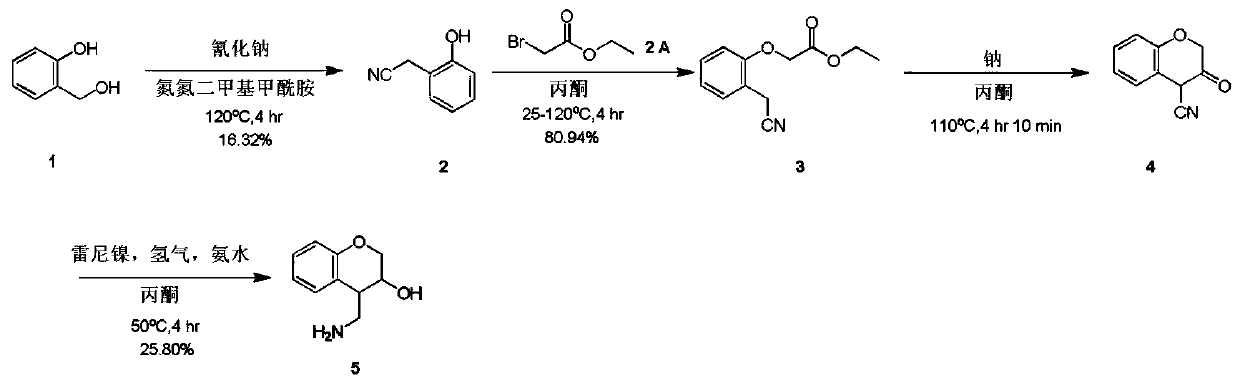
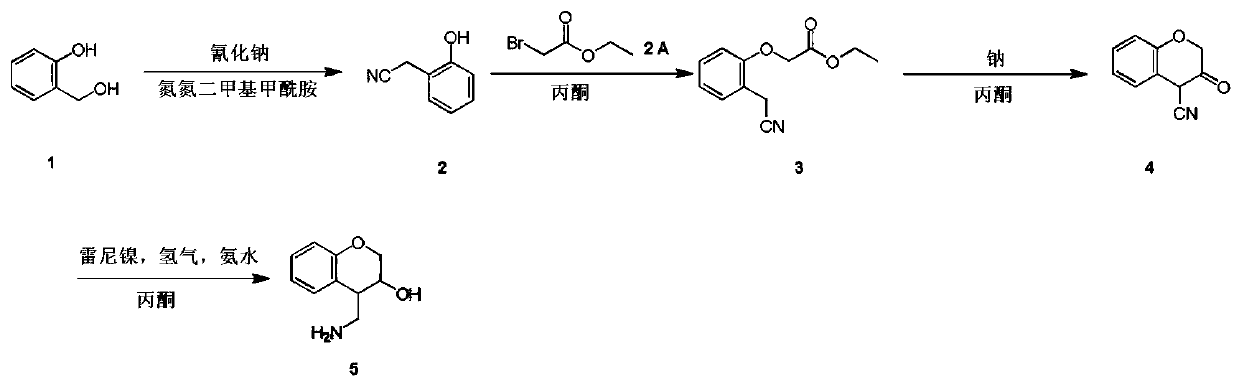
4-(aminomethyl)chromane-3-ol preparation method
A technology of aminomethyl and chromane, which is applied in the field of synthesis of 4-chroman-3-ol, can solve the problems of no suitable industrial synthesis method, and achieve the effects of convenient operation, easy reaction and short route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Example 1: a. Compound 1 (20.00 g, 161.11 mmol) and sodium cyanide (8.69 g, 177.22 mmol) were miscible in a nitrogen nitrogen dimethylformamide solution (200.00 mL). Control the temperature at 120°C and stir for 4 hours. TLC (volume ratio of petroleum ether / ethyl acetate = 2 / 1) indicated the end of the reaction. The reaction solution was poured into water (600 mL), and then the aqueous phase was extracted with ethyl acetate (100 mL * 6). The organic phases were combined, dried with sodium sulfate, filtered and concentrated by distillation under reduced pressure to obtain a crude product. The crude product was purified on a silica gel column (dichloromethane / methanol volume ratio = 10 / 1) to obtain compound 2 (3.5 g) as a yellow oil with a yield of 16.32%.
[0012] b. Dissolve compound 2 (1.50 g, 11.27 mmol) and ethyl bromoacetate (1.88 g, 11.27 mmol) in acetone (30.00 mL), then add potassium carbonate (4.67 g, 33.80 mmol) in batches, and the reaction solution Slowly rise...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com