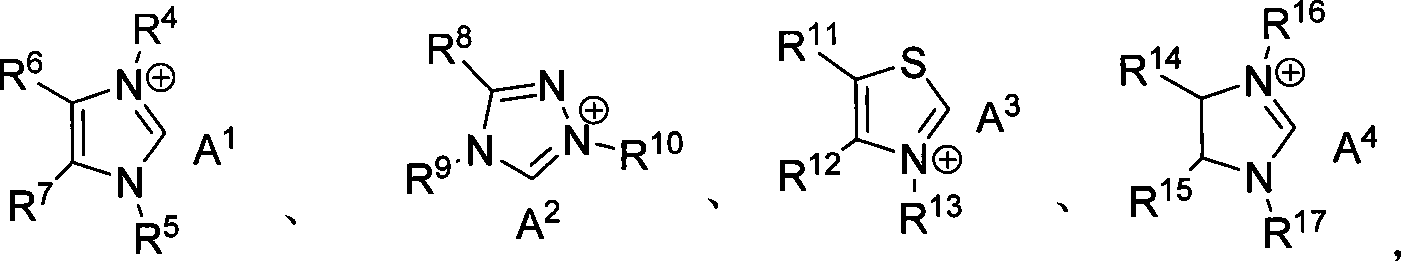
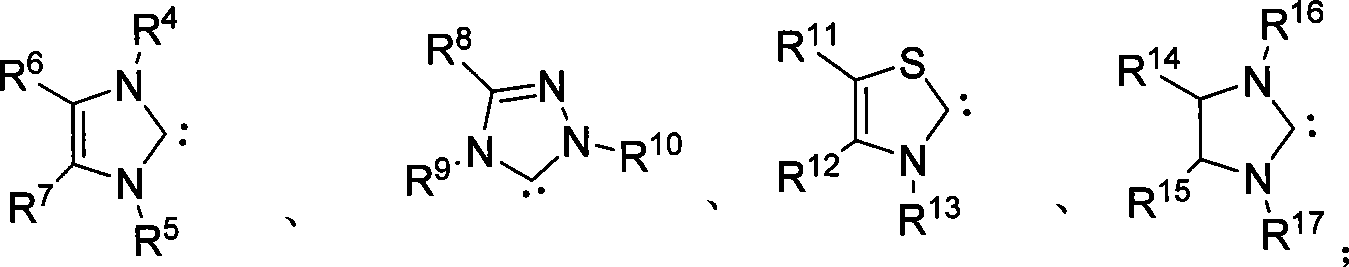
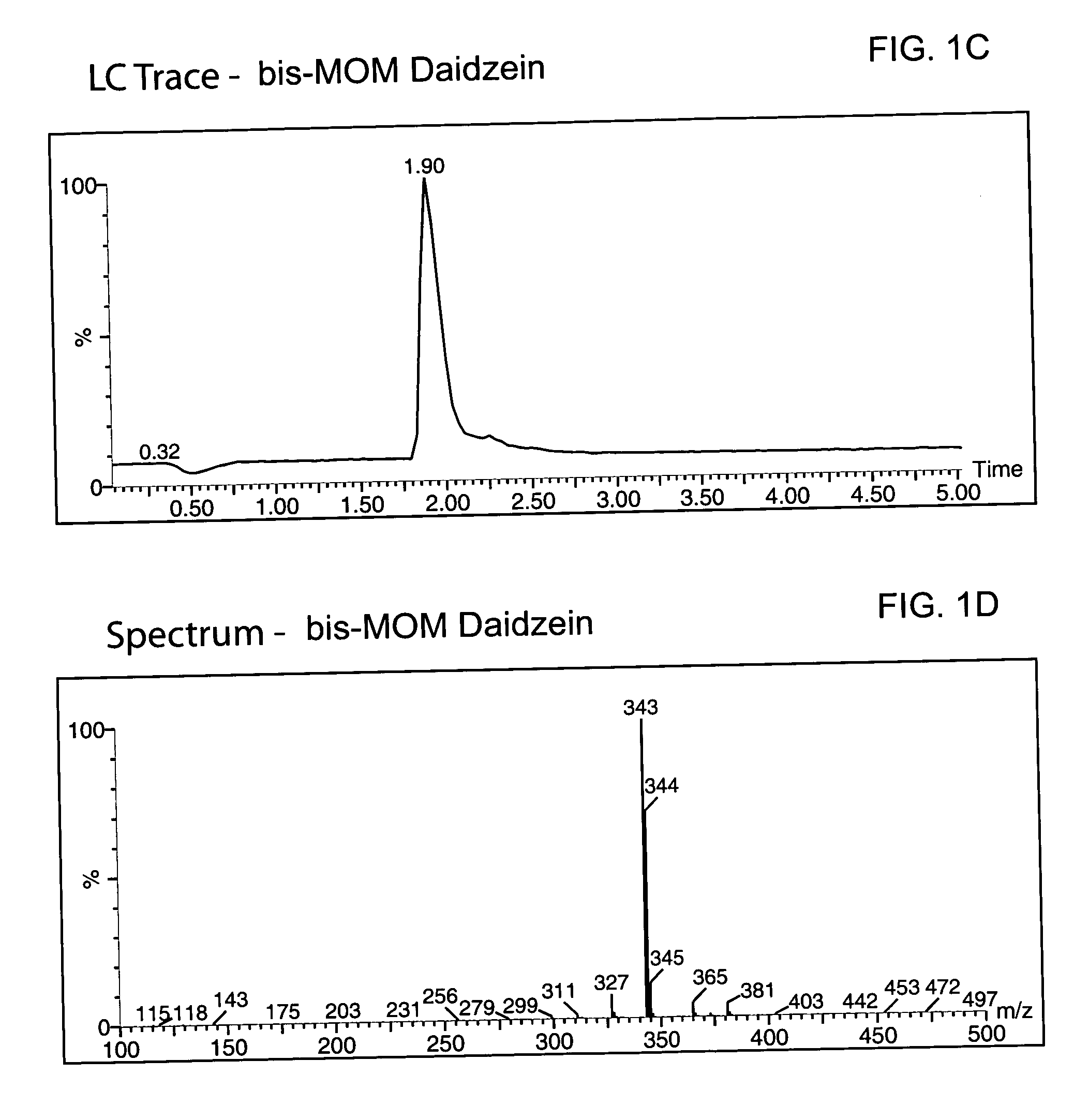
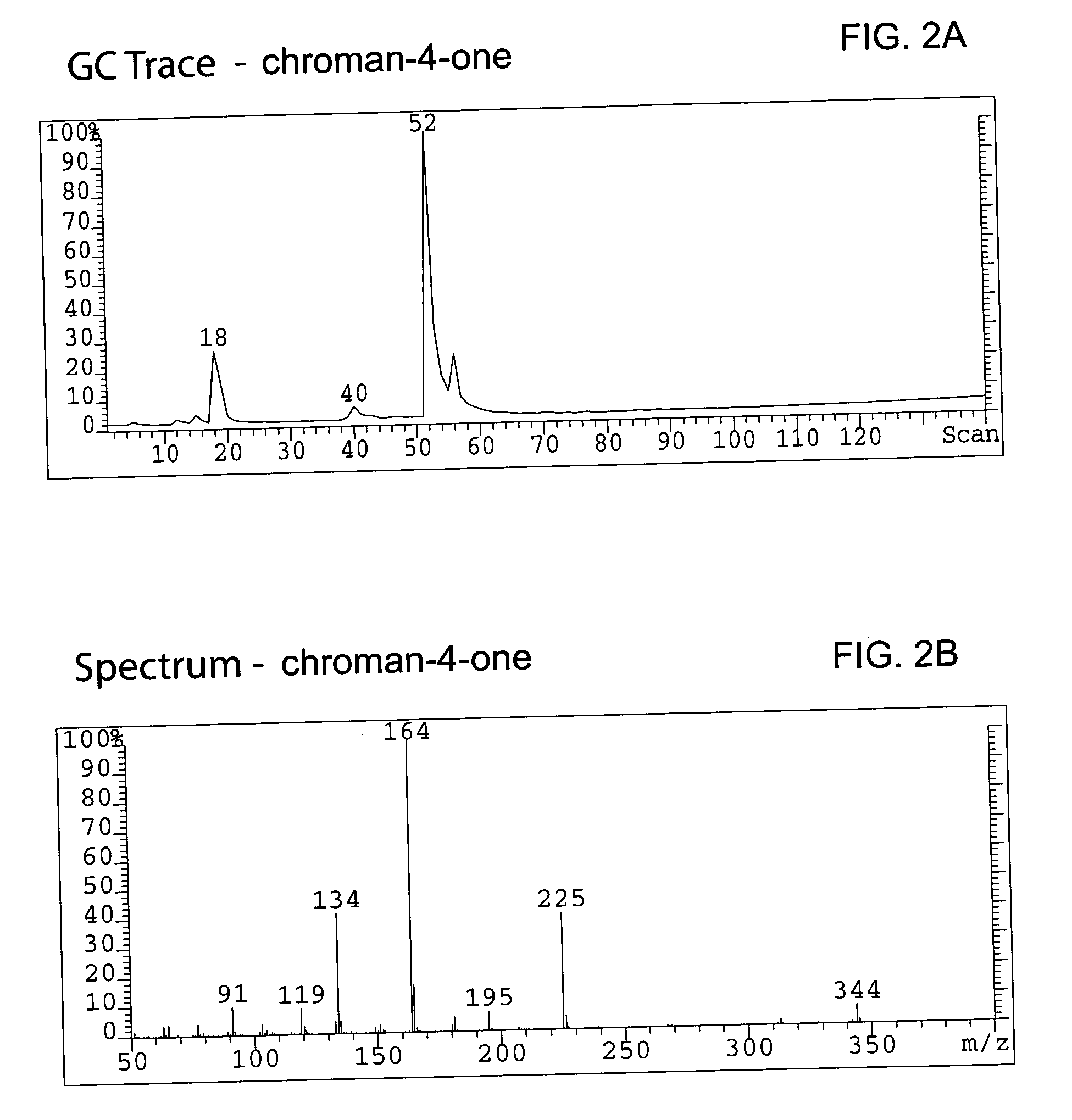
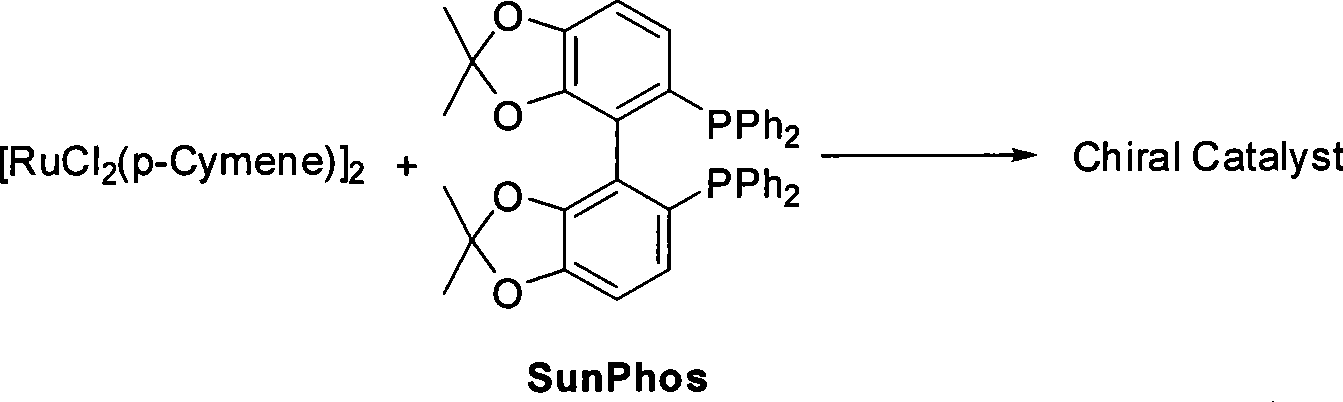
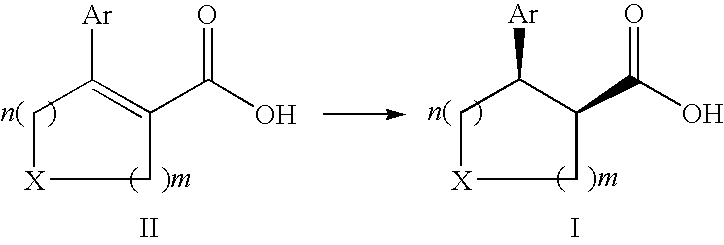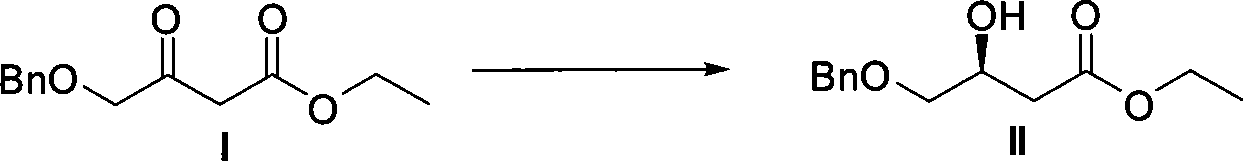Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1701 results about "Enantio selectivity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Medical Definition of enantioselectivity. : the degree to which one enantiomer of a chiral product is preferentially produced in a chemical reaction.
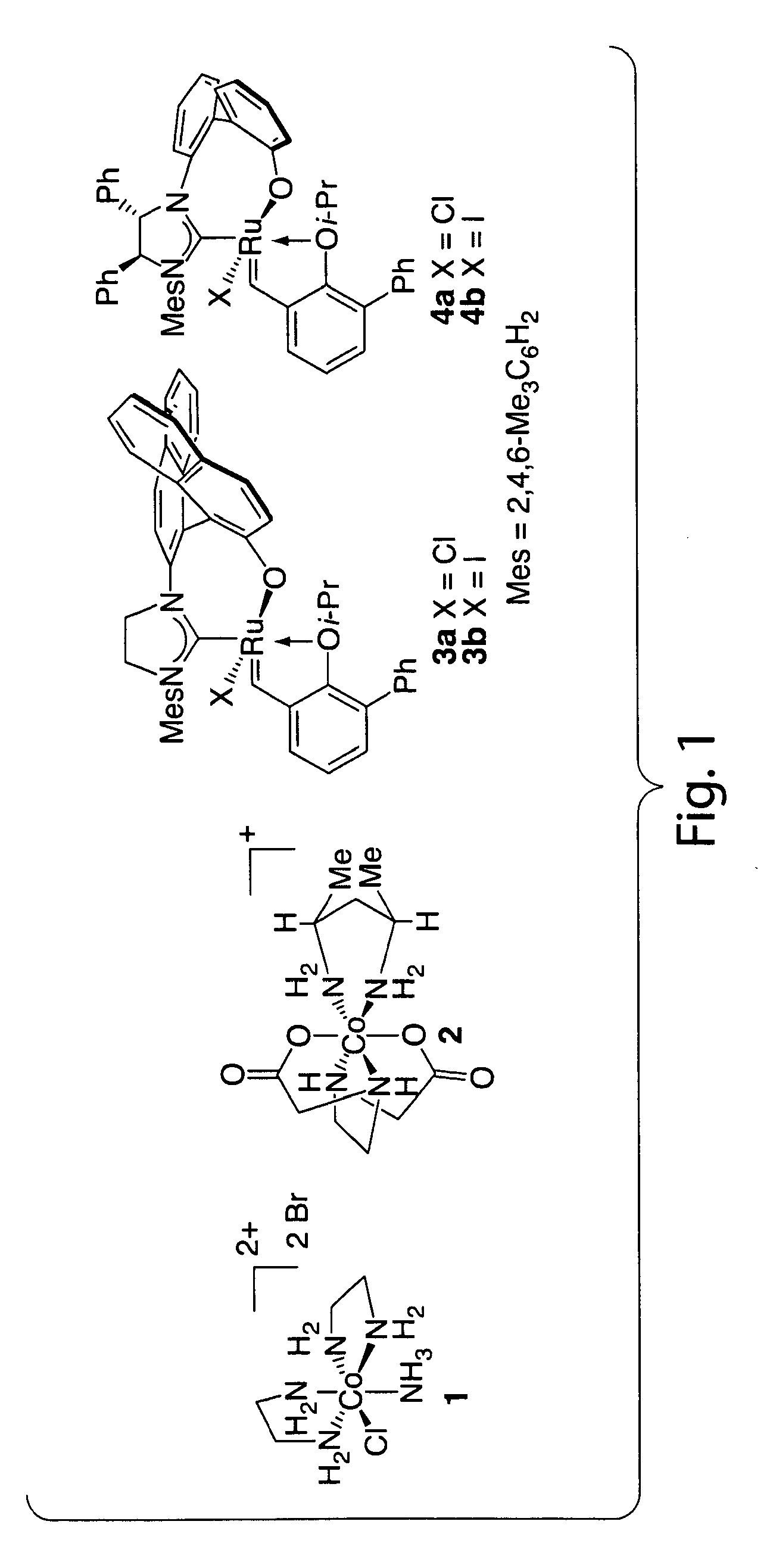
Catalysts for metathesis reactions including enantioselective olefin metathesis, and related methods
ActiveUS20110065915A1Silicon organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsChemical reactionEnantio selectivity
The present invention provides compositions comprising metal complexes, and related methods. In some embodiments, metal complexes of the invention may be useful as catalysts for chemical reactions, including metathesis reactions, wherein the catalysts exhibit enhanced activity and stereoselectivity. In some embodiments, the invention may advantageously provide metal complexes comprising a stereogenic metal atom. Such metal complexes may be useful in enantioselective catalysis.
Owner:BOSTON COLLEGE +1
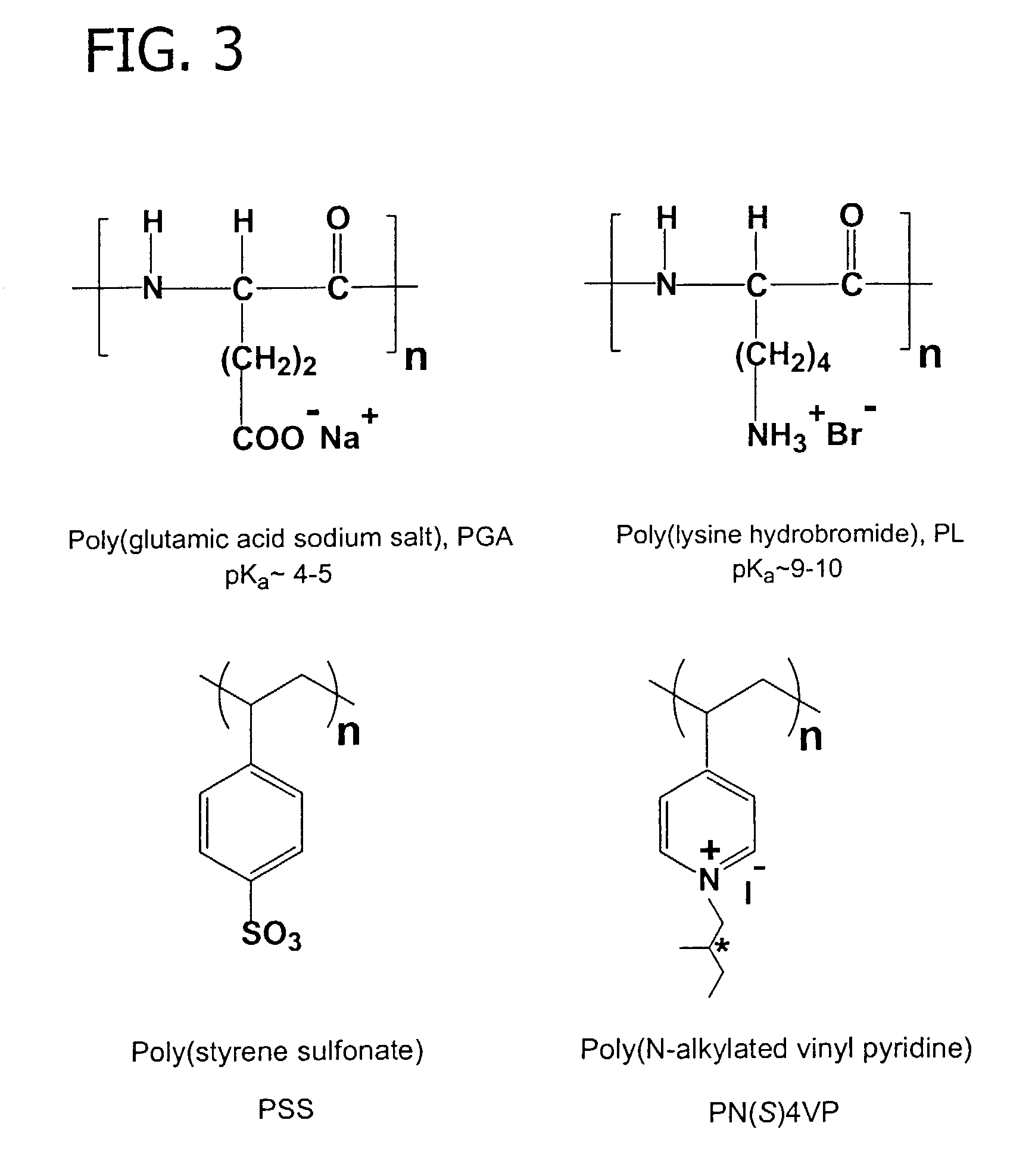
Polyelectrolyte complex films for analytical and membrane separation of chiral compounds
The present invention is directed to enantioselective polyelectrolyte complex films. Further, said films may be free or isolated membranes, or coatings on substrates such a porous substrates, capillary tubes, chromatographic packing material, and monolithic stationary phases and used to separate chiral compounds. The present invention is also directed to a method for forming such enantioselective polyelectrolyte complex films.
Owner:FLORIDA STATE UNIV RES FOUND INC
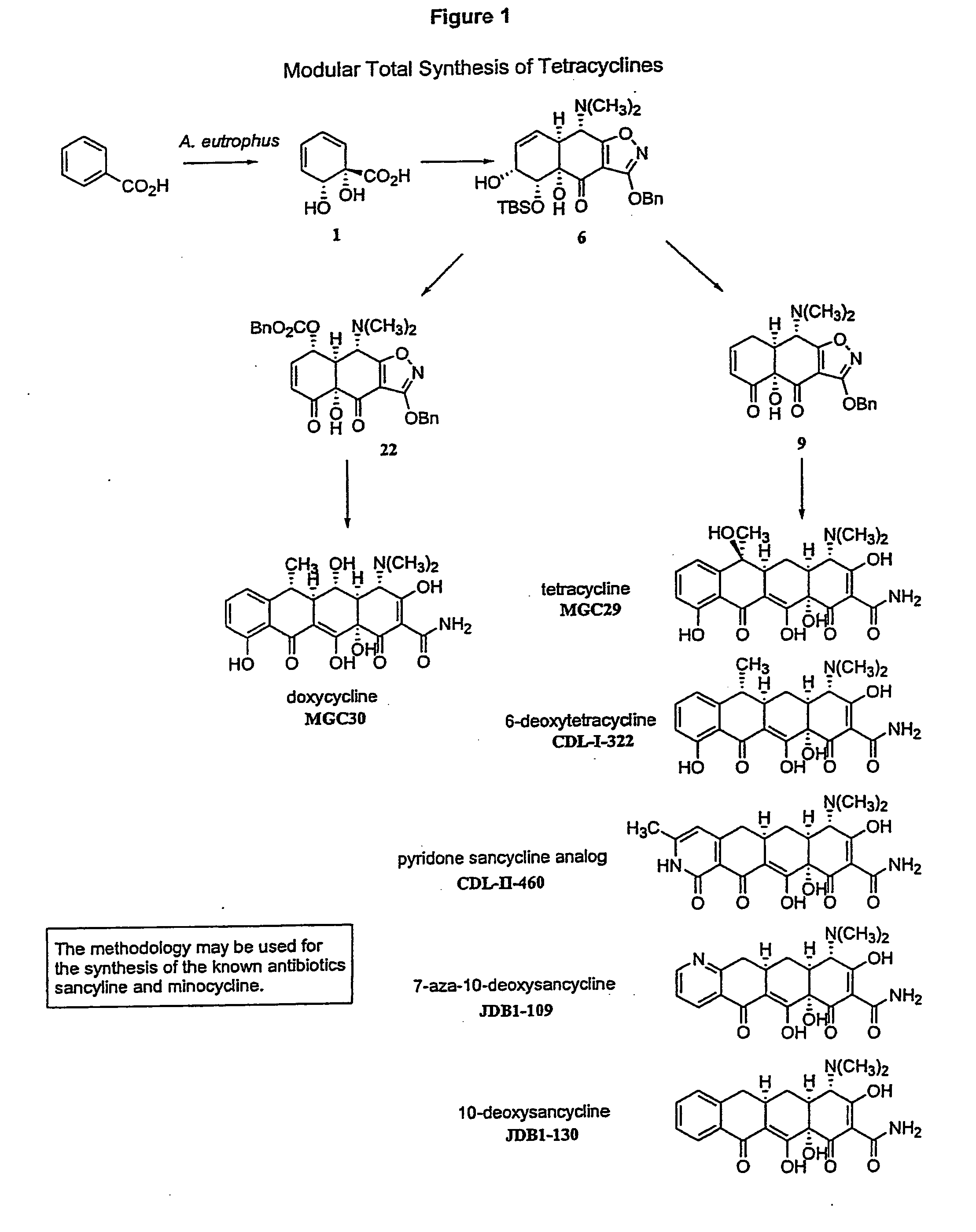
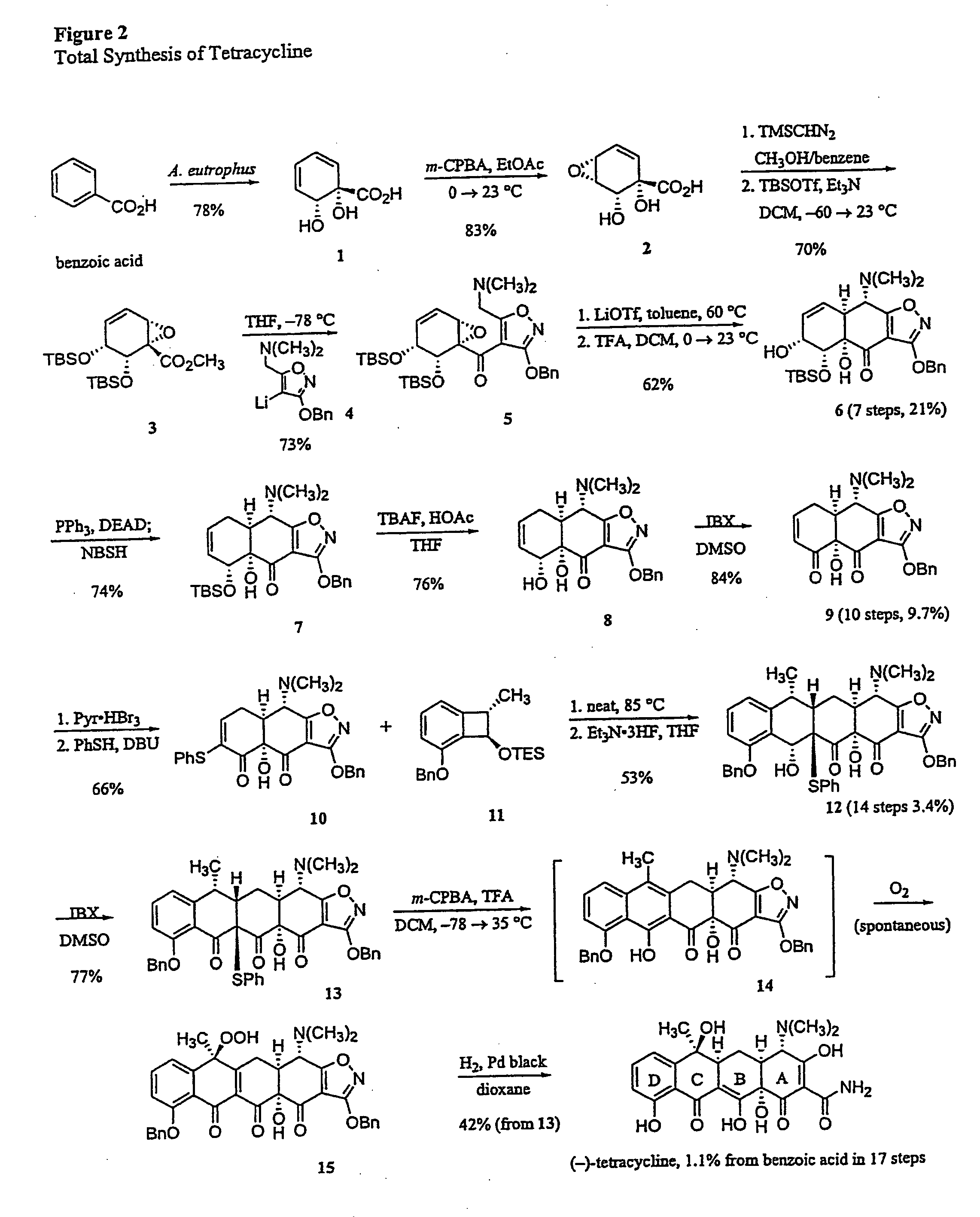
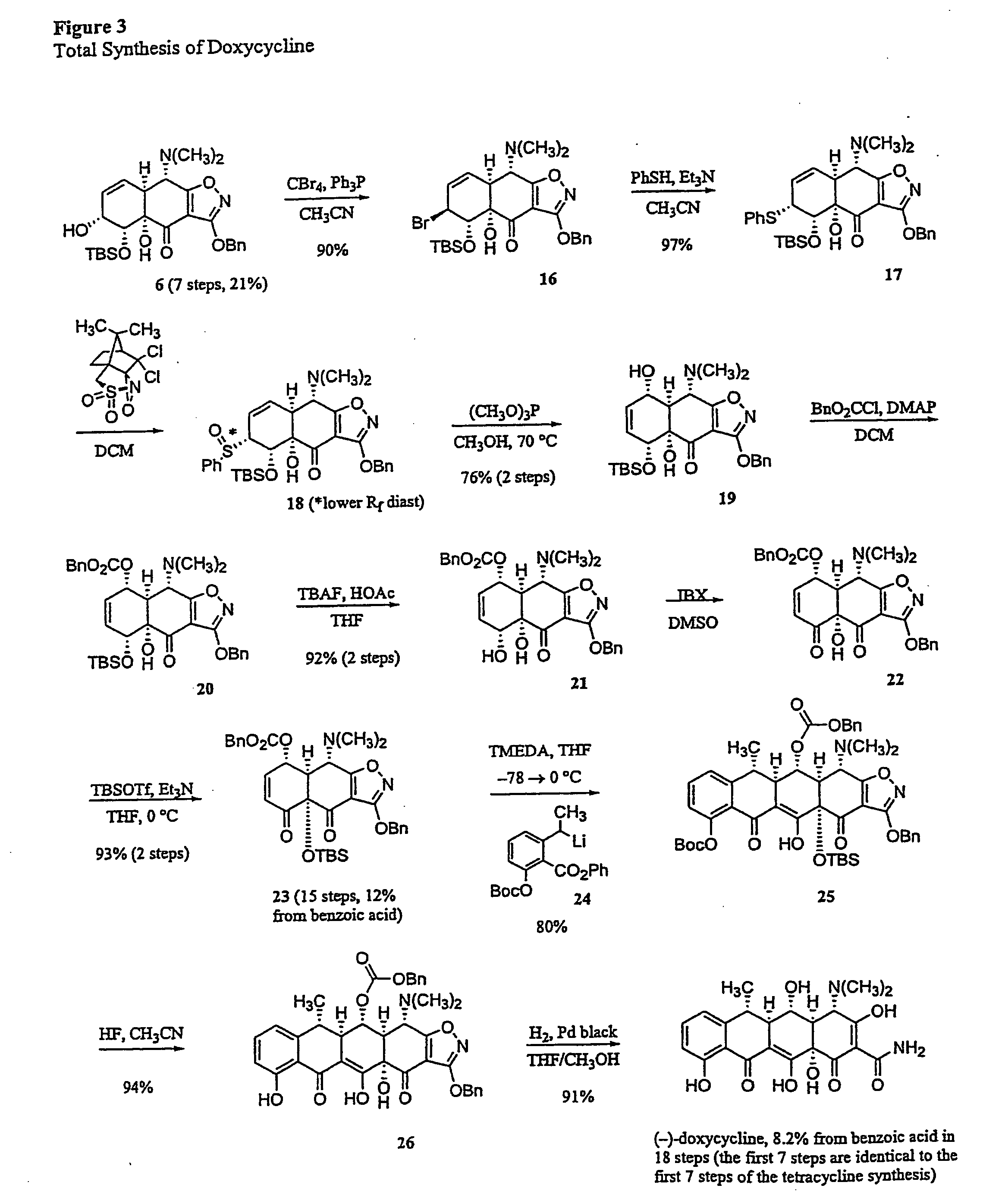
Synthesis of tetracyclines and analogues thereof
The tetracycline class of antibiotics has played a major role in the treatment of infectious diseases for the past 50 years. However, the increased use of the tetracyclines in human and veterinary medicine has led to resistance among many organisms previously susceptible to tetracycline antibiotics. The modular synthesis of tetracyclines and tetracycline analogs described provides an efficient and enantioselective route to a variety of tetracycline analogs and polycyclines previously inaccessible via earlier tetracycline syntheses and semi-synthetic methods. These analogs may be used as anti-microbial agents or anti-proliferative agents in the treatment of diseases of humans or other animals.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
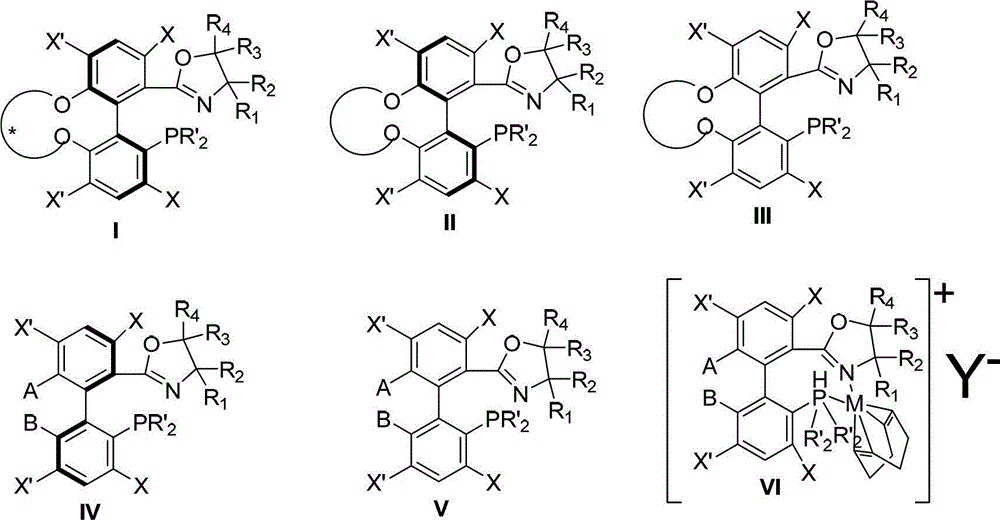
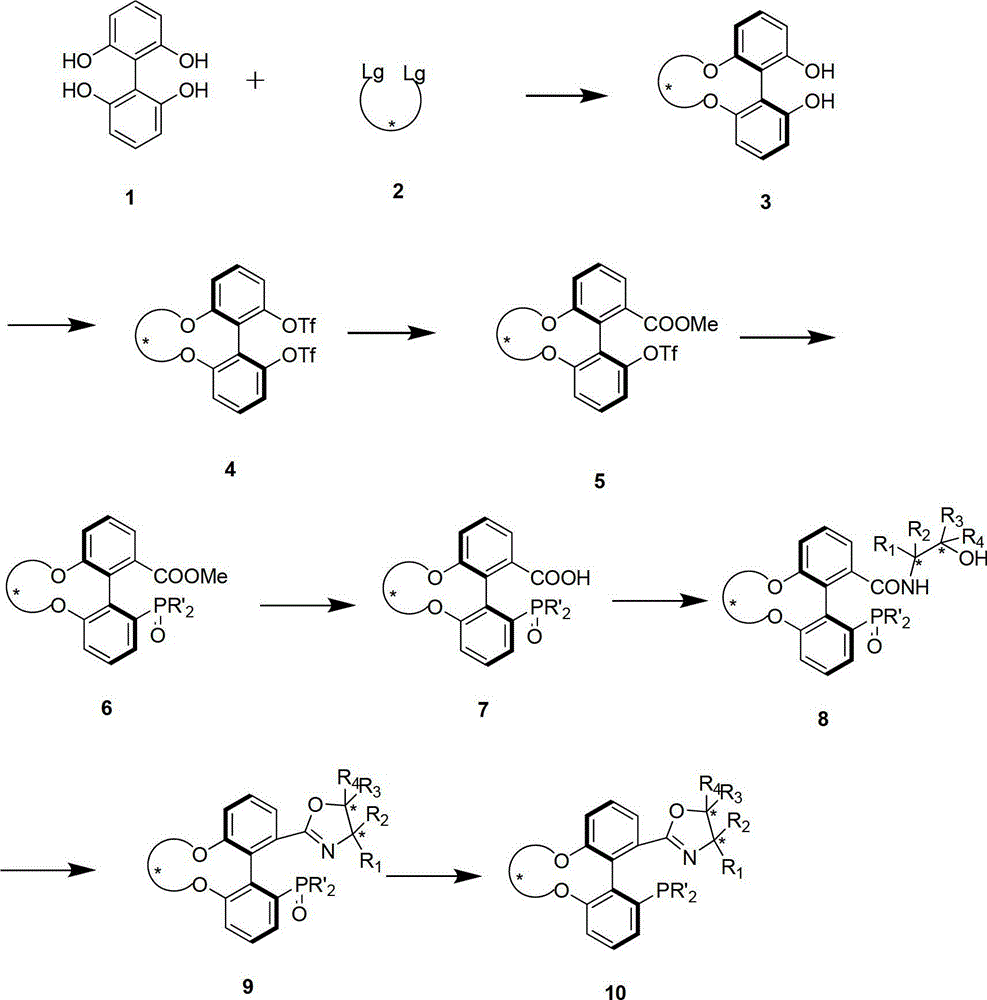
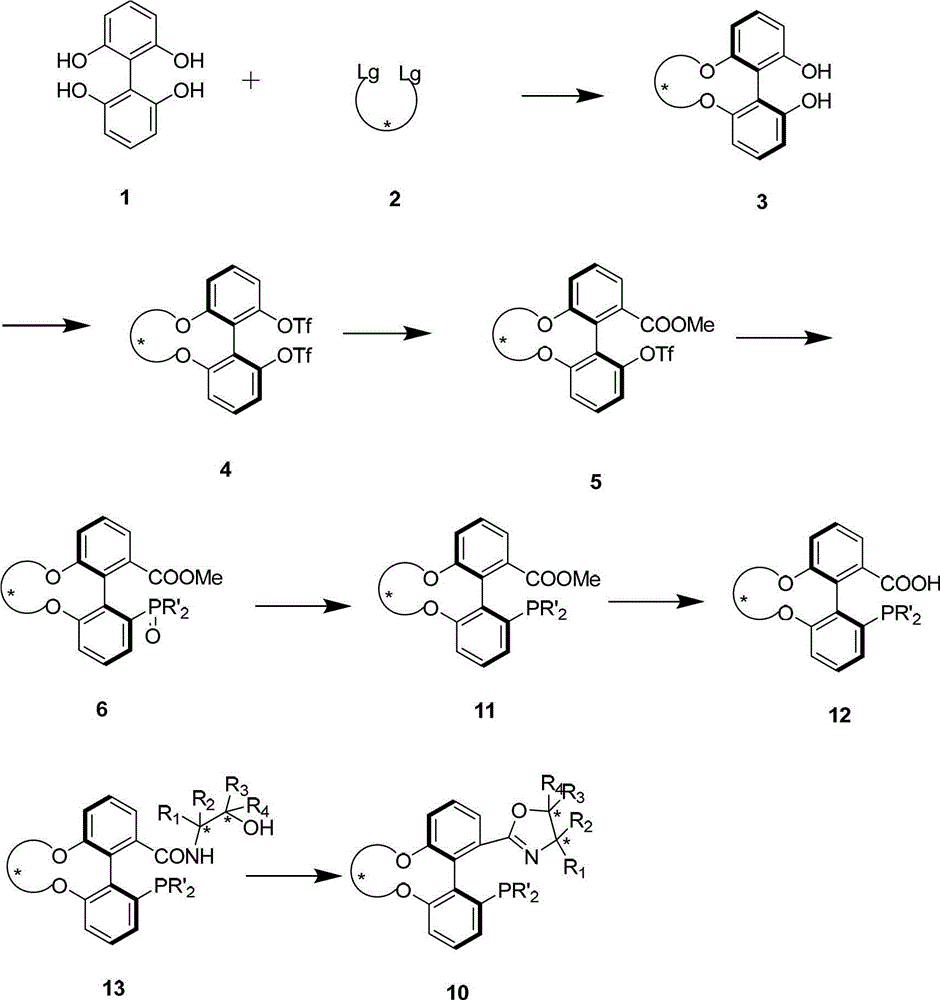
Spiro-diphosphine ligand
InactiveCN1439643AHigh stereoselectivityHigh enantioselectivityOrganic-compounds/hydrides/coordination-complexes catalystsGroup 5/15 element organic compoundsTrifluoromethanesulfonic anhydrideDiphosphines
A spirocyclo-biphosphine ligand is prepared from spirocyclo-biphenol through esterifying by trifluoro methylsulfonic acid anhydride, coupling with diaryloxyphosphine under catalysis of Pd, and reduction reacting on trichlorosilane. It includes d-and levo-spricyclo-biphosphine ligands, whose mixture is dl-spirocyclo-biphosphine ligand. It can be used for asymmetrical catalytic hydrogenation reaction of latent chiral ketone with high stereo selectivity and e.e. value up to 99.5%.
Owner:NANKAI UNIV
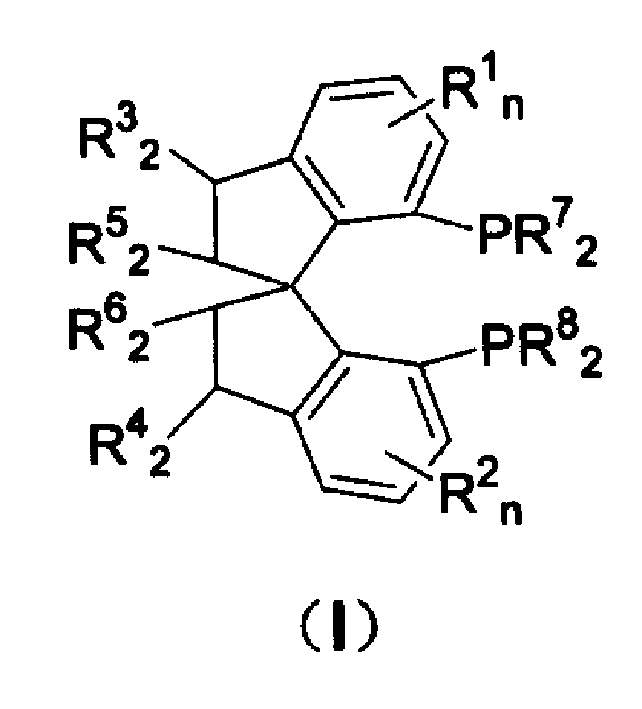
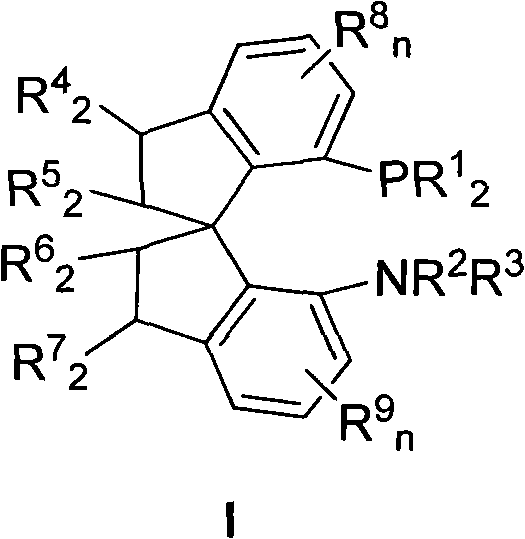
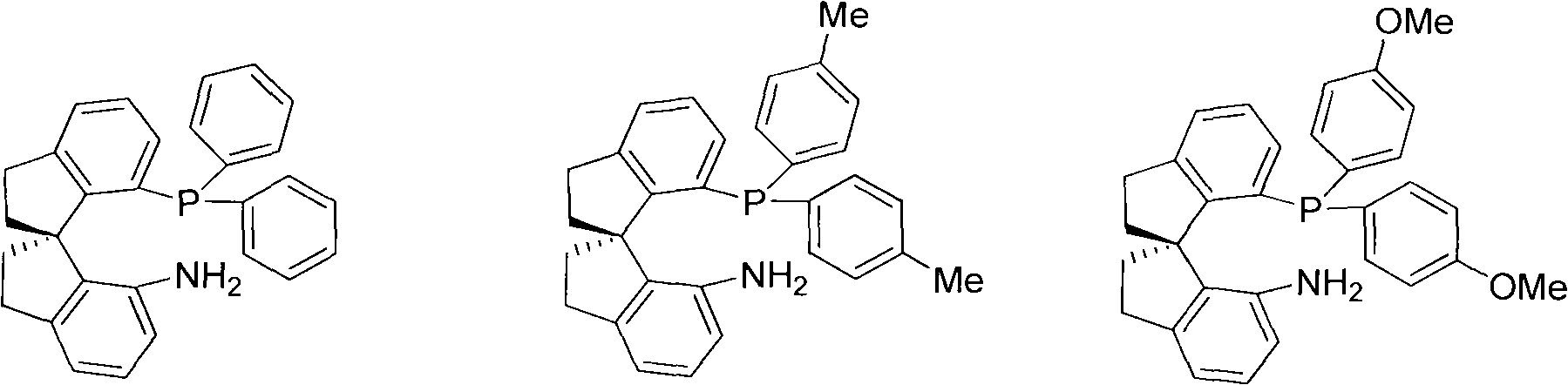
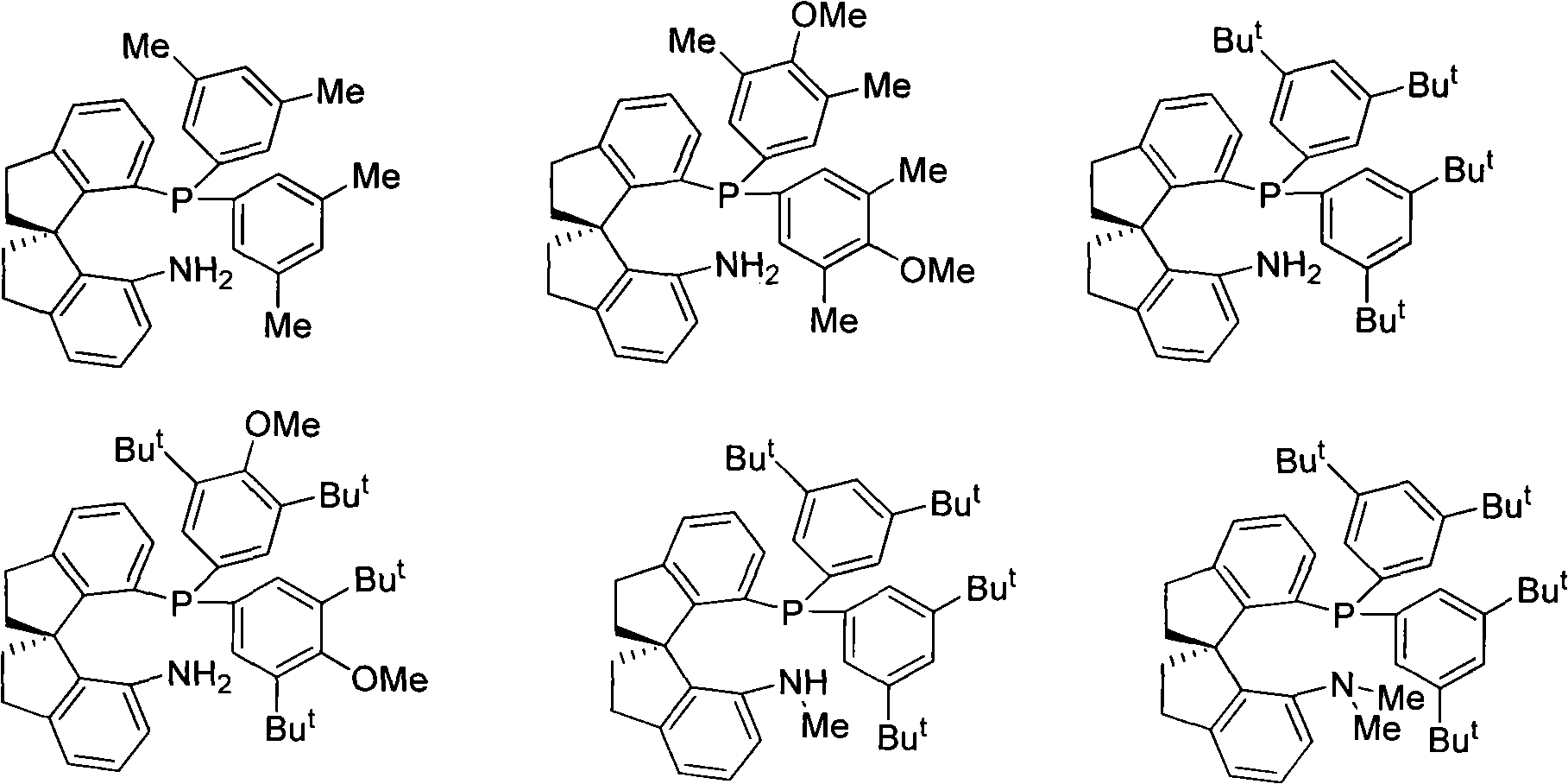
Chiral spiro aminophosphine ligand compound and synthesis method as well as application thereof
InactiveCN101671365AHigh reactivityHigh enantioselectivityOrganic compound preparationGroup 5/15 element organic compoundsIridiumSynthesis methods
The invention relates to a chiral spiro aminophosphine ligand compound and a synthesis method as well as an application thereof. The chiral spiro aminophosphine compound contains a compound with a structure shown in I, or a racemic body or an optical isomer thereof, or a catalyzed acceptable salt thereof, wherein the main structure characteristic is with a chiral spirobiindane matrix. The chiral spiro aminophosphine compound can be synthesized by taking 7-diaryl / phosphito-7'-carboxy-1, 1'-spirobiindane or substituted 7-diaryl / phosphito-7'-carboxy-1, 1'-spirobiindane as chiral starting materials, which has optical activity and spiro matrix. The chiral spiro aminophosphine compound can be used as a chiral ligand for an asymmetrically catalyzed hydrogenation of an iridium catalyzed carboxy compound and achieves high yield and enantioselectivity (97%ee). The reactive activity is also high and the consumption of the catalyst can be reduced to 0.01% molar.
Owner:NANKAI UNIV
Process for the preparation of chiral beta amino acid derivatives by asymmetric hydrogenation
ActiveUS7468459B2Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsMetalloleEnantiomer
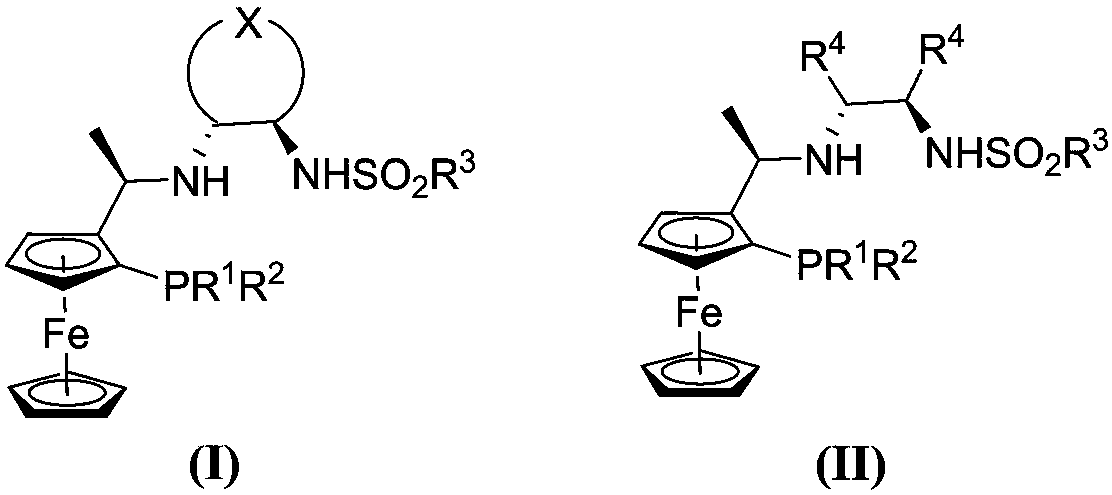
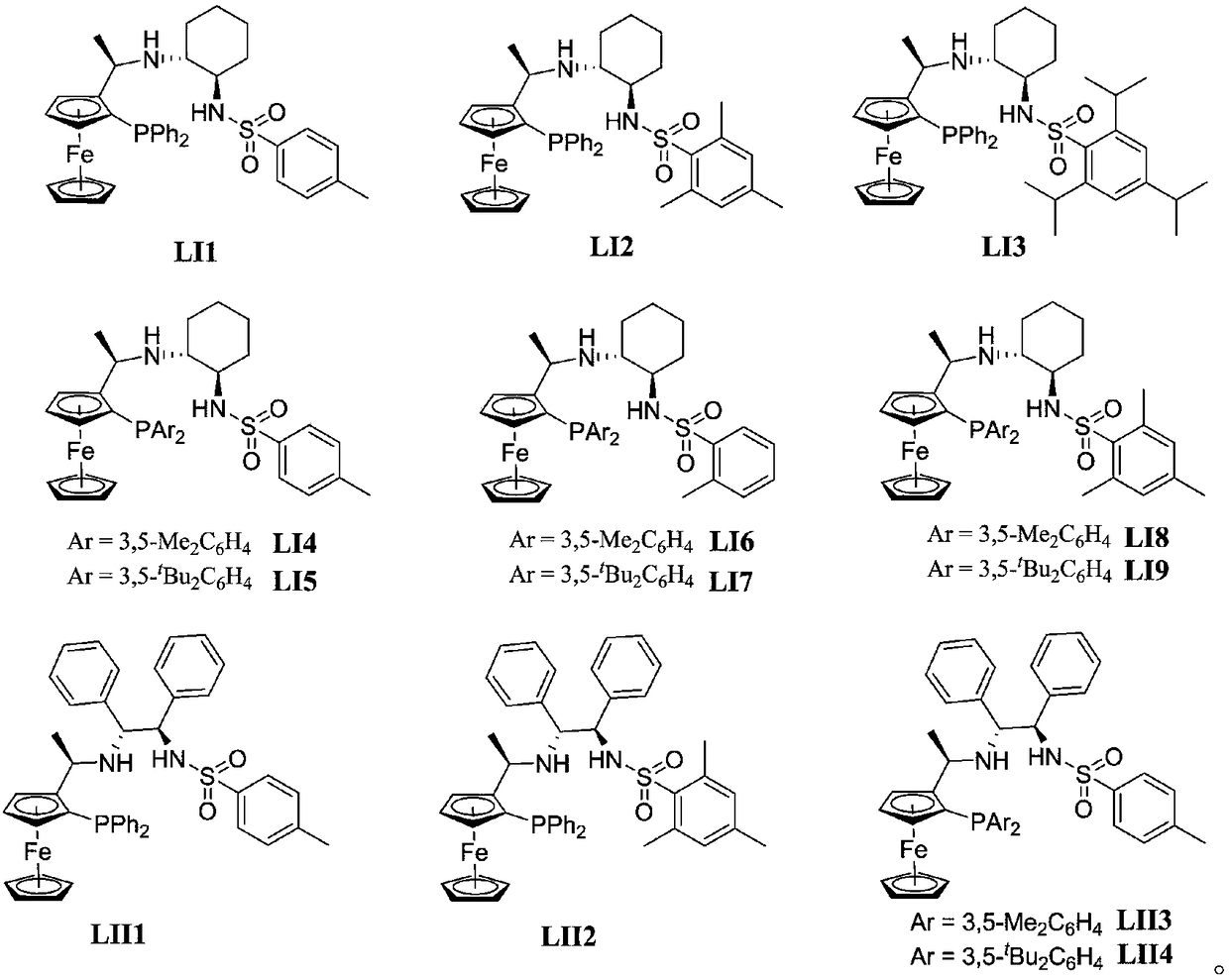
The present invention relates to a process for the efficient preparation of enantiomerically enriched beta amino acid derivatives which are useful in the asymmetric synthesis of biologically active molecules. The process comprises an enantioselective hydrogenation of a prochiral beta amino acrylic acid derivative substrate in the presence of a transition metal precursor complexed with a chiral ferrocenyl diphosphine ligand.
Owner:MERCK SHARP & DOHME LLC
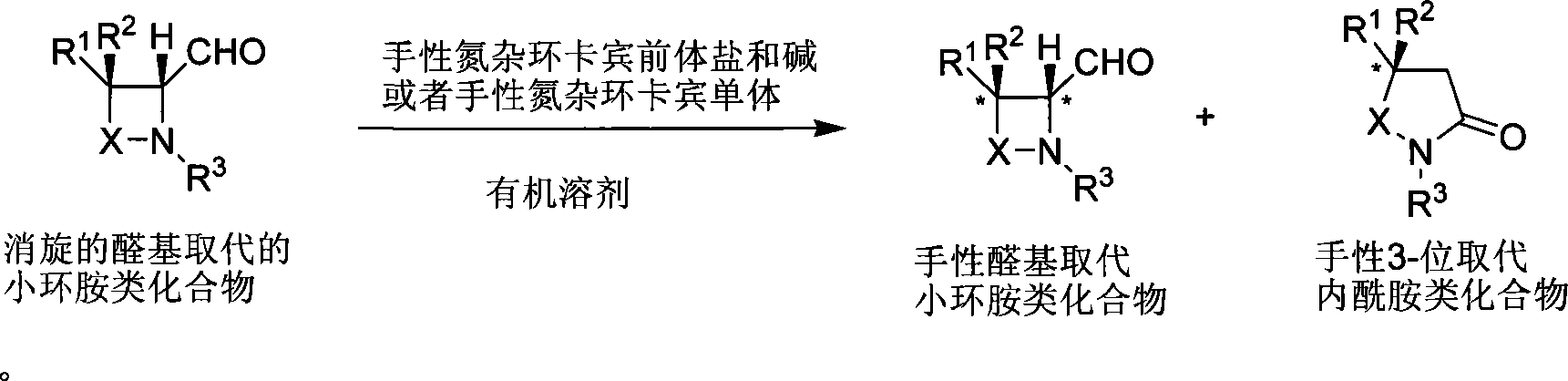
Method for synthesizing aldehyde substituted small ring amines compounds with high enantioselectivity and 3-substituted lactams compounds with optical activity
InactiveCN101125817AIncrease production capacityEasy to handleOrganic chemistryEnantio selectivityMetal
The invention provides a method of a racemoid aldehyde group-ubstituted small cyclic amine compound, a high efficiency high enantio-selectivity synthesized chiral aldehyde group-substituted small cyclic amine compound and the synthesis of 3-substituted lactam compound with certain optical activity. Compared with the existing method, the method has wide adaptive substrate, and the catalyst is easily acquired, the reaction condition is mild, the operation is simple and convenient and the reaction efficiency is high. No adding of any metal salt compound is required, thus facilitating the production and the processing of the medicine.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
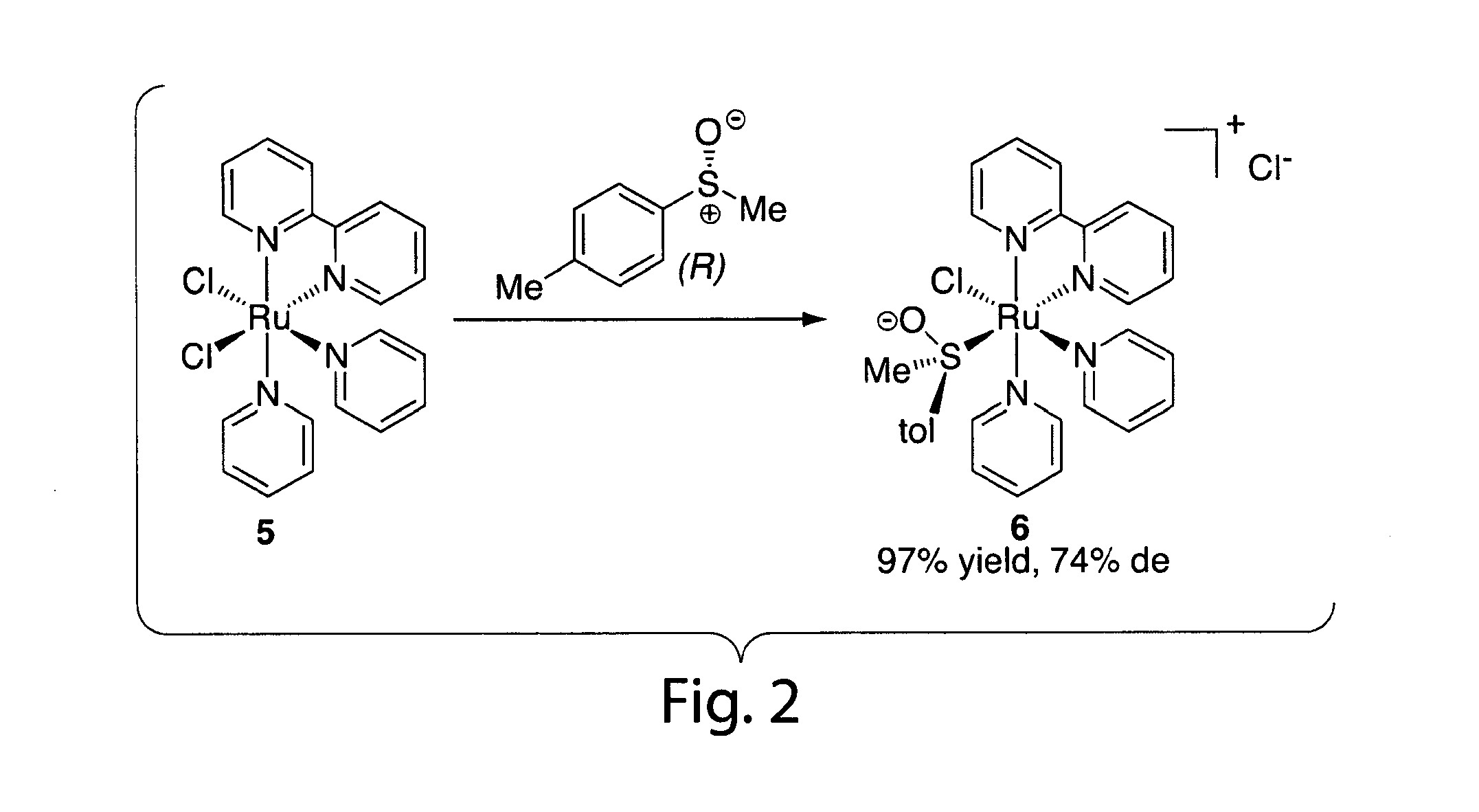
Heteroarylic-arylic diphosphines as chiral ligands
InactiveUS6153758ACarboxylic acid esters preparationNickel organic compoundsCarbon–carbon bondDiphosphines
PCT No. PCT / EP97 / 06358 Sec. 371 Date Apr. 18, 1999 Sec. 102(e) Date Apr. 18, 1999 PCT Filed Nov. 14, 1997 PCT Pub. No. WO98 / 22484 PCT Pub. Date May 28, 1998Diphosphines of a mixed heteroarylic-arylic type, wherein the phosphine group carrying backbone is constituted by the interconnection of a five-atom heteroaromatic ring and a carbocyclic aromatic ring, forming an atropoisomeric chiral system with a C1 symmetry. Said chiral diphosphines are advantageously used as ligands for the formation of chiral complexes with transition metals, in particular Ru, Rh, Pd, Ir, Ni. The so-obtained chiral complexes are used as chiral complexes are used as chiral catalysts for stereocontrolled reactions, in particular diastereo and enantioselective reduction reactions, hydroformylation reactions, hydrosilylation reactions, hydrocyanation reactions, double-bond isomerisation reactions, other reactions of carbon-carbon bond formation.
Owner:CHEMI SPA
Process for the preparation of enantiomerically enriched cyclic beta-aryl or heteroaryl carbocyclic acids
The present invention relates to a process for the preparation of cis substituted cyclic β-aryl or heteroaryl carboxylic acid derivatives in high diastereo- and enantioselectivity by enantioselective hydrogenation in accordance with the following schemewhereinX, Ar, n, and m are defined herein and corresponding salts thereof.
Owner:F HOFFMANN LA ROCHE & CO AG
Method for enantioselective hydrogenation of chromenes
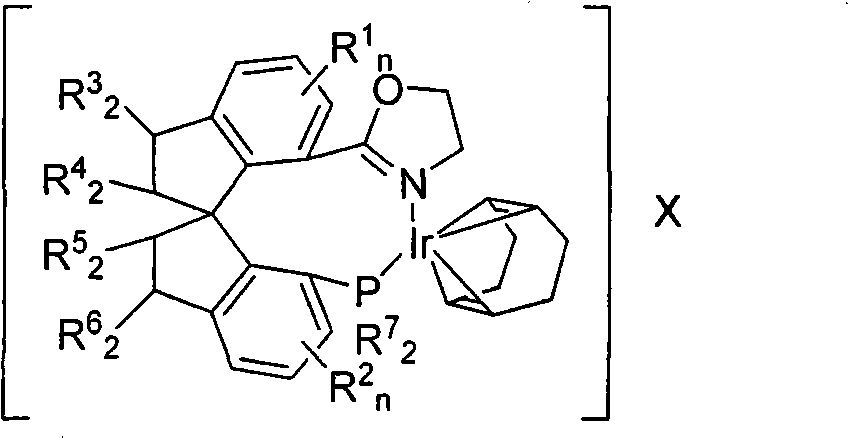
A method for preparing an enantiomeric chromane, by asymmetrically hydrogenating a chromene compound in the presence of an Ir catalyst having a chiral ligand. The method includes the enantioselective preparation of enantiomeric equol. A preferred Ir catalyst has a chiral phosphineoxazoline ligand. Enantiomeric chromanes of high stereoselective purity can be obtained.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI +1
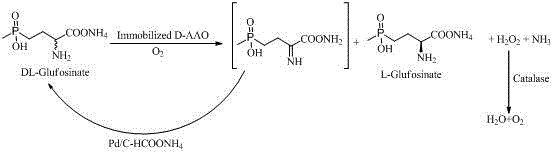
Enzyme-chemocatalysis racemization removing preparation method for L-glufosinate-ammonium
The invention discloses an enzyme-chemocatalysis racemization removing preparation method for L-glufosinate-ammonium. According to the method, a one-pot reaction manner is adopted, under the molecular oxygen, immobilization D-amino acid oxidase catalyzes D-enantiomer in an enantioselectivity mode into 2-imino-4-(hydroxy methyl phosphonyl) butyric acid in a dehydrogenation mode, and palladium-ammonium formate catalyzes 2-imino -4-(hydroxy methyl phosphonyl) butyric acid into DL-glufosinate-ammonium in an in-situ reduction mode. Hydrogen peroxide produced in the process is efficiently decomposed into water and oxygen through catalase. Complete reacemization removing of DL-glufosinate-ammonium and efficient preparing of L-glufosinate-ammonium are achieved through biological oxidation-chemical reduction circulation. The method has the advantages that the process is simple, cost is low, environmental friendliness is achieved, and energy is saved. High-concentration DL-glufosinate-ammonium can be converted into L-glufosinate-ammonium. The yield is 90%, the optical purity of the product is 99%, and the method is suitable for industrial production of L-glufosinate-ammonium.
Owner:重庆惠健生物科技有限公司
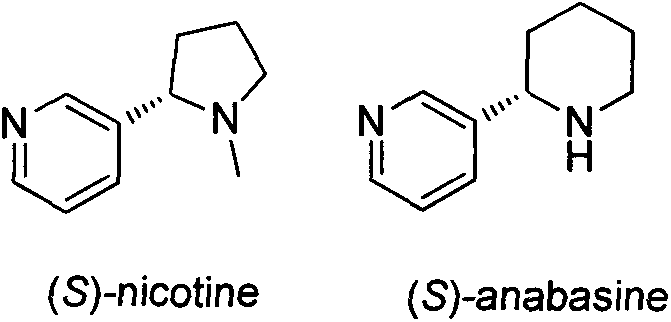
Asymmetric synthesis method for botanical pesticide nicotine and anabasine
ActiveCN104341390AHigh purityEasy to operateOrganic chemistryBulk chemical productionIridiumSynthesis methods
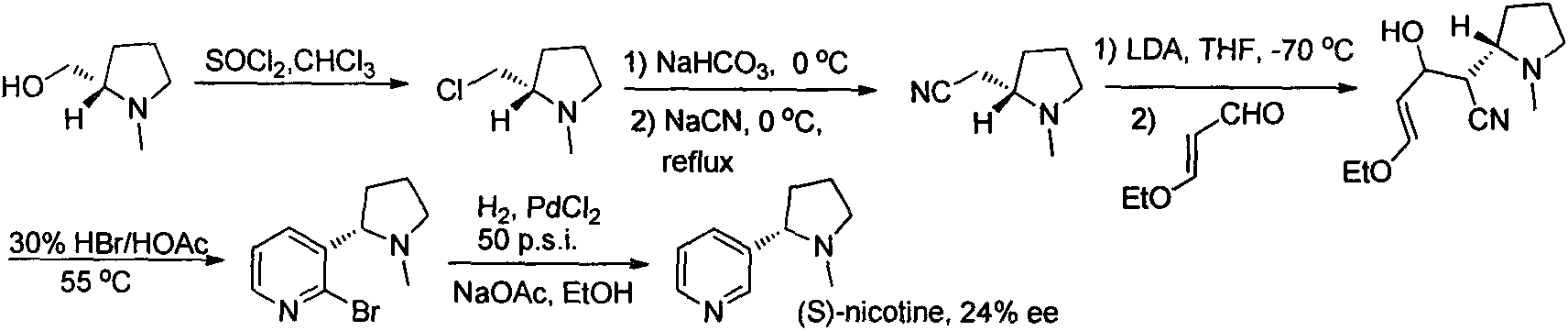
The invention relates to an asymmetric synthesis method for botanical pesticide nicotine and anabasine. The low-cost and easily acquired 2,5-dibromopyridine is taken as an initial raw material and is processed in two steps, so that the hydrogenation precursor annular imine is acquired; under the induction of the chiral catalyst, iridium-phosphine oxazoline, an important hydrogenated product intermediate is acquired through high enantioselectivity; the intermediate is processed in two steps, so that L-nicotine is acquired; the intermediate is converted into L-anabasine in one step. The asymmetric hydrogenation of the annular imine containing pyridine gene is taken as the key step of the method. According to the invention, the chiral catalyst, iridium-phosphine oxazoline, is used for catalyzing the asymmetric hydrogenation and the key intermediate with ultrahigh ee value is acquired, and then the methylation and reduction bromine-removing two-step reaction is performed for converting, so that the target products, natural nicotine and anabasine, are acquired. According to the invention, the operation is stable, the purity is high and the cost is low.
Owner:NANKAI UNIV
Process for preparing enantiomerically enriched amines
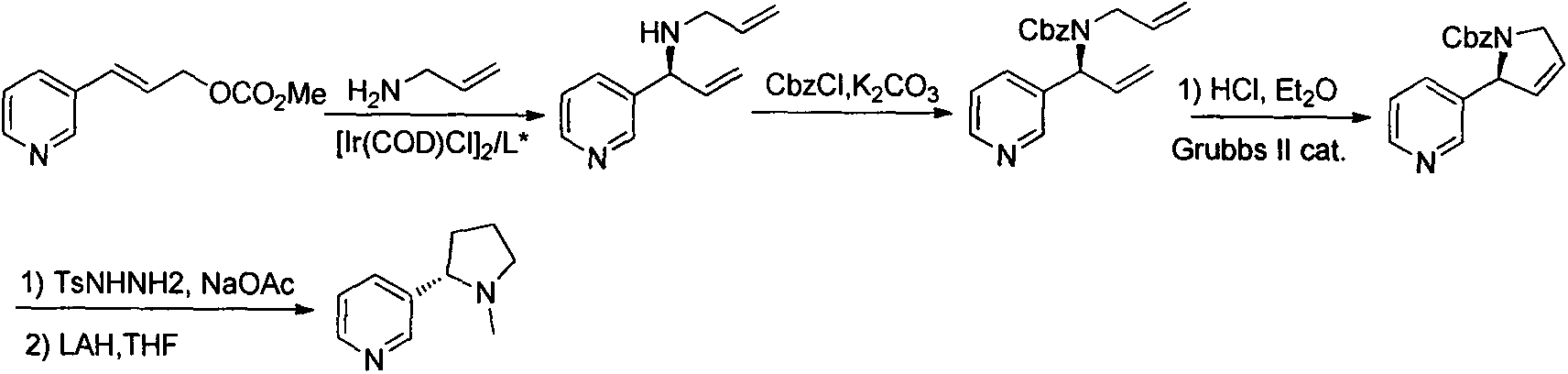
A process for preparing enantiomerically enriched amines by reacting a ketone with ammonia or an ammonium salt and a reducing agent in the presence of a catalytic system comprising the components:a) an amino acid transaminase,b) an alpha-amino acid which is a substrate of the amino acid transaminase,c) an amino acid dehydrogenase suitable for preparing the alpha-amino acid,d) NAD(P)+ ande) an NAD(P)+-reducing enzyme, which reacts NAD(P)+ with the reducing agent to give NAD(P)H.The process can be carried out with catalytic amounts of alpha-amino acid and NAD(P)+, and enables an enantioselective reductive amination of ketones.
Owner:EVONIK DEGUSSA GMBH
New process
ActiveCN105026361ALow number of reaction stepsLow costCarbamic acid derivatives preparationOrganic compound preparationOrganic chemistryEnantio selectivity
The invention relates to a new enantioselective process for producing useful intermediates for the manufacture of NEP inhibitors or prodrugs thereof, in particular NEP inhibitors comprising a γ-amino-δ-biphenyl-α-methylalkanoic acid, or acid ester, backbone.
Owner:SUZHOU NOVARTIS PHARMA TECHONOLOGY CO LTD +1
Enzymic method for the enantioselective reduction of keto compounds
ActiveUS20090017510A1Long stable timeHigh yieldBacteriaOxidoreductasesOrganic compoundEnantio selectivity
The invention relates to an enzymatic method for the enantioselective reduction of organic keto compounds to the corresponding chiral hydroxy compounds, an alcohol dehydrogenase from Lactobacillus minor and a method for the enantioselective production of (S)-hydroxy compounds from a racemate.
Owner:SCIENCES PO
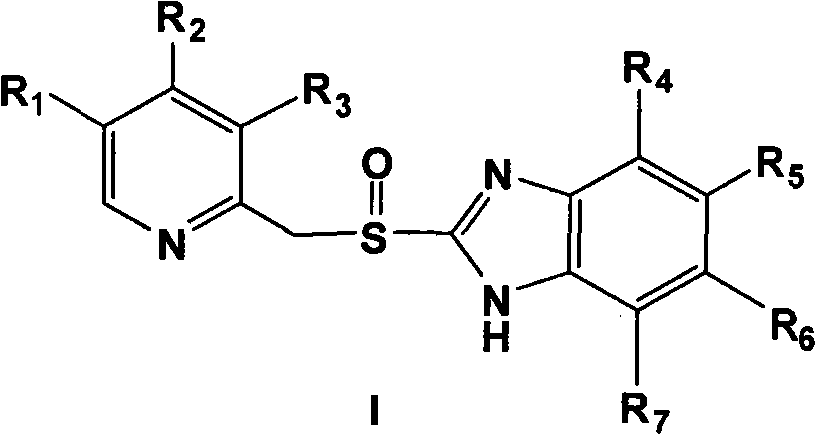
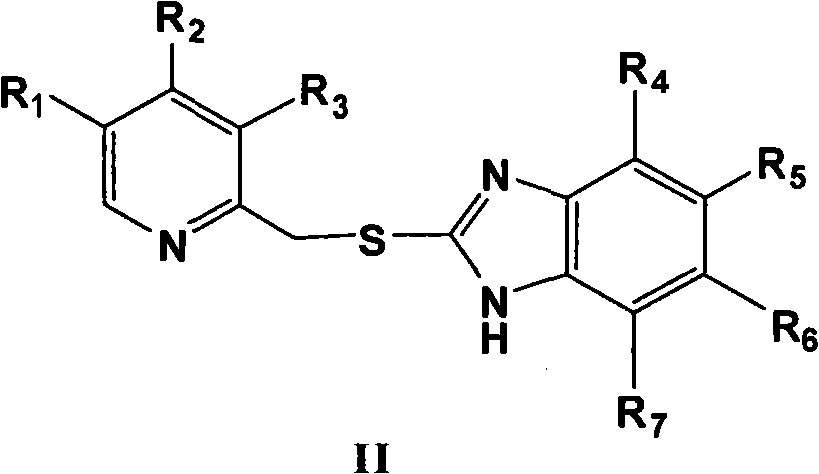
Novel method for preparing chiral sulphoxide compound
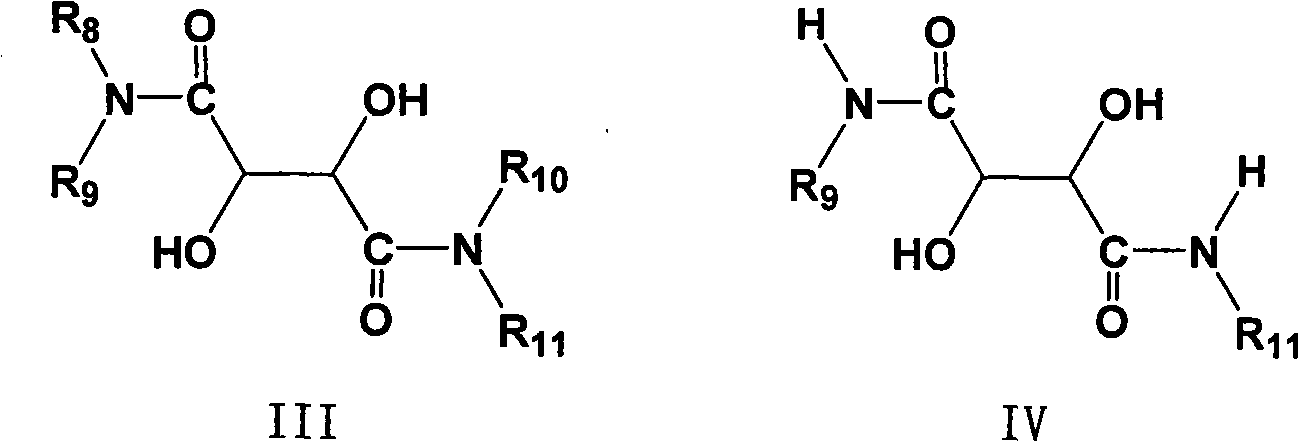
The invention provides a novel method for preparing optically pure substituted [(pyridyl methylene) sulfinyl]-1H-benzimidazole sulphoxide compound by enantioselective synthesis. The method requiring protection is to directly and asymmetrically oxidize prochiral thioether into a corresponding optically pure sulphoxide compound or a sulphoxide compound rich in single enantiomer by a mild and cheap oxidizing agent in the presence of a complex compound catalyst formed by an accessible and stable (+)- or (-)- tartaric acid diamide ligand shown in a general formula and titanium. Therefore, optically pure omeprazole, lansoprazole and pantoprazole can be obtained, wherein R8, R9, R10 and R11 are the same or different, and are selected from hydrogen, alkyl, aralkyl, aryl, organic polymers or a silica loading body.
Owner:CHENGDU ORGANIC CHEM CO LTD CHINESE ACAD OF SCI +1
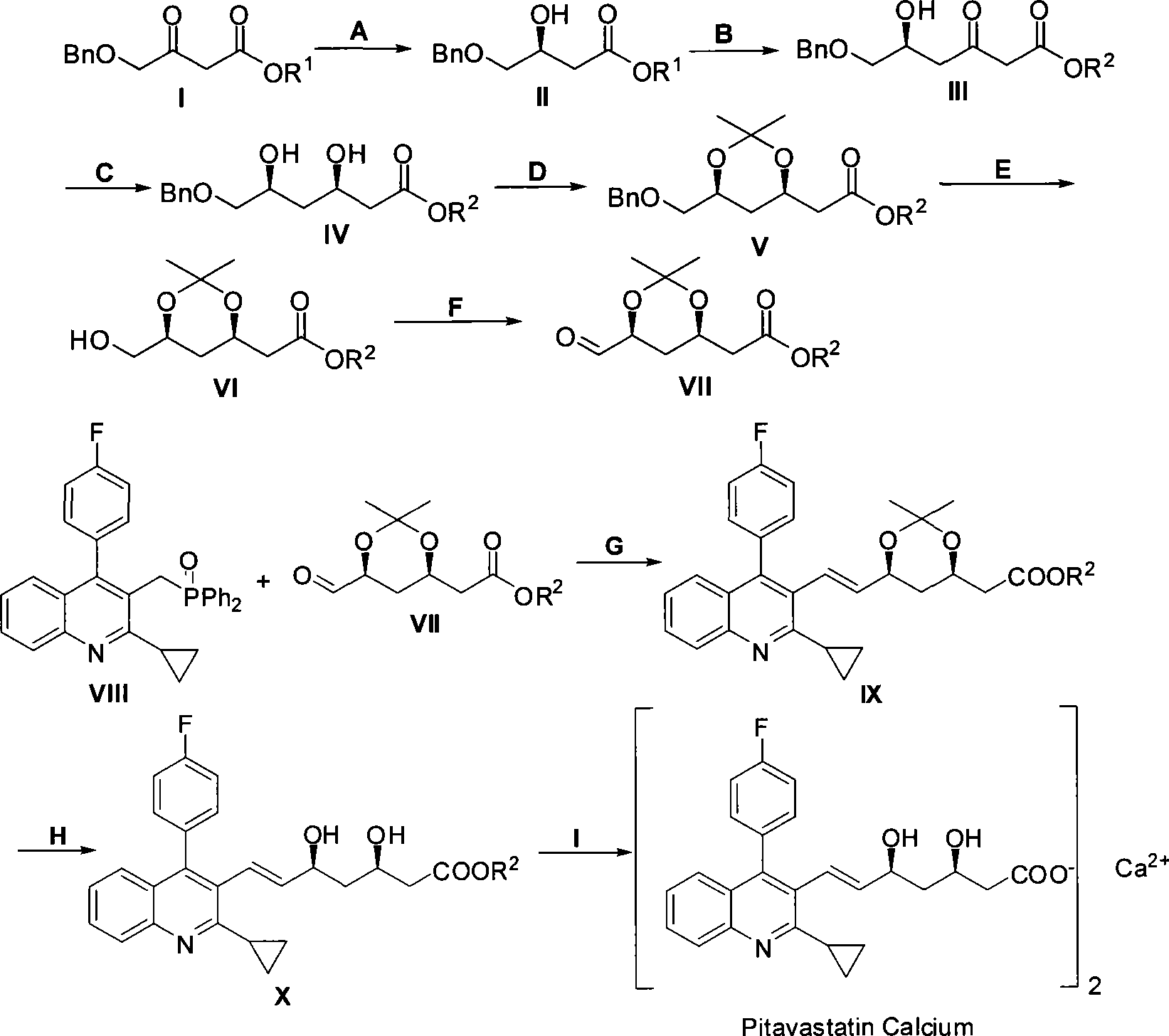
Method for preparing pitavastatin calcium raw material medicine using asymmetric hydrogenation
ActiveCN101386592ASolve the problem of expensive chiral side chainsMetabolism disorderAsymmetric synthesesClaisen condensationEthyl butyrate
The invention relates to a method for preparing a pitavastatin calcium raw material by asymmetric hydrogenation in the technical field of medicinal chemistry. The method comprises the following steps: performing catalytic hydrogenation on 4-benzyloxy-ethyl-acetoacetate I by using a chiral catalyst to prepare (S)-4-benzyloxy-3-hydroxy-ethyl-butyrate, and then obtaining the pitavastatin calcium through a series of reactions such as Claisen condensation, reduction, hydroxyl protection, removal of benzyl, oxidation and the like. In the invention, the highly-efficient chirality added value is realized by the use of catalytic amount of the chiral catalyst (wherein the ratio of substrate to the catalyst can reach 3, 000 to 1), and by performing the asymmetric hydrogenation and the reduction on non-chiral ketones to generate chiral alcohols compounds directly (wherein the enantioselectivity can be up to 94.3 percent).
Owner:JIANGSU WANBANG BIOPHARMLS +1
Process For The Preparation of (3R)-Hydroxybutyl (3R) -Hydroxybutyrate By Enzymatic Enantioselective Reduction Employing Lactobacillus Brevis Alcohol Dehydrogenase
ActiveUS20120064611A1Preparation from ketenes/polyketenesOxidoreductasesHydroxybutyric acidLactobacillus brevis
Owner:OXFORD UNIV INNOVATION LTD +1
Hydride reduction of alpha, beta-unsaturated carbonyl compounds using chiral organic catalysts
InactiveUS20060161024A1Lower Level RequirementsReduce compoundingOrganic compound preparationOrganic chemistry methodsGreek letter betaDihydropyridine
Nonmetallic, chiral organic catalysts are used to catalyze the 1,4-hydride reduction of an α,β-unsaturated carbonyl compound. The α,β-unsaturated carbonyl compound may be an aldehyde or cyclic ketone, and the hydride donor may be a dihydropyridine. The reaction is enantioselective, and proceeds with a variety of hydride donors, catalysts, and substrates. The invention also provides compositions effective in carrying out the 1,4-hydride addition of α,β-unsaturated carbonyl compounds.
Owner:CALIFORNIA INST OF TECH
Synthesis method of metaraminol bitartrate
ActiveCN103739504AEasy to controlFew synthetic stepsOrganic compound preparationCarboxylic acid salt preparationSynthesis methodsSpatial configuration
The invention discloses a synthesis method of metaraminol bitartrate, and in particular provides a method for synthesizing metaraminol bitartrate by using a chiral catalysis method. The synthesis method comprises the steps: catalyzing a chiral addition reaction of hydroxybenzaldehyde and nitroethane by using a chiral catalyst system consisting of cinchona alkaloid, copper acetate hydrate and less imidazole to obtain an addition product with a dominant required spatial configuration, and then reducing nitro by using hydrogen in the presence of Pd-C to obtain amine to obtain aramine, and salifying the aramine with L(+)-tartaric acid to obtain a final product metaraminol bitartrate. According to the synthesis method, an enzyme catalyst is prevented from being used, a raw material of the synthesis reaction is easily available, the chiral catalyst is easily purchased or prepared self, the synthesis steps are relatively less, the chiral control efficiency is higher, the enantioselectivity is high, the yield is good, the reaction operation is easily controlled, and is safe and reliable, and the foundation is laid for the later industrialized amplification production.
Owner:广州普星药业有限公司
Simple stereoselective synthesis method of sex pheromones of hyphantria cunea
The invention discloses a simple stereoselective synthesis method of sex pheromones of hyphantria cunea, which relates to epoxypropane compounds. The sex pheromones of hyphantria cunea III[(9S,10R)-9,10-epoxy-(3Z,6Z)-3,6-heneicosenediene] and IV[(9S,10R)-9,10-epoxy-(3Z,6Z)-3,6-heneicosenetriene] are prepared from cheap and readily available 2-propargyl alcohol serving as raw materials by eight steps with high efficiency, high enantioselectivity and high yield, wherein the total yield of (9S,10R)-III is 36 percent; the total yield of (9S,10R)-IV is 33 percent; and enantiomer excess e is more than 99 percent. The method has the advantages that: the operation and separation of each step are simple; the yield is high; all the adopted reagents are common reagents which are cheap and readily available; and the production line is short. The method is suitable for industrial production.
Owner:XIAMEN UNIV
Method for preparing cis-pinane by asymmetric catalytic hydrogenation of alpha-pinene
InactiveCN104003831AImprove conversion rateHigh enantioselectivityOrganic-compounds/hydrides/coordination-complexes catalystsHydrocarbon by hydrogenationNitrogen gasHigh pressure
The invention discloses a method for preparing cis-pinane by asymmetric catalytic hydrogenation of alpha-pinene, and belongs to the chemical engineering field. The method comprises the process steps: in a nitrogen atmosphere, heating RhCl3.3H2O to dissolve in an ethanol solution, and forming a solution A; dissolving newly recrystallized PPh3 in a deoxygenated ethanol, and forming a solution B; adding the solution B into the solution A, refluxing for a certain period of time, filtering under reduced pressure while being hot, washing with deoxygenated ether, carrying out vacuum drying to obtain a rhodium phosphine complex RhCl(PPh3)3; and adding the prepared rhodium phosphine complex RhCl(PPh3)3, an ionic liquid and alpha-pinene into a high-pressure reaction kettle according to a certain proportion, putting a cover and sealing, respectively replacing with nitrogen gas and hydrogen gas, carrying out pressure maintaining and leakage detection, and carrying out a reaction under certain conditions to prepare cis-pinane. The method has the advantages of mild reaction conditions, high conversion rate of alpha-pinene and high enantioselectivity of cis-pinane; the ionic liquid catalyst system is easily separated from the product and can be recycled; and the process flow is simple, and the energy consumption is low.
Owner:KUNMING UNIV OF SCI & TECH
Preparation method and application for phosphine-oxazoline ligand, and ionic metal complex, enantiomer or racemate thereof
InactiveCN102875601AThe synthesis method is simpleSynthetic method is economicalOrganic compound preparationGroup 5/15 element organic compoundsPlanar chiralityStructural formula
The invention discloses a preparation method and an application for phosphine-oxazoline ligand, and ionic metal complex, enantiomer or racemate thereof. The ligand and the ionic metal complex thereof have the following structural formulas. The phosphine ligand related by the invention employs biphenyl as a skeleton, and realizes completely transmission from planar chirality to axial chirality through an asymmetric desymmerization. The synthetic method is simple and economic, omits a common and complex chiral separation process in the preparation of the chiral ligand. The obtained chiral ligand has the advantages of high reactive activity, good enantiomorphous selectivity and the like in a model reaction.
Owner:SUN YAT SEN UNIV
A chiral nitrogen nitrogen phosphine tridentate ligand based on a ferrocene skeleton and an application thereof
ActiveCN108774271AHigh industrial application valueEasy to purifyOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsDouble bondHigh activity
The invention belongs to the technical field of asymmetric catalysis and specifically relates to a chiral nitrogen nitrogen phosphine tridentate ligand based on a ferrocene skeleton and an applicationthereof. A coordination compound is obtained by the disclosed chiral nitrogen nitrogen phosphine tridentate ligand base on the ferrocene skeleton complexed with a transition metal precursor, the complex is used as a precious metal catalyst, and is successfully applied to high efficiency asymmetric hydrogenation of aromatic ketone. Compared with other tridentate ligands, the ligand is simple to synthetize, is stable to water and air, and easy to prepare on a large scale, shows high activity and high enantioselectivity on carbon and oxygen double bond, and has greater implementation value and social and economic benefits.
Owner:ZHEJIANG UNIV OF TECH
Spiro phosphine-oxazoline and preparation method and application thereof
ActiveCN101565434AHigh activityHigh enantioselectivityRuthenium organic compoundsIndium organic compoundsIridiumIon exchange
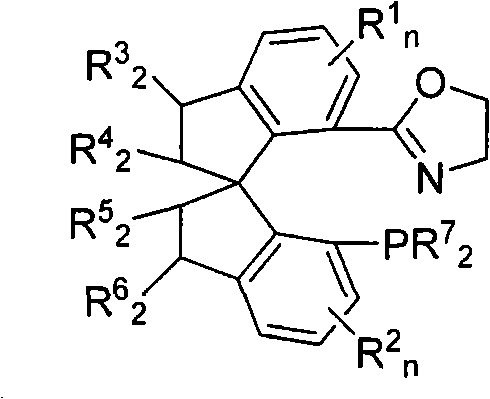
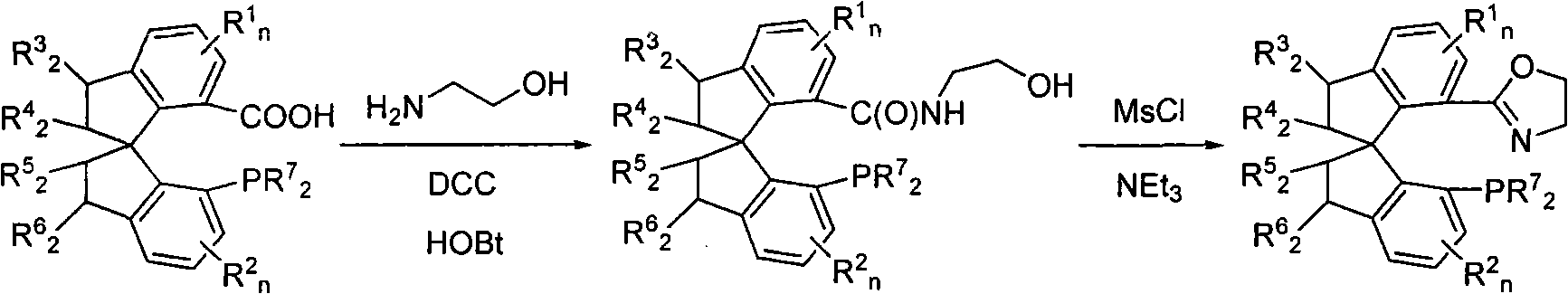
The invention belongs to spirophosphine-oxazoline and a preparation method and an application thereof, in particular to novel spirophosphine-oxazoline and a preparation method of iridium complex thereof. The novel spirophosphine-oxazoline is synthesized through two reactions by using substitutional 7-diarylphosphino-7'-carboxyl-1, 1'-spirobiindane as starting raw materials. Complexation and ion exchange are performed for the novel spirophosphine-oxazoline and iridium precursor, so as to obtain iridium / spirophosphine-oxazoline complex compound containing different anions. The invention overcomes the defects of the prior art, synthesizes the novel spirophosphine-oxazoline without substitutional group on oxazoline 4- position by using cheap aminoethanol as raw materials, can catalyze asymmetric hydrogenation reaction of Alpha-substituting acrylic acid, expresses higher activity and enantioselectivity and has higher research value and industrial prospect.
Owner:ZHEJIANG JIUZHOU PHARM CO LTD
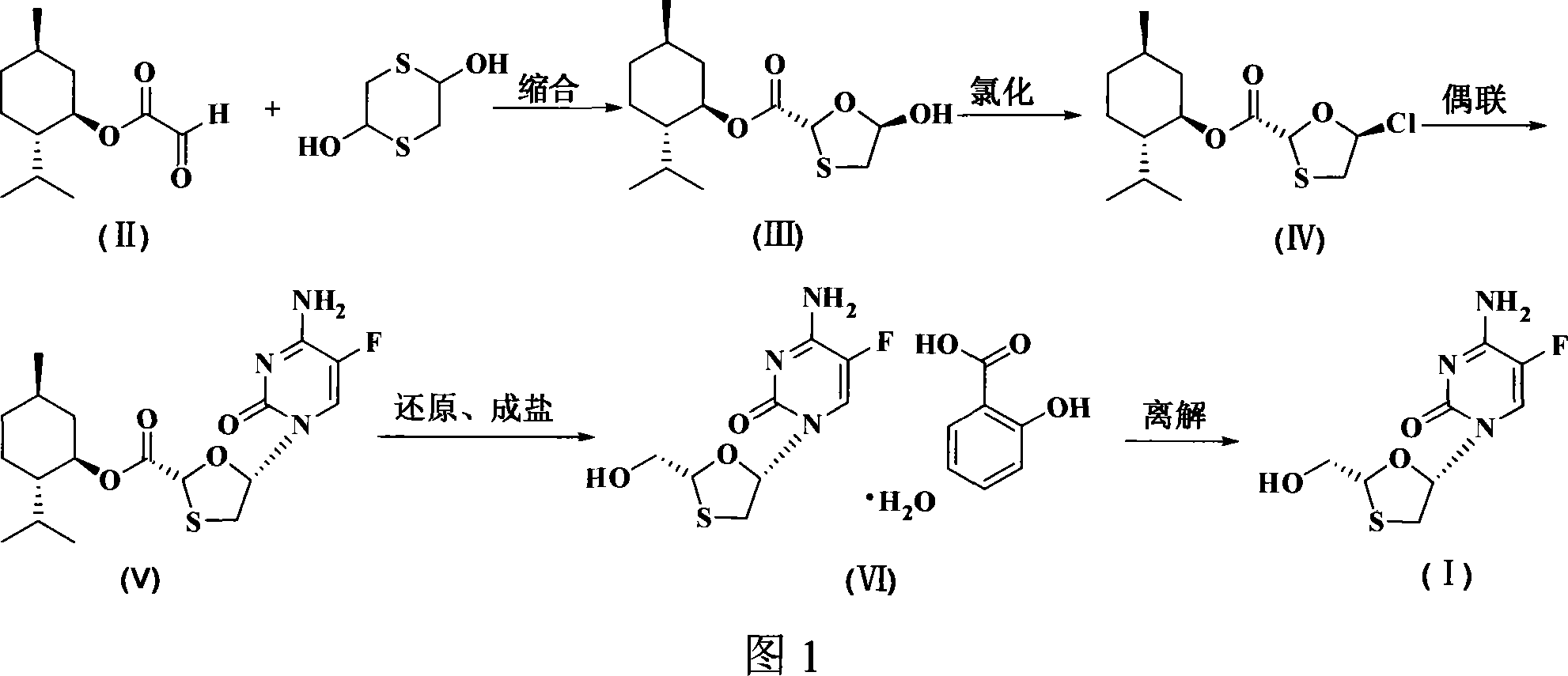
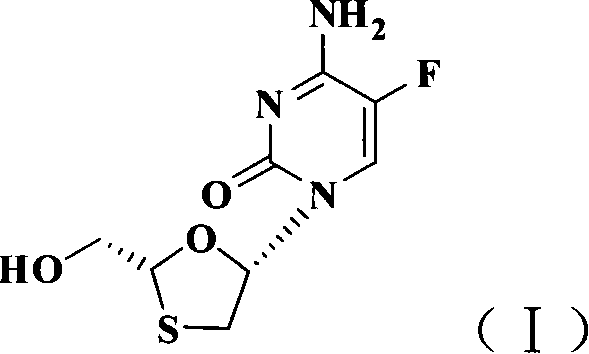
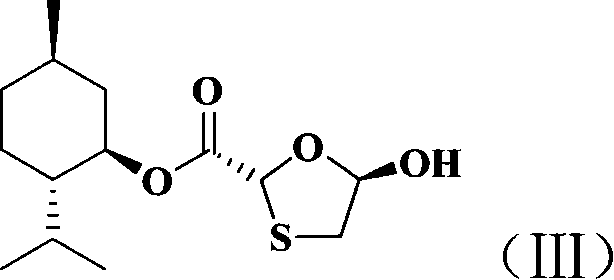
Non-enantioselective prepn process of emtricitabine
InactiveCN101066971AMild reaction conditionsSimple and fast operationOrganic chemistryDithianeAlcohol
The present invention discloses non-enantioselective preparation process of emtricitabine. The preparation process includes condensation of glyoxalic acid as the initial material and 2, 5-dihydroxy-1, 4-dithiane under the asymmetric induction of optically active alcohol ester to obtain trans-5-hydroxy-1, 3-oxythiacyclopentane-2-carboxylate; halogenating and coupling with silylated 5-flucytosine, and reducing to obtain initial product; reaction with salicylic acid to form salt, purification and separation to obtain optically pure emtricitabine. The present invention has mild reaction condition, simple operation, low cost, less environmental pollution and high product purity reaching medicinal standard, and is suitable for industrial production. The product is used in treating hepatitis B and AIDS.
Owner:葛建利
6-hydroxyl quinine quaternary ammonium salt asymmetric phase transfer catalyst, preparation method and application of 6-hydroxyl quinine quaternary ammonium salt asymmetry phase transfer catalyst
InactiveCN105457675AMild reaction conditionsSuitable for large-scale productionOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsOrganic synthesisReaction temperature
The invention discloses a 6-hydroxyl quinine quaternary ammonium salt asymmetric phase transfer catalyst, a preparation method and application of the 6-hydroxyl quinine quaternary ammonium salt asymmetry phase transfer catalyst and belongs to the field of organic synthesis. In a two-phase system of an oxidizing agent, an alkaline solution and an inert solvent, the 6-hydroxyl quinine quaternary ammonium salt asymmetric phase transfer catalyst converts a beta-dicarbonyl compound into a chiral alpha-hydroxyl-beta-dicarbonyl compound; the usage quantity of the chiral 6-hydroxyl quinine quaternary ammonium salt asymmetric phase transfer catalyst is 0.5-50 mol% of the beta-dicarbonyl compound, and under the mild condition that reaction temperature is -15 DEG C-65 DEG C, the yield of the chiral alpha-hydroxyl-beta-dicarbonyl compound is larger than or equal to 90%, and the enantioselectivity is higher than or equal to 70% ee. The method is mild in reaction condition, environmentally friendly, high in reaction efficiency and suitable for large-scale production and preparation.
Owner:DALIAN UNIV OF TECH
Method for Preparing 3-Hydroxy-4-Hydroxymethyl-Pyrrolidine Compounds
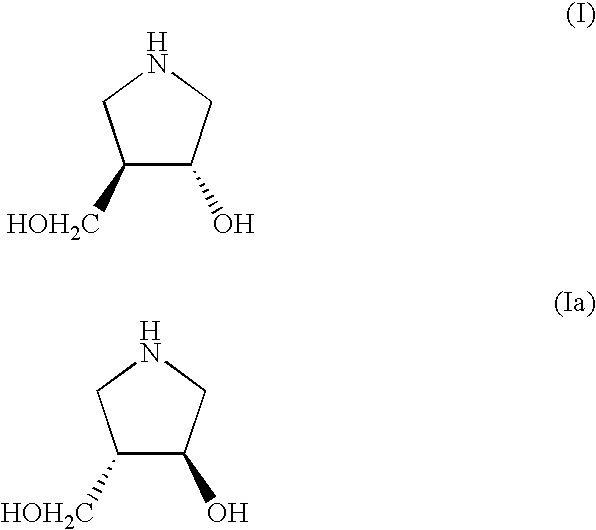
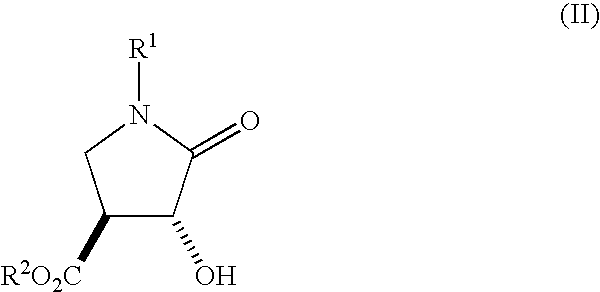
A process is disclosed for preparing (3R,4R)-3-hydroxy-4-hydroxymethylpyrrolidine, the compound of formula (I), or (3S,4S)-3-hydroxy-4-hydroxymethylpyrrolidine, the compound of formula (Ia) involving, as a key step, the enzyme-catalysed enantioselective hydrolysis of a racemic 3,4-trans-disubstituted pyrrolidinone compound of formula (II).
Owner:VICTORIA LINK LTD
Enantioselective resolution process for arylpropionic acid drugs from the racemic mixture
InactiveUS6093830AOrganic compound preparationOptically-active compound separationCelsius DegreeOrganic solvent
The invention relates to a novel non-catalytic enantioselective resolution process for the separation of enantiomer of arylpropionic acid drugs from the racemic mixture, which comprises dissolving the racemic mixture of the said drug an organic solvent, reacting this solution with an aqueous phase containing an ionic surfactant with or without a suitable co-surfactant, and an electrolyte in microemulsion / micellar / biphasic medium, reacting this mixture with an appropriate chiral amine at a temperature in the range of 0 to 70 degrees Celsius to obtain a diastereomeric salt, acid hydrolysing the diastereomeric salt to result in the pure enantiomer of the drug which is extracted by known methods.
Owner:COUNCIL OF SCI & IND RES
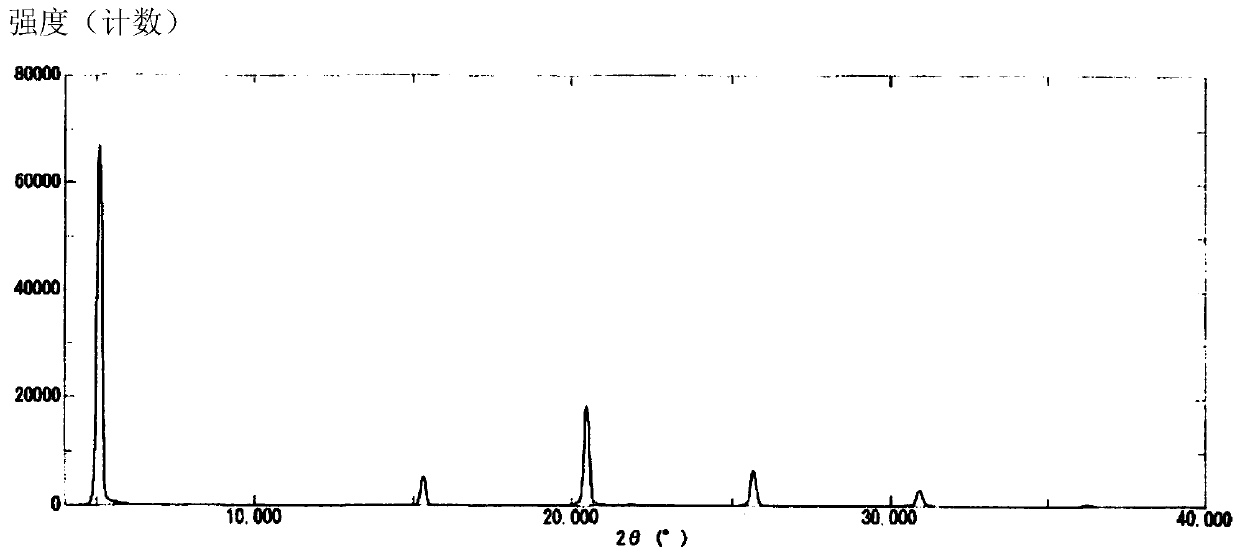
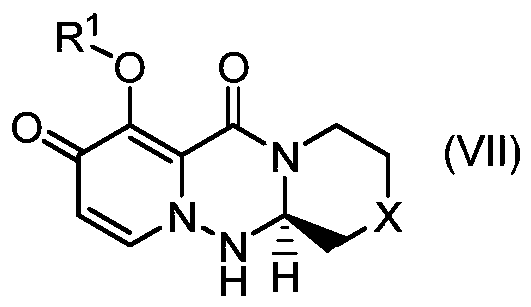
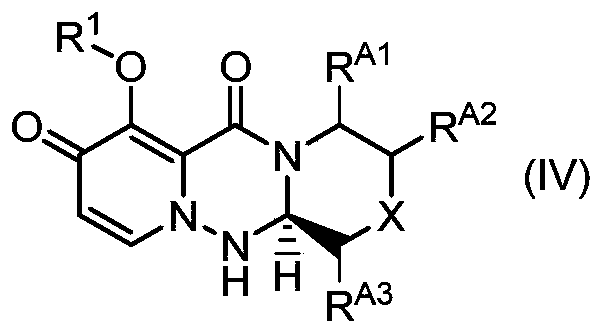
Method for stereoselectively producing substituted polycyclic pyridone derivative
ActiveCN111386276AOrganic active ingredientsOrganic chemistry methodsCombinatorial chemistryPerylene derivatives
The present invention provides a commercially applicable method for producing an intermediate of a substituted polycyclic pyridone derivative exhibiting cap-dependent endonuclease inhibitory activity.In the production method shown in the reaction formula (in the formula, the symbols are as defined in the description), by means of an intramolecular cyclisation reaction in which the stereochemistryof a compound shown in formula (III) or formula (VI) is controlled, a compound shown in formula (IV) and including an eliminable functional group on an asymmetric carbon is obtained, and said eliminable functional group is eliminated, whereby an optically active substituted 3-ring pyridone derivative shown in formula (VII) is highly enantioselectively obtained in a high yield.
Owner:SHIONOGI & CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com