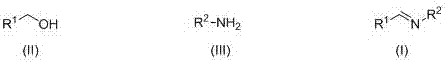Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
212 results about "Nonane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nonane is a linear alkane hydrocarbon with the chemical formula C₉H₂₀. It is a colorless, flammable liquid, occurring primarily in the component of the petroleum distillate fraction commonly called kerosene, which is used as a heating, tractor, and jet fuel. Nonane is also used as a solvent, distillation chaser, fuel additive, and a component in biodegradable detergents.
Method for reducing 8-benzyl-2,8-diazabicyclo[4,3,0]nonane and chiral isomer thereof
The invention discloses a method for reducing 8-benzyl-2,8-diazabicyclo[4,3,0]nonane and a chiral isomer thereof. The method comprises the steps of: taking 8-benzyl-7,9-dioxo2,8-diazabicyclo[4,3,0]nonane or (1S,6R)-8-benzyl-7,9-dioxo2,8-diazabicyclo[4,3,0]nonane as a raw material, and adopting a metal borohydride / BF3 reduction system for reduction to obtain a corresponding product, namely the 8-benzyl-2,8-diazabicyclo[4,3,0]nonane or (S,S)-8-benzyl-2,8-diazabicyclo[4,3,0]nonane. The method adopts the metal borohydride / BF3 reduction system for reduction, avoids the use of an expensive and dangerous reagent, namely lithium aluminum hydride, reduces production cost, improves the safety of the operation, and provides a safe and economical production method for industrial mass production of a moxifloxacin intermediate, namely the (S,S)-8-benzyl-2,8-diazabicyclo[4,3,0]nonane.
Owner:ZHEJIANG LIAOYUAN PHARM CO LTD
Benzoate salt of 4-(5-methyl-oxazolo[4,5-b]-pyridin-2-yl)-1,4-diazabicyclo[3.2.2]nonane
The present invention provides a benzoate salt of Formula I:Formula I is also known as 4-(5-methyloxazolo[4,5-b]pyridine-2-yl)-1,4-diazabicyclo[3.2.2]nonane. The benzoate salt of the invention is useful in the treatment of schizophrenia and Alzheimer's Disease. It is particularly of use in the treatment of cognitive deficits associated with schizophrenia, cognitive and attention deficit symptoms of Alzheimer's Disease, and neurodegeneration associated with Alzheimer's Disease.
Owner:PFIZER INC
Preparation of antimicrobial formulations using 7-oxa-2-thia-1,5-diazabicyclo[3.3.1]nonane-2,2-dione
ActiveUS7820651B2Improve efficacySimple wayOrganic chemistryPharmaceutical delivery mechanismNonaneMedicine
Use of 7-oxa-2-thia-1,5-diazabicyclo[3.3.1]nonane-2,2-dione (“cyclotaurolidin”) for the preparation of antimicrobial formulations, in particular antimicrobial solutions for technical or medical purposes and of aqueous lock solutions for catheters and port systems for preventing infections and sepsis of patients.
Owner:HERDEIS CLAUS +1
Compositions for chemical mechanical planarization of copper
InactiveUS6866792B2Other chemical processesSemiconductor/solid-state device manufacturingColloidal silicaNonane
The present invention relates chemical mechanical planarization (“CMP”) of copper surfaces and describes copper CMP slurries including an oxidizer, one or more hydroxylamine compounds and at least one abrasive. The hydroxylamine compositions can include hydroxylamine nitrate, hydroxylamine, hydroxylamine sulfate, hydroxyl ammonium salts and mixtures thereof. The oxidizers may further include citric acid as a complexing agent for copper. Sulfuric acid and / or nitric acid provide means for modifying the pH of the oxidizer so that the hydroxylamine chemistries are acidic. Some embodiments include corrosion inhibitors such as benzotriazole, 2,4-pentadione dioxime and / or 1,6-dioxaspiro[4,4] nonane 2,7-dione. Some embodiments also include a free radical inhibitor, advantageously hydrazine. Colloidal silica and milled alumina are used as typical abrasive components.
Owner:DUPONT AIR PRODS NANOMATERIALS +1
Solvent for treating polysilazane and method of treating polysilazane with the solvent
InactiveUS7344603B2Improve abilitiesAvoid adversely affecting the properties of a groundSemiconductor/solid-state device manufacturingDetergent compounding agentsAlkaneNonane
Polysilazane is treated with a single or mixed solvent comprising one or more members selected from the group consisting of xylene, anisole, decalin, cyclohexane, cyclohexene, methylcyclohexane, ethylcyclohexane, limonene, hexane, octane, nonane, decane, a C8-C11 alkane mixture, a C8-C11 aromatic hydrocarbon mixture, an aliphatic / alicyclic hydrocarbon mixture containing 5 to 25% by weight of C8 or more aromatic hydrocarbons, and dibutyl ether, wherein the number of 0.5 micron or more fine particles contained in 1 ml of the solvent is 50 or less. As the treatment of polysilazane, there are illustrated, for example, edge-rinsing and back rinsing of a polysilazane film formed by spin coating polysilazane on a semiconductor substrate. The water content of the solvent is preferably 100 ppm or less.
Owner:MERCK PATENT GMBH
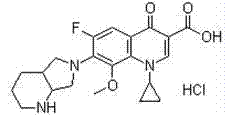
Preparation method of moxifloxacin hydrochloride
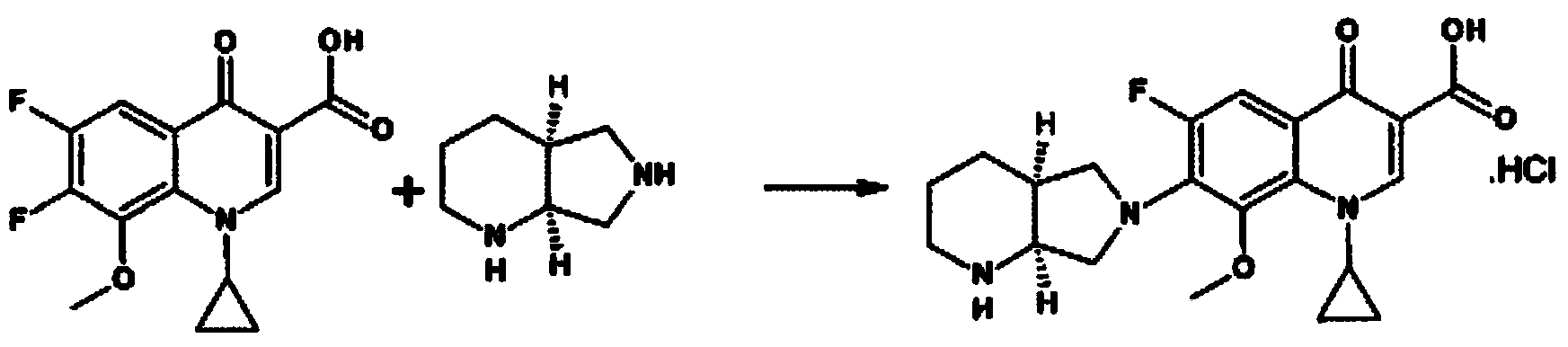
The invention discloses a preparation method of moxifloxacin hydrochloride, comprising the steps of: reacting the 1-cyclopropyl-6,7-difluoro-8-methoxy-4-oxo-1,4-dihydro-3-quinoline carboxylic acid and the S,S-2,8-diazabicyclo[4.3.0]nonane to prepare the moxifloxacin in the presence of organic base in organic solvent under the reaction temperature of 60 to 85 DEG C; separating the moxifloxacin, processing the moxifloxacin by concentrated hydrochloric acid in organic solvent under the reaction temperature of 60 to 85 DEG C to obtain the moxifloxacin hydrochloride.
Owner:JIANGSU CHIA TAI FENGHAI PHARMA
Alcohol oxidation catalyst and its preparation process
InactiveUS20080221331A1Higher catalytic turnoverLow costOrganic compound preparationCarbonyl compound preparationNonaneNitrogen
Owner:NISSAN CHEM IND LTD
Environmental-friendly drier and printing ink composition thereof
The invention discloses an environmental-friendly drier, a preparation method thereof and applications thereof. The drier is characterized in that: a reaction product of a ligand 3,7-diazabicyclo[3.3.1] nonane and a metal chloride is in a mixed crystal type or a solid-state solution type; and beneficial effects of the drier are that: the drier is free from metal cobalt having risks of "three cause", and has little bad influence on the environment and health; the drier is used for oxidation polymerization drying-type printing ink; and the ink film surface is free from skinning and wrinkling even when the printing ink layer is thick. According to the drier and the preparation method, the ratio of iron to manganese in a complex and the constitution of the ligand can be adjusted according to the drying condition of the printing ink so as to achieve an effect of adjusting the drying time of the printing ink. The ligand 3,7-diazabicyclo[3.3.1] nonane has a general chemical formula shown as follows.
Owner:CHINA BANKNOTE INK
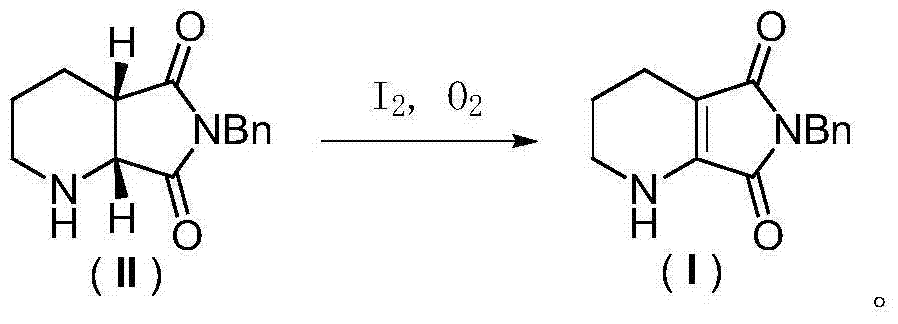
Process for producing racemic cis-8-benzyl-7, 9-dioxo-2, 8-diazabicyclo [4.3.0] nonyl hydride
InactiveCN101429199ANo sabotage involvedFast dehydrogenation reactionOrganic chemistryChemical recyclingNonaneDehydrogenation
The invention discloses a method for preparing racemic cis-8-benzyl-7, 9-dioxo-2, 8-diazabicyclo[4.3.0]nonane, which relates to a racemization method for (1R,6S)-cis-8-benzyl-7,9-dioxo-2,8-diazabicyclo[4.3.0]nonane. Firstly, the (1R,6S)-cis-8-benzyl-7,9-dioxo-2,8-diazabicyclo[4.3.0]nonane is refluxed for 3 to 10h in the presence of a manganese dioxide catalyst and a dehydrogenation solvent at a temperature of between 60 and 110 DEG C for an oxydehydrogenation reaction, and then obtained 6-benzyl-5,7-dioxo-1,2,3,4-tetrahydro-pyrido[3,4-b]pyridine reacts for 5 to 10 h in the presence of a Pd / C or PtO2 catalyst and a hydrogenation solvent at a temperature of between 70 and 100 DEG C at the pressure of between 5 and 9MPa and is reduced into the racemic cis-8-benzyl-7, 9-dioxo-2, 8-diazabicyclo[4.3.0]nonane. The method has the advantages of simple process, quick reaction, more than 90 percent of two-step overall yield, mild conditions, simple treatment after the reaction, no secondary reactions, and reutilized catalyst, and meets the requirement of environmental protection. The obtained product is the racemic cis-8-benzyl-7, 9-dioxo-2, 8-diazabicyclo[4.3.0]nonane, fully avoids the generation of trans-products, can be directly used for optical resolution, and remarkably improves the utilization rate of raw materials.
Owner:EAST CHINA NORMAL UNIV
Solvent for treating polysilazane and method of treating polysilazane with the solvent
InactiveUS20050027089A1Excellent propertyImprove trimming effectSemiconductor/solid-state device manufacturingDetergent compounding agentsAlkaneNonane
Polysilazane is treated with a single or mixed solvent comprising one or more members selected from the group consisting of xylene, anisole, decalin, cyclohexane, cyclohexene, methylcyclohexane, ethylcyclohexane, limonene, hexane, octane, nonane, decane, a C8-C11 alkane mixture, a C8-C11 aromatic hydrocarbon mixture, an aliphatic / alicyclic hydrocarbon mixture containing 5 to 25% by weight of C8 or more aromatic hydrocarbons, and dibutyl ether, wherein the number of 0.5 micron or more fine particles contained in 1 ml of the solvent is 50 or less. As the treatment of polysilazane, there are illustrated, for example, edge-rinsing and back rinsing of a polysilazane film formed by spin coating polysilazane on a semiconductor substrate. The water content of the solvent is preferably 100 ppm or less.
Owner:MERCK PATENT GMBH
Method for preparing high-purity moxifloxacin hydrochloride
The invention discloses a method for preparing high-purity moxifloxacin hydrochloride. The method includes the following steps: boric acid and acetic anhydride are adopted and subjected to a catalytic heating reaction at the presence of aluminium trichloride to generate B(OAc)3; B(OAc)3 and 1-cyclopropyl-6, 7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic acid ethyl ester are subjected to a heating reaction to obtain chelate; chelate and S,S-2,8-diazabicyclo[4.3.0] nonane are subjected to a nucleophilic substitution reaction at the presence of Et3N; after the reaction, a mixed solvent of petroleum ether, ethyl acetate and N, N-dimethyl formamide is used to remove impurities, and ethyl alcohol and chlorhydric acid treatments are carried out; cooling crystallization is carried out to obtain the moxifloxacin hydrochloride crude product; ethyl alcohol and an aqueous solution are used for crystallization according to the fact that the volume ratio of ethyl alcohol to the aqueous solution is 1:3, so that the high-purity moxifloxacin hydrochloride is obtained. According to the invention, the route is simple and convenient, the operation and post-treatment are simple, the yield is relatively high, the purity is high, and the method is suitable for industrialized production.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Welding and cutting gas and preparation method thereof
InactiveCN103320190AEasy to prepareNo pollution in the processGaseous fuelsNonaneRare earth metal compounds
The invention discloses a welding and cutting gas which consists of a natural gas and a gain agent, wherein the gain agent consists of a rare earth metal compound, a transition metallocene compound, methanol, toluene, methyl tertiary butyl ether, cyclohexane, nonane, decane and water. Compared with acetylene and propane, the welding and cutting gas is more economical, more energy-saving, more environment-friendly, safer and cleaner. The invention also provides a preparation method of the welding and cutting gas.
Owner:CANGXI COUNTY CHASE GAS INVESTMENT
Electrolytic solution for electric double layer capacitor, electric double layer capacitor using the same, and manufacturing method therefor
InactiveUS20120044614A1Reduce functionImprove leakageHybrid capacitor electrolytesHybrid capacitor electrodesNonaneSulfolane
Provided are an electrolytic solution for an electric double layer capacitor capable of providing an electric double layer capacitor having stable quality, an electric double layer capacitor using the electrolytic solution, and a manufacturing method for the electric double layer capacitor. The electrolytic solution includes a supporting electrolyte, sulfolane, and a linear sulfone. It is preferred that the electrolytic solution further include an organic fluorine compound. Further, it is preferred that the supporting electrolyte contain 5-azoniaspiro[4.4]nonane tetrafluoroborate, and the content of 5-azoniaspiro[4.4]nonane tetrafluoroborate be 1.5 to 3.6 mol / dm3.
Owner:SEIKO INSTR INC
Synthesis method of high-purity moxifloxacin hydrochloride
The invention provides a synthesis method of high-purity moxifloxacin hydrochloride, which comprises the following steps: protecting carbonyl of ethyl 1-cyclopropyl-6,7-difluoro-8-methoxy-1,4-dihydro-4-oxoquinolyl-3-carboxylate by using ethyl borate; performing condensation with (S,S)-2,8-diazabicyclo[4.3.0]nonane; deprotecting with hydrochloric acid to obtain a moxifloxacin hydrochloride crude product; dissolving the obtained moxifloxacin hydrochloride crude product with an alkali water solution, extracting with organic solvent, removing the organic phase, and adding hydrochloric acid into the water phase to regulate the pH value to acidity; crystallizing, filtering to obtain a filter cake, and recrystallizing the obtained filter cake with water-containing organic solvent; and finally, filtering, and drying to obtain the high-purity moxifloxacin hydrochloride. The product prepared by the method has the advantages of high purity, high yield and favorable reproducibility, and has very high industrial operation feasibility.
Owner:天津康鸿医药科技发展有限公司
Racemization method of (1R, 6S)-8-benzyl-7, 9-dioxo-2, 8- diazabicyclo (4, 3, 0) nonane
The invention relates to the field of a medical technology, and discloses a racemization method of (1R, 6S)-8-benzyl-7, 9-dioxo-2, 8- diazabicyclo (4, 3, 0) nonane. The method comprises the steps of (1) in ether solvent or polar aprotic solvent, carrying out oxidative dehydrogenation on the (1R, 6S)-8-benzyl-7, 9-dioxo-2, 8-diazabicyclo (4, 3, 0) nonane by taking elemental iodine and oxygen as co-oxidant to prepare 6-benzyl-5, 7-dioxo-1, 2, 3, 4-tetrahydron-pyrrolo (3, 4-b) pyrrole; (2) in ester solvent or alcohol solvent, carrying out catalytic hydrogenation on oxidative product by taking Pd / Al2O3 as a catalyst to obtain the racemic cis-8-benzyl-7, 9-dioxo-2, 8-diazabicyclo (4, 3, 0) nonane. The method is simple in technology, convenient to operate, mild in reaction conditions, etc.
Owner:SHAOXING UNIVERSITY
Environment-friendly transparent dewaxing liquid, preparation method thereof and application of environment-friendly transparent dewaxing liquid in preparing pathological section
The invention belongs to the field of pathological detection reagents, and particularly relates to environment-friendly transparent dewaxing liquid, a preparation method thereof and application of the environment-friendly transparent dewaxing liquid in preparing a pathological section. The environment-friendly transparent dewaxing liquid is prepared from the following raw materials by a mass ratio: 20-60% of white oil, 12-45% of nonane, 6-35% of octane, 5-20% of heptane, 4-25% of castor oil, 3-18% of pentanol and 2-9% of hexanol. The dewaxing liquid disclosed by the invention is simple in formula and reasonable in collocation, and can serve as a clarifier and a dewaxing agent used during preparation of the pathological section, so that a pathological section process is completely free from a xylene reagent.
Owner:河南新大阳生物技术有限公司
Biogenic Turbine And Diesel Fuel
The present invention provides fully renewable turbine and diesel fuels derived completely from biomass sources. In one embodiment the fully renewable turbine fuel is comprised of mesitylene and at least one alkane. Preferably, the turbine fuel comprises from about 50 to 99 wt % mesitylene and from about 1 to 50 wt % of at least one alkane. In another embodiment the diesel fuel comprises mesitylene, octadecane, and optionally octane or nonane. Preferably, the diesel fuel comprises from about 50 to 99 wt % mesitylene, and from about 1 to 50 wt % octadecane. These biomass derived fuels may be formulated to have a wide range of cetane values and differing freezing and boiling points.
Owner:SWIFT ENTERPRISES
Preparation method for liquid polyborosilazane
The invention relates to a preparation method for liquid polyborosilazane and belongs to the field of inorganic nonmetallic materials. The preparation method comprises the following steps: liquid polyborosilazane and 9-borabicyclo [3,3,1]-nonane are added in a solvent in inert atmosphere to obtain mixed solution, then the obtained mixed solution is hydroborated under the stirring condition, and then the solvent is removed after completion of hydroboration to obtain the liquid polyborosilazane. The preparation method solves the problems in the traditional polymer route that excessive cross-linking is easily caused in the hydroboration addition reaction, and solid polyborosilazane has poorer fluidity. The prepared liquid polyborosilazane has good fluidity and high ceramic yield and can be directly used for the high polymer infiltration and pyrolysis method for preparing SiBCN ceramic matrix composites.
Owner:XIAMEN UNIV
Mixed decyl mercaptans compositions and use thereof as mining chemical collectors
Disclosed herein is a process for the recovery of a metal from an ore using a collector composition. The process includes contacting the ore with the collector composition. The collector composition can include sulfur-containing compounds comprising (i) mercaptans comprising branched C10 mercaptans compounds selected from the group consisting of 5-methyl-1-mercapto-nonane, 3-propyl-1-mercapto-heptane, 4-ethyl-1-mercapto-octane, 2-butyl-1-mercapto-hexane, 5-methyl-2-mercapto-nonane, 3-propyl-2-mercapto-heptane, 4-ethyl-2-mercapto-octane, 5-methyl-5-mercapto-nonane, and combinations thereof; and (ii) sulfides comprising branched C20 sulfides represented by the structure R1—S—R2, wherein R1 and R2 are each independently a functional group derived from an olefin, wherein the olefin comprises 5-methyl-1-nonene, 3-propyl-1-heptene, 4-ethyl-1-octene, 2-butyl-1-hexene, or combinations thereof.
Owner:CHEVRON PHILLIPS CHEMICAL CO LP
Biogenic Turbine And Diesel Fuel
ActiveUS20110230686A1Increase energy contentHydrocarbon purification/separationBiofuelsAlkaneBoiling point
The present invention provides fully renewable turbine and diesel fuels created from biomass sources. In one embodiment, the fully renewable turbine fuel is comprised of mesitylene and at least one alkane. Preferably, the turbine fuel comprises from about 50 to 99 wt % mesitylene and from about 1 to 50 wt % of at least one alkane. In another embodiment the diesel fuel comprises mesitylene, octadecane, and optionally octane or nonane. Preferably, the diesel fuel comprises from about 50 to 99 wt % mesitylene, and from about 1 to 50 wt % octadecane. These biomass derived fuels may be formulated to have a wide range of cetane values and differing freezing and boiling points. A preferred biogenic turbine fuel comprises one or more synthetic paraffinic kerosenes (SPK) and / or hydroprocessed renewable jet (HRJ) fuel; and between about 8 to 25 vol % of mesitylene. Another preferred biogenic turbine fuel is a blend of about 50% petroleum-based fuel; and about 50% of one or more of synthetic paraffinic kerosenes (SPK) and / or hydroprocessed renewable jet fuel (HRJ), and mesitylene.
Owner:SWIFT ENTERPRISES
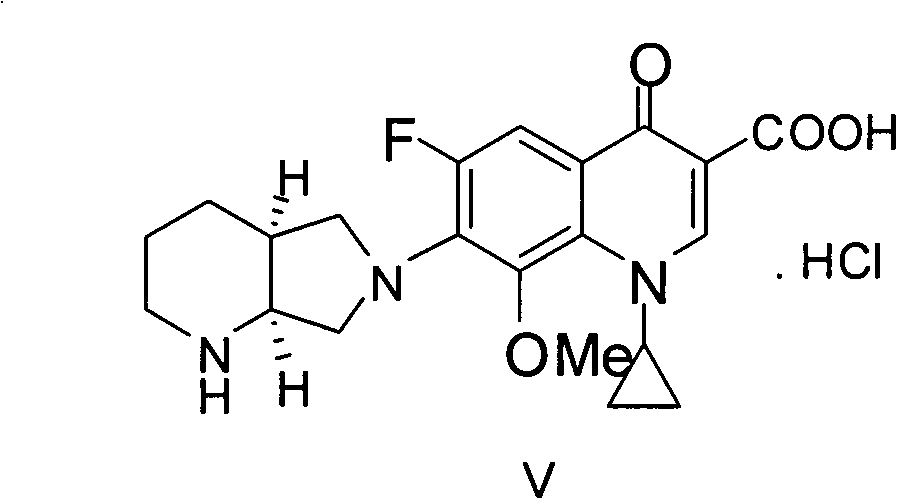
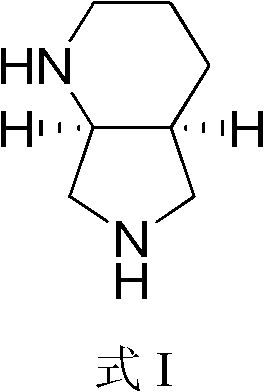
Preparation method of (S, S)-2, 8-diazabicyclo[4, 3, 0]nonane
The invention discloses a preparation method of (S, S)-2, 8-diazabicyclo[4, 3, 0]nonane and belongs to the field of the preparation method of a moxifloxacin intermediate. The preparation method comprises eight processes. A resolving process is carried out in initial of the preparation method, in the third process, an ester is resolved to form a chiral intermediate and then through a simple chemical reaction, the (S, S)-2, 8-diazabicyclo[4, 3, 0]nonane is prepared. The invention provides the preparation method utilizing an effective and economic synthesis route to prepare the high-chiral purity (S, S)-2, 8-diazabicyclo[4, 3, 0]nonane. The preparation method is free of an expensive resolving agent and greatly reduces a process cost. In the whole technology, the intermediate is not purified and the crude product can be directly used. The preparation method has simple processes and a high overall yield and can produce the product with chiral purity of 99%.
Owner:HEADING NANJING PHARMTECH CO LTD
Preparation method of moxifloxacin hydrochloride
The invention relates to a preparation method of moxifloxacin hydrochloride, which comprises the steps of: making 1-cyclopropyl-6,7-difluoro-8-methoxyl-1,4-dihydro-4-oxoquinoline-3-carboxylate-03,04-broron ester acetate and (S,S)-2,8-diazabicyclo[4.3.0] nonane as raw materials completely react in a solvent, then cooling, adding hydrochloric acid, regulating the pH value to 4-6, stirring and crystallizing for more than 10 minutes, then adding the hydrochloric acid, regulating pH value to 0.5-2, cooling to 0-40 DEG C, crystallizing, leaching, washing, and drying to prepare the moxifloxacin hydrochloride. The preparation method has the advantages of simple process, low cost, high yield and high purity, and is more suitable for industrialized production.
Owner:SICHUAN GOWELL PHARMA
Method for chiral synthesis of (S,S)-2-8-diazabicyclononane
The invention provides a method for chiral synthesis of (S,S)-2-8-diazabicyclo[4.3.0]nonane. A target product with a desired structure is prepared by using pyridine 2,3-diformate as a raw material and carrying out a four-step reaction comprising hydrogenation, resolution, aminolysis and reduction. According to the method, a synthetic route is simple and short, the step of resolution is carried out at an early stage, and reaction raw materials and reagents are saved; thus, production cost is reduced, and production efficiency is improved.
Owner:CHONGQING HUABANGSHENGKAI PHARM
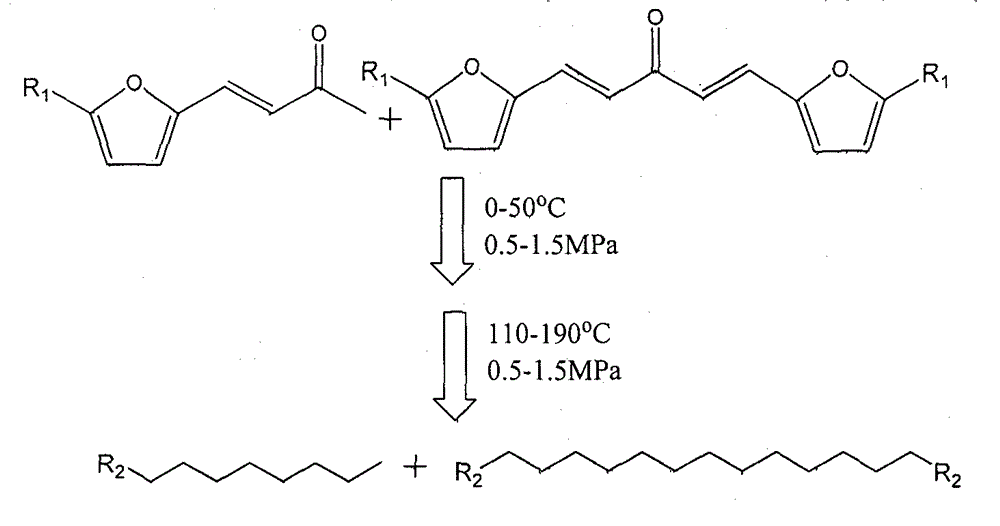
Novel technique for preparing long-chain alkane efficiently through multifunctional catalyst in one-step method
ActiveCN102850157BRedox activeExtend your lifeLiquid hydrocarbon mixture productionHydrocarbonsAlkaneNonane
The invention relates to a novel technique for preparing long-chain alkane efficiently through a multifunctional catalyst in a one-step method. The novel technique can be used for producing the long-chain alkane in one step with high selectively under a relative mild condition, and solve the problems of strict reaction condition, low energy efficiency and low alkane selectivity in a process of preparing the long-chain alkane by biomass derivatives. According to the technique, condensation products (C8, C9, C13 and C15) of a biomass derivative, namely furfural or HMF (Hydroxy Methyl Furfural), and acetone are taken as the raw materials, and by designing the three-center multifunctional catalyst of metal (I)-metal (II)-acid, the original two steps of independent reactions which require strict reaction conditions and need different catalysts to join in are combined into an one-step reaction which requires tender reaction conditions, so that the selectivity on corresponding alkane (octane, nonane, tridecane and pentadecane) is improved greatly, the highest yield can be 97%, and meanwhile, a step of separating the product from the catalyst is omitted, therefore, the energy efficiency of the while process is improved by a great step.
Owner:EAST CHINA UNIV OF SCI & TECH
One-dimensional organic semiconductor nano material and preparation method and application thereof
InactiveCN108586456AHigh fluorescence quantum yieldExcellent photoelectric propertiesOrganic chemistryFluorescence/phosphorescenceQuantum yieldNonane
The invention discloses a one-dimensional organic semiconductor nano material and a preparation method and an application thereof. The material employs a unsymmetrical perylene bisimide derivative monomer as a construction monomer, hydrophilic branched-chain alkyl (5-nonane) and pentafluorophenyl substituent are respectively introduced at two sides of perylene bisimide, and the one-dimensional organic semiconductor nano material can be obtained by self assembly preparation through Pi-Pi interacting of construction monomers. The one-dimensional organic semiconductor nano-material has the advantages of high fluorescence quantum yield, porous performance, large surface area, and good stability, greatly reduces the detection lowest limitation, has good anti-interference capability for an aggregate such as triphosgene and other common gases and organic solvent steam, and realizes specific and high-sensitivity detection on phosgene.
Owner:YANGTZE NORMAL UNIVERSITY
Novel preparation method of moxifloxacin hydrochloride
ActiveCN104230925AReduce generationReduce complicated stepsGroup 3/13 element organic compoundsNonaneEvaporation
The invention provides a method for preparing a moxifloxacin intermediate and moxifloxacin hydrochloride, which comprises the following steps: carrying out condensation reaction on main ring chelate disclosed as Formula (I) and (S,S)-2,8-diazabicyclo-[4.3.0]nonane in the presence of an acid acceptor in a solvent, acidifying for salification, crystallizing, filtering, washing and drying to obtain the moxifloxacin hydrochloride. The method is characterized in that the solvent in the condensation reaction is alcohol. The condensation reaction is carried out in the alcohol solvent at the controlled temperature of 30-80 DEG C (preferably lower temperature), so the method has the advantage of mild reaction conditions, greatly reduces the generation of impurities, saves the energy; the alcohol solvent can be directly acidified after sufficient reaction, thereby saving the complex step of removing acetonitrile by evaporation and greatly simplifying the steps; and the method is suitable for industrial production.
Owner:JIANGSU TIANYISHI PHARMA
Mixed decyl mercaptans compositions and use thereof as mining chemical collectors
Owner:CHEVRON PHILLIPS CHEMICAL CO LP
Processes for preparing 6,7,8-trihydroxy-1-(hydroxymethyl)-3-oxo-2-oxa-4-azabicyclo[3.3.1]nonane
Provided are processes for preparing 6,7,8-trihydroxy-1-(hydroxymethyl)-3-oxo-2-oxa-4-azabicyclo[3.3.1]nonane and processes for preparing valiolamine or its derivative using the same.
Owner:YUHAN
Method for industrial production of moxifloxacin side chain
InactiveCN103275078AReduce manufacturing costSuitable for industrial productionOrganic chemistryNonaneSide chain
The invention discloses a method for industrial production of moxifloxacin side chain. The method comprises the steps of A, dissolving 3-aldehyde pyridine-2-carboxylic acid and benzylamine in a mixed solvent of distilled water and an organic solvent, putting the above solution into an autoclave to carry out catalytic hydrogenation, and thus 6-benzyl-hexahydro-pyrrolo[3,4-b] pyridine-7-one is obtained; B. dissolving 6-benzyl-hexahydro-pyrrolo[3,4-b] pyridine-7-one in an organic solvent, adding a reducing agent in the solution to carry out a reduction reaction, and thus 6-benzyl-octahydro pyrrolo[3,4-b]pyridine is obtained; C. dissolving 6-benzyl-octahydro pyrrolo[3,4-b]pyridine in an organic solvent, adding D-(-)-tartaric acid to carry out resolution, and thus (s, s)-6-benzyl-octahydro-pyrrolo[3,4-b]pyridine is obtained; and D. dissolving (s, s)-6-benzyl-octahydro-pyrrolo[3,4-b]pyridine in an organic solvent, putting the solution in the autoclave to carry out catalytic hydrogenation for debenzylation, and thus (S,S)-2,8-diazabicyclo[4.3.0]nonane is obtained. The technical solution provided by the invention is simple and practical, low in cost and high in production efficiency, and is suitable for industrialized production.
Owner:SUZHOU MICRODIAG BIOLOGICALS
Method for preparing imine compound from alcohol and amine through catalytic oxidation
InactiveCN106938976ASimple and safe operationLow costSulfide preparationImino compound preparationNonaneOrganic solvent
The invention discloses a method for preparing an imine compound from alcohol and amine through catalytic oxidation. According to the method, an alcohol compound and an amine compound are used as reaction substrates, and a feeding mol ratio of the alcohol compound to the amine compound is 100: 100-60; 9-azabicyclo[3.3.1]nonane N-oxyl free radical is used as a catalyst, potassium hydroxide is used as an auxiliary agent, and a feeding mol ratio of the amine compound to the 9-azabicyclo[3.3.1]nonane N-oxyl free radical to potassium hydroxide is 100: 1-6: 10-50; air is used as an oxidizing agent, the reaction substrates are added into an organic solvent, and the mass of the used organic solvent is 2.5 to 5 times of the reaction substrate the amine compound; and a reaction is carried out at normal pressure at a temperature of 70 to 110 DEG C for 2 to 12 h, and aftertreatment is carried out after completion of the reaction so as to obtain the imine compound. The method provided by the invention is simple and safe to operate, reduces environmental cost due to usage of clean oxygen as the oxidizing agent and prevents the problem of transition-metal pollution by discarding usage of any transition-metal catalyst.
Owner:ZHEJIANG UNIV OF TECH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Method for reducing 8-benzyl-2,8-diazabicyclo[4,3,0]nonane and chiral isomer thereof Method for reducing 8-benzyl-2,8-diazabicyclo[4,3,0]nonane and chiral isomer thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d773f92e-9397-45a1-b63c-9d0abefeecf0/A2009101002270002C1.PNG)
![Method for reducing 8-benzyl-2,8-diazabicyclo[4,3,0]nonane and chiral isomer thereof Method for reducing 8-benzyl-2,8-diazabicyclo[4,3,0]nonane and chiral isomer thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d773f92e-9397-45a1-b63c-9d0abefeecf0/A20091010022700051.PNG)
![Method for reducing 8-benzyl-2,8-diazabicyclo[4,3,0]nonane and chiral isomer thereof Method for reducing 8-benzyl-2,8-diazabicyclo[4,3,0]nonane and chiral isomer thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d773f92e-9397-45a1-b63c-9d0abefeecf0/A20091010022700052.PNG)
![Benzoate salt of 4-(5-methyl-oxazolo[4,5-b]-pyridin-2-yl)-1,4-diazabicyclo[3.2.2]nonane Benzoate salt of 4-(5-methyl-oxazolo[4,5-b]-pyridin-2-yl)-1,4-diazabicyclo[3.2.2]nonane](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c5da3b43-0470-4f0a-855b-94384f136f61/US20080045512A1-20080221-D00000.png)
![Benzoate salt of 4-(5-methyl-oxazolo[4,5-b]-pyridin-2-yl)-1,4-diazabicyclo[3.2.2]nonane Benzoate salt of 4-(5-methyl-oxazolo[4,5-b]-pyridin-2-yl)-1,4-diazabicyclo[3.2.2]nonane](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c5da3b43-0470-4f0a-855b-94384f136f61/US20080045512A1-20080221-D00001.png)
![Benzoate salt of 4-(5-methyl-oxazolo[4,5-b]-pyridin-2-yl)-1,4-diazabicyclo[3.2.2]nonane Benzoate salt of 4-(5-methyl-oxazolo[4,5-b]-pyridin-2-yl)-1,4-diazabicyclo[3.2.2]nonane](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c5da3b43-0470-4f0a-855b-94384f136f61/US20080045512A1-20080221-D00002.png)
![Preparation of antimicrobial formulations using 7-oxa-2-thia-1,5-diazabicyclo[3.3.1]nonane-2,2-dione Preparation of antimicrobial formulations using 7-oxa-2-thia-1,5-diazabicyclo[3.3.1]nonane-2,2-dione](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a4452efb-1f49-42bf-a4c9-97cef896ab24/US07820651-20101026-C00001.png)
![Preparation of antimicrobial formulations using 7-oxa-2-thia-1,5-diazabicyclo[3.3.1]nonane-2,2-dione Preparation of antimicrobial formulations using 7-oxa-2-thia-1,5-diazabicyclo[3.3.1]nonane-2,2-dione](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a4452efb-1f49-42bf-a4c9-97cef896ab24/US07820651-20101026-C00002.png)
![Preparation of antimicrobial formulations using 7-oxa-2-thia-1,5-diazabicyclo[3.3.1]nonane-2,2-dione Preparation of antimicrobial formulations using 7-oxa-2-thia-1,5-diazabicyclo[3.3.1]nonane-2,2-dione](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a4452efb-1f49-42bf-a4c9-97cef896ab24/US07820651-20101026-C00003.png)









![Process for producing racemic cis-8-benzyl-7, 9-dioxo-2, 8-diazabicyclo [4.3.0] nonyl hydride Process for producing racemic cis-8-benzyl-7, 9-dioxo-2, 8-diazabicyclo [4.3.0] nonyl hydride](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7d7c4a43-459d-4b12-b68a-200cc23a9948/a200810042638d00031.PNG)
![Process for producing racemic cis-8-benzyl-7, 9-dioxo-2, 8-diazabicyclo [4.3.0] nonyl hydride Process for producing racemic cis-8-benzyl-7, 9-dioxo-2, 8-diazabicyclo [4.3.0] nonyl hydride](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7d7c4a43-459d-4b12-b68a-200cc23a9948/a200810042638d00041.PNG)
![Process for producing racemic cis-8-benzyl-7, 9-dioxo-2, 8-diazabicyclo [4.3.0] nonyl hydride Process for producing racemic cis-8-benzyl-7, 9-dioxo-2, 8-diazabicyclo [4.3.0] nonyl hydride](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7d7c4a43-459d-4b12-b68a-200cc23a9948/a200810042638d00051.PNG)






















![Preparation method of (S, S)-2, 8-diazabicyclo[4, 3, 0]nonane Preparation method of (S, S)-2, 8-diazabicyclo[4, 3, 0]nonane](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/02391988-7d43-4f83-9dc9-af83912b5570/HDA0000583691930000011.PNG)
![Preparation method of (S, S)-2, 8-diazabicyclo[4, 3, 0]nonane Preparation method of (S, S)-2, 8-diazabicyclo[4, 3, 0]nonane](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/02391988-7d43-4f83-9dc9-af83912b5570/HDA0000583691930000012.PNG)
![Preparation method of (S, S)-2, 8-diazabicyclo[4, 3, 0]nonane Preparation method of (S, S)-2, 8-diazabicyclo[4, 3, 0]nonane](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/02391988-7d43-4f83-9dc9-af83912b5570/HDA0000583691930000021.PNG)






![Processes for preparing 6,7,8-trihydroxy-1-(hydroxymethyl)-3-oxo-2-oxa-4-azabicyclo[3.3.1]nonane Processes for preparing 6,7,8-trihydroxy-1-(hydroxymethyl)-3-oxo-2-oxa-4-azabicyclo[3.3.1]nonane](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/4994ba3b-fdbc-4f7c-9dd7-dcd999e12ebd/A2006800116390002C1.PNG)
![Processes for preparing 6,7,8-trihydroxy-1-(hydroxymethyl)-3-oxo-2-oxa-4-azabicyclo[3.3.1]nonane Processes for preparing 6,7,8-trihydroxy-1-(hydroxymethyl)-3-oxo-2-oxa-4-azabicyclo[3.3.1]nonane](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/4994ba3b-fdbc-4f7c-9dd7-dcd999e12ebd/A20068001163900051.PNG)
![Processes for preparing 6,7,8-trihydroxy-1-(hydroxymethyl)-3-oxo-2-oxa-4-azabicyclo[3.3.1]nonane Processes for preparing 6,7,8-trihydroxy-1-(hydroxymethyl)-3-oxo-2-oxa-4-azabicyclo[3.3.1]nonane](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/4994ba3b-fdbc-4f7c-9dd7-dcd999e12ebd/A20068001163900061.PNG)