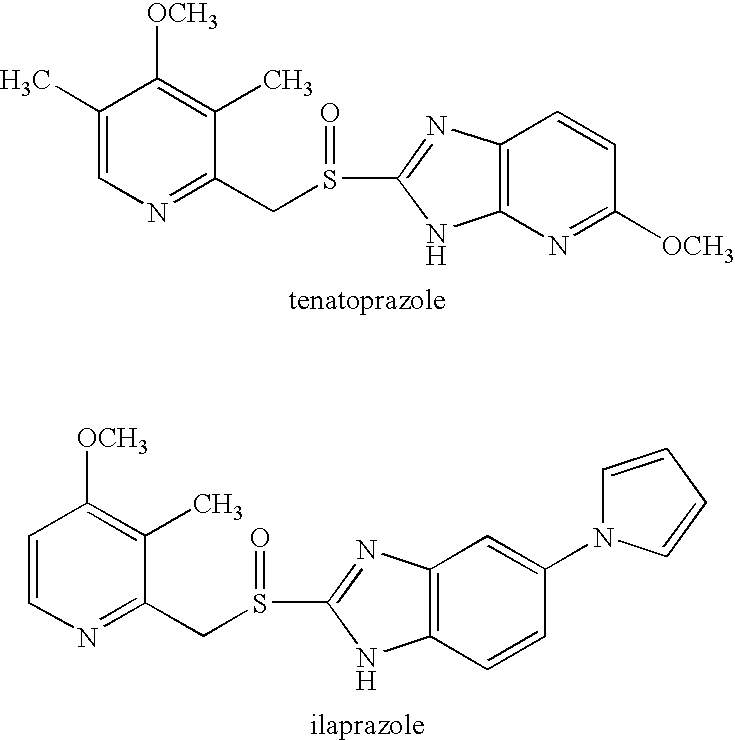
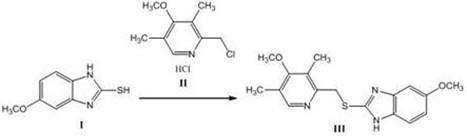
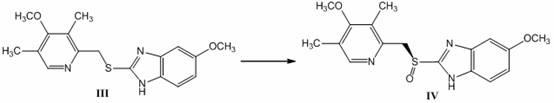
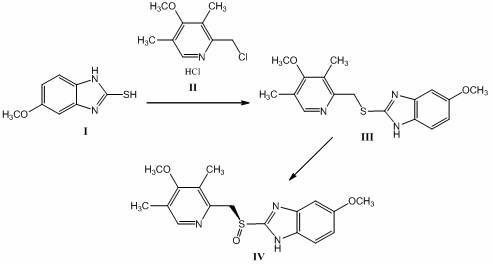
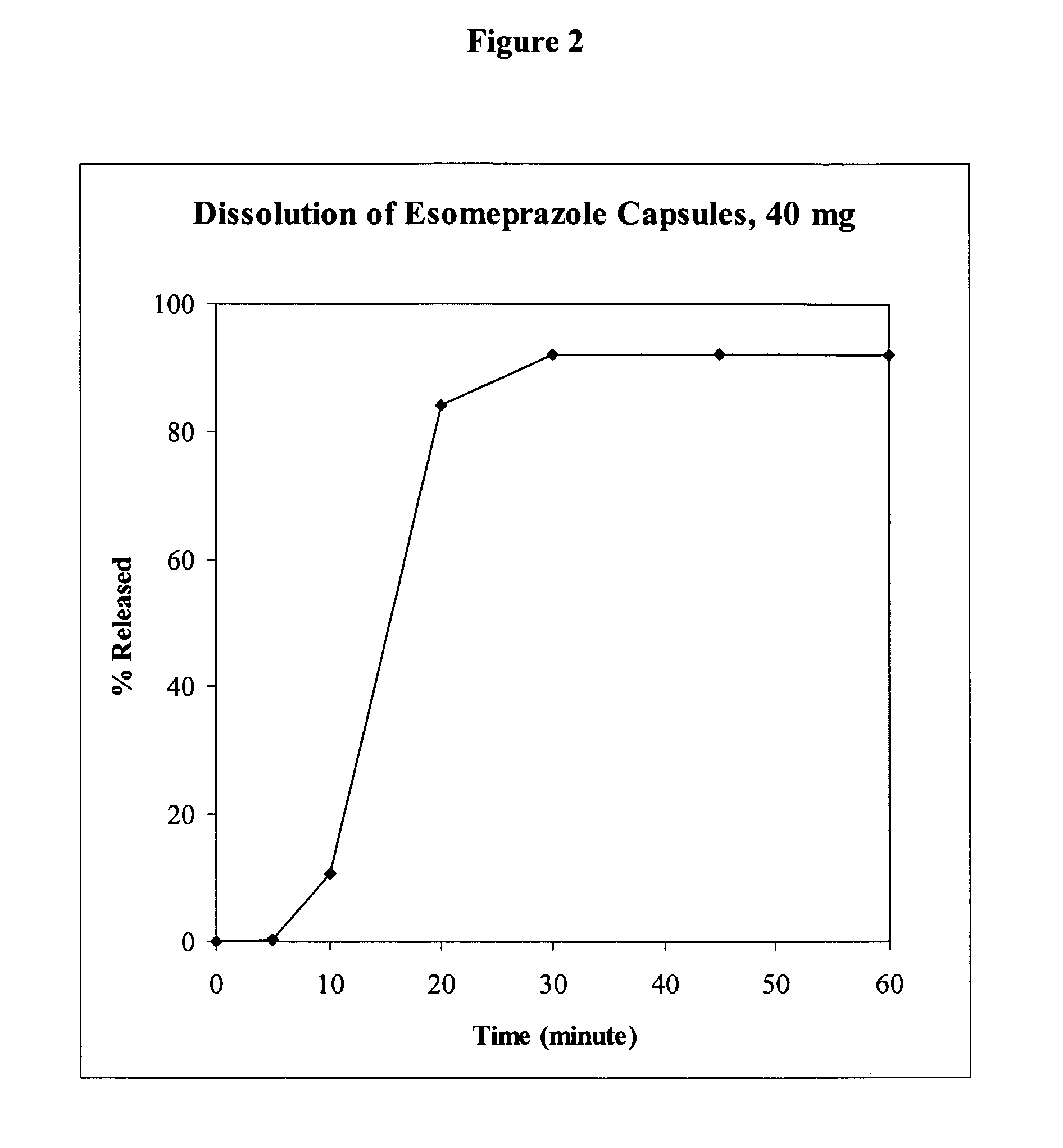
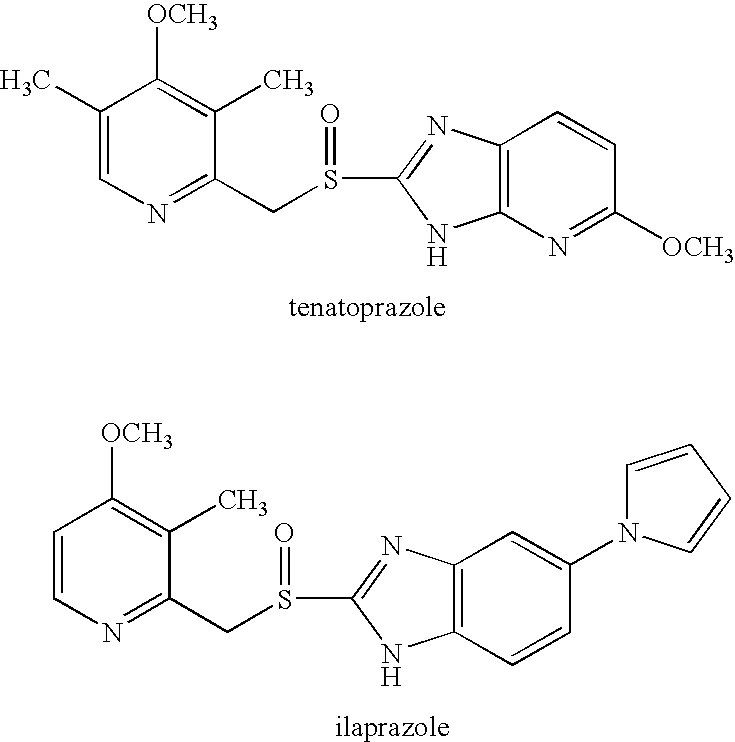
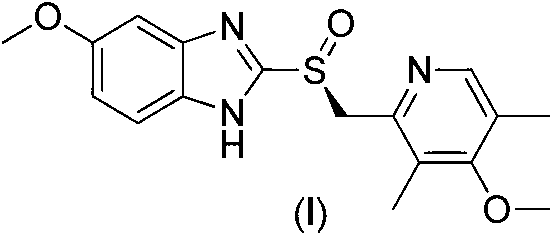
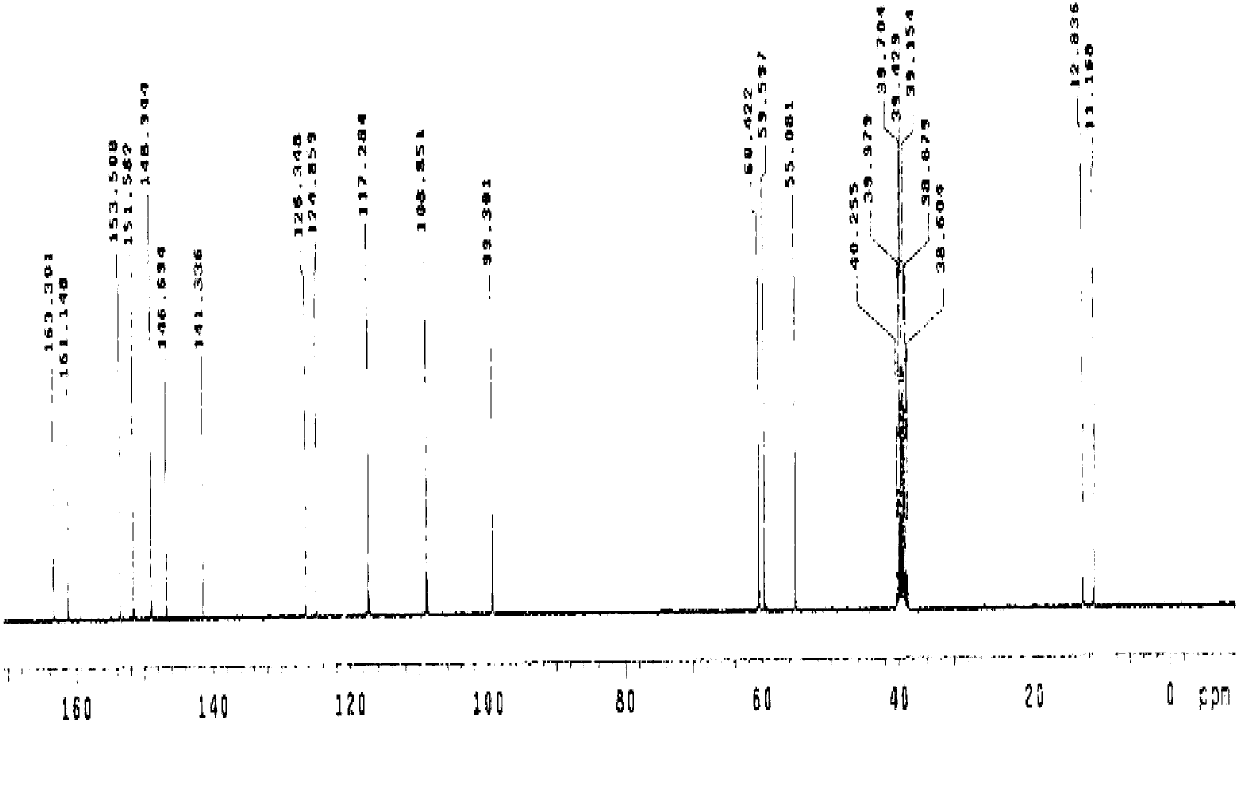
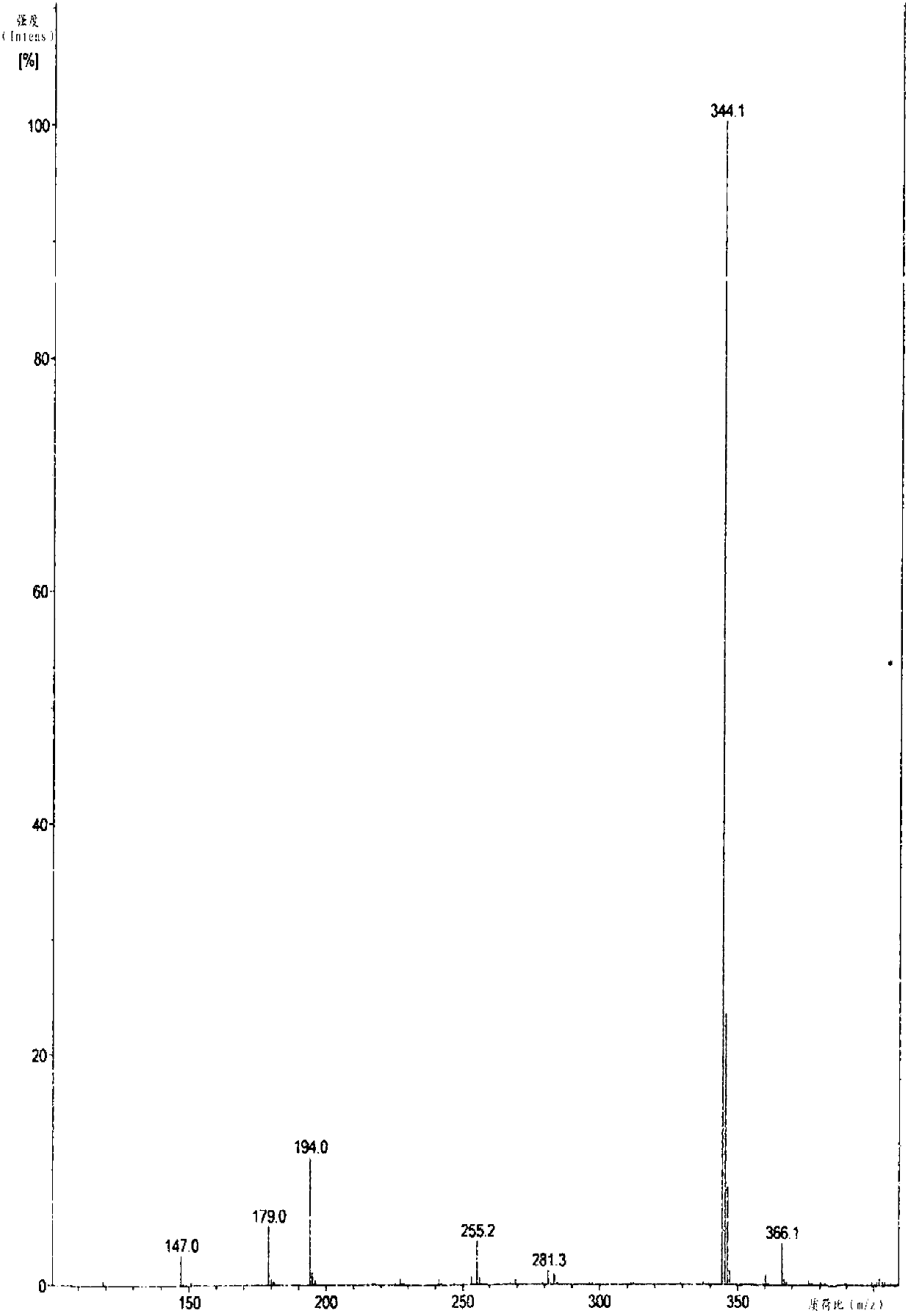
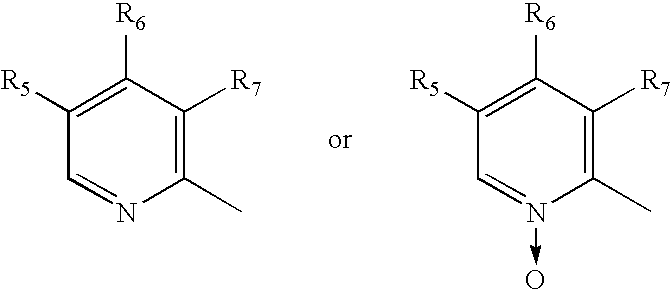
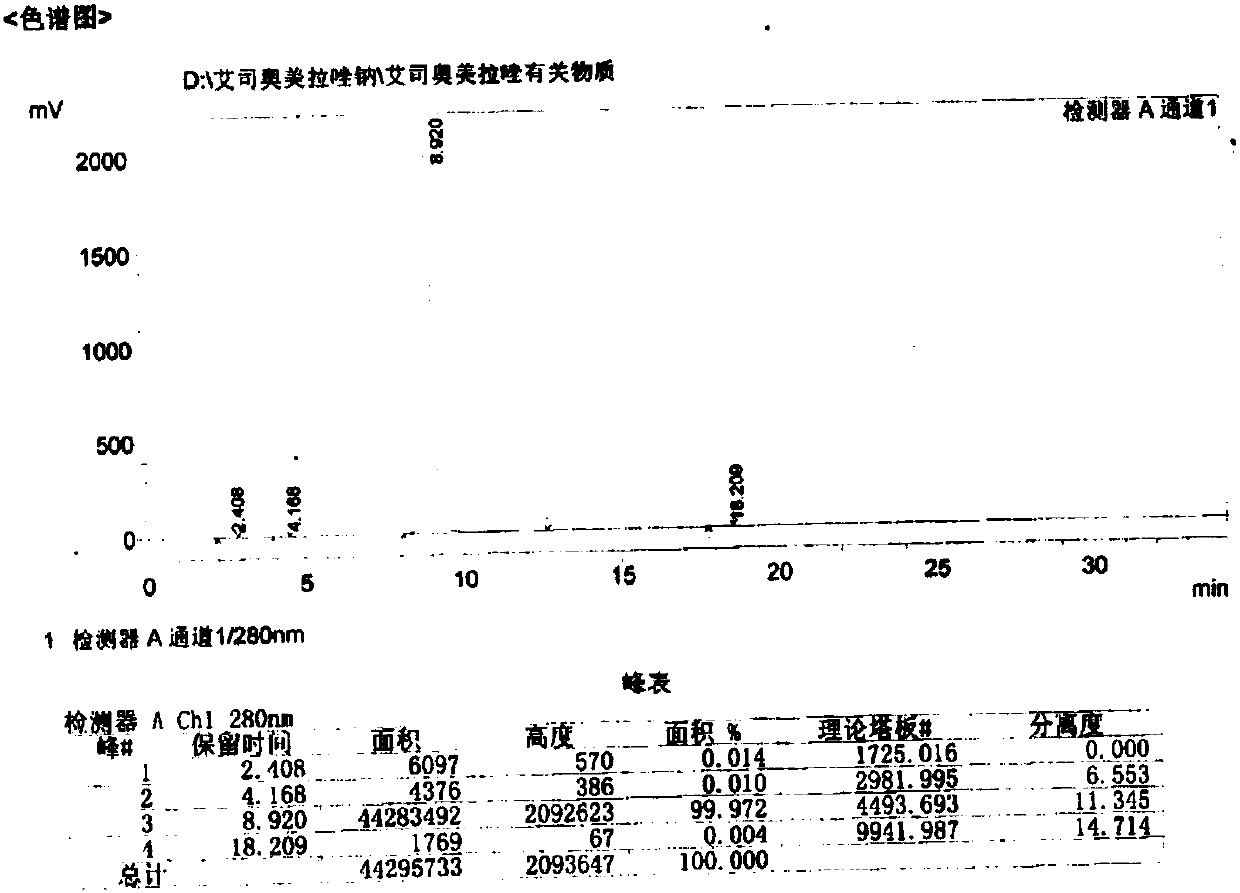
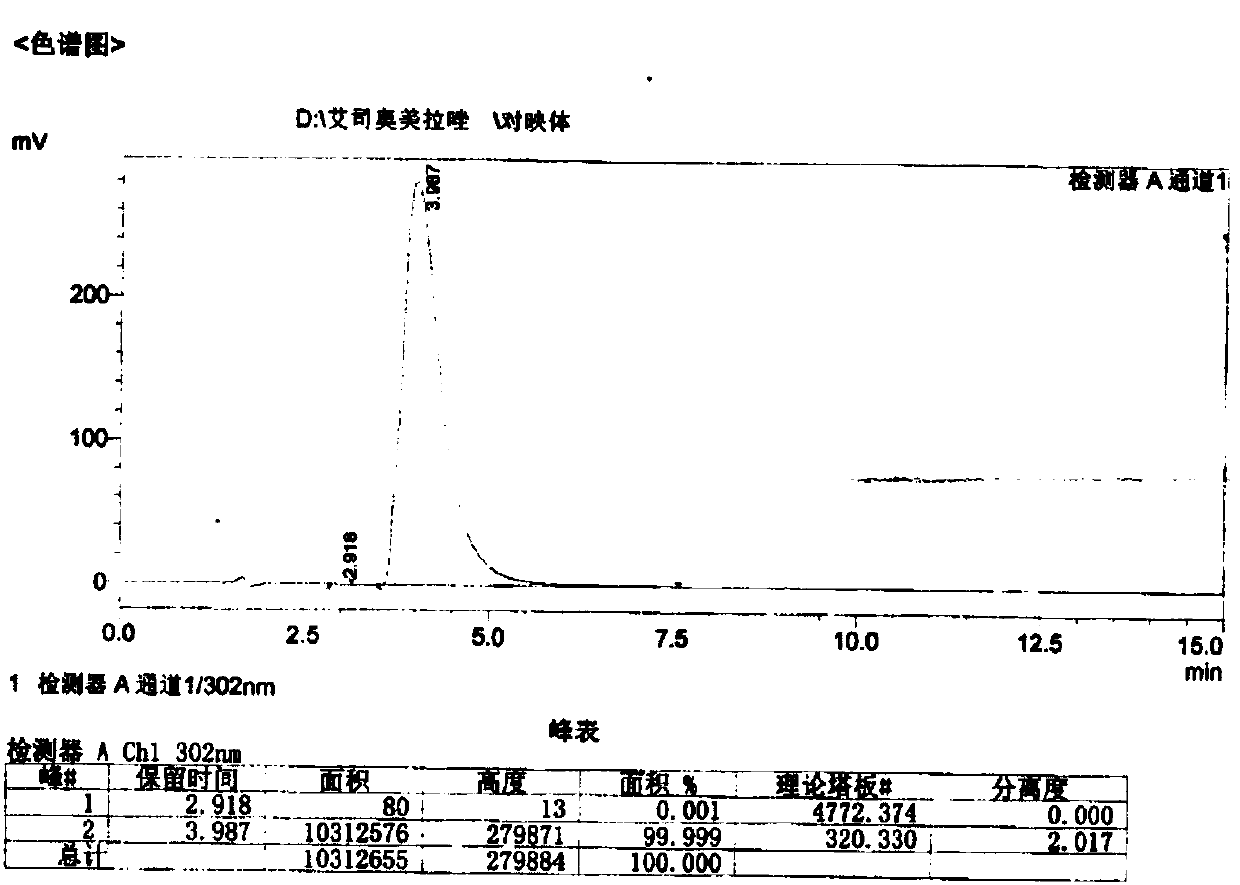
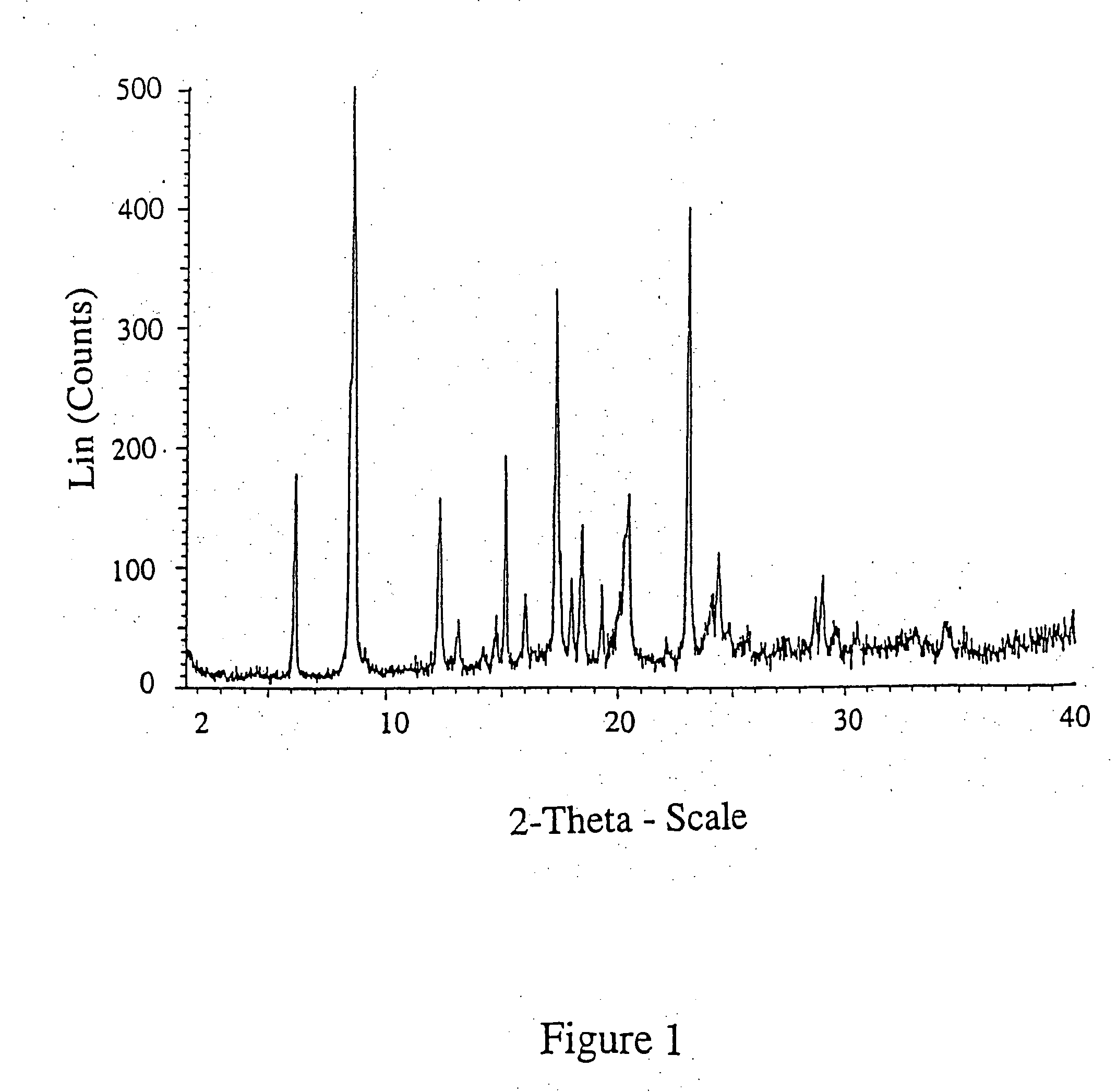
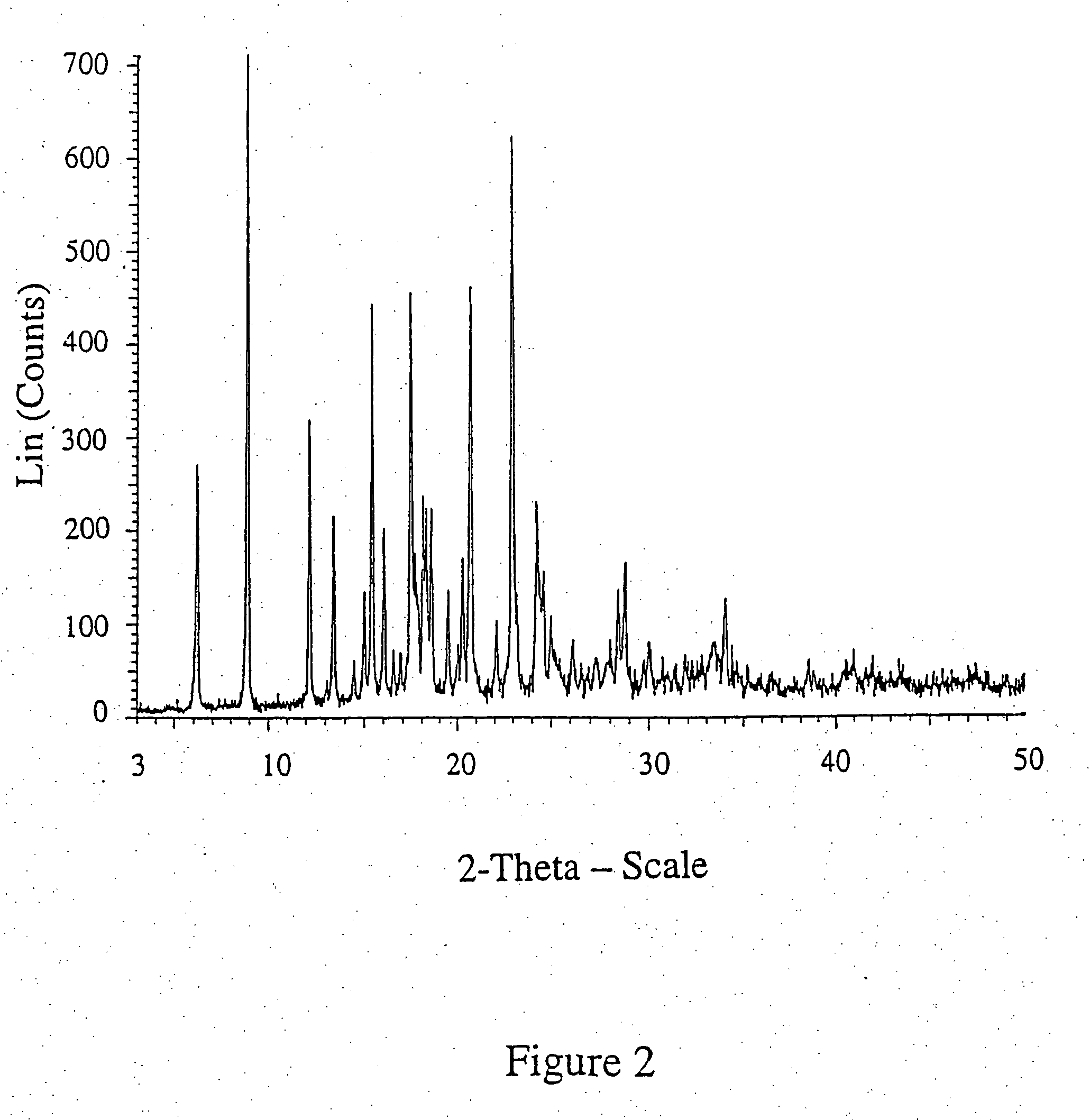
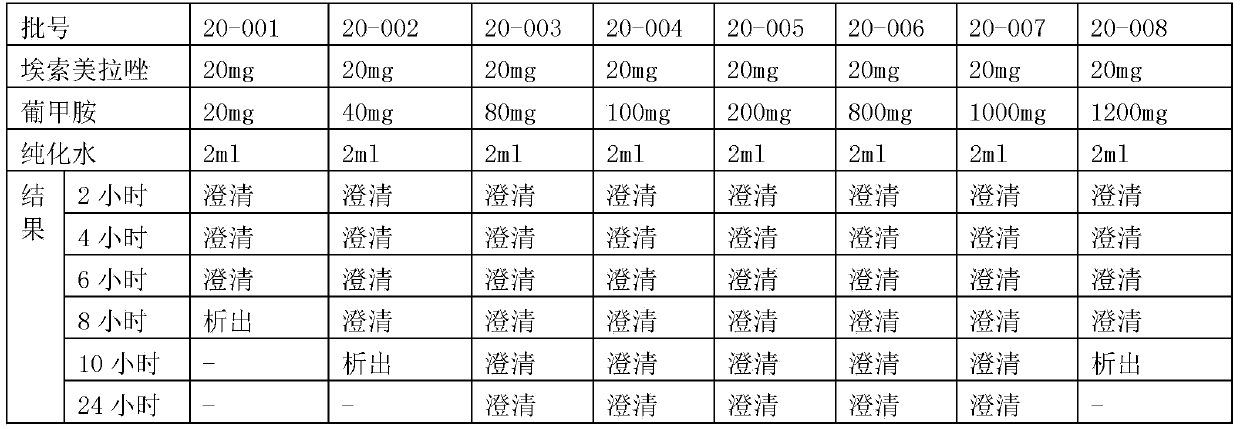
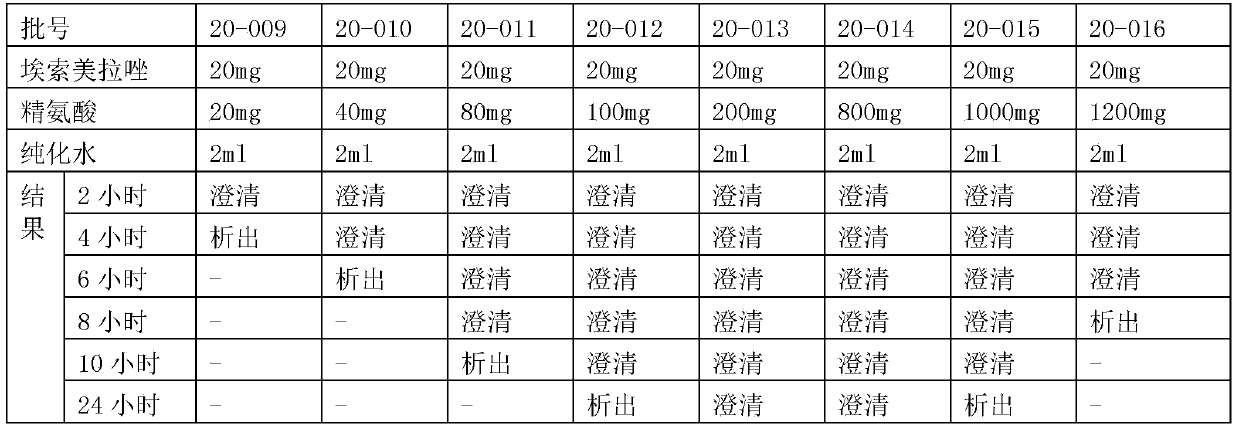
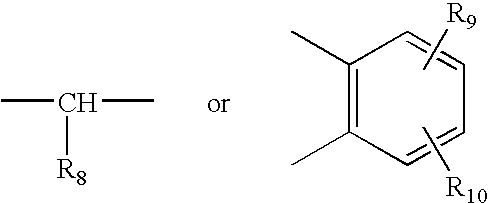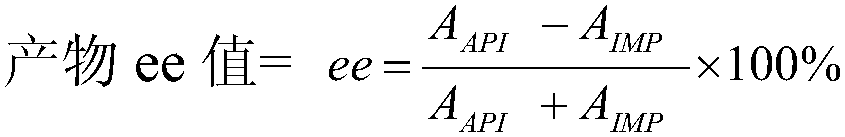Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
133 results about "Esomeprazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
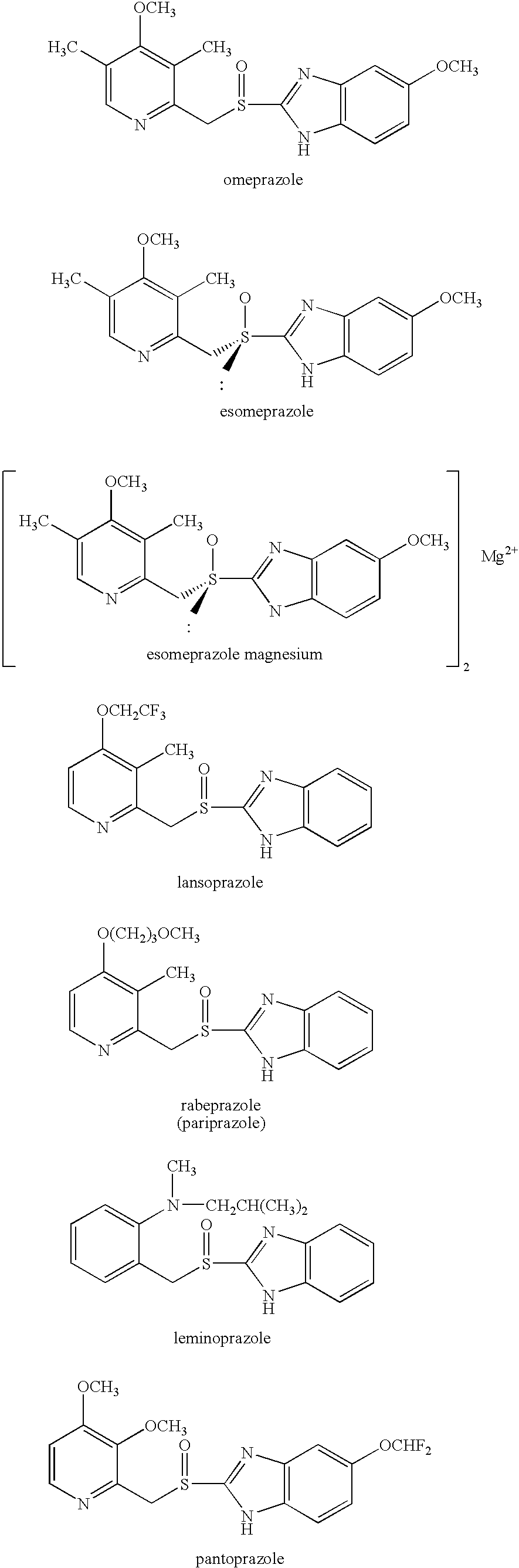
Esomeprazole is used to treat certain stomach and esophagus problems (such as acid reflux, ulcers).
New Combination Dosage Form
InactiveUS20070122470A1Salicyclic acid active ingredientsBiocideGastrointestinal complicationsSalicylic acid
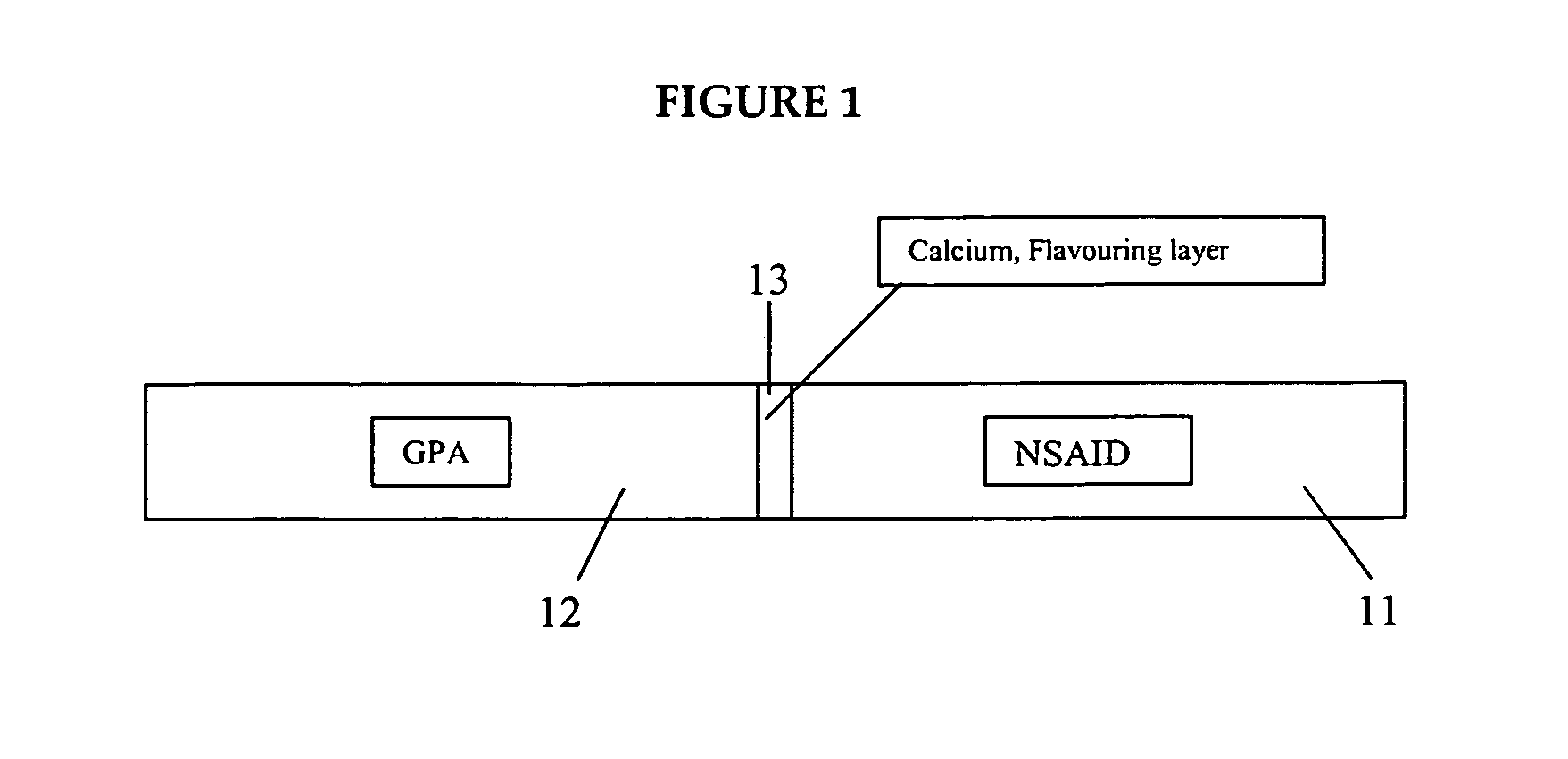
The present invention relates to an oral pharmaceutical preparation for use in the prevention and / or reduction of gastrointestinal complications associated with the use of acetyl salicylic acid. The present preparation comprises a fixed oral dosage form comprising a proton pump inhibitor in combination with acetyl salicylic acid. Furthermore, the present invention refers to a method for the manufacture thereof and the use thereof in medicine. The present invention also relates to a specific combination comprising esomeprazole, or an alkaline salt thereof or a hydrated form of any one of them, and acetyl salicylic acid for use as a medicament for the prevention of thromboembolic vascular events, such as myocardial infarction or stroke, and for the prevention and / or reduction of gastrointestinal complications associated with the use of acetyl salicylic acid.
Owner:ASTRAZENECA AB
Method for preparing high-purity esomeprazole
ActiveCN102584792AShort preparation timeReduce the difficulty of operationOrganic chemistryBiochemical engineeringCombinatorial chemistry
The invention discloses a method for synthesizing and refining salt of esomeprazole. According to the method, 2-sulfydryl-5-methoxyl-1H-benzimidazole is used as an initiative raw material for reaction, and a reaction condition is optimized, so that reaction is performed under a mild condition, and the content of impurities in the product is reduced effectively. After the synthesized product is refined further, the purity and enantiomer excess of the product are over 99 percent, so that the effect and safety of administration are improved.
Owner:NANJING YOUKE BIOLOGICAL MEDICAL RES
Pharmaceutical Compositions for the Coordinated Delivery of Naproxen and Esomeprazole
InactiveUS20100172983A1Reduce riskMinimize adverse effectsBiocideAntipyreticAnti arthriticUlcer care
The present disclosure is directed to drug dosage forms that release an agent that raises the pH of a patient's gastrointestinal tract, followed by a non-steroidal anti-inflammatory drug. The dosage form is designed so that the NSAID is not released until the intragastric pH has been raised to a safe level. The disclosure also encompasses methods of treating patients by administering this coordinated release, gastroprotective, antiarthritic / analgesic combination unit dosage form to achieve pain and symptom relief with a reduced risk of developing gastrointestinal damage such as ulcers, erosions and hemorrhages.
Owner:POZEN INC
Oral dosage forms
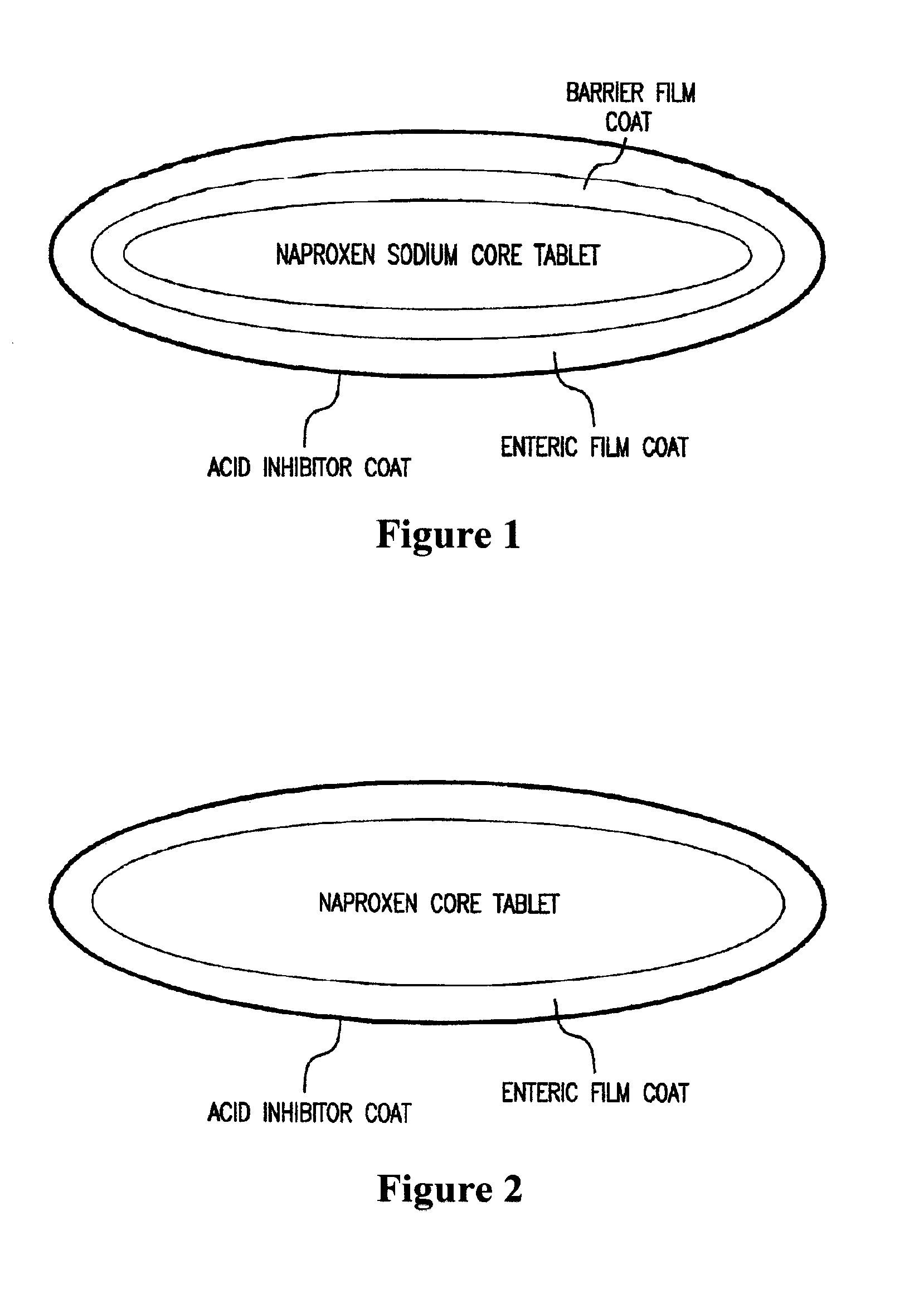
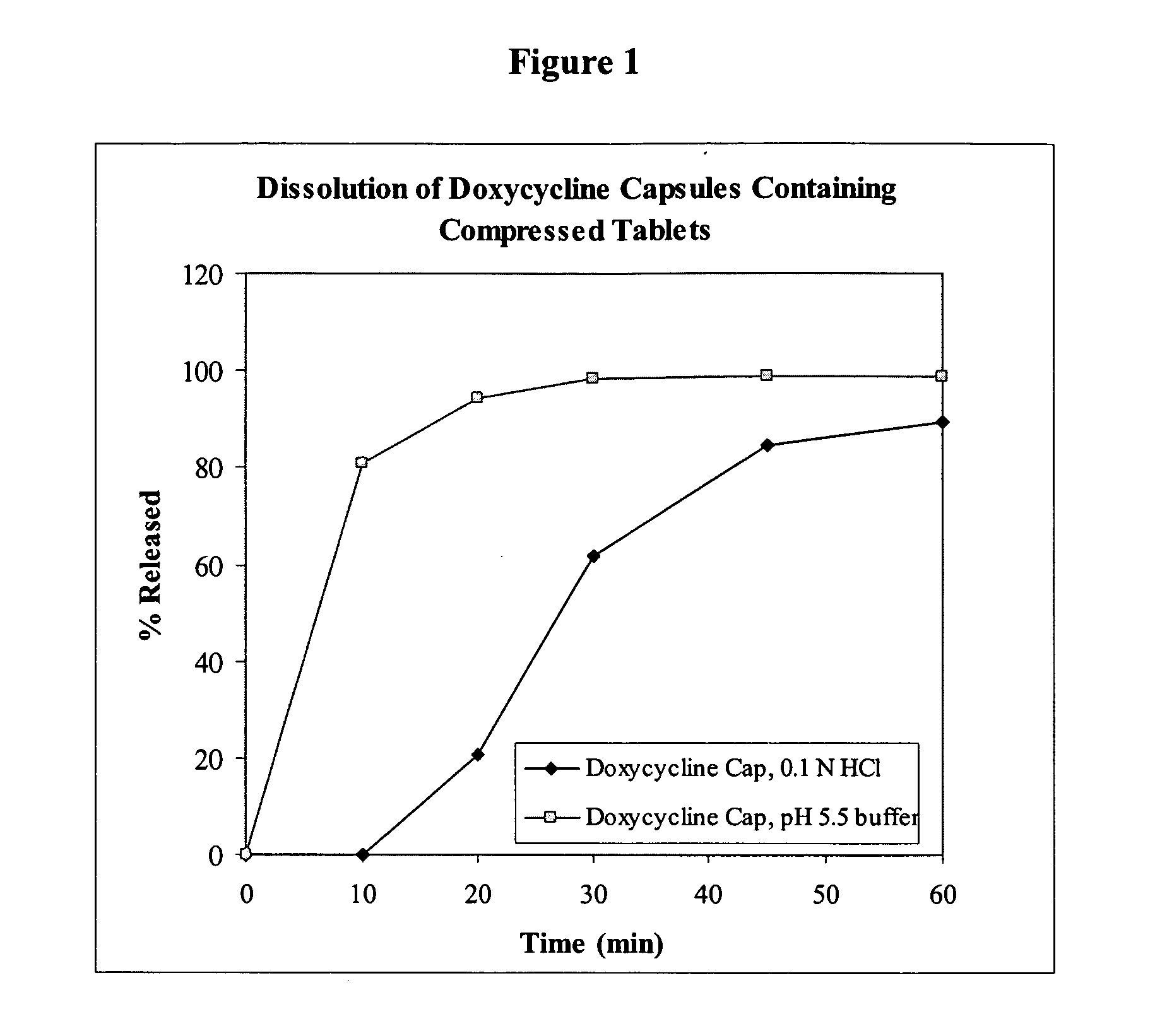
This invention relates to an oral dosage form of a pharmaceutically active ingredient comprising: (a) an outer capsule and (b) non-uniform pellets, having a non-uniform shape and / or size, contained within the capsule, wherein the pellets comprise a compressed powder comprising a pharmaceutically active ingredient. In one embodiment the active ingredient is selected from the group consisting of doxycycline, omeprazole, esomeprazole, and propafenone. Pharmaceutical formulations of the active ingredients as well as methods and tools for making the oral dosage form are also described.
Owner:PAR PHARMA
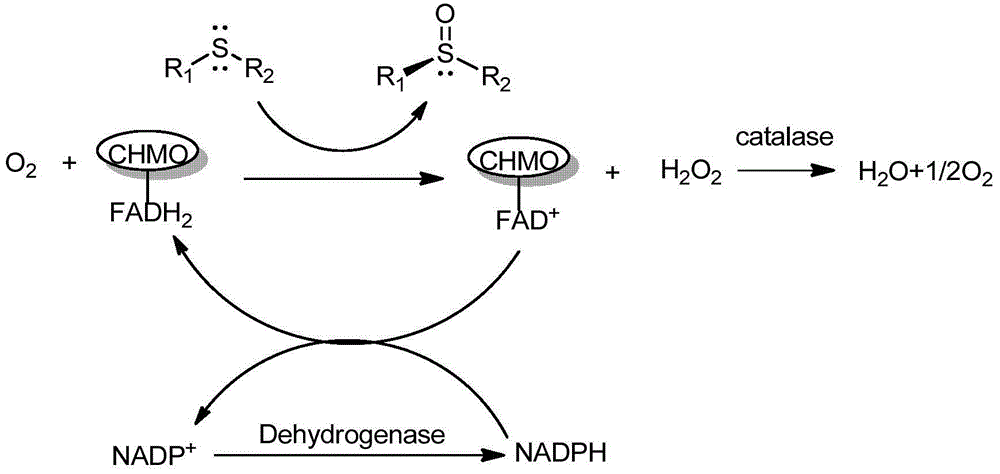
Cyclohexanone monooxygenase and application thereof in synthesis of esomeprazole
The invention provides application of a cyclohexanone monooxygenase characterized by high catalytic activity, high reaction yield, and environmental friendliness to asymmetric oxidation reaction to synthesize single-configuration prazole drugs. The invention also provides a gene of the cyclohexanone monooxygenase, a recombinant expression vector containing the gene, a recombinant expression transformant and an efficient preparation method of the cyclohexanone monooxygenase, and application of the cyclohexanone monooxygenase in catalysis of a prochiral thioether compound into single-configuration sulfoxide.
Owner:ABIOCHEM BIOTECH CO LTD
Oral Pharmaceutical Dosage Form Comprising as Active Ingredients a Proton Pump Inhibitor together with Acetyl Salicyclic Acid
InactiveUS20100178334A1Salicyclic acid active ingredientsBiocideSalicylic acidGastrointestinal complications
The present invention relates to an oral pharmaceutical preparation for use in the prevention and / or reduction of gastrointestinal complications associated with the use of acetyl salicylic acid. The present preparation comprises a fixed oral dosage form comprising a proton pump inhibitor in combination with acetyl salicylic acid. Furthermore, the present invention refers to a method for the manufacture thereof and the use thereof in medicine. The present invention also relates to a specific combination comprising esomeprazole, or an alkaline salt thereof or a hydrated form of any one of them, and acetyl salicylic acid for use as a medicament for the prevention of thromboembolic vascular events, such as myocardial infarction or stroke, and for the prevention and / or reduction of gastrointestinal complications associated with the use of acetyl salicylic acid.
Owner:ASTRAZENECA AB
Pharmaceutical formulation and process for its preparation
The present invention relates to a multiparticulate tablet with improved gastro-protection comprising at least a pharmaceutically active substance in the form of enteric coated particles, and a mixture of tableting excipients, wherein the said mixture of excipients comprising xylitol and / or maltitol, each in a directly compressible form, a disintegrating agent, a lubricant and at least one other diluent and the ratio of a) the xylitol and / or the maltitol to b) the other diluent(s) is less than 5 / 95 (weight / weight) and the result of the “test of integrity of the film” is greater than 95%, preferably greater than 97% and more preferably still greater than 99% and the result of the “release test” is greater than 90%, preferably greater than 95%. According to one embodiment of the invention, the active substance is omeprazole or esomeprazole. According to another embodiment the tablet is a disintegratable tablet, which disintegrate in the mouth with or without chewing. The invention also comprises a process for preparing the claim tablet and its use in medicine.
Owner:ASTRAZENECA AB
Esomeprazole and preparation method of magnesium trihydrate of esomeprazole
The invention provides esomeprazole and a preparation method of magnesium trihydrate of the esomeprazole. The preparation method includes the following steps of subjecting racemization omeprazole and inorganic base to acid-base neutralization reaction in an alcoholic solution to obtain racemization omeprazole sodium salt; dissolving omeprazole sodium salt, organic metal coordination agents, chelating agents and organic base in an organic solvent for complex reaction to obtain esomeprazole complex; subjecting S-mandelic acid and the esomeprazole complex to condensation reaction to obtain an esomeprazole mandelate compound; dissolving the esomeprazole mandelate compound in an acetone solution, and performing filtering to obtain S-omeprazole-S-mandelate compound; and suspending the S-omeprazole-S-mandelate compound in a first solvent to obtain a suspension solution, and adjusting potential of hydrogen (pH) of the suspension solution to be 8-10 to obtain the esomeprazole, wherein the first solvent includes 30-32v / v% of an alkaline aqueous solution and 68-70v / v% of an organic solvent. By means of the preparation method, the technical problem that the yield and the purity of the esomeprazole in prior art are low is solved.
Owner:HUNAN FANGSHENG PHARMACEUTICAL CO LTD
Novel process for substituted sulfoxides
The present invention relates to a process for preparing substituted sulfoxides either as a single enantiomer or in an enantiomerically enriched form. Thus, racemic omeprazole is reacted with (S)-camphorsulfonyl chloride to form a diastereomeric mixture and the diastereomers are separated by fractional crystallization, followed by deprotection to give esomeprazole.
Owner:HETERO DRUGS LTD
Pharmaceutical formulations comprising nsaid and proton pump inhibitor drugs
InactiveUS20100305163A1Increased riskAvoid erosionBiocideAntipyreticTherapeutic intentPharmaceutical formulation
Aspects of the invention relate to pharmaceutical formulations comprising an NSAID and acid reducer drug for therapeutic purposes, and methods of preparing the same. Further aspects of the invention relate to fixed dose pharmaceutical formulations comprising naproxen, or pharmaceutically acceptable salts thereof, and esomeprazole, or pharmaceutically acceptable salts thereof.
Owner:DR REDDYS LAB LTD +1
Medicated gumstick for treatment in anti-inflammatory conditions and prophylaxis against NSAID gastropathy
InactiveUS20070003490A1Promote absorptionMinimize contactAntipyreticAnalgesicsDiseaseMedicated chewing-gum
A stick of gum is provided containing therapeutic benefits of non-steroid anti-inflammatory drugs for inflammation in conditions such as arthritis, and also alleviates subsequent side effects of NSAID administration, as well as antacid effects from compounds such as an H2 antagonist (ranitidine, cimetidine, famotidine) and / or a proton pump inhibitor (such as lansoprazole, pantoprazole, omeprazole, esomeprazole or rabeprazole) and / or an acid pump antagonist selected from the group of soraprazan, AZD0865, YH1885 and CS-526.
Owner:MEDICAL FUTURES
Cyclohexanone monooxygenase and application thereof
ActiveCN108118035AHigh yieldLow priceBacteriaOxidoreductasesHigh concentrationCyclohexanone monooxygenase
The invention discloses cyclohexanone monooxygenase and an application thereof, in particular cyclohexanone monooxygenase obtained by site-specific mutagenesis and an application thereof. Compared with a SEQ ID NO: 1, the amino acid sequence of the cyclohexanone monooxygenase has gene mutation in at least one site as follows: serine Ser at the 386th site is mutated to asparagines Asn, and serine Ser at the 435th is mutated to threonine Thr. Experiments show that the cyclohexanone monooxygenase disclosed by the invention can catalytically convert a high concentration omeprazole thioether primerinto esomeprazole.
Owner:ZHEJIANG JINGXIN PHARMA +1
Preparation method of esomeprazole and preparation method of esomeprazole salt
The invention adopts 2-chloromethyl-4-nitryl-3, 5-dimethyl pyridine hydrochloride and 5-methoxyl-2-mercapto benzimidazole as starting materials to prepare esomeprazole salt by condensation, asymmetric oxidation and methoxidation. A preparation method has the advantages that the repeatability is good, the operation is simple, and the industrial production is easy; and the preparation conditions are moderate, the generation of impurities such as nitric oxide and sulphone is reduced in the preparation process, and the yield and the purity of the esomeprazole salt are increased.
Owner:SHANDONG UNIV +1
Pharmaceutical Compositions Comprising Amorphous Esomeprazole, Dosage Forms And Process Thereof
A stabilized pharmaceutical composition of benzimidazole compounds preferably amorphous form of esomeprazole and a process for preparing the same. The pharmaceutical compositions formulated into solid dosage forms preferably multiple unit tablet dosage forms and capsules and a method for preparing the same.
Owner:JUBILANT LIFE SCI
Process for substituted sulfoxides
The present invention relates to a process for preparing substituted sulfoxides either as a single enantiomer or in an enantiomerically enriched form. Thus, racemic omeprazole is reacted with (S)-camphorsulfonyl chloride to form a diastereomeric mixture and the diastereomers are separated by fractional crystallization, followed by deprotection to give esomeprazole.
Owner:HETERO DRUGS LTD
Medicine for treating gastroesophageal reflux disease and functional dyspepsia
InactiveCN101143143AImprove toleranceHigh synergistic effectDigestive systemSolution deliveryLansoprazoleRabeprazole
A combination preparation for remedying the gastroesophageal reflux disease (GERD) and the functional dyspepsia is characterized in that the prescription of the combination preparation consists of a proton pump depressor and a gastrointestinal power drug of itopride; the proton pump depressor is selected from one of a Pantoprazole, a Omeprazole, a Esomeprazole, a Lansoprazole, a Rabeprazole, a Tenatoprazole and a Leminorazole, wherein the Pantoprazole is preferential, and at the same time the neutral form of the basic salt of the proton pump depressor is also included, such as Naplus, Mg2plus, Ca2plus, Kplus or Li plus salt and a pure optical stereoisomer of the proton pump depressor or an active metabolite of the proton pump depressor; the gastrointestinal power drug is the itopride and a ramification of the itopride or one of the medicinal salts of the itopride; in the combination preparation, the weight ratio of the Pantoprazole and the itopride is 2 to 5 to 2 to 7. The invention has important affect for remedying the gastroesophageal reflux disease and the functional dyspepsia, and the preparation method of the invention is simple and convenient; the cost is low; the invention is fit for being orally taken by the patient; the invention has good conformance performance, high curative effect, low recrudescence rate and little adverse reaction.
Owner:沈阳东宇药业有限公司
Preparation method for esomeprazole
InactiveCN107892683AHigh yield and purityReduce generationOrganic chemistry methodsNitrogen oxideSulfone
The invention discloses a method for preparing esomeprazole, belonging to the technical field of medicine. According to the invention, 2-chloromethyl-4-methoxy-3,5-dimethylpyridine hydrochloride and 2-mercapto-5-methoxybenzimidazole are used as starting materials and are subjected to condensation and oxidation so as to prepare an esomeprazole salt. The method has good reproducibility, and is simple to operate, easy for realizing industrial production and mild in preparation conditions; and the production of impurity nitrogen oxide and sulfone (peroxide) during the preparation is reduced, and the yield and purity of the esomeprazole salt are improved.
Owner:JIANGSU ZHONGBANG PHARMA
Alkylammonium salts of omepazole and esomeprazole
The present invention relates to new salts of omeprazole and esomeprazole respectively, i.e. salts of 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)-methyl]sulfinyl]-1H-benzimidazole and the (S)-enantiomer thereof. More specifically, the present invention relates to alkylammonium salt of the compounds, formed by a reaction of omeprazole and esomeprazole respectively and an alkylamine with formula NR?1#191R?2#191R?3#191 wherein R?1#191 is a linear, branched or cyclic C?1#191-C?12#191-alkyl group, R?2#191 and R?3#191 are hydrogen. The present invention also relates to a process for preparing crystalline salts, a pharmaceutical preparation and a method for treatment of gastric related disorders by administering the compound of the invention.
Owner:ASTRAZENECA AB
Esomeprazole medicated pellet and preparing method thereof
ActiveCN102824316AAvoid wastingIncrease productivityOrganic active ingredientsDigestive systemPharmaceutical medicineMannitol
The invention discloses an esomeprazole medicated pellet and a preparing method of the esomeprazole medicated pellet. The esomeprazole medicated pellet is mainly prepared from 25-50% of esomeprazole raw material and ramification of the esomeprazole raw material, 20-50% of mannitol and 5-30% of polyvinylpolypyrrolidone according to weight percentage; and in addition, pharmaceutic adjuvant acceptable pharmaceutically is also contained. The ingredients of the esomeprazole medicated pellet are proportioned, and the esomeprazole medicated pellet product can be prepared by virtue of an extruding-rolling preparing method. Compared with the prior art, the technical scheme provided by the invention has the advantages that the efficiency is greatly improved, and waste of a large amount of time, manpower and material resources are avoided; and the method is simple, and the midbody of the obtained product preparation has the advantages of stable medicine, high reproducibility and the like.
Owner:ZHONGSHUAI PHARMA SCI & TECH CO LTD
Novel stereoselective synthesis of benzimidazole sulfoxides
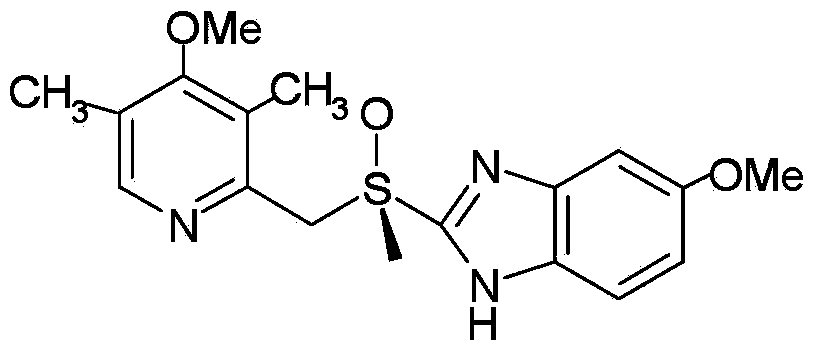
The present invention relates to a process for stereoselective synthesis of substituted sulfoxides either as a single enantiomer or in an enantiomerically enriched form. Thus, 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]thio]-1H-benzimidazole is reacted with (R)-camphorsulfonyl chloride to form a mixture of 1-(R)-camphorsulfonyl-5- (and 6-)methoxy-2-[(3,5-dimethyl-4-methoxy-2-pyridyl)methylthio]-1H-benzimidazole, oxidized to obtain a diastereomeric excess of 1-(R)-camphorsulfonyl-(5- and 6-)-methoxy-2-[(3,5-dimethyl-4-methoxy-2-pyridyl)methyl-(S)-sulfinyl]-1H-benzimidazole over 1-(R)-camphorsulfonyl-(5- and 6-)-methoxy-2-[(3,5-dimethyl-4-methoxy-2-pyridyl)methyl-(R)-sulfinyl]-1H-benzimidazole, the diastereomers are separated by fractional crystallization and the separated 1-(R)-camphorsulfonyl-(5- and 6-)-methoxy-2-[(3,5-dimethyl-4-methoxy-2-pyridyl)methyl-(S)-sulfinyl]-1H-benzimidazole is deprotected to give esomeprazole.
Owner:HETERO DRUGS LTD
Esomeprazole enteric pellet tablets and preparation method thereof
The invention relates to a preparation method for esomeprazole enteric pellet tablets. The method comprises the following steps of: preparing esomeprazole enteric pellets, and preparing mixed tablets of the pellets and excipient; the esomeprazole enteric pellets contain active pharmaceutical pellet cores, isolation layers and enteric coatings; the esomeprazole enteric pellets prepared by the method have certain elasticity and are pressureproof; the esomeprazole enteric pellets and the excipient are pressed to form tablets, so that the pellets can be isolated from air, and are more stable than pellet capsules; the validity period can reach over three years; and the esomeprazole enteric pellet tablets have the advantages of small size, dividable dose and the like, and the compliance of a patient is improved.
Owner:SHOUGUANG FUKANG PHARMA
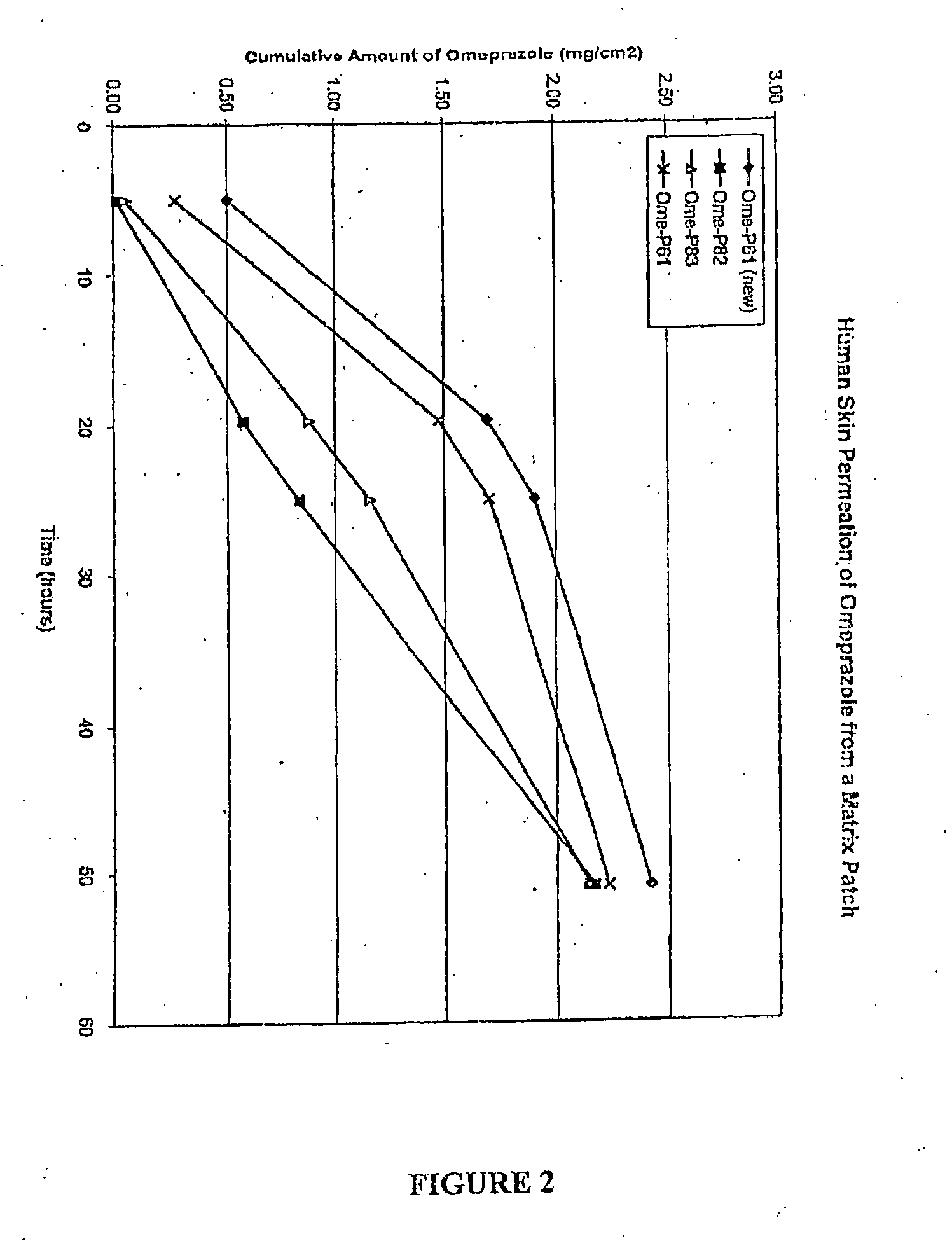
Transdermal Administration of Proton Pump Inhibitors
InactiveUS20080287502A1Increase ratingsImprove throughputBiocidePharmaceutical non-active ingredientsDepressantTransdermal
A method and composition for the transdermal administration of proton pump inhibitors such as substituted pyridyl methylsulfinyl benzimidazoles, and in particular, omeprazole, lansoprazole, esomeprazole, pantoprazole and raberprazole. The method and composition include the use of a hydroxide-releasing agent as a permeation enhancer to increase the flux of the protein pump inhibitor through a patient's skin or mucosal tissues and optionally also include the use of a carrier such as 1,3-butanediol, dipropylene glycol, and hexylene glycol.
Owner:DERMATRENDS INC
Method for preparing esomeprazole and salts thereof
The invention relates to a novel method for preparing esomeprazole and sodium salts, magnesium salts, lithium salts, potassium salts, calcium salts and ammonium salts thereof. The esomeprazole is prepared by oxidizing omeprazole sulfide; and an oxidant is a chiral camphorsulfonyloxaziridine compound. The method comprises the following steps of: dissolving the omeprazole sulfide into a solvent, adding an alkaline reagent, stirring for 30 to 90 minutes at room temperature, adjusting the temperature to -30 to 200 DEG C, adding the oxidant, namely the chiral camphorsulfonyloxaziridine compound, and stirring and reacting for 0.5 to 20 hours to obtain the esomeprazole. In the method, a chiral ligand and a metal compound are not required to be added, the introduction of heavy metals is avoided in the reaction, and the chiral camphorsulfonyloxaziridine compound which participates in the oxidation reaction can be recycled, so that the utilization rate of the oxidant is remarkably improved and the production cost is remarkably reduced; and the prepared esomeprazole in high in purity and yield.
Owner:刘强
Method for preparing esomeprazole impurity
The invention discloses a method for preparing an esomeprazole impurity. 5-methoxy-2-[(S)-[(4-chloro-3,5-dimethyl-2-pyridyl)methyl]sulfinyl]-1H-benzimidazole (II) is obtained through demethylation, halogenation, condensation and asymmetric oxidation by taking 2-chloromethyl-4-methoxy-3,5-dimethylpyridine hydrochloride as a starting raw material. According to the method disclosed by the invention,a synthesis route is simple and short, the starting raw material is easy to obtain, the reaction conditions are gentle, the operation is simple and convenient, and the impurity can be obtained withoutcolumn chromatography; the purity of the prepared impurity can be up to 99 percent or above, the ee (Enantiomeric Excess) value can be up to 99 percent or above, and the impurity can be used as reference substance in quality research.
Owner:珠海润都制药股份有限公司
New method for synthesizing esomeprazole through asymmetrically catalytic oxidation
The invention relates to a new method for synthesizing esomeprazole through asymmetrically catalytic oxidation. The method is characterized in that (S,S)-6,6'-dyhydroxy-2,2'-biphenyl dicarboxylate is used as a chiral ligand inducer, compounds containing molybdenum are used as catalyst, isopropyl hydrogen peroxide is used as oxidant, high-content and high-optical-purity esomeprazole which is a single enantiomer of omeprazole is obtained through room-temperature catalytic oxidation of prochiral thioether compounds, and corresponding alkali metal salts of esomeprazole can be further obtained through reaction with alkali inorganic salts. After the esomeprazole and the alkali metal salts thereof obtained by adopting the method are purified, the purity can reach more than 99 percent, the optical purity can reach more than 99.5 percent and the yield can reach more than 75 percent. The method can realize high utilization ratio of raw materials, is simple, convenient and feasible and is suitable for industrial mass production.
Owner:SHOUGUANG FUKANG PHARMA
Purification method of esomeprazole
InactiveCN102757421AEfficient purificationHigh purityOrganic chemistryPurification methodsDrug compound
The invention relates to a purification method of esomeprazole. According to the purification method, esomeprazole or salt thereof to be purified is transformed into potassium esomeprazole and then separated by crystallization, and if necessary, the crystallization product is transformed into esomeprazole or salt thereof. Compared with the conventional recrystallization method, the purification method can purify esomeprazole with high efficiency to obtain high-purity esomeprazole or salt thereof. The purification method is particularly suitable for purification of drug compounds with extremely strict requirements on the purity.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Production method of high-optical-purity esomeprazole
The invention belongs to the field of pharmacy and relates to a production method of high-optical-purity esomeprazole salt. The method takes m-chloro peroxy-sodium benzoate as an oxidizing agent and takes an organic solvent which is dissolved into water as a reaction solvent, so that the problem that the acidity of the m-chloro peroxy-sodium benzoate cannot be applied to preparing the esomeprazole is solved. The method is simple to operate and has a low cost; and the optical purify of the prepared product is high and the impurity content is low.
Owner:NANJING XUNAN PHARMA TECH
Pharmaceutical composition containing esomeprazole
InactiveCN101808622AImprove stabilityEasy to producePowder deliveryDigestive systemAlkali saltEsomeprazole
The present invention relates to esomeprazole free base or its alkali salt-containing composition which stability is improved and is easy to manufacture.
Owner:CTC BIO INC
Enzymatic preparation method of esomeprazole
InactiveCN108251465AImprove catalytic reaction efficiencyIncrease concentrationFermentationOmeprazolePhase-transfer catalyst
The invention discloses an enzymatic preparation method of esomeprazole. The enzymatic preparation method comprises: carrying out a reaction on omeprazole thioether as a substrate and an oxidizing agent in a solvent under the actions of monooxygenase, an auxiliary component and a phase transfer catalyst to generate esomeprazole. According to the present invention, with the enzymatic preparation method, esomeprazole is efficiently prepared; and by using the phase transfer catalyst, the efficiency of the enzyme catalytic reaction is improved, the reaction time is substantially reduced, the concentration of the reaction substrate is increased, the conversion rate can be up to 99.8%, the production cost is reduced, the single-batch yield is substantially improved, and the excellent industrialapplication value is provided.
Owner:ZHEJIANG JINGXIN PHARMA +1
Oral instant preparation of proton pump inhibitor and preparation method thereof
InactiveCN103386133AGreat tasteImprove bioavailabilityOrganic active ingredientsDigestive systemDrugs preparationsUser friendliness
The invention belongs to the field of drug preparation, provides a proton pump inhibitor and a preparation method thereof, and especially relates to an application of a water system of amine compound alkalizer in the preparation of stabilizer of esomeprazole free alkali. Besides, the invention provides a stable water system of esomeprazole free alkali, and the system is composed of amine compound alkalizer, esomeprazole free alkali and water medium. On the basis of the water system, an oral instant preparation of esomeprazole is prepared. The preparation has the advantages of stable quality, good taste, rapid absorption, quick effect and user-friendliness, can improve the compliance of the patients, and is especially suitable for the patients when the disease attacks with no water available. The preparation effectively solves the problem that esomeprazole is decomposed by acid, relieves the stimulation to the digestive tract, and increases the stability of the esomeprazole preparation and the safety of clinical application.
Owner:CHONGQING LUMMY PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com