Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31results about How to "No participation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
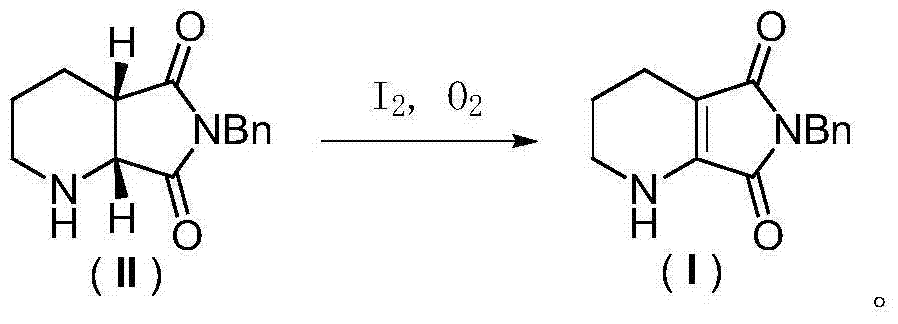
Racemization method of (1R, 6S)-8-benzyl-7, 9-dioxo-2, 8- diazabicyclo (4, 3, 0) nonane
The invention relates to the field of a medical technology, and discloses a racemization method of (1R, 6S)-8-benzyl-7, 9-dioxo-2, 8- diazabicyclo (4, 3, 0) nonane. The method comprises the steps of (1) in ether solvent or polar aprotic solvent, carrying out oxidative dehydrogenation on the (1R, 6S)-8-benzyl-7, 9-dioxo-2, 8-diazabicyclo (4, 3, 0) nonane by taking elemental iodine and oxygen as co-oxidant to prepare 6-benzyl-5, 7-dioxo-1, 2, 3, 4-tetrahydron-pyrrolo (3, 4-b) pyrrole; (2) in ester solvent or alcohol solvent, carrying out catalytic hydrogenation on oxidative product by taking Pd / Al2O3 as a catalyst to obtain the racemic cis-8-benzyl-7, 9-dioxo-2, 8-diazabicyclo (4, 3, 0) nonane. The method is simple in technology, convenient to operate, mild in reaction conditions, etc.
Owner:SHAOXING UNIVERSITY
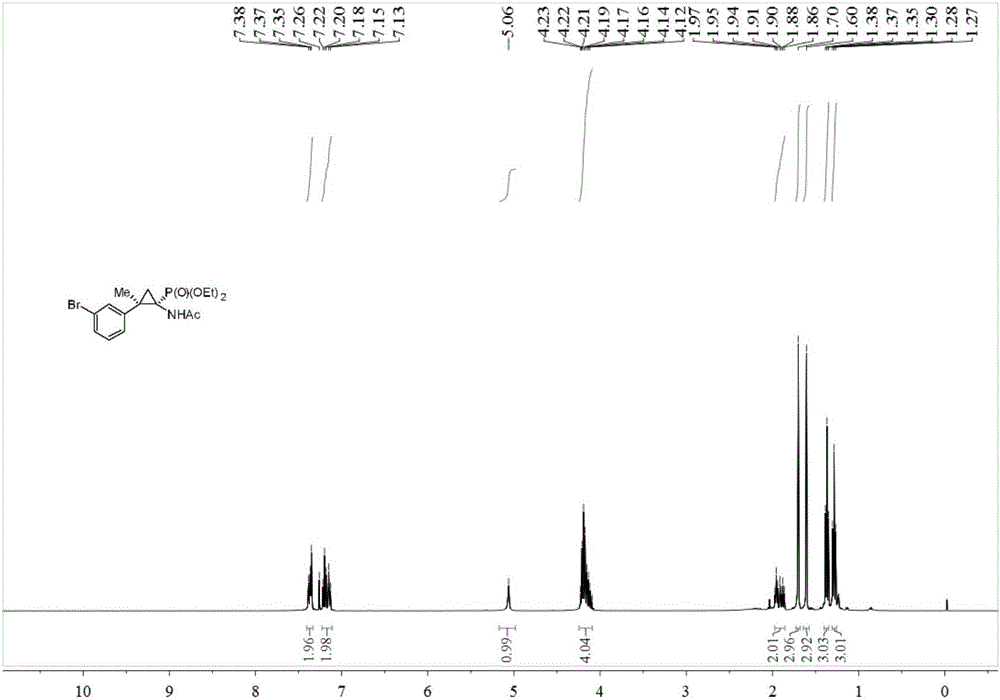
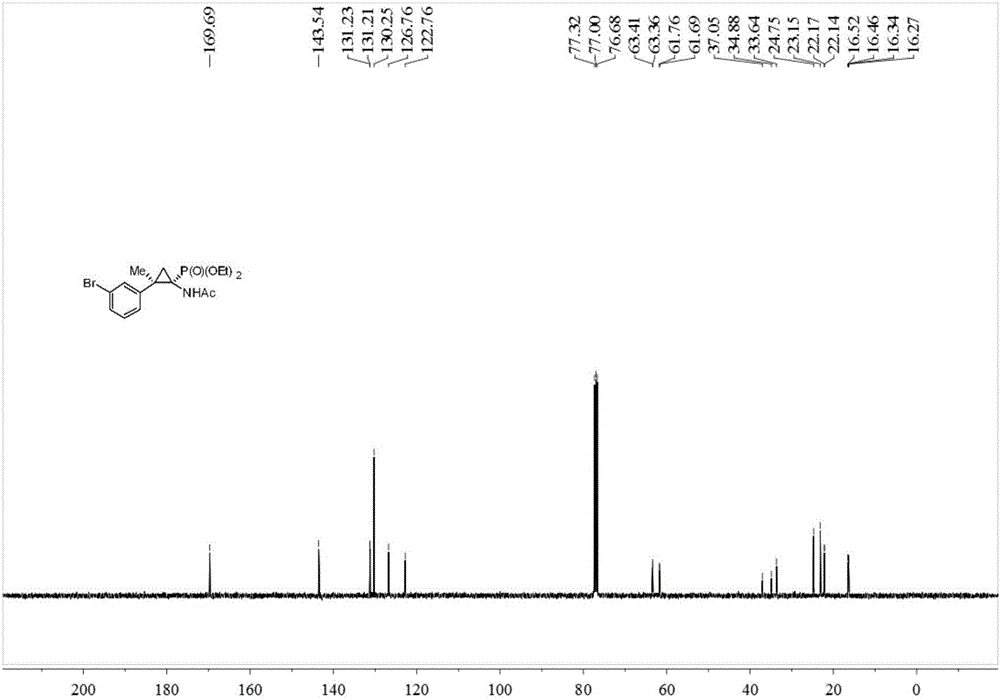
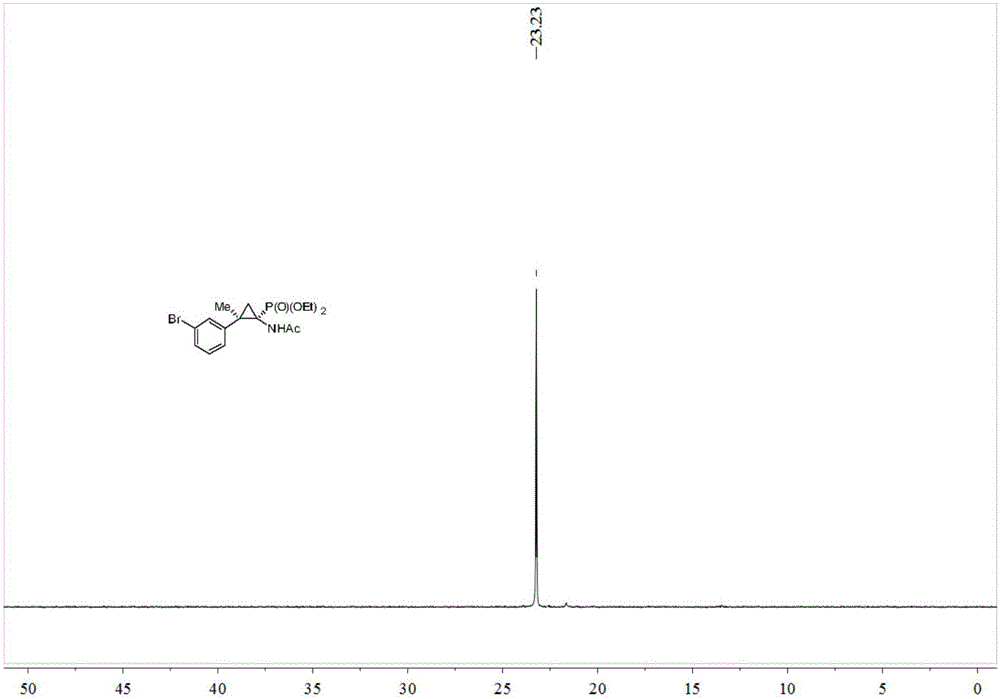
Synthesis method of cyclopropane phosphoramidate compound comprising continuous quaternary carbon center
InactiveCN106674276ANo participationRaw materials are easy to getGroup 5/15 element organic compoundsOrganic chemistry methodsChemical synthesisPhosphate
The invention belongs to the technical field of medicinal and chemical synthesis, and discloses a synthesis method of a cyclopropane phosphoramidate compound comprising a continuous quaternary carbon center. The synthesis method comprises the following steps of adding an N-tosylhydrazones compound, a phase transfer catalyst, an alkali and an organic solvent into a reactor, stirring at the room temperature for a certain period of time under the protection of inert atmosphere, then adding acetamido phosphate, heating to 60-100 DEG C, stirring to react for 10-24 hours, cooling to the room temperature after the reaction is finished, separating and purifying the product, so as to obtain the cyclopropane phosphoramidate compound comprising the continuous quaternary carbon center. According to the synthesis method, the raw materials are easy to get, the cost is low, the atom economy is high, the operation is safe and simple, no transition metal participates in the reaction, the method is environmentally friendly, is high in adaptability of the functional group and wide in adaptability of the substrate, and has an excellent industrial application prospect.
Owner:SOUTH CHINA UNIV OF TECH
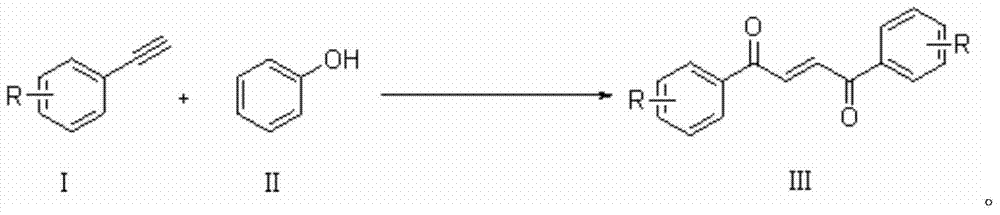
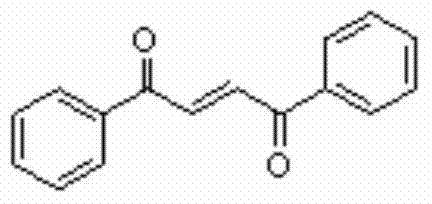
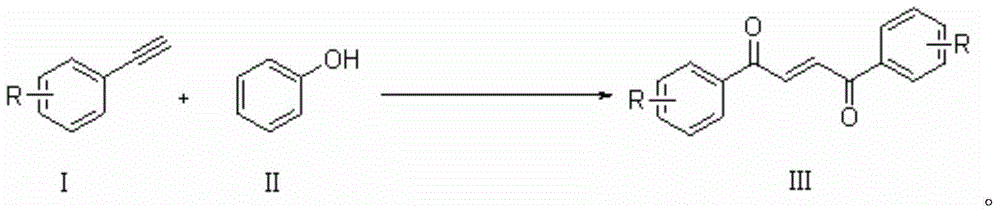
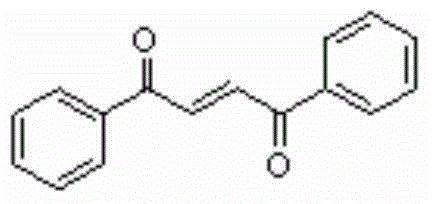
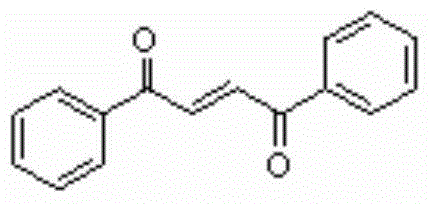
Method for synthesizing 1,4-butene diketone compound
ActiveCN104262122ASimple ingredientsRaw materials are easy to getOrganic compound preparationCarbonyl compound preparation by oxidationSolventSelectfluor
The invention belongs to the technical field of organic compound synthesis, provides a method for synthesizing a 1,4-butene diketone compound and aims to solve the problems that metal participates, materials are complex and the conditions are harsh in the method for synthesizing the 1,4-butene diketone compound at present. The synthesizing method comprises the step of reacting for 12-48h at 25-80 DEG C by using substituted aryne and phenol as initial materials, using 1-chloromethyl-4-fluoro-1,4-diazabicyclo[2.2.2] octane bi(tetrafluoroboric acid) salt (selectfluor) as an oxidant and using CH3CN as a solvent to prepare the 1,4-butene diketone compound. The invention provides a method for synthesizing the 1,4-butene diketone compound, which has the advantages that materials can be easily obtained, no metal participates and the reaction condition is moderate. The reaction formula is shown in the specification.
Owner:山东博洛德生物科技有限公司
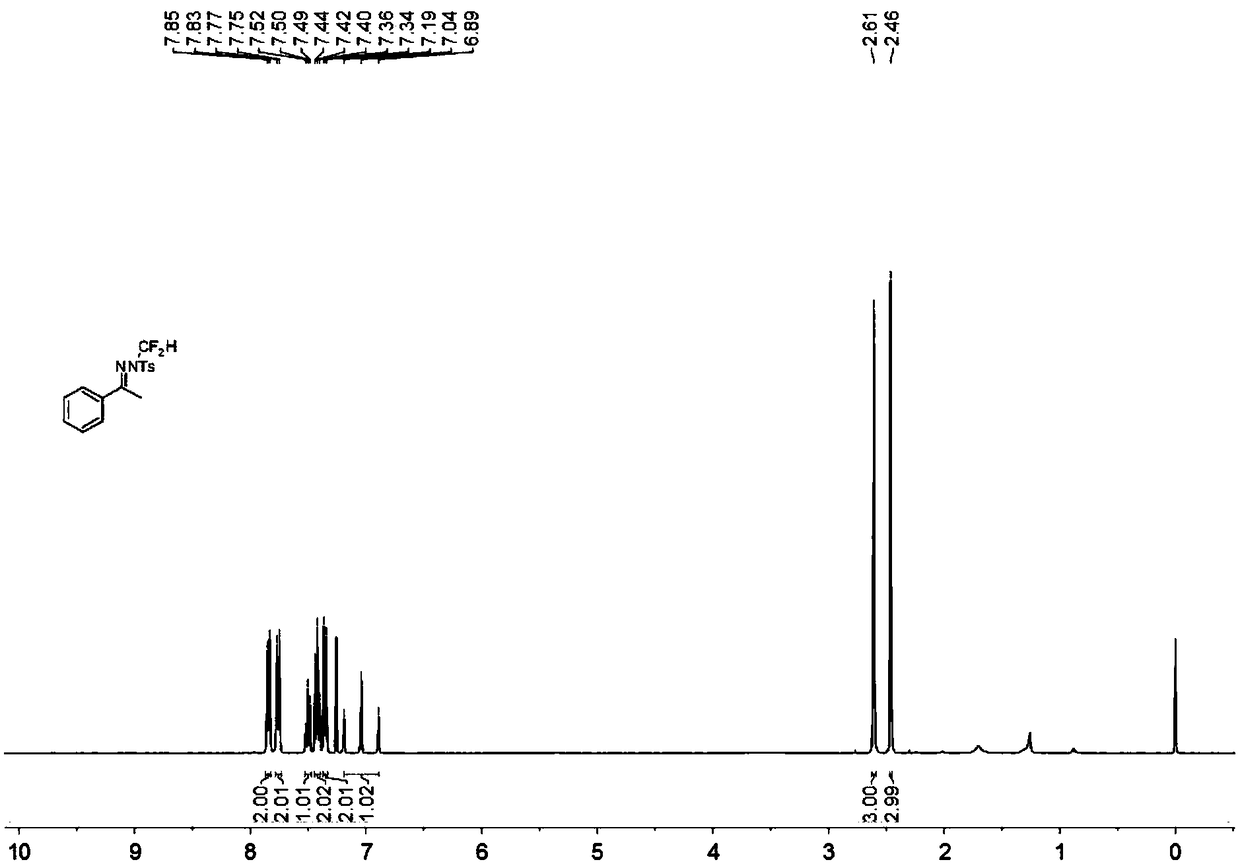
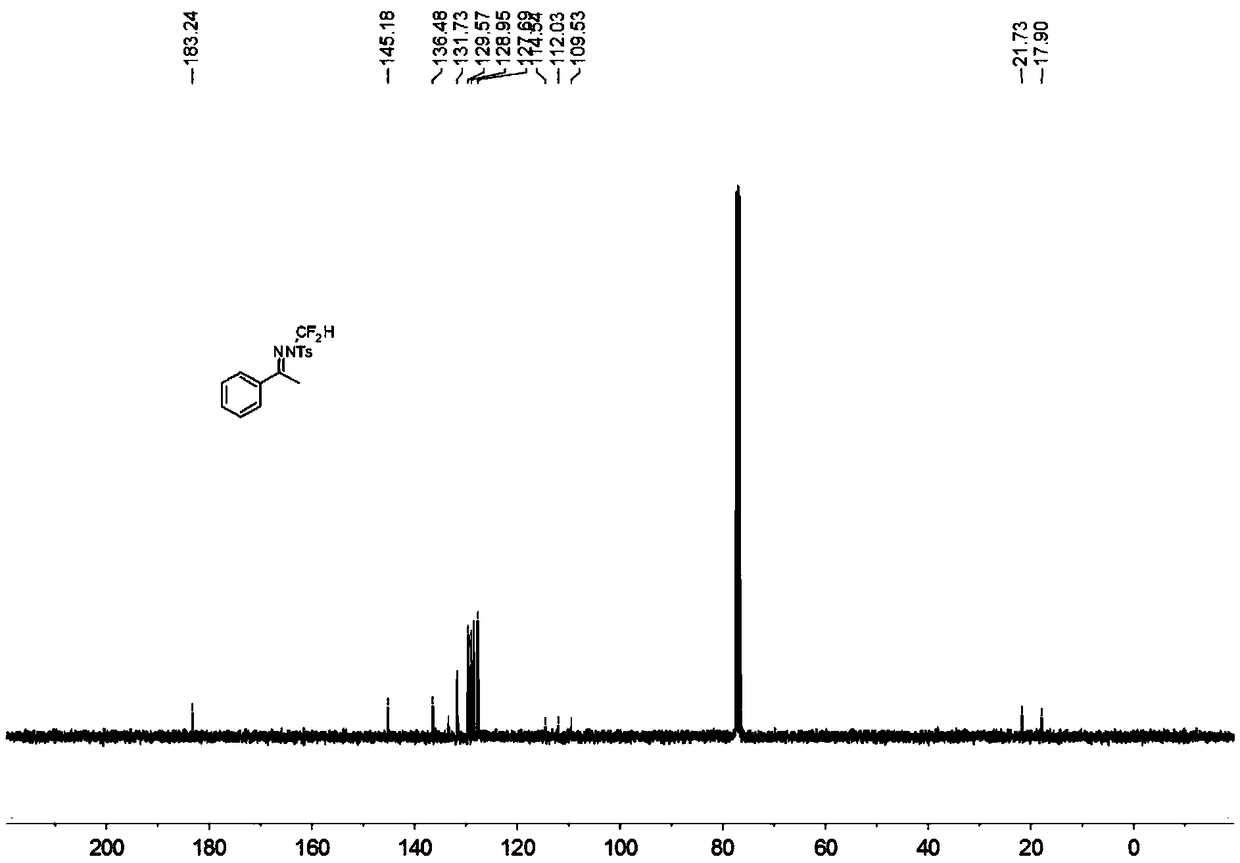
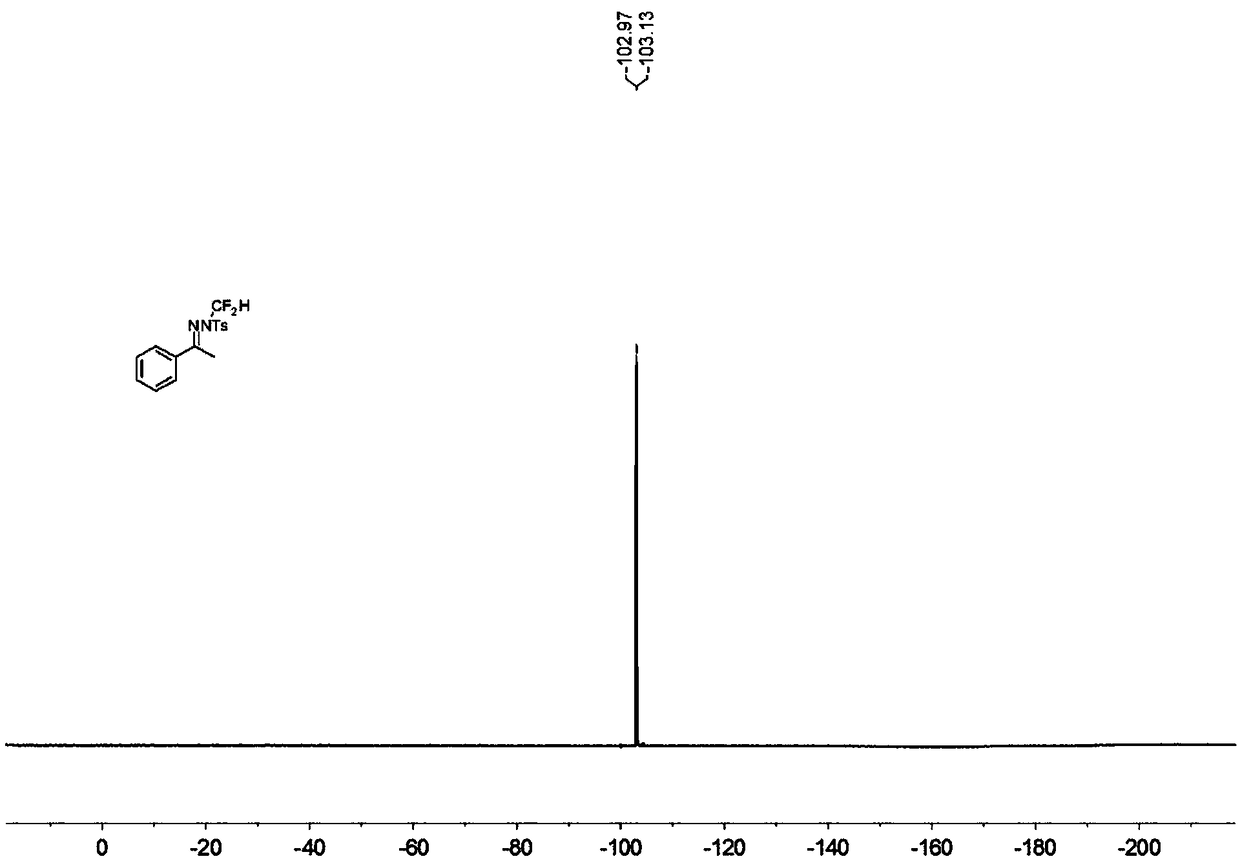
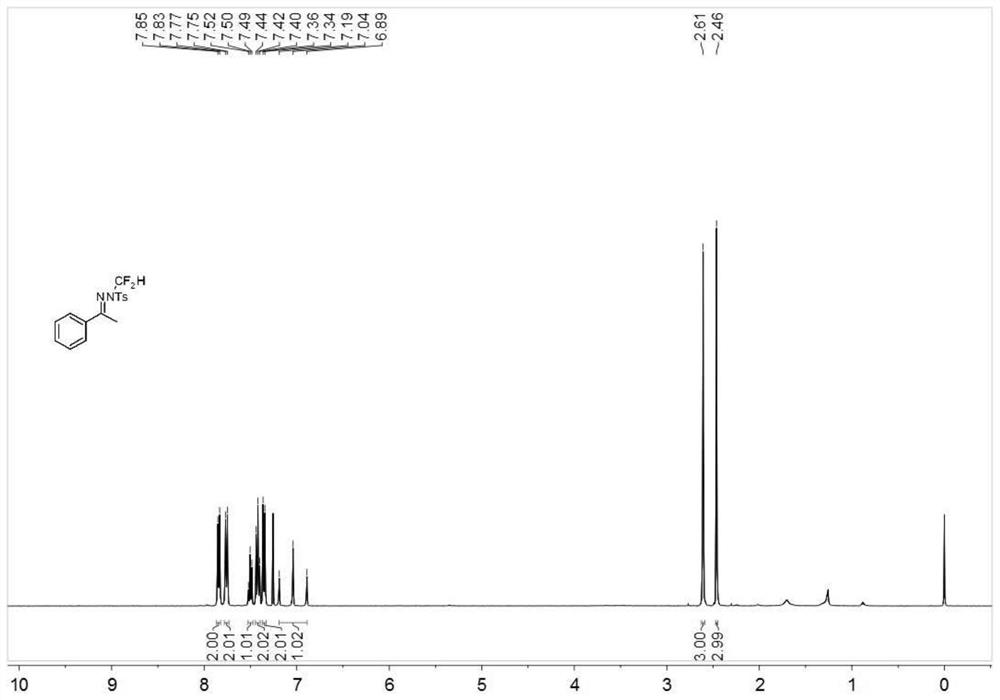
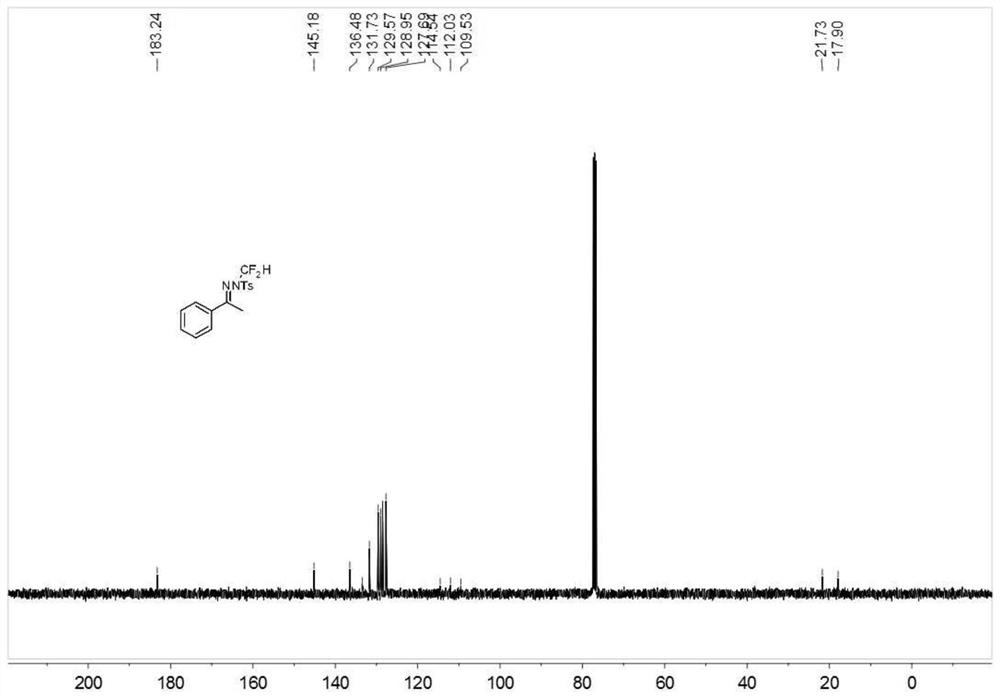
N-difluoromethyl hydrazone compound and synthesis method thereof
ActiveCN108794357ANo participationRaw materials are easy to getSulfonic acid amide preparationHydrazone preparationChemical synthesisHydrazone
The invention discloses an N-difluoromethyl hydrazone compound and a synthesis method thereof and belongs to the technical field of chemical synthesis of medicines. The synthesis method comprises thefollowing steps: putting a hydrazone compound, bromine difluoromethyl trimethyl silane, a phase transfer catalyst, an alkali and an organic solvent, heating to 25-110 DEG C, stirring to carry out reactions, and after the reactions are completed, cooling to the room temperature, and separating and purifying products, thereby obtaining the N-difluoromethyl hydrazone compound. The synthesis method disclosed by the invention is easy in raw material obtaining, low in cost, simple and safe to operate, free of metal, good in environment protection, high in functional group adaptability, high in substrate adaptability and good in industrial application prospect.
Owner:SOUTH CHINA UNIV OF TECH
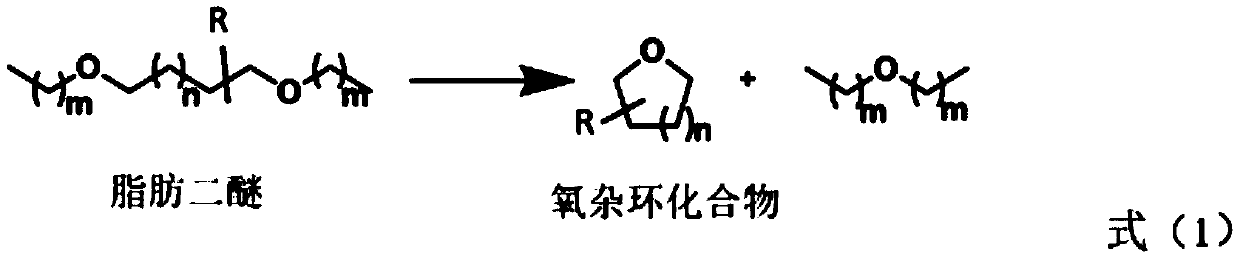
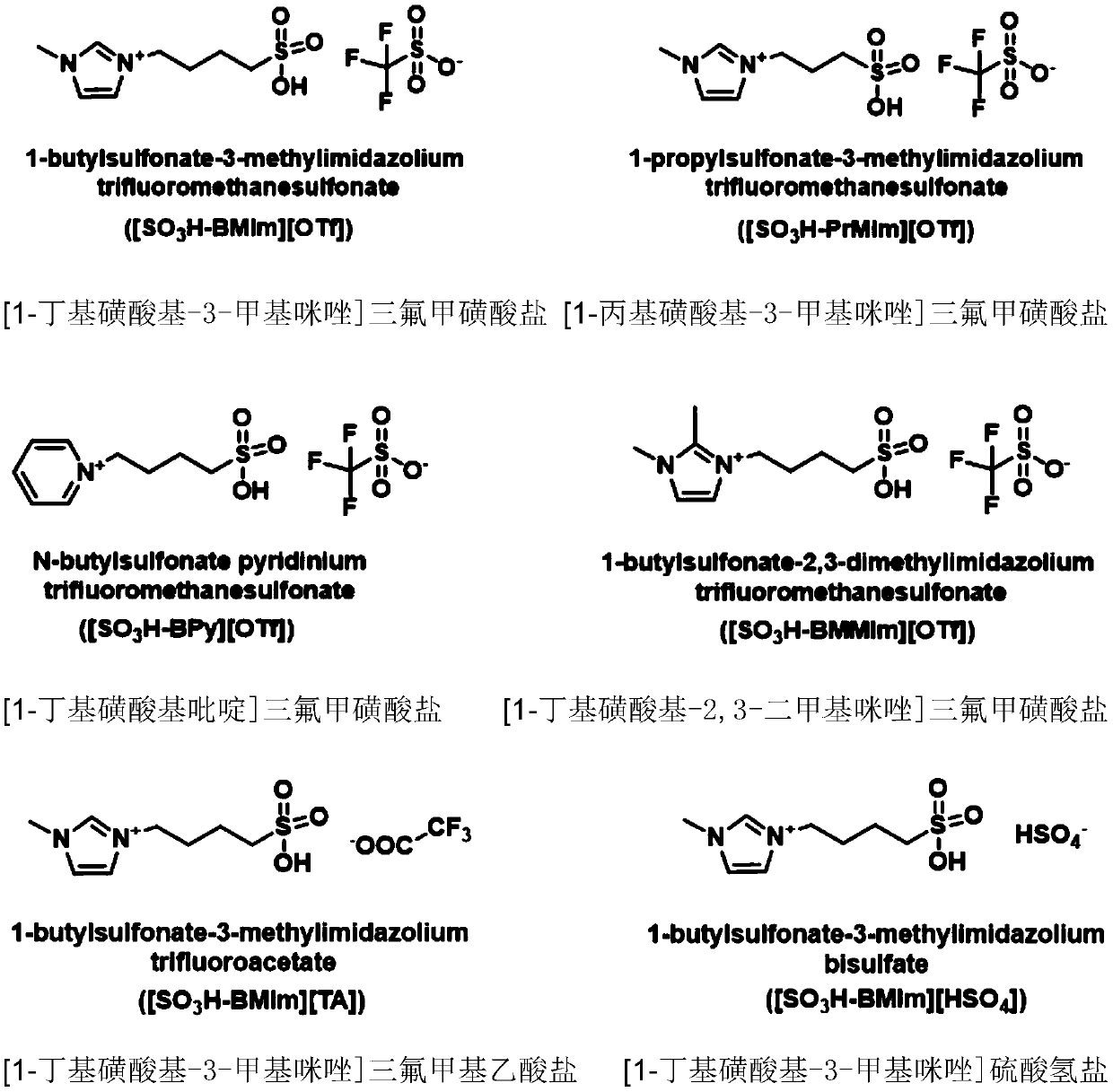
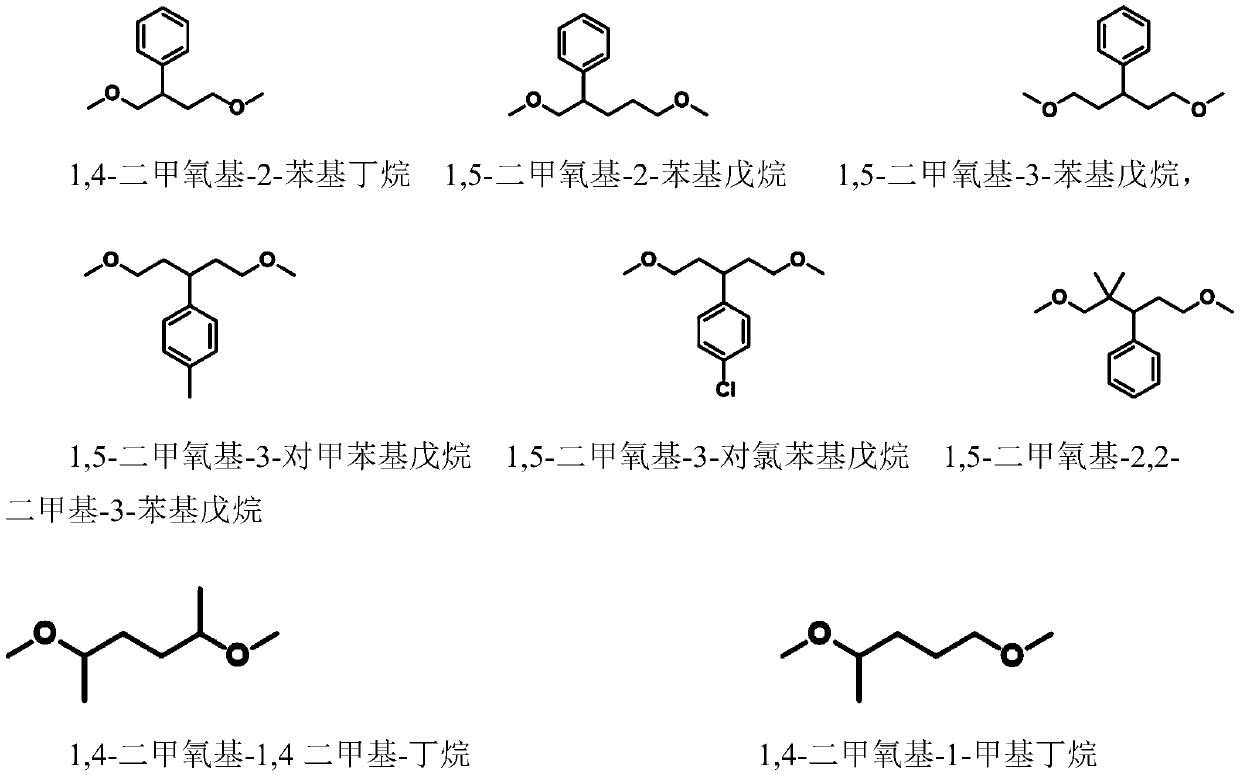
Novel method for preparing oxygen heterocyclic compound through ionic liquid catalysis
ActiveCN111440132AMild reaction conditionsNo metal involvedOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsSide productCombinatorial chemistry
The invention discloses a novel method for preparing an oxygen heterocyclic compound through ionic liquid catalysis. The ionic liquid catalytic system has the advantages of high efficiency, simplicity, mild reaction conditions, no metal participation, no by-product, simple separation and the like and can efficiently catalyze fatty diether metathesis cyclization to prepare an oxygen heterocyclic compound and has very high industrial application value.
Owner:INST OF CHEM CHINESE ACAD OF SCI
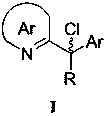
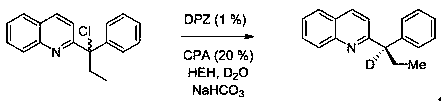
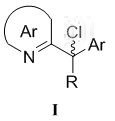
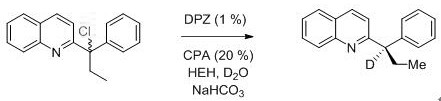
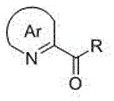
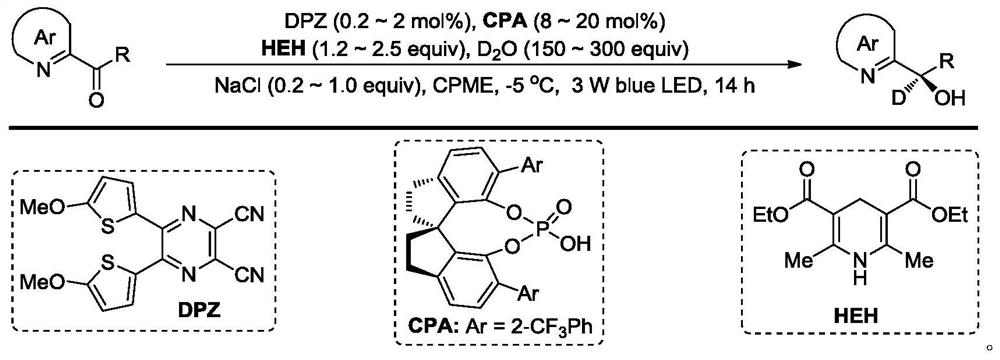
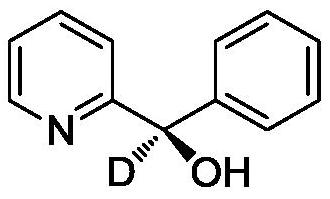
(R)-2-(alpha-deuterio-alpha-alkyl-alpha-aryl)azaaryl compounds as well as preparation method and application thereof
ActiveCN110003102AGreat application potentialNo participationOrganic chemistryAntineoplastic agentsSodium bicarbonateAryl
The invention provides a preparation method of (R)-2-(alpha-deuterio-alpha-alkyl-alpha-aryl)azaaryl compounds. The compounds are prepared by the following steps: dispersing one 2-(alpha-chloro-alpha-alkyl-alpha-aryl)aza arene, photosensitizer DPZ, chiral catalyst CPA, Hantzsch ester HEH, a deuterium source and sodium bicarbonate into an organic solvent, performing degassing treatment at a temperature not higher than -78 DEG C, placing the degassed product at 20-30 DEG C, performing a reaction for 20-40 min under irradiation of a blue light with powder of 3-10 W, and after the reaction is completed, performing separation, and performing purification to obtain one corresponding (R)-2-(alpha-deuterio-alpha-alkyl-alpha-aryl)azaaryl compound. According to the compounds provided by the invention, the enantiomeric excess of the obtained target product is about 90%, the deuteration rate is as high as 99% or more, the yield is high, and the method has mild reaction conditions and no pollution.
Owner:HENAN NORMAL UNIV
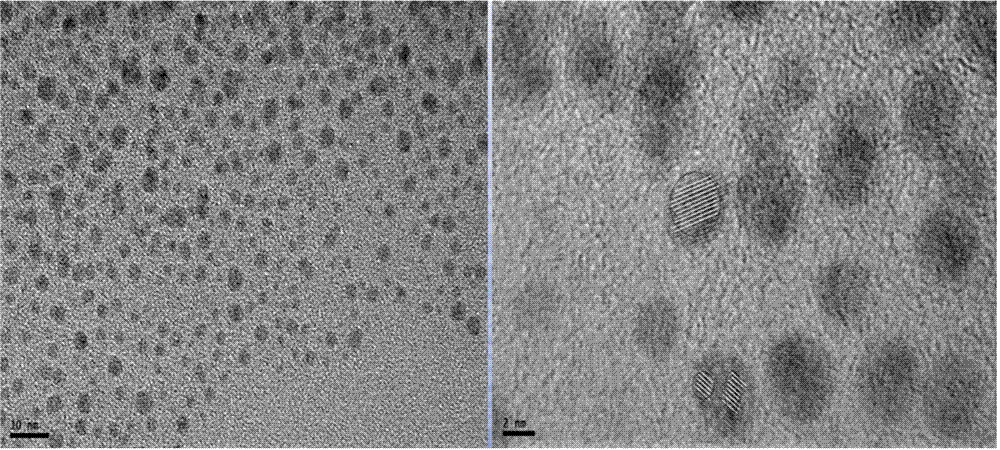
Method for preparing nanogold with size smaller than 5 nm through banana pulp extract
ActiveCN106623970ANo participationSimple stepsMaterial nanotechnologyTransportation and packagingWater bathsRoom temperature
The invention discloses a method for preparing nanogold with the size smaller than 5 nm through a banana pulp extract. The method comprises the steps that the banana pulp extract serves as a reducing agent and a stabilizer, chloroauric acid is reduced, by controlling the addition time and adding quantity of an additive sodium hydroxide in the reaction process, the reaction process of chloroauric acid and the reducing agent in a boiling water bath is controlled, the purpose of controlling the particle size of nanogold particles is achieved, and a spherical nanogold particle solution which is good in dispersibility and uniform in particle size and has the particle size being 1.5-4.9 nm is obtained. The method is mild in condition, short in reaction time, simple, easy to implement and low in cost, the reducing agent and the additive are harmless and free of pollution, the synthesized nanogold particles have the good stability at the room temperature in a salt solution, the method belongs to the environmentally-friendly synthesizing method, and the method is suitable for related application of the fields of medical science, pharmacy and the like.
Owner:XI'AN PETROLEUM UNIVERSITY
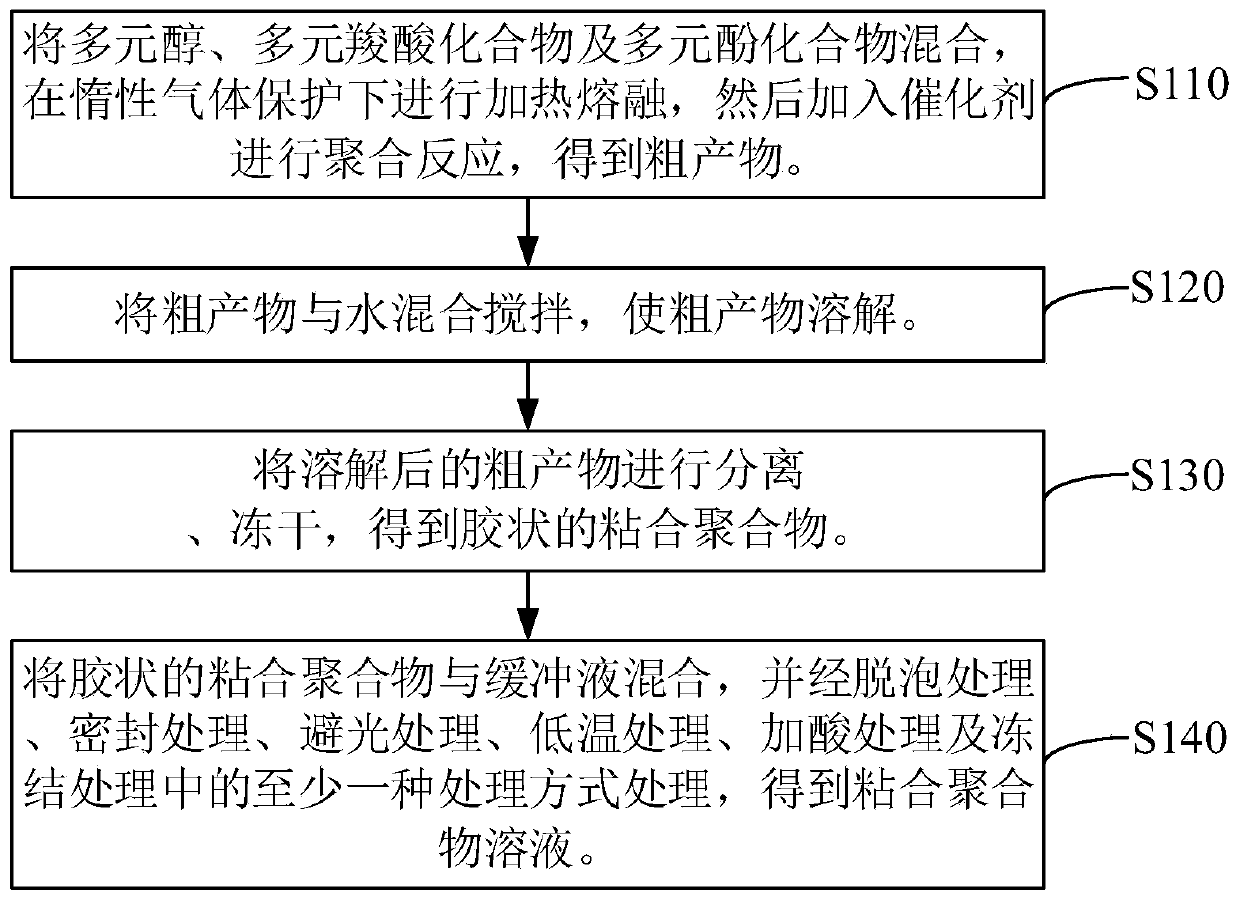
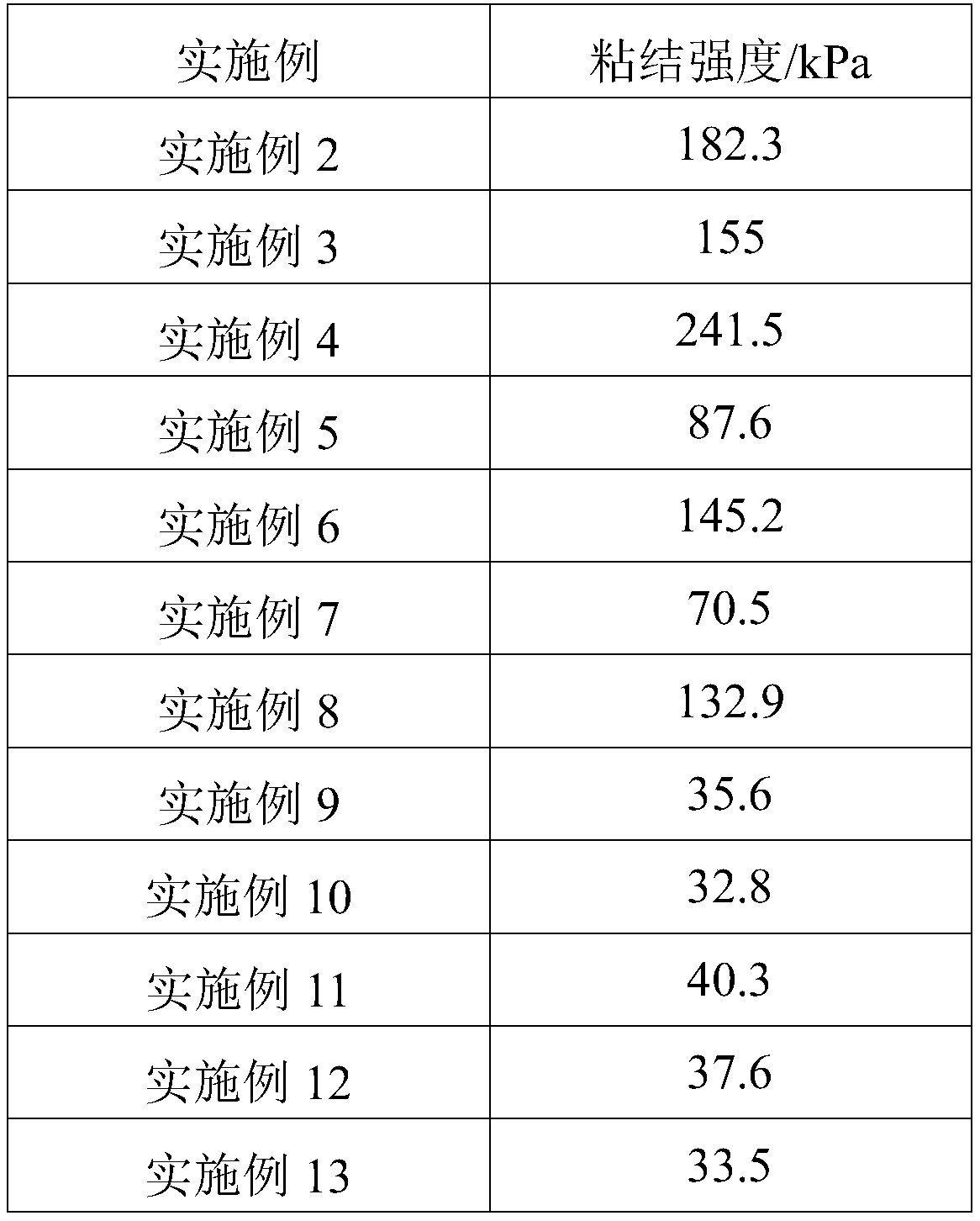
Bonding polymer, medical adhesive and preparation method of medical adhesive
ActiveCN111437430AEasy to prepareShort reaction timeSurgical adhesivesCompatibilizationPolymerization
The invention relates to a bonding polymer, a medical adhesive and a preparation method of the medical adhesive. The preparation method of the bonding polymer comprises the following steps of: mixingpolyhydric alcohols, a polycarboxylic acid compound and a polyphenol compound, heating and melting under the protection of inert gas, and then adding a catalyst for polymerization reaction to obtain acrude product, wherein the polyhydric alcohols comprise at least one of an alcohol compound containing multiple hydroxyl groups and an alcohol polymer containing multiple hydroxyl groups; mixing andstirring the crude product with water to dissolve the crude product; and separating and freeze-drying the dissolved crude product to obtain the colloidal bonding polymer. The preparation process of the bonding polymer is simple, and the prepared bonding polymer is good in biocompatibility and high in bonding strength, so that the bonding polymer can be used for preparing the medical adhesive.
Owner:SHENZHEN LANDO BIOMATERIALS
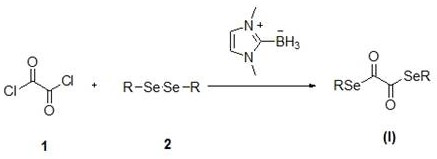
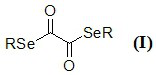
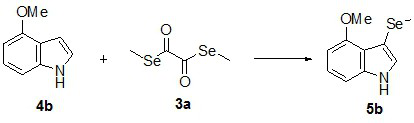
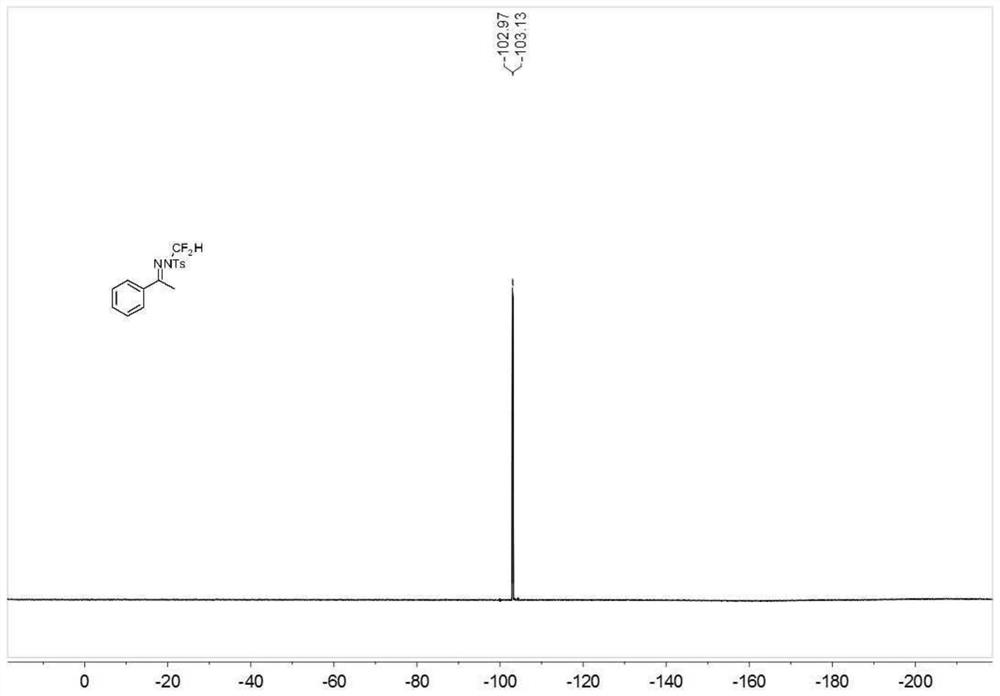
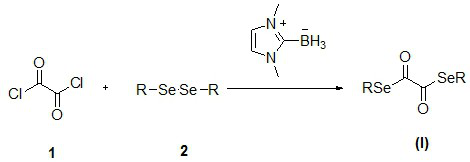
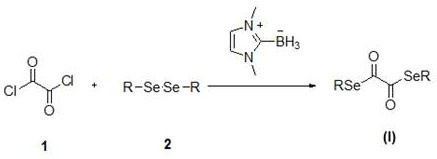
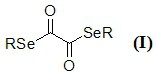
Novel diselenide oxalate compound and synthesis method and application thereof
ActiveCN112608262AEasy to storeAtom efficient and efficientOrganic chemistry methodsMethyl palmoxirateOxalyl chloride
The invention discloses a novel diselenide oxalate compound and a synthesis method and application thereof, and the preparation method is as follows: oxalyl chloride is used as an oxalylation reagent, dialkyl diselenide ether is used as a selenalkyl source, Nmethylimidazolium salt is used as an additive, acetonitrile is used as a solvent, and diselenide oxalate is synthesized under a heating condition. The preparation method has the advantages of simple operation, short steps, mild conditions, easy post-treatment, no metal participation and the like. The synthesized diselenide oxalate is a brand-new selenide ester compound, and selenylation of indole compounds can be realized under photocatalysis.
Owner:NORTHWEST UNIV(CN)
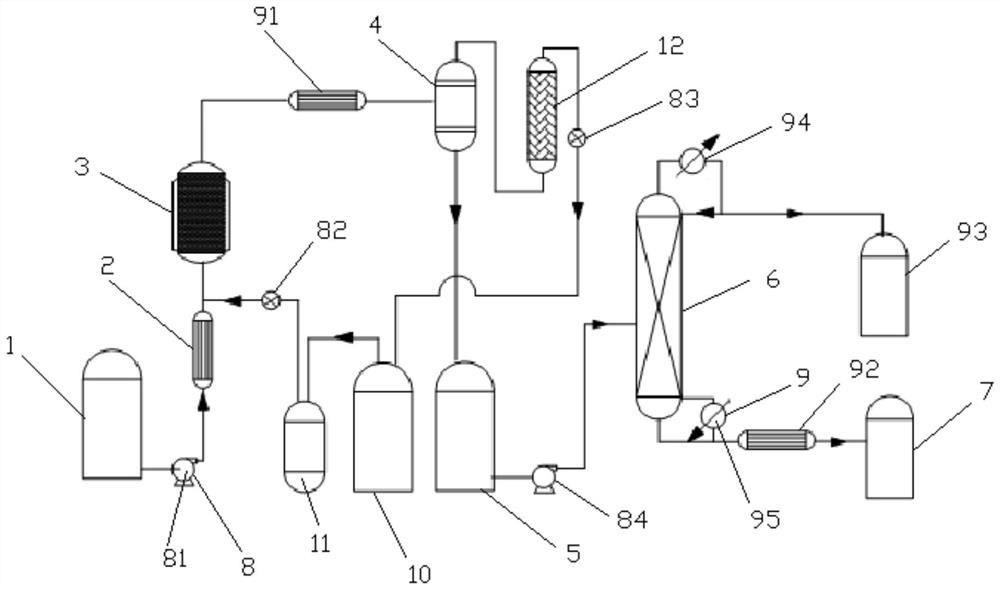
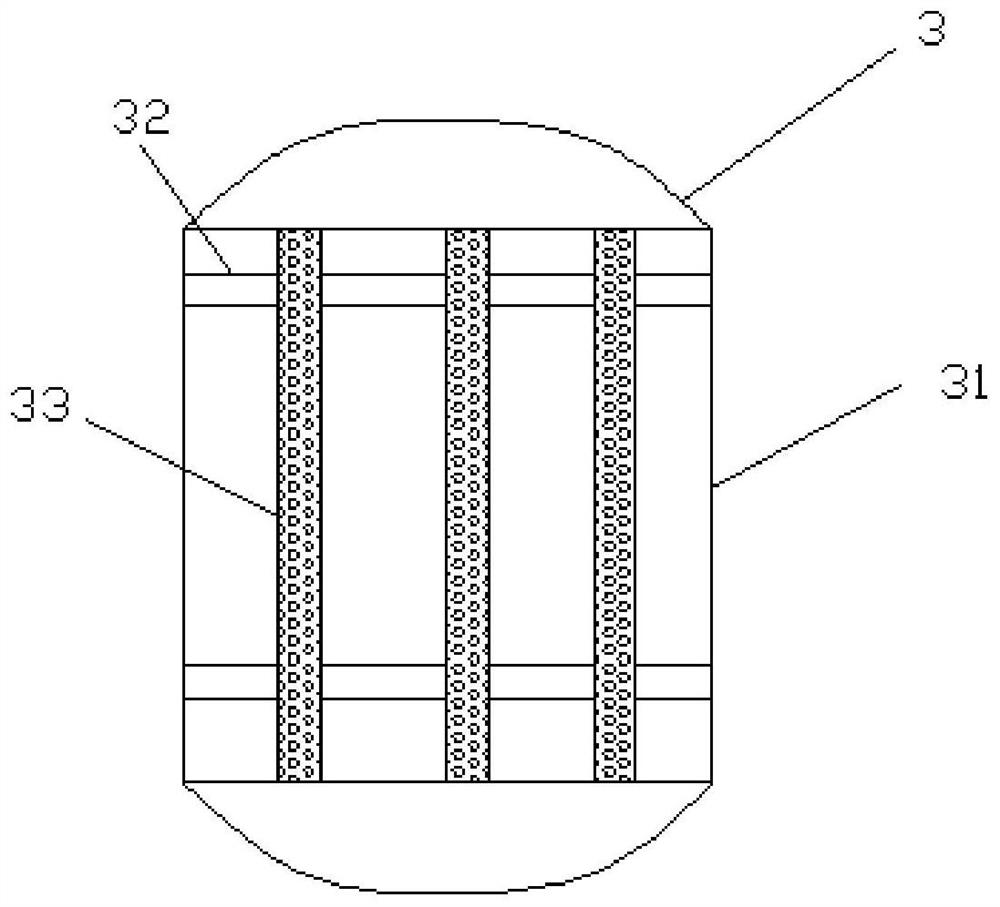
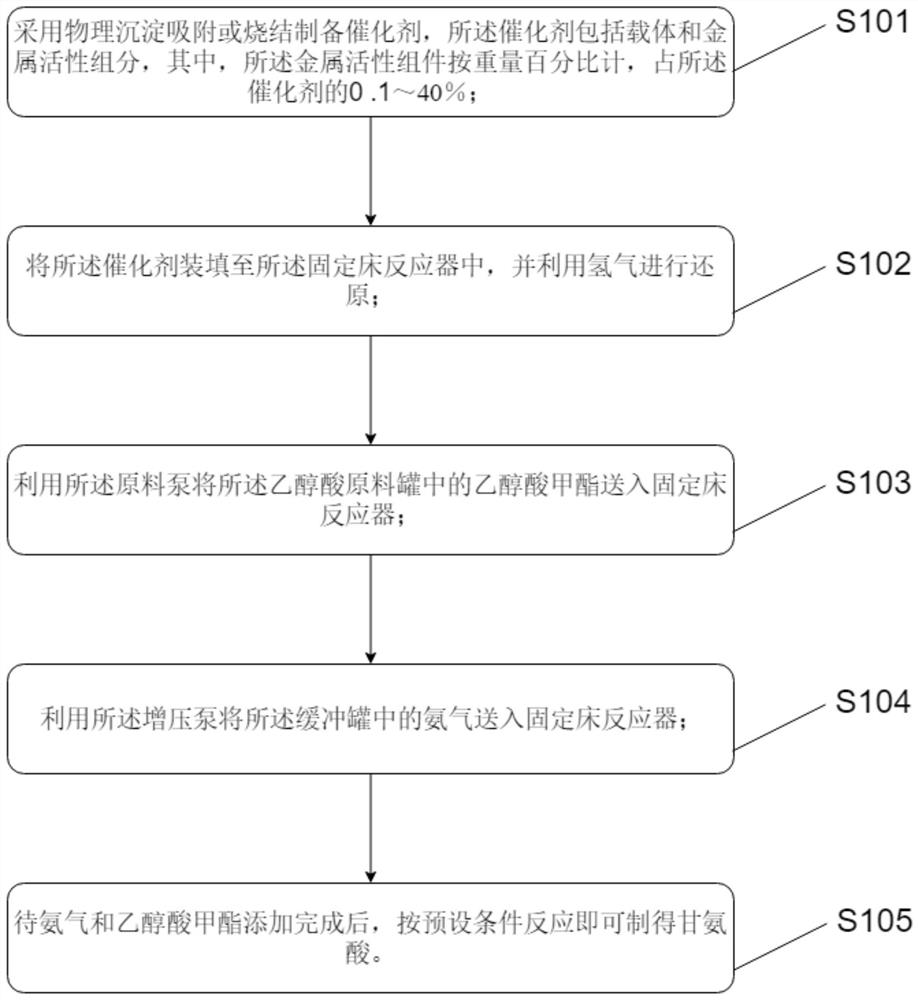
Glycine production process and device
PendingCN112358408AMild reaction conditionsHigh reaction conversion rateOrganic compound preparationAmino-carboxyl compound preparationPtru catalystVapor–liquid separator
The invention discloses a glycine production device, which comprises a methyl glycolate raw material tank, a preheater, a reactor, a gas-liquid separator, a first temporary storage tank, a product refining tower, a product tank, a pump set and an accessory set. An outlet of the methyl glycolate raw material tank is communicated with an inlet of the preheater through the pump set, an outlet of thepreheater is communicated with an inlet of the reactor, and the outlet of the reactor is communicated with the inlet of the gas-liquid separator through the accessory set. The invention also disclosesa glycine production process adopting the glycine production device. The process comprises the following steps: preparing a catalyst; adding methyl glycolate and ammonia gas; preparing glycine through reaction according to preset conditions. A new synthesis route is provided on the basis of an existing glycine synthesis route, methyl glycolate which is easy to obtain serves as a raw material, glycine is synthesized through catalytic ammonolysis, and compared with other routes, the route is mild in reaction condition, high in reaction conversion rate, free of participation of other solvents and small in emission.
Owner:江苏凯美普瑞工程技术有限公司
Monofluoromethylselenylation reagent as well as preparation method and application thereof
The invention discloses a monofluoromethylselenylation reagent represented by a structural general formula (I) as well as a preparation method and application thereof. The preparation method comprises the steps: by taking common aryl sodium sulfinate as an aryl sulfonylation reagent, selenium powder as a selenium source and monofluoromethane chloride as a monofluoromethylation reagent, firstly, carrying out selenylation reaction in an amine solvent at room temperature; and secondly, carrying out a monofluoromethylation reaction in an amide solvent to obtain the aryl sulfonic acid monofluoromethyl selenate compound. The preparation method disclosed by the invention has the advantages of simplicity in operation, short steps, mild conditions, easiness in post-treatment, no metal participation and the like, the prepared aryl sulfonic acid monofluoromethylselenate is a brand-new selenate compound, and the monofluoromethylselenylation reaction of the third site of an indole compound is successfully realized under photocatalysis.
Owner:NORTHWEST UNIV(CN)
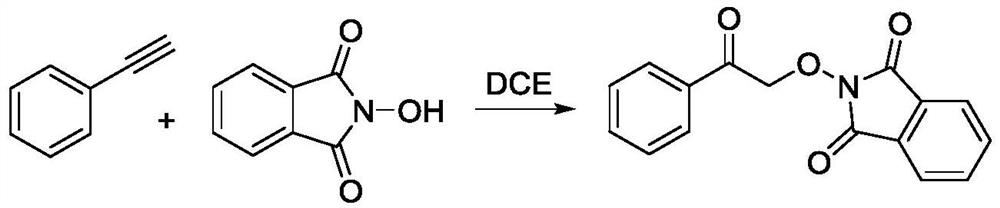
Method for preparing 2-(2-oxo-2-phenethoxy) isobenzyl-1, 3-diketone
InactiveCN111606876ANo participationMild reaction conditionsOrganic chemistryPtru catalystDistillation
The invention relates to a method for preparing 2-(2-oxo-2-phenylethoxy) isobenzyl-1, 3-diketone, which comprises the following steps: adding phenylacetylene and N-hydroxyphthalimide into a reactor, introducing oxygen into the reactor, adding 1, 2-dichloroethane, carrying out magnetic stirring, and putting the reactor into an oil bath pot to react; transferring the obtained reaction liquid into aseparating funnel, adding dichloromethane and water for extraction, and collecting a crude product of an organic phase after reduced pressure distillation; and carrying out column chromatography separation on the obtained crude product by adopting a system in which the ratio of ethyl acetate to petroleum ether is 1: 10, so as to obtain a white solid product of 2-(2-oxo-2-phenethoxy) isobenzyl-1, 3-diketone. The synthesis method has the advantages of mild reaction conditions, simple technological process, no participation of catalysts and oxidants and the like.
Owner:HUBEI UNIV OF TECH
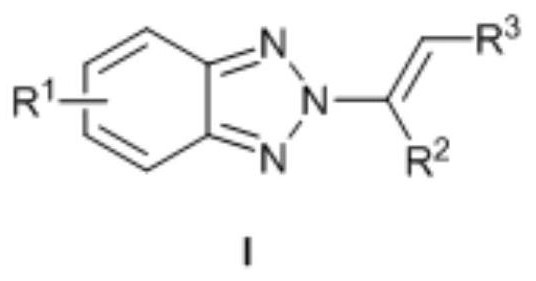
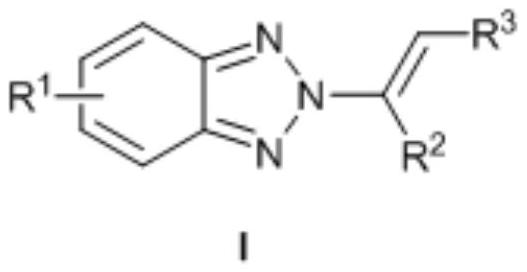
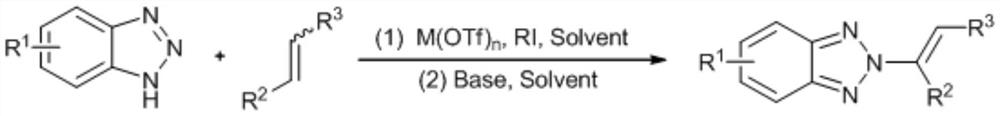
no 2 -Alkenyl benzotriazole derivatives and synthesis method thereof
ActiveCN109134392BNo participationSimple and mild reaction conditionsOrganic chemistryPtru catalystPotassium carbonate
provided by the invention N 2 -Alkenyl benzotriazole derivatives are compounds such as formula I:, with chloroform as solvent, M(OTf) n As a catalyst, react benzotriazole, N-iodosuccinimide and olefin at room temperature for 0.5-5h, then concentrate, use methanol as solvent and potassium carbonate as base, react at 50-70°C for 8-15h, The target compound can be obtained. A notable feature of this method is that the reaction conditions are simple and mild, no transition metals are involved, and the yield and N 2 ‑ Higher selectivity. Currently, the one-pot method directly builds N 2 The method of ‑alkenylbenzotriazole has not yet been reported, and the synthetic method is very simple and cheap, and is easy to operate and has reached more than 84%. N 2 ‑Selectivity, strong universality of response.
Owner:ZHOUKOU NORMAL UNIV +1
Fixed length silicon steel coil stacking device
PendingCN110775652AConvenient automatic stacking workNo participationConveyorsStacking articlesStructural engineeringWorkbench
The invention relates to a fixed length silicon steel coil stacking device. The silicon steel coil stacking device comprises a flow working table and a stacking working table which are located at thesame position as a horizontal plane, the flow working table and the stacking working table are arranged at intervals, the top positions of the flow working table and the stacking working table are equipped with top beams at intervals, the top beams are equipped with a lifting grabbing mechanism through a displacement mechanism, the output end of the lifting grabbing mechanism is corresponding to asilicon steel coil on the flow working table and the stacking working table correspondingly, and the silicon steel coil is in a circular ring structure. According to the fixed length silicon steel coil stacking device, the stacking efficiency is high, the stacking accuracy is high, the degree of automation is high, the operation difficulty is low, the safety factor is high, the personnel participation does not exist.
Owner:WUXI PUTIAN IRON CORE CO LTD
(r)-2-(α-deuterium-α-alkyl-α-aromatic) azaaryl compound and its preparation method and application
ActiveCN110003102BGreat application potentialNo participationOrganic chemistryAntineoplastic agentsSodium bicarbonateAryl
Owner:HENAN NORMAL UNIV
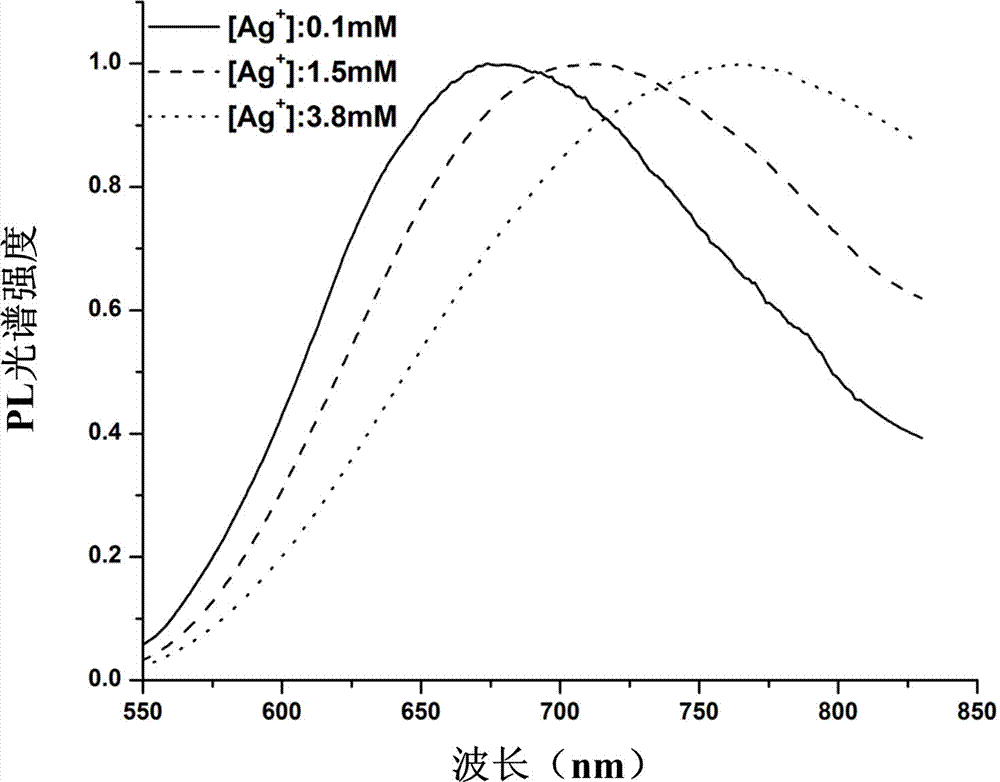
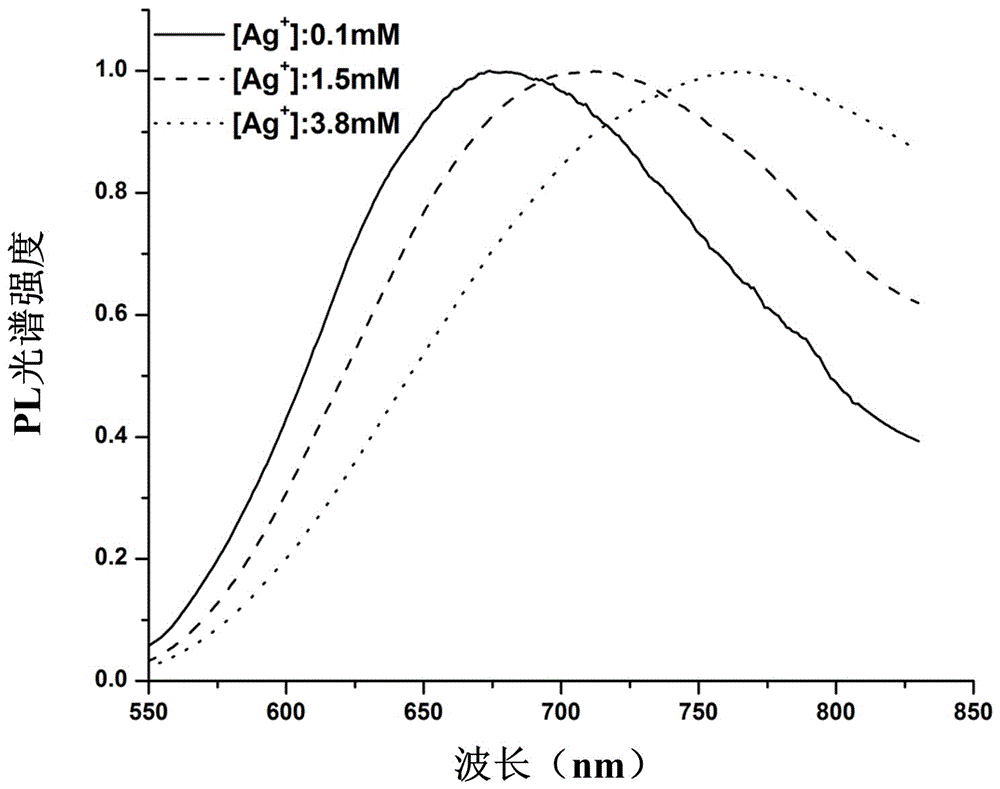
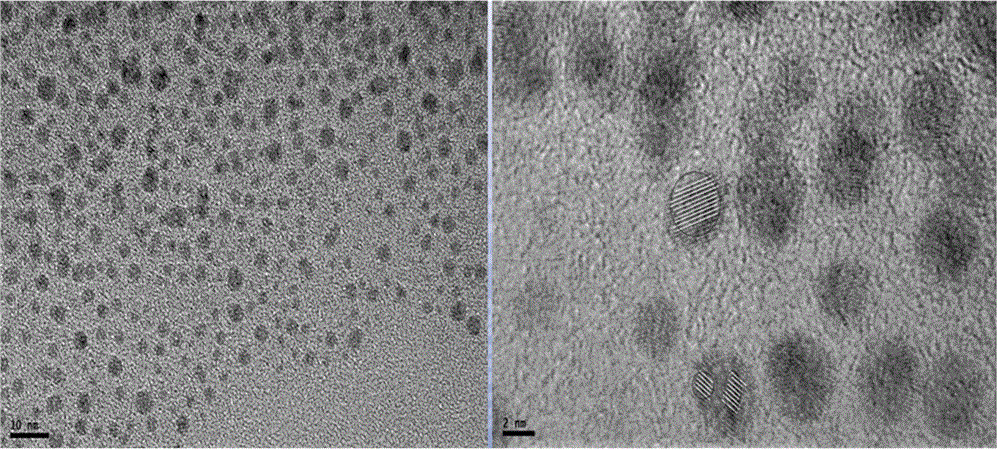
Silver nanocluster preparation method with hydrogen serving as reducing agent
The invention provides a silver nanocluster preparation method with hydrogen serving as a reducing agent. The silver nanocluster preparation method is characterized in that polyelectrolyte and silver salt react at a room temperature in a hydrogen atmosphere by taking the polyelectrolyte as a template and the hydrogen as the reducing agent so as to obtain a near-infrared emission silver nanocluster. The silver nanocluster preparation method has the advantages of low cost, simplicity, mild reacting conditions, no participation of highly-toxic substance and more economy and environmental protection, and the prepared silver nanocluster is excellent in fluorescence characteristic and stability.
Owner:EAST CHINA UNIV OF SCI & TECH
2-(α-deuterium-α-hydroxyl-α-aryl/alkyl) azaaromatic compound and its preparation method and application
ActiveCN110015985BGreat application potentialHigh yieldOrganic chemistryAntineoplastic agentsPtru catalystAcyl group
The present invention provides a kind of preparation method of 2-(α-deuterium-α-hydroxy-α-aryl / alkyl) azaaromatic compound, 2-aryl / alkanoyl azaaryl, photosensitizer DPZ, Han Stilbene ester HEH, chiral catalyst CPA, deuterium source and sodium chloride are dispersed in an organic solvent. Lamp irradiation, reaction 3~14 hours, after reaction finishes, separates and purifies and promptly obtains 2-(α-deuterium-α-hydroxyl-α-aryl / alkyl) azaaromatic compound; the enantiomer of the product obtained in the present invention The excess is about 90%, the deuterium substitution rate is over 90%, and the yield of some target products is as high as 99%, and the reaction conditions are mild, environmentally friendly, metal-free, less photocatalyst consumption, and short reaction time. It has laid a good foundation for chemical production.
Owner:HENAN NORMAL UNIV
A method for preparing nano-gold with a size less than 5nm by using banana pulp extract
ActiveCN106623970BNo participationSimple stepsMaterial nanotechnologyTransportation and packagingWater bathsSynthesis methods
The invention discloses a method for preparing nano-gold with a size less than 5nm by using banana pulp extract. The method uses the banana pulp extract as a reducing agent and a stabilizer to reduce gold tetrachlorate, and controls the amount of additive sodium hydroxide in the reaction process. The timing and amount of addition are used to control the reaction process of gold tetrachlorate and reducing agent in a boiling water bath, and then achieve the purpose of controlling the particle size of nano-gold particles, and obtain good dispersion, uniform particle size, and a particle size of 1.5 to 4.9 nm spherical gold nanoparticles solution. The method of the invention has mild conditions, short reaction time, simple operation, low cost, non-toxic and pollution-free reducing agent and additives, and the synthesized nano gold particles have good stability at room temperature and in salt solution, and belong The green synthesis method is suitable for related applications in the fields of medicine and pharmacy.
Owner:XI'AN PETROLEUM UNIVERSITY
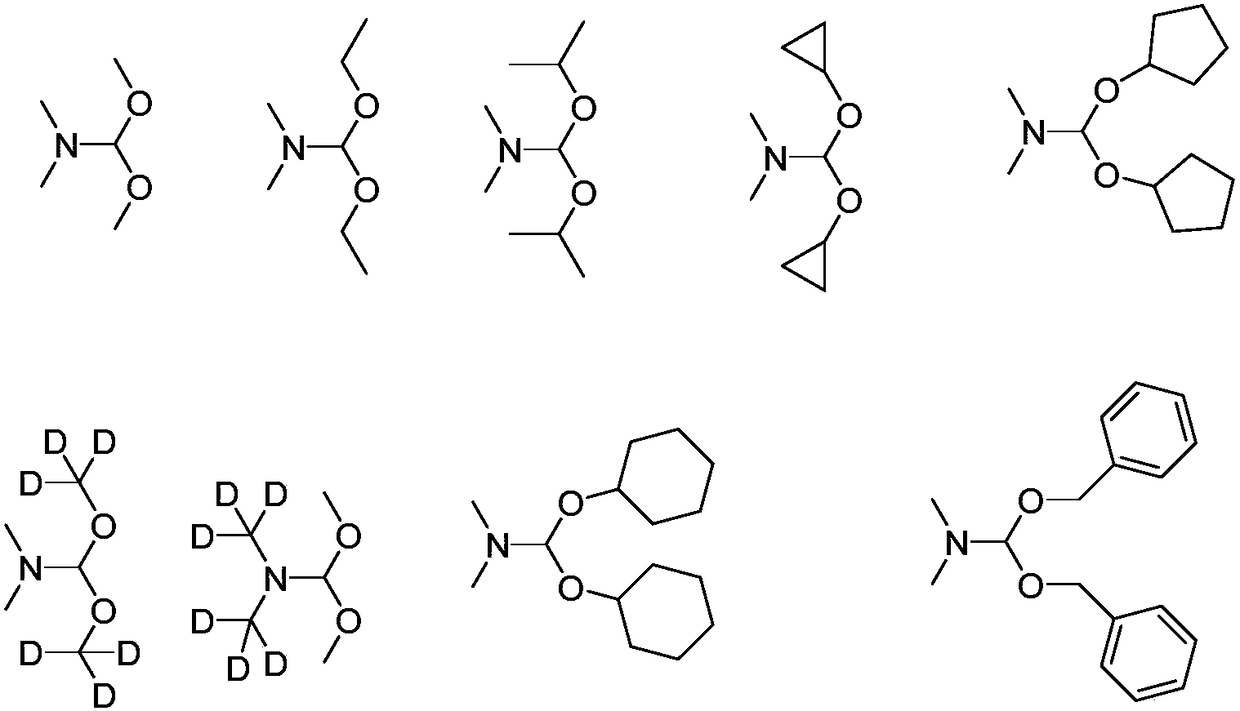
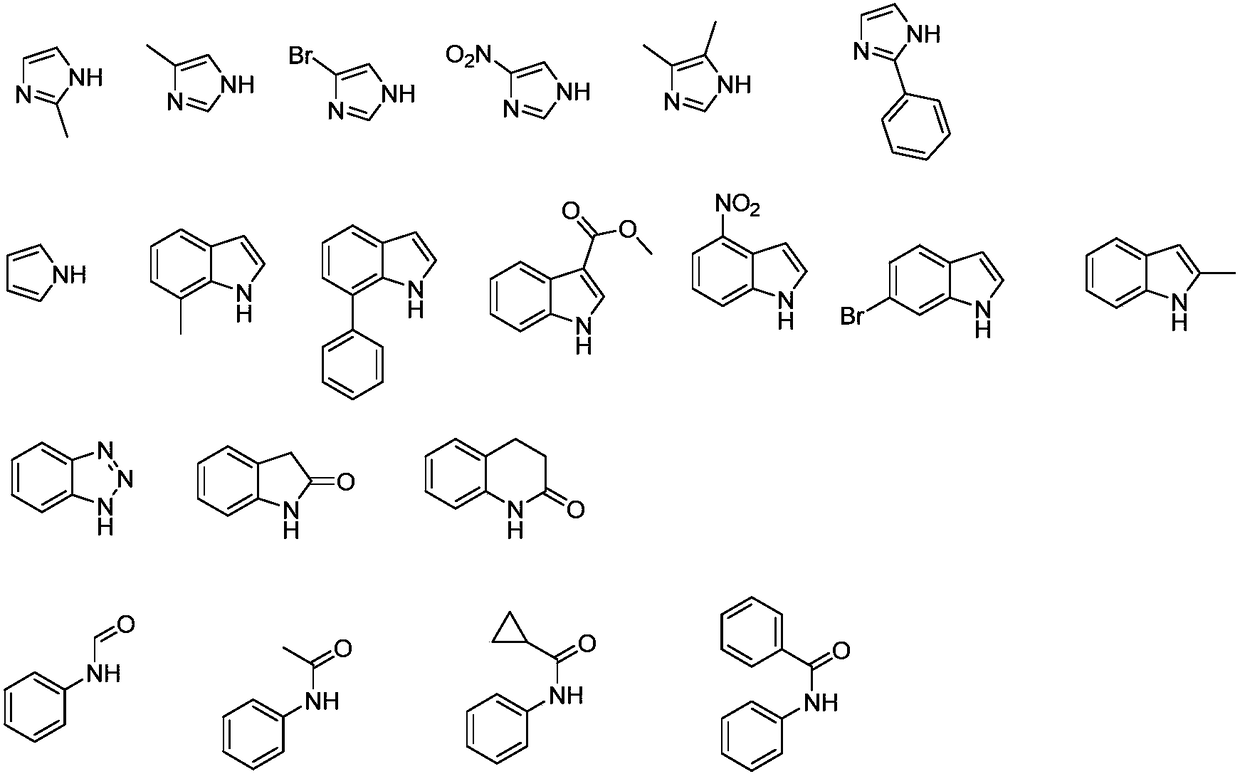
Alkylation method for nitrogen-hydrogen containing compounds and application thereof
ActiveCN108503583ANo participationMild reaction conditionsOrganic compound preparationAmino compound preparationN dimethylformamidePhotochemistry
The invention discloses an alkylation method for nitrogen-hydrogen containing compounds and an application thereof, belonging to the technical field of synthesis of organic compounds. The invention provides a series of methods for a nitrogen alkylation reaction of N-H containing heterocyclic compounds (II) with N,N-dimethylformamide dialkyl acetal as an alkyl source under the condition of no participation of metals, and a product with a hydrogen atom on a nitrogen atom substituted by R1 is obtained. The method provided by the invention has the advantages of highly-efficient reaction, high yield, simple treatment after the reaction, simple and convenient operation, mild reaction conditions, no participation of the metals, high tolerance of functional groups of a reaction substrate, wide range and easy preparation of the substrate, high reaction efficiency after amplification of the reaction, and applicability to large-scale industrial production.
Owner:JIANGNAN UNIV
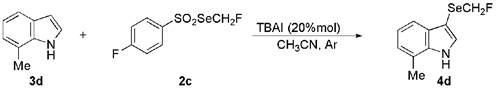
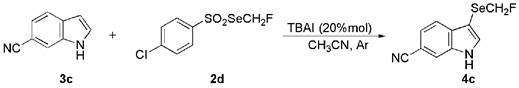
Sulfonylated indolo[1,2-a]quinoline compounds and preparation method thereof
ActiveCN109575015BNo participationMild reaction conditionsOrganic chemistryChemical industrySulfohydrazide
The invention discloses a sulfonylated indolo[1,2-a]quinoline compound and a preparation method thereof. The invention uses o-aryl alkynyl phenyl indole and aryl sulfonyl hydrazide as raw materials, Synthesis of sulfonylated indolo[1,2‑a]quinoline compounds under the action of ammonium iodide and tert-butyl hydroperoxide. The raw materials are cheap and easy to obtain, the reaction conditions are mild, the operation is simple, and the synthesis yield is high, which is beneficial to industrial production. Such derivatives have potential applications in the fields of chemical industry, medicine, etc., and the present invention provides a method for the synthesis of sulfonylated indolo[1,2-a]quinoline compounds for the first time.
Owner:ZHENGZHOU UNIV
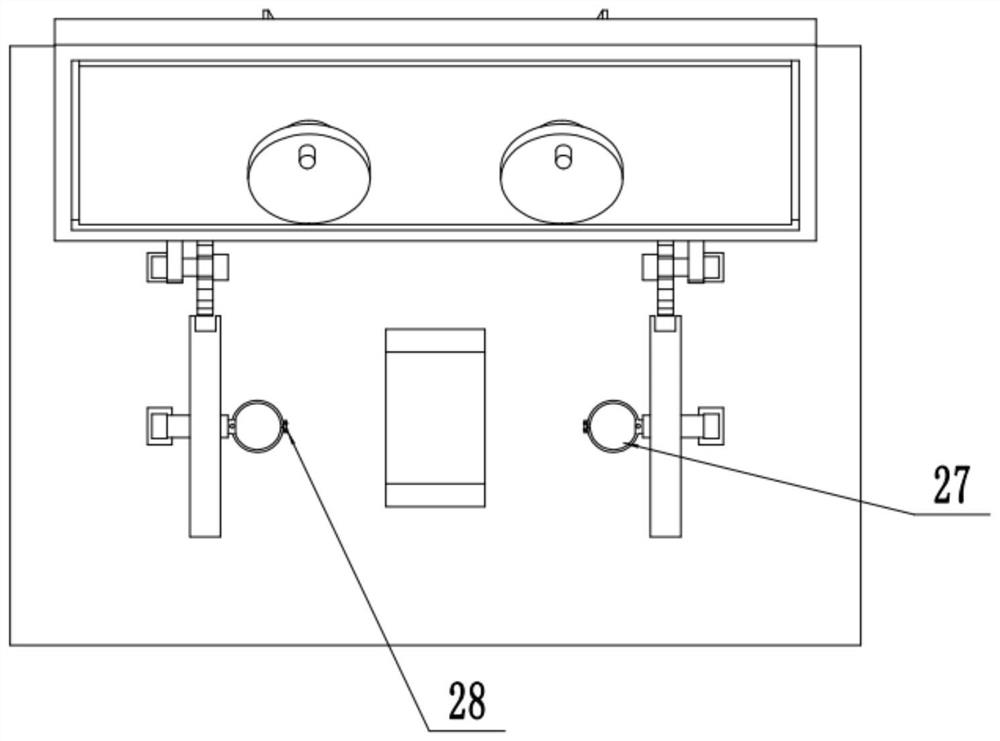
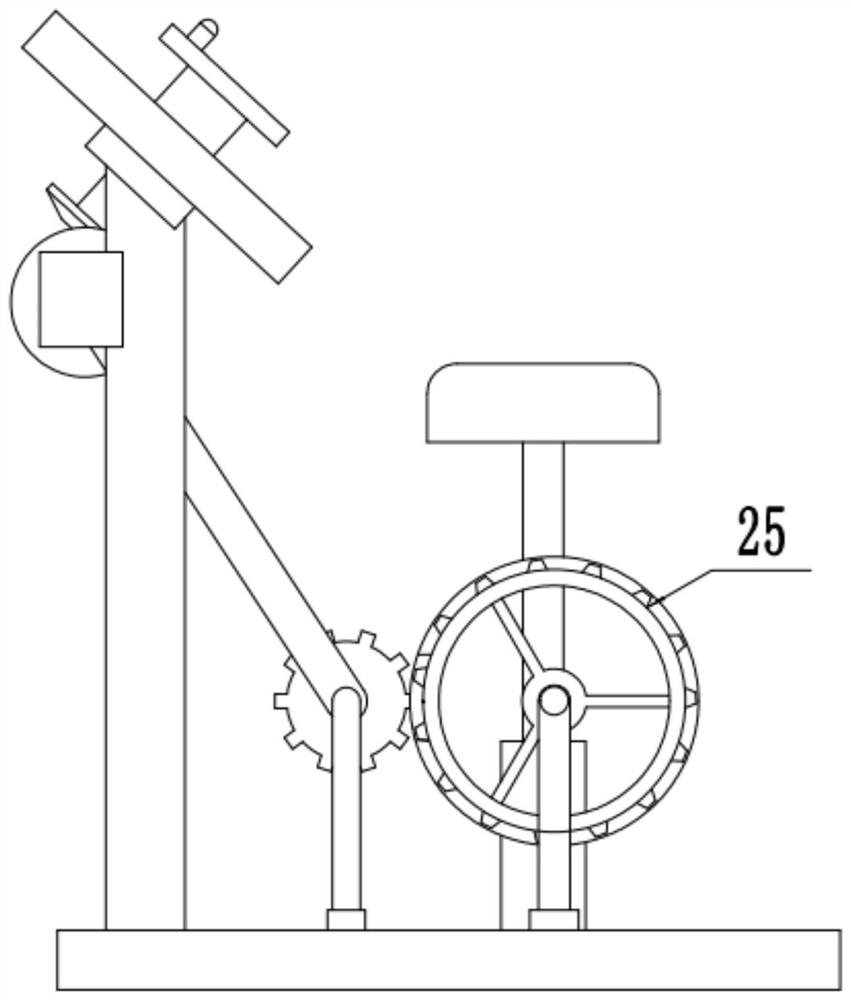
Hand-foot simultaneous exercising device for limb recovery of patient
InactiveCN111821661ANo participationCompact structureGymnastic exercisingChiropractic devicesPhysical medicine and rehabilitationArm exercise
The invention relates to the technical field of medical treatment. The invention discloses a hand-foot simultaneous exercising device for limb recovery of a patient. The hand-foot simultaneous exercising device comprises a base, the top of an arm exercising plate is bilaterally symmetrically and rotatably connected with two groups of turntables; the bottom of the turntable movably penetrates through the arm exercising plate through a rotating rod and is fixedly provided with a group of driving helical teeth; driven helical teeth are engaged with the sides, away from the left-right center lineof the base, of the driving helical teeth; a fixed rod is fixedly mounted on the rear side surface of the driven helical teeth; a group of gears which are supported above the base through supporting rods are rotatably connected to the right lower side of the fixed rod through a transmission belt; a circle of rotating groove is inwards formed in the side wall, facing the left-right center line direction of the base, of each fixing ring, a rotating gear ring is rotationally connected into the rotating groove, a meshing groove is formed in the side wall, located on the right side of the corresponding gear, of each fixing ring, and the gears and the rotating gear rings in the rotating grooves are conveniently meshed at the meshing grooves through the meshing grooves.
Owner:安徽华中机器人技术开发有限公司
A kind of n-difluoromethylhydrazone compound and its synthesis method
ActiveCN108794357BNo participationRaw materials are easy to getSulfonic acid amide preparationHydrazone preparationChemical synthesisPtru catalyst
The invention discloses an N-difluoromethyl hydrazone compound and a synthesis method thereof and belongs to the technical field of chemical synthesis of medicines. The synthesis method comprises thefollowing steps: putting a hydrazone compound, bromine difluoromethyl trimethyl silane, a phase transfer catalyst, an alkali and an organic solvent, heating to 25-110 DEG C, stirring to carry out reactions, and after the reactions are completed, cooling to the room temperature, and separating and purifying products, thereby obtaining the N-difluoromethyl hydrazone compound. The synthesis method disclosed by the invention is easy in raw material obtaining, low in cost, simple and safe to operate, free of metal, good in environment protection, high in functional group adaptability, high in substrate adaptability and good in industrial application prospect.
Owner:SOUTH CHINA UNIV OF TECH
Diselenide oxalate compound and its synthesis method and application
ActiveCN112608262BEasy to storeAtom efficient and efficientOrganic chemistry methodsMethyl palmoxirateOxalyl chloride
The invention discloses a new type of diselenide oxalate compound and its synthesis method and application. The preparation method uses oxalyl chloride as an oxalylating reagent, dialkyl diselenide as a selenyl source, and N-methylimidazolium salt As an additive, acetonitrile is used as a solvent, and diselenide oxalate is synthesized under heating conditions. The preparation method has the advantages of simple operation, short steps, mild conditions, easy post-processing and no metal participation. The synthesized diselenide oxalate is a new class of selenide compounds, which can realize the selenoalkylation of indole compounds under photocatalysis.
Owner:NORTHWEST UNIV
A racemization method of (1r,6s)-8-benzyl-7,9-dioxo-2,8-diazabicyclo[4,3,0]nonane
Owner:SHAOXING UNIVERSITY
A method for preparing silver clusters using hydrogen as a reducing agent
The invention provides a silver nanocluster preparation method with hydrogen serving as a reducing agent. The silver nanocluster preparation method is characterized in that polyelectrolyte and silver salt react at a room temperature in a hydrogen atmosphere by taking the polyelectrolyte as a template and the hydrogen as the reducing agent so as to obtain a near-infrared emission silver nanocluster. The silver nanocluster preparation method has the advantages of low cost, simplicity, mild reacting conditions, no participation of highly-toxic substance and more economy and environmental protection, and the prepared silver nanocluster is excellent in fluorescence characteristic and stability.
Owner:EAST CHINA UNIV OF SCI & TECH
A kind of synthetic method of 1,4-butenediones
ActiveCN104262122BNo participationActivated oxygenOrganic compound preparationCarbonyl compound preparation by oxidationSolventPhenol
The invention belongs to the technical field of organic compound synthesis, provides a method for synthesizing a 1,4-butene diketone compound and aims to solve the problems that metal participates, materials are complex and the conditions are harsh in the method for synthesizing the 1,4-butene diketone compound at present. The synthesizing method comprises the step of reacting for 12-48h at 25-80 DEG C by using substituted aryne and phenol as initial materials, using 1-chloromethyl-4-fluoro-1,4-diazabicyclo[2.2.2] octane bi(tetrafluoroboric acid) salt (selectfluor) as an oxidant and using CH3CN as a solvent to prepare the 1,4-butene diketone compound. The invention provides a method for synthesizing the 1,4-butene diketone compound, which has the advantages that materials can be easily obtained, no metal participates and the reaction condition is moderate. The reaction formula is shown in the specification.
Owner:山东博洛德生物科技有限公司
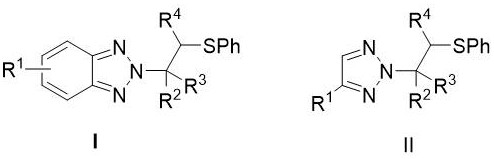
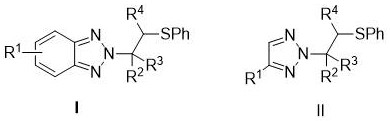
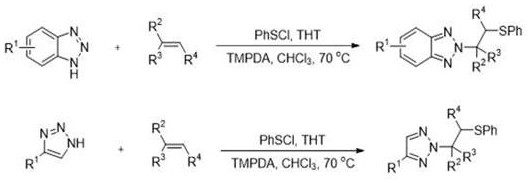
Synthesis method of N2-beta-sulfanyl triazole derivative
ActiveCN113754601ANo participationWide range of adaptabilityOrganic chemistry methodsSulfonyl chloridePtru catalyst
The invention provides a synthesis method of a N2-beta-sulfanyl triazole derivative, which comprises the steps that in a solvent I, under the action of tetrahydrothiophene as a catalyst or no catalyst, and under the condition of alkali or no alkali, triazole, a sulfur source and olefin react for 12-36 hours at the temperature of 50-80 DEG C, and a target compound is obtained after concentration and column chromatography, wherein the triazole is benzotriazole, a benzotriazole derivative, 1, 2, 3-triazole or a 1, 2, 3-triazole derivative, and the sulfur source is benzene sulfenyl chloride. The method has the advantages of simple and mild reaction conditions, no transition metal participation, wide substrate adaptability range, high yield and high N2-selectivity.
Owner:ZHOUKOU NORMAL UNIV
Sulfonylated indolo[1,2-a]quinoline compound and preparation method thereof
The invention discloses a sulfonylated indolo[1,2-a]quinoline compound and a preparation method thereof. O-arylalkynyl phenylindole and aryl sulfonyl hydrazide are subjected to the action of tetrabutyl ammonium iodide and tert-butyl hydroperoxide to synthesize the sulfonylated indolo[1,2-a]quinoline compound. The materials are low in price and easy to attain; the reaction conditions are mild, operation is simple, and synthetic yield is high; the compound herein is easy to industrially produce. The sulfonylated indolo[1,2-a]quinoline compound as a derivative is potentially applicable to the fields, such as chemical industry and medicine. The method to synthesize the sulfonylated indolo[1,2-a]quinoline compound is provided for the first time herein.
Owner:ZHENGZHOU UNIV
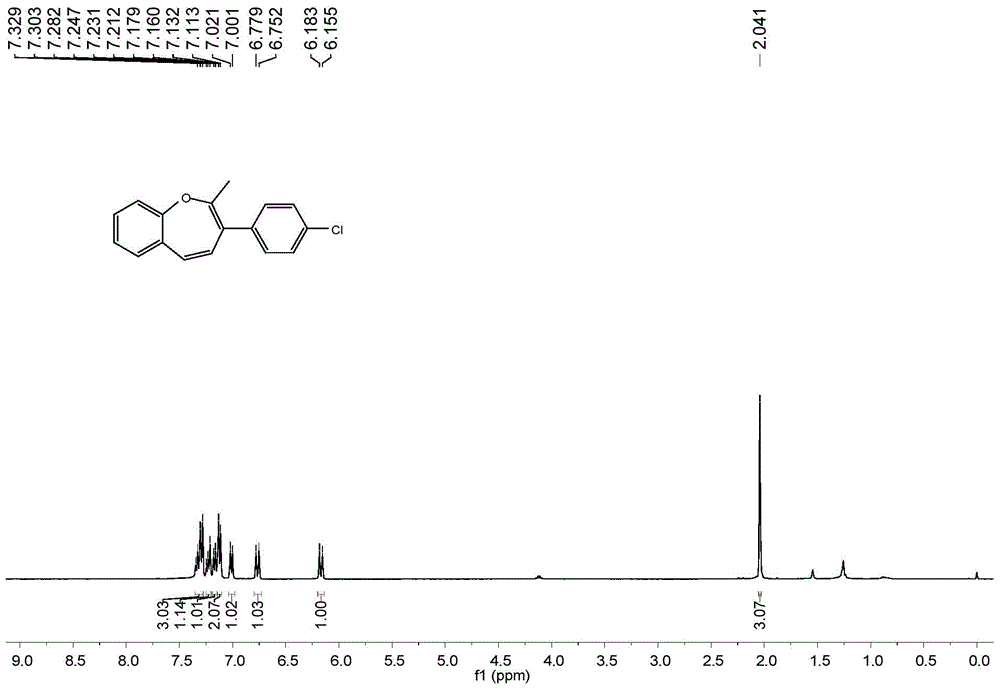
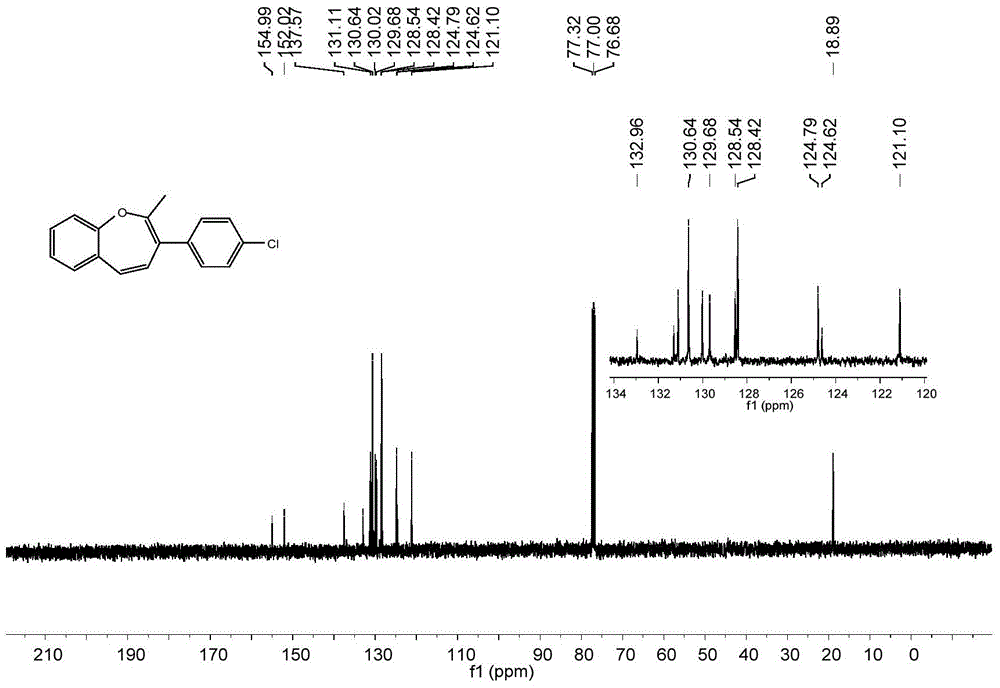
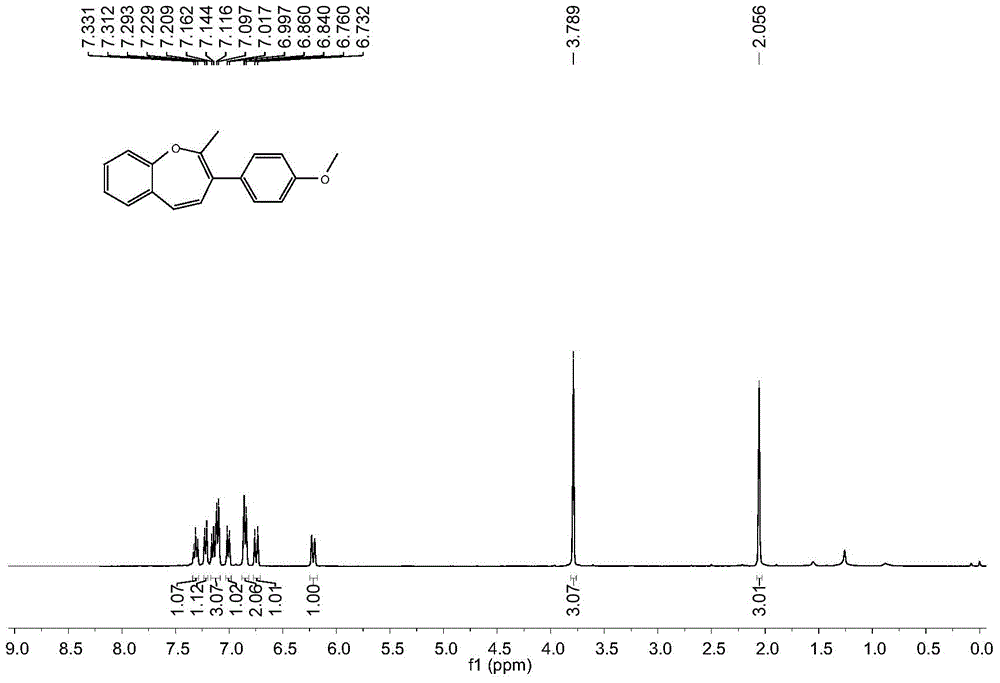
A kind of synthetic method of benzoxepin compound
The invention relates to a synthetic method of benzo oxepin compounds. The synthetic method comprises the following steps of: with an o-fluorobenzene acetylene compound and ketone as raw materials, alkali as an accelerant and an organic solvent as a solvent, increasing temperature to 80-150 DEG C under the protection of inert gas, and stirring to react for 6-24 hours; after the reaction is finished, cooling to room temperature, carrying out reduced pressure distillation and concentration to obtain a crude product, and then purifying through column chromatography to obtain the benzo oxepin compounds. The synthetic method of the benzo oxepin compounds, which is disclosed by the invention, has the advantages of safety and easiness in operation, good adaptability to a functional group, wide adaptability to substrates and environmental friendliness and is favorable for industrial production and widely applied to medicines and organic synthesis, and the raw materials are easily available and low in price.
Owner:SOUTH CHINA UNIV OF TECH
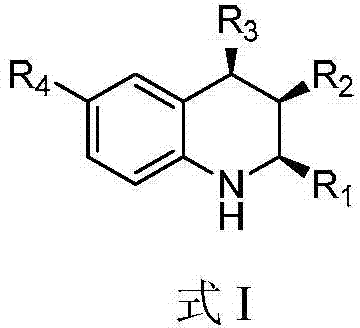
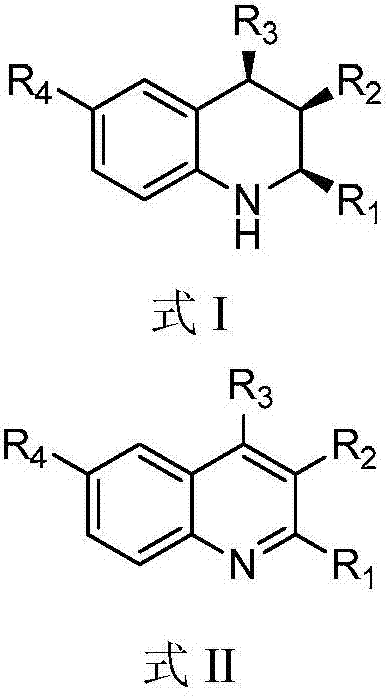
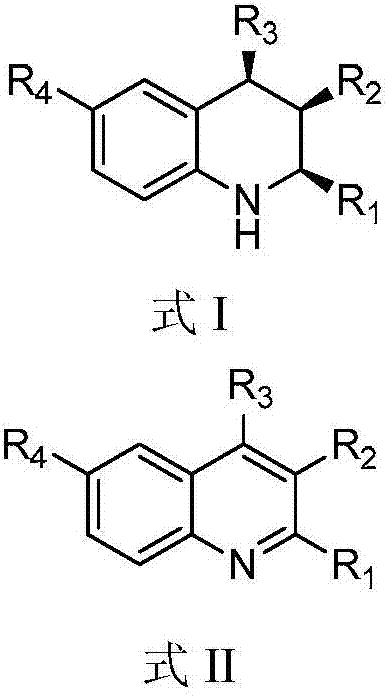
A kind of 1,2,3,4-tetrahydroquinoline derivative and preparation method thereof
ActiveCN104817496BStereoselectivity can be controlledEnantioselectivity can be controlledOrganic chemistryHydrogenChemical compound
The invention discloses a 1, 2, 3, 4-tetradroquinoline derivative and a method for preparing the same. The method for preparing the1, 2, 3, 4-tetradroquinoline derivative which can be a chemical compound shown by a formula I includes a step of enabling a chemical compound shown in a formula II and hydrogen to carry out reduction reaction under the catalysis effects of HB(C<6>F<5>)<2> and styrene to obtain the chemical compound shown in the formula I. Alternately, the method for preparing the 1, 2, 3, 4-tetradroquinoline derivative which can be an optically active chemical compound shown in a formula I includes a step of enabling the chemical compound shown in the formula II and hydrogen to carry out reduction reaction under the catalysis effects of the HB(C<6>F<5>)<2> and a chemical compound shown in a formula III to obtain the optically active chemical compound shown in the formula I. The 1, 2, 3, 4-tetradroquinoline derivative and the method have the advantages that the HB(C<6>F<5>)<2> and the styrene are used as catalysts, quinoline derivatives with different structures are used as substrates, accordingly, the high-yield all-cis 1, 2, 3, 4-tetradroquinoline derivative can be synthesized, and the stereoselectivity of products can be controlled; alternately, the HB(C<6>F<5>)<2> and chiral diene are used as catalysts, the quinoline derivatives with the different structures are used as the substrates, accordingly, the high-yield optically active 1, 2, 3, 4-tetradroquinoline derivative can be synthesized, and the enantioselectivity of products can be controlled.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com









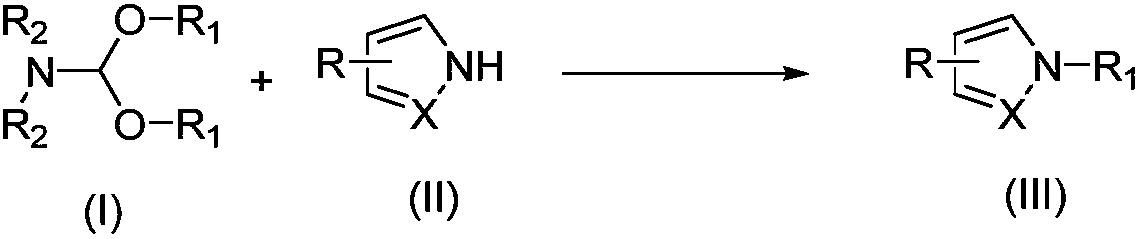
![Sulfonylated indolo[1,2-a]quinoline compounds and preparation method thereof Sulfonylated indolo[1,2-a]quinoline compounds and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/5218e891-e2f5-4b31-9445-b20b9ad6da5b/118824DEST_PATH_IMAGE005.png)
![Sulfonylated indolo[1,2-a]quinoline compounds and preparation method thereof Sulfonylated indolo[1,2-a]quinoline compounds and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/5218e891-e2f5-4b31-9445-b20b9ad6da5b/177730DEST_PATH_IMAGE009.png)
![Sulfonylated indolo[1,2-a]quinoline compounds and preparation method thereof Sulfonylated indolo[1,2-a]quinoline compounds and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/5218e891-e2f5-4b31-9445-b20b9ad6da5b/243775DEST_PATH_IMAGE006.png)
![A racemization method of (1r,6s)-8-benzyl-7,9-dioxo-2,8-diazabicyclo[4,3,0]nonane A racemization method of (1r,6s)-8-benzyl-7,9-dioxo-2,8-diazabicyclo[4,3,0]nonane](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8e9684b7-4576-4fa1-b780-285132757f08/BDA0000462153660000011.PNG)
![A racemization method of (1r,6s)-8-benzyl-7,9-dioxo-2,8-diazabicyclo[4,3,0]nonane A racemization method of (1r,6s)-8-benzyl-7,9-dioxo-2,8-diazabicyclo[4,3,0]nonane](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8e9684b7-4576-4fa1-b780-285132757f08/BDA0000462153660000021.PNG)
![A racemization method of (1r,6s)-8-benzyl-7,9-dioxo-2,8-diazabicyclo[4,3,0]nonane A racemization method of (1r,6s)-8-benzyl-7,9-dioxo-2,8-diazabicyclo[4,3,0]nonane](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8e9684b7-4576-4fa1-b780-285132757f08/BDA0000462153660000031.PNG)
![Sulfonylated indolo[1,2-a]quinoline compound and preparation method thereof Sulfonylated indolo[1,2-a]quinoline compound and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a9e609f4-c059-4d68-ab61-e08317597dab/118824DEST_PATH_IMAGE005.png)
![Sulfonylated indolo[1,2-a]quinoline compound and preparation method thereof Sulfonylated indolo[1,2-a]quinoline compound and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a9e609f4-c059-4d68-ab61-e08317597dab/132879DEST_PATH_IMAGE002.png)
![Sulfonylated indolo[1,2-a]quinoline compound and preparation method thereof Sulfonylated indolo[1,2-a]quinoline compound and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a9e609f4-c059-4d68-ab61-e08317597dab/177730DEST_PATH_IMAGE009.png)