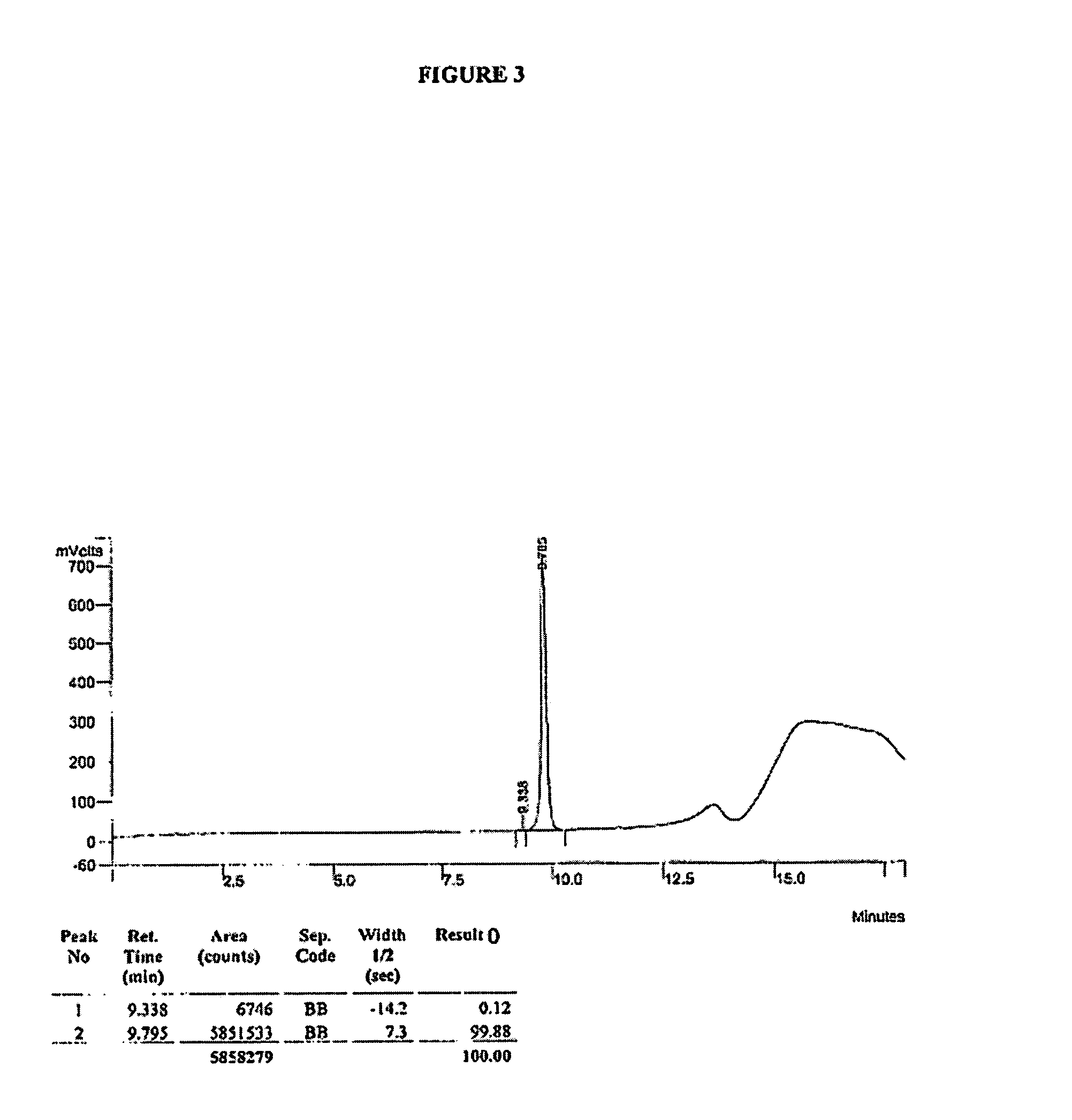
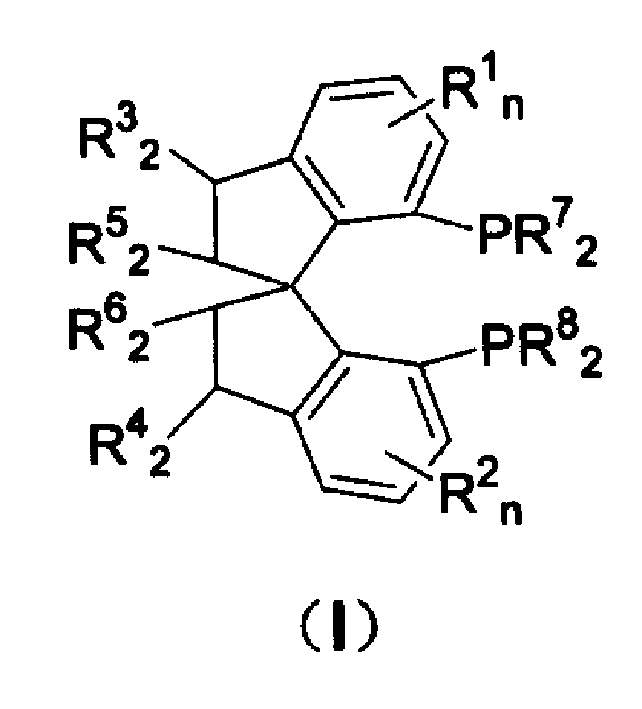
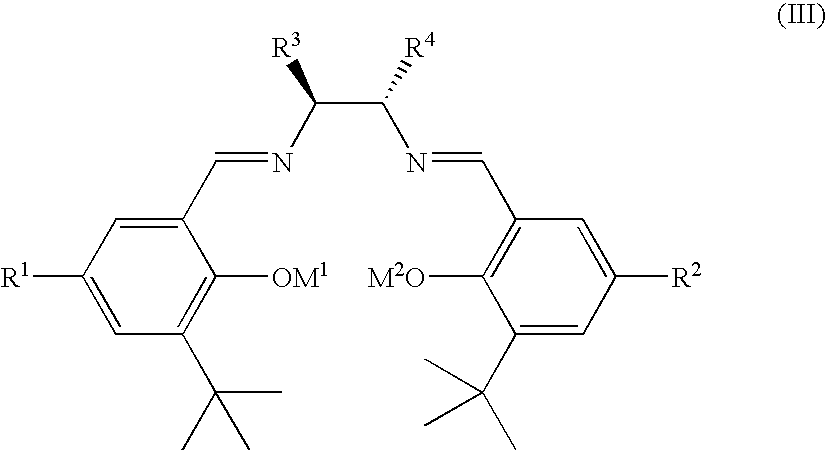
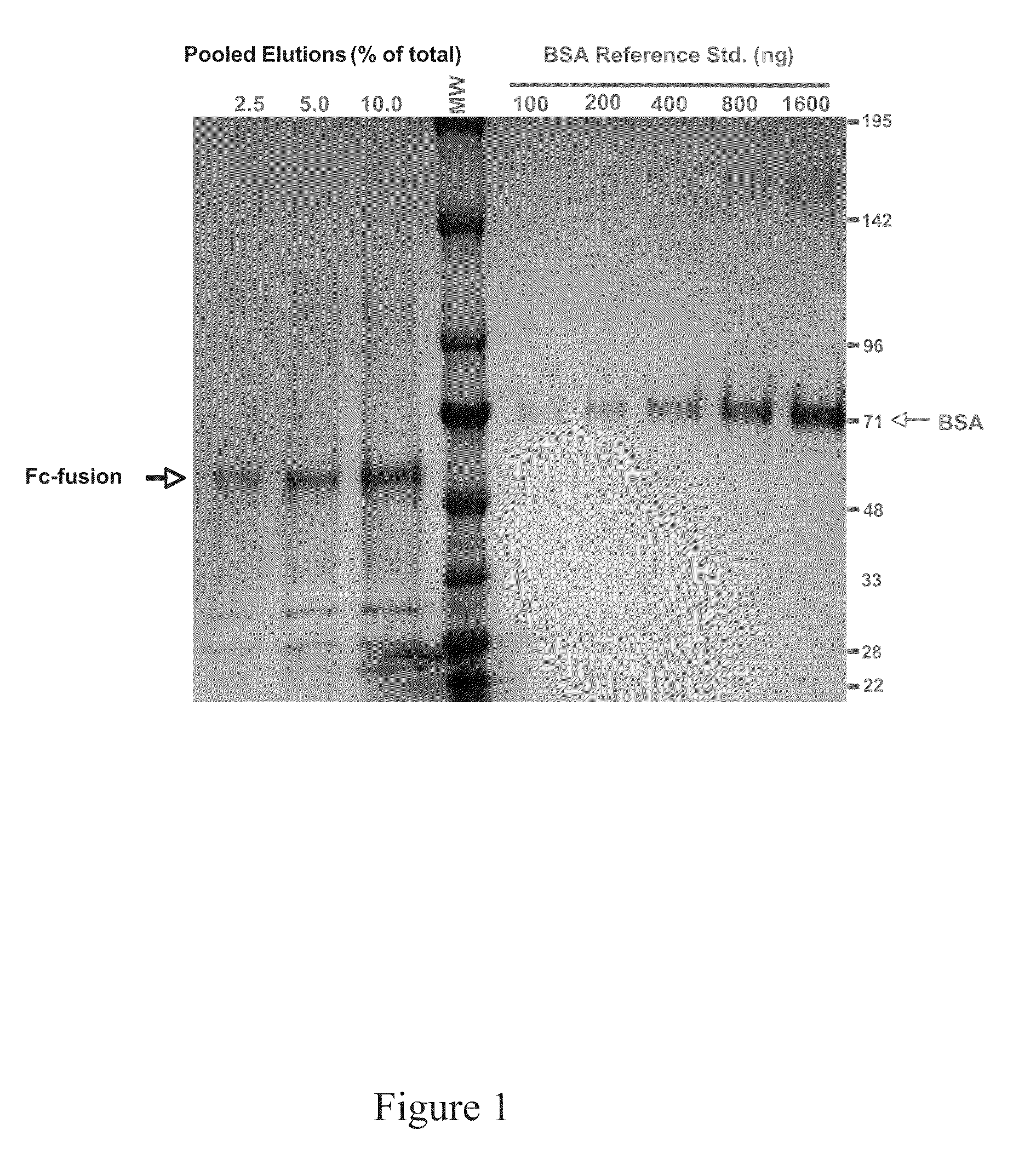
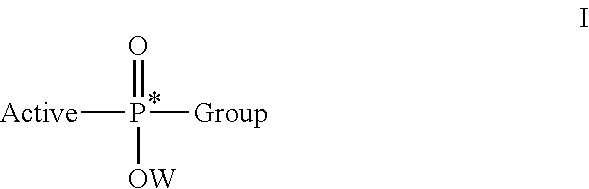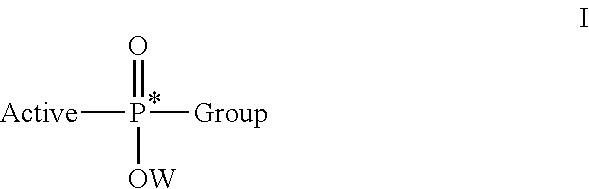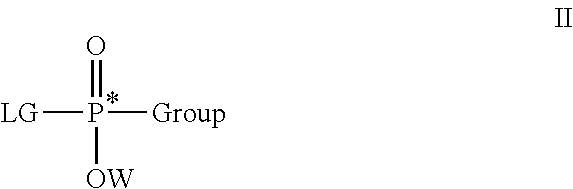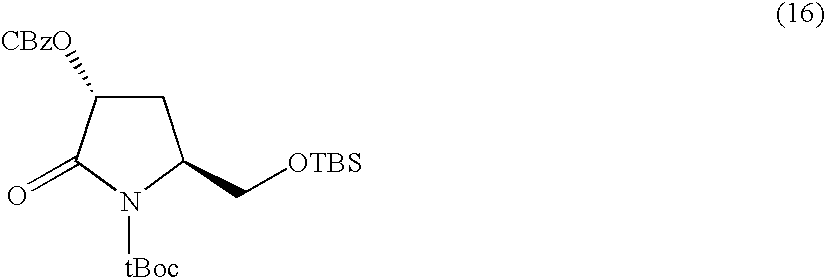Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2348 results about "Stereoselectivity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
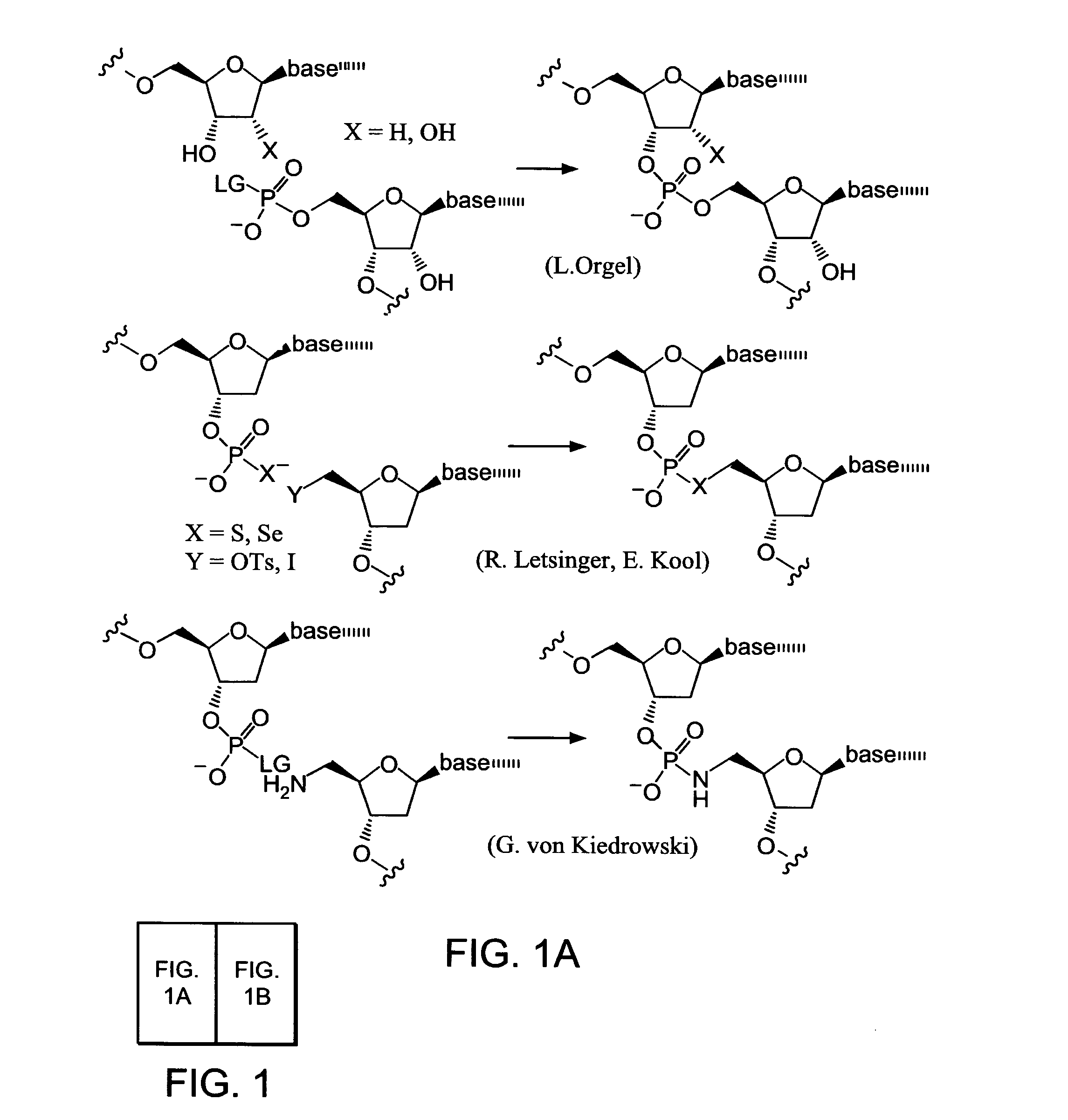
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non-stereospecific creation of a new stereocenter or during a non-stereospecific transformation of a pre-existing one. The selectivity arises from differences in steric effects and electronic effects in the mechanistic pathways leading to the different products. Stereoselectivity can vary in degree but it can never be total since the activation energy difference between the two pathways is finite. Both products are at least possible and merely differ in amount. However, in favorable cases, the minor stereoisomer may not be detectable by the analytic methods used.
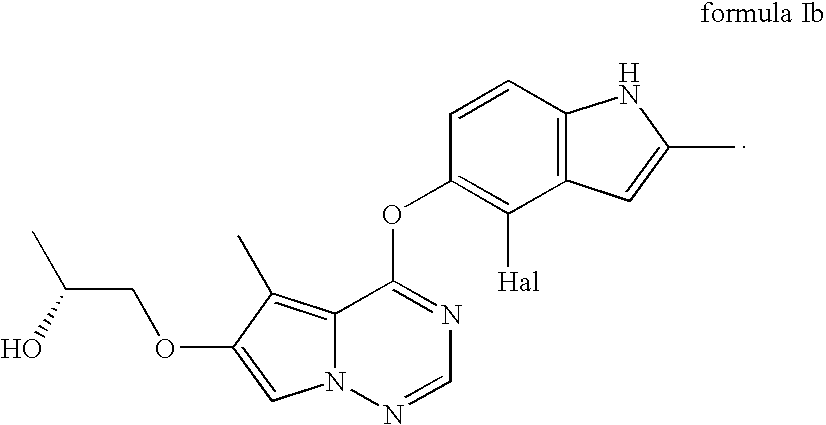
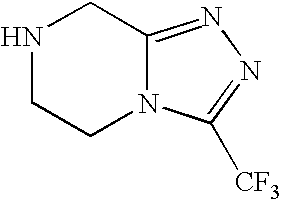
Stereoselective reduction process for the preparation of pyrrolotriazine compounds
The invention relates to a process for the enzymatic, stereoselective reduction of ketone compounds to provide chiral alcohols, for example the compound of formula Ib:
Owner:BRISTOL MYERS SQUIBB CO
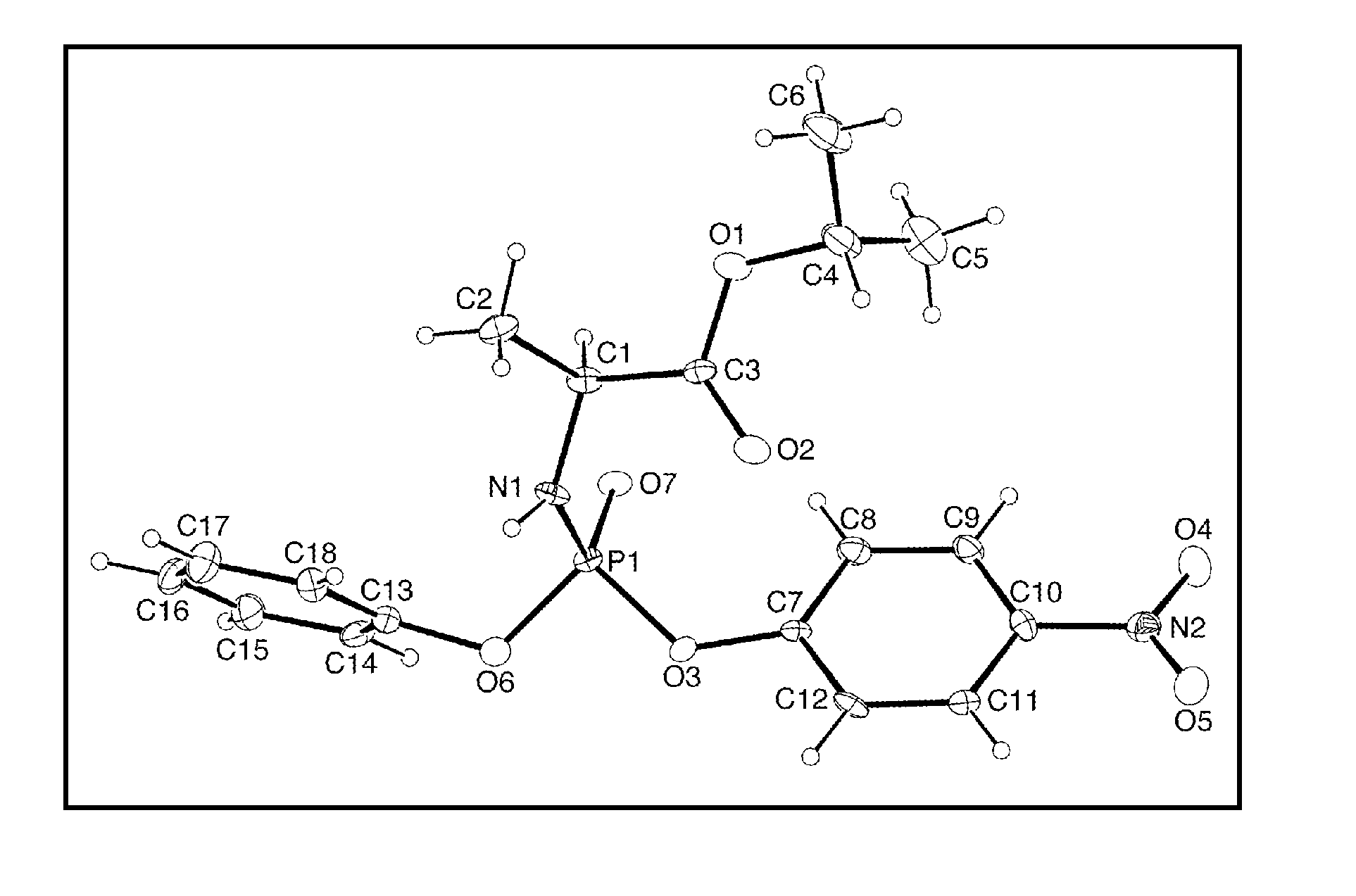
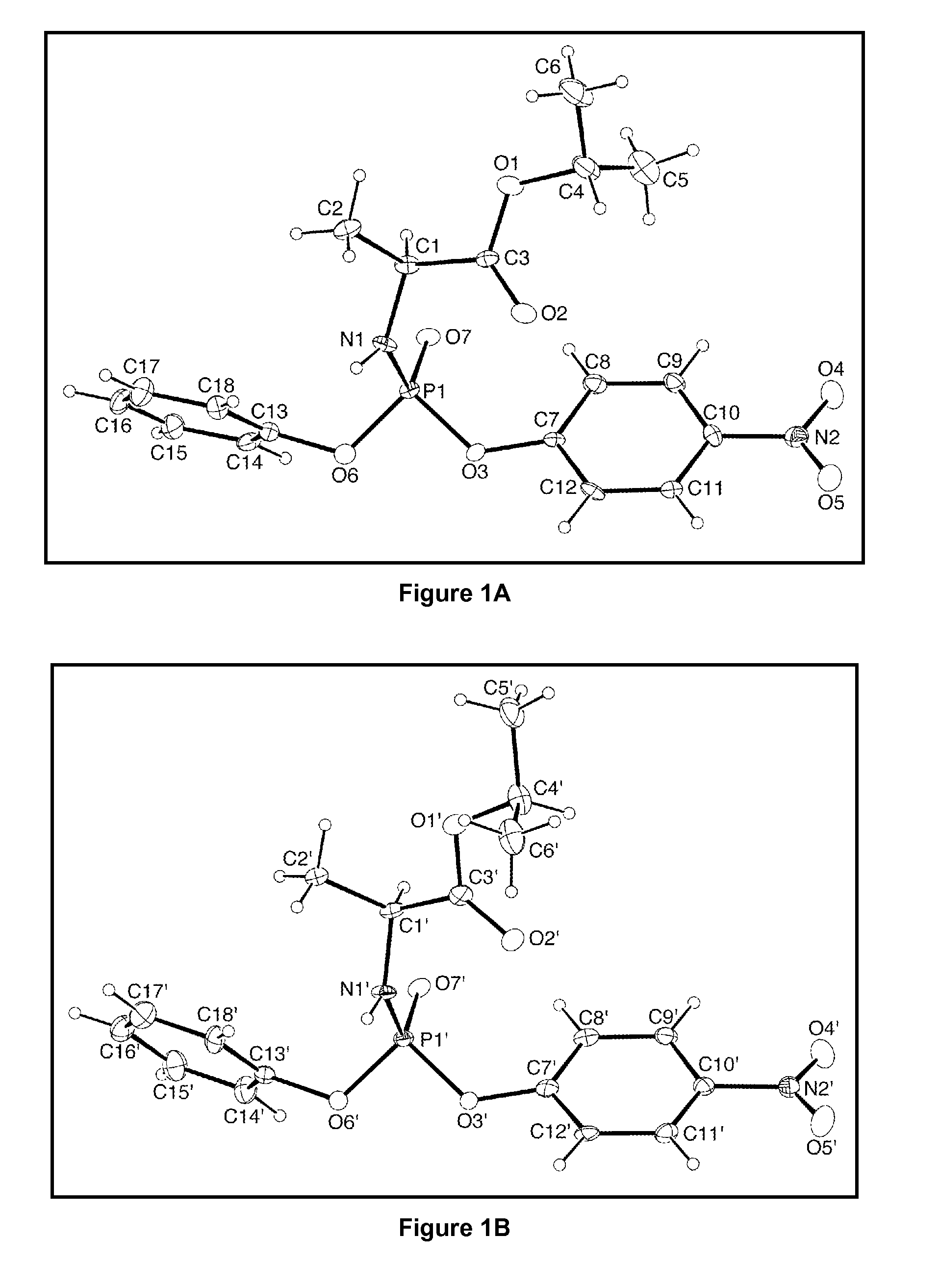
Stereoselective synthesis of phosphorus containing actives
ActiveUS20110245484A1Organic active ingredientsGroup 4/14 element organic compoundsOrganic chemistryStereoselectivity
Disclosed herein are phosphorus-containing actives, their use as actives for treating diseases, and a stereoselective process for preparing the same. Also disclosed herein are useful synthetic intermediates and processes for preparing the same.
Owner:GILEAD SCI INC
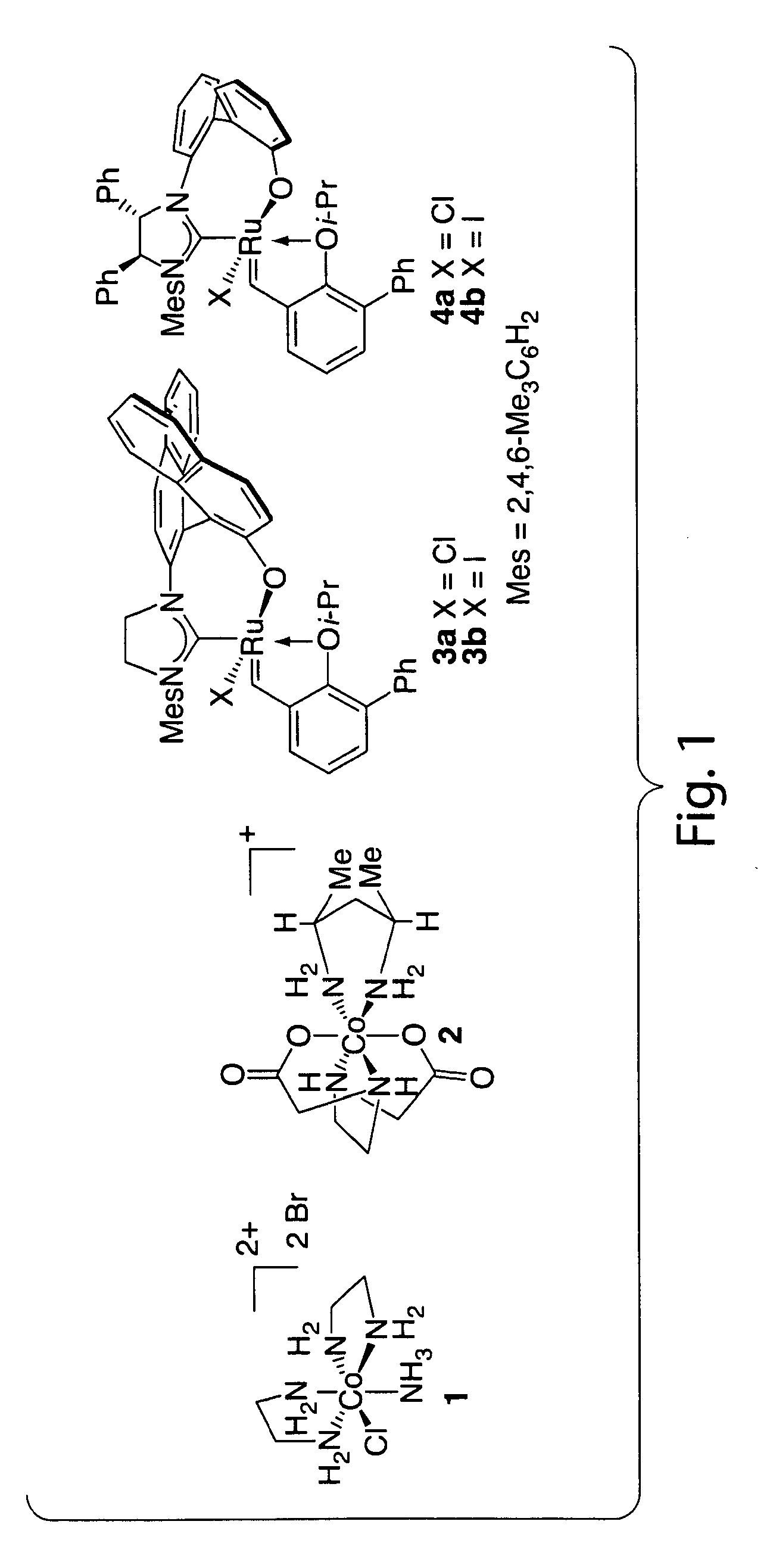
Catalysts for metathesis reactions including enantioselective olefin metathesis, and related methods
ActiveUS20110065915A1Silicon organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsChemical reactionEnantio selectivity
The present invention provides compositions comprising metal complexes, and related methods. In some embodiments, metal complexes of the invention may be useful as catalysts for chemical reactions, including metathesis reactions, wherein the catalysts exhibit enhanced activity and stereoselectivity. In some embodiments, the invention may advantageously provide metal complexes comprising a stereogenic metal atom. Such metal complexes may be useful in enantioselective catalysis.
Owner:BOSTON COLLEGE +1
Stereoselective Synthesis of Amino Acid Analogs for Tumor Imaging
InactiveUS20060292073A1Maximum service lifeOrganic compound preparationSulfonic acid esters preparation1-amino-3-fluorocyclobutane-1-carboxylic acidCyclobutane
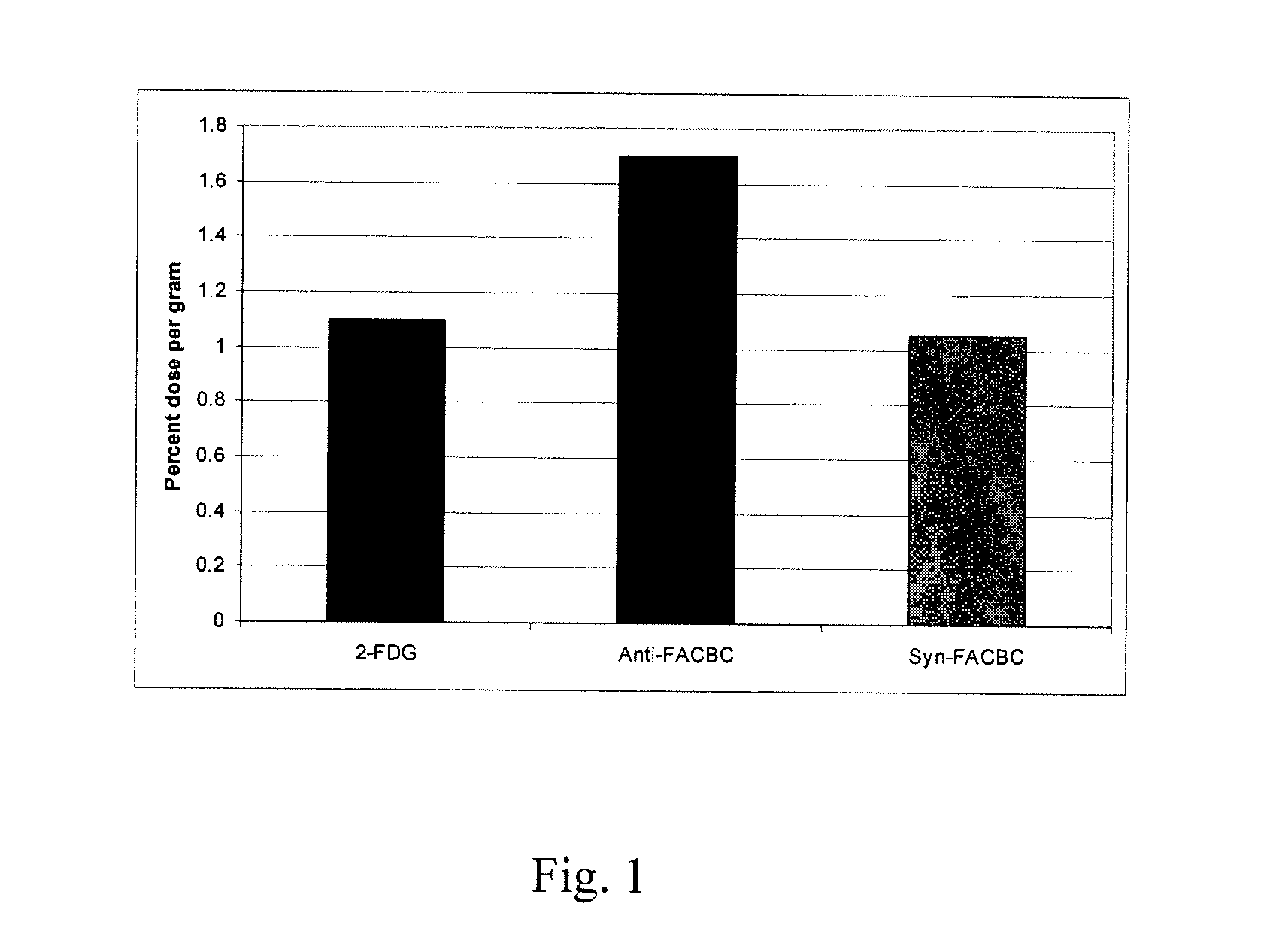
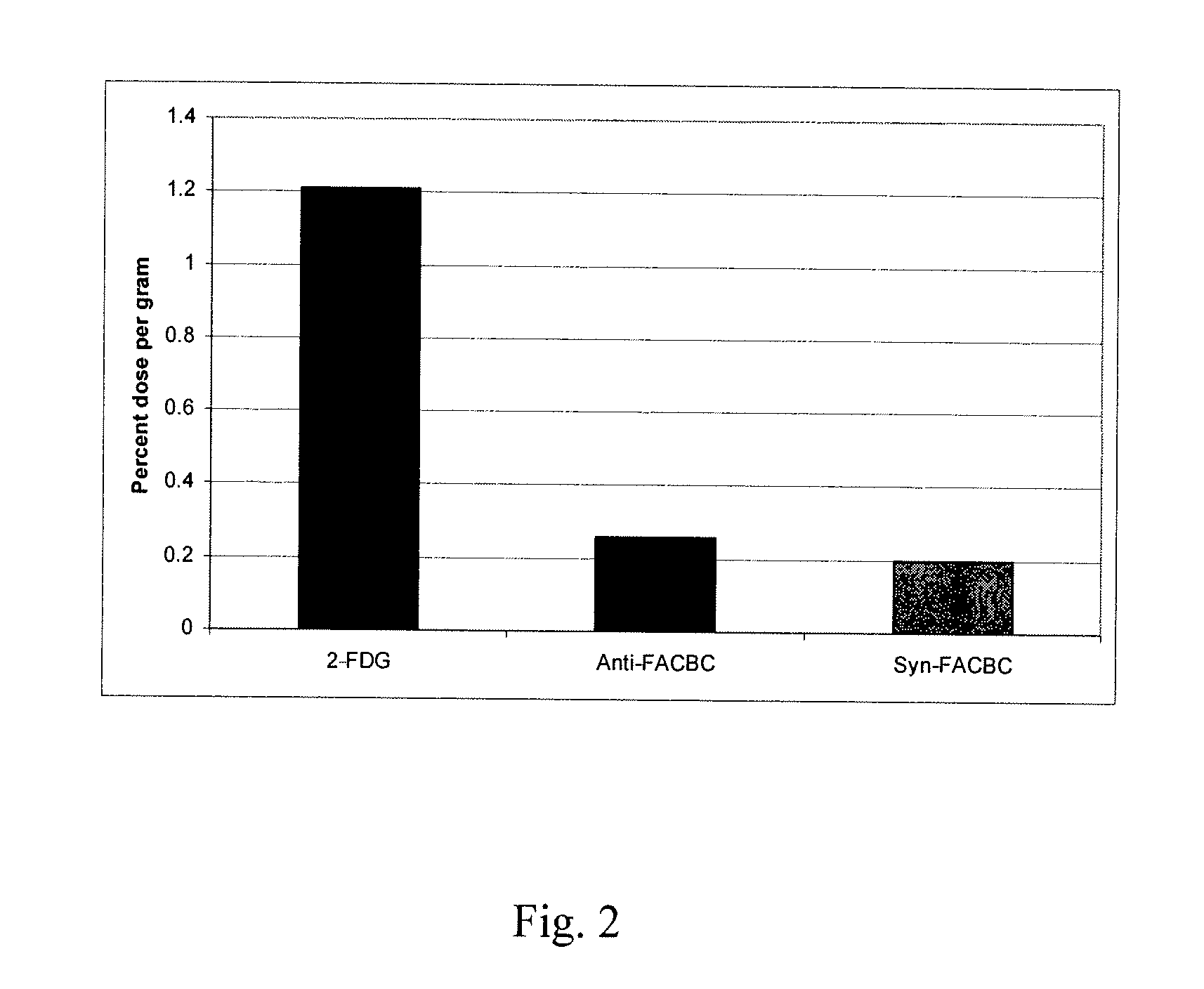
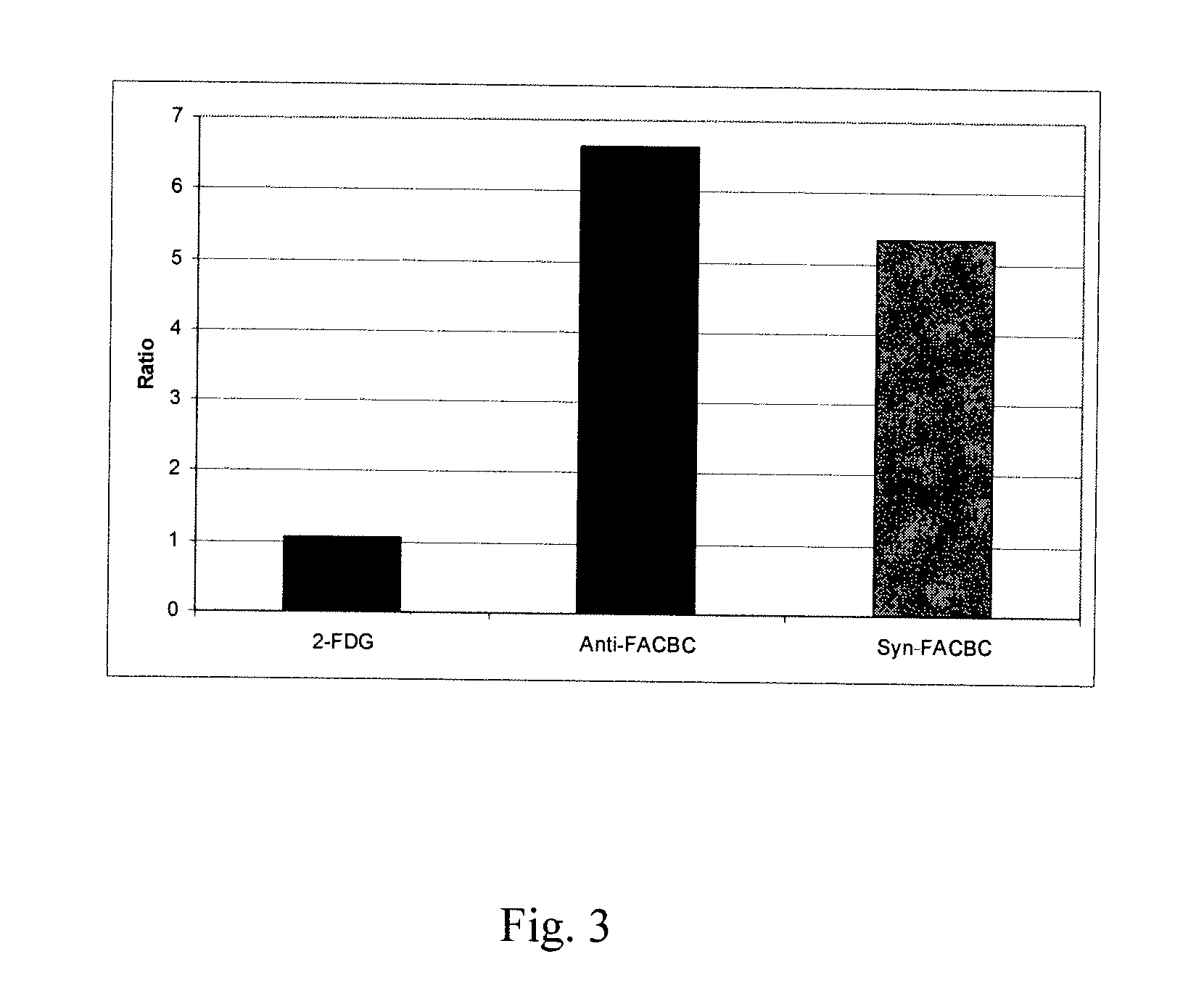
The radiolabeled non-natural amino acid 1-amino-3-cyclobutane-1-carboxylic acid (ACBC) and its analogs are candidate tumor imaging agents useful for positron emission tomography and single photon emission computed tomography due to their selective affinity for tumor cells. The present invention provides methods for stereo-selective synthesis of syn-ACBC analogs. The disclosed synthetic strategy is reliable and efficient and can be used to synthesize a gram quantity of various syn-isomers of the ACBC analogs, particularly, syn-[18F]-1-amino-3-fluorocyclobutane-1-carboxylic acid (FACBC) and syn-[123I]-1-amino-3-iodocyclobutane-1-carboxylic (IACBC) acid analogs.
Owner:EMORY UNIVERSITY
Producing optically active amino compounds
InactiveUS7169592B2Efficient and inexpensiveSuitable for useSugar derivativesBacteriaMicroorganismNucleotide
Owner:KANEKA CORP
Evolving new molecular function
InactiveUS7491494B2High selectivityEnhance covalent bond formationNucleotide librariesMicrobiological testing/measurementBio moleculesChemical reaction
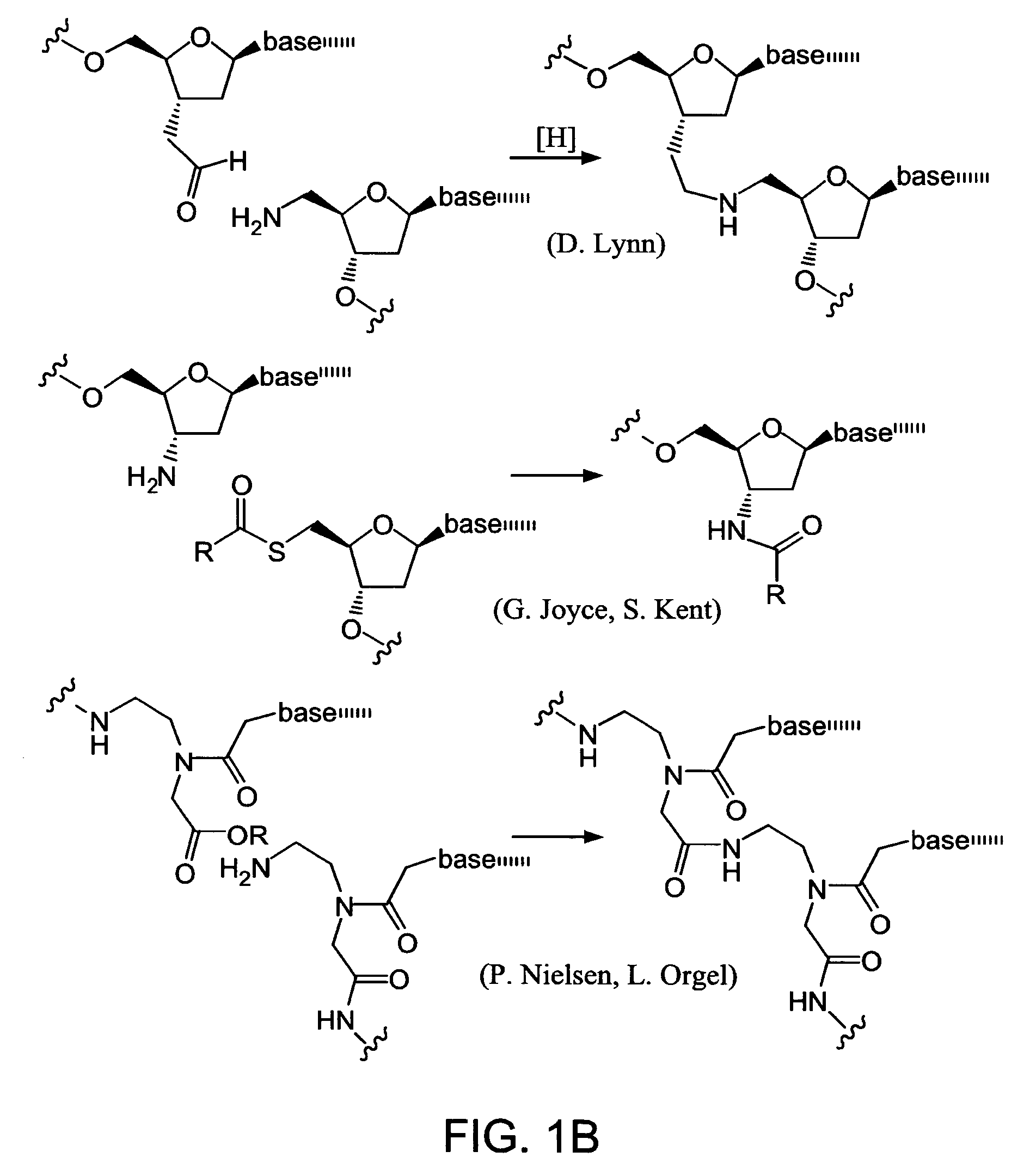
Nature evolves biological molecules such as proteins through iterated rounds of diversification, selection, and amplification. The power of Nature and the flexibility of organic synthesis are combined in nucleic acid-templated synthesis. The present invention provides a variety of template architectures for performing nucleic acid-templated synthesis, methods for increasing the selectivity of nucleic acid-templated reactions, methods for performing stereoselective nucleic acid-templated reactions, methods of selecting for reaction products resulting from nucleic acid-templated synthesis, and methods of identifying new chemical reactions based on nucleic acid-templated synthesis.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
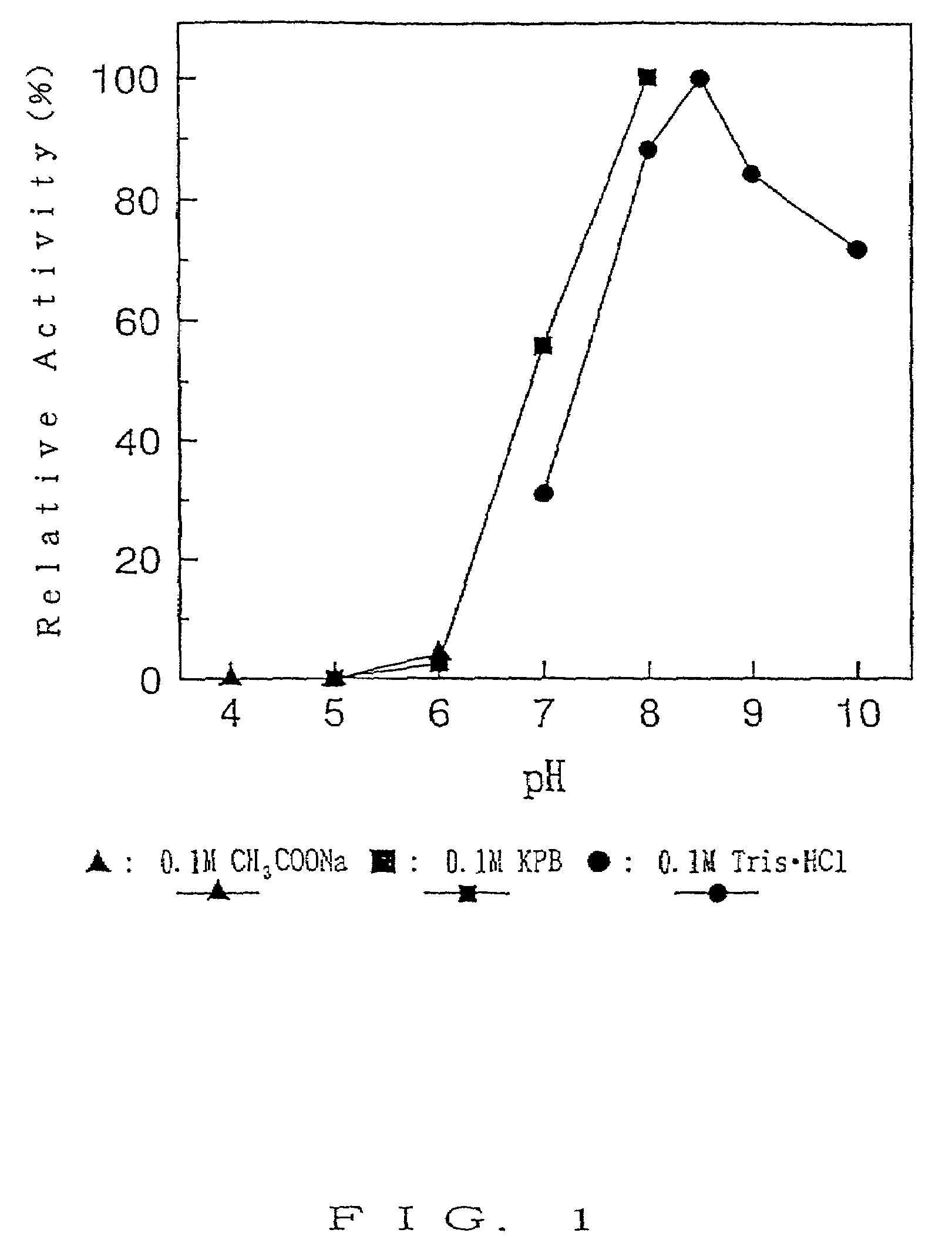
Method for screening stereoselective alpha-hydroxy acid dehydrogenase
InactiveCN102660631AOvercoming time-consuming and laboriousOvercoming demandsMicrobiological testing/measurementWater bathsAlpha hydroxy acid
The invention provides a method for screening stereoselective alpha-hydroxy acid dehydrogenase, comprising the following steps: (1) dissolving a sample to be detected containing alpha-hydroxy acid dehydrogenase in a buffer with pH of 6-9, adding an alpha-hydroxy acid chiral monomer as a substrate, and carrying out conversion reaction in water-bath at 20-50 DEG C; and (2) after the conversion reaction, adding the conversion solution in an FeCl3 developer solution to conduct color development reaction for 5-30 min, when the color development reaction finishes, judging the result according to the color appeared in the reaction solution. The method has no need to carry out derivatization on the substrate which is intend to screen, can rapidly identify the dehydrogenase activity and optical selectivity of the selected microbe by using simple colourimetry, has the advantages of simple screening flow, fast speed, low request for devices, strong versatility and the like, and offers great conveniences to the obtainment of optically pure products by using racemic mandelic acid and related derivatives as substrates to carry out resolution through biological enzymatic method.
Owner:ZHEJIANG UNIV OF TECH
Synthesis of clasto-lactacystin .beta.-lactone and analogs thereof
InactiveUS6849743B2High activity in inhibitionPrevent and reduce sizeBiocideOrganic compound preparationCombinatorial chemistryFormamide
The present invention is directed to an improved synthesis of clasto-lactacystin-β-lactone, and analogs thereof, that proceeds in fewer steps and in much greater overall yield than syntheses described in the prior art. The synthetic pathway relies upon a novel stereospecific synthesis of an oxazoline intermediate and a unique stereoselective addition of a formyl amide to the oxazoline. Also described are novel clasto-lactacystin-β-lactones, and analogs thereof and their use as proteasome inhibitors.
Owner:MILLENNIUM PHARMA INC
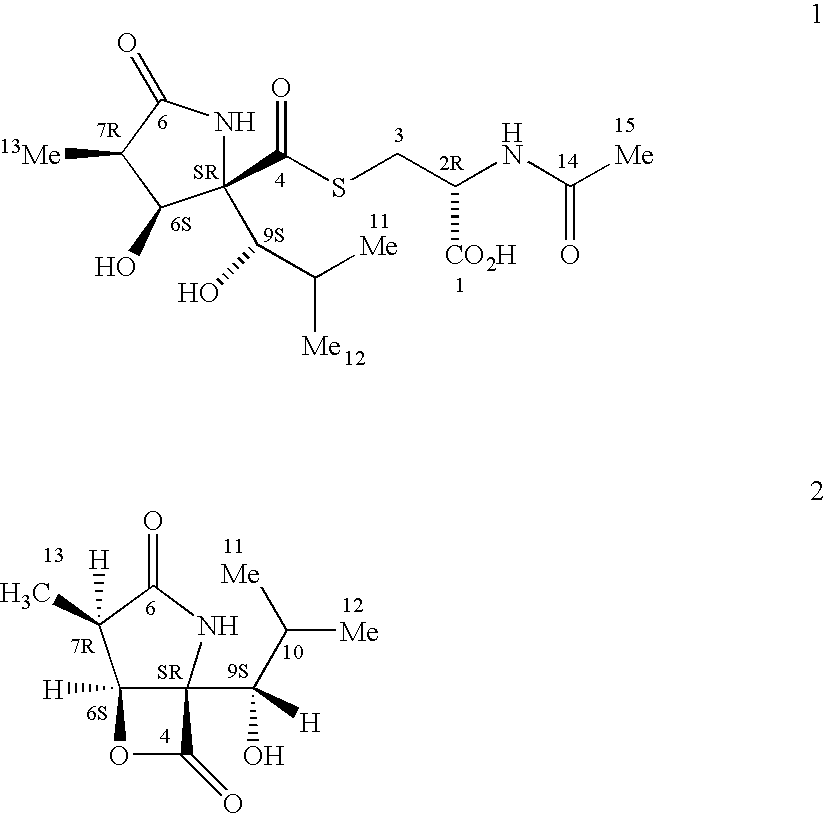
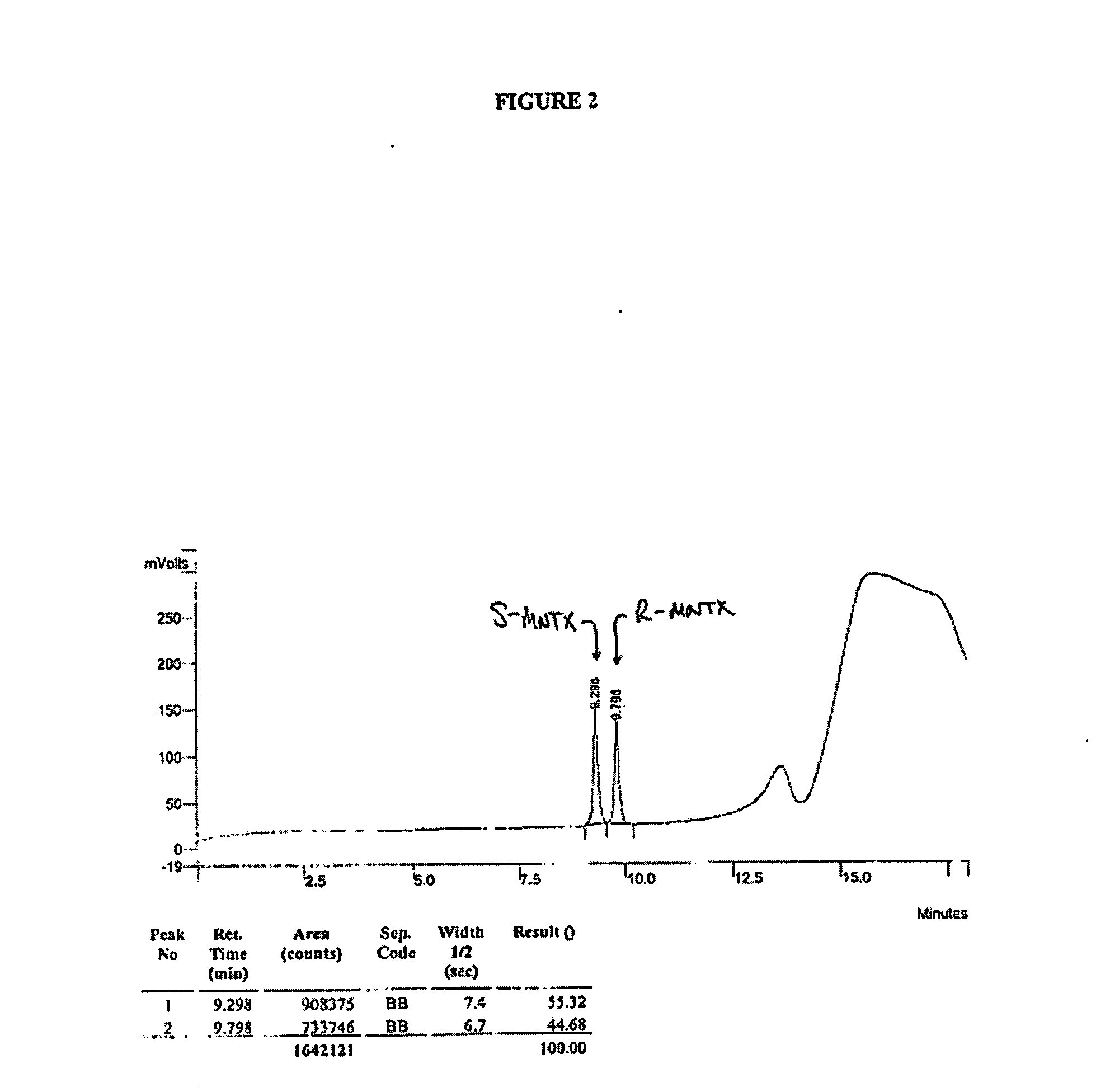
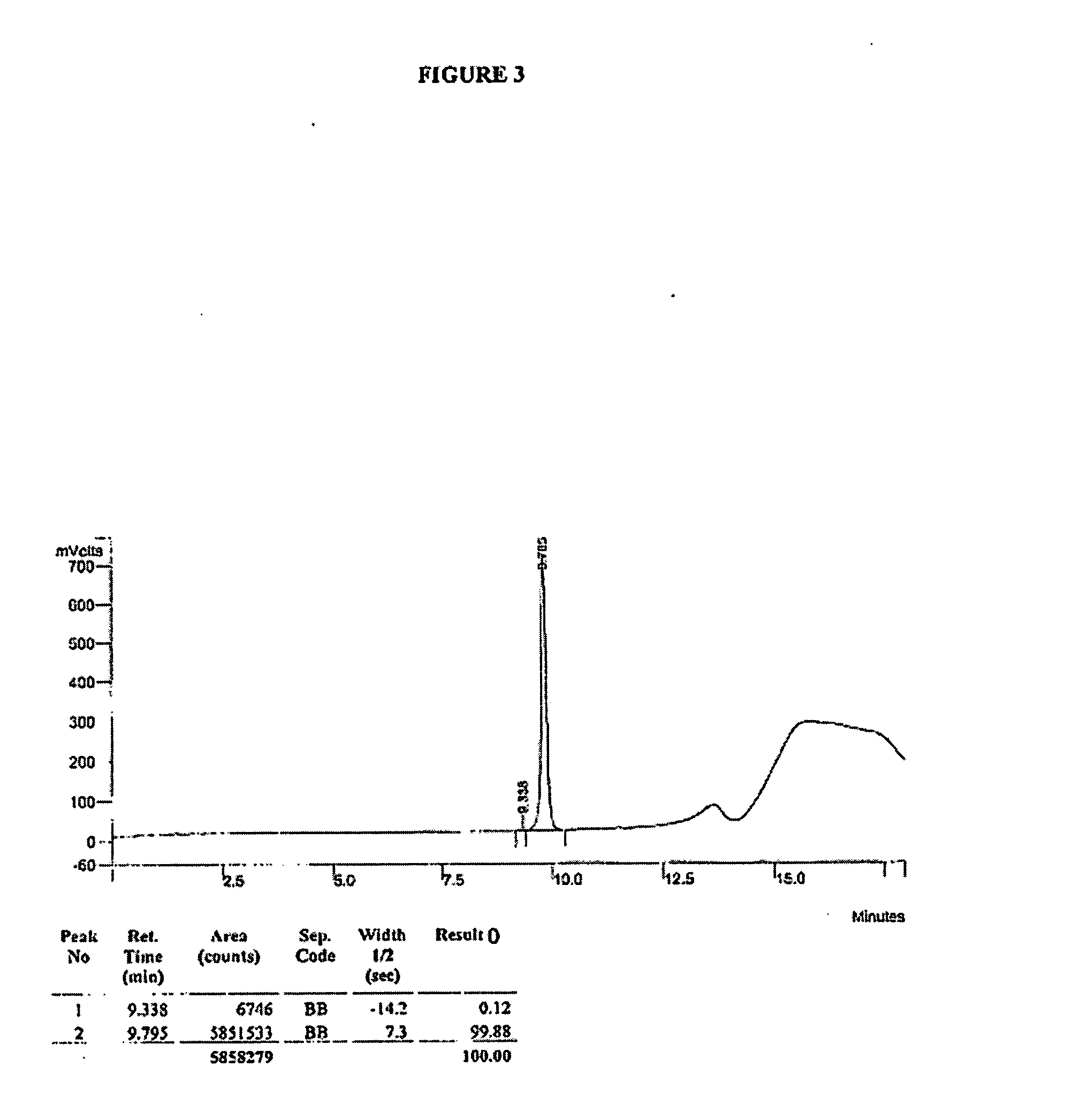
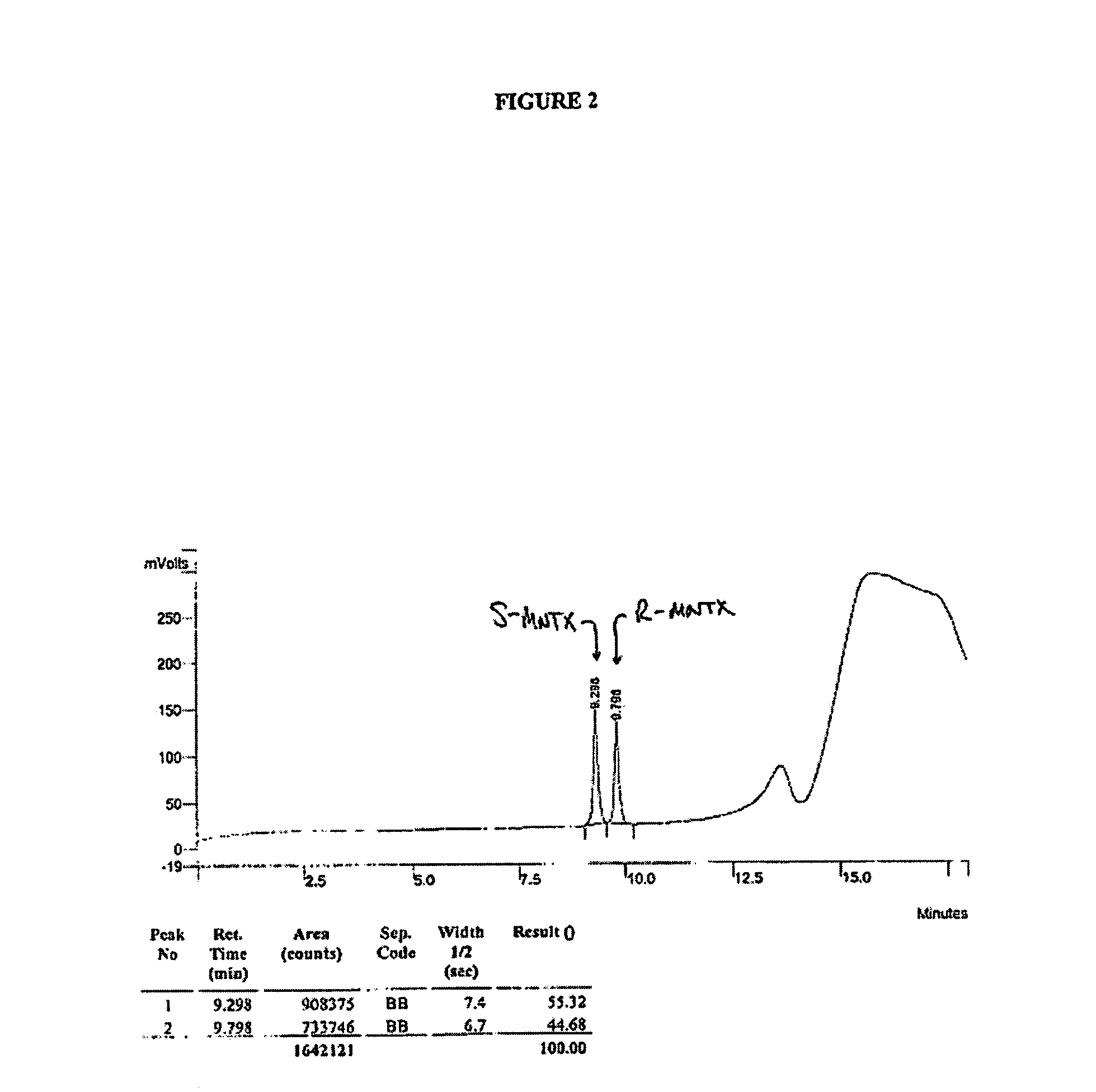
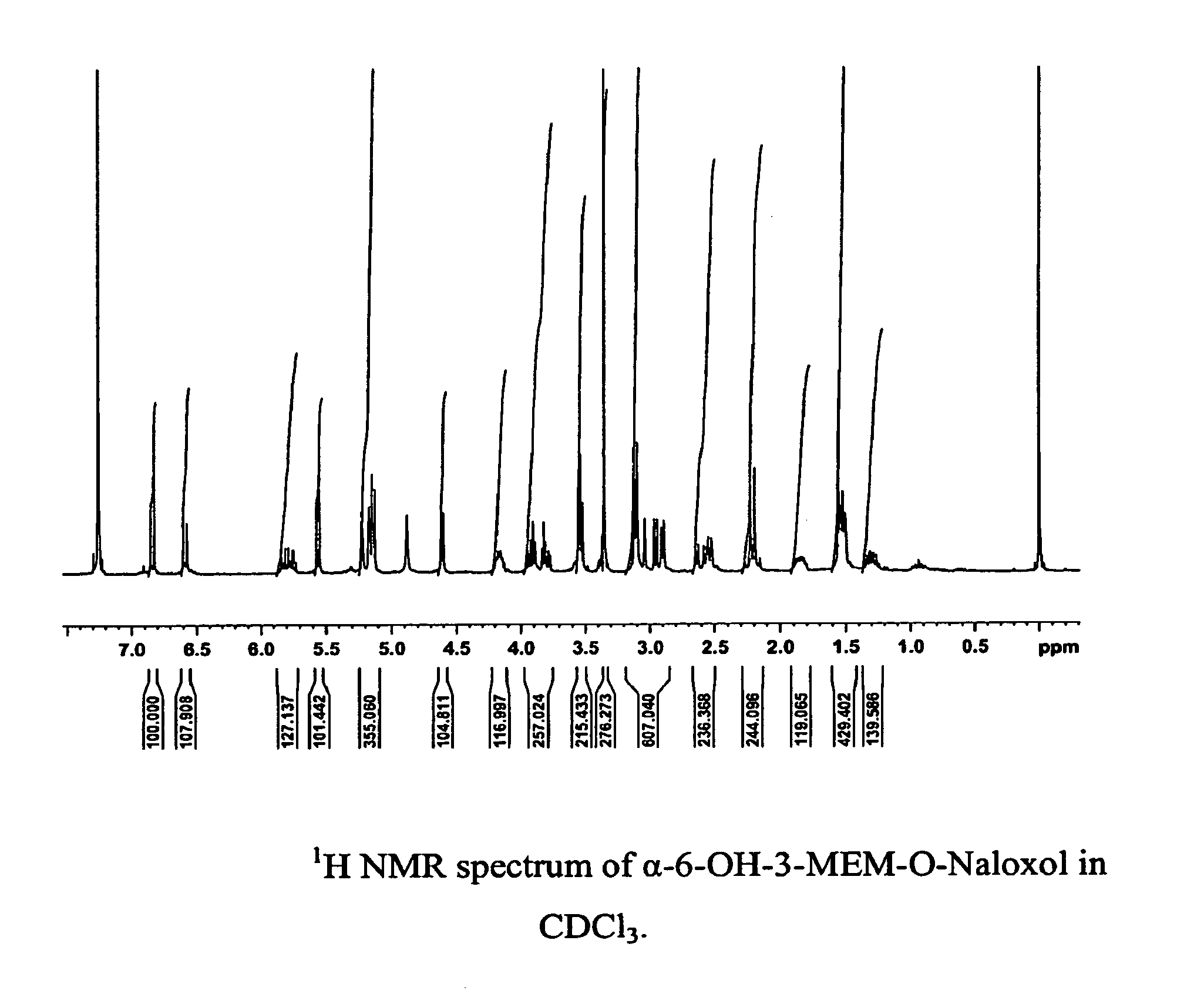
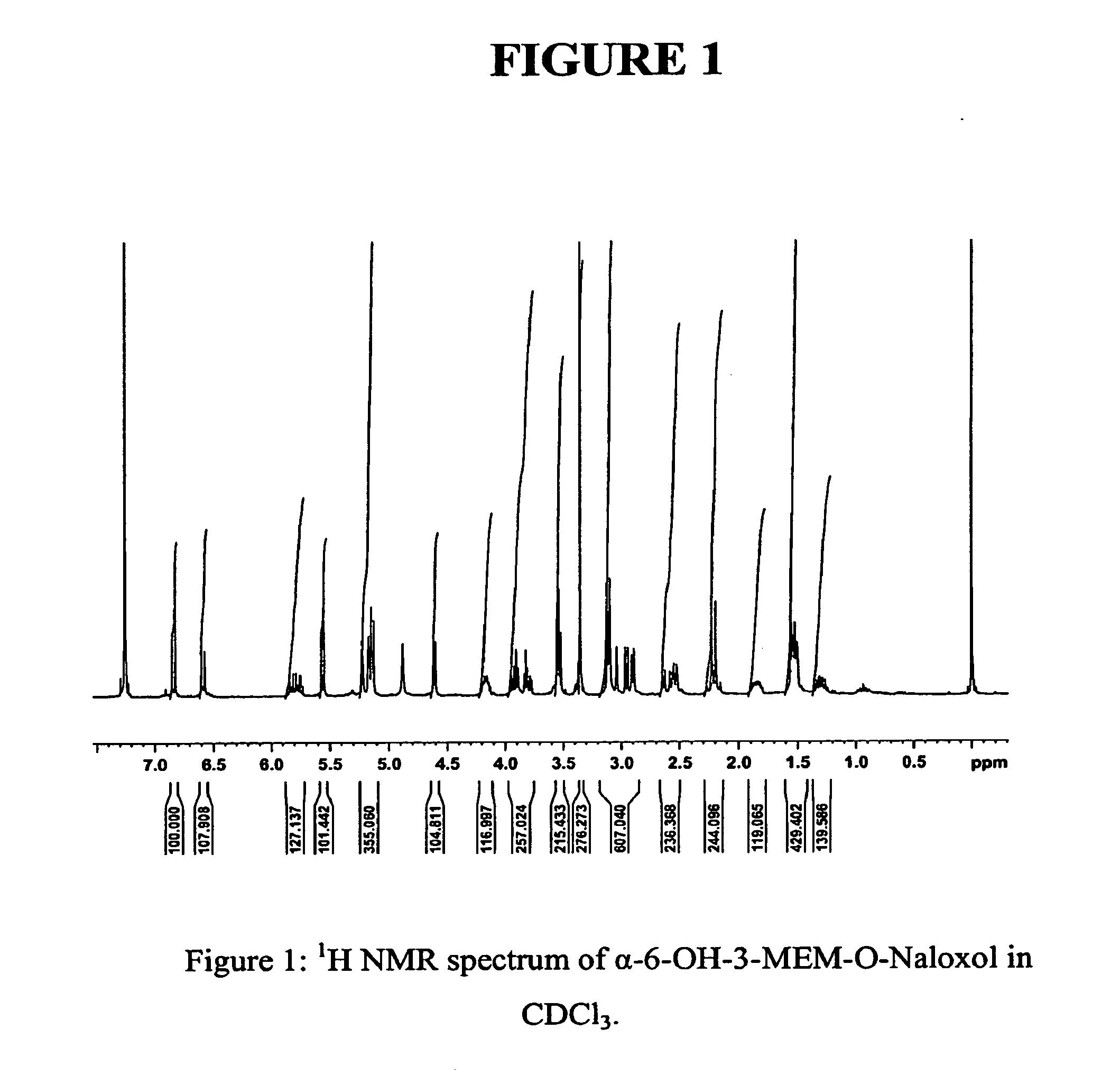
Synthesis of R-N-methylnaltrexone
InactiveUS20070099946A1Organic active ingredientsAntipyreticCombinatorial chemistryStereoselectivity
Owner:PROGENICS PHARMA INC
Evolving new molecular function
ActiveUS7771935B2High selectivityEnhance covalent bond formationMicrobiological testing/measurementFermentationChemical reactionOrganic synthesis
Nature evolves biological molecules such as proteins through iterated rounds of diversification, selection, and amplification. The power of Nature and the flexibility of organic synthesis are combined in nucleic acid-templated synthesis. The present invention provides a variety of template architectures for performing nucleic acid-templated synthesis, methods for increasing the selectivity of nucleic acid-templated reactions, methods for performing stereoselective nucleic acid-templated reactions, methods of selecting for reaction products resulting from nucleic acid-templated synthesis, and methods of identifying new chemical reactions based on nucleic acid-templated synthesis.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
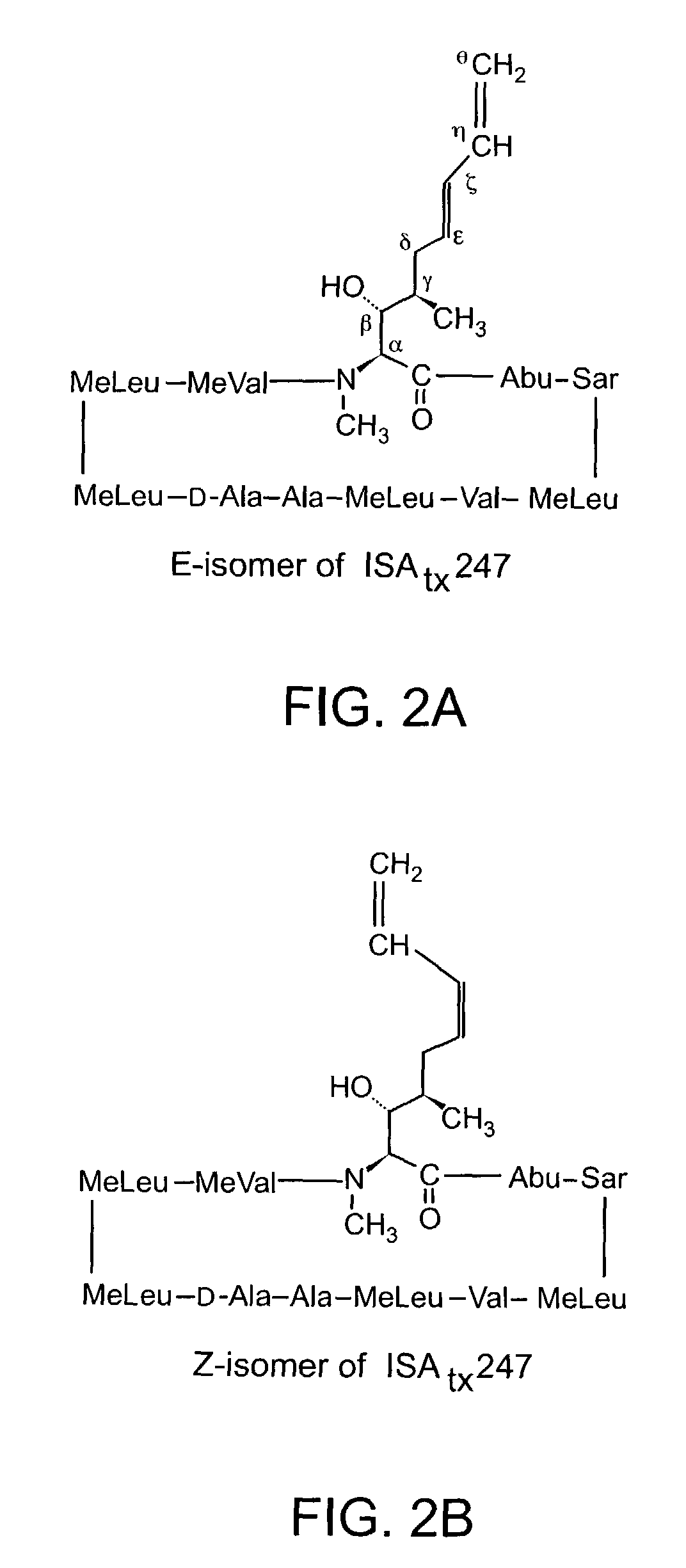
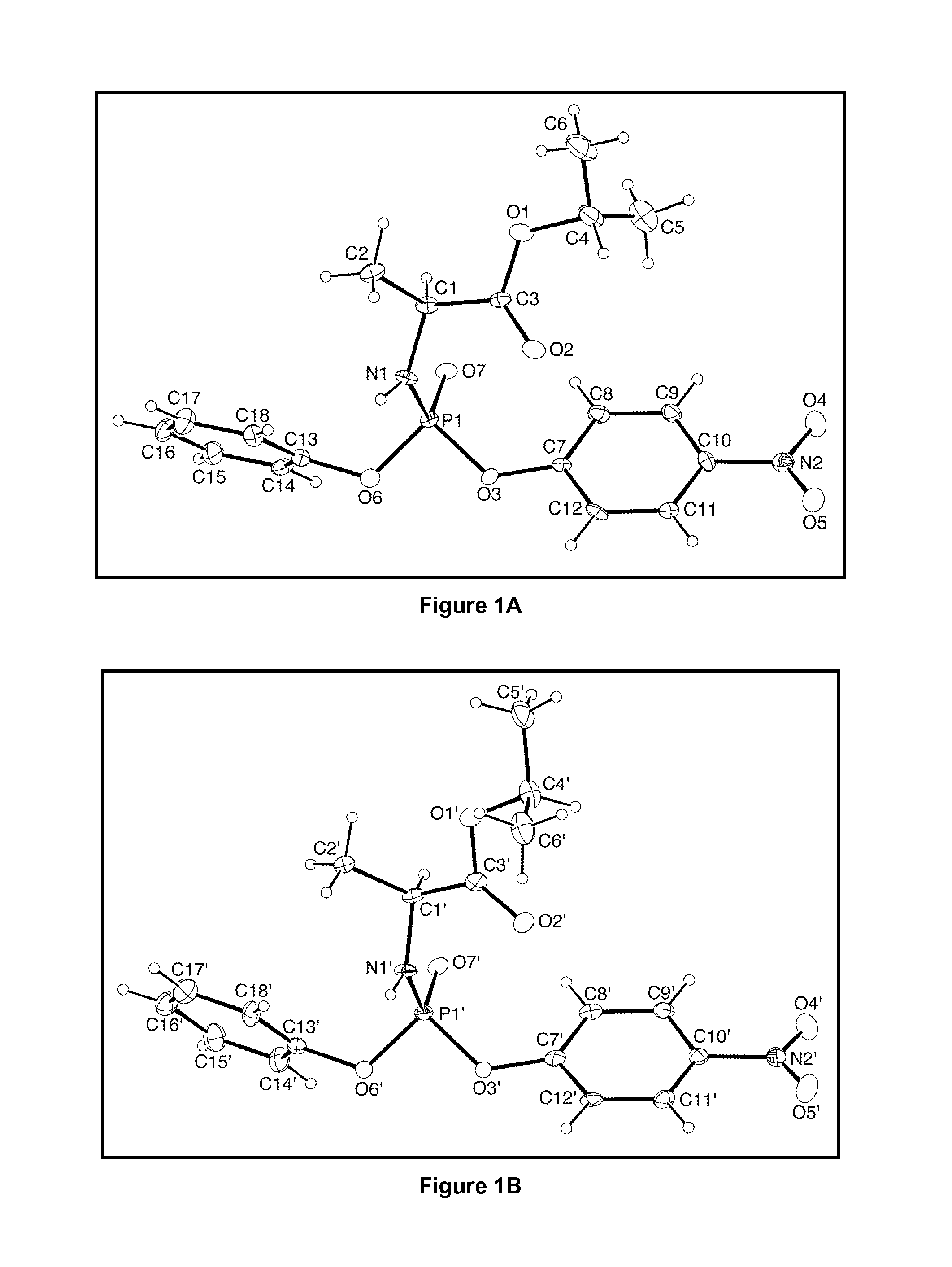
Cyclosporine analogue mixtures and their use as immunomodulating agents
ActiveUS6998385B2Potent immunosuppressantImprove effectivenessSenses disorderNervous disorderCyclosporinsImmunomodulating Agent
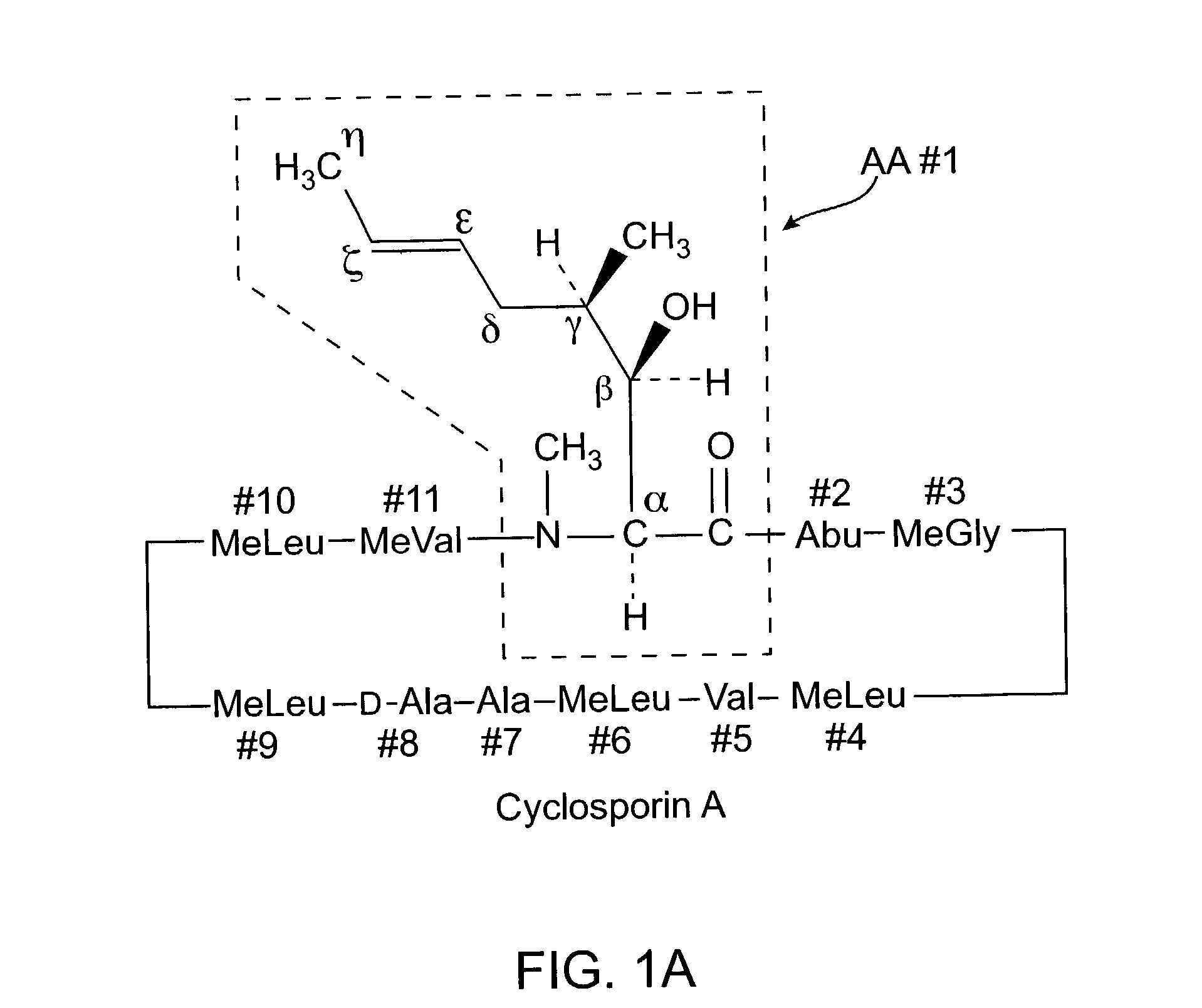
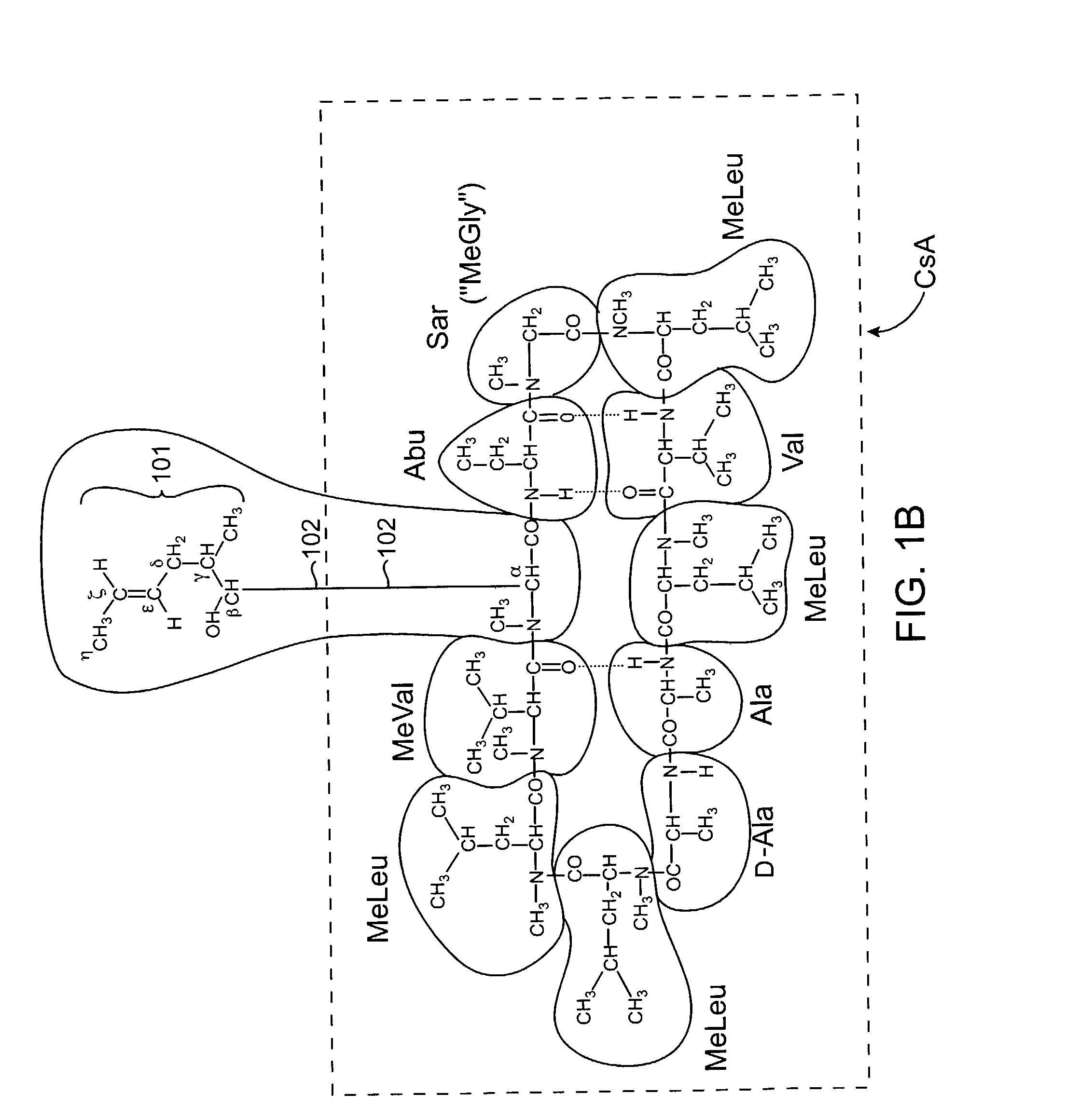
The invention is directed to isomeric mixtures of cyclosporine analogues that are structurally similar to cyclosporine A. The mixtures possess enhanced efficacy and reduced toxicity over the individual isomers and over naturally occurring and other presently known cyclosporines and cyclosporine derivatives. Embodiments of the present invention are directed toward cis and trans-isomers of cyclosporin A analogs referred to as ISATX247, and derivatives thereof. Mixtures of ISATX247 isomers exhibit a combination of enhanced potency and reduced toxicity over the naturally occurring and presently known cyclosporins. ISATX247 isomers and alkylated, arylated, and deuterated derivatives are synthesized by stereoselective pathways where the particular conditions of a reaction determine the degree of stereoselectivity. The ratio of isomers in a mixture may range from about 10 to 90 percent by weight of the (E)-isomer to about 90 to 10 percent by weight of the (Z)-isomer, based on the total weight of the mixture.
Owner:AURINIA PHARMA
Stereoselective synthesis of phosphorus containing actives
ActiveUS8859756B2Organic active ingredientsGroup 4/14 element organic compoundsOrganic chemistryStereoselectivity
Disclosed herein are phosphorus-containing actives, their use as actives for treating diseases, and a stereoselective process for preparing the same. Also disclosed herein are useful synthetic intermediates and processes for preparing the same.
Owner:GILEAD SCI INC
Process for manufacturing a glutamic acid derivative and a pyroglutamic acid derivative and a novel intermediate in the manufacture thereof
InactiveUS20060009394A1Easy to manufactureEfficient and easy manufactureNervous disorderOrganic compound preparationAlkylating antineoplastic agentHydroxyproline
Glutamic acid derivatives, in particular monatin, may be conveniently prepared by alkylating a 4-protected hydroxyl pyroglutamic acid derivative with an alkylating agent to prepare a 4-protected hydroxyl-4-alkylglutamic acid derivative, followed by the steps of hydrolysis and deprotection. The 4-protected hydroxyl pyroglutamic acid derivative is easy to produce from hydroxyproline. The 4-protected hydroxyl pyroglutamic acid derivative is particularly suitable for use in the efficient manufacture of monatin of high optical purity, since it can be alkylated selectively at the 4-position and stereoselectively and after its alkylation, it can easily be converted to a glutamic acid derivative.
Owner:AJINOMOTO CO INC
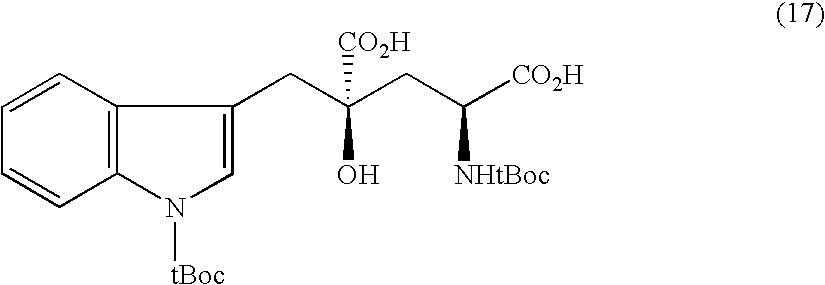
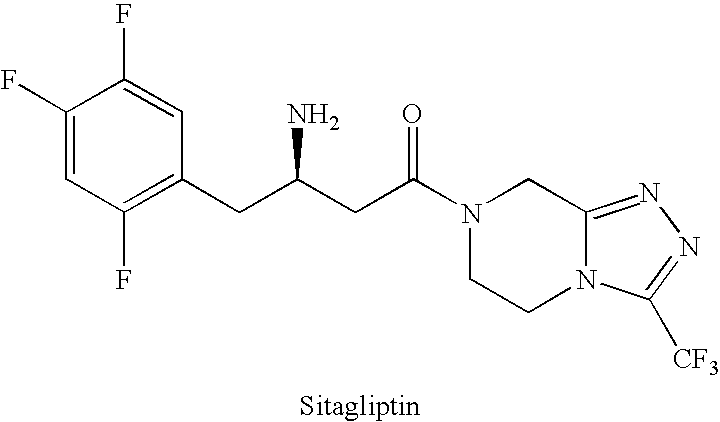
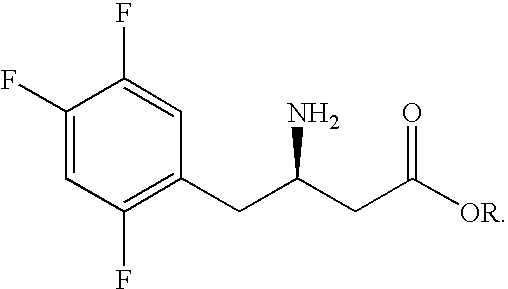
Preparation of sitagliptin intermediate
InactiveUS20090192326A1Organic compound preparationAmino-carboxyl compound preparationSitagliptinMedicinal chemistry
Intermediate compounds in the synthesis of Sitagliptin, 3-amino-4-(2,4,5-trifluorophenyl)but-2-enoic acid alkyl ester, and amino protected-3-amino-4-(2,4,5-trifluorophenyl)but-2-enoic acid alkyl ester, and the stereoselective reduction of these compound to give Synthon I, or the amino-protected Synthon I, are provided.
Owner:TEVA PHARM USA INC +1
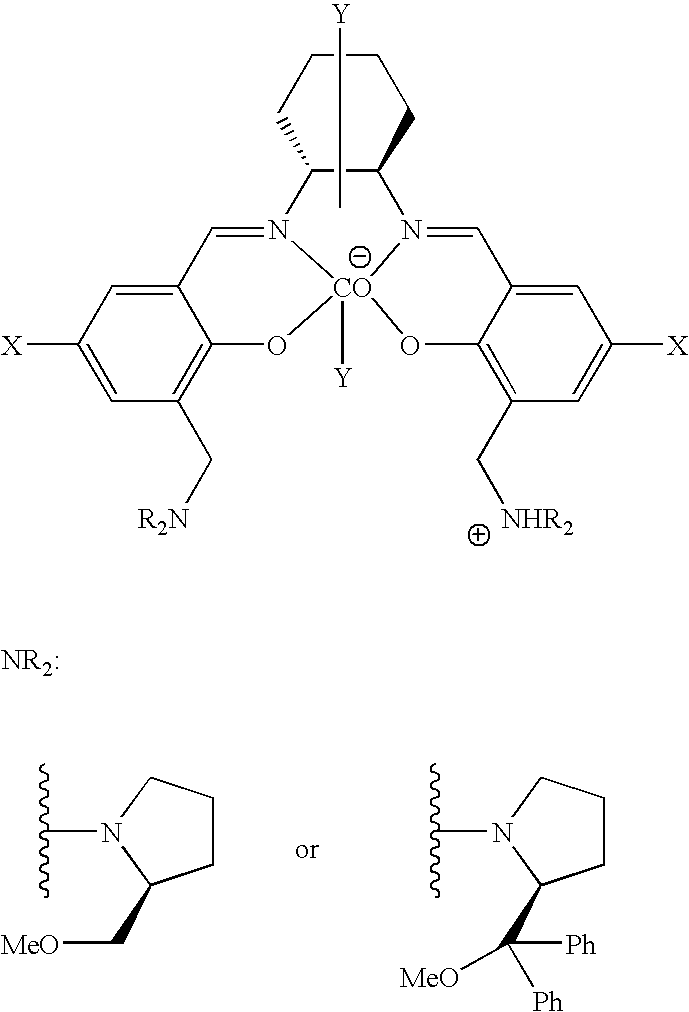
Stereoselective alternating copolymerization of epoxide with carbon dioxide
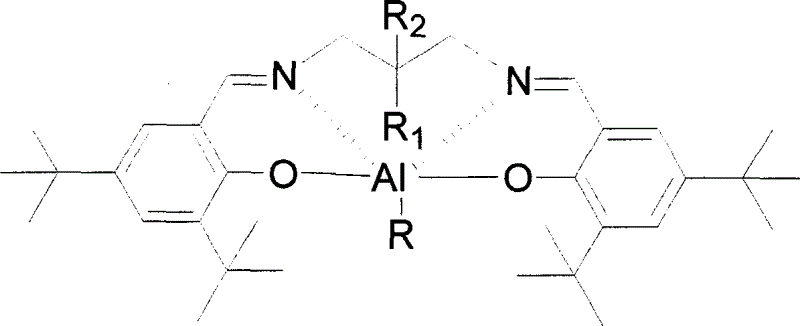
The present invention provides a method for manufacturing polycarbonate, which has a high conversion rate of a propylene oxide material into a polymer and can control stereoregularity of the macromolecular structure, and a catalytic compound for the manufacturing method.The manufacturing method of the present invention is a method for manufacturing a polycarbonate copolymer by copolymerizing an epoxide compound as a monomer with carbon dioxide in the presence of a planar tetracoordinate-type cobalt-Schiff base complex, wherein a ligand of the Schiff base is N,N′-bis(2-hydroxybenzylidene)ethylenediamine, N,N′-bis(2-hydroxybenzylidene)phenylenediamine, or a derivative thereof, and a methyl group substituted with an amino group having an asymmetrical carbon atom or an asymmetrical axis is introduced to the 3- and / or 3′-position of the benzene ring derived from the salicyl group. In addition, the catalytic compound of the present invention is a cobalt-Schiff base complex, wherein a methyl group substituted with an amino group having an asymmetrical carbon atom or an asymmetrical axis is introduced to the 3- and / or 3′-position of the salicyl group.
Owner:THE UNIV OF TOKYO
Synthesis of R-N-methylnaltrexone
Owner:PROGENICS PHARMA INC
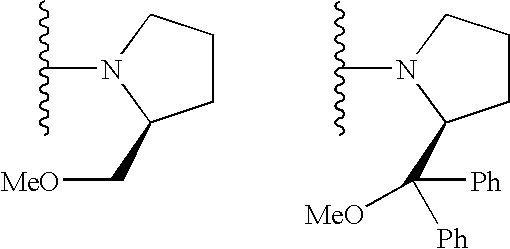
Spiro-diphosphine ligand
InactiveCN1439643AHigh stereoselectivityHigh enantioselectivityOrganic-compounds/hydrides/coordination-complexes catalystsGroup 5/15 element organic compoundsTrifluoromethanesulfonic anhydrideDiphosphines
A spirocyclo-biphosphine ligand is prepared from spirocyclo-biphenol through esterifying by trifluoro methylsulfonic acid anhydride, coupling with diaryloxyphosphine under catalysis of Pd, and reduction reacting on trichlorosilane. It includes d-and levo-spricyclo-biphosphine ligands, whose mixture is dl-spirocyclo-biphosphine ligand. It can be used for asymmetrical catalytic hydrogenation reaction of latent chiral ketone with high stereo selectivity and e.e. value up to 99.5%.
Owner:NANKAI UNIV
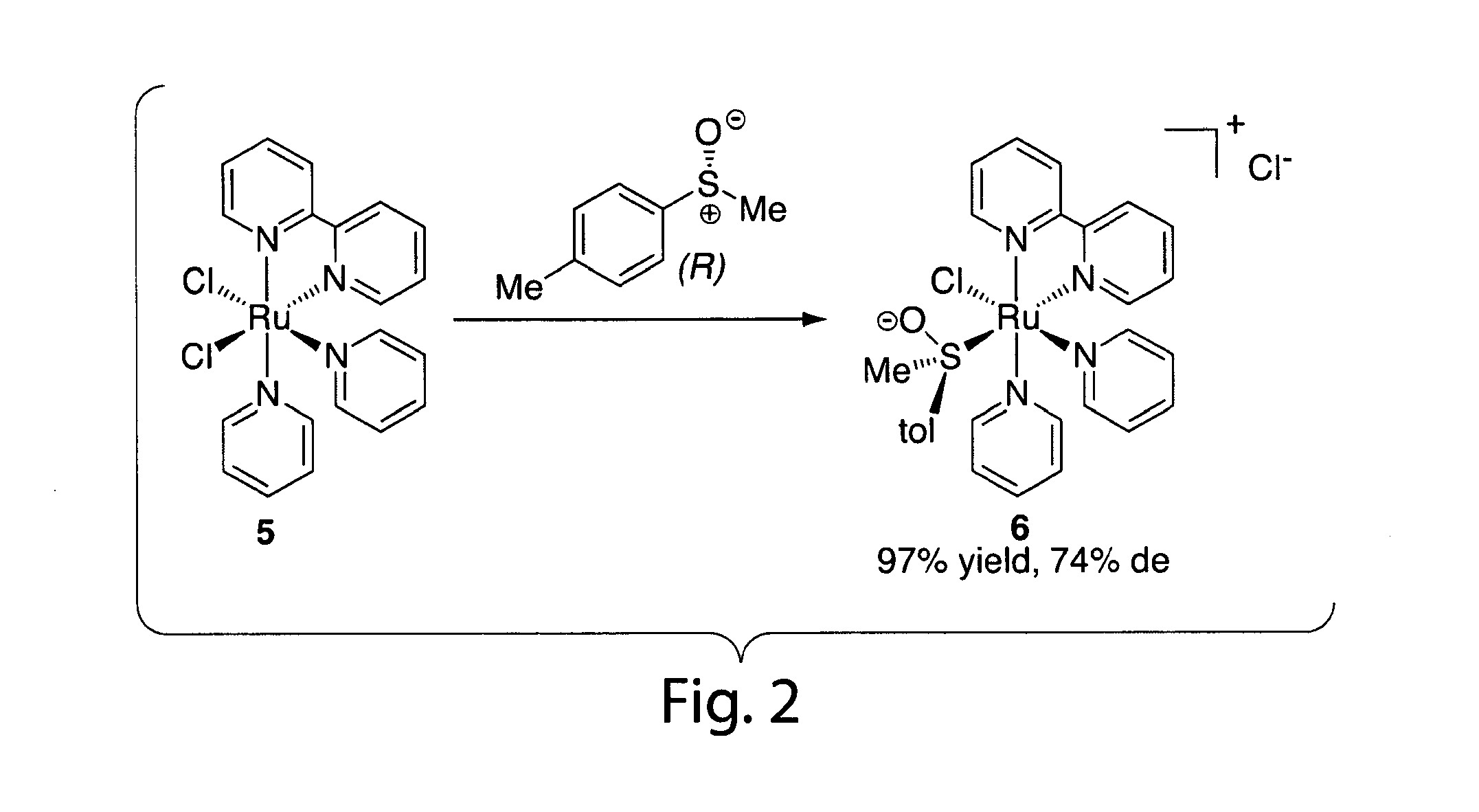
Ruthenium(II) catalysts for use in stereoselective cyclopropanations
ActiveUS7754902B2High stereoselectivityHigh yieldRuthenium organic compoundsCobalt organic compoundsProtonationSalen ligand
Chiral ruthenium catalysts comprising salen and alkenyl ligands are provided for stereoselective cyclopropanation, and methods of cyclopropanation are provided. The chiral ruthenium catalyst is prepared in situ by combining an alkenyl ligand, a deprotonated chiral salen ligand, and a ruthenium (II) metal. A preferred catalyst is prepared in situ by combining 2,3-dihydro-4-venylbenzofuran, deprotonated 1,2-cyclohexanediamino-N,N′-bis(3,5-di-t-butyl-salicylidene) and RuCl2(p-cymene)]2.
Owner:VANDA PHARMA INC
Production of fc-fusion polypeptides in eukaryotic algae
InactiveUS20110151515A1Increase serum stabilityEfficient separation and purificationUnicellular algaeVaccinesHeterologousProtein regulation
Methods and compositions are disclosed to engineer plastids comprising heterologous genes encoding immuno-activating domains fused to an extracellular domain (ECD) of a receptor or surface glycoprotein, a growth factor or an enzyme and produced within a subcellular organelle, such as a chloroplast. The immuno-activating domains may include those regions of a protein capable of modulating the interaction between immune effector cells via proteins containing stereoselective binding domains and specific ligands, such as the Fc regions of antibodies. The present disclosure also demonstrates the utility of plants, including green algae, for the production of complex multi-domain fusion proteins as soluble bioactive therapeutic agents.
Owner:SAPPHIRE ENERGY
Alteration of hydrolase genes and screening of the resulting libraries for the ability to catalyze specific reactions
The present invention relates to halocarbon and halohydrocarbon chemistry, including methods of dehalogenating halocarbons and halohydrocarbons to provide, inter alia, alcohols, polyols, and epoxides. In general, the methods involve reaction pathways catalyzed by altered hydrolase enzymes that can provide stereoselective or stereospecific reaction products. The invention also includes methods of providing altered nucleic acids that encode altered dehalogenase or other hydrolase enzymes. Additionally, the invention includes various reaction formats and kits.
Owner:MAXYGEN
Process for the catalytic hydrogenation of organic compounds and supported catalyst therefor
InactiveUS20020087036A1Extended shelf lifeConstant compositionOrganic compound preparationOrganic chemistry methodsRutheniumAsymmetric hydrogenation
A process for the catalytic hydrogenation of an organic compound, in particular a labile organic compound, in the presence of a support catalyst with a coating containing ruthenium as active metal and a total of 1.01 to 30 wt. % of active metals. Higher stereoselectivity and a greater catalyst shelf life may be obtained by using a support catalyst of which the oxide, carbide, nitride or siliceous support material has a BET (N2) surface area smaller than 10 m2 / g, particularly preferably 0.1 to 5 m2 / g, prior to loading with at least one active metal diatomaceous earth with a BET (N2) surface area greater than 2 m2 / g is excluded and the ruthenium content thereof makes up at least 50 wt. %, preferably at least 99 wt. % of the active metals. The process and the catalysts are particularly suitable for the hydrogenation of polyfunctional compounds such as hydroxycarbonyl compounds and aromatic amines.
Owner:DEGUSSA AG
Preparation method of catalyst component for polymerization reaction of olefins and catalyst thereof
The invention provides a method for preparing a catalyst component for the polymerization reaction of olefins, which comprises the following steps: (1) dissolving magnesium halide in a solvent system comprised of an organic epoxy compound, an organic phosphorus compound and an inert diluent to form uniform solution; (2) mixing a titanium compound with the uniform solution formed by the step (1) and adding a precipitant to form a mixture, or adding the precipitant into the uniform solution formed by the step (1) first and mixing the uniform solution with the titanium compound to form a mixture; and (3) heating the mixture obtained by the step (2), adding any selected electronic donor compound during heating or after heating, and washing the resulting product with an inert diluent to obtain a magnesium / titanium-containing solid material, wherein the precipitant is a glycol ester compound. The catalyst component has high polymerization activity and stereoselectivity and causes little polymer fine powder when used in the polymerization reaction of olefins.
Owner:CHINA PETROLEUM & CHEM CORP +1
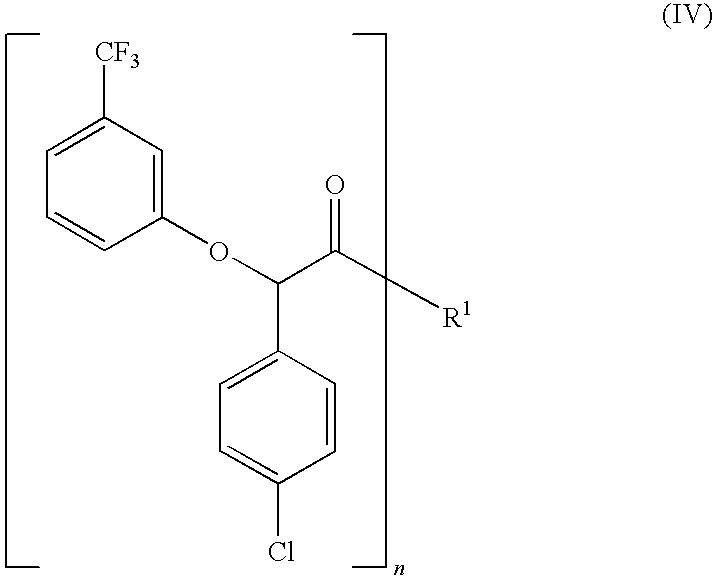
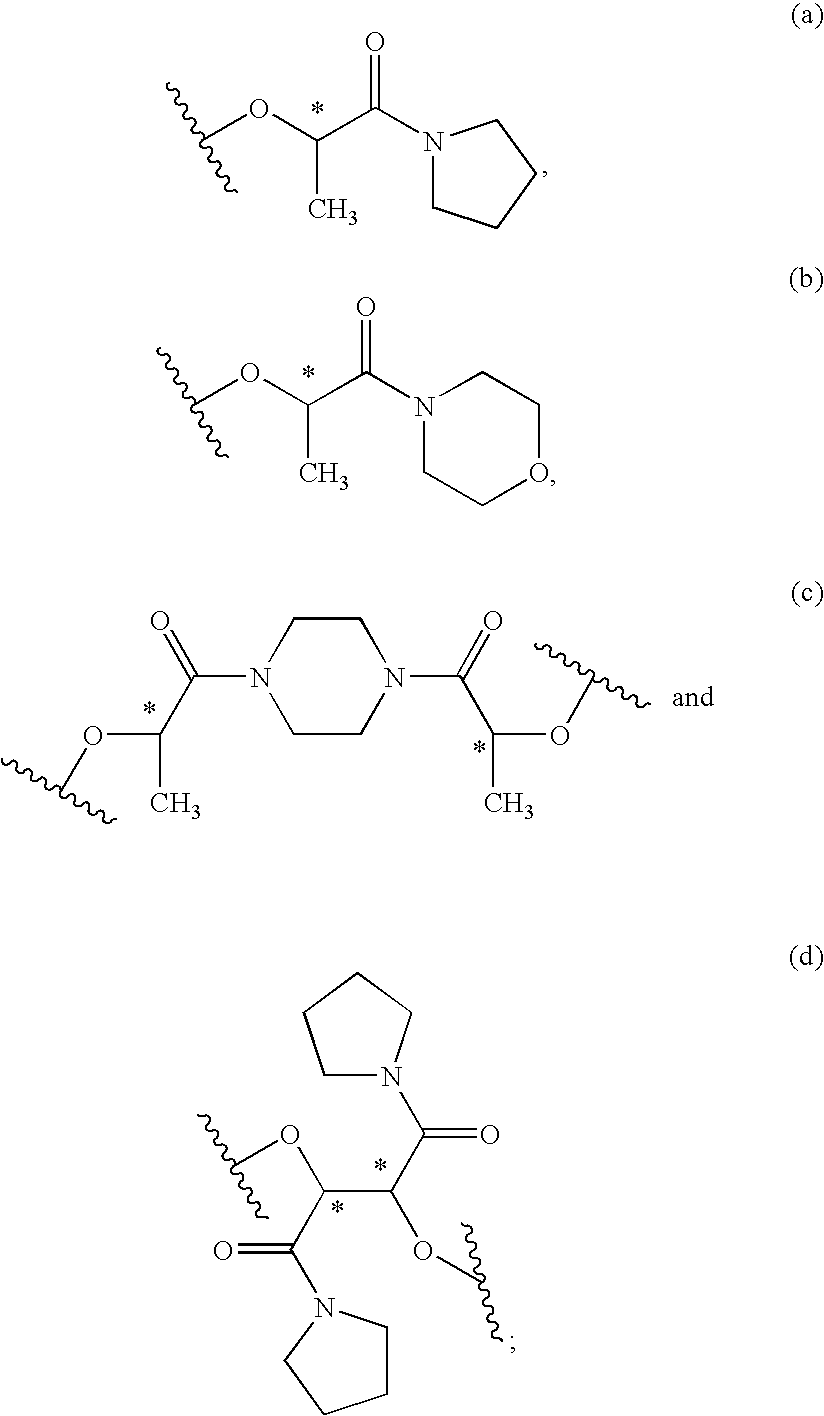
Process for the stereoselective preparation of (−)-halofenate and derivatives thereof
InactiveUS7714131B2High yieldHigh enantiomeric purityBiocideOrganic chemistryPhenylacetic acidHALOFENATE
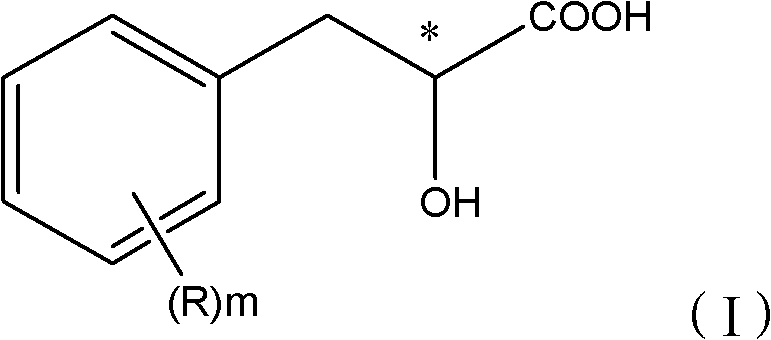
The present invention provides a compounds the formula (IV):and methods for producing an α-(phenoxy)phenylacetic acid compound of the formula:wherein R1 is a member selected from the group consisting of:each R2 is a member independently selected from the group consisting of (C1-C4)alkyl, halo, (C1-C4)haloalkyl, amino, (C1-C4)aminoalkyl, amido, (C1-C4)amidoalkyl, (C1-C4)sulfonylalkyl, (C1-C4)sulfamylalkyl, (C1-C4)alkoxy, (C1-C4)heteroalkyl, carboxy and nitro; the subscript n is 1 when R1 has the formula (a) or (b) and 2 when R1 has the formula (c) or (d); the subscript m is an integer of from 0 to 3; * indicates a carbon which is enriched in one stereoisomeric configuration; and the wavy line indicates the point of attachment of R1; and compounds.
Owner:DIATEX INC (US)
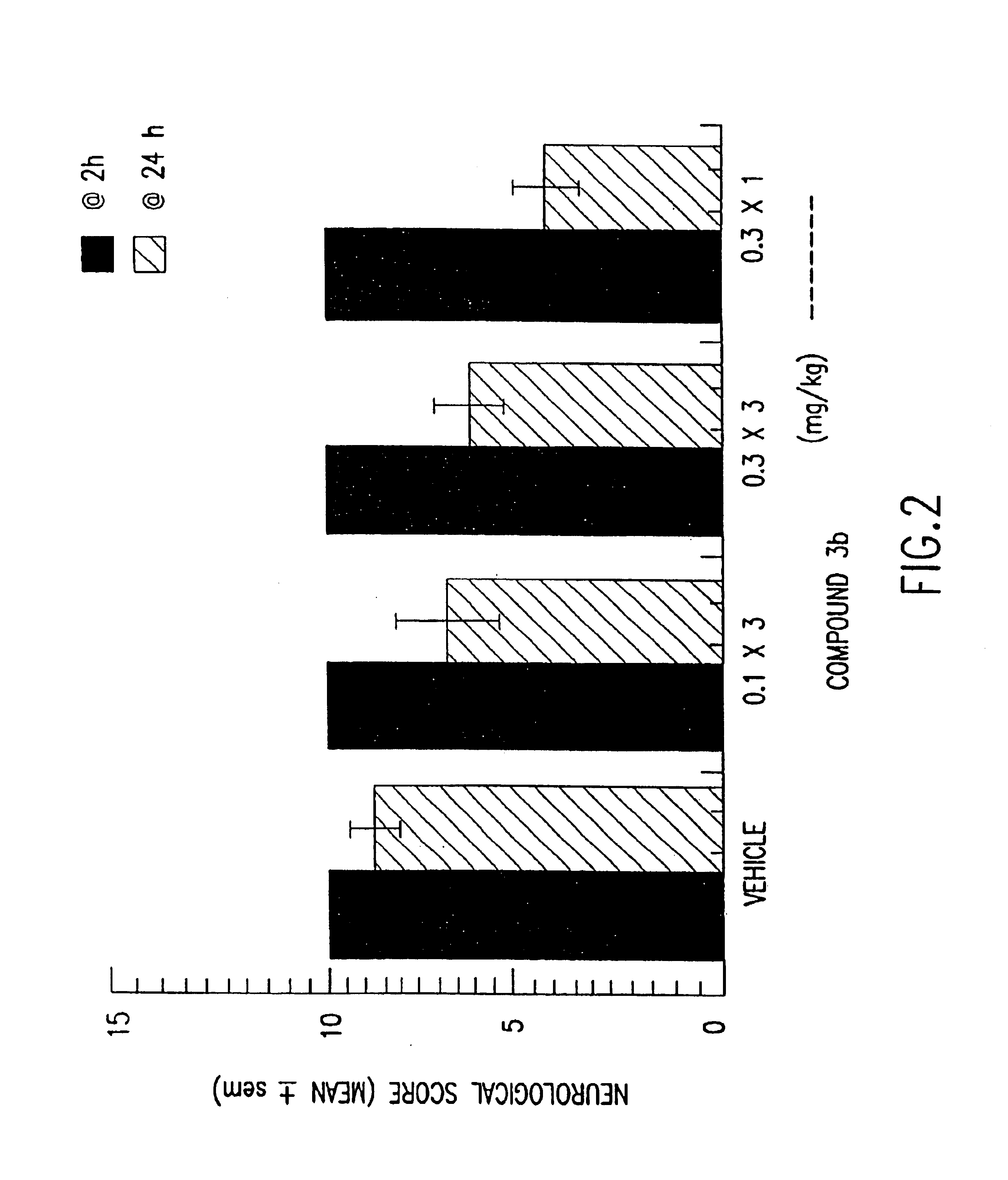
Stereoselective reduction of a morphinone
A synthetic method is provided, wherein the method comprises stereoselectively reducing a ketone of a morphinone to form a reduced morphinone and optionally covalently attaching a water soluble polymer to the reduced morphinone.
Owner:NEKTAR THERAPEUTICS INC
Synthesis of therapeutic and diagnostic drugs centered on regioselective and stereoselective ring opening of aziridinium ions
ActiveUS20130310555A1Short reaction stepsHigh yieldOrganic compound preparationThiol preparationRegioselectivityStereoselectivity
Owner:ILLINOIS INSTITUTE OF TECHNOLOGY
Method for producing H12MDA through hydrogenation reaction
ActiveCN101050184ALower conversion rateReduce contentOrganic compound preparationAmino compound preparationDiphenylmethaneNano catalyst
This invention discloses a method for preparing 4, 4'-diamino dicyclohexylmethane (H12MDA) from 4, 4'-diamino diphenylmethane (MDA) via hydrogenation catalyzed by supported nanoscale Ru catalyst. The method comprises: performing stereoselective hydrogenation on MDA at 100-180 deg.C and 4-10 MPa in an intermittent autoclave for 1-9 h to obtain H12MDA. The MDA conversion rate is near 100%, the H12MDA weight yield is near 100%, and the trans-H12MDA content is below 25%. After the supported nanoscale Ru catalyst is continuously reused for above 30 times without supplementation, the MDA conversion rate is still near 100%, and the trans-H12MDA content is below 27%.
Owner:WANHUA CHEMICAL (NINGBO) CO LTD
Chiral polymeric salen catalyst, and a process for preparing chiral compounds from racemic epoxides by using them
InactiveUS6903043B2Avoid deactivationEasy to produceRuthenium organic compoundsOrganic compound preparationDiolHydrolysis
The present invention relates to chiral salen catalysts and a process for preparing chiral compounds from racemic epoxides by using them. More particularly, the present invention is to provide a chiral polymeric salen catalyst and its use for producing chiral compounds such as chiral epoxides and chiral 1,2-diols economically in high yield and high optical purity by performing stereoselective hydrolysis or racemic epoxides.
Owner:RSTECH CO LTD
Gas-phase polymerization method and polymer of polybutene-1
The invention provides a gas-phase polymerization method of polybutene-1 for preparing high-quality butene-1 polymer, which comprises: performing homopolymerization of butene-1 serving as a monomer and a reaction medium or copolymerization of butene-1 and other alpha-olefins in a gas-phase polymerization reactor at a reaction temperature of 0 to 110 DEG C in the presence of a Ziegler-Natta catalyst with high stereoselectivity, and standing for 1 to 6 hours optimally. The method is simple in process, easy in operation and low in cost. The prepared polybutene-1 product is sphere-like or spherical particles and contains little fine powder, thereby contributing to the long-term operation o devices.
Owner:CHINA PETROLEUM & CHEM CORP +1
Catalyst for ring-opening polymerization of cyclic ester and preparation method thereof
The invention relates to a category of cyclic ester open loop polymerization catalyst and method for preparation, it also relates to the process for polymerizing racemic lactide by using these catalysts, wherein the catalysts are the product of the reaction between Schiff's base and aluminium triethide or aluminium isopropoxide, they exhibit stereoselectivity to the polymerization of racemic lactide, thus crystallinity polylactic acid can be obtained through polymerizing racemic lactide.
Owner:长春宸泰科技有限公司
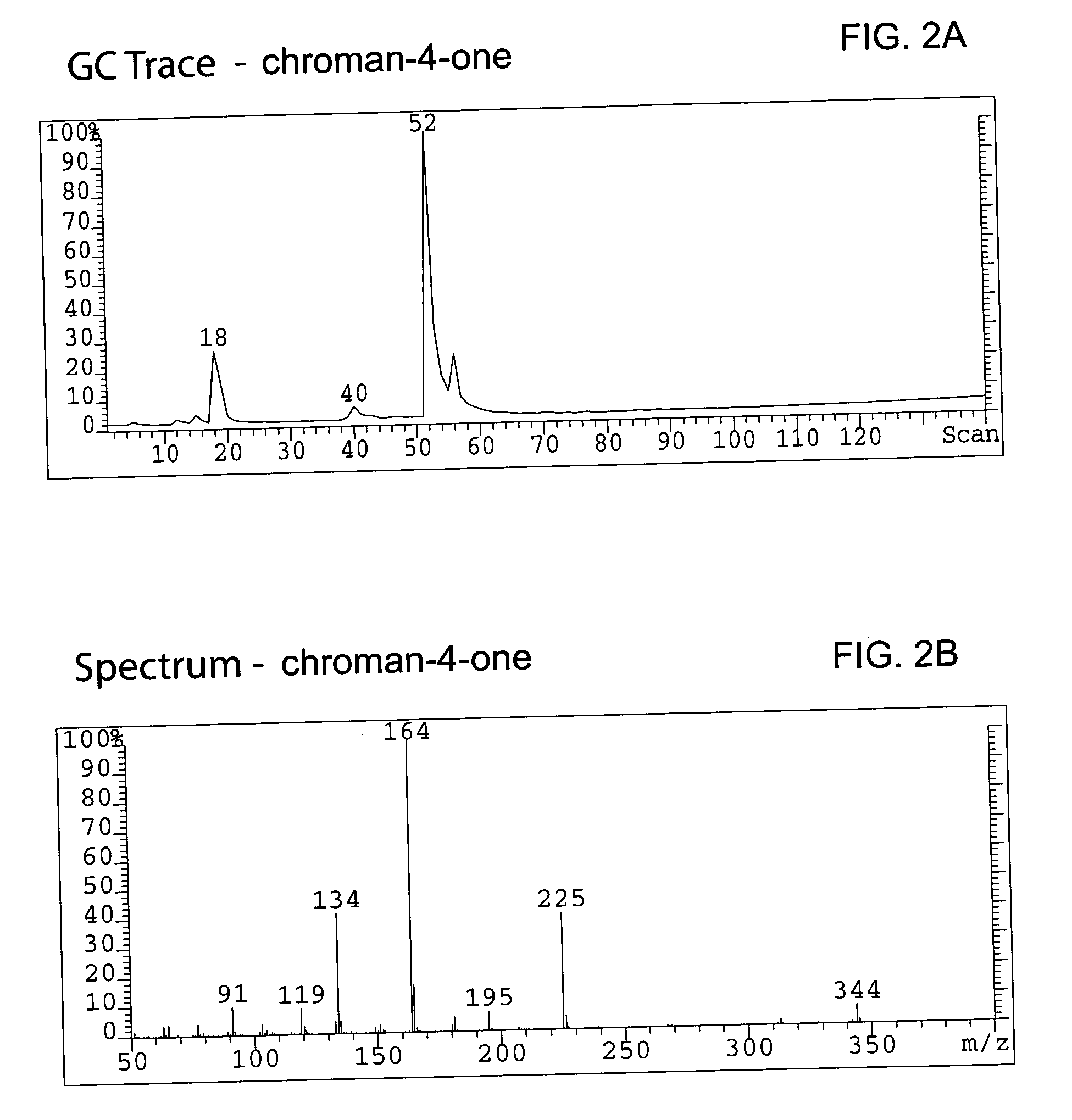
Method for enantioselective hydrogenation of chromenes
A method for preparing an enantiomeric chromane, by asymmetrically hydrogenating a chromene compound in the presence of an Ir catalyst having a chiral ligand. The method includes the enantioselective preparation of enantiomeric equol. A preferred Ir catalyst has a chiral phosphineoxazoline ligand. Enantiomeric chromanes of high stereoselective purity can be obtained.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com