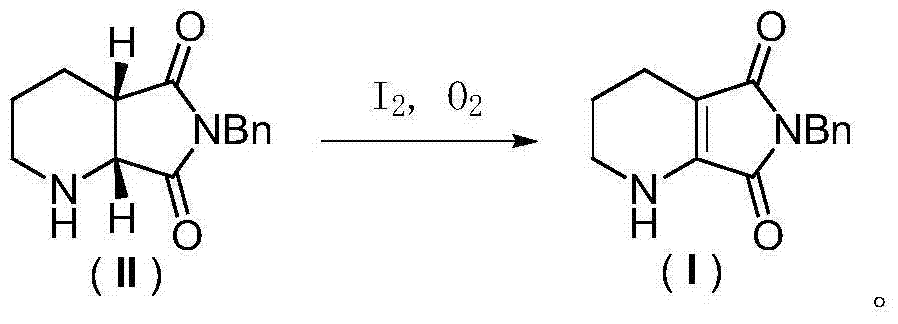
Racemization method of (1R, 6S)-8-benzyl-7, 9-dioxo-2, 8- diazabicyclo (4, 3, 0) nonane
A diazabicyclo and dioxo technology, applied in the field of medicine, can solve the problems of high energy consumption, heavy metal pollution, environmental pollution, etc., and achieve the effect of mild reaction conditions, no metal participation, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Oxidative dehydrogenation of (1R,6S)-8-benzyl-7,9-dioxo-2,8-diazabicyclo[4,3,0]nonane: Add 12.2g of (1R,6S)-8-benzyl-7,9-dioxo-2,8-diazabicyclo[4,3,0]nonane (II), 50mL dimethyl sulfoxide, 15.4g ( 1.2 equivalents) iodine element and oxygen balloon (1 bar), stirring at room temperature for 10 hours. After the reaction was completed, 100 mL of aqueous sodium thiosulfate solution with a concentration of 1 mol / L was added, the aqueous phase was extracted twice with 200 mL of ethyl acetate, the organic phases were combined and washed several times with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 11.0 g dark yellow solid. through 1 H-NMR analysis, this solid is highly pure 6-benzyl-5,7-dioxo-1,2,3,4-tetrahydro-pyrrolo[3,4-b]pyridine, and the productive rate is 91 %. product structure, 1 H-NMR and 13 C-NMR is as follows:
[0034]
[0035] 1 H-NMR (CDCl 3 ,400MHz)δppm7.29-7.22(m,5H),5.10(br s,1H),4.60(s,2H),...
Embodiment 2
[0038] Oxidative dehydrogenation of (1R,6S)-8-benzyl-7,9-dioxo-2,8-diazabicyclo[4,3,0]nonane: Add 12.2g of (1R,6S)-8-Benzyl-7,9-dioxo-2,8-diazabicyclo[4,3,0]nonane, 50 mL dimethylsulfoxide and 23.0 g (1.2 eq.) Simple iodine and oxygen balloons (1bar), stirred at room temperature for 10 hours. After the reaction was completed, 50 mL of saturated sodium thiosulfate aqueous solution was added, and the aqueous phase was extracted twice with 100 mL of ethyl acetate. The combined organic phases were washed several times with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 11.2 g of a dark yellow solid. through 1 H-NMR analysis, this solid is highly pure 6-benzyl-5,7-dioxo-1,2,3,4-tetrahydro-pyrrolo[3,4-b]pyridine, and the productive rate is 92 %.
Embodiment 3
[0040] Oxidative dehydrogenation of (1R,6S)-8-benzyl-7,9-dioxo-2,8-diazabicyclo[4,3,0]nonane: Add 12.2g of (1R,6S)-8-Benzyl-7,9-dioxo-2,8-diazabicyclo[4,3,0]nonane, 100mL ethyl acetate, 15.4g (1.2 equivalents) iodine and an oxygen bulb (1 bar), stirring at 40°C for 10 hours. After the reaction was completed, 100 mL of aqueous sodium thiosulfate solution with a concentration of 1 mol / L was added, the aqueous phase was extracted twice with 100 mL of ethyl acetate, the combined organic phases were washed once with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 8.5 g Dark yellow solid. through 1 According to H-NMR analysis, the solid was 6-benzyl-5,7-dioxo-1,2,3,4-tetrahydro-pyrrolo[3,4-b]pyridine, and the yield was 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com