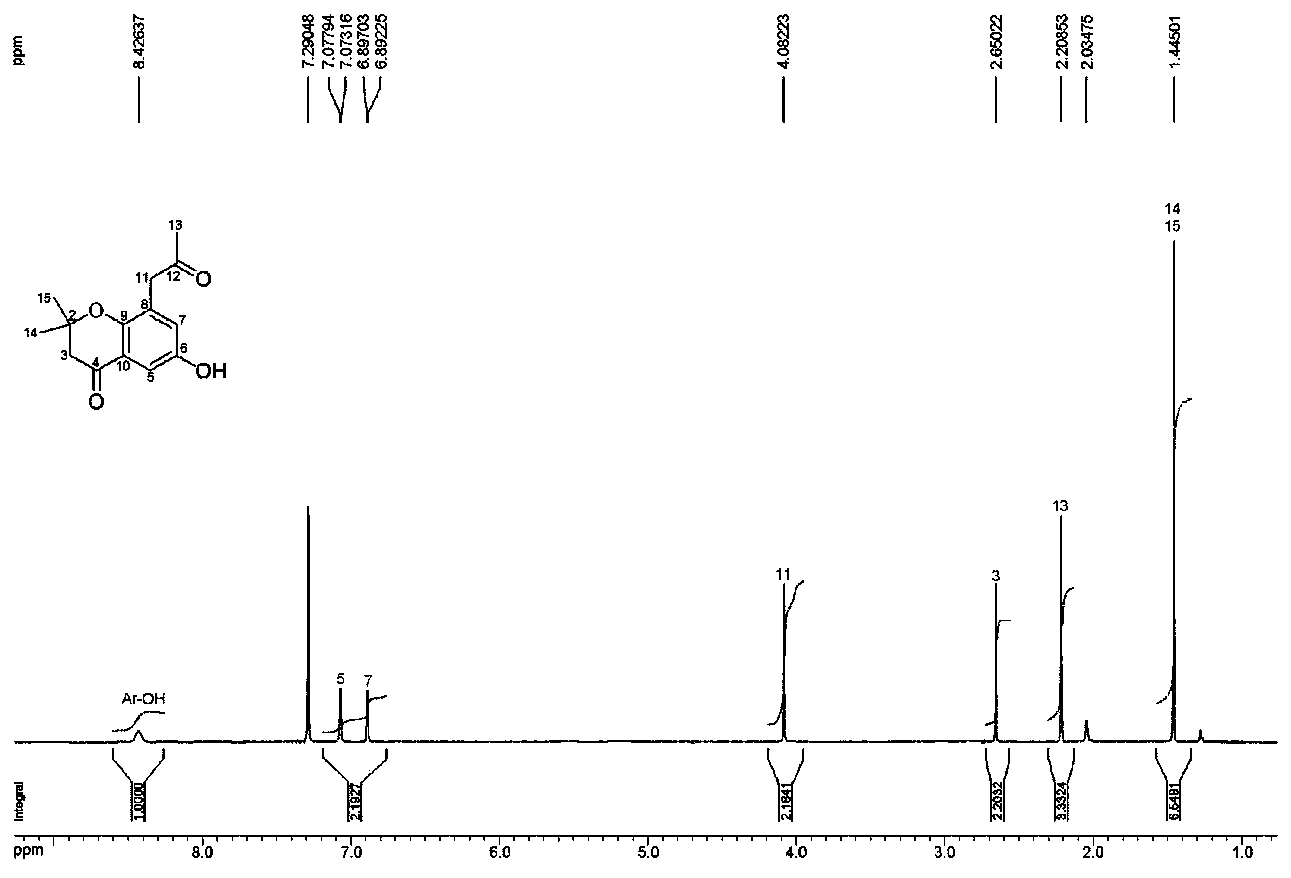
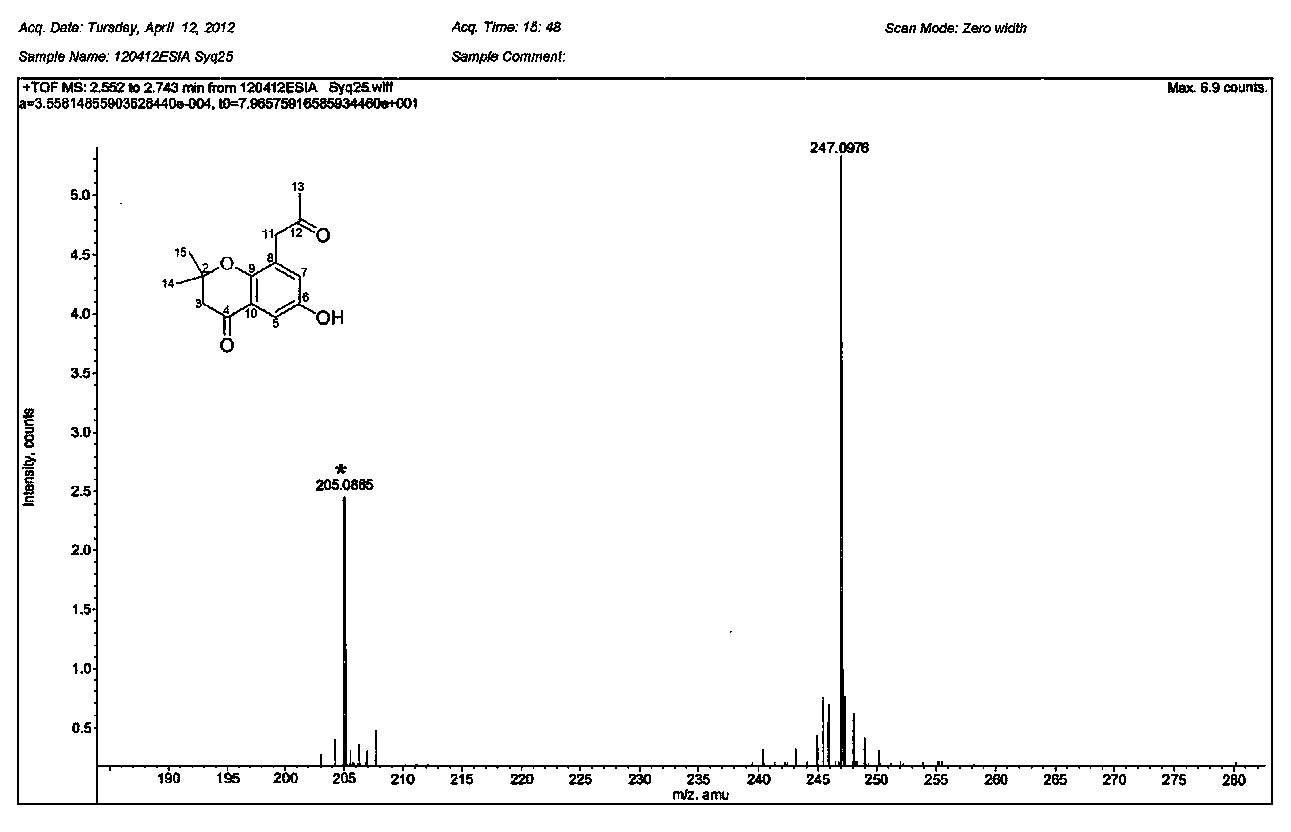
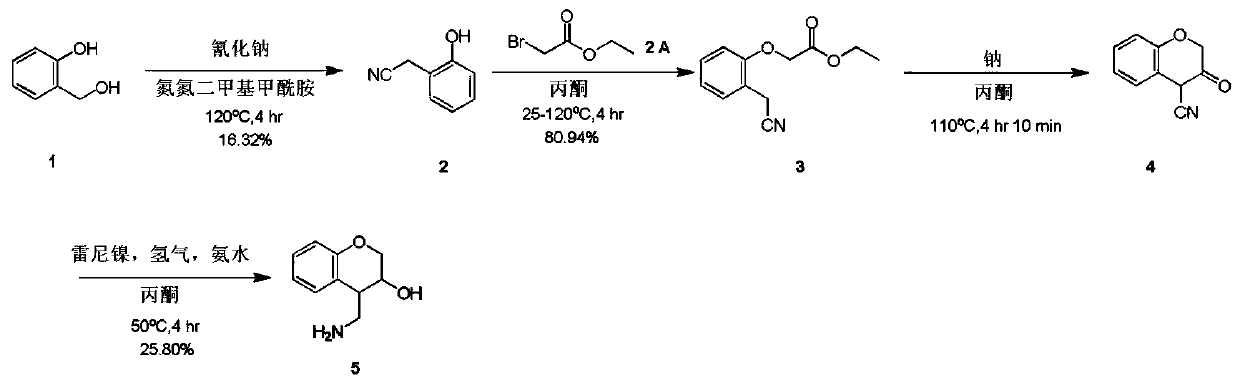
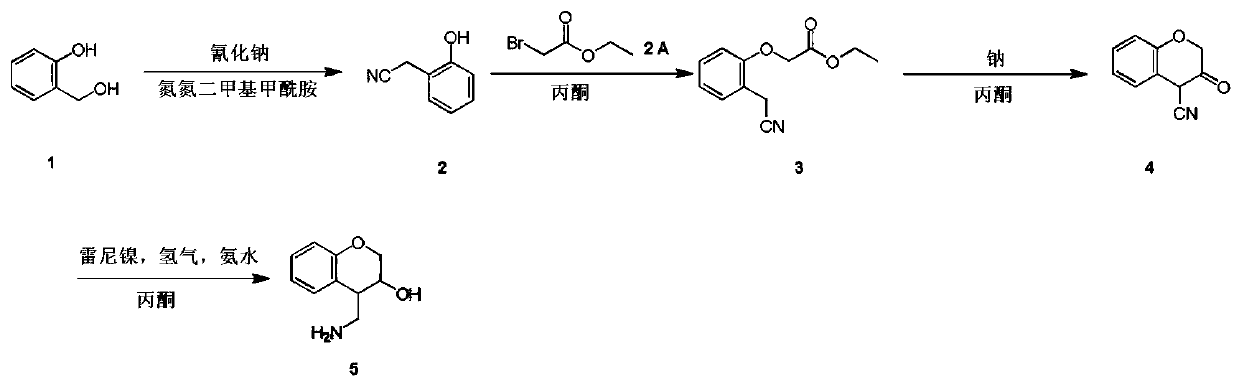
4-(aminomethyl)chromane-3-ol preparation method
The invention relates to a 4-(aminomethyl)chromane-3-ol preparation method. A purpose of the present invention is mainly to solve the technical problem that no suitable industrial synthesis method exists in the prior art. According to the present invention, the method comprises four steps, and comprises: generating a compound 2 from a compound 1 and a compound sodium cyanide in a solvent N,N-dimethylformamide, carrying out a reaction on the compound 2 and ethyl bromoacetate in acetone to obtain a compound 3, carrying out a reaction on the compound 3 and sodium in acetone to obtain a compound 4, and carrying out a reaction on the compound 4, ammonia water and Raney nickel in acetone under the condition of hydrogen introducing to obtain a final compound 5. According to the present invention,the obtained compound can be used as the intermediate or product for synthesis of a plurality of drugs.
Owner:上海合全医药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![Spiro[chromane-oxindole] derivatives, synthesis method and application thereof Spiro[chromane-oxindole] derivatives, synthesis method and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/06addaea-d8b2-4cb2-a1da-a227e057ab72/HDA0001133312770000011.png)
![Spiro[chromane-oxindole] derivatives, synthesis method and application thereof Spiro[chromane-oxindole] derivatives, synthesis method and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/06addaea-d8b2-4cb2-a1da-a227e057ab72/HDA0001133312770000012.png)
![Spiro[chromane-oxindole] derivatives, synthesis method and application thereof Spiro[chromane-oxindole] derivatives, synthesis method and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/06addaea-d8b2-4cb2-a1da-a227e057ab72/HDA0001133312770000021.png)






![Preparation method of 2,3-dihydro-1H-benzo[f] chromane-2-amine derivative Preparation method of 2,3-dihydro-1H-benzo[f] chromane-2-amine derivative](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0b1e74ab-c02a-4124-944b-952d7d938c3b/FDA0001592953780000011.png)
![Preparation method of 2,3-dihydro-1H-benzo[f] chromane-2-amine derivative Preparation method of 2,3-dihydro-1H-benzo[f] chromane-2-amine derivative](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0b1e74ab-c02a-4124-944b-952d7d938c3b/FDA0001592953780000012.png)
![Preparation method of 2,3-dihydro-1H-benzo[f] chromane-2-amine derivative Preparation method of 2,3-dihydro-1H-benzo[f] chromane-2-amine derivative](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0b1e74ab-c02a-4124-944b-952d7d938c3b/FDA0001592953780000013.png)