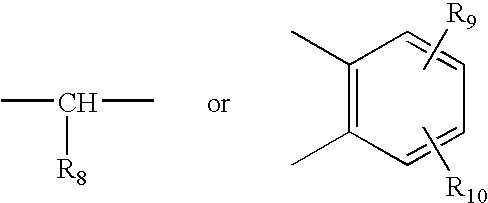Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
436 results about "Omeprazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
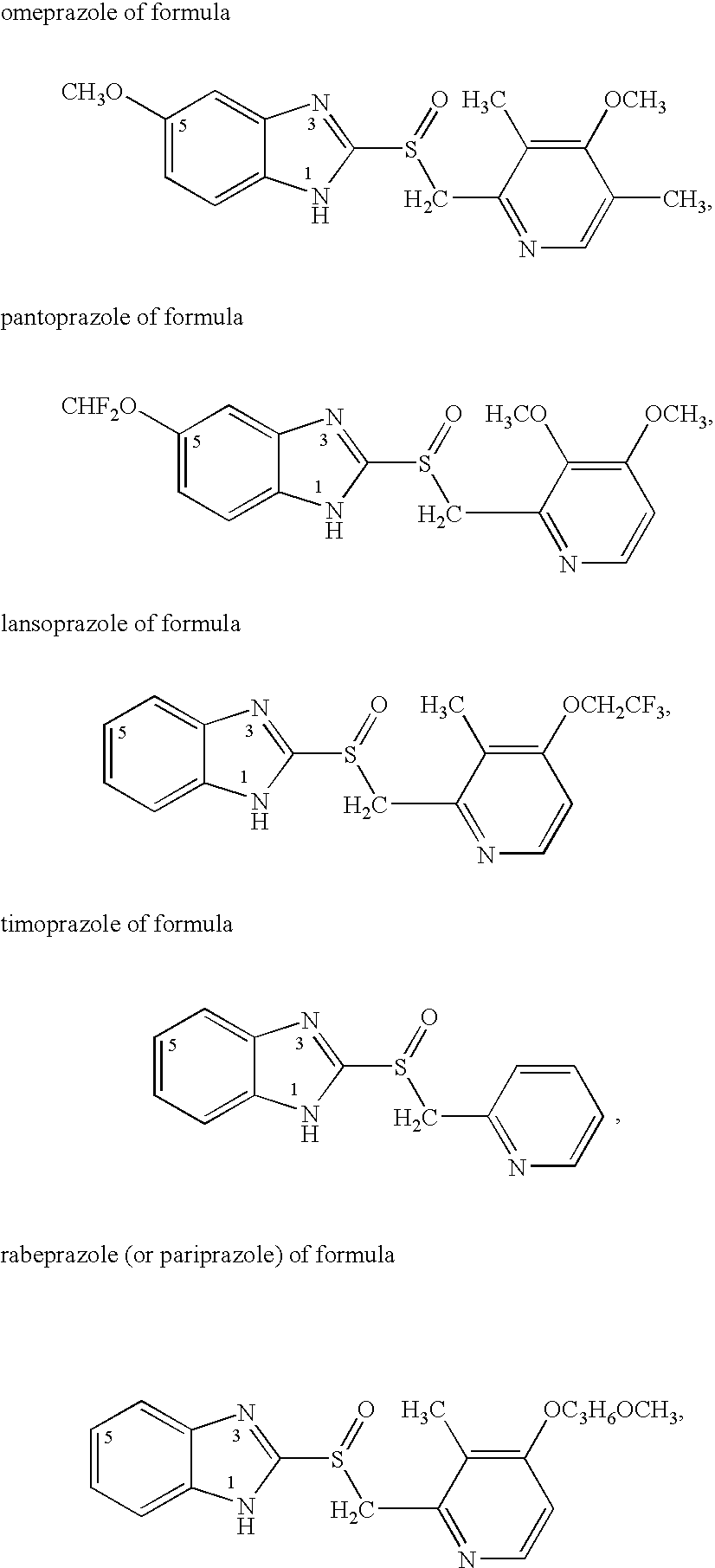
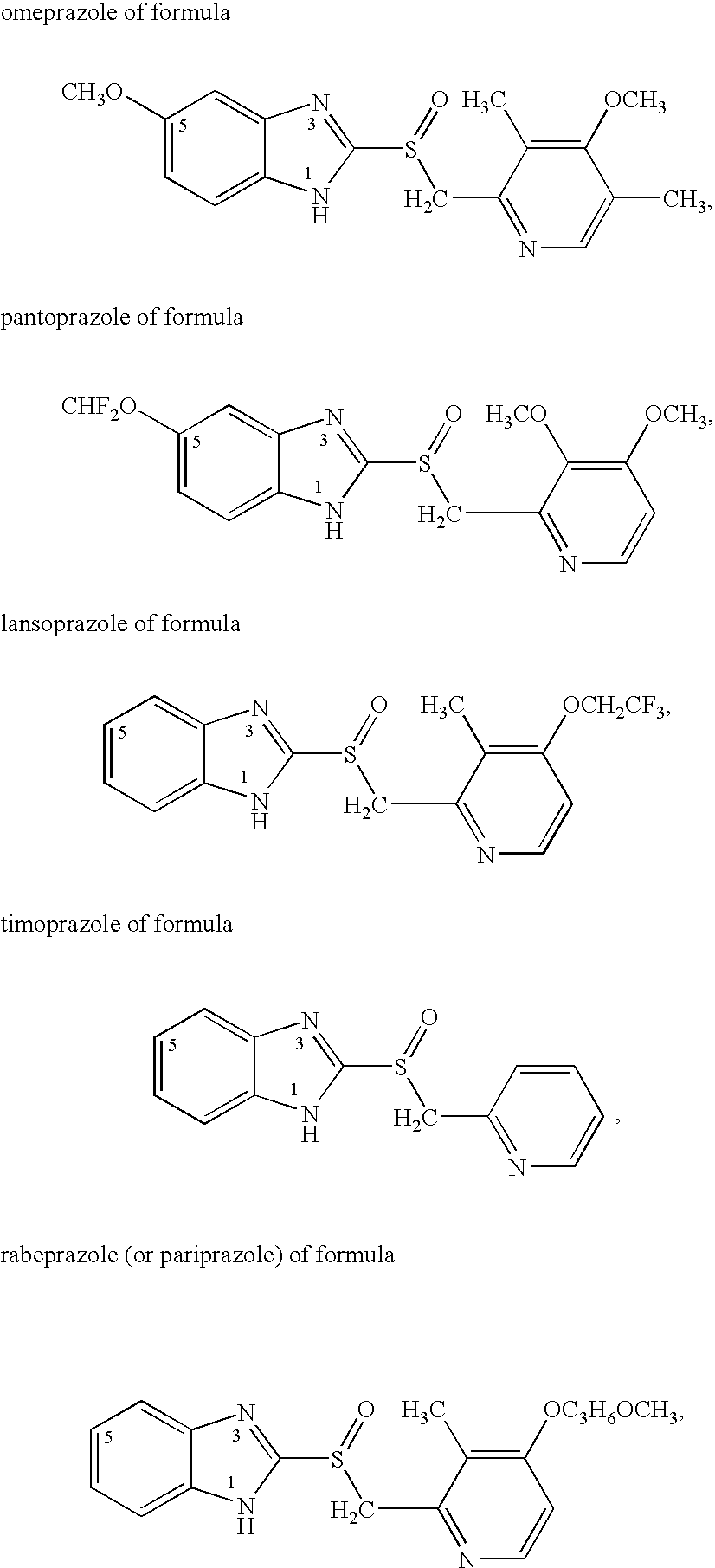
Omeprazole is used to treat certain stomach and esophagus problems (such as acid reflux, ulcers).
Compositions and methods for delivery of omeprazole plus acetylsalicylic acid
The present disclosure is directed to a method for delivering a pharmaceutical composition to a patient in need thereof, comprising: administering to said patient a pharmaceutical composition in unit dose form comprising acetylsalicylic acid, or pharmaceutically acceptable salt thereof, and omeprazole, or pharmaceutically acceptable salt thereof.
Owner:GENUS LIFESCI INC
Pharmaceutical formulation comprising omeprazole
InactiveUS6428810B1Reduce diffuseHigh film thicknessOrganic active ingredientsOrganic chemistryEnantiomerOmeprazole
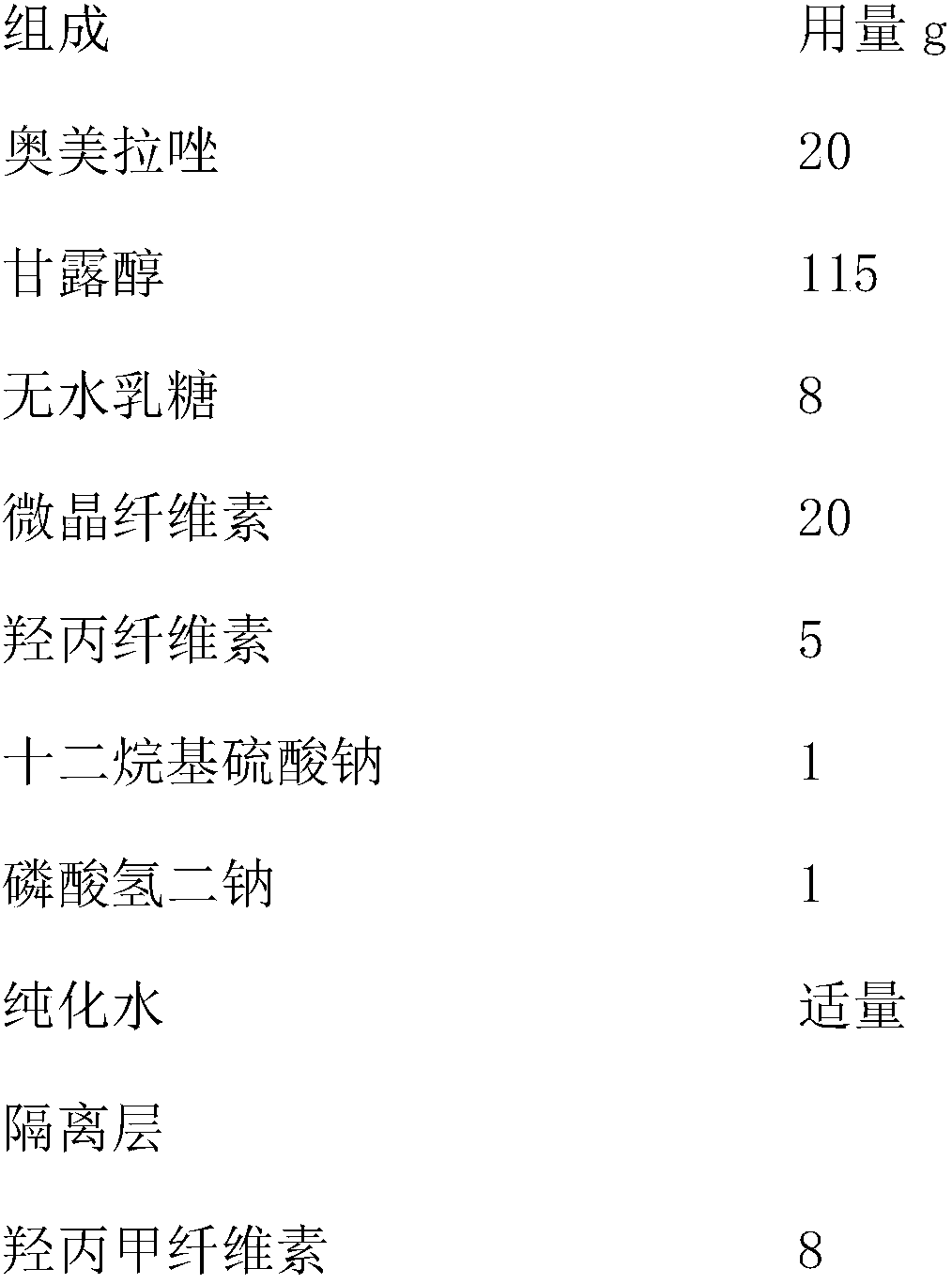
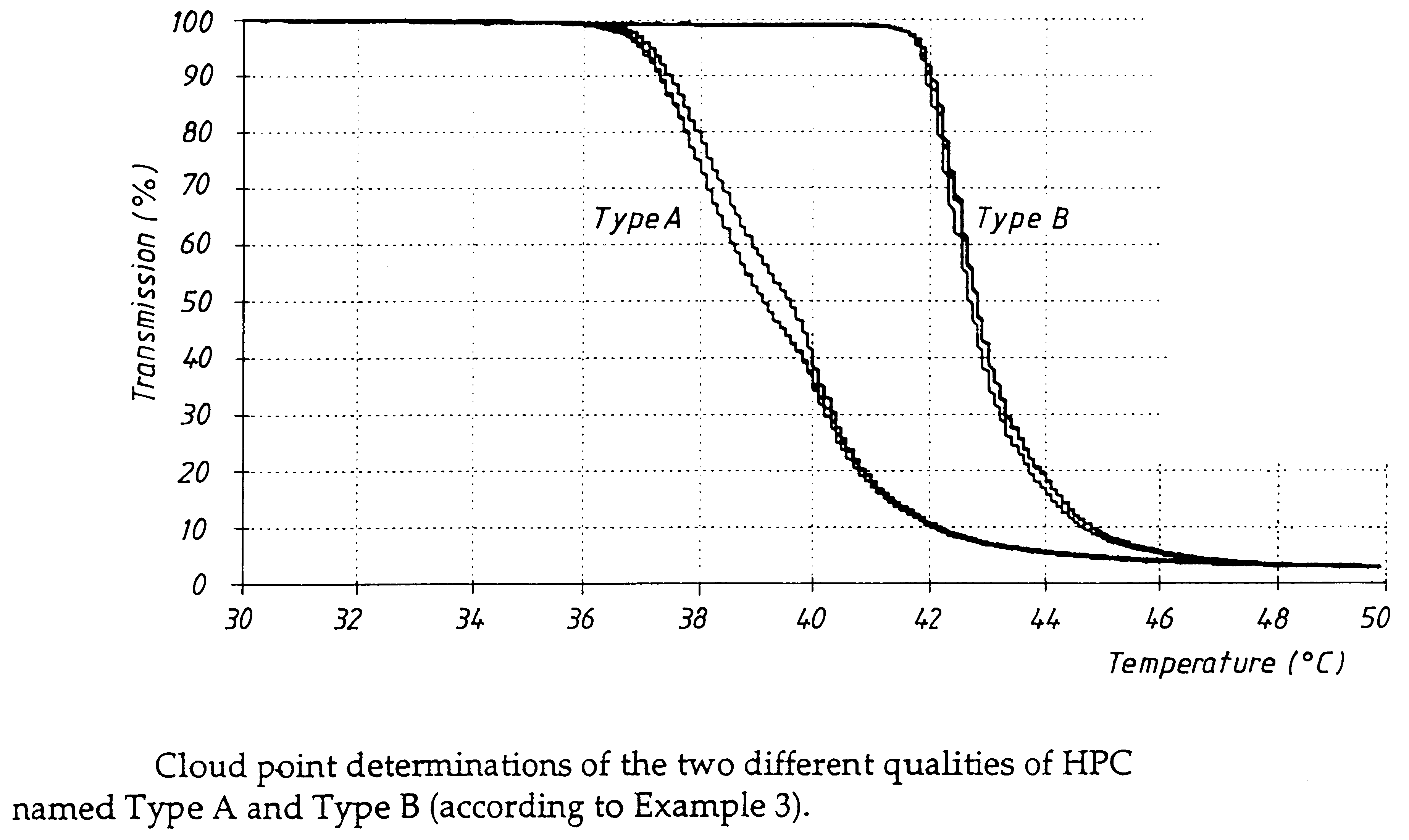
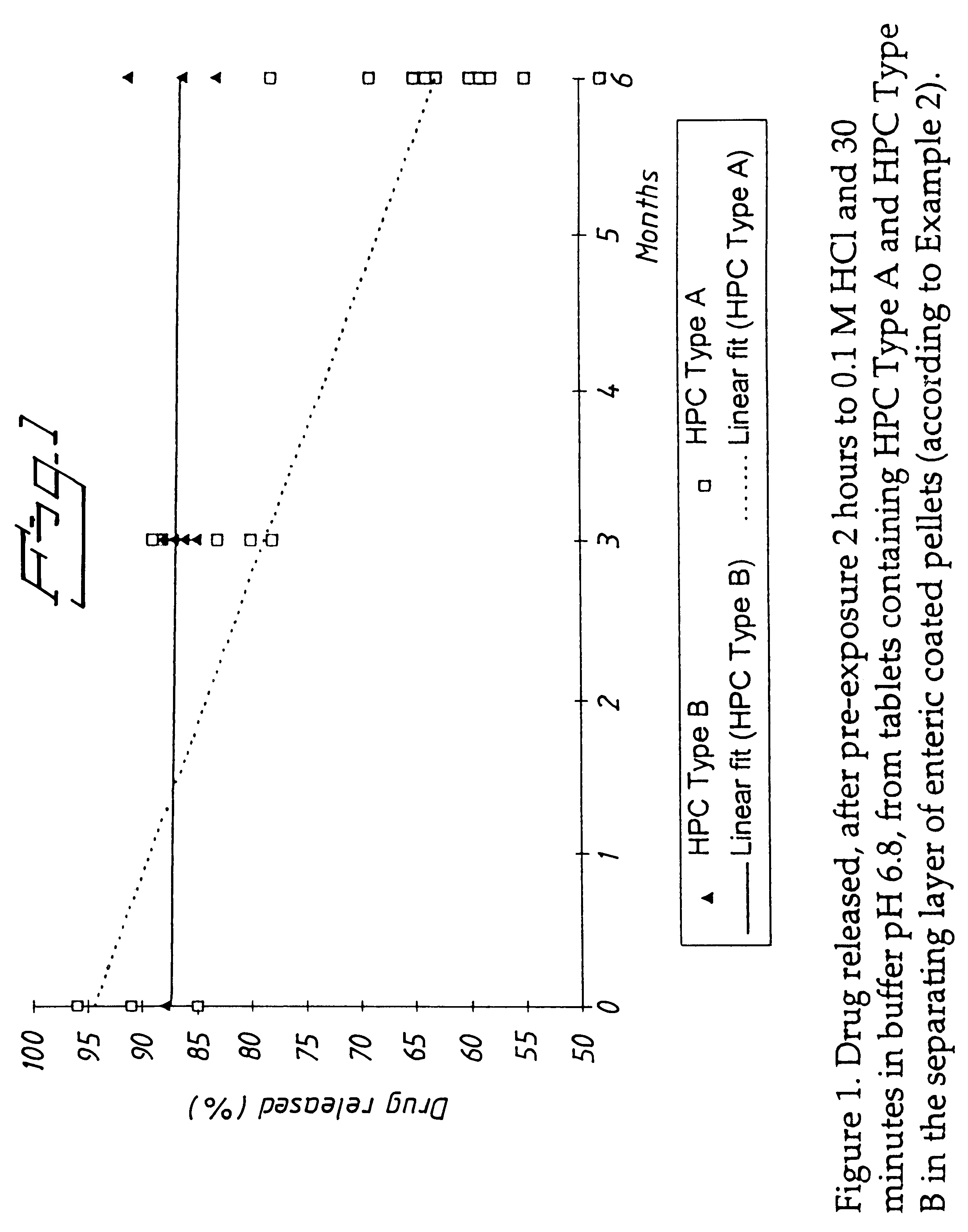
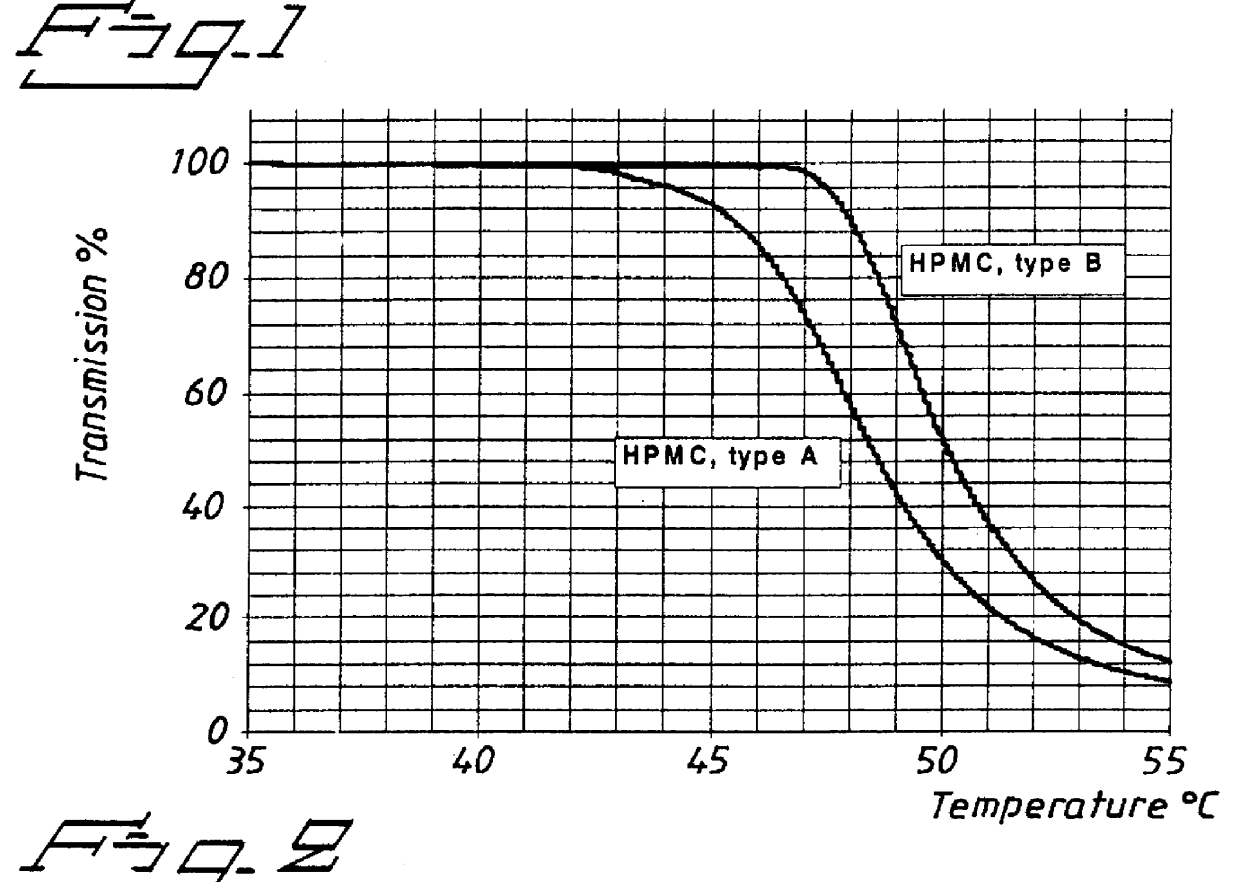
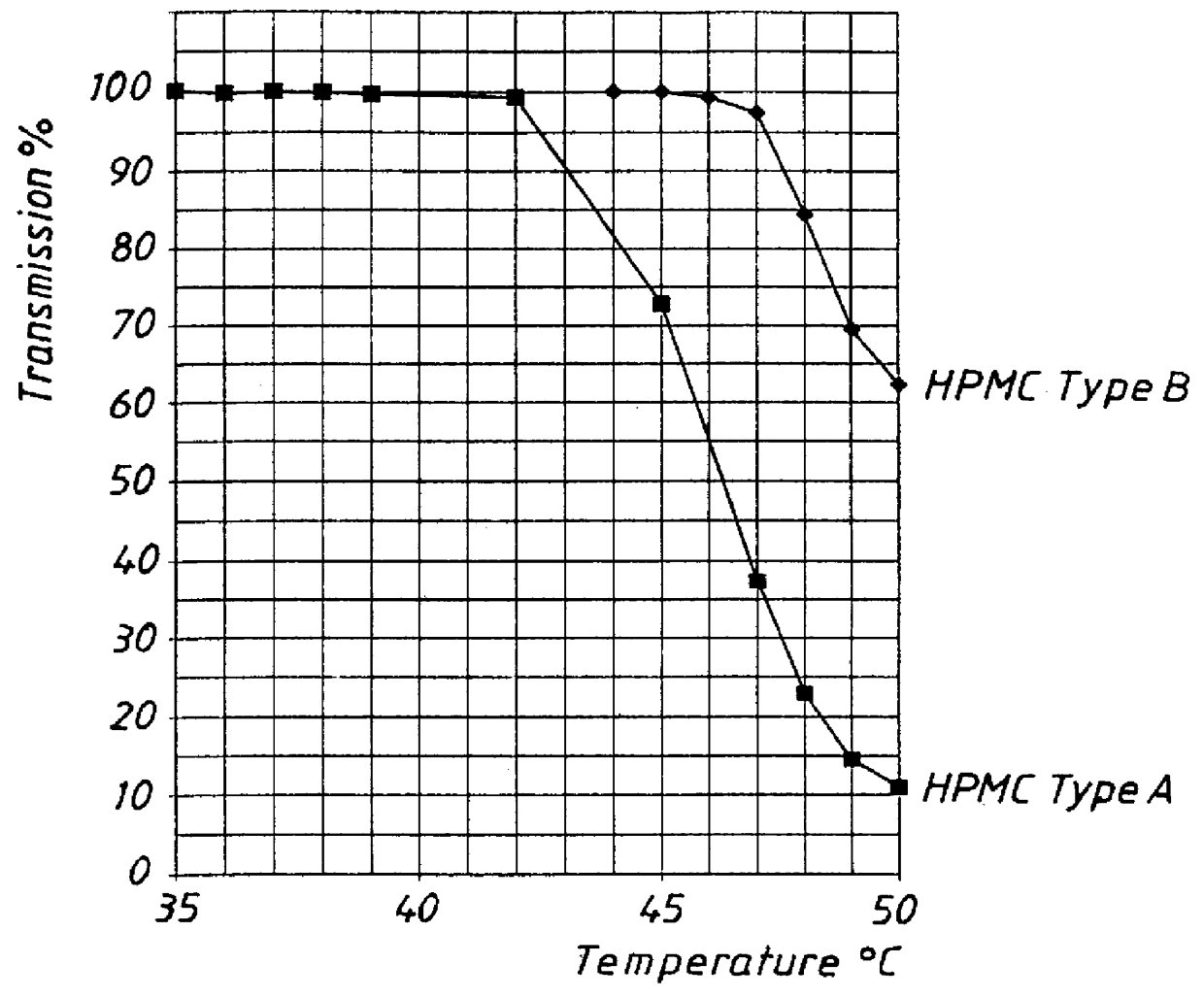
An enteric coated oral pharmaceutical formulation comprising as active ingredient a compound selected from the group of omeprazole, an alkaline salt of omeprazole, one of the single enantiomers of omeprazole and an alkaline salt of one of the single enantiomers of omeprazole, wherein the formulation comprises a core material that comprises the active ingredient and optionally an alkaline reacting compound, the active ingredient is in admixture with a pharmaceutically acceptable excipient, such as for instance a binding agent, and on said core material a separating layer and an enteric coating layer. A hydroxypropyl cellulose (HPC) with a specific cloud point is used in the manufacture of the claimed pharmaceutical formulations. Furthermore, the application describes the processes for their preparation and the use of the claimed formulations in medicine.
Owner:ASTRAZENECA AB
Enteric coated omeprazole pellets capsule and the preparing method thereof
The invention relates to an omeprazole enteric micropill capsule and a process for preparing the same. The content of the capsule is omeprazole enteric micropill including a blank pill core; an activity medicine layer having an alkaline component; a separator containing magnesia and titanium pigment; and an enteric coat layer. According to synergistic reaction, stability of the medicine is distinctly improved. The omeprazole enteric micropill capsule of the invention has period of validity of the preparation to three years or more.
Owner:HANGZHOU MINSHENG PHARM CO LTD
Pharmaceutical formulation of omeprazole
PCT No. PCT / SE98 / 00922 Sec. 371 Date Jun. 8, 1998 Sec. 102(e) Date Jun. 8, 1998 PCT Filed May 18, 1998 PCT Pub. No. WO98 / 53803 PCT Pub. Date Dec. 3, 1998An enteric coated oral pharmaceutical formulation comprising as active ingredient a compound selected from the group of omeprazole, an alkaline salt of omeprazole, the (-)-enantiomer of omeprazole and an alkaline salt of the (-)-enantiomer of omeprazole, wherein the formulation comprises a core material of the active ingredient and optionally an alkaline reacting compound, the active ingredient is in admixture with a pharmaceutically acceptable excipient, such as for instance a binding agent, and on said core material a separating layer and an enteric coating layer. A hydroxypropyl methylcellulose (HPMC) of low viscosity with a specific cloud point is used in the manufacture of pharmaceutical formulations. Furthermore, the application describes the processes for their preparation and the use of the claimed formualtions in medicine.
Owner:ASTRAZENECA AB
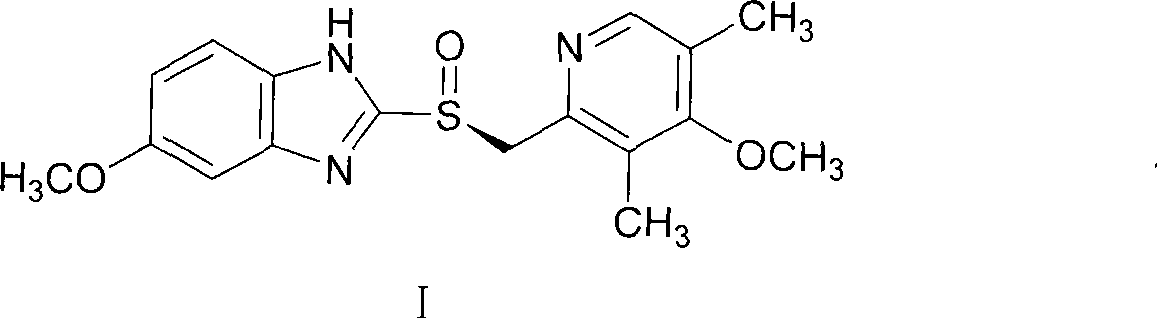
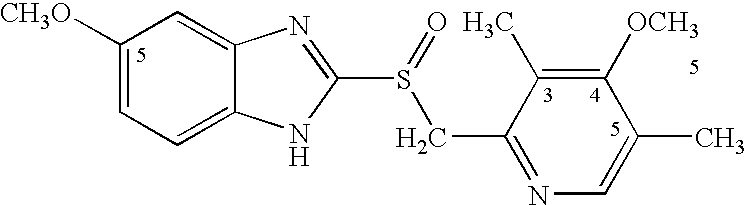
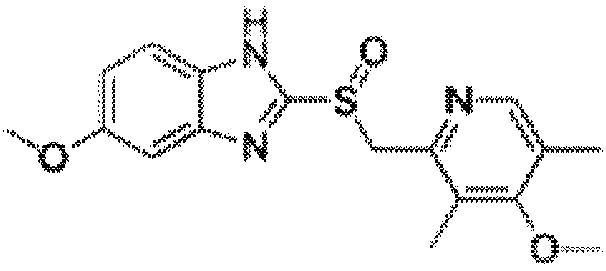
Potassium salt of (s)-omeprazole
The present invention relates to a novel form of 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)-methyl]sulfinyl]-1H-benzimidazole, known under the generic name omeprazole. More specifically, it relates to a novel crystalline form of the potassium salt of the (S)-enantiomer of 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole. The present invention also relates to processes for preparing such a form of the potassium salt of (S)-omeprazole and pharmaceutical compositions containing it.
Owner:ASTRAZENECA AB
Omeprazole formulation
A stable pharmaceutical composition of omeprazole for oral administration which consists essentially of: (a) a core of Omeprazole or a pharmaceutically equivalent salt, a filler and an alkaline material selected from the group consisting of lysing and arginine; and (b) a single layer of coating on said core which comprises a layer of an enteric coating agent which is applied from an organic solvent based system.
Owner:ANDRX PHARMA INC
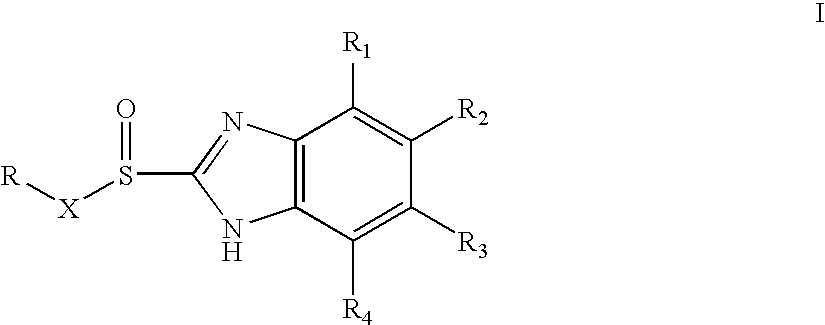
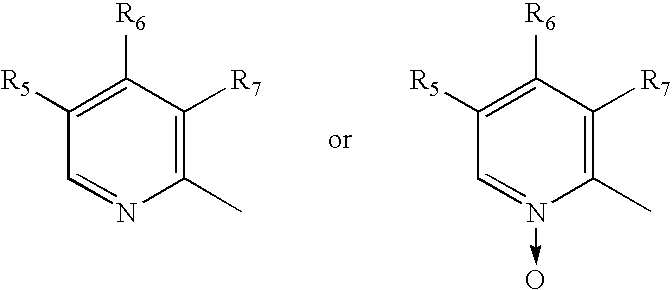
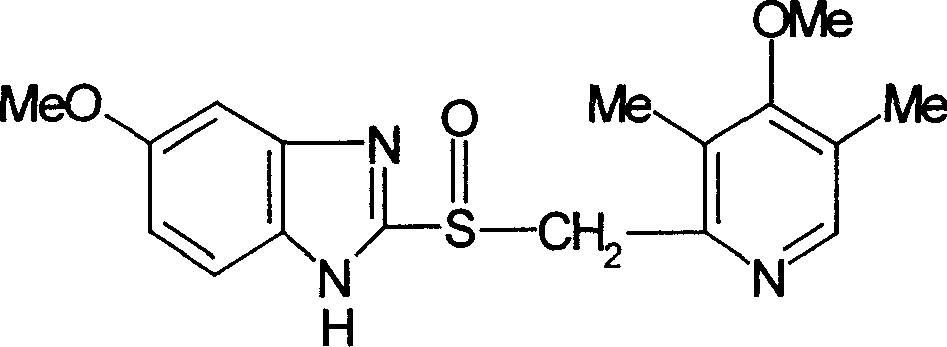
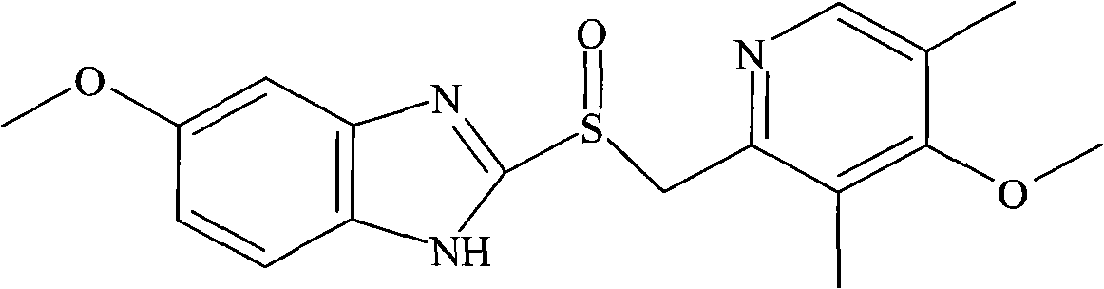
Process of synthesis of 5-methoxy- 2-[(4-methoxy-3,5-dimethy-2-pyridyl)methyl]sulfiny-1h-benzimidazole
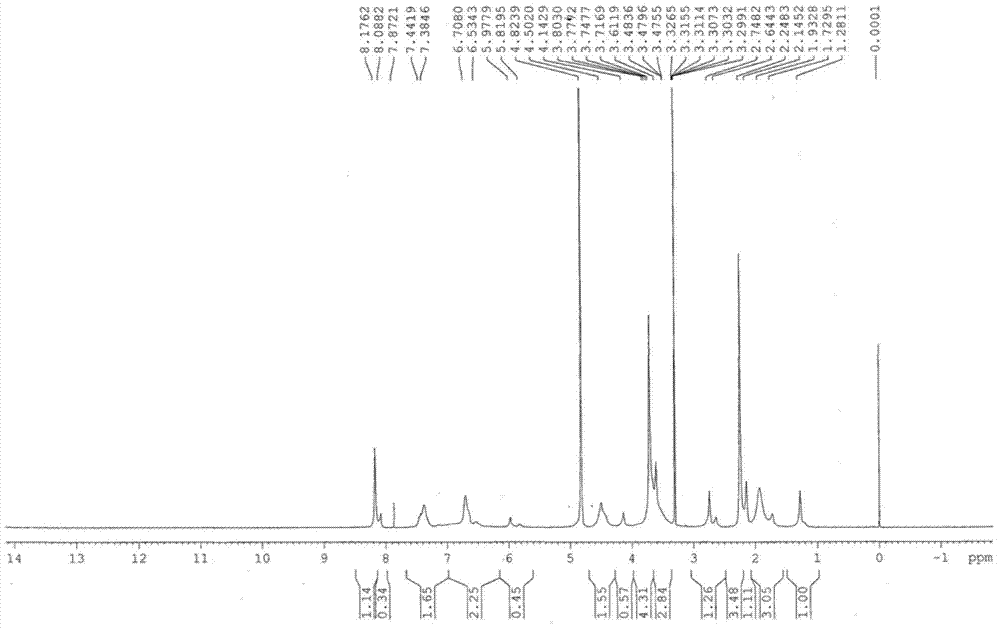
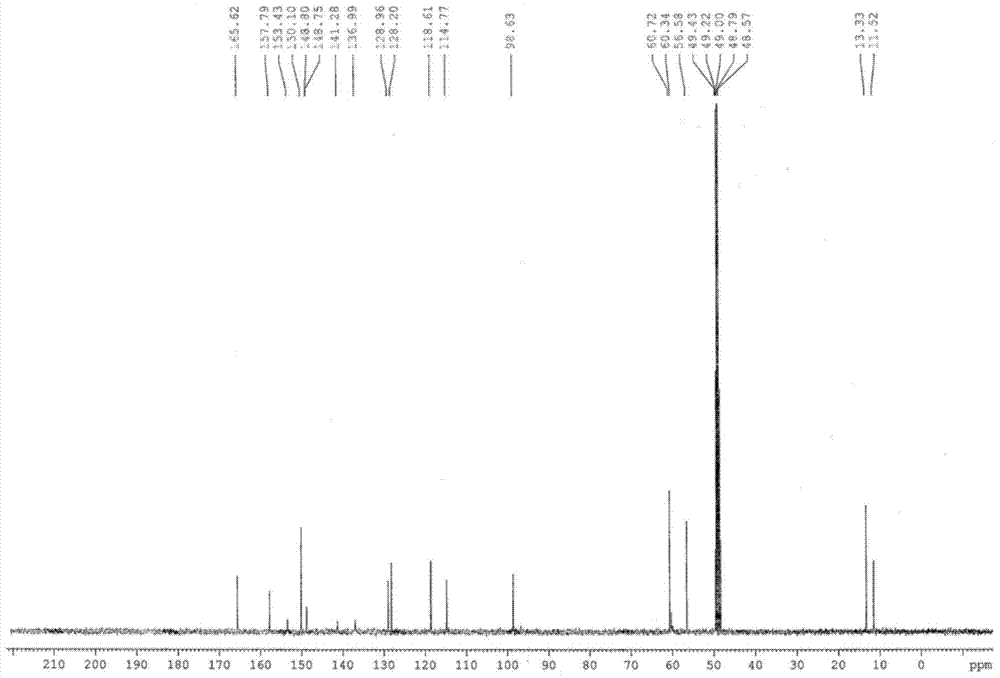
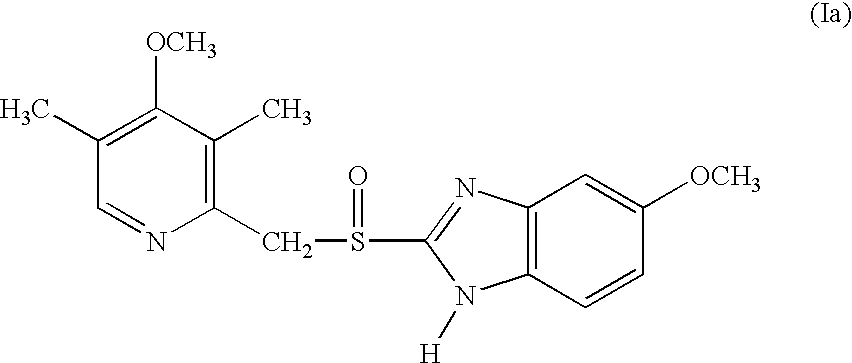
InactiveUS6268502B1Effect of the acidic medium on omeprazole decomposition isPoorly solubleOrganic active ingredientsDigestive systemAcetic acidBenzoic acid
An improved process of synthesis of 5-methoxy-2-[(4-methoxy-3,5-dimethyl-2-pyridyl)methyl]sulfinyl-1H-benzimidazole (omeprazole) by oxidation of 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridyl)methyl]thio]benzimidazole with 3-chloroperoxy-benzoic acid in ethyl acetate wherein omeprazole is poorly soluble, at a temperature between -10° C. and 5° C. is disclosed. The second step is a purification of the crude product by dissolution and reprecipitation of the final product.
Owner:LEK TOVARNA FARM & KEMICNIH IZDELKOV D D
Oral dosage forms
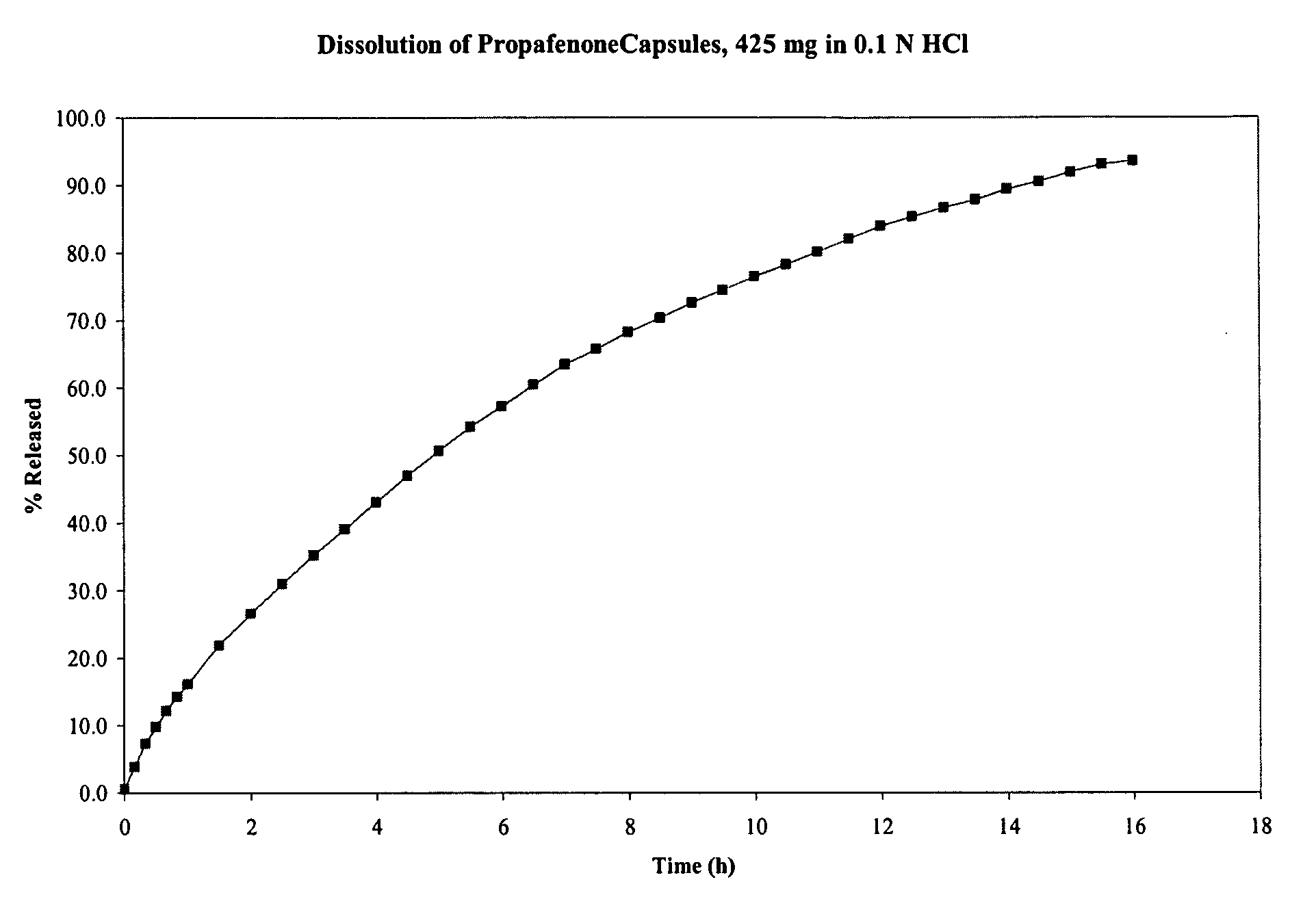
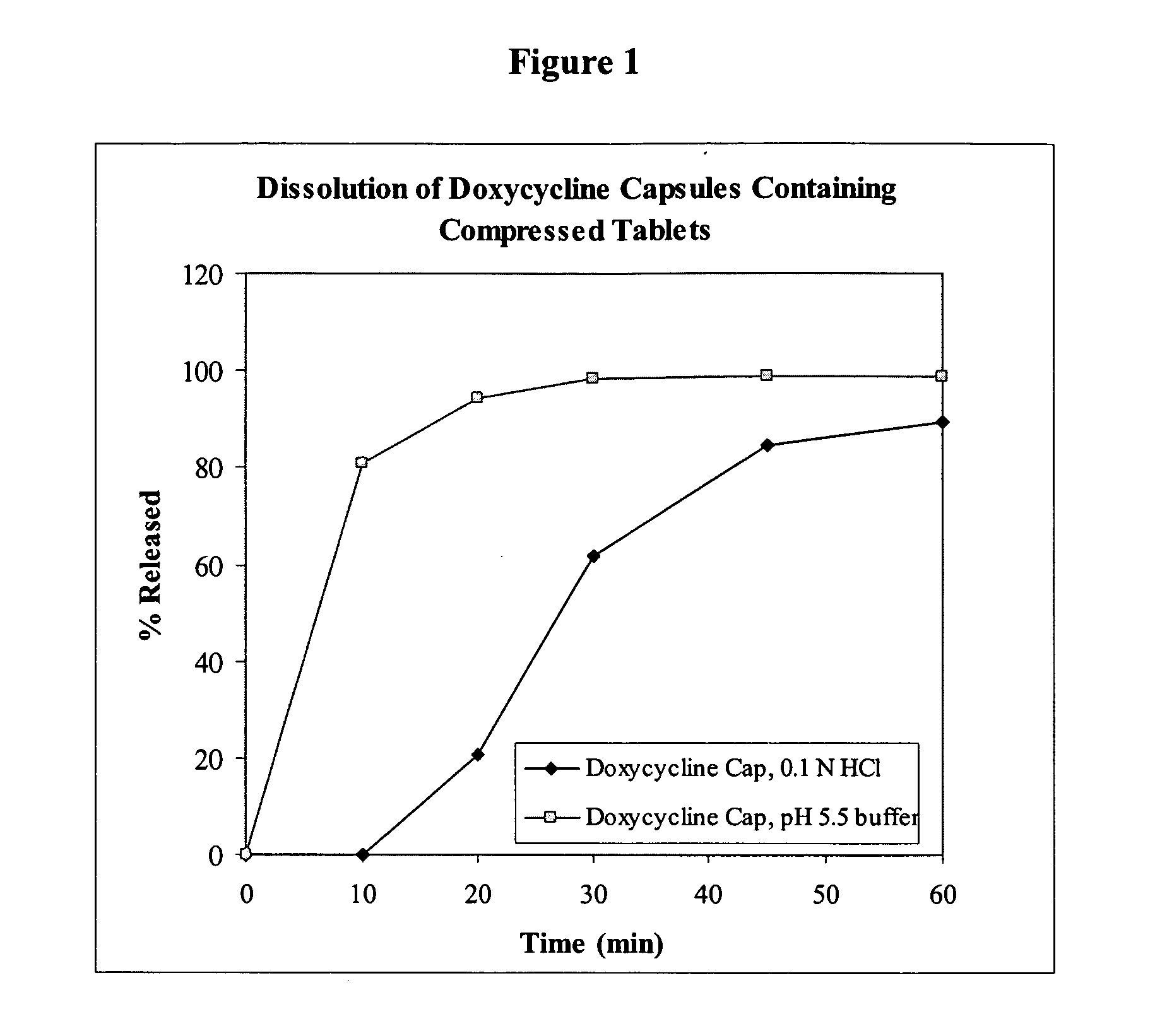
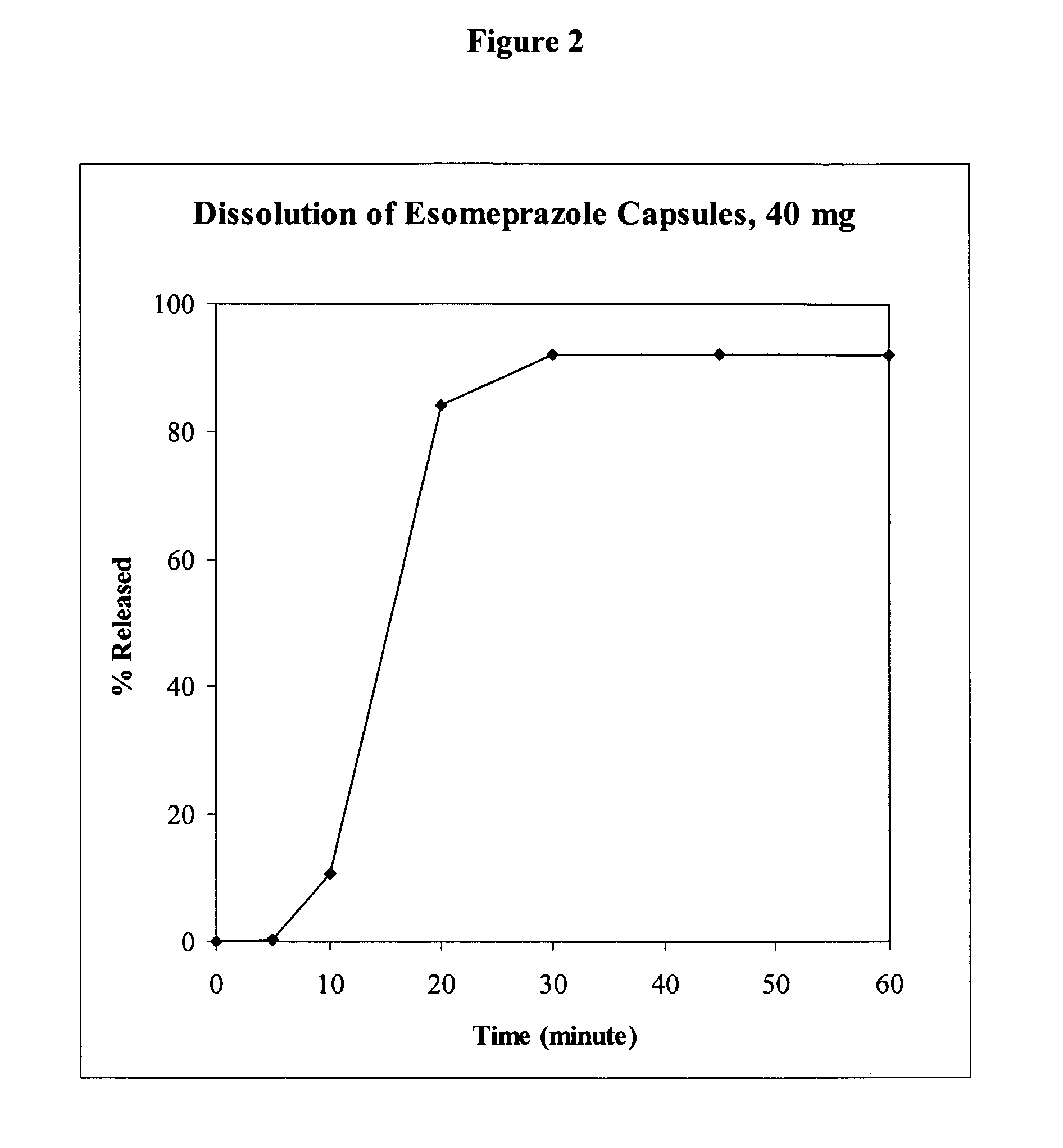
This invention relates to an oral dosage form of a pharmaceutically active ingredient comprising: (a) an outer capsule and (b) non-uniform pellets, having a non-uniform shape and / or size, contained within the capsule, wherein the pellets comprise a compressed powder comprising a pharmaceutically active ingredient. In one embodiment the active ingredient is selected from the group consisting of doxycycline, omeprazole, esomeprazole, and propafenone. Pharmaceutical formulations of the active ingredients as well as methods and tools for making the oral dosage form are also described.
Owner:PAR PHARMA
Compound preparation of omeprazol
InactiveCN101002769AQuick effectImprove stabilityPowder deliveryOrganic active ingredientsMedicineOmeprazole
An enteric or slowly-released composition of omeprazole is prepared from omeprazole, the buffer substance for neutralizing gastric acid and improving the stability of omeprazole, and the pharmacologically acceptable assistant.
Owner:GUANGDONG HUANAN PHARMACEUTICAL GROUP CO LTD
Solid oral formulations for combination therapy
InactiveUS20080085314A1Reduce gastric acid secretionPromote gastric acid secretionBiocideDrug compositionsRanitidineOmeprazole
A first, solid oral pharmaceutical composition includes an extended release acetaminophen, a non-steroidal anti-inflammatory drug, such as naproxen or ibuprofen, and a third drug capable of reducing gastric acid secretion, such as ranitidine or omeprazole. A second, solid oral pharmaceutical composition includes a non-steroidal anti-inflammatory drug and an agent for reducing gastric acid secretion.
Owner:POLY MED
Omeprazole composition and preparing process thereof
ActiveCN101120930AOvercome acidic degradationPH value is stableOrganic active ingredientsDigestive systemSodium bicarbonateMedicine
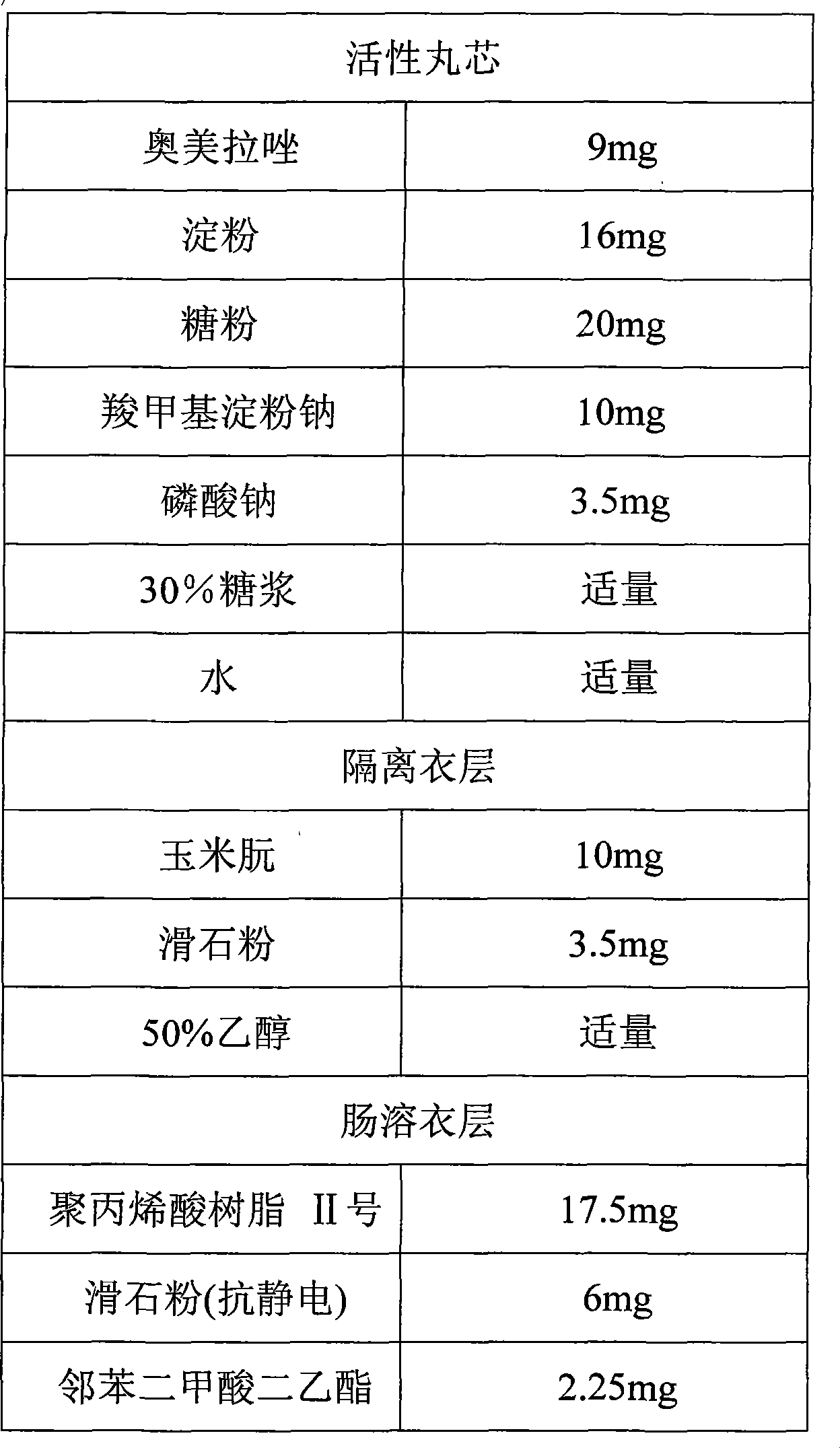
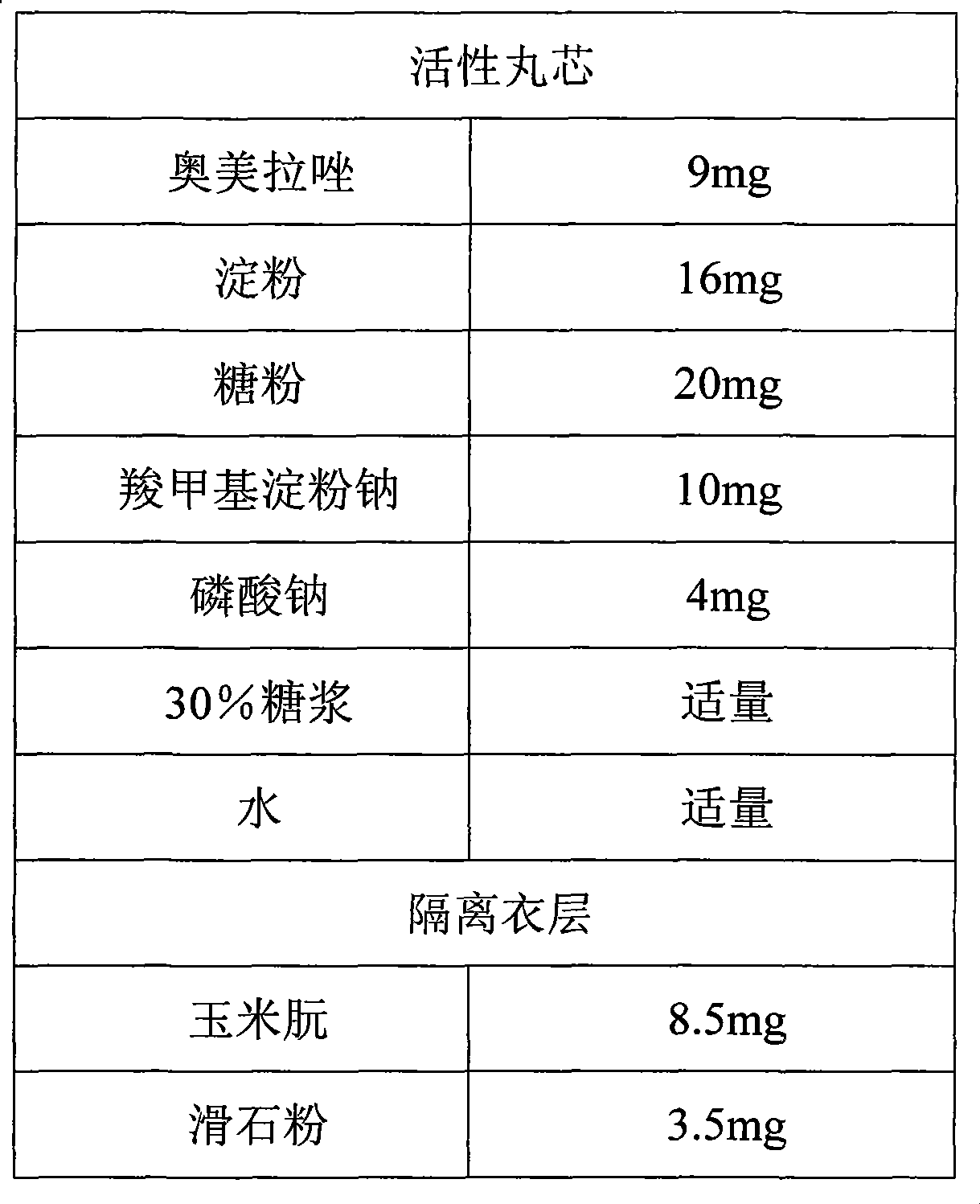
A compound of the omeprazole and the Sodium Bicarbonate comprise a plurality of enteric-coated pellets composed by the omeprazole and the Sodium Bicarbonate, the capsules composed by Sodium Bicarbonate of any mode. The enteric-coated pellets comprise a pellet core, an isolation layer and an enteric-coated layer. The present invention has the advantages that acid degradation of the omeprazole is resolved by the enteric-coated layer, which prolongs binding between the gastric wall and omeprazole. When Sodium Bicarbonate of any mode neutralizing the gastric acid reaches a certain PH value, the enteric-coated layer can disintegrate and omeprazole can be released. PH value in the stomach remains stable during the releasing process. Another advantage of the present invention is that the compound can be taken at any time when stomachache happens.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Novel formulation, omeprazole antacid complex-immediate release for rapid and sustained suppression of gastric acid
InactiveUS20050220870A1Reduce productionInhibit and reduce degradationBiocideDispersion deliveryImmediate releaseGastric fluid
The present invention is directed to methods, kits, combinations, and compositions for treating, preventing or reducing the risk of developing a gastrointestinal disorder or disease, or the symptoms associated with, or related to a gastrointestinal disorder or disease in a subject in need thereof. In one aspect, the present invention provides a pharmaceutical composition comprising a proton pump inhibiting agent and a buffering agent for oral administration and ingestion by a subject. Upon administration, the composition contacts the gastric fluid of the stomach and increases the gastric fluid pH of the stomach to a pH that substantially prevents or inhibits acid degradation of the proton pump inhibiting agent in the gastric fluid and allows a measurable serum concentration of the proton pump inhibiting agent to be absorbed into the blood serum of the subject.
Owner:SANTARUS
Pharmaceutical formulation and process for its preparation
The present invention relates to a multiparticulate tablet with improved gastro-protection comprising at least a pharmaceutically active substance in the form of enteric coated particles, and a mixture of tableting excipients, wherein the said mixture of excipients comprising xylitol and / or maltitol, each in a directly compressible form, a disintegrating agent, a lubricant and at least one other diluent and the ratio of a) the xylitol and / or the maltitol to b) the other diluent(s) is less than 5 / 95 (weight / weight) and the result of the “test of integrity of the film” is greater than 95%, preferably greater than 97% and more preferably still greater than 99% and the result of the “release test” is greater than 90%, preferably greater than 95%. According to one embodiment of the invention, the active substance is omeprazole or esomeprazole. According to another embodiment the tablet is a disintegratable tablet, which disintegrate in the mouth with or without chewing. The invention also comprises a process for preparing the claim tablet and its use in medicine.
Owner:ASTRAZENECA AB
Industrial method for refining esomeprazole sodium salt
The invention relates to an industrial method for refining esomeprazole sodium salt. The method is mainly characterized by comprising the following steps of: suspending the esomeprazole sodium salt in a hot poor solvent in an amount which is 1 to 10 times weight of the esomeprazole sodium salt, slowly adding a good solvent such as methanol and ethanol in an amount which is 0.5 to 1.5 times weightof the mixture, filtering while heat to obtain a clarified solution, cooling to room temperature, separating out a solid, filtering, washing and performing vacuum drying. The method is easy and convenient to implement, and inorganic impurities and organic impurities contained in the crude product of esomeprazole sodium salt, including a peroxide sulphone impurity and an R-esomeprazole impurity can be effectively removed. The esomeprazole sodium salt refined by the method has the content of over 99.5 percent, and has the single impurity content of less than 0.1 percent.
Owner:NANJING YOKO PHARMA
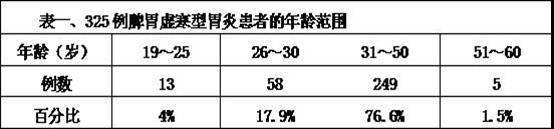
Preparation method of traditional Chinese medicine used for treating gastritis caused by insufficiency of spleen-yang
InactiveCN102366615ASmall side effectsEasy to makeAnthropod material medical ingredientsDigestive systemPolygonum fagopyrumSide effect
The invention belongs to the technical field of traditional Chinese medicine preparation method, and provides a preparation method of a traditional Chinese medicine used for treating gastritis caused by insufficiency of spleen-yang. According to prior arts, gastritis caused by insufficiency of spleen-yang is commonly treated by using omeprazole, which causes adverse reactions. The technical scheme of the invention is that: traditional Chinese medicines of ginseng, human milk, common jujube, common yam rhizome, wheat, different leaves pseudostellaria root tuber, balck-bone silky fowl, wild cabbage, dried longan pulp, largehead atractylodes rhizome, radix paeoniae alba, American ginseng, ligusticum, lotus seed, pilose asiabell root, sharpleaf galangal fruit, mongolian milkvetch root, Siberian solomonseal rhizome, nonglutinous rice, edible bird nest, dried ginger, bayberry, garden radish seed, carica papaya, roof iris rhizome, carrot, buckwheat, medicated leaven, cydonia oblonga mill, cerealose, honey, and licorice are soaked in water, and are decocted by using a mild fire; the obtained solution is filtered, a slag is removed, and the obtained medicine liquid is the traditional Chinese medicine used for treating gastritis caused by insufficiency of spleen-yang. The method is advantaged in that: the preparation method is simple; toxic and side-effects of the traditional Chinese medicine liquid are low; a treatment course is short; and a recovery rate is high. According to the invention, a good effect can be obtained with the cooperation of monarch and minister medicines. A traditional Chinese medicine preparation is adopted, such that adverse reactions, allergic reactions and toxic reactions caused by western medicines are avoided.
Owner:孟德芹
Omeprazole enteric coated pellets formulation and preparation method
The invention discloses an omeprazole enteric micropill preparation and a preparation method thereof. The preparation takes alkali salt containing omeprazole or a single antimer of the omeprazole as an active pill core, and contains a film isolating and coating layer and an enteric coating layer. The preparation takes zein as an isolating and coating material, thereby improving the stability of micropills. The preparation method uses alcohol as menstruum, thereby further improving the stability of the omeprazole micropills.
Owner:SHOUGUANG FUKANG PHARMA
Novel process for substituted sulfoxides
The present invention relates to a process for preparing substituted sulfoxides either as a single enantiomer or in an enantiomerically enriched form. Thus, racemic omeprazole is reacted with (S)-camphorsulfonyl chloride to form a diastereomeric mixture and the diastereomers are separated by fractional crystallization, followed by deprotection to give esomeprazole.
Owner:HETERO DRUGS LTD
Enteric omeprazole micropill and its preparing method
ActiveCN1985822ALow priceGood for healthOrganic active ingredientsDigestive systemClinical efficacyOmeprazole
The present invention discloses a kind of enteric omeprazole micropill preparation and its preparation process. The enteric omeprazole micropill preparation has core of omeprazole or its single antimer subsalt as the active component and excipient and middle isolating coating and enteric protecting layer to coat the core. The enteric omeprazole micropill preparation is prepared directly with the main medicine component and supplementary materials including magnesia as the stabilizer, hydroxypropylmethyl cellulose and talcum powder.
Owner:KAMP PHARMA
Cyclohexanone monooxygenase and application thereof
ActiveCN108118035AHigh yieldLow priceBacteriaOxidoreductasesHigh concentrationCyclohexanone monooxygenase
The invention discloses cyclohexanone monooxygenase and an application thereof, in particular cyclohexanone monooxygenase obtained by site-specific mutagenesis and an application thereof. Compared with a SEQ ID NO: 1, the amino acid sequence of the cyclohexanone monooxygenase has gene mutation in at least one site as follows: serine Ser at the 386th site is mutated to asparagines Asn, and serine Ser at the 435th is mutated to threonine Thr. Experiments show that the cyclohexanone monooxygenase disclosed by the invention can catalytically convert a high concentration omeprazole thioether primerinto esomeprazole.
Owner:ZHEJIANG JINGXIN PHARMA +1
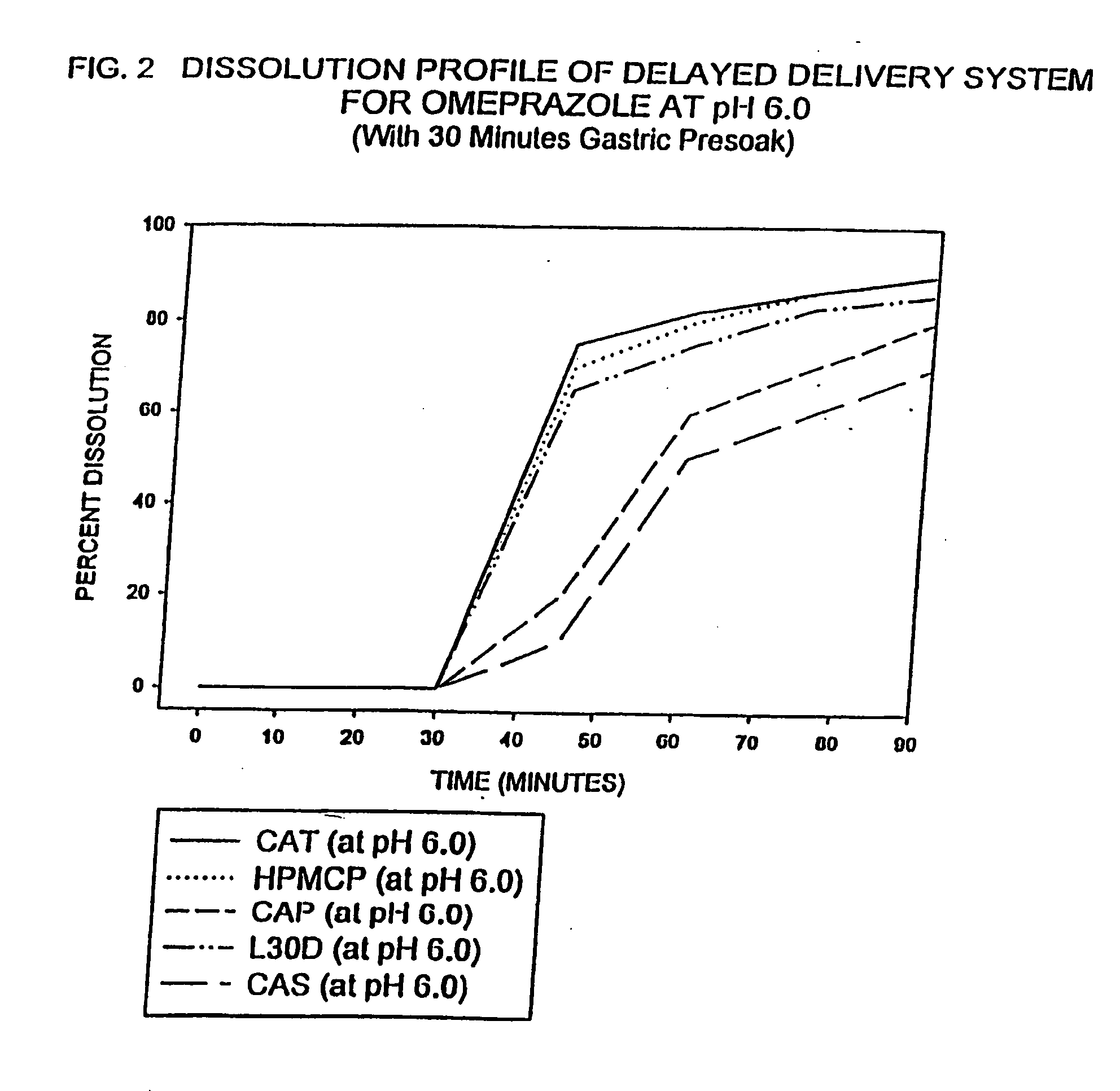
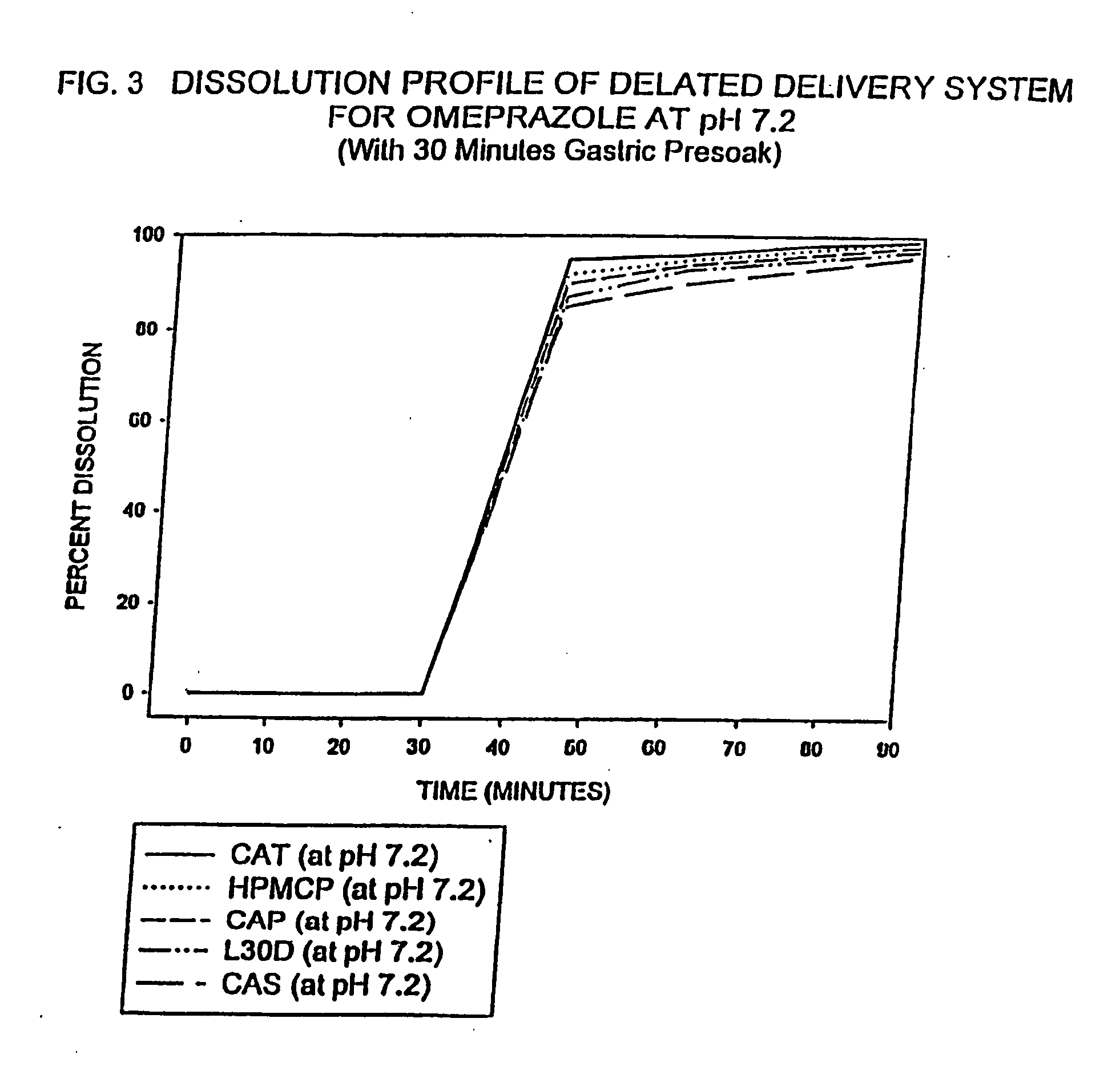
Delayed delivery system for acid-sensitive drugs
InactiveUS20050244497A1High cohesivenessImprove integrityGranular deliveryMicrocapsulesIntestinal fluidGastric fluid
The present invention relates to a delayed release drug delivery system containing omeprazole capable of site-specific delivery and pulsatile (bolus) kinetics for once-a-day dosage comprised of an alkaline core structure sequentially layered with suspensions of omeprazole; a separation barrier; and an enteric barrier. The separation barrier is coated with a pH-dependent enteric membrane, which is relatively insoluble in gastric fluid but rapidly to immediately soluble in intestinal fluid, whereby the drug is released in a pulsatile manner in the proximal segment of the gastrointestinal tract.
Owner:WOCKHARDT LTD
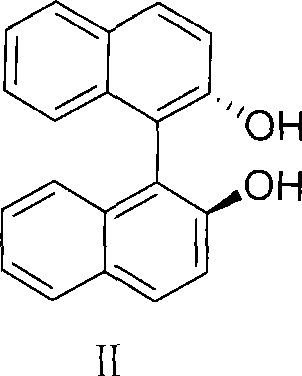
Method for preparing S-omeprazole and salt thereof by forming inclusion complex with (S)-(-)-1,1'-dinaphthalene-2,2'-diol
The invention provides a method for preparing S-Omeprazole and salt thereof by the resolution of racemic modification Omeprazole by an inclusion resolution method, the resolution regent of the inclusion resolution method is (S)-(-)-1, 1'-binaphthyl-2, 2'-diphenol, by adding S-Omeprazole and S-1, 1'-binaphthyl-2, 2'-diphenol inclusion complex seed grains at the temperature of 60 to 70 DEG C, the inclusion complex is obtained, then the inclusion complex is processed by the hydroxid aqueous solution of alkali metals to obtain the S-Omeprazole with high ee value, the S-Omeprazole and salt thereof obtained by the method have high ee value which is more than 99.2 percent, and the obtained S-Omeprazole has the color of kind of white, high yield and small pollution to the environment, and is suitable for industrial production.
Owner:安徽美诺华药物化学有限公司
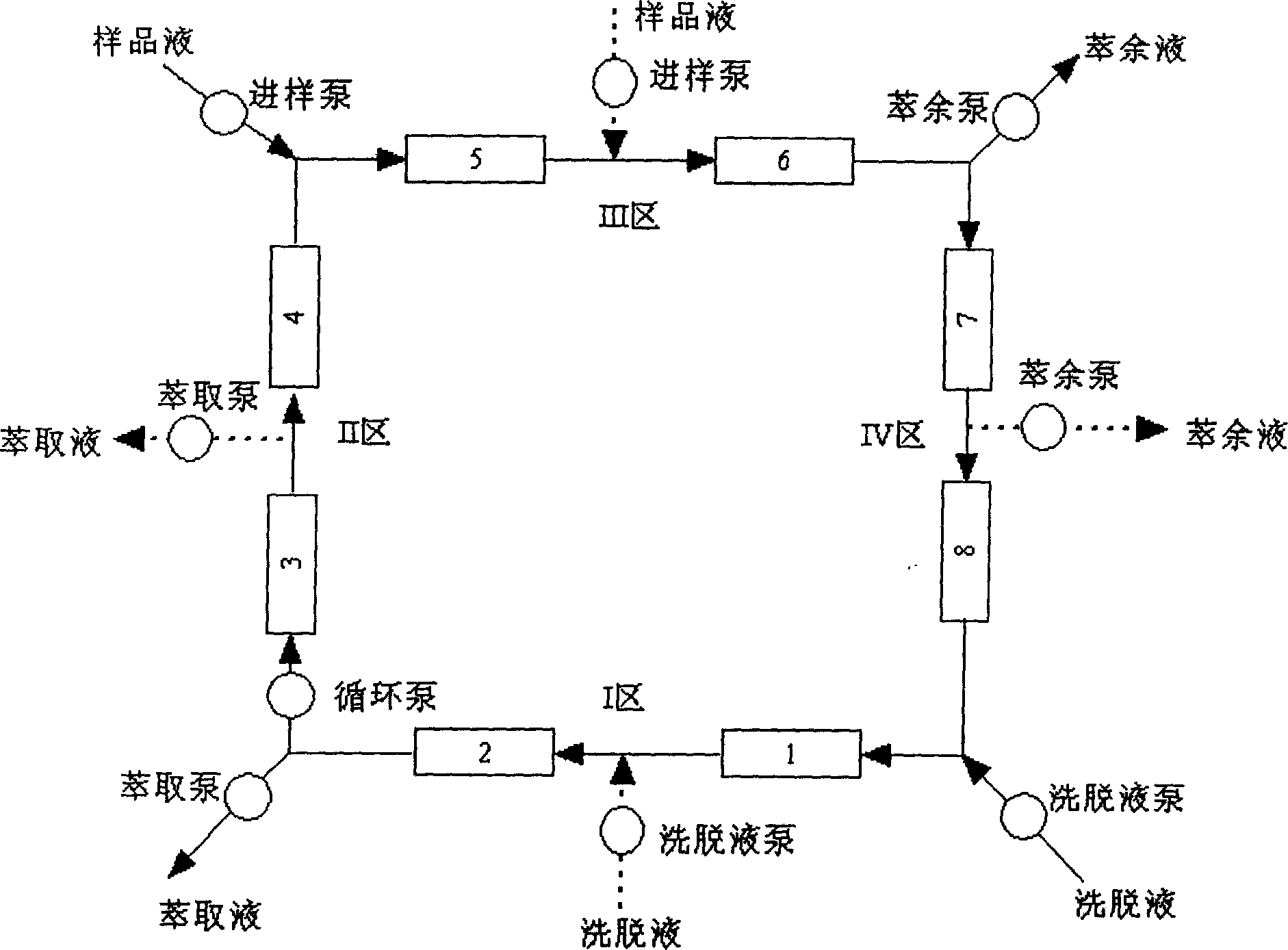
Simulated moving bed chromatography separating method of omeprazole antimer
InactiveCN1683368ASimple processContinuous productionOrganic chemistryDigestive systemCelluloseChromatographic separation
The present invention discloses the chromatographic separation process of omeprazole antimer in simulated mobile bed. In a simulated mobile bed chromatographic system with chiral fixed phase of cellulose triphenyl carbomate and flow phase of the mixture of ethanol, n-hexane and diethylamine, mixture of R-(+)-omeprazole and S-(-)-omeprazole is separated to obtain high purity S-(-)-omeprazole. Said separation process is a continuous process, so that the present invention has high automation, high production efficiency, low solvent consumption and no toxic solvent.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
Omeprazole enteric coated tablet and preparation method thereof
ActiveCN101744788ARapid dissolutionGood water solubilityOrganic active ingredientsDigestive systemPlasticizerDissolution
The invention provides an omeprazole enteric coated tablet and a preparation method thereof. The enteric coated tablet is formed by an inner tablet core and an outer enteric coating, wherein the inner tablet core takes omeprazole as active constituent. No protective isolating layer is arranged between the tablet core and the enteric coating. The inner tablet core is made of omeprazole cyclodextrin inclusion compound and other pharmaceutically acceptable auxiliary materials. The enteric coating contains no plasticizer and the dosage of the enteric coating accounts for 5 percent to 15 percent of the weight of the tablet. The invention has the advantages that the stability and the dissolution of omeprazole are improved, the dosage of basic materials and the dosage of all kinds of inert auxiliary materials are greatly reduced, omeprazole can be stably and rapidly released in the intestinal tract and the bioavailability is improved.
Owner:SHANDONG NEWTIME PHARMA
Compound omeprazole capsule, and preparation method and detection method thereof
ActiveCN103340892ASimple preparation processWon't breakOrganic active ingredientsComponent separationCross-linkSodium bicarbonate
The invention discloses a compound omeprazole capsule, and a preparation method and a detection method thereof. The capsule is prepared by: in parts by weight, 20 parts of omeprazole, 1100 parts of sodium bicarbonate, 30 parts of cross linked sodium carboxymethyl cellulose and proper amount of magnesium stearate; or the capsule is prepared by 40 parts of omeprazole, 1100 parts of sodium bicarbonate, 30 parts of cross linked sodium carboxymethyl cellulose and proper amount of magnesium stearate. Aiming at the deficiency of the prior art The formulation, the preparation technology and the detection method of the conventional compound omeprazole capsule are optimized, the compound omeprazole capsule of the invention exhibits more substantial curative effect for diseases comprising peptic ulcer, gastroesophageal reflux disease and the like caused by gastric hyperacidity; a systematic, integrated and effective component discrimination and content determination method is established, thereby the medicine quality is effectively controlled, and thus the clinic curative effect is guaranteed.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
Omeprazole quick-release solid preparation and preparation method thereof
ActiveCN101816641AImprove stabilityGood dissolution effectOrganic active ingredientsDigestive systemSodium bicarbonateDirect effects
The invention relates to the field of pharmaceutical preparations, in particular to an oral quick-release solid preparation containing omeprazole and a preparation method thereof. The preparation is a quick-acting proton pump inhibitor which is prepared from omeprazole, sodium bicarbonate and pharmaceutical auxiliary materials, wherein the sodium bicarbonate not only has the direct effect of inhibiting the secretion gastric acid, but also can prevent the omeprazole from being degraded by the gastric acid. The preparation of the invention has the advantages of quick action and long duration.
Owner:JILIN LIHUA DRUG
Omeprazole dosage form
InactiveUS20050266075A1Improve propertiesAlleviate acid sensitivityBiocideDigestive systemBenzimidazole derivativeActive component
The present relates to a stable pharmaceutical composition comprising as an active component thereof one or more known 2-[(2-pyridyl)]-methylsulphinyl]benzimidazole derivatives
Owner:PHARMASCIENCE INC
Preparation method for esomeprazole magnesium trihydrate
The invention discloses a preparation method for esomeprazole magnesium trihydrate, and the preparation method comprises the following steps: 1) taking omeprazole sulfide, then adding a chiral ligand, a catalyst and an organic solvent, heating and mixing for reaction, so as to form a chiral omeprazole sulfide compound; 2) adding an inorganic oxidant for oxidation reaction, and oxidizing omeprazole sulfide into esomeprazole; 3) adding inorganic base aqueous solution, extracting so as to enable the esomeprazole, obtained in the step 2), to form an esomeprazole inorganic salt, and dissolving the esomeprazole inorganic salt into the inorganic base aqueous solution layer; 4) adding inorganic magnesium salt into the inorganic base aqueous solution layer, stirring for reaction, and then carrying out centrifugation and drying, thus obtaining the esomeprazole magnesium trihydrate. The preparation method has the advantages of being high in product purity, high in yield, simple in technique, high-efficiency, environment-friendly, low in cost and the like.
Owner:珠海润都制药股份有限公司
Omeprazole enteric micro-pellet, capsule and preparation method of omeprazole enteric micro-pellet
ActiveCN107802612AImprove stabilityIncrease profitOrganic active ingredientsDigestive systemMedicineAdhesive
The invention relates to an omeprazole enteric micro-pellet, a capsule and a preparation method of the omeprazole enteric micro-pellet. The omeprazole enteric micro-pellet specifically comprises a pill core, an isolating layer coating and an enteric coating, wherein the pill core is prepared from the following components in percentage by weight: 10 to 13 percent of omeprazole, 82 to 87 percent offiller, 2 to 5 percent of an adhesive, 0.4 to 0.8 percent of a surfactant and 0.4 to 0.8 percent of an alkaline stabilizer; the isolating layer coating is prepared from hydroxypropyl methylcellulose and talcum powder of which the weight ratio is 4 to 1, and the coating weight gain rate is 5 to 10 percent; the enteric layer coating is prepared from eudragit L30D-55, triethyl citrate and talcum powder of which the weight ratio is 1 to 0.1 to 0.1, and the coating weight gain rate is 25 to 30 percent.
Owner:南京润瞳畅达医药科技有限公司
Method for preparing omeprazole enteric fast-release tablet
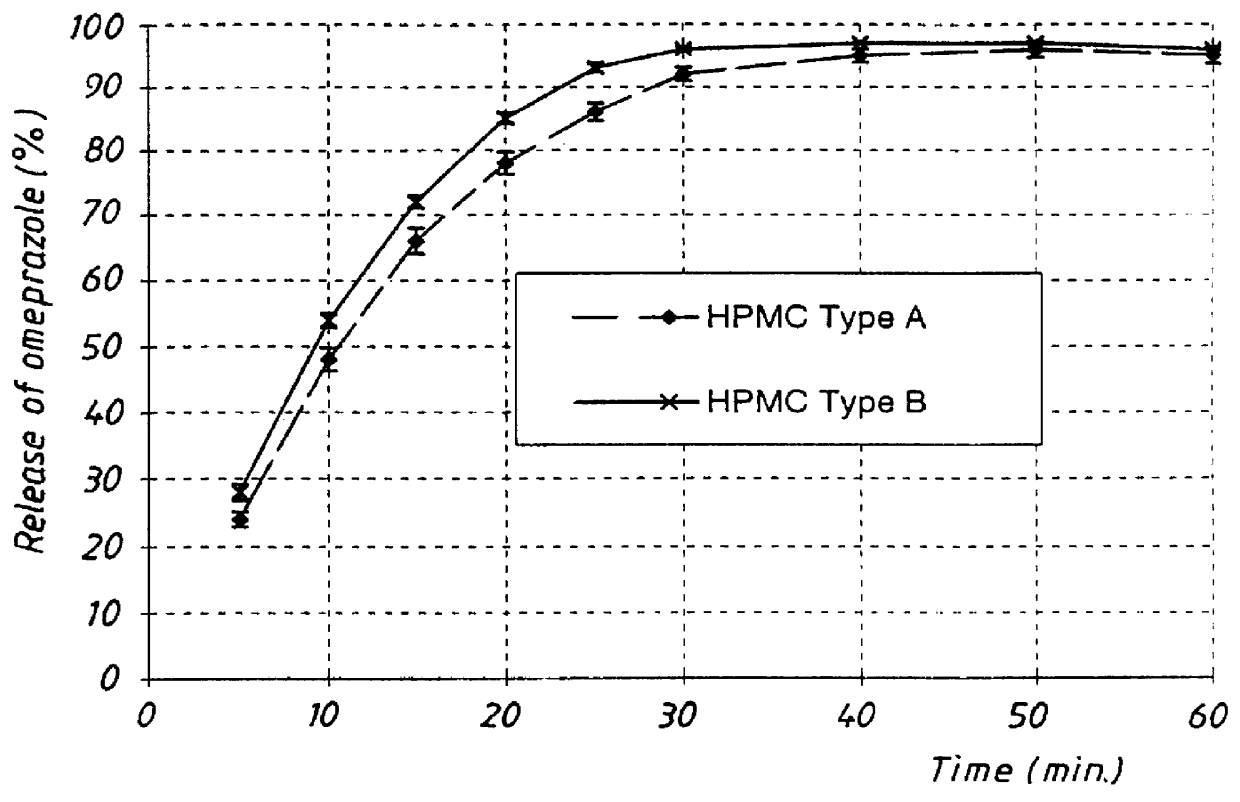
ActiveCN101579324AEvenly dispersedGood dispersionOrganic active ingredientsDigestive systemFast releaseMedicine
The invention relates to the field of pharmaceutical preparations, in particular to a method for preparing an omeprazole enteric fast-release tablet, which is characterized in that in the preparing process with a conventional method, omeprazole is dispersed in binding agent suspension solution, the mixture is granulated by a fluidized bed through top spraying, and the grain size is between 80 and 120 meshes. Besides, a sealing coat of the omeprazole enteric fast-release tablet comprises a first sealing coat and a second sealing coat, wherein the first sealing coat contains hydroxypropyl methylcellulose and a basifier, and the second sealing coat contains the hydroxypropyl methylcellulose, smoothers and titanium dioxide. An in vitro dissolution experiment shows that the omeprazole enteric fast-release preparation prepared by the method is dissolved for 100 percent at the 15th minute; and an acid resistance test shows that a coating tablet has no obvious change in two hours in an environment of which the pH is 1.2.
Owner:SUZHOU CHUNGHWA CHEM & PHARMA IND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Process of synthesis of 5-methoxy- 2-[(4-methoxy-3,5-dimethy-2-pyridyl)methyl]sulfiny-1h-benzimidazole Process of synthesis of 5-methoxy- 2-[(4-methoxy-3,5-dimethy-2-pyridyl)methyl]sulfiny-1h-benzimidazole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f7ed2a5c-6e2a-42c2-84c3-4ab262ab0a99/US06268502-20010731-C00001.png)
![Process of synthesis of 5-methoxy- 2-[(4-methoxy-3,5-dimethy-2-pyridyl)methyl]sulfiny-1h-benzimidazole Process of synthesis of 5-methoxy- 2-[(4-methoxy-3,5-dimethy-2-pyridyl)methyl]sulfiny-1h-benzimidazole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f7ed2a5c-6e2a-42c2-84c3-4ab262ab0a99/US06268502-20010731-C00002.png)