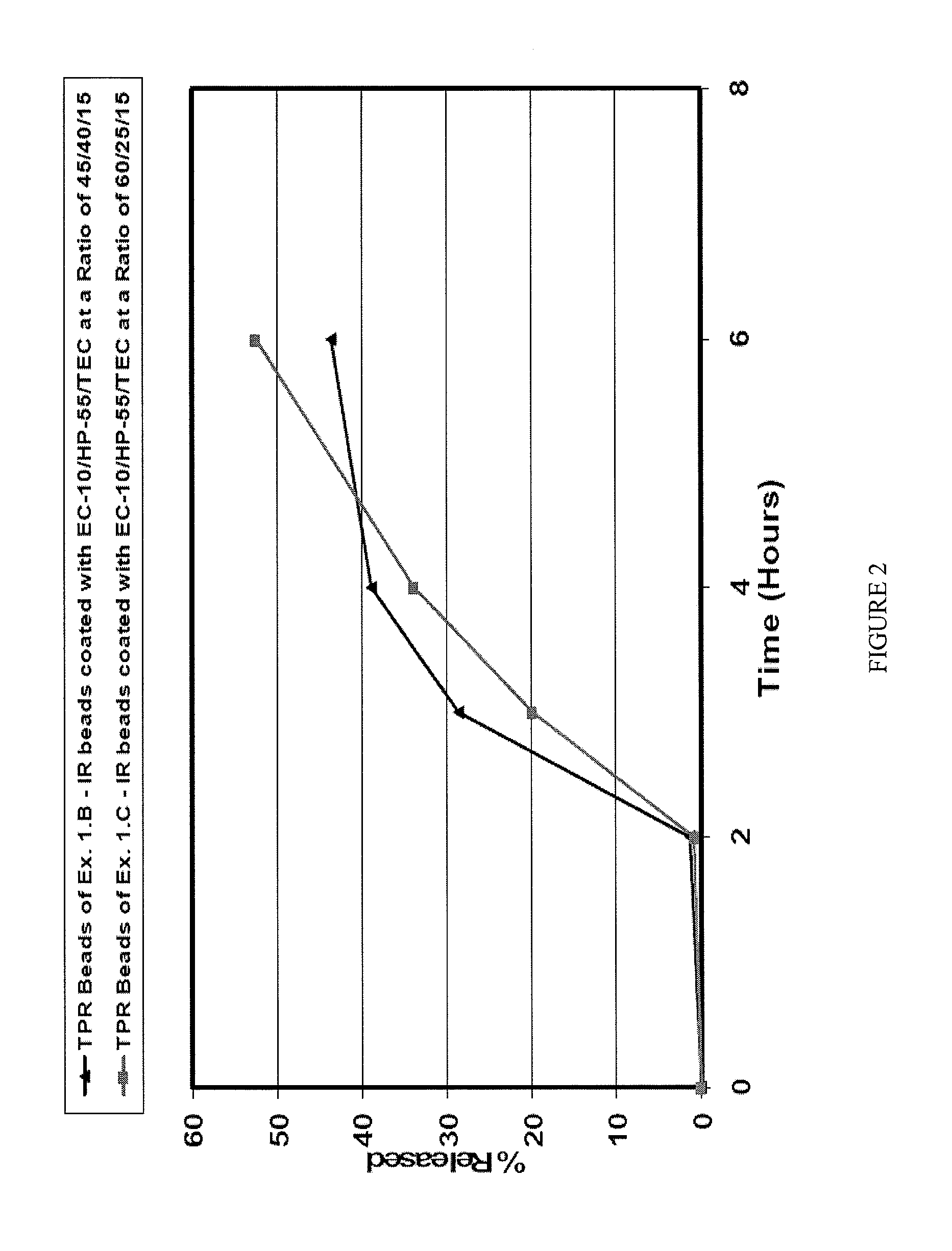
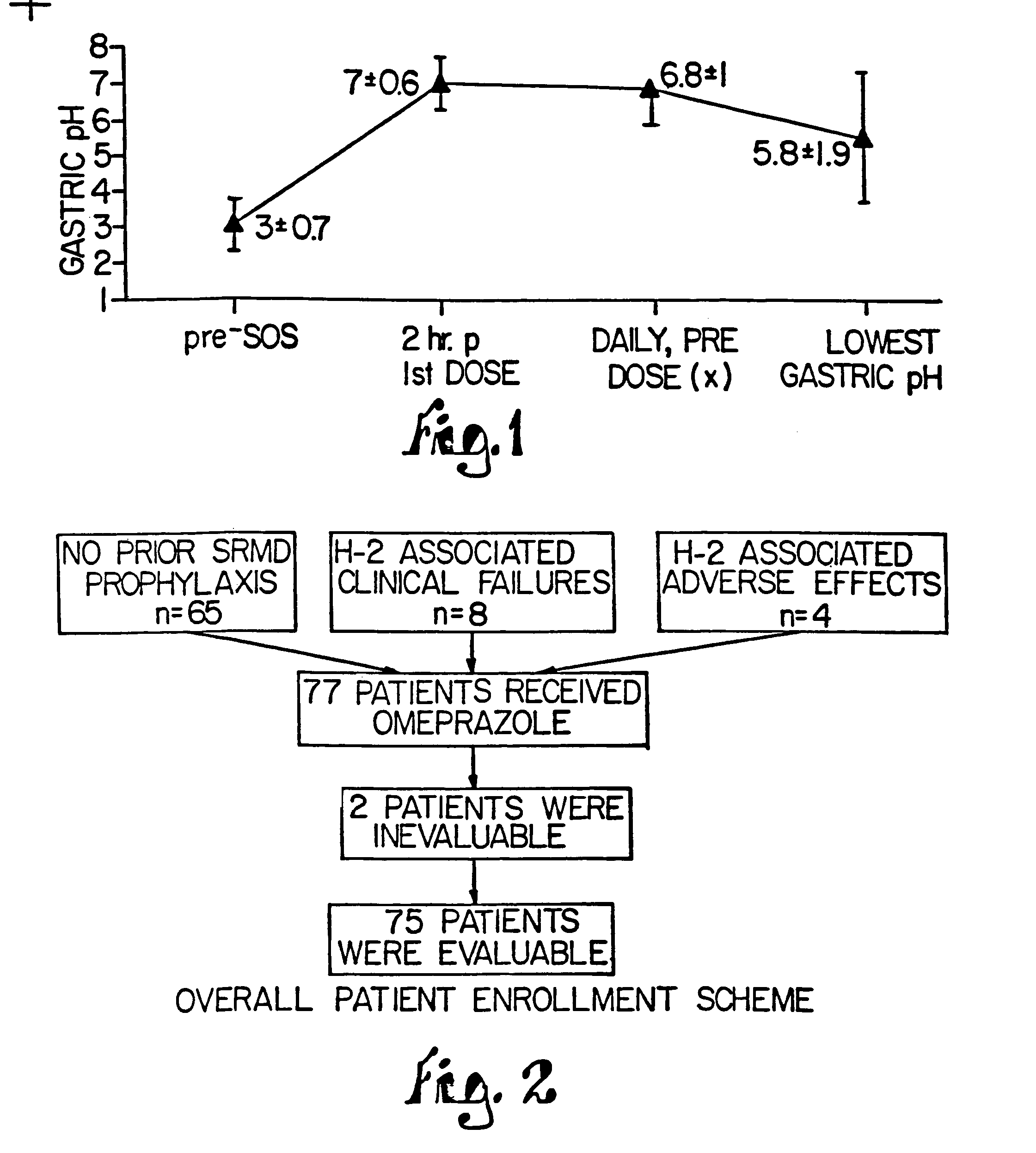
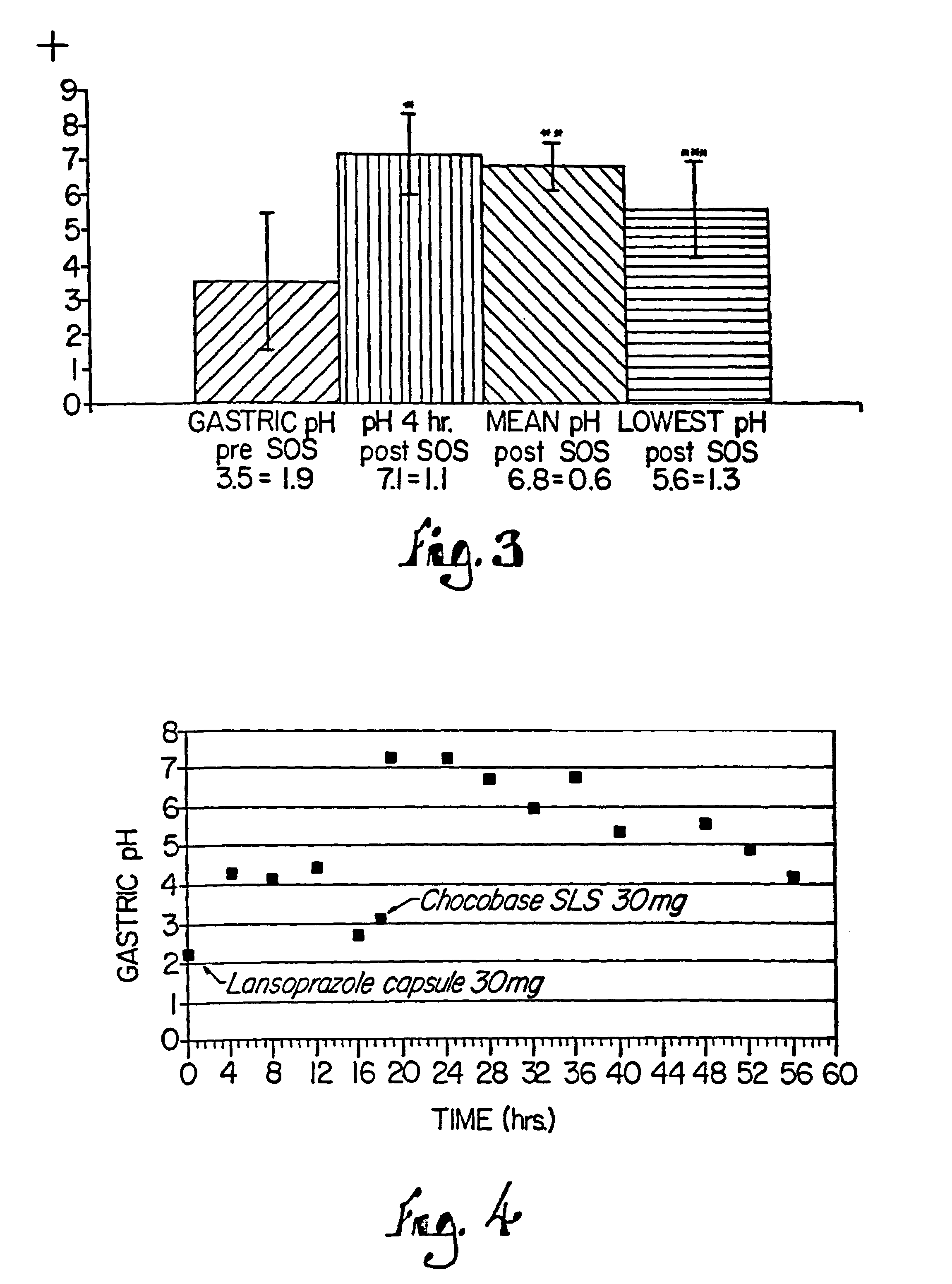
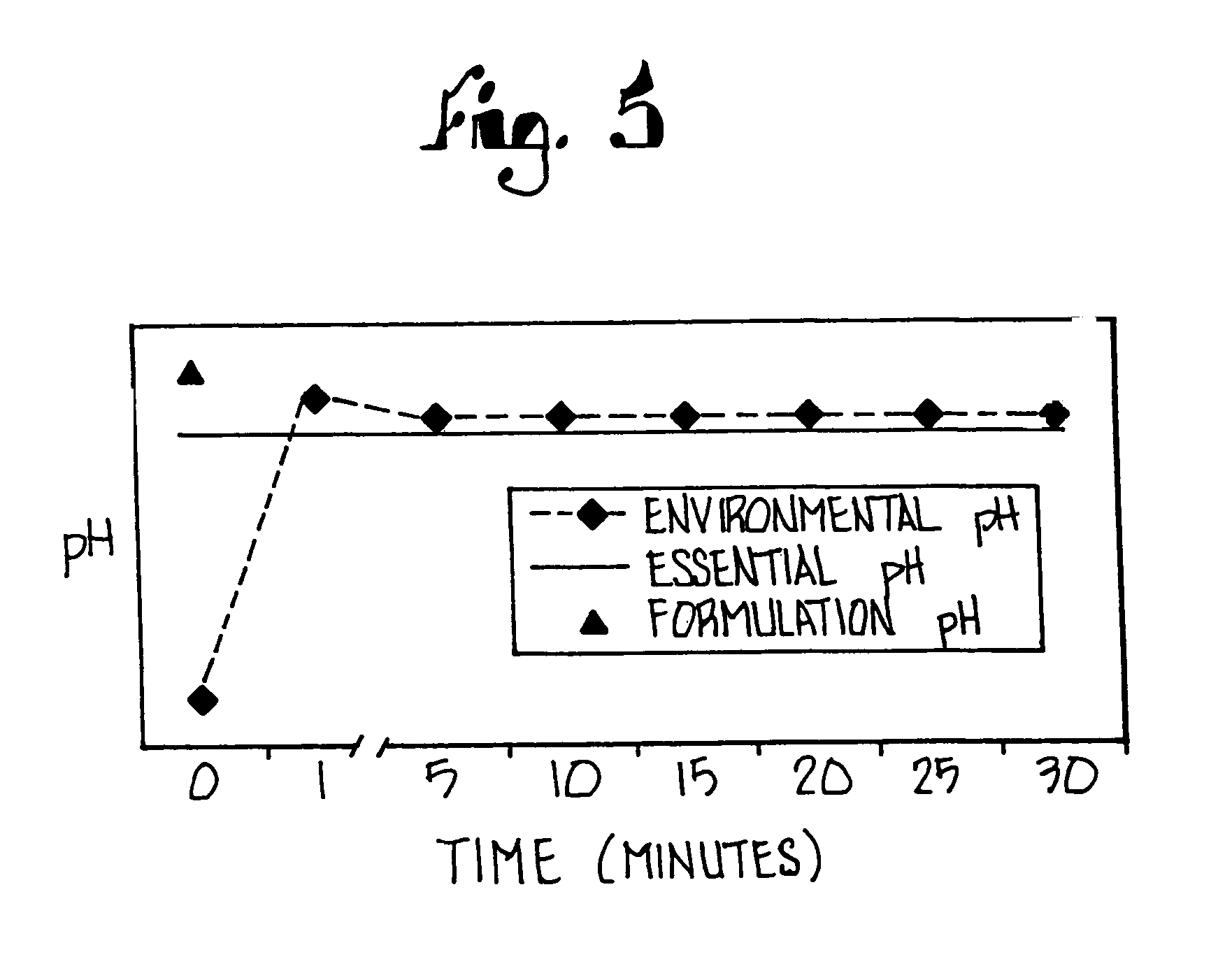
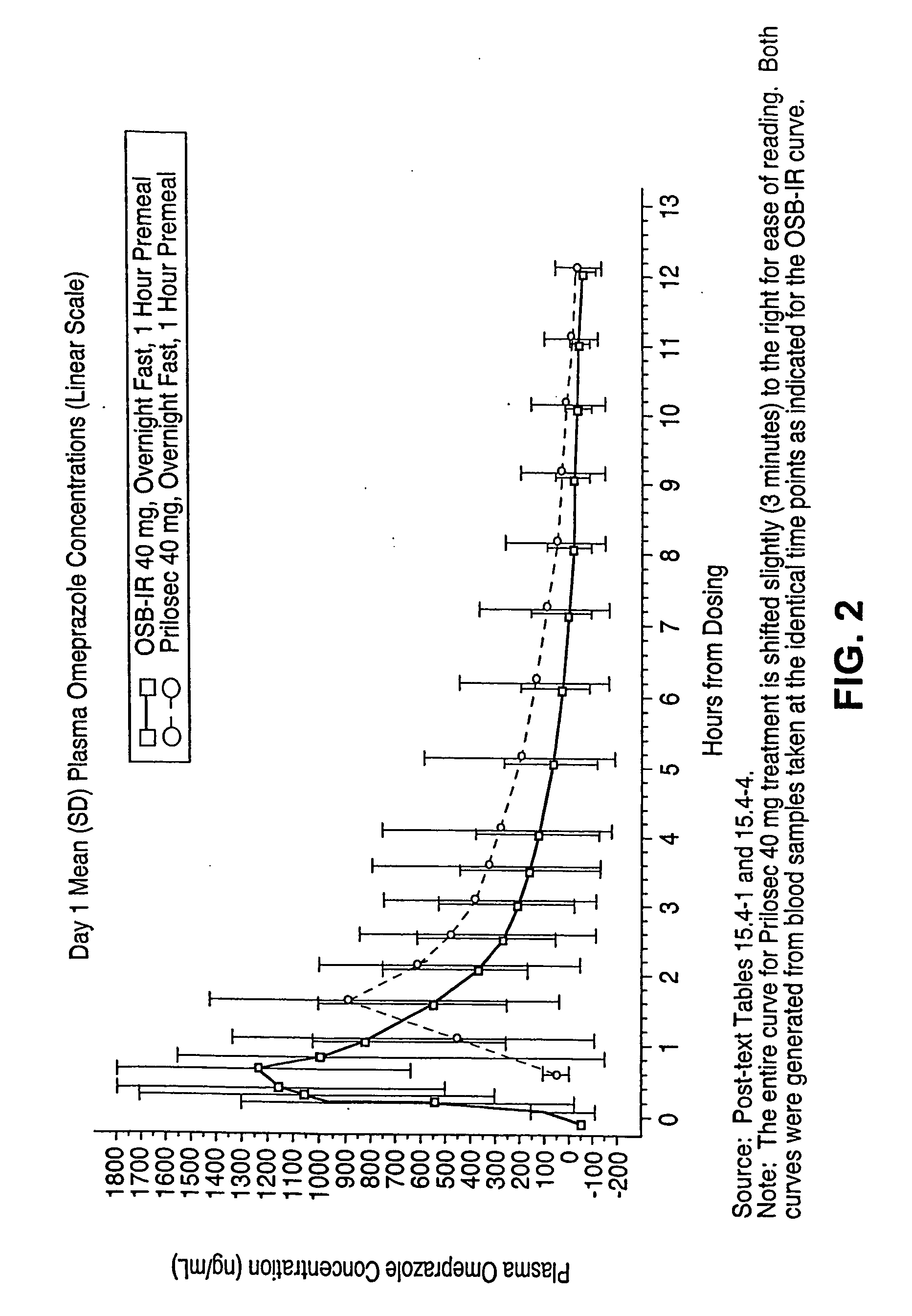
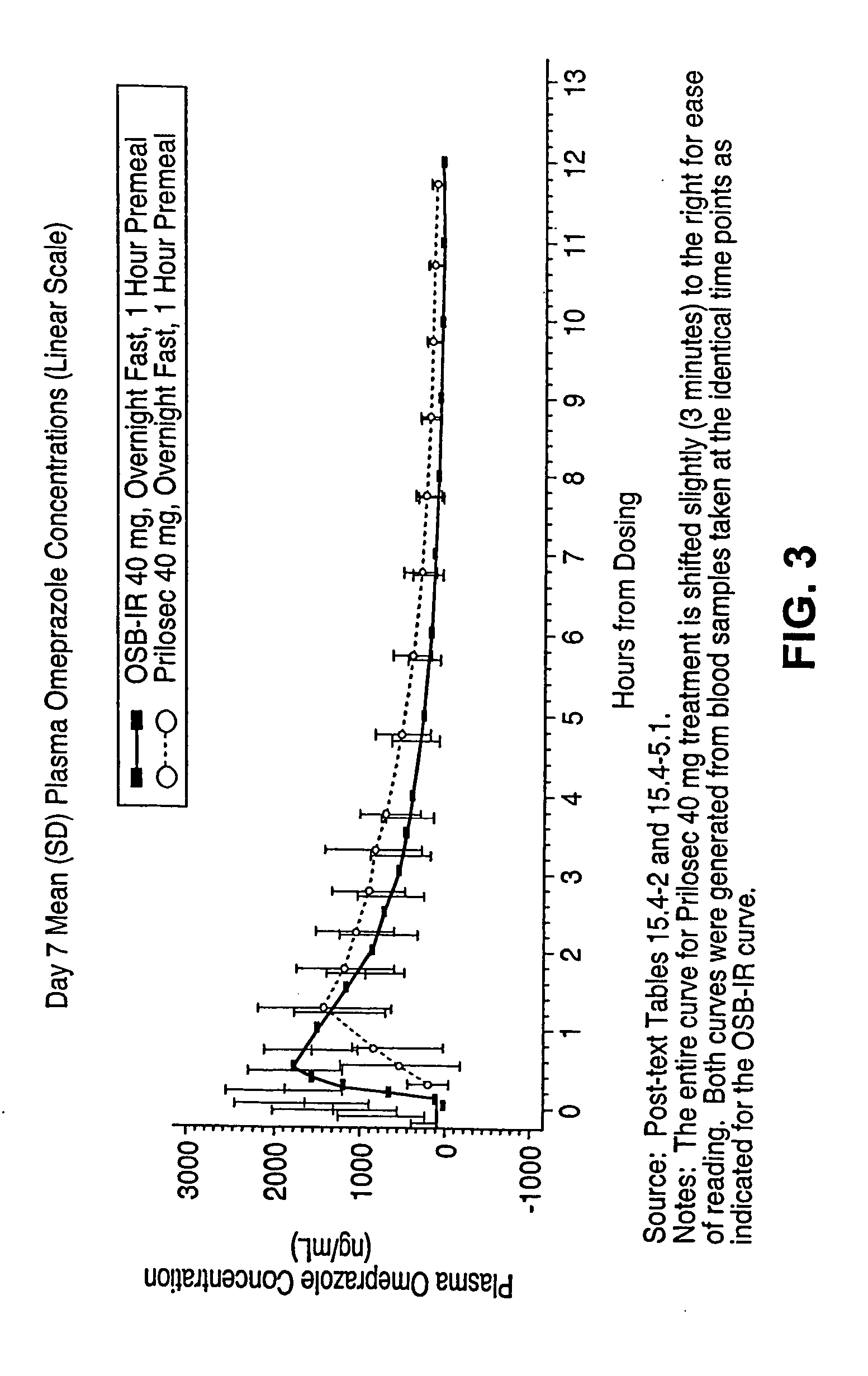
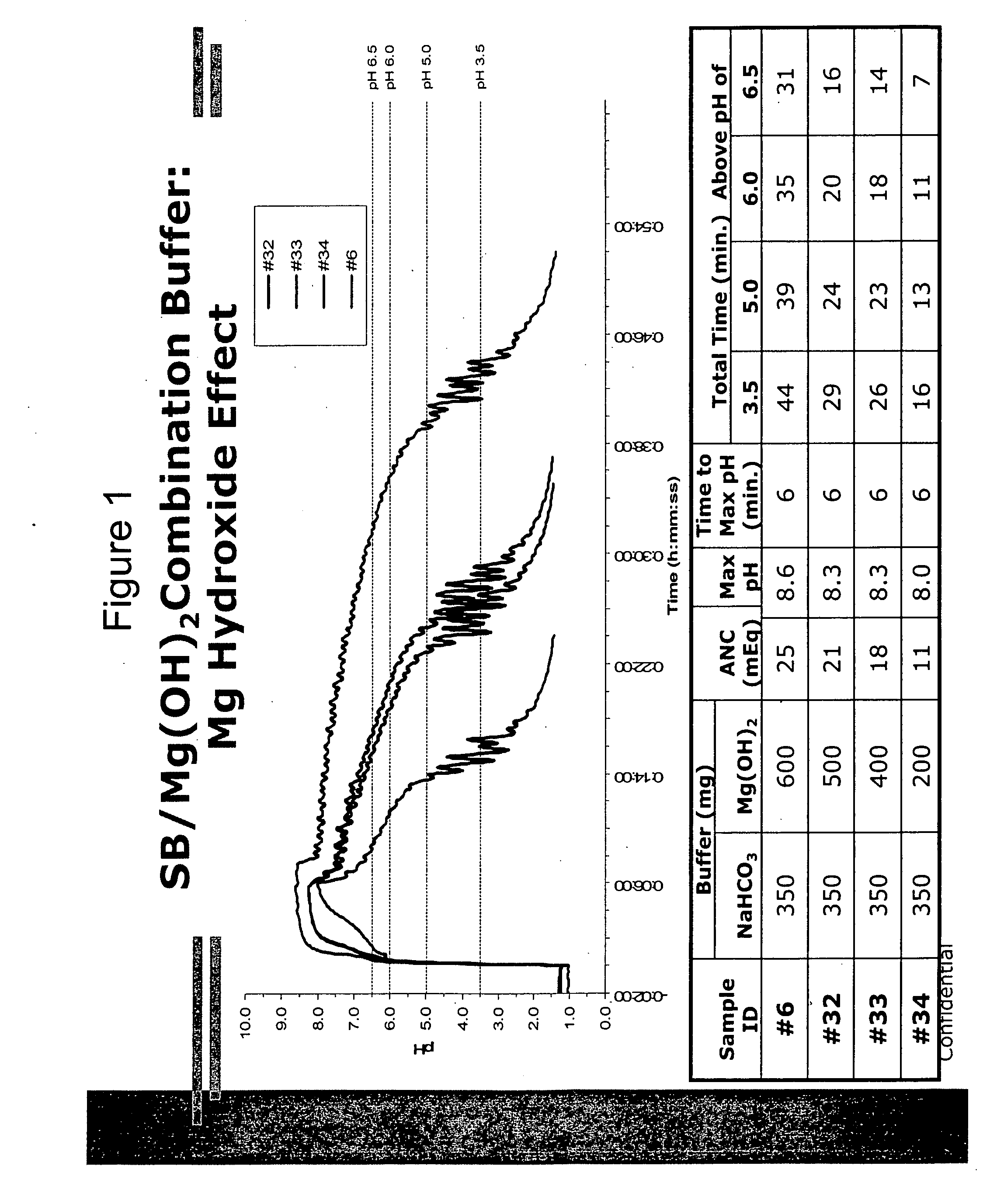
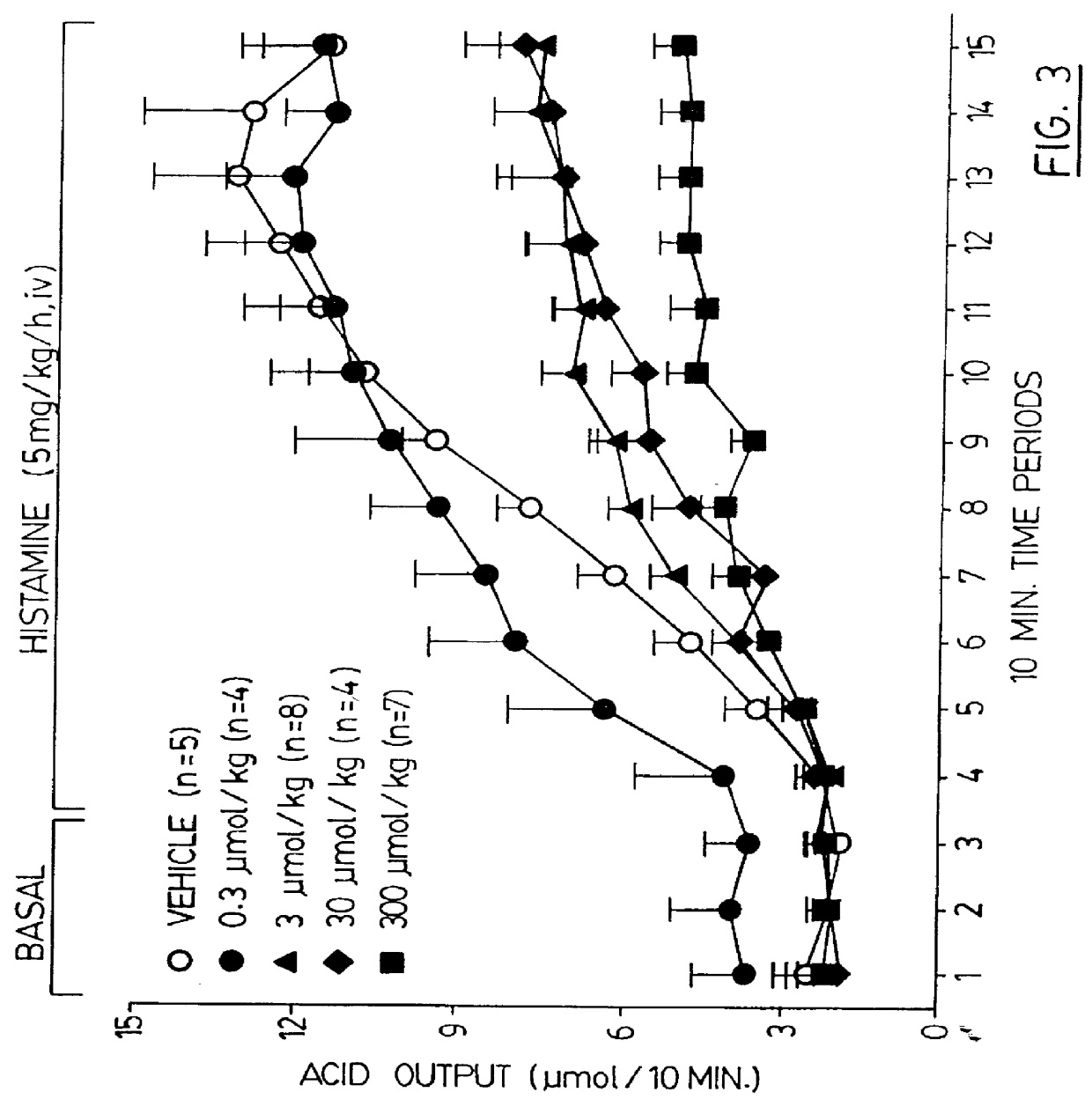
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
283 results about "Proton pump" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A proton pump is an integral membrane protein pump that builds up a proton gradient across a biological membrane. Mechanisms are based on energy-induced conformational changes of the protein structure or on the Q cycle.
Oral administration form for an acid liable active proton pump inhibitor
Novel administration form for acid-labile active compounds are described. The novel administration forms have no enteric layers and are suitable for oral administration.
Owner:TAKEDA GMBH
Neural stimulation device employing renewable chemical stimulation
A variety of neural stimulation devices are disclosed. The devices comprise an uptake component comprising means for selectively transporting a stimulating species into the device; a release component comprising means for releasing the stimulating species; and means for producing a concentration gradient of a second species. The concentration gradient of the second species provides energy to transport the stimulating species into the device. The stimulating species may be an ion, e.g., a potassium ion, or a neurotransmitter. In a preferred embodiment of the invention the stimulating species is a potassium ion. In a second preferred embodiment the stimulating species is dopamine. In certain embodiments of the invention countertransport across an uptake component comprising a synthetic ABA polymer membrane is achieved using a carboxylic acid crown ether. The gradient of the second species may be provided by means of a chemical reaction that takes place inside the device. The substrate for the chemical reaction is transported into the device from the external environment. In certain embodiments the neural stimulation device comprises light-sensitive elements that comprise light-sensitive proton pumps. The proton pumps translocate protons into the device in response to light, thereby triggering release of the stimulating species. In certain embodiments the neural stimulation device comprises electronic components that receive a signal and send an activating input to the device, thereby triggering release of the stimulating species.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +2
Pulsatile gastric retentive dosage forms
Dosage forms for delayed and pulsed release of therapeutic agents into the stomach are described. The dosage forms are gastric retentive dosage forms that achieve release of the therapeutic agent into the stomach and upper gastrointestinal tract subsequent to administration of the dosage form. The dosage forms find particular use in administration of acid-labile active agents such as proton pump inhibitors, and in treating gastric acid secretion such as gastro-esophageal reflux disease (GERD) and nocturnal acid breakthrough (NAB).
Owner:DEPOMED SYST INC
Controlled-release compositions comprising a proton pump inhibitor
The present invention relates to pharmaceutical compositions, and methods of preparing such compositions, comprising one or more populations of controlled-release particles comprising one or more proton pump inhibitors. The present invention also relates to pharmaceutical dosage forms, including orally disintegrating tablets, tablets, capsules, and methods for their preparation.
Owner:APTALIS PHARMA +1
Combination pain medication
InactiveUS20060177504A1Eliminate side effectsReduce riskBiocideSalicyclic acid active ingredientsSide effectMortality rate
This patent is an evolution of previous combination medication patents. Previous combination patents such as U.S. Pat. No. 6,613,354 which is a combination of an NSAID and Proton Pump Inhibitor. Thus the previous patents have covered gastrointestinal prophylaxis but none has covered both gastrointestinal and cardiovascular prophylaxis. This is likely because the cardiovascular side effects of NSAIDs were only recently discovered. This patent thus represents a leap in safety in a class of medication that is used by millions of Americans on a daily basis. This combination would thus decrease morbidity and mortality.
Owner:SUNDHARADAS RENJIT
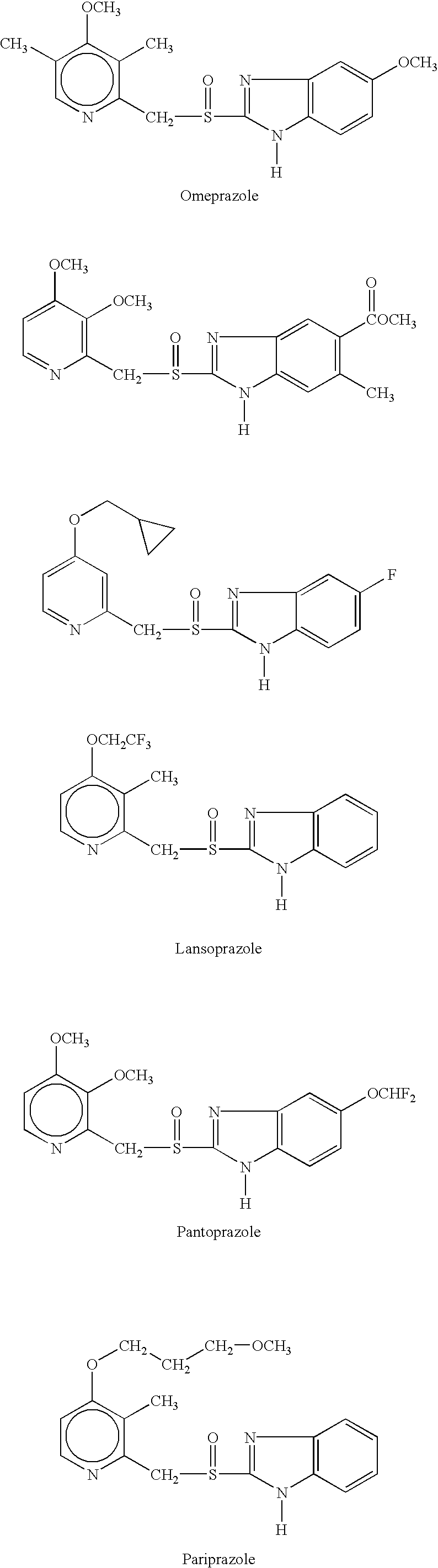
Substituted benzimidazole dosage forms and method of using same
InactiveUS7399772B2Easy to prepareImprove pharmacological activityBiocidePowder deliveryEffervescent PowderPharmaceutical formulation
The present invention relates to pharmaceutical preparations comprising substituted benzimidazole proton pump inhibitors. There is provided a liquid or solid pharmaceutical composition consisting of a proton pump inhibitor and at least one buffering agent. Also provided is a pharmaceutical composition further comprising a parietal cell activator, an anti-foaming agent, a flavoring agent and combinations thereof; a method for treating acid-related gastrointestinal disorders by administering a solid pharmaceutical composition; and, a kit for the preparation of a liquid oral pharmaceutical composition. Dosage forms include: liquid, powder, tablet, capsule, effervescent powder, effervescent tablet, pellets, and granules.
Owner:UNIVERSITY OF MISSOURI
Proton Pump Inhibitors
ActiveUS20080139639A1Improve isolationEasy to purifyBiocideOrganic chemistryAcyl groupPyrazolylchalcone
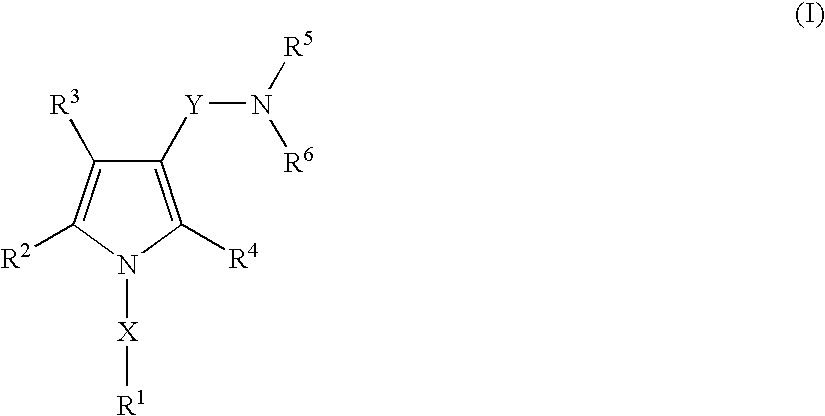
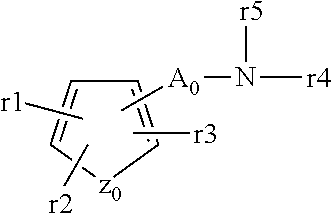
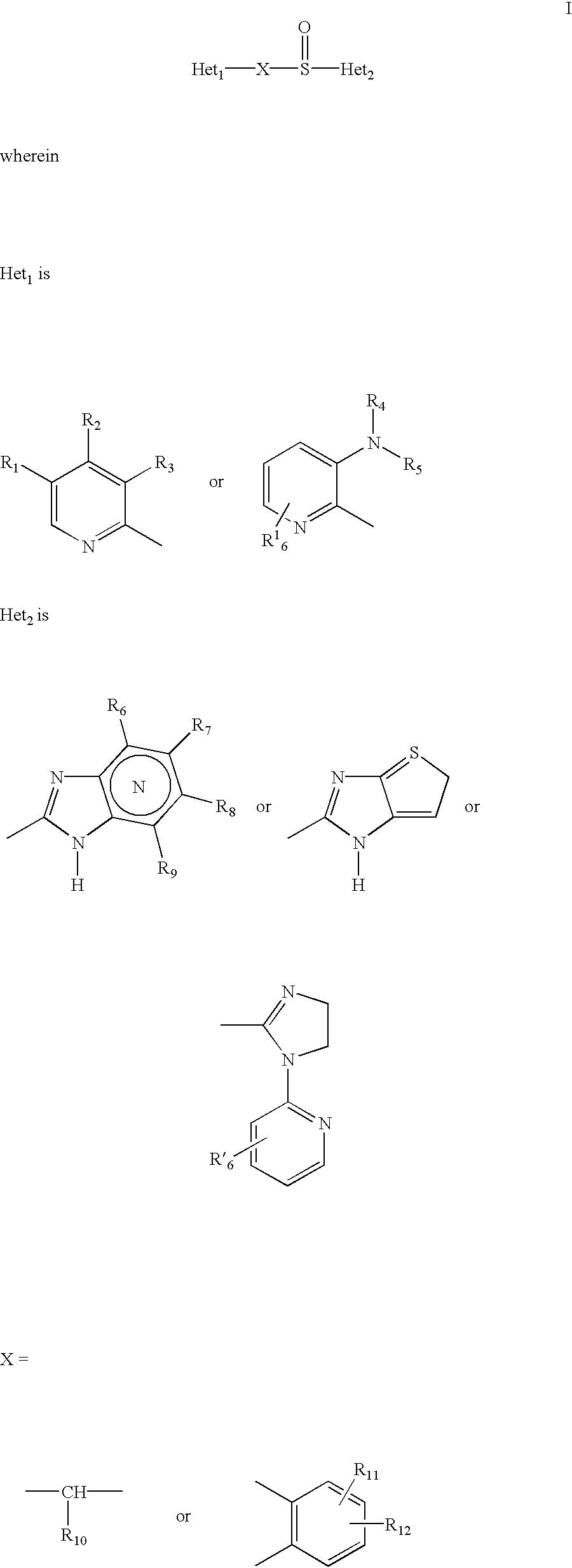
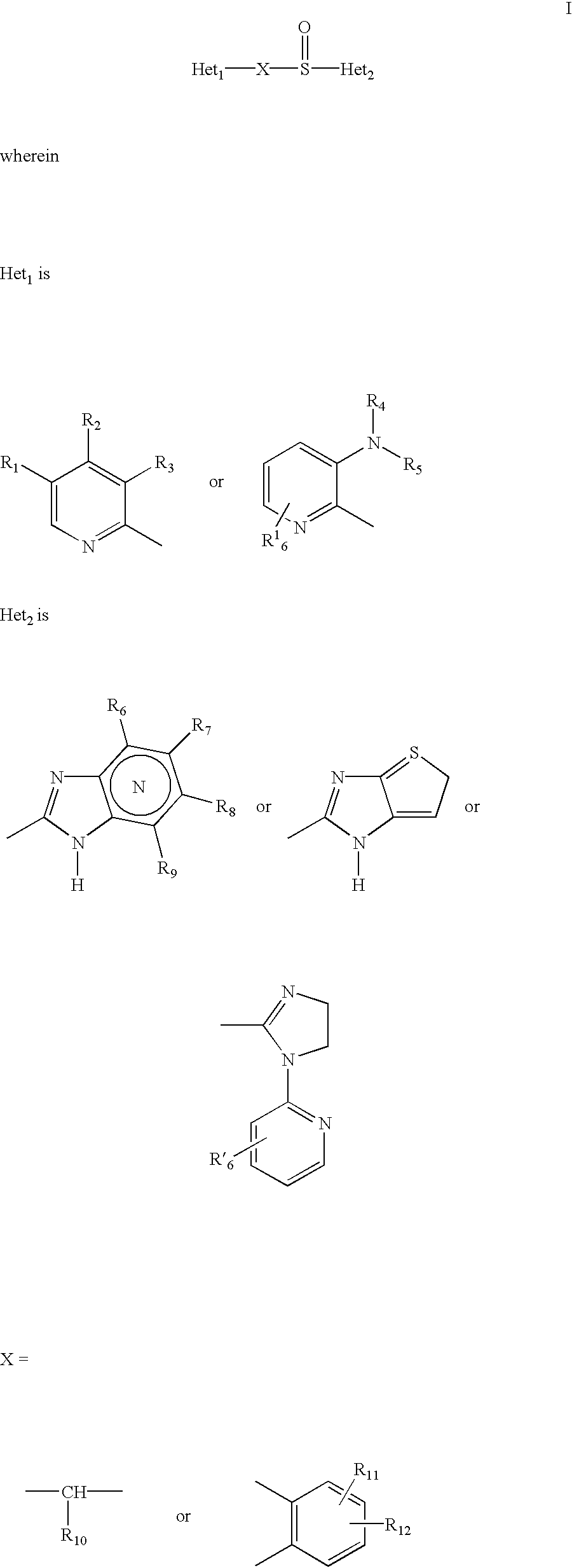
A proton pump inhibitor containing a compound represented by the formula (I)wherein X and Y are the same or different and each is a bond or a spacer having 1 to 20 carbon atoms in the main chain, R1 is an optionally substituted hydrocarbon group or an optionally substituted heterocyclic group, R2, R3 and R4 are the same or different and each is a hydrogen atom, an optionally substituted hydrocarbon group, an optionally substituted thienyl group, an optionally substituted benzo[b]thienyl group, an optionally substituted furyl group, an optionally substituted pyridyl group, an optionally substituted pyrazolyl group, an optionally substituted pyrimidinyl group, an acyl group, a halogen atom, a cyano group or a nitro group, R5 and R6 are the same or different and each is a hydrogen atom or an optionally substituted hydrocarbon group, which has a superior proton pump action and shows an antiulcer activity and the like after conversion to a proton pump inhibitor in the body, or a salt thereof. or a prodrug thereof is provided.
Owner:TAKEDA PHARMA CO LTD
Novel substituted benzimidazole dosage forms and method of using same
Disclosed herein are methods, kits, combinations, and compositions for treating gastric acid disorders employing pharmaceutical compositions comprising a proton pump inhibiting agent (PPI) and a buffering agent in a pharmaceutically acceptable carrier.
Owner:UNIVERSITY OF MISSOURI
Use of rifaximin for the prevention of aspiration pneumonia and/or sepsis
InactiveUS20060210592A1Organic active ingredientsDispersion deliveryAspiration pneumoniaEndocrinology
An oral preparation consisting of a non-systemic antibiotic and a proton pump blocker used for prevention of aspiration pneumonia and sepsis; and a method of prevention of aspiration pneumonia and / or sepsis by orally administering to a subject in need of such treatment a composition containing a therapeutically effective amount of rifaximin.
Owner:KODSI ROBERT E
New Combination Dosage Form
InactiveUS20070122470A1Salicyclic acid active ingredientsBiocideGastrointestinal complicationsSalicylic acid
The present invention relates to an oral pharmaceutical preparation for use in the prevention and / or reduction of gastrointestinal complications associated with the use of acetyl salicylic acid. The present preparation comprises a fixed oral dosage form comprising a proton pump inhibitor in combination with acetyl salicylic acid. Furthermore, the present invention refers to a method for the manufacture thereof and the use thereof in medicine. The present invention also relates to a specific combination comprising esomeprazole, or an alkaline salt thereof or a hydrated form of any one of them, and acetyl salicylic acid for use as a medicament for the prevention of thromboembolic vascular events, such as myocardial infarction or stroke, and for the prevention and / or reduction of gastrointestinal complications associated with the use of acetyl salicylic acid.
Owner:ASTRAZENECA AB
Composition and methods for inhibiting gastric acid secretion
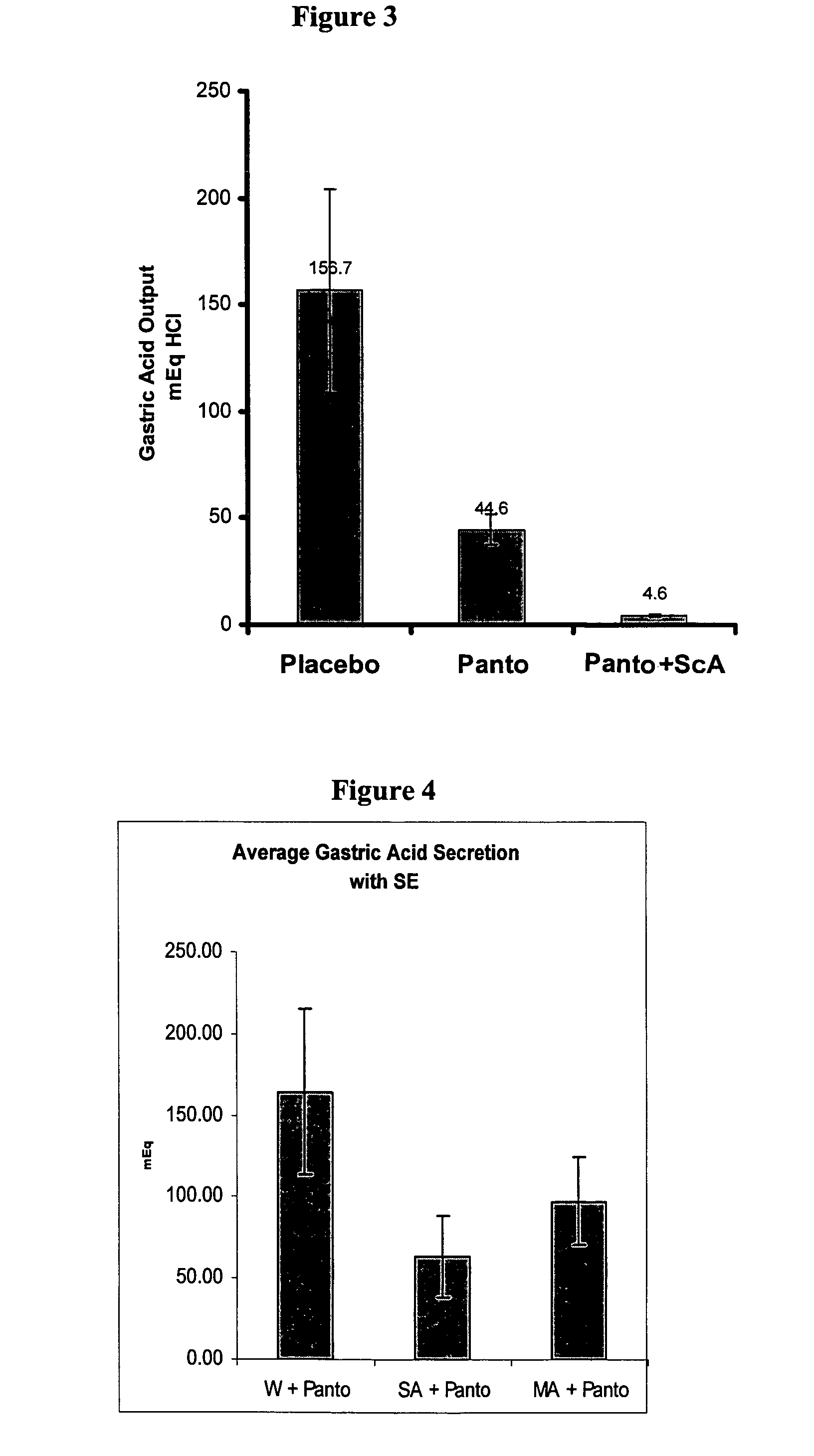
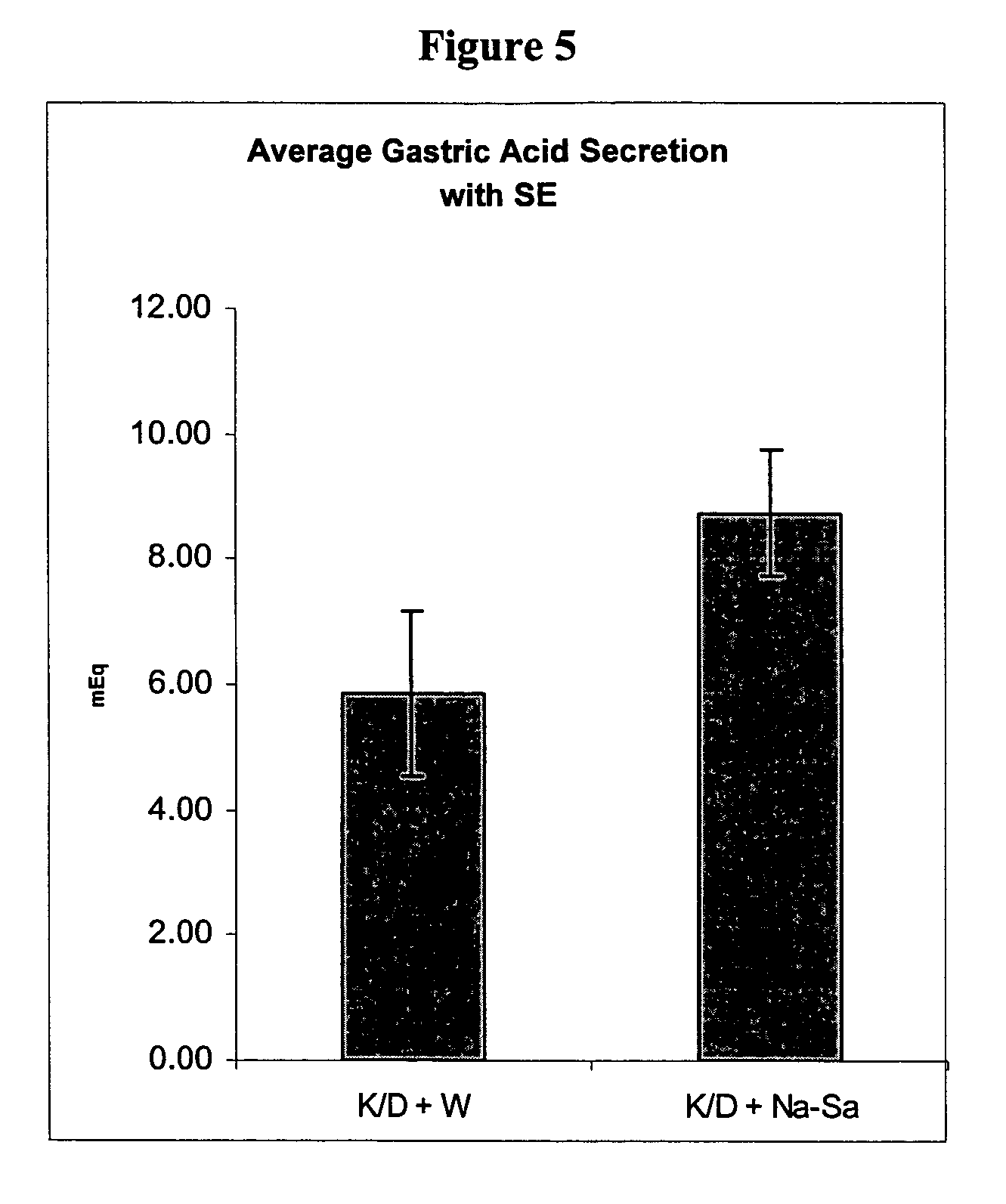
The present invention is related to oral compositions comprising an irreversible gastric H+ / K+-ATPase proton pump inhibitor (PPI) as a gastric acid secretion inhibitor and succinc acid as a parietal cell activator in the gastric lumen. The compositions of the present invention are capable of enhancing the anti-acid activity of PPI in the stomach. The present invention further relates to a method of using such compositions to reduce gastric acid secretion in a mammal.
Owner:VECTA
Esomeprazole sodium bicarbonate composition
InactiveCN102078616AImprove complianceQuick effectOrganic active ingredientsDigestive systemSodium bicarbonateEsomeprazole Sodium
The invention relates to an oral medicinal composition formulation for treating peptic ulcer. The composition comprises esomeprazole, antiacid sodium bicarbonate and a medicinally acceptable carrier, wherein the esomeprazole contains an acid-sensitive proton pump inhibitor; and the antiacid sodium bicarbonate can improve the stability of the proton pump inhibitor. The invention also relates to a preparation method of the medicinal composition. Two active ingredients are integrated into one fixed unit formulation, so that response is quick, a medicinal scheme is simplified in the process of treating the peptic ulcer, and the compliance of a patient is improved.
Owner:BEIJING HONGWAN PHARMA TECH
Controlled release composition
ActiveUS20060177509A1Reduce the amount requiredIncrease release rateDigestive systemPill deliveryControl releaseMedicine
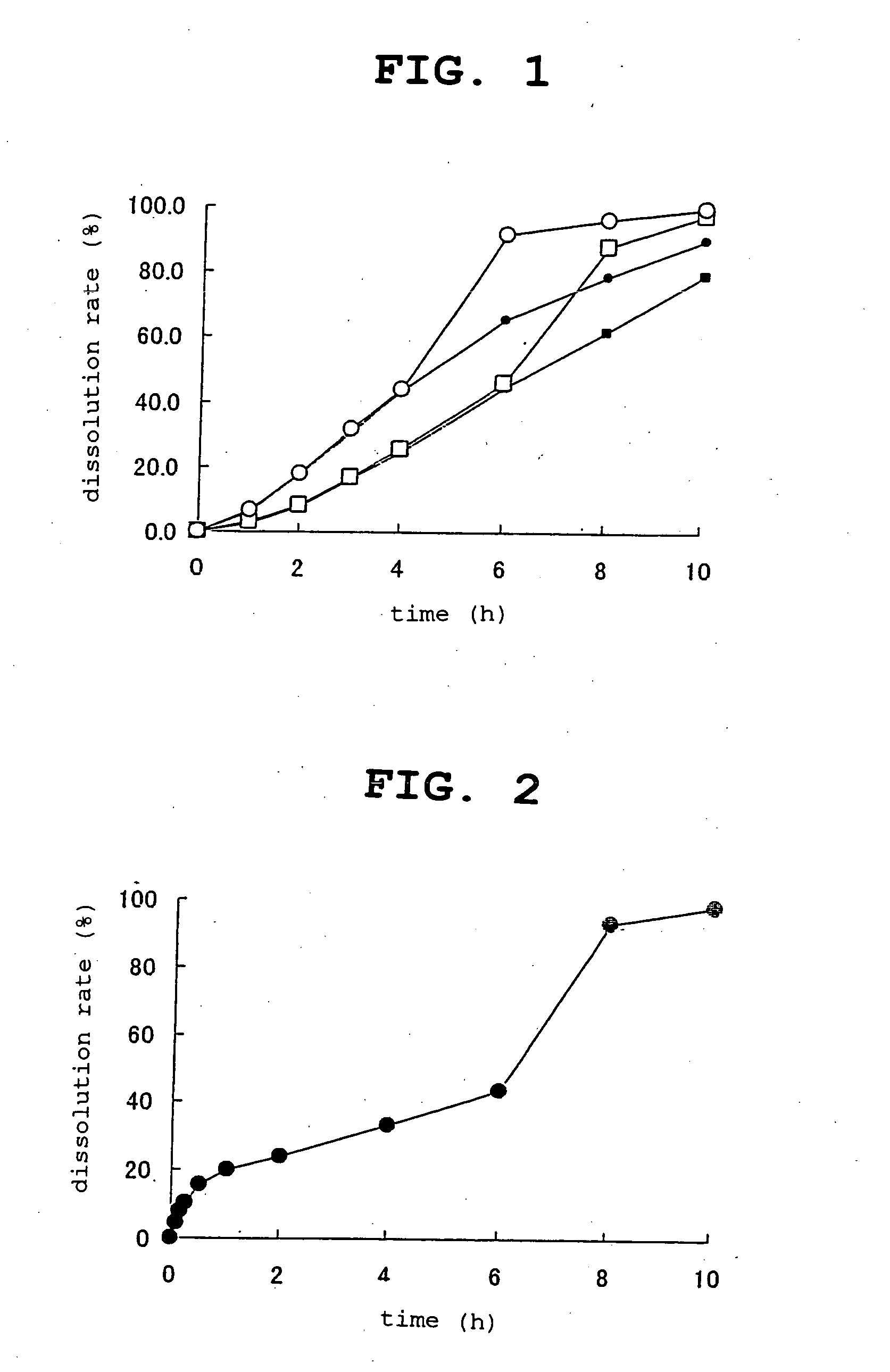
The present invention provides a controlled release composition showing release of an active ingredient (proton pump inhibitor) controlled in two or more steps at different release rates, which contains 1) a release-controlled part A capable of controlling release of the active ingredient to occur at a predetermined rate, 2) a release-controlled part B capable of controlling release of the active ingredient to occur at a predetermined rate lower than the release rate of the release-controlled part A, and where necessary, 3) a release-controlled part C capable of controlling release of the active ingredient to occur at a predetermined rate faster than the release rate of the release-controlled part B, wherein the release of the active ingredient from the release-controlled part B precedes the release of the active ingredient from the release-controlled part A (when release-controlled part C is contained, the release of the active ingredient from the release-controlled part C precedes the release of the active ingredient from the release-controlled part B).
Owner:TAKEDA PHARMA CO LTD
Proton pump inhibitors
Novel thiadiazole compounds are provided, which are effective as proton pumps inhibitors, useful in treating peptic ulcers by inhibition of the proton pump enzyme H+ / K+-ATPase. The compounds are 3-substituted 1,2,4-thiadiazolo [4,5- alpha ]benzimidazole and 3-substituted imidazo[1,2-d]-1,2,4-thiadiazoles corresponding to the general formula: where X and Z either represent an optionally substituted benzene ring fused to the diazole nucleus, or represent a variety of independent chemical groupings (hydrogen, lower alkyl, halo, etc.) and Y is selected from a wide range, e.g. heterocyclics and carbonyl groups.
Owner:KARIMIAN KHASHAYAR +5
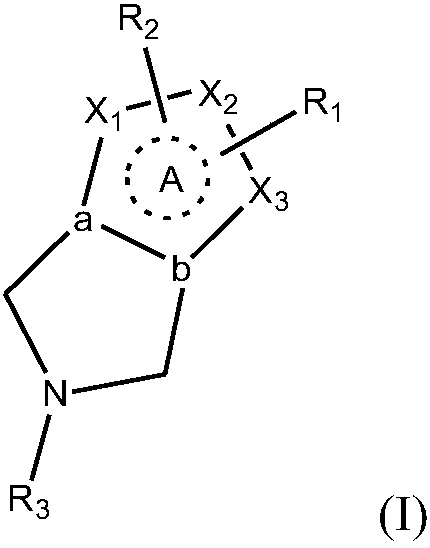
Aryl- or heteroaryl-sulfonyl compounds as acid secretion inhibitors
ActiveUS20110288040A1Low toxicityHigh expressionAntibacterial agentsBiocideAcyl groupProton-pump inhibitor
The present invention provides a compound having a superior acid secretion inhibitory action, an antiulcer activity and the like.A proton pump inhibitor containing a compound represented by the formula (I)wherein ring A is a saturated or unsaturated 5- or 6-membered ring group optionally having, as a ring-constituting atom besides carbon atom, 1 to 4 hetero atoms selected from a nitrogen atom, an oxygen atom and a sulfur atom, ring-constituting atoms X1 and X2 are each a carbon atom or a nitrogen atom, a ring-constituting atom X3 is a carbon atom, a nitrogen atom, an oxygen atom or a sulfur atom, R1 is an optionally substituted aryl group or an optionally substituted heteroaryl group, R2 is an optionally substituted alkyl group, an optionally substituted aryl group or an optionally substituted heteroaryl group, R3 is an aminomethyl group optionally substituted by 1 or 2 lower alkyl groups, which is a substituent on a ring-constituting atom other than X1, X2 and X3, and ring A optionally further has substituent(s) selected from a lower alkyl group, a halogen atom, a cyano group and an oxo group, or a salt thereof or a prodrug thereof.
Owner:TAKEDA PHARMA CO LTD
Medicine composition for treating diseases relating to gastric acid
InactiveCN101002939AQuick effectImprove stabilityPowder deliveryOrganic active ingredientsDiseaseDepressant
A composite medicine for treating the diseases associated with gastric acid contains the benzimidazole-type proton pump depressant, the buffer material for neutralizing acid and improving the stability of said proton pump depressant and pharmacologically acceptable assistants.
Owner:GUANGDONG HUANAN PHARMACEUTICAL GROUP CO LTD
Prevention and treatment of hypergastrinemia
Serum-associated hypergastrinemia is treated by administration of gastrin active or passive immunization. An anti-gastrin immunogenic composition containing a gastrin G17 or G34 peptide fragment which is amino acid spacer-linked to an immunogenic carrier, is administered so as to effectively neutralize the circulating gastrin hormone, and moreover, inhibit autocrine activity by progastrin such as Gly-extended G17, and amidated G17, in patients with pernicious anemia. Moreover, the method includes administration of a therapeutically effective amount of anti-G17 or anti-G34 antibodies which may be in humanized form. Finally, the method provides ameliorating treatment of hypergastrinemic effects of proton pump inhibitors or H2 histamine receptor blocking agents or antagonists, in addition to treatment of hypergastrinemia caused by diseases such as pernicious anemia.
Owner:CANCER ADVANCES INC
Azacycle-containing compound and preparation method and usage thereof
The invention discloses a compound or its salt shown in the formula (I) and usage of the compound as a potassium competitive acid blocker or a gastric acid secretion inhibitor. The compound has a proton pump (H+ / K+)-ATPase inhibitory effect and can be used for preventing, treating and inhibiting diseases related to gastric acid secretion, such as peptic ulcer, zollinger-ellison syndromes, gastritis, erosive esophagitis, reflux esophagitis and symptomatic gastroesophageal reflux diseases.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Pharmaceutical formulation and process
InactiveUS20030113375A1Digestive systemPharmaceutical non-active ingredientsWater solublePharmaceutical formulation
A new oral pharmaceutical dosage form comprising a core material that contains a proton pump inhibitor, one or more alkaline reacting compounds and optionally pharmaceutical excipients having a water soluble separating layer and an enteric coating layer. The core material as such is alkaline reacting and the separating layer between the alkaline reacting core material and the enteric coating layer is formed in situ as a water soluble salt between the alkaline reacting compound(s) and the enteric coating polymer. The invention also describes a new efficient process for the manufacture of such a dosage form comprising two functionally different layers in one manufacturing step, and its use in medicine.
Owner:ASTRAZENECA AB
Methods of treating gastro-esophogeal reflux disease using (-) norcisapride in combination with proton pump inhibitors or H2 receptor antagonists
InactiveUS6548518B2Prevent and alleviate symptomReducing and avoiding adverse effectBiocideDigestive systemNorcisapride5-HT3 receptor
Owner:SEPACOR INC
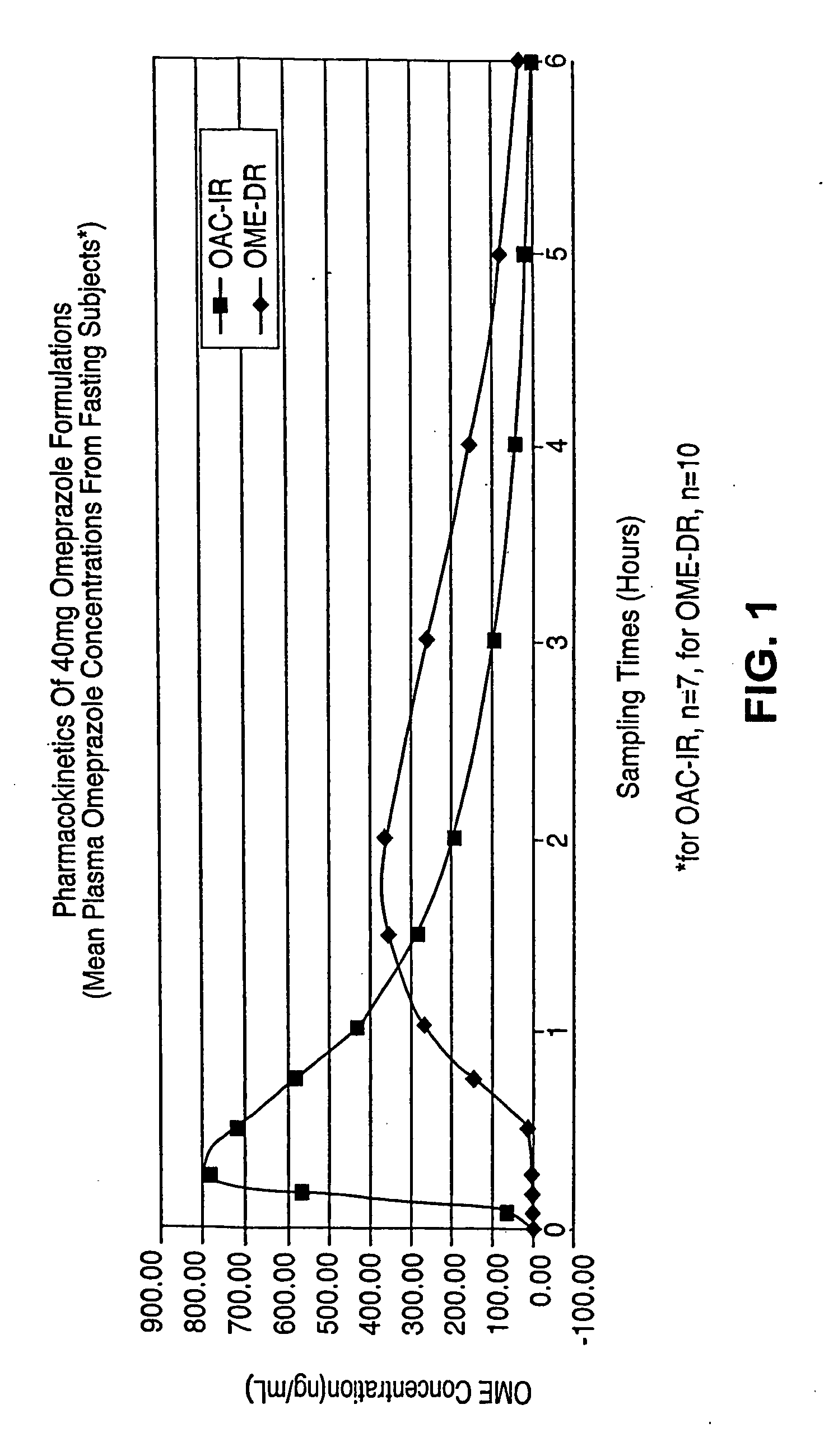
Novel formulation, omeprazole antacid complex-immediate release for rapid and sustained suppression of gastric acid
InactiveUS20050220870A1Reduce productionInhibit and reduce degradationBiocideDispersion deliveryImmediate releaseGastric fluid
The present invention is directed to methods, kits, combinations, and compositions for treating, preventing or reducing the risk of developing a gastrointestinal disorder or disease, or the symptoms associated with, or related to a gastrointestinal disorder or disease in a subject in need thereof. In one aspect, the present invention provides a pharmaceutical composition comprising a proton pump inhibiting agent and a buffering agent for oral administration and ingestion by a subject. Upon administration, the composition contacts the gastric fluid of the stomach and increases the gastric fluid pH of the stomach to a pH that substantially prevents or inhibits acid degradation of the proton pump inhibiting agent in the gastric fluid and allows a measurable serum concentration of the proton pump inhibiting agent to be absorbed into the blood serum of the subject.
Owner:SANTARUS
Dispersed tablet of proton pump inhibitor
InactiveCN101066251ASolve quality problemsSolve the costOrganic active ingredientsDigestive systemSodium bicarbonateDuodenal ulcer
The dispersed tablet of proton pump inhibitor is used for treating gastric ulcer, duodenal ulcer, stomal ulcer and other indications. It contains at least one kind of proton pump inhibitor and at least one kind of biologically acceptable buffering agent in the weight ratio of 1 to 10-200. The proton pump inhibitor is one selected from omeprazole, S-omeprazole, pantoprazole, lansoprazole, rabeprazole, leminoprazole, tenatoprazole and their salts. The biologically acceptable buffering agent is sodium bicarbonate, sodium carbonate, magnesium carbonate, etc or their mixture.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Pharmaceutical formulations useful for inhibiting acid secretion and methods for making and using them
The present invention relates to pharmaceutical formulations comprising at least one acid-labile proton pump inhibiting agent and at least one antacid, which have improved bioavailability, chemical stability, physical stability, dissolution profiles, disintegration times, safety, as well as other improved pharmacokinetic, pharmacodynamic, chemical and / or physical properties. The present invention is directed to methods, kits, combinations, and compositions for treating, preventing or reducing the risk of developing a gastrointestinal disorder or disease, or the symptoms associated with, or related to, a gastrointestinal disorder or disease in a subject in need thereof.
Owner:SANTARUS
Novel formulations of proton pump inhibitors and methods of using these formulations
InactiveUS20090092658A1Easy maintenanceTreat and prevents heartburnBiocidePowder deliveryGastrointestinal disorderDissolution
The present invention relates to combinations of a proton pump inhibiting agent and at least one buffering agent that have been found to possess improved bioavailability, chemical stability, physical stability, dissolution profiles, disintegration times, as well as other improved pharmacokinetic, pharmacodynamic, chemical and / or physical properties. The present invention is directed to methods, kits, combinations, and compositions for treating, preventing or reducing the risk of developing a gastrointestinal disorder or disease including nocturnal acid breakthrough, or the symptoms associated therewith
Owner:SANTARUS
Process for scavenging thiols
Thiols are trapped, and converted to disulfide compounds, by a process of reacting them with compounds containing a 1,2,4-thiadiazole ring structure carrying a substituent at position 3 of the thiadiazole ring, and being unsubstituted at position N-2. The process is useful pharmacologically, in inhibiting certain thiol-containing enzymes such as H+ / K+-ATPase (the proton pump), and industrially, in selective removal of thiol compounds from gas or liquid mixtures.
Owner:APOTEX TECH INC
Substituted benzimidazoles
InactiveUS20080255200A1Significant clinical effectReduce variationBiocideOrganic chemistryMedicinal chemistryBenzimidazole
Disclosed herein are substituted benzimidazole-based proton pump modulators of Formula I, processes of preparation thereof, pharmaceutical compositions thereof, and methods of use thereof.
Owner:AUSPEX PHARMA INC
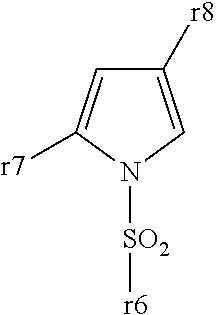
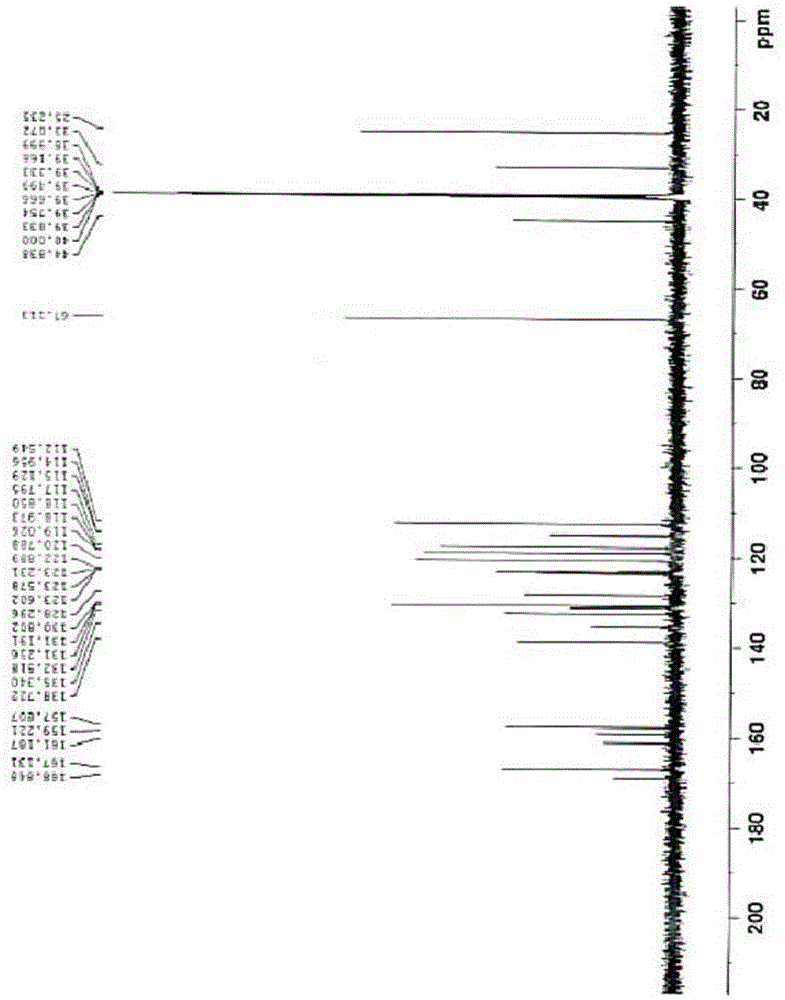
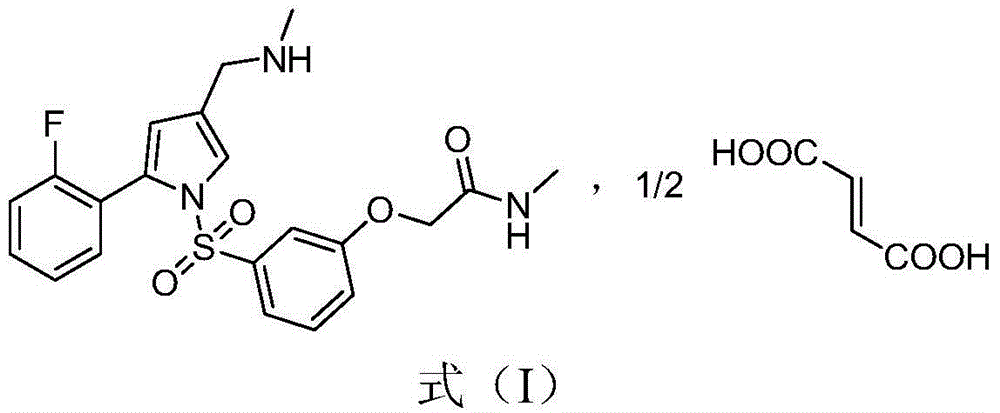
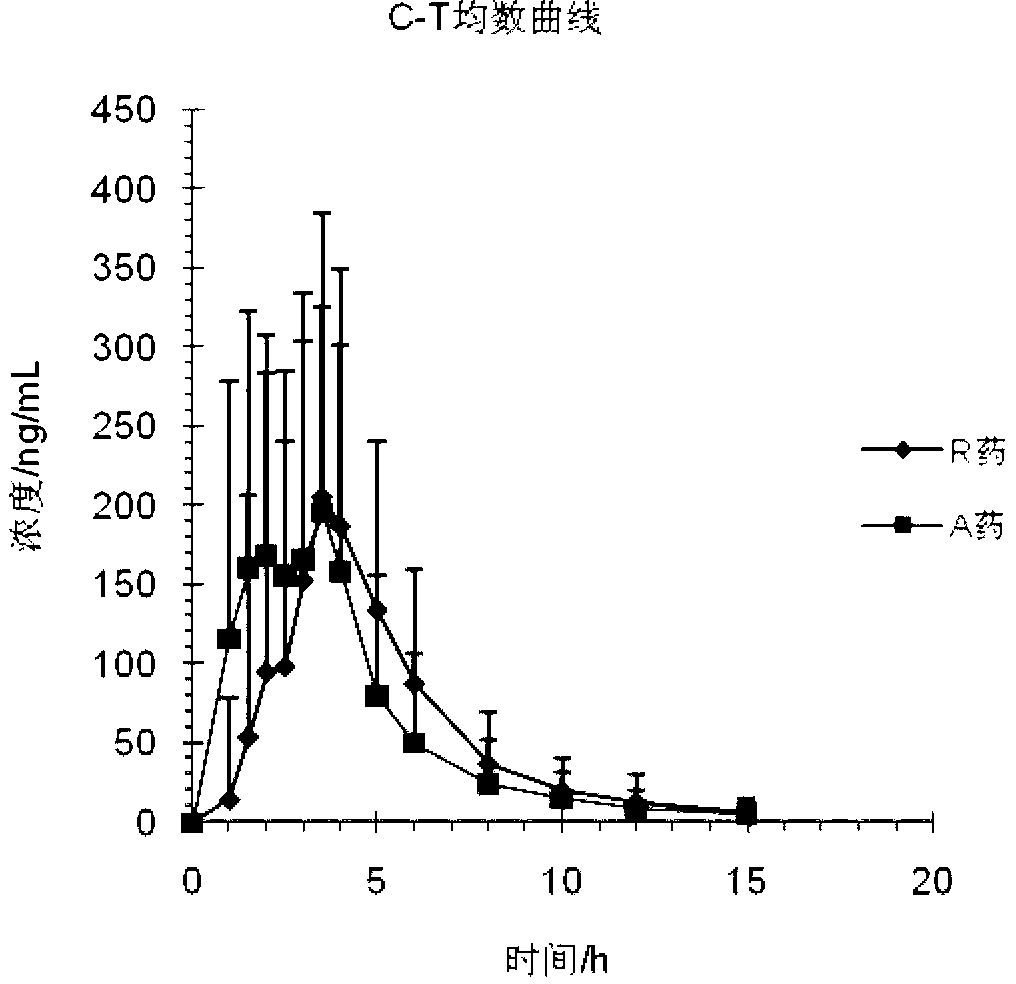
Pyrrole ring containing hemifumarate as proton pump inhibitor as well as intermediate and pharmaceutical application thereof
ActiveCN104447491AImprove stabilityImprove biological activityOrganic chemistryDigestive systemN-methylacetamideMethyl group
The invention discloses a pyrrole ring containing hemifumarate which serves as a proton pump inhibitor as well as an intermediate and pharmaceutical application thereof, and particularly discloses a pyrrole ring containing 2-(3-((2-(2-fluorophenyl)-4-((methylamino)methyl)-1H-pyrrol-1-yl)sulfonyl)phenoxy)-N-methylacetamide hemifumarate which serves as a proton pump inhibitor and has a formula (I) as shown in the specification, a method for synthesizing the same from 2-(3-((2-(2-fluorophenyl)-4-((methylamino)methyl)-1H-pyrrol-1-yl)sulfonyl)phenoxy)-N-methylacetamide and fumaric acid and an preparation intermediate of the 2-(3-((2-(2-fluorophenyl)-4-((methylamino)methyl)-1H-pyrrol-1-yl)sulfonyl)phenoxy)-N-methylacetamide hemifumarate. The pyrrole ring containing 2-(3-((2-(2-fluorophenyl)-4-((methylamino)methyl)-1H-pyrrol-1-yl)sulfonyl)phenoxy)-N-methylacetamide hemifumarate disclosed by the invention has good stability, excellent biological activity and a good gastric acid inhibition effect.
Owner:连云港恒运药业有限公司
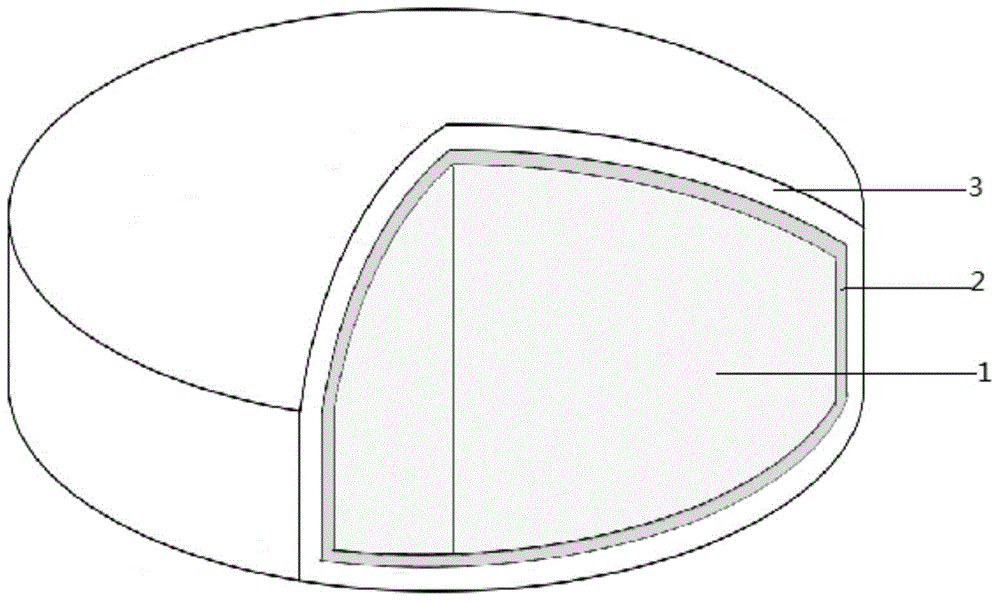
Enteric coating pellet of proton pump inhibitor
ActiveCN103340829AImprove stabilityImprove product qualityOrganic active ingredientsDigestive systemAlkalinityProton
The invention discloses an enteric coating pellet of a proton pump inhibitor. The enteric coating pellet comprises a blank pellet core, a drug carrying layer, an isolating layer and an enteric layer. The enteric coating pellet is characterized by comprising at least one alkali compound, wherein the weight of the alkali compound does not exceed 5% of the total weight of the pellet; the grain size D90 of the alkali compound is not greater than 75 microns. The enteric coating pellet preparation provided by the invention is capable of reducing the adsorption effect of the alkali compound to the drug and improving the release degree in vitro of the drug, and also capable of improving the alkalinity of the surrounding environment of the proton pump inhibitor and enhancing the stability of the drug.
Owner:珠海润都制药股份有限公司
Prodrugs of proton pump inhibitors
Owner:RGT UNIV OF CALIFORNIA +2
Preparation method of proton pump inhibitor enteric-coated tablet
ActiveCN104922086AImprove product qualityDigestive systemPharmaceutical delivery mechanismActive agentDissolution
The invention discloses a preparation method of a proton pump inhibitor enteric-coated tablet. The proton pump inhibitor enteric-coated tablet consists of a medicated tablet core, an isolating layer and an enteric-coated layer, wherein the medicated tablet core consists of active ingredients, filling agents, disintegrating agents, stabilizing agents, adhesion agents, surface active agents and lubricating agents; the isolating layer consists of film-forming agents, pore-foaming agents and hydrophobic materials; and the enteric-coated layer consists of an enteric-coated material, plasticizers, antisticking agents and light-screening agents. The formula and the preparation technology of the isolating layer are key and core technologies of controlling in-vitro release of drugs. By control on the formula and the preparation technology of the isolating layer and weight increment of the isolating layer, the releasing rate of the proton pump inhibitor enteric-coated tablet in different dissolution media with pH (potential of hydrogen) 1.2, pH 6.0, pH 6.8 and pH 8.0 and water. A prepared proton pump inhibitor enteric-coated tablet product is stable in quality and has a good market prospect.
Owner:珠海润都制药股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com