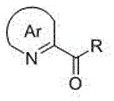
2-(α-deuterium-α-hydroxyl-α-aryl/alkyl) azaaromatic compound and its preparation method and application
A technology for aza aromatic hydrocarbons and compounds is applied in the field of 2-aza aromatic hydrocarbon compounds and their preparation, which can solve the problems of low stereoselectivity and the like, and achieve the effects of environmental friendliness, short reaction time and less amount of photocatalyst
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 5
[0024] The raw material 1-tert-butoxycarbonyl-2-benzoyl-benzimidazole used in Example 5, its synthesis can refer to the following documents: Sultan, S.; Rizvi, M.A.; Kumar, J.; Shah, B.A., Acyl Radicals from Terminal Alkynes:Photoredox-Catalyzed Acylation of Heteroarenes.Chemistry–A European Journal2018,24(42),10617-10620;
[0025] The synthesis of the raw materials used in the remaining examples can refer to the following documents: Wu, X.; Geng, X.; Zhao, P.; Zhang, J.; Gong, X.; Wu, Y.-d.; Wu, A.- X.,I 2 -Promoted Povarov-Type Reaction Using 1,4-Dithane-2,5-diol as an Ethylene Surrogate:Formal[4+2]Synthesis of Quinolines.Org.Lett.2017,19(7),1550-1553.
Embodiment 1
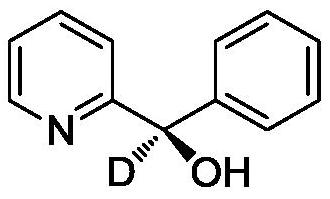
[0026] Embodiment 1: the preparation of 2-(α-deuterium-α-hydroxyl-α-phenyl)pyridine
[0027]
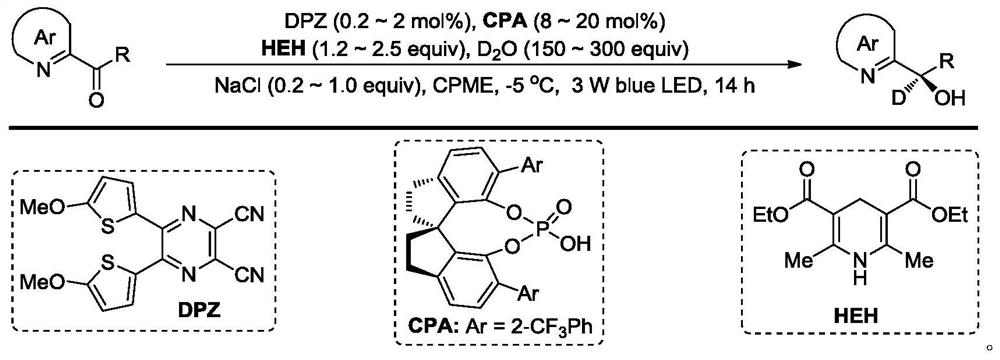
[0028] Preparation process: Weigh 2-benzoylpyridine (0.055g, 0.3mmol), DPZ (0.00053g, 0.0015mmol), Hans ester (0.11g, 0.45mmol), chiral spirocycle into a dry 25mL schlenk tube Phosphoric acid catalyst (0.018g, 0.003mmol), heavy water (0.8 ~ 1.6mL, 45 ~ 90mmol), sodium chloride (0.0035 ~ 0.0175g, 0.06mmol), continue to add 5mL of cyclopentyl methyl ether, at not higher than - At 78°C, use a vacuum pump to degas 2-3 times, each time for 5-10 minutes, then place it at -2-8°C, irradiate with a 3W blue light, and react for 3 hours. After the reaction, column chromatography separates ( Petroleum ether / ethyl acetate=20~4:1, volume ratio), rotary evaporation and concentration, vacuum drying (drying at 25°C for 1 hour), 2-(α-deuterium-α-hydroxyl-α-phenyl ) pyridine (0.056g, 0.3mmol), yield 96%, deuteration rate 91%. 1 H NMR (400MHz, CDCl 3 )δ8.60(d,J=4.8Hz,1H),7.65(td,J 1 =7.6Hz,J 2 =...
Embodiment 2
[0029] Embodiment 2: the preparation of 2-(α-deuterium-α-hydroxyl-α-phenyl) quinoline
[0030]
[0031] Preparation process: In a dry 25mL schlenk tube, weigh 2-benzoylquinoline (0.070g, 0.3mmol), DPZ (0.00053g, 0.0015mmol), Hansted ester (0.11g, 0.45mmol), chiral spiro Cyclic phosphoric acid catalyst (0.018g, 0.003mmol), heavy water (0.8~1.6mL, 45~90mmol), sodium chloride (0.0035~0.0175g, 0.06mmol), continue to add 5mL of cyclopentyl methyl ether, at no higher than At -78°C, use a vacuum pump to degas 2-3 times, each time for 5-10 minutes, and then place it at -2-8°C, irradiate with a 3W blue light, and react for 3 hours. After the reaction, separate by column chromatography (petroleum ether / ethyl acetate=20~4:1, volume ratio), concentrated by rotary evaporation, and vacuum dried (dried at 25°C for 1 hour) to obtain optically pure 2-(α-deuterium-α-hydroxyl-α- Phenyl)quinoline (0.068g, 0.279mmol), yield 96%, enantiomeric excess 91%, deuterated rate 91%. 1 H NMR (300MHz, C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com